User login
MS bears no effect on certain pregnancy complications, stillbirth, or congenital deformation
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
, according to a new study published online Feb. 3 in Neurology Clinical Practice. While pregnancy and childbirth are not regarded as conditions that engender high-risk pregnancy in the MS population, previous studies evaluating the effects of MS on pregnancy and parturition have yet to fully elucidate some outcomes for pregnant women and their babies in multiple sclerosis.
“Women with multiple sclerosis may be understandably concerned about the risk of pregnancy,” said Melinda Magyari, MD, PhD, a consultant at the University of Copenhagen. “While previous research has shown there is no higher risk of birth defect for babies born to women with MS, we wanted to find out if women with MS are at risk for a variety of pregnancy complications.”
MS is regarded as a progressive, neurological disease mediated by the immune system that demands careful consideration of numerous situations and life changes including family planning. The MS population is overwhelmingly female, as women account for three out of every four cases of MS. The majority of these women range from 20 to 40 years of age at the time of being diagnosed with MS. Despite the unknown risks of pregnancy-related complications and various perinatal complications in this patient population, women who have MS are not discouraged from conceiving.
Assessing pregnancy outcomes
This nationwide, population-based, cross-sectional study evaluated the pregnancies of 2,930 women with MS between Jan. 1, 1997, and Dec. 31, 2016, registered in the Danish Multiple Sclerosis Registry. The researchers compared pregnancy-related and prenatal outcomes to a 5% random sample of 56,958 randomly-selected pregnant women from Denmark’s general population who did not have MS. They found no differences in the risks associated with several pregnancy-related complications (e.g., preeclampsia, gestational diabetes, or placental complications), emergency Cesarean section (C-section), instrumental delivery, stillbirth, preterm birth, or congenital malformation. Apgar scores were low in both groups. A composite of various biometrics in newborns such as reflexes, muscle tone, and heart rate immediately following birth, the Apgar score is used to help assess the neonatal health, with a value of less than 7 considered low. Here, preterm birth is defined as delivery occurring before 37 weeks of gestation, and stillbirth describes a fetus born dead after 22 weeks of gestation.
Women in the MS cohort were more likely to have elective C-sections (odds ratio, 2.89 [95% confidence interval, 1.65-2.16]), induced labor (OR, 1.15 [95%CI, 1.01-1.31]) and have babies with low birth weight based on their gestational age (OR, 1.29 [95% CI, 1.04-1.60]). Nearly 30% of babies born in the cohort (n = 851) were born to mothers who had received disease-modifying therapy (DMT). Neonates exposed to DMT weighed an average of 116 g less than babies born to mothers who had not received DMT (3,378 g vs. 3,494 g) with a slightly lower gestational age (39 weeks as opposed to 40 weeks). However, babies born to mothers with MS were less likely to show signs of asphyxia (OR, 0.87 [95% CI, 0.78-0.97]) than the comparison cohort.
“We found overall, their pregnancies were just as healthy as those of the moms without MS,” Dr. Magyari said.
Comprehensive data
Denmark’s health care system has two key features that make it an attractive setting in which to conduct such a study – the first being its universal health care. The second advantage is that the country enacted several health registries in the 1970s and 1980s that enable the collection of more comprehensive data. For example, the Danish National Patient Register is a population-based registry that spans the entire nation, facilitating epidemiological research with what the study’s authors describe as “high generalizability.” Providing additional insights regarding the patient story helps add context to pregnancy and outcomes. Among the data collected on the women studied were demographics, contact information, and abortions, both spontaneous and medically induced. The country uses other databases and registries to capture additional data. For example, the Register of Legally Induced Abortions provides data regarding the context of medically induced abortions. In contrast, the Danish Medical Birth Registry provides context regarding specified variables regarding women’s pregnancies, delivery, and perinatal outcomes. Finally, the population’s education register offers information regarding patients’ educational history.
A key strength of this study is that the long duration of follow-up data from the Danish Medical Birth Registry, along with its comprehensive data collection, eliminates recall bias. Universal access to health care also improves the generalizability of data. A limitation of the study is its lack of data on maternal smoking and its effects on low gestational weights. The study also has some data gaps, including body mass index information missing from a large portion of the cohort. Finally, the sample size of newborns born to mothers who had received DMT therapy within the last 6 months of gestation was too underpowered to stratify based on first on first-line or second-line treatment.
Dr. Magyari served on scientific advisory boards for Biogen, Sanofi, Teva, Roche, Novartis, and Merck. She has also received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi, Genzyme, and has received research support and support for congress participation from Biogen, Genzyme, Teva, Roche, Merck, and Novartis. Coauthors disclosed various fees received from Merck, Novartis, Biogen, Roche, Sanofi Genzyme, and Teva.
FROM NEUROLOGY CLINICAL PRACTICE
Survey finds practice gaps in counseling women with hidradenitis suppurativa about pregnancy
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
FROM JAMA DERMATOLOGY
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
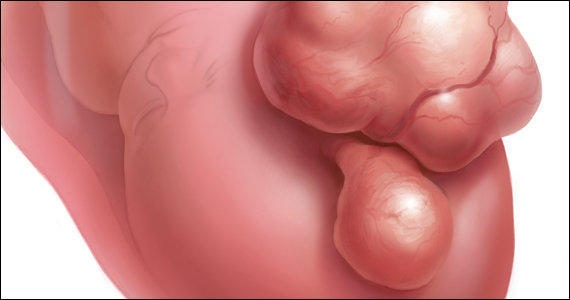
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
A case of BV during pregnancy: Best management approach
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
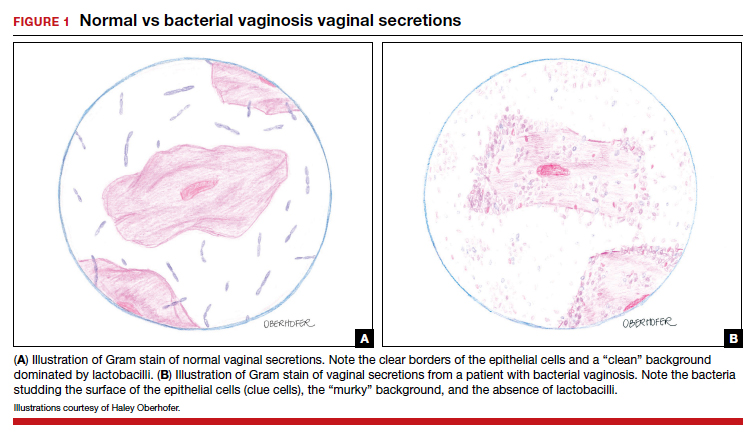
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.
BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
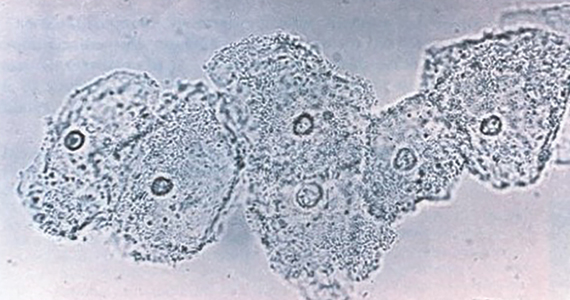
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.
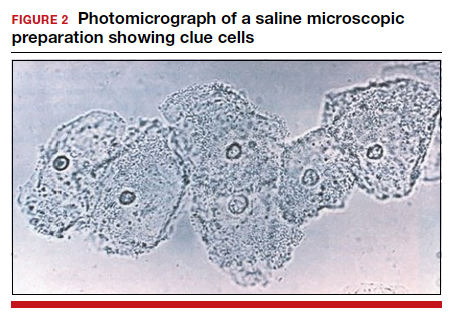
For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
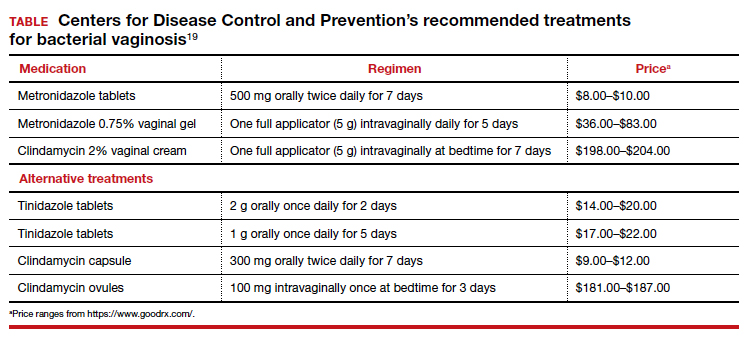
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.
Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
Practical obstetrics in pandemic times: Teamwork, flexibility, and creativity promote safety for patients and the care team
Practicing evidence-based medicine, as obstetricians know, is not always possible when one does not have evidence due to lack of data or long-term experience in pregnancy. During the COVID-19 pandemic, the evidence changed so rapidly that we were compelled to alter our strategy frequently as we learned more about the impact of this disease on our vulnerable patient population. The COVID-19 pandemic taught us that, in unprecedented times, centering the safety of the patient, her child, and the health care team requires quick thinking, flexibility, and above all effective communication between team members.
Here, I share our institutional experience in providing practical obstetric care through various stages of the still-evolving COVID-19 pandemic. We based our strategy on guidance from the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG),1,2 and the Society for Maternal-Fetal Medicine (SMFM).3-5 We were reminded yet again that the only constant is change and that timely but thoughtful adjustments were needed to keep up with the coronavirus.
Changes to prenatal care
Like many others, our institution has provided continued in-person outpatient prenatal care to both our low- and high-risk patients throughout each stage of the pandemic. While continuing to provide the necessary obstetric care, we made alterations to limit exposure and practice social distancing when possible.
Limiting patient support persons. One significant change was to restrict or limit support persons in the outpatient clinics based on guidelines reflecting community infection rates. Recognizing that this was not optimal for our patients’ emotional well-being, we needed to become more flexible in using technology to include family or support persons in prenatal visits and ultrasonography exams.
Altering test frequency. Using the guidance from SMFM,1 we changed the frequency of our antenatal testing and ultrasonography exams in the following ways: We increased the duration between indicated growth ultrasonography to every 4 weeks and decreased fetal antenatal testing to weekly, with twice-weekly testing continued for the highest-risk patients. Early first-trimester ultrasonography exams were limited and, when possible, delayed until after 10 to 12 weeks’ gestation or combined with other indications (nuchal translucency). Prenatal visits for low-risk patients were spaced out using existing models if the patient was amenable, especially in early pregnancy.
Adjusting staff assignments and using telehealth. In the early part of the pandemic, we divided into 2 groups to limit the number of clinicians at any one site: a dedicated group of outpatient clinicians who saw patients in the clinic only and a dedicated group of inpatient clinicians who staffed labor and delivery and the inpatient antepartum service. Additionally, our consultative maternal-fetal medicine service transitioned to a telehealth platform and performed the majority of consults remotely. Ultrasonography exams at various sites were read remotely and pertinent findings were communicated directly to patients via phone or the telehealth platform. Amniocentesis continued to be offered.
Responding to lower COVID-19 case numbers. When the number of COVID-19 cases decreased in the summer and fall of 2020, we returned to our prepandemic in-person practices, but we continued to offer telehealth visits as an option for patients who desired it. Patients were limited to one support person.
Shifting gears again. During the second surge of COVID-19 in our region, we used our experiences from the first to transition our practices to reduce in-person contact. Appointment frequency was decreased if appropriate, and we developed a tiered system of antenatal testing frequency based on risk factors. Visitors were again restricted, with exceptions made for extenuating circumstances. Consults were transitioned to telemedicine as appropriate and ultrasonography exams were read remotely when possible to limit exposures. Given the varied experiences with telemedicine and patient preferences, patients who desired in-person consult were (and are still) offered this option.
Some patients who were interested in telehealth but unable to access the technology were offered appointments via telehealth with the use of our clinic devices. Telemedicine increased our flexibility in offering consults as one provider could see patients at different office sites in one session. We continued our routine inpatient and outpatient coverage during this time as this kept our coverage options more flexible and expanded our obstetric backup plan in response to increased rates of community infection that affected both clinicians and patients.
Coordinating care for infected patients. One vital part of our prenatal care during the COVID-19 pandemic was to coordinate with our colleagues in medical specialties to provide outpatient care for patients with confirmed or suspected COVID-19 during their period of isolation or quarantine. Patients could be seen as outpatients in a dedicated space that used appropriate personal protective equipment (PPE) for not only prenatal care but also any needed in-person evaluation for COVID-19. Our obstetric clinicians and sonographers performed exams, antenatal testing (in the form of biophysical profiles), and indicated ultrasonography exams (such as umbilical artery Doppler studies and fetal growth assessments). This required a concerted effort and excellent communication between teams to provide the necessary care in the safest manner possible.
Continue to: Universal testing on labor and delivery...
Universal testing on labor and delivery
Not surprisingly, obstetric delivery volumes in our institution were not affected in the same way as elective surgery volumes. Our inpatient team continued to bring babies into the world at the same if not a higher rate than in prepandemic times. We continued elective inductions when space allowed. Our first COVID-19–positive patient was already at 40 weeks’ gestation when the result of her test, done due to exposure, was received. Creative effort among multiple specialties quickly developed her delivery plan, and she and her infant did well.
As data started coming out of the New York City obstetric experience, concern for preservation of the PPE supply and the potential for asymptomatic/presymptomatic patients led us, in consultation with our infectious disease colleagues, to institute universal testing for all antepartum and laboring patients. At first, all patients were tested on admission with our rapid in-house test. Eventually, we moved toward preoperative testing 3 to 5 days prior to scheduled cesarean deliveries in alignment with the surgical services when elective cases were reinstituted. Finally, we instituted preprocedure testing for all scheduled labor and delivery procedures, including inductions, cerclages, and fetal blood transfusions, while we still used rapid testing for patients who presented urgently or in labor.
We needed to address several considerations almost immediately after instituting universal testing, including:
- what to do in case of patient refusal to be tested
- which precautions to institute while awaiting test results
- potential postponement of elective delivery if a patient tested positive, and
- where best to deliver patients.
What we did at the beginning of the pandemic was not necessarily the same as we do in our current practice, and we expect that our procedures may need to change in the future. Derived from what we learned from others’ experience, we tailored our protocols to our own physical space, staffing capabilities, and testing limitations. We adjusted them often, with input from multiple services, based on updated policy, recommendation for isolation and quarantine durations, rates of community infection, and changes in the unit spaces. As with many things, one protocol did not fit every patient, necessitating case-by-case flexibility.
