User login
Case Studies in Toxicology: A Patchwork of Problems in Parkinson Patients
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
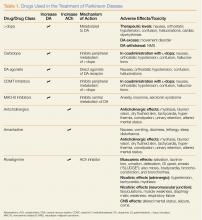
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
Case Report: Nasal Septal Abscess
Case
A 28-year-old woman with history of bipolar disorder and methamphetamine abuse presented to the ED complaining of nasal swelling and pain. She was unable to provide any medical history regarding the onset of her symptoms or other details, which the ED team attributed to her underlying psychiatric disorder. She denied nasal trauma, insufflation, or insertion of foreign bodies into the nasal cavity. When the patient’s mother was contacted, she stated her daughter’s symptoms, which she believed were secondary to a domestic-violence-related injury, had been present and evolving over the past 2 weeks. She also related that the patient had been treated at another ED 4 days earlier and discharged with oral antibiotics.
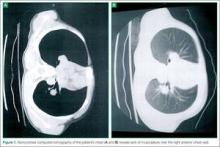
On physical examination, the bilateral nares were entirely occluded by soft-tissue swelling, with fluctuance on palpation. The area was erythematous, and there were pustules scattered throughout the local region (Figure 1). There was no evidence of spreading cellulitis. During the examination, the patient had a labile level of alertness that fluctuated between somnolence and agitation; however, she was arousable and had satisfactory airway guarding. Patient’s vital signs remained stable throughout evaluation and treatment in the ED. On physical examination, her pupils were equal bilaterally, extraocular movements were intact, and no neurological deficits were detected. A complete blood cell count showed leukocytosis, with a white blood cell count of 18,240/uL and a predominance (88.2%) of neutrophils. All other laboratory values were within normal limits.
Computed tomography (CT) of the face revealed prominent soft-tissue swelling involving the inferior portion of the nose (Figure 2). In addition to swelling and obstruction of the bilateral nares, heterogeneity was also noted within the affected tissues and thought to represent a fluid component.
After the procedure, the patient was admitted to the hospital for observation on the medical psychiatric unit where she received additional IV antibiotic therapy as well as a psychiatric consultation. After a 24-hour observation period, she was discharged on a one-week regimen of oral clindamycin and instructions for outpatient follow-up with OMFS for septal repair. Cultures taken during exploration were positive for pan-sensitive Staphylococcus aureus. The working diagnosis at discharge was bilateral septal abscess from untreated bilateral septal hematoma due to an unreported facial trauma.
Discussion
Nasal septal abscess, a rare complication of a nasal septal hematoma, is defined as a collection of pus between the cartilaginous or bony nasal septum and its normally applied mucoperichondrium or mucoperiosteum. Patients most commonly present with fluctuant, tender, bilateral, or unilateral nasal obstruction as a result of anterior nasal septum swelling. Other symptoms include localized pain, swelling, fever, headache, or perinasal tenderness.1 The external portion of the nose is swollen, erythematous, and tender, and the anterior nasal cavities are occluded by a smooth, round, deep red or grey swelling.2 In a review of pediatric patients with nasal septal abscess, the most common complaint was nasal congestion (95%). Other significant complaints were nasal pain (50%), fever (50%), and headache (5%).3,4
Nasal septal abscess is most commonly caused by a hematoma. Although trauma is typically associated with this condition, it is not the sole cause. Other etiology includes nasal surgery, a furuncle of the nasal vestibule, sinusitis, or, in rare cases, infection from a dental extraction.3
Staphylococcus aureus is the most common pathogen. Streptococcus and other anaerobes are less common, and pediatric patients are more susceptible to Haemophilus influenza than adults. Although rare, Psuedomonas and Klebsiella have also been reported.3
When nasal septal abscess is suspected, prior to drainage, the diagnosis should be confirmed by CT of the face and include the paranasal sinuses. Computed tomography is an excellent imaging tool for abscess detection and is the community standard for evaluation. Magnetic resonance imaging is not usually utilized (especially in the acute or ED setting) as it is unlikely to affect or alter initial management. In radiographs, nasal septal abscess typically appears as fluid collection with thin rim enhancement in the cartilaginous nasal septum5 (Figure 2). These findings can be missed on brain CT alone.5
In patients presenting several days from a related trauma, distinguishing uncomplicated septal hematoma from nasal septal abscess can be very difficult—though nasal septal abscesses tend to be larger and more painful. In addition, there may be inflammation of the overlying mucosa, occasionally with exudates. In untreated cases, infection can extend into the cavernous sinus causing intracranial infections or cavernous sinus thrombosis. The most common complication of septal abscess is cartilage necrosis that can result in nasal structural collapse and “saddle-nose” deformity. Complications, including meningitis, can develop quickly (ie, within 3 to 4 days).6
The structural complications associated with septal abscess result from the avascular nature of the septal cartilage, which receives blood from the adherent mucoperichondrium. Hematoma and abscess can expand and obstruct the blood vessels that supply the nasal cartilage. Pressure of the hematoma on the septum causes progressive avascular necrosis.6
Patients with confirmed nasal septal abscess should obtain otolaryngology or OMFS consultation in the ED. Due to the high risk of complications and need for follow up, immediate drainage should also be directed by otolaryngology or OMFS. All patients should be discharged on oral broad-spectrum antibiotics, with a referral to an otolaryngologist or OMFS within 24 hours for evaluation and possible removal of nasal packs.7
Dr Yusuf is an academic chief resident, John Peter Smith Emergency Medicine Residency Program, Fort Worth, Texas. Dr Kirk is associate residency director and ultrasound director, department of emergency medicine, John Peter Smith Health System, Fort Worth, Texas.
- Huang PH, Chiang YC, Yang TH, Chao PZ, Lee FP. Nasal septal abscess. Otolaryngol Head Neck Surg. 2006;135(2):335,336.
- Shapiro RS. Nasal septal abscess. Can Med Assoc J. 1978;119(11):1321-1323.
- Lo SH, Wang PC. Nasal septal abscess as a complication of laser inferior turbinectomy. Chang Gung Med J. 2004;27(5):390-393.
- Canty PA, Berkowitz RG. Hematoma and abscess of the nasal septum in children. Arch Otolaryngol Head Neck Surg. 1996;122(12):1373-1376.
- Debnam JM, Gillenwater AM, Ginsberg LE. Nasal septal abscess in patients with immunosuppression. Am J Neuroradiol. 2007;28(10):1878,1879.
- Friedman M, Landsberg R, Chiampas G. Nasal septal hematoma evacuation. In: Reichman EF, Simon RR, eds. Emergency Medicine Procedures. New York, NY: McGraw-Hill; 2004. http://www.accessemergencymedicine.com/content.aspx?aID=45644. Accessed March 20, 2014.
- Summers SM, Bey T. Epistaxis, nasal fractures, and rhinosinusitis. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011. http://www.accessemergencymedicine.com/content.aspx?aID=6388080. Accessed March 20, 2014.
Case
A 28-year-old woman with history of bipolar disorder and methamphetamine abuse presented to the ED complaining of nasal swelling and pain. She was unable to provide any medical history regarding the onset of her symptoms or other details, which the ED team attributed to her underlying psychiatric disorder. She denied nasal trauma, insufflation, or insertion of foreign bodies into the nasal cavity. When the patient’s mother was contacted, she stated her daughter’s symptoms, which she believed were secondary to a domestic-violence-related injury, had been present and evolving over the past 2 weeks. She also related that the patient had been treated at another ED 4 days earlier and discharged with oral antibiotics.
On physical examination, the bilateral nares were entirely occluded by soft-tissue swelling, with fluctuance on palpation. The area was erythematous, and there were pustules scattered throughout the local region (Figure 1). There was no evidence of spreading cellulitis. During the examination, the patient had a labile level of alertness that fluctuated between somnolence and agitation; however, she was arousable and had satisfactory airway guarding. Patient’s vital signs remained stable throughout evaluation and treatment in the ED. On physical examination, her pupils were equal bilaterally, extraocular movements were intact, and no neurological deficits were detected. A complete blood cell count showed leukocytosis, with a white blood cell count of 18,240/uL and a predominance (88.2%) of neutrophils. All other laboratory values were within normal limits.
Computed tomography (CT) of the face revealed prominent soft-tissue swelling involving the inferior portion of the nose (Figure 2). In addition to swelling and obstruction of the bilateral nares, heterogeneity was also noted within the affected tissues and thought to represent a fluid component.
After the procedure, the patient was admitted to the hospital for observation on the medical psychiatric unit where she received additional IV antibiotic therapy as well as a psychiatric consultation. After a 24-hour observation period, she was discharged on a one-week regimen of oral clindamycin and instructions for outpatient follow-up with OMFS for septal repair. Cultures taken during exploration were positive for pan-sensitive Staphylococcus aureus. The working diagnosis at discharge was bilateral septal abscess from untreated bilateral septal hematoma due to an unreported facial trauma.
Discussion
Nasal septal abscess, a rare complication of a nasal septal hematoma, is defined as a collection of pus between the cartilaginous or bony nasal septum and its normally applied mucoperichondrium or mucoperiosteum. Patients most commonly present with fluctuant, tender, bilateral, or unilateral nasal obstruction as a result of anterior nasal septum swelling. Other symptoms include localized pain, swelling, fever, headache, or perinasal tenderness.1 The external portion of the nose is swollen, erythematous, and tender, and the anterior nasal cavities are occluded by a smooth, round, deep red or grey swelling.2 In a review of pediatric patients with nasal septal abscess, the most common complaint was nasal congestion (95%). Other significant complaints were nasal pain (50%), fever (50%), and headache (5%).3,4
Nasal septal abscess is most commonly caused by a hematoma. Although trauma is typically associated with this condition, it is not the sole cause. Other etiology includes nasal surgery, a furuncle of the nasal vestibule, sinusitis, or, in rare cases, infection from a dental extraction.3
Staphylococcus aureus is the most common pathogen. Streptococcus and other anaerobes are less common, and pediatric patients are more susceptible to Haemophilus influenza than adults. Although rare, Psuedomonas and Klebsiella have also been reported.3
When nasal septal abscess is suspected, prior to drainage, the diagnosis should be confirmed by CT of the face and include the paranasal sinuses. Computed tomography is an excellent imaging tool for abscess detection and is the community standard for evaluation. Magnetic resonance imaging is not usually utilized (especially in the acute or ED setting) as it is unlikely to affect or alter initial management. In radiographs, nasal septal abscess typically appears as fluid collection with thin rim enhancement in the cartilaginous nasal septum5 (Figure 2). These findings can be missed on brain CT alone.5
In patients presenting several days from a related trauma, distinguishing uncomplicated septal hematoma from nasal septal abscess can be very difficult—though nasal septal abscesses tend to be larger and more painful. In addition, there may be inflammation of the overlying mucosa, occasionally with exudates. In untreated cases, infection can extend into the cavernous sinus causing intracranial infections or cavernous sinus thrombosis. The most common complication of septal abscess is cartilage necrosis that can result in nasal structural collapse and “saddle-nose” deformity. Complications, including meningitis, can develop quickly (ie, within 3 to 4 days).6
The structural complications associated with septal abscess result from the avascular nature of the septal cartilage, which receives blood from the adherent mucoperichondrium. Hematoma and abscess can expand and obstruct the blood vessels that supply the nasal cartilage. Pressure of the hematoma on the septum causes progressive avascular necrosis.6
Patients with confirmed nasal septal abscess should obtain otolaryngology or OMFS consultation in the ED. Due to the high risk of complications and need for follow up, immediate drainage should also be directed by otolaryngology or OMFS. All patients should be discharged on oral broad-spectrum antibiotics, with a referral to an otolaryngologist or OMFS within 24 hours for evaluation and possible removal of nasal packs.7
Dr Yusuf is an academic chief resident, John Peter Smith Emergency Medicine Residency Program, Fort Worth, Texas. Dr Kirk is associate residency director and ultrasound director, department of emergency medicine, John Peter Smith Health System, Fort Worth, Texas.
Case
A 28-year-old woman with history of bipolar disorder and methamphetamine abuse presented to the ED complaining of nasal swelling and pain. She was unable to provide any medical history regarding the onset of her symptoms or other details, which the ED team attributed to her underlying psychiatric disorder. She denied nasal trauma, insufflation, or insertion of foreign bodies into the nasal cavity. When the patient’s mother was contacted, she stated her daughter’s symptoms, which she believed were secondary to a domestic-violence-related injury, had been present and evolving over the past 2 weeks. She also related that the patient had been treated at another ED 4 days earlier and discharged with oral antibiotics.
On physical examination, the bilateral nares were entirely occluded by soft-tissue swelling, with fluctuance on palpation. The area was erythematous, and there were pustules scattered throughout the local region (Figure 1). There was no evidence of spreading cellulitis. During the examination, the patient had a labile level of alertness that fluctuated between somnolence and agitation; however, she was arousable and had satisfactory airway guarding. Patient’s vital signs remained stable throughout evaluation and treatment in the ED. On physical examination, her pupils were equal bilaterally, extraocular movements were intact, and no neurological deficits were detected. A complete blood cell count showed leukocytosis, with a white blood cell count of 18,240/uL and a predominance (88.2%) of neutrophils. All other laboratory values were within normal limits.
Computed tomography (CT) of the face revealed prominent soft-tissue swelling involving the inferior portion of the nose (Figure 2). In addition to swelling and obstruction of the bilateral nares, heterogeneity was also noted within the affected tissues and thought to represent a fluid component.
After the procedure, the patient was admitted to the hospital for observation on the medical psychiatric unit where she received additional IV antibiotic therapy as well as a psychiatric consultation. After a 24-hour observation period, she was discharged on a one-week regimen of oral clindamycin and instructions for outpatient follow-up with OMFS for septal repair. Cultures taken during exploration were positive for pan-sensitive Staphylococcus aureus. The working diagnosis at discharge was bilateral septal abscess from untreated bilateral septal hematoma due to an unreported facial trauma.
Discussion
Nasal septal abscess, a rare complication of a nasal septal hematoma, is defined as a collection of pus between the cartilaginous or bony nasal septum and its normally applied mucoperichondrium or mucoperiosteum. Patients most commonly present with fluctuant, tender, bilateral, or unilateral nasal obstruction as a result of anterior nasal septum swelling. Other symptoms include localized pain, swelling, fever, headache, or perinasal tenderness.1 The external portion of the nose is swollen, erythematous, and tender, and the anterior nasal cavities are occluded by a smooth, round, deep red or grey swelling.2 In a review of pediatric patients with nasal septal abscess, the most common complaint was nasal congestion (95%). Other significant complaints were nasal pain (50%), fever (50%), and headache (5%).3,4
Nasal septal abscess is most commonly caused by a hematoma. Although trauma is typically associated with this condition, it is not the sole cause. Other etiology includes nasal surgery, a furuncle of the nasal vestibule, sinusitis, or, in rare cases, infection from a dental extraction.3
Staphylococcus aureus is the most common pathogen. Streptococcus and other anaerobes are less common, and pediatric patients are more susceptible to Haemophilus influenza than adults. Although rare, Psuedomonas and Klebsiella have also been reported.3
When nasal septal abscess is suspected, prior to drainage, the diagnosis should be confirmed by CT of the face and include the paranasal sinuses. Computed tomography is an excellent imaging tool for abscess detection and is the community standard for evaluation. Magnetic resonance imaging is not usually utilized (especially in the acute or ED setting) as it is unlikely to affect or alter initial management. In radiographs, nasal septal abscess typically appears as fluid collection with thin rim enhancement in the cartilaginous nasal septum5 (Figure 2). These findings can be missed on brain CT alone.5
In patients presenting several days from a related trauma, distinguishing uncomplicated septal hematoma from nasal septal abscess can be very difficult—though nasal septal abscesses tend to be larger and more painful. In addition, there may be inflammation of the overlying mucosa, occasionally with exudates. In untreated cases, infection can extend into the cavernous sinus causing intracranial infections or cavernous sinus thrombosis. The most common complication of septal abscess is cartilage necrosis that can result in nasal structural collapse and “saddle-nose” deformity. Complications, including meningitis, can develop quickly (ie, within 3 to 4 days).6
The structural complications associated with septal abscess result from the avascular nature of the septal cartilage, which receives blood from the adherent mucoperichondrium. Hematoma and abscess can expand and obstruct the blood vessels that supply the nasal cartilage. Pressure of the hematoma on the septum causes progressive avascular necrosis.6
Patients with confirmed nasal septal abscess should obtain otolaryngology or OMFS consultation in the ED. Due to the high risk of complications and need for follow up, immediate drainage should also be directed by otolaryngology or OMFS. All patients should be discharged on oral broad-spectrum antibiotics, with a referral to an otolaryngologist or OMFS within 24 hours for evaluation and possible removal of nasal packs.7
Dr Yusuf is an academic chief resident, John Peter Smith Emergency Medicine Residency Program, Fort Worth, Texas. Dr Kirk is associate residency director and ultrasound director, department of emergency medicine, John Peter Smith Health System, Fort Worth, Texas.
- Huang PH, Chiang YC, Yang TH, Chao PZ, Lee FP. Nasal septal abscess. Otolaryngol Head Neck Surg. 2006;135(2):335,336.
- Shapiro RS. Nasal septal abscess. Can Med Assoc J. 1978;119(11):1321-1323.
- Lo SH, Wang PC. Nasal septal abscess as a complication of laser inferior turbinectomy. Chang Gung Med J. 2004;27(5):390-393.
- Canty PA, Berkowitz RG. Hematoma and abscess of the nasal septum in children. Arch Otolaryngol Head Neck Surg. 1996;122(12):1373-1376.
- Debnam JM, Gillenwater AM, Ginsberg LE. Nasal septal abscess in patients with immunosuppression. Am J Neuroradiol. 2007;28(10):1878,1879.
- Friedman M, Landsberg R, Chiampas G. Nasal septal hematoma evacuation. In: Reichman EF, Simon RR, eds. Emergency Medicine Procedures. New York, NY: McGraw-Hill; 2004. http://www.accessemergencymedicine.com/content.aspx?aID=45644. Accessed March 20, 2014.
- Summers SM, Bey T. Epistaxis, nasal fractures, and rhinosinusitis. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011. http://www.accessemergencymedicine.com/content.aspx?aID=6388080. Accessed March 20, 2014.
- Huang PH, Chiang YC, Yang TH, Chao PZ, Lee FP. Nasal septal abscess. Otolaryngol Head Neck Surg. 2006;135(2):335,336.
- Shapiro RS. Nasal septal abscess. Can Med Assoc J. 1978;119(11):1321-1323.
- Lo SH, Wang PC. Nasal septal abscess as a complication of laser inferior turbinectomy. Chang Gung Med J. 2004;27(5):390-393.
- Canty PA, Berkowitz RG. Hematoma and abscess of the nasal septum in children. Arch Otolaryngol Head Neck Surg. 1996;122(12):1373-1376.
- Debnam JM, Gillenwater AM, Ginsberg LE. Nasal septal abscess in patients with immunosuppression. Am J Neuroradiol. 2007;28(10):1878,1879.
- Friedman M, Landsberg R, Chiampas G. Nasal septal hematoma evacuation. In: Reichman EF, Simon RR, eds. Emergency Medicine Procedures. New York, NY: McGraw-Hill; 2004. http://www.accessemergencymedicine.com/content.aspx?aID=45644. Accessed March 20, 2014.
- Summers SM, Bey T. Epistaxis, nasal fractures, and rhinosinusitis. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011. http://www.accessemergencymedicine.com/content.aspx?aID=6388080. Accessed March 20, 2014.
Poland Syndrome: A Congenital Abnormality Mimicking a Traumatic Injury
Case
A 12-year-old boy presented to the ED via emergency medical services after he was struck by motor vehicle while skateboarding without a helmet or other safety equipment. He was thrown approximately 10 feet, but experienced no loss of consciousness, pain, or active bleeding at the site of the accident. Unaccompanied by family, he arrived to the ED fully immobilized on a long back board. His field vital signs were stable: blood pressure (BP), 100/65 mm Hg; heart rate (HR) 105 beats/minute; respiratory rate (RR), 22 breaths/minute; temperature, afebrile. Oxygen saturation was 100% on room air. The patient had an estimated Glasgow Coma Scale (GCS) of 14, with one point removed due to confusion.
Primary examination showed an intact airway with equal breath sounds bilaterally, and pulses were equal in all extremities with audible heart sounds. The patient was able to move all extremities, and showed no obvious deformities or bleeding. He was neurologically intact, with equal strength and sensation. He did, however, elicit some confusion during the examination, continuously stating it was “all his fault” and asking the medical staff where he was. This confusion persisted even after repeated reorientation. His vital signs remained stable, with slight tachycardia (BP, 105/67 mm hg; HR 100 beats/minute; RR, 17 breaths/minute; temperature, afebrile; pulse oxygen saturation, 99%). An abbreviated history revealed no allergies, medications, or past medical history. When questioned, the patient had no recollection of the accident or the last time he had eaten.
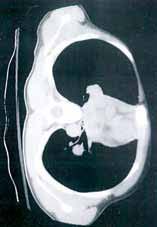
A secondary survey was significant for a small contusion/abrasion on the patient’s forehead but an otherwise normal head, ear, eyes, nose, and throat examination and no cervical c-spine tenderness. The patient denied any chest wall tenderness, but there was a dramatic palpable defect in the right chest wall, with profound asymmetry when compared to the left chest wall. No sharp, bony edges could be palpated, nor could any crepitance be felt. Breath sounds were reexamined and remained equal and nonlabored, and the patient continued to have a stable oxygen saturation of 99% on room air. The rest of the secondary survey was negative, and c-spine, pelvic, and portable chest X-rays were all negative for acute findings.
Due to the physical examination findings on the chest wall, a computed tomography (CT) scan of the chest was performed with contrast (Figure). The chest CT was normal, except for a lack of musculature over the right anterior chest wall. The patient’s mother arrived shortly after imaging studies, at which time he was reexamined. When interviewing his mother for further history, she stated that her son had been diagnosed with mild Poland Syndrome as a child, and that he has always had a chest deformity. All other studies, including a noncontrast CT of the brain, were normal. The child quickly improved during his 6-hour observation in the ED, and he was subsequently discharged home with the diagnosis of a concussion.
Discussion
Poland syndrome, also known as hand and ipsilateral thorax syndrome, is a rare congenital disorder with unknown etiology.1,2 The condition was first officially described in 1841 by Alfred Poland at Guy’s Hospital in London, though reports exist as early as 1826. Poland, a medical student, made the discovery while examining the cadaver of a hanged convict.
The occurrence of Poland syndrome is estimated to be from 1 in 25,000 to 1 in 75,000 to 100,000 by some reports,1-4 with a higher incidence in males than females (3:1 ratio) and 75% right-sided dominance.2 The syndrome is primarily described as unilateral, but there is one case report of suspected bilateral involvement.1 The components of the syndrome consist of aplasia of the sternal head of the pectoralis major muscle, hypoplasia of the pectoralis minor muscle, decreased development of breast and subcutaneous tissue, and a variety of ipsilateral hand abnormalities, including shortened carpels and phalanges, and syndactyly. The syndrome is quite variable, with different individuals eliciting combinations of the above components.
Poland syndrome was initially believed to be a nonfamilial disorder due to its sporadic nature, as illustrated by a case report of an isolated affected identical twin.3 However, enough cases of familial involvement have been reported that there is a proposed theory of an inheritable trait. Although over 250 patients with this syndrome have been described, there is no clear cause.2 The current theory of etiology is felt to be due to a lack of blood flow in the subclavian artery, or one of its branches, early in the development of the fetus, around the end of the sixth week of development. Individuals can have mild to severe manifestations, ranging as mild (eg, only pectoralis involvement), to severe (eg, rib hypoplasia, complete absence of ipsilateral hand, dextrocardia, lung herniation). Case reports of high functioning athletes with the disorder show that there is not necessarily functional impairment.
In addition to Poland syndrome, there are a number of congenital abnormalities that can also mimic traumatic chest injuries. Historically, surgeons have classified congenital wall deformities into one of five categories: Poland syndrome, pectus excavatum, pectus carinatum, sternal clefts, and generic skeletal and cartilage dysplasias (eg, absent ribs, rib torsion, vertebral anomalies).5-7 Of these categories, Poland syndrome, pectus excavatum, and some skeletal dysplasias cause anterior chest wall depression.5,6 Although these are examples of congenital thoracic wall abnormalities, one must also remember postoperative changes, which may also appear to be traumatic in origin. Examples of specific procedures are lumpectomy, mastectomy, rib resection, lung resection, or even cardiac surgery—all of which can alter the physical findings of the chest wall.
Conclusion
This report is an interesting case of an impaired patient presenting to the ED after a traumatic incident and unable to describe a past medical history of a congenital disorder. Although the patient was high functioning, as exemplified by his ability to complete normal adolescent activities such as skateboarding, he had a significant physical finding which appeared to correspond to the mechanism of his injury. He was initially thought to have a significant injury involving his chest wall, since secondary examination revealed a palpable defect. Although the patient was oxygenating well, and in no apparent distress, his altered mental status raised concerns about the accuracy of his report, with confusion and perseveration.
When a rare congenital abnormality imitates a traumatic condition, merely having the name of the condition—as we did when the family arrived—does not necessarily rule out the absence of a related deficit or injury. To better differentiate acute from preexisting physical deformities or deficits, one must gather and process multiple diagnostic clues. This is best accomplished by combining the presence or absence of symptoms (in this case, pain, dyspnea, or hemoptysis), physical examination findings (eg, ecchymosis, crepitance, flail segment), and supportive diagnostic tests (radiographs, CT, and echocardiograms). This approach will systematically eliminate or suggest acute traumatic diagnoses. With specific traumatic causes such as rib fracture, pneumothorax, or pulmonary contusion eliminated, one can expand the (nontraumatic) differential, keeping in mind the possibility of a congenital disorder.
Dr Martin is an emergency physician at Emergency Medical Associates of NY and NJ; and emergency medicine education director, Monmouth Medical Center, Long Branch, NJ.
Dr Martin reports no conflict of interest or financial arrangements.
- Fokin AA, Robicsek F. Poland syndrome revisited. Ann Thorac Surg. 2002;74(6):2218-2225
- Darian VB, Argenta LC, Pasyk KA. Familial Poland’s syndrome. Ann Plast Surg. 1989;23(6):531-537
- Stevens D, Fink B, Prevel C. Poland’s syndrome in one identical twin. J Pediatr Orthop. 2000;20(3):392-395.
- McGrath MH, Pomerantz J. Plastic surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:1935
- Spear SL, Pelletiere CV, Lee ES, Grotting JC. Anterior thoracic hypoplasia: a separate entity from Poland syndrome. Plast Reconstr Surg. 2004;113(1):
- Hodgkinson, DJ. Chest wall implants: their use for pectus excavatum, pectoralis muscle tears, Poland’s syndrome, and muscular insufficiency. Aesthetic Plast Surg. 1997;21(1):7-15.
- Hodgkinson, DJ. The management of anterior chest wall deformity in patients presenting for breast augmentation. Plast Reconstr Surg. 2002;109(5): 1714-1723.
Case
A 12-year-old boy presented to the ED via emergency medical services after he was struck by motor vehicle while skateboarding without a helmet or other safety equipment. He was thrown approximately 10 feet, but experienced no loss of consciousness, pain, or active bleeding at the site of the accident. Unaccompanied by family, he arrived to the ED fully immobilized on a long back board. His field vital signs were stable: blood pressure (BP), 100/65 mm Hg; heart rate (HR) 105 beats/minute; respiratory rate (RR), 22 breaths/minute; temperature, afebrile. Oxygen saturation was 100% on room air. The patient had an estimated Glasgow Coma Scale (GCS) of 14, with one point removed due to confusion.
Primary examination showed an intact airway with equal breath sounds bilaterally, and pulses were equal in all extremities with audible heart sounds. The patient was able to move all extremities, and showed no obvious deformities or bleeding. He was neurologically intact, with equal strength and sensation. He did, however, elicit some confusion during the examination, continuously stating it was “all his fault” and asking the medical staff where he was. This confusion persisted even after repeated reorientation. His vital signs remained stable, with slight tachycardia (BP, 105/67 mm hg; HR 100 beats/minute; RR, 17 breaths/minute; temperature, afebrile; pulse oxygen saturation, 99%). An abbreviated history revealed no allergies, medications, or past medical history. When questioned, the patient had no recollection of the accident or the last time he had eaten.
A secondary survey was significant for a small contusion/abrasion on the patient’s forehead but an otherwise normal head, ear, eyes, nose, and throat examination and no cervical c-spine tenderness. The patient denied any chest wall tenderness, but there was a dramatic palpable defect in the right chest wall, with profound asymmetry when compared to the left chest wall. No sharp, bony edges could be palpated, nor could any crepitance be felt. Breath sounds were reexamined and remained equal and nonlabored, and the patient continued to have a stable oxygen saturation of 99% on room air. The rest of the secondary survey was negative, and c-spine, pelvic, and portable chest X-rays were all negative for acute findings.
Due to the physical examination findings on the chest wall, a computed tomography (CT) scan of the chest was performed with contrast (Figure). The chest CT was normal, except for a lack of musculature over the right anterior chest wall. The patient’s mother arrived shortly after imaging studies, at which time he was reexamined. When interviewing his mother for further history, she stated that her son had been diagnosed with mild Poland Syndrome as a child, and that he has always had a chest deformity. All other studies, including a noncontrast CT of the brain, were normal. The child quickly improved during his 6-hour observation in the ED, and he was subsequently discharged home with the diagnosis of a concussion.
Discussion
Poland syndrome, also known as hand and ipsilateral thorax syndrome, is a rare congenital disorder with unknown etiology.1,2 The condition was first officially described in 1841 by Alfred Poland at Guy’s Hospital in London, though reports exist as early as 1826. Poland, a medical student, made the discovery while examining the cadaver of a hanged convict.
The occurrence of Poland syndrome is estimated to be from 1 in 25,000 to 1 in 75,000 to 100,000 by some reports,1-4 with a higher incidence in males than females (3:1 ratio) and 75% right-sided dominance.2 The syndrome is primarily described as unilateral, but there is one case report of suspected bilateral involvement.1 The components of the syndrome consist of aplasia of the sternal head of the pectoralis major muscle, hypoplasia of the pectoralis minor muscle, decreased development of breast and subcutaneous tissue, and a variety of ipsilateral hand abnormalities, including shortened carpels and phalanges, and syndactyly. The syndrome is quite variable, with different individuals eliciting combinations of the above components.
Poland syndrome was initially believed to be a nonfamilial disorder due to its sporadic nature, as illustrated by a case report of an isolated affected identical twin.3 However, enough cases of familial involvement have been reported that there is a proposed theory of an inheritable trait. Although over 250 patients with this syndrome have been described, there is no clear cause.2 The current theory of etiology is felt to be due to a lack of blood flow in the subclavian artery, or one of its branches, early in the development of the fetus, around the end of the sixth week of development. Individuals can have mild to severe manifestations, ranging as mild (eg, only pectoralis involvement), to severe (eg, rib hypoplasia, complete absence of ipsilateral hand, dextrocardia, lung herniation). Case reports of high functioning athletes with the disorder show that there is not necessarily functional impairment.
In addition to Poland syndrome, there are a number of congenital abnormalities that can also mimic traumatic chest injuries. Historically, surgeons have classified congenital wall deformities into one of five categories: Poland syndrome, pectus excavatum, pectus carinatum, sternal clefts, and generic skeletal and cartilage dysplasias (eg, absent ribs, rib torsion, vertebral anomalies).5-7 Of these categories, Poland syndrome, pectus excavatum, and some skeletal dysplasias cause anterior chest wall depression.5,6 Although these are examples of congenital thoracic wall abnormalities, one must also remember postoperative changes, which may also appear to be traumatic in origin. Examples of specific procedures are lumpectomy, mastectomy, rib resection, lung resection, or even cardiac surgery—all of which can alter the physical findings of the chest wall.
Conclusion
This report is an interesting case of an impaired patient presenting to the ED after a traumatic incident and unable to describe a past medical history of a congenital disorder. Although the patient was high functioning, as exemplified by his ability to complete normal adolescent activities such as skateboarding, he had a significant physical finding which appeared to correspond to the mechanism of his injury. He was initially thought to have a significant injury involving his chest wall, since secondary examination revealed a palpable defect. Although the patient was oxygenating well, and in no apparent distress, his altered mental status raised concerns about the accuracy of his report, with confusion and perseveration.
When a rare congenital abnormality imitates a traumatic condition, merely having the name of the condition—as we did when the family arrived—does not necessarily rule out the absence of a related deficit or injury. To better differentiate acute from preexisting physical deformities or deficits, one must gather and process multiple diagnostic clues. This is best accomplished by combining the presence or absence of symptoms (in this case, pain, dyspnea, or hemoptysis), physical examination findings (eg, ecchymosis, crepitance, flail segment), and supportive diagnostic tests (radiographs, CT, and echocardiograms). This approach will systematically eliminate or suggest acute traumatic diagnoses. With specific traumatic causes such as rib fracture, pneumothorax, or pulmonary contusion eliminated, one can expand the (nontraumatic) differential, keeping in mind the possibility of a congenital disorder.
Dr Martin is an emergency physician at Emergency Medical Associates of NY and NJ; and emergency medicine education director, Monmouth Medical Center, Long Branch, NJ.
Dr Martin reports no conflict of interest or financial arrangements.
Case
A 12-year-old boy presented to the ED via emergency medical services after he was struck by motor vehicle while skateboarding without a helmet or other safety equipment. He was thrown approximately 10 feet, but experienced no loss of consciousness, pain, or active bleeding at the site of the accident. Unaccompanied by family, he arrived to the ED fully immobilized on a long back board. His field vital signs were stable: blood pressure (BP), 100/65 mm Hg; heart rate (HR) 105 beats/minute; respiratory rate (RR), 22 breaths/minute; temperature, afebrile. Oxygen saturation was 100% on room air. The patient had an estimated Glasgow Coma Scale (GCS) of 14, with one point removed due to confusion.
Primary examination showed an intact airway with equal breath sounds bilaterally, and pulses were equal in all extremities with audible heart sounds. The patient was able to move all extremities, and showed no obvious deformities or bleeding. He was neurologically intact, with equal strength and sensation. He did, however, elicit some confusion during the examination, continuously stating it was “all his fault” and asking the medical staff where he was. This confusion persisted even after repeated reorientation. His vital signs remained stable, with slight tachycardia (BP, 105/67 mm hg; HR 100 beats/minute; RR, 17 breaths/minute; temperature, afebrile; pulse oxygen saturation, 99%). An abbreviated history revealed no allergies, medications, or past medical history. When questioned, the patient had no recollection of the accident or the last time he had eaten.
A secondary survey was significant for a small contusion/abrasion on the patient’s forehead but an otherwise normal head, ear, eyes, nose, and throat examination and no cervical c-spine tenderness. The patient denied any chest wall tenderness, but there was a dramatic palpable defect in the right chest wall, with profound asymmetry when compared to the left chest wall. No sharp, bony edges could be palpated, nor could any crepitance be felt. Breath sounds were reexamined and remained equal and nonlabored, and the patient continued to have a stable oxygen saturation of 99% on room air. The rest of the secondary survey was negative, and c-spine, pelvic, and portable chest X-rays were all negative for acute findings.
Due to the physical examination findings on the chest wall, a computed tomography (CT) scan of the chest was performed with contrast (Figure). The chest CT was normal, except for a lack of musculature over the right anterior chest wall. The patient’s mother arrived shortly after imaging studies, at which time he was reexamined. When interviewing his mother for further history, she stated that her son had been diagnosed with mild Poland Syndrome as a child, and that he has always had a chest deformity. All other studies, including a noncontrast CT of the brain, were normal. The child quickly improved during his 6-hour observation in the ED, and he was subsequently discharged home with the diagnosis of a concussion.
Discussion
Poland syndrome, also known as hand and ipsilateral thorax syndrome, is a rare congenital disorder with unknown etiology.1,2 The condition was first officially described in 1841 by Alfred Poland at Guy’s Hospital in London, though reports exist as early as 1826. Poland, a medical student, made the discovery while examining the cadaver of a hanged convict.
The occurrence of Poland syndrome is estimated to be from 1 in 25,000 to 1 in 75,000 to 100,000 by some reports,1-4 with a higher incidence in males than females (3:1 ratio) and 75% right-sided dominance.2 The syndrome is primarily described as unilateral, but there is one case report of suspected bilateral involvement.1 The components of the syndrome consist of aplasia of the sternal head of the pectoralis major muscle, hypoplasia of the pectoralis minor muscle, decreased development of breast and subcutaneous tissue, and a variety of ipsilateral hand abnormalities, including shortened carpels and phalanges, and syndactyly. The syndrome is quite variable, with different individuals eliciting combinations of the above components.
Poland syndrome was initially believed to be a nonfamilial disorder due to its sporadic nature, as illustrated by a case report of an isolated affected identical twin.3 However, enough cases of familial involvement have been reported that there is a proposed theory of an inheritable trait. Although over 250 patients with this syndrome have been described, there is no clear cause.2 The current theory of etiology is felt to be due to a lack of blood flow in the subclavian artery, or one of its branches, early in the development of the fetus, around the end of the sixth week of development. Individuals can have mild to severe manifestations, ranging as mild (eg, only pectoralis involvement), to severe (eg, rib hypoplasia, complete absence of ipsilateral hand, dextrocardia, lung herniation). Case reports of high functioning athletes with the disorder show that there is not necessarily functional impairment.
In addition to Poland syndrome, there are a number of congenital abnormalities that can also mimic traumatic chest injuries. Historically, surgeons have classified congenital wall deformities into one of five categories: Poland syndrome, pectus excavatum, pectus carinatum, sternal clefts, and generic skeletal and cartilage dysplasias (eg, absent ribs, rib torsion, vertebral anomalies).5-7 Of these categories, Poland syndrome, pectus excavatum, and some skeletal dysplasias cause anterior chest wall depression.5,6 Although these are examples of congenital thoracic wall abnormalities, one must also remember postoperative changes, which may also appear to be traumatic in origin. Examples of specific procedures are lumpectomy, mastectomy, rib resection, lung resection, or even cardiac surgery—all of which can alter the physical findings of the chest wall.
Conclusion
This report is an interesting case of an impaired patient presenting to the ED after a traumatic incident and unable to describe a past medical history of a congenital disorder. Although the patient was high functioning, as exemplified by his ability to complete normal adolescent activities such as skateboarding, he had a significant physical finding which appeared to correspond to the mechanism of his injury. He was initially thought to have a significant injury involving his chest wall, since secondary examination revealed a palpable defect. Although the patient was oxygenating well, and in no apparent distress, his altered mental status raised concerns about the accuracy of his report, with confusion and perseveration.
When a rare congenital abnormality imitates a traumatic condition, merely having the name of the condition—as we did when the family arrived—does not necessarily rule out the absence of a related deficit or injury. To better differentiate acute from preexisting physical deformities or deficits, one must gather and process multiple diagnostic clues. This is best accomplished by combining the presence or absence of symptoms (in this case, pain, dyspnea, or hemoptysis), physical examination findings (eg, ecchymosis, crepitance, flail segment), and supportive diagnostic tests (radiographs, CT, and echocardiograms). This approach will systematically eliminate or suggest acute traumatic diagnoses. With specific traumatic causes such as rib fracture, pneumothorax, or pulmonary contusion eliminated, one can expand the (nontraumatic) differential, keeping in mind the possibility of a congenital disorder.
Dr Martin is an emergency physician at Emergency Medical Associates of NY and NJ; and emergency medicine education director, Monmouth Medical Center, Long Branch, NJ.
Dr Martin reports no conflict of interest or financial arrangements.
- Fokin AA, Robicsek F. Poland syndrome revisited. Ann Thorac Surg. 2002;74(6):2218-2225
- Darian VB, Argenta LC, Pasyk KA. Familial Poland’s syndrome. Ann Plast Surg. 1989;23(6):531-537
- Stevens D, Fink B, Prevel C. Poland’s syndrome in one identical twin. J Pediatr Orthop. 2000;20(3):392-395.
- McGrath MH, Pomerantz J. Plastic surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:1935
- Spear SL, Pelletiere CV, Lee ES, Grotting JC. Anterior thoracic hypoplasia: a separate entity from Poland syndrome. Plast Reconstr Surg. 2004;113(1):
- Hodgkinson, DJ. Chest wall implants: their use for pectus excavatum, pectoralis muscle tears, Poland’s syndrome, and muscular insufficiency. Aesthetic Plast Surg. 1997;21(1):7-15.
- Hodgkinson, DJ. The management of anterior chest wall deformity in patients presenting for breast augmentation. Plast Reconstr Surg. 2002;109(5): 1714-1723.
- Fokin AA, Robicsek F. Poland syndrome revisited. Ann Thorac Surg. 2002;74(6):2218-2225
- Darian VB, Argenta LC, Pasyk KA. Familial Poland’s syndrome. Ann Plast Surg. 1989;23(6):531-537
- Stevens D, Fink B, Prevel C. Poland’s syndrome in one identical twin. J Pediatr Orthop. 2000;20(3):392-395.
- McGrath MH, Pomerantz J. Plastic surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:1935
- Spear SL, Pelletiere CV, Lee ES, Grotting JC. Anterior thoracic hypoplasia: a separate entity from Poland syndrome. Plast Reconstr Surg. 2004;113(1):
- Hodgkinson, DJ. Chest wall implants: their use for pectus excavatum, pectoralis muscle tears, Poland’s syndrome, and muscular insufficiency. Aesthetic Plast Surg. 1997;21(1):7-15.
- Hodgkinson, DJ. The management of anterior chest wall deformity in patients presenting for breast augmentation. Plast Reconstr Surg. 2002;109(5): 1714-1723.
Physicians are major source for frequent opioid misusers
Most people who misuse opioid pain relievers cite friends and relatives as their sources for the drugs, but more of the people who misuse these agents most often – those who take them from 200 to 365 days of the year – obtain their opioids from physicians’ prescriptions than from any other single source, according to a report published online March 3 in JAMA Internal Medicine.
"These results underscore the need for interventions targeting prescribing behaviors, in addition to those targeting medication sharing, selling, and diversion," the report’s authors warned.
It is a commonly cited statistic that most people who misuse opioid pain relievers obtain the drugs from family and friends for free, so many interventions to stop such misuse focus on patients. But few studies have examined whether the source of these drugs, and thus an appropriate target for interventions, might differ according to the frequency of misuse.
To study this issue, researchers analyzed data from the National Survey on Drug Use and Health, an annual survey that provides information on drug use among U.S. residents aged 12 years and older.
Survey data from 2008 through 2011 identified 11,018,735 respondents who said they misused an opioid pain reliever either by obtaining the drug without a prescription or by getting a prescription but taking the drug strictly because of the feeling or experience it provided. The source of the drug differed according to the frequency of use: As the days of use increased, the likelihood that the user obtained the drug from a friend or family member decreased, and the likelihood that he or she obtained the drug from a physician rose, said Christopher M. Jones, Pharm.D., and his associates at the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta.
Among people who misused opioids only 1-29 days of the year, 54.4% said they got them from a friend or relative for free – the most popular source. In contrast, only 18% of this patient group said the opioids were prescribed by one or more physicians.
But the percentage of patients who obtained misused opioids through physician prescriptions steadily rose with increasing use.
Among those who misused the drugs 200-365 days a year, the top source was physician prescriptions, 27.3% of users, followed by opioids obtained for free from friends or relatives, 26.4%. A total of 23.2% of users said they bought their opioids from friends or relatives, while another 15% of frequent users bought their drugs from dealers or strangers.
"This pattern is similar to that of patients in opioid treatment programs, who cite dealers and physicians as frequent sources," Dr. Jones and his associates said in a Research Letter to the Editor (JAMA Intern. Med. 2014 March 3 [doi:10.1001/jamainternmed.2013.12809]).
"Many abusers of opioid pain relievers are going directly to doctors for their drugs," CDC Director Tom Frieden commented in a statement. "Health care providers need to screen for abuse risk and prescribe judiciously by checking past records in state prescription drug monitoring programs. It’s time we stop the source and treat the troubled."
"The essential steps health care providers can take to curb this serious health problem include more judicious prescribing, use of prescription-drug–monitoring programs, and screening patients for abuse before prescribing opioids," the study authors noted.
The federal government is encouraging the development of abuse-deterrent opioid formulations, the CDC noted, and requiring companies that make extended-release and long-acting opioids to offer prescribers educational programs about the understanding the risks of opioid therapy; choosing, managing, and monitoring patients; and counseling patients on safe use of opioids.
The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
Most people who misuse opioid pain relievers cite friends and relatives as their sources for the drugs, but more of the people who misuse these agents most often – those who take them from 200 to 365 days of the year – obtain their opioids from physicians’ prescriptions than from any other single source, according to a report published online March 3 in JAMA Internal Medicine.
"These results underscore the need for interventions targeting prescribing behaviors, in addition to those targeting medication sharing, selling, and diversion," the report’s authors warned.
It is a commonly cited statistic that most people who misuse opioid pain relievers obtain the drugs from family and friends for free, so many interventions to stop such misuse focus on patients. But few studies have examined whether the source of these drugs, and thus an appropriate target for interventions, might differ according to the frequency of misuse.
To study this issue, researchers analyzed data from the National Survey on Drug Use and Health, an annual survey that provides information on drug use among U.S. residents aged 12 years and older.
Survey data from 2008 through 2011 identified 11,018,735 respondents who said they misused an opioid pain reliever either by obtaining the drug without a prescription or by getting a prescription but taking the drug strictly because of the feeling or experience it provided. The source of the drug differed according to the frequency of use: As the days of use increased, the likelihood that the user obtained the drug from a friend or family member decreased, and the likelihood that he or she obtained the drug from a physician rose, said Christopher M. Jones, Pharm.D., and his associates at the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta.
Among people who misused opioids only 1-29 days of the year, 54.4% said they got them from a friend or relative for free – the most popular source. In contrast, only 18% of this patient group said the opioids were prescribed by one or more physicians.
But the percentage of patients who obtained misused opioids through physician prescriptions steadily rose with increasing use.
Among those who misused the drugs 200-365 days a year, the top source was physician prescriptions, 27.3% of users, followed by opioids obtained for free from friends or relatives, 26.4%. A total of 23.2% of users said they bought their opioids from friends or relatives, while another 15% of frequent users bought their drugs from dealers or strangers.
"This pattern is similar to that of patients in opioid treatment programs, who cite dealers and physicians as frequent sources," Dr. Jones and his associates said in a Research Letter to the Editor (JAMA Intern. Med. 2014 March 3 [doi:10.1001/jamainternmed.2013.12809]).
"Many abusers of opioid pain relievers are going directly to doctors for their drugs," CDC Director Tom Frieden commented in a statement. "Health care providers need to screen for abuse risk and prescribe judiciously by checking past records in state prescription drug monitoring programs. It’s time we stop the source and treat the troubled."
"The essential steps health care providers can take to curb this serious health problem include more judicious prescribing, use of prescription-drug–monitoring programs, and screening patients for abuse before prescribing opioids," the study authors noted.
The federal government is encouraging the development of abuse-deterrent opioid formulations, the CDC noted, and requiring companies that make extended-release and long-acting opioids to offer prescribers educational programs about the understanding the risks of opioid therapy; choosing, managing, and monitoring patients; and counseling patients on safe use of opioids.
The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
Most people who misuse opioid pain relievers cite friends and relatives as their sources for the drugs, but more of the people who misuse these agents most often – those who take them from 200 to 365 days of the year – obtain their opioids from physicians’ prescriptions than from any other single source, according to a report published online March 3 in JAMA Internal Medicine.
"These results underscore the need for interventions targeting prescribing behaviors, in addition to those targeting medication sharing, selling, and diversion," the report’s authors warned.
It is a commonly cited statistic that most people who misuse opioid pain relievers obtain the drugs from family and friends for free, so many interventions to stop such misuse focus on patients. But few studies have examined whether the source of these drugs, and thus an appropriate target for interventions, might differ according to the frequency of misuse.
To study this issue, researchers analyzed data from the National Survey on Drug Use and Health, an annual survey that provides information on drug use among U.S. residents aged 12 years and older.
Survey data from 2008 through 2011 identified 11,018,735 respondents who said they misused an opioid pain reliever either by obtaining the drug without a prescription or by getting a prescription but taking the drug strictly because of the feeling or experience it provided. The source of the drug differed according to the frequency of use: As the days of use increased, the likelihood that the user obtained the drug from a friend or family member decreased, and the likelihood that he or she obtained the drug from a physician rose, said Christopher M. Jones, Pharm.D., and his associates at the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta.
Among people who misused opioids only 1-29 days of the year, 54.4% said they got them from a friend or relative for free – the most popular source. In contrast, only 18% of this patient group said the opioids were prescribed by one or more physicians.
But the percentage of patients who obtained misused opioids through physician prescriptions steadily rose with increasing use.
Among those who misused the drugs 200-365 days a year, the top source was physician prescriptions, 27.3% of users, followed by opioids obtained for free from friends or relatives, 26.4%. A total of 23.2% of users said they bought their opioids from friends or relatives, while another 15% of frequent users bought their drugs from dealers or strangers.
"This pattern is similar to that of patients in opioid treatment programs, who cite dealers and physicians as frequent sources," Dr. Jones and his associates said in a Research Letter to the Editor (JAMA Intern. Med. 2014 March 3 [doi:10.1001/jamainternmed.2013.12809]).
"Many abusers of opioid pain relievers are going directly to doctors for their drugs," CDC Director Tom Frieden commented in a statement. "Health care providers need to screen for abuse risk and prescribe judiciously by checking past records in state prescription drug monitoring programs. It’s time we stop the source and treat the troubled."
"The essential steps health care providers can take to curb this serious health problem include more judicious prescribing, use of prescription-drug–monitoring programs, and screening patients for abuse before prescribing opioids," the study authors noted.
The federal government is encouraging the development of abuse-deterrent opioid formulations, the CDC noted, and requiring companies that make extended-release and long-acting opioids to offer prescribers educational programs about the understanding the risks of opioid therapy; choosing, managing, and monitoring patients; and counseling patients on safe use of opioids.
The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
FROM JAMA INTERNAL MEDICINE
Major Finding: Opioid pain relievers were prescribed by a physician for 18% of people who used them only 1-29 days of the year, but that percentage steadily rose with increasing use, so that 27.3% of people who used the drugs 200-365 days/year obtained them via physician prescription, more than any other single source.
Data Source: An analysis of data on 11,018,735 survey respondents aged 12 years and older who reported misusing opioid pain relievers during a 4-year period.
Disclosures: The Centers for Disease Control and Prevention supported the study. No financial conflicts of interest were reported.
There’s No Place Like Home… for Carbon Monoxide Poisoning
Case
An 84-year-old woman with a history of hypertension and dyslipidemia and her husband, an 88-year-old man with a history of dementia and coronary artery disease, presented to the ED via EMS after neighbors discovered the woman lying on her living room floor, responding only to painful stimuli. Earlier in the evening, the same neighbors had helped the husband to bed after noticing that he had become lethargic. The EMS report indicated that a car had been left running in a closed garage of the patients’ home. The fire department identified an ambient carbon monoxide (CO) concentration of 88 ppm.
Upon arrival to the ED, the woman’s vital signs were: blood pressure (BP), 130/74 mm Hg; heart rate (HR), 63 beats/minute; respiratory rate (RR), 16 breaths/minute; temperature, 99°F. Oxygen saturation was 99% on room air. Her husband’s vital signs were: BP, 150/66 mm Hg; HR, 59 beats/minute; RR, 19 breaths/minute; temperature, 98°F; oxygen saturation was 98% on room air.
What is carbon monoxide poisoning?
Carbon monoxide is a colorless and odorless toxic gas produced by incomplete combustion of carbon-based fuel. Common sources in the United States include portable generators, gas-powered furnaces, cooking appliances, poorly ventilated home-heating systems, and motor vehicles (Box 1).1
Carbon monoxide is the leading cause of unintentional poisoning deaths in the United States,1 resulting in more than 20,000 ED visits and 2,000 hospital admissions. Nearly three-fourths of these deaths are due to exposures in the home, with more than half occurring during the months of November through February.2,3 The average cost of a hospital admission for confirmed CO poisoning is over $11,000, with a cumulative nationwide total cost of over $26 million per year. While the hospitalization rate for persons aged 18 to 44 years is only 6.7%, the admittance rates for persons aged 65 to 84 years and older than 85 years are 33% and 43%, respectively.3 Although there has been a slight decline in the incidence of CO poisoning over the past 10 years, it is still a public health concern (Figure 1).2
Who is most susceptible to motor vehicle-related carbon monoxide poisoning?
The US Centers for Disease Control and Prevention (CDC) reports that motor vehicles are the second most common source of CO exposure.4 A study of US news media reports covering a 2.5-year period revealed that 8% of such poisonings were the result of a motor vehicle left running in a garage—the overall mortality rate of which is suggested to be significantly higher than that of other sources of CO exposure.5
Approximately 430 deaths per year are caused by unintentional, nonfire-related CO poisoning,6 and the CDC reports the death rate is highest in persons older than age 65 years.1 The death rate from these exposures is more than three times higher in men than women (Figure 2).6 In addition, older patients are disproportionately affected: In US news media-reported cases of CO poisoning that included patient age, 29% occurred in persons older than age 80 years.5 Moreover, in approximately one-third of motor vehicle-related deaths due to CO poisoning, nearly all of patients older than age 80 years were found dead at the scene of exposure. These reports suggest that the elderly are at greater risk for CO exposure due to age-related cognitive changes, physical inability to escape a toxic environment once becoming symptomatic, and a greater susceptibility to poisoning due to comorbid conditions.5
Case Continued
The husband and wife’s initial carboxyhemoglobin concentrations in this case were 35% and 13%, respectively. Both were treated with hyperbaric oxygen (HBO) without complication. During their inpatient stay, the woman noted that their home did not have a CO detector.
What is the role of hyperbaric oxygen therapy as a treatment option for CO poisoning?
Hyperbaric oxygen therapy greatly accelerates the dissociation of hemoglobin from CO, reduces free radical-related cellular damage, and may have a role in preventing adverse neurological sequelae in the setting of CO poisoning. Although controversy exists, HBO therapy is generally indicated in select patients with elevated CO levels and abnormal neurological findings, cardiovascular findings, or persistent metabolic acidosis. While few ED patients with CO exposure receive HBO therapy, over 20% of patients requiring inpatient hospitalization receive treatment.3
What preventive measures can be taken to reduce motor vehicle-related CO poisoning?
The literature supports the enforcement of motor vehicle emissions standards and the proper use of home CO detectors as primary preventive strategies. Computerized data from the CDC, US Census Bureau, and US Environmental Protection Agency from 1968 to 1998 were used to evaluate the influence of national vehicle emissions policies on CO-related mortality. The Clean Air Act of 1970 set environmental limits on CO emissions from automobiles at 15.0 g/mile in 1975; the EPA further reduced this standard to 3.4 g/mile for automobiles manufactured after 1981. After the enforcement of standards set forth by the Clean Air Act and the introduction of the catalytic converter in 1975, CO emissions from automobiles decreased by an estimated 76.3%, and unintentional motor vehicle-related CO deaths declined by 81.3% (Figure 3).7 (Catalytic converters contain elements [eg, platinum] that catalyze the oxidation of CO to carbon dioxide.)
Since CO exposure occurs primarily in the home, the installation of battery-powered or battery-backed CO alarms—both in the home and garage—can prevent poisoning. These detectors are inexpensive and available at common retail stores. Unfortunately, despite the easy availability and access to CO detectors, only 39 states currently have legislation mandating their use, and approximately two-thirds of the states with existing legislation only require CO detectors in newly built structures.8
In 2010, the state of New York enacted legislation known as “Amanda’s Law,” (named after a teenaged girl whose death was caused by CO poisoning from a defective boiler) mandating CO detectors in all one- and two-family homes with heating sources that may emit CO or have attached garages. However, an industry survey in 2011 found that nearly half of New York families were not aware of this law.9 The two largest surveys on home CO detector use—those conducted by the US Census Bureau and CDC—estimate the national rate of having a working CO detector in a home is 32% to 40%, with a lower prevalence among those living in manufactured housing, renting a home, or living below the poverty level.10
What is the utilization of CO detectors by ED patients?
Case conclusion
After hospital admission and treatment, both patients were discharged on hospital day 2 with a return to a baseline mental status. Neither patient reported neurological sequelae or new cognitive changes when a follow up call was placed more than 6 months after HBO treatment. The couple furthermore reported that they installed a CO detector upon their return home.
Dr West is a resident, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York.
Dr McGregor is a resident, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York.
Dr Touger is an associate professor of clinical emergency medicine, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York. He is also medical director of the Jacobi Medical Center hyperbaric chamber.
Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at New York University School of Medicine and New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Centers for Disease Control and Prevention. Carbon monoxide-related deaths—United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56(50):1309-1312.
- Centers for Disease Control and Prevention. Carbon mononoxide exposures—United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2011;60(30):1014-1017.
- Iqbal S, Law HZ, Clower JH, Yip FY, Elixhauser A. Hospital burden of unintentional carbon monoxide poisoning in the United States, 2007. Am J Emerg Med. 2012;30(5):657-664.
- Centers for Disease Control and Prevention. Nonfatal, unintentional, non-fire-related carbon monoxide exposures—United States, 2004-2006. MMWR Morb Mortal Wkly Rep. 2008;57(33):896-899.
- Hampson NB. Residential carbon monoxide poisoning from motor vehicles. Am J Emerg Med. 2011;29(1):75-77.
- Centers for Disease Control and Prevention. Average annual number of deaths and death rates from unintentional, non-fire-related carbon monoxide poisoning, by sex and age group—United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2014;63(3):65.
- Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA. 2002;288(8):988-995.
- Carbon monoxide detectors: state statutes. National Conference of State Legislatures Web site. http://www.ncsl.org/research/environment-and-natural-resources/carbon-monoxide-detectors-state-statutes.aspx. Accessed March 11, 2014.
- Survey results: New York homeowners and the risk of carbon monoxide poisoning. Kidde Web site. http://www.kidde.com/PressRoom/Pages/SurveyResultsNYHomeownersCORisks.aspx. Accessed March 11, 2014.
- Iqbal S, Clower JH, King M, Bell J, Yip YF. National carbon monoxide poisoning surveillance framework and recent estimates. Public Health Rep. 2012;127(5):486-496.
- Johnson-Arbor K, Liebman DL, Carter EM. A survey of residential carbon monoxide detector utilization among Connecticut Emergency Department patients. Clin Toxicol (Phila). 2012;50(5):384-389.
Case
An 84-year-old woman with a history of hypertension and dyslipidemia and her husband, an 88-year-old man with a history of dementia and coronary artery disease, presented to the ED via EMS after neighbors discovered the woman lying on her living room floor, responding only to painful stimuli. Earlier in the evening, the same neighbors had helped the husband to bed after noticing that he had become lethargic. The EMS report indicated that a car had been left running in a closed garage of the patients’ home. The fire department identified an ambient carbon monoxide (CO) concentration of 88 ppm.
Upon arrival to the ED, the woman’s vital signs were: blood pressure (BP), 130/74 mm Hg; heart rate (HR), 63 beats/minute; respiratory rate (RR), 16 breaths/minute; temperature, 99°F. Oxygen saturation was 99% on room air. Her husband’s vital signs were: BP, 150/66 mm Hg; HR, 59 beats/minute; RR, 19 breaths/minute; temperature, 98°F; oxygen saturation was 98% on room air.
What is carbon monoxide poisoning?
Carbon monoxide is a colorless and odorless toxic gas produced by incomplete combustion of carbon-based fuel. Common sources in the United States include portable generators, gas-powered furnaces, cooking appliances, poorly ventilated home-heating systems, and motor vehicles (Box 1).1
Carbon monoxide is the leading cause of unintentional poisoning deaths in the United States,1 resulting in more than 20,000 ED visits and 2,000 hospital admissions. Nearly three-fourths of these deaths are due to exposures in the home, with more than half occurring during the months of November through February.2,3 The average cost of a hospital admission for confirmed CO poisoning is over $11,000, with a cumulative nationwide total cost of over $26 million per year. While the hospitalization rate for persons aged 18 to 44 years is only 6.7%, the admittance rates for persons aged 65 to 84 years and older than 85 years are 33% and 43%, respectively.3 Although there has been a slight decline in the incidence of CO poisoning over the past 10 years, it is still a public health concern (Figure 1).2
Who is most susceptible to motor vehicle-related carbon monoxide poisoning?
The US Centers for Disease Control and Prevention (CDC) reports that motor vehicles are the second most common source of CO exposure.4 A study of US news media reports covering a 2.5-year period revealed that 8% of such poisonings were the result of a motor vehicle left running in a garage—the overall mortality rate of which is suggested to be significantly higher than that of other sources of CO exposure.5
Approximately 430 deaths per year are caused by unintentional, nonfire-related CO poisoning,6 and the CDC reports the death rate is highest in persons older than age 65 years.1 The death rate from these exposures is more than three times higher in men than women (Figure 2).6 In addition, older patients are disproportionately affected: In US news media-reported cases of CO poisoning that included patient age, 29% occurred in persons older than age 80 years.5 Moreover, in approximately one-third of motor vehicle-related deaths due to CO poisoning, nearly all of patients older than age 80 years were found dead at the scene of exposure. These reports suggest that the elderly are at greater risk for CO exposure due to age-related cognitive changes, physical inability to escape a toxic environment once becoming symptomatic, and a greater susceptibility to poisoning due to comorbid conditions.5
Case Continued
The husband and wife’s initial carboxyhemoglobin concentrations in this case were 35% and 13%, respectively. Both were treated with hyperbaric oxygen (HBO) without complication. During their inpatient stay, the woman noted that their home did not have a CO detector.
What is the role of hyperbaric oxygen therapy as a treatment option for CO poisoning?
Hyperbaric oxygen therapy greatly accelerates the dissociation of hemoglobin from CO, reduces free radical-related cellular damage, and may have a role in preventing adverse neurological sequelae in the setting of CO poisoning. Although controversy exists, HBO therapy is generally indicated in select patients with elevated CO levels and abnormal neurological findings, cardiovascular findings, or persistent metabolic acidosis. While few ED patients with CO exposure receive HBO therapy, over 20% of patients requiring inpatient hospitalization receive treatment.3
What preventive measures can be taken to reduce motor vehicle-related CO poisoning?
The literature supports the enforcement of motor vehicle emissions standards and the proper use of home CO detectors as primary preventive strategies. Computerized data from the CDC, US Census Bureau, and US Environmental Protection Agency from 1968 to 1998 were used to evaluate the influence of national vehicle emissions policies on CO-related mortality. The Clean Air Act of 1970 set environmental limits on CO emissions from automobiles at 15.0 g/mile in 1975; the EPA further reduced this standard to 3.4 g/mile for automobiles manufactured after 1981. After the enforcement of standards set forth by the Clean Air Act and the introduction of the catalytic converter in 1975, CO emissions from automobiles decreased by an estimated 76.3%, and unintentional motor vehicle-related CO deaths declined by 81.3% (Figure 3).7 (Catalytic converters contain elements [eg, platinum] that catalyze the oxidation of CO to carbon dioxide.)
Since CO exposure occurs primarily in the home, the installation of battery-powered or battery-backed CO alarms—both in the home and garage—can prevent poisoning. These detectors are inexpensive and available at common retail stores. Unfortunately, despite the easy availability and access to CO detectors, only 39 states currently have legislation mandating their use, and approximately two-thirds of the states with existing legislation only require CO detectors in newly built structures.8
In 2010, the state of New York enacted legislation known as “Amanda’s Law,” (named after a teenaged girl whose death was caused by CO poisoning from a defective boiler) mandating CO detectors in all one- and two-family homes with heating sources that may emit CO or have attached garages. However, an industry survey in 2011 found that nearly half of New York families were not aware of this law.9 The two largest surveys on home CO detector use—those conducted by the US Census Bureau and CDC—estimate the national rate of having a working CO detector in a home is 32% to 40%, with a lower prevalence among those living in manufactured housing, renting a home, or living below the poverty level.10
What is the utilization of CO detectors by ED patients?
Case conclusion
After hospital admission and treatment, both patients were discharged on hospital day 2 with a return to a baseline mental status. Neither patient reported neurological sequelae or new cognitive changes when a follow up call was placed more than 6 months after HBO treatment. The couple furthermore reported that they installed a CO detector upon their return home.
Dr West is a resident, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York.
Dr McGregor is a resident, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York.
Dr Touger is an associate professor of clinical emergency medicine, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York. He is also medical director of the Jacobi Medical Center hyperbaric chamber.
Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at New York University School of Medicine and New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
An 84-year-old woman with a history of hypertension and dyslipidemia and her husband, an 88-year-old man with a history of dementia and coronary artery disease, presented to the ED via EMS after neighbors discovered the woman lying on her living room floor, responding only to painful stimuli. Earlier in the evening, the same neighbors had helped the husband to bed after noticing that he had become lethargic. The EMS report indicated that a car had been left running in a closed garage of the patients’ home. The fire department identified an ambient carbon monoxide (CO) concentration of 88 ppm.
Upon arrival to the ED, the woman’s vital signs were: blood pressure (BP), 130/74 mm Hg; heart rate (HR), 63 beats/minute; respiratory rate (RR), 16 breaths/minute; temperature, 99°F. Oxygen saturation was 99% on room air. Her husband’s vital signs were: BP, 150/66 mm Hg; HR, 59 beats/minute; RR, 19 breaths/minute; temperature, 98°F; oxygen saturation was 98% on room air.
What is carbon monoxide poisoning?
Carbon monoxide is a colorless and odorless toxic gas produced by incomplete combustion of carbon-based fuel. Common sources in the United States include portable generators, gas-powered furnaces, cooking appliances, poorly ventilated home-heating systems, and motor vehicles (Box 1).1
Carbon monoxide is the leading cause of unintentional poisoning deaths in the United States,1 resulting in more than 20,000 ED visits and 2,000 hospital admissions. Nearly three-fourths of these deaths are due to exposures in the home, with more than half occurring during the months of November through February.2,3 The average cost of a hospital admission for confirmed CO poisoning is over $11,000, with a cumulative nationwide total cost of over $26 million per year. While the hospitalization rate for persons aged 18 to 44 years is only 6.7%, the admittance rates for persons aged 65 to 84 years and older than 85 years are 33% and 43%, respectively.3 Although there has been a slight decline in the incidence of CO poisoning over the past 10 years, it is still a public health concern (Figure 1).2
Who is most susceptible to motor vehicle-related carbon monoxide poisoning?
The US Centers for Disease Control and Prevention (CDC) reports that motor vehicles are the second most common source of CO exposure.4 A study of US news media reports covering a 2.5-year period revealed that 8% of such poisonings were the result of a motor vehicle left running in a garage—the overall mortality rate of which is suggested to be significantly higher than that of other sources of CO exposure.5
Approximately 430 deaths per year are caused by unintentional, nonfire-related CO poisoning,6 and the CDC reports the death rate is highest in persons older than age 65 years.1 The death rate from these exposures is more than three times higher in men than women (Figure 2).6 In addition, older patients are disproportionately affected: In US news media-reported cases of CO poisoning that included patient age, 29% occurred in persons older than age 80 years.5 Moreover, in approximately one-third of motor vehicle-related deaths due to CO poisoning, nearly all of patients older than age 80 years were found dead at the scene of exposure. These reports suggest that the elderly are at greater risk for CO exposure due to age-related cognitive changes, physical inability to escape a toxic environment once becoming symptomatic, and a greater susceptibility to poisoning due to comorbid conditions.5
Case Continued
The husband and wife’s initial carboxyhemoglobin concentrations in this case were 35% and 13%, respectively. Both were treated with hyperbaric oxygen (HBO) without complication. During their inpatient stay, the woman noted that their home did not have a CO detector.
What is the role of hyperbaric oxygen therapy as a treatment option for CO poisoning?
Hyperbaric oxygen therapy greatly accelerates the dissociation of hemoglobin from CO, reduces free radical-related cellular damage, and may have a role in preventing adverse neurological sequelae in the setting of CO poisoning. Although controversy exists, HBO therapy is generally indicated in select patients with elevated CO levels and abnormal neurological findings, cardiovascular findings, or persistent metabolic acidosis. While few ED patients with CO exposure receive HBO therapy, over 20% of patients requiring inpatient hospitalization receive treatment.3
What preventive measures can be taken to reduce motor vehicle-related CO poisoning?
The literature supports the enforcement of motor vehicle emissions standards and the proper use of home CO detectors as primary preventive strategies. Computerized data from the CDC, US Census Bureau, and US Environmental Protection Agency from 1968 to 1998 were used to evaluate the influence of national vehicle emissions policies on CO-related mortality. The Clean Air Act of 1970 set environmental limits on CO emissions from automobiles at 15.0 g/mile in 1975; the EPA further reduced this standard to 3.4 g/mile for automobiles manufactured after 1981. After the enforcement of standards set forth by the Clean Air Act and the introduction of the catalytic converter in 1975, CO emissions from automobiles decreased by an estimated 76.3%, and unintentional motor vehicle-related CO deaths declined by 81.3% (Figure 3).7 (Catalytic converters contain elements [eg, platinum] that catalyze the oxidation of CO to carbon dioxide.)
Since CO exposure occurs primarily in the home, the installation of battery-powered or battery-backed CO alarms—both in the home and garage—can prevent poisoning. These detectors are inexpensive and available at common retail stores. Unfortunately, despite the easy availability and access to CO detectors, only 39 states currently have legislation mandating their use, and approximately two-thirds of the states with existing legislation only require CO detectors in newly built structures.8
In 2010, the state of New York enacted legislation known as “Amanda’s Law,” (named after a teenaged girl whose death was caused by CO poisoning from a defective boiler) mandating CO detectors in all one- and two-family homes with heating sources that may emit CO or have attached garages. However, an industry survey in 2011 found that nearly half of New York families were not aware of this law.9 The two largest surveys on home CO detector use—those conducted by the US Census Bureau and CDC—estimate the national rate of having a working CO detector in a home is 32% to 40%, with a lower prevalence among those living in manufactured housing, renting a home, or living below the poverty level.10
What is the utilization of CO detectors by ED patients?
Case conclusion
After hospital admission and treatment, both patients were discharged on hospital day 2 with a return to a baseline mental status. Neither patient reported neurological sequelae or new cognitive changes when a follow up call was placed more than 6 months after HBO treatment. The couple furthermore reported that they installed a CO detector upon their return home.
Dr West is a resident, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York.
Dr McGregor is a resident, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York.
Dr Touger is an associate professor of clinical emergency medicine, department of emergency medicine, Albert Einstein College of Medicine, Bronx, New York. He is also medical director of the Jacobi Medical Center hyperbaric chamber.
Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at New York University School of Medicine and New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Centers for Disease Control and Prevention. Carbon monoxide-related deaths—United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56(50):1309-1312.
- Centers for Disease Control and Prevention. Carbon mononoxide exposures—United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2011;60(30):1014-1017.
- Iqbal S, Law HZ, Clower JH, Yip FY, Elixhauser A. Hospital burden of unintentional carbon monoxide poisoning in the United States, 2007. Am J Emerg Med. 2012;30(5):657-664.
- Centers for Disease Control and Prevention. Nonfatal, unintentional, non-fire-related carbon monoxide exposures—United States, 2004-2006. MMWR Morb Mortal Wkly Rep. 2008;57(33):896-899.
- Hampson NB. Residential carbon monoxide poisoning from motor vehicles. Am J Emerg Med. 2011;29(1):75-77.
- Centers for Disease Control and Prevention. Average annual number of deaths and death rates from unintentional, non-fire-related carbon monoxide poisoning, by sex and age group—United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2014;63(3):65.
- Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA. 2002;288(8):988-995.
- Carbon monoxide detectors: state statutes. National Conference of State Legislatures Web site. http://www.ncsl.org/research/environment-and-natural-resources/carbon-monoxide-detectors-state-statutes.aspx. Accessed March 11, 2014.
- Survey results: New York homeowners and the risk of carbon monoxide poisoning. Kidde Web site. http://www.kidde.com/PressRoom/Pages/SurveyResultsNYHomeownersCORisks.aspx. Accessed March 11, 2014.
- Iqbal S, Clower JH, King M, Bell J, Yip YF. National carbon monoxide poisoning surveillance framework and recent estimates. Public Health Rep. 2012;127(5):486-496.
- Johnson-Arbor K, Liebman DL, Carter EM. A survey of residential carbon monoxide detector utilization among Connecticut Emergency Department patients. Clin Toxicol (Phila). 2012;50(5):384-389.
- Centers for Disease Control and Prevention. Carbon monoxide-related deaths—United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56(50):1309-1312.
- Centers for Disease Control and Prevention. Carbon mononoxide exposures—United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2011;60(30):1014-1017.
- Iqbal S, Law HZ, Clower JH, Yip FY, Elixhauser A. Hospital burden of unintentional carbon monoxide poisoning in the United States, 2007. Am J Emerg Med. 2012;30(5):657-664.
- Centers for Disease Control and Prevention. Nonfatal, unintentional, non-fire-related carbon monoxide exposures—United States, 2004-2006. MMWR Morb Mortal Wkly Rep. 2008;57(33):896-899.
- Hampson NB. Residential carbon monoxide poisoning from motor vehicles. Am J Emerg Med. 2011;29(1):75-77.
- Centers for Disease Control and Prevention. Average annual number of deaths and death rates from unintentional, non-fire-related carbon monoxide poisoning, by sex and age group—United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2014;63(3):65.
- Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA. 2002;288(8):988-995.
- Carbon monoxide detectors: state statutes. National Conference of State Legislatures Web site. http://www.ncsl.org/research/environment-and-natural-resources/carbon-monoxide-detectors-state-statutes.aspx. Accessed March 11, 2014.
- Survey results: New York homeowners and the risk of carbon monoxide poisoning. Kidde Web site. http://www.kidde.com/PressRoom/Pages/SurveyResultsNYHomeownersCORisks.aspx. Accessed March 11, 2014.
- Iqbal S, Clower JH, King M, Bell J, Yip YF. National carbon monoxide poisoning surveillance framework and recent estimates. Public Health Rep. 2012;127(5):486-496.
- Johnson-Arbor K, Liebman DL, Carter EM. A survey of residential carbon monoxide detector utilization among Connecticut Emergency Department patients. Clin Toxicol (Phila). 2012;50(5):384-389.
Pancytopenia warning added to label of hepatitis C drug boceprevir
A warning about cases of pancytopenia linked to the use of the oral hepatitis C antiviral drug boceprevir has been added to the drug’s label, the Food and Drug Administration has announced.
There have been postmarketing reports of "serious cases" of pancytopenia in people treated with boceprevir in combination with peginterferon alfa and ribavirin, according to the FDA. This information has been added to the "Warnings and Precautions" section of the label, with the recommendation that "complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at treatment weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate."
Approved in 2011, boceprevir is an HCV Ns3/4A protease inhibitor approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders. Boceprevir, in a 200-mg capsule formulation, is marketed as Victrelis by Merck Sharp & Dohme; the recommended dose is 800 mg, three times a day.
The boceprevir Medication Guide will also be updated with this information, according to the FDA.
Serious adverse events associated with boceprevir should be reported to the FDA at 800-332-1088 or at MedWatch.
A warning about cases of pancytopenia linked to the use of the oral hepatitis C antiviral drug boceprevir has been added to the drug’s label, the Food and Drug Administration has announced.
There have been postmarketing reports of "serious cases" of pancytopenia in people treated with boceprevir in combination with peginterferon alfa and ribavirin, according to the FDA. This information has been added to the "Warnings and Precautions" section of the label, with the recommendation that "complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at treatment weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate."
Approved in 2011, boceprevir is an HCV Ns3/4A protease inhibitor approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders. Boceprevir, in a 200-mg capsule formulation, is marketed as Victrelis by Merck Sharp & Dohme; the recommended dose is 800 mg, three times a day.
The boceprevir Medication Guide will also be updated with this information, according to the FDA.
Serious adverse events associated with boceprevir should be reported to the FDA at 800-332-1088 or at MedWatch.
A warning about cases of pancytopenia linked to the use of the oral hepatitis C antiviral drug boceprevir has been added to the drug’s label, the Food and Drug Administration has announced.
There have been postmarketing reports of "serious cases" of pancytopenia in people treated with boceprevir in combination with peginterferon alfa and ribavirin, according to the FDA. This information has been added to the "Warnings and Precautions" section of the label, with the recommendation that "complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at treatment weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate."
Approved in 2011, boceprevir is an HCV Ns3/4A protease inhibitor approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adults with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders. Boceprevir, in a 200-mg capsule formulation, is marketed as Victrelis by Merck Sharp & Dohme; the recommended dose is 800 mg, three times a day.
The boceprevir Medication Guide will also be updated with this information, according to the FDA.
Serious adverse events associated with boceprevir should be reported to the FDA at 800-332-1088 or at MedWatch.
Case Studies in Toxicology: The Acclaimed Zombie-Apocalypse Drug—Is it Just an Illusion?
Dr Takematsu is a senior fellow of medical toxicology, department of emergency medicine, New York University School of Medicine and New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at New York University School of Medicine and New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 50-year-old man with a 16-year history of injection heroin abuse presented to the ED complaining of ulcerative lesions on his right arm, which he stated had become worse over the past 3 months. He claimed the skin lesions, which involved his entire right arm, had grown “wider and deeper” since he started using the drug “Krokodil.” He further noted that he obtained the product from four different drug suppliers but did not know how it was prepared.
On presentation, his vital signs were: blood pressure, 135/78 mm Hg; heart rate, 92 beats/minute; respiratory rate, 14 breaths/minute; temperature, 98.2˚ F. Oxygen saturation was 100% on room air. Physical examination of the right arm was notable for broad ulcers that exposed fat tissue and muscle and involved the entire forearm circumferentially (Figure 1). There were no signs of acute infection such as erythema, warmth, or abscess formation. The remainder of the physical examination was unremarkable.
What is Krokodil?
The name Krokodil is derived from the Russian word for crocodile, and stems from the greenish, severely damaged “crocodile-like” skin lesions purportedly caused by subcutaneous injection of this opioid derivative. Krokodil is not a specific drug but rather it describes the product derived from an attempt to synthesize desomorphine, the core ingredient. Desomorphine is a short-acting opioid analogue that has a reported potency eight to 10 times greater than morphine (Figure 2).
Also called “Russian Magic,” Krokodil has been used in Russia since 2003 following the country’s major restrictions on the importation of heroin. Since desomorphine can be synthesized from codeine, which was available in over-the-counter medications in Russia until 2012, drug users have turned to it as an inexpensive heroin substitute. (The average cost of a codeine-containing product is about 120 Rubles [$4.00] per 10-pack.1)
The chemical process of synthesizing desomorphine typically involves mixing one to five packs of codeine-based analgesics1 with a solvent (eg, paint thinner, gasoline), along with iodine and red phosphorous, which can be obtained from the striking pads of matchboxes.2 This produces a yield equivalent to 500 Rubles of heroin, making it an attractive alternative to low-income drug users.1 However, the yields are poor, and the starting products vary based on the codeine source. No systemic analysis of Krokodil has been performed to assess the purity and concentration of desomorphine in the resulting product.
What are the risks of using Krokodil?
Krokodil is typically self-administered by either intravenous (IV) or subcutaneous (“skin-popping”) injection. Solvents and contaminants in the product can damage tissue with which they come into contact. Thus, IV use of this product causes venous scarring and collapse3; when venous access is exhausted, or following infiltration, the subcutaneous route may lead to necrosis and ulceration of the skin. These lesions are said to be used by illicit drug users as a “shooter’s patch” to inject drugs,4 leading to further skin damage. In addition to the local dermal effects, systemic inflammation results from the dissemination of the components of the product, causing neurological, solid-organ, and other effects.
Has Krokodil reached the United States?
Because of the horrifying appearance of the skin lesions, Krokodil has been called by many descriptive names such as the “flesh-eating drug” and “zombie-apocalypse drug,” which in turn has led to somewhat sensationalistic media reporting. There is, however, no clear evidence of entry of desomorphine or of Krokodil use in the United States, and most authorities feel either is unlikely to occur. This speculation is supported by the low cost and easy availability of heroin in the United States compared to Russia. Of note, although Russia banned the nonprescription sale of products containing codeine in November 2012, the ban has had minimal impact on the rate of necrotic skin lesions.
To date, none of the so-called cases of Krokodil-associated lesions reported in the United States have confirmatory analytical testing, and diagnoses have been based primarily on history or morphological features of the wound. Furthermore, no reference laboratories in the United States have identified desomorphine in any of their tested samples. Since the skin lesions are not pathognomonic of Krokodil use and share morphologic features with all forms of subcutaneous drug use, analytical confirmation is critical to identifying this as an emerging drug trend. Therefore, users of product sold or brewed as Krokodil may develop skin lesions regardless of desomorphine content due to contaminants in the injected product.
What causes the skin lesions?
Desomorphine itself is not likely the cause of the skin lesions. In its pure form, there is no reason to expect this compound would induce any specific changes in the skin that would lead to necrosis. In fact, similar chemical syntheses of desomorphine produced in other countries, such as in the Czech Republic and New Zealand, have not led to analogous skin lesions. Ulcerative skin damage is most likely caused by the inflammatory contaminants in Krokodil. Injection of solvents and other chemicals into the subcutaneous space lead to skin damage and provide a bed for the development of indolent infections. This mechanism is similar to that leading to epidemics, in which similar lesions have been associated with black-tar heroin injection and drug contamination with Bacillus anthracis.5,6
Some people, however, point out the severity of the skin lesions associated with the use of Krokodil compared to other injection drugs. This may be related to the need for frequent administration of Krokodil due to its short duration of action, in turn leading to repeated exposure to the impure solvents.
Treatment of associated lesions involves wound care, including debridement, topical care, and antibiotics; amputation may be required in severe cases.
Case conclusion
The patient in this case was taken to the operating room for debridement of the right arm. The pathology results of the tissue showed ulceration, abscess, acute and chronic inflamed granulation tissue, fibrosis, and necrosis. A magnetic resonance image of the arm showed no signs of osteomyelitis. A blood sample sent for analysis was negative for desomorphine. The patient was stable after the surgery, and was discharged with follow-up instructions and referral for drug abuse counseling.
- Grund JP, Latypov A, Harris M. Breaking worse: The emergence of krokodil and excessive injuries among people who inject drugs in Eurasia. Int J Drug Policy. 2013;24(4):265-274.
- Gahr M, Freudenmann RW, Hiemke C, Gunst IM, Connemann BJ, Schönfeldt-Lecuona C. Desomorphine goes “crocodile.” J Addict Dis. 2012;31(4):407-412.
- Pieper B, Kirsner RS, Templin TN, Birk TJ. Injection drug use: an understudied cause of venous disease. Arch Dermatol. 2007;143(10):1305-1309.
- Iyer S, Subramanian P, Pabari A. A devastating complication of “skin popping.” Surgeon. 2011;9(5):295-297.
- Dunbar NM, Harruff RC. Necrotizing fasciitis: manifestations, microbiology and connection with black tar heroin. J Forensic Sci. 2007;52(4):920-923.
- Grunow R, Klee SR, Beyer W, et al. Anthrax among heroin users in Europe possibly caused by same Bacillus anthracis strain since 2000. E Euro Surveill. 2013;18(13):pii=20437.
Dr Takematsu is a senior fellow of medical toxicology, department of emergency medicine, New York University School of Medicine and New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at New York University School of Medicine and New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 50-year-old man with a 16-year history of injection heroin abuse presented to the ED complaining of ulcerative lesions on his right arm, which he stated had become worse over the past 3 months. He claimed the skin lesions, which involved his entire right arm, had grown “wider and deeper” since he started using the drug “Krokodil.” He further noted that he obtained the product from four different drug suppliers but did not know how it was prepared.
On presentation, his vital signs were: blood pressure, 135/78 mm Hg; heart rate, 92 beats/minute; respiratory rate, 14 breaths/minute; temperature, 98.2˚ F. Oxygen saturation was 100% on room air. Physical examination of the right arm was notable for broad ulcers that exposed fat tissue and muscle and involved the entire forearm circumferentially (Figure 1). There were no signs of acute infection such as erythema, warmth, or abscess formation. The remainder of the physical examination was unremarkable.
What is Krokodil?
The name Krokodil is derived from the Russian word for crocodile, and stems from the greenish, severely damaged “crocodile-like” skin lesions purportedly caused by subcutaneous injection of this opioid derivative. Krokodil is not a specific drug but rather it describes the product derived from an attempt to synthesize desomorphine, the core ingredient. Desomorphine is a short-acting opioid analogue that has a reported potency eight to 10 times greater than morphine (Figure 2).
Also called “Russian Magic,” Krokodil has been used in Russia since 2003 following the country’s major restrictions on the importation of heroin. Since desomorphine can be synthesized from codeine, which was available in over-the-counter medications in Russia until 2012, drug users have turned to it as an inexpensive heroin substitute. (The average cost of a codeine-containing product is about 120 Rubles [$4.00] per 10-pack.1)
The chemical process of synthesizing desomorphine typically involves mixing one to five packs of codeine-based analgesics1 with a solvent (eg, paint thinner, gasoline), along with iodine and red phosphorous, which can be obtained from the striking pads of matchboxes.2 This produces a yield equivalent to 500 Rubles of heroin, making it an attractive alternative to low-income drug users.1 However, the yields are poor, and the starting products vary based on the codeine source. No systemic analysis of Krokodil has been performed to assess the purity and concentration of desomorphine in the resulting product.
What are the risks of using Krokodil?
Krokodil is typically self-administered by either intravenous (IV) or subcutaneous (“skin-popping”) injection. Solvents and contaminants in the product can damage tissue with which they come into contact. Thus, IV use of this product causes venous scarring and collapse3; when venous access is exhausted, or following infiltration, the subcutaneous route may lead to necrosis and ulceration of the skin. These lesions are said to be used by illicit drug users as a “shooter’s patch” to inject drugs,4 leading to further skin damage. In addition to the local dermal effects, systemic inflammation results from the dissemination of the components of the product, causing neurological, solid-organ, and other effects.
Has Krokodil reached the United States?
Because of the horrifying appearance of the skin lesions, Krokodil has been called by many descriptive names such as the “flesh-eating drug” and “zombie-apocalypse drug,” which in turn has led to somewhat sensationalistic media reporting. There is, however, no clear evidence of entry of desomorphine or of Krokodil use in the United States, and most authorities feel either is unlikely to occur. This speculation is supported by the low cost and easy availability of heroin in the United States compared to Russia. Of note, although Russia banned the nonprescription sale of products containing codeine in November 2012, the ban has had minimal impact on the rate of necrotic skin lesions.
To date, none of the so-called cases of Krokodil-associated lesions reported in the United States have confirmatory analytical testing, and diagnoses have been based primarily on history or morphological features of the wound. Furthermore, no reference laboratories in the United States have identified desomorphine in any of their tested samples. Since the skin lesions are not pathognomonic of Krokodil use and share morphologic features with all forms of subcutaneous drug use, analytical confirmation is critical to identifying this as an emerging drug trend. Therefore, users of product sold or brewed as Krokodil may develop skin lesions regardless of desomorphine content due to contaminants in the injected product.
What causes the skin lesions?
Desomorphine itself is not likely the cause of the skin lesions. In its pure form, there is no reason to expect this compound would induce any specific changes in the skin that would lead to necrosis. In fact, similar chemical syntheses of desomorphine produced in other countries, such as in the Czech Republic and New Zealand, have not led to analogous skin lesions. Ulcerative skin damage is most likely caused by the inflammatory contaminants in Krokodil. Injection of solvents and other chemicals into the subcutaneous space lead to skin damage and provide a bed for the development of indolent infections. This mechanism is similar to that leading to epidemics, in which similar lesions have been associated with black-tar heroin injection and drug contamination with Bacillus anthracis.5,6
Some people, however, point out the severity of the skin lesions associated with the use of Krokodil compared to other injection drugs. This may be related to the need for frequent administration of Krokodil due to its short duration of action, in turn leading to repeated exposure to the impure solvents.
Treatment of associated lesions involves wound care, including debridement, topical care, and antibiotics; amputation may be required in severe cases.
Case conclusion
The patient in this case was taken to the operating room for debridement of the right arm. The pathology results of the tissue showed ulceration, abscess, acute and chronic inflamed granulation tissue, fibrosis, and necrosis. A magnetic resonance image of the arm showed no signs of osteomyelitis. A blood sample sent for analysis was negative for desomorphine. The patient was stable after the surgery, and was discharged with follow-up instructions and referral for drug abuse counseling.
Dr Takematsu is a senior fellow of medical toxicology, department of emergency medicine, New York University School of Medicine and New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at New York University School of Medicine and New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 50-year-old man with a 16-year history of injection heroin abuse presented to the ED complaining of ulcerative lesions on his right arm, which he stated had become worse over the past 3 months. He claimed the skin lesions, which involved his entire right arm, had grown “wider and deeper” since he started using the drug “Krokodil.” He further noted that he obtained the product from four different drug suppliers but did not know how it was prepared.
On presentation, his vital signs were: blood pressure, 135/78 mm Hg; heart rate, 92 beats/minute; respiratory rate, 14 breaths/minute; temperature, 98.2˚ F. Oxygen saturation was 100% on room air. Physical examination of the right arm was notable for broad ulcers that exposed fat tissue and muscle and involved the entire forearm circumferentially (Figure 1). There were no signs of acute infection such as erythema, warmth, or abscess formation. The remainder of the physical examination was unremarkable.
What is Krokodil?
The name Krokodil is derived from the Russian word for crocodile, and stems from the greenish, severely damaged “crocodile-like” skin lesions purportedly caused by subcutaneous injection of this opioid derivative. Krokodil is not a specific drug but rather it describes the product derived from an attempt to synthesize desomorphine, the core ingredient. Desomorphine is a short-acting opioid analogue that has a reported potency eight to 10 times greater than morphine (Figure 2).
Also called “Russian Magic,” Krokodil has been used in Russia since 2003 following the country’s major restrictions on the importation of heroin. Since desomorphine can be synthesized from codeine, which was available in over-the-counter medications in Russia until 2012, drug users have turned to it as an inexpensive heroin substitute. (The average cost of a codeine-containing product is about 120 Rubles [$4.00] per 10-pack.1)
The chemical process of synthesizing desomorphine typically involves mixing one to five packs of codeine-based analgesics1 with a solvent (eg, paint thinner, gasoline), along with iodine and red phosphorous, which can be obtained from the striking pads of matchboxes.2 This produces a yield equivalent to 500 Rubles of heroin, making it an attractive alternative to low-income drug users.1 However, the yields are poor, and the starting products vary based on the codeine source. No systemic analysis of Krokodil has been performed to assess the purity and concentration of desomorphine in the resulting product.
What are the risks of using Krokodil?
Krokodil is typically self-administered by either intravenous (IV) or subcutaneous (“skin-popping”) injection. Solvents and contaminants in the product can damage tissue with which they come into contact. Thus, IV use of this product causes venous scarring and collapse3; when venous access is exhausted, or following infiltration, the subcutaneous route may lead to necrosis and ulceration of the skin. These lesions are said to be used by illicit drug users as a “shooter’s patch” to inject drugs,4 leading to further skin damage. In addition to the local dermal effects, systemic inflammation results from the dissemination of the components of the product, causing neurological, solid-organ, and other effects.
Has Krokodil reached the United States?
Because of the horrifying appearance of the skin lesions, Krokodil has been called by many descriptive names such as the “flesh-eating drug” and “zombie-apocalypse drug,” which in turn has led to somewhat sensationalistic media reporting. There is, however, no clear evidence of entry of desomorphine or of Krokodil use in the United States, and most authorities feel either is unlikely to occur. This speculation is supported by the low cost and easy availability of heroin in the United States compared to Russia. Of note, although Russia banned the nonprescription sale of products containing codeine in November 2012, the ban has had minimal impact on the rate of necrotic skin lesions.
To date, none of the so-called cases of Krokodil-associated lesions reported in the United States have confirmatory analytical testing, and diagnoses have been based primarily on history or morphological features of the wound. Furthermore, no reference laboratories in the United States have identified desomorphine in any of their tested samples. Since the skin lesions are not pathognomonic of Krokodil use and share morphologic features with all forms of subcutaneous drug use, analytical confirmation is critical to identifying this as an emerging drug trend. Therefore, users of product sold or brewed as Krokodil may develop skin lesions regardless of desomorphine content due to contaminants in the injected product.
What causes the skin lesions?
Desomorphine itself is not likely the cause of the skin lesions. In its pure form, there is no reason to expect this compound would induce any specific changes in the skin that would lead to necrosis. In fact, similar chemical syntheses of desomorphine produced in other countries, such as in the Czech Republic and New Zealand, have not led to analogous skin lesions. Ulcerative skin damage is most likely caused by the inflammatory contaminants in Krokodil. Injection of solvents and other chemicals into the subcutaneous space lead to skin damage and provide a bed for the development of indolent infections. This mechanism is similar to that leading to epidemics, in which similar lesions have been associated with black-tar heroin injection and drug contamination with Bacillus anthracis.5,6
Some people, however, point out the severity of the skin lesions associated with the use of Krokodil compared to other injection drugs. This may be related to the need for frequent administration of Krokodil due to its short duration of action, in turn leading to repeated exposure to the impure solvents.
Treatment of associated lesions involves wound care, including debridement, topical care, and antibiotics; amputation may be required in severe cases.
Case conclusion
The patient in this case was taken to the operating room for debridement of the right arm. The pathology results of the tissue showed ulceration, abscess, acute and chronic inflamed granulation tissue, fibrosis, and necrosis. A magnetic resonance image of the arm showed no signs of osteomyelitis. A blood sample sent for analysis was negative for desomorphine. The patient was stable after the surgery, and was discharged with follow-up instructions and referral for drug abuse counseling.
- Grund JP, Latypov A, Harris M. Breaking worse: The emergence of krokodil and excessive injuries among people who inject drugs in Eurasia. Int J Drug Policy. 2013;24(4):265-274.
- Gahr M, Freudenmann RW, Hiemke C, Gunst IM, Connemann BJ, Schönfeldt-Lecuona C. Desomorphine goes “crocodile.” J Addict Dis. 2012;31(4):407-412.
- Pieper B, Kirsner RS, Templin TN, Birk TJ. Injection drug use: an understudied cause of venous disease. Arch Dermatol. 2007;143(10):1305-1309.
- Iyer S, Subramanian P, Pabari A. A devastating complication of “skin popping.” Surgeon. 2011;9(5):295-297.
- Dunbar NM, Harruff RC. Necrotizing fasciitis: manifestations, microbiology and connection with black tar heroin. J Forensic Sci. 2007;52(4):920-923.
- Grunow R, Klee SR, Beyer W, et al. Anthrax among heroin users in Europe possibly caused by same Bacillus anthracis strain since 2000. E Euro Surveill. 2013;18(13):pii=20437.
- Grund JP, Latypov A, Harris M. Breaking worse: The emergence of krokodil and excessive injuries among people who inject drugs in Eurasia. Int J Drug Policy. 2013;24(4):265-274.
- Gahr M, Freudenmann RW, Hiemke C, Gunst IM, Connemann BJ, Schönfeldt-Lecuona C. Desomorphine goes “crocodile.” J Addict Dis. 2012;31(4):407-412.
- Pieper B, Kirsner RS, Templin TN, Birk TJ. Injection drug use: an understudied cause of venous disease. Arch Dermatol. 2007;143(10):1305-1309.
- Iyer S, Subramanian P, Pabari A. A devastating complication of “skin popping.” Surgeon. 2011;9(5):295-297.
- Dunbar NM, Harruff RC. Necrotizing fasciitis: manifestations, microbiology and connection with black tar heroin. J Forensic Sci. 2007;52(4):920-923.
- Grunow R, Klee SR, Beyer W, et al. Anthrax among heroin users in Europe possibly caused by same Bacillus anthracis strain since 2000. E Euro Surveill. 2013;18(13):pii=20437.
Case Studies in Toxicology: Tiny Bubbles (Or, the Dangers of Cleaning Your Fruit)
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
New definition of kidney injury is more predictive of mortality
The newly proposed consensus definition of acute kidney injury in patients with cirrhosis accurately predicts 30-day mortality and other adverse outcomes in this patient population much better than the current, more rigid definition would, according to a report in the December issue of Gastroenterology (doi:10.1053/j.gastro.2013.08.051).
In what they described as the largest prospective study of this topic to date, researchers found that the recently proposed, broader redefinition of acute kidney injury (AKI) correctly identified which patients were likely to die, develop severe complications such as organ failure, or require longer hospitalization, even when the AKI was transient and resolved completely after treatment.
Courtesy American Gastroenterological Association
More than half of the patients in this study who had episodes of AKI according to the new definition did not meet the criteria of the old definition. So using the new definition will help identify these high-risk patients at an earlier stage of renal dysfunction, "well before the stringent diagnostic criteria of [the old definition] are reached," when they will have a better treatment response, said Dr. Florence Wong of the division of gastroenterology, University of Toronto, and her associates.
The old definition of AKI required the presence of hepatorenal syndrome, with a serum creatinine level of greater than 2.5 mg/dL. This meant that patients with less severe renal dysfunction didn’t qualify and weren’t treated. But emerging evidence indicates that even mild degrees of renal dysfunction signal a poor prognosis, and that serum creatinine alone doesn’t accurately reflect renal dysfunction in advanced cirrhosis.
So the International Ascites Club and the Acute Dialysis Quality Initiative (ADQI) group proposed that acute kidney injury in cirrhosis should be redefined as an increase in serum creatinine level of 0.3 mg/dL or greater within 48 hours, or a 50% increase in serum creatinine level from a stable baseline reading within the previous 6 months, regardless of final serum creatinine level.
Dr. Wong and her colleagues assessed the new definition in a cohort of 337 cirrhotic patients treated during a 2-year period at 12 North American medical centers who were admitted with a bacterial infection (287 subjects) or who developed a bacterial infection during hospitalization (50 subjects). The most common infections were urinary tract infection (27% of patients), spontaneous bacterial peritonitis (21%), skin infection (14%), pneumonia (10%), and spontaneous bacteremia with no clear source of infection (9%).
Approximately half of these patients (49%) developed at least one episode of AKI during hospitalization. The 30-day mortality was significantly higher for those who developed AKI according to the new definition (34% mortality) than in those who did not (7% mortality), the investigators said.
Most patients who developed AKI had only a transient case, and their renal function completely recovered. Yet their subsequent mortality within 30 days was twice as high as that for patients who didn’t have any AKI.
The negative predictive value of the new definition of AKI was 93%, and the positive predictive value was 34%.
This study was supported in part by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Center for Research Resources. No financial conflicts of interest were reported.
Renal dysfunction in patients with cirrhosis is often associated with sepsis. This combination involves a very high probability of death. Recently, the concept of acute kidney injury has been proposed to be extended also to renal failure occurring in patients with cirrhosis. AKI should overcome limitations associated with a fixed creatinine threshold, ensure rapid identification of renal dysfunction, and allow timely treatment in patients with hepatorenal syndrome. However, AKI should also overcome the skepticism of those who wish not to abandon previous definitions.
The recent paper of Dr. Wong and her colleagues explored the impact of AKI in 337 hospitalized patients with cirrhosis. Two-hundred eighty-seven patients had bacterial infection at admission, and 93 developed it during hospitalization. Overall, 68 patients died from multiorgan failure, whereas only 7% of patients without AKI died. Mortality ranged from 15% in patients who recovered from AKI to 80% in those who did not. Moreover, 76 patients (23%) developed a second infection, often associated with invasive procedures! An elevated Model for End-Stage Liver Disease score and a second infection were factors independently associated with AKI. Accordingly, the development of AKI in cirrhosis, even if reversible, was shown to be a strong predictor of short survival.
These findings show that, in cirrhosis, even small creatinine changes (0.3 mg) are clinically relevant, and that AKI is probably a hallmark of hemodynamic instability with a risk of multiorgan failure and death. The altered hemodynamics in patients with cirrhosis cause central hypovolemia. Aiming at protecting our patients from infection and AKI, we should also pay more attention to clinical procedures that raise serum creatinine level.
Dr. Francesco Salerno is in the department of internal medicine, at the Policlinico IRCCS San Donato, University of Milan (Italy); Dr. Vincenzo La Mura is with the Fondazione IRCCS Ca'Granda, in the department of gastroenterologia-1 of the Hospital Maggiore Policlinico, Milan. They reported no relevant financial conflicts.
Renal dysfunction in patients with cirrhosis is often associated with sepsis. This combination involves a very high probability of death. Recently, the concept of acute kidney injury has been proposed to be extended also to renal failure occurring in patients with cirrhosis. AKI should overcome limitations associated with a fixed creatinine threshold, ensure rapid identification of renal dysfunction, and allow timely treatment in patients with hepatorenal syndrome. However, AKI should also overcome the skepticism of those who wish not to abandon previous definitions.
The recent paper of Dr. Wong and her colleagues explored the impact of AKI in 337 hospitalized patients with cirrhosis. Two-hundred eighty-seven patients had bacterial infection at admission, and 93 developed it during hospitalization. Overall, 68 patients died from multiorgan failure, whereas only 7% of patients without AKI died. Mortality ranged from 15% in patients who recovered from AKI to 80% in those who did not. Moreover, 76 patients (23%) developed a second infection, often associated with invasive procedures! An elevated Model for End-Stage Liver Disease score and a second infection were factors independently associated with AKI. Accordingly, the development of AKI in cirrhosis, even if reversible, was shown to be a strong predictor of short survival.
These findings show that, in cirrhosis, even small creatinine changes (0.3 mg) are clinically relevant, and that AKI is probably a hallmark of hemodynamic instability with a risk of multiorgan failure and death. The altered hemodynamics in patients with cirrhosis cause central hypovolemia. Aiming at protecting our patients from infection and AKI, we should also pay more attention to clinical procedures that raise serum creatinine level.
Dr. Francesco Salerno is in the department of internal medicine, at the Policlinico IRCCS San Donato, University of Milan (Italy); Dr. Vincenzo La Mura is with the Fondazione IRCCS Ca'Granda, in the department of gastroenterologia-1 of the Hospital Maggiore Policlinico, Milan. They reported no relevant financial conflicts.
Renal dysfunction in patients with cirrhosis is often associated with sepsis. This combination involves a very high probability of death. Recently, the concept of acute kidney injury has been proposed to be extended also to renal failure occurring in patients with cirrhosis. AKI should overcome limitations associated with a fixed creatinine threshold, ensure rapid identification of renal dysfunction, and allow timely treatment in patients with hepatorenal syndrome. However, AKI should also overcome the skepticism of those who wish not to abandon previous definitions.
The recent paper of Dr. Wong and her colleagues explored the impact of AKI in 337 hospitalized patients with cirrhosis. Two-hundred eighty-seven patients had bacterial infection at admission, and 93 developed it during hospitalization. Overall, 68 patients died from multiorgan failure, whereas only 7% of patients without AKI died. Mortality ranged from 15% in patients who recovered from AKI to 80% in those who did not. Moreover, 76 patients (23%) developed a second infection, often associated with invasive procedures! An elevated Model for End-Stage Liver Disease score and a second infection were factors independently associated with AKI. Accordingly, the development of AKI in cirrhosis, even if reversible, was shown to be a strong predictor of short survival.
These findings show that, in cirrhosis, even small creatinine changes (0.3 mg) are clinically relevant, and that AKI is probably a hallmark of hemodynamic instability with a risk of multiorgan failure and death. The altered hemodynamics in patients with cirrhosis cause central hypovolemia. Aiming at protecting our patients from infection and AKI, we should also pay more attention to clinical procedures that raise serum creatinine level.
Dr. Francesco Salerno is in the department of internal medicine, at the Policlinico IRCCS San Donato, University of Milan (Italy); Dr. Vincenzo La Mura is with the Fondazione IRCCS Ca'Granda, in the department of gastroenterologia-1 of the Hospital Maggiore Policlinico, Milan. They reported no relevant financial conflicts.
The newly proposed consensus definition of acute kidney injury in patients with cirrhosis accurately predicts 30-day mortality and other adverse outcomes in this patient population much better than the current, more rigid definition would, according to a report in the December issue of Gastroenterology (doi:10.1053/j.gastro.2013.08.051).
In what they described as the largest prospective study of this topic to date, researchers found that the recently proposed, broader redefinition of acute kidney injury (AKI) correctly identified which patients were likely to die, develop severe complications such as organ failure, or require longer hospitalization, even when the AKI was transient and resolved completely after treatment.
Courtesy American Gastroenterological Association
More than half of the patients in this study who had episodes of AKI according to the new definition did not meet the criteria of the old definition. So using the new definition will help identify these high-risk patients at an earlier stage of renal dysfunction, "well before the stringent diagnostic criteria of [the old definition] are reached," when they will have a better treatment response, said Dr. Florence Wong of the division of gastroenterology, University of Toronto, and her associates.
The old definition of AKI required the presence of hepatorenal syndrome, with a serum creatinine level of greater than 2.5 mg/dL. This meant that patients with less severe renal dysfunction didn’t qualify and weren’t treated. But emerging evidence indicates that even mild degrees of renal dysfunction signal a poor prognosis, and that serum creatinine alone doesn’t accurately reflect renal dysfunction in advanced cirrhosis.
So the International Ascites Club and the Acute Dialysis Quality Initiative (ADQI) group proposed that acute kidney injury in cirrhosis should be redefined as an increase in serum creatinine level of 0.3 mg/dL or greater within 48 hours, or a 50% increase in serum creatinine level from a stable baseline reading within the previous 6 months, regardless of final serum creatinine level.
Dr. Wong and her colleagues assessed the new definition in a cohort of 337 cirrhotic patients treated during a 2-year period at 12 North American medical centers who were admitted with a bacterial infection (287 subjects) or who developed a bacterial infection during hospitalization (50 subjects). The most common infections were urinary tract infection (27% of patients), spontaneous bacterial peritonitis (21%), skin infection (14%), pneumonia (10%), and spontaneous bacteremia with no clear source of infection (9%).
Approximately half of these patients (49%) developed at least one episode of AKI during hospitalization. The 30-day mortality was significantly higher for those who developed AKI according to the new definition (34% mortality) than in those who did not (7% mortality), the investigators said.
Most patients who developed AKI had only a transient case, and their renal function completely recovered. Yet their subsequent mortality within 30 days was twice as high as that for patients who didn’t have any AKI.
The negative predictive value of the new definition of AKI was 93%, and the positive predictive value was 34%.
This study was supported in part by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Center for Research Resources. No financial conflicts of interest were reported.
The newly proposed consensus definition of acute kidney injury in patients with cirrhosis accurately predicts 30-day mortality and other adverse outcomes in this patient population much better than the current, more rigid definition would, according to a report in the December issue of Gastroenterology (doi:10.1053/j.gastro.2013.08.051).
In what they described as the largest prospective study of this topic to date, researchers found that the recently proposed, broader redefinition of acute kidney injury (AKI) correctly identified which patients were likely to die, develop severe complications such as organ failure, or require longer hospitalization, even when the AKI was transient and resolved completely after treatment.
Courtesy American Gastroenterological Association
More than half of the patients in this study who had episodes of AKI according to the new definition did not meet the criteria of the old definition. So using the new definition will help identify these high-risk patients at an earlier stage of renal dysfunction, "well before the stringent diagnostic criteria of [the old definition] are reached," when they will have a better treatment response, said Dr. Florence Wong of the division of gastroenterology, University of Toronto, and her associates.
The old definition of AKI required the presence of hepatorenal syndrome, with a serum creatinine level of greater than 2.5 mg/dL. This meant that patients with less severe renal dysfunction didn’t qualify and weren’t treated. But emerging evidence indicates that even mild degrees of renal dysfunction signal a poor prognosis, and that serum creatinine alone doesn’t accurately reflect renal dysfunction in advanced cirrhosis.
So the International Ascites Club and the Acute Dialysis Quality Initiative (ADQI) group proposed that acute kidney injury in cirrhosis should be redefined as an increase in serum creatinine level of 0.3 mg/dL or greater within 48 hours, or a 50% increase in serum creatinine level from a stable baseline reading within the previous 6 months, regardless of final serum creatinine level.
Dr. Wong and her colleagues assessed the new definition in a cohort of 337 cirrhotic patients treated during a 2-year period at 12 North American medical centers who were admitted with a bacterial infection (287 subjects) or who developed a bacterial infection during hospitalization (50 subjects). The most common infections were urinary tract infection (27% of patients), spontaneous bacterial peritonitis (21%), skin infection (14%), pneumonia (10%), and spontaneous bacteremia with no clear source of infection (9%).
Approximately half of these patients (49%) developed at least one episode of AKI during hospitalization. The 30-day mortality was significantly higher for those who developed AKI according to the new definition (34% mortality) than in those who did not (7% mortality), the investigators said.
Most patients who developed AKI had only a transient case, and their renal function completely recovered. Yet their subsequent mortality within 30 days was twice as high as that for patients who didn’t have any AKI.
The negative predictive value of the new definition of AKI was 93%, and the positive predictive value was 34%.
This study was supported in part by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Center for Research Resources. No financial conflicts of interest were reported.
FROM GASTROENTEROLOGY
Major finding: 30-day mortality was significantly higher for those who developed acute kidney injury according to a new definition (34% mortality) than in those who did not (7% mortality).
Data source: A cohort study of 337 inpatients at 12 North American medical centers who had cirrhosis and a bacterial infection, half of whom developed AKI.
Disclosures: This study was supported in part by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Center for Research Resources. No financial conflicts of interest were reported.
Practice tips for opioid prescribing
Prescription opioids remain a profound clinical challenge, and we as prescribing practitioners have a significant amount of control over this epidemic. About 14,000 people die from prescription opioid overdoses each year in the United States. Our good intentions to alleviate patient suffering are inextricably bound to the reality of potential iatrogenic harm.
As the corpus of knowledge around best practices for safe prescribing has evolved, hopefully we are evolving our practice for our own patients. But for patients whom we inherit from other practitioners or for those who have been in our practice for years on high doses of opioid analgesics, we need to become comfortable telling them, "Things are different now." In order for us to feel comfortable saying this, though, we have to know what best practices are and how they are different from our current practices.
So what are best practices?
For opioid prescribing, best practices reduce the risk of overdose and misuse. Dr. Teryl Nuckols of the University of California, Los Angeles, and her colleagues published a systematic review of guideline recommendations related to mitigating the risk for accidental overdose and misuse (Ann. Intern. Med. 2013 Nov. 12 [doi: 10.7326/0003-4819-160-1-201401070-00732]). Thirteen guidelines informed the major conclusions, and all were published after 2009.
One risk mitigation strategy is having an upper dosing threshold that we do not violate. Evidence suggests that the risk for accidental overdose increases an estimated 1.9- to 3.1-fold with doses of 50- to 100-mg morphine equivalents, and even more dramatically with doses above 200 mg. Another strategy is to avoid prescribing methadone unless you are extremely comfortable with this medication, and to be careful with fentanyl because of unpredictable absorption with fever, exercise, or heat exposure.
Telling our patients that they can "have opioids or benzodiazepines, but not both" (especially if they are on more than 100 mg of morphine equivalents per day) can reduce risk, because 50% of accidental overdoses involve both. When switching opioids, reducing the dose of the equivalent opioids by 25%-50% is a safe practice, and free apps are available that can do this conversion for us.
Opioid contracts also may be helpful, and the use of screening tools (Current Opioid Misuse Measure) may inform our practice. Urine drug testing for the presence of the medication may be helpful to ensure adherence; the differential for a true negative is diversion, hoarding, or self-dosing leading to running out prematurely.
Concern about abuse is high, but data suggest that the absolute prevalence of this is low. Abuse occurs among 0.43%-3.27% of patients on chronic opioids, and addiction affects less than 0.05% (J. Pain Symptom Manage. 2008;35:214-28). Some experts suggest that trying to decipher abuse in the setting of chronic pain management with opioids is a "distinction without a difference."
Our main focus needs to remain on patient safety. We need to have honest conversations with our patients about mutual concerns and goals, and change discussions around appropriate dose reductions from "You want me to be in pain!" to "We need you to be safe."
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. He reports no conflicts of interest.
Prescription opioids remain a profound clinical challenge, and we as prescribing practitioners have a significant amount of control over this epidemic. About 14,000 people die from prescription opioid overdoses each year in the United States. Our good intentions to alleviate patient suffering are inextricably bound to the reality of potential iatrogenic harm.
As the corpus of knowledge around best practices for safe prescribing has evolved, hopefully we are evolving our practice for our own patients. But for patients whom we inherit from other practitioners or for those who have been in our practice for years on high doses of opioid analgesics, we need to become comfortable telling them, "Things are different now." In order for us to feel comfortable saying this, though, we have to know what best practices are and how they are different from our current practices.
So what are best practices?
For opioid prescribing, best practices reduce the risk of overdose and misuse. Dr. Teryl Nuckols of the University of California, Los Angeles, and her colleagues published a systematic review of guideline recommendations related to mitigating the risk for accidental overdose and misuse (Ann. Intern. Med. 2013 Nov. 12 [doi: 10.7326/0003-4819-160-1-201401070-00732]). Thirteen guidelines informed the major conclusions, and all were published after 2009.
One risk mitigation strategy is having an upper dosing threshold that we do not violate. Evidence suggests that the risk for accidental overdose increases an estimated 1.9- to 3.1-fold with doses of 50- to 100-mg morphine equivalents, and even more dramatically with doses above 200 mg. Another strategy is to avoid prescribing methadone unless you are extremely comfortable with this medication, and to be careful with fentanyl because of unpredictable absorption with fever, exercise, or heat exposure.
Telling our patients that they can "have opioids or benzodiazepines, but not both" (especially if they are on more than 100 mg of morphine equivalents per day) can reduce risk, because 50% of accidental overdoses involve both. When switching opioids, reducing the dose of the equivalent opioids by 25%-50% is a safe practice, and free apps are available that can do this conversion for us.
Opioid contracts also may be helpful, and the use of screening tools (Current Opioid Misuse Measure) may inform our practice. Urine drug testing for the presence of the medication may be helpful to ensure adherence; the differential for a true negative is diversion, hoarding, or self-dosing leading to running out prematurely.
Concern about abuse is high, but data suggest that the absolute prevalence of this is low. Abuse occurs among 0.43%-3.27% of patients on chronic opioids, and addiction affects less than 0.05% (J. Pain Symptom Manage. 2008;35:214-28). Some experts suggest that trying to decipher abuse in the setting of chronic pain management with opioids is a "distinction without a difference."
Our main focus needs to remain on patient safety. We need to have honest conversations with our patients about mutual concerns and goals, and change discussions around appropriate dose reductions from "You want me to be in pain!" to "We need you to be safe."
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. He reports no conflicts of interest.
Prescription opioids remain a profound clinical challenge, and we as prescribing practitioners have a significant amount of control over this epidemic. About 14,000 people die from prescription opioid overdoses each year in the United States. Our good intentions to alleviate patient suffering are inextricably bound to the reality of potential iatrogenic harm.
As the corpus of knowledge around best practices for safe prescribing has evolved, hopefully we are evolving our practice for our own patients. But for patients whom we inherit from other practitioners or for those who have been in our practice for years on high doses of opioid analgesics, we need to become comfortable telling them, "Things are different now." In order for us to feel comfortable saying this, though, we have to know what best practices are and how they are different from our current practices.
So what are best practices?
For opioid prescribing, best practices reduce the risk of overdose and misuse. Dr. Teryl Nuckols of the University of California, Los Angeles, and her colleagues published a systematic review of guideline recommendations related to mitigating the risk for accidental overdose and misuse (Ann. Intern. Med. 2013 Nov. 12 [doi: 10.7326/0003-4819-160-1-201401070-00732]). Thirteen guidelines informed the major conclusions, and all were published after 2009.
One risk mitigation strategy is having an upper dosing threshold that we do not violate. Evidence suggests that the risk for accidental overdose increases an estimated 1.9- to 3.1-fold with doses of 50- to 100-mg morphine equivalents, and even more dramatically with doses above 200 mg. Another strategy is to avoid prescribing methadone unless you are extremely comfortable with this medication, and to be careful with fentanyl because of unpredictable absorption with fever, exercise, or heat exposure.
Telling our patients that they can "have opioids or benzodiazepines, but not both" (especially if they are on more than 100 mg of morphine equivalents per day) can reduce risk, because 50% of accidental overdoses involve both. When switching opioids, reducing the dose of the equivalent opioids by 25%-50% is a safe practice, and free apps are available that can do this conversion for us.
Opioid contracts also may be helpful, and the use of screening tools (Current Opioid Misuse Measure) may inform our practice. Urine drug testing for the presence of the medication may be helpful to ensure adherence; the differential for a true negative is diversion, hoarding, or self-dosing leading to running out prematurely.
Concern about abuse is high, but data suggest that the absolute prevalence of this is low. Abuse occurs among 0.43%-3.27% of patients on chronic opioids, and addiction affects less than 0.05% (J. Pain Symptom Manage. 2008;35:214-28). Some experts suggest that trying to decipher abuse in the setting of chronic pain management with opioids is a "distinction without a difference."
Our main focus needs to remain on patient safety. We need to have honest conversations with our patients about mutual concerns and goals, and change discussions around appropriate dose reductions from "You want me to be in pain!" to "We need you to be safe."
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. He reports no conflicts of interest.