User login
For MD-IQ use only
Aaron Rodgers’s Panchakarma ‘cleanse’ is a dangerous play
Green Bay Packers quarterback Aaron Rodgers recently made headlines when he said he finished a 12-day detoxification process that is said to cleanse the body from within.
Registered dietitians who spoke to this news organizastion about the Panchakarma cleanse were quick to debunk it.
“There is no scientific evidence that supports a cleanse,” says Jessica DeGore, a registered dietitian and owner of Pittsburgh-based Dietitian Jess. “Our kidneys, GI systems, and liver all work to keep us healthy and rid us of toxins.”
The Panchakarma cleanse has roots in the ancient Indian alternative medicine approach known as ayurveda. Its 12-day approach includes such actions as self-induced vomiting; enemas; “nasya,” which means eliminating toxins through the nose; and even bloodletting in an effort to “detoxify” the blood.
All of it is misguided, says Alyssa Pike, a registered dietitian and senior manager of nutrition communications at the International Food Information Council.
“Certainly, there are medical procedures that require a fast of some kind for an extended period, and some choose to engage in a fast for religious or spiritual reasons,” she says, “but those are both very different from doing a voluntary ‘cleanse’ or ‘detox’ diet for purported health benefits.”
In fact, the idea that our bodies are full of “toxins” is simply incorrect.
“There isn’t a real medical definition of the word ‘toxins,’” says Ms. DeGore. “If you really had toxins in your body, you’d need emergency medical care, not a cleanse.”
Harmful advice
The entire notion of cleansing, whether Mr. Rodgers’s favored method or another, has gathered steam in the past few decades as celebrities like Gwyneth Paltrow peddle their favored methods for health.
“It’s easy for people to buy into these ideas when they see beautiful celebrities touting their methods for taking care of themselves,” says Ms. DeGore. “But behind the scenes, they receive support we can’t see or access to keep them well.”
Fans of Mr. Rodgers, Ms. Paltrow, and the like easily forget that these public figures have no medical credentials to support what they are pushing. And the celebrities often profit from their claims in the form of books and products related to them, leaving them anything but an unbiased resource.
In the case of Mr. Rodgers’s Panchakarma cleanse, there are real health risks in following its principles, says registered dietitian nutritionist Tiffany Godwin, director of nutrition and wellness at Connections Wellness Group.
“From a medical standpoint, engaging in activities such as induced vomiting, forced diarrhea, and enema use pose a high risk of extreme dehydration,” she says. “Dehydration can lead to fatigue, headaches, and dizziness at best. At worst, it can lead to seizures, kidney failure, coma, and death.”
Also, a cleanse that is designed to rid your body of toxins may introduce them to your body if you are using herbal medicines.
“Some of the products used in ayurvedic medicine contain herbs, metals, minerals, or other materials that may be harmful if used improperly,” Ms. Pike explains. “Ayurvedic medicines are regulated as dietary supplements rather than as drugs in the United States, so they are not required to meet the safety and efficacy standards for conventional medicines.”
When it comes to ayurveda, which is based on ancient writings that rely on a “natural” or holistic approach to physical and mental health, there is scant research or clinical trials in Western medical journals to support the approach. So people interested in following the practices should always consult with a doctor before trying them.
Mr. Rodgers’s approach includes a “nasal herbal remedy,” for instance.
“Tread very lightly with herbs and supplements,” advises Ms. DeGore. “We have the FDA to put drugs through a rigorous process before they approve them. These supplements are unregulated and don’t go through the same processes.”
Another danger is that when “cleansing,” you are starving your body of the nutrients it needs.
“When we vomit, or have diarrhea, we are not simply losing a mass amount of fluid from our bodies, but we are also losing essential electrolytes and minerals,” says Ms. Godwin.
Instead, say the registered dietitians, you can help your body by feeding it what it really needs.
“Eating plenty of fiber-rich foods such as fruits, veggies, beans, legumes, and whole grains, for example, keeps our GI tract moving and grooving, creating an ideal environment for our gut to use the useful things, and get rid of the not so useful,” says Ms. Godwin. “These systems can be compromised in different disease states, such as liver disease, kidney disease, and other GI disorders like Crohn’s or ulcerative colitis. With these disease states, however, cleanses can be even more harmful.”
Cleansing practices can also be a very slippery slope for people struggling with disordered eating.
“When celebrities promote these cleanses, they often bring in impressionable people,” says Ms. DeGore. “These approaches are stripping your body of nutritional needs and inducing disruptive behaviors. Ironically, they will slow down your metabolism, eventually leading to weight gain when you return to normal eating.”
With the Panchakarma cleanse, the 12-day length of cleansing is particularly alarming, says Ms. DeGore.
“Even after 5 days, you cannot think clearly and will have nasty side effects,” she says.
At the end of the day, whether it’s Mr. Rodgers, Ms. Paltrow, or another celebrity, all of the dietitians recommend steering clear of their advice when it comes to health and nutrition.
“Be wary of celebrities, influencers, or anyone who tries to persuade you to try an extreme cleanse or ‘too good to be true’ diet,” says Ms. Pike. “These can be dangerous for your health, physically and mentally.”
A version of this article first appeared on WebMD.com.
Green Bay Packers quarterback Aaron Rodgers recently made headlines when he said he finished a 12-day detoxification process that is said to cleanse the body from within.
Registered dietitians who spoke to this news organizastion about the Panchakarma cleanse were quick to debunk it.
“There is no scientific evidence that supports a cleanse,” says Jessica DeGore, a registered dietitian and owner of Pittsburgh-based Dietitian Jess. “Our kidneys, GI systems, and liver all work to keep us healthy and rid us of toxins.”
The Panchakarma cleanse has roots in the ancient Indian alternative medicine approach known as ayurveda. Its 12-day approach includes such actions as self-induced vomiting; enemas; “nasya,” which means eliminating toxins through the nose; and even bloodletting in an effort to “detoxify” the blood.
All of it is misguided, says Alyssa Pike, a registered dietitian and senior manager of nutrition communications at the International Food Information Council.
“Certainly, there are medical procedures that require a fast of some kind for an extended period, and some choose to engage in a fast for religious or spiritual reasons,” she says, “but those are both very different from doing a voluntary ‘cleanse’ or ‘detox’ diet for purported health benefits.”
In fact, the idea that our bodies are full of “toxins” is simply incorrect.
“There isn’t a real medical definition of the word ‘toxins,’” says Ms. DeGore. “If you really had toxins in your body, you’d need emergency medical care, not a cleanse.”
Harmful advice
The entire notion of cleansing, whether Mr. Rodgers’s favored method or another, has gathered steam in the past few decades as celebrities like Gwyneth Paltrow peddle their favored methods for health.
“It’s easy for people to buy into these ideas when they see beautiful celebrities touting their methods for taking care of themselves,” says Ms. DeGore. “But behind the scenes, they receive support we can’t see or access to keep them well.”
Fans of Mr. Rodgers, Ms. Paltrow, and the like easily forget that these public figures have no medical credentials to support what they are pushing. And the celebrities often profit from their claims in the form of books and products related to them, leaving them anything but an unbiased resource.
In the case of Mr. Rodgers’s Panchakarma cleanse, there are real health risks in following its principles, says registered dietitian nutritionist Tiffany Godwin, director of nutrition and wellness at Connections Wellness Group.
“From a medical standpoint, engaging in activities such as induced vomiting, forced diarrhea, and enema use pose a high risk of extreme dehydration,” she says. “Dehydration can lead to fatigue, headaches, and dizziness at best. At worst, it can lead to seizures, kidney failure, coma, and death.”
Also, a cleanse that is designed to rid your body of toxins may introduce them to your body if you are using herbal medicines.
“Some of the products used in ayurvedic medicine contain herbs, metals, minerals, or other materials that may be harmful if used improperly,” Ms. Pike explains. “Ayurvedic medicines are regulated as dietary supplements rather than as drugs in the United States, so they are not required to meet the safety and efficacy standards for conventional medicines.”
When it comes to ayurveda, which is based on ancient writings that rely on a “natural” or holistic approach to physical and mental health, there is scant research or clinical trials in Western medical journals to support the approach. So people interested in following the practices should always consult with a doctor before trying them.
Mr. Rodgers’s approach includes a “nasal herbal remedy,” for instance.
“Tread very lightly with herbs and supplements,” advises Ms. DeGore. “We have the FDA to put drugs through a rigorous process before they approve them. These supplements are unregulated and don’t go through the same processes.”
Another danger is that when “cleansing,” you are starving your body of the nutrients it needs.
“When we vomit, or have diarrhea, we are not simply losing a mass amount of fluid from our bodies, but we are also losing essential electrolytes and minerals,” says Ms. Godwin.
Instead, say the registered dietitians, you can help your body by feeding it what it really needs.
“Eating plenty of fiber-rich foods such as fruits, veggies, beans, legumes, and whole grains, for example, keeps our GI tract moving and grooving, creating an ideal environment for our gut to use the useful things, and get rid of the not so useful,” says Ms. Godwin. “These systems can be compromised in different disease states, such as liver disease, kidney disease, and other GI disorders like Crohn’s or ulcerative colitis. With these disease states, however, cleanses can be even more harmful.”
Cleansing practices can also be a very slippery slope for people struggling with disordered eating.
“When celebrities promote these cleanses, they often bring in impressionable people,” says Ms. DeGore. “These approaches are stripping your body of nutritional needs and inducing disruptive behaviors. Ironically, they will slow down your metabolism, eventually leading to weight gain when you return to normal eating.”
With the Panchakarma cleanse, the 12-day length of cleansing is particularly alarming, says Ms. DeGore.
“Even after 5 days, you cannot think clearly and will have nasty side effects,” she says.
At the end of the day, whether it’s Mr. Rodgers, Ms. Paltrow, or another celebrity, all of the dietitians recommend steering clear of their advice when it comes to health and nutrition.
“Be wary of celebrities, influencers, or anyone who tries to persuade you to try an extreme cleanse or ‘too good to be true’ diet,” says Ms. Pike. “These can be dangerous for your health, physically and mentally.”
A version of this article first appeared on WebMD.com.
Green Bay Packers quarterback Aaron Rodgers recently made headlines when he said he finished a 12-day detoxification process that is said to cleanse the body from within.
Registered dietitians who spoke to this news organizastion about the Panchakarma cleanse were quick to debunk it.
“There is no scientific evidence that supports a cleanse,” says Jessica DeGore, a registered dietitian and owner of Pittsburgh-based Dietitian Jess. “Our kidneys, GI systems, and liver all work to keep us healthy and rid us of toxins.”
The Panchakarma cleanse has roots in the ancient Indian alternative medicine approach known as ayurveda. Its 12-day approach includes such actions as self-induced vomiting; enemas; “nasya,” which means eliminating toxins through the nose; and even bloodletting in an effort to “detoxify” the blood.
All of it is misguided, says Alyssa Pike, a registered dietitian and senior manager of nutrition communications at the International Food Information Council.
“Certainly, there are medical procedures that require a fast of some kind for an extended period, and some choose to engage in a fast for religious or spiritual reasons,” she says, “but those are both very different from doing a voluntary ‘cleanse’ or ‘detox’ diet for purported health benefits.”
In fact, the idea that our bodies are full of “toxins” is simply incorrect.
“There isn’t a real medical definition of the word ‘toxins,’” says Ms. DeGore. “If you really had toxins in your body, you’d need emergency medical care, not a cleanse.”
Harmful advice
The entire notion of cleansing, whether Mr. Rodgers’s favored method or another, has gathered steam in the past few decades as celebrities like Gwyneth Paltrow peddle their favored methods for health.
“It’s easy for people to buy into these ideas when they see beautiful celebrities touting their methods for taking care of themselves,” says Ms. DeGore. “But behind the scenes, they receive support we can’t see or access to keep them well.”
Fans of Mr. Rodgers, Ms. Paltrow, and the like easily forget that these public figures have no medical credentials to support what they are pushing. And the celebrities often profit from their claims in the form of books and products related to them, leaving them anything but an unbiased resource.
In the case of Mr. Rodgers’s Panchakarma cleanse, there are real health risks in following its principles, says registered dietitian nutritionist Tiffany Godwin, director of nutrition and wellness at Connections Wellness Group.
“From a medical standpoint, engaging in activities such as induced vomiting, forced diarrhea, and enema use pose a high risk of extreme dehydration,” she says. “Dehydration can lead to fatigue, headaches, and dizziness at best. At worst, it can lead to seizures, kidney failure, coma, and death.”
Also, a cleanse that is designed to rid your body of toxins may introduce them to your body if you are using herbal medicines.
“Some of the products used in ayurvedic medicine contain herbs, metals, minerals, or other materials that may be harmful if used improperly,” Ms. Pike explains. “Ayurvedic medicines are regulated as dietary supplements rather than as drugs in the United States, so they are not required to meet the safety and efficacy standards for conventional medicines.”
When it comes to ayurveda, which is based on ancient writings that rely on a “natural” or holistic approach to physical and mental health, there is scant research or clinical trials in Western medical journals to support the approach. So people interested in following the practices should always consult with a doctor before trying them.
Mr. Rodgers’s approach includes a “nasal herbal remedy,” for instance.
“Tread very lightly with herbs and supplements,” advises Ms. DeGore. “We have the FDA to put drugs through a rigorous process before they approve them. These supplements are unregulated and don’t go through the same processes.”
Another danger is that when “cleansing,” you are starving your body of the nutrients it needs.
“When we vomit, or have diarrhea, we are not simply losing a mass amount of fluid from our bodies, but we are also losing essential electrolytes and minerals,” says Ms. Godwin.
Instead, say the registered dietitians, you can help your body by feeding it what it really needs.
“Eating plenty of fiber-rich foods such as fruits, veggies, beans, legumes, and whole grains, for example, keeps our GI tract moving and grooving, creating an ideal environment for our gut to use the useful things, and get rid of the not so useful,” says Ms. Godwin. “These systems can be compromised in different disease states, such as liver disease, kidney disease, and other GI disorders like Crohn’s or ulcerative colitis. With these disease states, however, cleanses can be even more harmful.”
Cleansing practices can also be a very slippery slope for people struggling with disordered eating.
“When celebrities promote these cleanses, they often bring in impressionable people,” says Ms. DeGore. “These approaches are stripping your body of nutritional needs and inducing disruptive behaviors. Ironically, they will slow down your metabolism, eventually leading to weight gain when you return to normal eating.”
With the Panchakarma cleanse, the 12-day length of cleansing is particularly alarming, says Ms. DeGore.
“Even after 5 days, you cannot think clearly and will have nasty side effects,” she says.
At the end of the day, whether it’s Mr. Rodgers, Ms. Paltrow, or another celebrity, all of the dietitians recommend steering clear of their advice when it comes to health and nutrition.
“Be wary of celebrities, influencers, or anyone who tries to persuade you to try an extreme cleanse or ‘too good to be true’ diet,” says Ms. Pike. “These can be dangerous for your health, physically and mentally.”
A version of this article first appeared on WebMD.com.
Can liquid biopsy predict oropharyngeal cancer recurrence?
PHOENIX – A liquid biopsy test may accurately predict recurrence of human papillomavirus (HPV)–driven oropharyngeal squamous cell carcinoma (OPSCC) earlier than standard clinical and imaging assessments, a new analysis indicates.
Of 80 patients who tested positive for circulating tumor tissue–modified viral (TTMV)-HPV DNA during surveillance, 74% (n = 59) had no other evidence of disease or had indeterminate disease status.
And of those patients, 93% (n = 55) “later had proven recurrent, metastatic disease on imaging and/or biopsy,” according to Glenn Hanna, MD, from the Dana-Farber Cancer Institute, Boston, who presented the results Feb. 24 at the 2022 Multidisciplinary Head and Neck Cancers Symposium.
“This is the first study to demonstrate broad clinical utility and validity of the biomarker in HPV-driven oropharyngeal cancer,” Dr. Hanna said in a press release.
Although patients with HPV-driven OPSCC generally have favorable outcomes, up to 25% will experience recurrence after treatment.
Post-treatment surveillance currently relies on physical examinations and imaging, but Dr. Hanna and colleagues wanted to determine whether a routine circulating cell-free TTMV-HPV DNA test could detect occult recurrence sooner.
Dr. Hanna and colleagues analyzed the records of 1,076 patients with HPV-driven OPSCC at 118 sites in the U.S. who had completed therapy more than 3 months previously and undergone an TTMV-HPV DNA test (NavDx, Naveris) between June 2020 and November 2021.
The results of the test, which used ultrasensitive digital droplet PCR to identify HPV subtypes 16, 18, 31, 33, and 35, were compared with subsequent clinical evidence of OPSCC via nasopharyngolaryngoscopy, radiologic evaluations, or tissue biopsy.
Approximately 7% of the patients tested positive (n = 80) for circulating TTMV-HPV DNA. Of those, 26.2% (n = 21) had known clinical recurrence, while 73.8% (n = 59) had no other evidence of disease or an intermediate disease status.
Among those with no clinical evidence of recurrence, 93.2% (n = 55) had their recurrence subsequently confirmed using imaging or biopsy. Of the 4 remaining patients, 2 had clinically suspicious lesions, and 2 had no other evidence of disease.
Overall, the data indicate that the biomarker test demonstrated a 95% positive predictive value (76 of 80 patients) for recurrence or persistence of HPV-driven OPSCC.
According to Dr. Hanna, a positive TTMV-HPV DNA test was the first indicator of recurrence for 72% of patients, and almost half of recurrences were detected more than 12 months after completing therapy.
“Incorporating a test for TTMV-HPV DNA into routine post-treatment follow-up can enable physicians to detect recurrent cancers earlier and allow us to start recommended interventions more quickly to improve outcomes,” Dr. Hanna said in the release.
The study was supported by Naveris, which developed the TTMV-HPV DNA test studied. Dr. Hanna declares relationships with Actuate Therapeutics, Altor BioScience, Bicara, BMS, GSK, Merck, Regeneron, Sanofi/Genzyme, and others.
A version of this article first appeared on Medscape.com.
PHOENIX – A liquid biopsy test may accurately predict recurrence of human papillomavirus (HPV)–driven oropharyngeal squamous cell carcinoma (OPSCC) earlier than standard clinical and imaging assessments, a new analysis indicates.
Of 80 patients who tested positive for circulating tumor tissue–modified viral (TTMV)-HPV DNA during surveillance, 74% (n = 59) had no other evidence of disease or had indeterminate disease status.
And of those patients, 93% (n = 55) “later had proven recurrent, metastatic disease on imaging and/or biopsy,” according to Glenn Hanna, MD, from the Dana-Farber Cancer Institute, Boston, who presented the results Feb. 24 at the 2022 Multidisciplinary Head and Neck Cancers Symposium.
“This is the first study to demonstrate broad clinical utility and validity of the biomarker in HPV-driven oropharyngeal cancer,” Dr. Hanna said in a press release.
Although patients with HPV-driven OPSCC generally have favorable outcomes, up to 25% will experience recurrence after treatment.
Post-treatment surveillance currently relies on physical examinations and imaging, but Dr. Hanna and colleagues wanted to determine whether a routine circulating cell-free TTMV-HPV DNA test could detect occult recurrence sooner.
Dr. Hanna and colleagues analyzed the records of 1,076 patients with HPV-driven OPSCC at 118 sites in the U.S. who had completed therapy more than 3 months previously and undergone an TTMV-HPV DNA test (NavDx, Naveris) between June 2020 and November 2021.
The results of the test, which used ultrasensitive digital droplet PCR to identify HPV subtypes 16, 18, 31, 33, and 35, were compared with subsequent clinical evidence of OPSCC via nasopharyngolaryngoscopy, radiologic evaluations, or tissue biopsy.
Approximately 7% of the patients tested positive (n = 80) for circulating TTMV-HPV DNA. Of those, 26.2% (n = 21) had known clinical recurrence, while 73.8% (n = 59) had no other evidence of disease or an intermediate disease status.
Among those with no clinical evidence of recurrence, 93.2% (n = 55) had their recurrence subsequently confirmed using imaging or biopsy. Of the 4 remaining patients, 2 had clinically suspicious lesions, and 2 had no other evidence of disease.
Overall, the data indicate that the biomarker test demonstrated a 95% positive predictive value (76 of 80 patients) for recurrence or persistence of HPV-driven OPSCC.
According to Dr. Hanna, a positive TTMV-HPV DNA test was the first indicator of recurrence for 72% of patients, and almost half of recurrences were detected more than 12 months after completing therapy.
“Incorporating a test for TTMV-HPV DNA into routine post-treatment follow-up can enable physicians to detect recurrent cancers earlier and allow us to start recommended interventions more quickly to improve outcomes,” Dr. Hanna said in the release.
The study was supported by Naveris, which developed the TTMV-HPV DNA test studied. Dr. Hanna declares relationships with Actuate Therapeutics, Altor BioScience, Bicara, BMS, GSK, Merck, Regeneron, Sanofi/Genzyme, and others.
A version of this article first appeared on Medscape.com.
PHOENIX – A liquid biopsy test may accurately predict recurrence of human papillomavirus (HPV)–driven oropharyngeal squamous cell carcinoma (OPSCC) earlier than standard clinical and imaging assessments, a new analysis indicates.
Of 80 patients who tested positive for circulating tumor tissue–modified viral (TTMV)-HPV DNA during surveillance, 74% (n = 59) had no other evidence of disease or had indeterminate disease status.
And of those patients, 93% (n = 55) “later had proven recurrent, metastatic disease on imaging and/or biopsy,” according to Glenn Hanna, MD, from the Dana-Farber Cancer Institute, Boston, who presented the results Feb. 24 at the 2022 Multidisciplinary Head and Neck Cancers Symposium.
“This is the first study to demonstrate broad clinical utility and validity of the biomarker in HPV-driven oropharyngeal cancer,” Dr. Hanna said in a press release.
Although patients with HPV-driven OPSCC generally have favorable outcomes, up to 25% will experience recurrence after treatment.
Post-treatment surveillance currently relies on physical examinations and imaging, but Dr. Hanna and colleagues wanted to determine whether a routine circulating cell-free TTMV-HPV DNA test could detect occult recurrence sooner.
Dr. Hanna and colleagues analyzed the records of 1,076 patients with HPV-driven OPSCC at 118 sites in the U.S. who had completed therapy more than 3 months previously and undergone an TTMV-HPV DNA test (NavDx, Naveris) between June 2020 and November 2021.
The results of the test, which used ultrasensitive digital droplet PCR to identify HPV subtypes 16, 18, 31, 33, and 35, were compared with subsequent clinical evidence of OPSCC via nasopharyngolaryngoscopy, radiologic evaluations, or tissue biopsy.
