User login
Improving Colorectal Cancer Screening via Mailed Fecal Immunochemical Testing in a Veterans Affairs Health System
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
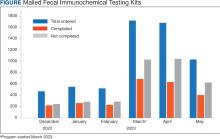
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
US Cancer Institute Studying Ivermectin's 'Ability to Kill Cancer Cells'
US Cancer Institute Studying Ivermectin's 'Ability to Kill Cancer Cells'
The National Cancer Institute (NCI), the federal research agency charged with leading the war against the nation’s second-largest killer, is studying ivermectin as a potential cancer treatment, according to its top official.
“There are enough reports of it, enough interest in it, that we actually did — ivermectin, in particular — did engage in sort of a better preclinical study of its properties and its ability to kill cancer cells,” said Anthony Letai, a physician the Trump administration appointed as NCI director in September.
Letai did not cite new evidence that might have prompted the institute to research the effectiveness of the antiparasitic drug against cancer. The drug, largely used to treat people or animals for infections caused by parasites, is a popular dewormer for horses.
“We’ll probably have those results in a few months,” Letai said. “So we are taking it seriously.”
He spoke about ivermectin at a January 30 event, “Reclaiming Science: The People’s NIH,” with National Institutes of Health (NIH) Director Jay Bhattacharya and other senior agency officials at Washington, DC’s Willard Hotel. The MAHA Institute hosted the discussion, framed by the “Make America Healthy Again” agenda of Health and Human Services (HSS) Secretary Robert F. Kennedy Jr. The National Cancer Institute is the largest of the NIH’s 27 branches.
During the COVID pandemic, ivermectin’s popularity surged as fringe medical groups promoted it as an effective treatment. Clinical trials have found it isn’t effective against COVID.
Ivermectin has become a symbol of resistance against the medical establishment among MAHA adherents and conservatives. Like-minded commentators and wellness and other online influencers have hyped — without evidence — ivermectin as a miracle cure for a host of diseases, including cancer. Trump officials have pointed to research on ivermectin as an example of the administration’s receptiveness to ideas the scientific establishment has rejected.
“If lots of people believe it and it’s moving public health, we as NIH have an obligation, again, to treat it seriously,” Bhattacharya said at the event. According to The Chronicle at Duke University, Bhattacharya recently said he wants the NIH to be “the research arm of MAHA.”
The decision by the world’s premier cancer research institute to study ivermectin as a cancer treatment has alarmed career scientists at the agency.
“I am shocked and appalled,” one NCI scientist said. “We are moving funds away from so much promising research in order to do a preclinical study based on nonscientific ideas. It’s absurd.”
KFF Health News granted the scientist and other NCI workers anonymity because they are not authorized to speak to the press and fear retaliation.
HHS and the National Cancer Institute did not answer KFF Health News’ questions on the amount of money the cancer institute is spending on the study, who is carrying it out, and whether there was new evidence that prompted NCI to look into ivermectin as an anticancer therapy. Emily Hilliard, an HHS spokesperson, said NIH is dedicated to “rigorous, gold-standard research,” something the administration has repeatedly professed.
A preclinical study is an early phase of research conducted in a lab to test whether a drug or treatment may be useful and to assess potential harms. These studies take place before human clinical trials.
The scientist questioned whether there is enough initial evidence to warrant NCI’s spending of taxpayer funds to investigate the drug’s potential as a cancer treatment.
The FDA has approved ivermectin for certain uses in humans and animals. Tablets are used to treat conditions caused by parasitic worms, and the FDA has approved ivermectin lotions to treat lice and rosacea. Two scientists involved in its discovery won the Nobel Prize in 2015, tied to the drug’s success in treating certain parasitic diseases.
The FDA has warned that large doses of ivermectin can be dangerous. Overdoses can cause seizures, comas, or death.
Kennedy, supporters of the MAHA movement, and some conservative commentators have promoted the idea that the government and pharmaceutical companies quashed ivermectin and other inexpensive, off-patent drugs because they’re not profitable for the drug industry.
“FDA’s war on public health is about to end,” Kennedy wrote in an October 2024 X post that has since gone viral. “This includes its aggressive suppression of psychedelics, peptides, stem cells, raw milk, hyperbaric therapies, chelating compounds, ivermectin, hydroxychloroquine, vitamins, clean foods, sunshine, exercise, nutraceuticals and anything else that advances human health and can’t be patented by Pharma.”
Previous laboratory research has shown that ivermectin could have anticancer effects because it promotes cell death and inhibits the growth of tumor cells. “It actually has been studied both with NIH funds and outside of NIH funds,” Letai said.
However, there is no evidence that ivermectin is safe and effective in treating cancer in humans. Preliminary data from a small clinical trial that gave ivermectin to patients with one type of metastatic breast cancer, in combination with immunotherapy, found no significant benefit from the addition of ivermectin.
Some physicians are concerned that patients will delay or forgo effective cancer treatments, or be harmed in other ways, if they believe unfounded claims that ivermectin can treat their disease.
“Many, many, many things work in a test tube. Quite a few things work in a mouse or a monkey. It still doesn’t mean it’s going to work in people,” said Jeffery Edenfield, executive medical director of oncology for the South Carolina-based Prisma Health Cancer Institute.
Edenfield said cancer patients ask him about ivermectin “regularly,” mostly because of what they see on social media. He said he persuaded a patient to stop using it, and a colleague recently had a patient who decided “to forgo highly effective standard therapy in favor of ivermectin.”
“People come to the discussion having largely already made up their mind,” Edenfield said. “We’re in this delicate time when there’s sort of a fundamental mistrust of medicine,” he added. “Some people are just not going to believe me. I just have to keep trying.”
A June letter by clinicians at Cincinnati Children’s Hospital Medical Center in Ohio detailed how an adolescent patient with metastatic bone cancer started taking ivermectin “after encountering social media posts touting its benefits.” The patient — who hadn’t been given a prescription by a clinician — experienced ivermectin-related neurotoxicity and had to seek emergency care because of nausea, fatigue, and other symptoms.
“We urge the pediatric oncology community to advocate for sensible health policy that prioritizes the well-being of our patients,” the clinicians wrote. The lack of evidence about ivermectin and cancer hasn’t stopped celebrities and online influencers from promoting the notion that the drug is a cure-all. On a January 2025 episode of Joe Rogan’s podcast, actor Mel Gibson claimed that a combination of drugs that included ivermectin cured 3friends with stage IV cancer. The episode has been viewed > 12 million times.
Lawmakers in a handful of states have made the drug available over the counter. And Florida — which, under Republican Governor Ron DeSantis, has become a hotbed for anti-vaccine policies and the spread of public health misinformation — announced last fall that the state plans to fund research to study the drug as a potential cancer treatment.
The Florida Department of Health did not respond to questions about that effort.
Letai, previously a Dana-Farber Cancer Institute oncologist, started at the National Cancer Institute after months of upheaval caused by Trump administration policies.
“What you’re hearing at the NIH now is an openness to ideas — even ideas that scientists would say, ‘Oh, there’s no way it could work’ — but nevertheless applying rigorous scientific methods to those ideas,” Bhattacharya said at the January 30 event.
A second NCI scientist, who was granted anonymity due to fear of retaliation, said the notion that NIH was not open to investigating the value of off-label drugs in cancer is “ridiculous.”
“This is not a new idea they came up with,” the scientist said.
Letai didn’t elaborate on whether NCI scientists are conducting the research or if it has directed funding to an outside institution. Three-fourths of the cancer institute’s research dollars go to outside scientists.
He also aimed to temper expectations.
“At least on a population level,” Letai said, “it’s not going to be a cure-all for cancer.”
The National Cancer Institute (NCI), the federal research agency charged with leading the war against the nation’s second-largest killer, is studying ivermectin as a potential cancer treatment, according to its top official.
“There are enough reports of it, enough interest in it, that we actually did — ivermectin, in particular — did engage in sort of a better preclinical study of its properties and its ability to kill cancer cells,” said Anthony Letai, a physician the Trump administration appointed as NCI director in September.
Letai did not cite new evidence that might have prompted the institute to research the effectiveness of the antiparasitic drug against cancer. The drug, largely used to treat people or animals for infections caused by parasites, is a popular dewormer for horses.
“We’ll probably have those results in a few months,” Letai said. “So we are taking it seriously.”
He spoke about ivermectin at a January 30 event, “Reclaiming Science: The People’s NIH,” with National Institutes of Health (NIH) Director Jay Bhattacharya and other senior agency officials at Washington, DC’s Willard Hotel. The MAHA Institute hosted the discussion, framed by the “Make America Healthy Again” agenda of Health and Human Services (HSS) Secretary Robert F. Kennedy Jr. The National Cancer Institute is the largest of the NIH’s 27 branches.
During the COVID pandemic, ivermectin’s popularity surged as fringe medical groups promoted it as an effective treatment. Clinical trials have found it isn’t effective against COVID.
Ivermectin has become a symbol of resistance against the medical establishment among MAHA adherents and conservatives. Like-minded commentators and wellness and other online influencers have hyped — without evidence — ivermectin as a miracle cure for a host of diseases, including cancer. Trump officials have pointed to research on ivermectin as an example of the administration’s receptiveness to ideas the scientific establishment has rejected.
“If lots of people believe it and it’s moving public health, we as NIH have an obligation, again, to treat it seriously,” Bhattacharya said at the event. According to The Chronicle at Duke University, Bhattacharya recently said he wants the NIH to be “the research arm of MAHA.”
The decision by the world’s premier cancer research institute to study ivermectin as a cancer treatment has alarmed career scientists at the agency.
“I am shocked and appalled,” one NCI scientist said. “We are moving funds away from so much promising research in order to do a preclinical study based on nonscientific ideas. It’s absurd.”
KFF Health News granted the scientist and other NCI workers anonymity because they are not authorized to speak to the press and fear retaliation.
HHS and the National Cancer Institute did not answer KFF Health News’ questions on the amount of money the cancer institute is spending on the study, who is carrying it out, and whether there was new evidence that prompted NCI to look into ivermectin as an anticancer therapy. Emily Hilliard, an HHS spokesperson, said NIH is dedicated to “rigorous, gold-standard research,” something the administration has repeatedly professed.
A preclinical study is an early phase of research conducted in a lab to test whether a drug or treatment may be useful and to assess potential harms. These studies take place before human clinical trials.
The scientist questioned whether there is enough initial evidence to warrant NCI’s spending of taxpayer funds to investigate the drug’s potential as a cancer treatment.
The FDA has approved ivermectin for certain uses in humans and animals. Tablets are used to treat conditions caused by parasitic worms, and the FDA has approved ivermectin lotions to treat lice and rosacea. Two scientists involved in its discovery won the Nobel Prize in 2015, tied to the drug’s success in treating certain parasitic diseases.
The FDA has warned that large doses of ivermectin can be dangerous. Overdoses can cause seizures, comas, or death.
Kennedy, supporters of the MAHA movement, and some conservative commentators have promoted the idea that the government and pharmaceutical companies quashed ivermectin and other inexpensive, off-patent drugs because they’re not profitable for the drug industry.
“FDA’s war on public health is about to end,” Kennedy wrote in an October 2024 X post that has since gone viral. “This includes its aggressive suppression of psychedelics, peptides, stem cells, raw milk, hyperbaric therapies, chelating compounds, ivermectin, hydroxychloroquine, vitamins, clean foods, sunshine, exercise, nutraceuticals and anything else that advances human health and can’t be patented by Pharma.”
Previous laboratory research has shown that ivermectin could have anticancer effects because it promotes cell death and inhibits the growth of tumor cells. “It actually has been studied both with NIH funds and outside of NIH funds,” Letai said.
However, there is no evidence that ivermectin is safe and effective in treating cancer in humans. Preliminary data from a small clinical trial that gave ivermectin to patients with one type of metastatic breast cancer, in combination with immunotherapy, found no significant benefit from the addition of ivermectin.
Some physicians are concerned that patients will delay or forgo effective cancer treatments, or be harmed in other ways, if they believe unfounded claims that ivermectin can treat their disease.
“Many, many, many things work in a test tube. Quite a few things work in a mouse or a monkey. It still doesn’t mean it’s going to work in people,” said Jeffery Edenfield, executive medical director of oncology for the South Carolina-based Prisma Health Cancer Institute.
Edenfield said cancer patients ask him about ivermectin “regularly,” mostly because of what they see on social media. He said he persuaded a patient to stop using it, and a colleague recently had a patient who decided “to forgo highly effective standard therapy in favor of ivermectin.”
“People come to the discussion having largely already made up their mind,” Edenfield said. “We’re in this delicate time when there’s sort of a fundamental mistrust of medicine,” he added. “Some people are just not going to believe me. I just have to keep trying.”
A June letter by clinicians at Cincinnati Children’s Hospital Medical Center in Ohio detailed how an adolescent patient with metastatic bone cancer started taking ivermectin “after encountering social media posts touting its benefits.” The patient — who hadn’t been given a prescription by a clinician — experienced ivermectin-related neurotoxicity and had to seek emergency care because of nausea, fatigue, and other symptoms.
“We urge the pediatric oncology community to advocate for sensible health policy that prioritizes the well-being of our patients,” the clinicians wrote. The lack of evidence about ivermectin and cancer hasn’t stopped celebrities and online influencers from promoting the notion that the drug is a cure-all. On a January 2025 episode of Joe Rogan’s podcast, actor Mel Gibson claimed that a combination of drugs that included ivermectin cured 3friends with stage IV cancer. The episode has been viewed > 12 million times.
Lawmakers in a handful of states have made the drug available over the counter. And Florida — which, under Republican Governor Ron DeSantis, has become a hotbed for anti-vaccine policies and the spread of public health misinformation — announced last fall that the state plans to fund research to study the drug as a potential cancer treatment.
The Florida Department of Health did not respond to questions about that effort.
Letai, previously a Dana-Farber Cancer Institute oncologist, started at the National Cancer Institute after months of upheaval caused by Trump administration policies.
“What you’re hearing at the NIH now is an openness to ideas — even ideas that scientists would say, ‘Oh, there’s no way it could work’ — but nevertheless applying rigorous scientific methods to those ideas,” Bhattacharya said at the January 30 event.
A second NCI scientist, who was granted anonymity due to fear of retaliation, said the notion that NIH was not open to investigating the value of off-label drugs in cancer is “ridiculous.”
“This is not a new idea they came up with,” the scientist said.
Letai didn’t elaborate on whether NCI scientists are conducting the research or if it has directed funding to an outside institution. Three-fourths of the cancer institute’s research dollars go to outside scientists.
He also aimed to temper expectations.
“At least on a population level,” Letai said, “it’s not going to be a cure-all for cancer.”
The National Cancer Institute (NCI), the federal research agency charged with leading the war against the nation’s second-largest killer, is studying ivermectin as a potential cancer treatment, according to its top official.
“There are enough reports of it, enough interest in it, that we actually did — ivermectin, in particular — did engage in sort of a better preclinical study of its properties and its ability to kill cancer cells,” said Anthony Letai, a physician the Trump administration appointed as NCI director in September.
Letai did not cite new evidence that might have prompted the institute to research the effectiveness of the antiparasitic drug against cancer. The drug, largely used to treat people or animals for infections caused by parasites, is a popular dewormer for horses.
“We’ll probably have those results in a few months,” Letai said. “So we are taking it seriously.”
He spoke about ivermectin at a January 30 event, “Reclaiming Science: The People’s NIH,” with National Institutes of Health (NIH) Director Jay Bhattacharya and other senior agency officials at Washington, DC’s Willard Hotel. The MAHA Institute hosted the discussion, framed by the “Make America Healthy Again” agenda of Health and Human Services (HSS) Secretary Robert F. Kennedy Jr. The National Cancer Institute is the largest of the NIH’s 27 branches.
During the COVID pandemic, ivermectin’s popularity surged as fringe medical groups promoted it as an effective treatment. Clinical trials have found it isn’t effective against COVID.
Ivermectin has become a symbol of resistance against the medical establishment among MAHA adherents and conservatives. Like-minded commentators and wellness and other online influencers have hyped — without evidence — ivermectin as a miracle cure for a host of diseases, including cancer. Trump officials have pointed to research on ivermectin as an example of the administration’s receptiveness to ideas the scientific establishment has rejected.
“If lots of people believe it and it’s moving public health, we as NIH have an obligation, again, to treat it seriously,” Bhattacharya said at the event. According to The Chronicle at Duke University, Bhattacharya recently said he wants the NIH to be “the research arm of MAHA.”
The decision by the world’s premier cancer research institute to study ivermectin as a cancer treatment has alarmed career scientists at the agency.
“I am shocked and appalled,” one NCI scientist said. “We are moving funds away from so much promising research in order to do a preclinical study based on nonscientific ideas. It’s absurd.”
KFF Health News granted the scientist and other NCI workers anonymity because they are not authorized to speak to the press and fear retaliation.
HHS and the National Cancer Institute did not answer KFF Health News’ questions on the amount of money the cancer institute is spending on the study, who is carrying it out, and whether there was new evidence that prompted NCI to look into ivermectin as an anticancer therapy. Emily Hilliard, an HHS spokesperson, said NIH is dedicated to “rigorous, gold-standard research,” something the administration has repeatedly professed.
A preclinical study is an early phase of research conducted in a lab to test whether a drug or treatment may be useful and to assess potential harms. These studies take place before human clinical trials.
The scientist questioned whether there is enough initial evidence to warrant NCI’s spending of taxpayer funds to investigate the drug’s potential as a cancer treatment.
The FDA has approved ivermectin for certain uses in humans and animals. Tablets are used to treat conditions caused by parasitic worms, and the FDA has approved ivermectin lotions to treat lice and rosacea. Two scientists involved in its discovery won the Nobel Prize in 2015, tied to the drug’s success in treating certain parasitic diseases.
The FDA has warned that large doses of ivermectin can be dangerous. Overdoses can cause seizures, comas, or death.
Kennedy, supporters of the MAHA movement, and some conservative commentators have promoted the idea that the government and pharmaceutical companies quashed ivermectin and other inexpensive, off-patent drugs because they’re not profitable for the drug industry.
“FDA’s war on public health is about to end,” Kennedy wrote in an October 2024 X post that has since gone viral. “This includes its aggressive suppression of psychedelics, peptides, stem cells, raw milk, hyperbaric therapies, chelating compounds, ivermectin, hydroxychloroquine, vitamins, clean foods, sunshine, exercise, nutraceuticals and anything else that advances human health and can’t be patented by Pharma.”
Previous laboratory research has shown that ivermectin could have anticancer effects because it promotes cell death and inhibits the growth of tumor cells. “It actually has been studied both with NIH funds and outside of NIH funds,” Letai said.
However, there is no evidence that ivermectin is safe and effective in treating cancer in humans. Preliminary data from a small clinical trial that gave ivermectin to patients with one type of metastatic breast cancer, in combination with immunotherapy, found no significant benefit from the addition of ivermectin.
Some physicians are concerned that patients will delay or forgo effective cancer treatments, or be harmed in other ways, if they believe unfounded claims that ivermectin can treat their disease.
“Many, many, many things work in a test tube. Quite a few things work in a mouse or a monkey. It still doesn’t mean it’s going to work in people,” said Jeffery Edenfield, executive medical director of oncology for the South Carolina-based Prisma Health Cancer Institute.
Edenfield said cancer patients ask him about ivermectin “regularly,” mostly because of what they see on social media. He said he persuaded a patient to stop using it, and a colleague recently had a patient who decided “to forgo highly effective standard therapy in favor of ivermectin.”
“People come to the discussion having largely already made up their mind,” Edenfield said. “We’re in this delicate time when there’s sort of a fundamental mistrust of medicine,” he added. “Some people are just not going to believe me. I just have to keep trying.”
A June letter by clinicians at Cincinnati Children’s Hospital Medical Center in Ohio detailed how an adolescent patient with metastatic bone cancer started taking ivermectin “after encountering social media posts touting its benefits.” The patient — who hadn’t been given a prescription by a clinician — experienced ivermectin-related neurotoxicity and had to seek emergency care because of nausea, fatigue, and other symptoms.
“We urge the pediatric oncology community to advocate for sensible health policy that prioritizes the well-being of our patients,” the clinicians wrote. The lack of evidence about ivermectin and cancer hasn’t stopped celebrities and online influencers from promoting the notion that the drug is a cure-all. On a January 2025 episode of Joe Rogan’s podcast, actor Mel Gibson claimed that a combination of drugs that included ivermectin cured 3friends with stage IV cancer. The episode has been viewed > 12 million times.
Lawmakers in a handful of states have made the drug available over the counter. And Florida — which, under Republican Governor Ron DeSantis, has become a hotbed for anti-vaccine policies and the spread of public health misinformation — announced last fall that the state plans to fund research to study the drug as a potential cancer treatment.
The Florida Department of Health did not respond to questions about that effort.
Letai, previously a Dana-Farber Cancer Institute oncologist, started at the National Cancer Institute after months of upheaval caused by Trump administration policies.
“What you’re hearing at the NIH now is an openness to ideas — even ideas that scientists would say, ‘Oh, there’s no way it could work’ — but nevertheless applying rigorous scientific methods to those ideas,” Bhattacharya said at the January 30 event.
A second NCI scientist, who was granted anonymity due to fear of retaliation, said the notion that NIH was not open to investigating the value of off-label drugs in cancer is “ridiculous.”
“This is not a new idea they came up with,” the scientist said.
Letai didn’t elaborate on whether NCI scientists are conducting the research or if it has directed funding to an outside institution. Three-fourths of the cancer institute’s research dollars go to outside scientists.
He also aimed to temper expectations.
“At least on a population level,” Letai said, “it’s not going to be a cure-all for cancer.”
US Cancer Institute Studying Ivermectin's 'Ability to Kill Cancer Cells'
US Cancer Institute Studying Ivermectin's 'Ability to Kill Cancer Cells'
Q&A: Why Are More Americans Under 50 Years of Age Dying of Colorectal Cancer?
Why Are More Americans Under Age 50 Dying of CRC?
First, the good news: Fewer Americans aged < 50 years are dying from cancer vs just a decade ago — reflecting progress in prevention, early detection, and treatment. There is, however, one big exception. Colorectal cancer mortality has been steadily inching up, and the disease now stands as the leading cause of cancer death in this age group, up from the fifth-leading in the early 1990s.
Those are the major findings of a recent study by the American Cancer Society (ACS), published as a research letter in JAMA.
Using SEER data, researchers found that the overall age-adjusted cancer death rate among Americans aged < 50 years dropped by 44% between 1990 and 2023 — from 25.5 to 14.2 per 100,000. And for 4 of the 5 leading causes of cancer death, there were mean annual declines from 2014 to 2023. The biggest change was in lung cancer deaths, which fell by an average of 5.7% per year. Meanwhile, leukemia and breast cancer deaths showed annual declines of 2.3% and 1.4%, respectively, despite rising incidences of both diseases among younger Americans.
The outlier is colorectal cancer, where mortality has been rising by about 1% per year since 2005. And it’s a pattern seen in both men and women.
Study coauthor Nikita Sandeep Wagle, PhD, MBBS, principal scientist, Cancer Surveillance Research at the ACS, and Arif Kamal, MD, ACS chief patient officer, discussed the research and its implications with Medscape Medical News.
Can you offer some possible reasons for the declining mortality in most of the cancers you studied?
Wagle: Mortality is going down for most of the cancers because we are getting better at finding cancers earlier and treating them more effectively. We have also seen improvements in screening, imaging, and therapy, and that means more people are being diagnosed at earlier stages and are surviving longer after diagnosis.
Regarding the rise in colorectal cancer mortality, do you think it's due to the rising incidence of early-onset colorectal cancer?
Kamal: Partially, but not completely, because the relationship between incidence and mortality is not always straightforward. For example, breast cancer incidence has been increasing, while mortality is going down. The rising mortality in people younger than 50 years is likely suggestive of more aggressive cancers being diagnosed — potentially secondary to environmental, dietary, or lifestyle factors. The colon is a unique organ because everything we put in our bodies passes through the colon, so food-based risk factors — for example, low fiber intake, red meat, and ultra-processed foods — are increasingly rising to the top as culprits.
Further, we know that only about 25% of people between the ages of 45 and 50 years are up to date with recommended colon cancer screenings, which can lead to later-stage diagnoses and thus higher mortality. So higher mortality speaks to the need to focus on lifestyle and diet changes and get more younger people to complete recommended cancer screenings.
Wagle: I think the “why” of your question is very important. Many researchers are trying to understand possible causes, such as diet, lifestyle, environmental factors, and genetics. But we cannot pinpoint one single cause. We need even more focus on research toward understanding the etiology of early-onset colorectal cancer.
What makes colorectal cancer different is that, unlike some other major cancers in this age group where mortality has declined despite rising incidence, roughly 3 in 4 colorectal cancers diagnosed in people younger than 50 years are [regional or distant], where the outcomes are worse.
Can you contextualize the rise in colorectal cancer mortality? What is the absolute rate among younger Americans now?
Wagle: It is around two deaths per 100,000 population in 2023 for people younger than 50 years. That number may not seem large, but the upward trend — a 1.1% annual increase from 2014 to 2023 — is concerning when you think about how overall mortality in this age group has dropped substantially over the past few decades. Colorectal cancer is moving in the opposite direction. I think the hopeful part is that it is also one of the most preventable cancers. Screening can stop cancer before it starts by removing precancerous polyps. Early-stage disease is highly treatable, and outcomes are better. That means better awareness and timely screening could make a real difference.
How can clinicians use this new information with regard to screening?
Wagle: For cancers with established screening guidelines, such as colorectal cancer, clinicians should continue to emphasize guideline-based screening and individualized risk assessment.
For colorectal cancer, screening now is recommended to start at age 45 for individuals at average risk, and earlier for [some], due to family history or other risk factors. Clinicians can use these findings to remind younger individuals that colorectal cancer is not only a disease of older adults and that screening at the recommended age can save lives.
In addition, red-flag symptoms such as persistent rectal bleeding, unexplained abdominal pain, difficulty in bowel movements, or signs of anemia should prompt appropriate evaluation in younger individuals.
Kamal: Clinicians should continue to emphasize timely completion of regular screening, starting at age 45 [for average-risk people]. Many still believe that the recommended starting age is 50 or that colonoscopy is the only way to get screened. Highlighting home-based screening options often helps patients make cancer screening logistically fit better into their busy lives.
Could you elaborate on the red-flag symptoms you mentioned, and what is an appropriate evaluation in younger individuals?
Kamal: Appropriate evaluation for any suspected bleeding — bright red or black and tarry — starts with an in-office evaluation by a primary care physician. Referral to a specialist, such as a gastroenterologist or surgeon, is done later, typically for direct visualization, such as with a colonoscopy. Rarely, imaging such as CT scans or ultrasounds is performed. Overall, because of the rising incidence of colon cancer in younger people, any concerning symptoms should be reported to a physician for an in-office evaluation as the first step.
Do these findings suggest that the starting age for average-risk people should be lowered—to age 40, for example?
Kamal: ACS screening guidelines for all cancers are part of an ongoing guideline development process by ACS scientists and volunteers. We monitor medical and scientific literature for new evidence that may support a change in current guidelines or the development of new guidelines and for information about cancer screening that should be conveyed to clinicians and target populations.
Keith Mulvihill is a freelance writer based in New York City.
A version of this article first appeared on Medscape.com.
First, the good news: Fewer Americans aged < 50 years are dying from cancer vs just a decade ago — reflecting progress in prevention, early detection, and treatment. There is, however, one big exception. Colorectal cancer mortality has been steadily inching up, and the disease now stands as the leading cause of cancer death in this age group, up from the fifth-leading in the early 1990s.
Those are the major findings of a recent study by the American Cancer Society (ACS), published as a research letter in JAMA.
Using SEER data, researchers found that the overall age-adjusted cancer death rate among Americans aged < 50 years dropped by 44% between 1990 and 2023 — from 25.5 to 14.2 per 100,000. And for 4 of the 5 leading causes of cancer death, there were mean annual declines from 2014 to 2023. The biggest change was in lung cancer deaths, which fell by an average of 5.7% per year. Meanwhile, leukemia and breast cancer deaths showed annual declines of 2.3% and 1.4%, respectively, despite rising incidences of both diseases among younger Americans.
The outlier is colorectal cancer, where mortality has been rising by about 1% per year since 2005. And it’s a pattern seen in both men and women.
Study coauthor Nikita Sandeep Wagle, PhD, MBBS, principal scientist, Cancer Surveillance Research at the ACS, and Arif Kamal, MD, ACS chief patient officer, discussed the research and its implications with Medscape Medical News.
Can you offer some possible reasons for the declining mortality in most of the cancers you studied?
Wagle: Mortality is going down for most of the cancers because we are getting better at finding cancers earlier and treating them more effectively. We have also seen improvements in screening, imaging, and therapy, and that means more people are being diagnosed at earlier stages and are surviving longer after diagnosis.
Regarding the rise in colorectal cancer mortality, do you think it's due to the rising incidence of early-onset colorectal cancer?
Kamal: Partially, but not completely, because the relationship between incidence and mortality is not always straightforward. For example, breast cancer incidence has been increasing, while mortality is going down. The rising mortality in people younger than 50 years is likely suggestive of more aggressive cancers being diagnosed — potentially secondary to environmental, dietary, or lifestyle factors. The colon is a unique organ because everything we put in our bodies passes through the colon, so food-based risk factors — for example, low fiber intake, red meat, and ultra-processed foods — are increasingly rising to the top as culprits.
Further, we know that only about 25% of people between the ages of 45 and 50 years are up to date with recommended colon cancer screenings, which can lead to later-stage diagnoses and thus higher mortality. So higher mortality speaks to the need to focus on lifestyle and diet changes and get more younger people to complete recommended cancer screenings.
Wagle: I think the “why” of your question is very important. Many researchers are trying to understand possible causes, such as diet, lifestyle, environmental factors, and genetics. But we cannot pinpoint one single cause. We need even more focus on research toward understanding the etiology of early-onset colorectal cancer.
What makes colorectal cancer different is that, unlike some other major cancers in this age group where mortality has declined despite rising incidence, roughly 3 in 4 colorectal cancers diagnosed in people younger than 50 years are [regional or distant], where the outcomes are worse.
Can you contextualize the rise in colorectal cancer mortality? What is the absolute rate among younger Americans now?
Wagle: It is around two deaths per 100,000 population in 2023 for people younger than 50 years. That number may not seem large, but the upward trend — a 1.1% annual increase from 2014 to 2023 — is concerning when you think about how overall mortality in this age group has dropped substantially over the past few decades. Colorectal cancer is moving in the opposite direction. I think the hopeful part is that it is also one of the most preventable cancers. Screening can stop cancer before it starts by removing precancerous polyps. Early-stage disease is highly treatable, and outcomes are better. That means better awareness and timely screening could make a real difference.
How can clinicians use this new information with regard to screening?
Wagle: For cancers with established screening guidelines, such as colorectal cancer, clinicians should continue to emphasize guideline-based screening and individualized risk assessment.
For colorectal cancer, screening now is recommended to start at age 45 for individuals at average risk, and earlier for [some], due to family history or other risk factors. Clinicians can use these findings to remind younger individuals that colorectal cancer is not only a disease of older adults and that screening at the recommended age can save lives.
In addition, red-flag symptoms such as persistent rectal bleeding, unexplained abdominal pain, difficulty in bowel movements, or signs of anemia should prompt appropriate evaluation in younger individuals.
Kamal: Clinicians should continue to emphasize timely completion of regular screening, starting at age 45 [for average-risk people]. Many still believe that the recommended starting age is 50 or that colonoscopy is the only way to get screened. Highlighting home-based screening options often helps patients make cancer screening logistically fit better into their busy lives.
Could you elaborate on the red-flag symptoms you mentioned, and what is an appropriate evaluation in younger individuals?
Kamal: Appropriate evaluation for any suspected bleeding — bright red or black and tarry — starts with an in-office evaluation by a primary care physician. Referral to a specialist, such as a gastroenterologist or surgeon, is done later, typically for direct visualization, such as with a colonoscopy. Rarely, imaging such as CT scans or ultrasounds is performed. Overall, because of the rising incidence of colon cancer in younger people, any concerning symptoms should be reported to a physician for an in-office evaluation as the first step.
Do these findings suggest that the starting age for average-risk people should be lowered—to age 40, for example?
Kamal: ACS screening guidelines for all cancers are part of an ongoing guideline development process by ACS scientists and volunteers. We monitor medical and scientific literature for new evidence that may support a change in current guidelines or the development of new guidelines and for information about cancer screening that should be conveyed to clinicians and target populations.
Keith Mulvihill is a freelance writer based in New York City.
A version of this article first appeared on Medscape.com.
First, the good news: Fewer Americans aged < 50 years are dying from cancer vs just a decade ago — reflecting progress in prevention, early detection, and treatment. There is, however, one big exception. Colorectal cancer mortality has been steadily inching up, and the disease now stands as the leading cause of cancer death in this age group, up from the fifth-leading in the early 1990s.
Those are the major findings of a recent study by the American Cancer Society (ACS), published as a research letter in JAMA.
Using SEER data, researchers found that the overall age-adjusted cancer death rate among Americans aged < 50 years dropped by 44% between 1990 and 2023 — from 25.5 to 14.2 per 100,000. And for 4 of the 5 leading causes of cancer death, there were mean annual declines from 2014 to 2023. The biggest change was in lung cancer deaths, which fell by an average of 5.7% per year. Meanwhile, leukemia and breast cancer deaths showed annual declines of 2.3% and 1.4%, respectively, despite rising incidences of both diseases among younger Americans.
The outlier is colorectal cancer, where mortality has been rising by about 1% per year since 2005. And it’s a pattern seen in both men and women.
Study coauthor Nikita Sandeep Wagle, PhD, MBBS, principal scientist, Cancer Surveillance Research at the ACS, and Arif Kamal, MD, ACS chief patient officer, discussed the research and its implications with Medscape Medical News.
Can you offer some possible reasons for the declining mortality in most of the cancers you studied?
Wagle: Mortality is going down for most of the cancers because we are getting better at finding cancers earlier and treating them more effectively. We have also seen improvements in screening, imaging, and therapy, and that means more people are being diagnosed at earlier stages and are surviving longer after diagnosis.
Regarding the rise in colorectal cancer mortality, do you think it's due to the rising incidence of early-onset colorectal cancer?
Kamal: Partially, but not completely, because the relationship between incidence and mortality is not always straightforward. For example, breast cancer incidence has been increasing, while mortality is going down. The rising mortality in people younger than 50 years is likely suggestive of more aggressive cancers being diagnosed — potentially secondary to environmental, dietary, or lifestyle factors. The colon is a unique organ because everything we put in our bodies passes through the colon, so food-based risk factors — for example, low fiber intake, red meat, and ultra-processed foods — are increasingly rising to the top as culprits.
Further, we know that only about 25% of people between the ages of 45 and 50 years are up to date with recommended colon cancer screenings, which can lead to later-stage diagnoses and thus higher mortality. So higher mortality speaks to the need to focus on lifestyle and diet changes and get more younger people to complete recommended cancer screenings.
Wagle: I think the “why” of your question is very important. Many researchers are trying to understand possible causes, such as diet, lifestyle, environmental factors, and genetics. But we cannot pinpoint one single cause. We need even more focus on research toward understanding the etiology of early-onset colorectal cancer.
What makes colorectal cancer different is that, unlike some other major cancers in this age group where mortality has declined despite rising incidence, roughly 3 in 4 colorectal cancers diagnosed in people younger than 50 years are [regional or distant], where the outcomes are worse.
Can you contextualize the rise in colorectal cancer mortality? What is the absolute rate among younger Americans now?
Wagle: It is around two deaths per 100,000 population in 2023 for people younger than 50 years. That number may not seem large, but the upward trend — a 1.1% annual increase from 2014 to 2023 — is concerning when you think about how overall mortality in this age group has dropped substantially over the past few decades. Colorectal cancer is moving in the opposite direction. I think the hopeful part is that it is also one of the most preventable cancers. Screening can stop cancer before it starts by removing precancerous polyps. Early-stage disease is highly treatable, and outcomes are better. That means better awareness and timely screening could make a real difference.
How can clinicians use this new information with regard to screening?
Wagle: For cancers with established screening guidelines, such as colorectal cancer, clinicians should continue to emphasize guideline-based screening and individualized risk assessment.
For colorectal cancer, screening now is recommended to start at age 45 for individuals at average risk, and earlier for [some], due to family history or other risk factors. Clinicians can use these findings to remind younger individuals that colorectal cancer is not only a disease of older adults and that screening at the recommended age can save lives.
In addition, red-flag symptoms such as persistent rectal bleeding, unexplained abdominal pain, difficulty in bowel movements, or signs of anemia should prompt appropriate evaluation in younger individuals.
Kamal: Clinicians should continue to emphasize timely completion of regular screening, starting at age 45 [for average-risk people]. Many still believe that the recommended starting age is 50 or that colonoscopy is the only way to get screened. Highlighting home-based screening options often helps patients make cancer screening logistically fit better into their busy lives.
Could you elaborate on the red-flag symptoms you mentioned, and what is an appropriate evaluation in younger individuals?
Kamal: Appropriate evaluation for any suspected bleeding — bright red or black and tarry — starts with an in-office evaluation by a primary care physician. Referral to a specialist, such as a gastroenterologist or surgeon, is done later, typically for direct visualization, such as with a colonoscopy. Rarely, imaging such as CT scans or ultrasounds is performed. Overall, because of the rising incidence of colon cancer in younger people, any concerning symptoms should be reported to a physician for an in-office evaluation as the first step.
Do these findings suggest that the starting age for average-risk people should be lowered—to age 40, for example?
Kamal: ACS screening guidelines for all cancers are part of an ongoing guideline development process by ACS scientists and volunteers. We monitor medical and scientific literature for new evidence that may support a change in current guidelines or the development of new guidelines and for information about cancer screening that should be conveyed to clinicians and target populations.
Keith Mulvihill is a freelance writer based in New York City.
A version of this article first appeared on Medscape.com.
Why Are More Americans Under Age 50 Dying of CRC?
Why Are More Americans Under Age 50 Dying of CRC?
Do Ultraprocessed Foods Impact Survival After Cancer?
Do Ultraprocessed Foods Impact Survival After Cancer?
Diets heavy in ultraprocessed foods (UPFs) are associated with earlier death in cancer survivors, a new study finds — though issues with the research design suggest that the findings should be taken with a grain of salt.
The study, published on February 4 in Cancer Epidemiology, Biomarkers & Prevention, is among the latest to point to health hazards from eating too many foods full of preservatives, dyes, and other industrially made ingredients.
These so-called UPFs have been linked to an increased risk for cancer, but whether they have any relationship to long-term survival after cancer has been unclear.
In the new study, of 802 adults with a previous cancer diagnosis, those in the top third for UPF consumption had a 48% higher rate of death from any cause over 15 years than those in the bottom third. Similarly, heavier UPF consumers had a 57% higher rate of death from cancer.
Those excess risks were seen after adjustment for numerous variables, including age, physical activity, BMI, smoking status, and socioeconomic indicators.
“Clinicians should encourage a shift toward fresh, minimally processed foods, [and] away from heavily industrially processed products,” said lead author Marialaura Bonaccio, PhD, of the Research Unit of Epidemiology and Prevention at IRCCS Neuromed in Pozzilli, Italy.
Oncologists not involved in the work said the findings support what researchers have suspected.
“UPFs have been linked to increased risk of obesity, diabetes, inflammation, cardiovascular disease, and...all-cause mortality and cardiovascular mortality,” said Urvi A. Shah, MD, a myeloma specialist who conducts nutrition research at Memorial Sloan Kettering Cancer Center in New York City. “However, there was limited data on cancer-specific mortality to date until this study.”
The findings also dovetail with recommendations on cancer prevention that emphasize diets rich in plant foods and low in processed foods, particularly those loaded with sugar, starch, and fat.
The study “may make oncologists think twice before assuring patients to ‘eat whatever you want, it doesn’t really matter’ because these investigators show that it does,” said Donald I. Abrams, MD, an integrative oncologist at the UCSF Osher Center for Integrative Health.
However, Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong in Wollongong, Australia, was not impressed by the analysis.
He pointed to several sources of potential bias and noted that the crude results actually showed that cancer survivors with the lowest UPF consumption had a higher rate of death than the heaviest consumers.
“The story of UPFs being bad is consistent with this data, but so is the story of UPFs being fine,” said Meyerowitz-Katz, who has written about prior research on the subject.
The broad questions of whether and how UPFs might be harming human health have been gaining research interest, partly because of their ubiquity. The foods reportedly make up about 60% of the typical American diet.
There’s no universal agreement on the precise definition of “ultraprocessed,” but researchers generally use the NOVA classification system, which assigns foods into one of four groups based on the level and purpose of processing. UPFs contain ingredients not found in the standard home kitchen (such as high-fructose corn syrup) and often have artificial flavors, colors, and other additives.
Examples of UPFs include the usual “junk food,” such as candy, soda, and processed meat, but many healthy-sounding products, such as flavored yogurts and plant-based milk, also qualify.
For their study, Bonaccio and her colleagues identified 802 cancer survivors from the Moli-sani cohort study (476 women and 326 men) who completed food-frequency questionnaires an average of 8 years postdiagnosis.
Using the NOVA system, the team calculated the amount of UPF in participants’ diets as both weight and energy ratios.
Over a median follow-up of nearly 15 years, there were 281 deaths. In the lowest third of UPF consumption (4.3% mean intake by weight), there were 3.3 deaths per 100 patient-years, compared with 2.4 per 100 patient-years in the highest UPF tertile (16.7% mean intake by weight). For cancer-specific deaths, those numbers were 1.5 and 1.4, respectively.
However, after adjustment for age and total energy intake, the top UPF-intake group showed significantly higher death rates. In the final model, which adjusted for > 20 variables, the hazard ratios for the highest versus lowest UPF consumption were 1.48 (95% CI, 1.07-2.03) for all-cause mortality and 1.57 (95% CI, 1.00-2.47) for cancer mortality.
To explore potential biological mechanisms, the researchers also analyzed certain biomarkers. They found that adjustment for inflammatory markers and resting heart rate at baseline attenuated the association between UPF and all-cause deaths by nearly 40%.
The authors acknowledged some limitations of their study, including its use of self-report and potential survivor bias.
But Meyerowitz-Katz found additional weak points. For one, he said the authors “downplayed” the impact of their analysis controlling for inflammation and heart rate.
“Inflammation and heart rate are both strong markers of future cancer risk,” Meyerowitz-Katz said. “In this cohort, there would be people who were already experiencing cancer recurrence, which is important to control for at baseline.”
He also highlighted a little-known but important issue in observational research called collider bias, which can create a false association between an exposure and outcome. In this study, he said, the researchers introduced “a huge potential for collider bias” by controlling for energy intake, because both UPF consumption and cancer recurrence are causally associated with energy intake.
Bonaccio called that particular critique “a fair methodological question” but defended her work.
She pointed out that study participants were long-term survivors, which reduces the chance that their calorie intake was mainly driven by active cancer or treatment side effects.
“And,” she said, “our models include a wide set of baseline covariates that capture major determinants of both mortality and dietary intake.”
For Bonaccio, the take-home message for patients remains the same: “Emphasizing simple, home-cooked meals and traditional dietary patterns might be especially beneficial during the survivorship phase.”
The two US experts agreed that overall diet quality is key, with limits on UPFs being part of that. They also noted that the average American’s diet contains substantially more UPFs than what was seen in this Italian study.
“I spend 20 minutes of my 60-minute new patient consult in integrative oncology advising patients to eat an organic, plant-based, antioxidant-rich, anti-inflammatory, real and whole-foods diet,” Abrams said.
For her part, Shah said that cancer survivors should aim to get at least 25-30 grams of dietary fiber daily. She also suggested they avoid particular types of UPF with little to no nutritional value, such as processed meats, sugar-laden beverages, and fast food.
The study received no commercial funding. Bonaccio, Abrams, and Meyerowitz-Katz reported no financial disclosures. Shah is principle investigator on the NUTRIVENTION trial and reported receiving research funding and/or personal fees from Celgene/BMS, Janssen, and Sanofi.
A version of this article first appeared on Medscape.com.
Diets heavy in ultraprocessed foods (UPFs) are associated with earlier death in cancer survivors, a new study finds — though issues with the research design suggest that the findings should be taken with a grain of salt.
The study, published on February 4 in Cancer Epidemiology, Biomarkers & Prevention, is among the latest to point to health hazards from eating too many foods full of preservatives, dyes, and other industrially made ingredients.
These so-called UPFs have been linked to an increased risk for cancer, but whether they have any relationship to long-term survival after cancer has been unclear.
In the new study, of 802 adults with a previous cancer diagnosis, those in the top third for UPF consumption had a 48% higher rate of death from any cause over 15 years than those in the bottom third. Similarly, heavier UPF consumers had a 57% higher rate of death from cancer.
Those excess risks were seen after adjustment for numerous variables, including age, physical activity, BMI, smoking status, and socioeconomic indicators.
“Clinicians should encourage a shift toward fresh, minimally processed foods, [and] away from heavily industrially processed products,” said lead author Marialaura Bonaccio, PhD, of the Research Unit of Epidemiology and Prevention at IRCCS Neuromed in Pozzilli, Italy.
Oncologists not involved in the work said the findings support what researchers have suspected.
“UPFs have been linked to increased risk of obesity, diabetes, inflammation, cardiovascular disease, and...all-cause mortality and cardiovascular mortality,” said Urvi A. Shah, MD, a myeloma specialist who conducts nutrition research at Memorial Sloan Kettering Cancer Center in New York City. “However, there was limited data on cancer-specific mortality to date until this study.”
The findings also dovetail with recommendations on cancer prevention that emphasize diets rich in plant foods and low in processed foods, particularly those loaded with sugar, starch, and fat.
The study “may make oncologists think twice before assuring patients to ‘eat whatever you want, it doesn’t really matter’ because these investigators show that it does,” said Donald I. Abrams, MD, an integrative oncologist at the UCSF Osher Center for Integrative Health.
However, Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong in Wollongong, Australia, was not impressed by the analysis.
He pointed to several sources of potential bias and noted that the crude results actually showed that cancer survivors with the lowest UPF consumption had a higher rate of death than the heaviest consumers.
“The story of UPFs being bad is consistent with this data, but so is the story of UPFs being fine,” said Meyerowitz-Katz, who has written about prior research on the subject.
The broad questions of whether and how UPFs might be harming human health have been gaining research interest, partly because of their ubiquity. The foods reportedly make up about 60% of the typical American diet.
There’s no universal agreement on the precise definition of “ultraprocessed,” but researchers generally use the NOVA classification system, which assigns foods into one of four groups based on the level and purpose of processing. UPFs contain ingredients not found in the standard home kitchen (such as high-fructose corn syrup) and often have artificial flavors, colors, and other additives.
Examples of UPFs include the usual “junk food,” such as candy, soda, and processed meat, but many healthy-sounding products, such as flavored yogurts and plant-based milk, also qualify.
For their study, Bonaccio and her colleagues identified 802 cancer survivors from the Moli-sani cohort study (476 women and 326 men) who completed food-frequency questionnaires an average of 8 years postdiagnosis.
Using the NOVA system, the team calculated the amount of UPF in participants’ diets as both weight and energy ratios.
Over a median follow-up of nearly 15 years, there were 281 deaths. In the lowest third of UPF consumption (4.3% mean intake by weight), there were 3.3 deaths per 100 patient-years, compared with 2.4 per 100 patient-years in the highest UPF tertile (16.7% mean intake by weight). For cancer-specific deaths, those numbers were 1.5 and 1.4, respectively.
However, after adjustment for age and total energy intake, the top UPF-intake group showed significantly higher death rates. In the final model, which adjusted for > 20 variables, the hazard ratios for the highest versus lowest UPF consumption were 1.48 (95% CI, 1.07-2.03) for all-cause mortality and 1.57 (95% CI, 1.00-2.47) for cancer mortality.
To explore potential biological mechanisms, the researchers also analyzed certain biomarkers. They found that adjustment for inflammatory markers and resting heart rate at baseline attenuated the association between UPF and all-cause deaths by nearly 40%.
The authors acknowledged some limitations of their study, including its use of self-report and potential survivor bias.
But Meyerowitz-Katz found additional weak points. For one, he said the authors “downplayed” the impact of their analysis controlling for inflammation and heart rate.
“Inflammation and heart rate are both strong markers of future cancer risk,” Meyerowitz-Katz said. “In this cohort, there would be people who were already experiencing cancer recurrence, which is important to control for at baseline.”
He also highlighted a little-known but important issue in observational research called collider bias, which can create a false association between an exposure and outcome. In this study, he said, the researchers introduced “a huge potential for collider bias” by controlling for energy intake, because both UPF consumption and cancer recurrence are causally associated with energy intake.
Bonaccio called that particular critique “a fair methodological question” but defended her work.
She pointed out that study participants were long-term survivors, which reduces the chance that their calorie intake was mainly driven by active cancer or treatment side effects.
“And,” she said, “our models include a wide set of baseline covariates that capture major determinants of both mortality and dietary intake.”
For Bonaccio, the take-home message for patients remains the same: “Emphasizing simple, home-cooked meals and traditional dietary patterns might be especially beneficial during the survivorship phase.”
The two US experts agreed that overall diet quality is key, with limits on UPFs being part of that. They also noted that the average American’s diet contains substantially more UPFs than what was seen in this Italian study.
“I spend 20 minutes of my 60-minute new patient consult in integrative oncology advising patients to eat an organic, plant-based, antioxidant-rich, anti-inflammatory, real and whole-foods diet,” Abrams said.
For her part, Shah said that cancer survivors should aim to get at least 25-30 grams of dietary fiber daily. She also suggested they avoid particular types of UPF with little to no nutritional value, such as processed meats, sugar-laden beverages, and fast food.
The study received no commercial funding. Bonaccio, Abrams, and Meyerowitz-Katz reported no financial disclosures. Shah is principle investigator on the NUTRIVENTION trial and reported receiving research funding and/or personal fees from Celgene/BMS, Janssen, and Sanofi.
A version of this article first appeared on Medscape.com.
Diets heavy in ultraprocessed foods (UPFs) are associated with earlier death in cancer survivors, a new study finds — though issues with the research design suggest that the findings should be taken with a grain of salt.
The study, published on February 4 in Cancer Epidemiology, Biomarkers & Prevention, is among the latest to point to health hazards from eating too many foods full of preservatives, dyes, and other industrially made ingredients.
These so-called UPFs have been linked to an increased risk for cancer, but whether they have any relationship to long-term survival after cancer has been unclear.
In the new study, of 802 adults with a previous cancer diagnosis, those in the top third for UPF consumption had a 48% higher rate of death from any cause over 15 years than those in the bottom third. Similarly, heavier UPF consumers had a 57% higher rate of death from cancer.
Those excess risks were seen after adjustment for numerous variables, including age, physical activity, BMI, smoking status, and socioeconomic indicators.
“Clinicians should encourage a shift toward fresh, minimally processed foods, [and] away from heavily industrially processed products,” said lead author Marialaura Bonaccio, PhD, of the Research Unit of Epidemiology and Prevention at IRCCS Neuromed in Pozzilli, Italy.
Oncologists not involved in the work said the findings support what researchers have suspected.
“UPFs have been linked to increased risk of obesity, diabetes, inflammation, cardiovascular disease, and...all-cause mortality and cardiovascular mortality,” said Urvi A. Shah, MD, a myeloma specialist who conducts nutrition research at Memorial Sloan Kettering Cancer Center in New York City. “However, there was limited data on cancer-specific mortality to date until this study.”
The findings also dovetail with recommendations on cancer prevention that emphasize diets rich in plant foods and low in processed foods, particularly those loaded with sugar, starch, and fat.
The study “may make oncologists think twice before assuring patients to ‘eat whatever you want, it doesn’t really matter’ because these investigators show that it does,” said Donald I. Abrams, MD, an integrative oncologist at the UCSF Osher Center for Integrative Health.
However, Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong in Wollongong, Australia, was not impressed by the analysis.
He pointed to several sources of potential bias and noted that the crude results actually showed that cancer survivors with the lowest UPF consumption had a higher rate of death than the heaviest consumers.
“The story of UPFs being bad is consistent with this data, but so is the story of UPFs being fine,” said Meyerowitz-Katz, who has written about prior research on the subject.
The broad questions of whether and how UPFs might be harming human health have been gaining research interest, partly because of their ubiquity. The foods reportedly make up about 60% of the typical American diet.
There’s no universal agreement on the precise definition of “ultraprocessed,” but researchers generally use the NOVA classification system, which assigns foods into one of four groups based on the level and purpose of processing. UPFs contain ingredients not found in the standard home kitchen (such as high-fructose corn syrup) and often have artificial flavors, colors, and other additives.
Examples of UPFs include the usual “junk food,” such as candy, soda, and processed meat, but many healthy-sounding products, such as flavored yogurts and plant-based milk, also qualify.
For their study, Bonaccio and her colleagues identified 802 cancer survivors from the Moli-sani cohort study (476 women and 326 men) who completed food-frequency questionnaires an average of 8 years postdiagnosis.
Using the NOVA system, the team calculated the amount of UPF in participants’ diets as both weight and energy ratios.
Over a median follow-up of nearly 15 years, there were 281 deaths. In the lowest third of UPF consumption (4.3% mean intake by weight), there were 3.3 deaths per 100 patient-years, compared with 2.4 per 100 patient-years in the highest UPF tertile (16.7% mean intake by weight). For cancer-specific deaths, those numbers were 1.5 and 1.4, respectively.
However, after adjustment for age and total energy intake, the top UPF-intake group showed significantly higher death rates. In the final model, which adjusted for > 20 variables, the hazard ratios for the highest versus lowest UPF consumption were 1.48 (95% CI, 1.07-2.03) for all-cause mortality and 1.57 (95% CI, 1.00-2.47) for cancer mortality.
To explore potential biological mechanisms, the researchers also analyzed certain biomarkers. They found that adjustment for inflammatory markers and resting heart rate at baseline attenuated the association between UPF and all-cause deaths by nearly 40%.
The authors acknowledged some limitations of their study, including its use of self-report and potential survivor bias.
But Meyerowitz-Katz found additional weak points. For one, he said the authors “downplayed” the impact of their analysis controlling for inflammation and heart rate.
“Inflammation and heart rate are both strong markers of future cancer risk,” Meyerowitz-Katz said. “In this cohort, there would be people who were already experiencing cancer recurrence, which is important to control for at baseline.”
He also highlighted a little-known but important issue in observational research called collider bias, which can create a false association between an exposure and outcome. In this study, he said, the researchers introduced “a huge potential for collider bias” by controlling for energy intake, because both UPF consumption and cancer recurrence are causally associated with energy intake.
Bonaccio called that particular critique “a fair methodological question” but defended her work.
She pointed out that study participants were long-term survivors, which reduces the chance that their calorie intake was mainly driven by active cancer or treatment side effects.
“And,” she said, “our models include a wide set of baseline covariates that capture major determinants of both mortality and dietary intake.”
For Bonaccio, the take-home message for patients remains the same: “Emphasizing simple, home-cooked meals and traditional dietary patterns might be especially beneficial during the survivorship phase.”
The two US experts agreed that overall diet quality is key, with limits on UPFs being part of that. They also noted that the average American’s diet contains substantially more UPFs than what was seen in this Italian study.
“I spend 20 minutes of my 60-minute new patient consult in integrative oncology advising patients to eat an organic, plant-based, antioxidant-rich, anti-inflammatory, real and whole-foods diet,” Abrams said.
For her part, Shah said that cancer survivors should aim to get at least 25-30 grams of dietary fiber daily. She also suggested they avoid particular types of UPF with little to no nutritional value, such as processed meats, sugar-laden beverages, and fast food.
The study received no commercial funding. Bonaccio, Abrams, and Meyerowitz-Katz reported no financial disclosures. Shah is principle investigator on the NUTRIVENTION trial and reported receiving research funding and/or personal fees from Celgene/BMS, Janssen, and Sanofi.
A version of this article first appeared on Medscape.com.
Do Ultraprocessed Foods Impact Survival After Cancer?
Do Ultraprocessed Foods Impact Survival After Cancer?
Alcohol and CRC: These Drinking Patterns May Influence Risk
Alcohol and CRC: These Drinking Patterns May Influence Risk
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Alcohol and CRC: These Drinking Patterns May Influence Risk
Alcohol and CRC: These Drinking Patterns May Influence Risk
New Insights on Treatment of Veterans With CLL From ASH 2025
New Insights on Treatment of Veterans With CLL From ASH 2025

Insights from phase 3 trials presented at the 2025 American Society of Hematology Annual Meeting may expand treatment options for veterans with chronic lymphocytic leukemia (CLL), as discussed by Dr Nicholas Burwick from University of Washington, Seattle.
Dr Burwick begins with the CLL17 trial examining continuous treatment vs fixed-duration therapy in previously untreated patients. The fixed-duration therapy showed noninferior results. Research pertaining to the veterans population in the phase 2 Benefit VA study may offer further insight on these results.
He next discusses the first study comparing the noncovalent BTKi pirtobrutinib to covalent ibrutinib in both treatment-naive patients and those with relapsed/refractory CLL. Pirtobrutinib demonstrated noninferiority in each subgroup.
Pirtobrutinib was compared to bendamustine plus rituximab in the treatment-naive setting in the next study, showing favorable progression-free survival and a notable trend in overall survival. These two trials could lead to use of a noncovalent BTKi as frontline therapy.
Dr Burwick then turns to 6-year follow-up in the SEQUOIA trial, in which zanubrutinib showed sustained superiority over bendamustine and rituximab. He notes that acalabrutinib is currently the preferred BTKi therapy for veterans with CLL.
Finally, he discusses a study examining combination acalabrutinib and venetoclax, to which obinutuzumab was added either early or late. The rate of infections was significantly higher in the early group, an issue of particular concern in the veterans population.
--
Nicholas R. Burwick, MD, VA Puget Sound Health Care System; Associate Professor, Department of Medicine, Division of Hematology, University of Washington, Seattle; President, AVAHO - Association of VA Hematology/Oncology
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.

Insights from phase 3 trials presented at the 2025 American Society of Hematology Annual Meeting may expand treatment options for veterans with chronic lymphocytic leukemia (CLL), as discussed by Dr Nicholas Burwick from University of Washington, Seattle.
Dr Burwick begins with the CLL17 trial examining continuous treatment vs fixed-duration therapy in previously untreated patients. The fixed-duration therapy showed noninferior results. Research pertaining to the veterans population in the phase 2 Benefit VA study may offer further insight on these results.
He next discusses the first study comparing the noncovalent BTKi pirtobrutinib to covalent ibrutinib in both treatment-naive patients and those with relapsed/refractory CLL. Pirtobrutinib demonstrated noninferiority in each subgroup.
Pirtobrutinib was compared to bendamustine plus rituximab in the treatment-naive setting in the next study, showing favorable progression-free survival and a notable trend in overall survival. These two trials could lead to use of a noncovalent BTKi as frontline therapy.
Dr Burwick then turns to 6-year follow-up in the SEQUOIA trial, in which zanubrutinib showed sustained superiority over bendamustine and rituximab. He notes that acalabrutinib is currently the preferred BTKi therapy for veterans with CLL.
Finally, he discusses a study examining combination acalabrutinib and venetoclax, to which obinutuzumab was added either early or late. The rate of infections was significantly higher in the early group, an issue of particular concern in the veterans population.
--
Nicholas R. Burwick, MD, VA Puget Sound Health Care System; Associate Professor, Department of Medicine, Division of Hematology, University of Washington, Seattle; President, AVAHO - Association of VA Hematology/Oncology
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.

Insights from phase 3 trials presented at the 2025 American Society of Hematology Annual Meeting may expand treatment options for veterans with chronic lymphocytic leukemia (CLL), as discussed by Dr Nicholas Burwick from University of Washington, Seattle.
Dr Burwick begins with the CLL17 trial examining continuous treatment vs fixed-duration therapy in previously untreated patients. The fixed-duration therapy showed noninferior results. Research pertaining to the veterans population in the phase 2 Benefit VA study may offer further insight on these results.
He next discusses the first study comparing the noncovalent BTKi pirtobrutinib to covalent ibrutinib in both treatment-naive patients and those with relapsed/refractory CLL. Pirtobrutinib demonstrated noninferiority in each subgroup.
Pirtobrutinib was compared to bendamustine plus rituximab in the treatment-naive setting in the next study, showing favorable progression-free survival and a notable trend in overall survival. These two trials could lead to use of a noncovalent BTKi as frontline therapy.
Dr Burwick then turns to 6-year follow-up in the SEQUOIA trial, in which zanubrutinib showed sustained superiority over bendamustine and rituximab. He notes that acalabrutinib is currently the preferred BTKi therapy for veterans with CLL.
Finally, he discusses a study examining combination acalabrutinib and venetoclax, to which obinutuzumab was added either early or late. The rate of infections was significantly higher in the early group, an issue of particular concern in the veterans population.
--
Nicholas R. Burwick, MD, VA Puget Sound Health Care System; Associate Professor, Department of Medicine, Division of Hematology, University of Washington, Seattle; President, AVAHO - Association of VA Hematology/Oncology
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.
New Insights on Treatment of Veterans With CLL From ASH 2025
New Insights on Treatment of Veterans With CLL From ASH 2025

When Does Spleen Size Signal Cancer Risk?
When Does Spleen Size Signal Cancer Risk?
TOPLINE:
Spleen volume larger than the 99th percentile was associated with an 11-fold increased risk for hematologic cancer compared with normal volumes, with 5-year risks as high as 46% among men aged 70 years or older. Significant risks for cirrhosis and liver cancer were also seen.
METHODOLOGY:
- Splenomegaly is often detected incidentally during imaging, but guidelines vary as to the threshold that should prompt evaluation — ranging from a spleen length of 120 mm to 150 mm. However, up to 21% of healthy individuals have spleen lengths > 120 mm, which could lead to unnecessary follow-up of low-risk patients.
- Researchers used data from two general population cohorts to evaluate the relative and absolute risks for hematologic cancer and liver disease (two common causes of spleen enlargement) among individuals with incidentally detected splenomegaly. They included 8459 Danish adults (57% female; median age, 61 years) and 38,607 UK adults (51.9% female; median age, 65 years) who underwent CT or MRI scans as part of study procedures.
- Spleen length and volume measurements were available from the Danish cohort, while only spleen volume was available from the UK group.
- Participants were followed for a median of 5 years after imaging to assess the incidence of hematologic cancers (both cohorts) and cirrhosis and liver cancer (UK cohort only). Hazard ratios were adjusted for age, sex, smoking status, alcohol consumption, comorbidities, and C-reactive protein levels.
TAKEAWAY:
- In the Danish cohort, the relative risk for any hematologic cancer was significantly increased among individuals with spleen lengths above the 99th percentile (≥ 135 mm) compared with those with spleen lengths in the 26th-74th percentile (hazard ratio [HR], 5.11; P < .001). Among individuals with a spleen length ≥ 140 mm, absolute 5-year risks reached 23% for men aged 70 years or older and 12% for women in that age group.
- Risks were even more pronounced for Danish adults with a spleen volume above the 99th percentile — > 433 mL. Relative to the 26th-74th percentile, their risk for any hematologic cancer was 11-fold higher (HR, 11.08; P < .001). Among people with a spleen volume ≥ 500 mL, 5-year risks reached 46% for men aged 70 years or older and 27% for women in that age group.
- Findings were similar in the UK cohort. Among individuals with a spleen volume above the 99th percentile (> 386 mL), the risk for hematologic cancer increased nearly 12-fold (HR, 11.82; P < .001). With a spleen volume ≥ 500 mL, 5-year risks reached 21% for men aged 70 years or older and 18% for women in that age group. Relative risks were also elevated — by 1.55-2.94 — among individuals in the 75th-99th percentile (199 mL-386 mL).
- The risks for liver disease began to rise substantially at a spleen volume ≥ 400 mL. Absolute 5-year risks for cirrhosis reached 10.8% for men and 9.3% for women aged 70 years or older with a spleen volume ≥ 500 mL. For liver cancer, 5-year risks reached 3.2% and 1.2% for men and women in that age group with a spleen volume ≥ 400 mL.
IN PRACTICE:
“To our knowledge, no previous studies have examined risk of hematologic cancers by spleen length or volume in incidentally detected splenomegaly,” the authors of the study wrote. “Risk was moderately increased at spleen length of 130-139 mm or spleen volume of 400-499 mL, where diagnostic workup may be considered, and more pronounced at spleen length of 140 mm or greater or spleen volume of 500 mL or greater, supporting that diagnostic workup may likely be relevant.”
They stressed, however, that the study participants were asymptomatic, and the underlying reason for imaging should always be considered.
SOURCE:
The study, led by Jens Helby, MD, PhD, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark, was published online in JAMA Oncology.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Boserup Foundation, Copenhagen University Hospital – Rigshospitalet, and Sanofi A/S. Helby reported having financial relationships with Sanofi and Disc Medicine. Additional disclosures are available in the full article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Spleen volume larger than the 99th percentile was associated with an 11-fold increased risk for hematologic cancer compared with normal volumes, with 5-year risks as high as 46% among men aged 70 years or older. Significant risks for cirrhosis and liver cancer were also seen.
METHODOLOGY:
- Splenomegaly is often detected incidentally during imaging, but guidelines vary as to the threshold that should prompt evaluation — ranging from a spleen length of 120 mm to 150 mm. However, up to 21% of healthy individuals have spleen lengths > 120 mm, which could lead to unnecessary follow-up of low-risk patients.
- Researchers used data from two general population cohorts to evaluate the relative and absolute risks for hematologic cancer and liver disease (two common causes of spleen enlargement) among individuals with incidentally detected splenomegaly. They included 8459 Danish adults (57% female; median age, 61 years) and 38,607 UK adults (51.9% female; median age, 65 years) who underwent CT or MRI scans as part of study procedures.
- Spleen length and volume measurements were available from the Danish cohort, while only spleen volume was available from the UK group.
- Participants were followed for a median of 5 years after imaging to assess the incidence of hematologic cancers (both cohorts) and cirrhosis and liver cancer (UK cohort only). Hazard ratios were adjusted for age, sex, smoking status, alcohol consumption, comorbidities, and C-reactive protein levels.
TAKEAWAY:
- In the Danish cohort, the relative risk for any hematologic cancer was significantly increased among individuals with spleen lengths above the 99th percentile (≥ 135 mm) compared with those with spleen lengths in the 26th-74th percentile (hazard ratio [HR], 5.11; P < .001). Among individuals with a spleen length ≥ 140 mm, absolute 5-year risks reached 23% for men aged 70 years or older and 12% for women in that age group.
- Risks were even more pronounced for Danish adults with a spleen volume above the 99th percentile — > 433 mL. Relative to the 26th-74th percentile, their risk for any hematologic cancer was 11-fold higher (HR, 11.08; P < .001). Among people with a spleen volume ≥ 500 mL, 5-year risks reached 46% for men aged 70 years or older and 27% for women in that age group.
- Findings were similar in the UK cohort. Among individuals with a spleen volume above the 99th percentile (> 386 mL), the risk for hematologic cancer increased nearly 12-fold (HR, 11.82; P < .001). With a spleen volume ≥ 500 mL, 5-year risks reached 21% for men aged 70 years or older and 18% for women in that age group. Relative risks were also elevated — by 1.55-2.94 — among individuals in the 75th-99th percentile (199 mL-386 mL).
- The risks for liver disease began to rise substantially at a spleen volume ≥ 400 mL. Absolute 5-year risks for cirrhosis reached 10.8% for men and 9.3% for women aged 70 years or older with a spleen volume ≥ 500 mL. For liver cancer, 5-year risks reached 3.2% and 1.2% for men and women in that age group with a spleen volume ≥ 400 mL.
IN PRACTICE:
“To our knowledge, no previous studies have examined risk of hematologic cancers by spleen length or volume in incidentally detected splenomegaly,” the authors of the study wrote. “Risk was moderately increased at spleen length of 130-139 mm or spleen volume of 400-499 mL, where diagnostic workup may be considered, and more pronounced at spleen length of 140 mm or greater or spleen volume of 500 mL or greater, supporting that diagnostic workup may likely be relevant.”
They stressed, however, that the study participants were asymptomatic, and the underlying reason for imaging should always be considered.
SOURCE:
The study, led by Jens Helby, MD, PhD, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark, was published online in JAMA Oncology.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Boserup Foundation, Copenhagen University Hospital – Rigshospitalet, and Sanofi A/S. Helby reported having financial relationships with Sanofi and Disc Medicine. Additional disclosures are available in the full article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Spleen volume larger than the 99th percentile was associated with an 11-fold increased risk for hematologic cancer compared with normal volumes, with 5-year risks as high as 46% among men aged 70 years or older. Significant risks for cirrhosis and liver cancer were also seen.
METHODOLOGY:
- Splenomegaly is often detected incidentally during imaging, but guidelines vary as to the threshold that should prompt evaluation — ranging from a spleen length of 120 mm to 150 mm. However, up to 21% of healthy individuals have spleen lengths > 120 mm, which could lead to unnecessary follow-up of low-risk patients.
- Researchers used data from two general population cohorts to evaluate the relative and absolute risks for hematologic cancer and liver disease (two common causes of spleen enlargement) among individuals with incidentally detected splenomegaly. They included 8459 Danish adults (57% female; median age, 61 years) and 38,607 UK adults (51.9% female; median age, 65 years) who underwent CT or MRI scans as part of study procedures.
- Spleen length and volume measurements were available from the Danish cohort, while only spleen volume was available from the UK group.
- Participants were followed for a median of 5 years after imaging to assess the incidence of hematologic cancers (both cohorts) and cirrhosis and liver cancer (UK cohort only). Hazard ratios were adjusted for age, sex, smoking status, alcohol consumption, comorbidities, and C-reactive protein levels.
TAKEAWAY:
- In the Danish cohort, the relative risk for any hematologic cancer was significantly increased among individuals with spleen lengths above the 99th percentile (≥ 135 mm) compared with those with spleen lengths in the 26th-74th percentile (hazard ratio [HR], 5.11; P < .001). Among individuals with a spleen length ≥ 140 mm, absolute 5-year risks reached 23% for men aged 70 years or older and 12% for women in that age group.
- Risks were even more pronounced for Danish adults with a spleen volume above the 99th percentile — > 433 mL. Relative to the 26th-74th percentile, their risk for any hematologic cancer was 11-fold higher (HR, 11.08; P < .001). Among people with a spleen volume ≥ 500 mL, 5-year risks reached 46% for men aged 70 years or older and 27% for women in that age group.
- Findings were similar in the UK cohort. Among individuals with a spleen volume above the 99th percentile (> 386 mL), the risk for hematologic cancer increased nearly 12-fold (HR, 11.82; P < .001). With a spleen volume ≥ 500 mL, 5-year risks reached 21% for men aged 70 years or older and 18% for women in that age group. Relative risks were also elevated — by 1.55-2.94 — among individuals in the 75th-99th percentile (199 mL-386 mL).
- The risks for liver disease began to rise substantially at a spleen volume ≥ 400 mL. Absolute 5-year risks for cirrhosis reached 10.8% for men and 9.3% for women aged 70 years or older with a spleen volume ≥ 500 mL. For liver cancer, 5-year risks reached 3.2% and 1.2% for men and women in that age group with a spleen volume ≥ 400 mL.
IN PRACTICE:
“To our knowledge, no previous studies have examined risk of hematologic cancers by spleen length or volume in incidentally detected splenomegaly,” the authors of the study wrote. “Risk was moderately increased at spleen length of 130-139 mm or spleen volume of 400-499 mL, where diagnostic workup may be considered, and more pronounced at spleen length of 140 mm or greater or spleen volume of 500 mL or greater, supporting that diagnostic workup may likely be relevant.”
They stressed, however, that the study participants were asymptomatic, and the underlying reason for imaging should always be considered.
SOURCE:
The study, led by Jens Helby, MD, PhD, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark, was published online in JAMA Oncology.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Boserup Foundation, Copenhagen University Hospital – Rigshospitalet, and Sanofi A/S. Helby reported having financial relationships with Sanofi and Disc Medicine. Additional disclosures are available in the full article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
When Does Spleen Size Signal Cancer Risk?
When Does Spleen Size Signal Cancer Risk?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
A New First-Line Option in BRAF-Mutant Metastatic CRC?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A randomized trial of 32 patients with advanced pancreatic cancer found that early access to medical cannabis patients' symptom burden, with minimal side effects.
METHODOLOGY:
- Patients with pancreatic cancer commonly experience moderate-to-severe pain, nausea, insomnia, and other symptoms that significantly affect their quality of life. Current management approaches are insufficient. Preliminary evidence suggests that medical cannabis has efficacy against multiple cancer-related symptoms, but high-quality data remain limited due to regulatory barriers.
- Researchers conducted a pilot randomized, waitlist-controlled trial involving 32 patients (median age, 71 years) with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma and at least one burdensome symptom.
- Patients were randomly assigned in a 1:1 ratio to early (0-8 weeks) or delayed (9-16 weeks) cannabis intervention through the Minnesota Medical Cannabis Program, which provided cannabis products and education in how to use them.
- Primary outcomes focused on feasibility, while secondary outcomes examined acceptability, changes in symptom burden, and quality of life in exploratory efficacy analyses.
TAKEAWAY:
- At baseline, patients reported a substantial moderate-to-severe symptom burden — most commonly insomnia (85%), pain (77%), and appetite loss (69%); 10 patients (31%) were using opioids.
- The study met all of its feasibility metrics, with 74% of the patients meeting enrollment eligibility and 81% complying with their random assignment. Patients in the arm with early cannabis access typically picked up their products 3 days after starting chemotherapy. Most used tablets or other oral cannabis formulations.
- At 8 weeks, patients in the early-access arm experienced numerically higher rates of improvement in pain (44% vs 20%; P = .35), appetite (56% vs 30%; P = .37), and insomnia (67% vs 30%; P = .18), as well as a reduction in opioid use. Their rates of potential cannabis side effects, including dry mouth, dizziness, and concentration problems, were lower compared with the waitlist group — possibly, the authors noted, due to their education to “start low, go slow.”
- Patients made a median of two trips to a cannabis dispensary during the study period, and most said that using cannabis was “easy” and “practical.”
IN PRACTICE:
“Early access to medical cannabis was associated with improvement in certain symptoms, such as insomnia, with minimal harms,” the authors wrote, adding that the research design offers a model collaboration between investigators and state cannabis programs.
“The encouraging preliminary efficacy and safety of cannabis in managing symptoms supports further exploration," they concluded.
SOURCE:
The study was led by Dylan Zylla, MD, MS, of HealthPartners Institute, Cancer Research Center, Minneapolis, Minnesota. It was presented on January 9 at the ASCO Gastrointestinal Cancers Symposium 2026 and simultaneously published in JCO Oncology Practice.
LIMITATIONS:
The trial was small and the 8-week primary study period precluded conclusions about longer-term benefits and safety. Generalizability may be limited as the trial was conducted in a single state with a predominantly urban and White patient population. Additionally, heterogeneity in state cannabis programs and laws may limit national applicability.
DISCLOSURES:
The study was supported by philanthropic support to the HealthPartners Cancer Research Center. Cannabis products were provided by Vireo Health (GreenGoods, Minnesota). Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Cannabis May Ease Symptoms in Advanced Pancreatic Cancer
Home Screening Cost-Effective for Anal Cancer
Home Screening Cost-Effective for Anal Cancer
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A recent analysis suggested that home-based screening for anal cancer is a cost-effective way to increase screening compared to clinic-based screening. The study found that a home-based approach led to higher participation rates (89.2% vs 74.2% for a clinic-based approach) among sexual and gender minority individuals and was cost-effective, costing $25.19 per additional individual screened when accounting for both direct and indirect costs and $132.36 per additional individual screened when only accounting for direct medical costs.
METHODOLOGY:
- Anal cancer screening is recommended for high-risk populations, such as sexual and gender minority individuals. However, it's unclear how cost-effective home-based self-sampling is compared to clinic-based screening.
- Researchers conducted an economic evaluation using data from a randomized clinical trial that included 240 sexual and gender minority individuals in Milwaukee from January 2020 to August 2022.
- Participants, aged ≥ 25 years, were randomized to either home-based self-sampling or clinic-based screening.
- Researchers evaluated direct home-based screening costs from the trial, and sourced clinic-based costs from the Medicare reimbursement schedule. Travel and time costs were determined from participant self-reports.
- The primary outcome was the incremental cost-effectiveness ratio (ICER), which was the additional cost needed to increase screening participation by one person. The researchers calculated ICERs from both a healthcare payer and societal perspective. The healthcare perspective included only direct medical costs and the societal perspective accounted for direct medical costs as well as indirect time and travel costs.
TAKEAWAY:
- Home-based screening led to higher participation rates than clinic-based screening—89.2% vs 74.2%—with 107 participants completing home-based screening compared with 89 participants doing clinic-based screening.
- The cost per participant was $64.18 for home-based screening and $60.40 for clinic-based screening from the societal perspective, and $61.91 for home-based screening and $42.06 for clinic-based screening from the healthcare payer perspective.
- With home-based screening, the ICER per additional screened participant was $25.19 from a societal perspective and $132.36 from a healthcare payer perspective.
- From the societal perspective, the probability that home-based screening was cost-effective compared with clinic-based screening was nearly 50% at a willingness-to-pay threshold of $25 and 99.99% at a threshold of $100. From the healthcare perspective, the probability was 3.8% at a threshold of $100 and 90.9% at a threshold of $200.
IN PRACTICE:
"These findings suggest that home-based screening promises to be a cost-effective option to enhance anal cancer screening participation," the study authors concluded.
SOURCE:
The study, led by Haluk Damgacioglu, PhD, Department of Public Health Sciences, Medical University of South Carolina in Charleston, South Carolina, was published online in JAMA Network Open.
LIMITATIONS:
The study was conducted in an urban setting where proximity to clinics may reduce structural barriers, potentially limiting generalizability to rural areas where longer travel distances and limited clinician availability could affect participation rates. The analysis did not include downstream steps such as follow-up clinic visits, confirmatory testing, treatment of precancerous lesions, or cancer prevention outcomes. While home-based screening participants were required to visit clinics for digital anal rectal examination to exclude prevalent anal cancer, these follow-up visit costs were not included in the cost-effectiveness analysis.
DISCLOSURES:
Elizabeth Chiao, PhD, reported receiving grants from the National Institutes of Health during the study. Jennifer S. Smith, PhD, MPH, disclosed receiving personal fees from Hologic, Inc., and materials for research purposes from Rovers Medical Devices. Ashish A. Deshmukh, PhD, MPH, reported receiving personal fees from Value Analytics Lab. Alan G. Nyitray, PhD, reported receiving grants from the National Cancer Institute and test kits from Copan Diagnostics.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Home Screening Cost-Effective for Anal Cancer
Home Screening Cost-Effective for Anal Cancer