User login
Bullous Pemphigoid Masquerading as a Prosthesis Allergy
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
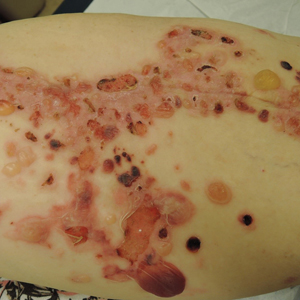
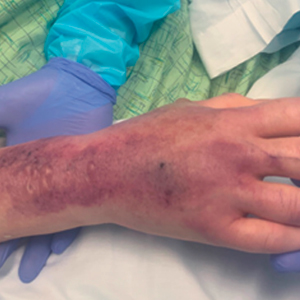
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.

Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.

Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.

Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
Practice Points
- Bullous pemphigoid frequently presents with urticarial plaques without classic tense blisters in the early phase of disease.
- The phenomenon of locus minoris resistentiae can lead to the presentation of bullous pemphigoid in locations traumatized by surgery.
- Bullous pemphigoid can present as urticarial plaques at surgery sites mimicking allergic contact dermatitis or reaction to surgical sutures or hardware.
Purpura Fulminans in an Asplenic Intravenous Drug User
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
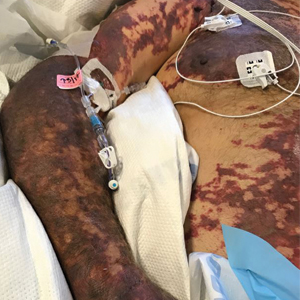
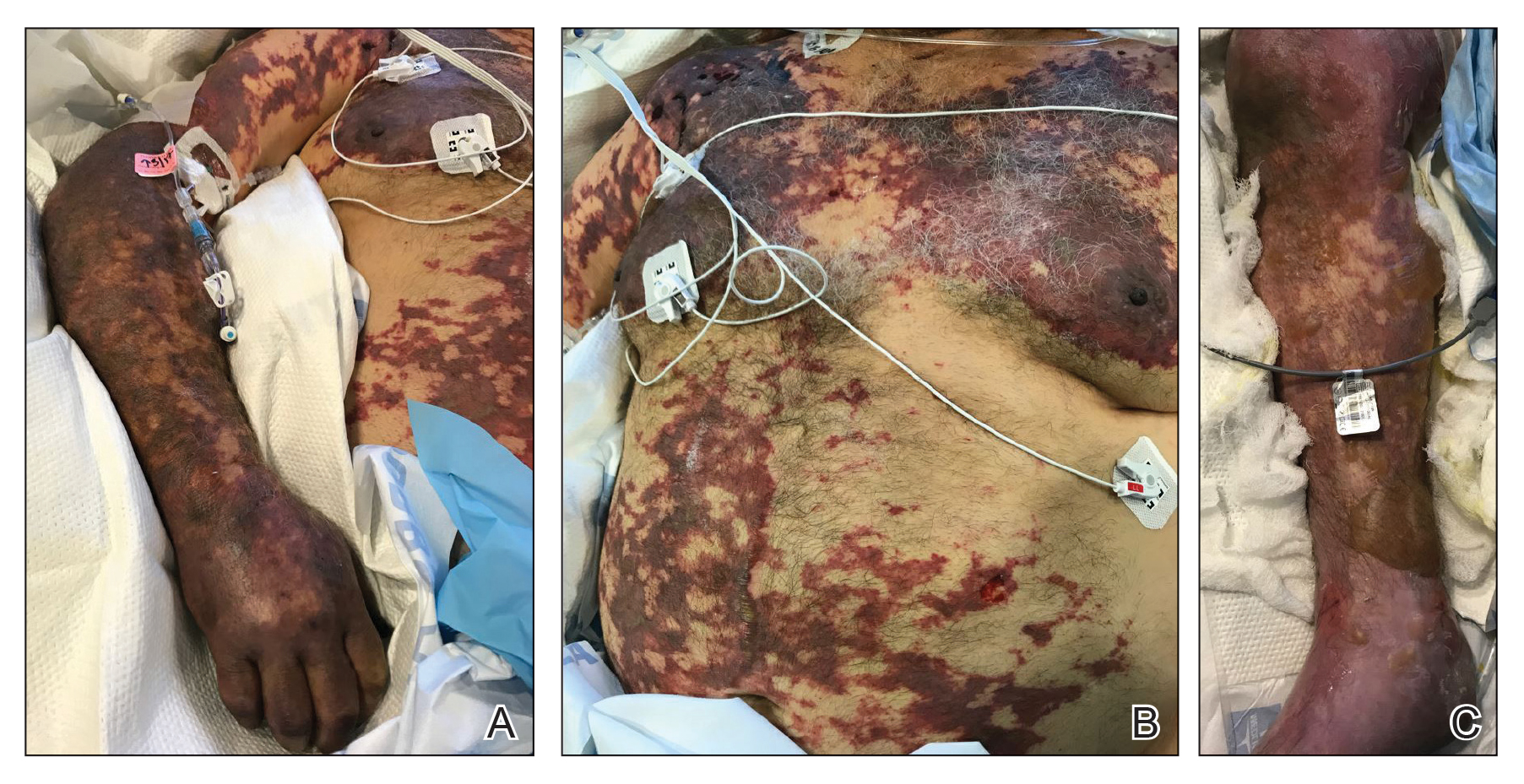
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
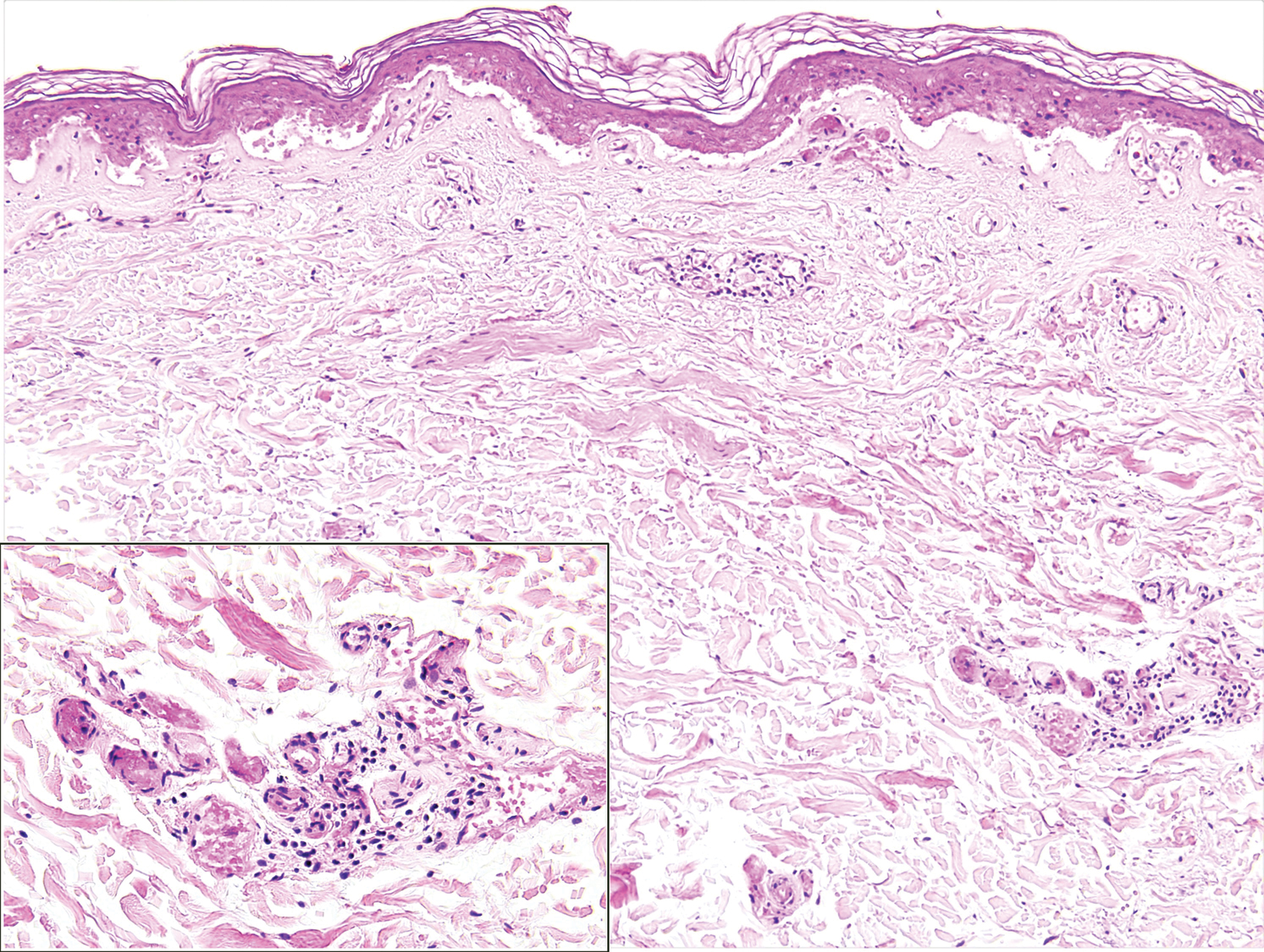
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
Practice Points
- Capnocytophaga species are fastidious, slow-growing microorganisms. It is important, therefore, to maintain a high degree of suspicion and alertthe microbiology laboratory to increase the likelihood of isolation.
- Patients should be cautioned regarding the need for prophylactic antibiotics in the event of an animal bite; asplenic patients are at particular risk for infection.
- In patients with severe purpura fulminans and a gangrenous limb, it is important to allow adequate time for demarcation of gangrene and not rush to amputation.
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
Enoxaparin-Induced Hemorrhagic Bullae at Sites of Trauma and Endothelial Pathology
To the Editor:
A 67-year-old man with diabetes mellitus was admitted to the hospital for exacerbation of congestive heart failure and atrial flutter with rapid ventricular response. He subsequently developed a non-ST segment elevation myocardial infarction and was started on subcutaneous enoxaparin 110 mg twice daily. On day 9 of hospitalization, small “blood blisters” on the legs were noted by the nurse, and dermatology was consulted.
Physical examination revealed tense hemorrhagic bullae with erythematous haloes scattered over the arms and legs and to a lesser extent on the trunk. The bullae were most concentrated at the surrounding subcutaneous injection sites of insulin and enoxaparin with secondary bruising (Figure 1). The lesions also were present on the legs, where pitting edema and capillaritis also were appreciated (Figure 2).
Laboratory workup for heparin-induced thrombocytopenia was negative. A diagnosis of enoxaparin-associated hemorrhagic bullae was made. Biopsy was recommended, but the patient declined based on anecdotal reports that the bullae typically self-resolve.
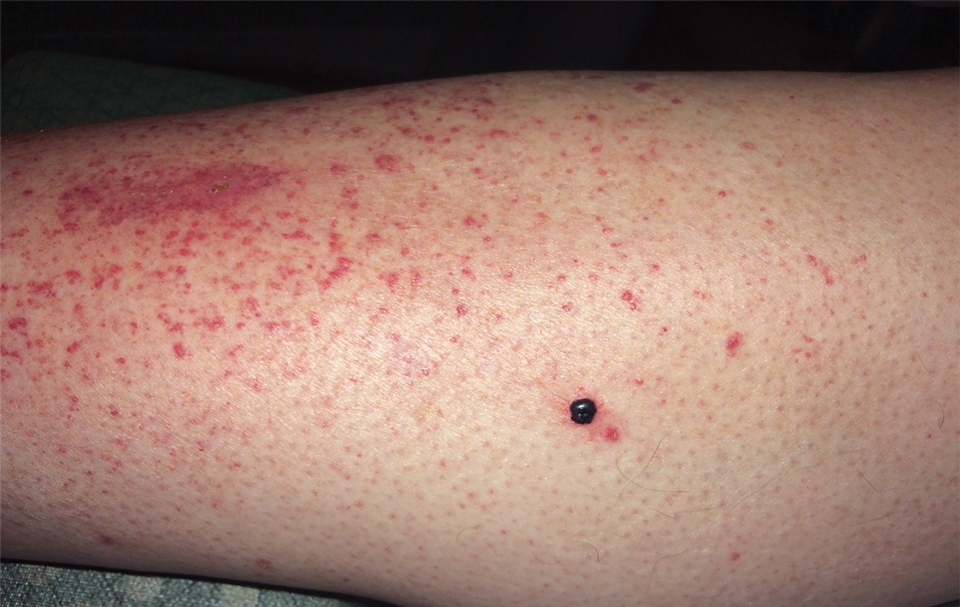
The enoxaparin was discontinued 7 days after the dermatology consultation, and the patient was transitioned to apixaban. A review of the medical record during the dermatology consultation revealed he had been on aspirin (81–385 mg/d) for 13 years prior to admission and had received prophylactic enoxaparin (40 mg/d) while hospitalized 2 and 7 years prior to the current episode of hemorrhagic bullae.
The patient declined outpatient dermatology follow-up; however, his cardiologist noted that the skin lesions had resolved at a 3-week postdischarge appointment. Approximately 5 months after discharge, the patient was re-treated by the cardiologist with enoxaparin 110 mg twice daily for 3 days to bridge to warfarin after he developed a deep vein thrombosis while taking apixaban. He did not develop hemorrhagic bullae upon retreatment with enoxaparin.
Heparin-induced hemorrhagic bullous dermatosis (HBD) has been associated with administration of both unfractionated and low-molecular-weight heparin.1 The condition typically develops 5 to 21 days after initiation of heparin as asymptomatic, purple-to-black bullae, sometimes with an erythematous halo.2,3 The arms and legs are the most common location, but the exact pathogenesis of the lesions remains unknown.3,4 Most cases resolve within weeks of discontinuing heparin, although some reports have suggested that discontinuation is unnecessary.3,4
Histopathologic analysis shows intraepidermal or subepidermal bullae with red blood cells and fibrin in the absence of vasculitis and intravascular thrombi.1,4 Immunofluorescence studies are negative.3 In a comprehensive review of HBD, the investigators hypothesized that the pathogenesis may be related to noninflammatory to pauci-inflammatory activation of basement membrane zone proteases or possibly epithelial or endothelial fragility in conjunction with trauma that causes disruption of the vascular endothelium (eg, subcutaneous injections, vasculitis).4
Our case is of particular interest because the bullae were strikingly limited to sites of subcutaneous injection and surrounding areas along with coexistent endothelial pathology on the lower legs (capillaritis and pitting edema). These clinical observations support trauma from the injections and altered endothelia as pathogenetic factors in HBD.
Of interest, our patient had 2 prior hospitalizations during which he received prophylactic enoxaparin and did not develop hemorrhagic bullae. Furthermore, repeat exposure to therapeutic dosing of enoxaparin with a shorter duration did not result in recurrence of HBD. This suggests that heparin dosing and duration of therapy also might be involved in the development of HBD.
Our hope is that future reports of HBD will address the presence or absence of coexistent cutaneous pathology, such as edema, stasis dermatitis, bruising, and capillaritis, along with heparin dosing, duration, and prior exposure to heparin treatment so that risk factors and pathogenesis can be further investigated. We also agree with Snow et al4 that HBD should be included as an outcome in future trials of heparin therapy.
- Komforti MK, Bressler ES, Selim MA, et al. A rare cutaneous manifestation of hemorrhagic bullae to low-molecular-weight heparin and fondaparinux: report of two cases: letter to the editor. J Cutan Pathol. 2017;44:104-106. doi:10.1111/cup.12821
- Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. JAMA Dermatol. 2013;149:871-872. doi:10.1001/jamadermatol.2013.3364a
- Gouveia AI, Lopes L, Soares-Almeida L, et al. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016;35:160-162. doi:10.3109/15569527.2015.1041033
- Snow SC, Pearson DR, Fathi R, et al. Heparin‐induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398. doi:10.1111/ced.13327
To the Editor:
A 67-year-old man with diabetes mellitus was admitted to the hospital for exacerbation of congestive heart failure and atrial flutter with rapid ventricular response. He subsequently developed a non-ST segment elevation myocardial infarction and was started on subcutaneous enoxaparin 110 mg twice daily. On day 9 of hospitalization, small “blood blisters” on the legs were noted by the nurse, and dermatology was consulted.
Physical examination revealed tense hemorrhagic bullae with erythematous haloes scattered over the arms and legs and to a lesser extent on the trunk. The bullae were most concentrated at the surrounding subcutaneous injection sites of insulin and enoxaparin with secondary bruising (Figure 1). The lesions also were present on the legs, where pitting edema and capillaritis also were appreciated (Figure 2).

Laboratory workup for heparin-induced thrombocytopenia was negative. A diagnosis of enoxaparin-associated hemorrhagic bullae was made. Biopsy was recommended, but the patient declined based on anecdotal reports that the bullae typically self-resolve.

The enoxaparin was discontinued 7 days after the dermatology consultation, and the patient was transitioned to apixaban. A review of the medical record during the dermatology consultation revealed he had been on aspirin (81–385 mg/d) for 13 years prior to admission and had received prophylactic enoxaparin (40 mg/d) while hospitalized 2 and 7 years prior to the current episode of hemorrhagic bullae.
The patient declined outpatient dermatology follow-up; however, his cardiologist noted that the skin lesions had resolved at a 3-week postdischarge appointment. Approximately 5 months after discharge, the patient was re-treated by the cardiologist with enoxaparin 110 mg twice daily for 3 days to bridge to warfarin after he developed a deep vein thrombosis while taking apixaban. He did not develop hemorrhagic bullae upon retreatment with enoxaparin.
Heparin-induced hemorrhagic bullous dermatosis (HBD) has been associated with administration of both unfractionated and low-molecular-weight heparin.1 The condition typically develops 5 to 21 days after initiation of heparin as asymptomatic, purple-to-black bullae, sometimes with an erythematous halo.2,3 The arms and legs are the most common location, but the exact pathogenesis of the lesions remains unknown.3,4 Most cases resolve within weeks of discontinuing heparin, although some reports have suggested that discontinuation is unnecessary.3,4
Histopathologic analysis shows intraepidermal or subepidermal bullae with red blood cells and fibrin in the absence of vasculitis and intravascular thrombi.1,4 Immunofluorescence studies are negative.3 In a comprehensive review of HBD, the investigators hypothesized that the pathogenesis may be related to noninflammatory to pauci-inflammatory activation of basement membrane zone proteases or possibly epithelial or endothelial fragility in conjunction with trauma that causes disruption of the vascular endothelium (eg, subcutaneous injections, vasculitis).4
Our case is of particular interest because the bullae were strikingly limited to sites of subcutaneous injection and surrounding areas along with coexistent endothelial pathology on the lower legs (capillaritis and pitting edema). These clinical observations support trauma from the injections and altered endothelia as pathogenetic factors in HBD.
Of interest, our patient had 2 prior hospitalizations during which he received prophylactic enoxaparin and did not develop hemorrhagic bullae. Furthermore, repeat exposure to therapeutic dosing of enoxaparin with a shorter duration did not result in recurrence of HBD. This suggests that heparin dosing and duration of therapy also might be involved in the development of HBD.
Our hope is that future reports of HBD will address the presence or absence of coexistent cutaneous pathology, such as edema, stasis dermatitis, bruising, and capillaritis, along with heparin dosing, duration, and prior exposure to heparin treatment so that risk factors and pathogenesis can be further investigated. We also agree with Snow et al4 that HBD should be included as an outcome in future trials of heparin therapy.
