User login
VIDEO: Despite toxicities, ibrutinib is beneficial for treatment-resistant graft-vs.-host disease
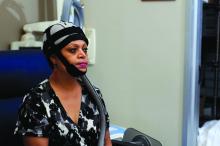
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Ibrutinib (420 mg) led to complete responses in one-third of patients with chronic, treatment-resistant graft-vs-host disease.
Major finding: No cardiotoxicities were observed, but 52% of patients had other serious adverse effects, such as sepsis, pyrexia, and pneumonia.
Data source: An open-label phase II study of 42 patients who developed chronic, treatment-resistant graft-vs.-host disease after undergoing allogeneic stem cell transplantation.
Disclosures: Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, reimbursement for travel and expenses, and research funding from Pharmacyclics.
Partnering with stakeholders using an example patient-reported outcomes project
Recently, researchers have been challenged to design methods that ensure that key constituents are partners in research, and not simply participants. Here we describe some innovative approaches we used to engage stakeholders. The approaches are drawn from a patient-centered outcomes research project, focusing on the graphic display of patient-reported outcomes (PROs) data. PROs represent patients’ perspectives on the impact of health, disease, and treatment, without interpretation by a clinician or anyone else. PROs include, among other things, patients’ assessments of their symptoms, their level of physical and psychosocial functioning, and health-related quality-of-life.1
As a first example of the key role of stakeholders in this project, input from cancer patients and clinicians, drawn from previous research, motivated us to ask whether there might be a “better way” to display PRO data when used to inform clinical practice. Specifically, even though cancer patients and clinicians endorse the importance of PRO data to promote patient-centered care, both groups report challenges using PROs in practice because of difficulty understanding what the PRO scores mean (eg, what is a good score or a bad score?; for individual patients, which scores should clinicians be concerned about?; for clinical trial PROs, what differences in PRO scores between treatments are clinically important?). The challenges in interpreting PRO data result in part from a large number of PRO measures (eg, one database includes more than 1,000 instruments)2 and no standards across PRO measures regarding how they are scored and scaled, or in how the data are presented.3 For example, on some PRO measures, higher scores represent better outcomes; on some PRO measures, lower scores represent better outcomes; and on some PRO measures, whether higher or lower scores represent better outcomes depends on the domain being measured. Further, some measures are scaled 0-100, with the extremes representing the best/worst scores possible, whereas others are normed to, for example, a population average of 50. Because of this variation, a score of 70 can have a completely different meaning depending on the PRO measure (or domain within a measure). As noted above, previous research has documented that this variation limits patients’ and clinicians’ understanding of the PRO scores, creating an important barrier to their use in practice.4-5
To address this stakeholder-driven research question, we undertook a three-part study to identify approaches for PRO data display that can be easily interpreted, regardless of scoring or scaling conventions, with the overall goal of improving patient and clinician understanding and use of PROs in oncology clinical practice. Part 1 of the study identified attributes of graphic displays of PRO data that are helpful and confusing.6 Part 2 involved developing improved PRO data presentation approaches.7 Part 3 evaluated the accuracy-of-interpretation and clarity of the developed approaches.8-10 The methods and findings of the three-part study are reported elsewhere;6-10 here, we describe the various approaches employed to engage stakeholders throughout the project.
As described above, the first reflection of stakeholder input was in the research question we asked. We then sought to identify the key stakeholder groups and ensure that they participated in each stage of the project. The relevant stakeholder groups we identified were: patients and their caregivers; health care providers (eg, oncologists, oncology nurses) who need to understand PRO data for their own consideration and for discussion with patients; and PRO researchers who develop, validate, and apply PRO measures.
Having identified these three key stakeholder groups, we sought to obtain broad representation of their perspectives. For example, we ensured that our investigative team included a cancer survivor, a cancer care provider, and PRO researchers. To supplement the stakeholder input from the investigative team, we formed a nine-member Stakeholder Advisory Board, with multiple representatives from each key constituency. We also aimed to be as broad as possible in the populations sampled for data collection. For example, we extended beyond the Johns Hopkins cancer center to include the Johns Hopkins Clinical Research Network, a consortium of academic and community health systems across the mid-Atlantic United States. Beyond the in-person data collection across the region, our study also included an internet survey of cancer patients/survivors, cancer care providers, and PRO researchers from across the United States and internationally. Taken together, these approaches improve the diversity of our sample and, thereby, the generalizability of our findings.
In addition to obtaining broad perspectives across stakeholder groups, we created genuine partnerships with the stakeholders to inform every aspect of the project. As described above, the study itself was motivated by feedback from cancer patients and clinicians regarding the challenges they experienced when trying to interpret PRO scores, and we therefore ensured that each stakeholder group contributed to the study’s design. Stakeholders also played a critical role in the conduct of the study. For example, in the first part of the study, we conducted one-on-one interviews with 50 cancer patients and 20 cancer clinicians to obtain their insights regarding attributes of current approaches for presenting PRO data that are helpful and confusing.6 At the completion of each interview, we asked participants whether they would be interested in partnering with the researchers in developing improved presentation formats in the next phase of the project. These volunteers were organized into work groups that reviewed the findings from the initial round of interviews with the investigative team, provided suggestions regarding candidate formats that could be used to improve presentation approaches, and helped pilot the internet survey.7 In this way, research participants had the opportunity to evolve into research partners, providing critically important input throughout the process.
The implementation and dissemination of findings is another area in which stakeholder partnership is particularly valuable. For example, several of our stakeholder partners have an advocacy background, which can be quite useful for conveying the project’s results in a compelling way. Other stakeholders, such as journal editors, are in a position to act directly to implement the study findings by, for example, adding best practices for presenting PRO data to their journal’s author instructions. Notably, some of the skills stakeholders bring come in addition to their role as stakeholders. For example, one of our patient stakeholders has a background in marketing, and this marketing expertise (completely separate from his patient experience) has helped the research team think about how to present data to broad audiences in a meaningful way.
In summary, this project has implemented stakeholder-driven approaches to address an important barrier to patient-centered cancer care. Several key lessons in stakeholder engagement have emerged from this experience. It is important to identify the key constituencies early on in the process. Involving stakeholders from the start enables them to play important roles in every aspect of the study, starting with study design conception. There are also innovative ways to integrate stakeholders in study conduct, such as our work groups of research participants who volunteered to partner with the research team to develop improved data presentation approaches. Implementation and dissemination is another area where stakeholders, based on their background and connections, can play a critical role. Throughout the process, it is valuable to challenge the project to obtain perspectives from as broad a range of stakeholders as possible. Finally, stakeholders have expertise beyond their stakeholder roles, and these skills can be quite valuable to the overall research agenda. In this project, our partnership with stakeholders has helped improve the presentation of PRO data to patients and providers, thereby improving the patient-centeredness of cancer care.
Acknowledgments
The PRO Data Presentation Stakeholder Advisory Board includes Neil K Aaronson, PhD (Netherlands Cancer Institute, Amsterdam); Patricia A Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center, Los Angeles, CA); Ravin Garg, MD (Anne Arundel Medical Center, Annapolis, MD); Michael Fisch, MD (MD Anderson Cancer Center, Houston, TX); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network, Washington, DC); Bryce B Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center, Chapel Hill, NC); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD); Ellen Stovall (National Coalition for Cancer Survivorship, Washington, DC [posthumous]); Matthew Zachary (Stupid Cancer, New York, NY).
