User login
Should clopidogrel be discontinued prior to open vascular procedures?
The continued use of perioperative clopidogrel is appropriate
Surgeons have always worried about bleeding risks for procedures we do. Complex vascular procedures are further complicated by the myriad of available antiplatelet agents designed to reduce ischemic events from cardiovascular disease burden at the expense of potential bleeding complications if antiplatelet medications are continued. Rather than relying on anecdotal reports by historical vignettes, let’s look at the evidence.
There probably is no other drug available in our vascular toolbox which has been studied more in the last 20 years than clopidogrel. Multiple randomized and double blinded studies such as CASPAR1 and CHARISMA2 have amplified what was known since the early CAPRIE trial in the 1990’s and that is that clopidogrel is safe when used as a single medication or as a dual agent with aspirin (duel antiplatelet therapy [DAPT]).
But not all our patients need DAPT. There is no level 1 evidence demonstrating the need for any antiplatelet therapy in the primary prevention of cardiovascular events for patients deemed at low or moderate risk of cardiovascular disease from a large meta-analysis review of six primary prevention trials encompassing over 95,000 patients.3
If our patients do present with vascular disease, current ACCP guidelines recommend single-agent antiplatelet medication (either ASA or clopidogrel) for symptomatic peripheral arterial disease (PAD) whether planning LE revascularization with bypass or via endovascular means with grade 1A evidence.4 This works fine for single-focus vascular disease and each antiplatelet agent have proponents but either works well.
That’s great, but what about all those sick cardiac patients we see the most of? First, CHARISMA subgroup analysis of patients with preexisting coronary and/or cerebrovascular disease demonstrate a 7.1% risk reduction in MI, cerebrovascular events, and cardiac ischemic deaths when continuing DAPT over aspirin alone, and similar risk reduction is found in PAD patients for endpoints of MI and ischemic cardiovascular events. Second, there was no significant difference in severe, fatal, or moderate bleeding in those receiving DAPT vs. aspirin alone with only minor bleeding increased using DAPT. Third, real-life practice echoes multiple trial experiences such as the Vascular Study Group of New England study group confirmed in reviewing 16 centers and 66 surgeons with more than 10,000 patients. Approximately 39% underwent major aortic or lower extremity bypass operations.
No statistical difference could be found for reoperation (P = .74), transfusion (P = .1) or operative type between DAPT or aspirin use alone.5 This is rediscovered once again by Saadeh and Sfeir in their prospective study of 647 major arterial procedures over 7 years finding no significant difference in reoperation for bleeding or bleeding mortality between DAPT vs. aspirin alone.6
So can we stop bashing clopidogrel as an evil agent of bleeding as Dr. Dalsing wishes to do? After all, he has been on record as stating, “I don’t know if our bleeding risk is worse or better … something we have to do to keep our grafts going.” Evidence tells us the benefits for continuing DAPT as seen in risk reduction in primary cardiovascular outcomes far outweigh the risk of minor bleeding associated with continued use.
Let the science dictate practice. Patients with low or moderate risk for cardiovascular disease need no antiplatelet medication unless undergoing PAD treatment where a single agent, either aspirin or clopidogrel alone, is sufficient. In those patients having a large cardiovascular burden of disease, combination of aspirin and clopidogrel improves survival benefit and reduces ischemic events without a significant risk of reoperation, transfusion, or bleeding-related mortality. As many of our patients require DAPT for drug eluting coronary stents, withholding clopidogrel preoperatively increases overall risk beyond acceptable limits. Improving surgical skills and paying attention to hemostasis during the operation will allow naysayers to achieve improved patient survival without fear of bleeding when continuing best medical therapy such as DAPT.
Gary Lemmon, MD, is professor of vascular surgery at Indiana University, Indianapolis, and chief, vascular surgery, Indianapolis VA Medical Center. He reported no relevant conflicts.
References
1. J Vasc Surg. 2010;52:825-33
2. Eur Heart J. 2009;30:192-201
3. Lancet. 2009;373:1849-604. Chest. 2012;141:e669s-90s
5. J Vasc Surg. 2011;54: 779-84
6. J Vasc Surg. 2013;58: 1586-92
The continued use of perioperative clopidogrel is debatable!
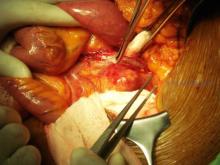
There are cases in which clopidogrel should not be discontinued for a needed vascular intervention. Delaying operation or maintaining clopidogrel during operation if your patient required a recent coronary stent is warranted unless you are willing to accept an acute coronary thrombosis.
However, in other cases, for example infrainguinal grafts, the risk of potential increased bleeding when adding clopidogrel to aspirin may outweigh potential improvements in graft patency. This is especially true of below-knee vein bypass grafts where data do not support improved patency. However, in the CASPAR trial, prosthetic graft patency did appear to be beneficial, but only in subgroup analysis.1
It is true that severe bleeding was not increased (intracranial hemorrhage, or hemodynamic compromise: 1 vs 2.7%, P = NS) but moderate bleeding (transfusion required: 0.7 vs 3.7%, P = .012) and mild bleeding (5.4 vs 12.1%, P = .004) was increased when this agent was used especially in vein graft surgery. This risk of bleeding was present even when clopidogrel was begun 2 or more days after surgery.1
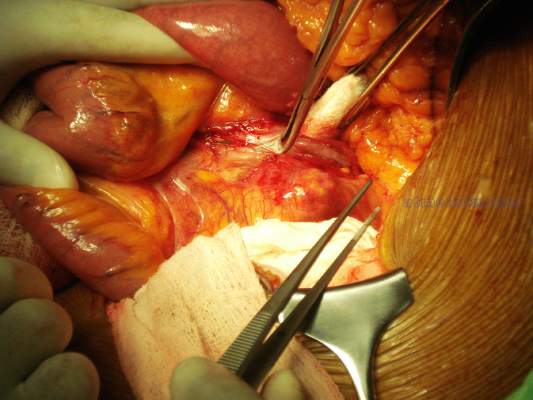
To complicate this decision, a Cochrane review did not consider subgroup analysis as statistically valid and so the authors considered infrainguinal graft patency as not improved with clopidogrel but bleeding risk was increased. One might even question the use of acetylsalicylic acid (ASA) for vein graft bypasses based on the results of this metanalysis.2 Carotid endarterectomy is a common vascular surgery procedure in which antiplatelet use has been evaluated in the real-world situation and with large cohorts. As is always the case when dealing with patient issues, the addition of one agent does not tell the entire story and patient demographics can have a significant influence on the outcome. A report from the Vascular Quality Initiative (VQI) database controlled for patient differences by propensity matching with more than 4,500 patients in each of the two groups; ASA vs. ASA + clopidogrel; demonstrated that major bleeding, defined as return to the OR for bleeding, was statistically more common with dual therapy (1.3% vs. 0.7%, P = .004).3
The addition of clopidogrel did statistically decrease the risk of ipsilateral TIA or stroke (0.8% vs. 1.2%, P = .02) but not the risk of death (0.2% vs. 0.3%, P = .3) or postoperative MI (1% vs. 0.8%, P = .4). Reoperation for bleeding is not inconsequential since in patients requiring this intervention, there is a significantly worse outcome in regard to stroke (3.7% vs. 0.8%, P = .001), MI (6.2% vs. 0.8%, P = .001), and death (2.5% vs. 0.2%,P = .001). Further drill down involving propensity score–matched analysis stratified by symptom status (asymptomatic vs. symptomatic) was quite interesting in that in only asymptomatic patients did the addition of clopidogrel actually demonstrate a statistically significant reduction in TIA or stroke, any stroke, or composite stroke/death. Symptomatic patients taking dual therapy demonstrated a slight reduction in TIA or stroke (1.4% vs. 1.7%, P = .6), any stroke (1.1% vs. 1.2%, P = .9) and composite stroke/death (1.2% vs. 1.5%, P = .5) but in no instance was statistical significance reached. The use of protamine did help to decrease the risk of bleeding.
Regarding the use of dual therapy during open aortic operations, an earlier report of the VQI database demonstrated no significant difference in bleeding risk statistically, but if one delves deeper the data indicate something different. In the majority of cases, vascular surgeons do not feel comfortable preforming this extensive dissection on dual therapy. Of the cases reported, 1,074 were preformed either free of either drug or only on ASA while 42 were on dual therapy and only 12 on clopidogrel only. In fact, in the conclusions, the authors note that they do not believe that conclusions regarding clopidogrel use in patient undergoing open abdominal aortic aneurysm repair can be drawn based on their results since the potential for a type II error was too great.4
It may be that our current level of sophistication is not sufficiently mature to determine the actual effect that clopidogrel is having on our patients. Clopidogrel, a thienopyridine, inhibits platelet activation by blocking the ADP-binding site for the P2Y12 receptor. Over 85% of ingested drug is metabolized into inactive metabolites while 15% is metabolized by the liver via a two-step oxidative process into the active thiol metabolite. Inter-individual variability in the antiplatelet response to thienopyridines is noted and partially caused by genetic mutations in the CP isoenzymes. Platelet reactivity testing is possible but most of the work has been conducted for those patients requiring coronary artery revascularization. Results of tailoring intervention to maximize therapeutic benefit and decrease the risk of bleeding have been inconsistent but, in some studies, appear to be promising.5 This approach may ultimately be found superior to determining how effective clopidogrel actually is in a particular case with some insight into the bleeding risk as well. With this determination, whether or not to hold clopidogrel perioperatively can be made with some science behind the decision.
Clearly, a blanket statement that the risk of bleeding should be accepted or ignored because of the demonstrated benefits of clopidogrel in patients requiring vascular surgery is not accurate. In some cases, there is no clear benefit, so eliminating the bleeding risk may well be the appropriate decision. The astute vascular surgeon understands the details of the written word in order to make an educated decision and understands that new information such as determining platelet reactivity may provide more clarity to such decisions in the future.
Michael C. Dalsing, MD, is chief of vascular surgery at Indiana University, Indianapolis. He reported no relevant conflicts.
References
1. J Vasc Surg. 2010;52:825-33
2. Cochrane Database Syst Rev. 2015, Issue 2. Art. No.: CD000535
The continued use of perioperative clopidogrel is appropriate
Surgeons have always worried about bleeding risks for procedures we do. Complex vascular procedures are further complicated by the myriad of available antiplatelet agents designed to reduce ischemic events from cardiovascular disease burden at the expense of potential bleeding complications if antiplatelet medications are continued. Rather than relying on anecdotal reports by historical vignettes, let’s look at the evidence.
There probably is no other drug available in our vascular toolbox which has been studied more in the last 20 years than clopidogrel. Multiple randomized and double blinded studies such as CASPAR1 and CHARISMA2 have amplified what was known since the early CAPRIE trial in the 1990’s and that is that clopidogrel is safe when used as a single medication or as a dual agent with aspirin (duel antiplatelet therapy [DAPT]).
But not all our patients need DAPT. There is no level 1 evidence demonstrating the need for any antiplatelet therapy in the primary prevention of cardiovascular events for patients deemed at low or moderate risk of cardiovascular disease from a large meta-analysis review of six primary prevention trials encompassing over 95,000 patients.3
If our patients do present with vascular disease, current ACCP guidelines recommend single-agent antiplatelet medication (either ASA or clopidogrel) for symptomatic peripheral arterial disease (PAD) whether planning LE revascularization with bypass or via endovascular means with grade 1A evidence.4 This works fine for single-focus vascular disease and each antiplatelet agent have proponents but either works well.
That’s great, but what about all those sick cardiac patients we see the most of? First, CHARISMA subgroup analysis of patients with preexisting coronary and/or cerebrovascular disease demonstrate a 7.1% risk reduction in MI, cerebrovascular events, and cardiac ischemic deaths when continuing DAPT over aspirin alone, and similar risk reduction is found in PAD patients for endpoints of MI and ischemic cardiovascular events. Second, there was no significant difference in severe, fatal, or moderate bleeding in those receiving DAPT vs. aspirin alone with only minor bleeding increased using DAPT. Third, real-life practice echoes multiple trial experiences such as the Vascular Study Group of New England study group confirmed in reviewing 16 centers and 66 surgeons with more than 10,000 patients. Approximately 39% underwent major aortic or lower extremity bypass operations.
No statistical difference could be found for reoperation (P = .74), transfusion (P = .1) or operative type between DAPT or aspirin use alone.5 This is rediscovered once again by Saadeh and Sfeir in their prospective study of 647 major arterial procedures over 7 years finding no significant difference in reoperation for bleeding or bleeding mortality between DAPT vs. aspirin alone.6
So can we stop bashing clopidogrel as an evil agent of bleeding as Dr. Dalsing wishes to do? After all, he has been on record as stating, “I don’t know if our bleeding risk is worse or better … something we have to do to keep our grafts going.” Evidence tells us the benefits for continuing DAPT as seen in risk reduction in primary cardiovascular outcomes far outweigh the risk of minor bleeding associated with continued use.
Let the science dictate practice. Patients with low or moderate risk for cardiovascular disease need no antiplatelet medication unless undergoing PAD treatment where a single agent, either aspirin or clopidogrel alone, is sufficient. In those patients having a large cardiovascular burden of disease, combination of aspirin and clopidogrel improves survival benefit and reduces ischemic events without a significant risk of reoperation, transfusion, or bleeding-related mortality. As many of our patients require DAPT for drug eluting coronary stents, withholding clopidogrel preoperatively increases overall risk beyond acceptable limits. Improving surgical skills and paying attention to hemostasis during the operation will allow naysayers to achieve improved patient survival without fear of bleeding when continuing best medical therapy such as DAPT.
Gary Lemmon, MD, is professor of vascular surgery at Indiana University, Indianapolis, and chief, vascular surgery, Indianapolis VA Medical Center. He reported no relevant conflicts.
References
1. J Vasc Surg. 2010;52:825-33
2. Eur Heart J. 2009;30:192-201
3. Lancet. 2009;373:1849-604. Chest. 2012;141:e669s-90s
5. J Vasc Surg. 2011;54: 779-84
6. J Vasc Surg. 2013;58: 1586-92
The continued use of perioperative clopidogrel is debatable!
There are cases in which clopidogrel should not be discontinued for a needed vascular intervention. Delaying operation or maintaining clopidogrel during operation if your patient required a recent coronary stent is warranted unless you are willing to accept an acute coronary thrombosis.
However, in other cases, for example infrainguinal grafts, the risk of potential increased bleeding when adding clopidogrel to aspirin may outweigh potential improvements in graft patency. This is especially true of below-knee vein bypass grafts where data do not support improved patency. However, in the CASPAR trial, prosthetic graft patency did appear to be beneficial, but only in subgroup analysis.1
It is true that severe bleeding was not increased (intracranial hemorrhage, or hemodynamic compromise: 1 vs 2.7%, P = NS) but moderate bleeding (transfusion required: 0.7 vs 3.7%, P = .012) and mild bleeding (5.4 vs 12.1%, P = .004) was increased when this agent was used especially in vein graft surgery. This risk of bleeding was present even when clopidogrel was begun 2 or more days after surgery.1
To complicate this decision, a Cochrane review did not consider subgroup analysis as statistically valid and so the authors considered infrainguinal graft patency as not improved with clopidogrel but bleeding risk was increased. One might even question the use of acetylsalicylic acid (ASA) for vein graft bypasses based on the results of this metanalysis.2 Carotid endarterectomy is a common vascular surgery procedure in which antiplatelet use has been evaluated in the real-world situation and with large cohorts. As is always the case when dealing with patient issues, the addition of one agent does not tell the entire story and patient demographics can have a significant influence on the outcome. A report from the Vascular Quality Initiative (VQI) database controlled for patient differences by propensity matching with more than 4,500 patients in each of the two groups; ASA vs. ASA + clopidogrel; demonstrated that major bleeding, defined as return to the OR for bleeding, was statistically more common with dual therapy (1.3% vs. 0.7%, P = .004).3
The addition of clopidogrel did statistically decrease the risk of ipsilateral TIA or stroke (0.8% vs. 1.2%, P = .02) but not the risk of death (0.2% vs. 0.3%, P = .3) or postoperative MI (1% vs. 0.8%, P = .4). Reoperation for bleeding is not inconsequential since in patients requiring this intervention, there is a significantly worse outcome in regard to stroke (3.7% vs. 0.8%, P = .001), MI (6.2% vs. 0.8%, P = .001), and death (2.5% vs. 0.2%,P = .001). Further drill down involving propensity score–matched analysis stratified by symptom status (asymptomatic vs. symptomatic) was quite interesting in that in only asymptomatic patients did the addition of clopidogrel actually demonstrate a statistically significant reduction in TIA or stroke, any stroke, or composite stroke/death. Symptomatic patients taking dual therapy demonstrated a slight reduction in TIA or stroke (1.4% vs. 1.7%, P = .6), any stroke (1.1% vs. 1.2%, P = .9) and composite stroke/death (1.2% vs. 1.5%, P = .5) but in no instance was statistical significance reached. The use of protamine did help to decrease the risk of bleeding.
Regarding the use of dual therapy during open aortic operations, an earlier report of the VQI database demonstrated no significant difference in bleeding risk statistically, but if one delves deeper the data indicate something different. In the majority of cases, vascular surgeons do not feel comfortable preforming this extensive dissection on dual therapy. Of the cases reported, 1,074 were preformed either free of either drug or only on ASA while 42 were on dual therapy and only 12 on clopidogrel only. In fact, in the conclusions, the authors note that they do not believe that conclusions regarding clopidogrel use in patient undergoing open abdominal aortic aneurysm repair can be drawn based on their results since the potential for a type II error was too great.4
It may be that our current level of sophistication is not sufficiently mature to determine the actual effect that clopidogrel is having on our patients. Clopidogrel, a thienopyridine, inhibits platelet activation by blocking the ADP-binding site for the P2Y12 receptor. Over 85% of ingested drug is metabolized into inactive metabolites while 15% is metabolized by the liver via a two-step oxidative process into the active thiol metabolite. Inter-individual variability in the antiplatelet response to thienopyridines is noted and partially caused by genetic mutations in the CP isoenzymes. Platelet reactivity testing is possible but most of the work has been conducted for those patients requiring coronary artery revascularization. Results of tailoring intervention to maximize therapeutic benefit and decrease the risk of bleeding have been inconsistent but, in some studies, appear to be promising.5 This approach may ultimately be found superior to determining how effective clopidogrel actually is in a particular case with some insight into the bleeding risk as well. With this determination, whether or not to hold clopidogrel perioperatively can be made with some science behind the decision.
Clearly, a blanket statement that the risk of bleeding should be accepted or ignored because of the demonstrated benefits of clopidogrel in patients requiring vascular surgery is not accurate. In some cases, there is no clear benefit, so eliminating the bleeding risk may well be the appropriate decision. The astute vascular surgeon understands the details of the written word in order to make an educated decision and understands that new information such as determining platelet reactivity may provide more clarity to such decisions in the future.
Michael C. Dalsing, MD, is chief of vascular surgery at Indiana University, Indianapolis. He reported no relevant conflicts.
