User login
Mechanism proposed for microvascular thrombosis in thrombotic thrombocytopenic purpura
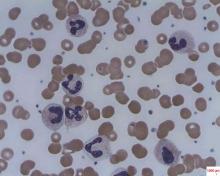
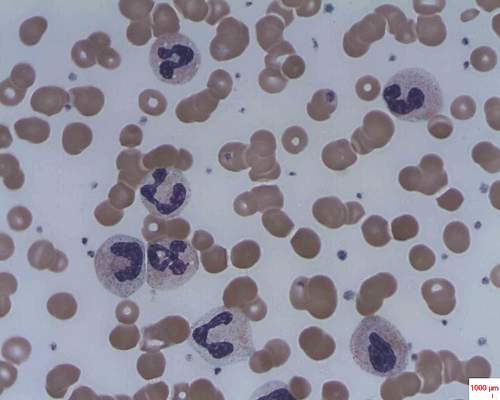
In patients with acquired autoimmune thrombotic thrombocytopenic purpura, elevated plasma levels of human neutrophil proteins 1-3 inhibit proteolytic cleavage of von Willebrand factor by ADAMTS13, Vikram G. Pillai, PhD, of the University of Alabama at Birmingham, and colleagues reported.
The finding may explain how inflammation triggers microvascular thrombosis in these patients and potentially others with immune thrombotic disorders, according to the researchers (Blood 2016;128:110-9).
They performed enzyme-linked immunosorbent assays and found markedly increased levels of plasma human neutrophil proteins (HNPs) 1-3 in most of the patients with acquired autoimmune thrombotic thrombocytopenic purpura (TTP). The median levels in the 19 patients were 170 ng/mL, compared with 23 ng/mL in 18 healthy controls, a statistically significant difference (P less than .0001).
Liquid chromatography plus tandem mass spectrometry similarly confirmed statistically significant increases in HNP1, HNP2, and HNP3 in patient samples (P less than .001).
Measures of HNPs 1-3 by both methods correlated well, and the researchers concluded that HNPs 1-3 likely inhibit ADAMTS13 activity by binding to the central A2 domain of von Willebrand factor and physically blocking ADAMTS13 binding.
The researchers had no relevant financial disclosures.
On Twitter @maryjodales
In patients with acquired autoimmune thrombotic thrombocytopenic purpura, elevated plasma levels of human neutrophil proteins 1-3 inhibit proteolytic cleavage of von Willebrand factor by ADAMTS13, Vikram G. Pillai, PhD, of the University of Alabama at Birmingham, and colleagues reported.
The finding may explain how inflammation triggers microvascular thrombosis in these patients and potentially others with immune thrombotic disorders, according to the researchers (Blood 2016;128:110-9).
They performed enzyme-linked immunosorbent assays and found markedly increased levels of plasma human neutrophil proteins (HNPs) 1-3 in most of the patients with acquired autoimmune thrombotic thrombocytopenic purpura (TTP). The median levels in the 19 patients were 170 ng/mL, compared with 23 ng/mL in 18 healthy controls, a statistically significant difference (P less than .0001).
Liquid chromatography plus tandem mass spectrometry similarly confirmed statistically significant increases in HNP1, HNP2, and HNP3 in patient samples (P less than .001).
Measures of HNPs 1-3 by both methods correlated well, and the researchers concluded that HNPs 1-3 likely inhibit ADAMTS13 activity by binding to the central A2 domain of von Willebrand factor and physically blocking ADAMTS13 binding.
The researchers had no relevant financial disclosures.
On Twitter @maryjodales
In patients with acquired autoimmune thrombotic thrombocytopenic purpura, elevated plasma levels of human neutrophil proteins 1-3 inhibit proteolytic cleavage of von Willebrand factor by ADAMTS13, Vikram G. Pillai, PhD, of the University of Alabama at Birmingham, and colleagues reported.
The finding may explain how inflammation triggers microvascular thrombosis in these patients and potentially others with immune thrombotic disorders, according to the researchers (Blood 2016;128:110-9).
They performed enzyme-linked immunosorbent assays and found markedly increased levels of plasma human neutrophil proteins (HNPs) 1-3 in most of the patients with acquired autoimmune thrombotic thrombocytopenic purpura (TTP). The median levels in the 19 patients were 170 ng/mL, compared with 23 ng/mL in 18 healthy controls, a statistically significant difference (P less than .0001).
Liquid chromatography plus tandem mass spectrometry similarly confirmed statistically significant increases in HNP1, HNP2, and HNP3 in patient samples (P less than .001).
Measures of HNPs 1-3 by both methods correlated well, and the researchers concluded that HNPs 1-3 likely inhibit ADAMTS13 activity by binding to the central A2 domain of von Willebrand factor and physically blocking ADAMTS13 binding.
The researchers had no relevant financial disclosures.
On Twitter @maryjodales
FROM BLOOD
Key clinical point: In patients with acquired autoimmune thrombotic thrombocytopenic purpura, elevated plasma levels of human neutrophil proteins 1-3 inhibit proteolytic cleavage of von Willebrand factor by ADAMTS13.
Major finding: The median levels of plasma human neutrophil proteins 1-3 in patients with acquired autoimmune TTP were 170 ng/mL, compared with 23 ng/mL in healthy controls, a statistically significant difference (P less than .0001).
Data source: Studies in 19 patients with TTP and 18 control subjects.
Disclosures: The researchers had no relevant financial disclosures.
Pediatric cancer survivors at increased risk for endocrine abnormalities
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
Patients who survived pediatric-onset cancer are at increased risk for developing or experiencing endocrine abnormalities.
Risk was significantly higher in survivors who underwent high-risk therapeutic exposures compared with survivors not so exposed. Moreover, the incidence and prevalence of endocrine abnormalities increased across the lifespan of survivors, reported Sogol Mostoufi-Moab, MD, of University of Pennsylvania, Philadelphia, and his associates (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2016.66.6545).
