User login
Case Studies in Toxicology: Hot as a Hare and Red as a Beet
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
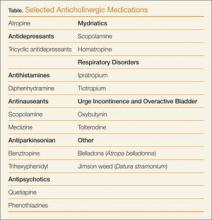
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
Letters to the Editor: Adverse Effects of Tramadol Overuse
Adverse Effects of Tramadol Overuse
To the Editor I very much enjoyed the review article “Appropriate Analgesic Use in the Emergency Department” (Emerg Med. 2014;46[6]:248-255). The discussion of tramadol neglected to mention the fact that overuse of Tramadol can precipitate hyponatremia and epileptic seizures. For this reason, Tramadol should be avoided in patients with seizure disorder. Similarly, other drugs like cyclobenzaprine and tricyclic antidepressants can provoke seizures. Tramadol should not be prescribed to patients on antidepressants or cyclobenzaprine.
William R. Prickett, MD
Author Affiliation: Medical Director,
City of Albuquerque, NM.
In Reply Thank you for your kind note regarding our article “Appropriate Analgesic Use in the Emergency Department.”
You are correct that tramadol use, most commonly in overdose situations or withdrawal following chronic use, has been associated with seizures. Unfortunately, the same is true for many of the narcotic medications we discussed. Seizures in the setting of opioid administration usually occur when given in high doses via the parenteral route. Care must be taken when administering any opioid medication to a patient with an underlying seizure disorder. Given the space limitations of our article, we were unable to discuss all of the adverse effects associated with analgesic use.
Francis L. Counselman, MD, CPE
Peter A. Byers, MD
Author Affiliations: Professor, Department of Emergency Medicine, Eastern Virginia Medical School and Emergency Physicians of Tidewater, Norfolk, VA (Counselman). Emergency physician, Presbyterian Medical Group, Albuquerque, NM (Byers).
Additional Therapy for Taxus Ingestion
To the Editor I read with interest your article “Death and Taxus” (Emerg Med. 2014;46[6]:256-259) as the taxus plant is so ubiquitous in community plantings. (We had a number of them in front of my childhood home, and I find it amazing that we never ate the berries.)
Regarding the treatment for yew berry intoxication, I did not see mention of lipid emulsion therapy for cardiac membrane stabilization. Any comments on this therapy? I have had good responses with lipid emulsion use in overdose scenarios with cardiovascular collapse where other agents, for example, bicarbonate infusion, have failed. Given the relative safety of the infusion as compared to the morbidity of the intoxication, it seems that intralipid therapy merits a mention.
Sarah Silver, MD
Author Affiliation: Attending Physician,
Meriter Emergency Department, Madison, WI.
In Reply We appreciate the thoughtful letter of Dr Silver. Indeed we believe there may be a role for intravenous lipid emulsion therapy in patients with Taxus (taxine) intoxication. The data supporting its benefit come from dozens of case reports and animal models, and the most convincing data derive from the treatment of bupivacaine toxicity. Lipid emulsion therapy is widely assumed to sequester lipophilic toxins within the circulating lipid emulsion and thereby assist in its removal from the affected organ. The Log P (a measure of lipid solubility) for taxine is similar to that for bupivacaine (both about 3)1 supporting its potential to be solubilized by the exogenously-administered lipid. There are no data from either experimental or clinical model to directly support its use, but as you suggest, the therapy is generally safe when administered appropriately.2 We therefore support its use in patients not otherwise responding to conventional therapy. Since lipid emulsion may also sequester medications administered therapeutically, such as amiodarone, appropriate caution should be observed.3
Lewis S. Nelson, MD
Author Affiliation: Professor, Department of Emergency Medicine
and Director of the Medical Toxicology Fellowship Program
at the New York University School of Medicine and the New York City Poison Control Center.
- http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@DOCNO+3541
- American College of Medical Toxicology. ACMT position statement: interim guidance for the use of lipid resuscitation therapy. J Med Toxicol. 2011;7(1):81,82. doi:10.1007/s13181-010-0125-3.
- Niiya T, Litonius E, Petäjä L, Neuvonen PJ, Rosenberg PH. Intravenous lipid emulsion sequesters amiodarone in plasma and eliminates its hypotensive action in pigs. Ann Emerg Med. 2010;56(4):402-408.e2. doi:10.1016/j.annemergmed.2010.06.001.
Adverse Effects of Tramadol Overuse
To the Editor I very much enjoyed the review article “Appropriate Analgesic Use in the Emergency Department” (Emerg Med. 2014;46[6]:248-255). The discussion of tramadol neglected to mention the fact that overuse of Tramadol can precipitate hyponatremia and epileptic seizures. For this reason, Tramadol should be avoided in patients with seizure disorder. Similarly, other drugs like cyclobenzaprine and tricyclic antidepressants can provoke seizures. Tramadol should not be prescribed to patients on antidepressants or cyclobenzaprine.
William R. Prickett, MD
Author Affiliation: Medical Director,
City of Albuquerque, NM.
In Reply Thank you for your kind note regarding our article “Appropriate Analgesic Use in the Emergency Department.”
You are correct that tramadol use, most commonly in overdose situations or withdrawal following chronic use, has been associated with seizures. Unfortunately, the same is true for many of the narcotic medications we discussed. Seizures in the setting of opioid administration usually occur when given in high doses via the parenteral route. Care must be taken when administering any opioid medication to a patient with an underlying seizure disorder. Given the space limitations of our article, we were unable to discuss all of the adverse effects associated with analgesic use.
Francis L. Counselman, MD, CPE
Peter A. Byers, MD
Author Affiliations: Professor, Department of Emergency Medicine, Eastern Virginia Medical School and Emergency Physicians of Tidewater, Norfolk, VA (Counselman). Emergency physician, Presbyterian Medical Group, Albuquerque, NM (Byers).
Additional Therapy for Taxus Ingestion
To the Editor I read with interest your article “Death and Taxus” (Emerg Med. 2014;46[6]:256-259) as the taxus plant is so ubiquitous in community plantings. (We had a number of them in front of my childhood home, and I find it amazing that we never ate the berries.)
Regarding the treatment for yew berry intoxication, I did not see mention of lipid emulsion therapy for cardiac membrane stabilization. Any comments on this therapy? I have had good responses with lipid emulsion use in overdose scenarios with cardiovascular collapse where other agents, for example, bicarbonate infusion, have failed. Given the relative safety of the infusion as compared to the morbidity of the intoxication, it seems that intralipid therapy merits a mention.
Sarah Silver, MD
Author Affiliation: Attending Physician,
Meriter Emergency Department, Madison, WI.
In Reply We appreciate the thoughtful letter of Dr Silver. Indeed we believe there may be a role for intravenous lipid emulsion therapy in patients with Taxus (taxine) intoxication. The data supporting its benefit come from dozens of case reports and animal models, and the most convincing data derive from the treatment of bupivacaine toxicity. Lipid emulsion therapy is widely assumed to sequester lipophilic toxins within the circulating lipid emulsion and thereby assist in its removal from the affected organ. The Log P (a measure of lipid solubility) for taxine is similar to that for bupivacaine (both about 3)1 supporting its potential to be solubilized by the exogenously-administered lipid. There are no data from either experimental or clinical model to directly support its use, but as you suggest, the therapy is generally safe when administered appropriately.2 We therefore support its use in patients not otherwise responding to conventional therapy. Since lipid emulsion may also sequester medications administered therapeutically, such as amiodarone, appropriate caution should be observed.3
Lewis S. Nelson, MD
Author Affiliation: Professor, Department of Emergency Medicine
and Director of the Medical Toxicology Fellowship Program
at the New York University School of Medicine and the New York City Poison Control Center.
Adverse Effects of Tramadol Overuse
To the Editor I very much enjoyed the review article “Appropriate Analgesic Use in the Emergency Department” (Emerg Med. 2014;46[6]:248-255). The discussion of tramadol neglected to mention the fact that overuse of Tramadol can precipitate hyponatremia and epileptic seizures. For this reason, Tramadol should be avoided in patients with seizure disorder. Similarly, other drugs like cyclobenzaprine and tricyclic antidepressants can provoke seizures. Tramadol should not be prescribed to patients on antidepressants or cyclobenzaprine.
William R. Prickett, MD
Author Affiliation: Medical Director,
City of Albuquerque, NM.
In Reply Thank you for your kind note regarding our article “Appropriate Analgesic Use in the Emergency Department.”
You are correct that tramadol use, most commonly in overdose situations or withdrawal following chronic use, has been associated with seizures. Unfortunately, the same is true for many of the narcotic medications we discussed. Seizures in the setting of opioid administration usually occur when given in high doses via the parenteral route. Care must be taken when administering any opioid medication to a patient with an underlying seizure disorder. Given the space limitations of our article, we were unable to discuss all of the adverse effects associated with analgesic use.
Francis L. Counselman, MD, CPE
Peter A. Byers, MD
Author Affiliations: Professor, Department of Emergency Medicine, Eastern Virginia Medical School and Emergency Physicians of Tidewater, Norfolk, VA (Counselman). Emergency physician, Presbyterian Medical Group, Albuquerque, NM (Byers).
Additional Therapy for Taxus Ingestion
To the Editor I read with interest your article “Death and Taxus” (Emerg Med. 2014;46[6]:256-259) as the taxus plant is so ubiquitous in community plantings. (We had a number of them in front of my childhood home, and I find it amazing that we never ate the berries.)
Regarding the treatment for yew berry intoxication, I did not see mention of lipid emulsion therapy for cardiac membrane stabilization. Any comments on this therapy? I have had good responses with lipid emulsion use in overdose scenarios with cardiovascular collapse where other agents, for example, bicarbonate infusion, have failed. Given the relative safety of the infusion as compared to the morbidity of the intoxication, it seems that intralipid therapy merits a mention.
Sarah Silver, MD
Author Affiliation: Attending Physician,
Meriter Emergency Department, Madison, WI.
In Reply We appreciate the thoughtful letter of Dr Silver. Indeed we believe there may be a role for intravenous lipid emulsion therapy in patients with Taxus (taxine) intoxication. The data supporting its benefit come from dozens of case reports and animal models, and the most convincing data derive from the treatment of bupivacaine toxicity. Lipid emulsion therapy is widely assumed to sequester lipophilic toxins within the circulating lipid emulsion and thereby assist in its removal from the affected organ. The Log P (a measure of lipid solubility) for taxine is similar to that for bupivacaine (both about 3)1 supporting its potential to be solubilized by the exogenously-administered lipid. There are no data from either experimental or clinical model to directly support its use, but as you suggest, the therapy is generally safe when administered appropriately.2 We therefore support its use in patients not otherwise responding to conventional therapy. Since lipid emulsion may also sequester medications administered therapeutically, such as amiodarone, appropriate caution should be observed.3
Lewis S. Nelson, MD
Author Affiliation: Professor, Department of Emergency Medicine
and Director of the Medical Toxicology Fellowship Program
at the New York University School of Medicine and the New York City Poison Control Center.
- http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@DOCNO+3541
- American College of Medical Toxicology. ACMT position statement: interim guidance for the use of lipid resuscitation therapy. J Med Toxicol. 2011;7(1):81,82. doi:10.1007/s13181-010-0125-3.
- Niiya T, Litonius E, Petäjä L, Neuvonen PJ, Rosenberg PH. Intravenous lipid emulsion sequesters amiodarone in plasma and eliminates its hypotensive action in pigs. Ann Emerg Med. 2010;56(4):402-408.e2. doi:10.1016/j.annemergmed.2010.06.001.
- http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@DOCNO+3541
- American College of Medical Toxicology. ACMT position statement: interim guidance for the use of lipid resuscitation therapy. J Med Toxicol. 2011;7(1):81,82. doi:10.1007/s13181-010-0125-3.
- Niiya T, Litonius E, Petäjä L, Neuvonen PJ, Rosenberg PH. Intravenous lipid emulsion sequesters amiodarone in plasma and eliminates its hypotensive action in pigs. Ann Emerg Med. 2010;56(4):402-408.e2. doi:10.1016/j.annemergmed.2010.06.001.
Heroin-related drug-poisoning deaths rose 110% from 2002 to 2011
The annual number of drug-poisoning deaths involving heroin more than doubled from 2002 to 2011, increasing from 2,089 to 4,397, the National Center for Health Statistics reported.
Over that period, the rate of heroin-related drug-poisoning deaths increased 171% among non-Hispanic whites aged 18-44 years – going from 1.4 per 100,000 population to 3.8 – and by 100% among non-Hispanic whites aged 45-64 years – from 0.7 to 1.4 per 100,000, according to the NCHS (MMWR 2014;63:595).
The rates for non-Hispanic blacks and Hispanics, by comparison, were stable. Non-Hispanic blacks were at 1.0 per 100,000 for younger adults and 2.2 per 100,000 for those aged 45-64 years in 2002 and remained there in 2011. The rate for Hispanics aged 18-44 years rose from 1.3 to 1.5, but the rate for 45- to 64-year-olds dropped from 2 to 1.9 per 100,000, according to mortality data from the National Vital Statistics System.

The annual number of drug-poisoning deaths involving heroin more than doubled from 2002 to 2011, increasing from 2,089 to 4,397, the National Center for Health Statistics reported.
Over that period, the rate of heroin-related drug-poisoning deaths increased 171% among non-Hispanic whites aged 18-44 years – going from 1.4 per 100,000 population to 3.8 – and by 100% among non-Hispanic whites aged 45-64 years – from 0.7 to 1.4 per 100,000, according to the NCHS (MMWR 2014;63:595).
The rates for non-Hispanic blacks and Hispanics, by comparison, were stable. Non-Hispanic blacks were at 1.0 per 100,000 for younger adults and 2.2 per 100,000 for those aged 45-64 years in 2002 and remained there in 2011. The rate for Hispanics aged 18-44 years rose from 1.3 to 1.5, but the rate for 45- to 64-year-olds dropped from 2 to 1.9 per 100,000, according to mortality data from the National Vital Statistics System.

The annual number of drug-poisoning deaths involving heroin more than doubled from 2002 to 2011, increasing from 2,089 to 4,397, the National Center for Health Statistics reported.
Over that period, the rate of heroin-related drug-poisoning deaths increased 171% among non-Hispanic whites aged 18-44 years – going from 1.4 per 100,000 population to 3.8 – and by 100% among non-Hispanic whites aged 45-64 years – from 0.7 to 1.4 per 100,000, according to the NCHS (MMWR 2014;63:595).
The rates for non-Hispanic blacks and Hispanics, by comparison, were stable. Non-Hispanic blacks were at 1.0 per 100,000 for younger adults and 2.2 per 100,000 for those aged 45-64 years in 2002 and remained there in 2011. The rate for Hispanics aged 18-44 years rose from 1.3 to 1.5, but the rate for 45- to 64-year-olds dropped from 2 to 1.9 per 100,000, according to mortality data from the National Vital Statistics System.

FROM MMWR
ECG predictors of cardiac events mandate troponin level testing in drug overdoses
DALLAS – The initial ECG is invaluable in predicting which emergency department patients with acute drug overdose will have a major cardiovascular event during hospitalization, a prospective study indicates.
"Based on our data, ECG evidence of ischemia or infarction really mandates sending for a troponin level in ED patients with overdose," Dr. Alex F. Manini said at the annual meeting of the Society for Academic Emergency Medicine.
The findings are important as "we’re currently undergoing the worst epidemic of drug overdoses in our nation’s history," observed Dr. Manini of the department of emergency medicine at Mount Sinai School of Medicine in New York. Poisoning is now the No. 1 cause of injury-related fatalities in the United States, and many patient series indicate 10%-15% of ED patients with an acute drug overdose experience a major cardiac event during their hospitalization.
Dr. Manini and his colleagues performed a study that validated the prognostic value of four high-risk features of the ED admission ECG in an acute drug overdose cohort: ectopy, a QTc interval of 500 msec or longer, non–sinus rhythm, and any evidence of ischemia or infarction.
Emergency physicians can readily identify those features without need for input from a cardiologist, he said.
In their study performed at two university EDs, 16% of 589 adults with acute drug overdoses experienced an acute MI, cardiogenic shock, dysrhythmia, or cardiac arrest during their hospitalization. The most common drug exposures were benzodiazepines, opioids, and acetaminophen.
Ectopy was associated with an 8.9-fold increased odds ratio for a major cardiovascular event. A QTc of 500 msec or longer was associated with an odds ratio of 11.2; a non–sinus rhythm, 8.9; and ischemia, 5.0.
The presence of one or more of these four ECG predictors was associated with 68% sensitivity and 69% specificity for a subsequent in-hospital cardiac event, with a negative predictive value of 91.9%. Dr. Manini called those sensitivity and specificity figures "modest." Thus, the ECG findings alone are not sufficient to exclude the likelihood of a cardiac event, although they certainly are useful in risk stratification. Future studies will seek to boost the predictive power by combining the ECG findings with other clinical tools, he said.
A QT dispersion of 50 msec or more also proved useful for prognosis, with an associated 2.2-fold increased risk of an in-hospital cardiac event. However, measuring QT dispersion is a fairly cumbersome process, and for this reason it needs further study before being introduced into clinical practice in busy EDs, Dr. Manini added.
In this study, any ECG evidence of ischemia or infarction – including ST depression or elevation, T wave inversion, or Q waves – had specificities of 91%-98% for an elevated troponin assay. In addition, ST depression was associated with a 6.4-fold increased odds ratio for in-hospital cardiac arrest.
The study was funded by the National Institute on Drug Abuse. Dr. Manini reported having no financial conflicts.
DALLAS – The initial ECG is invaluable in predicting which emergency department patients with acute drug overdose will have a major cardiovascular event during hospitalization, a prospective study indicates.
"Based on our data, ECG evidence of ischemia or infarction really mandates sending for a troponin level in ED patients with overdose," Dr. Alex F. Manini said at the annual meeting of the Society for Academic Emergency Medicine.
The findings are important as "we’re currently undergoing the worst epidemic of drug overdoses in our nation’s history," observed Dr. Manini of the department of emergency medicine at Mount Sinai School of Medicine in New York. Poisoning is now the No. 1 cause of injury-related fatalities in the United States, and many patient series indicate 10%-15% of ED patients with an acute drug overdose experience a major cardiac event during their hospitalization.
Dr. Manini and his colleagues performed a study that validated the prognostic value of four high-risk features of the ED admission ECG in an acute drug overdose cohort: ectopy, a QTc interval of 500 msec or longer, non–sinus rhythm, and any evidence of ischemia or infarction.
Emergency physicians can readily identify those features without need for input from a cardiologist, he said.
In their study performed at two university EDs, 16% of 589 adults with acute drug overdoses experienced an acute MI, cardiogenic shock, dysrhythmia, or cardiac arrest during their hospitalization. The most common drug exposures were benzodiazepines, opioids, and acetaminophen.
Ectopy was associated with an 8.9-fold increased odds ratio for a major cardiovascular event. A QTc of 500 msec or longer was associated with an odds ratio of 11.2; a non–sinus rhythm, 8.9; and ischemia, 5.0.
The presence of one or more of these four ECG predictors was associated with 68% sensitivity and 69% specificity for a subsequent in-hospital cardiac event, with a negative predictive value of 91.9%. Dr. Manini called those sensitivity and specificity figures "modest." Thus, the ECG findings alone are not sufficient to exclude the likelihood of a cardiac event, although they certainly are useful in risk stratification. Future studies will seek to boost the predictive power by combining the ECG findings with other clinical tools, he said.
A QT dispersion of 50 msec or more also proved useful for prognosis, with an associated 2.2-fold increased risk of an in-hospital cardiac event. However, measuring QT dispersion is a fairly cumbersome process, and for this reason it needs further study before being introduced into clinical practice in busy EDs, Dr. Manini added.
In this study, any ECG evidence of ischemia or infarction – including ST depression or elevation, T wave inversion, or Q waves – had specificities of 91%-98% for an elevated troponin assay. In addition, ST depression was associated with a 6.4-fold increased odds ratio for in-hospital cardiac arrest.
The study was funded by the National Institute on Drug Abuse. Dr. Manini reported having no financial conflicts.
DALLAS – The initial ECG is invaluable in predicting which emergency department patients with acute drug overdose will have a major cardiovascular event during hospitalization, a prospective study indicates.
"Based on our data, ECG evidence of ischemia or infarction really mandates sending for a troponin level in ED patients with overdose," Dr. Alex F. Manini said at the annual meeting of the Society for Academic Emergency Medicine.
The findings are important as "we’re currently undergoing the worst epidemic of drug overdoses in our nation’s history," observed Dr. Manini of the department of emergency medicine at Mount Sinai School of Medicine in New York. Poisoning is now the No. 1 cause of injury-related fatalities in the United States, and many patient series indicate 10%-15% of ED patients with an acute drug overdose experience a major cardiac event during their hospitalization.
Dr. Manini and his colleagues performed a study that validated the prognostic value of four high-risk features of the ED admission ECG in an acute drug overdose cohort: ectopy, a QTc interval of 500 msec or longer, non–sinus rhythm, and any evidence of ischemia or infarction.
Emergency physicians can readily identify those features without need for input from a cardiologist, he said.
In their study performed at two university EDs, 16% of 589 adults with acute drug overdoses experienced an acute MI, cardiogenic shock, dysrhythmia, or cardiac arrest during their hospitalization. The most common drug exposures were benzodiazepines, opioids, and acetaminophen.
Ectopy was associated with an 8.9-fold increased odds ratio for a major cardiovascular event. A QTc of 500 msec or longer was associated with an odds ratio of 11.2; a non–sinus rhythm, 8.9; and ischemia, 5.0.
The presence of one or more of these four ECG predictors was associated with 68% sensitivity and 69% specificity for a subsequent in-hospital cardiac event, with a negative predictive value of 91.9%. Dr. Manini called those sensitivity and specificity figures "modest." Thus, the ECG findings alone are not sufficient to exclude the likelihood of a cardiac event, although they certainly are useful in risk stratification. Future studies will seek to boost the predictive power by combining the ECG findings with other clinical tools, he said.
A QT dispersion of 50 msec or more also proved useful for prognosis, with an associated 2.2-fold increased risk of an in-hospital cardiac event. However, measuring QT dispersion is a fairly cumbersome process, and for this reason it needs further study before being introduced into clinical practice in busy EDs, Dr. Manini added.
In this study, any ECG evidence of ischemia or infarction – including ST depression or elevation, T wave inversion, or Q waves – had specificities of 91%-98% for an elevated troponin assay. In addition, ST depression was associated with a 6.4-fold increased odds ratio for in-hospital cardiac arrest.
The study was funded by the National Institute on Drug Abuse. Dr. Manini reported having no financial conflicts.
AT SAEM 2014
Key clinical point: Roughly 15% of adult ED patients with an acute drug overdose will experience a major cardiac event during their hospital stay. The ED admission ECG is helpful in risk stratification.
Major finding: Acute drug overdose patients with one or more of four key findings on their initial ECG in the ED – ectopy, a QTc interval of 500 msec or longer, non–sinus rhythm, or any evidence of ischemia or infarction – are at increased risk for a major cardiac event during their hospitalization.
Data source: This was a prospective study involving 589 adults with acute drug overdose in two university EDs.
Disclosures: The study was supported by the National Institute on Drug Abuse. The presenter reported having no financial conflicts.
Changing marijuana laws pose health challenges
The recent upsurge in medical and recreational marijuana laws is creating novel public health concerns for physicians, health advocates, and state regulators alike. Implications of the new laws include packaging risks, accessibility to children, dosage dangers, and the potential for greater drug-related traffic injuries.
Now "is really the time for public health [leaders] to engage on some of these issues" surrounding changes in state laws regarding marijuana use, Colorado Assistant Attorney General Eric Kuhn said during an American Society of Law, Medicine, and Ethics webinar on the expansion of medical marijuana laws. Twenty-one states and the District of Columbia have legalized medical marijuana, and eight other states are considering legislation in 2014. Two states – Colorado and Washington – have legalized recreational use of marijuana. Most recently, Florida legislators overwhelmingly voted in May to legalize a strain of marijuana for limited medicinal use. The bill is now in the hands of Florida Gov. Rick Scott.
Health challenges include how best to regulate the drug and to ensure that accessibility does not lead to related harms. Purity and packaging of marijuana are already posing significant worries for states that allow use of the drug, said Mr. Kuhn, who is a National Attorneys General Training and Research Institute/Robert Wood Johnson Foundation public health fellow and the author of a paper about public health issues related to marijuana legalization.
Marijuana samples "have been found to be contaminated with pesticides, herbicides, mold, fungus, bacteria, viruses and other contaminants," he said. Further, "a public health department can’t certify that a product, inherently adulterated with a schedule I substance, is pure."
"The emerging hazards of edibles [are] just beginning to get recognition," Gordon Smith, professor of epidemiology and public health at the University of Maryland, Baltimore, said during the webinar. Of particular concern is the inability to regulate dose. "The standard dose might be an eighth of a brownie. ... Who on earth eats an eighth of a brownie?
Edibles also pose driving risks. "You may start driving and feel fine and then a half an hour later, once the edible starts to get absorbed and have an effect, you start to become very intoxicated," Mr. Smith said. "You really can’t control the dosage."
Some marijuana packages have been labeled to resemble candy and have names similar to those of candy bars. A 2013 analysis of a large Colorado children’s hospital showed that before Sept. 30, 2009, there were zero cases of marijuana ingestion by pediatric patients. After that time, there were 14 cases of marijuana ingestions by children at the hospital, according to a study published in JAMA Pediatrics (2013;167:630-3). The study was conducted before the state’s January recreational marijuana law went into effect but after medical marijuana was decriminalized.
Physicians and health centers are integral to research and safety analyses of medical and recreational marijuana use, health experts said. For instance, more marijuana testing of injured patients by emergency care providers is necessary to determine the role cannabis is playing in traffic crashes, Mr. Smith said.
The recent upsurge in medical and recreational marijuana laws is creating novel public health concerns for physicians, health advocates, and state regulators alike. Implications of the new laws include packaging risks, accessibility to children, dosage dangers, and the potential for greater drug-related traffic injuries.
Now "is really the time for public health [leaders] to engage on some of these issues" surrounding changes in state laws regarding marijuana use, Colorado Assistant Attorney General Eric Kuhn said during an American Society of Law, Medicine, and Ethics webinar on the expansion of medical marijuana laws. Twenty-one states and the District of Columbia have legalized medical marijuana, and eight other states are considering legislation in 2014. Two states – Colorado and Washington – have legalized recreational use of marijuana. Most recently, Florida legislators overwhelmingly voted in May to legalize a strain of marijuana for limited medicinal use. The bill is now in the hands of Florida Gov. Rick Scott.
Health challenges include how best to regulate the drug and to ensure that accessibility does not lead to related harms. Purity and packaging of marijuana are already posing significant worries for states that allow use of the drug, said Mr. Kuhn, who is a National Attorneys General Training and Research Institute/Robert Wood Johnson Foundation public health fellow and the author of a paper about public health issues related to marijuana legalization.
Marijuana samples "have been found to be contaminated with pesticides, herbicides, mold, fungus, bacteria, viruses and other contaminants," he said. Further, "a public health department can’t certify that a product, inherently adulterated with a schedule I substance, is pure."
"The emerging hazards of edibles [are] just beginning to get recognition," Gordon Smith, professor of epidemiology and public health at the University of Maryland, Baltimore, said during the webinar. Of particular concern is the inability to regulate dose. "The standard dose might be an eighth of a brownie. ... Who on earth eats an eighth of a brownie?
Edibles also pose driving risks. "You may start driving and feel fine and then a half an hour later, once the edible starts to get absorbed and have an effect, you start to become very intoxicated," Mr. Smith said. "You really can’t control the dosage."
Some marijuana packages have been labeled to resemble candy and have names similar to those of candy bars. A 2013 analysis of a large Colorado children’s hospital showed that before Sept. 30, 2009, there were zero cases of marijuana ingestion by pediatric patients. After that time, there were 14 cases of marijuana ingestions by children at the hospital, according to a study published in JAMA Pediatrics (2013;167:630-3). The study was conducted before the state’s January recreational marijuana law went into effect but after medical marijuana was decriminalized.
Physicians and health centers are integral to research and safety analyses of medical and recreational marijuana use, health experts said. For instance, more marijuana testing of injured patients by emergency care providers is necessary to determine the role cannabis is playing in traffic crashes, Mr. Smith said.
The recent upsurge in medical and recreational marijuana laws is creating novel public health concerns for physicians, health advocates, and state regulators alike. Implications of the new laws include packaging risks, accessibility to children, dosage dangers, and the potential for greater drug-related traffic injuries.
Now "is really the time for public health [leaders] to engage on some of these issues" surrounding changes in state laws regarding marijuana use, Colorado Assistant Attorney General Eric Kuhn said during an American Society of Law, Medicine, and Ethics webinar on the expansion of medical marijuana laws. Twenty-one states and the District of Columbia have legalized medical marijuana, and eight other states are considering legislation in 2014. Two states – Colorado and Washington – have legalized recreational use of marijuana. Most recently, Florida legislators overwhelmingly voted in May to legalize a strain of marijuana for limited medicinal use. The bill is now in the hands of Florida Gov. Rick Scott.
Health challenges include how best to regulate the drug and to ensure that accessibility does not lead to related harms. Purity and packaging of marijuana are already posing significant worries for states that allow use of the drug, said Mr. Kuhn, who is a National Attorneys General Training and Research Institute/Robert Wood Johnson Foundation public health fellow and the author of a paper about public health issues related to marijuana legalization.
Marijuana samples "have been found to be contaminated with pesticides, herbicides, mold, fungus, bacteria, viruses and other contaminants," he said. Further, "a public health department can’t certify that a product, inherently adulterated with a schedule I substance, is pure."
"The emerging hazards of edibles [are] just beginning to get recognition," Gordon Smith, professor of epidemiology and public health at the University of Maryland, Baltimore, said during the webinar. Of particular concern is the inability to regulate dose. "The standard dose might be an eighth of a brownie. ... Who on earth eats an eighth of a brownie?
Edibles also pose driving risks. "You may start driving and feel fine and then a half an hour later, once the edible starts to get absorbed and have an effect, you start to become very intoxicated," Mr. Smith said. "You really can’t control the dosage."
Some marijuana packages have been labeled to resemble candy and have names similar to those of candy bars. A 2013 analysis of a large Colorado children’s hospital showed that before Sept. 30, 2009, there were zero cases of marijuana ingestion by pediatric patients. After that time, there were 14 cases of marijuana ingestions by children at the hospital, according to a study published in JAMA Pediatrics (2013;167:630-3). The study was conducted before the state’s January recreational marijuana law went into effect but after medical marijuana was decriminalized.
Physicians and health centers are integral to research and safety analyses of medical and recreational marijuana use, health experts said. For instance, more marijuana testing of injured patients by emergency care providers is necessary to determine the role cannabis is playing in traffic crashes, Mr. Smith said.
Case Studies in Toxicology: Death and Taxus
Case
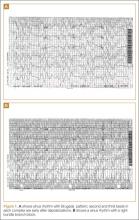
A 50-year-old man ingests two handfuls of small, red berries that he picked from a shrub in front of his apartment building, with the belief that they would have medicinal value. Two hours later, he developed abdominal cramping and vomited multiple times, followed shortly thereafter by profuse diaphoresis, lethargy, and ataxia. His concerned family brought him to the ED where his vital signs on presentation were: blood pressure (BP), 78/43 mm Hg; heart rate (HR), 50 beats/minute; respiratory rate (RR), 12 breaths/minute; temperature (T), 97.8°F. With the exception of bradycardia, the patient’s cardiac, pulmonary, and abdominal examinations were normal. His skin was diaphoretic, and he had no focal motor or sensory deficits or tremor. Initial laboratory values were: hemoglobin, 12.6 g/dL; sodium, 137 mEq/L; potassium, 4.6 mEq/L; bicarbonate, 20 mEq/L; blood urea nitrogen, 17 mg/dL; creatinine, 2.2 mg/dL; glucose, 288 mg/dL. The patient’s troponin I level was slightly elevated at 0.06 ng/mL; electrocardiogram (ECG) results are shown in Figure 1.
Why do plant poisonings occur?
There is the general belief that what is natural is not only healthful but also safe. This is clearly not true: cyanide, uranium, and king cobras are all natural but hardly safe. While most plants chosen for their purported medicinal properties are generally harmless in most patients when taken in low doses, there are plants that are sufficiently poisonous to be consequential with even relatively small exposures. Some people, often unknowingly vulnerable due to genetic or other causes, are uniquely susceptible to even minute doses.
Humans probably learned about plant toxicity early on—most likely the hard way. To this day, however, the Internet is replete with traditional and avant-garde natural healing remedies involving the use of naturally-derived plant products. These numerous bioactive compounds are often sold in plant form or as extracts, the latter being more concerning given their more concentrated formulation.
Plant misidentification is a common cause of poisoning, whether the intended use is for food or medicine. For example, some mistake “deadly nightshade” (Atropa belladonna) berries, which are deep blue, for blueberries, or pokeweed roots for horseradish roots due to their similar appearances.1
Alternatively, even when a plant is correctly identified, patients may experience adverse effects if they exceed the “therapeutic dose” (eg, dysrhythmia from aconite roots used in traditional Chinese medicine) or if the plant is improperly prepared (eg, hypoglycemia from consuming unripe ackee fruit).2 In addition, a toxic plant such as Jimson weed (Datura stramonium) or coca leaf extract may be intentionally ingested for its psychoactive hallucinatory effects.2 Although rare in the United States, in certain parts of Asia, persons intent on self-harm may consume toxic plants.1
When ingested, what plants cause bradycardia and hypotension, and why do these effects occur?
The two broad classes of plant-derived toxins that can cause these findings are cardioactive steroids and sodium channel active agents.
Cardioactive Steroids
There are numerous botanical sources of cardioactive steroids (sometimes called cardiac glycosides) such as Digitalis lanata, from which digoxin is derived; and Digitalis purpurea, the source of digitoxin. Poisoning by Digitalis spp, squill, lily of the valley, oleander, yellow oleander, and Cerbera manghas are clinically similar. Cardioactive steroids act pharmacologically to block the sodium-potassium ATPase pump on the myocardial cell membrane. This in turn increases intracellular sodium, which subsequently inhibits the exchange of extracellular sodium for intracellular calcium, leading to inotropy. Clinical manifestations of toxicity include nausea, vomiting, hyperkalemia, bradycardia, cardiac dysrhythmias, and occasionally hypotension—some of which can be life-threatening.
Sodium Channel Active Agents
Several plant toxins affect the flow of sodium by blocking or activating the sodium channel. Both effects alter the rate and strength of cardiac contraction, causing cardiac dysrhythmias.
Aconite is often used in traditional Chinese medicine. In North America, it is mainly derived from Aconitinum napellus, commonly called monkshood, helmet flower, or wolfsbane. It effectively holds open the voltage-dependent sodium channel, increasing cellular excitability. By prolonging the sodium current influx, neuronal and cardiac repolarization eventually slow due to sodium overload, leading to bradycardia and hypotension, as well as neurological effects. Its cardiotoxicity resembles that caused by cardiac glycosides, though a history of paresthesias or muscle weakness may help to differentiate the two toxins.
Veratrum spp include false hellebore, Indian poke, and California hellebore. These plants are occasionally mistaken for leeks (ramps) and can cause vomiting, bradycardia, and hypotension by a mechanism of action similar to aconitine.
Grayanotoxins, a group of diterpenoid toxins found in death camas, azalea, Rhododendron spp, and mountain laurel, can become concentrated in honey made from these plants. Depending on the specific toxin, they variably open or close the sodium channel. In addition to causing bradycardia and hypotension, patients may exhibit mental status changes (“mad honey” poisoning) and seizures.2
Case Continuation
After rapid infusion of 1-liter of normal saline, the patient’s BP was 80/63 mm Hg and HR was 52 beats/minute. His wife arrived to the ED 30-minutes later with a plastic bag containing the red berries the patient had ingested. The emergency physician identified them as Taxus baccata, or more commonly, yew berries. The patient stated that he ingested both the red fleshy aril and chewed the hard central seed.
How is cardiotoxicity from yew berries treated?
Within hours of ingestion, toxicity progresses from nausea, abdominal pain, paresthesias, and ataxia, to bradycardia, cardiac conduction delays, wide-complex ventricular dysrhythmias and mental status changes.3 Although toxicity of Taxus has been known since antiquity, no antidote exists. Ventricular dysrhythmias causing hemodynamic instability should be electrically cardioverted, although there is no evidence to support the safety or efficacy of such therapy. Since the serum, and therefore cardiac concentration of taxine will be identical after cardioversion to its value prior, recurrent dysrhythmias are common.1 Sodium bicarbonate has been inconsistently effective in the treatment of wide-complex tachydysrhythmias,4 but its use seems counterintuitive for most cases. There may be merit to raising the sodium gradient on an already sodium overloaded myocyte, but short-term gain may lead to unintended consequences. Success with antidysrhythmics has been limited: although amiodarone is often used to treat wide-complex tachydysrhythmias, its efficacy in Taxus toxicity has been conflicting.4-6
There have been a few reported cases of yew alkaloid crossreactivity with digoxin assays, suggesting that digoxin-specific antibody fragments may bind taxine.7 There is no evidence, however, that cardioactive steroids are present in yew, and empiric use of antidigoxin Fab-fragments cannot be recommended. A single case report demonstrated that hemodialysis was ineffective in the removal of taxines, likely due to the toxin’s large volume of distribution.8 As a last resort, extracorporeal life support with membrane oxygenation is described favorably in two cases of yew berry poisoning refractory to conventional therapy.9,10
Case Conclusion
The patient’s ECGs showed a morphologically abnormal rhythm, possibly with a Brugada pattern, which are representative of the dysrhythmias caused by taxine’s inhibitory effects on the sodium and calcium channels. Despite an attempt at electrical cardioversion, the dysrhythmia persisted. He was given intravenous boluses of fluids and started on an amiodarone infusion. The patient’s BP gradually improved over the following 2 hours, and the dysrhythmia resolved with hemodynamic improvement. The amiodarone infusion was then discontinued, and he was admitted to the hospital for further testing. Echocardiography, electrophysiology studies, and cardiac catheterization were all normal. The absence of structural, dysrhythmogenic, and ischemic abnormalities supported the toxic etiology of his hemodynamic aberrations. He was discharged from the hospital 3 days later without report of sequelae.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bruneton J. Toxic Plants; Dangerous to Humans and Animals. Paris, France: Lavoisier Publishing; 1999:4-752.
- Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. In: Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:1537-1560.
- Nelson LS, Shih RD, Balick MJ. Handbook of Poisonous and Injurious Plants. 2nd ed. New York, NY: Springer/New York Botanical Garden; 2007:288-290.
- Pierog J, Kane B, Kane K, Donovan JW. Management of isolated yew berry toxicity with sodium bicarbonate: a case report in treatment efficacy. J Med Toxicol. 2009;5(2):84-89.
- Jones R, Jones J, Causer J, Ewins D, Goenka N, Joseph F. Yew tree poisoning: a near-fatal lesson from history. Clin Med. 2011;11(2):173-175.
- Willaert W, Claessens P, Vankelecom B, Vanderheyden M. Intoxication with Taxus baccata: cardiac arrhythmias following yew leaves ingestion. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):511,512.
- Cummins RO, Haulman J, Quan L, Graves JR, Peterson D, Horan S. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med. 1990;19(1):38-43
- Dahlqvist M, Venzin R, König S, et al. Haemodialysis in Taxus baccata poisoning: a case report. QJM. 2012;105(4):359-361.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in severe Taxus baccata poisoning. Clin Toxicol. 2010;48(5):463-465.
- Soumagne N, Chauvet S, Chatellier D, Robert R, Charrière JM, Menu P. Treatment of yew leaf intoxication with extracorporeal circulation. Am J Emerg Med. 2011;29(3):354.e5-6.
Case
A 50-year-old man ingests two handfuls of small, red berries that he picked from a shrub in front of his apartment building, with the belief that they would have medicinal value. Two hours later, he developed abdominal cramping and vomited multiple times, followed shortly thereafter by profuse diaphoresis, lethargy, and ataxia. His concerned family brought him to the ED where his vital signs on presentation were: blood pressure (BP), 78/43 mm Hg; heart rate (HR), 50 beats/minute; respiratory rate (RR), 12 breaths/minute; temperature (T), 97.8°F. With the exception of bradycardia, the patient’s cardiac, pulmonary, and abdominal examinations were normal. His skin was diaphoretic, and he had no focal motor or sensory deficits or tremor. Initial laboratory values were: hemoglobin, 12.6 g/dL; sodium, 137 mEq/L; potassium, 4.6 mEq/L; bicarbonate, 20 mEq/L; blood urea nitrogen, 17 mg/dL; creatinine, 2.2 mg/dL; glucose, 288 mg/dL. The patient’s troponin I level was slightly elevated at 0.06 ng/mL; electrocardiogram (ECG) results are shown in Figure 1.
Why do plant poisonings occur?
There is the general belief that what is natural is not only healthful but also safe. This is clearly not true: cyanide, uranium, and king cobras are all natural but hardly safe. While most plants chosen for their purported medicinal properties are generally harmless in most patients when taken in low doses, there are plants that are sufficiently poisonous to be consequential with even relatively small exposures. Some people, often unknowingly vulnerable due to genetic or other causes, are uniquely susceptible to even minute doses.
Humans probably learned about plant toxicity early on—most likely the hard way. To this day, however, the Internet is replete with traditional and avant-garde natural healing remedies involving the use of naturally-derived plant products. These numerous bioactive compounds are often sold in plant form or as extracts, the latter being more concerning given their more concentrated formulation.
Plant misidentification is a common cause of poisoning, whether the intended use is for food or medicine. For example, some mistake “deadly nightshade” (Atropa belladonna) berries, which are deep blue, for blueberries, or pokeweed roots for horseradish roots due to their similar appearances.1
Alternatively, even when a plant is correctly identified, patients may experience adverse effects if they exceed the “therapeutic dose” (eg, dysrhythmia from aconite roots used in traditional Chinese medicine) or if the plant is improperly prepared (eg, hypoglycemia from consuming unripe ackee fruit).2 In addition, a toxic plant such as Jimson weed (Datura stramonium) or coca leaf extract may be intentionally ingested for its psychoactive hallucinatory effects.2 Although rare in the United States, in certain parts of Asia, persons intent on self-harm may consume toxic plants.1
When ingested, what plants cause bradycardia and hypotension, and why do these effects occur?
The two broad classes of plant-derived toxins that can cause these findings are cardioactive steroids and sodium channel active agents.
Cardioactive Steroids
There are numerous botanical sources of cardioactive steroids (sometimes called cardiac glycosides) such as Digitalis lanata, from which digoxin is derived; and Digitalis purpurea, the source of digitoxin. Poisoning by Digitalis spp, squill, lily of the valley, oleander, yellow oleander, and Cerbera manghas are clinically similar. Cardioactive steroids act pharmacologically to block the sodium-potassium ATPase pump on the myocardial cell membrane. This in turn increases intracellular sodium, which subsequently inhibits the exchange of extracellular sodium for intracellular calcium, leading to inotropy. Clinical manifestations of toxicity include nausea, vomiting, hyperkalemia, bradycardia, cardiac dysrhythmias, and occasionally hypotension—some of which can be life-threatening.
Sodium Channel Active Agents
Several plant toxins affect the flow of sodium by blocking or activating the sodium channel. Both effects alter the rate and strength of cardiac contraction, causing cardiac dysrhythmias.
Aconite is often used in traditional Chinese medicine. In North America, it is mainly derived from Aconitinum napellus, commonly called monkshood, helmet flower, or wolfsbane. It effectively holds open the voltage-dependent sodium channel, increasing cellular excitability. By prolonging the sodium current influx, neuronal and cardiac repolarization eventually slow due to sodium overload, leading to bradycardia and hypotension, as well as neurological effects. Its cardiotoxicity resembles that caused by cardiac glycosides, though a history of paresthesias or muscle weakness may help to differentiate the two toxins.
Veratrum spp include false hellebore, Indian poke, and California hellebore. These plants are occasionally mistaken for leeks (ramps) and can cause vomiting, bradycardia, and hypotension by a mechanism of action similar to aconitine.
Grayanotoxins, a group of diterpenoid toxins found in death camas, azalea, Rhododendron spp, and mountain laurel, can become concentrated in honey made from these plants. Depending on the specific toxin, they variably open or close the sodium channel. In addition to causing bradycardia and hypotension, patients may exhibit mental status changes (“mad honey” poisoning) and seizures.2
Case Continuation
After rapid infusion of 1-liter of normal saline, the patient’s BP was 80/63 mm Hg and HR was 52 beats/minute. His wife arrived to the ED 30-minutes later with a plastic bag containing the red berries the patient had ingested. The emergency physician identified them as Taxus baccata, or more commonly, yew berries. The patient stated that he ingested both the red fleshy aril and chewed the hard central seed.
How is cardiotoxicity from yew berries treated?
Within hours of ingestion, toxicity progresses from nausea, abdominal pain, paresthesias, and ataxia, to bradycardia, cardiac conduction delays, wide-complex ventricular dysrhythmias and mental status changes.3 Although toxicity of Taxus has been known since antiquity, no antidote exists. Ventricular dysrhythmias causing hemodynamic instability should be electrically cardioverted, although there is no evidence to support the safety or efficacy of such therapy. Since the serum, and therefore cardiac concentration of taxine will be identical after cardioversion to its value prior, recurrent dysrhythmias are common.1 Sodium bicarbonate has been inconsistently effective in the treatment of wide-complex tachydysrhythmias,4 but its use seems counterintuitive for most cases. There may be merit to raising the sodium gradient on an already sodium overloaded myocyte, but short-term gain may lead to unintended consequences. Success with antidysrhythmics has been limited: although amiodarone is often used to treat wide-complex tachydysrhythmias, its efficacy in Taxus toxicity has been conflicting.4-6
There have been a few reported cases of yew alkaloid crossreactivity with digoxin assays, suggesting that digoxin-specific antibody fragments may bind taxine.7 There is no evidence, however, that cardioactive steroids are present in yew, and empiric use of antidigoxin Fab-fragments cannot be recommended. A single case report demonstrated that hemodialysis was ineffective in the removal of taxines, likely due to the toxin’s large volume of distribution.8 As a last resort, extracorporeal life support with membrane oxygenation is described favorably in two cases of yew berry poisoning refractory to conventional therapy.9,10
Case Conclusion
The patient’s ECGs showed a morphologically abnormal rhythm, possibly with a Brugada pattern, which are representative of the dysrhythmias caused by taxine’s inhibitory effects on the sodium and calcium channels. Despite an attempt at electrical cardioversion, the dysrhythmia persisted. He was given intravenous boluses of fluids and started on an amiodarone infusion. The patient’s BP gradually improved over the following 2 hours, and the dysrhythmia resolved with hemodynamic improvement. The amiodarone infusion was then discontinued, and he was admitted to the hospital for further testing. Echocardiography, electrophysiology studies, and cardiac catheterization were all normal. The absence of structural, dysrhythmogenic, and ischemic abnormalities supported the toxic etiology of his hemodynamic aberrations. He was discharged from the hospital 3 days later without report of sequelae.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 50-year-old man ingests two handfuls of small, red berries that he picked from a shrub in front of his apartment building, with the belief that they would have medicinal value. Two hours later, he developed abdominal cramping and vomited multiple times, followed shortly thereafter by profuse diaphoresis, lethargy, and ataxia. His concerned family brought him to the ED where his vital signs on presentation were: blood pressure (BP), 78/43 mm Hg; heart rate (HR), 50 beats/minute; respiratory rate (RR), 12 breaths/minute; temperature (T), 97.8°F. With the exception of bradycardia, the patient’s cardiac, pulmonary, and abdominal examinations were normal. His skin was diaphoretic, and he had no focal motor or sensory deficits or tremor. Initial laboratory values were: hemoglobin, 12.6 g/dL; sodium, 137 mEq/L; potassium, 4.6 mEq/L; bicarbonate, 20 mEq/L; blood urea nitrogen, 17 mg/dL; creatinine, 2.2 mg/dL; glucose, 288 mg/dL. The patient’s troponin I level was slightly elevated at 0.06 ng/mL; electrocardiogram (ECG) results are shown in Figure 1.
Why do plant poisonings occur?
There is the general belief that what is natural is not only healthful but also safe. This is clearly not true: cyanide, uranium, and king cobras are all natural but hardly safe. While most plants chosen for their purported medicinal properties are generally harmless in most patients when taken in low doses, there are plants that are sufficiently poisonous to be consequential with even relatively small exposures. Some people, often unknowingly vulnerable due to genetic or other causes, are uniquely susceptible to even minute doses.
Humans probably learned about plant toxicity early on—most likely the hard way. To this day, however, the Internet is replete with traditional and avant-garde natural healing remedies involving the use of naturally-derived plant products. These numerous bioactive compounds are often sold in plant form or as extracts, the latter being more concerning given their more concentrated formulation.
Plant misidentification is a common cause of poisoning, whether the intended use is for food or medicine. For example, some mistake “deadly nightshade” (Atropa belladonna) berries, which are deep blue, for blueberries, or pokeweed roots for horseradish roots due to their similar appearances.1
Alternatively, even when a plant is correctly identified, patients may experience adverse effects if they exceed the “therapeutic dose” (eg, dysrhythmia from aconite roots used in traditional Chinese medicine) or if the plant is improperly prepared (eg, hypoglycemia from consuming unripe ackee fruit).2 In addition, a toxic plant such as Jimson weed (Datura stramonium) or coca leaf extract may be intentionally ingested for its psychoactive hallucinatory effects.2 Although rare in the United States, in certain parts of Asia, persons intent on self-harm may consume toxic plants.1
When ingested, what plants cause bradycardia and hypotension, and why do these effects occur?
The two broad classes of plant-derived toxins that can cause these findings are cardioactive steroids and sodium channel active agents.
Cardioactive Steroids
There are numerous botanical sources of cardioactive steroids (sometimes called cardiac glycosides) such as Digitalis lanata, from which digoxin is derived; and Digitalis purpurea, the source of digitoxin. Poisoning by Digitalis spp, squill, lily of the valley, oleander, yellow oleander, and Cerbera manghas are clinically similar. Cardioactive steroids act pharmacologically to block the sodium-potassium ATPase pump on the myocardial cell membrane. This in turn increases intracellular sodium, which subsequently inhibits the exchange of extracellular sodium for intracellular calcium, leading to inotropy. Clinical manifestations of toxicity include nausea, vomiting, hyperkalemia, bradycardia, cardiac dysrhythmias, and occasionally hypotension—some of which can be life-threatening.
Sodium Channel Active Agents
Several plant toxins affect the flow of sodium by blocking or activating the sodium channel. Both effects alter the rate and strength of cardiac contraction, causing cardiac dysrhythmias.
Aconite is often used in traditional Chinese medicine. In North America, it is mainly derived from Aconitinum napellus, commonly called monkshood, helmet flower, or wolfsbane. It effectively holds open the voltage-dependent sodium channel, increasing cellular excitability. By prolonging the sodium current influx, neuronal and cardiac repolarization eventually slow due to sodium overload, leading to bradycardia and hypotension, as well as neurological effects. Its cardiotoxicity resembles that caused by cardiac glycosides, though a history of paresthesias or muscle weakness may help to differentiate the two toxins.
Veratrum spp include false hellebore, Indian poke, and California hellebore. These plants are occasionally mistaken for leeks (ramps) and can cause vomiting, bradycardia, and hypotension by a mechanism of action similar to aconitine.
Grayanotoxins, a group of diterpenoid toxins found in death camas, azalea, Rhododendron spp, and mountain laurel, can become concentrated in honey made from these plants. Depending on the specific toxin, they variably open or close the sodium channel. In addition to causing bradycardia and hypotension, patients may exhibit mental status changes (“mad honey” poisoning) and seizures.2
Case Continuation
After rapid infusion of 1-liter of normal saline, the patient’s BP was 80/63 mm Hg and HR was 52 beats/minute. His wife arrived to the ED 30-minutes later with a plastic bag containing the red berries the patient had ingested. The emergency physician identified them as Taxus baccata, or more commonly, yew berries. The patient stated that he ingested both the red fleshy aril and chewed the hard central seed.
How is cardiotoxicity from yew berries treated?
Within hours of ingestion, toxicity progresses from nausea, abdominal pain, paresthesias, and ataxia, to bradycardia, cardiac conduction delays, wide-complex ventricular dysrhythmias and mental status changes.3 Although toxicity of Taxus has been known since antiquity, no antidote exists. Ventricular dysrhythmias causing hemodynamic instability should be electrically cardioverted, although there is no evidence to support the safety or efficacy of such therapy. Since the serum, and therefore cardiac concentration of taxine will be identical after cardioversion to its value prior, recurrent dysrhythmias are common.1 Sodium bicarbonate has been inconsistently effective in the treatment of wide-complex tachydysrhythmias,4 but its use seems counterintuitive for most cases. There may be merit to raising the sodium gradient on an already sodium overloaded myocyte, but short-term gain may lead to unintended consequences. Success with antidysrhythmics has been limited: although amiodarone is often used to treat wide-complex tachydysrhythmias, its efficacy in Taxus toxicity has been conflicting.4-6
There have been a few reported cases of yew alkaloid crossreactivity with digoxin assays, suggesting that digoxin-specific antibody fragments may bind taxine.7 There is no evidence, however, that cardioactive steroids are present in yew, and empiric use of antidigoxin Fab-fragments cannot be recommended. A single case report demonstrated that hemodialysis was ineffective in the removal of taxines, likely due to the toxin’s large volume of distribution.8 As a last resort, extracorporeal life support with membrane oxygenation is described favorably in two cases of yew berry poisoning refractory to conventional therapy.9,10
Case Conclusion
The patient’s ECGs showed a morphologically abnormal rhythm, possibly with a Brugada pattern, which are representative of the dysrhythmias caused by taxine’s inhibitory effects on the sodium and calcium channels. Despite an attempt at electrical cardioversion, the dysrhythmia persisted. He was given intravenous boluses of fluids and started on an amiodarone infusion. The patient’s BP gradually improved over the following 2 hours, and the dysrhythmia resolved with hemodynamic improvement. The amiodarone infusion was then discontinued, and he was admitted to the hospital for further testing. Echocardiography, electrophysiology studies, and cardiac catheterization were all normal. The absence of structural, dysrhythmogenic, and ischemic abnormalities supported the toxic etiology of his hemodynamic aberrations. He was discharged from the hospital 3 days later without report of sequelae.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bruneton J. Toxic Plants; Dangerous to Humans and Animals. Paris, France: Lavoisier Publishing; 1999:4-752.
- Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. In: Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:1537-1560.
- Nelson LS, Shih RD, Balick MJ. Handbook of Poisonous and Injurious Plants. 2nd ed. New York, NY: Springer/New York Botanical Garden; 2007:288-290.
- Pierog J, Kane B, Kane K, Donovan JW. Management of isolated yew berry toxicity with sodium bicarbonate: a case report in treatment efficacy. J Med Toxicol. 2009;5(2):84-89.
- Jones R, Jones J, Causer J, Ewins D, Goenka N, Joseph F. Yew tree poisoning: a near-fatal lesson from history. Clin Med. 2011;11(2):173-175.
- Willaert W, Claessens P, Vankelecom B, Vanderheyden M. Intoxication with Taxus baccata: cardiac arrhythmias following yew leaves ingestion. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):511,512.
- Cummins RO, Haulman J, Quan L, Graves JR, Peterson D, Horan S. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med. 1990;19(1):38-43
- Dahlqvist M, Venzin R, König S, et al. Haemodialysis in Taxus baccata poisoning: a case report. QJM. 2012;105(4):359-361.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in severe Taxus baccata poisoning. Clin Toxicol. 2010;48(5):463-465.
- Soumagne N, Chauvet S, Chatellier D, Robert R, Charrière JM, Menu P. Treatment of yew leaf intoxication with extracorporeal circulation. Am J Emerg Med. 2011;29(3):354.e5-6.
- Bruneton J. Toxic Plants; Dangerous to Humans and Animals. Paris, France: Lavoisier Publishing; 1999:4-752.
- Palmer ME, Betz JM. Plants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. In: Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:1537-1560.
- Nelson LS, Shih RD, Balick MJ. Handbook of Poisonous and Injurious Plants. 2nd ed. New York, NY: Springer/New York Botanical Garden; 2007:288-290.
- Pierog J, Kane B, Kane K, Donovan JW. Management of isolated yew berry toxicity with sodium bicarbonate: a case report in treatment efficacy. J Med Toxicol. 2009;5(2):84-89.
- Jones R, Jones J, Causer J, Ewins D, Goenka N, Joseph F. Yew tree poisoning: a near-fatal lesson from history. Clin Med. 2011;11(2):173-175.
- Willaert W, Claessens P, Vankelecom B, Vanderheyden M. Intoxication with Taxus baccata: cardiac arrhythmias following yew leaves ingestion. Pacing Clin Electrophysiol. 2002;25(4 Pt 1):511,512.
- Cummins RO, Haulman J, Quan L, Graves JR, Peterson D, Horan S. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med. 1990;19(1):38-43
- Dahlqvist M, Venzin R, König S, et al. Haemodialysis in Taxus baccata poisoning: a case report. QJM. 2012;105(4):359-361.
- Panzeri C, Bacis G, Ferri F, et al. Extracorporeal life support in severe Taxus baccata poisoning. Clin Toxicol. 2010;48(5):463-465.
- Soumagne N, Chauvet S, Chatellier D, Robert R, Charrière JM, Menu P. Treatment of yew leaf intoxication with extracorporeal circulation. Am J Emerg Med. 2011;29(3):354.e5-6.
Marked shift seen in demographics of heroin users seeking treatment
The demographics of heroin users have shifted profoundly in recent years, according to a retrospective analysis involving more than 2,700 people.
Heroin has migrated out of young minority populations in lower-class city neighborhoods, and users are now far more likely to be white, middle-class men and women in their late 20s living in suburban, small-town, or rural areas, wrote Theodore J. Cicero, Ph.D., of the department of psychiatry, Washington University, St. Louis (JAMA Psychiatry 2014 May 28 [doi:10.1001/jamapsychiatry.2014.366]).
Noting the paucity of systematic studies of the demographics of today’s heroin users, Dr. Cicero and his colleagues analyzed data from the ongoing Survey of Key Informants’ Patients (SKIP) program, in which 150 publicly and privately funded treatment centers (the key informants) recruit patients/clients to complete anonymous surveys about their substance use. The surveys cover all 48 contiguous states.
Dr. Cicero and his colleagues reviewed the survey responses of 2,797 self-reported heroin users entering treatment in 2010-2013. Most (86.4%) said they used heroin at least once a day, and many (66%) said they had concurrently abused prescription opioids during the preceding month.
Three-fourths of the respondents who began heroin use during the past decade said they had begun by abusing a prescription opioid, usually OxyContin. In contrast, about 80% of those who began using heroin in the 1960s and 1970s said they initiated their drug use with heroin itself. Fifty years ago, the average age at first use of heroin was 16 years; now it is 23 years.
A subset of 54 respondents who agreed to more detailed online interviews explained why they progressed from prescription opioids to heroin. A total of 98% said they considered the "high" from heroin to be superior to that from prescription opioids, and 94% said that heroin was far less expensive and far easier to obtain. In addition, one-third of this subgroup said that inhalation or injection is easier with heroin because it doesn’t require extraction, as prescription opioids do.
Nevertheless, if the cost, availability, and ease of use of the two agents were comparable, half of the 54 respondents said they would switch back to prescription opioids, which they described as offering a "cleaner" high and averting the legal problems associated with heroin, the investigators said.
Nearly 83% of the respondents who began heroin use in the 1960s or 1970s were men. In contrast, those who began heroin use during the past decade were approximately equally divided between men and women. Similarly, most who began using heroin in the 1960s and 1970s were nonwhite, while 90% of those who began use more recently were white.
It is important to note that this study population was not randomly selected and might not be representative of all current heroin users. These study subjects were entering treatment and had Internet access that enabled them to participate, so factors such as financial status, education level, family support, and court pressure affected their participation, Dr. Cicero and his associates said.
The SKIP database is supported by the Denver Health and Hospital Authority, which is funded by fees from 14 pharmaceutical firms. Dr. Cicero and his associates reported no financial conflicts of interest
The demographics of heroin users have shifted profoundly in recent years, according to a retrospective analysis involving more than 2,700 people.
Heroin has migrated out of young minority populations in lower-class city neighborhoods, and users are now far more likely to be white, middle-class men and women in their late 20s living in suburban, small-town, or rural areas, wrote Theodore J. Cicero, Ph.D., of the department of psychiatry, Washington University, St. Louis (JAMA Psychiatry 2014 May 28 [doi:10.1001/jamapsychiatry.2014.366]).
Noting the paucity of systematic studies of the demographics of today’s heroin users, Dr. Cicero and his colleagues analyzed data from the ongoing Survey of Key Informants’ Patients (SKIP) program, in which 150 publicly and privately funded treatment centers (the key informants) recruit patients/clients to complete anonymous surveys about their substance use. The surveys cover all 48 contiguous states.
Dr. Cicero and his colleagues reviewed the survey responses of 2,797 self-reported heroin users entering treatment in 2010-2013. Most (86.4%) said they used heroin at least once a day, and many (66%) said they had concurrently abused prescription opioids during the preceding month.
Three-fourths of the respondents who began heroin use during the past decade said they had begun by abusing a prescription opioid, usually OxyContin. In contrast, about 80% of those who began using heroin in the 1960s and 1970s said they initiated their drug use with heroin itself. Fifty years ago, the average age at first use of heroin was 16 years; now it is 23 years.
A subset of 54 respondents who agreed to more detailed online interviews explained why they progressed from prescription opioids to heroin. A total of 98% said they considered the "high" from heroin to be superior to that from prescription opioids, and 94% said that heroin was far less expensive and far easier to obtain. In addition, one-third of this subgroup said that inhalation or injection is easier with heroin because it doesn’t require extraction, as prescription opioids do.
Nevertheless, if the cost, availability, and ease of use of the two agents were comparable, half of the 54 respondents said they would switch back to prescription opioids, which they described as offering a "cleaner" high and averting the legal problems associated with heroin, the investigators said.
Nearly 83% of the respondents who began heroin use in the 1960s or 1970s were men. In contrast, those who began heroin use during the past decade were approximately equally divided between men and women. Similarly, most who began using heroin in the 1960s and 1970s were nonwhite, while 90% of those who began use more recently were white.
It is important to note that this study population was not randomly selected and might not be representative of all current heroin users. These study subjects were entering treatment and had Internet access that enabled them to participate, so factors such as financial status, education level, family support, and court pressure affected their participation, Dr. Cicero and his associates said.
The SKIP database is supported by the Denver Health and Hospital Authority, which is funded by fees from 14 pharmaceutical firms. Dr. Cicero and his associates reported no financial conflicts of interest
The demographics of heroin users have shifted profoundly in recent years, according to a retrospective analysis involving more than 2,700 people.
Heroin has migrated out of young minority populations in lower-class city neighborhoods, and users are now far more likely to be white, middle-class men and women in their late 20s living in suburban, small-town, or rural areas, wrote Theodore J. Cicero, Ph.D., of the department of psychiatry, Washington University, St. Louis (JAMA Psychiatry 2014 May 28 [doi:10.1001/jamapsychiatry.2014.366]).
Noting the paucity of systematic studies of the demographics of today’s heroin users, Dr. Cicero and his colleagues analyzed data from the ongoing Survey of Key Informants’ Patients (SKIP) program, in which 150 publicly and privately funded treatment centers (the key informants) recruit patients/clients to complete anonymous surveys about their substance use. The surveys cover all 48 contiguous states.
Dr. Cicero and his colleagues reviewed the survey responses of 2,797 self-reported heroin users entering treatment in 2010-2013. Most (86.4%) said they used heroin at least once a day, and many (66%) said they had concurrently abused prescription opioids during the preceding month.
Three-fourths of the respondents who began heroin use during the past decade said they had begun by abusing a prescription opioid, usually OxyContin. In contrast, about 80% of those who began using heroin in the 1960s and 1970s said they initiated their drug use with heroin itself. Fifty years ago, the average age at first use of heroin was 16 years; now it is 23 years.
A subset of 54 respondents who agreed to more detailed online interviews explained why they progressed from prescription opioids to heroin. A total of 98% said they considered the "high" from heroin to be superior to that from prescription opioids, and 94% said that heroin was far less expensive and far easier to obtain. In addition, one-third of this subgroup said that inhalation or injection is easier with heroin because it doesn’t require extraction, as prescription opioids do.
Nevertheless, if the cost, availability, and ease of use of the two agents were comparable, half of the 54 respondents said they would switch back to prescription opioids, which they described as offering a "cleaner" high and averting the legal problems associated with heroin, the investigators said.
Nearly 83% of the respondents who began heroin use in the 1960s or 1970s were men. In contrast, those who began heroin use during the past decade were approximately equally divided between men and women. Similarly, most who began using heroin in the 1960s and 1970s were nonwhite, while 90% of those who began use more recently were white.
It is important to note that this study population was not randomly selected and might not be representative of all current heroin users. These study subjects were entering treatment and had Internet access that enabled them to participate, so factors such as financial status, education level, family support, and court pressure affected their participation, Dr. Cicero and his associates said.
The SKIP database is supported by the Denver Health and Hospital Authority, which is funded by fees from 14 pharmaceutical firms. Dr. Cicero and his associates reported no financial conflicts of interest
FROM JAMA PSYCHIATRY
Key clinical point: Many heroin users transitioned to that drug from prescription opioids.
Major finding: In explaining why they progressed from prescription opioid to heroin use, 98% said the "high" from heroin was superior, 94% said heroin was far less expensive and far easier to obtain, and 31% said inhalation or injection is easier with heroin, because it doesn’t require extraction, as prescription opioids do.
Data source: A retrospective analysis of survey responses from 2,792 patients entering substance abuse treatment programs across the country for heroin dependence.
Disclosures: The SKIP database is supported by the Denver Health and Hospital Authority, which is funded by fees from 14 pharmaceutical firms. Dr. Cicero and his associates reported no financial conflicts of interest.
E-cigarettes trigger sharp rise in poison control center calls
Calls to U.S. poison control centers because of e-cigarette exposure increased from 1 per month in September 2010 to 215 per month in February 2014, according to a new study published in the April 3 edition of the Morbidity and Mortality Weekly Report.
"Calls about exposures to e-cigarettes, which were first marketed in the United States in 2007, now account for 41.7% of combined monthly e-cigarette and cigarette exposure calls to [poison control centers]," wrote the investigators, led by Dr. Kevin Chatham-Stephens of the CDC (MMWR Morb. Mortal. Wkly Rep. 2014;63:291-2).
Researchers from the Centers for Disease Control and Prevention analyzed data from 2,405 e-cigarette calls to poison control centers in all 50 states, the District of Columbia, and U.S. territories from September 2010 to February 2014. Although calls regarding overexposure are much more common with conventional tobacco products (16,248 calls over the same period of time), the investigators noted that 42% of the e-cigarette exposure calls involved people aged 20 years and older, whereas 94.9% of tobacco exposure calls involve children younger than 5 years.
In addition, health care facilities were responsible for significantly more of the e-cigarette exposure calls than for cigarette exposure calls, 12.8% vs. 5.9%. And callers were significantly more likely to report adverse health effects with e-cigarette exposures (57.8% of calls) than with cigarette exposures (36% of calls).
Poisoning cases can occur either from an exposure to the device itself or to the nicotine liquid contained in a small cartridge that the user inserts into the e-cigarette. Exposure to the liquid can occur through inhalation, ingestion, or absorption, and the most common adverse health effects in e-cigarette exposure calls were vomiting, nausea, and eye irritation.
“New data released today from the federal government confirms pediatricians’ concerns about e-cigarettes and their liquid nicotine refills: they are poisoning children at an alarming rate," Dr. James M. Perrin, president of the American Academy of Pediatrics, said in a statement.
“As pediatricians, we do everything in our power to keep our young patients safe from poisonous products, like household cleaners and prescription medications. Why should we act differently when it comes to liquid nicotine? The e-cigarette industry specifically targets children and teens with appealing flavors like cotton candy and gummy bear, and neither these products nor their liquid nicotine refills are currently regulated by the federal government," he said.
"Pediatricians call on the U.S. Department of Health and Human Services to convene Centers for Disease Control and Prevention, Food and Drug Administration, and other federal agencies and develop a national plan of action to keep children safe from e-cigarette poisoning. With more and more children being exposed to these dangerous products each month, we cannot afford to wait another day,” concluded Dr. Perrin, professor of pediatrics at Harvard Medical School, Boston.
Currently, e-cigarettes and their components that are marketed for therapeutic purposes such as smoking cessation are not regulated by the FDA Center for Tobacco Products, but are instead regulated by FDA Center for Drug Evaluation and Research.
*This article was updated 4/3/2014.
Calls to U.S. poison control centers because of e-cigarette exposure increased from 1 per month in September 2010 to 215 per month in February 2014, according to a new study published in the April 3 edition of the Morbidity and Mortality Weekly Report.
"Calls about exposures to e-cigarettes, which were first marketed in the United States in 2007, now account for 41.7% of combined monthly e-cigarette and cigarette exposure calls to [poison control centers]," wrote the investigators, led by Dr. Kevin Chatham-Stephens of the CDC (MMWR Morb. Mortal. Wkly Rep. 2014;63:291-2).
Researchers from the Centers for Disease Control and Prevention analyzed data from 2,405 e-cigarette calls to poison control centers in all 50 states, the District of Columbia, and U.S. territories from September 2010 to February 2014. Although calls regarding overexposure are much more common with conventional tobacco products (16,248 calls over the same period of time), the investigators noted that 42% of the e-cigarette exposure calls involved people aged 20 years and older, whereas 94.9% of tobacco exposure calls involve children younger than 5 years.
In addition, health care facilities were responsible for significantly more of the e-cigarette exposure calls than for cigarette exposure calls, 12.8% vs. 5.9%. And callers were significantly more likely to report adverse health effects with e-cigarette exposures (57.8% of calls) than with cigarette exposures (36% of calls).
Poisoning cases can occur either from an exposure to the device itself or to the nicotine liquid contained in a small cartridge that the user inserts into the e-cigarette. Exposure to the liquid can occur through inhalation, ingestion, or absorption, and the most common adverse health effects in e-cigarette exposure calls were vomiting, nausea, and eye irritation.
“New data released today from the federal government confirms pediatricians’ concerns about e-cigarettes and their liquid nicotine refills: they are poisoning children at an alarming rate," Dr. James M. Perrin, president of the American Academy of Pediatrics, said in a statement.
“As pediatricians, we do everything in our power to keep our young patients safe from poisonous products, like household cleaners and prescription medications. Why should we act differently when it comes to liquid nicotine? The e-cigarette industry specifically targets children and teens with appealing flavors like cotton candy and gummy bear, and neither these products nor their liquid nicotine refills are currently regulated by the federal government," he said.
"Pediatricians call on the U.S. Department of Health and Human Services to convene Centers for Disease Control and Prevention, Food and Drug Administration, and other federal agencies and develop a national plan of action to keep children safe from e-cigarette poisoning. With more and more children being exposed to these dangerous products each month, we cannot afford to wait another day,” concluded Dr. Perrin, professor of pediatrics at Harvard Medical School, Boston.
Currently, e-cigarettes and their components that are marketed for therapeutic purposes such as smoking cessation are not regulated by the FDA Center for Tobacco Products, but are instead regulated by FDA Center for Drug Evaluation and Research.
*This article was updated 4/3/2014.
Calls to U.S. poison control centers because of e-cigarette exposure increased from 1 per month in September 2010 to 215 per month in February 2014, according to a new study published in the April 3 edition of the Morbidity and Mortality Weekly Report.
"Calls about exposures to e-cigarettes, which were first marketed in the United States in 2007, now account for 41.7% of combined monthly e-cigarette and cigarette exposure calls to [poison control centers]," wrote the investigators, led by Dr. Kevin Chatham-Stephens of the CDC (MMWR Morb. Mortal. Wkly Rep. 2014;63:291-2).
Researchers from the Centers for Disease Control and Prevention analyzed data from 2,405 e-cigarette calls to poison control centers in all 50 states, the District of Columbia, and U.S. territories from September 2010 to February 2014. Although calls regarding overexposure are much more common with conventional tobacco products (16,248 calls over the same period of time), the investigators noted that 42% of the e-cigarette exposure calls involved people aged 20 years and older, whereas 94.9% of tobacco exposure calls involve children younger than 5 years.
In addition, health care facilities were responsible for significantly more of the e-cigarette exposure calls than for cigarette exposure calls, 12.8% vs. 5.9%. And callers were significantly more likely to report adverse health effects with e-cigarette exposures (57.8% of calls) than with cigarette exposures (36% of calls).
Poisoning cases can occur either from an exposure to the device itself or to the nicotine liquid contained in a small cartridge that the user inserts into the e-cigarette. Exposure to the liquid can occur through inhalation, ingestion, or absorption, and the most common adverse health effects in e-cigarette exposure calls were vomiting, nausea, and eye irritation.
“New data released today from the federal government confirms pediatricians’ concerns about e-cigarettes and their liquid nicotine refills: they are poisoning children at an alarming rate," Dr. James M. Perrin, president of the American Academy of Pediatrics, said in a statement.
“As pediatricians, we do everything in our power to keep our young patients safe from poisonous products, like household cleaners and prescription medications. Why should we act differently when it comes to liquid nicotine? The e-cigarette industry specifically targets children and teens with appealing flavors like cotton candy and gummy bear, and neither these products nor their liquid nicotine refills are currently regulated by the federal government," he said.
"Pediatricians call on the U.S. Department of Health and Human Services to convene Centers for Disease Control and Prevention, Food and Drug Administration, and other federal agencies and develop a national plan of action to keep children safe from e-cigarette poisoning. With more and more children being exposed to these dangerous products each month, we cannot afford to wait another day,” concluded Dr. Perrin, professor of pediatrics at Harvard Medical School, Boston.
Currently, e-cigarettes and their components that are marketed for therapeutic purposes such as smoking cessation are not regulated by the FDA Center for Tobacco Products, but are instead regulated by FDA Center for Drug Evaluation and Research.
*This article was updated 4/3/2014.
FROM MMWR
Naloxone autoinjector approved for caregiver use in treating opioid overdoses
A hand-held autoinjector that delivers a single dose of naloxone to be used in cases of suspected or known opioid overdoses has been approved by the Food and Drug Administration.
The treatment "is the first combination drug-device product designed to deliver a dose of naloxone for administration outside of a health care setting," said Dr. Bob Rappaport, director of the Division of Anesthesia, Analgesia, and Addiction Products in the FDA’s Center for Drug Evaluation and Research. "Making this product available could save lives by facilitating earlier use of the drug in emergency situations," he added in an FDA statement announcing the approval April 3.
The product’s application was granted priority review status by the agency and reviewed in only 15 weeks, FDA Commissioner Margaret A. Hamburg said in a separate statement.
The autoinjector will be marketed by kaléo, Inc., under the trade name Evzio. It can be administered subcutaneously or intramuscularly, and is intended to be administered by family members or caregivers in cases of suspected overdoses, the statement said. As with automated defibrillators, verbal instructions and visual clues regarding how to use the device are provided when activated. A trainer device is included with the product for people to become familiar with how to use the device.
The FDA statement notes that deaths due to drug overdoses are currently the leading cause of fatal injuries in the United States, and that in 2013, the Centers for Disease Control and Prevention reported that drug overdose deaths had increased steadily over 10 years.
While naloxone is the standard treatment for overdoses, it has been available for administration only with syringes by health care professionals.
The product is expected to be available though major pharmacies this summer, according to a kaléo statement announcing the approval.
A hand-held autoinjector that delivers a single dose of naloxone to be used in cases of suspected or known opioid overdoses has been approved by the Food and Drug Administration.
The treatment "is the first combination drug-device product designed to deliver a dose of naloxone for administration outside of a health care setting," said Dr. Bob Rappaport, director of the Division of Anesthesia, Analgesia, and Addiction Products in the FDA’s Center for Drug Evaluation and Research. "Making this product available could save lives by facilitating earlier use of the drug in emergency situations," he added in an FDA statement announcing the approval April 3.
The product’s application was granted priority review status by the agency and reviewed in only 15 weeks, FDA Commissioner Margaret A. Hamburg said in a separate statement.
The autoinjector will be marketed by kaléo, Inc., under the trade name Evzio. It can be administered subcutaneously or intramuscularly, and is intended to be administered by family members or caregivers in cases of suspected overdoses, the statement said. As with automated defibrillators, verbal instructions and visual clues regarding how to use the device are provided when activated. A trainer device is included with the product for people to become familiar with how to use the device.
The FDA statement notes that deaths due to drug overdoses are currently the leading cause of fatal injuries in the United States, and that in 2013, the Centers for Disease Control and Prevention reported that drug overdose deaths had increased steadily over 10 years.
While naloxone is the standard treatment for overdoses, it has been available for administration only with syringes by health care professionals.
The product is expected to be available though major pharmacies this summer, according to a kaléo statement announcing the approval.
A hand-held autoinjector that delivers a single dose of naloxone to be used in cases of suspected or known opioid overdoses has been approved by the Food and Drug Administration.
The treatment "is the first combination drug-device product designed to deliver a dose of naloxone for administration outside of a health care setting," said Dr. Bob Rappaport, director of the Division of Anesthesia, Analgesia, and Addiction Products in the FDA’s Center for Drug Evaluation and Research. "Making this product available could save lives by facilitating earlier use of the drug in emergency situations," he added in an FDA statement announcing the approval April 3.
The product’s application was granted priority review status by the agency and reviewed in only 15 weeks, FDA Commissioner Margaret A. Hamburg said in a separate statement.
The autoinjector will be marketed by kaléo, Inc., under the trade name Evzio. It can be administered subcutaneously or intramuscularly, and is intended to be administered by family members or caregivers in cases of suspected overdoses, the statement said. As with automated defibrillators, verbal instructions and visual clues regarding how to use the device are provided when activated. A trainer device is included with the product for people to become familiar with how to use the device.
The FDA statement notes that deaths due to drug overdoses are currently the leading cause of fatal injuries in the United States, and that in 2013, the Centers for Disease Control and Prevention reported that drug overdose deaths had increased steadily over 10 years.
While naloxone is the standard treatment for overdoses, it has been available for administration only with syringes by health care professionals.
The product is expected to be available though major pharmacies this summer, according to a kaléo statement announcing the approval.
Bites and Stings
Hymenoptera
The order Hymenoptera of the phylum Arthropoda can be divided into three subgroups that are medically relevant: (1) Apidae (Apids), which include the honeybee and bumblebee; (2) Vespidae, (Vespids) which include yellow jackets, hornets and wasps; and (3) Formicidae (ants).6
Bees and Wasps
The main allergens in Apid venom are phospholipase A2, hyaluronidase, and melittin. Melittin, the main component, is a membrane active polypeptide that causes degranulation of basophils and mast cells. The allergens in Vespid venom are phospholipase, hyaluronidase, and antigen 5. As all Hymenoptera share some of these components, cross-sensitization may occur and individuals may be allergic to more than one species.7
Occasionally after multiple stings, patients present with symptoms of a systemic toxic reaction. This is often seen in an Africanized bee attack. These so-called “killer bees” are hybrids of African bees that escaped from laboratories in Brazil in the 1950s and spread northward; they are found in most of the warmer US states. Their venom is not more toxic than that of any other bee, but Africanized honeybees are more aggressive and respond to a perceived threat in far greater numbers. The reaction that results from multiple stings is systemic and may resemble anaphylaxis. Common symptoms include nausea, vomiting, and diarrhea, as well as lightheadedness and syncope. Interestingly, urticaria and bronchospasm are not universally present, even though respiratory failure and cardiac arrest may occur. Other symptoms include renal failure with acute tubular necrosis, myoglobinuria or hemoglobinuria, hepatic failure, and disseminated intravascular coagulation (DIC).10,11 In addition, there have been reports of unusual reactions such as vasculitis, nephrosis, neuritis, encephalitis, and serum sickness. Late-appearing symptoms usually start several days to weeks after a sting and tend last for a prolonged period of time. Serum sickness tends to appear 5 to 14 days after exposure and consists of fever, malaise, headache, urticaria, lymphadenopathy, and polyarthritis.12 Of note, patients who have venom-induced serum sickness may be at risk for anaphylaxis after subsequent stings and may therefore be suitable candidates for venom immunotherapy.13
Anaphylaxis
The definition of anaphylaxis is not universally agreed upon. The American Academy of Allergy, Asthma and Immunology defines anaphylaxis as a serious allergic response that often involves swelling, hives, hypotension and, in severe cases, shock. A major difference between anaphylaxis and other allergic reactions is that anaphylaxis typically involves more than one body system.14 The clinical features of anaphylaxis from insect stings are the same as those from other causes, typically generalized urticaria, facial flushing, and angioedema. Abdominal cramping, nausea, vomiting, and diarrhea are also seen. Life-threatening symptoms include stridor, circulatory collapse with shock, and bronchospasm. Symptoms usually begin 10 to 20 minutes after a sting, and almost all will develop within 6 hours. Interestingly, symptoms may recur 8 to 12 hours after the initial reaction.15-18
Management
Immediate removal of the bee stinger is the most important principle as it precludes any further venom transfer. Traditional teaching recommended scraping the stinger out to avoid squeezing remaining venom into the tissues; however, involuntary muscle contractions of the gland continue after the stinger detaches, and the venom is quickly exhausted. Thus, immediate removal of the stinger is crucial, though the method of removal is now thought irrelevant.19
The mainstay of therapy for serious reactions is intramuscular (IM) epinephrine. The initial dosing is 0.3 to 0.5 mg (0.3 to 0.5 mL of 1:1000 concentration) in adults, and 0.01 mg/kg in children (maximum 0.3 mg). The injection should be IM and not subcutaneous, as IM dosing provides higher and more consistent and rapid peak blood epinephrine levels.20 Concomitant intravenous (IV) administration of standard antihistamines, often diphenhydramine 1 mg/kg (generally 25-50 mg) and histamine-2 receptor antagonists (typically ranitidine 50 mg) are also recommended. The administration of steroids (methylprednisolone 125 mg IV or prednisone 60 mg orally) is traditionally recommended and thought to help potentiate the effect of other interventional measures.20 Bronchospasm, if present, is treated with nebulized β-agonists (albuterol). Hypotension may develop and requires significant crystalloid infusion—often several liters. If hypotension persists despite adequate fluid replacement, vasopressor therapy is recommended.
If a patient does not respond to initial treatment and cardiovascular (CV) collapse is evident, IV infusion of epinephrine should be initiated. Epinephrine, 100 mcg (0.1 mg) IV, should be given as a 1:100,000 dilution. This can be done by placing epinephrine, 0.1 mg (0.1 mL of the 1:1000 dilution), in 10 mL of normal saline solution and infusing it over 5 to 10 minutes (a rate of 1 to 2 mL/min). If the patient is refractory to the initial bolus, then an epinephrine infusion can be started by placing epinephrine, 1 mg (1.0 mL of the 1:1000 dilution), in 500 mL of 5% dextrose in water or NS and administering at a rate of 1 to 4 mcg per minute (0.5 to 2 mL/min), titrating to effect.20 Antivenins have been studied for treatment, but none are commercially available at this time.21 Patients with anaphylaxis associated with severe signs and symptoms, including any evidence of CV collapse, should be admitted to the hospital for aggressive therapy and monitoring. Persons with mild-to-moderate reactions should be observed for 4 to 6 hours to monitor for late occurring symptoms. Outpatient therapy with antihistamines, oral steroids, and a prescription for an epinephrine auto-injector—including training on proper administration prior to discharge—are strongly recommended.22 Follow-up with an allergist is also indicated in patients with significant reactions, as skin testing and immunotherapy may be beneficial to prevent anaphylaxis during future exposures.
Ants
Fire ant venom is composed of an insoluble alkaloid, and crossreactivity with the venom of other Hymenopteras species does exist. Stings generally result in a papule, which evolves into a sterile pustule. Localized necrosis, scarring, and secondary infection can occur. Systemic reactions with angioedema and urticaria have been documented, which can sometimes lead to fatalities.24
Treatment
Treatment of fire ant stings begin and end with local wound care. If the reaction is systemic, a treatment plan similar to that outlined in the treatment section for bees and wasps is indicated.
Araneae
The order Araneae of the phylum Arthropoda includes over 34,000 species of spiders divided into 105 families. Of those, only half a dozen are medically relevant and only three are commonly encountered in the United States. These include Loxosceles (most notably, the brown recluse spider), Tegeneria (mainly the hobo brown spider) and Latrodectus (includes the black widow spider). True spiders have a worldwide distribution and tend to thrive in heavily populated areas, resulting in many biting episodes per year. Data from the AAPPC’s most recent annual report listed 9,255 single spider-bite exposures in 2012, with one associated death.3
Spiders are carnivores and use venom to paralyze their prey. They are generally not a threat to humans as their fangs are too small to penetrate human skin, and the amount of venom injected is too small to produce toxicity. Thus, reactions resulting from a spider bite are typically limited to a localized reaction. Fortunately, most bites only require supportive medical therapy.
Loxosceles
Loxosceles are present worldwide, but L reclusa (the “brown recluse spider”) accounts for a significant number of envenomations in the United States. The AAPCC’s 2012 data notes 1,365 cases of exposure to the brown recluse spider with 510 of those victims seeking medical care.3 In many instances, clinicians attribute necrotic bites to the brown recluse spider, however, confirmation is often lacking. Loxosceles are nocturnal, and they are found both indoors and outdoors—mostly in dark and dry areas such as basements, closets, and woodpiles. These spiders are shy, but may bite when threatened. Their venom contains enzymes, including hyaluronidase and sphingomyelinase. Though rare, wounds can become necrotic due to neutrophil activation, platelet aggregation, and thrombosis.25 The most common reaction to a Loxosceles bite is a mild painless erythematous lesion that becomes firm and generally heals over several days to weeks. In severe reactions, erythema, edema, and pruritus initially develop, followed within 24 to 72 hours by a hemorrhagic bulla surrounded by blanched skin. This leads to the “red, white, and blue sign” (ie, erythema, blanching, and ecchymosis). Infrequently, the ecchymotic area becomes necrotic and ulcerates in 3 to 5 days. The differential diagnoses should include necrotizing fasciitis, erythema chronicum migrans (from Borrelia-infected tick bites), and anthrax. Ulcerated lesions may result in significant cosmetic defect. Healing may take up to 2 weeks, and skin grafting is occasionally required.26
Treatment begins with the usual supportive measures, including analgesia, ice, elevation, and a light compression dressing. Antibiotics are not indicated, unless there are signs of secondary infection. Serial evaluation for wound checks should be arranged. If ulceration develops, surgical debridement may be required. The vast majority of bites heal with supportive care alone, and aggressive medical therapy is usually not warranted.27Patients with systemic manifestations should be admitted to the hospital for further care. There is no evidence-based literature to guide therapy. Many therapies have been tried with variable results and there remains no definitive standard of care.
Treatment regimens include antihistamines, antivenin, colchicine, dapsone, hyperbaric oxygen, cyproheptadine, surgical excision, and steroids.28 Dapsone continues to be widely advocated worldwide despite its known adverse effects—most notably hemolysis and methemoglobinemia. Antivenin administration has shown some promise in animal models, but its efficacy in humans is still unclear.29
Tegenaria
The Tegenaria agrestis or hobo spider is a native of Europe and central Asia and is only found in the northwest part of the United States. It is considered aggressive and tends to bite even with only mild provocation. The clinical presentation, inclusive of systemic reactions, is similar to that of the brown recluse spider. Similarly, there is no proven treatment. Surgical wound resection and skin grafting should be considered and is at times required.
Latrodectus.
Latrodectus, also known as widow spiders, are found worldwide. Five species are commonly found in the United States, but the black widow is the most well known. Only three of the species are actually black. Other varieties are typically brown or red. However, almost all Latrodectus spiders have a characteristic orange-red hourglass-shaped marking (Figure 3). Widow spiders aggressively defend their webs, and are most often found in woodpiles, basements, garages, and sheds. Most bites occur in the warmer months, between April and October.
Latrodectus antivenin is very effective, often resolving symptoms rapidly and reducing the duration of illness—even when administered up to 90 hours postenvenomation.32 This antivenin is derived from horse serum, and hypersensitivity reactions are possible. One death from anaphylaxis has been reported in the United States after antivenin was given undiluted via IV push; however, slow administration of diluted antivenin is considered safe.33
Diptera
The order Diptera of the phylum Arthropoda includes over 240,000 species. Among those, the mosquitoes and flies are the most medically relevant.
Mosquitoes
An actual mosquito bite itself causes minimal trauma and is not usually felt by the victim. However, the local anesthetic that is injected into the wound at the time of the attack causes local tissue damage. Mosquito bites can lead to both immediate and delayed reactions. Typical immediate reactions are of short duration and include edema, erythema, and pruritus. More severe reactions are extremely rare and consist of skin necrosis. Delayed skin reactions are fairly common, but tend to last longer, persisting for days or even weeks. Treatment is symptomatic, usually with antihistamines and NSAIDS.
Patients can acquire an allergy to mosquito saliva over time and develop increasingly pronounced edematous and pruritic lesions with subsequent bites. They can also experience fever, malaise, generalized edema, as well as severe nausea and vomiting.
Systemic or anaphylactic reactions are not known to occur. Instead, the greatest danger occurs with the transmission of life-threatening diseases. Malaria, yellow fever, dengue hemorrhagic fever, and different types of equine encephalitis are all transmitted by mosquito bites. One interesting newcomer to the United States is the West Nile virus (WNV), which has spread rapidly since its introduction in 2003. Over 1,850 cases were reported in 22 different states over the initial 8 months. Acute symptoms are mild in the majority of patients, but a small minority can experience fatal disease. Neurological symptoms include tumor, myoclonus, and Parkinsonism. An irreversible poliomyelitis-like syndrome may also develop. In addition to WNV, St Louis encephalitis and equine encephalitis also remain important pathogens in the United States.34 Prevention of bites is crucial and includes the use of pyrethroid-impregnated mosquito netting, repellents, and oral malaria prophylaxis. N,N-diethyl-3-methylbenzamide (DEET) remains the most effective mosquito repellent.35 Although toxic reactions are rare, they do occur and anaphylaxis has been reported. 36,37
Flies
Flies are blood-sucking insects that feed by stabbing and piercing their victim’s skin. Their bites always cause some degree of pain and pruritus. Allergic reactions are possible, though not as severe as those produced by Hymenoptera venom. Treatment is largely symptomatic with ice, oral antihistamine, analgesics. and topical or oral steroids as needed. Secondary bite infection is a concern and antibiotics are sometimes necessary.
Shiponaptera
The order Shinoptera includes fleas and lice. All produce very similar lesions, making diagnosis difficult. One concern with these bites is the development of secondary infections, especially in children. The skin should be washed with soap and water. Calamine, cool soaks, and oral or topical antihistamine may all be helpful to reduce symptoms.
Fleas
With fleas, as with mosquitoes, there is additionally a concern for transmission of life-threatening diseases. Fleas transmit bubonic plague, endemic typhus, brucellosis, melioidosis, and erysipeloid. Fortunately, effective oral and injectable formulations for both dogs and cats are now available to control fleas on most family pets.
Lice
Hemiptera
Lepidoptera
The order Lepidoptera includes butterflies and moths and their caterpillars. Symptoms that result from contact with this class of insects are referred to as lepidopterism. Caterpillars have hair or spines for protection, which are also sometimes connected to a venom gland. Contact with these spines usually causes localized skin irritation and pruritus. Megalopyge opercularis, also known as the “puss caterpillar,” is mainly found in the southeastern United States and accounts for the majority of envenomation cases in this country. Intense local burning pain is typical at the site of contact and is followed by a grid-like pattern of hemorrhagic papules, which appear 2 to 3 hours after exposure and may last for several days. Regional lymphadenopathy is common. Other symptoms include headache, fever, hypotension, and convulsions. No deaths have ever been reported.
As there is no antivenin available for lepidopterism, treatment is mostly supportive. If spines are visible following contact, they should be removed with adhesive tape. Antihistamines and steroids may be used for symptom control. In patients with hypotension, IV fluids and IV epinephrine may be required.43
Coleoptera
The order Coleoptera includes a large number of beetles, though clinically significant envenomation occurs only with blister beetles. There are over 1,500 species of blister beetles worldwide, approximately 2,002 of which are in the United States. The blister beetle responsible for most of the medically significant envenomations is Cantharis vesicatoria—also known as “Spanish fly.” Of note, the Spanish fly is not naturally found in the United States.
Cantharidin-containing substances are sometimes used medicinally in wart removal preparations and are also sold for their purported aphrodisiac effects (the associated vascular congestion and urethral inflammation are interpreted as enhanced sexuality). Transdermal absorption or ingestion may lead to systemic toxicity with severe vomiting, hematemesis, abdominal pain, diarrhea, hematuria, renal failure, etc. Death has been reported after large ingestions.
Treatment is largely supportive. The skin should be irrigated thoroughly after exposure, followed by local wound care. Patients who present after ingestion should be admitted to the hospital for further treatment and care.47
Conclusion
Knowledge of a vast array of stinging insects and spiders is important for any clinician, but the appropriate evaluation and treatment of bites and envenomations are crucial for EPs. Most exposures can be treated with supportive care, while others require in-depth knowledge and clinical expertise.
Dr Deljoui is a former resident, department of emergency medicine, Eastern Virginia Medical School, Norfolk; and current critical care fellow, University of Maryland, Baltimore.
Dr Knapp is an associate professor and residency program director, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
- White J. Bites and stings from venomous animals: a global overview. The Drug Monit. 2000;22(1):65-68.
- Oten EJ. Venomous animal injuries. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:794-807.
- Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila). 2013;51(10):949-1229. doi:10.3109/15563650.2013.863906. https://aapcc.s3.amazonaws.com/pdfs/annual_reports/2012_NPDS_Annual_Report.pdf. Accessed April 2, 2014.
- Liberman P, Camargo CA, Bohike K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596-602.
- Barnard JH. Studies of 400 Hymenoptera sting deaths in the United States. J Allergy Clin Immunol. 1973;52(5):259-264.
- Frazier CA. Insect Allergy: Allergic and Toxic Reactions to Insects and Other Arthropods. 2nd Ed. St Louis, MO: WH Green; 1987:421.
- King TP, Spangfort MD. Structure and biology of stinging insect venom allergens. Int Arch Allergy Immunol. 2000;123(2):99-106.
- Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol.2002;2(4);341-346.
- Mauriello PM, Barde SH. Natural history of large local reactions from stinging insects. J Allergy Clin Immunol. 1984;74(4 Pt 1):494-498.
- Díaz-Sánchez CL, Lifshitz-Guinzberg A, Ignacio-Ibarra G, Halabe-Cherem J, Quinones-Galvan A. Survival after massive (>2,000) Africanized honey bee stings. Arch Intern Med. 1998;158(8):925-927.
- Elston DM. Life-threatening stings, bites, infestations and parasitic diseases. Clin Dermatol. 2005;23(2):164-170.
- Lazoglu AH1, Boglioli LR, Taff ML, Rosenbluth M, Macris NT. Serum sickness reaction following multiple insect stings. Ann Allergy Asthma Immunol. 1995;75(6 Pt 1):522-524.
- Reisman RE, Livingston A. Late-onset allergic reactions, including serum sickness, after insect stings. J Allergy Clin Immunol. 1989;84(3);331-337.
- Anaphylaxis. American Academy of Allergy, Asthma & Immunology Web site. http://www.aaaai.org/conditions-and-treatments/conditions-a-to-zsearch/anaphylaxis.aspx. Accessed April 2, 2014.
- Brown H, Benton HS. Allergy to the Hymenoptera. V. Clinical study of 400 patients. Arch Intern Med. 1970;125(4):665-669.
- Frazier CA. Allergic reactions to insect stings: a review of 180 cases. South Med J. 1964;57;1023-1034.
- Mueller HL. Further experiences with severe allergic reactions to insect stings. N Engl J Med. 1959;161:374-377.
- Lockey RF, Turkeltaub PC, Baird-Warren IA, et al. The Hymenoptera venom study I, 1979-1982: demographics and history-sting data. J Allergy Clin Immunol. 1988;82(3 Pt 1):370-381.
- Schneir AB, Clark RF. Bites and stings. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011:chap120;585-596.
- Rowe BH, Gaeta T. Anaphylaxis, acute allergic reactions, and angioedema. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011:chap 6;52-54.
- Jones RG1, Corteling RL, Bhogal G, Landon J. A novel Fab-based antivenom for the treatment of mass bee attacks. Am J Trop Med Hyg. 1999;61(3):361-366.
- National Institutes of Health, US Department of Health and Human Services, National Insitute of Allergy and Infectious Diseases. Guidelines for the Diagnosis and Management of Food Allergy in the United States. Summary of the NIAID-Sponsored Expert Panel Report. Bethesda, MD: National Institutes of Health; 2010. NIH Publication No. 11-7700. http://www.niaid.nih.gov/topics/foodAllergy/clinical/Documents/FAGuidelinesExecSummary.pdf. Accessed April 2, 2014.
- Kemp SF, deShazo RD, Moffitt JE, Williams DF, Buhner WA 2nd. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105(4):683-691.
- Fernández-Meléndez S, Miranda A, García-González JJ, Barber D, Lombardero M. Anaphylaxis caused by imported red fire ant stings in Málaga, Spain. J Investig Allergol Immunol. 2007;17(1):48,49.
- Swanson DL. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med. 2005;352(7):700-707.
- Saucier JR. Arachnid envenomation. Emerg Med Clin North Am. 2004;22(2):405-422.
- Wright SW, Wrenn KD, Murray L, Seger D. Clinical presentation and outcome of brown recluse spider bite. Ann Emerg Med. 1997;30(1):28-32.
- Phillips S, Kohn M, Baker D, et al. Therapy of brown spider envenomation: a controlled trial of hyperbaric oxygen, dapsone, and cyproheptadine. Ann Emerg Med. 1995;25(3):363-368.
- Pauli I, Puka J, Gubert IC, Minozzo JC. The efficacy of antivenom in loxoscelism treatment. Toxicon. 2006;48(2):123-127.
- Ushkaryov YA, Volynski KE, Ashton AC. The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon. 2004;43(5):527-542.
- Clark RF, Wethern-Kestner S, Vance MV, Gerkin R. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21(7):782-787.
- O’Malley GF, Dart RC, Kuffner EF. Successful treatment of latrodectism with antivenom after 90 hours. N Engl J Med. 1999;340(8):657.
- Clark RF. The safety and efficacy of antivenin Latrodectus mactans. J Toxicol Clin Toxicol. 2001;39(2):125-127.
- Sejvar JJ, Haddad MB, Tierney BC. Neurologic manifestations and outcome of West Nile virus infection [published correction appears in JAMA. 2003;290(10):1318]. JAMA. 2003;290(4):511-515.
- Brown M, Herbert AA. Insect repellents: an overview. J Am Acad Dermatol. 1997;36(2 Pt 1):243-249.
- Fradin MS. Mosquitoes and mosquito repellents: a clinician’s quide. Ann Intern Med. 1998;128(11):931-940.
- Miller JD. Anaphylaxis associated with insect repellent. N Engl J Med. 1982;307(21):1341,1342.
- Spach DH, Kanter AS, Dougherty MJ, et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332(7): 424-428.
- Jackson LA, Spach DH, Kippen DA, et al. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J Infect Dis. 1996;173(4):1023-1026.
- Sundnes KO. Epidemic of louse-borne relapsing fever in Ethiopia. Lancet. 1993;342(8881):1213-1215.
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7(1):6. http://escholarship.org/uc/item/59k2m8wt. Accessed April 2, 2014.
- Stucki A, Ludwig R. Images in clinical medicine. Bedbug bites. N Engl J Med. 2008; 359:10)1047.
- Kuspis DA, Rawlins JE, Krenzelok EP. Human exposures to stinging caterpillars: Lophocampa caryae exposures. Am J Emerg Med. 2001;19(5):396-398.
- Moed L, Shwayder TA, 0.Chang MW. Cantharidin revisited: a blistering defense of an ancient medicine. Arch Dermatol. 2001;137(10):1357-1360.
Hymenoptera
The order Hymenoptera of the phylum Arthropoda can be divided into three subgroups that are medically relevant: (1) Apidae (Apids), which include the honeybee and bumblebee; (2) Vespidae, (Vespids) which include yellow jackets, hornets and wasps; and (3) Formicidae (ants).6
Bees and Wasps
The main allergens in Apid venom are phospholipase A2, hyaluronidase, and melittin. Melittin, the main component, is a membrane active polypeptide that causes degranulation of basophils and mast cells. The allergens in Vespid venom are phospholipase, hyaluronidase, and antigen 5. As all Hymenoptera share some of these components, cross-sensitization may occur and individuals may be allergic to more than one species.7
Occasionally after multiple stings, patients present with symptoms of a systemic toxic reaction. This is often seen in an Africanized bee attack. These so-called “killer bees” are hybrids of African bees that escaped from laboratories in Brazil in the 1950s and spread northward; they are found in most of the warmer US states. Their venom is not more toxic than that of any other bee, but Africanized honeybees are more aggressive and respond to a perceived threat in far greater numbers. The reaction that results from multiple stings is systemic and may resemble anaphylaxis. Common symptoms include nausea, vomiting, and diarrhea, as well as lightheadedness and syncope. Interestingly, urticaria and bronchospasm are not universally present, even though respiratory failure and cardiac arrest may occur. Other symptoms include renal failure with acute tubular necrosis, myoglobinuria or hemoglobinuria, hepatic failure, and disseminated intravascular coagulation (DIC).10,11 In addition, there have been reports of unusual reactions such as vasculitis, nephrosis, neuritis, encephalitis, and serum sickness. Late-appearing symptoms usually start several days to weeks after a sting and tend last for a prolonged period of time. Serum sickness tends to appear 5 to 14 days after exposure and consists of fever, malaise, headache, urticaria, lymphadenopathy, and polyarthritis.12 Of note, patients who have venom-induced serum sickness may be at risk for anaphylaxis after subsequent stings and may therefore be suitable candidates for venom immunotherapy.13
Anaphylaxis
The definition of anaphylaxis is not universally agreed upon. The American Academy of Allergy, Asthma and Immunology defines anaphylaxis as a serious allergic response that often involves swelling, hives, hypotension and, in severe cases, shock. A major difference between anaphylaxis and other allergic reactions is that anaphylaxis typically involves more than one body system.14 The clinical features of anaphylaxis from insect stings are the same as those from other causes, typically generalized urticaria, facial flushing, and angioedema. Abdominal cramping, nausea, vomiting, and diarrhea are also seen. Life-threatening symptoms include stridor, circulatory collapse with shock, and bronchospasm. Symptoms usually begin 10 to 20 minutes after a sting, and almost all will develop within 6 hours. Interestingly, symptoms may recur 8 to 12 hours after the initial reaction.15-18
Management
Immediate removal of the bee stinger is the most important principle as it precludes any further venom transfer. Traditional teaching recommended scraping the stinger out to avoid squeezing remaining venom into the tissues; however, involuntary muscle contractions of the gland continue after the stinger detaches, and the venom is quickly exhausted. Thus, immediate removal of the stinger is crucial, though the method of removal is now thought irrelevant.19
The mainstay of therapy for serious reactions is intramuscular (IM) epinephrine. The initial dosing is 0.3 to 0.5 mg (0.3 to 0.5 mL of 1:1000 concentration) in adults, and 0.01 mg/kg in children (maximum 0.3 mg). The injection should be IM and not subcutaneous, as IM dosing provides higher and more consistent and rapid peak blood epinephrine levels.20 Concomitant intravenous (IV) administration of standard antihistamines, often diphenhydramine 1 mg/kg (generally 25-50 mg) and histamine-2 receptor antagonists (typically ranitidine 50 mg) are also recommended. The administration of steroids (methylprednisolone 125 mg IV or prednisone 60 mg orally) is traditionally recommended and thought to help potentiate the effect of other interventional measures.20 Bronchospasm, if present, is treated with nebulized β-agonists (albuterol). Hypotension may develop and requires significant crystalloid infusion—often several liters. If hypotension persists despite adequate fluid replacement, vasopressor therapy is recommended.
If a patient does not respond to initial treatment and cardiovascular (CV) collapse is evident, IV infusion of epinephrine should be initiated. Epinephrine, 100 mcg (0.1 mg) IV, should be given as a 1:100,000 dilution. This can be done by placing epinephrine, 0.1 mg (0.1 mL of the 1:1000 dilution), in 10 mL of normal saline solution and infusing it over 5 to 10 minutes (a rate of 1 to 2 mL/min). If the patient is refractory to the initial bolus, then an epinephrine infusion can be started by placing epinephrine, 1 mg (1.0 mL of the 1:1000 dilution), in 500 mL of 5% dextrose in water or NS and administering at a rate of 1 to 4 mcg per minute (0.5 to 2 mL/min), titrating to effect.20 Antivenins have been studied for treatment, but none are commercially available at this time.21 Patients with anaphylaxis associated with severe signs and symptoms, including any evidence of CV collapse, should be admitted to the hospital for aggressive therapy and monitoring. Persons with mild-to-moderate reactions should be observed for 4 to 6 hours to monitor for late occurring symptoms. Outpatient therapy with antihistamines, oral steroids, and a prescription for an epinephrine auto-injector—including training on proper administration prior to discharge—are strongly recommended.22 Follow-up with an allergist is also indicated in patients with significant reactions, as skin testing and immunotherapy may be beneficial to prevent anaphylaxis during future exposures.
Ants
Fire ant venom is composed of an insoluble alkaloid, and crossreactivity with the venom of other Hymenopteras species does exist. Stings generally result in a papule, which evolves into a sterile pustule. Localized necrosis, scarring, and secondary infection can occur. Systemic reactions with angioedema and urticaria have been documented, which can sometimes lead to fatalities.24
Treatment
Treatment of fire ant stings begin and end with local wound care. If the reaction is systemic, a treatment plan similar to that outlined in the treatment section for bees and wasps is indicated.
Araneae
The order Araneae of the phylum Arthropoda includes over 34,000 species of spiders divided into 105 families. Of those, only half a dozen are medically relevant and only three are commonly encountered in the United States. These include Loxosceles (most notably, the brown recluse spider), Tegeneria (mainly the hobo brown spider) and Latrodectus (includes the black widow spider). True spiders have a worldwide distribution and tend to thrive in heavily populated areas, resulting in many biting episodes per year. Data from the AAPPC’s most recent annual report listed 9,255 single spider-bite exposures in 2012, with one associated death.3
Spiders are carnivores and use venom to paralyze their prey. They are generally not a threat to humans as their fangs are too small to penetrate human skin, and the amount of venom injected is too small to produce toxicity. Thus, reactions resulting from a spider bite are typically limited to a localized reaction. Fortunately, most bites only require supportive medical therapy.
Loxosceles
Loxosceles are present worldwide, but L reclusa (the “brown recluse spider”) accounts for a significant number of envenomations in the United States. The AAPCC’s 2012 data notes 1,365 cases of exposure to the brown recluse spider with 510 of those victims seeking medical care.3 In many instances, clinicians attribute necrotic bites to the brown recluse spider, however, confirmation is often lacking. Loxosceles are nocturnal, and they are found both indoors and outdoors—mostly in dark and dry areas such as basements, closets, and woodpiles. These spiders are shy, but may bite when threatened. Their venom contains enzymes, including hyaluronidase and sphingomyelinase. Though rare, wounds can become necrotic due to neutrophil activation, platelet aggregation, and thrombosis.25 The most common reaction to a Loxosceles bite is a mild painless erythematous lesion that becomes firm and generally heals over several days to weeks. In severe reactions, erythema, edema, and pruritus initially develop, followed within 24 to 72 hours by a hemorrhagic bulla surrounded by blanched skin. This leads to the “red, white, and blue sign” (ie, erythema, blanching, and ecchymosis). Infrequently, the ecchymotic area becomes necrotic and ulcerates in 3 to 5 days. The differential diagnoses should include necrotizing fasciitis, erythema chronicum migrans (from Borrelia-infected tick bites), and anthrax. Ulcerated lesions may result in significant cosmetic defect. Healing may take up to 2 weeks, and skin grafting is occasionally required.26
Treatment begins with the usual supportive measures, including analgesia, ice, elevation, and a light compression dressing. Antibiotics are not indicated, unless there are signs of secondary infection. Serial evaluation for wound checks should be arranged. If ulceration develops, surgical debridement may be required. The vast majority of bites heal with supportive care alone, and aggressive medical therapy is usually not warranted.27Patients with systemic manifestations should be admitted to the hospital for further care. There is no evidence-based literature to guide therapy. Many therapies have been tried with variable results and there remains no definitive standard of care.
Treatment regimens include antihistamines, antivenin, colchicine, dapsone, hyperbaric oxygen, cyproheptadine, surgical excision, and steroids.28 Dapsone continues to be widely advocated worldwide despite its known adverse effects—most notably hemolysis and methemoglobinemia. Antivenin administration has shown some promise in animal models, but its efficacy in humans is still unclear.29
Tegenaria
The Tegenaria agrestis or hobo spider is a native of Europe and central Asia and is only found in the northwest part of the United States. It is considered aggressive and tends to bite even with only mild provocation. The clinical presentation, inclusive of systemic reactions, is similar to that of the brown recluse spider. Similarly, there is no proven treatment. Surgical wound resection and skin grafting should be considered and is at times required.
Latrodectus.
Latrodectus, also known as widow spiders, are found worldwide. Five species are commonly found in the United States, but the black widow is the most well known. Only three of the species are actually black. Other varieties are typically brown or red. However, almost all Latrodectus spiders have a characteristic orange-red hourglass-shaped marking (Figure 3). Widow spiders aggressively defend their webs, and are most often found in woodpiles, basements, garages, and sheds. Most bites occur in the warmer months, between April and October.
Latrodectus antivenin is very effective, often resolving symptoms rapidly and reducing the duration of illness—even when administered up to 90 hours postenvenomation.32 This antivenin is derived from horse serum, and hypersensitivity reactions are possible. One death from anaphylaxis has been reported in the United States after antivenin was given undiluted via IV push; however, slow administration of diluted antivenin is considered safe.33
Diptera
The order Diptera of the phylum Arthropoda includes over 240,000 species. Among those, the mosquitoes and flies are the most medically relevant.
Mosquitoes
An actual mosquito bite itself causes minimal trauma and is not usually felt by the victim. However, the local anesthetic that is injected into the wound at the time of the attack causes local tissue damage. Mosquito bites can lead to both immediate and delayed reactions. Typical immediate reactions are of short duration and include edema, erythema, and pruritus. More severe reactions are extremely rare and consist of skin necrosis. Delayed skin reactions are fairly common, but tend to last longer, persisting for days or even weeks. Treatment is symptomatic, usually with antihistamines and NSAIDS.
Patients can acquire an allergy to mosquito saliva over time and develop increasingly pronounced edematous and pruritic lesions with subsequent bites. They can also experience fever, malaise, generalized edema, as well as severe nausea and vomiting.
Systemic or anaphylactic reactions are not known to occur. Instead, the greatest danger occurs with the transmission of life-threatening diseases. Malaria, yellow fever, dengue hemorrhagic fever, and different types of equine encephalitis are all transmitted by mosquito bites. One interesting newcomer to the United States is the West Nile virus (WNV), which has spread rapidly since its introduction in 2003. Over 1,850 cases were reported in 22 different states over the initial 8 months. Acute symptoms are mild in the majority of patients, but a small minority can experience fatal disease. Neurological symptoms include tumor, myoclonus, and Parkinsonism. An irreversible poliomyelitis-like syndrome may also develop. In addition to WNV, St Louis encephalitis and equine encephalitis also remain important pathogens in the United States.34 Prevention of bites is crucial and includes the use of pyrethroid-impregnated mosquito netting, repellents, and oral malaria prophylaxis. N,N-diethyl-3-methylbenzamide (DEET) remains the most effective mosquito repellent.35 Although toxic reactions are rare, they do occur and anaphylaxis has been reported. 36,37
Flies
Flies are blood-sucking insects that feed by stabbing and piercing their victim’s skin. Their bites always cause some degree of pain and pruritus. Allergic reactions are possible, though not as severe as those produced by Hymenoptera venom. Treatment is largely symptomatic with ice, oral antihistamine, analgesics. and topical or oral steroids as needed. Secondary bite infection is a concern and antibiotics are sometimes necessary.
Shiponaptera
The order Shinoptera includes fleas and lice. All produce very similar lesions, making diagnosis difficult. One concern with these bites is the development of secondary infections, especially in children. The skin should be washed with soap and water. Calamine, cool soaks, and oral or topical antihistamine may all be helpful to reduce symptoms.
Fleas
With fleas, as with mosquitoes, there is additionally a concern for transmission of life-threatening diseases. Fleas transmit bubonic plague, endemic typhus, brucellosis, melioidosis, and erysipeloid. Fortunately, effective oral and injectable formulations for both dogs and cats are now available to control fleas on most family pets.
Lice
Hemiptera
Lepidoptera
The order Lepidoptera includes butterflies and moths and their caterpillars. Symptoms that result from contact with this class of insects are referred to as lepidopterism. Caterpillars have hair or spines for protection, which are also sometimes connected to a venom gland. Contact with these spines usually causes localized skin irritation and pruritus. Megalopyge opercularis, also known as the “puss caterpillar,” is mainly found in the southeastern United States and accounts for the majority of envenomation cases in this country. Intense local burning pain is typical at the site of contact and is followed by a grid-like pattern of hemorrhagic papules, which appear 2 to 3 hours after exposure and may last for several days. Regional lymphadenopathy is common. Other symptoms include headache, fever, hypotension, and convulsions. No deaths have ever been reported.
As there is no antivenin available for lepidopterism, treatment is mostly supportive. If spines are visible following contact, they should be removed with adhesive tape. Antihistamines and steroids may be used for symptom control. In patients with hypotension, IV fluids and IV epinephrine may be required.43
Coleoptera
The order Coleoptera includes a large number of beetles, though clinically significant envenomation occurs only with blister beetles. There are over 1,500 species of blister beetles worldwide, approximately 2,002 of which are in the United States. The blister beetle responsible for most of the medically significant envenomations is Cantharis vesicatoria—also known as “Spanish fly.” Of note, the Spanish fly is not naturally found in the United States.
Cantharidin-containing substances are sometimes used medicinally in wart removal preparations and are also sold for their purported aphrodisiac effects (the associated vascular congestion and urethral inflammation are interpreted as enhanced sexuality). Transdermal absorption or ingestion may lead to systemic toxicity with severe vomiting, hematemesis, abdominal pain, diarrhea, hematuria, renal failure, etc. Death has been reported after large ingestions.
Treatment is largely supportive. The skin should be irrigated thoroughly after exposure, followed by local wound care. Patients who present after ingestion should be admitted to the hospital for further treatment and care.47
Conclusion
Knowledge of a vast array of stinging insects and spiders is important for any clinician, but the appropriate evaluation and treatment of bites and envenomations are crucial for EPs. Most exposures can be treated with supportive care, while others require in-depth knowledge and clinical expertise.
Dr Deljoui is a former resident, department of emergency medicine, Eastern Virginia Medical School, Norfolk; and current critical care fellow, University of Maryland, Baltimore.
Dr Knapp is an associate professor and residency program director, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
Hymenoptera
The order Hymenoptera of the phylum Arthropoda can be divided into three subgroups that are medically relevant: (1) Apidae (Apids), which include the honeybee and bumblebee; (2) Vespidae, (Vespids) which include yellow jackets, hornets and wasps; and (3) Formicidae (ants).6
Bees and Wasps
The main allergens in Apid venom are phospholipase A2, hyaluronidase, and melittin. Melittin, the main component, is a membrane active polypeptide that causes degranulation of basophils and mast cells. The allergens in Vespid venom are phospholipase, hyaluronidase, and antigen 5. As all Hymenoptera share some of these components, cross-sensitization may occur and individuals may be allergic to more than one species.7
Occasionally after multiple stings, patients present with symptoms of a systemic toxic reaction. This is often seen in an Africanized bee attack. These so-called “killer bees” are hybrids of African bees that escaped from laboratories in Brazil in the 1950s and spread northward; they are found in most of the warmer US states. Their venom is not more toxic than that of any other bee, but Africanized honeybees are more aggressive and respond to a perceived threat in far greater numbers. The reaction that results from multiple stings is systemic and may resemble anaphylaxis. Common symptoms include nausea, vomiting, and diarrhea, as well as lightheadedness and syncope. Interestingly, urticaria and bronchospasm are not universally present, even though respiratory failure and cardiac arrest may occur. Other symptoms include renal failure with acute tubular necrosis, myoglobinuria or hemoglobinuria, hepatic failure, and disseminated intravascular coagulation (DIC).10,11 In addition, there have been reports of unusual reactions such as vasculitis, nephrosis, neuritis, encephalitis, and serum sickness. Late-appearing symptoms usually start several days to weeks after a sting and tend last for a prolonged period of time. Serum sickness tends to appear 5 to 14 days after exposure and consists of fever, malaise, headache, urticaria, lymphadenopathy, and polyarthritis.12 Of note, patients who have venom-induced serum sickness may be at risk for anaphylaxis after subsequent stings and may therefore be suitable candidates for venom immunotherapy.13
Anaphylaxis
The definition of anaphylaxis is not universally agreed upon. The American Academy of Allergy, Asthma and Immunology defines anaphylaxis as a serious allergic response that often involves swelling, hives, hypotension and, in severe cases, shock. A major difference between anaphylaxis and other allergic reactions is that anaphylaxis typically involves more than one body system.14 The clinical features of anaphylaxis from insect stings are the same as those from other causes, typically generalized urticaria, facial flushing, and angioedema. Abdominal cramping, nausea, vomiting, and diarrhea are also seen. Life-threatening symptoms include stridor, circulatory collapse with shock, and bronchospasm. Symptoms usually begin 10 to 20 minutes after a sting, and almost all will develop within 6 hours. Interestingly, symptoms may recur 8 to 12 hours after the initial reaction.15-18
Management
Immediate removal of the bee stinger is the most important principle as it precludes any further venom transfer. Traditional teaching recommended scraping the stinger out to avoid squeezing remaining venom into the tissues; however, involuntary muscle contractions of the gland continue after the stinger detaches, and the venom is quickly exhausted. Thus, immediate removal of the stinger is crucial, though the method of removal is now thought irrelevant.19
The mainstay of therapy for serious reactions is intramuscular (IM) epinephrine. The initial dosing is 0.3 to 0.5 mg (0.3 to 0.5 mL of 1:1000 concentration) in adults, and 0.01 mg/kg in children (maximum 0.3 mg). The injection should be IM and not subcutaneous, as IM dosing provides higher and more consistent and rapid peak blood epinephrine levels.20 Concomitant intravenous (IV) administration of standard antihistamines, often diphenhydramine 1 mg/kg (generally 25-50 mg) and histamine-2 receptor antagonists (typically ranitidine 50 mg) are also recommended. The administration of steroids (methylprednisolone 125 mg IV or prednisone 60 mg orally) is traditionally recommended and thought to help potentiate the effect of other interventional measures.20 Bronchospasm, if present, is treated with nebulized β-agonists (albuterol). Hypotension may develop and requires significant crystalloid infusion—often several liters. If hypotension persists despite adequate fluid replacement, vasopressor therapy is recommended.
If a patient does not respond to initial treatment and cardiovascular (CV) collapse is evident, IV infusion of epinephrine should be initiated. Epinephrine, 100 mcg (0.1 mg) IV, should be given as a 1:100,000 dilution. This can be done by placing epinephrine, 0.1 mg (0.1 mL of the 1:1000 dilution), in 10 mL of normal saline solution and infusing it over 5 to 10 minutes (a rate of 1 to 2 mL/min). If the patient is refractory to the initial bolus, then an epinephrine infusion can be started by placing epinephrine, 1 mg (1.0 mL of the 1:1000 dilution), in 500 mL of 5% dextrose in water or NS and administering at a rate of 1 to 4 mcg per minute (0.5 to 2 mL/min), titrating to effect.20 Antivenins have been studied for treatment, but none are commercially available at this time.21 Patients with anaphylaxis associated with severe signs and symptoms, including any evidence of CV collapse, should be admitted to the hospital for aggressive therapy and monitoring. Persons with mild-to-moderate reactions should be observed for 4 to 6 hours to monitor for late occurring symptoms. Outpatient therapy with antihistamines, oral steroids, and a prescription for an epinephrine auto-injector—including training on proper administration prior to discharge—are strongly recommended.22 Follow-up with an allergist is also indicated in patients with significant reactions, as skin testing and immunotherapy may be beneficial to prevent anaphylaxis during future exposures.
Ants
Fire ant venom is composed of an insoluble alkaloid, and crossreactivity with the venom of other Hymenopteras species does exist. Stings generally result in a papule, which evolves into a sterile pustule. Localized necrosis, scarring, and secondary infection can occur. Systemic reactions with angioedema and urticaria have been documented, which can sometimes lead to fatalities.24
Treatment
Treatment of fire ant stings begin and end with local wound care. If the reaction is systemic, a treatment plan similar to that outlined in the treatment section for bees and wasps is indicated.
Araneae
The order Araneae of the phylum Arthropoda includes over 34,000 species of spiders divided into 105 families. Of those, only half a dozen are medically relevant and only three are commonly encountered in the United States. These include Loxosceles (most notably, the brown recluse spider), Tegeneria (mainly the hobo brown spider) and Latrodectus (includes the black widow spider). True spiders have a worldwide distribution and tend to thrive in heavily populated areas, resulting in many biting episodes per year. Data from the AAPPC’s most recent annual report listed 9,255 single spider-bite exposures in 2012, with one associated death.3
Spiders are carnivores and use venom to paralyze their prey. They are generally not a threat to humans as their fangs are too small to penetrate human skin, and the amount of venom injected is too small to produce toxicity. Thus, reactions resulting from a spider bite are typically limited to a localized reaction. Fortunately, most bites only require supportive medical therapy.
Loxosceles
Loxosceles are present worldwide, but L reclusa (the “brown recluse spider”) accounts for a significant number of envenomations in the United States. The AAPCC’s 2012 data notes 1,365 cases of exposure to the brown recluse spider with 510 of those victims seeking medical care.3 In many instances, clinicians attribute necrotic bites to the brown recluse spider, however, confirmation is often lacking. Loxosceles are nocturnal, and they are found both indoors and outdoors—mostly in dark and dry areas such as basements, closets, and woodpiles. These spiders are shy, but may bite when threatened. Their venom contains enzymes, including hyaluronidase and sphingomyelinase. Though rare, wounds can become necrotic due to neutrophil activation, platelet aggregation, and thrombosis.25 The most common reaction to a Loxosceles bite is a mild painless erythematous lesion that becomes firm and generally heals over several days to weeks. In severe reactions, erythema, edema, and pruritus initially develop, followed within 24 to 72 hours by a hemorrhagic bulla surrounded by blanched skin. This leads to the “red, white, and blue sign” (ie, erythema, blanching, and ecchymosis). Infrequently, the ecchymotic area becomes necrotic and ulcerates in 3 to 5 days. The differential diagnoses should include necrotizing fasciitis, erythema chronicum migrans (from Borrelia-infected tick bites), and anthrax. Ulcerated lesions may result in significant cosmetic defect. Healing may take up to 2 weeks, and skin grafting is occasionally required.26
Treatment begins with the usual supportive measures, including analgesia, ice, elevation, and a light compression dressing. Antibiotics are not indicated, unless there are signs of secondary infection. Serial evaluation for wound checks should be arranged. If ulceration develops, surgical debridement may be required. The vast majority of bites heal with supportive care alone, and aggressive medical therapy is usually not warranted.27Patients with systemic manifestations should be admitted to the hospital for further care. There is no evidence-based literature to guide therapy. Many therapies have been tried with variable results and there remains no definitive standard of care.
Treatment regimens include antihistamines, antivenin, colchicine, dapsone, hyperbaric oxygen, cyproheptadine, surgical excision, and steroids.28 Dapsone continues to be widely advocated worldwide despite its known adverse effects—most notably hemolysis and methemoglobinemia. Antivenin administration has shown some promise in animal models, but its efficacy in humans is still unclear.29
Tegenaria
The Tegenaria agrestis or hobo spider is a native of Europe and central Asia and is only found in the northwest part of the United States. It is considered aggressive and tends to bite even with only mild provocation. The clinical presentation, inclusive of systemic reactions, is similar to that of the brown recluse spider. Similarly, there is no proven treatment. Surgical wound resection and skin grafting should be considered and is at times required.
Latrodectus.
Latrodectus, also known as widow spiders, are found worldwide. Five species are commonly found in the United States, but the black widow is the most well known. Only three of the species are actually black. Other varieties are typically brown or red. However, almost all Latrodectus spiders have a characteristic orange-red hourglass-shaped marking (Figure 3). Widow spiders aggressively defend their webs, and are most often found in woodpiles, basements, garages, and sheds. Most bites occur in the warmer months, between April and October.
Latrodectus antivenin is very effective, often resolving symptoms rapidly and reducing the duration of illness—even when administered up to 90 hours postenvenomation.32 This antivenin is derived from horse serum, and hypersensitivity reactions are possible. One death from anaphylaxis has been reported in the United States after antivenin was given undiluted via IV push; however, slow administration of diluted antivenin is considered safe.33
Diptera
The order Diptera of the phylum Arthropoda includes over 240,000 species. Among those, the mosquitoes and flies are the most medically relevant.
Mosquitoes
An actual mosquito bite itself causes minimal trauma and is not usually felt by the victim. However, the local anesthetic that is injected into the wound at the time of the attack causes local tissue damage. Mosquito bites can lead to both immediate and delayed reactions. Typical immediate reactions are of short duration and include edema, erythema, and pruritus. More severe reactions are extremely rare and consist of skin necrosis. Delayed skin reactions are fairly common, but tend to last longer, persisting for days or even weeks. Treatment is symptomatic, usually with antihistamines and NSAIDS.
Patients can acquire an allergy to mosquito saliva over time and develop increasingly pronounced edematous and pruritic lesions with subsequent bites. They can also experience fever, malaise, generalized edema, as well as severe nausea and vomiting.
Systemic or anaphylactic reactions are not known to occur. Instead, the greatest danger occurs with the transmission of life-threatening diseases. Malaria, yellow fever, dengue hemorrhagic fever, and different types of equine encephalitis are all transmitted by mosquito bites. One interesting newcomer to the United States is the West Nile virus (WNV), which has spread rapidly since its introduction in 2003. Over 1,850 cases were reported in 22 different states over the initial 8 months. Acute symptoms are mild in the majority of patients, but a small minority can experience fatal disease. Neurological symptoms include tumor, myoclonus, and Parkinsonism. An irreversible poliomyelitis-like syndrome may also develop. In addition to WNV, St Louis encephalitis and equine encephalitis also remain important pathogens in the United States.34 Prevention of bites is crucial and includes the use of pyrethroid-impregnated mosquito netting, repellents, and oral malaria prophylaxis. N,N-diethyl-3-methylbenzamide (DEET) remains the most effective mosquito repellent.35 Although toxic reactions are rare, they do occur and anaphylaxis has been reported. 36,37
Flies
Flies are blood-sucking insects that feed by stabbing and piercing their victim’s skin. Their bites always cause some degree of pain and pruritus. Allergic reactions are possible, though not as severe as those produced by Hymenoptera venom. Treatment is largely symptomatic with ice, oral antihistamine, analgesics. and topical or oral steroids as needed. Secondary bite infection is a concern and antibiotics are sometimes necessary.
Shiponaptera
The order Shinoptera includes fleas and lice. All produce very similar lesions, making diagnosis difficult. One concern with these bites is the development of secondary infections, especially in children. The skin should be washed with soap and water. Calamine, cool soaks, and oral or topical antihistamine may all be helpful to reduce symptoms.
Fleas
With fleas, as with mosquitoes, there is additionally a concern for transmission of life-threatening diseases. Fleas transmit bubonic plague, endemic typhus, brucellosis, melioidosis, and erysipeloid. Fortunately, effective oral and injectable formulations for both dogs and cats are now available to control fleas on most family pets.
Lice
Hemiptera
Lepidoptera
The order Lepidoptera includes butterflies and moths and their caterpillars. Symptoms that result from contact with this class of insects are referred to as lepidopterism. Caterpillars have hair or spines for protection, which are also sometimes connected to a venom gland. Contact with these spines usually causes localized skin irritation and pruritus. Megalopyge opercularis, also known as the “puss caterpillar,” is mainly found in the southeastern United States and accounts for the majority of envenomation cases in this country. Intense local burning pain is typical at the site of contact and is followed by a grid-like pattern of hemorrhagic papules, which appear 2 to 3 hours after exposure and may last for several days. Regional lymphadenopathy is common. Other symptoms include headache, fever, hypotension, and convulsions. No deaths have ever been reported.
As there is no antivenin available for lepidopterism, treatment is mostly supportive. If spines are visible following contact, they should be removed with adhesive tape. Antihistamines and steroids may be used for symptom control. In patients with hypotension, IV fluids and IV epinephrine may be required.43
Coleoptera
The order Coleoptera includes a large number of beetles, though clinically significant envenomation occurs only with blister beetles. There are over 1,500 species of blister beetles worldwide, approximately 2,002 of which are in the United States. The blister beetle responsible for most of the medically significant envenomations is Cantharis vesicatoria—also known as “Spanish fly.” Of note, the Spanish fly is not naturally found in the United States.
Cantharidin-containing substances are sometimes used medicinally in wart removal preparations and are also sold for their purported aphrodisiac effects (the associated vascular congestion and urethral inflammation are interpreted as enhanced sexuality). Transdermal absorption or ingestion may lead to systemic toxicity with severe vomiting, hematemesis, abdominal pain, diarrhea, hematuria, renal failure, etc. Death has been reported after large ingestions.
Treatment is largely supportive. The skin should be irrigated thoroughly after exposure, followed by local wound care. Patients who present after ingestion should be admitted to the hospital for further treatment and care.47
Conclusion
Knowledge of a vast array of stinging insects and spiders is important for any clinician, but the appropriate evaluation and treatment of bites and envenomations are crucial for EPs. Most exposures can be treated with supportive care, while others require in-depth knowledge and clinical expertise.
Dr Deljoui is a former resident, department of emergency medicine, Eastern Virginia Medical School, Norfolk; and current critical care fellow, University of Maryland, Baltimore.
Dr Knapp is an associate professor and residency program director, department of emergency medicine, Eastern Virginia Medical School, Norfolk.
- White J. Bites and stings from venomous animals: a global overview. The Drug Monit. 2000;22(1):65-68.
- Oten EJ. Venomous animal injuries. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:794-807.
- Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila). 2013;51(10):949-1229. doi:10.3109/15563650.2013.863906. https://aapcc.s3.amazonaws.com/pdfs/annual_reports/2012_NPDS_Annual_Report.pdf. Accessed April 2, 2014.
- Liberman P, Camargo CA, Bohike K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596-602.
- Barnard JH. Studies of 400 Hymenoptera sting deaths in the United States. J Allergy Clin Immunol. 1973;52(5):259-264.
- Frazier CA. Insect Allergy: Allergic and Toxic Reactions to Insects and Other Arthropods. 2nd Ed. St Louis, MO: WH Green; 1987:421.
- King TP, Spangfort MD. Structure and biology of stinging insect venom allergens. Int Arch Allergy Immunol. 2000;123(2):99-106.
- Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol.2002;2(4);341-346.
- Mauriello PM, Barde SH. Natural history of large local reactions from stinging insects. J Allergy Clin Immunol. 1984;74(4 Pt 1):494-498.
- Díaz-Sánchez CL, Lifshitz-Guinzberg A, Ignacio-Ibarra G, Halabe-Cherem J, Quinones-Galvan A. Survival after massive (>2,000) Africanized honey bee stings. Arch Intern Med. 1998;158(8):925-927.
- Elston DM. Life-threatening stings, bites, infestations and parasitic diseases. Clin Dermatol. 2005;23(2):164-170.
- Lazoglu AH1, Boglioli LR, Taff ML, Rosenbluth M, Macris NT. Serum sickness reaction following multiple insect stings. Ann Allergy Asthma Immunol. 1995;75(6 Pt 1):522-524.
- Reisman RE, Livingston A. Late-onset allergic reactions, including serum sickness, after insect stings. J Allergy Clin Immunol. 1989;84(3);331-337.
- Anaphylaxis. American Academy of Allergy, Asthma & Immunology Web site. http://www.aaaai.org/conditions-and-treatments/conditions-a-to-zsearch/anaphylaxis.aspx. Accessed April 2, 2014.
- Brown H, Benton HS. Allergy to the Hymenoptera. V. Clinical study of 400 patients. Arch Intern Med. 1970;125(4):665-669.
- Frazier CA. Allergic reactions to insect stings: a review of 180 cases. South Med J. 1964;57;1023-1034.
- Mueller HL. Further experiences with severe allergic reactions to insect stings. N Engl J Med. 1959;161:374-377.
- Lockey RF, Turkeltaub PC, Baird-Warren IA, et al. The Hymenoptera venom study I, 1979-1982: demographics and history-sting data. J Allergy Clin Immunol. 1988;82(3 Pt 1):370-381.
- Schneir AB, Clark RF. Bites and stings. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011:chap120;585-596.
- Rowe BH, Gaeta T. Anaphylaxis, acute allergic reactions, and angioedema. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011:chap 6;52-54.
- Jones RG1, Corteling RL, Bhogal G, Landon J. A novel Fab-based antivenom for the treatment of mass bee attacks. Am J Trop Med Hyg. 1999;61(3):361-366.
- National Institutes of Health, US Department of Health and Human Services, National Insitute of Allergy and Infectious Diseases. Guidelines for the Diagnosis and Management of Food Allergy in the United States. Summary of the NIAID-Sponsored Expert Panel Report. Bethesda, MD: National Institutes of Health; 2010. NIH Publication No. 11-7700. http://www.niaid.nih.gov/topics/foodAllergy/clinical/Documents/FAGuidelinesExecSummary.pdf. Accessed April 2, 2014.
- Kemp SF, deShazo RD, Moffitt JE, Williams DF, Buhner WA 2nd. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105(4):683-691.
- Fernández-Meléndez S, Miranda A, García-González JJ, Barber D, Lombardero M. Anaphylaxis caused by imported red fire ant stings in Málaga, Spain. J Investig Allergol Immunol. 2007;17(1):48,49.
- Swanson DL. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med. 2005;352(7):700-707.
- Saucier JR. Arachnid envenomation. Emerg Med Clin North Am. 2004;22(2):405-422.
- Wright SW, Wrenn KD, Murray L, Seger D. Clinical presentation and outcome of brown recluse spider bite. Ann Emerg Med. 1997;30(1):28-32.
- Phillips S, Kohn M, Baker D, et al. Therapy of brown spider envenomation: a controlled trial of hyperbaric oxygen, dapsone, and cyproheptadine. Ann Emerg Med. 1995;25(3):363-368.
- Pauli I, Puka J, Gubert IC, Minozzo JC. The efficacy of antivenom in loxoscelism treatment. Toxicon. 2006;48(2):123-127.
- Ushkaryov YA, Volynski KE, Ashton AC. The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon. 2004;43(5):527-542.
- Clark RF, Wethern-Kestner S, Vance MV, Gerkin R. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21(7):782-787.
- O’Malley GF, Dart RC, Kuffner EF. Successful treatment of latrodectism with antivenom after 90 hours. N Engl J Med. 1999;340(8):657.
- Clark RF. The safety and efficacy of antivenin Latrodectus mactans. J Toxicol Clin Toxicol. 2001;39(2):125-127.
- Sejvar JJ, Haddad MB, Tierney BC. Neurologic manifestations and outcome of West Nile virus infection [published correction appears in JAMA. 2003;290(10):1318]. JAMA. 2003;290(4):511-515.
- Brown M, Herbert AA. Insect repellents: an overview. J Am Acad Dermatol. 1997;36(2 Pt 1):243-249.
- Fradin MS. Mosquitoes and mosquito repellents: a clinician’s quide. Ann Intern Med. 1998;128(11):931-940.
- Miller JD. Anaphylaxis associated with insect repellent. N Engl J Med. 1982;307(21):1341,1342.
- Spach DH, Kanter AS, Dougherty MJ, et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332(7): 424-428.
- Jackson LA, Spach DH, Kippen DA, et al. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J Infect Dis. 1996;173(4):1023-1026.
- Sundnes KO. Epidemic of louse-borne relapsing fever in Ethiopia. Lancet. 1993;342(8881):1213-1215.
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7(1):6. http://escholarship.org/uc/item/59k2m8wt. Accessed April 2, 2014.
- Stucki A, Ludwig R. Images in clinical medicine. Bedbug bites. N Engl J Med. 2008; 359:10)1047.
- Kuspis DA, Rawlins JE, Krenzelok EP. Human exposures to stinging caterpillars: Lophocampa caryae exposures. Am J Emerg Med. 2001;19(5):396-398.
- Moed L, Shwayder TA, 0.Chang MW. Cantharidin revisited: a blistering defense of an ancient medicine. Arch Dermatol. 2001;137(10):1357-1360.
- White J. Bites and stings from venomous animals: a global overview. The Drug Monit. 2000;22(1):65-68.
- Oten EJ. Venomous animal injuries. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:794-807.
- Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila). 2013;51(10):949-1229. doi:10.3109/15563650.2013.863906. https://aapcc.s3.amazonaws.com/pdfs/annual_reports/2012_NPDS_Annual_Report.pdf. Accessed April 2, 2014.
- Liberman P, Camargo CA, Bohike K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596-602.
- Barnard JH. Studies of 400 Hymenoptera sting deaths in the United States. J Allergy Clin Immunol. 1973;52(5):259-264.
- Frazier CA. Insect Allergy: Allergic and Toxic Reactions to Insects and Other Arthropods. 2nd Ed. St Louis, MO: WH Green; 1987:421.
- King TP, Spangfort MD. Structure and biology of stinging insect venom allergens. Int Arch Allergy Immunol. 2000;123(2):99-106.
- Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol.2002;2(4);341-346.
- Mauriello PM, Barde SH. Natural history of large local reactions from stinging insects. J Allergy Clin Immunol. 1984;74(4 Pt 1):494-498.
- Díaz-Sánchez CL, Lifshitz-Guinzberg A, Ignacio-Ibarra G, Halabe-Cherem J, Quinones-Galvan A. Survival after massive (>2,000) Africanized honey bee stings. Arch Intern Med. 1998;158(8):925-927.
- Elston DM. Life-threatening stings, bites, infestations and parasitic diseases. Clin Dermatol. 2005;23(2):164-170.
- Lazoglu AH1, Boglioli LR, Taff ML, Rosenbluth M, Macris NT. Serum sickness reaction following multiple insect stings. Ann Allergy Asthma Immunol. 1995;75(6 Pt 1):522-524.
- Reisman RE, Livingston A. Late-onset allergic reactions, including serum sickness, after insect stings. J Allergy Clin Immunol. 1989;84(3);331-337.
- Anaphylaxis. American Academy of Allergy, Asthma & Immunology Web site. http://www.aaaai.org/conditions-and-treatments/conditions-a-to-zsearch/anaphylaxis.aspx. Accessed April 2, 2014.
- Brown H, Benton HS. Allergy to the Hymenoptera. V. Clinical study of 400 patients. Arch Intern Med. 1970;125(4):665-669.
- Frazier CA. Allergic reactions to insect stings: a review of 180 cases. South Med J. 1964;57;1023-1034.
- Mueller HL. Further experiences with severe allergic reactions to insect stings. N Engl J Med. 1959;161:374-377.
- Lockey RF, Turkeltaub PC, Baird-Warren IA, et al. The Hymenoptera venom study I, 1979-1982: demographics and history-sting data. J Allergy Clin Immunol. 1988;82(3 Pt 1):370-381.
- Schneir AB, Clark RF. Bites and stings. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011:chap120;585-596.
- Rowe BH, Gaeta T. Anaphylaxis, acute allergic reactions, and angioedema. In: Tintinalli JE, Stapczynski JS, Ma OJ, Cline DM, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw-Hill; 2011:chap 6;52-54.
- Jones RG1, Corteling RL, Bhogal G, Landon J. A novel Fab-based antivenom for the treatment of mass bee attacks. Am J Trop Med Hyg. 1999;61(3):361-366.
- National Institutes of Health, US Department of Health and Human Services, National Insitute of Allergy and Infectious Diseases. Guidelines for the Diagnosis and Management of Food Allergy in the United States. Summary of the NIAID-Sponsored Expert Panel Report. Bethesda, MD: National Institutes of Health; 2010. NIH Publication No. 11-7700. http://www.niaid.nih.gov/topics/foodAllergy/clinical/Documents/FAGuidelinesExecSummary.pdf. Accessed April 2, 2014.
- Kemp SF, deShazo RD, Moffitt JE, Williams DF, Buhner WA 2nd. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105(4):683-691.
- Fernández-Meléndez S, Miranda A, García-González JJ, Barber D, Lombardero M. Anaphylaxis caused by imported red fire ant stings in Málaga, Spain. J Investig Allergol Immunol. 2007;17(1):48,49.
- Swanson DL. Bites of brown recluse spiders and suspected necrotic arachnidism. N Engl J Med. 2005;352(7):700-707.
- Saucier JR. Arachnid envenomation. Emerg Med Clin North Am. 2004;22(2):405-422.
- Wright SW, Wrenn KD, Murray L, Seger D. Clinical presentation and outcome of brown recluse spider bite. Ann Emerg Med. 1997;30(1):28-32.
- Phillips S, Kohn M, Baker D, et al. Therapy of brown spider envenomation: a controlled trial of hyperbaric oxygen, dapsone, and cyproheptadine. Ann Emerg Med. 1995;25(3):363-368.
- Pauli I, Puka J, Gubert IC, Minozzo JC. The efficacy of antivenom in loxoscelism treatment. Toxicon. 2006;48(2):123-127.
- Ushkaryov YA, Volynski KE, Ashton AC. The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon. 2004;43(5):527-542.
- Clark RF, Wethern-Kestner S, Vance MV, Gerkin R. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21(7):782-787.
- O’Malley GF, Dart RC, Kuffner EF. Successful treatment of latrodectism with antivenom after 90 hours. N Engl J Med. 1999;340(8):657.
- Clark RF. The safety and efficacy of antivenin Latrodectus mactans. J Toxicol Clin Toxicol. 2001;39(2):125-127.
- Sejvar JJ, Haddad MB, Tierney BC. Neurologic manifestations and outcome of West Nile virus infection [published correction appears in JAMA. 2003;290(10):1318]. JAMA. 2003;290(4):511-515.
- Brown M, Herbert AA. Insect repellents: an overview. J Am Acad Dermatol. 1997;36(2 Pt 1):243-249.
- Fradin MS. Mosquitoes and mosquito repellents: a clinician’s quide. Ann Intern Med. 1998;128(11):931-940.
- Miller JD. Anaphylaxis associated with insect repellent. N Engl J Med. 1982;307(21):1341,1342.
- Spach DH, Kanter AS, Dougherty MJ, et al. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332(7): 424-428.
- Jackson LA, Spach DH, Kippen DA, et al. Seroprevalence to Bartonella quintana among patients at a community clinic in downtown Seattle. J Infect Dis. 1996;173(4):1023-1026.
- Sundnes KO. Epidemic of louse-borne relapsing fever in Ethiopia. Lancet. 1993;342(8881):1213-1215.
- Vetter R. Kissing bugs (Triatoma) and the skin. Dermatol Online J. 2001;7(1):6. http://escholarship.org/uc/item/59k2m8wt. Accessed April 2, 2014.
- Stucki A, Ludwig R. Images in clinical medicine. Bedbug bites. N Engl J Med. 2008; 359:10)1047.
- Kuspis DA, Rawlins JE, Krenzelok EP. Human exposures to stinging caterpillars: Lophocampa caryae exposures. Am J Emerg Med. 2001;19(5):396-398.
- Moed L, Shwayder TA, 0.Chang MW. Cantharidin revisited: a blistering defense of an ancient medicine. Arch Dermatol. 2001;137(10):1357-1360.