References
1. J Vasc Surg. 2010;52:825-33
2. Cochrane Database Syst Rev. 2015, Issue 2. Art. No.: CD000535
The continued use of perioperative clopidogrel is appropriate
Surgeons have always worried about bleeding risks for procedures we do. Complex vascular procedures are further complicated by the myriad of available antiplatelet agents designed to reduce ischemic events from cardiovascular disease burden at the expense of potential bleeding complications if antiplatelet medications are continued. Rather than relying on anecdotal reports by historical vignettes, let’s look at the evidence.
There probably is no other drug available in our vascular toolbox which has been studied more in the last 20 years than clopidogrel. Multiple randomized and double blinded studies such as CASPAR1 and CHARISMA2 have amplified what was known since the early CAPRIE trial in the 1990’s and that is that clopidogrel is safe when used as a single medication or as a dual agent with aspirin (duel antiplatelet therapy [DAPT]).
But not all our patients need DAPT. There is no level 1 evidence demonstrating the need for any antiplatelet therapy in the primary prevention of cardiovascular events for patients deemed at low or moderate risk of cardiovascular disease from a large meta-analysis review of six primary prevention trials encompassing over 95,000 patients.3
If our patients do present with vascular disease, current ACCP guidelines recommend single-agent antiplatelet medication (either ASA or clopidogrel) for symptomatic peripheral arterial disease (PAD) whether planning LE revascularization with bypass or via endovascular means with grade 1A evidence.4 This works fine for single-focus vascular disease and each antiplatelet agent have proponents but either works well.
That’s great, but what about all those sick cardiac patients we see the most of? First, CHARISMA subgroup analysis of patients with preexisting coronary and/or cerebrovascular disease demonstrate a 7.1% risk reduction in MI, cerebrovascular events, and cardiac ischemic deaths when continuing DAPT over aspirin alone, and similar risk reduction is found in PAD patients for endpoints of MI and ischemic cardiovascular events. Second, there was no significant difference in severe, fatal, or moderate bleeding in those receiving DAPT vs. aspirin alone with only minor bleeding increased using DAPT. Third, real-life practice echoes multiple trial experiences such as the Vascular Study Group of New England study group confirmed in reviewing 16 centers and 66 surgeons with more than 10,000 patients. Approximately 39% underwent major aortic or lower extremity bypass operations.
No statistical difference could be found for reoperation (P = .74), transfusion (P = .1) or operative type between DAPT or aspirin use alone.5 This is rediscovered once again by Saadeh and Sfeir in their prospective study of 647 major arterial procedures over 7 years finding no significant difference in reoperation for bleeding or bleeding mortality between DAPT vs. aspirin alone.6
So can we stop bashing clopidogrel as an evil agent of bleeding as Dr. Dalsing wishes to do? After all, he has been on record as stating, “I don’t know if our bleeding risk is worse or better … something we have to do to keep our grafts going.” Evidence tells us the benefits for continuing DAPT as seen in risk reduction in primary cardiovascular outcomes far outweigh the risk of minor bleeding associated with continued use.
Let the science dictate practice. Patients with low or moderate risk for cardiovascular disease need no antiplatelet medication unless undergoing PAD treatment where a single agent, either aspirin or clopidogrel alone, is sufficient. In those patients having a large cardiovascular burden of disease, combination of aspirin and clopidogrel improves survival benefit and reduces ischemic events without a significant risk of reoperation, transfusion, or bleeding-related mortality. As many of our patients require DAPT for drug eluting coronary stents, withholding clopidogrel preoperatively increases overall risk beyond acceptable limits. Improving surgical skills and paying attention to hemostasis during the operation will allow naysayers to achieve improved patient survival without fear of bleeding when continuing best medical therapy such as DAPT.
Gary Lemmon, MD, is professor of vascular surgery at Indiana University, Indianapolis, and chief, vascular surgery, Indianapolis VA Medical Center. He reported no relevant conflicts.
References
1. J Vasc Surg. 2010;52:825-33
2. Eur Heart J. 2009;30:192-201
3. Lancet. 2009;373:1849-604. Chest. 2012;141:e669s-90s
5. J Vasc Surg. 2011;54: 779-84
6. J Vasc Surg. 2013;58: 1586-92
The continued use of perioperative clopidogrel is debatable!
There are cases in which clopidogrel should not be discontinued for a needed vascular intervention. Delaying operation or maintaining clopidogrel during operation if your patient required a recent coronary stent is warranted unless you are willing to accept an acute coronary thrombosis.
However, in other cases, for example infrainguinal grafts, the risk of potential increased bleeding when adding clopidogrel to aspirin may outweigh potential improvements in graft patency. This is especially true of below-knee vein bypass grafts where data do not support improved patency. However, in the CASPAR trial, prosthetic graft patency did appear to be beneficial, but only in subgroup analysis.1
It is true that severe bleeding was not increased (intracranial hemorrhage, or hemodynamic compromise: 1 vs 2.7%, P = NS) but moderate bleeding (transfusion required: 0.7 vs 3.7%, P = .012) and mild bleeding (5.4 vs 12.1%, P = .004) was increased when this agent was used especially in vein graft surgery. This risk of bleeding was present even when clopidogrel was begun 2 or more days after surgery.1
To complicate this decision, a Cochrane review did not consider subgroup analysis as statistically valid and so the authors considered infrainguinal graft patency as not improved with clopidogrel but bleeding risk was increased. One might even question the use of acetylsalicylic acid (ASA) for vein graft bypasses based on the results of this metanalysis.2 Carotid endarterectomy is a common vascular surgery procedure in which antiplatelet use has been evaluated in the real-world situation and with large cohorts. As is always the case when dealing with patient issues, the addition of one agent does not tell the entire story and patient demographics can have a significant influence on the outcome. A report from the Vascular Quality Initiative (VQI) database controlled for patient differences by propensity matching with more than 4,500 patients in each of the two groups; ASA vs. ASA + clopidogrel; demonstrated that major bleeding, defined as return to the OR for bleeding, was statistically more common with dual therapy (1.3% vs. 0.7%, P = .004).3
The addition of clopidogrel did statistically decrease the risk of ipsilateral TIA or stroke (0.8% vs. 1.2%, P = .02) but not the risk of death (0.2% vs. 0.3%, P = .3) or postoperative MI (1% vs. 0.8%, P = .4). Reoperation for bleeding is not inconsequential since in patients requiring this intervention, there is a significantly worse outcome in regard to stroke (3.7% vs. 0.8%, P = .001), MI (6.2% vs. 0.8%, P = .001), and death (2.5% vs. 0.2%,P = .001). Further drill down involving propensity score–matched analysis stratified by symptom status (asymptomatic vs. symptomatic) was quite interesting in that in only asymptomatic patients did the addition of clopidogrel actually demonstrate a statistically significant reduction in TIA or stroke, any stroke, or composite stroke/death. Symptomatic patients taking dual therapy demonstrated a slight reduction in TIA or stroke (1.4% vs. 1.7%, P = .6), any stroke (1.1% vs. 1.2%, P = .9) and composite stroke/death (1.2% vs. 1.5%, P = .5) but in no instance was statistical significance reached. The use of protamine did help to decrease the risk of bleeding.
Regarding the use of dual therapy during open aortic operations, an earlier report of the VQI database demonstrated no significant difference in bleeding risk statistically, but if one delves deeper the data indicate something different. In the majority of cases, vascular surgeons do not feel comfortable preforming this extensive dissection on dual therapy. Of the cases reported, 1,074 were preformed either free of either drug or only on ASA while 42 were on dual therapy and only 12 on clopidogrel only. In fact, in the conclusions, the authors note that they do not believe that conclusions regarding clopidogrel use in patient undergoing open abdominal aortic aneurysm repair can be drawn based on their results since the potential for a type II error was too great.4
It may be that our current level of sophistication is not sufficiently mature to determine the actual effect that clopidogrel is having on our patients. Clopidogrel, a thienopyridine, inhibits platelet activation by blocking the ADP-binding site for the P2Y12 receptor. Over 85% of ingested drug is metabolized into inactive metabolites while 15% is metabolized by the liver via a two-step oxidative process into the active thiol metabolite. Inter-individual variability in the antiplatelet response to thienopyridines is noted and partially caused by genetic mutations in the CP isoenzymes. Platelet reactivity testing is possible but most of the work has been conducted for those patients requiring coronary artery revascularization. Results of tailoring intervention to maximize therapeutic benefit and decrease the risk of bleeding have been inconsistent but, in some studies, appear to be promising.5 This approach may ultimately be found superior to determining how effective clopidogrel actually is in a particular case with some insight into the bleeding risk as well. With this determination, whether or not to hold clopidogrel perioperatively can be made with some science behind the decision.
Clearly, a blanket statement that the risk of bleeding should be accepted or ignored because of the demonstrated benefits of clopidogrel in patients requiring vascular surgery is not accurate. In some cases, there is no clear benefit, so eliminating the bleeding risk may well be the appropriate decision. The astute vascular surgeon understands the details of the written word in order to make an educated decision and understands that new information such as determining platelet reactivity may provide more clarity to such decisions in the future.
Michael C. Dalsing, MD, is chief of vascular surgery at Indiana University, Indianapolis. He reported no relevant conflicts.
References
1. J Vasc Surg. 2010;52:825-33
2. Cochrane Database Syst Rev. 2015, Issue 2. Art. No.: CD000535
The new NOACs are generally the best bet
New NOACs have largely replaced the need for vitamin K antagonists
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation that Campbell and Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs) began. Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice. Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared with VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs. Additionally, NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low molecular weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities which may influence drug effect and clearance.
Lastly, it should be mentioned that the pharmacologic benefits of NOACs are not only beneficial from a patient perspective, but also from a health care systems standpoint as their use may provide an opportunity to deliver more cost-effective care. Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared with warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that, at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs versus VKAs or placebos for the management of nonvalvular atrial fibrillation (AF), venous thromboembolism (VTE), and as adjunctive therapy for patients with acute coronary syndrome.6 Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation where NOACs have significant reductions in stroke, intracranial hemorrhage, and all-cause mortality, compared with warfarin while displaying variable effects with regards to gastrointestinal bleeding.6,7
In patients with VTE, NOACs have been found to have similar efficacy, compared with VKAs, with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6 Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, it should be noted that NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon the class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life-threatening bleeding complications. Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monocolonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently in phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9 Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable, compared with VKAs, and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusions
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the health care system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Madhukar S. Patel, MD, and Elliot L. Chaikof, MD, are from the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported having no conflicts of interest.
References
1. J Am Vet Med Assoc 1924;64:553-575
3. Hematology Am Soc Hematol Educ Program 2013;2013:464-470
4. Eur Heart J 2013;34:2094-2106
6. Nat Rev Cardiol 2014;11:693-703
8. N Engl J Med 2015;373:511-520
9. N Engl J Med 2014;371:2141-2142
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
Recently, several new oral anticoagulants (NOACs) have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once or twice daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute VTE and AF.
Dabigatran and edoxaban
Similar to warfarin, dabigatran and edoxaban require the use of a LMWH or UFH “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent which can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke, compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique amongst the NOACs, have been tested for extended therapy of acute deep vein thrombosis after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding, compared with placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding, compared with warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels or reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects. With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published the times when it might be useful to obtain levels. These times include:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.7
Currently, there exists no commercially available reversal agent for any of the NOACs, and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials are lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
Currently there are no national guidelines or large scale studies to guide bridging NOACs for procedures.
The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double-edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important in order to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that despite all the benefits, they also each carry a risk of bleeding as they all target portions of the coagulation mechanism. We caution that, as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Thomas Wakefield, MD, is the Stanley Professor of Vascular Surgery; head, section of vascular surgery; and director, Samuel and Jean Frankel Cardiovascular Center. Andrea Obi, MD, is a vascular surgery fellow and Dawn Coleman MD, is the program director, section of vascular surgery, all at the University of Michigan, Ann Arbor. They reported having no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-2352
2. J Vasc Surg: Venous and Lymphatic Disorders. 2013;1:418-426
3. N Engl J Med 2013;369:1406-1415
4. N Engl J Med 2010;363:2499-2510
5. N Engl J Med 2013;368:699-708
6. Arteriosclerosis, thrombosis, and vascular biology 2015;35:1056-1065
7. J Thrombosis and Haemostasis 2013;11:756-760
New NOACs have largely replaced the need for vitamin K antagonists
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation that Campbell and Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs) began. Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice. Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared with VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs. Additionally, NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low molecular weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities which may influence drug effect and clearance.
Lastly, it should be mentioned that the pharmacologic benefits of NOACs are not only beneficial from a patient perspective, but also from a health care systems standpoint as their use may provide an opportunity to deliver more cost-effective care. Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared with warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that, at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs versus VKAs or placebos for the management of nonvalvular atrial fibrillation (AF), venous thromboembolism (VTE), and as adjunctive therapy for patients with acute coronary syndrome.6 Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation where NOACs have significant reductions in stroke, intracranial hemorrhage, and all-cause mortality, compared with warfarin while displaying variable effects with regards to gastrointestinal bleeding.6,7
In patients with VTE, NOACs have been found to have similar efficacy, compared with VKAs, with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6 Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, it should be noted that NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon the class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life-threatening bleeding complications. Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monocolonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently in phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9 Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable, compared with VKAs, and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusions
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the health care system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Madhukar S. Patel, MD, and Elliot L. Chaikof, MD, are from the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported having no conflicts of interest.
References
1. J Am Vet Med Assoc 1924;64:553-575
3. Hematology Am Soc Hematol Educ Program 2013;2013:464-470
4. Eur Heart J 2013;34:2094-2106
6. Nat Rev Cardiol 2014;11:693-703
8. N Engl J Med 2015;373:511-520
9. N Engl J Med 2014;371:2141-2142
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
Recently, several new oral anticoagulants (NOACs) have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once or twice daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute VTE and AF.
Dabigatran and edoxaban
Similar to warfarin, dabigatran and edoxaban require the use of a LMWH or UFH “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent which can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke, compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique amongst the NOACs, have been tested for extended therapy of acute deep vein thrombosis after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding, compared with placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding, compared with warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels or reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects. With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published the times when it might be useful to obtain levels. These times include:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.7
Currently, there exists no commercially available reversal agent for any of the NOACs, and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials are lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
Currently there are no national guidelines or large scale studies to guide bridging NOACs for procedures.
The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double-edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important in order to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that despite all the benefits, they also each carry a risk of bleeding as they all target portions of the coagulation mechanism. We caution that, as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Thomas Wakefield, MD, is the Stanley Professor of Vascular Surgery; head, section of vascular surgery; and director, Samuel and Jean Frankel Cardiovascular Center. Andrea Obi, MD, is a vascular surgery fellow and Dawn Coleman MD, is the program director, section of vascular surgery, all at the University of Michigan, Ann Arbor. They reported having no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-2352
2. J Vasc Surg: Venous and Lymphatic Disorders. 2013;1:418-426
3. N Engl J Med 2013;369:1406-1415
4. N Engl J Med 2010;363:2499-2510
5. N Engl J Med 2013;368:699-708
6. Arteriosclerosis, thrombosis, and vascular biology 2015;35:1056-1065
7. J Thrombosis and Haemostasis 2013;11:756-760
New NOACs have largely replaced the need for vitamin K antagonists
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation that Campbell and Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs) began. Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice. Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared with VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs. Additionally, NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low molecular weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities which may influence drug effect and clearance.
Lastly, it should be mentioned that the pharmacologic benefits of NOACs are not only beneficial from a patient perspective, but also from a health care systems standpoint as their use may provide an opportunity to deliver more cost-effective care. Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared with warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that, at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs versus VKAs or placebos for the management of nonvalvular atrial fibrillation (AF), venous thromboembolism (VTE), and as adjunctive therapy for patients with acute coronary syndrome.6 Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation where NOACs have significant reductions in stroke, intracranial hemorrhage, and all-cause mortality, compared with warfarin while displaying variable effects with regards to gastrointestinal bleeding.6,7
In patients with VTE, NOACs have been found to have similar efficacy, compared with VKAs, with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6 Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, it should be noted that NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon the class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life-threatening bleeding complications. Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monocolonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently in phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9 Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable, compared with VKAs, and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusions
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the health care system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Madhukar S. Patel, MD, and Elliot L. Chaikof, MD, are from the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported having no conflicts of interest.
References
1. J Am Vet Med Assoc 1924;64:553-575
3. Hematology Am Soc Hematol Educ Program 2013;2013:464-470
4. Eur Heart J 2013;34:2094-2106
6. Nat Rev Cardiol 2014;11:693-703
8. N Engl J Med 2015;373:511-520
9. N Engl J Med 2014;371:2141-2142
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
Recently, several new oral anticoagulants (NOACs) have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once or twice daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute VTE and AF.
Dabigatran and edoxaban
Similar to warfarin, dabigatran and edoxaban require the use of a LMWH or UFH “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent which can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke, compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique amongst the NOACs, have been tested for extended therapy of acute deep vein thrombosis after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding, compared with placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding, compared with warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels or reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects. With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published the times when it might be useful to obtain levels. These times include:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.7
Currently, there exists no commercially available reversal agent for any of the NOACs, and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials are lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
Currently there are no national guidelines or large scale studies to guide bridging NOACs for procedures.
The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double-edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important in order to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that despite all the benefits, they also each carry a risk of bleeding as they all target portions of the coagulation mechanism. We caution that, as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Thomas Wakefield, MD, is the Stanley Professor of Vascular Surgery; head, section of vascular surgery; and director, Samuel and Jean Frankel Cardiovascular Center. Andrea Obi, MD, is a vascular surgery fellow and Dawn Coleman MD, is the program director, section of vascular surgery, all at the University of Michigan, Ann Arbor. They reported having no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-2352
2. J Vasc Surg: Venous and Lymphatic Disorders. 2013;1:418-426
3. N Engl J Med 2013;369:1406-1415
4. N Engl J Med 2010;363:2499-2510
5. N Engl J Med 2013;368:699-708
6. Arteriosclerosis, thrombosis, and vascular biology 2015;35:1056-1065
7. J Thrombosis and Haemostasis 2013;11:756-760
Commentary: INR instability in the NOAC era
Progress in the development of new oral anticoagulants (NOACs), as well as agents for their reversal, has lowered the threshold to use these therapeutics as first line agents for the management of nonvalvular atrial fibrillation and venous thromboembolism.1,2 Despite this increase in adoption, however, debate persists as to whether patients chronically maintained on vitamin K antagonists (VKAs), such as warfarin, should be switched to NOACs. The recently published research letter by Pokorney et al. assessed the stability of international normalized ratios (INRs) in patients on long-term warfarin therapy in order to address this question.3
Specifically, prospective registry data from 3,749 patients with at least three INR values in the first 6 months of therapy as well as six or more in the following year were included. Patients were deemed stable if 80% or more of their INRs were in a therapeutic range defined as an INR between 2 and 3.3 During the initiation period, only one in four patients taking warfarin had a stable INR.3 Furthermore, stability in the first 6 months was found to have limited ability to predict stability in the subsequent year (concordance index of 0.61). With regard to time in therapeutic range (TTR), only 32% of patients had a TTR of greater than 80% during the first 6 months with less than half (42%) of these patients able to maintain this in the following year.
Findings from Pokorney et al. add to the growing body of literature demonstrating the difficulty of achieving and maintaining a therapeutic INR while on warfarin therapy.4-7 Clinically, these findings are important, as deviations from TTR have been shown to be associated with increased risk of bleeding and thrombosis as well as increased health care costs.8-10 Mechanistically, patient factors such as differences in vitamin K consumption, comorbid conditions, drug-drug interactions, and medication compliance, as well as genetic differences that impact drug metabolism undoubtedly contribute to the variation of INR noted in patients on warfarin therapy.
Attempts to improve stability have included the administration of low-dose oral vitamin K. However, recent data from a multicenter randomized control trial suggests that while such therapy may help to decrease extreme variations in INR, it does not lead to an increased TTR.11 Furthermore, while significant work has been conducted in identifying specific gene variants, such as CYP2C9 and VKORC, which encode cytochrome P450 and vitamin K epoxide reductase enzymes, respectively, economic analyses suggest that testing for these gene variants would not be cost-effective.12 Additionally, clinical prediction tools, which incorporate important patient factors to help guide anticoagulation explain less than 10% of TTR variability.4
Nonetheless, some caution is warranted in the interpretation of the results reported by Pokorney and his colleagues. The proportion of registry patients treated with warfarin who had a low TTR was much lower than that previously reported by the pivotal U.S. trials of NOACs (55%-68%) and significantly lower than the results of a recent nationwide Swedish registry involving 40,449 patients.13
In the Swedish registry, the mean individual TTR was 70% with more than half the patients having a TTR of 70% or more, emphasizing the importance of health care system effects. Moreover, regardless of whether a patient is on warfarin or a NOAC, patients with a lower TTR have higher rates of diabetes, chronic obstructive pulmonary disease, heart failure, and renal failure, which may contribute to the need for additional therapies that may influence TTR.
For example, INR may be increased by ciprofloxacin or omeprazole when taken with warfarin, and CYP3A4 and P-glycoprotein (P-gp) inducers and inhibitors can result in an increased or decreased anticoagulation effect when used with NOACs. Recent reports have also highlighted variability in the safety of NOACs, particularly among patients with renal or liver insufficiency, African Americans, or patients with a prior history of GI bleeding.14-16 For these subgroups, determining NOAC activity to improve clinical safety of these agents is difficult.
PT or INR testing is largely insensitive or otherwise highly variable and the blood draw time relative to the most recent dose significantly influences the measured level of anti-Xa activity. Importantly, socioeconomic factors and family support systems also influence TTR, as important determinants of access to needed drugs or the ability to sustain related costs over time.
Taken together, prior INR stability on warfarin therapy does not ensure continued stability and, as a consequence, long-term warfarin therapy requires close monitoring in order to remain effective. To this end, further development of point-of-care coagulometers for self-testing and self-management, which have been found to be acceptable and preferred by patients, should be pursued.17 Similarly, attempts to decrease INR variability through research on optimizing computer assisted dosing programs remains warranted.18 NOACs offer an advantage over warfarin therapy in that they have a more predictable pharmacokinetic profile, which precludes the need for routine monitoring of anticoagulation parameters. However, many of the same factors, which influence TTR for warfarin do so for NOACs; NOACs have increased bleeding risk in comparison to warfarin for a number of demographic groups; and the high cost of NOACs may influence patient compliance.
Accordingly, until further data is available, consideration of the conversion of a patient on warfarin with a low TTR to a NOAC should be individualized.
Madhukar S. Patel, MD, is a general surgeon at the Department of Surgery, Massachusetts General Hospital, Boston, and Elliot L. Chaikof, MD, is Surgeon-in-Chief, Beth Israel Deaconess Medical Center, and Chairman, Roberta and Stephen R. Weiner Department of Surgery, Johnson and Johnson Professor of Surgery, Harvard Medical School. Dr. Chaikof is also an associate editor for Vascular Specialist. They have no relevant conflicts.
References
2. Nat Rev Cardiol. 2014;11:693-703.
5. J Thromb Haemost. 2010;8:2182-91.
6. Thromb Haemost. 2009;101:552-6.
7. Am J Cardiovasc Drugs. 2015;15:205-11.
8. Circ Cardiovasc Qual Outcomes. 2008;1:84-91.
10. J Med Econ. 2015;18:333-40.
11. Thromb Haemost. 2016;116:480-5.
12. Ann Intern Med. 2009;150:73-83.
13. JAMA Cardiol. 2016;1:172-80.
14. N Engl J Med. 2013;369:2093-104.
15. JAMA Intern Med. 2015;175:18-24.
16. J Am Coll Cardiol. 2014;63:891-900.
Progress in the development of new oral anticoagulants (NOACs), as well as agents for their reversal, has lowered the threshold to use these therapeutics as first line agents for the management of nonvalvular atrial fibrillation and venous thromboembolism.1,2 Despite this increase in adoption, however, debate persists as to whether patients chronically maintained on vitamin K antagonists (VKAs), such as warfarin, should be switched to NOACs. The recently published research letter by Pokorney et al. assessed the stability of international normalized ratios (INRs) in patients on long-term warfarin therapy in order to address this question.3
Specifically, prospective registry data from 3,749 patients with at least three INR values in the first 6 months of therapy as well as six or more in the following year were included. Patients were deemed stable if 80% or more of their INRs were in a therapeutic range defined as an INR between 2 and 3.3 During the initiation period, only one in four patients taking warfarin had a stable INR.3 Furthermore, stability in the first 6 months was found to have limited ability to predict stability in the subsequent year (concordance index of 0.61). With regard to time in therapeutic range (TTR), only 32% of patients had a TTR of greater than 80% during the first 6 months with less than half (42%) of these patients able to maintain this in the following year.
Findings from Pokorney et al. add to the growing body of literature demonstrating the difficulty of achieving and maintaining a therapeutic INR while on warfarin therapy.4-7 Clinically, these findings are important, as deviations from TTR have been shown to be associated with increased risk of bleeding and thrombosis as well as increased health care costs.8-10 Mechanistically, patient factors such as differences in vitamin K consumption, comorbid conditions, drug-drug interactions, and medication compliance, as well as genetic differences that impact drug metabolism undoubtedly contribute to the variation of INR noted in patients on warfarin therapy.
Attempts to improve stability have included the administration of low-dose oral vitamin K. However, recent data from a multicenter randomized control trial suggests that while such therapy may help to decrease extreme variations in INR, it does not lead to an increased TTR.11 Furthermore, while significant work has been conducted in identifying specific gene variants, such as CYP2C9 and VKORC, which encode cytochrome P450 and vitamin K epoxide reductase enzymes, respectively, economic analyses suggest that testing for these gene variants would not be cost-effective.12 Additionally, clinical prediction tools, which incorporate important patient factors to help guide anticoagulation explain less than 10% of TTR variability.4
Nonetheless, some caution is warranted in the interpretation of the results reported by Pokorney and his colleagues. The proportion of registry patients treated with warfarin who had a low TTR was much lower than that previously reported by the pivotal U.S. trials of NOACs (55%-68%) and significantly lower than the results of a recent nationwide Swedish registry involving 40,449 patients.13
In the Swedish registry, the mean individual TTR was 70% with more than half the patients having a TTR of 70% or more, emphasizing the importance of health care system effects. Moreover, regardless of whether a patient is on warfarin or a NOAC, patients with a lower TTR have higher rates of diabetes, chronic obstructive pulmonary disease, heart failure, and renal failure, which may contribute to the need for additional therapies that may influence TTR.
For example, INR may be increased by ciprofloxacin or omeprazole when taken with warfarin, and CYP3A4 and P-glycoprotein (P-gp) inducers and inhibitors can result in an increased or decreased anticoagulation effect when used with NOACs. Recent reports have also highlighted variability in the safety of NOACs, particularly among patients with renal or liver insufficiency, African Americans, or patients with a prior history of GI bleeding.14-16 For these subgroups, determining NOAC activity to improve clinical safety of these agents is difficult.
PT or INR testing is largely insensitive or otherwise highly variable and the blood draw time relative to the most recent dose significantly influences the measured level of anti-Xa activity. Importantly, socioeconomic factors and family support systems also influence TTR, as important determinants of access to needed drugs or the ability to sustain related costs over time.
Taken together, prior INR stability on warfarin therapy does not ensure continued stability and, as a consequence, long-term warfarin therapy requires close monitoring in order to remain effective. To this end, further development of point-of-care coagulometers for self-testing and self-management, which have been found to be acceptable and preferred by patients, should be pursued.17 Similarly, attempts to decrease INR variability through research on optimizing computer assisted dosing programs remains warranted.18 NOACs offer an advantage over warfarin therapy in that they have a more predictable pharmacokinetic profile, which precludes the need for routine monitoring of anticoagulation parameters. However, many of the same factors, which influence TTR for warfarin do so for NOACs; NOACs have increased bleeding risk in comparison to warfarin for a number of demographic groups; and the high cost of NOACs may influence patient compliance.
Accordingly, until further data is available, consideration of the conversion of a patient on warfarin with a low TTR to a NOAC should be individualized.
Madhukar S. Patel, MD, is a general surgeon at the Department of Surgery, Massachusetts General Hospital, Boston, and Elliot L. Chaikof, MD, is Surgeon-in-Chief, Beth Israel Deaconess Medical Center, and Chairman, Roberta and Stephen R. Weiner Department of Surgery, Johnson and Johnson Professor of Surgery, Harvard Medical School. Dr. Chaikof is also an associate editor for Vascular Specialist. They have no relevant conflicts.
References
2. Nat Rev Cardiol. 2014;11:693-703.
5. J Thromb Haemost. 2010;8:2182-91.
6. Thromb Haemost. 2009;101:552-6.
7. Am J Cardiovasc Drugs. 2015;15:205-11.
8. Circ Cardiovasc Qual Outcomes. 2008;1:84-91.
10. J Med Econ. 2015;18:333-40.
11. Thromb Haemost. 2016;116:480-5.
12. Ann Intern Med. 2009;150:73-83.
13. JAMA Cardiol. 2016;1:172-80.
14. N Engl J Med. 2013;369:2093-104.
15. JAMA Intern Med. 2015;175:18-24.
16. J Am Coll Cardiol. 2014;63:891-900.
Progress in the development of new oral anticoagulants (NOACs), as well as agents for their reversal, has lowered the threshold to use these therapeutics as first line agents for the management of nonvalvular atrial fibrillation and venous thromboembolism.1,2 Despite this increase in adoption, however, debate persists as to whether patients chronically maintained on vitamin K antagonists (VKAs), such as warfarin, should be switched to NOACs. The recently published research letter by Pokorney et al. assessed the stability of international normalized ratios (INRs) in patients on long-term warfarin therapy in order to address this question.3
Specifically, prospective registry data from 3,749 patients with at least three INR values in the first 6 months of therapy as well as six or more in the following year were included. Patients were deemed stable if 80% or more of their INRs were in a therapeutic range defined as an INR between 2 and 3.3 During the initiation period, only one in four patients taking warfarin had a stable INR.3 Furthermore, stability in the first 6 months was found to have limited ability to predict stability in the subsequent year (concordance index of 0.61). With regard to time in therapeutic range (TTR), only 32% of patients had a TTR of greater than 80% during the first 6 months with less than half (42%) of these patients able to maintain this in the following year.
Findings from Pokorney et al. add to the growing body of literature demonstrating the difficulty of achieving and maintaining a therapeutic INR while on warfarin therapy.4-7 Clinically, these findings are important, as deviations from TTR have been shown to be associated with increased risk of bleeding and thrombosis as well as increased health care costs.8-10 Mechanistically, patient factors such as differences in vitamin K consumption, comorbid conditions, drug-drug interactions, and medication compliance, as well as genetic differences that impact drug metabolism undoubtedly contribute to the variation of INR noted in patients on warfarin therapy.
Attempts to improve stability have included the administration of low-dose oral vitamin K. However, recent data from a multicenter randomized control trial suggests that while such therapy may help to decrease extreme variations in INR, it does not lead to an increased TTR.11 Furthermore, while significant work has been conducted in identifying specific gene variants, such as CYP2C9 and VKORC, which encode cytochrome P450 and vitamin K epoxide reductase enzymes, respectively, economic analyses suggest that testing for these gene variants would not be cost-effective.12 Additionally, clinical prediction tools, which incorporate important patient factors to help guide anticoagulation explain less than 10% of TTR variability.4
Nonetheless, some caution is warranted in the interpretation of the results reported by Pokorney and his colleagues. The proportion of registry patients treated with warfarin who had a low TTR was much lower than that previously reported by the pivotal U.S. trials of NOACs (55%-68%) and significantly lower than the results of a recent nationwide Swedish registry involving 40,449 patients.13
In the Swedish registry, the mean individual TTR was 70% with more than half the patients having a TTR of 70% or more, emphasizing the importance of health care system effects. Moreover, regardless of whether a patient is on warfarin or a NOAC, patients with a lower TTR have higher rates of diabetes, chronic obstructive pulmonary disease, heart failure, and renal failure, which may contribute to the need for additional therapies that may influence TTR.
For example, INR may be increased by ciprofloxacin or omeprazole when taken with warfarin, and CYP3A4 and P-glycoprotein (P-gp) inducers and inhibitors can result in an increased or decreased anticoagulation effect when used with NOACs. Recent reports have also highlighted variability in the safety of NOACs, particularly among patients with renal or liver insufficiency, African Americans, or patients with a prior history of GI bleeding.14-16 For these subgroups, determining NOAC activity to improve clinical safety of these agents is difficult.
PT or INR testing is largely insensitive or otherwise highly variable and the blood draw time relative to the most recent dose significantly influences the measured level of anti-Xa activity. Importantly, socioeconomic factors and family support systems also influence TTR, as important determinants of access to needed drugs or the ability to sustain related costs over time.
Taken together, prior INR stability on warfarin therapy does not ensure continued stability and, as a consequence, long-term warfarin therapy requires close monitoring in order to remain effective. To this end, further development of point-of-care coagulometers for self-testing and self-management, which have been found to be acceptable and preferred by patients, should be pursued.17 Similarly, attempts to decrease INR variability through research on optimizing computer assisted dosing programs remains warranted.18 NOACs offer an advantage over warfarin therapy in that they have a more predictable pharmacokinetic profile, which precludes the need for routine monitoring of anticoagulation parameters. However, many of the same factors, which influence TTR for warfarin do so for NOACs; NOACs have increased bleeding risk in comparison to warfarin for a number of demographic groups; and the high cost of NOACs may influence patient compliance.
Accordingly, until further data is available, consideration of the conversion of a patient on warfarin with a low TTR to a NOAC should be individualized.
Madhukar S. Patel, MD, is a general surgeon at the Department of Surgery, Massachusetts General Hospital, Boston, and Elliot L. Chaikof, MD, is Surgeon-in-Chief, Beth Israel Deaconess Medical Center, and Chairman, Roberta and Stephen R. Weiner Department of Surgery, Johnson and Johnson Professor of Surgery, Harvard Medical School. Dr. Chaikof is also an associate editor for Vascular Specialist. They have no relevant conflicts.
References
2. Nat Rev Cardiol. 2014;11:693-703.
5. J Thromb Haemost. 2010;8:2182-91.
6. Thromb Haemost. 2009;101:552-6.
7. Am J Cardiovasc Drugs. 2015;15:205-11.
8. Circ Cardiovasc Qual Outcomes. 2008;1:84-91.
10. J Med Econ. 2015;18:333-40.
11. Thromb Haemost. 2016;116:480-5.
12. Ann Intern Med. 2009;150:73-83.
13. JAMA Cardiol. 2016;1:172-80.
14. N Engl J Med. 2013;369:2093-104.
15. JAMA Intern Med. 2015;175:18-24.
16. J Am Coll Cardiol. 2014;63:891-900.
Vascular surgeons assisting nonvascular colleagues require depth/breadth of experience
Vascular surgeons called upon to provide intraoperative assistance should be prepared to undertake a wide range of repairs.
Nonvascular surgery patients who required any vascular repair had a higher incidence of the primary endpoint of death, myocardial infarction, or unplanned return to the operating room within 30 days post surgery. In addition, such cases accounted for almost 7% of the operative volume of the hospital’s vascular surgery service, according to the results of a retrospective record review of all 533 patients who underwent nonvascular surgery requiring intraoperative assistance by a vascular surgeon at Northwestern Memorial Hospital, Chicago, between January 2010 and June 2014.
After excluding 28 trauma patients and 226 who required placement of an inferior vena cava filter only, the remaining cohort of 299 patients were assessed. This cohort represented 6.9% of the entire operative output of the vascular surgery service at the hospital during the period assessed. The cohort comprised 49.5% men and a had mean patient age of 56.4 years, according to Tadaki M. Tomita, MD, and his colleagues at Northwestern University, Chicago.
Intraoperative assistance was requested by 12 different surgical subspecialties during the period studied, with the most common being neurosurgery (33.8%), orthopedic surgery (26.4%), urology (15.7%), and surgical oncology (6.7%). The major vascular surgeon participation by indications were spine exposure (52%), vascular reconstruction (19%), vascular control without hemorrhage (14.4%), and control of hemorrhage (14.4%), according to a report published online in JAMA Surgery (2016 Aug 3. doi: 10.1001/jamasurg.2016.2247).
For the entire cohort, 110 patients (36.8%) required vascular repairs, with 13 bypasses (4.4%), 18 patch angioplasties (6.0%), and 79 primary repairs (26.4%) performed; 64 cases were venous (21.4%) and 43 arterial (14.7%). The anatomic distribution in patients requiring vascular repair was 72.7% truncal and 27.4% peripheral.
Patients who required any vascular repair had a significantly higher incidence of the primary endpoint than did patients who did not require vascular repair (17.4% vs. 7.9%; P = .01), with five deaths, 16 MIs, and 20 unplanned returns to the OR.
“Vascular surgeons are often called on by nonvascular surgeons for assistance in the OR in a variety of clinical situations and anatomic locations,” the researchers stated. The vascular surgeon in all cases performed an open surgical exposure and open repair was performed in all cases that required vascular repair.
“While most consultations occurred preoperatively, a high proportion of emergent cases that are more likely to require vascular repair demonstrates the importance of having vascular surgeons immediately available at the hospital. To continue providing this valuable service, vascular trainees will need to continue to learn the full breadth of anatomic exposures and open vascular reconstructions,” the researchers concluded.
The authors reported that they had no disclosures.
Vascular surgeons called upon to provide intraoperative assistance should be prepared to undertake a wide range of repairs.
Nonvascular surgery patients who required any vascular repair had a higher incidence of the primary endpoint of death, myocardial infarction, or unplanned return to the operating room within 30 days post surgery. In addition, such cases accounted for almost 7% of the operative volume of the hospital’s vascular surgery service, according to the results of a retrospective record review of all 533 patients who underwent nonvascular surgery requiring intraoperative assistance by a vascular surgeon at Northwestern Memorial Hospital, Chicago, between January 2010 and June 2014.
After excluding 28 trauma patients and 226 who required placement of an inferior vena cava filter only, the remaining cohort of 299 patients were assessed. This cohort represented 6.9% of the entire operative output of the vascular surgery service at the hospital during the period assessed. The cohort comprised 49.5% men and a had mean patient age of 56.4 years, according to Tadaki M. Tomita, MD, and his colleagues at Northwestern University, Chicago.
Intraoperative assistance was requested by 12 different surgical subspecialties during the period studied, with the most common being neurosurgery (33.8%), orthopedic surgery (26.4%), urology (15.7%), and surgical oncology (6.7%). The major vascular surgeon participation by indications were spine exposure (52%), vascular reconstruction (19%), vascular control without hemorrhage (14.4%), and control of hemorrhage (14.4%), according to a report published online in JAMA Surgery (2016 Aug 3. doi: 10.1001/jamasurg.2016.2247).
For the entire cohort, 110 patients (36.8%) required vascular repairs, with 13 bypasses (4.4%), 18 patch angioplasties (6.0%), and 79 primary repairs (26.4%) performed; 64 cases were venous (21.4%) and 43 arterial (14.7%). The anatomic distribution in patients requiring vascular repair was 72.7% truncal and 27.4% peripheral.
Patients who required any vascular repair had a significantly higher incidence of the primary endpoint than did patients who did not require vascular repair (17.4% vs. 7.9%; P = .01), with five deaths, 16 MIs, and 20 unplanned returns to the OR.
“Vascular surgeons are often called on by nonvascular surgeons for assistance in the OR in a variety of clinical situations and anatomic locations,” the researchers stated. The vascular surgeon in all cases performed an open surgical exposure and open repair was performed in all cases that required vascular repair.
“While most consultations occurred preoperatively, a high proportion of emergent cases that are more likely to require vascular repair demonstrates the importance of having vascular surgeons immediately available at the hospital. To continue providing this valuable service, vascular trainees will need to continue to learn the full breadth of anatomic exposures and open vascular reconstructions,” the researchers concluded.
The authors reported that they had no disclosures.
Vascular surgeons called upon to provide intraoperative assistance should be prepared to undertake a wide range of repairs.
Nonvascular surgery patients who required any vascular repair had a higher incidence of the primary endpoint of death, myocardial infarction, or unplanned return to the operating room within 30 days post surgery. In addition, such cases accounted for almost 7% of the operative volume of the hospital’s vascular surgery service, according to the results of a retrospective record review of all 533 patients who underwent nonvascular surgery requiring intraoperative assistance by a vascular surgeon at Northwestern Memorial Hospital, Chicago, between January 2010 and June 2014.
After excluding 28 trauma patients and 226 who required placement of an inferior vena cava filter only, the remaining cohort of 299 patients were assessed. This cohort represented 6.9% of the entire operative output of the vascular surgery service at the hospital during the period assessed. The cohort comprised 49.5% men and a had mean patient age of 56.4 years, according to Tadaki M. Tomita, MD, and his colleagues at Northwestern University, Chicago.
Intraoperative assistance was requested by 12 different surgical subspecialties during the period studied, with the most common being neurosurgery (33.8%), orthopedic surgery (26.4%), urology (15.7%), and surgical oncology (6.7%). The major vascular surgeon participation by indications were spine exposure (52%), vascular reconstruction (19%), vascular control without hemorrhage (14.4%), and control of hemorrhage (14.4%), according to a report published online in JAMA Surgery (2016 Aug 3. doi: 10.1001/jamasurg.2016.2247).
For the entire cohort, 110 patients (36.8%) required vascular repairs, with 13 bypasses (4.4%), 18 patch angioplasties (6.0%), and 79 primary repairs (26.4%) performed; 64 cases were venous (21.4%) and 43 arterial (14.7%). The anatomic distribution in patients requiring vascular repair was 72.7% truncal and 27.4% peripheral.
Patients who required any vascular repair had a significantly higher incidence of the primary endpoint than did patients who did not require vascular repair (17.4% vs. 7.9%; P = .01), with five deaths, 16 MIs, and 20 unplanned returns to the OR.
“Vascular surgeons are often called on by nonvascular surgeons for assistance in the OR in a variety of clinical situations and anatomic locations,” the researchers stated. The vascular surgeon in all cases performed an open surgical exposure and open repair was performed in all cases that required vascular repair.
“While most consultations occurred preoperatively, a high proportion of emergent cases that are more likely to require vascular repair demonstrates the importance of having vascular surgeons immediately available at the hospital. To continue providing this valuable service, vascular trainees will need to continue to learn the full breadth of anatomic exposures and open vascular reconstructions,” the researchers concluded.
The authors reported that they had no disclosures.
FROM JAMA SURGERY
Key clinical point: Intraoperative assistance of vascular surgeons in nonvascular procedures accounted for nearly 7% of vascular work at a single institution and uniformly required open repair.
Major finding: Patients who required any intraoperative vascular repair had a higher incidence of the primary endpoint of death, myocardial infarction, or unplanned return to the operating room within 30 days post surgery.
Data source: The study was a retrospective review of all 299 patients undergoing nonvascular surgery who required intraoperative vascular surgery assistance at a single institution between January 2010 and June 2014.
Disclosures: The authors reported that they had no disclosures.
NSQIP Study: Symptomatic AAAs have a twofold increased periop mortality risk over asymptomatic
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
A recent small study suggested that, in the age of endovascular aortic aneurysm repair (EVAR), the mortality rates between symptomatic and asymptomatic abdominal aortic aneurysm (AAA) repair have become similar, according to Peter A. Soden, MD, of Beth Deaconess Medical Center, Boston, and his colleagues. However, in their large database study, Dr. Soden and his colleagues found that outcomes for the repair of abdominal aortic aneurysms were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
The researchers assessed all patients undergoing endovascular and open AAA repair in the 2011-2013 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data set, according to a report published in the August issue of the Journal of Vascular Surgery.
Symptomatic AAA was defined as lack of evidence of rupture but with the presence of abdominal or back pain or symptoms from local compression by the aneurysm causing early satiety, hydronephrosis, or deep vein thrombosis. Ruptured aneurysms were divided into two categories: hypotensive (defined as systolic blood pressure less than 90 mmm Hg or drop of greater than 40 mm HG from baseline) and nonhypotensive (J Vasc Surg. 2016;64:297-305).
There were numerous demographic and comorbidity differences between asymptomatic and symptomatic patients and between symptomatic and ruptured patients, with a general trend of increasing of commodities and factors influencing operative risk.
The final study included 5,502 patients undergoing repair of infrarenal (85%; 92% EVAR) or juxtarenal (15%;20% EVAR) aneurysms. These differences in the use of EVAR were statistically significant.
This population comprised 4,495 asymptomatic patients (82% EVAR), 455 symptomatic patients (69% EVAR), and 552 ruptured patients (52% EVAR).
The overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (5.1% vs. 1.9%; P less than .001).Similarly, for EVAR, the overall 30-day mortality rate was significantly higher in symptomatic over asymptomatic patients (3.8% vs. 1.4%; P less than .001). For open repair, there was no significant difference in mortality (7.7% vs. 4.3%) between symptomatic and asymptomatic patients, respectively.
Multivariate analysis showed that symptomatic patients had twice the operative mortality as asymptomatic patients (odds ratio, 2.1). A symptomatic aneurysm was also predictive of a major adverse event (OR, 1.5). Ruptured aneurysms had a significant nearly sevenfold increase in mortality risk vs. symptomatic aneurysms (OR, 6.5) and a fivefold increase of risk of a major adverse event (OR 5.1), with all ORs within their 95% confidence interval levels).
“In this large contemporary study of symptomatic AAA patients, in which the majority were treated with EVAR, we found that symptomatic patients have twice the perioperative mortality compared with asymptomatic patients. Despite this, we also find a reduction in perioperative mortality for symptomatic aneurysms compared with prior reports in which the majority were treated with open repair, and we believe this supports an EVAR-first approach for symptomatic aneurysms with suitable anatomy,” the researchers concluded.
The authors reported that they had no relevant disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: Outcomes for repair of abdominal aortic aneurysm repair were increasingly worse from asymptomatic to symptomatic to ruptured AAA.
Major finding: Patients with symptomatic AAA had a twofold increased risk of perioperative mortality, compared with patients with asymptomatic AAA undergoing repair.
Data source: The study assessed all patients undergoing AAA repair in the 2011-2013 American College of Surgeons NSQIP data set.
Disclosures: The authors reported that they had no relevant disclosures.
Abdominal compartment syndrome – a common adverse event after rAAA repair
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The authors of this paper provide compelling data demonstrating the seriousness of abdominal compartment syndrome developing following abdominal aortic surgery. Recognition of this complication is therefore mandatory and techniques to relieve it should be instituted immediately. In my practice, I have successfully used the Wittmann patch, but recently I switched to the VAC (vacuum-assisted closure) device. Following an endovascular approach for a ruptured AAA, patients may require concomitant exploration for retrograde bleeding sources, such as an inferior mesenteric artery or large lumbars that will continue to bleed unless ligated.
Dr. Russell Samson is the medical editor of Vascular Specialist.
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
Abdominal compartment syndrome (ACS) was common after ruptured abdominal aortic aneurysm (AAA) repair, with a similar incidence for both open surgical and endovascular repair (EVAR), according to a report published in the European Journal of Vascular and Endovascular Surgery.
Samuel Ersryd, a doctoral student, and his colleagues at Uppsala (Sweden) University performed their study to determine the contemporary incidence, treatment, and outcomes of ACS after AAA repair.
The analysis included 6,634 patients in the Swedish vascular registry who were treated for abdominal aortic aneurysm repair at 31 institutions from May 2008 to December 2013. The mean patient age was 72.8 years, and 16.6% were women. There were 5,271 intact AAA (iAAA) repairs and 1,341 ruptured AAA (rAAA repairs). A total of 41.9% of iAAA repairs were open, as were 72.0% of the rAAA repairs (Eur J Vasc Endovasc Surg. 2016;52:158-65).The study found an incidence of ACS in the rAAA group of 6.8% after open surgery and 6.9% after EVAR.
The morbidity and mortality rates for iAAA and rAAA with ACS were “devastating” in both groups, according to the authors.
Mortality at 90 days for patients with ACS after rAAA was 58.7%, twice that of patients without ACS. In patients with iAAA repair with ACS, the 90-day mortality was 19.2%, six times higher than for those without ACS.
Prophylactic open abdomen treatment was performed in 10.7% of open-surgery patients.
The researchers found no differences in mortality among patients in either group that developed ACS, whether they were treated with decompression laparotomy or not.
Age, sex, and perioperative comorbidities were not associated with ACS, Mr. Ersryd and his associates said. Within the rAAA group, however, ACS was associated with the lowest measured preoperative blood pressure and with preoperative unconsciousness. In addition, ACS was more common in both the iAAA and rAAA groups after perioperative bleeding greater than 5 L, in the iAAA group after reimplantation of a renal artery, and in the rAAA group after the use of balloon occlusion after EVAR. In those patients operated on for iAAA, the risk of developing ACS was 8.1% in patients who had perioperative bleeding greater than 5 L, compared with only 0.8% if bleeding was less than 5 L (P less than .001).
“With such poor results among patients who developed ACS, prevention is the obvious key to success. Massive transfusion protocols and permissive hypotension in patients with ongoing bleeding are important, as well as being restrictive with crystalloids,” the authors said. In addition, they recommended a proactive strategy treating intra-abdominal hypertension with medical therapy, effective pain relief, and neuromuscular blockade as important preventive measures.
“ACS is associated with a devastating effect on outcome after surgery for both ruptured and intact AAA. There was no difference in outcome among those who developed ACS, depending on whether the primary treatment had been performed with an open or endovascular technique,” the researchers concluded.
The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
On Twitter @VascularTweets
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Abdominal compartment syndrome is a dangerous and frequent complication after treatment of ruptured abdominal aortic aneurysms.
Major finding: After ruptured AAA repair, ACS developed in 6.8% of patients with open repair and 6.9% of patients with EVAR.
Data source: Researchers performed a population-based study using the Swedish vascular registry and the Swedish national population registry.
Disclosures: The authors reported they had no conflicts of interest and the study was funded by the Swedish Research Council and Uppsala University.
Should patients with stable ischemic heart disease undergo revascularization?
The answer is less clear for these patients than for patients with acute coronary syndromes. In the latter group, percutaneous or surgical revascularization reduces the rates of morbidity and mortality, whereas in patients with stable ischemic heart disease, benefits may be limited to the improvement of angina. Certain markers and criteria may help us in this decision, and trials are ongoing.
Of importance, all patients with coronary artery disease should receive guideline-directed medical therapy as tolerated, regardless of whether they undergo revascularization.
MEDICAL THERAPY FOR ALL
In all the relevant trials, patients with stable ischemic heart disease in both the revascularization groups and the unrevascularized groups received guideline-directed medical therapy. Current guidelines1 give class I recommendations (ie, treatment should be given) for:
- Lipid management
- Blood pressure management
- Physical activity
- Weight management
- Smoking cessation
- Antiplatelet therapy
- Beta-blockers for patients with normal left ventricular function after an acute coronary syndrome event, and for those with an ejection fraction of 40% or less
- Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients who have hypertension, diabetes mellitus, a left ventricular ejection fraction of 40% or less, or chronic kidney disease
- Annual influenza vaccination
- Anti-ischemic medications (beta-blockers, calcium channel blockers, nitrates) for relief of symptoms.
REVASCULARIZATION FOR SOME?
Results of the studies outlined below will help in deciding when to use guideline-directed medical therapy alone or medical therapy plus revascularization.
COURAGE trial: No added benefit in patients at low risk
The findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE), published in 2007, suggested that in select patients, percutaneous coronary intervention for stable coronary artery disease was no better than guideline-directed medical therapy alone for reducing the outcomes of death, myocardial infarction, or hospitalization for acute coronary syndrome.2
Of note, however, is that the 2,287 patients included in COURAGE were a low-risk subset of the more than 35,000 patients initially evaluated. The investigators reviewed the patients’ coronary angiograms before enrollment, and thus many patients with complex or high-risk anatomy were likely excluded based on an a priori assessment of angiographic images.
Also, coronary stent technology has substantially improved since COURAGE (which primarily used bare-metal stents and early drug-eluting stents), and this brings into question whether the results are applicable to current patients.
Moreover, in subsequent substudies from COURAGE, revascularization significantly improved symptoms of angina and quality-of-life scores compared with medical therapy alone.3,4
Also important is that more than one-third of the patients in the medical therapy group crossed over to revascularization during the study, most often for worsening symptoms of angina.
Regardless of its limitations, COURAGE played an important role in delineating the use of guideline-directed medical therapy alone in certain low-risk patients and sparked debate about when and if to revascularize other patients.
BARI 2D trial: CABG may benefit those with diabetes
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, published in 2009, aimed to find out if revascularization in patients with stable ischemic heart disease and diabetes was beneficial compared with medical therapy alone.5
While it was not designed to directly compare percutaneous coronary intervention vs coronary artery bypass grafting (CABG), it did find that medical therapy plus CABG might reduce the rate of adverse cardiovascular events in this population compared with medical therapy alone or medical therapy plus percutaneous intervention.
As with COURAGE, however, the patients in the medical therapy group in BARI 2D also had a high rate of crossover to revascularization, primarily driven by worsening anginal symptoms.
FREEDOM and the 2014 updated guideline
Based on the findings of BARI 2D and those of FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease),6 the American College of Cardiology and American Heart Association updated their recommendations in 2014.7 This focused update states that for patients with diabetes and multivessel coronary artery disease, if revascularization is likely to improve survival (for example, in three-vessel disease or complex two-vessel disease involving the proximal left anterior descending artery), then CABG should be performed if a left internal mammary artery graft can be anastomosed to the left anterior descending artery. Otherwise, percutaneous coronary intervention should be reserved for those patients with diabetes and high-risk or complex multivessel coronary artery disease who are not good surgical candidates.
FAME 2 trial: Fractional flow reserve as a guide
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial,8 published in 2012, evaluated whether clinical outcomes differ between patients who undergo percutaneous revascularization plus medical therapy and those who are treated with medical therapy alone, using fractional flow reserve as a means to determine which stenoses should be considered for intervention. Fractional flow reserve performed during invasive angiography determines the ratio of intracoronary pressure to aortic pressure using a wire advanced across a coronary obstruction.
FAME 2 found a markedly lower incidence of the primary composite end point of death, myocardial infarction, and urgent revascularization with randomization to percutaneous revascularization plus medical therapy compared with medical therapy only (4.3% vs 12.7%, P = .001) in patients with a fractional flow reserve less than 0.80 (considered a hemodynamically significant obstruction). The trial was stopped early because of the markedly different outcomes.
Of note, however, the reduction in adverse clinical outcomes was driven primarily by a reduction in urgent revascularizations in those treated with percutaneous coronary intervention in the revascularization arm. Regardless, using fractional flow reserve to guide whether obstructive coronary lesions should be treated with percutaneous coronary intervention has appropriately become a mainstay in interventional cardiology.
Stress testing
Noninvasive stress testing has played a role in helping to guide revascularization decisions in stable ischemic heart disease. In particular, revascularization in the setting of greater than 10% ischemia on perfusion imaging has been associated with a lower risk of cardiac death than in those who were revascularized with an ischemic burden less than 10%.9
A substudy of COURAGE found that percutaneous coronary intervention reduced ischemia to a greater degree than medical therapy alone on serial nuclear stress tests in patients with stable ischemic heart disease.10 In this substudy, when both groups were combined, the investigators also found that there were fewer adverse events in those who had an overall reduction of ischemia regardless of treatment strategy.
ISCHEMIA: Revascularize those with ischemia?
While COURAGE, BARI 2D, and FAME 2 suggested that early revascularization for low-risk patients with coronary artery disease does not confer a benefit over medical treatment alone with regard to hard clinical end points, it remains unclear whether an early revascularization strategy is advantageous in patients with stable ischemic heart disease who have at least a moderate amount of ischemia on noninvasive stress testing.
The ongoing ISCHEMIA (International Study of Comparative Effectiveness With Medical and Invasive Approaches) trial will help to answer that question. In this study, 8,000 patients with stable angina and at least moderate ischemia on noninvasive stress testing are being randomized before coronary angiography either to guideline-directed medical therapy plus revascularization (percutaneous or surgical) or to medical therapy alone.11 The ISCHEMIA study population reflects current practice more closely than the previous studies discussed above in its inclusion of fractional flow reserve and later-generation drug-eluting stents.
The results of ISCHEMIA will be an important piece of the puzzle to answer whether patients with stable ischemic heart disease benefit from revascularization in terms of cardiovascular mortality or myocardial infarction (the primary end point of the study).