Delivery considerations
To answer some of the above questions, all patients who declined testing, were awaiting test results while in labor, or were in triage were placed in droplet and contact isolation on our unit, a practice we continue currently. Given the concern of potential aerosolization during the second stage of labor or during intubation, for any patients in those categories who required delivery, we limited the number of staff in their rooms as possible. Additional pediatric staff waited in close proximity of the room and were ready to come in if needed depending on fetal complications and gestational age. For delivery, all team members used full special pathogens precautions (N95 masks, face shields, gowns, and gloves).
Patients who were asymptomatic and tested negative for COVID-19 had and continue to have routine care from a PPE (standard gowns, gloves, face mask, and eye protection) and health care team perspective. We have allowed visitation of one support person per hospital stay for these patients throughout the pandemic.
For the majority of our experience during the pandemic, adult patients who tested positive for COVID-19 were cohorted within dedicated negative pressure units of varying levels of care. As these units included the same intensive care unit (ICU) we utilized in non-COVID times for critical obstetric patients, we had already operationalized their use and they were wired for our electronic fetal monitoring system. These rooms are adjacent to the main operating room (OR) complex, which allows for transition to a dedicated COVID-19 OR for cesarean delivery. We worked with the primary COVID-19 team, ICU team, anesthesia, and neonatal ICU team to develop a written protocol that detailed the care for our COVID-19–positive laboring and postpartum patients in this critical care COVID-19 unit.
For a time, admitted COVID-19–positive patients were not permitted to have support persons. The health care team therefore stepped in to be the patients’ support during the delivery of their child. Care of these patients required a great deal of coordination and communication between teams as well as the addition of a dedicated obstetric physician—separate from the regular labor and delivery team—assigned to care for these patients.
For pregnant patients in the emergency room or in the intermediate or floor COVID-19 units, portable fetal monitors and ultrasonography equipment were used for obstetric consults, fetal testing, and obstetrical ultrasonography as appropriate based on gestational age and medical conditions. Again, communication between teams was essential to provide seamless and timely patient care. Patients usually were admitted to the COVID-19 teams with maternal-fetal medicine or obstetric consult teams following daily; they were admitted and transferred to the ICU COVID-19 unit if delivery was necessary. To limit exposures whenever possible, coordinated care (such as exams and telephone evaluation) was performed outside of the room with the nursing and primary teams.
Continue to: Staying flexible to the changing COVID-19 environment...
Staying flexible to the changing
COVID-19 environment
Postponed in-person visits. Whenever possible, deliveries that were not medically indicated and in-person outpatient care visits were postponed until isolation/quarantine precautions could be lifted to avoid the need for special pathogens precautions, separation of mother and infant, and visitor restrictions. We did not postpone any medically indicated deliveries or appropriate care due to COVID-19 alone. As the CDC guidelines changed regarding the timing of infectivity, we had to continually re-evaluate when a patient could return to regular outpatient care instead of the COVID-19 clinic and/or be delivered.
Mother-infant separation. As outlined in an article we wrote with our pediatric colleagues, originally all infants were immediately separated from their COVID-19–positive mothers, and delayed cord clamping was not performed.6 We adjusted our protocols as experience and data grew regarding the risk of transmission to the newborn from asymptomatic mothers and as updated recommendations were made by ACOG and the CDC. Currently, if desired, asymptomatic mothers are not separated from their well term infants. We practice our standard delayed cord clamping technique for all patients. Masking, hand hygiene, and physical distancing are used to reduce the risk of infection transmission. Breastfeeding is encouraged if the patient desires it, either directly using precautions or supported via pumping.
Reduced workplace exposure. Along with many others, we are even more cognizant of reducing the risk of workplace exposure; thus, we conduct our daily multidisciplinary huddle and physician transition of care sign-outs. We use multiple rooms for our larger group with secure video chats, and we limit huddles to a single representative from each specialty.
Medication protocols. Early in the pandemic in our area, we limited antenatal corticosteroids for fetal lung maturity to patients who were at less than 34 weeks’ gestation, per ACOG recommendations, carefully considering necessity in the critically ill. Now, we continue to administer antenatal steroids according to our usual protocols up to 36 6/7 weeks, per ACOG and SMFM recommendations, regardless of illness severity.7 Nonsteroidal anti-inflammatory drug use, once limited in COVID-19–positive patients, are now used again. Additionally, we had a comprehensive venous thromboembolism (VTE) prophylaxis protocol for our obstetric patients, and we have added special consideration for prophylaxis for patients with moderate to severe illness or other VTE risk factors. While we do not perform routine circumcisions on infants of COVID-19–positive mothers, we have a process in place to provide that service after discharge when isolation precautions are lifted.
Labor accommodations. As COVID-19 cases increased in our hospital during recent months, we made one more significant change in our care protocols. To open up space in the ICU, we moved our care for asymptomatic COVID-19–positive laboring patients to our new labor and delivery unit with implemented special pathogens precautions. This is not revolutionary; many other hospitals did not have the same capability we did with our existing collaboration with the ICU for critical obstetric care. However, this change again required communication and collaboration among multiple care teams, agreement on the qualifications for delivery on labor and delivery versus in the ICU, and physical alteration of our unit to accommodate additional isolation precautions.
Visitor policy. Another change is that we have opened up the visitor policy to welcome an asymptomatic support person for the COVID-19–positive labor patient, giving special attention to adherence to isolation precautions. Our staff members have embraced this change as they have everything else, with cautious optimism and focus on keeping both the patients and the health care team safe. Our moderate to severely ill patients continue to be cared for in the COVID-19 unit in close collaboration with our infectious disease and ICU colleagues.
It’s all about teamwork
I hope I have given a clear example of our approach to providing obstetric care in the ever-changing landscape of the COVID-19 pandemic. We embraced this period of necessary change as practically and safely as possible for both our patients and our health care workers. We learned multiple times along the way that what seemed to be a good idea was not feasible, or not the ideal option, or that COVID-19 had changed the rules of the game again. Our team met daily if not more frequently, as we found we had to constantly adapt and change to each new challenge or new clinical scenario. When we struggled, it generally related to a gap in communication.
I am privileged to work with a dedicated, selfless, multidisciplinary team that rose to the occasion. They had the focused goal to provide the highest quality and safety in obstetric care while offering compassion and empathy for the experience of having a baby during a pandemic. ●
The author would like to acknowledge Danielle Prentice, DO, and Jaimie Maines, MD, for their manuscript review.
- The requirement for reduced in-person contact due to the COVID-19 pandemic challenged our traditional obstetric care models. This led us to comprehensively incorporate technology for communication with patients and their families and to significantly alter how, where, and when we delivered prenatal care.
- Both patients and clinicians needed to adjust to the impact of these changes, especially concerning visitor policies.
- Early incorporation of universal COVID-19 testing for labor and antepartum patients was initially instituted to improve patient and staff safety and to preserve PPE. However, it quickly led to the need for various protocols for both anticipated and unanticipated clinical scenarios.
- As new data emerged and the number of cases fluctuated throughout the pandemic, our approach and protocols necessitated flexibility: Our strategy for maternal and neonatal care early in the pandemic was not the same as our current approach, and it will likely change several more times before we are done.
- One of the biggest challenges to our care team was maintaining standards of excellence and safety in obstetric care while also adhering to the physical barriers of isolation precautions and maintaining vigilance to reduce exposure risk during our routine workflow.
- The physical and operational specifics of our institution determined our approach to obstetric care during COVID-19, in part because halfway through the pandemic we moved our maternity unit from the adult hospital to a new center within our children’s hospital.
- The frequent changes in the knowledge of and recommendations for COVID-19 highlighted the importance of maintaining multidisciplinary communication on a daily, if not more frequent, basis.
- American College of Obstetricians and Gynecologists. Practice advisory: novel coronavirus 2019 (COVID-19): summary of key updates (December 14, 2020). https://www.acog.org/clinical /clinical-guidance/practice-advisory/articles/2020/03/novel -coronavirus-2019. Accessed January 28, 2021.
- American College of Obstetricians and Gynecologists. COVID19 FAQs for obstetrician-gynecologists, obstetrics. Washington, DC: ACOG; 2020. https://www.acog.org/clinical-information /physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Updated November 23, 2020. https: //s3.amazonaws.com/cdn.smfm.org/media/2589/COVID19 -What_MFMs_need_to_know_revision_11-23-20_final.pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Updated January 7, 2021. https://s3.amazonaws.com/cdn.smfm.org /media/2668/SMFM_COVID_Management_of_COVID_pos _preg_patients_1-7-21_(final).pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. COVID-19 ultrasound clinical practice suggestions. Updated October 20, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2550 /Ultrasound_Covid19_Suggestions_10-20-20_(final).pdf. Accessed January 28, 2020.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987-996.
- American College of Obstetricians and Gynecologists. Committee opinion no 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102-e109.
Practicing evidence-based medicine, as obstetricians know, is not always possible when one does not have evidence due to lack of data or long-term experience in pregnancy. During the COVID-19 pandemic, the evidence changed so rapidly that we were compelled to alter our strategy frequently as we learned more about the impact of this disease on our vulnerable patient population. The COVID-19 pandemic taught us that, in unprecedented times, centering the safety of the patient, her child, and the health care team requires quick thinking, flexibility, and above all effective communication between team members.
Here, I share our institutional experience in providing practical obstetric care through various stages of the still-evolving COVID-19 pandemic. We based our strategy on guidance from the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG),1,2 and the Society for Maternal-Fetal Medicine (SMFM).3-5 We were reminded yet again that the only constant is change and that timely but thoughtful adjustments were needed to keep up with the coronavirus.
Changes to prenatal care
Like many others, our institution has provided continued in-person outpatient prenatal care to both our low- and high-risk patients throughout each stage of the pandemic. While continuing to provide the necessary obstetric care, we made alterations to limit exposure and practice social distancing when possible.
Limiting patient support persons. One significant change was to restrict or limit support persons in the outpatient clinics based on guidelines reflecting community infection rates. Recognizing that this was not optimal for our patients’ emotional well-being, we needed to become more flexible in using technology to include family or support persons in prenatal visits and ultrasonography exams.
Altering test frequency. Using the guidance from SMFM,1 we changed the frequency of our antenatal testing and ultrasonography exams in the following ways: We increased the duration between indicated growth ultrasonography to every 4 weeks and decreased fetal antenatal testing to weekly, with twice-weekly testing continued for the highest-risk patients. Early first-trimester ultrasonography exams were limited and, when possible, delayed until after 10 to 12 weeks’ gestation or combined with other indications (nuchal translucency). Prenatal visits for low-risk patients were spaced out using existing models if the patient was amenable, especially in early pregnancy.
Adjusting staff assignments and using telehealth. In the early part of the pandemic, we divided into 2 groups to limit the number of clinicians at any one site: a dedicated group of outpatient clinicians who saw patients in the clinic only and a dedicated group of inpatient clinicians who staffed labor and delivery and the inpatient antepartum service. Additionally, our consultative maternal-fetal medicine service transitioned to a telehealth platform and performed the majority of consults remotely. Ultrasonography exams at various sites were read remotely and pertinent findings were communicated directly to patients via phone or the telehealth platform. Amniocentesis continued to be offered.
Responding to lower COVID-19 case numbers. When the number of COVID-19 cases decreased in the summer and fall of 2020, we returned to our prepandemic in-person practices, but we continued to offer telehealth visits as an option for patients who desired it. Patients were limited to one support person.
Shifting gears again. During the second surge of COVID-19 in our region, we used our experiences from the first to transition our practices to reduce in-person contact. Appointment frequency was decreased if appropriate, and we developed a tiered system of antenatal testing frequency based on risk factors. Visitors were again restricted, with exceptions made for extenuating circumstances. Consults were transitioned to telemedicine as appropriate and ultrasonography exams were read remotely when possible to limit exposures. Given the varied experiences with telemedicine and patient preferences, patients who desired in-person consult were (and are still) offered this option.
Some patients who were interested in telehealth but unable to access the technology were offered appointments via telehealth with the use of our clinic devices. Telemedicine increased our flexibility in offering consults as one provider could see patients at different office sites in one session. We continued our routine inpatient and outpatient coverage during this time as this kept our coverage options more flexible and expanded our obstetric backup plan in response to increased rates of community infection that affected both clinicians and patients.
Coordinating care for infected patients. One vital part of our prenatal care during the COVID-19 pandemic was to coordinate with our colleagues in medical specialties to provide outpatient care for patients with confirmed or suspected COVID-19 during their period of isolation or quarantine. Patients could be seen as outpatients in a dedicated space that used appropriate personal protective equipment (PPE) for not only prenatal care but also any needed in-person evaluation for COVID-19. Our obstetric clinicians and sonographers performed exams, antenatal testing (in the form of biophysical profiles), and indicated ultrasonography exams (such as umbilical artery Doppler studies and fetal growth assessments). This required a concerted effort and excellent communication between teams to provide the necessary care in the safest manner possible.
Continue to: Universal testing on labor and delivery...
Universal testing on labor and delivery
Not surprisingly, obstetric delivery volumes in our institution were not affected in the same way as elective surgery volumes. Our inpatient team continued to bring babies into the world at the same if not a higher rate than in prepandemic times. We continued elective inductions when space allowed. Our first COVID-19–positive patient was already at 40 weeks’ gestation when the result of her test, done due to exposure, was received. Creative effort among multiple specialties quickly developed her delivery plan, and she and her infant did well.
As data started coming out of the New York City obstetric experience, concern for preservation of the PPE supply and the potential for asymptomatic/presymptomatic patients led us, in consultation with our infectious disease colleagues, to institute universal testing for all antepartum and laboring patients. At first, all patients were tested on admission with our rapid in-house test. Eventually, we moved toward preoperative testing 3 to 5 days prior to scheduled cesarean deliveries in alignment with the surgical services when elective cases were reinstituted. Finally, we instituted preprocedure testing for all scheduled labor and delivery procedures, including inductions, cerclages, and fetal blood transfusions, while we still used rapid testing for patients who presented urgently or in labor.
We needed to address several considerations almost immediately after instituting universal testing, including:
- what to do in case of patient refusal to be tested
- which precautions to institute while awaiting test results
- potential postponement of elective delivery if a patient tested positive, and
- where best to deliver patients.
What we did at the beginning of the pandemic was not necessarily the same as we do in our current practice, and we expect that our procedures may need to change in the future. Derived from what we learned from others’ experience, we tailored our protocols to our own physical space, staffing capabilities, and testing limitations. We adjusted them often, with input from multiple services, based on updated policy, recommendation for isolation and quarantine durations, rates of community infection, and changes in the unit spaces. As with many things, one protocol did not fit every patient, necessitating case-by-case flexibility.
