User login
AGA publishes care pathway for IBD in pregnancy
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
FROM THE AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Sperm quality linked to some recurrent pregnancy loss
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
REPORTING FROM ENDO 2019
2018 FDA-approved new drugs
In 2018, the Food and Drug Administration approved a record 58 new drugs for humans. One of these agents, Annovera (segesterone acetate and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol-mknl), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. The agents with molecular weights (MW) less than 1,000 probably cross the placenta, but nearly all, regardless of MW, will cross in the second half of pregnancy.
There is no human pregnancy data for these agents, but there are five drugs included in pregnancy registries. However, it will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in a reference that I coauthored (“Should pregnant women be included in phase 4 clinical drug trials?” Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). The article makes a strong argument for including some drugs in these trials.
Amyloidosis
Onpattro (patisiran) is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The drug caused embryo-fetal death and reduced fetal body weight in rabbits at doses also associated with maternal toxicity. No developmental toxicity was observed in rats.
Anti-infectives
Aemcolo (rifamycin), which has a MW of 720, is indicated for treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli. No adverse fetal effects were observed in rats and rabbits that received close to human doses.
Krintafel (tafenoquine) is an antimalarial agent that is used to prevent relapse in patients who are receiving appropriate antimalarial therapy for Plasmodium vivax infection. The drug may cause hemolytic anemia in a fetus deficient in glucose-6-phosphate dehydrogenase. In rabbits, the drug caused dose-related abortions and maternal toxicity was observed in rabbits and rats. Treatment with this drug in pregnancy is not recommended, according to the manufacturer.
Tpoxx (tecovirimat monohydrate), which has a MW of about 394, is indicated for the treatment of smallpox disease. The drug did not cause embryo-fetal toxicity in pregnant mice and rabbits, but the maximum exposure in rabbits was only 0.4 times the human exposure.
Xofluza (baloxavir marboxil), which has a MW of about 572, is a prodrug that is converted by hydrolysis to baloxavir. It is indicated for the treatment of acute uncomplicated influenza. No adverse developmental effects were observed in rats and rabbits.
Zemdri (plazomicin), which has a MW of about 593, is an aminoglycoside indicated for the treatment of complicated urinary tract infections including pyelonephritis. The drug did not cause fetal harm in rats and rabbits at doses that did not cause maternal toxicity; however, prolonged use of an aminoglycoside (such as streptomycin) has caused irreversible, bilateral congenital deafness in children exposed in utero to prolonged use and is a potential complication.
Three new drugs in 2018 are indicated for treating HIV-1:
Biktarvy is a three-drug combination that includes bictegravir, emtricitabine, and tenofovir. The latter two drugs are included in the 11th edition of my book (“Drugs in Pregnancy and Lactation,” 11th ed. [Riverwoods, Ill.: Wolters Kluwer, 2017) and are not included here. Both are classified as compatible in pregnancy. Bictegravir has a MW of about 471. No adverse embryo-fetal effects in rats and rabbits were observed with this agent.
Trogarzo (ibalizumab-uiyk), which has a MW of about 150,000, is a monoclonal antibody antiretroviral agent used in combination with other antiretrovirals. There are no animal data. Although the MW is very high, monoclonal antibodies are transported across the placenta as pregnancy progresses.
Pifeltro (doravirine), which has a MW of about 426, is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents for the treatment of HIV-1. The drug caused no significant toxicologic effects on embryo-fetal rats and rabbits.
If Biktarvy, Pifeltro, or Trogarzo are used in pregnancy, health care providers are encouraged to register the patient in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
There are three new agents in the tetracycline class.
Nuzyra (omadacycline), which has a MW of about 729, is for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.
Seysara (sarecycline), which has a MW of about 524, is for inflammatory lesions of nonnodular, moderate to severe acne vulgaris.
Xerava (eravacycline), which has a MW of about 632, is for complicated intra-abdominal infection.
The various dose-related toxicities observed with the three drugs in rats and rabbits included maternal deaths; increased postimplantation loss; reduced fetal body weights; delays in skeletal ossification; and fetal malformations of the skeleton, heart, and lung. Use of these drugs in the last half of pregnancy may cause permanent discoloration of the teeth and enamel hypoplasia, as well as inhibition of bone growth.
Antilipemic agents
Crysvita (burosumab-twza), which has a MW of about 147,000, is a fibroblast growth factor–blocking antibody indicated for the treatment of X-linked hypophosphatemia. In pregnant cynomolgus monkeys, doses slightly higher than the human dose were not teratogenic. The drug was detected in fetal serum indicating that it crossed the monkey placenta.
Tegsedi (inotersen), which has a MW of about 7,601, is an amyloidosis inhibitor used for polyneuropathy of hereditary transthyretin-mediated amyloidosis. It is available only through a restricted program. The drug was not teratogenic in mice and rabbits; however, it does decrease vitamin A levels, so supplementation with the vitamin is recommended.
Antineoplastics
The manufacturers recommend avoiding these drugs during pregnancy. Effective contraception should be used.
Daurismo (glasdegib), which has a MW of about 491, is a hedgehog pathway inhibitor indicated in combination with low-dose cytarabine for newly diagnosed acute myeloid leukemia (AML). The drug caused embryotoxicity, fetotoxicity, and teratogenicity in rats and rabbits at doses less than the human dose.
Erleada (apalutamide), which has a MW of about 477, is an androgen receptor inhibitor indicated for nonmetastatic, castration-resistant prostate cancer. Animal studies were not conducted because the drug should not be used in females.
Elzonris (tagraxofusp-erzs), which has a MW of 57,695, is a cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm. Animal studies have not been conducted.
Lumoxiti (moxetumomab pasudotox–tdfk) which as a MW of about 63,000, is indicated for relapsed or refractory hairy cell leukemia. Studies have not been conducted in pregnant animals. Two life-threatening outcomes have occurred with the drug: capillary leak syndrome and hemolytic uremic syndrome. The drug should be discontinued if either occurs.
Lutathera (lutetium Lu 177 dotatate), which has a MW of about 1,610, is a radiolabeled somatostatin analogue given as a single intravenous dose every 8 weeks for four doses for the treatment of gastroenteropancreatic neuroendocrine tumors. Reproductive studies in animals have not been conducted. However, all radiopharmaceuticals have the potential to cause embryo-fetal harm. They also can cause infertility in males and females.
Talzenna (talazoparib), which has a MW of about 553, is a poly (ADP-ribose) polymerase inhibitor indicated for the treatment of certain types of breast cancer. At doses much less then the human dose, the drug caused fetal malformations and embryo-fetal death in rats.
Tibsovo (ivosidenib), which has a MW of 583, is an isocitrate dehydrogenase 1 inhibitor used for patients with relapsed or refractory AML. The drug caused embryo-fetal toxicity in rats and rabbits at doses slighter higher than the human dose.
There are seven new kinase inhibitors.
Braftovi (encorafenib), which has a MW of 540, is indicated in combination with Mektovi for patients with a specific type of metastatic melanoma. The drug caused embryo-fetal toxicity in rats and rabbits.
Copiktra (duvelisib), which has a MW of about 435, is indicated for treatment of chronic lymphocytic leukemia and follicular lymphoma. In rats and rabbits, the drug caused embryo-fetal death, lower fetal weights, and malformations.
Lorbrena (lorlatinib), which has a MW of about 406, is given for the treatment of metastatic non–small cell lung cancer. In rats and rabbits, the drug caused abortions, decreased fetal body weight, and major malformations.
Mektovi (binimetinib), which has a MW of about 441, is used in combination with Braftovi for patients with a specific type of melanoma. The drug was embryotoxic and abortifacient in rabbits.
Vitrakvi (larotrectinib), which has a MW of about 527, is used for patients with solid tumors. Studies in rats revealed fetal anasarca (extreme generalized edema) and omphalocele in rabbits.
Vizimpro (dacomitinib), which has a MW of about 488, is indicated for metastatic non–small cell lung cancer. The drug caused embryo-fetal toxicity in rats and mice.
Xospata (gilteritinib), which has a MW of about 1,222, is indicated for relapsed or refractory AML. In rats, the drug caused embryo-fetal death, suppressed fetal growth, and caused multiple malformations.
Three drugs are classified as monoclonal antibodies.
Gamifant (emapalumab), which has a MW of about 148,000, is indicated for primary hemophagocytic lymphohistiocytosis. A murine surrogate antimouse antibody was given to pregnant mice throughout gestation and no fetal harm was observed.
Libtayo (cemiplimab-rwlc), which has a MW of 146,000, is indicated for patients with metastatic or locally advanced cutaneous squamous cell carcinoma. Animal reproduction studies have not been conducted; however, based on its mechanism, increased rates of abortion or stillbirth may occur if the drug is used in human pregnancy.
Poteligeo (mogamulizumab-kpkc), which has a MW of about 149,000, is given for relapsed/refractory mycosis fungoides or Sézary syndrome. In pregnant monkeys, there was no embryo-fetal lethality, teratogenicity, fetal growth restriction, spontaneous abortion, or increased fetal death.
Central nervous system
There are three antimigraine agents that are monoclonal antibodies given as a subcutaneous injection.
Aimovig (erenumab-aooe), which has a MW of about 150,000, caused no adverse effects in monkey offspring.
Ajovy (fremanezumab-vfrm), which has a MW of about 148,000, had no adverse effect in rat and rabbit offspring.
Emgality (galcanezumab-gnlm), which has a MW of about 147,000, produced no adverse effects in rat and rabbit offspring.
Diacomit (stiripentol), which has a MW of about 234, is an anticonvulsant used to treat seizures associated with Dravet syndrome. The drug caused severe embryo-fetal toxicity in mice, rabbits, and rats. The drug is included in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Patients can enroll themselves by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Epidiolex (cannabidiol), which has a MW of about 314, is an anticonvulsant indicated for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. In pregnant rats, doses up to about 16 times the recommended human dose (RHD) caused no embryo-fetal adverse effects. The drug caused decreased fetal body weights, increased fetal structural variations, and maternal toxicity when the drug was given to pregnant rabbits throughout organogenesis. The no-effect dose for embryo-fetal toxicity was less than the human dose. Patients can enroll themselves in the NAAED Pregnancy Registry by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Firdapse (amifampridine), a potassium channel blocker with a MW of about 201, is used for the treatment of Lambert-Eaton myasthenic syndrome. No adverse effects on embryo-fetal development were observed in rats and rabbits given the drug throughout organogenesis. However, in rats given the drug throughout pregnancy and lactation, there was an increase in stillbirths and pup deaths, reduced pup weight, and delayed sexual development in female pups.
Lucemyra (lofexidine), which has a MW of about 296, is used to mitigate opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. The drug caused severe toxicity in the fetuses of rats and rabbits.
Olumiant (baricitinib), which has a MW of about 371, is a Janus kinase inhibitor indicated for the treatment of rheumatoid arthritis. The drug was teratogenic in pregnant rats given doses about 20 times greater than the maximum RHD based on area under the curve. In rabbits, embryo death and rib anomalies were observed with doses 84 times greater than the maximum RHD, but no developmental toxicity was seen with doses 12 times greater than the maximum RHD.
