User login
Infection, readmission linked after open lower-extremity procedures
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
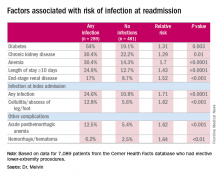
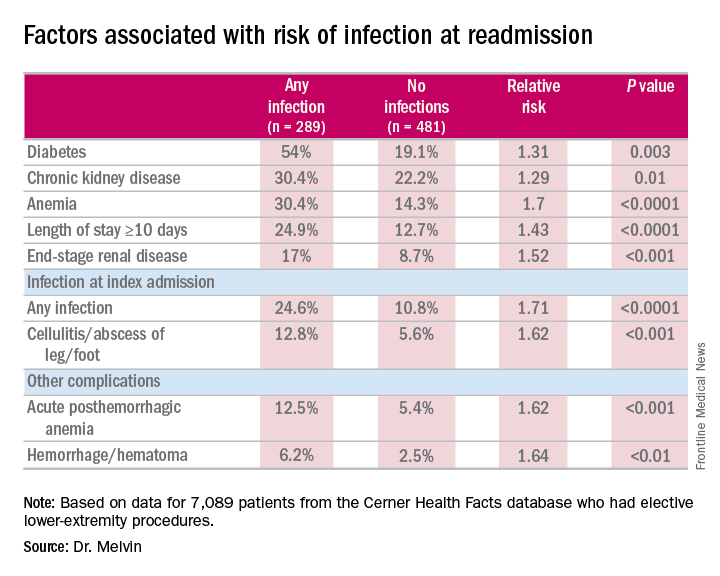
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Extended hospital stay and other factors can help identify patients at greatest risk for readmission due to infection.
Major finding: More than one-third of readmissions from lower-extremity procedures are the result of infections.
Data source: 7,089 elective lower extremity procedures selected from the Cerner Health Facts database.
Disclosures: Dr. Melvin reported having no financial disclosures.
Can carotid interventions affect cognitive function?
COLUMBUS, OHIO – The primary goal of carotid artery revascularization is to prevent stroke, heart attack or death, but carotid artery stenting and carotid endarterectomy may also cause changes in cognitive skills, according Raghu Motaganahalli, MD, of the Indiana University, Indianapolis.
“What about cognitive dysfunction as a result of carotid artery stenting (CAS) or carotid endarterectomy (CEA)?” Dr. Motaganahalli asked at the annual meeting of the Midwestern Vascular Surgical Society. “I think this is real, that there’s some truth to the matter. The question is how much and what domains of cognitive functions are affected?”
“Cerebrovascular hemodynamics status plays a role in cognitive function, but we need a better understanding of cerebrovascular hemodynamic failure and either improvement or decline of cognitive function after CAS or CEA,” he said.
A review of published trials shows that 10%-20% of patients who have either CAS or CEA have some degree of cognitive dysfunction as early as a day after the procedure. “It’s not a small number, compared to stoke, risk of myocardial infarction and death,” he said.
Some series have reported up to 40% of patients showed some cognitive dysfunction, and post–carotid endarterectomy cognitive dysfunction has been associated with early death, Dr. Motaganahalli said.
Cognitive dysfunction manifests in various forms, ranging from level of consciousness and memory to mood and ability to make calculations. Although the Mini-Mental State Examination Global Cognitive Assessment tool provides a method for evaluating cognitive function, “There is no uniformly accepted neurocognition test,” Dr. Motaganahalli said. That explains the wide variability of findings among published studies.
Vascular surgeons take a somewhat casual approach to their patients’ cognitive abilities after carotid revascularization, Dr. Motaganahalli said. “We don’t evaluate their memory and their cognitive functions on post-op day one; we just look to see whether they have neurologic dysfunction up front and that they’re capable of going home after that.”
But predicting in advance which patients are predisposed to cognitive decline after the procedures is difficult, he said. He cited a systematic review of 32 studies published between 1990-2007 that showed variable results (Stroke. 2008;39:3116-27): 11 studies during 1990-2005 suggested cognition actually improved after CEA; 9 studies during 1994-2006 suggested the opposite; 4 trials during 1992-2005 suggested no change in cognition after CEA; 5 studies during 2003-2007 showed improvement in cognition after CAS; and 3 trials comparing CAS and CEA and cognition found no differences in how the two procedures affect cognition.
Dr. Motaganahalli also cited a systematic review of 37 studies, 18 of which examined CEA, 12 CAS and seven compared CEA and CAS, found that either cognitive improvement or impairment for CEA and CAS separately were 10–15% of patients (Cerebrovasc Dis Extra. 2014;4:132-48).
“We have 69 papers that looked at cognitive function alone, but unfortunately, we don’t know whether cognitive function really improved based on this data set,” he said. “None of them are making the argument so clearly that there is cognitive improvement after revascularization.”
The variability in study findings can be due to differences in methodologies, the types of psychometric tests used, statistical analyses and the timing of cognitive assessments, Dr. Motaganahalli said.
Cognitive impairment after stroke caused by carotid disease is better understood than is cognitive impairment in the absence of a major stroke, Dr. Motaganahalli said.
“The mechanisms of how carotid disease can cause the cognitive impairment are threefold: It could be microembolism and hypoperfusion, which together can cause white matter disease and thereby some cognitive dysfunction in the long term,” he said (Neuroimaging Clin N Am. 2007 Aug;17:313-24).
Functional neurons may be a biomarker of cognitive outcome, he said. Hypoperfusion of functional neurons may lead to hypofunctional neurons, which can increase cerebral blood flow and cerebral metabolic rate for oxygen (CMRO2), and thus improve cognition. However, when additional variables are introduced to the hypofunctional neurons – such as microembolism, white matter disease, and prolonged hypoperfusion – that can lead to neuronal infarction that, while increasing cerebral blood flow, causes no change in CMRO2 and, thus, no cognitive improvement. The interval between hypofunctional neurons and neuronal infarction “is the time to do the revascularization, as long as you can demonstrate that there may be some truth to matter that it influences cognition,” Dr. Motaganahalli said.
While vascular surgeons may not be able to predict who will have cognitive decline after carotid interventions, “There are some pointers for possibly picking those patients who may benefit,” Dr. Motaganahalli said.
That choice of patients revolves around recognizing that chronic ischemia induces and increases the severity of cognitive dysfunction. Therefore, incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease may provide an opportunity to extend the goals of carotid artery revascularization to include preventing or reversing cognitive decline, he said.
Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical.
COLUMBUS, OHIO – The primary goal of carotid artery revascularization is to prevent stroke, heart attack or death, but carotid artery stenting and carotid endarterectomy may also cause changes in cognitive skills, according Raghu Motaganahalli, MD, of the Indiana University, Indianapolis.
“What about cognitive dysfunction as a result of carotid artery stenting (CAS) or carotid endarterectomy (CEA)?” Dr. Motaganahalli asked at the annual meeting of the Midwestern Vascular Surgical Society. “I think this is real, that there’s some truth to the matter. The question is how much and what domains of cognitive functions are affected?”
“Cerebrovascular hemodynamics status plays a role in cognitive function, but we need a better understanding of cerebrovascular hemodynamic failure and either improvement or decline of cognitive function after CAS or CEA,” he said.
A review of published trials shows that 10%-20% of patients who have either CAS or CEA have some degree of cognitive dysfunction as early as a day after the procedure. “It’s not a small number, compared to stoke, risk of myocardial infarction and death,” he said.
Some series have reported up to 40% of patients showed some cognitive dysfunction, and post–carotid endarterectomy cognitive dysfunction has been associated with early death, Dr. Motaganahalli said.