Delivery considerations
To answer some of the above questions, all patients who declined testing, were awaiting test results while in labor, or were in triage were placed in droplet and contact isolation on our unit, a practice we continue currently. Given the concern of potential aerosolization during the second stage of labor or during intubation, for any patients in those categories who required delivery, we limited the number of staff in their rooms as possible. Additional pediatric staff waited in close proximity of the room and were ready to come in if needed depending on fetal complications and gestational age. For delivery, all team members used full special pathogens precautions (N95 masks, face shields, gowns, and gloves).
Patients who were asymptomatic and tested negative for COVID-19 had and continue to have routine care from a PPE (standard gowns, gloves, face mask, and eye protection) and health care team perspective. We have allowed visitation of one support person per hospital stay for these patients throughout the pandemic.
For the majority of our experience during the pandemic, adult patients who tested positive for COVID-19 were cohorted within dedicated negative pressure units of varying levels of care. As these units included the same intensive care unit (ICU) we utilized in non-COVID times for critical obstetric patients, we had already operationalized their use and they were wired for our electronic fetal monitoring system. These rooms are adjacent to the main operating room (OR) complex, which allows for transition to a dedicated COVID-19 OR for cesarean delivery. We worked with the primary COVID-19 team, ICU team, anesthesia, and neonatal ICU team to develop a written protocol that detailed the care for our COVID-19–positive laboring and postpartum patients in this critical care COVID-19 unit.
For a time, admitted COVID-19–positive patients were not permitted to have support persons. The health care team therefore stepped in to be the patients’ support during the delivery of their child. Care of these patients required a great deal of coordination and communication between teams as well as the addition of a dedicated obstetric physician—separate from the regular labor and delivery team—assigned to care for these patients.
For pregnant patients in the emergency room or in the intermediate or floor COVID-19 units, portable fetal monitors and ultrasonography equipment were used for obstetric consults, fetal testing, and obstetrical ultrasonography as appropriate based on gestational age and medical conditions. Again, communication between teams was essential to provide seamless and timely patient care. Patients usually were admitted to the COVID-19 teams with maternal-fetal medicine or obstetric consult teams following daily; they were admitted and transferred to the ICU COVID-19 unit if delivery was necessary. To limit exposures whenever possible, coordinated care (such as exams and telephone evaluation) was performed outside of the room with the nursing and primary teams.
Continue to: Staying flexible to the changing COVID-19 environment...
Staying flexible to the changing
COVID-19 environment
Postponed in-person visits. Whenever possible, deliveries that were not medically indicated and in-person outpatient care visits were postponed until isolation/quarantine precautions could be lifted to avoid the need for special pathogens precautions, separation of mother and infant, and visitor restrictions. We did not postpone any medically indicated deliveries or appropriate care due to COVID-19 alone. As the CDC guidelines changed regarding the timing of infectivity, we had to continually re-evaluate when a patient could return to regular outpatient care instead of the COVID-19 clinic and/or be delivered.
Mother-infant separation. As outlined in an article we wrote with our pediatric colleagues, originally all infants were immediately separated from their COVID-19–positive mothers, and delayed cord clamping was not performed.6 We adjusted our protocols as experience and data grew regarding the risk of transmission to the newborn from asymptomatic mothers and as updated recommendations were made by ACOG and the CDC. Currently, if desired, asymptomatic mothers are not separated from their well term infants. We practice our standard delayed cord clamping technique for all patients. Masking, hand hygiene, and physical distancing are used to reduce the risk of infection transmission. Breastfeeding is encouraged if the patient desires it, either directly using precautions or supported via pumping.
Reduced workplace exposure. Along with many others, we are even more cognizant of reducing the risk of workplace exposure; thus, we conduct our daily multidisciplinary huddle and physician transition of care sign-outs. We use multiple rooms for our larger group with secure video chats, and we limit huddles to a single representative from each specialty.
Medication protocols. Early in the pandemic in our area, we limited antenatal corticosteroids for fetal lung maturity to patients who were at less than 34 weeks’ gestation, per ACOG recommendations, carefully considering necessity in the critically ill. Now, we continue to administer antenatal steroids according to our usual protocols up to 36 6/7 weeks, per ACOG and SMFM recommendations, regardless of illness severity.7 Nonsteroidal anti-inflammatory drug use, once limited in COVID-19–positive patients, are now used again. Additionally, we had a comprehensive venous thromboembolism (VTE) prophylaxis protocol for our obstetric patients, and we have added special consideration for prophylaxis for patients with moderate to severe illness or other VTE risk factors. While we do not perform routine circumcisions on infants of COVID-19–positive mothers, we have a process in place to provide that service after discharge when isolation precautions are lifted.
Labor accommodations. As COVID-19 cases increased in our hospital during recent months, we made one more significant change in our care protocols. To open up space in the ICU, we moved our care for asymptomatic COVID-19–positive laboring patients to our new labor and delivery unit with implemented special pathogens precautions. This is not revolutionary; many other hospitals did not have the same capability we did with our existing collaboration with the ICU for critical obstetric care. However, this change again required communication and collaboration among multiple care teams, agreement on the qualifications for delivery on labor and delivery versus in the ICU, and physical alteration of our unit to accommodate additional isolation precautions.
Visitor policy. Another change is that we have opened up the visitor policy to welcome an asymptomatic support person for the COVID-19–positive labor patient, giving special attention to adherence to isolation precautions. Our staff members have embraced this change as they have everything else, with cautious optimism and focus on keeping both the patients and the health care team safe. Our moderate to severely ill patients continue to be cared for in the COVID-19 unit in close collaboration with our infectious disease and ICU colleagues.
It’s all about teamwork
I hope I have given a clear example of our approach to providing obstetric care in the ever-changing landscape of the COVID-19 pandemic. We embraced this period of necessary change as practically and safely as possible for both our patients and our health care workers. We learned multiple times along the way that what seemed to be a good idea was not feasible, or not the ideal option, or that COVID-19 had changed the rules of the game again. Our team met daily if not more frequently, as we found we had to constantly adapt and change to each new challenge or new clinical scenario. When we struggled, it generally related to a gap in communication.
I am privileged to work with a dedicated, selfless, multidisciplinary team that rose to the occasion. They had the focused goal to provide the highest quality and safety in obstetric care while offering compassion and empathy for the experience of having a baby during a pandemic. ●
The author would like to acknowledge Danielle Prentice, DO, and Jaimie Maines, MD, for their manuscript review.
- The requirement for reduced in-person contact due to the COVID-19 pandemic challenged our traditional obstetric care models. This led us to comprehensively incorporate technology for communication with patients and their families and to significantly alter how, where, and when we delivered prenatal care.
- Both patients and clinicians needed to adjust to the impact of these changes, especially concerning visitor policies.
- Early incorporation of universal COVID-19 testing for labor and antepartum patients was initially instituted to improve patient and staff safety and to preserve PPE. However, it quickly led to the need for various protocols for both anticipated and unanticipated clinical scenarios.
- As new data emerged and the number of cases fluctuated throughout the pandemic, our approach and protocols necessitated flexibility: Our strategy for maternal and neonatal care early in the pandemic was not the same as our current approach, and it will likely change several more times before we are done.
- One of the biggest challenges to our care team was maintaining standards of excellence and safety in obstetric care while also adhering to the physical barriers of isolation precautions and maintaining vigilance to reduce exposure risk during our routine workflow.
- The physical and operational specifics of our institution determined our approach to obstetric care during COVID-19, in part because halfway through the pandemic we moved our maternity unit from the adult hospital to a new center within our children’s hospital.
- The frequent changes in the knowledge of and recommendations for COVID-19 highlighted the importance of maintaining multidisciplinary communication on a daily, if not more frequent, basis.
Practicing evidence-based medicine, as obstetricians know, is not always possible when one does not have evidence due to lack of data or long-term experience in pregnancy. During the COVID-19 pandemic, the evidence changed so rapidly that we were compelled to alter our strategy frequently as we learned more about the impact of this disease on our vulnerable patient population. The COVID-19 pandemic taught us that, in unprecedented times, centering the safety of the patient, her child, and the health care team requires quick thinking, flexibility, and above all effective communication between team members.
Here, I share our institutional experience in providing practical obstetric care through various stages of the still-evolving COVID-19 pandemic. We based our strategy on guidance from the Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG),1,2 and the Society for Maternal-Fetal Medicine (SMFM).3-5 We were reminded yet again that the only constant is change and that timely but thoughtful adjustments were needed to keep up with the coronavirus.
Changes to prenatal care
Like many others, our institution has provided continued in-person outpatient prenatal care to both our low- and high-risk patients throughout each stage of the pandemic. While continuing to provide the necessary obstetric care, we made alterations to limit exposure and practice social distancing when possible.
Limiting patient support persons. One significant change was to restrict or limit support persons in the outpatient clinics based on guidelines reflecting community infection rates. Recognizing that this was not optimal for our patients’ emotional well-being, we needed to become more flexible in using technology to include family or support persons in prenatal visits and ultrasonography exams.
Altering test frequency. Using the guidance from SMFM,1 we changed the frequency of our antenatal testing and ultrasonography exams in the following ways: We increased the duration between indicated growth ultrasonography to every 4 weeks and decreased fetal antenatal testing to weekly, with twice-weekly testing continued for the highest-risk patients. Early first-trimester ultrasonography exams were limited and, when possible, delayed until after 10 to 12 weeks’ gestation or combined with other indications (nuchal translucency). Prenatal visits for low-risk patients were spaced out using existing models if the patient was amenable, especially in early pregnancy.
Adjusting staff assignments and using telehealth. In the early part of the pandemic, we divided into 2 groups to limit the number of clinicians at any one site: a dedicated group of outpatient clinicians who saw patients in the clinic only and a dedicated group of inpatient clinicians who staffed labor and delivery and the inpatient antepartum service. Additionally, our consultative maternal-fetal medicine service transitioned to a telehealth platform and performed the majority of consults remotely. Ultrasonography exams at various sites were read remotely and pertinent findings were communicated directly to patients via phone or the telehealth platform. Amniocentesis continued to be offered.
Responding to lower COVID-19 case numbers. When the number of COVID-19 cases decreased in the summer and fall of 2020, we returned to our prepandemic in-person practices, but we continued to offer telehealth visits as an option for patients who desired it. Patients were limited to one support person.
Shifting gears again. During the second surge of COVID-19 in our region, we used our experiences from the first to transition our practices to reduce in-person contact. Appointment frequency was decreased if appropriate, and we developed a tiered system of antenatal testing frequency based on risk factors. Visitors were again restricted, with exceptions made for extenuating circumstances. Consults were transitioned to telemedicine as appropriate and ultrasonography exams were read remotely when possible to limit exposures. Given the varied experiences with telemedicine and patient preferences, patients who desired in-person consult were (and are still) offered this option.
Some patients who were interested in telehealth but unable to access the technology were offered appointments via telehealth with the use of our clinic devices. Telemedicine increased our flexibility in offering consults as one provider could see patients at different office sites in one session. We continued our routine inpatient and outpatient coverage during this time as this kept our coverage options more flexible and expanded our obstetric backup plan in response to increased rates of community infection that affected both clinicians and patients.
Coordinating care for infected patients. One vital part of our prenatal care during the COVID-19 pandemic was to coordinate with our colleagues in medical specialties to provide outpatient care for patients with confirmed or suspected COVID-19 during their period of isolation or quarantine. Patients could be seen as outpatients in a dedicated space that used appropriate personal protective equipment (PPE) for not only prenatal care but also any needed in-person evaluation for COVID-19. Our obstetric clinicians and sonographers performed exams, antenatal testing (in the form of biophysical profiles), and indicated ultrasonography exams (such as umbilical artery Doppler studies and fetal growth assessments). This required a concerted effort and excellent communication between teams to provide the necessary care in the safest manner possible.
Continue to: Universal testing on labor and delivery...
Universal testing on labor and delivery
Not surprisingly, obstetric delivery volumes in our institution were not affected in the same way as elective surgery volumes. Our inpatient team continued to bring babies into the world at the same if not a higher rate than in prepandemic times. We continued elective inductions when space allowed. Our first COVID-19–positive patient was already at 40 weeks’ gestation when the result of her test, done due to exposure, was received. Creative effort among multiple specialties quickly developed her delivery plan, and she and her infant did well.
As data started coming out of the New York City obstetric experience, concern for preservation of the PPE supply and the potential for asymptomatic/presymptomatic patients led us, in consultation with our infectious disease colleagues, to institute universal testing for all antepartum and laboring patients. At first, all patients were tested on admission with our rapid in-house test. Eventually, we moved toward preoperative testing 3 to 5 days prior to scheduled cesarean deliveries in alignment with the surgical services when elective cases were reinstituted. Finally, we instituted preprocedure testing for all scheduled labor and delivery procedures, including inductions, cerclages, and fetal blood transfusions, while we still used rapid testing for patients who presented urgently or in labor.
We needed to address several considerations almost immediately after instituting universal testing, including:
- what to do in case of patient refusal to be tested
- which precautions to institute while awaiting test results
- potential postponement of elective delivery if a patient tested positive, and
- where best to deliver patients.
What we did at the beginning of the pandemic was not necessarily the same as we do in our current practice, and we expect that our procedures may need to change in the future. Derived from what we learned from others’ experience, we tailored our protocols to our own physical space, staffing capabilities, and testing limitations. We adjusted them often, with input from multiple services, based on updated policy, recommendation for isolation and quarantine durations, rates of community infection, and changes in the unit spaces. As with many things, one protocol did not fit every patient, necessitating case-by-case flexibility.
Delivery considerations
To answer some of the above questions, all patients who declined testing, were awaiting test results while in labor, or were in triage were placed in droplet and contact isolation on our unit, a practice we continue currently. Given the concern of potential aerosolization during the second stage of labor or during intubation, for any patients in those categories who required delivery, we limited the number of staff in their rooms as possible. Additional pediatric staff waited in close proximity of the room and were ready to come in if needed depending on fetal complications and gestational age. For delivery, all team members used full special pathogens precautions (N95 masks, face shields, gowns, and gloves).
Patients who were asymptomatic and tested negative for COVID-19 had and continue to have routine care from a PPE (standard gowns, gloves, face mask, and eye protection) and health care team perspective. We have allowed visitation of one support person per hospital stay for these patients throughout the pandemic.
For the majority of our experience during the pandemic, adult patients who tested positive for COVID-19 were cohorted within dedicated negative pressure units of varying levels of care. As these units included the same intensive care unit (ICU) we utilized in non-COVID times for critical obstetric patients, we had already operationalized their use and they were wired for our electronic fetal monitoring system. These rooms are adjacent to the main operating room (OR) complex, which allows for transition to a dedicated COVID-19 OR for cesarean delivery. We worked with the primary COVID-19 team, ICU team, anesthesia, and neonatal ICU team to develop a written protocol that detailed the care for our COVID-19–positive laboring and postpartum patients in this critical care COVID-19 unit.
For a time, admitted COVID-19–positive patients were not permitted to have support persons. The health care team therefore stepped in to be the patients’ support during the delivery of their child. Care of these patients required a great deal of coordination and communication between teams as well as the addition of a dedicated obstetric physician—separate from the regular labor and delivery team—assigned to care for these patients.
For pregnant patients in the emergency room or in the intermediate or floor COVID-19 units, portable fetal monitors and ultrasonography equipment were used for obstetric consults, fetal testing, and obstetrical ultrasonography as appropriate based on gestational age and medical conditions. Again, communication between teams was essential to provide seamless and timely patient care. Patients usually were admitted to the COVID-19 teams with maternal-fetal medicine or obstetric consult teams following daily; they were admitted and transferred to the ICU COVID-19 unit if delivery was necessary. To limit exposures whenever possible, coordinated care (such as exams and telephone evaluation) was performed outside of the room with the nursing and primary teams.
Continue to: Staying flexible to the changing COVID-19 environment...
Staying flexible to the changing
COVID-19 environment
Postponed in-person visits. Whenever possible, deliveries that were not medically indicated and in-person outpatient care visits were postponed until isolation/quarantine precautions could be lifted to avoid the need for special pathogens precautions, separation of mother and infant, and visitor restrictions. We did not postpone any medically indicated deliveries or appropriate care due to COVID-19 alone. As the CDC guidelines changed regarding the timing of infectivity, we had to continually re-evaluate when a patient could return to regular outpatient care instead of the COVID-19 clinic and/or be delivered.
Mother-infant separation. As outlined in an article we wrote with our pediatric colleagues, originally all infants were immediately separated from their COVID-19–positive mothers, and delayed cord clamping was not performed.6 We adjusted our protocols as experience and data grew regarding the risk of transmission to the newborn from asymptomatic mothers and as updated recommendations were made by ACOG and the CDC. Currently, if desired, asymptomatic mothers are not separated from their well term infants. We practice our standard delayed cord clamping technique for all patients. Masking, hand hygiene, and physical distancing are used to reduce the risk of infection transmission. Breastfeeding is encouraged if the patient desires it, either directly using precautions or supported via pumping.
Reduced workplace exposure. Along with many others, we are even more cognizant of reducing the risk of workplace exposure; thus, we conduct our daily multidisciplinary huddle and physician transition of care sign-outs. We use multiple rooms for our larger group with secure video chats, and we limit huddles to a single representative from each specialty.
Medication protocols. Early in the pandemic in our area, we limited antenatal corticosteroids for fetal lung maturity to patients who were at less than 34 weeks’ gestation, per ACOG recommendations, carefully considering necessity in the critically ill. Now, we continue to administer antenatal steroids according to our usual protocols up to 36 6/7 weeks, per ACOG and SMFM recommendations, regardless of illness severity.7 Nonsteroidal anti-inflammatory drug use, once limited in COVID-19–positive patients, are now used again. Additionally, we had a comprehensive venous thromboembolism (VTE) prophylaxis protocol for our obstetric patients, and we have added special consideration for prophylaxis for patients with moderate to severe illness or other VTE risk factors. While we do not perform routine circumcisions on infants of COVID-19–positive mothers, we have a process in place to provide that service after discharge when isolation precautions are lifted.
Labor accommodations. As COVID-19 cases increased in our hospital during recent months, we made one more significant change in our care protocols. To open up space in the ICU, we moved our care for asymptomatic COVID-19–positive laboring patients to our new labor and delivery unit with implemented special pathogens precautions. This is not revolutionary; many other hospitals did not have the same capability we did with our existing collaboration with the ICU for critical obstetric care. However, this change again required communication and collaboration among multiple care teams, agreement on the qualifications for delivery on labor and delivery versus in the ICU, and physical alteration of our unit to accommodate additional isolation precautions.
Visitor policy. Another change is that we have opened up the visitor policy to welcome an asymptomatic support person for the COVID-19–positive labor patient, giving special attention to adherence to isolation precautions. Our staff members have embraced this change as they have everything else, with cautious optimism and focus on keeping both the patients and the health care team safe. Our moderate to severely ill patients continue to be cared for in the COVID-19 unit in close collaboration with our infectious disease and ICU colleagues.
It’s all about teamwork
I hope I have given a clear example of our approach to providing obstetric care in the ever-changing landscape of the COVID-19 pandemic. We embraced this period of necessary change as practically and safely as possible for both our patients and our health care workers. We learned multiple times along the way that what seemed to be a good idea was not feasible, or not the ideal option, or that COVID-19 had changed the rules of the game again. Our team met daily if not more frequently, as we found we had to constantly adapt and change to each new challenge or new clinical scenario. When we struggled, it generally related to a gap in communication.
I am privileged to work with a dedicated, selfless, multidisciplinary team that rose to the occasion. They had the focused goal to provide the highest quality and safety in obstetric care while offering compassion and empathy for the experience of having a baby during a pandemic. ●
The author would like to acknowledge Danielle Prentice, DO, and Jaimie Maines, MD, for their manuscript review.
- The requirement for reduced in-person contact due to the COVID-19 pandemic challenged our traditional obstetric care models. This led us to comprehensively incorporate technology for communication with patients and their families and to significantly alter how, where, and when we delivered prenatal care.
- Both patients and clinicians needed to adjust to the impact of these changes, especially concerning visitor policies.
- Early incorporation of universal COVID-19 testing for labor and antepartum patients was initially instituted to improve patient and staff safety and to preserve PPE. However, it quickly led to the need for various protocols for both anticipated and unanticipated clinical scenarios.
- As new data emerged and the number of cases fluctuated throughout the pandemic, our approach and protocols necessitated flexibility: Our strategy for maternal and neonatal care early in the pandemic was not the same as our current approach, and it will likely change several more times before we are done.
- One of the biggest challenges to our care team was maintaining standards of excellence and safety in obstetric care while also adhering to the physical barriers of isolation precautions and maintaining vigilance to reduce exposure risk during our routine workflow.
- The physical and operational specifics of our institution determined our approach to obstetric care during COVID-19, in part because halfway through the pandemic we moved our maternity unit from the adult hospital to a new center within our children’s hospital.
- The frequent changes in the knowledge of and recommendations for COVID-19 highlighted the importance of maintaining multidisciplinary communication on a daily, if not more frequent, basis.
- American College of Obstetricians and Gynecologists. Practice advisory: novel coronavirus 2019 (COVID-19): summary of key updates (December 14, 2020). https://www.acog.org/clinical /clinical-guidance/practice-advisory/articles/2020/03/novel -coronavirus-2019. Accessed January 28, 2021.
- American College of Obstetricians and Gynecologists. COVID19 FAQs for obstetrician-gynecologists, obstetrics. Washington, DC: ACOG; 2020. https://www.acog.org/clinical-information /physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Updated November 23, 2020. https: //s3.amazonaws.com/cdn.smfm.org/media/2589/COVID19 -What_MFMs_need_to_know_revision_11-23-20_final.pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Updated January 7, 2021. https://s3.amazonaws.com/cdn.smfm.org /media/2668/SMFM_COVID_Management_of_COVID_pos _preg_patients_1-7-21_(final).pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. COVID-19 ultrasound clinical practice suggestions. Updated October 20, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2550 /Ultrasound_Covid19_Suggestions_10-20-20_(final).pdf. Accessed January 28, 2020.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987-996.
- American College of Obstetricians and Gynecologists. Committee opinion no 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102-e109.
- American College of Obstetricians and Gynecologists. Practice advisory: novel coronavirus 2019 (COVID-19): summary of key updates (December 14, 2020). https://www.acog.org/clinical /clinical-guidance/practice-advisory/articles/2020/03/novel -coronavirus-2019. Accessed January 28, 2021.
- American College of Obstetricians and Gynecologists. COVID19 FAQs for obstetrician-gynecologists, obstetrics. Washington, DC: ACOG; 2020. https://www.acog.org/clinical-information /physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Coronavirus (COVID19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Updated November 23, 2020. https: //s3.amazonaws.com/cdn.smfm.org/media/2589/COVID19 -What_MFMs_need_to_know_revision_11-23-20_final.pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. Management considerations for pregnant patients with COVID-19. Updated January 7, 2021. https://s3.amazonaws.com/cdn.smfm.org /media/2668/SMFM_COVID_Management_of_COVID_pos _preg_patients_1-7-21_(final).pdf. Accessed January 28, 2021.
- Society for Maternal-Fetal Medicine. COVID-19 ultrasound clinical practice suggestions. Updated October 20, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2550 /Ultrasound_Covid19_Suggestions_10-20-20_(final).pdf. Accessed January 28, 2020.
- Amatya S, Corr TE, Gandhi CK, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987-996.
- American College of Obstetricians and Gynecologists. Committee opinion no 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130:e102-e109.
Product update: Breast biopsy system, tamponade mini-sponge, ovulation prediction device and app
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
2021 Update on fertility
In this Update, we discuss several aspects of infertility and emerging technologic advances in treatment. We review an important infertility fact sheet recently issued by the World Health Organization (WHO) that provides a succinct overview of infertility causes, the rights of infertility patients, treatment challenges, and advocacy efforts. In addition, we discuss what the infertility literature reveals about reducing multiple birth rates and the technologic, financial, and social factors involved. Finally, we look at the molecular progress made in germline-editing technology and the myriad complications involved in its potential future translation to clinical phenotyping.
WHO recognizes the burden of infertility and addresses fertility care needs
World Health Organization (WHO). Infertility fact sheet. September 14, 2020. https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed January 24, 2021.
The WHO published its first comprehensive infertility fact sheet in September 2020. This document is important because it validates infertility as a high-burden disease and disability that diminishes quality of life for up to 186 million individuals globally. The infertility fact sheet is a comprehensive yet focused quick read that addresses the causes of infertility, why infertility is important, challenges, and the WHO response.
Factors in infertility
Infertility is caused by different factors in women and men, yet sometimes it is unexplained, and its relative importance can vary from country to country. For women, tubal disorders (for example, postinfectious), uterine problems (fibroids, congenital), endometriosis, ovarian disorders (polycystic ovary syndrome, ovulation disorders), and endocrine imbalances are the most common factors.
For men, causes of infertility include obstruction of the reproductive tract (as after injuries or infection); hormonal disorders in the hypothalamus, pituitary, and/or testicles (for example, low testosterone); testicular failure to produce sperm (such as after cancer treatment); and abnormal sperm function and quality (low count, motility, or morphology).
Environmental and lifestyle factors— including smoking, obesity, alcohol, or toxins—can affect fertility.
Continue to: Recognizing all individuals’ fertility rights...
Recognizing all individuals’ fertility rights
The WHO infertility fact sheet makes strong statements, recognizing that individuals and couples have the right to decide the number, timing, and spacing of their children. Addressing infertility is therefore an important part of realizing the right of individuals and couples to found a family. This includes heterosexual couples, same-sex partners, older persons, individuals not in sexual relationships who might require infertility management and fertility care services, and notably marginalized populations.
Addressing infertility also can help mitigate gender inequality, which has significant negative social impacts on the lives of infertile individuals, especially women. Fertility education is important to reduce the fear of infertility and contraception use in those wanting pregnancy in the future.
In most countries the biggest challenges are availability, access, and quality of interventions to address infertility. This includes the United States, where only 1 in 4 individuals receive the fertility care they need. Lack of prioritization, ineffective public health strategies, inadequate funding, and costs are barriers. Health policies need to recognize that infertility is a disease that often can be prevented, thereby reducing future costs. Comprehensive awareness and education programs, laws and policies that regulate and ensure access and the human rights of all involved, are essential.
Advocacy efforts
To address infertility and fertility care, the WHO is committed to:
- collaborate with partners on epidemiologic and etiologic research
- facilitate policy dialogue globally to frame infertility within a legal and policy framework
- support generation of data on the burden of infertility
- develop guidelines
- produce other documents of standards
- collaborate with all stakeholders to strengthen political commitment and health system capacity, and
- provide country-level technical support to develop or strengthen policies and services.
For your practice, this means that infertility is recognized as a disease that should receive its appropriate share of health care resources. Infertility and fertility care are the right of every individual according to their desires to found a family. Besides providing the best care you can to all your patients, including referring them when necessary, all health care clinicians should advocate on behalf of their patients to payors, policy makers, and the public the need to provide equitable laws, resources, and funding for infertility and fertility care.
Every person has the right to infertility and fertility care as endorsed by the recent WHO infertility fact sheet. To address this high-burden disease, all women’s health care clinicians should be aware of, equitably diagnose and treat, refer as necessary, and advocate for infertile individuals.
Continue to: Lessons learned in reducing multiple pregnancy rates in infertility treatment...
Lessons learned in reducing multiple pregnancy rates in infertility treatment
Views and reviews section. Fertil Steril. 2020;114:671- 672; 673-679; 680-689; 690-714; 715-721.
In the October 2020 issue of Fertility and Sterility, the Views and Reviews section included 5 articles on avoiding multiple live birth rates (LBRs) in assisted reproductive technologies (ART).1-5 International experts provided a comprehensive review of global multiple LBRs and their associated negative impact on maternal and perinatal outcomes, reasons for global variability, strategies to reduce multiples, single embryo transfer, and implications of funding and reporting. These international comparisons and recommendations are helpful and applicable to infertility care in the United States.3
The rise of multiple birth rates
During the first decade of in vitro fertilization (IVF), live birth rates were low, increasing to 14% in 1990. Multiple embryos needed to be transferred so that even these LBRs could be obtained. In the 1990s, however, laboratory technology improved rapidly, with increased implantation rates and subsequent rapid increases in LBR, but also with increased multiple birth rates (MBRs).
In the United States, clinic-specific reporting helped create competition among clinics for the best LBRs, and this led to MBRs of 30% and higher. Numerous studies documented the associated significantly increased morbidity and mortality of both mothers and babies. Similar situations occurred in many other countries while some, especially Nordic nations, Australia, New Zealand, and Japan, had twin rates of less than 10% or even 5% since the early 2000s. So why the difference?
The higher MBR is due largely to the transfer of more than one embryo. The immediate solution is therefore always to perform elective single embryo transfer (eSET). However, numerous factors affect the decision to perform eSET or not, and this ideal is far from being achieved. Older women, those with longer duration of infertility and/or failed treatment, often feel a time pressure and want to transfer more embryos. Of course, biologically this is reasonable because the number and quality of their embryos is lower. While attempts have been made to assess embryo quality with preimplantation genetic testing for aneuploidy, evidence that this increases the LBR is controversial except possibly in women aged 35 to 38 years. This is especially true when the cumulative LBR, that is, the number of live births after transfer of all embryos from an egg retrieval cycle, is the measured outcome.
The major factor that determines the frequency of eSET is financial. Affordability is the out-of-pocket cost (after insurance or other subsidy) as a percentage of disposable income, and it is the most important factor that determines whether eSET is performed. Less affordable treatment creates a financial incentive to transfer more than one embryo to maximize the pregnancy rates in fewer cycles.5 Other factors include whether the effectiveness of treatment, that is, LBR, is emphasized over safety, that is, MBR. In the United States, the Society for Assisted Reproductive Technology now reports cumulative LBR, singleton and multiple LBR, and preterm births as outcomes, thereby increasing the emphasis on eSET.
Sociologic, cultural, and religious factors also can affect the frequency of eSET. Even within the United States, great variation exists in values and beliefs regarding infertility treatment. It can be challenging to determine who makes decisions: the patient alone, the physician, the payor, professional guidelines, or laws. In many countries, including the United States, it is an amalgam of these.
Setting new goals
If the goal is to reduce the MBR, what should that rate be? In the past few years, the MBR in the United States has been reduced to approximately 10%. It is reasonable now to set a goal of 5% in the next several years. To do this, we can learn from countries that have been successful. The United States already has very high-quality clinical and laboratory services, knowledgeable physicians, and a reasonable regulatory environment. Improved technology, specifically embryo selection for transfer, and focus on adherence to established embryo transfer guidelines could help.
Many would argue that eSET essentially should be performed always in women younger than age 40 and in all women of any age with a known euploid embryo. The major problem that drives multiples is the lack of affordability, which can be addressed by increased subsidies from payors. Increased subsidies can result from legislative mandates or societal pressures on employers, either of which could be associated with requirements for eSET and/or reduced MBRs.
In your practice, you can now reassure your infertility patients that cumulative LBRs are excellent in the United States and that the risk of multiple pregnancy has been reduced dramatically. This should encourage more patients to accept and take advantage of this successful technology that has resulted in the birth of millions of babies globally. Further reduction of the MBR to 5% should be possible within a few years through education and advocacy by women’s health care clinicians that results in increased subsidies and more affordable IVF.
The multiple birth rate in ART has been reduced to 10% in the United States through an increased understanding of the complex factors that affect embryo transfer practices globally. Further progress will depend primarily on increased subsidies that make ART more affordable.
Continue to: Genetics and ART...
Genetics and ART: Selection versus correction
Adashi EY, Cohen IG. The case for remedial germline editing—the long-term view. JAMA. 2020;323:1762-1763.
Rosenbaum L. The future of gene editing—toward scientific and social consensus. N Engl J Med. 2019;380:971-975.
Cyranoski D. The CRISPR-baby scandal: what’s next for human gene-editing. Nature. 2019;566:440-442.
de Wert G, Pennings G, Clarke A, et al; European Society of Human Genetics and European Society of Human Reproduction and Embryology. Human germline gene editing: recommendations of ESHG and ESHRE. Hum Reprod Open. 2018;hox025.
Following the completion of the Human Genome Project in 2003 and major technologic advancements in the subsequent years, the field of human genetics became the focal point of convergence for several distinct but interrelated disciplines: bioinformatics, computational biology, and sequencing technologies. As the result, individual human genomes can now be sequenced at a single base pair level, and with higher fidelity, at a fraction of the original cost and at a much faster speed.
This molecular progress, however, has not been accompanied by an equivalent clinical progress, because in a significant number of cases a defined and predictable clinical phenotype cannot be attributed to a detected molecular genotype. This has resulted in an overabundance of variants of uncertain significance. Variable expressivity, incomplete penetrance, epigenetics, mosaicism, and the polygenic nature of many human traits further complicate reliable interpretation and prognostication of the colossal amount of molecular genetic data that are being generated by the above-mentioned technologic advances.
Considering these limitations, at this juncture it is crucial to acknowledge that any attempts to prematurely commercialize these preclinical and research studies (such as polygenic risk scores for embryos) are perilous and have the potential to cause significant harm in terms of unnecessary stress and anxiety for intended parents as well as the potential for yet-unmapped societal and legal implications.
However, it is just a matter of time until more accurate clinical phenotyping catches up with molecular genotyping. As we get closer to this next historic milestone, precision medicine in the postnatal life (with regard to both diagnostics and therapeutics) and preimplantation genetic testing (PGT) at the prenatal stage for a much wider spectrum of conditions—including both monogenic and polygenic traits—may indeed become a reality.
The potential of germline editing
Specifically regarding PGT (which requires IVF), it is important to recognize that due to the limited and nonrenewable endowment of human oocytes (ovarian reserve), combined with the detrimental impact of advancing age on the quality of the remaining cohort as manifested by a higher risk of aneuploidy, the current clinical practice of trying to “select” a nonaffected embryo can be very inefficient. As a result, the intended parents pursuing such treatments may need to undergo multiple cycles of ovarian stimulation and oocyte retrieval.
A potential solution for genes associated with known diseases is the prospect of remedial germline editing by CRISPR–Cas9 technology or its future descendants. This would take advantage of the existing embryos to try to “correct” the defective gene instead of trying to “select” a normal embryo. These technologies are still in the early stages of development and are remotely distant from clinical applications. On the other hand, although germline gene editing, if actualized, would be a monumental breakthrough in the history of genetics and medicine, we must be cognizant of its serious legal, societal, and ethical ramifications, which are currently unknown. Furthermore, even at the biologic and technical level, the technology still is not advanced enough to reliably rule out off-target modifications, and the unintended clinical consequences of the on-target corrections have not been studied either.
Regulation of genetic modifications
Due to these myriad concerns and the lack of an existing appropriate regulatory framework and oversight for such interventions, current US law (since December 2015, through provisions in annual federal appropriations laws passed by Congress and renewed annually thereafter) bars the US Food and Drug Administration from considering any clinical trial application “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Notably, this moratorium also prohibits mitochondrial replacement technology (MRT), which is a less controversial and relatively better-studied innovation.
Mitochondrial genetic disorders caused by the mutations in mitochondrial DNA (versus nuclear DNA) are amenable to a specific treatment strategy aimed at substituting the defective maternal mitochondrial genome with the mitochondrial genome of an unaffected donor oocyte. This can be achieved via either pronuclear transfer, which involves isolation and transfer of the male and female pronuclei from an affected embryo to an enucleated normal donor embryo, or maternal spindle transfer, which involves isolation and transfer of the metaphase II spindle complex of an affected oocyte to an enucleated disease-free donor egg. It is noteworthy that in 2015 in the United Kingdom, Parliament expanded the definition of “permitted eggs and embryos” to include those “where unhealthy mitochondrial DNA is replaced by healthy mitochondrial DNA from a donor.” This thereby allows the UK Human Fertilisation and Embryology Authority to formally direct and oversee clinical trials in MRT.
Summing up
Although the future of assisted human reproduction cannot be clearly outlined at this time, it is likely to be radically different from the current state given these emerging applications at the intersection of ART and diagnostic and therapeutic genetics. To ensure that exploring this uncharted territory will ultimately be in the interest of humankind and civilization, proper regulatory oversight—after careful consideration of all ethical, societal, and legal implications—needs to be developed for all preclinical and clinical research in this field. Participatory public engagement must be an integrated part of this process. ●
The field of human genetics has already transformed medicine. However, the convergence of the interrelated disciplines of bioinformatics, computational biology, sequencing technologies, and CRISPR–Cas9 technology is creating incredible new advances that will bring great benefits but also major societal challenges.
- Farquhar C. Avoiding multiple pregnancies in assisted reproductive technologies: transferring one embryo at a time should be the norm. Fertil Steril. 2020;114:671-672.
- Bergh C, Kamath MS, Wang R, et al. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673-679.
- Adamson GD, Norman RJ. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil Steril. 2020;114:680-689.
- Eapen A, Ryan GL, Ten Eyck P, et al. Current evidence supporting a goal of singletons: a review of maternal and neonatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2020;114: 690-714.
- Chambers GM, Keller E, Choi S, et al. Funding and public reporting strategies for reducing multiple pregnancy from fertility treatments. Fertil Steril. 2020;114:715-721.
In this Update, we discuss several aspects of infertility and emerging technologic advances in treatment. We review an important infertility fact sheet recently issued by the World Health Organization (WHO) that provides a succinct overview of infertility causes, the rights of infertility patients, treatment challenges, and advocacy efforts. In addition, we discuss what the infertility literature reveals about reducing multiple birth rates and the technologic, financial, and social factors involved. Finally, we look at the molecular progress made in germline-editing technology and the myriad complications involved in its potential future translation to clinical phenotyping.
WHO recognizes the burden of infertility and addresses fertility care needs
World Health Organization (WHO). Infertility fact sheet. September 14, 2020. https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed January 24, 2021.
The WHO published its first comprehensive infertility fact sheet in September 2020. This document is important because it validates infertility as a high-burden disease and disability that diminishes quality of life for up to 186 million individuals globally. The infertility fact sheet is a comprehensive yet focused quick read that addresses the causes of infertility, why infertility is important, challenges, and the WHO response.
Factors in infertility
Infertility is caused by different factors in women and men, yet sometimes it is unexplained, and its relative importance can vary from country to country. For women, tubal disorders (for example, postinfectious), uterine problems (fibroids, congenital), endometriosis, ovarian disorders (polycystic ovary syndrome, ovulation disorders), and endocrine imbalances are the most common factors.
For men, causes of infertility include obstruction of the reproductive tract (as after injuries or infection); hormonal disorders in the hypothalamus, pituitary, and/or testicles (for example, low testosterone); testicular failure to produce sperm (such as after cancer treatment); and abnormal sperm function and quality (low count, motility, or morphology).
Environmental and lifestyle factors— including smoking, obesity, alcohol, or toxins—can affect fertility.
Continue to: Recognizing all individuals’ fertility rights...
Recognizing all individuals’ fertility rights
The WHO infertility fact sheet makes strong statements, recognizing that individuals and couples have the right to decide the number, timing, and spacing of their children. Addressing infertility is therefore an important part of realizing the right of individuals and couples to found a family. This includes heterosexual couples, same-sex partners, older persons, individuals not in sexual relationships who might require infertility management and fertility care services, and notably marginalized populations.
Addressing infertility also can help mitigate gender inequality, which has significant negative social impacts on the lives of infertile individuals, especially women. Fertility education is important to reduce the fear of infertility and contraception use in those wanting pregnancy in the future.
In most countries the biggest challenges are availability, access, and quality of interventions to address infertility. This includes the United States, where only 1 in 4 individuals receive the fertility care they need. Lack of prioritization, ineffective public health strategies, inadequate funding, and costs are barriers. Health policies need to recognize that infertility is a disease that often can be prevented, thereby reducing future costs. Comprehensive awareness and education programs, laws and policies that regulate and ensure access and the human rights of all involved, are essential.
Advocacy efforts
To address infertility and fertility care, the WHO is committed to:
- collaborate with partners on epidemiologic and etiologic research
- facilitate policy dialogue globally to frame infertility within a legal and policy framework
- support generation of data on the burden of infertility
- develop guidelines
- produce other documents of standards
- collaborate with all stakeholders to strengthen political commitment and health system capacity, and
- provide country-level technical support to develop or strengthen policies and services.
For your practice, this means that infertility is recognized as a disease that should receive its appropriate share of health care resources. Infertility and fertility care are the right of every individual according to their desires to found a family. Besides providing the best care you can to all your patients, including referring them when necessary, all health care clinicians should advocate on behalf of their patients to payors, policy makers, and the public the need to provide equitable laws, resources, and funding for infertility and fertility care.
Every person has the right to infertility and fertility care as endorsed by the recent WHO infertility fact sheet. To address this high-burden disease, all women’s health care clinicians should be aware of, equitably diagnose and treat, refer as necessary, and advocate for infertile individuals.
Continue to: Lessons learned in reducing multiple pregnancy rates in infertility treatment...
Lessons learned in reducing multiple pregnancy rates in infertility treatment
Views and reviews section. Fertil Steril. 2020;114:671- 672; 673-679; 680-689; 690-714; 715-721.
In the October 2020 issue of Fertility and Sterility, the Views and Reviews section included 5 articles on avoiding multiple live birth rates (LBRs) in assisted reproductive technologies (ART).1-5 International experts provided a comprehensive review of global multiple LBRs and their associated negative impact on maternal and perinatal outcomes, reasons for global variability, strategies to reduce multiples, single embryo transfer, and implications of funding and reporting. These international comparisons and recommendations are helpful and applicable to infertility care in the United States.3
The rise of multiple birth rates
During the first decade of in vitro fertilization (IVF), live birth rates were low, increasing to 14% in 1990. Multiple embryos needed to be transferred so that even these LBRs could be obtained. In the 1990s, however, laboratory technology improved rapidly, with increased implantation rates and subsequent rapid increases in LBR, but also with increased multiple birth rates (MBRs).
In the United States, clinic-specific reporting helped create competition among clinics for the best LBRs, and this led to MBRs of 30% and higher. Numerous studies documented the associated significantly increased morbidity and mortality of both mothers and babies. Similar situations occurred in many other countries while some, especially Nordic nations, Australia, New Zealand, and Japan, had twin rates of less than 10% or even 5% since the early 2000s. So why the difference?
The higher MBR is due largely to the transfer of more than one embryo. The immediate solution is therefore always to perform elective single embryo transfer (eSET). However, numerous factors affect the decision to perform eSET or not, and this ideal is far from being achieved. Older women, those with longer duration of infertility and/or failed treatment, often feel a time pressure and want to transfer more embryos. Of course, biologically this is reasonable because the number and quality of their embryos is lower. While attempts have been made to assess embryo quality with preimplantation genetic testing for aneuploidy, evidence that this increases the LBR is controversial except possibly in women aged 35 to 38 years. This is especially true when the cumulative LBR, that is, the number of live births after transfer of all embryos from an egg retrieval cycle, is the measured outcome.
The major factor that determines the frequency of eSET is financial. Affordability is the out-of-pocket cost (after insurance or other subsidy) as a percentage of disposable income, and it is the most important factor that determines whether eSET is performed. Less affordable treatment creates a financial incentive to transfer more than one embryo to maximize the pregnancy rates in fewer cycles.5 Other factors include whether the effectiveness of treatment, that is, LBR, is emphasized over safety, that is, MBR. In the United States, the Society for Assisted Reproductive Technology now reports cumulative LBR, singleton and multiple LBR, and preterm births as outcomes, thereby increasing the emphasis on eSET.
Sociologic, cultural, and religious factors also can affect the frequency of eSET. Even within the United States, great variation exists in values and beliefs regarding infertility treatment. It can be challenging to determine who makes decisions: the patient alone, the physician, the payor, professional guidelines, or laws. In many countries, including the United States, it is an amalgam of these.
Setting new goals
If the goal is to reduce the MBR, what should that rate be? In the past few years, the MBR in the United States has been reduced to approximately 10%. It is reasonable now to set a goal of 5% in the next several years. To do this, we can learn from countries that have been successful. The United States already has very high-quality clinical and laboratory services, knowledgeable physicians, and a reasonable regulatory environment. Improved technology, specifically embryo selection for transfer, and focus on adherence to established embryo transfer guidelines could help.
Many would argue that eSET essentially should be performed always in women younger than age 40 and in all women of any age with a known euploid embryo. The major problem that drives multiples is the lack of affordability, which can be addressed by increased subsidies from payors. Increased subsidies can result from legislative mandates or societal pressures on employers, either of which could be associated with requirements for eSET and/or reduced MBRs.
In your practice, you can now reassure your infertility patients that cumulative LBRs are excellent in the United States and that the risk of multiple pregnancy has been reduced dramatically. This should encourage more patients to accept and take advantage of this successful technology that has resulted in the birth of millions of babies globally. Further reduction of the MBR to 5% should be possible within a few years through education and advocacy by women’s health care clinicians that results in increased subsidies and more affordable IVF.
The multiple birth rate in ART has been reduced to 10% in the United States through an increased understanding of the complex factors that affect embryo transfer practices globally. Further progress will depend primarily on increased subsidies that make ART more affordable.
Continue to: Genetics and ART...
Genetics and ART: Selection versus correction
Adashi EY, Cohen IG. The case for remedial germline editing—the long-term view. JAMA. 2020;323:1762-1763.
Rosenbaum L. The future of gene editing—toward scientific and social consensus. N Engl J Med. 2019;380:971-975.
Cyranoski D. The CRISPR-baby scandal: what’s next for human gene-editing. Nature. 2019;566:440-442.
de Wert G, Pennings G, Clarke A, et al; European Society of Human Genetics and European Society of Human Reproduction and Embryology. Human germline gene editing: recommendations of ESHG and ESHRE. Hum Reprod Open. 2018;hox025.
Following the completion of the Human Genome Project in 2003 and major technologic advancements in the subsequent years, the field of human genetics became the focal point of convergence for several distinct but interrelated disciplines: bioinformatics, computational biology, and sequencing technologies. As the result, individual human genomes can now be sequenced at a single base pair level, and with higher fidelity, at a fraction of the original cost and at a much faster speed.
This molecular progress, however, has not been accompanied by an equivalent clinical progress, because in a significant number of cases a defined and predictable clinical phenotype cannot be attributed to a detected molecular genotype. This has resulted in an overabundance of variants of uncertain significance. Variable expressivity, incomplete penetrance, epigenetics, mosaicism, and the polygenic nature of many human traits further complicate reliable interpretation and prognostication of the colossal amount of molecular genetic data that are being generated by the above-mentioned technologic advances.
Considering these limitations, at this juncture it is crucial to acknowledge that any attempts to prematurely commercialize these preclinical and research studies (such as polygenic risk scores for embryos) are perilous and have the potential to cause significant harm in terms of unnecessary stress and anxiety for intended parents as well as the potential for yet-unmapped societal and legal implications.
However, it is just a matter of time until more accurate clinical phenotyping catches up with molecular genotyping. As we get closer to this next historic milestone, precision medicine in the postnatal life (with regard to both diagnostics and therapeutics) and preimplantation genetic testing (PGT) at the prenatal stage for a much wider spectrum of conditions—including both monogenic and polygenic traits—may indeed become a reality.
The potential of germline editing
Specifically regarding PGT (which requires IVF), it is important to recognize that due to the limited and nonrenewable endowment of human oocytes (ovarian reserve), combined with the detrimental impact of advancing age on the quality of the remaining cohort as manifested by a higher risk of aneuploidy, the current clinical practice of trying to “select” a nonaffected embryo can be very inefficient. As a result, the intended parents pursuing such treatments may need to undergo multiple cycles of ovarian stimulation and oocyte retrieval.
A potential solution for genes associated with known diseases is the prospect of remedial germline editing by CRISPR–Cas9 technology or its future descendants. This would take advantage of the existing embryos to try to “correct” the defective gene instead of trying to “select” a normal embryo. These technologies are still in the early stages of development and are remotely distant from clinical applications. On the other hand, although germline gene editing, if actualized, would be a monumental breakthrough in the history of genetics and medicine, we must be cognizant of its serious legal, societal, and ethical ramifications, which are currently unknown. Furthermore, even at the biologic and technical level, the technology still is not advanced enough to reliably rule out off-target modifications, and the unintended clinical consequences of the on-target corrections have not been studied either.
Regulation of genetic modifications
Due to these myriad concerns and the lack of an existing appropriate regulatory framework and oversight for such interventions, current US law (since December 2015, through provisions in annual federal appropriations laws passed by Congress and renewed annually thereafter) bars the US Food and Drug Administration from considering any clinical trial application “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Notably, this moratorium also prohibits mitochondrial replacement technology (MRT), which is a less controversial and relatively better-studied innovation.
Mitochondrial genetic disorders caused by the mutations in mitochondrial DNA (versus nuclear DNA) are amenable to a specific treatment strategy aimed at substituting the defective maternal mitochondrial genome with the mitochondrial genome of an unaffected donor oocyte. This can be achieved via either pronuclear transfer, which involves isolation and transfer of the male and female pronuclei from an affected embryo to an enucleated normal donor embryo, or maternal spindle transfer, which involves isolation and transfer of the metaphase II spindle complex of an affected oocyte to an enucleated disease-free donor egg. It is noteworthy that in 2015 in the United Kingdom, Parliament expanded the definition of “permitted eggs and embryos” to include those “where unhealthy mitochondrial DNA is replaced by healthy mitochondrial DNA from a donor.” This thereby allows the UK Human Fertilisation and Embryology Authority to formally direct and oversee clinical trials in MRT.
Summing up
Although the future of assisted human reproduction cannot be clearly outlined at this time, it is likely to be radically different from the current state given these emerging applications at the intersection of ART and diagnostic and therapeutic genetics. To ensure that exploring this uncharted territory will ultimately be in the interest of humankind and civilization, proper regulatory oversight—after careful consideration of all ethical, societal, and legal implications—needs to be developed for all preclinical and clinical research in this field. Participatory public engagement must be an integrated part of this process. ●
The field of human genetics has already transformed medicine. However, the convergence of the interrelated disciplines of bioinformatics, computational biology, sequencing technologies, and CRISPR–Cas9 technology is creating incredible new advances that will bring great benefits but also major societal challenges.
In this Update, we discuss several aspects of infertility and emerging technologic advances in treatment. We review an important infertility fact sheet recently issued by the World Health Organization (WHO) that provides a succinct overview of infertility causes, the rights of infertility patients, treatment challenges, and advocacy efforts. In addition, we discuss what the infertility literature reveals about reducing multiple birth rates and the technologic, financial, and social factors involved. Finally, we look at the molecular progress made in germline-editing technology and the myriad complications involved in its potential future translation to clinical phenotyping.
WHO recognizes the burden of infertility and addresses fertility care needs
World Health Organization (WHO). Infertility fact sheet. September 14, 2020. https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed January 24, 2021.
The WHO published its first comprehensive infertility fact sheet in September 2020. This document is important because it validates infertility as a high-burden disease and disability that diminishes quality of life for up to 186 million individuals globally. The infertility fact sheet is a comprehensive yet focused quick read that addresses the causes of infertility, why infertility is important, challenges, and the WHO response.
Factors in infertility
Infertility is caused by different factors in women and men, yet sometimes it is unexplained, and its relative importance can vary from country to country. For women, tubal disorders (for example, postinfectious), uterine problems (fibroids, congenital), endometriosis, ovarian disorders (polycystic ovary syndrome, ovulation disorders), and endocrine imbalances are the most common factors.
For men, causes of infertility include obstruction of the reproductive tract (as after injuries or infection); hormonal disorders in the hypothalamus, pituitary, and/or testicles (for example, low testosterone); testicular failure to produce sperm (such as after cancer treatment); and abnormal sperm function and quality (low count, motility, or morphology).
Environmental and lifestyle factors— including smoking, obesity, alcohol, or toxins—can affect fertility.
Continue to: Recognizing all individuals’ fertility rights...
Recognizing all individuals’ fertility rights
The WHO infertility fact sheet makes strong statements, recognizing that individuals and couples have the right to decide the number, timing, and spacing of their children. Addressing infertility is therefore an important part of realizing the right of individuals and couples to found a family. This includes heterosexual couples, same-sex partners, older persons, individuals not in sexual relationships who might require infertility management and fertility care services, and notably marginalized populations.
Addressing infertility also can help mitigate gender inequality, which has significant negative social impacts on the lives of infertile individuals, especially women. Fertility education is important to reduce the fear of infertility and contraception use in those wanting pregnancy in the future.
In most countries the biggest challenges are availability, access, and quality of interventions to address infertility. This includes the United States, where only 1 in 4 individuals receive the fertility care they need. Lack of prioritization, ineffective public health strategies, inadequate funding, and costs are barriers. Health policies need to recognize that infertility is a disease that often can be prevented, thereby reducing future costs. Comprehensive awareness and education programs, laws and policies that regulate and ensure access and the human rights of all involved, are essential.
Advocacy efforts
To address infertility and fertility care, the WHO is committed to:
- collaborate with partners on epidemiologic and etiologic research
- facilitate policy dialogue globally to frame infertility within a legal and policy framework
- support generation of data on the burden of infertility
- develop guidelines
- produce other documents of standards
- collaborate with all stakeholders to strengthen political commitment and health system capacity, and
- provide country-level technical support to develop or strengthen policies and services.
For your practice, this means that infertility is recognized as a disease that should receive its appropriate share of health care resources. Infertility and fertility care are the right of every individual according to their desires to found a family. Besides providing the best care you can to all your patients, including referring them when necessary, all health care clinicians should advocate on behalf of their patients to payors, policy makers, and the public the need to provide equitable laws, resources, and funding for infertility and fertility care.
Every person has the right to infertility and fertility care as endorsed by the recent WHO infertility fact sheet. To address this high-burden disease, all women’s health care clinicians should be aware of, equitably diagnose and treat, refer as necessary, and advocate for infertile individuals.
Continue to: Lessons learned in reducing multiple pregnancy rates in infertility treatment...
Lessons learned in reducing multiple pregnancy rates in infertility treatment
Views and reviews section. Fertil Steril. 2020;114:671- 672; 673-679; 680-689; 690-714; 715-721.
In the October 2020 issue of Fertility and Sterility, the Views and Reviews section included 5 articles on avoiding multiple live birth rates (LBRs) in assisted reproductive technologies (ART).1-5 International experts provided a comprehensive review of global multiple LBRs and their associated negative impact on maternal and perinatal outcomes, reasons for global variability, strategies to reduce multiples, single embryo transfer, and implications of funding and reporting. These international comparisons and recommendations are helpful and applicable to infertility care in the United States.3
The rise of multiple birth rates
During the first decade of in vitro fertilization (IVF), live birth rates were low, increasing to 14% in 1990. Multiple embryos needed to be transferred so that even these LBRs could be obtained. In the 1990s, however, laboratory technology improved rapidly, with increased implantation rates and subsequent rapid increases in LBR, but also with increased multiple birth rates (MBRs).
In the United States, clinic-specific reporting helped create competition among clinics for the best LBRs, and this led to MBRs of 30% and higher. Numerous studies documented the associated significantly increased morbidity and mortality of both mothers and babies. Similar situations occurred in many other countries while some, especially Nordic nations, Australia, New Zealand, and Japan, had twin rates of less than 10% or even 5% since the early 2000s. So why the difference?
The higher MBR is due largely to the transfer of more than one embryo. The immediate solution is therefore always to perform elective single embryo transfer (eSET). However, numerous factors affect the decision to perform eSET or not, and this ideal is far from being achieved. Older women, those with longer duration of infertility and/or failed treatment, often feel a time pressure and want to transfer more embryos. Of course, biologically this is reasonable because the number and quality of their embryos is lower. While attempts have been made to assess embryo quality with preimplantation genetic testing for aneuploidy, evidence that this increases the LBR is controversial except possibly in women aged 35 to 38 years. This is especially true when the cumulative LBR, that is, the number of live births after transfer of all embryos from an egg retrieval cycle, is the measured outcome.
The major factor that determines the frequency of eSET is financial. Affordability is the out-of-pocket cost (after insurance or other subsidy) as a percentage of disposable income, and it is the most important factor that determines whether eSET is performed. Less affordable treatment creates a financial incentive to transfer more than one embryo to maximize the pregnancy rates in fewer cycles.5 Other factors include whether the effectiveness of treatment, that is, LBR, is emphasized over safety, that is, MBR. In the United States, the Society for Assisted Reproductive Technology now reports cumulative LBR, singleton and multiple LBR, and preterm births as outcomes, thereby increasing the emphasis on eSET.
Sociologic, cultural, and religious factors also can affect the frequency of eSET. Even within the United States, great variation exists in values and beliefs regarding infertility treatment. It can be challenging to determine who makes decisions: the patient alone, the physician, the payor, professional guidelines, or laws. In many countries, including the United States, it is an amalgam of these.
Setting new goals
If the goal is to reduce the MBR, what should that rate be? In the past few years, the MBR in the United States has been reduced to approximately 10%. It is reasonable now to set a goal of 5% in the next several years. To do this, we can learn from countries that have been successful. The United States already has very high-quality clinical and laboratory services, knowledgeable physicians, and a reasonable regulatory environment. Improved technology, specifically embryo selection for transfer, and focus on adherence to established embryo transfer guidelines could help.
Many would argue that eSET essentially should be performed always in women younger than age 40 and in all women of any age with a known euploid embryo. The major problem that drives multiples is the lack of affordability, which can be addressed by increased subsidies from payors. Increased subsidies can result from legislative mandates or societal pressures on employers, either of which could be associated with requirements for eSET and/or reduced MBRs.
In your practice, you can now reassure your infertility patients that cumulative LBRs are excellent in the United States and that the risk of multiple pregnancy has been reduced dramatically. This should encourage more patients to accept and take advantage of this successful technology that has resulted in the birth of millions of babies globally. Further reduction of the MBR to 5% should be possible within a few years through education and advocacy by women’s health care clinicians that results in increased subsidies and more affordable IVF.
The multiple birth rate in ART has been reduced to 10% in the United States through an increased understanding of the complex factors that affect embryo transfer practices globally. Further progress will depend primarily on increased subsidies that make ART more affordable.
Continue to: Genetics and ART...
Genetics and ART: Selection versus correction
Adashi EY, Cohen IG. The case for remedial germline editing—the long-term view. JAMA. 2020;323:1762-1763.
Rosenbaum L. The future of gene editing—toward scientific and social consensus. N Engl J Med. 2019;380:971-975.
Cyranoski D. The CRISPR-baby scandal: what’s next for human gene-editing. Nature. 2019;566:440-442.
de Wert G, Pennings G, Clarke A, et al; European Society of Human Genetics and European Society of Human Reproduction and Embryology. Human germline gene editing: recommendations of ESHG and ESHRE. Hum Reprod Open. 2018;hox025.
Following the completion of the Human Genome Project in 2003 and major technologic advancements in the subsequent years, the field of human genetics became the focal point of convergence for several distinct but interrelated disciplines: bioinformatics, computational biology, and sequencing technologies. As the result, individual human genomes can now be sequenced at a single base pair level, and with higher fidelity, at a fraction of the original cost and at a much faster speed.
This molecular progress, however, has not been accompanied by an equivalent clinical progress, because in a significant number of cases a defined and predictable clinical phenotype cannot be attributed to a detected molecular genotype. This has resulted in an overabundance of variants of uncertain significance. Variable expressivity, incomplete penetrance, epigenetics, mosaicism, and the polygenic nature of many human traits further complicate reliable interpretation and prognostication of the colossal amount of molecular genetic data that are being generated by the above-mentioned technologic advances.
Considering these limitations, at this juncture it is crucial to acknowledge that any attempts to prematurely commercialize these preclinical and research studies (such as polygenic risk scores for embryos) are perilous and have the potential to cause significant harm in terms of unnecessary stress and anxiety for intended parents as well as the potential for yet-unmapped societal and legal implications.
However, it is just a matter of time until more accurate clinical phenotyping catches up with molecular genotyping. As we get closer to this next historic milestone, precision medicine in the postnatal life (with regard to both diagnostics and therapeutics) and preimplantation genetic testing (PGT) at the prenatal stage for a much wider spectrum of conditions—including both monogenic and polygenic traits—may indeed become a reality.
The potential of germline editing
Specifically regarding PGT (which requires IVF), it is important to recognize that due to the limited and nonrenewable endowment of human oocytes (ovarian reserve), combined with the detrimental impact of advancing age on the quality of the remaining cohort as manifested by a higher risk of aneuploidy, the current clinical practice of trying to “select” a nonaffected embryo can be very inefficient. As a result, the intended parents pursuing such treatments may need to undergo multiple cycles of ovarian stimulation and oocyte retrieval.
A potential solution for genes associated with known diseases is the prospect of remedial germline editing by CRISPR–Cas9 technology or its future descendants. This would take advantage of the existing embryos to try to “correct” the defective gene instead of trying to “select” a normal embryo. These technologies are still in the early stages of development and are remotely distant from clinical applications. On the other hand, although germline gene editing, if actualized, would be a monumental breakthrough in the history of genetics and medicine, we must be cognizant of its serious legal, societal, and ethical ramifications, which are currently unknown. Furthermore, even at the biologic and technical level, the technology still is not advanced enough to reliably rule out off-target modifications, and the unintended clinical consequences of the on-target corrections have not been studied either.
Regulation of genetic modifications
Due to these myriad concerns and the lack of an existing appropriate regulatory framework and oversight for such interventions, current US law (since December 2015, through provisions in annual federal appropriations laws passed by Congress and renewed annually thereafter) bars the US Food and Drug Administration from considering any clinical trial application “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Notably, this moratorium also prohibits mitochondrial replacement technology (MRT), which is a less controversial and relatively better-studied innovation.
Mitochondrial genetic disorders caused by the mutations in mitochondrial DNA (versus nuclear DNA) are amenable to a specific treatment strategy aimed at substituting the defective maternal mitochondrial genome with the mitochondrial genome of an unaffected donor oocyte. This can be achieved via either pronuclear transfer, which involves isolation and transfer of the male and female pronuclei from an affected embryo to an enucleated normal donor embryo, or maternal spindle transfer, which involves isolation and transfer of the metaphase II spindle complex of an affected oocyte to an enucleated disease-free donor egg. It is noteworthy that in 2015 in the United Kingdom, Parliament expanded the definition of “permitted eggs and embryos” to include those “where unhealthy mitochondrial DNA is replaced by healthy mitochondrial DNA from a donor.” This thereby allows the UK Human Fertilisation and Embryology Authority to formally direct and oversee clinical trials in MRT.
Summing up
Although the future of assisted human reproduction cannot be clearly outlined at this time, it is likely to be radically different from the current state given these emerging applications at the intersection of ART and diagnostic and therapeutic genetics. To ensure that exploring this uncharted territory will ultimately be in the interest of humankind and civilization, proper regulatory oversight—after careful consideration of all ethical, societal, and legal implications—needs to be developed for all preclinical and clinical research in this field. Participatory public engagement must be an integrated part of this process. ●
The field of human genetics has already transformed medicine. However, the convergence of the interrelated disciplines of bioinformatics, computational biology, sequencing technologies, and CRISPR–Cas9 technology is creating incredible new advances that will bring great benefits but also major societal challenges.
- Farquhar C. Avoiding multiple pregnancies in assisted reproductive technologies: transferring one embryo at a time should be the norm. Fertil Steril. 2020;114:671-672.
- Bergh C, Kamath MS, Wang R, et al. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673-679.
- Adamson GD, Norman RJ. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil Steril. 2020;114:680-689.
- Eapen A, Ryan GL, Ten Eyck P, et al. Current evidence supporting a goal of singletons: a review of maternal and neonatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2020;114: 690-714.
- Chambers GM, Keller E, Choi S, et al. Funding and public reporting strategies for reducing multiple pregnancy from fertility treatments. Fertil Steril. 2020;114:715-721.
- Farquhar C. Avoiding multiple pregnancies in assisted reproductive technologies: transferring one embryo at a time should be the norm. Fertil Steril. 2020;114:671-672.
- Bergh C, Kamath MS, Wang R, et al. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673-679.
- Adamson GD, Norman RJ. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil Steril. 2020;114:680-689.
- Eapen A, Ryan GL, Ten Eyck P, et al. Current evidence supporting a goal of singletons: a review of maternal and neonatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2020;114: 690-714.
- Chambers GM, Keller E, Choi S, et al. Funding and public reporting strategies for reducing multiple pregnancy from fertility treatments. Fertil Steril. 2020;114:715-721.
Metformin tied to longer gestation in women with preterm preeclampsia
Metformin extended gestation by nearly a week in women with preterm preeclampsia and was also linked to a shorter neonatal hospital stay, according to findings from a study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
The causes of preeclampsia have continued to elude researchers, but most agree the placenta plays a key role, explained Cathy Cluver, PhD, director of the preeclampsia research unit and an associate professor at Stellenbosch University, Cape Town. Past trials have tested sildenafil, antithrombin, pravastatin, and esomeprazole, but the drugs either did not show promise, had unacceptable side effects, or need further study.
“This trial provides proof of concept that preterm preeclampsia can be treated and that we can slow the progression of preterm preeclampsia,” Dr. Cluver said.
In this trial, the researchers enrolled 180 women with preterm preeclampsia between 26 and 31 weeks of gestation. All the women were taking hypertensives. They were randomly assigned to receive 3 g oral metformin XR or placebo daily. The intention-to-treat analysis included 87 women who received metformin and 84 who received placebo, with baseline characteristics similar in both groups.
Women in the metformin group gave birth a median 16.2 days after randomization, which was 6.7 days longer than the 9.5 days postrandomization delivery of women in the placebo group. The differences, however, narrowly missed statistical significance (P =.056).
But when the researchers took compliance and dose into account, the effect of the metformin increased, showing a dose-dependent effect, and did reach statistical significance. Among the 147 women who continued treatment until delivery, those in the metformin group delivered a median 8.4 days later than those in the placebo group (16.2 vs. 7.4 days; P =.026). Further, when the analysis was further restricted to just the 100 women who continued taking the full dose until delivery, the difference was even greater (16.2 vs. 4.8 days; P =.008). In accordance with the safety profile of metformin, women taking the drug had more diarrhea and a trend toward more nausea than those taking the placebo.
There were no differences between the groups in composite maternal or neonatal outcomes, but the infants were an average 136 g (4.8 ounces) heavier in the metformin group, albeit the difference did not reach statistical significance. The 6-day–shorter neonatal stay at the study site facility for infants of the metformin group also did not reach statistical significance, but there was a significant difference between the groups on overall stay, including transfers to other facilities. Infants in the metformin group averaged 26 days vs. 34 days for infants in the placebo group (P =.007).
“We have shown that metformin XR may be a treatment for preterm preeclampsia. We now plan to do a larger study to hopefully confirm these findings, which will be powered to both prolongation of pregnancy and neonatal outcomes,” Dr. Cluver told this news organization. “We have also shown that one can prolong pregnancy in preterm preeclampsia, and we hope that this will encourage others in our field to continue researching therapeutics for preterm preeclampsia.”
In response to questions from attendees, Dr. Cluver reported that her team did not collect histological data from placentas in this study, and lack of funding is limiting their ability to evaluate longer-term outcomes.
The findings of prolonged gestation were certainly exciting, but they warrant caution before any changes in clinical practice, Michelle Y. Owens, MD, professor and chief of maternal-fetal medicine at the University of Mississippi Medical Center, Jackson, said in an interview.
“While the findings of this study are promising, the sample size was small, the dosing exceeds what we typically use in the U.S., and this was undertaken in Cape Town, South Africa, all of which may render this study less generalizable to our population and others across the globe,” said Dr. Owens, who moderated the oral abstract session.
She also pointed out a possible conflicting effect on birth weight brought on by using metformin to extend gestation.
“If larger studies are undertaken, I believe it is quite possible that, with extended gestation, there will be bigger babies,” she said. “However, metformin also helps control blood glucose and in so doing, may contribute to lower birth weights over time, compared with women not exposed to the drug.”
Dr. Cluver and Dr. Owens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This story was updated on 2/9/2021.
Metformin extended gestation by nearly a week in women with preterm preeclampsia and was also linked to a shorter neonatal hospital stay, according to findings from a study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
The causes of preeclampsia have continued to elude researchers, but most agree the placenta plays a key role, explained Cathy Cluver, PhD, director of the preeclampsia research unit and an associate professor at Stellenbosch University, Cape Town. Past trials have tested sildenafil, antithrombin, pravastatin, and esomeprazole, but the drugs either did not show promise, had unacceptable side effects, or need further study.
“This trial provides proof of concept that preterm preeclampsia can be treated and that we can slow the progression of preterm preeclampsia,” Dr. Cluver said.
In this trial, the researchers enrolled 180 women with preterm preeclampsia between 26 and 31 weeks of gestation. All the women were taking hypertensives. They were randomly assigned to receive 3 g oral metformin XR or placebo daily. The intention-to-treat analysis included 87 women who received metformin and 84 who received placebo, with baseline characteristics similar in both groups.
Women in the metformin group gave birth a median 16.2 days after randomization, which was 6.7 days longer than the 9.5 days postrandomization delivery of women in the placebo group. The differences, however, narrowly missed statistical significance (P =.056).
But when the researchers took compliance and dose into account, the effect of the metformin increased, showing a dose-dependent effect, and did reach statistical significance. Among the 147 women who continued treatment until delivery, those in the metformin group delivered a median 8.4 days later than those in the placebo group (16.2 vs. 7.4 days; P =.026). Further, when the analysis was further restricted to just the 100 women who continued taking the full dose until delivery, the difference was even greater (16.2 vs. 4.8 days; P =.008). In accordance with the safety profile of metformin, women taking the drug had more diarrhea and a trend toward more nausea than those taking the placebo.
There were no differences between the groups in composite maternal or neonatal outcomes, but the infants were an average 136 g (4.8 ounces) heavier in the metformin group, albeit the difference did not reach statistical significance. The 6-day–shorter neonatal stay at the study site facility for infants of the metformin group also did not reach statistical significance, but there was a significant difference between the groups on overall stay, including transfers to other facilities. Infants in the metformin group averaged 26 days vs. 34 days for infants in the placebo group (P =.007).
“We have shown that metformin XR may be a treatment for preterm preeclampsia. We now plan to do a larger study to hopefully confirm these findings, which will be powered to both prolongation of pregnancy and neonatal outcomes,” Dr. Cluver told this news organization. “We have also shown that one can prolong pregnancy in preterm preeclampsia, and we hope that this will encourage others in our field to continue researching therapeutics for preterm preeclampsia.”
In response to questions from attendees, Dr. Cluver reported that her team did not collect histological data from placentas in this study, and lack of funding is limiting their ability to evaluate longer-term outcomes.
The findings of prolonged gestation were certainly exciting, but they warrant caution before any changes in clinical practice, Michelle Y. Owens, MD, professor and chief of maternal-fetal medicine at the University of Mississippi Medical Center, Jackson, said in an interview.
“While the findings of this study are promising, the sample size was small, the dosing exceeds what we typically use in the U.S., and this was undertaken in Cape Town, South Africa, all of which may render this study less generalizable to our population and others across the globe,” said Dr. Owens, who moderated the oral abstract session.
She also pointed out a possible conflicting effect on birth weight brought on by using metformin to extend gestation.
“If larger studies are undertaken, I believe it is quite possible that, with extended gestation, there will be bigger babies,” she said. “However, metformin also helps control blood glucose and in so doing, may contribute to lower birth weights over time, compared with women not exposed to the drug.”
Dr. Cluver and Dr. Owens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This story was updated on 2/9/2021.
Metformin extended gestation by nearly a week in women with preterm preeclampsia and was also linked to a shorter neonatal hospital stay, according to findings from a study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
The causes of preeclampsia have continued to elude researchers, but most agree the placenta plays a key role, explained Cathy Cluver, PhD, director of the preeclampsia research unit and an associate professor at Stellenbosch University, Cape Town. Past trials have tested sildenafil, antithrombin, pravastatin, and esomeprazole, but the drugs either did not show promise, had unacceptable side effects, or need further study.
“This trial provides proof of concept that preterm preeclampsia can be treated and that we can slow the progression of preterm preeclampsia,” Dr. Cluver said.
In this trial, the researchers enrolled 180 women with preterm preeclampsia between 26 and 31 weeks of gestation. All the women were taking hypertensives. They were randomly assigned to receive 3 g oral metformin XR or placebo daily. The intention-to-treat analysis included 87 women who received metformin and 84 who received placebo, with baseline characteristics similar in both groups.
Women in the metformin group gave birth a median 16.2 days after randomization, which was 6.7 days longer than the 9.5 days postrandomization delivery of women in the placebo group. The differences, however, narrowly missed statistical significance (P =.056).
But when the researchers took compliance and dose into account, the effect of the metformin increased, showing a dose-dependent effect, and did reach statistical significance. Among the 147 women who continued treatment until delivery, those in the metformin group delivered a median 8.4 days later than those in the placebo group (16.2 vs. 7.4 days; P =.026). Further, when the analysis was further restricted to just the 100 women who continued taking the full dose until delivery, the difference was even greater (16.2 vs. 4.8 days; P =.008). In accordance with the safety profile of metformin, women taking the drug had more diarrhea and a trend toward more nausea than those taking the placebo.
There were no differences between the groups in composite maternal or neonatal outcomes, but the infants were an average 136 g (4.8 ounces) heavier in the metformin group, albeit the difference did not reach statistical significance. The 6-day–shorter neonatal stay at the study site facility for infants of the metformin group also did not reach statistical significance, but there was a significant difference between the groups on overall stay, including transfers to other facilities. Infants in the metformin group averaged 26 days vs. 34 days for infants in the placebo group (P =.007).
“We have shown that metformin XR may be a treatment for preterm preeclampsia. We now plan to do a larger study to hopefully confirm these findings, which will be powered to both prolongation of pregnancy and neonatal outcomes,” Dr. Cluver told this news organization. “We have also shown that one can prolong pregnancy in preterm preeclampsia, and we hope that this will encourage others in our field to continue researching therapeutics for preterm preeclampsia.”
In response to questions from attendees, Dr. Cluver reported that her team did not collect histological data from placentas in this study, and lack of funding is limiting their ability to evaluate longer-term outcomes.
The findings of prolonged gestation were certainly exciting, but they warrant caution before any changes in clinical practice, Michelle Y. Owens, MD, professor and chief of maternal-fetal medicine at the University of Mississippi Medical Center, Jackson, said in an interview.
“While the findings of this study are promising, the sample size was small, the dosing exceeds what we typically use in the U.S., and this was undertaken in Cape Town, South Africa, all of which may render this study less generalizable to our population and others across the globe,” said Dr. Owens, who moderated the oral abstract session.
She also pointed out a possible conflicting effect on birth weight brought on by using metformin to extend gestation.
“If larger studies are undertaken, I believe it is quite possible that, with extended gestation, there will be bigger babies,” she said. “However, metformin also helps control blood glucose and in so doing, may contribute to lower birth weights over time, compared with women not exposed to the drug.”
Dr. Cluver and Dr. Owens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*This story was updated on 2/9/2021.
COVID-19 in pregnancy tied to hypertension, preeclampsia
Having COVID-19 during pregnancy is linked to a significantly increased risk for gestational hypertension and preeclampsia compared with not having COVID-19 while pregnant, according to findings from a retrospective study presented Jan. 28 at the Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“This was not entirely surprising given that inflammation has been implicated in the pathogenesis of both hypertensive disorders of pregnancy and COVID-19 infection and thus may serve to exacerbate each other,” Nigel Madden, MD, a resident physician in the ob.gyn. department at Columbia University, New York. , told this news organization after she presented the results.
Hypertensive disorders of pregnancy occur in 10%-15% of all pregnancies and are the leading cause of maternal and perinatal morbidity and mortality worldwide, Dr. Madden told attendees of the meeting. Although it’s not clear what causes hypertensive diseases in pregnancy generally, “it is possible that the acute inflammatory state of the COVID infection may incite or exacerbate hypertensive disease of pregnancy,” Dr. Madden said.
The researchers conducted a retrospective chart review of 1,715 patients who had a singleton pregnancy and who underwent routine nasal polymerase chain reaction testing at admission to one institution’s labor and delivery department between March and June 2020. The researchers excluded patients who had a history of chronic hypertension.
Overall, 10% of the patients tested positive for COVID-19 (n = 167), and 90% tested negative (n = 1,548). There were several differences at baseline between the groups. Those who tested positive tended to be younger, with an average age of 28, compared with an average age of 31 years for the group that tested negative. The group that tested negative also had a higher proportion of mothers aged 35 and older (P < .01). There were also significant differences in the racial makeup of the groups. Half of those in the COVID-positive group reported “other” for their race. The biggest baseline disparity between the groups was with regard to insurance type: 73% of those who tested positive for COVID-19 used Medicaid; only 36% of patients in the COVID-negative group used Medicaid. Those with private insurance were more likely to test negative (43%) than positive (25%) (P < .01).
The researchers defined gestational hypertension as having a systolic blood pressure greater than or equal to 140 mm Hg or a diastolic blood pressure greater than or equal to 90 mm Hg on two occasions at least 4 hours apart. A preeclampsia diagnosis required elevated blood pressure (using the same definition as for hypertension) as well as proteinuria, characterized by a protein/creatine ratio greater than or equal to 0.3 mg/dL or greater than or equal to 300 mg of protein on a 24-hour urine collection. Preeclampsia with severe features required prespecified laboratory abnormalities, pulmonary edema, or symptoms of headache, vision changes, chest pain, shortness of breath, or right upper quadrant pain.
More than twice as many patients with COVID had a hypertensive disorder of pregnancy (18%) as those who tested negative (8%). The patients who were COVID positive were significantly more likely than those who tested negative to have gestational hypertension and preeclampsia without severe features. Rates of preeclampsia with severe features were not significantly different between the groups.
The severity of hypertensive disease did not differ between the groups. Limitations of the study included its retrospective design, the small number of COVID-positive patients, and the fact that it was conducted at a single institution in New York. However, the study population was diverse, and it was conducted during the height of infections at the epicenter of the COVID-19 pandemic.
“This was a study of great clinical significance,” said Kim Boggess, MD, of the University of North Carolina at Chapel Hill, while moderating the session. “I would argue that you guys in New York are the best poised to answer some of the questions that need to be answered as it relates to the effect of coronavirus infection in pregnancy.”
Dr. Boggess asked whether the study examined associations related to the severity of COVID-19. Only 10 of the patients were symptomatic, Dr. Madden said, and only one of those patients developed preeclampsia with severe features.
Michelle Y. Owens, MD, professor and chief of maternal fetal medicine at the University of Mississippi Medical Center, Jackson, who also moderated the session, said in an interview that the findings call for physicians to remain vigilant about evaluating patients who test positive for COVID-19 for hypertensive disease and disorders.
“Additionally, these women should be educated about hypertensive disorders and the common symptoms to facilitate early diagnosis and treatment when indicated,” Dr. Owens said. “I believe this is of particular interest in those women who are not severely affected by COVID, as these changes may occur while they are undergoing quarantine or being monitored remotely. This amplifies the need for remote assessment or home monitoring of maternal blood pressures.”
Dr. Madden, Dr. Boggess, and Dr. Owens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Having COVID-19 during pregnancy is linked to a significantly increased risk for gestational hypertension and preeclampsia compared with not having COVID-19 while pregnant, according to findings from a retrospective study presented Jan. 28 at the Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“This was not entirely surprising given that inflammation has been implicated in the pathogenesis of both hypertensive disorders of pregnancy and COVID-19 infection and thus may serve to exacerbate each other,” Nigel Madden, MD, a resident physician in the ob.gyn. department at Columbia University, New York. , told this news organization after she presented the results.
Hypertensive disorders of pregnancy occur in 10%-15% of all pregnancies and are the leading cause of maternal and perinatal morbidity and mortality worldwide, Dr. Madden told attendees of the meeting. Although it’s not clear what causes hypertensive diseases in pregnancy generally, “it is possible that the acute inflammatory state of the COVID infection may incite or exacerbate hypertensive disease of pregnancy,” Dr. Madden said.
The researchers conducted a retrospective chart review of 1,715 patients who had a singleton pregnancy and who underwent routine nasal polymerase chain reaction testing at admission to one institution’s labor and delivery department between March and June 2020. The researchers excluded patients who had a history of chronic hypertension.
Overall, 10% of the patients tested positive for COVID-19 (n = 167), and 90% tested negative (n = 1,548). There were several differences at baseline between the groups. Those who tested positive tended to be younger, with an average age of 28, compared with an average age of 31 years for the group that tested negative. The group that tested negative also had a higher proportion of mothers aged 35 and older (P < .01). There were also significant differences in the racial makeup of the groups. Half of those in the COVID-positive group reported “other” for their race. The biggest baseline disparity between the groups was with regard to insurance type: 73% of those who tested positive for COVID-19 used Medicaid; only 36% of patients in the COVID-negative group used Medicaid. Those with private insurance were more likely to test negative (43%) than positive (25%) (P < .01).
The researchers defined gestational hypertension as having a systolic blood pressure greater than or equal to 140 mm Hg or a diastolic blood pressure greater than or equal to 90 mm Hg on two occasions at least 4 hours apart. A preeclampsia diagnosis required elevated blood pressure (using the same definition as for hypertension) as well as proteinuria, characterized by a protein/creatine ratio greater than or equal to 0.3 mg/dL or greater than or equal to 300 mg of protein on a 24-hour urine collection. Preeclampsia with severe features required prespecified laboratory abnormalities, pulmonary edema, or symptoms of headache, vision changes, chest pain, shortness of breath, or right upper quadrant pain.
More than twice as many patients with COVID had a hypertensive disorder of pregnancy (18%) as those who tested negative (8%). The patients who were COVID positive were significantly more likely than those who tested negative to have gestational hypertension and preeclampsia without severe features. Rates of preeclampsia with severe features were not significantly different between the groups.
The severity of hypertensive disease did not differ between the groups. Limitations of the study included its retrospective design, the small number of COVID-positive patients, and the fact that it was conducted at a single institution in New York. However, the study population was diverse, and it was conducted during the height of infections at the epicenter of the COVID-19 pandemic.
“This was a study of great clinical significance,” said Kim Boggess, MD, of the University of North Carolina at Chapel Hill, while moderating the session. “I would argue that you guys in New York are the best poised to answer some of the questions that need to be answered as it relates to the effect of coronavirus infection in pregnancy.”
Dr. Boggess asked whether the study examined associations related to the severity of COVID-19. Only 10 of the patients were symptomatic, Dr. Madden said, and only one of those patients developed preeclampsia with severe features.
Michelle Y. Owens, MD, professor and chief of maternal fetal medicine at the University of Mississippi Medical Center, Jackson, who also moderated the session, said in an interview that the findings call for physicians to remain vigilant about evaluating patients who test positive for COVID-19 for hypertensive disease and disorders.
“Additionally, these women should be educated about hypertensive disorders and the common symptoms to facilitate early diagnosis and treatment when indicated,” Dr. Owens said. “I believe this is of particular interest in those women who are not severely affected by COVID, as these changes may occur while they are undergoing quarantine or being monitored remotely. This amplifies the need for remote assessment or home monitoring of maternal blood pressures.”
Dr. Madden, Dr. Boggess, and Dr. Owens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Having COVID-19 during pregnancy is linked to a significantly increased risk for gestational hypertension and preeclampsia compared with not having COVID-19 while pregnant, according to findings from a retrospective study presented Jan. 28 at the Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“This was not entirely surprising given that inflammation has been implicated in the pathogenesis of both hypertensive disorders of pregnancy and COVID-19 infection and thus may serve to exacerbate each other,” Nigel Madden, MD, a resident physician in the ob.gyn. department at Columbia University, New York. , told this news organization after she presented the results.
Hypertensive disorders of pregnancy occur in 10%-15% of all pregnancies and are the leading cause of maternal and perinatal morbidity and mortality worldwide, Dr. Madden told attendees of the meeting. Although it’s not clear what causes hypertensive diseases in pregnancy generally, “it is possible that the acute inflammatory state of the COVID infection may incite or exacerbate hypertensive disease of pregnancy,” Dr. Madden said.
The researchers conducted a retrospective chart review of 1,715 patients who had a singleton pregnancy and who underwent routine nasal polymerase chain reaction testing at admission to one institution’s labor and delivery department between March and June 2020. The researchers excluded patients who had a history of chronic hypertension.
Overall, 10% of the patients tested positive for COVID-19 (n = 167), and 90% tested negative (n = 1,548). There were several differences at baseline between the groups. Those who tested positive tended to be younger, with an average age of 28, compared with an average age of 31 years for the group that tested negative. The group that tested negative also had a higher proportion of mothers aged 35 and older (P < .01). There were also significant differences in the racial makeup of the groups. Half of those in the COVID-positive group reported “other” for their race. The biggest baseline disparity between the groups was with regard to insurance type: 73% of those who tested positive for COVID-19 used Medicaid; only 36% of patients in the COVID-negative group used Medicaid. Those with private insurance were more likely to test negative (43%) than positive (25%) (P < .01).
The researchers defined gestational hypertension as having a systolic blood pressure greater than or equal to 140 mm Hg or a diastolic blood pressure greater than or equal to 90 mm Hg on two occasions at least 4 hours apart. A preeclampsia diagnosis required elevated blood pressure (using the same definition as for hypertension) as well as proteinuria, characterized by a protein/creatine ratio greater than or equal to 0.3 mg/dL or greater than or equal to 300 mg of protein on a 24-hour urine collection. Preeclampsia with severe features required prespecified laboratory abnormalities, pulmonary edema, or symptoms of headache, vision changes, chest pain, shortness of breath, or right upper quadrant pain.
More than twice as many patients with COVID had a hypertensive disorder of pregnancy (18%) as those who tested negative (8%). The patients who were COVID positive were significantly more likely than those who tested negative to have gestational hypertension and preeclampsia without severe features. Rates of preeclampsia with severe features were not significantly different between the groups.
The severity of hypertensive disease did not differ between the groups. Limitations of the study included its retrospective design, the small number of COVID-positive patients, and the fact that it was conducted at a single institution in New York. However, the study population was diverse, and it was conducted during the height of infections at the epicenter of the COVID-19 pandemic.
“This was a study of great clinical significance,” said Kim Boggess, MD, of the University of North Carolina at Chapel Hill, while moderating the session. “I would argue that you guys in New York are the best poised to answer some of the questions that need to be answered as it relates to the effect of coronavirus infection in pregnancy.”
Dr. Boggess asked whether the study examined associations related to the severity of COVID-19. Only 10 of the patients were symptomatic, Dr. Madden said, and only one of those patients developed preeclampsia with severe features.
Michelle Y. Owens, MD, professor and chief of maternal fetal medicine at the University of Mississippi Medical Center, Jackson, who also moderated the session, said in an interview that the findings call for physicians to remain vigilant about evaluating patients who test positive for COVID-19 for hypertensive disease and disorders.
“Additionally, these women should be educated about hypertensive disorders and the common symptoms to facilitate early diagnosis and treatment when indicated,” Dr. Owens said. “I believe this is of particular interest in those women who are not severely affected by COVID, as these changes may occur while they are undergoing quarantine or being monitored remotely. This amplifies the need for remote assessment or home monitoring of maternal blood pressures.”
Dr. Madden, Dr. Boggess, and Dr. Owens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Algorithm trims time to treatment of acute hypertension in pregnancy
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY