User login
Betamethasone Dipropionate Spray 0.05% Alleviates Troublesome Symptoms of Plaque Psoriasis
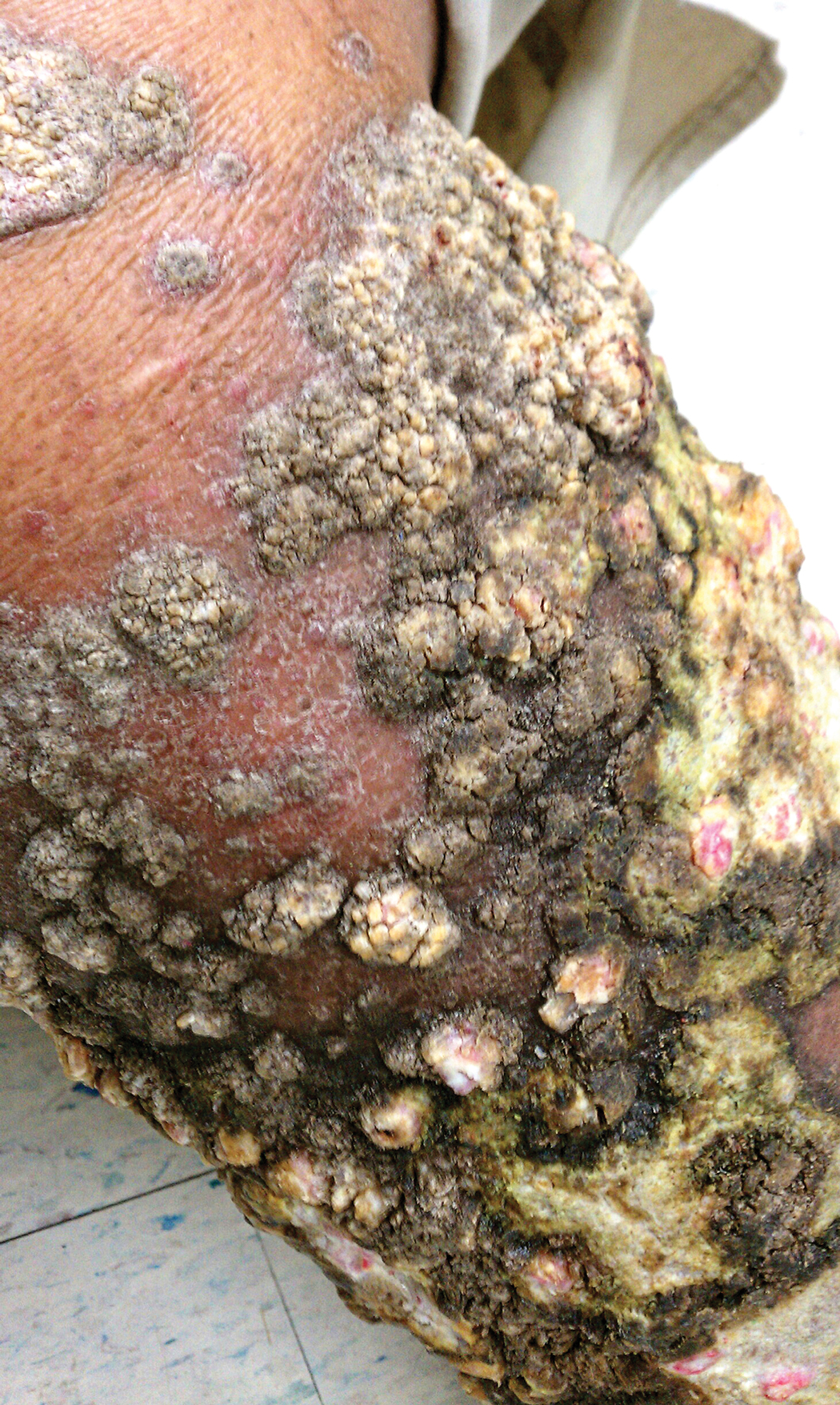
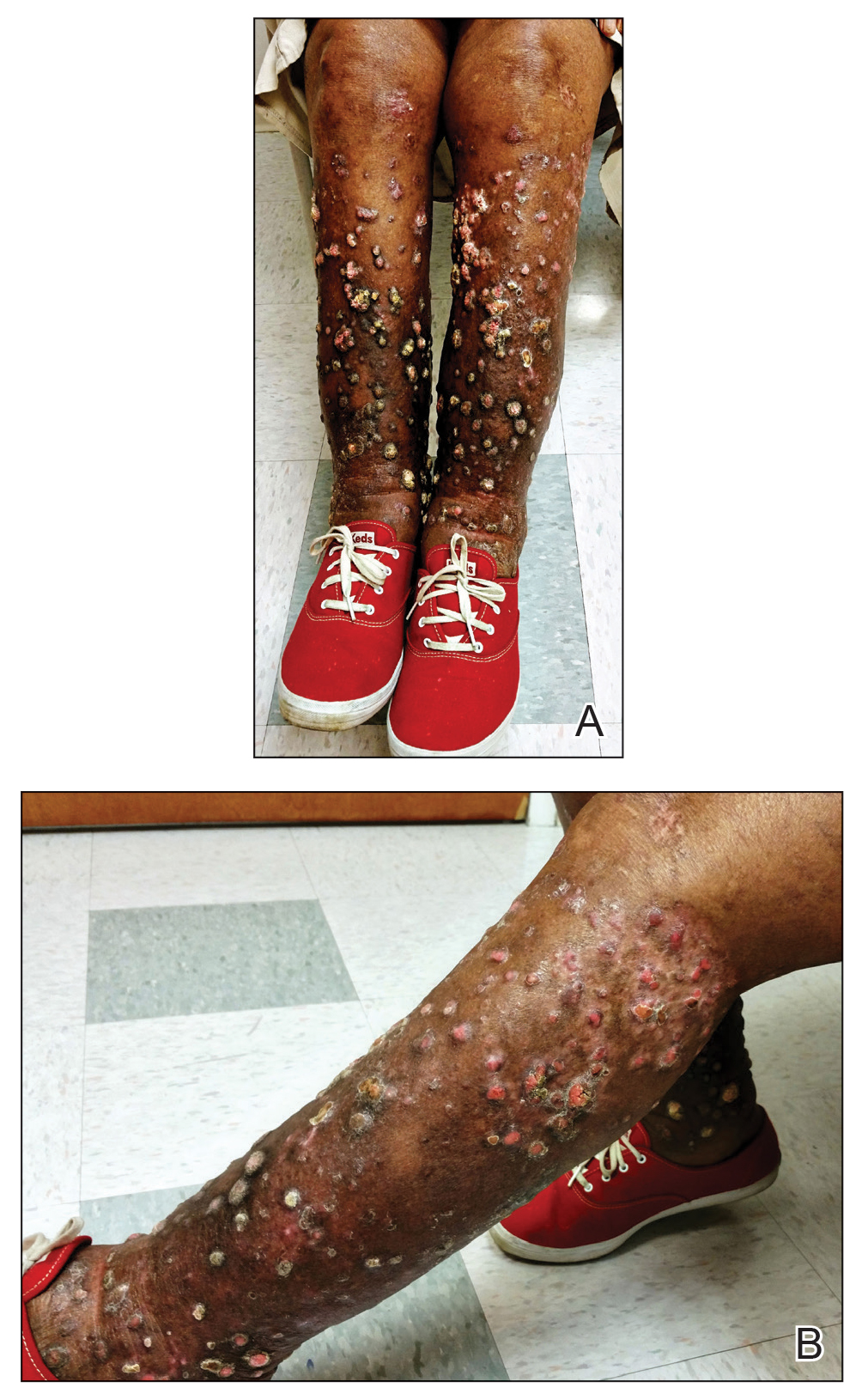
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
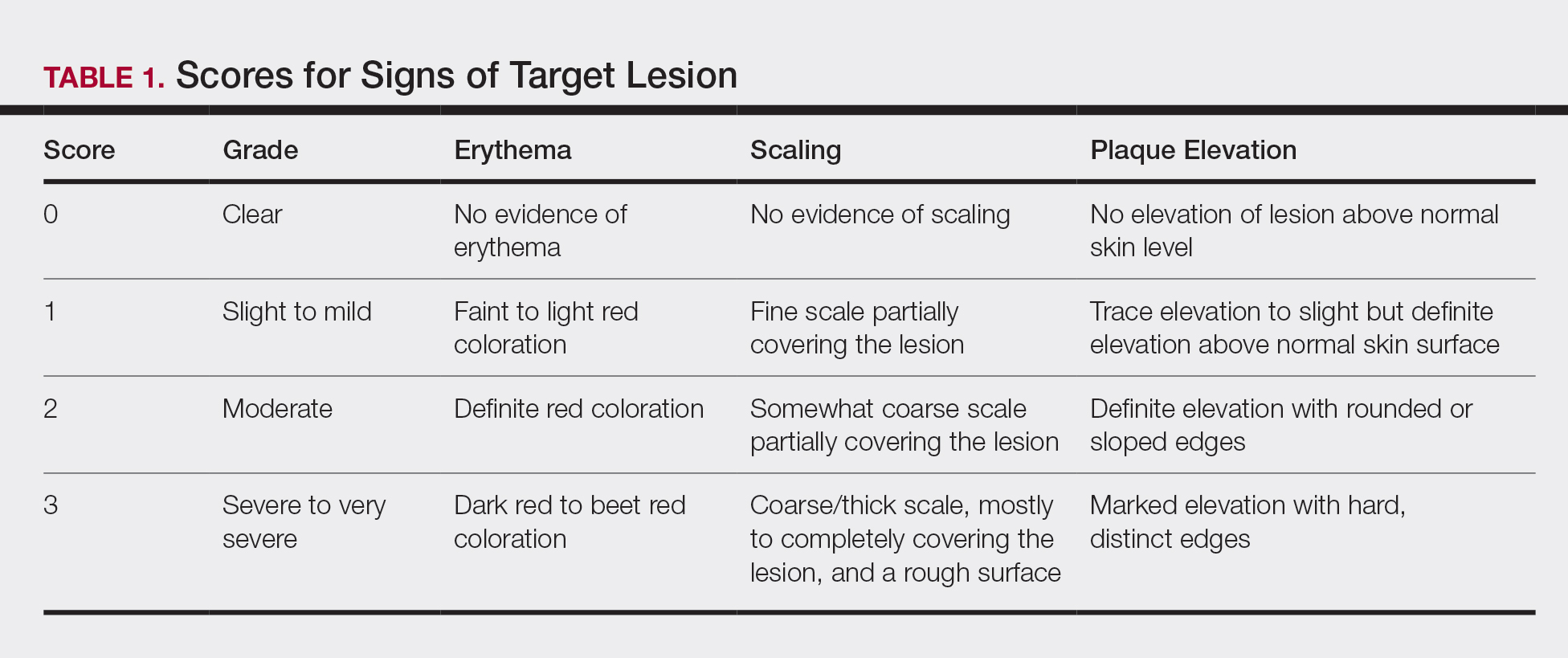
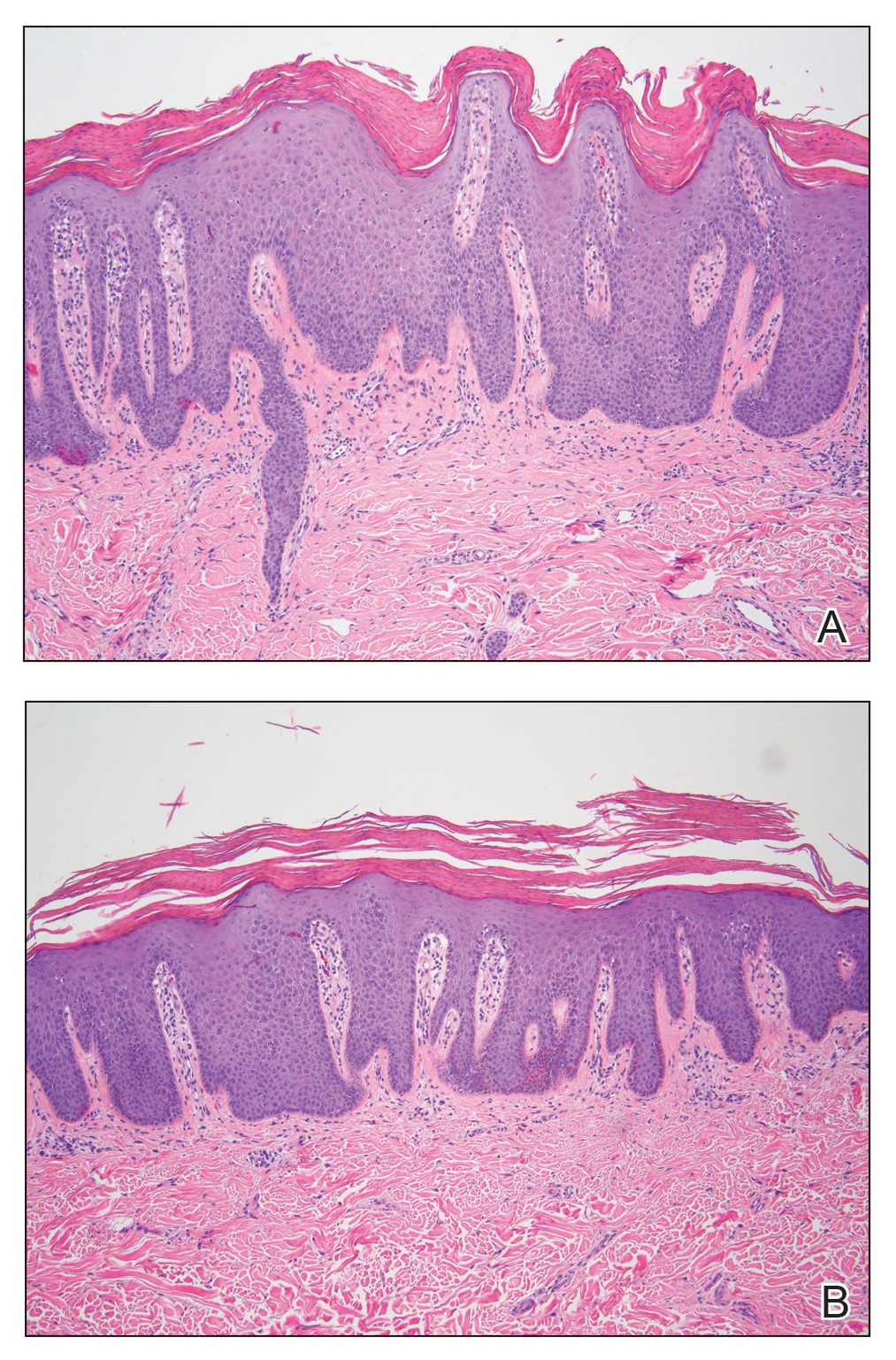
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
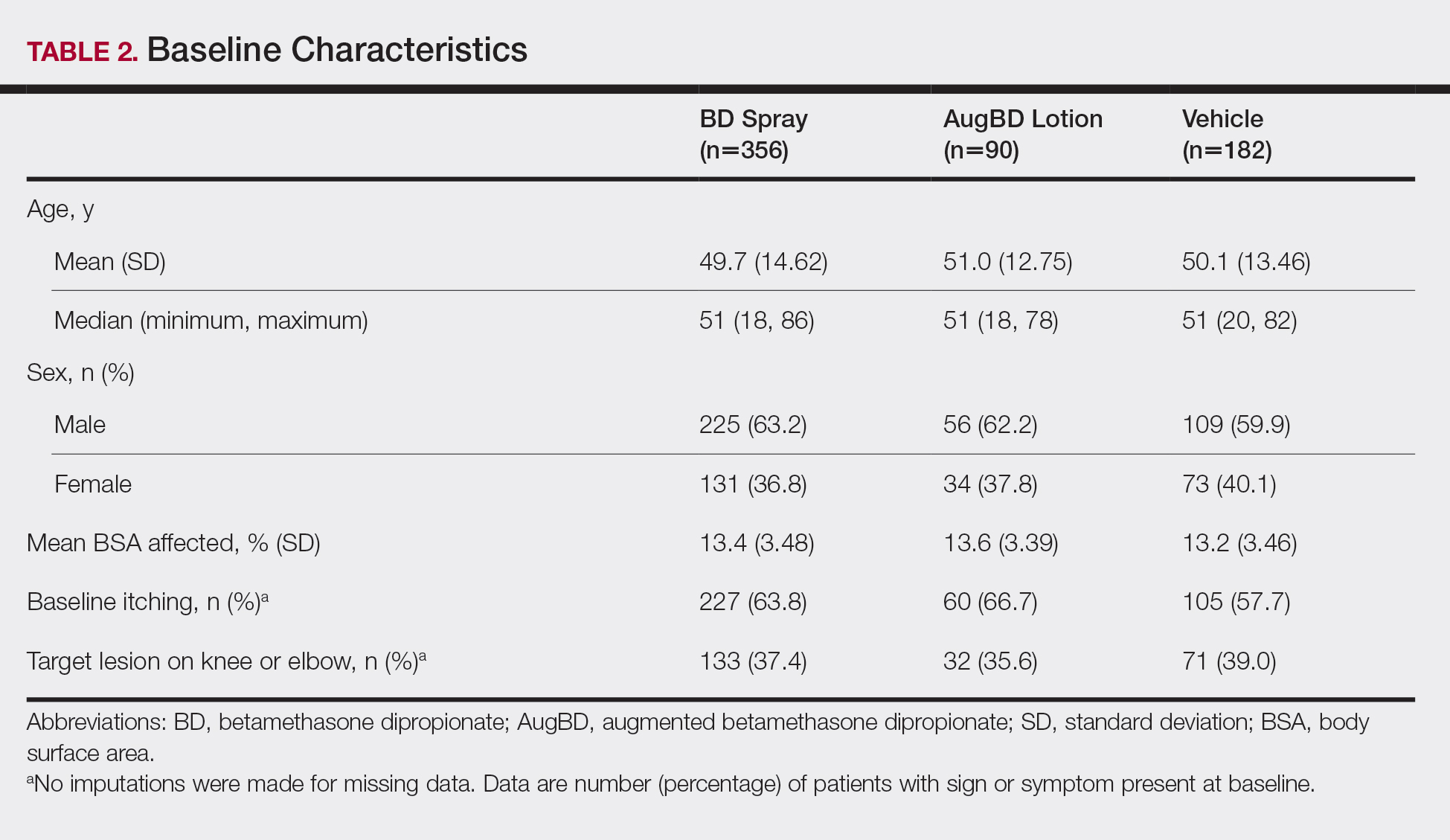
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
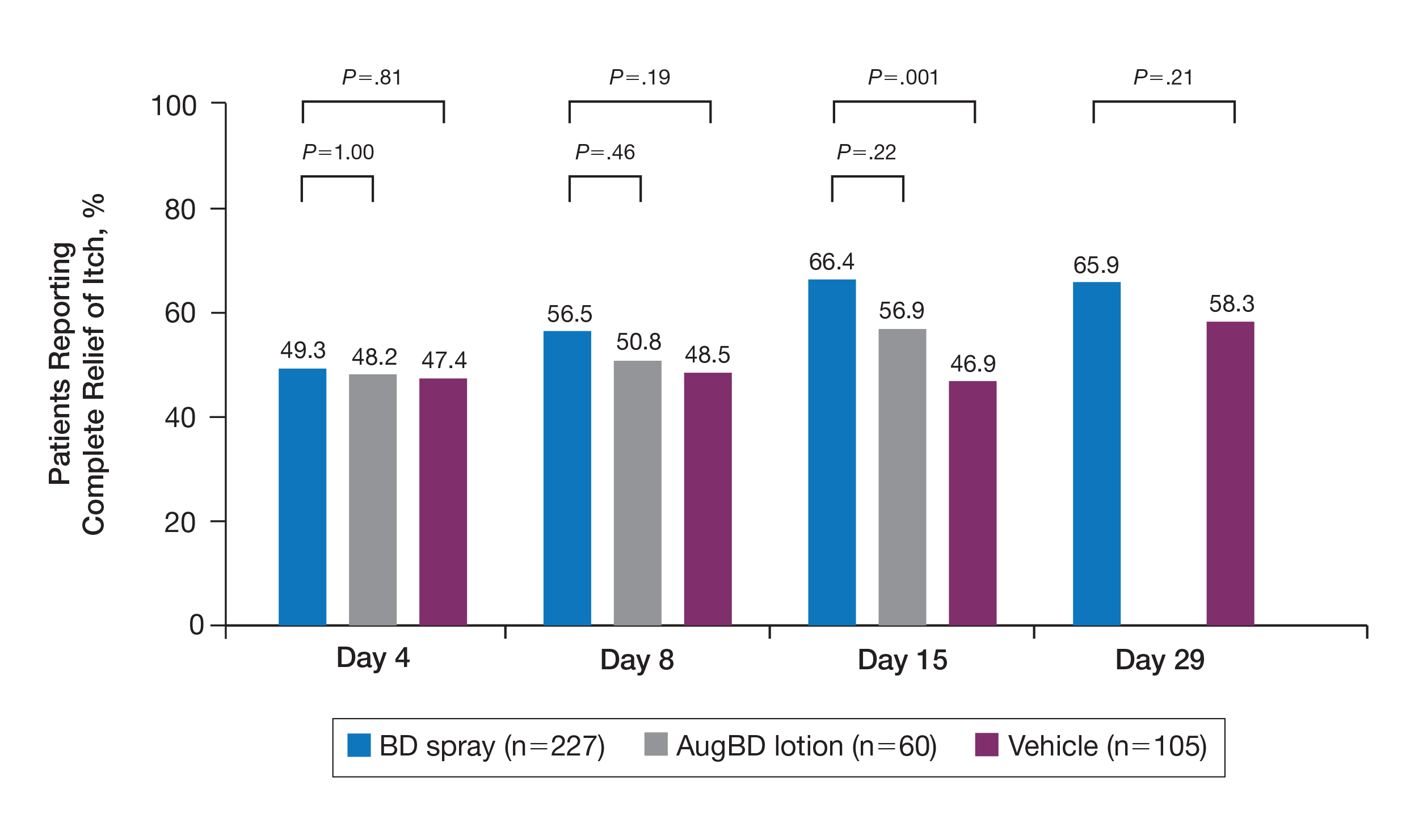
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
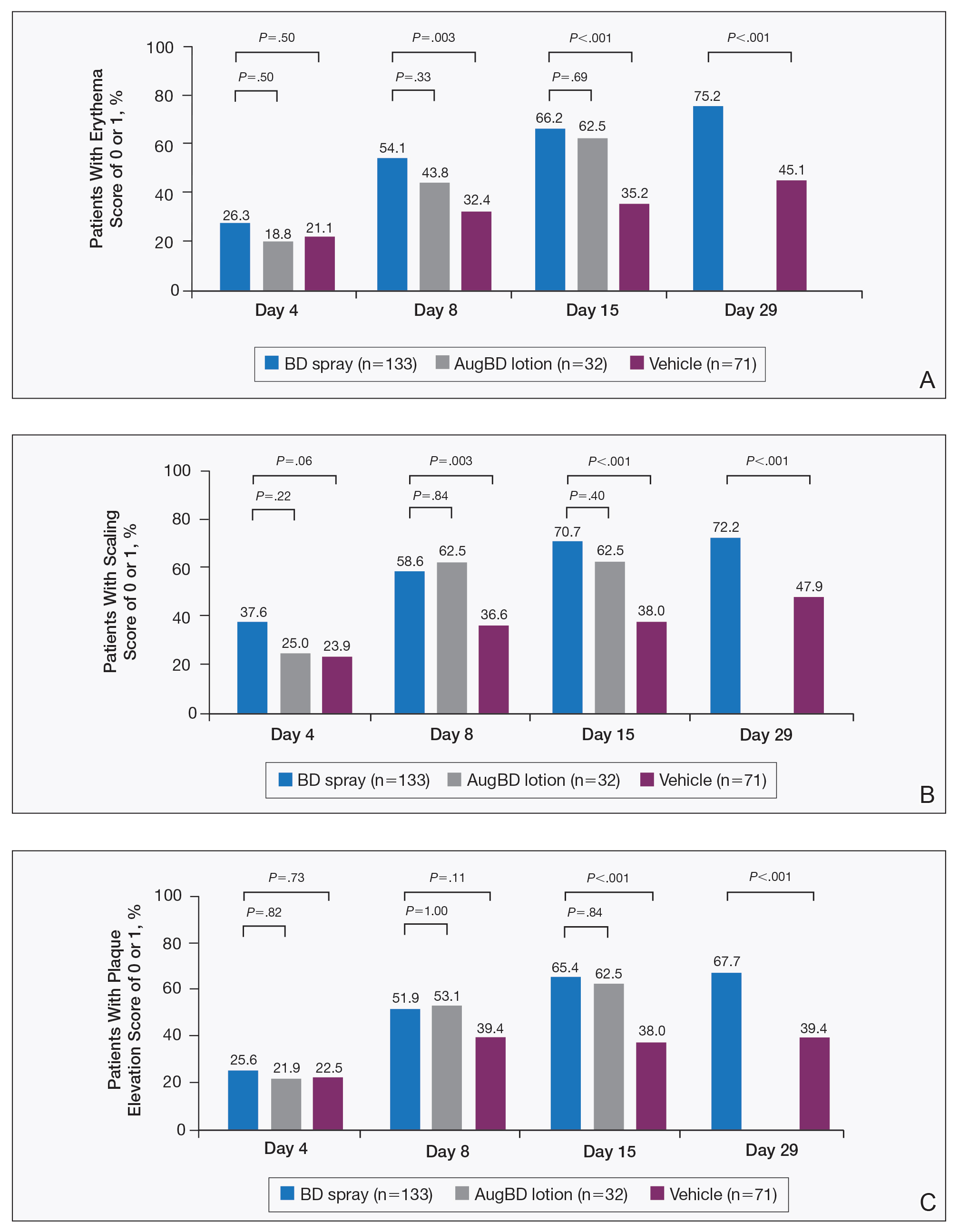
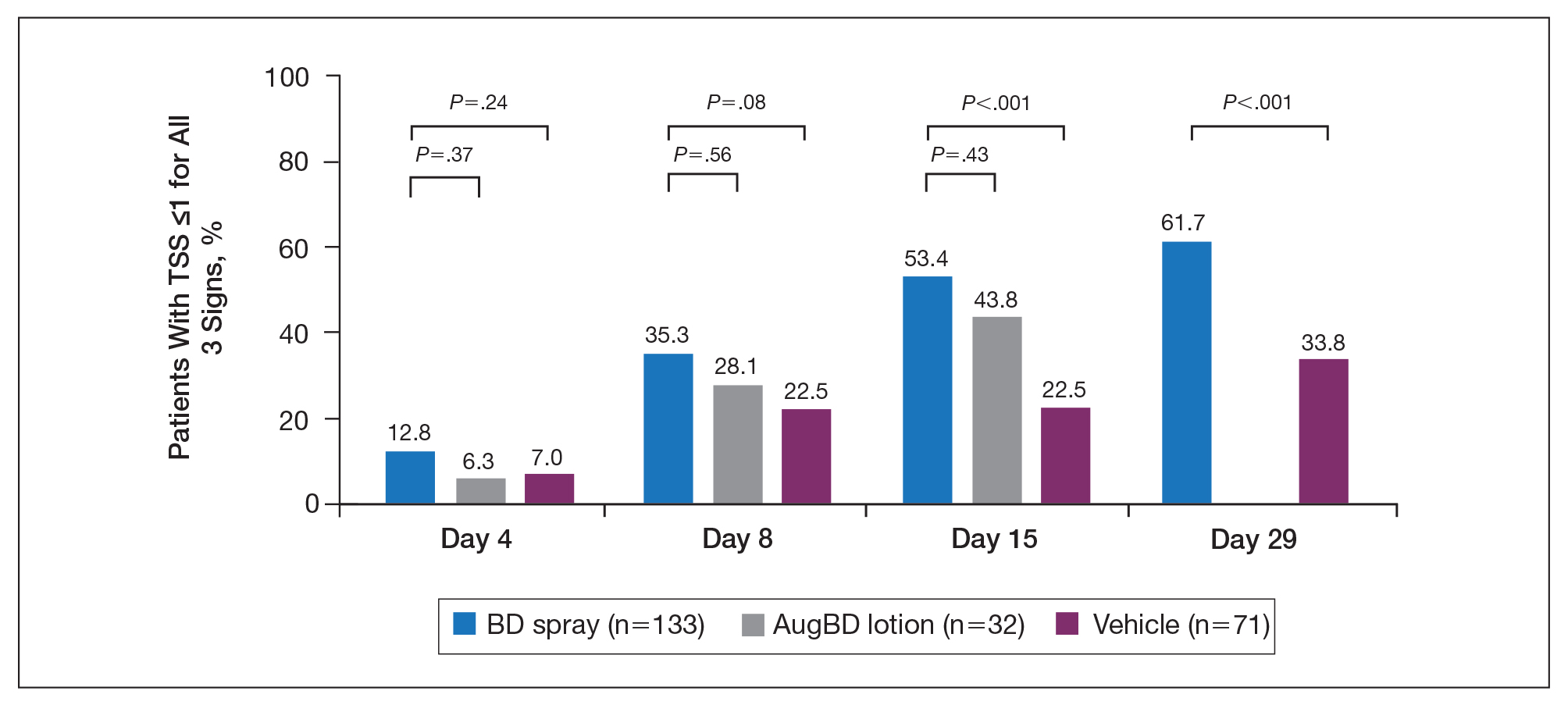
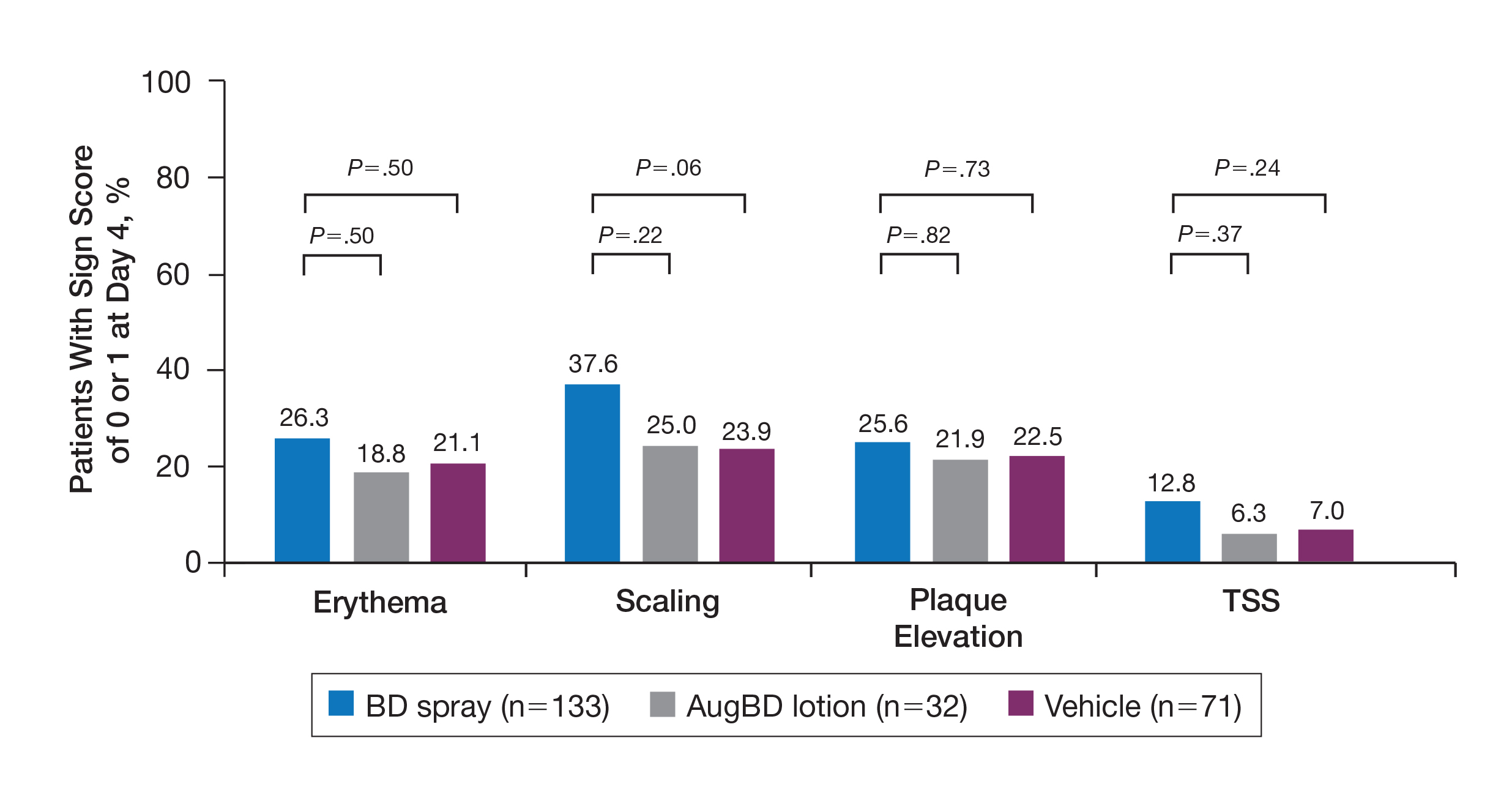
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
Psoriasis affects approximately 2% to 3% of the US population and is characterized by plaques that are red, scaly, and elevated.1 Cutaneous symptoms of the disease are described by patients as itching, burning, and stinging sensations. Large multinational and US surveys