User login
Black women show heightened risk for depression after early pregnancy loss
Black women are significantly more likely than non-Black women to develop major depression within a month of early pregnancy loss, based on data from a secondary analysis of 300 women.
Approximately 25% of women experience a pregnancy loss, and many of these women are at increased risk for psychological problems including major depression, wrote Jade M. Shorter, MD, of Stanford (Calif.) University, and colleagues.
Data from previous studies show that Black women experience higher rates of perinatal depression, compared with other racial groups, and that stress and adverse childhood experiences also are higher among Black individuals, they noted.
“Based on data showing higher rates of pregnancy loss, perinatal depression, and perceived stress in Black women, we hypothesized that the odds of having risk for major depression or high perceived stress 30 days after miscarriage treatment would be higher in Black participants when compared with non-Black participants,” they wrote.
In a study published in Obstetrics & Gynecology, the researchers conducted a secondary analysis of 300 women aged 18 years and older with nonviable intrauterine pregnancy between 5 and 12 weeks’ gestation who were part of a larger randomized trial conducted between May 2014 and April 2017. The women were randomized to medical treatment of either mifepristone 200 mg orally plus misoprostol 800 mcg vaginally after 24 hours or the usual treatment of misoprostol 800 mcg vaginally.
Depression was assessed using the Center for Epidemiological Studies–Depression scale, Perceived Stress Scale, and Adverse Childhood Experience scale. Adverse childhood experience data were collected at baseline; stress and depression data were collected at baseline and at 30 days after treatment.
A total of 120 participants self-identified as Black and 155 self-identified as non-Black.
Depression risk doubles in Black women
At 30 days after treatment for early pregnancy loss, 24% of women met criteria for major depression, including 57% of Black women and 43% of non-Black women. The odds of depression were twice as high among Black women, compared with non-Black women (odds ratio 2.02), and Black women were more likely to be younger, have lower levels of education, and have public insurance, compared with non-Black women.
The association between Black race and increased risk for depression at 30 days after treatment persisted after controlling for factors including parity, baseline depression, and adverse childhood experiences, the researchers noted.
The study findings were limited by several factors, including the potential for different depression risk in those from the original study who did and did not participate in the secondary analysis and by the use of the original Adverse Childhood Experience survey, which may not reflect the range of adversity faced by different demographic groups, the researchers noted. However, the results were strengthened by the collection of 30-day outcome data in the clinical setting and by the diverse study population.
“These findings should be not be used to stigmatize Black women; instead, it is important to consider the complex systemic factors, such as structural racism, that are the root causes of disparate health outcomes,” and to support appropriate mental health resources and interventions for all women who experience early pregnancy loss, the researchers emphasized.
Recognize risks, reduce barriers
“Early pregnancy loss is unfortunately a common event that affects 15%-20% of pregnancies,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
However, “the mental health impact of early pregnancy loss is understudied, and as a result mental health disorders often go unnoticed and untreated,” she said.
Growing evidence shows that Black women in particular are at greater risk for chronic stressors that affect their overall health. “Black women are more likely to be exposed to trauma in their lifetime, such as physical and emotional abuse, neglect, and household instability, all of which predispose women to mental health disorders such as depression. Untreated maternal depression has an impact on future pregnancy outcomes such as increasing the risk of having a preterm delivery and/or delivering a low-birth-weight baby, outcomes where Black women are at disproportionately high risk in comparison to non-Black women,” Dr. Krishna said.
“This study found that the risk for depression after an early pregnancy loss is twice as high for Black women in comparison to non-Black women. The findings of this study further underscore the fact that Black women are at disproportionate high risk for poor maternal and pregnancy outcomes,” Dr. Krishna added.
“Structural racism is a major barrier to caring for the health of Black women. To care for the health of Black women we must overcome racial and ethnic disparities. Addressing disparities involves a multitiered approach, including identifying and addressing implicit bias in health care and improving access to health care for women of color,” she said.
“Additional research is needed in identifying at-risk women and mental health interventions that can improve the mental well-being of women after adverse pregnancy outcomes such as early pregnancy loss,” Dr. Krishna concluded.
The study was supported by the Society of Family Planning Research Fund. Lead author Dr. Shorter had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose.
SOURCE: Shorter JM et al. Obstet Gynecol. 2020 Dec 3. doi: 10.1097/AOG.0000000000004212.
Black women are significantly more likely than non-Black women to develop major depression within a month of early pregnancy loss, based on data from a secondary analysis of 300 women.
Approximately 25% of women experience a pregnancy loss, and many of these women are at increased risk for psychological problems including major depression, wrote Jade M. Shorter, MD, of Stanford (Calif.) University, and colleagues.
Data from previous studies show that Black women experience higher rates of perinatal depression, compared with other racial groups, and that stress and adverse childhood experiences also are higher among Black individuals, they noted.
“Based on data showing higher rates of pregnancy loss, perinatal depression, and perceived stress in Black women, we hypothesized that the odds of having risk for major depression or high perceived stress 30 days after miscarriage treatment would be higher in Black participants when compared with non-Black participants,” they wrote.
In a study published in Obstetrics & Gynecology, the researchers conducted a secondary analysis of 300 women aged 18 years and older with nonviable intrauterine pregnancy between 5 and 12 weeks’ gestation who were part of a larger randomized trial conducted between May 2014 and April 2017. The women were randomized to medical treatment of either mifepristone 200 mg orally plus misoprostol 800 mcg vaginally after 24 hours or the usual treatment of misoprostol 800 mcg vaginally.
Depression was assessed using the Center for Epidemiological Studies–Depression scale, Perceived Stress Scale, and Adverse Childhood Experience scale. Adverse childhood experience data were collected at baseline; stress and depression data were collected at baseline and at 30 days after treatment.
A total of 120 participants self-identified as Black and 155 self-identified as non-Black.
Depression risk doubles in Black women
At 30 days after treatment for early pregnancy loss, 24% of women met criteria for major depression, including 57% of Black women and 43% of non-Black women. The odds of depression were twice as high among Black women, compared with non-Black women (odds ratio 2.02), and Black women were more likely to be younger, have lower levels of education, and have public insurance, compared with non-Black women.
The association between Black race and increased risk for depression at 30 days after treatment persisted after controlling for factors including parity, baseline depression, and adverse childhood experiences, the researchers noted.
The study findings were limited by several factors, including the potential for different depression risk in those from the original study who did and did not participate in the secondary analysis and by the use of the original Adverse Childhood Experience survey, which may not reflect the range of adversity faced by different demographic groups, the researchers noted. However, the results were strengthened by the collection of 30-day outcome data in the clinical setting and by the diverse study population.
“These findings should be not be used to stigmatize Black women; instead, it is important to consider the complex systemic factors, such as structural racism, that are the root causes of disparate health outcomes,” and to support appropriate mental health resources and interventions for all women who experience early pregnancy loss, the researchers emphasized.
Recognize risks, reduce barriers
“Early pregnancy loss is unfortunately a common event that affects 15%-20% of pregnancies,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
However, “the mental health impact of early pregnancy loss is understudied, and as a result mental health disorders often go unnoticed and untreated,” she said.
Growing evidence shows that Black women in particular are at greater risk for chronic stressors that affect their overall health. “Black women are more likely to be exposed to trauma in their lifetime, such as physical and emotional abuse, neglect, and household instability, all of which predispose women to mental health disorders such as depression. Untreated maternal depression has an impact on future pregnancy outcomes such as increasing the risk of having a preterm delivery and/or delivering a low-birth-weight baby, outcomes where Black women are at disproportionately high risk in comparison to non-Black women,” Dr. Krishna said.
“This study found that the risk for depression after an early pregnancy loss is twice as high for Black women in comparison to non-Black women. The findings of this study further underscore the fact that Black women are at disproportionate high risk for poor maternal and pregnancy outcomes,” Dr. Krishna added.
“Structural racism is a major barrier to caring for the health of Black women. To care for the health of Black women we must overcome racial and ethnic disparities. Addressing disparities involves a multitiered approach, including identifying and addressing implicit bias in health care and improving access to health care for women of color,” she said.
“Additional research is needed in identifying at-risk women and mental health interventions that can improve the mental well-being of women after adverse pregnancy outcomes such as early pregnancy loss,” Dr. Krishna concluded.
The study was supported by the Society of Family Planning Research Fund. Lead author Dr. Shorter had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose.
SOURCE: Shorter JM et al. Obstet Gynecol. 2020 Dec 3. doi: 10.1097/AOG.0000000000004212.
Black women are significantly more likely than non-Black women to develop major depression within a month of early pregnancy loss, based on data from a secondary analysis of 300 women.
Approximately 25% of women experience a pregnancy loss, and many of these women are at increased risk for psychological problems including major depression, wrote Jade M. Shorter, MD, of Stanford (Calif.) University, and colleagues.
Data from previous studies show that Black women experience higher rates of perinatal depression, compared with other racial groups, and that stress and adverse childhood experiences also are higher among Black individuals, they noted.
“Based on data showing higher rates of pregnancy loss, perinatal depression, and perceived stress in Black women, we hypothesized that the odds of having risk for major depression or high perceived stress 30 days after miscarriage treatment would be higher in Black participants when compared with non-Black participants,” they wrote.
In a study published in Obstetrics & Gynecology, the researchers conducted a secondary analysis of 300 women aged 18 years and older with nonviable intrauterine pregnancy between 5 and 12 weeks’ gestation who were part of a larger randomized trial conducted between May 2014 and April 2017. The women were randomized to medical treatment of either mifepristone 200 mg orally plus misoprostol 800 mcg vaginally after 24 hours or the usual treatment of misoprostol 800 mcg vaginally.
Depression was assessed using the Center for Epidemiological Studies–Depression scale, Perceived Stress Scale, and Adverse Childhood Experience scale. Adverse childhood experience data were collected at baseline; stress and depression data were collected at baseline and at 30 days after treatment.
A total of 120 participants self-identified as Black and 155 self-identified as non-Black.
Depression risk doubles in Black women
At 30 days after treatment for early pregnancy loss, 24% of women met criteria for major depression, including 57% of Black women and 43% of non-Black women. The odds of depression were twice as high among Black women, compared with non-Black women (odds ratio 2.02), and Black women were more likely to be younger, have lower levels of education, and have public insurance, compared with non-Black women.
The association between Black race and increased risk for depression at 30 days after treatment persisted after controlling for factors including parity, baseline depression, and adverse childhood experiences, the researchers noted.
The study findings were limited by several factors, including the potential for different depression risk in those from the original study who did and did not participate in the secondary analysis and by the use of the original Adverse Childhood Experience survey, which may not reflect the range of adversity faced by different demographic groups, the researchers noted. However, the results were strengthened by the collection of 30-day outcome data in the clinical setting and by the diverse study population.
“These findings should be not be used to stigmatize Black women; instead, it is important to consider the complex systemic factors, such as structural racism, that are the root causes of disparate health outcomes,” and to support appropriate mental health resources and interventions for all women who experience early pregnancy loss, the researchers emphasized.
Recognize risks, reduce barriers
“Early pregnancy loss is unfortunately a common event that affects 15%-20% of pregnancies,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
However, “the mental health impact of early pregnancy loss is understudied, and as a result mental health disorders often go unnoticed and untreated,” she said.
Growing evidence shows that Black women in particular are at greater risk for chronic stressors that affect their overall health. “Black women are more likely to be exposed to trauma in their lifetime, such as physical and emotional abuse, neglect, and household instability, all of which predispose women to mental health disorders such as depression. Untreated maternal depression has an impact on future pregnancy outcomes such as increasing the risk of having a preterm delivery and/or delivering a low-birth-weight baby, outcomes where Black women are at disproportionately high risk in comparison to non-Black women,” Dr. Krishna said.
“This study found that the risk for depression after an early pregnancy loss is twice as high for Black women in comparison to non-Black women. The findings of this study further underscore the fact that Black women are at disproportionate high risk for poor maternal and pregnancy outcomes,” Dr. Krishna added.
“Structural racism is a major barrier to caring for the health of Black women. To care for the health of Black women we must overcome racial and ethnic disparities. Addressing disparities involves a multitiered approach, including identifying and addressing implicit bias in health care and improving access to health care for women of color,” she said.
“Additional research is needed in identifying at-risk women and mental health interventions that can improve the mental well-being of women after adverse pregnancy outcomes such as early pregnancy loss,” Dr. Krishna concluded.
The study was supported by the Society of Family Planning Research Fund. Lead author Dr. Shorter had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose.
SOURCE: Shorter JM et al. Obstet Gynecol. 2020 Dec 3. doi: 10.1097/AOG.0000000000004212.
FROM OBSTETRICS & GYNECOLOGY
Outpatient penicillin allergy testing found safe in pregnancy
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
FROM OBSTETRICS & GYNECOLOGY
Higher dose maximizes effects of magnesium sulfate for obese women
Obese women may benefit from a higher dose of magnesium sulfate to protect against preeclampsia, based on data from a randomized trial.
Pharmacokinetic models have shown that, “in women who received a 4-g intravenous loading dose followed by a 2-g/h IV maintenance dose, obese women took approximately twice as long as women of mean body weight in the sample to achieve these previously accepted therapeutic serum magnesium concentrations,” which suggests the need for alternate dosing based on body mass index, wrote Kathleen F. Brookfield, MD, of Oregon Health & Science University, Portland, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 37 women aged 15-45 years with a BMI of 35 kg/m2 or higher who were at least 32 weeks’ gestation to receive the standard Zuspan regimen of magnesium sulfate (4 g intravenous loading dose, followed by a 1-g/hour infusion) or to higher dosing (6 g IV loading dose, followed by a 2-g/hour infusion).
Higher dose increases effectiveness
Serum magnesium concentrations were measured at baseline, and after administration of magnesium sulfate at 1 hour, 4 hours, and delivery; the primary outcome was the proportion of women with subtherapeutic serum magnesium concentrations (less than 4.8 mg/dL) 4 hours after administration.
After 4 hours, the average magnesium sulfate concentrations were significantly higher for women in the high-dose group vs. the standard group (4.41 mg/dL vs. 3.53 mg/dL). In addition, 100% of women in the standard group had subtherapeutic serum magnesium concentrations compared with 63% of the high-dose group.
No significant differences in maternal side effects or neonatal outcomes occurred between the groups. However, rates of nausea and flushing were higher in the higher dose group, compared with the standard group (10.5% vs. 5.5% and 5.2% vs. 0%, respectively).
The study findings were limited by several factors including the lack of statistical power to evaluate clinical outcomes and lack of generalizability to extremely obese patients, as well as to settings in which the higher-dose regimen is already the standard treatment, the researchers noted. However, the results were strengthened by the use of prospective pharmacokinetic data to determine dosing.
The researchers also noted that the study was not powered to examine preeclampsia as an outcome “and there is no evidence to date to suggest women in the United States with higher BMIs are more likely to experience eclampsia,” they said. “Therefore, we caution against universally applying the study findings to obese women without also considering the potential for increased toxicity with higher dosing regimens,” they added.
Current results may not affect practice
The study objectives are unclear, as they do not change the dosing for magnesium sulfate already in use, said Baha M. Sibai, MD, of the University of Texas Health Science Center at Houston, in an interview.
Dr. Sibai said he was not surprised by the findings. “This information has been known for almost 30 years as to serum levels with different dosing irrespective of BMI,” he said. Based on current evidence, Dr. Sibai advised clinicians “not to change your practice, since there are no therapeutic levels for preventing seizures.” In fact, “the largest trial that included 10,000 women showed no difference in the rate of eclampsia between 4 grams loading with 1 g/hour [magnesium sulfate] and 6 g loading and 2 g/hour,” he explained.
Future research should focus on different outcomes, said Dr. Sibai. “The outcome should be eclampsia and not serum levels. This requires studying over 6,000 women,” he emphasized.
The study was supported by the National Institutes of Health Loan Repayment Program and a Mission Support Award from Oregon Health & Science University to Dr. Brookfield and by the Oregon Clinical & Translational Research Institute grant. Dr. Brookfield also disclosed funding from the World Health Organization. Dr. Sibai had no financial conflicts to disclose.
SOURCE: Brookfield KF et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004137.
Obese women may benefit from a higher dose of magnesium sulfate to protect against preeclampsia, based on data from a randomized trial.
Pharmacokinetic models have shown that, “in women who received a 4-g intravenous loading dose followed by a 2-g/h IV maintenance dose, obese women took approximately twice as long as women of mean body weight in the sample to achieve these previously accepted therapeutic serum magnesium concentrations,” which suggests the need for alternate dosing based on body mass index, wrote Kathleen F. Brookfield, MD, of Oregon Health & Science University, Portland, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 37 women aged 15-45 years with a BMI of 35 kg/m2 or higher who were at least 32 weeks’ gestation to receive the standard Zuspan regimen of magnesium sulfate (4 g intravenous loading dose, followed by a 1-g/hour infusion) or to higher dosing (6 g IV loading dose, followed by a 2-g/hour infusion).
Higher dose increases effectiveness
Serum magnesium concentrations were measured at baseline, and after administration of magnesium sulfate at 1 hour, 4 hours, and delivery; the primary outcome was the proportion of women with subtherapeutic serum magnesium concentrations (less than 4.8 mg/dL) 4 hours after administration.
After 4 hours, the average magnesium sulfate concentrations were significantly higher for women in the high-dose group vs. the standard group (4.41 mg/dL vs. 3.53 mg/dL). In addition, 100% of women in the standard group had subtherapeutic serum magnesium concentrations compared with 63% of the high-dose group.
No significant differences in maternal side effects or neonatal outcomes occurred between the groups. However, rates of nausea and flushing were higher in the higher dose group, compared with the standard group (10.5% vs. 5.5% and 5.2% vs. 0%, respectively).
The study findings were limited by several factors including the lack of statistical power to evaluate clinical outcomes and lack of generalizability to extremely obese patients, as well as to settings in which the higher-dose regimen is already the standard treatment, the researchers noted. However, the results were strengthened by the use of prospective pharmacokinetic data to determine dosing.
The researchers also noted that the study was not powered to examine preeclampsia as an outcome “and there is no evidence to date to suggest women in the United States with higher BMIs are more likely to experience eclampsia,” they said. “Therefore, we caution against universally applying the study findings to obese women without also considering the potential for increased toxicity with higher dosing regimens,” they added.
Current results may not affect practice
The study objectives are unclear, as they do not change the dosing for magnesium sulfate already in use, said Baha M. Sibai, MD, of the University of Texas Health Science Center at Houston, in an interview.
Dr. Sibai said he was not surprised by the findings. “This information has been known for almost 30 years as to serum levels with different dosing irrespective of BMI,” he said. Based on current evidence, Dr. Sibai advised clinicians “not to change your practice, since there are no therapeutic levels for preventing seizures.” In fact, “the largest trial that included 10,000 women showed no difference in the rate of eclampsia between 4 grams loading with 1 g/hour [magnesium sulfate] and 6 g loading and 2 g/hour,” he explained.
Future research should focus on different outcomes, said Dr. Sibai. “The outcome should be eclampsia and not serum levels. This requires studying over 6,000 women,” he emphasized.
The study was supported by the National Institutes of Health Loan Repayment Program and a Mission Support Award from Oregon Health & Science University to Dr. Brookfield and by the Oregon Clinical & Translational Research Institute grant. Dr. Brookfield also disclosed funding from the World Health Organization. Dr. Sibai had no financial conflicts to disclose.
SOURCE: Brookfield KF et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004137.
Obese women may benefit from a higher dose of magnesium sulfate to protect against preeclampsia, based on data from a randomized trial.
Pharmacokinetic models have shown that, “in women who received a 4-g intravenous loading dose followed by a 2-g/h IV maintenance dose, obese women took approximately twice as long as women of mean body weight in the sample to achieve these previously accepted therapeutic serum magnesium concentrations,” which suggests the need for alternate dosing based on body mass index, wrote Kathleen F. Brookfield, MD, of Oregon Health & Science University, Portland, and colleagues.
In a study published in Obstetrics & Gynecology, the researchers randomized 37 women aged 15-45 years with a BMI of 35 kg/m2 or higher who were at least 32 weeks’ gestation to receive the standard Zuspan regimen of magnesium sulfate (4 g intravenous loading dose, followed by a 1-g/hour infusion) or to higher dosing (6 g IV loading dose, followed by a 2-g/hour infusion).
Higher dose increases effectiveness
Serum magnesium concentrations were measured at baseline, and after administration of magnesium sulfate at 1 hour, 4 hours, and delivery; the primary outcome was the proportion of women with subtherapeutic serum magnesium concentrations (less than 4.8 mg/dL) 4 hours after administration.
After 4 hours, the average magnesium sulfate concentrations were significantly higher for women in the high-dose group vs. the standard group (4.41 mg/dL vs. 3.53 mg/dL). In addition, 100% of women in the standard group had subtherapeutic serum magnesium concentrations compared with 63% of the high-dose group.
No significant differences in maternal side effects or neonatal outcomes occurred between the groups. However, rates of nausea and flushing were higher in the higher dose group, compared with the standard group (10.5% vs. 5.5% and 5.2% vs. 0%, respectively).
The study findings were limited by several factors including the lack of statistical power to evaluate clinical outcomes and lack of generalizability to extremely obese patients, as well as to settings in which the higher-dose regimen is already the standard treatment, the researchers noted. However, the results were strengthened by the use of prospective pharmacokinetic data to determine dosing.
The researchers also noted that the study was not powered to examine preeclampsia as an outcome “and there is no evidence to date to suggest women in the United States with higher BMIs are more likely to experience eclampsia,” they said. “Therefore, we caution against universally applying the study findings to obese women without also considering the potential for increased toxicity with higher dosing regimens,” they added.
Current results may not affect practice
The study objectives are unclear, as they do not change the dosing for magnesium sulfate already in use, said Baha M. Sibai, MD, of the University of Texas Health Science Center at Houston, in an interview.
Dr. Sibai said he was not surprised by the findings. “This information has been known for almost 30 years as to serum levels with different dosing irrespective of BMI,” he said. Based on current evidence, Dr. Sibai advised clinicians “not to change your practice, since there are no therapeutic levels for preventing seizures.” In fact, “the largest trial that included 10,000 women showed no difference in the rate of eclampsia between 4 grams loading with 1 g/hour [magnesium sulfate] and 6 g loading and 2 g/hour,” he explained.
Future research should focus on different outcomes, said Dr. Sibai. “The outcome should be eclampsia and not serum levels. This requires studying over 6,000 women,” he emphasized.
The study was supported by the National Institutes of Health Loan Repayment Program and a Mission Support Award from Oregon Health & Science University to Dr. Brookfield and by the Oregon Clinical & Translational Research Institute grant. Dr. Brookfield also disclosed funding from the World Health Organization. Dr. Sibai had no financial conflicts to disclose.
SOURCE: Brookfield KF et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004137.
FROM OBSTETRICS & GYNECOLOGY
Home pregnancy tests—Is ectopic always on your mind?
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
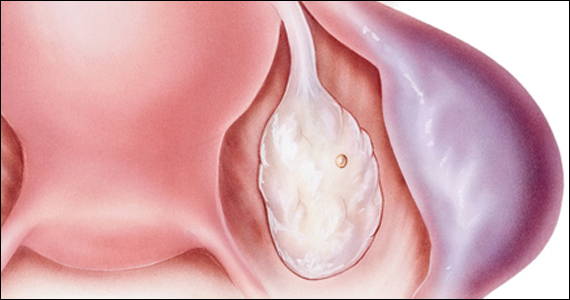
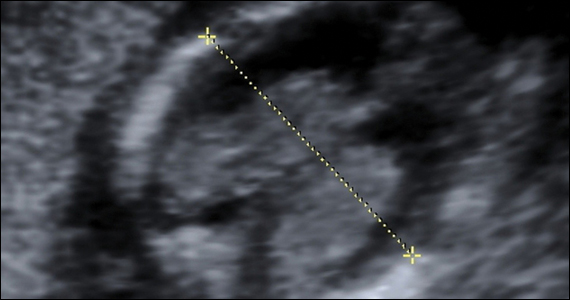
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
2021 Update on obstetrics
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
When ultrasonography reveals a fetal abdominal wall defect
CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.
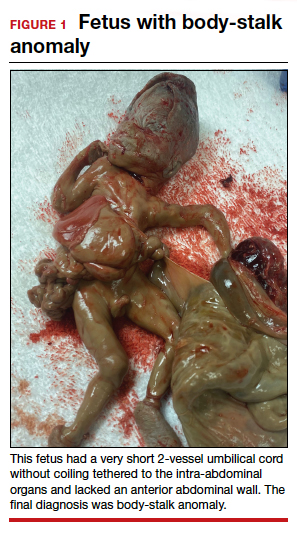
Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.
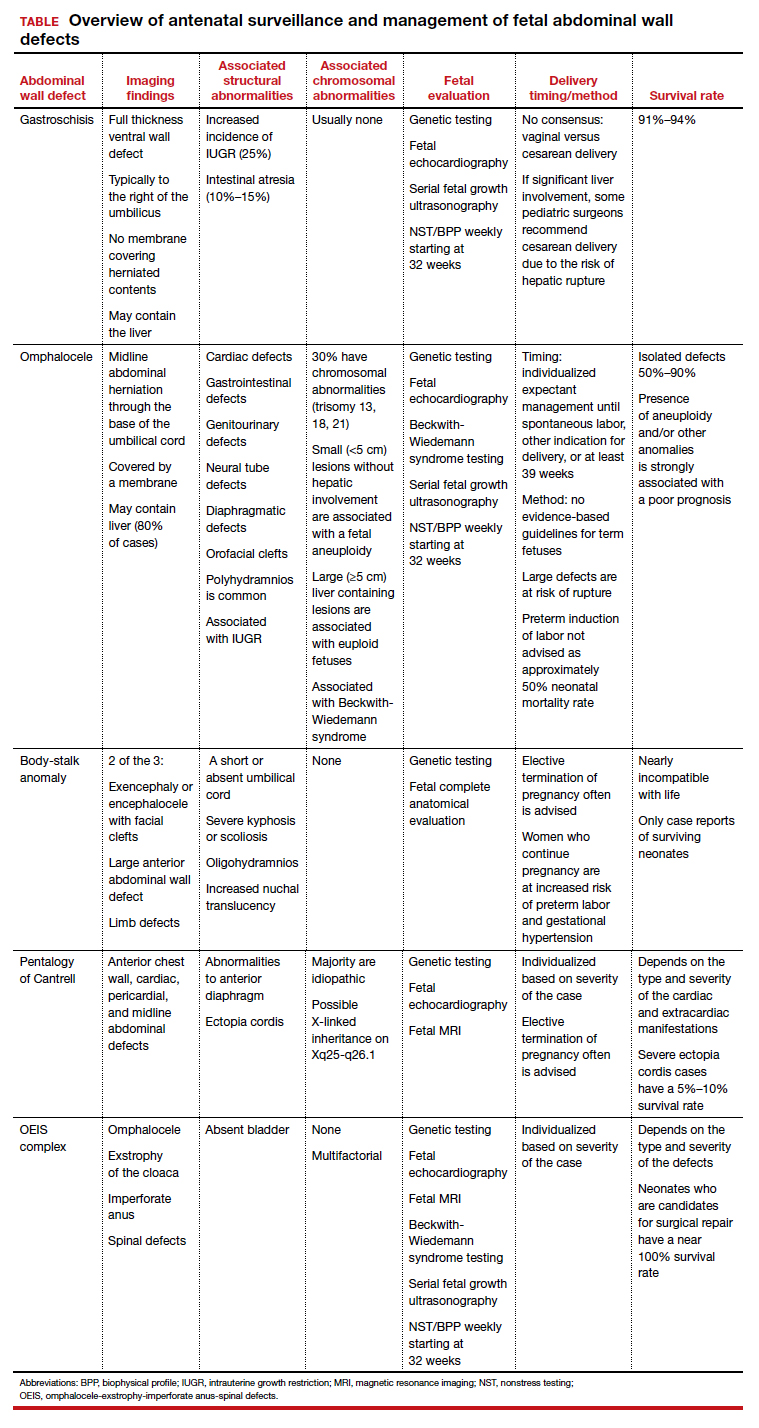
Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10
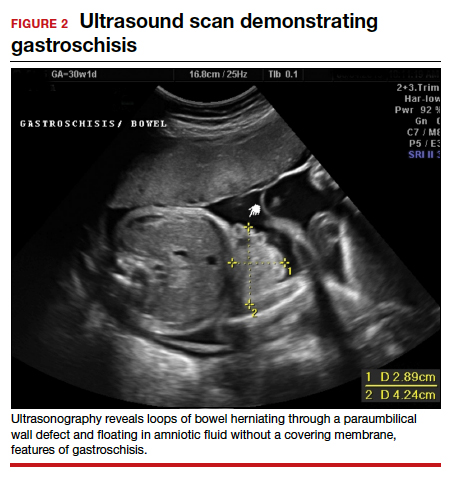
Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29
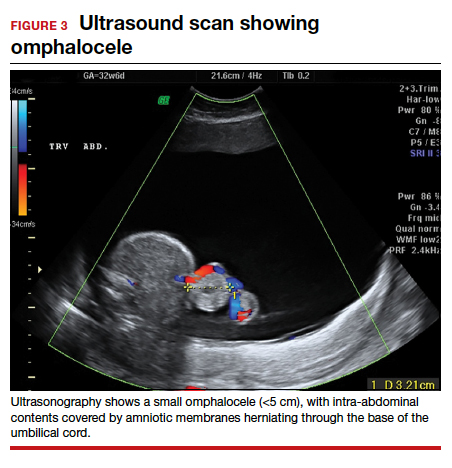
Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.

CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.

Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.

Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10

Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29

Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.

CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.

Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.

Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10

Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29

Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.
Optimizing the use of oxytocin on labor and delivery

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.
Is it safe to be pregnant during the COVID-19 pandemic?
Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.

Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●

Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.
Racial, ethnic disparities in maternal mortality, morbidity persist
Racial and ethnic disparities in maternal and infant outcomes persist in the United States, with Black women being 3-4 times more likely to die of pregnancy-related causes, compared with Latina and non-Latina white women, Elizabeth Howell, MD, said in a presentation at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Location matters, too, and ethnic disparities appear to transcend class, said Dr. Howell of Penn Medicine, Philadelphia. In New York City, for example, Black women are 8-12 times more likely to die than white women regardless of educational attainment.
Dr. Howell cited the definitions of health equity and health disparities as defined by Paula Braveman, MD, in 2014 in the journal Public Health Reports, as follows: “Health equity means social justice in health (i.e., no one is denied the possibility to be healthy for belonging to a group that has historically been economically/socially disadvantaged. Health disparities are the metric we use to measure progress toward achieving health equity.”
Structural racism and discrimination contribute to disparities in maternal and infant morbidity and mortality in several ways, she said. Patient factors include sociodemographics, age, education, poverty, insurance, marital status, language, and literacy. In addition, a patient’s knowledge, beliefs, and health behaviors, as well as stress and self-efficacy are involved. Community factors such as crime, poverty, and community support play a role.
“These factors contribute to the health status of a woman when she becomes pregnant,” Dr. Howell said. “These factors contribute as the woman goes through the health system.”
Then provider factors that impact maternal and infant morbidity and mortality include knowledge, experience, implicit bias, cultural humility, and communication; these factors affect the quality and delivery of neonatal care, and can impact outcomes, Dr. Howell said.
“It is really important to note that many of these pregnancy-related deaths are thought to be preventable,” she said. “They are often caused by delays in diagnosis, problems with communication, and other system failures. Site care has received a great deal of attention” in recent years, the ob.gyn. noted.
How hospital quality contributes to health disparities
Dr. Howell shared data from a pair of National Institutes of Health–funded parallel group studies she conducted at New York City hospitals to investigate the contribution of hospital quality to health disparities in severe maternal morbidity and very preterm birth (prior to 32 weeks).
The researchers used vital statistics linked with discharge abstracts for all New York City deliveries between 2011-2013 and 2010-2014. They conducted a logistic regression analysis and ranked hospitals based on metrics of severe maternal morbidity and very preterm birth, and assessed differences by race in each delivery location.
Overall, Black women were almost three times as likely and Latina women were almost twice as likely as White women to experience some type of severe maternal morbidity, with rates of 4.2%, 2.7%, and 1.5%, respectively.
The researchers also ranked hospitals, and found a wide variation; women delivering in the lowest-ranked hospitals had six times the rate of severe maternal morbidity. They also conducted a simulation/thought exercise and determined that the hospital of delivery accounted for approximately 48% of the disparity in severe maternal morbidity between Black and White women.
Results were similar in the parallel study of very preterm birth rates in New York City hospitals, which were 32%, 28%, and 23% for Black, Latina, and White women, respectively.
The researchers also conducted interviews with personnel including chief medical officers, neonatal ICU directors, nurses, and respiratory therapists. The final phase of the research, which is ongoing, is the dissemination of the information, said Dr. Howell.
Overall, the high-performing hospitals were more likely to focus on standards and standardized care, stronger nurse/physician communication, greater awareness of the potential impact of racism on care, and greater sharing of performance data.
Women who participated in focus groups reported a range of experiences, but women of color were likely to report poor communication, feeling traumatized, and not being heard.
Study implications
Dr. Howell discussed the implications of her study in a question and answer session. “It is incredibly important for us to think about all the levers that we have to address disparities.”
“It is a complex web of factors, but quality of care is one of those mechanisms, and it is something we can do something about,” she noted.
In response to a question about whether women should know the rates of adverse outcomes at various hospitals, she said, “I think we have a responsibility to come up with quality of care measures that are informative to the women we care for.”
Much of obstetric quality issues focus on overuse of resources, “but that doesn’t help us reduce disparities,” she said.
Dr. Howell had no financial conflicts to disclose.
Racial and ethnic disparities in maternal and infant outcomes persist in the United States, with Black women being 3-4 times more likely to die of pregnancy-related causes, compared with Latina and non-Latina white women, Elizabeth Howell, MD, said in a presentation at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Location matters, too, and ethnic disparities appear to transcend class, said Dr. Howell of Penn Medicine, Philadelphia. In New York City, for example, Black women are 8-12 times more likely to die than white women regardless of educational attainment.
Dr. Howell cited the definitions of health equity and health disparities as defined by Paula Braveman, MD, in 2014 in the journal Public Health Reports, as follows: “Health equity means social justice in health (i.e., no one is denied the possibility to be healthy for belonging to a group that has historically been economically/socially disadvantaged. Health disparities are the metric we use to measure progress toward achieving health equity.”
Structural racism and discrimination contribute to disparities in maternal and infant morbidity and mortality in several ways, she said. Patient factors include sociodemographics, age, education, poverty, insurance, marital status, language, and literacy. In addition, a patient’s knowledge, beliefs, and health behaviors, as well as stress and self-efficacy are involved. Community factors such as crime, poverty, and community support play a role.
“These factors contribute to the health status of a woman when she becomes pregnant,” Dr. Howell said. “These factors contribute as the woman goes through the health system.”
Then provider factors that impact maternal and infant morbidity and mortality include knowledge, experience, implicit bias, cultural humility, and communication; these factors affect the quality and delivery of neonatal care, and can impact outcomes, Dr. Howell said.
“It is really important to note that many of these pregnancy-related deaths are thought to be preventable,” she said. “They are often caused by delays in diagnosis, problems with communication, and other system failures. Site care has received a great deal of attention” in recent years, the ob.gyn. noted.
How hospital quality contributes to health disparities
Dr. Howell shared data from a pair of National Institutes of Health–funded parallel group studies she conducted at New York City hospitals to investigate the contribution of hospital quality to health disparities in severe maternal morbidity and very preterm birth (prior to 32 weeks).
The researchers used vital statistics linked with discharge abstracts for all New York City deliveries between 2011-2013 and 2010-2014. They conducted a logistic regression analysis and ranked hospitals based on metrics of severe maternal morbidity and very preterm birth, and assessed differences by race in each delivery location.
Overall, Black women were almost three times as likely and Latina women were almost twice as likely as White women to experience some type of severe maternal morbidity, with rates of 4.2%, 2.7%, and 1.5%, respectively.
The researchers also ranked hospitals, and found a wide variation; women delivering in the lowest-ranked hospitals had six times the rate of severe maternal morbidity. They also conducted a simulation/thought exercise and determined that the hospital of delivery accounted for approximately 48% of the disparity in severe maternal morbidity between Black and White women.
Results were similar in the parallel study of very preterm birth rates in New York City hospitals, which were 32%, 28%, and 23% for Black, Latina, and White women, respectively.
The researchers also conducted interviews with personnel including chief medical officers, neonatal ICU directors, nurses, and respiratory therapists. The final phase of the research, which is ongoing, is the dissemination of the information, said Dr. Howell.
Overall, the high-performing hospitals were more likely to focus on standards and standardized care, stronger nurse/physician communication, greater awareness of the potential impact of racism on care, and greater sharing of performance data.
Women who participated in focus groups reported a range of experiences, but women of color were likely to report poor communication, feeling traumatized, and not being heard.
Study implications
Dr. Howell discussed the implications of her study in a question and answer session. “It is incredibly important for us to think about all the levers that we have to address disparities.”
“It is a complex web of factors, but quality of care is one of those mechanisms, and it is something we can do something about,” she noted.
In response to a question about whether women should know the rates of adverse outcomes at various hospitals, she said, “I think we have a responsibility to come up with quality of care measures that are informative to the women we care for.”
Much of obstetric quality issues focus on overuse of resources, “but that doesn’t help us reduce disparities,” she said.
Dr. Howell had no financial conflicts to disclose.
Racial and ethnic disparities in maternal and infant outcomes persist in the United States, with Black women being 3-4 times more likely to die of pregnancy-related causes, compared with Latina and non-Latina white women, Elizabeth Howell, MD, said in a presentation at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Location matters, too, and ethnic disparities appear to transcend class, said Dr. Howell of Penn Medicine, Philadelphia. In New York City, for example, Black women are 8-12 times more likely to die than white women regardless of educational attainment.
Dr. Howell cited the definitions of health equity and health disparities as defined by Paula Braveman, MD, in 2014 in the journal Public Health Reports, as follows: “Health equity means social justice in health (i.e., no one is denied the possibility to be healthy for belonging to a group that has historically been economically/socially disadvantaged. Health disparities are the metric we use to measure progress toward achieving health equity.”
Structural racism and discrimination contribute to disparities in maternal and infant morbidity and mortality in several ways, she said. Patient factors include sociodemographics, age, education, poverty, insurance, marital status, language, and literacy. In addition, a patient’s knowledge, beliefs, and health behaviors, as well as stress and self-efficacy are involved. Community factors such as crime, poverty, and community support play a role.
“These factors contribute to the health status of a woman when she becomes pregnant,” Dr. Howell said. “These factors contribute as the woman goes through the health system.”
Then provider factors that impact maternal and infant morbidity and mortality include knowledge, experience, implicit bias, cultural humility, and communication; these factors affect the quality and delivery of neonatal care, and can impact outcomes, Dr. Howell said.
“It is really important to note that many of these pregnancy-related deaths are thought to be preventable,” she said. “They are often caused by delays in diagnosis, problems with communication, and other system failures. Site care has received a great deal of attention” in recent years, the ob.gyn. noted.
How hospital quality contributes to health disparities
Dr. Howell shared data from a pair of National Institutes of Health–funded parallel group studies she conducted at New York City hospitals to investigate the contribution of hospital quality to health disparities in severe maternal morbidity and very preterm birth (prior to 32 weeks).
The researchers used vital statistics linked with discharge abstracts for all New York City deliveries between 2011-2013 and 2010-2014. They conducted a logistic regression analysis and ranked hospitals based on metrics of severe maternal morbidity and very preterm birth, and assessed differences by race in each delivery location.
Overall, Black women were almost three times as likely and Latina women were almost twice as likely as White women to experience some type of severe maternal morbidity, with rates of 4.2%, 2.7%, and 1.5%, respectively.
The researchers also ranked hospitals, and found a wide variation; women delivering in the lowest-ranked hospitals had six times the rate of severe maternal morbidity. They also conducted a simulation/thought exercise and determined that the hospital of delivery accounted for approximately 48% of the disparity in severe maternal morbidity between Black and White women.
Results were similar in the parallel study of very preterm birth rates in New York City hospitals, which were 32%, 28%, and 23% for Black, Latina, and White women, respectively.
The researchers also conducted interviews with personnel including chief medical officers, neonatal ICU directors, nurses, and respiratory therapists. The final phase of the research, which is ongoing, is the dissemination of the information, said Dr. Howell.
Overall, the high-performing hospitals were more likely to focus on standards and standardized care, stronger nurse/physician communication, greater awareness of the potential impact of racism on care, and greater sharing of performance data.
Women who participated in focus groups reported a range of experiences, but women of color were likely to report poor communication, feeling traumatized, and not being heard.
Study implications
Dr. Howell discussed the implications of her study in a question and answer session. “It is incredibly important for us to think about all the levers that we have to address disparities.”
“It is a complex web of factors, but quality of care is one of those mechanisms, and it is something we can do something about,” she noted.
In response to a question about whether women should know the rates of adverse outcomes at various hospitals, she said, “I think we have a responsibility to come up with quality of care measures that are informative to the women we care for.”
Much of obstetric quality issues focus on overuse of resources, “but that doesn’t help us reduce disparities,” she said.
Dr. Howell had no financial conflicts to disclose.
FROM ACOG 2020
Microplastics permeate human placentas
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
FROM ENVIRONMENT INTERNATIONAL