User login
Which breast cancer surgery leads to better quality of life?
Women diagnosed with early breast cancer facing surgery often have a choice of having all of their breast or only a part of the breast removed.
A new study shows that a patient’s satisfaction with their breasts at 10 years after surgery is similar for both groups of women.
However, superior psychosocial and sexual well-being at 10 years after surgery was reported by women who underwent breast-conserving surgery and adjuvant radiation therapy (RT), compared with those who underwent mastectomy and reconstruction.
“These findings may inform preference-sensitive decision-making for women with early-stage breast cancer,” write the authors, led by Benjamin D. Smith, MD, department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
The study was published online in JAMA Surgery.
These findings have important implications for patient decision-making, given that more women eligible for breast-conserving surgery are opting for a mastectomy, say Sudheer Vemuru, MD, from the University of Colorado at Denver, Aurora, and colleagues, writing in an accompanying editorial.
“Overall, the preponderance of evidence suggests superior short-term and long-term patient-reported outcomes in patients with early-stage breast cancer undergoing breast conserving surgery compared with mastectomy,” they comment.
Study details
For their study, Dr. Smith and colleagues conducted a comparative effectiveness research study using data from the Texas Cancer Registry and identified women diagnosed with stage 0-II breast cancer and treated with breast-conserving surgery or mastectomy and reconstruction between 2006 and 2008.
A total of 647 patients were included in their analysis (40%; 356 had undergone breast-conserving surgery; 291 had undergone mastectomy and reconstruction), 551 (85.2%) confirmed treatment with breast-conserving surgery with RT (n = 315) or mastectomy and reconstruction without RT (n = 236).
The median age of the cohort was 53 years and the median time from diagnosis to survey was 10.3 years. Mastectomy and reconstruction were more common among women who were White, younger, node positive, had larger tumors, had bilateral breast cancer, received chemotherapy, and had higher income.
The primary outcome was patient satisfaction with their breasts, as measured with the BREAST-Q patient-reported outcome measure. Secondary outcomes included physical well-being, psychosocial well-being, and sexual well-being. The EuroQol Health-Related Quality of Life 5-Dimension, 3-Level gaged health utility, and local therapy decisional regret was measured via the Decisional Regret Scale.
Using breast-conserving surgery plus RT as the referent, the authors did not find any significant differences in breast satisfaction, physical well-being, health utility, or decisional regret among the study cohorts: breast satisfaction: effect size, 2.71 (P = .30); physical well-being: effect size, –1.80 (P = .36); health utility: effect size, –0.003 (P = .83); and decisional regret: effect size, 1.32 (P = .61).
However, psychosocial well-being (effect size, –8.61; P < .001) and sexual well-being (effect size, –10.68; P < .001) were significantly worse among women who had undergone mastectomy and reconstruction without RT.
They noted that interactions of race and ethnicity and age by treatment group were not significant for reported satisfaction with breast outcomes. But the findings “indicated that the burden of poor long-term QOL outcomes was greater among younger individuals, those with lower educational attainment and income, and certain racial and ethnic minority populations,” they write. “These findings suggest that opportunities exist to enhance equity in the long-term QOL of individuals with breast cancer.”
The editorialists note that previous studies have also found diminished quality of life following mastectomy compared with breast-conserving surgery. However, most of these prior studies included patients undergoing breast-conserving surgery without RT, patients undergoing mastectomy without reconstruction, and patients undergoing mastectomy with RT.
In contrast, this latest study “directly compared breast-conserving surgery with RT vs. mastectomy and reconstruction without RT to avoid those potential confounders,” they point out.
The study was supported by grants from the National Cancer Institute and other bodies. Several of the study authors disclosed relationships with industry and/or with nonprofit organizations. The full list can be found with the original article. Editorialist Clara Lee, MD, reported receiving grants from the Agency for Healthcare Research and Quality during the conduct of the study.
A version of this article first appeared on Medscape.com.
Women diagnosed with early breast cancer facing surgery often have a choice of having all of their breast or only a part of the breast removed.
A new study shows that a patient’s satisfaction with their breasts at 10 years after surgery is similar for both groups of women.
However, superior psychosocial and sexual well-being at 10 years after surgery was reported by women who underwent breast-conserving surgery and adjuvant radiation therapy (RT), compared with those who underwent mastectomy and reconstruction.
“These findings may inform preference-sensitive decision-making for women with early-stage breast cancer,” write the authors, led by Benjamin D. Smith, MD, department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
The study was published online in JAMA Surgery.
These findings have important implications for patient decision-making, given that more women eligible for breast-conserving surgery are opting for a mastectomy, say Sudheer Vemuru, MD, from the University of Colorado at Denver, Aurora, and colleagues, writing in an accompanying editorial.
“Overall, the preponderance of evidence suggests superior short-term and long-term patient-reported outcomes in patients with early-stage breast cancer undergoing breast conserving surgery compared with mastectomy,” they comment.
Study details
For their study, Dr. Smith and colleagues conducted a comparative effectiveness research study using data from the Texas Cancer Registry and identified women diagnosed with stage 0-II breast cancer and treated with breast-conserving surgery or mastectomy and reconstruction between 2006 and 2008.
A total of 647 patients were included in their analysis (40%; 356 had undergone breast-conserving surgery; 291 had undergone mastectomy and reconstruction), 551 (85.2%) confirmed treatment with breast-conserving surgery with RT (n = 315) or mastectomy and reconstruction without RT (n = 236).
The median age of the cohort was 53 years and the median time from diagnosis to survey was 10.3 years. Mastectomy and reconstruction were more common among women who were White, younger, node positive, had larger tumors, had bilateral breast cancer, received chemotherapy, and had higher income.
The primary outcome was patient satisfaction with their breasts, as measured with the BREAST-Q patient-reported outcome measure. Secondary outcomes included physical well-being, psychosocial well-being, and sexual well-being. The EuroQol Health-Related Quality of Life 5-Dimension, 3-Level gaged health utility, and local therapy decisional regret was measured via the Decisional Regret Scale.
Using breast-conserving surgery plus RT as the referent, the authors did not find any significant differences in breast satisfaction, physical well-being, health utility, or decisional regret among the study cohorts: breast satisfaction: effect size, 2.71 (P = .30); physical well-being: effect size, –1.80 (P = .36); health utility: effect size, –0.003 (P = .83); and decisional regret: effect size, 1.32 (P = .61).
However, psychosocial well-being (effect size, –8.61; P < .001) and sexual well-being (effect size, –10.68; P < .001) were significantly worse among women who had undergone mastectomy and reconstruction without RT.
They noted that interactions of race and ethnicity and age by treatment group were not significant for reported satisfaction with breast outcomes. But the findings “indicated that the burden of poor long-term QOL outcomes was greater among younger individuals, those with lower educational attainment and income, and certain racial and ethnic minority populations,” they write. “These findings suggest that opportunities exist to enhance equity in the long-term QOL of individuals with breast cancer.”
The editorialists note that previous studies have also found diminished quality of life following mastectomy compared with breast-conserving surgery. However, most of these prior studies included patients undergoing breast-conserving surgery without RT, patients undergoing mastectomy without reconstruction, and patients undergoing mastectomy with RT.
In contrast, this latest study “directly compared breast-conserving surgery with RT vs. mastectomy and reconstruction without RT to avoid those potential confounders,” they point out.
The study was supported by grants from the National Cancer Institute and other bodies. Several of the study authors disclosed relationships with industry and/or with nonprofit organizations. The full list can be found with the original article. Editorialist Clara Lee, MD, reported receiving grants from the Agency for Healthcare Research and Quality during the conduct of the study.
A version of this article first appeared on Medscape.com.
Women diagnosed with early breast cancer facing surgery often have a choice of having all of their breast or only a part of the breast removed.
A new study shows that a patient’s satisfaction with their breasts at 10 years after surgery is similar for both groups of women.
However, superior psychosocial and sexual well-being at 10 years after surgery was reported by women who underwent breast-conserving surgery and adjuvant radiation therapy (RT), compared with those who underwent mastectomy and reconstruction.
“These findings may inform preference-sensitive decision-making for women with early-stage breast cancer,” write the authors, led by Benjamin D. Smith, MD, department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
The study was published online in JAMA Surgery.
These findings have important implications for patient decision-making, given that more women eligible for breast-conserving surgery are opting for a mastectomy, say Sudheer Vemuru, MD, from the University of Colorado at Denver, Aurora, and colleagues, writing in an accompanying editorial.
“Overall, the preponderance of evidence suggests superior short-term and long-term patient-reported outcomes in patients with early-stage breast cancer undergoing breast conserving surgery compared with mastectomy,” they comment.
Study details
For their study, Dr. Smith and colleagues conducted a comparative effectiveness research study using data from the Texas Cancer Registry and identified women diagnosed with stage 0-II breast cancer and treated with breast-conserving surgery or mastectomy and reconstruction between 2006 and 2008.
A total of 647 patients were included in their analysis (40%; 356 had undergone breast-conserving surgery; 291 had undergone mastectomy and reconstruction), 551 (85.2%) confirmed treatment with breast-conserving surgery with RT (n = 315) or mastectomy and reconstruction without RT (n = 236).
The median age of the cohort was 53 years and the median time from diagnosis to survey was 10.3 years. Mastectomy and reconstruction were more common among women who were White, younger, node positive, had larger tumors, had bilateral breast cancer, received chemotherapy, and had higher income.
The primary outcome was patient satisfaction with their breasts, as measured with the BREAST-Q patient-reported outcome measure. Secondary outcomes included physical well-being, psychosocial well-being, and sexual well-being. The EuroQol Health-Related Quality of Life 5-Dimension, 3-Level gaged health utility, and local therapy decisional regret was measured via the Decisional Regret Scale.
Using breast-conserving surgery plus RT as the referent, the authors did not find any significant differences in breast satisfaction, physical well-being, health utility, or decisional regret among the study cohorts: breast satisfaction: effect size, 2.71 (P = .30); physical well-being: effect size, –1.80 (P = .36); health utility: effect size, –0.003 (P = .83); and decisional regret: effect size, 1.32 (P = .61).
However, psychosocial well-being (effect size, –8.61; P < .001) and sexual well-being (effect size, –10.68; P < .001) were significantly worse among women who had undergone mastectomy and reconstruction without RT.
They noted that interactions of race and ethnicity and age by treatment group were not significant for reported satisfaction with breast outcomes. But the findings “indicated that the burden of poor long-term QOL outcomes was greater among younger individuals, those with lower educational attainment and income, and certain racial and ethnic minority populations,” they write. “These findings suggest that opportunities exist to enhance equity in the long-term QOL of individuals with breast cancer.”
The editorialists note that previous studies have also found diminished quality of life following mastectomy compared with breast-conserving surgery. However, most of these prior studies included patients undergoing breast-conserving surgery without RT, patients undergoing mastectomy without reconstruction, and patients undergoing mastectomy with RT.
In contrast, this latest study “directly compared breast-conserving surgery with RT vs. mastectomy and reconstruction without RT to avoid those potential confounders,” they point out.
The study was supported by grants from the National Cancer Institute and other bodies. Several of the study authors disclosed relationships with industry and/or with nonprofit organizations. The full list can be found with the original article. Editorialist Clara Lee, MD, reported receiving grants from the Agency for Healthcare Research and Quality during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Fetuses suffer the effects of poverty in the womb
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH
Cheap and noninvasive: Detecting HPV in sanitary pads
A cell phone rings in a red-brick bungalow in a village in India. A woman on the other end of the phone tells Ms. SK, a community health worker, that menstruation has started. Ms. SK guns her scooter through the dusty streets for 15 minutes in 30° C (86° F) heat.
A 32-year-old woman, waiting in the shade of a blue corrugated-iron roof, hands over a green polythene bag. Ms. SK whisks the package to the local health center and tucks it into a –20° C freezer. The following week, it will ride in dry ice to the National Institute for Research in Reproductive and Child Health Laboratory in Mumbai for human papillomavirus (HPV) testing.
This moment in rural India at first glance appears to have little relevance to wealthy countries such as the United States.
However, public health officials in both countries are trying to solve the same problem: how to prevent unnecessary deaths from cervical cancer by reaching women who have never or rarely been screened.
The United States has more in common with India than it may care to admit.
“In the U.S., we still have pockets of disparities that actually have incidence rates [of cervical cancer] comparable to many low- and middle-income countries,” said Vikrant Sahasrabuddhe, MBBS, DrPh, MPH, of the National Cancer Institute, where he heads the HPV and cervical cancer prevention clinical research program for the National Institutes of Health.
The incidence of cervical cancer in India is approximately 19 per 100,000 women. For the past 15 years incidence in the United States has stalled at approximately 7 per 100,000.
In India, there are no organized screening programs and most cervical cancer is regional or distant metastatic at diagnosis.
In the United States, 52% of new cases are advanced, and half of these are among women who have never or rarely been screened.
“There is a critical need for new strategies to reach this population,” Dr. Sahasrabuddhe said. “We absolutely have to do something out of the box creatively.”
Almost all cervical cancers are triggered by HPV, most commonly high-risk HPV-16 and HPV-18, although there are more than 200 types. HPV testing is taking over from cytology (Papanicolaou test) for secondary prevention of cervical cancer.
The trial of screening for HPV in menstrual pads that is ongoing in India was the brainchild of Atul Budukh, PhD, a government public health researcher and professor at the Centre for Cancer Epidemiology, Tata Memorial Centre, Mumbai.
Dr. Budukh’s eyes were opened to the scale of the problem when he participated in a cluster-randomized trial funded by the Bill and Melinda Gates Foundation. The study, published in 2009 in the New England Journal of Medicine, involved 131,746 rural women in the Osmanabad district of India.
A team of researchers from India and France compared outcomes for women over 8 years after cervical screening by HPV, cytology, or visual inspection with acetic acid. The control group was usual care, where women were advised how to seek screening at local hospitals. Women who screened positive were referred for colposcopy, biopsy, and treatment.
Over the 8-year follow-up, advanced cervical cancer was found in twice as many women left to their own devices, compared with women who had HPV testing during the study (82 vs. 39; hazard ratio for HPV, 0.47; 95% confidence interval, 0.32-0.69).
Similarly, cervical cancer deaths in the control group were nearly two times higher than among the women who were screened for HPV in the study (64 vs. 34; HR for HPV, 0.52; 95% CI, 0.33-0.83).
The study proved that rural Indian women were dying unnecessarily because they weren’t seeking cervical screening. And education wasn’t the problem.
“When we go and educate [a rural woman] about ... risk factors and the need to undergo screening, she understands it very well,” said Dr. Budukh. “She is ready to come but her priority is her bread and butter – she will lose her daily wages.”
Dr. Budukh and his team negotiated with local employers so that women could come to screening clinics, but they soon realized this wasn’t scalable.
One year after the NEJM publication, Dr. Budukh found what he was looking for.
A team of Hong Kong clinicians, headed by Sze Chuen Cesar Wong of the Hong Kong Cancer Institute, published a paper in 2010 in the Journal of Clinical Microbiology showing that menstrual pads provide reliable HPV results in women with and without cervical disease.
The Hong Kong team tested sanitary napkins for HPV from 235 of their patients with cervical intraepithelial neoplasia or condyloma acuminatum before and after treatment. Samples were compared with those from 323 women without cervical disease; for HPV in sanitary napkins the sensitivity was 82.8%, specificity was 93.1%, and positive and negative predictive values were 90% and 87.9%, respectively.
The authors pointed out that menstrual pad testing was the only truly noninvasive approach to HPV screening versus the other self-sampling methods such as tampons and cytobrushes. Also, these self-sampling tests require specialized liquid-based transport media. A menstrual pad needs only a plastic bag.
Dr. Budukh had his at-home solution for the hard-working rural women of India.
With funding from the Indian government, Dr. Budukh’s team put together a validation trial that ran from 2013 to 2016 in 18 rural villages in two separate districts: Ahmednagar and Pune.
Local health workers went house to house to recruit women and get family buy-in for this culturally delicate project. Participants were instructed to use their regular sanitary protection – most commonly a washable cloth – and told to call the health worker on the first day of menstruation. Health workers gave each woman a Ziploc bag for the pad and, for privacy, an outer polythene sac.
In Ahmednagar, all women who provided their pad also got screened with Hybrid Capture 2 (HC2; Qiagen) by a mobile screening unit. In Pune, only the positive cases underwent HC2. Screening was also extended to anyone who requested it, but these people were not included in the final analysis.
Genomic DNA was extracted from three 5 mm–sized punches in the pad using a commercial kit, QIAamp DNA Micro, and the quality and purity of the DNA checked by Implen NanoPhotometer.
The team followed the same protocol for PCR HPV assay as the team from Hong Kong.
The results were published in the European Journal of Cancer Prevention in 2018.
The concordance rate for a positive result between the menstrual pad sample and conventional HPV sampling was 98.8% for Ahmednagar and 95.2% for samples from Pune. The sensitivity for the first study was 83% and the specificity 99% – similar to that for the women in Hong Kong. The second study had lower sensitivity and specificity (67% and 88%), partly because of poor storage as a result of frequent power cuts.
The total cost per woman was $30.78.
“I was very excited when we saw the results,” Dr. Budukh recalled. “That day I couldn’t sleep ... such a wonderful result! I was excited to start the next phase immediately.”
Dr. Budukh has applied to the Indian government for funding for a larger trial involving 3,000 women. If successful, he hopes such evidence would be sufficient to convince the Indian government to make menstrual pad screening standard procedure for the 390 million women who live in India’s countryside.
Testing never-screened women for cervical cancer using menstrual pads appears to be relatively reliable, convenient, private, noninvasive, and incredibly cheap.
So who else has tried it?
The first published account of HPV in menstrual blood was a 2003 study by Tommy Tong and colleagues at the Princess Margaret Hospital in Hong Kong. The authors heralded, with lamentable optimism, “a new paradigm in cervical cancer screening.”
In the following 20 years, just six more studies appeared: two from Dr. Budukh’s field trial in India and four from hospital-based pilot studies in Hong Kong (in 2010 and 2018), South Korea (in 2016), and mainland China (in 2021). All these studies, although small, were published in top-flight journals and demonstrate high concordance between conventional high-risk HPV testing and menstrual-blood tests.
This news organization tried to find a U.S. thought-leader who had heard of the approach.
Elizabeth Fontham, MPH, DrPh, is the founding dean of the school of public health at Louisiana State University Health Center in New Orleans, and president of the American Cancer Society. Dr. Fontham said in an email that she had “no plans to evaluate the impact related to menstrual pads, but perhaps others have looked into that.”
Joy Melnikow, MD, MPH, was first author on the evidence synthesis driving the current cervical cancer screening recommendations from the U.S. Preventive Services Task Force. When asked about menstrual pad testing for HPV, she said she had “not heard of it before.”
The USPSTF guidelines don’t mention sanitary pads but acknowledge that “self-collection may be one strategy for increasing screening rates among populations where they are currently low.”
The USPSTF methodology excludes data from countries that don’t match the United States on the Human Development Index “or [are] not applicable to U.S. clinical settings or populations.” (Presumably, data from Hong Kong and South Korea would qualify; Indian data would not.)
Dr. Sahasrabuddhe of the NCI hadn’t heard of menstrual pad testing either, but he has a different explanation for lack of interest in this approach – or, indeed, any form of self-sampling for cervical cancer screening – in the United States.
“We have not seen movement happen in this space for years. ... If there is one intervention that we can simplify, that still has not been made widely available, it is self-sampling ... [but] we don’t have [Food and Drug Administration] approval for it,” Dr. Sahasrabuddhe said.
“Our system, at least in the U.S., is based on industry manufacturers seeking an approval for a particular way of collection and then clinicians and clinical-guideline bodies signing on. ... For a lot of reasons industry has shied away over the past several years, so far, at least, on seeking approval for self-sampling-based approaches,” he commented.
Dr. Sahasrabuddhe aims to change that. He heads a new NCI-led initiative called “The Last Mile,” a nationwide clinical trial supported by federal agencies, industry partners, and professional societies. The goal is to validate self-sampled HPV testing as non-inferior to specimens collected by providers. The team is currently finalizing the methodology of the study, so Dr. Sahasrabuddhe could not share the self-sampling methods that will be on trial, nor the industry partners who have signed up.
The following tests are approved in the United States for physician-collected HPV screening: Hybrid Capture 2, used in the Indian studies (Qiagen); cobas HPV (Roche); Aptima (Hologic); Cervista (Hologic); and Onclarity (Becton Dickinson).
Dr. Sahasrabuddhe said that, while a sanitary pad in a Ziploc bag is unlikely to make the grade for The Last Mile study, he doesn’t totally dismiss their potential and said the NCI is always open to new ideas.
“We are not supporting anybody specifically for menstrual pad-based collection device development,” Dr. Sahasrabuddhe said, “But if they fulfill other criteria for a small business–based grant application, they absolutely are welcome to apply for NCI funding for this.”
Said Dr. Melnikow: “Pre-COVID, the head of [the World Health Organization] said that we could eliminate cervical cancer from the globe and that we have the tools to do that now. And he’s right.”
Dr. Budukh, Dr. Melnikow, and Dr. Sahasrabuddhe disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A cell phone rings in a red-brick bungalow in a village in India. A woman on the other end of the phone tells Ms. SK, a community health worker, that menstruation has started. Ms. SK guns her scooter through the dusty streets for 15 minutes in 30° C (86° F) heat.
A 32-year-old woman, waiting in the shade of a blue corrugated-iron roof, hands over a green polythene bag. Ms. SK whisks the package to the local health center and tucks it into a –20° C freezer. The following week, it will ride in dry ice to the National Institute for Research in Reproductive and Child Health Laboratory in Mumbai for human papillomavirus (HPV) testing.
This moment in rural India at first glance appears to have little relevance to wealthy countries such as the United States.
However, public health officials in both countries are trying to solve the same problem: how to prevent unnecessary deaths from cervical cancer by reaching women who have never or rarely been screened.
The United States has more in common with India than it may care to admit.
“In the U.S., we still have pockets of disparities that actually have incidence rates [of cervical cancer] comparable to many low- and middle-income countries,” said Vikrant Sahasrabuddhe, MBBS, DrPh, MPH, of the National Cancer Institute, where he heads the HPV and cervical cancer prevention clinical research program for the National Institutes of Health.
The incidence of cervical cancer in India is approximately 19 per 100,000 women. For the past 15 years incidence in the United States has stalled at approximately 7 per 100,000.
In India, there are no organized screening programs and most cervical cancer is regional or distant metastatic at diagnosis.
In the United States, 52% of new cases are advanced, and half of these are among women who have never or rarely been screened.
“There is a critical need for new strategies to reach this population,” Dr. Sahasrabuddhe said. “We absolutely have to do something out of the box creatively.”
Almost all cervical cancers are triggered by HPV, most commonly high-risk HPV-16 and HPV-18, although there are more than 200 types. HPV testing is taking over from cytology (Papanicolaou test) for secondary prevention of cervical cancer.
The trial of screening for HPV in menstrual pads that is ongoing in India was the brainchild of Atul Budukh, PhD, a government public health researcher and professor at the Centre for Cancer Epidemiology, Tata Memorial Centre, Mumbai.
Dr. Budukh’s eyes were opened to the scale of the problem when he participated in a cluster-randomized trial funded by the Bill and Melinda Gates Foundation. The study, published in 2009 in the New England Journal of Medicine, involved 131,746 rural women in the Osmanabad district of India.
A team of researchers from India and France compared outcomes for women over 8 years after cervical screening by HPV, cytology, or visual inspection with acetic acid. The control group was usual care, where women were advised how to seek screening at local hospitals. Women who screened positive were referred for colposcopy, biopsy, and treatment.
Over the 8-year follow-up, advanced cervical cancer was found in twice as many women left to their own devices, compared with women who had HPV testing during the study (82 vs. 39; hazard ratio for HPV, 0.47; 95% confidence interval, 0.32-0.69).
Similarly, cervical cancer deaths in the control group were nearly two times higher than among the women who were screened for HPV in the study (64 vs. 34; HR for HPV, 0.52; 95% CI, 0.33-0.83).
The study proved that rural Indian women were dying unnecessarily because they weren’t seeking cervical screening. And education wasn’t the problem.
“When we go and educate [a rural woman] about ... risk factors and the need to undergo screening, she understands it very well,” said Dr. Budukh. “She is ready to come but her priority is her bread and butter – she will lose her daily wages.”
Dr. Budukh and his team negotiated with local employers so that women could come to screening clinics, but they soon realized this wasn’t scalable.
One year after the NEJM publication, Dr. Budukh found what he was looking for.
A team of Hong Kong clinicians, headed by Sze Chuen Cesar Wong of the Hong Kong Cancer Institute, published a paper in 2010 in the Journal of Clinical Microbiology showing that menstrual pads provide reliable HPV results in women with and without cervical disease.
The Hong Kong team tested sanitary napkins for HPV from 235 of their patients with cervical intraepithelial neoplasia or condyloma acuminatum before and after treatment. Samples were compared with those from 323 women without cervical disease; for HPV in sanitary napkins the sensitivity was 82.8%, specificity was 93.1%, and positive and negative predictive values were 90% and 87.9%, respectively.
The authors pointed out that menstrual pad testing was the only truly noninvasive approach to HPV screening versus the other self-sampling methods such as tampons and cytobrushes. Also, these self-sampling tests require specialized liquid-based transport media. A menstrual pad needs only a plastic bag.
Dr. Budukh had his at-home solution for the hard-working rural women of India.
With funding from the Indian government, Dr. Budukh’s team put together a validation trial that ran from 2013 to 2016 in 18 rural villages in two separate districts: Ahmednagar and Pune.
Local health workers went house to house to recruit women and get family buy-in for this culturally delicate project. Participants were instructed to use their regular sanitary protection – most commonly a washable cloth – and told to call the health worker on the first day of menstruation. Health workers gave each woman a Ziploc bag for the pad and, for privacy, an outer polythene sac.
In Ahmednagar, all women who provided their pad also got screened with Hybrid Capture 2 (HC2; Qiagen) by a mobile screening unit. In Pune, only the positive cases underwent HC2. Screening was also extended to anyone who requested it, but these people were not included in the final analysis.
Genomic DNA was extracted from three 5 mm–sized punches in the pad using a commercial kit, QIAamp DNA Micro, and the quality and purity of the DNA checked by Implen NanoPhotometer.
The team followed the same protocol for PCR HPV assay as the team from Hong Kong.
The results were published in the European Journal of Cancer Prevention in 2018.
The concordance rate for a positive result between the menstrual pad sample and conventional HPV sampling was 98.8% for Ahmednagar and 95.2% for samples from Pune. The sensitivity for the first study was 83% and the specificity 99% – similar to that for the women in Hong Kong. The second study had lower sensitivity and specificity (67% and 88%), partly because of poor storage as a result of frequent power cuts.
The total cost per woman was $30.78.
“I was very excited when we saw the results,” Dr. Budukh recalled. “That day I couldn’t sleep ... such a wonderful result! I was excited to start the next phase immediately.”
Dr. Budukh has applied to the Indian government for funding for a larger trial involving 3,000 women. If successful, he hopes such evidence would be sufficient to convince the Indian government to make menstrual pad screening standard procedure for the 390 million women who live in India’s countryside.
Testing never-screened women for cervical cancer using menstrual pads appears to be relatively reliable, convenient, private, noninvasive, and incredibly cheap.
So who else has tried it?
The first published account of HPV in menstrual blood was a 2003 study by Tommy Tong and colleagues at the Princess Margaret Hospital in Hong Kong. The authors heralded, with lamentable optimism, “a new paradigm in cervical cancer screening.”
In the following 20 years, just six more studies appeared: two from Dr. Budukh’s field trial in India and four from hospital-based pilot studies in Hong Kong (in 2010 and 2018), South Korea (in 2016), and mainland China (in 2021). All these studies, although small, were published in top-flight journals and demonstrate high concordance between conventional high-risk HPV testing and menstrual-blood tests.
