User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
Long-term opioid use substantial in elderly adults prior to total joint replacement
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Long-term opioid use is highly prevalent among older adults with osteoarthritis who underwent total joint replacement.
Major finding: Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama.
Study details: An observational cohort study including 358,121 Medicare enrollees with advanced osteoarthritis.
Disclosures: Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Coauthors provided disclosures related to a number of pharmaceutical companies.
Source: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
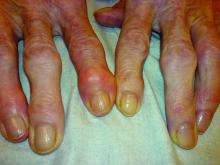
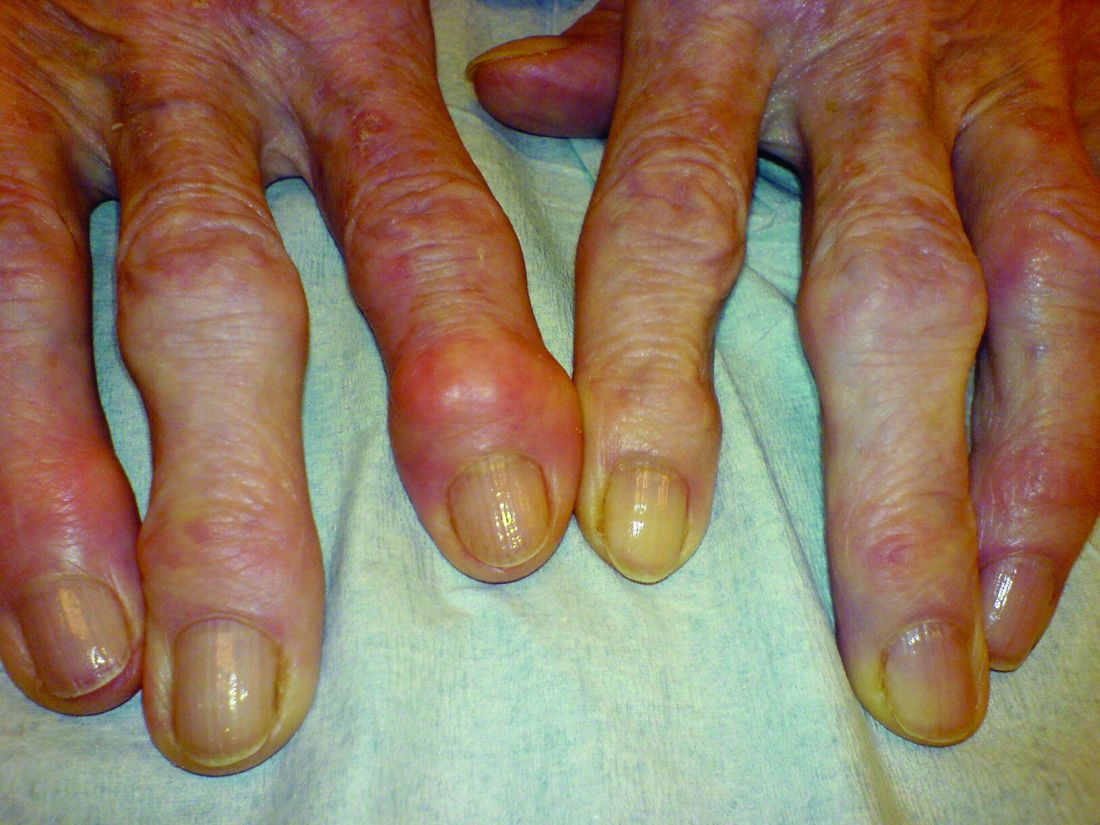
Heberden’s nodes linked to knee OA progression
according to a review of 575 participants in a substudy of the Osteoarthritis Initiative cohort.
After assessing Heberden’s nodes (HNs) – bony enlargements of the last finger joint – and knee MRI findings at baseline and 24 months, the investigators found that HNs were associated with periarticular bone area expansion in the knee. The investigators reported their findings in Arthritis & Rheumatology.
Comparing the 395 subjects with HNs with the 180 without, there was more periarticular bone area expansion among HN patients at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83), especially in the medial femur (aOR, 1.49; 95% CI, 1.05-2.13), lateral femur (aOR, 2.51; 95% CI, 1.58-3.97), femoral notch (aOR, 1.37; 95% CI, 1.02-1.84), and lateral trochlea (aOR, 1.44; 95% CI, 1.08-1.9). The comparisons were adjusted for age, sex, body mass index, and bone remodeling agent use.
“The presence of Heberden’s nodes in a physical examination is associated with a distinct pattern of worsening of osteoarthritis-related structural damage in the knee joint,” lead investigator Arya Haj-Mirzaian, MD, a radiologist and postdoctoral fellow at Johns Hopkins University, Baltimore, said in a press release.
However, HNs were also associated with less worsening of knee osteophytes, especially at the femoral end of the knee joint (aOR, 0.54; 95% CI, 0.31-0.95); the finding seemed to contradict the overall picture of worsening knee osteoarthritis with HNs.
“Although osteophytes are thought to be a late secondary sequel or compensatory repair mechanism in OA and indicator of advanced knee OA, less worsening in osteophytes’ score ... may propose that less ossification is involved in the pathophysiology of knee OA in the presence of HNs,” the investigators wrote. It’s a subject for future research.
Patients with HNs were older, more often female, and had a lower frequency for other knee OA risk factors, such as excessive body mass index and knee injury. Patients with gout were excluded.
There was no external funding, and the investigators reported no disclosures.
SOURCE: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
according to a review of 575 participants in a substudy of the Osteoarthritis Initiative cohort.
After assessing Heberden’s nodes (HNs) – bony enlargements of the last finger joint – and knee MRI findings at baseline and 24 months, the investigators found that HNs were associated with periarticular bone area expansion in the knee. The investigators reported their findings in Arthritis & Rheumatology.
Comparing the 395 subjects with HNs with the 180 without, there was more periarticular bone area expansion among HN patients at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83), especially in the medial femur (aOR, 1.49; 95% CI, 1.05-2.13), lateral femur (aOR, 2.51; 95% CI, 1.58-3.97), femoral notch (aOR, 1.37; 95% CI, 1.02-1.84), and lateral trochlea (aOR, 1.44; 95% CI, 1.08-1.9). The comparisons were adjusted for age, sex, body mass index, and bone remodeling agent use.
“The presence of Heberden’s nodes in a physical examination is associated with a distinct pattern of worsening of osteoarthritis-related structural damage in the knee joint,” lead investigator Arya Haj-Mirzaian, MD, a radiologist and postdoctoral fellow at Johns Hopkins University, Baltimore, said in a press release.
However, HNs were also associated with less worsening of knee osteophytes, especially at the femoral end of the knee joint (aOR, 0.54; 95% CI, 0.31-0.95); the finding seemed to contradict the overall picture of worsening knee osteoarthritis with HNs.
“Although osteophytes are thought to be a late secondary sequel or compensatory repair mechanism in OA and indicator of advanced knee OA, less worsening in osteophytes’ score ... may propose that less ossification is involved in the pathophysiology of knee OA in the presence of HNs,” the investigators wrote. It’s a subject for future research.
Patients with HNs were older, more often female, and had a lower frequency for other knee OA risk factors, such as excessive body mass index and knee injury. Patients with gout were excluded.
There was no external funding, and the investigators reported no disclosures.
SOURCE: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
according to a review of 575 participants in a substudy of the Osteoarthritis Initiative cohort.
After assessing Heberden’s nodes (HNs) – bony enlargements of the last finger joint – and knee MRI findings at baseline and 24 months, the investigators found that HNs were associated with periarticular bone area expansion in the knee. The investigators reported their findings in Arthritis & Rheumatology.
Comparing the 395 subjects with HNs with the 180 without, there was more periarticular bone area expansion among HN patients at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83), especially in the medial femur (aOR, 1.49; 95% CI, 1.05-2.13), lateral femur (aOR, 2.51; 95% CI, 1.58-3.97), femoral notch (aOR, 1.37; 95% CI, 1.02-1.84), and lateral trochlea (aOR, 1.44; 95% CI, 1.08-1.9). The comparisons were adjusted for age, sex, body mass index, and bone remodeling agent use.
“The presence of Heberden’s nodes in a physical examination is associated with a distinct pattern of worsening of osteoarthritis-related structural damage in the knee joint,” lead investigator Arya Haj-Mirzaian, MD, a radiologist and postdoctoral fellow at Johns Hopkins University, Baltimore, said in a press release.
However, HNs were also associated with less worsening of knee osteophytes, especially at the femoral end of the knee joint (aOR, 0.54; 95% CI, 0.31-0.95); the finding seemed to contradict the overall picture of worsening knee osteoarthritis with HNs.
“Although osteophytes are thought to be a late secondary sequel or compensatory repair mechanism in OA and indicator of advanced knee OA, less worsening in osteophytes’ score ... may propose that less ossification is involved in the pathophysiology of knee OA in the presence of HNs,” the investigators wrote. It’s a subject for future research.
Patients with HNs were older, more often female, and had a lower frequency for other knee OA risk factors, such as excessive body mass index and knee injury. Patients with gout were excluded.
There was no external funding, and the investigators reported no disclosures.
SOURCE: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Heberden’s nodes may be an indicator of knee OA progression.
Major finding: There was more periarticular bone area expansion among patients with Heberden’s nodes at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83).
Study details: A substudy of 575 participants in the Osteoarthritis Initiative cohort
Disclosures: There was no external funding, and the investigators reported no disclosures.
Source: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
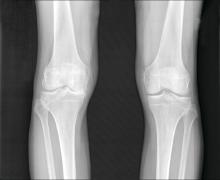
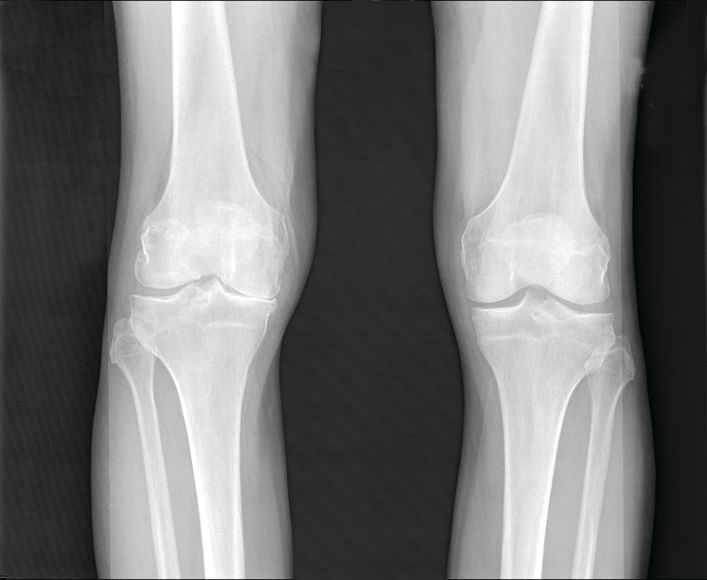
Knee pathologies, including multiple meniscal tears, predict accelerated OA
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: One year before the knee OA index visit, more than 75% of patients with accelerated knee OA had meniscal damage in at least two regions.
Study details: The data come from 375 adults with typical knee OA, accelerated knee OA, or no knee OA in the longitudinal Osteoarthritis Initiative cohort study.
Disclosures: The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
Source: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Canakinumab reduces arthroplasty rates
CHICAGO – Canakinumab, a human monoclonal antibody targeting interleukin-1 beta, was associated with an eye-popping 45% relative risk reduction in the rate of total knee or hip replacement in a prespecified secondary analysis of the landmark CANTOS trial, Matthias Schieker, MD, reported at the annual meeting of the American College of Rheumatology.
For the broader composite endpoint of all osteoarthritis-related adverse events, including new-onset OA or worsening of symptoms in those with OA at baseline, the relative risk reduction was 23% in patients randomized to canakinumab rather than placebo. For CANTOS participants who already had OA at baseline, the relative risk reduction was 31%, according to Dr. Schieker, who is head of the joint, bone, and tendon disease group at the Novartis Institute for Biomedical Research in Basel, Switzerland, and professor of regenerative medicine at the University of Munich.
CANTOS (the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was designed as a massive phase 3 secondary cardiovascular prevention trial. It included 10,061 patients with a history of acute MI and an elevated high-sensitivity C-reactive protein (hsCRP) level of 2 mg/L or more who were randomized double blind to subcutaneous canakinumab at 50, 150, or 300 mg or placebo given once every 3 months. During a median 3.7 years of prospective follow-up, patients in the 150-mg group had a highly significant 17% reduction relative to placebo in the risk of the composite efficacy endpoint comprising cardiovascular death, MI, stroke, or hospitalization for unstable angina resulting in urgent coronary revascularization (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Since this result was achieved with a 39% reduction in CRP, compared with placebo, and involved no lipid-lowering effect, it was hailed in the cardiology world as the long-awaited proof of the inflammatory hypothesis of atherosclerotic cardiovascular disease.
CANTOS has proved to be the gift that keeps on giving. Secondary analyses of the study data have found statistically significant reductions in the incidence of and mortality caused by lung cancer in the coronary disease patients on canakinumab, as well as a decreased risk of developing gout. Moreover, the CANTOS investigators, well aware that there are no approved therapies to prevent disease progression in OA, had the foresight to prospectively collect data on OA-related symptoms and outcomes.
At baseline, 15.6% of CANTOS participants had a history of OA. During follow-up, patients in that subgroup had a 3.4% incidence of total knee replacement or total hip replacement if they had been assigned to canakinumab, compared with a 6.3% incidence if they got placebo. In the full 10,000-plus CANTOS cohort, the arthroplasty rates were 0.8% and 1.4%, respectively.
The combined rate of OA-related adverse events in the full CANTOS cohort was 5.4% with canakinumab and 7.0% with placebo. In the subgroup with baseline OA, the rates were 14.5% and 20.8%.
Canakinumab is marketed by Novartis as Ilaris and is already approved for cryopyrin-associated periodic syndromes, familial Mediterranean fever, juvenile idiopathic arthritis, and other rare autoimmune inflammatory diseases. Based upon the positive primary outcomes of the CANTOS trial, Novartis applied to the Food and Drug Administration for a major expanded indication of the IL-1B inhibitor for cardiovascular risk reduction. However, the regulatory agency has turned down that bid.
Although the CANTOS OA-related outcomes data caused quite a stir at the meeting, Dr. Schieker said in an interview that the impressive findings didn’t really come as a surprise to him.
“I think everyone in the field has assumed that IL-1 plays a role in OA. That idea has been around for quite a long time, but until now no effects could be shown in OA. We were lucky to have an enriched population with elevated hsCRP that was so large and followed for so long that we could finally show these relative risk reductions,” he explained.
SOURCE: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
CHICAGO – Canakinumab, a human monoclonal antibody targeting interleukin-1 beta, was associated with an eye-popping 45% relative risk reduction in the rate of total knee or hip replacement in a prespecified secondary analysis of the landmark CANTOS trial, Matthias Schieker, MD, reported at the annual meeting of the American College of Rheumatology.
For the broader composite endpoint of all osteoarthritis-related adverse events, including new-onset OA or worsening of symptoms in those with OA at baseline, the relative risk reduction was 23% in patients randomized to canakinumab rather than placebo. For CANTOS participants who already had OA at baseline, the relative risk reduction was 31%, according to Dr. Schieker, who is head of the joint, bone, and tendon disease group at the Novartis Institute for Biomedical Research in Basel, Switzerland, and professor of regenerative medicine at the University of Munich.
CANTOS (the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was designed as a massive phase 3 secondary cardiovascular prevention trial. It included 10,061 patients with a history of acute MI and an elevated high-sensitivity C-reactive protein (hsCRP) level of 2 mg/L or more who were randomized double blind to subcutaneous canakinumab at 50, 150, or 300 mg or placebo given once every 3 months. During a median 3.7 years of prospective follow-up, patients in the 150-mg group had a highly significant 17% reduction relative to placebo in the risk of the composite efficacy endpoint comprising cardiovascular death, MI, stroke, or hospitalization for unstable angina resulting in urgent coronary revascularization (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Since this result was achieved with a 39% reduction in CRP, compared with placebo, and involved no lipid-lowering effect, it was hailed in the cardiology world as the long-awaited proof of the inflammatory hypothesis of atherosclerotic cardiovascular disease.
CANTOS has proved to be the gift that keeps on giving. Secondary analyses of the study data have found statistically significant reductions in the incidence of and mortality caused by lung cancer in the coronary disease patients on canakinumab, as well as a decreased risk of developing gout. Moreover, the CANTOS investigators, well aware that there are no approved therapies to prevent disease progression in OA, had the foresight to prospectively collect data on OA-related symptoms and outcomes.
At baseline, 15.6% of CANTOS participants had a history of OA. During follow-up, patients in that subgroup had a 3.4% incidence of total knee replacement or total hip replacement if they had been assigned to canakinumab, compared with a 6.3% incidence if they got placebo. In the full 10,000-plus CANTOS cohort, the arthroplasty rates were 0.8% and 1.4%, respectively.
The combined rate of OA-related adverse events in the full CANTOS cohort was 5.4% with canakinumab and 7.0% with placebo. In the subgroup with baseline OA, the rates were 14.5% and 20.8%.
Canakinumab is marketed by Novartis as Ilaris and is already approved for cryopyrin-associated periodic syndromes, familial Mediterranean fever, juvenile idiopathic arthritis, and other rare autoimmune inflammatory diseases. Based upon the positive primary outcomes of the CANTOS trial, Novartis applied to the Food and Drug Administration for a major expanded indication of the IL-1B inhibitor for cardiovascular risk reduction. However, the regulatory agency has turned down that bid.
Although the CANTOS OA-related outcomes data caused quite a stir at the meeting, Dr. Schieker said in an interview that the impressive findings didn’t really come as a surprise to him.
“I think everyone in the field has assumed that IL-1 plays a role in OA. That idea has been around for quite a long time, but until now no effects could be shown in OA. We were lucky to have an enriched population with elevated hsCRP that was so large and followed for so long that we could finally show these relative risk reductions,” he explained.
SOURCE: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
CHICAGO – Canakinumab, a human monoclonal antibody targeting interleukin-1 beta, was associated with an eye-popping 45% relative risk reduction in the rate of total knee or hip replacement in a prespecified secondary analysis of the landmark CANTOS trial, Matthias Schieker, MD, reported at the annual meeting of the American College of Rheumatology.
For the broader composite endpoint of all osteoarthritis-related adverse events, including new-onset OA or worsening of symptoms in those with OA at baseline, the relative risk reduction was 23% in patients randomized to canakinumab rather than placebo. For CANTOS participants who already had OA at baseline, the relative risk reduction was 31%, according to Dr. Schieker, who is head of the joint, bone, and tendon disease group at the Novartis Institute for Biomedical Research in Basel, Switzerland, and professor of regenerative medicine at the University of Munich.
CANTOS (the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) was designed as a massive phase 3 secondary cardiovascular prevention trial. It included 10,061 patients with a history of acute MI and an elevated high-sensitivity C-reactive protein (hsCRP) level of 2 mg/L or more who were randomized double blind to subcutaneous canakinumab at 50, 150, or 300 mg or placebo given once every 3 months. During a median 3.7 years of prospective follow-up, patients in the 150-mg group had a highly significant 17% reduction relative to placebo in the risk of the composite efficacy endpoint comprising cardiovascular death, MI, stroke, or hospitalization for unstable angina resulting in urgent coronary revascularization (N Engl J Med. 2017 Sep 21;377[12]:1119-31).
Since this result was achieved with a 39% reduction in CRP, compared with placebo, and involved no lipid-lowering effect, it was hailed in the cardiology world as the long-awaited proof of the inflammatory hypothesis of atherosclerotic cardiovascular disease.
CANTOS has proved to be the gift that keeps on giving. Secondary analyses of the study data have found statistically significant reductions in the incidence of and mortality caused by lung cancer in the coronary disease patients on canakinumab, as well as a decreased risk of developing gout. Moreover, the CANTOS investigators, well aware that there are no approved therapies to prevent disease progression in OA, had the foresight to prospectively collect data on OA-related symptoms and outcomes.
At baseline, 15.6% of CANTOS participants had a history of OA. During follow-up, patients in that subgroup had a 3.4% incidence of total knee replacement or total hip replacement if they had been assigned to canakinumab, compared with a 6.3% incidence if they got placebo. In the full 10,000-plus CANTOS cohort, the arthroplasty rates were 0.8% and 1.4%, respectively.
The combined rate of OA-related adverse events in the full CANTOS cohort was 5.4% with canakinumab and 7.0% with placebo. In the subgroup with baseline OA, the rates were 14.5% and 20.8%.
Canakinumab is marketed by Novartis as Ilaris and is already approved for cryopyrin-associated periodic syndromes, familial Mediterranean fever, juvenile idiopathic arthritis, and other rare autoimmune inflammatory diseases. Based upon the positive primary outcomes of the CANTOS trial, Novartis applied to the Food and Drug Administration for a major expanded indication of the IL-1B inhibitor for cardiovascular risk reduction. However, the regulatory agency has turned down that bid.
Although the CANTOS OA-related outcomes data caused quite a stir at the meeting, Dr. Schieker said in an interview that the impressive findings didn’t really come as a surprise to him.
“I think everyone in the field has assumed that IL-1 plays a role in OA. That idea has been around for quite a long time, but until now no effects could be shown in OA. We were lucky to have an enriched population with elevated hsCRP that was so large and followed for so long that we could finally show these relative risk reductions,” he explained.
SOURCE: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Patients on the IL-1B inhibitor canakinumab for secondary cardiovascular prevention also experienced a 45% risk reduction in total knee or total hip replacement, compared with placebo.
Study details: This was a prespecified secondary analysis of OA-related outcomes in the 10,061 participants in the randomized, double-blind CANTOS trial.
Disclosures: The presenter is an employee of Novartis, which markets canakinumab and sponsored CANTOS.
Source: Schieker M et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 445.
A case of cold burn reported with whole-body cryotherapy
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
FROM JAAD CASE REPORTS
Intra-articular Wnt inhibitor for knee OA sails through phase 2
CHICAGO – A single intra-articular injection of a novel drug known for now as SMO4690 resulted in statistically significant and clinically meaningful improvements in pain and physical function through 6 months of follow-up in a phase 2b study including 695 patients with moderately to severely symptomatic knee OA, Yusuf Yazici, MD, reported at the annual meeting of the American College of Rheumatology.
Based on the encouraging results of this and another large phase 2 study, two pivotal phase 3, randomized clinical trials are due to start in the spring of 2019. If the results are positive, SMO4690 could become the first approved disease-modifying OA drug (DMOAD), something that’s been a long-sought, high-priority goal in rheumatology, noted Dr. Yazici, chief medical officer at San Diego–based Samumed, which is developing the drug, and a rheumatologist at New York University.
SMO4690 is a small molecule inhibitor of the Wnt signaling pathway. The drug has two distinct mechanisms of action for treatment of knee OA: It has an anti-inflammatory effect and it protects cartilage from degeneration, as demonstrated by a clinically significant improvement in joint space width by x-ray, compared with placebo at 12 months of follow-up after a single baseline injection in the earlier 455-patient, phase 2 study. Also, animal studies suggest SMO4690 generates cartilage, an exciting possibility now being evaluated in two ongoing serial MRI studies in knee OA patients.
Dr. Yazici presented patient-reported outcomes from the 695-patient, 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 2b study, which evaluated four different concentrations of SMO4690. The 0.07- and 0.23-mg per 2-mL injection doses proved significantly better than placebo at 12 weeks – the primary endpoint – for all patient-reported outcomes.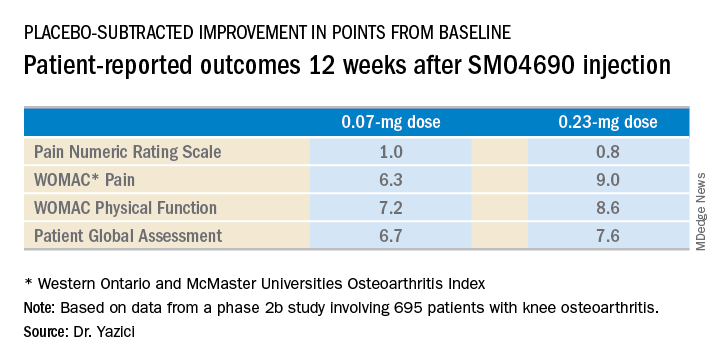
The 0.23-mg dose of SMO4690 remained superior to placebo for all four patient-reported outcomes at subsequent assessments at weeks 16, 20, and 24. The 0.07-mg dose remained significantly better than placebo through week 20.
As in the earlier phase 2 study, SMO4690 raised no significant safety concerns. Adverse events were similar in type and frequency in the active-treatment and placebo groups.
The study was sponsored by Samumed, where Dr. Yazici is employed as chief medical officer.
SOURCE: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
CHICAGO – A single intra-articular injection of a novel drug known for now as SMO4690 resulted in statistically significant and clinically meaningful improvements in pain and physical function through 6 months of follow-up in a phase 2b study including 695 patients with moderately to severely symptomatic knee OA, Yusuf Yazici, MD, reported at the annual meeting of the American College of Rheumatology.
Based on the encouraging results of this and another large phase 2 study, two pivotal phase 3, randomized clinical trials are due to start in the spring of 2019. If the results are positive, SMO4690 could become the first approved disease-modifying OA drug (DMOAD), something that’s been a long-sought, high-priority goal in rheumatology, noted Dr. Yazici, chief medical officer at San Diego–based Samumed, which is developing the drug, and a rheumatologist at New York University.
SMO4690 is a small molecule inhibitor of the Wnt signaling pathway. The drug has two distinct mechanisms of action for treatment of knee OA: It has an anti-inflammatory effect and it protects cartilage from degeneration, as demonstrated by a clinically significant improvement in joint space width by x-ray, compared with placebo at 12 months of follow-up after a single baseline injection in the earlier 455-patient, phase 2 study. Also, animal studies suggest SMO4690 generates cartilage, an exciting possibility now being evaluated in two ongoing serial MRI studies in knee OA patients.
Dr. Yazici presented patient-reported outcomes from the 695-patient, 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 2b study, which evaluated four different concentrations of SMO4690. The 0.07- and 0.23-mg per 2-mL injection doses proved significantly better than placebo at 12 weeks – the primary endpoint – for all patient-reported outcomes.
The 0.23-mg dose of SMO4690 remained superior to placebo for all four patient-reported outcomes at subsequent assessments at weeks 16, 20, and 24. The 0.07-mg dose remained significantly better than placebo through week 20.
As in the earlier phase 2 study, SMO4690 raised no significant safety concerns. Adverse events were similar in type and frequency in the active-treatment and placebo groups.
The study was sponsored by Samumed, where Dr. Yazici is employed as chief medical officer.
SOURCE: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
CHICAGO – A single intra-articular injection of a novel drug known for now as SMO4690 resulted in statistically significant and clinically meaningful improvements in pain and physical function through 6 months of follow-up in a phase 2b study including 695 patients with moderately to severely symptomatic knee OA, Yusuf Yazici, MD, reported at the annual meeting of the American College of Rheumatology.
Based on the encouraging results of this and another large phase 2 study, two pivotal phase 3, randomized clinical trials are due to start in the spring of 2019. If the results are positive, SMO4690 could become the first approved disease-modifying OA drug (DMOAD), something that’s been a long-sought, high-priority goal in rheumatology, noted Dr. Yazici, chief medical officer at San Diego–based Samumed, which is developing the drug, and a rheumatologist at New York University.
SMO4690 is a small molecule inhibitor of the Wnt signaling pathway. The drug has two distinct mechanisms of action for treatment of knee OA: It has an anti-inflammatory effect and it protects cartilage from degeneration, as demonstrated by a clinically significant improvement in joint space width by x-ray, compared with placebo at 12 months of follow-up after a single baseline injection in the earlier 455-patient, phase 2 study. Also, animal studies suggest SMO4690 generates cartilage, an exciting possibility now being evaluated in two ongoing serial MRI studies in knee OA patients.
Dr. Yazici presented patient-reported outcomes from the 695-patient, 24-week, multicenter, randomized, placebo-controlled, double-blind, phase 2b study, which evaluated four different concentrations of SMO4690. The 0.07- and 0.23-mg per 2-mL injection doses proved significantly better than placebo at 12 weeks – the primary endpoint – for all patient-reported outcomes.
The 0.23-mg dose of SMO4690 remained superior to placebo for all four patient-reported outcomes at subsequent assessments at weeks 16, 20, and 24. The 0.07-mg dose remained significantly better than placebo through week 20.
As in the earlier phase 2 study, SMO4690 raised no significant safety concerns. Adverse events were similar in type and frequency in the active-treatment and placebo groups.
The study was sponsored by Samumed, where Dr. Yazici is employed as chief medical officer.
SOURCE: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The 0.23-mg dose of SMO4690 proved superior to placebo for four key patient-reported outcomes.
Study details: This was a 24-week, randomized, multicenter, placebo-controlled, double-blind, phase 2b study in 695 patients with moderately to severely symptomatic knee OA.
Disclosures: The presenter is chief medical officer at Samumed, the study sponsor.
Source: Yazici Y et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract L03.
Nerve growth factor antibody cuts OA pain with low AEs
CHICAGO – The nerve growth factor antibody tanezumab met its primary efficacy endpoints for improving pain and physical function in patients with knee or hip OA while also showing a low incidence of the drug’s most concerning adverse effect in the first results from a phase 3 study of a drug in this new class.
The placebo-controlled, multicenter study enrolled 696 U.S. OA patients with moderate to severe knee or hip pain and showed that two subcutaneous injections of the humanized antibody tanezumab spaced 8 weeks apart led to statistically significant improvements relative to placebo for pain, physical function, and patient global assessment of OA, Thomas J. Schnitzer, MD, said at the annual meeting of the American College of Rheumatology.
The primary efficacy measurements occurred 8 weeks following the second subcutaneous injection. The study included two active treatment arms, and all the efficacy measures responses showed consistent, “modest” improvements in the patients who received a 2.5 mg injection followed by a 5 mg injection, compared with those who received two 2.5 mg injections.
For the safety analysis the researchers followed patients out to 24 weeks after they received their final injection. The main adverse event of interest was rapidly progressive OA (RPOA), which occurred in five patients who received two 2.5-mg dosages and in one patient who received the 2.5 mg followed by 5 mg regimen; no RPOA occurred among placebo patients. The 1.3% incidence of RPOA among all 494 patients who received tanezumab “aligned with expectations based on the risk mitigation procedures used,” said Dr. Schnitzer, a rheumatologist and professor of medicine, anesthesiology, and physical medicine and rehabilitation at Northwestern University, Chicago. No patient developed primary osteonecrosis.
Two patients who developed RPOA then underwent total joint replacement. The overall rate of total joint replacement was 4 among placebo patients, all involving knees, and 24 among patients treated with tanezumab, including 12 knee replacements and 12 hip replacements. Blinded adjudication determined that 26 of the total 28 joint replacements resulted from “normal” OA progression.
The rates of all adverse events, serious adverse events, and adverse events leading to treatment discontinuation were low and similar in the three treatment arms.
Earlier clinical studies of tanezumab had signaled a problem with RPOA (Arthritis Rheumatol. 2016 Feb;68[2]:382-91), which led the Food and Drug Administration to order a temporary halt to clinical testing of all nerve growth factor antagonists in 2010 that the agency then lifted in 2015 (Clin Exp Rheumatol. 2017 Sept-Oct;35[suppl 107]:85-7). In 2017, the FDA gave clinical development of tanezumab “fast-track” status. The results that Dr. Schnitzer reported represent the first outcomes from several phase 3 studies that the companies developing tanezumab are now running and that collectively include about 7,000 total patients, according to a written statement from the developing companies. The companies released an initial statement about the current results in July 2018.
The study reported by Dr. Schnitzer enrolled patients with OA of the knee or hip at several U.S. sites. For the primary endpoint of mean change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, the results at 16 weeks were –2.6 units in the placebo patients and –3.4 units among patients who received the higher dosage. For the primary outcome of mean change in WOMAC physical function score, the results were –2.6 in the placebo arm and –3.5 in patients on the higher dosage. For the primary outcome of change in patient’s global assessment of their OA, the results were –0.65 in the placebo patients and –0.90 in those on the highest dosage. All three between-group differences were statistically significant.
A key secondary outcome was the percentage of patients having at least a 50% reduction in their WOMAC pain score, which occurred in 38% of the placebo patients and in 57% on the higher tanezumab dosage, a statistically significant difference, Dr. Schnitzer reported. A final efficacy finding was the percentage of patients who had a 50% or greater pain reduction at week 16 who did not have a pain response at week 8, a parameter that could reflect incremental benefit from the larger, second tanezumab dose. This outcome occurred in 19% of placebo patients and in 22% of patients who received two 2.5-mg doses of tanezumab; among those who received a 5-mg dose after the first 2.5-mg dose, the rate was 33%.
The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
SOURCE: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(Suppl 10) Abstract L20.
CHICAGO – The nerve growth factor antibody tanezumab met its primary efficacy endpoints for improving pain and physical function in patients with knee or hip OA while also showing a low incidence of the drug’s most concerning adverse effect in the first results from a phase 3 study of a drug in this new class.
The placebo-controlled, multicenter study enrolled 696 U.S. OA patients with moderate to severe knee or hip pain and showed that two subcutaneous injections of the humanized antibody tanezumab spaced 8 weeks apart led to statistically significant improvements relative to placebo for pain, physical function, and patient global assessment of OA, Thomas J. Schnitzer, MD, said at the annual meeting of the American College of Rheumatology.
The primary efficacy measurements occurred 8 weeks following the second subcutaneous injection. The study included two active treatment arms, and all the efficacy measures responses showed consistent, “modest” improvements in the patients who received a 2.5 mg injection followed by a 5 mg injection, compared with those who received two 2.5 mg injections.
For the safety analysis the researchers followed patients out to 24 weeks after they received their final injection. The main adverse event of interest was rapidly progressive OA (RPOA), which occurred in five patients who received two 2.5-mg dosages and in one patient who received the 2.5 mg followed by 5 mg regimen; no RPOA occurred among placebo patients. The 1.3% incidence of RPOA among all 494 patients who received tanezumab “aligned with expectations based on the risk mitigation procedures used,” said Dr. Schnitzer, a rheumatologist and professor of medicine, anesthesiology, and physical medicine and rehabilitation at Northwestern University, Chicago. No patient developed primary osteonecrosis.
Two patients who developed RPOA then underwent total joint replacement. The overall rate of total joint replacement was 4 among placebo patients, all involving knees, and 24 among patients treated with tanezumab, including 12 knee replacements and 12 hip replacements. Blinded adjudication determined that 26 of the total 28 joint replacements resulted from “normal” OA progression.
The rates of all adverse events, serious adverse events, and adverse events leading to treatment discontinuation were low and similar in the three treatment arms.
Earlier clinical studies of tanezumab had signaled a problem with RPOA (Arthritis Rheumatol. 2016 Feb;68[2]:382-91), which led the Food and Drug Administration to order a temporary halt to clinical testing of all nerve growth factor antagonists in 2010 that the agency then lifted in 2015 (Clin Exp Rheumatol. 2017 Sept-Oct;35[suppl 107]:85-7). In 2017, the FDA gave clinical development of tanezumab “fast-track” status. The results that Dr. Schnitzer reported represent the first outcomes from several phase 3 studies that the companies developing tanezumab are now running and that collectively include about 7,000 total patients, according to a written statement from the developing companies. The companies released an initial statement about the current results in July 2018.
The study reported by Dr. Schnitzer enrolled patients with OA of the knee or hip at several U.S. sites. For the primary endpoint of mean change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, the results at 16 weeks were –2.6 units in the placebo patients and –3.4 units among patients who received the higher dosage. For the primary outcome of mean change in WOMAC physical function score, the results were –2.6 in the placebo arm and –3.5 in patients on the higher dosage. For the primary outcome of change in patient’s global assessment of their OA, the results were –0.65 in the placebo patients and –0.90 in those on the highest dosage. All three between-group differences were statistically significant.
A key secondary outcome was the percentage of patients having at least a 50% reduction in their WOMAC pain score, which occurred in 38% of the placebo patients and in 57% on the higher tanezumab dosage, a statistically significant difference, Dr. Schnitzer reported. A final efficacy finding was the percentage of patients who had a 50% or greater pain reduction at week 16 who did not have a pain response at week 8, a parameter that could reflect incremental benefit from the larger, second tanezumab dose. This outcome occurred in 19% of placebo patients and in 22% of patients who received two 2.5-mg doses of tanezumab; among those who received a 5-mg dose after the first 2.5-mg dose, the rate was 33%.
The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
SOURCE: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(Suppl 10) Abstract L20.
CHICAGO – The nerve growth factor antibody tanezumab met its primary efficacy endpoints for improving pain and physical function in patients with knee or hip OA while also showing a low incidence of the drug’s most concerning adverse effect in the first results from a phase 3 study of a drug in this new class.
The placebo-controlled, multicenter study enrolled 696 U.S. OA patients with moderate to severe knee or hip pain and showed that two subcutaneous injections of the humanized antibody tanezumab spaced 8 weeks apart led to statistically significant improvements relative to placebo for pain, physical function, and patient global assessment of OA, Thomas J. Schnitzer, MD, said at the annual meeting of the American College of Rheumatology.
The primary efficacy measurements occurred 8 weeks following the second subcutaneous injection. The study included two active treatment arms, and all the efficacy measures responses showed consistent, “modest” improvements in the patients who received a 2.5 mg injection followed by a 5 mg injection, compared with those who received two 2.5 mg injections.
For the safety analysis the researchers followed patients out to 24 weeks after they received their final injection. The main adverse event of interest was rapidly progressive OA (RPOA), which occurred in five patients who received two 2.5-mg dosages and in one patient who received the 2.5 mg followed by 5 mg regimen; no RPOA occurred among placebo patients. The 1.3% incidence of RPOA among all 494 patients who received tanezumab “aligned with expectations based on the risk mitigation procedures used,” said Dr. Schnitzer, a rheumatologist and professor of medicine, anesthesiology, and physical medicine and rehabilitation at Northwestern University, Chicago. No patient developed primary osteonecrosis.
Two patients who developed RPOA then underwent total joint replacement. The overall rate of total joint replacement was 4 among placebo patients, all involving knees, and 24 among patients treated with tanezumab, including 12 knee replacements and 12 hip replacements. Blinded adjudication determined that 26 of the total 28 joint replacements resulted from “normal” OA progression.
The rates of all adverse events, serious adverse events, and adverse events leading to treatment discontinuation were low and similar in the three treatment arms.
Earlier clinical studies of tanezumab had signaled a problem with RPOA (Arthritis Rheumatol. 2016 Feb;68[2]:382-91), which led the Food and Drug Administration to order a temporary halt to clinical testing of all nerve growth factor antagonists in 2010 that the agency then lifted in 2015 (Clin Exp Rheumatol. 2017 Sept-Oct;35[suppl 107]:85-7). In 2017, the FDA gave clinical development of tanezumab “fast-track” status. The results that Dr. Schnitzer reported represent the first outcomes from several phase 3 studies that the companies developing tanezumab are now running and that collectively include about 7,000 total patients, according to a written statement from the developing companies. The companies released an initial statement about the current results in July 2018.
The study reported by Dr. Schnitzer enrolled patients with OA of the knee or hip at several U.S. sites. For the primary endpoint of mean change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, the results at 16 weeks were –2.6 units in the placebo patients and –3.4 units among patients who received the higher dosage. For the primary outcome of mean change in WOMAC physical function score, the results were –2.6 in the placebo arm and –3.5 in patients on the higher dosage. For the primary outcome of change in patient’s global assessment of their OA, the results were –0.65 in the placebo patients and –0.90 in those on the highest dosage. All three between-group differences were statistically significant.
A key secondary outcome was the percentage of patients having at least a 50% reduction in their WOMAC pain score, which occurred in 38% of the placebo patients and in 57% on the higher tanezumab dosage, a statistically significant difference, Dr. Schnitzer reported. A final efficacy finding was the percentage of patients who had a 50% or greater pain reduction at week 16 who did not have a pain response at week 8, a parameter that could reflect incremental benefit from the larger, second tanezumab dose. This outcome occurred in 19% of placebo patients and in 22% of patients who received two 2.5-mg doses of tanezumab; among those who received a 5-mg dose after the first 2.5-mg dose, the rate was 33%.
The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
SOURCE: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(Suppl 10) Abstract L20.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The Western Ontario and McMaster Universities Osteoarthritis Index pain score fell by an average –3.4 points with maximum tanezumab treatment and by –2.6 points with placebo.
Study details: A multicenter, randomized study with 696 patients with moderate to severe OA of the knee or hip and moderate to severe pain.
Disclosures: The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
Source: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(suppl 10) Abstract L20.
Total knee replacement risk soars after arthroscopic surgery for meniscal tear
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Risk of total knee replacement is five times higher after arthroscopic partial meniscectomy.
Major finding: Patients randomized to arthroscopic partial meniscectomy were 400% more likely to subsequently undergo total knee replacement than were those randomized to physical therapy alone.
Study details: This was a presentation of the 5-year follow-up results in 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy.
Disclosures: The presenter reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
Source: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
Brisk walking may decrease TKR risk in OA
CHICAGO – according to a new analysis of data from the National Institutes of Health-sponsored Osteoarthritis Initiative.

Whether walking increases or decreases the risk of structural deterioration and total knee replacement (TKR) in patients with knee osteoarthritis has been a controversial topic marked by conflicting data. That’s probably because prior studies haven’t taken into account walking intensity, Hiral Master said at the annual meeting of the American College of Rheumatology.
Ms. Master, a PhD candidate in physical therapy at the University of Delaware, Newark, presented a study of 1,854 patients with knee osteoarthritis who participated in the Osteoarthritis Initiative, all of whom had worn an accelerometer. This permitted calculation of time spent walking at various intensities. Subjects spent an average of 459 minutes per day not walking and 8 minutes walking at moderate to vigorous intensity, defined as a cadence of more than 100 steps per minute.
During 5 years of follow-up, the incidence of TKR was 6%. In this video interview, Ms. Master explains that patients who replaced 5 minutes of not walking with 5 minutes of brisk walking daily had an adjusted 14% reduction in the risk of TKR. A dose-response was evident, with more minutes of moderate to vigorous walking being associated with progressively larger reductions in the risk of this major surgery. Walking at a cadence of less than 100 steps per minute, regardless of duration, was nonprotective.
SOURCE: Master H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1166.
CHICAGO – according to a new analysis of data from the National Institutes of Health-sponsored Osteoarthritis Initiative.

Whether walking increases or decreases the risk of structural deterioration and total knee replacement (TKR) in patients with knee osteoarthritis has been a controversial topic marked by conflicting data. That’s probably because prior studies haven’t taken into account walking intensity, Hiral Master said at the annual meeting of the American College of Rheumatology.
Ms. Master, a PhD candidate in physical therapy at the University of Delaware, Newark, presented a study of 1,854 patients with knee osteoarthritis who participated in the Osteoarthritis Initiative, all of whom had worn an accelerometer. This permitted calculation of time spent walking at various intensities. Subjects spent an average of 459 minutes per day not walking and 8 minutes walking at moderate to vigorous intensity, defined as a cadence of more than 100 steps per minute.
During 5 years of follow-up, the incidence of TKR was 6%. In this video interview, Ms. Master explains that patients who replaced 5 minutes of not walking with 5 minutes of brisk walking daily had an adjusted 14% reduction in the risk of TKR. A dose-response was evident, with more minutes of moderate to vigorous walking being associated with progressively larger reductions in the risk of this major surgery. Walking at a cadence of less than 100 steps per minute, regardless of duration, was nonprotective.
SOURCE: Master H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1166.
CHICAGO – according to a new analysis of data from the National Institutes of Health-sponsored Osteoarthritis Initiative.

Whether walking increases or decreases the risk of structural deterioration and total knee replacement (TKR) in patients with knee osteoarthritis has been a controversial topic marked by conflicting data. That’s probably because prior studies haven’t taken into account walking intensity, Hiral Master said at the annual meeting of the American College of Rheumatology.
Ms. Master, a PhD candidate in physical therapy at the University of Delaware, Newark, presented a study of 1,854 patients with knee osteoarthritis who participated in the Osteoarthritis Initiative, all of whom had worn an accelerometer. This permitted calculation of time spent walking at various intensities. Subjects spent an average of 459 minutes per day not walking and 8 minutes walking at moderate to vigorous intensity, defined as a cadence of more than 100 steps per minute.
During 5 years of follow-up, the incidence of TKR was 6%. In this video interview, Ms. Master explains that patients who replaced 5 minutes of not walking with 5 minutes of brisk walking daily had an adjusted 14% reduction in the risk of TKR. A dose-response was evident, with more minutes of moderate to vigorous walking being associated with progressively larger reductions in the risk of this major surgery. Walking at a cadence of less than 100 steps per minute, regardless of duration, was nonprotective.
SOURCE: Master H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 1166.
REPORTING FROM THE ACR ANNUAL MEETING
Risk score validated for major NSAID adverse events
CHICAGO – Researchers have derived and validated a 10-item formula to estimate a patient’s risk for developing a major adverse event while on NSAID treatment.
The calculator could “help guide use of NSAIDs in clinical practice,” said Daniel H. Solomon, MD, at the annual meeting of the American College of Rheumatology. Although he called for further validation of the risk-score formula using other databases, he noted that it uses readily available data and could easily be calculated with standard inputs in an electronic medical record. The formula predicts the risk for a major adverse effect during 1 year of daily NSAID use.
Dr. Solomon and his associates devised the risk-score calculator with data collected in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial, a safety study designed to test whether treatment with celecoxib was noninferior to treatment with naproxen or ibuprofen for producing cardiovascular adverse events, a hypothesis proven by the study’s results (N Engl J Med. 2016 Dec 29;375[26]:2519-29). They had full data available for 23,950 of the more than 24,000 enrolled patients. The patients averaged 63 years old, just over a third were men, their average body mass index was 31 kg/m2, and 90% had osteoarthritis and 10% had rheumatoid arthritis. The study enrolled patients with an elevated risk for a cardiovascular event, so 63% had hypertension and 36% had diabetes.
The adverse events included as possible outcomes estimated by the formula were all-cause death, major adverse cardiovascular events, clinically significant GI events, or renal insufficiency or failure. The investigators used data from more than 15,000 patients enrolled during the first 4 years of the study to derive the risk-score formula, and data from the nearly 9,000 patients enrolled during the next 5 years to validate it.
The analysis identified and validated 10 baseline items that, when plugged into the formula, calculated a predicted rate for the subsequent development of a major averse event during 1 year of NSAID treatment. The 10 parameters are: age, sex, known cardiovascular disease, hypertension, diabetes, current cigarette use, on treatment with a statin, baseline serum creatinine level, rheumatoid arthritis, and hematocrit.
As examples of the accuracy of the prediction score, Dr. Solomon reported that, among the patients with a predicted risk for a major adverse event of less than 1%, the observed rate was 0.4%; among people with a predicted rate of 1%-4%, the observed rate was 1.7%; and among those with a predicted risk of more than 4% the observed rate was 5.6%. Major cardiovascular events were the most common type of adverse events observed among the nearly 24,000 patients enrolled in PRECISION. A total of 5% of the patients fell into the lowest risk category, with a risk of less than 1%; 70% were in the intermediate risk category, with a predicted risk of 1%-4%; and 25% had a predicted risk of more than 4%, reported Dr. Solomon, a professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston.
Age is a major driver of risk, he noted. A patient who is at least 65 years old would have a greater than 1% risk for an adverse event regardless of the other nine risk factors in the scoring formula.
PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
SOURCE: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
CHICAGO – Researchers have derived and validated a 10-item formula to estimate a patient’s risk for developing a major adverse event while on NSAID treatment.
The calculator could “help guide use of NSAIDs in clinical practice,” said Daniel H. Solomon, MD, at the annual meeting of the American College of Rheumatology. Although he called for further validation of the risk-score formula using other databases, he noted that it uses readily available data and could easily be calculated with standard inputs in an electronic medical record. The formula predicts the risk for a major adverse effect during 1 year of daily NSAID use.
Dr. Solomon and his associates devised the risk-score calculator with data collected in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial, a safety study designed to test whether treatment with celecoxib was noninferior to treatment with naproxen or ibuprofen for producing cardiovascular adverse events, a hypothesis proven by the study’s results (N Engl J Med. 2016 Dec 29;375[26]:2519-29). They had full data available for 23,950 of the more than 24,000 enrolled patients. The patients averaged 63 years old, just over a third were men, their average body mass index was 31 kg/m2, and 90% had osteoarthritis and 10% had rheumatoid arthritis. The study enrolled patients with an elevated risk for a cardiovascular event, so 63% had hypertension and 36% had diabetes.
The adverse events included as possible outcomes estimated by the formula were all-cause death, major adverse cardiovascular events, clinically significant GI events, or renal insufficiency or failure. The investigators used data from more than 15,000 patients enrolled during the first 4 years of the study to derive the risk-score formula, and data from the nearly 9,000 patients enrolled during the next 5 years to validate it.
The analysis identified and validated 10 baseline items that, when plugged into the formula, calculated a predicted rate for the subsequent development of a major averse event during 1 year of NSAID treatment. The 10 parameters are: age, sex, known cardiovascular disease, hypertension, diabetes, current cigarette use, on treatment with a statin, baseline serum creatinine level, rheumatoid arthritis, and hematocrit.
As examples of the accuracy of the prediction score, Dr. Solomon reported that, among the patients with a predicted risk for a major adverse event of less than 1%, the observed rate was 0.4%; among people with a predicted rate of 1%-4%, the observed rate was 1.7%; and among those with a predicted risk of more than 4% the observed rate was 5.6%. Major cardiovascular events were the most common type of adverse events observed among the nearly 24,000 patients enrolled in PRECISION. A total of 5% of the patients fell into the lowest risk category, with a risk of less than 1%; 70% were in the intermediate risk category, with a predicted risk of 1%-4%; and 25% had a predicted risk of more than 4%, reported Dr. Solomon, a professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston.
Age is a major driver of risk, he noted. A patient who is at least 65 years old would have a greater than 1% risk for an adverse event regardless of the other nine risk factors in the scoring formula.
PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
SOURCE: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
CHICAGO – Researchers have derived and validated a 10-item formula to estimate a patient’s risk for developing a major adverse event while on NSAID treatment.
The calculator could “help guide use of NSAIDs in clinical practice,” said Daniel H. Solomon, MD, at the annual meeting of the American College of Rheumatology. Although he called for further validation of the risk-score formula using other databases, he noted that it uses readily available data and could easily be calculated with standard inputs in an electronic medical record. The formula predicts the risk for a major adverse effect during 1 year of daily NSAID use.
Dr. Solomon and his associates devised the risk-score calculator with data collected in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial, a safety study designed to test whether treatment with celecoxib was noninferior to treatment with naproxen or ibuprofen for producing cardiovascular adverse events, a hypothesis proven by the study’s results (N Engl J Med. 2016 Dec 29;375[26]:2519-29). They had full data available for 23,950 of the more than 24,000 enrolled patients. The patients averaged 63 years old, just over a third were men, their average body mass index was 31 kg/m2, and 90% had osteoarthritis and 10% had rheumatoid arthritis. The study enrolled patients with an elevated risk for a cardiovascular event, so 63% had hypertension and 36% had diabetes.
The adverse events included as possible outcomes estimated by the formula were all-cause death, major adverse cardiovascular events, clinically significant GI events, or renal insufficiency or failure. The investigators used data from more than 15,000 patients enrolled during the first 4 years of the study to derive the risk-score formula, and data from the nearly 9,000 patients enrolled during the next 5 years to validate it.
The analysis identified and validated 10 baseline items that, when plugged into the formula, calculated a predicted rate for the subsequent development of a major averse event during 1 year of NSAID treatment. The 10 parameters are: age, sex, known cardiovascular disease, hypertension, diabetes, current cigarette use, on treatment with a statin, baseline serum creatinine level, rheumatoid arthritis, and hematocrit.
As examples of the accuracy of the prediction score, Dr. Solomon reported that, among the patients with a predicted risk for a major adverse event of less than 1%, the observed rate was 0.4%; among people with a predicted rate of 1%-4%, the observed rate was 1.7%; and among those with a predicted risk of more than 4% the observed rate was 5.6%. Major cardiovascular events were the most common type of adverse events observed among the nearly 24,000 patients enrolled in PRECISION. A total of 5% of the patients fell into the lowest risk category, with a risk of less than 1%; 70% were in the intermediate risk category, with a predicted risk of 1%-4%; and 25% had a predicted risk of more than 4%, reported Dr. Solomon, a professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston.
Age is a major driver of risk, he noted. A patient who is at least 65 years old would have a greater than 1% risk for an adverse event regardless of the other nine risk factors in the scoring formula.
PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
SOURCE: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Five percent of patients had a predicted risk below 1%; 70% had 1%-4% risk; 25% had greater than 4% risk.
Study details: Derivation and validation of the risk score used data from 23,950 patients in the PRECISION trial.
Disclosures: PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
Source: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).