Approximately 7% of the patients tested positive (n = 80) for circulating TTMV-HPV DNA. Of those, 26.2% (n = 21) had known clinical recurrence, while 73.8% (n = 59) had no other evidence of disease or an intermediate disease status.
Among those with no clinical evidence of recurrence, 93.2% (n = 55) had their recurrence subsequently confirmed using imaging or biopsy. Of the 4 remaining patients, 2 had clinically suspicious lesions, and 2 had no other evidence of disease.
Overall, the data indicate that the biomarker test demonstrated a 95% positive predictive value (76 of 80 patients) for recurrence or persistence of HPV-driven OPSCC.
According to Dr. Hanna, a positive TTMV-HPV DNA test was the first indicator of recurrence for 72% of patients, and almost half of recurrences were detected more than 12 months after completing therapy.
“Incorporating a test for TTMV-HPV DNA into routine post-treatment follow-up can enable physicians to detect recurrent cancers earlier and allow us to start recommended interventions more quickly to improve outcomes,” Dr. Hanna said in the release.
The study was supported by Naveris, which developed the TTMV-HPV DNA test studied. Dr. Hanna declares relationships with Actuate Therapeutics, Altor BioScience, Bicara, BMS, GSK, Merck, Regeneron, Sanofi/Genzyme, and others.
A version of this article first appeared on Medscape.com.
Tastier chocolate may be healthier chocolate
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Practicing across state lines: A challenge for telemental health
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
I was taught to think clinically first and legally second. There are moments when following every regulation is clearly detrimental to the well-being of both the patient and the medical community at large, and these challenges have been highlighted by issues with telemental health during the pandemic.
A friend emailed me with a problem: He has a son who is a traveling nurse and is currently in psychotherapy. The therapist has, in accordance with licensing requirements, told his son that she can not see him when assignments take him to any state where she is not licensed. The patient needs to physically be in the same state where the clinician holds a license, technically for every appointment. The nursing assignments last for 3 months and he will be going to a variety of states. Does he really need to get a new therapist every 90 days?
The logistics seem mind-boggling in a time when there is a shortage of mental health professionals, and there are often long wait lists to get care. And even if it was all easy, I’ll point out that working with a therapist is a bit different then going to an urgent care center to have sutures removed or to obtain antibiotics for strep throat: The relationship is not easily interchangeable, and I know of no one who would think it clinically optimal for anyone to change psychotherapists every 3 months. The traveling nurse does not just need to find a “provider” in each state, he needs to find one he is comfortable with and he will have to spend several sessions relaying his history and forming a new therapeutic alliance. And given the ambiguities of psychotherapy, he would optimally see therapists who do not make conflicting interpretations or recommendations. Mind-boggling. And while none of us are irreplaceable, it feels heartless to tell someone who is traveling to provide medical care to others during a pandemic that they can’t have mental health care when our technology would allow for it.
In the “old days” it was simpler: Patients came to the office and both the patient and the clinician were physically located in the same state, even if the patient resided in another state and commuted hours to the appointment. Telemental health was done in select rural areas or in military settings, and most physicians did not consider the option for video visits, much less full video treatment. For the average practitioner, issues of location were not relevant. The exception was for college students who might reside in one state and see a psychiatrist or therapist in another, but typically everyone was comfortable taking a break from therapy when the patient could not meet with the therapist in person. If psychiatrists were having phone or video sessions with out-of-state patients on an occasional basis, it may have been because there was less scrutiny and it was less obvious that this was not permitted.
When the pandemic forced treatment to go online, the issues changed. At the beginning, issues related to state licensing were waived. Now each state has a different requirement with regard to out-of-state physicians; some allow their residents to be seen, while others require the physician to get licensed in their state and the process may or may not be costly or arduous for the provider. The regulations change frequently, and can be quite confusing to follow. Since psychiatry is a shortage field, many psychiatrists are not looking to have more patients from other states and are not motivated to apply for extra licenses.
Life as a practicing psychiatrist has been a moving target: I reopened my practice for some in-person visits for vaccinated patients in June 2021, then closed it when the Omicron surge seemed too risky, and I’ll be reopening soon. Patients, too, have had unpredictable lives.
For the practitioner who is following the rules precisely, the issues can be sticky. It may be fine to have Zoom visits with a patient who lives across the street, but not with the elderly patient who has to drive 90 minutes across a state line, and it’s always fine to have a video session with a patient in Guam. If a patient signs on for a video visit with a doctor licensed in Maine and announces there will be a visit to a brother in Michigan, does the clinician abruptly end the session? Does he charge for the then missed appointment, and don’t we feel this is a waste of the psychiatrist’s time when appointments are limited?
If college students started with therapists in their home states when universities shut down in the spring of 2020, must they now try to get treatment in the states where their college campuses are located? What if the university has a long wait for services, there are no local psychiatrists taking on new patients, or the student feels he is making good progress with the doctor he is working with? And how do we even know for sure where our patients are located? Are we obligated to ask for a precise location at the beginning of each session? What if patients do not offer their locations, or lie about where they are?
Oddly, the issue is with the location of the patient; the doctor can be anywhere as long as the patient’s body is in a state where he or she is licensed. And it has never been a problem to send prescriptions to pharmacies in other states, though this seems to me the essence of practicing across state lines.
In the State of the Union Address on March 1, President Biden had a hefty agenda: The Russian invasion of Ukraine, a global pandemic, spiraling inflation, and for the first time in a SOTU address, our president discussed a strategy to address our National Mental Health Crisis. The fact sheet released by the White House details many long-awaited changes to increase the mental health workforce to address shortages, instituting a “988” crisis line to initiate “someone to call, someone to respond, and somewhere for every American in crisis to go.” The proposals call for a sweeping reform in providing access to services, strengthening parity, and improving community, veterans, and university services – and the Biden administration specifically addresses telemental health. “To maintain continuity of access, the Administration will work with Congress to ensure coverage of tele-behavioral health across health plans, and support appropriate delivery of telemedicine across state lines.”
This is good news, as it’s time we concentrated on allowing for access to care in a consumer-oriented way. It may let us focus on offering good clinical care and not focus on following outdated regulations. Hopefully, those who want help will be able to access it, and perhaps soon a traveling nurse will be permitted to get mental health care with continuity of treatment.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
Dressing in blue
On the first Friday in March, it has become an annual tradition to dress in blue to promote colorectal cancer awareness. Twitter feeds are filled with photos of members of our gastroenterology community (sometimes entire endoscopy units!) swathed in various shades of blue. This tradition was started in the mid-2000’s by a patient diagnosed with early-onset colorectal cancer who planned a fund raiser at her daughter’s elementary school where students were encouraged to wear a blue outfit and make a $1 donation to support awareness of this deadly but preventable cancer. What was once a local effort has now grown into a national phenomenon, and a powerful opportunity for the medical community to educate patients, friends, and family regarding risk factors for colorectal cancer and the importance of timely and effective screening. But while raising awareness is vital, it is only an initial step in the complex process of optimizing delivery of screening services and improving cancer outcomes through prevention and early detection.
In this month’s issue of GIHN, we report on a study from Cancer demonstrating the effectiveness of Spanish-speaking patient navigators in boosting colorectal cancer screening rates among Hispanic patients. We also highlight a quality improvement initiative at a large academic medical center demonstrating the impact of an electronic “primer” message delivered through the patient portal on screening completion rates in a mailed fecal immunochemical test outreach program. Finally, in this month’s Practice Management Toolbox column, Dr. Brill and Dr. Lieberman advise us on how to prepare for upcoming coverage changes impacting screening colonoscopy – a result of AGA’s tireless efforts to eliminate financial barriers impeding access to colorectal cancer screening.
As always, thank you for being a dedicated reader and please stay safe out there. Better days are ahead.
Megan A. Adams, MD, JD, MSc
Editor in Chief
On the first Friday in March, it has become an annual tradition to dress in blue to promote colorectal cancer awareness. Twitter feeds are filled with photos of members of our gastroenterology community (sometimes entire endoscopy units!) swathed in various shades of blue. This tradition was started in the mid-2000’s by a patient diagnosed with early-onset colorectal cancer who planned a fund raiser at her daughter’s elementary school where students were encouraged to wear a blue outfit and make a $1 donation to support awareness of this deadly but preventable cancer. What was once a local effort has now grown into a national phenomenon, and a powerful opportunity for the medical community to educate patients, friends, and family regarding risk factors for colorectal cancer and the importance of timely and effective screening. But while raising awareness is vital, it is only an initial step in the complex process of optimizing delivery of screening services and improving cancer outcomes through prevention and early detection.
In this month’s issue of GIHN, we report on a study from Cancer demonstrating the effectiveness of Spanish-speaking patient navigators in boosting colorectal cancer screening rates among Hispanic patients. We also highlight a quality improvement initiative at a large academic medical center demonstrating the impact of an electronic “primer” message delivered through the patient portal on screening completion rates in a mailed fecal immunochemical test outreach program. Finally, in this month’s Practice Management Toolbox column, Dr. Brill and Dr. Lieberman advise us on how to prepare for upcoming coverage changes impacting screening colonoscopy – a result of AGA’s tireless efforts to eliminate financial barriers impeding access to colorectal cancer screening.
As always, thank you for being a dedicated reader and please stay safe out there. Better days are ahead.
Megan A. Adams, MD, JD, MSc
Editor in Chief
On the first Friday in March, it has become an annual tradition to dress in blue to promote colorectal cancer awareness. Twitter feeds are filled with photos of members of our gastroenterology community (sometimes entire endoscopy units!) swathed in various shades of blue. This tradition was started in the mid-2000’s by a patient diagnosed with early-onset colorectal cancer who planned a fund raiser at her daughter’s elementary school where students were encouraged to wear a blue outfit and make a $1 donation to support awareness of this deadly but preventable cancer. What was once a local effort has now grown into a national phenomenon, and a powerful opportunity for the medical community to educate patients, friends, and family regarding risk factors for colorectal cancer and the importance of timely and effective screening. But while raising awareness is vital, it is only an initial step in the complex process of optimizing delivery of screening services and improving cancer outcomes through prevention and early detection.
In this month’s issue of GIHN, we report on a study from Cancer demonstrating the effectiveness of Spanish-speaking patient navigators in boosting colorectal cancer screening rates among Hispanic patients. We also highlight a quality improvement initiative at a large academic medical center demonstrating the impact of an electronic “primer” message delivered through the patient portal on screening completion rates in a mailed fecal immunochemical test outreach program. Finally, in this month’s Practice Management Toolbox column, Dr. Brill and Dr. Lieberman advise us on how to prepare for upcoming coverage changes impacting screening colonoscopy – a result of AGA’s tireless efforts to eliminate financial barriers impeding access to colorectal cancer screening.
As always, thank you for being a dedicated reader and please stay safe out there. Better days are ahead.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Autism spectrum disorder: Keys to early detection and accurate diagnosis
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.
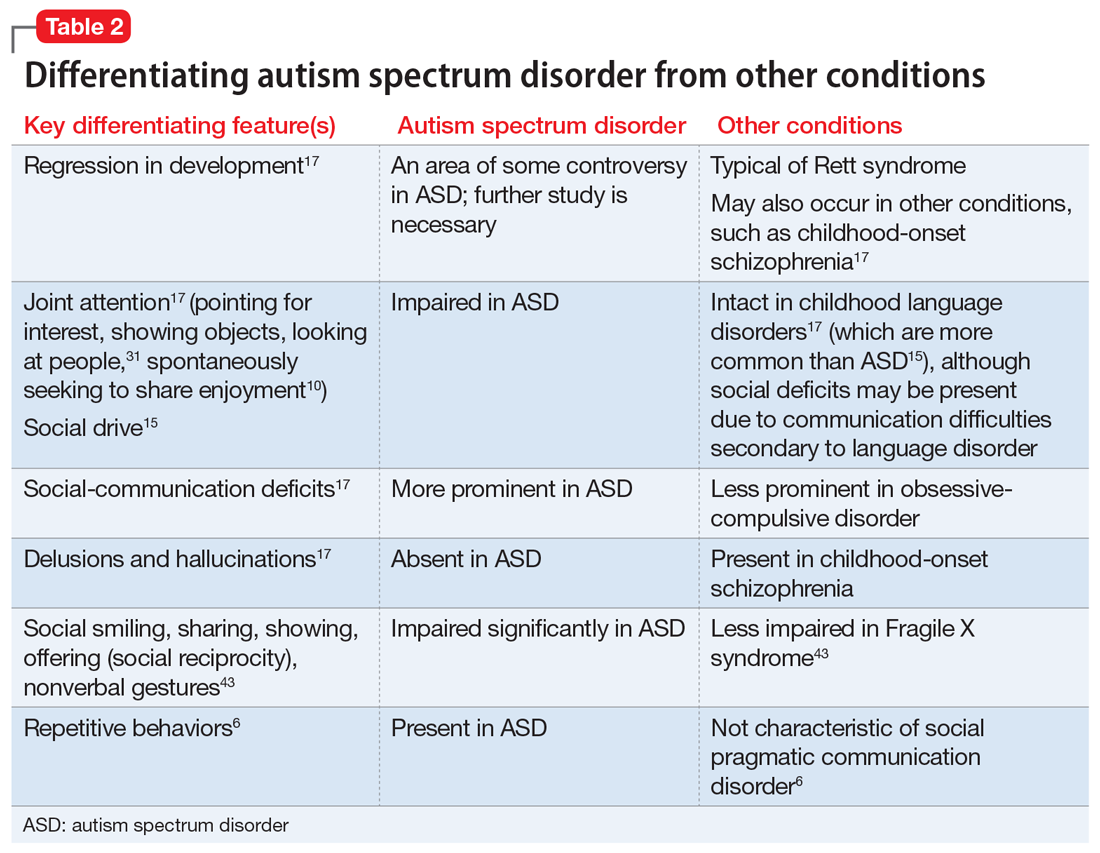
Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.

Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
FIRST OF 2 PARTS
Autism spectrum disorder (ASD) is a complex, heterogenous neurodevelopmental disorder with genetic and environmental underpinnings, and an onset early in life.1-9 It affects social communication, cognition, and sensory-motor domains, and manifests as deficits in social reciprocity, repetitive behavior, restricted range of interests, and sensory sensitivities.6,10-14 In recent years, the prevalence of ASD has been increasing.3,6,10 A large percentage of individuals with ASD experience significant social deficits in adulthood,10 which often leads to isolation, depressive symptoms, and poor occupational and relationship functioning.15,16 Interventions in early childhood can result in significant and lasting changes in outcome and in functioning of individuals with ASD.
This article provides an update on various aspects of ASD diagnosis, with the goal of equipping clinicians with knowledge to help make an accurate ASD diagnosis at an early stage. Part 1 focuses on early detection and diagnosis, while Part 2 will describe treatment strategies.
Benefits of early detection
Substantial research has established that early intervention confers substantial benefits for outcomes among children with ASD.2,3,5,6,9,13,14,16-22 Earlier age of intervention correlates with greater developmental gain and symptom reduction.21,23 The atypical neural development responsible for ASD likely occurs much earlier than the behavioral manifestations of this disorder, which implies that there is a crucial period to intervene before behavioral features emerge.1 This necessitates early recognition of ASD,9,17 and the need for further research to find novel ways to detect ASD earlier.
In the United States, children with ASD are diagnosed with the disorder on average between age 3 and 4 years.6,24 However, evidence suggests there may be a prodromal phase for ASD during the first several months of life, wherein infants and toddlers exhibit developmentally inadequate communication and social skills and/or unusual behaviors.18 Behavioral signs suggestive of ASD may be evident as early as infancy, and commonly earlier than age 18 months.1,17,19 Problems with sleeping and eating may be evident in early childhood.19 Deficits in joint attention may be evident as early as age 6 months to 8 months. Research suggests that a diagnosis of ASD by trained, expert professionals is likely to be accurate at the age of 2, and even as early as 18 months.6,24
In a prospective study, Anderson et al25 found that 9% of children who were diagnosed with ASD at age 2 no longer met the diagnostic criteria for ASD by adulthood.6 Those who no longer met ASD criteria were more likely to have received early intervention, had a verbal IQ ≥70, and had experienced a larger decrease in repetitive behaviors between ages 2 and 3, compared with other youth in this study who had a verbal IQ ≥70. One of the limitations of this study was a small sample size (85 participants); larger, randomized studies are needed to replicate these findings.25
Continue to: Characteristics of ASD...
Characteristics of ASD
Table 16,8,10,13,15,26-29 outlines various characteristics of ASD, which may manifest in varying degrees among children with the condition.
Speech/language. Speech helps to facilitate bonding between parents and an infant by offering a soothing, pleasurable, and reinforcing experience.30 More than 50% of children with ASD have language delays or deficits that persist throughout adulthood.13 The extent of these language deficits varies; in general, the more severe the speech/language deficits, the more severe the long-term symptoms.13 Language deficits in young children with ASD tend to be of both the expressive and receptive type, with onset in infancy, which suggests that neural processes predate the emergence of behavioral symptoms of ASD, and also that early language deficits/delays could be a marker for or indicator of future risk of ASD.13 Individuals with ASD also have been noted to have limitations in orienting or attending to human voices.13,30
Facial recognition. Evidence has linked ASD with deficits in facial recognition that emerge in the first few months of life.2 Earlier studies have found that lack of attention to others’ faces was the strongest distinguishing factor between 1-year-olds with ASD and typically developing 1-year-olds.2,31 A recent study that used EEG to compare facial emotion recognition in boys with ASD vs typically developing boys found that boys with ASD exhibited significantly lower sensitivity to angry and fearful faces.27
Other features. A 2020 study (N = 37) found that compared with typically developing children, those with ASD show less “interactional synchrony’’ (a dynamic process in which the timing of children and caregivers’ behaviors [specifically, vocalizations and movements] become mutually coordinated) with both familiar and unfamiliar adults.32 These researchers concluded that impairment in interactional synchrony may be linked to social communication deficits in ASD.32
A recent study (N = 98) evaluated “sluggish cognitive tempo” in 3 groups of children: children with attention-deficit/hyperactivity disorder (ADHD), children with ASD, and children with both ADHD and ASD.33 It found that children with ASD exhibited sluggish cognitive tempo at levels similar to those of the other 2 groups, and indicated that sluggish cognitive tempo may be linked with “social and global impairment above and beyond” the impairment associated with ASD.
Understanding early aberrations in neurobiologic processes in ASD can help develop biomarkers for early recognition of ASD, as well as guide the development of targeted interventions and treatments (Box1-3,7-9,12,13,30,35-39).
Box
Compared with individuals who do not have autism spectrum disorder (ASD), individuals with ASD exhibit anatomical differences in the brain that can be seen on MRI.9,35 Brain regions affected in ASD include the frontal gyrus, temporal gyrus, cingulate gyrus, postcentral gyrus, precuneus, caudate, and hippocampus.9 Some studies have found anomalous structural neural characteristics in infants, such as in the uncinate fasciculus, that correlated with later joint attention challenges, while others have found aberrations in the corpus callosum(responsible for transfer of procedural learning between the hemispheres, and oculomotor response)and internal capsule (responsible for sensorimotor function, as well as other functions) in children with ASD.12
Widespread white matter anomalies have been noted in ASD.12,35,36 In a 2-year longitudinal study that used diffusion tensor imaging, Li et al35 found that preschool children with ASD experience overgrowth of the uncinate fasciculus, which is one of the brain regions implicated in socioemotional processing, and concluded that this overgrowth correlated with ASD severity.35 Andrews et al37 used diffusion-weighted MRI to examine white matter in 127 preschool children. They found that compared with typically developing children, children with ASD exhibited altered white matter microstructure.37
Research suggests that developing representations of the reward value of social stimuli may be challenging for children with ASD.2 Abrams et al30 used resting-state functional brain MRI to evaluate children with typical development and children with highfunctioning, “verbally fluent” ASD. They found that the children with ASD exhibited lower functional connectivity between voice-specific left hemisphere posterior superior temporal sulcus and areas representing the reward circuitry.30 This study also found that children with ASD had underconnectivity between the right hemisphere posterior superior temporal sulcus (which deals with speech prosody) and areas known for emotion-linked associative learning, the orbitofrontal cortex and amygdala.30 These findings are thought to align with the social motivation theory of ASD.13,30,38
The extent of underconnectivity between these systems was found to determine the severity of communication challenges in high-functioning children with ASD.30 One MRI study observed lower gray matter volume in the voice-selective bilateral superior temporal sulcus in children age approximately 9 to 11 years with ASD.39
Neural systems responsible for facial recognition (particularly the right fusiform gyrus and other brain areas) have been shown to exist or begin “very early in life,” which suggests that impaired face recognition may be an early marker of ASD.2 In addition to problems with visual scanning, preferential attention to (and visual sensitivity to) biological motion is a forerunner for the development of social interactions in infants, specifically in regard to being able to detect and recognize emotion, which is considered vital for attachment.7,8 Impaired biological motion perception has been found in very young children with ASD.7,8 This presents an important avenue/potential biomarker for further research to better understand neurobiologic processes underlying atypical development at an earlier age.3,8
Early neural biomarkers for ASD
Nonlinear EEG values may serve as an early neurobiomarker for detecting ASD in young children.1 Because it is relatively inexpensive and convenient, EEG may be highly useful for detecting ASD.1 A study that compared EEG results of 99 infants who had siblings with ASD and 89 low-risk controls from age 3 months to 36 months found that nonlinear EEG measurements predicted with high accuracy later diagnosis of ASD, and were strongly correlated with later Autism Diagnostic Observation Schedule scores.1
Continue to: A complex differential diagnosis...
A complex differential diagnosis
The differential diagnosis of ASD warrants careful attention and consideration to rule out other developmental and psychiatric conditions.
Intellectual disability (ID). DSM-5 diagnostic criteria for ASD necessitate that disturbances are not better explained by ID or global developmental delay and that deficits should exceed impairment consistent with the level of intellectual disability.28 Still, ASD is often overdiagnosed in children with ID.28 Research suggests phenotypic and genetic overlap between ID and ASD.28 Social functioning is often impaired in patients with ID; the greater the severity of ID, the greater the degree of social deficits.28 In approximately 30% of cases, ASD and ID are comorbid.6 This overlap and comorbidity can pose a challenge, particularly due to the inherent complexities involved in assessment and differentiation.28 When ID is present in ASD, there is a greater degree of social-communication deficits.6 It may be difficult to assess for ASD symptoms in children with severe ID.28 Although there is no minimum age or developmental level below which ASD should not be diagnosed, some studies have started to use minimum criteria for diagnosis, such as a nonverbal mental age of 18 months.28,40 Commonly used tests for ASD have much lower specificity when used for children with nonverbal age <15 months.28 It would make sense, then, that the presence of ID might significantly affect the results of these diagnostic tests.28
Other conditions that need to be ruled out include language disorders, hearing loss, rare genetic neurodevelopmental disorders (eg, Fragile X syndrome,3 Rett syndrome6), childhood-onset schizophrenia, obsessive-compulsive disorder, attachment disorders, and other conditions.18 ASD may be overdiagnosed in children with genetic disorders such as Angelman syndrome.41 In a systematic review, Moss and Howlin42 recommended caution when evaluating ASD-like behavioral symptoms in children with genetic syndromes and severe ID. On the other hand, some research has observed that individuals with Fragile X syndrome may exhibit symptoms that meet criteria for ASD.6,43 McDuffie et al43 used the Autism Diagnostic Interview-Revised (ADI-R) to compare boys with Fragile X syndrome who also met criteria for ASD with boys with nonsyndromic ASD. Those in the former group had lesser impairment in social smiling, offering, showing, and nonverbal gestures, but had more complex mannerisms, compared with boys in the latter group.43
Milder manifestations of ASD may be more challenging to diagnose,1 particularly in children age <3 and those with above-average cognition.6 Generally, in the case of a patient with ASD, parents find that the child did not have a period of typical development, or unusual behaviors were evident early on.17
ASD can be comorbid with ADHD. The presence of ADHD may mask or delay the diagnosis of ASD in children.6 In children with both ASD and ADHD, studies have found greater reduction in social and adaptive functioning compared with children with ADHD alone.44
Table 26,10,15,17,31,43 highlights some of the features that can be used to distinguish ASD from other conditions.

Continue to: Screening and diagnosis...
Screening and diagnosis
A medical workup is the first step to rule out other potential conditions that could be masquerading as ASD.17 Obtain a comprehensive history from parents/caregivers, particularly regarding social, behavioral, movement, sensory, and developmental aspects. In addition, audiologic testing is an essential step. Consider genetic testing, particularly if any dysmorphic features and/or ID are present, both of which confer additional risk for a genetic syndrome.6 A physical exam to detect any neurologic anomalies, organ dysfunction, and body dysmorphic features should be conducted.6
The Modified Checklist for Autism in Toddlers–Revised (MCHAT-R) is a commonly used, validated parental screening survey for ASD.5,6 Research has shown that this survey has <50% specificity.5A recent American Academy of Pediatrics Clinical Report recommended universal screening for ASD at pediatric visits at age 18 months and at 24 months, in addition to developmental screening for all children at routine pediatric visits at age 9, 18, and 30 months.6,19
Screening tools such as the Modified Checklist for Autism in Toddlers with Follow-Up (M-CHAT/F) can be integrated into routine primary health care. In a large (N = 25,999) study, Guthrie et al45 used M-CHAT/F to conduct universal, primary care–based screening in young children. They found that the positive predictive value of M-CHAT/F was lower among girls, children of color, and those from lower-income households. There is a need for development of screening tools with higher accuracy and sensitivity for identifying young children with ASD regardless of their ethnic or socioeconomic background, and also for children older than 30 months.5,6,45
Definitive diagnosis of ASD is ideally done by a multidisciplinary team46 using established gold standard measures such as the ADOS (Autism Diagnostic Observation Schedule) and ADI-R.47 Such multidisciplinary teams usually include a child psychiatrist, child psychologist, speech therapist, occupational therapist, school educator, and developmental pediatrician. However, because there are long wait times to receive this type of diagnosis in the United States,6 in the interest of not missing the critical window of early intervention, physicians who suspect a patient may have ASD should refer the child and family for appropriate educational and behavioral interventions as early as possible, rather than waiting for definitive testing.6
ADI-R has limitations in distinguishing ASD from other conditions, especially in very young children, and particularly in distinguishing ASD from childhood-onset schizophrenia.47 Similarly, ADOS, which is a semi-structured, standardized, observation assessment tool, also has limitations, including generating false-positive results, which can make it difficult to distinguish children and adolescents with developmental disabilities from those with ASD.47 However, in combination, these 2 tools are generally efficacious.47 Further research is warranted to develop and fine-tune definitive diagnostic tools with greater sensitivity and specificity.
A newer measure—the Autism Parent Screen for Infants (APSI) questionnaire—has been shown to be effective in detecting early signs predictive of ASD in high-risk infants (eg, siblings of children with ASD), and has potential as an early screening tool.48,49
Part 2 of this article will review nonpharmacologic and pharmacologic treatments for patients with ASD.
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
1. Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8(1):6828. doi:10.1038/s41598-018-24318-x
2. Dawson G, Carver L, Meltzoff AN, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700-717. doi:10.1111/1467-8624.00433
3. Frye RE, Vassall S, Kaur G, et al. Emerging biomarkers in autism spectrum disorder: a systematic review. Ann Transl Med. 2019;7(23):792. doi:10.21037/atm.2019.11.5
4. Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110(52):20953-20958. doi:10.1073/pnas.1312857110
5. Hicks SD, Carpenter RL, Wagner KE, et al. Saliva microRNA differentiates children with autism from peers with typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2020;59(2):296-308.
6. Hyman SL, Levy SE, Myers SM, et al; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi:10.1542/peds.2019-3447
7. Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107(49):21223-1228. doi:10.1073/pnas.1010412107
8. Klin A, Lin DJ, Gorrindo P, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257-261. doi:10.1038/nature07868
9. Chen T, Chen Y, Yuan M, et al. Towards developing a practical artificial intelligence tool for diagnosing and evaluating autism spectrum disorder: a study using multicenter ABIDE II datasets. JMIR Med Inform. 2020;8(5):e15767. doi:10.2196/15767
10. Maglione MA, Gans D, Das L, et al; Technical Expert Panel, & HRSA Autism Intervention Research – Behavioral (AIR‐B) Network. Nonmedical interventions for children with ASD: recommended guidelines and further research needs. Pediatrics. 2012;30(Suppl 2), S169-S178.
11. Monz BU, Houghton R, Law K, et al. Treatment patterns in children with autism in the United States. Autism Res. 2019;12(3):5170-526. doi:10.1002/aur.2070
12. Shukla DK, Keehn B, Lincoln AJ, et al. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1269-1278.e12782. doi:10.1016/j.jaac.2010.08.018
13. Sperdin HF, Schaer M. Aberrant development of speech processing in young children with autism: new insights from neuroimaging biomarkers. Front Neurosci. 2016;10:393. doi: 10.3389/fnins.2016.00393
14. Zwaigenbaum L, Brian JA, Ip A. Early detection for autism spectrum disorder in young children. Paediatr Child Health. 2019;24(7):424-443. doi:10.1093/pch/pxz119
15. Simms MD, Jin XM. Autism, language disorder, and social (pragmatic) communication disorder: DSM-V and differential diagnoses. Pediatr Rev. 2015;36(8):355-363. doi:10.1542/pir.36-8-355
16. Su Maw S, Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for autism spectrum disorder in preschool-aged children: a systematic review and meta-analysis. Heliyon. 2018;4(9):e00763. doi:10.1016/j.heliyon.2018.e00763
17. Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 2014;53(2):237-257.
18. Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30(1):25-39. doi:10.1080/09540261.2018.1432574
19. Lipkin PH, Macias MM; Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145(1)e20193449. doi:10.1542/peds.2019-3449
20. Pickles A, Le Couteur A, Leadbitter K, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. 2016;388:2501-2509.
21. Rogers SJ, Estes A, Lord C, et al. Effects of a brief early start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1052-1065. doi:10.1016/j.jaac.2012.08.003
22. Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411-2428. doi:10.1007/s10803-015-2407-8
23. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. 2018;47(6):497-514.
24. Zwaigenbaum L, Bryson SE, Brian J, et al. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res. 2016;9(7):790-800. doi:10.1002/aur.1585
25. Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485-494. doi:10.1111/jcpp.12178
26. Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65(8):946-954. doi:10.1001/archpsyc.65.8.946
27. Van der Donck S, Dzhelyova M, Vettori S, et al. Rapid neural categorization of angry and fearful faces is specifically impaired in boys with autism spectrum disorder. J Child Psychol Psychiatry. 2020;61(9):1019-1029. doi:10.1111/jcpp.13201
28. Thurm A, Farmer C, Salzman E, et al. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry. 2019;10:526. doi:10.3389/fpsyt.2019.00526
29. Kuno-Fujita A, Iwabuchi T, Wakusawa K, et al. Sensory processing patterns and fusiform activity during face processing in autism spectrum disorder. Autism Res. 2020;13(5):741-750. doi: 10.1002/aur.2283
30. Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060-12065. doi:10.1073/pnas.1302982110
31. Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24(3):247-257.
32. Zampella CJ, Csumitta KD, Simon E, et al. Interactional synchrony and its association with social and communication ability in children with and without autism spectrum disorder. J Autism Dev Disord. 2020;50(9):3195-3206. doi:10.1007/s10803-020-04412-8
33. McFayden T, Jarrett MA, White SW, et al. Sluggish cognitive tempo in autism spectrum disorder, ADHD, and their comorbidity: implications for impairment. J Clin Child Adolesc Psychol. 2020:1-8. doi:10.1080/15374416.2020.1716365
34. Baribeau DA, Vigod S, Pullenayegum E, et al. Repetitive behavior severity as an early indicator of risk for elevated anxiety symptoms in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2020;59(7):890-899.e3. doi:10.1016/j.jaac.2019.08.478
35. Li Y, Zhou Z, Chang C, et al. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry. 2019;19(1):399. doi:10.1186/s12888-019-2391-1
36. Payabvash S, Palacios EM, Owen JP, et al. White matter connectome edge density in children with autism spectrum disorders: potential imaging biomarkers using machine-learning models. Brain Connect. 2019;9(2):209-220. doi:10.1089/brain.2018.0658
37. Andrews DS, Lee JK, Solomon M, et al. A diffusion-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodev Disord. 2019;11(1):32. doi:10.1186/s11689-019-9291-z
38. Chevallier C, Kohls G, Troiani V, et al. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231-239. doi:10.1016/j.tics.2012.02.007
39. Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364-369. doi:10.1016/j.neuroimage.2004.06.016
40. Lord C, Petkova E, Hus V, et al. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gen Psychiatry. 2012;69(3):306-313. doi:10.1001/archgenpsychiatry.2011.148
41. Trillingsgaard A, ØStergaard JR. Autism in Angelman syndrome: an exploration of comorbidity. Autism. 2004;8(2):163-174.
42. Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852-873. doi:10.1111/j.1365-2788.2009.01197.x
43. McDuffie A, Thurman AJ, Hagerman RJ, et al. Symptoms of autism in males with Fragile X syndrome: a comparison to nonsyndromic ASD using current ADI-R scores. J Autism Dev Disord. 2015;45(7):1925-1937. doi:10.1007/s10803-013-2013-6
44. Ashwood KL, Tye C, Azadi B, et al. Brief report: adaptive functioning in children with ASD, ADHD and ASD + ADHD. J Autism Dev Disord. 2015;45(7):2235-4222. doi:10.1007/s10803-014-2352-y
45. Guthrie W, Wallis K, Bennett A, et al. Accuracy of autism screening in a large pediatric network. Pediatrics. 2019;144(4): e20183963. doi:10.1542/peds.2018-3963
46. Brian JA, Zwaigenbaum L, Ip A. Standards of diagnostic assessment for autism spectrum disorder. Paediatr Child Health. 2019;24(7):444-460. doi:10.1093/pch/pxz117
47. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: interests, limits and openings. Encephale. 2019;45(5):441-448. doi:10.1016/j.encep.2019.07.002
48. Sacrey LR, Zwaigenbaum L, Bryson S, et al. Screening for behavioral signs of autism spectrum disorder in 9-month-old infant siblings. J Autism Dev Disord. 2021;51(3):839-848. doi:10.1007/s10803-020-04371-0
49. Sacrey LR, Bryson S, Zwaigenbaum L, et al. The autism parent screen for infants: predicting risk of autism spectrum disorder based on parent-reported behavior observed at 6-24 months of age. Autism. 2018;22(3):322-334
Differentiating pediatric schizotypal disorder from schizophrenia and autism
Schizotypal disorder is a complex condition that is characterized by cognitive-perceptual impairments, oddness, disorganization, and interpersonal difficulties. It often is unrecognized or underdiagnosed. In DSM-5, schizotypal disorder is categorized a personality disorder, but it is also considered part of the schizophrenia spectrum disorders.1 The diagnostic criteria for schizotypal disorder are outlined in the Table.1,2

Although schizotypal disorder has a lifetime prevalence of approximately 4% in the general population of the United States,2 it can present during childhood or adolescence and may be overlooked in the differential diagnosis for psychotic symptoms in pediatric patients.3 Schizotypal disorder of childhood (SDC) can present with significant overlap with several pediatric diagnoses, including schizophrenia spectrum disorders and autism spectrum disorder (ASD), all of which may include psychotic symptoms and difficulties in interpersonal relationships. This overlap, combined with the lack of awareness of schizotypal disorder, can pose a diagnostic challenge. Better recognition of SDC could result in earlier and more effective treatment. In this article, we provide tips for differentiating SDC from childhood-onset schizophrenia and from ASD.
Differentiating SDC from schizophrenia
SDC may be mistaken for childhood-onset schizophrenia due to its perceptual disturbances (which may be interpreted as visual or auditory hallucinations), bizarre fantasies (which may be mistaken for overt delusions), paranoia, and odd behavior. Two ways to distinguish SDC from childhood schizophrenia are by clinical course and by severity of negative psychotic symptoms.
SDC tends to have an overall stable clinical course,4 with patients experiencing periods of time when they exhibit a more normal mental status complemented by fluctuations in symptom severity, which are exacerbated by stressors and followed by a return to baseline.3 SDC psychotic symptoms are predominantly positive, and patients typically do not demonstrate negative features beyond social difficulties. Childhood-onset schizophrenia is typically progressive and disabling, with worsening severity over time, and is much more likely to incorporate prominent negative symptoms.3
Differentiating SDC from ASD
SDC also demonstrates considerable diagnostic overlap with ASD, especially with regards to inappropriate affect; odd thinking, behavior, and speech; and social difficulties. Further complicating the diagnosis, ASD and SDC are comorbid in approximately 40% of ASD cases.3,5 The Melbourne Assessment of Schizotypy in Kids demonstrates validity in diagnosing schizotypal disorder in patients with comorbid ASD.5,6 For clinicians without easy access to advanced testing, 2 ways to distinguish SDC from ASD are the content of the odd behavior and thoughts, and the patient’s reaction to social deficits.
In SDC, odd behavior and thoughts most often revolve around daydreaming and a focus on “elaborate inner fantasies.”3,6 Unlike in ASD, in patients with SDC, behaviors don’t typically involve stereotyped mannerisms, the patient is unlikely to have rigid interests (apart from their fantasies), and there is not a particular focus on detail in the external world.3,6 Notably, imaginary companions are common in SDC; children with ASD are less likely to have an imaginary companion compared with children with SDC or those with no psychiatric diagnosis.6 Patients with SDC have social difficulties (often due to social anxiety stemming from their paranoia) but usually seek out interaction and are bothered by alienation, while patients with ASD may have less interest in social engagement.6
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Pulay AJ, Stinson FS, Dawson DA, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV schizotypal personality disorder: results from the wave 2 national epidemiologic survey on alcohol and related conditions. Prim Care Companion J Clin Psychiatry. 2009;11(2):53-67. doi:10.4088/pcc.08m00679
3. Tonge BJ, Testa R, Díaz-Arteche C, et al. Schizotypal disorder in children—a neglected diagnosis. Schizophrenia Bulletin Open. 2020;1(1):sgaa048. doi:10.1093/schizbullopen/sgaa048
4. Asarnow JR. Childhood-onset schizotypal disorder: a follow-up study and comparison with childhood-onset schizophrenia. J Child Adolesc Psychopharmacol. 2005;15(3):395-402.
5. Jones HP, Testa RR, Ross N, et al. The Melbourne Assessment of Schizotypy in Kids: a useful measure of childhood schizotypal personality disorder. Biomed Res Int. 2015;2015:635732. doi:10.1155/2015/635732
6. Poletti M, Raballo A. Childhood schizotypal features vs. high-functioning autism spectrum disorder: developmental overlaps and phenomenological differences. Schizophr Res. 2020;223:53-58. doi:10.1016/j.schres.2020.09.027
Schizotypal disorder is a complex condition that is characterized by cognitive-perceptual impairments, oddness, disorganization, and interpersonal difficulties. It often is unrecognized or underdiagnosed. In DSM-5, schizotypal disorder is categorized a personality disorder, but it is also considered part of the schizophrenia spectrum disorders.1 The diagnostic criteria for schizotypal disorder are outlined in the Table.1,2

Although schizotypal disorder has a lifetime prevalence of approximately 4% in the general population of the United States,2 it can present during childhood or adolescence and may be overlooked in the differential diagnosis for psychotic symptoms in pediatric patients.3 Schizotypal disorder of childhood (SDC) can present with significant overlap with several pediatric diagnoses, including schizophrenia spectrum disorders and autism spectrum disorder (ASD), all of which may include psychotic symptoms and difficulties in interpersonal relationships. This overlap, combined with the lack of awareness of schizotypal disorder, can pose a diagnostic challenge. Better recognition of SDC could result in earlier and more effective treatment. In this article, we provide tips for differentiating SDC from childhood-onset schizophrenia and from ASD.
Differentiating SDC from schizophrenia
SDC may be mistaken for childhood-onset schizophrenia due to its perceptual disturbances (which may be interpreted as visual or auditory hallucinations), bizarre fantasies (which may be mistaken for overt delusions), paranoia, and odd behavior. Two ways to distinguish SDC from childhood schizophrenia are by clinical course and by severity of negative psychotic symptoms.
SDC tends to have an overall stable clinical course,4 with patients experiencing periods of time when they exhibit a more normal mental status complemented by fluctuations in symptom severity, which are exacerbated by stressors and followed by a return to baseline.3 SDC psychotic symptoms are predominantly positive, and patients typically do not demonstrate negative features beyond social difficulties. Childhood-onset schizophrenia is typically progressive and disabling, with worsening severity over time, and is much more likely to incorporate prominent negative symptoms.3
Differentiating SDC from ASD
SDC also demonstrates considerable diagnostic overlap with ASD, especially with regards to inappropriate affect; odd thinking, behavior, and speech; and social difficulties. Further complicating the diagnosis, ASD and SDC are comorbid in approximately 40% of ASD cases.3,5 The Melbourne Assessment of Schizotypy in Kids demonstrates validity in diagnosing schizotypal disorder in patients with comorbid ASD.5,6 For clinicians without easy access to advanced testing, 2 ways to distinguish SDC from ASD are the content of the odd behavior and thoughts, and the patient’s reaction to social deficits.
In SDC, odd behavior and thoughts most often revolve around daydreaming and a focus on “elaborate inner fantasies.”3,6 Unlike in ASD, in patients with SDC, behaviors don’t typically involve stereotyped mannerisms, the patient is unlikely to have rigid interests (apart from their fantasies), and there is not a particular focus on detail in the external world.3,6 Notably, imaginary companions are common in SDC; children with ASD are less likely to have an imaginary companion compared with children with SDC or those with no psychiatric diagnosis.6 Patients with SDC have social difficulties (often due to social anxiety stemming from their paranoia) but usually seek out interaction and are bothered by alienation, while patients with ASD may have less interest in social engagement.6
Schizotypal disorder is a complex condition that is characterized by cognitive-perceptual impairments, oddness, disorganization, and interpersonal difficulties. It often is unrecognized or underdiagnosed. In DSM-5, schizotypal disorder is categorized a personality disorder, but it is also considered part of the schizophrenia spectrum disorders.1 The diagnostic criteria for schizotypal disorder are outlined in the Table.1,2

Although schizotypal disorder has a lifetime prevalence of approximately 4% in the general population of the United States,2 it can present during childhood or adolescence and may be overlooked in the differential diagnosis for psychotic symptoms in pediatric patients.3 Schizotypal disorder of childhood (SDC) can present with significant overlap with several pediatric diagnoses, including schizophrenia spectrum disorders and autism spectrum disorder (ASD), all of which may include psychotic symptoms and difficulties in interpersonal relationships. This overlap, combined with the lack of awareness of schizotypal disorder, can pose a diagnostic challenge. Better recognition of SDC could result in earlier and more effective treatment. In this article, we provide tips for differentiating SDC from childhood-onset schizophrenia and from ASD.
Differentiating SDC from schizophrenia
SDC may be mistaken for childhood-onset schizophrenia due to its perceptual disturbances (which may be interpreted as visual or auditory hallucinations), bizarre fantasies (which may be mistaken for overt delusions), paranoia, and odd behavior. Two ways to distinguish SDC from childhood schizophrenia are by clinical course and by severity of negative psychotic symptoms.
SDC tends to have an overall stable clinical course,4 with patients experiencing periods of time when they exhibit a more normal mental status complemented by fluctuations in symptom severity, which are exacerbated by stressors and followed by a return to baseline.3 SDC psychotic symptoms are predominantly positive, and patients typically do not demonstrate negative features beyond social difficulties. Childhood-onset schizophrenia is typically progressive and disabling, with worsening severity over time, and is much more likely to incorporate prominent negative symptoms.3
Differentiating SDC from ASD
SDC also demonstrates considerable diagnostic overlap with ASD, especially with regards to inappropriate affect; odd thinking, behavior, and speech; and social difficulties. Further complicating the diagnosis, ASD and SDC are comorbid in approximately 40% of ASD cases.3,5 The Melbourne Assessment of Schizotypy in Kids demonstrates validity in diagnosing schizotypal disorder in patients with comorbid ASD.5,6 For clinicians without easy access to advanced testing, 2 ways to distinguish SDC from ASD are the content of the odd behavior and thoughts, and the patient’s reaction to social deficits.
In SDC, odd behavior and thoughts most often revolve around daydreaming and a focus on “elaborate inner fantasies.”3,6 Unlike in ASD, in patients with SDC, behaviors don’t typically involve stereotyped mannerisms, the patient is unlikely to have rigid interests (apart from their fantasies), and there is not a particular focus on detail in the external world.3,6 Notably, imaginary companions are common in SDC; children with ASD are less likely to have an imaginary companion compared with children with SDC or those with no psychiatric diagnosis.6 Patients with SDC have social difficulties (often due to social anxiety stemming from their paranoia) but usually seek out interaction and are bothered by alienation, while patients with ASD may have less interest in social engagement.6
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Pulay AJ, Stinson FS, Dawson DA, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV schizotypal personality disorder: results from the wave 2 national epidemiologic survey on alcohol and related conditions. Prim Care Companion J Clin Psychiatry. 2009;11(2):53-67. doi:10.4088/pcc.08m00679
3. Tonge BJ, Testa R, Díaz-Arteche C, et al. Schizotypal disorder in children—a neglected diagnosis. Schizophrenia Bulletin Open. 2020;1(1):sgaa048. doi:10.1093/schizbullopen/sgaa048
4. Asarnow JR. Childhood-onset schizotypal disorder: a follow-up study and comparison with childhood-onset schizophrenia. J Child Adolesc Psychopharmacol. 2005;15(3):395-402.
5. Jones HP, Testa RR, Ross N, et al. The Melbourne Assessment of Schizotypy in Kids: a useful measure of childhood schizotypal personality disorder. Biomed Res Int. 2015;2015:635732. doi:10.1155/2015/635732
6. Poletti M, Raballo A. Childhood schizotypal features vs. high-functioning autism spectrum disorder: developmental overlaps and phenomenological differences. Schizophr Res. 2020;223:53-58. doi:10.1016/j.schres.2020.09.027
1. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
2. Pulay AJ, Stinson FS, Dawson DA, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV schizotypal personality disorder: results from the wave 2 national epidemiologic survey on alcohol and related conditions. Prim Care Companion J Clin Psychiatry. 2009;11(2):53-67. doi:10.4088/pcc.08m00679
3. Tonge BJ, Testa R, Díaz-Arteche C, et al. Schizotypal disorder in children—a neglected diagnosis. Schizophrenia Bulletin Open. 2020;1(1):sgaa048. doi:10.1093/schizbullopen/sgaa048
4. Asarnow JR. Childhood-onset schizotypal disorder: a follow-up study and comparison with childhood-onset schizophrenia. J Child Adolesc Psychopharmacol. 2005;15(3):395-402.
5. Jones HP, Testa RR, Ross N, et al. The Melbourne Assessment of Schizotypy in Kids: a useful measure of childhood schizotypal personality disorder. Biomed Res Int. 2015;2015:635732. doi:10.1155/2015/635732
6. Poletti M, Raballo A. Childhood schizotypal features vs. high-functioning autism spectrum disorder: developmental overlaps and phenomenological differences. Schizophr Res. 2020;223:53-58. doi:10.1016/j.schres.2020.09.027
How to say ‘no’ to inappropriate patient requests
Although we may want to say “yes” when our patients ask us for certain medications, work excuses, etc, often it is more appropriate to say “no” because the conditions do not support those requests. Saying no to a patient usually is not a comfortable experience, but we should not say yes to avoid hurting their feelings, damaging our rapport with them, or having them post potential negative reviews about us. For many of us, saying no is a skill that does not come naturally. For some, bluntly telling a patient no may work, but this approach is more likely to be ineffective. At the same time, saying no in an equivocal manner may weaken our patients’ confidence in us and could be displeasing for both our patients and us.1,2
We should say no in an “effective, professional manner that fosters good patient care and preserves the therapeutic relationship, while supporting physician well-being.”1 In this article, I provide practical tips for saying no to inappropriate patient requests in an emphatic manner so that we can feel more empowered and less uncomfortable.
Acknowledge and analyze your discomfort.
Before saying no, recognize that you are feeling uncomfortable with your patient’s inappropriate request. This uncomfortable feeling is a probable cue that there is likely no appropriate context for their request, ie, saying yes would be poor medical care, illegal, against policy, etc.1,3 In most cases, you should be able to identify the reason(s) your patient’s request feels inappropriate and uncomfortable.
Gather information and provide an explanation.
Ask your patient for more information about their request so you can determine if there are any underlying factors and if any additional information is needed.3 Once you decide to say no, explain why. Your explanation should be brief, because lengthy explanations might create room for debate (which could be exhausting and/or time-consuming), lead to giving in to their inappropriate request, and/or lead them to become more frustrated and misunderstood.1
Be empathetic, and re-establish rapport.
After declining a patient’s request, you may have to use empathy to re-establish rapport if it has been damaged. After being told no, your patient may feel frustrated or powerless. Acknowledge their feelings with statements such as “I know this is not want you wanted to hear” or “I can see you are irritated.”Accept your patient’s negative emotions, rather than minimizing them or trying to fix them.1,3
1. Kane M, Chambliss ML. Getting to no: how to respond to inappropriate patient requests. Fam Prac Manag. 2018;25(1):25-30.
2. Paterniti DA, Facher TL, Cipri CS, et al. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388.
3. Huben-Kearney A. Just say no to certain patient requests—and here’s how. Psychiatric News. 2021;56(2):13.
Although we may want to say “yes” when our patients ask us for certain medications, work excuses, etc, often it is more appropriate to say “no” because the conditions do not support those requests. Saying no to a patient usually is not a comfortable experience, but we should not say yes to avoid hurting their feelings, damaging our rapport with them, or having them post potential negative reviews about us. For many of us, saying no is a skill that does not come naturally. For some, bluntly telling a patient no may work, but this approach is more likely to be ineffective. At the same time, saying no in an equivocal manner may weaken our patients’ confidence in us and could be displeasing for both our patients and us.1,2
We should say no in an “effective, professional manner that fosters good patient care and preserves the therapeutic relationship, while supporting physician well-being.”1 In this article, I provide practical tips for saying no to inappropriate patient requests in an emphatic manner so that we can feel more empowered and less uncomfortable.
Acknowledge and analyze your discomfort.
Before saying no, recognize that you are feeling uncomfortable with your patient’s inappropriate request. This uncomfortable feeling is a probable cue that there is likely no appropriate context for their request, ie, saying yes would be poor medical care, illegal, against policy, etc.1,3 In most cases, you should be able to identify the reason(s) your patient’s request feels inappropriate and uncomfortable.
Gather information and provide an explanation.
Ask your patient for more information about their request so you can determine if there are any underlying factors and if any additional information is needed.3 Once you decide to say no, explain why. Your explanation should be brief, because lengthy explanations might create room for debate (which could be exhausting and/or time-consuming), lead to giving in to their inappropriate request, and/or lead them to become more frustrated and misunderstood.1
Be empathetic, and re-establish rapport.
After declining a patient’s request, you may have to use empathy to re-establish rapport if it has been damaged. After being told no, your patient may feel frustrated or powerless. Acknowledge their feelings with statements such as “I know this is not want you wanted to hear” or “I can see you are irritated.”Accept your patient’s negative emotions, rather than minimizing them or trying to fix them.1,3
Although we may want to say “yes” when our patients ask us for certain medications, work excuses, etc, often it is more appropriate to say “no” because the conditions do not support those requests. Saying no to a patient usually is not a comfortable experience, but we should not say yes to avoid hurting their feelings, damaging our rapport with them, or having them post potential negative reviews about us. For many of us, saying no is a skill that does not come naturally. For some, bluntly telling a patient no may work, but this approach is more likely to be ineffective. At the same time, saying no in an equivocal manner may weaken our patients’ confidence in us and could be displeasing for both our patients and us.1,2
We should say no in an “effective, professional manner that fosters good patient care and preserves the therapeutic relationship, while supporting physician well-being.”1 In this article, I provide practical tips for saying no to inappropriate patient requests in an emphatic manner so that we can feel more empowered and less uncomfortable.
Acknowledge and analyze your discomfort.
Before saying no, recognize that you are feeling uncomfortable with your patient’s inappropriate request. This uncomfortable feeling is a probable cue that there is likely no appropriate context for their request, ie, saying yes would be poor medical care, illegal, against policy, etc.1,3 In most cases, you should be able to identify the reason(s) your patient’s request feels inappropriate and uncomfortable.
Gather information and provide an explanation.
Ask your patient for more information about their request so you can determine if there are any underlying factors and if any additional information is needed.3 Once you decide to say no, explain why. Your explanation should be brief, because lengthy explanations might create room for debate (which could be exhausting and/or time-consuming), lead to giving in to their inappropriate request, and/or lead them to become more frustrated and misunderstood.1
Be empathetic, and re-establish rapport.
After declining a patient’s request, you may have to use empathy to re-establish rapport if it has been damaged. After being told no, your patient may feel frustrated or powerless. Acknowledge their feelings with statements such as “I know this is not want you wanted to hear” or “I can see you are irritated.”Accept your patient’s negative emotions, rather than minimizing them or trying to fix them.1,3
1. Kane M, Chambliss ML. Getting to no: how to respond to inappropriate patient requests. Fam Prac Manag. 2018;25(1):25-30.
2. Paterniti DA, Facher TL, Cipri CS, et al. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388.
3. Huben-Kearney A. Just say no to certain patient requests—and here’s how. Psychiatric News. 2021;56(2):13.
1. Kane M, Chambliss ML. Getting to no: how to respond to inappropriate patient requests. Fam Prac Manag. 2018;25(1):25-30.
2. Paterniti DA, Facher TL, Cipri CS, et al. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388.
3. Huben-Kearney A. Just say no to certain patient requests—and here’s how. Psychiatric News. 2021;56(2):13.
Closing your practice: What to consider
Closing your practice can be a stressful experience, and it requires careful planning. The process requires numerous steps, such as informing your staff, notifying your patients, closing accounts with your vendors and suppliers, storing medical records, and following applicable federal and state laws for dissolving your practice.1,2 Many of these steps may require consulting with an attorney, an accountant, and your malpractice insurance carrier.1,2 Although the recommendations I provide in this article are not exhaustive, when faced with closing your practice, be sure to consider the following factors.
Notify staff and patients.
Select a date to close your practice that will allow you to stop taking new patients, provides adequate leeway for your staff to find new employment and for you to hire temporary staff if needed, ensures you meet your obligations to your staff, such as payroll, and gives you time to set up appropriate continuity of care for your patients. In addition to verbally notifying your patients of your practice’s closing, inform them in writing (whether hand-delivered or via certified mail with return receipt) of the date of the practice’s closure, reason for the closure, cancellation of scheduled appointments after the closure date, referral options, and how they can obtain a copy of their medical records.1,2 Make sure your patients have an adequate supply of their medications before the closure.
Notify other parties.
Inform all suppliers, vendors, contracted service providers, insurance broker(s) for your practice, and payers (including Medicare and Medicaid, if applicable) of your intent to close your practice.1,2 Provide payers with a forwarding address to send payments that resolve after your practice closes, and request final invoices from vendors and suppliers so you can close your accounts with them. If you don’t own the building in which your practice is located, notify the building management in accordance with the provisions of your lease.1,2 Give cancellation notices to utilities and ancillary services (eg, labs, imaging facilities) to which you refer your patients, and notify facilities where you are credentialed and have admitting privileges.1,2 Inform your state medical licensing board, your state’s controlled substance division, and the Drug Enforcement Administration, because these agencies have requirements regarding changing the status of your medical license (if you decide to retire), continuing or surrendering your state and federal controlled substance registration, and disposal of prescription medications and prescription pads.1,2 Contact your local post office and delivery services with your change of address.
Address other considerations.