Orilissa (elagolix), which has a MW of about 654, is a gonadotropin-releasing hormone receptor antagonist indicated for the management of pain associated with endometriosis. The drug caused abortions in rats and rabbits. Because the drug may increase the risk of early pregnancy loss, the manufacturer classifies it as contraindicated in pregnancy.
Dermatologic agents
Ilumya (tildrakizumab), which has a MW of about 147,000, is given by subcutaneous injection for the treatment of moderate to severe plaque psoriasis. When given during organogenesis in monkeys, no maternal or embryo-fetal toxicities were observed. However, when given throughout pregnancy a few neonatal deaths occurred, but the clinical significance of these nonclinical findings were unknown.
Fabry disease
Galafold (migalastat), which has a MW of about 200, is an alpha-galactosidase A pharmacologic chaperone indicated for the treatment of Fabry disease. Three pregnant women with Fabry disease were exposed to the drug in clinical studies but no information was provided on the pregnancy outcomes. No adverse developmental effects were observed in pregnant rats and rabbits.
Gastrointestinal agents
Akynzeo (netupitant or fosnetupitant palonosetron), which have MWs of about 579, 333, and 762, respectively, is available as an oral capsule (netupitant + palonosetron) and as an intravenous formulation (fosnetupitant + palonosetron). They are indicated, in combination with dexamethasone, for the prevention of nausea and vomiting related to cancer chemotherapy. Netupitant and fosnetupitant produced no embryo-fetal adverse effects in rats but were toxic to rabbit embryos. Palonosetron caused no embryo-fetal adverse effects in rats and rabbits.
Motegrity (prucalopride), which has a MW of about 486, is indicated for chronic idiopathic constipation. No adverse embryo-fetal developmental effects were observed in rats and rabbits.
Hematologic agents
Doptelet (avatrombopag), which has a MW of about 766, is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. No embryo-fetal effects were observed in rats, but in rabbits the drug was associated with spontaneous abortions.
Lokelma (sodium zirconium cyclosilicate) is a nonabsorbed zirconium silicate that exchanges potassium for hydrogen and sodium. Animal studies have not been conducted. Because it is not absorbed, it is not expected to result in fetal exposure to the drug.
Mulpleta (lusutrombopag), which has a MW of about 592, a thrombopoietin receptor agonist, is indicated for the treatment of thrombocytopenia in patients with chronic liver disease. High levels of the drug in pregnant rats were associated with adverse developmental outcomes. No adverse embryo-fetal effects were seen in pregnant rabbits.
Palynziq (pegvaliase-pqpz), which has a MW of about 1,000,000, is a phenylalanine-metabolizing enzyme indicated to reduce blood phenylalanine concentrations in patients with phenylketonuria. In pregnant rats, the drug caused an increase in skeletal variations. In rabbits, the drug caused a high incidence of multiple malformations.
Takhzyro (lanadelumab-flyo), which has a MW of about 49,000, is a monoclonal antibody indicated for prophylaxis to prevent attacks of hereditary angioedema. The drug caused no fetal harm in monkeys.
Tavalisse (fostamatinib disodium hexahydrate), which has a MW of about 733, is a kinase inhibitor used for the treatment of thrombocytopenia. In pregnant rats and rabbits, the drug caused adverse developmental outcomes including embryo-fetal mortality, lower fetal weights, and structural anomalies.
Ultomiris (ravulizumab), which has a MW of about 148,000, is a humanized monoclonal antibody indicated for adult patients with paroxysmal nocturnal hemoglobinuria. In mice, the drug was associated with increased rates of developmental abnormalities and an increased rate of dead and moribund offspring.
Immunologic agent
Revcovi (elapegademase-lvlr), which has a MW of about 113,000, is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency. Animal studies in pregnancy have not been conducted.
Nutrient/Nutritional supplement
Fish oil is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis. Animal reproduction studies have not been conducted. It is doubtful if this product will be used in pregnancy.
Ophthalmic – nerve growth factor
Oxervate (cenegermin-bkbj), which has a MW of 13,266, is a solution that contains 118 amino acids. It is a recombinant human nerve growth factor indicated for neurotrophic keratitis. In rats and rabbits given the drug during organogenesis, there was a slight increase in postimplantation loss at doses greater than or equal to 267 times the human dose.
Respiratory drugs
Symdeko (tezacaftor + ivacaftor), which have MWs of about 521 and 392, is indicated for the treatment of patients with cystic fibrosis who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator gene. There were no adverse developmental effects in pregnant rats and rabbits when the drugs were used separately or combined.
Yupelri (revefenacin), which has a MW of about 598, is an anticholinergic drug. It is an inhaled solution for the maintenance treatment of chronic obstructive pulmonary disease. In rats and rabbits, doses that were about 209 times the RHD produced no evidence of fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs reported no relevant financial disclosures. Email him at [email protected].
In 2018, the Food and Drug Administration approved a record 58 new drugs for humans. One of these agents, Annovera (segesterone acetate and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol-mknl), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. The agents with molecular weights (MW) less than 1,000 probably cross the placenta, but nearly all, regardless of MW, will cross in the second half of pregnancy.
There is no human pregnancy data for these agents, but there are five drugs included in pregnancy registries. However, it will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in a reference that I coauthored (“Should pregnant women be included in phase 4 clinical drug trials?” Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). The article makes a strong argument for including some drugs in these trials.
Amyloidosis
Onpattro (patisiran) is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The drug caused embryo-fetal death and reduced fetal body weight in rabbits at doses also associated with maternal toxicity. No developmental toxicity was observed in rats.
Anti-infectives
Aemcolo (rifamycin), which has a MW of 720, is indicated for treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli. No adverse fetal effects were observed in rats and rabbits that received close to human doses.
Krintafel (tafenoquine) is an antimalarial agent that is used to prevent relapse in patients who are receiving appropriate antimalarial therapy for Plasmodium vivax infection. The drug may cause hemolytic anemia in a fetus deficient in glucose-6-phosphate dehydrogenase. In rabbits, the drug caused dose-related abortions and maternal toxicity was observed in rabbits and rats. Treatment with this drug in pregnancy is not recommended, according to the manufacturer.
Tpoxx (tecovirimat monohydrate), which has a MW of about 394, is indicated for the treatment of smallpox disease. The drug did not cause embryo-fetal toxicity in pregnant mice and rabbits, but the maximum exposure in rabbits was only 0.4 times the human exposure.
Xofluza (baloxavir marboxil), which has a MW of about 572, is a prodrug that is converted by hydrolysis to baloxavir. It is indicated for the treatment of acute uncomplicated influenza. No adverse developmental effects were observed in rats and rabbits.
Zemdri (plazomicin), which has a MW of about 593, is an aminoglycoside indicated for the treatment of complicated urinary tract infections including pyelonephritis. The drug did not cause fetal harm in rats and rabbits at doses that did not cause maternal toxicity; however, prolonged use of an aminoglycoside (such as streptomycin) has caused irreversible, bilateral congenital deafness in children exposed in utero to prolonged use and is a potential complication.
Three new drugs in 2018 are indicated for treating HIV-1:
Biktarvy is a three-drug combination that includes bictegravir, emtricitabine, and tenofovir. The latter two drugs are included in the 11th edition of my book (“Drugs in Pregnancy and Lactation,” 11th ed. [Riverwoods, Ill.: Wolters Kluwer, 2017) and are not included here. Both are classified as compatible in pregnancy. Bictegravir has a MW of about 471. No adverse embryo-fetal effects in rats and rabbits were observed with this agent.
Trogarzo (ibalizumab-uiyk), which has a MW of about 150,000, is a monoclonal antibody antiretroviral agent used in combination with other antiretrovirals. There are no animal data. Although the MW is very high, monoclonal antibodies are transported across the placenta as pregnancy progresses.
Pifeltro (doravirine), which has a MW of about 426, is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents for the treatment of HIV-1. The drug caused no significant toxicologic effects on embryo-fetal rats and rabbits.
If Biktarvy, Pifeltro, or Trogarzo are used in pregnancy, health care providers are encouraged to register the patient in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
There are three new agents in the tetracycline class.
Nuzyra (omadacycline), which has a MW of about 729, is for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.
Seysara (sarecycline), which has a MW of about 524, is for inflammatory lesions of nonnodular, moderate to severe acne vulgaris.
Xerava (eravacycline), which has a MW of about 632, is for complicated intra-abdominal infection.
The various dose-related toxicities observed with the three drugs in rats and rabbits included maternal deaths; increased postimplantation loss; reduced fetal body weights; delays in skeletal ossification; and fetal malformations of the skeleton, heart, and lung. Use of these drugs in the last half of pregnancy may cause permanent discoloration of the teeth and enamel hypoplasia, as well as inhibition of bone growth.
Antilipemic agents
Crysvita (burosumab-twza), which has a MW of about 147,000, is a fibroblast growth factor–blocking antibody indicated for the treatment of X-linked hypophosphatemia. In pregnant cynomolgus monkeys, doses slightly higher than the human dose were not teratogenic. The drug was detected in fetal serum indicating that it crossed the monkey placenta.
Tegsedi (inotersen), which has a MW of about 7,601, is an amyloidosis inhibitor used for polyneuropathy of hereditary transthyretin-mediated amyloidosis. It is available only through a restricted program. The drug was not teratogenic in mice and rabbits; however, it does decrease vitamin A levels, so supplementation with the vitamin is recommended.
Antineoplastics
The manufacturers recommend avoiding these drugs during pregnancy. Effective contraception should be used.
Daurismo (glasdegib), which has a MW of about 491, is a hedgehog pathway inhibitor indicated in combination with low-dose cytarabine for newly diagnosed acute myeloid leukemia (AML). The drug caused embryotoxicity, fetotoxicity, and teratogenicity in rats and rabbits at doses less than the human dose.
Erleada (apalutamide), which has a MW of about 477, is an androgen receptor inhibitor indicated for nonmetastatic, castration-resistant prostate cancer. Animal studies were not conducted because the drug should not be used in females.
Elzonris (tagraxofusp-erzs), which has a MW of 57,695, is a cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm. Animal studies have not been conducted.
Lumoxiti (moxetumomab pasudotox–tdfk) which as a MW of about 63,000, is indicated for relapsed or refractory hairy cell leukemia. Studies have not been conducted in pregnant animals. Two life-threatening outcomes have occurred with the drug: capillary leak syndrome and hemolytic uremic syndrome. The drug should be discontinued if either occurs.
Lutathera (lutetium Lu 177 dotatate), which has a MW of about 1,610, is a radiolabeled somatostatin analogue given as a single intravenous dose every 8 weeks for four doses for the treatment of gastroenteropancreatic neuroendocrine tumors. Reproductive studies in animals have not been conducted. However, all radiopharmaceuticals have the potential to cause embryo-fetal harm. They also can cause infertility in males and females.
Talzenna (talazoparib), which has a MW of about 553, is a poly (ADP-ribose) polymerase inhibitor indicated for the treatment of certain types of breast cancer. At doses much less then the human dose, the drug caused fetal malformations and embryo-fetal death in rats.
Tibsovo (ivosidenib), which has a MW of 583, is an isocitrate dehydrogenase 1 inhibitor used for patients with relapsed or refractory AML. The drug caused embryo-fetal toxicity in rats and rabbits at doses slighter higher than the human dose.
There are seven new kinase inhibitors.
Braftovi (encorafenib), which has a MW of 540, is indicated in combination with Mektovi for patients with a specific type of metastatic melanoma. The drug caused embryo-fetal toxicity in rats and rabbits.
Copiktra (duvelisib), which has a MW of about 435, is indicated for treatment of chronic lymphocytic leukemia and follicular lymphoma. In rats and rabbits, the drug caused embryo-fetal death, lower fetal weights, and malformations.
Lorbrena (lorlatinib), which has a MW of about 406, is given for the treatment of metastatic non–small cell lung cancer. In rats and rabbits, the drug caused abortions, decreased fetal body weight, and major malformations.
Mektovi (binimetinib), which has a MW of about 441, is used in combination with Braftovi for patients with a specific type of melanoma. The drug was embryotoxic and abortifacient in rabbits.
Vitrakvi (larotrectinib), which has a MW of about 527, is used for patients with solid tumors. Studies in rats revealed fetal anasarca (extreme generalized edema) and omphalocele in rabbits.
Vizimpro (dacomitinib), which has a MW of about 488, is indicated for metastatic non–small cell lung cancer. The drug caused embryo-fetal toxicity in rats and mice.
Xospata (gilteritinib), which has a MW of about 1,222, is indicated for relapsed or refractory AML. In rats, the drug caused embryo-fetal death, suppressed fetal growth, and caused multiple malformations.
Three drugs are classified as monoclonal antibodies.
Gamifant (emapalumab), which has a MW of about 148,000, is indicated for primary hemophagocytic lymphohistiocytosis. A murine surrogate antimouse antibody was given to pregnant mice throughout gestation and no fetal harm was observed.
Libtayo (cemiplimab-rwlc), which has a MW of 146,000, is indicated for patients with metastatic or locally advanced cutaneous squamous cell carcinoma. Animal reproduction studies have not been conducted; however, based on its mechanism, increased rates of abortion or stillbirth may occur if the drug is used in human pregnancy.
Poteligeo (mogamulizumab-kpkc), which has a MW of about 149,000, is given for relapsed/refractory mycosis fungoides or Sézary syndrome. In pregnant monkeys, there was no embryo-fetal lethality, teratogenicity, fetal growth restriction, spontaneous abortion, or increased fetal death.
Central nervous system
There are three antimigraine agents that are monoclonal antibodies given as a subcutaneous injection.
Aimovig (erenumab-aooe), which has a MW of about 150,000, caused no adverse effects in monkey offspring.
Ajovy (fremanezumab-vfrm), which has a MW of about 148,000, had no adverse effect in rat and rabbit offspring.
Emgality (galcanezumab-gnlm), which has a MW of about 147,000, produced no adverse effects in rat and rabbit offspring.
Diacomit (stiripentol), which has a MW of about 234, is an anticonvulsant used to treat seizures associated with Dravet syndrome. The drug caused severe embryo-fetal toxicity in mice, rabbits, and rats. The drug is included in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Patients can enroll themselves by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Epidiolex (cannabidiol), which has a MW of about 314, is an anticonvulsant indicated for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. In pregnant rats, doses up to about 16 times the recommended human dose (RHD) caused no embryo-fetal adverse effects. The drug caused decreased fetal body weights, increased fetal structural variations, and maternal toxicity when the drug was given to pregnant rabbits throughout organogenesis. The no-effect dose for embryo-fetal toxicity was less than the human dose. Patients can enroll themselves in the NAAED Pregnancy Registry by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Firdapse (amifampridine), a potassium channel blocker with a MW of about 201, is used for the treatment of Lambert-Eaton myasthenic syndrome. No adverse effects on embryo-fetal development were observed in rats and rabbits given the drug throughout organogenesis. However, in rats given the drug throughout pregnancy and lactation, there was an increase in stillbirths and pup deaths, reduced pup weight, and delayed sexual development in female pups.
Lucemyra (lofexidine), which has a MW of about 296, is used to mitigate opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. The drug caused severe toxicity in the fetuses of rats and rabbits.
Olumiant (baricitinib), which has a MW of about 371, is a Janus kinase inhibitor indicated for the treatment of rheumatoid arthritis. The drug was teratogenic in pregnant rats given doses about 20 times greater than the maximum RHD based on area under the curve. In rabbits, embryo death and rib anomalies were observed with doses 84 times greater than the maximum RHD, but no developmental toxicity was seen with doses 12 times greater than the maximum RHD.
Orilissa (elagolix), which has a MW of about 654, is a gonadotropin-releasing hormone receptor antagonist indicated for the management of pain associated with endometriosis. The drug caused abortions in rats and rabbits. Because the drug may increase the risk of early pregnancy loss, the manufacturer classifies it as contraindicated in pregnancy.
Dermatologic agents
Ilumya (tildrakizumab), which has a MW of about 147,000, is given by subcutaneous injection for the treatment of moderate to severe plaque psoriasis. When given during organogenesis in monkeys, no maternal or embryo-fetal toxicities were observed. However, when given throughout pregnancy a few neonatal deaths occurred, but the clinical significance of these nonclinical findings were unknown.
Fabry disease
Galafold (migalastat), which has a MW of about 200, is an alpha-galactosidase A pharmacologic chaperone indicated for the treatment of Fabry disease. Three pregnant women with Fabry disease were exposed to the drug in clinical studies but no information was provided on the pregnancy outcomes. No adverse developmental effects were observed in pregnant rats and rabbits.
Gastrointestinal agents
Akynzeo (netupitant or fosnetupitant palonosetron), which have MWs of about 579, 333, and 762, respectively, is available as an oral capsule (netupitant + palonosetron) and as an intravenous formulation (fosnetupitant + palonosetron). They are indicated, in combination with dexamethasone, for the prevention of nausea and vomiting related to cancer chemotherapy. Netupitant and fosnetupitant produced no embryo-fetal adverse effects in rats but were toxic to rabbit embryos. Palonosetron caused no embryo-fetal adverse effects in rats and rabbits.
Motegrity (prucalopride), which has a MW of about 486, is indicated for chronic idiopathic constipation. No adverse embryo-fetal developmental effects were observed in rats and rabbits.
Hematologic agents
Doptelet (avatrombopag), which has a MW of about 766, is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. No embryo-fetal effects were observed in rats, but in rabbits the drug was associated with spontaneous abortions.
Lokelma (sodium zirconium cyclosilicate) is a nonabsorbed zirconium silicate that exchanges potassium for hydrogen and sodium. Animal studies have not been conducted. Because it is not absorbed, it is not expected to result in fetal exposure to the drug.
Mulpleta (lusutrombopag), which has a MW of about 592, a thrombopoietin receptor agonist, is indicated for the treatment of thrombocytopenia in patients with chronic liver disease. High levels of the drug in pregnant rats were associated with adverse developmental outcomes. No adverse embryo-fetal effects were seen in pregnant rabbits.
Palynziq (pegvaliase-pqpz), which has a MW of about 1,000,000, is a phenylalanine-metabolizing enzyme indicated to reduce blood phenylalanine concentrations in patients with phenylketonuria. In pregnant rats, the drug caused an increase in skeletal variations. In rabbits, the drug caused a high incidence of multiple malformations.
Takhzyro (lanadelumab-flyo), which has a MW of about 49,000, is a monoclonal antibody indicated for prophylaxis to prevent attacks of hereditary angioedema. The drug caused no fetal harm in monkeys.
Tavalisse (fostamatinib disodium hexahydrate), which has a MW of about 733, is a kinase inhibitor used for the treatment of thrombocytopenia. In pregnant rats and rabbits, the drug caused adverse developmental outcomes including embryo-fetal mortality, lower fetal weights, and structural anomalies.
Ultomiris (ravulizumab), which has a MW of about 148,000, is a humanized monoclonal antibody indicated for adult patients with paroxysmal nocturnal hemoglobinuria. In mice, the drug was associated with increased rates of developmental abnormalities and an increased rate of dead and moribund offspring.
Immunologic agent
Revcovi (elapegademase-lvlr), which has a MW of about 113,000, is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency. Animal studies in pregnancy have not been conducted.
Nutrient/Nutritional supplement
Fish oil is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis. Animal reproduction studies have not been conducted. It is doubtful if this product will be used in pregnancy.
Ophthalmic – nerve growth factor
Oxervate (cenegermin-bkbj), which has a MW of 13,266, is a solution that contains 118 amino acids. It is a recombinant human nerve growth factor indicated for neurotrophic keratitis. In rats and rabbits given the drug during organogenesis, there was a slight increase in postimplantation loss at doses greater than or equal to 267 times the human dose.
Respiratory drugs
Symdeko (tezacaftor + ivacaftor), which have MWs of about 521 and 392, is indicated for the treatment of patients with cystic fibrosis who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator gene. There were no adverse developmental effects in pregnant rats and rabbits when the drugs were used separately or combined.
Yupelri (revefenacin), which has a MW of about 598, is an anticholinergic drug. It is an inhaled solution for the maintenance treatment of chronic obstructive pulmonary disease. In rats and rabbits, doses that were about 209 times the RHD produced no evidence of fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs reported no relevant financial disclosures. Email him at [email protected].
In 2018, the Food and Drug Administration approved a record 58 new drugs for humans. One of these agents, Annovera (segesterone acetate and ethinyl estradiol), is a vaginal ring to prevent pregnancy and is not relevant in this article. A second drug, Asparlas (calaspargase pegol-mknl), indicated to treat acute lymphoblastic leukemia, has not yet been released by its manufacturer. The agents with molecular weights (MW) less than 1,000 probably cross the placenta, but nearly all, regardless of MW, will cross in the second half of pregnancy.
There is no human pregnancy data for these agents, but there are five drugs included in pregnancy registries. However, it will take some time before the outcomes of these drugs are published. The routine absence of pregnancy data for most drugs was pointed out in a reference that I coauthored (“Should pregnant women be included in phase 4 clinical drug trials?” Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). The article makes a strong argument for including some drugs in these trials.
Amyloidosis
Onpattro (patisiran) is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The drug caused embryo-fetal death and reduced fetal body weight in rabbits at doses also associated with maternal toxicity. No developmental toxicity was observed in rats.
Anti-infectives
Aemcolo (rifamycin), which has a MW of 720, is indicated for treatment of travelers’ diarrhea caused by noninvasive strains of Escherichia coli. No adverse fetal effects were observed in rats and rabbits that received close to human doses.
Krintafel (tafenoquine) is an antimalarial agent that is used to prevent relapse in patients who are receiving appropriate antimalarial therapy for Plasmodium vivax infection. The drug may cause hemolytic anemia in a fetus deficient in glucose-6-phosphate dehydrogenase. In rabbits, the drug caused dose-related abortions and maternal toxicity was observed in rabbits and rats. Treatment with this drug in pregnancy is not recommended, according to the manufacturer.
Tpoxx (tecovirimat monohydrate), which has a MW of about 394, is indicated for the treatment of smallpox disease. The drug did not cause embryo-fetal toxicity in pregnant mice and rabbits, but the maximum exposure in rabbits was only 0.4 times the human exposure.
Xofluza (baloxavir marboxil), which has a MW of about 572, is a prodrug that is converted by hydrolysis to baloxavir. It is indicated for the treatment of acute uncomplicated influenza. No adverse developmental effects were observed in rats and rabbits.
Zemdri (plazomicin), which has a MW of about 593, is an aminoglycoside indicated for the treatment of complicated urinary tract infections including pyelonephritis. The drug did not cause fetal harm in rats and rabbits at doses that did not cause maternal toxicity; however, prolonged use of an aminoglycoside (such as streptomycin) has caused irreversible, bilateral congenital deafness in children exposed in utero to prolonged use and is a potential complication.
Three new drugs in 2018 are indicated for treating HIV-1:
Biktarvy is a three-drug combination that includes bictegravir, emtricitabine, and tenofovir. The latter two drugs are included in the 11th edition of my book (“Drugs in Pregnancy and Lactation,” 11th ed. [Riverwoods, Ill.: Wolters Kluwer, 2017) and are not included here. Both are classified as compatible in pregnancy. Bictegravir has a MW of about 471. No adverse embryo-fetal effects in rats and rabbits were observed with this agent.
Trogarzo (ibalizumab-uiyk), which has a MW of about 150,000, is a monoclonal antibody antiretroviral agent used in combination with other antiretrovirals. There are no animal data. Although the MW is very high, monoclonal antibodies are transported across the placenta as pregnancy progresses.
Pifeltro (doravirine), which has a MW of about 426, is a nonnucleoside reverse transcriptase inhibitor used in combination with other antiretroviral agents for the treatment of HIV-1. The drug caused no significant toxicologic effects on embryo-fetal rats and rabbits.
If Biktarvy, Pifeltro, or Trogarzo are used in pregnancy, health care providers are encouraged to register the patient in the Antiretroviral Pregnancy Registry by calling 1-800-258-4263.
There are three new agents in the tetracycline class.
Nuzyra (omadacycline), which has a MW of about 729, is for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.
Seysara (sarecycline), which has a MW of about 524, is for inflammatory lesions of nonnodular, moderate to severe acne vulgaris.
Xerava (eravacycline), which has a MW of about 632, is for complicated intra-abdominal infection.
The various dose-related toxicities observed with the three drugs in rats and rabbits included maternal deaths; increased postimplantation loss; reduced fetal body weights; delays in skeletal ossification; and fetal malformations of the skeleton, heart, and lung. Use of these drugs in the last half of pregnancy may cause permanent discoloration of the teeth and enamel hypoplasia, as well as inhibition of bone growth.
Antilipemic agents
Crysvita (burosumab-twza), which has a MW of about 147,000, is a fibroblast growth factor–blocking antibody indicated for the treatment of X-linked hypophosphatemia. In pregnant cynomolgus monkeys, doses slightly higher than the human dose were not teratogenic. The drug was detected in fetal serum indicating that it crossed the monkey placenta.
Tegsedi (inotersen), which has a MW of about 7,601, is an amyloidosis inhibitor used for polyneuropathy of hereditary transthyretin-mediated amyloidosis. It is available only through a restricted program. The drug was not teratogenic in mice and rabbits; however, it does decrease vitamin A levels, so supplementation with the vitamin is recommended.
Antineoplastics
The manufacturers recommend avoiding these drugs during pregnancy. Effective contraception should be used.
Daurismo (glasdegib), which has a MW of about 491, is a hedgehog pathway inhibitor indicated in combination with low-dose cytarabine for newly diagnosed acute myeloid leukemia (AML). The drug caused embryotoxicity, fetotoxicity, and teratogenicity in rats and rabbits at doses less than the human dose.
Erleada (apalutamide), which has a MW of about 477, is an androgen receptor inhibitor indicated for nonmetastatic, castration-resistant prostate cancer. Animal studies were not conducted because the drug should not be used in females.
Elzonris (tagraxofusp-erzs), which has a MW of 57,695, is a cytotoxin indicated for the treatment of blastic plasmacytoid dendritic cell neoplasm. Animal studies have not been conducted.
Lumoxiti (moxetumomab pasudotox–tdfk) which as a MW of about 63,000, is indicated for relapsed or refractory hairy cell leukemia. Studies have not been conducted in pregnant animals. Two life-threatening outcomes have occurred with the drug: capillary leak syndrome and hemolytic uremic syndrome. The drug should be discontinued if either occurs.
Lutathera (lutetium Lu 177 dotatate), which has a MW of about 1,610, is a radiolabeled somatostatin analogue given as a single intravenous dose every 8 weeks for four doses for the treatment of gastroenteropancreatic neuroendocrine tumors. Reproductive studies in animals have not been conducted. However, all radiopharmaceuticals have the potential to cause embryo-fetal harm. They also can cause infertility in males and females.
Talzenna (talazoparib), which has a MW of about 553, is a poly (ADP-ribose) polymerase inhibitor indicated for the treatment of certain types of breast cancer. At doses much less then the human dose, the drug caused fetal malformations and embryo-fetal death in rats.
Tibsovo (ivosidenib), which has a MW of 583, is an isocitrate dehydrogenase 1 inhibitor used for patients with relapsed or refractory AML. The drug caused embryo-fetal toxicity in rats and rabbits at doses slighter higher than the human dose.
There are seven new kinase inhibitors.
Braftovi (encorafenib), which has a MW of 540, is indicated in combination with Mektovi for patients with a specific type of metastatic melanoma. The drug caused embryo-fetal toxicity in rats and rabbits.
Copiktra (duvelisib), which has a MW of about 435, is indicated for treatment of chronic lymphocytic leukemia and follicular lymphoma. In rats and rabbits, the drug caused embryo-fetal death, lower fetal weights, and malformations.
Lorbrena (lorlatinib), which has a MW of about 406, is given for the treatment of metastatic non–small cell lung cancer. In rats and rabbits, the drug caused abortions, decreased fetal body weight, and major malformations.
Mektovi (binimetinib), which has a MW of about 441, is used in combination with Braftovi for patients with a specific type of melanoma. The drug was embryotoxic and abortifacient in rabbits.
Vitrakvi (larotrectinib), which has a MW of about 527, is used for patients with solid tumors. Studies in rats revealed fetal anasarca (extreme generalized edema) and omphalocele in rabbits.
Vizimpro (dacomitinib), which has a MW of about 488, is indicated for metastatic non–small cell lung cancer. The drug caused embryo-fetal toxicity in rats and mice.
Xospata (gilteritinib), which has a MW of about 1,222, is indicated for relapsed or refractory AML. In rats, the drug caused embryo-fetal death, suppressed fetal growth, and caused multiple malformations.
Three drugs are classified as monoclonal antibodies.
Gamifant (emapalumab), which has a MW of about 148,000, is indicated for primary hemophagocytic lymphohistiocytosis. A murine surrogate antimouse antibody was given to pregnant mice throughout gestation and no fetal harm was observed.
Libtayo (cemiplimab-rwlc), which has a MW of 146,000, is indicated for patients with metastatic or locally advanced cutaneous squamous cell carcinoma. Animal reproduction studies have not been conducted; however, based on its mechanism, increased rates of abortion or stillbirth may occur if the drug is used in human pregnancy.
Poteligeo (mogamulizumab-kpkc), which has a MW of about 149,000, is given for relapsed/refractory mycosis fungoides or Sézary syndrome. In pregnant monkeys, there was no embryo-fetal lethality, teratogenicity, fetal growth restriction, spontaneous abortion, or increased fetal death.
Central nervous system
There are three antimigraine agents that are monoclonal antibodies given as a subcutaneous injection.
Aimovig (erenumab-aooe), which has a MW of about 150,000, caused no adverse effects in monkey offspring.
Ajovy (fremanezumab-vfrm), which has a MW of about 148,000, had no adverse effect in rat and rabbit offspring.
Emgality (galcanezumab-gnlm), which has a MW of about 147,000, produced no adverse effects in rat and rabbit offspring.
Diacomit (stiripentol), which has a MW of about 234, is an anticonvulsant used to treat seizures associated with Dravet syndrome. The drug caused severe embryo-fetal toxicity in mice, rabbits, and rats. The drug is included in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Patients can enroll themselves by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Epidiolex (cannabidiol), which has a MW of about 314, is an anticonvulsant indicated for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. In pregnant rats, doses up to about 16 times the recommended human dose (RHD) caused no embryo-fetal adverse effects. The drug caused decreased fetal body weights, increased fetal structural variations, and maternal toxicity when the drug was given to pregnant rabbits throughout organogenesis. The no-effect dose for embryo-fetal toxicity was less than the human dose. Patients can enroll themselves in the NAAED Pregnancy Registry by calling the toll-free number 1-888-233-2334 or visiting http://aedpregnancyregistry.org/.
Firdapse (amifampridine), a potassium channel blocker with a MW of about 201, is used for the treatment of Lambert-Eaton myasthenic syndrome. No adverse effects on embryo-fetal development were observed in rats and rabbits given the drug throughout organogenesis. However, in rats given the drug throughout pregnancy and lactation, there was an increase in stillbirths and pup deaths, reduced pup weight, and delayed sexual development in female pups.
Lucemyra (lofexidine), which has a MW of about 296, is used to mitigate opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. The drug caused severe toxicity in the fetuses of rats and rabbits.
Olumiant (baricitinib), which has a MW of about 371, is a Janus kinase inhibitor indicated for the treatment of rheumatoid arthritis. The drug was teratogenic in pregnant rats given doses about 20 times greater than the maximum RHD based on area under the curve. In rabbits, embryo death and rib anomalies were observed with doses 84 times greater than the maximum RHD, but no developmental toxicity was seen with doses 12 times greater than the maximum RHD.
Orilissa (elagolix), which has a MW of about 654, is a gonadotropin-releasing hormone receptor antagonist indicated for the management of pain associated with endometriosis. The drug caused abortions in rats and rabbits. Because the drug may increase the risk of early pregnancy loss, the manufacturer classifies it as contraindicated in pregnancy.
Dermatologic agents
Ilumya (tildrakizumab), which has a MW of about 147,000, is given by subcutaneous injection for the treatment of moderate to severe plaque psoriasis. When given during organogenesis in monkeys, no maternal or embryo-fetal toxicities were observed. However, when given throughout pregnancy a few neonatal deaths occurred, but the clinical significance of these nonclinical findings were unknown.
Fabry disease
Galafold (migalastat), which has a MW of about 200, is an alpha-galactosidase A pharmacologic chaperone indicated for the treatment of Fabry disease. Three pregnant women with Fabry disease were exposed to the drug in clinical studies but no information was provided on the pregnancy outcomes. No adverse developmental effects were observed in pregnant rats and rabbits.
Gastrointestinal agents
Akynzeo (netupitant or fosnetupitant palonosetron), which have MWs of about 579, 333, and 762, respectively, is available as an oral capsule (netupitant + palonosetron) and as an intravenous formulation (fosnetupitant + palonosetron). They are indicated, in combination with dexamethasone, for the prevention of nausea and vomiting related to cancer chemotherapy. Netupitant and fosnetupitant produced no embryo-fetal adverse effects in rats but were toxic to rabbit embryos. Palonosetron caused no embryo-fetal adverse effects in rats and rabbits.
Motegrity (prucalopride), which has a MW of about 486, is indicated for chronic idiopathic constipation. No adverse embryo-fetal developmental effects were observed in rats and rabbits.
Hematologic agents
Doptelet (avatrombopag), which has a MW of about 766, is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. No embryo-fetal effects were observed in rats, but in rabbits the drug was associated with spontaneous abortions.
Lokelma (sodium zirconium cyclosilicate) is a nonabsorbed zirconium silicate that exchanges potassium for hydrogen and sodium. Animal studies have not been conducted. Because it is not absorbed, it is not expected to result in fetal exposure to the drug.
Mulpleta (lusutrombopag), which has a MW of about 592, a thrombopoietin receptor agonist, is indicated for the treatment of thrombocytopenia in patients with chronic liver disease. High levels of the drug in pregnant rats were associated with adverse developmental outcomes. No adverse embryo-fetal effects were seen in pregnant rabbits.
Palynziq (pegvaliase-pqpz), which has a MW of about 1,000,000, is a phenylalanine-metabolizing enzyme indicated to reduce blood phenylalanine concentrations in patients with phenylketonuria. In pregnant rats, the drug caused an increase in skeletal variations. In rabbits, the drug caused a high incidence of multiple malformations.
Takhzyro (lanadelumab-flyo), which has a MW of about 49,000, is a monoclonal antibody indicated for prophylaxis to prevent attacks of hereditary angioedema. The drug caused no fetal harm in monkeys.
Tavalisse (fostamatinib disodium hexahydrate), which has a MW of about 733, is a kinase inhibitor used for the treatment of thrombocytopenia. In pregnant rats and rabbits, the drug caused adverse developmental outcomes including embryo-fetal mortality, lower fetal weights, and structural anomalies.
Ultomiris (ravulizumab), which has a MW of about 148,000, is a humanized monoclonal antibody indicated for adult patients with paroxysmal nocturnal hemoglobinuria. In mice, the drug was associated with increased rates of developmental abnormalities and an increased rate of dead and moribund offspring.
Immunologic agent
Revcovi (elapegademase-lvlr), which has a MW of about 113,000, is a recombinant adenosine deaminase indicated for the treatment of adenosine deaminase severe combined immune deficiency. Animal studies in pregnancy have not been conducted.
Nutrient/Nutritional supplement
Fish oil is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis. Animal reproduction studies have not been conducted. It is doubtful if this product will be used in pregnancy.
Ophthalmic – nerve growth factor
Oxervate (cenegermin-bkbj), which has a MW of 13,266, is a solution that contains 118 amino acids. It is a recombinant human nerve growth factor indicated for neurotrophic keratitis. In rats and rabbits given the drug during organogenesis, there was a slight increase in postimplantation loss at doses greater than or equal to 267 times the human dose.
Respiratory drugs
Symdeko (tezacaftor + ivacaftor), which have MWs of about 521 and 392, is indicated for the treatment of patients with cystic fibrosis who are homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator gene. There were no adverse developmental effects in pregnant rats and rabbits when the drugs were used separately or combined.
Yupelri (revefenacin), which has a MW of about 598, is an anticholinergic drug. It is an inhaled solution for the maintenance treatment of chronic obstructive pulmonary disease. In rats and rabbits, doses that were about 209 times the RHD produced no evidence of fetal harm.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs reported no relevant financial disclosures. Email him at [email protected].
Postpartum hypertension
When managing our pregnant patients, we often might be tempted to view the delivery of the baby as the conclusion of prenatal care. For many women, the baby’s birth coincides with a resolution of health conditions that they may have experienced during pregnancy, including edema, gestational diabetes, and hypertensive disorders. However, the postpartum period remains a critical time in the health of the mother. Indeed, the weeks immediately following parturition often are colloquially referred to as the fourth trimester, further emphasizing the importance of appropriate patient management and care during this time.
One of the key health conditions we must monitor in the immediate postpartum period is hypertension. According to a 2018 report compiling data from nine of the Centers for Disease Control and Prevention’s Maternal Mortality Review Committees, hypertensive disorders accounted for approximately 9.3% of pregnancy-related maternal deaths within 42 days after delivery (http://reviewtoaction.org/Report_from_Nine_MMRCs). Although women who have hypertensive disorders during pregnancy are at risk for complications after giving birth, women without gestational hypertension, preeclampsia, or eclampsia can experience these conditions post partum at a rate between 0.3% and 27.5% (Am J Obstet Gynecol 2012 Jun;206[6]:470-5). Therefore, we cannot assume that a patient with an uncomplicated pregnancy is completely “in the clear” after delivery.
Despite these somewhat grim statistics, With vigilant monitoring and strong communication with our patients, ob.gyns. can reduce the risks of these complications from occurring, more quickly resolve symptoms as they might arise, and significantly improve the health and well-being of new mothers in the fourth trimester.
The importance of caring for all of our patients along the continuum of pregnancy, especially as it pertains to monitoring and preventing postpartum hypertension, is the focus of the third and final installment of this Master Class series on hypertension in pregnancy authored by Dr. Baha Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
When managing our pregnant patients, we often might be tempted to view the delivery of the baby as the conclusion of prenatal care. For many women, the baby’s birth coincides with a resolution of health conditions that they may have experienced during pregnancy, including edema, gestational diabetes, and hypertensive disorders. However, the postpartum period remains a critical time in the health of the mother. Indeed, the weeks immediately following parturition often are colloquially referred to as the fourth trimester, further emphasizing the importance of appropriate patient management and care during this time.
One of the key health conditions we must monitor in the immediate postpartum period is hypertension. According to a 2018 report compiling data from nine of the Centers for Disease Control and Prevention’s Maternal Mortality Review Committees, hypertensive disorders accounted for approximately 9.3% of pregnancy-related maternal deaths within 42 days after delivery (http://reviewtoaction.org/Report_from_Nine_MMRCs). Although women who have hypertensive disorders during pregnancy are at risk for complications after giving birth, women without gestational hypertension, preeclampsia, or eclampsia can experience these conditions post partum at a rate between 0.3% and 27.5% (Am J Obstet Gynecol 2012 Jun;206[6]:470-5). Therefore, we cannot assume that a patient with an uncomplicated pregnancy is completely “in the clear” after delivery.
Despite these somewhat grim statistics, With vigilant monitoring and strong communication with our patients, ob.gyns. can reduce the risks of these complications from occurring, more quickly resolve symptoms as they might arise, and significantly improve the health and well-being of new mothers in the fourth trimester.
The importance of caring for all of our patients along the continuum of pregnancy, especially as it pertains to monitoring and preventing postpartum hypertension, is the focus of the third and final installment of this Master Class series on hypertension in pregnancy authored by Dr. Baha Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
When managing our pregnant patients, we often might be tempted to view the delivery of the baby as the conclusion of prenatal care. For many women, the baby’s birth coincides with a resolution of health conditions that they may have experienced during pregnancy, including edema, gestational diabetes, and hypertensive disorders. However, the postpartum period remains a critical time in the health of the mother. Indeed, the weeks immediately following parturition often are colloquially referred to as the fourth trimester, further emphasizing the importance of appropriate patient management and care during this time.
One of the key health conditions we must monitor in the immediate postpartum period is hypertension. According to a 2018 report compiling data from nine of the Centers for Disease Control and Prevention’s Maternal Mortality Review Committees, hypertensive disorders accounted for approximately 9.3% of pregnancy-related maternal deaths within 42 days after delivery (http://reviewtoaction.org/Report_from_Nine_MMRCs). Although women who have hypertensive disorders during pregnancy are at risk for complications after giving birth, women without gestational hypertension, preeclampsia, or eclampsia can experience these conditions post partum at a rate between 0.3% and 27.5% (Am J Obstet Gynecol 2012 Jun;206[6]:470-5). Therefore, we cannot assume that a patient with an uncomplicated pregnancy is completely “in the clear” after delivery.
Despite these somewhat grim statistics, With vigilant monitoring and strong communication with our patients, ob.gyns. can reduce the risks of these complications from occurring, more quickly resolve symptoms as they might arise, and significantly improve the health and well-being of new mothers in the fourth trimester.
The importance of caring for all of our patients along the continuum of pregnancy, especially as it pertains to monitoring and preventing postpartum hypertension, is the focus of the third and final installment of this Master Class series on hypertension in pregnancy authored by Dr. Baha Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Recognition, evaluation, and management of postpartum hypertension
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.
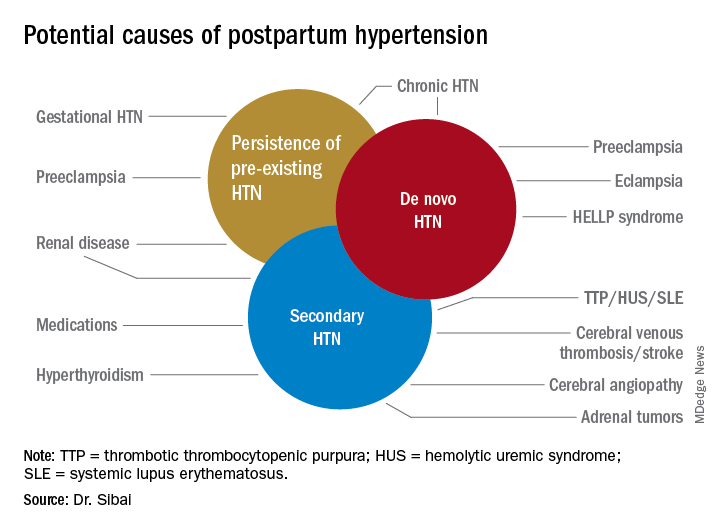
Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.

Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Postpartum hypertension has a host of potential causes, some of which may be benign (such as the persistence of mild gestational hypertension or mild chronic hypertension) whereas others (such as severe de novo preeclampsia-eclampsia and HELLP syndrome [a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and a low platelet count]) can be life threatening.

Postpartum hypertension may occur secondary to lupus, hyperthyroidism, hemolytic uremic syndrome, stroke, and other conditions, which means that we must have a high index of suspicion for secondary dangerous causes of hypertension when evaluating such women.
With monitoring, reporting, and prompt evaluation of symptoms in the postpartum period – and with patient education on signs and symptoms of severe hypertension and preeclampsia (PE) – we can expect to avoid a range of potential maternal complications, from hypertensive encephalopathy, liver hemorrhage, renal failure, and the development of eclampsia, ischemic stroke/cerebral hemorrhage, pulmonary edema, and cardiomyopathy.
Most women with gestational hypertension (GHTN) become normotensive during the first week post partum, but in women who develop PE during pregnancy, hypertension often takes longer to resolve. Some of these women may have an initial decrease in blood pressure immediately post partum followed by development of hypertension again between days 3 and 6. Therefore, This can be achieved either in-hospital, through home BP monitoring, or with in-office visits.
In addition, all women – including those who did not have hypertension during their pregnancies – should be educated about the signs and symptoms of severe hypertension or PE and instructed to report these to a medical provider in a timely fashion. Severe hypertension or PE with severe features may develop for the first time during the postpartum period either before or after hospital discharge. It is important to appreciate, moreover, that approximately 25%-40% of cases of eclampsia develop in the postpartum period with onset ranging from 2 days to 6 weeks after delivery. Moreover, almost one-third of women who develop the HELLP syndrome do so during the postpartum period.
Management of persistent hypertension
The most common causes for persistent hypertension beyond 48 hours after delivery are GHTN, PE, or chronic hypertension. Initial management will depend on history, clinical findings, presence or absence of associated symptoms, results of laboratory findings (urine protein, platelet count, liver enzymes, serum creatinine, and electrolytes), and response to prior treatment of hypertension.
Certain medications that frequently are prescribed in the postpartum period, such as ergonovine and decongestants, should be discontinued if they are being used. These agents can aggravate preexisting hypertension or result in new-onset hypertension if used in large or frequent doses. Their use also may be associated with cerebral symptoms, nausea, and vomiting.
Subsequent management includes close observation until resolution of hypertension and associated symptoms. If the patient has hypertension only with no symptoms, no proteinuria, and normal laboratory findings, BP control is the focus; antihypertensives are used if systolic BP remains persistently greater than or equal to 150 mm Hg and/or if diastolic BP persists at greater than or equal to 100 mm Hg. Intravenous boluses of either labetalol or hydralazine or oral rapid-acting nifedipine are used initially if systolic BP is greater than or equal to 160 mm Hg or diastolic BP greater than or equal to 110 mm Hg persists for at least 30 minutes. This is followed by oral medication to keep systolic BP less than 150 mg Hg and diastolic BP less than 100 mm Hg.
For patients with persistent hypertension after GHTN or PE, I recommend oral long-acting nifedipine XL (30 mg every 12 hours) or oral labetalol (200 mg every 8-12 hours). Compared with labetalol, oral nifedipine is associated with improved renal blood flow with resultant diuresis, which makes it the drug of choice in women with volume overload. In some, it is necessary to switch to a new agent such as an angiotensin-converting enzyme (ACE) inhibitor; an ACE inhibitor is the drug of choice in those with pregestational diabetes mellitus, renal disease, or cardiomyopathy. In addition, thiazide or loop diuretics may be needed in women with circulatory overload and in those with pulmonary edema. Antihypertensives such as nifedipine, labetalol, furosemide, captopril, and enalapril are compatible with breastfeeding.
If the BP remains less than 150 mm Hg (systolic) and/or less than 100 mm Hg (diastolic) for 24 hours, and there are no maternal symptoms, the patient may be discharged home with instructions for daily BP measurements (self or by a visiting nurse) and the reporting of symptoms until her next visit in 1 week. Antihypertensives then are discontinued if the BP remains below the hypertensive levels for at least 48 hours. This may take 1 or several weeks to achieve.
Women with PE with severe features should receive close monitoring of BP and of symptoms during the immediate postpartum period, as well as accurate measurements of fluid intake, urinary output, and weight gain. These women often have received large amounts of IV fluids during labor as a result of prehydration before epidural analgesia, as well as IV fluids administered during the use of oxytocin and magnesium sulfate in labor and post partum. Mobilization of extracellular fluid also leads to increased intravascular volume. As a result, women who have PE with severe features – particularly those with abnormal renal function, capillary leak, or early-onset disease – are at increased risk for pulmonary edema and exacerbation of severe hypertension.
Careful evaluation of the volume of IV fluids, oral intake, blood products, urine output, respiratory symptoms, and vital signs is advised. Patients who develop tachycardia or respiratory symptoms such as dry cough, shortness of breath, or orthopnea also should be monitored with pulse oximetry and frequent chest auscultation, as well as chest x-ray.
New-onset severe symptoms
Because severe hypertension or PE with severe features may develop for the first time during the postpartum period, postpartum women – and the medical providers and personnel who respond to patient phone calls – should be well educated about the signs and symptoms of severe hypertension or PE. These include new-onset severe headaches that do not respond to maximum doses of analgesics, persistent severe visual changes, and new-onset epigastric pain with nausea and vomiting, dyspnea, orthopnea, shortness of breath, or palpitations. These women are at increased risk for eclampsia, pulmonary edema, stroke, and thromboembolism; these women require careful evaluation and potential hospitalization.
Severe new onset of persistent headaches and/or visual symptoms. Women with hypertension in association with new-onset persistent headaches and/or visual changes should be suspected to have severe PE. Patients who have hypertension with seizure should be initially treated as having eclampsia and should receive brain imaging to rule out other etiologies. Magnesium sulfate therapy must be initiated promptly for seizure prophylaxis and/or treatment. In addition, intravenous antihypertensive medications are recommended to lower BP to the desired goal while considering an alternative cause for the cerebral symptoms.
Women presenting with hypertension in association with refractory and/or thunderclap headaches, visual disturbances, or neurologic deficits should be evaluated for possible cerebrovascular complications such as reversible cerebral vasoconstriction syndrome (RCVS), cerebral venous thrombosis, or stroke. These women will require selective diagnostic neuroimaging and consultation with neurology and/or neurosurgery. Such an evaluation may include CT scan for hemorrhage, MRI for detection of vasogenic edema and/or ischemia or infarction, cerebral angiography for diagnosis of RCVS, and cerebral venography for detection of cerebral venous thrombosis. Subsequent treatment will depend on the etiology.
Severe new-onset epigastric/right upper quadrant pain with nausea and vomiting. Women with persistent nausea, vomiting, or epigastric pain should be evaluated for HELLP syndrome because up to 30% who develop the syndrome do so post partum. The time of onset of clinical and laboratory findings ranges from 1 to 7 days post partum. Women are managed as they are before delivery, with the use of magnesium sulfate, antihypertensives, and close monitoring of vital signs and laboratory values.
In general, patients with HELLP syndrome will demonstrate an improvement in clinical and laboratory findings within 72 hours after treatment. If there is either no improvement or a deterioration in these findings, then it is important to consult with appropriate specialists for evaluation and subsequent management of possible rare syndromes such as acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, or exacerbation of lupus.
Severe new-onset shortness of breath, dyspnea, orthopnea, or palpitations. Women with these symptoms in the postpartum period should be evaluated for possible pulmonary edema, pulmonary embolism, or peripartum cardiomyopathy. Women with postpartum hypertension are at risk for pulmonary edema with onset at 3-6 days after delivery. Diagnosis is confirmed by physical exam (tachycardia, tachypnea), presence of rales on lung exam, pulse oximetry (oxygen saturation less than 93%), and chest x-ray, and echocardiography to exclude other etiologies. Treatment of pulmonary edema includes oxygen supplementation, 40 mg IV furosemide, control of severe hypertension, fluid restriction, and supportive care.
Pulmonary embolism usually is confirmed by chest CT angiography and managed with therapeutic anticoagulation. Peripartum cardiomyopathy is diagnosed by echocardiography revealing left ventricular systolic dysfunction (ejection fraction less than 45%, dilated left ventricle). Treatment includes IV furosemide, use of a vasodilator, and ACE inhibitor therapy.
Remote prognosis
Recent research suggests that women who develop PE may be at increased risk for future cardiovascular disease such as heart failure, coronary artery disease, and stroke later in life. Indeed, many of the risk factors and pathophysiologic abnormalities of PE are similar to those of coronary artery disease.
The American College of Obstetricians and Gynecologists and the American Heart Association recommend that women with PE receive close observation in the postpartum period and careful evaluation in the first year after delivery to identify those who could benefit from early intervention to prevent subsequent cardiovascular disease. In general, when pregnancies are complicated by PE, there are opportunities for lifestyle and risk factor modification.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston
Swedish strategies improve survival for premature infants
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
The wide variation in outcomes for preterm birth worldwide raise questions as to whether the success seen in Sweden is possible in other countries, Matthew A. Rysavy, MD, PhD, and Danielle E. Y. Ehret, MD, MPH, wrote in an accompanying editorial. Considerations include population demographics, current guidelines, gestational age at which intensive care is offered, and the nature of follow-up care.
Sweden already has low rates of perinatal mortality, and current guidelines call for use of antenatal corticosteroids for births at 22 weeks’ gestation. By contrast, in the United States, antenatal corticosteroids are not recommended until 23-24 weeks’ gestation, they noted.
The editorialists called particular attention to the improvements in survival at 1 year and survival without major morbidity at 22-23 weeks’ gestation. “At 22 weeks’ gestation, the stillbirth rate decreased from 65% of all births during 2004-2007 to 35% during 2014-2016, with a reciprocal increase in live births.”
Although the results are promising, and show the possibilities for improving survival, the editorialists wrote that ongoing follow up of children born prematurely is essential to inform future research, as “much remains unknown about the later-life effects of being born so early and of the therapies used to sustain life after birth.”
Dr. Rysavy is affiliated with the department of pediatrics at the University of Iowa, Iowa City; Dr. Ehret is affiliated with the department of pediatrics at the University of Vermont, Burlington. This is a summary of their editorial accompanying the article by Norman et al. (JAMA. 2019 Mar 26;321:1163-64). They reported no financial conflicts.
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
based on data from a study of two cohorts including 2,205 births at 22-26 weeks’ gestational age.
The impact of the recommendations has not been well studied, wrote Mikael Norman, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues in JAMA. To determine the impact, the researchers compared data from 1,009 births at 22-26 weeks’ gestational age during 2004-2007 with 1,196 births at 22-26 weeks’ gestational age during 2014-2016, after the implementation of guidelines on “centralization of care, antenatal corticosteroid treatment, mode of delivery, a neonatologist attending at the birth, and resuscitation of infants delivered at 22, 23, and 24 weeks’ gestational age.”
The 1-year survival increased from 70% during 2004-2007 to 77% during 2014-2016; a significant improvement (P = .003).
In addition, 1-year survival with no major morbidity improved significantly between the two time periods, from 32% to 38%, respectively (P = .008).
The mothers and infants were part of EXPRESS (Extremely Preterm Infants in Sweden Study), a national, population-based, prospective cohort study. In most cases, gestational age was determined by routine antenatal ultrasound early in the second trimester, or by date of embryo transfer in cases of in vitro fertilization. The primary outcome was survival at the age of 1 year; the secondary outcome was survival at 1 year with no major neonatal comorbidity.
Among premature infants who survived at 1 year, some conditions were significantly more prevalent in the earlier birth cohort, compared with the later cohort, notably cystic periventricular leukomalacia (6% vs. 2%), any bronchopulmonary dysplasia (73% vs. 62%), and severe bronchopulmonary dysplasia (25% vs. 14%).
Although the proportion of premature births with a neonatologist attending was similar between the two cohorts, significantly more premature infants were born outside of university hospitals and transported to a level III neonatal ICU after birth during 2004-2007, compared with 2014-2016, the researchers noted.
The study findings were limited by several factors including the retrospective design of the second cohort, inability to account for fetal losses prior to 22 weeks’ gestational age, lack of data on the causes of fetal and infant deaths, potentially unknown confounding variables that impacted infant survival, and the small sample size of some gestational age groups, the researchers noted. However, the results show improvements in 1-year survival in the wake of specific guidelines on perinatal management.
Dr. Norman reported receiving grants from the Swedish Heart Lung Foundation and the H2020/European Union, as well as personal fees from a Swedish medical journal, the Swedish patient insurance, Liber, Studentlitteratur, and AbbVie. The study was funded by the Swedish Order of Freemasons’ Foundation for Children’s Welfare.
SOURCE: Norman M et al. JAMA. 2019;321:1188-99.
FROM JAMA
Algorithm ruled out PE, averts radiation exposure in pregnant women
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
FROM The New England Journal of Medicine
No birth rate gains from levothyroxine in pregnancy
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Treatment with levothyroxine does not improve the live birth rate in women with thyroid peroxidase antibodies before conception, according to data presented at the annual meeting of the Endocrine Society.
Until now, the evidence for the use of levothyroxine in pregnant women with thyroid peroxidase antibodies but normal thyroid function has been inconclusive, Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and her coauthors said in a paper published simultaneously with the meeting presentation March 23 in the New England Journal of Medicine.
Previous studies have shown that women with thyroid peroxidase antibodies but normal thyroid function have a nearly fourfold higher risk of miscarriage and twofold higher risk of preterm birth, compared with women who don’t have the antibodies.
In the new double-blind study, 952 women with thyroid peroxidase antibodies, normal thyroid function, and a history of miscarriage or infertility were randomized either to daily 50 mcg levothyroxine or placebo, taken from conception to the end of pregnancy.
The rate of pregnancy was similar in the levothyroxine and placebo groups (56.6% vs. 58.3%, respectively), as was the live birth rate (37.4% vs. 37.9%), despite the observation that the levothyroxine group had consistently lower serum thyrotropin and higher free T4 concentrations than did the placebo group.
There were also no significant differences between the two groups in secondary outcomes of miscarriage, preterm birth, or neonatal outcomes such as birth weight.
Researchers also saw no statistically significant differences in the rate of serious adverse events or in the number of women who showed abnormal results on thyroid function tests.
The authors noted that the dosage of levothyroxine used in the study was fixed, leaving the possibility that “the dose may need to be adjusted depending on the participant’s body weight, thyroid peroxidase antibody level, or thyrotropin concentration.”
Existing guidelines from the American Thyroid Association acknowledge the lack of evidence in favor of levothyroxine decreasing the risk of pregnancy loss. However, the guidelines also state that it can be considered in antibody-positive, euthyroid women with a history of loss, “given its potential benefits in comparison with its minimal risk.”
The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
SOURCE: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
FROM ENDO 2019
Key clinical point:
Major finding: Pregnancy rates and outcomes were similar in women treated with levothyroxine and those treated with placebo.
Study details: Double-blind, randomized, placebo-controlled trial in 952 women.
Disclosures: The study was supported by the National Institute for Health Research. No conflicts of interest were declared.
Source: Dhillon-Smith R et al. N Engl J Med. 2019 March 23. doi: 10.1056/NEJMoa1812537
Postcesarean pain relief better on nonopioid regimen
LAS VEGAS – Women who had cesarean delivery and received a nonopioid pain control regimen at hospital discharge had lower pain scores by 4 weeks post partum than those who also received opioids, according to study results shared during a fellows session at the meeting presented by the Society for Maternal-Fetal Medicine.
At 2-4 weeks post partum, the mean pain score on a visual analog scale (VAS) was 12/100 mm for women on the nonopioid regimen, compared with 16/100 mm for women who received opioids, using an intention-to-treat analysis. The median pain score for those in the nonopioid arm was 0, compared with 6 for those in the opioid arm.
The findings surprised Jenifer Dinis, MD, a maternal-fetal medicine fellow at the University of Texas, Houston, and her collaborators, because they had hypothesized merely that the two groups would have similar pain scores 2-4 weeks after delivery.
Although women in the nonopioid arm were able to obtain a rescue hydrocodone prescription through the study, and some women obtained opioids from their private physician, they still used less than half as much opioid medication as women in the opioid arm (21 versus 43 morphine milligram equivalents, P less than .01).
However, women in the nonopioid arm did not use significantly more ibuprofen or acetaminophen, and there was no difference in patient satisfaction with the outpatient postpartum analgesic regimen between study arms. Somnolence was more common in the opioid arm (P = .03); no other medication side effects were significantly more common in one group than the other.
Overall, 22 of 76 (29%) women in the nonopioid arm took any opioids after discharge, compared with 59/81 (73%) in the opioid arm (P less than .01).
After cesarean delivery, the 170 participating women had an inpatient pain control regimen determined by their primary ob.gyn., Dr. Dinis said in her presentation. Patients were randomized 1:1 to their outpatient analgesia regimens on postoperative day 2 or 3, with appropriate prescriptions placed in patient charts. Participants received either a nonopioid regimen with prescriptions for 60 ibuprofen tablets (600 mg) and 60 acetaminophen tablets (325 mg), or to an opioid regimen that included ibuprofen plus hydrocodone/acetaminophen 5 (325 mg) 1-2 tablets every 4 hours.
Pain scores were assessed between 2 and 4 weeks after delivery, either at an in-person appointment or by means of a phone call and a provided email link.
The single-site study was designed as a parallel-group equivalence trial, to show noninferiority of one pain control regimen over the other. Women between the ages of 18 and 50 years were included if they had a cesarean delivery; both English- and Spanish-speaking women were enrolled.
Allowing for attrition and crossover, Dr. Dinis and her colleagues enrolled 85 patients per study arm to achieve sufficient statistical power to detect the difference needed. The investigators planned both an intention-to-treat and a per-protocol analysis in their registered clinical trial.
Postpartum pain assessments were not obtained for 12 patients in the nonopioid group, and 9 in the opioid group, leaving 73 and 76 patients in each group for the per-protocol analysis, respectively.
At baseline, patients were a mean 28 years old, and a little over a quarter (28%) were nulliparous. Participants were overall about half African American and 34%-40% Hispanic. Over half (62%-72%) received Medicaid; most women (62%-75%) had body mass indices of 30 kg/m2 or more.
The mean gestational age at delivery was a little more than 36 weeks, with about half of deliveries being the participant’s first cesarean delivery. About 90% of women had a Pfannenstiel skin incision, with a low transverse uterine incision.
Patients were aware of their allocation, and the study results aren’t applicable to women with opioid or benzodiazepine use disorder, she noted. However, the study was pragmatic, included all types of cesarean deliveries, and was adequately powered to detect “the smallest clinically significant difference.”
Dr. Dinis reported no outside sources of funding and no conflicts of interest.
SOURCE: Dinis J et al. Am J Obstet Gynecol. 2019 Jan;220(1):S34, Abstract 42.
LAS VEGAS – Women who had cesarean delivery and received a nonopioid pain control regimen at hospital discharge had lower pain scores by 4 weeks post partum than those who also received opioids, according to study results shared during a fellows session at the meeting presented by the Society for Maternal-Fetal Medicine.
At 2-4 weeks post partum, the mean pain score on a visual analog scale (VAS) was 12/100 mm for women on the nonopioid regimen, compared with 16/100 mm for women who received opioids, using an intention-to-treat analysis. The median pain score for those in the nonopioid arm was 0, compared with 6 for those in the opioid arm.
The findings surprised Jenifer Dinis, MD, a maternal-fetal medicine fellow at the University of Texas, Houston, and her collaborators, because they had hypothesized merely that the two groups would have similar pain scores 2-4 weeks after delivery.
Although women in the nonopioid arm were able to obtain a rescue hydrocodone prescription through the study, and some women obtained opioids from their private physician, they still used less than half as much opioid medication as women in the opioid arm (21 versus 43 morphine milligram equivalents, P less than .01).
However, women in the nonopioid arm did not use significantly more ibuprofen or acetaminophen, and there was no difference in patient satisfaction with the outpatient postpartum analgesic regimen between study arms. Somnolence was more common in the opioid arm (P = .03); no other medication side effects were significantly more common in one group than the other.
Overall, 22 of 76 (29%) women in the nonopioid arm took any opioids after discharge, compared with 59/81 (73%) in the opioid arm (P less than .01).
After cesarean delivery, the 170 participating women had an inpatient pain control regimen determined by their primary ob.gyn., Dr. Dinis said in her presentation. Patients were randomized 1:1 to their outpatient analgesia regimens on postoperative day 2 or 3, with appropriate prescriptions placed in patient charts. Participants received either a nonopioid regimen with prescriptions for 60 ibuprofen tablets (600 mg) and 60 acetaminophen tablets (325 mg), or to an opioid regimen that included ibuprofen plus hydrocodone/acetaminophen 5 (325 mg) 1-2 tablets every 4 hours.
Pain scores were assessed between 2 and 4 weeks after delivery, either at an in-person appointment or by means of a phone call and a provided email link.
The single-site study was designed as a parallel-group equivalence trial, to show noninferiority of one pain control regimen over the other. Women between the ages of 18 and 50 years were included if they had a cesarean delivery; both English- and Spanish-speaking women were enrolled.
Allowing for attrition and crossover, Dr. Dinis and her colleagues enrolled 85 patients per study arm to achieve sufficient statistical power to detect the difference needed. The investigators planned both an intention-to-treat and a per-protocol analysis in their registered clinical trial.
Postpartum pain assessments were not obtained for 12 patients in the nonopioid group, and 9 in the opioid group, leaving 73 and 76 patients in each group for the per-protocol analysis, respectively.
At baseline, patients were a mean 28 years old, and a little over a quarter (28%) were nulliparous. Participants were overall about half African American and 34%-40% Hispanic. Over half (62%-72%) received Medicaid; most women (62%-75%) had body mass indices of 30 kg/m2 or more.
The mean gestational age at delivery was a little more than 36 weeks, with about half of deliveries being the participant’s first cesarean delivery. About 90% of women had a Pfannenstiel skin incision, with a low transverse uterine incision.
Patients were aware of their allocation, and the study results aren’t applicable to women with opioid or benzodiazepine use disorder, she noted. However, the study was pragmatic, included all types of cesarean deliveries, and was adequately powered to detect “the smallest clinically significant difference.”
Dr. Dinis reported no outside sources of funding and no conflicts of interest.
SOURCE: Dinis J et al. Am J Obstet Gynecol. 2019 Jan;220(1):S34, Abstract 42.
LAS VEGAS – Women who had cesarean delivery and received a nonopioid pain control regimen at hospital discharge had lower pain scores by 4 weeks post partum than those who also received opioids, according to study results shared during a fellows session at the meeting presented by the Society for Maternal-Fetal Medicine.
At 2-4 weeks post partum, the mean pain score on a visual analog scale (VAS) was 12/100 mm for women on the nonopioid regimen, compared with 16/100 mm for women who received opioids, using an intention-to-treat analysis. The median pain score for those in the nonopioid arm was 0, compared with 6 for those in the opioid arm.
The findings surprised Jenifer Dinis, MD, a maternal-fetal medicine fellow at the University of Texas, Houston, and her collaborators, because they had hypothesized merely that the two groups would have similar pain scores 2-4 weeks after delivery.
Although women in the nonopioid arm were able to obtain a rescue hydrocodone prescription through the study, and some women obtained opioids from their private physician, they still used less than half as much opioid medication as women in the opioid arm (21 versus 43 morphine milligram equivalents, P less than .01).
However, women in the nonopioid arm did not use significantly more ibuprofen or acetaminophen, and there was no difference in patient satisfaction with the outpatient postpartum analgesic regimen between study arms. Somnolence was more common in the opioid arm (P = .03); no other medication side effects were significantly more common in one group than the other.
Overall, 22 of 76 (29%) women in the nonopioid arm took any opioids after discharge, compared with 59/81 (73%) in the opioid arm (P less than .01).
After cesarean delivery, the 170 participating women had an inpatient pain control regimen determined by their primary ob.gyn., Dr. Dinis said in her presentation. Patients were randomized 1:1 to their outpatient analgesia regimens on postoperative day 2 or 3, with appropriate prescriptions placed in patient charts. Participants received either a nonopioid regimen with prescriptions for 60 ibuprofen tablets (600 mg) and 60 acetaminophen tablets (325 mg), or to an opioid regimen that included ibuprofen plus hydrocodone/acetaminophen 5 (325 mg) 1-2 tablets every 4 hours.
Pain scores were assessed between 2 and 4 weeks after delivery, either at an in-person appointment or by means of a phone call and a provided email link.
The single-site study was designed as a parallel-group equivalence trial, to show noninferiority of one pain control regimen over the other. Women between the ages of 18 and 50 years were included if they had a cesarean delivery; both English- and Spanish-speaking women were enrolled.
Allowing for attrition and crossover, Dr. Dinis and her colleagues enrolled 85 patients per study arm to achieve sufficient statistical power to detect the difference needed. The investigators planned both an intention-to-treat and a per-protocol analysis in their registered clinical trial.
Postpartum pain assessments were not obtained for 12 patients in the nonopioid group, and 9 in the opioid group, leaving 73 and 76 patients in each group for the per-protocol analysis, respectively.
At baseline, patients were a mean 28 years old, and a little over a quarter (28%) were nulliparous. Participants were overall about half African American and 34%-40% Hispanic. Over half (62%-72%) received Medicaid; most women (62%-75%) had body mass indices of 30 kg/m2 or more.
The mean gestational age at delivery was a little more than 36 weeks, with about half of deliveries being the participant’s first cesarean delivery. About 90% of women had a Pfannenstiel skin incision, with a low transverse uterine incision.
Patients were aware of their allocation, and the study results aren’t applicable to women with opioid or benzodiazepine use disorder, she noted. However, the study was pragmatic, included all types of cesarean deliveries, and was adequately powered to detect “the smallest clinically significant difference.”
Dr. Dinis reported no outside sources of funding and no conflicts of interest.
SOURCE: Dinis J et al. Am J Obstet Gynecol. 2019 Jan;220(1):S34, Abstract 42.
REPORTING FROM THE PREGNANCY MEETING
Successful external cephalic version more likely in taller, leaner women
LAS VEGAS – according to data shared in a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Race/ethnicity also had an impact on the likelihood that external cephalic version (ECV) would be successful.
In an interview, first author Ashley E. Skeith, a medical student at Oregon Health and Sciences University, Portland, said that various characteristics of a pregnancy may affect the success of ECV, but it wasn’t known which maternal characteristics might be associated with greater success of the maneuver.
She and her collaborators found that rates of success were high overall, but that 84% of women 68 inches or taller had successful ECVs, compared with 78% of women less than 60 inches tall. Rates were 82% and 83% for women 60-64 inches and 64-68 inches tall, respectively (adjusted odds ratio, 1.03; P less than .001).
The retrospective cohort study used data from 18,896 women who had singleton, breech, term gestations for whom ECV was attempted. Variables extracted from the medical record included maternal age, height, race, and prepregnancy body mass index (BMI).
For analysis, maternal BMI was grouped into four categories: underweight (BMI, less than 18.5 kg/m2), normal weight (BMI, 18.5-24.9 kg/m2), overweight (BMI, 25-29 kg/m2), and obese (BMI, greater than 30 kg/m2).
Women who were normal weight had the highest likelihood of a successful ECV, at 86%, followed by underweight women at 85%. Women who were overweight and obese had lower success rates, at 82% and 78%, respectively (aOR, 0.86; P less than .001).
Compared with white women, black women had an aOR of 0.60 for successful ECV (P less than .001). The aOR for successful ECV for Asian women was 0.71; for Hispanic women, the aOR was 0.82. American Indian and Alaska Native women were slightly more likely to have successful ECV than white women, but the difference was not significant after statistical adjustment.
Neither advanced maternal age (greater than 35 years) nor adolescent pregnancy were associated with decreased likelihood of successful ECV.
Potential confounders included maternal education level and insurance status, how much weight was gained during pregnancy, whether an epidural was administered, and whether the mother had diabetes. Multivariable regression analysis adjusted for these variables, said Ms. Skeith.
“External cephalic version is a safe procedure that reduces risk of cesarean delivery,” wrote Ms. Skeith and her colleagues. “Though fetal positioning and analgesia have been considered in the prediction of ECV success, [these] data [suggest] that maternal stature and race/ethnicity could be incorporated into potential prediction tools.”
Ms. Skeith reported no outside sources of funding or conflicts of interest.
SOURCE: Skeith AE et al. Am J Obstet Gynecol. 2019 Jan;220(1):S445-7, Abstract 674.
LAS VEGAS – according to data shared in a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Race/ethnicity also had an impact on the likelihood that external cephalic version (ECV) would be successful.
In an interview, first author Ashley E. Skeith, a medical student at Oregon Health and Sciences University, Portland, said that various characteristics of a pregnancy may affect the success of ECV, but it wasn’t known which maternal characteristics might be associated with greater success of the maneuver.
She and her collaborators found that rates of success were high overall, but that 84% of women 68 inches or taller had successful ECVs, compared with 78% of women less than 60 inches tall. Rates were 82% and 83% for women 60-64 inches and 64-68 inches tall, respectively (adjusted odds ratio, 1.03; P less than .001).
The retrospective cohort study used data from 18,896 women who had singleton, breech, term gestations for whom ECV was attempted. Variables extracted from the medical record included maternal age, height, race, and prepregnancy body mass index (BMI).
For analysis, maternal BMI was grouped into four categories: underweight (BMI, less than 18.5 kg/m2), normal weight (BMI, 18.5-24.9 kg/m2), overweight (BMI, 25-29 kg/m2), and obese (BMI, greater than 30 kg/m2).
Women who were normal weight had the highest likelihood of a successful ECV, at 86%, followed by underweight women at 85%. Women who were overweight and obese had lower success rates, at 82% and 78%, respectively (aOR, 0.86; P less than .001).
Compared with white women, black women had an aOR of 0.60 for successful ECV (P less than .001). The aOR for successful ECV for Asian women was 0.71; for Hispanic women, the aOR was 0.82. American Indian and Alaska Native women were slightly more likely to have successful ECV than white women, but the difference was not significant after statistical adjustment.
Neither advanced maternal age (greater than 35 years) nor adolescent pregnancy were associated with decreased likelihood of successful ECV.
Potential confounders included maternal education level and insurance status, how much weight was gained during pregnancy, whether an epidural was administered, and whether the mother had diabetes. Multivariable regression analysis adjusted for these variables, said Ms. Skeith.
“External cephalic version is a safe procedure that reduces risk of cesarean delivery,” wrote Ms. Skeith and her colleagues. “Though fetal positioning and analgesia have been considered in the prediction of ECV success, [these] data [suggest] that maternal stature and race/ethnicity could be incorporated into potential prediction tools.”
Ms. Skeith reported no outside sources of funding or conflicts of interest.
SOURCE: Skeith AE et al. Am J Obstet Gynecol. 2019 Jan;220(1):S445-7, Abstract 674.
LAS VEGAS – according to data shared in a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Race/ethnicity also had an impact on the likelihood that external cephalic version (ECV) would be successful.
In an interview, first author Ashley E. Skeith, a medical student at Oregon Health and Sciences University, Portland, said that various characteristics of a pregnancy may affect the success of ECV, but it wasn’t known which maternal characteristics might be associated with greater success of the maneuver.
She and her collaborators found that rates of success were high overall, but that 84% of women 68 inches or taller had successful ECVs, compared with 78% of women less than 60 inches tall. Rates were 82% and 83% for women 60-64 inches and 64-68 inches tall, respectively (adjusted odds ratio, 1.03; P less than .001).
The retrospective cohort study used data from 18,896 women who had singleton, breech, term gestations for whom ECV was attempted. Variables extracted from the medical record included maternal age, height, race, and prepregnancy body mass index (BMI).
For analysis, maternal BMI was grouped into four categories: underweight (BMI, less than 18.5 kg/m2), normal weight (BMI, 18.5-24.9 kg/m2), overweight (BMI, 25-29 kg/m2), and obese (BMI, greater than 30 kg/m2).
Women who were normal weight had the highest likelihood of a successful ECV, at 86%, followed by underweight women at 85%. Women who were overweight and obese had lower success rates, at 82% and 78%, respectively (aOR, 0.86; P less than .001).
Compared with white women, black women had an aOR of 0.60 for successful ECV (P less than .001). The aOR for successful ECV for Asian women was 0.71; for Hispanic women, the aOR was 0.82. American Indian and Alaska Native women were slightly more likely to have successful ECV than white women, but the difference was not significant after statistical adjustment.
Neither advanced maternal age (greater than 35 years) nor adolescent pregnancy were associated with decreased likelihood of successful ECV.
Potential confounders included maternal education level and insurance status, how much weight was gained during pregnancy, whether an epidural was administered, and whether the mother had diabetes. Multivariable regression analysis adjusted for these variables, said Ms. Skeith.
“External cephalic version is a safe procedure that reduces risk of cesarean delivery,” wrote Ms. Skeith and her colleagues. “Though fetal positioning and analgesia have been considered in the prediction of ECV success, [these] data [suggest] that maternal stature and race/ethnicity could be incorporated into potential prediction tools.”
Ms. Skeith reported no outside sources of funding or conflicts of interest.
SOURCE: Skeith AE et al. Am J Obstet Gynecol. 2019 Jan;220(1):S445-7, Abstract 674.
REPORTING FROM THE PREGNANCY MEETING