User login
Successful accelerated taper for sleeping aid
THE CASE
A 49-year-old man with chronic insomnia was referred to the pharmacist authors (LF and DP) to initiate and manage the tapering of nightly zolpidem use. Per chart review, the patient had complaints of insomnia for more than 30 years. His care had been transferred to a Nebraska clinic 5 years earlier, with a medication list that included zolpidem controlled release (CR) 12.5 mg nightly. Since then, multiple interventions to achieve cessation had been tried, including counseling on sleep hygiene, adjunct antidepressant use, and abrupt discontinuation. Each of these methods was unsuccessful. So, his family physician (SS) reached out to the pharmacist authors (LF and DP).
THE APPROACH
Due to the patient’s long history of zolpidem use, a lack of literature on the topic, and worry for withdrawal symptoms, a taper schedule was designed utilizing various benzodiazepine taper resources for guidance. The proposed taper utilized 5-mg immediate release (IR) tablets to ensure ease of tapering. The taper ranged from 20% to 43% weekly reductions based on the ability to split the zolpidem tablet in half.
DISCUSSION
Zolpidem is a sedative-hypnotic medication indicated for the treatment of insomnia when used at therapeutic dosing (ie, 5 to 10 mg nightly). Anecdotal efficacy, accompanied by weak chronic insomnia guideline recommendations, has led prescribers to use zolpidem as a chronic medication to treat insomnia.1,2 There is evidence of dependence and possible seizures from supratherapeutic zolpidem doses in the hundreds of milligrams, raising safety concerns regarding abuse, dependence, and withdrawal seizures in chronic use.2,3
Additionally, there is limited evidence regarding the appropriate process of discontinuing zolpidem after chronic use.2 Often a taper schedule—similar to those used with benzodiazepine medications—is used as a reference for discontinuation.1 The hypothetical goal of a taper is to prevent withdrawal effects such as rebound insomnia, anxiety, palpitations, and seizures.3 However, an extended taper may not actually be necessary with chronic zolpidem patients.
Tapering with minimal adverse effects
Pharmacokinetic and pharmacodynamic studies have suggested minimal, if not complete, absence of rebound or withdrawal effects with short-term zolpidem use.4 The same appears to be true of patients with long-term use. In a study, Roehrs and colleagues5 explored whether long-term treatment (defined as 8 months) caused rebound insomnia upon abrupt withdrawal. The investigators concluded that people with primary insomnia do not experience rebound insomnia or withdrawal symptoms with chronic, therapeutic dosing.
Another study involving 92 elderly patients on long-term treatment of zolpidem (defined as > 1 month, with average around 9.9 ± 6.2 years) experienced only 1 or 2 nights of rebound insomnia during a month-long taper.1,6 Following that, they experienced improvements in initiation and staying asleep.
A possible explanation for the lack of dependence or withdrawal symptoms in patients chronically treated with zolpidem is the pharmacokinetic profile. While the selectivity of the binding sites differentiates this medication from benzodiazepines, the additional fact of a short half-life, and no repeated dosing throughout the day, likely limit the risk of experiencing withdrawal symptoms.1 The daily periods of minimal zolpidem exposure in the body may limit the amount of physical dependence.
Continue to: Discontinuation of zolpidem
Discontinuation of zolpidem
The 49-year-old man had a history of failed abrupt discontinuation of zolpidem in the past (without noted withdrawal symptoms). Thus, various benzodiazepine taper resources were consulted to develop a taper schedule.
We switched our patient from the zolpidem CR 12.5 mg nightly to 10 mg of the IR formulation, and the pharmacists proposed 20% to 43% weekly decreases in dosing based on dosage strengths. At the initial 3-day follow-up (having taken 10 mg nightly for 3 days), the patient reported a quicker onset of sleep but an inability to sleep through the night. The patient denied withdrawal symptoms or any significant impact to his daily routines. These results encouraged a progression to the next step of the taper. For the next 9 days, the patient took 5 mg nightly, rather than the pharmacist-advised dosing of alternating 5 mg and 10 mg nightly, and reported similar outcomes at his next visit.
This success led to the discontinuation of scheduled zolpidem. The patient was also given a prescription of 2.5 mg, as needed, if insomnia rebounded. No adverse effects were noted despite the accelerated taper. Based on patient response and motivation, the taper had progressed more quickly than scheduled, resulting in 3 days of 10 mg, 9 days of 5 mg, and 1 final day of 2.5 mg that was used when the patient had trouble falling asleep. At the 6-month follow-up, the patient informed the physician that he had neither experienced insomnia nor used any further medication.
THE TAKEAWAY
This case documents a successfully accelerated taper for a patient with a chronic history (> 5 years) of zolpidem use. Although withdrawal is often patient specific, this case suggests the risk is low despite the chronic usage. This further adds to the literature suggesting against the need for an extended taper, and possibly a taper at all, when using recommended doses of chronic zolpidem. This is a significant difference compared to past practices that drew from literature-based benzodiazepine tapers.6 This case serves as an observational point of reference for clinicians who are assisting patients with chronic zolpidem tapers.
CORRESPONDENCE
Logan Franck, PharmD, 986145 Nebraska Medical Center, Omaha, NE 68198-6145; [email protected]
1. Lähteenmäki R, Neuvonen PJ, Puustinen J, et al. Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol. 2019;124:330-340. doi: 10.1111/bcpt.13144
2. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
3. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. 2014;16:e19926. doi: 10.5812/ircmj.19926
4. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142-153. doi: 10.2165/00003088-199529030-00002
5. Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088-1095. doi: 10.1177/0269881111424455
6. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295-301. doi: 10.1111/j.1365-2125.2012.04418.x
THE CASE
A 49-year-old man with chronic insomnia was referred to the pharmacist authors (LF and DP) to initiate and manage the tapering of nightly zolpidem use. Per chart review, the patient had complaints of insomnia for more than 30 years. His care had been transferred to a Nebraska clinic 5 years earlier, with a medication list that included zolpidem controlled release (CR) 12.5 mg nightly. Since then, multiple interventions to achieve cessation had been tried, including counseling on sleep hygiene, adjunct antidepressant use, and abrupt discontinuation. Each of these methods was unsuccessful. So, his family physician (SS) reached out to the pharmacist authors (LF and DP).
THE APPROACH
Due to the patient’s long history of zolpidem use, a lack of literature on the topic, and worry for withdrawal symptoms, a taper schedule was designed utilizing various benzodiazepine taper resources for guidance. The proposed taper utilized 5-mg immediate release (IR) tablets to ensure ease of tapering. The taper ranged from 20% to 43% weekly reductions based on the ability to split the zolpidem tablet in half.
DISCUSSION
Zolpidem is a sedative-hypnotic medication indicated for the treatment of insomnia when used at therapeutic dosing (ie, 5 to 10 mg nightly). Anecdotal efficacy, accompanied by weak chronic insomnia guideline recommendations, has led prescribers to use zolpidem as a chronic medication to treat insomnia.1,2 There is evidence of dependence and possible seizures from supratherapeutic zolpidem doses in the hundreds of milligrams, raising safety concerns regarding abuse, dependence, and withdrawal seizures in chronic use.2,3
Additionally, there is limited evidence regarding the appropriate process of discontinuing zolpidem after chronic use.2 Often a taper schedule—similar to those used with benzodiazepine medications—is used as a reference for discontinuation.1 The hypothetical goal of a taper is to prevent withdrawal effects such as rebound insomnia, anxiety, palpitations, and seizures.3 However, an extended taper may not actually be necessary with chronic zolpidem patients.
Tapering with minimal adverse effects
Pharmacokinetic and pharmacodynamic studies have suggested minimal, if not complete, absence of rebound or withdrawal effects with short-term zolpidem use.4 The same appears to be true of patients with long-term use. In a study, Roehrs and colleagues5 explored whether long-term treatment (defined as 8 months) caused rebound insomnia upon abrupt withdrawal. The investigators concluded that people with primary insomnia do not experience rebound insomnia or withdrawal symptoms with chronic, therapeutic dosing.
Another study involving 92 elderly patients on long-term treatment of zolpidem (defined as > 1 month, with average around 9.9 ± 6.2 years) experienced only 1 or 2 nights of rebound insomnia during a month-long taper.1,6 Following that, they experienced improvements in initiation and staying asleep.
A possible explanation for the lack of dependence or withdrawal symptoms in patients chronically treated with zolpidem is the pharmacokinetic profile. While the selectivity of the binding sites differentiates this medication from benzodiazepines, the additional fact of a short half-life, and no repeated dosing throughout the day, likely limit the risk of experiencing withdrawal symptoms.1 The daily periods of minimal zolpidem exposure in the body may limit the amount of physical dependence.
Continue to: Discontinuation of zolpidem
Discontinuation of zolpidem
The 49-year-old man had a history of failed abrupt discontinuation of zolpidem in the past (without noted withdrawal symptoms). Thus, various benzodiazepine taper resources were consulted to develop a taper schedule.
We switched our patient from the zolpidem CR 12.5 mg nightly to 10 mg of the IR formulation, and the pharmacists proposed 20% to 43% weekly decreases in dosing based on dosage strengths. At the initial 3-day follow-up (having taken 10 mg nightly for 3 days), the patient reported a quicker onset of sleep but an inability to sleep through the night. The patient denied withdrawal symptoms or any significant impact to his daily routines. These results encouraged a progression to the next step of the taper. For the next 9 days, the patient took 5 mg nightly, rather than the pharmacist-advised dosing of alternating 5 mg and 10 mg nightly, and reported similar outcomes at his next visit.
This success led to the discontinuation of scheduled zolpidem. The patient was also given a prescription of 2.5 mg, as needed, if insomnia rebounded. No adverse effects were noted despite the accelerated taper. Based on patient response and motivation, the taper had progressed more quickly than scheduled, resulting in 3 days of 10 mg, 9 days of 5 mg, and 1 final day of 2.5 mg that was used when the patient had trouble falling asleep. At the 6-month follow-up, the patient informed the physician that he had neither experienced insomnia nor used any further medication.
THE TAKEAWAY
This case documents a successfully accelerated taper for a patient with a chronic history (> 5 years) of zolpidem use. Although withdrawal is often patient specific, this case suggests the risk is low despite the chronic usage. This further adds to the literature suggesting against the need for an extended taper, and possibly a taper at all, when using recommended doses of chronic zolpidem. This is a significant difference compared to past practices that drew from literature-based benzodiazepine tapers.6 This case serves as an observational point of reference for clinicians who are assisting patients with chronic zolpidem tapers.
CORRESPONDENCE
Logan Franck, PharmD, 986145 Nebraska Medical Center, Omaha, NE 68198-6145; [email protected]
THE CASE
A 49-year-old man with chronic insomnia was referred to the pharmacist authors (LF and DP) to initiate and manage the tapering of nightly zolpidem use. Per chart review, the patient had complaints of insomnia for more than 30 years. His care had been transferred to a Nebraska clinic 5 years earlier, with a medication list that included zolpidem controlled release (CR) 12.5 mg nightly. Since then, multiple interventions to achieve cessation had been tried, including counseling on sleep hygiene, adjunct antidepressant use, and abrupt discontinuation. Each of these methods was unsuccessful. So, his family physician (SS) reached out to the pharmacist authors (LF and DP).
THE APPROACH
Due to the patient’s long history of zolpidem use, a lack of literature on the topic, and worry for withdrawal symptoms, a taper schedule was designed utilizing various benzodiazepine taper resources for guidance. The proposed taper utilized 5-mg immediate release (IR) tablets to ensure ease of tapering. The taper ranged from 20% to 43% weekly reductions based on the ability to split the zolpidem tablet in half.
DISCUSSION
Zolpidem is a sedative-hypnotic medication indicated for the treatment of insomnia when used at therapeutic dosing (ie, 5 to 10 mg nightly). Anecdotal efficacy, accompanied by weak chronic insomnia guideline recommendations, has led prescribers to use zolpidem as a chronic medication to treat insomnia.1,2 There is evidence of dependence and possible seizures from supratherapeutic zolpidem doses in the hundreds of milligrams, raising safety concerns regarding abuse, dependence, and withdrawal seizures in chronic use.2,3
Additionally, there is limited evidence regarding the appropriate process of discontinuing zolpidem after chronic use.2 Often a taper schedule—similar to those used with benzodiazepine medications—is used as a reference for discontinuation.1 The hypothetical goal of a taper is to prevent withdrawal effects such as rebound insomnia, anxiety, palpitations, and seizures.3 However, an extended taper may not actually be necessary with chronic zolpidem patients.
Tapering with minimal adverse effects
Pharmacokinetic and pharmacodynamic studies have suggested minimal, if not complete, absence of rebound or withdrawal effects with short-term zolpidem use.4 The same appears to be true of patients with long-term use. In a study, Roehrs and colleagues5 explored whether long-term treatment (defined as 8 months) caused rebound insomnia upon abrupt withdrawal. The investigators concluded that people with primary insomnia do not experience rebound insomnia or withdrawal symptoms with chronic, therapeutic dosing.
Another study involving 92 elderly patients on long-term treatment of zolpidem (defined as > 1 month, with average around 9.9 ± 6.2 years) experienced only 1 or 2 nights of rebound insomnia during a month-long taper.1,6 Following that, they experienced improvements in initiation and staying asleep.
A possible explanation for the lack of dependence or withdrawal symptoms in patients chronically treated with zolpidem is the pharmacokinetic profile. While the selectivity of the binding sites differentiates this medication from benzodiazepines, the additional fact of a short half-life, and no repeated dosing throughout the day, likely limit the risk of experiencing withdrawal symptoms.1 The daily periods of minimal zolpidem exposure in the body may limit the amount of physical dependence.
Continue to: Discontinuation of zolpidem
Discontinuation of zolpidem
The 49-year-old man had a history of failed abrupt discontinuation of zolpidem in the past (without noted withdrawal symptoms). Thus, various benzodiazepine taper resources were consulted to develop a taper schedule.
We switched our patient from the zolpidem CR 12.5 mg nightly to 10 mg of the IR formulation, and the pharmacists proposed 20% to 43% weekly decreases in dosing based on dosage strengths. At the initial 3-day follow-up (having taken 10 mg nightly for 3 days), the patient reported a quicker onset of sleep but an inability to sleep through the night. The patient denied withdrawal symptoms or any significant impact to his daily routines. These results encouraged a progression to the next step of the taper. For the next 9 days, the patient took 5 mg nightly, rather than the pharmacist-advised dosing of alternating 5 mg and 10 mg nightly, and reported similar outcomes at his next visit.
This success led to the discontinuation of scheduled zolpidem. The patient was also given a prescription of 2.5 mg, as needed, if insomnia rebounded. No adverse effects were noted despite the accelerated taper. Based on patient response and motivation, the taper had progressed more quickly than scheduled, resulting in 3 days of 10 mg, 9 days of 5 mg, and 1 final day of 2.5 mg that was used when the patient had trouble falling asleep. At the 6-month follow-up, the patient informed the physician that he had neither experienced insomnia nor used any further medication.
THE TAKEAWAY
This case documents a successfully accelerated taper for a patient with a chronic history (> 5 years) of zolpidem use. Although withdrawal is often patient specific, this case suggests the risk is low despite the chronic usage. This further adds to the literature suggesting against the need for an extended taper, and possibly a taper at all, when using recommended doses of chronic zolpidem. This is a significant difference compared to past practices that drew from literature-based benzodiazepine tapers.6 This case serves as an observational point of reference for clinicians who are assisting patients with chronic zolpidem tapers.
CORRESPONDENCE
Logan Franck, PharmD, 986145 Nebraska Medical Center, Omaha, NE 68198-6145; [email protected]
1. Lähteenmäki R, Neuvonen PJ, Puustinen J, et al. Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol. 2019;124:330-340. doi: 10.1111/bcpt.13144
2. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
3. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. 2014;16:e19926. doi: 10.5812/ircmj.19926
4. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142-153. doi: 10.2165/00003088-199529030-00002
5. Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088-1095. doi: 10.1177/0269881111424455
6. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295-301. doi: 10.1111/j.1365-2125.2012.04418.x
1. Lähteenmäki R, Neuvonen PJ, Puustinen J, et al. Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol. 2019;124:330-340. doi: 10.1111/bcpt.13144
2. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
3. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. 2014;16:e19926. doi: 10.5812/ircmj.19926
4. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142-153. doi: 10.2165/00003088-199529030-00002
5. Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088-1095. doi: 10.1177/0269881111424455
6. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295-301. doi: 10.1111/j.1365-2125.2012.04418.x
What to do about pandemic PTSD
When the COVID-19 pandemic engulfed the nation well over a year ago, Rebecca Hendrickson, MD, PhD, immersed herself in the shell-shocking revelations that clinicians began posting on social media. The accounts offered just a snapshot of the pandemic’s heavy psychological toll, and Dr. Hendrickson, a psychiatrist at the University of Washington in Seattle and an expert in posttraumatic stress disorder (PTSD), wanted to know more.
She and her colleagues devised a survey to assess the impact of several pandemic-related factors, including increased work hours, social distancing restrictions, and lack of adequate personal protective equipment.
What began as a survey of health care workers soon expanded in scope. Of the more than 600 survey respondents to date, health care workers account for about 60%, while the rest are first responders – police officers, firefighters, paramedics, and emergency medical technicians – and nonclinical personnel, such as security guards and office staff, in health care settings. The respondents range in age from 19 to 72, and hail from all regions of the country.
“Our findings were really striking,” Dr. Hendrickson said, “including very high rates of thoughts of suicide and thoughts of leaving one’s current field, which were both strongly linked to COVID-19–related occupational stress exposure.”
The distress stemmed from a multitude of factors. Among the most demoralizing: witnessing patients die in isolation and being stretched thin to provide optimal care for all patients amid an unrelenting onslaught of COVID-19 cases, she said. For some health care workers, living in the garage or basement – to avoid infecting family members with the virus – also wore on their psyches.
Of all health care workers in the study, more than three-quarters reported symptoms that fell within the clinical range for depression (76%) and anxiety (78%). More than 25% noted that they had lost a family member or close colleague to the virus.
Dr. Hendrickson, who works with military veterans at the VA Puget Sound Hospital System’s Mental Illness Research, Education, and Clinical Center and its PTSD outpatient clinic, hadn’t expected the experience of loss to be so pervasive. She said the sheer number of people who “crossed the threshold” into despair concerned her deeply.
Signs and symptoms of PTSD
PTSD’s prevalence among health care workers has always been variable, said Jessica Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry at Washington University in St. Louis.
As a psychiatrist who sees health care workers in her clinical practice, Dr. Gold has noted poor baseline mental health, including depression and trauma. Significant data have pointed to a relatively higher suicide rate among physicians than among the general population. These problems have been compounded by COVID-19.
“It has been an unrelenting series of new stressors,” she said, citing lack of resources; a feeling of being unable to help; and the high frequency of risk of death to patients, family and friends, and the caregivers themselves as just as few examples. “It is very likely going to increase our baseline trauma, and honestly, I don’t know that we can predict how. To me, ”
PTSD can manifest itself in health care workers in several different ways. A few commonalities Dr. Gold has observed are sleep disruption (including insomnia and nightmares), work avoidance by taking disability or quitting, irritability or other changes in mood, trouble concentrating, and hypervigilance.
She said she has seen physical manifestations of trauma – such as body pain, stomachaches, and teeth grinding, which “you might not realize are at all related to trauma but ultimately are.” Sometimes, she added, “people have panic attacks on the way to work or right when they get to work, or are thinking about work.”
Dr. Gold noted that different types of treatment, such as cognitive-behavioral therapy and eye movement desensitization and reprocessing (EMDR), can be effective for PTSD. Medication is often necessary because of comorbid anxiety, depression, or eating disorders, said Dr. Gold, who is conducting a study on the pandemic’s effects on medical students.
The difficulties in isolating COVID-19 as a contributor
Not all researchers are convinced that a causal relationship has been established between the pandemic and worsening mental health among those in the health care sector.
With provider burnout being a long-standing concern in medicine, Ankur A. Butala, MD, assistant professor of neurology, psychiatry, and behavioral sciences at the Johns Hopkins University, Baltimore, said he remains a bit skeptical that acute stressors during the pandemic amounted to a uniquely potent driving force that can be extrapolated and quantified in a study.
“It’s hard to interpret a chronic, rolling, ongoing trauma like COVID-19 against tools or scales developed to investigate symptoms from a singular and acute trauma, like a school shooting or a [military] firefight,” Dr. Butala said.
In addition, he noted a reluctance to generalizing results from a study in which participants were recruited via social media as opposed to research methods involving more rigorous selection protocols.
Although Dr. Hendrickson acknowledged the study’s limitations, she said her team nonetheless found strong correlations between COVID-19-related stressors and self-reported struggles in completing work-related tasks, as well as increasing thoughts of leaving one’s current field. They adjusted for previous lifetime trauma exposure, age, gender, and a personal history of contracting COVID-19.
The underlying premise of the study could be confirmed with repeated surveys over time, Dr. Butala said, as the COVID-19 pandemic evolves and the vaccination effort unfolds.
Follow-up surveys are being sent to participants every 2 weeks and every 3 months to gauge their mood, for a total follow-up period of 9 months per individual. New participants are still welcome. “We will continue to enroll as long as it seems relevant,” Dr. Hendrickson said.
Carol S. North, MD, MPE, who has added to the growing research on the pandemic’s toll on mental health, noted that because symptom scales do not provide psychiatric diagnoses, it is difficult to attribute the prevalence of psychiatric disorders to the pandemic. Dr. North is chair and professor of crisis psychiatry at UT Southwestern Medical Center in Dallas, and director of the program in trauma and disaster at VA North Texas Health Care System.
The DSM-5 criteria exclude naturally occurring illness, such as a virus (even during a pandemic) as a qualifying trauma for the diagnosis of PTSD. According to current criteria by the American Psychiatric Association, COVID-19 and the pandemic are not defined as trauma, Dr. North said, while noting that “just because it’s not trauma or PTSD does not mean that the pandemic should be discounted as not stressful; people are finding it very stressful.”
Identifying the exact source of distress would still be difficult, Dr. North said, as the pandemic has produced severe economic consequences and prolonged social isolation, as well as occurring alongside nationwide protests over racial and ethnic divisions. Studies to date haven’t effectively separated out for these stressors, making it impossible to weigh their relative impact.
Furthermore, “most of us face many other stressors in our daily lives, such as grief, losses, broken relationships, and personal failures,” she said. “All of these may contribute to psychological distress, and research is needed to determine how much was a product of the virus, other aspects of the pandemic, or unrelated life stressors.”
A rallying cry for new interventions
Despite such doubts, a growing number of studies are reporting that health care workers and first responders are experiencing intensified PTSD, depression, anxiety, and insomnia as a result of the pandemic, said Hrayr Pierre Attarian, MD, professor of neurology at Northwestern University, Chicago. These results should act as a rallying cry for implementing more policies tailored to prevent burnout, he said.
“What we are seeing during this terrible pandemic is burnout on steroids,” said Dr. Attarian, medical director of Northwestern’s Center for Sleep Disorders. There are already high burnout rates, “so this should be doubly important.”
Rooting out this problem starts at the institutional level, but merely advising providers to “be well” wouldn’t make inroads. “There needs to be fluid dialogue between health care workers and the leadership,” he said.
Among his proposed remedies: Access to confidential and free mental health resources, increased administrative support, flexible hours, respect for work-life balance, and forgiveness for occasional errors that don’t result in harm.
“Sometimes even the perception that a mistake has been made is taken as proof of guilt,” Dr. Attarian said. “It is not conducive to wellness. Extra income does not replace a nurturing work environment.”
Furthermore, “as a profession, we must stop glorifying ‘overwork.’ We must stop wearing ‘lack of sleep’ as badge of honor,” he said. “Sleep is a biological imperative like self-preservation, hunger, and thirst. When we don’t sleep anxiety, pain, and depression get amplified. Our perception of distress is off, as is our judgment.”
The Federation of State Physician Health Programs provides a directory that physicians can use for referrals to confidential consultation or treatment.
Christopher Bundy, MD, MPH, executive medical director of Washington Physicians Health Program in Seattle, has been following Dr. Hendrickson’s longitudinal study with keen interest. As president of the Federation of State Physician Health Programs, he hopes to translate the findings into practice.
“Obviously, the COVID-19 pandemic has been a ‘black swan’ in terms of workforce sustainability issues,” Dr. Bundy said, citing “high rates of burnout, disillusionment, and dissatisfaction.” He sees some similarities with his former role in treating war veterans.
“The invisible wounds of combat, the psychological scars don’t really become apparent until after you’re out of the war zone,” said Dr. Bundy, clinical associate professor of psychiatry at the University of Washington.
Likewise, he expects the “emotional chickens will come home to roost as the pandemic subsides.” Until then, “people are just focused on survival, and in doing their jobs and protecting their patients.” Eventually, “their own wounds inside the pandemic will take hold.”
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic engulfed the nation well over a year ago, Rebecca Hendrickson, MD, PhD, immersed herself in the shell-shocking revelations that clinicians began posting on social media. The accounts offered just a snapshot of the pandemic’s heavy psychological toll, and Dr. Hendrickson, a psychiatrist at the University of Washington in Seattle and an expert in posttraumatic stress disorder (PTSD), wanted to know more.
She and her colleagues devised a survey to assess the impact of several pandemic-related factors, including increased work hours, social distancing restrictions, and lack of adequate personal protective equipment.
What began as a survey of health care workers soon expanded in scope. Of the more than 600 survey respondents to date, health care workers account for about 60%, while the rest are first responders – police officers, firefighters, paramedics, and emergency medical technicians – and nonclinical personnel, such as security guards and office staff, in health care settings. The respondents range in age from 19 to 72, and hail from all regions of the country.
“Our findings were really striking,” Dr. Hendrickson said, “including very high rates of thoughts of suicide and thoughts of leaving one’s current field, which were both strongly linked to COVID-19–related occupational stress exposure.”
The distress stemmed from a multitude of factors. Among the most demoralizing: witnessing patients die in isolation and being stretched thin to provide optimal care for all patients amid an unrelenting onslaught of COVID-19 cases, she said. For some health care workers, living in the garage or basement – to avoid infecting family members with the virus – also wore on their psyches.
Of all health care workers in the study, more than three-quarters reported symptoms that fell within the clinical range for depression (76%) and anxiety (78%). More than 25% noted that they had lost a family member or close colleague to the virus.
Dr. Hendrickson, who works with military veterans at the VA Puget Sound Hospital System’s Mental Illness Research, Education, and Clinical Center and its PTSD outpatient clinic, hadn’t expected the experience of loss to be so pervasive. She said the sheer number of people who “crossed the threshold” into despair concerned her deeply.
Signs and symptoms of PTSD
PTSD’s prevalence among health care workers has always been variable, said Jessica Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry at Washington University in St. Louis.
As a psychiatrist who sees health care workers in her clinical practice, Dr. Gold has noted poor baseline mental health, including depression and trauma. Significant data have pointed to a relatively higher suicide rate among physicians than among the general population. These problems have been compounded by COVID-19.
“It has been an unrelenting series of new stressors,” she said, citing lack of resources; a feeling of being unable to help; and the high frequency of risk of death to patients, family and friends, and the caregivers themselves as just as few examples. “It is very likely going to increase our baseline trauma, and honestly, I don’t know that we can predict how. To me, ”
PTSD can manifest itself in health care workers in several different ways. A few commonalities Dr. Gold has observed are sleep disruption (including insomnia and nightmares), work avoidance by taking disability or quitting, irritability or other changes in mood, trouble concentrating, and hypervigilance.
She said she has seen physical manifestations of trauma – such as body pain, stomachaches, and teeth grinding, which “you might not realize are at all related to trauma but ultimately are.” Sometimes, she added, “people have panic attacks on the way to work or right when they get to work, or are thinking about work.”
Dr. Gold noted that different types of treatment, such as cognitive-behavioral therapy and eye movement desensitization and reprocessing (EMDR), can be effective for PTSD. Medication is often necessary because of comorbid anxiety, depression, or eating disorders, said Dr. Gold, who is conducting a study on the pandemic’s effects on medical students.
The difficulties in isolating COVID-19 as a contributor
Not all researchers are convinced that a causal relationship has been established between the pandemic and worsening mental health among those in the health care sector.
With provider burnout being a long-standing concern in medicine, Ankur A. Butala, MD, assistant professor of neurology, psychiatry, and behavioral sciences at the Johns Hopkins University, Baltimore, said he remains a bit skeptical that acute stressors during the pandemic amounted to a uniquely potent driving force that can be extrapolated and quantified in a study.
“It’s hard to interpret a chronic, rolling, ongoing trauma like COVID-19 against tools or scales developed to investigate symptoms from a singular and acute trauma, like a school shooting or a [military] firefight,” Dr. Butala said.
In addition, he noted a reluctance to generalizing results from a study in which participants were recruited via social media as opposed to research methods involving more rigorous selection protocols.
Although Dr. Hendrickson acknowledged the study’s limitations, she said her team nonetheless found strong correlations between COVID-19-related stressors and self-reported struggles in completing work-related tasks, as well as increasing thoughts of leaving one’s current field. They adjusted for previous lifetime trauma exposure, age, gender, and a personal history of contracting COVID-19.
The underlying premise of the study could be confirmed with repeated surveys over time, Dr. Butala said, as the COVID-19 pandemic evolves and the vaccination effort unfolds.
Follow-up surveys are being sent to participants every 2 weeks and every 3 months to gauge their mood, for a total follow-up period of 9 months per individual. New participants are still welcome. “We will continue to enroll as long as it seems relevant,” Dr. Hendrickson said.
Carol S. North, MD, MPE, who has added to the growing research on the pandemic’s toll on mental health, noted that because symptom scales do not provide psychiatric diagnoses, it is difficult to attribute the prevalence of psychiatric disorders to the pandemic. Dr. North is chair and professor of crisis psychiatry at UT Southwestern Medical Center in Dallas, and director of the program in trauma and disaster at VA North Texas Health Care System.
The DSM-5 criteria exclude naturally occurring illness, such as a virus (even during a pandemic) as a qualifying trauma for the diagnosis of PTSD. According to current criteria by the American Psychiatric Association, COVID-19 and the pandemic are not defined as trauma, Dr. North said, while noting that “just because it’s not trauma or PTSD does not mean that the pandemic should be discounted as not stressful; people are finding it very stressful.”
Identifying the exact source of distress would still be difficult, Dr. North said, as the pandemic has produced severe economic consequences and prolonged social isolation, as well as occurring alongside nationwide protests over racial and ethnic divisions. Studies to date haven’t effectively separated out for these stressors, making it impossible to weigh their relative impact.
Furthermore, “most of us face many other stressors in our daily lives, such as grief, losses, broken relationships, and personal failures,” she said. “All of these may contribute to psychological distress, and research is needed to determine how much was a product of the virus, other aspects of the pandemic, or unrelated life stressors.”
A rallying cry for new interventions
Despite such doubts, a growing number of studies are reporting that health care workers and first responders are experiencing intensified PTSD, depression, anxiety, and insomnia as a result of the pandemic, said Hrayr Pierre Attarian, MD, professor of neurology at Northwestern University, Chicago. These results should act as a rallying cry for implementing more policies tailored to prevent burnout, he said.
“What we are seeing during this terrible pandemic is burnout on steroids,” said Dr. Attarian, medical director of Northwestern’s Center for Sleep Disorders. There are already high burnout rates, “so this should be doubly important.”
Rooting out this problem starts at the institutional level, but merely advising providers to “be well” wouldn’t make inroads. “There needs to be fluid dialogue between health care workers and the leadership,” he said.
Among his proposed remedies: Access to confidential and free mental health resources, increased administrative support, flexible hours, respect for work-life balance, and forgiveness for occasional errors that don’t result in harm.
“Sometimes even the perception that a mistake has been made is taken as proof of guilt,” Dr. Attarian said. “It is not conducive to wellness. Extra income does not replace a nurturing work environment.”
Furthermore, “as a profession, we must stop glorifying ‘overwork.’ We must stop wearing ‘lack of sleep’ as badge of honor,” he said. “Sleep is a biological imperative like self-preservation, hunger, and thirst. When we don’t sleep anxiety, pain, and depression get amplified. Our perception of distress is off, as is our judgment.”
The Federation of State Physician Health Programs provides a directory that physicians can use for referrals to confidential consultation or treatment.
Christopher Bundy, MD, MPH, executive medical director of Washington Physicians Health Program in Seattle, has been following Dr. Hendrickson’s longitudinal study with keen interest. As president of the Federation of State Physician Health Programs, he hopes to translate the findings into practice.
“Obviously, the COVID-19 pandemic has been a ‘black swan’ in terms of workforce sustainability issues,” Dr. Bundy said, citing “high rates of burnout, disillusionment, and dissatisfaction.” He sees some similarities with his former role in treating war veterans.
“The invisible wounds of combat, the psychological scars don’t really become apparent until after you’re out of the war zone,” said Dr. Bundy, clinical associate professor of psychiatry at the University of Washington.
Likewise, he expects the “emotional chickens will come home to roost as the pandemic subsides.” Until then, “people are just focused on survival, and in doing their jobs and protecting their patients.” Eventually, “their own wounds inside the pandemic will take hold.”
A version of this article first appeared on Medscape.com.
When the COVID-19 pandemic engulfed the nation well over a year ago, Rebecca Hendrickson, MD, PhD, immersed herself in the shell-shocking revelations that clinicians began posting on social media. The accounts offered just a snapshot of the pandemic’s heavy psychological toll, and Dr. Hendrickson, a psychiatrist at the University of Washington in Seattle and an expert in posttraumatic stress disorder (PTSD), wanted to know more.
She and her colleagues devised a survey to assess the impact of several pandemic-related factors, including increased work hours, social distancing restrictions, and lack of adequate personal protective equipment.
What began as a survey of health care workers soon expanded in scope. Of the more than 600 survey respondents to date, health care workers account for about 60%, while the rest are first responders – police officers, firefighters, paramedics, and emergency medical technicians – and nonclinical personnel, such as security guards and office staff, in health care settings. The respondents range in age from 19 to 72, and hail from all regions of the country.
“Our findings were really striking,” Dr. Hendrickson said, “including very high rates of thoughts of suicide and thoughts of leaving one’s current field, which were both strongly linked to COVID-19–related occupational stress exposure.”
The distress stemmed from a multitude of factors. Among the most demoralizing: witnessing patients die in isolation and being stretched thin to provide optimal care for all patients amid an unrelenting onslaught of COVID-19 cases, she said. For some health care workers, living in the garage or basement – to avoid infecting family members with the virus – also wore on their psyches.
Of all health care workers in the study, more than three-quarters reported symptoms that fell within the clinical range for depression (76%) and anxiety (78%). More than 25% noted that they had lost a family member or close colleague to the virus.
Dr. Hendrickson, who works with military veterans at the VA Puget Sound Hospital System’s Mental Illness Research, Education, and Clinical Center and its PTSD outpatient clinic, hadn’t expected the experience of loss to be so pervasive. She said the sheer number of people who “crossed the threshold” into despair concerned her deeply.
Signs and symptoms of PTSD
PTSD’s prevalence among health care workers has always been variable, said Jessica Gold, MD, assistant professor and director of wellness, engagement, and outreach in the department of psychiatry at Washington University in St. Louis.
As a psychiatrist who sees health care workers in her clinical practice, Dr. Gold has noted poor baseline mental health, including depression and trauma. Significant data have pointed to a relatively higher suicide rate among physicians than among the general population. These problems have been compounded by COVID-19.
“It has been an unrelenting series of new stressors,” she said, citing lack of resources; a feeling of being unable to help; and the high frequency of risk of death to patients, family and friends, and the caregivers themselves as just as few examples. “It is very likely going to increase our baseline trauma, and honestly, I don’t know that we can predict how. To me, ”
PTSD can manifest itself in health care workers in several different ways. A few commonalities Dr. Gold has observed are sleep disruption (including insomnia and nightmares), work avoidance by taking disability or quitting, irritability or other changes in mood, trouble concentrating, and hypervigilance.
She said she has seen physical manifestations of trauma – such as body pain, stomachaches, and teeth grinding, which “you might not realize are at all related to trauma but ultimately are.” Sometimes, she added, “people have panic attacks on the way to work or right when they get to work, or are thinking about work.”
Dr. Gold noted that different types of treatment, such as cognitive-behavioral therapy and eye movement desensitization and reprocessing (EMDR), can be effective for PTSD. Medication is often necessary because of comorbid anxiety, depression, or eating disorders, said Dr. Gold, who is conducting a study on the pandemic’s effects on medical students.
The difficulties in isolating COVID-19 as a contributor
Not all researchers are convinced that a causal relationship has been established between the pandemic and worsening mental health among those in the health care sector.
With provider burnout being a long-standing concern in medicine, Ankur A. Butala, MD, assistant professor of neurology, psychiatry, and behavioral sciences at the Johns Hopkins University, Baltimore, said he remains a bit skeptical that acute stressors during the pandemic amounted to a uniquely potent driving force that can be extrapolated and quantified in a study.
“It’s hard to interpret a chronic, rolling, ongoing trauma like COVID-19 against tools or scales developed to investigate symptoms from a singular and acute trauma, like a school shooting or a [military] firefight,” Dr. Butala said.
In addition, he noted a reluctance to generalizing results from a study in which participants were recruited via social media as opposed to research methods involving more rigorous selection protocols.
Although Dr. Hendrickson acknowledged the study’s limitations, she said her team nonetheless found strong correlations between COVID-19-related stressors and self-reported struggles in completing work-related tasks, as well as increasing thoughts of leaving one’s current field. They adjusted for previous lifetime trauma exposure, age, gender, and a personal history of contracting COVID-19.
The underlying premise of the study could be confirmed with repeated surveys over time, Dr. Butala said, as the COVID-19 pandemic evolves and the vaccination effort unfolds.
Follow-up surveys are being sent to participants every 2 weeks and every 3 months to gauge their mood, for a total follow-up period of 9 months per individual. New participants are still welcome. “We will continue to enroll as long as it seems relevant,” Dr. Hendrickson said.
Carol S. North, MD, MPE, who has added to the growing research on the pandemic’s toll on mental health, noted that because symptom scales do not provide psychiatric diagnoses, it is difficult to attribute the prevalence of psychiatric disorders to the pandemic. Dr. North is chair and professor of crisis psychiatry at UT Southwestern Medical Center in Dallas, and director of the program in trauma and disaster at VA North Texas Health Care System.
The DSM-5 criteria exclude naturally occurring illness, such as a virus (even during a pandemic) as a qualifying trauma for the diagnosis of PTSD. According to current criteria by the American Psychiatric Association, COVID-19 and the pandemic are not defined as trauma, Dr. North said, while noting that “just because it’s not trauma or PTSD does not mean that the pandemic should be discounted as not stressful; people are finding it very stressful.”
Identifying the exact source of distress would still be difficult, Dr. North said, as the pandemic has produced severe economic consequences and prolonged social isolation, as well as occurring alongside nationwide protests over racial and ethnic divisions. Studies to date haven’t effectively separated out for these stressors, making it impossible to weigh their relative impact.
Furthermore, “most of us face many other stressors in our daily lives, such as grief, losses, broken relationships, and personal failures,” she said. “All of these may contribute to psychological distress, and research is needed to determine how much was a product of the virus, other aspects of the pandemic, or unrelated life stressors.”
A rallying cry for new interventions
Despite such doubts, a growing number of studies are reporting that health care workers and first responders are experiencing intensified PTSD, depression, anxiety, and insomnia as a result of the pandemic, said Hrayr Pierre Attarian, MD, professor of neurology at Northwestern University, Chicago. These results should act as a rallying cry for implementing more policies tailored to prevent burnout, he said.
“What we are seeing during this terrible pandemic is burnout on steroids,” said Dr. Attarian, medical director of Northwestern’s Center for Sleep Disorders. There are already high burnout rates, “so this should be doubly important.”
Rooting out this problem starts at the institutional level, but merely advising providers to “be well” wouldn’t make inroads. “There needs to be fluid dialogue between health care workers and the leadership,” he said.
Among his proposed remedies: Access to confidential and free mental health resources, increased administrative support, flexible hours, respect for work-life balance, and forgiveness for occasional errors that don’t result in harm.
“Sometimes even the perception that a mistake has been made is taken as proof of guilt,” Dr. Attarian said. “It is not conducive to wellness. Extra income does not replace a nurturing work environment.”
Furthermore, “as a profession, we must stop glorifying ‘overwork.’ We must stop wearing ‘lack of sleep’ as badge of honor,” he said. “Sleep is a biological imperative like self-preservation, hunger, and thirst. When we don’t sleep anxiety, pain, and depression get amplified. Our perception of distress is off, as is our judgment.”
The Federation of State Physician Health Programs provides a directory that physicians can use for referrals to confidential consultation or treatment.
Christopher Bundy, MD, MPH, executive medical director of Washington Physicians Health Program in Seattle, has been following Dr. Hendrickson’s longitudinal study with keen interest. As president of the Federation of State Physician Health Programs, he hopes to translate the findings into practice.
“Obviously, the COVID-19 pandemic has been a ‘black swan’ in terms of workforce sustainability issues,” Dr. Bundy said, citing “high rates of burnout, disillusionment, and dissatisfaction.” He sees some similarities with his former role in treating war veterans.
“The invisible wounds of combat, the psychological scars don’t really become apparent until after you’re out of the war zone,” said Dr. Bundy, clinical associate professor of psychiatry at the University of Washington.
Likewise, he expects the “emotional chickens will come home to roost as the pandemic subsides.” Until then, “people are just focused on survival, and in doing their jobs and protecting their patients.” Eventually, “their own wounds inside the pandemic will take hold.”
A version of this article first appeared on Medscape.com.
Do adolescents develop CNS autoimmunity after COVID-19?
Recent research suggests that some pediatric patients who develop neuropsychiatric symptoms from COVID-19 may have intrathecal antineural SARS-CoV-2 autoantibodies, which may hint at central nervous system (CNS) autoimmunity in these patients.
“Overall, these findings indicate that severe neuropsychiatric symptoms can occur in the setting of pediatric COVID-19, including patients who lack many of the cardinal systemic features,” Christopher M. Bartley, MD, PhD, of the Weill Institute for Neurosciences at the University of California, San Francisco, and colleagues wrote in their study. “These data highlight the possibility of SARS-CoV-2 neuroinvasion and/or CNS autoimmunity in pediatric patients with COVID-19 and neuropsychiatric symptoms.”
In a case series published Oct. 25 in JAMA Neurology (doi: 10.1001/jamaneurol.2021.3821), Dr. Bartley and colleagues examined three pediatric patients who were infected with SARS-CoV-2 and, over a period of 5 months in 2020, were admitted to the hospital – where they received a neurology consultation for “subacute, functionally impairing behavioral changes.”
Patient 1 had a history of unspecified anxiety and depression, and was admitted for erratic behavior, paranoia-like fears, social withdrawal, and insomnia. The patient did not respond to treatment with risperidone and gabapentin, and was readmitted soon after discharge, then treated with olanzapine followed by a transition to valproate and lorazepam. It was found the patient had cerebrospinal fluid (CSF) abnormalities in the form of elevated protein levels, and an elevated IgG index, and was given intravenous immunoglobulin followed by IV methylprednisolone. While symptoms such as paranoia improved and the patient was able to better organize thoughts after 5 days, other symptoms such as delusions and hyperreflexia persisted for at least 1 month before resolving, and some symptoms, such as lability, did not resolve before discharge.
Patient 2 had a history of motor tics and anxiety, but showed signs of insomnia, mood lability, impaired concentration, difficulty finding words, and problems completing homework following a SARS-CoV-2 infection. The patient’s father previously had been diagnosed with COVID-19 and the patient developed respiratory symptoms and fever; an IgG serology test later confirmed a SARS-CoV-2 infection. The patient went on to experience internal preoccupation, aggression, and suicidal ideation. The patients was treated with aripiprazole and risperidone, but did not respond, and was admitted to the hospital. As with patient 1, patient 2 had CSF abnormalities in the form of elevated protein levels, and responded to IV methylprednisolone, with working memory and bradyphrenia improving. However, the patient developed insomnia, extreme anxiety, suicidal ideation, aggression, and sadness after discharge, and was readmitted. The patient was treated with IV immunoglobulin, and discharged with quetiapine and lithium.
“Six months later, although improved from initial presentation, the patient required academic accommodations and continued to endorse forgetfulness and attention difficulties. The patient’s chronic tics and anxiety were unchanged,” Dr. Bartley and colleagues wrote.
Patient 3 had no psychiatric history but started to demonstrate “odd behavior, including repetitive behaviors, anorexia, and insomnia” following a SARS-CoV-2 infection. After being hospitalized, the patient showed signs of “ideomotor apraxia, abulia, disorganized behavior, agitation, and diffusely brisk reflexes” and had a high white blood cell count, creatine kinase level, and C-reactive protein level. CSF was also abnormal for this patient, with three unique oligoclonal bands identified. The patient was treated with lorazepam and olanzapine, did not receive immunotherapy, and was discharged without psychiatric medications after 4 days.
When the researchers performed testing on each of the three patients, they found intrathecal anti–SARS-CoV-2 IgG and immunostained mouse brain tissue, and “a diverse set of candidate autoantigens by human phage immunoprecipitation sequencing” in patient 1 and patient 2. In comparison, patient 3 “neither appreciably immunostained nor enriched candidates by human phage immunoprecipitation sequencing,” the researchers said.
“ and the potential for immunotherapy in some,” Dr. Bartley and colleagues concluded.
Potential of CNS autoimmunity
Evan J. Kyzar, MD, PhD, a resident physician in psychiatry at New York State Psychiatric Institute in New York Presbyterian–Columbia Campus, said in an interview that the results of the case series show some pediatric patients with neuropsychiatric symptoms can have anti-SARS-CoV-2 antibodies after viral clearance.
“Interestingly, some of the patients in this study also had antibodies in the CSF that targeted native proteins, demonstrating that COVID-19 may lead to autoimmunity directed at the brain,” he said. “This study increases our knowledge of how COVID-19 interacts with the nervous system and how autoimmune mechanisms might be contributing to at least a portion of patients with neuropsychiatric symptoms during acute infection, and possibly even after viral clearance.”
Dr. Kyzar noted that the immunological methods in the study were “cutting-edge” and the validation exploring the immune responses was detailed, but was limited because of the small sample size.
“[T]he researchers are using similar techniques to explore psychiatric disorders such as depression and schizophrenia to determine if some patients diagnosed with these conditions may have CNS-targeting autoantibodies that contribute to their symptoms and clinical presentation,” Dr. Kyzar said. “This work has the potential to discover novel neuroimmune mechanisms contributing to neuropsychiatric disease and offer possible pathways for the discovery of new treatments.”
The authors reported financial relationships with Allen & Company, the Chan Zuckerberg Initiative, National Institutes of Health, Novartis, Public Health Company, Roche/Genentech, Sandler Foundation, and Takeda in the form of grants and personal fees. They reported funding and/or support from the Brain Research Foundation, Hanna H. Gray Fellowship, Howard Hughes Medical Institute, John A. Watson Scholar Program, Latinx Center of Excellence, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, President’s Postdoctoral Fellowship Program, and Shared Instrumentation grant. Dr. Kyzar reported no relevant financial disclosures.
Recent research suggests that some pediatric patients who develop neuropsychiatric symptoms from COVID-19 may have intrathecal antineural SARS-CoV-2 autoantibodies, which may hint at central nervous system (CNS) autoimmunity in these patients.
“Overall, these findings indicate that severe neuropsychiatric symptoms can occur in the setting of pediatric COVID-19, including patients who lack many of the cardinal systemic features,” Christopher M. Bartley, MD, PhD, of the Weill Institute for Neurosciences at the University of California, San Francisco, and colleagues wrote in their study. “These data highlight the possibility of SARS-CoV-2 neuroinvasion and/or CNS autoimmunity in pediatric patients with COVID-19 and neuropsychiatric symptoms.”
In a case series published Oct. 25 in JAMA Neurology (doi: 10.1001/jamaneurol.2021.3821), Dr. Bartley and colleagues examined three pediatric patients who were infected with SARS-CoV-2 and, over a period of 5 months in 2020, were admitted to the hospital – where they received a neurology consultation for “subacute, functionally impairing behavioral changes.”
Patient 1 had a history of unspecified anxiety and depression, and was admitted for erratic behavior, paranoia-like fears, social withdrawal, and insomnia. The patient did not respond to treatment with risperidone and gabapentin, and was readmitted soon after discharge, then treated with olanzapine followed by a transition to valproate and lorazepam. It was found the patient had cerebrospinal fluid (CSF) abnormalities in the form of elevated protein levels, and an elevated IgG index, and was given intravenous immunoglobulin followed by IV methylprednisolone. While symptoms such as paranoia improved and the patient was able to better organize thoughts after 5 days, other symptoms such as delusions and hyperreflexia persisted for at least 1 month before resolving, and some symptoms, such as lability, did not resolve before discharge.
Patient 2 had a history of motor tics and anxiety, but showed signs of insomnia, mood lability, impaired concentration, difficulty finding words, and problems completing homework following a SARS-CoV-2 infection. The patient’s father previously had been diagnosed with COVID-19 and the patient developed respiratory symptoms and fever; an IgG serology test later confirmed a SARS-CoV-2 infection. The patient went on to experience internal preoccupation, aggression, and suicidal ideation. The patients was treated with aripiprazole and risperidone, but did not respond, and was admitted to the hospital. As with patient 1, patient 2 had CSF abnormalities in the form of elevated protein levels, and responded to IV methylprednisolone, with working memory and bradyphrenia improving. However, the patient developed insomnia, extreme anxiety, suicidal ideation, aggression, and sadness after discharge, and was readmitted. The patient was treated with IV immunoglobulin, and discharged with quetiapine and lithium.
“Six months later, although improved from initial presentation, the patient required academic accommodations and continued to endorse forgetfulness and attention difficulties. The patient’s chronic tics and anxiety were unchanged,” Dr. Bartley and colleagues wrote.
Patient 3 had no psychiatric history but started to demonstrate “odd behavior, including repetitive behaviors, anorexia, and insomnia” following a SARS-CoV-2 infection. After being hospitalized, the patient showed signs of “ideomotor apraxia, abulia, disorganized behavior, agitation, and diffusely brisk reflexes” and had a high white blood cell count, creatine kinase level, and C-reactive protein level. CSF was also abnormal for this patient, with three unique oligoclonal bands identified. The patient was treated with lorazepam and olanzapine, did not receive immunotherapy, and was discharged without psychiatric medications after 4 days.
When the researchers performed testing on each of the three patients, they found intrathecal anti–SARS-CoV-2 IgG and immunostained mouse brain tissue, and “a diverse set of candidate autoantigens by human phage immunoprecipitation sequencing” in patient 1 and patient 2. In comparison, patient 3 “neither appreciably immunostained nor enriched candidates by human phage immunoprecipitation sequencing,” the researchers said.
“ and the potential for immunotherapy in some,” Dr. Bartley and colleagues concluded.
Potential of CNS autoimmunity
Evan J. Kyzar, MD, PhD, a resident physician in psychiatry at New York State Psychiatric Institute in New York Presbyterian–Columbia Campus, said in an interview that the results of the case series show some pediatric patients with neuropsychiatric symptoms can have anti-SARS-CoV-2 antibodies after viral clearance.
“Interestingly, some of the patients in this study also had antibodies in the CSF that targeted native proteins, demonstrating that COVID-19 may lead to autoimmunity directed at the brain,” he said. “This study increases our knowledge of how COVID-19 interacts with the nervous system and how autoimmune mechanisms might be contributing to at least a portion of patients with neuropsychiatric symptoms during acute infection, and possibly even after viral clearance.”
Dr. Kyzar noted that the immunological methods in the study were “cutting-edge” and the validation exploring the immune responses was detailed, but was limited because of the small sample size.
“[T]he researchers are using similar techniques to explore psychiatric disorders such as depression and schizophrenia to determine if some patients diagnosed with these conditions may have CNS-targeting autoantibodies that contribute to their symptoms and clinical presentation,” Dr. Kyzar said. “This work has the potential to discover novel neuroimmune mechanisms contributing to neuropsychiatric disease and offer possible pathways for the discovery of new treatments.”
The authors reported financial relationships with Allen & Company, the Chan Zuckerberg Initiative, National Institutes of Health, Novartis, Public Health Company, Roche/Genentech, Sandler Foundation, and Takeda in the form of grants and personal fees. They reported funding and/or support from the Brain Research Foundation, Hanna H. Gray Fellowship, Howard Hughes Medical Institute, John A. Watson Scholar Program, Latinx Center of Excellence, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, President’s Postdoctoral Fellowship Program, and Shared Instrumentation grant. Dr. Kyzar reported no relevant financial disclosures.
Recent research suggests that some pediatric patients who develop neuropsychiatric symptoms from COVID-19 may have intrathecal antineural SARS-CoV-2 autoantibodies, which may hint at central nervous system (CNS) autoimmunity in these patients.
“Overall, these findings indicate that severe neuropsychiatric symptoms can occur in the setting of pediatric COVID-19, including patients who lack many of the cardinal systemic features,” Christopher M. Bartley, MD, PhD, of the Weill Institute for Neurosciences at the University of California, San Francisco, and colleagues wrote in their study. “These data highlight the possibility of SARS-CoV-2 neuroinvasion and/or CNS autoimmunity in pediatric patients with COVID-19 and neuropsychiatric symptoms.”
In a case series published Oct. 25 in JAMA Neurology (doi: 10.1001/jamaneurol.2021.3821), Dr. Bartley and colleagues examined three pediatric patients who were infected with SARS-CoV-2 and, over a period of 5 months in 2020, were admitted to the hospital – where they received a neurology consultation for “subacute, functionally impairing behavioral changes.”
Patient 1 had a history of unspecified anxiety and depression, and was admitted for erratic behavior, paranoia-like fears, social withdrawal, and insomnia. The patient did not respond to treatment with risperidone and gabapentin, and was readmitted soon after discharge, then treated with olanzapine followed by a transition to valproate and lorazepam. It was found the patient had cerebrospinal fluid (CSF) abnormalities in the form of elevated protein levels, and an elevated IgG index, and was given intravenous immunoglobulin followed by IV methylprednisolone. While symptoms such as paranoia improved and the patient was able to better organize thoughts after 5 days, other symptoms such as delusions and hyperreflexia persisted for at least 1 month before resolving, and some symptoms, such as lability, did not resolve before discharge.
Patient 2 had a history of motor tics and anxiety, but showed signs of insomnia, mood lability, impaired concentration, difficulty finding words, and problems completing homework following a SARS-CoV-2 infection. The patient’s father previously had been diagnosed with COVID-19 and the patient developed respiratory symptoms and fever; an IgG serology test later confirmed a SARS-CoV-2 infection. The patient went on to experience internal preoccupation, aggression, and suicidal ideation. The patients was treated with aripiprazole and risperidone, but did not respond, and was admitted to the hospital. As with patient 1, patient 2 had CSF abnormalities in the form of elevated protein levels, and responded to IV methylprednisolone, with working memory and bradyphrenia improving. However, the patient developed insomnia, extreme anxiety, suicidal ideation, aggression, and sadness after discharge, and was readmitted. The patient was treated with IV immunoglobulin, and discharged with quetiapine and lithium.
“Six months later, although improved from initial presentation, the patient required academic accommodations and continued to endorse forgetfulness and attention difficulties. The patient’s chronic tics and anxiety were unchanged,” Dr. Bartley and colleagues wrote.
Patient 3 had no psychiatric history but started to demonstrate “odd behavior, including repetitive behaviors, anorexia, and insomnia” following a SARS-CoV-2 infection. After being hospitalized, the patient showed signs of “ideomotor apraxia, abulia, disorganized behavior, agitation, and diffusely brisk reflexes” and had a high white blood cell count, creatine kinase level, and C-reactive protein level. CSF was also abnormal for this patient, with three unique oligoclonal bands identified. The patient was treated with lorazepam and olanzapine, did not receive immunotherapy, and was discharged without psychiatric medications after 4 days.
When the researchers performed testing on each of the three patients, they found intrathecal anti–SARS-CoV-2 IgG and immunostained mouse brain tissue, and “a diverse set of candidate autoantigens by human phage immunoprecipitation sequencing” in patient 1 and patient 2. In comparison, patient 3 “neither appreciably immunostained nor enriched candidates by human phage immunoprecipitation sequencing,” the researchers said.
“ and the potential for immunotherapy in some,” Dr. Bartley and colleagues concluded.
Potential of CNS autoimmunity
Evan J. Kyzar, MD, PhD, a resident physician in psychiatry at New York State Psychiatric Institute in New York Presbyterian–Columbia Campus, said in an interview that the results of the case series show some pediatric patients with neuropsychiatric symptoms can have anti-SARS-CoV-2 antibodies after viral clearance.
“Interestingly, some of the patients in this study also had antibodies in the CSF that targeted native proteins, demonstrating that COVID-19 may lead to autoimmunity directed at the brain,” he said. “This study increases our knowledge of how COVID-19 interacts with the nervous system and how autoimmune mechanisms might be contributing to at least a portion of patients with neuropsychiatric symptoms during acute infection, and possibly even after viral clearance.”
Dr. Kyzar noted that the immunological methods in the study were “cutting-edge” and the validation exploring the immune responses was detailed, but was limited because of the small sample size.
“[T]he researchers are using similar techniques to explore psychiatric disorders such as depression and schizophrenia to determine if some patients diagnosed with these conditions may have CNS-targeting autoantibodies that contribute to their symptoms and clinical presentation,” Dr. Kyzar said. “This work has the potential to discover novel neuroimmune mechanisms contributing to neuropsychiatric disease and offer possible pathways for the discovery of new treatments.”
The authors reported financial relationships with Allen & Company, the Chan Zuckerberg Initiative, National Institutes of Health, Novartis, Public Health Company, Roche/Genentech, Sandler Foundation, and Takeda in the form of grants and personal fees. They reported funding and/or support from the Brain Research Foundation, Hanna H. Gray Fellowship, Howard Hughes Medical Institute, John A. Watson Scholar Program, Latinx Center of Excellence, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, President’s Postdoctoral Fellowship Program, and Shared Instrumentation grant. Dr. Kyzar reported no relevant financial disclosures.
FROM JAMA NEUROLOGY
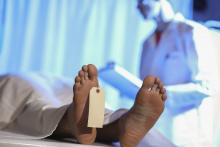
Step right up, folks, for a public dissection
The greatest autopsy on Earth?
The LOTME staff would like to apologize in advance. The following item contains historical facts.
P.T. Barnum is a rather controversial figure in American history. The greatest show on Earth was certainly popular in its day. However, Barnum got his start in 1835 by leasing a slave named Joyce Heth, an elderly Black woman who told vivid stories of caring for a young George Washington. He toured her around the country, advertising her as a 160-year-old woman who served as George Washington’s nanny. When Ms. Heth died the next year, Barnum sold tickets to the autopsy, charging the equivalent of $30 in today’s money.
When a doctor announced that Ms. Heth was actually 75-80 when she died, it caused great controversy in the press and ruined Barnum’s career. Wait, no, that’s not right. The opposite, actually. He weathered the storm, built his famous circus, and never again committed a hoax.
It’s difficult to quantify how wrong publicly dissecting a person and charging people to see said dissection is, but that was almost 200 years ago. At the very least, we can say that such terrible behavior is firmly in the distant past.
Oh wait.
David Saunders, a 98-year-old veteran of World War II and the Korean War, donated his body to science. His body, however, was purchased by DeathScience.org from a medical lab – with the buyer supposedly misleading the medical lab about its intentions, which was for use at the traveling Oddities and Curiosities Expo. Tickets went for up to $500 each to witness the public autopsy of Mr. Saunders’ body, which took place at a Marriott in Portland, Ore. It promised to be an exciting, all-day event from 9 a.m. to 4 p.m., with a break for lunch, of course. You can’t have an autopsy without a catered lunch.
Another public autopsy event was scheduled in Seattle but canceled after news of the first event broke. Oh, and for that extra little kick, Mr. Saunders died from COVID-19, meaning that all those paying customers were exposed.
P.T. Barnum is probably rolling over in his grave right now. His autopsy tickets were a bargain.
Go ahead, have that soda before math
We should all know by now that sugary drinks are bad, even artificially sweetened ones. It might not always stop us from drinking them, but we know the deal. But what if sugary drinks like soda could be helpful for girls in school?
You read that right. We said girls. A soda before class might have boys bouncing off the walls, but not girls. A recent study showed that not only was girls’ behavior unaffected by having a sugary drink, their math skills even improved.
Researchers analyzed the behavior of 4- to 6-year-old children before and after having a sugary drink. The sugar rush was actually calming for girls and helped them perform better with numerical skills, but the opposite was true for boys. “Our study is the first to provide large-scale experimental evidence on the impact of sugary drinks on preschool children. The results clearly indicate a causal impact of sugary drinks on children’s behavior and test scores,” Fritz Schiltz, PhD, said in a written statement.
This probably isn’t the green light to have as many sugary drinks as you want, but it might be interesting to see how your work is affected after a soda.
Chicken nuggets and the meat paradox
Two young children are fighting over the last chicken nugget when an adult comes in to see what’s going on.
Liam: Vegetable!
Olivia: Meat!
Liam: Chicken nuggets are vegetables!
Olivia: No, dorkface! They’re meat.
Caregiver: Good news, kids. You’re both right.
Olivia: How can we both be right?
At this point, a woman enters the room. She’s wearing a white lab coat, so she must be a scientist.
Dr. Scientist: You can’t both be right, Olivia. You are being fed a serving of the meat paradox. That’s why Liam here doesn’t know that chicken nuggets are made of chicken, which is a form of meat. Sadly, he’s not the only one.
In a recent study, scientists from Furman University in Greenville, S.C., found that 38% of 176 children aged 4-7 years thought that chicken nuggets were vegetables and more than 46% identified French fries as animal based.
Olivia: Did our caregiver lie to us, Dr. Scientist?
Dr. Scientist: Yes, Olivia. The researchers I mentioned explained that “many people experience unease while eating meat. Omnivores eat foods that entail animal suffering and death while at the same time endorsing the compassionate treatment of animals.” That’s the meat paradox.
Liam: What else did they say, Dr. Scientist?
Dr. Scientist: Over 70% of those children said that cows and pigs were not edible and 5% thought that cats and horses were. The investigators wrote “that children and youth should be viewed as agents of environmental change” in the future, but suggested that parents need to bring honesty to the table.
Caregiver: How did you get in here anyway? And how do you know their names?
Dr. Scientist: I’ve been rooting through your garbage for years. All in the name of science, of course.
Bedtimes aren’t just for children
There are multiple ways to prevent heart disease, but what if it could be as easy as switching your bedtime? A recent study in European Heart Journal–Digital Health suggests that there’s a sweet spot when it comes to sleep timing.
Through smartwatch-like devices, researchers measured the sleep-onset and wake-up times for 7 days in 88,026 participants aged 43-79 years. After 5.7 years of follow-up to see if anyone had a heart attack, stroke, or any other cardiovascular event, 3.6% developed some kind of cardiovascular disease.
Those who went to bed between 10 p.m. and 11 p.m. had a lower risk of developing heart disease. The risk was 25% higher for subjects who went to bed at midnight or later, 24% higher for bedtimes before 10 p.m., and 12% higher for bedtimes between 11 p.m. and midnight.
So, why can you go to bed before “The Tonight Show” and lower your cardiovascular risk but not before the nightly news? Well, it has something to do with your body’s natural clock.
“The optimum time to go to sleep is at a specific point in the body’s 24-hour cycle and deviations may be detrimental to health. The riskiest time was after midnight, potentially because it may reduce the likelihood of seeing morning light, which resets the body clock,” said study author Dr. David Plans of the University of Exeter, England.
Although a sleep schedule is preferred, it isn’t realistic all the time for those in certain occupations who might have to resort to other methods to keep their circadian clocks ticking optimally for their health. But if all it takes is prescribing a sleep time to reduce heart disease on a massive scale it would make a great “low-cost public health target.”
So bedtimes aren’t just for children.
The greatest autopsy on Earth?
The LOTME staff would like to apologize in advance. The following item contains historical facts.
P.T. Barnum is a rather controversial figure in American history. The greatest show on Earth was certainly popular in its day. However, Barnum got his start in 1835 by leasing a slave named Joyce Heth, an elderly Black woman who told vivid stories of caring for a young George Washington. He toured her around the country, advertising her as a 160-year-old woman who served as George Washington’s nanny. When Ms. Heth died the next year, Barnum sold tickets to the autopsy, charging the equivalent of $30 in today’s money.
When a doctor announced that Ms. Heth was actually 75-80 when she died, it caused great controversy in the press and ruined Barnum’s career. Wait, no, that’s not right. The opposite, actually. He weathered the storm, built his famous circus, and never again committed a hoax.
It’s difficult to quantify how wrong publicly dissecting a person and charging people to see said dissection is, but that was almost 200 years ago. At the very least, we can say that such terrible behavior is firmly in the distant past.
Oh wait.
David Saunders, a 98-year-old veteran of World War II and the Korean War, donated his body to science. His body, however, was purchased by DeathScience.org from a medical lab – with the buyer supposedly misleading the medical lab about its intentions, which was for use at the traveling Oddities and Curiosities Expo. Tickets went for up to $500 each to witness the public autopsy of Mr. Saunders’ body, which took place at a Marriott in Portland, Ore. It promised to be an exciting, all-day event from 9 a.m. to 4 p.m., with a break for lunch, of course. You can’t have an autopsy without a catered lunch.
Another public autopsy event was scheduled in Seattle but canceled after news of the first event broke. Oh, and for that extra little kick, Mr. Saunders died from COVID-19, meaning that all those paying customers were exposed.
P.T. Barnum is probably rolling over in his grave right now. His autopsy tickets were a bargain.
Go ahead, have that soda before math
We should all know by now that sugary drinks are bad, even artificially sweetened ones. It might not always stop us from drinking them, but we know the deal. But what if sugary drinks like soda could be helpful for girls in school?
You read that right. We said girls. A soda before class might have boys bouncing off the walls, but not girls. A recent study showed that not only was girls’ behavior unaffected by having a sugary drink, their math skills even improved.
Researchers analyzed the behavior of 4- to 6-year-old children before and after having a sugary drink. The sugar rush was actually calming for girls and helped them perform better with numerical skills, but the opposite was true for boys. “Our study is the first to provide large-scale experimental evidence on the impact of sugary drinks on preschool children. The results clearly indicate a causal impact of sugary drinks on children’s behavior and test scores,” Fritz Schiltz, PhD, said in a written statement.
This probably isn’t the green light to have as many sugary drinks as you want, but it might be interesting to see how your work is affected after a soda.
Chicken nuggets and the meat paradox
Two young children are fighting over the last chicken nugget when an adult comes in to see what’s going on.
Liam: Vegetable!
Olivia: Meat!
Liam: Chicken nuggets are vegetables!
Olivia: No, dorkface! They’re meat.
Caregiver: Good news, kids. You’re both right.
Olivia: How can we both be right?
At this point, a woman enters the room. She’s wearing a white lab coat, so she must be a scientist.
Dr. Scientist: You can’t both be right, Olivia. You are being fed a serving of the meat paradox. That’s why Liam here doesn’t know that chicken nuggets are made of chicken, which is a form of meat. Sadly, he’s not the only one.
In a recent study, scientists from Furman University in Greenville, S.C., found that 38% of 176 children aged 4-7 years thought that chicken nuggets were vegetables and more than 46% identified French fries as animal based.
Olivia: Did our caregiver lie to us, Dr. Scientist?
Dr. Scientist: Yes, Olivia. The researchers I mentioned explained that “many people experience unease while eating meat. Omnivores eat foods that entail animal suffering and death while at the same time endorsing the compassionate treatment of animals.” That’s the meat paradox.
Liam: What else did they say, Dr. Scientist?
Dr. Scientist: Over 70% of those children said that cows and pigs were not edible and 5% thought that cats and horses were. The investigators wrote “that children and youth should be viewed as agents of environmental change” in the future, but suggested that parents need to bring honesty to the table.
Caregiver: How did you get in here anyway? And how do you know their names?
Dr. Scientist: I’ve been rooting through your garbage for years. All in the name of science, of course.
Bedtimes aren’t just for children
There are multiple ways to prevent heart disease, but what if it could be as easy as switching your bedtime? A recent study in European Heart Journal–Digital Health suggests that there’s a sweet spot when it comes to sleep timing.
Through smartwatch-like devices, researchers measured the sleep-onset and wake-up times for 7 days in 88,026 participants aged 43-79 years. After 5.7 years of follow-up to see if anyone had a heart attack, stroke, or any other cardiovascular event, 3.6% developed some kind of cardiovascular disease.
Those who went to bed between 10 p.m. and 11 p.m. had a lower risk of developing heart disease. The risk was 25% higher for subjects who went to bed at midnight or later, 24% higher for bedtimes before 10 p.m., and 12% higher for bedtimes between 11 p.m. and midnight.
So, why can you go to bed before “The Tonight Show” and lower your cardiovascular risk but not before the nightly news? Well, it has something to do with your body’s natural clock.
“The optimum time to go to sleep is at a specific point in the body’s 24-hour cycle and deviations may be detrimental to health. The riskiest time was after midnight, potentially because it may reduce the likelihood of seeing morning light, which resets the body clock,” said study author Dr. David Plans of the University of Exeter, England.
Although a sleep schedule is preferred, it isn’t realistic all the time for those in certain occupations who might have to resort to other methods to keep their circadian clocks ticking optimally for their health. But if all it takes is prescribing a sleep time to reduce heart disease on a massive scale it would make a great “low-cost public health target.”
So bedtimes aren’t just for children.
The greatest autopsy on Earth?
The LOTME staff would like to apologize in advance. The following item contains historical facts.
P.T. Barnum is a rather controversial figure in American history. The greatest show on Earth was certainly popular in its day. However, Barnum got his start in 1835 by leasing a slave named Joyce Heth, an elderly Black woman who told vivid stories of caring for a young George Washington. He toured her around the country, advertising her as a 160-year-old woman who served as George Washington’s nanny. When Ms. Heth died the next year, Barnum sold tickets to the autopsy, charging the equivalent of $30 in today’s money.
When a doctor announced that Ms. Heth was actually 75-80 when she died, it caused great controversy in the press and ruined Barnum’s career. Wait, no, that’s not right. The opposite, actually. He weathered the storm, built his famous circus, and never again committed a hoax.
It’s difficult to quantify how wrong publicly dissecting a person and charging people to see said dissection is, but that was almost 200 years ago. At the very least, we can say that such terrible behavior is firmly in the distant past.
Oh wait.
David Saunders, a 98-year-old veteran of World War II and the Korean War, donated his body to science. His body, however, was purchased by DeathScience.org from a medical lab – with the buyer supposedly misleading the medical lab about its intentions, which was for use at the traveling Oddities and Curiosities Expo. Tickets went for up to $500 each to witness the public autopsy of Mr. Saunders’ body, which took place at a Marriott in Portland, Ore. It promised to be an exciting, all-day event from 9 a.m. to 4 p.m., with a break for lunch, of course. You can’t have an autopsy without a catered lunch.
Another public autopsy event was scheduled in Seattle but canceled after news of the first event broke. Oh, and for that extra little kick, Mr. Saunders died from COVID-19, meaning that all those paying customers were exposed.
P.T. Barnum is probably rolling over in his grave right now. His autopsy tickets were a bargain.
Go ahead, have that soda before math
We should all know by now that sugary drinks are bad, even artificially sweetened ones. It might not always stop us from drinking them, but we know the deal. But what if sugary drinks like soda could be helpful for girls in school?
You read that right. We said girls. A soda before class might have boys bouncing off the walls, but not girls. A recent study showed that not only was girls’ behavior unaffected by having a sugary drink, their math skills even improved.
Researchers analyzed the behavior of 4- to 6-year-old children before and after having a sugary drink. The sugar rush was actually calming for girls and helped them perform better with numerical skills, but the opposite was true for boys. “Our study is the first to provide large-scale experimental evidence on the impact of sugary drinks on preschool children. The results clearly indicate a causal impact of sugary drinks on children’s behavior and test scores,” Fritz Schiltz, PhD, said in a written statement.
This probably isn’t the green light to have as many sugary drinks as you want, but it might be interesting to see how your work is affected after a soda.
Chicken nuggets and the meat paradox
Two young children are fighting over the last chicken nugget when an adult comes in to see what’s going on.
Liam: Vegetable!
Olivia: Meat!
Liam: Chicken nuggets are vegetables!
Olivia: No, dorkface! They’re meat.
Caregiver: Good news, kids. You’re both right.
Olivia: How can we both be right?
At this point, a woman enters the room. She’s wearing a white lab coat, so she must be a scientist.
Dr. Scientist: You can’t both be right, Olivia. You are being fed a serving of the meat paradox. That’s why Liam here doesn’t know that chicken nuggets are made of chicken, which is a form of meat. Sadly, he’s not the only one.
In a recent study, scientists from Furman University in Greenville, S.C., found that 38% of 176 children aged 4-7 years thought that chicken nuggets were vegetables and more than 46% identified French fries as animal based.
Olivia: Did our caregiver lie to us, Dr. Scientist?
Dr. Scientist: Yes, Olivia. The researchers I mentioned explained that “many people experience unease while eating meat. Omnivores eat foods that entail animal suffering and death while at the same time endorsing the compassionate treatment of animals.” That’s the meat paradox.
Liam: What else did they say, Dr. Scientist?
Dr. Scientist: Over 70% of those children said that cows and pigs were not edible and 5% thought that cats and horses were. The investigators wrote “that children and youth should be viewed as agents of environmental change” in the future, but suggested that parents need to bring honesty to the table.
Caregiver: How did you get in here anyway? And how do you know their names?
Dr. Scientist: I’ve been rooting through your garbage for years. All in the name of science, of course.
Bedtimes aren’t just for children
There are multiple ways to prevent heart disease, but what if it could be as easy as switching your bedtime? A recent study in European Heart Journal–Digital Health suggests that there’s a sweet spot when it comes to sleep timing.
Through smartwatch-like devices, researchers measured the sleep-onset and wake-up times for 7 days in 88,026 participants aged 43-79 years. After 5.7 years of follow-up to see if anyone had a heart attack, stroke, or any other cardiovascular event, 3.6% developed some kind of cardiovascular disease.
Those who went to bed between 10 p.m. and 11 p.m. had a lower risk of developing heart disease. The risk was 25% higher for subjects who went to bed at midnight or later, 24% higher for bedtimes before 10 p.m., and 12% higher for bedtimes between 11 p.m. and midnight.
So, why can you go to bed before “The Tonight Show” and lower your cardiovascular risk but not before the nightly news? Well, it has something to do with your body’s natural clock.
“The optimum time to go to sleep is at a specific point in the body’s 24-hour cycle and deviations may be detrimental to health. The riskiest time was after midnight, potentially because it may reduce the likelihood of seeing morning light, which resets the body clock,” said study author Dr. David Plans of the University of Exeter, England.
Although a sleep schedule is preferred, it isn’t realistic all the time for those in certain occupations who might have to resort to other methods to keep their circadian clocks ticking optimally for their health. But if all it takes is prescribing a sleep time to reduce heart disease on a massive scale it would make a great “low-cost public health target.”
So bedtimes aren’t just for children.
Pandemic and sleep: Increased stress, lack of exercise and insomnia
While working as a registered nurse on inpatient Stroke and Generalized Rehabilitation unit, she pursued for a degree in Adult and Gerontology Primary Care degree. She currently practices at UW Medicine/Harborview Medical Center for Sleep Medicine treating a variety of sleep disorders. She strives to provide quality and safe care to her patients.
1. According to the American Academy of Sleep Medicine, even in normal times, 30 to 35 % of the US population contends with acute, or short-term insomnia. As a board-certified nurse practitioner focusing on treating sleep disorders among older adults, can you discuss whether that percentage has increased during the coronavirus (COVID-19) pandemic, and if so, what would you say are the underlying reasons or causes?
As a sleep medicine nurse practitioner at UW (University of Washington) Medicine, I have seen quite a few patients with sleep disorders including acute and chronic insomnia. Since the start of the COVID-19 pandemic there has been a noticeable increase in poor-sleep complaints -- the data indicate a 37% increase in the rate of clinical insomnia since the pandemic started.
Stress can worsen insomnia, and the pandemic has negatively affected most if not everyone’s life. It has changed lifestyles through social distancing, mask mandates, and stay-at-home orders. Many have been forced to balance working from home with household duties; parents are supervising their children’s schooling. This disruption in the workday environment and workload can be hard to manage. The uncertainty of the pandemic has increased worries – health related and financially related. Ready access to media can also increase stress. Moreover, the lack of structure in a person’s day can cause many problems. Working from home, quarantining, living a more sedentary lifestyle, losing a job, losing socialization, including attending events, all can cause a disruption in a person’s daily routine and induce later bed- and wake-up times. This disruption to the body’s biological or circadian rhythm can reduce sleep quality and INCREASE phase-delay insomnia. Moreover, the pandemic has been especially hard on people’s mental health. One CDC study showed that 40% of adults are struggling with adverse mental health and substance-use issues due to COVID. Also, 13.3% of adults have responded to surveys saying they’ve started or increased their use of substances. As the pandemic continues, acute insomnia will likely turn into chronic insomnia.
2. How can increased stress and lack of exercise cause insomnia? What risk factors contribute to lack of sleep and impact our overall health?
The incidence of anxiety disorder and depressive disorder has increased significantly as compared to pre-pandemic rates. Psychological stress, especially at bedtime, increases psychophysiological arousal. The hypothalamic- pituitary- adrenal (HPA) axis responds to stress by releasing cortisol. HPA activation is associated with poorer sleep quality – it increases sleep latency, frequency of awakening, decreases in slow-wave sleep, and degrades overall sleep efficiency. The result of poor quality and fragmented sleep can further activate the HPA axis, causing a positive feedback loop.
A deterrent to poor sleep is physical activity. It greatly improves sleep by improving sleep efficiency, decreasing light sleep, increasing REM sleep, and regulating circadian rhythm. Lack of physical activity has been associated with increased sleep problems such as daytime sleepiness, an insufficient amount of sleep, snoring, sleep apnea symptoms, and restless sleep. And poor sleep further reduces physical activity which perpetuates the problem. The pandemic’s effect on physical activity is significant. It has caused people to stay home more often and therefore decreases in levels of exercise. and increased sedentary lifestyle. More than half of the adults in this country do not meet federal guidelines for aerobic physical activity.
Sleep deprivation can be dangerous, as sleepiness increases the likelihood of major occupational and road traffic accidents. Being awake for at least 18 hours is equivalent to having a blood alcohol content of 0.05% to 0.10% for 24 hours. Chronic sleep deprivation, defined as getting, on average, fewer than 7 hours per night negatively affects all systems of the body. Sleep deprivation therefore reduces quality of life and can reduce life expectancy.
Cardiovascular – Sleep deprivation can increase excessive heart age and reduce heart rate recovery after exercise. It is also linked to increases in heart rate, blood pressure, and death from cardiovascular issues.
Respiratory – Even one night of sleep deprivation can increase respiratory load. Studies have shown an association between sleep apnea and sleep deprivation. Sleep deprivation and respiratory disorders can perpetuate each other.
Neurologic – Sleep is crucial in brain development. Lack of sleep is associated with low grade neuroinflammation, memory and cognitive function decline, and acceleration of Alzheimer’s disease. Sleep deprivation can increase pain sensitivity, the risk of stroke, aggressive behavior, cognitive instability, hyperactivity, and socialization problems.
Endocrine – Sleep deprivation increases appetite stimulation causing excessive food intake and weight gain. It can also impair metabolism, which leads to obesity and insulin resistance.
Reproductive – Studies on sleep deprivation and the human reproduction system are limited. A study in male rats shows a relation between less sleep and overall lower reproductive health such as alteration of spermatic function, “decreased sexual behavior, lower testosterone level, and lower sperm viability level”. Studies also show renal dysfunction and high blood pressure in the offspring of sleep deprived rats in the last week of pregnancy.
3. Please discuss coronasomnia and its symptoms. Also, will you discuss your thoughts on the diagnosis and provide examples of the types of stressors associated with coronasomnia.
Coronasomnia is the term used to describe the increase in sleep problems associated with the COVID-19 pandemic. Coronasomnia is associated with increased sleep onset, maintenance insomnia, delayed sleep schedule, nocturnal awakening, sleep deprivation, and worsened pre-existing sleep issues. The worst insomnia and psychological symptoms are among those who are in the center of the pandemic, such as frontline workers and people living in areas more impacted by COVID-19.
During the pandemic, anxiety, depression, stress, and poor sleep have significantly increased. Anxiety and depression can be accompanied by intrusive thoughts which interfere with falling asleep. Patients with depression have a twofold risk of sleep disruption. Lack of daily routine may be associated with an increase in poor dental hygiene, such as lower rates of flossing and brushing. There’s also an increased rate in snacking (weight gain) and avoidance of visits to the dentists.
More time at home leads to more time spent on TV or social media. Increased screen time and media use at night, especially close to bedtime, are linked to poorer sleep. Blue light emitted by electronic devices can suppress the release of melatonin, making it more difficult to fall asleep. In addition, viewing or listening to content that is distressing or exciting right before bedtime negatively affects sleep quality. Following pandemic news for more than 3 hours a day has been found to be associated with increased levels of anxiety.
Health care providers are especially susceptible to coronasomnia. Those who work directly with COVID -19 patients are twice as likely to report disrupted sleep, anxiety, and depression. An increased work and patient load, the shortage of both fellow providers and supplies, all contribute to increased anxiety and disrupted sleep. Poor sleep, especially coupled with longer work hours and shift work, are associated with a worsened immune system and poor work performance.
4. In looking at the overall challenges pertaining to pandemic-induced sleep problems, what are your guideline recommendations to help ensure we sleep well during this outbreak?
Poor sleep can be detrimental to physical and mental health, and poor sleep hygiene practices can significantly impact sleep quality. Below are some general sleep-hygiene recommendations.
Caffeine – Caffeine consumed close to bedtime can disrupt sleep. Caffeine should be avoided 6 hours prior to bedtime. Everyone’s tolerance to caffeine is different so timing and caffeine dosage may need to be individually tailored.
Alcohol – Alcohol consumed close to bedtime can decrease sleep latency. However, it increases arousal during the second half of the night. It can also worsen snoring and sleep apnea. The effect can be alcohol level dependent.
Exercise – Regular exercise, as already discussed, is linked to better sleep quality. It is typically recommended to exercise earlier in the day; research has shown conflicting results on nighttime exercise. One study of patients with insomnia who exercised at night showed that aerobic exercise of moderate intensity improved polysomnography patient-reported sleep latency, and total sleep time.
Routine – An irregular sleep schedule is associated with poor sleep and daytime sleepiness. Following a consistent sleep schedule promotes stable circadian rhythm. A familiar relaxing routine should be established before bedtime.
Stress – To lower stress, patients should be advised to schedule brief meditation sessions so they can reflect on stressful situations. Patients also should limit the amount of exposure to pandemic news. Writing down and talking about stress, relaxation, and mindfulness techniques may reduce stress. However, stress and anxiety significantly differ case by case and interventions from health care providers may be needed.
Time in bed – Limit the amount of time in bed only for sleep and sex. Limit the use of electronics before bed and avoid use of electronics in bed. Turning off devices or silencing notifications can all help in reducing sleep disruption.
Cognitive behavioral therapy for insomnia (CBT-I) should be considered for patients with chronic insomnia. This therapy often includes sleep hygiene education, sleep restriction therapy, and relaxation training. Benefits of CBT-I treatment are long-term and reduce the need for additional pharmacologic therapies.
While many patients are experiencing insomnia these days, other underlying sleep disorders also should be considered. Patients should be evaluated to see if a sleep specialist is needed to diagnose and treat their sleep disorders.
Sleep Foundation. Sleep Guidelines and Help During the COVID-19 Pandemic. .Apr 7, 2021.
Morin CM, Carrier C. The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021: 77: 346–347. doi: 10.1016/j.sleep.2020.06.005
Pengpid S, Peltzer K. Sedentary Behaviour and 12 Sleep Problem Indicators among Middle-Aged and Elderly Adults in South Africa. Int J Environ Res Public Health. 2019 Apr; 16(8): 1422.
Czeisler M É, Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic — United States, June 24–30, 2020 | MMWR Weekly. Aug 14, 2020. 69(32);1049–1057.
van Dalfsen JH, Markus, CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA)axis reactivity: A systematic review. Sleep Medicine Reviews. June 2018, 187-194. doi.org/10.1016/j.smrv.2017.10.002
Nicolaides NC, et al, eds. Axis and Sleep. Endotext - NCBI Bookshelf. South Dartmouth, MA. 2000- https://www.ncbi.nlm.nih.gov/books/NBK278943/
Issa FG and Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982 Apr; 45: pp 353–359.
Liewa SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Medicine. January 2021, pp 192-204.
Sleep Foundation. Coronasomnia: Definition, Symptoms, and Solutions | Sleep Foundation. Apr 14, 2021. https://www.sleepfoundation.org/covid-19-and-sleep/coronasomnia
American Association of Endodontists. Survey Reveals COVID-19 is a Major Factor in Americans’ Failing Dental Health | American Association of Endodontists (aae.org). Mar 4, 2021.
Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. April 4, 2020. doi.org/10.1111/jsr.13052 https://onlinelibrary.wiley.com/doi/10.1111/jsr.13052
CDC. Drowsy Driving- Sleep and Sleep Disorders. Mar 17, 2017. https://www.cdc.gov/sleep/about_sleep/drowsy_driving.html
Dolezal, BA, Neufeld, EV, Boland DM. Interrelationship between Sleep and Exercise: A Systematic Review. Adv Prev Med. 2017; 2017: 1364387. doi: 10.1155/2017/1364387
Irish LA, Kline, CE, Heather E. Gunn HE, et al. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence.Sleep Med Rev. 2015 Aug; 22: 23–36.doi: 10.1016/j.smrv.2014.10.001
Edinger JD, Arnedt JT, Suzanne M. Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. Feb. 1, 2021.
While working as a registered nurse on inpatient Stroke and Generalized Rehabilitation unit, she pursued for a degree in Adult and Gerontology Primary Care degree. She currently practices at UW Medicine/Harborview Medical Center for Sleep Medicine treating a variety of sleep disorders. She strives to provide quality and safe care to her patients.
1. According to the American Academy of Sleep Medicine, even in normal times, 30 to 35 % of the US population contends with acute, or short-term insomnia. As a board-certified nurse practitioner focusing on treating sleep disorders among older adults, can you discuss whether that percentage has increased during the coronavirus (COVID-19) pandemic, and if so, what would you say are the underlying reasons or causes?
As a sleep medicine nurse practitioner at UW (University of Washington) Medicine, I have seen quite a few patients with sleep disorders including acute and chronic insomnia. Since the start of the COVID-19 pandemic there has been a noticeable increase in poor-sleep complaints -- the data indicate a 37% increase in the rate of clinical insomnia since the pandemic started.
Stress can worsen insomnia, and the pandemic has negatively affected most if not everyone’s life. It has changed lifestyles through social distancing, mask mandates, and stay-at-home orders. Many have been forced to balance working from home with household duties; parents are supervising their children’s schooling. This disruption in the workday environment and workload can be hard to manage. The uncertainty of the pandemic has increased worries – health related and financially related. Ready access to media can also increase stress. Moreover, the lack of structure in a person’s day can cause many problems. Working from home, quarantining, living a more sedentary lifestyle, losing a job, losing socialization, including attending events, all can cause a disruption in a person’s daily routine and induce later bed- and wake-up times. This disruption to the body’s biological or circadian rhythm can reduce sleep quality and INCREASE phase-delay insomnia. Moreover, the pandemic has been especially hard on people’s mental health. One CDC study showed that 40% of adults are struggling with adverse mental health and substance-use issues due to COVID. Also, 13.3% of adults have responded to surveys saying they’ve started or increased their use of substances. As the pandemic continues, acute insomnia will likely turn into chronic insomnia.
2. How can increased stress and lack of exercise cause insomnia? What risk factors contribute to lack of sleep and impact our overall health?
The incidence of anxiety disorder and depressive disorder has increased significantly as compared to pre-pandemic rates. Psychological stress, especially at bedtime, increases psychophysiological arousal. The hypothalamic- pituitary- adrenal (HPA) axis responds to stress by releasing cortisol. HPA activation is associated with poorer sleep quality – it increases sleep latency, frequency of awakening, decreases in slow-wave sleep, and degrades overall sleep efficiency. The result of poor quality and fragmented sleep can further activate the HPA axis, causing a positive feedback loop.
A deterrent to poor sleep is physical activity. It greatly improves sleep by improving sleep efficiency, decreasing light sleep, increasing REM sleep, and regulating circadian rhythm. Lack of physical activity has been associated with increased sleep problems such as daytime sleepiness, an insufficient amount of sleep, snoring, sleep apnea symptoms, and restless sleep. And poor sleep further reduces physical activity which perpetuates the problem. The pandemic’s effect on physical activity is significant. It has caused people to stay home more often and therefore decreases in levels of exercise. and increased sedentary lifestyle. More than half of the adults in this country do not meet federal guidelines for aerobic physical activity.
Sleep deprivation can be dangerous, as sleepiness increases the likelihood of major occupational and road traffic accidents. Being awake for at least 18 hours is equivalent to having a blood alcohol content of 0.05% to 0.10% for 24 hours. Chronic sleep deprivation, defined as getting, on average, fewer than 7 hours per night negatively affects all systems of the body. Sleep deprivation therefore reduces quality of life and can reduce life expectancy.
Cardiovascular – Sleep deprivation can increase excessive heart age and reduce heart rate recovery after exercise. It is also linked to increases in heart rate, blood pressure, and death from cardiovascular issues.
Respiratory – Even one night of sleep deprivation can increase respiratory load. Studies have shown an association between sleep apnea and sleep deprivation. Sleep deprivation and respiratory disorders can perpetuate each other.
Neurologic – Sleep is crucial in brain development. Lack of sleep is associated with low grade neuroinflammation, memory and cognitive function decline, and acceleration of Alzheimer’s disease. Sleep deprivation can increase pain sensitivity, the risk of stroke, aggressive behavior, cognitive instability, hyperactivity, and socialization problems.
Endocrine – Sleep deprivation increases appetite stimulation causing excessive food intake and weight gain. It can also impair metabolism, which leads to obesity and insulin resistance.
Reproductive – Studies on sleep deprivation and the human reproduction system are limited. A study in male rats shows a relation between less sleep and overall lower reproductive health such as alteration of spermatic function, “decreased sexual behavior, lower testosterone level, and lower sperm viability level”. Studies also show renal dysfunction and high blood pressure in the offspring of sleep deprived rats in the last week of pregnancy.
3. Please discuss coronasomnia and its symptoms. Also, will you discuss your thoughts on the diagnosis and provide examples of the types of stressors associated with coronasomnia.
Coronasomnia is the term used to describe the increase in sleep problems associated with the COVID-19 pandemic. Coronasomnia is associated with increased sleep onset, maintenance insomnia, delayed sleep schedule, nocturnal awakening, sleep deprivation, and worsened pre-existing sleep issues. The worst insomnia and psychological symptoms are among those who are in the center of the pandemic, such as frontline workers and people living in areas more impacted by COVID-19.
During the pandemic, anxiety, depression, stress, and poor sleep have significantly increased. Anxiety and depression can be accompanied by intrusive thoughts which interfere with falling asleep. Patients with depression have a twofold risk of sleep disruption. Lack of daily routine may be associated with an increase in poor dental hygiene, such as lower rates of flossing and brushing. There’s also an increased rate in snacking (weight gain) and avoidance of visits to the dentists.
More time at home leads to more time spent on TV or social media. Increased screen time and media use at night, especially close to bedtime, are linked to poorer sleep. Blue light emitted by electronic devices can suppress the release of melatonin, making it more difficult to fall asleep. In addition, viewing or listening to content that is distressing or exciting right before bedtime negatively affects sleep quality. Following pandemic news for more than 3 hours a day has been found to be associated with increased levels of anxiety.
Health care providers are especially susceptible to coronasomnia. Those who work directly with COVID -19 patients are twice as likely to report disrupted sleep, anxiety, and depression. An increased work and patient load, the shortage of both fellow providers and supplies, all contribute to increased anxiety and disrupted sleep. Poor sleep, especially coupled with longer work hours and shift work, are associated with a worsened immune system and poor work performance.
4. In looking at the overall challenges pertaining to pandemic-induced sleep problems, what are your guideline recommendations to help ensure we sleep well during this outbreak?
Poor sleep can be detrimental to physical and mental health, and poor sleep hygiene practices can significantly impact sleep quality. Below are some general sleep-hygiene recommendations.
Caffeine – Caffeine consumed close to bedtime can disrupt sleep. Caffeine should be avoided 6 hours prior to bedtime. Everyone’s tolerance to caffeine is different so timing and caffeine dosage may need to be individually tailored.
Alcohol – Alcohol consumed close to bedtime can decrease sleep latency. However, it increases arousal during the second half of the night. It can also worsen snoring and sleep apnea. The effect can be alcohol level dependent.
Exercise – Regular exercise, as already discussed, is linked to better sleep quality. It is typically recommended to exercise earlier in the day; research has shown conflicting results on nighttime exercise. One study of patients with insomnia who exercised at night showed that aerobic exercise of moderate intensity improved polysomnography patient-reported sleep latency, and total sleep time.
Routine – An irregular sleep schedule is associated with poor sleep and daytime sleepiness. Following a consistent sleep schedule promotes stable circadian rhythm. A familiar relaxing routine should be established before bedtime.
Stress – To lower stress, patients should be advised to schedule brief meditation sessions so they can reflect on stressful situations. Patients also should limit the amount of exposure to pandemic news. Writing down and talking about stress, relaxation, and mindfulness techniques may reduce stress. However, stress and anxiety significantly differ case by case and interventions from health care providers may be needed.
Time in bed – Limit the amount of time in bed only for sleep and sex. Limit the use of electronics before bed and avoid use of electronics in bed. Turning off devices or silencing notifications can all help in reducing sleep disruption.
Cognitive behavioral therapy for insomnia (CBT-I) should be considered for patients with chronic insomnia. This therapy often includes sleep hygiene education, sleep restriction therapy, and relaxation training. Benefits of CBT-I treatment are long-term and reduce the need for additional pharmacologic therapies.
While many patients are experiencing insomnia these days, other underlying sleep disorders also should be considered. Patients should be evaluated to see if a sleep specialist is needed to diagnose and treat their sleep disorders.
While working as a registered nurse on inpatient Stroke and Generalized Rehabilitation unit, she pursued for a degree in Adult and Gerontology Primary Care degree. She currently practices at UW Medicine/Harborview Medical Center for Sleep Medicine treating a variety of sleep disorders. She strives to provide quality and safe care to her patients.
1. According to the American Academy of Sleep Medicine, even in normal times, 30 to 35 % of the US population contends with acute, or short-term insomnia. As a board-certified nurse practitioner focusing on treating sleep disorders among older adults, can you discuss whether that percentage has increased during the coronavirus (COVID-19) pandemic, and if so, what would you say are the underlying reasons or causes?
As a sleep medicine nurse practitioner at UW (University of Washington) Medicine, I have seen quite a few patients with sleep disorders including acute and chronic insomnia. Since the start of the COVID-19 pandemic there has been a noticeable increase in poor-sleep complaints -- the data indicate a 37% increase in the rate of clinical insomnia since the pandemic started.
Stress can worsen insomnia, and the pandemic has negatively affected most if not everyone’s life. It has changed lifestyles through social distancing, mask mandates, and stay-at-home orders. Many have been forced to balance working from home with household duties; parents are supervising their children’s schooling. This disruption in the workday environment and workload can be hard to manage. The uncertainty of the pandemic has increased worries – health related and financially related. Ready access to media can also increase stress. Moreover, the lack of structure in a person’s day can cause many problems. Working from home, quarantining, living a more sedentary lifestyle, losing a job, losing socialization, including attending events, all can cause a disruption in a person’s daily routine and induce later bed- and wake-up times. This disruption to the body’s biological or circadian rhythm can reduce sleep quality and INCREASE phase-delay insomnia. Moreover, the pandemic has been especially hard on people’s mental health. One CDC study showed that 40% of adults are struggling with adverse mental health and substance-use issues due to COVID. Also, 13.3% of adults have responded to surveys saying they’ve started or increased their use of substances. As the pandemic continues, acute insomnia will likely turn into chronic insomnia.
2. How can increased stress and lack of exercise cause insomnia? What risk factors contribute to lack of sleep and impact our overall health?
The incidence of anxiety disorder and depressive disorder has increased significantly as compared to pre-pandemic rates. Psychological stress, especially at bedtime, increases psychophysiological arousal. The hypothalamic- pituitary- adrenal (HPA) axis responds to stress by releasing cortisol. HPA activation is associated with poorer sleep quality – it increases sleep latency, frequency of awakening, decreases in slow-wave sleep, and degrades overall sleep efficiency. The result of poor quality and fragmented sleep can further activate the HPA axis, causing a positive feedback loop.
A deterrent to poor sleep is physical activity. It greatly improves sleep by improving sleep efficiency, decreasing light sleep, increasing REM sleep, and regulating circadian rhythm. Lack of physical activity has been associated with increased sleep problems such as daytime sleepiness, an insufficient amount of sleep, snoring, sleep apnea symptoms, and restless sleep. And poor sleep further reduces physical activity which perpetuates the problem. The pandemic’s effect on physical activity is significant. It has caused people to stay home more often and therefore decreases in levels of exercise. and increased sedentary lifestyle. More than half of the adults in this country do not meet federal guidelines for aerobic physical activity.
Sleep deprivation can be dangerous, as sleepiness increases the likelihood of major occupational and road traffic accidents. Being awake for at least 18 hours is equivalent to having a blood alcohol content of 0.05% to 0.10% for 24 hours. Chronic sleep deprivation, defined as getting, on average, fewer than 7 hours per night negatively affects all systems of the body. Sleep deprivation therefore reduces quality of life and can reduce life expectancy.
Cardiovascular – Sleep deprivation can increase excessive heart age and reduce heart rate recovery after exercise. It is also linked to increases in heart rate, blood pressure, and death from cardiovascular issues.
Respiratory – Even one night of sleep deprivation can increase respiratory load. Studies have shown an association between sleep apnea and sleep deprivation. Sleep deprivation and respiratory disorders can perpetuate each other.
Neurologic – Sleep is crucial in brain development. Lack of sleep is associated with low grade neuroinflammation, memory and cognitive function decline, and acceleration of Alzheimer’s disease. Sleep deprivation can increase pain sensitivity, the risk of stroke, aggressive behavior, cognitive instability, hyperactivity, and socialization problems.
Endocrine – Sleep deprivation increases appetite stimulation causing excessive food intake and weight gain. It can also impair metabolism, which leads to obesity and insulin resistance.
Reproductive – Studies on sleep deprivation and the human reproduction system are limited. A study in male rats shows a relation between less sleep and overall lower reproductive health such as alteration of spermatic function, “decreased sexual behavior, lower testosterone level, and lower sperm viability level”. Studies also show renal dysfunction and high blood pressure in the offspring of sleep deprived rats in the last week of pregnancy.
3. Please discuss coronasomnia and its symptoms. Also, will you discuss your thoughts on the diagnosis and provide examples of the types of stressors associated with coronasomnia.
Coronasomnia is the term used to describe the increase in sleep problems associated with the COVID-19 pandemic. Coronasomnia is associated with increased sleep onset, maintenance insomnia, delayed sleep schedule, nocturnal awakening, sleep deprivation, and worsened pre-existing sleep issues. The worst insomnia and psychological symptoms are among those who are in the center of the pandemic, such as frontline workers and people living in areas more impacted by COVID-19.
During the pandemic, anxiety, depression, stress, and poor sleep have significantly increased. Anxiety and depression can be accompanied by intrusive thoughts which interfere with falling asleep. Patients with depression have a twofold risk of sleep disruption. Lack of daily routine may be associated with an increase in poor dental hygiene, such as lower rates of flossing and brushing. There’s also an increased rate in snacking (weight gain) and avoidance of visits to the dentists.
More time at home leads to more time spent on TV or social media. Increased screen time and media use at night, especially close to bedtime, are linked to poorer sleep. Blue light emitted by electronic devices can suppress the release of melatonin, making it more difficult to fall asleep. In addition, viewing or listening to content that is distressing or exciting right before bedtime negatively affects sleep quality. Following pandemic news for more than 3 hours a day has been found to be associated with increased levels of anxiety.
Health care providers are especially susceptible to coronasomnia. Those who work directly with COVID -19 patients are twice as likely to report disrupted sleep, anxiety, and depression. An increased work and patient load, the shortage of both fellow providers and supplies, all contribute to increased anxiety and disrupted sleep. Poor sleep, especially coupled with longer work hours and shift work, are associated with a worsened immune system and poor work performance.
4. In looking at the overall challenges pertaining to pandemic-induced sleep problems, what are your guideline recommendations to help ensure we sleep well during this outbreak?
Poor sleep can be detrimental to physical and mental health, and poor sleep hygiene practices can significantly impact sleep quality. Below are some general sleep-hygiene recommendations.
Caffeine – Caffeine consumed close to bedtime can disrupt sleep. Caffeine should be avoided 6 hours prior to bedtime. Everyone’s tolerance to caffeine is different so timing and caffeine dosage may need to be individually tailored.
Alcohol – Alcohol consumed close to bedtime can decrease sleep latency. However, it increases arousal during the second half of the night. It can also worsen snoring and sleep apnea. The effect can be alcohol level dependent.
Exercise – Regular exercise, as already discussed, is linked to better sleep quality. It is typically recommended to exercise earlier in the day; research has shown conflicting results on nighttime exercise. One study of patients with insomnia who exercised at night showed that aerobic exercise of moderate intensity improved polysomnography patient-reported sleep latency, and total sleep time.
Routine – An irregular sleep schedule is associated with poor sleep and daytime sleepiness. Following a consistent sleep schedule promotes stable circadian rhythm. A familiar relaxing routine should be established before bedtime.
Stress – To lower stress, patients should be advised to schedule brief meditation sessions so they can reflect on stressful situations. Patients also should limit the amount of exposure to pandemic news. Writing down and talking about stress, relaxation, and mindfulness techniques may reduce stress. However, stress and anxiety significantly differ case by case and interventions from health care providers may be needed.
Time in bed – Limit the amount of time in bed only for sleep and sex. Limit the use of electronics before bed and avoid use of electronics in bed. Turning off devices or silencing notifications can all help in reducing sleep disruption.
Cognitive behavioral therapy for insomnia (CBT-I) should be considered for patients with chronic insomnia. This therapy often includes sleep hygiene education, sleep restriction therapy, and relaxation training. Benefits of CBT-I treatment are long-term and reduce the need for additional pharmacologic therapies.
While many patients are experiencing insomnia these days, other underlying sleep disorders also should be considered. Patients should be evaluated to see if a sleep specialist is needed to diagnose and treat their sleep disorders.
Sleep Foundation. Sleep Guidelines and Help During the COVID-19 Pandemic. .Apr 7, 2021.
Morin CM, Carrier C. The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021: 77: 346–347. doi: 10.1016/j.sleep.2020.06.005
Pengpid S, Peltzer K. Sedentary Behaviour and 12 Sleep Problem Indicators among Middle-Aged and Elderly Adults in South Africa. Int J Environ Res Public Health. 2019 Apr; 16(8): 1422.
Czeisler M É, Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic — United States, June 24–30, 2020 | MMWR Weekly. Aug 14, 2020. 69(32);1049–1057.
van Dalfsen JH, Markus, CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA)axis reactivity: A systematic review. Sleep Medicine Reviews. June 2018, 187-194. doi.org/10.1016/j.smrv.2017.10.002
Nicolaides NC, et al, eds. Axis and Sleep. Endotext - NCBI Bookshelf. South Dartmouth, MA. 2000- https://www.ncbi.nlm.nih.gov/books/NBK278943/
Issa FG and Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982 Apr; 45: pp 353–359.
Liewa SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Medicine. January 2021, pp 192-204.
Sleep Foundation. Coronasomnia: Definition, Symptoms, and Solutions | Sleep Foundation. Apr 14, 2021. https://www.sleepfoundation.org/covid-19-and-sleep/coronasomnia
American Association of Endodontists. Survey Reveals COVID-19 is a Major Factor in Americans’ Failing Dental Health | American Association of Endodontists (aae.org). Mar 4, 2021.
Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. April 4, 2020. doi.org/10.1111/jsr.13052 https://onlinelibrary.wiley.com/doi/10.1111/jsr.13052
CDC. Drowsy Driving- Sleep and Sleep Disorders. Mar 17, 2017. https://www.cdc.gov/sleep/about_sleep/drowsy_driving.html
Dolezal, BA, Neufeld, EV, Boland DM. Interrelationship between Sleep and Exercise: A Systematic Review. Adv Prev Med. 2017; 2017: 1364387. doi: 10.1155/2017/1364387
Irish LA, Kline, CE, Heather E. Gunn HE, et al. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence.Sleep Med Rev. 2015 Aug; 22: 23–36.doi: 10.1016/j.smrv.2014.10.001
Edinger JD, Arnedt JT, Suzanne M. Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. Feb. 1, 2021.
Sleep Foundation. Sleep Guidelines and Help During the COVID-19 Pandemic. .Apr 7, 2021.
Morin CM, Carrier C. The acute effects of the COVID-19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021: 77: 346–347. doi: 10.1016/j.sleep.2020.06.005
Pengpid S, Peltzer K. Sedentary Behaviour and 12 Sleep Problem Indicators among Middle-Aged and Elderly Adults in South Africa. Int J Environ Res Public Health. 2019 Apr; 16(8): 1422.
Czeisler M É, Lane RI, Petrosky E, et al. Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic — United States, June 24–30, 2020 | MMWR Weekly. Aug 14, 2020. 69(32);1049–1057.
van Dalfsen JH, Markus, CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA)axis reactivity: A systematic review. Sleep Medicine Reviews. June 2018, 187-194. doi.org/10.1016/j.smrv.2017.10.002
Nicolaides NC, et al, eds. Axis and Sleep. Endotext - NCBI Bookshelf. South Dartmouth, MA. 2000- https://www.ncbi.nlm.nih.gov/books/NBK278943/
Issa FG and Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982 Apr; 45: pp 353–359.
Liewa SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Medicine. January 2021, pp 192-204.
Sleep Foundation. Coronasomnia: Definition, Symptoms, and Solutions | Sleep Foundation. Apr 14, 2021. https://www.sleepfoundation.org/covid-19-and-sleep/coronasomnia
American Association of Endodontists. Survey Reveals COVID-19 is a Major Factor in Americans’ Failing Dental Health | American Association of Endodontists (aae.org). Mar 4, 2021.
Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. April 4, 2020. doi.org/10.1111/jsr.13052 https://onlinelibrary.wiley.com/doi/10.1111/jsr.13052
CDC. Drowsy Driving- Sleep and Sleep Disorders. Mar 17, 2017. https://www.cdc.gov/sleep/about_sleep/drowsy_driving.html
Dolezal, BA, Neufeld, EV, Boland DM. Interrelationship between Sleep and Exercise: A Systematic Review. Adv Prev Med. 2017; 2017: 1364387. doi: 10.1155/2017/1364387
Irish LA, Kline, CE, Heather E. Gunn HE, et al. The Role of Sleep Hygiene in Promoting Public Health: A Review of Empirical Evidence.Sleep Med Rev. 2015 Aug; 22: 23–36.doi: 10.1016/j.smrv.2014.10.001
Edinger JD, Arnedt JT, Suzanne M. Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. Feb. 1, 2021.
Sleep time ‘sweet spot’ to slow cognitive decline identified?
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis, said in an interview.
The study, published online Oct. 20, 2021, in the journal Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, AD, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of AD, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid-beta, a hallmark sign of AD.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University.
Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta 42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR greater than 0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of AD pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale–Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both).
The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta 42 ratio, apo E four-allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of AD could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis, said in an interview.
The study, published online Oct. 20, 2021, in the journal Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, AD, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of AD, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid-beta, a hallmark sign of AD.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University.
Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta 42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR greater than 0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of AD pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale–Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both).
The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta 42 ratio, apo E four-allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of AD could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis, said in an interview.
The study, published online Oct. 20, 2021, in the journal Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, AD, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of AD, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid-beta, a hallmark sign of AD.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University.
Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta 42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR greater than 0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of AD pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale–Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both).
The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta 42 ratio, apo E four-allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of AD could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis use: Messages remain mixed across diagnoses
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
FROM PSYCHOPHARMACOLOGY UPDATE
Sleep time ‘sweet spot’ to slow cognitive decline identified?
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
From Brain
Sleep apnea has many faces
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Sleep problems in mental illness highly pervasive
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.