Cognitive dysfunction manifests in various forms, ranging from level of consciousness and memory to mood and ability to make calculations. Although the Mini-Mental State Examination Global Cognitive Assessment tool provides a method for evaluating cognitive function, “There is no uniformly accepted neurocognition test,” Dr. Motaganahalli said. That explains the wide variability of findings among published studies.
Vascular surgeons take a somewhat casual approach to their patients’ cognitive abilities after carotid revascularization, Dr. Motaganahalli said. “We don’t evaluate their memory and their cognitive functions on post-op day one; we just look to see whether they have neurologic dysfunction up front and that they’re capable of going home after that.”
But predicting in advance which patients are predisposed to cognitive decline after the procedures is difficult, he said. He cited a systematic review of 32 studies published between 1990-2007 that showed variable results (Stroke. 2008;39:3116-27): 11 studies during 1990-2005 suggested cognition actually improved after CEA; 9 studies during 1994-2006 suggested the opposite; 4 trials during 1992-2005 suggested no change in cognition after CEA; 5 studies during 2003-2007 showed improvement in cognition after CAS; and 3 trials comparing CAS and CEA and cognition found no differences in how the two procedures affect cognition.
Dr. Motaganahalli also cited a systematic review of 37 studies, 18 of which examined CEA, 12 CAS and seven compared CEA and CAS, found that either cognitive improvement or impairment for CEA and CAS separately were 10–15% of patients (Cerebrovasc Dis Extra. 2014;4:132-48).
“We have 69 papers that looked at cognitive function alone, but unfortunately, we don’t know whether cognitive function really improved based on this data set,” he said. “None of them are making the argument so clearly that there is cognitive improvement after revascularization.”
The variability in study findings can be due to differences in methodologies, the types of psychometric tests used, statistical analyses and the timing of cognitive assessments, Dr. Motaganahalli said.
Cognitive impairment after stroke caused by carotid disease is better understood than is cognitive impairment in the absence of a major stroke, Dr. Motaganahalli said.
“The mechanisms of how carotid disease can cause the cognitive impairment are threefold: It could be microembolism and hypoperfusion, which together can cause white matter disease and thereby some cognitive dysfunction in the long term,” he said (Neuroimaging Clin N Am. 2007 Aug;17:313-24).
Functional neurons may be a biomarker of cognitive outcome, he said. Hypoperfusion of functional neurons may lead to hypofunctional neurons, which can increase cerebral blood flow and cerebral metabolic rate for oxygen (CMRO2), and thus improve cognition. However, when additional variables are introduced to the hypofunctional neurons – such as microembolism, white matter disease, and prolonged hypoperfusion – that can lead to neuronal infarction that, while increasing cerebral blood flow, causes no change in CMRO2 and, thus, no cognitive improvement. The interval between hypofunctional neurons and neuronal infarction “is the time to do the revascularization, as long as you can demonstrate that there may be some truth to matter that it influences cognition,” Dr. Motaganahalli said.
While vascular surgeons may not be able to predict who will have cognitive decline after carotid interventions, “There are some pointers for possibly picking those patients who may benefit,” Dr. Motaganahalli said.
That choice of patients revolves around recognizing that chronic ischemia induces and increases the severity of cognitive dysfunction. Therefore, incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease may provide an opportunity to extend the goals of carotid artery revascularization to include preventing or reversing cognitive decline, he said.
Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical.
COLUMBUS, OHIO – The primary goal of carotid artery revascularization is to prevent stroke, heart attack or death, but carotid artery stenting and carotid endarterectomy may also cause changes in cognitive skills, according Raghu Motaganahalli, MD, of the Indiana University, Indianapolis.
“What about cognitive dysfunction as a result of carotid artery stenting (CAS) or carotid endarterectomy (CEA)?” Dr. Motaganahalli asked at the annual meeting of the Midwestern Vascular Surgical Society. “I think this is real, that there’s some truth to the matter. The question is how much and what domains of cognitive functions are affected?”
“Cerebrovascular hemodynamics status plays a role in cognitive function, but we need a better understanding of cerebrovascular hemodynamic failure and either improvement or decline of cognitive function after CAS or CEA,” he said.
A review of published trials shows that 10%-20% of patients who have either CAS or CEA have some degree of cognitive dysfunction as early as a day after the procedure. “It’s not a small number, compared to stoke, risk of myocardial infarction and death,” he said.
Some series have reported up to 40% of patients showed some cognitive dysfunction, and post–carotid endarterectomy cognitive dysfunction has been associated with early death, Dr. Motaganahalli said.
Cognitive dysfunction manifests in various forms, ranging from level of consciousness and memory to mood and ability to make calculations. Although the Mini-Mental State Examination Global Cognitive Assessment tool provides a method for evaluating cognitive function, “There is no uniformly accepted neurocognition test,” Dr. Motaganahalli said. That explains the wide variability of findings among published studies.
Vascular surgeons take a somewhat casual approach to their patients’ cognitive abilities after carotid revascularization, Dr. Motaganahalli said. “We don’t evaluate their memory and their cognitive functions on post-op day one; we just look to see whether they have neurologic dysfunction up front and that they’re capable of going home after that.”
But predicting in advance which patients are predisposed to cognitive decline after the procedures is difficult, he said. He cited a systematic review of 32 studies published between 1990-2007 that showed variable results (Stroke. 2008;39:3116-27): 11 studies during 1990-2005 suggested cognition actually improved after CEA; 9 studies during 1994-2006 suggested the opposite; 4 trials during 1992-2005 suggested no change in cognition after CEA; 5 studies during 2003-2007 showed improvement in cognition after CAS; and 3 trials comparing CAS and CEA and cognition found no differences in how the two procedures affect cognition.
Dr. Motaganahalli also cited a systematic review of 37 studies, 18 of which examined CEA, 12 CAS and seven compared CEA and CAS, found that either cognitive improvement or impairment for CEA and CAS separately were 10–15% of patients (Cerebrovasc Dis Extra. 2014;4:132-48).
“We have 69 papers that looked at cognitive function alone, but unfortunately, we don’t know whether cognitive function really improved based on this data set,” he said. “None of them are making the argument so clearly that there is cognitive improvement after revascularization.”
The variability in study findings can be due to differences in methodologies, the types of psychometric tests used, statistical analyses and the timing of cognitive assessments, Dr. Motaganahalli said.
Cognitive impairment after stroke caused by carotid disease is better understood than is cognitive impairment in the absence of a major stroke, Dr. Motaganahalli said.
“The mechanisms of how carotid disease can cause the cognitive impairment are threefold: It could be microembolism and hypoperfusion, which together can cause white matter disease and thereby some cognitive dysfunction in the long term,” he said (Neuroimaging Clin N Am. 2007 Aug;17:313-24).
Functional neurons may be a biomarker of cognitive outcome, he said. Hypoperfusion of functional neurons may lead to hypofunctional neurons, which can increase cerebral blood flow and cerebral metabolic rate for oxygen (CMRO2), and thus improve cognition. However, when additional variables are introduced to the hypofunctional neurons – such as microembolism, white matter disease, and prolonged hypoperfusion – that can lead to neuronal infarction that, while increasing cerebral blood flow, causes no change in CMRO2 and, thus, no cognitive improvement. The interval between hypofunctional neurons and neuronal infarction “is the time to do the revascularization, as long as you can demonstrate that there may be some truth to matter that it influences cognition,” Dr. Motaganahalli said.
While vascular surgeons may not be able to predict who will have cognitive decline after carotid interventions, “There are some pointers for possibly picking those patients who may benefit,” Dr. Motaganahalli said.
