User login
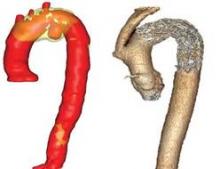
High-dose steroids tame post-EVAR inflammation
BOSTON – Preoperative high-dose methylprednisolone dramatically reduced inflammation and enhanced recovery after endovascular aortic repair without increased morbidity in a prospective, double-blinded randomized study.
The primary outcome of modified systemic inflammatory response syndrome (SIRS) developed in 92% on placebo and 27% given methylprednisolone (relative risk, 0.29; P less than .001).
The effect was particularly striking in patients with three or more SIRS criteria, with an overall number needed to treat of 1.5, Dr. Henrik Kehlet said at the annual meeting of the American Surgical Association.
Tempering the postoperative inflammatory response with methylprednisolone also significantly trimmed hospital stays from 3 to 2 days (P less than .001) and morphine requirements from 10 mg to 0 mg (P less than .001).
Medical morbidity was reduced in the methylprednisolone group, but not significantly (23% vs. 36%; P = .1), as was surgical morbidity (20% vs. 21%; P = 1.0).
Importantly, there was no sign of an increase in diagnosed (23% vs. 17%) or treated (1% vs. 3%) endoleaks on computed tomography scan among 146 patients evaluable at 3 months’ follow-up, said Dr. Kehlet, professor of perioperative therapy and head of the surgical pathophysiology section at Rigshospitalet, Copenhagen University, and the oft-described "father" of rapid recovery.
About 40%-60% of patients undergoing endovascular aortic repair (EVAR) will develop postoperative SIRS. Although there is limited surgical injury with the minimally invasive procedure, there is still a stress response because the prosthesis releases proinflammatory mediators from the thrombus in the aortic aneurysm, he explained.
Invited discussant Dr. Basil Pruitt of the division of trauma at the University of Texas Health Science Center, San Antonio, observed that the findings take on added import in light of a 7,500-patient study presented at the recent American College of Cardiology meeting showing that methylprednisolone conferred no significant benefit and was associated with a 15% increased risk of postoperative heart attack plus death when given during cardiac surgery with cardiopulmonary bypass. He also questioned whether the glucocorticosteroid lowered the metabolic rate, as this would impair wound healing, or induced hypoglycemia or glucose intolerance.
"Do those findings and the concerns noted above simply define patient groups in whom methylprednisolone should not be infused preoperatively or, alternatively, do they define a threshold of physiologic or operative insult beyond which the effect of methylprednisolone is outweighed by the magnitude of injury and the associated systemic inflammatory response syndrome?" he asked.
Dr. Kehlet responded that he was unable to determine why that particular abstract found increased morbidity, but noted that the same authors previously published a systematic review (Eur. Heart J. 2008;29:2592-600) supporting reduced morbidity with steroid use in cardiopulmonary bypass.
He added, "The effect may depend on the type of injury, as you mention; cardiopulmonary bypass [is] a very special injury with many mediators of the stress response, compared to other surgical operations. We need more studies. And, finally, if you want to find out about the real outcomes, you must integrate the pharmacologic intervention with an optimized, fast-track setup."
Dr. Kehlet said they did not measure metabolic rate, but that no clinical problem was observed in the limited number of diabetics (n = 22) in their study. This finding is also supported by a huge, multicenter Dutch trial, in which intraoperative high-dose dexamethasone was associated with higher postoperative glucose levels in cardiopulmonary bypass patients, but had no effect on diabetes in subgroup analyses or on the primary endpoint of major morbidity at 30 days (JAMA 2012;308:1761-7).
For Dr. Kehlet's single-center, double-blinded study, 153 patients undergoing EVAR for abdominal aortic aneurysm were randomly assigned to receive a preoperative single dose of placebo or what was described as a single "old-fashioned sepsis dose of methylprednisolone" at 30 mg/kg. The two groups were similar at baseline with respect to age, comorbidities, aneurysm size, thrombus volume, EVAR procedures, and blood loss.
A modified version of SIRS was assessed during the first 4 days after surgery and defined by two or more of the following criteria: fever of more than 38° C (100.4° F) or less than 36° C (96.8° F), heart rate of more than 90 beats per minute, respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension of less than 4.3 kPa (32.25 mm Hg), and a C-reactive protein (CRP) level of more than 75 mg/L. The traditional fourth SIRS criterion of leukocytosis was replaced with CRP level because glucocorticoids always lead to leukocytosis, Dr. Kehlet noted.
Among 150 evaluable patients, high-dose methylprednisolone almost completely eliminated the proinflammatory activities of interleukin (IL)-6 (186 pg/mL vs. 20 mg/dL; P less than .001), IL-8, and CRP.
Among other inflammatory parameters, methylprednisolone reduced soluble tumor necrosis factor receptor 1 levels, but did not modify the d-dimer response, metalloproteinase-9, or myeloperoxidase, he said.
By 3 months, mortality was similar at 3% in the methylprednisolone group and 1% in the placebo group (P less than .5).
"What we don’t know is what the optimal dose-response relationship is because we used a very high dose and, of course, this was only 150 patients, so the safety issues cannot be answered based on this study, but the results are promising for future large-scale studies in this interesting operation," Dr. Kehlet concluded.
Dr. Kehlet and his coauthors reported no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 134th annual meeting, April 2014, in Boston, is anticipated to be published in the Annals of Surgery, pending editorial review.
BOSTON – Preoperative high-dose methylprednisolone dramatically reduced inflammation and enhanced recovery after endovascular aortic repair without increased morbidity in a prospective, double-blinded randomized study.
The primary outcome of modified systemic inflammatory response syndrome (SIRS) developed in 92% on placebo and 27% given methylprednisolone (relative risk, 0.29; P less than .001).
The effect was particularly striking in patients with three or more SIRS criteria, with an overall number needed to treat of 1.5, Dr. Henrik Kehlet said at the annual meeting of the American Surgical Association.
Tempering the postoperative inflammatory response with methylprednisolone also significantly trimmed hospital stays from 3 to 2 days (P less than .001) and morphine requirements from 10 mg to 0 mg (P less than .001).
Medical morbidity was reduced in the methylprednisolone group, but not significantly (23% vs. 36%; P = .1), as was surgical morbidity (20% vs. 21%; P = 1.0).
Importantly, there was no sign of an increase in diagnosed (23% vs. 17%) or treated (1% vs. 3%) endoleaks on computed tomography scan among 146 patients evaluable at 3 months’ follow-up, said Dr. Kehlet, professor of perioperative therapy and head of the surgical pathophysiology section at Rigshospitalet, Copenhagen University, and the oft-described "father" of rapid recovery.
About 40%-60% of patients undergoing endovascular aortic repair (EVAR) will develop postoperative SIRS. Although there is limited surgical injury with the minimally invasive procedure, there is still a stress response because the prosthesis releases proinflammatory mediators from the thrombus in the aortic aneurysm, he explained.
Invited discussant Dr. Basil Pruitt of the division of trauma at the University of Texas Health Science Center, San Antonio, observed that the findings take on added import in light of a 7,500-patient study presented at the recent American College of Cardiology meeting showing that methylprednisolone conferred no significant benefit and was associated with a 15% increased risk of postoperative heart attack plus death when given during cardiac surgery with cardiopulmonary bypass. He also questioned whether the glucocorticosteroid lowered the metabolic rate, as this would impair wound healing, or induced hypoglycemia or glucose intolerance.
"Do those findings and the concerns noted above simply define patient groups in whom methylprednisolone should not be infused preoperatively or, alternatively, do they define a threshold of physiologic or operative insult beyond which the effect of methylprednisolone is outweighed by the magnitude of injury and the associated systemic inflammatory response syndrome?" he asked.
Dr. Kehlet responded that he was unable to determine why that particular abstract found increased morbidity, but noted that the same authors previously published a systematic review (Eur. Heart J. 2008;29:2592-600) supporting reduced morbidity with steroid use in cardiopulmonary bypass.
He added, "The effect may depend on the type of injury, as you mention; cardiopulmonary bypass [is] a very special injury with many mediators of the stress response, compared to other surgical operations. We need more studies. And, finally, if you want to find out about the real outcomes, you must integrate the pharmacologic intervention with an optimized, fast-track setup."
Dr. Kehlet said they did not measure metabolic rate, but that no clinical problem was observed in the limited number of diabetics (n = 22) in their study. This finding is also supported by a huge, multicenter Dutch trial, in which intraoperative high-dose dexamethasone was associated with higher postoperative glucose levels in cardiopulmonary bypass patients, but had no effect on diabetes in subgroup analyses or on the primary endpoint of major morbidity at 30 days (JAMA 2012;308:1761-7).
For Dr. Kehlet's single-center, double-blinded study, 153 patients undergoing EVAR for abdominal aortic aneurysm were randomly assigned to receive a preoperative single dose of placebo or what was described as a single "old-fashioned sepsis dose of methylprednisolone" at 30 mg/kg. The two groups were similar at baseline with respect to age, comorbidities, aneurysm size, thrombus volume, EVAR procedures, and blood loss.
A modified version of SIRS was assessed during the first 4 days after surgery and defined by two or more of the following criteria: fever of more than 38° C (100.4° F) or less than 36° C (96.8° F), heart rate of more than 90 beats per minute, respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension of less than 4.3 kPa (32.25 mm Hg), and a C-reactive protein (CRP) level of more than 75 mg/L. The traditional fourth SIRS criterion of leukocytosis was replaced with CRP level because glucocorticoids always lead to leukocytosis, Dr. Kehlet noted.
Among 150 evaluable patients, high-dose methylprednisolone almost completely eliminated the proinflammatory activities of interleukin (IL)-6 (186 pg/mL vs. 20 mg/dL; P less than .001), IL-8, and CRP.
Among other inflammatory parameters, methylprednisolone reduced soluble tumor necrosis factor receptor 1 levels, but did not modify the d-dimer response, metalloproteinase-9, or myeloperoxidase, he said.
By 3 months, mortality was similar at 3% in the methylprednisolone group and 1% in the placebo group (P less than .5).
"What we don’t know is what the optimal dose-response relationship is because we used a very high dose and, of course, this was only 150 patients, so the safety issues cannot be answered based on this study, but the results are promising for future large-scale studies in this interesting operation," Dr. Kehlet concluded.
Dr. Kehlet and his coauthors reported no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 134th annual meeting, April 2014, in Boston, is anticipated to be published in the Annals of Surgery, pending editorial review.
BOSTON – Preoperative high-dose methylprednisolone dramatically reduced inflammation and enhanced recovery after endovascular aortic repair without increased morbidity in a prospective, double-blinded randomized study.
The primary outcome of modified systemic inflammatory response syndrome (SIRS) developed in 92% on placebo and 27% given methylprednisolone (relative risk, 0.29; P less than .001).
The effect was particularly striking in patients with three or more SIRS criteria, with an overall number needed to treat of 1.5, Dr. Henrik Kehlet said at the annual meeting of the American Surgical Association.
Tempering the postoperative inflammatory response with methylprednisolone also significantly trimmed hospital stays from 3 to 2 days (P less than .001) and morphine requirements from 10 mg to 0 mg (P less than .001).
Medical morbidity was reduced in the methylprednisolone group, but not significantly (23% vs. 36%; P = .1), as was surgical morbidity (20% vs. 21%; P = 1.0).
Importantly, there was no sign of an increase in diagnosed (23% vs. 17%) or treated (1% vs. 3%) endoleaks on computed tomography scan among 146 patients evaluable at 3 months’ follow-up, said Dr. Kehlet, professor of perioperative therapy and head of the surgical pathophysiology section at Rigshospitalet, Copenhagen University, and the oft-described "father" of rapid recovery.
About 40%-60% of patients undergoing endovascular aortic repair (EVAR) will develop postoperative SIRS. Although there is limited surgical injury with the minimally invasive procedure, there is still a stress response because the prosthesis releases proinflammatory mediators from the thrombus in the aortic aneurysm, he explained.
Invited discussant Dr. Basil Pruitt of the division of trauma at the University of Texas Health Science Center, San Antonio, observed that the findings take on added import in light of a 7,500-patient study presented at the recent American College of Cardiology meeting showing that methylprednisolone conferred no significant benefit and was associated with a 15% increased risk of postoperative heart attack plus death when given during cardiac surgery with cardiopulmonary bypass. He also questioned whether the glucocorticosteroid lowered the metabolic rate, as this would impair wound healing, or induced hypoglycemia or glucose intolerance.
"Do those findings and the concerns noted above simply define patient groups in whom methylprednisolone should not be infused preoperatively or, alternatively, do they define a threshold of physiologic or operative insult beyond which the effect of methylprednisolone is outweighed by the magnitude of injury and the associated systemic inflammatory response syndrome?" he asked.
Dr. Kehlet responded that he was unable to determine why that particular abstract found increased morbidity, but noted that the same authors previously published a systematic review (Eur. Heart J. 2008;29:2592-600) supporting reduced morbidity with steroid use in cardiopulmonary bypass.
He added, "The effect may depend on the type of injury, as you mention; cardiopulmonary bypass [is] a very special injury with many mediators of the stress response, compared to other surgical operations. We need more studies. And, finally, if you want to find out about the real outcomes, you must integrate the pharmacologic intervention with an optimized, fast-track setup."
Dr. Kehlet said they did not measure metabolic rate, but that no clinical problem was observed in the limited number of diabetics (n = 22) in their study. This finding is also supported by a huge, multicenter Dutch trial, in which intraoperative high-dose dexamethasone was associated with higher postoperative glucose levels in cardiopulmonary bypass patients, but had no effect on diabetes in subgroup analyses or on the primary endpoint of major morbidity at 30 days (JAMA 2012;308:1761-7).
For Dr. Kehlet's single-center, double-blinded study, 153 patients undergoing EVAR for abdominal aortic aneurysm were randomly assigned to receive a preoperative single dose of placebo or what was described as a single "old-fashioned sepsis dose of methylprednisolone" at 30 mg/kg. The two groups were similar at baseline with respect to age, comorbidities, aneurysm size, thrombus volume, EVAR procedures, and blood loss.
A modified version of SIRS was assessed during the first 4 days after surgery and defined by two or more of the following criteria: fever of more than 38° C (100.4° F) or less than 36° C (96.8° F), heart rate of more than 90 beats per minute, respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension of less than 4.3 kPa (32.25 mm Hg), and a C-reactive protein (CRP) level of more than 75 mg/L. The traditional fourth SIRS criterion of leukocytosis was replaced with CRP level because glucocorticoids always lead to leukocytosis, Dr. Kehlet noted.
Among 150 evaluable patients, high-dose methylprednisolone almost completely eliminated the proinflammatory activities of interleukin (IL)-6 (186 pg/mL vs. 20 mg/dL; P less than .001), IL-8, and CRP.
Among other inflammatory parameters, methylprednisolone reduced soluble tumor necrosis factor receptor 1 levels, but did not modify the d-dimer response, metalloproteinase-9, or myeloperoxidase, he said.
By 3 months, mortality was similar at 3% in the methylprednisolone group and 1% in the placebo group (P less than .5).
"What we don’t know is what the optimal dose-response relationship is because we used a very high dose and, of course, this was only 150 patients, so the safety issues cannot be answered based on this study, but the results are promising for future large-scale studies in this interesting operation," Dr. Kehlet concluded.
Dr. Kehlet and his coauthors reported no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 134th annual meeting, April 2014, in Boston, is anticipated to be published in the Annals of Surgery, pending editorial review.
AT ASA 2014
Major finding: Systemic inflammatory response syndrome developed in 92% on placebo and 27% given methylprednisolone (relative risk, 0.29; P less than .001).
Data source: A single-center, randomized double-blinded study in 153 patients undergoing EVAR for abdominal aortic aneurysm.
Disclosures: Dr. Kehlet and his coauthors reported no financial disclosures.
VIDEO: Rethink the VTE prophylaxis mantra
LAS VEGAS – Venous thromboembolism prophylaxis – a sine qua non of the Joint Commission and others – doesn’t seem to prevent deep vein thrombosis or pulmonary embolism in hospitalized medical patients, but it does make them more likely to bleed, according to investigators from the Michigan Hospital Medicine Safety Consortium.
The findings are prompting one of those investigators to reassess his own approach. In an interview at the Society of Hospital Medicine’s 2014 meeting, Dr. Scott Kaatz, chief quality officer at Hurley Medical Center in Flint, Mich., told us how he’s thinking a bit differently these days when it comes to VTE prophylaxis in medical inpatients.
LAS VEGAS – Venous thromboembolism prophylaxis – a sine qua non of the Joint Commission and others – doesn’t seem to prevent deep vein thrombosis or pulmonary embolism in hospitalized medical patients, but it does make them more likely to bleed, according to investigators from the Michigan Hospital Medicine Safety Consortium.
The findings are prompting one of those investigators to reassess his own approach. In an interview at the Society of Hospital Medicine’s 2014 meeting, Dr. Scott Kaatz, chief quality officer at Hurley Medical Center in Flint, Mich., told us how he’s thinking a bit differently these days when it comes to VTE prophylaxis in medical inpatients.
LAS VEGAS – Venous thromboembolism prophylaxis – a sine qua non of the Joint Commission and others – doesn’t seem to prevent deep vein thrombosis or pulmonary embolism in hospitalized medical patients, but it does make them more likely to bleed, according to investigators from the Michigan Hospital Medicine Safety Consortium.
The findings are prompting one of those investigators to reassess his own approach. In an interview at the Society of Hospital Medicine’s 2014 meeting, Dr. Scott Kaatz, chief quality officer at Hurley Medical Center in Flint, Mich., told us how he’s thinking a bit differently these days when it comes to VTE prophylaxis in medical inpatients.
AT HOSPITAL MEDICINE 2014
Post procedure clopidogrel cuts amputation rates
CHICAGO – Clopidogrel use after endovascular lower-extremity revascularization was significantly associated with 1-year freedom from amputation and survival, but only 38% of the Medicare population was on the drug post intervention in a large retrospective analysis.
Patients with the most severe peripheral vascular disease, ulceration, or gangrene benefited the most from post revascularization clopidogrel (Plavix), but were the least likely to be using the drug, Dr. Mark L. Janzen said at the annual meeting of the Midwestern Vascular Surgical Society.
The analysis involved 14,353 patients, 65 years and older, who underwent lower-extremity revascularization in 2007 and 2008 and were identified from the Medicare Provider Analysis and Review files using ICD-9 codes. Of these, 5,697 had claudication (50%), 1,467 rest pain (10%), and 7,189 ulceration or gangrene (50%).
Overall amputation rates for patients started on clopidogrel right after the procedure were significantly lower than for nonusers at 30 days (10.34% vs. 14.1%), 90 days (14.1% vs. 18.7%), and 1 year (19.7% vs. 24.1%; all P values less than .0001), said Dr. Janzen of the University of Missouri Hospitals and Clinics, Columbia.
Among patients with ulceration or gangrene, limb loss was 21.2% and 26.6% with and without clopidogrel at 30 days, 28.5% and 34.8% at 90 days (P less than .0001), and 38.2% and 43.5% at 1 year.
Only 35.7% of patients with ulceration or gangrene were on post-procedure clopidogrel, compared with 37.4% with rest pain and 40.4% with claudication, according to National Drug Code Directory and Medicare Part D files. Combination therapy with aspirin and clopidogrel or other antiplatelet therapies was not evaluated.
Interestingly, clopidogrel did not significantly affect amputation rates in patients with claudication or rest pain, Dr. Janzen said.
Multivariate logistic regression analyses adjusted for patient demographics, comorbidities, and disease severity, confirmed that clopidogrel nonusers were more likely than users to undergo amputation at 30 days (odds ratio, 1.28), 90 days (OR, 1.29), and 1 year (OR, 1.16).
In the ulceration/gangrene subgroup, failure to use clopidogrel increased the odds of amputation (OR, 1.2) "to levels approaching renal failure (OR, 1.24) and diabetes (OR, 1.6), two known risk factors for below-the-knee amputation," he observed.
Cox regression analyses revealed a 20% higher hazard of death at 1 year among all patients for nonusers than for clopidogrel users (hazard ratio 1.2; P less than .0001).
During a discussion of the study, Dr. Sean Patrick Lyden of the Cleveland Clinic, questioned the impact of end-stage renal disease (ESRD) on the results, remarking that ESRD is probably the strongest predictor of limb loss in patients with ulceration or gangrene and that clopidogrel is typically not given to ESRD patients because the pharmacodynamics are unknown.
Dr. Janzen said it was a valid point, but that the study did not look specifically at ESRD.
This line of questioning was picked up by another attendee, who asked whether amputation rates were calculated for patients with ESRD and diabetes. Although the analysis did not look at outcomes for this lethal combination of comorbidities, Dr. Janzen said a prior study reported a 5-year amputation rate of about 27% in patients with peripheral vascular disease and diabetes (Diabetes Care 2003;26:491-4).
Dr. Iraklis Pipinos, professor of surgery at the University of Nebraska Medical Center in Omaha, commented that "This is a fantastic study and a tremendously important question."
He went on to ask whether the patients with ulceration or gangrene underwent additional lower extremity revascularization procedures, observing that the 1-year amputation rates were quite high in this subgroup.
Dr. Janzen said the study was not designed to determine rates of procedure repetition, although it was noted that overall clopidogrel users were less likely to convert to open bypass early in their post-procedure course.
Finally, the audience asked about the optimal duration of post-procedure clopidogrel, with one attendee observing that it’s not unusual for patients to discontinue clopidogrel 6 weeks after an endovascular revascularization procedure. Dr. Janzen said new cardiology trends recommend that patients with both bare metal and drug-eluting stents remain on clopidogrel for a full year after stent placement and that the optimal duration for patients undergoing endovascular lower limb revascularization is unknown and hopefully will be answered in properly powered randomized trials.
Dr. Janzen and his coauthors reported having no financial disclosures.
CHICAGO – Clopidogrel use after endovascular lower-extremity revascularization was significantly associated with 1-year freedom from amputation and survival, but only 38% of the Medicare population was on the drug post intervention in a large retrospective analysis.
Patients with the most severe peripheral vascular disease, ulceration, or gangrene benefited the most from post revascularization clopidogrel (Plavix), but were the least likely to be using the drug, Dr. Mark L. Janzen said at the annual meeting of the Midwestern Vascular Surgical Society.