This news organization tried to find a U.S. thought-leader who had heard of the approach.
Elizabeth Fontham, MPH, DrPh, is the founding dean of the school of public health at Louisiana State University Health Center in New Orleans, and president of the American Cancer Society. Dr. Fontham said in an email that she had “no plans to evaluate the impact related to menstrual pads, but perhaps others have looked into that.”
Joy Melnikow, MD, MPH, was first author on the evidence synthesis driving the current cervical cancer screening recommendations from the U.S. Preventive Services Task Force. When asked about menstrual pad testing for HPV, she said she had “not heard of it before.”
The USPSTF guidelines don’t mention sanitary pads but acknowledge that “self-collection may be one strategy for increasing screening rates among populations where they are currently low.”
The USPSTF methodology excludes data from countries that don’t match the United States on the Human Development Index “or [are] not applicable to U.S. clinical settings or populations.” (Presumably, data from Hong Kong and South Korea would qualify; Indian data would not.)
Dr. Sahasrabuddhe of the NCI hadn’t heard of menstrual pad testing either, but he has a different explanation for lack of interest in this approach – or, indeed, any form of self-sampling for cervical cancer screening – in the United States.
“We have not seen movement happen in this space for years. ... If there is one intervention that we can simplify, that still has not been made widely available, it is self-sampling ... [but] we don’t have [Food and Drug Administration] approval for it,” Dr. Sahasrabuddhe said.
“Our system, at least in the U.S., is based on industry manufacturers seeking an approval for a particular way of collection and then clinicians and clinical-guideline bodies signing on. ... For a lot of reasons industry has shied away over the past several years, so far, at least, on seeking approval for self-sampling-based approaches,” he commented.
Dr. Sahasrabuddhe aims to change that. He heads a new NCI-led initiative called “The Last Mile,” a nationwide clinical trial supported by federal agencies, industry partners, and professional societies. The goal is to validate self-sampled HPV testing as non-inferior to specimens collected by providers. The team is currently finalizing the methodology of the study, so Dr. Sahasrabuddhe could not share the self-sampling methods that will be on trial, nor the industry partners who have signed up.
The following tests are approved in the United States for physician-collected HPV screening: Hybrid Capture 2, used in the Indian studies (Qiagen); cobas HPV (Roche); Aptima (Hologic); Cervista (Hologic); and Onclarity (Becton Dickinson).
Dr. Sahasrabuddhe said that, while a sanitary pad in a Ziploc bag is unlikely to make the grade for The Last Mile study, he doesn’t totally dismiss their potential and said the NCI is always open to new ideas.
“We are not supporting anybody specifically for menstrual pad-based collection device development,” Dr. Sahasrabuddhe said, “But if they fulfill other criteria for a small business–based grant application, they absolutely are welcome to apply for NCI funding for this.”
Said Dr. Melnikow: “Pre-COVID, the head of [the World Health Organization] said that we could eliminate cervical cancer from the globe and that we have the tools to do that now. And he’s right.”
Dr. Budukh, Dr. Melnikow, and Dr. Sahasrabuddhe disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A cell phone rings in a red-brick bungalow in a village in India. A woman on the other end of the phone tells Ms. SK, a community health worker, that menstruation has started. Ms. SK guns her scooter through the dusty streets for 15 minutes in 30° C (86° F) heat.
A 32-year-old woman, waiting in the shade of a blue corrugated-iron roof, hands over a green polythene bag. Ms. SK whisks the package to the local health center and tucks it into a –20° C freezer. The following week, it will ride in dry ice to the National Institute for Research in Reproductive and Child Health Laboratory in Mumbai for human papillomavirus (HPV) testing.
This moment in rural India at first glance appears to have little relevance to wealthy countries such as the United States.
However, public health officials in both countries are trying to solve the same problem: how to prevent unnecessary deaths from cervical cancer by reaching women who have never or rarely been screened.
The United States has more in common with India than it may care to admit.
“In the U.S., we still have pockets of disparities that actually have incidence rates [of cervical cancer] comparable to many low- and middle-income countries,” said Vikrant Sahasrabuddhe, MBBS, DrPh, MPH, of the National Cancer Institute, where he heads the HPV and cervical cancer prevention clinical research program for the National Institutes of Health.
The incidence of cervical cancer in India is approximately 19 per 100,000 women. For the past 15 years incidence in the United States has stalled at approximately 7 per 100,000.
In India, there are no organized screening programs and most cervical cancer is regional or distant metastatic at diagnosis.
In the United States, 52% of new cases are advanced, and half of these are among women who have never or rarely been screened.
“There is a critical need for new strategies to reach this population,” Dr. Sahasrabuddhe said. “We absolutely have to do something out of the box creatively.”
Almost all cervical cancers are triggered by HPV, most commonly high-risk HPV-16 and HPV-18, although there are more than 200 types. HPV testing is taking over from cytology (Papanicolaou test) for secondary prevention of cervical cancer.
The trial of screening for HPV in menstrual pads that is ongoing in India was the brainchild of Atul Budukh, PhD, a government public health researcher and professor at the Centre for Cancer Epidemiology, Tata Memorial Centre, Mumbai.
Dr. Budukh’s eyes were opened to the scale of the problem when he participated in a cluster-randomized trial funded by the Bill and Melinda Gates Foundation. The study, published in 2009 in the New England Journal of Medicine, involved 131,746 rural women in the Osmanabad district of India.
A team of researchers from India and France compared outcomes for women over 8 years after cervical screening by HPV, cytology, or visual inspection with acetic acid. The control group was usual care, where women were advised how to seek screening at local hospitals. Women who screened positive were referred for colposcopy, biopsy, and treatment.
Over the 8-year follow-up, advanced cervical cancer was found in twice as many women left to their own devices, compared with women who had HPV testing during the study (82 vs. 39; hazard ratio for HPV, 0.47; 95% confidence interval, 0.32-0.69).
Similarly, cervical cancer deaths in the control group were nearly two times higher than among the women who were screened for HPV in the study (64 vs. 34; HR for HPV, 0.52; 95% CI, 0.33-0.83).
The study proved that rural Indian women were dying unnecessarily because they weren’t seeking cervical screening. And education wasn’t the problem.
“When we go and educate [a rural woman] about ... risk factors and the need to undergo screening, she understands it very well,” said Dr. Budukh. “She is ready to come but her priority is her bread and butter – she will lose her daily wages.”
Dr. Budukh and his team negotiated with local employers so that women could come to screening clinics, but they soon realized this wasn’t scalable.
One year after the NEJM publication, Dr. Budukh found what he was looking for.
A team of Hong Kong clinicians, headed by Sze Chuen Cesar Wong of the Hong Kong Cancer Institute, published a paper in 2010 in the Journal of Clinical Microbiology showing that menstrual pads provide reliable HPV results in women with and without cervical disease.
The Hong Kong team tested sanitary napkins for HPV from 235 of their patients with cervical intraepithelial neoplasia or condyloma acuminatum before and after treatment. Samples were compared with those from 323 women without cervical disease; for HPV in sanitary napkins the sensitivity was 82.8%, specificity was 93.1%, and positive and negative predictive values were 90% and 87.9%, respectively.
The authors pointed out that menstrual pad testing was the only truly noninvasive approach to HPV screening versus the other self-sampling methods such as tampons and cytobrushes. Also, these self-sampling tests require specialized liquid-based transport media. A menstrual pad needs only a plastic bag.
Dr. Budukh had his at-home solution for the hard-working rural women of India.
With funding from the Indian government, Dr. Budukh’s team put together a validation trial that ran from 2013 to 2016 in 18 rural villages in two separate districts: Ahmednagar and Pune.
Local health workers went house to house to recruit women and get family buy-in for this culturally delicate project. Participants were instructed to use their regular sanitary protection – most commonly a washable cloth – and told to call the health worker on the first day of menstruation. Health workers gave each woman a Ziploc bag for the pad and, for privacy, an outer polythene sac.
In Ahmednagar, all women who provided their pad also got screened with Hybrid Capture 2 (HC2; Qiagen) by a mobile screening unit. In Pune, only the positive cases underwent HC2. Screening was also extended to anyone who requested it, but these people were not included in the final analysis.
Genomic DNA was extracted from three 5 mm–sized punches in the pad using a commercial kit, QIAamp DNA Micro, and the quality and purity of the DNA checked by Implen NanoPhotometer.
The team followed the same protocol for PCR HPV assay as the team from Hong Kong.
The results were published in the European Journal of Cancer Prevention in 2018.
The concordance rate for a positive result between the menstrual pad sample and conventional HPV sampling was 98.8% for Ahmednagar and 95.2% for samples from Pune. The sensitivity for the first study was 83% and the specificity 99% – similar to that for the women in Hong Kong. The second study had lower sensitivity and specificity (67% and 88%), partly because of poor storage as a result of frequent power cuts.
The total cost per woman was $30.78.
“I was very excited when we saw the results,” Dr. Budukh recalled. “That day I couldn’t sleep ... such a wonderful result! I was excited to start the next phase immediately.”
Dr. Budukh has applied to the Indian government for funding for a larger trial involving 3,000 women. If successful, he hopes such evidence would be sufficient to convince the Indian government to make menstrual pad screening standard procedure for the 390 million women who live in India’s countryside.
Testing never-screened women for cervical cancer using menstrual pads appears to be relatively reliable, convenient, private, noninvasive, and incredibly cheap.
So who else has tried it?
The first published account of HPV in menstrual blood was a 2003 study by Tommy Tong and colleagues at the Princess Margaret Hospital in Hong Kong. The authors heralded, with lamentable optimism, “a new paradigm in cervical cancer screening.”
In the following 20 years, just six more studies appeared: two from Dr. Budukh’s field trial in India and four from hospital-based pilot studies in Hong Kong (in 2010 and 2018), South Korea (in 2016), and mainland China (in 2021). All these studies, although small, were published in top-flight journals and demonstrate high concordance between conventional high-risk HPV testing and menstrual-blood tests.
This news organization tried to find a U.S. thought-leader who had heard of the approach.
Elizabeth Fontham, MPH, DrPh, is the founding dean of the school of public health at Louisiana State University Health Center in New Orleans, and president of the American Cancer Society. Dr. Fontham said in an email that she had “no plans to evaluate the impact related to menstrual pads, but perhaps others have looked into that.”
Joy Melnikow, MD, MPH, was first author on the evidence synthesis driving the current cervical cancer screening recommendations from the U.S. Preventive Services Task Force. When asked about menstrual pad testing for HPV, she said she had “not heard of it before.”
The USPSTF guidelines don’t mention sanitary pads but acknowledge that “self-collection may be one strategy for increasing screening rates among populations where they are currently low.”
The USPSTF methodology excludes data from countries that don’t match the United States on the Human Development Index “or [are] not applicable to U.S. clinical settings or populations.” (Presumably, data from Hong Kong and South Korea would qualify; Indian data would not.)
Dr. Sahasrabuddhe of the NCI hadn’t heard of menstrual pad testing either, but he has a different explanation for lack of interest in this approach – or, indeed, any form of self-sampling for cervical cancer screening – in the United States.
“We have not seen movement happen in this space for years. ... If there is one intervention that we can simplify, that still has not been made widely available, it is self-sampling ... [but] we don’t have [Food and Drug Administration] approval for it,” Dr. Sahasrabuddhe said.
“Our system, at least in the U.S., is based on industry manufacturers seeking an approval for a particular way of collection and then clinicians and clinical-guideline bodies signing on. ... For a lot of reasons industry has shied away over the past several years, so far, at least, on seeking approval for self-sampling-based approaches,” he commented.
Dr. Sahasrabuddhe aims to change that. He heads a new NCI-led initiative called “The Last Mile,” a nationwide clinical trial supported by federal agencies, industry partners, and professional societies. The goal is to validate self-sampled HPV testing as non-inferior to specimens collected by providers. The team is currently finalizing the methodology of the study, so Dr. Sahasrabuddhe could not share the self-sampling methods that will be on trial, nor the industry partners who have signed up.
The following tests are approved in the United States for physician-collected HPV screening: Hybrid Capture 2, used in the Indian studies (Qiagen); cobas HPV (Roche); Aptima (Hologic); Cervista (Hologic); and Onclarity (Becton Dickinson).
Dr. Sahasrabuddhe said that, while a sanitary pad in a Ziploc bag is unlikely to make the grade for The Last Mile study, he doesn’t totally dismiss their potential and said the NCI is always open to new ideas.
“We are not supporting anybody specifically for menstrual pad-based collection device development,” Dr. Sahasrabuddhe said, “But if they fulfill other criteria for a small business–based grant application, they absolutely are welcome to apply for NCI funding for this.”
Said Dr. Melnikow: “Pre-COVID, the head of [the World Health Organization] said that we could eliminate cervical cancer from the globe and that we have the tools to do that now. And he’s right.”
Dr. Budukh, Dr. Melnikow, and Dr. Sahasrabuddhe disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unraveling primary ovarian insufficiency
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
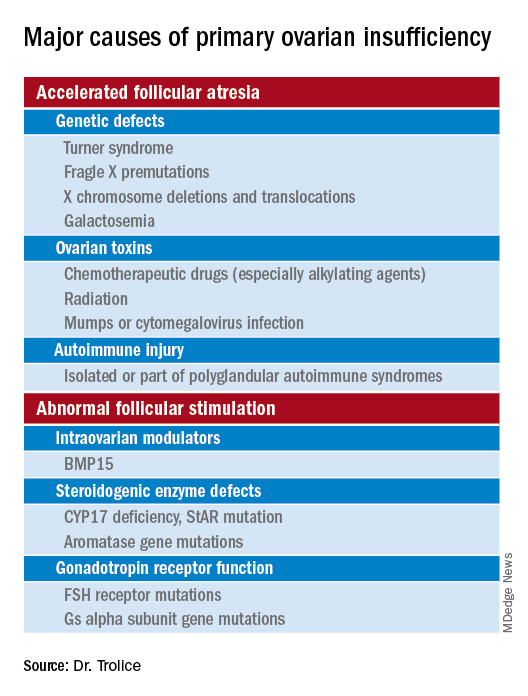
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years
Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Persistent problem: High C-section rates plague the South
All along, Julia Maeda knew she wanted to have her baby naturally. For her, that meant in a hospital, vaginally, without an epidural for pain relief.
This was her first pregnancy. And although she is a nurse, she was working with cancer patients at the time, not with laboring mothers or babies. “I really didn’t know what I was getting into,” said Ms. Maeda, now 32. “I didn’t do much preparation.”
Her home state of Mississippi has the highest cesarean section rate in the United States – nearly 4 in 10 women who give birth there deliver their babies via C-section. Almost 2 weeks past her due date in 2019, Ms. Maeda became one of them after her doctor came to her bedside while she was in labor.
“‘You’re not in distress, and your baby is not in distress – but we don’t want you to get that way, so we need to think about a C-section,’” she recalled her doctor saying. “I was totally defeated. I just gave in.”
C-sections are sometimes necessary and even lifesaving, but public health experts have long contended that too many performed in the U.S. aren’t. They argue it is major surgery accompanied by significant risk and a high price tag.
Overall, 31.8% of all births in the U.S. were C-sections in 2020, just a slight tick up from 31.7% the year before, according to the latest data from the Centers for Disease Control and Prevention. But that’s close to the peak in 2009, when it was 32.9%. And the rates are far higher in many states, especially across the South.
These high C-section rates have persisted – and in some states, such as Alabama and Kentucky, even grown slightly – despite continual calls to reduce them. And although the pandemic presented new challenges for pregnant women, research suggests that the U.S. C-section rate was unaffected by COVID. Instead, obstetricians and other health experts say the high rate is an intractable problem.
Some states, such as California and New Jersey, have reduced their rates through a variety of strategies, including sharing C-section data with doctors and hospitals. But change has proved difficult elsewhere, especially in the South and in Texas, where women are generally less healthy heading into their pregnancies and maternal and infant health problems are among the highest in the United States.
“We have to restructure how we think about C-sections,” said Veronica Gillispie-Bell, MD, an ob.gyn. who is medical director of the Louisiana Perinatal Quality Collaborative, Kenner, La., a group of 43 birthing hospitals focused on lowering Louisiana’s C-section rate. “It’s a lifesaving technique, but it’s also not without risks.”
She said C-sections, like any operation, create scar tissue, including in the uterus, which may complicate future pregnancies or abdominal surgeries. C-sections also typically lead to an extended hospital stay and recovery period and increase the chance of infection. Babies face risks, too. In rare cases, they can be nicked or cut during an incision.
Although C-sections are sometimes necessary, public health leaders say these surgeries have been overused in many places. Black women, particularly, are more likely to give birth by C-section than any other racial group in the country. Often, hospitals and even regions have wide, unexplained variations in rates.
“If you were delivering in Miami-Dade County, you had a 75% greater chance of having a cesarean than in northern Florida,” said William Sappenfield, MD, an ob.gyn. and epidemiologist at the University of South Florida, Tampa, who has studied the state’s high C-section rate.
Some physicians say their rates are driven by mothers who request the procedure, not by doctors. But Rebekah Gee, MD, an ob.gyn. at Louisiana State University Healthcare Network, New Orleans, and former secretary of the Louisiana Department of Health, said she saw C-section rates go dramatically up at 4 and 5 p.m. – around the time when doctors tend to want to go home.
She led several initiatives to improve birth outcomes in Louisiana, including leveling Medicaid payment rates to hospitals for vaginal deliveries and C-sections. In most places, C-sections are significantly more expensive than vaginal deliveries, making high C-section rates not only a concern for expectant mothers but also for taxpayers.
Medicaid pays for 60% of all births in Louisiana, according to KFF, and about half of all births in most Southern states, compared with 42% nationally. That’s one reason some states – including Louisiana, Tennessee, and Minnesota – have tried to tackle high C-section rates by changing how much Medicaid pays for them. But payment reform alone isn’t enough, Dr. Gee said.
“There was a guy in central Louisiana who was doing more C-sections and early elective deliveries than anyone in the U.S.,” she said. “When you have a culture like that, it’s hard to shift from it.”
Linda Schwimmer, president and CEO of the New Jersey Health Care Quality Institute, said many hospitals and doctors don’t even know their C-section rates. Sharing this data with doctors and hospitals – and making it public – made some providers uncomfortable, she said, but it ultimately worked. New Jersey’s C-section rate among first-time, low-risk mothers dropped from 33.1% in 2013 to 26.7% 6 years later once the state began sharing these data, among other initiatives.
The New Jersey Health Care Quality Institute and other groups like it around the country focus on reducing a subset of C-sections called “nulliparous, term, singleton, vertex” C-sections, or surgeries on first-time, full-term moms giving birth to a single infant who is positioned head-down in the uterus.
NTSV C-sections are important to track because women who have a C-section during their first pregnancy face a 90% chance of having another in subsequent pregnancies. Across the U.S., the rate for these C-sections was 25.9% in 2020 and 25.6% in 2019.
Elliott Main, MD, a maternal-fetal specialist at Stanford (Calif.) University and the medical director of the California Maternal Quality Care Collaborative, coauthored a paper, published in JAMA last year, that outlines interventions the collaborative took that lowered California’s NTSV C-Section rate from 26.0% in 2014 to 22.8% in 2019. Nationally, the rate was unchanged during that period.
Allowing women to labor for longer stretches of time before resorting to surgery is important, he said.
The cervix must be 10 cm dilated before a woman gives birth. The threshold for “active labor” used to be when the cervix was dilated at least 4 cm. In more recent years, though, the onset of active labor has been changed to 5-6 cm.
“People show up at the hospital too early,” said Toni Hill, president of the Mississippi Midwives Alliance. “If you show up to the hospital at 2-3 centimeters, you can be at 2-3 centimeters for weeks. I don’t even consider that labor.”
Too often, she said, women at an early stage of labor end up being induced and deliver via C-section.
“It’s almost like, at this point, C-sections are being handed out like lollipops,” said LA’Patricia Washington, a doula based in Jackson, Miss. Doulas are trained, nonmedical workers who help parents before, during, and after delivery.
Ms. Washington works with a nonprofit group, the Jackson Safer Childbirth Experience, that pays for doulas to help expectant mothers in the region. Some state Medicaid programs, such as New Jersey’s, reimburse for services by doulas because research shows they can reduce C-section rates. California has been trying to roll out the same benefit for its Medicaid members.
In 2020, when Julia Maeda became pregnant again, she paid out-of-pocket for a doula to attend the birth. The experience of having her son via C-section the previous year had been “emotionally and psychologically traumatic,” Ms. Maeda said.
She told her ob.gyn. that she wanted a VBAC, short for “vaginal birth after cesarean.” But, she said, “he just shook his head and said, ‘That’s not a good idea.’”
She had VBAC anyway. Ms. Maeda credits her doula with making it happen.
“Maybe just her presence relayed to the nursing staff that this was something I was serious about,” Ms. Maeda said. “They want you to have your baby during business hours. And biology doesn’t work that way.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
All along, Julia Maeda knew she wanted to have her baby naturally. For her, that meant in a hospital, vaginally, without an epidural for pain relief.
This was her first pregnancy. And although she is a nurse, she was working with cancer patients at the time, not with laboring mothers or babies. “I really didn’t know what I was getting into,” said Ms. Maeda, now 32. “I didn’t do much preparation.”
Her home state of Mississippi has the highest cesarean section rate in the United States – nearly 4 in 10 women who give birth there deliver their babies via C-section. Almost 2 weeks past her due date in 2019, Ms. Maeda became one of them after her doctor came to her bedside while she was in labor.
“‘You’re not in distress, and your baby is not in distress – but we don’t want you to get that way, so we need to think about a C-section,’” she recalled her doctor saying. “I was totally defeated. I just gave in.”
C-sections are sometimes necessary and even lifesaving, but public health experts have long contended that too many performed in the U.S. aren’t. They argue it is major surgery accompanied by significant risk and a high price tag.
Overall, 31.8% of all births in the U.S. were C-sections in 2020, just a slight tick up from 31.7% the year before, according to the latest data from the Centers for Disease Control and Prevention. But that’s close to the peak in 2009, when it was 32.9%. And the rates are far higher in many states, especially across the South.
These high C-section rates have persisted – and in some states, such as Alabama and Kentucky, even grown slightly – despite continual calls to reduce them. And although the pandemic presented new challenges for pregnant women, research suggests that the U.S. C-section rate was unaffected by COVID. Instead, obstetricians and other health experts say the high rate is an intractable problem.
Some states, such as California and New Jersey, have reduced their rates through a variety of strategies, including sharing C-section data with doctors and hospitals. But change has proved difficult elsewhere, especially in the South and in Texas, where women are generally less healthy heading into their pregnancies and maternal and infant health problems are among the highest in the United States.
“We have to restructure how we think about C-sections,” said Veronica Gillispie-Bell, MD, an ob.gyn. who is medical director of the Louisiana Perinatal Quality Collaborative, Kenner, La., a group of 43 birthing hospitals focused on lowering Louisiana’s C-section rate. “It’s a lifesaving technique, but it’s also not without risks.”
She said C-sections, like any operation, create scar tissue, including in the uterus, which may complicate future pregnancies or abdominal surgeries. C-sections also typically lead to an extended hospital stay and recovery period and increase the chance of infection. Babies face risks, too. In rare cases, they can be nicked or cut during an incision.
Although C-sections are sometimes necessary, public health leaders say these surgeries have been overused in many places. Black women, particularly, are more likely to give birth by C-section than any other racial group in the country. Often, hospitals and even regions have wide, unexplained variations in rates.
“If you were delivering in Miami-Dade County, you had a 75% greater chance of having a cesarean than in northern Florida,” said William Sappenfield, MD, an ob.gyn. and epidemiologist at the University of South Florida, Tampa, who has studied the state’s high C-section rate.
Some physicians say their rates are driven by mothers who request the procedure, not by doctors. But Rebekah Gee, MD, an ob.gyn. at Louisiana State University Healthcare Network, New Orleans, and former secretary of the Louisiana Department of Health, said she saw C-section rates go dramatically up at 4 and 5 p.m. – around the time when doctors tend to want to go home.
She led several initiatives to improve birth outcomes in Louisiana, including leveling Medicaid payment rates to hospitals for vaginal deliveries and C-sections. In most places, C-sections are significantly more expensive than vaginal deliveries, making high C-section rates not only a concern for expectant mothers but also for taxpayers.
Medicaid pays for 60% of all births in Louisiana, according to KFF, and about half of all births in most Southern states, compared with 42% nationally. That’s one reason some states – including Louisiana, Tennessee, and Minnesota – have tried to tackle high C-section rates by changing how much Medicaid pays for them. But payment reform alone isn’t enough, Dr. Gee said.
“There was a guy in central Louisiana who was doing more C-sections and early elective deliveries than anyone in the U.S.,” she said. “When you have a culture like that, it’s hard to shift from it.”
Linda Schwimmer, president and CEO of the New Jersey Health Care Quality Institute, said many hospitals and doctors don’t even know their C-section rates. Sharing this data with doctors and hospitals – and making it public – made some providers uncomfortable, she said, but it ultimately worked. New Jersey’s C-section rate among first-time, low-risk mothers dropped from 33.1% in 2013 to 26.7% 6 years later once the state began sharing these data, among other initiatives.
The New Jersey Health Care Quality Institute and other groups like it around the country focus on reducing a subset of C-sections called “nulliparous, term, singleton, vertex” C-sections, or surgeries on first-time, full-term moms giving birth to a single infant who is positioned head-down in the uterus.
NTSV C-sections are important to track because women who have a C-section during their first pregnancy face a 90% chance of having another in subsequent pregnancies. Across the U.S., the rate for these C-sections was 25.9% in 2020 and 25.6% in 2019.
Elliott Main, MD, a maternal-fetal specialist at Stanford (Calif.) University and the medical director of the California Maternal Quality Care Collaborative, coauthored a paper, published in JAMA last year, that outlines interventions the collaborative took that lowered California’s NTSV C-Section rate from 26.0% in 2014 to 22.8% in 2019. Nationally, the rate was unchanged during that period.
Allowing women to labor for longer stretches of time before resorting to surgery is important, he said.
The cervix must be 10 cm dilated before a woman gives birth. The threshold for “active labor” used to be when the cervix was dilated at least 4 cm. In more recent years, though, the onset of active labor has been changed to 5-6 cm.
“People show up at the hospital too early,” said Toni Hill, president of the Mississippi Midwives Alliance. “If you show up to the hospital at 2-3 centimeters, you can be at 2-3 centimeters for weeks. I don’t even consider that labor.”
Too often, she said, women at an early stage of labor end up being induced and deliver via C-section.
“It’s almost like, at this point, C-sections are being handed out like lollipops,” said LA’Patricia Washington, a doula based in Jackson, Miss. Doulas are trained, nonmedical workers who help parents before, during, and after delivery.
Ms. Washington works with a nonprofit group, the Jackson Safer Childbirth Experience, that pays for doulas to help expectant mothers in the region. Some state Medicaid programs, such as New Jersey’s, reimburse for services by doulas because research shows they can reduce C-section rates. California has been trying to roll out the same benefit for its Medicaid members.
In 2020, when Julia Maeda became pregnant again, she paid out-of-pocket for a doula to attend the birth. The experience of having her son via C-section the previous year had been “emotionally and psychologically traumatic,” Ms. Maeda said.
She told her ob.gyn. that she wanted a VBAC, short for “vaginal birth after cesarean.” But, she said, “he just shook his head and said, ‘That’s not a good idea.’”
She had VBAC anyway. Ms. Maeda credits her doula with making it happen.
“Maybe just her presence relayed to the nursing staff that this was something I was serious about,” Ms. Maeda said. “They want you to have your baby during business hours. And biology doesn’t work that way.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
All along, Julia Maeda knew she wanted to have her baby naturally. For her, that meant in a hospital, vaginally, without an epidural for pain relief.
This was her first pregnancy. And although she is a nurse, she was working with cancer patients at the time, not with laboring mothers or babies. “I really didn’t know what I was getting into,” said Ms. Maeda, now 32. “I didn’t do much preparation.”
Her home state of Mississippi has the highest cesarean section rate in the United States – nearly 4 in 10 women who give birth there deliver their babies via C-section. Almost 2 weeks past her due date in 2019, Ms. Maeda became one of them after her doctor came to her bedside while she was in labor.
“‘You’re not in distress, and your baby is not in distress – but we don’t want you to get that way, so we need to think about a C-section,’” she recalled her doctor saying. “I was totally defeated. I just gave in.”
C-sections are sometimes necessary and even lifesaving, but public health experts have long contended that too many performed in the U.S. aren’t. They argue it is major surgery accompanied by significant risk and a high price tag.
Overall, 31.8% of all births in the U.S. were C-sections in 2020, just a slight tick up from 31.7% the year before, according to the latest data from the Centers for Disease Control and Prevention. But that’s close to the peak in 2009, when it was 32.9%. And the rates are far higher in many states, especially across the South.
These high C-section rates have persisted – and in some states, such as Alabama and Kentucky, even grown slightly – despite continual calls to reduce them. And although the pandemic presented new challenges for pregnant women, research suggests that the U.S. C-section rate was unaffected by COVID. Instead, obstetricians and other health experts say the high rate is an intractable problem.
Some states, such as California and New Jersey, have reduced their rates through a variety of strategies, including sharing C-section data with doctors and hospitals. But change has proved difficult elsewhere, especially in the South and in Texas, where women are generally less healthy heading into their pregnancies and maternal and infant health problems are among the highest in the United States.
“We have to restructure how we think about C-sections,” said Veronica Gillispie-Bell, MD, an ob.gyn. who is medical director of the Louisiana Perinatal Quality Collaborative, Kenner, La., a group of 43 birthing hospitals focused on lowering Louisiana’s C-section rate. “It’s a lifesaving technique, but it’s also not without risks.”
She said C-sections, like any operation, create scar tissue, including in the uterus, which may complicate future pregnancies or abdominal surgeries. C-sections also typically lead to an extended hospital stay and recovery period and increase the chance of infection. Babies face risks, too. In rare cases, they can be nicked or cut during an incision.
Although C-sections are sometimes necessary, public health leaders say these surgeries have been overused in many places. Black women, particularly, are more likely to give birth by C-section than any other racial group in the country. Often, hospitals and even regions have wide, unexplained variations in rates.
“If you were delivering in Miami-Dade County, you had a 75% greater chance of having a cesarean than in northern Florida,” said William Sappenfield, MD, an ob.gyn. and epidemiologist at the University of South Florida, Tampa, who has studied the state’s high C-section rate.
Some physicians say their rates are driven by mothers who request the procedure, not by doctors. But Rebekah Gee, MD, an ob.gyn. at Louisiana State University Healthcare Network, New Orleans, and former secretary of the Louisiana Department of Health, said she saw C-section rates go dramatically up at 4 and 5 p.m. – around the time when doctors tend to want to go home.
She led several initiatives to improve birth outcomes in Louisiana, including leveling Medicaid payment rates to hospitals for vaginal deliveries and C-sections. In most places, C-sections are significantly more expensive than vaginal deliveries, making high C-section rates not only a concern for expectant mothers but also for taxpayers.
Medicaid pays for 60% of all births in Louisiana, according to KFF, and about half of all births in most Southern states, compared with 42% nationally. That’s one reason some states – including Louisiana, Tennessee, and Minnesota – have tried to tackle high C-section rates by changing how much Medicaid pays for them. But payment reform alone isn’t enough, Dr. Gee said.
“There was a guy in central Louisiana who was doing more C-sections and early elective deliveries than anyone in the U.S.,” she said. “When you have a culture like that, it’s hard to shift from it.”
Linda Schwimmer, president and CEO of the New Jersey Health Care Quality Institute, said many hospitals and doctors don’t even know their C-section rates. Sharing this data with doctors and hospitals – and making it public – made some providers uncomfortable, she said, but it ultimately worked. New Jersey’s C-section rate among first-time, low-risk mothers dropped from 33.1% in 2013 to 26.7% 6 years later once the state began sharing these data, among other initiatives.
The New Jersey Health Care Quality Institute and other groups like it around the country focus on reducing a subset of C-sections called “nulliparous, term, singleton, vertex” C-sections, or surgeries on first-time, full-term moms giving birth to a single infant who is positioned head-down in the uterus.
NTSV C-sections are important to track because women who have a C-section during their first pregnancy face a 90% chance of having another in subsequent pregnancies. Across the U.S., the rate for these C-sections was 25.9% in 2020 and 25.6% in 2019.
Elliott Main, MD, a maternal-fetal specialist at Stanford (Calif.) University and the medical director of the California Maternal Quality Care Collaborative, coauthored a paper, published in JAMA last year, that outlines interventions the collaborative took that lowered California’s NTSV C-Section rate from 26.0% in 2014 to 22.8% in 2019. Nationally, the rate was unchanged during that period.
Allowing women to labor for longer stretches of time before resorting to surgery is important, he said.
The cervix must be 10 cm dilated before a woman gives birth. The threshold for “active labor” used to be when the cervix was dilated at least 4 cm. In more recent years, though, the onset of active labor has been changed to 5-6 cm.
“People show up at the hospital too early,” said Toni Hill, president of the Mississippi Midwives Alliance. “If you show up to the hospital at 2-3 centimeters, you can be at 2-3 centimeters for weeks. I don’t even consider that labor.”
Too often, she said, women at an early stage of labor end up being induced and deliver via C-section.
“It’s almost like, at this point, C-sections are being handed out like lollipops,” said LA’Patricia Washington, a doula based in Jackson, Miss. Doulas are trained, nonmedical workers who help parents before, during, and after delivery.
Ms. Washington works with a nonprofit group, the Jackson Safer Childbirth Experience, that pays for doulas to help expectant mothers in the region. Some state Medicaid programs, such as New Jersey’s, reimburse for services by doulas because research shows they can reduce C-section rates. California has been trying to roll out the same benefit for its Medicaid members.
In 2020, when Julia Maeda became pregnant again, she paid out-of-pocket for a doula to attend the birth. The experience of having her son via C-section the previous year had been “emotionally and psychologically traumatic,” Ms. Maeda said.
She told her ob.gyn. that she wanted a VBAC, short for “vaginal birth after cesarean.” But, she said, “he just shook his head and said, ‘That’s not a good idea.’”
She had VBAC anyway. Ms. Maeda credits her doula with making it happen.
“Maybe just her presence relayed to the nursing staff that this was something I was serious about,” Ms. Maeda said. “They want you to have your baby during business hours. And biology doesn’t work that way.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Managing Heavy Menstrual Bleeding Associated with Fibroids
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Diagnosing PTSD: Heart rate variability may help
, according to a study published in Frontiers in Psychiatry.
It is estimated that between 8% and 15% of clinically recognized pregnancies and up to 30% of all pregnancies result in miscarriage – a loss that can be devastating for everyone. There are limited data on the strength of the association between perinatal loss and subsequent common mental health disorders, such as anxiety, depression, and PTSD. The prevalence of PTSD among this group is still unknown, and one of the factors that contribute to the absence of data is that diagnostic evaluation is subjective.
To address this issue, researchers from Anhembi Morumbi University (UAM) in São José dos Campos, Brazil, along with teams in the United States and United Arab Emirates (UAE), investigated biomarkers for the severity of PTSD. The hope is that the research will enable psychiatrists to assess women who experience pregnancy loss more objectively. Study author Ovidiu Constantin Baltatu, MD, PhD, a professor at Brazil’s UAM and the UAE’s Khalifa University, spoke to this news organization about the study.
Under the guidance of Dr. Baltatu, psychologist Cláudia de Faria Cardoso carried out the research as part of her studies in biomedical engineering at UAM. Fifty-three women were recruited; the average age of the cohort was 33 years. All participants had a history of at least one perinatal loss. Pregnancy loss intervals ranged from less than 40 days to more than 6 months.
Participants completed a clinical interview and a questionnaire; PTSD symptoms were assessed on the basis of criteria in the DSM-5. The instrument used for the assessment was the Brazilian version of the Post-traumatic Stress Disorder Checklist (PCL-5). In addition, to evaluate general autonomic dysfunction, patients completed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire.
HRV was assessed during a deep breathing test using an HRV scanner system with wireless electrocardiography that enabled real-time data analysis and visualization. The investigators examined the following HRV measures: standard deviation (SD) of normal R-R wave intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent normal R wave intervals, and the number of all R-R intervals in which the change in consecutive normal sinus intervals exceeds 50 ms divided by the total number of R-R intervals measured.
Of the 53 participants, 25 had been diagnosed with pregnancy loss–induced PTSD. The results indicated a significant association between PCL-5 scores and HRV indices. The SDNN index effectively distinguished between patients with PTSD and those without.
To Dr. Baltatu, HRV indices reflect dysfunction of the autonomic nervous system (ANS), one of the major neural pathways activated by stress.
Although the deep breathing test has been around for a long time, it’s not widely used in current clinical practice, he said. According to him, maximum and minimum heart rates during breathing at six cycles per minute can typically be used to calculate the inspiratory-to-expiratory ratio, thus providing an indication of ANS function. “Our group introduced the study of HRV during deep breathing test, which is a step forward,” he said.
The methodology used by the team was well received by the participants. “With the deep breathing test, the women were able to look at a screen and see real-time graphics displaying the stress that they were experiencing after having suffered trauma. This visualization of objective measures was perceived as an improved care,” said Dr. Baltatu.
In general, HRV provides a more objective means of diagnosing PTSD. “Normally, PTSD is assessed through a questionnaire and an interview with psychologists,” said Dr. Baltatu. The subjectivity of the assessment is one of the main factors associated with the underdiagnosis of this condition, he explained.
It is important to remember that other factors, such as a lack of awareness about the problem, also hinder the diagnosis of PTSD in this population, Dr. Baltatu added. Women who have had a miscarriage often don’t think that their symptoms may result from PTSD. This fact highlights why it is so important that hospitals have a clinical psychologist on staff. In addition, Dr. Baltatu pointed out that a woman who experiences a pregnancy loss usually has negative memories of the hospital and is therefore reluctant to reach out for professional help. “In our study, all psychological care and assessments took place outside of a hospital setting, which the participants seemed to appreciate,” he emphasized.
Dr. Baltatu and his team are conducting follow-up research. The preliminary results indicate that the biomarkers identified in the study are promising in the assessment of patients’ clinical progress. This finding may reflect the fact that the HRV indices have proven useful not only in diagnosing but also in monitoring women in treatment, because they are able to identify which patients are responding better to treatment.
A version of this article first appeared on Medscape.com.
, according to a study published in Frontiers in Psychiatry.
It is estimated that between 8% and 15% of clinically recognized pregnancies and up to 30% of all pregnancies result in miscarriage – a loss that can be devastating for everyone. There are limited data on the strength of the association between perinatal loss and subsequent common mental health disorders, such as anxiety, depression, and PTSD. The prevalence of PTSD among this group is still unknown, and one of the factors that contribute to the absence of data is that diagnostic evaluation is subjective.
To address this issue, researchers from Anhembi Morumbi University (UAM) in São José dos Campos, Brazil, along with teams in the United States and United Arab Emirates (UAE), investigated biomarkers for the severity of PTSD. The hope is that the research will enable psychiatrists to assess women who experience pregnancy loss more objectively. Study author Ovidiu Constantin Baltatu, MD, PhD, a professor at Brazil’s UAM and the UAE’s Khalifa University, spoke to this news organization about the study.
Under the guidance of Dr. Baltatu, psychologist Cláudia de Faria Cardoso carried out the research as part of her studies in biomedical engineering at UAM. Fifty-three women were recruited; the average age of the cohort was 33 years. All participants had a history of at least one perinatal loss. Pregnancy loss intervals ranged from less than 40 days to more than 6 months.
Participants completed a clinical interview and a questionnaire; PTSD symptoms were assessed on the basis of criteria in the DSM-5. The instrument used for the assessment was the Brazilian version of the Post-traumatic Stress Disorder Checklist (PCL-5). In addition, to evaluate general autonomic dysfunction, patients completed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire.
HRV was assessed during a deep breathing test using an HRV scanner system with wireless electrocardiography that enabled real-time data analysis and visualization. The investigators examined the following HRV measures: standard deviation (SD) of normal R-R wave intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent normal R wave intervals, and the number of all R-R intervals in which the change in consecutive normal sinus intervals exceeds 50 ms divided by the total number of R-R intervals measured.
Of the 53 participants, 25 had been diagnosed with pregnancy loss–induced PTSD. The results indicated a significant association between PCL-5 scores and HRV indices. The SDNN index effectively distinguished between patients with PTSD and those without.
To Dr. Baltatu, HRV indices reflect dysfunction of the autonomic nervous system (ANS), one of the major neural pathways activated by stress.
Although the deep breathing test has been around for a long time, it’s not widely used in current clinical practice, he said. According to him, maximum and minimum heart rates during breathing at six cycles per minute can typically be used to calculate the inspiratory-to-expiratory ratio, thus providing an indication of ANS function. “Our group introduced the study of HRV during deep breathing test, which is a step forward,” he said.
The methodology used by the team was well received by the participants. “With the deep breathing test, the women were able to look at a screen and see real-time graphics displaying the stress that they were experiencing after having suffered trauma. This visualization of objective measures was perceived as an improved care,” said Dr. Baltatu.
In general, HRV provides a more objective means of diagnosing PTSD. “Normally, PTSD is assessed through a questionnaire and an interview with psychologists,” said Dr. Baltatu. The subjectivity of the assessment is one of the main factors associated with the underdiagnosis of this condition, he explained.
It is important to remember that other factors, such as a lack of awareness about the problem, also hinder the diagnosis of PTSD in this population, Dr. Baltatu added. Women who have had a miscarriage often don’t think that their symptoms may result from PTSD. This fact highlights why it is so important that hospitals have a clinical psychologist on staff. In addition, Dr. Baltatu pointed out that a woman who experiences a pregnancy loss usually has negative memories of the hospital and is therefore reluctant to reach out for professional help. “In our study, all psychological care and assessments took place outside of a hospital setting, which the participants seemed to appreciate,” he emphasized.
Dr. Baltatu and his team are conducting follow-up research. The preliminary results indicate that the biomarkers identified in the study are promising in the assessment of patients’ clinical progress. This finding may reflect the fact that the HRV indices have proven useful not only in diagnosing but also in monitoring women in treatment, because they are able to identify which patients are responding better to treatment.
A version of this article first appeared on Medscape.com.
, according to a study published in Frontiers in Psychiatry.
It is estimated that between 8% and 15% of clinically recognized pregnancies and up to 30% of all pregnancies result in miscarriage – a loss that can be devastating for everyone. There are limited data on the strength of the association between perinatal loss and subsequent common mental health disorders, such as anxiety, depression, and PTSD. The prevalence of PTSD among this group is still unknown, and one of the factors that contribute to the absence of data is that diagnostic evaluation is subjective.
To address this issue, researchers from Anhembi Morumbi University (UAM) in São José dos Campos, Brazil, along with teams in the United States and United Arab Emirates (UAE), investigated biomarkers for the severity of PTSD. The hope is that the research will enable psychiatrists to assess women who experience pregnancy loss more objectively. Study author Ovidiu Constantin Baltatu, MD, PhD, a professor at Brazil’s UAM and the UAE’s Khalifa University, spoke to this news organization about the study.
Under the guidance of Dr. Baltatu, psychologist Cláudia de Faria Cardoso carried out the research as part of her studies in biomedical engineering at UAM. Fifty-three women were recruited; the average age of the cohort was 33 years. All participants had a history of at least one perinatal loss. Pregnancy loss intervals ranged from less than 40 days to more than 6 months.
Participants completed a clinical interview and a questionnaire; PTSD symptoms were assessed on the basis of criteria in the DSM-5. The instrument used for the assessment was the Brazilian version of the Post-traumatic Stress Disorder Checklist (PCL-5). In addition, to evaluate general autonomic dysfunction, patients completed the Composite Autonomic Symptom Score 31 (COMPASS-31) questionnaire.
HRV was assessed during a deep breathing test using an HRV scanner system with wireless electrocardiography that enabled real-time data analysis and visualization. The investigators examined the following HRV measures: standard deviation (SD) of normal R-R wave intervals (SDNN), square root of the mean of the sum of the squares of differences between adjacent normal R wave intervals, and the number of all R-R intervals in which the change in consecutive normal sinus intervals exceeds 50 ms divided by the total number of R-R intervals measured.
Of the 53 participants, 25 had been diagnosed with pregnancy loss–induced PTSD. The results indicated a significant association between PCL-5 scores and HRV indices. The SDNN index effectively distinguished between patients with PTSD and those without.
To Dr. Baltatu, HRV indices reflect dysfunction of the autonomic nervous system (ANS), one of the major neural pathways activated by stress.
Although the deep breathing test has been around for a long time, it’s not widely used in current clinical practice, he said. According to him, maximum and minimum heart rates during breathing at six cycles per minute can typically be used to calculate the inspiratory-to-expiratory ratio, thus providing an indication of ANS function. “Our group introduced the study of HRV during deep breathing test, which is a step forward,” he said.
The methodology used by the team was well received by the participants. “With the deep breathing test, the women were able to look at a screen and see real-time graphics displaying the stress that they were experiencing after having suffered trauma. This visualization of objective measures was perceived as an improved care,” said Dr. Baltatu.
In general, HRV provides a more objective means of diagnosing PTSD. “Normally, PTSD is assessed through a questionnaire and an interview with psychologists,” said Dr. Baltatu. The subjectivity of the assessment is one of the main factors associated with the underdiagnosis of this condition, he explained.
It is important to remember that other factors, such as a lack of awareness about the problem, also hinder the diagnosis of PTSD in this population, Dr. Baltatu added. Women who have had a miscarriage often don’t think that their symptoms may result from PTSD. This fact highlights why it is so important that hospitals have a clinical psychologist on staff. In addition, Dr. Baltatu pointed out that a woman who experiences a pregnancy loss usually has negative memories of the hospital and is therefore reluctant to reach out for professional help. “In our study, all psychological care and assessments took place outside of a hospital setting, which the participants seemed to appreciate,” he emphasized.
Dr. Baltatu and his team are conducting follow-up research. The preliminary results indicate that the biomarkers identified in the study are promising in the assessment of patients’ clinical progress. This finding may reflect the fact that the HRV indices have proven useful not only in diagnosing but also in monitoring women in treatment, because they are able to identify which patients are responding better to treatment.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PSYCHIATRY
Babies die as congenital syphilis continues a decade-long surge across the U.S.
For a decade, the number of babies born with syphilis in the United States has surged, undeterred. Data released Apr. 12 by the Centers for Disease Control and Prevention shows just how dire the outbreak has become.
In 2012, 332 babies were born infected with the disease. In 2021, that number had climbed nearly sevenfold, to at least 2,268, according to preliminary estimates. And 166 of those babies died.
About 7% of babies diagnosed with syphilis in recent years have died; thousands of others born with the disease have faced problems that include brain and bone malformations, blindness, and organ damage.
For public health officials, the situation is all the more heartbreaking, considering that congenital syphilis rates reached near-historic modern lows from 2000 to 2012 amid ambitious prevention and education efforts. By 2020, following a sharp erosion in funding and attention, the nationwide case rate was more than seven times that of 2012.
“The really depressing thing about it is we had this thing virtually eradicated back in the year 2000,” said William Andrews, a public information officer for Oklahoma’s sexual health and harm reduction service. “Now it’s back with a vengeance. We are really trying to get the message out that sexual health is health. It’s nothing to be ashamed of.”
Even as caseloads soar, the CDC budget for STD prevention – the primary funding source for most public health departments – has been largely stagnant for two decades, its purchasing power dragged even lower by inflation.
The CDC report on STD trends provides official data on congenital syphilis cases for 2020, as well as preliminary case counts for 2021 that are expected to increase. CDC data shows that congenital syphilis rates in 2020 continued to climb in already overwhelmed states like Texas, California, and Nevada and that the disease is now present in almost every state in the nation. All but three states – Maine, New Hampshire, and Vermont – reported congenital syphilis cases in 2020.
From 2011 to 2020, congenital syphilis resulted in 633 documented stillbirths and infant deaths, according to the new CDC data.
Preventing congenital syphilis – the term used when syphilis is transferred to a fetus in utero – is from a medical standpoint exceedingly simple: If a pregnant woman is diagnosed at least a month before giving birth, just a few shots of penicillin have a near-perfect cure rate for mother and baby. But funding cuts and competing priorities in the nation’s fragmented public health care system have vastly narrowed access to such services.
The reasons pregnant people with syphilis go undiagnosed or untreated vary geographically, according to data collected by states and analyzed by the CDC.
In Western states, the largest share of cases involve women who have received little to no prenatal care and aren’t tested for syphilis until they give birth. Many have substance use disorders, primarily related to methamphetamines. “They’ve felt a lot of judgment and stigma by the medical community,” said Stephanie Pierce, MD, a maternal fetal medicine specialist at the University of Oklahoma, Oklahoma City, who runs a clinic for women with high-risk pregnancies.
In Southern states, a CDC study of 2018 data found that the largest share of congenital syphilis cases were among women who had been tested and diagnosed but hadn’t received treatment. That year, among Black moms who gave birth to a baby with syphilis, 37% had not been treated adequately even though they’d received a timely diagnosis. Among white moms, that number was 24%. Longstanding racism in medical care, poverty, transportation issues, poorly funded public health departments, and crowded clinics whose employees are too overworked to follow up with patients all contribute to the problem, according to infectious disease experts.
Doctors are also noticing a growing number of women who are treated for syphilis but reinfected during pregnancy. Amid rising cases and stagnant resources, some states have focused disease investigations on pregnant women of childbearing age; they can no longer prioritize treating sexual partners who are also infected.
Eric McGrath, MD, a pediatric infectious disease specialist at Wayne State University, Detroit, said that he’d seen several newborns in recent years whose mothers had been treated for syphilis but then were re-exposed during pregnancy by partners who hadn’t been treated.
Treating a newborn baby for syphilis isn’t trivial. Penicillin carries little risk, but delivering it to a baby often involves a lumbar puncture and other painful procedures. And treatment typically means keeping the baby in the hospital for 10 days, interrupting an important time for family bonding.
Dr. McGrath has seen a couple of babies in his career who weren’t diagnosed or treated at birth and later came to him with full-blown syphilis complications, including full-body rashes and inflamed livers. It was an awful experience he doesn’t want to repeat. The preferred course, he said, is to spare the baby the ordeal and treat parents early in the pregnancy.
But in some places, providers aren’t routinely testing for syphilis. Although most states mandate testing at some point during pregnancy, as of last year just 14 required it for everyone in the third trimester. The CDC recommends third-trimester testing in areas with high rates of syphilis, a growing share of the United States.
After Arizona declared a statewide outbreak in 2018, state health officials wanted to know whether widespread testing in the third trimester could have prevented infections. Looking at 18 months of data, analysts found that nearly three-quarters of the more than 200 pregnant women diagnosed with syphilis in 2017 and the first half of 2018 got treatment. That left 57 babies born with syphilis, nine of whom died. The analysts estimated that a third of the infections could have been prevented with testing in the third trimester.
Based on the numbers they saw in those 18 months, officials estimated that screening all women on Medicaid in the third trimester would cost the state $113,300 annually, and that treating all cases of syphilis that screening would catch could be done for just $113. Factoring in the hospitalization costs for infected infants, the officials concluded the additional testing would save the state money.
And yet prevention money has been hard to come by. Taking inflation into account, CDC prevention funding for STDs has fallen 41% since 2003, according to an analysis by the National Coalition of STD Directors. That’s even as cases have risen, leaving public health departments saddled with more work and far less money.
Janine Waters, STD program manager for the state of New Mexico, has watched the unraveling. When Ms. Waters started her career more than 20 years ago, she and her colleagues followed up on every case of chlamydia, gonorrhea, and syphilis reported, not only making sure that people got treatment but also getting in touch with their sexual partners, with the aim of stopping the spread of infection. In a 2019 interview with Kaiser Health News, she said her team was struggling to keep up with syphilis alone, even as they registered with dread congenital syphilis cases surging in neighboring Texas and Arizona.
By 2020, New Mexico had the highest rate of congenital syphilis in the country.
The COVID-19 pandemic drained the remaining resources. Half of health departments across the country discontinued STD fieldwork altogether, diverting their resources to COVID. In California, which for years has struggled with high rates of congenital syphilis, three-quarters of local health departments dispatched more than half of their STD staffers to work on COVID.
As the pandemic ebbs – at least in the short term – many public health departments are turning their attention back to syphilis and other diseases. And they are doing it with reinforcements. Although the Biden administration’s proposed STD prevention budget for 2023 remains flat, the American Rescue Plan Act included $200 million to help health departments boost contact tracing and surveillance for covid and other infectious diseases. Many departments are funneling that money toward STDs.
The money is an infusion that state health officials say will make a difference. But when taking inflation into account, it essentially brings STD prevention funding back to what it was in 2003, said Stephanie Arnold Pang of the National Coalition of STD Directors. And the American Rescue Plan money doesn’t cover some aspects of STD prevention, including clinical services.
The coalition wants to revive dedicated STD clinics, where people can drop in for testing and treatment at little to no cost. Advocates say that would fill a void that has plagued treatment efforts since public clinics closed en masse in the wake of the 2008 recession.
Texas, battling its own pervasive outbreak, will use its share of American Rescue Plan money to fill 94 new positions focused on various aspects of STD prevention. Those hires will bolster a range of measures the state put in place before the pandemic, including an updated data system to track infections, review boards in major cities that examine what went wrong for every case of congenital syphilis, and a requirement that providers test for syphilis during the third trimester of pregnancy. The suite of interventions seems to be working, but it could be a while before cases go down, said Amy Carter, the state’s congenital syphilis coordinator.
“The growth didn’t happen overnight,” Ms. Carter said. “So our prevention efforts aren’t going to have a direct impact overnight either.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation
For a decade, the number of babies born with syphilis in the United States has surged, undeterred. Data released Apr. 12 by the Centers for Disease Control and Prevention shows just how dire the outbreak has become.
In 2012, 332 babies were born infected with the disease. In 2021, that number had climbed nearly sevenfold, to at least 2,268, according to preliminary estimates. And 166 of those babies died.
About 7% of babies diagnosed with syphilis in recent years have died; thousands of others born with the disease have faced problems that include brain and bone malformations, blindness, and organ damage.
For public health officials, the situation is all the more heartbreaking, considering that congenital syphilis rates reached near-historic modern lows from 2000 to 2012 amid ambitious prevention and education efforts. By 2020, following a sharp erosion in funding and attention, the nationwide case rate was more than seven times that of 2012.
“The really depressing thing about it is we had this thing virtually eradicated back in the year 2000,” said William Andrews, a public information officer for Oklahoma’s sexual health and harm reduction service. “Now it’s back with a vengeance. We are really trying to get the message out that sexual health is health. It’s nothing to be ashamed of.”
Even as caseloads soar, the CDC budget for STD prevention – the primary funding source for most public health departments – has been largely stagnant for two decades, its purchasing power dragged even lower by inflation.
The CDC report on STD trends provides official data on congenital syphilis cases for 2020, as well as preliminary case counts for 2021 that are expected to increase. CDC data shows that congenital syphilis rates in 2020 continued to climb in already overwhelmed states like Texas, California, and Nevada and that the disease is now present in almost every state in the nation. All but three states – Maine, New Hampshire, and Vermont – reported congenital syphilis cases in 2020.
From 2011 to 2020, congenital syphilis resulted in 633 documented stillbirths and infant deaths, according to the new CDC data.
Preventing congenital syphilis – the term used when syphilis is transferred to a fetus in utero – is from a medical standpoint exceedingly simple: If a pregnant woman is diagnosed at least a month before giving birth, just a few shots of penicillin have a near-perfect cure rate for mother and baby. But funding cuts and competing priorities in the nation’s fragmented public health care system have vastly narrowed access to such services.
The reasons pregnant people with syphilis go undiagnosed or untreated vary geographically, according to data collected by states and analyzed by the CDC.
In Western states, the largest share of cases involve women who have received little to no prenatal care and aren’t tested for syphilis until they give birth. Many have substance use disorders, primarily related to methamphetamines. “They’ve felt a lot of judgment and stigma by the medical community,” said Stephanie Pierce, MD, a maternal fetal medicine specialist at the University of Oklahoma, Oklahoma City, who runs a clinic for women with high-risk pregnancies.
In Southern states, a CDC study of 2018 data found that the largest share of congenital syphilis cases were among women who had been tested and diagnosed but hadn’t received treatment. That year, among Black moms who gave birth to a baby with syphilis, 37% had not been treated adequately even though they’d received a timely diagnosis. Among white moms, that number was 24%. Longstanding racism in medical care, poverty, transportation issues, poorly funded public health departments, and crowded clinics whose employees are too overworked to follow up with patients all contribute to the problem, according to infectious disease experts.
Doctors are also noticing a growing number of women who are treated for syphilis but reinfected during pregnancy. Amid rising cases and stagnant resources, some states have focused disease investigations on pregnant women of childbearing age; they can no longer prioritize treating sexual partners who are also infected.
Eric McGrath, MD, a pediatric infectious disease specialist at Wayne State University, Detroit, said that he’d seen several newborns in recent years whose mothers had been treated for syphilis but then were re-exposed during pregnancy by partners who hadn’t been treated.
Treating a newborn baby for syphilis isn’t trivial. Penicillin carries little risk, but delivering it to a baby often involves a lumbar puncture and other painful procedures. And treatment typically means keeping the baby in the hospital for 10 days, interrupting an important time for family bonding.
Dr. McGrath has seen a couple of babies in his career who weren’t diagnosed or treated at birth and later came to him with full-blown syphilis complications, including full-body rashes and inflamed livers. It was an awful experience he doesn’t want to repeat. The preferred course, he said, is to spare the baby the ordeal and treat parents early in the pregnancy.
But in some places, providers aren’t routinely testing for syphilis. Although most states mandate testing at some point during pregnancy, as of last year just 14 required it for everyone in the third trimester. The CDC recommends third-trimester testing in areas with high rates of syphilis, a growing share of the United States.
After Arizona declared a statewide outbreak in 2018, state health officials wanted to know whether widespread testing in the third trimester could have prevented infections. Looking at 18 months of data, analysts found that nearly three-quarters of the more than 200 pregnant women diagnosed with syphilis in 2017 and the first half of 2018 got treatment. That left 57 babies born with syphilis, nine of whom died. The analysts estimated that a third of the infections could have been prevented with testing in the third trimester.
Based on the numbers they saw in those 18 months, officials estimated that screening all women on Medicaid in the third trimester would cost the state $113,300 annually, and that treating all cases of syphilis that screening would catch could be done for just $113. Factoring in the hospitalization costs for infected infants, the officials concluded the additional testing would save the state money.
And yet prevention money has been hard to come by. Taking inflation into account, CDC prevention funding for STDs has fallen 41% since 2003, according to an analysis by the National Coalition of STD Directors. That’s even as cases have risen, leaving public health departments saddled with more work and far less money.
Janine Waters, STD program manager for the state of New Mexico, has watched the unraveling. When Ms. Waters started her career more than 20 years ago, she and her colleagues followed up on every case of chlamydia, gonorrhea, and syphilis reported, not only making sure that people got treatment but also getting in touch with their sexual partners, with the aim of stopping the spread of infection. In a 2019 interview with Kaiser Health News, she said her team was struggling to keep up with syphilis alone, even as they registered with dread congenital syphilis cases surging in neighboring Texas and Arizona.
By 2020, New Mexico had the highest rate of congenital syphilis in the country.
The COVID-19 pandemic drained the remaining resources. Half of health departments across the country discontinued STD fieldwork altogether, diverting their resources to COVID. In California, which for years has struggled with high rates of congenital syphilis, three-quarters of local health departments dispatched more than half of their STD staffers to work on COVID.
As the pandemic ebbs – at least in the short term – many public health departments are turning their attention back to syphilis and other diseases. And they are doing it with reinforcements. Although the Biden administration’s proposed STD prevention budget for 2023 remains flat, the American Rescue Plan Act included $200 million to help health departments boost contact tracing and surveillance for covid and other infectious diseases. Many departments are funneling that money toward STDs.
The money is an infusion that state health officials say will make a difference. But when taking inflation into account, it essentially brings STD prevention funding back to what it was in 2003, said Stephanie Arnold Pang of the National Coalition of STD Directors. And the American Rescue Plan money doesn’t cover some aspects of STD prevention, including clinical services.
The coalition wants to revive dedicated STD clinics, where people can drop in for testing and treatment at little to no cost. Advocates say that would fill a void that has plagued treatment efforts since public clinics closed en masse in the wake of the 2008 recession.
Texas, battling its own pervasive outbreak, will use its share of American Rescue Plan money to fill 94 new positions focused on various aspects of STD prevention. Those hires will bolster a range of measures the state put in place before the pandemic, including an updated data system to track infections, review boards in major cities that examine what went wrong for every case of congenital syphilis, and a requirement that providers test for syphilis during the third trimester of pregnancy. The suite of interventions seems to be working, but it could be a while before cases go down, said Amy Carter, the state’s congenital syphilis coordinator.
“The growth didn’t happen overnight,” Ms. Carter said. “So our prevention efforts aren’t going to have a direct impact overnight either.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation
For a decade, the number of babies born with syphilis in the United States has surged, undeterred. Data released Apr. 12 by the Centers for Disease Control and Prevention shows just how dire the outbreak has become.
In 2012, 332 babies were born infected with the disease. In 2021, that number had climbed nearly sevenfold, to at least 2,268, according to preliminary estimates. And 166 of those babies died.
About 7% of babies diagnosed with syphilis in recent years have died; thousands of others born with the disease have faced problems that include brain and bone malformations, blindness, and organ damage.
For public health officials, the situation is all the more heartbreaking, considering that congenital syphilis rates reached near-historic modern lows from 2000 to 2012 amid ambitious prevention and education efforts. By 2020, following a sharp erosion in funding and attention, the nationwide case rate was more than seven times that of 2012.
“The really depressing thing about it is we had this thing virtually eradicated back in the year 2000,” said William Andrews, a public information officer for Oklahoma’s sexual health and harm reduction service. “Now it’s back with a vengeance. We are really trying to get the message out that sexual health is health. It’s nothing to be ashamed of.”
Even as caseloads soar, the CDC budget for STD prevention – the primary funding source for most public health departments – has been largely stagnant for two decades, its purchasing power dragged even lower by inflation.
The CDC report on STD trends provides official data on congenital syphilis cases for 2020, as well as preliminary case counts for 2021 that are expected to increase. CDC data shows that congenital syphilis rates in 2020 continued to climb in already overwhelmed states like Texas, California, and Nevada and that the disease is now present in almost every state in the nation. All but three states – Maine, New Hampshire, and Vermont – reported congenital syphilis cases in 2020.
From 2011 to 2020, congenital syphilis resulted in 633 documented stillbirths and infant deaths, according to the new CDC data.
Preventing congenital syphilis – the term used when syphilis is transferred to a fetus in utero – is from a medical standpoint exceedingly simple: If a pregnant woman is diagnosed at least a month before giving birth, just a few shots of penicillin have a near-perfect cure rate for mother and baby. But funding cuts and competing priorities in the nation’s fragmented public health care system have vastly narrowed access to such services.
The reasons pregnant people with syphilis go undiagnosed or untreated vary geographically, according to data collected by states and analyzed by the CDC.
In Western states, the largest share of cases involve women who have received little to no prenatal care and aren’t tested for syphilis until they give birth. Many have substance use disorders, primarily related to methamphetamines. “They’ve felt a lot of judgment and stigma by the medical community,” said Stephanie Pierce, MD, a maternal fetal medicine specialist at the University of Oklahoma, Oklahoma City, who runs a clinic for women with high-risk pregnancies.
In Southern states, a CDC study of 2018 data found that the largest share of congenital syphilis cases were among women who had been tested and diagnosed but hadn’t received treatment. That year, among Black moms who gave birth to a baby with syphilis, 37% had not been treated adequately even though they’d received a timely diagnosis. Among white moms, that number was 24%. Longstanding racism in medical care, poverty, transportation issues, poorly funded public health departments, and crowded clinics whose employees are too overworked to follow up with patients all contribute to the problem, according to infectious disease experts.
Doctors are also noticing a growing number of women who are treated for syphilis but reinfected during pregnancy. Amid rising cases and stagnant resources, some states have focused disease investigations on pregnant women of childbearing age; they can no longer prioritize treating sexual partners who are also infected.
Eric McGrath, MD, a pediatric infectious disease specialist at Wayne State University, Detroit, said that he’d seen several newborns in recent years whose mothers had been treated for syphilis but then were re-exposed during pregnancy by partners who hadn’t been treated.
Treating a newborn baby for syphilis isn’t trivial. Penicillin carries little risk, but delivering it to a baby often involves a lumbar puncture and other painful procedures. And treatment typically means keeping the baby in the hospital for 10 days, interrupting an important time for family bonding.
Dr. McGrath has seen a couple of babies in his career who weren’t diagnosed or treated at birth and later came to him with full-blown syphilis complications, including full-body rashes and inflamed livers. It was an awful experience he doesn’t want to repeat. The preferred course, he said, is to spare the baby the ordeal and treat parents early in the pregnancy.
But in some places, providers aren’t routinely testing for syphilis. Although most states mandate testing at some point during pregnancy, as of last year just 14 required it for everyone in the third trimester. The CDC recommends third-trimester testing in areas with high rates of syphilis, a growing share of the United States.
After Arizona declared a statewide outbreak in 2018, state health officials wanted to know whether widespread testing in the third trimester could have prevented infections. Looking at 18 months of data, analysts found that nearly three-quarters of the more than 200 pregnant women diagnosed with syphilis in 2017 and the first half of 2018 got treatment. That left 57 babies born with syphilis, nine of whom died. The analysts estimated that a third of the infections could have been prevented with testing in the third trimester.
Based on the numbers they saw in those 18 months, officials estimated that screening all women on Medicaid in the third trimester would cost the state $113,300 annually, and that treating all cases of syphilis that screening would catch could be done for just $113. Factoring in the hospitalization costs for infected infants, the officials concluded the additional testing would save the state money.
And yet prevention money has been hard to come by. Taking inflation into account, CDC prevention funding for STDs has fallen 41% since 2003, according to an analysis by the National Coalition of STD Directors. That’s even as cases have risen, leaving public health departments saddled with more work and far less money.
Janine Waters, STD program manager for the state of New Mexico, has watched the unraveling. When Ms. Waters started her career more than 20 years ago, she and her colleagues followed up on every case of chlamydia, gonorrhea, and syphilis reported, not only making sure that people got treatment but also getting in touch with their sexual partners, with the aim of stopping the spread of infection. In a 2019 interview with Kaiser Health News, she said her team was struggling to keep up with syphilis alone, even as they registered with dread congenital syphilis cases surging in neighboring Texas and Arizona.
By 2020, New Mexico had the highest rate of congenital syphilis in the country.
The COVID-19 pandemic drained the remaining resources. Half of health departments across the country discontinued STD fieldwork altogether, diverting their resources to COVID. In California, which for years has struggled with high rates of congenital syphilis, three-quarters of local health departments dispatched more than half of their STD staffers to work on COVID.
As the pandemic ebbs – at least in the short term – many public health departments are turning their attention back to syphilis and other diseases. And they are doing it with reinforcements. Although the Biden administration’s proposed STD prevention budget for 2023 remains flat, the American Rescue Plan Act included $200 million to help health departments boost contact tracing and surveillance for covid and other infectious diseases. Many departments are funneling that money toward STDs.
The money is an infusion that state health officials say will make a difference. But when taking inflation into account, it essentially brings STD prevention funding back to what it was in 2003, said Stephanie Arnold Pang of the National Coalition of STD Directors. And the American Rescue Plan money doesn’t cover some aspects of STD prevention, including clinical services.
The coalition wants to revive dedicated STD clinics, where people can drop in for testing and treatment at little to no cost. Advocates say that would fill a void that has plagued treatment efforts since public clinics closed en masse in the wake of the 2008 recession.
Texas, battling its own pervasive outbreak, will use its share of American Rescue Plan money to fill 94 new positions focused on various aspects of STD prevention. Those hires will bolster a range of measures the state put in place before the pandemic, including an updated data system to track infections, review boards in major cities that examine what went wrong for every case of congenital syphilis, and a requirement that providers test for syphilis during the third trimester of pregnancy. The suite of interventions seems to be working, but it could be a while before cases go down, said Amy Carter, the state’s congenital syphilis coordinator.
“The growth didn’t happen overnight,” Ms. Carter said. “So our prevention efforts aren’t going to have a direct impact overnight either.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation
Racial and ethnic disparities persist in pregnant women with gestational diabetes
Between 2014 and 2020, the frequency of adverse pregnancy outcomes in the United States increased among women with gestational diabetes, with persisting differences in adverse outcomes by race and ethnicity, according to a report in JAMA
“[Previous] population-based studies on racial and ethnic disparities in gestational diabetes have focused on differences in the rate of diagnosis, rather than adverse pregnancy outcomes,” lead author Kartik K. Venkatesh, MD, PhD, of Ohio State University, Columbus, and colleagues explained.
The researchers conducted a cross-sectional, descriptive study to evaluate whether the frequency of adverse pregnancy outcomes with gestational diabetes changed over time and whether the risk of these outcomes differed by maternal race and ethnicity.
The data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics Natality Files. Exposures of interest were year of delivery, as well as race and ethnicity.
Results
The study cohort included 1,560,822 pregnant women with gestational diabetes aged 15-44 years. Among the study participants the mean age was 31 years (standard deviation, 5.5 years) and the majority were White (48%), followed by Hispanic/Latina (27%), Asian/Pacific Islander (13%), and Black (12%).
There was a significant increase in the overall frequency of transfusion (8.0%; 95% confidence interval, 3.8%-12.4%), preeclampsia or gestational hypertension (4.2%; 95% CI, 3.3%-5.2%), NICU admission (1.0%; 95% CI, 0.3%-1.7%), and preterm birth at less than 37 weeks (0.9%; 95% CI, 0.3%-1.5%) from 2014 to 2020 for these women and their infants.
In addition, there was a significant decrease in the following outcomes: macrosomia (–4.7%; 95% CI, –5.3% to –4.0%), cesarean delivery (–1.4%; 95% CI, –1.7% to –1.1%), primary cesarean delivery (–1.2%; 95% CI, –1.5% to –0.9%), and large for gestational age (–2.3%; 95% CI, –2.8% to –1.8%), but there was no significant differences in maternal ICU admission and small-for-gestational-age infants.
From 2014 through 2020, differences in adverse outcomes by race and ethnicity persisted; in comparison with Whites, Black participants were at significantly higher risk of all evaluated outcomes, except for macrosomia and large for gestational age.
Hispanic/Latina and Asian/Pacific Islander individuals were also at significantly higher risk of preterm birth, NICU admission, maternal ICU admission, and small for gestational age. Furthermore, American Indian participants were at significantly higher risk of all evaluated outcomes, except for cesarean delivery and small for gestational age.
Results in context
Health policy researcher Felicia Hill-Briggs, PhD, at the Feinstein Institutes for Medical Research in Manhasset, N.Y. commented: “Two alarming trends highlighted by this study: 1) Racial and ethnic inequities in adverse gestational diabetes outcomes; and 2) the rising rates of gestational diabetes overall – both must and can be halted.”
“Optimizing medical management of gestational diabetes, whether through improved access to diabetes care in pregnancy, behavioral interventions, and pharmacotherapy can decrease the risk of adverse pregnancy outcomes,” Dr. Venkatesh commented. “It is possible that the equitable delivery of these interventions to address glycemic control could decrease racial and ethnic disparities in adverse pregnancy outcomes among individuals with gestational diabetes.”
Dr. Venkatesh and his colleagues acknowledged that a key limitation of the study was the use of administrative data; thus, inferences on maternal care improvements could not be determined.
“Further research could focus on greater understanding of racial and ethnic differences in the management of gestational diabetes,” the researchers concluded.
This study was supported by the Care Innovation and Community Improvement Program at Ohio State University. One author reported receiving grants from the National Institutes of Health outside of this study. The other authors reported no relevant disclosures. Dr. Hill-Briggs had no relevant disclosures.
Between 2014 and 2020, the frequency of adverse pregnancy outcomes in the United States increased among women with gestational diabetes, with persisting differences in adverse outcomes by race and ethnicity, according to a report in JAMA
“[Previous] population-based studies on racial and ethnic disparities in gestational diabetes have focused on differences in the rate of diagnosis, rather than adverse pregnancy outcomes,” lead author Kartik K. Venkatesh, MD, PhD, of Ohio State University, Columbus, and colleagues explained.
The researchers conducted a cross-sectional, descriptive study to evaluate whether the frequency of adverse pregnancy outcomes with gestational diabetes changed over time and whether the risk of these outcomes differed by maternal race and ethnicity.
The data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics Natality Files. Exposures of interest were year of delivery, as well as race and ethnicity.
Results
The study cohort included 1,560,822 pregnant women with gestational diabetes aged 15-44 years. Among the study participants the mean age was 31 years (standard deviation, 5.5 years) and the majority were White (48%), followed by Hispanic/Latina (27%), Asian/Pacific Islander (13%), and Black (12%).
There was a significant increase in the overall frequency of transfusion (8.0%; 95% confidence interval, 3.8%-12.4%), preeclampsia or gestational hypertension (4.2%; 95% CI, 3.3%-5.2%), NICU admission (1.0%; 95% CI, 0.3%-1.7%), and preterm birth at less than 37 weeks (0.9%; 95% CI, 0.3%-1.5%) from 2014 to 2020 for these women and their infants.
In addition, there was a significant decrease in the following outcomes: macrosomia (–4.7%; 95% CI, –5.3% to –4.0%), cesarean delivery (–1.4%; 95% CI, –1.7% to –1.1%), primary cesarean delivery (–1.2%; 95% CI, –1.5% to –0.9%), and large for gestational age (–2.3%; 95% CI, –2.8% to –1.8%), but there was no significant differences in maternal ICU admission and small-for-gestational-age infants.
From 2014 through 2020, differences in adverse outcomes by race and ethnicity persisted; in comparison with Whites, Black participants were at significantly higher risk of all evaluated outcomes, except for macrosomia and large for gestational age.
Hispanic/Latina and Asian/Pacific Islander individuals were also at significantly higher risk of preterm birth, NICU admission, maternal ICU admission, and small for gestational age. Furthermore, American Indian participants were at significantly higher risk of all evaluated outcomes, except for cesarean delivery and small for gestational age.
Results in context
Health policy researcher Felicia Hill-Briggs, PhD, at the Feinstein Institutes for Medical Research in Manhasset, N.Y. commented: “Two alarming trends highlighted by this study: 1) Racial and ethnic inequities in adverse gestational diabetes outcomes; and 2) the rising rates of gestational diabetes overall – both must and can be halted.”
“Optimizing medical management of gestational diabetes, whether through improved access to diabetes care in pregnancy, behavioral interventions, and pharmacotherapy can decrease the risk of adverse pregnancy outcomes,” Dr. Venkatesh commented. “It is possible that the equitable delivery of these interventions to address glycemic control could decrease racial and ethnic disparities in adverse pregnancy outcomes among individuals with gestational diabetes.”
Dr. Venkatesh and his colleagues acknowledged that a key limitation of the study was the use of administrative data; thus, inferences on maternal care improvements could not be determined.
“Further research could focus on greater understanding of racial and ethnic differences in the management of gestational diabetes,” the researchers concluded.
This study was supported by the Care Innovation and Community Improvement Program at Ohio State University. One author reported receiving grants from the National Institutes of Health outside of this study. The other authors reported no relevant disclosures. Dr. Hill-Briggs had no relevant disclosures.
Between 2014 and 2020, the frequency of adverse pregnancy outcomes in the United States increased among women with gestational diabetes, with persisting differences in adverse outcomes by race and ethnicity, according to a report in JAMA
“[Previous] population-based studies on racial and ethnic disparities in gestational diabetes have focused on differences in the rate of diagnosis, rather than adverse pregnancy outcomes,” lead author Kartik K. Venkatesh, MD, PhD, of Ohio State University, Columbus, and colleagues explained.
The researchers conducted a cross-sectional, descriptive study to evaluate whether the frequency of adverse pregnancy outcomes with gestational diabetes changed over time and whether the risk of these outcomes differed by maternal race and ethnicity.
The data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics Natality Files. Exposures of interest were year of delivery, as well as race and ethnicity.
Results
The study cohort included 1,560,822 pregnant women with gestational diabetes aged 15-44 years. Among the study participants the mean age was 31 years (standard deviation, 5.5 years) and the majority were White (48%), followed by Hispanic/Latina (27%), Asian/Pacific Islander (13%), and Black (12%).
There was a significant increase in the overall frequency of transfusion (8.0%; 95% confidence interval, 3.8%-12.4%), preeclampsia or gestational hypertension (4.2%; 95% CI, 3.3%-5.2%), NICU admission (1.0%; 95% CI, 0.3%-1.7%), and preterm birth at less than 37 weeks (0.9%; 95% CI, 0.3%-1.5%) from 2014 to 2020 for these women and their infants.
In addition, there was a significant decrease in the following outcomes: macrosomia (–4.7%; 95% CI, –5.3% to –4.0%), cesarean delivery (–1.4%; 95% CI, –1.7% to –1.1%), primary cesarean delivery (–1.2%; 95% CI, –1.5% to –0.9%), and large for gestational age (–2.3%; 95% CI, –2.8% to –1.8%), but there was no significant differences in maternal ICU admission and small-for-gestational-age infants.
From 2014 through 2020, differences in adverse outcomes by race and ethnicity persisted; in comparison with Whites, Black participants were at significantly higher risk of all evaluated outcomes, except for macrosomia and large for gestational age.
Hispanic/Latina and Asian/Pacific Islander individuals were also at significantly higher risk of preterm birth, NICU admission, maternal ICU admission, and small for gestational age. Furthermore, American Indian participants were at significantly higher risk of all evaluated outcomes, except for cesarean delivery and small for gestational age.
Results in context
Health policy researcher Felicia Hill-Briggs, PhD, at the Feinstein Institutes for Medical Research in Manhasset, N.Y. commented: “Two alarming trends highlighted by this study: 1) Racial and ethnic inequities in adverse gestational diabetes outcomes; and 2) the rising rates of gestational diabetes overall – both must and can be halted.”
“Optimizing medical management of gestational diabetes, whether through improved access to diabetes care in pregnancy, behavioral interventions, and pharmacotherapy can decrease the risk of adverse pregnancy outcomes,” Dr. Venkatesh commented. “It is possible that the equitable delivery of these interventions to address glycemic control could decrease racial and ethnic disparities in adverse pregnancy outcomes among individuals with gestational diabetes.”
Dr. Venkatesh and his colleagues acknowledged that a key limitation of the study was the use of administrative data; thus, inferences on maternal care improvements could not be determined.
“Further research could focus on greater understanding of racial and ethnic differences in the management of gestational diabetes,” the researchers concluded.
This study was supported by the Care Innovation and Community Improvement Program at Ohio State University. One author reported receiving grants from the National Institutes of Health outside of this study. The other authors reported no relevant disclosures. Dr. Hill-Briggs had no relevant disclosures.
FROM JAMA
‘Forever chemicals’ exposures may compound diabetes risk
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women in midlife exposed to combinations of perfluoroalkyl and polyfluoroalkyl substances (PFASs), dubbed “forever and everywhere chemicals”, are at increased risk of developing diabetes, similar to the magnitude of risk associated with overweight and even greater than the risk associated with smoking, new research shows.
“This is the first study to examine the joint effect of PFAS on incident diabetes,” first author Sung Kyun Park, ScD, MPH, told this news organization.
“We showed that multiple PFAS as mixtures have larger effects than individual PFAS,” said Dr. Park, of the department of epidemiology, School of Public Health, University of Michigan, Ann Arbor.
The results suggest that, “given that 1.5 million Americans are newly diagnosed with diabetes each year in the USA, approximately 370,000 new cases of diabetes annually in the U.S. are attributable to PFAS exposure,” Dr. Park and authors note in the study, published in Diabetologia.
However, Kevin McConway, PhD, emeritus professor of applied statistics, The Open University, U.K., told the UK Science Media Centre: “[Some] doubt about cause still remains. Yes, this study does show that PFAS may increase diabetes risk in middle-aged women, but it certainly can’t rule out other explanations for its findings.”
Is there any way to reduce exposure?
PFASs, known to be ubiquitous in the environment and also often dubbed “endocrine-disrupting” chemicals, have structures similar to fatty acids. They have been detected in the blood of most people and linked to health concerns including pre-eclampsia, altered levels of liver enzymes, inflammation, and altered lipid and glucose metabolism.
Sources of PFAS exposure can run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
The authors note a recent Consumer Reports investigation of 118 food packaging products, for instance, which reported finding PFAS chemicals in the packaging of every fast-food chain and retailer examined, including Burger King, McDonald’s, and even more health-focused chains, such as Trader Joe’s.
While efforts to pressure industry to limit PFAS in products are ongoing, Dr. Park asserted that “PFAS exposure reduction at the individual-level is very limited, so a more important way is to change policies and to limit PFAS in the air, drinking water, and foods, etc.”
“It is impossible to completely avoid exposure to PFAS, but I think it is important to acknowledge such sources and change our mindset,” he said.
In terms of clinical practice, the authors add that “it is also important for clinicians to be aware of PFAS as unrecognized risk factors for diabetes and to be prepared to counsel patients in terms of sources of exposure and potential health effects.”
Prospective findings from the SWAN-MPS study
The findings come from a prospective study of 1,237 women, with a median age of 49.4 years, who were diabetes-free upon entering the Study of Women’s Health Across the Nation – Multi-Pollutant Study (SWAN-MPS) between 1999 and 2000 and followed until 2017.
Blood samples taken throughout the study were analyzed for serum concentrations of seven PFASs.
Over the study period, there were 102 cases of incident diabetes, representing a rate of 6 cases per 1,000 person-years. Type of diabetes was not determined, but given the age of study participants, most were assumed to have type 2 diabetes, Dr. Park and colleagues note.
After adjustment for key confounders including race/ethnicity, smoking status, alcohol consumption, total energy intake, physical activity, menopausal status, and body mass index (BMI), those in the highest tertile of exposure to a combination of all seven of the PFASs were significantly more likely to develop diabetes, compared with those in the lowest tertile for exposure (hazard ratio, 2.62).
This risk was greater than that seen with individual PFASs (HR, 1.36-1.85), suggesting a potential additive or synergistic effect of multiple PFASs on diabetes risk.
The association between the combined exposure to PFASs among the highest versus lowest tertile was similar to the risk of diabetes developing among those with overweight (BMI 25-< 30 kg/m2) versus normal weight (HR, 2.89) and higher than the risk among current versus never smokers (HR, 2.30).
“Our findings suggest that PFAS may be an important risk factor for diabetes that has a substantial public health impact,” the authors say.
“Given the widespread exposure to PFAS in the general population, the expected benefit of reducing exposure to these ubiquitous chemicals might be considerable,” they emphasize.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA







