User login
Renal injury taints proximal AAA repair
CHICAGO – Proximal abdominal aortic aneurysm repair can be achieved with a low perioperative mortality of about 3%, although renal dysfunction remains an Achilles’ heel, according to a 27-year review involving 245 patients.
In all, 60% of patients had postoperative acute kidney injury (AKI), 28% had persistent AKI at discharge, and 1.6% were discharged on hemodialysis. Persistent AKI at discharge was also associated with reduced long-term survival, Dr. Loay Kabbani said at the annual meeting of the Midwestern Vascular Surgical Society.
AKI stage was based on the Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease/Acute Kidney Injury Network (AKIN) classification system, with a change in serum creatinine of more than 0.3 mg/dL considered positive for injury. This is a much more sensitive criterion than what was used in previous studies, but is the new standard for reporting renal injury.
"This data should assist in establishing a benchmark for endovascular repair of these complex aneurysms," said Dr. Kabbani, a vascular surgeon at Henry Ford Hospital in Detroit.
The investigators identified 245 patients who underwent proximal AAA repair between 1986 and February 2013 at Henry Ford Hospital for juxtarenal (127), suprarenal (68), or type IV thoracoabdominal AAAs (50). The average aneurysm size was 6.4 cm (range, 3.1-14 cm), and mean preoperative estimated glomerular filtration rate was 62 mL/min per 1.73 m2 (range, 14-166 mL/min per 1.73 m2).
Most patients were male (69%), white (88.5%), and hypertensive (84%); 55% had chronic kidney disease based on a preoperative estimated glomerular filtration rate below 60 mL/min per 1.73 m2. Their average age was 71 years.
For most patients, the approach was retroperitoneal (48%) or thoracoabdominal (30%); the clamp was placed supraceliac (58%); and tube grafts were used (64%).
In-hospital mortality was 2.9% (7 patients) and 30-day mortality 3.3% (8 patients), Dr. Kabbani said.
Postoperative complications were AKI in 144 patients (60%), major pulmonary complications in 55 (22%), myocardial infarction in 9 (4%), return to the operating room for postoperative bleeding in 7 (3%), neurologic complications in 7 (3%), and bowel ischemia in 5 (2%).
Specifically, postoperative AKI was stage 1 (mild) in 35%, stage 2 (moderate) in 15%, and stage 3 (severe) in 10%, improving to 12%, 4%, and 3%, respectively, at discharge.
Though preoperative comorbidities were similar between patients with juxtarenal, suprarenal, and type IV aneurysms, postoperative complications increased significantly as the aneurysm extended more proximally (31% vs. 50% vs. 72%; P < .0001), Dr. Kabbani said.
Rates of AKI at discharge also followed the same pattern (18.2% vs. 35.4% vs. 43.8%; P less than .0012).
After a median follow-up of 54 months, the 5-year survival estimate was 70% and 10-year survival 43% based on a review of electronic medical records and the Social Security Death Index.
Cox regression analyses revealed no difference in long-term survival based on aneurysm type, but significantly worse survival based on increased aneurysm size (hazard ratio, 1.1; P = .01), preoperative chronic obstructive pulmonary disease (HR, 1.8; P = .002), congestive heart failure (HR, 3.5; P less than .001), history of stroke (HR, 1.9; P = .04), and persistent stage 2 (HR, 5.29; P = .001) and stage 3 AKI at discharge (HR, 2.71; P = .014), he said.
During a discussion of the results, Dr. Kabbani said the hospital usually uses mannitol (12.5-25 g) for renal protection and cold renal perfusion in select patients, although the latter did not jump out as a risk factor for CKI. The data were not broken down to see whether use of renal bypass during surgery affected outcomes for specific AAA types. Some attendees countered that renal protection should be used for all patients undergoing proximal AAA repair.
Dr. Kabbani reported having no financial disclosures.
CHICAGO – Proximal abdominal aortic aneurysm repair can be achieved with a low perioperative mortality of about 3%, although renal dysfunction remains an Achilles’ heel, according to a 27-year review involving 245 patients.
In all, 60% of patients had postoperative acute kidney injury (AKI), 28% had persistent AKI at discharge, and 1.6% were discharged on hemodialysis. Persistent AKI at discharge was also associated with reduced long-term survival, Dr. Loay Kabbani said at the annual meeting of the Midwestern Vascular Surgical Society.
AKI stage was based on the Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease/Acute Kidney Injury Network (AKIN) classification system, with a change in serum creatinine of more than 0.3 mg/dL considered positive for injury. This is a much more sensitive criterion than what was used in previous studies, but is the new standard for reporting renal injury.
"This data should assist in establishing a benchmark for endovascular repair of these complex aneurysms," said Dr. Kabbani, a vascular surgeon at Henry Ford Hospital in Detroit.
The investigators identified 245 patients who underwent proximal AAA repair between 1986 and February 2013 at Henry Ford Hospital for juxtarenal (127), suprarenal (68), or type IV thoracoabdominal AAAs (50). The average aneurysm size was 6.4 cm (range, 3.1-14 cm), and mean preoperative estimated glomerular filtration rate was 62 mL/min per 1.73 m2 (range, 14-166 mL/min per 1.73 m2).
Most patients were male (69%), white (88.5%), and hypertensive (84%); 55% had chronic kidney disease based on a preoperative estimated glomerular filtration rate below 60 mL/min per 1.73 m2. Their average age was 71 years.
For most patients, the approach was retroperitoneal (48%) or thoracoabdominal (30%); the clamp was placed supraceliac (58%); and tube grafts were used (64%).
In-hospital mortality was 2.9% (7 patients) and 30-day mortality 3.3% (8 patients), Dr. Kabbani said.
Postoperative complications were AKI in 144 patients (60%), major pulmonary complications in 55 (22%), myocardial infarction in 9 (4%), return to the operating room for postoperative bleeding in 7 (3%), neurologic complications in 7 (3%), and bowel ischemia in 5 (2%).
Specifically, postoperative AKI was stage 1 (mild) in 35%, stage 2 (moderate) in 15%, and stage 3 (severe) in 10%, improving to 12%, 4%, and 3%, respectively, at discharge.
Though preoperative comorbidities were similar between patients with juxtarenal, suprarenal, and type IV aneurysms, postoperative complications increased significantly as the aneurysm extended more proximally (31% vs. 50% vs. 72%; P < .0001), Dr. Kabbani said.
Rates of AKI at discharge also followed the same pattern (18.2% vs. 35.4% vs. 43.8%; P less than .0012).
After a median follow-up of 54 months, the 5-year survival estimate was 70% and 10-year survival 43% based on a review of electronic medical records and the Social Security Death Index.
Cox regression analyses revealed no difference in long-term survival based on aneurysm type, but significantly worse survival based on increased aneurysm size (hazard ratio, 1.1; P = .01), preoperative chronic obstructive pulmonary disease (HR, 1.8; P = .002), congestive heart failure (HR, 3.5; P less than .001), history of stroke (HR, 1.9; P = .04), and persistent stage 2 (HR, 5.29; P = .001) and stage 3 AKI at discharge (HR, 2.71; P = .014), he said.
During a discussion of the results, Dr. Kabbani said the hospital usually uses mannitol (12.5-25 g) for renal protection and cold renal perfusion in select patients, although the latter did not jump out as a risk factor for CKI. The data were not broken down to see whether use of renal bypass during surgery affected outcomes for specific AAA types. Some attendees countered that renal protection should be used for all patients undergoing proximal AAA repair.
Dr. Kabbani reported having no financial disclosures.
CHICAGO – Proximal abdominal aortic aneurysm repair can be achieved with a low perioperative mortality of about 3%, although renal dysfunction remains an Achilles’ heel, according to a 27-year review involving 245 patients.
In all, 60% of patients had postoperative acute kidney injury (AKI), 28% had persistent AKI at discharge, and 1.6% were discharged on hemodialysis. Persistent AKI at discharge was also associated with reduced long-term survival, Dr. Loay Kabbani said at the annual meeting of the Midwestern Vascular Surgical Society.
AKI stage was based on the Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease/Acute Kidney Injury Network (AKIN) classification system, with a change in serum creatinine of more than 0.3 mg/dL considered positive for injury. This is a much more sensitive criterion than what was used in previous studies, but is the new standard for reporting renal injury.
"This data should assist in establishing a benchmark for endovascular repair of these complex aneurysms," said Dr. Kabbani, a vascular surgeon at Henry Ford Hospital in Detroit.
The investigators identified 245 patients who underwent proximal AAA repair between 1986 and February 2013 at Henry Ford Hospital for juxtarenal (127), suprarenal (68), or type IV thoracoabdominal AAAs (50). The average aneurysm size was 6.4 cm (range, 3.1-14 cm), and mean preoperative estimated glomerular filtration rate was 62 mL/min per 1.73 m2 (range, 14-166 mL/min per 1.73 m2).
Most patients were male (69%), white (88.5%), and hypertensive (84%); 55% had chronic kidney disease based on a preoperative estimated glomerular filtration rate below 60 mL/min per 1.73 m2. Their average age was 71 years.
For most patients, the approach was retroperitoneal (48%) or thoracoabdominal (30%); the clamp was placed supraceliac (58%); and tube grafts were used (64%).
In-hospital mortality was 2.9% (7 patients) and 30-day mortality 3.3% (8 patients), Dr. Kabbani said.
Postoperative complications were AKI in 144 patients (60%), major pulmonary complications in 55 (22%), myocardial infarction in 9 (4%), return to the operating room for postoperative bleeding in 7 (3%), neurologic complications in 7 (3%), and bowel ischemia in 5 (2%).
Specifically, postoperative AKI was stage 1 (mild) in 35%, stage 2 (moderate) in 15%, and stage 3 (severe) in 10%, improving to 12%, 4%, and 3%, respectively, at discharge.
Though preoperative comorbidities were similar between patients with juxtarenal, suprarenal, and type IV aneurysms, postoperative complications increased significantly as the aneurysm extended more proximally (31% vs. 50% vs. 72%; P < .0001), Dr. Kabbani said.
Rates of AKI at discharge also followed the same pattern (18.2% vs. 35.4% vs. 43.8%; P less than .0012).
After a median follow-up of 54 months, the 5-year survival estimate was 70% and 10-year survival 43% based on a review of electronic medical records and the Social Security Death Index.
Cox regression analyses revealed no difference in long-term survival based on aneurysm type, but significantly worse survival based on increased aneurysm size (hazard ratio, 1.1; P = .01), preoperative chronic obstructive pulmonary disease (HR, 1.8; P = .002), congestive heart failure (HR, 3.5; P less than .001), history of stroke (HR, 1.9; P = .04), and persistent stage 2 (HR, 5.29; P = .001) and stage 3 AKI at discharge (HR, 2.71; P = .014), he said.
During a discussion of the results, Dr. Kabbani said the hospital usually uses mannitol (12.5-25 g) for renal protection and cold renal perfusion in select patients, although the latter did not jump out as a risk factor for CKI. The data were not broken down to see whether use of renal bypass during surgery affected outcomes for specific AAA types. Some attendees countered that renal protection should be used for all patients undergoing proximal AAA repair.
Dr. Kabbani reported having no financial disclosures.
AT MIDWESTERN VASCULAR 2013
Major finding: Perioperative mortality was 2.9%, but 60% of patients had postoperative acute kidney injury.
Data source: A retrospective analysis of 245 patients undergoing open proximal abdominal aortic aneurysm repair.
Disclosures: Dr. Kabbani reported having no financial disclosures.
First-in-man bioengineered graft proves enduring for vascular access
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: The 6-month enduring patency rate of an investigational tissue-engineered vascular graft for hemodialysis access was 100%, markedly better than rates achievable with synthetic PTFE grafts, the current benchmark technology.
Data source: An initial report from an ongoing prospective first-in-man study in which, to date, 28 patients with end-stage renal disease have been implanted with a novel tissue-engineered vascular graft for use as a hemodialysis access.
Disclosures: The study was funded by the Department of Defense and Humacyte. The presenter is a consultant to the company.
DVT risk higher in cardiac and vascular surgery patients
WASHINGTON – Cardiac and vascular surgery patients are at higher risk for deep vein thrombosis than are general surgery patients, according to data presented at the annual clinical congress of the American College of Surgeons.
In a retrospective analysis of 2,669,772 patients with a median age of 64 years, 43% of whom were males, in the ACS-National Surgery Quality Improvement Program (NSQIP) during 2005-2009, Dr. Faisal Aziz of Penn State Hershey (Pa.) Heart and Vascular Institute and his colleagues sought to determine the actual rate of deep vein thrombosis (DVT) during revascularization procedures, compared with general surgery. They also investigated the relationship between the type of operation and the DVT incidence rate.
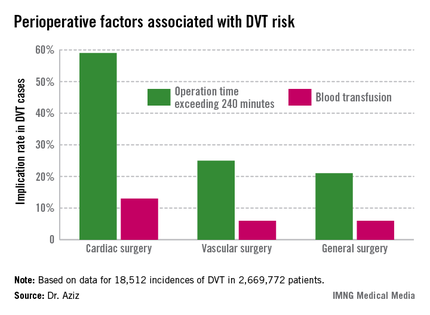
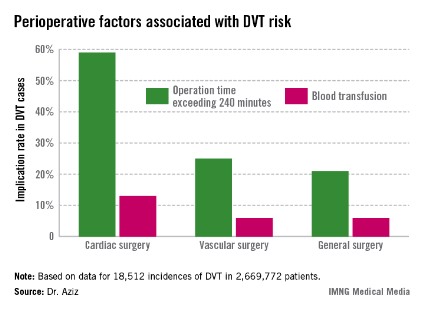
The Agency for Healthcare Research and Quality considers the incidence rate of DVT a patient safety indicator. Dr. Aziz cited data indicating that one in four patients who develop DVT postoperatively before discharge has an additional venous thromboembolic event–related event in the subsequent 21 months requiring hospitalization, at a cost of approximately $15,000, or roughly 21% higher than the original DVT event (J. Manag. Care. Pharm. 2007;13:475-86).
The researchers sorted patients according to DVT risk factors such as age, gender, body mass index over 40 kg/m2, and whether the surgery was acute. They then assessed intraoperative factors such as total time to completion and its American Society of Anesthesiology score. They then considered the postoperative factors associated with DVT, such as blood transfusions, return to the operating room, deep wound infection, cardiac arrest, and mortality.
Dr. Aziz and his team determined that there were 18,512 incidences of DVT, equaling 0.69% of all patients studied. Of those, 0.66% occurred during general surgery, 2.08% occurred during cardiac surgery, and 1% occurred during vascular surgery.
"The implications of our study are that, contrary to popular belief, the incidence of postoperative DVT is actually higher after cardiac surgery and vascular surgery procedures," he said.
The cardiac surgery procedures associated with the highest DVT incidence rate were tricuspid valve replacement (8%), thoracic endovascular aortic repair (5%), thoracic aortic graft replacement (4%), and pericardial window (4%).
In a comparison of cardiac procedures, tricuspid valve replacement vs. aortic valve replacement had a risk ratio of 3.5 (P < .001). In tricuspid valve replacement vs. coronary artery bypass, the former had a risk ratio of 11.24 (P < .001).
Vascular surgeries with the highest DVT incidence rates were peripheral bypass (1%), amputation (trans-metatarsal, 0.75%; below knee, 1%; above the knee, 1%), and ruptured aortic aneurysms (3.5%), Dr. Aziz reported.
Intra-and postoperative factors associated with DVT risk included operation times exceeding 240 minutes and previous DVT. Compared with 21% of general surgery patients, operation time was implicated in 59% of cardiac surgery patients (relative risk, 2.72; P < .001) and 25% of vascular surgery patients (RR, 1.14; P <.001). Blood transfusions affected 13% of cardiac surgery patients (RR, 2.3; P < .001), 6% of vascular surgery patients (RR, 1.3; P < .001), and 6% of general surgery patients.
Compared with 24% for general surgery patients, returning to the operating room was implicated in 27% of cardiac patients (RR, 1.4; P = .27) and 32% of vascular surgery patients (RR, 1.3; P < .001).
"Procedures and perioperative factors associated with high risk of postoperative DVT should be identified, and adequate DVT prophylaxis should be ensured for these patients," he concluded.
Dr. Aziz and his associates had no disclosures.
WASHINGTON – Cardiac and vascular surgery patients are at higher risk for deep vein thrombosis than are general surgery patients, according to data presented at the annual clinical congress of the American College of Surgeons.
In a retrospective analysis of 2,669,772 patients with a median age of 64 years, 43% of whom were males, in the ACS-National Surgery Quality Improvement Program (NSQIP) during 2005-2009, Dr. Faisal Aziz of Penn State Hershey (Pa.) Heart and Vascular Institute and his colleagues sought to determine the actual rate of deep vein thrombosis (DVT) during revascularization procedures, compared with general surgery. They also investigated the relationship between the type of operation and the DVT incidence rate.

The Agency for Healthcare Research and Quality considers the incidence rate of DVT a patient safety indicator. Dr. Aziz cited data indicating that one in four patients who develop DVT postoperatively before discharge has an additional venous thromboembolic event–related event in the subsequent 21 months requiring hospitalization, at a cost of approximately $15,000, or roughly 21% higher than the original DVT event (J. Manag. Care. Pharm. 2007;13:475-86).
The researchers sorted patients according to DVT risk factors such as age, gender, body mass index over 40 kg/m2, and whether the surgery was acute. They then assessed intraoperative factors such as total time to completion and its American Society of Anesthesiology score. They then considered the postoperative factors associated with DVT, such as blood transfusions, return to the operating room, deep wound infection, cardiac arrest, and mortality.
Dr. Aziz and his team determined that there were 18,512 incidences of DVT, equaling 0.69% of all patients studied. Of those, 0.66% occurred during general surgery, 2.08% occurred during cardiac surgery, and 1% occurred during vascular surgery.
"The implications of our study are that, contrary to popular belief, the incidence of postoperative DVT is actually higher after cardiac surgery and vascular surgery procedures," he said.
The cardiac surgery procedures associated with the highest DVT incidence rate were tricuspid valve replacement (8%), thoracic endovascular aortic repair (5%), thoracic aortic graft replacement (4%), and pericardial window (4%).
In a comparison of cardiac procedures, tricuspid valve replacement vs. aortic valve replacement had a risk ratio of 3.5 (P < .001). In tricuspid valve replacement vs. coronary artery bypass, the former had a risk ratio of 11.24 (P < .001).
Vascular surgeries with the highest DVT incidence rates were peripheral bypass (1%), amputation (trans-metatarsal, 0.75%; below knee, 1%; above the knee, 1%), and ruptured aortic aneurysms (3.5%), Dr. Aziz reported.
Intra-and postoperative factors associated with DVT risk included operation times exceeding 240 minutes and previous DVT. Compared with 21% of general surgery patients, operation time was implicated in 59% of cardiac surgery patients (relative risk, 2.72; P < .001) and 25% of vascular surgery patients (RR, 1.14; P <.001). Blood transfusions affected 13% of cardiac surgery patients (RR, 2.3; P < .001), 6% of vascular surgery patients (RR, 1.3; P < .001), and 6% of general surgery patients.
Compared with 24% for general surgery patients, returning to the operating room was implicated in 27% of cardiac patients (RR, 1.4; P = .27) and 32% of vascular surgery patients (RR, 1.3; P < .001).
"Procedures and perioperative factors associated with high risk of postoperative DVT should be identified, and adequate DVT prophylaxis should be ensured for these patients," he concluded.
Dr. Aziz and his associates had no disclosures.
WASHINGTON – Cardiac and vascular surgery patients are at higher risk for deep vein thrombosis than are general surgery patients, according to data presented at the annual clinical congress of the American College of Surgeons.
In a retrospective analysis of 2,669,772 patients with a median age of 64 years, 43% of whom were males, in the ACS-National Surgery Quality Improvement Program (NSQIP) during 2005-2009, Dr. Faisal Aziz of Penn State Hershey (Pa.) Heart and Vascular Institute and his colleagues sought to determine the actual rate of deep vein thrombosis (DVT) during revascularization procedures, compared with general surgery. They also investigated the relationship between the type of operation and the DVT incidence rate.

The Agency for Healthcare Research and Quality considers the incidence rate of DVT a patient safety indicator. Dr. Aziz cited data indicating that one in four patients who develop DVT postoperatively before discharge has an additional venous thromboembolic event–related event in the subsequent 21 months requiring hospitalization, at a cost of approximately $15,000, or roughly 21% higher than the original DVT event (J. Manag. Care. Pharm. 2007;13:475-86).
The researchers sorted patients according to DVT risk factors such as age, gender, body mass index over 40 kg/m2, and whether the surgery was acute. They then assessed intraoperative factors such as total time to completion and its American Society of Anesthesiology score. They then considered the postoperative factors associated with DVT, such as blood transfusions, return to the operating room, deep wound infection, cardiac arrest, and mortality.
Dr. Aziz and his team determined that there were 18,512 incidences of DVT, equaling 0.69% of all patients studied. Of those, 0.66% occurred during general surgery, 2.08% occurred during cardiac surgery, and 1% occurred during vascular surgery.
"The implications of our study are that, contrary to popular belief, the incidence of postoperative DVT is actually higher after cardiac surgery and vascular surgery procedures," he said.
The cardiac surgery procedures associated with the highest DVT incidence rate were tricuspid valve replacement (8%), thoracic endovascular aortic repair (5%), thoracic aortic graft replacement (4%), and pericardial window (4%).
In a comparison of cardiac procedures, tricuspid valve replacement vs. aortic valve replacement had a risk ratio of 3.5 (P < .001). In tricuspid valve replacement vs. coronary artery bypass, the former had a risk ratio of 11.24 (P < .001).
Vascular surgeries with the highest DVT incidence rates were peripheral bypass (1%), amputation (trans-metatarsal, 0.75%; below knee, 1%; above the knee, 1%), and ruptured aortic aneurysms (3.5%), Dr. Aziz reported.
Intra-and postoperative factors associated with DVT risk included operation times exceeding 240 minutes and previous DVT. Compared with 21% of general surgery patients, operation time was implicated in 59% of cardiac surgery patients (relative risk, 2.72; P < .001) and 25% of vascular surgery patients (RR, 1.14; P <.001). Blood transfusions affected 13% of cardiac surgery patients (RR, 2.3; P < .001), 6% of vascular surgery patients (RR, 1.3; P < .001), and 6% of general surgery patients.
Compared with 24% for general surgery patients, returning to the operating room was implicated in 27% of cardiac patients (RR, 1.4; P = .27) and 32% of vascular surgery patients (RR, 1.3; P < .001).
"Procedures and perioperative factors associated with high risk of postoperative DVT should be identified, and adequate DVT prophylaxis should be ensured for these patients," he concluded.
Dr. Aziz and his associates had no disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: Of the 2,669,772 patients studied, 18,512 (0.69%) had DVTs during surgery. The rate was 0.66% for general surgery, 2.08% for cardiac surgery, and 1% for vascular surgery.
Data source: Retrospective analysis of NSQIP 2005-2009 database analyzed according to surgical specialty.
Disclosures: Dr. Aziz and his associates had no disclosures.
Early VTE prophylaxis found safe in blunt abdominal injuries
WASHINGTON – Nonoperative prophylaxis for blunt solid abdominal organ trauma was found safe when given 48 hours post injury, according to data presented at the annual clinical congress of the American College of Surgeons.
Because updated guidelines for nonoperative management of solid abdominal organ injuries do not state an optimal time for initiation of prophylaxis, investigators, including presenter Caitlyn Harrison, a third-year medical student at the University of Arizona, Tucson, sought to determine how soon is too soon in this patient population.
Theorizing that there would be no difference in bleeding complications and failure rates associated with early venous thromboembolism (VTE) prevention, the investigators reviewed 7 years of patient data (2005-2011) from a single trauma center to compare the safety of early (less than 48 hours), intermediate (48-72 hours), and late (more than 72 hours) initiation of unfractionated heparin (5,000 units, subcutaneously, every 8 hours) in blunt abdominal injury patients.
Included for review were patients whose abdominal injuries were equal to or greater than 3 on the Abbreviated Injury Scale (AIS). Patients with head injuries that scored 3 or greater on the AIS and those who had been transferred were excluded.
A total of 116 patients were matched according to whether they had received early (n = 58; 67.2% male; mean age, 40 years), intermediate (n = 29; 69% male; mean age, 44.3 years), or late (n = 29; 72.4% male; mean age, 45 years) initiation of VTE prophylaxis.
They also were matched according to organs injured. The investigators found a preponderance of splenic injuries: 41.4% in the early group, 37.9% in the intermediate, and 45.2% in the late group.
The grade of injury and laboratory values including blood pressure and injury severity also were measured.
The researchers found that none of the patients in the intermediate or late groups reached the primary outcome of the need for a post-treatment blood transfusion, although 3.2% of the early group did, Ms. Harrison said.
No patients in any of the three groups required an operative intervention after nonoperative management, although 1.7% of the early group did require embolization, as did 3.4% each of the intermediate and late groups.
Similarly, while thromboembolisms were not found to have occurred in the early group, they did occur in the intermediate and late groups at a rate of 3.4% each. No mortality was recorded as an outcome in any of the three groups, she said, concluding that "early VTE prophylaxis is safe."
Ms. Harrison reported no relevant disclosures.
WASHINGTON – Nonoperative prophylaxis for blunt solid abdominal organ trauma was found safe when given 48 hours post injury, according to data presented at the annual clinical congress of the American College of Surgeons.
Because updated guidelines for nonoperative management of solid abdominal organ injuries do not state an optimal time for initiation of prophylaxis, investigators, including presenter Caitlyn Harrison, a third-year medical student at the University of Arizona, Tucson, sought to determine how soon is too soon in this patient population.
Theorizing that there would be no difference in bleeding complications and failure rates associated with early venous thromboembolism (VTE) prevention, the investigators reviewed 7 years of patient data (2005-2011) from a single trauma center to compare the safety of early (less than 48 hours), intermediate (48-72 hours), and late (more than 72 hours) initiation of unfractionated heparin (5,000 units, subcutaneously, every 8 hours) in blunt abdominal injury patients.
Included for review were patients whose abdominal injuries were equal to or greater than 3 on the Abbreviated Injury Scale (AIS). Patients with head injuries that scored 3 or greater on the AIS and those who had been transferred were excluded.
A total of 116 patients were matched according to whether they had received early (n = 58; 67.2% male; mean age, 40 years), intermediate (n = 29; 69% male; mean age, 44.3 years), or late (n = 29; 72.4% male; mean age, 45 years) initiation of VTE prophylaxis.
They also were matched according to organs injured. The investigators found a preponderance of splenic injuries: 41.4% in the early group, 37.9% in the intermediate, and 45.2% in the late group.
The grade of injury and laboratory values including blood pressure and injury severity also were measured.
The researchers found that none of the patients in the intermediate or late groups reached the primary outcome of the need for a post-treatment blood transfusion, although 3.2% of the early group did, Ms. Harrison said.
No patients in any of the three groups required an operative intervention after nonoperative management, although 1.7% of the early group did require embolization, as did 3.4% each of the intermediate and late groups.
Similarly, while thromboembolisms were not found to have occurred in the early group, they did occur in the intermediate and late groups at a rate of 3.4% each. No mortality was recorded as an outcome in any of the three groups, she said, concluding that "early VTE prophylaxis is safe."
Ms. Harrison reported no relevant disclosures.
WASHINGTON – Nonoperative prophylaxis for blunt solid abdominal organ trauma was found safe when given 48 hours post injury, according to data presented at the annual clinical congress of the American College of Surgeons.
Because updated guidelines for nonoperative management of solid abdominal organ injuries do not state an optimal time for initiation of prophylaxis, investigators, including presenter Caitlyn Harrison, a third-year medical student at the University of Arizona, Tucson, sought to determine how soon is too soon in this patient population.
Theorizing that there would be no difference in bleeding complications and failure rates associated with early venous thromboembolism (VTE) prevention, the investigators reviewed 7 years of patient data (2005-2011) from a single trauma center to compare the safety of early (less than 48 hours), intermediate (48-72 hours), and late (more than 72 hours) initiation of unfractionated heparin (5,000 units, subcutaneously, every 8 hours) in blunt abdominal injury patients.
Included for review were patients whose abdominal injuries were equal to or greater than 3 on the Abbreviated Injury Scale (AIS). Patients with head injuries that scored 3 or greater on the AIS and those who had been transferred were excluded.
A total of 116 patients were matched according to whether they had received early (n = 58; 67.2% male; mean age, 40 years), intermediate (n = 29; 69% male; mean age, 44.3 years), or late (n = 29; 72.4% male; mean age, 45 years) initiation of VTE prophylaxis.
They also were matched according to organs injured. The investigators found a preponderance of splenic injuries: 41.4% in the early group, 37.9% in the intermediate, and 45.2% in the late group.
The grade of injury and laboratory values including blood pressure and injury severity also were measured.
The researchers found that none of the patients in the intermediate or late groups reached the primary outcome of the need for a post-treatment blood transfusion, although 3.2% of the early group did, Ms. Harrison said.
No patients in any of the three groups required an operative intervention after nonoperative management, although 1.7% of the early group did require embolization, as did 3.4% each of the intermediate and late groups.
Similarly, while thromboembolisms were not found to have occurred in the early group, they did occur in the intermediate and late groups at a rate of 3.4% each. No mortality was recorded as an outcome in any of the three groups, she said, concluding that "early VTE prophylaxis is safe."
Ms. Harrison reported no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: Thromboembolic prophylaxis was found safe in blunt abdominal injury, when administered at either 48, 48-72, or 72 hours post injury.
Data source: Review of 116 blunt solid organ injury patients managed non-operatively at a single trauma center between 2005-2011.
Disclosures: Ms. Harrison reported no relevant disclosures.
Amputations/revascularization top vascular readmission list
CHICAGO – Lower-extremity revascularization or amputation was among the strongest predictors of 30-day vascular surgery readmission in what is being described as the largest single-center review in this setting to date.
Lower-extremity revascularization and amputations made up 63% of unplanned readmissions, though rates for endovascular lower-extremity revascularization were almost half that of open revascularization (8.2% vs. 15%).
Notably, below-knee amputations fared the worst, with a 30-day unplanned readmission rate of 24%, compared with 13.3% for above-knee amputations and 16.4% for foot amputation.
"Amputations and open lower-extremity revascularization had the highest rates of readmission in this analysis and therefore we need to focus our efforts and find additional postoperative [management] strategies for these two subgroups," Dr. Travis L. Engelbert said at the annual meeting of the annual meeting of the Midwestern Vascular Surgical Society.*
The analysis involved 2,505 patients who underwent vascular surgery at the University of Wisconsin Hospitals and Clinics in Madison from 2009 to mid-2013. The overall readmission rate was 9.7% (n = 244).
Of these, 147 patients (60.2%) were readmitted to the vascular surgery service.
The most common readmitting diagnosis was wound complication or infection in 37%, said Dr. Engelbert, a vascular surgeon at the university.
Patients whose index admission was urgent rather than elective had significantly higher readmission rates (14.6% vs. 6.9%; P less than .001), as did those living remotely rather than inside Dane County, where the university is located (12% vs. 8.8%; P = .02).
Not surprisingly, higher illness severity, as calculated using the 3M APR DRG software, was significantly associated with readmission (15.6% high vs. 4.3% low severity; P less than .001).
Patients who were readmitted had a longer initial length of stay (LOS) (8.5 days vs. 6.1 days; P less than .01), and were more likely to have an ICU admission (18.3% vs. 9.5% without ICU stay; P less than .05), he reported.
Based on insurance status, patients covered by Medicaid (16.8%) and Medicare (10%) were most likely to have an unplanned readmission, followed by fee-for-service patients (9.5%), self-pay (8%), and HMO (5.5%) patients (P = .02).
Dr. Engelbert observed that vascular surgery outcomes have come under scrutiny and that there has been some discussion of cutbacks in Medicare reimbursement given its high rates of readmission.
"This is already starting to happen for certain medical patient populations and if this were to happen, it would significantly affect a vascular service’s practice because a majority of our patients are covered by Medicare and have a higher readmission rate," he said.
The analysis suggests that vascular surgeons may also want to pay closer attention to discharge destination for their patients. Readmission rates were about three times higher for patients discharged to a rehabilitation facility or skilled nursing facility than for those discharged home (19.2% and 16.2% vs. 6.2%; P less than .01).
"The discharge destination matters," Dr. Engelbert said. "... we need to have improved coordination between hospitals and postdischarge destinations. And, we also might need to look at how these patients are cared for and if they are discharged to the appropriate level of care when they’re discharged to these skilled nursing and rehabilitation facilities."
The effects of discharge destination (odds ratio, 1.54 skilled nursing facility), index length of stay (OR, 1.03), insurance (OR, 0.43 HMO), and lower-extremity revascularization or amputation (OR, 2.35) persisted in multivariable logistic regression analysis that controlled for age, sex, race, proximity to hospital, clinic follow-up time, urgent vs. elective admission, insurance type, procedure type, length of stay, and discharge destination.
When asked by the audience what the university has done to reduce its vascular readmission rates, Dr. Engelbert said they have looked at using in-patient swabs to reduce infection and dedicated vascular nurse practitioners or case managers to ensure patients are being discharged to the appropriate level of care.
"I think further efforts need to focus on how we can reduce outpatient complications, through closer and quicker follow-up perhaps, as well as ways to use technology to monitor patients," he said.
One example of this is the use of outpatient remote wound analysis using smartphone photograph technology.
"Wound complications and subsequent readmissions are frequent, costly, and a significant burden to the patients," Dr. Engelbert said in an interview. "Hopefully, this method will reduce the severity of wound complications if they can be caught and treated at an earlier stage with digital photograph analysis."
One audience member argued that the vast majority of vascular problems could be cared for outpatient, but that vascular surgeons frequently aren’t told their patients have been readmitted until after they’ve been in the hospital for 2 or 3 days.
Dr. Engelbert reported having no financial disclosures. A coauthor reported grant funding from Abbott, Cook, Covidien, Endologix, Gore, and the National Institutes of Health.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
CHICAGO – Lower-extremity revascularization or amputation was among the strongest predictors of 30-day vascular surgery readmission in what is being described as the largest single-center review in this setting to date.
Lower-extremity revascularization and amputations made up 63% of unplanned readmissions, though rates for endovascular lower-extremity revascularization were almost half that of open revascularization (8.2% vs. 15%).
Notably, below-knee amputations fared the worst, with a 30-day unplanned readmission rate of 24%, compared with 13.3% for above-knee amputations and 16.4% for foot amputation.
"Amputations and open lower-extremity revascularization had the highest rates of readmission in this analysis and therefore we need to focus our efforts and find additional postoperative [management] strategies for these two subgroups," Dr. Travis L. Engelbert said at the annual meeting of the annual meeting of the Midwestern Vascular Surgical Society.*
The analysis involved 2,505 patients who underwent vascular surgery at the University of Wisconsin Hospitals and Clinics in Madison from 2009 to mid-2013. The overall readmission rate was 9.7% (n = 244).
Of these, 147 patients (60.2%) were readmitted to the vascular surgery service.
The most common readmitting diagnosis was wound complication or infection in 37%, said Dr. Engelbert, a vascular surgeon at the university.
Patients whose index admission was urgent rather than elective had significantly higher readmission rates (14.6% vs. 6.9%; P less than .001), as did those living remotely rather than inside Dane County, where the university is located (12% vs. 8.8%; P = .02).
Not surprisingly, higher illness severity, as calculated using the 3M APR DRG software, was significantly associated with readmission (15.6% high vs. 4.3% low severity; P less than .001).
Patients who were readmitted had a longer initial length of stay (LOS) (8.5 days vs. 6.1 days; P less than .01), and were more likely to have an ICU admission (18.3% vs. 9.5% without ICU stay; P less than .05), he reported.
Based on insurance status, patients covered by Medicaid (16.8%) and Medicare (10%) were most likely to have an unplanned readmission, followed by fee-for-service patients (9.5%), self-pay (8%), and HMO (5.5%) patients (P = .02).
Dr. Engelbert observed that vascular surgery outcomes have come under scrutiny and that there has been some discussion of cutbacks in Medicare reimbursement given its high rates of readmission.
"This is already starting to happen for certain medical patient populations and if this were to happen, it would significantly affect a vascular service’s practice because a majority of our patients are covered by Medicare and have a higher readmission rate," he said.
The analysis suggests that vascular surgeons may also want to pay closer attention to discharge destination for their patients. Readmission rates were about three times higher for patients discharged to a rehabilitation facility or skilled nursing facility than for those discharged home (19.2% and 16.2% vs. 6.2%; P less than .01).
"The discharge destination matters," Dr. Engelbert said. "... we need to have improved coordination between hospitals and postdischarge destinations. And, we also might need to look at how these patients are cared for and if they are discharged to the appropriate level of care when they’re discharged to these skilled nursing and rehabilitation facilities."
The effects of discharge destination (odds ratio, 1.54 skilled nursing facility), index length of stay (OR, 1.03), insurance (OR, 0.43 HMO), and lower-extremity revascularization or amputation (OR, 2.35) persisted in multivariable logistic regression analysis that controlled for age, sex, race, proximity to hospital, clinic follow-up time, urgent vs. elective admission, insurance type, procedure type, length of stay, and discharge destination.
When asked by the audience what the university has done to reduce its vascular readmission rates, Dr. Engelbert said they have looked at using in-patient swabs to reduce infection and dedicated vascular nurse practitioners or case managers to ensure patients are being discharged to the appropriate level of care.
"I think further efforts need to focus on how we can reduce outpatient complications, through closer and quicker follow-up perhaps, as well as ways to use technology to monitor patients," he said.
One example of this is the use of outpatient remote wound analysis using smartphone photograph technology.
"Wound complications and subsequent readmissions are frequent, costly, and a significant burden to the patients," Dr. Engelbert said in an interview. "Hopefully, this method will reduce the severity of wound complications if they can be caught and treated at an earlier stage with digital photograph analysis."
One audience member argued that the vast majority of vascular problems could be cared for outpatient, but that vascular surgeons frequently aren’t told their patients have been readmitted until after they’ve been in the hospital for 2 or 3 days.
Dr. Engelbert reported having no financial disclosures. A coauthor reported grant funding from Abbott, Cook, Covidien, Endologix, Gore, and the National Institutes of Health.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
CHICAGO – Lower-extremity revascularization or amputation was among the strongest predictors of 30-day vascular surgery readmission in what is being described as the largest single-center review in this setting to date.
Lower-extremity revascularization and amputations made up 63% of unplanned readmissions, though rates for endovascular lower-extremity revascularization were almost half that of open revascularization (8.2% vs. 15%).
Notably, below-knee amputations fared the worst, with a 30-day unplanned readmission rate of 24%, compared with 13.3% for above-knee amputations and 16.4% for foot amputation.
"Amputations and open lower-extremity revascularization had the highest rates of readmission in this analysis and therefore we need to focus our efforts and find additional postoperative [management] strategies for these two subgroups," Dr. Travis L. Engelbert said at the annual meeting of the annual meeting of the Midwestern Vascular Surgical Society.*
The analysis involved 2,505 patients who underwent vascular surgery at the University of Wisconsin Hospitals and Clinics in Madison from 2009 to mid-2013. The overall readmission rate was 9.7% (n = 244).
Of these, 147 patients (60.2%) were readmitted to the vascular surgery service.
The most common readmitting diagnosis was wound complication or infection in 37%, said Dr. Engelbert, a vascular surgeon at the university.
Patients whose index admission was urgent rather than elective had significantly higher readmission rates (14.6% vs. 6.9%; P less than .001), as did those living remotely rather than inside Dane County, where the university is located (12% vs. 8.8%; P = .02).
Not surprisingly, higher illness severity, as calculated using the 3M APR DRG software, was significantly associated with readmission (15.6% high vs. 4.3% low severity; P less than .001).
Patients who were readmitted had a longer initial length of stay (LOS) (8.5 days vs. 6.1 days; P less than .01), and were more likely to have an ICU admission (18.3% vs. 9.5% without ICU stay; P less than .05), he reported.
Based on insurance status, patients covered by Medicaid (16.8%) and Medicare (10%) were most likely to have an unplanned readmission, followed by fee-for-service patients (9.5%), self-pay (8%), and HMO (5.5%) patients (P = .02).
Dr. Engelbert observed that vascular surgery outcomes have come under scrutiny and that there has been some discussion of cutbacks in Medicare reimbursement given its high rates of readmission.
"This is already starting to happen for certain medical patient populations and if this were to happen, it would significantly affect a vascular service’s practice because a majority of our patients are covered by Medicare and have a higher readmission rate," he said.
The analysis suggests that vascular surgeons may also want to pay closer attention to discharge destination for their patients. Readmission rates were about three times higher for patients discharged to a rehabilitation facility or skilled nursing facility than for those discharged home (19.2% and 16.2% vs. 6.2%; P less than .01).
"The discharge destination matters," Dr. Engelbert said. "... we need to have improved coordination between hospitals and postdischarge destinations. And, we also might need to look at how these patients are cared for and if they are discharged to the appropriate level of care when they’re discharged to these skilled nursing and rehabilitation facilities."
The effects of discharge destination (odds ratio, 1.54 skilled nursing facility), index length of stay (OR, 1.03), insurance (OR, 0.43 HMO), and lower-extremity revascularization or amputation (OR, 2.35) persisted in multivariable logistic regression analysis that controlled for age, sex, race, proximity to hospital, clinic follow-up time, urgent vs. elective admission, insurance type, procedure type, length of stay, and discharge destination.
When asked by the audience what the university has done to reduce its vascular readmission rates, Dr. Engelbert said they have looked at using in-patient swabs to reduce infection and dedicated vascular nurse practitioners or case managers to ensure patients are being discharged to the appropriate level of care.
"I think further efforts need to focus on how we can reduce outpatient complications, through closer and quicker follow-up perhaps, as well as ways to use technology to monitor patients," he said.
One example of this is the use of outpatient remote wound analysis using smartphone photograph technology.
"Wound complications and subsequent readmissions are frequent, costly, and a significant burden to the patients," Dr. Engelbert said in an interview. "Hopefully, this method will reduce the severity of wound complications if they can be caught and treated at an earlier stage with digital photograph analysis."
One audience member argued that the vast majority of vascular problems could be cared for outpatient, but that vascular surgeons frequently aren’t told their patients have been readmitted until after they’ve been in the hospital for 2 or 3 days.
Dr. Engelbert reported having no financial disclosures. A coauthor reported grant funding from Abbott, Cook, Covidien, Endologix, Gore, and the National Institutes of Health.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
FROM THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGICAL SOCIETY
Major finding: The 30-day vascular readmission rate was 9.7%, with amputation/lower-limb revascularization comprising 63% of readmissions.
Data source: A retrospective review of 2,505 patients undergoing vascular surgery at a single institution.
Disclosures: Dr. Engelbert reported having no financial disclosures. A coauthor reported grant funding from Abbott, Cook, Covidien, Endologix, Gore, and the National Institutes of Health.
Endovascular coiling aids pelvic congestion syndrome
CHICAGO – Endovascular coiling should be offered to women with pelvic congestion syndrome as an effective treatment.
"The technical success rate is high, pain scores were significantly improved, and most importantly, the patient satisfaction with resolution of their symptoms is very high," Dr. Axel Thors said at the annual meeting of the Midwestern Vascular Surgical Society*.
He reported on a 4-year review involving 15 women with pelvic congestion syndrome (PCS) who underwent endovenous coil embolization (n = 14) or stenting of the iliac vein (n = 1).
The diagnosis of PCS was made clinically by the presence of chronic pelvic pain for 6 months or more, sensations of pelvic fullness, dyspareunia, or perineal varicosities. There was no evidence of nutcracker syndrome or perirenal varicosities. Other pathologies had been previously ruled out.
"By the time these women got to us, we were probably the last provider they had seen and they had all undergone extensive evaluation for their pelvic pain, all the way from their primary providers to the ob.gyns.," said Dr. Thors of Ohio State University, Columbus.
Their average age was 36 years. Fourteen patients had a previous pregnancy, with an average parity of two.
Twelve patients presented with symptomatic vulvar varices and three with imaging or laproscopic findings of tubo-ovarian varices. All had complaints of chronic pelvic pain.
"Lower extremity venous insufficiency was closely associated with the incidence [of PCS], as was chronic dyspareunia," Dr. Thors said.
Gonadal vein venograms were performed during normal breath and the Valsalva maneuver. Embolization was performed if there was gonadal vein incompetence, congestion of the ovarian venous plexus, uterine venous congestion, cross-pelvic congestion, or marked enlargement of gonadal veins (minimum 6 mm). The average venality size was 7.3 mm.
In all, 13 gonadal veins were embolized with an average of three coils, ranging in size from 6 mm to 12 mm, Dr. Thors said.
Four gonadal veins were occluded using an Amplatzer plug (range 12-18 mm). One iliac vein was stented with a 16 mm by 60 mm stent.
Lower-extremity venous insufficiency was treated with ablation and subsequently followed clinically, he said.
Pain scores on a 10-point visual analog scale declined significantly from baseline for eight evaluable patients for pelvic pain (9.3 vs. 1.8), dyspareunia (8.875 vs. 1.5), painful vulvar varices (9.2 vs. 1.2), and lower extremity venous insufficiency (7 vs. 1), he said.
Two patients had recurrence, and their baseline pain score of 1.2 increased to 4.0 after a mean of 21 months.
All eight patients reported that they were "satisfied" or "very satisfied" with their procedure.
"Patients with chronic pelvic pain, vulvar varices, multiparity, and lower extremity venous insufficiency should be offered endovascular evaluation and treatment," Dr. Thors concluded.
Audience members said that the study represents an important concept in the management of these patients. It is a validation of a very old treatment that sometimes is not offered because of a lack of knowledge or perceived lack of data. A 2012 Agency for Healthcare Research and Quality review estimated that outpatient management of chronic pelvic pain cost $1.2 billion annually. The AHRQ review of 36 studies concluded that there is insufficient evidence to demonstrate the effectiveness of surgical approaches for chronic pelvic pain.
Dr. Thors and his coauthors reported having no financial disclosures.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
CHICAGO – Endovascular coiling should be offered to women with pelvic congestion syndrome as an effective treatment.
"The technical success rate is high, pain scores were significantly improved, and most importantly, the patient satisfaction with resolution of their symptoms is very high," Dr. Axel Thors said at the annual meeting of the Midwestern Vascular Surgical Society*.
He reported on a 4-year review involving 15 women with pelvic congestion syndrome (PCS) who underwent endovenous coil embolization (n = 14) or stenting of the iliac vein (n = 1).
The diagnosis of PCS was made clinically by the presence of chronic pelvic pain for 6 months or more, sensations of pelvic fullness, dyspareunia, or perineal varicosities. There was no evidence of nutcracker syndrome or perirenal varicosities. Other pathologies had been previously ruled out.
"By the time these women got to us, we were probably the last provider they had seen and they had all undergone extensive evaluation for their pelvic pain, all the way from their primary providers to the ob.gyns.," said Dr. Thors of Ohio State University, Columbus.
Their average age was 36 years. Fourteen patients had a previous pregnancy, with an average parity of two.
Twelve patients presented with symptomatic vulvar varices and three with imaging or laproscopic findings of tubo-ovarian varices. All had complaints of chronic pelvic pain.
"Lower extremity venous insufficiency was closely associated with the incidence [of PCS], as was chronic dyspareunia," Dr. Thors said.
Gonadal vein venograms were performed during normal breath and the Valsalva maneuver. Embolization was performed if there was gonadal vein incompetence, congestion of the ovarian venous plexus, uterine venous congestion, cross-pelvic congestion, or marked enlargement of gonadal veins (minimum 6 mm). The average venality size was 7.3 mm.
In all, 13 gonadal veins were embolized with an average of three coils, ranging in size from 6 mm to 12 mm, Dr. Thors said.
Four gonadal veins were occluded using an Amplatzer plug (range 12-18 mm). One iliac vein was stented with a 16 mm by 60 mm stent.
Lower-extremity venous insufficiency was treated with ablation and subsequently followed clinically, he said.
Pain scores on a 10-point visual analog scale declined significantly from baseline for eight evaluable patients for pelvic pain (9.3 vs. 1.8), dyspareunia (8.875 vs. 1.5), painful vulvar varices (9.2 vs. 1.2), and lower extremity venous insufficiency (7 vs. 1), he said.
Two patients had recurrence, and their baseline pain score of 1.2 increased to 4.0 after a mean of 21 months.
All eight patients reported that they were "satisfied" or "very satisfied" with their procedure.
"Patients with chronic pelvic pain, vulvar varices, multiparity, and lower extremity venous insufficiency should be offered endovascular evaluation and treatment," Dr. Thors concluded.
Audience members said that the study represents an important concept in the management of these patients. It is a validation of a very old treatment that sometimes is not offered because of a lack of knowledge or perceived lack of data. A 2012 Agency for Healthcare Research and Quality review estimated that outpatient management of chronic pelvic pain cost $1.2 billion annually. The AHRQ review of 36 studies concluded that there is insufficient evidence to demonstrate the effectiveness of surgical approaches for chronic pelvic pain.
Dr. Thors and his coauthors reported having no financial disclosures.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
CHICAGO – Endovascular coiling should be offered to women with pelvic congestion syndrome as an effective treatment.
"The technical success rate is high, pain scores were significantly improved, and most importantly, the patient satisfaction with resolution of their symptoms is very high," Dr. Axel Thors said at the annual meeting of the Midwestern Vascular Surgical Society*.
He reported on a 4-year review involving 15 women with pelvic congestion syndrome (PCS) who underwent endovenous coil embolization (n = 14) or stenting of the iliac vein (n = 1).
The diagnosis of PCS was made clinically by the presence of chronic pelvic pain for 6 months or more, sensations of pelvic fullness, dyspareunia, or perineal varicosities. There was no evidence of nutcracker syndrome or perirenal varicosities. Other pathologies had been previously ruled out.
"By the time these women got to us, we were probably the last provider they had seen and they had all undergone extensive evaluation for their pelvic pain, all the way from their primary providers to the ob.gyns.," said Dr. Thors of Ohio State University, Columbus.
Their average age was 36 years. Fourteen patients had a previous pregnancy, with an average parity of two.
Twelve patients presented with symptomatic vulvar varices and three with imaging or laproscopic findings of tubo-ovarian varices. All had complaints of chronic pelvic pain.
"Lower extremity venous insufficiency was closely associated with the incidence [of PCS], as was chronic dyspareunia," Dr. Thors said.
Gonadal vein venograms were performed during normal breath and the Valsalva maneuver. Embolization was performed if there was gonadal vein incompetence, congestion of the ovarian venous plexus, uterine venous congestion, cross-pelvic congestion, or marked enlargement of gonadal veins (minimum 6 mm). The average venality size was 7.3 mm.
In all, 13 gonadal veins were embolized with an average of three coils, ranging in size from 6 mm to 12 mm, Dr. Thors said.
Four gonadal veins were occluded using an Amplatzer plug (range 12-18 mm). One iliac vein was stented with a 16 mm by 60 mm stent.
Lower-extremity venous insufficiency was treated with ablation and subsequently followed clinically, he said.
Pain scores on a 10-point visual analog scale declined significantly from baseline for eight evaluable patients for pelvic pain (9.3 vs. 1.8), dyspareunia (8.875 vs. 1.5), painful vulvar varices (9.2 vs. 1.2), and lower extremity venous insufficiency (7 vs. 1), he said.
Two patients had recurrence, and their baseline pain score of 1.2 increased to 4.0 after a mean of 21 months.
All eight patients reported that they were "satisfied" or "very satisfied" with their procedure.
"Patients with chronic pelvic pain, vulvar varices, multiparity, and lower extremity venous insufficiency should be offered endovascular evaluation and treatment," Dr. Thors concluded.
Audience members said that the study represents an important concept in the management of these patients. It is a validation of a very old treatment that sometimes is not offered because of a lack of knowledge or perceived lack of data. A 2012 Agency for Healthcare Research and Quality review estimated that outpatient management of chronic pelvic pain cost $1.2 billion annually. The AHRQ review of 36 studies concluded that there is insufficient evidence to demonstrate the effectiveness of surgical approaches for chronic pelvic pain.
Dr. Thors and his coauthors reported having no financial disclosures.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
FROM THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGICAL SOCIETY
Local anesthesia improves hemodynamic stability during carotid endarterectomy
CHICAGO – Patients undergoing carotid endarterectomy with cervical block anesthesia had fewer hemodynamic fluctuations and required less vasoactive medications than those under general anesthesia in a retrospective evaluation.
"Under cervical block anesthesia, carotid endarterectomy can be performed with a better hemodynamic profile," Dr. Marika Y. Gassner, a resident with Henry Ford Macomb Hospital, Clinton Township, Mich., said at the annual meeting of the Midwestern Vascular Surgical Society*.
The practice switched in 2003 from using general anesthesia for the majority of carotid endarterectomy to performing more than 90% of cases under local cervical block anesthesia (CBA). Exceptions include patients who are extremely nervous, unable to communicate in English, or who have plaque extending above C2.
The investigators organized the retrospective cohort study after initial observations suggested patients under CBA had less intraoperative hypotension or fluctuations in mean arterial pressure below 65 mm Hg. Vasoactive therapy demands were also lower. For example, anesthesia records showed that several doses of beta-blockers and ephedrine were required for a patient under general anesthesia, while a patient under CBA had only a single dose of midazolam (Versed) early in the procedure, she said.
Other advantages of CBA include continuous feedback on neurologic status/cerebral perfusion, endotracheal intubation not required, shorter operative times, and reduced use of shunts, Dr. Gassner said.
The analysis included 651 patients who underwent carotid endarterectomy by a single surgeon at two suburban teaching hospitals, with 397 under general anesthesia (GA) and 254 under CBA.
The CBA and GA groups were similar in age (71.26 vs. 70.97 years) and incidence of coronary artery disease (57% vs. 56%), hypertension (77% vs. 75%), and renal failure (3.5% vs. 4.0%). The GA group, however, had significantly more females (39% vs. 46.6%), and a higher incidence of chronic obstructive pulmonary disease (16% vs. 23%), nicotine abuse, (50% vs. 63%), and symptomatic patients (41.3% vs. 54%).
The incidence of intraoperative hypotension (systolic BP less than 100 mm HG) was 0.52% with CBA and 17.84% with GA (P less than .001), Dr. Gassner said.
Mean arterial pressure changes of 20% or more per patient occurred in 20% and 41%, respectively (P less than .001).
Vasopressors were required during surgery in 51.13% of the GA group and 36.22% of the CBA group (P = .0002), and antihypertensive medications in 64% and 73.6% (P = .0085). Drugs from both categories were required by significantly fewer CBA patients (22.5% vs. 33.75%; P = .045), she said.
There were no deaths in either group. Postoperative complications were higher in the GA than the CBA group including myocardial infarction (4 vs. 0 events), stroke (6 vs. 0 events), hematoma (7 vs. 2 events), and return to the OR (7 vs. 0 events). The difference did not reach statistical significance because of the sample size, Dr. Gassner said.
Earlier in the presentation, she observed that there was no difference in the primary composite endpoint of stroke, myocardial infarction, or death at 30 days in the randomized GALA (general anaesthesia vs. local anaesthesia for carotid surgery) trial conducted at 95 centers in 24 countries (Lancet 2008;372:2132-42).
"If they couldn’t find it [a survival advantage] in GALA with 3,500 patients, we couldn’t find it here," Dr. Gassner said, adding that a randomized trial powered to look at late mortality is needed.
The group has no current plans to conduct such a study or perform a cost analysis, although a subsequent analysis of the GALA data revealed a cost savings of about $283 favoring carotid endarterectomy using local anesthesia (Br. J. Surg. 2010;97:1218-25).
Dr. Gassner and her coauthors reported having no financial disclosures.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
CHICAGO – Patients undergoing carotid endarterectomy with cervical block anesthesia had fewer hemodynamic fluctuations and required less vasoactive medications than those under general anesthesia in a retrospective evaluation.
"Under cervical block anesthesia, carotid endarterectomy can be performed with a better hemodynamic profile," Dr. Marika Y. Gassner, a resident with Henry Ford Macomb Hospital, Clinton Township, Mich., said at the annual meeting of the Midwestern Vascular Surgical Society*.
The practice switched in 2003 from using general anesthesia for the majority of carotid endarterectomy to performing more than 90% of cases under local cervical block anesthesia (CBA). Exceptions include patients who are extremely nervous, unable to communicate in English, or who have plaque extending above C2.
The investigators organized the retrospective cohort study after initial observations suggested patients under CBA had less intraoperative hypotension or fluctuations in mean arterial pressure below 65 mm Hg. Vasoactive therapy demands were also lower. For example, anesthesia records showed that several doses of beta-blockers and ephedrine were required for a patient under general anesthesia, while a patient under CBA had only a single dose of midazolam (Versed) early in the procedure, she said.
Other advantages of CBA include continuous feedback on neurologic status/cerebral perfusion, endotracheal intubation not required, shorter operative times, and reduced use of shunts, Dr. Gassner said.
The analysis included 651 patients who underwent carotid endarterectomy by a single surgeon at two suburban teaching hospitals, with 397 under general anesthesia (GA) and 254 under CBA.
The CBA and GA groups were similar in age (71.26 vs. 70.97 years) and incidence of coronary artery disease (57% vs. 56%), hypertension (77% vs. 75%), and renal failure (3.5% vs. 4.0%). The GA group, however, had significantly more females (39% vs. 46.6%), and a higher incidence of chronic obstructive pulmonary disease (16% vs. 23%), nicotine abuse, (50% vs. 63%), and symptomatic patients (41.3% vs. 54%).
The incidence of intraoperative hypotension (systolic BP less than 100 mm HG) was 0.52% with CBA and 17.84% with GA (P less than .001), Dr. Gassner said.
Mean arterial pressure changes of 20% or more per patient occurred in 20% and 41%, respectively (P less than .001).
Vasopressors were required during surgery in 51.13% of the GA group and 36.22% of the CBA group (P = .0002), and antihypertensive medications in 64% and 73.6% (P = .0085). Drugs from both categories were required by significantly fewer CBA patients (22.5% vs. 33.75%; P = .045), she said.
There were no deaths in either group. Postoperative complications were higher in the GA than the CBA group including myocardial infarction (4 vs. 0 events), stroke (6 vs. 0 events), hematoma (7 vs. 2 events), and return to the OR (7 vs. 0 events). The difference did not reach statistical significance because of the sample size, Dr. Gassner said.
Earlier in the presentation, she observed that there was no difference in the primary composite endpoint of stroke, myocardial infarction, or death at 30 days in the randomized GALA (general anaesthesia vs. local anaesthesia for carotid surgery) trial conducted at 95 centers in 24 countries (Lancet 2008;372:2132-42).
"If they couldn’t find it [a survival advantage] in GALA with 3,500 patients, we couldn’t find it here," Dr. Gassner said, adding that a randomized trial powered to look at late mortality is needed.
The group has no current plans to conduct such a study or perform a cost analysis, although a subsequent analysis of the GALA data revealed a cost savings of about $283 favoring carotid endarterectomy using local anesthesia (Br. J. Surg. 2010;97:1218-25).
Dr. Gassner and her coauthors reported having no financial disclosures.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
CHICAGO – Patients undergoing carotid endarterectomy with cervical block anesthesia had fewer hemodynamic fluctuations and required less vasoactive medications than those under general anesthesia in a retrospective evaluation.
"Under cervical block anesthesia, carotid endarterectomy can be performed with a better hemodynamic profile," Dr. Marika Y. Gassner, a resident with Henry Ford Macomb Hospital, Clinton Township, Mich., said at the annual meeting of the Midwestern Vascular Surgical Society*.
The practice switched in 2003 from using general anesthesia for the majority of carotid endarterectomy to performing more than 90% of cases under local cervical block anesthesia (CBA). Exceptions include patients who are extremely nervous, unable to communicate in English, or who have plaque extending above C2.
The investigators organized the retrospective cohort study after initial observations suggested patients under CBA had less intraoperative hypotension or fluctuations in mean arterial pressure below 65 mm Hg. Vasoactive therapy demands were also lower. For example, anesthesia records showed that several doses of beta-blockers and ephedrine were required for a patient under general anesthesia, while a patient under CBA had only a single dose of midazolam (Versed) early in the procedure, she said.
Other advantages of CBA include continuous feedback on neurologic status/cerebral perfusion, endotracheal intubation not required, shorter operative times, and reduced use of shunts, Dr. Gassner said.
The analysis included 651 patients who underwent carotid endarterectomy by a single surgeon at two suburban teaching hospitals, with 397 under general anesthesia (GA) and 254 under CBA.
The CBA and GA groups were similar in age (71.26 vs. 70.97 years) and incidence of coronary artery disease (57% vs. 56%), hypertension (77% vs. 75%), and renal failure (3.5% vs. 4.0%). The GA group, however, had significantly more females (39% vs. 46.6%), and a higher incidence of chronic obstructive pulmonary disease (16% vs. 23%), nicotine abuse, (50% vs. 63%), and symptomatic patients (41.3% vs. 54%).
The incidence of intraoperative hypotension (systolic BP less than 100 mm HG) was 0.52% with CBA and 17.84% with GA (P less than .001), Dr. Gassner said.
Mean arterial pressure changes of 20% or more per patient occurred in 20% and 41%, respectively (P less than .001).
Vasopressors were required during surgery in 51.13% of the GA group and 36.22% of the CBA group (P = .0002), and antihypertensive medications in 64% and 73.6% (P = .0085). Drugs from both categories were required by significantly fewer CBA patients (22.5% vs. 33.75%; P = .045), she said.
There were no deaths in either group. Postoperative complications were higher in the GA than the CBA group including myocardial infarction (4 vs. 0 events), stroke (6 vs. 0 events), hematoma (7 vs. 2 events), and return to the OR (7 vs. 0 events). The difference did not reach statistical significance because of the sample size, Dr. Gassner said.
Earlier in the presentation, she observed that there was no difference in the primary composite endpoint of stroke, myocardial infarction, or death at 30 days in the randomized GALA (general anaesthesia vs. local anaesthesia for carotid surgery) trial conducted at 95 centers in 24 countries (Lancet 2008;372:2132-42).
"If they couldn’t find it [a survival advantage] in GALA with 3,500 patients, we couldn’t find it here," Dr. Gassner said, adding that a randomized trial powered to look at late mortality is needed.
The group has no current plans to conduct such a study or perform a cost analysis, although a subsequent analysis of the GALA data revealed a cost savings of about $283 favoring carotid endarterectomy using local anesthesia (Br. J. Surg. 2010;97:1218-25).
Dr. Gassner and her coauthors reported having no financial disclosures.
*CORRECTION, 10/29/2013: An earlier version of this article misstated the name of the annual meeting of the Midwestern Vascular Surgical Society.
FROM THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGICAL SOCIETY
Major finding: The incidence of intraoperative hypotension was 0.52% with cervical block anesthesia and 17.84% with general anesthesia.
Data source: Retrospective review of 651 patients undergoing carotid endarterectomy.
Disclosures: Dr. Gassner and her coauthors reported having no financial disclosures.
Edwards to start U.S. trial of SAPIEN 3
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
The Food and Drug Administration has given Edwards Lifesciences the green light to start a clinical trial on the SAPIEN 3 transcatheter aortic heart valve, which is a more advanced and improved version of the currently available SAPIEN valve.
"This is very exciting news," said Dr. Augusto Pichard, director of cardiac intervention and structural heart disease at MedStar Heart Institute in Washington, D.C. "One of the major concerns we have is vascular access. The current FDA-approved [SAPIEN] valve is large and sometimes doesn’t fit, or produces serious vascular complications," Dr. Pichard said in an interview. "The new valve has much smaller delivery size, making it very likely that many more patients would benefit from the procedure."
SAPIEN 3 also has a fabric cuff to reduce paravalvular leak, which is one of the major determinants of the procedure's long-term outcomes.
Under FDA’s conditional Investigational Device Exemption (IDE), Edwards will enroll up to 500 high-risk or inoperable patients with severe symptomatic aortic stenosis. The valve can be placed through transfemoral, transapical, or transaortic approach, and patients will be followed up for 1 year.
In the United States, the SAPIEN valve has been the first and only approved transcatheter aortic valve for transcatheter aortic valve replacement (TAVR), and several clinical trials by Medtronic are underway. But the valve technology has been moving forward elsewhere.
"The U.S. has lagged behind the rest of the world in access to new TAVR technology," Dr. John Carroll, a member of the STS/ACC TVT Registry Steering Committee and director of interventional cardiology and professor of medicine at the University of Colorado Hospital, Aurora, said in an interview. "This IDE being launched potentially allows us to start the process, bringing better and safer technologies to our patients."
Although the indications for SAPIEN 3 are the same as the currently used SAPIEN valve, experts say that the smaller SAPIEN 3 would benefit more patients.
"It’s been frustrating not to be able to treat many patients," said Dr. Carroll. "In TVT registry, we’ve gathered data on the initial U.S. experiences, with over 7,000 patients, and we certainly see a need for technology improvement, both smaller deliver systems and trying to eliminate paravalvular leak."
Edwards noted that the SAPIEN 3 valve is still an investigational device and is not commercially available in any country, but results from initial trials have been promising.
Dr. Pichard and Dr. Carroll said that they have observed the SAPIEN 3 placement on several occasions – overseas or via live case transmissions. They said the procedure for SAPIEN 3 is similar to the SAPIEN valve, and it appears to be easier.
Both cardiologists have been involved in Edwards’ PARTNER trials and said that they expected the patient enrollment to be rapid. They were not sure if Edwards will include their hospitals in the trial. After 1-year follow-up of the patients, the company will give the data to the FDA for a final decision.
The IDE is one of the key hurdles to initiating a clinical trial, which can potentially lead to FDA approval and commercial release. The process, if all goes as expected, could take at least 2 years.
Dr. Carroll said that he had no disclosures other than being involved in the PARTNER trial. Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves.
On Twitter @NaseemSMiller
Tools help predict VTE after abdominal, thoracic surgery
Two simple-to-use calculation tools, which physicians can use to predict separately the 30-day risk of postoperative venous thromboembolism in hospital and after discharge, have been developed. Researchers based the two nomograms on the results of a retrospective analysis of more than 450,000 thoracic and abdominal surgical patients in the American College of Surgeons National Surgical Quality Improvement Program database.
"Substantial variation exists in the incidence of VTE [venous thromboembolism] and VTEDC [venous thromboembolism after hospital discharge] after abdominal or thoracic surgery, depending on patient and procedural factors," wrote Dr. Robert Canter, Dr. Dhruvil R. Shah, and colleagues at the University of California, Davis. They analyzed these factors to determine statistically significant risks in order to construct the predictive nomograms, according to their report published in the July issue of the Journal of Surgical Research. Dr. Canter is the senior author of the study and associate professor of surgery in the Division of Surgical Oncology at UC Davis.*
The authors used data obtained from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) to construct a nomogram by which physicians could use a ruler and draw lines from each separate risk factor on a no-yes gridline to a point score for that factor. By adding the point results for all factors, the total number of points could then be used to calculate the 30-day risk of developing a VTE. A separate nomogram using the same process was constructed for calculating the risk of a VTEDC.
The researchers assessed 471,867 patients who underwent inpatient abdominal or thoracic operations between 2005 and 2010. Patients who underwent primary vascular and spine operations were excluded. The overall unadjusted, 30-day incidence of postoperative VT) in these patients was 1.5% and that of VTEDC was 0.5%, and the annual incidence rates remained unchanged over the period studied. The median time to VTE was 9 days and the median time to VTEDC was 17 days, and these also did not change over the study period.
The majority of patients were white (75.5%) and men (59%), with a mean age of 54 years and a mean body mass index of 31 kg/m2; 93% of the patients were functionally independent.
Comorbidities included smoking (19%), history of preoperative infection or sepsis (16.5%), diabetes (15%), and a variety of cardiovascular conditions or procedures (9%). The vast majority of operations were abdominal (98%), with most performed on the gastrointestinal tract (51.5%). Thoracic operations accounted for 2.1%. Minimally invasive (excluding bariatric) surgery was performed in 37% of cases, with 20% of all operations performed for cancer. A total of 11% of patients experienced one or more major postoperative complications within 30 days of the operation, including post discharge.
Multivariate analysis showed that the significant predictors of both VTE and VTEDC were age, body mass index, presence of postoperative infection, operation for cancer, procedure type (primarily splenectomy), multivisceral resection, and nonbariatric laparoscopic surgery. Significant factors predicting VTE but not VTEDC were a history of chronic obstructive pulmonary disease, disseminated cancer, and emergent operation (J. Surg. Res. 2013;183:462-71).
In addition to the nomogram, the researchers used internally developed concordance indices to measure the probability of concordance between the predicted and measured outcomes and found that these were 0.77 for VTE and 0.67 for VTEDC. They acknowledge that the lower value for VTEDC is a probable indicator of unknown and uncaptured risk factors, possibly including socioeconomic status, a change in postoperative functional status,
Limitations of the study included those inherent to using the ACS-NSQUIP database, which meant that they could not identify patients who had a prior history of VTE or central line placement, both of which have been cited as VTE risk factors, according to the researchers. In addition, asymptomatic VTE events and events for which patients did not receive anticoagulation were not captured in the database.
"Although these nomograms require external validation to ensure reproducibility, these tools will aid clinicians, researchers, and administrators in determining which subset of patients undergoing abdominal or general thoracic general surgical operations are at highest risk for postoperative VTE and VTEDC. Our data may allow for more targeted quality improvement interventions to reduce VTE and VTEDC" in these patients, the researchers concluded.
The authors disclosed no conflicts that the editors of the journal determined should be reported in the article.
*UPDATED: This story was updated to include Dr. Canter's information and photo.
Two simple-to-use calculation tools, which physicians can use to predict separately the 30-day risk of postoperative venous thromboembolism in hospital and after discharge, have been developed. Researchers based the two nomograms on the results of a retrospective analysis of more than 450,000 thoracic and abdominal surgical patients in the American College of Surgeons National Surgical Quality Improvement Program database.
"Substantial variation exists in the incidence of VTE [venous thromboembolism] and VTEDC [venous thromboembolism after hospital discharge] after abdominal or thoracic surgery, depending on patient and procedural factors," wrote Dr. Robert Canter, Dr. Dhruvil R. Shah, and colleagues at the University of California, Davis. They analyzed these factors to determine statistically significant risks in order to construct the predictive nomograms, according to their report published in the July issue of the Journal of Surgical Research. Dr. Canter is the senior author of the study and associate professor of surgery in the Division of Surgical Oncology at UC Davis.*
The authors used data obtained from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) to construct a nomogram by which physicians could use a ruler and draw lines from each separate risk factor on a no-yes gridline to a point score for that factor. By adding the point results for all factors, the total number of points could then be used to calculate the 30-day risk of developing a VTE. A separate nomogram using the same process was constructed for calculating the risk of a VTEDC.
The researchers assessed 471,867 patients who underwent inpatient abdominal or thoracic operations between 2005 and 2010. Patients who underwent primary vascular and spine operations were excluded. The overall unadjusted, 30-day incidence of postoperative VT) in these patients was 1.5% and that of VTEDC was 0.5%, and the annual incidence rates remained unchanged over the period studied. The median time to VTE was 9 days and the median time to VTEDC was 17 days, and these also did not change over the study period.
The majority of patients were white (75.5%) and men (59%), with a mean age of 54 years and a mean body mass index of 31 kg/m2; 93% of the patients were functionally independent.
Comorbidities included smoking (19%), history of preoperative infection or sepsis (16.5%), diabetes (15%), and a variety of cardiovascular conditions or procedures (9%). The vast majority of operations were abdominal (98%), with most performed on the gastrointestinal tract (51.5%). Thoracic operations accounted for 2.1%. Minimally invasive (excluding bariatric) surgery was performed in 37% of cases, with 20% of all operations performed for cancer. A total of 11% of patients experienced one or more major postoperative complications within 30 days of the operation, including post discharge.
Multivariate analysis showed that the significant predictors of both VTE and VTEDC were age, body mass index, presence of postoperative infection, operation for cancer, procedure type (primarily splenectomy), multivisceral resection, and nonbariatric laparoscopic surgery. Significant factors predicting VTE but not VTEDC were a history of chronic obstructive pulmonary disease, disseminated cancer, and emergent operation (J. Surg. Res. 2013;183:462-71).
In addition to the nomogram, the researchers used internally developed concordance indices to measure the probability of concordance between the predicted and measured outcomes and found that these were 0.77 for VTE and 0.67 for VTEDC. They acknowledge that the lower value for VTEDC is a probable indicator of unknown and uncaptured risk factors, possibly including socioeconomic status, a change in postoperative functional status,
Limitations of the study included those inherent to using the ACS-NSQUIP database, which meant that they could not identify patients who had a prior history of VTE or central line placement, both of which have been cited as VTE risk factors, according to the researchers. In addition, asymptomatic VTE events and events for which patients did not receive anticoagulation were not captured in the database.
"Although these nomograms require external validation to ensure reproducibility, these tools will aid clinicians, researchers, and administrators in determining which subset of patients undergoing abdominal or general thoracic general surgical operations are at highest risk for postoperative VTE and VTEDC. Our data may allow for more targeted quality improvement interventions to reduce VTE and VTEDC" in these patients, the researchers concluded.
The authors disclosed no conflicts that the editors of the journal determined should be reported in the article.
*UPDATED: This story was updated to include Dr. Canter's information and photo.
Two simple-to-use calculation tools, which physicians can use to predict separately the 30-day risk of postoperative venous thromboembolism in hospital and after discharge, have been developed. Researchers based the two nomograms on the results of a retrospective analysis of more than 450,000 thoracic and abdominal surgical patients in the American College of Surgeons National Surgical Quality Improvement Program database.
"Substantial variation exists in the incidence of VTE [venous thromboembolism] and VTEDC [venous thromboembolism after hospital discharge] after abdominal or thoracic surgery, depending on patient and procedural factors," wrote Dr. Robert Canter, Dr. Dhruvil R. Shah, and colleagues at the University of California, Davis. They analyzed these factors to determine statistically significant risks in order to construct the predictive nomograms, according to their report published in the July issue of the Journal of Surgical Research. Dr. Canter is the senior author of the study and associate professor of surgery in the Division of Surgical Oncology at UC Davis.*
The authors used data obtained from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) to construct a nomogram by which physicians could use a ruler and draw lines from each separate risk factor on a no-yes gridline to a point score for that factor. By adding the point results for all factors, the total number of points could then be used to calculate the 30-day risk of developing a VTE. A separate nomogram using the same process was constructed for calculating the risk of a VTEDC.
The researchers assessed 471,867 patients who underwent inpatient abdominal or thoracic operations between 2005 and 2010. Patients who underwent primary vascular and spine operations were excluded. The overall unadjusted, 30-day incidence of postoperative VT) in these patients was 1.5% and that of VTEDC was 0.5%, and the annual incidence rates remained unchanged over the period studied. The median time to VTE was 9 days and the median time to VTEDC was 17 days, and these also did not change over the study period.
The majority of patients were white (75.5%) and men (59%), with a mean age of 54 years and a mean body mass index of 31 kg/m2; 93% of the patients were functionally independent.
Comorbidities included smoking (19%), history of preoperative infection or sepsis (16.5%), diabetes (15%), and a variety of cardiovascular conditions or procedures (9%). The vast majority of operations were abdominal (98%), with most performed on the gastrointestinal tract (51.5%). Thoracic operations accounted for 2.1%. Minimally invasive (excluding bariatric) surgery was performed in 37% of cases, with 20% of all operations performed for cancer. A total of 11% of patients experienced one or more major postoperative complications within 30 days of the operation, including post discharge.
Multivariate analysis showed that the significant predictors of both VTE and VTEDC were age, body mass index, presence of postoperative infection, operation for cancer, procedure type (primarily splenectomy), multivisceral resection, and nonbariatric laparoscopic surgery. Significant factors predicting VTE but not VTEDC were a history of chronic obstructive pulmonary disease, disseminated cancer, and emergent operation (J. Surg. Res. 2013;183:462-71).
In addition to the nomogram, the researchers used internally developed concordance indices to measure the probability of concordance between the predicted and measured outcomes and found that these were 0.77 for VTE and 0.67 for VTEDC. They acknowledge that the lower value for VTEDC is a probable indicator of unknown and uncaptured risk factors, possibly including socioeconomic status, a change in postoperative functional status,
Limitations of the study included those inherent to using the ACS-NSQUIP database, which meant that they could not identify patients who had a prior history of VTE or central line placement, both of which have been cited as VTE risk factors, according to the researchers. In addition, asymptomatic VTE events and events for which patients did not receive anticoagulation were not captured in the database.
"Although these nomograms require external validation to ensure reproducibility, these tools will aid clinicians, researchers, and administrators in determining which subset of patients undergoing abdominal or general thoracic general surgical operations are at highest risk for postoperative VTE and VTEDC. Our data may allow for more targeted quality improvement interventions to reduce VTE and VTEDC" in these patients, the researchers concluded.
The authors disclosed no conflicts that the editors of the journal determined should be reported in the article.
*UPDATED: This story was updated to include Dr. Canter's information and photo.
FROM THE JOURNAL OF SURGICAL RESEARCH
AMPLIFY: Apixaban beat warfarin on safety in acute VTE
AMSTERDAM – In patients with acute venous thromboembolism, 6 months of treatment with the oral-anticoagulant apixaban was as effective as was standard therapy with subcutaneous enoxaparin for a week followed by oral warfarin, and apixaban caused significantly fewer major bleeding complications in a randomized, multicenter trial with more than 5,000 patients.
But in addition to apixaban’s sterling individual performance in this pivotal trial, which seems to clear the way for the drug to eventually receive a labeled indication for acute venous thromboembolism, the results also appeared to further anoint the new, oral anticoagulant roster of dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis) as the thrombotic-disease troika to be reckoned with, the newcomers whose time has come.
Ever since rivaroxaban became the first of the trio to gain acute VTE labeling, last November, physicians who manage patients with acute VTE had to wrestle with the question of how to integrate this option into their practices. The new findings on apixaban suggest that physicians will soon have to think about deciding between rivaroxaban and apixaban for this indication. And since recent results from other major trials also established dabigatran as the equal of warfarin for efficacy when treating acute VTE patients and with superior safety, dabigatran’s entry into acute VTE management seems imminent (N. Engl. J. Med. 2013;368:709-18).
Propelling this new anticoagulant era are the indications of efficacy that’s equivalent with heparin, but safer, and with far easier drug delivery as the need for anticoagulation clinics and regular measurement of international normalized ratio (INR) is eliminated by all three new drugs.
"An oral regimen without laboratory monitoring will simplify therapy," Dr. Giancarlo Agnelli noted when he presented the new apixaban findings at the congress of the International Society on Thrombosis and Haemostasis. Concurrently with his report at the meeting, the results were published online (N. Engl. J. Med. 2013;doi:10.1056/nejmoa1302507).
"I think the argument is overwhelming" to use one of the new drugs instead of warfarin. "They are oral drugs where you do not need a blood draw every 2 or 3 weeks, they are a lot easier to use, and they are at least as good as warfarin and at least as safe," said Dr. Frits R. Rosendaal, professor of clinical epidemiology in hemostasis and thrombosis at Leiden (The Netherlands) University.
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial randomized 5,400 acute VTE patients at 358 centers in 28 countries. Patients received either apixaban starting with a 10-mg b.i.d. dosage for 7 days, followed by a dosage of 5 mg b.i.d. for 6 months, or enoxaparin at a dosage of 1 mg/kg every 12 hours for a median of 7 days followed by warfarin for 6 months with a target INR of 2.0-3.0.
The study’s primary efficacy endpoint was the combined rate of recurrent, symptomatic VTE or death related to VTE. This occurred in 59 of 2,609 patients (2.3%) who received apixaban, and in 71 of 2,635 (2.7%) patients who received enoxaparin followed by warfarin. These results met the study’s prespecified criterion for apixaban’s noninferiority to standard treatment reported Dr. Agnelli, professor of medicine at the University of Perugia, Italy.
Major bleeding events occurred in 15 of 2,676 (0.6%) patients on apixaban and in 49 of 2,689 (1.8%) patients on enoxaparin and warfarin, a statistically significant difference. A composite safety outcome of major bleeds plus clinically relevant nonmajor bleeds occurred in 4.3% of the apixaban patients and in 9.7% of the patients on standard therapy, a statistically significant difference. Aside from bleeding events, the rates of all other adverse events were similar in the two treatment arms.
The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
The new anticoagulant era for patients with acute venous thromboembolism began late last year, when the Food and Drug Administration gave rivaroxaban this new labeled indication. Each physician who enters this new era, by opting to prescribe rivaroxaban instead of warfarin, needs to carefully think through which types of patients the evidence supports treating.
Clinicians who manage patients with acute venous thromboembolism (VTE) need to decide how to bring rivaroxaban into their practices, and eventually they will need to make similar decisions about dabigatran and apixaban once those drugs receive an acute VTE indication.

|
|
A few weeks after rivaroxaban’s approval for acute VTE, I got together with my colleagues at the University of Vermont in Burlington who manage these patients to draw up a framework for how we’ll decide which patients should be treated with rivaroxaban and which ones we should still treat with warfarin. I included the six main elements of that framework in an editorial I wrote about the AMPLIFY results reported by Dr. Agnelli (N. Engl. J. Med. 2013;doi:10.1056/nejme1307413).
One factor we urged clinicians to keep in mind was that the new-anticoagulant trials use selected patients. For example, the AMPLIFY trial excluded patients with provoked VTE because they are usually not treated for 6 months, the treatment duration studied in the trial. The new drugs are not suitable for every patient; patients on dialysis shouldn’t get them because these agents all involve renal clearance. Very old patients, those aged beyond 80 years, are an uncertain group because the trials have so far included few such patients, but on the other hand, eliminating the need for regular anticoagulation monitoring may make the new drugs more suitable for some elderly patients. Patients with cancer are another uncertain subgroup because of their low representation in the trials. My colleagues and I have for the time being decided not to use rivaroxaban on cancer patients with acute VTE because we judged the current evidence base too limited.
Rivaroxaban, apixaban, and dabigatran each have their own unique list of drug interactions and contraindications. None of them has as many caveats as warfarin, but it’s still essential to be familiar with each drug’s label and which clinical situations to avoid.
Another issue is how likely a patient will be to adhere to the prescribed regimen. A limitation of the new drugs is their short half-life. A patient just needs to miss a couple of doses and the anticoagulation effect starts to fade. In contrast, warfarin’s effect lingers much longer; a patient on a stable warfarin regimen can go several days without treatment before the international normalized ratio begins to fall out of the target range. Plus, patients on the new drugs provide physicians with no compliance feedback from monitoring results. That means patients should only be started on a new oral anticoagulant if they understand this limitation and the need for commitment to full compliance. There are also issues of cost and insurance coverage with the new drugs. Some patients have insurers that require preapproval for treatment of VTE with rivaroxaban, but then the payers deny the preapproval when we request it.
Warfarin has been the top anticoagulant for the past 60 years, much longer than my entire career in medicine. Physicians now face the challenge of applying the results from carefully controlled studies of the new anticoagulants – where selected cohorts of patients received their care from a small number of anticoagulation management experts – to a much broader and more diverse spectrum of patients in a variety of clinical settings. How exactly that gets done needs careful consideration.
Dr. Mary Cushman is a hematologist and professor of medicine at the University of Vermont in Burlington. She made these comments in an interview. She said that she had no disclosures.
The new anticoagulant era for patients with acute venous thromboembolism began late last year, when the Food and Drug Administration gave rivaroxaban this new labeled indication. Each physician who enters this new era, by opting to prescribe rivaroxaban instead of warfarin, needs to carefully think through which types of patients the evidence supports treating.
Clinicians who manage patients with acute venous thromboembolism (VTE) need to decide how to bring rivaroxaban into their practices, and eventually they will need to make similar decisions about dabigatran and apixaban once those drugs receive an acute VTE indication.

|
|
A few weeks after rivaroxaban’s approval for acute VTE, I got together with my colleagues at the University of Vermont in Burlington who manage these patients to draw up a framework for how we’ll decide which patients should be treated with rivaroxaban and which ones we should still treat with warfarin. I included the six main elements of that framework in an editorial I wrote about the AMPLIFY results reported by Dr. Agnelli (N. Engl. J. Med. 2013;doi:10.1056/nejme1307413).
One factor we urged clinicians to keep in mind was that the new-anticoagulant trials use selected patients. For example, the AMPLIFY trial excluded patients with provoked VTE because they are usually not treated for 6 months, the treatment duration studied in the trial. The new drugs are not suitable for every patient; patients on dialysis shouldn’t get them because these agents all involve renal clearance. Very old patients, those aged beyond 80 years, are an uncertain group because the trials have so far included few such patients, but on the other hand, eliminating the need for regular anticoagulation monitoring may make the new drugs more suitable for some elderly patients. Patients with cancer are another uncertain subgroup because of their low representation in the trials. My colleagues and I have for the time being decided not to use rivaroxaban on cancer patients with acute VTE because we judged the current evidence base too limited.
Rivaroxaban, apixaban, and dabigatran each have their own unique list of drug interactions and contraindications. None of them has as many caveats as warfarin, but it’s still essential to be familiar with each drug’s label and which clinical situations to avoid.
Another issue is how likely a patient will be to adhere to the prescribed regimen. A limitation of the new drugs is their short half-life. A patient just needs to miss a couple of doses and the anticoagulation effect starts to fade. In contrast, warfarin’s effect lingers much longer; a patient on a stable warfarin regimen can go several days without treatment before the international normalized ratio begins to fall out of the target range. Plus, patients on the new drugs provide physicians with no compliance feedback from monitoring results. That means patients should only be started on a new oral anticoagulant if they understand this limitation and the need for commitment to full compliance. There are also issues of cost and insurance coverage with the new drugs. Some patients have insurers that require preapproval for treatment of VTE with rivaroxaban, but then the payers deny the preapproval when we request it.
Warfarin has been the top anticoagulant for the past 60 years, much longer than my entire career in medicine. Physicians now face the challenge of applying the results from carefully controlled studies of the new anticoagulants – where selected cohorts of patients received their care from a small number of anticoagulation management experts – to a much broader and more diverse spectrum of patients in a variety of clinical settings. How exactly that gets done needs careful consideration.
Dr. Mary Cushman is a hematologist and professor of medicine at the University of Vermont in Burlington. She made these comments in an interview. She said that she had no disclosures.
The new anticoagulant era for patients with acute venous thromboembolism began late last year, when the Food and Drug Administration gave rivaroxaban this new labeled indication. Each physician who enters this new era, by opting to prescribe rivaroxaban instead of warfarin, needs to carefully think through which types of patients the evidence supports treating.
Clinicians who manage patients with acute venous thromboembolism (VTE) need to decide how to bring rivaroxaban into their practices, and eventually they will need to make similar decisions about dabigatran and apixaban once those drugs receive an acute VTE indication.

|
|
A few weeks after rivaroxaban’s approval for acute VTE, I got together with my colleagues at the University of Vermont in Burlington who manage these patients to draw up a framework for how we’ll decide which patients should be treated with rivaroxaban and which ones we should still treat with warfarin. I included the six main elements of that framework in an editorial I wrote about the AMPLIFY results reported by Dr. Agnelli (N. Engl. J. Med. 2013;doi:10.1056/nejme1307413).
One factor we urged clinicians to keep in mind was that the new-anticoagulant trials use selected patients. For example, the AMPLIFY trial excluded patients with provoked VTE because they are usually not treated for 6 months, the treatment duration studied in the trial. The new drugs are not suitable for every patient; patients on dialysis shouldn’t get them because these agents all involve renal clearance. Very old patients, those aged beyond 80 years, are an uncertain group because the trials have so far included few such patients, but on the other hand, eliminating the need for regular anticoagulation monitoring may make the new drugs more suitable for some elderly patients. Patients with cancer are another uncertain subgroup because of their low representation in the trials. My colleagues and I have for the time being decided not to use rivaroxaban on cancer patients with acute VTE because we judged the current evidence base too limited.
Rivaroxaban, apixaban, and dabigatran each have their own unique list of drug interactions and contraindications. None of them has as many caveats as warfarin, but it’s still essential to be familiar with each drug’s label and which clinical situations to avoid.
Another issue is how likely a patient will be to adhere to the prescribed regimen. A limitation of the new drugs is their short half-life. A patient just needs to miss a couple of doses and the anticoagulation effect starts to fade. In contrast, warfarin’s effect lingers much longer; a patient on a stable warfarin regimen can go several days without treatment before the international normalized ratio begins to fall out of the target range. Plus, patients on the new drugs provide physicians with no compliance feedback from monitoring results. That means patients should only be started on a new oral anticoagulant if they understand this limitation and the need for commitment to full compliance. There are also issues of cost and insurance coverage with the new drugs. Some patients have insurers that require preapproval for treatment of VTE with rivaroxaban, but then the payers deny the preapproval when we request it.
Warfarin has been the top anticoagulant for the past 60 years, much longer than my entire career in medicine. Physicians now face the challenge of applying the results from carefully controlled studies of the new anticoagulants – where selected cohorts of patients received their care from a small number of anticoagulation management experts – to a much broader and more diverse spectrum of patients in a variety of clinical settings. How exactly that gets done needs careful consideration.
Dr. Mary Cushman is a hematologist and professor of medicine at the University of Vermont in Burlington. She made these comments in an interview. She said that she had no disclosures.
AMSTERDAM – In patients with acute venous thromboembolism, 6 months of treatment with the oral-anticoagulant apixaban was as effective as was standard therapy with subcutaneous enoxaparin for a week followed by oral warfarin, and apixaban caused significantly fewer major bleeding complications in a randomized, multicenter trial with more than 5,000 patients.
But in addition to apixaban’s sterling individual performance in this pivotal trial, which seems to clear the way for the drug to eventually receive a labeled indication for acute venous thromboembolism, the results also appeared to further anoint the new, oral anticoagulant roster of dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis) as the thrombotic-disease troika to be reckoned with, the newcomers whose time has come.
Ever since rivaroxaban became the first of the trio to gain acute VTE labeling, last November, physicians who manage patients with acute VTE had to wrestle with the question of how to integrate this option into their practices. The new findings on apixaban suggest that physicians will soon have to think about deciding between rivaroxaban and apixaban for this indication. And since recent results from other major trials also established dabigatran as the equal of warfarin for efficacy when treating acute VTE patients and with superior safety, dabigatran’s entry into acute VTE management seems imminent (N. Engl. J. Med. 2013;368:709-18).
Propelling this new anticoagulant era are the indications of efficacy that’s equivalent with heparin, but safer, and with far easier drug delivery as the need for anticoagulation clinics and regular measurement of international normalized ratio (INR) is eliminated by all three new drugs.
"An oral regimen without laboratory monitoring will simplify therapy," Dr. Giancarlo Agnelli noted when he presented the new apixaban findings at the congress of the International Society on Thrombosis and Haemostasis. Concurrently with his report at the meeting, the results were published online (N. Engl. J. Med. 2013;doi:10.1056/nejmoa1302507).
"I think the argument is overwhelming" to use one of the new drugs instead of warfarin. "They are oral drugs where you do not need a blood draw every 2 or 3 weeks, they are a lot easier to use, and they are at least as good as warfarin and at least as safe," said Dr. Frits R. Rosendaal, professor of clinical epidemiology in hemostasis and thrombosis at Leiden (The Netherlands) University.
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial randomized 5,400 acute VTE patients at 358 centers in 28 countries. Patients received either apixaban starting with a 10-mg b.i.d. dosage for 7 days, followed by a dosage of 5 mg b.i.d. for 6 months, or enoxaparin at a dosage of 1 mg/kg every 12 hours for a median of 7 days followed by warfarin for 6 months with a target INR of 2.0-3.0.
The study’s primary efficacy endpoint was the combined rate of recurrent, symptomatic VTE or death related to VTE. This occurred in 59 of 2,609 patients (2.3%) who received apixaban, and in 71 of 2,635 (2.7%) patients who received enoxaparin followed by warfarin. These results met the study’s prespecified criterion for apixaban’s noninferiority to standard treatment reported Dr. Agnelli, professor of medicine at the University of Perugia, Italy.
Major bleeding events occurred in 15 of 2,676 (0.6%) patients on apixaban and in 49 of 2,689 (1.8%) patients on enoxaparin and warfarin, a statistically significant difference. A composite safety outcome of major bleeds plus clinically relevant nonmajor bleeds occurred in 4.3% of the apixaban patients and in 9.7% of the patients on standard therapy, a statistically significant difference. Aside from bleeding events, the rates of all other adverse events were similar in the two treatment arms.
The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
AMSTERDAM – In patients with acute venous thromboembolism, 6 months of treatment with the oral-anticoagulant apixaban was as effective as was standard therapy with subcutaneous enoxaparin for a week followed by oral warfarin, and apixaban caused significantly fewer major bleeding complications in a randomized, multicenter trial with more than 5,000 patients.
But in addition to apixaban’s sterling individual performance in this pivotal trial, which seems to clear the way for the drug to eventually receive a labeled indication for acute venous thromboembolism, the results also appeared to further anoint the new, oral anticoagulant roster of dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis) as the thrombotic-disease troika to be reckoned with, the newcomers whose time has come.
Ever since rivaroxaban became the first of the trio to gain acute VTE labeling, last November, physicians who manage patients with acute VTE had to wrestle with the question of how to integrate this option into their practices. The new findings on apixaban suggest that physicians will soon have to think about deciding between rivaroxaban and apixaban for this indication. And since recent results from other major trials also established dabigatran as the equal of warfarin for efficacy when treating acute VTE patients and with superior safety, dabigatran’s entry into acute VTE management seems imminent (N. Engl. J. Med. 2013;368:709-18).
Propelling this new anticoagulant era are the indications of efficacy that’s equivalent with heparin, but safer, and with far easier drug delivery as the need for anticoagulation clinics and regular measurement of international normalized ratio (INR) is eliminated by all three new drugs.
"An oral regimen without laboratory monitoring will simplify therapy," Dr. Giancarlo Agnelli noted when he presented the new apixaban findings at the congress of the International Society on Thrombosis and Haemostasis. Concurrently with his report at the meeting, the results were published online (N. Engl. J. Med. 2013;doi:10.1056/nejmoa1302507).
"I think the argument is overwhelming" to use one of the new drugs instead of warfarin. "They are oral drugs where you do not need a blood draw every 2 or 3 weeks, they are a lot easier to use, and they are at least as good as warfarin and at least as safe," said Dr. Frits R. Rosendaal, professor of clinical epidemiology in hemostasis and thrombosis at Leiden (The Netherlands) University.
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial randomized 5,400 acute VTE patients at 358 centers in 28 countries. Patients received either apixaban starting with a 10-mg b.i.d. dosage for 7 days, followed by a dosage of 5 mg b.i.d. for 6 months, or enoxaparin at a dosage of 1 mg/kg every 12 hours for a median of 7 days followed by warfarin for 6 months with a target INR of 2.0-3.0.
The study’s primary efficacy endpoint was the combined rate of recurrent, symptomatic VTE or death related to VTE. This occurred in 59 of 2,609 patients (2.3%) who received apixaban, and in 71 of 2,635 (2.7%) patients who received enoxaparin followed by warfarin. These results met the study’s prespecified criterion for apixaban’s noninferiority to standard treatment reported Dr. Agnelli, professor of medicine at the University of Perugia, Italy.
Major bleeding events occurred in 15 of 2,676 (0.6%) patients on apixaban and in 49 of 2,689 (1.8%) patients on enoxaparin and warfarin, a statistically significant difference. A composite safety outcome of major bleeds plus clinically relevant nonmajor bleeds occurred in 4.3% of the apixaban patients and in 9.7% of the patients on standard therapy, a statistically significant difference. Aside from bleeding events, the rates of all other adverse events were similar in the two treatment arms.
The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
AT THE 2013 ISTH CONGRESS
Major finding: Major bleeds occurred in 0.6% of patients on apixaban and 1.8% of patients on warfarin, with similar efficacy.
Data source: The AMPLIFY study, which randomized 5,400 patients with acute venous thromboembolism to treatment with apixaban or enoxaparin followed by warfarin for 6 months.
Disclosures: The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.