The analysis involved 14,353 patients, 65 years and older, who underwent lower-extremity revascularization in 2007 and 2008 and were identified from the Medicare Provider Analysis and Review files using ICD-9 codes. Of these, 5,697 had claudication (50%), 1,467 rest pain (10%), and 7,189 ulceration or gangrene (50%).
Overall amputation rates for patients started on clopidogrel right after the procedure were significantly lower than for nonusers at 30 days (10.34% vs. 14.1%), 90 days (14.1% vs. 18.7%), and 1 year (19.7% vs. 24.1%; all P values less than .0001), said Dr. Janzen of the University of Missouri Hospitals and Clinics, Columbia.
Among patients with ulceration or gangrene, limb loss was 21.2% and 26.6% with and without clopidogrel at 30 days, 28.5% and 34.8% at 90 days (P less than .0001), and 38.2% and 43.5% at 1 year.
Only 35.7% of patients with ulceration or gangrene were on post-procedure clopidogrel, compared with 37.4% with rest pain and 40.4% with claudication, according to National Drug Code Directory and Medicare Part D files. Combination therapy with aspirin and clopidogrel or other antiplatelet therapies was not evaluated.
Interestingly, clopidogrel did not significantly affect amputation rates in patients with claudication or rest pain, Dr. Janzen said.
Multivariate logistic regression analyses adjusted for patient demographics, comorbidities, and disease severity, confirmed that clopidogrel nonusers were more likely than users to undergo amputation at 30 days (odds ratio, 1.28), 90 days (OR, 1.29), and 1 year (OR, 1.16).
In the ulceration/gangrene subgroup, failure to use clopidogrel increased the odds of amputation (OR, 1.2) "to levels approaching renal failure (OR, 1.24) and diabetes (OR, 1.6), two known risk factors for below-the-knee amputation," he observed.
Cox regression analyses revealed a 20% higher hazard of death at 1 year among all patients for nonusers than for clopidogrel users (hazard ratio 1.2; P less than .0001).
During a discussion of the study, Dr. Sean Patrick Lyden of the Cleveland Clinic, questioned the impact of end-stage renal disease (ESRD) on the results, remarking that ESRD is probably the strongest predictor of limb loss in patients with ulceration or gangrene and that clopidogrel is typically not given to ESRD patients because the pharmacodynamics are unknown.
Dr. Janzen said it was a valid point, but that the study did not look specifically at ESRD.
This line of questioning was picked up by another attendee, who asked whether amputation rates were calculated for patients with ESRD and diabetes. Although the analysis did not look at outcomes for this lethal combination of comorbidities, Dr. Janzen said a prior study reported a 5-year amputation rate of about 27% in patients with peripheral vascular disease and diabetes (Diabetes Care 2003;26:491-4).
Dr. Iraklis Pipinos, professor of surgery at the University of Nebraska Medical Center in Omaha, commented that "This is a fantastic study and a tremendously important question."
He went on to ask whether the patients with ulceration or gangrene underwent additional lower extremity revascularization procedures, observing that the 1-year amputation rates were quite high in this subgroup.
Dr. Janzen said the study was not designed to determine rates of procedure repetition, although it was noted that overall clopidogrel users were less likely to convert to open bypass early in their post-procedure course.
Finally, the audience asked about the optimal duration of post-procedure clopidogrel, with one attendee observing that it’s not unusual for patients to discontinue clopidogrel 6 weeks after an endovascular revascularization procedure. Dr. Janzen said new cardiology trends recommend that patients with both bare metal and drug-eluting stents remain on clopidogrel for a full year after stent placement and that the optimal duration for patients undergoing endovascular lower limb revascularization is unknown and hopefully will be answered in properly powered randomized trials.
Dr. Janzen and his coauthors reported having no financial disclosures.
CHICAGO – Clopidogrel use after endovascular lower-extremity revascularization was significantly associated with 1-year freedom from amputation and survival, but only 38% of the Medicare population was on the drug post intervention in a large retrospective analysis.
Patients with the most severe peripheral vascular disease, ulceration, or gangrene benefited the most from post revascularization clopidogrel (Plavix), but were the least likely to be using the drug, Dr. Mark L. Janzen said at the annual meeting of the Midwestern Vascular Surgical Society.
The analysis involved 14,353 patients, 65 years and older, who underwent lower-extremity revascularization in 2007 and 2008 and were identified from the Medicare Provider Analysis and Review files using ICD-9 codes. Of these, 5,697 had claudication (50%), 1,467 rest pain (10%), and 7,189 ulceration or gangrene (50%).
Overall amputation rates for patients started on clopidogrel right after the procedure were significantly lower than for nonusers at 30 days (10.34% vs. 14.1%), 90 days (14.1% vs. 18.7%), and 1 year (19.7% vs. 24.1%; all P values less than .0001), said Dr. Janzen of the University of Missouri Hospitals and Clinics, Columbia.
Among patients with ulceration or gangrene, limb loss was 21.2% and 26.6% with and without clopidogrel at 30 days, 28.5% and 34.8% at 90 days (P less than .0001), and 38.2% and 43.5% at 1 year.
Only 35.7% of patients with ulceration or gangrene were on post-procedure clopidogrel, compared with 37.4% with rest pain and 40.4% with claudication, according to National Drug Code Directory and Medicare Part D files. Combination therapy with aspirin and clopidogrel or other antiplatelet therapies was not evaluated.
Interestingly, clopidogrel did not significantly affect amputation rates in patients with claudication or rest pain, Dr. Janzen said.
Multivariate logistic regression analyses adjusted for patient demographics, comorbidities, and disease severity, confirmed that clopidogrel nonusers were more likely than users to undergo amputation at 30 days (odds ratio, 1.28), 90 days (OR, 1.29), and 1 year (OR, 1.16).
In the ulceration/gangrene subgroup, failure to use clopidogrel increased the odds of amputation (OR, 1.2) "to levels approaching renal failure (OR, 1.24) and diabetes (OR, 1.6), two known risk factors for below-the-knee amputation," he observed.
Cox regression analyses revealed a 20% higher hazard of death at 1 year among all patients for nonusers than for clopidogrel users (hazard ratio 1.2; P less than .0001).
During a discussion of the study, Dr. Sean Patrick Lyden of the Cleveland Clinic, questioned the impact of end-stage renal disease (ESRD) on the results, remarking that ESRD is probably the strongest predictor of limb loss in patients with ulceration or gangrene and that clopidogrel is typically not given to ESRD patients because the pharmacodynamics are unknown.
Dr. Janzen said it was a valid point, but that the study did not look specifically at ESRD.
This line of questioning was picked up by another attendee, who asked whether amputation rates were calculated for patients with ESRD and diabetes. Although the analysis did not look at outcomes for this lethal combination of comorbidities, Dr. Janzen said a prior study reported a 5-year amputation rate of about 27% in patients with peripheral vascular disease and diabetes (Diabetes Care 2003;26:491-4).
Dr. Iraklis Pipinos, professor of surgery at the University of Nebraska Medical Center in Omaha, commented that "This is a fantastic study and a tremendously important question."
He went on to ask whether the patients with ulceration or gangrene underwent additional lower extremity revascularization procedures, observing that the 1-year amputation rates were quite high in this subgroup.
Dr. Janzen said the study was not designed to determine rates of procedure repetition, although it was noted that overall clopidogrel users were less likely to convert to open bypass early in their post-procedure course.
Finally, the audience asked about the optimal duration of post-procedure clopidogrel, with one attendee observing that it’s not unusual for patients to discontinue clopidogrel 6 weeks after an endovascular revascularization procedure. Dr. Janzen said new cardiology trends recommend that patients with both bare metal and drug-eluting stents remain on clopidogrel for a full year after stent placement and that the optimal duration for patients undergoing endovascular lower limb revascularization is unknown and hopefully will be answered in properly powered randomized trials.
Dr. Janzen and his coauthors reported having no financial disclosures.
AT MIDWESTERN VASCULAR 2013
Key clinical point: Post procedure clopidogrel use was associated with a lower risk of amputation at 1 year for all patients and for patients with ulceration or gangrene.
Major finding: Cox regression analyses revealed a 20% higher hazard of death at 1 year among all patients for nonusers than for clopidogrel users (hazard ratio 1.2; P less than .0001).
Data source: Retrospective analysis of 14,353 patients undergoing endovascular lower-extremity revascularization.
Disclosures: Dr. Janzen and his coauthors reported having no financial disclosures.
VIDEO: Drug cocktail beats single agent in subarachnoid hemorrhage vasospasm treatment
SAN DIEGO - An intra-arterial infusion drug cocktail of nicardipine, verapamil, and nitroglycerin appears to work better than nicardipine or verapamil alone – the usual approach – to open up cerebral vasospasms after subarachnoid hemorrhage. The early results are so promising that lead investigator Dr. Peng Roc Chen, a cerebrovascular neurosurgeon at the University of Texas, Houston, and his colleagues are following up with a randomized controlled trial. Dr. Chen explained the project to us at this year’s International Stroke Conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO - An intra-arterial infusion drug cocktail of nicardipine, verapamil, and nitroglycerin appears to work better than nicardipine or verapamil alone – the usual approach – to open up cerebral vasospasms after subarachnoid hemorrhage. The early results are so promising that lead investigator Dr. Peng Roc Chen, a cerebrovascular neurosurgeon at the University of Texas, Houston, and his colleagues are following up with a randomized controlled trial. Dr. Chen explained the project to us at this year’s International Stroke Conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO - An intra-arterial infusion drug cocktail of nicardipine, verapamil, and nitroglycerin appears to work better than nicardipine or verapamil alone – the usual approach – to open up cerebral vasospasms after subarachnoid hemorrhage. The early results are so promising that lead investigator Dr. Peng Roc Chen, a cerebrovascular neurosurgeon at the University of Texas, Houston, and his colleagues are following up with a randomized controlled trial. Dr. Chen explained the project to us at this year’s International Stroke Conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Vasodilator cocktail beats single-agent infusion for subarachnoid hemorrhage vasospasm
SAN DIEGO – Cerebral vasospasms open up, on average, 34.9% more when patients are infused with an intra-arterial cocktail of nicardipine, verapamil, and nitroglycerin, instead of the usual approach of nicardipine or verapamil alone, according to a retrospective study from the University of Texas, Houston.
Investigators there compared the cocktail to single-agent infusions in patients with vasospasms due to aneurysmal subarachnoid hemorrhages, after the offending aneurysms had been clipped or coiled.
Fifty-four patients with 116 spasmed vessels were infused with verapamil 10 mg or nicardipine 5 mg per vascular territory. Another 50 patients with 106 spasmed vessels were infused with verapamil 10 mg, nicardipine 5 mg, and nitroglycerin 200 mcg per vascular territory. The patients underwent repeat cerebral angiography at least 15 minutes after treatment.
In addition to having greater average dilation, the cocktail group had significantly greater improvement in the ratio of arterial lumen diameter before and after treatment than did the single-agent group (45.8% vs. 10.9%, respectively). The effect was independent of age.
There was a trend toward more modified Rankin Scale (mRS) scores of 0-2 in the multiple-agent group, but it was not statistically significant. Lead investigator Dr. Peng Roc Chen, a cerebrovascular neurosurgeon at the University of Texas, Houston, has launched a prospective randomized trial with his colleagues to investigate the matter further. Nine medical centers have signed up so far, and they are looking for more.
