User login
Recurrent pregnancy loss and inherited thrombophilias: Does low molecular weight heparin improve the live birth rate?
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
Quenby S, Booth K, Hiller L, et al; ALIFE2 Block Writing Committee and ALIFE2 Investigators. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023;402:54-61. doi:10.1016/S0140-6736(23)00693-1.
EXPERT COMMENTARY
“Follow the evidence to where it leads, even if the conclusion is uncomfortable.”
—Steven James, author
Women with RPL have endured overzealous evaluations and management despite a lack of proven efficacy. From alloimmune testing that results in paternal leukocyte immunization1 and the long-entrusted metroplasty for a septate uterus recently put under fire2 to the “hammer and nail” approach of preimplantation genetic testing for embryo aneuploid screening,3 patients have been subjected to unsubstantiated treatments.
While the evaluation of RPL has evolved, guidelines from the American Society for Reproductive Medicine (ASRM), American College of Obstetricians and Gynecologists (ACOG), and Royal College of Obstetricians and Gynaecologists (RCOG) do not recommend testing for inherited thrombophilias outside of a history for venous thromboembolism.4-6 These 3 societies support treating acquired thrombophilias that represent the antiphospholipid antibody syndrome.
Citing insufficient evidence for reducing adverse pregnancy outcomes, ACOG recommends the use of prophylactic- or intermediate-dose LMWH or unfractionated heparin (UFH) for patients with “high-risk” thrombophilias only to prevent venous thromboembolism during pregnancy and continuing postpartum.4 (High-risk thrombophilias are defined as factor V Leiden homozygosity, prothrombin gene G20210A mutation homozygosity, heterozygosity for both factor V Leiden homozygosity and prothrombin gene G20210A mutation, or an antithrombin deficiency.4)
To determine the impact of LMWH treatment versus no treatment on live birth rate, Quenby and colleagues conducted a prospective randomized controlled trial of women with RPL and inherited thrombophilias (the ALIFE2 trial). This was a follow-up to their 2010 randomized controlled trial that demonstrated no effect of LMWH with low-dose aspirin versus low-dose aspirin alone compared with placebo in women with unexplained RPL.7
 PHOTO: BETAVERSO/SHUTTERSTOCK
PHOTO: BETAVERSO/SHUTTERSTOCK

Continue to: Details of the study...
Details of the study
The ALIFE2 study took place over 8 years and involved 5 countries, including the United States, with the 2 main centers in the Netherlands and the United Kingdom. Women eligible for the study were aged 18 to 42 years, had an inherited thrombophilia (confirmed by 2 tests), experienced recurrent miscarriages (2 or more consecutive miscarriages, nonconsecutive miscarriages, or intrauterine fetal deaths, irrespective of gestational age), and were less than 7 weeks’ estimated gestational age. Study patients were randomly allocated with a positive pregnancy test to either surveillance or LMWH treatment, which was continued throughout pregnancy.
The primary outcome was live birth rate, and secondary outcomes were a history of miscarriage, ectopic pregnancy, and obstetric complications. A total of 164 women were allocated to LMWH plus standard care, and 162 women to standard care alone. LMWH was shown to be safe without major/minor bleeding or maternal heparin-induced thrombocytopenia.
The statistical calculation was by “intention to treat,” which considers all enrolled participants, including those who dropped out of the study, as opposed to a “per protocol” analysis in which only patients who completed the study were analyzed.
Results. Primary outcome data were available for 320 participants. Of the 162 women in the LMWH-treated group, 116 (72%) had live birth rates, as did 112 (71%) of 158 in the standard care group. There was no significant difference between groups (OR, 1.04; 95% CI, 0.64–1.68).
Study strengths and limitations
The outcome of the ALIFE2 study is consistent with that of a Cochrane review that found insufficient evidence for improved live birth rate in patients with RPL and inherited thrombophilias treated with LMWH versus low-dose aspirin. Of their review of the studies at low risk of bias, only 1 was placebo controlled.8
This study by Quenby and colleagues was well designed and ensured a sufficient number of enrolled participants to comply with their power analysis. However, by beginning LMWH at 7 weeks’ gestation, patients may not have received a therapeutic benefit as opposed to initiation of treatment with a positive pregnancy test. The authors did not describe when testing for thrombophilias occurred or explain the protocol and reason for repeat testing.
Study limitations included a deviation from protocol in the standard care group, which was the initiation of LMWH after 7 weeks’ gestation. In the standard care group, 30 participants received LMWH, 18 of whom started heparin treatment before 12 weeks of gestation. The other 12 participants received LMWH after 12 weeks’ gestation, and 6 of those 12 started after 28 weeks’ gestation, since they were determined to need LMWH for thromboprophylaxis according to RCOG guidelines. While this had the potential to influence outcomes, only 18 of 162 (11%) patients were involved.
The authors did not define RPL based on a clinical versus a biochemical pregnancy loss as the latter is more common and is without agreed upon criteria for testing. Additionally, a lack of patient masking to medication could play an undetermined role in affecting the outcome. ●
This elegant, and vital, randomized controlled trial provides double take-home messages: There is no value in testing for inherited thrombophilias in RPL, as they occur in a similar prevalence in the general population, and there is no significant difference in live birth rate from LMWH treatment in women with RPL and inherited thrombophilias compared with surveillance. Consequently, the increased cost of medication and testing can be averted.
MARK P. TROLICE, MD, MBA
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
- Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2014; CD000112. doi:10.1002/14651858.CD000112
- Trolice MP. The septate uterus and metroplasty—another dogma under siege. Fertil Steril. 2021;116:693-694. doi:10.1016/j.fertnstert.2021.06.063
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. doi:10.1093 /humrep/deab194
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 197: inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34. doi:10.1097 /AOG.0000000000002703
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103-1111. doi:10.1016/j.fertnstert.2012.06.048
- Regan L, Rai R, Saravelos S, et al; Royal College of Obstetricians and Gynaecologists. Recurrent Miscarriage Green‐top Guideline No. 17. BJOG. June 19, 2023. doi:10.1111/1471 -0528.17515
- Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586-1596. doi:10.1056 /NEJMoa1000641
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;CD004734. doi:10.1002/14651858.CD004734 .pub4
U.S. maternal mortality crisis grows, yet deaths seem preventable
On June 2, 2019, 35-year-old Anne Hutchinson gave birth to her first child, Lillian. There were no problems with the pregnancy or the birth at Fairview Hospital, which is part of the Cleveland Clinic system.
But 2 days after the birth, she had shortness of breath and couldn’t lie down and breathe.
“My mom’s a nurse, and she was like, ‘You need to go to the hospital immediately,’” Ms. Hutchinson said. When she was admitted to the hospital, there were suddenly “10 doctors in the room.”
Ms. Hutchinson was diagnosed with peripartum cardiomyopathy, a weakness of the heart muscle. She had heart failure. The seriousness of heart failure is measured by the ejection fraction, or the percentage of blood the heart pumps out. Normal is 50%-70%. Ms. Hutchinson’s ejection fraction was 20%.
She was put on medication, left the hospital after 5 days, and her ejection fraction eventually rose to 35%. But she was still at risk for sudden cardiac death.
“The cardiologist said to me, ‘You probably can’t have any more children.’ My heart did not bounce back,” Ms. Hutchinson said.
By the end of 2019, her cardiologist determined that she needed an internal cardiac defibrillator, which monitors the heartbeat and delivers electric shocks to restore the heart’s normal rhythm when needed.
By 2020, when Ms. Hutchinson’s ejection fraction was near normal, she decided that she wanted another child.
“I had a daughter. She was beautiful and amazing. But I felt like I wanted to have a sibling for her,” she says. Yet when her cardiologist at Fairview Hospital heard the plan, she told her getting pregnant again “would be like Russian roulette.”
Ms. Hutchinson is one of a growing number of women whose medical condition puts them at high risk of death during and after giving birth. An estimated 30% of maternal deaths in the United States result from cardiovascular disease – a problem that has become more common with increases in diabetes and obesity.
And, in some women, hypertension can develop suddenly during pregnancy. This is called preeclampsia and is increasing in the United States, particularly in Black women. In rare cases, it can become the life-threatening condition eclampsia, with seizures and death.
Three-time Olympic medalist and world champion sprinter Tori Bowie was found dead in June of apparent complications of pregnancy. The medical examiner’s office in Orange County, Fla., said she was believed to have been in her 8th month of pregnancy and may have died of eclampsia.
Heart conditions in pregnant women are one of a long list of reasons why the United States has the highest maternal mortality rate of any developed country. But the risk is marked by significant racial differences, with death rates three times higher in Black women, compared with White women.
What troubles many experts is that it is estimated that 80% of these deaths are preventable.
“That is a ridiculous number,” said Melissa A. Simon, MD, MPH, director of the Center for Health Equity Transformation at Northwestern Medicine in Evanston, Ill.. “For a health care system in a country that is so high-resourced and high-income, for 8 out of 10 deaths for moms who are pregnant [to be preventable], that’s absolutely unacceptable.”
Pregnant women are not only at risk of death from cardiovascular complications, but other types of problems, including hemorrhage, thrombotic embolism, and infection.
But experts now are focusing attention on nonmedical reasons for maternal mortality, such as racial disparities and the fundamental issue of whether women are telling doctors about their symptoms but are not being heard.
The government has acknowledged the depth of this problem with the Centers for Disease Control and Prevention’s “Hear Her” campaign, which includes videos of women who describe how their health professionals did not take their concerns seriously.
In one such video, a woman named Sanari says 2 days after the birth of her second child, she started developing soreness.
“By day 3, it just didn’t feel right. I asked the nurses, explained my symptoms and that I was having crazy pains, and they assured me it was just gas,” she says on the video.
Sanari described how she started to have odorous discharge and ended up in an emergency room at a different hospital. Health care providers found a large abscess on her uterus.
“I’m glad I didn’t stop at no, and I’m glad someone finally heard me – someone finally listened to me,” she said.
“Hear Her” featured another woman named Lindsay, who had preeclampsia in her first pregnancy and began to get symptoms during her second pregnancy.
She describes how she voiced her concerns to her doctors, saying, “sometimes it would be, ‘Oh, you’re pregnant and your feet are supposed to swell. … It’s just fine.’ But I didn’t feel fine.”
The campaign aims to raise awareness of warning signs that require fast medical attention to prevent pregnancy-related deaths.
But Shanna Cox, associate director of the CDC’s Division of Reproductive Health, said the agency has collected many stories of women who died or nearly died because their concerns were not being addressed properly.
Ms. Cox says another part of the campaign “is really focused on health care providers and listening … to their patients, providing that respectful patient-centered care to be sure that all their concerns are addressed.”
And some experts believe the thinking has shifted even more dramatically.
“We’ve moved from beyond the days of blaming the individual, the birth person, or the woman, to say you haven’t done this, you haven’t come into health care, you are not taking care of yourself, you aren’t keeping your appointments,” says Laurie Zephyrin, MD, MPH, vice president of the Commonwealth Fund, a private foundation in New York dedicated to improving health care.
Dr. Zephyrin says the health care system falls short of providing equitable, quality care. “There’s data that shows Black people receive worse care than White people for about 40% of quality measures,” she said.
These disparities have led to the formation of organizations like National Birth Equity Collaborative, an advocacy group in New Orleans working to improve maternal care for Black patients.
Carmen Green, vice president of research and strategy, said institutional racism has been embedded into some health care providers.
“They have this hierarchy that teaches them they have to manage, they have to control, they have to direct the medical experience, and that is just not how birthing works,” she said.
She used the example of the birth experience as a car ride, where the mothers have been in the backseat with the doctor driving. “We want the birthing person in the driving seat and want [them] to be respected as a person who is deciding where that destination is going,” Ms. Green said.
She says health providers often “blame the mamas” based on assumptions, stereotypes, and biases against low-income people.
So how is American medicine responding to the medical and social causes of maternal mortality?
This news organization surveyed 10 medical centers ranked by U.S. News & World Report as the country’s top facilities for obstetrics. They were asked what programs they had and studies they had done to try to reduce maternal mortality, improve racial disparities, and target cardiovascular causes of maternal mortality.
One of the most extensive programs was founded at the Stanford School of Medicine in Stanford, Calif., in 2006. The California Maternal Quality Care Collaborative includes 200 hospitals in the state committed to ending preventable maternal mortality and racial disparities.
Nine hospitals in the collaborative have started programs to reduce hemorrhages, manage high blood pressure disorders, and reduce the rate of cesarean deliveries. All are important reasons for maternal mortality.
These programs helped bring about a 62% reduction in California’s maternal mortality rate from 2006 to 2016. And 2023 figures show that California has the lowest maternal mortality rate of any state.
Alabama has the sixth highest rate of maternal mortality in the nation. The University of Alabama at Birmingham wants to address the racial disparities in maternal mortality with a cooperative called the P3 EQUATE Network.
The network is part of a $20 million program by the American Heart Association to gain greater understanding of the disproportionate effect of maternal mortality on Black and Native American people.
The program works with pregnant and postpartum women “to discover ways to reduce racism and social problems that contribute to poor health outcomes.”
In addition to collaborative efforts, the survey found maternal mortality programs at all the top medical centers.
NewYork-Presbyterian Hospital has a Mothers Center that provides specialized care to pregnant women with complications.
The University of Chicago Medical Center established a program called “Systematic Treatment and Management of Postpartum Hypertension” that includes patient and staff education, standardized hospital discharge instructions, and a follow-up in a postpartum hypertension clinic.
A 2021 study found that the program had helped increase the number of postpartum women who correctly follow blood pressure control guidance.
A program called MOMS Navigation at Northwell Health in Long Island, N.Y., provides support to high-risk mothers. The program decreased 30-day readmission rates for all patients by 50% and for Black birthing patients by 60%. Reducing readmission is an important measure for reducing complications.
Vanderbilt University Medical Center in Nashville has what it calls the first-of-its-kind educational podcasts Healthy Mom Healthy Baby, where 30% of the content is devoted to health disparities.
And several centers, including Brigham and Women’s Hospital in Boston and NewYork-Presbyterian, make sure mothers have access to doulas – professional support people trained in the needs of the family during pregnancy and childbirth.
The survey found that 9 of the 10 centers have obstetric programs devoted to cardiac care, including the University of Chicago, Stanford Medicine, UCLA, and the Cleveland Clinic.
But the survey results raise the question: How can we have these programs and research at our best obstetrics centers devoted to reducing maternal mortality and have the highest rate of all developed countries?
“Maternal mortality largely falls on pregnant and birthing persons who do not intersect with, nor are touched, by the best obstetrical care centers in the country,” Dr. Simon said.
Unfortunately, she said, the pregnant people who face “high maternal mortality rates … face all the access-to-care barriers and do not have the privilege of birthing or accessing care at top centers.”
Anne Hutchinson believed going to a top center – the Cleveland Clinic – would give her a good chance of safely delivering a second child.
Karlee Hoffman, DO, a cardiologist in the hospital’s cardio-obstetric high-risk clinic, said Ms. Hutchinson “came to me, she was determined to have another child, and she said, ‘Please help me do this. I’m doing it regardless. So, I would really like your support in moving forward,’” Dr. Hoffman recalls.
Ms. Hutchinson said Cleveland Clinic doctors told her she had a 20%-30% chance of peripartum cardiomyopathy again if she had a second child. If that happened, the risks “ranged from mild decompensation of my heart function to death,“ she said.
Ms. Hutchinson and her husband decided to go ahead with the pregnancy. Her parents cried when they found out. But Ms. Hutchinson says she was confident in the cardio obstetric team at Cleveland Clinic.
Her fertility medicine raised the possibility of multiple births, which would be a definite threat to her life. Her heart failure medicine, Entresto, could not be used during pregnancy, so her doctors put her on older medicines.
She got pregnant in June 2022 and developed gestational diabetes, which can affect pregnancy because of raised blood sugar. Another potential risk. She was carefully monitored by the specialists and hospitalized once.
At 37 weeks, she was induced and had a forceps delivery. On Feb. 15, 2023, her second daughter, Charlotte, was born.
Ms. Hutchinson was asked to write about how she felt when she delivered Charlotte:
“I am not sure how to put into words the love, joy, and elation that I felt holding Charlotte for the first time. As I write this, I have tears of joy in my eyes thinking of that moment. I had prayed for her for so long and after being told I couldn’t or shouldn’t have any more children.”
“I felt that Charlotte and I were forever bonded in triumph from that moment on. We did it and made it out alive! And our family was now complete. I have so much joy watching the love that is growing between Charlotte and Lillian. Life is truly amazing, and I am forever grateful to have them.”
A version of this article first appeared on WebMD.com.
On June 2, 2019, 35-year-old Anne Hutchinson gave birth to her first child, Lillian. There were no problems with the pregnancy or the birth at Fairview Hospital, which is part of the Cleveland Clinic system.
But 2 days after the birth, she had shortness of breath and couldn’t lie down and breathe.
“My mom’s a nurse, and she was like, ‘You need to go to the hospital immediately,’” Ms. Hutchinson said. When she was admitted to the hospital, there were suddenly “10 doctors in the room.”
Ms. Hutchinson was diagnosed with peripartum cardiomyopathy, a weakness of the heart muscle. She had heart failure. The seriousness of heart failure is measured by the ejection fraction, or the percentage of blood the heart pumps out. Normal is 50%-70%. Ms. Hutchinson’s ejection fraction was 20%.
She was put on medication, left the hospital after 5 days, and her ejection fraction eventually rose to 35%. But she was still at risk for sudden cardiac death.
“The cardiologist said to me, ‘You probably can’t have any more children.’ My heart did not bounce back,” Ms. Hutchinson said.
By the end of 2019, her cardiologist determined that she needed an internal cardiac defibrillator, which monitors the heartbeat and delivers electric shocks to restore the heart’s normal rhythm when needed.
By 2020, when Ms. Hutchinson’s ejection fraction was near normal, she decided that she wanted another child.
“I had a daughter. She was beautiful and amazing. But I felt like I wanted to have a sibling for her,” she says. Yet when her cardiologist at Fairview Hospital heard the plan, she told her getting pregnant again “would be like Russian roulette.”
Ms. Hutchinson is one of a growing number of women whose medical condition puts them at high risk of death during and after giving birth. An estimated 30% of maternal deaths in the United States result from cardiovascular disease – a problem that has become more common with increases in diabetes and obesity.
And, in some women, hypertension can develop suddenly during pregnancy. This is called preeclampsia and is increasing in the United States, particularly in Black women. In rare cases, it can become the life-threatening condition eclampsia, with seizures and death.
Three-time Olympic medalist and world champion sprinter Tori Bowie was found dead in June of apparent complications of pregnancy. The medical examiner’s office in Orange County, Fla., said she was believed to have been in her 8th month of pregnancy and may have died of eclampsia.
Heart conditions in pregnant women are one of a long list of reasons why the United States has the highest maternal mortality rate of any developed country. But the risk is marked by significant racial differences, with death rates three times higher in Black women, compared with White women.
What troubles many experts is that it is estimated that 80% of these deaths are preventable.
“That is a ridiculous number,” said Melissa A. Simon, MD, MPH, director of the Center for Health Equity Transformation at Northwestern Medicine in Evanston, Ill.. “For a health care system in a country that is so high-resourced and high-income, for 8 out of 10 deaths for moms who are pregnant [to be preventable], that’s absolutely unacceptable.”
Pregnant women are not only at risk of death from cardiovascular complications, but other types of problems, including hemorrhage, thrombotic embolism, and infection.
But experts now are focusing attention on nonmedical reasons for maternal mortality, such as racial disparities and the fundamental issue of whether women are telling doctors about their symptoms but are not being heard.
The government has acknowledged the depth of this problem with the Centers for Disease Control and Prevention’s “Hear Her” campaign, which includes videos of women who describe how their health professionals did not take their concerns seriously.
In one such video, a woman named Sanari says 2 days after the birth of her second child, she started developing soreness.
“By day 3, it just didn’t feel right. I asked the nurses, explained my symptoms and that I was having crazy pains, and they assured me it was just gas,” she says on the video.
Sanari described how she started to have odorous discharge and ended up in an emergency room at a different hospital. Health care providers found a large abscess on her uterus.
“I’m glad I didn’t stop at no, and I’m glad someone finally heard me – someone finally listened to me,” she said.
“Hear Her” featured another woman named Lindsay, who had preeclampsia in her first pregnancy and began to get symptoms during her second pregnancy.
She describes how she voiced her concerns to her doctors, saying, “sometimes it would be, ‘Oh, you’re pregnant and your feet are supposed to swell. … It’s just fine.’ But I didn’t feel fine.”
The campaign aims to raise awareness of warning signs that require fast medical attention to prevent pregnancy-related deaths.
But Shanna Cox, associate director of the CDC’s Division of Reproductive Health, said the agency has collected many stories of women who died or nearly died because their concerns were not being addressed properly.
Ms. Cox says another part of the campaign “is really focused on health care providers and listening … to their patients, providing that respectful patient-centered care to be sure that all their concerns are addressed.”
And some experts believe the thinking has shifted even more dramatically.
“We’ve moved from beyond the days of blaming the individual, the birth person, or the woman, to say you haven’t done this, you haven’t come into health care, you are not taking care of yourself, you aren’t keeping your appointments,” says Laurie Zephyrin, MD, MPH, vice president of the Commonwealth Fund, a private foundation in New York dedicated to improving health care.
Dr. Zephyrin says the health care system falls short of providing equitable, quality care. “There’s data that shows Black people receive worse care than White people for about 40% of quality measures,” she said.
These disparities have led to the formation of organizations like National Birth Equity Collaborative, an advocacy group in New Orleans working to improve maternal care for Black patients.
Carmen Green, vice president of research and strategy, said institutional racism has been embedded into some health care providers.
“They have this hierarchy that teaches them they have to manage, they have to control, they have to direct the medical experience, and that is just not how birthing works,” she said.
She used the example of the birth experience as a car ride, where the mothers have been in the backseat with the doctor driving. “We want the birthing person in the driving seat and want [them] to be respected as a person who is deciding where that destination is going,” Ms. Green said.
She says health providers often “blame the mamas” based on assumptions, stereotypes, and biases against low-income people.
So how is American medicine responding to the medical and social causes of maternal mortality?
This news organization surveyed 10 medical centers ranked by U.S. News & World Report as the country’s top facilities for obstetrics. They were asked what programs they had and studies they had done to try to reduce maternal mortality, improve racial disparities, and target cardiovascular causes of maternal mortality.
One of the most extensive programs was founded at the Stanford School of Medicine in Stanford, Calif., in 2006. The California Maternal Quality Care Collaborative includes 200 hospitals in the state committed to ending preventable maternal mortality and racial disparities.
Nine hospitals in the collaborative have started programs to reduce hemorrhages, manage high blood pressure disorders, and reduce the rate of cesarean deliveries. All are important reasons for maternal mortality.
These programs helped bring about a 62% reduction in California’s maternal mortality rate from 2006 to 2016. And 2023 figures show that California has the lowest maternal mortality rate of any state.
Alabama has the sixth highest rate of maternal mortality in the nation. The University of Alabama at Birmingham wants to address the racial disparities in maternal mortality with a cooperative called the P3 EQUATE Network.
The network is part of a $20 million program by the American Heart Association to gain greater understanding of the disproportionate effect of maternal mortality on Black and Native American people.
The program works with pregnant and postpartum women “to discover ways to reduce racism and social problems that contribute to poor health outcomes.”
In addition to collaborative efforts, the survey found maternal mortality programs at all the top medical centers.
NewYork-Presbyterian Hospital has a Mothers Center that provides specialized care to pregnant women with complications.
The University of Chicago Medical Center established a program called “Systematic Treatment and Management of Postpartum Hypertension” that includes patient and staff education, standardized hospital discharge instructions, and a follow-up in a postpartum hypertension clinic.
A 2021 study found that the program had helped increase the number of postpartum women who correctly follow blood pressure control guidance.
A program called MOMS Navigation at Northwell Health in Long Island, N.Y., provides support to high-risk mothers. The program decreased 30-day readmission rates for all patients by 50% and for Black birthing patients by 60%. Reducing readmission is an important measure for reducing complications.
Vanderbilt University Medical Center in Nashville has what it calls the first-of-its-kind educational podcasts Healthy Mom Healthy Baby, where 30% of the content is devoted to health disparities.
And several centers, including Brigham and Women’s Hospital in Boston and NewYork-Presbyterian, make sure mothers have access to doulas – professional support people trained in the needs of the family during pregnancy and childbirth.
The survey found that 9 of the 10 centers have obstetric programs devoted to cardiac care, including the University of Chicago, Stanford Medicine, UCLA, and the Cleveland Clinic.
But the survey results raise the question: How can we have these programs and research at our best obstetrics centers devoted to reducing maternal mortality and have the highest rate of all developed countries?
“Maternal mortality largely falls on pregnant and birthing persons who do not intersect with, nor are touched, by the best obstetrical care centers in the country,” Dr. Simon said.
Unfortunately, she said, the pregnant people who face “high maternal mortality rates … face all the access-to-care barriers and do not have the privilege of birthing or accessing care at top centers.”
Anne Hutchinson believed going to a top center – the Cleveland Clinic – would give her a good chance of safely delivering a second child.
Karlee Hoffman, DO, a cardiologist in the hospital’s cardio-obstetric high-risk clinic, said Ms. Hutchinson “came to me, she was determined to have another child, and she said, ‘Please help me do this. I’m doing it regardless. So, I would really like your support in moving forward,’” Dr. Hoffman recalls.
Ms. Hutchinson said Cleveland Clinic doctors told her she had a 20%-30% chance of peripartum cardiomyopathy again if she had a second child. If that happened, the risks “ranged from mild decompensation of my heart function to death,“ she said.
Ms. Hutchinson and her husband decided to go ahead with the pregnancy. Her parents cried when they found out. But Ms. Hutchinson says she was confident in the cardio obstetric team at Cleveland Clinic.
Her fertility medicine raised the possibility of multiple births, which would be a definite threat to her life. Her heart failure medicine, Entresto, could not be used during pregnancy, so her doctors put her on older medicines.
She got pregnant in June 2022 and developed gestational diabetes, which can affect pregnancy because of raised blood sugar. Another potential risk. She was carefully monitored by the specialists and hospitalized once.
At 37 weeks, she was induced and had a forceps delivery. On Feb. 15, 2023, her second daughter, Charlotte, was born.
Ms. Hutchinson was asked to write about how she felt when she delivered Charlotte:
“I am not sure how to put into words the love, joy, and elation that I felt holding Charlotte for the first time. As I write this, I have tears of joy in my eyes thinking of that moment. I had prayed for her for so long and after being told I couldn’t or shouldn’t have any more children.”
“I felt that Charlotte and I were forever bonded in triumph from that moment on. We did it and made it out alive! And our family was now complete. I have so much joy watching the love that is growing between Charlotte and Lillian. Life is truly amazing, and I am forever grateful to have them.”
A version of this article first appeared on WebMD.com.
On June 2, 2019, 35-year-old Anne Hutchinson gave birth to her first child, Lillian. There were no problems with the pregnancy or the birth at Fairview Hospital, which is part of the Cleveland Clinic system.
But 2 days after the birth, she had shortness of breath and couldn’t lie down and breathe.
“My mom’s a nurse, and she was like, ‘You need to go to the hospital immediately,’” Ms. Hutchinson said. When she was admitted to the hospital, there were suddenly “10 doctors in the room.”
Ms. Hutchinson was diagnosed with peripartum cardiomyopathy, a weakness of the heart muscle. She had heart failure. The seriousness of heart failure is measured by the ejection fraction, or the percentage of blood the heart pumps out. Normal is 50%-70%. Ms. Hutchinson’s ejection fraction was 20%.
She was put on medication, left the hospital after 5 days, and her ejection fraction eventually rose to 35%. But she was still at risk for sudden cardiac death.
“The cardiologist said to me, ‘You probably can’t have any more children.’ My heart did not bounce back,” Ms. Hutchinson said.
By the end of 2019, her cardiologist determined that she needed an internal cardiac defibrillator, which monitors the heartbeat and delivers electric shocks to restore the heart’s normal rhythm when needed.
By 2020, when Ms. Hutchinson’s ejection fraction was near normal, she decided that she wanted another child.
“I had a daughter. She was beautiful and amazing. But I felt like I wanted to have a sibling for her,” she says. Yet when her cardiologist at Fairview Hospital heard the plan, she told her getting pregnant again “would be like Russian roulette.”
Ms. Hutchinson is one of a growing number of women whose medical condition puts them at high risk of death during and after giving birth. An estimated 30% of maternal deaths in the United States result from cardiovascular disease – a problem that has become more common with increases in diabetes and obesity.
And, in some women, hypertension can develop suddenly during pregnancy. This is called preeclampsia and is increasing in the United States, particularly in Black women. In rare cases, it can become the life-threatening condition eclampsia, with seizures and death.
Three-time Olympic medalist and world champion sprinter Tori Bowie was found dead in June of apparent complications of pregnancy. The medical examiner’s office in Orange County, Fla., said she was believed to have been in her 8th month of pregnancy and may have died of eclampsia.
Heart conditions in pregnant women are one of a long list of reasons why the United States has the highest maternal mortality rate of any developed country. But the risk is marked by significant racial differences, with death rates three times higher in Black women, compared with White women.
What troubles many experts is that it is estimated that 80% of these deaths are preventable.
“That is a ridiculous number,” said Melissa A. Simon, MD, MPH, director of the Center for Health Equity Transformation at Northwestern Medicine in Evanston, Ill.. “For a health care system in a country that is so high-resourced and high-income, for 8 out of 10 deaths for moms who are pregnant [to be preventable], that’s absolutely unacceptable.”
Pregnant women are not only at risk of death from cardiovascular complications, but other types of problems, including hemorrhage, thrombotic embolism, and infection.
But experts now are focusing attention on nonmedical reasons for maternal mortality, such as racial disparities and the fundamental issue of whether women are telling doctors about their symptoms but are not being heard.
The government has acknowledged the depth of this problem with the Centers for Disease Control and Prevention’s “Hear Her” campaign, which includes videos of women who describe how their health professionals did not take their concerns seriously.
In one such video, a woman named Sanari says 2 days after the birth of her second child, she started developing soreness.
“By day 3, it just didn’t feel right. I asked the nurses, explained my symptoms and that I was having crazy pains, and they assured me it was just gas,” she says on the video.
Sanari described how she started to have odorous discharge and ended up in an emergency room at a different hospital. Health care providers found a large abscess on her uterus.
“I’m glad I didn’t stop at no, and I’m glad someone finally heard me – someone finally listened to me,” she said.
“Hear Her” featured another woman named Lindsay, who had preeclampsia in her first pregnancy and began to get symptoms during her second pregnancy.
She describes how she voiced her concerns to her doctors, saying, “sometimes it would be, ‘Oh, you’re pregnant and your feet are supposed to swell. … It’s just fine.’ But I didn’t feel fine.”
The campaign aims to raise awareness of warning signs that require fast medical attention to prevent pregnancy-related deaths.
But Shanna Cox, associate director of the CDC’s Division of Reproductive Health, said the agency has collected many stories of women who died or nearly died because their concerns were not being addressed properly.
Ms. Cox says another part of the campaign “is really focused on health care providers and listening … to their patients, providing that respectful patient-centered care to be sure that all their concerns are addressed.”
And some experts believe the thinking has shifted even more dramatically.
“We’ve moved from beyond the days of blaming the individual, the birth person, or the woman, to say you haven’t done this, you haven’t come into health care, you are not taking care of yourself, you aren’t keeping your appointments,” says Laurie Zephyrin, MD, MPH, vice president of the Commonwealth Fund, a private foundation in New York dedicated to improving health care.
Dr. Zephyrin says the health care system falls short of providing equitable, quality care. “There’s data that shows Black people receive worse care than White people for about 40% of quality measures,” she said.
These disparities have led to the formation of organizations like National Birth Equity Collaborative, an advocacy group in New Orleans working to improve maternal care for Black patients.
Carmen Green, vice president of research and strategy, said institutional racism has been embedded into some health care providers.
“They have this hierarchy that teaches them they have to manage, they have to control, they have to direct the medical experience, and that is just not how birthing works,” she said.
She used the example of the birth experience as a car ride, where the mothers have been in the backseat with the doctor driving. “We want the birthing person in the driving seat and want [them] to be respected as a person who is deciding where that destination is going,” Ms. Green said.
She says health providers often “blame the mamas” based on assumptions, stereotypes, and biases against low-income people.
So how is American medicine responding to the medical and social causes of maternal mortality?
This news organization surveyed 10 medical centers ranked by U.S. News & World Report as the country’s top facilities for obstetrics. They were asked what programs they had and studies they had done to try to reduce maternal mortality, improve racial disparities, and target cardiovascular causes of maternal mortality.
One of the most extensive programs was founded at the Stanford School of Medicine in Stanford, Calif., in 2006. The California Maternal Quality Care Collaborative includes 200 hospitals in the state committed to ending preventable maternal mortality and racial disparities.
Nine hospitals in the collaborative have started programs to reduce hemorrhages, manage high blood pressure disorders, and reduce the rate of cesarean deliveries. All are important reasons for maternal mortality.
These programs helped bring about a 62% reduction in California’s maternal mortality rate from 2006 to 2016. And 2023 figures show that California has the lowest maternal mortality rate of any state.
Alabama has the sixth highest rate of maternal mortality in the nation. The University of Alabama at Birmingham wants to address the racial disparities in maternal mortality with a cooperative called the P3 EQUATE Network.
The network is part of a $20 million program by the American Heart Association to gain greater understanding of the disproportionate effect of maternal mortality on Black and Native American people.
The program works with pregnant and postpartum women “to discover ways to reduce racism and social problems that contribute to poor health outcomes.”
In addition to collaborative efforts, the survey found maternal mortality programs at all the top medical centers.
NewYork-Presbyterian Hospital has a Mothers Center that provides specialized care to pregnant women with complications.
The University of Chicago Medical Center established a program called “Systematic Treatment and Management of Postpartum Hypertension” that includes patient and staff education, standardized hospital discharge instructions, and a follow-up in a postpartum hypertension clinic.
A 2021 study found that the program had helped increase the number of postpartum women who correctly follow blood pressure control guidance.
A program called MOMS Navigation at Northwell Health in Long Island, N.Y., provides support to high-risk mothers. The program decreased 30-day readmission rates for all patients by 50% and for Black birthing patients by 60%. Reducing readmission is an important measure for reducing complications.
Vanderbilt University Medical Center in Nashville has what it calls the first-of-its-kind educational podcasts Healthy Mom Healthy Baby, where 30% of the content is devoted to health disparities.
And several centers, including Brigham and Women’s Hospital in Boston and NewYork-Presbyterian, make sure mothers have access to doulas – professional support people trained in the needs of the family during pregnancy and childbirth.
The survey found that 9 of the 10 centers have obstetric programs devoted to cardiac care, including the University of Chicago, Stanford Medicine, UCLA, and the Cleveland Clinic.
But the survey results raise the question: How can we have these programs and research at our best obstetrics centers devoted to reducing maternal mortality and have the highest rate of all developed countries?
“Maternal mortality largely falls on pregnant and birthing persons who do not intersect with, nor are touched, by the best obstetrical care centers in the country,” Dr. Simon said.
Unfortunately, she said, the pregnant people who face “high maternal mortality rates … face all the access-to-care barriers and do not have the privilege of birthing or accessing care at top centers.”
Anne Hutchinson believed going to a top center – the Cleveland Clinic – would give her a good chance of safely delivering a second child.
Karlee Hoffman, DO, a cardiologist in the hospital’s cardio-obstetric high-risk clinic, said Ms. Hutchinson “came to me, she was determined to have another child, and she said, ‘Please help me do this. I’m doing it regardless. So, I would really like your support in moving forward,’” Dr. Hoffman recalls.
Ms. Hutchinson said Cleveland Clinic doctors told her she had a 20%-30% chance of peripartum cardiomyopathy again if she had a second child. If that happened, the risks “ranged from mild decompensation of my heart function to death,“ she said.
Ms. Hutchinson and her husband decided to go ahead with the pregnancy. Her parents cried when they found out. But Ms. Hutchinson says she was confident in the cardio obstetric team at Cleveland Clinic.
Her fertility medicine raised the possibility of multiple births, which would be a definite threat to her life. Her heart failure medicine, Entresto, could not be used during pregnancy, so her doctors put her on older medicines.
She got pregnant in June 2022 and developed gestational diabetes, which can affect pregnancy because of raised blood sugar. Another potential risk. She was carefully monitored by the specialists and hospitalized once.
At 37 weeks, she was induced and had a forceps delivery. On Feb. 15, 2023, her second daughter, Charlotte, was born.
Ms. Hutchinson was asked to write about how she felt when she delivered Charlotte:
“I am not sure how to put into words the love, joy, and elation that I felt holding Charlotte for the first time. As I write this, I have tears of joy in my eyes thinking of that moment. I had prayed for her for so long and after being told I couldn’t or shouldn’t have any more children.”
“I felt that Charlotte and I were forever bonded in triumph from that moment on. We did it and made it out alive! And our family was now complete. I have so much joy watching the love that is growing between Charlotte and Lillian. Life is truly amazing, and I am forever grateful to have them.”
A version of this article first appeared on WebMD.com.
Study evaluating in utero treatment for hypohidrotic ectodermal dysplasia seeks enrollees
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
Oxycodone tied to persistent use only after vaginal delivery
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Fast-acting postpartum depression drug is effective
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Woman with transplanted uterus gives birth to boy
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
Pregnancy risks elevated in women with chronic pancreatitis
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Number of cervical cancer screenings linked to higher preterm birth risk
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
FROM JAMA HEALTH FORUM
Total cesarean delivery rates in the US, 2022
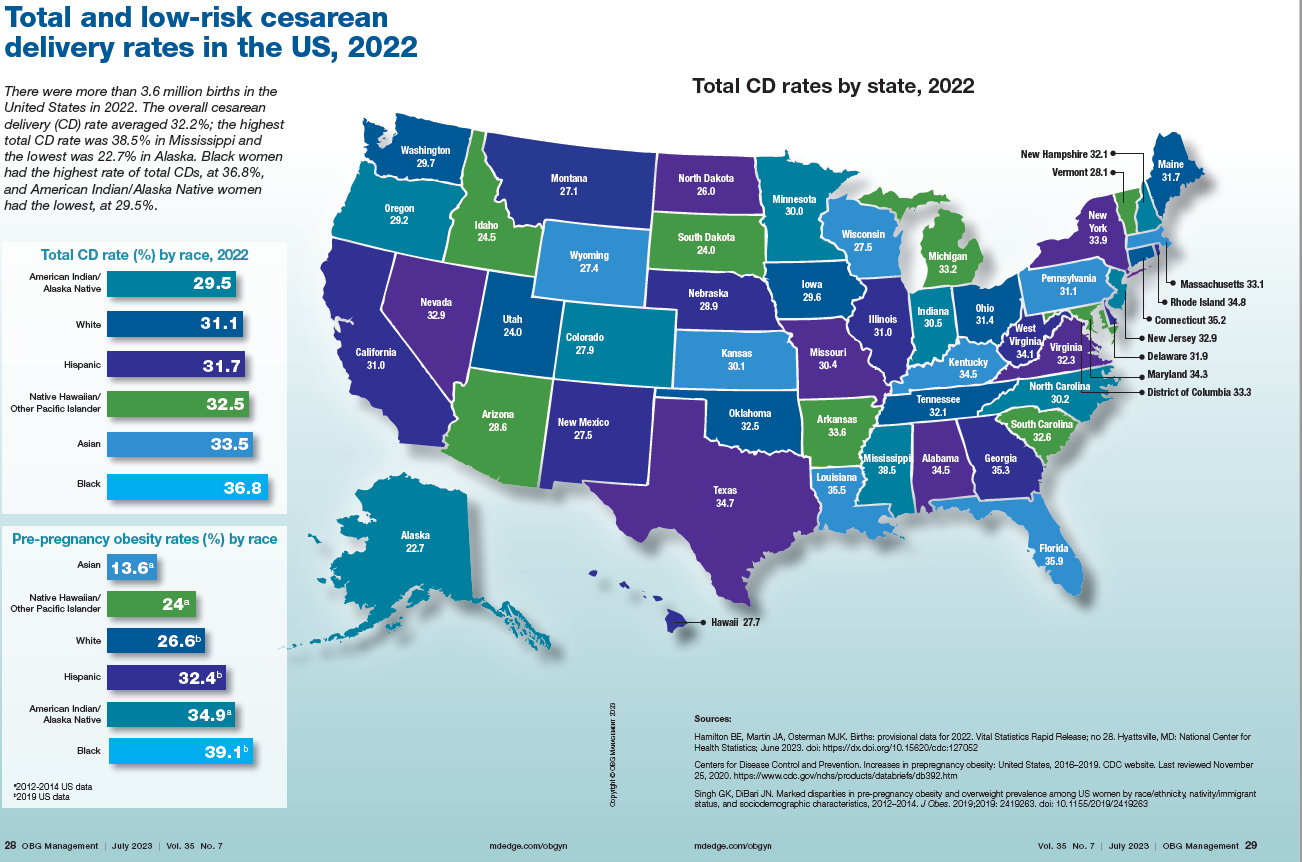


News & Perspectives from Ob.Gyn. News
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.
REPRODUCTIVE ROUNDS
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
Performed properly, office hysteroscopy can transform your practice by accurately, gently, and safely assessing the uterine cavity as well as assessing tubal patency.
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively. Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope. Further, infection rates and vasovagal events were far lower with hysteroscopy.
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity. Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
https://www.mdedge.com/obgyn/reproductive-rounds
FEATURE
Is ChatGPT a friend or foe of medical publishing?
Researchers may use artificial intelligence (AI) language models such as ChatGPT to write and revise scientific manuscripts, according to a new announcement from the International Committee of Medical Journal Editors. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said.
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
Continue to: FROM THE JOURNALS...
FROM THE JOURNALS
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
“Masking in interactions between patients and health care personnel should continue to receive serious consideration as a patient safety measure,” Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
https://www.mdedge.com/obgyn/covid-19-updates
CONFERENCE COVERAGE
A ‘one-stop shop’: New guidance on hormones and aging
A new statement from the Endocrine Society on hormones and aging highlights the differences between normal aging and disease, and when treatment is and isn’t appropriate.
The idea of the statement “is to be complete, but also to clarify some misunderstandings. ...We tried to be very clear in the language about what we know, where we can go, where we shouldn’t go, and what we still need to learn,” statement coauthor Cynthia A. Stuenkel, MD, of the University of California, San Diego, said in an interview.
The document is divided into nine parts or axes: growth hormone, adrenal, ovarian, testicular, thyroid, osteoporosis, vitamin D deficiency, type 2 diabetes, and water metabolism. Each section covers natural history and observational data in older individuals, available therapies, clinical trial data on efficacy and safety in older individuals, bulleted “key points,” and research gaps.
“Hormones and Aging: An Endocrine Society Scientific Statement” was presented at the annual meeting of the Endocrine Society and published online in the Journal of Clinical Endocrinology & Metabolism.
During a press briefing, writing group chair Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia, said the goal is to “provide a really concise summary across each of these areas. ... There are multiple hormonal changes that occur with age, so we really couldn’t limit ourselves to just one gland or the few that we commonly think about. We wanted to cover all the axes.”
The statement tackles several controversial areas, including hormone therapy for menopausal symptoms in women and hypogonadal symptoms in men and diabetes treatment goals in older adults.
“Hormones have these almost mythical qualities to some people. ... ‘If I just had my hormones back the way they were, it would all work out.’ What we want to do is make sure that patients are being treated appropriately and that their symptoms are being heard and managed and ascribed to the appropriate problems and not necessarily to hormonal problems when they are not. ... Part of what we need to do is [provide] the evidence that we have, which includes evidence of when not to prescribe as well as [when] to prescribe,” Dr. Cappola said.