User login
Correction: Update on VTE
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
In the article, “Update on the management of venous thromboembolism” (Bartholomew JR, Cleve Clin J Med 2017; 84[suppl 3]:39–46), 2 sentences in the text regarding dose reduction for body weight have errors. The corrected sentences follow:
On page 42, left column, the last 5 lines should read: “The recommended dose should be reduced to 2.5 mg twice daily in patients that meet 2 of the following criteria: age 80 or older; body weight of 60 kg or less; or with a serum creatinine 1.5 mg/dL or greater.”
And on page 42, right column, the sentence 10 lines from the top should read: “Edoxaban is given orally at 60 mg once daily but reduced to 30 mg once daily if the CrCL is 30 mL/min to 50 mL/min, if body weight is 60 kg or less, or with use of certain P-glycoprotein inhibitors.”
Promising outcomes of thrombolysis for caval extension of iliofemoral DVT
CHICAGO – Caval extension of an acute iliofemoral deep vein thrombosis paradoxically portends better treatment outcomes than does thrombolysis of a DVT without involvement of the inferior vena cava, according to Rabih A. Chaer, MD, professor of surgery at the University of Pittsburgh.
This finding from a retrospective analysis of the University of Pittsburgh experience might seem counterintuitive. After all, caval extension clearly indicates a greater clot burden. One possible explanation: Clearing a thrombus from a large vessel, such as the inferior vena cava (IVC), provides an added protective effect. Also, since the caval segments don’t have valves – their flow is based upon negative pressure in the chest – they may not contribute as much to postthrombotic morbidity to the same extent as do thrombosed iliofemoral segments, Dr. Chaer speculated at a symposium on vascular surgery sponsored by Northwestern University.
The impetus for Dr. Chaer and coinvestigators to review the Pittsburgh experience was a lack of clarity in the literature as to the effect IVC thrombosis has on thrombolysis outcomes in patients with acute iliofemoral DVT. Even though caval thrombus extension is present in up to 22% of patients with iliofemoral DVT, current guidelines issued by the American College of Chest Physicians, the American Heart Association, and the Society for Vascular Surgery don’t address the distinction between iliofemoral DVT with and without IVC extension in regard to the occurrence of postthrombotic syndrome (PTS), the most common complication of DVT.
The incidence of PTS in patients whose iliofemoral DVT is treated by anticoagulation and compression alone is up to 50%. Mounting evidence indicates that catheter-directed thrombolysis and pharmacomechanical thrombolysis aimed at achieving early thrombus removal and symptom relief help maintain valvular competence and reduce the risk of PTS, the surgeon noted.
PTS is diagnosed using the validated Villalta scale, which incorporates clinical signs including pain on calf compression, skin edema and redness, and ulcers, as well as symptoms such as leg cramping, heaviness, itching, and paresthesia.
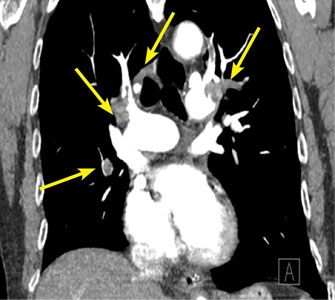
The Pittsburgh series included 102 consecutive patients treated with various combinations of catheter-directed or pharmacomechanical thrombolysis in 127 limbs with acute iliofemoral thrombosis. In 46 patients, the thrombus extended into the IVC, all the way up to the renal veins in most cases.
The groups with and without caval extension were similar in terms of age and prevalence of malignancy, hypercoagulable state, and clot age. However, a history of previous DVT was significantly more common in the group with IVC thrombus. Also, more than 60% of patients with caval extension got an IVC filter, a rate more than 10-fold greater than that in patients without caval extension.
In this series, caval thrombosis had no effect on the technical success of thrombolysis. The technical success rate –defined as at least 50% clot lysis – was 89% in both groups. Rates of recurrent DVT within 30 days were similar in the two groups as well: 11% in the caval thrombosis group and 14% in the noncaval group. At 2 years postintervention, 77%-78% of patients in both groups remained free of DVT recurrence. The rate of PTS – defined by a Villalta score of 5 or more – at 2 years was 34% in the noncaval group, which was significantly higher than the 11% rate in patients with IVC thrombus extension. Ultrasound-identified valve reflux was present in 51% of the noncaval group at 2 years, compared with 51% of the noncaval group.
On multivariate analysis, incomplete clot lysis was associated with nearly a 23-fold increased risk of recurrent DVT and a 5.6-fold increased risk of PTS. Caval involvement was independently associated with a 78% reduction in PTS risk.
The Society for Vascular Surgery’s guidelines recommend pharmacomechanical thrombolysis over catheter-directed thrombolysis if the expertise is available. The Pittsburgh experience speaks to the worth of that recommendation.
“Pharmacomechanical techniques can be advantageous. They can expedite the lysis process by clearing most of the clot. In our series, 20 patients were treated with pharmacomechanical techniques in a single session,” Dr. Chaer noted.
The use of IVC filters in the setting of caval extension of iliofemoral DVT is controversial, according to the surgeon: A thrombus that gets trapped in the filter is tough to remove, precluding successful recanalization.
“One-third of the patients in our series got a filter, but we’ve become more conservative nowadays. We don’t use filters anymore. But I think those patients who might benefit from an IVC filter are those who present with a PE [pulmonary embolism], because that’s telling you they might develop another PE, as well as those patients in whom pharmacomechanical thrombolysis is anticipated because we’ve seen that those patients are also more likely to develop a PE,” he said.
The University of Pittsburgh study on the effect of IVC thrombus extension has been published (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:385-91).
Dr. Chaer reported serving as a paid speaker for Boston Scientific.
SOURCE: Chaer RA. Northwestern Vascular Symposium 2017.
CHICAGO – Caval extension of an acute iliofemoral deep vein thrombosis paradoxically portends better treatment outcomes than does thrombolysis of a DVT without involvement of the inferior vena cava, according to Rabih A. Chaer, MD, professor of surgery at the University of Pittsburgh.
This finding from a retrospective analysis of the University of Pittsburgh experience might seem counterintuitive. After all, caval extension clearly indicates a greater clot burden. One possible explanation: Clearing a thrombus from a large vessel, such as the inferior vena cava (IVC), provides an added protective effect. Also, since the caval segments don’t have valves – their flow is based upon negative pressure in the chest – they may not contribute as much to postthrombotic morbidity to the same extent as do thrombosed iliofemoral segments, Dr. Chaer speculated at a symposium on vascular surgery sponsored by Northwestern University.
The impetus for Dr. Chaer and coinvestigators to review the Pittsburgh experience was a lack of clarity in the literature as to the effect IVC thrombosis has on thrombolysis outcomes in patients with acute iliofemoral DVT. Even though caval thrombus extension is present in up to 22% of patients with iliofemoral DVT, current guidelines issued by the American College of Chest Physicians, the American Heart Association, and the Society for Vascular Surgery don’t address the distinction between iliofemoral DVT with and without IVC extension in regard to the occurrence of postthrombotic syndrome (PTS), the most common complication of DVT.
The incidence of PTS in patients whose iliofemoral DVT is treated by anticoagulation and compression alone is up to 50%. Mounting evidence indicates that catheter-directed thrombolysis and pharmacomechanical thrombolysis aimed at achieving early thrombus removal and symptom relief help maintain valvular competence and reduce the risk of PTS, the surgeon noted.
PTS is diagnosed using the validated Villalta scale, which incorporates clinical signs including pain on calf compression, skin edema and redness, and ulcers, as well as symptoms such as leg cramping, heaviness, itching, and paresthesia.
The Pittsburgh series included 102 consecutive patients treated with various combinations of catheter-directed or pharmacomechanical thrombolysis in 127 limbs with acute iliofemoral thrombosis. In 46 patients, the thrombus extended into the IVC, all the way up to the renal veins in most cases.
The groups with and without caval extension were similar in terms of age and prevalence of malignancy, hypercoagulable state, and clot age. However, a history of previous DVT was significantly more common in the group with IVC thrombus. Also, more than 60% of patients with caval extension got an IVC filter, a rate more than 10-fold greater than that in patients without caval extension.
In this series, caval thrombosis had no effect on the technical success of thrombolysis. The technical success rate –defined as at least 50% clot lysis – was 89% in both groups. Rates of recurrent DVT within 30 days were similar in the two groups as well: 11% in the caval thrombosis group and 14% in the noncaval group. At 2 years postintervention, 77%-78% of patients in both groups remained free of DVT recurrence. The rate of PTS – defined by a Villalta score of 5 or more – at 2 years was 34% in the noncaval group, which was significantly higher than the 11% rate in patients with IVC thrombus extension. Ultrasound-identified valve reflux was present in 51% of the noncaval group at 2 years, compared with 51% of the noncaval group.
On multivariate analysis, incomplete clot lysis was associated with nearly a 23-fold increased risk of recurrent DVT and a 5.6-fold increased risk of PTS. Caval involvement was independently associated with a 78% reduction in PTS risk.
The Society for Vascular Surgery’s guidelines recommend pharmacomechanical thrombolysis over catheter-directed thrombolysis if the expertise is available. The Pittsburgh experience speaks to the worth of that recommendation.
“Pharmacomechanical techniques can be advantageous. They can expedite the lysis process by clearing most of the clot. In our series, 20 patients were treated with pharmacomechanical techniques in a single session,” Dr. Chaer noted.
The use of IVC filters in the setting of caval extension of iliofemoral DVT is controversial, according to the surgeon: A thrombus that gets trapped in the filter is tough to remove, precluding successful recanalization.
“One-third of the patients in our series got a filter, but we’ve become more conservative nowadays. We don’t use filters anymore. But I think those patients who might benefit from an IVC filter are those who present with a PE [pulmonary embolism], because that’s telling you they might develop another PE, as well as those patients in whom pharmacomechanical thrombolysis is anticipated because we’ve seen that those patients are also more likely to develop a PE,” he said.
The University of Pittsburgh study on the effect of IVC thrombus extension has been published (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:385-91).
Dr. Chaer reported serving as a paid speaker for Boston Scientific.
SOURCE: Chaer RA. Northwestern Vascular Symposium 2017.
CHICAGO – Caval extension of an acute iliofemoral deep vein thrombosis paradoxically portends better treatment outcomes than does thrombolysis of a DVT without involvement of the inferior vena cava, according to Rabih A. Chaer, MD, professor of surgery at the University of Pittsburgh.
This finding from a retrospective analysis of the University of Pittsburgh experience might seem counterintuitive. After all, caval extension clearly indicates a greater clot burden. One possible explanation: Clearing a thrombus from a large vessel, such as the inferior vena cava (IVC), provides an added protective effect. Also, since the caval segments don’t have valves – their flow is based upon negative pressure in the chest – they may not contribute as much to postthrombotic morbidity to the same extent as do thrombosed iliofemoral segments, Dr. Chaer speculated at a symposium on vascular surgery sponsored by Northwestern University.
The impetus for Dr. Chaer and coinvestigators to review the Pittsburgh experience was a lack of clarity in the literature as to the effect IVC thrombosis has on thrombolysis outcomes in patients with acute iliofemoral DVT. Even though caval thrombus extension is present in up to 22% of patients with iliofemoral DVT, current guidelines issued by the American College of Chest Physicians, the American Heart Association, and the Society for Vascular Surgery don’t address the distinction between iliofemoral DVT with and without IVC extension in regard to the occurrence of postthrombotic syndrome (PTS), the most common complication of DVT.
The incidence of PTS in patients whose iliofemoral DVT is treated by anticoagulation and compression alone is up to 50%. Mounting evidence indicates that catheter-directed thrombolysis and pharmacomechanical thrombolysis aimed at achieving early thrombus removal and symptom relief help maintain valvular competence and reduce the risk of PTS, the surgeon noted.
PTS is diagnosed using the validated Villalta scale, which incorporates clinical signs including pain on calf compression, skin edema and redness, and ulcers, as well as symptoms such as leg cramping, heaviness, itching, and paresthesia.
The Pittsburgh series included 102 consecutive patients treated with various combinations of catheter-directed or pharmacomechanical thrombolysis in 127 limbs with acute iliofemoral thrombosis. In 46 patients, the thrombus extended into the IVC, all the way up to the renal veins in most cases.
The groups with and without caval extension were similar in terms of age and prevalence of malignancy, hypercoagulable state, and clot age. However, a history of previous DVT was significantly more common in the group with IVC thrombus. Also, more than 60% of patients with caval extension got an IVC filter, a rate more than 10-fold greater than that in patients without caval extension.
In this series, caval thrombosis had no effect on the technical success of thrombolysis. The technical success rate –defined as at least 50% clot lysis – was 89% in both groups. Rates of recurrent DVT within 30 days were similar in the two groups as well: 11% in the caval thrombosis group and 14% in the noncaval group. At 2 years postintervention, 77%-78% of patients in both groups remained free of DVT recurrence. The rate of PTS – defined by a Villalta score of 5 or more – at 2 years was 34% in the noncaval group, which was significantly higher than the 11% rate in patients with IVC thrombus extension. Ultrasound-identified valve reflux was present in 51% of the noncaval group at 2 years, compared with 51% of the noncaval group.
On multivariate analysis, incomplete clot lysis was associated with nearly a 23-fold increased risk of recurrent DVT and a 5.6-fold increased risk of PTS. Caval involvement was independently associated with a 78% reduction in PTS risk.
The Society for Vascular Surgery’s guidelines recommend pharmacomechanical thrombolysis over catheter-directed thrombolysis if the expertise is available. The Pittsburgh experience speaks to the worth of that recommendation.
“Pharmacomechanical techniques can be advantageous. They can expedite the lysis process by clearing most of the clot. In our series, 20 patients were treated with pharmacomechanical techniques in a single session,” Dr. Chaer noted.
The use of IVC filters in the setting of caval extension of iliofemoral DVT is controversial, according to the surgeon: A thrombus that gets trapped in the filter is tough to remove, precluding successful recanalization.
“One-third of the patients in our series got a filter, but we’ve become more conservative nowadays. We don’t use filters anymore. But I think those patients who might benefit from an IVC filter are those who present with a PE [pulmonary embolism], because that’s telling you they might develop another PE, as well as those patients in whom pharmacomechanical thrombolysis is anticipated because we’ve seen that those patients are also more likely to develop a PE,” he said.
The University of Pittsburgh study on the effect of IVC thrombus extension has been published (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:385-91).
Dr. Chaer reported serving as a paid speaker for Boston Scientific.
SOURCE: Chaer RA. Northwestern Vascular Symposium 2017.
REPORTING FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Know risk factors for ischemic colitis after AAA repair
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CHICAGO – Postoperative ischemic colitis after abdominal aortic aneurysm (AAA) repair is a feared, potentially devastating complication with a mortality approaching 50%, but early diagnosis can mitigate that risk, Roy M. Fujitani, MD, said at a symposium on vascular surgery sponsored by Northwestern University in Chicago.
The most common etiology of ischemic colitis following AAA repair is hypoperfusion of the mesenteric vasculature leading to nonocclusive ischemia. Caught early – in the initial hyperactive phase of colonic ischemia – the complication is typically transient and can be managed medically without further sequelae. Improvement is generally noted within a day or 2, with complete resolution within 1-2 weeks.
The earliest indicator that a patient is in the hyperactive phase of ischemic colitis following completion of an AAA repair can be defecation while still on the operating table.
“When you’ve just completed an operation and the patient has a bowel movement right on the operating table, that always makes me very, very concerned because of the likelihood of an associated ischemic colitis,” the surgeon noted.
A conscious patient in the first phase of ischemic colitis will describe an urgent desire to defecate, along with crampy pain and loose bowel movements with or without blood in the stool.
In the second, paralytic phase of ischemic colitis, the pain diminishes in intensity but becomes more continuous and diffuse, usually in the lateral borders of the abdomen. The abdomen becomes distended and much more tender, and there are no bowel sounds.
In patients whose ischemic colitis has been misdiagnosed or undiagnosed, the shock phase comes next. This is marked by massive fluid, protein, and electrolyte loss through the gangrenous mucosa. The result is severe dehydration, metabolic acidosis, and hypovolemic shock.
Nonocclusive colonic ischemia most often affects the watershed areas of the colon, such as the Sudeck point at the rectosigmoid junction.
The two other etiologies of ischemic colitis occurring as a complication of AAA repair are acute arterial occlusion, typically caused by iatrogenic embolization from a proximal source, often during endovascular aneurysm repair (EVAR), or rarely, venous thrombosis.
Making the diagnosis
When a patient is suspected of having ischemic colitis, one of the easiest ways of advancing toward a diagnosis is to obtain an abdominal plain x-ray, which classically shows thumb printing indicative of submucosal edema. CT with IV contrast typically shows bowel wall thickening, pericolonic fat stranding, and – most significantly – there may be free air within the colonic wall, an indicator of more advanced ischemia that occurs shortly before transmural gangrenous changes.
Colonoscopy is, however, the mainstay of diagnosis. It should be performed in any patient where postoperative ischemic colitis is suspected.
Ischemic colitis risk factors and outcomes
Dr. Fujitani was senior author of the largest ever study of risk factors for and outcomes of postoperative ischemic colitis in patients undergoing contemporary methods of open and endovascular AAA repair. This retrospective analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database included 3,486 patients who underwent AAA repair in U.S. hospitals during 2011-2012. Twelve percent had an open repair, while the other 88% underwent EVAR.
The incidence of postoperative ischemic colitis was 2.2%. The median time of diagnosis was on postoperative day 2. The rate was nearly threefold higher in the open repair group: 5.2% versus 1.8%. However, the open-repair group had a higher rate of emergency admission, ruptured aneurysm before surgery, and other high-risk features. Upon multivariate analysis, the adjusted risk of postoperative ischemic colitis was no longer significantly different in the open-repair and EVAR groups.
The mean hospital length of stay in patients with postoperative ischemic colitis was 20 days, compared with 5 days in those without the complication. The unadjusted in-hospital mortality rate in patients with ischemic colitis was 39% versus 4% in those without ischemic colitis.
Of the 75 patients who developed postoperative ischemic colitis, 37 were managed medically, 38 surgically.
“What was quite surprising was that there was a 56.8% in-hospital mortality in the surgically treated patients. The point being that if you end up having ischemic colitis, there’s a 50% chance you’ll end up requiring an operation, and if you do undergo an operation you have more than a 50% chance of succumbing from the process,” Dr. Fujitani observed.
Dr. Fujitani and his coinvestigators scrutinized a plethora of potential risk factors for postoperative ischemic colitis. Six emerged as significant upon multivariate analysis: ruptured aneurysm before surgery, with an associated adjusted 4.1-fold increased risk; need for intra- or postoperative transfusion, with a 6-fold increased risk; renal failure requiring dialysis, with a 3.9-fold risk; proximal extension of the aneurysm, with a 2.2-fold elevation in risk; diabetes, with a 1.9-fold risk; and female sex, with an adjusted 1.75-fold increased risk (J Vasc Surg. 2016 Apr;63[4]:866-72).
Of note, these risk factors are largely unmodifiable, which underscores the importance of vigorous surveillance for possible signs of ischemic colitis during the first 4 days after AAA repair, especially in patients with multiple risk factors, Dr. Fujitani said.
Also, careful intraoperative assessment of the collateral mesenteric vascular anatomy is important in assessing a patient’s risk for postoperative ischemic colitis. This assessment should include the superior and inferior mesenteric arteries, as well as the celiac and internal iliac arteries. It’s worth bearing in mind that, even though collateral flow may appear adequate, it can be affected by hypovolemia, hypotension, or low cardiac output, the surgeon continued.
In the NSQIP data analysis, no patients who underwent reimplantation of the inferior mesenteric artery during open repair developed postoperative ischemic colitis. While this is an encouraging finding, the numbers were too small to draw definitive conclusions as to whether reimplantation of the artery is protective. It’s an important issue for further study, though, since so few of the recognized risk factors for the complication are modifiable, Dr. Fujitani noted.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CHICAGO – Postoperative ischemic colitis after abdominal aortic aneurysm (AAA) repair is a feared, potentially devastating complication with a mortality approaching 50%, but early diagnosis can mitigate that risk, Roy M. Fujitani, MD, said at a symposium on vascular surgery sponsored by Northwestern University in Chicago.
The most common etiology of ischemic colitis following AAA repair is hypoperfusion of the mesenteric vasculature leading to nonocclusive ischemia. Caught early – in the initial hyperactive phase of colonic ischemia – the complication is typically transient and can be managed medically without further sequelae. Improvement is generally noted within a day or 2, with complete resolution within 1-2 weeks.
The earliest indicator that a patient is in the hyperactive phase of ischemic colitis following completion of an AAA repair can be defecation while still on the operating table.
“When you’ve just completed an operation and the patient has a bowel movement right on the operating table, that always makes me very, very concerned because of the likelihood of an associated ischemic colitis,” the surgeon noted.
A conscious patient in the first phase of ischemic colitis will describe an urgent desire to defecate, along with crampy pain and loose bowel movements with or without blood in the stool.
In the second, paralytic phase of ischemic colitis, the pain diminishes in intensity but becomes more continuous and diffuse, usually in the lateral borders of the abdomen. The abdomen becomes distended and much more tender, and there are no bowel sounds.
In patients whose ischemic colitis has been misdiagnosed or undiagnosed, the shock phase comes next. This is marked by massive fluid, protein, and electrolyte loss through the gangrenous mucosa. The result is severe dehydration, metabolic acidosis, and hypovolemic shock.
Nonocclusive colonic ischemia most often affects the watershed areas of the colon, such as the Sudeck point at the rectosigmoid junction.
The two other etiologies of ischemic colitis occurring as a complication of AAA repair are acute arterial occlusion, typically caused by iatrogenic embolization from a proximal source, often during endovascular aneurysm repair (EVAR), or rarely, venous thrombosis.