Single-agent infusion is standard practice in the United States, usually with verapamil, but it doesn’t work well, "and there’s no conclusive literature suggesting the best intra-arterial infusion regimen for vasospasm, particularly when balloon angioplasty is not feasible," Dr. Chen said at the International Stroke Conference, sponsored by the American Heart Association.
The team hoped for synergistic effects by combining commonly used intra-arterial vasodilators with different mechanisms of action, while avoiding the cardiovascular instability that comes with high-dose infusion of single agents. Verapamil and nicardipine are both calcium-channel blockers, but they work on different receptors, he said.
At discharge, 24 (44.4%) patients in the single-agent group had an mRS score of 0-2, 28 (51.9%) had an mRS score of 3-5, and 2 (3.7%) had died. At 3 months, 25 of the 34 patients not lost to follow-up (73.5%) had an mRS score of 0-2, seven (20.6%) had an mRS score of 3-5, and 2 (5.9%) had died.
In the multiagent group, 31 (62%) had an mRS score of 0-2 at discharge, 16 (32%) had an mRS score of 3-5, and 3 patients (6%) had died. At 3 months, 29 of the 36 patients not lost to follow-up (80.6%) had an mRS score of 0-2, 4 (11.1%) had an mRS score of 3-5, and 3 (8.3%) had died.
Small numbers and follow-up loss may have contributed to the lack of outcome significance. It’s also possible that subarachnoid hemorrhage drove outcomes, regardless of vasospasm treatment. In any case, multiagent infusion is now the standard approach at Dr. Chen’s medical center; the single-agent patients were historical controls, he said.
Sixteen (29.6%) patients in the single-agent arm needed additional treatment, either repeat infusions or balloon angioplasties. Twenty-two (44%) needed additional treatment in the multiple-agent group (P = .128).
Dr. Chen did not report patient demographics, but said there were no differences in post-treatment blood pressures, heart rate changes, or intracranial pressures between the two treatment groups. Patients who had balloon angioplasties before vasodilation were excluded from the study.
Verapamil and nicardipine were infused at a rate of 1 mg/min and nitroglycerin, at a rate of 100 mcg/min. In the single-agent arm, the investigators found no differences in vessel diameter change between verapamil and nicardipine.
The investigators reported having no relevant financial disclosures.

|
|
We’ve been learning that combining therapies seems to be better than giving a single agent. Whether this will translate into something we’ll be using down the line remains to be seen. We have to look at the safety of giving the three together. There’s a possibility, perhaps, that you could increase the diameter of the vessel too much, to the point where the patient will have edema, or you drop the pressure too much. Those issues may not have been picked up retrospectively.
Dr. Jose Suarez is head of the section of vascular neurology and neurocritical care at Baylor College of Medicine, Houston. He reported having no relevant financial disclosures.

|
|
We’ve been learning that combining therapies seems to be better than giving a single agent. Whether this will translate into something we’ll be using down the line remains to be seen. We have to look at the safety of giving the three together. There’s a possibility, perhaps, that you could increase the diameter of the vessel too much, to the point where the patient will have edema, or you drop the pressure too much. Those issues may not have been picked up retrospectively.
Dr. Jose Suarez is head of the section of vascular neurology and neurocritical care at Baylor College of Medicine, Houston. He reported having no relevant financial disclosures.

|
|
We’ve been learning that combining therapies seems to be better than giving a single agent. Whether this will translate into something we’ll be using down the line remains to be seen. We have to look at the safety of giving the three together. There’s a possibility, perhaps, that you could increase the diameter of the vessel too much, to the point where the patient will have edema, or you drop the pressure too much. Those issues may not have been picked up retrospectively.
Dr. Jose Suarez is head of the section of vascular neurology and neurocritical care at Baylor College of Medicine, Houston. He reported having no relevant financial disclosures.
SAN DIEGO – Cerebral vasospasms open up, on average, 34.9% more when patients are infused with an intra-arterial cocktail of nicardipine, verapamil, and nitroglycerin, instead of the usual approach of nicardipine or verapamil alone, according to a retrospective study from the University of Texas, Houston.
Investigators there compared the cocktail to single-agent infusions in patients with vasospasms due to aneurysmal subarachnoid hemorrhages, after the offending aneurysms had been clipped or coiled.
Fifty-four patients with 116 spasmed vessels were infused with verapamil 10 mg or nicardipine 5 mg per vascular territory. Another 50 patients with 106 spasmed vessels were infused with verapamil 10 mg, nicardipine 5 mg, and nitroglycerin 200 mcg per vascular territory. The patients underwent repeat cerebral angiography at least 15 minutes after treatment.
In addition to having greater average dilation, the cocktail group had significantly greater improvement in the ratio of arterial lumen diameter before and after treatment than did the single-agent group (45.8% vs. 10.9%, respectively). The effect was independent of age.
There was a trend toward more modified Rankin Scale (mRS) scores of 0-2 in the multiple-agent group, but it was not statistically significant. Lead investigator Dr. Peng Roc Chen, a cerebrovascular neurosurgeon at the University of Texas, Houston, has launched a prospective randomized trial with his colleagues to investigate the matter further. Nine medical centers have signed up so far, and they are looking for more.
Single-agent infusion is standard practice in the United States, usually with verapamil, but it doesn’t work well, "and there’s no conclusive literature suggesting the best intra-arterial infusion regimen for vasospasm, particularly when balloon angioplasty is not feasible," Dr. Chen said at the International Stroke Conference, sponsored by the American Heart Association.
The team hoped for synergistic effects by combining commonly used intra-arterial vasodilators with different mechanisms of action, while avoiding the cardiovascular instability that comes with high-dose infusion of single agents. Verapamil and nicardipine are both calcium-channel blockers, but they work on different receptors, he said.
At discharge, 24 (44.4%) patients in the single-agent group had an mRS score of 0-2, 28 (51.9%) had an mRS score of 3-5, and 2 (3.7%) had died. At 3 months, 25 of the 34 patients not lost to follow-up (73.5%) had an mRS score of 0-2, seven (20.6%) had an mRS score of 3-5, and 2 (5.9%) had died.
In the multiagent group, 31 (62%) had an mRS score of 0-2 at discharge, 16 (32%) had an mRS score of 3-5, and 3 patients (6%) had died. At 3 months, 29 of the 36 patients not lost to follow-up (80.6%) had an mRS score of 0-2, 4 (11.1%) had an mRS score of 3-5, and 3 (8.3%) had died.
Small numbers and follow-up loss may have contributed to the lack of outcome significance. It’s also possible that subarachnoid hemorrhage drove outcomes, regardless of vasospasm treatment. In any case, multiagent infusion is now the standard approach at Dr. Chen’s medical center; the single-agent patients were historical controls, he said.
Sixteen (29.6%) patients in the single-agent arm needed additional treatment, either repeat infusions or balloon angioplasties. Twenty-two (44%) needed additional treatment in the multiple-agent group (P = .128).
Dr. Chen did not report patient demographics, but said there were no differences in post-treatment blood pressures, heart rate changes, or intracranial pressures between the two treatment groups. Patients who had balloon angioplasties before vasodilation were excluded from the study.
Verapamil and nicardipine were infused at a rate of 1 mg/min and nitroglycerin, at a rate of 100 mcg/min. In the single-agent arm, the investigators found no differences in vessel diameter change between verapamil and nicardipine.
The investigators reported having no relevant financial disclosures.
SAN DIEGO – Cerebral vasospasms open up, on average, 34.9% more when patients are infused with an intra-arterial cocktail of nicardipine, verapamil, and nitroglycerin, instead of the usual approach of nicardipine or verapamil alone, according to a retrospective study from the University of Texas, Houston.
Investigators there compared the cocktail to single-agent infusions in patients with vasospasms due to aneurysmal subarachnoid hemorrhages, after the offending aneurysms had been clipped or coiled.
Fifty-four patients with 116 spasmed vessels were infused with verapamil 10 mg or nicardipine 5 mg per vascular territory. Another 50 patients with 106 spasmed vessels were infused with verapamil 10 mg, nicardipine 5 mg, and nitroglycerin 200 mcg per vascular territory. The patients underwent repeat cerebral angiography at least 15 minutes after treatment.
In addition to having greater average dilation, the cocktail group had significantly greater improvement in the ratio of arterial lumen diameter before and after treatment than did the single-agent group (45.8% vs. 10.9%, respectively). The effect was independent of age.
There was a trend toward more modified Rankin Scale (mRS) scores of 0-2 in the multiple-agent group, but it was not statistically significant. Lead investigator Dr. Peng Roc Chen, a cerebrovascular neurosurgeon at the University of Texas, Houston, has launched a prospective randomized trial with his colleagues to investigate the matter further. Nine medical centers have signed up so far, and they are looking for more.
Single-agent infusion is standard practice in the United States, usually with verapamil, but it doesn’t work well, "and there’s no conclusive literature suggesting the best intra-arterial infusion regimen for vasospasm, particularly when balloon angioplasty is not feasible," Dr. Chen said at the International Stroke Conference, sponsored by the American Heart Association.
The team hoped for synergistic effects by combining commonly used intra-arterial vasodilators with different mechanisms of action, while avoiding the cardiovascular instability that comes with high-dose infusion of single agents. Verapamil and nicardipine are both calcium-channel blockers, but they work on different receptors, he said.
At discharge, 24 (44.4%) patients in the single-agent group had an mRS score of 0-2, 28 (51.9%) had an mRS score of 3-5, and 2 (3.7%) had died. At 3 months, 25 of the 34 patients not lost to follow-up (73.5%) had an mRS score of 0-2, seven (20.6%) had an mRS score of 3-5, and 2 (5.9%) had died.
In the multiagent group, 31 (62%) had an mRS score of 0-2 at discharge, 16 (32%) had an mRS score of 3-5, and 3 patients (6%) had died. At 3 months, 29 of the 36 patients not lost to follow-up (80.6%) had an mRS score of 0-2, 4 (11.1%) had an mRS score of 3-5, and 3 (8.3%) had died.
Small numbers and follow-up loss may have contributed to the lack of outcome significance. It’s also possible that subarachnoid hemorrhage drove outcomes, regardless of vasospasm treatment. In any case, multiagent infusion is now the standard approach at Dr. Chen’s medical center; the single-agent patients were historical controls, he said.
Sixteen (29.6%) patients in the single-agent arm needed additional treatment, either repeat infusions or balloon angioplasties. Twenty-two (44%) needed additional treatment in the multiple-agent group (P = .128).
Dr. Chen did not report patient demographics, but said there were no differences in post-treatment blood pressures, heart rate changes, or intracranial pressures between the two treatment groups. Patients who had balloon angioplasties before vasodilation were excluded from the study.
Verapamil and nicardipine were infused at a rate of 1 mg/min and nitroglycerin, at a rate of 100 mcg/min. In the single-agent arm, the investigators found no differences in vessel diameter change between verapamil and nicardipine.
The investigators reported having no relevant financial disclosures.
AT THE INTERNATIONAL STROKE CONFERENCE
Major finding: Patients who received nicardipine, verapamil, and nitroglycerin had significantly greater improvement in the ratio of arterial lumen diameter before and after treatment than did those who received verapamil or nicardipine alone (45.8% vs. 10.9%, respectively).
Data Source: A retrospective study of 50 patients infused with an intra-arterial vasodilator cocktail and 54 infused with verapamil or nicardipine.
Disclosures: The investigators reported having no relevant financial disclosures.