have reported pruritus as patients’ most bothersome symptom, with scaling/flaking reported as the second most bothersome.2,3 Reported incidence rates for itching range from 60.4% to 98.3%, with at least half of these patients reporting daily or constant pruritus.2,4-7 Consequent effects on quality of life include impaired sleep,6 difficulty concentrating, lower sex drive, and depression.7 Despite these findings, pruritus is rarely included in the efficacy assessments of psoriasis treatments. In addition, 2 of the most commonly reported but difficult-to-treat locations for plaques are the outside of the elbows (45%) and the knees (32%),1,2,8 areas where the stratum corneum typically is thicker, less hydrated, and less likely to absorb topical products.9-11 Clinical studies have not focused specifically on these areas when assessing treatments.
Topical corticosteroids have been the mainstay of psoriasis therapy for decades because of their anti-inflammatory and antiproliferative properties.7 One large multinational physician survey indicated that 75% of patients are prescribed topical steroids,12 which are important for first-line treatment and are often maintained as adjunctive therapy in combination with other treatments for patients with extensive disease or recalcitrant lesions.13 Topical corticosteroids are ranked into different classes based on their vasoconstrictor assay (VCA), a measure of skin blanching used as a marker for vasoconstriction. Topical agents with VCA ratings of mid-potency or superpotency are generally recommended for initial therapy, with superpotent agents required for the treatment of thick chronic plaques. However, longer durations of use may contribute to systemic absorption and adverse events.13 The vehicle composition is important for corticosteroid delivery and retention at the site of pathology, contributing to the efficacy of the steroid.13,14 Selecting the appropriate steroid and vehicle is important to maximize efficacy and minimize adverse events.
Betamethasone dipropionate (BD) spray 0.05% is an emollient formulation of 0.05% BD that can be sprayed onto psoriatic plaques. The BD spray formulation was designed to penetrate the stratum corneum and be retained within the dermis and epidermis, the site of T-cell activity that drives the psoriatic disease process.14 In 2 phase 3 studies, BD spray demonstrated the ability to reduce the signs of plaque psoriasis with indication of improvement by day 4.15,16 These studies also showed improvement in the local cutaneous symptoms of itching, burning and stinging, and pain. As a mid-potent steroid, BD spray displays less systemic absorption but similar efficacy compared to a superpotent augmented BD (AugBD) lotion in relieving the signs and symptoms of plaque psoriasis.15-17
The objective of the current investigation was to assess the ability of BD spray to relieve itching and to clear plaque psoriasis on the knees and elbows utilizing post hoc analyses of the 2 phase 3 trials. The goal of these analyses was to demonstrate BD spray as effective at relieving the most troublesome signs and symptoms affecting patients with plaque psoriasis.
Methods
Two phase 3 studies were conducted to demonstrate the efficacy and safety of BD spray.15,16 The design of the studies was similar15,16 to allow the data to be pooled for post hoc analyses.
Both were US multicenter, randomized, vehicle-controlled, double-blind, parallel-group studies comparing the safety and efficacy of BD spray 0.05% (Sernivo, Promius Pharma) with its vehicle formulation spray (identical to BD spray, but lacking the active steroid component).15,16 One of the studies also compared BD spray with an AugBD lotion 0.05% (Diprolene,Merck & Co). Adults with moderate plaque psoriasis (investigator global assessment of 3; 10%–20% body surface area) were randomized to apply BD spray, vehicle spray, or AugBD lotion (1 study only) twice daily to all affected areas, excluding the face, scalp, and intertriginous areas for 28 days (BD spray and vehicle) or 14 days (AugBD lotion, per product label).15
Assessments
Two post hoc analyses were conducted on data pooled from the 2 phase 3 trials: (1) incidence of itching, and (2) total sign score (TSS) for lesions located on the knees and elbows.
Itching
Itching was assessed proactively by asking patients if they were experiencing itching (yes/no) at each visit (baseline and days 4, 8, 15, and 29) or had experienced itching since their last visit. As itching could be an adverse event of topical application, application-site pruritus was also recorded.
Total Sign Score
For each patient, a target plaque was selected that was representative of their psoriasis. The plaque was assessed on a 3-point grading scale for each of 3 key signs of plaque psoriasis: erythema, scaling, and plaque elevation (Table 1) at baseline and days 4, 8, 15, and 29. Total sign score was calculated by summing the scores for these 3 signs, resulting in a score ranging from 0 to 9. Treatment success was measured as (1) achieving a score of 0 or 1 (ie, reducing the plaque to clear or slight to mild) for the individual signs of erythema, scaling, and plaque elevation; and (2) achieving a TSS of 0 or 1 for all 3 signs—erythema, scaling, and plaque elevation—for each target lesion. Total sign score was assessed proactively for all patients.15,16 The post hoc analysis reported here examined patients whose target lesion was located on either the knee or the elbow.
Statistical Analyses
Because both study protocols were identical, data were pooled from the 2 phase 3 trials. All statistical analyses were performed using SAS software (SAS Institute). Two-sided hypothesis testing was conducted for all analyses using a significance level of P=.05. Post hoc analyses used Fisher exact test. No imputations were made for missing data.
Statistical analyses of itching compared the incidence of itching at each assessment time point (baseline and days 4, 8, 15, and 29) between BD spray and vehicle and between BD spray and AugBD lotion. Additional analysis included a statistical test on the incidence of itching in the subgroup of patients who reported itching at baseline.
Statistical analyses for the knees and elbows included only patients with their target lesion located on either the knee or the elbow. Analyses compared BD spray with vehicle and BD spray with AugBD lotion at days 4, 8, 15, and 29. Comparison with AugBD lotion treatment was up to day 14 only, consistent with application time limits in the AugBD lotion product label.18
Results
These analyses included data from the 628 patients enrolled in the 2 phase 3 trials. Patients had similar baseline characteristics across treatment groups (Table 2). Itching was the most common cutaneous symptom at baseline, reported by almost two-thirds (n=392, 62.4%) of patients. Of the 628 patients, 236 (37.6%) had a target lesion located on the elbow or knee selected for assessment. The mean baseline body surface area was 13% to 14% across groups.
A post hoc analysis was performed on the subgroup of patients who reported itching at baseline (N=392)(eFigure 1). For these patients, almost half were itch free by day 4 across all groups (49.3% BD spray, 48.2% AugBD lotion, and 47.4% vehicle). By the end of treatment, 65.9% of patients using BD spray and 58.3% of patients using vehicle were itch free at day 29, with 56.9% of AugBD lotion patients itch free at day 15.
Application-site pruritus recorded as a treatment-emergent adverse event was seen in low numbers and was similar in proportion between the 2 steroid treatments (7.7% BD spray, 6.7% AugBD lotion, and 14.4% vehicle).
Psoriasis Individual Sign Scores for Knee and Elbow Plaques
Target lesions located on the knee or elbow represented 37.6% of all target lesions assessed. Efficacy analysis of the pooled data on knee and elbow lesions revealed that BD spray was similar to AugBD lotion in reducing sign scores to 0 or 1 (Figures 1 and 2).
The percentage of patients reporting improvements in erythema, scaling, and plaque elevation scores at day 4
The proportion of patients achieving treatment success (defined as a score of 0 or 1) was comparable for the2 products on day 15 for erythema (66.2% BD spray vs 62.5% AugBD lotion), scaling (70.7% BD spray vs 62.5% AugBD lotion), and plaque elevation (65.4% BD spray vs 62.5% AugBD lotion)(Figure 1). From day 8, BD spray reduced erythema and scaling in significantly more patients than vehicle (P=.003 for both), and BD spray reduced erythema, scaling, and plaque elevation in more patients than vehicle from day 15 (P<.001 for all). No statistically significant difference was found between BD spray and AugBD lotion on erythema, scaling, and plaque elevation scores.
Total Sign Score
Total sign score results showed that the mean percentage of patients achieving a TSS of 0 or 1 for all signs for lesions located on the knees or elbows was numerically higher for BD spray vs AugBD lotion at day 4, but this difference was not statistically significant (Figure 2). Day 15 outcomes for TSS also showed a numerically greater success rate for BD spray, but again this difference was not statistically significant (53.4% BD spray vs 43.8% AugBD lotion). At days 15 and 29, significantly more patients treated with BD spray achieved TSS of 0 or 1 for all 3 signs compared to those treated with vehicle (P<.001). Improvement in TSS with BD spray continued through to day 29 of the study.
Comment
In these post hoc analyses, mid-potency BD spray demonstrated early relief of itching and early efficacy in the treatment of psoriasis plaques on the elbows and knees with minimal systemic absorption and a low rate of adverse events.
Betamethasone dipropionate spray and its vehicle formulation relieved psoriatic itching with similar efficacy to the superpotent AugBD steroid lotion. Notably, relief was rapid, with approximately half of responding patients reporting relief of itching by day 4. The results seen with vehicle suggest that the emollient formulation of BD spray is responsible for hydrating dry skin, contributing to the relief of this cutaneous symptom. Dry skin can exacerbate itching, and emollients are recognized as being able to alleviate itching by hydrating and soothing the skin.7
The second set of post hoc analyses reported here demonstrated that BD spray was efficacious in clearing the signs of psoriatic lesions on the difficult-to-treat areas of the knees and elbows. Efficacy with BD spray was similar to the superpotent steroid AugBD lotion, with no statistical difference between the 2 products at any time point. Betamethasone dipropionate spray was significantly more effective than its vehicle in reducing the signs of erythema and scaling from day 8 and plaque elevation from day 15.
Rapid relief of symptoms is important for patient comfort and to improve treatment adherence. These analyses showed that by day 4, BD spray resulted in numerically higher percentages of patients achieving a score of 0 or 1 for the individual signs of erythema, scaling, and plaque elevation compared to AugBD lotion. Of particular note, 37.6% of patients treated with BD spray had scaling scores of clear or almost clear by day 4 compared to 25.0% of patients treated with AugBD lotion. Scaling has been consistently reported as the second most bothersome symptom experienced by patients2,3 and has been shown to be associated with decreased quality of life and work productivity.19
Betamethasone dipropionate spray has a rationally designed vehicle, with the formulation selected specifically to maximize penetration of the product through the stratum corneum and retention of BD steroid in the epidermis and upper dermis while reducing absorption into the systemic circulation.14 The reduced absorption into the systemic circulation leads to less vasoconstriction; fewer adverse events; and a “medium potent” VCA designation compared to the “superpotent” designation of the AugBD formulation, despite containing the same active ingredient.
These analyses demonstrate that BD spray is effective at addressing 2 symptoms that patients with psoriasis consider most bothersome: itching and scaling. Notably, BD spray was able to achieve these results rapidly, with many patients experiencing improvements in 4 days. In these analyses, mid-potent BD spray demonstrated similar efficacy to AugBD lotion, a superpotent steroid formulation.
This analysis is limited by being post hoc. Although the statistical methodology is valid, the AugBD lotion arm of the analyses was relatively small compared with the BD spray and vehicle arms, as it was only included in 1 of 2 studies pooled.
Conclusion
Mid-potency BD spray effectively improved the symptom of itching and cleared hard-to-treat lesions on knees and elbows with efficacy similar to a superpotent AugBD formulation but with less systemic absorption. Improvements were seen in erythema, scaling, and plaque elevation. Reductions in psoriatic signs were observed as early as day 4, with continued improvement seen throughout the study period. These findings provide evidence that BD spray can rapidly relieve 2 of the most troublesome symptoms affecting patients with psoriasis (itching and scaling), potentially improving quality of life.
Acknowledgments
The authors wish to thank Alix Bennett, PhD, formerly of Promius Pharma, a subsidiary of Dr. Reddy’s Laboratories, Inc (Princeton, New Jersey), and Jodie Macoun, PhD, of CUBE Information (Katonah, New York), for their review and assistance with the preparation of this manuscript. Manuscript preparation was supported by Promius Pharma (Princeton, New Jersey)(DRL #866).
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
- About psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis. Accessed October 1, 2019.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e1-30.
- Pariser D, Schenkel B, Carter C, et al; Psoriasis Patient Interview Study Group. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27:19-26.
- Dickison P, Swain G, Peek JJ, et al. Itching for answers: prevalence and severity of pruritus in psoriasis. Australas J Dermatol. 2018;59:206-209.
- Bahali AG, Onsun N, Su O, et al. The relationship between pruritus and clinical variables in patients with psoriasis. An Bras Dermatol. 2017;92:470-473.
- Prignano F, Ricceri F, Pescitelli L, et al. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9-13.
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227-233.
- Queille-Roussel C, Rosen M, Clonier F, et al. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37:355-361.
- Betesil [package insert]. Lodi, Italy: IBSA Pharmaceutici Italia S.r.I; 2013.
- Cannavò SP, Guarneri F, Giuffrida R, et al. Evaluation of cutaneous surface parameters in psoriatic patients. Skin Res Technol. 2017;23:41-47.
- Egawa M, Arimoto H, Hirao T, et al. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: consideration of measurement depth. Appl Spectrosc. 2006;60:24-28.
- van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002-2010.
- Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Kircik L, Okumu F, Kandavilli S, et al. Rational vehicle design ensures targeted cutaneous steroid delivery. J Clin Aesthet Dermatol. 2017;10:12-19.
- Fowler JF Jr, Herbert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Stein Gold L, Jackson JM, Knuckles ML, et al. Improvement in extensive moderate plaque psoriasis with a novel emollient spray formulation of betamethasone dipropionate 0.05. J Drugs Dermatol. 2016;15:334-342.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mild-to-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Diprolene Lotion (augmented betamethasone dipropionate 0.05%) [package insert]. Kenilworth, NJ: Schering Corporation; 1999.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21. pii:13030/qt1x16v3dg.
Practice Points
- Pruritus is one of the most bothersome symptoms of psoriasis; plaques located on the knees and elbows remain hard to treat.
- Topical corticosteroids are the initial form of treatment of localized plaque psoriasis.
- The choice of vehicle can change the penetration of the medication, alter the efficacy, and minimize side effects of the drug.
- Betamethasone dipropionate spray 0.05% is a mid-potent corticosteroid that provides fast symptom relief and early efficacy in clearing plaques, similar to a high-potency topical corticosteroid but with less potential for systemic absorption and adverse events.
Tumor Necrosis Factor Inhibitors May Reduce Cardiovascular Morbidity in Patients With Psoriasis
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
Psoriasis: A look back over the past 50 years, and forward to next steps
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
REPORTING FROM ACR 2019
The Lowdown on Low-Dose Naltrexone
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
Resident Pearl
- Low-dose naltrexone is an alternative antiinflammatory treatment to consider in patients with inflammatory skin diseases, with a minimal side-effect profile.
Bimekizumab elevates psoriasis therapy
MADRID – Renowned dermatologic clinical trialist Kim A. Papp, MD, PhD, is known to pick his words carefully, and the word he uses to describe the quality of life improvement documented in psoriasis patients treated with the novel investigational humanized monoclonal antibody bimekizumab is “phenomenal.”
Dr. Papp was lead investigator in the previously reported phase 2b multicenter BE ABLE 1 trial, in which 250 patients with moderate to severe chronic plaque psoriasis were randomized double-blind to various doses of bimekizumab or placebo every 4 weeks for 12 weeks (J Am Acad Dermatol. 2018 Aug;79[2]:277-86.e10. doi: 10.1016/j.jaad.2018.03.037). He was also lead investigator in the 48-week phase 2b BE ABLE 2 extension study. He presented the 60-week quality-of-life BE ABLE 2 results for the first time at the annual congress of the European Academy of Dermatology and Venereology.
“Small numbers, but the results are nonetheless very compelling,” said Dr. Papp, president and founder of Probity Medical Research in Waterloo, Ont.
Bimekizumab is unique in that it selectively neutralizes both interleukin-17A and -17F, two closely related proinflammatory cytokines which, when upregulated, synergize with other proinflammatory cytokines to drive psoriasis and other immune-mediated inflammatory diseases. In contrast, secukinumab (Cosentyx) and ixekizumab (Taltz) specifically inhibit only IL-17A, and brodalumab (Siliq) targets the IL-17 receptor A. The bimekizumab clinical trials program – a work in progress – aims to demonstrate that dual neutralization of IL-17A and -17F provides a more complete therapeutic approach in psoriasis, with greater efficacy and fewer safety concerns than with current biologics, the dermatologist explained.
In BE ABLE 1, the primary endpoint of at least a 90% reduction in Psoriasis Area and Severity Index (PASI90) response was achieved at week 12 in 46%-79% of patients randomized to bimekizumab in dose-dependent fashion. Those PASI90 responses were maintained with additional treatment out to week 60 in BE ABLE 2 in 80%-100% of patients.
Dr. Papp’s focus at EADV 2019 was on the quality-of-life improvement achieved in bimekizumab-treated patients, a benefit not captured by PASI scores. For this purpose, he and coinvestigators turned to the Dermatology Life Quality Index (DLQI), measured in structured fashion every 4 weeks out to week 60.
“We often forget that even though we’re looking at the patient from the outside, what’s really important is how well they respond to our treatments internally. The DLQI is not a perfect tool, but it’s the best tool we have available. It gives us a fairly good survey of the various domains that affect patients’ day-to-day living,” he said.
In BE ABLE 1, the proportion of week-12 PASI90 responders achieving a DLQI of 0 or 1 – indicative of essentially no disease impact on quality of life – increased rapidly up until week 8. At week 12, 70%-100% of the PASI90 responders in the various treatment arms had a DLQI of 0 or 1. This quality-of-life improvement, like the PASI90 response, proved durable: When the week-12 PASI90 responders were assessed at week 60 in BE ABLE 2, 76%-93% of them had a DLQI of 0 or 1.
The improvements in quality of life correlated with clinical response. BE ABLE enrollees had an average PASI score of 19 at baseline. Overall, 79% of those with an absolute PASI score of 0 at week 12 had a DLQI of 0 or 1 at that time, as did 95% of those with a PASI of 0 at week 60. A PASI of 1 was associated with a 77% likelihood of a DLQI of 0 or 1 at week 12 and an 82% rate at week 60. In contrast, patients with an absolute PASI of 2-4 at week 12 had a 46% rate of DLQI 0/1, and those with a PASI 2-4 at week 60 had a 50% chance of having a DLQI of 0/1.
Phase 3 clinical trials of bimekizumab totaling several thousand psoriasis patients are ongoing.
The BE ABLE trials were sponsored by UCB Pharma. Dr. Papp reported serving as a consultant to and/or recipient of research grants from UCB and dozens of other pharmaceutical companies.
SOURCE: Papp KA. EADV 2019 Abstract FC02.02.
MADRID – Renowned dermatologic clinical trialist Kim A. Papp, MD, PhD, is known to pick his words carefully, and the word he uses to describe the quality of life improvement documented in psoriasis patients treated with the novel investigational humanized monoclonal antibody bimekizumab is “phenomenal.”
Dr. Papp was lead investigator in the previously reported phase 2b multicenter BE ABLE 1 trial, in which 250 patients with moderate to severe chronic plaque psoriasis were randomized double-blind to various doses of bimekizumab or placebo every 4 weeks for 12 weeks (J Am Acad Dermatol. 2018 Aug;79[2]:277-86.e10. doi: 10.1016/j.jaad.2018.03.037). He was also lead investigator in the 48-week phase 2b BE ABLE 2 extension study. He presented the 60-week quality-of-life BE ABLE 2 results for the first time at the annual congress of the European Academy of Dermatology and Venereology.
“Small numbers, but the results are nonetheless very compelling,” said Dr. Papp, president and founder of Probity Medical Research in Waterloo, Ont.
Bimekizumab is unique in that it selectively neutralizes both interleukin-17A and -17F, two closely related proinflammatory cytokines which, when upregulated, synergize with other proinflammatory cytokines to drive psoriasis and other immune-mediated inflammatory diseases. In contrast, secukinumab (Cosentyx) and ixekizumab (Taltz) specifically inhibit only IL-17A, and brodalumab (Siliq) targets the IL-17 receptor A. The bimekizumab clinical trials program – a work in progress – aims to demonstrate that dual neutralization of IL-17A and -17F provides a more complete therapeutic approach in psoriasis, with greater efficacy and fewer safety concerns than with current biologics, the dermatologist explained.
In BE ABLE 1, the primary endpoint of at least a 90% reduction in Psoriasis Area and Severity Index (PASI90) response was achieved at week 12 in 46%-79% of patients randomized to bimekizumab in dose-dependent fashion. Those PASI90 responses were maintained with additional treatment out to week 60 in BE ABLE 2 in 80%-100% of patients.
Dr. Papp’s focus at EADV 2019 was on the quality-of-life improvement achieved in bimekizumab-treated patients, a benefit not captured by PASI scores. For this purpose, he and coinvestigators turned to the Dermatology Life Quality Index (DLQI), measured in structured fashion every 4 weeks out to week 60.
“We often forget that even though we’re looking at the patient from the outside, what’s really important is how well they respond to our treatments internally. The DLQI is not a perfect tool, but it’s the best tool we have available. It gives us a fairly good survey of the various domains that affect patients’ day-to-day living,” he said.
In BE ABLE 1, the proportion of week-12 PASI90 responders achieving a DLQI of 0 or 1 – indicative of essentially no disease impact on quality of life – increased rapidly up until week 8. At week 12, 70%-100% of the PASI90 responders in the various treatment arms had a DLQI of 0 or 1. This quality-of-life improvement, like the PASI90 response, proved durable: When the week-12 PASI90 responders were assessed at week 60 in BE ABLE 2, 76%-93% of them had a DLQI of 0 or 1.
The improvements in quality of life correlated with clinical response. BE ABLE enrollees had an average PASI score of 19 at baseline. Overall, 79% of those with an absolute PASI score of 0 at week 12 had a DLQI of 0 or 1 at that time, as did 95% of those with a PASI of 0 at week 60. A PASI of 1 was associated with a 77% likelihood of a DLQI of 0 or 1 at week 12 and an 82% rate at week 60. In contrast, patients with an absolute PASI of 2-4 at week 12 had a 46% rate of DLQI 0/1, and those with a PASI 2-4 at week 60 had a 50% chance of having a DLQI of 0/1.
Phase 3 clinical trials of bimekizumab totaling several thousand psoriasis patients are ongoing.
The BE ABLE trials were sponsored by UCB Pharma. Dr. Papp reported serving as a consultant to and/or recipient of research grants from UCB and dozens of other pharmaceutical companies.
SOURCE: Papp KA. EADV 2019 Abstract FC02.02.
MADRID – Renowned dermatologic clinical trialist Kim A. Papp, MD, PhD, is known to pick his words carefully, and the word he uses to describe the quality of life improvement documented in psoriasis patients treated with the novel investigational humanized monoclonal antibody bimekizumab is “phenomenal.”
Dr. Papp was lead investigator in the previously reported phase 2b multicenter BE ABLE 1 trial, in which 250 patients with moderate to severe chronic plaque psoriasis were randomized double-blind to various doses of bimekizumab or placebo every 4 weeks for 12 weeks (J Am Acad Dermatol. 2018 Aug;79[2]:277-86.e10. doi: 10.1016/j.jaad.2018.03.037). He was also lead investigator in the 48-week phase 2b BE ABLE 2 extension study. He presented the 60-week quality-of-life BE ABLE 2 results for the first time at the annual congress of the European Academy of Dermatology and Venereology.
“Small numbers, but the results are nonetheless very compelling,” said Dr. Papp, president and founder of Probity Medical Research in Waterloo, Ont.
Bimekizumab is unique in that it selectively neutralizes both interleukin-17A and -17F, two closely related proinflammatory cytokines which, when upregulated, synergize with other proinflammatory cytokines to drive psoriasis and other immune-mediated inflammatory diseases. In contrast, secukinumab (Cosentyx) and ixekizumab (Taltz) specifically inhibit only IL-17A, and brodalumab (Siliq) targets the IL-17 receptor A. The bimekizumab clinical trials program – a work in progress – aims to demonstrate that dual neutralization of IL-17A and -17F provides a more complete therapeutic approach in psoriasis, with greater efficacy and fewer safety concerns than with current biologics, the dermatologist explained.
In BE ABLE 1, the primary endpoint of at least a 90% reduction in Psoriasis Area and Severity Index (PASI90) response was achieved at week 12 in 46%-79% of patients randomized to bimekizumab in dose-dependent fashion. Those PASI90 responses were maintained with additional treatment out to week 60 in BE ABLE 2 in 80%-100% of patients.
Dr. Papp’s focus at EADV 2019 was on the quality-of-life improvement achieved in bimekizumab-treated patients, a benefit not captured by PASI scores. For this purpose, he and coinvestigators turned to the Dermatology Life Quality Index (DLQI), measured in structured fashion every 4 weeks out to week 60.
“We often forget that even though we’re looking at the patient from the outside, what’s really important is how well they respond to our treatments internally. The DLQI is not a perfect tool, but it’s the best tool we have available. It gives us a fairly good survey of the various domains that affect patients’ day-to-day living,” he said.
In BE ABLE 1, the proportion of week-12 PASI90 responders achieving a DLQI of 0 or 1 – indicative of essentially no disease impact on quality of life – increased rapidly up until week 8. At week 12, 70%-100% of the PASI90 responders in the various treatment arms had a DLQI of 0 or 1. This quality-of-life improvement, like the PASI90 response, proved durable: When the week-12 PASI90 responders were assessed at week 60 in BE ABLE 2, 76%-93% of them had a DLQI of 0 or 1.
The improvements in quality of life correlated with clinical response. BE ABLE enrollees had an average PASI score of 19 at baseline. Overall, 79% of those with an absolute PASI score of 0 at week 12 had a DLQI of 0 or 1 at that time, as did 95% of those with a PASI of 0 at week 60. A PASI of 1 was associated with a 77% likelihood of a DLQI of 0 or 1 at week 12 and an 82% rate at week 60. In contrast, patients with an absolute PASI of 2-4 at week 12 had a 46% rate of DLQI 0/1, and those with a PASI 2-4 at week 60 had a 50% chance of having a DLQI of 0/1.
Phase 3 clinical trials of bimekizumab totaling several thousand psoriasis patients are ongoing.
The BE ABLE trials were sponsored by UCB Pharma. Dr. Papp reported serving as a consultant to and/or recipient of research grants from UCB and dozens of other pharmaceutical companies.
SOURCE: Papp KA. EADV 2019 Abstract FC02.02.
REPORTING FROM THE EADV CONGRESS
The Ketogenic Diet and Dermatology: A Primer on Current Literature
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
Practice Points
- The ketogenic diet has been employed since antiquity for varying ailments and has a good safety and efficacy profile if administered by a knowledgeable provider.
- New literature is showing promising potential roles for the ketogenic diet as an adjunctive therapy, particularly in the realm of inflammatory disorders, metabolic diseases, and malignancy.
- The dermatologist should be aware of this diet because it is gaining popularity with physicians and patients alike. Dermatologists also should know how it can potentially benefit a number of patients with dermatologic diseases based on small clinical trials, population studies, and basic science research.
Meta-analysis provides safety data on IL-17, IL-23 inhibitors
according to the results of a meta-analysis of 44 studies.
While associated with more adverse events than with placebo, IL-17 and IL-23 inhibitors are “generally well-tolerated and considered safe,” but the extent of adverse events and the existence of a possible drug class effect “have not been fully investigated,” wrote Nikolai D. Loft, MD, of the department of dermatology and allergy at Herlev and Gentofte Hospital in Hellerup, Denmark, and colleagues.
In a study published in the Journal of the European Academy of Dermatology and Venereology, the researchers identified phase 3 studies with data on adverse event reports in patients with psoriasis and psoriatic arthritis who were treated with either IL-17 inhibitors (brodalumab, ixekizumab, or secukinumab) or IL-23 inhibitors (guselkumab, risankizumab, or tildrakizumab).
Overall, across all treatments, the proportion of patients with reports of any adverse events ranged from 0.49 to 0.57 in short-term studies (12-16 weeks) and from 0.83 to 0.93 with long-term treatment (52 weeks). In a pooled analysis, the proportion of patients with any adverse events was 0.57, 0.52, 0.72, and 0.81, at 12, 16, 24, and 52 weeks, respectively.
The most common adverse events across all treatments were infections, nasopharyngitis, and headaches. Among those on ixekizumab, injection-site reactions was one of the most common adverse events reported, in nearly 16% of patients after 52 weeks of treatment, the authors noted.
Fewer adverse events were reported in patients on IL-23 inhibitors, compared with those on IL-17 inhibitors. The proportion of patients reporting serious adverse events was “low,” the researchers wrote. Patients on tildrakizumab had the lowest proportion of any adverse events overall, based on short-term data over 12-16 weeks.
No significant differences emerged in reported adverse events across IL-17 inhibitors after 52 weeks.
Other findings included a higher prevalence of Candida infections among those treated with IL-17 inhibitors after 12-16 weeks and 24 weeks, compared with those on placebo, but the infections, described as mild to moderate, did not result in drug discontinuation, the authors noted. The potential risk of inflammatory bowel disease (IBD) associated with IL-17 inhibitors has been raised as a concern, but in their analysis, “IBD was very rare and after 12 weeks no difference between active treatments and placebo was seen.”
The study findings were limited by several factors, including incomplete data for interdrug comparison, varying time points for safety measures, differences in dosing in clinical trials than the approved dosing, and lack of longer-term follow-up data for most of the treatments, the researchers noted. However, the analysis was strengthened by the inclusion of phase 3 studies with both short-and long-term data, and “overall, IL-17 and IL-23 inhibitors appear to be well-tolerated with good safety profiles.”
Dr. Loft disclosed serving as an honorary speaker for Eli Lilly; other coauthors disclosed relationships with multiple companies; two authors reported no conflicts of interest. There were no funding sources for the study listed.
SOURCE: Loft ND et al. J Eur Acad Dermatol Venereol 2019 Nov 13. doi: 10.1111/jdv.16073.
according to the results of a meta-analysis of 44 studies.
While associated with more adverse events than with placebo, IL-17 and IL-23 inhibitors are “generally well-tolerated and considered safe,” but the extent of adverse events and the existence of a possible drug class effect “have not been fully investigated,” wrote Nikolai D. Loft, MD, of the department of dermatology and allergy at Herlev and Gentofte Hospital in Hellerup, Denmark, and colleagues.
In a study published in the Journal of the European Academy of Dermatology and Venereology, the researchers identified phase 3 studies with data on adverse event reports in patients with psoriasis and psoriatic arthritis who were treated with either IL-17 inhibitors (brodalumab, ixekizumab, or secukinumab) or IL-23 inhibitors (guselkumab, risankizumab, or tildrakizumab).
Overall, across all treatments, the proportion of patients with reports of any adverse events ranged from 0.49 to 0.57 in short-term studies (12-16 weeks) and from 0.83 to 0.93 with long-term treatment (52 weeks). In a pooled analysis, the proportion of patients with any adverse events was 0.57, 0.52, 0.72, and 0.81, at 12, 16, 24, and 52 weeks, respectively.
The most common adverse events across all treatments were infections, nasopharyngitis, and headaches. Among those on ixekizumab, injection-site reactions was one of the most common adverse events reported, in nearly 16% of patients after 52 weeks of treatment, the authors noted.
Fewer adverse events were reported in patients on IL-23 inhibitors, compared with those on IL-17 inhibitors. The proportion of patients reporting serious adverse events was “low,” the researchers wrote. Patients on tildrakizumab had the lowest proportion of any adverse events overall, based on short-term data over 12-16 weeks.
No significant differences emerged in reported adverse events across IL-17 inhibitors after 52 weeks.
Other findings included a higher prevalence of Candida infections among those treated with IL-17 inhibitors after 12-16 weeks and 24 weeks, compared with those on placebo, but the infections, described as mild to moderate, did not result in drug discontinuation, the authors noted. The potential risk of inflammatory bowel disease (IBD) associated with IL-17 inhibitors has been raised as a concern, but in their analysis, “IBD was very rare and after 12 weeks no difference between active treatments and placebo was seen.”
The study findings were limited by several factors, including incomplete data for interdrug comparison, varying time points for safety measures, differences in dosing in clinical trials than the approved dosing, and lack of longer-term follow-up data for most of the treatments, the researchers noted. However, the analysis was strengthened by the inclusion of phase 3 studies with both short-and long-term data, and “overall, IL-17 and IL-23 inhibitors appear to be well-tolerated with good safety profiles.”
Dr. Loft disclosed serving as an honorary speaker for Eli Lilly; other coauthors disclosed relationships with multiple companies; two authors reported no conflicts of interest. There were no funding sources for the study listed.
SOURCE: Loft ND et al. J Eur Acad Dermatol Venereol 2019 Nov 13. doi: 10.1111/jdv.16073.
according to the results of a meta-analysis of 44 studies.
While associated with more adverse events than with placebo, IL-17 and IL-23 inhibitors are “generally well-tolerated and considered safe,” but the extent of adverse events and the existence of a possible drug class effect “have not been fully investigated,” wrote Nikolai D. Loft, MD, of the department of dermatology and allergy at Herlev and Gentofte Hospital in Hellerup, Denmark, and colleagues.
In a study published in the Journal of the European Academy of Dermatology and Venereology, the researchers identified phase 3 studies with data on adverse event reports in patients with psoriasis and psoriatic arthritis who were treated with either IL-17 inhibitors (brodalumab, ixekizumab, or secukinumab) or IL-23 inhibitors (guselkumab, risankizumab, or tildrakizumab).
Overall, across all treatments, the proportion of patients with reports of any adverse events ranged from 0.49 to 0.57 in short-term studies (12-16 weeks) and from 0.83 to 0.93 with long-term treatment (52 weeks). In a pooled analysis, the proportion of patients with any adverse events was 0.57, 0.52, 0.72, and 0.81, at 12, 16, 24, and 52 weeks, respectively.
The most common adverse events across all treatments were infections, nasopharyngitis, and headaches. Among those on ixekizumab, injection-site reactions was one of the most common adverse events reported, in nearly 16% of patients after 52 weeks of treatment, the authors noted.
Fewer adverse events were reported in patients on IL-23 inhibitors, compared with those on IL-17 inhibitors. The proportion of patients reporting serious adverse events was “low,” the researchers wrote. Patients on tildrakizumab had the lowest proportion of any adverse events overall, based on short-term data over 12-16 weeks.
No significant differences emerged in reported adverse events across IL-17 inhibitors after 52 weeks.
Other findings included a higher prevalence of Candida infections among those treated with IL-17 inhibitors after 12-16 weeks and 24 weeks, compared with those on placebo, but the infections, described as mild to moderate, did not result in drug discontinuation, the authors noted. The potential risk of inflammatory bowel disease (IBD) associated with IL-17 inhibitors has been raised as a concern, but in their analysis, “IBD was very rare and after 12 weeks no difference between active treatments and placebo was seen.”
The study findings were limited by several factors, including incomplete data for interdrug comparison, varying time points for safety measures, differences in dosing in clinical trials than the approved dosing, and lack of longer-term follow-up data for most of the treatments, the researchers noted. However, the analysis was strengthened by the inclusion of phase 3 studies with both short-and long-term data, and “overall, IL-17 and IL-23 inhibitors appear to be well-tolerated with good safety profiles.”
Dr. Loft disclosed serving as an honorary speaker for Eli Lilly; other coauthors disclosed relationships with multiple companies; two authors reported no conflicts of interest. There were no funding sources for the study listed.
SOURCE: Loft ND et al. J Eur Acad Dermatol Venereol 2019 Nov 13. doi: 10.1111/jdv.16073.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Ultrasound improves specificity of psoriatic arthritis referrals
The use of ultrasound in screening for psoriatic arthritis in patients with psoriasis could reduce the number of unnecessary referrals to rheumatologists, according to a research letter published in the British Journal of Dermatology.
Up to one-third of patients with psoriasis have underlying psoriatic arthritis (PsA), but half of all patients with psoriasis experience nonspecific musculoskeletal complaints.
“Different screening tools have been developed for the dermatology practice to distinguish patients with a higher likelihood of having PsA; however, the low specificities of these tools limit their use in clinical practice,” wrote Dilek Solmaz, MD, and colleagues at the University of Ottawa.
In this prospective study, 51 patients with psoriasis were screened for referral to a rheumatologist using the Early Arthritis for Psoriatic Patients and Psoriasis Epidemiology Screening Tool questionnaires. They also underwent a limited ultrasound scanning of wrists, hands, feet, and the most painful joint, which was reviewed by experienced rheumatologists.
A dermatologist was asked to make a decision on referral based on the questionnaire data alone, then invited to revisit that decision after viewing the ultrasound results. When basing their decision on the questionnaires only, the dermatologist decided to refer 92% of patients to a rheumatologist. Of these patients, 40% were subsequently diagnosed with PsA, which represented a sensitivity of 95% but specificity of just 9%.
After reviewing the ultrasound data, the dermatologist revised their recommendations and only referred 43% of patients. Of these, 68% were later diagnosed with psoriatic arthritis. Among the patients who were not referred after the ultrasound review, five were diagnosed with PsA, but two had isolated axial involvement with no peripheral joint disease. Excluding these two cases, the sensitivity decreased to 88% but specificity increased to 77%.
“Screening tools in psoriasis that have high sensitivities usually have low specificities, which means a higher number of patients to be referred to rheumatology than needed,” the authors wrote. “Our study demonstrated that a musculoskeletal [ultrasound] based on a predefined protocol improves the referrals made to rheumatology.”
The authors did note that the ultrasounds were reviewed by experienced rheumatologists, so the results might not be generalizable to less-experienced sonographers without experience in musculoskeletal disorders.
The study was funded by AbbVie. One author declared receiving funding for a fellowship from UCB. Two authors declared honoraria and advisory consultancies with the pharmaceutical sector, including AbbVie.
SOURCE: Solmaz D et al. Br J Dermatol. 2019 Nov 28. doi: 10.1111/bjd.18515.
The use of ultrasound in screening for psoriatic arthritis in patients with psoriasis could reduce the number of unnecessary referrals to rheumatologists, according to a research letter published in the British Journal of Dermatology.
Up to one-third of patients with psoriasis have underlying psoriatic arthritis (PsA), but half of all patients with psoriasis experience nonspecific musculoskeletal complaints.
“Different screening tools have been developed for the dermatology practice to distinguish patients with a higher likelihood of having PsA; however, the low specificities of these tools limit their use in clinical practice,” wrote Dilek Solmaz, MD, and colleagues at the University of Ottawa.
In this prospective study, 51 patients with psoriasis were screened for referral to a rheumatologist using the Early Arthritis for Psoriatic Patients and Psoriasis Epidemiology Screening Tool questionnaires. They also underwent a limited ultrasound scanning of wrists, hands, feet, and the most painful joint, which was reviewed by experienced rheumatologists.
A dermatologist was asked to make a decision on referral based on the questionnaire data alone, then invited to revisit that decision after viewing the ultrasound results. When basing their decision on the questionnaires only, the dermatologist decided to refer 92% of patients to a rheumatologist. Of these patients, 40% were subsequently diagnosed with PsA, which represented a sensitivity of 95% but specificity of just 9%.
After reviewing the ultrasound data, the dermatologist revised their recommendations and only referred 43% of patients. Of these, 68% were later diagnosed with psoriatic arthritis. Among the patients who were not referred after the ultrasound review, five were diagnosed with PsA, but two had isolated axial involvement with no peripheral joint disease. Excluding these two cases, the sensitivity decreased to 88% but specificity increased to 77%.
“Screening tools in psoriasis that have high sensitivities usually have low specificities, which means a higher number of patients to be referred to rheumatology than needed,” the authors wrote. “Our study demonstrated that a musculoskeletal [ultrasound] based on a predefined protocol improves the referrals made to rheumatology.”
The authors did note that the ultrasounds were reviewed by experienced rheumatologists, so the results might not be generalizable to less-experienced sonographers without experience in musculoskeletal disorders.
The study was funded by AbbVie. One author declared receiving funding for a fellowship from UCB. Two authors declared honoraria and advisory consultancies with the pharmaceutical sector, including AbbVie.
SOURCE: Solmaz D et al. Br J Dermatol. 2019 Nov 28. doi: 10.1111/bjd.18515.
The use of ultrasound in screening for psoriatic arthritis in patients with psoriasis could reduce the number of unnecessary referrals to rheumatologists, according to a research letter published in the British Journal of Dermatology.
Up to one-third of patients with psoriasis have underlying psoriatic arthritis (PsA), but half of all patients with psoriasis experience nonspecific musculoskeletal complaints.
“Different screening tools have been developed for the dermatology practice to distinguish patients with a higher likelihood of having PsA; however, the low specificities of these tools limit their use in clinical practice,” wrote Dilek Solmaz, MD, and colleagues at the University of Ottawa.
In this prospective study, 51 patients with psoriasis were screened for referral to a rheumatologist using the Early Arthritis for Psoriatic Patients and Psoriasis Epidemiology Screening Tool questionnaires. They also underwent a limited ultrasound scanning of wrists, hands, feet, and the most painful joint, which was reviewed by experienced rheumatologists.
A dermatologist was asked to make a decision on referral based on the questionnaire data alone, then invited to revisit that decision after viewing the ultrasound results. When basing their decision on the questionnaires only, the dermatologist decided to refer 92% of patients to a rheumatologist. Of these patients, 40% were subsequently diagnosed with PsA, which represented a sensitivity of 95% but specificity of just 9%.
After reviewing the ultrasound data, the dermatologist revised their recommendations and only referred 43% of patients. Of these, 68% were later diagnosed with psoriatic arthritis. Among the patients who were not referred after the ultrasound review, five were diagnosed with PsA, but two had isolated axial involvement with no peripheral joint disease. Excluding these two cases, the sensitivity decreased to 88% but specificity increased to 77%.
“Screening tools in psoriasis that have high sensitivities usually have low specificities, which means a higher number of patients to be referred to rheumatology than needed,” the authors wrote. “Our study demonstrated that a musculoskeletal [ultrasound] based on a predefined protocol improves the referrals made to rheumatology.”
The authors did note that the ultrasounds were reviewed by experienced rheumatologists, so the results might not be generalizable to less-experienced sonographers without experience in musculoskeletal disorders.
The study was funded by AbbVie. One author declared receiving funding for a fellowship from UCB. Two authors declared honoraria and advisory consultancies with the pharmaceutical sector, including AbbVie.
SOURCE: Solmaz D et al. Br J Dermatol. 2019 Nov 28. doi: 10.1111/bjd.18515.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Verrucous Psoriasis Treated With Methotrexate and Acitretin Combination Therapy
To the Editor:
A 76-year-old woman with venous insufficiency presented with numerous thick, hyperkeratotic, confluent papules and plaques involving both legs and thighs as well as the lower back. She initially developed lesions on the distal legs, which progressed to involve the thighs and lower back, slowly enlarging over 7 years (Figure 1). The eruption was associated with pruritus and was profoundly malodorous. The patient had been unsuccessfully treated with triamcinolone ointment, bleach baths, and several courses of oral antibiotics. Her history was remarkable for marked venous insufficiency and mild anemia, with a hemoglobin level of 11.9 g/dL (reference range, 14.0–17.5 g/dL). She had no other abnormalities on a comprehensive blood test, basic metabolic panel, or liver function test.
A punch biopsy specimen from the left lower back was obtained and demonstrated papillomatous psoriasiform epidermal hyperplasia with broad parakeratosis, few intracorneal neutrophils, hypogranulosis, and suprapapillary thinning (Figure 2). She was initially treated with oral methotrexate (20 mg weekly), resulting in partial improvement of plaques and complete resolution of pruritus and malodor. After 15 months of treatment with methotrexate, low-dose methotrexate (10 mg weekly) in combination with acitretin 25 mg daily was started, resulting in further improvement of hyperkeratosis (Figure 3). The patient also was given a compounded corticosteroid ointment containing liquor carbonis detergens, salicylic acid, and fluocinonide ointment, achieving minor additional benefit. Comprehensive metabolic panel, lipid panel, and liver function tests were obtained quarterly. Hemoglobin levels remained low, similar to baseline (11.3–12.5 g/dL), while all other values were within reference range. The patient tolerated treatment well, reporting mild dryness of lips on review of systems, which was attributed to acitretin and was treated with emollients.
Verrucous psoriasis is an uncommon variant of psoriasis that presents as localized annular, erythrodermic, or drug-induced disease, as reported in a patient with preexisting psoriasis after interferon treatment of hepatitis C.1,2 It is characterized by symmetric hypertrophic verrucous plaques that may have an erythematous base and involve the legs, arms, trunk, and dorsal aspect of the hands3; malodor is frequent.1 Histopathologically, overlapping features of verruca vulgaris and psoriasis have been described. Specifically, lesions display typical psoriasiform changes, including parakeratosis, epidermal acanthosis with elongation of rete ridges, suprapapillary thinning, epidermal hypogranulosis, dilated or tortuous capillaries, and neutrophil collections in the stratum corneum (Munro microabscesses) or stratum spinosum (spongiform pustules of Kogoj).3 Additional findings of papillomatosis and epithelial buttressing are highly suggestive of verrucous psoriasis,3 though epithelial buttressing is not universally present.4-6 Similarly, although eosinophils and plasma cells have been described in some patients with verrucous psoriasis, this finding has not been consistently reported.4-6 Our biopsy specimen (Figure 2) lacks the epithelial buttressing but does exhibit subtle papillomatous hyperplasia consistent with the diagnosis of psoriasis.
The etiology of this entity is unknown. An association with diabetes mellitus, pulmonary disease, lymphatic circulation disorders, and immunosuppression has been proposed. Others have reported repeated trauma as contributing to the pathogenesis.1 For our patient, trauma secondary to scratching, long-standing venous insufficiency, and neglect likely contributed to the development of verrucous plaques.
The diagnosis of verrucous psoriasis can be challenging because of its similarity to several other entities, including verruca vulgaris; epidermal nevus; and squamous cell carcinoma, particularly verrucous carcinoma.4,6,7 The diagnosis has been less challenging in areas where prior typical psoriatic lesions evolved into a verrucous morphology. Our patient presented a diagnostic challenge and draws attention to this unique variant of psoriasis that could easily be misdiagnosed and lead to inappropriate treatment.
Verrucous psoriasis can be recalcitrant to therapy. Although studies addressing treatment modalities are lacking, several recommendations can be derived from case reports and our patient. The use of topical therapies, including topical corticosteroids (eg, fluocinonide, clobetasol, halobetasol), keratolytic agents (eg, urea, salicylic acid), and calcipotriene, provide only minimal improvement when used as monotherapy.1 Better success has been reported with systemic therapies, mainly methotrexate and acitretin, with anecdotal reports favoring the use of oral retinoids.1,6 Conversely, biologic medications such as etanercept, ustekinumab, adalimumab, and infliximab have only provided a partial response.1 Combination therapies including intralesional triamcinolone plus methotrexate4 or methotrexate plus acitretin, as in our patient, seem to provide additional benefit. Methotrexate and acitretin combination therapy has traditionally been avoided because of the risk for hepatotoxicity. However, a case series has demonstrated a moderate safety profile with concurrent use of these drugs in treatment-resistant psoriasis.8 In our case, clinical response was most pronounced with combination therapy of methotrexate 10 mg weekly and acitretin 25 mg daily. Thus, strong consideration should be given for combination methotrexate-acitretin therapy in patients with recalcitrant verrucous psoriasis who lack comorbid conditions.
We present a case of verrucous psoriasis, a variant of psoriasis characterized by hypertrophic plaques. We propose that venous insufficiency and long-standing untreated disease was instrumental to the development of these lesions. Furthermore, retinoids, particularly in combination with methotrexate, provided the most benefit for our patient.
Acknowledgment
We thank Stephen Somach, MD (Cleveland, Ohio), for his help interpreting the microscopic findings in our biopsy specimen. He received no compensation.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Scavo S, Gurrera A, Mazzaglia C, et al. Verrucous psoriasis in a patient with chronic C hepatitis treated with interferon. Clin Drug Investig. 2004;24:427-429.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris [abstract]. J Am Acad Dermatol. 2013;68(suppl 1):AB218.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Larsen F, Susa JS, Cockerell CJ, et al. Case of multiple verrucous carcinomas responding to treatment with acetretin more likely to have been a case of verrucous psoriasis. J Am Acad Dermatol. 2007;57:534-535.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Lowenthal KE, Horn PJ, Kalb RE. Concurrent use of methotrexate and acitretin revisited. J Dermatolog Treat. 2008;19:22-26.
To the Editor:
A 76-year-old woman with venous insufficiency presented with numerous thick, hyperkeratotic, confluent papules and plaques involving both legs and thighs as well as the lower back. She initially developed lesions on the distal legs, which progressed to involve the thighs and lower back, slowly enlarging over 7 years (Figure 1). The eruption was associated with pruritus and was profoundly malodorous. The patient had been unsuccessfully treated with triamcinolone ointment, bleach baths, and several courses of oral antibiotics. Her history was remarkable for marked venous insufficiency and mild anemia, with a hemoglobin level of 11.9 g/dL (reference range, 14.0–17.5 g/dL). She had no other abnormalities on a comprehensive blood test, basic metabolic panel, or liver function test.
A punch biopsy specimen from the left lower back was obtained and demonstrated papillomatous psoriasiform epidermal hyperplasia with broad parakeratosis, few intracorneal neutrophils, hypogranulosis, and suprapapillary thinning (Figure 2). She was initially treated with oral methotrexate (20 mg weekly), resulting in partial improvement of plaques and complete resolution of pruritus and malodor. After 15 months of treatment with methotrexate, low-dose methotrexate (10 mg weekly) in combination with acitretin 25 mg daily was started, resulting in further improvement of hyperkeratosis (Figure 3). The patient also was given a compounded corticosteroid ointment containing liquor carbonis detergens, salicylic acid, and fluocinonide ointment, achieving minor additional benefit. Comprehensive metabolic panel, lipid panel, and liver function tests were obtained quarterly. Hemoglobin levels remained low, similar to baseline (11.3–12.5 g/dL), while all other values were within reference range. The patient tolerated treatment well, reporting mild dryness of lips on review of systems, which was attributed to acitretin and was treated with emollients.
Verrucous psoriasis is an uncommon variant of psoriasis that presents as localized annular, erythrodermic, or drug-induced disease, as reported in a patient with preexisting psoriasis after interferon treatment of hepatitis C.1,2 It is characterized by symmetric hypertrophic verrucous plaques that may have an erythematous base and involve the legs, arms, trunk, and dorsal aspect of the hands3; malodor is frequent.1 Histopathologically, overlapping features of verruca vulgaris and psoriasis have been described. Specifically, lesions display typical psoriasiform changes, including parakeratosis, epidermal acanthosis with elongation of rete ridges, suprapapillary thinning, epidermal hypogranulosis, dilated or tortuous capillaries, and neutrophil collections in the stratum corneum (Munro microabscesses) or stratum spinosum (spongiform pustules of Kogoj).3 Additional findings of papillomatosis and epithelial buttressing are highly suggestive of verrucous psoriasis,3 though epithelial buttressing is not universally present.4-6 Similarly, although eosinophils and plasma cells have been described in some patients with verrucous psoriasis, this finding has not been consistently reported.4-6 Our biopsy specimen (Figure 2) lacks the epithelial buttressing but does exhibit subtle papillomatous hyperplasia consistent with the diagnosis of psoriasis.
The etiology of this entity is unknown. An association with diabetes mellitus, pulmonary disease, lymphatic circulation disorders, and immunosuppression has been proposed. Others have reported repeated trauma as contributing to the pathogenesis.1 For our patient, trauma secondary to scratching, long-standing venous insufficiency, and neglect likely contributed to the development of verrucous plaques.
The diagnosis of verrucous psoriasis can be challenging because of its similarity to several other entities, including verruca vulgaris; epidermal nevus; and squamous cell carcinoma, particularly verrucous carcinoma.4,6,7 The diagnosis has been less challenging in areas where prior typical psoriatic lesions evolved into a verrucous morphology. Our patient presented a diagnostic challenge and draws attention to this unique variant of psoriasis that could easily be misdiagnosed and lead to inappropriate treatment.
Verrucous psoriasis can be recalcitrant to therapy. Although studies addressing treatment modalities are lacking, several recommendations can be derived from case reports and our patient. The use of topical therapies, including topical corticosteroids (eg, fluocinonide, clobetasol, halobetasol), keratolytic agents (eg, urea, salicylic acid), and calcipotriene, provide only minimal improvement when used as monotherapy.1 Better success has been reported with systemic therapies, mainly methotrexate and acitretin, with anecdotal reports favoring the use of oral retinoids.1,6 Conversely, biologic medications such as etanercept, ustekinumab, adalimumab, and infliximab have only provided a partial response.1 Combination therapies including intralesional triamcinolone plus methotrexate4 or methotrexate plus acitretin, as in our patient, seem to provide additional benefit. Methotrexate and acitretin combination therapy has traditionally been avoided because of the risk for hepatotoxicity. However, a case series has demonstrated a moderate safety profile with concurrent use of these drugs in treatment-resistant psoriasis.8 In our case, clinical response was most pronounced with combination therapy of methotrexate 10 mg weekly and acitretin 25 mg daily. Thus, strong consideration should be given for combination methotrexate-acitretin therapy in patients with recalcitrant verrucous psoriasis who lack comorbid conditions.
We present a case of verrucous psoriasis, a variant of psoriasis characterized by hypertrophic plaques. We propose that venous insufficiency and long-standing untreated disease was instrumental to the development of these lesions. Furthermore, retinoids, particularly in combination with methotrexate, provided the most benefit for our patient.
Acknowledgment
We thank Stephen Somach, MD (Cleveland, Ohio), for his help interpreting the microscopic findings in our biopsy specimen. He received no compensation.
To the Editor:
A 76-year-old woman with venous insufficiency presented with numerous thick, hyperkeratotic, confluent papules and plaques involving both legs and thighs as well as the lower back. She initially developed lesions on the distal legs, which progressed to involve the thighs and lower back, slowly enlarging over 7 years (Figure 1). The eruption was associated with pruritus and was profoundly malodorous. The patient had been unsuccessfully treated with triamcinolone ointment, bleach baths, and several courses of oral antibiotics. Her history was remarkable for marked venous insufficiency and mild anemia, with a hemoglobin level of 11.9 g/dL (reference range, 14.0–17.5 g/dL). She had no other abnormalities on a comprehensive blood test, basic metabolic panel, or liver function test.
A punch biopsy specimen from the left lower back was obtained and demonstrated papillomatous psoriasiform epidermal hyperplasia with broad parakeratosis, few intracorneal neutrophils, hypogranulosis, and suprapapillary thinning (Figure 2). She was initially treated with oral methotrexate (20 mg weekly), resulting in partial improvement of plaques and complete resolution of pruritus and malodor. After 15 months of treatment with methotrexate, low-dose methotrexate (10 mg weekly) in combination with acitretin 25 mg daily was started, resulting in further improvement of hyperkeratosis (Figure 3). The patient also was given a compounded corticosteroid ointment containing liquor carbonis detergens, salicylic acid, and fluocinonide ointment, achieving minor additional benefit. Comprehensive metabolic panel, lipid panel, and liver function tests were obtained quarterly. Hemoglobin levels remained low, similar to baseline (11.3–12.5 g/dL), while all other values were within reference range. The patient tolerated treatment well, reporting mild dryness of lips on review of systems, which was attributed to acitretin and was treated with emollients.
Verrucous psoriasis is an uncommon variant of psoriasis that presents as localized annular, erythrodermic, or drug-induced disease, as reported in a patient with preexisting psoriasis after interferon treatment of hepatitis C.1,2 It is characterized by symmetric hypertrophic verrucous plaques that may have an erythematous base and involve the legs, arms, trunk, and dorsal aspect of the hands3; malodor is frequent.1 Histopathologically, overlapping features of verruca vulgaris and psoriasis have been described. Specifically, lesions display typical psoriasiform changes, including parakeratosis, epidermal acanthosis with elongation of rete ridges, suprapapillary thinning, epidermal hypogranulosis, dilated or tortuous capillaries, and neutrophil collections in the stratum corneum (Munro microabscesses) or stratum spinosum (spongiform pustules of Kogoj).3 Additional findings of papillomatosis and epithelial buttressing are highly suggestive of verrucous psoriasis,3 though epithelial buttressing is not universally present.4-6 Similarly, although eosinophils and plasma cells have been described in some patients with verrucous psoriasis, this finding has not been consistently reported.4-6 Our biopsy specimen (Figure 2) lacks the epithelial buttressing but does exhibit subtle papillomatous hyperplasia consistent with the diagnosis of psoriasis.
The etiology of this entity is unknown. An association with diabetes mellitus, pulmonary disease, lymphatic circulation disorders, and immunosuppression has been proposed. Others have reported repeated trauma as contributing to the pathogenesis.1 For our patient, trauma secondary to scratching, long-standing venous insufficiency, and neglect likely contributed to the development of verrucous plaques.
The diagnosis of verrucous psoriasis can be challenging because of its similarity to several other entities, including verruca vulgaris; epidermal nevus; and squamous cell carcinoma, particularly verrucous carcinoma.4,6,7 The diagnosis has been less challenging in areas where prior typical psoriatic lesions evolved into a verrucous morphology. Our patient presented a diagnostic challenge and draws attention to this unique variant of psoriasis that could easily be misdiagnosed and lead to inappropriate treatment.
Verrucous psoriasis can be recalcitrant to therapy. Although studies addressing treatment modalities are lacking, several recommendations can be derived from case reports and our patient. The use of topical therapies, including topical corticosteroids (eg, fluocinonide, clobetasol, halobetasol), keratolytic agents (eg, urea, salicylic acid), and calcipotriene, provide only minimal improvement when used as monotherapy.1 Better success has been reported with systemic therapies, mainly methotrexate and acitretin, with anecdotal reports favoring the use of oral retinoids.1,6 Conversely, biologic medications such as etanercept, ustekinumab, adalimumab, and infliximab have only provided a partial response.1 Combination therapies including intralesional triamcinolone plus methotrexate4 or methotrexate plus acitretin, as in our patient, seem to provide additional benefit. Methotrexate and acitretin combination therapy has traditionally been avoided because of the risk for hepatotoxicity. However, a case series has demonstrated a moderate safety profile with concurrent use of these drugs in treatment-resistant psoriasis.8 In our case, clinical response was most pronounced with combination therapy of methotrexate 10 mg weekly and acitretin 25 mg daily. Thus, strong consideration should be given for combination methotrexate-acitretin therapy in patients with recalcitrant verrucous psoriasis who lack comorbid conditions.
We present a case of verrucous psoriasis, a variant of psoriasis characterized by hypertrophic plaques. We propose that venous insufficiency and long-standing untreated disease was instrumental to the development of these lesions. Furthermore, retinoids, particularly in combination with methotrexate, provided the most benefit for our patient.
Acknowledgment
We thank Stephen Somach, MD (Cleveland, Ohio), for his help interpreting the microscopic findings in our biopsy specimen. He received no compensation.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Scavo S, Gurrera A, Mazzaglia C, et al. Verrucous psoriasis in a patient with chronic C hepatitis treated with interferon. Clin Drug Investig. 2004;24:427-429.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris [abstract]. J Am Acad Dermatol. 2013;68(suppl 1):AB218.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Larsen F, Susa JS, Cockerell CJ, et al. Case of multiple verrucous carcinomas responding to treatment with acetretin more likely to have been a case of verrucous psoriasis. J Am Acad Dermatol. 2007;57:534-535.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Lowenthal KE, Horn PJ, Kalb RE. Concurrent use of methotrexate and acitretin revisited. J Dermatolog Treat. 2008;19:22-26.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Scavo S, Gurrera A, Mazzaglia C, et al. Verrucous psoriasis in a patient with chronic C hepatitis treated with interferon. Clin Drug Investig. 2004;24:427-429.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris [abstract]. J Am Acad Dermatol. 2013;68(suppl 1):AB218.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Larsen F, Susa JS, Cockerell CJ, et al. Case of multiple verrucous carcinomas responding to treatment with acetretin more likely to have been a case of verrucous psoriasis. J Am Acad Dermatol. 2007;57:534-535.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Lowenthal KE, Horn PJ, Kalb RE. Concurrent use of methotrexate and acitretin revisited. J Dermatolog Treat. 2008;19:22-26.
Practice Points
- Verrucous psoriasis in an uncommon but recalcitrant-to-treatment variant of psoriasis that is characterized by hypertrophic plaques.
- The diagnosis of verrucous psoriasis is challenging, as it can mimic other entities such as verruca vulgaris and squamous cell carcinoma.
- Although the etiology of this entity is unknown, an association with diabetes mellitus, pulmonary disease, lymphatic circulation disorders, and immunosuppression has been described.
- The combination of methotrexate and acitretin is a safe and effective option for these patients in the absence of comorbid conditions.