User login
COVID-19 prompts ‘democratization’ of cancer trials
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
FROM AACR: COVID-19 and Cancer
The march of immunotherapy continues at ESMO 2020
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The use of immunotherapy for upper gastrointestinal tumors and renal cancer, ALK- and EGFR-targeted agents in non–small cell lung cancer (NSCLC), and the next step in personalized prostate cancer management will all be subjects of headlining presentations at the ESMO Virtual Congress 2020.
The conference will, like so many other major events, be held online this year because of the COVID-19 pandemic.
John B. Haanen, PhD, ESMO 2020 scientific chair, who is from the Netherlands Cancer Institute, Amsterdam, the Netherlands, told Medscape Medical News that, because the congress is being held online this year, fewer abstracts were submitted; nevertheless, “We were very happy to see ... that the quality was very good.”
The number of submissions was not the only problem the organizing committee had to face in transforming the ESMO Congress into a virtual meeting.
They were unable to fit the scientific and educational programs together and so have had to split them over two consecutive weekends. Moreover, many of the sessions were highly interactive and needed to be either adapted or omitted.
“So the program is somewhat different,” Haanen said. He noted that “the presentations were also made shorter, especially on the educational sessions, because...we can’t expect people to sit behind screens for hours listening to long presentations.”
He added: “That was out of the question.”
Haanen is nevertheless hopeful that the virtual meeting will be “very exciting.”
Solange Peters, MD, PhD, ESMO president, who is also affiliated with the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, said in a press conference that it was a “sacrifice” to move ESMO 2020 online and that “there were very sad moments” when deciding on the content.
However, there were some benefits from the change.
She said that all of the ESMO meetings this year have seen “huge” increases in the number of attendees and the geographical span or reach of each of the conferences.
“So suddenly you also realize that, what is one of the missions of ESMO being to convey education globally ... was probably better reached, better achieved with the virtual format,” she commented.
Presidential symposia
Turning to the program, Haanen first picked out the third presidential symposium, which will be held on Monday, September 21. This will focus entirely on upper gastrointestinal tumors in both the adjuvant and metastatic setting.
He said that in recent years, “very little progress has been made” in this area, with treatment mostly consisting of chemotherapy and chemoradiotherapy.
However, this year’s presentations will explore the addition of immunotherapy either to chemotherapy or as an adjuvant treatment following completion of standard-of-care treatment for local disease.
Haanen said that the results will be “very interesting ... and may change current practice,” something that “is very important for both doctors and their patients.”
On Saturday, September 19, the first presidential symposium will include two presentations on lung cancer that Haanen said will offer some “exciting new [results] that I am sure will change clinical practice.”
One will be on the CROWN phase 3 trial comparing lorlatinib and crizotinib in the first-line treatment of patients with advanced ALK-positive NSCLC.
The other will present results on central nervous system disease recurrence from the ADAURA phase 3 trial of osimertinib adjuvant therapy in patients with resected EGFR-mutated NSCLC.
The same session will also see new data in advanced renal cell carcinoma from CheckMate 9ER, in which the c-Met and VEGFR2 inhibitor cabozantinib (Cabometyx) was combined with nivolumab (Opdivo) and compared to sunitinib (Sutent) in untreated patients.
“Last year, there were already some exciting results of the combination of axitinib [Inlyta], either with pembrolizumab [Keytruda] or with avelumab [Bavencio]...in the first-line setting in metastatic clear cell renal cell cancer,” Hannen said.
“Clearly there was a survival advantage over the standard of care, sunitinib,” he added.
This year, not only will efficacy data from CheckMate 9ER be presented but also quality-of-life results.
“That’s very important, because what everybody is afraid of is that, by adding drugs, you always get more impact on the quality of life, but you will see that the quality-of-life results are very exciting,” he said.
The second presidential symposium will feature studies on prostate cancer, notably the phase 3 IPATential150 trial of abiraterone (Zytiga) plus either ipatasertib or placebo in metastatic castration-resistant prostate cancer.
Ipatasertib targets Akt, and Haanen said that “by adding it to, let’s say, standard-of-care treatment ... the question of course of will be, Does that have a better outcome?”
He believes the results will be a “very nice illustration” that prostate cancer management is heading in the direction of personalized treatment.
Alongside the presidential symposia, there will be a number of proffered paper sessions on the latest results in all aspects of oncology, including results from the ASCENT trial in triple-negative breast cancer, as well as a dedicated COVID-19 track.
Haanen said that the ESMO Virtual Congress 2020, coming after the AACR and ASCO annual meetings, has the “advantage” of being able to present the latest outcomes of patients treated with chemotherapy and immunotherapy against the backdrop of the pandemic.
This will include a study from the ESMO Resilience Task Force on the impact of COVID-19 on oncology professionals both in terms of their personal distress and burnout and their job performance.
“I think that is very important,” Haanen said, “especially because the whole thing with COVID-19 is not yet over, and everybody is preparing for a second wave in the fall and winter.
“It may help us give us clues on how we can protect ourselves or each other to prevent burnout or other problems that we as healthcare caregivers face in this difficult period.”
Next year
For next year, Peters remains hopeful that the ESMO 2021 meeting will take place as planned in Paris.
She anticipates that, indeed, ESMO meetings will be able to take place from spring next year.
This will depend on a vaccine for COVID-19 becoming widely available, although oncologists in some countries may still not be able to travel.
This means “starting probably with hybrid formats, with some of the faculty being on site and some not, [and] the same thing for the attendees,” Peters said.
She suggested that, for ESMO Congress 2021 to work as an on-site meeting, it will require at least half or two-thirds of the originally anticipated number of attendees.
“I hope that Paris next year will happen,” Peters said, adding that it “will probably happen with less attendees – that’s fine, but [still] with a large number of faculty and attendees.”
The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FROM ESMO 2020
First U.S. trial to test aerosolized chemotherapy in advanced cancers
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.
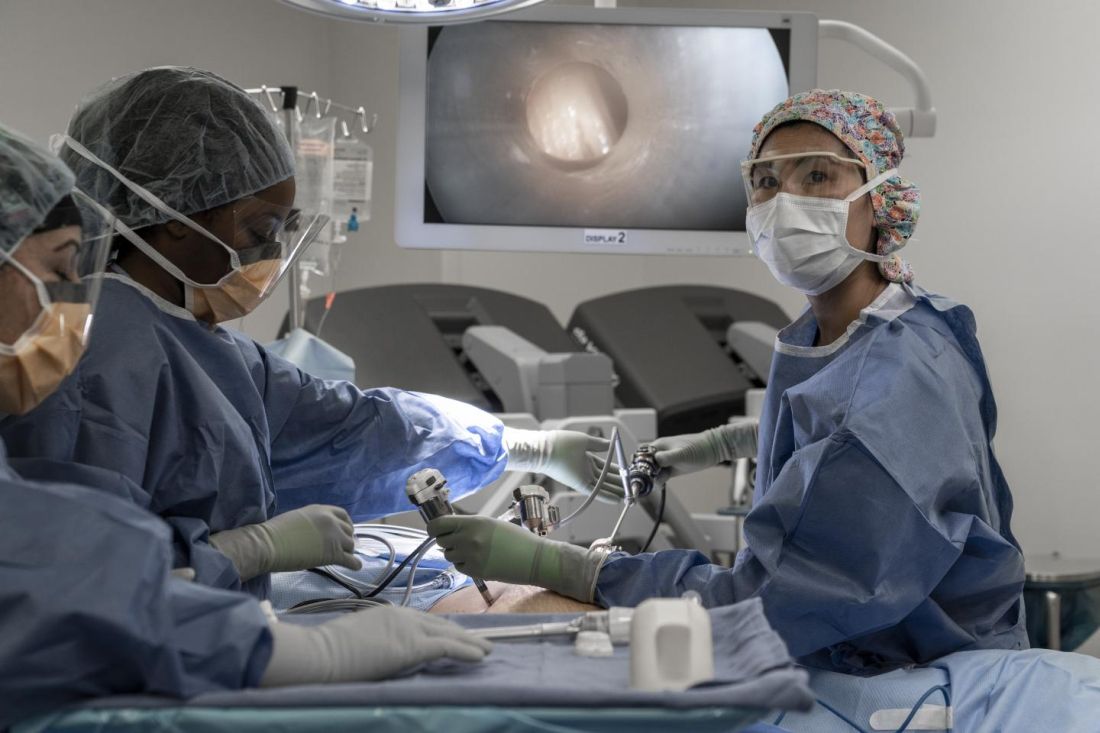
The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
Hair dye and cancer study ‘offers some reassurance’
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Findings limited to White women in United States
Findings limited to White women in United States
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The largest study of its kind has found no positive association between personal use of permanent hair dye and the risk for most cancers and cancer mortality.
The findings come from the Nurses’ Health Study, an ongoing prospective cohort study of more than 117,000 women who have been followed for 36 years and who did not have cancer at baseline.
The findings were published online on September 2 in the BMJ.
The results “offer some reassurance against concerns that personal use of permanent hair dyes might be associated with increased cancer risk or mortality,” write the investigators, with first author Yin Zhang, PhD, of Harvard Medical School, Boston.
The findings, which are limited to White women in the United States, indicate correlation, not causation, the authors emphasize.
Nevertheless, the researchers found an increased risk for some cancers among hair dye users, especially with greater cumulative dose (200 or more uses during the study period). The risk was increased for basal cell carcinoma, breast cancer (specifically, estrogen receptor negative [ER–], progesterone receptor negative [PR–], and hormone receptor negative [ER–, PR–]), and ovarian cancer.
A British expert not involved in the study dismissed these findings. “The reported associations are very weak, and, given the number of associations reported in this manuscript, they are very likely to be chance findings,” commented Paul Pharoah, PhD, professor of cancer epidemiology at the University of Cambridge (England).
“For the cancers where an increase in risk is reported, the results are not compelling. Even if they were real findings, the associations may not be cause-and-effect, and, even if they were causal associations, the magnitude of the effects are so small that any risk would be trivial.
“In short, none of the findings reported in this manuscript suggest that women who use hair dye are putting themselves at increased risk of cancer,” he stated.
A U.S. researcher who has previously coauthored a study suggesting an association between hair dye and breast cancer agreed that the increases in risk reported in this current study are “small.” But they are “of interest,” especially for breast and ovarian cancer, said Alexandra White, PhD, of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, N.C.
Hair dyes include compounds that “are not just potential carcinogens but also act as endocrine disruptors,” she said in an interview.
“In both breast and ovarian cancer, we know that hormones play an important part in the etiology ... so it’s biologically plausible that you would see [these associations in the current study],” added Dr. White, who was approached for comment.
However, she added that, even with the “modest” 20%-28% increase in the relative risk for certain breast cancers linked to a heavy cumulative dose of dyes in the current study, “there doesn’t seem to be any strong association with any cancer type.”
But she also pointed out that the most outstanding risk association was among ER–/PR– breast cancers, which are the “most aggressive and difficult to treat,” and thus the new findings are “important.”
Dr. White is the lead author of a 2019 study that received a lot of media attention because it rang an alarm bell about hair dyes and breast cancer risk.
That study concluded that ever using permanent hair dye or hair straighteners was associated with a higher risk for breast cancer than never using them and that this higher risk was especially associated with Black women. However, the study participants were from the prospective Sister Study. The participants in that study had no history of breast cancer, but they each had at least one sister who did. This family history of breast cancer may represent selection bias.
With changes in the 1980s, even safer now?
The study of hair dyes and cancer has “major public health implications” because the use of hair dye is widespread, Dr. Zhang and colleagues write in their article. They estimate that 50% to 80% of women and 10% of men aged 40 years and older in the United States and Europe use hair dye.
Permanent hair dyes “pose the greatest potential concern,” they stated, adding that these account for approximately 80% of hair dyes used in the United States and Europe and an even higher percentage in Asia.
The International Agency for Research on Cancer classifies occupational exposure to hair dyes as probably carcinogenic, but the carcinogenicity resulting from personal use of hair dyes is not classifiable – thus, there is no warning about at-home usage.
Notably, there was “a huge and very important” change in hair dye ingredients in the 1980s after the Food and Drug Administration warned about some chemicals in permanent hair dyes and the cosmetic industry altered their formulas, lead author Dr. Zhang said.
However, the researchers could not analyze use before and after the changes because not enough women reported first use of permanent hair dye after 1980 (only 1890 of 117,200 participants).
“We could expect that the current ingredients should make it safer,” Dr. Zhang said.
Study details
The researchers report that ever-users of permanent hair dyes had no significant increases in risk for solid cancers (n = 20,805; hazard ratio, 0.98, 95% confidence interval, 0.96-1.01) or hematopoietic cancers overall (n = 1,807; HR, 1.00; 95% CI, 0.91-1.10) compared with nonusers.
Additionally, ever-users did not have an increased risk for most specific cancers or cancer-related death (n = 4,860; HR, 0.96; 95% CI, 0.91-1.02).
As noted above, there were some exceptions.
Basal cell carcinoma risk was slightly increased for ever-users (n = 22,560; HR, 1.05; 95% CI, 1.02-1.08). Cumulative dose (a calculation of duration and frequency) was positively associated with risk for ER– breast cancer, PR– breast cancer, ER–/PR– breast cancer, and ovarian cancer, with risk rising in accordance with the total amount of dye.
Notably, at a cumulative dose of ≥200 uses, there was a 20% increase in the relative risk for ER- breast cancer (n = 1521; HR, 1.20; 95% CI, 1.02-1.41; P value for trend, .03). At the same cumulative dose, there was a 28% increase in the relative risk for ER-/PR- breast cancer (n = 1287; HR, 1.28, 95% CI, 1.08-1.52; P value for trend, .006).
In addition, an increased risk for Hodgkin lymphoma was observed, but only for women with naturally dark hair (the calculation was based on 70 women, 24 of whom had dark hair).
In a press statement, senior author Eva Schernhammer, PhD, of Harvard and the Medical University of Vienna, said the results “justify further prospective validation.”
She also explained that there are many variables to consider in this research, including different populations and countries, different susceptibility genotypes, different exposure settings (personal use vs. occupational exposure), and different colors of the permanent hair dyes used (dark dyes vs. light dyes).
Geographic location is a particularly important variable, suggested the study authors.
They pointed out that Europe, but not the United States, banned some individual hair dye ingredients that were considered carcinogenic during both the 1980s and 2000s. One country has even tighter oversight: “The most restrictive regulation of hair dyes exists in Japan, where cosmetic products are considered equivalent to drugs.”
The study was funded by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The study authors and Dr. White have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
VTE, sepsis risk increased among COVID-19 patients with cancer
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
FROM AACR: COVID-19 AND CANCER
First guideline on NGS testing in cancer, from ESMO
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Selpercatinib ‘poised to alter the landscape’ of RET+ cancers
Clinical data for the first-ever RET inhibitor, selpercatinib (Retevmo), show efficacy in two groups of patients with cancer – those with RET fusion–positive non–small cell lung cancer (NSCLC), and those with RET-mutant medullary thyroid cancer (MTC).
The drug showed “very good efficacy and also very good tolerability” in both groups, said lead author Lori J. Wirth, MD, medical director of head and neck cancers, Massachusetts General Hospital Cancer Center, Boston, in a statement.
“The response rates are high, responses are very durable, and overall, the drug does not cause a lot of toxicity,” she said.
“If you have a clean, RET-specific inhibitor such as selpercatinib, then you can really pound down RET very strongly and hit the driver alteration much harder, with a better side effect profile,” Dr. Wirth added.
Both groups of patients were part of the phase 1/2 LIBRETTO-001 study, which served as the basis for the recent accelerated approval of selpercatinib by the Food and Drug Administration.
Data from LIBRETTO-001 were published in the New England Journal of Medicine as two articles, one on NSCLC patients and one on MTC patients.
There has been a “remarkable increase” in the number of targeted agents that are effective in treating patients with advanced cancers that harbor specific genomic alterations, commented Razelle Kurzrock, MD, from the University of California, San Diego, in an accompanying editorial.
Selpercatinib, a potent RET inhibitor, “is now poised to alter the landscape of another genomic subgroup – RET-altered cancers,” she wrote.
Multikinase inhibitors such as vandetanib and cabozantinib have ancillary RET-inhibitor activity and are also active against RET-driven cancers. But these drugs are limited by off-target side effects, Dr. Krurzrock pointed out. “In contrast, next-generation, highly potent, and selective RET inhibitors such as selpercatinib offer the potential for improved efficacy and a more satisfactory side effect profile.”
In both parts of the study, selpercatinib produced durable responses in a majority of patients. Only about 3% of patients discontinued taking selpercatinib because of drug-related adverse events.
Taken together, these results show that selpercatinib “had marked and durable antitumor activity in most patients with RET-altered thyroid cancer or NSCLC,” wrote Dr. Krurzrock. “RET abnormalities now join other genomic alterations such as NTRK fusions, tumor mutational burden, and deficient mismatchrepair genes across cancers and ALK, BRAF, EGFR, MET, and ROS1 alterations in NSCLC that warrant molecular screening strategies.”
Results in patients with RET-mutated NSCLC
All patients enrolled in the LIBRETTO-001 trial received selpercatinib 160 mg orally twice daily until disease progression or unacceptable toxicity occurred.
Of 105 patients with NSCLC who had received at least one platinum-based chemotherapy regimen, the objective response rate was 64%. The median duration of response was 17.5 months.
At a median follow-up of 12.1 months, 63% of the responses were ongoing.
The cohort included 39 treatment-naive patients, among whom the response rate was even higher, at 85%; 90% of the responses were ongoing at 6 months. In addition, 11 patients had measurable central nervous system metastasis at study enrollment. Of this group, 91% achieved an intracranial response.
Common adverse events of grade 3 or higher included hypertension (in 14% of the patients), an increase in ALT level (in 12%), an increase in AST level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). The drug was discontinued in 12 patients because of a drug-related adverse event.
Results in patients with RET-mutated MTC
Efficacy for MTC was evaluated in 55 patients with advanced or metastatic RET-mutant MTC who had previously been treated with cabozantinib, vandetanib, or both. The objective response rate was 69%. The 1-year progression-free survival rate was 82%.
For the 88 patients who had not previously received vandetanib or cabozantinib, the response rate was 73%. The 1-year progression-free survival rate was 92%.
In a subgroup of 19 patients with previously treated RET fusion–positive thyroid cancer, 79% responded to the therapy; 1-year progression-free survival was 64%.
The most common adverse events of grade 3 or higher were hypertension (in 21% of the patients), an increase in ALT level (in 11%), an increase in AST level (in 9%), hyponatremia (in 8%), and diarrhea (in 6%). Selpercatinib was discontinued by 12 patients because of drug-related adverse events.
The study was funded by Loxo Oncology (a wholly owned subsidiary of Eli Lilly) and by grants from the National Institutes of Health and the University of Texas MD Anderson Cancer Center. Kurzrock and Wirth report relationships with numerous pharmaceutical companies, as listed in the journal article.
This article first appeared on Medscape.com.
Clinical data for the first-ever RET inhibitor, selpercatinib (Retevmo), show efficacy in two groups of patients with cancer – those with RET fusion–positive non–small cell lung cancer (NSCLC), and those with RET-mutant medullary thyroid cancer (MTC).
The drug showed “very good efficacy and also very good tolerability” in both groups, said lead author Lori J. Wirth, MD, medical director of head and neck cancers, Massachusetts General Hospital Cancer Center, Boston, in a statement.
“The response rates are high, responses are very durable, and overall, the drug does not cause a lot of toxicity,” she said.
“If you have a clean, RET-specific inhibitor such as selpercatinib, then you can really pound down RET very strongly and hit the driver alteration much harder, with a better side effect profile,” Dr. Wirth added.
Both groups of patients were part of the phase 1/2 LIBRETTO-001 study, which served as the basis for the recent accelerated approval of selpercatinib by the Food and Drug Administration.
Data from LIBRETTO-001 were published in the New England Journal of Medicine as two articles, one on NSCLC patients and one on MTC patients.
There has been a “remarkable increase” in the number of targeted agents that are effective in treating patients with advanced cancers that harbor specific genomic alterations, commented Razelle Kurzrock, MD, from the University of California, San Diego, in an accompanying editorial.
Selpercatinib, a potent RET inhibitor, “is now poised to alter the landscape of another genomic subgroup – RET-altered cancers,” she wrote.
Multikinase inhibitors such as vandetanib and cabozantinib have ancillary RET-inhibitor activity and are also active against RET-driven cancers. But these drugs are limited by off-target side effects, Dr. Krurzrock pointed out. “In contrast, next-generation, highly potent, and selective RET inhibitors such as selpercatinib offer the potential for improved efficacy and a more satisfactory side effect profile.”
In both parts of the study, selpercatinib produced durable responses in a majority of patients. Only about 3% of patients discontinued taking selpercatinib because of drug-related adverse events.
Taken together, these results show that selpercatinib “had marked and durable antitumor activity in most patients with RET-altered thyroid cancer or NSCLC,” wrote Dr. Krurzrock. “RET abnormalities now join other genomic alterations such as NTRK fusions, tumor mutational burden, and deficient mismatchrepair genes across cancers and ALK, BRAF, EGFR, MET, and ROS1 alterations in NSCLC that warrant molecular screening strategies.”
Results in patients with RET-mutated NSCLC
All patients enrolled in the LIBRETTO-001 trial received selpercatinib 160 mg orally twice daily until disease progression or unacceptable toxicity occurred.
Of 105 patients with NSCLC who had received at least one platinum-based chemotherapy regimen, the objective response rate was 64%. The median duration of response was 17.5 months.
At a median follow-up of 12.1 months, 63% of the responses were ongoing.
The cohort included 39 treatment-naive patients, among whom the response rate was even higher, at 85%; 90% of the responses were ongoing at 6 months. In addition, 11 patients had measurable central nervous system metastasis at study enrollment. Of this group, 91% achieved an intracranial response.
Common adverse events of grade 3 or higher included hypertension (in 14% of the patients), an increase in ALT level (in 12%), an increase in AST level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). The drug was discontinued in 12 patients because of a drug-related adverse event.
Results in patients with RET-mutated MTC
Efficacy for MTC was evaluated in 55 patients with advanced or metastatic RET-mutant MTC who had previously been treated with cabozantinib, vandetanib, or both. The objective response rate was 69%. The 1-year progression-free survival rate was 82%.
For the 88 patients who had not previously received vandetanib or cabozantinib, the response rate was 73%. The 1-year progression-free survival rate was 92%.
In a subgroup of 19 patients with previously treated RET fusion–positive thyroid cancer, 79% responded to the therapy; 1-year progression-free survival was 64%.
The most common adverse events of grade 3 or higher were hypertension (in 21% of the patients), an increase in ALT level (in 11%), an increase in AST level (in 9%), hyponatremia (in 8%), and diarrhea (in 6%). Selpercatinib was discontinued by 12 patients because of drug-related adverse events.
The study was funded by Loxo Oncology (a wholly owned subsidiary of Eli Lilly) and by grants from the National Institutes of Health and the University of Texas MD Anderson Cancer Center. Kurzrock and Wirth report relationships with numerous pharmaceutical companies, as listed in the journal article.
This article first appeared on Medscape.com.
Clinical data for the first-ever RET inhibitor, selpercatinib (Retevmo), show efficacy in two groups of patients with cancer – those with RET fusion–positive non–small cell lung cancer (NSCLC), and those with RET-mutant medullary thyroid cancer (MTC).
The drug showed “very good efficacy and also very good tolerability” in both groups, said lead author Lori J. Wirth, MD, medical director of head and neck cancers, Massachusetts General Hospital Cancer Center, Boston, in a statement.
“The response rates are high, responses are very durable, and overall, the drug does not cause a lot of toxicity,” she said.
“If you have a clean, RET-specific inhibitor such as selpercatinib, then you can really pound down RET very strongly and hit the driver alteration much harder, with a better side effect profile,” Dr. Wirth added.
Both groups of patients were part of the phase 1/2 LIBRETTO-001 study, which served as the basis for the recent accelerated approval of selpercatinib by the Food and Drug Administration.
Data from LIBRETTO-001 were published in the New England Journal of Medicine as two articles, one on NSCLC patients and one on MTC patients.
There has been a “remarkable increase” in the number of targeted agents that are effective in treating patients with advanced cancers that harbor specific genomic alterations, commented Razelle Kurzrock, MD, from the University of California, San Diego, in an accompanying editorial.
Selpercatinib, a potent RET inhibitor, “is now poised to alter the landscape of another genomic subgroup – RET-altered cancers,” she wrote.
Multikinase inhibitors such as vandetanib and cabozantinib have ancillary RET-inhibitor activity and are also active against RET-driven cancers. But these drugs are limited by off-target side effects, Dr. Krurzrock pointed out. “In contrast, next-generation, highly potent, and selective RET inhibitors such as selpercatinib offer the potential for improved efficacy and a more satisfactory side effect profile.”
In both parts of the study, selpercatinib produced durable responses in a majority of patients. Only about 3% of patients discontinued taking selpercatinib because of drug-related adverse events.
Taken together, these results show that selpercatinib “had marked and durable antitumor activity in most patients with RET-altered thyroid cancer or NSCLC,” wrote Dr. Krurzrock. “RET abnormalities now join other genomic alterations such as NTRK fusions, tumor mutational burden, and deficient mismatchrepair genes across cancers and ALK, BRAF, EGFR, MET, and ROS1 alterations in NSCLC that warrant molecular screening strategies.”
Results in patients with RET-mutated NSCLC
All patients enrolled in the LIBRETTO-001 trial received selpercatinib 160 mg orally twice daily until disease progression or unacceptable toxicity occurred.
Of 105 patients with NSCLC who had received at least one platinum-based chemotherapy regimen, the objective response rate was 64%. The median duration of response was 17.5 months.
At a median follow-up of 12.1 months, 63% of the responses were ongoing.
The cohort included 39 treatment-naive patients, among whom the response rate was even higher, at 85%; 90% of the responses were ongoing at 6 months. In addition, 11 patients had measurable central nervous system metastasis at study enrollment. Of this group, 91% achieved an intracranial response.
Common adverse events of grade 3 or higher included hypertension (in 14% of the patients), an increase in ALT level (in 12%), an increase in AST level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). The drug was discontinued in 12 patients because of a drug-related adverse event.
Results in patients with RET-mutated MTC
Efficacy for MTC was evaluated in 55 patients with advanced or metastatic RET-mutant MTC who had previously been treated with cabozantinib, vandetanib, or both. The objective response rate was 69%. The 1-year progression-free survival rate was 82%.
For the 88 patients who had not previously received vandetanib or cabozantinib, the response rate was 73%. The 1-year progression-free survival rate was 92%.
In a subgroup of 19 patients with previously treated RET fusion–positive thyroid cancer, 79% responded to the therapy; 1-year progression-free survival was 64%.
The most common adverse events of grade 3 or higher were hypertension (in 21% of the patients), an increase in ALT level (in 11%), an increase in AST level (in 9%), hyponatremia (in 8%), and diarrhea (in 6%). Selpercatinib was discontinued by 12 patients because of drug-related adverse events.
The study was funded by Loxo Oncology (a wholly owned subsidiary of Eli Lilly) and by grants from the National Institutes of Health and the University of Texas MD Anderson Cancer Center. Kurzrock and Wirth report relationships with numerous pharmaceutical companies, as listed in the journal article.
This article first appeared on Medscape.com.
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Tailored messaging needed to get cancer screening back on track
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.