The authors thank The Johns Hopkins Clinical Research Network site investigators and staff and, in particular, the patients and clinicians who participated in this project.
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. Drs Snyder and Smith are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funders had no role in the study design; data collection, analysis, or interpretation; writing; or decision to submit.
1. Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522-531.
2. PROQOLID, the Patient-Reported Outcome and Quality of Life Instruments Database. https://eprovide.mapi-trust.org/. Accessed November 10, 2016.
3. Brundage MD, Snyder CF. Patient-reported outcomes in clinical practice: using standards to break down barriers. Clin Invest. 2012;2(4):343-346.
4. Brundage M, Bass B, Jolie R, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. 2011;20(7):979-985.
5. Snyder CF, Jensen R, Courtin SO, et al. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18(7):793-800.
6. Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF. PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24(10):2457-2472.
7. Smith KC, Brundage MD, Tolbert E, Little EA, Bantug ET, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer. 2016;24(10):4149-4157.
8. Snyder CF, Smith KC, Bantug ET, Tolbert EE, Blackford AL, Brundage MD. PRO Data Presentation Stakeholder Advisory Board. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123(10):1848-1859.
9. Brundage M, Blackford A, Tolbert E, Smith K, Bantug E, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Presenting comparative study PRO results to clinicians and researchers: beyond the eye of the beholder. Qual Life Res. 2017 Nov 2 [Epub ahead of print].
10. Tolbert E, Snyder C, Bantug E, Blackford A, Brundage M. PRO Data Presentation Stakeholder Advisory Board. Graphing group-level data from research studies for presentation to patients in educational materials and decision aids. Qual Life Res. 2016;25(suppl 1):17.
Recently, researchers have been challenged to design methods that ensure that key constituents are partners in research, and not simply participants. Here we describe some innovative approaches we used to engage stakeholders. The approaches are drawn from a patient-centered outcomes research project, focusing on the graphic display of patient-reported outcomes (PROs) data. PROs represent patients’ perspectives on the impact of health, disease, and treatment, without interpretation by a clinician or anyone else. PROs include, among other things, patients’ assessments of their symptoms, their level of physical and psychosocial functioning, and health-related quality-of-life.1
As a first example of the key role of stakeholders in this project, input from cancer patients and clinicians, drawn from previous research, motivated us to ask whether there might be a “better way” to display PRO data when used to inform clinical practice. Specifically, even though cancer patients and clinicians endorse the importance of PRO data to promote patient-centered care, both groups report challenges using PROs in practice because of difficulty understanding what the PRO scores mean (eg, what is a good score or a bad score?; for individual patients, which scores should clinicians be concerned about?; for clinical trial PROs, what differences in PRO scores between treatments are clinically important?). The challenges in interpreting PRO data result in part from a large number of PRO measures (eg, one database includes more than 1,000 instruments)2 and no standards across PRO measures regarding how they are scored and scaled, or in how the data are presented.3 For example, on some PRO measures, higher scores represent better outcomes; on some PRO measures, lower scores represent better outcomes; and on some PRO measures, whether higher or lower scores represent better outcomes depends on the domain being measured. Further, some measures are scaled 0-100, with the extremes representing the best/worst scores possible, whereas others are normed to, for example, a population average of 50. Because of this variation, a score of 70 can have a completely different meaning depending on the PRO measure (or domain within a measure). As noted above, previous research has documented that this variation limits patients’ and clinicians’ understanding of the PRO scores, creating an important barrier to their use in practice.4-5
To address this stakeholder-driven research question, we undertook a three-part study to identify approaches for PRO data display that can be easily interpreted, regardless of scoring or scaling conventions, with the overall goal of improving patient and clinician understanding and use of PROs in oncology clinical practice. Part 1 of the study identified attributes of graphic displays of PRO data that are helpful and confusing.6 Part 2 involved developing improved PRO data presentation approaches.7 Part 3 evaluated the accuracy-of-interpretation and clarity of the developed approaches.8-10 The methods and findings of the three-part study are reported elsewhere;6-10 here, we describe the various approaches employed to engage stakeholders throughout the project.
As described above, the first reflection of stakeholder input was in the research question we asked. We then sought to identify the key stakeholder groups and ensure that they participated in each stage of the project. The relevant stakeholder groups we identified were: patients and their caregivers; health care providers (eg, oncologists, oncology nurses) who need to understand PRO data for their own consideration and for discussion with patients; and PRO researchers who develop, validate, and apply PRO measures.
Having identified these three key stakeholder groups, we sought to obtain broad representation of their perspectives. For example, we ensured that our investigative team included a cancer survivor, a cancer care provider, and PRO researchers. To supplement the stakeholder input from the investigative team, we formed a nine-member Stakeholder Advisory Board, with multiple representatives from each key constituency. We also aimed to be as broad as possible in the populations sampled for data collection. For example, we extended beyond the Johns Hopkins cancer center to include the Johns Hopkins Clinical Research Network, a consortium of academic and community health systems across the mid-Atlantic United States. Beyond the in-person data collection across the region, our study also included an internet survey of cancer patients/survivors, cancer care providers, and PRO researchers from across the United States and internationally. Taken together, these approaches improve the diversity of our sample and, thereby, the generalizability of our findings.
In addition to obtaining broad perspectives across stakeholder groups, we created genuine partnerships with the stakeholders to inform every aspect of the project. As described above, the study itself was motivated by feedback from cancer patients and clinicians regarding the challenges they experienced when trying to interpret PRO scores, and we therefore ensured that each stakeholder group contributed to the study’s design. Stakeholders also played a critical role in the conduct of the study. For example, in the first part of the study, we conducted one-on-one interviews with 50 cancer patients and 20 cancer clinicians to obtain their insights regarding attributes of current approaches for presenting PRO data that are helpful and confusing.6 At the completion of each interview, we asked participants whether they would be interested in partnering with the researchers in developing improved presentation formats in the next phase of the project. These volunteers were organized into work groups that reviewed the findings from the initial round of interviews with the investigative team, provided suggestions regarding candidate formats that could be used to improve presentation approaches, and helped pilot the internet survey.7 In this way, research participants had the opportunity to evolve into research partners, providing critically important input throughout the process.
The implementation and dissemination of findings is another area in which stakeholder partnership is particularly valuable. For example, several of our stakeholder partners have an advocacy background, which can be quite useful for conveying the project’s results in a compelling way. Other stakeholders, such as journal editors, are in a position to act directly to implement the study findings by, for example, adding best practices for presenting PRO data to their journal’s author instructions. Notably, some of the skills stakeholders bring come in addition to their role as stakeholders. For example, one of our patient stakeholders has a background in marketing, and this marketing expertise (completely separate from his patient experience) has helped the research team think about how to present data to broad audiences in a meaningful way.
In summary, this project has implemented stakeholder-driven approaches to address an important barrier to patient-centered cancer care. Several key lessons in stakeholder engagement have emerged from this experience. It is important to identify the key constituencies early on in the process. Involving stakeholders from the start enables them to play important roles in every aspect of the study, starting with study design conception. There are also innovative ways to integrate stakeholders in study conduct, such as our work groups of research participants who volunteered to partner with the research team to develop improved data presentation approaches. Implementation and dissemination is another area where stakeholders, based on their background and connections, can play a critical role. Throughout the process, it is valuable to challenge the project to obtain perspectives from as broad a range of stakeholders as possible. Finally, stakeholders have expertise beyond their stakeholder roles, and these skills can be quite valuable to the overall research agenda. In this project, our partnership with stakeholders has helped improve the presentation of PRO data to patients and providers, thereby improving the patient-centeredness of cancer care.
Acknowledgments
The PRO Data Presentation Stakeholder Advisory Board includes Neil K Aaronson, PhD (Netherlands Cancer Institute, Amsterdam); Patricia A Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center, Los Angeles, CA); Ravin Garg, MD (Anne Arundel Medical Center, Annapolis, MD); Michael Fisch, MD (MD Anderson Cancer Center, Houston, TX); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network, Washington, DC); Bryce B Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center, Chapel Hill, NC); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD); Ellen Stovall (National Coalition for Cancer Survivorship, Washington, DC [posthumous]); Matthew Zachary (Stupid Cancer, New York, NY).
The authors thank The Johns Hopkins Clinical Research Network site investigators and staff and, in particular, the patients and clinicians who participated in this project.
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. Drs Snyder and Smith are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funders had no role in the study design; data collection, analysis, or interpretation; writing; or decision to submit.
Recently, researchers have been challenged to design methods that ensure that key constituents are partners in research, and not simply participants. Here we describe some innovative approaches we used to engage stakeholders. The approaches are drawn from a patient-centered outcomes research project, focusing on the graphic display of patient-reported outcomes (PROs) data. PROs represent patients’ perspectives on the impact of health, disease, and treatment, without interpretation by a clinician or anyone else. PROs include, among other things, patients’ assessments of their symptoms, their level of physical and psychosocial functioning, and health-related quality-of-life.1
As a first example of the key role of stakeholders in this project, input from cancer patients and clinicians, drawn from previous research, motivated us to ask whether there might be a “better way” to display PRO data when used to inform clinical practice. Specifically, even though cancer patients and clinicians endorse the importance of PRO data to promote patient-centered care, both groups report challenges using PROs in practice because of difficulty understanding what the PRO scores mean (eg, what is a good score or a bad score?; for individual patients, which scores should clinicians be concerned about?; for clinical trial PROs, what differences in PRO scores between treatments are clinically important?). The challenges in interpreting PRO data result in part from a large number of PRO measures (eg, one database includes more than 1,000 instruments)2 and no standards across PRO measures regarding how they are scored and scaled, or in how the data are presented.3 For example, on some PRO measures, higher scores represent better outcomes; on some PRO measures, lower scores represent better outcomes; and on some PRO measures, whether higher or lower scores represent better outcomes depends on the domain being measured. Further, some measures are scaled 0-100, with the extremes representing the best/worst scores possible, whereas others are normed to, for example, a population average of 50. Because of this variation, a score of 70 can have a completely different meaning depending on the PRO measure (or domain within a measure). As noted above, previous research has documented that this variation limits patients’ and clinicians’ understanding of the PRO scores, creating an important barrier to their use in practice.4-5
To address this stakeholder-driven research question, we undertook a three-part study to identify approaches for PRO data display that can be easily interpreted, regardless of scoring or scaling conventions, with the overall goal of improving patient and clinician understanding and use of PROs in oncology clinical practice. Part 1 of the study identified attributes of graphic displays of PRO data that are helpful and confusing.6 Part 2 involved developing improved PRO data presentation approaches.7 Part 3 evaluated the accuracy-of-interpretation and clarity of the developed approaches.8-10 The methods and findings of the three-part study are reported elsewhere;6-10 here, we describe the various approaches employed to engage stakeholders throughout the project.
As described above, the first reflection of stakeholder input was in the research question we asked. We then sought to identify the key stakeholder groups and ensure that they participated in each stage of the project. The relevant stakeholder groups we identified were: patients and their caregivers; health care providers (eg, oncologists, oncology nurses) who need to understand PRO data for their own consideration and for discussion with patients; and PRO researchers who develop, validate, and apply PRO measures.
Having identified these three key stakeholder groups, we sought to obtain broad representation of their perspectives. For example, we ensured that our investigative team included a cancer survivor, a cancer care provider, and PRO researchers. To supplement the stakeholder input from the investigative team, we formed a nine-member Stakeholder Advisory Board, with multiple representatives from each key constituency. We also aimed to be as broad as possible in the populations sampled for data collection. For example, we extended beyond the Johns Hopkins cancer center to include the Johns Hopkins Clinical Research Network, a consortium of academic and community health systems across the mid-Atlantic United States. Beyond the in-person data collection across the region, our study also included an internet survey of cancer patients/survivors, cancer care providers, and PRO researchers from across the United States and internationally. Taken together, these approaches improve the diversity of our sample and, thereby, the generalizability of our findings.
In addition to obtaining broad perspectives across stakeholder groups, we created genuine partnerships with the stakeholders to inform every aspect of the project. As described above, the study itself was motivated by feedback from cancer patients and clinicians regarding the challenges they experienced when trying to interpret PRO scores, and we therefore ensured that each stakeholder group contributed to the study’s design. Stakeholders also played a critical role in the conduct of the study. For example, in the first part of the study, we conducted one-on-one interviews with 50 cancer patients and 20 cancer clinicians to obtain their insights regarding attributes of current approaches for presenting PRO data that are helpful and confusing.6 At the completion of each interview, we asked participants whether they would be interested in partnering with the researchers in developing improved presentation formats in the next phase of the project. These volunteers were organized into work groups that reviewed the findings from the initial round of interviews with the investigative team, provided suggestions regarding candidate formats that could be used to improve presentation approaches, and helped pilot the internet survey.7 In this way, research participants had the opportunity to evolve into research partners, providing critically important input throughout the process.
The implementation and dissemination of findings is another area in which stakeholder partnership is particularly valuable. For example, several of our stakeholder partners have an advocacy background, which can be quite useful for conveying the project’s results in a compelling way. Other stakeholders, such as journal editors, are in a position to act directly to implement the study findings by, for example, adding best practices for presenting PRO data to their journal’s author instructions. Notably, some of the skills stakeholders bring come in addition to their role as stakeholders. For example, one of our patient stakeholders has a background in marketing, and this marketing expertise (completely separate from his patient experience) has helped the research team think about how to present data to broad audiences in a meaningful way.
In summary, this project has implemented stakeholder-driven approaches to address an important barrier to patient-centered cancer care. Several key lessons in stakeholder engagement have emerged from this experience. It is important to identify the key constituencies early on in the process. Involving stakeholders from the start enables them to play important roles in every aspect of the study, starting with study design conception. There are also innovative ways to integrate stakeholders in study conduct, such as our work groups of research participants who volunteered to partner with the research team to develop improved data presentation approaches. Implementation and dissemination is another area where stakeholders, based on their background and connections, can play a critical role. Throughout the process, it is valuable to challenge the project to obtain perspectives from as broad a range of stakeholders as possible. Finally, stakeholders have expertise beyond their stakeholder roles, and these skills can be quite valuable to the overall research agenda. In this project, our partnership with stakeholders has helped improve the presentation of PRO data to patients and providers, thereby improving the patient-centeredness of cancer care.
Acknowledgments
The PRO Data Presentation Stakeholder Advisory Board includes Neil K Aaronson, PhD (Netherlands Cancer Institute, Amsterdam); Patricia A Ganz, MD (University of California-Los Angeles and Jonsson Comprehensive Cancer Center, Los Angeles, CA); Ravin Garg, MD (Anne Arundel Medical Center, Annapolis, MD); Michael Fisch, MD (MD Anderson Cancer Center, Houston, TX); Vanessa Hoffman, MPH (Bladder Cancer Advocacy Network, Washington, DC); Bryce B Reeve, PhD (University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center, Chapel Hill, NC); Eden Stotsky-Himelfarb (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD); Ellen Stovall (National Coalition for Cancer Survivorship, Washington, DC [posthumous]); Matthew Zachary (Stupid Cancer, New York, NY).
The authors thank The Johns Hopkins Clinical Research Network site investigators and staff and, in particular, the patients and clinicians who participated in this project.
Supported by a Patient-Centered Outcomes Research Institute (PCORI) Award (R-1410-24904). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or methodology committee. Drs Snyder and Smith are members of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (P30 CA 006973). The funders had no role in the study design; data collection, analysis, or interpretation; writing; or decision to submit.
1. Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522-531.
2. PROQOLID, the Patient-Reported Outcome and Quality of Life Instruments Database. https://eprovide.mapi-trust.org/. Accessed November 10, 2016.
3. Brundage MD, Snyder CF. Patient-reported outcomes in clinical practice: using standards to break down barriers. Clin Invest. 2012;2(4):343-346.
4. Brundage M, Bass B, Jolie R, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. 2011;20(7):979-985.
5. Snyder CF, Jensen R, Courtin SO, et al. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18(7):793-800.
6. Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF. PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24(10):2457-2472.
7. Smith KC, Brundage MD, Tolbert E, Little EA, Bantug ET, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer. 2016;24(10):4149-4157.
8. Snyder CF, Smith KC, Bantug ET, Tolbert EE, Blackford AL, Brundage MD. PRO Data Presentation Stakeholder Advisory Board. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123(10):1848-1859.
9. Brundage M, Blackford A, Tolbert E, Smith K, Bantug E, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Presenting comparative study PRO results to clinicians and researchers: beyond the eye of the beholder. Qual Life Res. 2017 Nov 2 [Epub ahead of print].
10. Tolbert E, Snyder C, Bantug E, Blackford A, Brundage M. PRO Data Presentation Stakeholder Advisory Board. Graphing group-level data from research studies for presentation to patients in educational materials and decision aids. Qual Life Res. 2016;25(suppl 1):17.
1. Acquadro C, Berzon R, Dubois D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the Patient-Reported Outcomes (PRO) Harmonization Group meeting at the Food and Drug Administration, February 16, 2001. Value Health. 2003;6(5):522-531.
2. PROQOLID, the Patient-Reported Outcome and Quality of Life Instruments Database. https://eprovide.mapi-trust.org/. Accessed November 10, 2016.
3. Brundage MD, Snyder CF. Patient-reported outcomes in clinical practice: using standards to break down barriers. Clin Invest. 2012;2(4):343-346.
4. Brundage M, Bass B, Jolie R, et al. A knowledge translation challenge: clinical use of quality of life data from cancer clinical trials. Qual Life Res. 2011;20(7):979-985.
5. Snyder CF, Jensen R, Courtin SO, et al. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18(7):793-800.
6. Brundage M, Smith KC, Little EA, Bantug ET, Snyder CF. PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Qual Life Res. 2015;24(10):2457-2472.
7. Smith KC, Brundage MD, Tolbert E, Little EA, Bantug ET, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Engaging stakeholders to improve presentation of patient-reported outcomes data in clinical practice. Support Care Cancer. 2016;24(10):4149-4157.
8. Snyder CF, Smith KC, Bantug ET, Tolbert EE, Blackford AL, Brundage MD. PRO Data Presentation Stakeholder Advisory Board. What do these scores mean? Presenting patient-reported outcomes data to patients and clinicians to improve interpretability. Cancer. 2017;123(10):1848-1859.
9. Brundage M, Blackford A, Tolbert E, Smith K, Bantug E, Snyder C. PRO Data Presentation Stakeholder Advisory Board. Presenting comparative study PRO results to clinicians and researchers: beyond the eye of the beholder. Qual Life Res. 2017 Nov 2 [Epub ahead of print].
10. Tolbert E, Snyder C, Bantug E, Blackford A, Brundage M. PRO Data Presentation Stakeholder Advisory Board. Graphing group-level data from research studies for presentation to patients in educational materials and decision aids. Qual Life Res. 2016;25(suppl 1):17.
Unicentric Castleman disease disguised as a pancreatic neoplasm
Castleman disease or angiofollicular lymph node hyperplasia is an uncommon cause of an incidental abdominal mass found on imaging. The etiology of Castleman disease is relatively unknown, however, it is thought to be primarily associated with an oversecretion of interleukin-6. The oversecretion of this pro-inflammatory cytokine leads to lymph node hyperplasia. Castleman disease can be classified into 2 categories: unicentric or multicentric. Most cases of unicentric Castleman disease are asymptomatic and are found on routine imaging. It is found predominately in middle-aged persons of equal sex and is managed primarily by surgical resection. We present here a case of a peripancreatic mass diagnosed by surgical excision as Castleman disease, hyaline vascular type.
Click on the PDF icon at the top of this introduction to read the full article.
Castleman disease or angiofollicular lymph node hyperplasia is an uncommon cause of an incidental abdominal mass found on imaging. The etiology of Castleman disease is relatively unknown, however, it is thought to be primarily associated with an oversecretion of interleukin-6. The oversecretion of this pro-inflammatory cytokine leads to lymph node hyperplasia. Castleman disease can be classified into 2 categories: unicentric or multicentric. Most cases of unicentric Castleman disease are asymptomatic and are found on routine imaging. It is found predominately in middle-aged persons of equal sex and is managed primarily by surgical resection. We present here a case of a peripancreatic mass diagnosed by surgical excision as Castleman disease, hyaline vascular type.
Click on the PDF icon at the top of this introduction to read the full article.
Castleman disease or angiofollicular lymph node hyperplasia is an uncommon cause of an incidental abdominal mass found on imaging. The etiology of Castleman disease is relatively unknown, however, it is thought to be primarily associated with an oversecretion of interleukin-6. The oversecretion of this pro-inflammatory cytokine leads to lymph node hyperplasia. Castleman disease can be classified into 2 categories: unicentric or multicentric. Most cases of unicentric Castleman disease are asymptomatic and are found on routine imaging. It is found predominately in middle-aged persons of equal sex and is managed primarily by surgical resection. We present here a case of a peripancreatic mass diagnosed by surgical excision as Castleman disease, hyaline vascular type.
Click on the PDF icon at the top of this introduction to read the full article.
Paraneoplastic Isaacs syndrome leading to diagnosis of small-cell lung cancer
Paraneoplastic Isaacs syndrome is a rare disorder with distinct clinical and electromyographic characteristics. It is a consequence of neoplastic process that is not directly caused by the tumor itself, but usually mediated by immune response primarily against the tumor and neural tissues are damaged owing to bystander effect. Paraneoplastic neurologic disorders may precede cancer diagnosis. Here we report the case of 75-year-old woman who presented with numbness, tingling sensation, and weakness of lower extremities, and was diagnosed with Isaacs syndrome and subsequently small-cell lung cancer. Plasmapheresis and treatment of small-cell lung cancer produced signficant symptoms improvement. We also conduct a complete review of the published case reports and case series of Isaacs syndrome of paraneoplastic etiology, which usually has good response to carbamazepine and to specfic treatment of underlying neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
Paraneoplastic Isaacs syndrome is a rare disorder with distinct clinical and electromyographic characteristics. It is a consequence of neoplastic process that is not directly caused by the tumor itself, but usually mediated by immune response primarily against the tumor and neural tissues are damaged owing to bystander effect. Paraneoplastic neurologic disorders may precede cancer diagnosis. Here we report the case of 75-year-old woman who presented with numbness, tingling sensation, and weakness of lower extremities, and was diagnosed with Isaacs syndrome and subsequently small-cell lung cancer. Plasmapheresis and treatment of small-cell lung cancer produced signficant symptoms improvement. We also conduct a complete review of the published case reports and case series of Isaacs syndrome of paraneoplastic etiology, which usually has good response to carbamazepine and to specfic treatment of underlying neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
Paraneoplastic Isaacs syndrome is a rare disorder with distinct clinical and electromyographic characteristics. It is a consequence of neoplastic process that is not directly caused by the tumor itself, but usually mediated by immune response primarily against the tumor and neural tissues are damaged owing to bystander effect. Paraneoplastic neurologic disorders may precede cancer diagnosis. Here we report the case of 75-year-old woman who presented with numbness, tingling sensation, and weakness of lower extremities, and was diagnosed with Isaacs syndrome and subsequently small-cell lung cancer. Plasmapheresis and treatment of small-cell lung cancer produced signficant symptoms improvement. We also conduct a complete review of the published case reports and case series of Isaacs syndrome of paraneoplastic etiology, which usually has good response to carbamazepine and to specfic treatment of underlying neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
Quality of life after surgery for pleural malignant mesothelioma – methodological considerations
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a dearth of literature on patient quality of life (QoL) after treatment for malignant pleural mesothelioma (MPM).
Objectives To review the literature on QoL after surgery for MPM and assess differences in quality of life between patients who have extrapleural pneumonectomy (EPP) and those who have pleurectomy and decortication (P-D).
Methods We retrieved and reviewed original research studies on quality of life after mesothelioma surgery. They had been published from January 1990 through June 2016, and included 15 articles and 12 datasets for a total of 523 patients.
Results QoL data was available for 102 EPP patients and 296 P-D patients. Two studies directly compared QoL outcomes between the 2 techniques. Symptoms, lung function parameters, and physical and social functioning were still compromised 6 months after surgery. However, P-D patients fared better than did EPP patients across QoL measures.
Limitations The amount of available literature is small, and the studies are heterogeneous.
Conclusions QoL is better for a longer period of time in patients who undergo P-D, compared with those who have EPP. Given the need for multimodality therapy for MPM and the aggressive nature of the disease, QoL outcomes should be strongly considered when choosing type of surgery for mesothelioma.
Click on the PDF icon at the top of this introduction to read the full article.
Social support needs among patients with advanced breast cancer: sensitivity trumps substance
Background The importance of social support for cancer patients has been established in previous studies. However, much of the existing research has identified associations between general measures of social support and various health indicators. Nevertheless, some research has begun to suggest the utility of more nuanced understandings of how patients receive and use social support.
Objective To examine the roles of nondirective (ie, support that accepts recipients’ feelings and is cooperative with their plans) and directive support (ie, support that prescribes “correct” choices and feelings) as well as social support needs and desires among patients with advanced breast cancer.
Methods We conducted semi-structured interviews (qualitative method) with 8 patients with stage IV breast cancer to collect qualitative information about the disease-related challenges they faced, the support they received from their families and medical teams, and the appropriateness of directive and nondirective support. In addition, we used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess clinically relevant cut-offs for anxiety and depression and the 16-item Social Support Inventory to assess the provision of nondirective and directive social support to the patients (quantitative method).
Results Qualitative findings suggested that there was considerable variability among patients’ reports of social support provided by family, friends, and the medical team. From the qualitative data, patients reported directive support as more useful in times of acute need and emphasized the importance of supportive systems rather than supportive persons in providing emotional support. From the quantitative data, patients reported nondirective support as more typical of support received from both family and medical teams than directive support. On the HADS, 1 patient had a score of 9 on the anxiety subscale, above the score of 7 that is for mild anxiety. No patients scored above the criterion for mild depression, also a score of 7.
Limitations Very small sample limits the ability to generalize findings.
Conclusions The right type of support for patients with advanced breast cancer is contingent on a range of variables, which suggests that the key characteristic of support may not be any particular feature, but the nuanced adjustment of its content and style of delivery to the patient’s circumstances.
Funding Peers for Progress
Click on the PDF icon at the top of this introduction to read the full article.
Background The importance of social support for cancer patients has been established in previous studies. However, much of the existing research has identified associations between general measures of social support and various health indicators. Nevertheless, some research has begun to suggest the utility of more nuanced understandings of how patients receive and use social support.
Objective To examine the roles of nondirective (ie, support that accepts recipients’ feelings and is cooperative with their plans) and directive support (ie, support that prescribes “correct” choices and feelings) as well as social support needs and desires among patients with advanced breast cancer.
Methods We conducted semi-structured interviews (qualitative method) with 8 patients with stage IV breast cancer to collect qualitative information about the disease-related challenges they faced, the support they received from their families and medical teams, and the appropriateness of directive and nondirective support. In addition, we used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess clinically relevant cut-offs for anxiety and depression and the 16-item Social Support Inventory to assess the provision of nondirective and directive social support to the patients (quantitative method).
Results Qualitative findings suggested that there was considerable variability among patients’ reports of social support provided by family, friends, and the medical team. From the qualitative data, patients reported directive support as more useful in times of acute need and emphasized the importance of supportive systems rather than supportive persons in providing emotional support. From the quantitative data, patients reported nondirective support as more typical of support received from both family and medical teams than directive support. On the HADS, 1 patient had a score of 9 on the anxiety subscale, above the score of 7 that is for mild anxiety. No patients scored above the criterion for mild depression, also a score of 7.
Limitations Very small sample limits the ability to generalize findings.
Conclusions The right type of support for patients with advanced breast cancer is contingent on a range of variables, which suggests that the key characteristic of support may not be any particular feature, but the nuanced adjustment of its content and style of delivery to the patient’s circumstances.
Funding Peers for Progress
Click on the PDF icon at the top of this introduction to read the full article.
Background The importance of social support for cancer patients has been established in previous studies. However, much of the existing research has identified associations between general measures of social support and various health indicators. Nevertheless, some research has begun to suggest the utility of more nuanced understandings of how patients receive and use social support.
Objective To examine the roles of nondirective (ie, support that accepts recipients’ feelings and is cooperative with their plans) and directive support (ie, support that prescribes “correct” choices and feelings) as well as social support needs and desires among patients with advanced breast cancer.
Methods We conducted semi-structured interviews (qualitative method) with 8 patients with stage IV breast cancer to collect qualitative information about the disease-related challenges they faced, the support they received from their families and medical teams, and the appropriateness of directive and nondirective support. In addition, we used the 14-item Hospital Anxiety and Depression Scale (HADS) to assess clinically relevant cut-offs for anxiety and depression and the 16-item Social Support Inventory to assess the provision of nondirective and directive social support to the patients (quantitative method).
Results Qualitative findings suggested that there was considerable variability among patients’ reports of social support provided by family, friends, and the medical team. From the qualitative data, patients reported directive support as more useful in times of acute need and emphasized the importance of supportive systems rather than supportive persons in providing emotional support. From the quantitative data, patients reported nondirective support as more typical of support received from both family and medical teams than directive support. On the HADS, 1 patient had a score of 9 on the anxiety subscale, above the score of 7 that is for mild anxiety. No patients scored above the criterion for mild depression, also a score of 7.
Limitations Very small sample limits the ability to generalize findings.
Conclusions The right type of support for patients with advanced breast cancer is contingent on a range of variables, which suggests that the key characteristic of support may not be any particular feature, but the nuanced adjustment of its content and style of delivery to the patient’s circumstances.
Funding Peers for Progress
Click on the PDF icon at the top of this introduction to read the full article.
Patients’ retrospective assessment of palliative chemotherapy for lung or gastrointestinal cancers
Background Decision-making about palliative chemotherapy is complex because treatment goals include increased survival, symptom control, and functional improvement.
Objective To examine whether retrospective assessment by chemotherapy-experienced patients could inform decision-making support for future patients.
Methods 51 patients with thoracic or gastrointestinal malignancy, with no further systemic treatment options, completed the Functional Assessment of Chronic Illness Therapy–Treatment Satisfaction (FACIT-TS) survey and answered free-text questions about their past decisions about therapy.
Results FACIT-TS subscale of treatment effectiveness showed 36% of 49 eligible patients rating effectiveness as being worse than expected, 25% as expected, 37% better. 51% found side effects worse than expected, 19% as expected, and 28% better than expected. Textual analysis of survey responses indicated the majority of patients stood by their decision to take chemotherapy but wished they’d had more information about what to expect. Overall, 55% found chemotherapy to have been worthwhile, 37% not, 8% were undecided.
Limitations Accrual was slower than expected, in part because of a lack of awareness by patients that there were no further chemotherapy options available to them. Selection bias may have favored enrolment from teams open to soliciting patient feedback.
Conclusions Although the majority of patients stood by their decisions about palliative chemotherapy based on their understanding of the therapy at the time of making their decisions, there is a discrepancy between initial expectations about chemotherapy and retrospective assessment of chemotherapy effectiveness and side effects. The introduction of end-of-treatment feedback surveys as a routine quality assurance procedure should be considered.
Click on the PDF icon at the top of this introduction to read the full article.
Background Decision-making about palliative chemotherapy is complex because treatment goals include increased survival, symptom control, and functional improvement.
Objective To examine whether retrospective assessment by chemotherapy-experienced patients could inform decision-making support for future patients.
Methods 51 patients with thoracic or gastrointestinal malignancy, with no further systemic treatment options, completed the Functional Assessment of Chronic Illness Therapy–Treatment Satisfaction (FACIT-TS) survey and answered free-text questions about their past decisions about therapy.
Results FACIT-TS subscale of treatment effectiveness showed 36% of 49 eligible patients rating effectiveness as being worse than expected, 25% as expected, 37% better. 51% found side effects worse than expected, 19% as expected, and 28% better than expected. Textual analysis of survey responses indicated the majority of patients stood by their decision to take chemotherapy but wished they’d had more information about what to expect. Overall, 55% found chemotherapy to have been worthwhile, 37% not, 8% were undecided.
Limitations Accrual was slower than expected, in part because of a lack of awareness by patients that there were no further chemotherapy options available to them. Selection bias may have favored enrolment from teams open to soliciting patient feedback.
Conclusions Although the majority of patients stood by their decisions about palliative chemotherapy based on their understanding of the therapy at the time of making their decisions, there is a discrepancy between initial expectations about chemotherapy and retrospective assessment of chemotherapy effectiveness and side effects. The introduction of end-of-treatment feedback surveys as a routine quality assurance procedure should be considered.
Click on the PDF icon at the top of this introduction to read the full article.
Background Decision-making about palliative chemotherapy is complex because treatment goals include increased survival, symptom control, and functional improvement.
Objective To examine whether retrospective assessment by chemotherapy-experienced patients could inform decision-making support for future patients.
Methods 51 patients with thoracic or gastrointestinal malignancy, with no further systemic treatment options, completed the Functional Assessment of Chronic Illness Therapy–Treatment Satisfaction (FACIT-TS) survey and answered free-text questions about their past decisions about therapy.
Results FACIT-TS subscale of treatment effectiveness showed 36% of 49 eligible patients rating effectiveness as being worse than expected, 25% as expected, 37% better. 51% found side effects worse than expected, 19% as expected, and 28% better than expected. Textual analysis of survey responses indicated the majority of patients stood by their decision to take chemotherapy but wished they’d had more information about what to expect. Overall, 55% found chemotherapy to have been worthwhile, 37% not, 8% were undecided.
Limitations Accrual was slower than expected, in part because of a lack of awareness by patients that there were no further chemotherapy options available to them. Selection bias may have favored enrolment from teams open to soliciting patient feedback.
Conclusions Although the majority of patients stood by their decisions about palliative chemotherapy based on their understanding of the therapy at the time of making their decisions, there is a discrepancy between initial expectations about chemotherapy and retrospective assessment of chemotherapy effectiveness and side effects. The introduction of end-of-treatment feedback surveys as a routine quality assurance procedure should be considered.
Click on the PDF icon at the top of this introduction to read the full article.
Stronger together: how to implement oncology and palliative care co-management
Outpatient palliative care is increasingly delivered through co-management, a collaborative model of care that enables palliative care clinicians and oncologists to coordinate efforts. Here, we offer a distillation of our experience with co-management at a large teaching hospital. We describe three strategies to implement co-management: a shared understanding of each subspecialty, a shared framework to help patients cultivate prognostic awareness, and a shared vision for the clinical goals. We hope that this synthesis will foster the development of co-management.
Click on the PDF icon at the top of this introduction to read the full article.
Outpatient palliative care is increasingly delivered through co-management, a collaborative model of care that enables palliative care clinicians and oncologists to coordinate efforts. Here, we offer a distillation of our experience with co-management at a large teaching hospital. We describe three strategies to implement co-management: a shared understanding of each subspecialty, a shared framework to help patients cultivate prognostic awareness, and a shared vision for the clinical goals. We hope that this synthesis will foster the development of co-management.
Click on the PDF icon at the top of this introduction to read the full article.
Outpatient palliative care is increasingly delivered through co-management, a collaborative model of care that enables palliative care clinicians and oncologists to coordinate efforts. Here, we offer a distillation of our experience with co-management at a large teaching hospital. We describe three strategies to implement co-management: a shared understanding of each subspecialty, a shared framework to help patients cultivate prognostic awareness, and a shared vision for the clinical goals. We hope that this synthesis will foster the development of co-management.
Click on the PDF icon at the top of this introduction to read the full article.
VIDEO: Improved QOL an added benefit of pembrolizumab for NSCLC patients
VIENNA – Patients with metastatic non–small-cell lung cancer with high levels of PD-L1 who received first-line pembrolizumab treatment had clinically meaningful improvement in their quality of life, compared with patients randomized to chemotherapy, in a prespecified secondary analysis of data from the drug’s pivotal trial.
This boost in quality of life as well as other measures of health status add to the pivotal trial’s primary finding of significantly increased progression-free survival compared with chemotherapy, as well as previously-reported secondary findings of superior overall survival, objective response rate, and safety with pembrolizumab compared with chemotherapy (N Engl J Med. 2016 Nov 10;375[19]:1823-33), Julie R. Brahmer, MD, said at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
The primary endpoint of the Study of Pembrolizumab Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non–Small Cell Lung Cancer (KEYNOTE-024) showed an average 4.3-month increase in progression-free survival with pembrolizumab immunotherapy, compared with a standard chemotherapy regimen.
Improved quality of life on top of improved efficacy and safety is an important added benefit from pembrolizumab that should further spur its widespread adoption as first-line treatment for approved patients, Dr. Brahmer said in a video interview.
“When you talk about improving efficacy by months, patients and physicians want to also see improved quality of life,” said Dr. Brahmer, director of thoracic oncology at the Johns Hopkins Kimmel Cancer Center in Baltimore. “If symptoms are not improved or there are a ton of side effects with the treatment then use might be low.”
Based on its performance in KEYNOTE-024, pembrolizumab (Keytruda) received Food and Drug Administration approval on Oct. 24, 2016, as first-line treatment for patients with metastatic non–small-cell lung cancer that has a tumor proportion score of at least 50% for programmed death ligand 1 (PD-L1). Pembrolizumab is a monoclonal antibody that binds and blocks PD-1, the immune-cell receptor that tumor-cell PD-L1 binds to make immune cells less active. Other new immune checkpoint inhibitor drugs that act by blocking PD-1 or PD-L1 have shown similar quality of life benefits, she noted.
Routine availability of pembrolizumab as initial treatment for patients who have tumors with this level of PD-L1 expression (and also have no EGFR or ALK genomic aberrations) is shifting practice, Dr. Brahmer said.
“It’s catching on. The limitation right now is making sure patients get tested” for their PD-L1 tumor proportion score at the time they are first diagnosed. “Medical oncologists need to educate pathologists that we need this testing automatically, upfront. It’s not there yet,” she said.
Patients also are enthused. “There is a lot of chemo-exhaustion among patients. They are looking for something different, and something that uses their immune system makes sense.” But only about one quarter of patients have tumors with this level of PD-L1 expression; the others must start chemotherapy first before trying immunotherapy, unless they have an EGFR mutation. Out-of-pocket cost for pembrolizumab is also a major issues for many patients, she said.
KEYNOTE-024 randomized 305 patients at 102 international sites and followed patients for a median of 11 months. Dr. Brahmer and her associates made two primary analyses of patient-reported outcomes. One was measurement of global health status at 15 weeks after the start of treatment using the European Organization for the Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire designed to assess quality of life. Weighted averaging of the EORTC QCQ-C30 scores showed a mean improvement of 7.8 points (P = .002) in the pembrolizumab patients compared with the chemotherapy patients, a difference Dr. Brahmer called “clinically meaningful” as well as statistically significant.
A second analysis of patient-reported outcomes used a second EORTC instrument, the QLC-LC13, which combines assessment of cough, chest pain, and dyspnea. Treatment with pembrolizumab significantly reduced the time to deterioration as measured by this questionnaire by a relative 34%, (P = .029).
A third analysis looked at 15 individual function or symptom domains that make up the QLQ-C30. In general, these showed more improvements with pembrolizumab than with chemotherapy. One notable subcategory was fatigue, which showed significant improvement with pembrolizumab compared with a small worsening with chemotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
This is a very nice analysis using a well-validated group of instruments to assess quality of life. The researchers also achieved a high level of compliance, with 79%-85% of patients completing the quality-of life questionnaire at 15 weeks, when the primary measure of health status occurred.
The mean difference of the weighted global health status score of 7.8 points between the pembrolizumab and chemotherapy patients was a little less than a minimally important difference, but in the context of a randomized, controlled trial this difference probably tells us that there is health status improvement in the pembrolizumab patients. In addition, the individual symptom and function domains showed that in general pembrolizumab performed better than chemotherapy.
Michael Boyer, MD , is professor of medicine at the University of Sydney and a thoracic oncologist and chief clinical officer of the Chris O’Brien Lifehouse in Sydney. He has received research support from Merck and from Pfizer, Roche, Eli Lilly, BMS, AstraZeneca, and Clovis. He made these comments as designated discussant for the report.
This is a very nice analysis using a well-validated group of instruments to assess quality of life. The researchers also achieved a high level of compliance, with 79%-85% of patients completing the quality-of life questionnaire at 15 weeks, when the primary measure of health status occurred.
The mean difference of the weighted global health status score of 7.8 points between the pembrolizumab and chemotherapy patients was a little less than a minimally important difference, but in the context of a randomized, controlled trial this difference probably tells us that there is health status improvement in the pembrolizumab patients. In addition, the individual symptom and function domains showed that in general pembrolizumab performed better than chemotherapy.
Michael Boyer, MD , is professor of medicine at the University of Sydney and a thoracic oncologist and chief clinical officer of the Chris O’Brien Lifehouse in Sydney. He has received research support from Merck and from Pfizer, Roche, Eli Lilly, BMS, AstraZeneca, and Clovis. He made these comments as designated discussant for the report.
This is a very nice analysis using a well-validated group of instruments to assess quality of life. The researchers also achieved a high level of compliance, with 79%-85% of patients completing the quality-of life questionnaire at 15 weeks, when the primary measure of health status occurred.
The mean difference of the weighted global health status score of 7.8 points between the pembrolizumab and chemotherapy patients was a little less than a minimally important difference, but in the context of a randomized, controlled trial this difference probably tells us that there is health status improvement in the pembrolizumab patients. In addition, the individual symptom and function domains showed that in general pembrolizumab performed better than chemotherapy.
Michael Boyer, MD , is professor of medicine at the University of Sydney and a thoracic oncologist and chief clinical officer of the Chris O’Brien Lifehouse in Sydney. He has received research support from Merck and from Pfizer, Roche, Eli Lilly, BMS, AstraZeneca, and Clovis. He made these comments as designated discussant for the report.
VIENNA – Patients with metastatic non–small-cell lung cancer with high levels of PD-L1 who received first-line pembrolizumab treatment had clinically meaningful improvement in their quality of life, compared with patients randomized to chemotherapy, in a prespecified secondary analysis of data from the drug’s pivotal trial.
This boost in quality of life as well as other measures of health status add to the pivotal trial’s primary finding of significantly increased progression-free survival compared with chemotherapy, as well as previously-reported secondary findings of superior overall survival, objective response rate, and safety with pembrolizumab compared with chemotherapy (N Engl J Med. 2016 Nov 10;375[19]:1823-33), Julie R. Brahmer, MD, said at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
The primary endpoint of the Study of Pembrolizumab Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non–Small Cell Lung Cancer (KEYNOTE-024) showed an average 4.3-month increase in progression-free survival with pembrolizumab immunotherapy, compared with a standard chemotherapy regimen.
Improved quality of life on top of improved efficacy and safety is an important added benefit from pembrolizumab that should further spur its widespread adoption as first-line treatment for approved patients, Dr. Brahmer said in a video interview.
“When you talk about improving efficacy by months, patients and physicians want to also see improved quality of life,” said Dr. Brahmer, director of thoracic oncology at the Johns Hopkins Kimmel Cancer Center in Baltimore. “If symptoms are not improved or there are a ton of side effects with the treatment then use might be low.”
Based on its performance in KEYNOTE-024, pembrolizumab (Keytruda) received Food and Drug Administration approval on Oct. 24, 2016, as first-line treatment for patients with metastatic non–small-cell lung cancer that has a tumor proportion score of at least 50% for programmed death ligand 1 (PD-L1). Pembrolizumab is a monoclonal antibody that binds and blocks PD-1, the immune-cell receptor that tumor-cell PD-L1 binds to make immune cells less active. Other new immune checkpoint inhibitor drugs that act by blocking PD-1 or PD-L1 have shown similar quality of life benefits, she noted.
Routine availability of pembrolizumab as initial treatment for patients who have tumors with this level of PD-L1 expression (and also have no EGFR or ALK genomic aberrations) is shifting practice, Dr. Brahmer said.
“It’s catching on. The limitation right now is making sure patients get tested” for their PD-L1 tumor proportion score at the time they are first diagnosed. “Medical oncologists need to educate pathologists that we need this testing automatically, upfront. It’s not there yet,” she said.
Patients also are enthused. “There is a lot of chemo-exhaustion among patients. They are looking for something different, and something that uses their immune system makes sense.” But only about one quarter of patients have tumors with this level of PD-L1 expression; the others must start chemotherapy first before trying immunotherapy, unless they have an EGFR mutation. Out-of-pocket cost for pembrolizumab is also a major issues for many patients, she said.
KEYNOTE-024 randomized 305 patients at 102 international sites and followed patients for a median of 11 months. Dr. Brahmer and her associates made two primary analyses of patient-reported outcomes. One was measurement of global health status at 15 weeks after the start of treatment using the European Organization for the Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire designed to assess quality of life. Weighted averaging of the EORTC QCQ-C30 scores showed a mean improvement of 7.8 points (P = .002) in the pembrolizumab patients compared with the chemotherapy patients, a difference Dr. Brahmer called “clinically meaningful” as well as statistically significant.
A second analysis of patient-reported outcomes used a second EORTC instrument, the QLC-LC13, which combines assessment of cough, chest pain, and dyspnea. Treatment with pembrolizumab significantly reduced the time to deterioration as measured by this questionnaire by a relative 34%, (P = .029).
A third analysis looked at 15 individual function or symptom domains that make up the QLQ-C30. In general, these showed more improvements with pembrolizumab than with chemotherapy. One notable subcategory was fatigue, which showed significant improvement with pembrolizumab compared with a small worsening with chemotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
VIENNA – Patients with metastatic non–small-cell lung cancer with high levels of PD-L1 who received first-line pembrolizumab treatment had clinically meaningful improvement in their quality of life, compared with patients randomized to chemotherapy, in a prespecified secondary analysis of data from the drug’s pivotal trial.
This boost in quality of life as well as other measures of health status add to the pivotal trial’s primary finding of significantly increased progression-free survival compared with chemotherapy, as well as previously-reported secondary findings of superior overall survival, objective response rate, and safety with pembrolizumab compared with chemotherapy (N Engl J Med. 2016 Nov 10;375[19]:1823-33), Julie R. Brahmer, MD, said at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
The primary endpoint of the Study of Pembrolizumab Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non–Small Cell Lung Cancer (KEYNOTE-024) showed an average 4.3-month increase in progression-free survival with pembrolizumab immunotherapy, compared with a standard chemotherapy regimen.
Improved quality of life on top of improved efficacy and safety is an important added benefit from pembrolizumab that should further spur its widespread adoption as first-line treatment for approved patients, Dr. Brahmer said in a video interview.
“When you talk about improving efficacy by months, patients and physicians want to also see improved quality of life,” said Dr. Brahmer, director of thoracic oncology at the Johns Hopkins Kimmel Cancer Center in Baltimore. “If symptoms are not improved or there are a ton of side effects with the treatment then use might be low.”
Based on its performance in KEYNOTE-024, pembrolizumab (Keytruda) received Food and Drug Administration approval on Oct. 24, 2016, as first-line treatment for patients with metastatic non–small-cell lung cancer that has a tumor proportion score of at least 50% for programmed death ligand 1 (PD-L1). Pembrolizumab is a monoclonal antibody that binds and blocks PD-1, the immune-cell receptor that tumor-cell PD-L1 binds to make immune cells less active. Other new immune checkpoint inhibitor drugs that act by blocking PD-1 or PD-L1 have shown similar quality of life benefits, she noted.
Routine availability of pembrolizumab as initial treatment for patients who have tumors with this level of PD-L1 expression (and also have no EGFR or ALK genomic aberrations) is shifting practice, Dr. Brahmer said.
“It’s catching on. The limitation right now is making sure patients get tested” for their PD-L1 tumor proportion score at the time they are first diagnosed. “Medical oncologists need to educate pathologists that we need this testing automatically, upfront. It’s not there yet,” she said.
Patients also are enthused. “There is a lot of chemo-exhaustion among patients. They are looking for something different, and something that uses their immune system makes sense.” But only about one quarter of patients have tumors with this level of PD-L1 expression; the others must start chemotherapy first before trying immunotherapy, unless they have an EGFR mutation. Out-of-pocket cost for pembrolizumab is also a major issues for many patients, she said.
KEYNOTE-024 randomized 305 patients at 102 international sites and followed patients for a median of 11 months. Dr. Brahmer and her associates made two primary analyses of patient-reported outcomes. One was measurement of global health status at 15 weeks after the start of treatment using the European Organization for the Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire designed to assess quality of life. Weighted averaging of the EORTC QCQ-C30 scores showed a mean improvement of 7.8 points (P = .002) in the pembrolizumab patients compared with the chemotherapy patients, a difference Dr. Brahmer called “clinically meaningful” as well as statistically significant.
A second analysis of patient-reported outcomes used a second EORTC instrument, the QLC-LC13, which combines assessment of cough, chest pain, and dyspnea. Treatment with pembrolizumab significantly reduced the time to deterioration as measured by this questionnaire by a relative 34%, (P = .029).
A third analysis looked at 15 individual function or symptom domains that make up the QLQ-C30. In general, these showed more improvements with pembrolizumab than with chemotherapy. One notable subcategory was fatigue, which showed significant improvement with pembrolizumab compared with a small worsening with chemotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT WCLC 2016
Key clinical point:
Major finding: The weighted average change from baseline in QLQ-C30 was 7.8 points higher in pembrolizumab patients compared with chemotherapy patients.
Data source: KEYNOTE-024, a multicenter, international randomized trial comprising 305 patients.
Disclosures: Merck, which markets pembrolizumab (Keytruda), sponsored KEYNOTE-024. Dr. Brahmer has served on an advisory board for Merck.
Cooling device reduces breast cancer–related alopecia during chemotherapy
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
AT SABCS 2016
Key clinical point:
Major finding: 50.5% of treated patients experienced no more than grade 1 alopecia (less than 50% hair loss), compared with 0% of control subjects.
Data source: The prospective, randomized SCALP trial involving 182 women.
Disclosures: Dr. Nangia reported receiving research funding from Paxman, sponsor of the study, to her institution.