Making the diagnosis
When a patient is suspected of having ischemic colitis, one of the easiest ways of advancing toward a diagnosis is to obtain an abdominal plain x-ray, which classically shows thumb printing indicative of submucosal edema. CT with IV contrast typically shows bowel wall thickening, pericolonic fat stranding, and – most significantly – there may be free air within the colonic wall, an indicator of more advanced ischemia that occurs shortly before transmural gangrenous changes.
Colonoscopy is, however, the mainstay of diagnosis. It should be performed in any patient where postoperative ischemic colitis is suspected.
Ischemic colitis risk factors and outcomes
Dr. Fujitani was senior author of the largest ever study of risk factors for and outcomes of postoperative ischemic colitis in patients undergoing contemporary methods of open and endovascular AAA repair. This retrospective analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database included 3,486 patients who underwent AAA repair in U.S. hospitals during 2011-2012. Twelve percent had an open repair, while the other 88% underwent EVAR.
The incidence of postoperative ischemic colitis was 2.2%. The median time of diagnosis was on postoperative day 2. The rate was nearly threefold higher in the open repair group: 5.2% versus 1.8%. However, the open-repair group had a higher rate of emergency admission, ruptured aneurysm before surgery, and other high-risk features. Upon multivariate analysis, the adjusted risk of postoperative ischemic colitis was no longer significantly different in the open-repair and EVAR groups.
The mean hospital length of stay in patients with postoperative ischemic colitis was 20 days, compared with 5 days in those without the complication. The unadjusted in-hospital mortality rate in patients with ischemic colitis was 39% versus 4% in those without ischemic colitis.
Of the 75 patients who developed postoperative ischemic colitis, 37 were managed medically, 38 surgically.
“What was quite surprising was that there was a 56.8% in-hospital mortality in the surgically treated patients. The point being that if you end up having ischemic colitis, there’s a 50% chance you’ll end up requiring an operation, and if you do undergo an operation you have more than a 50% chance of succumbing from the process,” Dr. Fujitani observed.
Dr. Fujitani and his coinvestigators scrutinized a plethora of potential risk factors for postoperative ischemic colitis. Six emerged as significant upon multivariate analysis: ruptured aneurysm before surgery, with an associated adjusted 4.1-fold increased risk; need for intra- or postoperative transfusion, with a 6-fold increased risk; renal failure requiring dialysis, with a 3.9-fold risk; proximal extension of the aneurysm, with a 2.2-fold elevation in risk; diabetes, with a 1.9-fold risk; and female sex, with an adjusted 1.75-fold increased risk (J Vasc Surg. 2016 Apr;63[4]:866-72).
Of note, these risk factors are largely unmodifiable, which underscores the importance of vigorous surveillance for possible signs of ischemic colitis during the first 4 days after AAA repair, especially in patients with multiple risk factors, Dr. Fujitani said.
Also, careful intraoperative assessment of the collateral mesenteric vascular anatomy is important in assessing a patient’s risk for postoperative ischemic colitis. This assessment should include the superior and inferior mesenteric arteries, as well as the celiac and internal iliac arteries. It’s worth bearing in mind that, even though collateral flow may appear adequate, it can be affected by hypovolemia, hypotension, or low cardiac output, the surgeon continued.
In the NSQIP data analysis, no patients who underwent reimplantation of the inferior mesenteric artery during open repair developed postoperative ischemic colitis. While this is an encouraging finding, the numbers were too small to draw definitive conclusions as to whether reimplantation of the artery is protective. It’s an important issue for further study, though, since so few of the recognized risk factors for the complication are modifiable, Dr. Fujitani noted.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
CHICAGO – Postoperative ischemic colitis after abdominal aortic aneurysm (AAA) repair is a feared, potentially devastating complication with a mortality approaching 50%, but early diagnosis can mitigate that risk, Roy M. Fujitani, MD, said at a symposium on vascular surgery sponsored by Northwestern University in Chicago.
The most common etiology of ischemic colitis following AAA repair is hypoperfusion of the mesenteric vasculature leading to nonocclusive ischemia. Caught early – in the initial hyperactive phase of colonic ischemia – the complication is typically transient and can be managed medically without further sequelae. Improvement is generally noted within a day or 2, with complete resolution within 1-2 weeks.
The earliest indicator that a patient is in the hyperactive phase of ischemic colitis following completion of an AAA repair can be defecation while still on the operating table.
“When you’ve just completed an operation and the patient has a bowel movement right on the operating table, that always makes me very, very concerned because of the likelihood of an associated ischemic colitis,” the surgeon noted.
A conscious patient in the first phase of ischemic colitis will describe an urgent desire to defecate, along with crampy pain and loose bowel movements with or without blood in the stool.
In the second, paralytic phase of ischemic colitis, the pain diminishes in intensity but becomes more continuous and diffuse, usually in the lateral borders of the abdomen. The abdomen becomes distended and much more tender, and there are no bowel sounds.
In patients whose ischemic colitis has been misdiagnosed or undiagnosed, the shock phase comes next. This is marked by massive fluid, protein, and electrolyte loss through the gangrenous mucosa. The result is severe dehydration, metabolic acidosis, and hypovolemic shock.
Nonocclusive colonic ischemia most often affects the watershed areas of the colon, such as the Sudeck point at the rectosigmoid junction.
The two other etiologies of ischemic colitis occurring as a complication of AAA repair are acute arterial occlusion, typically caused by iatrogenic embolization from a proximal source, often during endovascular aneurysm repair (EVAR), or rarely, venous thrombosis.
Making the diagnosis
When a patient is suspected of having ischemic colitis, one of the easiest ways of advancing toward a diagnosis is to obtain an abdominal plain x-ray, which classically shows thumb printing indicative of submucosal edema. CT with IV contrast typically shows bowel wall thickening, pericolonic fat stranding, and – most significantly – there may be free air within the colonic wall, an indicator of more advanced ischemia that occurs shortly before transmural gangrenous changes.
Colonoscopy is, however, the mainstay of diagnosis. It should be performed in any patient where postoperative ischemic colitis is suspected.
Ischemic colitis risk factors and outcomes
Dr. Fujitani was senior author of the largest ever study of risk factors for and outcomes of postoperative ischemic colitis in patients undergoing contemporary methods of open and endovascular AAA repair. This retrospective analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database included 3,486 patients who underwent AAA repair in U.S. hospitals during 2011-2012. Twelve percent had an open repair, while the other 88% underwent EVAR.
The incidence of postoperative ischemic colitis was 2.2%. The median time of diagnosis was on postoperative day 2. The rate was nearly threefold higher in the open repair group: 5.2% versus 1.8%. However, the open-repair group had a higher rate of emergency admission, ruptured aneurysm before surgery, and other high-risk features. Upon multivariate analysis, the adjusted risk of postoperative ischemic colitis was no longer significantly different in the open-repair and EVAR groups.
The mean hospital length of stay in patients with postoperative ischemic colitis was 20 days, compared with 5 days in those without the complication. The unadjusted in-hospital mortality rate in patients with ischemic colitis was 39% versus 4% in those without ischemic colitis.
Of the 75 patients who developed postoperative ischemic colitis, 37 were managed medically, 38 surgically.
“What was quite surprising was that there was a 56.8% in-hospital mortality in the surgically treated patients. The point being that if you end up having ischemic colitis, there’s a 50% chance you’ll end up requiring an operation, and if you do undergo an operation you have more than a 50% chance of succumbing from the process,” Dr. Fujitani observed.
Dr. Fujitani and his coinvestigators scrutinized a plethora of potential risk factors for postoperative ischemic colitis. Six emerged as significant upon multivariate analysis: ruptured aneurysm before surgery, with an associated adjusted 4.1-fold increased risk; need for intra- or postoperative transfusion, with a 6-fold increased risk; renal failure requiring dialysis, with a 3.9-fold risk; proximal extension of the aneurysm, with a 2.2-fold elevation in risk; diabetes, with a 1.9-fold risk; and female sex, with an adjusted 1.75-fold increased risk (J Vasc Surg. 2016 Apr;63[4]:866-72).
Of note, these risk factors are largely unmodifiable, which underscores the importance of vigorous surveillance for possible signs of ischemic colitis during the first 4 days after AAA repair, especially in patients with multiple risk factors, Dr. Fujitani said.
Also, careful intraoperative assessment of the collateral mesenteric vascular anatomy is important in assessing a patient’s risk for postoperative ischemic colitis. This assessment should include the superior and inferior mesenteric arteries, as well as the celiac and internal iliac arteries. It’s worth bearing in mind that, even though collateral flow may appear adequate, it can be affected by hypovolemia, hypotension, or low cardiac output, the surgeon continued.
In the NSQIP data analysis, no patients who underwent reimplantation of the inferior mesenteric artery during open repair developed postoperative ischemic colitis. While this is an encouraging finding, the numbers were too small to draw definitive conclusions as to whether reimplantation of the artery is protective. It’s an important issue for further study, though, since so few of the recognized risk factors for the complication are modifiable, Dr. Fujitani noted.
He reported having no financial conflicts regarding his presentation.
PCI for stable angina: A missed opportunity for shared decision-making
Multiple randomized controlled trials have compared percutaneous coronary intervention (PCI) vs optimal medical therapy for patients with chronic stable angina. All have consistently shown that PCI does not reduce the risk of death or even myocardial infarction (MI) but that it may relieve angina temporarily. Nevertheless, PCI is still commonly performed for patients with stable coronary disease, often in the absence of angina, and patients mistakenly believe the procedure is life-saving. Cardiologists may not be aware of patients’ misperceptions, or worse, may encourage them. In either case, if patients do not understand the benefits of the procedure, they cannot give informed consent.
This article reviews the pathophysiology of coronary artery disease, evidence from clinical trials of the value of PCI for chronic stable angina, patient and physician perceptions of PCI, and ways to promote patient-centered, shared decision-making.
CLINICAL CASE: EXERTIONAL ANGINA
While climbing 4 flights of stairs, a 55-year-old man noticed tightness in his chest, which lasted for 5 minutes and resolved spontaneously. Several weeks later, when visiting his primary care physician, he mentioned the episode. He had had no symptoms in the interim, but the physician ordered an exercise stress test.
Six minutes into a standard Bruce protocol, the patient experienced the same chest tightness, accompanied by 1-mm ST-segment depressions in leads II, III, and aVF. He was then referred to a cardiologist, who recommended catheterization.
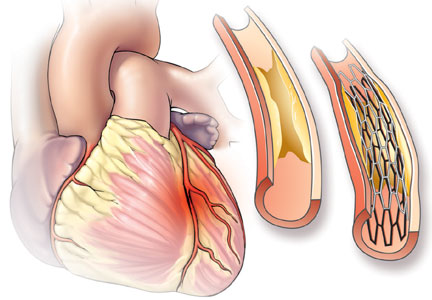
Catheterization demonstrated a 95% stenosis of the right coronary artery with nonsignificant stenoses of the left anterior descending and circumflex arteries. A drug-eluting stent was placed in the right coronary artery, with no residual stenosis.
Did this intervention likely prevent an MI and perhaps save the man’s life?
HOW MYOCARDIAL INFARCTION HAPPENS
Understanding the pathogenesis of MI is critical to having realistic expectations of the benefits of stent placement.
Doctors often describe coronary atherosclerosis as a plumbing problem, where deposits of cholesterol and fat build up in arterial walls, clogging the pipes and eventually causing a heart attack. This analogy, which has been around since the 1950s, is easy to for patients to grasp and has been popularized in the press and internalized by the public—as one patient with a 95% stenosis put it, “I was 95% dead.” In that model, angioplasty and stenting can resolve the blockage and “fix” the problem, much as a plumber can clear your pipes with a Roto-Rooter.
Despite the visual appeal of this model,1 it doesn’t accurately convey what we know about the pathophysiology of coronary artery disease. Instead of a gradual buildup of fatty deposits, low-density lipoprotein cholesterol particles infiltrate arterial walls and trigger an inflammatory reaction as they are engulfed by macrophages, leading to a cascade of cytokines and recruitment of more inflammatory cells.2 This immune response can eventually cause the rupture of the plaque’s fibrous cap, triggering thrombosis and infarction, often at a site of insignificant stenosis.
In this new model, coronary artery disease is primarily a problem of inflammation distributed throughout the vasculature, rather than a mechanical problem localized to the site of a significant stenosis.
Significant stenosis does not equal unstable plaque
Not all plaques are equally likely to rupture. Stable plaques tend to be long-standing and calcified, with a thick fibrous cap. A stable plaque causing a 95% stenosis may cause symptoms with exertion, but it is unlikely to cause infarction.3 Conversely, rupture-prone plaques may cause little stenosis, but a large and dangerous plaque may be lurking beneath the thin fibrous cap.
Relying on angiography can be misleading. Treating all significant stenoses improves blood flow, but does not reduce the risk of infarction, because infarction most often occurs in areas where the lumen is not obstructed. A plaque causing only 30% stenosis can suddenly rupture, causing thrombosis and complete occlusion.
The current model explains why PCI is no better than optimal medical therapy (ie, risk factor modification, antiplatelet therapy with aspirin, and a statin). Diet, exercise, smoking cessation, and statins target inflammatory processes and lower low-density lipoprotein cholesterol levels, while aspirin prevents platelet aggregation, among other likely actions.
The model also explains why coronary artery bypass grafting reduces the risk of MI and death in patients with left main or 3-vessel disease. A patient with generalized coronary artery disease has multiple lesions, many of which do not cause significant stenoses. PCI corrects only a single stenosis, whereas coronary artery bypass grafting circumvents all the vulnerable plaques in a vessel.
THE LANDMARK COURAGE TRIAL
Published in 2007, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial4 randomized more than 2,000 patients to receive either optimal medical therapy plus PCI or optimal medical therapy alone. The primary outcome was a composite of death from any cause and nonfatal MI. Patients were followed for at least 3 years, and some for as long as 7 years.
There was an initial small upward spike in the primary outcome in the PCI arm due to periprocedural events. By 5 years, the outcomes of the 2 arms converged and then stayed the same for up to 15 years.5 The authors concluded that PCI conferred no benefit over optimal medical therapy in the risk of death or MI.
Some doctors dismiss the study because of its stringent entry criteria—of 35,539 patients assessed, only 3,071 met the eligibility criteria. However, the entry criteria were meant to identify patients most likely to benefit from PCI. Many patients who undergo PCI today would not have qualified for the study because they lack objective evidence of ischemia.6 To enroll, patients needed a proximal stenosis of at least 70% and objective evidence of ischemia or a coronary stenosis of more than 80% and classic angina. Exclusion criteria disqualified few patients: Canadian Cardiovascular Society class IV angina (ie, angina evoked from minimal activity or at rest); a markedly positive stress test (substantial ST-segment depression or hypotension during stage I of the Bruce protocol); refractory heart failure or cardiogenic shock; an ejection fraction of less than 30%; revascularization within the past 6 months; and coronary anatomy unsuitable for PCI.
OTHER TRIALS SUPPORT COURAGE FINDINGS
Although COURAGE was hailed as a landmark trial, it largely supported the results of previous studies. A meta-analysis of PCI vs optimal medical therapy published in 2005 found no significant differences in death, cardiac death, MI, or nonfatal MI.7 MI was actually slightly more common in the PCI group due to the increased risk of MI during the periprocedural period.
Nor has the evidence from COURAGE discouraged additional studies of the same topic. Despite consistent findings that fit with our understanding of coronary disease as inflammation, we continue to conduct studies aimed at addressing significant stenosis, as if that was the problem. Thus, there have been studies of angioplasty alone, followed by studies of bare-metal stents and then drug-eluting stents.
In 2009, Trikalinos et al published a review of 61 randomized controlled trials comprising more than 25,000 patients with stable coronary disease and comparing medical therapy and angioplasty in its various forms over the previous 20 years.8 In all direct and indirect comparisons of PCI and medical therapy, there were no improvements in rates of death or MI.
Even so, the studies continue. The most recent “improvement” was the addition of fractional flow reserve, which served as the inclusion criterion for the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.9 In that study, patients with at least 1 stenosis with a fractional flow reserve less than 0.80 were randomized to PCI plus medical therapy or to medical therapy alone. The primary end point was a composite of death from any cause, MI, and urgent revascularization. Unfortunately, the study was stopped early when the primary end point was met due to a reduction in the need for urgent revascularization. There was no reduction in the rate of MI (hazard ratio 1.05, 95% confidence interval 0.51–2.19).
The reduction in urgent revascularization has also been shown consistently in past studies, but this is the weakest outcome measure because it does not equate to a reduction in the rate of MI. There is no demonstrable harm to putting off stent placement, even in functionally significant arteries, and most patients do not require a stent, even in the future.
In summary, the primary benefit of getting a stent now is a reduced likelihood of needing one later.
PCI MAY IMPROVE ANGINA FASTER
Another important finding of the COURAGE trial was that PCI improved symptoms more than optimal medical therapy.10 This is not surprising, because angina is often a direct result of a significant stenosis. What was unexpected was that even after PCI, most patients were not symptom-free. At 1 month, significantly more PCI patients were angina-free (42%) than were medical patients (33%). This translates into an absolute risk reduction of 9% or a number needed to treat of 11 to prevent 1 case of angina.
Patients in both groups improved over time, and after 3 years, the difference between the 2 groups was no longer significant: 59% in the PCI group vs 56% in the medical therapy group were angina-free.
A more recent study has raised the possibility that the improvement in angina with PCI is primarily a placebo effect. Researchers in the United Kingdom randomized patients with stable angina and at least a 70% stenosis of one vessel to either PCI or sham PCI, in which they threaded the catheter but did not deploy the stent.11 All patients received aggressive antianginal therapy before the procedure. At 6 weeks, there was improvement in angina in both groups, but no statistically significant difference between them in either exercise time or angina. Approximately half the patients in each group improved by at least 1 grade on the Canadian Cardiovascular Society angina classification, and more than 20% improved 2 grades.
This finding is not without precedent. Ligation of the internal mammary arteries, a popular treatment for angina in the 1950s, often provided dramatic relief of symptoms, until it was proven to be no better than a sham operation.12,13 More recently, a placebo-controlled trial of percutaneous laser myocardial revascularization also failed to show improvement over a sham treatment, despite promising results from a phase 1 trial.14 Together, these studies emphasize the subjective nature of angina as an outcome and call into question the routine use of PCI to relieve it.
PCI ENTAILS RISK
PCI entails a small but not inconsequential risk. During the procedure, 2% of patients develop bleeding or blood vessel damage, and another 1% die or have an MI or a stroke. In the first year after stent placement, 3% of patients have a bleeding event from the antiplatelet therapy needed for the stent, and an additional 2% develop a clot in the stent that leads to MI.15
INFORMED CONSENT IS CRITICAL
As demonstrated above, for patients with stable angina, the only evidence-based benefit of PCI over optimal medical therapy is that symptoms may respond faster. At the same time, there are costs and risks associated with the procedure. Because symptoms are subjective, patients should play a key role in deciding whether PCI is appropriate for them.
The American Medical Association states that a physician providing any treatment or procedure should disclose and discuss with patients the risks and benefits. Unfortunately, a substantial body of evidence demonstrates that this is not occurring in practice.
Patients and cardiologists have conflicting beliefs about PCI
Studies over the past 20 years demonstrate that patients with chronic stable angina consistently overestimate the benefits of PCI, with 71% to 88% believing that it will reduce their chance of death.16–19 Patients also understand that PCI can relieve their symptoms, though no study seems to have assessed the perceived magnitude of this benefit.
In contrast, when cardiologists were asked about the benefits their patients could expect from PCI, only 20% said that it would reduce mortality and 25% said it would prevent MI.18 These are still surprisingly high percentages, since the study was conducted after the COURAGE trial.
Nevertheless, these differences in perception show that cardiologists fail to successfully communicate the benefits of the procedure to their patients. Without complete information, patients cannot make informed decisions.
Cardiologists’ reasons for performing PCI
If PCI cannot improve hard outcomes like MI or death in stable coronary disease, why do cardiologists continue to perform it so frequently?
Soon after the COURAGE trial, Lin et al conducted focus groups with cardiologists to find out.20 Some said that they doubted the clinical trial evidence, given the reduction in the cardiac mortality rate over the past 30 years. Others remarked that their overriding goal is to stamp out ischemia, and that once a lesion is found by catheterization, one must proceed with PCI. This has been termed the “oculostenotic reflex,” ie, the interventionist sees coronary artery disease and immediately places a stent.
Atreya et al found objective evidence of this practice.21 In a 2016 study of 207 patients with obstructive lesions amenable to PCI, the only factors associated with medical management were those that increased the risk of the procedure: age, chronic kidney disease, distal location of the lesion, and type C lesions (the most difficult ones to treat by PCI). More important, evidence of ischemia, presence of angina, and being on optimal medical therapy or maximal antianginal therapy were not associated with PCI.
When surveyed, cardiologists offered reasons similar to those identified by Lin et al, including a positive stress test (70%) and significant myocardium at risk (50%).18 Optimal medical therapy failure was cited less often (40%). Over 30% identified relief of chest pain for patients who were not prescribed optimal medical therapy. Another 30% said that patient anxiety contributed to their decision, but patients who reported anxiety were not more likely to get PCI than those who did not.
True informed consent rarely occurs
Surveys of patients and recordings of doctor visits suggest that doctors often discuss the risks of the procedure but rarely accurately describe the benefits or mention alternative treatments, including optimal medical therapy.
Fowler et al22 surveyed 472 Medicare patients who had undergone PCI in the past year about their consent discussion, particularly regarding alternative options. Only 6% of patients recalled discussing medication as a serious option with their doctor.
In 2 published studies,23,24 we analyzed recorded conversations between doctors and patients in which angiography and PCI were discussed.
In a qualitative assessment of how cardiologists presented the rationale for PCI to patients,23 we observed that cardiologists gave an accurate presentation of the benefits in only 5% of cases. In 13% of the conversations the benefits were explicitly overstated (eg, “If you don’t do it [angiogram/PCI], what could happen? Well, you could…have a heart attack involving that area which can lead to a sudden death”). In another 35% of cases, physicians offered an implicit overstatement of the benefit by saying they could “fix” the problem (eg, “So that’s where we start thinking, Well maybe we better try to fix that [blockage]”), without specifically stating that fixing the problem would offer any benefit. Patients were left to fill in the blanks. Conversations frequently focused on the rationale for performing PCI (eg, ischemia on a stress test) and a description of the procedure, rather than on the risks and benefits.
In a quantitative study of the same data set, we assessed how often physicians addressed the 7 elements of informed decision-making as defined by Braddock et al.24
- Explaining the patient’s role in decision-making (ie, that the patient has a choice to make) was present in half of the conversations. Sometimes a doctor would simply say, “The next step is to perform catheterization.”
- Discussion of clinical issues (eg, having a blockage, stress test results) was performed in almost every case, demonstrating physicians’ comfort with that element.
- Discussing treatment alternatives occurred in only 1 in 4 conversations. This was more frequent than previously reported, and appeared most often when patients expressed hesitancy about proceeding to PCI.
- Discussing pros and cons of the alternatives was done in 42%.
- Uncertainty about the procedure (eg, that it might not relieve the angina) was expressed in only 10% of conversations.
- Assessment of patient understanding was done in 65% of cases. This included even minimal efforts (eg, “Do you have any questions?”). More advanced methods such as teach-back were never used.
- Exploration of patient preferences (eg, asking patients which treatment they prefer, or attempting to understand how angina affects a patient’s life) the final element, occurred in 73% of conversations.
Only 3% of the conversations contained all 7 elements. Even using a more relaxed definition of 3 critical elements (ie, discussing clinical issues, treatment alternatives, and pros and cons), only 13% of conversations included them all.
Discussion affects decisions
Informed decision-making is not only important because of its ethical implications. Offering patients more information was associated with their choosing not to have PCI. The probability of a patient undergoing PCI was negatively associated with 3 specific elements of informed decision-making. Patients were less likely to choose PCI if the patient’s role in decision-making was discussed (61% vs 86% chose PCI, P < .03); if alternatives were discussed (31% vs 89% chose PCI, P < .01); and if uncertainties were discussed (17% vs 80% chose PCI, P < .01).
There was also a linear relationship between the total number of elements discussed and the probability of choosing PCI: it ranged from 100% of patients choosing PCI when just 1 element was present to 3% of patients choosing PCI when all 7 elements were present. The relationship is not entirely causal, since doctors were more likely to talk about alternatives and risks if patients hesitated and raised questions. Cautious patients received more information.
From these observational studies, we know that physicians do not generally communicate the benefits of PCI, and patients make incorrect assumptions about the benefits they can expect. We know that those who receive more information are less likely to choose PCI, but what would happen if patients were randomly assigned to receive complete information?
An online survey
We conducted an online survey of more than 1,000 participants over age 50 who had never undergone PCI, asking them to imagine visiting a cardiologist after having a positive stress test for stable chest pain.25 Three intervention groups read different scenarios couched as information provided by their cardiologist:
- The “standard care” group received no specific information about the effects of PCI on the risk of myocardial infarction
- The “specific information” group was specifically told that PCI does not reduce the risk of myocardial infarction
- The “explanatory information” group was told how medications work and why PCI does not reduce the risk of myocardial infarction.
All 3 groups received information about the risks of PCI, its role in reducing angina, and the risks and benefits of optimal medical therapy.
After reading their scenario, all participants completed an identical questionnaire, which asked if they would opt for PCI, medical therapy, or both. Overall, 55% chose PCI, ranging from 70% in the standard care group to 46% in the group given explanatory information. Rates in the specific-information and explanatory-information groups were not statistically different from each other, but both were significantly different from that in the standard-care group. Interestingly, the more information patients were given about PCI, the more likely they were to choose optimal medical therapy.
After reading the scenario, participants were also asked if PCI would “prevent a heart attack.” Of those who received standard care, 71% endorsed that belief, which is remarkably similar to studies of real patients who have received standard care. In contrast, only 39% of those given specific information and 31% given explanatory information held that belief. Moreover, the belief that PCI prevented MI was the strongest predictor of choosing PCI (odds ratio 5.82, 95% confidence interval 4.13–8.26).25
Interestingly, 52% of the standard care group falsely remembered that the doctor had told them that PCI would prevent an MI, even though the doctor said nothing about it one way or the other. It appears that participants were projecting their own beliefs onto the encounter. This highlights the importance of providing full information to patients who are considering this procedure.
TOWARD SHARED DECISION-MAKING
Shared decision-making is a process in which physicians enter into a partnership with a patient, offer information, elicit the patient’s preferences, and then come to a decision in concert with the patient.
Although many decisions can and should involve elements of shared decision-making, the decision to proceed with PCI for stable angina is particularly well-suited to shared decision-making. This is because the benefit of PCI depends on the value a patient attaches to being free of angina sooner. Since there is no difference in the risk of MI or death, the patient must decide if the risks of the procedure and the inconvenience of taking dual antiplatelet therapy are worth the benefit of improving symptoms faster. Presumably, patients who have more severe symptoms or experienced side effects from antianginal therapy would be more likely to choose PCI.
Despite having substantial experience educating patients, most physicians are unfamiliar with the process of shared decision-making. In particular, the process of eliciting preferences is often overlooked.
To address this issue, researchers at the Mayo Clinic developed a decision aid that compares PCI plus optimal medical therapy vs optimal medical therapy alone in an easily understandable information card.15 On one side, the 2 options are clearly stated, with the magnitude of symptom improvement over time graphically illustrated and the statement, “NO DIFFERENCE in heart attack or death,” prominently displayed. The back of the card discusses the risks of each option in easily understood tables.
The decision aid was compared with standard care in a randomized trial involving patients who were referred for catheterization and possible PCI.26 The decision aid improved patients’ overall knowledge about PCI. In particular, 60% of those who used the decision aid knew that PCI did not prevent death or MI vs 40% of usual-care patients—results similar to those of the online experiment.
Interestingly, the decision about whether to undergo PCI did not differ significantly between the 2 groups, although there was a trend toward more patients in the decision-aid group choosing medical therapy alone (53%) vs the standard-care patients (39%).
To understand why the decision aid did not make more of a difference, the investigators performed qualitative interviews of the cardiologists in the study.27 One theme was the timing of the intervention. Patients using the decision aid had already been referred for catheterization, and some felt the process should have occurred earlier. Engaging in shared decision-making with a general cardiologist before referral could help to improve the quality of patient decisions.
Cardiologists also noted the difficulty in changing their work flow to incorporate the decision aid. Although some embraced the idea of shared decision-making, others were concerned that many patients could not participate, and there was confusion about the difference between an educational tool, which could be used by a patient alone, and a decision aid, which is meant to generate discussion between the doctor and patient. Some expressed interest in using the tool in the future.
These findings serve to emphasize that providing information alone is not enough. If the physician does not “buy in” to the idea of shared decision-making, it will not occur.
PRACTICE IMPLICATIONS
Based on the pathophysiology of coronary artery disease and the results of multiple randomized controlled trials, it is evident that PCI does not prevent heart attacks in patients with chronic stable angina. However, most patients who undergo PCI are unaware of this and therefore do not truly give informed consent. In the absence of explicit information to the contrary, most patients with stable angina assume that PCI prevents MI and thus are biased toward choosing PCI.
Even minimal amounts of explicit information can partially overcome that bias and influence decision-making. In particular, explaining why PCI does not prevent MI was the most effective means of overcoming the bias.
To this end, shared decision aids may help physicians to engage in shared decision-making. Shared decision-making is most likely to occur if physicians are trained in the concept of shared decision-making, are committed to practicing it, and can fit it into their work flow. Ideally, this would occur in the office of a general cardiologist before referral for PCI.
For those practicing in accountable-care organizations, Medicare has recently introduced the shared decision-making model for 6 preference-sensitive conditions, including stable ischemic heart disease. Participants in this program will have the opportunity to receive payments for shared decision-making services and to share in any savings that result from reduced use of resources. Use of these tools holds the promise for providing more patient-centered care at lower cost.
- Jones DS. Visions of a cure. Visualization, clinical trials, and controversies in cardiac therapeutics, 1968–1998. Isis 2000; 91:504–541.
- Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695.
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364:226–235.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 2015; 373:1937–1946.
- Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA 2008; 300:1765–1773.
- Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005; 111:2906–2912.
- Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet 2009; 373:911–918.
- De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Al-Lamee R, Thompson D, Dehbi H-M, et al, on behalf of the ORBITA Investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. Published online November 2, 2017. http://dx.doi.org/10.1016/S0140-6736(17)32714-9. Accessed November 10, 2017.
- Cobb LA, Thomas GI, Dillard DH, et al. An evaluation of internal mammary-artery ligation by a double-blind technic. N Engl J Med 1959; 260:1115–1118.
- Dimond EG, Fittle F, Crockett JE. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am J Cardiol 1960; 5:483-486.
- Leon MB, Kornowski R, Downey WE, et al. A blinded, randomized placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 2005; 46:1812–1819.
- Coylewright M, Shepel K, Leblanc A, et al. Shared decision making in patients with stable coronary artery disease: PCI choice. PLoS One 2012; 7:e49827.
- Holmboe ES, Fiellin DA, Cusanelli E, Remetz M, Krumholz HM. Perceptions of benefit and risk of patients undergoing first-time elective percutaneous coronary revascularization. J Gen Intern Med 2000; 15:632–637.
- Kee F, McDonald P, Gaffney B. Risks and benefits of coronary angioplasty: the patients perspective: a preliminary study. Qual Health Care 1997; 6:131–139.
- Rothberg MB, Sivalingam SK, Ashraf J, et al. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med 2010; 153:307–313.
- Whittle J, Conigliaro J, Good CB, Kelley ME, Skanderson M. Understanding of the benefits of coronary revascularization procedures among patients who are offered such procedures. Am Heart J 2007; 154:662–668.
- Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med 2007; 167:1604–1609.
- Atreya AR, Sivalingam SK, Arora S, et al. Predictors of medical management in patients undergoing elective cardiac catheterization for chronic ischemic heart disease. Clin Cardiol 2016; 39:207–214.
- Fowler FJ Jr, Gallagher PM, Bynum JP, Barry MJ, Lucas FL, Skinner JS. Decision-making process reported by Medicare patients who had coronary artery stenting or surgery for prostate cancer. J Gen Intern Med 2012; 27:911–916.
- Goff SL, Mazor KM, Ting HH, Kleppel R, Rothberg MB. How cardiologists present the benefits of percutaneous coronary interventions to patients with stable angina: a qualitative analysis. JAMA Intern Med 2014; 174:1614–1621.
- Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA 1999; 282:2313–2320.
- Rothberg MB, Scherer L, Kashef MA, et al. The effect of information presentation on beliefs about the benefits of elective percutaneous coronary intervention. JAMA Intern Med 2014; 174:1623–1629.
- Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016; 9:767–776.
- Coylewright M, O’Neill ES, Dick S, Grande SW. PCI choice: cardiovascular clinicians’ perceptions of shared decision making in stable coronary artery disease. Patient Educ Couns 2017; 100:1136–1143.
Multiple randomized controlled trials have compared percutaneous coronary intervention (PCI) vs optimal medical therapy for patients with chronic stable angina. All have consistently shown that PCI does not reduce the risk of death or even myocardial infarction (MI) but that it may relieve angina temporarily. Nevertheless, PCI is still commonly performed for patients with stable coronary disease, often in the absence of angina, and patients mistakenly believe the procedure is life-saving. Cardiologists may not be aware of patients’ misperceptions, or worse, may encourage them. In either case, if patients do not understand the benefits of the procedure, they cannot give informed consent.
This article reviews the pathophysiology of coronary artery disease, evidence from clinical trials of the value of PCI for chronic stable angina, patient and physician perceptions of PCI, and ways to promote patient-centered, shared decision-making.
CLINICAL CASE: EXERTIONAL ANGINA
While climbing 4 flights of stairs, a 55-year-old man noticed tightness in his chest, which lasted for 5 minutes and resolved spontaneously. Several weeks later, when visiting his primary care physician, he mentioned the episode. He had had no symptoms in the interim, but the physician ordered an exercise stress test.
Six minutes into a standard Bruce protocol, the patient experienced the same chest tightness, accompanied by 1-mm ST-segment depressions in leads II, III, and aVF. He was then referred to a cardiologist, who recommended catheterization.
Catheterization demonstrated a 95% stenosis of the right coronary artery with nonsignificant stenoses of the left anterior descending and circumflex arteries. A drug-eluting stent was placed in the right coronary artery, with no residual stenosis.
Did this intervention likely prevent an MI and perhaps save the man’s life?
HOW MYOCARDIAL INFARCTION HAPPENS
Understanding the pathogenesis of MI is critical to having realistic expectations of the benefits of stent placement.
Doctors often describe coronary atherosclerosis as a plumbing problem, where deposits of cholesterol and fat build up in arterial walls, clogging the pipes and eventually causing a heart attack. This analogy, which has been around since the 1950s, is easy to for patients to grasp and has been popularized in the press and internalized by the public—as one patient with a 95% stenosis put it, “I was 95% dead.” In that model, angioplasty and stenting can resolve the blockage and “fix” the problem, much as a plumber can clear your pipes with a Roto-Rooter.
Despite the visual appeal of this model,1 it doesn’t accurately convey what we know about the pathophysiology of coronary artery disease. Instead of a gradual buildup of fatty deposits, low-density lipoprotein cholesterol particles infiltrate arterial walls and trigger an inflammatory reaction as they are engulfed by macrophages, leading to a cascade of cytokines and recruitment of more inflammatory cells.2 This immune response can eventually cause the rupture of the plaque’s fibrous cap, triggering thrombosis and infarction, often at a site of insignificant stenosis.
In this new model, coronary artery disease is primarily a problem of inflammation distributed throughout the vasculature, rather than a mechanical problem localized to the site of a significant stenosis.
Significant stenosis does not equal unstable plaque
Not all plaques are equally likely to rupture. Stable plaques tend to be long-standing and calcified, with a thick fibrous cap. A stable plaque causing a 95% stenosis may cause symptoms with exertion, but it is unlikely to cause infarction.3 Conversely, rupture-prone plaques may cause little stenosis, but a large and dangerous plaque may be lurking beneath the thin fibrous cap.
Relying on angiography can be misleading. Treating all significant stenoses improves blood flow, but does not reduce the risk of infarction, because infarction most often occurs in areas where the lumen is not obstructed. A plaque causing only 30% stenosis can suddenly rupture, causing thrombosis and complete occlusion.
The current model explains why PCI is no better than optimal medical therapy (ie, risk factor modification, antiplatelet therapy with aspirin, and a statin). Diet, exercise, smoking cessation, and statins target inflammatory processes and lower low-density lipoprotein cholesterol levels, while aspirin prevents platelet aggregation, among other likely actions.
The model also explains why coronary artery bypass grafting reduces the risk of MI and death in patients with left main or 3-vessel disease. A patient with generalized coronary artery disease has multiple lesions, many of which do not cause significant stenoses. PCI corrects only a single stenosis, whereas coronary artery bypass grafting circumvents all the vulnerable plaques in a vessel.
THE LANDMARK COURAGE TRIAL
Published in 2007, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial4 randomized more than 2,000 patients to receive either optimal medical therapy plus PCI or optimal medical therapy alone. The primary outcome was a composite of death from any cause and nonfatal MI. Patients were followed for at least 3 years, and some for as long as 7 years.
There was an initial small upward spike in the primary outcome in the PCI arm due to periprocedural events. By 5 years, the outcomes of the 2 arms converged and then stayed the same for up to 15 years.5 The authors concluded that PCI conferred no benefit over optimal medical therapy in the risk of death or MI.
Some doctors dismiss the study because of its stringent entry criteria—of 35,539 patients assessed, only 3,071 met the eligibility criteria. However, the entry criteria were meant to identify patients most likely to benefit from PCI. Many patients who undergo PCI today would not have qualified for the study because they lack objective evidence of ischemia.6 To enroll, patients needed a proximal stenosis of at least 70% and objective evidence of ischemia or a coronary stenosis of more than 80% and classic angina. Exclusion criteria disqualified few patients: Canadian Cardiovascular Society class IV angina (ie, angina evoked from minimal activity or at rest); a markedly positive stress test (substantial ST-segment depression or hypotension during stage I of the Bruce protocol); refractory heart failure or cardiogenic shock; an ejection fraction of less than 30%; revascularization within the past 6 months; and coronary anatomy unsuitable for PCI.
OTHER TRIALS SUPPORT COURAGE FINDINGS
Although COURAGE was hailed as a landmark trial, it largely supported the results of previous studies. A meta-analysis of PCI vs optimal medical therapy published in 2005 found no significant differences in death, cardiac death, MI, or nonfatal MI.7 MI was actually slightly more common in the PCI group due to the increased risk of MI during the periprocedural period.
Nor has the evidence from COURAGE discouraged additional studies of the same topic. Despite consistent findings that fit with our understanding of coronary disease as inflammation, we continue to conduct studies aimed at addressing significant stenosis, as if that was the problem. Thus, there have been studies of angioplasty alone, followed by studies of bare-metal stents and then drug-eluting stents.
In 2009, Trikalinos et al published a review of 61 randomized controlled trials comprising more than 25,000 patients with stable coronary disease and comparing medical therapy and angioplasty in its various forms over the previous 20 years.8 In all direct and indirect comparisons of PCI and medical therapy, there were no improvements in rates of death or MI.
Even so, the studies continue. The most recent “improvement” was the addition of fractional flow reserve, which served as the inclusion criterion for the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.9 In that study, patients with at least 1 stenosis with a fractional flow reserve less than 0.80 were randomized to PCI plus medical therapy or to medical therapy alone. The primary end point was a composite of death from any cause, MI, and urgent revascularization. Unfortunately, the study was stopped early when the primary end point was met due to a reduction in the need for urgent revascularization. There was no reduction in the rate of MI (hazard ratio 1.05, 95% confidence interval 0.51–2.19).
The reduction in urgent revascularization has also been shown consistently in past studies, but this is the weakest outcome measure because it does not equate to a reduction in the rate of MI. There is no demonstrable harm to putting off stent placement, even in functionally significant arteries, and most patients do not require a stent, even in the future.
In summary, the primary benefit of getting a stent now is a reduced likelihood of needing one later.
PCI MAY IMPROVE ANGINA FASTER
Another important finding of the COURAGE trial was that PCI improved symptoms more than optimal medical therapy.10 This is not surprising, because angina is often a direct result of a significant stenosis. What was unexpected was that even after PCI, most patients were not symptom-free. At 1 month, significantly more PCI patients were angina-free (42%) than were medical patients (33%). This translates into an absolute risk reduction of 9% or a number needed to treat of 11 to prevent 1 case of angina.
Patients in both groups improved over time, and after 3 years, the difference between the 2 groups was no longer significant: 59% in the PCI group vs 56% in the medical therapy group were angina-free.
A more recent study has raised the possibility that the improvement in angina with PCI is primarily a placebo effect. Researchers in the United Kingdom randomized patients with stable angina and at least a 70% stenosis of one vessel to either PCI or sham PCI, in which they threaded the catheter but did not deploy the stent.11 All patients received aggressive antianginal therapy before the procedure. At 6 weeks, there was improvement in angina in both groups, but no statistically significant difference between them in either exercise time or angina. Approximately half the patients in each group improved by at least 1 grade on the Canadian Cardiovascular Society angina classification, and more than 20% improved 2 grades.
This finding is not without precedent. Ligation of the internal mammary arteries, a popular treatment for angina in the 1950s, often provided dramatic relief of symptoms, until it was proven to be no better than a sham operation.12,13 More recently, a placebo-controlled trial of percutaneous laser myocardial revascularization also failed to show improvement over a sham treatment, despite promising results from a phase 1 trial.14 Together, these studies emphasize the subjective nature of angina as an outcome and call into question the routine use of PCI to relieve it.
PCI ENTAILS RISK
PCI entails a small but not inconsequential risk. During the procedure, 2% of patients develop bleeding or blood vessel damage, and another 1% die or have an MI or a stroke. In the first year after stent placement, 3% of patients have a bleeding event from the antiplatelet therapy needed for the stent, and an additional 2% develop a clot in the stent that leads to MI.15
INFORMED CONSENT IS CRITICAL
As demonstrated above, for patients with stable angina, the only evidence-based benefit of PCI over optimal medical therapy is that symptoms may respond faster. At the same time, there are costs and risks associated with the procedure. Because symptoms are subjective, patients should play a key role in deciding whether PCI is appropriate for them.
The American Medical Association states that a physician providing any treatment or procedure should disclose and discuss with patients the risks and benefits. Unfortunately, a substantial body of evidence demonstrates that this is not occurring in practice.
Patients and cardiologists have conflicting beliefs about PCI
Studies over the past 20 years demonstrate that patients with chronic stable angina consistently overestimate the benefits of PCI, with 71% to 88% believing that it will reduce their chance of death.16–19 Patients also understand that PCI can relieve their symptoms, though no study seems to have assessed the perceived magnitude of this benefit.
In contrast, when cardiologists were asked about the benefits their patients could expect from PCI, only 20% said that it would reduce mortality and 25% said it would prevent MI.18 These are still surprisingly high percentages, since the study was conducted after the COURAGE trial.
Nevertheless, these differences in perception show that cardiologists fail to successfully communicate the benefits of the procedure to their patients. Without complete information, patients cannot make informed decisions.
Cardiologists’ reasons for performing PCI
If PCI cannot improve hard outcomes like MI or death in stable coronary disease, why do cardiologists continue to perform it so frequently?
