User login
For MD-IQ use only
New year, new hopes
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Finding common purpose, or else
I am composing this editorial 4 days after the U.S. Capitol was invaded and 10 days before the presidential inauguration. It is impossible to ignore what is happening in our country, but I hesitate to add my thoughts to the overwhelming sea of opinions circulating in standard media, social media, and the dark web. I hope, as do many, that we return to a civil discourse, recognize the voices of all people, respect each other, and return to a belief in science and facts.
SARS-CoV-2 has devastated the world and will continue to cause preventable deaths until we adopt stricter mitigation measures, vaccinate most people, and develop widespread immunity. We are gaining immense knowledge about this virus, and as gastroenterologists, we are on the front lines in many aspects. A recent article in American Journal of Gastroenterology, among others, emphasized that mild GI symptoms may be the only presenting complaint for people with COVID-19. Responses to COVID-19, such as limits on elective procedures and social distancing, have upended our endoscopic processes and even altered the business models of GI practice. We will never go back to pre-COVID models.
The front page of this month’s GI & Hepatology News features important articles for our practice. One article delves into an extensive guideline from the American Gastroenterological Association on medical management of colonic diverticulitis. In another article, they also describe how efforts to encourage our patients with nonalcoholic fatty liver disease to exercise and manage their diet can make a real difference in their health. Finally, another explores how and why your immunocompromised patients (including those with inflammatory bowel disease) should and can be safely vaccinated for COVID-19.
Meanwhile, we need civility, science, and community. Without common purpose, we will experience the William Forster Lloyd’s Tragedy of the Commons. Incivility has economic and emotional costs, according to the Harvard Business Review. “Weathering,” the deterioration of Black women’s health over time that’s related to continued socioeconomic disadvantage, has multigenerational impacts; for example the Department of Health & Human Services reports that infant mortality among African American women is 2.3 times that of non-Hispanic Whites. Late effects of redlining continue to cause economic, health, and emotional harms (Badger E. “How Redlining’s Racist Effects Lasted for Decades” The New York Times. 2017 Aug 24).
“If Men were angels, no government would be necessary,” James Madison wrote. “In framing a government which is to be administered by men over men, the great difficulty lies in this: you must first enable the government to control the governed; and the next place, oblige it to control itself.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
I am composing this editorial 4 days after the U.S. Capitol was invaded and 10 days before the presidential inauguration. It is impossible to ignore what is happening in our country, but I hesitate to add my thoughts to the overwhelming sea of opinions circulating in standard media, social media, and the dark web. I hope, as do many, that we return to a civil discourse, recognize the voices of all people, respect each other, and return to a belief in science and facts.
SARS-CoV-2 has devastated the world and will continue to cause preventable deaths until we adopt stricter mitigation measures, vaccinate most people, and develop widespread immunity. We are gaining immense knowledge about this virus, and as gastroenterologists, we are on the front lines in many aspects. A recent article in American Journal of Gastroenterology, among others, emphasized that mild GI symptoms may be the only presenting complaint for people with COVID-19. Responses to COVID-19, such as limits on elective procedures and social distancing, have upended our endoscopic processes and even altered the business models of GI practice. We will never go back to pre-COVID models.
The front page of this month’s GI & Hepatology News features important articles for our practice. One article delves into an extensive guideline from the American Gastroenterological Association on medical management of colonic diverticulitis. In another article, they also describe how efforts to encourage our patients with nonalcoholic fatty liver disease to exercise and manage their diet can make a real difference in their health. Finally, another explores how and why your immunocompromised patients (including those with inflammatory bowel disease) should and can be safely vaccinated for COVID-19.
Meanwhile, we need civility, science, and community. Without common purpose, we will experience the William Forster Lloyd’s Tragedy of the Commons. Incivility has economic and emotional costs, according to the Harvard Business Review. “Weathering,” the deterioration of Black women’s health over time that’s related to continued socioeconomic disadvantage, has multigenerational impacts; for example the Department of Health & Human Services reports that infant mortality among African American women is 2.3 times that of non-Hispanic Whites. Late effects of redlining continue to cause economic, health, and emotional harms (Badger E. “How Redlining’s Racist Effects Lasted for Decades” The New York Times. 2017 Aug 24).
“If Men were angels, no government would be necessary,” James Madison wrote. “In framing a government which is to be administered by men over men, the great difficulty lies in this: you must first enable the government to control the governed; and the next place, oblige it to control itself.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
I am composing this editorial 4 days after the U.S. Capitol was invaded and 10 days before the presidential inauguration. It is impossible to ignore what is happening in our country, but I hesitate to add my thoughts to the overwhelming sea of opinions circulating in standard media, social media, and the dark web. I hope, as do many, that we return to a civil discourse, recognize the voices of all people, respect each other, and return to a belief in science and facts.
SARS-CoV-2 has devastated the world and will continue to cause preventable deaths until we adopt stricter mitigation measures, vaccinate most people, and develop widespread immunity. We are gaining immense knowledge about this virus, and as gastroenterologists, we are on the front lines in many aspects. A recent article in American Journal of Gastroenterology, among others, emphasized that mild GI symptoms may be the only presenting complaint for people with COVID-19. Responses to COVID-19, such as limits on elective procedures and social distancing, have upended our endoscopic processes and even altered the business models of GI practice. We will never go back to pre-COVID models.
The front page of this month’s GI & Hepatology News features important articles for our practice. One article delves into an extensive guideline from the American Gastroenterological Association on medical management of colonic diverticulitis. In another article, they also describe how efforts to encourage our patients with nonalcoholic fatty liver disease to exercise and manage their diet can make a real difference in their health. Finally, another explores how and why your immunocompromised patients (including those with inflammatory bowel disease) should and can be safely vaccinated for COVID-19.
Meanwhile, we need civility, science, and community. Without common purpose, we will experience the William Forster Lloyd’s Tragedy of the Commons. Incivility has economic and emotional costs, according to the Harvard Business Review. “Weathering,” the deterioration of Black women’s health over time that’s related to continued socioeconomic disadvantage, has multigenerational impacts; for example the Department of Health & Human Services reports that infant mortality among African American women is 2.3 times that of non-Hispanic Whites. Late effects of redlining continue to cause economic, health, and emotional harms (Badger E. “How Redlining’s Racist Effects Lasted for Decades” The New York Times. 2017 Aug 24).
“If Men were angels, no government would be necessary,” James Madison wrote. “In framing a government which is to be administered by men over men, the great difficulty lies in this: you must first enable the government to control the governed; and the next place, oblige it to control itself.”
John I. Allen, MD, MBA, AGAF
Editor in Chief
Update on feeding tubes: Indications and troubleshooting complications
Introduction
Gastroenterologists are in a unique position to manage individuals with feeding tubes as their training underscores principles in digestion, absorption, nutrition support, and enteral tube placement. Adequate management of individuals with feeding tubes and, importantly, the complications that arise from feeding tube use and placement require a basic understanding of intestinal anatomy and physiology. Therefore, gastroenterologists are well suited to both place and manage individuals with feeding tubes in the long term.
Indications for tube feeding
When deciding on the appropriate route for artificial nutrition support, the first decision to be made is enteral access versus parenteral nutrition support. Enteral nutrition confers multiple benefits, including preservation of the mucosal lining, reductions in complicated infections, decreased costs, and improved patient compliance. All attempts at adequate enteral access should be made before deciding on the use of parenteral nutrition. Following the clinical decision to pursue artificial means of nutrition support and enteral access, the next common decision is the anticipated duration of nutrition support. Generally, the oral or nasal tubes are used for short durations (i.e., less than 4 weeks) with percutaneous placement into the stomach or small intestine for longer-term feeding (i.e., percutaneous endoscopic gastrostomy [PEG] or percutaneous endoscopic jejunostomy [PEJ]).
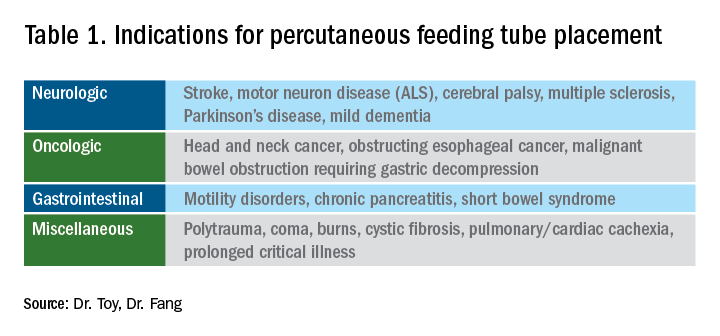
The most general indication for nutrition support is an inability to maintain adequate nutritional needs with oral intake alone. General categories of inadequate oral intake include neurologic disorders, malignancy, and gastrointestinal conditions affecting digestion and absorption (Table 1). Absolute and relative contraindications to PEG placement are listed in Table 2. If an endoscopic placement is not possible, alternative means of placement (i.e., surgery or interventional radiology) can be considered to avoid the consequences of prolonged malnutrition. In-hospital mortality following PEG placement has decreased 40% over the last 10 years, which can be attributed to improved patient selection, enhanced discharge practices, and exclusion of patients with the highest comorbidity and mortality rates, like those with advanced dementia or terminal cancer.1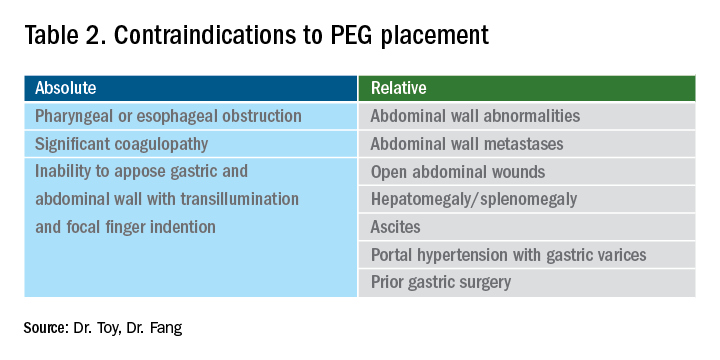
PEG placement in patients with dementia is controversial, with previous studies not demonstrating improved outcomes and association with high mortality rates,2 so the practice is currently not recommended by the American Geriatrics Society in individuals with advanced dementia.3 However, a large Japanese study showed that careful selection of patients with mild dementia to undergo gastrostomy increased independence fourfold; therefore, multidisciplinary involvement is often necessary in the decision to pursue artificial means of nutrition support in this population.4
The recent coronavirus disease 2019 (COVID-19) pandemic has placed additional strains on endoscopic placement and has highlighted the effect of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) on GI symptoms. A recent meta-analysis showed an overall incidence of GI symptoms of 17.6% in the following conditions in decreasing order of prevalence: anorexia, diarrhea, nausea, vomiting, and abdominal discomfort.5 In addition, the prolonged ventilatory requirements among a subset of individuals with the most severe COVID-19 results in extended periods of nutrition support via enteral tube placements. In individuals with ICU-acquired weakness and discharge to long-term care facilities, the placement of percutaneous endoscopic tubes may be required, although with the additional consideration of the need for an aerosolizing procedure. Delay of placement has been advocated, in addition to appropriate personal protective equipment, in order to ensure safe placement for the endoscopy staff.6
Types of feeding tubes
After deciding to feed a patient enterally and determining the anticipated duration of enteral support, the next decision is to determine the most appropriate location of feeding delivery: into the stomach or the small bowel. Gastric feeding is advantageous most commonly because of its increased capacity, allowing for larger volumes to be delivered over shorter durations. However, in the setting of postsurgical anatomy, gastroparesis, or obstructing tumors/pancreatic inflammation, distal delivery of tube feeds may be required into the jejunum. Additionally, percutaneous tubes placed into the stomach can have extenders into the small bowel (GJ tubes) to allow for feeding into the small bowel and decompression or delivery of medications into the stomach.
In general, gastric feeding is preferred over small bowel feeding as PEG tubes are more stable and have fewer complications than either PEG-J or direct PEJ tubes. Gastrostomy tubes are generally shorter and larger in diameter making them less likely to clog. PEG-J tubes have separate lumens for gastric and small intestinal access, but the smaller-bore jejunal extension tubes are more likely to clog or become dislodged. While direct PEJ is shown to have higher rates of tube patency and decreased rates of endoscopic re-intervention, compared with PEG-J,7 one limitation of a direct PEJ is difficulty in placement and site selection, which can be performed with a pediatric colonoscope or balloon enteroscopy system. Most commonly, this procedure is performed under general anesthesia.
In the case of a critically ill patient in the ICU, it is recommended to start enteral nutrition within 24-48 hours of arrival to avoid complications of prolonged calorie deficits. Nasally inserted feeding tubes (e.g., Cortrak, Avanos Medical Devices, Alpharetta, Ga.) are most commonly used at the bedside and can be placed blindly using electromagnetic image guidance, radiographically, or endoscopy. However, the small caliber of nasoenteric tubes comes with the common complication of clogging, which can be overcome with slightly larger bore gastric feeding tubes. If gastric feeding is not tolerated (e.g., in the case of vomiting, witnessed aspiration), small bowel feeding should be initiated and can be a more durable form of enteral feeding with fewer interruptions as feedings do not need to be held for procedures or symptomatic gastric intolerance. In clinical areas of question, or if there is a concern for intolerance of enteral feeding, a short trial with nasogastric or nasojejunal tube placement should be performed before a more definitive percutaneous placement.
With respect to percutaneous tubes, important characteristics to choose are the size (diameter in French units), type of internal retention device, and external appearance of the tube (standard or low profile). All percutaneous tubes contain an external retention device (i.e., bumper) that fits against the skin and an internal retention device that is either a balloon or plastic dome or funnel that prevents the tube from becoming dislodged. Balloon retention tubes require replacement every 3-6 months, while nonballoon tubes generally require replacement annually in order to prevent the plastic from cracking, which can make removal complicated. Low-profile tubes have an external cap, which, when opened, allows for extension tubing to be securely attached while in use and detached while not in use. Low-profile tubes are often preferred among younger, active patients and those with adequate dexterity to allow for attachment of the external extension tubing. These tubes are most often inserted as a replacement for an initially endoscopically placed tube, although one-step systems for initial placement are available. The size of the low-profile tube is chosen based on the size of the existing PEG tube and by measuring the length of the stoma tract using specialized measuring devices.8 Patients and caregivers can also be trained to replace balloon-type tubes on their own to limit complications of displaced or cracked tubes. Low-profile tubes are commercially available for both gastric placement and gastric placement with extension into the small bowel, which often requires fluoroscopy for secure placement.
All percutaneous enteral tubes are being transitioned to the ENfit connector system, which prevents connections from the enteral system to nonenteral systems (namely intravenous lines, chest tubes) and vice versa. Tubing misconnections have been rarely reported, and the EnFIT system is designed to prevent such misadventures that have resulted in serious complications and even mortality.9 Adapter devices are available that may be required for patients with feeding tubes who have not been transitioned yet. Most commonly with new tube placements and replacements, patients and providers will have to become familiar with the new syringes and feeding bags required with EnFIT connectors.
Gastrostomy placement can be considered a higher-risk endoscopic procedure. One complicating factor is the increased use of antiplatelet and anticoagulant therapies in individuals with a history of neurologic insults. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend that coumadin be held 5 days before the procedure and bridged with heparin if the patient is at high risk of thromboembolic complications. For patients on dual anti-platelet therapy, thienopyridines like clopidogrel are often stopped 5-7 days prior to procedure with continuation of aspirin,10 but there are more recent data that PEG insertion is safe with continued use of DAPT.11 Direct-acting anticoagulants (DOACs) are often stopped 24-48 hours prior to procedure and then restarted 48 hours after tube placement, but this is dependent on the half-life of the specific DOAC and the patient’s renal function. Patients with decreased creatinine clearance may need to hold the DOAC up to 3-4 days prior to the procedure. In this situation, referring to ASGE guidelines and consultation with a hematologist or managing anti-coagulation clinic is advised.10
Troubleshooting complications
Nasoenteric tubes: One of the most common and irritating complications with nasoenteric feeding tubes is clogging. To prevent clogging, the tube should be flushed frequently.12 At least 30 mL of free water should be used to flush the tube every 4-8 hours for continuous feedings or before and after bolus feeding. Additionally, 15-30 mL of water should be given with each separate medication administration, and if possible, medication administration via small-bore small bowel feeding tubes should be avoided.12 Water flushing is especially important with small-caliber tubes and pumps that deliver both feeding and water flushes. It is available for small bowel feeding in order to allow for programmed water delivery.
Warm water flushes can also help unclog the tube,12 and additional pharmacologic and mechanical devices have been promoted for clogged tubes. One common technique is mixing pancreatic enzymes (Viokase) with a crushed 325-mg tablet of nonenteric coated sodium bicarbonate and 5 mL of water to create a solution that has the alkaline properties allowing for both pancreatic enzyme activation and clog dissolution. Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) catheter can be placed into longer feeding tubes to directly infuse the activated agent to the site of the clog.13 If water and enzymes are not successful in unclogging the tube, commercially available brushes can help remove clogs. The TubeClear® system (Actuated Medical, Bellefonte, Penna) has a single-use stem that is connected to AC power to create a jackhammerlike movement to remove clogs in longer nasoenteral and gastrojejunal tubes.
PEG tubes (short-term complications): Procedural and immediate postprocedural complications include bleeding, aspiration, pneumoperitoneum, and perforation. Pneumoperitoneum occurs in approximately 50% of cases and is generally clinically insignificant. The risk of pneumoperitoneum can be reduced by using CO2 insufflation.14 If the patient develops systemic signs of infection or peritoneal signs, CT scan with oral contrast is warranted for further evaluation and to assess for inadvertent perforation of overlying bowel or dislodged tube. Aspiration during or following endoscopy is another common complication of PEG placement and risk factors include over-sedation, supine positioning, advanced age, and neurologic dysfunction. This risk can be mitigated by avoiding over-sedation, immediately aspirating gastric contents when the stomach is reached, and avoiding excessive insufflation.15 In addition, elevating the head of the bed during the procedure and dedicating an assistant to perform oral suctioning during the entire procedure is recommended.
PEG tubes (long-term complications): More delayed complications of PEG insertion include wound infection, buried bumper syndrome, tumor seeding, peristomal leakage, and tube dislodgement. The prevalence of wound infection is 5%- 25%,16 and randomized controlled trials have demonstrated the efficacy of a single dose of an IV antibiotic (i.e., cephalosporin) in those not already receiving a broad spectrum antibiotic and administered prophylactically before tube placement.17 The significance of this reduction is such that antibiotic administration before tube placement should be considered a quality measure for the procedure. A small amount of redness around the tube site (less than 5 mm) is typical, but extension of erythema, warmth, tenderness, purulent drainage, or systemic symptoms is consistent with infection and warrants additional antibiotic administration. Minor infections can be treated with local antiseptics and oral antibiotics, and early intervention is important to prevent need for hospital admission, systemic antibiotics, and even surgical debridement.
Peristomal leakage is reported in approximately 1%-2% of patients.18 Photographs of the site can be very useful in evaluating and managing peristomal leakage and infections. Interventions include reducing gastric secretions with proton pump inhibitors and management of the skin with barrier creams, such as zinc oxide (Calmoseptine®) ointment. Placement of a larger-diameter tube only enlarges the stoma track and worsens the leakage. In such cases, thorough evaluations for delayed gastric emptying (gastroparesis), distal obstruction, or constipation should be performed and managed accordingly. Opiates are common contributors to constipation and delayed gastric emptying and often require reduction in use or directed antagonist therapy to reduce leaking. Continuous feeding over bolus feedings and delivering nutrition distally into the small bowel (PEG-J placement) can improve leaking from gastrostomy tubes. Additional means of management include stabilizing the tube by replacing a traditional tube with a low-profile tube or using right-angle external bumpers. If all measures fail, removing the tube and allowing for stomal closure can be attempted,16 although this option often requires parenteral nutrition support to prevent prolonged periods of inadequate nutrition.
Buried bumper syndrome (BBS) occurs in 1.5%-8.8% of PEG placements and is a common late complication of PEG placement, although early reports have been described.18 The development of BBS occurs when the internal bumper migrates from the gastric lumen through and into the stomach or abdominal wall. It occurs more frequently with solid nonballoon retention tubes and is caused by excessive compression of the external bumper against the skin and abdominal wall. Patients with BBS usually present with an immobile catheter, resistance with feeds (because of a closure of the stomach wall around the internal portion of the gastrostomy tube), abdominal pain, or peristomal leakage. Physicians should be aware of and assess tubes for BBS, in particular when replacing an immobile tube (cannot be pushed into the free stomach lumen) or when there is difficulty in flushing water into the tube. This complication can be easily prevented by allowing a minimum of 0.5-1.0 cm (1 finger breadth) between the external bumper and the abdominal wall. In particular, patients and caregivers should be warned that if the patient gains significant amounts of weight, the outer bumper will need to be loosened. Once BBS is diagnosed, the PEG tube requires removal and replacement as it can cause bleeding, infection, or fasciitis. The general steps to replacement include endoscopic removal of the existing tube and replacement of new PEG in the existing tract as long as the BBS is not severe. In most cases a replacement tube can be pulled into place using the pull-PEG technique at the same gastrostomy site as long as the stoma tract can be cannulated with a wire after the existing tube is removed.
Similar to nasoenteric tubes, PEG tubes can become clogged, although this complication is infrequent. The primary steps for prevention include adequately flushing with water before and after feeds and ensuring that all medications are liquid or well crushed and dissolved before instilling. Timely tube replacement also ensures that the internal portions of the gastrostomy tube remain free of debris. Management is similar to that of unclogging nasoenteral tubes, as discussed above, and specific commercial declogging devices for PEG tubes include the Bionix Declogger® (Bionix Development Corp., Toledo, Ohio) and the Bard® PEG cleaning brush (Bard Peripheral Vascular Inc., Tempe, Ariz.). The Bionix system has a plastic stem with a screw and thread design that will remove clogs in 14-24 French PEG tubes, while the Bard brush has a flexible nylon stem with soft bristles at the end to prevent mucosal injury and can be used for prophylaxis against clogs, as well as removing clogs themselves.12
Lastly, a rare but important complication of PEG placement is tumor seeding of the PEG site in patients with active head and neck or upper gastrointestinal cancer.19 The presumed mechanism is shearing of tumor cells as the PEG is pulled through the upper aerodigestive tract and through the wall of the stomach, as prior studies have demonstrated frequent seeding of tubes and incision sites as shown by brushing the tube for malignant cells after tube placement.20 It is important to recognize this complication and not misdiagnose it as granulation tissue, infection, or bleeding as the spread of the cancer generally portends a poor prognosis. Therefore, it is best to use a PEG insertion technique that does not involve pulling or pushing the PEG through the upper aerodigestive tract in patients with active cancer and instead place tubes via an external approach by colleagues in interventional radiology or via direct surgical placement.
Conclusion
Gastroenterologists occupy a unique role in evaluation, diagnosis, and management of patients requiring enteral feeding. In addition, they are best equipped to place, prevent, and manage complications of tube feeding. For this reason, it is imperative that gastroenterologists familiarize themselves with indications for enteral tubes and types of enteral tubes available, as well as the identification and management of common complications. Comprehensive understanding of these concepts will augment the practicing gastroenterologist’s ability to manage patients requiring enteral nutrition support with confidence.
References
1. Stein DJ et al. Dig Dis Sci. 2020 Jun 19. doi: 10.1007/s10620-020-06396-y.
2. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
3. Dietrich CG, Schoppmeyer K. World J Gastroenterol. 2020;26(20):2464-71.
4. Suzuki Y et al. T Gastroenterology Res.2012 Feb;5(1):10-20.
5. Cheung KS et al. Gastroenterology. 2020 Jul;159(1):81-95.
6. Micic D et al. Am J Gastroenterol. 2020 Sep;115(9):1367-70.
7. Fan AC et al. Gastrointest Endosc. 2002;56(6):890-4.
8. Tang SJ. Video J Encycl GI Endosc. 2014;2(2):70-3.
9. Guenter P, Lyman B. Nutr Clin Pract. 2016;31(6):769-72.
10. Acosta RD et al. Gastrointest Endosc. 2016;83(1):3-16.
11. Richter JA et al. Gastrointest Endosc. 2011;74(1):22-34.
12. Boullata JI et al. JPEN. 2017;41(1):15-103.
13. McClave SA. Tech Gastrointest Endosc. 2021;3(1):62-8.
14. Murphy CJ et al. Endosc Int Open. 2016;4(3):E292. doi: 10.1053/tgie.2001.19915.
15. Lynch CR et al. Pract Gastroenterology. 2004;28:66-77.
16. Hucl T et al. Best Pract Res Clin Gastroenterol. 2016;30(5):769-81. doi: 10.1016/j.bpg.2016.10.002.
17. Jafri NS et al. Aliment Pharmacol & Therapeut. 2007;25(6):647-56. doi: 10.1111/j.1365-2036.2007.03247.x.
18. Blumenstein I et al. World J Gastroenterol. 2014;20(26):8505-24. doi: 10.3748/wjg.v20.i26.8505.
19. Fung E et al. Surgical Endosc. 2017;31(9):3623-7. doi: 10.1007/s00464-016-5394-8.
20. Ellrichmann M et al. Endoscopy. 2013;45(07):526-31. doi: 10.1055/s-0033-1344023.
Dr. Toy is with the department of internal medicine at the University of Utah, Salt Lake City. Dr. Fang is with the division of gastroenterology and hepatology at the University of Utah.
Introduction
Gastroenterologists are in a unique position to manage individuals with feeding tubes as their training underscores principles in digestion, absorption, nutrition support, and enteral tube placement. Adequate management of individuals with feeding tubes and, importantly, the complications that arise from feeding tube use and placement require a basic understanding of intestinal anatomy and physiology. Therefore, gastroenterologists are well suited to both place and manage individuals with feeding tubes in the long term.
Indications for tube feeding
When deciding on the appropriate route for artificial nutrition support, the first decision to be made is enteral access versus parenteral nutrition support. Enteral nutrition confers multiple benefits, including preservation of the mucosal lining, reductions in complicated infections, decreased costs, and improved patient compliance. All attempts at adequate enteral access should be made before deciding on the use of parenteral nutrition. Following the clinical decision to pursue artificial means of nutrition support and enteral access, the next common decision is the anticipated duration of nutrition support. Generally, the oral or nasal tubes are used for short durations (i.e., less than 4 weeks) with percutaneous placement into the stomach or small intestine for longer-term feeding (i.e., percutaneous endoscopic gastrostomy [PEG] or percutaneous endoscopic jejunostomy [PEJ]).

The most general indication for nutrition support is an inability to maintain adequate nutritional needs with oral intake alone. General categories of inadequate oral intake include neurologic disorders, malignancy, and gastrointestinal conditions affecting digestion and absorption (Table 1). Absolute and relative contraindications to PEG placement are listed in Table 2. If an endoscopic placement is not possible, alternative means of placement (i.e., surgery or interventional radiology) can be considered to avoid the consequences of prolonged malnutrition. In-hospital mortality following PEG placement has decreased 40% over the last 10 years, which can be attributed to improved patient selection, enhanced discharge practices, and exclusion of patients with the highest comorbidity and mortality rates, like those with advanced dementia or terminal cancer.1
PEG placement in patients with dementia is controversial, with previous studies not demonstrating improved outcomes and association with high mortality rates,2 so the practice is currently not recommended by the American Geriatrics Society in individuals with advanced dementia.3 However, a large Japanese study showed that careful selection of patients with mild dementia to undergo gastrostomy increased independence fourfold; therefore, multidisciplinary involvement is often necessary in the decision to pursue artificial means of nutrition support in this population.4
The recent coronavirus disease 2019 (COVID-19) pandemic has placed additional strains on endoscopic placement and has highlighted the effect of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) on GI symptoms. A recent meta-analysis showed an overall incidence of GI symptoms of 17.6% in the following conditions in decreasing order of prevalence: anorexia, diarrhea, nausea, vomiting, and abdominal discomfort.5 In addition, the prolonged ventilatory requirements among a subset of individuals with the most severe COVID-19 results in extended periods of nutrition support via enteral tube placements. In individuals with ICU-acquired weakness and discharge to long-term care facilities, the placement of percutaneous endoscopic tubes may be required, although with the additional consideration of the need for an aerosolizing procedure. Delay of placement has been advocated, in addition to appropriate personal protective equipment, in order to ensure safe placement for the endoscopy staff.6
Types of feeding tubes
After deciding to feed a patient enterally and determining the anticipated duration of enteral support, the next decision is to determine the most appropriate location of feeding delivery: into the stomach or the small bowel. Gastric feeding is advantageous most commonly because of its increased capacity, allowing for larger volumes to be delivered over shorter durations. However, in the setting of postsurgical anatomy, gastroparesis, or obstructing tumors/pancreatic inflammation, distal delivery of tube feeds may be required into the jejunum. Additionally, percutaneous tubes placed into the stomach can have extenders into the small bowel (GJ tubes) to allow for feeding into the small bowel and decompression or delivery of medications into the stomach.
In general, gastric feeding is preferred over small bowel feeding as PEG tubes are more stable and have fewer complications than either PEG-J or direct PEJ tubes. Gastrostomy tubes are generally shorter and larger in diameter making them less likely to clog. PEG-J tubes have separate lumens for gastric and small intestinal access, but the smaller-bore jejunal extension tubes are more likely to clog or become dislodged. While direct PEJ is shown to have higher rates of tube patency and decreased rates of endoscopic re-intervention, compared with PEG-J,7 one limitation of a direct PEJ is difficulty in placement and site selection, which can be performed with a pediatric colonoscope or balloon enteroscopy system. Most commonly, this procedure is performed under general anesthesia.
In the case of a critically ill patient in the ICU, it is recommended to start enteral nutrition within 24-48 hours of arrival to avoid complications of prolonged calorie deficits. Nasally inserted feeding tubes (e.g., Cortrak, Avanos Medical Devices, Alpharetta, Ga.) are most commonly used at the bedside and can be placed blindly using electromagnetic image guidance, radiographically, or endoscopy. However, the small caliber of nasoenteric tubes comes with the common complication of clogging, which can be overcome with slightly larger bore gastric feeding tubes. If gastric feeding is not tolerated (e.g., in the case of vomiting, witnessed aspiration), small bowel feeding should be initiated and can be a more durable form of enteral feeding with fewer interruptions as feedings do not need to be held for procedures or symptomatic gastric intolerance. In clinical areas of question, or if there is a concern for intolerance of enteral feeding, a short trial with nasogastric or nasojejunal tube placement should be performed before a more definitive percutaneous placement.
With respect to percutaneous tubes, important characteristics to choose are the size (diameter in French units), type of internal retention device, and external appearance of the tube (standard or low profile). All percutaneous tubes contain an external retention device (i.e., bumper) that fits against the skin and an internal retention device that is either a balloon or plastic dome or funnel that prevents the tube from becoming dislodged. Balloon retention tubes require replacement every 3-6 months, while nonballoon tubes generally require replacement annually in order to prevent the plastic from cracking, which can make removal complicated. Low-profile tubes have an external cap, which, when opened, allows for extension tubing to be securely attached while in use and detached while not in use. Low-profile tubes are often preferred among younger, active patients and those with adequate dexterity to allow for attachment of the external extension tubing. These tubes are most often inserted as a replacement for an initially endoscopically placed tube, although one-step systems for initial placement are available. The size of the low-profile tube is chosen based on the size of the existing PEG tube and by measuring the length of the stoma tract using specialized measuring devices.8 Patients and caregivers can also be trained to replace balloon-type tubes on their own to limit complications of displaced or cracked tubes. Low-profile tubes are commercially available for both gastric placement and gastric placement with extension into the small bowel, which often requires fluoroscopy for secure placement.
All percutaneous enteral tubes are being transitioned to the ENfit connector system, which prevents connections from the enteral system to nonenteral systems (namely intravenous lines, chest tubes) and vice versa. Tubing misconnections have been rarely reported, and the EnFIT system is designed to prevent such misadventures that have resulted in serious complications and even mortality.9 Adapter devices are available that may be required for patients with feeding tubes who have not been transitioned yet. Most commonly with new tube placements and replacements, patients and providers will have to become familiar with the new syringes and feeding bags required with EnFIT connectors.
Gastrostomy placement can be considered a higher-risk endoscopic procedure. One complicating factor is the increased use of antiplatelet and anticoagulant therapies in individuals with a history of neurologic insults. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend that coumadin be held 5 days before the procedure and bridged with heparin if the patient is at high risk of thromboembolic complications. For patients on dual anti-platelet therapy, thienopyridines like clopidogrel are often stopped 5-7 days prior to procedure with continuation of aspirin,10 but there are more recent data that PEG insertion is safe with continued use of DAPT.11 Direct-acting anticoagulants (DOACs) are often stopped 24-48 hours prior to procedure and then restarted 48 hours after tube placement, but this is dependent on the half-life of the specific DOAC and the patient’s renal function. Patients with decreased creatinine clearance may need to hold the DOAC up to 3-4 days prior to the procedure. In this situation, referring to ASGE guidelines and consultation with a hematologist or managing anti-coagulation clinic is advised.10
Troubleshooting complications
Nasoenteric tubes: One of the most common and irritating complications with nasoenteric feeding tubes is clogging. To prevent clogging, the tube should be flushed frequently.12 At least 30 mL of free water should be used to flush the tube every 4-8 hours for continuous feedings or before and after bolus feeding. Additionally, 15-30 mL of water should be given with each separate medication administration, and if possible, medication administration via small-bore small bowel feeding tubes should be avoided.12 Water flushing is especially important with small-caliber tubes and pumps that deliver both feeding and water flushes. It is available for small bowel feeding in order to allow for programmed water delivery.
Warm water flushes can also help unclog the tube,12 and additional pharmacologic and mechanical devices have been promoted for clogged tubes. One common technique is mixing pancreatic enzymes (Viokase) with a crushed 325-mg tablet of nonenteric coated sodium bicarbonate and 5 mL of water to create a solution that has the alkaline properties allowing for both pancreatic enzyme activation and clog dissolution. Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) catheter can be placed into longer feeding tubes to directly infuse the activated agent to the site of the clog.13 If water and enzymes are not successful in unclogging the tube, commercially available brushes can help remove clogs. The TubeClear® system (Actuated Medical, Bellefonte, Penna) has a single-use stem that is connected to AC power to create a jackhammerlike movement to remove clogs in longer nasoenteral and gastrojejunal tubes.
PEG tubes (short-term complications): Procedural and immediate postprocedural complications include bleeding, aspiration, pneumoperitoneum, and perforation. Pneumoperitoneum occurs in approximately 50% of cases and is generally clinically insignificant. The risk of pneumoperitoneum can be reduced by using CO2 insufflation.14 If the patient develops systemic signs of infection or peritoneal signs, CT scan with oral contrast is warranted for further evaluation and to assess for inadvertent perforation of overlying bowel or dislodged tube. Aspiration during or following endoscopy is another common complication of PEG placement and risk factors include over-sedation, supine positioning, advanced age, and neurologic dysfunction. This risk can be mitigated by avoiding over-sedation, immediately aspirating gastric contents when the stomach is reached, and avoiding excessive insufflation.15 In addition, elevating the head of the bed during the procedure and dedicating an assistant to perform oral suctioning during the entire procedure is recommended.
PEG tubes (long-term complications): More delayed complications of PEG insertion include wound infection, buried bumper syndrome, tumor seeding, peristomal leakage, and tube dislodgement. The prevalence of wound infection is 5%- 25%,16 and randomized controlled trials have demonstrated the efficacy of a single dose of an IV antibiotic (i.e., cephalosporin) in those not already receiving a broad spectrum antibiotic and administered prophylactically before tube placement.17 The significance of this reduction is such that antibiotic administration before tube placement should be considered a quality measure for the procedure. A small amount of redness around the tube site (less than 5 mm) is typical, but extension of erythema, warmth, tenderness, purulent drainage, or systemic symptoms is consistent with infection and warrants additional antibiotic administration. Minor infections can be treated with local antiseptics and oral antibiotics, and early intervention is important to prevent need for hospital admission, systemic antibiotics, and even surgical debridement.
Peristomal leakage is reported in approximately 1%-2% of patients.18 Photographs of the site can be very useful in evaluating and managing peristomal leakage and infections. Interventions include reducing gastric secretions with proton pump inhibitors and management of the skin with barrier creams, such as zinc oxide (Calmoseptine®) ointment. Placement of a larger-diameter tube only enlarges the stoma track and worsens the leakage. In such cases, thorough evaluations for delayed gastric emptying (gastroparesis), distal obstruction, or constipation should be performed and managed accordingly. Opiates are common contributors to constipation and delayed gastric emptying and often require reduction in use or directed antagonist therapy to reduce leaking. Continuous feeding over bolus feedings and delivering nutrition distally into the small bowel (PEG-J placement) can improve leaking from gastrostomy tubes. Additional means of management include stabilizing the tube by replacing a traditional tube with a low-profile tube or using right-angle external bumpers. If all measures fail, removing the tube and allowing for stomal closure can be attempted,16 although this option often requires parenteral nutrition support to prevent prolonged periods of inadequate nutrition.
Buried bumper syndrome (BBS) occurs in 1.5%-8.8% of PEG placements and is a common late complication of PEG placement, although early reports have been described.18 The development of BBS occurs when the internal bumper migrates from the gastric lumen through and into the stomach or abdominal wall. It occurs more frequently with solid nonballoon retention tubes and is caused by excessive compression of the external bumper against the skin and abdominal wall. Patients with BBS usually present with an immobile catheter, resistance with feeds (because of a closure of the stomach wall around the internal portion of the gastrostomy tube), abdominal pain, or peristomal leakage. Physicians should be aware of and assess tubes for BBS, in particular when replacing an immobile tube (cannot be pushed into the free stomach lumen) or when there is difficulty in flushing water into the tube. This complication can be easily prevented by allowing a minimum of 0.5-1.0 cm (1 finger breadth) between the external bumper and the abdominal wall. In particular, patients and caregivers should be warned that if the patient gains significant amounts of weight, the outer bumper will need to be loosened. Once BBS is diagnosed, the PEG tube requires removal and replacement as it can cause bleeding, infection, or fasciitis. The general steps to replacement include endoscopic removal of the existing tube and replacement of new PEG in the existing tract as long as the BBS is not severe. In most cases a replacement tube can be pulled into place using the pull-PEG technique at the same gastrostomy site as long as the stoma tract can be cannulated with a wire after the existing tube is removed.
Similar to nasoenteric tubes, PEG tubes can become clogged, although this complication is infrequent. The primary steps for prevention include adequately flushing with water before and after feeds and ensuring that all medications are liquid or well crushed and dissolved before instilling. Timely tube replacement also ensures that the internal portions of the gastrostomy tube remain free of debris. Management is similar to that of unclogging nasoenteral tubes, as discussed above, and specific commercial declogging devices for PEG tubes include the Bionix Declogger® (Bionix Development Corp., Toledo, Ohio) and the Bard® PEG cleaning brush (Bard Peripheral Vascular Inc., Tempe, Ariz.). The Bionix system has a plastic stem with a screw and thread design that will remove clogs in 14-24 French PEG tubes, while the Bard brush has a flexible nylon stem with soft bristles at the end to prevent mucosal injury and can be used for prophylaxis against clogs, as well as removing clogs themselves.12
Lastly, a rare but important complication of PEG placement is tumor seeding of the PEG site in patients with active head and neck or upper gastrointestinal cancer.19 The presumed mechanism is shearing of tumor cells as the PEG is pulled through the upper aerodigestive tract and through the wall of the stomach, as prior studies have demonstrated frequent seeding of tubes and incision sites as shown by brushing the tube for malignant cells after tube placement.20 It is important to recognize this complication and not misdiagnose it as granulation tissue, infection, or bleeding as the spread of the cancer generally portends a poor prognosis. Therefore, it is best to use a PEG insertion technique that does not involve pulling or pushing the PEG through the upper aerodigestive tract in patients with active cancer and instead place tubes via an external approach by colleagues in interventional radiology or via direct surgical placement.
Conclusion
Gastroenterologists occupy a unique role in evaluation, diagnosis, and management of patients requiring enteral feeding. In addition, they are best equipped to place, prevent, and manage complications of tube feeding. For this reason, it is imperative that gastroenterologists familiarize themselves with indications for enteral tubes and types of enteral tubes available, as well as the identification and management of common complications. Comprehensive understanding of these concepts will augment the practicing gastroenterologist’s ability to manage patients requiring enteral nutrition support with confidence.
References
1. Stein DJ et al. Dig Dis Sci. 2020 Jun 19. doi: 10.1007/s10620-020-06396-y.
2. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
3. Dietrich CG, Schoppmeyer K. World J Gastroenterol. 2020;26(20):2464-71.
4. Suzuki Y et al. T Gastroenterology Res.2012 Feb;5(1):10-20.
5. Cheung KS et al. Gastroenterology. 2020 Jul;159(1):81-95.
6. Micic D et al. Am J Gastroenterol. 2020 Sep;115(9):1367-70.
7. Fan AC et al. Gastrointest Endosc. 2002;56(6):890-4.
8. Tang SJ. Video J Encycl GI Endosc. 2014;2(2):70-3.
9. Guenter P, Lyman B. Nutr Clin Pract. 2016;31(6):769-72.
10. Acosta RD et al. Gastrointest Endosc. 2016;83(1):3-16.
11. Richter JA et al. Gastrointest Endosc. 2011;74(1):22-34.
12. Boullata JI et al. JPEN. 2017;41(1):15-103.
13. McClave SA. Tech Gastrointest Endosc. 2021;3(1):62-8.
14. Murphy CJ et al. Endosc Int Open. 2016;4(3):E292. doi: 10.1053/tgie.2001.19915.
15. Lynch CR et al. Pract Gastroenterology. 2004;28:66-77.
16. Hucl T et al. Best Pract Res Clin Gastroenterol. 2016;30(5):769-81. doi: 10.1016/j.bpg.2016.10.002.
17. Jafri NS et al. Aliment Pharmacol & Therapeut. 2007;25(6):647-56. doi: 10.1111/j.1365-2036.2007.03247.x.
18. Blumenstein I et al. World J Gastroenterol. 2014;20(26):8505-24. doi: 10.3748/wjg.v20.i26.8505.
19. Fung E et al. Surgical Endosc. 2017;31(9):3623-7. doi: 10.1007/s00464-016-5394-8.
20. Ellrichmann M et al. Endoscopy. 2013;45(07):526-31. doi: 10.1055/s-0033-1344023.
Dr. Toy is with the department of internal medicine at the University of Utah, Salt Lake City. Dr. Fang is with the division of gastroenterology and hepatology at the University of Utah.
Introduction
Gastroenterologists are in a unique position to manage individuals with feeding tubes as their training underscores principles in digestion, absorption, nutrition support, and enteral tube placement. Adequate management of individuals with feeding tubes and, importantly, the complications that arise from feeding tube use and placement require a basic understanding of intestinal anatomy and physiology. Therefore, gastroenterologists are well suited to both place and manage individuals with feeding tubes in the long term.
Indications for tube feeding
When deciding on the appropriate route for artificial nutrition support, the first decision to be made is enteral access versus parenteral nutrition support. Enteral nutrition confers multiple benefits, including preservation of the mucosal lining, reductions in complicated infections, decreased costs, and improved patient compliance. All attempts at adequate enteral access should be made before deciding on the use of parenteral nutrition. Following the clinical decision to pursue artificial means of nutrition support and enteral access, the next common decision is the anticipated duration of nutrition support. Generally, the oral or nasal tubes are used for short durations (i.e., less than 4 weeks) with percutaneous placement into the stomach or small intestine for longer-term feeding (i.e., percutaneous endoscopic gastrostomy [PEG] or percutaneous endoscopic jejunostomy [PEJ]).

The most general indication for nutrition support is an inability to maintain adequate nutritional needs with oral intake alone. General categories of inadequate oral intake include neurologic disorders, malignancy, and gastrointestinal conditions affecting digestion and absorption (Table 1). Absolute and relative contraindications to PEG placement are listed in Table 2. If an endoscopic placement is not possible, alternative means of placement (i.e., surgery or interventional radiology) can be considered to avoid the consequences of prolonged malnutrition. In-hospital mortality following PEG placement has decreased 40% over the last 10 years, which can be attributed to improved patient selection, enhanced discharge practices, and exclusion of patients with the highest comorbidity and mortality rates, like those with advanced dementia or terminal cancer.1
PEG placement in patients with dementia is controversial, with previous studies not demonstrating improved outcomes and association with high mortality rates,2 so the practice is currently not recommended by the American Geriatrics Society in individuals with advanced dementia.3 However, a large Japanese study showed that careful selection of patients with mild dementia to undergo gastrostomy increased independence fourfold; therefore, multidisciplinary involvement is often necessary in the decision to pursue artificial means of nutrition support in this population.4
The recent coronavirus disease 2019 (COVID-19) pandemic has placed additional strains on endoscopic placement and has highlighted the effect of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) on GI symptoms. A recent meta-analysis showed an overall incidence of GI symptoms of 17.6% in the following conditions in decreasing order of prevalence: anorexia, diarrhea, nausea, vomiting, and abdominal discomfort.5 In addition, the prolonged ventilatory requirements among a subset of individuals with the most severe COVID-19 results in extended periods of nutrition support via enteral tube placements. In individuals with ICU-acquired weakness and discharge to long-term care facilities, the placement of percutaneous endoscopic tubes may be required, although with the additional consideration of the need for an aerosolizing procedure. Delay of placement has been advocated, in addition to appropriate personal protective equipment, in order to ensure safe placement for the endoscopy staff.6
Types of feeding tubes
After deciding to feed a patient enterally and determining the anticipated duration of enteral support, the next decision is to determine the most appropriate location of feeding delivery: into the stomach or the small bowel. Gastric feeding is advantageous most commonly because of its increased capacity, allowing for larger volumes to be delivered over shorter durations. However, in the setting of postsurgical anatomy, gastroparesis, or obstructing tumors/pancreatic inflammation, distal delivery of tube feeds may be required into the jejunum. Additionally, percutaneous tubes placed into the stomach can have extenders into the small bowel (GJ tubes) to allow for feeding into the small bowel and decompression or delivery of medications into the stomach.
In general, gastric feeding is preferred over small bowel feeding as PEG tubes are more stable and have fewer complications than either PEG-J or direct PEJ tubes. Gastrostomy tubes are generally shorter and larger in diameter making them less likely to clog. PEG-J tubes have separate lumens for gastric and small intestinal access, but the smaller-bore jejunal extension tubes are more likely to clog or become dislodged. While direct PEJ is shown to have higher rates of tube patency and decreased rates of endoscopic re-intervention, compared with PEG-J,7 one limitation of a direct PEJ is difficulty in placement and site selection, which can be performed with a pediatric colonoscope or balloon enteroscopy system. Most commonly, this procedure is performed under general anesthesia.
In the case of a critically ill patient in the ICU, it is recommended to start enteral nutrition within 24-48 hours of arrival to avoid complications of prolonged calorie deficits. Nasally inserted feeding tubes (e.g., Cortrak, Avanos Medical Devices, Alpharetta, Ga.) are most commonly used at the bedside and can be placed blindly using electromagnetic image guidance, radiographically, or endoscopy. However, the small caliber of nasoenteric tubes comes with the common complication of clogging, which can be overcome with slightly larger bore gastric feeding tubes. If gastric feeding is not tolerated (e.g., in the case of vomiting, witnessed aspiration), small bowel feeding should be initiated and can be a more durable form of enteral feeding with fewer interruptions as feedings do not need to be held for procedures or symptomatic gastric intolerance. In clinical areas of question, or if there is a concern for intolerance of enteral feeding, a short trial with nasogastric or nasojejunal tube placement should be performed before a more definitive percutaneous placement.
With respect to percutaneous tubes, important characteristics to choose are the size (diameter in French units), type of internal retention device, and external appearance of the tube (standard or low profile). All percutaneous tubes contain an external retention device (i.e., bumper) that fits against the skin and an internal retention device that is either a balloon or plastic dome or funnel that prevents the tube from becoming dislodged. Balloon retention tubes require replacement every 3-6 months, while nonballoon tubes generally require replacement annually in order to prevent the plastic from cracking, which can make removal complicated. Low-profile tubes have an external cap, which, when opened, allows for extension tubing to be securely attached while in use and detached while not in use. Low-profile tubes are often preferred among younger, active patients and those with adequate dexterity to allow for attachment of the external extension tubing. These tubes are most often inserted as a replacement for an initially endoscopically placed tube, although one-step systems for initial placement are available. The size of the low-profile tube is chosen based on the size of the existing PEG tube and by measuring the length of the stoma tract using specialized measuring devices.8 Patients and caregivers can also be trained to replace balloon-type tubes on their own to limit complications of displaced or cracked tubes. Low-profile tubes are commercially available for both gastric placement and gastric placement with extension into the small bowel, which often requires fluoroscopy for secure placement.
All percutaneous enteral tubes are being transitioned to the ENfit connector system, which prevents connections from the enteral system to nonenteral systems (namely intravenous lines, chest tubes) and vice versa. Tubing misconnections have been rarely reported, and the EnFIT system is designed to prevent such misadventures that have resulted in serious complications and even mortality.9 Adapter devices are available that may be required for patients with feeding tubes who have not been transitioned yet. Most commonly with new tube placements and replacements, patients and providers will have to become familiar with the new syringes and feeding bags required with EnFIT connectors.
Gastrostomy placement can be considered a higher-risk endoscopic procedure. One complicating factor is the increased use of antiplatelet and anticoagulant therapies in individuals with a history of neurologic insults. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend that coumadin be held 5 days before the procedure and bridged with heparin if the patient is at high risk of thromboembolic complications. For patients on dual anti-platelet therapy, thienopyridines like clopidogrel are often stopped 5-7 days prior to procedure with continuation of aspirin,10 but there are more recent data that PEG insertion is safe with continued use of DAPT.11 Direct-acting anticoagulants (DOACs) are often stopped 24-48 hours prior to procedure and then restarted 48 hours after tube placement, but this is dependent on the half-life of the specific DOAC and the patient’s renal function. Patients with decreased creatinine clearance may need to hold the DOAC up to 3-4 days prior to the procedure. In this situation, referring to ASGE guidelines and consultation with a hematologist or managing anti-coagulation clinic is advised.10
Troubleshooting complications
Nasoenteric tubes: One of the most common and irritating complications with nasoenteric feeding tubes is clogging. To prevent clogging, the tube should be flushed frequently.12 At least 30 mL of free water should be used to flush the tube every 4-8 hours for continuous feedings or before and after bolus feeding. Additionally, 15-30 mL of water should be given with each separate medication administration, and if possible, medication administration via small-bore small bowel feeding tubes should be avoided.12 Water flushing is especially important with small-caliber tubes and pumps that deliver both feeding and water flushes. It is available for small bowel feeding in order to allow for programmed water delivery.
Warm water flushes can also help unclog the tube,12 and additional pharmacologic and mechanical devices have been promoted for clogged tubes. One common technique is mixing pancreatic enzymes (Viokase) with a crushed 325-mg tablet of nonenteric coated sodium bicarbonate and 5 mL of water to create a solution that has the alkaline properties allowing for both pancreatic enzyme activation and clog dissolution. Additionally, an endoscopic retrograde cholangiopancreatography (ERCP) catheter can be placed into longer feeding tubes to directly infuse the activated agent to the site of the clog.13 If water and enzymes are not successful in unclogging the tube, commercially available brushes can help remove clogs. The TubeClear® system (Actuated Medical, Bellefonte, Penna) has a single-use stem that is connected to AC power to create a jackhammerlike movement to remove clogs in longer nasoenteral and gastrojejunal tubes.
PEG tubes (short-term complications): Procedural and immediate postprocedural complications include bleeding, aspiration, pneumoperitoneum, and perforation. Pneumoperitoneum occurs in approximately 50% of cases and is generally clinically insignificant. The risk of pneumoperitoneum can be reduced by using CO2 insufflation.14 If the patient develops systemic signs of infection or peritoneal signs, CT scan with oral contrast is warranted for further evaluation and to assess for inadvertent perforation of overlying bowel or dislodged tube. Aspiration during or following endoscopy is another common complication of PEG placement and risk factors include over-sedation, supine positioning, advanced age, and neurologic dysfunction. This risk can be mitigated by avoiding over-sedation, immediately aspirating gastric contents when the stomach is reached, and avoiding excessive insufflation.15 In addition, elevating the head of the bed during the procedure and dedicating an assistant to perform oral suctioning during the entire procedure is recommended.
PEG tubes (long-term complications): More delayed complications of PEG insertion include wound infection, buried bumper syndrome, tumor seeding, peristomal leakage, and tube dislodgement. The prevalence of wound infection is 5%- 25%,16 and randomized controlled trials have demonstrated the efficacy of a single dose of an IV antibiotic (i.e., cephalosporin) in those not already receiving a broad spectrum antibiotic and administered prophylactically before tube placement.17 The significance of this reduction is such that antibiotic administration before tube placement should be considered a quality measure for the procedure. A small amount of redness around the tube site (less than 5 mm) is typical, but extension of erythema, warmth, tenderness, purulent drainage, or systemic symptoms is consistent with infection and warrants additional antibiotic administration. Minor infections can be treated with local antiseptics and oral antibiotics, and early intervention is important to prevent need for hospital admission, systemic antibiotics, and even surgical debridement.
Peristomal leakage is reported in approximately 1%-2% of patients.18 Photographs of the site can be very useful in evaluating and managing peristomal leakage and infections. Interventions include reducing gastric secretions with proton pump inhibitors and management of the skin with barrier creams, such as zinc oxide (Calmoseptine®) ointment. Placement of a larger-diameter tube only enlarges the stoma track and worsens the leakage. In such cases, thorough evaluations for delayed gastric emptying (gastroparesis), distal obstruction, or constipation should be performed and managed accordingly. Opiates are common contributors to constipation and delayed gastric emptying and often require reduction in use or directed antagonist therapy to reduce leaking. Continuous feeding over bolus feedings and delivering nutrition distally into the small bowel (PEG-J placement) can improve leaking from gastrostomy tubes. Additional means of management include stabilizing the tube by replacing a traditional tube with a low-profile tube or using right-angle external bumpers. If all measures fail, removing the tube and allowing for stomal closure can be attempted,16 although this option often requires parenteral nutrition support to prevent prolonged periods of inadequate nutrition.
Buried bumper syndrome (BBS) occurs in 1.5%-8.8% of PEG placements and is a common late complication of PEG placement, although early reports have been described.18 The development of BBS occurs when the internal bumper migrates from the gastric lumen through and into the stomach or abdominal wall. It occurs more frequently with solid nonballoon retention tubes and is caused by excessive compression of the external bumper against the skin and abdominal wall. Patients with BBS usually present with an immobile catheter, resistance with feeds (because of a closure of the stomach wall around the internal portion of the gastrostomy tube), abdominal pain, or peristomal leakage. Physicians should be aware of and assess tubes for BBS, in particular when replacing an immobile tube (cannot be pushed into the free stomach lumen) or when there is difficulty in flushing water into the tube. This complication can be easily prevented by allowing a minimum of 0.5-1.0 cm (1 finger breadth) between the external bumper and the abdominal wall. In particular, patients and caregivers should be warned that if the patient gains significant amounts of weight, the outer bumper will need to be loosened. Once BBS is diagnosed, the PEG tube requires removal and replacement as it can cause bleeding, infection, or fasciitis. The general steps to replacement include endoscopic removal of the existing tube and replacement of new PEG in the existing tract as long as the BBS is not severe. In most cases a replacement tube can be pulled into place using the pull-PEG technique at the same gastrostomy site as long as the stoma tract can be cannulated with a wire after the existing tube is removed.
Similar to nasoenteric tubes, PEG tubes can become clogged, although this complication is infrequent. The primary steps for prevention include adequately flushing with water before and after feeds and ensuring that all medications are liquid or well crushed and dissolved before instilling. Timely tube replacement also ensures that the internal portions of the gastrostomy tube remain free of debris. Management is similar to that of unclogging nasoenteral tubes, as discussed above, and specific commercial declogging devices for PEG tubes include the Bionix Declogger® (Bionix Development Corp., Toledo, Ohio) and the Bard® PEG cleaning brush (Bard Peripheral Vascular Inc., Tempe, Ariz.). The Bionix system has a plastic stem with a screw and thread design that will remove clogs in 14-24 French PEG tubes, while the Bard brush has a flexible nylon stem with soft bristles at the end to prevent mucosal injury and can be used for prophylaxis against clogs, as well as removing clogs themselves.12
Lastly, a rare but important complication of PEG placement is tumor seeding of the PEG site in patients with active head and neck or upper gastrointestinal cancer.19 The presumed mechanism is shearing of tumor cells as the PEG is pulled through the upper aerodigestive tract and through the wall of the stomach, as prior studies have demonstrated frequent seeding of tubes and incision sites as shown by brushing the tube for malignant cells after tube placement.20 It is important to recognize this complication and not misdiagnose it as granulation tissue, infection, or bleeding as the spread of the cancer generally portends a poor prognosis. Therefore, it is best to use a PEG insertion technique that does not involve pulling or pushing the PEG through the upper aerodigestive tract in patients with active cancer and instead place tubes via an external approach by colleagues in interventional radiology or via direct surgical placement.
Conclusion
Gastroenterologists occupy a unique role in evaluation, diagnosis, and management of patients requiring enteral feeding. In addition, they are best equipped to place, prevent, and manage complications of tube feeding. For this reason, it is imperative that gastroenterologists familiarize themselves with indications for enteral tubes and types of enteral tubes available, as well as the identification and management of common complications. Comprehensive understanding of these concepts will augment the practicing gastroenterologist’s ability to manage patients requiring enteral nutrition support with confidence.
References
1. Stein DJ et al. Dig Dis Sci. 2020 Jun 19. doi: 10.1007/s10620-020-06396-y.
2. American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. J Am Geriatr Soc. 2014;62(8):1590-3.
3. Dietrich CG, Schoppmeyer K. World J Gastroenterol. 2020;26(20):2464-71.
4. Suzuki Y et al. T Gastroenterology Res.2012 Feb;5(1):10-20.
5. Cheung KS et al. Gastroenterology. 2020 Jul;159(1):81-95.
6. Micic D et al. Am J Gastroenterol. 2020 Sep;115(9):1367-70.
7. Fan AC et al. Gastrointest Endosc. 2002;56(6):890-4.
8. Tang SJ. Video J Encycl GI Endosc. 2014;2(2):70-3.
9. Guenter P, Lyman B. Nutr Clin Pract. 2016;31(6):769-72.
10. Acosta RD et al. Gastrointest Endosc. 2016;83(1):3-16.
11. Richter JA et al. Gastrointest Endosc. 2011;74(1):22-34.
12. Boullata JI et al. JPEN. 2017;41(1):15-103.
13. McClave SA. Tech Gastrointest Endosc. 2021;3(1):62-8.
14. Murphy CJ et al. Endosc Int Open. 2016;4(3):E292. doi: 10.1053/tgie.2001.19915.
15. Lynch CR et al. Pract Gastroenterology. 2004;28:66-77.
16. Hucl T et al. Best Pract Res Clin Gastroenterol. 2016;30(5):769-81. doi: 10.1016/j.bpg.2016.10.002.
17. Jafri NS et al. Aliment Pharmacol & Therapeut. 2007;25(6):647-56. doi: 10.1111/j.1365-2036.2007.03247.x.
18. Blumenstein I et al. World J Gastroenterol. 2014;20(26):8505-24. doi: 10.3748/wjg.v20.i26.8505.
19. Fung E et al. Surgical Endosc. 2017;31(9):3623-7. doi: 10.1007/s00464-016-5394-8.
20. Ellrichmann M et al. Endoscopy. 2013;45(07):526-31. doi: 10.1055/s-0033-1344023.
Dr. Toy is with the department of internal medicine at the University of Utah, Salt Lake City. Dr. Fang is with the division of gastroenterology and hepatology at the University of Utah.
How productivity influences compensation in private practice
When starting a career in gastroenterology, physicians tend to work in the hospital, where there is usually high demand for services and productivity goals are easy to meet. This is a little different in private GI groups, where it takes some time to build up your patient base. This might be a significant concern for young physicians considering private practice. But understanding the role that productivity plays in compensation packages can help in choosing the right group to join.
While compensation models may differ from practice to practice, there is usually a base salary provided with a productivity bonus. Some practices may use productivity along with other measures to determine when a physician is eligible to become a partner in the practice. Partnership is often accompanied with the benefits of ancillary services ownership such as ambulatory surgery centers (ASCs) and anesthesia, pathology, and infusion services.
How is productivity measured?
Most practices utilize relative value units (RVUs), a standard used by Medicare to determine the amount to pay physicians according to their productivity. Most public and private payers are utilizing the RVU system first developed for Medicare as a useful, time-saving way to handle physician payments. The RVU defines the volume of work doctors perform for all procedures and services covered under the Medicare Physician Fee Schedule.
The Medicare Physician Payment System has three components:
• The geographic practice cost indices (GPCIs)
• Relative value units (RVUs)
• A conversion factor
It is important to understand the types of RVUs that exist to understand how to calculate them properly – these include the following categories:
• Physician work, which accounts for the time and effort to perform a procedure.
• Practice expense, which is for the costs of nonphysician labor such as rent and supplies.
• Global fees, which includes fees for initial visits, follow-ups, and practice expense, and applies during a predetermined length of time known as the “global periods,” primarily for major surgeries.
• Malpractice expense, such as costs for professional liability insurance.
There is no specific dollar amount attached to an RVU because RVUs are part of a resource-based relative value scale (RBRVS) which uses RVUs to relate medical procedures to each other. Payment for physician work is based on whether the procedure is performed in an ASC or hospital outpatient department or in an office. A separate facility fee payment is made to the ASC or hospital outpatient department for procedures performed there. Other elements include skills and the amount of time needed to perform a procedure. Calculating the reimbursement from an RVU involves several components and a significant amount of complex math.
Meeting goals while building a practice
For many young physicians working in the hospital where patients are plentiful, it might seem daunting to build your practice with productivity goals. Practices should, and many do, design their initial productivity plans to minimum or mean RVUs for young physicians rather than someone 10 years into practice. Younger physicians have fellowship and training, but it takes years to become highly efficient with time and productivity. It’s important for everyone involved to set attainable benchmarks.
The practice should also do its best to support your efforts to grow your patient base. While you should be expected to develop relationships with referring physicians, you’ll benefit from the practice’s marketing efforts. When new patients come in, they usually go to newly hired physicians because more senior physicians are booked weeks or months in advance.
Practice administrators also work hard to time new hires to overlap with expected retirements. Senior partners will always have follow-up colonoscopies and associates will need to take on these cases as their colleagues retire. In some practices, younger physicians are expected to take the hospital on call schedules or respond to emergency department calls, so it shouldn’t be difficult to meet productivity goals.
And once you become a partner and are further along on in your career, your productivity plan will change. Some groups have productivity-based compensation, which allows more senior partners to work when they want to – as long as they are meeting the productivity rates that will cover their portion of the practice expenses.
If a physician is consistently not meeting productivity measures, a practice may exercise the right to terminate the relationship, but this is rare. More often, physicians meet their productivity levels and receive certain bonuses for exceeding their goals. In most practices, the partners you work with will know if you aren’t meeting your goals. In most cases, they will take on a mentorship role to help you succeed.
Ask questions, be engaged
Another thing to be aware of is that all practices worth joining make sure productivity plans do not violate the Stark Law, anti-kickback statutes, or other regulations. A huge red flag to look out for is a productivity plan that is based on the number of procedures – it should never be tied to volume.
It’s also best to consider how often the productivity plan is measured. It might be a red flag if it is measured weekly or monthly or if there are heavy consequences for not meeting RVU goals. Most groups look at productivity on a quarterly basis and integrate those discussions into a standard review process.
The successful early-career GIs we interview in our practices are those who are interested in understanding the ins and outs of our practices and what they can achieve through practicing independently. The practices worth joining will likewise be interested in discussing your level of entrepreneurship, the opportunities for you to grow in your career, and what it takes to be on the track to partner.
Dr. Baig is a practicing gastroenterologist at Allied Digestive Care in New Jersey and is the chair of communications for the Digestive Health Physicians Association (DHPA); Mr. Harlen is the president of PE Practice Solutions and immediate past chief operating officer of Capital Digestive Care in Maryland. He is the executive director of DHPA.
When starting a career in gastroenterology, physicians tend to work in the hospital, where there is usually high demand for services and productivity goals are easy to meet. This is a little different in private GI groups, where it takes some time to build up your patient base. This might be a significant concern for young physicians considering private practice. But understanding the role that productivity plays in compensation packages can help in choosing the right group to join.
While compensation models may differ from practice to practice, there is usually a base salary provided with a productivity bonus. Some practices may use productivity along with other measures to determine when a physician is eligible to become a partner in the practice. Partnership is often accompanied with the benefits of ancillary services ownership such as ambulatory surgery centers (ASCs) and anesthesia, pathology, and infusion services.
How is productivity measured?
Most practices utilize relative value units (RVUs), a standard used by Medicare to determine the amount to pay physicians according to their productivity. Most public and private payers are utilizing the RVU system first developed for Medicare as a useful, time-saving way to handle physician payments. The RVU defines the volume of work doctors perform for all procedures and services covered under the Medicare Physician Fee Schedule.
The Medicare Physician Payment System has three components:
• The geographic practice cost indices (GPCIs)
• Relative value units (RVUs)
• A conversion factor
It is important to understand the types of RVUs that exist to understand how to calculate them properly – these include the following categories:
• Physician work, which accounts for the time and effort to perform a procedure.
• Practice expense, which is for the costs of nonphysician labor such as rent and supplies.
• Global fees, which includes fees for initial visits, follow-ups, and practice expense, and applies during a predetermined length of time known as the “global periods,” primarily for major surgeries.
• Malpractice expense, such as costs for professional liability insurance.
There is no specific dollar amount attached to an RVU because RVUs are part of a resource-based relative value scale (RBRVS) which uses RVUs to relate medical procedures to each other. Payment for physician work is based on whether the procedure is performed in an ASC or hospital outpatient department or in an office. A separate facility fee payment is made to the ASC or hospital outpatient department for procedures performed there. Other elements include skills and the amount of time needed to perform a procedure. Calculating the reimbursement from an RVU involves several components and a significant amount of complex math.
Meeting goals while building a practice
For many young physicians working in the hospital where patients are plentiful, it might seem daunting to build your practice with productivity goals. Practices should, and many do, design their initial productivity plans to minimum or mean RVUs for young physicians rather than someone 10 years into practice. Younger physicians have fellowship and training, but it takes years to become highly efficient with time and productivity. It’s important for everyone involved to set attainable benchmarks.
The practice should also do its best to support your efforts to grow your patient base. While you should be expected to develop relationships with referring physicians, you’ll benefit from the practice’s marketing efforts. When new patients come in, they usually go to newly hired physicians because more senior physicians are booked weeks or months in advance.
Practice administrators also work hard to time new hires to overlap with expected retirements. Senior partners will always have follow-up colonoscopies and associates will need to take on these cases as their colleagues retire. In some practices, younger physicians are expected to take the hospital on call schedules or respond to emergency department calls, so it shouldn’t be difficult to meet productivity goals.
And once you become a partner and are further along on in your career, your productivity plan will change. Some groups have productivity-based compensation, which allows more senior partners to work when they want to – as long as they are meeting the productivity rates that will cover their portion of the practice expenses.
If a physician is consistently not meeting productivity measures, a practice may exercise the right to terminate the relationship, but this is rare. More often, physicians meet their productivity levels and receive certain bonuses for exceeding their goals. In most practices, the partners you work with will know if you aren’t meeting your goals. In most cases, they will take on a mentorship role to help you succeed.
Ask questions, be engaged
Another thing to be aware of is that all practices worth joining make sure productivity plans do not violate the Stark Law, anti-kickback statutes, or other regulations. A huge red flag to look out for is a productivity plan that is based on the number of procedures – it should never be tied to volume.
It’s also best to consider how often the productivity plan is measured. It might be a red flag if it is measured weekly or monthly or if there are heavy consequences for not meeting RVU goals. Most groups look at productivity on a quarterly basis and integrate those discussions into a standard review process.
The successful early-career GIs we interview in our practices are those who are interested in understanding the ins and outs of our practices and what they can achieve through practicing independently. The practices worth joining will likewise be interested in discussing your level of entrepreneurship, the opportunities for you to grow in your career, and what it takes to be on the track to partner.
Dr. Baig is a practicing gastroenterologist at Allied Digestive Care in New Jersey and is the chair of communications for the Digestive Health Physicians Association (DHPA); Mr. Harlen is the president of PE Practice Solutions and immediate past chief operating officer of Capital Digestive Care in Maryland. He is the executive director of DHPA.
When starting a career in gastroenterology, physicians tend to work in the hospital, where there is usually high demand for services and productivity goals are easy to meet. This is a little different in private GI groups, where it takes some time to build up your patient base. This might be a significant concern for young physicians considering private practice. But understanding the role that productivity plays in compensation packages can help in choosing the right group to join.
While compensation models may differ from practice to practice, there is usually a base salary provided with a productivity bonus. Some practices may use productivity along with other measures to determine when a physician is eligible to become a partner in the practice. Partnership is often accompanied with the benefits of ancillary services ownership such as ambulatory surgery centers (ASCs) and anesthesia, pathology, and infusion services.
How is productivity measured?
Most practices utilize relative value units (RVUs), a standard used by Medicare to determine the amount to pay physicians according to their productivity. Most public and private payers are utilizing the RVU system first developed for Medicare as a useful, time-saving way to handle physician payments. The RVU defines the volume of work doctors perform for all procedures and services covered under the Medicare Physician Fee Schedule.
The Medicare Physician Payment System has three components:
• The geographic practice cost indices (GPCIs)
• Relative value units (RVUs)
• A conversion factor
It is important to understand the types of RVUs that exist to understand how to calculate them properly – these include the following categories:
• Physician work, which accounts for the time and effort to perform a procedure.
• Practice expense, which is for the costs of nonphysician labor such as rent and supplies.
• Global fees, which includes fees for initial visits, follow-ups, and practice expense, and applies during a predetermined length of time known as the “global periods,” primarily for major surgeries.
• Malpractice expense, such as costs for professional liability insurance.
There is no specific dollar amount attached to an RVU because RVUs are part of a resource-based relative value scale (RBRVS) which uses RVUs to relate medical procedures to each other. Payment for physician work is based on whether the procedure is performed in an ASC or hospital outpatient department or in an office. A separate facility fee payment is made to the ASC or hospital outpatient department for procedures performed there. Other elements include skills and the amount of time needed to perform a procedure. Calculating the reimbursement from an RVU involves several components and a significant amount of complex math.
Meeting goals while building a practice
For many young physicians working in the hospital where patients are plentiful, it might seem daunting to build your practice with productivity goals. Practices should, and many do, design their initial productivity plans to minimum or mean RVUs for young physicians rather than someone 10 years into practice. Younger physicians have fellowship and training, but it takes years to become highly efficient with time and productivity. It’s important for everyone involved to set attainable benchmarks.
The practice should also do its best to support your efforts to grow your patient base. While you should be expected to develop relationships with referring physicians, you’ll benefit from the practice’s marketing efforts. When new patients come in, they usually go to newly hired physicians because more senior physicians are booked weeks or months in advance.
Practice administrators also work hard to time new hires to overlap with expected retirements. Senior partners will always have follow-up colonoscopies and associates will need to take on these cases as their colleagues retire. In some practices, younger physicians are expected to take the hospital on call schedules or respond to emergency department calls, so it shouldn’t be difficult to meet productivity goals.
And once you become a partner and are further along on in your career, your productivity plan will change. Some groups have productivity-based compensation, which allows more senior partners to work when they want to – as long as they are meeting the productivity rates that will cover their portion of the practice expenses.
If a physician is consistently not meeting productivity measures, a practice may exercise the right to terminate the relationship, but this is rare. More often, physicians meet their productivity levels and receive certain bonuses for exceeding their goals. In most practices, the partners you work with will know if you aren’t meeting your goals. In most cases, they will take on a mentorship role to help you succeed.
Ask questions, be engaged
Another thing to be aware of is that all practices worth joining make sure productivity plans do not violate the Stark Law, anti-kickback statutes, or other regulations. A huge red flag to look out for is a productivity plan that is based on the number of procedures – it should never be tied to volume.
It’s also best to consider how often the productivity plan is measured. It might be a red flag if it is measured weekly or monthly or if there are heavy consequences for not meeting RVU goals. Most groups look at productivity on a quarterly basis and integrate those discussions into a standard review process.
The successful early-career GIs we interview in our practices are those who are interested in understanding the ins and outs of our practices and what they can achieve through practicing independently. The practices worth joining will likewise be interested in discussing your level of entrepreneurship, the opportunities for you to grow in your career, and what it takes to be on the track to partner.
Dr. Baig is a practicing gastroenterologist at Allied Digestive Care in New Jersey and is the chair of communications for the Digestive Health Physicians Association (DHPA); Mr. Harlen is the president of PE Practice Solutions and immediate past chief operating officer of Capital Digestive Care in Maryland. He is the executive director of DHPA.
Dermatology history: University Hospital ‘Saint Louis,’ Paris
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at [email protected].
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at [email protected].
The Hospital “Saint Louis” was founded in 1607 by King Henry IV of France to relieve overcrowding of Parisian hospitals during the plague epidemic of 1605-1606. He named it Saint-Louis in memory of his grandfather, King Louis IX.
Today, the Hospital Saint-Louis, a registered historic monument, is used for administrative activities.

Since 1980, a modern building has hosted all the activities of the University Hospital Center, which belongs to the University of Paris 7.

In addition to dermatology, the main departments include hematology and bone marrow transplantation, hemato-oncology, general surgery, endocrinology, gastroenterology, clinical immunology, internal medicine, and nephrology. Saint-Louis Hospital employs 2,500 people, including a medical staff of 1,000. It houses the Institute Inserm U976 – a public research unit that is part of the National Health and Medical Research Institute, which focuses on human immunology, physiopathology and immunotherapy – as well as the René-Touraine Foundation, a private non-profit organization that brings together dermatologists, scientists, pharmaceutical companies, and health authorities to support therapeutic progress in dermatology.
Saint-Louis Hospital is known for its long tradition in hematology; it is the site of the first successful allogeneic bone marrow transplant in 1958, performed by Georges Mathé, MD, Professor Jean Bernard, and one of the recipients of the 1980 Nobel Prize in Medicine, Professor Jean Dausset. The hospital is known for not only its activity in dermatology care and research (such as oncodermatology and inflammatory diseases) but also its long tradition of teaching in dermatology and venereology.
Over the last four centuries, great physicians have practiced their art here and many professors, and clinicians at Saint-Louis Hospital have authored publications and developed manuals of dermatology that have been translated across five continents. Many diseases and semiology signs in dermatology were first described by physicians from this hospital, their names familiar to dermatologists worldwide: Jean-Louis-Marc Alibert, MD; Jean Guillaume Auguste Lugol, MD; Laurent-Théodore Biett, MD; Pierre-Antoine-Ernest Bazin, MD; Pierre Louis Alphée Cazenave, MD; François Henri Hallopeau, MD; Léon Lortat-Jacob, MD; Henri-Alexandre Danlos, MD; Ernest Besnier, MD; Jean Baptiste Emile Vidal, MD; Ferdinand-Jean Darier, MD; Louis Brocq, MD; Bernard Felix Duperrat, MD; Gaston Auguste Milian, MD; Albert Sézary, MD; Achille Civatte, MD; Raymond Sabouraud, MD; Henri Gougerot, MD; Albert Touraine, MD; Arnault Tzanck, MD; and Robert Degos, MD, among others.
The Henri-Feulard library – known as the “Dermatology Wax Museum” – is a fascinating place that houses the world’s largest collection of 4,807 wax casts dedicated to teaching skin diseases and venereal diseases.

The library next to the museum contains numerous outstanding ancient works on dermatology and sexually transmitted diseases, including first issues of dermatology journals from the 19th century and rare dermatology textbooks published in the last 2 centuries.
The recently renovated museum hosts national and international dermatological meetings and is also where the hospital’s dermatology staff meets weekly.
In the dermatology department at Saint-Louis Hospital, patient care is provided in two hospital areas with 18 beds each and a day hospital with 8 beds for patients with inflammatory and dysimmune dermatoses, including a special room with a bathtub for the management of patients with severe genodermatoses. The department is a referral center for genodermatoses and a dedicated center for autoimmune bullous diseases.
Patients with all types of skin tumors, particularly melanomas, carcinomas, sarcomas, and cutaneous lymphomas, are treated at the oncodermatology center, which has a 10-bed day hospital and a very active consultation service. The Saint-Louis Hospital dermatology department is also a National Reference Center for cutaneous lymphomas, providing four Multidisciplinary Consultation Meetings, a national MCM for cutaneous lymphomas, and a multidisciplinary MCM for the diagnosis and treatment of side effects of new targeted therapies and immunotherapies for cancers.
The dermatology polyclinic, an outpatient clinic, provides 54,000 consultations per year. It includes a very active general consultation service, including a wide variety of specialized consultations for atopic dermatitis, psoriasis, hand dermatitis, hidradenitis suppurativa, internal medicine/dermatology, bullous diseases, keloids, angiomas, leprosy, genodermatoses, and medical mycology.
Anonymous, free screening services are available at the Sexually Transmitted Diseases Center through “CeGIDD,” a free center for HIV/AIDS screening and specialized consultations in venereology and mucosal pathologies.
The surgical activity of the department is provided in the Center of Dermo-Surgery. Dedicated medical and paramedical consultations ensure the management of ulcers and therapeutic baths for patients and families with refractory scabies.
The technical platform includes an allergology consultation, a phototherapy center, a Fotofinder diagnosis, a photodynamic therapy unit, and a confocal microscopy unit. The department, completed in September 2019 with a laser center with four devices, also works in close collaboration with the Sabouraud Center, created by Dr. Sabouraud and dedicated to the investigation and treatment of scalp diseases.
We are absolutely aware that working in a hospital so rich in past personalities and discoveries and part of the history of dermatology is not only a huge honor requiring a special commitment to continue the tradition of research and excellence in dermatology initiated hundreds of years ago, but also an important responsibility to focus all our efforts on teaching dermatology to next generations in France and around the world. It is also our responsibility to pursue this historic tradition of excellence by developing dynamic translational research activities that lead to innovations in the field of dermatology.
Professor Bagot is head of the dermatology department of University Hospital Saint-Louis, Paris. Dr. Ionescu is a specialist in dermatology and venereology in the department of dermatology at University Hospital Saint-Louis in Paris and is a member of the Dermatology News editorial board. Write to them at [email protected].
COVID-19 may alter gut microbiota
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
FROM GUT
Expert highlights advances in DRESS
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
FROM THE EADV CONGRESS
CDC: 20% of people in the U.S. are infected with an STD
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
FROM SEXUALLY TRANSMITTED DISEASES
First monthly injectable HIV treatment approved by FDA
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
NEWS FROM THE FDA
Pediatric HM highlights from the 2020 State of Hospital Medicine Report
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.











