User login
Joint guidelines favor antibody testing for certain Lyme disease manifestations
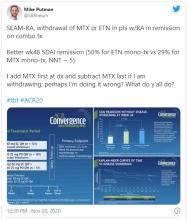
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
FROM CLINICAL INFECTIOUS DISEASES
How Twitter amplifies my doctor and human voice
When I graduated from residency in 2007, Facebook had just become “a thing,” and my cohort decided to use it to keep in touch. These days, Twitter seems to be the social media platform of choice for health care professionals.
When I started on Twitter a few years ago, it was in reaction to the current political climate. I wanted to keep track of what my favorite thinkers were writing. I was anonymous and tweeted about politics mostly. My husband was my only follower for a while.
I deanonymized when, at last year’s American College of Rheumatology meeting, I presented a poster and wanted to reach a wider audience. I could have created two different personas on Twitter, like many doctors apparently do. Initially, I resisted doing that because I am frankly too lazy to keep track of two different social media profiles, but now I resist because I see my profession as an extension of my political self, and have no problem with using my (very low) profile to amplify both my doctor voice and my human voice.
Professionally, Twitter is rewarding. It is a space for networking and for promoting one’s work. It is a fantastic learning format, as evidenced by the popularity of tweetorials. The international consortium that has worked to collect information on rheumatology patients with COVID started as an idea on Twitter. The fact that ACR Convergence 2020 abstracts are now available? I only know because of the #ACRambassadors that I follow.
But I find that I cannot separate who I am from what I do. As a rheumatologist, I build long-term relationships with patients. I cannot care for their medical conditions in isolation without also concerning myself with their nonmedical circumstances. For that reason, I have opinions that one might call humanist, and I suspect that I am not alone among rheumatologists.
I can think of three areas, broadly construed but with huge overlaps, that concern me a great deal.
First, there are things that affect all physicians: race and gender discrimination in the workplace; advancement of women in science, technology, engineering, or math; Medicare reimbursement; COVID-19 preparedness; immigration issues (an issue near and dear to me, as I am an immigrant and a foreign medical graduate); and federal funding (including funding for training programs and community health centers, funding for the National Institutes of Health, and funding for stem cell research).
Then there are the things that affect rheumatologists in particular. Access to medications and procedures is one thing. (I did say these categories hugely overlap.) If you›ve ever tried to prescribe even a drug as old as oral cyclophosphamide, you’ll have experienced the difficulty of getting it for Medicare patients. Patients who need biologics are limited by insurance contracts with pharmaceutical companies, but also by requirements such as step therapy. I am all varieties of annoyed, incredulous, and apologetic that when a patient asks me how much a treatment will cost him/her, I do not have an answer.
Speaking of pricing, don’t even get me started on pharmaceutical company price gouging. Yes, the H.P. Acthar gel may be the most egregious offender among rheumatology medications, but it’s easy to not prescribe a drug that costs $80,000 a vial and which does not do much more than prednisone does. On the other hand, I remember a time when colchicine cost $0.10 cents a pill and patients did not have to jump through hoops to get it.
And what of reproductive freedom? Our patients rely on us for advice about their childbearing options, including birth control, in vitro fertilization, and pregnancy termination.
Finally, and most important, the things that affect me most are the issues that affect patients. The lowest-hanging fruit here is the abject incompetence of the federal response to the ongoing pandemic. How many of our patients’ lives have been lost or adversely affected? And what of coverage for preexisting conditions for the vast majority of our patients, whose illnesses are chronic?
While we’re at it, the fact of health insurance being tied to employment, something that seemingly no other country in the developed world does, makes living with chronic conditions outright scary, doesn’t it? It isn’t quite so easy to remain employed when one cannot get the right medications for RA.
I could go on. Gun violence and health care disparities, vaccine denialism, coverage for mental health issues, LGBTQ rights, refugee rights, police brutality … there is a seemingly endless list of things to care about. It’s exhausting.
While I do use my Twitter account to learn from colleagues and to promote work that interests me, my primary aim is to participate in civil society as a person. Critics will use “stay in your lane” as shorthand to say x professionals should stick to x (actors to acting, musicians to music, athletes to sports). If only I could. But my humanity won’t let me. Aristotle said man is a political animal; even the venerable New England Journal of Medicine has found it impossible to keep silent.
Karmela Kim Chan, MD, is an assistant professor at Weill Cornell Medicine, New York, and an attending physician at the Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center, both in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a past columnist for MDedge Rheumatology, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice.
A version of this article originally appeared on Medscape.com.
When I graduated from residency in 2007, Facebook had just become “a thing,” and my cohort decided to use it to keep in touch. These days, Twitter seems to be the social media platform of choice for health care professionals.
When I started on Twitter a few years ago, it was in reaction to the current political climate. I wanted to keep track of what my favorite thinkers were writing. I was anonymous and tweeted about politics mostly. My husband was my only follower for a while.
I deanonymized when, at last year’s American College of Rheumatology meeting, I presented a poster and wanted to reach a wider audience. I could have created two different personas on Twitter, like many doctors apparently do. Initially, I resisted doing that because I am frankly too lazy to keep track of two different social media profiles, but now I resist because I see my profession as an extension of my political self, and have no problem with using my (very low) profile to amplify both my doctor voice and my human voice.
Professionally, Twitter is rewarding. It is a space for networking and for promoting one’s work. It is a fantastic learning format, as evidenced by the popularity of tweetorials. The international consortium that has worked to collect information on rheumatology patients with COVID started as an idea on Twitter. The fact that ACR Convergence 2020 abstracts are now available? I only know because of the #ACRambassadors that I follow.
But I find that I cannot separate who I am from what I do. As a rheumatologist, I build long-term relationships with patients. I cannot care for their medical conditions in isolation without also concerning myself with their nonmedical circumstances. For that reason, I have opinions that one might call humanist, and I suspect that I am not alone among rheumatologists.
I can think of three areas, broadly construed but with huge overlaps, that concern me a great deal.
First, there are things that affect all physicians: race and gender discrimination in the workplace; advancement of women in science, technology, engineering, or math; Medicare reimbursement; COVID-19 preparedness; immigration issues (an issue near and dear to me, as I am an immigrant and a foreign medical graduate); and federal funding (including funding for training programs and community health centers, funding for the National Institutes of Health, and funding for stem cell research).
Then there are the things that affect rheumatologists in particular. Access to medications and procedures is one thing. (I did say these categories hugely overlap.) If you›ve ever tried to prescribe even a drug as old as oral cyclophosphamide, you’ll have experienced the difficulty of getting it for Medicare patients. Patients who need biologics are limited by insurance contracts with pharmaceutical companies, but also by requirements such as step therapy. I am all varieties of annoyed, incredulous, and apologetic that when a patient asks me how much a treatment will cost him/her, I do not have an answer.
Speaking of pricing, don’t even get me started on pharmaceutical company price gouging. Yes, the H.P. Acthar gel may be the most egregious offender among rheumatology medications, but it’s easy to not prescribe a drug that costs $80,000 a vial and which does not do much more than prednisone does. On the other hand, I remember a time when colchicine cost $0.10 cents a pill and patients did not have to jump through hoops to get it.
And what of reproductive freedom? Our patients rely on us for advice about their childbearing options, including birth control, in vitro fertilization, and pregnancy termination.
Finally, and most important, the things that affect me most are the issues that affect patients. The lowest-hanging fruit here is the abject incompetence of the federal response to the ongoing pandemic. How many of our patients’ lives have been lost or adversely affected? And what of coverage for preexisting conditions for the vast majority of our patients, whose illnesses are chronic?
While we’re at it, the fact of health insurance being tied to employment, something that seemingly no other country in the developed world does, makes living with chronic conditions outright scary, doesn’t it? It isn’t quite so easy to remain employed when one cannot get the right medications for RA.
I could go on. Gun violence and health care disparities, vaccine denialism, coverage for mental health issues, LGBTQ rights, refugee rights, police brutality … there is a seemingly endless list of things to care about. It’s exhausting.
While I do use my Twitter account to learn from colleagues and to promote work that interests me, my primary aim is to participate in civil society as a person. Critics will use “stay in your lane” as shorthand to say x professionals should stick to x (actors to acting, musicians to music, athletes to sports). If only I could. But my humanity won’t let me. Aristotle said man is a political animal; even the venerable New England Journal of Medicine has found it impossible to keep silent.
Karmela Kim Chan, MD, is an assistant professor at Weill Cornell Medicine, New York, and an attending physician at the Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center, both in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a past columnist for MDedge Rheumatology, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice.
A version of this article originally appeared on Medscape.com.
When I graduated from residency in 2007, Facebook had just become “a thing,” and my cohort decided to use it to keep in touch. These days, Twitter seems to be the social media platform of choice for health care professionals.
When I started on Twitter a few years ago, it was in reaction to the current political climate. I wanted to keep track of what my favorite thinkers were writing. I was anonymous and tweeted about politics mostly. My husband was my only follower for a while.
I deanonymized when, at last year’s American College of Rheumatology meeting, I presented a poster and wanted to reach a wider audience. I could have created two different personas on Twitter, like many doctors apparently do. Initially, I resisted doing that because I am frankly too lazy to keep track of two different social media profiles, but now I resist because I see my profession as an extension of my political self, and have no problem with using my (very low) profile to amplify both my doctor voice and my human voice.
Professionally, Twitter is rewarding. It is a space for networking and for promoting one’s work. It is a fantastic learning format, as evidenced by the popularity of tweetorials. The international consortium that has worked to collect information on rheumatology patients with COVID started as an idea on Twitter. The fact that ACR Convergence 2020 abstracts are now available? I only know because of the #ACRambassadors that I follow.
But I find that I cannot separate who I am from what I do. As a rheumatologist, I build long-term relationships with patients. I cannot care for their medical conditions in isolation without also concerning myself with their nonmedical circumstances. For that reason, I have opinions that one might call humanist, and I suspect that I am not alone among rheumatologists.
I can think of three areas, broadly construed but with huge overlaps, that concern me a great deal.
First, there are things that affect all physicians: race and gender discrimination in the workplace; advancement of women in science, technology, engineering, or math; Medicare reimbursement; COVID-19 preparedness; immigration issues (an issue near and dear to me, as I am an immigrant and a foreign medical graduate); and federal funding (including funding for training programs and community health centers, funding for the National Institutes of Health, and funding for stem cell research).
Then there are the things that affect rheumatologists in particular. Access to medications and procedures is one thing. (I did say these categories hugely overlap.) If you›ve ever tried to prescribe even a drug as old as oral cyclophosphamide, you’ll have experienced the difficulty of getting it for Medicare patients. Patients who need biologics are limited by insurance contracts with pharmaceutical companies, but also by requirements such as step therapy. I am all varieties of annoyed, incredulous, and apologetic that when a patient asks me how much a treatment will cost him/her, I do not have an answer.
Speaking of pricing, don’t even get me started on pharmaceutical company price gouging. Yes, the H.P. Acthar gel may be the most egregious offender among rheumatology medications, but it’s easy to not prescribe a drug that costs $80,000 a vial and which does not do much more than prednisone does. On the other hand, I remember a time when colchicine cost $0.10 cents a pill and patients did not have to jump through hoops to get it.
And what of reproductive freedom? Our patients rely on us for advice about their childbearing options, including birth control, in vitro fertilization, and pregnancy termination.
Finally, and most important, the things that affect me most are the issues that affect patients. The lowest-hanging fruit here is the abject incompetence of the federal response to the ongoing pandemic. How many of our patients’ lives have been lost or adversely affected? And what of coverage for preexisting conditions for the vast majority of our patients, whose illnesses are chronic?
While we’re at it, the fact of health insurance being tied to employment, something that seemingly no other country in the developed world does, makes living with chronic conditions outright scary, doesn’t it? It isn’t quite so easy to remain employed when one cannot get the right medications for RA.
I could go on. Gun violence and health care disparities, vaccine denialism, coverage for mental health issues, LGBTQ rights, refugee rights, police brutality … there is a seemingly endless list of things to care about. It’s exhausting.
While I do use my Twitter account to learn from colleagues and to promote work that interests me, my primary aim is to participate in civil society as a person. Critics will use “stay in your lane” as shorthand to say x professionals should stick to x (actors to acting, musicians to music, athletes to sports). If only I could. But my humanity won’t let me. Aristotle said man is a political animal; even the venerable New England Journal of Medicine has found it impossible to keep silent.
Karmela Kim Chan, MD, is an assistant professor at Weill Cornell Medicine, New York, and an attending physician at the Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center, both in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a past columnist for MDedge Rheumatology, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice.
A version of this article originally appeared on Medscape.com.
Colchicine a case study for what’s wrong with U.S. drug pricing
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Real acupuncture beat sham for osteoarthritis knee pain
Electro-acupuncture resulted in significant improvement in pain and function, compared with sham acupuncture, in a randomized trial of more than 400 adults with knee OA.
The socioeconomic burden of knee OA (KOA) remains high, and will likely increase with the aging population and rising rates of obesity, wrote first author Jian-Feng Tu, MD, PhD, of Beijing University of Chinese Medicine and colleagues. “Since no disease-modifying pharmaceutical agents have been approved, current KOA treatments are mainly symptomatic,” and identifying new therapies in addition to pharmacological agents or surgery is a research priority, they added. The research on acupuncture as a treatment for KOA has increased, but remains controversial as researchers attempt to determine the number of sessions needed for effectiveness.
In a study published in Arthritis & Rheumatology, the researchers recruited 480 adults aged 45-75 years with confirmed KOA who reported knee pain for longer than 6 months. Participants were randomized to three groups: electroacupuncture (EA), manual acupuncture (MA), or sham acupuncture (SA). Each group received three treatment sessions per week. In all groups, electrodes were attached to selected acupuncture needles, but the current was turned on only in the EA treatment group.
The primary outcome was the response rate after 8 weeks of treatment, defined as patients who achieved the minimal clinically important improvement (MCII) on both the Numeric Rating Scale and the Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
Overall, response rates at 8 weeks were 60.3%, 58.6%, and 47.3% for the EA, MA, and SA groups, respectively.
Between-group differences were statistically significant for EA versus SA (13%, P = .0234) but not for MA versus SA (11.3%, P = .0507) at 8 weeks; however, both EA and MA groups showed significantly higher response rates, compared with the SA group at 16 and 26 weeks. “Although a clinically meaningful response rate for KOA is not available in the literature, the difference of 11.3%, which indicates the number needed to treat of 9, is acceptable in clinical practices,” the researchers noted.
Adverse events occurred in 11.5% of the EA group, 14.2% of the MA group, and 10.8% of the SA group, and included subcutaneous hematoma, post-needling pain, and pantalgia. All adverse events related to acupuncture resolved within a week and none were serious, the researchers wrote.
The study findings were limited by several factors, including the potential burden on patients of three sessions per week, the limited study population of patients with radiologic grades of II or III only, the use of self-reports, and the lack of blinding for outcome assessors, the researchers noted.
However, the results show persistent effects in reducing pain and improving function with EA or MA, compared with SA, the researchers wrote. The findings were strengthened by “adequate dosage of acupuncture, the use of the primary outcome at an individual level, and the rigorous methodology.” The use of the MCII in the primary outcome “can provide patients and policy makers with more straightforward information to decide whether a treatment should be used.”
Optimal dosing questions remain
Current options for managing KOA are limited by factors that include low efficacy and unwanted side effects, while joint replacements increase the burden on health care systems, wrote David J. Hunter, MBBS, PhD, of the University of Sydney, and Richard E. Harris, PhD, of the University of Michigan, Ann Arbor, in an accompanying editorial. “In this context, development of new treatments or identification of efficacy of existing therapies to address the huge unmet need of pain are strongly desired.” Acupuncture continues to gain popularity in North and South America, but its efficacy for pain and KOA remain controversial.
The question of dose is challenging when assessing acupuncture because the optimal dose and how to classify it remains unknown. “In this study, the authors used three treatments a week, which is more frequent than typical studies done in the West and potentially may not be feasible in some health care settings. A recent systematic review suggests that treatment frequency matters and a dose of three sessions per week may be superior to less frequent treatment,” they emphasized. Acupuncture is generally considered to be safe, but many health systems do not reimburse for it. Patients may have large out-of-pocket expenses because of the number of visits required, which may be a barrier to further implementation in practice.
“Acupuncture is already widely practiced and readily available in many countries and health care systems,” the editorialists said. However, “more research is needed in the areas of dose-response relationships, effects of blinding the acupuncturist, feasibility of three times weekly regimens, and clarifying the mechanism of effect, particularly given the persistence of benefit.”
The study was funded by Beijing Municipal Science & Technology Commission and Beijing Municipal Administration of Hospitals. The researchers had no financial conflicts to disclose. Dr. Hunter disclosed support from a National Health and Medical Research Council Investigator Grant and providing consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly, and Pfizer.
SOURCE: Tu J-F et al. Arthritis Rheumatol. 2020 Nov 10. doi: 10.1002/art.41584.
Electro-acupuncture resulted in significant improvement in pain and function, compared with sham acupuncture, in a randomized trial of more than 400 adults with knee OA.
The socioeconomic burden of knee OA (KOA) remains high, and will likely increase with the aging population and rising rates of obesity, wrote first author Jian-Feng Tu, MD, PhD, of Beijing University of Chinese Medicine and colleagues. “Since no disease-modifying pharmaceutical agents have been approved, current KOA treatments are mainly symptomatic,” and identifying new therapies in addition to pharmacological agents or surgery is a research priority, they added. The research on acupuncture as a treatment for KOA has increased, but remains controversial as researchers attempt to determine the number of sessions needed for effectiveness.
In a study published in Arthritis & Rheumatology, the researchers recruited 480 adults aged 45-75 years with confirmed KOA who reported knee pain for longer than 6 months. Participants were randomized to three groups: electroacupuncture (EA), manual acupuncture (MA), or sham acupuncture (SA). Each group received three treatment sessions per week. In all groups, electrodes were attached to selected acupuncture needles, but the current was turned on only in the EA treatment group.
The primary outcome was the response rate after 8 weeks of treatment, defined as patients who achieved the minimal clinically important improvement (MCII) on both the Numeric Rating Scale and the Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
Overall, response rates at 8 weeks were 60.3%, 58.6%, and 47.3% for the EA, MA, and SA groups, respectively.
Between-group differences were statistically significant for EA versus SA (13%, P = .0234) but not for MA versus SA (11.3%, P = .0507) at 8 weeks; however, both EA and MA groups showed significantly higher response rates, compared with the SA group at 16 and 26 weeks. “Although a clinically meaningful response rate for KOA is not available in the literature, the difference of 11.3%, which indicates the number needed to treat of 9, is acceptable in clinical practices,” the researchers noted.
Adverse events occurred in 11.5% of the EA group, 14.2% of the MA group, and 10.8% of the SA group, and included subcutaneous hematoma, post-needling pain, and pantalgia. All adverse events related to acupuncture resolved within a week and none were serious, the researchers wrote.
The study findings were limited by several factors, including the potential burden on patients of three sessions per week, the limited study population of patients with radiologic grades of II or III only, the use of self-reports, and the lack of blinding for outcome assessors, the researchers noted.
However, the results show persistent effects in reducing pain and improving function with EA or MA, compared with SA, the researchers wrote. The findings were strengthened by “adequate dosage of acupuncture, the use of the primary outcome at an individual level, and the rigorous methodology.” The use of the MCII in the primary outcome “can provide patients and policy makers with more straightforward information to decide whether a treatment should be used.”
Optimal dosing questions remain
Current options for managing KOA are limited by factors that include low efficacy and unwanted side effects, while joint replacements increase the burden on health care systems, wrote David J. Hunter, MBBS, PhD, of the University of Sydney, and Richard E. Harris, PhD, of the University of Michigan, Ann Arbor, in an accompanying editorial. “In this context, development of new treatments or identification of efficacy of existing therapies to address the huge unmet need of pain are strongly desired.” Acupuncture continues to gain popularity in North and South America, but its efficacy for pain and KOA remain controversial.
The question of dose is challenging when assessing acupuncture because the optimal dose and how to classify it remains unknown. “In this study, the authors used three treatments a week, which is more frequent than typical studies done in the West and potentially may not be feasible in some health care settings. A recent systematic review suggests that treatment frequency matters and a dose of three sessions per week may be superior to less frequent treatment,” they emphasized. Acupuncture is generally considered to be safe, but many health systems do not reimburse for it. Patients may have large out-of-pocket expenses because of the number of visits required, which may be a barrier to further implementation in practice.
“Acupuncture is already widely practiced and readily available in many countries and health care systems,” the editorialists said. However, “more research is needed in the areas of dose-response relationships, effects of blinding the acupuncturist, feasibility of three times weekly regimens, and clarifying the mechanism of effect, particularly given the persistence of benefit.”
The study was funded by Beijing Municipal Science & Technology Commission and Beijing Municipal Administration of Hospitals. The researchers had no financial conflicts to disclose. Dr. Hunter disclosed support from a National Health and Medical Research Council Investigator Grant and providing consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly, and Pfizer.
SOURCE: Tu J-F et al. Arthritis Rheumatol. 2020 Nov 10. doi: 10.1002/art.41584.
Electro-acupuncture resulted in significant improvement in pain and function, compared with sham acupuncture, in a randomized trial of more than 400 adults with knee OA.
The socioeconomic burden of knee OA (KOA) remains high, and will likely increase with the aging population and rising rates of obesity, wrote first author Jian-Feng Tu, MD, PhD, of Beijing University of Chinese Medicine and colleagues. “Since no disease-modifying pharmaceutical agents have been approved, current KOA treatments are mainly symptomatic,” and identifying new therapies in addition to pharmacological agents or surgery is a research priority, they added. The research on acupuncture as a treatment for KOA has increased, but remains controversial as researchers attempt to determine the number of sessions needed for effectiveness.
In a study published in Arthritis & Rheumatology, the researchers recruited 480 adults aged 45-75 years with confirmed KOA who reported knee pain for longer than 6 months. Participants were randomized to three groups: electroacupuncture (EA), manual acupuncture (MA), or sham acupuncture (SA). Each group received three treatment sessions per week. In all groups, electrodes were attached to selected acupuncture needles, but the current was turned on only in the EA treatment group.
The primary outcome was the response rate after 8 weeks of treatment, defined as patients who achieved the minimal clinically important improvement (MCII) on both the Numeric Rating Scale and the Western Ontario and McMaster Universities Osteoarthritis Index function subscale.
Overall, response rates at 8 weeks were 60.3%, 58.6%, and 47.3% for the EA, MA, and SA groups, respectively.
Between-group differences were statistically significant for EA versus SA (13%, P = .0234) but not for MA versus SA (11.3%, P = .0507) at 8 weeks; however, both EA and MA groups showed significantly higher response rates, compared with the SA group at 16 and 26 weeks. “Although a clinically meaningful response rate for KOA is not available in the literature, the difference of 11.3%, which indicates the number needed to treat of 9, is acceptable in clinical practices,” the researchers noted.
Adverse events occurred in 11.5% of the EA group, 14.2% of the MA group, and 10.8% of the SA group, and included subcutaneous hematoma, post-needling pain, and pantalgia. All adverse events related to acupuncture resolved within a week and none were serious, the researchers wrote.
The study findings were limited by several factors, including the potential burden on patients of three sessions per week, the limited study population of patients with radiologic grades of II or III only, the use of self-reports, and the lack of blinding for outcome assessors, the researchers noted.
However, the results show persistent effects in reducing pain and improving function with EA or MA, compared with SA, the researchers wrote. The findings were strengthened by “adequate dosage of acupuncture, the use of the primary outcome at an individual level, and the rigorous methodology.” The use of the MCII in the primary outcome “can provide patients and policy makers with more straightforward information to decide whether a treatment should be used.”
Optimal dosing questions remain
Current options for managing KOA are limited by factors that include low efficacy and unwanted side effects, while joint replacements increase the burden on health care systems, wrote David J. Hunter, MBBS, PhD, of the University of Sydney, and Richard E. Harris, PhD, of the University of Michigan, Ann Arbor, in an accompanying editorial. “In this context, development of new treatments or identification of efficacy of existing therapies to address the huge unmet need of pain are strongly desired.” Acupuncture continues to gain popularity in North and South America, but its efficacy for pain and KOA remain controversial.
The question of dose is challenging when assessing acupuncture because the optimal dose and how to classify it remains unknown. “In this study, the authors used three treatments a week, which is more frequent than typical studies done in the West and potentially may not be feasible in some health care settings. A recent systematic review suggests that treatment frequency matters and a dose of three sessions per week may be superior to less frequent treatment,” they emphasized. Acupuncture is generally considered to be safe, but many health systems do not reimburse for it. Patients may have large out-of-pocket expenses because of the number of visits required, which may be a barrier to further implementation in practice.
“Acupuncture is already widely practiced and readily available in many countries and health care systems,” the editorialists said. However, “more research is needed in the areas of dose-response relationships, effects of blinding the acupuncturist, feasibility of three times weekly regimens, and clarifying the mechanism of effect, particularly given the persistence of benefit.”
The study was funded by Beijing Municipal Science & Technology Commission and Beijing Municipal Administration of Hospitals. The researchers had no financial conflicts to disclose. Dr. Hunter disclosed support from a National Health and Medical Research Council Investigator Grant and providing consulting advice for Merck Serono, TLC Bio, Tissuegene, Lilly, and Pfizer.
SOURCE: Tu J-F et al. Arthritis Rheumatol. 2020 Nov 10. doi: 10.1002/art.41584.
FROM ARTHRITIS & RHEUMATOLOGY
Cognitive Behavioral Therapy Plus Placebo Is Inferior to NSAID Therapy for Arthritis Pain
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Stopping methotrexate, staying on etanercept provides best RA outcomes after remission
In patients with RA whose disease is well controlled by methotrexate combined with etanercept, withdrawal of methotrexate led to long-term outcomes that were nearly as good as continuation of combination therapy. The finding comes from the randomized, controlled SEAM-RA trial that sought to address weaknesses of previous studies. It included a long lead-in time with stringent criteria to ensure that participants had very good disease control.
Both the American College of Rheumatology and the European League Against Rheumatism recommend tapering medication in RA patients who are in long-term remission, but there is no established optimal strategy.
“There have been some prior RA trials that have looked at therapy reduction or withdrawal, but most did not use a very stringent definition of how well people were when they began. Were they in remission, or only in low disease activity?” said Jeffrey R. Curtis, MD, during a presentation of the results at the virtual annual meeting of the ACR. The study was also published online Nov. 18 in Arthritis & Rheumatology.
Stringent remission criteria
The key feature of the trial was the 6-month run-in period, when subjects were taking 50 mg etanercept once per week and 10-25 mg of oral methotrexate once per week, and had to complete at least three visits. They were excluded from the ensuing randomization if they had a Simplified Disease Activity Index (SDAI) score >3.3 and ≤11 at two or more visits, had an SDAI >11 at any time during the run-in period, or had an SDAI >3.3 at the third run-in visit.
“We [wanted them] to be doing quite well for a long period of time. That was empirically confirmed under observation as part of the lead-in period, and even before that, the clinical investigator had to affirm that they believed the patient was doing well for 6 or more months even before they were screened to enter the trial,” said Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
Once enrolled in the trial, patients were randomized 2:2:1 to continuing etanercept only (n = 101), continuing methotrexate only (n = 101), or continuing both medications (n = 51). Patients were eligible for rescue after randomization if they had an SDAI score >11 at any time, SDAI between 3.3 and 11 on three separate visits, or between 3.3 and 11 at two consecutive visits at least 2 weeks apart. About three-quarters of patients in each treatment arm were female, with a mean age of about 55 years, and 82%-91% were White.
Good remission recovery with rescue therapy
At week 48, 28.7% of the methotrexate-only group were in remission (SDAI ≤3.3), compared with 49.5% of the etanercept-only group (P = .004) and 52.9% of the combination group (P = .006). Time to disease worsening was shorter in the methotrexate-only group (median, 198 days) than in the etanercept-only group (median, not estimable; P < .001) and the combined therapy group (median, not estimable; P < .001).
The researchers also found that most patients who underwent rescue therapy once again achieved remission, including 71% of the methotrexate-only group, 75% of the etanercept-only group, and 80% of the combination therapy group. There was no between-group differences in the time required to reattain remission.
The high rate of remission recovery was a good sign, Dr. Curtis said. “To me as a clinician, the risk to try [withdrawing a medication] is quite low because the likelihood you can regain where you were before is quite good. It’s obviously more successful if you stop methotrexate and continue etanercept than if you do the reverse, but to me, this is quite a practical trial, and in fact the rigor of the inclusion criteria are much more like the patients I’m talking to about stopping therapy than some of the past studies in this regard. I think it’s quite useful in terms of generalizability. We want people that are doing this well or close to it before we take away medication.”
Positive reactions from rheumatologists
The reaction from the viewing audience was also positive. “I think this study fills a big data gap for what we do in clinical practice,” wrote Janet Pope, MD, in comments during the session.
Dr. Pope, who is a professor of medicine at the University of Western Ontario and head of rheumatology at St. Joseph’s Health Centre, both in London, said that the results build on previous work, including the CAMEO study, which showed that discontinuation of methotrexate in patients taking methotrexate and etanercept failed to achieve noninferiority to continuation of both medications, and the PRIZE study, which showed that continuing combination therapy at a reduced dose led to better outcomes than did switching to methotrexate alone or placebo. “This may be for some patients what they prefer if they don’t tolerate methotrexate,” she added.
“It’s wonderful to have these data to counsel patients. This is something we face every day,” wrote Elizabeth Wahl, MD, who is an acting assistant professor at the University of Washington, Seattle, and acting chief of rheumatology at the VA Puget Sound Healthcare System.
The study was funded by Amgen. Dr. Curtis has received grants or research support from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB. Dr. Pope consults for a variety of pharmaceutical companies. Dr. Wahl has no relevant financial disclosures.
SOURCE: Curtis JR et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 0939.
In patients with RA whose disease is well controlled by methotrexate combined with etanercept, withdrawal of methotrexate led to long-term outcomes that were nearly as good as continuation of combination therapy. The finding comes from the randomized, controlled SEAM-RA trial that sought to address weaknesses of previous studies. It included a long lead-in time with stringent criteria to ensure that participants had very good disease control.
Both the American College of Rheumatology and the European League Against Rheumatism recommend tapering medication in RA patients who are in long-term remission, but there is no established optimal strategy.
“There have been some prior RA trials that have looked at therapy reduction or withdrawal, but most did not use a very stringent definition of how well people were when they began. Were they in remission, or only in low disease activity?” said Jeffrey R. Curtis, MD, during a presentation of the results at the virtual annual meeting of the ACR. The study was also published online Nov. 18 in Arthritis & Rheumatology.
Stringent remission criteria
The key feature of the trial was the 6-month run-in period, when subjects were taking 50 mg etanercept once per week and 10-25 mg of oral methotrexate once per week, and had to complete at least three visits. They were excluded from the ensuing randomization if they had a Simplified Disease Activity Index (SDAI) score >3.3 and ≤11 at two or more visits, had an SDAI >11 at any time during the run-in period, or had an SDAI >3.3 at the third run-in visit.
“We [wanted them] to be doing quite well for a long period of time. That was empirically confirmed under observation as part of the lead-in period, and even before that, the clinical investigator had to affirm that they believed the patient was doing well for 6 or more months even before they were screened to enter the trial,” said Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
Once enrolled in the trial, patients were randomized 2:2:1 to continuing etanercept only (n = 101), continuing methotrexate only (n = 101), or continuing both medications (n = 51). Patients were eligible for rescue after randomization if they had an SDAI score >11 at any time, SDAI between 3.3 and 11 on three separate visits, or between 3.3 and 11 at two consecutive visits at least 2 weeks apart. About three-quarters of patients in each treatment arm were female, with a mean age of about 55 years, and 82%-91% were White.
Good remission recovery with rescue therapy
At week 48, 28.7% of the methotrexate-only group were in remission (SDAI ≤3.3), compared with 49.5% of the etanercept-only group (P = .004) and 52.9% of the combination group (P = .006). Time to disease worsening was shorter in the methotrexate-only group (median, 198 days) than in the etanercept-only group (median, not estimable; P < .001) and the combined therapy group (median, not estimable; P < .001).
The researchers also found that most patients who underwent rescue therapy once again achieved remission, including 71% of the methotrexate-only group, 75% of the etanercept-only group, and 80% of the combination therapy group. There was no between-group differences in the time required to reattain remission.
The high rate of remission recovery was a good sign, Dr. Curtis said. “To me as a clinician, the risk to try [withdrawing a medication] is quite low because the likelihood you can regain where you were before is quite good. It’s obviously more successful if you stop methotrexate and continue etanercept than if you do the reverse, but to me, this is quite a practical trial, and in fact the rigor of the inclusion criteria are much more like the patients I’m talking to about stopping therapy than some of the past studies in this regard. I think it’s quite useful in terms of generalizability. We want people that are doing this well or close to it before we take away medication.”
Positive reactions from rheumatologists
The reaction from the viewing audience was also positive. “I think this study fills a big data gap for what we do in clinical practice,” wrote Janet Pope, MD, in comments during the session.
Dr. Pope, who is a professor of medicine at the University of Western Ontario and head of rheumatology at St. Joseph’s Health Centre, both in London, said that the results build on previous work, including the CAMEO study, which showed that discontinuation of methotrexate in patients taking methotrexate and etanercept failed to achieve noninferiority to continuation of both medications, and the PRIZE study, which showed that continuing combination therapy at a reduced dose led to better outcomes than did switching to methotrexate alone or placebo. “This may be for some patients what they prefer if they don’t tolerate methotrexate,” she added.
“It’s wonderful to have these data to counsel patients. This is something we face every day,” wrote Elizabeth Wahl, MD, who is an acting assistant professor at the University of Washington, Seattle, and acting chief of rheumatology at the VA Puget Sound Healthcare System.
The study was funded by Amgen. Dr. Curtis has received grants or research support from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB. Dr. Pope consults for a variety of pharmaceutical companies. Dr. Wahl has no relevant financial disclosures.
SOURCE: Curtis JR et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 0939.
In patients with RA whose disease is well controlled by methotrexate combined with etanercept, withdrawal of methotrexate led to long-term outcomes that were nearly as good as continuation of combination therapy. The finding comes from the randomized, controlled SEAM-RA trial that sought to address weaknesses of previous studies. It included a long lead-in time with stringent criteria to ensure that participants had very good disease control.
Both the American College of Rheumatology and the European League Against Rheumatism recommend tapering medication in RA patients who are in long-term remission, but there is no established optimal strategy.
“There have been some prior RA trials that have looked at therapy reduction or withdrawal, but most did not use a very stringent definition of how well people were when they began. Were they in remission, or only in low disease activity?” said Jeffrey R. Curtis, MD, during a presentation of the results at the virtual annual meeting of the ACR. The study was also published online Nov. 18 in Arthritis & Rheumatology.
Stringent remission criteria
The key feature of the trial was the 6-month run-in period, when subjects were taking 50 mg etanercept once per week and 10-25 mg of oral methotrexate once per week, and had to complete at least three visits. They were excluded from the ensuing randomization if they had a Simplified Disease Activity Index (SDAI) score >3.3 and ≤11 at two or more visits, had an SDAI >11 at any time during the run-in period, or had an SDAI >3.3 at the third run-in visit.
“We [wanted them] to be doing quite well for a long period of time. That was empirically confirmed under observation as part of the lead-in period, and even before that, the clinical investigator had to affirm that they believed the patient was doing well for 6 or more months even before they were screened to enter the trial,” said Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham.
Once enrolled in the trial, patients were randomized 2:2:1 to continuing etanercept only (n = 101), continuing methotrexate only (n = 101), or continuing both medications (n = 51). Patients were eligible for rescue after randomization if they had an SDAI score >11 at any time, SDAI between 3.3 and 11 on three separate visits, or between 3.3 and 11 at two consecutive visits at least 2 weeks apart. About three-quarters of patients in each treatment arm were female, with a mean age of about 55 years, and 82%-91% were White.
Good remission recovery with rescue therapy
At week 48, 28.7% of the methotrexate-only group were in remission (SDAI ≤3.3), compared with 49.5% of the etanercept-only group (P = .004) and 52.9% of the combination group (P = .006). Time to disease worsening was shorter in the methotrexate-only group (median, 198 days) than in the etanercept-only group (median, not estimable; P < .001) and the combined therapy group (median, not estimable; P < .001).
The researchers also found that most patients who underwent rescue therapy once again achieved remission, including 71% of the methotrexate-only group, 75% of the etanercept-only group, and 80% of the combination therapy group. There was no between-group differences in the time required to reattain remission.
The high rate of remission recovery was a good sign, Dr. Curtis said. “To me as a clinician, the risk to try [withdrawing a medication] is quite low because the likelihood you can regain where you were before is quite good. It’s obviously more successful if you stop methotrexate and continue etanercept than if you do the reverse, but to me, this is quite a practical trial, and in fact the rigor of the inclusion criteria are much more like the patients I’m talking to about stopping therapy than some of the past studies in this regard. I think it’s quite useful in terms of generalizability. We want people that are doing this well or close to it before we take away medication.”
Positive reactions from rheumatologists
The reaction from the viewing audience was also positive. “I think this study fills a big data gap for what we do in clinical practice,” wrote Janet Pope, MD, in comments during the session.
Dr. Pope, who is a professor of medicine at the University of Western Ontario and head of rheumatology at St. Joseph’s Health Centre, both in London, said that the results build on previous work, including the CAMEO study, which showed that discontinuation of methotrexate in patients taking methotrexate and etanercept failed to achieve noninferiority to continuation of both medications, and the PRIZE study, which showed that continuing combination therapy at a reduced dose led to better outcomes than did switching to methotrexate alone or placebo. “This may be for some patients what they prefer if they don’t tolerate methotrexate,” she added.
“It’s wonderful to have these data to counsel patients. This is something we face every day,” wrote Elizabeth Wahl, MD, who is an acting assistant professor at the University of Washington, Seattle, and acting chief of rheumatology at the VA Puget Sound Healthcare System.
The study was funded by Amgen. Dr. Curtis has received grants or research support from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Janssen, Lilly, Myriad, Pfizer, Regeneron, Roche, and UCB. Dr. Pope consults for a variety of pharmaceutical companies. Dr. Wahl has no relevant financial disclosures.
SOURCE: Curtis JR et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 0939.
FROM ACR 2020
Slow taper off antimalarial is best to avoid lupus flare during remission
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
Slowly tapering off – or remaining on – antimalarial medications can help prevent disease flare in patients with systemic lupus erythematosus (SLE) who’ve achieved clinical remission for at least a year, according to a new study that was presented at the virtual annual meeting of the American College of Rheumatology.
“Except in the setting of toxicity, cessation of antimalarial medication in patients with disease quiescence is feasible using a slow taper,” lead author Danaë Papachristos, MBBS, said during an oral abstract presentation at the online meeting. Dr. Papachristos conducted the research while a clinical and research fellow at the University of Toronto’s lupus clinic, but is now a consultant rheumatologist at the Wesley Hospital in Brisbane, Queensland, Australia.
To investigate flare in patients with SLE who were on or recently off antimalarial medications (AMs), the researchers identified 1,573 potential participants from a long-term observational cohort study at the university’s lupus clinic. From that larger group, 88 cases – patients who achieved clinical remission for at least a year and stopped taking AMs – were matched to at least one control – patients who also achieved remission and continued on medication. Most cases were also matched to a second control, bringing the total number to 173. All patients had at least 2 years of follow-up.
Flare was defined as any increase in the SLEDAI-2K score, with major flare defined as an increase of 4 or more. Patients in the case group were roughly 44 years old, compared with an average age of 46 in the control group. Both groups were almost entirely female and largely white. Reasons for withdrawal in the case group included self-cessation, disease quiescence, and retinal, mucocutaneous, or cardiac toxicities. Twenty participants in the case group reported AM toxicity, compared with four controls.
Dr. Papachristos noted in her presentation that the toxicity disparity was expected, “because controls are those who continue their medication, and most patients who have toxicity will stop their medication.”
Disease flare occurred in 61.4% of cases, compared with 45.1% of controls (P = .002), with the most common types being cutaneous and musculoskeletal flares. After multivariate analysis, the risk of flare more than doubled for those who ceased AMs (odds ratio, 2.26; 95% confidence interval, 1.24-4.11; P = .008). More than half of the cases (n = 46) restarted AMs after withdrawal, which was largely due to disease flare. Of the patients who restarted due to flare, 88% either recaptured control or improved, and the remaining 12% had further flares.
Of the 88 patients in the case group, 51 abruptly withdrew AMs while 37 tapered off. Patients who tapered had fewer flares (45.9%), compared with patients who withdrew abruptly (72.6%). After multivariate analysis, the risk of flare more than tripled for the abrupt withdrawal group (OR, 3.42; 95% CI, 1.26-9.26; P = .016). Fewer patients who tapered later restarted AMs, compared with the abrupt withdrawal group (37.8% vs. 62.7%; P = .02).
When asked about other differences in medications between the two groups, Dr. Papachristos answered: “We didn’t look into that specifically. We did look at those patients who were on prednisone and on any immunosuppression, although we didn’t look at specific therapies. Those variables were adjusted for in the analysis, and it didn’t make any difference if patients were on immunosuppression or prednisone at the point of index date.
“But we would like to look into the different forms of immunosuppression,” she added, “just to see if that made any difference.”
Withdrawing hydroxychloroquine in older patients
Older patients with SLE who discontinue their use of hydroxychloroquine (HCQ) are also not at increased risk of disease flare, according to a retrospective chart review from rheumatologists Ruth Fernandez-Ruiz, MD, and Peter M. Izmirly, MD, of New York University (Arthritis Res Ther. 2020;22:191. doi: 10.1186/s13075-020-02282-0).
“We wanted to focus on older patients who may have a lower risk of flaring and a higher risk of side effects from the drug,” Dr. Fernandez-Ruiz said in an interview.
The doctors embarked on the study after noticing eye and heart toxicities in certain older patients. They matched 26 lupus patients who had been on HCQ for at least 5 years before discontinuing the drug with 32 control patients who remained on HCQ, ultimately finding that withdrawal had no effect on their risk of lupus flares within a year.
“After starting a drug, the second question most people ask, after ‘What are the side effects?’ is ‘How long do I have to be on this?’ ” Dr. Izmirly said in an interview. “These patients are having side effects associated with long-term HCQ use. And we were noticing that, after you stop the drug, despite what you’re taught, they weren’t flaring.”
Only five patients from each group – 19.2% of the withdrawal group and 15.6% of the continuation group – experienced a flare (OR, 1.28; 95% CI, 0.31-5.30; P = .73). Most of the flares were cutaneous and musculoskeletal in nature, and no severe flares occurred in either group.
“On each side, the overall flare rate was not that high, and they were all relatively mild,” Dr. Izmirly said.
The two doctors acknowledged their study’s smaller sample size, compared with the study by Papachristos and colleagues, along with the advanced age of their patient population, which limits the generalizability of their findings. “We selected patients who had a very low disease activity to begin with, and who were older,” Dr. Fernandez-Ruiz noted.
That said, they reinforced the scarcity of existing research on this subset of lupus patients, one that will only continue to grow.
“Older [patients with] lupus,” Dr. Izmirly said, are “an understudied demographic.”
One of the authors of the study presented at ACR 2020 acknowledged receiving research support and consulting fees from various pharmaceutical companies. The HCQ study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its authors declared no conflicts of interest.
SOURCE: Papachristos D et al. Arthritis Rheumatol. 2020;72(suppl 10). Abstract 0983.
FROM ACR 2020
Debate: After methotrexate failure, is JAK inhibitor or biologic next?
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.
Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.

Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
What is the next step in treatment after a person with rheumatoid arthritis fails to adequately respond to methotrexate – a Janus kinase (JAK) inhibitor or a biologic? That was the focus of a lively debate at the virtual annual meeting of the American College of Rheumatology.

Vibeke Strand, MD, argued that JAK inhibitors offer the distinct advantage of a faster clinical response than biologics, meaning that decisions to change therapy based on nonresponse or adverse effects can be made earlier in a treatment plan.
Michael Weinblatt, MD, countered that the faster-response advantage is offset by potential adverse events associated with the JAK inhibitors, including increased risk of herpes zoster infection, venous thromboembolism (VTE), and arterial thromboembolism (ATE). He suggested switching patients to a biologic instead.
In addition, the debate was held just days before the ACR released a proposed guideline for the management of RA. This update to the 2015 guidance is the first to prioritize the order of RA treatments, emphasizing that clinicians should maximize methotrexate therapy before switching RA patients to a JAK inhibitor or a biologic. Release of the full guidelines is pending, and it remains unclear if the ACR provides any guidance regarding the “jakinib” versus biologic decision.
Interestingly, the debate did not hinge on any differences in efficacy. Both speakers pointed to similar efficacy between anti–tumor necrosis factor (TNF) agents and JAK inhibitors, and despite working on different pathways, among the individual JAK inhibitors as well.
Is timing of the essence?
Knowing whether a person with RA responds to a JAK inhibitor more quickly than to a biologic is a major advantage, said Dr. Strand, of the division of immunology and rheumatology at Stanford (Calif.) University. “The argument that I am making is that patients are more responsive if treated earlier in the disease process and they are less treatment-experienced.”
Dr. Strand said the advantages extend to remission as well. “When patients are aware of early improvements, their adherence is increased. Remission is more likely because it occurs earlier.”
“I will certainly grant it to Vibeke that jakinibs work much faster,” said Dr. Weinblatt, chair of rheumatology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School in Boston. However, he added, “my bias is that you give patients an anti-TNF therapy first, and if they are not responding by 12 weeks, you move on to another class of drugs, perhaps even the jakinibs.”
Herpes zoster risk
Dr. Strand and Dr. Weinblatt addressed potential adverse events associated with both classes of agents. For the JAK inhibitors, concerns include herpes zoster infections, increased VTE and ATE incidence, and largely unknown risks during pregnancy and lactation. For the anti-TNF agents, safety concerns include reactivation of tuberculosis, fungal infections, demyelinating syndrome, and skin cancer.
With the shortest half-life of any therapeutic class in rheumatology, adverse events with JAK inhibitors often can resolve quickly, Dr. Strand said.
The increased risk of herpes zoster is important, she added, “but we have a recombinant vaccination that works. It’s quite effective.”
Dr. Weinblatt pointed out that all the JAK inhibitors carry this increase herpes zoster risk, which is related to their mechanism of action. There is a catch with the vaccine, however, he added. The vaccine is approved for treatment of patients 50 years and older. For younger people with RA starting a JAK inhibitor, the cost is out-of-pocket.
Evaluating risk of emboli
The incidence of VTE is about two times higher among people with RA, compared with the general population, Dr. Strand said, with research suggesting the majority of risk resides among people with a previous event. However, she added, an emerging profile of thromboembolic events associated with JAK inhibitors is “considered a class effect by the FDA.”
One exception in the JAK inhibitor class could be tofacitinib (Xeljanz), which might carry less risk because “most of the data with tofacitinib are quite good,” Dr. Weinblatt said. One study presented at ACR 2018, for example, showed a similar VTE rate between tofacitinib and TNF inhibitors.
Nevertheless, the FDA issued a boxed warning in July 2019 about elevated risks of blood clots and death at a higher dose of tofacitinib. The concerns stem from an open-label, endpoint-driven study mandated by the FDA to explore major adverse cardiac events. “There was a clinically important and statistically significant occurrence of pulmonary embolism and VTE, and an increase in mortality in the 10-mg dose group as opposed to the anti-TNF therapies,” Dr. Weinblatt said. As a result, the FDA requested patients on the 10-mg twice-daily dose be transitioned to the 5-mg twice-daily dose.
The package labeling for the JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) feature warnings about increased risk for thromboembolic events. Furthermore, the labeling for filgotinib, a JAK inhibitor in development that received a complete response letter from the FDA in August 2020, is expected to carry the same warning.An unanswered question remains on why this class of agents potentially increases risk of thromboembolism. “We’re all uncomfortable because there is no known mechanism of JAK inhibition that should lead to this,” he added. Another unresolved issue is whether or not patients prescribed a JAK inhibitor should also be prescribed an anticoagulant.
Anti-TNF adverse events
Infections, primarily reactivation of tuberculosis and an increased risk for fungal disease, are concerns with the anti-TNF agents. However, the risk is not restricted to this class. “Greater risk of infection is seen with all our immune-modulating therapies,” Dr. Weinblatt said.
Rare adverse events include demyelinating syndromes, hematologic toxicity, and a worsening of heart failure in some cases.
“Despite a concern about malignancy, the only defined cancer reported over 22 years of use was skin cancer,” he said. “It took more than a decade of ongoing registry data for skin cancer to be identified. It was not noted in randomized, placebo-controlled trials.”
Potential pregnancy concerns
When it comes to risk during reproduction, “there is a clear difference,” Dr. Weinblatt said. “We know anti-TNF therapy can be used safely in pregnant women. We know they can conceive on them and maintain them during pregnancy. They can also breastfeed on them.”
“Frankly, I’m not so ‘gung ho’ on TNF inhibitor safety in pregnancy and lactation with exception of certolizumab, which doesn’t result in high levels of antibody in the placenta or the mother’s milk,” Dr. Strand said.
The 2020 ACR Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases states that all anti-TNF therapies can be used during pregnancy and lactation, Dr. Weinblatt said. “Although I agree certolizumab has the best safety profile, all of them can be used.”
“The same is not true with the jakinibs,” he added, pointing to warnings that women of reproductive age should use contraception while on JAK inhibitors and for 4 weeks after stopping treatment.
However, Dr. Strand defended the pregnancy risk with JAK inhibitors. She cited two publications, including a 2016 study where researchers evaluated the safety of tofacitinib during pregnancy in women with rheumatoid arthritis and psoriasis. “There was only one possible deformity, a pulmonary stenosis,” Dr. Strand said. “Essentially, the majority of patients delivered healthy babies. There was very little difference from what we know occurs in RA otherwise.”
A 2018 study assessed pregnancy outcomes with tofacitinib among people with ulcerative colitis, “again showing the majority of patients had normal deliveries.”
“There just aren’t enough data,” Dr. Weinblatt said. “Perhaps in 5 years, we will reach same conclusion with the jakinibs.”
Differences in cost?
“We have not benefited yet from the biosimilar costs. But in Europe, the cost of an adalimumab or etanercept biosimilar is about $5,000, versus about $50,000 to $60,000 in the U.S. for the JAK inhibitors,” Dr. Weinblatt said. “So there are major cost savings with biosimilars.”
“I can’t understand cost at all for our drugs,” he continued. “They’re not rational, and the price increases are clearly not rational. Potentially, a small molecule is going to be a lot easier to produce than a biologic, so you could argue that generic jakinibs ought to be less. But in the United States we have a distorted pricing model.”
“Until that changes, I don’t think we can predict [future costs]. One could predict that generics and biosimilars will be less than the orginators,” Dr. Weinblatt said.
“It is really criminal we don’t have biosimilars for most of our TNFs, but that is the way it is,” Dr. Strand said.
Summary statements
“The JAK-inhibitor class is an exciting development for rheumatology and a broad variety of autoimmune diseases,” Dr. Strand said.
“In rheumatoid arthritis, they should be used early,” she added. “Based on phase 3 trials, responses are better in progressively earlier disease with less treatment-experienced patients.”
She pointed out that many patients like the convenience of the oral JAK inhibitors.
Dr. Weinblatt stated the 22 years of clinical experience with the anti-TNF class versus about 8 years with jakinibs favors the biologics. “Virtually every approved drug has been tested versus methotrexate, in early studies, long-term studies, and most importantly, in reduction and withdrawal studies, which are not available with the JAK inhibitors.”
Anti-TNFs have impressive effects on clinical disease activity, functional outcomes, and radiographic progression, Dr. Weinblatt said. They work in early and longstanding disease among patients who are disease-modifying antirheumatic drug naive and after multiple DMARD failures, he added.
Adding up the vote
The question was: Should JAK inhibitors be used before TNF inhibitors? The results showed 69%-31% in favor of anti-TNF agents.
“So the majority are more comfortable using TNFs,” said debate moderator Elizabeth Wahl, MD, of the department of rheumatology at VA Puget Sound Healthcare System and the University of Washington in Seattle. Regarding a switch to JAK inhibitors, she interpreted the poll numbers to mean, “we are not there yet, it takes years and years of safety data.”
Both Dr. Strand and Dr. Weinblatt disclosed numerous financial relationships with pharmaceutical companies that market RA drugs.
FROM ACR 2020
Sjögren’s symptom clusters may identify treatment options
Patients with Sjögren’s syndrome can be categorized into four distinct symptom clusters – independent of age, sex, and some disease manifestations – that may both improve symptom relief and aid in the development of targeted therapies, investigators reported.
Analysis of data from a survey conducted by the Sjögren’s Foundation identified four symptom clusters based on the grouping of five common characteristics: anxiety, depression, pain, fatigue, and dryness, Sara S. McCoy, MD, of the University of Wisconsin–Madison, and colleagues reported.
“Verification of features unique to each Sjögren’s cluster might provide guidance for future cluster-targeted therapy,” Dr. McCoy said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
The dearth of Food and Drug Administration–approved disease-modifying therapies for Sjögren’s syndrome can be attributed in part to the small number of patients with extraglandular disease manifestations, the heterogeneity of disease, and the failure of available therapy to improve common symptoms such as fatigue, dryness, quality-of-life decrements, anxiety and depression, she said.
Symptom clusters verify smaller study’s findings
Dr. McCoy and colleagues explored whether symptom clusters identified in a 2019 study from the United Kingdom would apply to a larger U.S. population.
In the U.K. study, Jessica R. Tarn, PhD, and colleagues performed a hierarchical cluster analysis to identify subgroups among 608 patients in the U.K. Primary Sjögren’s Syndrome Registry and in 396 patients in two independent validation cohorts from Norway and France.
They identified four subgroups they categorized as low symptom burden, high symptom burden, dryness dominant with fatigue, and pain dominant with fatigue, and reported that the groups showed significant difference in serum and transcriptomic markers.
In the U.S. study, McCoy et al. sought to verify the symptom-based cluster and report on differences in key measures between the groups. They used data from a survey by the Sjögren’s Foundation of 2,961 adults with self-reported Sjögren’s syndrome. The investigators then used an unsupervised hierarchical clustering method to identify the optimal phenotypically similar clusters based on patient-reported severity of anxiety (from never to daily), depression (never to daily), pain on a visual analog scale (0 to 10), fatigue on a VAS, and dryness on a VAS. They collected data on demographics, medications, quality of life, and Sjögren’s-specific symptom frequency and systemic manifestations within each cluster, and identified cluster differences controlled for age, sex, race, and Social Security disability.
They identified four symptom-based clusters from 2,806 participants for whom complete data on the five key symptoms were available:
- Cluster 1 patients (prevalence, 30%) had high symptom burden in all categories.
- Cluster 2 patients (22%) had high anxiety and depression (22%), with some fatigue.
- Cluster 3 patients (34%) had predominant high dryness and fatigue.
- Cluster 4 patients (14%) had low symptom burden.
“We found that clusters differed in Sjögren’s-specific symptoms,” Dr. McCoy said.
For example, patients in the high-symptom-burden cluster had, as the name implies, an overall higher burden of symptoms among all major ocular, oral, and other dryness symptoms, as well as systemic organ system symptoms, whereas patients in the low-symptom-burden group consistently had the lowest levels of symptoms across the spectrum.
“We also noticed significant differences in systemic medication use. High symptom burden and high dryness and fatigue had higher use of systemic therapies targeting dryness, as might be expected,” she said.
The highest corticosteroid use was in the high-symptom-burden group, while hydroxychloroquine use was highest in the high-anxiety/depression group. Antidepressant use was also high in these two groups.
In addition, 35% of patients in the high-symptom-burden group used prescription opioid analgesics, compared with just 7% in the low-symptom-burden group.
The categories from low to high symptom burden also significantly correlated with quality-of-life measures, including Social Security Disability enrollment, emotional burden of disease, effects of disease on independence, and effects of Sjögren’s on relationships with family and friends (P < .001 for all).
Systemic manifestations of disease differed significantly among the groups for inflammatory arthritis, interstitial lung disease, and neuropathy, but there were no significant differences in the incidence of leukopenia or lymphoma.
The investigators plan to perform symptom-based cluster analysis with validated Sjögren’s syndrome populations, and propose studies to define phenotypic features of distinct clusters “to better define subsets of this heterogeneous disease, and ultimately inform targeted therapy,” Dr. McCoy concluded.
Opportunity to tailor practice and research
During the question-and-answer period following the presentation, Gabriela Hernandez-Molina, MD, of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City, commented that “fatigue and pain might be also attributed to other comorbidities in these patients such as fibromyalgia,” and asked Dr. McCoy to comment on that.
“That’s exactly what we’re driving at,” Dr. McCoy replied. “Fatigue and pain frequently affect how patients experience their disease, and it would be beneficial to take this into account when we evaluate patients, and also for the studies that we’re performing, as well as future ‘-omic’ studies – transcriptomics and what-not – there’s potential here to take that type of patient we frequently see and try to tailor our clinical practice and our research to clearly what we’re seeing in practice, which is these other comorbidities.”
Dana DiRenzo, MD, from Johns Hopkins University, Baltimore, who moderated the session, asked how the information is changing the management of patients with Sjögren’s syndrome in clinic.
Dr. McCoy said that the study was based on self-reported data that can introduce bias, and that she and colleagues plan to validate the results before applying them to clinical care.
The study was supported by grants from the National Institutes of Health and the University of Wisconsin. Dr. McCoy disclosed consulting fees from Novartis and Bristol-Myers Squibb. Dr. Hernandez-Molina and Dr. DiRenzo reported no relevant disclosures.
SOURCE: McCoy SS et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 1504.
Patients with Sjögren’s syndrome can be categorized into four distinct symptom clusters – independent of age, sex, and some disease manifestations – that may both improve symptom relief and aid in the development of targeted therapies, investigators reported.
Analysis of data from a survey conducted by the Sjögren’s Foundation identified four symptom clusters based on the grouping of five common characteristics: anxiety, depression, pain, fatigue, and dryness, Sara S. McCoy, MD, of the University of Wisconsin–Madison, and colleagues reported.
“Verification of features unique to each Sjögren’s cluster might provide guidance for future cluster-targeted therapy,” Dr. McCoy said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
The dearth of Food and Drug Administration–approved disease-modifying therapies for Sjögren’s syndrome can be attributed in part to the small number of patients with extraglandular disease manifestations, the heterogeneity of disease, and the failure of available therapy to improve common symptoms such as fatigue, dryness, quality-of-life decrements, anxiety and depression, she said.
Symptom clusters verify smaller study’s findings
Dr. McCoy and colleagues explored whether symptom clusters identified in a 2019 study from the United Kingdom would apply to a larger U.S. population.
In the U.K. study, Jessica R. Tarn, PhD, and colleagues performed a hierarchical cluster analysis to identify subgroups among 608 patients in the U.K. Primary Sjögren’s Syndrome Registry and in 396 patients in two independent validation cohorts from Norway and France.
They identified four subgroups they categorized as low symptom burden, high symptom burden, dryness dominant with fatigue, and pain dominant with fatigue, and reported that the groups showed significant difference in serum and transcriptomic markers.
In the U.S. study, McCoy et al. sought to verify the symptom-based cluster and report on differences in key measures between the groups. They used data from a survey by the Sjögren’s Foundation of 2,961 adults with self-reported Sjögren’s syndrome. The investigators then used an unsupervised hierarchical clustering method to identify the optimal phenotypically similar clusters based on patient-reported severity of anxiety (from never to daily), depression (never to daily), pain on a visual analog scale (0 to 10), fatigue on a VAS, and dryness on a VAS. They collected data on demographics, medications, quality of life, and Sjögren’s-specific symptom frequency and systemic manifestations within each cluster, and identified cluster differences controlled for age, sex, race, and Social Security disability.
They identified four symptom-based clusters from 2,806 participants for whom complete data on the five key symptoms were available:
- Cluster 1 patients (prevalence, 30%) had high symptom burden in all categories.
- Cluster 2 patients (22%) had high anxiety and depression (22%), with some fatigue.
- Cluster 3 patients (34%) had predominant high dryness and fatigue.
- Cluster 4 patients (14%) had low symptom burden.
“We found that clusters differed in Sjögren’s-specific symptoms,” Dr. McCoy said.
For example, patients in the high-symptom-burden cluster had, as the name implies, an overall higher burden of symptoms among all major ocular, oral, and other dryness symptoms, as well as systemic organ system symptoms, whereas patients in the low-symptom-burden group consistently had the lowest levels of symptoms across the spectrum.
“We also noticed significant differences in systemic medication use. High symptom burden and high dryness and fatigue had higher use of systemic therapies targeting dryness, as might be expected,” she said.
The highest corticosteroid use was in the high-symptom-burden group, while hydroxychloroquine use was highest in the high-anxiety/depression group. Antidepressant use was also high in these two groups.
In addition, 35% of patients in the high-symptom-burden group used prescription opioid analgesics, compared with just 7% in the low-symptom-burden group.
The categories from low to high symptom burden also significantly correlated with quality-of-life measures, including Social Security Disability enrollment, emotional burden of disease, effects of disease on independence, and effects of Sjögren’s on relationships with family and friends (P < .001 for all).
Systemic manifestations of disease differed significantly among the groups for inflammatory arthritis, interstitial lung disease, and neuropathy, but there were no significant differences in the incidence of leukopenia or lymphoma.
The investigators plan to perform symptom-based cluster analysis with validated Sjögren’s syndrome populations, and propose studies to define phenotypic features of distinct clusters “to better define subsets of this heterogeneous disease, and ultimately inform targeted therapy,” Dr. McCoy concluded.
Opportunity to tailor practice and research
During the question-and-answer period following the presentation, Gabriela Hernandez-Molina, MD, of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City, commented that “fatigue and pain might be also attributed to other comorbidities in these patients such as fibromyalgia,” and asked Dr. McCoy to comment on that.
“That’s exactly what we’re driving at,” Dr. McCoy replied. “Fatigue and pain frequently affect how patients experience their disease, and it would be beneficial to take this into account when we evaluate patients, and also for the studies that we’re performing, as well as future ‘-omic’ studies – transcriptomics and what-not – there’s potential here to take that type of patient we frequently see and try to tailor our clinical practice and our research to clearly what we’re seeing in practice, which is these other comorbidities.”
Dana DiRenzo, MD, from Johns Hopkins University, Baltimore, who moderated the session, asked how the information is changing the management of patients with Sjögren’s syndrome in clinic.
Dr. McCoy said that the study was based on self-reported data that can introduce bias, and that she and colleagues plan to validate the results before applying them to clinical care.
The study was supported by grants from the National Institutes of Health and the University of Wisconsin. Dr. McCoy disclosed consulting fees from Novartis and Bristol-Myers Squibb. Dr. Hernandez-Molina and Dr. DiRenzo reported no relevant disclosures.
SOURCE: McCoy SS et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 1504.
Patients with Sjögren’s syndrome can be categorized into four distinct symptom clusters – independent of age, sex, and some disease manifestations – that may both improve symptom relief and aid in the development of targeted therapies, investigators reported.
Analysis of data from a survey conducted by the Sjögren’s Foundation identified four symptom clusters based on the grouping of five common characteristics: anxiety, depression, pain, fatigue, and dryness, Sara S. McCoy, MD, of the University of Wisconsin–Madison, and colleagues reported.
“Verification of features unique to each Sjögren’s cluster might provide guidance for future cluster-targeted therapy,” Dr. McCoy said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
The dearth of Food and Drug Administration–approved disease-modifying therapies for Sjögren’s syndrome can be attributed in part to the small number of patients with extraglandular disease manifestations, the heterogeneity of disease, and the failure of available therapy to improve common symptoms such as fatigue, dryness, quality-of-life decrements, anxiety and depression, she said.
Symptom clusters verify smaller study’s findings
Dr. McCoy and colleagues explored whether symptom clusters identified in a 2019 study from the United Kingdom would apply to a larger U.S. population.
In the U.K. study, Jessica R. Tarn, PhD, and colleagues performed a hierarchical cluster analysis to identify subgroups among 608 patients in the U.K. Primary Sjögren’s Syndrome Registry and in 396 patients in two independent validation cohorts from Norway and France.
They identified four subgroups they categorized as low symptom burden, high symptom burden, dryness dominant with fatigue, and pain dominant with fatigue, and reported that the groups showed significant difference in serum and transcriptomic markers.
In the U.S. study, McCoy et al. sought to verify the symptom-based cluster and report on differences in key measures between the groups. They used data from a survey by the Sjögren’s Foundation of 2,961 adults with self-reported Sjögren’s syndrome. The investigators then used an unsupervised hierarchical clustering method to identify the optimal phenotypically similar clusters based on patient-reported severity of anxiety (from never to daily), depression (never to daily), pain on a visual analog scale (0 to 10), fatigue on a VAS, and dryness on a VAS. They collected data on demographics, medications, quality of life, and Sjögren’s-specific symptom frequency and systemic manifestations within each cluster, and identified cluster differences controlled for age, sex, race, and Social Security disability.
They identified four symptom-based clusters from 2,806 participants for whom complete data on the five key symptoms were available:
- Cluster 1 patients (prevalence, 30%) had high symptom burden in all categories.
- Cluster 2 patients (22%) had high anxiety and depression (22%), with some fatigue.
- Cluster 3 patients (34%) had predominant high dryness and fatigue.
- Cluster 4 patients (14%) had low symptom burden.
“We found that clusters differed in Sjögren’s-specific symptoms,” Dr. McCoy said.
For example, patients in the high-symptom-burden cluster had, as the name implies, an overall higher burden of symptoms among all major ocular, oral, and other dryness symptoms, as well as systemic organ system symptoms, whereas patients in the low-symptom-burden group consistently had the lowest levels of symptoms across the spectrum.
“We also noticed significant differences in systemic medication use. High symptom burden and high dryness and fatigue had higher use of systemic therapies targeting dryness, as might be expected,” she said.
The highest corticosteroid use was in the high-symptom-burden group, while hydroxychloroquine use was highest in the high-anxiety/depression group. Antidepressant use was also high in these two groups.
In addition, 35% of patients in the high-symptom-burden group used prescription opioid analgesics, compared with just 7% in the low-symptom-burden group.
The categories from low to high symptom burden also significantly correlated with quality-of-life measures, including Social Security Disability enrollment, emotional burden of disease, effects of disease on independence, and effects of Sjögren’s on relationships with family and friends (P < .001 for all).
Systemic manifestations of disease differed significantly among the groups for inflammatory arthritis, interstitial lung disease, and neuropathy, but there were no significant differences in the incidence of leukopenia or lymphoma.
The investigators plan to perform symptom-based cluster analysis with validated Sjögren’s syndrome populations, and propose studies to define phenotypic features of distinct clusters “to better define subsets of this heterogeneous disease, and ultimately inform targeted therapy,” Dr. McCoy concluded.
Opportunity to tailor practice and research
During the question-and-answer period following the presentation, Gabriela Hernandez-Molina, MD, of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City, commented that “fatigue and pain might be also attributed to other comorbidities in these patients such as fibromyalgia,” and asked Dr. McCoy to comment on that.
“That’s exactly what we’re driving at,” Dr. McCoy replied. “Fatigue and pain frequently affect how patients experience their disease, and it would be beneficial to take this into account when we evaluate patients, and also for the studies that we’re performing, as well as future ‘-omic’ studies – transcriptomics and what-not – there’s potential here to take that type of patient we frequently see and try to tailor our clinical practice and our research to clearly what we’re seeing in practice, which is these other comorbidities.”
Dana DiRenzo, MD, from Johns Hopkins University, Baltimore, who moderated the session, asked how the information is changing the management of patients with Sjögren’s syndrome in clinic.
Dr. McCoy said that the study was based on self-reported data that can introduce bias, and that she and colleagues plan to validate the results before applying them to clinical care.
The study was supported by grants from the National Institutes of Health and the University of Wisconsin. Dr. McCoy disclosed consulting fees from Novartis and Bristol-Myers Squibb. Dr. Hernandez-Molina and Dr. DiRenzo reported no relevant disclosures.
SOURCE: McCoy SS et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 1504.
FROM ACR 2020
Methotrexate and hydroxychloroquine split on cardiovascular outcomes in RA
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
No significant differences in major adverse cardiovascular events (MACE) emerged between methotrexate and hydroxychloroquine (HCQ) treatment in a comparison of adults 65 years or older with rheumatoid arthritis. However, researchers reported some elevation in risk for stroke in the methotrexate group and for myocardial infarction and heart failure in the HCQ group.
The primary outcome, a composite of MI, stroke, or cardiovascular death, had an incidence of 23.39 per 1,000 person-years in the methotrexate group versus 24.33 in the HCQ group in this observational study of nearly 60,000 people.
“These results suggest an importance of looking at different individual events of cardiovascular disease rather than the whole ‘CV’ disease only,” Seoyoung Kim, MD, said in an interview. “The other important thing is that the mortality was not significantly different between the two groups.”
For example, the researchers reported 256 cardiovascular-related deaths in the methotrexate group and 263 such deaths in the HCQ cohort.
Addressing a recognized risk
“It is well known that patients with rheumatoid arthritis have excessive morbidity and mortality,” Dr. Kim, of the division of rheumatology at Brigham and Women’s Hospital and associate professor of medicine at Harvard Medical School in Boston, said at the virtual annual meeting of the American College of Rheumatology.
Among prior studies in this area, the Cardiovascular Inflammation Reduction Trial (CIRT) found no significant reduction in cardiovascular events among people taking methotrexate versus placebo. However, the study of 4,786 people was not specific to RA, Dr. Kim said. The lack of efficacy on this endpoint prompted researchers to stop CIRT early.
“So what does the conclusion of the CIRT trial mean for rheumatoid arthritis patients?” Dr. Kim asked.
To find out, she and colleagues compared risk of MACE among participants newly starting either methotrexate or HCQ. The study included 59,329 people aged 65 and older who were identified through Medicare claims data from 2008 to 2016. Mean age was 74 years, and 80% were women.
The investigators used propensity score matching to control for multiple covariates for demographics, other medications, and comorbidities. Use of other medications was similar between groups, including glucocorticoids, NSAIDs, and statins. Baseline cardiovascular morbidities likewise were well balanced, Dr. Kim said.
The hazard ratio for the primary MACE outcome was 0.96 (95% confidence interval, 0.86-1.08).
Secondary outcomes
MI was less common in the methotrexate group, for example, with an incidence of 8.49 per 1,000 person-years versus 10.68 per 1,000 person-years in the HCQ cohort. This finding was statically significant, Dr. Kim said, with a hazard ratio of 0.80 favoring methotrexate.
Heart failure also occurred less often in the methotrexate cohort, with an incidence rate of 8.57 per 1,000 person-years versus a rate of 14.24 in the HCQ group. The hazard ratio again favored methotrexate at 0.60.
In contrast, strokes were more common with methotrexate than with (incidence of 7.94 vs. 6.01 per 1,000 person-years).
Another secondary outcome, all-cause mortality, was not significantly different between groups. There were 821 deaths in the methotrexate group (28.65 per 1,000 person-years) and 796 deaths in the HCQ group (31.33 per 1,000 person-years).
Studying causality next?
Session moderator Maya Buch, MD, PhD, professor of rheumatology at the University of Manchester (England), asked Dr. Kim why she found significant differences in some secondary outcomes but not the primary composite endpoint.
“When we think of cardiovascular diseases, we tend to think of them all developing through the same mechanism. But perhaps the exact mechanism might not be identical,” Dr. Kim replied. The findings do not suggest causality because the study was observational, she added, “but maybe this will lead to a randomized, controlled trial.”
When asked for comment, Dr. Buch said that the study was “interesting” and “suggestive of differences in type of MACE between the two drugs evaluated,” but that there should be caution in interpreting the findings because of the lack of detailed information on RA disease and activity in claims databases, in addition to other factors, even though the investigators made adjustments for known differences through propensity score matching.
The division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital supported the study. Dr. Kim has received support for Brigham and Women’s Hospital for unrelated research from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb. Several other coauthors reported having financial relationships with pharmaceutical companies that make drugs for RA. Dr. Buch had no relevant disclosures.
SOURCE: He M et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 1993.
FROM ACR 2020