Set up a medical record retention and destruction plan in accordance with state and federal regulations, arrange for the safe storage for both paper and electronic medical records, and make sure storage facilities have experience handling confidential, Health Insurance Portability and Accountability Act (HIPAA)-sensitive patient information.1,2 In addition, establish a process for permanently deleting all HIPAA-sensitive patient information from any equipment that you don’t intend to keep.1,2
1. Funicelli AM. Risk management checklist when closing your practice. Psychiatric News. 2020;55(23):11.
2. American Academy of Family Physicians. Closing your practice checklist. Accessed January 21, 2022. https://www.aafp.org/dam/AAFP/documents/practice_management/admin_staffing/ClosingPracticeChecklist.pdf
Closing your practice can be a stressful experience, and it requires careful planning. The process requires numerous steps, such as informing your staff, notifying your patients, closing accounts with your vendors and suppliers, storing medical records, and following applicable federal and state laws for dissolving your practice.1,2 Many of these steps may require consulting with an attorney, an accountant, and your malpractice insurance carrier.1,2 Although the recommendations I provide in this article are not exhaustive, when faced with closing your practice, be sure to consider the following factors.
Notify staff and patients.
Select a date to close your practice that will allow you to stop taking new patients, provides adequate leeway for your staff to find new employment and for you to hire temporary staff if needed, ensures you meet your obligations to your staff, such as payroll, and gives you time to set up appropriate continuity of care for your patients. In addition to verbally notifying your patients of your practice’s closing, inform them in writing (whether hand-delivered or via certified mail with return receipt) of the date of the practice’s closure, reason for the closure, cancellation of scheduled appointments after the closure date, referral options, and how they can obtain a copy of their medical records.1,2 Make sure your patients have an adequate supply of their medications before the closure.
Notify other parties.
Inform all suppliers, vendors, contracted service providers, insurance broker(s) for your practice, and payers (including Medicare and Medicaid, if applicable) of your intent to close your practice.1,2 Provide payers with a forwarding address to send payments that resolve after your practice closes, and request final invoices from vendors and suppliers so you can close your accounts with them. If you don’t own the building in which your practice is located, notify the building management in accordance with the provisions of your lease.1,2 Give cancellation notices to utilities and ancillary services (eg, labs, imaging facilities) to which you refer your patients, and notify facilities where you are credentialed and have admitting privileges.1,2 Inform your state medical licensing board, your state’s controlled substance division, and the Drug Enforcement Administration, because these agencies have requirements regarding changing the status of your medical license (if you decide to retire), continuing or surrendering your state and federal controlled substance registration, and disposal of prescription medications and prescription pads.1,2 Contact your local post office and delivery services with your change of address.
Address other considerations.
Set up a medical record retention and destruction plan in accordance with state and federal regulations, arrange for the safe storage for both paper and electronic medical records, and make sure storage facilities have experience handling confidential, Health Insurance Portability and Accountability Act (HIPAA)-sensitive patient information.1,2 In addition, establish a process for permanently deleting all HIPAA-sensitive patient information from any equipment that you don’t intend to keep.1,2
Closing your practice can be a stressful experience, and it requires careful planning. The process requires numerous steps, such as informing your staff, notifying your patients, closing accounts with your vendors and suppliers, storing medical records, and following applicable federal and state laws for dissolving your practice.1,2 Many of these steps may require consulting with an attorney, an accountant, and your malpractice insurance carrier.1,2 Although the recommendations I provide in this article are not exhaustive, when faced with closing your practice, be sure to consider the following factors.
Notify staff and patients.
Select a date to close your practice that will allow you to stop taking new patients, provides adequate leeway for your staff to find new employment and for you to hire temporary staff if needed, ensures you meet your obligations to your staff, such as payroll, and gives you time to set up appropriate continuity of care for your patients. In addition to verbally notifying your patients of your practice’s closing, inform them in writing (whether hand-delivered or via certified mail with return receipt) of the date of the practice’s closure, reason for the closure, cancellation of scheduled appointments after the closure date, referral options, and how they can obtain a copy of their medical records.1,2 Make sure your patients have an adequate supply of their medications before the closure.
Notify other parties.
Inform all suppliers, vendors, contracted service providers, insurance broker(s) for your practice, and payers (including Medicare and Medicaid, if applicable) of your intent to close your practice.1,2 Provide payers with a forwarding address to send payments that resolve after your practice closes, and request final invoices from vendors and suppliers so you can close your accounts with them. If you don’t own the building in which your practice is located, notify the building management in accordance with the provisions of your lease.1,2 Give cancellation notices to utilities and ancillary services (eg, labs, imaging facilities) to which you refer your patients, and notify facilities where you are credentialed and have admitting privileges.1,2 Inform your state medical licensing board, your state’s controlled substance division, and the Drug Enforcement Administration, because these agencies have requirements regarding changing the status of your medical license (if you decide to retire), continuing or surrendering your state and federal controlled substance registration, and disposal of prescription medications and prescription pads.1,2 Contact your local post office and delivery services with your change of address.
Address other considerations.
Set up a medical record retention and destruction plan in accordance with state and federal regulations, arrange for the safe storage for both paper and electronic medical records, and make sure storage facilities have experience handling confidential, Health Insurance Portability and Accountability Act (HIPAA)-sensitive patient information.1,2 In addition, establish a process for permanently deleting all HIPAA-sensitive patient information from any equipment that you don’t intend to keep.1,2
1. Funicelli AM. Risk management checklist when closing your practice. Psychiatric News. 2020;55(23):11.
2. American Academy of Family Physicians. Closing your practice checklist. Accessed January 21, 2022. https://www.aafp.org/dam/AAFP/documents/practice_management/admin_staffing/ClosingPracticeChecklist.pdf
1. Funicelli AM. Risk management checklist when closing your practice. Psychiatric News. 2020;55(23):11.
2. American Academy of Family Physicians. Closing your practice checklist. Accessed January 21, 2022. https://www.aafp.org/dam/AAFP/documents/practice_management/admin_staffing/ClosingPracticeChecklist.pdf
What's your diagnosis?
Mucous membrane pemphigoid with esophageal web stricture.
Additional laboratory examination showed that his serum anti-BP180 antibody level was high (11.7 U/mL; normal range, <9.0 U/mL). Biopsy specimens taken from the laryngopharyngeal erosion showed subepithelial blister formation and it was consistent with pemphigoid pathologically (Figure D). He did not have cutaneous lesions and was diagnosed with mucous membrane pemphigoid (MMP). After performing endoscopic dilation, prednisolone (20 mg/d) was administered orally. Three months after starting the prednisolone treatment, follow-up endoscopy showed improvements of the laryngopharyngeal erosions (Figure E) and esophageal blister on the web. However, esophageal narrowing remained, and thus endoscopic balloon dilation was performed (Figure F-H). Three months after the dilation, the narrowing improved (Figure I).
MMP is an autoimmune blistering disease that induces the formation of mucous membrane subepithelial bullae. Basement membrane zone components such as collagen XVII (also known as BP180) are targets of autoantibodies in MMP. Symptomatic esophageal involvement affects 5.4% of patients with MMP and dysphagia is the most frequent symptom.1 Endoscopic findings include erosion, web stricture, subepithelial hematomas, and scars.2,3 Endoscopic dilation is sometimes necessary for the treatment of severe esophageal strictures.1
References
1. Zehou O et al. Br J Dermatol. 2017 Oct;177(4):1074-85.
2. Sallout H et al. Gastrointest Endosc. 2000 Sep;52(3):429-33.
3. Gaspar R et al. Gastrointest Endosc. 2017 Aug;86(2):400-2.
Mucous membrane pemphigoid with esophageal web stricture.
Additional laboratory examination showed that his serum anti-BP180 antibody level was high (11.7 U/mL; normal range, <9.0 U/mL). Biopsy specimens taken from the laryngopharyngeal erosion showed subepithelial blister formation and it was consistent with pemphigoid pathologically (Figure D). He did not have cutaneous lesions and was diagnosed with mucous membrane pemphigoid (MMP). After performing endoscopic dilation, prednisolone (20 mg/d) was administered orally. Three months after starting the prednisolone treatment, follow-up endoscopy showed improvements of the laryngopharyngeal erosions (Figure E) and esophageal blister on the web. However, esophageal narrowing remained, and thus endoscopic balloon dilation was performed (Figure F-H). Three months after the dilation, the narrowing improved (Figure I).

MMP is an autoimmune blistering disease that induces the formation of mucous membrane subepithelial bullae. Basement membrane zone components such as collagen XVII (also known as BP180) are targets of autoantibodies in MMP. Symptomatic esophageal involvement affects 5.4% of patients with MMP and dysphagia is the most frequent symptom.1 Endoscopic findings include erosion, web stricture, subepithelial hematomas, and scars.2,3 Endoscopic dilation is sometimes necessary for the treatment of severe esophageal strictures.1
References
1. Zehou O et al. Br J Dermatol. 2017 Oct;177(4):1074-85.
2. Sallout H et al. Gastrointest Endosc. 2000 Sep;52(3):429-33.
3. Gaspar R et al. Gastrointest Endosc. 2017 Aug;86(2):400-2.
Mucous membrane pemphigoid with esophageal web stricture.
Additional laboratory examination showed that his serum anti-BP180 antibody level was high (11.7 U/mL; normal range, <9.0 U/mL). Biopsy specimens taken from the laryngopharyngeal erosion showed subepithelial blister formation and it was consistent with pemphigoid pathologically (Figure D). He did not have cutaneous lesions and was diagnosed with mucous membrane pemphigoid (MMP). After performing endoscopic dilation, prednisolone (20 mg/d) was administered orally. Three months after starting the prednisolone treatment, follow-up endoscopy showed improvements of the laryngopharyngeal erosions (Figure E) and esophageal blister on the web. However, esophageal narrowing remained, and thus endoscopic balloon dilation was performed (Figure F-H). Three months after the dilation, the narrowing improved (Figure I).

MMP is an autoimmune blistering disease that induces the formation of mucous membrane subepithelial bullae. Basement membrane zone components such as collagen XVII (also known as BP180) are targets of autoantibodies in MMP. Symptomatic esophageal involvement affects 5.4% of patients with MMP and dysphagia is the most frequent symptom.1 Endoscopic findings include erosion, web stricture, subepithelial hematomas, and scars.2,3 Endoscopic dilation is sometimes necessary for the treatment of severe esophageal strictures.1
References
1. Zehou O et al. Br J Dermatol. 2017 Oct;177(4):1074-85.
2. Sallout H et al. Gastrointest Endosc. 2000 Sep;52(3):429-33.
3. Gaspar R et al. Gastrointest Endosc. 2017 Aug;86(2):400-2.
A 70-year-old man with a history of rectal cancer was referred to our clinic for chronic dysphagia and odynophagia. He did not have fevers or an allergic history. Physical examination was unremarkable except for multiple erosions in the oral cavity. Upper gastrointestinal endoscopy revealed multiple erosions in the palate and laryngopharynx (Figure A), a web stricture in the cervical esophagus (Figure B), and multiple scars in the thoracic esophagus.
Laboratory examination showed normal results including a normal white blood cell count (8010/mcL; eosinophils 360/mcL), hemoglobin level (14.0 g/dL), mean corpuscular volume (97.8 fL), serum iron level (140 mcg/dL), and ferritin level (50.5 mg/L). His dysphagia gradually worsened and he finally could not take pills nor solid food. Two weeks after the first endoscopy, a second endoscopic examination was performed and it showed exacerbation of esophageal stricture and appearance of a bloody blister (Figure C).
What is the diagnosis?
Previously published in Gastroenterology