Studies in additional subsets
It is important to recognize that there are additional subsets of patients with stable ischemic heart disease (those with multivessel disease, left main coronary disease, or low ejection fractions, for example) who have been studied to help determine when and how to perform revascularization. In addition, there are guidelines12 for both interventional cardiologists and cardiac surgeons that help delineate which patients should undergo revascularization. While a complete review is beyond the scope of this discussion, three trials are worth mentioning:
The Coronary Artery Surgery Study (CASS)13 revealed that revascularization in left main coronary artery disease is associated with lower mortality rates than medical therapy alone. This study, along with others, eventually led to recommendations for revascularization to be performed in all patients with significant left main coronary disease, regardless of symptoms or stress test findings.14,15
The Surgical Treatment for Ischemic Heart Failure (STICH) trial16 found that patients with a low ejection fraction (< 35%) and ischemic heart disease had no difference in all-cause mortality rates when treated with CABG plus medical therapy compared with medical therapy alone (although the study’s design has been heavily criticized).
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) study17 found that CABG was associated with fewer adverse events in three-vessel coronary artery disease or complex left main coronary artery disease compared with percutaneous coronary intervention. The study used early-generation paclitaxel drug-eluting stents that are no longer used in contemporary practice. This study established the SYNTAX score, which is often used to help make revascularization decisions. A low SYNTAX score of 0 to 22 (meaning less-severe coronary artery disease) was associated with equivalent outcomes for both percutaneous coronary intervention and CABG. Thus, even if there is multivessel disease or left main disease, if the SYNTAX score is low, then percutaneous coronary intervention is an acceptable method for revascularization with similar results as for CABG.
A TEAM APPROACH
Due to the complexity of stable ischemic heart disease and the subtleties of managing these patients, a multidisciplinary “heart team” approach may be the best way to navigate treating stable ischemic heart disease via revascularization or with medical therapy alone. The heart team approach could take advantage of the particular expertise that the primary care physician, cardiologist, interventional cardiologist, and cardiac surgeon provide.
The upcoming results of studies such as the ISCHEMIA trial will help to provide additional guidance for these teams in long-term management of patients with stable ischemic heart disease.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012; 126:e354–e471.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Blankenship J, Marshall JJ, Pinto DS, et al; Society for Cardiovascular Angiography and Interventions. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81:243–249.
- BARI 2D Study Group; Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Farkouh ME, Domanski M, Sleep LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:1929–1949.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998; 97:535–543.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Stone GW, Hochman JS, Williams DO, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol 2016; 67:81–99.
- Patel M, Dehmer G, Hirshfeld J, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol 2012; 59:857–881.
- Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990; 82:1629–1646.
- Hillis L, Smith P, Anderson J, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine G, Bates E, Blankenship J, et al. 2011 ACCF/AHA guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Velazquez EJ, Lee KL, Deja MA, et al, for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364:1607–1616.
- Serruys PW, Morice M-C, Kappetein AP, et al, for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
The answer is less clear for these patients than for patients with acute coronary syndromes. In the latter group, percutaneous or surgical revascularization reduces the rates of morbidity and mortality, whereas in patients with stable ischemic heart disease, benefits may be limited to the improvement of angina. Certain markers and criteria may help us in this decision, and trials are ongoing.
Of importance, all patients with coronary artery disease should receive guideline-directed medical therapy as tolerated, regardless of whether they undergo revascularization.
MEDICAL THERAPY FOR ALL
In all the relevant trials, patients with stable ischemic heart disease in both the revascularization groups and the unrevascularized groups received guideline-directed medical therapy. Current guidelines1 give class I recommendations (ie, treatment should be given) for:
- Lipid management
- Blood pressure management
- Physical activity
- Weight management
- Smoking cessation
- Antiplatelet therapy
- Beta-blockers for patients with normal left ventricular function after an acute coronary syndrome event, and for those with an ejection fraction of 40% or less
- Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients who have hypertension, diabetes mellitus, a left ventricular ejection fraction of 40% or less, or chronic kidney disease
- Annual influenza vaccination
- Anti-ischemic medications (beta-blockers, calcium channel blockers, nitrates) for relief of symptoms.
REVASCULARIZATION FOR SOME?
Results of the studies outlined below will help in deciding when to use guideline-directed medical therapy alone or medical therapy plus revascularization.
COURAGE trial: No added benefit in patients at low risk
The findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE), published in 2007, suggested that in select patients, percutaneous coronary intervention for stable coronary artery disease was no better than guideline-directed medical therapy alone for reducing the outcomes of death, myocardial infarction, or hospitalization for acute coronary syndrome.2
Of note, however, is that the 2,287 patients included in COURAGE were a low-risk subset of the more than 35,000 patients initially evaluated. The investigators reviewed the patients’ coronary angiograms before enrollment, and thus many patients with complex or high-risk anatomy were likely excluded based on an a priori assessment of angiographic images.
Also, coronary stent technology has substantially improved since COURAGE (which primarily used bare-metal stents and early drug-eluting stents), and this brings into question whether the results are applicable to current patients.
Moreover, in subsequent substudies from COURAGE, revascularization significantly improved symptoms of angina and quality-of-life scores compared with medical therapy alone.3,4
Also important is that more than one-third of the patients in the medical therapy group crossed over to revascularization during the study, most often for worsening symptoms of angina.
Regardless of its limitations, COURAGE played an important role in delineating the use of guideline-directed medical therapy alone in certain low-risk patients and sparked debate about when and if to revascularize other patients.
BARI 2D trial: CABG may benefit those with diabetes
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, published in 2009, aimed to find out if revascularization in patients with stable ischemic heart disease and diabetes was beneficial compared with medical therapy alone.5
While it was not designed to directly compare percutaneous coronary intervention vs coronary artery bypass grafting (CABG), it did find that medical therapy plus CABG might reduce the rate of adverse cardiovascular events in this population compared with medical therapy alone or medical therapy plus percutaneous intervention.
As with COURAGE, however, the patients in the medical therapy group in BARI 2D also had a high rate of crossover to revascularization, primarily driven by worsening anginal symptoms.
FREEDOM and the 2014 updated guideline
Based on the findings of BARI 2D and those of FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease),6 the American College of Cardiology and American Heart Association updated their recommendations in 2014.7 This focused update states that for patients with diabetes and multivessel coronary artery disease, if revascularization is likely to improve survival (for example, in three-vessel disease or complex two-vessel disease involving the proximal left anterior descending artery), then CABG should be performed if a left internal mammary artery graft can be anastomosed to the left anterior descending artery. Otherwise, percutaneous coronary intervention should be reserved for those patients with diabetes and high-risk or complex multivessel coronary artery disease who are not good surgical candidates.
FAME 2 trial: Fractional flow reserve as a guide
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial,8 published in 2012, evaluated whether clinical outcomes differ between patients who undergo percutaneous revascularization plus medical therapy and those who are treated with medical therapy alone, using fractional flow reserve as a means to determine which stenoses should be considered for intervention. Fractional flow reserve performed during invasive angiography determines the ratio of intracoronary pressure to aortic pressure using a wire advanced across a coronary obstruction.
FAME 2 found a markedly lower incidence of the primary composite end point of death, myocardial infarction, and urgent revascularization with randomization to percutaneous revascularization plus medical therapy compared with medical therapy only (4.3% vs 12.7%, P = .001) in patients with a fractional flow reserve less than 0.80 (considered a hemodynamically significant obstruction). The trial was stopped early because of the markedly different outcomes.
Of note, however, the reduction in adverse clinical outcomes was driven primarily by a reduction in urgent revascularizations in those treated with percutaneous coronary intervention in the revascularization arm. Regardless, using fractional flow reserve to guide whether obstructive coronary lesions should be treated with percutaneous coronary intervention has appropriately become a mainstay in interventional cardiology.
Stress testing
Noninvasive stress testing has played a role in helping to guide revascularization decisions in stable ischemic heart disease. In particular, revascularization in the setting of greater than 10% ischemia on perfusion imaging has been associated with a lower risk of cardiac death than in those who were revascularized with an ischemic burden less than 10%.9
A substudy of COURAGE found that percutaneous coronary intervention reduced ischemia to a greater degree than medical therapy alone on serial nuclear stress tests in patients with stable ischemic heart disease.10 In this substudy, when both groups were combined, the investigators also found that there were fewer adverse events in those who had an overall reduction of ischemia regardless of treatment strategy.
ISCHEMIA: Revascularize those with ischemia?
While COURAGE, BARI 2D, and FAME 2 suggested that early revascularization for low-risk patients with coronary artery disease does not confer a benefit over medical treatment alone with regard to hard clinical end points, it remains unclear whether an early revascularization strategy is advantageous in patients with stable ischemic heart disease who have at least a moderate amount of ischemia on noninvasive stress testing.
The ongoing ISCHEMIA (International Study of Comparative Effectiveness With Medical and Invasive Approaches) trial will help to answer that question. In this study, 8,000 patients with stable angina and at least moderate ischemia on noninvasive stress testing are being randomized before coronary angiography either to guideline-directed medical therapy plus revascularization (percutaneous or surgical) or to medical therapy alone.11 The ISCHEMIA study population reflects current practice more closely than the previous studies discussed above in its inclusion of fractional flow reserve and later-generation drug-eluting stents.
The results of ISCHEMIA will be an important piece of the puzzle to answer whether patients with stable ischemic heart disease benefit from revascularization in terms of cardiovascular mortality or myocardial infarction (the primary end point of the study).
Studies in additional subsets
It is important to recognize that there are additional subsets of patients with stable ischemic heart disease (those with multivessel disease, left main coronary disease, or low ejection fractions, for example) who have been studied to help determine when and how to perform revascularization. In addition, there are guidelines12 for both interventional cardiologists and cardiac surgeons that help delineate which patients should undergo revascularization. While a complete review is beyond the scope of this discussion, three trials are worth mentioning:
The Coronary Artery Surgery Study (CASS)13 revealed that revascularization in left main coronary artery disease is associated with lower mortality rates than medical therapy alone. This study, along with others, eventually led to recommendations for revascularization to be performed in all patients with significant left main coronary disease, regardless of symptoms or stress test findings.14,15
The Surgical Treatment for Ischemic Heart Failure (STICH) trial16 found that patients with a low ejection fraction (< 35%) and ischemic heart disease had no difference in all-cause mortality rates when treated with CABG plus medical therapy compared with medical therapy alone (although the study’s design has been heavily criticized).
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) study17 found that CABG was associated with fewer adverse events in three-vessel coronary artery disease or complex left main coronary artery disease compared with percutaneous coronary intervention. The study used early-generation paclitaxel drug-eluting stents that are no longer used in contemporary practice. This study established the SYNTAX score, which is often used to help make revascularization decisions. A low SYNTAX score of 0 to 22 (meaning less-severe coronary artery disease) was associated with equivalent outcomes for both percutaneous coronary intervention and CABG. Thus, even if there is multivessel disease or left main disease, if the SYNTAX score is low, then percutaneous coronary intervention is an acceptable method for revascularization with similar results as for CABG.
A TEAM APPROACH
Due to the complexity of stable ischemic heart disease and the subtleties of managing these patients, a multidisciplinary “heart team” approach may be the best way to navigate treating stable ischemic heart disease via revascularization or with medical therapy alone. The heart team approach could take advantage of the particular expertise that the primary care physician, cardiologist, interventional cardiologist, and cardiac surgeon provide.
The upcoming results of studies such as the ISCHEMIA trial will help to provide additional guidance for these teams in long-term management of patients with stable ischemic heart disease.
The answer is less clear for these patients than for patients with acute coronary syndromes. In the latter group, percutaneous or surgical revascularization reduces the rates of morbidity and mortality, whereas in patients with stable ischemic heart disease, benefits may be limited to the improvement of angina. Certain markers and criteria may help us in this decision, and trials are ongoing.
Of importance, all patients with coronary artery disease should receive guideline-directed medical therapy as tolerated, regardless of whether they undergo revascularization.
MEDICAL THERAPY FOR ALL
In all the relevant trials, patients with stable ischemic heart disease in both the revascularization groups and the unrevascularized groups received guideline-directed medical therapy. Current guidelines1 give class I recommendations (ie, treatment should be given) for:
- Lipid management
- Blood pressure management
- Physical activity
- Weight management
- Smoking cessation
- Antiplatelet therapy
- Beta-blockers for patients with normal left ventricular function after an acute coronary syndrome event, and for those with an ejection fraction of 40% or less
- Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients who have hypertension, diabetes mellitus, a left ventricular ejection fraction of 40% or less, or chronic kidney disease
- Annual influenza vaccination
- Anti-ischemic medications (beta-blockers, calcium channel blockers, nitrates) for relief of symptoms.
REVASCULARIZATION FOR SOME?
Results of the studies outlined below will help in deciding when to use guideline-directed medical therapy alone or medical therapy plus revascularization.
COURAGE trial: No added benefit in patients at low risk
The findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE), published in 2007, suggested that in select patients, percutaneous coronary intervention for stable coronary artery disease was no better than guideline-directed medical therapy alone for reducing the outcomes of death, myocardial infarction, or hospitalization for acute coronary syndrome.2
Of note, however, is that the 2,287 patients included in COURAGE were a low-risk subset of the more than 35,000 patients initially evaluated. The investigators reviewed the patients’ coronary angiograms before enrollment, and thus many patients with complex or high-risk anatomy were likely excluded based on an a priori assessment of angiographic images.
Also, coronary stent technology has substantially improved since COURAGE (which primarily used bare-metal stents and early drug-eluting stents), and this brings into question whether the results are applicable to current patients.
Moreover, in subsequent substudies from COURAGE, revascularization significantly improved symptoms of angina and quality-of-life scores compared with medical therapy alone.3,4
Also important is that more than one-third of the patients in the medical therapy group crossed over to revascularization during the study, most often for worsening symptoms of angina.
Regardless of its limitations, COURAGE played an important role in delineating the use of guideline-directed medical therapy alone in certain low-risk patients and sparked debate about when and if to revascularize other patients.
BARI 2D trial: CABG may benefit those with diabetes
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, published in 2009, aimed to find out if revascularization in patients with stable ischemic heart disease and diabetes was beneficial compared with medical therapy alone.5
While it was not designed to directly compare percutaneous coronary intervention vs coronary artery bypass grafting (CABG), it did find that medical therapy plus CABG might reduce the rate of adverse cardiovascular events in this population compared with medical therapy alone or medical therapy plus percutaneous intervention.
As with COURAGE, however, the patients in the medical therapy group in BARI 2D also had a high rate of crossover to revascularization, primarily driven by worsening anginal symptoms.
FREEDOM and the 2014 updated guideline
Based on the findings of BARI 2D and those of FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease),6 the American College of Cardiology and American Heart Association updated their recommendations in 2014.7 This focused update states that for patients with diabetes and multivessel coronary artery disease, if revascularization is likely to improve survival (for example, in three-vessel disease or complex two-vessel disease involving the proximal left anterior descending artery), then CABG should be performed if a left internal mammary artery graft can be anastomosed to the left anterior descending artery. Otherwise, percutaneous coronary intervention should be reserved for those patients with diabetes and high-risk or complex multivessel coronary artery disease who are not good surgical candidates.
FAME 2 trial: Fractional flow reserve as a guide
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial,8 published in 2012, evaluated whether clinical outcomes differ between patients who undergo percutaneous revascularization plus medical therapy and those who are treated with medical therapy alone, using fractional flow reserve as a means to determine which stenoses should be considered for intervention. Fractional flow reserve performed during invasive angiography determines the ratio of intracoronary pressure to aortic pressure using a wire advanced across a coronary obstruction.
FAME 2 found a markedly lower incidence of the primary composite end point of death, myocardial infarction, and urgent revascularization with randomization to percutaneous revascularization plus medical therapy compared with medical therapy only (4.3% vs 12.7%, P = .001) in patients with a fractional flow reserve less than 0.80 (considered a hemodynamically significant obstruction). The trial was stopped early because of the markedly different outcomes.
Of note, however, the reduction in adverse clinical outcomes was driven primarily by a reduction in urgent revascularizations in those treated with percutaneous coronary intervention in the revascularization arm. Regardless, using fractional flow reserve to guide whether obstructive coronary lesions should be treated with percutaneous coronary intervention has appropriately become a mainstay in interventional cardiology.
Stress testing
Noninvasive stress testing has played a role in helping to guide revascularization decisions in stable ischemic heart disease. In particular, revascularization in the setting of greater than 10% ischemia on perfusion imaging has been associated with a lower risk of cardiac death than in those who were revascularized with an ischemic burden less than 10%.9
A substudy of COURAGE found that percutaneous coronary intervention reduced ischemia to a greater degree than medical therapy alone on serial nuclear stress tests in patients with stable ischemic heart disease.10 In this substudy, when both groups were combined, the investigators also found that there were fewer adverse events in those who had an overall reduction of ischemia regardless of treatment strategy.
ISCHEMIA: Revascularize those with ischemia?
While COURAGE, BARI 2D, and FAME 2 suggested that early revascularization for low-risk patients with coronary artery disease does not confer a benefit over medical treatment alone with regard to hard clinical end points, it remains unclear whether an early revascularization strategy is advantageous in patients with stable ischemic heart disease who have at least a moderate amount of ischemia on noninvasive stress testing.
The ongoing ISCHEMIA (International Study of Comparative Effectiveness With Medical and Invasive Approaches) trial will help to answer that question. In this study, 8,000 patients with stable angina and at least moderate ischemia on noninvasive stress testing are being randomized before coronary angiography either to guideline-directed medical therapy plus revascularization (percutaneous or surgical) or to medical therapy alone.11 The ISCHEMIA study population reflects current practice more closely than the previous studies discussed above in its inclusion of fractional flow reserve and later-generation drug-eluting stents.
The results of ISCHEMIA will be an important piece of the puzzle to answer whether patients with stable ischemic heart disease benefit from revascularization in terms of cardiovascular mortality or myocardial infarction (the primary end point of the study).
Studies in additional subsets
It is important to recognize that there are additional subsets of patients with stable ischemic heart disease (those with multivessel disease, left main coronary disease, or low ejection fractions, for example) who have been studied to help determine when and how to perform revascularization. In addition, there are guidelines12 for both interventional cardiologists and cardiac surgeons that help delineate which patients should undergo revascularization. While a complete review is beyond the scope of this discussion, three trials are worth mentioning:
The Coronary Artery Surgery Study (CASS)13 revealed that revascularization in left main coronary artery disease is associated with lower mortality rates than medical therapy alone. This study, along with others, eventually led to recommendations for revascularization to be performed in all patients with significant left main coronary disease, regardless of symptoms or stress test findings.14,15
The Surgical Treatment for Ischemic Heart Failure (STICH) trial16 found that patients with a low ejection fraction (< 35%) and ischemic heart disease had no difference in all-cause mortality rates when treated with CABG plus medical therapy compared with medical therapy alone (although the study’s design has been heavily criticized).
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) study17 found that CABG was associated with fewer adverse events in three-vessel coronary artery disease or complex left main coronary artery disease compared with percutaneous coronary intervention. The study used early-generation paclitaxel drug-eluting stents that are no longer used in contemporary practice. This study established the SYNTAX score, which is often used to help make revascularization decisions. A low SYNTAX score of 0 to 22 (meaning less-severe coronary artery disease) was associated with equivalent outcomes for both percutaneous coronary intervention and CABG. Thus, even if there is multivessel disease or left main disease, if the SYNTAX score is low, then percutaneous coronary intervention is an acceptable method for revascularization with similar results as for CABG.
A TEAM APPROACH
Due to the complexity of stable ischemic heart disease and the subtleties of managing these patients, a multidisciplinary “heart team” approach may be the best way to navigate treating stable ischemic heart disease via revascularization or with medical therapy alone. The heart team approach could take advantage of the particular expertise that the primary care physician, cardiologist, interventional cardiologist, and cardiac surgeon provide.
The upcoming results of studies such as the ISCHEMIA trial will help to provide additional guidance for these teams in long-term management of patients with stable ischemic heart disease.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012; 126:e354–e471.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Blankenship J, Marshall JJ, Pinto DS, et al; Society for Cardiovascular Angiography and Interventions. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81:243–249.
- BARI 2D Study Group; Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Farkouh ME, Domanski M, Sleep LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:1929–1949.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998; 97:535–543.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Stone GW, Hochman JS, Williams DO, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol 2016; 67:81–99.
- Patel M, Dehmer G, Hirshfeld J, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol 2012; 59:857–881.
- Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990; 82:1629–1646.
- Hillis L, Smith P, Anderson J, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine G, Bates E, Blankenship J, et al. 2011 ACCF/AHA guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Velazquez EJ, Lee KL, Deja MA, et al, for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364:1607–1616.
- Serruys PW, Morice M-C, Kappetein AP, et al, for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012; 126:e354–e471.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Blankenship J, Marshall JJ, Pinto DS, et al; Society for Cardiovascular Angiography and Interventions. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81:243–249.
- BARI 2D Study Group; Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Farkouh ME, Domanski M, Sleep LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:1929–1949.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998; 97:535–543.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Stone GW, Hochman JS, Williams DO, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol 2016; 67:81–99.
- Patel M, Dehmer G, Hirshfeld J, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol 2012; 59:857–881.
- Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990; 82:1629–1646.
- Hillis L, Smith P, Anderson J, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine G, Bates E, Blankenship J, et al. 2011 ACCF/AHA guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Velazquez EJ, Lee KL, Deja MA, et al, for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364:1607–1616.
- Serruys PW, Morice M-C, Kappetein AP, et al, for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
Thrombotic thrombocytopenic purpura: The role of ADAMTS13
A breakthrough in understanding the pathogenesis of thrombotic thrombocytopenic purpura (TTP) came with the discovery of ADAMTS13 (an abbreviation for “a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13”), a plasma protein that cleaves von Willebrand factor, which interacts with platelets to promote blood clotting. If ADAMTS13 is lacking, unusually large multimers of von Willebrand factor can accumulate and trigger intravascular platelet aggregation and microthrombosis, causing the signs and symptoms of TTP.1–3
This knowledge has practical applications: we can now measure ADAMTS13 activity, ADAMTS13 inhibitor, and antibodies against ADAMTS13 to help us diagnose TTP and distinguish it from other forms of thrombotic microangiopathy, such as hemolytic-uremic syndrome, that have similar symptoms but require different treatment.
Using case studies, this article describes typical presentations of acute and relapsing TTP; the role of laboratory testing, including the ADAMTS13 assay; how to distinguish TTP from other conditions that present similarly; and how to manage this condition.
A HIGH RISK OF DEATH WITHOUT PLASMA EXCHANGE
TTP is characterized by disseminated microthrombi composed of agglutinated platelets and von Willebrand factor in small vessels. Tissue damage by microthrombi can cause thrombocytopenia (platelet deficiency), microangiopathic hemolytic anemia (loss of red blood cells caused by destructive conditions in small vessels), and multiorgan failure.1
Untreated TTP has a mortality rate of about 90%.1 As shown in Case 1, Case 2, and Table 1, rapid diagnosis and prompt initiation of daily therapeutic plasma exchange can improve this grave outlook.4

ADAMTS13 DEFICIENCY CAN BE ACQUIRED OR CONGENITAL
Two major forms of TTP with ADAMTS13 deficiency and microvascular thrombosis are recognized:
Acquired TTP, the more common form, peaks in incidence between ages 30 and 50.2,5 It more often affects women, particularly during and after pregnancy (its estimated prevalence is 1 in 25,000 pregnancies), and African Americans.6 Acquired TTP may be:
- Primary (idiopathic or autoantibody-mediated), associated with severely decreased ADAMTS13 and the presence of ultra-large von Willebrand factor multimers, or
- Secondary (23%–67% of cases), arising from a variety of conditions, including autoimmune disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), solid organ or hematopoietic cell transplant, malignancy, drugs, and pregnancy (Table 2).1,5–8 Secondary TTP has a worse prognosis than idiopathic TTP.5,9
Congenital TTP (Upshaw-Shulman syndrome) is a rare autosomal-recessive disease caused by compound heterozygous or homozygous mutations of the ADAMTS13 gene, producing nonfunctional ADAMTS13 protein. Patients have severely deficient ADAMTS13 activity but usually do not develop autoantibodies. There is a high risk of chronic, relapsing episodes; identified triggers include pregnancy and heavy alcohol intake.2,10 About half of patients with congenital TTP have an early onset, usually presenting with acute TTP between birth and age 5, and about half have a late onset, usually remaining without symptoms until age 20 to 40.
THE CLINICAL PICTURE OF TTP IS NOT ALWAYS CLASSIC
TTP is primarily diagnosed clinically, but diagnosis is often difficult because of various nonspecific symptoms. Typical TTP presents with the “classic pentad”:
- Severe thrombocytopenia (70%–100% of patients)
- Microangiopathic hemolytic anemia with multiple schistocytes (70%–100%) (Figure 1)
- Neurologic involvement (50%–90%)
- Renal abnormalities (about 50%)
- Fever (25%).
However, the entire picture often does not emerge in a single patient.2,6 Waiting for the entire pentad to develop before diagnosing TTP can have grave clinical consequences,1,2,5 and the presence of thrombocytopenia and unexplained microangiopathic hemolytic anemia are considered clinically sufficient to suspect TTP.5
Neurologic symptoms usually fluctuate. They can include mild abnormalities such as weakness, dizziness, headache, blurred vision, ataxia, and transient mental status changes, as well as severe abnormalities including stroke, seizure, and coma.2,6
Most patients have normal findings on computed tomography and magnetic resonance imaging at the onset of neurologic symptoms or with a history of TTP. Some patients (8%–39%) show reversible acute brain lesions, including ischemic changes.11–13
Other signs and symptoms may result from multiorgan failure due to microthrombosis; ischemia in retinal, coronary, and abdominal circulations; and unconjugated hyperbilirubinemia.2
Atypical presentations. About 18% of patients have cardiac involvement from microvascular occlusion, with arrhythmia, angina, or congestive heart failure. Abdominal pain and pancreatitis occur in 5% to 13%, and visual disturbances in 8% to 10%.
Patients with an atypical presentation may not have laboratory evidence of microangiopathic hemolytic anemia, but an ADAMTS13 assay will show severely decreased activity. Therapeutic plasma exchange can improve atypical symptoms.2,3,10,14,15
ADAMTS13 ASSAY IS KEY TO DIAGNOSIS
Laboratory evidence typically includes hemolytic anemia (reticulocytosis, schistocytes, elevated indirect bilirubin, reduced haptoglobin, elevated lactate dehydrogenase) and thrombocytopenia.3 There are no significant abnormalities in prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen, or D-dimer level.
Measuring the levels of ADAMTS13 activity, ADAMTS13 inhibitor, and ADAMTS13 antibody is becoming standard to confirm the diagnosis of TTP, to determine if it is congenital or acquired, and to distinguish it from thrombocytopenic conditions such as hemolytic-uremic syndrome, idiopathic thrombocytopenic purpura, and heparin-induced thrombocytopenia.4,5 A newer ADAMTS13 assay based on fluorescence energy transfer (FRET) technology with a synthetic amino acid-von Willebrand factor peptide substrate has a faster turnaround time and less test variability.6,16,17 This FRET assay can give the result of ADAMTS13 activity within 2 hours. In comparison, the assay based on multimeric von Willebrand factor takes 2 to 3 days, and mass spectrometry to measure the cleavage products of a synthetic von Willebrand factor molecule takes about 4 hours.3,10,16
About two-thirds of patients with the clinical diagnosis of idiopathic TTP have ADAMTS13 activity levels lower than 10%.5,14,18 In the appropriate clinical setting, this threshold level is highly sensitive (89%–100%) and specific (99%–100%) in differentiating TTP from other thrombotic angiopathies.2,3,18
Note: The ADAMTS13 assay was needed for early correct diagnosis in Case 1 and Case 2.
Inhibitors provide more clues
Autoantibodies can be classified according to whether they inhibit ADAMTS13 activity.
Neutralizing inhibitors. Most cases of acquired, idiopathic TTP with severe ADAMTS13 deficiency are related to circulating autoantibodies that neutralize ADAMTS13 activity. This ADAMTS13 inhibitor level is obtained by measuring residual ADAMTS13 activity after mixing equal amounts of patient plasma with normal pooled plasma. ADAMTS13 inhibitor is detectable in 44% to 93% of patients with severely deficient ADAMTS13 activity.3,6,19
Nonneutralizing inhibitors. From 10% to 15% of patients with TTP with severe ADAMTS13 deficiency lack ADAMTS13 autoantibodies measured by enzyme immunoassay but have nonneutralizing immunoglobulin G (IgG) or IgM autoantibodies. In such cases, ADAMTS13 deficiency may be related to increased antibody-mediated clearance or other unknown mechanisms.
Neutralizing inhibitors and nonneutralizing inhibitors may be present simultaneously in some patients.3,10,19,20
Blood factors affect ADAMTS13 activity
Specimen factors can affect ADAMTS13 activity and antibody levels.
Hemoglobin is a potent inhibitor of ADAMTS13, so an elevated plasma level of free hemoglobin (> 2 g/dL) can reduce ADAMTS13 activity, as can hyperbilirubinemia (> 15 mg/dL).
High levels of endogenous von Willebrand factor, lipids, thrombin, or other proteases that may cleave ADAMTS13 can also reduce ADAMTS13 activity.3 Conversely, recent plasma exchange or transfusion can mask the diagnosis of TTP because of false normalization of ADAMTS13 activity. In addition, ADAMTS13 autoantibody can be detected in other immune-mediated disorders (eg, systemic lupus erythematosus, antiphospholipid syndrome), and hypergammaglobulinemia, as well as in 10% to 15% of healthy individuals.19
CONSIDER OTHER CONDITIONS
Before diagnosing TTP, other conditions causing thrombocytopenia and hemolytic anemia should be excluded by taking a careful clinical, laboratory, and medication history (Table 2). Of these conditions, the most challenging to differentiate from TTP—and often indistinguishable from it at presentation—is hemolytic-uremic syndrome (Table 3).
Hemolytic-uremic syndrome
Hemolytic-uremic syndrome presents with a triad of thrombocytopenia, acute renal failure, and microangiopathic hemolytic anemia, with increased lactate dehydrogenase levels. Renal dysfunction from ischemia or tissue injury by microvascular thrombi predominates. Hemolytic-uremic syndrome most often occurs in children and is often related to hemorrhagic enterocolitis caused by infection with Escherichia coli O157:H7 or Shigella species (90%–95% of cases).1,2,5
From 5% to 10% of cases of hemolytic- uremic syndrome are atypical. These cases are not associated with diarrhea, and many are caused by genetic mutations that result in chronic excessive complement activation. Implicated genes regulate complement regulator factor H (20%–30% of cases) or CD46 (10%) and other cofactors, or autoantibodies against factor H (10%), which affect the alternate complement pathway.6,21–23
Initial therapeutic plasma exchange is commonly undertaken for atypical hemolytic- uremic syndrome, particularly for patients at risk of rapid progression to end-stage renal failure. But despite such treatment, about 60% of these patients die or develop permanent renal damage within 1 year.2,3,24
Eculizumab, a monoclonal antibody against complement component C5, has been approved by the US Food and Drug Administration for atypical hemolytic-uremic syndrome and may improve quality of life.25–27
PLASMA EXCHANGE IS THE MAINSTAY OF THERAPY
In 2012, the British Society for Haematology published revised guidelines for managing TTP and other thrombotic microangiopathies.28
Acquired idiopathic TTP with reduced ADAMTS13 activity requires immediate therapeutic plasma exchange. Daily plasma exchange combines plasmapheresis to remove circulating ultralarge von Willebrand factor-platelet strings and autoantibodies against ADAMTS13, and infusion of fresh-frozen plasma to replace ADAMTS13.18 This procedure is the mainstay of therapy and brings 70% to 90% of patients with idiopathic TTP to remission.1,2,5,6 However, the optimal duration of daily plasma exchange and the number of procedures required is highly variable according to clinical condition. Therapeutic plasma exchange can also cause plasma-related adverse reactions.9,28 Congenital TTP requires plasma infusion or exchange depending on the patient’s severity of ADAMTS13 deficiency.
Corticosteroids are used in combination with daily therapeutic plasma exchange, although evidence from controlled trials of their efficacy in this setting is lacking. Patients with severely decreased ADAMTS13 activity or low titers of ADAMTS13 autoantibodies tend to respond to the therapy.5,8,29
An ADAMTS13 assay with a short turn-around time can help guide the decision to initiate therapeutic plasma exchange. However, if there is a strong clinical suspicion of TTP, plasma exchange should be initiated immediately without waiting for test results.5,30 Monitoring ADAMTS13 activity or inhibitor during initial plasma exchange therapy has had conflicting results in several studies and is generally not recommended for patients with acquired TTP.8,30,31
RELAPSE IS COMMON
About 20% to 50% of patients with idiopathic TTP experience a relapse (Case 2). Most relapses occur within the first 2 years after the initial episode, with an estimated risk of 43% for relapse at 7.5 years.5,9
Factors that predict a higher risk of relapse include persistently severely decreased ADAMTS13 activity, positive inhibitor, and high titers of autoantibodies to ADAMTS13 during symptomatic TTP. During clinical remission, persistence of autoantibodies also indicates increased risk.1,3,5,6,9
Patients who have a relapse and whose disease is refractory to therapeutic plasma exchange (10%–20% of cases) have been treated with corticosteroids, splenectomy, or immunosuppressive agents (cyclosporine, azathioprine, or cyclophosphamide) with varying rates of success. Rituximab (monoclonal anti-CD20) has recently been used as second-line therapy in refractory or relapsing immune-mediated TTP or idiopathic TTP with neurologic or cardiac symptoms associated with a poor prognosis. Therapy including rituximab results in improved response and progression-free survival.32 Other potential therapies, including recombinant active ADAMTS13, are under investigation.9,23,28,30,33,34
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2004; 1:407–423.
- Shenkman B, Einav Y. Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: diagnosis and classification. Autoimmun Rev 2014; 13:584–586.
- Shah N, Sarode R. Thrombotic thrombocytopenic purpura-what is new? J Clin Apher 2013; 28:30–35.
- Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher 2012; 27:221–226.
- George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012; 1:604–609.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 2013; 163:514–519.
- Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 2012; 157:501–503.
- Mannucci PM, Peyvandi F. TTP and ADAMTS13: when Is testing appropriate? Hematology Am Soc Hematol Educ Program 2007; 1:121–126.
- Chaturved S, Carcioppolo D, Zhang L, McCar KR. Management and outcomes of patients with TTP: analysis of 100 cases at a single institution. Am J Hematol 2013; 88:560–565.
- Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost 2010; 8:631–640.
- Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol 2011; 86:87–89.
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica 2001; 86:1194–1199.
- Kwaan HC, Boggio LN. The clinical spectrum of thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2005; 31:673–680.
- Sarode R. Atypical presentations of thrombotic thrombocytopenic purpura: a review. J Clin Apher 2009; 24:47–52.
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis 2000; 35:E3.
- Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 2006; 4:1146–1148.
- Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost 2006; 4:698–699.
- Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med 2014; 138:546–549.
- Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005; 106:1262–1267.
- Rogers HJ, Kottke-Marchant K. ADAMTS13 evaluation for thrombotic thrombocytopenic purpura. Pathology Innovations, Pathology and Laboratory Medicine Institute. Cleveland Clinic, Fall 2014:6–9.
- Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111:1512–1514.
- Diamante Chiodini B, Davin JC, Corazza F, et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 2014; 133:e1764–e1768.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH; working party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2009; 148:37–47.
- Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36:673–681.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol 2014; 295323:1–7.
- Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol 2011; 26:621–624.
- Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O. Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 2015; 30:783–789.
- Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158:323–325.
- Abassi E, Yawn D, Leveque E, Nolasco L, Lopez J, Moake J. Correlation of ADAMTS-13 activity with response to plasma exchange in patients diagnosed with thrombotic thrombocytopenic purpura (Abstract #3921). Blood 2004; 104:242a.
- Blombery P, Scully M. Management of thrombocytic thrombocytopenic purpura: current perspectives. J Blood Med 2014; 5:15–23.
- Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion 2015; 55:18–24.
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood 2016; 127:2952–2953.
- Chapman K, Yuen S. Therapy for thrombotic thrombocytopenic purpura: past, present and future. Semin Thromb Hemost 2014; 40:34–40.
- Heidel F, Lipka DB, von Auer C, Huber C, Schrarrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007; 97:228–233.
A breakthrough in understanding the pathogenesis of thrombotic thrombocytopenic purpura (TTP) came with the discovery of ADAMTS13 (an abbreviation for “a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13”), a plasma protein that cleaves von Willebrand factor, which interacts with platelets to promote blood clotting. If ADAMTS13 is lacking, unusually large multimers of von Willebrand factor can accumulate and trigger intravascular platelet aggregation and microthrombosis, causing the signs and symptoms of TTP.1–3
This knowledge has practical applications: we can now measure ADAMTS13 activity, ADAMTS13 inhibitor, and antibodies against ADAMTS13 to help us diagnose TTP and distinguish it from other forms of thrombotic microangiopathy, such as hemolytic-uremic syndrome, that have similar symptoms but require different treatment.
Using case studies, this article describes typical presentations of acute and relapsing TTP; the role of laboratory testing, including the ADAMTS13 assay; how to distinguish TTP from other conditions that present similarly; and how to manage this condition.
A HIGH RISK OF DEATH WITHOUT PLASMA EXCHANGE
TTP is characterized by disseminated microthrombi composed of agglutinated platelets and von Willebrand factor in small vessels. Tissue damage by microthrombi can cause thrombocytopenia (platelet deficiency), microangiopathic hemolytic anemia (loss of red blood cells caused by destructive conditions in small vessels), and multiorgan failure.1
Untreated TTP has a mortality rate of about 90%.1 As shown in Case 1, Case 2, and Table 1, rapid diagnosis and prompt initiation of daily therapeutic plasma exchange can improve this grave outlook.4


ADAMTS13 DEFICIENCY CAN BE ACQUIRED OR CONGENITAL
Two major forms of TTP with ADAMTS13 deficiency and microvascular thrombosis are recognized:
Acquired TTP, the more common form, peaks in incidence between ages 30 and 50.2,5 It more often affects women, particularly during and after pregnancy (its estimated prevalence is 1 in 25,000 pregnancies), and African Americans.6 Acquired TTP may be:
- Primary (idiopathic or autoantibody-mediated), associated with severely decreased ADAMTS13 and the presence of ultra-large von Willebrand factor multimers, or
- Secondary (23%–67% of cases), arising from a variety of conditions, including autoimmune disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), solid organ or hematopoietic cell transplant, malignancy, drugs, and pregnancy (Table 2).1,5–8 Secondary TTP has a worse prognosis than idiopathic TTP.5,9
Congenital TTP (Upshaw-Shulman syndrome) is a rare autosomal-recessive disease caused by compound heterozygous or homozygous mutations of the ADAMTS13 gene, producing nonfunctional ADAMTS13 protein. Patients have severely deficient ADAMTS13 activity but usually do not develop autoantibodies. There is a high risk of chronic, relapsing episodes; identified triggers include pregnancy and heavy alcohol intake.2,10 About half of patients with congenital TTP have an early onset, usually presenting with acute TTP between birth and age 5, and about half have a late onset, usually remaining without symptoms until age 20 to 40.
THE CLINICAL PICTURE OF TTP IS NOT ALWAYS CLASSIC
TTP is primarily diagnosed clinically, but diagnosis is often difficult because of various nonspecific symptoms. Typical TTP presents with the “classic pentad”:
- Severe thrombocytopenia (70%–100% of patients)
- Microangiopathic hemolytic anemia with multiple schistocytes (70%–100%) (Figure 1)
- Neurologic involvement (50%–90%)
- Renal abnormalities (about 50%)
- Fever (25%).
However, the entire picture often does not emerge in a single patient.2,6 Waiting for the entire pentad to develop before diagnosing TTP can have grave clinical consequences,1,2,5 and the presence of thrombocytopenia and unexplained microangiopathic hemolytic anemia are considered clinically sufficient to suspect TTP.5
Neurologic symptoms usually fluctuate. They can include mild abnormalities such as weakness, dizziness, headache, blurred vision, ataxia, and transient mental status changes, as well as severe abnormalities including stroke, seizure, and coma.2,6
Most patients have normal findings on computed tomography and magnetic resonance imaging at the onset of neurologic symptoms or with a history of TTP. Some patients (8%–39%) show reversible acute brain lesions, including ischemic changes.11–13
Other signs and symptoms may result from multiorgan failure due to microthrombosis; ischemia in retinal, coronary, and abdominal circulations; and unconjugated hyperbilirubinemia.2
Atypical presentations. About 18% of patients have cardiac involvement from microvascular occlusion, with arrhythmia, angina, or congestive heart failure. Abdominal pain and pancreatitis occur in 5% to 13%, and visual disturbances in 8% to 10%.
Patients with an atypical presentation may not have laboratory evidence of microangiopathic hemolytic anemia, but an ADAMTS13 assay will show severely decreased activity. Therapeutic plasma exchange can improve atypical symptoms.2,3,10,14,15
ADAMTS13 ASSAY IS KEY TO DIAGNOSIS
Laboratory evidence typically includes hemolytic anemia (reticulocytosis, schistocytes, elevated indirect bilirubin, reduced haptoglobin, elevated lactate dehydrogenase) and thrombocytopenia.3 There are no significant abnormalities in prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen, or D-dimer level.
Measuring the levels of ADAMTS13 activity, ADAMTS13 inhibitor, and ADAMTS13 antibody is becoming standard to confirm the diagnosis of TTP, to determine if it is congenital or acquired, and to distinguish it from thrombocytopenic conditions such as hemolytic-uremic syndrome, idiopathic thrombocytopenic purpura, and heparin-induced thrombocytopenia.4,5 A newer ADAMTS13 assay based on fluorescence energy transfer (FRET) technology with a synthetic amino acid-von Willebrand factor peptide substrate has a faster turnaround time and less test variability.6,16,17 This FRET assay can give the result of ADAMTS13 activity within 2 hours. In comparison, the assay based on multimeric von Willebrand factor takes 2 to 3 days, and mass spectrometry to measure the cleavage products of a synthetic von Willebrand factor molecule takes about 4 hours.3,10,16
About two-thirds of patients with the clinical diagnosis of idiopathic TTP have ADAMTS13 activity levels lower than 10%.5,14,18 In the appropriate clinical setting, this threshold level is highly sensitive (89%–100%) and specific (99%–100%) in differentiating TTP from other thrombotic angiopathies.2,3,18
Note: The ADAMTS13 assay was needed for early correct diagnosis in Case 1 and Case 2.
Inhibitors provide more clues
Autoantibodies can be classified according to whether they inhibit ADAMTS13 activity.
Neutralizing inhibitors. Most cases of acquired, idiopathic TTP with severe ADAMTS13 deficiency are related to circulating autoantibodies that neutralize ADAMTS13 activity. This ADAMTS13 inhibitor level is obtained by measuring residual ADAMTS13 activity after mixing equal amounts of patient plasma with normal pooled plasma. ADAMTS13 inhibitor is detectable in 44% to 93% of patients with severely deficient ADAMTS13 activity.3,6,19
Nonneutralizing inhibitors. From 10% to 15% of patients with TTP with severe ADAMTS13 deficiency lack ADAMTS13 autoantibodies measured by enzyme immunoassay but have nonneutralizing immunoglobulin G (IgG) or IgM autoantibodies. In such cases, ADAMTS13 deficiency may be related to increased antibody-mediated clearance or other unknown mechanisms.
Neutralizing inhibitors and nonneutralizing inhibitors may be present simultaneously in some patients.3,10,19,20
Blood factors affect ADAMTS13 activity
Specimen factors can affect ADAMTS13 activity and antibody levels.
Hemoglobin is a potent inhibitor of ADAMTS13, so an elevated plasma level of free hemoglobin (> 2 g/dL) can reduce ADAMTS13 activity, as can hyperbilirubinemia (> 15 mg/dL).
High levels of endogenous von Willebrand factor, lipids, thrombin, or other proteases that may cleave ADAMTS13 can also reduce ADAMTS13 activity.3 Conversely, recent plasma exchange or transfusion can mask the diagnosis of TTP because of false normalization of ADAMTS13 activity. In addition, ADAMTS13 autoantibody can be detected in other immune-mediated disorders (eg, systemic lupus erythematosus, antiphospholipid syndrome), and hypergammaglobulinemia, as well as in 10% to 15% of healthy individuals.19
CONSIDER OTHER CONDITIONS
Before diagnosing TTP, other conditions causing thrombocytopenia and hemolytic anemia should be excluded by taking a careful clinical, laboratory, and medication history (Table 2). Of these conditions, the most challenging to differentiate from TTP—and often indistinguishable from it at presentation—is hemolytic-uremic syndrome (Table 3).
Hemolytic-uremic syndrome
Hemolytic-uremic syndrome presents with a triad of thrombocytopenia, acute renal failure, and microangiopathic hemolytic anemia, with increased lactate dehydrogenase levels. Renal dysfunction from ischemia or tissue injury by microvascular thrombi predominates. Hemolytic-uremic syndrome most often occurs in children and is often related to hemorrhagic enterocolitis caused by infection with Escherichia coli O157:H7 or Shigella species (90%–95% of cases).1,2,5
From 5% to 10% of cases of hemolytic- uremic syndrome are atypical. These cases are not associated with diarrhea, and many are caused by genetic mutations that result in chronic excessive complement activation. Implicated genes regulate complement regulator factor H (20%–30% of cases) or CD46 (10%) and other cofactors, or autoantibodies against factor H (10%), which affect the alternate complement pathway.6,21–23
Initial therapeutic plasma exchange is commonly undertaken for atypical hemolytic- uremic syndrome, particularly for patients at risk of rapid progression to end-stage renal failure. But despite such treatment, about 60% of these patients die or develop permanent renal damage within 1 year.2,3,24
Eculizumab, a monoclonal antibody against complement component C5, has been approved by the US Food and Drug Administration for atypical hemolytic-uremic syndrome and may improve quality of life.25–27
PLASMA EXCHANGE IS THE MAINSTAY OF THERAPY
In 2012, the British Society for Haematology published revised guidelines for managing TTP and other thrombotic microangiopathies.28
Acquired idiopathic TTP with reduced ADAMTS13 activity requires immediate therapeutic plasma exchange. Daily plasma exchange combines plasmapheresis to remove circulating ultralarge von Willebrand factor-platelet strings and autoantibodies against ADAMTS13, and infusion of fresh-frozen plasma to replace ADAMTS13.18 This procedure is the mainstay of therapy and brings 70% to 90% of patients with idiopathic TTP to remission.1,2,5,6 However, the optimal duration of daily plasma exchange and the number of procedures required is highly variable according to clinical condition. Therapeutic plasma exchange can also cause plasma-related adverse reactions.9,28 Congenital TTP requires plasma infusion or exchange depending on the patient’s severity of ADAMTS13 deficiency.
Corticosteroids are used in combination with daily therapeutic plasma exchange, although evidence from controlled trials of their efficacy in this setting is lacking. Patients with severely decreased ADAMTS13 activity or low titers of ADAMTS13 autoantibodies tend to respond to the therapy.5,8,29
An ADAMTS13 assay with a short turn-around time can help guide the decision to initiate therapeutic plasma exchange. However, if there is a strong clinical suspicion of TTP, plasma exchange should be initiated immediately without waiting for test results.5,30 Monitoring ADAMTS13 activity or inhibitor during initial plasma exchange therapy has had conflicting results in several studies and is generally not recommended for patients with acquired TTP.8,30,31
RELAPSE IS COMMON
About 20% to 50% of patients with idiopathic TTP experience a relapse (Case 2). Most relapses occur within the first 2 years after the initial episode, with an estimated risk of 43% for relapse at 7.5 years.5,9
Factors that predict a higher risk of relapse include persistently severely decreased ADAMTS13 activity, positive inhibitor, and high titers of autoantibodies to ADAMTS13 during symptomatic TTP. During clinical remission, persistence of autoantibodies also indicates increased risk.1,3,5,6,9
Patients who have a relapse and whose disease is refractory to therapeutic plasma exchange (10%–20% of cases) have been treated with corticosteroids, splenectomy, or immunosuppressive agents (cyclosporine, azathioprine, or cyclophosphamide) with varying rates of success. Rituximab (monoclonal anti-CD20) has recently been used as second-line therapy in refractory or relapsing immune-mediated TTP or idiopathic TTP with neurologic or cardiac symptoms associated with a poor prognosis. Therapy including rituximab results in improved response and progression-free survival.32 Other potential therapies, including recombinant active ADAMTS13, are under investigation.9,23,28,30,33,34
A breakthrough in understanding the pathogenesis of thrombotic thrombocytopenic purpura (TTP) came with the discovery of ADAMTS13 (an abbreviation for “a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13”), a plasma protein that cleaves von Willebrand factor, which interacts with platelets to promote blood clotting. If ADAMTS13 is lacking, unusually large multimers of von Willebrand factor can accumulate and trigger intravascular platelet aggregation and microthrombosis, causing the signs and symptoms of TTP.1–3
This knowledge has practical applications: we can now measure ADAMTS13 activity, ADAMTS13 inhibitor, and antibodies against ADAMTS13 to help us diagnose TTP and distinguish it from other forms of thrombotic microangiopathy, such as hemolytic-uremic syndrome, that have similar symptoms but require different treatment.
Using case studies, this article describes typical presentations of acute and relapsing TTP; the role of laboratory testing, including the ADAMTS13 assay; how to distinguish TTP from other conditions that present similarly; and how to manage this condition.
A HIGH RISK OF DEATH WITHOUT PLASMA EXCHANGE
TTP is characterized by disseminated microthrombi composed of agglutinated platelets and von Willebrand factor in small vessels. Tissue damage by microthrombi can cause thrombocytopenia (platelet deficiency), microangiopathic hemolytic anemia (loss of red blood cells caused by destructive conditions in small vessels), and multiorgan failure.1
Untreated TTP has a mortality rate of about 90%.1 As shown in Case 1, Case 2, and Table 1, rapid diagnosis and prompt initiation of daily therapeutic plasma exchange can improve this grave outlook.4


ADAMTS13 DEFICIENCY CAN BE ACQUIRED OR CONGENITAL
Two major forms of TTP with ADAMTS13 deficiency and microvascular thrombosis are recognized:
Acquired TTP, the more common form, peaks in incidence between ages 30 and 50.2,5 It more often affects women, particularly during and after pregnancy (its estimated prevalence is 1 in 25,000 pregnancies), and African Americans.6 Acquired TTP may be:
- Primary (idiopathic or autoantibody-mediated), associated with severely decreased ADAMTS13 and the presence of ultra-large von Willebrand factor multimers, or
- Secondary (23%–67% of cases), arising from a variety of conditions, including autoimmune disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), solid organ or hematopoietic cell transplant, malignancy, drugs, and pregnancy (Table 2).1,5–8 Secondary TTP has a worse prognosis than idiopathic TTP.5,9
Congenital TTP (Upshaw-Shulman syndrome) is a rare autosomal-recessive disease caused by compound heterozygous or homozygous mutations of the ADAMTS13 gene, producing nonfunctional ADAMTS13 protein. Patients have severely deficient ADAMTS13 activity but usually do not develop autoantibodies. There is a high risk of chronic, relapsing episodes; identified triggers include pregnancy and heavy alcohol intake.2,10 About half of patients with congenital TTP have an early onset, usually presenting with acute TTP between birth and age 5, and about half have a late onset, usually remaining without symptoms until age 20 to 40.
THE CLINICAL PICTURE OF TTP IS NOT ALWAYS CLASSIC
TTP is primarily diagnosed clinically, but diagnosis is often difficult because of various nonspecific symptoms. Typical TTP presents with the “classic pentad”:
- Severe thrombocytopenia (70%–100% of patients)
- Microangiopathic hemolytic anemia with multiple schistocytes (70%–100%) (Figure 1)
- Neurologic involvement (50%–90%)
- Renal abnormalities (about 50%)
- Fever (25%).
However, the entire picture often does not emerge in a single patient.2,6 Waiting for the entire pentad to develop before diagnosing TTP can have grave clinical consequences,1,2,5 and the presence of thrombocytopenia and unexplained microangiopathic hemolytic anemia are considered clinically sufficient to suspect TTP.5
Neurologic symptoms usually fluctuate. They can include mild abnormalities such as weakness, dizziness, headache, blurred vision, ataxia, and transient mental status changes, as well as severe abnormalities including stroke, seizure, and coma.2,6
Most patients have normal findings on computed tomography and magnetic resonance imaging at the onset of neurologic symptoms or with a history of TTP. Some patients (8%–39%) show reversible acute brain lesions, including ischemic changes.11–13
Other signs and symptoms may result from multiorgan failure due to microthrombosis; ischemia in retinal, coronary, and abdominal circulations; and unconjugated hyperbilirubinemia.2
Atypical presentations. About 18% of patients have cardiac involvement from microvascular occlusion, with arrhythmia, angina, or congestive heart failure. Abdominal pain and pancreatitis occur in 5% to 13%, and visual disturbances in 8% to 10%.
Patients with an atypical presentation may not have laboratory evidence of microangiopathic hemolytic anemia, but an ADAMTS13 assay will show severely decreased activity. Therapeutic plasma exchange can improve atypical symptoms.2,3,10,14,15
ADAMTS13 ASSAY IS KEY TO DIAGNOSIS
Laboratory evidence typically includes hemolytic anemia (reticulocytosis, schistocytes, elevated indirect bilirubin, reduced haptoglobin, elevated lactate dehydrogenase) and thrombocytopenia.3 There are no significant abnormalities in prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen, or D-dimer level.
Measuring the levels of ADAMTS13 activity, ADAMTS13 inhibitor, and ADAMTS13 antibody is becoming standard to confirm the diagnosis of TTP, to determine if it is congenital or acquired, and to distinguish it from thrombocytopenic conditions such as hemolytic-uremic syndrome, idiopathic thrombocytopenic purpura, and heparin-induced thrombocytopenia.4,5 A newer ADAMTS13 assay based on fluorescence energy transfer (FRET) technology with a synthetic amino acid-von Willebrand factor peptide substrate has a faster turnaround time and less test variability.6,16,17 This FRET assay can give the result of ADAMTS13 activity within 2 hours. In comparison, the assay based on multimeric von Willebrand factor takes 2 to 3 days, and mass spectrometry to measure the cleavage products of a synthetic von Willebrand factor molecule takes about 4 hours.3,10,16
About two-thirds of patients with the clinical diagnosis of idiopathic TTP have ADAMTS13 activity levels lower than 10%.5,14,18 In the appropriate clinical setting, this threshold level is highly sensitive (89%–100%) and specific (99%–100%) in differentiating TTP from other thrombotic angiopathies.2,3,18
Note: The ADAMTS13 assay was needed for early correct diagnosis in Case 1 and Case 2.
Inhibitors provide more clues
Autoantibodies can be classified according to whether they inhibit ADAMTS13 activity.
Neutralizing inhibitors. Most cases of acquired, idiopathic TTP with severe ADAMTS13 deficiency are related to circulating autoantibodies that neutralize ADAMTS13 activity. This ADAMTS13 inhibitor level is obtained by measuring residual ADAMTS13 activity after mixing equal amounts of patient plasma with normal pooled plasma. ADAMTS13 inhibitor is detectable in 44% to 93% of patients with severely deficient ADAMTS13 activity.3,6,19
Nonneutralizing inhibitors. From 10% to 15% of patients with TTP with severe ADAMTS13 deficiency lack ADAMTS13 autoantibodies measured by enzyme immunoassay but have nonneutralizing immunoglobulin G (IgG) or IgM autoantibodies. In such cases, ADAMTS13 deficiency may be related to increased antibody-mediated clearance or other unknown mechanisms.
Neutralizing inhibitors and nonneutralizing inhibitors may be present simultaneously in some patients.3,10,19,20
Blood factors affect ADAMTS13 activity
Specimen factors can affect ADAMTS13 activity and antibody levels.
Hemoglobin is a potent inhibitor of ADAMTS13, so an elevated plasma level of free hemoglobin (> 2 g/dL) can reduce ADAMTS13 activity, as can hyperbilirubinemia (> 15 mg/dL).
High levels of endogenous von Willebrand factor, lipids, thrombin, or other proteases that may cleave ADAMTS13 can also reduce ADAMTS13 activity.3 Conversely, recent plasma exchange or transfusion can mask the diagnosis of TTP because of false normalization of ADAMTS13 activity. In addition, ADAMTS13 autoantibody can be detected in other immune-mediated disorders (eg, systemic lupus erythematosus, antiphospholipid syndrome), and hypergammaglobulinemia, as well as in 10% to 15% of healthy individuals.19
CONSIDER OTHER CONDITIONS
Before diagnosing TTP, other conditions causing thrombocytopenia and hemolytic anemia should be excluded by taking a careful clinical, laboratory, and medication history (Table 2). Of these conditions, the most challenging to differentiate from TTP—and often indistinguishable from it at presentation—is hemolytic-uremic syndrome (Table 3).
Hemolytic-uremic syndrome
Hemolytic-uremic syndrome presents with a triad of thrombocytopenia, acute renal failure, and microangiopathic hemolytic anemia, with increased lactate dehydrogenase levels. Renal dysfunction from ischemia or tissue injury by microvascular thrombi predominates. Hemolytic-uremic syndrome most often occurs in children and is often related to hemorrhagic enterocolitis caused by infection with Escherichia coli O157:H7 or Shigella species (90%–95% of cases).1,2,5
From 5% to 10% of cases of hemolytic- uremic syndrome are atypical. These cases are not associated with diarrhea, and many are caused by genetic mutations that result in chronic excessive complement activation. Implicated genes regulate complement regulator factor H (20%–30% of cases) or CD46 (10%) and other cofactors, or autoantibodies against factor H (10%), which affect the alternate complement pathway.6,21–23
Initial therapeutic plasma exchange is commonly undertaken for atypical hemolytic- uremic syndrome, particularly for patients at risk of rapid progression to end-stage renal failure. But despite such treatment, about 60% of these patients die or develop permanent renal damage within 1 year.2,3,24
Eculizumab, a monoclonal antibody against complement component C5, has been approved by the US Food and Drug Administration for atypical hemolytic-uremic syndrome and may improve quality of life.25–27
PLASMA EXCHANGE IS THE MAINSTAY OF THERAPY
In 2012, the British Society for Haematology published revised guidelines for managing TTP and other thrombotic microangiopathies.28
Acquired idiopathic TTP with reduced ADAMTS13 activity requires immediate therapeutic plasma exchange. Daily plasma exchange combines plasmapheresis to remove circulating ultralarge von Willebrand factor-platelet strings and autoantibodies against ADAMTS13, and infusion of fresh-frozen plasma to replace ADAMTS13.18 This procedure is the mainstay of therapy and brings 70% to 90% of patients with idiopathic TTP to remission.1,2,5,6 However, the optimal duration of daily plasma exchange and the number of procedures required is highly variable according to clinical condition. Therapeutic plasma exchange can also cause plasma-related adverse reactions.9,28 Congenital TTP requires plasma infusion or exchange depending on the patient’s severity of ADAMTS13 deficiency.
Corticosteroids are used in combination with daily therapeutic plasma exchange, although evidence from controlled trials of their efficacy in this setting is lacking. Patients with severely decreased ADAMTS13 activity or low titers of ADAMTS13 autoantibodies tend to respond to the therapy.5,8,29
An ADAMTS13 assay with a short turn-around time can help guide the decision to initiate therapeutic plasma exchange. However, if there is a strong clinical suspicion of TTP, plasma exchange should be initiated immediately without waiting for test results.5,30 Monitoring ADAMTS13 activity or inhibitor during initial plasma exchange therapy has had conflicting results in several studies and is generally not recommended for patients with acquired TTP.8,30,31
RELAPSE IS COMMON
About 20% to 50% of patients with idiopathic TTP experience a relapse (Case 2). Most relapses occur within the first 2 years after the initial episode, with an estimated risk of 43% for relapse at 7.5 years.5,9
Factors that predict a higher risk of relapse include persistently severely decreased ADAMTS13 activity, positive inhibitor, and high titers of autoantibodies to ADAMTS13 during symptomatic TTP. During clinical remission, persistence of autoantibodies also indicates increased risk.1,3,5,6,9
Patients who have a relapse and whose disease is refractory to therapeutic plasma exchange (10%–20% of cases) have been treated with corticosteroids, splenectomy, or immunosuppressive agents (cyclosporine, azathioprine, or cyclophosphamide) with varying rates of success. Rituximab (monoclonal anti-CD20) has recently been used as second-line therapy in refractory or relapsing immune-mediated TTP or idiopathic TTP with neurologic or cardiac symptoms associated with a poor prognosis. Therapy including rituximab results in improved response and progression-free survival.32 Other potential therapies, including recombinant active ADAMTS13, are under investigation.9,23,28,30,33,34
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2004; 1:407–423.
- Shenkman B, Einav Y. Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: diagnosis and classification. Autoimmun Rev 2014; 13:584–586.
- Shah N, Sarode R. Thrombotic thrombocytopenic purpura-what is new? J Clin Apher 2013; 28:30–35.
- Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher 2012; 27:221–226.
- George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012; 1:604–609.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 2013; 163:514–519.
- Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 2012; 157:501–503.
- Mannucci PM, Peyvandi F. TTP and ADAMTS13: when Is testing appropriate? Hematology Am Soc Hematol Educ Program 2007; 1:121–126.
- Chaturved S, Carcioppolo D, Zhang L, McCar KR. Management and outcomes of patients with TTP: analysis of 100 cases at a single institution. Am J Hematol 2013; 88:560–565.
- Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost 2010; 8:631–640.
- Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol 2011; 86:87–89.
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica 2001; 86:1194–1199.
- Kwaan HC, Boggio LN. The clinical spectrum of thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2005; 31:673–680.
- Sarode R. Atypical presentations of thrombotic thrombocytopenic purpura: a review. J Clin Apher 2009; 24:47–52.
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis 2000; 35:E3.
- Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 2006; 4:1146–1148.
- Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost 2006; 4:698–699.
- Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med 2014; 138:546–549.
- Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005; 106:1262–1267.
- Rogers HJ, Kottke-Marchant K. ADAMTS13 evaluation for thrombotic thrombocytopenic purpura. Pathology Innovations, Pathology and Laboratory Medicine Institute. Cleveland Clinic, Fall 2014:6–9.
- Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111:1512–1514.
- Diamante Chiodini B, Davin JC, Corazza F, et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 2014; 133:e1764–e1768.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH; working party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2009; 148:37–47.
- Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36:673–681.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol 2014; 295323:1–7.
- Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol 2011; 26:621–624.
- Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O. Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 2015; 30:783–789.
- Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158:323–325.
- Abassi E, Yawn D, Leveque E, Nolasco L, Lopez J, Moake J. Correlation of ADAMTS-13 activity with response to plasma exchange in patients diagnosed with thrombotic thrombocytopenic purpura (Abstract #3921). Blood 2004; 104:242a.
- Blombery P, Scully M. Management of thrombocytic thrombocytopenic purpura: current perspectives. J Blood Med 2014; 5:15–23.
- Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion 2015; 55:18–24.
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood 2016; 127:2952–2953.
- Chapman K, Yuen S. Therapy for thrombotic thrombocytopenic purpura: past, present and future. Semin Thromb Hemost 2014; 40:34–40.
- Heidel F, Lipka DB, von Auer C, Huber C, Schrarrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007; 97:228–233.
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2004; 1:407–423.
- Shenkman B, Einav Y. Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: diagnosis and classification. Autoimmun Rev 2014; 13:584–586.
- Shah N, Sarode R. Thrombotic thrombocytopenic purpura-what is new? J Clin Apher 2013; 28:30–35.
- Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher 2012; 27:221–226.
- George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012; 1:604–609.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 2013; 163:514–519.
- Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 2012; 157:501–503.
- Mannucci PM, Peyvandi F. TTP and ADAMTS13: when Is testing appropriate? Hematology Am Soc Hematol Educ Program 2007; 1:121–126.
- Chaturved S, Carcioppolo D, Zhang L, McCar KR. Management and outcomes of patients with TTP: analysis of 100 cases at a single institution. Am J Hematol 2013; 88:560–565.
- Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost 2010; 8:631–640.
- Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol 2011; 86:87–89.
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica 2001; 86:1194–1199.
- Kwaan HC, Boggio LN. The clinical spectrum of thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2005; 31:673–680.
- Sarode R. Atypical presentations of thrombotic thrombocytopenic purpura: a review. J Clin Apher 2009; 24:47–52.
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis 2000; 35:E3.
- Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 2006; 4:1146–1148.
- Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost 2006; 4:698–699.
- Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med 2014; 138:546–549.
- Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005; 106:1262–1267.
- Rogers HJ, Kottke-Marchant K. ADAMTS13 evaluation for thrombotic thrombocytopenic purpura. Pathology Innovations, Pathology and Laboratory Medicine Institute. Cleveland Clinic, Fall 2014:6–9.
- Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111:1512–1514.
- Diamante Chiodini B, Davin JC, Corazza F, et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 2014; 133:e1764–e1768.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH; working party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2009; 148:37–47.
- Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36:673–681.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol 2014; 295323:1–7.
- Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol 2011; 26:621–624.
- Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O. Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 2015; 30:783–789.
- Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158:323–325.
- Abassi E, Yawn D, Leveque E, Nolasco L, Lopez J, Moake J. Correlation of ADAMTS-13 activity with response to plasma exchange in patients diagnosed with thrombotic thrombocytopenic purpura (Abstract #3921). Blood 2004; 104:242a.
- Blombery P, Scully M. Management of thrombocytic thrombocytopenic purpura: current perspectives. J Blood Med 2014; 5:15–23.
- Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion 2015; 55:18–24.
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood 2016; 127:2952–2953.
- Chapman K, Yuen S. Therapy for thrombotic thrombocytopenic purpura: past, present and future. Semin Thromb Hemost 2014; 40:34–40.
- Heidel F, Lipka DB, von Auer C, Huber C, Schrarrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007; 97:228–233.
KEY POINTS
- Symptoms of TTP are usually neurologic but can also be cardiac or abdominal. Thrombocytopenia and unexplained microangiopathic hemolytic anemia are sufficient to highly suspect the disease.
- In the appropriate clinical setting, an ADAMTS13 activity level lower than 10% is highly indicative of TTP.
- ADAMTS13 inhibitor and ADAMTS13 antibody assays provide more diagnostic clues. ADAMTS13 antibody is generally absent in the congenital form.
- The ADAMTS13 assay can help distinguish TTP from hemolytic-uremic syndrome, which presents similarly but typically involves normal or only mildly reduced ADAMTS13 activity.
- A strong clinical suspicion of TTP warrants immediate initiation of therapeutic plasma exchange without waiting for ADAMTS13 test results.
Postop delirium linked to greater long-term cognitive decline
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
FROM ALZHEIMER’S & DEMENTIA
Below-knee angioplasty for limb salvage: Keep it simple
PARIS – Below-the-knee plain balloon angioplasty is an effective strategy for limb-salvage in patients with critical limb ischemia who otherwise face the prospect of a major amputation, Ana P. Mollon, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Mollon of Posadas National Hospital in Buenos Aires, presented a retrospective series of 82 consecutive patients who underwent below-the-knee percutaneous angioplasty for critical limb ischemia with multivessel involvement. The amputation-free survival rate at a mean of 15.1 months of follow-up was 88%.
Sixty of the 82 patients had triple-artery involvement below the knee. The other 22 had two involved arteries. As is typical in patients with critical limb ischemia, comorbid conditions were common: Seventy-five patients had diabetes, 58 were hypertensive, and 48 were current smokers.
Of the 124 arteries treated by Dr. Mollon and coworkers, the posterior tibial artery was addressed in 41% of cases, the anterior tibial artery in 39%, and the peroneal artery in 18%. Two percent of patients received dilatation of plantar arch lesions.
Seventy percent of treated lesions were total occlusions, nearly half of which were more than 5 cm in length.
The treatment was plain balloon angioplasty in 78% of cases, drug-coated balloons in 12%, bare metal stenting in 7%, and drug-eluting stents in 3%. An antegrade approach was used in 95% of cases, and the remainder received a dual antegrade/retrograde approach.
Roughly 80% of patients were Rutherford category 5 or 6 before treatment. At 12 months post angioplasty, most patients were category 1 or 2, and about one-quarter were category 5 or 6.
Angioplasty was unsuccessful in restoring straight line flow in six patients.
All 10 patients who underwent a major amputation had triple-vessel involvement below the knee; in 9 of the 10, interventionalists were able to treat one of the three severely diseased arteries. Five of the 10 amputees had osteomyelitis.
Session chair Flavio Ribichini, MD, applauded Dr. Mollon and her Argentine colleagues for their predominant use of plain balloon angioplasty in this setting.
“I absolutely share your view on this. It’s the simplest and cheapest approach. The point is that you’re saving the foot now. It’s not that important what’s going to happen in 1 year. I don’t think it makes sense to use drug-coated balloons in this setting. It’s much more sensible to use a simple procedure and see how it goes,” said Dr. Ribichini, professor of cardiovascular medicine at the University of Verona (Italy).
Dr. Mollon said that several years ago her group briefly turned to the use of drug-coated balloons for below-the-knee limb salvage, but they soon switched back to plain balloon angioplasty because they didn’t see any advantage in patient outcomes with the more elaborate technology.
Discussant Benjamin Honton, MD, of the Pasteur Clinic in Toulouse, France, said, “We, too, have been disappointed with the drug-coated balloon, especially in the posterior tibial artery.”
Dr. Mollon reported having no financial conflicts.
PARIS – Below-the-knee plain balloon angioplasty is an effective strategy for limb-salvage in patients with critical limb ischemia who otherwise face the prospect of a major amputation, Ana P. Mollon, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Mollon of Posadas National Hospital in Buenos Aires, presented a retrospective series of 82 consecutive patients who underwent below-the-knee percutaneous angioplasty for critical limb ischemia with multivessel involvement. The amputation-free survival rate at a mean of 15.1 months of follow-up was 88%.
Sixty of the 82 patients had triple-artery involvement below the knee. The other 22 had two involved arteries. As is typical in patients with critical limb ischemia, comorbid conditions were common: Seventy-five patients had diabetes, 58 were hypertensive, and 48 were current smokers.
Of the 124 arteries treated by Dr. Mollon and coworkers, the posterior tibial artery was addressed in 41% of cases, the anterior tibial artery in 39%, and the peroneal artery in 18%. Two percent of patients received dilatation of plantar arch lesions.
Seventy percent of treated lesions were total occlusions, nearly half of which were more than 5 cm in length.
The treatment was plain balloon angioplasty in 78% of cases, drug-coated balloons in 12%, bare metal stenting in 7%, and drug-eluting stents in 3%. An antegrade approach was used in 95% of cases, and the remainder received a dual antegrade/retrograde approach.
Roughly 80% of patients were Rutherford category 5 or 6 before treatment. At 12 months post angioplasty, most patients were category 1 or 2, and about one-quarter were category 5 or 6.
Angioplasty was unsuccessful in restoring straight line flow in six patients.
All 10 patients who underwent a major amputation had triple-vessel involvement below the knee; in 9 of the 10, interventionalists were able to treat one of the three severely diseased arteries. Five of the 10 amputees had osteomyelitis.
Session chair Flavio Ribichini, MD, applauded Dr. Mollon and her Argentine colleagues for their predominant use of plain balloon angioplasty in this setting.
“I absolutely share your view on this. It’s the simplest and cheapest approach. The point is that you’re saving the foot now. It’s not that important what’s going to happen in 1 year. I don’t think it makes sense to use drug-coated balloons in this setting. It’s much more sensible to use a simple procedure and see how it goes,” said Dr. Ribichini, professor of cardiovascular medicine at the University of Verona (Italy).
Dr. Mollon said that several years ago her group briefly turned to the use of drug-coated balloons for below-the-knee limb salvage, but they soon switched back to plain balloon angioplasty because they didn’t see any advantage in patient outcomes with the more elaborate technology.
Discussant Benjamin Honton, MD, of the Pasteur Clinic in Toulouse, France, said, “We, too, have been disappointed with the drug-coated balloon, especially in the posterior tibial artery.”
Dr. Mollon reported having no financial conflicts.
PARIS – Below-the-knee plain balloon angioplasty is an effective strategy for limb-salvage in patients with critical limb ischemia who otherwise face the prospect of a major amputation, Ana P. Mollon, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Mollon of Posadas National Hospital in Buenos Aires, presented a retrospective series of 82 consecutive patients who underwent below-the-knee percutaneous angioplasty for critical limb ischemia with multivessel involvement. The amputation-free survival rate at a mean of 15.1 months of follow-up was 88%.
Sixty of the 82 patients had triple-artery involvement below the knee. The other 22 had two involved arteries. As is typical in patients with critical limb ischemia, comorbid conditions were common: Seventy-five patients had diabetes, 58 were hypertensive, and 48 were current smokers.
Of the 124 arteries treated by Dr. Mollon and coworkers, the posterior tibial artery was addressed in 41% of cases, the anterior tibial artery in 39%, and the peroneal artery in 18%. Two percent of patients received dilatation of plantar arch lesions.
Seventy percent of treated lesions were total occlusions, nearly half of which were more than 5 cm in length.
The treatment was plain balloon angioplasty in 78% of cases, drug-coated balloons in 12%, bare metal stenting in 7%, and drug-eluting stents in 3%. An antegrade approach was used in 95% of cases, and the remainder received a dual antegrade/retrograde approach.
Roughly 80% of patients were Rutherford category 5 or 6 before treatment. At 12 months post angioplasty, most patients were category 1 or 2, and about one-quarter were category 5 or 6.
Angioplasty was unsuccessful in restoring straight line flow in six patients.
All 10 patients who underwent a major amputation had triple-vessel involvement below the knee; in 9 of the 10, interventionalists were able to treat one of the three severely diseased arteries. Five of the 10 amputees had osteomyelitis.
Session chair Flavio Ribichini, MD, applauded Dr. Mollon and her Argentine colleagues for their predominant use of plain balloon angioplasty in this setting.
“I absolutely share your view on this. It’s the simplest and cheapest approach. The point is that you’re saving the foot now. It’s not that important what’s going to happen in 1 year. I don’t think it makes sense to use drug-coated balloons in this setting. It’s much more sensible to use a simple procedure and see how it goes,” said Dr. Ribichini, professor of cardiovascular medicine at the University of Verona (Italy).
Dr. Mollon said that several years ago her group briefly turned to the use of drug-coated balloons for below-the-knee limb salvage, but they soon switched back to plain balloon angioplasty because they didn’t see any advantage in patient outcomes with the more elaborate technology.
Discussant Benjamin Honton, MD, of the Pasteur Clinic in Toulouse, France, said, “We, too, have been disappointed with the drug-coated balloon, especially in the posterior tibial artery.”
Dr. Mollon reported having no financial conflicts.
AT EUROPCR 2016
Key clinical point: Experts agree that plain balloon angioplasty is the way to go for limb salvage in patients with critical limb ischemia.
Major finding: The amputation-free survival rate in a consecutive series of patients who underwent below-the-knee angioplasty for critical limb ischemia was 88% at a mean of 15.1 months of follow-up.
Data source: A retrospective case series comprising 82 consecutive patients.
Disclosures: The presenter reported having no financial conflicts.