A total of 14,290 patients met the study’s eligibility requirements, which included a diagnosis of cancer before age 21 years and 5-year survival following diagnosis. Cancer diagnoses included leukemia, Hodgkin and non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, sarcoma, bone malignancy, and central nervous system malignancy. Baseline and follow-up questionnaires collected endocrine-related outcomes of interest, demographic information, and medical histories for both cancer survivors and their siblings (n = 4,031). For survivors, median age at diagnosis was 6 years and median age at last follow-up was 32 years. For siblings, median age at last follow-up was 34 years.
Overall 44% of cancer survivors had at least one endocrinopathy, 16.7% had at least two, and 6.6% had three or more. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Specifically, thyroid disorders were more frequent among cancer survivors than among their siblings: underactive thyroid (hazard ratio, 2.2; 95% confidence interval, 1.8-2.7), overactive thyroid (HR, 2.4; 95% CI, 1.7-3.3), thyroid nodules (HR, 3.9; 95% CI, 2.9-5.4), and thyroid cancer (HR 2.5; 95% CI, 1.2-5.3).
Compared to their siblings, cancer survivors showed increased risk of developing diabetes (RR, 1.8; 95% CI, 1.4-2.3).
Among survivors, those exposed to high-risk therapies (defined by the Children’s Oncology Group’s Long-Term Follow-Up Guidelinesfor Survivors of Childhood, Adolescent, and Young Adult Cancers) were at a greater risk of developing primary hypothyroidism (HR, 6.6; 95% CI, 5.6-7.8) central hypothyroidism (HR, 3.9; 95% CI, 2.9-5.2), an overactive thyroid (HR, 1.8; 95% CI, 1.2-2.8), thyroid nodules (HR, 6.3; 95% CI, 5.2-7.5), and thyroid cancer (HR, 9.2; 95% CI, 6.2-13.7) compared with survivors not so exposed.
The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of pediatric-onset cancer are at increased risk for developing endocrine abnormalities.
Major finding: Overall, 44% of childhood cancer survivors had at least one endocrinopathy. Survivors of Hodgkin lymphoma had the highest frequency of endocrine abnormality (60.1%) followed by survivors of CNS malignancy (54%), leukemia (45.6%), sarcoma (41.3%), non-Hodgkin lymphoma (39.7%), and neuroblastoma (31.9%).
Data source: A multi-institutional retrospective study of 14,290 men and women who survived pediatric cancer.
Disclosures: The National Cancer Institute, the Cancer Center Support Grant, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital funded the study. Dr. Mostoufi-Moab and nine other investigators had no disclosures to report. Two investigators reported receiving financial compensation or honoraria from Merck or Sandoz.
Diabetes management in cancer patients
In an interview with Dr David Henry, Editor-in-Chief of The Journal of Community and Supportive Oncology, Dr Todd Brown reviews the several different classes of the hyperglycemic management drugs (apart from insulin) focusing on how they work and how they should be used in the management of diabetes in patients with cancer. Dr Henry is vice-chair of the Department of Medicine and Clinical Professor of Medicine at Pennsylvania Hospital, in Philadelphia, and Dr Brown is associate professor of Medicine and Epidemiology in the Division of Endocrinology, Diabetes, and Metabolism at the Johns Hopkins University in Baltimore, Maryland.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In an interview with Dr David Henry, Editor-in-Chief of The Journal of Community and Supportive Oncology, Dr Todd Brown reviews the several different classes of the hyperglycemic management drugs (apart from insulin) focusing on how they work and how they should be used in the management of diabetes in patients with cancer. Dr Henry is vice-chair of the Department of Medicine and Clinical Professor of Medicine at Pennsylvania Hospital, in Philadelphia, and Dr Brown is associate professor of Medicine and Epidemiology in the Division of Endocrinology, Diabetes, and Metabolism at the Johns Hopkins University in Baltimore, Maryland.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In an interview with Dr David Henry, Editor-in-Chief of The Journal of Community and Supportive Oncology, Dr Todd Brown reviews the several different classes of the hyperglycemic management drugs (apart from insulin) focusing on how they work and how they should be used in the management of diabetes in patients with cancer. Dr Henry is vice-chair of the Department of Medicine and Clinical Professor of Medicine at Pennsylvania Hospital, in Philadelphia, and Dr Brown is associate professor of Medicine and Epidemiology in the Division of Endocrinology, Diabetes, and Metabolism at the Johns Hopkins University in Baltimore, Maryland.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
Cisplatin-based chemo may be linked to hearing loss
In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss, according to the results of the large, multicenter Platinum Study.
For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred, Robert Frisina, PhD, of the University of South Florida, Tampa, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8822).
A total of 488 men with adult-onset germ cell tumors who were treated with cisplatin-based chemotherapy were consented into this study, and completed questionnaires concerning neurotoxic symptoms, lifestyle habits, and medication use. Each patient underwent bone-conduction and speech-conducting threshold testing. Pure-tone air conduction thresholds were obtained bilaterally at speech frequency range (0.25 to 12 kHz). Classification of hearing loss and assessment of severity followed standardized criteria as defined by the American Speech-Language-Hearing Association. Median age at cancer diagnosis was 31 years; the median interval between chemotherapy and audiometric testing was 4.25 years. Median cumulative cisplatin dose was 400 mg/m2. Increasing cumulative cisplatin dose was associated with increasing (worse) hearing thresholds at 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P less than .001), 10 kHz (P less than .001), and 12 kHz (P = .0013) after correcting for age.
Cumulative cisplatin doses above 300 mg/m2 were associated with more severe hearing loss, compared with doses less than 300 mg/m2 (odds ratio, 1.59; 95% confidence interval, 1.14-2.21; P = .0066).
Conductive hearing loss in the middle ear was not associated with drug exposure dosage levels. Hypertension was identified as a risk factor for hearing loss as impaired overall hearing threshold was significantly associated with hypertension when correcting for age and cisplatin dose (n = 60, P = .0066).
“Because alterations in the highly successful [germ cell tumor] regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity,” investigators wrote. They also suggested that cancer patients treated with cisplatin should be careful to avoid noise exposure, ototoxic drugs, and other factors that could further increase damage.
This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss products. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
On Twitter @jessnicolecraig
In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss, according to the results of the large, multicenter Platinum Study.
For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred, Robert Frisina, PhD, of the University of South Florida, Tampa, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8822).
A total of 488 men with adult-onset germ cell tumors who were treated with cisplatin-based chemotherapy were consented into this study, and completed questionnaires concerning neurotoxic symptoms, lifestyle habits, and medication use. Each patient underwent bone-conduction and speech-conducting threshold testing. Pure-tone air conduction thresholds were obtained bilaterally at speech frequency range (0.25 to 12 kHz). Classification of hearing loss and assessment of severity followed standardized criteria as defined by the American Speech-Language-Hearing Association. Median age at cancer diagnosis was 31 years; the median interval between chemotherapy and audiometric testing was 4.25 years. Median cumulative cisplatin dose was 400 mg/m2. Increasing cumulative cisplatin dose was associated with increasing (worse) hearing thresholds at 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P less than .001), 10 kHz (P less than .001), and 12 kHz (P = .0013) after correcting for age.
Cumulative cisplatin doses above 300 mg/m2 were associated with more severe hearing loss, compared with doses less than 300 mg/m2 (odds ratio, 1.59; 95% confidence interval, 1.14-2.21; P = .0066).
Conductive hearing loss in the middle ear was not associated with drug exposure dosage levels. Hypertension was identified as a risk factor for hearing loss as impaired overall hearing threshold was significantly associated with hypertension when correcting for age and cisplatin dose (n = 60, P = .0066).
“Because alterations in the highly successful [germ cell tumor] regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity,” investigators wrote. They also suggested that cancer patients treated with cisplatin should be careful to avoid noise exposure, ototoxic drugs, and other factors that could further increase damage.
This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss products. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
On Twitter @jessnicolecraig
In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss, according to the results of the large, multicenter Platinum Study.
For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred, Robert Frisina, PhD, of the University of South Florida, Tampa, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8822).
A total of 488 men with adult-onset germ cell tumors who were treated with cisplatin-based chemotherapy were consented into this study, and completed questionnaires concerning neurotoxic symptoms, lifestyle habits, and medication use. Each patient underwent bone-conduction and speech-conducting threshold testing. Pure-tone air conduction thresholds were obtained bilaterally at speech frequency range (0.25 to 12 kHz). Classification of hearing loss and assessment of severity followed standardized criteria as defined by the American Speech-Language-Hearing Association. Median age at cancer diagnosis was 31 years; the median interval between chemotherapy and audiometric testing was 4.25 years. Median cumulative cisplatin dose was 400 mg/m2. Increasing cumulative cisplatin dose was associated with increasing (worse) hearing thresholds at 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P less than .001), 10 kHz (P less than .001), and 12 kHz (P = .0013) after correcting for age.
Cumulative cisplatin doses above 300 mg/m2 were associated with more severe hearing loss, compared with doses less than 300 mg/m2 (odds ratio, 1.59; 95% confidence interval, 1.14-2.21; P = .0066).
Conductive hearing loss in the middle ear was not associated with drug exposure dosage levels. Hypertension was identified as a risk factor for hearing loss as impaired overall hearing threshold was significantly associated with hypertension when correcting for age and cisplatin dose (n = 60, P = .0066).
“Because alterations in the highly successful [germ cell tumor] regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity,” investigators wrote. They also suggested that cancer patients treated with cisplatin should be careful to avoid noise exposure, ototoxic drugs, and other factors that could further increase damage.
This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss products. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In male patients with adult-onset germ cell tumors, cisplatin-based chemotherapy may be associated with hearing loss.
Major finding: For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold occurred.
Data source: A multicenter study of 488 men with adult-onset germ cell tumors.
Disclosures: This study was funded by the National Cancer Institute. Dr. Frisina reported holding patents related to hearing loss. Six other investigators reported serving in advisory roles, receiving financial compensation or honoraria from multiple pharmaceutical and biomedical companies.
Nearly 200 practices expected to participate in Oncology Care Model
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
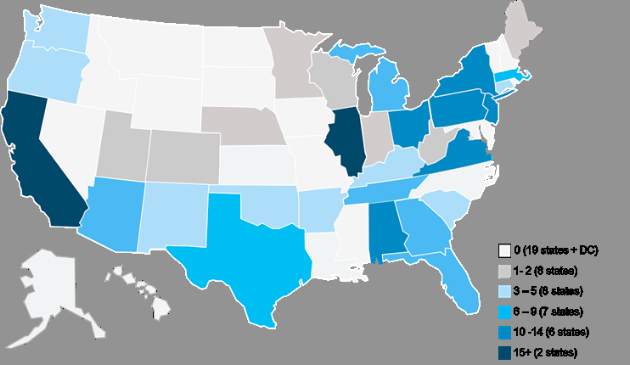
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
The Department of Health and Human Services has announced that nearly 200 physician group practices – more than double the number expected – and 17 health insurance companies will be participating in the Oncology Care Model, a voluntary payment and care delivery model developed by the CMS Innovation Center and advanced by the Affordable Care Act. The 5-year program, designed to improve cancer care by providing financial incentives to physician practices that provide effective treatment, is set to begin July 1, 2016.
The Medicare arm of the Oncology Care Model (OCM) will include more than 3,200 oncologists and will cover approximately 155,000 Medicare beneficiaries nationwide, according to a written statement from the HHS.
“CMS is thrilled with how many physician groups chose to be a part of the Oncology Care Model,” Patrick Conway, MD, CMS principal deputy administrator and chief medical officer, said in the statement.
Physician practices from 31 states will be participating, with the highest levels of provider participation in Alabama, California, Illinois, New Jersey, New York, Ohio, Pennsylvania, and Virginia, according to an analysis conducted by Avalere Health, a Washington, DC–based health care consulting firm.
The CMS first announced the OCM project in February 2015 and originally aimed to have 100 physician practices participating in the first-ever oncology-specific payment reform model.
“Based on feedback from the medical, consumer, and business communities, we are launching this new model of care to support clinicians’ work with their patients,” HHS Secretary Sylvia M. Burwell said in a written statement in February 2015.
“We aim to provide Medicare beneficiaries struggling with cancer with high-quality care around the clock and to reward doctors for the value, not volume, of care they provide. Improving the way we pay providers and deliver care to patients will result in healthier people,” she said.
The OCM encourages practices to improve care and lower costs through episodic and performance-based payments that reward high-quality patient care. It is a multipayer model that includes Medicare’s fee-for-service (OCM-FFS) and commercial payers.
OCM participants will receive regular OCM-FFS payments during the model. To create incentives to improve the quality of care, reimbursement will include a monthly payment of $160 per beneficiary for delivery of OCM enhanced services, and a performance-based payment for OCM episodes, according to a CMS fact sheet.
An OCM-FFS episode begins on the date of initial Part B or D chemotherapy claim and includes all Medicare Part A and B (and some Part D) services received during the episode period which lasts 6 months. Beneficiaries who receive chemotherapy after the end of an episode will begin a new episode.
Enhanced services include patient navigation, a care plan based on the Institute of Medicine care management report, patient access 24 hours a day, 7 days a week, and treatment with therapies that are consistent with nationally recognized clinical guidelines.
View the complete list of participating practices at https://innovation.cms.gov/initiatives/Oncology-Care.
On Twitter @jessnicolecraig
Group-based psychosocial services: assessing outpatient oncology needs
Background Group-based services can improve quality-of-life outcomes for oncology patients.
Objective To assess patient preferences for supportive and wellness programming to better meet patient needs and allocate resources.
Methods Patients from 3 cancer centers in New York City completed a 15-item questionnaire about their interest in educational topics (wellness, nutrition, legal issues, etc) and services (support groups, lectures, and exercise programs).
Results 311 patients participated in the survey. Mean age was 59 years, and 74% were women. The most common cancer was breast (40%), followed by genitourinary (15%). Women preferred wellness workshops most, followed by informative sessions; men most preferred informative sessions, followed equally by posttreatment support and wellness workshops. Older age was related to an increased likelihood of group attendance. Overall, 68% of participants reported that they would be likely to attend groups. For lectures, nutrition was of greatest interest for men (43%) and women (34%), followed by anxiety management (17% and 18%, respectively). Overall, 64% of participants reported that they would be likely to attend a lecture. A majority of respondents (54%) expressed a desire for exercise programs.
Limitations Generalizability to all cancer centers is limited, because data was not tracked on those who refused to complete the questionnaire.
Conclusions Obtaining patient feedback on psychosocial programs is imperative for understanding patient preferences and developing effective support programming.
Click on the PDF icon at the top of this introduction to read the full article.
Background Group-based services can improve quality-of-life outcomes for oncology patients.
Objective To assess patient preferences for supportive and wellness programming to better meet patient needs and allocate resources.
Methods Patients from 3 cancer centers in New York City completed a 15-item questionnaire about their interest in educational topics (wellness, nutrition, legal issues, etc) and services (support groups, lectures, and exercise programs).
Results 311 patients participated in the survey. Mean age was 59 years, and 74% were women. The most common cancer was breast (40%), followed by genitourinary (15%). Women preferred wellness workshops most, followed by informative sessions; men most preferred informative sessions, followed equally by posttreatment support and wellness workshops. Older age was related to an increased likelihood of group attendance. Overall, 68% of participants reported that they would be likely to attend groups. For lectures, nutrition was of greatest interest for men (43%) and women (34%), followed by anxiety management (17% and 18%, respectively). Overall, 64% of participants reported that they would be likely to attend a lecture. A majority of respondents (54%) expressed a desire for exercise programs.
Limitations Generalizability to all cancer centers is limited, because data was not tracked on those who refused to complete the questionnaire.
Conclusions Obtaining patient feedback on psychosocial programs is imperative for understanding patient preferences and developing effective support programming.
Click on the PDF icon at the top of this introduction to read the full article.
Background Group-based services can improve quality-of-life outcomes for oncology patients.
Objective To assess patient preferences for supportive and wellness programming to better meet patient needs and allocate resources.
Methods Patients from 3 cancer centers in New York City completed a 15-item questionnaire about their interest in educational topics (wellness, nutrition, legal issues, etc) and services (support groups, lectures, and exercise programs).
Results 311 patients participated in the survey. Mean age was 59 years, and 74% were women. The most common cancer was breast (40%), followed by genitourinary (15%). Women preferred wellness workshops most, followed by informative sessions; men most preferred informative sessions, followed equally by posttreatment support and wellness workshops. Older age was related to an increased likelihood of group attendance. Overall, 68% of participants reported that they would be likely to attend groups. For lectures, nutrition was of greatest interest for men (43%) and women (34%), followed by anxiety management (17% and 18%, respectively). Overall, 64% of participants reported that they would be likely to attend a lecture. A majority of respondents (54%) expressed a desire for exercise programs.
Limitations Generalizability to all cancer centers is limited, because data was not tracked on those who refused to complete the questionnaire.
Conclusions Obtaining patient feedback on psychosocial programs is imperative for understanding patient preferences and developing effective support programming.
Click on the PDF icon at the top of this introduction to read the full article.
The impact of a nurse practitioner-led symptom clinic on emergency department use in cancer patients
Background Emergency department (ED) use and hospitalization is distressing to cancer patients and drives up the cost of health care. A growing body of evidence demonstrates that more than half of those visits may be avoidable.
Objective To examine the impact of a nurse practitioner (NP)-led, physician-supervised, outpatient symptom management clinic on ED use.
Methods We conducted a retrospective review of ED encounters to quantify the frequency of ED use by oncology patients at a community cancer institute 6 months before (October 2012-March 2013) and after (April-September 2013) the initiation of an NP-staffed symptom management clinic.
Results The highest use of the ED and supportive clinic was among patients with advanced cancer, most commonly with lung or breast cancer, who were receiving cytotoxic chemotherapy. Uncontrolled symptoms of shortness of breath, pain, weakness, fever, nausea, vomiting, and diarrhea commonly led to ED visits. Despite instituting the NP-staffed symptom management clinic to manage those symptoms, there was a 17.9% increase in ED use. However, of the patients seen by the NP, 95% may have avoided hospitalization.
Limitations Retrospective study
Conclusions Our study identifies a high-risk population of patients who use the ED frequently. NP-led clinics could aggressively manage the symptom burden of these patients and potentially reduce ED visits as other studies have demonstrated. Although our study did not directly demonstrate this, we have identified weaknesses of care delivery in our clinic that could be optimized. In addition, we have demonstrated that the majority of patients seen for acute symptoms by an NP avoided an ED visit.
Click on the PDF icon at the top of this introduction to read the full article.
Background Emergency department (ED) use and hospitalization is distressing to cancer patients and drives up the cost of health care. A growing body of evidence demonstrates that more than half of those visits may be avoidable.
Objective To examine the impact of a nurse practitioner (NP)-led, physician-supervised, outpatient symptom management clinic on ED use.
Methods We conducted a retrospective review of ED encounters to quantify the frequency of ED use by oncology patients at a community cancer institute 6 months before (October 2012-March 2013) and after (April-September 2013) the initiation of an NP-staffed symptom management clinic.
Results The highest use of the ED and supportive clinic was among patients with advanced cancer, most commonly with lung or breast cancer, who were receiving cytotoxic chemotherapy. Uncontrolled symptoms of shortness of breath, pain, weakness, fever, nausea, vomiting, and diarrhea commonly led to ED visits. Despite instituting the NP-staffed symptom management clinic to manage those symptoms, there was a 17.9% increase in ED use. However, of the patients seen by the NP, 95% may have avoided hospitalization.
Limitations Retrospective study
Conclusions Our study identifies a high-risk population of patients who use the ED frequently. NP-led clinics could aggressively manage the symptom burden of these patients and potentially reduce ED visits as other studies have demonstrated. Although our study did not directly demonstrate this, we have identified weaknesses of care delivery in our clinic that could be optimized. In addition, we have demonstrated that the majority of patients seen for acute symptoms by an NP avoided an ED visit.
Click on the PDF icon at the top of this introduction to read the full article.
Background Emergency department (ED) use and hospitalization is distressing to cancer patients and drives up the cost of health care. A growing body of evidence demonstrates that more than half of those visits may be avoidable.
Objective To examine the impact of a nurse practitioner (NP)-led, physician-supervised, outpatient symptom management clinic on ED use.
Methods We conducted a retrospective review of ED encounters to quantify the frequency of ED use by oncology patients at a community cancer institute 6 months before (October 2012-March 2013) and after (April-September 2013) the initiation of an NP-staffed symptom management clinic.
Results The highest use of the ED and supportive clinic was among patients with advanced cancer, most commonly with lung or breast cancer, who were receiving cytotoxic chemotherapy. Uncontrolled symptoms of shortness of breath, pain, weakness, fever, nausea, vomiting, and diarrhea commonly led to ED visits. Despite instituting the NP-staffed symptom management clinic to manage those symptoms, there was a 17.9% increase in ED use. However, of the patients seen by the NP, 95% may have avoided hospitalization.
Limitations Retrospective study
Conclusions Our study identifies a high-risk population of patients who use the ED frequently. NP-led clinics could aggressively manage the symptom burden of these patients and potentially reduce ED visits as other studies have demonstrated. Although our study did not directly demonstrate this, we have identified weaknesses of care delivery in our clinic that could be optimized. In addition, we have demonstrated that the majority of patients seen for acute symptoms by an NP avoided an ED visit.
Click on the PDF icon at the top of this introduction to read the full article.
Quality versus queasy: neurokinin 1 receptor antagonist use in moderately emetogenic chemotherapy
Background The American Society of Clinical Oncology (ASCO) launched the Quality Oncology Practice Initiative (QOPI) program in 2010 to promote quality cancer care. The association has subsequently influenced the use of neurokinin 1 (NK-1) receptor antagonists through articles published in peer-reviewed publications and its Choosing Wisely campaign.
Objective To explore the rationale behind the use of NK-1 receptor antagonists in clinical practice.
Methods We distributed an anonymous 12-question online survey to 650 medical oncologists in 5 states, inquiring about their use of these agents. A total of 155 responses were analyzed.
Results QOPI-certified physicians were significantly more likely than noncertified physicians to use NK-1 receptor antagonists with moderately emetogenic regimens, including weekly cisplatin for head and neck cancer (82.6% vs 27.0%, respectively; P < .001), cervical and bladder cancer (81.4% vs 32.7%, P < .001), and with CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone) with or without rituximab in lymphoma (81.4 vs 17.3%, P < .001). The majority of QOPI-certified physicians reported using these agents for the sole purpose of maintaining QOPI certification (80.0%-86.0%). Certified physicians were also significantly more likely to appropriately prescribe NK-1 antagonists with highly emetogenic chemotherapy.
Limitations Responder bias; short survey that precludes detailed analysis; small sample size may limit generalizability to the field of medical oncology.
Conclusion Our data demonstrate that providers in QOPI-certified practices are significantly more likely than those in noncertified practices to prescribe NK-1 receptor antagonists. Certified physicians report that satisfying ASCO-QOPI requirements is their primary motivation for offering these agents.
Click on the PDF icon at the top of this introduction to read the full article.
Background The American Society of Clinical Oncology (ASCO) launched the Quality Oncology Practice Initiative (QOPI) program in 2010 to promote quality cancer care. The association has subsequently influenced the use of neurokinin 1 (NK-1) receptor antagonists through articles published in peer-reviewed publications and its Choosing Wisely campaign.
Objective To explore the rationale behind the use of NK-1 receptor antagonists in clinical practice.
Methods We distributed an anonymous 12-question online survey to 650 medical oncologists in 5 states, inquiring about their use of these agents. A total of 155 responses were analyzed.
Results QOPI-certified physicians were significantly more likely than noncertified physicians to use NK-1 receptor antagonists with moderately emetogenic regimens, including weekly cisplatin for head and neck cancer (82.6% vs 27.0%, respectively; P < .001), cervical and bladder cancer (81.4% vs 32.7%, P < .001), and with CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone) with or without rituximab in lymphoma (81.4 vs 17.3%, P < .001). The majority of QOPI-certified physicians reported using these agents for the sole purpose of maintaining QOPI certification (80.0%-86.0%). Certified physicians were also significantly more likely to appropriately prescribe NK-1 antagonists with highly emetogenic chemotherapy.
Limitations Responder bias; short survey that precludes detailed analysis; small sample size may limit generalizability to the field of medical oncology.
Conclusion Our data demonstrate that providers in QOPI-certified practices are significantly more likely than those in noncertified practices to prescribe NK-1 receptor antagonists. Certified physicians report that satisfying ASCO-QOPI requirements is their primary motivation for offering these agents.
Click on the PDF icon at the top of this introduction to read the full article.
Background The American Society of Clinical Oncology (ASCO) launched the Quality Oncology Practice Initiative (QOPI) program in 2010 to promote quality cancer care. The association has subsequently influenced the use of neurokinin 1 (NK-1) receptor antagonists through articles published in peer-reviewed publications and its Choosing Wisely campaign.
Objective To explore the rationale behind the use of NK-1 receptor antagonists in clinical practice.
Methods We distributed an anonymous 12-question online survey to 650 medical oncologists in 5 states, inquiring about their use of these agents. A total of 155 responses were analyzed.
Results QOPI-certified physicians were significantly more likely than noncertified physicians to use NK-1 receptor antagonists with moderately emetogenic regimens, including weekly cisplatin for head and neck cancer (82.6% vs 27.0%, respectively; P < .001), cervical and bladder cancer (81.4% vs 32.7%, P < .001), and with CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone) with or without rituximab in lymphoma (81.4 vs 17.3%, P < .001). The majority of QOPI-certified physicians reported using these agents for the sole purpose of maintaining QOPI certification (80.0%-86.0%). Certified physicians were also significantly more likely to appropriately prescribe NK-1 antagonists with highly emetogenic chemotherapy.
Limitations Responder bias; short survey that precludes detailed analysis; small sample size may limit generalizability to the field of medical oncology.
Conclusion Our data demonstrate that providers in QOPI-certified practices are significantly more likely than those in noncertified practices to prescribe NK-1 receptor antagonists. Certified physicians report that satisfying ASCO-QOPI requirements is their primary motivation for offering these agents.
Click on the PDF icon at the top of this introduction to read the full article.
Racial bias linked with shorter, less-supportive appointments with black patients
Higher implicit racial bias among non-black oncologists was associated with aspects of their interactions and care of non-black patients, investigators report.
In a study of 18 non-black oncologists and 112 black patients, oncologists who were higher in implicit racial bias had shorter interactions with the black patients, and those interactions were rated less supportive by observers and patients. Higher implicit bias was also associated with more patient difficulty remembering contents of the interaction, Louis A. Penner, Ph.D., of Karmanos Cancer Institute, Detroit, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.66.3658).
“We acknowledge it is unlikely racial bias alone [that] is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patients’ health-related attitudes also contribute to racial disparities in cancer treatment. However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored,” wrote Dr. Penner and his associates.
For the study, oncologists completed the Implicit Association Test that measured implicit racial bias prior to professional interaction. Patients also completed a baseline questionnaire prior to their appointment with an oncologist. Patients and physicians then had an appointment to discuss initial treatment for a current cancer. Patient questionnaires measuring perception of oncologist, the interaction, and recommended treatments, and physician questionnaires measuring patient participation were completed following the appointment. In addition, 96 of 112 appointments were videotaped and reviewed by four – two black and two white – researchers to assess various aspects of the physician-patient interaction.
Bivariate multilevel models revealed that higher implicit racial bias among oncologists was significantly associated to shorter appointment times (P = .02) and decreased use of supportive communication (P less than .01 when controlling for physician age). Implicit racial bias was not significantly correlated to talk-time ratio (P = .27) nor to the extent to which oncologists involved their patients in treatment decisions (P = .22).
Higher implicit racial bias was associated with patients experiencing greater difficulty remembering conversation contents (P less than .01) and patients perceiving the conversation as being less patient-centered (P = .01). Higher implicit racial bias was not significantly correlated to patient’s perception of treatment plans discussed (P = .19), post-visit distress (P = .57), or trust in their oncologist (P = .08).
This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of multiple companies.
On Twitter @jessnicolecraig
Higher implicit racial bias among non-black oncologists was associated with aspects of their interactions and care of non-black patients, investigators report.
In a study of 18 non-black oncologists and 112 black patients, oncologists who were higher in implicit racial bias had shorter interactions with the black patients, and those interactions were rated less supportive by observers and patients. Higher implicit bias was also associated with more patient difficulty remembering contents of the interaction, Louis A. Penner, Ph.D., of Karmanos Cancer Institute, Detroit, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.66.3658).
“We acknowledge it is unlikely racial bias alone [that] is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patients’ health-related attitudes also contribute to racial disparities in cancer treatment. However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored,” wrote Dr. Penner and his associates.
For the study, oncologists completed the Implicit Association Test that measured implicit racial bias prior to professional interaction. Patients also completed a baseline questionnaire prior to their appointment with an oncologist. Patients and physicians then had an appointment to discuss initial treatment for a current cancer. Patient questionnaires measuring perception of oncologist, the interaction, and recommended treatments, and physician questionnaires measuring patient participation were completed following the appointment. In addition, 96 of 112 appointments were videotaped and reviewed by four – two black and two white – researchers to assess various aspects of the physician-patient interaction.
Bivariate multilevel models revealed that higher implicit racial bias among oncologists was significantly associated to shorter appointment times (P = .02) and decreased use of supportive communication (P less than .01 when controlling for physician age). Implicit racial bias was not significantly correlated to talk-time ratio (P = .27) nor to the extent to which oncologists involved their patients in treatment decisions (P = .22).
Higher implicit racial bias was associated with patients experiencing greater difficulty remembering conversation contents (P less than .01) and patients perceiving the conversation as being less patient-centered (P = .01). Higher implicit racial bias was not significantly correlated to patient’s perception of treatment plans discussed (P = .19), post-visit distress (P = .57), or trust in their oncologist (P = .08).
This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of multiple companies.
On Twitter @jessnicolecraig
Higher implicit racial bias among non-black oncologists was associated with aspects of their interactions and care of non-black patients, investigators report.
In a study of 18 non-black oncologists and 112 black patients, oncologists who were higher in implicit racial bias had shorter interactions with the black patients, and those interactions were rated less supportive by observers and patients. Higher implicit bias was also associated with more patient difficulty remembering contents of the interaction, Louis A. Penner, Ph.D., of Karmanos Cancer Institute, Detroit, and his associates reported (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.66.3658).
“We acknowledge it is unlikely racial bias alone [that] is the major source of the well-documented, widespread racial disparities in cancer treatment. Factors such as patient socioeconomic status, limited access to high-quality health care, and patients’ health-related attitudes also contribute to racial disparities in cancer treatment. However, our data suggest that oncologist implicit racial bias may uniquely contribute to these disparities and should be further explored,” wrote Dr. Penner and his associates.
For the study, oncologists completed the Implicit Association Test that measured implicit racial bias prior to professional interaction. Patients also completed a baseline questionnaire prior to their appointment with an oncologist. Patients and physicians then had an appointment to discuss initial treatment for a current cancer. Patient questionnaires measuring perception of oncologist, the interaction, and recommended treatments, and physician questionnaires measuring patient participation were completed following the appointment. In addition, 96 of 112 appointments were videotaped and reviewed by four – two black and two white – researchers to assess various aspects of the physician-patient interaction.
Bivariate multilevel models revealed that higher implicit racial bias among oncologists was significantly associated to shorter appointment times (P = .02) and decreased use of supportive communication (P less than .01 when controlling for physician age). Implicit racial bias was not significantly correlated to talk-time ratio (P = .27) nor to the extent to which oncologists involved their patients in treatment decisions (P = .22).
Higher implicit racial bias was associated with patients experiencing greater difficulty remembering conversation contents (P less than .01) and patients perceiving the conversation as being less patient-centered (P = .01). Higher implicit racial bias was not significantly correlated to patient’s perception of treatment plans discussed (P = .19), post-visit distress (P = .57), or trust in their oncologist (P = .08).
This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of multiple companies.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Higher implicit racial bias among oncologists was linked with less-supportive care for black patients.
Major finding: Higher oncologist racial bias was significantly associated to shorter appointment times (P = .02) and less-supportive communication (P less than .01).
Data source: A randomized study involving 18 non-black oncologists and 112 black patients.
Disclosures: This study was funded by the National Cancer Institute and the research advisory committee of the Southeast Michigan Partners Against Cancer. Eleven investigators had no relevant disclosures to report. The three other investigators reported serving in advisory roles for, receiving financial compensation or honoraria from, or participating in the speakers bureau of Eli Lilly, Albrecht Pharmaceutical Consulting, GE Healthcare, and Karyopharm Therapeutics.
Symptoms linger after ‘successful’ gyn. cancer therapy
SAN DIEGO – Fully half of gynecologic cancer survivors who are done with treatment and cancer free nonetheless report having moderate to severe symptoms 2 and even 5 years after diagnosis, Dr. Michelle Rowland reported at the annual meeting of the Society of Gynecologic Oncology.
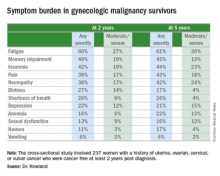
The most common symptoms in her cross-sectional study of 237 women with a history of uterine, ovarian, cervical, or vulvar cancer who were cancer free and at least 2 years post diagnosis were fatigue, insomnia, pain, memory impairment, and neuropathy, according to Dr. Rowland, a gynecologic oncology fellow at the University of Oklahoma in Oklahoma City.
She found the high prevalence of fatigue in gynecologic cancer survivors so distant from treatment particularly unexpected.
“The fact that 60% of women report some degree of fatigue 2 and 5 years out from their gynecologic cancer diagnosis was surprising to me,” she said in an interview. “Fatigue is something we usually think of as being related either to the treatment that they’re getting or to the cancer itself. But these women don’t have cancer anymore and are not being treated.”
The cross-sectional study included 237 patients who completed a self-assessment symptom questionnaire during a university outpatient gynecologic oncology clinic for ongoing routine disease surveillance. All were believed to be cancer free and none were receiving treatment 2 or more years post diagnosis. Seventy-seven of the women were 5 or more years out from diagnosis. The prevalence and self-rated severity of symptoms on a 0-10 scale were similar in the 2- and 5-year survivor groups.
One-quarter of the 2-year survivors reported having three or more moderate to severe symptoms as defined by a rating of 4-10. So did 29% of the 5-year survivors.
Three-quarters of women reported having one or more symptom.
In an effort to identify predictors of high symptom burden, Dr. Rowland and coinvestigators conducted a multivariate logistic regression analysis controlling for tumor stage, disease, site, race, and all forms of cancer therapy received. Prior chemotherapy proved to be an independent risk factor for high symptom burden at 2 years, while prior radiation therapy predicted high symptom burden at 5 or more years. Of note, cancer type was not predictive.
Roughly 40% of subjects reported currently being on medications for chronic pain, 11% were taking antianxiety drugs, and a similar proportion were using sleep aids.
Dr. Rowland concluded that long-term follow-up of gynecologic cancer survivors should include a symptom assessment. Survivor clinics, which are becoming increasingly common, offer a way to specifically address ongoing symptoms.
She reported having no financial conflicts regarding her study.
SAN DIEGO – Fully half of gynecologic cancer survivors who are done with treatment and cancer free nonetheless report having moderate to severe symptoms 2 and even 5 years after diagnosis, Dr. Michelle Rowland reported at the annual meeting of the Society of Gynecologic Oncology.
The most common symptoms in her cross-sectional study of 237 women with a history of uterine, ovarian, cervical, or vulvar cancer who were cancer free and at least 2 years post diagnosis were fatigue, insomnia, pain, memory impairment, and neuropathy, according to Dr. Rowland, a gynecologic oncology fellow at the University of Oklahoma in Oklahoma City.
She found the high prevalence of fatigue in gynecologic cancer survivors so distant from treatment particularly unexpected.
“The fact that 60% of women report some degree of fatigue 2 and 5 years out from their gynecologic cancer diagnosis was surprising to me,” she said in an interview. “Fatigue is something we usually think of as being related either to the treatment that they’re getting or to the cancer itself. But these women don’t have cancer anymore and are not being treated.”
The cross-sectional study included 237 patients who completed a self-assessment symptom questionnaire during a university outpatient gynecologic oncology clinic for ongoing routine disease surveillance. All were believed to be cancer free and none were receiving treatment 2 or more years post diagnosis. Seventy-seven of the women were 5 or more years out from diagnosis. The prevalence and self-rated severity of symptoms on a 0-10 scale were similar in the 2- and 5-year survivor groups.
One-quarter of the 2-year survivors reported having three or more moderate to severe symptoms as defined by a rating of 4-10. So did 29% of the 5-year survivors.
Three-quarters of women reported having one or more symptom.
In an effort to identify predictors of high symptom burden, Dr. Rowland and coinvestigators conducted a multivariate logistic regression analysis controlling for tumor stage, disease, site, race, and all forms of cancer therapy received. Prior chemotherapy proved to be an independent risk factor for high symptom burden at 2 years, while prior radiation therapy predicted high symptom burden at 5 or more years. Of note, cancer type was not predictive.
Roughly 40% of subjects reported currently being on medications for chronic pain, 11% were taking antianxiety drugs, and a similar proportion were using sleep aids.
Dr. Rowland concluded that long-term follow-up of gynecologic cancer survivors should include a symptom assessment. Survivor clinics, which are becoming increasingly common, offer a way to specifically address ongoing symptoms.
She reported having no financial conflicts regarding her study.
SAN DIEGO – Fully half of gynecologic cancer survivors who are done with treatment and cancer free nonetheless report having moderate to severe symptoms 2 and even 5 years after diagnosis, Dr. Michelle Rowland reported at the annual meeting of the Society of Gynecologic Oncology.
The most common symptoms in her cross-sectional study of 237 women with a history of uterine, ovarian, cervical, or vulvar cancer who were cancer free and at least 2 years post diagnosis were fatigue, insomnia, pain, memory impairment, and neuropathy, according to Dr. Rowland, a gynecologic oncology fellow at the University of Oklahoma in Oklahoma City.
She found the high prevalence of fatigue in gynecologic cancer survivors so distant from treatment particularly unexpected.
“The fact that 60% of women report some degree of fatigue 2 and 5 years out from their gynecologic cancer diagnosis was surprising to me,” she said in an interview. “Fatigue is something we usually think of as being related either to the treatment that they’re getting or to the cancer itself. But these women don’t have cancer anymore and are not being treated.”
The cross-sectional study included 237 patients who completed a self-assessment symptom questionnaire during a university outpatient gynecologic oncology clinic for ongoing routine disease surveillance. All were believed to be cancer free and none were receiving treatment 2 or more years post diagnosis. Seventy-seven of the women were 5 or more years out from diagnosis. The prevalence and self-rated severity of symptoms on a 0-10 scale were similar in the 2- and 5-year survivor groups.
One-quarter of the 2-year survivors reported having three or more moderate to severe symptoms as defined by a rating of 4-10. So did 29% of the 5-year survivors.
Three-quarters of women reported having one or more symptom.
In an effort to identify predictors of high symptom burden, Dr. Rowland and coinvestigators conducted a multivariate logistic regression analysis controlling for tumor stage, disease, site, race, and all forms of cancer therapy received. Prior chemotherapy proved to be an independent risk factor for high symptom burden at 2 years, while prior radiation therapy predicted high symptom burden at 5 or more years. Of note, cancer type was not predictive.
Roughly 40% of subjects reported currently being on medications for chronic pain, 11% were taking antianxiety drugs, and a similar proportion were using sleep aids.
Dr. Rowland concluded that long-term follow-up of gynecologic cancer survivors should include a symptom assessment. Survivor clinics, which are becoming increasingly common, offer a way to specifically address ongoing symptoms.
She reported having no financial conflicts regarding her study.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Long-term follow-up of gynecologic cancer survivors should include assessment of cancer- or treatment-related symptoms.
Major finding: One-quarter of cancer-free patients reported three or more lingering symptoms of moderate to severe intensity 2 years after diagnosis.
Data source: This cross-sectional study utilized a structured questionnaire to evaluate the types and severity of symptoms present in 237 former gynecologic cancer patients who were off treatment and cancer free at least 2 years after diagnosis.
Disclosures: The study presenter reported having no financial conflicts of interest.