User login
Urgent recall for Penumbra JET 7 Xtra Flex reperfusion catheters
“All users should stop using this device, and facilities should remove these devices from inventory,” the recall notice, posted on the U.S. Food and Drug Administration website, advises.
The recall covers the JET 7 Xtra Flex catheter, which was cleared for use in June 2019, and the JET 7MAX configuration (which includes the JET 7 Xtra Flex catheter and MAX delivery device), which was cleared in February of this year.
The recall does not apply to the Penumbra JET 7 reperfusion catheter with standard tip.
The FDA says it has received over 200 medical device reports (MDRs) associated with the JET 7 Xtra Flex catheter, including reports of deaths, serious injuries, and malfunctions.
Twenty of these MDRs describe 14 unique patient deaths. Other MDRs describe serious patient injury, such as vessel damage, hemorrhage, and cerebral infarction.
Device malfunctions described in the reports include ballooning, expansion, rupture, breakage or complete separation, and exposure of internal support coils near the distal tip region of the JET 7 Xtra Flex catheter.
According to the FDA, bench testing by the manufacturer, in which the catheter distal tip is plugged and pressurized to failure, indicates that the JET 7 Xtra Flex catheter is not able to withstand the same burst pressures to failure as the manufacturer’s other large-bore aspiration catheters used to remove thrombus for patients with acute ischemic stroke.
Penumbra’s urgent medical device recall letter advises health care providers and facilities to remove and quarantine all unused devices covered by this recall, to complete the product identification and return form, and to return all products to Penumbra in accordance with instructions provided.
For questions regarding this recall, contact Penumbra customer service by phone at 888-272-4606 or by email at [email protected].
A version of this article first appeared on Medscape.com.
“All users should stop using this device, and facilities should remove these devices from inventory,” the recall notice, posted on the U.S. Food and Drug Administration website, advises.
The recall covers the JET 7 Xtra Flex catheter, which was cleared for use in June 2019, and the JET 7MAX configuration (which includes the JET 7 Xtra Flex catheter and MAX delivery device), which was cleared in February of this year.
The recall does not apply to the Penumbra JET 7 reperfusion catheter with standard tip.
The FDA says it has received over 200 medical device reports (MDRs) associated with the JET 7 Xtra Flex catheter, including reports of deaths, serious injuries, and malfunctions.
Twenty of these MDRs describe 14 unique patient deaths. Other MDRs describe serious patient injury, such as vessel damage, hemorrhage, and cerebral infarction.
Device malfunctions described in the reports include ballooning, expansion, rupture, breakage or complete separation, and exposure of internal support coils near the distal tip region of the JET 7 Xtra Flex catheter.
According to the FDA, bench testing by the manufacturer, in which the catheter distal tip is plugged and pressurized to failure, indicates that the JET 7 Xtra Flex catheter is not able to withstand the same burst pressures to failure as the manufacturer’s other large-bore aspiration catheters used to remove thrombus for patients with acute ischemic stroke.
Penumbra’s urgent medical device recall letter advises health care providers and facilities to remove and quarantine all unused devices covered by this recall, to complete the product identification and return form, and to return all products to Penumbra in accordance with instructions provided.
For questions regarding this recall, contact Penumbra customer service by phone at 888-272-4606 or by email at [email protected].
A version of this article first appeared on Medscape.com.
“All users should stop using this device, and facilities should remove these devices from inventory,” the recall notice, posted on the U.S. Food and Drug Administration website, advises.
The recall covers the JET 7 Xtra Flex catheter, which was cleared for use in June 2019, and the JET 7MAX configuration (which includes the JET 7 Xtra Flex catheter and MAX delivery device), which was cleared in February of this year.
The recall does not apply to the Penumbra JET 7 reperfusion catheter with standard tip.
The FDA says it has received over 200 medical device reports (MDRs) associated with the JET 7 Xtra Flex catheter, including reports of deaths, serious injuries, and malfunctions.
Twenty of these MDRs describe 14 unique patient deaths. Other MDRs describe serious patient injury, such as vessel damage, hemorrhage, and cerebral infarction.
Device malfunctions described in the reports include ballooning, expansion, rupture, breakage or complete separation, and exposure of internal support coils near the distal tip region of the JET 7 Xtra Flex catheter.
According to the FDA, bench testing by the manufacturer, in which the catheter distal tip is plugged and pressurized to failure, indicates that the JET 7 Xtra Flex catheter is not able to withstand the same burst pressures to failure as the manufacturer’s other large-bore aspiration catheters used to remove thrombus for patients with acute ischemic stroke.
Penumbra’s urgent medical device recall letter advises health care providers and facilities to remove and quarantine all unused devices covered by this recall, to complete the product identification and return form, and to return all products to Penumbra in accordance with instructions provided.
For questions regarding this recall, contact Penumbra customer service by phone at 888-272-4606 or by email at [email protected].
A version of this article first appeared on Medscape.com.
Disabling stroke reduced with ticagrelor after minor stroke, TIA
Additional results from the THALES trial have shown that 1 month’s dual antiplatelet therapy with ticagrelor (Brilinta; Astra Zeneca) plus aspirin is associated with a reduction in disabling stroke, compared with aspirin alone in patients with minor stroke or high-risk transient ischemic attack (TIA).
Primary results of the THALES trial, published earlier this year in the New England Journal of Medicine, showed a reduction in the primary endpoint of stroke or death within 30 days with the combination of ticagrelor plus aspirin versus aspirin alone, although this was accompanied by an increase in bleeding. In terms of risk/benefit, the main results showed that for every 1,000 patients treatment with ticagrelor on top of aspirin would prevent 11 strokes or deaths at the cost of four severe hemorrhages.
The current exploratory analysis, which focuses on the severity of the strokes occurring in the trial, was published online Nov. 7 in JAMA Neurology to coincide with its presentation at the European Stroke Organisation-World Stroke Organization Conference 2020.
Results showed that, compared with aspirin alone, ticagrelor plus aspirin significantly reduced the 30-day risk for disabling stroke or death (4.0% versus 4.7%), and the total disability burden (the shift analysis of the distribution of modified Rankin scale) following subsequent ischemic stroke was reduced by a significant 23%.
“This new information on disabling stroke underlines the importance of getting patients on dual antiplatelet therapy quickly after a TIA or mild stroke,” said principal investigator of the THALES trial, S. Claiborne Johnston, MD, PhD.
Dr. Johnston, who is dean of Dell Medical School at the University of Texas at Austin, added: “It’s reassuring that ticagrelor has this effect, which was pretty robust. An accompanying editorial to the THALES publication in the NEJM incorrectly stated that ticagrelor did not reduce risk of disabling stroke, so it is good to be able to correct that misconception with this new data.”
Lead author of the exploratory analysis, Pierre Amarenco, MD, professor of neurology at Bichat University Hospital, Paris, added: “The main results showed that ticagrelor on top of aspirin reduced stroke but now we have new information showing reduction in disabling stroke. Obviously, these are the most important types of stroke to prevent. These are the strokes that will impact patients functionally.”
The THALES trial included 11,016 patients with a noncardioembolic, nonsevere ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 5) or high-risk TIA, of whom 10,803 had modified Rankin Scale (mRS) functional score recorded at 30 days.
They were randomized within 24 hours of symptom onset to ticagrelor (180-mg loading dose on day 1 followed by 90 mg twice daily for 1 month) or placebo. All patients received aspirin (300-325 mg on day 1 followed by 75-100 mg daily for 1 month).
In the new analysis, time to occurrence of disabling stroke (mRS greater than 1) or death within 30 days occurred in 221 of 5,511 patients (4.0%) randomized to ticagrelor and in 260 of 5,478 patients (4.7%) randomized to placebo (hazard ratio, 0.83; P = .04).
The ordinal analysis of mRS in patients with recurrent stroke showed a shift of the disability burden following a recurrent ischemic stroke in favor of ticagrelor (odds ratio, 0.77; P = .002).
Factors associated with disability were baseline NIHSS score of 4-5, ipsilateral stenosis of at least 30%, Asian race/ethnicity, older age, and higher systolic blood pressure.
Asked how the current results compared with observations reported in the main NEJM paper of similar incidences of disability (mRS > 1) in the two groups, Dr. Johnston explained that the result in the original paper looked at disability in the overall population, not just those who went on to have a stroke during follow-up.
“The problem with looking at overall disability is that most of it is actually from the index stroke (the one that led to the patient being enrolled in the trial). That creates a lot of noise that overwhelms the benefit in reducing disability due to new stroke, the thing we really care about and the subject of the new paper,” he commented.
Ticagrelor or clopidogrel?
Ticagrelor now becomes the second antiplatelet agent to have shown benefits on top of aspirin in the minor stroke and high-risk TIA population. Clopidogrel also showed a reduction in major ischemic events in the POINT trial as well as in the Chinese CHANCE trial in similar populations.
Dr. Amarenco pointed out, however, that until now the only treatment that has been shown to reduce disabling stroke in the minor stroke/high risk TIA population in a single trial is aspirin. “The CHANCE and POINT trials of clopidogrel did not show a reduction in disabling stroke individually but this was observed when the trials were combined,” he noted.
“Clinicians will now have to choose between ticagrelor and clopidogrel. We don’t have a head-to-head comparison yet but ticagrelor is effective in all patients whereas clopidogrel may not be as effective in the large subgroup of patients who carry the loss of function gene which make up about 20% of the western population and about 40% of the Asian population,” he said.
“It is very important in the acute phase of stroke to know that the antiplatelet drug is immediately effective as the risk of a recurrent event is highest in the first few hours and days.”
Dr. Amarenco acknowledged that some hospitals may favor clopidogrel because of cost, as it is available generically so is much cheaper than ticagrelor. “But we are only talking about 30 days of treatment, so cost is not too much of an issue,” he pointed out.
The Food and Drug Administration recently approved use of ticagrelor in this indication on the basis of the THALES study.
“It is great news that vascular neurologists now have a new player for reducing future stroke in these patients,” Dr. Amarenco said. Clopidogrel is not approved for this indication but is recommended in American Heart Association/American Stroke Association guidelines, he added.
Dr. Johnston, who was also the lead investigator of the POINT trial with clopidogrel, suggested that it is more important to get patients on dual-antiplatelet therapy rather than worrying too much about which agent to use. “I think we can use aspirin plus either ticagrelor or clopidogrel. The effect on disabling stroke was not significant in POINT but it did reach significance in a meta-analysis combining POINT and CHANCE,” he noted.
He said that choosing between ticagrelor and clopidogrel is tricky without head-to-head data. “Differences in the studied populations makes direct comparison of the trials unwise,” he stressed.
Dr. Johnston pointed out that neither of the clopidogrel trials included moderate strokes (NIHSS scores of 4 and 5) in their study population. “We only have data on ticagrelor for this important group, which accounted for 30% of the THALES study population,” he noted.
“Some people are concerned about the limited efficacy of clopidogrel in large subgroups of patients who do not metabolize it to its active form, but on the flip side, clopidogrel is cheaper – though a 21- to 30-day course [of ticagrelor] probably isn’t that costly – and has more data in combination with aspirin,” he added.
Dr. Johnston said that the approval of ticagrelor for this new indication was “reassuring,” and “provides some air cover for practitioners given the risks of hemorrhage.” He added: “We didn’t bother with an FDA submission after POINT because it was an NIH-sponsored trial. The drug company normally prioritizes regulatory approvals for marketing purposes but their interests were limited because clopidogrel has exceeded its patent life.”
Cost-utility analyses are not yet available, but Dr. Johnston noted: “I suspect both drugs will have substantial benefits and be cost saving. Stroke is expensive, particularly disabling stroke.”
Dr. Johnston said that the more important message is: “Get these people on dual-antiplatelet therapy as soon as possible. Too many patients are not getting the right treatment immediately after symptom onset. We have lots of work to do here.”
Reassuring information
Commenting on the research, J. David Spence, MD, professor of neurology at the Robarts Research Institute, London, Ont., who was not involved in the THALES trial, said this new analysis provided useful and important information that should reassure and encourage clinicians to use dual-antiplatelet therapy in this patient population.
He pointed out that the shift analysis gives the most clinically relevant results. “While the number of patients with a disabling stroke defined as an mRS greater than 1 is lower in the ticagrelor group, I am much more interested in the effect on more severe disability levels – those with an mRS score of 3 or more. Those are the disabilities that we really want to prevent. And from examining the shift analysis distribution, we can see that these more severe disabilities are being reduced with ticagrelor.”
Dr. Spence believes the benefit/risk ratio of dual-antiplatelet therapy could be further improved by better control of blood pressure. “The absolute risk of severe hemorrhage was low in this study, but in my view, most of this could have been prevented by better control of hypertension, as 20 of the 28 severe hemorrhages in the ticagrelor group were intracranial bleeds which can be significantly reduced by good blood pressure control.
“In my view, the increased risk of hemorrhage with dual-antiplatelet therapy should not be regarded as inevitable; it can be virtually eliminated with better medical care,” he stated.
Another outside commentator, Peter Rothwell, MD, PhD, professor of neurology, University of Oxford (England), also believes this is an important paper. “The main NEJM report presented the data on overall disability, but did not present a clear analysis of the effect of ticagrelor plus aspirin on disabling recurrent stroke, but disability in all patients is mainly determined by nonvascular premorbid disability and by the effects of the initial prerandomization stroke. It was highly unlikely that ticagrelor plus aspirin would change these pretrial factors. The only thing that treatment could change was the severity of any posttreatment recurrent stroke, which it did,” he said.
“There is evidence that aspirin plus clopidogrel has the same effect on disabling recurrent stroke. So we now know that ticagrelor plus aspirin also has this effect, which informs consideration of the relative merits of the two treatment strategies,” Dr. Rothwell added.
The THALES trial was sponsored by Astra Zeneca. Dr. Johnston reports support from Sanofi and AstraZeneca outside the submitted work. Dr. Amarenco reports grants and personal fees from AstraZeneca and Bristol-Myers Squibb during the conduct of the study.
A version of this article originally appeared on Medscape.com.
Additional results from the THALES trial have shown that 1 month’s dual antiplatelet therapy with ticagrelor (Brilinta; Astra Zeneca) plus aspirin is associated with a reduction in disabling stroke, compared with aspirin alone in patients with minor stroke or high-risk transient ischemic attack (TIA).
Primary results of the THALES trial, published earlier this year in the New England Journal of Medicine, showed a reduction in the primary endpoint of stroke or death within 30 days with the combination of ticagrelor plus aspirin versus aspirin alone, although this was accompanied by an increase in bleeding. In terms of risk/benefit, the main results showed that for every 1,000 patients treatment with ticagrelor on top of aspirin would prevent 11 strokes or deaths at the cost of four severe hemorrhages.
The current exploratory analysis, which focuses on the severity of the strokes occurring in the trial, was published online Nov. 7 in JAMA Neurology to coincide with its presentation at the European Stroke Organisation-World Stroke Organization Conference 2020.
Results showed that, compared with aspirin alone, ticagrelor plus aspirin significantly reduced the 30-day risk for disabling stroke or death (4.0% versus 4.7%), and the total disability burden (the shift analysis of the distribution of modified Rankin scale) following subsequent ischemic stroke was reduced by a significant 23%.
“This new information on disabling stroke underlines the importance of getting patients on dual antiplatelet therapy quickly after a TIA or mild stroke,” said principal investigator of the THALES trial, S. Claiborne Johnston, MD, PhD.
Dr. Johnston, who is dean of Dell Medical School at the University of Texas at Austin, added: “It’s reassuring that ticagrelor has this effect, which was pretty robust. An accompanying editorial to the THALES publication in the NEJM incorrectly stated that ticagrelor did not reduce risk of disabling stroke, so it is good to be able to correct that misconception with this new data.”
Lead author of the exploratory analysis, Pierre Amarenco, MD, professor of neurology at Bichat University Hospital, Paris, added: “The main results showed that ticagrelor on top of aspirin reduced stroke but now we have new information showing reduction in disabling stroke. Obviously, these are the most important types of stroke to prevent. These are the strokes that will impact patients functionally.”
The THALES trial included 11,016 patients with a noncardioembolic, nonsevere ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 5) or high-risk TIA, of whom 10,803 had modified Rankin Scale (mRS) functional score recorded at 30 days.
They were randomized within 24 hours of symptom onset to ticagrelor (180-mg loading dose on day 1 followed by 90 mg twice daily for 1 month) or placebo. All patients received aspirin (300-325 mg on day 1 followed by 75-100 mg daily for 1 month).
In the new analysis, time to occurrence of disabling stroke (mRS greater than 1) or death within 30 days occurred in 221 of 5,511 patients (4.0%) randomized to ticagrelor and in 260 of 5,478 patients (4.7%) randomized to placebo (hazard ratio, 0.83; P = .04).
The ordinal analysis of mRS in patients with recurrent stroke showed a shift of the disability burden following a recurrent ischemic stroke in favor of ticagrelor (odds ratio, 0.77; P = .002).
Factors associated with disability were baseline NIHSS score of 4-5, ipsilateral stenosis of at least 30%, Asian race/ethnicity, older age, and higher systolic blood pressure.
Asked how the current results compared with observations reported in the main NEJM paper of similar incidences of disability (mRS > 1) in the two groups, Dr. Johnston explained that the result in the original paper looked at disability in the overall population, not just those who went on to have a stroke during follow-up.
“The problem with looking at overall disability is that most of it is actually from the index stroke (the one that led to the patient being enrolled in the trial). That creates a lot of noise that overwhelms the benefit in reducing disability due to new stroke, the thing we really care about and the subject of the new paper,” he commented.
Ticagrelor or clopidogrel?
Ticagrelor now becomes the second antiplatelet agent to have shown benefits on top of aspirin in the minor stroke and high-risk TIA population. Clopidogrel also showed a reduction in major ischemic events in the POINT trial as well as in the Chinese CHANCE trial in similar populations.
Dr. Amarenco pointed out, however, that until now the only treatment that has been shown to reduce disabling stroke in the minor stroke/high risk TIA population in a single trial is aspirin. “The CHANCE and POINT trials of clopidogrel did not show a reduction in disabling stroke individually but this was observed when the trials were combined,” he noted.
“Clinicians will now have to choose between ticagrelor and clopidogrel. We don’t have a head-to-head comparison yet but ticagrelor is effective in all patients whereas clopidogrel may not be as effective in the large subgroup of patients who carry the loss of function gene which make up about 20% of the western population and about 40% of the Asian population,” he said.
“It is very important in the acute phase of stroke to know that the antiplatelet drug is immediately effective as the risk of a recurrent event is highest in the first few hours and days.”
Dr. Amarenco acknowledged that some hospitals may favor clopidogrel because of cost, as it is available generically so is much cheaper than ticagrelor. “But we are only talking about 30 days of treatment, so cost is not too much of an issue,” he pointed out.
The Food and Drug Administration recently approved use of ticagrelor in this indication on the basis of the THALES study.
“It is great news that vascular neurologists now have a new player for reducing future stroke in these patients,” Dr. Amarenco said. Clopidogrel is not approved for this indication but is recommended in American Heart Association/American Stroke Association guidelines, he added.
Dr. Johnston, who was also the lead investigator of the POINT trial with clopidogrel, suggested that it is more important to get patients on dual-antiplatelet therapy rather than worrying too much about which agent to use. “I think we can use aspirin plus either ticagrelor or clopidogrel. The effect on disabling stroke was not significant in POINT but it did reach significance in a meta-analysis combining POINT and CHANCE,” he noted.
He said that choosing between ticagrelor and clopidogrel is tricky without head-to-head data. “Differences in the studied populations makes direct comparison of the trials unwise,” he stressed.
Dr. Johnston pointed out that neither of the clopidogrel trials included moderate strokes (NIHSS scores of 4 and 5) in their study population. “We only have data on ticagrelor for this important group, which accounted for 30% of the THALES study population,” he noted.
“Some people are concerned about the limited efficacy of clopidogrel in large subgroups of patients who do not metabolize it to its active form, but on the flip side, clopidogrel is cheaper – though a 21- to 30-day course [of ticagrelor] probably isn’t that costly – and has more data in combination with aspirin,” he added.
Dr. Johnston said that the approval of ticagrelor for this new indication was “reassuring,” and “provides some air cover for practitioners given the risks of hemorrhage.” He added: “We didn’t bother with an FDA submission after POINT because it was an NIH-sponsored trial. The drug company normally prioritizes regulatory approvals for marketing purposes but their interests were limited because clopidogrel has exceeded its patent life.”
Cost-utility analyses are not yet available, but Dr. Johnston noted: “I suspect both drugs will have substantial benefits and be cost saving. Stroke is expensive, particularly disabling stroke.”
Dr. Johnston said that the more important message is: “Get these people on dual-antiplatelet therapy as soon as possible. Too many patients are not getting the right treatment immediately after symptom onset. We have lots of work to do here.”
Reassuring information
Commenting on the research, J. David Spence, MD, professor of neurology at the Robarts Research Institute, London, Ont., who was not involved in the THALES trial, said this new analysis provided useful and important information that should reassure and encourage clinicians to use dual-antiplatelet therapy in this patient population.
He pointed out that the shift analysis gives the most clinically relevant results. “While the number of patients with a disabling stroke defined as an mRS greater than 1 is lower in the ticagrelor group, I am much more interested in the effect on more severe disability levels – those with an mRS score of 3 or more. Those are the disabilities that we really want to prevent. And from examining the shift analysis distribution, we can see that these more severe disabilities are being reduced with ticagrelor.”
Dr. Spence believes the benefit/risk ratio of dual-antiplatelet therapy could be further improved by better control of blood pressure. “The absolute risk of severe hemorrhage was low in this study, but in my view, most of this could have been prevented by better control of hypertension, as 20 of the 28 severe hemorrhages in the ticagrelor group were intracranial bleeds which can be significantly reduced by good blood pressure control.
“In my view, the increased risk of hemorrhage with dual-antiplatelet therapy should not be regarded as inevitable; it can be virtually eliminated with better medical care,” he stated.
Another outside commentator, Peter Rothwell, MD, PhD, professor of neurology, University of Oxford (England), also believes this is an important paper. “The main NEJM report presented the data on overall disability, but did not present a clear analysis of the effect of ticagrelor plus aspirin on disabling recurrent stroke, but disability in all patients is mainly determined by nonvascular premorbid disability and by the effects of the initial prerandomization stroke. It was highly unlikely that ticagrelor plus aspirin would change these pretrial factors. The only thing that treatment could change was the severity of any posttreatment recurrent stroke, which it did,” he said.
“There is evidence that aspirin plus clopidogrel has the same effect on disabling recurrent stroke. So we now know that ticagrelor plus aspirin also has this effect, which informs consideration of the relative merits of the two treatment strategies,” Dr. Rothwell added.
The THALES trial was sponsored by Astra Zeneca. Dr. Johnston reports support from Sanofi and AstraZeneca outside the submitted work. Dr. Amarenco reports grants and personal fees from AstraZeneca and Bristol-Myers Squibb during the conduct of the study.
A version of this article originally appeared on Medscape.com.
Additional results from the THALES trial have shown that 1 month’s dual antiplatelet therapy with ticagrelor (Brilinta; Astra Zeneca) plus aspirin is associated with a reduction in disabling stroke, compared with aspirin alone in patients with minor stroke or high-risk transient ischemic attack (TIA).
Primary results of the THALES trial, published earlier this year in the New England Journal of Medicine, showed a reduction in the primary endpoint of stroke or death within 30 days with the combination of ticagrelor plus aspirin versus aspirin alone, although this was accompanied by an increase in bleeding. In terms of risk/benefit, the main results showed that for every 1,000 patients treatment with ticagrelor on top of aspirin would prevent 11 strokes or deaths at the cost of four severe hemorrhages.
The current exploratory analysis, which focuses on the severity of the strokes occurring in the trial, was published online Nov. 7 in JAMA Neurology to coincide with its presentation at the European Stroke Organisation-World Stroke Organization Conference 2020.
Results showed that, compared with aspirin alone, ticagrelor plus aspirin significantly reduced the 30-day risk for disabling stroke or death (4.0% versus 4.7%), and the total disability burden (the shift analysis of the distribution of modified Rankin scale) following subsequent ischemic stroke was reduced by a significant 23%.
“This new information on disabling stroke underlines the importance of getting patients on dual antiplatelet therapy quickly after a TIA or mild stroke,” said principal investigator of the THALES trial, S. Claiborne Johnston, MD, PhD.
Dr. Johnston, who is dean of Dell Medical School at the University of Texas at Austin, added: “It’s reassuring that ticagrelor has this effect, which was pretty robust. An accompanying editorial to the THALES publication in the NEJM incorrectly stated that ticagrelor did not reduce risk of disabling stroke, so it is good to be able to correct that misconception with this new data.”
Lead author of the exploratory analysis, Pierre Amarenco, MD, professor of neurology at Bichat University Hospital, Paris, added: “The main results showed that ticagrelor on top of aspirin reduced stroke but now we have new information showing reduction in disabling stroke. Obviously, these are the most important types of stroke to prevent. These are the strokes that will impact patients functionally.”
The THALES trial included 11,016 patients with a noncardioembolic, nonsevere ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≤ 5) or high-risk TIA, of whom 10,803 had modified Rankin Scale (mRS) functional score recorded at 30 days.
They were randomized within 24 hours of symptom onset to ticagrelor (180-mg loading dose on day 1 followed by 90 mg twice daily for 1 month) or placebo. All patients received aspirin (300-325 mg on day 1 followed by 75-100 mg daily for 1 month).
In the new analysis, time to occurrence of disabling stroke (mRS greater than 1) or death within 30 days occurred in 221 of 5,511 patients (4.0%) randomized to ticagrelor and in 260 of 5,478 patients (4.7%) randomized to placebo (hazard ratio, 0.83; P = .04).
The ordinal analysis of mRS in patients with recurrent stroke showed a shift of the disability burden following a recurrent ischemic stroke in favor of ticagrelor (odds ratio, 0.77; P = .002).
Factors associated with disability were baseline NIHSS score of 4-5, ipsilateral stenosis of at least 30%, Asian race/ethnicity, older age, and higher systolic blood pressure.
Asked how the current results compared with observations reported in the main NEJM paper of similar incidences of disability (mRS > 1) in the two groups, Dr. Johnston explained that the result in the original paper looked at disability in the overall population, not just those who went on to have a stroke during follow-up.
“The problem with looking at overall disability is that most of it is actually from the index stroke (the one that led to the patient being enrolled in the trial). That creates a lot of noise that overwhelms the benefit in reducing disability due to new stroke, the thing we really care about and the subject of the new paper,” he commented.
Ticagrelor or clopidogrel?
Ticagrelor now becomes the second antiplatelet agent to have shown benefits on top of aspirin in the minor stroke and high-risk TIA population. Clopidogrel also showed a reduction in major ischemic events in the POINT trial as well as in the Chinese CHANCE trial in similar populations.
Dr. Amarenco pointed out, however, that until now the only treatment that has been shown to reduce disabling stroke in the minor stroke/high risk TIA population in a single trial is aspirin. “The CHANCE and POINT trials of clopidogrel did not show a reduction in disabling stroke individually but this was observed when the trials were combined,” he noted.
“Clinicians will now have to choose between ticagrelor and clopidogrel. We don’t have a head-to-head comparison yet but ticagrelor is effective in all patients whereas clopidogrel may not be as effective in the large subgroup of patients who carry the loss of function gene which make up about 20% of the western population and about 40% of the Asian population,” he said.
“It is very important in the acute phase of stroke to know that the antiplatelet drug is immediately effective as the risk of a recurrent event is highest in the first few hours and days.”
Dr. Amarenco acknowledged that some hospitals may favor clopidogrel because of cost, as it is available generically so is much cheaper than ticagrelor. “But we are only talking about 30 days of treatment, so cost is not too much of an issue,” he pointed out.
The Food and Drug Administration recently approved use of ticagrelor in this indication on the basis of the THALES study.
“It is great news that vascular neurologists now have a new player for reducing future stroke in these patients,” Dr. Amarenco said. Clopidogrel is not approved for this indication but is recommended in American Heart Association/American Stroke Association guidelines, he added.
Dr. Johnston, who was also the lead investigator of the POINT trial with clopidogrel, suggested that it is more important to get patients on dual-antiplatelet therapy rather than worrying too much about which agent to use. “I think we can use aspirin plus either ticagrelor or clopidogrel. The effect on disabling stroke was not significant in POINT but it did reach significance in a meta-analysis combining POINT and CHANCE,” he noted.
He said that choosing between ticagrelor and clopidogrel is tricky without head-to-head data. “Differences in the studied populations makes direct comparison of the trials unwise,” he stressed.
Dr. Johnston pointed out that neither of the clopidogrel trials included moderate strokes (NIHSS scores of 4 and 5) in their study population. “We only have data on ticagrelor for this important group, which accounted for 30% of the THALES study population,” he noted.
“Some people are concerned about the limited efficacy of clopidogrel in large subgroups of patients who do not metabolize it to its active form, but on the flip side, clopidogrel is cheaper – though a 21- to 30-day course [of ticagrelor] probably isn’t that costly – and has more data in combination with aspirin,” he added.
Dr. Johnston said that the approval of ticagrelor for this new indication was “reassuring,” and “provides some air cover for practitioners given the risks of hemorrhage.” He added: “We didn’t bother with an FDA submission after POINT because it was an NIH-sponsored trial. The drug company normally prioritizes regulatory approvals for marketing purposes but their interests were limited because clopidogrel has exceeded its patent life.”
Cost-utility analyses are not yet available, but Dr. Johnston noted: “I suspect both drugs will have substantial benefits and be cost saving. Stroke is expensive, particularly disabling stroke.”
Dr. Johnston said that the more important message is: “Get these people on dual-antiplatelet therapy as soon as possible. Too many patients are not getting the right treatment immediately after symptom onset. We have lots of work to do here.”
Reassuring information
Commenting on the research, J. David Spence, MD, professor of neurology at the Robarts Research Institute, London, Ont., who was not involved in the THALES trial, said this new analysis provided useful and important information that should reassure and encourage clinicians to use dual-antiplatelet therapy in this patient population.
He pointed out that the shift analysis gives the most clinically relevant results. “While the number of patients with a disabling stroke defined as an mRS greater than 1 is lower in the ticagrelor group, I am much more interested in the effect on more severe disability levels – those with an mRS score of 3 or more. Those are the disabilities that we really want to prevent. And from examining the shift analysis distribution, we can see that these more severe disabilities are being reduced with ticagrelor.”
Dr. Spence believes the benefit/risk ratio of dual-antiplatelet therapy could be further improved by better control of blood pressure. “The absolute risk of severe hemorrhage was low in this study, but in my view, most of this could have been prevented by better control of hypertension, as 20 of the 28 severe hemorrhages in the ticagrelor group were intracranial bleeds which can be significantly reduced by good blood pressure control.
“In my view, the increased risk of hemorrhage with dual-antiplatelet therapy should not be regarded as inevitable; it can be virtually eliminated with better medical care,” he stated.
Another outside commentator, Peter Rothwell, MD, PhD, professor of neurology, University of Oxford (England), also believes this is an important paper. “The main NEJM report presented the data on overall disability, but did not present a clear analysis of the effect of ticagrelor plus aspirin on disabling recurrent stroke, but disability in all patients is mainly determined by nonvascular premorbid disability and by the effects of the initial prerandomization stroke. It was highly unlikely that ticagrelor plus aspirin would change these pretrial factors. The only thing that treatment could change was the severity of any posttreatment recurrent stroke, which it did,” he said.
“There is evidence that aspirin plus clopidogrel has the same effect on disabling recurrent stroke. So we now know that ticagrelor plus aspirin also has this effect, which informs consideration of the relative merits of the two treatment strategies,” Dr. Rothwell added.
The THALES trial was sponsored by Astra Zeneca. Dr. Johnston reports support from Sanofi and AstraZeneca outside the submitted work. Dr. Amarenco reports grants and personal fees from AstraZeneca and Bristol-Myers Squibb during the conduct of the study.
A version of this article originally appeared on Medscape.com.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.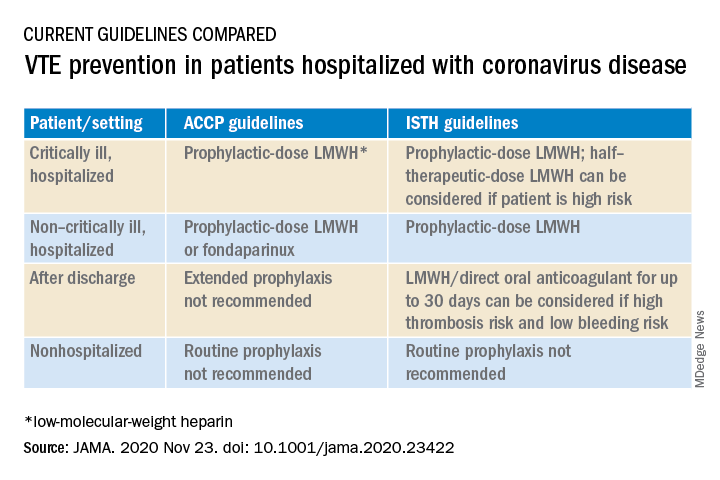
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
Blood pressure treatment reduces bleeding in ICH
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ESO-WSO CONFERENCE 2020
Statins beneficial in elderly, guidelines should be strengthened
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Marijuana use tied to repeat MI, stroke after percutaneous coronary intervention
in separate studies.
Rhushik Bhuva, MD, presented the recurrent-MI results from a national U.S. study, and Sang Gune K. Yoo, MD, presented the PCI study, which used data from a Michigan cohort. The studies were presented at the American Heart Association scientific sessions.
Both studies “add to our accumulating knowledge of the cardiovascular risks of marijuana,” Ersilia M. DeFilippis, MD, a cardiology fellow at Columbia University Irvine Medical Center, New York, who was not involved with this research, said in an interview.
Dr. DeFilippis and the two study authors say clinicians and patients need to be more aware of cardiovascular risks from smoking marijuana, and they call for more patient screening, counseling, and research.
Need for screening and counseling
Marijuana is a Schedule 1 controlled substance in the United States, which makes it illegal to conduct rigorous controlled trials of marijuana products. Existing knowledge is therefore based on observational studies, Dr. DeFilippis noted.
She was lead author of a review of marijuana use by patients with cardiovascular disease. The review was published in the Journal of the American College of Cardiology. An AHA scientific statement about marijuana and cardiovascular health was published in Circulation.
Both documents drew attention to risks from marijuana use in patients with cardiovascular disease.
Until more data are available, “I think it is absolutely critical” that cardiologists and general providers screen patients for marijuana use, “either at the time of their MI or ideally prior to that, when they are making a cardiovascular risk assessment,” said Dr. DeFilippis.
That is also the time to “counsel patients, especially those who have had an MI, about risks associated with continuing to use marijuana.”
Importantly, providers and patients need to be aware that “cannabinoids, through the cytochrome P450 system, can interact with well-known cardiovascular medications, which we know provide benefit in the post-MI period,” she added. “For example, marijuana can interfere with beta-blockers, statins, antiarrhythmics, and certain anticoagulants.”
Dr. Bhuva, a cardiology fellow with the Wright Center for Community Health, Scranton, Pa., said that it is “concerning” that “recurrent heart attacks and cardiac interventions [were] higher among cannabis users, even though they were younger and had fewer risk factors for heart disease.
“Spreading awareness regarding the potential risk of recurrent heart attacks in middle-aged, African American, and male cannabis users and screening them at an earlier age for potential risk factors of future heart attacks should be encouraged among clinicians,” he urged in a statement from the AHA.
Dr. Yoo, an internal medicine resident at the University of Michigan, Ann Arbor, pointed out that, in their study of patients who underwent PCI after MI or because they had coronary artery disease, those who smoked or vaped marijuana were younger and were more likely to be male. They were less likely to have traditional cardiovascular risk factors except for smoking tobacco, which was highly prevalent.
After propensity matching, patients who used marijuana had a 1.5-fold increased risk of in-hospital bleeding and an 11-fold higher risk for in-hospital stroke following PCI.
However, the absolute number of strokes in PCI was small, and the confidence interval was wide (indicating a large uncertainty), Dr. Yoo said in an interview.
These risks “should not deter patients from undergoing these [lifesaving] procedures,” he said; however, clinicians should be aware of these risks with marijuana use and should screen and counsel patients about this.
Hospitalized patients with prior MI
Dr. Bhuva and colleagues identified patients from the National Inpatient Sample who were hospitalized in the United States from 2007 to 2014 and who had experienced a prior MI and had undergone revascularization with PCI or coronary artery bypass grafting (CABG).
There were about 8 million hospital stays per year. The database did not specify the type of marijuana that patients used.
During the 8-year study period, many states legalized or decriminalized medical and/or recreational marijuana, and marijuana use increased steadily, from 0.2% to 0.7%.
Compared with nonusers, those who used marijuana were younger (median age, 53 vs. 72 years), and there were more men (77% vs. 62%) or Black persons (34% vs. 10%) (all P < .001). Fewer marijuana users had hypertension (72% vs. 75%), diabetes (24% vs. 33%), or dyslipidemia (51% vs. 58%) (all P < .001). More marijuana users underwent a repeat MI (67% vs. 41%).
On the other hand, marijuana users, who were younger and healthier than the other patients, were less likely to die during hospitalization for a recurrent MI (0.8% vs. 2.5%), and their hospital costs were lower.
The researchers acknowledged that study limitations include lack of information about marijuana type (smoked, edible, medicinal, or recreational) or dose, as well as the time from marijuana use to cardiac event.
In-Hospital outcomes after PCI
Dr. Yoo and colleagues analyzed data from patients who underwent PCI from Jan. 1, 2013, to Oct. 1, 2016, at Michigan’s 48 nonfederal hospitals, which are part of the Blue Cross Blue Shield Michigan Cardiovascular Consortium PCI registry.
In this cohort, 3,970 patients (3.5%) had smoked or vaped marijuana in the month prior to PCI, and 109,507 patients had not done so. The marijuana users were younger (mean age, 54 vs. 66 years) and were more likely to be male (79% vs. 67%) and to smoke cigarettes (73% vs. 27%).
They were less likely to have hypertension, type 2 diabetes, dyslipidemia, cerebrovascular disease, or prior CABG and were equally likely to have had a prior MI (36%).
Compared with nonusers, marijuana users were more likely to present with non–ST-elevation MI (30% vs. 23%) or ST-elevation MI (27% vs. 16%) and were less likely to present with angina.
Using propensity score matching, the researchers matched 3,803 marijuana users with the same number of nonusers.
In the matched cohort, patients who used marijuana had a greater risk of in-hospital bleeding (adjusted odds ratio, 1.54; 95% confidence interval, 1.20-1.97; P < .001) or stroke (aOR, 11.01; 95% CI, 1.32-91.67; P = .026) following PCI.
Marijuana users had a lower risk for acute kidney injury (2.2% vs. 2.9%; P = .007). Transfusion and mortality rates were similar in both groups.
The researchers acknowledged study limitations, including the fact that it did not include marijuana edibles, that the results may not be generalizable, and that marijuana use is now likely more common in Michigan following legalization of recreational marijuana in 2018.
Dr. Bhuva, Dr. Yoo, and Dr. DeFilippis have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
in separate studies.
Rhushik Bhuva, MD, presented the recurrent-MI results from a national U.S. study, and Sang Gune K. Yoo, MD, presented the PCI study, which used data from a Michigan cohort. The studies were presented at the American Heart Association scientific sessions.
Both studies “add to our accumulating knowledge of the cardiovascular risks of marijuana,” Ersilia M. DeFilippis, MD, a cardiology fellow at Columbia University Irvine Medical Center, New York, who was not involved with this research, said in an interview.
Dr. DeFilippis and the two study authors say clinicians and patients need to be more aware of cardiovascular risks from smoking marijuana, and they call for more patient screening, counseling, and research.
Need for screening and counseling
Marijuana is a Schedule 1 controlled substance in the United States, which makes it illegal to conduct rigorous controlled trials of marijuana products. Existing knowledge is therefore based on observational studies, Dr. DeFilippis noted.
She was lead author of a review of marijuana use by patients with cardiovascular disease. The review was published in the Journal of the American College of Cardiology. An AHA scientific statement about marijuana and cardiovascular health was published in Circulation.
Both documents drew attention to risks from marijuana use in patients with cardiovascular disease.
Until more data are available, “I think it is absolutely critical” that cardiologists and general providers screen patients for marijuana use, “either at the time of their MI or ideally prior to that, when they are making a cardiovascular risk assessment,” said Dr. DeFilippis.
That is also the time to “counsel patients, especially those who have had an MI, about risks associated with continuing to use marijuana.”
Importantly, providers and patients need to be aware that “cannabinoids, through the cytochrome P450 system, can interact with well-known cardiovascular medications, which we know provide benefit in the post-MI period,” she added. “For example, marijuana can interfere with beta-blockers, statins, antiarrhythmics, and certain anticoagulants.”
Dr. Bhuva, a cardiology fellow with the Wright Center for Community Health, Scranton, Pa., said that it is “concerning” that “recurrent heart attacks and cardiac interventions [were] higher among cannabis users, even though they were younger and had fewer risk factors for heart disease.
“Spreading awareness regarding the potential risk of recurrent heart attacks in middle-aged, African American, and male cannabis users and screening them at an earlier age for potential risk factors of future heart attacks should be encouraged among clinicians,” he urged in a statement from the AHA.
Dr. Yoo, an internal medicine resident at the University of Michigan, Ann Arbor, pointed out that, in their study of patients who underwent PCI after MI or because they had coronary artery disease, those who smoked or vaped marijuana were younger and were more likely to be male. They were less likely to have traditional cardiovascular risk factors except for smoking tobacco, which was highly prevalent.
After propensity matching, patients who used marijuana had a 1.5-fold increased risk of in-hospital bleeding and an 11-fold higher risk for in-hospital stroke following PCI.
However, the absolute number of strokes in PCI was small, and the confidence interval was wide (indicating a large uncertainty), Dr. Yoo said in an interview.
These risks “should not deter patients from undergoing these [lifesaving] procedures,” he said; however, clinicians should be aware of these risks with marijuana use and should screen and counsel patients about this.
Hospitalized patients with prior MI
Dr. Bhuva and colleagues identified patients from the National Inpatient Sample who were hospitalized in the United States from 2007 to 2014 and who had experienced a prior MI and had undergone revascularization with PCI or coronary artery bypass grafting (CABG).
There were about 8 million hospital stays per year. The database did not specify the type of marijuana that patients used.
During the 8-year study period, many states legalized or decriminalized medical and/or recreational marijuana, and marijuana use increased steadily, from 0.2% to 0.7%.
Compared with nonusers, those who used marijuana were younger (median age, 53 vs. 72 years), and there were more men (77% vs. 62%) or Black persons (34% vs. 10%) (all P < .001). Fewer marijuana users had hypertension (72% vs. 75%), diabetes (24% vs. 33%), or dyslipidemia (51% vs. 58%) (all P < .001). More marijuana users underwent a repeat MI (67% vs. 41%).
On the other hand, marijuana users, who were younger and healthier than the other patients, were less likely to die during hospitalization for a recurrent MI (0.8% vs. 2.5%), and their hospital costs were lower.
The researchers acknowledged that study limitations include lack of information about marijuana type (smoked, edible, medicinal, or recreational) or dose, as well as the time from marijuana use to cardiac event.
In-Hospital outcomes after PCI
Dr. Yoo and colleagues analyzed data from patients who underwent PCI from Jan. 1, 2013, to Oct. 1, 2016, at Michigan’s 48 nonfederal hospitals, which are part of the Blue Cross Blue Shield Michigan Cardiovascular Consortium PCI registry.
In this cohort, 3,970 patients (3.5%) had smoked or vaped marijuana in the month prior to PCI, and 109,507 patients had not done so. The marijuana users were younger (mean age, 54 vs. 66 years) and were more likely to be male (79% vs. 67%) and to smoke cigarettes (73% vs. 27%).
They were less likely to have hypertension, type 2 diabetes, dyslipidemia, cerebrovascular disease, or prior CABG and were equally likely to have had a prior MI (36%).
Compared with nonusers, marijuana users were more likely to present with non–ST-elevation MI (30% vs. 23%) or ST-elevation MI (27% vs. 16%) and were less likely to present with angina.
Using propensity score matching, the researchers matched 3,803 marijuana users with the same number of nonusers.
In the matched cohort, patients who used marijuana had a greater risk of in-hospital bleeding (adjusted odds ratio, 1.54; 95% confidence interval, 1.20-1.97; P < .001) or stroke (aOR, 11.01; 95% CI, 1.32-91.67; P = .026) following PCI.
Marijuana users had a lower risk for acute kidney injury (2.2% vs. 2.9%; P = .007). Transfusion and mortality rates were similar in both groups.
The researchers acknowledged study limitations, including the fact that it did not include marijuana edibles, that the results may not be generalizable, and that marijuana use is now likely more common in Michigan following legalization of recreational marijuana in 2018.
Dr. Bhuva, Dr. Yoo, and Dr. DeFilippis have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
in separate studies.
Rhushik Bhuva, MD, presented the recurrent-MI results from a national U.S. study, and Sang Gune K. Yoo, MD, presented the PCI study, which used data from a Michigan cohort. The studies were presented at the American Heart Association scientific sessions.
Both studies “add to our accumulating knowledge of the cardiovascular risks of marijuana,” Ersilia M. DeFilippis, MD, a cardiology fellow at Columbia University Irvine Medical Center, New York, who was not involved with this research, said in an interview.
Dr. DeFilippis and the two study authors say clinicians and patients need to be more aware of cardiovascular risks from smoking marijuana, and they call for more patient screening, counseling, and research.
Need for screening and counseling
Marijuana is a Schedule 1 controlled substance in the United States, which makes it illegal to conduct rigorous controlled trials of marijuana products. Existing knowledge is therefore based on observational studies, Dr. DeFilippis noted.
She was lead author of a review of marijuana use by patients with cardiovascular disease. The review was published in the Journal of the American College of Cardiology. An AHA scientific statement about marijuana and cardiovascular health was published in Circulation.
Both documents drew attention to risks from marijuana use in patients with cardiovascular disease.
Until more data are available, “I think it is absolutely critical” that cardiologists and general providers screen patients for marijuana use, “either at the time of their MI or ideally prior to that, when they are making a cardiovascular risk assessment,” said Dr. DeFilippis.
That is also the time to “counsel patients, especially those who have had an MI, about risks associated with continuing to use marijuana.”
Importantly, providers and patients need to be aware that “cannabinoids, through the cytochrome P450 system, can interact with well-known cardiovascular medications, which we know provide benefit in the post-MI period,” she added. “For example, marijuana can interfere with beta-blockers, statins, antiarrhythmics, and certain anticoagulants.”
Dr. Bhuva, a cardiology fellow with the Wright Center for Community Health, Scranton, Pa., said that it is “concerning” that “recurrent heart attacks and cardiac interventions [were] higher among cannabis users, even though they were younger and had fewer risk factors for heart disease.
“Spreading awareness regarding the potential risk of recurrent heart attacks in middle-aged, African American, and male cannabis users and screening them at an earlier age for potential risk factors of future heart attacks should be encouraged among clinicians,” he urged in a statement from the AHA.
Dr. Yoo, an internal medicine resident at the University of Michigan, Ann Arbor, pointed out that, in their study of patients who underwent PCI after MI or because they had coronary artery disease, those who smoked or vaped marijuana were younger and were more likely to be male. They were less likely to have traditional cardiovascular risk factors except for smoking tobacco, which was highly prevalent.
After propensity matching, patients who used marijuana had a 1.5-fold increased risk of in-hospital bleeding and an 11-fold higher risk for in-hospital stroke following PCI.
However, the absolute number of strokes in PCI was small, and the confidence interval was wide (indicating a large uncertainty), Dr. Yoo said in an interview.
These risks “should not deter patients from undergoing these [lifesaving] procedures,” he said; however, clinicians should be aware of these risks with marijuana use and should screen and counsel patients about this.
Hospitalized patients with prior MI
Dr. Bhuva and colleagues identified patients from the National Inpatient Sample who were hospitalized in the United States from 2007 to 2014 and who had experienced a prior MI and had undergone revascularization with PCI or coronary artery bypass grafting (CABG).
There were about 8 million hospital stays per year. The database did not specify the type of marijuana that patients used.
During the 8-year study period, many states legalized or decriminalized medical and/or recreational marijuana, and marijuana use increased steadily, from 0.2% to 0.7%.
Compared with nonusers, those who used marijuana were younger (median age, 53 vs. 72 years), and there were more men (77% vs. 62%) or Black persons (34% vs. 10%) (all P < .001). Fewer marijuana users had hypertension (72% vs. 75%), diabetes (24% vs. 33%), or dyslipidemia (51% vs. 58%) (all P < .001). More marijuana users underwent a repeat MI (67% vs. 41%).
On the other hand, marijuana users, who were younger and healthier than the other patients, were less likely to die during hospitalization for a recurrent MI (0.8% vs. 2.5%), and their hospital costs were lower.
The researchers acknowledged that study limitations include lack of information about marijuana type (smoked, edible, medicinal, or recreational) or dose, as well as the time from marijuana use to cardiac event.
In-Hospital outcomes after PCI
Dr. Yoo and colleagues analyzed data from patients who underwent PCI from Jan. 1, 2013, to Oct. 1, 2016, at Michigan’s 48 nonfederal hospitals, which are part of the Blue Cross Blue Shield Michigan Cardiovascular Consortium PCI registry.
In this cohort, 3,970 patients (3.5%) had smoked or vaped marijuana in the month prior to PCI, and 109,507 patients had not done so. The marijuana users were younger (mean age, 54 vs. 66 years) and were more likely to be male (79% vs. 67%) and to smoke cigarettes (73% vs. 27%).
They were less likely to have hypertension, type 2 diabetes, dyslipidemia, cerebrovascular disease, or prior CABG and were equally likely to have had a prior MI (36%).
Compared with nonusers, marijuana users were more likely to present with non–ST-elevation MI (30% vs. 23%) or ST-elevation MI (27% vs. 16%) and were less likely to present with angina.
Using propensity score matching, the researchers matched 3,803 marijuana users with the same number of nonusers.
In the matched cohort, patients who used marijuana had a greater risk of in-hospital bleeding (adjusted odds ratio, 1.54; 95% confidence interval, 1.20-1.97; P < .001) or stroke (aOR, 11.01; 95% CI, 1.32-91.67; P = .026) following PCI.
Marijuana users had a lower risk for acute kidney injury (2.2% vs. 2.9%; P = .007). Transfusion and mortality rates were similar in both groups.
The researchers acknowledged study limitations, including the fact that it did not include marijuana edibles, that the results may not be generalizable, and that marijuana use is now likely more common in Michigan following legalization of recreational marijuana in 2018.
Dr. Bhuva, Dr. Yoo, and Dr. DeFilippis have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From AHA 2020
Factor XI inhibitor–based anticoagulation strategies gain ground
according to Jeffrey I. Weitz, MD.
These strategies could pick up where direct-acting oral anticoagulants leave off, he suggested during a presentation at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
“We all know that the direct oral anticoagulants – the DOACs – are an advance over vitamin K antagonists,” said Dr. Weitz, professor of medicine and biochemistry at McMaster University, Hamilton, Ontario.
Not only are DOACs at least as effective as vitamin K antagonists such as warfarin for stroke prevention in atrial fibrillation or for treatment of venous thromboembolism (VTE), but they also reduce intracranial bleeding and major bleeding risk in those settings, respectively, and they are more convenient to administer because they can be delivered using fixed doses without the need for coagulation monitoring, he added.
Still, new targets are needed, he said, explaining that, although DOACs moved closer to the goal of attenuating thrombosis without increasing the risk of bleeding, annual rates of major bleeding remain at 2%-3% in the atrial fibrillation population, and rates of major and clinically relevant nonmajor bleeding are about 10%.
“The fear of bleeding leads to underuse of anticoagulants for eligible patients with atrial fibrillation and inappropriate use of low-dose [non–vitamin K antagonist oral anticoagulant] regimens, which can leave patients unprotected from thrombotic complications,” he said.
Factor XI
That’s where Factor XI (FXI) may come in, Dr. Weitz said.
Current anticoagulants target enzymes, including FXa or thrombin, in the common pathway of coagulation, but the intrinsic pathway at the level of FXI and FXII has attracted attention in recent years.
The intrinsic pathway is activated when blood comes into contact with medical devices like stents, mechanical heart valves, or central venous catheters, but evidence also suggests that it plays a role in clot stabilization and growth, he explained, noting additional evidence of attenuation of thrombosis in mice deficient in FXI or FXII and in animals with FXI or FXII inhibitors.
“There is no bleeding with congenital FXII deficiency, and patients with FXI deficiency rarely have spontaneous bleeding, although they can bleed with surgery or trauma,” he noted. “Therefore, the promise of contact pathway inhibition is that we can attenuate thrombosis with little or no disruption of hemostasis.”
The initiators of the intrinsic pathway are naturally occurring polyphosphates that can activate FXI and FXII, promote platelet activation, and lead to thrombosis. A number of agents are being investigated to target these enzymes – particularly FXI, for which the strongest epidemiological and other evidence of its link with thrombosis exists. He noted that FXI deficiency appears protective against deep-vein thrombosis (DVT) and ischemic stroke, whereas high levels are linked with an increased risk of venous and arterial thrombosis.
Investigative strategies include the use of antisense oligonucleotides to reduce hepatic synthesis of FXI, aptamers to bind FXI and block its activity, antibodies to bind FXI and block its activation or activity, and small molecules to bind reversibly to the active site of FXI and block its activity “much like the DOACs block the activity of FXa or thrombin.”
“We have to remember that the DOACs have taken over from vitamin K antagonists, like warfarin, for many indications, and as they go generic their uptake will increase even further,” Dr. Weitz said. “When we compare the FXI inhibitors with existing anticoagulants, we don’t necessarily want to go up against the DOACs – we’re looking for indications where [DOACs] have yet to be tested or may be unsafe.”
Potential indications include the following:
Prevention of major adverse cardiovascular events in patients with end-stage renal disease with or without atrial fibrillation.
Provision of a safer platform for antiplatelet therapy in patients with acute coronary syndrome.
Secondary stroke prevention.
Prevention or treatment of cancer-associated VTE.
Prevention of thrombosis associated with central venous catheters, left ventricular assist devices, or mechanical heart valves.
Agents in development
Of the FXI inhibitors in development, ISIS-FXIRx, an antisense oligonucleotide against FXI, is furthest along. In a study published in Blood, ISIS-FXIRx produced a dose-dependent and sustained reduction in FXI levels in healthy volunteers, and in a later randomized study published in The New England Journal of Medicine, it significantly reduced the incidence of DVT in patients undergoing voluntary total knee arthroplasty (30.4% with enoxaparin vs. 4.2% with ISIS-FXIRx at a dose of 300 mg). Bleeding rates were 8.3% and 2.6%, respectively.
The findings showed the potential for reducing thrombosis without increasing bleeding by targeting FXI, Dr. Weitz said, adding that ISIS-FXIRx was also evaluated in a small study of patients with end-stage renal disease undergoing hemodialysis and was shown to produce a dose-dependent reduction in FXI levels and to reduce the incidence of category 3 and 4 clotting in the air trap and dialyzer, compared with placebo, when given in addition to heparin.
This suggests that FXI knockdown can attenuate device-associated clotting to a greater extent than heparin alone, Dr. Weitz said.
The FXIa-directed inhibitory antibody osocimab has also been evaluated in both healthy volunteers and in patients undergoing total knee arthroplasty. In a 2019 study of healthy volunteers, a single IV injection showed a dose-dependent pharmacokinetic profile and produced FXI inhibition for about 1 month, and in the FOXTROT trial published in January in JAMA by Dr. Weitz and colleagues, osocimab was shown to reduce the incidence of symptomatic VTE, asymptomatic DVT, and VTE-related death up to day 10-13 after total knee arthroplasty.
Osocimab at doses ranging from 0.3-1.8 mg/kg given postoperatively or preoperatively were noninferior to enoxaparin (rates of 15.7%-23.7% vs. 26.3%), and osocimab at a preoperative dose of 1.8 mg/kg was superior to both enoxaparin and apixaban (11.5% vs. 26.3% and 14.5%, respectively), he said.
Bleeding rates ranged from 0%-5% with osocimab, compared with 6% with enoxaparin and 2% with apixaban
Ongoing studies
Currently ongoing studies of FXI-directed anticoagulation strategies include a study comparing ISIS-FXIRx with placebo in 200 patients with end-stage renal disease, a study comparing osocimab with placebo in 600 patients with end-stage renal disease, and a study comparing abelacimab – an antibody that binds to FXI and prevents its activation by either FXIIa or thrombin, with enoxaparin in 700 patients undergoing total knee arthroplasty, Dr. Weitz said.
Additionally, there is “considerable activity” with small molecule inhibitors of FXIa, including a phase 2, placebo-controlled, dose-ranging study looking at the novel JNG-7003/BMS-986177 agent for secondary stroke/transient ischemic attack prevention in 2,500 patients and a phase 2 study comparing it with enoxaparin for postoperative thromboprophylaxis in 1,200 patients undergoing total knee arthroplasty.
Parallel phase 2 studies are also underway to compare the novel BAY-2433334 small molecule inhibitor with placebo for stroke/transient ischemic attack prevention, with apixaban for atrial fibrillation, and for prevention of major adverse cardiovascular events in patients with acute MI.
These ongoing trials will help determine the risk-benefit profile of FXI inhibitors he said.
Session comoderator Anne Rose, PharmD, pharmacy coordinator at the University of Wisconsin, Madison, noted that these types of agents have been discussed “for quite some time” and asked whether they will be available for use in clinical practice in the near future.
Dr. Weitz predicted it will be at least a few years. The studies are just now moving to phase 2b and will still need to be evaluated in phase 3 trials and for appropriate new indications, he said.
Dr. Weitz reported research support from Canadian Institutes of Health research, Heart and Stroke Foundation, and Canadian Fund for Innovation, and he is a consultant and/or scientific advisory board member for Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Pfizer, Portola, Servier , and Thetherex.
according to Jeffrey I. Weitz, MD.
These strategies could pick up where direct-acting oral anticoagulants leave off, he suggested during a presentation at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
“We all know that the direct oral anticoagulants – the DOACs – are an advance over vitamin K antagonists,” said Dr. Weitz, professor of medicine and biochemistry at McMaster University, Hamilton, Ontario.
Not only are DOACs at least as effective as vitamin K antagonists such as warfarin for stroke prevention in atrial fibrillation or for treatment of venous thromboembolism (VTE), but they also reduce intracranial bleeding and major bleeding risk in those settings, respectively, and they are more convenient to administer because they can be delivered using fixed doses without the need for coagulation monitoring, he added.
Still, new targets are needed, he said, explaining that, although DOACs moved closer to the goal of attenuating thrombosis without increasing the risk of bleeding, annual rates of major bleeding remain at 2%-3% in the atrial fibrillation population, and rates of major and clinically relevant nonmajor bleeding are about 10%.
“The fear of bleeding leads to underuse of anticoagulants for eligible patients with atrial fibrillation and inappropriate use of low-dose [non–vitamin K antagonist oral anticoagulant] regimens, which can leave patients unprotected from thrombotic complications,” he said.
Factor XI
That’s where Factor XI (FXI) may come in, Dr. Weitz said.
Current anticoagulants target enzymes, including FXa or thrombin, in the common pathway of coagulation, but the intrinsic pathway at the level of FXI and FXII has attracted attention in recent years.
The intrinsic pathway is activated when blood comes into contact with medical devices like stents, mechanical heart valves, or central venous catheters, but evidence also suggests that it plays a role in clot stabilization and growth, he explained, noting additional evidence of attenuation of thrombosis in mice deficient in FXI or FXII and in animals with FXI or FXII inhibitors.
“There is no bleeding with congenital FXII deficiency, and patients with FXI deficiency rarely have spontaneous bleeding, although they can bleed with surgery or trauma,” he noted. “Therefore, the promise of contact pathway inhibition is that we can attenuate thrombosis with little or no disruption of hemostasis.”
The initiators of the intrinsic pathway are naturally occurring polyphosphates that can activate FXI and FXII, promote platelet activation, and lead to thrombosis. A number of agents are being investigated to target these enzymes – particularly FXI, for which the strongest epidemiological and other evidence of its link with thrombosis exists. He noted that FXI deficiency appears protective against deep-vein thrombosis (DVT) and ischemic stroke, whereas high levels are linked with an increased risk of venous and arterial thrombosis.
Investigative strategies include the use of antisense oligonucleotides to reduce hepatic synthesis of FXI, aptamers to bind FXI and block its activity, antibodies to bind FXI and block its activation or activity, and small molecules to bind reversibly to the active site of FXI and block its activity “much like the DOACs block the activity of FXa or thrombin.”
“We have to remember that the DOACs have taken over from vitamin K antagonists, like warfarin, for many indications, and as they go generic their uptake will increase even further,” Dr. Weitz said. “When we compare the FXI inhibitors with existing anticoagulants, we don’t necessarily want to go up against the DOACs – we’re looking for indications where [DOACs] have yet to be tested or may be unsafe.”
Potential indications include the following:
Prevention of major adverse cardiovascular events in patients with end-stage renal disease with or without atrial fibrillation.
Provision of a safer platform for antiplatelet therapy in patients with acute coronary syndrome.
Secondary stroke prevention.
Prevention or treatment of cancer-associated VTE.
Prevention of thrombosis associated with central venous catheters, left ventricular assist devices, or mechanical heart valves.
Agents in development
Of the FXI inhibitors in development, ISIS-FXIRx, an antisense oligonucleotide against FXI, is furthest along. In a study published in Blood, ISIS-FXIRx produced a dose-dependent and sustained reduction in FXI levels in healthy volunteers, and in a later randomized study published in The New England Journal of Medicine, it significantly reduced the incidence of DVT in patients undergoing voluntary total knee arthroplasty (30.4% with enoxaparin vs. 4.2% with ISIS-FXIRx at a dose of 300 mg). Bleeding rates were 8.3% and 2.6%, respectively.
The findings showed the potential for reducing thrombosis without increasing bleeding by targeting FXI, Dr. Weitz said, adding that ISIS-FXIRx was also evaluated in a small study of patients with end-stage renal disease undergoing hemodialysis and was shown to produce a dose-dependent reduction in FXI levels and to reduce the incidence of category 3 and 4 clotting in the air trap and dialyzer, compared with placebo, when given in addition to heparin.
This suggests that FXI knockdown can attenuate device-associated clotting to a greater extent than heparin alone, Dr. Weitz said.
The FXIa-directed inhibitory antibody osocimab has also been evaluated in both healthy volunteers and in patients undergoing total knee arthroplasty. In a 2019 study of healthy volunteers, a single IV injection showed a dose-dependent pharmacokinetic profile and produced FXI inhibition for about 1 month, and in the FOXTROT trial published in January in JAMA by Dr. Weitz and colleagues, osocimab was shown to reduce the incidence of symptomatic VTE, asymptomatic DVT, and VTE-related death up to day 10-13 after total knee arthroplasty.
Osocimab at doses ranging from 0.3-1.8 mg/kg given postoperatively or preoperatively were noninferior to enoxaparin (rates of 15.7%-23.7% vs. 26.3%), and osocimab at a preoperative dose of 1.8 mg/kg was superior to both enoxaparin and apixaban (11.5% vs. 26.3% and 14.5%, respectively), he said.
Bleeding rates ranged from 0%-5% with osocimab, compared with 6% with enoxaparin and 2% with apixaban
Ongoing studies
Currently ongoing studies of FXI-directed anticoagulation strategies include a study comparing ISIS-FXIRx with placebo in 200 patients with end-stage renal disease, a study comparing osocimab with placebo in 600 patients with end-stage renal disease, and a study comparing abelacimab – an antibody that binds to FXI and prevents its activation by either FXIIa or thrombin, with enoxaparin in 700 patients undergoing total knee arthroplasty, Dr. Weitz said.
Additionally, there is “considerable activity” with small molecule inhibitors of FXIa, including a phase 2, placebo-controlled, dose-ranging study looking at the novel JNG-7003/BMS-986177 agent for secondary stroke/transient ischemic attack prevention in 2,500 patients and a phase 2 study comparing it with enoxaparin for postoperative thromboprophylaxis in 1,200 patients undergoing total knee arthroplasty.
Parallel phase 2 studies are also underway to compare the novel BAY-2433334 small molecule inhibitor with placebo for stroke/transient ischemic attack prevention, with apixaban for atrial fibrillation, and for prevention of major adverse cardiovascular events in patients with acute MI.
These ongoing trials will help determine the risk-benefit profile of FXI inhibitors he said.
Session comoderator Anne Rose, PharmD, pharmacy coordinator at the University of Wisconsin, Madison, noted that these types of agents have been discussed “for quite some time” and asked whether they will be available for use in clinical practice in the near future.
Dr. Weitz predicted it will be at least a few years. The studies are just now moving to phase 2b and will still need to be evaluated in phase 3 trials and for appropriate new indications, he said.
Dr. Weitz reported research support from Canadian Institutes of Health research, Heart and Stroke Foundation, and Canadian Fund for Innovation, and he is a consultant and/or scientific advisory board member for Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Pfizer, Portola, Servier , and Thetherex.
according to Jeffrey I. Weitz, MD.
These strategies could pick up where direct-acting oral anticoagulants leave off, he suggested during a presentation at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
“We all know that the direct oral anticoagulants – the DOACs – are an advance over vitamin K antagonists,” said Dr. Weitz, professor of medicine and biochemistry at McMaster University, Hamilton, Ontario.
Not only are DOACs at least as effective as vitamin K antagonists such as warfarin for stroke prevention in atrial fibrillation or for treatment of venous thromboembolism (VTE), but they also reduce intracranial bleeding and major bleeding risk in those settings, respectively, and they are more convenient to administer because they can be delivered using fixed doses without the need for coagulation monitoring, he added.
Still, new targets are needed, he said, explaining that, although DOACs moved closer to the goal of attenuating thrombosis without increasing the risk of bleeding, annual rates of major bleeding remain at 2%-3% in the atrial fibrillation population, and rates of major and clinically relevant nonmajor bleeding are about 10%.
“The fear of bleeding leads to underuse of anticoagulants for eligible patients with atrial fibrillation and inappropriate use of low-dose [non–vitamin K antagonist oral anticoagulant] regimens, which can leave patients unprotected from thrombotic complications,” he said.
Factor XI
That’s where Factor XI (FXI) may come in, Dr. Weitz said.
Current anticoagulants target enzymes, including FXa or thrombin, in the common pathway of coagulation, but the intrinsic pathway at the level of FXI and FXII has attracted attention in recent years.
The intrinsic pathway is activated when blood comes into contact with medical devices like stents, mechanical heart valves, or central venous catheters, but evidence also suggests that it plays a role in clot stabilization and growth, he explained, noting additional evidence of attenuation of thrombosis in mice deficient in FXI or FXII and in animals with FXI or FXII inhibitors.
“There is no bleeding with congenital FXII deficiency, and patients with FXI deficiency rarely have spontaneous bleeding, although they can bleed with surgery or trauma,” he noted. “Therefore, the promise of contact pathway inhibition is that we can attenuate thrombosis with little or no disruption of hemostasis.”
The initiators of the intrinsic pathway are naturally occurring polyphosphates that can activate FXI and FXII, promote platelet activation, and lead to thrombosis. A number of agents are being investigated to target these enzymes – particularly FXI, for which the strongest epidemiological and other evidence of its link with thrombosis exists. He noted that FXI deficiency appears protective against deep-vein thrombosis (DVT) and ischemic stroke, whereas high levels are linked with an increased risk of venous and arterial thrombosis.
Investigative strategies include the use of antisense oligonucleotides to reduce hepatic synthesis of FXI, aptamers to bind FXI and block its activity, antibodies to bind FXI and block its activation or activity, and small molecules to bind reversibly to the active site of FXI and block its activity “much like the DOACs block the activity of FXa or thrombin.”
“We have to remember that the DOACs have taken over from vitamin K antagonists, like warfarin, for many indications, and as they go generic their uptake will increase even further,” Dr. Weitz said. “When we compare the FXI inhibitors with existing anticoagulants, we don’t necessarily want to go up against the DOACs – we’re looking for indications where [DOACs] have yet to be tested or may be unsafe.”
Potential indications include the following:
Prevention of major adverse cardiovascular events in patients with end-stage renal disease with or without atrial fibrillation.
Provision of a safer platform for antiplatelet therapy in patients with acute coronary syndrome.
Secondary stroke prevention.
Prevention or treatment of cancer-associated VTE.
Prevention of thrombosis associated with central venous catheters, left ventricular assist devices, or mechanical heart valves.
Agents in development
Of the FXI inhibitors in development, ISIS-FXIRx, an antisense oligonucleotide against FXI, is furthest along. In a study published in Blood, ISIS-FXIRx produced a dose-dependent and sustained reduction in FXI levels in healthy volunteers, and in a later randomized study published in The New England Journal of Medicine, it significantly reduced the incidence of DVT in patients undergoing voluntary total knee arthroplasty (30.4% with enoxaparin vs. 4.2% with ISIS-FXIRx at a dose of 300 mg). Bleeding rates were 8.3% and 2.6%, respectively.
The findings showed the potential for reducing thrombosis without increasing bleeding by targeting FXI, Dr. Weitz said, adding that ISIS-FXIRx was also evaluated in a small study of patients with end-stage renal disease undergoing hemodialysis and was shown to produce a dose-dependent reduction in FXI levels and to reduce the incidence of category 3 and 4 clotting in the air trap and dialyzer, compared with placebo, when given in addition to heparin.
This suggests that FXI knockdown can attenuate device-associated clotting to a greater extent than heparin alone, Dr. Weitz said.
The FXIa-directed inhibitory antibody osocimab has also been evaluated in both healthy volunteers and in patients undergoing total knee arthroplasty. In a 2019 study of healthy volunteers, a single IV injection showed a dose-dependent pharmacokinetic profile and produced FXI inhibition for about 1 month, and in the FOXTROT trial published in January in JAMA by Dr. Weitz and colleagues, osocimab was shown to reduce the incidence of symptomatic VTE, asymptomatic DVT, and VTE-related death up to day 10-13 after total knee arthroplasty.
Osocimab at doses ranging from 0.3-1.8 mg/kg given postoperatively or preoperatively were noninferior to enoxaparin (rates of 15.7%-23.7% vs. 26.3%), and osocimab at a preoperative dose of 1.8 mg/kg was superior to both enoxaparin and apixaban (11.5% vs. 26.3% and 14.5%, respectively), he said.
Bleeding rates ranged from 0%-5% with osocimab, compared with 6% with enoxaparin and 2% with apixaban
Ongoing studies
Currently ongoing studies of FXI-directed anticoagulation strategies include a study comparing ISIS-FXIRx with placebo in 200 patients with end-stage renal disease, a study comparing osocimab with placebo in 600 patients with end-stage renal disease, and a study comparing abelacimab – an antibody that binds to FXI and prevents its activation by either FXIIa or thrombin, with enoxaparin in 700 patients undergoing total knee arthroplasty, Dr. Weitz said.
Additionally, there is “considerable activity” with small molecule inhibitors of FXIa, including a phase 2, placebo-controlled, dose-ranging study looking at the novel JNG-7003/BMS-986177 agent for secondary stroke/transient ischemic attack prevention in 2,500 patients and a phase 2 study comparing it with enoxaparin for postoperative thromboprophylaxis in 1,200 patients undergoing total knee arthroplasty.
Parallel phase 2 studies are also underway to compare the novel BAY-2433334 small molecule inhibitor with placebo for stroke/transient ischemic attack prevention, with apixaban for atrial fibrillation, and for prevention of major adverse cardiovascular events in patients with acute MI.
These ongoing trials will help determine the risk-benefit profile of FXI inhibitors he said.
Session comoderator Anne Rose, PharmD, pharmacy coordinator at the University of Wisconsin, Madison, noted that these types of agents have been discussed “for quite some time” and asked whether they will be available for use in clinical practice in the near future.
Dr. Weitz predicted it will be at least a few years. The studies are just now moving to phase 2b and will still need to be evaluated in phase 3 trials and for appropriate new indications, he said.
Dr. Weitz reported research support from Canadian Institutes of Health research, Heart and Stroke Foundation, and Canadian Fund for Innovation, and he is a consultant and/or scientific advisory board member for Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Pfizer, Portola, Servier , and Thetherex.
FROM THE THSNA BIENNIAL SUMMIT
Local hospitals still have a role in treating severe stroke
a new study has shown.
In the RACECAT trial, functional outcomes were similar for patients suspected of having a large-vessel occlusion stroke who were located in areas not currently served by a comprehensive stroke center, whether they were first taken to a local primary stroke center or whether they were transported over a longer distance to a comprehensive center.
“Under the particular conditions in our study where we had a very well-organized system, a ‘mothership’ transfer protocol for patients with suspected large-vessel occlusion has not proven superior over the ‘drip-and-ship’ protocol, but the opposite is also true,” lead investigator Marc Ribo, MD, concluded.
Dr. Ribo, assistant professor of neurology at Hospital Vall d’Hebron, Barcelona, presented the RACECAT results at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Dr. Ribo said in an interview that there is a feeling among the stroke community that patients with a suspected large-vessel occlusion should be transferred directly to a comprehensive stroke center capable of performing endovascular thrombectomy, even if there is a nearer, smaller primary stroke center where patients are usually taken first for thrombolysis.
“Many stroke neurologists believe we are losing time by sending patients with severe stroke to a local hospital and that we should skip this step, but this is controversial area,” he commented. “Our findings suggest that we should not automatically bypass local stroke centers.”
Dr. Ribo pointed out that the local centers performed very well in the study, with very fast “in/out” times for patients who were subsequently transferred for thrombectomy.
“On the basis of our results, we recommend that if a local stroke center can perform well like ours did – if they are within the time indicators for treating and transferring patients – then they should keep receiving these patients. But if they are not performing well in this regard, then they should probably be bypassed,” he commented.
The RACECAT trial was well received by stroke experts at an ESO-WSO 2020 press conference at which it was discussed.
Stefan Kiechl, MD, Medical University Innsbruck (Austria), described the trial as “outstanding,” adding: “It has addressed a very important question. It is a big achievement in stroke medicine.”
Patrik Michel, MD, Lausanne (Switzerland) University Hospital, said that “this is a very important and highly sophisticated trial in terms of design and execution. The message is that it doesn’t matter which pathway is used, but it is important to have a well-organized network with highly trained paramedics.”
RACECAT
The RACECAT trial was conducted in the Catalonia region of Spain. Twenty-seven hospitals participated, including 7 comprehensive stroke centers and 20 local stroke centers.
The trial included stroke patients with suspected large-vessel occlusion stroke, as determined on the basis of evaluation by paramedics using the criteria of a Rapid Arterial Occlusion Evaluation (RACE) scale score above 4 and on the basis of a call to a vascular neurologist. For inclusion in the study, patients had to be in a geographical area not served by a comprehensive stroke center and to have an estimated arrival time to a comprehensive center of less than 7 hours from symptom onset in order that thrombectomy would be possible.
Of 7,475 stroke code patients evaluated, 1,401 met the inclusion criteria and were randomly assigned to be transferred to a local hospital or to a comprehensive stroke center farther away.
Baseline characteristics were similar between the two groups. The patients had severe strokes with an average National Institutes of Health Stroke Scale score of 17. It was later confirmed that 46% of the patients enrolled in the study had a large-vessel occlusion stroke.
Results showed that time from symptom onset to hospital arrival was 142 minutes for those taken to a local center and 216 minutes for those taken to a comprehensive stroke center. Of those taken to a local hospital, 86% arrived within 4 hours of symptom onset and so were potential candidates for thrombolysis, compared with 76% of those taken to a comprehensive center.
Of the patients taken to a local hospital, 60% were given thrombolysis versus 43% of those taken immediately to a comprehensive center. On the other hand, 50% of patients who were taken directly to a comprehensive center underwent thrombectomy, compared with 40% who were first taken to a local center.
For patients who received thrombolysis, time to tissue plasminogen activator administration was 120 minutes for those treated at a local hospital versus 155 minutes for those taken directly to a comprehensive center.
For patients who received thrombectomy, time from symptom onset to groin puncture was 270 minutes if they were first taken to a local hospital and were then transferred, versus 214 minutes for those taken directly to the comprehensive center.
The primary efficacy endpoint was functional outcome using Modified Rankin Scale (mRS) shift analysis at 90 days for ischemic stroke patients. This showed a “completely flat” result, Dr. Ribo reported, with an adjusted hazard ratio of 1.029 for patients taken to a comprehensive center in comparison with those taken to a local center.
“There was absolutely no trend towards benefit in one group over the other,” he said.
What about hemorrhagic stroke?
The study also evaluated functional outcomes for the whole population enrolled. “If we make the decision just based on thrombectomy-eligible patients, we may harm the rest of the patients, so we did this study to look at the whole population of severe stroke patients,” Dr. Ribo said.
Of the study population, 25% of patients were found to have had a hemorrhagic stroke.
“The problem is, at the prehospital level, it is impossible to know if a patient is having a large-vessel occlusion ischemic stroke or a hemorrhagic stroke,” Dr. Ribo explained. “We have to make a decision for the whole population, and while a longer transport time to get to a comprehensive stroke center might help a patient with a large-vessel occlusion ischemic stroke, it might not be so appropriate for patients with a hemorrhagic stroke who need to have their blood pressure stabilized as soon as possible.”
For the whole population, the mRS shift analysis at 90 days was also neutral, with an aHR of 0.965.
When considering only patients with hemorrhagic stroke, the adjusted hazard ratio for the mRS shift analysis at 90 days was 1.216, which was still nonsignificant (95% confidence interval, 0.864-1.709). This included a nonsignificant increase in mortality among those taken directly to a comprehensive center.
“If we had better tools for a certain diagnosis in the field, then we could consider taking large-vessel occlusion ischemic stroke patients to a comprehensive center and hemorrhagic stroke patients to the local stroke center, but so far, we don’t have this option apart from a few places using mobile stroke units with CT scanners,” Dr. Ribo noted.
Transfer times to comprehensive centers in the study ranged from 30 minutes to 2.5 hours. “There might well be a difference in outcomes for short and long transfers, and we may be able to offer different transfer protocols in these different situations, and we are looking at that, but the study was only stopped in June, and we haven’t had a chance to analyze those results yet,” Dr. Ribo added.
Complications during transport occurred in 0.5% of those taken to a local hospital and in 1% of those taken directly to a comprehensive center. “We were concerned about complications with longer transfers, but these numbers are quite low. Intubations were very low – just one patient taken to a local center, versus three or four in the longer transfer group,” he added.
For both local and comprehensive centers, treatment times were impressive in the study. For local hospitals, the average in/out time was just 60 minutes for patients who went to a comprehensive center; for patients receiving thrombolysis, the average door to needle time was around 30 minutes.
Time to thrombectomy in the comprehensive center for patients transferred from a local hospital was also very fast, with an average door to groin puncture time of less than 40 minutes. “This shows we have a very well-oiled system,” Dr. Ribo said.
“There is always going to be a balance between a quicker time to thrombolysis by taking a patient to the closest hospital but a quicker time to thrombectomy if patients are taken straight to the comprehensive center,” he concluded. “But in our system, where we are achieving fast treatment and transfer times, our results show that patients had timely access to reperfusion therapies regardless of transfer protocol, and under these circumstances, it is fine for the emergency services to take stroke patients to the closest stroke center.”
Results applicable elsewhere?
During the discussion at an ESO-WSO 2020 press conference, other experts pointed out that the Catalonia group is a leader in this field, being the pioneers of the RACE score used in this study for paramedics to identify suspected large-vessel occlusions. This led to questions about the applicability of the results.
“The performance by paramedics was very good using the RACE scale, and the performance times were very impressive. Are these results applicable elsewhere?” Dr. Kiechl asked.
Dr. Ribo said the combination of the RACE score and a call with a vascular neurologist was of “great value” in identifying appropriate patients. Half of the patients selected in this way for the trial were confirmed to have a large-vessel occlusion. “That is a good result,” he added.
He noted that the performance of the local hospitals improved dramatically during the study. “They had an incentive to work on their times. They could have lost most of their stroke patients if their results came out worse. We told them they had an opportunity to show that they have a role in treating these patients, and they took that opportunity.”
Dr. Ribo said there were lessons here for those involved in acute stroke care. “When creating stroke transfer policies in local networks, the performances of individual centers need to be taken into account. If primary stroke centers are motivated and can work in a well-coordinated way and perform to within the recommended times, then they can keep receiving stroke code patients. This should be possible in most developed countries.”
Noting that the in/out time of 60 minutes at local hospitals was “very impressive,” Dr. Kiechl asked how such fast times were achieved.
Dr. Ribo responded that, to a great extent, this was because of ambulance staff. “We have trained the paramedics to anticipate a second transfer after delivering the patient to the local hospital so they can prepare for this rather than waiting for a second call.”
Dr. Ribo pointed out that there were other advantages in taking patients to local centers first. “For those that do not need to be transferred on, they will be closer to relatives. It is very difficult for the family if the patient is hundreds of miles away. And there may be a cost advantage. We did look at costs, but haven’t got that data yet.”
He said: “If local stroke centers do not treat so many stroke code patients, they will lose their expertise, and that will be detrimental to the remaining patients who are taken there. We want to try to maintain a good standard of stroke care across a decent spread of hospitals—not just a couple of major comprehensive centers,” he added.
Commenting on the study, Jesse Dawson, MD, University of Glasgow, who was chair of the plenary session at which the study was presented, said: “RACECAT is very interesting but needs a lot of thought to dissect. My takeaway is that we know that time to reperfusion is key, and we need to get these times as low as possible, but we don’t need to chase a particular care pathway. Thus, if your country/geography suits ‘drip and ship’ better, this is acceptable. If direct to endovascular is possible or you are close to such a center, then this is ideal. But within those paradigms, be as fast as possible.”
He added that results of the subgroups with regard to transfer time will be helpful.
The RACECAT study was funded by Fundacio Ictus Malaltia Vascular.
A version of this article originally appeared on Medscape.com.
a new study has shown.
In the RACECAT trial, functional outcomes were similar for patients suspected of having a large-vessel occlusion stroke who were located in areas not currently served by a comprehensive stroke center, whether they were first taken to a local primary stroke center or whether they were transported over a longer distance to a comprehensive center.
“Under the particular conditions in our study where we had a very well-organized system, a ‘mothership’ transfer protocol for patients with suspected large-vessel occlusion has not proven superior over the ‘drip-and-ship’ protocol, but the opposite is also true,” lead investigator Marc Ribo, MD, concluded.
Dr. Ribo, assistant professor of neurology at Hospital Vall d’Hebron, Barcelona, presented the RACECAT results at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Dr. Ribo said in an interview that there is a feeling among the stroke community that patients with a suspected large-vessel occlusion should be transferred directly to a comprehensive stroke center capable of performing endovascular thrombectomy, even if there is a nearer, smaller primary stroke center where patients are usually taken first for thrombolysis.
“Many stroke neurologists believe we are losing time by sending patients with severe stroke to a local hospital and that we should skip this step, but this is controversial area,” he commented. “Our findings suggest that we should not automatically bypass local stroke centers.”
Dr. Ribo pointed out that the local centers performed very well in the study, with very fast “in/out” times for patients who were subsequently transferred for thrombectomy.
“On the basis of our results, we recommend that if a local stroke center can perform well like ours did – if they are within the time indicators for treating and transferring patients – then they should keep receiving these patients. But if they are not performing well in this regard, then they should probably be bypassed,” he commented.
The RACECAT trial was well received by stroke experts at an ESO-WSO 2020 press conference at which it was discussed.
Stefan Kiechl, MD, Medical University Innsbruck (Austria), described the trial as “outstanding,” adding: “It has addressed a very important question. It is a big achievement in stroke medicine.”
Patrik Michel, MD, Lausanne (Switzerland) University Hospital, said that “this is a very important and highly sophisticated trial in terms of design and execution. The message is that it doesn’t matter which pathway is used, but it is important to have a well-organized network with highly trained paramedics.”
RACECAT
The RACECAT trial was conducted in the Catalonia region of Spain. Twenty-seven hospitals participated, including 7 comprehensive stroke centers and 20 local stroke centers.
The trial included stroke patients with suspected large-vessel occlusion stroke, as determined on the basis of evaluation by paramedics using the criteria of a Rapid Arterial Occlusion Evaluation (RACE) scale score above 4 and on the basis of a call to a vascular neurologist. For inclusion in the study, patients had to be in a geographical area not served by a comprehensive stroke center and to have an estimated arrival time to a comprehensive center of less than 7 hours from symptom onset in order that thrombectomy would be possible.
Of 7,475 stroke code patients evaluated, 1,401 met the inclusion criteria and were randomly assigned to be transferred to a local hospital or to a comprehensive stroke center farther away.
Baseline characteristics were similar between the two groups. The patients had severe strokes with an average National Institutes of Health Stroke Scale score of 17. It was later confirmed that 46% of the patients enrolled in the study had a large-vessel occlusion stroke.
Results showed that time from symptom onset to hospital arrival was 142 minutes for those taken to a local center and 216 minutes for those taken to a comprehensive stroke center. Of those taken to a local hospital, 86% arrived within 4 hours of symptom onset and so were potential candidates for thrombolysis, compared with 76% of those taken to a comprehensive center.
Of the patients taken to a local hospital, 60% were given thrombolysis versus 43% of those taken immediately to a comprehensive center. On the other hand, 50% of patients who were taken directly to a comprehensive center underwent thrombectomy, compared with 40% who were first taken to a local center.
For patients who received thrombolysis, time to tissue plasminogen activator administration was 120 minutes for those treated at a local hospital versus 155 minutes for those taken directly to a comprehensive center.
For patients who received thrombectomy, time from symptom onset to groin puncture was 270 minutes if they were first taken to a local hospital and were then transferred, versus 214 minutes for those taken directly to the comprehensive center.
The primary efficacy endpoint was functional outcome using Modified Rankin Scale (mRS) shift analysis at 90 days for ischemic stroke patients. This showed a “completely flat” result, Dr. Ribo reported, with an adjusted hazard ratio of 1.029 for patients taken to a comprehensive center in comparison with those taken to a local center.
“There was absolutely no trend towards benefit in one group over the other,” he said.
What about hemorrhagic stroke?
The study also evaluated functional outcomes for the whole population enrolled. “If we make the decision just based on thrombectomy-eligible patients, we may harm the rest of the patients, so we did this study to look at the whole population of severe stroke patients,” Dr. Ribo said.
Of the study population, 25% of patients were found to have had a hemorrhagic stroke.
“The problem is, at the prehospital level, it is impossible to know if a patient is having a large-vessel occlusion ischemic stroke or a hemorrhagic stroke,” Dr. Ribo explained. “We have to make a decision for the whole population, and while a longer transport time to get to a comprehensive stroke center might help a patient with a large-vessel occlusion ischemic stroke, it might not be so appropriate for patients with a hemorrhagic stroke who need to have their blood pressure stabilized as soon as possible.”
For the whole population, the mRS shift analysis at 90 days was also neutral, with an aHR of 0.965.
When considering only patients with hemorrhagic stroke, the adjusted hazard ratio for the mRS shift analysis at 90 days was 1.216, which was still nonsignificant (95% confidence interval, 0.864-1.709). This included a nonsignificant increase in mortality among those taken directly to a comprehensive center.
“If we had better tools for a certain diagnosis in the field, then we could consider taking large-vessel occlusion ischemic stroke patients to a comprehensive center and hemorrhagic stroke patients to the local stroke center, but so far, we don’t have this option apart from a few places using mobile stroke units with CT scanners,” Dr. Ribo noted.
Transfer times to comprehensive centers in the study ranged from 30 minutes to 2.5 hours. “There might well be a difference in outcomes for short and long transfers, and we may be able to offer different transfer protocols in these different situations, and we are looking at that, but the study was only stopped in June, and we haven’t had a chance to analyze those results yet,” Dr. Ribo added.
Complications during transport occurred in 0.5% of those taken to a local hospital and in 1% of those taken directly to a comprehensive center. “We were concerned about complications with longer transfers, but these numbers are quite low. Intubations were very low – just one patient taken to a local center, versus three or four in the longer transfer group,” he added.
For both local and comprehensive centers, treatment times were impressive in the study. For local hospitals, the average in/out time was just 60 minutes for patients who went to a comprehensive center; for patients receiving thrombolysis, the average door to needle time was around 30 minutes.
Time to thrombectomy in the comprehensive center for patients transferred from a local hospital was also very fast, with an average door to groin puncture time of less than 40 minutes. “This shows we have a very well-oiled system,” Dr. Ribo said.
“There is always going to be a balance between a quicker time to thrombolysis by taking a patient to the closest hospital but a quicker time to thrombectomy if patients are taken straight to the comprehensive center,” he concluded. “But in our system, where we are achieving fast treatment and transfer times, our results show that patients had timely access to reperfusion therapies regardless of transfer protocol, and under these circumstances, it is fine for the emergency services to take stroke patients to the closest stroke center.”
Results applicable elsewhere?
During the discussion at an ESO-WSO 2020 press conference, other experts pointed out that the Catalonia group is a leader in this field, being the pioneers of the RACE score used in this study for paramedics to identify suspected large-vessel occlusions. This led to questions about the applicability of the results.
“The performance by paramedics was very good using the RACE scale, and the performance times were very impressive. Are these results applicable elsewhere?” Dr. Kiechl asked.
Dr. Ribo said the combination of the RACE score and a call with a vascular neurologist was of “great value” in identifying appropriate patients. Half of the patients selected in this way for the trial were confirmed to have a large-vessel occlusion. “That is a good result,” he added.
He noted that the performance of the local hospitals improved dramatically during the study. “They had an incentive to work on their times. They could have lost most of their stroke patients if their results came out worse. We told them they had an opportunity to show that they have a role in treating these patients, and they took that opportunity.”
Dr. Ribo said there were lessons here for those involved in acute stroke care. “When creating stroke transfer policies in local networks, the performances of individual centers need to be taken into account. If primary stroke centers are motivated and can work in a well-coordinated way and perform to within the recommended times, then they can keep receiving stroke code patients. This should be possible in most developed countries.”
Noting that the in/out time of 60 minutes at local hospitals was “very impressive,” Dr. Kiechl asked how such fast times were achieved.
Dr. Ribo responded that, to a great extent, this was because of ambulance staff. “We have trained the paramedics to anticipate a second transfer after delivering the patient to the local hospital so they can prepare for this rather than waiting for a second call.”
Dr. Ribo pointed out that there were other advantages in taking patients to local centers first. “For those that do not need to be transferred on, they will be closer to relatives. It is very difficult for the family if the patient is hundreds of miles away. And there may be a cost advantage. We did look at costs, but haven’t got that data yet.”
He said: “If local stroke centers do not treat so many stroke code patients, they will lose their expertise, and that will be detrimental to the remaining patients who are taken there. We want to try to maintain a good standard of stroke care across a decent spread of hospitals—not just a couple of major comprehensive centers,” he added.
Commenting on the study, Jesse Dawson, MD, University of Glasgow, who was chair of the plenary session at which the study was presented, said: “RACECAT is very interesting but needs a lot of thought to dissect. My takeaway is that we know that time to reperfusion is key, and we need to get these times as low as possible, but we don’t need to chase a particular care pathway. Thus, if your country/geography suits ‘drip and ship’ better, this is acceptable. If direct to endovascular is possible or you are close to such a center, then this is ideal. But within those paradigms, be as fast as possible.”
He added that results of the subgroups with regard to transfer time will be helpful.
The RACECAT study was funded by Fundacio Ictus Malaltia Vascular.
A version of this article originally appeared on Medscape.com.
a new study has shown.
In the RACECAT trial, functional outcomes were similar for patients suspected of having a large-vessel occlusion stroke who were located in areas not currently served by a comprehensive stroke center, whether they were first taken to a local primary stroke center or whether they were transported over a longer distance to a comprehensive center.
“Under the particular conditions in our study where we had a very well-organized system, a ‘mothership’ transfer protocol for patients with suspected large-vessel occlusion has not proven superior over the ‘drip-and-ship’ protocol, but the opposite is also true,” lead investigator Marc Ribo, MD, concluded.
Dr. Ribo, assistant professor of neurology at Hospital Vall d’Hebron, Barcelona, presented the RACECAT results at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Dr. Ribo said in an interview that there is a feeling among the stroke community that patients with a suspected large-vessel occlusion should be transferred directly to a comprehensive stroke center capable of performing endovascular thrombectomy, even if there is a nearer, smaller primary stroke center where patients are usually taken first for thrombolysis.
“Many stroke neurologists believe we are losing time by sending patients with severe stroke to a local hospital and that we should skip this step, but this is controversial area,” he commented. “Our findings suggest that we should not automatically bypass local stroke centers.”
Dr. Ribo pointed out that the local centers performed very well in the study, with very fast “in/out” times for patients who were subsequently transferred for thrombectomy.
“On the basis of our results, we recommend that if a local stroke center can perform well like ours did – if they are within the time indicators for treating and transferring patients – then they should keep receiving these patients. But if they are not performing well in this regard, then they should probably be bypassed,” he commented.
The RACECAT trial was well received by stroke experts at an ESO-WSO 2020 press conference at which it was discussed.
Stefan Kiechl, MD, Medical University Innsbruck (Austria), described the trial as “outstanding,” adding: “It has addressed a very important question. It is a big achievement in stroke medicine.”
Patrik Michel, MD, Lausanne (Switzerland) University Hospital, said that “this is a very important and highly sophisticated trial in terms of design and execution. The message is that it doesn’t matter which pathway is used, but it is important to have a well-organized network with highly trained paramedics.”
RACECAT
The RACECAT trial was conducted in the Catalonia region of Spain. Twenty-seven hospitals participated, including 7 comprehensive stroke centers and 20 local stroke centers.
The trial included stroke patients with suspected large-vessel occlusion stroke, as determined on the basis of evaluation by paramedics using the criteria of a Rapid Arterial Occlusion Evaluation (RACE) scale score above 4 and on the basis of a call to a vascular neurologist. For inclusion in the study, patients had to be in a geographical area not served by a comprehensive stroke center and to have an estimated arrival time to a comprehensive center of less than 7 hours from symptom onset in order that thrombectomy would be possible.
Of 7,475 stroke code patients evaluated, 1,401 met the inclusion criteria and were randomly assigned to be transferred to a local hospital or to a comprehensive stroke center farther away.
Baseline characteristics were similar between the two groups. The patients had severe strokes with an average National Institutes of Health Stroke Scale score of 17. It was later confirmed that 46% of the patients enrolled in the study had a large-vessel occlusion stroke.
Results showed that time from symptom onset to hospital arrival was 142 minutes for those taken to a local center and 216 minutes for those taken to a comprehensive stroke center. Of those taken to a local hospital, 86% arrived within 4 hours of symptom onset and so were potential candidates for thrombolysis, compared with 76% of those taken to a comprehensive center.
Of the patients taken to a local hospital, 60% were given thrombolysis versus 43% of those taken immediately to a comprehensive center. On the other hand, 50% of patients who were taken directly to a comprehensive center underwent thrombectomy, compared with 40% who were first taken to a local center.
For patients who received thrombolysis, time to tissue plasminogen activator administration was 120 minutes for those treated at a local hospital versus 155 minutes for those taken directly to a comprehensive center.
For patients who received thrombectomy, time from symptom onset to groin puncture was 270 minutes if they were first taken to a local hospital and were then transferred, versus 214 minutes for those taken directly to the comprehensive center.
The primary efficacy endpoint was functional outcome using Modified Rankin Scale (mRS) shift analysis at 90 days for ischemic stroke patients. This showed a “completely flat” result, Dr. Ribo reported, with an adjusted hazard ratio of 1.029 for patients taken to a comprehensive center in comparison with those taken to a local center.
“There was absolutely no trend towards benefit in one group over the other,” he said.
What about hemorrhagic stroke?
The study also evaluated functional outcomes for the whole population enrolled. “If we make the decision just based on thrombectomy-eligible patients, we may harm the rest of the patients, so we did this study to look at the whole population of severe stroke patients,” Dr. Ribo said.
Of the study population, 25% of patients were found to have had a hemorrhagic stroke.
“The problem is, at the prehospital level, it is impossible to know if a patient is having a large-vessel occlusion ischemic stroke or a hemorrhagic stroke,” Dr. Ribo explained. “We have to make a decision for the whole population, and while a longer transport time to get to a comprehensive stroke center might help a patient with a large-vessel occlusion ischemic stroke, it might not be so appropriate for patients with a hemorrhagic stroke who need to have their blood pressure stabilized as soon as possible.”
For the whole population, the mRS shift analysis at 90 days was also neutral, with an aHR of 0.965.
When considering only patients with hemorrhagic stroke, the adjusted hazard ratio for the mRS shift analysis at 90 days was 1.216, which was still nonsignificant (95% confidence interval, 0.864-1.709). This included a nonsignificant increase in mortality among those taken directly to a comprehensive center.
“If we had better tools for a certain diagnosis in the field, then we could consider taking large-vessel occlusion ischemic stroke patients to a comprehensive center and hemorrhagic stroke patients to the local stroke center, but so far, we don’t have this option apart from a few places using mobile stroke units with CT scanners,” Dr. Ribo noted.
Transfer times to comprehensive centers in the study ranged from 30 minutes to 2.5 hours. “There might well be a difference in outcomes for short and long transfers, and we may be able to offer different transfer protocols in these different situations, and we are looking at that, but the study was only stopped in June, and we haven’t had a chance to analyze those results yet,” Dr. Ribo added.
Complications during transport occurred in 0.5% of those taken to a local hospital and in 1% of those taken directly to a comprehensive center. “We were concerned about complications with longer transfers, but these numbers are quite low. Intubations were very low – just one patient taken to a local center, versus three or four in the longer transfer group,” he added.
For both local and comprehensive centers, treatment times were impressive in the study. For local hospitals, the average in/out time was just 60 minutes for patients who went to a comprehensive center; for patients receiving thrombolysis, the average door to needle time was around 30 minutes.
Time to thrombectomy in the comprehensive center for patients transferred from a local hospital was also very fast, with an average door to groin puncture time of less than 40 minutes. “This shows we have a very well-oiled system,” Dr. Ribo said.
“There is always going to be a balance between a quicker time to thrombolysis by taking a patient to the closest hospital but a quicker time to thrombectomy if patients are taken straight to the comprehensive center,” he concluded. “But in our system, where we are achieving fast treatment and transfer times, our results show that patients had timely access to reperfusion therapies regardless of transfer protocol, and under these circumstances, it is fine for the emergency services to take stroke patients to the closest stroke center.”
Results applicable elsewhere?
During the discussion at an ESO-WSO 2020 press conference, other experts pointed out that the Catalonia group is a leader in this field, being the pioneers of the RACE score used in this study for paramedics to identify suspected large-vessel occlusions. This led to questions about the applicability of the results.
“The performance by paramedics was very good using the RACE scale, and the performance times were very impressive. Are these results applicable elsewhere?” Dr. Kiechl asked.
Dr. Ribo said the combination of the RACE score and a call with a vascular neurologist was of “great value” in identifying appropriate patients. Half of the patients selected in this way for the trial were confirmed to have a large-vessel occlusion. “That is a good result,” he added.
He noted that the performance of the local hospitals improved dramatically during the study. “They had an incentive to work on their times. They could have lost most of their stroke patients if their results came out worse. We told them they had an opportunity to show that they have a role in treating these patients, and they took that opportunity.”
Dr. Ribo said there were lessons here for those involved in acute stroke care. “When creating stroke transfer policies in local networks, the performances of individual centers need to be taken into account. If primary stroke centers are motivated and can work in a well-coordinated way and perform to within the recommended times, then they can keep receiving stroke code patients. This should be possible in most developed countries.”
Noting that the in/out time of 60 minutes at local hospitals was “very impressive,” Dr. Kiechl asked how such fast times were achieved.
Dr. Ribo responded that, to a great extent, this was because of ambulance staff. “We have trained the paramedics to anticipate a second transfer after delivering the patient to the local hospital so they can prepare for this rather than waiting for a second call.”
Dr. Ribo pointed out that there were other advantages in taking patients to local centers first. “For those that do not need to be transferred on, they will be closer to relatives. It is very difficult for the family if the patient is hundreds of miles away. And there may be a cost advantage. We did look at costs, but haven’t got that data yet.”
He said: “If local stroke centers do not treat so many stroke code patients, they will lose their expertise, and that will be detrimental to the remaining patients who are taken there. We want to try to maintain a good standard of stroke care across a decent spread of hospitals—not just a couple of major comprehensive centers,” he added.
Commenting on the study, Jesse Dawson, MD, University of Glasgow, who was chair of the plenary session at which the study was presented, said: “RACECAT is very interesting but needs a lot of thought to dissect. My takeaway is that we know that time to reperfusion is key, and we need to get these times as low as possible, but we don’t need to chase a particular care pathway. Thus, if your country/geography suits ‘drip and ship’ better, this is acceptable. If direct to endovascular is possible or you are close to such a center, then this is ideal. But within those paradigms, be as fast as possible.”
He added that results of the subgroups with regard to transfer time will be helpful.
The RACECAT study was funded by Fundacio Ictus Malaltia Vascular.
A version of this article originally appeared on Medscape.com.
FROM ESO-WSO 2020
Methotrexate and hydroxychloroquine split on cardiovascular outcomes in RA
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
FROM ACR 2020
Proinflammatory dietary pattern linked to higher CV risk
Dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease (CVD) and stroke in a new pooled analysis of three prospective cohort studies.
The analysis included 210,145 U.S. women and men followed for up to 32 years in the Nurses’ Health Studies I and II and the Health Professionals Follow-up Study.
After adjustment for use of anti-inflammatory medications and CVD risk factors, those whose dietary pattern ranked in the highest quintile of inflammatory potential had a 38% higher risk of CVD (hazard ratio comparing highest with lowest quintiles, 1.38), a 46% higher risk of coronary heart disease (HR, 1.46), and a 28% higher risk of stroke (HR, 1.28) (all P for trend < .001).
Jun Li, MD, PhD, and colleagues at Harvard School of Public Health and Harvard Medical School, Boston, published the findings of their study in the Nov. 10 issue of the Journal of the American College of Cardiology.
The inflammatory potential of a diet was assessed using a food-based, dietary index called the “empirical dietary inflammatory pattern” or EDIP.
In an interview, Dr. Li explained that the EDIP was developed 4 years ago by many of the same authors involved with this study, including nutrition heavyweights Walter C. Willett, MD, DrPH, and Frank B. Hu, MD, PhD, both from Harvard.
“We summarized all the foods people eat into 39 defined food groups and did a reduced-rank regression analysis that looked at these 39 food groups and three inflammatory markers – interleukin-6, C-reactive protein, and tumor necrosis factor–alpha receptor 2. We found 18 food groups that are most predictive of these biomarkers, and the EDIP was calculated as the weighted sum of these 18 food groups.”
Individuals who had higher intakes of green-leafy vegetables (kale, spinach, arugula), dark-yellow vegetables (pumpkin, yellow peppers, carrots), whole grains, fruits, tea, coffee and wine had lower long-term CVD risk than those with higher intakes of red meat, processed meat, organ meat, refined carbohydrates, and sweetened beverages.
The associations were consistent across cohorts and between sexes and remained significant in multiple sensitivity analysis that adjusted for alcohol consumption, smoking pack-years, use of lipid-lowering and antihypertensive medications, sodium intake, and blood pressure.
In a secondary analysis, diets with higher inflammatory potential were also associated with significantly higher biomarker levels indicative of more systemic, vascular, and metabolic inflammation, as well as less favorable lipid profiles.
“We wanted to be able to provide guidance on dietary patterns and food combinations,” said Dr. Li. “If you tell people to eat more polyunsaturated fats instead of saturated fat or trans fat, most people don’t know what foods are higher and lower in those nutrients. Also, many foods have different nutrients – some of which are good and some of which are bad – so we wanted to help people find the foods with the higher proportion of healthy nutrients rather than point out specific nutrients to avoid.”
Researchers used prospectively gathered data from the Nurses’ Health Studies I and II starting from 1984 and from the Health Professionals Follow-up Study. After excluding participants with missing diet information or previously diagnosed heart disease, stroke or cancer, over 210,000 participants were included in the analysis. Participants completed a survey every 4 years to ascertain dietary intake.
Prevention, not treatment
In an editorial comment, Ramon Estruch, MD, PhD, from the Hospital Clinic in Barcelona, and colleagues suggested that it might be time for better dietary guidelines.
“A better knowledge of health protection provided by different foods and dietary patterns, mainly their anti-inflammatory properties, should provide the basis for designing even healthier dietary patterns to protect against heart disease,” the editorialists wrote.
They added extra-virgin olive oil, fatty fish, and tomatoes to the list of foods with “established anti-inflammatory activity.”
In a comment, Dr. Estruch said the findings of this new study are confirmatory of the PREDIMED trial, which showed a reduction in risk of major CV events in individuals at high cardiovascular risk assigned to an anti-inflammatory Mediterranean diet pattern supplemented with extra-virgin olive oil or nuts as compared with those assigned to a reduced-fat diet.
“The study of Jun Li et al. confirms that an anti-inflammatory diet is useful to prevent cardiovascular events and, more important, that healthy dietary patterns may be even healthier if subjects increase consumption of foods with the highest anti-inflammatory potential,” he said, adding that “mechanistic explanations add plausibility to the results of observational studies.”
Dr. Estruch was the principal investigator of PREDIMED. This trial was originally published in 2013 and then retracted and republished in 2018, with some required corrections, but the results had not materially changed.
Dr. Li is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Boston Nutrition Obesity Research Center. Dr. Estruch disclosed no financial relationships relevant to the contents of this article.
A version of this article originally appeared on Medscape.com.
Dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease (CVD) and stroke in a new pooled analysis of three prospective cohort studies.
The analysis included 210,145 U.S. women and men followed for up to 32 years in the Nurses’ Health Studies I and II and the Health Professionals Follow-up Study.
After adjustment for use of anti-inflammatory medications and CVD risk factors, those whose dietary pattern ranked in the highest quintile of inflammatory potential had a 38% higher risk of CVD (hazard ratio comparing highest with lowest quintiles, 1.38), a 46% higher risk of coronary heart disease (HR, 1.46), and a 28% higher risk of stroke (HR, 1.28) (all P for trend < .001).
Jun Li, MD, PhD, and colleagues at Harvard School of Public Health and Harvard Medical School, Boston, published the findings of their study in the Nov. 10 issue of the Journal of the American College of Cardiology.
The inflammatory potential of a diet was assessed using a food-based, dietary index called the “empirical dietary inflammatory pattern” or EDIP.
In an interview, Dr. Li explained that the EDIP was developed 4 years ago by many of the same authors involved with this study, including nutrition heavyweights Walter C. Willett, MD, DrPH, and Frank B. Hu, MD, PhD, both from Harvard.
“We summarized all the foods people eat into 39 defined food groups and did a reduced-rank regression analysis that looked at these 39 food groups and three inflammatory markers – interleukin-6, C-reactive protein, and tumor necrosis factor–alpha receptor 2. We found 18 food groups that are most predictive of these biomarkers, and the EDIP was calculated as the weighted sum of these 18 food groups.”
Individuals who had higher intakes of green-leafy vegetables (kale, spinach, arugula), dark-yellow vegetables (pumpkin, yellow peppers, carrots), whole grains, fruits, tea, coffee and wine had lower long-term CVD risk than those with higher intakes of red meat, processed meat, organ meat, refined carbohydrates, and sweetened beverages.
The associations were consistent across cohorts and between sexes and remained significant in multiple sensitivity analysis that adjusted for alcohol consumption, smoking pack-years, use of lipid-lowering and antihypertensive medications, sodium intake, and blood pressure.
In a secondary analysis, diets with higher inflammatory potential were also associated with significantly higher biomarker levels indicative of more systemic, vascular, and metabolic inflammation, as well as less favorable lipid profiles.
“We wanted to be able to provide guidance on dietary patterns and food combinations,” said Dr. Li. “If you tell people to eat more polyunsaturated fats instead of saturated fat or trans fat, most people don’t know what foods are higher and lower in those nutrients. Also, many foods have different nutrients – some of which are good and some of which are bad – so we wanted to help people find the foods with the higher proportion of healthy nutrients rather than point out specific nutrients to avoid.”
Researchers used prospectively gathered data from the Nurses’ Health Studies I and II starting from 1984 and from the Health Professionals Follow-up Study. After excluding participants with missing diet information or previously diagnosed heart disease, stroke or cancer, over 210,000 participants were included in the analysis. Participants completed a survey every 4 years to ascertain dietary intake.
Prevention, not treatment
In an editorial comment, Ramon Estruch, MD, PhD, from the Hospital Clinic in Barcelona, and colleagues suggested that it might be time for better dietary guidelines.
“A better knowledge of health protection provided by different foods and dietary patterns, mainly their anti-inflammatory properties, should provide the basis for designing even healthier dietary patterns to protect against heart disease,” the editorialists wrote.
They added extra-virgin olive oil, fatty fish, and tomatoes to the list of foods with “established anti-inflammatory activity.”
In a comment, Dr. Estruch said the findings of this new study are confirmatory of the PREDIMED trial, which showed a reduction in risk of major CV events in individuals at high cardiovascular risk assigned to an anti-inflammatory Mediterranean diet pattern supplemented with extra-virgin olive oil or nuts as compared with those assigned to a reduced-fat diet.
“The study of Jun Li et al. confirms that an anti-inflammatory diet is useful to prevent cardiovascular events and, more important, that healthy dietary patterns may be even healthier if subjects increase consumption of foods with the highest anti-inflammatory potential,” he said, adding that “mechanistic explanations add plausibility to the results of observational studies.”
Dr. Estruch was the principal investigator of PREDIMED. This trial was originally published in 2013 and then retracted and republished in 2018, with some required corrections, but the results had not materially changed.
Dr. Li is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Boston Nutrition Obesity Research Center. Dr. Estruch disclosed no financial relationships relevant to the contents of this article.
A version of this article originally appeared on Medscape.com.
Dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease (CVD) and stroke in a new pooled analysis of three prospective cohort studies.
The analysis included 210,145 U.S. women and men followed for up to 32 years in the Nurses’ Health Studies I and II and the Health Professionals Follow-up Study.
After adjustment for use of anti-inflammatory medications and CVD risk factors, those whose dietary pattern ranked in the highest quintile of inflammatory potential had a 38% higher risk of CVD (hazard ratio comparing highest with lowest quintiles, 1.38), a 46% higher risk of coronary heart disease (HR, 1.46), and a 28% higher risk of stroke (HR, 1.28) (all P for trend < .001).
Jun Li, MD, PhD, and colleagues at Harvard School of Public Health and Harvard Medical School, Boston, published the findings of their study in the Nov. 10 issue of the Journal of the American College of Cardiology.
The inflammatory potential of a diet was assessed using a food-based, dietary index called the “empirical dietary inflammatory pattern” or EDIP.
In an interview, Dr. Li explained that the EDIP was developed 4 years ago by many of the same authors involved with this study, including nutrition heavyweights Walter C. Willett, MD, DrPH, and Frank B. Hu, MD, PhD, both from Harvard.
“We summarized all the foods people eat into 39 defined food groups and did a reduced-rank regression analysis that looked at these 39 food groups and three inflammatory markers – interleukin-6, C-reactive protein, and tumor necrosis factor–alpha receptor 2. We found 18 food groups that are most predictive of these biomarkers, and the EDIP was calculated as the weighted sum of these 18 food groups.”
Individuals who had higher intakes of green-leafy vegetables (kale, spinach, arugula), dark-yellow vegetables (pumpkin, yellow peppers, carrots), whole grains, fruits, tea, coffee and wine had lower long-term CVD risk than those with higher intakes of red meat, processed meat, organ meat, refined carbohydrates, and sweetened beverages.
The associations were consistent across cohorts and between sexes and remained significant in multiple sensitivity analysis that adjusted for alcohol consumption, smoking pack-years, use of lipid-lowering and antihypertensive medications, sodium intake, and blood pressure.
In a secondary analysis, diets with higher inflammatory potential were also associated with significantly higher biomarker levels indicative of more systemic, vascular, and metabolic inflammation, as well as less favorable lipid profiles.
“We wanted to be able to provide guidance on dietary patterns and food combinations,” said Dr. Li. “If you tell people to eat more polyunsaturated fats instead of saturated fat or trans fat, most people don’t know what foods are higher and lower in those nutrients. Also, many foods have different nutrients – some of which are good and some of which are bad – so we wanted to help people find the foods with the higher proportion of healthy nutrients rather than point out specific nutrients to avoid.”
Researchers used prospectively gathered data from the Nurses’ Health Studies I and II starting from 1984 and from the Health Professionals Follow-up Study. After excluding participants with missing diet information or previously diagnosed heart disease, stroke or cancer, over 210,000 participants were included in the analysis. Participants completed a survey every 4 years to ascertain dietary intake.
Prevention, not treatment
In an editorial comment, Ramon Estruch, MD, PhD, from the Hospital Clinic in Barcelona, and colleagues suggested that it might be time for better dietary guidelines.
“A better knowledge of health protection provided by different foods and dietary patterns, mainly their anti-inflammatory properties, should provide the basis for designing even healthier dietary patterns to protect against heart disease,” the editorialists wrote.
They added extra-virgin olive oil, fatty fish, and tomatoes to the list of foods with “established anti-inflammatory activity.”
In a comment, Dr. Estruch said the findings of this new study are confirmatory of the PREDIMED trial, which showed a reduction in risk of major CV events in individuals at high cardiovascular risk assigned to an anti-inflammatory Mediterranean diet pattern supplemented with extra-virgin olive oil or nuts as compared with those assigned to a reduced-fat diet.
“The study of Jun Li et al. confirms that an anti-inflammatory diet is useful to prevent cardiovascular events and, more important, that healthy dietary patterns may be even healthier if subjects increase consumption of foods with the highest anti-inflammatory potential,” he said, adding that “mechanistic explanations add plausibility to the results of observational studies.”
Dr. Estruch was the principal investigator of PREDIMED. This trial was originally published in 2013 and then retracted and republished in 2018, with some required corrections, but the results had not materially changed.
Dr. Li is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Boston Nutrition Obesity Research Center. Dr. Estruch disclosed no financial relationships relevant to the contents of this article.
A version of this article originally appeared on Medscape.com.