To the Editor:
A 67-year-old man with diabetes mellitus was admitted to the hospital for exacerbation of congestive heart failure and atrial flutter with rapid ventricular response. He subsequently developed a non-ST segment elevation myocardial infarction and was started on subcutaneous enoxaparin 110 mg twice daily. On day 9 of hospitalization, small “blood blisters” on the legs were noted by the nurse, and dermatology was consulted.
Physical examination revealed tense hemorrhagic bullae with erythematous haloes scattered over the arms and legs and to a lesser extent on the trunk. The bullae were most concentrated at the surrounding subcutaneous injection sites of insulin and enoxaparin with secondary bruising (Figure 1). The lesions also were present on the legs, where pitting edema and capillaritis also were appreciated (Figure 2).

Laboratory workup for heparin-induced thrombocytopenia was negative. A diagnosis of enoxaparin-associated hemorrhagic bullae was made. Biopsy was recommended, but the patient declined based on anecdotal reports that the bullae typically self-resolve.

The enoxaparin was discontinued 7 days after the dermatology consultation, and the patient was transitioned to apixaban. A review of the medical record during the dermatology consultation revealed he had been on aspirin (81–385 mg/d) for 13 years prior to admission and had received prophylactic enoxaparin (40 mg/d) while hospitalized 2 and 7 years prior to the current episode of hemorrhagic bullae.
The patient declined outpatient dermatology follow-up; however, his cardiologist noted that the skin lesions had resolved at a 3-week postdischarge appointment. Approximately 5 months after discharge, the patient was re-treated by the cardiologist with enoxaparin 110 mg twice daily for 3 days to bridge to warfarin after he developed a deep vein thrombosis while taking apixaban. He did not develop hemorrhagic bullae upon retreatment with enoxaparin.
Heparin-induced hemorrhagic bullous dermatosis (HBD) has been associated with administration of both unfractionated and low-molecular-weight heparin.1 The condition typically develops 5 to 21 days after initiation of heparin as asymptomatic, purple-to-black bullae, sometimes with an erythematous halo.2,3 The arms and legs are the most common location, but the exact pathogenesis of the lesions remains unknown.3,4 Most cases resolve within weeks of discontinuing heparin, although some reports have suggested that discontinuation is unnecessary.3,4
Histopathologic analysis shows intraepidermal or subepidermal bullae with red blood cells and fibrin in the absence of vasculitis and intravascular thrombi.1,4 Immunofluorescence studies are negative.3 In a comprehensive review of HBD, the investigators hypothesized that the pathogenesis may be related to noninflammatory to pauci-inflammatory activation of basement membrane zone proteases or possibly epithelial or endothelial fragility in conjunction with trauma that causes disruption of the vascular endothelium (eg, subcutaneous injections, vasculitis).4
Our case is of particular interest because the bullae were strikingly limited to sites of subcutaneous injection and surrounding areas along with coexistent endothelial pathology on the lower legs (capillaritis and pitting edema). These clinical observations support trauma from the injections and altered endothelia as pathogenetic factors in HBD.
Of interest, our patient had 2 prior hospitalizations during which he received prophylactic enoxaparin and did not develop hemorrhagic bullae. Furthermore, repeat exposure to therapeutic dosing of enoxaparin with a shorter duration did not result in recurrence of HBD. This suggests that heparin dosing and duration of therapy also might be involved in the development of HBD.
Our hope is that future reports of HBD will address the presence or absence of coexistent cutaneous pathology, such as edema, stasis dermatitis, bruising, and capillaritis, along with heparin dosing, duration, and prior exposure to heparin treatment so that risk factors and pathogenesis can be further investigated. We also agree with Snow et al4 that HBD should be included as an outcome in future trials of heparin therapy.
- Komforti MK, Bressler ES, Selim MA, et al. A rare cutaneous manifestation of hemorrhagic bullae to low-molecular-weight heparin and fondaparinux: report of two cases: letter to the editor. J Cutan Pathol. 2017;44:104-106. doi:10.1111/cup.12821
- Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. JAMA Dermatol. 2013;149:871-872. doi:10.1001/jamadermatol.2013.3364a
- Gouveia AI, Lopes L, Soares-Almeida L, et al. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016;35:160-162. doi:10.3109/15569527.2015.1041033
- Snow SC, Pearson DR, Fathi R, et al. Heparin‐induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398. doi:10.1111/ced.13327
- Komforti MK, Bressler ES, Selim MA, et al. A rare cutaneous manifestation of hemorrhagic bullae to low-molecular-weight heparin and fondaparinux: report of two cases: letter to the editor. J Cutan Pathol. 2017;44:104-106. doi:10.1111/cup.12821
- Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. JAMA Dermatol. 2013;149:871-872. doi:10.1001/jamadermatol.2013.3364a
- Gouveia AI, Lopes L, Soares-Almeida L, et al. Bullous hemorrhagic dermatosis induced by enoxaparin. Cutan Ocul Toxicol. 2016;35:160-162. doi:10.3109/15569527.2015.1041033
- Snow SC, Pearson DR, Fathi R, et al. Heparin‐induced haemorrhagic bullous dermatosis. Clin Exp Dermatol. 2018;43:393-398. doi:10.1111/ced.13327
Vetiver: More than a pleasant aroma?