That choice of patients revolves around recognizing that chronic ischemia induces and increases the severity of cognitive dysfunction. Therefore, incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease may provide an opportunity to extend the goals of carotid artery revascularization to include preventing or reversing cognitive decline, he said.
Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical.
AT THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGERY SOCIETY
Key clinical point: Incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease could expand the goals of revascularization to encompass cognitive decline.
Major finding: Cerebrovascular hemodynamic status plays a role in cognitive function after carotid artery interventions, but the mechanisms of either improvement or decline need better understanding.
Data source: Systematic review of 32 papers on neurocognition after carotid interventions published between 1990-2007 and analysis of 37 studies of CAS or CEA or both published since 2007.
Disclosures: Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical Inc.
Study identifies SSI risk factors after open LEB
COLUMBUS, OHIO – A study of vascular procedures at 35 Michigan hospitals has identified three risk factors for surgical site infection after lower-extremity bypass that hospitals and vascular surgery teams may be able to modify.
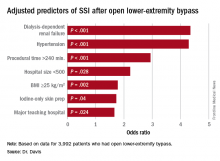
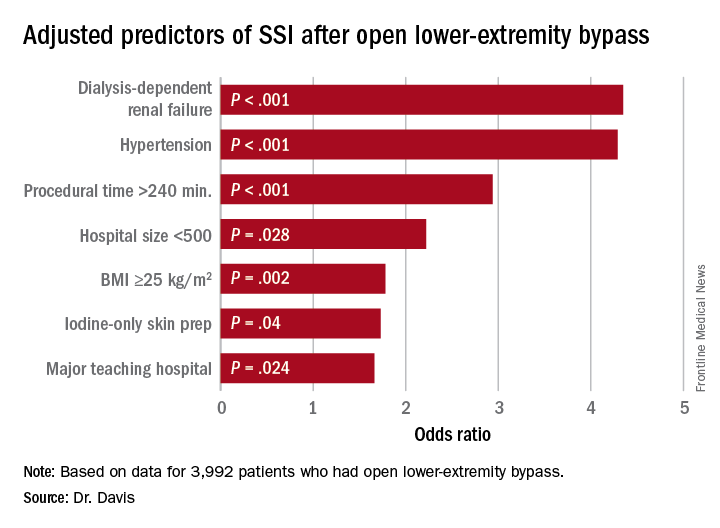
“Patients who had iodine-only skin antiseptic preparation, a high-peak intraoperative glucose, or long operative times were more likely to have substantially increased risk for surgical site infection (SSI),” Frank Davis, MD, of the University of Michigan said in reporting the study results at the annual meeting of the Midwestern Vascular Surgical Society. Those risk factors are modifiable, Dr. Davis said.
“Specific attention needs to be served moving forward in attempts to decrease the risk of SSI for lower-extremity bypass,” Dr. Davis said. “The incidence of SSI in our cohort across the state of Michigan was approximately 9.2%, and for those who did develop a SSI, there was a substantial increase in 30-day morbidity.”
Patients who had an SSI were more than three times more likely to have a major amputation (9% vs. 2.3%) than those without, and more than five times more likely to have a reoperation (3.9% vs. 0.7%), Dr. Davis said.
“With regard to preoperative symptomatology, those with lower peripheral artery questionnaire scores, resting pain, or acute ischemia were more likely to develop SSI postoperatively,” Dr. Davis said. “Patients who underwent an interim coronal bypass had a significant increase of SSI in comparison to all other bypass configurations.”
He also noted that major teaching hospitals or hospitals with 500 or fewer beds had higher rates of SSI.
“Targeted improvements in preoperative care may decrease complications and improve vascular patient outcomes,” Dr. Davis said.
Dr. Davis had no relationships to disclose.
COLUMBUS, OHIO – A study of vascular procedures at 35 Michigan hospitals has identified three risk factors for surgical site infection after lower-extremity bypass that hospitals and vascular surgery teams may be able to modify.
“Patients who had iodine-only skin antiseptic preparation, a high-peak intraoperative glucose, or long operative times were more likely to have substantially increased risk for surgical site infection (SSI),” Frank Davis, MD, of the University of Michigan said in reporting the study results at the annual meeting of the Midwestern Vascular Surgical Society. Those risk factors are modifiable, Dr. Davis said.
“Specific attention needs to be served moving forward in attempts to decrease the risk of SSI for lower-extremity bypass,” Dr. Davis said. “The incidence of SSI in our cohort across the state of Michigan was approximately 9.2%, and for those who did develop a SSI, there was a substantial increase in 30-day morbidity.”
Patients who had an SSI were more than three times more likely to have a major amputation (9% vs. 2.3%) than those without, and more than five times more likely to have a reoperation (3.9% vs. 0.7%), Dr. Davis said.
“With regard to preoperative symptomatology, those with lower peripheral artery questionnaire scores, resting pain, or acute ischemia were more likely to develop SSI postoperatively,” Dr. Davis said. “Patients who underwent an interim coronal bypass had a significant increase of SSI in comparison to all other bypass configurations.”
He also noted that major teaching hospitals or hospitals with 500 or fewer beds had higher rates of SSI.
“Targeted improvements in preoperative care may decrease complications and improve vascular patient outcomes,” Dr. Davis said.
Dr. Davis had no relationships to disclose.
COLUMBUS, OHIO – A study of vascular procedures at 35 Michigan hospitals has identified three risk factors for surgical site infection after lower-extremity bypass that hospitals and vascular surgery teams may be able to modify.
“Patients who had iodine-only skin antiseptic preparation, a high-peak intraoperative glucose, or long operative times were more likely to have substantially increased risk for surgical site infection (SSI),” Frank Davis, MD, of the University of Michigan said in reporting the study results at the annual meeting of the Midwestern Vascular Surgical Society. Those risk factors are modifiable, Dr. Davis said.
“Specific attention needs to be served moving forward in attempts to decrease the risk of SSI for lower-extremity bypass,” Dr. Davis said. “The incidence of SSI in our cohort across the state of Michigan was approximately 9.2%, and for those who did develop a SSI, there was a substantial increase in 30-day morbidity.”
Patients who had an SSI were more than three times more likely to have a major amputation (9% vs. 2.3%) than those without, and more than five times more likely to have a reoperation (3.9% vs. 0.7%), Dr. Davis said.
“With regard to preoperative symptomatology, those with lower peripheral artery questionnaire scores, resting pain, or acute ischemia were more likely to develop SSI postoperatively,” Dr. Davis said. “Patients who underwent an interim coronal bypass had a significant increase of SSI in comparison to all other bypass configurations.”
He also noted that major teaching hospitals or hospitals with 500 or fewer beds had higher rates of SSI.
“Targeted improvements in preoperative care may decrease complications and improve vascular patient outcomes,” Dr. Davis said.
Dr. Davis had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Study identified three key modifiable risk factors in surgical site infection (SSI) open after lower-extremity bypass (LEB).
Major finding: Incidence of SSI was 9.2% in the study cohort.
Data source: Blue Cross Blue Shield Michigan Vascular Intervention Collaborative database of 3,992 open LEB operations at 35 centers from January 2012 to June 2015.
Disclosures: Dr. Davis reported having no financial disclosures.
Left ventricular thrombosis can still complicate acute myocardial infarction
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
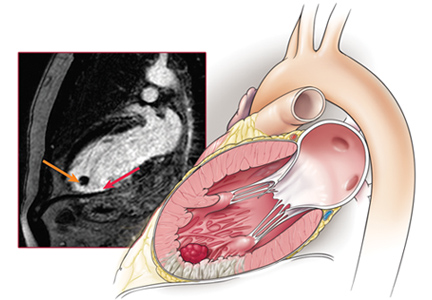
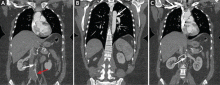
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
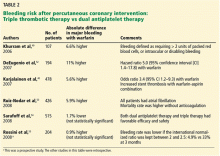
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
AAA screening showed no mortality reduction in new trial
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
These new data will not change the finding of robust reduction in AAA-related mortality from screening seen in all previous meta-analyses. However, the most recently updated meta-analyses now reveal the small reduction in all-cause mortality with screening to be statistically significant.
So although the findings of the Western Australian trial remain negative and raise some concerns about screening, their aggregation with other studies does not change the overall conclusions that screening substantially reduced AAA-related mortality and also resulted in a statistically significant reduction in all-cause mortality. Restricting screening to men who have smoked (the strongest risk factor for AAA) further lowers cost and increases efficiency.
Frank A. Lederle, MD, is from the Center for Chronic Disease Outcomes Research at the Veterans Affairs Medical Center. These comments are taken from an accompanying editorial (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6663). No conflicts of interest were declared.
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
In contrast to previous studies, screening for abdominal aortic aneurysms in older men does not appear to have a significant effect on overall mortality, according to a prospective, randomized study.
Mortality from ruptured AAA remains high in older men, which has prompted four previous large randomized trials to explore whether screening men aged 65 years and older might reduce mortality.
Writing in the October 31 online edition of JAMA Internal Medicine, the authors reported the long-term outcomes of an Australian population-based trial of screening for abdominal aortic aneurysms in 49,801 men aged 64-83 years, of whom 19 249 were invited to screening and 12,203 of those underwent screening (isrctn.org Identifier: ISRCTN16171472).
After a mean 12.8 years of follow-up, there was a non-significant 9% lower mortality in the invited screening group compared to the control group and a non-significant 8% lower mortality among men aged 65-74 years.
Overall, there were 90 deaths from ruptured AAA in the screening group and 98 in the control group (JAMA Internal Medicine 2016, October 31. DOI:10.1001/jamainternmed.2016.6633).
The prevalence of abdominal aortic aneurysms with a diameter at or above 30 mm was 6.6% in men aged 65-74, and 0.4% for those with a diameter of 55 mm or above.
While the rate of ruptured abdominal aortic aneurysms was significantly lower in the invited group compared to the control group (72 vs. 99, P = .04), the 30-day mortality after surgery for rupture was higher in the invited group compared to the control group (61.5% vs. 43.2%).
Screening had no meaningful impact on the risk of all-cause, cardiovascular, and other mortality, but men who had smoked had a higher risk of rupture and of death from a rupture than those who had never smoked, regardless of screening status.
The rate of total elective operations was significantly higher in the invited group compared to controls (536 vs. 414, P < .001), mainly in the first year after screening.
The authors calculated that to prevent one death from a ruptured abdominal aortic aneurysm in five years, 4784 men aged 64-83 years or 3290 men aged 65-74 years would need to be invited for screening.
While the strength of the study was that it was truly population-based – using the electoral roll – the authors said the lack of a benefit from screening was likely due to the relatively low rate of rupture and death from AAA, as well as a high rate of elective surgery for this condition, in the control group.
The non-significant 8% reduction in mortality observed in the study was significantly less than the 42% and 66% reductions seen in previous trials with a similar length of follow-up.
The authors suggested this may also have been related to a lower fraction of invited men participating in screening, but pointed out that the incidence of AAA in men is declining.
“The reason for the decrease in incidence and prevalence is multifactorial but is probably driven by differences in rates of smoking and cessation because the relative risk for AAA events is 3- to 6-fold higher in smokers compared with non-smokers,” they wrote.
The authors said selective screening of smokers or ex-smokers may be more effective, but pointed out that this approach would miss around one-quarter of aneurysms. However they suggested more targeted screening may yet achieve a benefit.
“The small overall benefit of population-wide screening does not mean that finding AAAs in suitable older men is not worthwhile because deaths from AAAs in men who actually attended for screening were halved by early detection and successful treatment.”
The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
Key clinical point:
Major finding: Men invited to undergo screening for abdominal aortic aneurysms had a non-significant 9% lower mortality compared to a control group.
Data source: Prospective, population-based randomized controlled trial in 49,801 men aged 64-83 years.
Disclosures: The study was supported by the National Health and Medical Research Council Project. The authors reported that they had no conflicts of interest.
Midterm results of thoracic stenting for acute type B dissection promising
LAS VEGAS – Patients with acute, complicated type B aortic dissections are reported to have a greater than 50% likelihood of dying from their condition. Three-year results of the Valiant thoracic stent graft in the treatment of these dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%, according to Ali Azizzadeh, MD.
Dr. Azizzadeh presented the midterm results of the Medtronic Dissection US IDE trial of endovascular treatment with the Valiant Captivia thoracic stent graft (Medtronic) in acute, complicated type B aortic dissection patients at the 2016 Vascular Interventional Advances meeting.
One-year outcomes of the trial were reported last year in the Annals of Thoracic Surgery (2015 Sep;100:802-9).
Dr. Azizzadeh is a vascular surgeon at the Memorial Hermann Heart and Vascular Institute, Houston.
Between June 2010 and May 2012, 50 patients with acute, complicated type B aortic dissection were enrolled at 16 clinical sites in the United States in this multicenter, prospective, nonrandomized trial with a planned 5-year follow-up.
The primary safety endpoint was all-cause mortality within 30 days from the index procedure.
A total of 28 patients completed their 3-year follow-up. Through 3 years, there were no postindex ruptures or conversions to open surgical repair reported in the trial.
At 3 years, true lumen diameter over the stented region (or endograft segment) remained stable or increased in 92.3% of patients, according to Dr. Azizzadeh. False lumen diameter remained stable or decreased in 69.3% of patients, and the false lumen was partially or completely thrombosed in 75% of patients.
One death (from sepsis) occurred between years 2 and 3; and was adjudicated by the clinical events committee as unrelated to the device, the procedure, or the dissection.
Although these midterm results are encouraging, said Dr. Azizzadeh, longer-term outcomes are needed to assess the durability of the stent graft in this indication.
The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
LAS VEGAS – Patients with acute, complicated type B aortic dissections are reported to have a greater than 50% likelihood of dying from their condition. Three-year results of the Valiant thoracic stent graft in the treatment of these dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%, according to Ali Azizzadeh, MD.
Dr. Azizzadeh presented the midterm results of the Medtronic Dissection US IDE trial of endovascular treatment with the Valiant Captivia thoracic stent graft (Medtronic) in acute, complicated type B aortic dissection patients at the 2016 Vascular Interventional Advances meeting.
One-year outcomes of the trial were reported last year in the Annals of Thoracic Surgery (2015 Sep;100:802-9).
Dr. Azizzadeh is a vascular surgeon at the Memorial Hermann Heart and Vascular Institute, Houston.
Between June 2010 and May 2012, 50 patients with acute, complicated type B aortic dissection were enrolled at 16 clinical sites in the United States in this multicenter, prospective, nonrandomized trial with a planned 5-year follow-up.
The primary safety endpoint was all-cause mortality within 30 days from the index procedure.
A total of 28 patients completed their 3-year follow-up. Through 3 years, there were no postindex ruptures or conversions to open surgical repair reported in the trial.
At 3 years, true lumen diameter over the stented region (or endograft segment) remained stable or increased in 92.3% of patients, according to Dr. Azizzadeh. False lumen diameter remained stable or decreased in 69.3% of patients, and the false lumen was partially or completely thrombosed in 75% of patients.
One death (from sepsis) occurred between years 2 and 3; and was adjudicated by the clinical events committee as unrelated to the device, the procedure, or the dissection.
Although these midterm results are encouraging, said Dr. Azizzadeh, longer-term outcomes are needed to assess the durability of the stent graft in this indication.
The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
LAS VEGAS – Patients with acute, complicated type B aortic dissections are reported to have a greater than 50% likelihood of dying from their condition. Three-year results of the Valiant thoracic stent graft in the treatment of these dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%, according to Ali Azizzadeh, MD.
Dr. Azizzadeh presented the midterm results of the Medtronic Dissection US IDE trial of endovascular treatment with the Valiant Captivia thoracic stent graft (Medtronic) in acute, complicated type B aortic dissection patients at the 2016 Vascular Interventional Advances meeting.
One-year outcomes of the trial were reported last year in the Annals of Thoracic Surgery (2015 Sep;100:802-9).
Dr. Azizzadeh is a vascular surgeon at the Memorial Hermann Heart and Vascular Institute, Houston.
Between June 2010 and May 2012, 50 patients with acute, complicated type B aortic dissection were enrolled at 16 clinical sites in the United States in this multicenter, prospective, nonrandomized trial with a planned 5-year follow-up.
The primary safety endpoint was all-cause mortality within 30 days from the index procedure.
A total of 28 patients completed their 3-year follow-up. Through 3 years, there were no postindex ruptures or conversions to open surgical repair reported in the trial.
At 3 years, true lumen diameter over the stented region (or endograft segment) remained stable or increased in 92.3% of patients, according to Dr. Azizzadeh. False lumen diameter remained stable or decreased in 69.3% of patients, and the false lumen was partially or completely thrombosed in 75% of patients.
One death (from sepsis) occurred between years 2 and 3; and was adjudicated by the clinical events committee as unrelated to the device, the procedure, or the dissection.
Although these midterm results are encouraging, said Dr. Azizzadeh, longer-term outcomes are needed to assess the durability of the stent graft in this indication.
The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
AT VIVA16 LAS VEGAS
Key clinical point:
Major finding: Three-year results of the Valiant thoracic stent graft in the treatment of acute type B dissections showed freedom from all-cause mortality of 79.4%, and a freedom from dissection-related mortality of 90%.
Data source: Midterm results were presented from the multicenter, prospective, nonrandomized Medtronic Dissection US IDE trial.
Disclosures: The trial was sponsored by Medtronic. Dr. Azizzadeh has consulted for and received research/trial funding from W.L. Gore & Associates and Medtronic.
Fast-Track EVAR protocol shown safe, effective, and cheaper
LAS VEGAS – A ‘fast-track” stenting method for endovascular abdominal aortic aneurysm repair (EVAR) enabled patients to be treated safely and effectively without general anesthetic or ICU admission, and with next-day discharge, Zvonimir Krajcer, MD, said at the 2016 Vascular Interventional Advances meeting.
Dr. Krajcer discussed the final results of the Prospective LIFE Registry, which followed 250 patients treated with the Ovation Abdominal Stent Graft platform, who had suitable femoral arteries to allow the use of the Perclose ProGlide Suture-Mediated Closure (SMC) System.
For the Fast-Track patients, vascular access, stent graft delivery, and deployment were successful. The Fast-Track EVAR protocol was successfully completed in 216 (87%) of the patients. In comparing the Fast-Track cohort (n = 216) to the non–Fast-Track cohort (n = 34), procedure time was found to be 84 vs. 110 minutes, the use of general anesthesia was 0% vs. 18%, and the need for ICU stay was 0% vs. 32%. Hospital stay for the two groups was 1.2 vs. 1.9 days, respectively.
Quality of life score improvement from baseline to 30 days as assessed via the EQ-5D questionnaire, was significantly greater in the Fast-Track patients, compared with the EVAR controls, said Dr. Krajcer, an interventional cardiologist at Texas Heart Institute and St. Luke’s Hospital, Houston, and a clinical professor of medicine at Baylor College of Medicine.
To determine adverse events, patients were followed through 1 month after treatment. The researchers found no device- or procedure-related major adverse events, abdominal aortic aneurysm (AAA) ruptures, surgical conversions, or AAA-related secondary interventions. One patient in the fast-track group died from acute respiratory failure. Overall, for the Fast-Track and control groups, the freedom from type I/III endoleak was 99% and 100%, respectively.
Dr. Krajcer reported that the 30-day hospital readmission rate in the LIFE study was 1.6%, compared to 8% reported for EVAR from the American College of Surgeons National Surgical Quality Improvement Program.
The economic analysis was performed comparing the Fast-Track patients to a control group, which consisted of a database of 8,306 patients treated with elective infrarenal EVAR at 3,750 U.S. hospitals based on inpatient discharge between 2012 and 2015. The researchers calculated costs related to access, anesthesia, ICU stay, and hospital stay.
The Fast-Track protocol showed $21,000 in perioperative cost savings relative to standard EVAR, largely driven by differences in hospital stay costs, according to Dr. Krajcer.
“Fast-track EVAR using the Ovation Prime stent graft is safe and feasible and lowers perioperative costs,” said Dr. Krajcer. “Our results warrant the establishment of a Fast-Track EVAR protocol in experienced EVAR centers,” he concluded.
Dr. Krajcer had nothing to disclose.
LAS VEGAS – A ‘fast-track” stenting method for endovascular abdominal aortic aneurysm repair (EVAR) enabled patients to be treated safely and effectively without general anesthetic or ICU admission, and with next-day discharge, Zvonimir Krajcer, MD, said at the 2016 Vascular Interventional Advances meeting.
Dr. Krajcer discussed the final results of the Prospective LIFE Registry, which followed 250 patients treated with the Ovation Abdominal Stent Graft platform, who had suitable femoral arteries to allow the use of the Perclose ProGlide Suture-Mediated Closure (SMC) System.
For the Fast-Track patients, vascular access, stent graft delivery, and deployment were successful. The Fast-Track EVAR protocol was successfully completed in 216 (87%) of the patients. In comparing the Fast-Track cohort (n = 216) to the non–Fast-Track cohort (n = 34), procedure time was found to be 84 vs. 110 minutes, the use of general anesthesia was 0% vs. 18%, and the need for ICU stay was 0% vs. 32%. Hospital stay for the two groups was 1.2 vs. 1.9 days, respectively.
Quality of life score improvement from baseline to 30 days as assessed via the EQ-5D questionnaire, was significantly greater in the Fast-Track patients, compared with the EVAR controls, said Dr. Krajcer, an interventional cardiologist at Texas Heart Institute and St. Luke’s Hospital, Houston, and a clinical professor of medicine at Baylor College of Medicine.
To determine adverse events, patients were followed through 1 month after treatment. The researchers found no device- or procedure-related major adverse events, abdominal aortic aneurysm (AAA) ruptures, surgical conversions, or AAA-related secondary interventions. One patient in the fast-track group died from acute respiratory failure. Overall, for the Fast-Track and control groups, the freedom from type I/III endoleak was 99% and 100%, respectively.
Dr. Krajcer reported that the 30-day hospital readmission rate in the LIFE study was 1.6%, compared to 8% reported for EVAR from the American College of Surgeons National Surgical Quality Improvement Program.
The economic analysis was performed comparing the Fast-Track patients to a control group, which consisted of a database of 8,306 patients treated with elective infrarenal EVAR at 3,750 U.S. hospitals based on inpatient discharge between 2012 and 2015. The researchers calculated costs related to access, anesthesia, ICU stay, and hospital stay.
The Fast-Track protocol showed $21,000 in perioperative cost savings relative to standard EVAR, largely driven by differences in hospital stay costs, according to Dr. Krajcer.
“Fast-track EVAR using the Ovation Prime stent graft is safe and feasible and lowers perioperative costs,” said Dr. Krajcer. “Our results warrant the establishment of a Fast-Track EVAR protocol in experienced EVAR centers,” he concluded.
Dr. Krajcer had nothing to disclose.
LAS VEGAS – A ‘fast-track” stenting method for endovascular abdominal aortic aneurysm repair (EVAR) enabled patients to be treated safely and effectively without general anesthetic or ICU admission, and with next-day discharge, Zvonimir Krajcer, MD, said at the 2016 Vascular Interventional Advances meeting.
Dr. Krajcer discussed the final results of the Prospective LIFE Registry, which followed 250 patients treated with the Ovation Abdominal Stent Graft platform, who had suitable femoral arteries to allow the use of the Perclose ProGlide Suture-Mediated Closure (SMC) System.
For the Fast-Track patients, vascular access, stent graft delivery, and deployment were successful. The Fast-Track EVAR protocol was successfully completed in 216 (87%) of the patients. In comparing the Fast-Track cohort (n = 216) to the non–Fast-Track cohort (n = 34), procedure time was found to be 84 vs. 110 minutes, the use of general anesthesia was 0% vs. 18%, and the need for ICU stay was 0% vs. 32%. Hospital stay for the two groups was 1.2 vs. 1.9 days, respectively.
Quality of life score improvement from baseline to 30 days as assessed via the EQ-5D questionnaire, was significantly greater in the Fast-Track patients, compared with the EVAR controls, said Dr. Krajcer, an interventional cardiologist at Texas Heart Institute and St. Luke’s Hospital, Houston, and a clinical professor of medicine at Baylor College of Medicine.
To determine adverse events, patients were followed through 1 month after treatment. The researchers found no device- or procedure-related major adverse events, abdominal aortic aneurysm (AAA) ruptures, surgical conversions, or AAA-related secondary interventions. One patient in the fast-track group died from acute respiratory failure. Overall, for the Fast-Track and control groups, the freedom from type I/III endoleak was 99% and 100%, respectively.
Dr. Krajcer reported that the 30-day hospital readmission rate in the LIFE study was 1.6%, compared to 8% reported for EVAR from the American College of Surgeons National Surgical Quality Improvement Program.
The economic analysis was performed comparing the Fast-Track patients to a control group, which consisted of a database of 8,306 patients treated with elective infrarenal EVAR at 3,750 U.S. hospitals based on inpatient discharge between 2012 and 2015. The researchers calculated costs related to access, anesthesia, ICU stay, and hospital stay.
The Fast-Track protocol showed $21,000 in perioperative cost savings relative to standard EVAR, largely driven by differences in hospital stay costs, according to Dr. Krajcer.
“Fast-track EVAR using the Ovation Prime stent graft is safe and feasible and lowers perioperative costs,” said Dr. Krajcer. “Our results warrant the establishment of a Fast-Track EVAR protocol in experienced EVAR centers,” he concluded.
Dr. Krajcer had nothing to disclose.
AT VIVA16
Key clinical point:
Major finding: Quality of life score improvement from baseline to 30 days was significantly greater in the Fast-Track patients, compared with the EVAR controls.
Data source: The researchers assessed 250 patients in the Prospective LIFE Registry and compared results to more than 8,000 patients in an EVAR database as controls.
Disclosures: Dr. Krajcer had nothing to disclose.
Nonatherosclerotic limb ischemia: Prompt evaluation and diagnosis
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
KEY POINTS
- A high index of suspicion should be maintained to recognize symptoms consistent with limb ischemia in a younger patient in the absence of the usual atherosclerosis risk factors.
- A workup for most conditions includes noninvasive vascular ultrasonography to detect and quantify limb ischemia.
- Prompt referral for surgical or endovascular treatment is necessary for optimal limb salvage.
Benefits of early endovascular thrombectomy outlined in five trials
For patients with large-vessel ischemic stroke, endovascular thrombectomy produces better functional outcomes at 90 days than does optimal medical therapy, as long as the procedure is started within 7.3 hours of symptom onset, according to a report published online Sept. 27 in JAMA.
The benefit of thrombectomy was greatest when the procedure was begun under 2 hours from symptom onset, and it became nonsignificant after 7 hours and 18 minutes elapsed. This emphasizes “the importance of programs to enhance patient awareness, out-of-hospital care, and in-hospital management to shorten symptom onset-to-treatment times,” wrote Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates.
Five major randomized trials have demonstrated the benefit of second-generation endovascular recanalization therapies over medical therapy in this patient population, but uncertainties persist regarding the timing of the intervention. For example, practice guidelines in the United States recommend thrombectomy until 6 hours after symptom onset, but the Food and Drug Administration allows thrombectomy devices to be used up to 8 hours after symptom onset and Canadian guidelines recommend the procedure for selected patients up to 12 hours after symptom onset.
The investigators for the five trials formed the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration to pool their individual patient data and perform a meta-analysis to clarify the issue of timing. They assessed patients’ functional independence at 90 days using the modified Rankin Score (mRS). The study participants included 634 patients who had been randomly assigned to endovascular thrombectomy and 653 randomly assigned to medical therapy.
The intervention correlated with a substantially lower degree of patient disability at 90 days than did medical therapy: the mean mRS was 2.9 in the thrombectomy group and 3.6 in the medical therapy group. In addition, increasing delays in treatment were associated with higher levels of residual disability in the thrombectomy group but not in the medical therapy group, the investigators reported.
“Based on the current study, and assuming the findings are generalizable to the population of patients with acute ischemic stroke due to large-vessel occlusion, among every 1,000 patients achieving substantial endovascular reperfusion, for every 15-minute faster ED door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence (mRS 0-2),” Dr. Saver and his associates wrote (JAMA. 2016;316[12]:1279-88).
These findings reinforce current recommendations to attempt endovascular thrombectomy when the procedure can be initiated within 6 hours of symptom onset, and they also “provide evidence that potentially supports strengthening of the recommendation for treatment from 6 through 7.3 hours after symptom onset,” they added.
No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
Earlier thrombectomy has such a profound effect on stroke patients’ outcomes that substantial changes in the current medical system are warranted to shorten these times even further. In this study, median time from symptom onset to randomization was approximately 3 hours, median time to arterial puncture was approximately 4 hours, and median time to reperfusion was nearly 5 hours.
Reducing the number of patients who are transferred from community hospitals to facilities with stroke centers would shorten door-to-reperfusion time a great deal. It is estimated that direct transport to stroke centers would allow endovascular thrombectomy for an additional 13% of stroke patients. Telemedicine, mobile stroke units, and out-of-hospital administration of tissue plasminogen activator are other possibilities that should be investigated.
Steven Warach, MD, PhD, and S. Claiborne Johnston, MD, PhD, are with the University of Texas at Austin. They reported having no relevant financial disclosures. Dr. Warach and Dr. Johnston made these remarks in an editorial (JAMA. 2016;316[12]:1265-6) accompanying Dr. Saver’s report.
Earlier thrombectomy has such a profound effect on stroke patients’ outcomes that substantial changes in the current medical system are warranted to shorten these times even further. In this study, median time from symptom onset to randomization was approximately 3 hours, median time to arterial puncture was approximately 4 hours, and median time to reperfusion was nearly 5 hours.
Reducing the number of patients who are transferred from community hospitals to facilities with stroke centers would shorten door-to-reperfusion time a great deal. It is estimated that direct transport to stroke centers would allow endovascular thrombectomy for an additional 13% of stroke patients. Telemedicine, mobile stroke units, and out-of-hospital administration of tissue plasminogen activator are other possibilities that should be investigated.
Steven Warach, MD, PhD, and S. Claiborne Johnston, MD, PhD, are with the University of Texas at Austin. They reported having no relevant financial disclosures. Dr. Warach and Dr. Johnston made these remarks in an editorial (JAMA. 2016;316[12]:1265-6) accompanying Dr. Saver’s report.
Earlier thrombectomy has such a profound effect on stroke patients’ outcomes that substantial changes in the current medical system are warranted to shorten these times even further. In this study, median time from symptom onset to randomization was approximately 3 hours, median time to arterial puncture was approximately 4 hours, and median time to reperfusion was nearly 5 hours.
Reducing the number of patients who are transferred from community hospitals to facilities with stroke centers would shorten door-to-reperfusion time a great deal. It is estimated that direct transport to stroke centers would allow endovascular thrombectomy for an additional 13% of stroke patients. Telemedicine, mobile stroke units, and out-of-hospital administration of tissue plasminogen activator are other possibilities that should be investigated.
Steven Warach, MD, PhD, and S. Claiborne Johnston, MD, PhD, are with the University of Texas at Austin. They reported having no relevant financial disclosures. Dr. Warach and Dr. Johnston made these remarks in an editorial (JAMA. 2016;316[12]:1265-6) accompanying Dr. Saver’s report.
For patients with large-vessel ischemic stroke, endovascular thrombectomy produces better functional outcomes at 90 days than does optimal medical therapy, as long as the procedure is started within 7.3 hours of symptom onset, according to a report published online Sept. 27 in JAMA.
The benefit of thrombectomy was greatest when the procedure was begun under 2 hours from symptom onset, and it became nonsignificant after 7 hours and 18 minutes elapsed. This emphasizes “the importance of programs to enhance patient awareness, out-of-hospital care, and in-hospital management to shorten symptom onset-to-treatment times,” wrote Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates.
Five major randomized trials have demonstrated the benefit of second-generation endovascular recanalization therapies over medical therapy in this patient population, but uncertainties persist regarding the timing of the intervention. For example, practice guidelines in the United States recommend thrombectomy until 6 hours after symptom onset, but the Food and Drug Administration allows thrombectomy devices to be used up to 8 hours after symptom onset and Canadian guidelines recommend the procedure for selected patients up to 12 hours after symptom onset.
The investigators for the five trials formed the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration to pool their individual patient data and perform a meta-analysis to clarify the issue of timing. They assessed patients’ functional independence at 90 days using the modified Rankin Score (mRS). The study participants included 634 patients who had been randomly assigned to endovascular thrombectomy and 653 randomly assigned to medical therapy.
The intervention correlated with a substantially lower degree of patient disability at 90 days than did medical therapy: the mean mRS was 2.9 in the thrombectomy group and 3.6 in the medical therapy group. In addition, increasing delays in treatment were associated with higher levels of residual disability in the thrombectomy group but not in the medical therapy group, the investigators reported.
“Based on the current study, and assuming the findings are generalizable to the population of patients with acute ischemic stroke due to large-vessel occlusion, among every 1,000 patients achieving substantial endovascular reperfusion, for every 15-minute faster ED door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence (mRS 0-2),” Dr. Saver and his associates wrote (JAMA. 2016;316[12]:1279-88).
These findings reinforce current recommendations to attempt endovascular thrombectomy when the procedure can be initiated within 6 hours of symptom onset, and they also “provide evidence that potentially supports strengthening of the recommendation for treatment from 6 through 7.3 hours after symptom onset,” they added.
No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
For patients with large-vessel ischemic stroke, endovascular thrombectomy produces better functional outcomes at 90 days than does optimal medical therapy, as long as the procedure is started within 7.3 hours of symptom onset, according to a report published online Sept. 27 in JAMA.
The benefit of thrombectomy was greatest when the procedure was begun under 2 hours from symptom onset, and it became nonsignificant after 7 hours and 18 minutes elapsed. This emphasizes “the importance of programs to enhance patient awareness, out-of-hospital care, and in-hospital management to shorten symptom onset-to-treatment times,” wrote Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates.
Five major randomized trials have demonstrated the benefit of second-generation endovascular recanalization therapies over medical therapy in this patient population, but uncertainties persist regarding the timing of the intervention. For example, practice guidelines in the United States recommend thrombectomy until 6 hours after symptom onset, but the Food and Drug Administration allows thrombectomy devices to be used up to 8 hours after symptom onset and Canadian guidelines recommend the procedure for selected patients up to 12 hours after symptom onset.
The investigators for the five trials formed the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration to pool their individual patient data and perform a meta-analysis to clarify the issue of timing. They assessed patients’ functional independence at 90 days using the modified Rankin Score (mRS). The study participants included 634 patients who had been randomly assigned to endovascular thrombectomy and 653 randomly assigned to medical therapy.
The intervention correlated with a substantially lower degree of patient disability at 90 days than did medical therapy: the mean mRS was 2.9 in the thrombectomy group and 3.6 in the medical therapy group. In addition, increasing delays in treatment were associated with higher levels of residual disability in the thrombectomy group but not in the medical therapy group, the investigators reported.
“Based on the current study, and assuming the findings are generalizable to the population of patients with acute ischemic stroke due to large-vessel occlusion, among every 1,000 patients achieving substantial endovascular reperfusion, for every 15-minute faster ED door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence (mRS 0-2),” Dr. Saver and his associates wrote (JAMA. 2016;316[12]:1279-88).
These findings reinforce current recommendations to attempt endovascular thrombectomy when the procedure can be initiated within 6 hours of symptom onset, and they also “provide evidence that potentially supports strengthening of the recommendation for treatment from 6 through 7.3 hours after symptom onset,” they added.
No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Endovascular thrombectomy started within 7.3 hours of symptom onset for large-vessel ischemic stroke produces better outcomes than does optimal medical therapy.
Major finding: For every 15-minute shorter door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence.
Data source: A meta-analysis of pooled data from five randomized clinical trials involving 1,287 patients.
Disclosures: No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
Blacks found to have higher amputation rates than whites
COLUMBUS, OHIO – The disparity in amputation rates between blacks and non-Hispanic whites in Chicago was higher than the national average and up to five times greater on the predominantly black South Side than on the predominantly white North Side of the city.
The reasons for this disparity are unclear, but a recent study of white and black Chicagoans has shown that the rates at which the races self-report claudication do not differ, suggesting that better utilization of public health resources in the black community could potentially reduce this disparity.
Reporting at the annual meeting of the Midwestern Vascular Surgery Society, lead investigator Samantha Minc, MD, of Rush University in Chicago, said the difference in amputation rates may be traced to later recognition of disease in nonwhites.
“Possible reasons for late recognition of disease include lack of access to care, communication issues with doctors, education issues, or the patient and/or caregiver and primary care provider having difficulty accessing specialists,” Dr. Minc said. “Another often-cited explanation for the late recognition is the possibility of differences in disease symptom manifestations in nonwhites, which is what we chose to study.”
Investigators from Rush University analyzed data from a combined cohort of Chicago residents age 65 years and older from two studies of aging: The Rush Memory and Aging Project and the Minority Aging Research Study.
Because of the segregated nature of Chicago – the North Side is mostly white, the South Side mostly black and the West Side mostly Hispanic – prior research has coupled census data with the Illinois Department of Public Health database to analyze care patterns. This data showed that the disparity in amputation rates among groups in Chicago was higher than the national average and up to five times greater on the South Side, compared with the North, Dr. Minc said.
The latest study matched blacks and whites from both studies 1:2 based on age, education, gender, and length of follow-up. In all, a cohort of 2,487 subjects was generated: 801 blacks and 1,686 non-Hispanic whites. Among blacks, 6.5% reported claudication at baseline, compared with 5.9% of whites, and at any point of the study 19.5% of blacks and 19.6% of non-Hispanic whites reported claudication, differences that were not statistically significant.
Blacks did have higher rates of diabetes, 27% vs. 12.6%, were more likely to have used tobacco, more likely to be female, and were slightly younger on average, but other demographic factors – such as income or rates of congestive heart failure and coronary artery disease – were not significantly different, Dr. Minc said.
The study findings will inform the Chicago Department of Public Health’s Healthy Chicago 2.0 initiative to reduce the disparity in amputation rates between whites and blacks by 10% by 2020.
Dr. Minc acknowledged limitations of the study, among them that participants were volunteers, aged 65 years and older, and 72% female, that Hispanics were excluded, and that the use of the Modified Rose/World Health Organization questionnaire to detect claudication is “specific, but not very sensitive.”
“Our findings suggest that the delay in recognition in disease in nonwhites is not due to a racial differences in disease manifestation or symptomatology and that addressing socioeconomic issues, such as access to care, communication, and education may reduce the racial disparity in amputation rates,” Dr. Minc said. “Public health resources should focus on early recognition of the disease in higher-risk groups to decrease this disparity.”
Dr. Minc and her coauthors had no relationships to disclose.
COLUMBUS, OHIO – The disparity in amputation rates between blacks and non-Hispanic whites in Chicago was higher than the national average and up to five times greater on the predominantly black South Side than on the predominantly white North Side of the city.
The reasons for this disparity are unclear, but a recent study of white and black Chicagoans has shown that the rates at which the races self-report claudication do not differ, suggesting that better utilization of public health resources in the black community could potentially reduce this disparity.
Reporting at the annual meeting of the Midwestern Vascular Surgery Society, lead investigator Samantha Minc, MD, of Rush University in Chicago, said the difference in amputation rates may be traced to later recognition of disease in nonwhites.
“Possible reasons for late recognition of disease include lack of access to care, communication issues with doctors, education issues, or the patient and/or caregiver and primary care provider having difficulty accessing specialists,” Dr. Minc said. “Another often-cited explanation for the late recognition is the possibility of differences in disease symptom manifestations in nonwhites, which is what we chose to study.”
Investigators from Rush University analyzed data from a combined cohort of Chicago residents age 65 years and older from two studies of aging: The Rush Memory and Aging Project and the Minority Aging Research Study.
Because of the segregated nature of Chicago – the North Side is mostly white, the South Side mostly black and the West Side mostly Hispanic – prior research has coupled census data with the Illinois Department of Public Health database to analyze care patterns. This data showed that the disparity in amputation rates among groups in Chicago was higher than the national average and up to five times greater on the South Side, compared with the North, Dr. Minc said.
The latest study matched blacks and whites from both studies 1:2 based on age, education, gender, and length of follow-up. In all, a cohort of 2,487 subjects was generated: 801 blacks and 1,686 non-Hispanic whites. Among blacks, 6.5% reported claudication at baseline, compared with 5.9% of whites, and at any point of the study 19.5% of blacks and 19.6% of non-Hispanic whites reported claudication, differences that were not statistically significant.
Blacks did have higher rates of diabetes, 27% vs. 12.6%, were more likely to have used tobacco, more likely to be female, and were slightly younger on average, but other demographic factors – such as income or rates of congestive heart failure and coronary artery disease – were not significantly different, Dr. Minc said.
The study findings will inform the Chicago Department of Public Health’s Healthy Chicago 2.0 initiative to reduce the disparity in amputation rates between whites and blacks by 10% by 2020.
Dr. Minc acknowledged limitations of the study, among them that participants were volunteers, aged 65 years and older, and 72% female, that Hispanics were excluded, and that the use of the Modified Rose/World Health Organization questionnaire to detect claudication is “specific, but not very sensitive.”
“Our findings suggest that the delay in recognition in disease in nonwhites is not due to a racial differences in disease manifestation or symptomatology and that addressing socioeconomic issues, such as access to care, communication, and education may reduce the racial disparity in amputation rates,” Dr. Minc said. “Public health resources should focus on early recognition of the disease in higher-risk groups to decrease this disparity.”
Dr. Minc and her coauthors had no relationships to disclose.
COLUMBUS, OHIO – The disparity in amputation rates between blacks and non-Hispanic whites in Chicago was higher than the national average and up to five times greater on the predominantly black South Side than on the predominantly white North Side of the city.
The reasons for this disparity are unclear, but a recent study of white and black Chicagoans has shown that the rates at which the races self-report claudication do not differ, suggesting that better utilization of public health resources in the black community could potentially reduce this disparity.
Reporting at the annual meeting of the Midwestern Vascular Surgery Society, lead investigator Samantha Minc, MD, of Rush University in Chicago, said the difference in amputation rates may be traced to later recognition of disease in nonwhites.
“Possible reasons for late recognition of disease include lack of access to care, communication issues with doctors, education issues, or the patient and/or caregiver and primary care provider having difficulty accessing specialists,” Dr. Minc said. “Another often-cited explanation for the late recognition is the possibility of differences in disease symptom manifestations in nonwhites, which is what we chose to study.”
Investigators from Rush University analyzed data from a combined cohort of Chicago residents age 65 years and older from two studies of aging: The Rush Memory and Aging Project and the Minority Aging Research Study.
Because of the segregated nature of Chicago – the North Side is mostly white, the South Side mostly black and the West Side mostly Hispanic – prior research has coupled census data with the Illinois Department of Public Health database to analyze care patterns. This data showed that the disparity in amputation rates among groups in Chicago was higher than the national average and up to five times greater on the South Side, compared with the North, Dr. Minc said.
The latest study matched blacks and whites from both studies 1:2 based on age, education, gender, and length of follow-up. In all, a cohort of 2,487 subjects was generated: 801 blacks and 1,686 non-Hispanic whites. Among blacks, 6.5% reported claudication at baseline, compared with 5.9% of whites, and at any point of the study 19.5% of blacks and 19.6% of non-Hispanic whites reported claudication, differences that were not statistically significant.
Blacks did have higher rates of diabetes, 27% vs. 12.6%, were more likely to have used tobacco, more likely to be female, and were slightly younger on average, but other demographic factors – such as income or rates of congestive heart failure and coronary artery disease – were not significantly different, Dr. Minc said.
The study findings will inform the Chicago Department of Public Health’s Healthy Chicago 2.0 initiative to reduce the disparity in amputation rates between whites and blacks by 10% by 2020.
Dr. Minc acknowledged limitations of the study, among them that participants were volunteers, aged 65 years and older, and 72% female, that Hispanics were excluded, and that the use of the Modified Rose/World Health Organization questionnaire to detect claudication is “specific, but not very sensitive.”
“Our findings suggest that the delay in recognition in disease in nonwhites is not due to a racial differences in disease manifestation or symptomatology and that addressing socioeconomic issues, such as access to care, communication, and education may reduce the racial disparity in amputation rates,” Dr. Minc said. “Public health resources should focus on early recognition of the disease in higher-risk groups to decrease this disparity.”
Dr. Minc and her coauthors had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Amputation rates in Chicago were up to five times greater on the predominantly black South Side of the city than on the predominantly white North Side of the city.
Major finding: Disparities in amputation rates suggest a need to address access to care and improve communication and education with regard to amputation risks in the nonwhite communities.
Data source: 2,487 subjects from the Rush Memory and Aging Project and the Minority Aging Research Study.
Disclosures: Dr. Minc and her coauthors had no relationships to disclose.