User login
Flu vaccination associated with reduced stroke risk
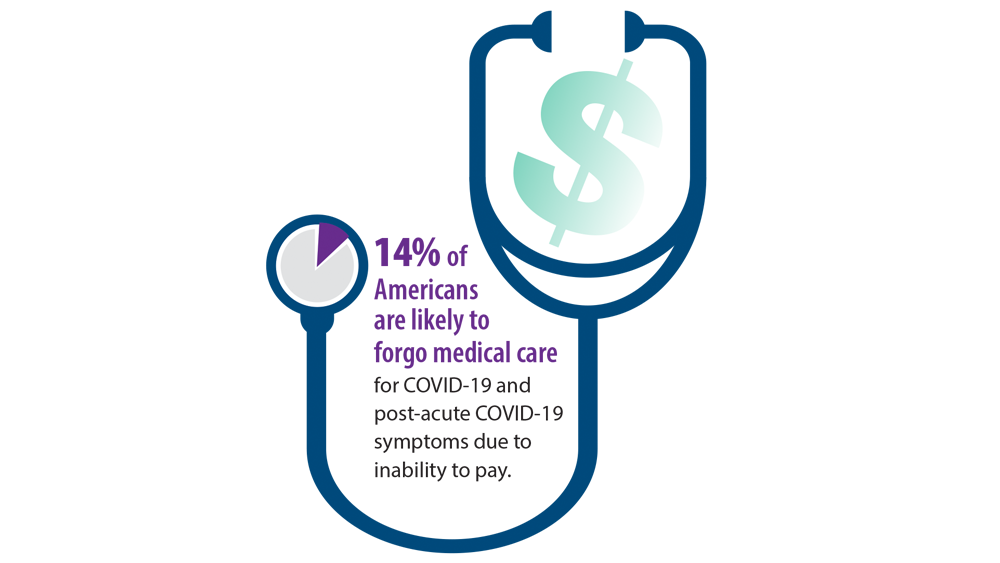
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM LANCET PUBLIC HEALTH
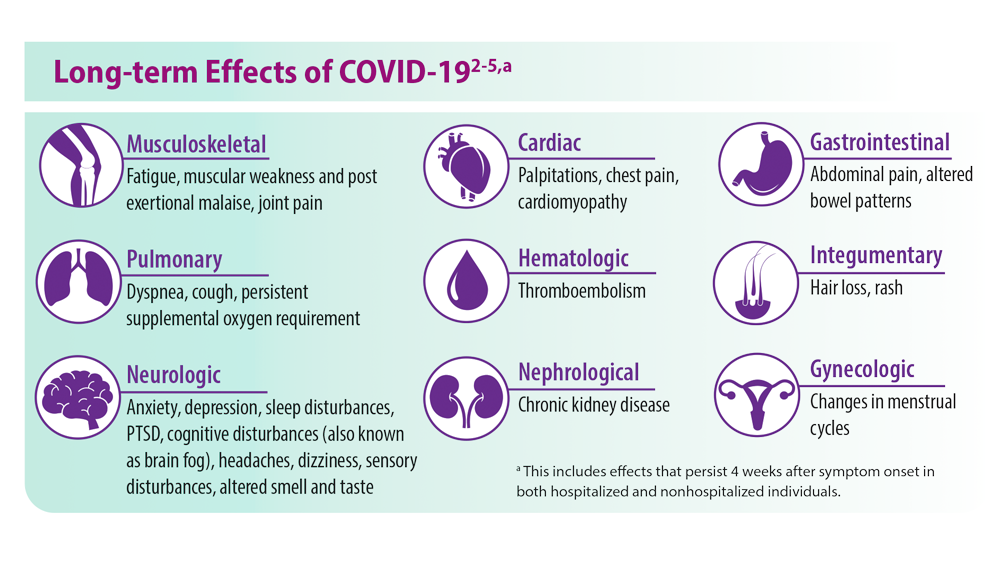
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
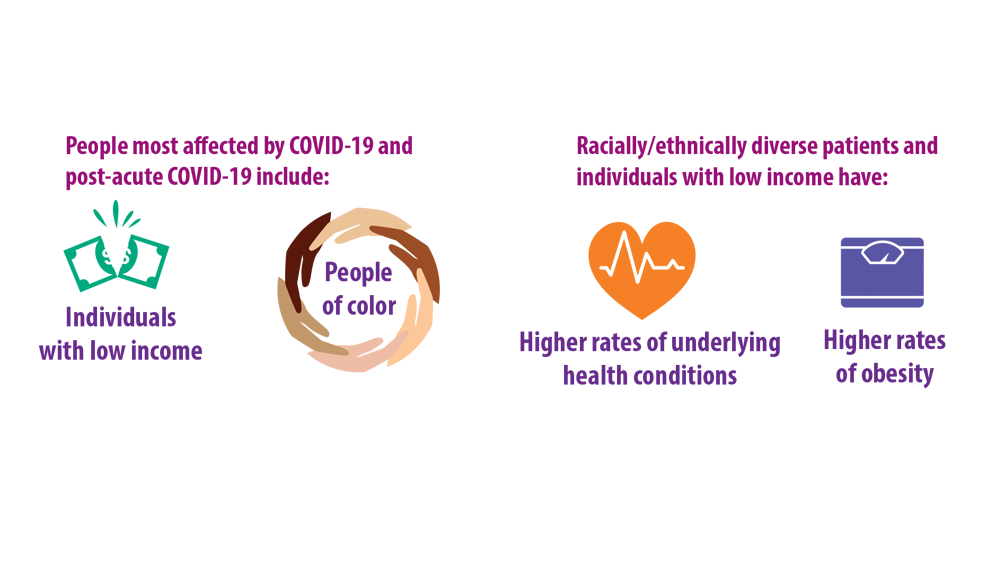
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Buzz kill: Lung damage looks worse in pot smokers
Scans of the lungs of pot users have turned up an alarming surprise:
“There’s a public perception that marijuana is safe,” said Giselle Revah, MD, a radiologist at the University of Ottawa. “This study is raising concern that this might not be true.”
Dr. Revah said she can often tell immediately if a CT scan is from a heavy or long-time cigarette smoker. But with the legalization and increased use of marijuana in Canada and many U.S. states, she began to wonder what cannabis use does to the lungs and whether she would be able to differentiate its effects from those of cigarette smoking.
She and her colleagues retrospectively examined chest CT scans from 56 marijuana smokers and compared them to scans of 57 nonsmokers and 33 users of tobacco alone.
Emphysema was significantly more common among marijuana smokers (75%) than among nonsmokers (5%). When matched for age and sex, 93% of marijuana smokers had emphysema, vs. 67% of those who smoked tobacco only (P = .009).
Without age matching, rates of emphysema remained slightly higher among the marijuana users (75% vs. 67%), although the difference was no longer statistically significant. Yet more than 40% of the marijuana group was younger than 50 years, and all of the tobacco-only users were 50 or older – meaning that marijuana smokers may develop lung damage earlier or with less exposure, Dr. Revah said.
Dr. Revah added that her colleagues in family medicine have said the findings match their clinical experience. “In their practices, they have younger patients with emphysema,” she said.
Marijuana smokers also showed higher rates of airway inflammation, including bronchial thickening, bronchiectasis, and mucoid impaction, with and without sex- and age-matching, the researchers found.
The findings are “not even a little bit surprising,” according to Alan Kaplan, MD, a family physician in Ontario who has expertise in respiratory health. He is the author of a 2021 review on cannabis and lung health.
In an editorial accompanying the journal article by Dr. Revah and colleagues , pulmonary experts noted that the new data give context to a recent uptick in referrals for nontraumatic pneumothorax. The authors said they had received 22 of these referrals during the past 2 years but that they had received only 6 between 2012 and 2020. “Many, but not all, of these patients have a documented history of marijuana use,” they wrote.
One reason for the additional damage may be the way marijuana is inhaled, Dr. Kaplan said. Marijuana smokers “take a big breath in, and they really push it into lungs and hold pressure on it, which may actually cause alveoli to distend over time.”
Because most marijuana smokers in the study also smoked cigarettes, whether the observed damage was caused by marijuana alone or occurred through a synergy with tobacco is impossible to discern, Dr. Revah said.
Still, the results are striking, she said, because the marijuana group was compared to tobacco users who had an extensive smoking history – 25 to 100 pack-years – and who were from a high-risk lung cancer screening program.
Dr. Revah and her colleagues are now conducting a larger, prospective study to see whether they can confirm their findings.
“The message to physicians is to ask about cannabis smoking,” Dr. Kaplan said. In the past, people have been reluctant to admit to using cannabis. Even with legalization, they may be slow to tell their physicians. But clinicians should still try to identify frequent users, especially those who are predisposed for lung conditions. If they intend to use the drug, the advice should be, “There are safer ways to use cannabis,” he said.
Dr. Revah and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Scans of the lungs of pot users have turned up an alarming surprise:
“There’s a public perception that marijuana is safe,” said Giselle Revah, MD, a radiologist at the University of Ottawa. “This study is raising concern that this might not be true.”
Dr. Revah said she can often tell immediately if a CT scan is from a heavy or long-time cigarette smoker. But with the legalization and increased use of marijuana in Canada and many U.S. states, she began to wonder what cannabis use does to the lungs and whether she would be able to differentiate its effects from those of cigarette smoking.
She and her colleagues retrospectively examined chest CT scans from 56 marijuana smokers and compared them to scans of 57 nonsmokers and 33 users of tobacco alone.
Emphysema was significantly more common among marijuana smokers (75%) than among nonsmokers (5%). When matched for age and sex, 93% of marijuana smokers had emphysema, vs. 67% of those who smoked tobacco only (P = .009).
Without age matching, rates of emphysema remained slightly higher among the marijuana users (75% vs. 67%), although the difference was no longer statistically significant. Yet more than 40% of the marijuana group was younger than 50 years, and all of the tobacco-only users were 50 or older – meaning that marijuana smokers may develop lung damage earlier or with less exposure, Dr. Revah said.
Dr. Revah added that her colleagues in family medicine have said the findings match their clinical experience. “In their practices, they have younger patients with emphysema,” she said.
Marijuana smokers also showed higher rates of airway inflammation, including bronchial thickening, bronchiectasis, and mucoid impaction, with and without sex- and age-matching, the researchers found.
The findings are “not even a little bit surprising,” according to Alan Kaplan, MD, a family physician in Ontario who has expertise in respiratory health. He is the author of a 2021 review on cannabis and lung health.
In an editorial accompanying the journal article by Dr. Revah and colleagues , pulmonary experts noted that the new data give context to a recent uptick in referrals for nontraumatic pneumothorax. The authors said they had received 22 of these referrals during the past 2 years but that they had received only 6 between 2012 and 2020. “Many, but not all, of these patients have a documented history of marijuana use,” they wrote.
One reason for the additional damage may be the way marijuana is inhaled, Dr. Kaplan said. Marijuana smokers “take a big breath in, and they really push it into lungs and hold pressure on it, which may actually cause alveoli to distend over time.”
Because most marijuana smokers in the study also smoked cigarettes, whether the observed damage was caused by marijuana alone or occurred through a synergy with tobacco is impossible to discern, Dr. Revah said.
Still, the results are striking, she said, because the marijuana group was compared to tobacco users who had an extensive smoking history – 25 to 100 pack-years – and who were from a high-risk lung cancer screening program.
Dr. Revah and her colleagues are now conducting a larger, prospective study to see whether they can confirm their findings.
“The message to physicians is to ask about cannabis smoking,” Dr. Kaplan said. In the past, people have been reluctant to admit to using cannabis. Even with legalization, they may be slow to tell their physicians. But clinicians should still try to identify frequent users, especially those who are predisposed for lung conditions. If they intend to use the drug, the advice should be, “There are safer ways to use cannabis,” he said.
Dr. Revah and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Scans of the lungs of pot users have turned up an alarming surprise:
“There’s a public perception that marijuana is safe,” said Giselle Revah, MD, a radiologist at the University of Ottawa. “This study is raising concern that this might not be true.”
Dr. Revah said she can often tell immediately if a CT scan is from a heavy or long-time cigarette smoker. But with the legalization and increased use of marijuana in Canada and many U.S. states, she began to wonder what cannabis use does to the lungs and whether she would be able to differentiate its effects from those of cigarette smoking.
She and her colleagues retrospectively examined chest CT scans from 56 marijuana smokers and compared them to scans of 57 nonsmokers and 33 users of tobacco alone.
Emphysema was significantly more common among marijuana smokers (75%) than among nonsmokers (5%). When matched for age and sex, 93% of marijuana smokers had emphysema, vs. 67% of those who smoked tobacco only (P = .009).
Without age matching, rates of emphysema remained slightly higher among the marijuana users (75% vs. 67%), although the difference was no longer statistically significant. Yet more than 40% of the marijuana group was younger than 50 years, and all of the tobacco-only users were 50 or older – meaning that marijuana smokers may develop lung damage earlier or with less exposure, Dr. Revah said.
Dr. Revah added that her colleagues in family medicine have said the findings match their clinical experience. “In their practices, they have younger patients with emphysema,” she said.
Marijuana smokers also showed higher rates of airway inflammation, including bronchial thickening, bronchiectasis, and mucoid impaction, with and without sex- and age-matching, the researchers found.
The findings are “not even a little bit surprising,” according to Alan Kaplan, MD, a family physician in Ontario who has expertise in respiratory health. He is the author of a 2021 review on cannabis and lung health.
In an editorial accompanying the journal article by Dr. Revah and colleagues , pulmonary experts noted that the new data give context to a recent uptick in referrals for nontraumatic pneumothorax. The authors said they had received 22 of these referrals during the past 2 years but that they had received only 6 between 2012 and 2020. “Many, but not all, of these patients have a documented history of marijuana use,” they wrote.
One reason for the additional damage may be the way marijuana is inhaled, Dr. Kaplan said. Marijuana smokers “take a big breath in, and they really push it into lungs and hold pressure on it, which may actually cause alveoli to distend over time.”
Because most marijuana smokers in the study also smoked cigarettes, whether the observed damage was caused by marijuana alone or occurred through a synergy with tobacco is impossible to discern, Dr. Revah said.
Still, the results are striking, she said, because the marijuana group was compared to tobacco users who had an extensive smoking history – 25 to 100 pack-years – and who were from a high-risk lung cancer screening program.
Dr. Revah and her colleagues are now conducting a larger, prospective study to see whether they can confirm their findings.
“The message to physicians is to ask about cannabis smoking,” Dr. Kaplan said. In the past, people have been reluctant to admit to using cannabis. Even with legalization, they may be slow to tell their physicians. But clinicians should still try to identify frequent users, especially those who are predisposed for lung conditions. If they intend to use the drug, the advice should be, “There are safer ways to use cannabis,” he said.
Dr. Revah and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY
Keeping up with the evidence (and the residents)
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
Lung cancer screening: New evidence, updated guidance
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
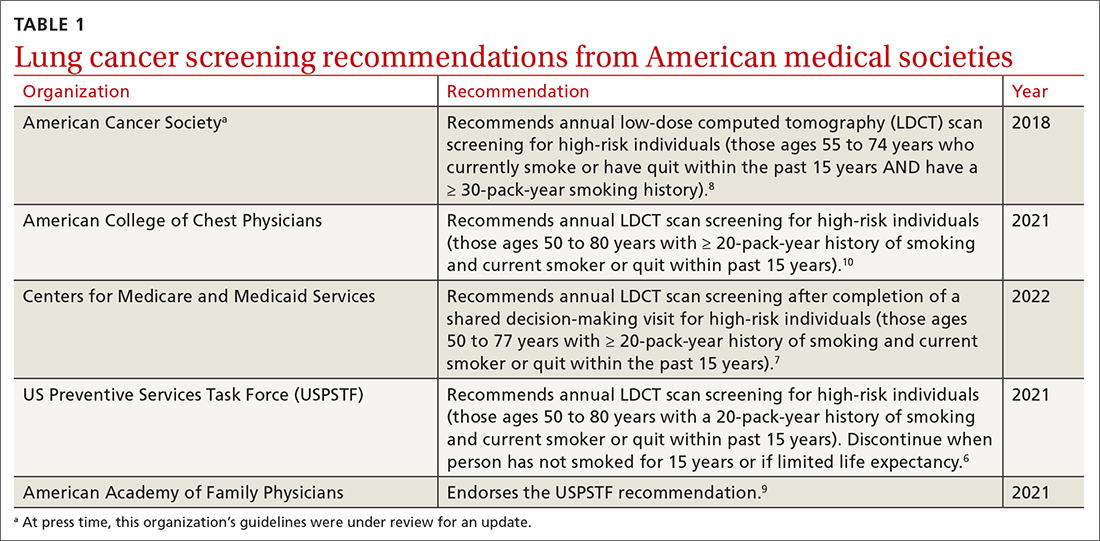
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.
The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.
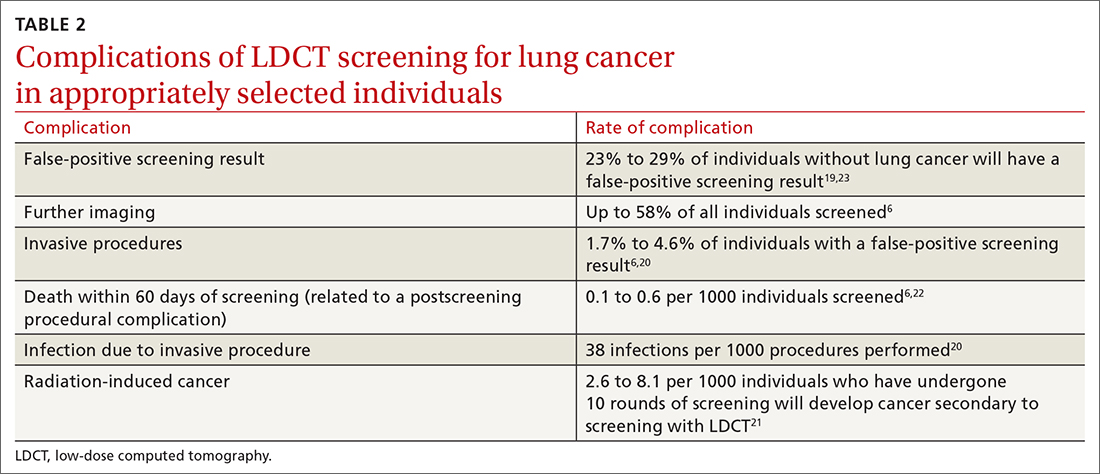
The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
PRACTICE RECOMMENDATIONS
› Recommend annual lung cancer screening for all highrisk adults ages 50 to 80 years using low-dose computed tomography. A
› Do not pursue lung cancer screening in patients who quit smoking ≥ 15 years ago, have a health problem that limits their life expectancy, or are unwilling to undergo lung surgery. A
› Recommend varenicline as first-line pharmacotherapy for smokers who would like to quit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Combination therapy shows mixed results for scleroderma-related lung disease
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
AT ACR 2022
Rituximab ‘a reasonable alternative to cyclophosphamide’ to improve ILD-CTD
PHILADELPHIA – In the first controlled clinical trial to compare the two drugs, rituximab and cyclophosphamide were similarly effective in improving lung function in patients with interstitial lung disease (ILD) associated with idiopathic inflammatory myositis and mixed connective tissue disease (CTD). The findings also revealed some nuanced findings that could help clarify which drug to use in specific patients.
“We feel that rituximab is a reasonable alternative to cyclophosphamide as a treatment in patients with these diseases,” said Toby Maher, MD, of the University of Southern California, Los Angeles, who presented results of an analysis of three disease subgroups from the RECITAL (Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease) study at the annual meeting of the American College of Rheumatology.
“We didn’t show it to be better, so I think you can reasonably choose between the two, but rituximab almost certainly has the advantage of being safer and better tolerated than cyclophosphamide,” Dr. Maher said in an interview. The findings were published simultaneously in The Lancet Respiratory Medicine.
Double-blind, double-dummy
RECITAL is a phase 2b, randomized, controlled trial to test the hypothesis that intravenous rituximab would be superior to cyclophosphamide for ILD-associated CTD.
The study included adults with three separate diagnoses: myositis (n = 44), mixed CTD (n = 16), and systemic sclerosis (SSc, n = 37). The study was done in the United Kingdom when Dr. Maher was with Imperial College London.
Patients in the rituximab group received 1,000 mg of IV treatment at baseline and 2 weeks, then placebo treatment every 4 weeks to week 20. Cyclophosphamide patients received 600 mg/m2 of body surface area intravenously every 4 weeks for six doses.
“When we designed this study there was limited evidence for any treatment for any disease associated with ILD,” Dr. Maher said. “But cyclophosphamide brings with it many challenges. It can be poorly tolerated and carries issues like infertility and risk of bladder cancer.”
Improved lung function
While the study failed to meet its primary endpoint – superiority of rituximab versus cyclophosphamide – it did show that both drugs led to improvement in lung function, measured by the rate of change in forced vital capacity (FVC), as well as quality of life measures, Dr. Maher said.
“Overall by week 48, we saw about a 5% improvement in FVC in the cyclophosphamide group and approximately a 4% improvement in FVC from baseline in the rituximab group, suggesting that both drugs almost certainly had a positive benefit in this patient group,” he said.
But secondary outcomes varied somewhat across the different disease groups. Patients with SSc saw a slight deterioration with cyclophosphamide in the modified Rodnan skin score at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab (–3.4 ± 8.1 units).
“One area where we did see a difference was in the number of adverse events,” Dr. Maher said. “They were fewer in the rituximab arm – namely gastrointestinal disorders [and] nausea, which we saw quite frequently following cyclophosphamide. Also, they had fewer headaches, which we saw quite frequently following cyclophosphamide.”
Rituximab patients also had fewer infusion reactions, but the number of infections was similar between the two treatment groups, he said.
“The patient group that responded best to treatment was the myositis group,” Dr. Maher said in his presentation. “Cyclophosphamide actually appears to be more effective than rituximab in improving their disease. By the end of 48 weeks, the cyclophosphamide group actually gained about 400 mL in FVC, so a close to 20% improvement.”
The rituximab group had “a little bit of a drop-off” in efficacy from weeks 24 to 48, although the trial didn’t repeat dosing at 6 months, “which is what perhaps one might do in clinical practice,” he said.
Oliver Distler, MD, chair of rheumatology at the University Hospital Zürich, raised questions about concurrent corticosteroid use in study patients that may have caused a “spillover” in the study’s efficacy analysis. But Dr. Maher noted that steroid use was balanced in all treatment arms. Patients in the cyclophosphamide arm averaged 42.9 mg of hydrocortisone daily versus 37.6 mg daily in the rituximab arm. That represents a 12.3% reduction in steroid exposure for the latter.
Dr. Distler noted that the myositis population represented the bulk of those study patients on steroids. “So in the myositis subanalysis we do see a combination of high-dose steroid plus cyclophosphamide and rituximab.”
Dr. Maher disclosed relationships with Boehringer Ingelheim, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Trevi, CSL Behring, Pliant and Veracyte. Dr. Distler disclosed relationships with numerous pharmaceutical companies.
PHILADELPHIA – In the first controlled clinical trial to compare the two drugs, rituximab and cyclophosphamide were similarly effective in improving lung function in patients with interstitial lung disease (ILD) associated with idiopathic inflammatory myositis and mixed connective tissue disease (CTD). The findings also revealed some nuanced findings that could help clarify which drug to use in specific patients.
“We feel that rituximab is a reasonable alternative to cyclophosphamide as a treatment in patients with these diseases,” said Toby Maher, MD, of the University of Southern California, Los Angeles, who presented results of an analysis of three disease subgroups from the RECITAL (Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease) study at the annual meeting of the American College of Rheumatology.
“We didn’t show it to be better, so I think you can reasonably choose between the two, but rituximab almost certainly has the advantage of being safer and better tolerated than cyclophosphamide,” Dr. Maher said in an interview. The findings were published simultaneously in The Lancet Respiratory Medicine.
Double-blind, double-dummy
RECITAL is a phase 2b, randomized, controlled trial to test the hypothesis that intravenous rituximab would be superior to cyclophosphamide for ILD-associated CTD.
The study included adults with three separate diagnoses: myositis (n = 44), mixed CTD (n = 16), and systemic sclerosis (SSc, n = 37). The study was done in the United Kingdom when Dr. Maher was with Imperial College London.
Patients in the rituximab group received 1,000 mg of IV treatment at baseline and 2 weeks, then placebo treatment every 4 weeks to week 20. Cyclophosphamide patients received 600 mg/m2 of body surface area intravenously every 4 weeks for six doses.
“When we designed this study there was limited evidence for any treatment for any disease associated with ILD,” Dr. Maher said. “But cyclophosphamide brings with it many challenges. It can be poorly tolerated and carries issues like infertility and risk of bladder cancer.”
Improved lung function
While the study failed to meet its primary endpoint – superiority of rituximab versus cyclophosphamide – it did show that both drugs led to improvement in lung function, measured by the rate of change in forced vital capacity (FVC), as well as quality of life measures, Dr. Maher said.
“Overall by week 48, we saw about a 5% improvement in FVC in the cyclophosphamide group and approximately a 4% improvement in FVC from baseline in the rituximab group, suggesting that both drugs almost certainly had a positive benefit in this patient group,” he said.
But secondary outcomes varied somewhat across the different disease groups. Patients with SSc saw a slight deterioration with cyclophosphamide in the modified Rodnan skin score at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab (–3.4 ± 8.1 units).
“One area where we did see a difference was in the number of adverse events,” Dr. Maher said. “They were fewer in the rituximab arm – namely gastrointestinal disorders [and] nausea, which we saw quite frequently following cyclophosphamide. Also, they had fewer headaches, which we saw quite frequently following cyclophosphamide.”
Rituximab patients also had fewer infusion reactions, but the number of infections was similar between the two treatment groups, he said.
“The patient group that responded best to treatment was the myositis group,” Dr. Maher said in his presentation. “Cyclophosphamide actually appears to be more effective than rituximab in improving their disease. By the end of 48 weeks, the cyclophosphamide group actually gained about 400 mL in FVC, so a close to 20% improvement.”
The rituximab group had “a little bit of a drop-off” in efficacy from weeks 24 to 48, although the trial didn’t repeat dosing at 6 months, “which is what perhaps one might do in clinical practice,” he said.
Oliver Distler, MD, chair of rheumatology at the University Hospital Zürich, raised questions about concurrent corticosteroid use in study patients that may have caused a “spillover” in the study’s efficacy analysis. But Dr. Maher noted that steroid use was balanced in all treatment arms. Patients in the cyclophosphamide arm averaged 42.9 mg of hydrocortisone daily versus 37.6 mg daily in the rituximab arm. That represents a 12.3% reduction in steroid exposure for the latter.
Dr. Distler noted that the myositis population represented the bulk of those study patients on steroids. “So in the myositis subanalysis we do see a combination of high-dose steroid plus cyclophosphamide and rituximab.”
Dr. Maher disclosed relationships with Boehringer Ingelheim, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Trevi, CSL Behring, Pliant and Veracyte. Dr. Distler disclosed relationships with numerous pharmaceutical companies.
PHILADELPHIA – In the first controlled clinical trial to compare the two drugs, rituximab and cyclophosphamide were similarly effective in improving lung function in patients with interstitial lung disease (ILD) associated with idiopathic inflammatory myositis and mixed connective tissue disease (CTD). The findings also revealed some nuanced findings that could help clarify which drug to use in specific patients.
“We feel that rituximab is a reasonable alternative to cyclophosphamide as a treatment in patients with these diseases,” said Toby Maher, MD, of the University of Southern California, Los Angeles, who presented results of an analysis of three disease subgroups from the RECITAL (Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease) study at the annual meeting of the American College of Rheumatology.
“We didn’t show it to be better, so I think you can reasonably choose between the two, but rituximab almost certainly has the advantage of being safer and better tolerated than cyclophosphamide,” Dr. Maher said in an interview. The findings were published simultaneously in The Lancet Respiratory Medicine.
Double-blind, double-dummy
RECITAL is a phase 2b, randomized, controlled trial to test the hypothesis that intravenous rituximab would be superior to cyclophosphamide for ILD-associated CTD.
The study included adults with three separate diagnoses: myositis (n = 44), mixed CTD (n = 16), and systemic sclerosis (SSc, n = 37). The study was done in the United Kingdom when Dr. Maher was with Imperial College London.
Patients in the rituximab group received 1,000 mg of IV treatment at baseline and 2 weeks, then placebo treatment every 4 weeks to week 20. Cyclophosphamide patients received 600 mg/m2 of body surface area intravenously every 4 weeks for six doses.
“When we designed this study there was limited evidence for any treatment for any disease associated with ILD,” Dr. Maher said. “But cyclophosphamide brings with it many challenges. It can be poorly tolerated and carries issues like infertility and risk of bladder cancer.”
Improved lung function
While the study failed to meet its primary endpoint – superiority of rituximab versus cyclophosphamide – it did show that both drugs led to improvement in lung function, measured by the rate of change in forced vital capacity (FVC), as well as quality of life measures, Dr. Maher said.
“Overall by week 48, we saw about a 5% improvement in FVC in the cyclophosphamide group and approximately a 4% improvement in FVC from baseline in the rituximab group, suggesting that both drugs almost certainly had a positive benefit in this patient group,” he said.
But secondary outcomes varied somewhat across the different disease groups. Patients with SSc saw a slight deterioration with cyclophosphamide in the modified Rodnan skin score at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab (–3.4 ± 8.1 units).
“One area where we did see a difference was in the number of adverse events,” Dr. Maher said. “They were fewer in the rituximab arm – namely gastrointestinal disorders [and] nausea, which we saw quite frequently following cyclophosphamide. Also, they had fewer headaches, which we saw quite frequently following cyclophosphamide.”
Rituximab patients also had fewer infusion reactions, but the number of infections was similar between the two treatment groups, he said.
“The patient group that responded best to treatment was the myositis group,” Dr. Maher said in his presentation. “Cyclophosphamide actually appears to be more effective than rituximab in improving their disease. By the end of 48 weeks, the cyclophosphamide group actually gained about 400 mL in FVC, so a close to 20% improvement.”
The rituximab group had “a little bit of a drop-off” in efficacy from weeks 24 to 48, although the trial didn’t repeat dosing at 6 months, “which is what perhaps one might do in clinical practice,” he said.
Oliver Distler, MD, chair of rheumatology at the University Hospital Zürich, raised questions about concurrent corticosteroid use in study patients that may have caused a “spillover” in the study’s efficacy analysis. But Dr. Maher noted that steroid use was balanced in all treatment arms. Patients in the cyclophosphamide arm averaged 42.9 mg of hydrocortisone daily versus 37.6 mg daily in the rituximab arm. That represents a 12.3% reduction in steroid exposure for the latter.
Dr. Distler noted that the myositis population represented the bulk of those study patients on steroids. “So in the myositis subanalysis we do see a combination of high-dose steroid plus cyclophosphamide and rituximab.”
Dr. Maher disclosed relationships with Boehringer Ingelheim, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Trevi, CSL Behring, Pliant and Veracyte. Dr. Distler disclosed relationships with numerous pharmaceutical companies.
AT ACR 2022
The Long Arc of Justice for Veteran Benefits
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
EHR-based thromboembolism risk tool boosted prophylaxis
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
AT AHA 2022
Post-COVID-19 Effects
- Centers for Disease Control and Prevention. COVID data tracker. Updated August 19, 2022. Accessed August 22, 2022. https://covid.cdc.gov/covid-data-tracker
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi:10.1038/s41591-021-01283-z
- Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated May 5, 2022. Accessed June 6, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-termeffects/index.html
- Ghazanfar H, Kandhi S, Shin D, et al. Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus. 2022;14(3):e23333. doi:10.7759/cureus.23333
- Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270-273. doi:10.1016/j.ajog.2021.09.016
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi:10.7326/M20-5661
- Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036-3039. doi:10.1007/s11606-020-06042-3
- Centers for Disease Control and Prevention. COVID data tracker. Updated August 19, 2022. Accessed August 22, 2022. https://covid.cdc.gov/covid-data-tracker
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi:10.1038/s41591-021-01283-z
- Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated May 5, 2022. Accessed June 6, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-termeffects/index.html
- Ghazanfar H, Kandhi S, Shin D, et al. Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus. 2022;14(3):e23333. doi:10.7759/cureus.23333
- Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270-273. doi:10.1016/j.ajog.2021.09.016
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi:10.7326/M20-5661
- Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036-3039. doi:10.1007/s11606-020-06042-3
- Centers for Disease Control and Prevention. COVID data tracker. Updated August 19, 2022. Accessed August 22, 2022. https://covid.cdc.gov/covid-data-tracker
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi:10.1038/s41591-021-01283-z
- Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated May 5, 2022. Accessed June 6, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-termeffects/index.html
- Ghazanfar H, Kandhi S, Shin D, et al. Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus. 2022;14(3):e23333. doi:10.7759/cureus.23333
- Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270-273. doi:10.1016/j.ajog.2021.09.016
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi:10.7326/M20-5661
- Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036-3039. doi:10.1007/s11606-020-06042-3