Soon after the COURAGE trial, Lin et al conducted focus groups with cardiologists to find out.20 Some said that they doubted the clinical trial evidence, given the reduction in the cardiac mortality rate over the past 30 years. Others remarked that their overriding goal is to stamp out ischemia, and that once a lesion is found by catheterization, one must proceed with PCI. This has been termed the “oculostenotic reflex,” ie, the interventionist sees coronary artery disease and immediately places a stent.
Atreya et al found objective evidence of this practice.21 In a 2016 study of 207 patients with obstructive lesions amenable to PCI, the only factors associated with medical management were those that increased the risk of the procedure: age, chronic kidney disease, distal location of the lesion, and type C lesions (the most difficult ones to treat by PCI). More important, evidence of ischemia, presence of angina, and being on optimal medical therapy or maximal antianginal therapy were not associated with PCI.
When surveyed, cardiologists offered reasons similar to those identified by Lin et al, including a positive stress test (70%) and significant myocardium at risk (50%).18 Optimal medical therapy failure was cited less often (40%). Over 30% identified relief of chest pain for patients who were not prescribed optimal medical therapy. Another 30% said that patient anxiety contributed to their decision, but patients who reported anxiety were not more likely to get PCI than those who did not.
True informed consent rarely occurs
Surveys of patients and recordings of doctor visits suggest that doctors often discuss the risks of the procedure but rarely accurately describe the benefits or mention alternative treatments, including optimal medical therapy.
Fowler et al22 surveyed 472 Medicare patients who had undergone PCI in the past year about their consent discussion, particularly regarding alternative options. Only 6% of patients recalled discussing medication as a serious option with their doctor.
In 2 published studies,23,24 we analyzed recorded conversations between doctors and patients in which angiography and PCI were discussed.
In a qualitative assessment of how cardiologists presented the rationale for PCI to patients,23 we observed that cardiologists gave an accurate presentation of the benefits in only 5% of cases. In 13% of the conversations the benefits were explicitly overstated (eg, “If you don’t do it [angiogram/PCI], what could happen? Well, you could…have a heart attack involving that area which can lead to a sudden death”). In another 35% of cases, physicians offered an implicit overstatement of the benefit by saying they could “fix” the problem (eg, “So that’s where we start thinking, Well maybe we better try to fix that [blockage]”), without specifically stating that fixing the problem would offer any benefit. Patients were left to fill in the blanks. Conversations frequently focused on the rationale for performing PCI (eg, ischemia on a stress test) and a description of the procedure, rather than on the risks and benefits.
In a quantitative study of the same data set, we assessed how often physicians addressed the 7 elements of informed decision-making as defined by Braddock et al.24
- Explaining the patient’s role in decision-making (ie, that the patient has a choice to make) was present in half of the conversations. Sometimes a doctor would simply say, “The next step is to perform catheterization.”
- Discussion of clinical issues (eg, having a blockage, stress test results) was performed in almost every case, demonstrating physicians’ comfort with that element.
- Discussing treatment alternatives occurred in only 1 in 4 conversations. This was more frequent than previously reported, and appeared most often when patients expressed hesitancy about proceeding to PCI.
- Discussing pros and cons of the alternatives was done in 42%.
- Uncertainty about the procedure (eg, that it might not relieve the angina) was expressed in only 10% of conversations.
- Assessment of patient understanding was done in 65% of cases. This included even minimal efforts (eg, “Do you have any questions?”). More advanced methods such as teach-back were never used.
- Exploration of patient preferences (eg, asking patients which treatment they prefer, or attempting to understand how angina affects a patient’s life) the final element, occurred in 73% of conversations.
Only 3% of the conversations contained all 7 elements. Even using a more relaxed definition of 3 critical elements (ie, discussing clinical issues, treatment alternatives, and pros and cons), only 13% of conversations included them all.
Discussion affects decisions
Informed decision-making is not only important because of its ethical implications. Offering patients more information was associated with their choosing not to have PCI. The probability of a patient undergoing PCI was negatively associated with 3 specific elements of informed decision-making. Patients were less likely to choose PCI if the patient’s role in decision-making was discussed (61% vs 86% chose PCI, P < .03); if alternatives were discussed (31% vs 89% chose PCI, P < .01); and if uncertainties were discussed (17% vs 80% chose PCI, P < .01).
There was also a linear relationship between the total number of elements discussed and the probability of choosing PCI: it ranged from 100% of patients choosing PCI when just 1 element was present to 3% of patients choosing PCI when all 7 elements were present. The relationship is not entirely causal, since doctors were more likely to talk about alternatives and risks if patients hesitated and raised questions. Cautious patients received more information.
From these observational studies, we know that physicians do not generally communicate the benefits of PCI, and patients make incorrect assumptions about the benefits they can expect. We know that those who receive more information are less likely to choose PCI, but what would happen if patients were randomly assigned to receive complete information?
An online survey
We conducted an online survey of more than 1,000 participants over age 50 who had never undergone PCI, asking them to imagine visiting a cardiologist after having a positive stress test for stable chest pain.25 Three intervention groups read different scenarios couched as information provided by their cardiologist:
- The “standard care” group received no specific information about the effects of PCI on the risk of myocardial infarction
- The “specific information” group was specifically told that PCI does not reduce the risk of myocardial infarction
- The “explanatory information” group was told how medications work and why PCI does not reduce the risk of myocardial infarction.
All 3 groups received information about the risks of PCI, its role in reducing angina, and the risks and benefits of optimal medical therapy.
After reading their scenario, all participants completed an identical questionnaire, which asked if they would opt for PCI, medical therapy, or both. Overall, 55% chose PCI, ranging from 70% in the standard care group to 46% in the group given explanatory information. Rates in the specific-information and explanatory-information groups were not statistically different from each other, but both were significantly different from that in the standard-care group. Interestingly, the more information patients were given about PCI, the more likely they were to choose optimal medical therapy.
After reading the scenario, participants were also asked if PCI would “prevent a heart attack.” Of those who received standard care, 71% endorsed that belief, which is remarkably similar to studies of real patients who have received standard care. In contrast, only 39% of those given specific information and 31% given explanatory information held that belief. Moreover, the belief that PCI prevented MI was the strongest predictor of choosing PCI (odds ratio 5.82, 95% confidence interval 4.13–8.26).25
Interestingly, 52% of the standard care group falsely remembered that the doctor had told them that PCI would prevent an MI, even though the doctor said nothing about it one way or the other. It appears that participants were projecting their own beliefs onto the encounter. This highlights the importance of providing full information to patients who are considering this procedure.
TOWARD SHARED DECISION-MAKING
Shared decision-making is a process in which physicians enter into a partnership with a patient, offer information, elicit the patient’s preferences, and then come to a decision in concert with the patient.
Although many decisions can and should involve elements of shared decision-making, the decision to proceed with PCI for stable angina is particularly well-suited to shared decision-making. This is because the benefit of PCI depends on the value a patient attaches to being free of angina sooner. Since there is no difference in the risk of MI or death, the patient must decide if the risks of the procedure and the inconvenience of taking dual antiplatelet therapy are worth the benefit of improving symptoms faster. Presumably, patients who have more severe symptoms or experienced side effects from antianginal therapy would be more likely to choose PCI.
Despite having substantial experience educating patients, most physicians are unfamiliar with the process of shared decision-making. In particular, the process of eliciting preferences is often overlooked.
To address this issue, researchers at the Mayo Clinic developed a decision aid that compares PCI plus optimal medical therapy vs optimal medical therapy alone in an easily understandable information card.15 On one side, the 2 options are clearly stated, with the magnitude of symptom improvement over time graphically illustrated and the statement, “NO DIFFERENCE in heart attack or death,” prominently displayed. The back of the card discusses the risks of each option in easily understood tables.
The decision aid was compared with standard care in a randomized trial involving patients who were referred for catheterization and possible PCI.26 The decision aid improved patients’ overall knowledge about PCI. In particular, 60% of those who used the decision aid knew that PCI did not prevent death or MI vs 40% of usual-care patients—results similar to those of the online experiment.
Interestingly, the decision about whether to undergo PCI did not differ significantly between the 2 groups, although there was a trend toward more patients in the decision-aid group choosing medical therapy alone (53%) vs the standard-care patients (39%).
To understand why the decision aid did not make more of a difference, the investigators performed qualitative interviews of the cardiologists in the study.27 One theme was the timing of the intervention. Patients using the decision aid had already been referred for catheterization, and some felt the process should have occurred earlier. Engaging in shared decision-making with a general cardiologist before referral could help to improve the quality of patient decisions.
Cardiologists also noted the difficulty in changing their work flow to incorporate the decision aid. Although some embraced the idea of shared decision-making, others were concerned that many patients could not participate, and there was confusion about the difference between an educational tool, which could be used by a patient alone, and a decision aid, which is meant to generate discussion between the doctor and patient. Some expressed interest in using the tool in the future.
These findings serve to emphasize that providing information alone is not enough. If the physician does not “buy in” to the idea of shared decision-making, it will not occur.
PRACTICE IMPLICATIONS
Based on the pathophysiology of coronary artery disease and the results of multiple randomized controlled trials, it is evident that PCI does not prevent heart attacks in patients with chronic stable angina. However, most patients who undergo PCI are unaware of this and therefore do not truly give informed consent. In the absence of explicit information to the contrary, most patients with stable angina assume that PCI prevents MI and thus are biased toward choosing PCI.
Even minimal amounts of explicit information can partially overcome that bias and influence decision-making. In particular, explaining why PCI does not prevent MI was the most effective means of overcoming the bias.
To this end, shared decision aids may help physicians to engage in shared decision-making. Shared decision-making is most likely to occur if physicians are trained in the concept of shared decision-making, are committed to practicing it, and can fit it into their work flow. Ideally, this would occur in the office of a general cardiologist before referral for PCI.
For those practicing in accountable-care organizations, Medicare has recently introduced the shared decision-making model for 6 preference-sensitive conditions, including stable ischemic heart disease. Participants in this program will have the opportunity to receive payments for shared decision-making services and to share in any savings that result from reduced use of resources. Use of these tools holds the promise for providing more patient-centered care at lower cost.
Multiple randomized controlled trials have compared percutaneous coronary intervention (PCI) vs optimal medical therapy for patients with chronic stable angina. All have consistently shown that PCI does not reduce the risk of death or even myocardial infarction (MI) but that it may relieve angina temporarily. Nevertheless, PCI is still commonly performed for patients with stable coronary disease, often in the absence of angina, and patients mistakenly believe the procedure is life-saving. Cardiologists may not be aware of patients’ misperceptions, or worse, may encourage them. In either case, if patients do not understand the benefits of the procedure, they cannot give informed consent.
This article reviews the pathophysiology of coronary artery disease, evidence from clinical trials of the value of PCI for chronic stable angina, patient and physician perceptions of PCI, and ways to promote patient-centered, shared decision-making.
CLINICAL CASE: EXERTIONAL ANGINA
While climbing 4 flights of stairs, a 55-year-old man noticed tightness in his chest, which lasted for 5 minutes and resolved spontaneously. Several weeks later, when visiting his primary care physician, he mentioned the episode. He had had no symptoms in the interim, but the physician ordered an exercise stress test.
Six minutes into a standard Bruce protocol, the patient experienced the same chest tightness, accompanied by 1-mm ST-segment depressions in leads II, III, and aVF. He was then referred to a cardiologist, who recommended catheterization.
Catheterization demonstrated a 95% stenosis of the right coronary artery with nonsignificant stenoses of the left anterior descending and circumflex arteries. A drug-eluting stent was placed in the right coronary artery, with no residual stenosis.
Did this intervention likely prevent an MI and perhaps save the man’s life?
HOW MYOCARDIAL INFARCTION HAPPENS
Understanding the pathogenesis of MI is critical to having realistic expectations of the benefits of stent placement.
Doctors often describe coronary atherosclerosis as a plumbing problem, where deposits of cholesterol and fat build up in arterial walls, clogging the pipes and eventually causing a heart attack. This analogy, which has been around since the 1950s, is easy to for patients to grasp and has been popularized in the press and internalized by the public—as one patient with a 95% stenosis put it, “I was 95% dead.” In that model, angioplasty and stenting can resolve the blockage and “fix” the problem, much as a plumber can clear your pipes with a Roto-Rooter.
Despite the visual appeal of this model,1 it doesn’t accurately convey what we know about the pathophysiology of coronary artery disease. Instead of a gradual buildup of fatty deposits, low-density lipoprotein cholesterol particles infiltrate arterial walls and trigger an inflammatory reaction as they are engulfed by macrophages, leading to a cascade of cytokines and recruitment of more inflammatory cells.2 This immune response can eventually cause the rupture of the plaque’s fibrous cap, triggering thrombosis and infarction, often at a site of insignificant stenosis.
In this new model, coronary artery disease is primarily a problem of inflammation distributed throughout the vasculature, rather than a mechanical problem localized to the site of a significant stenosis.
Significant stenosis does not equal unstable plaque
Not all plaques are equally likely to rupture. Stable plaques tend to be long-standing and calcified, with a thick fibrous cap. A stable plaque causing a 95% stenosis may cause symptoms with exertion, but it is unlikely to cause infarction.3 Conversely, rupture-prone plaques may cause little stenosis, but a large and dangerous plaque may be lurking beneath the thin fibrous cap.
Relying on angiography can be misleading. Treating all significant stenoses improves blood flow, but does not reduce the risk of infarction, because infarction most often occurs in areas where the lumen is not obstructed. A plaque causing only 30% stenosis can suddenly rupture, causing thrombosis and complete occlusion.
The current model explains why PCI is no better than optimal medical therapy (ie, risk factor modification, antiplatelet therapy with aspirin, and a statin). Diet, exercise, smoking cessation, and statins target inflammatory processes and lower low-density lipoprotein cholesterol levels, while aspirin prevents platelet aggregation, among other likely actions.
The model also explains why coronary artery bypass grafting reduces the risk of MI and death in patients with left main or 3-vessel disease. A patient with generalized coronary artery disease has multiple lesions, many of which do not cause significant stenoses. PCI corrects only a single stenosis, whereas coronary artery bypass grafting circumvents all the vulnerable plaques in a vessel.
THE LANDMARK COURAGE TRIAL
Published in 2007, the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial4 randomized more than 2,000 patients to receive either optimal medical therapy plus PCI or optimal medical therapy alone. The primary outcome was a composite of death from any cause and nonfatal MI. Patients were followed for at least 3 years, and some for as long as 7 years.
There was an initial small upward spike in the primary outcome in the PCI arm due to periprocedural events. By 5 years, the outcomes of the 2 arms converged and then stayed the same for up to 15 years.5 The authors concluded that PCI conferred no benefit over optimal medical therapy in the risk of death or MI.
Some doctors dismiss the study because of its stringent entry criteria—of 35,539 patients assessed, only 3,071 met the eligibility criteria. However, the entry criteria were meant to identify patients most likely to benefit from PCI. Many patients who undergo PCI today would not have qualified for the study because they lack objective evidence of ischemia.6 To enroll, patients needed a proximal stenosis of at least 70% and objective evidence of ischemia or a coronary stenosis of more than 80% and classic angina. Exclusion criteria disqualified few patients: Canadian Cardiovascular Society class IV angina (ie, angina evoked from minimal activity or at rest); a markedly positive stress test (substantial ST-segment depression or hypotension during stage I of the Bruce protocol); refractory heart failure or cardiogenic shock; an ejection fraction of less than 30%; revascularization within the past 6 months; and coronary anatomy unsuitable for PCI.
OTHER TRIALS SUPPORT COURAGE FINDINGS
Although COURAGE was hailed as a landmark trial, it largely supported the results of previous studies. A meta-analysis of PCI vs optimal medical therapy published in 2005 found no significant differences in death, cardiac death, MI, or nonfatal MI.7 MI was actually slightly more common in the PCI group due to the increased risk of MI during the periprocedural period.
Nor has the evidence from COURAGE discouraged additional studies of the same topic. Despite consistent findings that fit with our understanding of coronary disease as inflammation, we continue to conduct studies aimed at addressing significant stenosis, as if that was the problem. Thus, there have been studies of angioplasty alone, followed by studies of bare-metal stents and then drug-eluting stents.
In 2009, Trikalinos et al published a review of 61 randomized controlled trials comprising more than 25,000 patients with stable coronary disease and comparing medical therapy and angioplasty in its various forms over the previous 20 years.8 In all direct and indirect comparisons of PCI and medical therapy, there were no improvements in rates of death or MI.
Even so, the studies continue. The most recent “improvement” was the addition of fractional flow reserve, which served as the inclusion criterion for the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.9 In that study, patients with at least 1 stenosis with a fractional flow reserve less than 0.80 were randomized to PCI plus medical therapy or to medical therapy alone. The primary end point was a composite of death from any cause, MI, and urgent revascularization. Unfortunately, the study was stopped early when the primary end point was met due to a reduction in the need for urgent revascularization. There was no reduction in the rate of MI (hazard ratio 1.05, 95% confidence interval 0.51–2.19).
The reduction in urgent revascularization has also been shown consistently in past studies, but this is the weakest outcome measure because it does not equate to a reduction in the rate of MI. There is no demonstrable harm to putting off stent placement, even in functionally significant arteries, and most patients do not require a stent, even in the future.
In summary, the primary benefit of getting a stent now is a reduced likelihood of needing one later.
PCI MAY IMPROVE ANGINA FASTER
Another important finding of the COURAGE trial was that PCI improved symptoms more than optimal medical therapy.10 This is not surprising, because angina is often a direct result of a significant stenosis. What was unexpected was that even after PCI, most patients were not symptom-free. At 1 month, significantly more PCI patients were angina-free (42%) than were medical patients (33%). This translates into an absolute risk reduction of 9% or a number needed to treat of 11 to prevent 1 case of angina.
Patients in both groups improved over time, and after 3 years, the difference between the 2 groups was no longer significant: 59% in the PCI group vs 56% in the medical therapy group were angina-free.
A more recent study has raised the possibility that the improvement in angina with PCI is primarily a placebo effect. Researchers in the United Kingdom randomized patients with stable angina and at least a 70% stenosis of one vessel to either PCI or sham PCI, in which they threaded the catheter but did not deploy the stent.11 All patients received aggressive antianginal therapy before the procedure. At 6 weeks, there was improvement in angina in both groups, but no statistically significant difference between them in either exercise time or angina. Approximately half the patients in each group improved by at least 1 grade on the Canadian Cardiovascular Society angina classification, and more than 20% improved 2 grades.
This finding is not without precedent. Ligation of the internal mammary arteries, a popular treatment for angina in the 1950s, often provided dramatic relief of symptoms, until it was proven to be no better than a sham operation.12,13 More recently, a placebo-controlled trial of percutaneous laser myocardial revascularization also failed to show improvement over a sham treatment, despite promising results from a phase 1 trial.14 Together, these studies emphasize the subjective nature of angina as an outcome and call into question the routine use of PCI to relieve it.
PCI ENTAILS RISK
PCI entails a small but not inconsequential risk. During the procedure, 2% of patients develop bleeding or blood vessel damage, and another 1% die or have an MI or a stroke. In the first year after stent placement, 3% of patients have a bleeding event from the antiplatelet therapy needed for the stent, and an additional 2% develop a clot in the stent that leads to MI.15
INFORMED CONSENT IS CRITICAL
As demonstrated above, for patients with stable angina, the only evidence-based benefit of PCI over optimal medical therapy is that symptoms may respond faster. At the same time, there are costs and risks associated with the procedure. Because symptoms are subjective, patients should play a key role in deciding whether PCI is appropriate for them.
The American Medical Association states that a physician providing any treatment or procedure should disclose and discuss with patients the risks and benefits. Unfortunately, a substantial body of evidence demonstrates that this is not occurring in practice.
Patients and cardiologists have conflicting beliefs about PCI
Studies over the past 20 years demonstrate that patients with chronic stable angina consistently overestimate the benefits of PCI, with 71% to 88% believing that it will reduce their chance of death.16–19 Patients also understand that PCI can relieve their symptoms, though no study seems to have assessed the perceived magnitude of this benefit.
In contrast, when cardiologists were asked about the benefits their patients could expect from PCI, only 20% said that it would reduce mortality and 25% said it would prevent MI.18 These are still surprisingly high percentages, since the study was conducted after the COURAGE trial.
Nevertheless, these differences in perception show that cardiologists fail to successfully communicate the benefits of the procedure to their patients. Without complete information, patients cannot make informed decisions.
Cardiologists’ reasons for performing PCI
If PCI cannot improve hard outcomes like MI or death in stable coronary disease, why do cardiologists continue to perform it so frequently?
Soon after the COURAGE trial, Lin et al conducted focus groups with cardiologists to find out.20 Some said that they doubted the clinical trial evidence, given the reduction in the cardiac mortality rate over the past 30 years. Others remarked that their overriding goal is to stamp out ischemia, and that once a lesion is found by catheterization, one must proceed with PCI. This has been termed the “oculostenotic reflex,” ie, the interventionist sees coronary artery disease and immediately places a stent.
Atreya et al found objective evidence of this practice.21 In a 2016 study of 207 patients with obstructive lesions amenable to PCI, the only factors associated with medical management were those that increased the risk of the procedure: age, chronic kidney disease, distal location of the lesion, and type C lesions (the most difficult ones to treat by PCI). More important, evidence of ischemia, presence of angina, and being on optimal medical therapy or maximal antianginal therapy were not associated with PCI.
When surveyed, cardiologists offered reasons similar to those identified by Lin et al, including a positive stress test (70%) and significant myocardium at risk (50%).18 Optimal medical therapy failure was cited less often (40%). Over 30% identified relief of chest pain for patients who were not prescribed optimal medical therapy. Another 30% said that patient anxiety contributed to their decision, but patients who reported anxiety were not more likely to get PCI than those who did not.
True informed consent rarely occurs
Surveys of patients and recordings of doctor visits suggest that doctors often discuss the risks of the procedure but rarely accurately describe the benefits or mention alternative treatments, including optimal medical therapy.