.
Multiple revascularization ups risk of amputation, death
The risk of amputation and death appears to increase as the number of revascularization procedures increases, according to findings from a retrospective analysis of data.
The amputation risk was present among patients who underwent percutaneous transluminal angioplasty (PTA) only, as well as among subsets of patients who underwent lower extremity bypass (LEB) only, reported Dr. Alexander T. Hawkins of the Center for Surgery and Public Health, Boston, and his colleagues.
Among 11,190 patients with critical limb ischemia who underwent one, two, three, four, or five or more revascularization procedures, the 1-year estimated amputation rates were 23.3%, 27.1%, 30.3%, 26.7%, and 28.6%, and the 1-year estimated mortality rates were 18.7%, 21.1%, 26.3%, 23.6%, and 32.1%, respectively, the investigators reported. The findings were published in the January issue of Annals of Vascular Surgery.
The risk of amputation increased significantly for those with two vs. one revascularization procedures (hazard ratio, 1.22) and for those with three vs. two procedures (HR, 1.33). The risk for death at 1 year also increased significantly among those with two vs. one procedure (HR, 1.18) (Ann. Vasc. Surg. 2014;28:35-47).
Similar trends for amputation were seen in the PTA-only (1: 24.5%; 2: 26.1%; 3: 27.9%; 4: 31.3%; 5+: 26.8%), and LEB-only (1: 26.0%; 2: 32.5%; 3+: 45.5%) groups. "The increases did not appear to be exponential," they noted.
No changes were seen in the PTA-only and LEB-only groups with respect to 1-year estimates of in-hospital death.
A subgroup analysis further showed that timing between procedures was significantly associated with 1-year amputation risk; the risk was 27.2% for a 1-7 day interval, 36.4% for 8 days to 1 month, 19.4% for 1-6 months; and 22.2% for 6 months or more.
"There was also a difference in 1-year amputation rates between bypass patients who underwent bypass first and who underwent PTA followed by bypass" (21.8% vs. 30.7%), the researchers wrote.
Study subjects were adult patients with a mean age of 71 years who underwent revascularization between July 2007 and December 2009. The patients, including 6,225 men (55.9%), were identified from the California State Inpatient Database and had a high burden of comorbidities; 55.2% abused tobacco, 64.9% had coronary artery disease, 51.3% had hypertension, and 68% had diabetes.
Though limited by factors inherent in the use of an administrative database (such as potential inconsistencies in coding accuracy) and in a nonrandomized study (subject to confounding), the findings nonetheless provide "novel and useful information on the increasing risk of amputation and death in patients undergoing multiple revascularization procedures," the investigators said.
They stressed that they are "by no means making the claim that secondary revascularization is inappropriate," but rather, that they are presenting the risks associated with further procedures in an effort to inform the decision-making process.
Critical limb ischemia confers a high risk of limb loss without treatment, they said, noting that 16%-50% of revascularized patients require secondary revascularization. A "major proportion" of these patients will require further procedures, they noted.
"We emphasize continued communication between clinicians and patients on the true risks and benefits of these procedures," they concluded.
Dr. Hawkins and his coauthor, Dr. Stuart Lipsitz, are supported by a grant from the Brigham and Women’s Center for Surgery and Public Health Arthur Tracy Cabot Fellowship. Dr. Hawkins is also supported by the NIH NHLBI T32 Harvard/Longwood Vascular Surgery Training Program. Another author, Dr. Maria J. Schaumeier, is supported by a grant from the Freiwillige Akademische Gesellschaft, Basel, Switzerland.
The risk of amputation and death appears to increase as the number of revascularization procedures increases, according to findings from a retrospective analysis of data.
The amputation risk was present among patients who underwent percutaneous transluminal angioplasty (PTA) only, as well as among subsets of patients who underwent lower extremity bypass (LEB) only, reported Dr. Alexander T. Hawkins of the Center for Surgery and Public Health, Boston, and his colleagues.
Among 11,190 patients with critical limb ischemia who underwent one, two, three, four, or five or more revascularization procedures, the 1-year estimated amputation rates were 23.3%, 27.1%, 30.3%, 26.7%, and 28.6%, and the 1-year estimated mortality rates were 18.7%, 21.1%, 26.3%, 23.6%, and 32.1%, respectively, the investigators reported. The findings were published in the January issue of Annals of Vascular Surgery.
The risk of amputation increased significantly for those with two vs. one revascularization procedures (hazard ratio, 1.22) and for those with three vs. two procedures (HR, 1.33). The risk for death at 1 year also increased significantly among those with two vs. one procedure (HR, 1.18) (Ann. Vasc. Surg. 2014;28:35-47).
Similar trends for amputation were seen in the PTA-only (1: 24.5%; 2: 26.1%; 3: 27.9%; 4: 31.3%; 5+: 26.8%), and LEB-only (1: 26.0%; 2: 32.5%; 3+: 45.5%) groups. "The increases did not appear to be exponential," they noted.
No changes were seen in the PTA-only and LEB-only groups with respect to 1-year estimates of in-hospital death.
A subgroup analysis further showed that timing between procedures was significantly associated with 1-year amputation risk; the risk was 27.2% for a 1-7 day interval, 36.4% for 8 days to 1 month, 19.4% for 1-6 months; and 22.2% for 6 months or more.
"There was also a difference in 1-year amputation rates between bypass patients who underwent bypass first and who underwent PTA followed by bypass" (21.8% vs. 30.7%), the researchers wrote.
Study subjects were adult patients with a mean age of 71 years who underwent revascularization between July 2007 and December 2009. The patients, including 6,225 men (55.9%), were identified from the California State Inpatient Database and had a high burden of comorbidities; 55.2% abused tobacco, 64.9% had coronary artery disease, 51.3% had hypertension, and 68% had diabetes.
Though limited by factors inherent in the use of an administrative database (such as potential inconsistencies in coding accuracy) and in a nonrandomized study (subject to confounding), the findings nonetheless provide "novel and useful information on the increasing risk of amputation and death in patients undergoing multiple revascularization procedures," the investigators said.
They stressed that they are "by no means making the claim that secondary revascularization is inappropriate," but rather, that they are presenting the risks associated with further procedures in an effort to inform the decision-making process.
Critical limb ischemia confers a high risk of limb loss without treatment, they said, noting that 16%-50% of revascularized patients require secondary revascularization. A "major proportion" of these patients will require further procedures, they noted.
"We emphasize continued communication between clinicians and patients on the true risks and benefits of these procedures," they concluded.
Dr. Hawkins and his coauthor, Dr. Stuart Lipsitz, are supported by a grant from the Brigham and Women’s Center for Surgery and Public Health Arthur Tracy Cabot Fellowship. Dr. Hawkins is also supported by the NIH NHLBI T32 Harvard/Longwood Vascular Surgery Training Program. Another author, Dr. Maria J. Schaumeier, is supported by a grant from the Freiwillige Akademische Gesellschaft, Basel, Switzerland.
The risk of amputation and death appears to increase as the number of revascularization procedures increases, according to findings from a retrospective analysis of data.
The amputation risk was present among patients who underwent percutaneous transluminal angioplasty (PTA) only, as well as among subsets of patients who underwent lower extremity bypass (LEB) only, reported Dr. Alexander T. Hawkins of the Center for Surgery and Public Health, Boston, and his colleagues.
Among 11,190 patients with critical limb ischemia who underwent one, two, three, four, or five or more revascularization procedures, the 1-year estimated amputation rates were 23.3%, 27.1%, 30.3%, 26.7%, and 28.6%, and the 1-year estimated mortality rates were 18.7%, 21.1%, 26.3%, 23.6%, and 32.1%, respectively, the investigators reported. The findings were published in the January issue of Annals of Vascular Surgery.
The risk of amputation increased significantly for those with two vs. one revascularization procedures (hazard ratio, 1.22) and for those with three vs. two procedures (HR, 1.33). The risk for death at 1 year also increased significantly among those with two vs. one procedure (HR, 1.18) (Ann. Vasc. Surg. 2014;28:35-47).
Similar trends for amputation were seen in the PTA-only (1: 24.5%; 2: 26.1%; 3: 27.9%; 4: 31.3%; 5+: 26.8%), and LEB-only (1: 26.0%; 2: 32.5%; 3+: 45.5%) groups. "The increases did not appear to be exponential," they noted.
No changes were seen in the PTA-only and LEB-only groups with respect to 1-year estimates of in-hospital death.
A subgroup analysis further showed that timing between procedures was significantly associated with 1-year amputation risk; the risk was 27.2% for a 1-7 day interval, 36.4% for 8 days to 1 month, 19.4% for 1-6 months; and 22.2% for 6 months or more.
"There was also a difference in 1-year amputation rates between bypass patients who underwent bypass first and who underwent PTA followed by bypass" (21.8% vs. 30.7%), the researchers wrote.
Study subjects were adult patients with a mean age of 71 years who underwent revascularization between July 2007 and December 2009. The patients, including 6,225 men (55.9%), were identified from the California State Inpatient Database and had a high burden of comorbidities; 55.2% abused tobacco, 64.9% had coronary artery disease, 51.3% had hypertension, and 68% had diabetes.
Though limited by factors inherent in the use of an administrative database (such as potential inconsistencies in coding accuracy) and in a nonrandomized study (subject to confounding), the findings nonetheless provide "novel and useful information on the increasing risk of amputation and death in patients undergoing multiple revascularization procedures," the investigators said.
They stressed that they are "by no means making the claim that secondary revascularization is inappropriate," but rather, that they are presenting the risks associated with further procedures in an effort to inform the decision-making process.
Critical limb ischemia confers a high risk of limb loss without treatment, they said, noting that 16%-50% of revascularized patients require secondary revascularization. A "major proportion" of these patients will require further procedures, they noted.
"We emphasize continued communication between clinicians and patients on the true risks and benefits of these procedures," they concluded.
Dr. Hawkins and his coauthor, Dr. Stuart Lipsitz, are supported by a grant from the Brigham and Women’s Center for Surgery and Public Health Arthur Tracy Cabot Fellowship. Dr. Hawkins is also supported by the NIH NHLBI T32 Harvard/Longwood Vascular Surgery Training Program. Another author, Dr. Maria J. Schaumeier, is supported by a grant from the Freiwillige Akademische Gesellschaft, Basel, Switzerland.
FROM ANNALS OF VASCULAR SURGERY
Major finding: Amputation risk increased significantly for those with two revascularization procedures vs. one procedure (hazard ratio, 1.22) and for those with three vs. two procedures (HR, 1.33). The risk for death increased significantly among those with two vs. one procedure (HR, 1.18).
Data source: A retrospective analysis of 11,190 patients in an administrative database.
Disclosures: Dr. Hawkins and his coauthor, Dr. Stuart Lipsitz, are supported by a grant from the Brigham and Women’s Center for Surgery and Public Health Arthur Tracy Cabot Fellowship. Dr. Hawkins is also supported by the NIH NHLBI T32 Harvard/Longwood Vascular Surgery Training Program. Another author, Dr. Maria J. Schaumeier, is supported by a grant from the Freiwillige Akademische Gesellschaft, Basel, Switzerland.
Anatomy’s role is elusive in carotid stenting risk
CHICAGO – Pinning down the anatomic characteristics that increase the risk of stroke with carotid artery stenting continues to be a challenge, as demonstrated by a database study and literature review presented at a symposium on vascular surgery sponsored by Northwestern University.
"I am absolutely convinced that certain anatomic and plaque characteristics increase the risk of a stroke for patients with carotid artery stenting," Dr. Melina R. Kibbe said in presenting her study. "The studies are not consistent in the literature, but there is a pattern."
She presented a prospective database review that evaluated no less than a dozen anatomic variables in 381 carotid arteries stented at the university from 2001 to 2010. The mostly male (75%), asymptomatic (70%), and moderate- to high-risk cohort had an average age of 70.5 years.
Within 30 days of carotid stenting, there were six strokes and eight transient ischemic attacks (TIAs), for an overall neurologic event rate of 3.7%, she said. Three patients had a heart attack, and two died.
The risk of perioperative stroke or TIA was significantly increased only in patients with a higher degree of internal carotid artery (ICA) stenosis (87% stenosis vs. 81% stenosis; P = .03).
It also trended higher, but fell short of significance, in those with greater arch calcification (P = .06).
Surprisingly, no statistical association was found between neurologic events and arch type (P = .16), despite the clinical belief that increasing arch type can be associated with more difficulty accessing the target lesion, said Dr. Kibbe, professor of vascular surgery and surgical research at Northwestern University, Chicago.
Other variables with trends that failed to reach statistical significance included internal to common carotid artery angulation, tortuous carotid artery, ipsilateral external carotid artery (ECA) stenosis, plaque calcification, and lesion length.
Turning to the literature for more answers, Dr. Kibbe and her colleagues performed a review of eight carotid stenting studies between 1993 and 2013. These studies included SAPPHIRE, which demonstrated an association between type II and III arches and increased stroke risk. Two other studies implicated type II, type III, and bovine arches, while three other studies, including a systematic review that incorporated the EVA-3S study (Stroke 2011;42:380-8), found no association between arch type and stroke.
Still, Dr. Kibbe said she’s convinced no two patients are alike when it comes to arterial anatomy and called for data from pivotal trials like CREST to be reanalyzed for stroke, based on arch type and plaque characteristics.
"Even better, I’d like to see a prospective, randomized study that excludes patients for carotid artery stenting based on their anatomy; for example, excluding all type III arches," she said. "My own personal bias is that patients with type III arches should not have carotid stenting. We don’t need to push the envelope."
Interestingly, no study in the review has shown ICA stenosis to be a risk factor for stroke or TIA, although lesions with a higher degree of stenosis would be expected to contribute to perioperative stroke risk.
Arch calcification, ECA stenosis, and plaque calcification also failed to register as risk factors, despite their potential to increase the risk of embolization with wire or catheter manipulation, Dr. Kibbe observed.
Two studies found that lesions longer than 1 cm or 1.5 cm were predictive of stroke, while one study (Catheter Cardiovasc. Interv. 2012;80:321-8) linked stroke with ICA tortuosity, defined as a distal ICA angle of more than 60 degrees.
Conventional thinking would suggest that right-sided carotid stenting would also confer a greater stroke risk because selecting the right ICA requires crossing the orifice of the left ICA, but only the SAPPHIRE trial reported more strokes with right-sided lesions, she said.
Finally, Dr. Kibbe highlighted an anatomic scoring system for carotid artery stenting developed by an international panel of seven vascular surgeons and five interventional radiologists (Stroke 2009;40:1698-703). Based on expert consensus, the greatest risk factor is type III arch, followed in descending order of risk by arch atheroma, diseased common carotid artery, ECA disease, angulated distal ICA, bovine arch, and pinhole stenosis. The resulting color-coded, traffic-light scoring system looks rather busy, but is relatively easy to use and provides guidance on carotid stenting suitability for the novice physician, she said.
Dr. Kibbe reported having no financial disclosures.
CHICAGO – Pinning down the anatomic characteristics that increase the risk of stroke with carotid artery stenting continues to be a challenge, as demonstrated by a database study and literature review presented at a symposium on vascular surgery sponsored by Northwestern University.
"I am absolutely convinced that certain anatomic and plaque characteristics increase the risk of a stroke for patients with carotid artery stenting," Dr. Melina R. Kibbe said in presenting her study. "The studies are not consistent in the literature, but there is a pattern."
She presented a prospective database review that evaluated no less than a dozen anatomic variables in 381 carotid arteries stented at the university from 2001 to 2010. The mostly male (75%), asymptomatic (70%), and moderate- to high-risk cohort had an average age of 70.5 years.
Within 30 days of carotid stenting, there were six strokes and eight transient ischemic attacks (TIAs), for an overall neurologic event rate of 3.7%, she said. Three patients had a heart attack, and two died.
The risk of perioperative stroke or TIA was significantly increased only in patients with a higher degree of internal carotid artery (ICA) stenosis (87% stenosis vs. 81% stenosis; P = .03).
It also trended higher, but fell short of significance, in those with greater arch calcification (P = .06).
Surprisingly, no statistical association was found between neurologic events and arch type (P = .16), despite the clinical belief that increasing arch type can be associated with more difficulty accessing the target lesion, said Dr. Kibbe, professor of vascular surgery and surgical research at Northwestern University, Chicago.
Other variables with trends that failed to reach statistical significance included internal to common carotid artery angulation, tortuous carotid artery, ipsilateral external carotid artery (ECA) stenosis, plaque calcification, and lesion length.
Turning to the literature for more answers, Dr. Kibbe and her colleagues performed a review of eight carotid stenting studies between 1993 and 2013. These studies included SAPPHIRE, which demonstrated an association between type II and III arches and increased stroke risk. Two other studies implicated type II, type III, and bovine arches, while three other studies, including a systematic review that incorporated the EVA-3S study (Stroke 2011;42:380-8), found no association between arch type and stroke.
Still, Dr. Kibbe said she’s convinced no two patients are alike when it comes to arterial anatomy and called for data from pivotal trials like CREST to be reanalyzed for stroke, based on arch type and plaque characteristics.
"Even better, I’d like to see a prospective, randomized study that excludes patients for carotid artery stenting based on their anatomy; for example, excluding all type III arches," she said. "My own personal bias is that patients with type III arches should not have carotid stenting. We don’t need to push the envelope."
Interestingly, no study in the review has shown ICA stenosis to be a risk factor for stroke or TIA, although lesions with a higher degree of stenosis would be expected to contribute to perioperative stroke risk.
Arch calcification, ECA stenosis, and plaque calcification also failed to register as risk factors, despite their potential to increase the risk of embolization with wire or catheter manipulation, Dr. Kibbe observed.
Two studies found that lesions longer than 1 cm or 1.5 cm were predictive of stroke, while one study (Catheter Cardiovasc. Interv. 2012;80:321-8) linked stroke with ICA tortuosity, defined as a distal ICA angle of more than 60 degrees.
Conventional thinking would suggest that right-sided carotid stenting would also confer a greater stroke risk because selecting the right ICA requires crossing the orifice of the left ICA, but only the SAPPHIRE trial reported more strokes with right-sided lesions, she said.
Finally, Dr. Kibbe highlighted an anatomic scoring system for carotid artery stenting developed by an international panel of seven vascular surgeons and five interventional radiologists (Stroke 2009;40:1698-703). Based on expert consensus, the greatest risk factor is type III arch, followed in descending order of risk by arch atheroma, diseased common carotid artery, ECA disease, angulated distal ICA, bovine arch, and pinhole stenosis. The resulting color-coded, traffic-light scoring system looks rather busy, but is relatively easy to use and provides guidance on carotid stenting suitability for the novice physician, she said.
Dr. Kibbe reported having no financial disclosures.
CHICAGO – Pinning down the anatomic characteristics that increase the risk of stroke with carotid artery stenting continues to be a challenge, as demonstrated by a database study and literature review presented at a symposium on vascular surgery sponsored by Northwestern University.
"I am absolutely convinced that certain anatomic and plaque characteristics increase the risk of a stroke for patients with carotid artery stenting," Dr. Melina R. Kibbe said in presenting her study. "The studies are not consistent in the literature, but there is a pattern."
She presented a prospective database review that evaluated no less than a dozen anatomic variables in 381 carotid arteries stented at the university from 2001 to 2010. The mostly male (75%), asymptomatic (70%), and moderate- to high-risk cohort had an average age of 70.5 years.
Within 30 days of carotid stenting, there were six strokes and eight transient ischemic attacks (TIAs), for an overall neurologic event rate of 3.7%, she said. Three patients had a heart attack, and two died.
The risk of perioperative stroke or TIA was significantly increased only in patients with a higher degree of internal carotid artery (ICA) stenosis (87% stenosis vs. 81% stenosis; P = .03).
It also trended higher, but fell short of significance, in those with greater arch calcification (P = .06).
Surprisingly, no statistical association was found between neurologic events and arch type (P = .16), despite the clinical belief that increasing arch type can be associated with more difficulty accessing the target lesion, said Dr. Kibbe, professor of vascular surgery and surgical research at Northwestern University, Chicago.
Other variables with trends that failed to reach statistical significance included internal to common carotid artery angulation, tortuous carotid artery, ipsilateral external carotid artery (ECA) stenosis, plaque calcification, and lesion length.
Turning to the literature for more answers, Dr. Kibbe and her colleagues performed a review of eight carotid stenting studies between 1993 and 2013. These studies included SAPPHIRE, which demonstrated an association between type II and III arches and increased stroke risk. Two other studies implicated type II, type III, and bovine arches, while three other studies, including a systematic review that incorporated the EVA-3S study (Stroke 2011;42:380-8), found no association between arch type and stroke.
Still, Dr. Kibbe said she’s convinced no two patients are alike when it comes to arterial anatomy and called for data from pivotal trials like CREST to be reanalyzed for stroke, based on arch type and plaque characteristics.
"Even better, I’d like to see a prospective, randomized study that excludes patients for carotid artery stenting based on their anatomy; for example, excluding all type III arches," she said. "My own personal bias is that patients with type III arches should not have carotid stenting. We don’t need to push the envelope."
Interestingly, no study in the review has shown ICA stenosis to be a risk factor for stroke or TIA, although lesions with a higher degree of stenosis would be expected to contribute to perioperative stroke risk.
Arch calcification, ECA stenosis, and plaque calcification also failed to register as risk factors, despite their potential to increase the risk of embolization with wire or catheter manipulation, Dr. Kibbe observed.
Two studies found that lesions longer than 1 cm or 1.5 cm were predictive of stroke, while one study (Catheter Cardiovasc. Interv. 2012;80:321-8) linked stroke with ICA tortuosity, defined as a distal ICA angle of more than 60 degrees.
Conventional thinking would suggest that right-sided carotid stenting would also confer a greater stroke risk because selecting the right ICA requires crossing the orifice of the left ICA, but only the SAPPHIRE trial reported more strokes with right-sided lesions, she said.
Finally, Dr. Kibbe highlighted an anatomic scoring system for carotid artery stenting developed by an international panel of seven vascular surgeons and five interventional radiologists (Stroke 2009;40:1698-703). Based on expert consensus, the greatest risk factor is type III arch, followed in descending order of risk by arch atheroma, diseased common carotid artery, ECA disease, angulated distal ICA, bovine arch, and pinhole stenosis. The resulting color-coded, traffic-light scoring system looks rather busy, but is relatively easy to use and provides guidance on carotid stenting suitability for the novice physician, she said.
Dr. Kibbe reported having no financial disclosures.
EXPERT ANALYSIS AT A NORTHWESTERN VASCULAR SYMPOSIUM
Major finding: The risk of perioperative stroke or TIA was significantly increased only in patients with a higher degree of internal carotid artery (ICA) stenosis (87% stenosis vs. 81% stenosis; P = .03).
Data source: A retrospective study of 381 carotid arteries and an analysis of eight published studies.
Disclosures: Dr. Kibbe reported having no financial disclosures.
Routinely screen relatives when thoracic aortic aneurysm disease presents before age 60
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: One in seven patients presenting with thoracic aortic disease in a large series had thoracic aortic aneurysm and dissection, or TAAD, an under-recognized, asymptomatic condition characterized by cystic medial necrosis and a strong genetic component.
Data source: This was a review of 1,276 patients who presented to a tertiary center with thoracic aortic disease. Systemic screening of first-degree relatives of patients with familial TAAD turned up two additional cases per proband.
Disclosures: The study was conducted free of commercial support. The presenter reported having no financial conflicts.
Zenith AAA fenestrated graft: 30-day outcomes match those seen in trial
CHICAGO – Real-world 30-day outcomes with the Zenith fenestrated endovascular graft compared well with those in the U.S. fenestrated trial for the treatment of juxtarenal aortic aneurysms, based on a multicenter, retrospective study of 57 patients.
The good results were seen despite higher rates of comorbidities and more challenging anatomy in the patients treated post approval. But "these are short-term outcomes and, ultimately, we’re going to need to know the long-term durability of this type of repair," Dr. Chandu Vemuri said at the annual meeting of the Midwestern Vascular Surgical Society.
The Zenith fenestrated AAA endovascular graft (zFEN) has been commercially available outside the United States since November 2002, and it gained federal approval in April 2012 based on the results of the pivotal U.S. fenestrated trial (USFT). The graft is indicated for the treatment of patients with abdominal aortic or aortoiliac aneurysms that have an infrarenal aortic neck at least 4 mm in length.
Notably, 47% of patients in the current analysis did not meet the USFT anatomic criteria of a 4- to 15-mm infrarenal neck. There were also significantly more mesenteric stents used in this group than in the USFT (13 vs. 0; P < .05), reflecting the higher anatomic complexity of these patients, said Dr. Vemuri, of Washington University, St. Louis.
Compared with the 42 USFT patients, study patients also had significantly higher rates of preoperative coronary artery disease (79% vs. 52%), myocardial infarction (60% vs. 24%), and renal insufficiency (26% vs. 9.5%); all P < .05).
Dr. Vemuri was quick to point out, however, that the results of the study may not broadly translate. The trial was conducted at institutions that had a high aortic volume, and involved highly experienced surgeons, many of whom took part in the USFT and were familiar with the zFEN device.
The technical success rate, with regard to aneurysm exclusion, was 100% in both the study and the USFT. In two study patients, the left renal artery (RA) fenestration was not able to be aligned. One patient had a kinked renal stent that was successfully restented, and one patient was converted to a unibody prosthesis with femoral-femoral bypass and an iliac plug, he said.
Other 30-day outcomes were similar between the study and USFT patients, including mortality (1 vs. 0), renal insufficiency (4 vs. 0), postoperative dialysis (1 vs. 0), and endoleak (10 vs. 9). Two of the 10 endoleaks were type III and 8 were type II.
The most common graft configuration was a scallop of the superior mesenteric artery (SMA) and bilateral RA fenestration in 40 patients, followed by bilateral RA fenestrations alone in 8, and a celiac scallop, SMA fenestration, and bilateral RA fenestration in 3, Dr. Vemuri observed.
One patient each had the following: right RA scallop plus left RA fenestration, right RA fenestration, right accessory RA fenestration, left RA fenestration, celiac scallop and SMA fenestration with a right RA fenestration, and an SMA scallop with left RA fenestration.
Total operative time averaged 250 minutes.
Audience members pressed Dr. Vemuri for details on the 47% of patients who did not meet the USFT anatomic criteria and whether the current cohort was really anatomically challenging or just consisted of on-label cases that were harder than usual.
Dr. Vemuri responded that the intervention was off-label in 27% of patients who did not have the minimum 4-mm infrarenal neck. One graft configuration was more complex than in the device’s instructions for use (IFU) document. All of the patients had a sufficient infrarenal neck for appropriate seal.
"I don’t think the purpose of this study is to endorse use outside the IFU of the device, but to report that when people do, this is what happens," he added.
Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
CHICAGO – Real-world 30-day outcomes with the Zenith fenestrated endovascular graft compared well with those in the U.S. fenestrated trial for the treatment of juxtarenal aortic aneurysms, based on a multicenter, retrospective study of 57 patients.
The good results were seen despite higher rates of comorbidities and more challenging anatomy in the patients treated post approval. But "these are short-term outcomes and, ultimately, we’re going to need to know the long-term durability of this type of repair," Dr. Chandu Vemuri said at the annual meeting of the Midwestern Vascular Surgical Society.
The Zenith fenestrated AAA endovascular graft (zFEN) has been commercially available outside the United States since November 2002, and it gained federal approval in April 2012 based on the results of the pivotal U.S. fenestrated trial (USFT). The graft is indicated for the treatment of patients with abdominal aortic or aortoiliac aneurysms that have an infrarenal aortic neck at least 4 mm in length.
Notably, 47% of patients in the current analysis did not meet the USFT anatomic criteria of a 4- to 15-mm infrarenal neck. There were also significantly more mesenteric stents used in this group than in the USFT (13 vs. 0; P < .05), reflecting the higher anatomic complexity of these patients, said Dr. Vemuri, of Washington University, St. Louis.
Compared with the 42 USFT patients, study patients also had significantly higher rates of preoperative coronary artery disease (79% vs. 52%), myocardial infarction (60% vs. 24%), and renal insufficiency (26% vs. 9.5%); all P < .05).
Dr. Vemuri was quick to point out, however, that the results of the study may not broadly translate. The trial was conducted at institutions that had a high aortic volume, and involved highly experienced surgeons, many of whom took part in the USFT and were familiar with the zFEN device.
The technical success rate, with regard to aneurysm exclusion, was 100% in both the study and the USFT. In two study patients, the left renal artery (RA) fenestration was not able to be aligned. One patient had a kinked renal stent that was successfully restented, and one patient was converted to a unibody prosthesis with femoral-femoral bypass and an iliac plug, he said.
Other 30-day outcomes were similar between the study and USFT patients, including mortality (1 vs. 0), renal insufficiency (4 vs. 0), postoperative dialysis (1 vs. 0), and endoleak (10 vs. 9). Two of the 10 endoleaks were type III and 8 were type II.
The most common graft configuration was a scallop of the superior mesenteric artery (SMA) and bilateral RA fenestration in 40 patients, followed by bilateral RA fenestrations alone in 8, and a celiac scallop, SMA fenestration, and bilateral RA fenestration in 3, Dr. Vemuri observed.
One patient each had the following: right RA scallop plus left RA fenestration, right RA fenestration, right accessory RA fenestration, left RA fenestration, celiac scallop and SMA fenestration with a right RA fenestration, and an SMA scallop with left RA fenestration.
Total operative time averaged 250 minutes.
Audience members pressed Dr. Vemuri for details on the 47% of patients who did not meet the USFT anatomic criteria and whether the current cohort was really anatomically challenging or just consisted of on-label cases that were harder than usual.
Dr. Vemuri responded that the intervention was off-label in 27% of patients who did not have the minimum 4-mm infrarenal neck. One graft configuration was more complex than in the device’s instructions for use (IFU) document. All of the patients had a sufficient infrarenal neck for appropriate seal.
"I don’t think the purpose of this study is to endorse use outside the IFU of the device, but to report that when people do, this is what happens," he added.
Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
CHICAGO – Real-world 30-day outcomes with the Zenith fenestrated endovascular graft compared well with those in the U.S. fenestrated trial for the treatment of juxtarenal aortic aneurysms, based on a multicenter, retrospective study of 57 patients.
The good results were seen despite higher rates of comorbidities and more challenging anatomy in the patients treated post approval. But "these are short-term outcomes and, ultimately, we’re going to need to know the long-term durability of this type of repair," Dr. Chandu Vemuri said at the annual meeting of the Midwestern Vascular Surgical Society.
The Zenith fenestrated AAA endovascular graft (zFEN) has been commercially available outside the United States since November 2002, and it gained federal approval in April 2012 based on the results of the pivotal U.S. fenestrated trial (USFT). The graft is indicated for the treatment of patients with abdominal aortic or aortoiliac aneurysms that have an infrarenal aortic neck at least 4 mm in length.
Notably, 47% of patients in the current analysis did not meet the USFT anatomic criteria of a 4- to 15-mm infrarenal neck. There were also significantly more mesenteric stents used in this group than in the USFT (13 vs. 0; P < .05), reflecting the higher anatomic complexity of these patients, said Dr. Vemuri, of Washington University, St. Louis.
Compared with the 42 USFT patients, study patients also had significantly higher rates of preoperative coronary artery disease (79% vs. 52%), myocardial infarction (60% vs. 24%), and renal insufficiency (26% vs. 9.5%); all P < .05).
Dr. Vemuri was quick to point out, however, that the results of the study may not broadly translate. The trial was conducted at institutions that had a high aortic volume, and involved highly experienced surgeons, many of whom took part in the USFT and were familiar with the zFEN device.
The technical success rate, with regard to aneurysm exclusion, was 100% in both the study and the USFT. In two study patients, the left renal artery (RA) fenestration was not able to be aligned. One patient had a kinked renal stent that was successfully restented, and one patient was converted to a unibody prosthesis with femoral-femoral bypass and an iliac plug, he said.
Other 30-day outcomes were similar between the study and USFT patients, including mortality (1 vs. 0), renal insufficiency (4 vs. 0), postoperative dialysis (1 vs. 0), and endoleak (10 vs. 9). Two of the 10 endoleaks were type III and 8 were type II.
The most common graft configuration was a scallop of the superior mesenteric artery (SMA) and bilateral RA fenestration in 40 patients, followed by bilateral RA fenestrations alone in 8, and a celiac scallop, SMA fenestration, and bilateral RA fenestration in 3, Dr. Vemuri observed.
One patient each had the following: right RA scallop plus left RA fenestration, right RA fenestration, right accessory RA fenestration, left RA fenestration, celiac scallop and SMA fenestration with a right RA fenestration, and an SMA scallop with left RA fenestration.
Total operative time averaged 250 minutes.
Audience members pressed Dr. Vemuri for details on the 47% of patients who did not meet the USFT anatomic criteria and whether the current cohort was really anatomically challenging or just consisted of on-label cases that were harder than usual.
Dr. Vemuri responded that the intervention was off-label in 27% of patients who did not have the minimum 4-mm infrarenal neck. One graft configuration was more complex than in the device’s instructions for use (IFU) document. All of the patients had a sufficient infrarenal neck for appropriate seal.
"I don’t think the purpose of this study is to endorse use outside the IFU of the device, but to report that when people do, this is what happens," he added.
Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
AT MIDWESTERN VASCULAR 2013
Major finding: The technical success rate was 100%.
Data source: Retrospective study of 57 patients with juxtarenal aortic aneurysms treated post approval with the Zenith fenestrated endovascular graft.
Disclosures: Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
Aortic arch repair outcomes held acceptable
CHICAGO – Repair of the isolated nontraumatic aortic arch aneurysm can be performed with acceptable early and late results, based on a 20-year review of 137 patients.
Early mortality was seen in nine patients (6.6%), and was similarly split between endovascular and open repair (7.3% vs. 6.5%, respectively).
Five-year survival did not differ between the endovascular- and open-repair groups (86% vs. 70%; P = .57), although the risk of reintervention was significantly higher with an endovascular strategy (94% vs. 77.5%; P = .02).
"These data support ongoing efforts to develop branched endografts specifically tailored for complex and difficult-to-treat pathology to potentially reduce the morbidity of therapy for this population," Dr. Himanshu J. Patel said at the annual meeting of the Midwestern Vascular Surgical Society.
Marginal landing zones and the need for complex arch debranching procedures to extend landing zones and facilitate repair can hinder endovascular repair, though open aortic repair is challenged by the frequent need for hypothermic circulatory arrest. Early and late results with either option are ill defined for this pathology, observed Dr. Patel, with the Cardiovascular Center, University of Michigan, Ann Arbor.
To investigate these outcomes, the authors evaluated data from 1993 to 2013 for 137 patients with nontraumatic aortic aneurysms located between the left and right pulmonary artery; 93 patients underwent open repair and 44 had an endovascular or hybrid repair.
The pathology was saccular in 39%, dissection in 11%, pseudoaneurysm in 18%, and anomalous arch in 11%. Rupture was identified in 14%. The average patient age was 59 years, and 46% were male.
In the open-repair group, 84 patients underwent a posterolateral thoracotomy and 9 had a median sternotomy. Extracorporeal perfusion was used in all, and 80 required deep hypothermic arrest.
In the endovascular group, the proximal extent of surgery was classified as Ishimaru zone 0 in 10 patients, zone 1 in 1, and zone 2 in 33. Treatment included full arch debranching and stent graft repair for zone 0 and zone 2 patients, respectively. A chimney stent of the carotid artery and left subclavian arterial bypass was used for the zone 1 patient.
Although the institutional preference is to utilize left subclavian artery (LSCA) revascularization for all, 76% of endovascular patients requiring LSCA coverage underwent preoperative bypass, Dr. Patel observed.
Significant differences existed at baseline between the two groups, with the endovascular group almost a full decade older than the open group (65 vs. 56 years). The endovascular group was also more likely to have saccular pathology (57% vs. 30%), and less likely to have a history of tobacco use (12% vs. 84%) or prior arch repair (16% vs. 36%).
Early morbidity included stroke in 6.6%, spinal cord ischemia in 0.7%, need for dialysis in 3.6%, and tracheostomy in 7.2%, Dr. Patel said.
The composite endpoint of stroke or early mortality was independently predicted by increasing age (odds ratio, 1.05; P = .055) and use of a hybrid procedure (OR, 6.4; P = .01).
Two-year freedom from aortic rupture or reintervention was 78% in the open group and 94% in the endovascular group (P = .02).
After a mean follow-up of 66 months, four patients had died of stroke, two from myocardial infarction, one from a proximal anastomosis disruption/rupture, and one from a type A dissection/rupture, Dr. Patel said.
The average survival was 145 months and 5-year survival rate was 59%.
Independent predictors of late mortality were increasing age (hazard ratio, 1.06; P = .001), peripheral vascular disease (HR, 2.4; P = .024), postoperative stroke (HR, 6.4; P less than .001), and postoperative dialysis (HR, 11.4; P less than .001). Notably, repair type was not predictive (P = .22), Dr. Patel said.
He cautioned the audience, however, that "cerebrovascular accidents remain an important cause of early and late mortality."
Dr. Patel reported consulting fees from W.L. Gore and Terumo.
CHICAGO – Repair of the isolated nontraumatic aortic arch aneurysm can be performed with acceptable early and late results, based on a 20-year review of 137 patients.
Early mortality was seen in nine patients (6.6%), and was similarly split between endovascular and open repair (7.3% vs. 6.5%, respectively).
Five-year survival did not differ between the endovascular- and open-repair groups (86% vs. 70%; P = .57), although the risk of reintervention was significantly higher with an endovascular strategy (94% vs. 77.5%; P = .02).
"These data support ongoing efforts to develop branched endografts specifically tailored for complex and difficult-to-treat pathology to potentially reduce the morbidity of therapy for this population," Dr. Himanshu J. Patel said at the annual meeting of the Midwestern Vascular Surgical Society.
Marginal landing zones and the need for complex arch debranching procedures to extend landing zones and facilitate repair can hinder endovascular repair, though open aortic repair is challenged by the frequent need for hypothermic circulatory arrest. Early and late results with either option are ill defined for this pathology, observed Dr. Patel, with the Cardiovascular Center, University of Michigan, Ann Arbor.
To investigate these outcomes, the authors evaluated data from 1993 to 2013 for 137 patients with nontraumatic aortic aneurysms located between the left and right pulmonary artery; 93 patients underwent open repair and 44 had an endovascular or hybrid repair.
The pathology was saccular in 39%, dissection in 11%, pseudoaneurysm in 18%, and anomalous arch in 11%. Rupture was identified in 14%. The average patient age was 59 years, and 46% were male.
In the open-repair group, 84 patients underwent a posterolateral thoracotomy and 9 had a median sternotomy. Extracorporeal perfusion was used in all, and 80 required deep hypothermic arrest.
In the endovascular group, the proximal extent of surgery was classified as Ishimaru zone 0 in 10 patients, zone 1 in 1, and zone 2 in 33. Treatment included full arch debranching and stent graft repair for zone 0 and zone 2 patients, respectively. A chimney stent of the carotid artery and left subclavian arterial bypass was used for the zone 1 patient.
Although the institutional preference is to utilize left subclavian artery (LSCA) revascularization for all, 76% of endovascular patients requiring LSCA coverage underwent preoperative bypass, Dr. Patel observed.
Significant differences existed at baseline between the two groups, with the endovascular group almost a full decade older than the open group (65 vs. 56 years). The endovascular group was also more likely to have saccular pathology (57% vs. 30%), and less likely to have a history of tobacco use (12% vs. 84%) or prior arch repair (16% vs. 36%).
Early morbidity included stroke in 6.6%, spinal cord ischemia in 0.7%, need for dialysis in 3.6%, and tracheostomy in 7.2%, Dr. Patel said.
The composite endpoint of stroke or early mortality was independently predicted by increasing age (odds ratio, 1.05; P = .055) and use of a hybrid procedure (OR, 6.4; P = .01).
Two-year freedom from aortic rupture or reintervention was 78% in the open group and 94% in the endovascular group (P = .02).
After a mean follow-up of 66 months, four patients had died of stroke, two from myocardial infarction, one from a proximal anastomosis disruption/rupture, and one from a type A dissection/rupture, Dr. Patel said.
The average survival was 145 months and 5-year survival rate was 59%.
Independent predictors of late mortality were increasing age (hazard ratio, 1.06; P = .001), peripheral vascular disease (HR, 2.4; P = .024), postoperative stroke (HR, 6.4; P less than .001), and postoperative dialysis (HR, 11.4; P less than .001). Notably, repair type was not predictive (P = .22), Dr. Patel said.
He cautioned the audience, however, that "cerebrovascular accidents remain an important cause of early and late mortality."
Dr. Patel reported consulting fees from W.L. Gore and Terumo.
CHICAGO – Repair of the isolated nontraumatic aortic arch aneurysm can be performed with acceptable early and late results, based on a 20-year review of 137 patients.
Early mortality was seen in nine patients (6.6%), and was similarly split between endovascular and open repair (7.3% vs. 6.5%, respectively).
Five-year survival did not differ between the endovascular- and open-repair groups (86% vs. 70%; P = .57), although the risk of reintervention was significantly higher with an endovascular strategy (94% vs. 77.5%; P = .02).
"These data support ongoing efforts to develop branched endografts specifically tailored for complex and difficult-to-treat pathology to potentially reduce the morbidity of therapy for this population," Dr. Himanshu J. Patel said at the annual meeting of the Midwestern Vascular Surgical Society.
Marginal landing zones and the need for complex arch debranching procedures to extend landing zones and facilitate repair can hinder endovascular repair, though open aortic repair is challenged by the frequent need for hypothermic circulatory arrest. Early and late results with either option are ill defined for this pathology, observed Dr. Patel, with the Cardiovascular Center, University of Michigan, Ann Arbor.
To investigate these outcomes, the authors evaluated data from 1993 to 2013 for 137 patients with nontraumatic aortic aneurysms located between the left and right pulmonary artery; 93 patients underwent open repair and 44 had an endovascular or hybrid repair.
The pathology was saccular in 39%, dissection in 11%, pseudoaneurysm in 18%, and anomalous arch in 11%. Rupture was identified in 14%. The average patient age was 59 years, and 46% were male.
In the open-repair group, 84 patients underwent a posterolateral thoracotomy and 9 had a median sternotomy. Extracorporeal perfusion was used in all, and 80 required deep hypothermic arrest.
In the endovascular group, the proximal extent of surgery was classified as Ishimaru zone 0 in 10 patients, zone 1 in 1, and zone 2 in 33. Treatment included full arch debranching and stent graft repair for zone 0 and zone 2 patients, respectively. A chimney stent of the carotid artery and left subclavian arterial bypass was used for the zone 1 patient.
Although the institutional preference is to utilize left subclavian artery (LSCA) revascularization for all, 76% of endovascular patients requiring LSCA coverage underwent preoperative bypass, Dr. Patel observed.
Significant differences existed at baseline between the two groups, with the endovascular group almost a full decade older than the open group (65 vs. 56 years). The endovascular group was also more likely to have saccular pathology (57% vs. 30%), and less likely to have a history of tobacco use (12% vs. 84%) or prior arch repair (16% vs. 36%).
Early morbidity included stroke in 6.6%, spinal cord ischemia in 0.7%, need for dialysis in 3.6%, and tracheostomy in 7.2%, Dr. Patel said.
The composite endpoint of stroke or early mortality was independently predicted by increasing age (odds ratio, 1.05; P = .055) and use of a hybrid procedure (OR, 6.4; P = .01).
Two-year freedom from aortic rupture or reintervention was 78% in the open group and 94% in the endovascular group (P = .02).
After a mean follow-up of 66 months, four patients had died of stroke, two from myocardial infarction, one from a proximal anastomosis disruption/rupture, and one from a type A dissection/rupture, Dr. Patel said.
The average survival was 145 months and 5-year survival rate was 59%.
Independent predictors of late mortality were increasing age (hazard ratio, 1.06; P = .001), peripheral vascular disease (HR, 2.4; P = .024), postoperative stroke (HR, 6.4; P less than .001), and postoperative dialysis (HR, 11.4; P less than .001). Notably, repair type was not predictive (P = .22), Dr. Patel said.
He cautioned the audience, however, that "cerebrovascular accidents remain an important cause of early and late mortality."
Dr. Patel reported consulting fees from W.L. Gore and Terumo.
AT MIDWESTERN VASCULAR 2013
Major finding: Five-year survival was 86% in the endovascular group and 70% in the open group (P = .57).
Data source: A retrospective study of 137 patients undergoing isolated aortic arch repair between 1993 and 2013.
Disclosures: Dr. Patel reported consulting fees from W.L. Gore and Terumo.