User login
FDA approves metoclopramide nasal spray for diabetic gastroparesis
The Food and Drug Administration has approved a new formulation of metoclopramide for relief of symptoms of diabetic gastroparesis in adults.
The product, called Gimoti (Evoke Pharma) delivers metoclopramide through nasal administration, offering an advantage over oral administration, which can be impeded because of slowed stomach emptying, the company said in an announcement of the approval. The delivery system provides 15 mg metoclopramide in each 70-mcL spray, which can be taken 30 minutes before each meal and at bedtime for 2-8 weeks, depending on symptomatic response, according to Gimoti’s prescribing information.
Metoclopramide, a dopamine-2 antagonist, has been available for 4 decades in oral and injection formulations. It carries a risk of developing tardive dyskinesia – a serious, often-irreversible movement disorder – that increases with duration of treatment. Therefore, use of the drug should not exceed 12 weeks. Other contraindications include a history of tardive dyskinesia, when stimulation of GI motility might be dangerous, pheochromocytoma and catecholamine-releasing paragangliomas, and epilepsy.
Henry Parkman, MD, who was involved with clinical trials leading to the approval, explained in the Evoke statement that “patients with gastroparesis suffer from characteristic symptoms such as nausea, abdominal pain, bloating, early satiety, as well as vomiting which can be severe and debilitating. These patients often have erratic absorption of orally administered drugs because of delayed gastric emptying.
“Unlike oral medications, Gimoti is administered nasally, bypassing the diseased GI track, allowing the drug to enter the bloodstream directly and therefore may provide predictable delivery of the therapy,” adds Dr. Parkman, chair and director of the Gastroenterology Motility Laboratory at Temple University, Philadelphia.
Gimoti will be available commercially in the fourth quarter of this year, according to Evoke.
The Food and Drug Administration has approved a new formulation of metoclopramide for relief of symptoms of diabetic gastroparesis in adults.
The product, called Gimoti (Evoke Pharma) delivers metoclopramide through nasal administration, offering an advantage over oral administration, which can be impeded because of slowed stomach emptying, the company said in an announcement of the approval. The delivery system provides 15 mg metoclopramide in each 70-mcL spray, which can be taken 30 minutes before each meal and at bedtime for 2-8 weeks, depending on symptomatic response, according to Gimoti’s prescribing information.
Metoclopramide, a dopamine-2 antagonist, has been available for 4 decades in oral and injection formulations. It carries a risk of developing tardive dyskinesia – a serious, often-irreversible movement disorder – that increases with duration of treatment. Therefore, use of the drug should not exceed 12 weeks. Other contraindications include a history of tardive dyskinesia, when stimulation of GI motility might be dangerous, pheochromocytoma and catecholamine-releasing paragangliomas, and epilepsy.
Henry Parkman, MD, who was involved with clinical trials leading to the approval, explained in the Evoke statement that “patients with gastroparesis suffer from characteristic symptoms such as nausea, abdominal pain, bloating, early satiety, as well as vomiting which can be severe and debilitating. These patients often have erratic absorption of orally administered drugs because of delayed gastric emptying.
“Unlike oral medications, Gimoti is administered nasally, bypassing the diseased GI track, allowing the drug to enter the bloodstream directly and therefore may provide predictable delivery of the therapy,” adds Dr. Parkman, chair and director of the Gastroenterology Motility Laboratory at Temple University, Philadelphia.
Gimoti will be available commercially in the fourth quarter of this year, according to Evoke.
The Food and Drug Administration has approved a new formulation of metoclopramide for relief of symptoms of diabetic gastroparesis in adults.
The product, called Gimoti (Evoke Pharma) delivers metoclopramide through nasal administration, offering an advantage over oral administration, which can be impeded because of slowed stomach emptying, the company said in an announcement of the approval. The delivery system provides 15 mg metoclopramide in each 70-mcL spray, which can be taken 30 minutes before each meal and at bedtime for 2-8 weeks, depending on symptomatic response, according to Gimoti’s prescribing information.
Metoclopramide, a dopamine-2 antagonist, has been available for 4 decades in oral and injection formulations. It carries a risk of developing tardive dyskinesia – a serious, often-irreversible movement disorder – that increases with duration of treatment. Therefore, use of the drug should not exceed 12 weeks. Other contraindications include a history of tardive dyskinesia, when stimulation of GI motility might be dangerous, pheochromocytoma and catecholamine-releasing paragangliomas, and epilepsy.
Henry Parkman, MD, who was involved with clinical trials leading to the approval, explained in the Evoke statement that “patients with gastroparesis suffer from characteristic symptoms such as nausea, abdominal pain, bloating, early satiety, as well as vomiting which can be severe and debilitating. These patients often have erratic absorption of orally administered drugs because of delayed gastric emptying.
“Unlike oral medications, Gimoti is administered nasally, bypassing the diseased GI track, allowing the drug to enter the bloodstream directly and therefore may provide predictable delivery of the therapy,” adds Dr. Parkman, chair and director of the Gastroenterology Motility Laboratory at Temple University, Philadelphia.
Gimoti will be available commercially in the fourth quarter of this year, according to Evoke.
AGA Clinical Practice Update: Functional heartburn
Recognizing the presence of functional heartburn is vital to prevent unnecessary acid-suppressive therapy and invasive antireflux treatments, which are ineffective and “might even lead to harm,” cautions a new clinical practice update from the American Gastroenterological Association.
Proton pump inhibitors (PPIs) “have no therapeutic value in functional heartburn,” unless patients also have gastroesophageal reflux disease (GERD), Ronnie Fass, MD, of MetroHealth System in Cleveland, and coauthors wrote in Gastroenterology. If clinical work-up finds no clear evidence of GERD, “an attempt to discontinue PPI therapy is warranted,” they added. Likewise, antireflux surgery and endoscopic treatments for GERD “have no therapeutic benefit in functional heartburn and should not be recommended.” However, histamine2 receptor antagonists (H2RAs) “may have an independent benefit in functional heartburn from an esophageal pain modulatory effect.”
Heartburn consists of burning or discomfort that radiates retrosternally from the epigastrium. Patients may report reflux, regurgitation, chest pain or discomfort, fullness, water brash, belching, or a sour and bitter taste in the mouth. Functional heartburn is heartburn that persists after at least 3 months of maximal (double-dose) PPIs taken before meals. Confirming functional heartburn requires high-resolution manometry to rule out major esophageal motor disorders, esophageal endoscopy with biopsy to rule out structural abnormalities and mucosal disorders (e.g., erosive esophagitis, Barrett’s esophagus, and eosinophilic esophagitis), and either pH monitoring while off PPI therapy or pH-impedance monitoring on therapy if patients have proven GERD. According to the clinical practice update, pH studies should document physiological acid exposure in the distal esophagus that is unlinked to symptoms (i.e., both a negative symptom index and a negative symptom association probability).
Functional heartburn resembles GERD, but symptoms are unrelated to acid exposure. Balloon distension studies indicate that patients with functional heartburn experience both esophageal and rectal hypersensitivity. Anxiety and mood disorders also are highly prevalent, and patients “will likely not improve unless esophageal perception and underlying affective disorders are adequately managed,” Dr. Fass and coauthors emphasized.
In keeping with this approach, limited evidence from clinical trials supports the first-line use of neuromodulator therapies, including selective serotonin reuptake inhibitors, tricyclic antidepressants, the serotonin 4 receptor antagonist tegaserod, and H2RAs (e.g., cimetidine, famotidine, nizatidine). The only SSRI studied thus far in functional heartburn is fluoxetine. In a placebo-controlled trial of patients with normal endoscopy and heartburn that had not responded to once-daily PPI therapy, 6 weeks of fluoxetine (20 mg daily) significantly outperformed double-dose omeprazole (P < .001) for the primary endpoint of heartburn-free days. “This superior therapeutic effect of fluoxetine was seen only in the subset of patients with a normal pH test,” the experts noted.
In another placebo-controlled trial, the neuromodulator tegaserod (a serotonin 5-HT4 receptor partial agonist) significantly improved tolerance of esophageal pressure during balloon distension and significantly decreased heartburn, regurgitation, and associated distress among patients with functional heartburn. Melatonin, which “also has a pain modulatory effect in the gastrointestinal tract,” significantly improved symptom-related quality of life, compared with nortriptyline and placebo in a randomized, three-arm trial. The patients on melatonin received a 6-mg dose at bedtime for 3 months.
Acupuncture and hypnotherapy also have shown benefit in small studies of functional heartburn patients and may be appropriate as monotherapy or adjunctive treatment, according to the clinical practice update. In a small randomized study, 10 acupuncture sessions delivered over 4 weeks significantly improved daytime and nighttime heartburn and acid regurgitation scores, compared with double-dose PPI. “Mean general health score was significantly improved only in those receiving acupuncture,” the experts noted. Hypnotherapy, the only psychological intervention to have been studied in functional heartburn, was associated with significant improvements in symptoms, visceral anxiety, and quality of life in an uncontrolled study of nine patients.
Although the overall prevalence of functional heartburn is unclear, it has been detected in 21%-39% of PPI-refractory patients evaluated with pH-impedance monitoring, Dr. Fass and associates wrote. Because functional heartburn and GERD can co-occur, some patients with functional heartburn may develop long-term complications of GERD, such as Barrett’s esophagus or peptic stricture. However, the experts noted, “this is anticipated to be rare, and the vast majority of patients with functional heartburn will have compromised quality of life, rather than organic complications over time.
Dr. Fass reported receiving consulting, research, and speaking fees from Ironwood, Takeda, and Salix, among other pharmaceutical companies; Dr. Zerbib received consulting fees from Reckitt Benckiser; and Dr. Gyawali received teaching and consulting fees from Medtronic, Diversatek, and Ironwood.
SOURCE: Fass R et al. Gastroenterology. 2020 Feb 1. doi: 10.1053/j.gastro.2020.01.034.
This story was updated on 6/11/2020.
Recognizing the presence of functional heartburn is vital to prevent unnecessary acid-suppressive therapy and invasive antireflux treatments, which are ineffective and “might even lead to harm,” cautions a new clinical practice update from the American Gastroenterological Association.
Proton pump inhibitors (PPIs) “have no therapeutic value in functional heartburn,” unless patients also have gastroesophageal reflux disease (GERD), Ronnie Fass, MD, of MetroHealth System in Cleveland, and coauthors wrote in Gastroenterology. If clinical work-up finds no clear evidence of GERD, “an attempt to discontinue PPI therapy is warranted,” they added. Likewise, antireflux surgery and endoscopic treatments for GERD “have no therapeutic benefit in functional heartburn and should not be recommended.” However, histamine2 receptor antagonists (H2RAs) “may have an independent benefit in functional heartburn from an esophageal pain modulatory effect.”
Heartburn consists of burning or discomfort that radiates retrosternally from the epigastrium. Patients may report reflux, regurgitation, chest pain or discomfort, fullness, water brash, belching, or a sour and bitter taste in the mouth. Functional heartburn is heartburn that persists after at least 3 months of maximal (double-dose) PPIs taken before meals. Confirming functional heartburn requires high-resolution manometry to rule out major esophageal motor disorders, esophageal endoscopy with biopsy to rule out structural abnormalities and mucosal disorders (e.g., erosive esophagitis, Barrett’s esophagus, and eosinophilic esophagitis), and either pH monitoring while off PPI therapy or pH-impedance monitoring on therapy if patients have proven GERD. According to the clinical practice update, pH studies should document physiological acid exposure in the distal esophagus that is unlinked to symptoms (i.e., both a negative symptom index and a negative symptom association probability).
Functional heartburn resembles GERD, but symptoms are unrelated to acid exposure. Balloon distension studies indicate that patients with functional heartburn experience both esophageal and rectal hypersensitivity. Anxiety and mood disorders also are highly prevalent, and patients “will likely not improve unless esophageal perception and underlying affective disorders are adequately managed,” Dr. Fass and coauthors emphasized.
In keeping with this approach, limited evidence from clinical trials supports the first-line use of neuromodulator therapies, including selective serotonin reuptake inhibitors, tricyclic antidepressants, the serotonin 4 receptor antagonist tegaserod, and H2RAs (e.g., cimetidine, famotidine, nizatidine). The only SSRI studied thus far in functional heartburn is fluoxetine. In a placebo-controlled trial of patients with normal endoscopy and heartburn that had not responded to once-daily PPI therapy, 6 weeks of fluoxetine (20 mg daily) significantly outperformed double-dose omeprazole (P < .001) for the primary endpoint of heartburn-free days. “This superior therapeutic effect of fluoxetine was seen only in the subset of patients with a normal pH test,” the experts noted.
In another placebo-controlled trial, the neuromodulator tegaserod (a serotonin 5-HT4 receptor partial agonist) significantly improved tolerance of esophageal pressure during balloon distension and significantly decreased heartburn, regurgitation, and associated distress among patients with functional heartburn. Melatonin, which “also has a pain modulatory effect in the gastrointestinal tract,” significantly improved symptom-related quality of life, compared with nortriptyline and placebo in a randomized, three-arm trial. The patients on melatonin received a 6-mg dose at bedtime for 3 months.
Acupuncture and hypnotherapy also have shown benefit in small studies of functional heartburn patients and may be appropriate as monotherapy or adjunctive treatment, according to the clinical practice update. In a small randomized study, 10 acupuncture sessions delivered over 4 weeks significantly improved daytime and nighttime heartburn and acid regurgitation scores, compared with double-dose PPI. “Mean general health score was significantly improved only in those receiving acupuncture,” the experts noted. Hypnotherapy, the only psychological intervention to have been studied in functional heartburn, was associated with significant improvements in symptoms, visceral anxiety, and quality of life in an uncontrolled study of nine patients.
Although the overall prevalence of functional heartburn is unclear, it has been detected in 21%-39% of PPI-refractory patients evaluated with pH-impedance monitoring, Dr. Fass and associates wrote. Because functional heartburn and GERD can co-occur, some patients with functional heartburn may develop long-term complications of GERD, such as Barrett’s esophagus or peptic stricture. However, the experts noted, “this is anticipated to be rare, and the vast majority of patients with functional heartburn will have compromised quality of life, rather than organic complications over time.
Dr. Fass reported receiving consulting, research, and speaking fees from Ironwood, Takeda, and Salix, among other pharmaceutical companies; Dr. Zerbib received consulting fees from Reckitt Benckiser; and Dr. Gyawali received teaching and consulting fees from Medtronic, Diversatek, and Ironwood.
SOURCE: Fass R et al. Gastroenterology. 2020 Feb 1. doi: 10.1053/j.gastro.2020.01.034.
This story was updated on 6/11/2020.
Recognizing the presence of functional heartburn is vital to prevent unnecessary acid-suppressive therapy and invasive antireflux treatments, which are ineffective and “might even lead to harm,” cautions a new clinical practice update from the American Gastroenterological Association.
Proton pump inhibitors (PPIs) “have no therapeutic value in functional heartburn,” unless patients also have gastroesophageal reflux disease (GERD), Ronnie Fass, MD, of MetroHealth System in Cleveland, and coauthors wrote in Gastroenterology. If clinical work-up finds no clear evidence of GERD, “an attempt to discontinue PPI therapy is warranted,” they added. Likewise, antireflux surgery and endoscopic treatments for GERD “have no therapeutic benefit in functional heartburn and should not be recommended.” However, histamine2 receptor antagonists (H2RAs) “may have an independent benefit in functional heartburn from an esophageal pain modulatory effect.”
Heartburn consists of burning or discomfort that radiates retrosternally from the epigastrium. Patients may report reflux, regurgitation, chest pain or discomfort, fullness, water brash, belching, or a sour and bitter taste in the mouth. Functional heartburn is heartburn that persists after at least 3 months of maximal (double-dose) PPIs taken before meals. Confirming functional heartburn requires high-resolution manometry to rule out major esophageal motor disorders, esophageal endoscopy with biopsy to rule out structural abnormalities and mucosal disorders (e.g., erosive esophagitis, Barrett’s esophagus, and eosinophilic esophagitis), and either pH monitoring while off PPI therapy or pH-impedance monitoring on therapy if patients have proven GERD. According to the clinical practice update, pH studies should document physiological acid exposure in the distal esophagus that is unlinked to symptoms (i.e., both a negative symptom index and a negative symptom association probability).
Functional heartburn resembles GERD, but symptoms are unrelated to acid exposure. Balloon distension studies indicate that patients with functional heartburn experience both esophageal and rectal hypersensitivity. Anxiety and mood disorders also are highly prevalent, and patients “will likely not improve unless esophageal perception and underlying affective disorders are adequately managed,” Dr. Fass and coauthors emphasized.
In keeping with this approach, limited evidence from clinical trials supports the first-line use of neuromodulator therapies, including selective serotonin reuptake inhibitors, tricyclic antidepressants, the serotonin 4 receptor antagonist tegaserod, and H2RAs (e.g., cimetidine, famotidine, nizatidine). The only SSRI studied thus far in functional heartburn is fluoxetine. In a placebo-controlled trial of patients with normal endoscopy and heartburn that had not responded to once-daily PPI therapy, 6 weeks of fluoxetine (20 mg daily) significantly outperformed double-dose omeprazole (P < .001) for the primary endpoint of heartburn-free days. “This superior therapeutic effect of fluoxetine was seen only in the subset of patients with a normal pH test,” the experts noted.
In another placebo-controlled trial, the neuromodulator tegaserod (a serotonin 5-HT4 receptor partial agonist) significantly improved tolerance of esophageal pressure during balloon distension and significantly decreased heartburn, regurgitation, and associated distress among patients with functional heartburn. Melatonin, which “also has a pain modulatory effect in the gastrointestinal tract,” significantly improved symptom-related quality of life, compared with nortriptyline and placebo in a randomized, three-arm trial. The patients on melatonin received a 6-mg dose at bedtime for 3 months.
Acupuncture and hypnotherapy also have shown benefit in small studies of functional heartburn patients and may be appropriate as monotherapy or adjunctive treatment, according to the clinical practice update. In a small randomized study, 10 acupuncture sessions delivered over 4 weeks significantly improved daytime and nighttime heartburn and acid regurgitation scores, compared with double-dose PPI. “Mean general health score was significantly improved only in those receiving acupuncture,” the experts noted. Hypnotherapy, the only psychological intervention to have been studied in functional heartburn, was associated with significant improvements in symptoms, visceral anxiety, and quality of life in an uncontrolled study of nine patients.
Although the overall prevalence of functional heartburn is unclear, it has been detected in 21%-39% of PPI-refractory patients evaluated with pH-impedance monitoring, Dr. Fass and associates wrote. Because functional heartburn and GERD can co-occur, some patients with functional heartburn may develop long-term complications of GERD, such as Barrett’s esophagus or peptic stricture. However, the experts noted, “this is anticipated to be rare, and the vast majority of patients with functional heartburn will have compromised quality of life, rather than organic complications over time.
Dr. Fass reported receiving consulting, research, and speaking fees from Ironwood, Takeda, and Salix, among other pharmaceutical companies; Dr. Zerbib received consulting fees from Reckitt Benckiser; and Dr. Gyawali received teaching and consulting fees from Medtronic, Diversatek, and Ironwood.
SOURCE: Fass R et al. Gastroenterology. 2020 Feb 1. doi: 10.1053/j.gastro.2020.01.034.
This story was updated on 6/11/2020.
FROM GASTROENTEROLOGY
AGA Guideline: Management of eosinophilic esophagitis
Patients with eosinophilic esophagitis should receive topical steroids instead of oral steroids or no treatment, according to new recommendations from the American Gastroenterological Association and the Joint Task Force on Allergy-Immunology Practice Parameters.
In a pooled analysis of eight double-blind clinical trials, monotherapy with topical budesonide or topical fluticasone was about 61% more likely than placebo to produce histologic remissions in patients with eosinophilic esophagitis (relative risk of failure to achieve remission, 0.39; 95% confidence interval, 0.26-0.58), wrote Ikuo Hirano, MD, of Northwestern University, Chicago. Although these trials differed methodologically, the results were robust enough to warrant a strong recommendation for topical steroids, wrote Dr. Hirano and coauthors of the guidelines, published in Gastroenterology (doi: 10.1053/j.gastro.2020.02.038). “[T]he same inhaled steroid agents are considered very safe for use in children and adults with asthma and are routinely used in [its] primary management,” they noted.
All other recommendations in the guidelines are graded as conditional, reflecting a lack of high-quality supporting evidence. For example, one only study to date has compared topical and oral steroids for patients with eosinophilic esophagitis. In this pediatric trial, children benefited similarly from fluticasone (two puffs four times daily) and oral prednisone (1 mg/kg twice daily), but prednisone caused side effects (weight gain and cushingoid appearance) in 40% of patients, while topical steroids caused oral candidiasis (thrush) in only 15% of patients. Similarities between pediatric and adult eosinophilic esophagitis support the use of topical versus oral steroids in both groups, the guidelines conclude.
Eosinophilic esophagitis tends to be chronic and can progress to recurrent dysphagia, esophageal impactions, and stricture if left untreated. For this reason, the guidelines call for remitted patients to stay on topical steroids as maintenance therapy despite “very low confidence in the estimated benefits of [any type of] long-term therapy.” In a very small trial, 1 year of low-dose budesonide maintenance therapy (0.25 mg twice daily) outperformed placebo, but only 36% of patients maintained less than 5 eosinophils per high power field. Other studies have produced mixed results. Pending more data, the guidelines call maintenance treatment with topical steroids, proton pump inhibitors, and elimination diets “reasonable options” that comprise “a preference-sensitive area of management.”
Dietary interventions for eosinophilic esophagitis include the elemental diet (amino acid–based formulas), the empiric six-food elimination diet, and eliminating foods based on allergy testing. The guidelines cite moderate-quality evidence for the elemental diet, which induced histologic remissions (less than than 15 eosinophils per high power field) in nearly 94% of patients in six single-arm observational studies (in contrast, the rate of histologic failure with placebo is nearly 87%). However, patients may struggle to adhere to both the elemental diet and the six-food elimination diet, which has less supporting evidence. Hence, patients “may reasonably decline” these treatment options and “may prefer alternative medical or dietary therapies” to a diet exclusively based on food allergens, tests for which are potentially inaccurate, the guidelines state.
Esophageal dilation is recommended for patients with stricture based on a systematic review in which 87% of patients improved with this therapy. However, dilation “does not address the esophageal inflammation associated with eosinophilic esophagitis,” and the “assumption that no clinical improvement would occur if dilation was not performed likely overestimates [its] treatment benefit, given the reported symptom-placebo response noted in controlled trials,” according to the guidelines. Moreover, the evidence for dilation “was considered low quality due to the retrospective, single-arm design of all but one of the reports, and the lack of a standard definition for what constitutes clinical improvement.”
Anti-IgE therapy is not recommended – it failed to improve symptoms or esophageal eosinophilia in the only trial conducted to date. Because of a lack of evidence, the guidelines state that patients should receive only montelukast, cromolyn sodium, immunomodulators, anti–tumor necrosis factor (anti-TNF) therapies, or therapies targeting interleukin (IL)-5, IL-13, or IL-4 in the context of a clinical trial.
Eosinophilic esophagitis is triggered by exposure to food antigens and often overlaps with other atopic conditions, such as asthma, eczema, and allergic rhinitis. It has no approved treatments in the United States, although in 2018, the European Medicines Agency approved a budesonide tablet formulation.
The guideline authors disclosed no conflicts of interest.
SOURCE: Hirano I et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.02.038.
Patients with eosinophilic esophagitis should receive topical steroids instead of oral steroids or no treatment, according to new recommendations from the American Gastroenterological Association and the Joint Task Force on Allergy-Immunology Practice Parameters.
In a pooled analysis of eight double-blind clinical trials, monotherapy with topical budesonide or topical fluticasone was about 61% more likely than placebo to produce histologic remissions in patients with eosinophilic esophagitis (relative risk of failure to achieve remission, 0.39; 95% confidence interval, 0.26-0.58), wrote Ikuo Hirano, MD, of Northwestern University, Chicago. Although these trials differed methodologically, the results were robust enough to warrant a strong recommendation for topical steroids, wrote Dr. Hirano and coauthors of the guidelines, published in Gastroenterology (doi: 10.1053/j.gastro.2020.02.038). “[T]he same inhaled steroid agents are considered very safe for use in children and adults with asthma and are routinely used in [its] primary management,” they noted.
All other recommendations in the guidelines are graded as conditional, reflecting a lack of high-quality supporting evidence. For example, one only study to date has compared topical and oral steroids for patients with eosinophilic esophagitis. In this pediatric trial, children benefited similarly from fluticasone (two puffs four times daily) and oral prednisone (1 mg/kg twice daily), but prednisone caused side effects (weight gain and cushingoid appearance) in 40% of patients, while topical steroids caused oral candidiasis (thrush) in only 15% of patients. Similarities between pediatric and adult eosinophilic esophagitis support the use of topical versus oral steroids in both groups, the guidelines conclude.
Eosinophilic esophagitis tends to be chronic and can progress to recurrent dysphagia, esophageal impactions, and stricture if left untreated. For this reason, the guidelines call for remitted patients to stay on topical steroids as maintenance therapy despite “very low confidence in the estimated benefits of [any type of] long-term therapy.” In a very small trial, 1 year of low-dose budesonide maintenance therapy (0.25 mg twice daily) outperformed placebo, but only 36% of patients maintained less than 5 eosinophils per high power field. Other studies have produced mixed results. Pending more data, the guidelines call maintenance treatment with topical steroids, proton pump inhibitors, and elimination diets “reasonable options” that comprise “a preference-sensitive area of management.”
Dietary interventions for eosinophilic esophagitis include the elemental diet (amino acid–based formulas), the empiric six-food elimination diet, and eliminating foods based on allergy testing. The guidelines cite moderate-quality evidence for the elemental diet, which induced histologic remissions (less than than 15 eosinophils per high power field) in nearly 94% of patients in six single-arm observational studies (in contrast, the rate of histologic failure with placebo is nearly 87%). However, patients may struggle to adhere to both the elemental diet and the six-food elimination diet, which has less supporting evidence. Hence, patients “may reasonably decline” these treatment options and “may prefer alternative medical or dietary therapies” to a diet exclusively based on food allergens, tests for which are potentially inaccurate, the guidelines state.
Esophageal dilation is recommended for patients with stricture based on a systematic review in which 87% of patients improved with this therapy. However, dilation “does not address the esophageal inflammation associated with eosinophilic esophagitis,” and the “assumption that no clinical improvement would occur if dilation was not performed likely overestimates [its] treatment benefit, given the reported symptom-placebo response noted in controlled trials,” according to the guidelines. Moreover, the evidence for dilation “was considered low quality due to the retrospective, single-arm design of all but one of the reports, and the lack of a standard definition for what constitutes clinical improvement.”
Anti-IgE therapy is not recommended – it failed to improve symptoms or esophageal eosinophilia in the only trial conducted to date. Because of a lack of evidence, the guidelines state that patients should receive only montelukast, cromolyn sodium, immunomodulators, anti–tumor necrosis factor (anti-TNF) therapies, or therapies targeting interleukin (IL)-5, IL-13, or IL-4 in the context of a clinical trial.
Eosinophilic esophagitis is triggered by exposure to food antigens and often overlaps with other atopic conditions, such as asthma, eczema, and allergic rhinitis. It has no approved treatments in the United States, although in 2018, the European Medicines Agency approved a budesonide tablet formulation.
The guideline authors disclosed no conflicts of interest.
SOURCE: Hirano I et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.02.038.
Patients with eosinophilic esophagitis should receive topical steroids instead of oral steroids or no treatment, according to new recommendations from the American Gastroenterological Association and the Joint Task Force on Allergy-Immunology Practice Parameters.
In a pooled analysis of eight double-blind clinical trials, monotherapy with topical budesonide or topical fluticasone was about 61% more likely than placebo to produce histologic remissions in patients with eosinophilic esophagitis (relative risk of failure to achieve remission, 0.39; 95% confidence interval, 0.26-0.58), wrote Ikuo Hirano, MD, of Northwestern University, Chicago. Although these trials differed methodologically, the results were robust enough to warrant a strong recommendation for topical steroids, wrote Dr. Hirano and coauthors of the guidelines, published in Gastroenterology (doi: 10.1053/j.gastro.2020.02.038). “[T]he same inhaled steroid agents are considered very safe for use in children and adults with asthma and are routinely used in [its] primary management,” they noted.
All other recommendations in the guidelines are graded as conditional, reflecting a lack of high-quality supporting evidence. For example, one only study to date has compared topical and oral steroids for patients with eosinophilic esophagitis. In this pediatric trial, children benefited similarly from fluticasone (two puffs four times daily) and oral prednisone (1 mg/kg twice daily), but prednisone caused side effects (weight gain and cushingoid appearance) in 40% of patients, while topical steroids caused oral candidiasis (thrush) in only 15% of patients. Similarities between pediatric and adult eosinophilic esophagitis support the use of topical versus oral steroids in both groups, the guidelines conclude.
Eosinophilic esophagitis tends to be chronic and can progress to recurrent dysphagia, esophageal impactions, and stricture if left untreated. For this reason, the guidelines call for remitted patients to stay on topical steroids as maintenance therapy despite “very low confidence in the estimated benefits of [any type of] long-term therapy.” In a very small trial, 1 year of low-dose budesonide maintenance therapy (0.25 mg twice daily) outperformed placebo, but only 36% of patients maintained less than 5 eosinophils per high power field. Other studies have produced mixed results. Pending more data, the guidelines call maintenance treatment with topical steroids, proton pump inhibitors, and elimination diets “reasonable options” that comprise “a preference-sensitive area of management.”
Dietary interventions for eosinophilic esophagitis include the elemental diet (amino acid–based formulas), the empiric six-food elimination diet, and eliminating foods based on allergy testing. The guidelines cite moderate-quality evidence for the elemental diet, which induced histologic remissions (less than than 15 eosinophils per high power field) in nearly 94% of patients in six single-arm observational studies (in contrast, the rate of histologic failure with placebo is nearly 87%). However, patients may struggle to adhere to both the elemental diet and the six-food elimination diet, which has less supporting evidence. Hence, patients “may reasonably decline” these treatment options and “may prefer alternative medical or dietary therapies” to a diet exclusively based on food allergens, tests for which are potentially inaccurate, the guidelines state.
Esophageal dilation is recommended for patients with stricture based on a systematic review in which 87% of patients improved with this therapy. However, dilation “does not address the esophageal inflammation associated with eosinophilic esophagitis,” and the “assumption that no clinical improvement would occur if dilation was not performed likely overestimates [its] treatment benefit, given the reported symptom-placebo response noted in controlled trials,” according to the guidelines. Moreover, the evidence for dilation “was considered low quality due to the retrospective, single-arm design of all but one of the reports, and the lack of a standard definition for what constitutes clinical improvement.”
Anti-IgE therapy is not recommended – it failed to improve symptoms or esophageal eosinophilia in the only trial conducted to date. Because of a lack of evidence, the guidelines state that patients should receive only montelukast, cromolyn sodium, immunomodulators, anti–tumor necrosis factor (anti-TNF) therapies, or therapies targeting interleukin (IL)-5, IL-13, or IL-4 in the context of a clinical trial.
Eosinophilic esophagitis is triggered by exposure to food antigens and often overlaps with other atopic conditions, such as asthma, eczema, and allergic rhinitis. It has no approved treatments in the United States, although in 2018, the European Medicines Agency approved a budesonide tablet formulation.
The guideline authors disclosed no conflicts of interest.
SOURCE: Hirano I et al. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.02.038.
FROM GASTROENTEROLOGY
Cyclic vomiting syndrome: A GI primer
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS
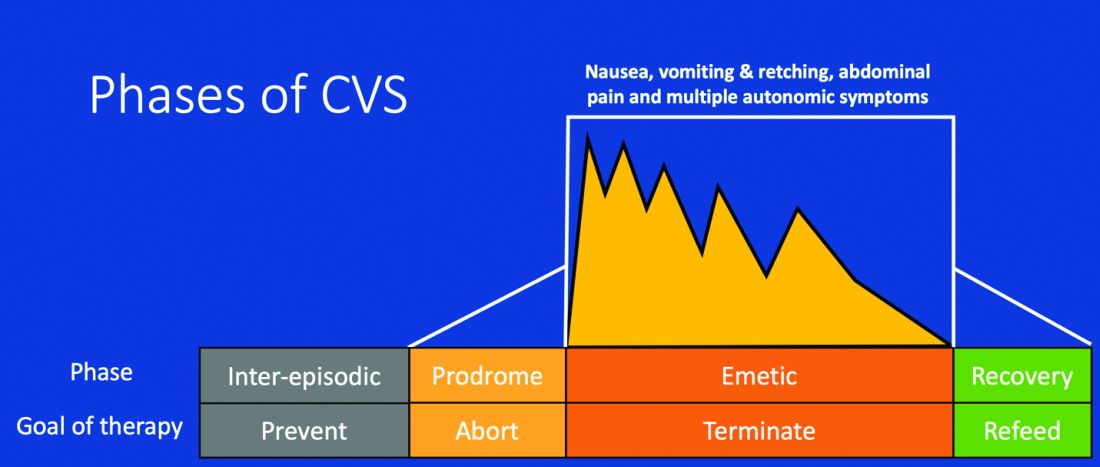
Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22
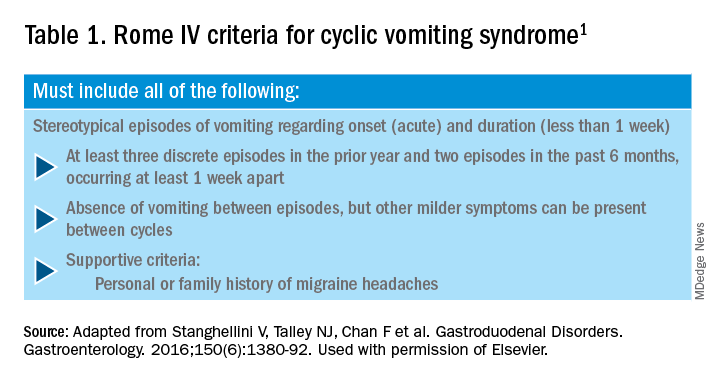
CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.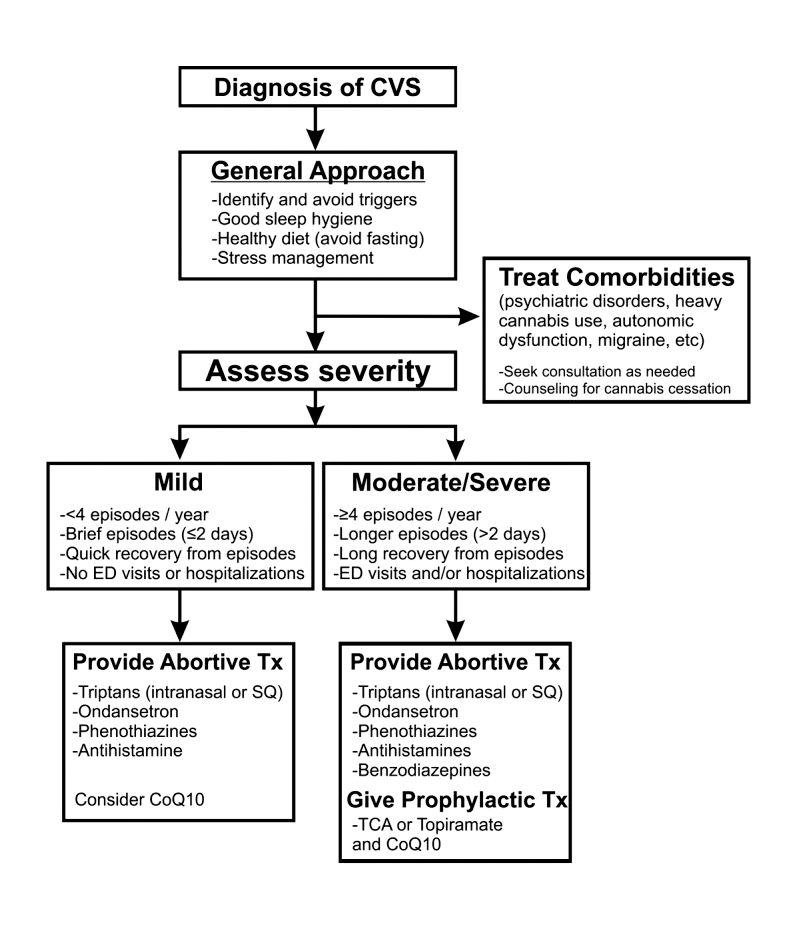
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
Introduction
Cyclic vomiting syndrome (CVS) is a chronic disorder of gut-brain interaction (DGBI) and is characterized by recurrent episodes of severe nausea, vomiting, and often, abdominal pain. Patients are usually asymptomatic in between episodes.1 CVS was considered a pediatric disease but is now known to be as common in adults. The prevalence of CVS in adults was 2% in a recent population-based study.2 Patients are predominantly white. Both males and females are affected with some studies showing a female preponderance. The mean age of onset is 5 years in children and 35 years in adults.3
The etiology of CVS is not known, but various hypotheses have been proposed. Zaki et al. showed that two mitochondrial DNA polymorphisms 16519T and 3010A were associated with a 17-fold increased odds of having CVS in children.4 These polymorphisms were not associated with CVS in adults.5 Alterations in the brain-gut axis also have been shown in CVS. Functional neuroimaging studies demonstrate that patients with CVS displayed increased connectivity between insula and salience networks with concomitant decrease in connectivity to somatosensory networks.6 Recent data also indicate that the endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal axis are implicated in CVS with an increase in serum endocannabinoid concentration during an episode.7 The same study also showed a significant increase in salivary cortisol in CVS patients who used cannabis. Further, single nucleotide polymorphisms (SNPs) in the gene that encodes for the cannabinoid receptor type 1 (CB1R) are implicated in CVS.8 The CB1R is part of the ECS and is densely expressed in brain areas involved in emesis, such as the dorsal vagal complex consisting of the area postrema (AP), nucleus of the solitary tract (NTS), and also the dorsal motor nucleus of the vagus.9 Wasilewski et al. showed an increased risk of CVS among individuals with AG and GG genotypes of CNR1 rs806380 (P less than .01), whereas the CC genotype of CNR1 rs806368 was associated with a decreased risk of CVS (P less than .05).8 CB1R agonists – endocannabinoids and tetrahydrocannabinol (THC) – have acute antiemetic and anxiolytic effects.9-11 The apparent paradoxical effects of cannabis in this patient population are yet to be explained and need further study.
Diagnosis and clinical features of CVS

Figure 1: Phases of Cyclic Vomiting Syndrome12
Adapted from Fleisher DR, Gornowicz B, Adams K, Burch R, Feldman EJ. Cyclic Vomiting Syndrome in 41 adults: The illness, the patients, and problems of management. BMC Med 2005;3:20. This work is licensed under the Creative Commons Attribution 4.0 International License https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, distribution, modification, and reproduction in any medium.
CVS consists of four phases which include the a) prodromal phase, b) the episodic phase, c) recovery phase, and d) the interepisodic phase; and was first described by David Fleisher.12 The phases of CVS are important for clinicians and patients alike as they have therapeutic implications. The administration of abortive medications during a prodrome can terminate an episode. The phases of CVS are shown above.
Most patients (~ 93%) have a prodromal phase. Symptoms during this phase can include nausea, abdominal pain, diaphoresis, fatigue, weakness, hot flashes, chills, shivering, increased thirst, loss of appetite, burping, lightheadedness, and paresthesia.13 Some patients report a sense of impending doom and many have symptoms consistent with panic. If untreated, this progresses to the emetic phase and patients have unrelenting nausea, retching, vomiting, and other symptoms. During an episode, patients may vomit up to 20 times per hour and the episode may last several hours to days. During this phase, patients are sometimes described as being in a “conscious coma” and exhibit lethargy, listlessness, withdrawal, and sometimes disorientation.14,15 The emetic phase is followed by the recovery phase, during which symptoms subside and patients are able to resume oral intake. Patients are usually asymptomatic between episodes but ~ 30% can have interepisodic nausea and dyspepsia. In some patients, episodes become progressively longer and the interepisodic phase is considerably shortened and patients have a “coalescence of symptoms.”12 It is important to elicit a thorough history in all patients with vomiting in order to make an accurate diagnosis of CVS since coalescence of symptoms only occurs over a period of time. Episodes often are triggered by psychological stress, both positive and negative. Common triggers can include positive events such as birthdays, holidays, and negative ones like examinations, the death of a loved one, etc. Sleep deprivation and physical exhaustion also can trigger an episode.12
CVS remains a clinical diagnosis since there are no biomarkers. While there is a lack of data on the optimal work-up in these patients, experts recommend an upper endoscopy or upper GI series in order to rule out alternative gastric and intestinal pathology (e.g., malrotation with volvulus).16 Of note, a gastric-emptying study is not recommended as part of the routine work-up as per recent guidelines because of the poor specificity of this test in establishing a diagnosis of CVS.16 Biochemical testing including a complete blood count, serum electrolytes, serum glucose, liver panel, and urinalysis is also warranted. Any additional testing is indicated when clinical features suggest an alternative diagnosis. For instance, neurologic symptoms might warrant a cranial MRI to exclude an intracerebral tumor or other lesions of the brain.

The severity and unpredictable nature of symptoms makes it difficult for some patients to attend school or work; one study found that 32% of patients with CVS were completely disabled.12 Despite increasing awareness of this disorder, patients often are misdiagnosed. The prevalence of CVS in an outpatient gastroenterology clinic in the United Kingdom was 11% and was markedly underdiagnosed in the community.17 Only 5% of patients who were subsequently diagnosed with CVS were initially diagnosed accurately by their referring physician despite meeting criteria for the disorder.17 A subset of patients with CVS even undergo futile surgeries.13 Fleisher et al. noted that 30% of a 41-patient cohort underwent cholecystectomy for CVS symptoms without any improvement in disease.12 Prompt diagnosis and appropriate therapy is essential to improve patient outcomes and improve quality of life.
CVS is associated with various comorbidities such as migraine, anxiety, depression and dysautonomia, which can further impair quality of life.18,19 Approximately 70% of CVS patients report a personal or family history of migraine. Anxiety and depression affects nearly half of patients with CVS.13 Cannabis use is significantly more prevalent among patients with CVS than patients without CVS.20
Role of cannabis in CVS
The role of cannabis in the pathogenesis of symptoms in CVS is controversial. While cannabis has antiemetic properties, there is a strong link between its use and CVS. The use of cannabis has increased over the past decade with increasing legalization.21 Several studies have shown that 40%-80% of patients with CVS use cannabis.22,23 Following this, cannabinoid hyperemesis syndrome (CHS) was coined as a separate entity based on this statistical association, though there are no data to support the notion that cannabis causes vomiting.24,25 CHS has clinical features that are indistinguishable from CVS except for the chronic heavy cannabis use. A peculiar bathing behavior called “compulsive hot-water bathing” has been described and was thought to be pathognomonic of cannabis use.26 During an episode, patients will take multiple hot showers/baths, which temporarily alleviate their symptoms. Many patients even report running out of hot water and sometimes check into a hotel for a continuous supply of hot water. A small number of patients may sustain burns from the hot-water bathing. However, studies show that this hot-water bathing behavior also is seen in about 50% of patents with CVS who do not use cannabis.22

CHS is now defined by Rome IV criteria, which include episodes of nausea and vomiting similar to CVS preceded by chronic, heavy cannabis use. Patients must have complete resolution of symptoms following cessation.1 A recent systematic review of 376 cases of purported CHS showed that only 59 (15.7%) met Rome IV criteria for this disorder.27 This is because of considerable heterogeneity in how the diagnosis of CHS was made and the lack of standard diagnostic criteria at the time. Some cases of CHS were diagnosed merely based on an association of vomiting, hot-water bathing, and cannabis use.28 Only a minority of patients (71,19%) had a duration of follow-up more than 4 weeks, which would make it impossible to establish a diagnosis of CHS. A period of at least a year or a duration of time that spans at least three episodes is generally recommended to determine if abstinence from cannabis causes a true resolution of symptoms.27 Whether CHS is a separate entity or a subtype of CVS remains to be determined. The paradoxical effects of cannabis may happen because of the use of highly potent cannabis products that are currently in use. A complete discussion of the role of cannabis in CVS is beyond the scope of this article, and the reader is referred to a recent systematic review and discussion.27
Treatment
CVS should be treated based on a biopsychosocial model with a multidisciplinary team that includes a gastroenterologist with knowledge of CVS, primary care physician, psychologist, psychiatrist, and sleep specialist if needed.16 Initiating prophylactic treatment is based on the severity of disease. An algorithm for the treatment of CVS based on severity of symptoms is shown below.
Figure 2. Adapted and reprinted by permission from the Licensor: Springer Nature, Current Treatment Options in Gastroenterology, Bhandari S, Venkatesan T. Novel Treatments for Cyclic Vomiting Syndrome: Beyond Ondansetron and Amitriptyline, 14:495-506, Copyright 2016.
Patients who have mild disease (defined as fewer than four episodes/year, episodes lasting up to 2 days, quick recovery from episodes, or episodes not requiring ED care or hospitalization) are usually prescribed abortive medications.16 These medications are best administered during the prodromal phase and can prevent progression to the emetic phase. Medications used for aborting episodes include sumatriptan (20 mg intranasal or 6 mg subcutaneous), ondansetron (8 mg sublingual), and diphenhydramine (25-50 mg).30,31 This combination can help abort symptoms and potentially avoid ED visits or hospitalizations. Patients with moderate-to-severe CVS are offered prophylactic therapy in addition to abortive therapy.16
Recent guidelines recommend tricyclic antidepressants (TCAs) as the first-line agent in the prophylaxis of CVS episodes. Data from 14 studies determined that 70% (413/600) of patients responded partially or completely to TCAs.16 An open-label study of 46 patients by Hejazi et al. noted a decline in the number of CVS episodes from 17 to 3, in the duration of a CVS episode from 6 to 2 days, and in the number of ED visits/ hospitalizations from 15 to 3.3.32Amitriptyline should be started at 25 mg at night and titrated up by 10-25 mg each week to minimize emergence of side effects. The mean effective dose is 75-100 mg or 1.0-1.5 mg/kg. An EKG should be checked at baseline and during titration to monitor the QT interval. Unfortunately, side effects from TCAs are quite common and include cognitive impairment, drowsiness, dryness of mouth, weight gain, constipation, and mood changes, which may warrant dose reduction or discontinuation. Antiepileptics such as topiramate, mitochondrial supplements such as Coenzyme Q10 and riboflavin are alternative prophylactic agents in CVS.33 Aprepitant, a newer NK1 receptor antagonist has been found to be effective in refractory CVS.34 In addition to pharmacotherapy, addressing comorbid conditions such as anxiety and depression and counseling patients to abstain from heavy cannabis use is also important to achieve good health care outcomes.
In summary, CVS is a common, chronic functional GI disorder with episodic nausea, vomiting, and often, abdominal pain. Symptoms can be disabling, and prompt diagnosis and therapy is important. CVS is associated with multiple comorbid conditions such as migraine, anxiety and depression, and a biopsychosocial model of care is essential. Medications such as amitriptyline are effective in the prophylaxis of CVS, but side effects hamper their use. Recent recommendations for management of CVS have been published.16 Cannabis is frequently used by patients for symptom relief but use of high potency products may cause worsening of symptoms or unmask symptoms in genetically predisposed individuals.23 Studies to elucidate the pathophysiology of CVS should help in the development of better therapies.
Dr. Mooers is PGY-2, an internal medicine resident in the department of medicine, Medical College of Wisconsin, Milwaukee; Dr. Venkatesan is professor of medicine, division of gastroenterology and hepatology, department of medicine, Medical College of Wisconsin, Milwaukee. The authors have no conflicts to disclose.
References
1. Stanghellini V et al. Gastroenterology. 2016;150:1380-92.
2. Aziz I et al. Clin Gastroenterol Hepatol. 2019 Apr;17(5):878-86.
3. Kovacic K et al. Curr Gastroenterol Rep. 2018;20(10):46.
4. Zaki EA et al. Cephalalgia. 2009;29:719-28.
5. Venkatesan T et al. BMC Gastroenterol. 2014;14:181.
6. Ellingsen DM et al. Neurogastroenterol Motil. 2017;29 (6)e13004 9.
7. Venkatesan T et al. Neurogastroenterol Motil. 2016;28:1409-18.
8. Wasilewski A et al. Am J Gastroenterol. 2017;112:933-9.
9. van Sickle MD et al. Am J Physiol Gastrointest Liver Physiol 2003;285:G566-76.
10. Parker LA et al. Br J Pharmacol. 2011;163:1411-22.
11. van Sickle MD et al. Gastroenterology. 2001;121:767-74.
12. Fleisher DR et al. BMC Med. 2005;3:20.
13. Kumar N et al. BMC Gastroenterol. 2012;12:52.
14. Li BU et al. J Pediatr Gastroenterol Nutr. 2008;47:379-93.
15. Bhandari S et al. Clin Auton Res. 2018 Apr;28(2):203-9.
16. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. doi: 10.1111/nmo.13604.
17. Sagar RC et al. Neurogastroenterol Motil. 2018;30. doi: 10.1111/nmo.13174.
18. Taranukha T et al. Neurogastroenterol Motil. 2018 Apr;30(4):e13245. doi: 10.1111/nmo.13245.
19. Bhandari S and Venkatesan T. Dig Dis Sci. 2017;62:2035-44.
20. Choung RS et al. Neurogastroenterol Motil. 2012;24:20-6, e21. doi: 10.1111/j.1365-2982.2011.01791.x.
21. Bhandari S et al. Intern Med J. 2019 May;49(5):649-55.
22. Venkatesan T et al. Exp Brain Res. 2014; 232:2563-70.
23. Venkatesan T et al. Clin Gastroenterol Hepatol. 2019 Jul 25. doi: 10.1016/j.cgh.2019.07.039.
24. Simonetto DA et al. Mayo Clin Proc. 2012;87:114-9.
25. Wallace EA et al. South Med J. 2011;104:659-64.
26. Allen JH et al. Gut. 2004;53:1566-70.
27. Venkatesan T et al. Neurogastroenterol Motil. 2019;31 Suppl 2:e13606. doi: 10.1111/nmo.13606.
28. Habboushe J et al. Basic Clin Pharmacol Toxicol. 2018;122:660-2.
29. Bhandari S and Venkatesan T. Curr Treat Options Gastroenterol. 2016;14:495-506.
30. Hikita T et al. Cephalalgia. 2011;31:504-7.
31. Fuseau E et al. Clin Pharmacokinet 2002;41:801-11.
32. Hejazi RA et al. J Clin Gastroenterol. 2010;44:18-21.
33. Sezer OB and Sezer T. J Neurogastroenterol Motil. 2016;22:656-60.
34. Cristofori F et al. Aliment Pharmacol Ther. 2014;40:309-17.
FDA calls for market removal of ranitidine
A problem with both branded and generic over-the-counter and prescription forms, from the market.
The NDMA contamination does not stem from a manufacturing concern, but rather the levels have been found to increase over time depending on how the ranitidine is stored.
In particular, the FDA found through product testing that the NDMA impurity developed over time when the ranitidine was stored above room temperature.
“The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA,” FDA said in a statement announcing the call for product withdrawal.
The FDA has been investigating NDMA contamination since September 2019 when the agency first announced the contamination in ranitidine. Manufacturers have been withdrawing their products from the market since the first reports of contamination surfaced. Despite these recalls, there were still ranitidine products on the market, according to an FDA spokesperson, necessitating the further action taken by the agency.
In addition to products being removed from the market, FDA is asking consumers to discard any ranitidine products they may have.
“There are still questions about how the impurity is formed in ranitidine over time during storage,” Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, said during an April 1 conference call with reporters announcing the withdrawal request. “For example, what impact does the drug packaging have on the development or the specific formulation have on the development of NDMA.”
She said the issue may be fixable over time, and the agency is open to reformulations that demonstrate that ranitidine is stable over time and under various storage conditions.
Dr. Woodcock stressed that the products at the point of manufacture do not have unacceptable levels of NDMA.
“This is a market withdrawal, this is not a recall because technically the products are okay. They met all their specs,” she said. “It is only when they are subjected generally to heat stress do they manifest higher levels” of NDMA.
“Clearly, we can’t have products on the market that if they are stored under conditions consumers might store them under that they would become unacceptable.”
Dr. Woodcock said FDA is not withdrawing approvals for the products, but manufacturers would need to show the product remains stable under normal storage conditions.
This article was updated 4/7/20.
A problem with both branded and generic over-the-counter and prescription forms, from the market.
The NDMA contamination does not stem from a manufacturing concern, but rather the levels have been found to increase over time depending on how the ranitidine is stored.
In particular, the FDA found through product testing that the NDMA impurity developed over time when the ranitidine was stored above room temperature.
“The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA,” FDA said in a statement announcing the call for product withdrawal.
The FDA has been investigating NDMA contamination since September 2019 when the agency first announced the contamination in ranitidine. Manufacturers have been withdrawing their products from the market since the first reports of contamination surfaced. Despite these recalls, there were still ranitidine products on the market, according to an FDA spokesperson, necessitating the further action taken by the agency.
In addition to products being removed from the market, FDA is asking consumers to discard any ranitidine products they may have.
“There are still questions about how the impurity is formed in ranitidine over time during storage,” Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, said during an April 1 conference call with reporters announcing the withdrawal request. “For example, what impact does the drug packaging have on the development or the specific formulation have on the development of NDMA.”
She said the issue may be fixable over time, and the agency is open to reformulations that demonstrate that ranitidine is stable over time and under various storage conditions.
Dr. Woodcock stressed that the products at the point of manufacture do not have unacceptable levels of NDMA.
“This is a market withdrawal, this is not a recall because technically the products are okay. They met all their specs,” she said. “It is only when they are subjected generally to heat stress do they manifest higher levels” of NDMA.
“Clearly, we can’t have products on the market that if they are stored under conditions consumers might store them under that they would become unacceptable.”
Dr. Woodcock said FDA is not withdrawing approvals for the products, but manufacturers would need to show the product remains stable under normal storage conditions.
This article was updated 4/7/20.
A problem with both branded and generic over-the-counter and prescription forms, from the market.
The NDMA contamination does not stem from a manufacturing concern, but rather the levels have been found to increase over time depending on how the ranitidine is stored.
In particular, the FDA found through product testing that the NDMA impurity developed over time when the ranitidine was stored above room temperature.
“The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA,” FDA said in a statement announcing the call for product withdrawal.
The FDA has been investigating NDMA contamination since September 2019 when the agency first announced the contamination in ranitidine. Manufacturers have been withdrawing their products from the market since the first reports of contamination surfaced. Despite these recalls, there were still ranitidine products on the market, according to an FDA spokesperson, necessitating the further action taken by the agency.
In addition to products being removed from the market, FDA is asking consumers to discard any ranitidine products they may have.
“There are still questions about how the impurity is formed in ranitidine over time during storage,” Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, said during an April 1 conference call with reporters announcing the withdrawal request. “For example, what impact does the drug packaging have on the development or the specific formulation have on the development of NDMA.”
She said the issue may be fixable over time, and the agency is open to reformulations that demonstrate that ranitidine is stable over time and under various storage conditions.
Dr. Woodcock stressed that the products at the point of manufacture do not have unacceptable levels of NDMA.
“This is a market withdrawal, this is not a recall because technically the products are okay. They met all their specs,” she said. “It is only when they are subjected generally to heat stress do they manifest higher levels” of NDMA.
“Clearly, we can’t have products on the market that if they are stored under conditions consumers might store them under that they would become unacceptable.”
Dr. Woodcock said FDA is not withdrawing approvals for the products, but manufacturers would need to show the product remains stable under normal storage conditions.
This article was updated 4/7/20.
Microbiome studies may require correction for PPI use
Microbiome studies should be correcting statistics to account for proton pump inhibitor (PPI) use, according to a leading expert.
After antibiotics, PPIs are the leading cause of microbiome variance in both research and general populations, and these alterations could have a range of consequences, reported Rinse K. Weersma, MD, PhD, of the University of Groningen (the Netherlands).
About 20% of people are taking a PPI, Dr. Weersma said at the annual Gut Microbiota for Health World Summit, noting that, in countries such as the United States and the United Kingdom, this figure may be higher.
“There’s chronic use of proton pump inhibitors in the population on a massive scale,” Dr. Weersma said.
To complicate matters, estimates suggest that 25%-70% of people who are taking PPIs have no appropriate indication. While this issue is partly because of increasing over-the-counter usage, physicians are also contributing to the problem by prescribing PPIs without adequate follow-up.
“The number of people using proton pump inhibitors is steadily increasing,” Dr. Weersma said. “The number of people getting them prescribed is relatively stable. The problem is, we never stop.”
According to Dr. Weersma, a growing body of research shows that PPI use may increase the risk of developing other conditions. Although many of these relationships are correlative, some are now widely accepted as causal. Most notable and clinically relevant, Dr. Weersma said, are enteric infections. Clostridioides difficile–associated diarrhea, for instance, is 65% more common among PPI users.
While the mechanisms behind this susceptibility to infection are uncertain, Dr. Weersma suggested that the most likely cause is “oralization” of the gut microbiome caused by loss of the acid barrier, which introduces upper gastrointestinal bacteria, or oral bacteria, into the lower intestines.
Perhaps more relevant to clinical trials, PPIs may also influence the safety and efficacy of drugs.
“There is a lot of interaction between the gut microbiome and a lot of drugs,” Dr. Weersma said at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility. “We really don’t know a lot about this at the moment.”
He went on to explain that bidirectional interactions between drugs and the microbiome may actually present clinical opportunities.
“This is a field that people currently call pharmacomicrobiomics,” Dr. Weersma said. “This is very intriguing, of course, because everyone knows about pharmacogenomics ... which lets you stratify your patients, but you cannot intervene; you cannot change your genetic background to increase efficacy or avoid toxicity. But in fact, with the microbiome, we could modulate the microbiome and improve bioavailability, for example.”
Conversely, Dr. Weersma pointed out that PPI use may be interfering with drug efficacy to a life-altering degree.
He cited a recent study by Chalabi and colleagues, which found that PPI use affected responses to immune checkpoint inhibitors (Ann Oncol. 2020 Jan 16. doi: 10.1016/j.annonc.2020.01.006). Among 169 patients with lung cancer who were treated with atezolizumab, overall survival was significantly lower in PPI users (9.6 vs. 14.5 months; P = .001).
A number of other clinical implications are also possible, Dr. Weersma said, although these require further investigation. For example, a 2019 study by Stark and colleagues suggested that childhood use of PPIs may increase obesity risk.
“[There are] no microbiome data here,” Dr. Weersma said, “but it makes you think.”
While considering the downsides of PPIs, Dr. Weersma also emphasized their importance in clinical practice. “[Proton pump inhibitors] are very great drugs. They are cheap, they are safe, they are very effective. So if you have evidence-based indications to use proton pump inhibitors, you should definitely use them and not stop them.”
Dr. Weersma called for responsible use of PPIs, and suggested that clinicians need to prepare for pushback from patients, who, after stopping PPIs, may experience a temporary resurgence of symptoms because of acid rebound.
“You have to make them aware [of acid rebound],” Dr. Weersma said. “Say: ‘Wait 2 or 3 weeks and this rebound is gone.’ We should say that way, way, way more often.”
But clinicians shouldn’t bear the burden of responsible usage alone, Dr. Weersma said.
“There’s a role for clinicians, patients, and regulatory bodies also, to think about the massive use of proton pump inhibitors now and in the future.”
In the discussion that followed the presentation, a summit attendee brought up the realities of clinical practice before PPIs, when patients frequently had gastrointestinal bleeding secondary to nonsteroidal anti-inflammatory use. In response, Dr. Weersma again emphasized that PPIs play a critical role for many patients. After once more encouraging responsible use, Dr. Weersma expressed concern about the risks involved in conveying his message; not only to the medical community, but also to the general public.
“This is a very difficult message [to deliver],” Dr. Weersma said. “In the Netherlands this was taken up by the media and the news, so my email inbox exploded. It’s difficult to get this nuance right.”
Dr. Weersma disclosed relationships with Takeda, Johnson & Johnson, Ferring, and others.
Microbiome studies should be correcting statistics to account for proton pump inhibitor (PPI) use, according to a leading expert.
After antibiotics, PPIs are the leading cause of microbiome variance in both research and general populations, and these alterations could have a range of consequences, reported Rinse K. Weersma, MD, PhD, of the University of Groningen (the Netherlands).
About 20% of people are taking a PPI, Dr. Weersma said at the annual Gut Microbiota for Health World Summit, noting that, in countries such as the United States and the United Kingdom, this figure may be higher.
“There’s chronic use of proton pump inhibitors in the population on a massive scale,” Dr. Weersma said.
To complicate matters, estimates suggest that 25%-70% of people who are taking PPIs have no appropriate indication. While this issue is partly because of increasing over-the-counter usage, physicians are also contributing to the problem by prescribing PPIs without adequate follow-up.
“The number of people using proton pump inhibitors is steadily increasing,” Dr. Weersma said. “The number of people getting them prescribed is relatively stable. The problem is, we never stop.”
According to Dr. Weersma, a growing body of research shows that PPI use may increase the risk of developing other conditions. Although many of these relationships are correlative, some are now widely accepted as causal. Most notable and clinically relevant, Dr. Weersma said, are enteric infections. Clostridioides difficile–associated diarrhea, for instance, is 65% more common among PPI users.
While the mechanisms behind this susceptibility to infection are uncertain, Dr. Weersma suggested that the most likely cause is “oralization” of the gut microbiome caused by loss of the acid barrier, which introduces upper gastrointestinal bacteria, or oral bacteria, into the lower intestines.
Perhaps more relevant to clinical trials, PPIs may also influence the safety and efficacy of drugs.
“There is a lot of interaction between the gut microbiome and a lot of drugs,” Dr. Weersma said at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility. “We really don’t know a lot about this at the moment.”
He went on to explain that bidirectional interactions between drugs and the microbiome may actually present clinical opportunities.
“This is a field that people currently call pharmacomicrobiomics,” Dr. Weersma said. “This is very intriguing, of course, because everyone knows about pharmacogenomics ... which lets you stratify your patients, but you cannot intervene; you cannot change your genetic background to increase efficacy or avoid toxicity. But in fact, with the microbiome, we could modulate the microbiome and improve bioavailability, for example.”
Conversely, Dr. Weersma pointed out that PPI use may be interfering with drug efficacy to a life-altering degree.
He cited a recent study by Chalabi and colleagues, which found that PPI use affected responses to immune checkpoint inhibitors (Ann Oncol. 2020 Jan 16. doi: 10.1016/j.annonc.2020.01.006). Among 169 patients with lung cancer who were treated with atezolizumab, overall survival was significantly lower in PPI users (9.6 vs. 14.5 months; P = .001).
A number of other clinical implications are also possible, Dr. Weersma said, although these require further investigation. For example, a 2019 study by Stark and colleagues suggested that childhood use of PPIs may increase obesity risk.
“[There are] no microbiome data here,” Dr. Weersma said, “but it makes you think.”
While considering the downsides of PPIs, Dr. Weersma also emphasized their importance in clinical practice. “[Proton pump inhibitors] are very great drugs. They are cheap, they are safe, they are very effective. So if you have evidence-based indications to use proton pump inhibitors, you should definitely use them and not stop them.”
Dr. Weersma called for responsible use of PPIs, and suggested that clinicians need to prepare for pushback from patients, who, after stopping PPIs, may experience a temporary resurgence of symptoms because of acid rebound.
“You have to make them aware [of acid rebound],” Dr. Weersma said. “Say: ‘Wait 2 or 3 weeks and this rebound is gone.’ We should say that way, way, way more often.”
But clinicians shouldn’t bear the burden of responsible usage alone, Dr. Weersma said.
“There’s a role for clinicians, patients, and regulatory bodies also, to think about the massive use of proton pump inhibitors now and in the future.”
In the discussion that followed the presentation, a summit attendee brought up the realities of clinical practice before PPIs, when patients frequently had gastrointestinal bleeding secondary to nonsteroidal anti-inflammatory use. In response, Dr. Weersma again emphasized that PPIs play a critical role for many patients. After once more encouraging responsible use, Dr. Weersma expressed concern about the risks involved in conveying his message; not only to the medical community, but also to the general public.
“This is a very difficult message [to deliver],” Dr. Weersma said. “In the Netherlands this was taken up by the media and the news, so my email inbox exploded. It’s difficult to get this nuance right.”
Dr. Weersma disclosed relationships with Takeda, Johnson & Johnson, Ferring, and others.
Microbiome studies should be correcting statistics to account for proton pump inhibitor (PPI) use, according to a leading expert.
After antibiotics, PPIs are the leading cause of microbiome variance in both research and general populations, and these alterations could have a range of consequences, reported Rinse K. Weersma, MD, PhD, of the University of Groningen (the Netherlands).
About 20% of people are taking a PPI, Dr. Weersma said at the annual Gut Microbiota for Health World Summit, noting that, in countries such as the United States and the United Kingdom, this figure may be higher.
“There’s chronic use of proton pump inhibitors in the population on a massive scale,” Dr. Weersma said.
To complicate matters, estimates suggest that 25%-70% of people who are taking PPIs have no appropriate indication. While this issue is partly because of increasing over-the-counter usage, physicians are also contributing to the problem by prescribing PPIs without adequate follow-up.
“The number of people using proton pump inhibitors is steadily increasing,” Dr. Weersma said. “The number of people getting them prescribed is relatively stable. The problem is, we never stop.”
According to Dr. Weersma, a growing body of research shows that PPI use may increase the risk of developing other conditions. Although many of these relationships are correlative, some are now widely accepted as causal. Most notable and clinically relevant, Dr. Weersma said, are enteric infections. Clostridioides difficile–associated diarrhea, for instance, is 65% more common among PPI users.
While the mechanisms behind this susceptibility to infection are uncertain, Dr. Weersma suggested that the most likely cause is “oralization” of the gut microbiome caused by loss of the acid barrier, which introduces upper gastrointestinal bacteria, or oral bacteria, into the lower intestines.
Perhaps more relevant to clinical trials, PPIs may also influence the safety and efficacy of drugs.
“There is a lot of interaction between the gut microbiome and a lot of drugs,” Dr. Weersma said at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility. “We really don’t know a lot about this at the moment.”
He went on to explain that bidirectional interactions between drugs and the microbiome may actually present clinical opportunities.
“This is a field that people currently call pharmacomicrobiomics,” Dr. Weersma said. “This is very intriguing, of course, because everyone knows about pharmacogenomics ... which lets you stratify your patients, but you cannot intervene; you cannot change your genetic background to increase efficacy or avoid toxicity. But in fact, with the microbiome, we could modulate the microbiome and improve bioavailability, for example.”
Conversely, Dr. Weersma pointed out that PPI use may be interfering with drug efficacy to a life-altering degree.
He cited a recent study by Chalabi and colleagues, which found that PPI use affected responses to immune checkpoint inhibitors (Ann Oncol. 2020 Jan 16. doi: 10.1016/j.annonc.2020.01.006). Among 169 patients with lung cancer who were treated with atezolizumab, overall survival was significantly lower in PPI users (9.6 vs. 14.5 months; P = .001).
A number of other clinical implications are also possible, Dr. Weersma said, although these require further investigation. For example, a 2019 study by Stark and colleagues suggested that childhood use of PPIs may increase obesity risk.
“[There are] no microbiome data here,” Dr. Weersma said, “but it makes you think.”
While considering the downsides of PPIs, Dr. Weersma also emphasized their importance in clinical practice. “[Proton pump inhibitors] are very great drugs. They are cheap, they are safe, they are very effective. So if you have evidence-based indications to use proton pump inhibitors, you should definitely use them and not stop them.”
Dr. Weersma called for responsible use of PPIs, and suggested that clinicians need to prepare for pushback from patients, who, after stopping PPIs, may experience a temporary resurgence of symptoms because of acid rebound.
“You have to make them aware [of acid rebound],” Dr. Weersma said. “Say: ‘Wait 2 or 3 weeks and this rebound is gone.’ We should say that way, way, way more often.”
But clinicians shouldn’t bear the burden of responsible usage alone, Dr. Weersma said.
“There’s a role for clinicians, patients, and regulatory bodies also, to think about the massive use of proton pump inhibitors now and in the future.”
In the discussion that followed the presentation, a summit attendee brought up the realities of clinical practice before PPIs, when patients frequently had gastrointestinal bleeding secondary to nonsteroidal anti-inflammatory use. In response, Dr. Weersma again emphasized that PPIs play a critical role for many patients. After once more encouraging responsible use, Dr. Weersma expressed concern about the risks involved in conveying his message; not only to the medical community, but also to the general public.
“This is a very difficult message [to deliver],” Dr. Weersma said. “In the Netherlands this was taken up by the media and the news, so my email inbox exploded. It’s difficult to get this nuance right.”
Dr. Weersma disclosed relationships with Takeda, Johnson & Johnson, Ferring, and others.
EXPERT ANALYSIS FROM GMFH 2020
GERD symptoms affect one in three Americans
For most patients, proton pump inhibitors do not control symptoms of gastroesophageal reflux disease, according to the findings of a large population-based survey study.
In all, 31% of respondents reported gastroesophageal reflux disease (GERD) symptoms within the past week, and 54% of those on proton pump inhibitors (PPIs) had breakthrough symptoms, said Sean D. Delshad, MD, MBA. In all, 54% of patients on PPIs for GERD reported having breakthrough symptoms of heartburn or regurgitation. Novel treatments are needed for patients with PPI-refractory symptoms of GERD, he and his associates wrote in Gastroenterology.
Prior population-based U.S. studies have reported a lower prevalence (16%-28%) of weekly or monthly GERD symptoms, noted Dr. Delshad of the Cedars-Sinai Center for Outcomes Research and Education in Los Angeles. However, the study cohorts do not reflect current U.S. demographics — two were 82%-90% white and the third was 43% African American. The most recent data also were collected approximately 15 years ago, the researchers noted.
For the study, they deployed a mobile app that guides users through an automated, online assessment of GI symptoms called AEGIS. Respondents were asked to select any GERD symptoms they had ever experienced and any symptoms they had experienced in the past week. Options included heartburn, acid reflux, gastroesophageal reflux, abdominal pain, bloating or gas, constipation, diarrhea, disrupted swallowing, fecal incontinence, nausea and vomiting, and “no symptoms.” All 71,812 respondents were recruited by a research firm and surveyed during a 3-week period in 2015.
In all, 44% of respondents reported having ever had heartburn, acid reflux, or gastroesophageal reflux, and 31% reported having GERD symptoms in the past week. In all, 55% of respondents who had ever experienced GERD symptoms were on PPIs, 24% were on histamine2 receptor blockers, and 24% were on antacid agents.
Among more than 3,000 participants on daily PPIs, 54% had persistent symptoms of GERD, which compares with the results of prior community-based studies, the investigators wrote. Current GERD symptoms and PPI-refractory GERD were especially prevalent among women, non-Hispanic whites, and individuals with comorbidities such as irritable bowel syndrome, diabetes, Crohn’s disease, and endometriosis.
In an adjusted analysis, Latinos were 2.44 times more likely to have PPI-refractory GERD ,compared with non-Hispanic whites. “The reason behind this finding is unclear but may be secondary to physiologic or even cultural etiologies,” the researchers wrote.
The more independent and functional middle-aged and older adults are more likely to respond to online surveys. Furthermore, although incentives were used to reduce participation bias, calling the tool a “GI Survey” could have made those with GI symptoms more likely to respond. The survey also did not assess if respondents were taking PPIs correctly or if they had made behavioral changes to mitigate GERD.
This study was sponsored by Ironwood Pharmaceuticals, whose bile acid sequestrant IW-3718 is in late-phase development as an add-on to PPI therapy for patients with persistent GERD. Dr. Delshad reported having no relevant conflicts of interest, but two coinvestigators disclosed consulting relationships with Ironwood Pharmaceuticals.
SOURCE: Delshad SD et al. Gastroenterology. 2019 Dec 10. doi: 10.1053/j.gastro.2019.12.014.
Heartburn is a common symptom and is ubiquitously attributed to gastroesophageal reflux disease (GERD) among patients and clinicians. However, it is important to note that although most patients with GERD do have heartburn and/or regurgitation, many patients with these symptoms do not have GERD.
When the authors used a more precise definition of GERD based on the modified Montreal classification, they found that only 18% of the study population met the criteria for the disease. This is similar to prevalence of GERD reported in North America by other studies. The authors also found that, among patients on daily proton pump inhibitors (PPIs), 54% still reported persistent reflux symptoms.
Although this highlights the need for future research into developing other therapeutic modalities for GERD (such as bile acid sequestrants), most of the patients that are “PPI refractory” have lack of response because of a functional esophageal disorder. This is highlighted by the similar risk factors for functional heartburn and the PPI-refractory group in this study: younger individuals, women, and participants with irritable bowel syndrome.
Dhyanesh A. Patel, MD, is an assistant professor of medicine at the Center for Esophageal Disorders, Vanderbilt University Medical Center, Nashville, Tenn. He reported that he has no conflicts of interest.
Heartburn is a common symptom and is ubiquitously attributed to gastroesophageal reflux disease (GERD) among patients and clinicians. However, it is important to note that although most patients with GERD do have heartburn and/or regurgitation, many patients with these symptoms do not have GERD.
When the authors used a more precise definition of GERD based on the modified Montreal classification, they found that only 18% of the study population met the criteria for the disease. This is similar to prevalence of GERD reported in North America by other studies. The authors also found that, among patients on daily proton pump inhibitors (PPIs), 54% still reported persistent reflux symptoms.
Although this highlights the need for future research into developing other therapeutic modalities for GERD (such as bile acid sequestrants), most of the patients that are “PPI refractory” have lack of response because of a functional esophageal disorder. This is highlighted by the similar risk factors for functional heartburn and the PPI-refractory group in this study: younger individuals, women, and participants with irritable bowel syndrome.
Dhyanesh A. Patel, MD, is an assistant professor of medicine at the Center for Esophageal Disorders, Vanderbilt University Medical Center, Nashville, Tenn. He reported that he has no conflicts of interest.
Heartburn is a common symptom and is ubiquitously attributed to gastroesophageal reflux disease (GERD) among patients and clinicians. However, it is important to note that although most patients with GERD do have heartburn and/or regurgitation, many patients with these symptoms do not have GERD.
When the authors used a more precise definition of GERD based on the modified Montreal classification, they found that only 18% of the study population met the criteria for the disease. This is similar to prevalence of GERD reported in North America by other studies. The authors also found that, among patients on daily proton pump inhibitors (PPIs), 54% still reported persistent reflux symptoms.
Although this highlights the need for future research into developing other therapeutic modalities for GERD (such as bile acid sequestrants), most of the patients that are “PPI refractory” have lack of response because of a functional esophageal disorder. This is highlighted by the similar risk factors for functional heartburn and the PPI-refractory group in this study: younger individuals, women, and participants with irritable bowel syndrome.
Dhyanesh A. Patel, MD, is an assistant professor of medicine at the Center for Esophageal Disorders, Vanderbilt University Medical Center, Nashville, Tenn. He reported that he has no conflicts of interest.
For most patients, proton pump inhibitors do not control symptoms of gastroesophageal reflux disease, according to the findings of a large population-based survey study.
In all, 31% of respondents reported gastroesophageal reflux disease (GERD) symptoms within the past week, and 54% of those on proton pump inhibitors (PPIs) had breakthrough symptoms, said Sean D. Delshad, MD, MBA. In all, 54% of patients on PPIs for GERD reported having breakthrough symptoms of heartburn or regurgitation. Novel treatments are needed for patients with PPI-refractory symptoms of GERD, he and his associates wrote in Gastroenterology.
Prior population-based U.S. studies have reported a lower prevalence (16%-28%) of weekly or monthly GERD symptoms, noted Dr. Delshad of the Cedars-Sinai Center for Outcomes Research and Education in Los Angeles. However, the study cohorts do not reflect current U.S. demographics — two were 82%-90% white and the third was 43% African American. The most recent data also were collected approximately 15 years ago, the researchers noted.
For the study, they deployed a mobile app that guides users through an automated, online assessment of GI symptoms called AEGIS. Respondents were asked to select any GERD symptoms they had ever experienced and any symptoms they had experienced in the past week. Options included heartburn, acid reflux, gastroesophageal reflux, abdominal pain, bloating or gas, constipation, diarrhea, disrupted swallowing, fecal incontinence, nausea and vomiting, and “no symptoms.” All 71,812 respondents were recruited by a research firm and surveyed during a 3-week period in 2015.
In all, 44% of respondents reported having ever had heartburn, acid reflux, or gastroesophageal reflux, and 31% reported having GERD symptoms in the past week. In all, 55% of respondents who had ever experienced GERD symptoms were on PPIs, 24% were on histamine2 receptor blockers, and 24% were on antacid agents.
Among more than 3,000 participants on daily PPIs, 54% had persistent symptoms of GERD, which compares with the results of prior community-based studies, the investigators wrote. Current GERD symptoms and PPI-refractory GERD were especially prevalent among women, non-Hispanic whites, and individuals with comorbidities such as irritable bowel syndrome, diabetes, Crohn’s disease, and endometriosis.
In an adjusted analysis, Latinos were 2.44 times more likely to have PPI-refractory GERD ,compared with non-Hispanic whites. “The reason behind this finding is unclear but may be secondary to physiologic or even cultural etiologies,” the researchers wrote.
The more independent and functional middle-aged and older adults are more likely to respond to online surveys. Furthermore, although incentives were used to reduce participation bias, calling the tool a “GI Survey” could have made those with GI symptoms more likely to respond. The survey also did not assess if respondents were taking PPIs correctly or if they had made behavioral changes to mitigate GERD.
This study was sponsored by Ironwood Pharmaceuticals, whose bile acid sequestrant IW-3718 is in late-phase development as an add-on to PPI therapy for patients with persistent GERD. Dr. Delshad reported having no relevant conflicts of interest, but two coinvestigators disclosed consulting relationships with Ironwood Pharmaceuticals.
SOURCE: Delshad SD et al. Gastroenterology. 2019 Dec 10. doi: 10.1053/j.gastro.2019.12.014.
For most patients, proton pump inhibitors do not control symptoms of gastroesophageal reflux disease, according to the findings of a large population-based survey study.
In all, 31% of respondents reported gastroesophageal reflux disease (GERD) symptoms within the past week, and 54% of those on proton pump inhibitors (PPIs) had breakthrough symptoms, said Sean D. Delshad, MD, MBA. In all, 54% of patients on PPIs for GERD reported having breakthrough symptoms of heartburn or regurgitation. Novel treatments are needed for patients with PPI-refractory symptoms of GERD, he and his associates wrote in Gastroenterology.
Prior population-based U.S. studies have reported a lower prevalence (16%-28%) of weekly or monthly GERD symptoms, noted Dr. Delshad of the Cedars-Sinai Center for Outcomes Research and Education in Los Angeles. However, the study cohorts do not reflect current U.S. demographics — two were 82%-90% white and the third was 43% African American. The most recent data also were collected approximately 15 years ago, the researchers noted.
For the study, they deployed a mobile app that guides users through an automated, online assessment of GI symptoms called AEGIS. Respondents were asked to select any GERD symptoms they had ever experienced and any symptoms they had experienced in the past week. Options included heartburn, acid reflux, gastroesophageal reflux, abdominal pain, bloating or gas, constipation, diarrhea, disrupted swallowing, fecal incontinence, nausea and vomiting, and “no symptoms.” All 71,812 respondents were recruited by a research firm and surveyed during a 3-week period in 2015.
In all, 44% of respondents reported having ever had heartburn, acid reflux, or gastroesophageal reflux, and 31% reported having GERD symptoms in the past week. In all, 55% of respondents who had ever experienced GERD symptoms were on PPIs, 24% were on histamine2 receptor blockers, and 24% were on antacid agents.
Among more than 3,000 participants on daily PPIs, 54% had persistent symptoms of GERD, which compares with the results of prior community-based studies, the investigators wrote. Current GERD symptoms and PPI-refractory GERD were especially prevalent among women, non-Hispanic whites, and individuals with comorbidities such as irritable bowel syndrome, diabetes, Crohn’s disease, and endometriosis.
In an adjusted analysis, Latinos were 2.44 times more likely to have PPI-refractory GERD ,compared with non-Hispanic whites. “The reason behind this finding is unclear but may be secondary to physiologic or even cultural etiologies,” the researchers wrote.
The more independent and functional middle-aged and older adults are more likely to respond to online surveys. Furthermore, although incentives were used to reduce participation bias, calling the tool a “GI Survey” could have made those with GI symptoms more likely to respond. The survey also did not assess if respondents were taking PPIs correctly or if they had made behavioral changes to mitigate GERD.
This study was sponsored by Ironwood Pharmaceuticals, whose bile acid sequestrant IW-3718 is in late-phase development as an add-on to PPI therapy for patients with persistent GERD. Dr. Delshad reported having no relevant conflicts of interest, but two coinvestigators disclosed consulting relationships with Ironwood Pharmaceuticals.
SOURCE: Delshad SD et al. Gastroenterology. 2019 Dec 10. doi: 10.1053/j.gastro.2019.12.014.
FROM GASTROENTEROLOGY
COVID-19 update: Transmission 5% or less among close contacts
The transmission rate of coronavirus disease 2019 (COVID-19) was 1%-5% among 38,000 Chinese people in close contact with infected patients, according to the chief epidemiologist of the Chinese Centers for Disease Control and Prevention, Beijing, Zunyou Wu, MD, PhD, who gave an update on the epidemic at the Conference on Retroviruses & Opportunistic Infections.
The rate of spread to family members – the driver of the infection in China – was 10% early in the outbreak, but fell to 3% with quicker recognition and isolation. The overall numbers are lower than might have been expected, and an important insight for clinicians trying to contain the outbreak in the United States.
, but their ability to spread the infection dropped after that, Dr. Wu and others said at a special COVID-19 session at the meeting, which was scheduled to be in Boston, but was held online instead because of concerns about spreading the virus. The session has been posted.
Transmission from presymptomatic people is rare. Shedding persists to some degree for 7-12 days in mild/moderate cases, but 2 weeks or more in severe cases.
Dr. Wu said the numbers in China are moving in the right direction, which means that containment efforts there have worked.
The virus emerged in Wuhan, the capital of Hubei province in central China, in connection with a wildlife food market in December 2019. Bats are thought to be the reservoir, with perhaps an intermediate step between civet cats and raccoon dogs. Officials shut down the market.
Essentially, the entire population of China, more than a billion people, was told to stay home for 10 days to interrupt the transmission cycle after the virus spread throughout the country in a few weeks, and almost 60 million people in Hubei were put behind a cordon sanitaire, where they have been for 50 days and will remain “for a while,” Dr. Wu said.
It’s led to a steep drop in new cases and deaths in China since mid-February; both are now more common outside China than inside, and international numbers are lower than they were at the peak in China.
Meanwhile, there’s been no evidence of perinatal transmission; the virus has not been detected in amniotic fluid, cord blood, neonatal throat swabs, or breast milk. Maternal morbidity appears to be similar to uninfected women. “The data around pregnancy are reassuring,” said John Brooks, MD, chief medical officers for HIV/AIDS prevention at the Centers for Disease Control and Prevention, Atlanta, who has been involved with CDC’s containment efforts.
There’s no data yet for immunocompromised people, but for people with HIV, he said, “we think the risk of severe illness would be greater” with lower CD4 counts and unsuppressed viral loads. “People living with HIV should take precautions against this new virus,” including having at least a 30-day supply of HIV medications; keeping up flu and pneumonia vaccinations; and having a care plan if quarantined. Setting up telemedicine might be a good idea.
The usual incubation period for COVID-19 is 4-6 days but can be longer. Recovery time is about 2 weeks in mild cases and 3-6 weeks in more severe cases. People who die do so within 2 months of symptom onset.
The most common symptoms among hospitalized patients in China are fever, dry cough, fatigue, and headache. Truly asymptomatic cases are not common; most go on to develop symptoms. There have been reports of diarrhea before other symptoms by a day or two, but it’s probably a red herring. The virus has been isolated from stool, but there is no evidence of fecal-oral transmission, Dr. Wu said.
Eighty percent of COVID-19 cases are mild or moderate and most patients recover spontaneously, especially middle aged and younger people. There is no meaningful difference in distribution between the sexes.
There are limited pediatric data perhaps due to underreporting, “but we know [children] experience milder illness than adults,” the CDC’s Dr. Brooks said.
He pegged the latest case fatality estimate at 0.5% to 3.5%, which is considerably higher than seasonal flu, but might well drop as more mild cases are detected and added to the denominator, he said.
For now, death rates top 5% in adults over 60 years old and climb further with increasing age, approaching 16% in people 80 years or older. Patients with hypertension, diabetes, cardiovascular disease, and chronic respiratory illness are at increased risk. The ultimate cause of death is acute respiratory distress syndrome, said Ralph Baric, PhD, a coronavirus expert and epidemiology professor at the University of North Carolina, Chapel Hill, who also presented at the meeting.
Several drug and vaccine candidates are under study for the infection. An intriguing possibility is that angiotensin converting enzyme (ACE) inhibitors might help. Hypertension is a known risk factor for severe infection; the virus makes use of ACE receptor pathways to infect airway epithelial cells; and there have been reports of ACE inhibitors having effect against the virus that caused severe acute respiratory syndrome (SARS), another coronavirus outbreak in 2003.
“I think it’s a very good idea to go back and re-explore use of these drugs,” Dr. Baric said.
The presenters didn’t have any relevant disclosures.
The transmission rate of coronavirus disease 2019 (COVID-19) was 1%-5% among 38,000 Chinese people in close contact with infected patients, according to the chief epidemiologist of the Chinese Centers for Disease Control and Prevention, Beijing, Zunyou Wu, MD, PhD, who gave an update on the epidemic at the Conference on Retroviruses & Opportunistic Infections.
The rate of spread to family members – the driver of the infection in China – was 10% early in the outbreak, but fell to 3% with quicker recognition and isolation. The overall numbers are lower than might have been expected, and an important insight for clinicians trying to contain the outbreak in the United States.
, but their ability to spread the infection dropped after that, Dr. Wu and others said at a special COVID-19 session at the meeting, which was scheduled to be in Boston, but was held online instead because of concerns about spreading the virus. The session has been posted.
Transmission from presymptomatic people is rare. Shedding persists to some degree for 7-12 days in mild/moderate cases, but 2 weeks or more in severe cases.
Dr. Wu said the numbers in China are moving in the right direction, which means that containment efforts there have worked.
The virus emerged in Wuhan, the capital of Hubei province in central China, in connection with a wildlife food market in December 2019. Bats are thought to be the reservoir, with perhaps an intermediate step between civet cats and raccoon dogs. Officials shut down the market.
Essentially, the entire population of China, more than a billion people, was told to stay home for 10 days to interrupt the transmission cycle after the virus spread throughout the country in a few weeks, and almost 60 million people in Hubei were put behind a cordon sanitaire, where they have been for 50 days and will remain “for a while,” Dr. Wu said.
It’s led to a steep drop in new cases and deaths in China since mid-February; both are now more common outside China than inside, and international numbers are lower than they were at the peak in China.
Meanwhile, there’s been no evidence of perinatal transmission; the virus has not been detected in amniotic fluid, cord blood, neonatal throat swabs, or breast milk. Maternal morbidity appears to be similar to uninfected women. “The data around pregnancy are reassuring,” said John Brooks, MD, chief medical officers for HIV/AIDS prevention at the Centers for Disease Control and Prevention, Atlanta, who has been involved with CDC’s containment efforts.
There’s no data yet for immunocompromised people, but for people with HIV, he said, “we think the risk of severe illness would be greater” with lower CD4 counts and unsuppressed viral loads. “People living with HIV should take precautions against this new virus,” including having at least a 30-day supply of HIV medications; keeping up flu and pneumonia vaccinations; and having a care plan if quarantined. Setting up telemedicine might be a good idea.
The usual incubation period for COVID-19 is 4-6 days but can be longer. Recovery time is about 2 weeks in mild cases and 3-6 weeks in more severe cases. People who die do so within 2 months of symptom onset.
The most common symptoms among hospitalized patients in China are fever, dry cough, fatigue, and headache. Truly asymptomatic cases are not common; most go on to develop symptoms. There have been reports of diarrhea before other symptoms by a day or two, but it’s probably a red herring. The virus has been isolated from stool, but there is no evidence of fecal-oral transmission, Dr. Wu said.
Eighty percent of COVID-19 cases are mild or moderate and most patients recover spontaneously, especially middle aged and younger people. There is no meaningful difference in distribution between the sexes.
There are limited pediatric data perhaps due to underreporting, “but we know [children] experience milder illness than adults,” the CDC’s Dr. Brooks said.
He pegged the latest case fatality estimate at 0.5% to 3.5%, which is considerably higher than seasonal flu, but might well drop as more mild cases are detected and added to the denominator, he said.
For now, death rates top 5% in adults over 60 years old and climb further with increasing age, approaching 16% in people 80 years or older. Patients with hypertension, diabetes, cardiovascular disease, and chronic respiratory illness are at increased risk. The ultimate cause of death is acute respiratory distress syndrome, said Ralph Baric, PhD, a coronavirus expert and epidemiology professor at the University of North Carolina, Chapel Hill, who also presented at the meeting.
Several drug and vaccine candidates are under study for the infection. An intriguing possibility is that angiotensin converting enzyme (ACE) inhibitors might help. Hypertension is a known risk factor for severe infection; the virus makes use of ACE receptor pathways to infect airway epithelial cells; and there have been reports of ACE inhibitors having effect against the virus that caused severe acute respiratory syndrome (SARS), another coronavirus outbreak in 2003.
“I think it’s a very good idea to go back and re-explore use of these drugs,” Dr. Baric said.
The presenters didn’t have any relevant disclosures.
The transmission rate of coronavirus disease 2019 (COVID-19) was 1%-5% among 38,000 Chinese people in close contact with infected patients, according to the chief epidemiologist of the Chinese Centers for Disease Control and Prevention, Beijing, Zunyou Wu, MD, PhD, who gave an update on the epidemic at the Conference on Retroviruses & Opportunistic Infections.
The rate of spread to family members – the driver of the infection in China – was 10% early in the outbreak, but fell to 3% with quicker recognition and isolation. The overall numbers are lower than might have been expected, and an important insight for clinicians trying to contain the outbreak in the United States.
, but their ability to spread the infection dropped after that, Dr. Wu and others said at a special COVID-19 session at the meeting, which was scheduled to be in Boston, but was held online instead because of concerns about spreading the virus. The session has been posted.
Transmission from presymptomatic people is rare. Shedding persists to some degree for 7-12 days in mild/moderate cases, but 2 weeks or more in severe cases.
Dr. Wu said the numbers in China are moving in the right direction, which means that containment efforts there have worked.
The virus emerged in Wuhan, the capital of Hubei province in central China, in connection with a wildlife food market in December 2019. Bats are thought to be the reservoir, with perhaps an intermediate step between civet cats and raccoon dogs. Officials shut down the market.
Essentially, the entire population of China, more than a billion people, was told to stay home for 10 days to interrupt the transmission cycle after the virus spread throughout the country in a few weeks, and almost 60 million people in Hubei were put behind a cordon sanitaire, where they have been for 50 days and will remain “for a while,” Dr. Wu said.
It’s led to a steep drop in new cases and deaths in China since mid-February; both are now more common outside China than inside, and international numbers are lower than they were at the peak in China.
Meanwhile, there’s been no evidence of perinatal transmission; the virus has not been detected in amniotic fluid, cord blood, neonatal throat swabs, or breast milk. Maternal morbidity appears to be similar to uninfected women. “The data around pregnancy are reassuring,” said John Brooks, MD, chief medical officers for HIV/AIDS prevention at the Centers for Disease Control and Prevention, Atlanta, who has been involved with CDC’s containment efforts.
There’s no data yet for immunocompromised people, but for people with HIV, he said, “we think the risk of severe illness would be greater” with lower CD4 counts and unsuppressed viral loads. “People living with HIV should take precautions against this new virus,” including having at least a 30-day supply of HIV medications; keeping up flu and pneumonia vaccinations; and having a care plan if quarantined. Setting up telemedicine might be a good idea.
The usual incubation period for COVID-19 is 4-6 days but can be longer. Recovery time is about 2 weeks in mild cases and 3-6 weeks in more severe cases. People who die do so within 2 months of symptom onset.
The most common symptoms among hospitalized patients in China are fever, dry cough, fatigue, and headache. Truly asymptomatic cases are not common; most go on to develop symptoms. There have been reports of diarrhea before other symptoms by a day or two, but it’s probably a red herring. The virus has been isolated from stool, but there is no evidence of fecal-oral transmission, Dr. Wu said.
Eighty percent of COVID-19 cases are mild or moderate and most patients recover spontaneously, especially middle aged and younger people. There is no meaningful difference in distribution between the sexes.
There are limited pediatric data perhaps due to underreporting, “but we know [children] experience milder illness than adults,” the CDC’s Dr. Brooks said.
He pegged the latest case fatality estimate at 0.5% to 3.5%, which is considerably higher than seasonal flu, but might well drop as more mild cases are detected and added to the denominator, he said.
For now, death rates top 5% in adults over 60 years old and climb further with increasing age, approaching 16% in people 80 years or older. Patients with hypertension, diabetes, cardiovascular disease, and chronic respiratory illness are at increased risk. The ultimate cause of death is acute respiratory distress syndrome, said Ralph Baric, PhD, a coronavirus expert and epidemiology professor at the University of North Carolina, Chapel Hill, who also presented at the meeting.
Several drug and vaccine candidates are under study for the infection. An intriguing possibility is that angiotensin converting enzyme (ACE) inhibitors might help. Hypertension is a known risk factor for severe infection; the virus makes use of ACE receptor pathways to infect airway epithelial cells; and there have been reports of ACE inhibitors having effect against the virus that caused severe acute respiratory syndrome (SARS), another coronavirus outbreak in 2003.
“I think it’s a very good idea to go back and re-explore use of these drugs,” Dr. Baric said.
The presenters didn’t have any relevant disclosures.
FROM CROI 2020
Some infected patients could show COVID-19 symptoms after quarantine
Although a 14-day quarantine after exposure to novel coronavirus is “well supported” by evidence, some infected individuals will not become symptomatic until after that period, according to authors of a recent analysis published in Annals of Internal Medicine.
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will develop symptoms by day 12 of the infection, which is within the 14-day period of active monitoring currently recommended by the Centers for Disease Control and Prevention, the authors wrote.
However, an estimated 101 out of 10,000 cases could become symptomatic after the end of that 14-day monitoring period, they cautioned.
“Our analyses do not preclude that estimate from being higher,” said the investigators, led by Stephen A. Lauer, PhD, MD, of Johns Hopkins Bloomberg School of Public Health, Baltimore.
The analysis, based on 181 confirmed cases of coronavirus disease 2019 (COVID-19) that were documented outside of the outbreak epicenter, Wuhan, China, makes “more conservative assumptions” about the window of symptom onset and potential for continued exposure, compared with analyses in previous studies, the researchers wrote.
The estimated incubation period for SARS-CoV-2 in the 181-patient study was a median of 5.1 days, which is comparable with previous estimates based on COVID-19 cases outside of Wuhan and consistent with other known human coronavirus diseases, such as SARS, which had a reported mean incubation period of 5 days, Dr. Lauer and colleagues noted.
Symptoms developed within 11.5 days for 97.5% of patients in the study.
Whether it’s acceptable to have 101 out of 10,000 cases becoming symptomatic beyond the recommended quarantine window depends on two factors, according to the authors. The first is the expected infection risk in the population that is being monitored, and the second is “judgment about the cost of missing cases,” wrote the authors.
In an interview, Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau, Oceanside, N.Y., said that in practical terms, the results suggest that the majority of patients with COVID-19 will be identified within 14 days, with an “outside chance” of an infected individual leaving quarantine and transmitting virus for a short period of time before becoming symptomatic.
“I think the proper message to give those patients [who are asymptomatic upon leaving quarantine] is, ‘after 14 days, we’re pretty sure you’re out of the woods, but should you get any symptoms, immediately requarantine yourself and seek medical care,” he said.
Study coauthor Kyra H. Grantz, a doctoral graduate student at the Johns Hopkins Bloomberg School of Public Health, said that extending a quarantine beyond 14 days might be considered in the highest-risk scenarios, though the benefits of doing so would have to be weighed against the costs to public health and to the individuals under quarantine.
“Our estimate of the incubation period definitely supports the 14-day recommendation that the CDC has been using,” she said in an interview.
Dr. Grantz emphasized that the estimate of 101 out of 10,000 cases developing symptoms after day 14 of active monitoring – representing the 99th percentile of cases – assumes the “most conservative, worst-case scenario” in a population that is fully infected.
“If you’re looking at a following a cohort of 1,000 people whom you think may have been exposed, only a certain percentage will be infected, and only a certain percentage of those will even develop symptoms – before we get to this idea of how many people would we miss,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
SOURCE: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi:10.1101/2020.02.02.20020016.
Although a 14-day quarantine after exposure to novel coronavirus is “well supported” by evidence, some infected individuals will not become symptomatic until after that period, according to authors of a recent analysis published in Annals of Internal Medicine.
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will develop symptoms by day 12 of the infection, which is within the 14-day period of active monitoring currently recommended by the Centers for Disease Control and Prevention, the authors wrote.
However, an estimated 101 out of 10,000 cases could become symptomatic after the end of that 14-day monitoring period, they cautioned.
“Our analyses do not preclude that estimate from being higher,” said the investigators, led by Stephen A. Lauer, PhD, MD, of Johns Hopkins Bloomberg School of Public Health, Baltimore.
The analysis, based on 181 confirmed cases of coronavirus disease 2019 (COVID-19) that were documented outside of the outbreak epicenter, Wuhan, China, makes “more conservative assumptions” about the window of symptom onset and potential for continued exposure, compared with analyses in previous studies, the researchers wrote.
The estimated incubation period for SARS-CoV-2 in the 181-patient study was a median of 5.1 days, which is comparable with previous estimates based on COVID-19 cases outside of Wuhan and consistent with other known human coronavirus diseases, such as SARS, which had a reported mean incubation period of 5 days, Dr. Lauer and colleagues noted.
Symptoms developed within 11.5 days for 97.5% of patients in the study.
Whether it’s acceptable to have 101 out of 10,000 cases becoming symptomatic beyond the recommended quarantine window depends on two factors, according to the authors. The first is the expected infection risk in the population that is being monitored, and the second is “judgment about the cost of missing cases,” wrote the authors.
In an interview, Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau, Oceanside, N.Y., said that in practical terms, the results suggest that the majority of patients with COVID-19 will be identified within 14 days, with an “outside chance” of an infected individual leaving quarantine and transmitting virus for a short period of time before becoming symptomatic.
“I think the proper message to give those patients [who are asymptomatic upon leaving quarantine] is, ‘after 14 days, we’re pretty sure you’re out of the woods, but should you get any symptoms, immediately requarantine yourself and seek medical care,” he said.
Study coauthor Kyra H. Grantz, a doctoral graduate student at the Johns Hopkins Bloomberg School of Public Health, said that extending a quarantine beyond 14 days might be considered in the highest-risk scenarios, though the benefits of doing so would have to be weighed against the costs to public health and to the individuals under quarantine.
“Our estimate of the incubation period definitely supports the 14-day recommendation that the CDC has been using,” she said in an interview.
Dr. Grantz emphasized that the estimate of 101 out of 10,000 cases developing symptoms after day 14 of active monitoring – representing the 99th percentile of cases – assumes the “most conservative, worst-case scenario” in a population that is fully infected.
“If you’re looking at a following a cohort of 1,000 people whom you think may have been exposed, only a certain percentage will be infected, and only a certain percentage of those will even develop symptoms – before we get to this idea of how many people would we miss,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
SOURCE: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi:10.1101/2020.02.02.20020016.
Although a 14-day quarantine after exposure to novel coronavirus is “well supported” by evidence, some infected individuals will not become symptomatic until after that period, according to authors of a recent analysis published in Annals of Internal Medicine.
Most individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will develop symptoms by day 12 of the infection, which is within the 14-day period of active monitoring currently recommended by the Centers for Disease Control and Prevention, the authors wrote.
However, an estimated 101 out of 10,000 cases could become symptomatic after the end of that 14-day monitoring period, they cautioned.
“Our analyses do not preclude that estimate from being higher,” said the investigators, led by Stephen A. Lauer, PhD, MD, of Johns Hopkins Bloomberg School of Public Health, Baltimore.
The analysis, based on 181 confirmed cases of coronavirus disease 2019 (COVID-19) that were documented outside of the outbreak epicenter, Wuhan, China, makes “more conservative assumptions” about the window of symptom onset and potential for continued exposure, compared with analyses in previous studies, the researchers wrote.
The estimated incubation period for SARS-CoV-2 in the 181-patient study was a median of 5.1 days, which is comparable with previous estimates based on COVID-19 cases outside of Wuhan and consistent with other known human coronavirus diseases, such as SARS, which had a reported mean incubation period of 5 days, Dr. Lauer and colleagues noted.
Symptoms developed within 11.5 days for 97.5% of patients in the study.
Whether it’s acceptable to have 101 out of 10,000 cases becoming symptomatic beyond the recommended quarantine window depends on two factors, according to the authors. The first is the expected infection risk in the population that is being monitored, and the second is “judgment about the cost of missing cases,” wrote the authors.
In an interview, Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau, Oceanside, N.Y., said that in practical terms, the results suggest that the majority of patients with COVID-19 will be identified within 14 days, with an “outside chance” of an infected individual leaving quarantine and transmitting virus for a short period of time before becoming symptomatic.
“I think the proper message to give those patients [who are asymptomatic upon leaving quarantine] is, ‘after 14 days, we’re pretty sure you’re out of the woods, but should you get any symptoms, immediately requarantine yourself and seek medical care,” he said.
Study coauthor Kyra H. Grantz, a doctoral graduate student at the Johns Hopkins Bloomberg School of Public Health, said that extending a quarantine beyond 14 days might be considered in the highest-risk scenarios, though the benefits of doing so would have to be weighed against the costs to public health and to the individuals under quarantine.
“Our estimate of the incubation period definitely supports the 14-day recommendation that the CDC has been using,” she said in an interview.
Dr. Grantz emphasized that the estimate of 101 out of 10,000 cases developing symptoms after day 14 of active monitoring – representing the 99th percentile of cases – assumes the “most conservative, worst-case scenario” in a population that is fully infected.
“If you’re looking at a following a cohort of 1,000 people whom you think may have been exposed, only a certain percentage will be infected, and only a certain percentage of those will even develop symptoms – before we get to this idea of how many people would we miss,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
SOURCE: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi:10.1101/2020.02.02.20020016.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Some individuals who are infected with the novel coronavirus could become symptomatic after the active 14-day quarantine period.
Major finding: The median incubation period was 5.1 days, with 97.5% of patients developing symptoms within 11.5 days, implying that 101 of every 10,000 cases (99th percentile) would develop symptoms beyond the quarantine period.
Study details: Analysis of 181 confirmed COVID-19 cases identified outside of the outbreak epicenter, Wuhan, China.
Disclosures: The study was supported by the U.S. Centers for Disease Control and Prevention, the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, and the Alexander von Humboldt Foundation. Four authors reported disclosures related to those entities, and the remaining five reported no conflicts of interest.
Source: Lauer SA et al. Ann Intern Med. 2020 Mar 9. doi: 10.1101/2020.02.02.20020016.
CMS issues guidance on containing spread of coronavirus
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”