Fowler et al22 surveyed 472 Medicare patients who had undergone PCI in the past year about their consent discussion, particularly regarding alternative options. Only 6% of patients recalled discussing medication as a serious option with their doctor.
In 2 published studies,23,24 we analyzed recorded conversations between doctors and patients in which angiography and PCI were discussed.
In a qualitative assessment of how cardiologists presented the rationale for PCI to patients,23 we observed that cardiologists gave an accurate presentation of the benefits in only 5% of cases. In 13% of the conversations the benefits were explicitly overstated (eg, “If you don’t do it [angiogram/PCI], what could happen? Well, you could…have a heart attack involving that area which can lead to a sudden death”). In another 35% of cases, physicians offered an implicit overstatement of the benefit by saying they could “fix” the problem (eg, “So that’s where we start thinking, Well maybe we better try to fix that [blockage]”), without specifically stating that fixing the problem would offer any benefit. Patients were left to fill in the blanks. Conversations frequently focused on the rationale for performing PCI (eg, ischemia on a stress test) and a description of the procedure, rather than on the risks and benefits.
In a quantitative study of the same data set, we assessed how often physicians addressed the 7 elements of informed decision-making as defined by Braddock et al.24
- Explaining the patient’s role in decision-making (ie, that the patient has a choice to make) was present in half of the conversations. Sometimes a doctor would simply say, “The next step is to perform catheterization.”
- Discussion of clinical issues (eg, having a blockage, stress test results) was performed in almost every case, demonstrating physicians’ comfort with that element.
- Discussing treatment alternatives occurred in only 1 in 4 conversations. This was more frequent than previously reported, and appeared most often when patients expressed hesitancy about proceeding to PCI.
- Discussing pros and cons of the alternatives was done in 42%.
- Uncertainty about the procedure (eg, that it might not relieve the angina) was expressed in only 10% of conversations.
- Assessment of patient understanding was done in 65% of cases. This included even minimal efforts (eg, “Do you have any questions?”). More advanced methods such as teach-back were never used.
- Exploration of patient preferences (eg, asking patients which treatment they prefer, or attempting to understand how angina affects a patient’s life) the final element, occurred in 73% of conversations.
Only 3% of the conversations contained all 7 elements. Even using a more relaxed definition of 3 critical elements (ie, discussing clinical issues, treatment alternatives, and pros and cons), only 13% of conversations included them all.
Discussion affects decisions
Informed decision-making is not only important because of its ethical implications. Offering patients more information was associated with their choosing not to have PCI. The probability of a patient undergoing PCI was negatively associated with 3 specific elements of informed decision-making. Patients were less likely to choose PCI if the patient’s role in decision-making was discussed (61% vs 86% chose PCI, P < .03); if alternatives were discussed (31% vs 89% chose PCI, P < .01); and if uncertainties were discussed (17% vs 80% chose PCI, P < .01).
There was also a linear relationship between the total number of elements discussed and the probability of choosing PCI: it ranged from 100% of patients choosing PCI when just 1 element was present to 3% of patients choosing PCI when all 7 elements were present. The relationship is not entirely causal, since doctors were more likely to talk about alternatives and risks if patients hesitated and raised questions. Cautious patients received more information.
From these observational studies, we know that physicians do not generally communicate the benefits of PCI, and patients make incorrect assumptions about the benefits they can expect. We know that those who receive more information are less likely to choose PCI, but what would happen if patients were randomly assigned to receive complete information?
An online survey
We conducted an online survey of more than 1,000 participants over age 50 who had never undergone PCI, asking them to imagine visiting a cardiologist after having a positive stress test for stable chest pain.25 Three intervention groups read different scenarios couched as information provided by their cardiologist:
- The “standard care” group received no specific information about the effects of PCI on the risk of myocardial infarction
- The “specific information” group was specifically told that PCI does not reduce the risk of myocardial infarction
- The “explanatory information” group was told how medications work and why PCI does not reduce the risk of myocardial infarction.
All 3 groups received information about the risks of PCI, its role in reducing angina, and the risks and benefits of optimal medical therapy.
After reading their scenario, all participants completed an identical questionnaire, which asked if they would opt for PCI, medical therapy, or both. Overall, 55% chose PCI, ranging from 70% in the standard care group to 46% in the group given explanatory information. Rates in the specific-information and explanatory-information groups were not statistically different from each other, but both were significantly different from that in the standard-care group. Interestingly, the more information patients were given about PCI, the more likely they were to choose optimal medical therapy.
After reading the scenario, participants were also asked if PCI would “prevent a heart attack.” Of those who received standard care, 71% endorsed that belief, which is remarkably similar to studies of real patients who have received standard care. In contrast, only 39% of those given specific information and 31% given explanatory information held that belief. Moreover, the belief that PCI prevented MI was the strongest predictor of choosing PCI (odds ratio 5.82, 95% confidence interval 4.13–8.26).25
Interestingly, 52% of the standard care group falsely remembered that the doctor had told them that PCI would prevent an MI, even though the doctor said nothing about it one way or the other. It appears that participants were projecting their own beliefs onto the encounter. This highlights the importance of providing full information to patients who are considering this procedure.
TOWARD SHARED DECISION-MAKING
Shared decision-making is a process in which physicians enter into a partnership with a patient, offer information, elicit the patient’s preferences, and then come to a decision in concert with the patient.
Although many decisions can and should involve elements of shared decision-making, the decision to proceed with PCI for stable angina is particularly well-suited to shared decision-making. This is because the benefit of PCI depends on the value a patient attaches to being free of angina sooner. Since there is no difference in the risk of MI or death, the patient must decide if the risks of the procedure and the inconvenience of taking dual antiplatelet therapy are worth the benefit of improving symptoms faster. Presumably, patients who have more severe symptoms or experienced side effects from antianginal therapy would be more likely to choose PCI.
Despite having substantial experience educating patients, most physicians are unfamiliar with the process of shared decision-making. In particular, the process of eliciting preferences is often overlooked.
To address this issue, researchers at the Mayo Clinic developed a decision aid that compares PCI plus optimal medical therapy vs optimal medical therapy alone in an easily understandable information card.15 On one side, the 2 options are clearly stated, with the magnitude of symptom improvement over time graphically illustrated and the statement, “NO DIFFERENCE in heart attack or death,” prominently displayed. The back of the card discusses the risks of each option in easily understood tables.
The decision aid was compared with standard care in a randomized trial involving patients who were referred for catheterization and possible PCI.26 The decision aid improved patients’ overall knowledge about PCI. In particular, 60% of those who used the decision aid knew that PCI did not prevent death or MI vs 40% of usual-care patients—results similar to those of the online experiment.
Interestingly, the decision about whether to undergo PCI did not differ significantly between the 2 groups, although there was a trend toward more patients in the decision-aid group choosing medical therapy alone (53%) vs the standard-care patients (39%).
To understand why the decision aid did not make more of a difference, the investigators performed qualitative interviews of the cardiologists in the study.27 One theme was the timing of the intervention. Patients using the decision aid had already been referred for catheterization, and some felt the process should have occurred earlier. Engaging in shared decision-making with a general cardiologist before referral could help to improve the quality of patient decisions.
Cardiologists also noted the difficulty in changing their work flow to incorporate the decision aid. Although some embraced the idea of shared decision-making, others were concerned that many patients could not participate, and there was confusion about the difference between an educational tool, which could be used by a patient alone, and a decision aid, which is meant to generate discussion between the doctor and patient. Some expressed interest in using the tool in the future.
These findings serve to emphasize that providing information alone is not enough. If the physician does not “buy in” to the idea of shared decision-making, it will not occur.
PRACTICE IMPLICATIONS
Based on the pathophysiology of coronary artery disease and the results of multiple randomized controlled trials, it is evident that PCI does not prevent heart attacks in patients with chronic stable angina. However, most patients who undergo PCI are unaware of this and therefore do not truly give informed consent. In the absence of explicit information to the contrary, most patients with stable angina assume that PCI prevents MI and thus are biased toward choosing PCI.
Even minimal amounts of explicit information can partially overcome that bias and influence decision-making. In particular, explaining why PCI does not prevent MI was the most effective means of overcoming the bias.
To this end, shared decision aids may help physicians to engage in shared decision-making. Shared decision-making is most likely to occur if physicians are trained in the concept of shared decision-making, are committed to practicing it, and can fit it into their work flow. Ideally, this would occur in the office of a general cardiologist before referral for PCI.
For those practicing in accountable-care organizations, Medicare has recently introduced the shared decision-making model for 6 preference-sensitive conditions, including stable ischemic heart disease. Participants in this program will have the opportunity to receive payments for shared decision-making services and to share in any savings that result from reduced use of resources. Use of these tools holds the promise for providing more patient-centered care at lower cost.
- Jones DS. Visions of a cure. Visualization, clinical trials, and controversies in cardiac therapeutics, 1968–1998. Isis 2000; 91:504–541.
- Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695.
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364:226–235.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 2015; 373:1937–1946.
- Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA 2008; 300:1765–1773.
- Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005; 111:2906–2912.
- Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet 2009; 373:911–918.
- De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Al-Lamee R, Thompson D, Dehbi H-M, et al, on behalf of the ORBITA Investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. Published online November 2, 2017. http://dx.doi.org/10.1016/S0140-6736(17)32714-9. Accessed November 10, 2017.
- Cobb LA, Thomas GI, Dillard DH, et al. An evaluation of internal mammary-artery ligation by a double-blind technic. N Engl J Med 1959; 260:1115–1118.
- Dimond EG, Fittle F, Crockett JE. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am J Cardiol 1960; 5:483-486.
- Leon MB, Kornowski R, Downey WE, et al. A blinded, randomized placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 2005; 46:1812–1819.
- Coylewright M, Shepel K, Leblanc A, et al. Shared decision making in patients with stable coronary artery disease: PCI choice. PLoS One 2012; 7:e49827.
- Holmboe ES, Fiellin DA, Cusanelli E, Remetz M, Krumholz HM. Perceptions of benefit and risk of patients undergoing first-time elective percutaneous coronary revascularization. J Gen Intern Med 2000; 15:632–637.
- Kee F, McDonald P, Gaffney B. Risks and benefits of coronary angioplasty: the patients perspective: a preliminary study. Qual Health Care 1997; 6:131–139.
- Rothberg MB, Sivalingam SK, Ashraf J, et al. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med 2010; 153:307–313.
- Whittle J, Conigliaro J, Good CB, Kelley ME, Skanderson M. Understanding of the benefits of coronary revascularization procedures among patients who are offered such procedures. Am Heart J 2007; 154:662–668.
- Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med 2007; 167:1604–1609.
- Atreya AR, Sivalingam SK, Arora S, et al. Predictors of medical management in patients undergoing elective cardiac catheterization for chronic ischemic heart disease. Clin Cardiol 2016; 39:207–214.
- Fowler FJ Jr, Gallagher PM, Bynum JP, Barry MJ, Lucas FL, Skinner JS. Decision-making process reported by Medicare patients who had coronary artery stenting or surgery for prostate cancer. J Gen Intern Med 2012; 27:911–916.
- Goff SL, Mazor KM, Ting HH, Kleppel R, Rothberg MB. How cardiologists present the benefits of percutaneous coronary interventions to patients with stable angina: a qualitative analysis. JAMA Intern Med 2014; 174:1614–1621.
- Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA 1999; 282:2313–2320.
- Rothberg MB, Scherer L, Kashef MA, et al. The effect of information presentation on beliefs about the benefits of elective percutaneous coronary intervention. JAMA Intern Med 2014; 174:1623–1629.
- Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016; 9:767–776.
- Coylewright M, O’Neill ES, Dick S, Grande SW. PCI choice: cardiovascular clinicians’ perceptions of shared decision making in stable coronary artery disease. Patient Educ Couns 2017; 100:1136–1143.
- Jones DS. Visions of a cure. Visualization, clinical trials, and controversies in cardiac therapeutics, 1968–1998. Isis 2000; 91:504–541.
- Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695.
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364:226–235.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Sedlis SP, Hartigan PM, Teo KK, et al. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 2015; 373:1937–1946.
- Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA 2008; 300:1765–1773.
- Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005; 111:2906–2912.
- Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet 2009; 373:911–918.
- De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Al-Lamee R, Thompson D, Dehbi H-M, et al, on behalf of the ORBITA Investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. Published online November 2, 2017. http://dx.doi.org/10.1016/S0140-6736(17)32714-9. Accessed November 10, 2017.
- Cobb LA, Thomas GI, Dillard DH, et al. An evaluation of internal mammary-artery ligation by a double-blind technic. N Engl J Med 1959; 260:1115–1118.
- Dimond EG, Fittle F, Crockett JE. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am J Cardiol 1960; 5:483-486.
- Leon MB, Kornowski R, Downey WE, et al. A blinded, randomized placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 2005; 46:1812–1819.
- Coylewright M, Shepel K, Leblanc A, et al. Shared decision making in patients with stable coronary artery disease: PCI choice. PLoS One 2012; 7:e49827.
- Holmboe ES, Fiellin DA, Cusanelli E, Remetz M, Krumholz HM. Perceptions of benefit and risk of patients undergoing first-time elective percutaneous coronary revascularization. J Gen Intern Med 2000; 15:632–637.
- Kee F, McDonald P, Gaffney B. Risks and benefits of coronary angioplasty: the patients perspective: a preliminary study. Qual Health Care 1997; 6:131–139.
- Rothberg MB, Sivalingam SK, Ashraf J, et al. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med 2010; 153:307–313.
- Whittle J, Conigliaro J, Good CB, Kelley ME, Skanderson M. Understanding of the benefits of coronary revascularization procedures among patients who are offered such procedures. Am Heart J 2007; 154:662–668.
- Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med 2007; 167:1604–1609.
- Atreya AR, Sivalingam SK, Arora S, et al. Predictors of medical management in patients undergoing elective cardiac catheterization for chronic ischemic heart disease. Clin Cardiol 2016; 39:207–214.
- Fowler FJ Jr, Gallagher PM, Bynum JP, Barry MJ, Lucas FL, Skinner JS. Decision-making process reported by Medicare patients who had coronary artery stenting or surgery for prostate cancer. J Gen Intern Med 2012; 27:911–916.
- Goff SL, Mazor KM, Ting HH, Kleppel R, Rothberg MB. How cardiologists present the benefits of percutaneous coronary interventions to patients with stable angina: a qualitative analysis. JAMA Intern Med 2014; 174:1614–1621.
- Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA 1999; 282:2313–2320.
- Rothberg MB, Scherer L, Kashef MA, et al. The effect of information presentation on beliefs about the benefits of elective percutaneous coronary intervention. JAMA Intern Med 2014; 174:1623–1629.
- Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016; 9:767–776.
- Coylewright M, O’Neill ES, Dick S, Grande SW. PCI choice: cardiovascular clinicians’ perceptions of shared decision making in stable coronary artery disease. Patient Educ Couns 2017; 100:1136–1143.
KEY POINTS
- For patients with stable angina pectoris, PCI does not prevent myocardial infarction or death.
- Optimal medical therapy with aspirin and a statin can reduce the risk of myocardial infarction and should be recommended for all patients with stable angina, regardless of whether they undergo PCI.
- PCI improves symptoms of angina faster than medical therapy alone, but more than half of patients will be free of angina in about 2 years with either option.
- In the absence of information to the contrary, most patients and some doctors assume that PCI is life-saving and are biased towards choosing it. As a result, patients are rarely able to give true informed consent to undergo PCI.
Having the COURAGE to include PCI in shared decision-making for stable angina
Invented by Andreas Grüntzig in 1977, percutaneous coronary intervention (PCI) has revolutionized the management of coronary artery disease.1 Initially, PCI was more attractive than conventional revascularization with coronary artery bypass grafting because it was less invasive, but as time went on PCI acquired its own evidence base of improved clinical outcomes. In fact, for ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and cardiogenic shock, there is clear evidence that PCI saves lives in both the short and long term.2,3
But PCI is also used widely in stable coronary artery disease, and in contrast to the clear-cut benefit in the acute conditions noted above, a series of reports culminating in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial has shown that PCI in stable coronary artery disease does not reduce the risk of death or of subsequent myocardial infarction.4,5 Cardiologists have heeded the COURAGE trial findings in their clinical decision-making, and the rate of PCI for stable coronary artery disease dropped by 60% from 2006 to 2011.6
In an article in this issue,7 Dr. Michael Rothberg describes a 55-year-old man who develops new-onset angina and then undergoes a Bruce protocol stress test that is stopped at 6 minutes due to chest pain and ST-segment depression. Dr. Rothberg argues that, based on COURAGE trial data, this patient and other patients with stable coronary artery disease should not be treated with PCI but instead should receive optimal medical therapy.
KEY ISSUES ABOUT THE COURAGE TRIAL
To understand the applicability of the results of the COURAGE trial to patient care, it is important to examine a number of key issues about this trial.
First, COURAGE enrolled a narrow group of patients with stable coronary artery disease and excluded many common patient subgroups, such as those with heart failure, severe anginal symptoms, or left main artery stenosis, who would benefit from revascularization.5 Specifically, for every 100 patients enrolled in COURAGE, 161 were excluded for having heart failure, 39 were excluded for class IV angina, and 31 were excluded for left main stenosis.
Second, although COURAGE has been described as a trial of PCI vs optimal medical therapy, it was not. Rather, it was a trial of optimal medical therapy with PCI first vs optimal medical therapy with crossover PCI if medical therapy failed.5 The crossover rate was not insubstantial: 16.1% of the patients in the medical therapy group underwent PCI by the end of the first year, increasing to 32.6% at a median of 4.6 years of follow-up.5,7 And patients with more intense and frequent angina and resulting worse quality of life were the ones who required crossover PCI.8
Third, it has been proposed that patients with suspicious cardiac symptoms or abnormal stress test findings can be managed with optimal medical therapy initially, based on the COURAGE findings. However, the COURAGE trial required diagnostic angiography both to confirm underlying coronary artery disease and to exclude left main disease.5 Thus, regardless of one’s position on the role of PCI in stable coronary artery disease, diagnostic investigation by cardiac catheterization or computed tomographic angiography to confirm the presence or absence of coronary artery disease remains mandatory.
Fourth, optimal medical therapy was prescribed by the trial’s protocol, so that one would expect that both treatment groups received similar levels of optimal medical therapy. However, the optimal medical therapy group required more medications to achieve the same outcome as the PCI group.5
Finally, although it has been reported that the COURAGE trial showed no benefit for PCI, in fact, for the outcome of symptom relief, initial PCI was clearly superior to optimal medical therapy beginning at 3 months and extending out to 24 months—a result for which the magnitude of benefit is underestimated due to the occurrence of crossover PCI.9 In particular, women and patients with a high frequency of angina derived improvement in angina-related quality of life from PCI compared with optimal medical therapy.8,10
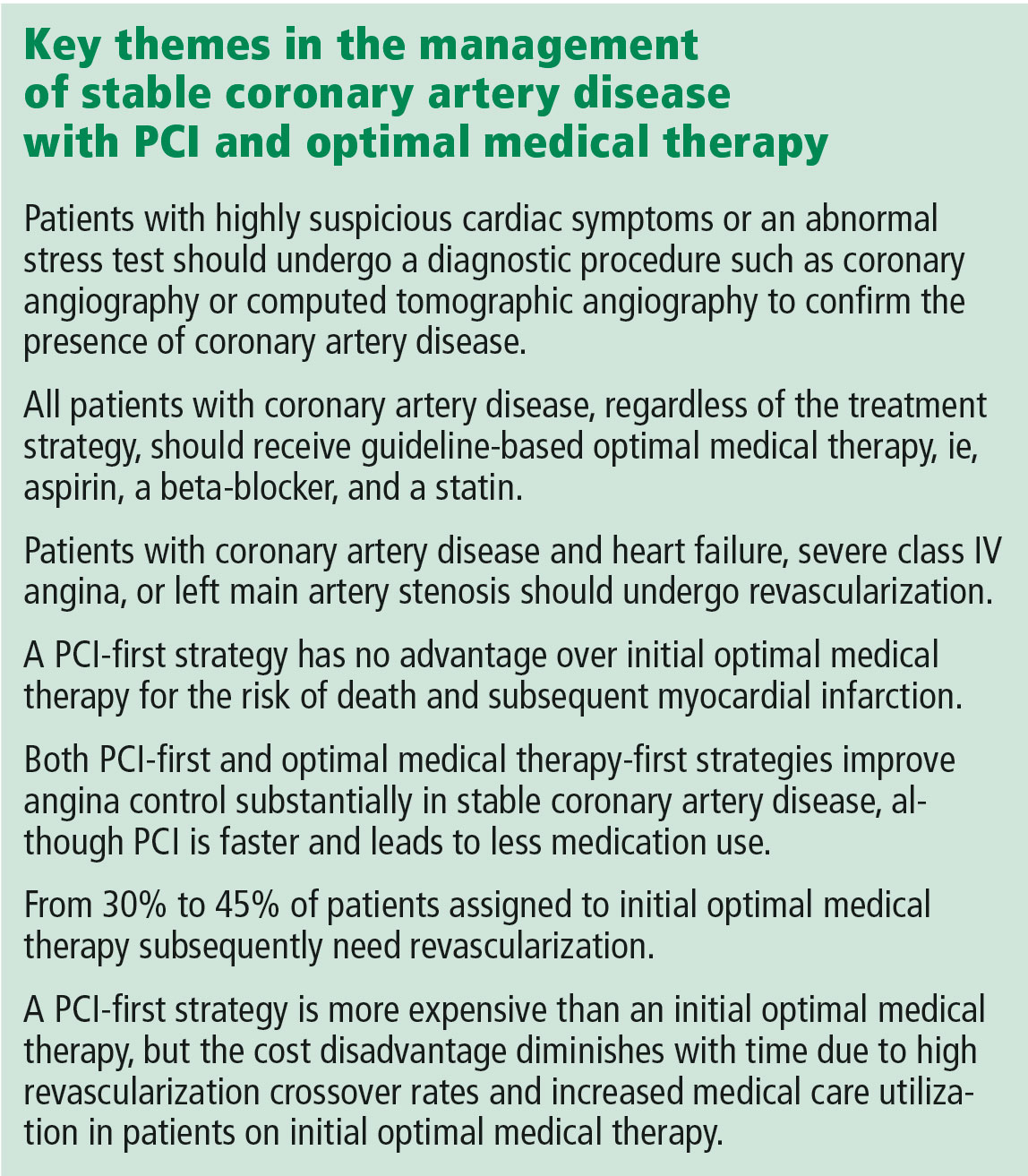
A MORE NUANCED INTERPRETATION
For these reasons, the role of PCI in stable coronary disease is more nuanced than simply stating that the COURAGE trial results were “negative” for PCI. It is more accurate to say that in selected patients with moderate symptoms of angina and without heart failure or left main artery disease, a PCI-first strategy has no advantage over an optimal medical treatment-first strategy for the risk of death and myocardial infarction but does lead to earlier angina relief and less long-term need for medication. In addition, in up to one-third of cases, an optimal medical treatment-first strategy fails and requires crossover to PCI.5
Dr. Rothberg is correct in highlighting the crucial importance of optimal medical therapy in the management of stable coronary artery disease. In fact, cardiologists strive to prescribe optimal medical treatment for all coronary artery disease patients irrespective of treatment strategy. However, 3 important issues in his analysis need to be highlighted.
Controlling symptoms is important, and we should not underrate it. The patient described in Dr. Rothberg’s article could exercise for only 6 minutes on a Bruce treadmill test, indicating a quite limited functional capacity of only 5.8 metabolic equivalents of the task (METs).11 (A healthy 55-year-old man should be able to achieve 10.5 METs.12) Inability to achieve 6 METs precludes the ability to dance, to ride a bike at a moderate pace, or to go on a hike.13 For many patients, these limitations are serious and important concerns for their lifestyle and quality of life. PCI has been shown to be superior to medical therapy in improving functional capacity, improving it by 20% vs 2% in one trial14 and 26% vs 7% in a second trial.14 Patients undergoing PCI were twice as likely to have a greater than 2-minute increase in exercise capacity.15 Recognizing the importance of symptom control in stable coronary artery disease is patient-centered care.
Patient decision-making is complicated, and we should not assume that patients choose PCI primarily to reduce their risk of death. A randomized trial showed that patients continued to select PCI as initial treatment even when they clearly knew that it would not prevent death or myocardial infarction.16 As noted above, patients may value earlier symptom relief, particularly if their angina is frequent or limiting. In addition, patients strongly desire to minimize medical therapy and may be willing to trade decreased life expectancy to reduce the need to take medications.17 Finally, some patients may want to be able to continue to participate in certain lifestyle activities.
PCI is expensive, but less so over the long run. With a PCI-first strategy, costs are front-loaded, and studies with short-term follow-up show a marked increase in cost. However, long-term follow-up shows that the cost differences diminish dramatically due to high rates of crossover to revascularization and increased medical care in the optimal medical therapy arm. The cumulative lifetime costs in the COURAGE trial with a PCI-first strategy, although statistically significant, were only 10% higher than with the optimal medical treatment-first strategy ($99,820 vs $90,370).18 Therefore, substantial long-term cost-savings by shifting from an initial PCI strategy to initial optimal medical therapy are unlikely to be delivered when measured over the long term.
NEWER TRIALS SUPPORT A BALANCED APPROACH
The most recent studies of the management of stable coronary artery disease support a balanced approach.
The ORBITA trial (Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina), on one hand, showed limited benefit of PCI vs medical therapy in patients with single-vessel coronary artery disease, preserved functional capacity, and mild symptoms.19 There was no significant improvement in exercise capacity or angina frequency, although baseline angina frequency after medical stabilization was quite low.
The FAME 2 trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation), on the other hand, studied patients with positive fractional flow reserve coronary artery disease (ie, using an invasive technique to confirm the hemodynamic significance of the coronary stenosis) and showed markedly better outcomes with PCI than with medical therapy.20 Specifically, the PCI-first group had improved quality of life and dramatically less need for urgent revascularization.
Furthermore, as in the COURAGE trial, the optimal medical therapy group had a high crossover rate to PCI (44.2%), leading to the complete elimination of the early cost advantage of medical therapy by 3 years. The initial costs with PCI vs medical therapy were $9,944 vs $4,439 (P < .001); the 3-year costs were $16,792 vs $16,737 (P = .94).
For these reasons, a balanced approach to recommending PCI first vs optimal medical treatment first remains the best strategy.
TOWARD PATIENT-CENTERED CARE
For the 55-year-old patient in Dr. Rothberg’s article, the first step in making an appropriate decision would be to understand the severity of symptoms relative to the patient’s lifestyle. The second step is to assess the patient’s interest in an invasive procedure such as PCI relative to optimal medical therapy, as the patient may have a strong preference for one option or the other.
Finally, with the understanding that there is no difference in hard end points of myocardial infarction and death, a balanced discussion of the advantages and disadvantages of both PCI and optimal medical therapy would be needed. For PCI, advantages include earlier symptom control and improved quality of the life, particularly if symptoms are severe, with disadvantages of an invasive procedure with its attendant risks. For optimal medical therapy, advantages include improved symptom control and avoidance of an invasive procedure, while disadvantages include increased medication use and a high rate of eventual crossover to PCI. This important discussion integrating both patient and medical perspectives ultimately leads to the best decision for the individual patient.
A patient-centered approach to clinical decision-making mandates inclusion of PCI first as an option in the management of stable coronary artery disease. After confirming the patient has coronary artery disease, patients with heart failure, class IV angina at rest, or left main artery stenosis should be referred for revascularization. In the remaining patients with confirmed coronary artery disease and moderate angina symptoms, either PCI first or optimal medical therapy first is an appropriate initial strategy that considers coronary anatomy, symptom burden, and patient desires.
- Meier B. The first patient to undergo coronary angioplasty—23-year follow-up. N Engl J Med 2001; 344:144–145.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Fox KAA, Clayton TC, Damman P, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010; 55:2435–2445.
- Katritsis DG, Ioannidis JPA. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005; 111:2906–2912.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Bangalore S, Gupta N, Généreux P, Guo Y, Pancholy S, Feit F. Trend in percutaneous coronary intervention volume following the COURAGE and BARI-2D trials: insight from over 8.1 million percutaneous coronary interventions. Int J Cardiol 2015; 183:6–10.
- Rothberg MB. PCI for stable angina: a missed opportunity for shared decision-making. Cleve Clin J Med 2018; 85:105–121.
- Spertus JA, Maron DJ, Cohen DJ, et al. Frequency, predictors, and consequences of crossing over to revascularization within 12 months of randomization to optimal medical therapy in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial. Circ Cardiovasc Qual Outcomes 2013; 6:409–418.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Acharjee S, Teo KK, Jacobs AK, et al. Optimal medical therapy with or without percutaneous coronary intervention in women with stable coronary disease: a pre-specified subset analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation (COURAGE) trial. Am Heart J 2016; 173:108–117.
- Foster C, Jackson AS, Pollock ML, et al. Generalized equations for predicting functional capacity from treadmill performance. Am Heart J 1984; 107:1229–1234.
- Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol 1993; 22:175–182.
- Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol 1990; 13:555–565.
- Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007; 297:1985–1991.
- Strauss WE, Fortin T, Hartigan P, Folland ED, Parisi AF. A comparison of quality of life scores in patients with angina pectoris after angioplasty compared with after medical therapy. Outcomes of a randomized clinical trial. Veterans Affairs Study of Angioplasty Compared to Medical Therapy Investigators. Circulation 1995; 92:1710–1719.
- Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016; 9:767–776.
- Fontana M, Asaria P, Moraldo M, et al. Patient-accessible tool for shared decision making in cardiovascular primary prevention: balancing longevity benefits against medication disutility. Circulation 2014; 129:2539–2546.
- Weintraub WS, Boden WE, Zhang Z, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes 2008; 1:12–20.
- Al-Lamee R, Thompson D, Dehbi H-M, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet November 2, 2017; doi:10.1016/S0140-6736(17)32714-9.
- Fearon WF, Nishi T, Bruyne BD, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation November 2017; doi:10.1161/CIRCULATIONAHA.117.031907.
Invented by Andreas Grüntzig in 1977, percutaneous coronary intervention (PCI) has revolutionized the management of coronary artery disease.1 Initially, PCI was more attractive than conventional revascularization with coronary artery bypass grafting because it was less invasive, but as time went on PCI acquired its own evidence base of improved clinical outcomes. In fact, for ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and cardiogenic shock, there is clear evidence that PCI saves lives in both the short and long term.2,3
But PCI is also used widely in stable coronary artery disease, and in contrast to the clear-cut benefit in the acute conditions noted above, a series of reports culminating in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial has shown that PCI in stable coronary artery disease does not reduce the risk of death or of subsequent myocardial infarction.4,5 Cardiologists have heeded the COURAGE trial findings in their clinical decision-making, and the rate of PCI for stable coronary artery disease dropped by 60% from 2006 to 2011.6
In an article in this issue,7 Dr. Michael Rothberg describes a 55-year-old man who develops new-onset angina and then undergoes a Bruce protocol stress test that is stopped at 6 minutes due to chest pain and ST-segment depression. Dr. Rothberg argues that, based on COURAGE trial data, this patient and other patients with stable coronary artery disease should not be treated with PCI but instead should receive optimal medical therapy.
KEY ISSUES ABOUT THE COURAGE TRIAL
To understand the applicability of the results of the COURAGE trial to patient care, it is important to examine a number of key issues about this trial.
First, COURAGE enrolled a narrow group of patients with stable coronary artery disease and excluded many common patient subgroups, such as those with heart failure, severe anginal symptoms, or left main artery stenosis, who would benefit from revascularization.5 Specifically, for every 100 patients enrolled in COURAGE, 161 were excluded for having heart failure, 39 were excluded for class IV angina, and 31 were excluded for left main stenosis.
Second, although COURAGE has been described as a trial of PCI vs optimal medical therapy, it was not. Rather, it was a trial of optimal medical therapy with PCI first vs optimal medical therapy with crossover PCI if medical therapy failed.5 The crossover rate was not insubstantial: 16.1% of the patients in the medical therapy group underwent PCI by the end of the first year, increasing to 32.6% at a median of 4.6 years of follow-up.5,7 And patients with more intense and frequent angina and resulting worse quality of life were the ones who required crossover PCI.8
Third, it has been proposed that patients with suspicious cardiac symptoms or abnormal stress test findings can be managed with optimal medical therapy initially, based on the COURAGE findings. However, the COURAGE trial required diagnostic angiography both to confirm underlying coronary artery disease and to exclude left main disease.5 Thus, regardless of one’s position on the role of PCI in stable coronary artery disease, diagnostic investigation by cardiac catheterization or computed tomographic angiography to confirm the presence or absence of coronary artery disease remains mandatory.
Fourth, optimal medical therapy was prescribed by the trial’s protocol, so that one would expect that both treatment groups received similar levels of optimal medical therapy. However, the optimal medical therapy group required more medications to achieve the same outcome as the PCI group.5
Finally, although it has been reported that the COURAGE trial showed no benefit for PCI, in fact, for the outcome of symptom relief, initial PCI was clearly superior to optimal medical therapy beginning at 3 months and extending out to 24 months—a result for which the magnitude of benefit is underestimated due to the occurrence of crossover PCI.9 In particular, women and patients with a high frequency of angina derived improvement in angina-related quality of life from PCI compared with optimal medical therapy.8,10

A MORE NUANCED INTERPRETATION
For these reasons, the role of PCI in stable coronary disease is more nuanced than simply stating that the COURAGE trial results were “negative” for PCI. It is more accurate to say that in selected patients with moderate symptoms of angina and without heart failure or left main artery disease, a PCI-first strategy has no advantage over an optimal medical treatment-first strategy for the risk of death and myocardial infarction but does lead to earlier angina relief and less long-term need for medication. In addition, in up to one-third of cases, an optimal medical treatment-first strategy fails and requires crossover to PCI.5
Dr. Rothberg is correct in highlighting the crucial importance of optimal medical therapy in the management of stable coronary artery disease. In fact, cardiologists strive to prescribe optimal medical treatment for all coronary artery disease patients irrespective of treatment strategy. However, 3 important issues in his analysis need to be highlighted.
Controlling symptoms is important, and we should not underrate it. The patient described in Dr. Rothberg’s article could exercise for only 6 minutes on a Bruce treadmill test, indicating a quite limited functional capacity of only 5.8 metabolic equivalents of the task (METs).11 (A healthy 55-year-old man should be able to achieve 10.5 METs.12) Inability to achieve 6 METs precludes the ability to dance, to ride a bike at a moderate pace, or to go on a hike.13 For many patients, these limitations are serious and important concerns for their lifestyle and quality of life. PCI has been shown to be superior to medical therapy in improving functional capacity, improving it by 20% vs 2% in one trial14 and 26% vs 7% in a second trial.14 Patients undergoing PCI were twice as likely to have a greater than 2-minute increase in exercise capacity.15 Recognizing the importance of symptom control in stable coronary artery disease is patient-centered care.
Patient decision-making is complicated, and we should not assume that patients choose PCI primarily to reduce their risk of death. A randomized trial showed that patients continued to select PCI as initial treatment even when they clearly knew that it would not prevent death or myocardial infarction.16 As noted above, patients may value earlier symptom relief, particularly if their angina is frequent or limiting. In addition, patients strongly desire to minimize medical therapy and may be willing to trade decreased life expectancy to reduce the need to take medications.17 Finally, some patients may want to be able to continue to participate in certain lifestyle activities.
PCI is expensive, but less so over the long run. With a PCI-first strategy, costs are front-loaded, and studies with short-term follow-up show a marked increase in cost. However, long-term follow-up shows that the cost differences diminish dramatically due to high rates of crossover to revascularization and increased medical care in the optimal medical therapy arm. The cumulative lifetime costs in the COURAGE trial with a PCI-first strategy, although statistically significant, were only 10% higher than with the optimal medical treatment-first strategy ($99,820 vs $90,370).18 Therefore, substantial long-term cost-savings by shifting from an initial PCI strategy to initial optimal medical therapy are unlikely to be delivered when measured over the long term.
NEWER TRIALS SUPPORT A BALANCED APPROACH
The most recent studies of the management of stable coronary artery disease support a balanced approach.
The ORBITA trial (Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina), on one hand, showed limited benefit of PCI vs medical therapy in patients with single-vessel coronary artery disease, preserved functional capacity, and mild symptoms.19 There was no significant improvement in exercise capacity or angina frequency, although baseline angina frequency after medical stabilization was quite low.
The FAME 2 trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation), on the other hand, studied patients with positive fractional flow reserve coronary artery disease (ie, using an invasive technique to confirm the hemodynamic significance of the coronary stenosis) and showed markedly better outcomes with PCI than with medical therapy.20 Specifically, the PCI-first group had improved quality of life and dramatically less need for urgent revascularization.
Furthermore, as in the COURAGE trial, the optimal medical therapy group had a high crossover rate to PCI (44.2%), leading to the complete elimination of the early cost advantage of medical therapy by 3 years. The initial costs with PCI vs medical therapy were $9,944 vs $4,439 (P < .001); the 3-year costs were $16,792 vs $16,737 (P = .94).
For these reasons, a balanced approach to recommending PCI first vs optimal medical treatment first remains the best strategy.
TOWARD PATIENT-CENTERED CARE
For the 55-year-old patient in Dr. Rothberg’s article, the first step in making an appropriate decision would be to understand the severity of symptoms relative to the patient’s lifestyle. The second step is to assess the patient’s interest in an invasive procedure such as PCI relative to optimal medical therapy, as the patient may have a strong preference for one option or the other.
Finally, with the understanding that there is no difference in hard end points of myocardial infarction and death, a balanced discussion of the advantages and disadvantages of both PCI and optimal medical therapy would be needed. For PCI, advantages include earlier symptom control and improved quality of the life, particularly if symptoms are severe, with disadvantages of an invasive procedure with its attendant risks. For optimal medical therapy, advantages include improved symptom control and avoidance of an invasive procedure, while disadvantages include increased medication use and a high rate of eventual crossover to PCI. This important discussion integrating both patient and medical perspectives ultimately leads to the best decision for the individual patient.
A patient-centered approach to clinical decision-making mandates inclusion of PCI first as an option in the management of stable coronary artery disease. After confirming the patient has coronary artery disease, patients with heart failure, class IV angina at rest, or left main artery stenosis should be referred for revascularization. In the remaining patients with confirmed coronary artery disease and moderate angina symptoms, either PCI first or optimal medical therapy first is an appropriate initial strategy that considers coronary anatomy, symptom burden, and patient desires.
Invented by Andreas Grüntzig in 1977, percutaneous coronary intervention (PCI) has revolutionized the management of coronary artery disease.1 Initially, PCI was more attractive than conventional revascularization with coronary artery bypass grafting because it was less invasive, but as time went on PCI acquired its own evidence base of improved clinical outcomes. In fact, for ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and cardiogenic shock, there is clear evidence that PCI saves lives in both the short and long term.2,3
But PCI is also used widely in stable coronary artery disease, and in contrast to the clear-cut benefit in the acute conditions noted above, a series of reports culminating in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial has shown that PCI in stable coronary artery disease does not reduce the risk of death or of subsequent myocardial infarction.4,5 Cardiologists have heeded the COURAGE trial findings in their clinical decision-making, and the rate of PCI for stable coronary artery disease dropped by 60% from 2006 to 2011.6
In an article in this issue,7 Dr. Michael Rothberg describes a 55-year-old man who develops new-onset angina and then undergoes a Bruce protocol stress test that is stopped at 6 minutes due to chest pain and ST-segment depression. Dr. Rothberg argues that, based on COURAGE trial data, this patient and other patients with stable coronary artery disease should not be treated with PCI but instead should receive optimal medical therapy.
KEY ISSUES ABOUT THE COURAGE TRIAL
To understand the applicability of the results of the COURAGE trial to patient care, it is important to examine a number of key issues about this trial.
First, COURAGE enrolled a narrow group of patients with stable coronary artery disease and excluded many common patient subgroups, such as those with heart failure, severe anginal symptoms, or left main artery stenosis, who would benefit from revascularization.5 Specifically, for every 100 patients enrolled in COURAGE, 161 were excluded for having heart failure, 39 were excluded for class IV angina, and 31 were excluded for left main stenosis.
Second, although COURAGE has been described as a trial of PCI vs optimal medical therapy, it was not. Rather, it was a trial of optimal medical therapy with PCI first vs optimal medical therapy with crossover PCI if medical therapy failed.5 The crossover rate was not insubstantial: 16.1% of the patients in the medical therapy group underwent PCI by the end of the first year, increasing to 32.6% at a median of 4.6 years of follow-up.5,7 And patients with more intense and frequent angina and resulting worse quality of life were the ones who required crossover PCI.8
Third, it has been proposed that patients with suspicious cardiac symptoms or abnormal stress test findings can be managed with optimal medical therapy initially, based on the COURAGE findings. However, the COURAGE trial required diagnostic angiography both to confirm underlying coronary artery disease and to exclude left main disease.5 Thus, regardless of one’s position on the role of PCI in stable coronary artery disease, diagnostic investigation by cardiac catheterization or computed tomographic angiography to confirm the presence or absence of coronary artery disease remains mandatory.
Fourth, optimal medical therapy was prescribed by the trial’s protocol, so that one would expect that both treatment groups received similar levels of optimal medical therapy. However, the optimal medical therapy group required more medications to achieve the same outcome as the PCI group.5
Finally, although it has been reported that the COURAGE trial showed no benefit for PCI, in fact, for the outcome of symptom relief, initial PCI was clearly superior to optimal medical therapy beginning at 3 months and extending out to 24 months—a result for which the magnitude of benefit is underestimated due to the occurrence of crossover PCI.9 In particular, women and patients with a high frequency of angina derived improvement in angina-related quality of life from PCI compared with optimal medical therapy.8,10

A MORE NUANCED INTERPRETATION
For these reasons, the role of PCI in stable coronary disease is more nuanced than simply stating that the COURAGE trial results were “negative” for PCI. It is more accurate to say that in selected patients with moderate symptoms of angina and without heart failure or left main artery disease, a PCI-first strategy has no advantage over an optimal medical treatment-first strategy for the risk of death and myocardial infarction but does lead to earlier angina relief and less long-term need for medication. In addition, in up to one-third of cases, an optimal medical treatment-first strategy fails and requires crossover to PCI.5
Dr. Rothberg is correct in highlighting the crucial importance of optimal medical therapy in the management of stable coronary artery disease. In fact, cardiologists strive to prescribe optimal medical treatment for all coronary artery disease patients irrespective of treatment strategy. However, 3 important issues in his analysis need to be highlighted.
Controlling symptoms is important, and we should not underrate it. The patient described in Dr. Rothberg’s article could exercise for only 6 minutes on a Bruce treadmill test, indicating a quite limited functional capacity of only 5.8 metabolic equivalents of the task (METs).11 (A healthy 55-year-old man should be able to achieve 10.5 METs.12) Inability to achieve 6 METs precludes the ability to dance, to ride a bike at a moderate pace, or to go on a hike.13 For many patients, these limitations are serious and important concerns for their lifestyle and quality of life. PCI has been shown to be superior to medical therapy in improving functional capacity, improving it by 20% vs 2% in one trial14 and 26% vs 7% in a second trial.14 Patients undergoing PCI were twice as likely to have a greater than 2-minute increase in exercise capacity.15 Recognizing the importance of symptom control in stable coronary artery disease is patient-centered care.
Patient decision-making is complicated, and we should not assume that patients choose PCI primarily to reduce their risk of death. A randomized trial showed that patients continued to select PCI as initial treatment even when they clearly knew that it would not prevent death or myocardial infarction.16 As noted above, patients may value earlier symptom relief, particularly if their angina is frequent or limiting. In addition, patients strongly desire to minimize medical therapy and may be willing to trade decreased life expectancy to reduce the need to take medications.17 Finally, some patients may want to be able to continue to participate in certain lifestyle activities.
PCI is expensive, but less so over the long run. With a PCI-first strategy, costs are front-loaded, and studies with short-term follow-up show a marked increase in cost. However, long-term follow-up shows that the cost differences diminish dramatically due to high rates of crossover to revascularization and increased medical care in the optimal medical therapy arm. The cumulative lifetime costs in the COURAGE trial with a PCI-first strategy, although statistically significant, were only 10% higher than with the optimal medical treatment-first strategy ($99,820 vs $90,370).18 Therefore, substantial long-term cost-savings by shifting from an initial PCI strategy to initial optimal medical therapy are unlikely to be delivered when measured over the long term.
NEWER TRIALS SUPPORT A BALANCED APPROACH
The most recent studies of the management of stable coronary artery disease support a balanced approach.
The ORBITA trial (Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina), on one hand, showed limited benefit of PCI vs medical therapy in patients with single-vessel coronary artery disease, preserved functional capacity, and mild symptoms.19 There was no significant improvement in exercise capacity or angina frequency, although baseline angina frequency after medical stabilization was quite low.
The FAME 2 trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation), on the other hand, studied patients with positive fractional flow reserve coronary artery disease (ie, using an invasive technique to confirm the hemodynamic significance of the coronary stenosis) and showed markedly better outcomes with PCI than with medical therapy.20 Specifically, the PCI-first group had improved quality of life and dramatically less need for urgent revascularization.
Furthermore, as in the COURAGE trial, the optimal medical therapy group had a high crossover rate to PCI (44.2%), leading to the complete elimination of the early cost advantage of medical therapy by 3 years. The initial costs with PCI vs medical therapy were $9,944 vs $4,439 (P < .001); the 3-year costs were $16,792 vs $16,737 (P = .94).
For these reasons, a balanced approach to recommending PCI first vs optimal medical treatment first remains the best strategy.
TOWARD PATIENT-CENTERED CARE
For the 55-year-old patient in Dr. Rothberg’s article, the first step in making an appropriate decision would be to understand the severity of symptoms relative to the patient’s lifestyle. The second step is to assess the patient’s interest in an invasive procedure such as PCI relative to optimal medical therapy, as the patient may have a strong preference for one option or the other.
Finally, with the understanding that there is no difference in hard end points of myocardial infarction and death, a balanced discussion of the advantages and disadvantages of both PCI and optimal medical therapy would be needed. For PCI, advantages include earlier symptom control and improved quality of the life, particularly if symptoms are severe, with disadvantages of an invasive procedure with its attendant risks. For optimal medical therapy, advantages include improved symptom control and avoidance of an invasive procedure, while disadvantages include increased medication use and a high rate of eventual crossover to PCI. This important discussion integrating both patient and medical perspectives ultimately leads to the best decision for the individual patient.
A patient-centered approach to clinical decision-making mandates inclusion of PCI first as an option in the management of stable coronary artery disease. After confirming the patient has coronary artery disease, patients with heart failure, class IV angina at rest, or left main artery stenosis should be referred for revascularization. In the remaining patients with confirmed coronary artery disease and moderate angina symptoms, either PCI first or optimal medical therapy first is an appropriate initial strategy that considers coronary anatomy, symptom burden, and patient desires.
- Meier B. The first patient to undergo coronary angioplasty—23-year follow-up. N Engl J Med 2001; 344:144–145.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Fox KAA, Clayton TC, Damman P, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010; 55:2435–2445.
- Katritsis DG, Ioannidis JPA. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005; 111:2906–2912.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Bangalore S, Gupta N, Généreux P, Guo Y, Pancholy S, Feit F. Trend in percutaneous coronary intervention volume following the COURAGE and BARI-2D trials: insight from over 8.1 million percutaneous coronary interventions. Int J Cardiol 2015; 183:6–10.
- Rothberg MB. PCI for stable angina: a missed opportunity for shared decision-making. Cleve Clin J Med 2018; 85:105–121.
- Spertus JA, Maron DJ, Cohen DJ, et al. Frequency, predictors, and consequences of crossing over to revascularization within 12 months of randomization to optimal medical therapy in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial. Circ Cardiovasc Qual Outcomes 2013; 6:409–418.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Acharjee S, Teo KK, Jacobs AK, et al. Optimal medical therapy with or without percutaneous coronary intervention in women with stable coronary disease: a pre-specified subset analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation (COURAGE) trial. Am Heart J 2016; 173:108–117.
- Foster C, Jackson AS, Pollock ML, et al. Generalized equations for predicting functional capacity from treadmill performance. Am Heart J 1984; 107:1229–1234.
- Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol 1993; 22:175–182.
- Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol 1990; 13:555–565.
- Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007; 297:1985–1991.
- Strauss WE, Fortin T, Hartigan P, Folland ED, Parisi AF. A comparison of quality of life scores in patients with angina pectoris after angioplasty compared with after medical therapy. Outcomes of a randomized clinical trial. Veterans Affairs Study of Angioplasty Compared to Medical Therapy Investigators. Circulation 1995; 92:1710–1719.
- Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016; 9:767–776.
- Fontana M, Asaria P, Moraldo M, et al. Patient-accessible tool for shared decision making in cardiovascular primary prevention: balancing longevity benefits against medication disutility. Circulation 2014; 129:2539–2546.
- Weintraub WS, Boden WE, Zhang Z, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes 2008; 1:12–20.
- Al-Lamee R, Thompson D, Dehbi H-M, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet November 2, 2017; doi:10.1016/S0140-6736(17)32714-9.
- Fearon WF, Nishi T, Bruyne BD, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation November 2017; doi:10.1161/CIRCULATIONAHA.117.031907.
- Meier B. The first patient to undergo coronary angioplasty—23-year follow-up. N Engl J Med 2001; 344:144–145.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Fox KAA, Clayton TC, Damman P, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010; 55:2435–2445.
- Katritsis DG, Ioannidis JPA. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005; 111:2906–2912.
- Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Bangalore S, Gupta N, Généreux P, Guo Y, Pancholy S, Feit F. Trend in percutaneous coronary intervention volume following the COURAGE and BARI-2D trials: insight from over 8.1 million percutaneous coronary interventions. Int J Cardiol 2015; 183:6–10.
- Rothberg MB. PCI for stable angina: a missed opportunity for shared decision-making. Cleve Clin J Med 2018; 85:105–121.
- Spertus JA, Maron DJ, Cohen DJ, et al. Frequency, predictors, and consequences of crossing over to revascularization within 12 months of randomization to optimal medical therapy in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial. Circ Cardiovasc Qual Outcomes 2013; 6:409–418.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Acharjee S, Teo KK, Jacobs AK, et al. Optimal medical therapy with or without percutaneous coronary intervention in women with stable coronary disease: a pre-specified subset analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation (COURAGE) trial. Am Heart J 2016; 173:108–117.
- Foster C, Jackson AS, Pollock ML, et al. Generalized equations for predicting functional capacity from treadmill performance. Am Heart J 1984; 107:1229–1234.
- Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol 1993; 22:175–182.
- Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol 1990; 13:555–565.
- Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007; 297:1985–1991.
- Strauss WE, Fortin T, Hartigan P, Folland ED, Parisi AF. A comparison of quality of life scores in patients with angina pectoris after angioplasty compared with after medical therapy. Outcomes of a randomized clinical trial. Veterans Affairs Study of Angioplasty Compared to Medical Therapy Investigators. Circulation 1995; 92:1710–1719.
- Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016; 9:767–776.
- Fontana M, Asaria P, Moraldo M, et al. Patient-accessible tool for shared decision making in cardiovascular primary prevention: balancing longevity benefits against medication disutility. Circulation 2014; 129:2539–2546.
- Weintraub WS, Boden WE, Zhang Z, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes 2008; 1:12–20.
- Al-Lamee R, Thompson D, Dehbi H-M, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet November 2, 2017; doi:10.1016/S0140-6736(17)32714-9.
- Fearon WF, Nishi T, Bruyne BD, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation November 2017; doi:10.1161/CIRCULATIONAHA.117.031907.
A 75-year-old with abdominal pain, hypoxia, and weak pulses in the left leg
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
A 75-year-old man presented to the emergency department for evaluation of abdominal pain. He had stage 3 chronic obstructive pulmonary disease (COPD), with a forced expiratory volume in 1 second of 33%.
PREVIOUS HOSPITALIZATION
Aside from his COPD, he had been healthy until 1 month earlier, when he had been hospitalized because of shortness of breath and chest pressure with exertion. His troponin T level had been elevated, peaking at 0.117 ng/mL (reference range 0–0.029).
Left heart catheterization had shown no significant coronary artery disease. A myocardial bridge of the distal left anterior descending coronary artery had been seen, so that the artery appeared to be narrowed by 50% to 60% with ventricular contraction. But this was not thought to have been the cause of his presentation.
On discharge, he required oxygen 4 L/min by nasal cannula. Previously, he had not needed supplemental oxygen.
CURRENT PRESENTATION
The patient described persistent and severe periumbilical abdominal pain during the previous day. It was not associated with eating, and he denied diarrhea, constipation, hematemesis, hematochezia, bright red blood per rectum, or melena. He continued to describe persistent shortness of breath and pleuritic chest pain. His vital signs were as follows:
- Heart rate 104 beats per minute
- Respiratory rate 16 to 20 breaths per minute
- Blood pressure 101–142/62–84 mm Hg
- Oxygen saturation 78% on room air.
His laboratory findings on presentation are shown in Table 1, and his electrocardiogram is shown in Figure 1.
WHAT DOES HIS ELECTROCARDIOGRAM SHOW?
1. Which of the following is the most accurate description of this patient’s electrocardiogram?
- Sinus tachycardia, peaked P waves (P pulmonale) in lead II, and T-wave inversions in the right precordial leads
- Sinus tachycardia and left bundle branch block
- Sinus tachycardia and poor R-wave progression
- Sinus tachycardia and ST elevation in the precordial leads
Our patient’s electrocardiogram shows sinus tachycardia, P pulmonale, T-wave inversion in the right precordial leads (V1–V3), and biphasic T waves in lead V4,, which suggest right ventricular strain.
The rhythm most commonly seen in patients with pulmonary embolism is sinus tachycardia, followed by nonspecific ST-segment or T-wave abnormalities. In one series of patients with acute pulmonary embolism, the classic findings of P pulmonale, right ventricular hypertrophy, right axis deviation, and right bundle branch block were rare (< 6%).1 Thus, these classic findings are not sensitive for the diagnosis of pulmonary embolism, and their absence does not rule it out.
Further studies for our patient
Transthoracic echocardiography was performed to look for evidence of right ventricular strain secondary to the pulmonary embolism.
ECHOCARDIOGRAPHIC SIGNS OF PULMONARY EMBOLISM
2. Which of the following findings on transthoracic echocardiography would not suggest acute pulmonary embolism?
- Midright ventricular wall hypokinesis with apical sparing
- Severe tricuspid regurgitation
- Left ventricular dilation
- Lack of respiratory variation of the inferior vena cava
- Septal wall motion toward the left ventricle
Left ventricular dilation does not suggest acute pulmonary embolism. Echocardiograms of patients with acute submassive pulmonary embolism typically show evidence of right ventricular strain, such as the other entities listed above (midright ventricular hypokinesis with apical sparing, severe tricuspid regurgitation, lack of respiratory variation of the inferior vena cava, and septal wall motion toward the left ventricle).
The degree of right ventricular dysfunction is related to the extent of acute pulmonary vascular occlusion and aids in risk-stratification of patients with acute pulmonary embolism. Midright ventricular wall hypokinesis with apical sparing has been termed the McConnell sign.2
In our patient, transthoracic echocardiography showed:
- Normal left ventricular ejection fraction
- Mild diastolic dysfunction
- Right ventricular dilation with moderately decreased right ventricular systolic function and apical sparing
- Right ventricular systolic pressure 54 mm Hg, consistent with moderate pulmonary hypertension
- Right atrial pressure 10 mm Hg
- No inspiratory collapse of a dilated inferior vena cava
- Mild tricuspid valve regurgitation.
CLASSIFICATION OF ACUTE PULMONARY EMBOLISM
3. Given the above information, how would you classify the patient’s pulmonary embolism?
- Massive
- Submassive
- Low-risk
- Clinically stable
The patient’s pulmonary embolism is submassive.
Historically, the classification of pulmonary embolism was determined by the angiographic thrombus burden. However, this has limited utility because clinical factors (eg, hypotension on initial presentation) have been shown to be better predictors of short-term mortality risk.3
Our patient is characterized as having a submassive pulmonary embolism based on elevated biomarkers (troponin T, N-terminal pro-B-type natriuretic peptide) and right ventricular dysfunction in the absence of hypotension.
ULTRASONOGRAPHY FOR DIAGNOSIS OF DEEP VEIN THROMBOSIS
Venous duplex ultrasonography has become the standard for diagnosis of lower extremity deep vein thrombosis. However, its quality and diagnostic accuracy depend on the skill of the person performing the examination. It is further limited by certain patient characteristics, including severe obesity, edema, and wounds and dressings at the site being examined.5
Our patient underwent duplex ultrasonography of the lower extremities, which demonstrated acute proximal and calf deep vein thrombosis in the right femoral, popliteal, and peroneal veins and no deep vein thrombosis in the left leg.
RISK STRATIFICATION IN ACUTE PULMONARY EMBOLISM
Multiple models exist to estimate the risk of complications in patients with acute pulmonary embolism.
The Bova score6 is based on the following factors:
- Systolic blood pressure 90–100 mm Hg (2 points) (patients with systolic blood pressure lower than 90 mm Hg were excluded from the study from which this score was derived)
- Cardiac troponin elevation (2 points)
- Right ventricular dysfunction on echocardiography or computed tomography (2 points)
- Heart rate 100 beats/min or greater (1 point).
A total score of 0, 1, or 2 (stage I) denotes low risk, 3 or 4 points (stage II) intermediate risk, and more than 4 points (stage III) high risk.
The PESI score (Pulmonary Embolism Severity Index)7 is based on:
- Age (1 point per year)
- Sex (10 points for being male)
- Heart rate 110 per minute or greater (20 points)
- Cancer (30 points)
- Heart failure (10 points)
- Chronic lung disease (10 points)
- Systolic blood pressure less than 100 mm Hg (30 points)
- Respiratory rate at least 30 per minute (20 points)
- Temperature less than 36ºC (20 points)
- Altered mental status (60 points)
- Arterial oxygen saturation less than 90% (20 points).
The total score is broken down into 5 classes: I (< 65 points), II (65–85), III (86–105), IV (106–125), and V (> 126). Classes I and II are low risk, and the higher ones are high risk.
The simplified PESI score8 was developed to more rapidly risk-stratify patients and has been found to be similar to the PESI score in prognostic accuracy. Patients get 1 point for each of the following:
- Age over 80
- Cancer
- Chronic cardiopulmonary disease (heart failure or chronic lung disease)
- Heart rate 110 per minute or greater
- Systolic blood pressure less than 100 mm Hg
- Arterial oxygen saturation less than 90%.
A total score of 0 is low risk; anything higher is high risk.
Back to our patient
Our patient had proximal and calf deep vein thrombosis of the right leg, bilateral submassive pulmonary emboli with associated biomarker elevation and right ventricular dysfunction, and left renal artery thrombosis with infarction. Using the PESI score, his risk of death in the next 30 days was 13.7% and his 30-day risk of a complicated course was 27%. Using the Bova score, his 30-day risk of death was 15.5% and his 30-day risk of a complicated course was 29.2%.6,7
Notably, the patient’s right ventricular function had also been impaired on the echocardiogram performed during his admission 1 month previously. On transthoracic echocardiography during the current admission, the patient was found to have a similar degree of right ventricular dysfunction. This finding, along with the oxygen requirement that developed during the earlier admission, suggested that his pulmonary embolism may have been subacute and that the diagnosis may have been missed during the earlier hospital stay.
The patient was treated with unfractionated heparin. After the hospital’s multidisciplinary pulmonary embolism response team discussed and weighed the above factors, they recommended to not pursue thrombolytic therapy or inferior vena cava filter placement.
Of note, the patient’s pulses in the left lower extremity continued to be weak but palpable, and the left leg was cooler to touch than the right leg.
ASSESSING PERIPHERAL ARTERY DISEASE
4. How should the finding of weak pulses in this patient’s left leg be initially investigated?
- Computed tomographic angiography with runoff
- Ankle-brachial indices with pulse-volume recordings
- Arterial duplex ultrasonography
- Magnetic resonance angiography of the lower extremities
The ankle-brachial index is the initial diagnostic test for assessment of pulse abnormalities and for diagnosis of lower-extremity peripheral artery disease. It is calculated by dividing the higher of the ankle systolic pressures (posterior tibial or dorsalis pedis) by the higher of the 2 brachial pressures (left or right).9 Normal values are between 1.00 and 1.40.
Ankle-brachial indices in our patient
Our patient underwent measurement of his brachial, dorsalis pedis, and posterior tibial artery systolic pressures using blood pressure cuffs and continuous-wave Doppler. Ankle pulse-volume recordings were also obtained.
Given the patient’s poor renal function and concern for acute renal infarction, we thought it best to avoid iodinated or gadolinium contrast, such as with magnetic resonance or computed tomographic angiography.
Segmental leg pressures and pulse-volume recordings can be performed to help localize the level of arterial disease in the extremities, but were not done in this case because of the extensive deep vein thrombosis in the right leg.10,11
Arterial ultrasonography in our patient
Arterial duplex ultrasonography was performed to help determine the location of arterial disease. It showed patent arteries in the right leg. In the left lower extremity there was slow, monophasic blood flow in the distal superficial femoral artery. The popliteal artery was occluded. The posterior tibial artery was occluded at the origin, with reconstitution distally. The peroneal artery was occluded throughout. The anterior tibial artery was patent throughout. The ultrasonographic findings were thought to be suspicious for arterial thromboembolism.
WHAT CAN CAUSE BOTH ARTERIAL AND VENOUS THROMBOSIS?
5. Given that the patient had both arterial thrombosis (renal artery, lower-extremity arteries) and venous thromboembolism (deep vein thrombosis and pulmonary embolism), which of the following would be included in the differential diagnosis?
- Antiphospholipid antibody syndrome
- Protein C or protein S deficiency
- Malignancy
- Paradoxical embolization
- Factor V Leiden mutation
Correct answers include antiphospholipid antibody syndrome, malignancy, and paradoxical embolization.
The differential diagnosis for concomitant venous and arterial thrombosis is broad,12 and includes the following:
- Structural factors: patent foramen ovale, popliteal artery aneurysm
- Malignancy
- Inflammatory diseases: Behçet disease, Buerger disease, inflammatory bowel disease, antiphospholipid antibody syndrome, elevated lipoprotein(a), elevated homocysteine
- Hematologic diseases: myelodysplastic syndrome, disseminated intravascular coagulation, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia.
Traditional risk factors for venous thromboembolism include protein C deficiency, protein S deficiency, factor V Leiden mutation, the prothrombin G20210A gene mutation, and others. These are relatively minor risk factors for venous thrombosis and do not pose a risk for arterial thrombosis.12 In contrast, antiphospholipid antibody syndrome and malignancy pose a risk for both venous and arterial thrombosis. Paradoxical embolism is a mechanism by which arterial thrombosis (emboli) can develop in the setting of existing venous thrombosis.12
Our patient underwent testing for antiphospholipid antibodies and lupus anticoagulant, and he was encouraged to undergo age-appropriate cancer screening as an outpatient.12
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
Antiphospholipid antibody syndrome is defined by both clinical and laboratory criteria. Clinical symptoms include vascular thrombosis (arterial, venous, or both) and pregnancy-related complications.13
Laboratory criteria require the presence of antiphospholipid antibodies or lupus anticoagulant. These must be confirmed with repeat testing in 12 weeks. Antiphospholipid antibodies are detected by an enzyme-linked immunosorbent assay; laboratory assessment for the presence of lupus anticoagulant is a stepwise process and relies on 4 criteria:
- There should be prolongation of a phospholipid-dependent clotting test (eg, activated partial thromboplastin time, dilute Russell viper venom time test).
- There must be evidence of an inhibitory activity with mixing study.
- The inhibitor must exhibit phospholipid dependence; that is, with more phospholipid there is shortening of clotting time.
- Specific inhibitors must be excluded, including factor VIII and anticoagulant drugs such as heparin.14–17
Clinically, one should consider antiphospholipid antibody syndrome in patients who have arterial thrombosis, a history of pregnancy morbidity, or unexplained prolongation of activated partial thromboplastin time.13
Antiphospholipid antibodies may be present in up to a quarter of patients with venous thromboembolism, but it is persistent positivity of antibody assays that is associated with increased future risk of venous thromboembolism.19 Of note, the risk of venous thromboembolism in patients with confirmed antiphospholipid antibody syndrome is 10 times higher than in the general population.20
ANTIPHOSPHOLIPID ANTIBODIES ARE NOT ALL THE SAME
6. Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?
- Anti-beta-2-glycoprotein I IgG
- Lupus anticoagulant
- Antiphosphatidylserine
- Anticardiolipin IgM
- Anticardiolipin IgG
The correct answer is antiphosphatidylserine.15
Antiphospholipid antibodies are directed against a portion of select plasma proteins that are uncovered upon phospholipid binding. While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.15,21
PARADOXICAL EMBOLISM
Patent foramen ovale, a communication between the right and left atrium in the interatrial septum, is associated with an increased risk of paradoxical embolization. The prevalence of patent foramen ovale is estimated to be 27% to 29% in the general population.22 Noncerebral systemic paradoxical embolism occurs less frequently than cerebral embolism, accounting for approximately 5% to 10% of paradoxical emboli.22
To evaluate for patent foramen ovale, transthoracic echocardiography is performed with a bubble (agitated saline contrast) study to assess for interatrial shunting. Transesophageal echocardiography or transcranial Doppler bubble studies may also be performed.