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
Skin ulcers can pose tricky diagnostic challenges
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
FROM PDA 2021
Novel diabetic foot ulcer cream shows promise in phase 3 trial
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Exsanguinating the truth about dragon’s blood in cosmeceuticals
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
An Algorithm for Managing Spitting Sutures
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
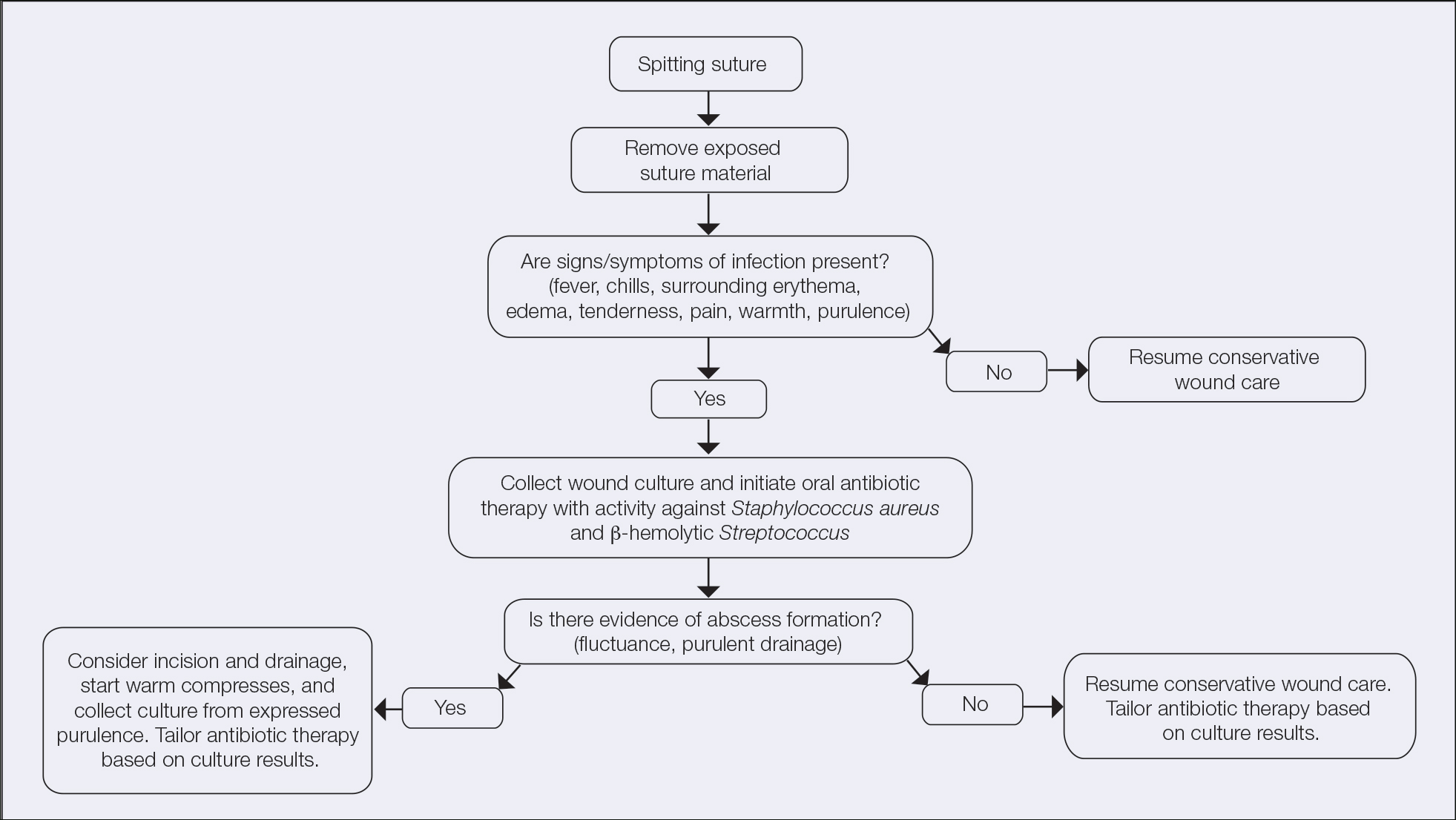
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).

Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.

Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).

Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.

- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
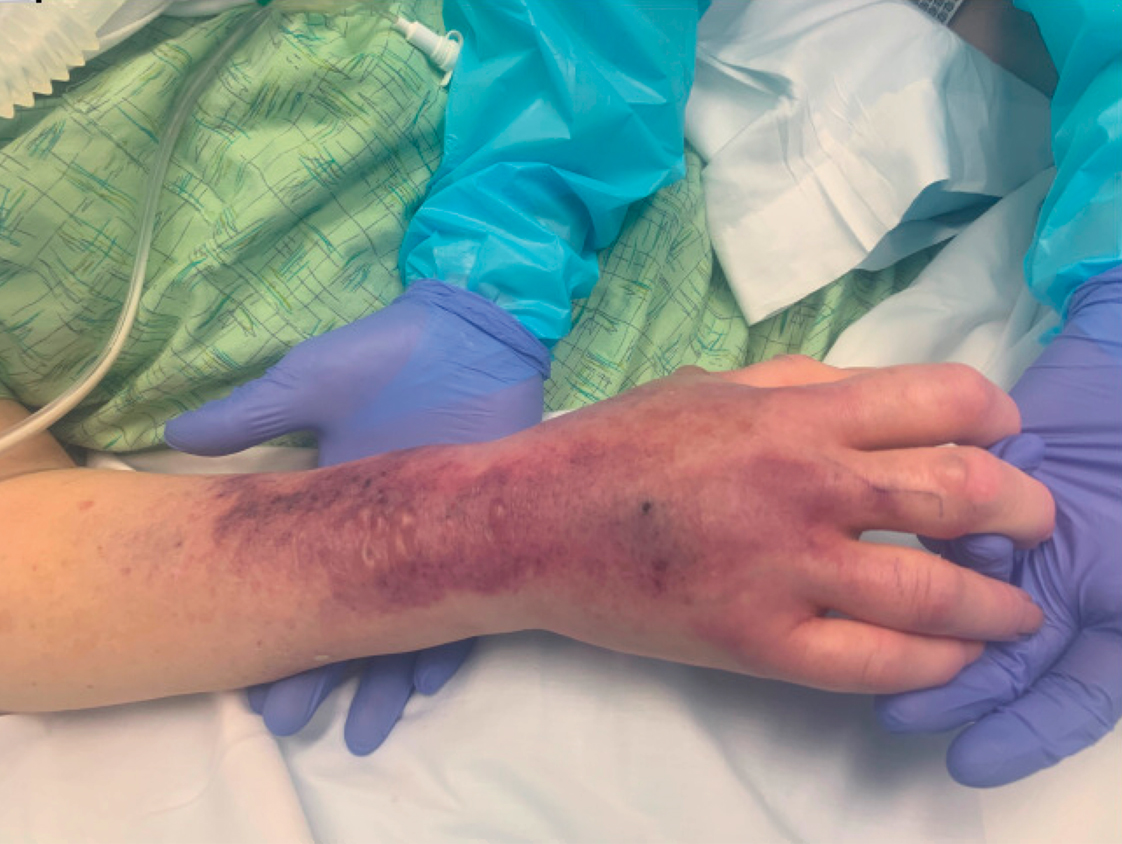
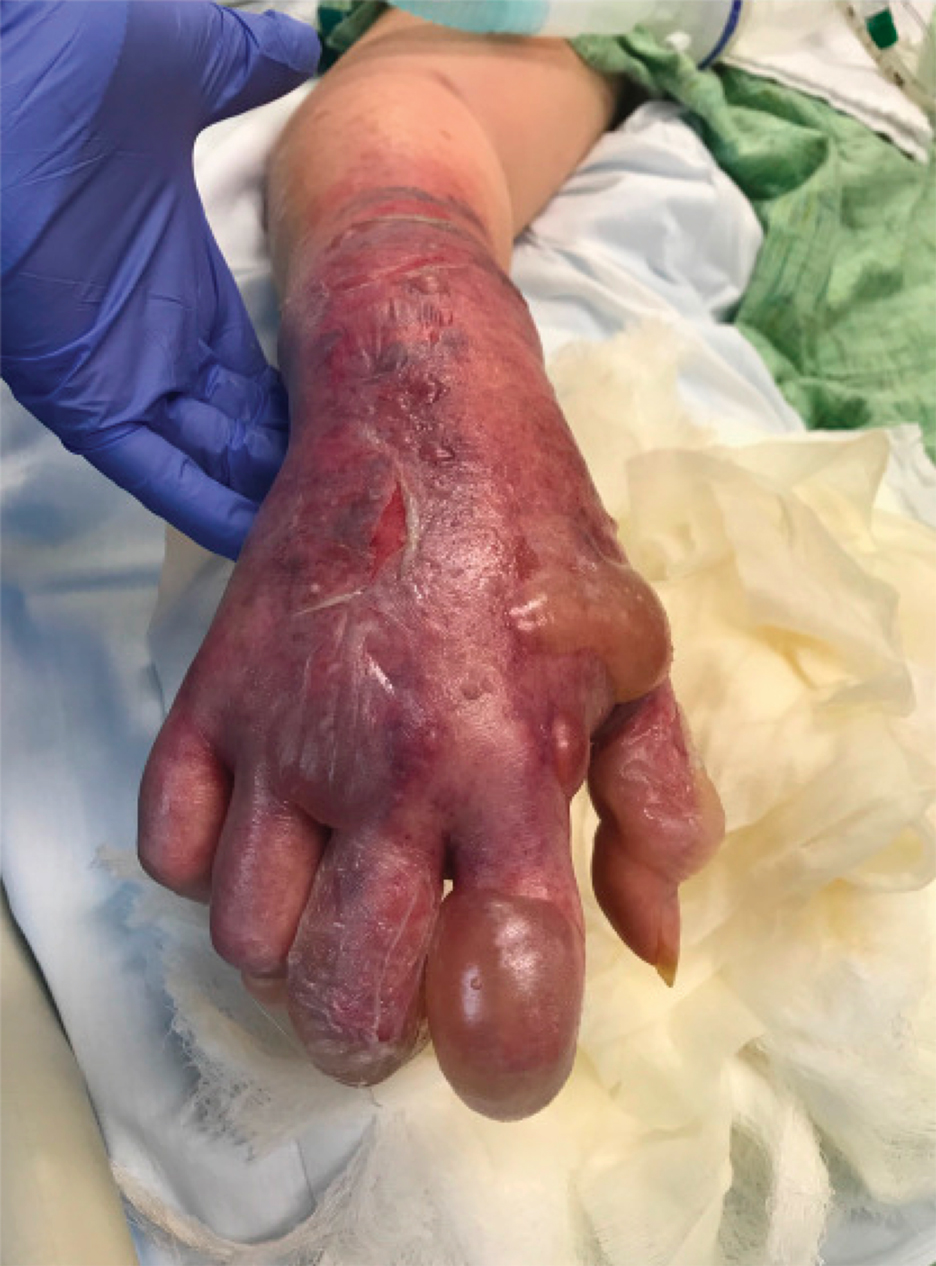
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.

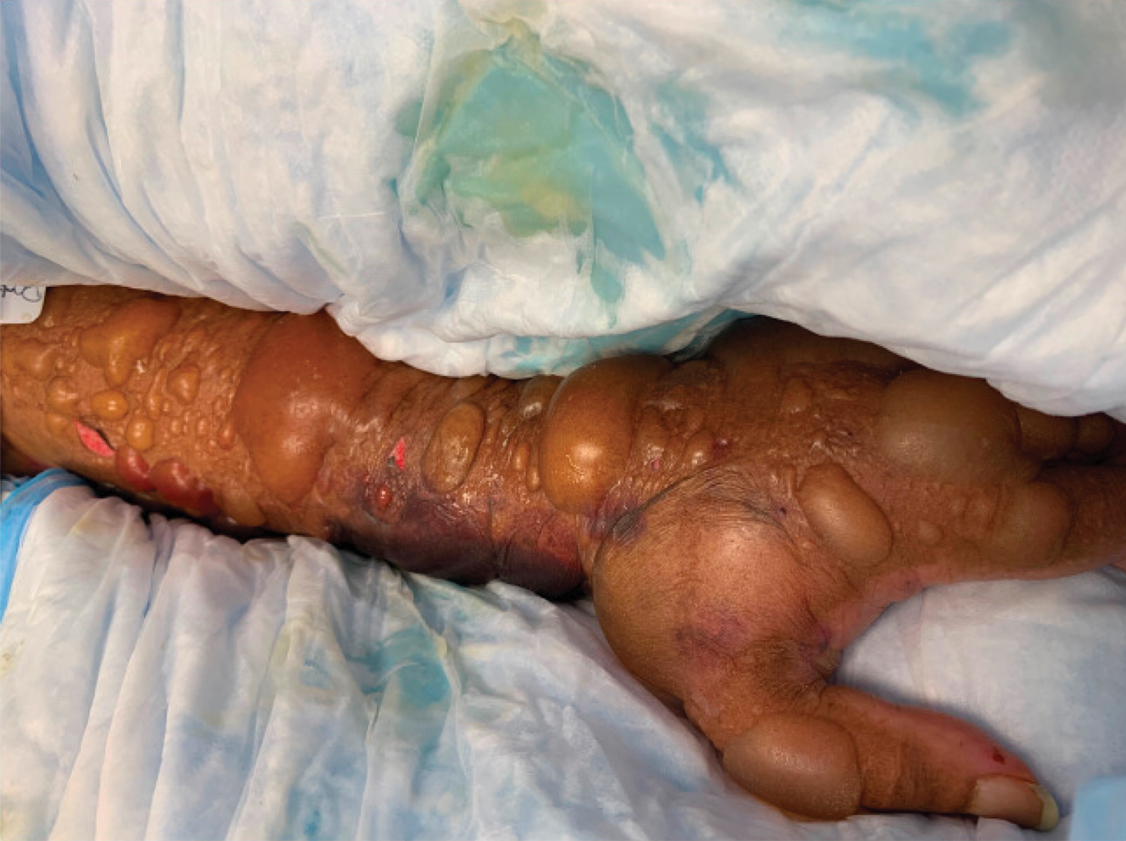
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.