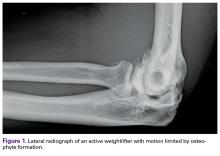
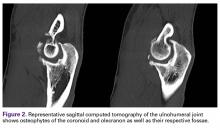
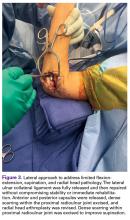
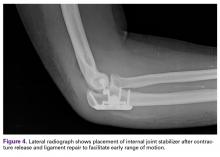
User login
Superior Capsular Reconstruction: Clinical Outcomes After Minimum 2-Year Follow-Up
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
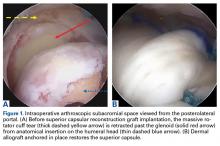
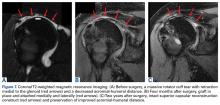
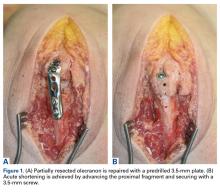
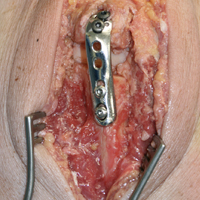
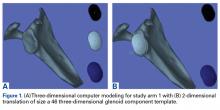
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
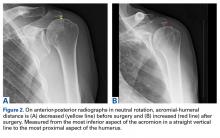
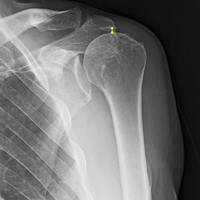
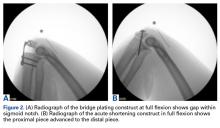
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
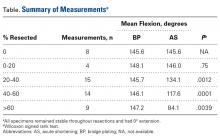
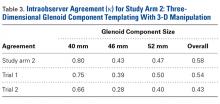
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
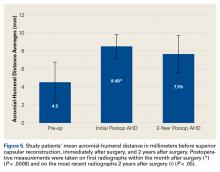
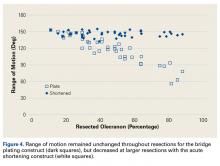
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
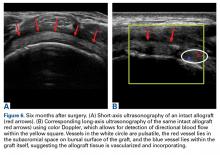
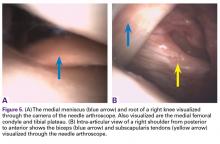
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
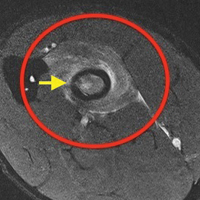
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
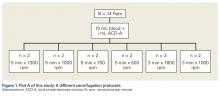
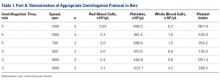
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
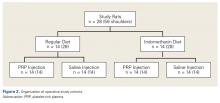
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
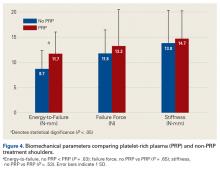
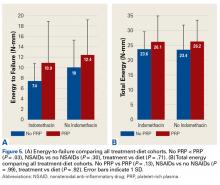
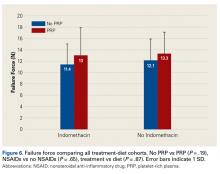
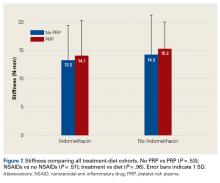
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Acute Shortening Versus Bridging Plate for Highly Comminuted Olecranon Fractures
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Radial Shaft Stress Fracture in a Major League Pitcher
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Reverse Shoulder Arthroplasty and Latissimus Dorsi Tendon Transfer
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Reliability of 3-Dimensional Glenoid Component Templating and Correlation to Intraoperative Component Selection
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
In-Office Diagnostic Needle Arthroscopy: Understanding the Potential Value for the US Healthcare System
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
5 Points on Stiff Elbow
Take-Home Points
- Proper patient selection is critical as extensive postoperative rehabilitation is required to obtain an excellent outcome.
- Open and arthroscopic approaches are effective treatment options for elbow contractures.
- Elbow stability must be restored to obtain a successful outcome.
- Knowledge of neurovascular anatomy is essential to prevent neurologic complications.
- Prophylactic ulnar nerve release should be considered, especially in patients with limited flexion.
Elbow stiffness has several etiologies, posttraumatic being the most common. Elbow stiffness can have debilitating functional effects necessitating treatment. In a biomechanical study of normal elbow function, Morrey and colleagues1 determined that a flexion extension arc of 100° (30°-130°) and a forearm rotation arc of 100° (50° pronation-50° supination) are required in 90% of activities of daily living. Similarly, elbow flexion of <105° was poorly tolerated, whereas patients could easier adapt to flexion contractures up to 40°.2
The goal of initial evaluation should be to establish the cause of the contracture and the patient’s functional demands and ability to cooperate in the extensive postoperative rehabilitation that is essential in achieving an excellent functional outcome. In a thorough clinical examination, the clinician must note skin, range of motion (ROM), ligamentous stability, and neurovascular structures and give special attention to ulnar nerve function and symptoms. Mid-arc pain suggests additional intra-articular pathology, as stiffness typically causes pain only at the limits of motion as osteophytes impinge and soft tissue is under maximal tension. Routine elbow radiographs are required in all cases, and computed tomography (CT) can be useful in evaluating osseous sources of contracture. Suspected ligamentous instability and cartilaginous defects particularly in the setting of mid-arc pain are best evaluated with magnetic resonance imaging.3
In this 5-point review, we evaluate treatment options as well as rehabilitation protocols in the management of elbow stiffness.
1 Anatomy of Contracture: The Usual Suspects
The cause of elbow stiffness is incompletely understood. Several posited contributing factors include biology, complex intra-articular anatomy, capsular distention favoring a flexed position, and tenuous postoperative fixation necessitating prolonged immobilization. Identifying intrinsic and extrinsic anatomical sources of stiffness can help guide treatment.4 Intrinsic pathology includes intra-articular malunion, osteophytes, loose bodies, and adhesions; extrinsic pathology includes soft-tissue contracture, heterotopic ossification, and extra-articular malunion.
Compared with the normal elbow, the capsule becomes thickened and fibrotic and thereby prevents motion. Severe contractures, and extension contractures in particular, may require release of the posterior medial capsule and the posterior medial collateral ligament (MCL) to regain motion. In a series of 42 patients with flexion <100°, Park and colleagues5 noted that all patients required release of the posterior band of the MCL to regain flexion. Other muscular impediments to motion include contracture of the brachialis and scarring of the triceps to the posterior humerus. Scarring of the triceps to the humerus can limit flexion.
In the posttrauma setting, intra-articular and extra-articular malunion must be considered. Extension malunion of the distal humerus can reduce flexion,6 and shortening with compromise of the olecranon and coronoid fossae can limit both flexion and extension.
Last, heterotopic ossification and osteophytes should be assessed as potential causes of limited ROM. Both the coronoid process and the olecranon can develop osteophytes, and their respective fossae should be assessed with CT. Posterior impingement is rare at the tip of the olecranon; it occurs because of "widening" of the olecranon by "Mickey Mouse ear" osteophytes and bony encroachment along the medial and lateral columns. Thus, the olecranon must be narrowed and the fossa widened and deepened.
In case of concomitant ligament instability, we prefer to reconstruct the ligament first, and then perform contracture release as a staged procedure. We favor a staged approach because the rehabilitation regimens for instability and contracture release are diametrically opposed: Instability requires immobilization, and contracture release requires immediate motion. Last, incision placement and ulnar nerve management are crucial in minimizing the potential complications of the second procedure.
2 Nonoperative Treatment
In the absence of significant bony impediments to motion—such as heterotopic ossification or malunion—initial treatment should commence with nonoperative therapy. Therapy should be initiated as soon as concern for stiffness arises in order to prevent contracture. Initial nonoperative treatment can also serve as an important litmus test of postoperative adherence. Adequate patient relaxation is crucial in avoiding co-contracture resisting stretching forces. Passive ROM exercises use sustained force to allow time-dependent stress relaxation to increase tissue length as well as fatigue antagonist muscles. In addition, hold-and-relax techniques apply isometric resistance to induce relaxation of antagonist muscles.7 Active ROM should emphasize triceps isolation and elbow extension to prevent scarring of the triceps to the posterior humerus.
Corrective splinting can be an effective adjuvant to physiotherapy. Static progressive turnbuckle splints was described as an effective treatment for both elbow flexion and extension contractures, effecting an average 43° increase in elbow motion in a series of 15 patients.8 Similarly, Gelinas and colleagues9 noted improvement among 22 patients treated with turnbuckle splinting for an average of 4.5 months. In addition, serial extension splints may be used in the treatment of elbow flexion contractures.
3 Open Contacture Release and Surgical Approach
When nonoperative therapies fail to restore the functional arc of motion, patients with flexion contractures or extension contractures of >30° may be indicated for contracture release. Surgical approach should be determined by meticulous preoperative planning that notes prior incisions and CT findings. It can be helpful to organize common offending structures and their effects on flexion and extension (Table).
A medial over-the-top approach uses the medial supracondylar ridge as a landmark, subperiosteally reflecting the brachialis anteriorly.10 The ulnar nerve is neurolyzed and protected posteriorly. The flexor-pronator mass is split distally and elevated along with the brachialis as a single sleeve of muscle. The coronal plane of dissection should be the anterior half of the lateral epicondyle to avoid injury to the MCL. Large Bennett or Hohmann retractors can hinge on the lateral border of the humerus and provide clear visualization of the anterior capsule and the ulnohumeral joint. Exposure of the radiocapitellar joint is possible, but this joint is very deep in the operative field, and caution should be taken excising the anterolateral capsule because of the risk of radial nerve injury. The ulnar nerve can be temporarily transposed anteriorly to dissect posteriorly along the supracondylar ridge of the humerus. The triceps is reflected off the distal humerus. Occasionally, the posterior band of the MCL must be resected in severe extension contractures. If possible, the anterior bundle should be preserved. With this approach, the anterior capsule, distal humerus, coronoid process, posterior MCL, posterior capsule, and triceps can be addressed. The zone anterior to the radial head and the anterolateral and posterolateral capsule cannot be safely exposed with a medial approach. As described by Wada and colleagues,11 a primarily medial approach resulted in an average 64° increase in arc of motion.
an internal joint stabilizer (Skeletal Dynamics) (Figure 4) and to initiate motion therapy immediately. External fixation (hinged or unhinged is rarely used in our practice.
4 Arthroscopic Contracture Release and Technique
Recently, arthroscopic elbow contracture release, a technically demanding but effective treatment option, has gained popularity. Knowledge of neurovascular anatomy is a prerequisite to the prevention of devastating neurologic complications (ulnar, median, and radial nerve transections have been described14,15). Relative contraindications include extensive heterotopic ossification, ulnar nerve transposition, and limited arthroscopic experience. Functional improvements as well as average 26° to 42° increases in arc of motion have been described with arthroscopic release.16-18 In thin-framed patients with dense elbow capsular scarring (severe loss of elbow motion with hard block) and small joint space, arthroscopic release and particularly arthroscope insertion are notoriously difficult.
The patient may be placed in the prone, lateral decubitus, or supine position, depending on surgeon preference (Figure 5). Before surgery, portals and the ulnar nerve should be carefully outlined.19
We prefer to start by entering the posterior compartment and using the shaver to create a working space. All bone work and resectioning should be performed before capsular resection. After the joint and the olecranon fossa are identified, soft-tissue and bony débridement of the olecranon and the fossa can be performed. Care should be taken to protect the ulnar nerve when the posteromedial corner or medial gutter is approached.
5 Additional Considerations
After surgery, the elbow is immobilized in maximal extension and supination with an anterior splint, and therapy is initiated either immediately or after temporary immobilization.16,19,20 Regional anesthesia is crucial in obtaining adequate pain control and establishing an immediate postoperative therapy program. The utility of continuous passive motion (CPM) in postoperative protocols is controversial. A retrospective case-control study of 32 patients matched on age, diagnosis, and contraction severity found no benefit of CPM use, and increased costs and hospital length of stay, leading the authors to recommend against CPM use.20
Neurovascular risks are associated with both open and arthroscopic elbow contracture release. Particularly concerning is the risk of traction ulnar neuropathy, described in upward of 20% of patients.21 Anatomical studies have found decreases in cubital tunnel and ulnar nerve area as elbow flexion increases with corresponding increased intraneural pressure,22 leading some authors to recommend prophylactic ulnar nerve release with limited preoperative flexion.15 Nevertheless, despite transposition, ulnar nerve symptoms were noted in 8 of 40 patients who underwent open contracture release for posttraumatic loss of elbow flexion.5 In a retrospective review of 164 open and arthroscopic elbow contracture releases, Williams and colleagues21 noted an 8.1% rate of postoperative new-onset ulnar nerve symptoms. The rate of ulnar neuropathy was nonsignificantly elevated among patients with preoperative flexion of <100° (15.2% vs 3.7%; P = .057). Recently, a retrospective review of 564 consecutive arthroscopic contracture releases found a significantly higher rate of delayed-onset ulnar neuritis among patients without prophylactic ulnar nerve decompression or transposition (11% vs 3%; P < .001).23 Further analysis revealed that, compared with decompression, ulnar nerve transposition did not offer additional benefit but was associated with a significantly higher rate of wound complications (19% vs 4%; P = .03). We favor prophylactic release, particularly in the setting of preoperative extension contracture. For open contracture release from the lateral approach, however, we do not routinely release the ulnar nerve unless there were preoperative symptoms.
Although open and arthroscopic contracture releases can provide durable outcomes in the setting of painless elbow stiffness, options are more limited in the treatment of the painful stiff elbow. Total elbow arthroplasty remains an option in low-demand elderly patients but is not without significant risk of complications.24 In addition, durability concerns and postoperative restrictions make total elbow arthroplasty less attractive to younger patients. Interposition arthroplasty may be indicated as a salvage procedure in the treatment of a young or high-demand patient with a stiff painful elbow.25 Elbow stability is crucial in obtaining a successful outcome, and data on optimal graft choices are limited.
Conclusion
Elbow stiffness, a common complication of trauma, significantly impairs activities of daily living. Early after trauma, therapy should be initiated to prevent contracture. In the absence of symptomatic arthritis, both open and arthroscopic contracture releases are effective surgical treatments in properly selected and motivated patients. Although more research is needed to establish the optimal surgical approach, severity and anatomical cause of contracture should guide decisions as to which approach to use. Having a thorough understanding of neurovascular anatomy and of prophylactic ulnar nerve decompression in the setting of limited preoperative flexion can mitigate complications.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Hotchkiss RN. Elbow contracture. In: Green DP, Rotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. New York, NY: Churchill-Livingstone; 2005:667-682.
3. Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19(2):13-19.
4. Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601-618.
5. Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692-2699.
6. Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg Am. 2009;34(7):
1256-1260.
7. Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300-309.
8. Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092-1095.
9. Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74-78.
10. Hotchkiss RN, Kasparyan GN. The medial "over the top" approach to the elbow. Tech Orthop. 2000;15(2):105-112.
11. Wada T, Ishii S, Usui M, Miyano S. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Br. 2000;82(1):68-73.
12. Husband JB, Hastings H. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353-1358.
13. Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603-1605.
14. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83(1):25-34.
16. Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11(6):624-629.
17. Ćefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434-439.
18. Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842-849.
19. Sahajpal D, Choi T, Wright TW. Arthroscopic release of the stiff elbow. J Hand Surg. 2009;34(3):540-544.
20. Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg. 2007;32(10):1605-1623.
21. Williams BG, Sotereanos DG, Baratz ME, Jarrett CD, Venouziou AI, Miller MC. The contracted elbow: is ulnar nerve release necessary? J Shoulder Elbow Surg. 2012;21(12):
1632-1636.
22. Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492-501.
23. Blonna D, O’Driscoll SW. Delayed-onset ulnar neuritis after release of elbow contracture: preventive strategies derived from a study of 563 cases. Arthroscopy. 2014;30(8):947-956.
24. Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg. 2000;82(9):1260-1268.
25. Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181-188.
Take-Home Points
- Proper patient selection is critical as extensive postoperative rehabilitation is required to obtain an excellent outcome.
- Open and arthroscopic approaches are effective treatment options for elbow contractures.
- Elbow stability must be restored to obtain a successful outcome.
- Knowledge of neurovascular anatomy is essential to prevent neurologic complications.
- Prophylactic ulnar nerve release should be considered, especially in patients with limited flexion.
Elbow stiffness has several etiologies, posttraumatic being the most common. Elbow stiffness can have debilitating functional effects necessitating treatment. In a biomechanical study of normal elbow function, Morrey and colleagues1 determined that a flexion extension arc of 100° (30°-130°) and a forearm rotation arc of 100° (50° pronation-50° supination) are required in 90% of activities of daily living. Similarly, elbow flexion of <105° was poorly tolerated, whereas patients could easier adapt to flexion contractures up to 40°.2
The goal of initial evaluation should be to establish the cause of the contracture and the patient’s functional demands and ability to cooperate in the extensive postoperative rehabilitation that is essential in achieving an excellent functional outcome. In a thorough clinical examination, the clinician must note skin, range of motion (ROM), ligamentous stability, and neurovascular structures and give special attention to ulnar nerve function and symptoms. Mid-arc pain suggests additional intra-articular pathology, as stiffness typically causes pain only at the limits of motion as osteophytes impinge and soft tissue is under maximal tension. Routine elbow radiographs are required in all cases, and computed tomography (CT) can be useful in evaluating osseous sources of contracture. Suspected ligamentous instability and cartilaginous defects particularly in the setting of mid-arc pain are best evaluated with magnetic resonance imaging.3
In this 5-point review, we evaluate treatment options as well as rehabilitation protocols in the management of elbow stiffness.
1 Anatomy of Contracture: The Usual Suspects
The cause of elbow stiffness is incompletely understood. Several posited contributing factors include biology, complex intra-articular anatomy, capsular distention favoring a flexed position, and tenuous postoperative fixation necessitating prolonged immobilization. Identifying intrinsic and extrinsic anatomical sources of stiffness can help guide treatment.4 Intrinsic pathology includes intra-articular malunion, osteophytes, loose bodies, and adhesions; extrinsic pathology includes soft-tissue contracture, heterotopic ossification, and extra-articular malunion.
Compared with the normal elbow, the capsule becomes thickened and fibrotic and thereby prevents motion. Severe contractures, and extension contractures in particular, may require release of the posterior medial capsule and the posterior medial collateral ligament (MCL) to regain motion. In a series of 42 patients with flexion <100°, Park and colleagues5 noted that all patients required release of the posterior band of the MCL to regain flexion. Other muscular impediments to motion include contracture of the brachialis and scarring of the triceps to the posterior humerus. Scarring of the triceps to the humerus can limit flexion.
In the posttrauma setting, intra-articular and extra-articular malunion must be considered. Extension malunion of the distal humerus can reduce flexion,6 and shortening with compromise of the olecranon and coronoid fossae can limit both flexion and extension.
Last, heterotopic ossification and osteophytes should be assessed as potential causes of limited ROM. Both the coronoid process and the olecranon can develop osteophytes, and their respective fossae should be assessed with CT. Posterior impingement is rare at the tip of the olecranon; it occurs because of "widening" of the olecranon by "Mickey Mouse ear" osteophytes and bony encroachment along the medial and lateral columns. Thus, the olecranon must be narrowed and the fossa widened and deepened.
In case of concomitant ligament instability, we prefer to reconstruct the ligament first, and then perform contracture release as a staged procedure. We favor a staged approach because the rehabilitation regimens for instability and contracture release are diametrically opposed: Instability requires immobilization, and contracture release requires immediate motion. Last, incision placement and ulnar nerve management are crucial in minimizing the potential complications of the second procedure.
2 Nonoperative Treatment
In the absence of significant bony impediments to motion—such as heterotopic ossification or malunion—initial treatment should commence with nonoperative therapy. Therapy should be initiated as soon as concern for stiffness arises in order to prevent contracture. Initial nonoperative treatment can also serve as an important litmus test of postoperative adherence. Adequate patient relaxation is crucial in avoiding co-contracture resisting stretching forces. Passive ROM exercises use sustained force to allow time-dependent stress relaxation to increase tissue length as well as fatigue antagonist muscles. In addition, hold-and-relax techniques apply isometric resistance to induce relaxation of antagonist muscles.7 Active ROM should emphasize triceps isolation and elbow extension to prevent scarring of the triceps to the posterior humerus.
Corrective splinting can be an effective adjuvant to physiotherapy. Static progressive turnbuckle splints was described as an effective treatment for both elbow flexion and extension contractures, effecting an average 43° increase in elbow motion in a series of 15 patients.8 Similarly, Gelinas and colleagues9 noted improvement among 22 patients treated with turnbuckle splinting for an average of 4.5 months. In addition, serial extension splints may be used in the treatment of elbow flexion contractures.
3 Open Contacture Release and Surgical Approach
When nonoperative therapies fail to restore the functional arc of motion, patients with flexion contractures or extension contractures of >30° may be indicated for contracture release. Surgical approach should be determined by meticulous preoperative planning that notes prior incisions and CT findings. It can be helpful to organize common offending structures and their effects on flexion and extension (Table).
A medial over-the-top approach uses the medial supracondylar ridge as a landmark, subperiosteally reflecting the brachialis anteriorly.10 The ulnar nerve is neurolyzed and protected posteriorly. The flexor-pronator mass is split distally and elevated along with the brachialis as a single sleeve of muscle. The coronal plane of dissection should be the anterior half of the lateral epicondyle to avoid injury to the MCL. Large Bennett or Hohmann retractors can hinge on the lateral border of the humerus and provide clear visualization of the anterior capsule and the ulnohumeral joint. Exposure of the radiocapitellar joint is possible, but this joint is very deep in the operative field, and caution should be taken excising the anterolateral capsule because of the risk of radial nerve injury. The ulnar nerve can be temporarily transposed anteriorly to dissect posteriorly along the supracondylar ridge of the humerus. The triceps is reflected off the distal humerus. Occasionally, the posterior band of the MCL must be resected in severe extension contractures. If possible, the anterior bundle should be preserved. With this approach, the anterior capsule, distal humerus, coronoid process, posterior MCL, posterior capsule, and triceps can be addressed. The zone anterior to the radial head and the anterolateral and posterolateral capsule cannot be safely exposed with a medial approach. As described by Wada and colleagues,11 a primarily medial approach resulted in an average 64° increase in arc of motion.
an internal joint stabilizer (Skeletal Dynamics) (Figure 4) and to initiate motion therapy immediately. External fixation (hinged or unhinged is rarely used in our practice.
4 Arthroscopic Contracture Release and Technique
Recently, arthroscopic elbow contracture release, a technically demanding but effective treatment option, has gained popularity. Knowledge of neurovascular anatomy is a prerequisite to the prevention of devastating neurologic complications (ulnar, median, and radial nerve transections have been described14,15). Relative contraindications include extensive heterotopic ossification, ulnar nerve transposition, and limited arthroscopic experience. Functional improvements as well as average 26° to 42° increases in arc of motion have been described with arthroscopic release.16-18 In thin-framed patients with dense elbow capsular scarring (severe loss of elbow motion with hard block) and small joint space, arthroscopic release and particularly arthroscope insertion are notoriously difficult.
The patient may be placed in the prone, lateral decubitus, or supine position, depending on surgeon preference (Figure 5). Before surgery, portals and the ulnar nerve should be carefully outlined.19
We prefer to start by entering the posterior compartment and using the shaver to create a working space. All bone work and resectioning should be performed before capsular resection. After the joint and the olecranon fossa are identified, soft-tissue and bony débridement of the olecranon and the fossa can be performed. Care should be taken to protect the ulnar nerve when the posteromedial corner or medial gutter is approached.
5 Additional Considerations
After surgery, the elbow is immobilized in maximal extension and supination with an anterior splint, and therapy is initiated either immediately or after temporary immobilization.16,19,20 Regional anesthesia is crucial in obtaining adequate pain control and establishing an immediate postoperative therapy program. The utility of continuous passive motion (CPM) in postoperative protocols is controversial. A retrospective case-control study of 32 patients matched on age, diagnosis, and contraction severity found no benefit of CPM use, and increased costs and hospital length of stay, leading the authors to recommend against CPM use.20
Neurovascular risks are associated with both open and arthroscopic elbow contracture release. Particularly concerning is the risk of traction ulnar neuropathy, described in upward of 20% of patients.21 Anatomical studies have found decreases in cubital tunnel and ulnar nerve area as elbow flexion increases with corresponding increased intraneural pressure,22 leading some authors to recommend prophylactic ulnar nerve release with limited preoperative flexion.15 Nevertheless, despite transposition, ulnar nerve symptoms were noted in 8 of 40 patients who underwent open contracture release for posttraumatic loss of elbow flexion.5 In a retrospective review of 164 open and arthroscopic elbow contracture releases, Williams and colleagues21 noted an 8.1% rate of postoperative new-onset ulnar nerve symptoms. The rate of ulnar neuropathy was nonsignificantly elevated among patients with preoperative flexion of <100° (15.2% vs 3.7%; P = .057). Recently, a retrospective review of 564 consecutive arthroscopic contracture releases found a significantly higher rate of delayed-onset ulnar neuritis among patients without prophylactic ulnar nerve decompression or transposition (11% vs 3%; P < .001).23 Further analysis revealed that, compared with decompression, ulnar nerve transposition did not offer additional benefit but was associated with a significantly higher rate of wound complications (19% vs 4%; P = .03). We favor prophylactic release, particularly in the setting of preoperative extension contracture. For open contracture release from the lateral approach, however, we do not routinely release the ulnar nerve unless there were preoperative symptoms.
Although open and arthroscopic contracture releases can provide durable outcomes in the setting of painless elbow stiffness, options are more limited in the treatment of the painful stiff elbow. Total elbow arthroplasty remains an option in low-demand elderly patients but is not without significant risk of complications.24 In addition, durability concerns and postoperative restrictions make total elbow arthroplasty less attractive to younger patients. Interposition arthroplasty may be indicated as a salvage procedure in the treatment of a young or high-demand patient with a stiff painful elbow.25 Elbow stability is crucial in obtaining a successful outcome, and data on optimal graft choices are limited.
Conclusion
Elbow stiffness, a common complication of trauma, significantly impairs activities of daily living. Early after trauma, therapy should be initiated to prevent contracture. In the absence of symptomatic arthritis, both open and arthroscopic contracture releases are effective surgical treatments in properly selected and motivated patients. Although more research is needed to establish the optimal surgical approach, severity and anatomical cause of contracture should guide decisions as to which approach to use. Having a thorough understanding of neurovascular anatomy and of prophylactic ulnar nerve decompression in the setting of limited preoperative flexion can mitigate complications.
Take-Home Points
- Proper patient selection is critical as extensive postoperative rehabilitation is required to obtain an excellent outcome.
- Open and arthroscopic approaches are effective treatment options for elbow contractures.
- Elbow stability must be restored to obtain a successful outcome.
- Knowledge of neurovascular anatomy is essential to prevent neurologic complications.
- Prophylactic ulnar nerve release should be considered, especially in patients with limited flexion.
Elbow stiffness has several etiologies, posttraumatic being the most common. Elbow stiffness can have debilitating functional effects necessitating treatment. In a biomechanical study of normal elbow function, Morrey and colleagues1 determined that a flexion extension arc of 100° (30°-130°) and a forearm rotation arc of 100° (50° pronation-50° supination) are required in 90% of activities of daily living. Similarly, elbow flexion of <105° was poorly tolerated, whereas patients could easier adapt to flexion contractures up to 40°.2
The goal of initial evaluation should be to establish the cause of the contracture and the patient’s functional demands and ability to cooperate in the extensive postoperative rehabilitation that is essential in achieving an excellent functional outcome. In a thorough clinical examination, the clinician must note skin, range of motion (ROM), ligamentous stability, and neurovascular structures and give special attention to ulnar nerve function and symptoms. Mid-arc pain suggests additional intra-articular pathology, as stiffness typically causes pain only at the limits of motion as osteophytes impinge and soft tissue is under maximal tension. Routine elbow radiographs are required in all cases, and computed tomography (CT) can be useful in evaluating osseous sources of contracture. Suspected ligamentous instability and cartilaginous defects particularly in the setting of mid-arc pain are best evaluated with magnetic resonance imaging.3
In this 5-point review, we evaluate treatment options as well as rehabilitation protocols in the management of elbow stiffness.
1 Anatomy of Contracture: The Usual Suspects
The cause of elbow stiffness is incompletely understood. Several posited contributing factors include biology, complex intra-articular anatomy, capsular distention favoring a flexed position, and tenuous postoperative fixation necessitating prolonged immobilization. Identifying intrinsic and extrinsic anatomical sources of stiffness can help guide treatment.4 Intrinsic pathology includes intra-articular malunion, osteophytes, loose bodies, and adhesions; extrinsic pathology includes soft-tissue contracture, heterotopic ossification, and extra-articular malunion.
Compared with the normal elbow, the capsule becomes thickened and fibrotic and thereby prevents motion. Severe contractures, and extension contractures in particular, may require release of the posterior medial capsule and the posterior medial collateral ligament (MCL) to regain motion. In a series of 42 patients with flexion <100°, Park and colleagues5 noted that all patients required release of the posterior band of the MCL to regain flexion. Other muscular impediments to motion include contracture of the brachialis and scarring of the triceps to the posterior humerus. Scarring of the triceps to the humerus can limit flexion.
In the posttrauma setting, intra-articular and extra-articular malunion must be considered. Extension malunion of the distal humerus can reduce flexion,6 and shortening with compromise of the olecranon and coronoid fossae can limit both flexion and extension.
Last, heterotopic ossification and osteophytes should be assessed as potential causes of limited ROM. Both the coronoid process and the olecranon can develop osteophytes, and their respective fossae should be assessed with CT. Posterior impingement is rare at the tip of the olecranon; it occurs because of "widening" of the olecranon by "Mickey Mouse ear" osteophytes and bony encroachment along the medial and lateral columns. Thus, the olecranon must be narrowed and the fossa widened and deepened.
In case of concomitant ligament instability, we prefer to reconstruct the ligament first, and then perform contracture release as a staged procedure. We favor a staged approach because the rehabilitation regimens for instability and contracture release are diametrically opposed: Instability requires immobilization, and contracture release requires immediate motion. Last, incision placement and ulnar nerve management are crucial in minimizing the potential complications of the second procedure.
2 Nonoperative Treatment
In the absence of significant bony impediments to motion—such as heterotopic ossification or malunion—initial treatment should commence with nonoperative therapy. Therapy should be initiated as soon as concern for stiffness arises in order to prevent contracture. Initial nonoperative treatment can also serve as an important litmus test of postoperative adherence. Adequate patient relaxation is crucial in avoiding co-contracture resisting stretching forces. Passive ROM exercises use sustained force to allow time-dependent stress relaxation to increase tissue length as well as fatigue antagonist muscles. In addition, hold-and-relax techniques apply isometric resistance to induce relaxation of antagonist muscles.7 Active ROM should emphasize triceps isolation and elbow extension to prevent scarring of the triceps to the posterior humerus.
Corrective splinting can be an effective adjuvant to physiotherapy. Static progressive turnbuckle splints was described as an effective treatment for both elbow flexion and extension contractures, effecting an average 43° increase in elbow motion in a series of 15 patients.8 Similarly, Gelinas and colleagues9 noted improvement among 22 patients treated with turnbuckle splinting for an average of 4.5 months. In addition, serial extension splints may be used in the treatment of elbow flexion contractures.
3 Open Contacture Release and Surgical Approach
When nonoperative therapies fail to restore the functional arc of motion, patients with flexion contractures or extension contractures of >30° may be indicated for contracture release. Surgical approach should be determined by meticulous preoperative planning that notes prior incisions and CT findings. It can be helpful to organize common offending structures and their effects on flexion and extension (Table).
A medial over-the-top approach uses the medial supracondylar ridge as a landmark, subperiosteally reflecting the brachialis anteriorly.10 The ulnar nerve is neurolyzed and protected posteriorly. The flexor-pronator mass is split distally and elevated along with the brachialis as a single sleeve of muscle. The coronal plane of dissection should be the anterior half of the lateral epicondyle to avoid injury to the MCL. Large Bennett or Hohmann retractors can hinge on the lateral border of the humerus and provide clear visualization of the anterior capsule and the ulnohumeral joint. Exposure of the radiocapitellar joint is possible, but this joint is very deep in the operative field, and caution should be taken excising the anterolateral capsule because of the risk of radial nerve injury. The ulnar nerve can be temporarily transposed anteriorly to dissect posteriorly along the supracondylar ridge of the humerus. The triceps is reflected off the distal humerus. Occasionally, the posterior band of the MCL must be resected in severe extension contractures. If possible, the anterior bundle should be preserved. With this approach, the anterior capsule, distal humerus, coronoid process, posterior MCL, posterior capsule, and triceps can be addressed. The zone anterior to the radial head and the anterolateral and posterolateral capsule cannot be safely exposed with a medial approach. As described by Wada and colleagues,11 a primarily medial approach resulted in an average 64° increase in arc of motion.
an internal joint stabilizer (Skeletal Dynamics) (Figure 4) and to initiate motion therapy immediately. External fixation (hinged or unhinged is rarely used in our practice.
4 Arthroscopic Contracture Release and Technique
Recently, arthroscopic elbow contracture release, a technically demanding but effective treatment option, has gained popularity. Knowledge of neurovascular anatomy is a prerequisite to the prevention of devastating neurologic complications (ulnar, median, and radial nerve transections have been described14,15). Relative contraindications include extensive heterotopic ossification, ulnar nerve transposition, and limited arthroscopic experience. Functional improvements as well as average 26° to 42° increases in arc of motion have been described with arthroscopic release.16-18 In thin-framed patients with dense elbow capsular scarring (severe loss of elbow motion with hard block) and small joint space, arthroscopic release and particularly arthroscope insertion are notoriously difficult.
The patient may be placed in the prone, lateral decubitus, or supine position, depending on surgeon preference (Figure 5). Before surgery, portals and the ulnar nerve should be carefully outlined.19
We prefer to start by entering the posterior compartment and using the shaver to create a working space. All bone work and resectioning should be performed before capsular resection. After the joint and the olecranon fossa are identified, soft-tissue and bony débridement of the olecranon and the fossa can be performed. Care should be taken to protect the ulnar nerve when the posteromedial corner or medial gutter is approached.
5 Additional Considerations
After surgery, the elbow is immobilized in maximal extension and supination with an anterior splint, and therapy is initiated either immediately or after temporary immobilization.16,19,20 Regional anesthesia is crucial in obtaining adequate pain control and establishing an immediate postoperative therapy program. The utility of continuous passive motion (CPM) in postoperative protocols is controversial. A retrospective case-control study of 32 patients matched on age, diagnosis, and contraction severity found no benefit of CPM use, and increased costs and hospital length of stay, leading the authors to recommend against CPM use.20
Neurovascular risks are associated with both open and arthroscopic elbow contracture release. Particularly concerning is the risk of traction ulnar neuropathy, described in upward of 20% of patients.21 Anatomical studies have found decreases in cubital tunnel and ulnar nerve area as elbow flexion increases with corresponding increased intraneural pressure,22 leading some authors to recommend prophylactic ulnar nerve release with limited preoperative flexion.15 Nevertheless, despite transposition, ulnar nerve symptoms were noted in 8 of 40 patients who underwent open contracture release for posttraumatic loss of elbow flexion.5 In a retrospective review of 164 open and arthroscopic elbow contracture releases, Williams and colleagues21 noted an 8.1% rate of postoperative new-onset ulnar nerve symptoms. The rate of ulnar neuropathy was nonsignificantly elevated among patients with preoperative flexion of <100° (15.2% vs 3.7%; P = .057). Recently, a retrospective review of 564 consecutive arthroscopic contracture releases found a significantly higher rate of delayed-onset ulnar neuritis among patients without prophylactic ulnar nerve decompression or transposition (11% vs 3%; P < .001).23 Further analysis revealed that, compared with decompression, ulnar nerve transposition did not offer additional benefit but was associated with a significantly higher rate of wound complications (19% vs 4%; P = .03). We favor prophylactic release, particularly in the setting of preoperative extension contracture. For open contracture release from the lateral approach, however, we do not routinely release the ulnar nerve unless there were preoperative symptoms.
Although open and arthroscopic contracture releases can provide durable outcomes in the setting of painless elbow stiffness, options are more limited in the treatment of the painful stiff elbow. Total elbow arthroplasty remains an option in low-demand elderly patients but is not without significant risk of complications.24 In addition, durability concerns and postoperative restrictions make total elbow arthroplasty less attractive to younger patients. Interposition arthroplasty may be indicated as a salvage procedure in the treatment of a young or high-demand patient with a stiff painful elbow.25 Elbow stability is crucial in obtaining a successful outcome, and data on optimal graft choices are limited.
Conclusion
Elbow stiffness, a common complication of trauma, significantly impairs activities of daily living. Early after trauma, therapy should be initiated to prevent contracture. In the absence of symptomatic arthritis, both open and arthroscopic contracture releases are effective surgical treatments in properly selected and motivated patients. Although more research is needed to establish the optimal surgical approach, severity and anatomical cause of contracture should guide decisions as to which approach to use. Having a thorough understanding of neurovascular anatomy and of prophylactic ulnar nerve decompression in the setting of limited preoperative flexion can mitigate complications.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Hotchkiss RN. Elbow contracture. In: Green DP, Rotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. New York, NY: Churchill-Livingstone; 2005:667-682.
3. Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19(2):13-19.
4. Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601-618.
5. Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692-2699.
6. Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg Am. 2009;34(7):
1256-1260.
7. Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300-309.
8. Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092-1095.
9. Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74-78.
10. Hotchkiss RN, Kasparyan GN. The medial "over the top" approach to the elbow. Tech Orthop. 2000;15(2):105-112.
11. Wada T, Ishii S, Usui M, Miyano S. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Br. 2000;82(1):68-73.
12. Husband JB, Hastings H. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353-1358.
13. Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603-1605.
14. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83(1):25-34.
16. Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11(6):624-629.
17. Ćefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434-439.
18. Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842-849.
19. Sahajpal D, Choi T, Wright TW. Arthroscopic release of the stiff elbow. J Hand Surg. 2009;34(3):540-544.
20. Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg. 2007;32(10):1605-1623.
21. Williams BG, Sotereanos DG, Baratz ME, Jarrett CD, Venouziou AI, Miller MC. The contracted elbow: is ulnar nerve release necessary? J Shoulder Elbow Surg. 2012;21(12):
1632-1636.
22. Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492-501.
23. Blonna D, O’Driscoll SW. Delayed-onset ulnar neuritis after release of elbow contracture: preventive strategies derived from a study of 563 cases. Arthroscopy. 2014;30(8):947-956.
24. Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg. 2000;82(9):1260-1268.
25. Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181-188.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Hotchkiss RN. Elbow contracture. In: Green DP, Rotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. New York, NY: Churchill-Livingstone; 2005:667-682.
3. Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19(2):13-19.
4. Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601-618.
5. Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692-2699.
6. Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg Am. 2009;34(7):
1256-1260.
7. Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300-309.
8. Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092-1095.
9. Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74-78.
10. Hotchkiss RN, Kasparyan GN. The medial "over the top" approach to the elbow. Tech Orthop. 2000;15(2):105-112.
11. Wada T, Ishii S, Usui M, Miyano S. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Br. 2000;82(1):68-73.
12. Husband JB, Hastings H. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353-1358.
13. Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603-1605.
14. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83(1):25-34.
16. Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11(6):624-629.
17. Ćefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434-439.
18. Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842-849.
19. Sahajpal D, Choi T, Wright TW. Arthroscopic release of the stiff elbow. J Hand Surg. 2009;34(3):540-544.
20. Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg. 2007;32(10):1605-1623.
21. Williams BG, Sotereanos DG, Baratz ME, Jarrett CD, Venouziou AI, Miller MC. The contracted elbow: is ulnar nerve release necessary? J Shoulder Elbow Surg. 2012;21(12):
1632-1636.
22. Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492-501.
23. Blonna D, O’Driscoll SW. Delayed-onset ulnar neuritis after release of elbow contracture: preventive strategies derived from a study of 563 cases. Arthroscopy. 2014;30(8):947-956.
24. Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg. 2000;82(9):1260-1268.
25. Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181-188.
Pronator Teres Myotendinous Tear
I read with interest the article "Pronator Teres Myotendinous Tear" by Drs. Qayyum, Villacis, and Jobin (Am J Orthop. 2017;46(2):E105-E107), and I commend the authors for their interesting exploration of this unusual injury.
I would like to note that this pathology was previously reported by our group in 2015.1 We now have a 2-year follow-up on this patient, and he has remained asymptomatic since his return to golf. Since this article was published, we have been contacted by 3 patients (one of whom is a radiologist who interpreted his own magnetic resonance imaging) describing similar mechanisms of injury, symptoms, imaging findings, and recovery with nonoperative management. This suggests that pronator teres rupture may have been previously unrecognized or underreported.
It is interesting that this patient was injured when his club stuck in the ground while our patient reported taking only a small divot during his injury. From these differing mechanisms it is unclear whether forceful contraction or sudden loading is the largest risk factor for obtaining this injury, and this could be a point for further research. As awareness of this injury pattern spreads, we look forward to seeing larger series and establishing the success rate of nonoperative treatment and the risk factors for its failure.
Brooks W. Ficke, MD
Brent A. Ponce, MD
Birmingham, AL
Authors' Response
We appreciate Dr. Ficke’s comments regarding his experience treating pronator teres injuries and agree that they are likely under-recognized and possibly underreported. We are uncertain which mechanisms during the golf swing strains the pronator teres to the point of injury, but it may be a combination of muscular fatigue, forceful contraction, and sudden resistance to concentric loading during the club striking the ground. In our experience, these injuries do appear to heal without observable deficit. Our patient is back golfing regularly without any arm symptoms and actually had an improvement in his golf handicap this season.
Charles M. Jobin, MD
Usama Qayyum, MBBS
Diego Villacis, MD
New York, NY
1. Ficke BW, Larrison MC, Ponce BA. Isolated rupture of the pronator teres in an amateur golfer: a case report. Int J Orthop. 2015;2(6):481-483.
I read with interest the article "Pronator Teres Myotendinous Tear" by Drs. Qayyum, Villacis, and Jobin (Am J Orthop. 2017;46(2):E105-E107), and I commend the authors for their interesting exploration of this unusual injury.
I would like to note that this pathology was previously reported by our group in 2015.1 We now have a 2-year follow-up on this patient, and he has remained asymptomatic since his return to golf. Since this article was published, we have been contacted by 3 patients (one of whom is a radiologist who interpreted his own magnetic resonance imaging) describing similar mechanisms of injury, symptoms, imaging findings, and recovery with nonoperative management. This suggests that pronator teres rupture may have been previously unrecognized or underreported.
It is interesting that this patient was injured when his club stuck in the ground while our patient reported taking only a small divot during his injury. From these differing mechanisms it is unclear whether forceful contraction or sudden loading is the largest risk factor for obtaining this injury, and this could be a point for further research. As awareness of this injury pattern spreads, we look forward to seeing larger series and establishing the success rate of nonoperative treatment and the risk factors for its failure.
Brooks W. Ficke, MD
Brent A. Ponce, MD
Birmingham, AL
Authors' Response
We appreciate Dr. Ficke’s comments regarding his experience treating pronator teres injuries and agree that they are likely under-recognized and possibly underreported. We are uncertain which mechanisms during the golf swing strains the pronator teres to the point of injury, but it may be a combination of muscular fatigue, forceful contraction, and sudden resistance to concentric loading during the club striking the ground. In our experience, these injuries do appear to heal without observable deficit. Our patient is back golfing regularly without any arm symptoms and actually had an improvement in his golf handicap this season.
Charles M. Jobin, MD
Usama Qayyum, MBBS
Diego Villacis, MD
New York, NY
I read with interest the article "Pronator Teres Myotendinous Tear" by Drs. Qayyum, Villacis, and Jobin (Am J Orthop. 2017;46(2):E105-E107), and I commend the authors for their interesting exploration of this unusual injury.
I would like to note that this pathology was previously reported by our group in 2015.1 We now have a 2-year follow-up on this patient, and he has remained asymptomatic since his return to golf. Since this article was published, we have been contacted by 3 patients (one of whom is a radiologist who interpreted his own magnetic resonance imaging) describing similar mechanisms of injury, symptoms, imaging findings, and recovery with nonoperative management. This suggests that pronator teres rupture may have been previously unrecognized or underreported.
It is interesting that this patient was injured when his club stuck in the ground while our patient reported taking only a small divot during his injury. From these differing mechanisms it is unclear whether forceful contraction or sudden loading is the largest risk factor for obtaining this injury, and this could be a point for further research. As awareness of this injury pattern spreads, we look forward to seeing larger series and establishing the success rate of nonoperative treatment and the risk factors for its failure.
Brooks W. Ficke, MD
Brent A. Ponce, MD
Birmingham, AL
Authors' Response
We appreciate Dr. Ficke’s comments regarding his experience treating pronator teres injuries and agree that they are likely under-recognized and possibly underreported. We are uncertain which mechanisms during the golf swing strains the pronator teres to the point of injury, but it may be a combination of muscular fatigue, forceful contraction, and sudden resistance to concentric loading during the club striking the ground. In our experience, these injuries do appear to heal without observable deficit. Our patient is back golfing regularly without any arm symptoms and actually had an improvement in his golf handicap this season.
Charles M. Jobin, MD
Usama Qayyum, MBBS
Diego Villacis, MD
New York, NY
1. Ficke BW, Larrison MC, Ponce BA. Isolated rupture of the pronator teres in an amateur golfer: a case report. Int J Orthop. 2015;2(6):481-483.
1. Ficke BW, Larrison MC, Ponce BA. Isolated rupture of the pronator teres in an amateur golfer: a case report. Int J Orthop. 2015;2(6):481-483.
Patient Preference Before and After Arthroscopic Rotator Cuff Repair: Which Is More Important, Pain Relief or Strength Return?
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.