Although patent foramen ovale is most commonly associated with cerebral embolism, peripheral emboli can occur. Some research suggests that this may be a more common cause of arterial thromboembolism in younger patients. There have also been reports of other sites of systemic embolization, including the renal artery.12
Back to our patient
Initial antiphospholipid antibody testing was positive for lupus anticoagulant. Anticardiolipin and anti-beta-2-glycoprotein I antibodies were not detected.
Transesophageal echocardiography revealed a patent foramen ovale with a highly mobile atrial septum (atrial septal aneurysm).
The patient was treated with intravenous unfractionated heparin with bridging to warfarin with a target international normalized ratio (INR) of 2 to 3. His renal artery infarction and his lower-extremity arterial thromboembolic event were conservatively managed. His respiratory status improved, and he no longer required supplemental oxygen. His creatinine peaked at 1.7 mg/dL during his admission and improved to 1.2 mg/dL before he was discharged.
At follow-up, repeat echocardiography showed that his right ventricular systolic pressure had improved (decreased) to 37 mm Hg from 54 mm Hg. Repeat confirmatory testing was positive for lupus anticoagulant 12 weeks later. He has been maintained on warfarin with an INR goal of 2 to 3 as well as low-dose aspirin with plans for long-term anticoagulation. We decided to keep the patient on anticoagulation indefinitely with warfarin; he was not a candidate for a direct oral anticoagulant, given limited data on the use of these agents in the setting of lupus anticoagulant and antiphospholipid antibody syndrome.
SUMMARY OF CASE
In summary, this patient was a 75-year-old man with COPD who presented with abdominal pain. He was noted to have a left renal infarction, extensive unprovoked lower-extremity deep vein thrombosis with pulmonary emboli, and lower limb arterial thromboembolism.
He also had an underlying hypercoagulable state—antiphospholipid antibody syndrome—that predisposed him to both arterial and venous thrombosis. He was ultimately found to have a patent foramen ovale, which further increased the risk of arterial thrombosis by facilitating paradoxical embolization of venous thrombi. It is not certain whether the renal infarction and leg artery thrombi were due to paradoxical embolism or to in situ thrombosis, but we believe that it was most likely paradoxical embolization.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100:598–603.
- Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13:43–51.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Gornik HL, Sharma AM. Duplex ultrasound in the diagnosis of lower-extremity deep venous thrombosis. Circulation 2014; 129:917–921.
- Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218.
- Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. J Intern Med 2007; 261:597–604.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Kim ES, Wattanakit K, Gornik HL. Using the ankle-brachial index to diagnose peripheral artery disease and assess cardiovascular risk. Cleve Clin J Med 2012; 79:651–661.
- Jaff MR. Lower extremity arterial disease. Diagnostic aspects. Cardiol Clin 2002; 20:491–500.
- Rooke TW, Hirsch AT, Misra S, et al; American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:1555–1570.
- Lichtin A, Bartholomew J. The coagulation consult: a case-based guide. New York, NY: Springer; 2014.
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346:752–763.
- Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost 1995; 74:1185–1190.
- Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Nichols WL, Kottke-Marchant K, Ledford-Kraemer MR, Homburger HA, Cardel LK. Lupus anticoagulants, antiphospholipid antibodies, and antiphospholipid syndrome. In: Kottke-Marchant K, Davis BH, editors. Laboratory Hematology Practice. Hoboken, New Jersey: Blackwell Publishing, Ltd.; 2012:509–525.
- Houghton DE, Moll S. Antiphospholipid antibodies. Vasc Med 2017; 22:545–550.
- Roldan V, Lecumberri R, Muñoz-Torrero JFS, et al; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res 2009; 124:174–177.
- Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G. Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 1998; 7:15–22.
- Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med 1990; 112:682–698.
- Thompson T, Evans W. Paradoxical embolism. QJM 1930; os-23:135–150.
DEFUSE 3: Thrombectomy time window broadens
LOS ANGELES – The final results of the DEFUSE 3 trial are in, and the results are unequivocal: Thrombectomy performed 6-16 hours after the stroke patient was last known to be well was associated with dramatically improved outcomes in 90-day death and disability.
It has long been said that time is brain. Time is still vital, but some strokes progress at a slower rate. “We have the opportunity to treat these patients in a time window that we never thought was possible,” Gregory W. Albers, MD, said in presenting the findings at the International Stroke Conference sponsored by the American Heart Association.
The subjects included those with proximal middle-cerebral artery or internal-carotid artery occlusion and infarcts of 70 mL or less in size, and a ratio of the volume of ischemic tissue on perfusion imaging to infarct tissue of 1.8 or higher. Both DAWN and DEFUSE 3 used the RAPID software from iSchemaView to assess infarct volume.
The DEFUSE 3 results aren’t a surprise, as the trial was stopped early after an interim analysis showed efficacy. But they are immediately practice changing. “This will perhaps be a once in a lifetime situation where a study gets published and within 2 hours gets incorporated into new guidelines,” said Dr. Albers, lead author of the study, who is the Coyote Foundation Professor, neurology and neurological sciences, and professor, by courtesy, of neurosurgery at the Stanford (Calif.) University Medical Center. Minutes later at the press conference, it was announced that the results of both DEFUSE 3 and DAWN had indeed been included in the guidelines.
The DEFUSE (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) 3 trial included 182 patients from 38 U.S. centers who were recruited during May 2016–May 2017. Of these, 92 were randomized to thrombectomy and standard medical therapy, and 90 to standard medical therapy only.
Patients who underwent thrombectomy were more likely to have a favorable distribution of disability scores on the modified Rankin scale at 90 days (unadjusted odds ratio, 2.77; adjusted OR, 3.36; both P less than .001). “The odds ratio [of 2.77] was the largest ever reported for a thrombectomy study,” Dr. Albers said, and the ISC audience erupted into spontaneous applause. “We couldn’t be happier,” he responded.
Nearly half (45%) of patients in the thrombectomy group scored as functionally independent at 90 days (Rankin score 0-2), compared with 17% in the medical-therapy group (risk ratio, 2.67; P less than .001). Mortality was also lower in the intervention group (14% vs. 26%; P = .05).
The rates of symptomatic intracranial hemorrhage (7% thrombectomy, 4% medical therapy only) did not differ significantly between the two groups. Serious complications occurred in 43% of patients in the thrombectomy group, compared with 53% in the medical therapy–only group (P = .018).
A subanalysis showed consistent benefit of thrombectomy, even in patients with a longer elapsed time between stroke onset and randomization, while the patients who received medical therapy had worse outcomes as more time passed. In 50 patients in the under 9-hour group, 40% of those who received thrombectomy were functionally independent at 9 weeks, compared with 28% in the medical therapy–only group. Among 72 patients in the 9- to 12-hour group, 50% were functionally independent (vs. 17%), and in the greater than 12-hour group, 42% (7%).
The study filled up rapidly, at about twice the rate that the researchers anticipated, and that suggests that the procedure could find broad use. “It shows that these patients are not difficult to find,” said Dr. Albers.
The National Institutes of Health funded the study. Dr. Albers has a financial stake in iSchemaView and is on the scientific advisory board for iSchemaView and Medtronic.
The DEFUSE 3 results were published online concurrently with Dr. Albers’s presentation (N Engl J Med. 2018 Jan 24; doi: 10.1056/nejmoa1713973).
SOURCE: Albers G et al. ISC 2018
LOS ANGELES – The final results of the DEFUSE 3 trial are in, and the results are unequivocal: Thrombectomy performed 6-16 hours after the stroke patient was last known to be well was associated with dramatically improved outcomes in 90-day death and disability.
It has long been said that time is brain. Time is still vital, but some strokes progress at a slower rate. “We have the opportunity to treat these patients in a time window that we never thought was possible,” Gregory W. Albers, MD, said in presenting the findings at the International Stroke Conference sponsored by the American Heart Association.
The subjects included those with proximal middle-cerebral artery or internal-carotid artery occlusion and infarcts of 70 mL or less in size, and a ratio of the volume of ischemic tissue on perfusion imaging to infarct tissue of 1.8 or higher. Both DAWN and DEFUSE 3 used the RAPID software from iSchemaView to assess infarct volume.
The DEFUSE 3 results aren’t a surprise, as the trial was stopped early after an interim analysis showed efficacy. But they are immediately practice changing. “This will perhaps be a once in a lifetime situation where a study gets published and within 2 hours gets incorporated into new guidelines,” said Dr. Albers, lead author of the study, who is the Coyote Foundation Professor, neurology and neurological sciences, and professor, by courtesy, of neurosurgery at the Stanford (Calif.) University Medical Center. Minutes later at the press conference, it was announced that the results of both DEFUSE 3 and DAWN had indeed been included in the guidelines.
The DEFUSE (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) 3 trial included 182 patients from 38 U.S. centers who were recruited during May 2016–May 2017. Of these, 92 were randomized to thrombectomy and standard medical therapy, and 90 to standard medical therapy only.
Patients who underwent thrombectomy were more likely to have a favorable distribution of disability scores on the modified Rankin scale at 90 days (unadjusted odds ratio, 2.77; adjusted OR, 3.36; both P less than .001). “The odds ratio [of 2.77] was the largest ever reported for a thrombectomy study,” Dr. Albers said, and the ISC audience erupted into spontaneous applause. “We couldn’t be happier,” he responded.
Nearly half (45%) of patients in the thrombectomy group scored as functionally independent at 90 days (Rankin score 0-2), compared with 17% in the medical-therapy group (risk ratio, 2.67; P less than .001). Mortality was also lower in the intervention group (14% vs. 26%; P = .05).
The rates of symptomatic intracranial hemorrhage (7% thrombectomy, 4% medical therapy only) did not differ significantly between the two groups. Serious complications occurred in 43% of patients in the thrombectomy group, compared with 53% in the medical therapy–only group (P = .018).
A subanalysis showed consistent benefit of thrombectomy, even in patients with a longer elapsed time between stroke onset and randomization, while the patients who received medical therapy had worse outcomes as more time passed. In 50 patients in the under 9-hour group, 40% of those who received thrombectomy were functionally independent at 9 weeks, compared with 28% in the medical therapy–only group. Among 72 patients in the 9- to 12-hour group, 50% were functionally independent (vs. 17%), and in the greater than 12-hour group, 42% (7%).
The study filled up rapidly, at about twice the rate that the researchers anticipated, and that suggests that the procedure could find broad use. “It shows that these patients are not difficult to find,” said Dr. Albers.
The National Institutes of Health funded the study. Dr. Albers has a financial stake in iSchemaView and is on the scientific advisory board for iSchemaView and Medtronic.
The DEFUSE 3 results were published online concurrently with Dr. Albers’s presentation (N Engl J Med. 2018 Jan 24; doi: 10.1056/nejmoa1713973).
SOURCE: Albers G et al. ISC 2018
LOS ANGELES – The final results of the DEFUSE 3 trial are in, and the results are unequivocal: Thrombectomy performed 6-16 hours after the stroke patient was last known to be well was associated with dramatically improved outcomes in 90-day death and disability.
It has long been said that time is brain. Time is still vital, but some strokes progress at a slower rate. “We have the opportunity to treat these patients in a time window that we never thought was possible,” Gregory W. Albers, MD, said in presenting the findings at the International Stroke Conference sponsored by the American Heart Association.
The subjects included those with proximal middle-cerebral artery or internal-carotid artery occlusion and infarcts of 70 mL or less in size, and a ratio of the volume of ischemic tissue on perfusion imaging to infarct tissue of 1.8 or higher. Both DAWN and DEFUSE 3 used the RAPID software from iSchemaView to assess infarct volume.
The DEFUSE 3 results aren’t a surprise, as the trial was stopped early after an interim analysis showed efficacy. But they are immediately practice changing. “This will perhaps be a once in a lifetime situation where a study gets published and within 2 hours gets incorporated into new guidelines,” said Dr. Albers, lead author of the study, who is the Coyote Foundation Professor, neurology and neurological sciences, and professor, by courtesy, of neurosurgery at the Stanford (Calif.) University Medical Center. Minutes later at the press conference, it was announced that the results of both DEFUSE 3 and DAWN had indeed been included in the guidelines.
The DEFUSE (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) 3 trial included 182 patients from 38 U.S. centers who were recruited during May 2016–May 2017. Of these, 92 were randomized to thrombectomy and standard medical therapy, and 90 to standard medical therapy only.
Patients who underwent thrombectomy were more likely to have a favorable distribution of disability scores on the modified Rankin scale at 90 days (unadjusted odds ratio, 2.77; adjusted OR, 3.36; both P less than .001). “The odds ratio [of 2.77] was the largest ever reported for a thrombectomy study,” Dr. Albers said, and the ISC audience erupted into spontaneous applause. “We couldn’t be happier,” he responded.
Nearly half (45%) of patients in the thrombectomy group scored as functionally independent at 90 days (Rankin score 0-2), compared with 17% in the medical-therapy group (risk ratio, 2.67; P less than .001). Mortality was also lower in the intervention group (14% vs. 26%; P = .05).
The rates of symptomatic intracranial hemorrhage (7% thrombectomy, 4% medical therapy only) did not differ significantly between the two groups. Serious complications occurred in 43% of patients in the thrombectomy group, compared with 53% in the medical therapy–only group (P = .018).
A subanalysis showed consistent benefit of thrombectomy, even in patients with a longer elapsed time between stroke onset and randomization, while the patients who received medical therapy had worse outcomes as more time passed. In 50 patients in the under 9-hour group, 40% of those who received thrombectomy were functionally independent at 9 weeks, compared with 28% in the medical therapy–only group. Among 72 patients in the 9- to 12-hour group, 50% were functionally independent (vs. 17%), and in the greater than 12-hour group, 42% (7%).
The study filled up rapidly, at about twice the rate that the researchers anticipated, and that suggests that the procedure could find broad use. “It shows that these patients are not difficult to find,” said Dr. Albers.
The National Institutes of Health funded the study. Dr. Albers has a financial stake in iSchemaView and is on the scientific advisory board for iSchemaView and Medtronic.
The DEFUSE 3 results were published online concurrently with Dr. Albers’s presentation (N Engl J Med. 2018 Jan 24; doi: 10.1056/nejmoa1713973).
SOURCE: Albers G et al. ISC 2018
REPORTING FROM ISC 2018
Key clinical point: Stroke patients with clinical imaging mismatch had significantly better 90-day disability outcomes with thrombectomy.
Major finding: The odds ratio of a favorable outcome at 90 days was 2.77.
Data source: DEFUSE 3, a multicenter, randomized, controlled trial in 182 stroke patients.
Disclosures: The National Institutes of Health funded the study. Dr. Albers has a financial stake in iSchemaView and is on the scientific advisor board for iSchemaView and Medtronic.
Source: Albers G. et al. ISC 2018
Transcatheter aortic valve-in-ring for mitral disease a winner
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
REPORTING FROM TCT 2017
Key clinical point: .
Major finding: Thirty-day all-cause mortality following a transcatheter valve-in-ring procedure in unacceptably high surgical-risk patients with severe mitral valve disease due to a failing annuloplasty ring was 6.8%.
Study details: This prospective observational study included 60 patients who underwent transcatheter mitral valve replacement for severe mitral valve disease, half due to a failed annuloplasty ring and half secondary to mitral annular calcification.
Disclosures: The MITRAL trial was partially supported by Edwards Lifesciences. The study presenter reported receiving a research grant from the company.
Source: Guerrero M. No abstract.
Pendulum swings on mesenteric venous thrombosis treatment
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Treatment of isolated acute mesenteric venous thrombosis remains a topic of controversy, with no established guidelines available, Thomas S. Maldonado, MD, observed at a symposium on vascular surgery sponsored by Northwestern University.
“There has been a pendulum swing. Earlier on there was a lot of excitement about surgical thrombectomy, then we tended to become more nonoperative and conservative, using just anticoagulation. But in recent years endovascular therapy has been gaining some traction and shows good preliminary results,” according to Dr. Maldonado, professor of surgery at New York University.
Today MVT accounts for 1 in 1,000 emergency department visits and 6%-9% of cases of acute mesenteric ischemia. Dr. Maldonado cited two reasons for the increasing incidence. One is the widespread recognition that contrast-enhanced helical CT is the diagnostic imaging method of choice; it is being employed more liberally because of its ready availability and overall 95%-100% accuracy, which allows for rapid and reliable diagnosis with precise location of the thrombus.
The other factor is that bariatric surgery is booming. While the most common local etiologies of the hypercoagulable state predisposing to MVT remain cancer and intra-abdominal inflammatory diseases such as pancreatitis, there is no doubt that laparoscopic bariatric surgery is emerging as another contributing factor, according to the surgeon.
Diagnosis
MVT is an insidious and lethal disease. In most series, it has a mortality of at least 25%, and it doesn’t appear to be going down in recent years. This is probably because of difficulty in making a prompt diagnosis before bowel ischemia occurs. Multiple studies show that onset of symptoms typically occurs 6-14 days before patients present for care.
“I think this is really the Achilles heel of this diagnosis – that it can be delayed. The diagnosis can be elusive. There is no constellation of signs or symptoms that is pathognomonic for MVT. This is where prompt recognition and a CT scan can really play an important role,” Dr. Maldonado said.
He and a coworker conducted a review of 37 studies on MVT published in 1997-2016 which underscored the challenges in making a prompt diagnosis. The most common presenting symptom was nonspecific abdominal pain out of proportion to findings on physical exam. Other possible symptoms included anorexia, nausea, vomiting, constipation, and/or passage of blood through the anus. The disease occurred most often in men aged 40-60. A history of unprovoked venous thromboembolism was often present (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:501-7).
The three-phase CT scan – arterial, venous, and delayed venous – not only locates the thrombus with precision, it also shows whether the occlusion is partial or complete, which is important information prognostically (see below). The scan also provides information on bowel ischemia with at least 90% sensitivity and specificity. Bowel compromise shows up on CT as a thickened bowel wall with dilated lumen, mesenteric fat stranding, and ascites.
CT imaging has become so useful for rapid diagnosis of MVT that duplex ultrasound, although considerably less costly and radiation-free, has become relegated to a secondary role. At most centers its use is restricted to follow-up surveillance to assess for thrombus resolution and vascular recanalization after the acute episode has been treated. Duplex ultrasound simply can’t match CT in the crucial task of assessment for bowel ischemia.
Treatment
The mainstay of treatment in patients with MVT without bowel ischemia is medical management: immediate anticoagulation with unfractionated or low-molecular-weight heparin bridging to warfarin, bowel rest, aggressive fluid resuscitation, and correction of electrolyte imbalances. Most patients with nonocclusive MVT and no ischemic bowel can be managed in this way without surgical intervention. The newer oral anticoagulants haven’t yet been studied in patients with MVT.
How long to continue oral anticoagulation is an unresolved issue. In Dr. Maldonado’s literature review, the median duration was 90 days. In his own practice, anticoagulants aren’t stopped until duplex ultrasound demonstrates recanalization of the mesenteric venous system. If residual thrombus is present or a patient has an underlying hypercoagulable state, treatment continues indefinitely.
In a series of 50 noncirrhotic MVT patients treated at New York University using various strategies, 19, or 38%, were completely recanalized. Recurrence of MVT after successful treatment occurred in only 2 of these 19 patients, in both cases upon discontinuation of anticoagulation.
“It speaks to the issue of length of treatment – or should it be discontinued at all?” the surgeon said.
Open surgical thrombectomy has fallen into disfavor because the thrombus tends to recur within 7 days post surgery. It is now best reserved for patients with acute MVT with a contraindication to thrombolytic therapy, such as cirrhosis or recent major surgery, according to Dr. Maldonado.
Multiple patient series using endovascular catheter-directed thrombolytic therapy with a transhepatic, transvenous, transarterial, or combined approach have reported high rates of successful recanalization – even in the 90% range – with low recurrence rates and fewer bowel resections than with anticoagulation alone.
Indeed, Dr. Maldonado and his fellow vascular surgeons at New York University have recently developed a management algorithm whereby patients with occlusive MVT and no bowel ischemia undergo catheter-directed thrombolysis provided there are no contraindications, such as uncontrolled hypertension or a recent hemorrhagic stroke. The surgeons will also seriously consider catheter-directed lytic therapy in MVT patients with bowel ischemia who show no improvement after laparotomy, bowel resection, and open thrombectomy.
Prognosis
A retrospective review by Dr. Maldonado and coinvestigators of 80 noncirrhotic patients with MVT managed at New York University raised a red flag regarding the high risk of portal hypertension as a long-term sequela. At a median follow-up of 480 days, fully half of patients with imaging results available displayed radiographic features of portal hypertension, although as yet none had developed frank clinical manifestations of cirrhosis.
The investigators identified two predictors of portal hypertension. One was complete as opposed to partial thrombosis at the initial event. Complete thrombosis was present in 73% of patients who eventually developed portal hypertension, compared with 43% of those who didn’t. The other predictor was lack of successful recanalization: only 37% of patients who developed portal hypertension were successfully recanalized, compared with a 65% recanalization rate in those who remained free of this long-term complication (J Vasc Surg Venous Lymphat Disord. 2016 Oct;4[4]:400-6).
These observations raise the possibility that initial complete thrombosis of the mesenteric vein and nonrecanalization with medical therapy might tip the balance in favor of endovascular lytic therapy as a potential means of preventing later portal hypertension.
“I don’t think we know the answer, but there’s certainly room for research,” Dr. Maldonado observed.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Carotid stenting isn’t safer than endarterectomy with contralateral carotid occlusion
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM