User login
Findings question value of pessary for pelvic organ prolapse
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
The standard nonsurgical treatment for pelvic organ prolapse does not appear to work as well as surgery to correct the problem, Dutch researchers have found.
Pelvic organ prolapse is an uncomfortable condition, causing a troublesome vaginal bulge, often accompanied by urinary, bowel, or sexual dysfunction. Between 3% and 6% of women develop symptomatic prolapse, with the highest incidence in women aged 60-69 years – a fast-growing demographic.
Although many women choose surgical treatment, the American College of Obstetricians and Gynecologists recommends that women be offered a vaginal pessary as a noninvasive alternative, despite inconsistent data from observational studies on their effectiveness.
Lisa van der Vaart, MD, a doctoral student in ob.gyn. at the University of Amsterdam and the lead author of the new study, published in JAMA, said that differences in outcome measures, small sample size, and lack of long-term follow-up have bedeviled previous comparisons of the two techniques.
“We thought it was very important to perform a randomized control trial on this subject to improve counseling to women who suffer from symptomatic pelvic organ prolapse,” Dr. van der Vaart said.
She and her colleagues conducted a noninferiority randomized clinical trial that recruited 1,605 women with stage II or higher prolapse who were referred to specialty care at 21 hospitals in the Netherlands between 2015 and 2019. Of the 440 women who agreed to participate in the trial, 218 received a pessary, a device inserted into the vagina that provides support to tissues displaced by prolapse, and 222 underwent surgery.
The primary outcome was subjective improvement using a standardized questionnaire at 24 months; women were asked to rank their symptoms on a seven-point scale, and subjective improvement was defined as a response of much better or very much better.
“We saw a substantial amount of improvement in both groups,” Dr. van der Vaart said in an interview.
After 24 months of follow-up, outcome data were available for 173 women in the pessary group and 162 in the surgery group. For this intention-to treat population, 76.3% in the pessary group and 81.5% in the surgery group reported improvement.
Results were similar for the smaller group of participants who completed the study per protocol, without crossing over to a treatment to which they had not been allocated.
However, neither the intention-to-treat nor per-protocol analysis met the prespecified criteria for noninferiority, suggesting that use of a vaginal pessary is not equivalent to surgery.
The study also found differences in adverse events. Among women randomly assigned to surgery, 9% suffered a postoperative urinary tract infection, and 5.4% underwent additional therapy, such as pessary or repeat operation.
But use of a pessary also had downsides. The most common adverse event was discomfort (42.7%), and by 24 months, 60% of the participants in the pessary group had discontinued use.
Dr. van der Vaart said that she was surprised by the high number of women assigned to the pessary group who later elected to undergo surgery. “Women should be told that their chance of crossing over to a surgical intervention is quite high – more than 50% do eventually end up having surgery.”
Cheryl Iglesia, MD, director of the National Center for Advanced Pelvic Surgery at MedStar Health and professor of obstetrics and gynecology and urology at Georgetown University, both in Washington, was also struck by the high crossover rate. “We’ve had the same pessaries probably for the last 100 years,” she said. “We need to get better.”
Dr. Iglesia welcomed new approaches to making vaginal pessaries that are custom designed for each woman’s unique anatomy using 3D printing and pointed to promising initial clinical trials of disposable pessaries. With the aging of the population and demand for treatment of prolapse increasing, she cited a need for better nonsurgical alternatives: “We have a work-force issue and may not have enough adequately trained urogynecologists to meet the demand for prolapse repairs as our population ages.”
The study was funded by a grant from ZonMW, a Dutch governmental health care organization. Dr. van der Vaart reported grants from ZonMW during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA
Focus on menopause
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
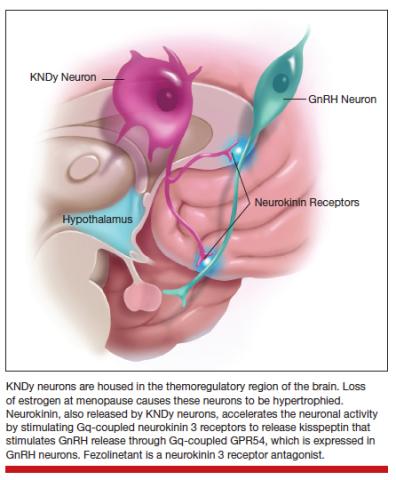
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
OBG Management caught up with Drs. Jan Shifren and Genevieve Neal-Perry while they were attending the annual meeting of The North American Menopause Society (NAMS), held October 12-15, 2022, in Atlanta, Georgia. Dr. Shifren presented on the “Ins and Outs of Hormone Therapy,” while Dr. Neal-Perry focused on “Menopause Physiology.”
Evaluating symptomatic patients for appropriate hormone therapy
OBG Management: In your presentation to the group at the NAMS meeting, you described a 51-year-old patient with the principal symptoms of frequent hot flashes and night sweats, sleep disruption, fatigue, irritability, vaginal dryness, and dyspareunia. As she reported already trying several lifestyle modification approaches, what are your questions for her to determine whether hormone therapy (HT), systemic or low-dose vaginal, is advisable?
Jan Shifren, MD: As with every patient, you need to begin with a thorough history and confirm her physical exam is up to date. If there are concerns related to genitourinary symptoms of menopause (GSM), then a pelvic exam is indicated. This patient is a healthy menopausal woman with bothersome hot flashes, night sweats, and vaginal dryness. Sleep disruption from night sweats is likely the cause of her fatigue and irritability, and her dyspareunia due to atrophic vulvovaginal changes. The principal indication for systemic HT is bothersome vasomotor symptoms (VMS), and a healthy woman who is under age 60 or within 10 years of the onset of menopause is generally a very good candidate for hormones. For this healthy 51-year-old with bothersome VMS unresponsive to lifestyle modification, the benefits of HT should outweigh potential risks. As low-dose vaginal estrogen therapy is minimally absorbed and very safe, this would be recommended instead of systemic HT if her only menopause symptoms were vaginal dryness and dyspareunia.
HT types and formulations
OBG Management: For this patient, low-dose vaginal estrogen is appropriate. In general, how do you decide on recommendations for combination therapy or estrogen only, and what formulations and dosages do you recommend?
Dr. Shifren: Any woman with a uterus needs to take a progestogen together with estrogen to protect her uterus from estrogen-induced endometrial overgrowth. With low dose vaginal estrogen therapy, however, concurrent progestogen is not needed.
Continue to: Estrogen options...
Estrogen options. I ask my patients about their preferences, but I typically recommend transdermal or non-oral estradiol formulations for my menopausal patients. The most commonly prescribed non-oral menopausal estrogen is the patch—as they are convenient, come in a wide range of doses, and are generic and generally affordable. There are also US Food and Drug Administration (FDA)–approved transdermal gels and creams, and a vaginal ring that provides systemic estrogen, but these options are typically more expensive than the patch. All non-oral estrogen formulations are composed of estradiol, which is especially nice for a patient preferring “bioidentical HT.”
Many of our patients like the idea that they are using “natural” HT. I inform them that bioidentical is a marketing term rather than a medical term, but if their goal is to take the same hormones that their ovaries made when they were younger, they should use FDA-approved formulations of estradiol and progesterone for their menopausal HT symptoms. I do not recommend compounded bioidentical HT due to concerns regarding product quality and safety. The combination of FDA-approved estradiol patches and oral micronized progesterone provides a high quality, carefully regulated bioidentical HT regimen. For women greatly preferring an oral estrogen, oral estradiol with micronized progesterone is an option.
In addition to patient preference for natural HT, the reasons that I encourage women to consider the estradiol transdermal patch for their menopausal HT include:
- no increased risk of venous thromboembolic events when physiologically dosed menopausal estradiol therapy is provided by a skin patch (observational data).1 With oral estrogens, even when dosed for menopause, VTE risk increases, as coagulation factors increase due to the first-pass hepatic effect. This does not occur with non-oral menopausal estrogens.
- no increased risk of gallbladder disease, which occurs with oral estrogen therapy (observational data)2
- possibly lower risk of stroke when low-dose menopausal HT is provided via skin patch (observational data)3
- convenience—the patches are changed once or twice weekly
- wide range of doses available, which optimizes identifying the lowest effective dose and decreasing the dose over time.
Progestogen options. Progestogens may be given daily or cyclically. Use of daily progestogen typically results in amenorrhea, which is preferred by most women. Cyclic use of a progestogen for 12-14 days each month results in a monthly withdrawal bleed, which is a good option for a woman experiencing bothersome breakthrough bleeding with daily progestogen. Use of a progestogen-releasing IUD is an off-label alternative for endometrial protection with menopausal HT. As discussed earlier, as many women prefer bioidentical HT, one of our preferred regimens is to provide transdermal estradiol with FDA-approved oral micronized progesterone. There are several patches that combine estradiol with a progestogen, but there is not a lot of dosing flexibility and product choice. There also is an approved product available that combines oral estradiol and micronized progesterone in one tablet.
Scheduling follow-up
OBG Management: Now that you have started the opening case patient on HT, how often are you going to monitor her for treatment?
Dr. Shifren: Women will not experience maximum efficacy for hot flash relief from their estrogen therapy for 3 months, so I typically see a patient back at 3 to 4 months to assess side effects and symptom control. I encourage women to reach out sooner if they are having a bothersome side effect. Once she is doing well on an HT regimen, we assess risks and benefits of ongoing treatment annually. The goal is to be certain she is on the lowest dose of estrogen that treats her symptoms, and we slowly decrease the estrogen dose over time.
Breast cancer risk
OBG Management: In your presentation, you mentioned that the risk of breast cancer does not increase appreciably with short-term use of HT. Is it possible to define short term?
Dr. Shifren: In the Women’s Health Initiative (WHI), a large double-blind, randomized, placebo-controlled trial of menopausal HT, there was a slight increase in breast cancer risk after approximately 4 to 5 years of use in women using estrogen with progestogen.4 I share with patients that this increased risk is about the same as that of obesity or drinking more than 1 alcoholic beverage daily. As an increased risk of breast cancer does not occur for several years, a woman may be able to take hormones for bothersome symptoms, feel well, and slowly come off without incurring significant breast cancer risk. In the WHI, there was no increase in breast cancer risk in women without a uterus randomized to estrogen alone.
Regarding cardiovascular risk, in the WHI, an increased risk of cardiovascular events generally was not seen in healthy women younger than age 60 and within 10 years of the onset of menopause.5 Benefits of HT may not outweigh risks for women with significant underlying cardiovascular risk factors, even if they are younger and close to menopause onset.
Continue to: The importance of shared decision making...
The importance of shared decision making
Dr. Shifren: As with any important health care decision, women should be involved in an individualized discussion of risks and benefits, with shared decision making about whether HT is the right choice. Women also should be involved in ongoing decisions regarding HT formulation, dose, and duration of use.
A nonhormonal option for hot flashes
OBG Management: How many women experience VMS around the time of menopause?
Dr. Genevieve Neal-Perry, MD, PhD: About 60% to 70% of individuals will experience hot flashes around the time of the menopause.6 Of those, about 40% are what we would call moderate to severe hot flashes—which are typically the most disruptive in terms of quality of life.7 The window of time in which they are likely to have them, at typically their most intense timeframe, is 2 years before the final menstrual period and the year after.7 In terms of the average duration, however, it’s about 7 years, which is a lot longer than what we previously thought.8 Moreover, there are disparities in that women of color, particularly African American women, can have them as long as 10 years.8
OBG Management: Can you explain why the VMS occur, and specifically around the time of menopause?
Dr. Neal-Perry: For many years we did not understand the basic biology of hot flashes. When you think about it, it’s completely amazing—when half of our population experiences hot flashes, and we don’t understand why, and we don’t have therapy that specifically targets hot flashes.
What we now know from work completed by Naomi Rance, in particular, is that a specific region of the brain, the hypothalamus, exhibited changes in number of neurons that seemed to be increased in size in menopausal people and smaller in size in people who were not menopausal.9 That started the journey to understanding the biology, and eventual mechanism, of hot flashes. It took about 10-15 years before we really began to understand why.
What we know now is that estrogen, a hormone that is made by the ovaries, activates and inactivates neurons located in the hypothalamus, a brain region that controls our thermoregulation—the way your body perceives temperature. The hypothalamus controls your response to temperature, either you experience chills or you dissipate heat by vasodilating (hot flush) and sweating.
The thermoregulatory region of the hypothalamus houses cells that receive messages from KNDy neurons, neurons also located in the hypothalamus that express kisspeptin, neurokinin, and dynorphin. Importantly, KNDy neurons express estrogen receptors. (The way that I like to think about estrogen and estrogen receptors is that estrogen is like the ball and the receptor is like the catcher’s mitt.) When estrogen interacts with this receptor, it keeps KNDy neurons quiet. But the increased variability and loss of estrogen that occurs around the time of menopause “disinhibits” KNDy neurons—meaning that they are no longer being reined in by estrogen. In response to decreased estrogen regulation, KNDy neurons become hypertrophied with neurotransmitters and more active. Specifically, KNDy neurons release neurokinin, a neuropeptide that self-stimulates KNDy neurons and activates neurons in the thermoregulatory zone of the brain—it’s a speed-forward feed-backward mechanism. The thermoregulatory neurons interpret this signal as “I feel hot,” and the body begins a series of functions to cool things down.
Continue to: Treatments that act on the thermoregulatory region
Treatments that act on the thermoregulatory region
Dr. Neal-Perry: I have described what happens in the brain around the time of menopause, and what triggers those hot flashes.
Estrogen. The reason that estrogen worked to treat the hot flashes is because estrogen inhibits and calms the neurons that become hyperactive during the menopause.
Fezolinetant. Fezolinetant is unique because it specifically targets the hormone receptor that triggers hot flashes, the neurokinin receptor. Fezolinetant is a nonhormone therapy that not only reduces the activity of KNDy neurons but also blocks the effects of neurons in the thermoregulatory zone, thereby reducing the sensation of the hot flashes. We are in such a special time in medical history for individuals who experience hot flashes because now we understand the basic biology of hot flashes, and we can generate targeted therapy to manage hot flashes—that is for both individuals who identify as women and individuals who identify as men, because both experience hot flashes.
OBG Management: Is there a particular threshold of hot flash symptoms that is considered important to treat, or is treatment based on essentially the bother to patients?
Dr. Neal-Perry: Treatment is solely based on if it bothers the patient. But we do know that people who have lots of bothersome hot flashes have a higher risk for heart disease and may have sleep disruption, reduced cognitive function, and poorer quality of life. Sleep dysfunction can impact the ability to think and function and can put those affected at increased risk for accidents.
For people who are having these symptoms that are disruptive to their life, you do want to treat them. You might say, “Well, we’ve had estrogen, why not use estrogen,” right? Well estrogen works very well, but there are lots of people who can’t use estrogen—individuals who have breast cancer, blood clotting disorders, significant heart disease, or diabetes. Then there are just some people who don’t feel comfortable using estrogen.
We have had a huge gap in care for individuals who experience hot flashes and who are ineligible for menopausal HT. While there are other nonhormonal options, they often have side effects like sexual dysfunction, hypersomnolence, or insomnia. Some people choose not to use these nonhormonal treatments because the side effects are worse for them than to trying to manage the hot flashes. The introduction to a nonhormonal therapy that is effective and does not have lots of side effects is exciting and will be welcomed by many who have not found relief.
OBG Management: Is fezolinetant available now for patients?
Dr. Neal-Perry: It is not available yet. Hopefully, it will be approved within the next year. Astellas recently completed a double blind randomized cross over design phase 3 study that found fezolinetant is highly effective for the management of hot flashes and that it has a low side effect profile.10 Fezolinetant’s most common side effect was COVID-19, a reflection of the fact that the trial was done during the COVID pandemic. The other most common side effect was headache. Everything else was minimal.
Other drugs in the same class as fezolinetant have been under development for the management of hot flashes; however, they encountered liver function challenges, and studies were stopped. Fezolinetant did not cause liver dysfunction.
Hot flash modifiers
OBG Management: Referring to that neuropathway, are there physiologic differences among women who do and do not experience hot flashes, and are there particular mechanisms that may protect patients against being bothered by hot flashes?
Dr. Neal-Perry: Well, there are some things that we can control, and there are things that we cannot control (like our genetic background). Some of the processes that are important for estrogen receptor function and estrogen metabolism, as well as some other receptor systems, can work differently. When estrogen metabolism is slightly different, it could result in reduced estrogen receptor activity and more hot flashes. Then there are some receptor polymorphisms that can increase or reduce the risk for hot flashes—the genetic piece.11
There are things that can modify your risk for hot flashes and the duration of hot flashes. Individuals who are obese or smoke may experience more hot flashes. Women of color, especially African American women, tend to have hot flashes occur earlier in their reproductive life and last for a longer duration; hot flashes may occur up to 2 years before menopause, last for more than 10 years, and be more disruptive. By contrast, Asian women tend to report fewer and less disruptive hot flashes.8
OBG Management: If fezolinetant were to be FDA approved, will there be particular patients that it will most appropriate for, since it is an estrogen alternative?
Dr. Neal-Perry: Yes, there may be different patients who might benefit from fezolinetant. This will depend on what the situation is—patients who have breast cancer, poorly controlled diabetes, or heart disease, and those patients who prefer not to use estrogen will benefit from fezolinetant, as we are going to look for other treatment options for those individuals. It will be important for medical providers to listen to their patients and understand the medical background of that individual to really define what is the best next step for the management of their hot flashes.
This is an exciting time for individuals affected by menopausal hot flashes; to understand the biology of hot flashes gives us real opportunities to bridge gaps around how to manage them. Individuals who experience hot flashes will know that they don’t have to suffer, that there are other options that are safe, that can help meet their needs and put them in a better place. ●
Excerpted from the presentation, “Do you see me? Culturally responsive care in menopause,” by Makeba Williams, MD, NCMP, at The North American Menopause Society meeting in Atlanta, Georgia, October 12-15, 2022.
Dr. Williams is Vice Chair of Professional Development and Wellness, Associate Professor, Washington University School of Medicine
The Study of Women’s Health Across the Nation (SWAN) challenged the notion that there is a universal menopausal experience.1 Up until that time, we had been using this universal experience that is based largely on the experiences of White women and applying that data to the experiences of women of color. Other research has shown that African American women have poorer quality of life and health status, and that they receive less treatment for a number of conditions.2,3
In a recent review of more than 20 years of literature, we found only 17 articles that met the inclusion criteria, reflecting the invisibility of African American women and other ethnic and racial minorities in the menopause literature and research. Key findings included that African American women1,4:
- experience an earlier age of onset of menopause
- have higher rates of premature menopause and early menopause, which is a risk factor for cardiovascular disease
- experience a longer time of the menopausal transition, with variability in the average age of menopause onset
- overall report lower rates of vaginal symptoms
- are less likely to report sleep disturbances than White women or Hispanic women, but more likely to report these symptoms than Asian women
- experience a higher prevalence, frequency, and severity of vasomotor symptoms (VMS), and were more bothered by those symptoms
− 48.4 years in the Healthy Women’s Study
− 50.9 years in the Penn Ovarian Aging Study
− 51.4 years in SWAN
- reported lower educational attainment, experiencing more socioeconomic disadvantage and exposure to more adverse life effects
- receive less treatment for VMS, hypertension, and depression, and are less likely to be prescribed statin drugs
- experience more discrimination
- use cigarettes and tobacco more, but are less likely to use alcohol and less likely to have physical activity.
Cultural influences on menopause
Im and colleagues have published many studies looking at cultural influences on African American, Hispanic, and Asian American women, and comparing them to White women.5 Notable differences were found regarding education level, family income, employment, number of children, and greater perceived health (which is associated with fewer menopausal symptoms). They identified 5 qualitative ideas:
- Positive acceptance. Minority women, or racial and ethnic women, perceived the transition to menopause more positively, and generally took on a posture of acceptance, reporting feeling liberated from many of the challenges associated with the reproductive period. In addition, many associated a greater sense of maturity and respect within their communities with the natural aging process.
- Optimism. Ethnic women tended to embrace menopause, using humor and laughter to express emotions during stressful life changes. This runs counter to many of the perspectives reported by White women, who often viewed the menopausal transition and aging negatively, as we equate aging with the loss of youthfulness in the United States.
- Unique, not universal. Most of the ethnic minority women thought that there was something unique about their menopausal experiences, and that they were influenced by immigration transition, financial situations, etc. Many White woman perceived that the menopausal experience was shared among all women.
- Closed, not open. There were differences in how we talk about symptoms, or whether or not we talk about them at all. Ethnic women tended to be silent about their symptoms. By contrast, White women tended to be more open and talkative and communicative about their symptoms.
- Minimizing, not controlling. No symptom management was the strategy of choice for most women. Minority women tended to manage their symptoms by tolerating and normalizing them. Only those women with the most serious symptoms sought out medication for temporary relief. Some expressed a tendency to downplay their symptoms because many of them had more important things that they were dealing with in their lives.
What is an individual social identity?
An individual social identity reflects the many groups to which one belongs. It is how one shows up, and yet it is much more than how they physically show up. When you pass your eye on patients, you are only seeing the tip of the iceberg. The full social identity of a patient resides below the surface. Social identity is complex, on a continuum, and can change depending on time and place. How we prioritize our social identities may change, depending on the context and the situation.
Our intersecting social identities give rise to our cultural identity, and it is through the prism of intersectionality that we can understand the ways in which our social identities converge to give rise to disparities in health care in midlife and menopausal women. Holding space for cultural identity, we can impact how our patients are perceiving their menopause, how they are moving through decision making about taking care of themselves in menopause. And we can provide more responsive care to their cultural identities, and hopefully at the end of the day we reduce some of these disparities that we are seeing in our menopausal patients and also are reducing our unconscious bias in our patient interactions.
Culturally responsive care
There are several components to home in on when we are trying to provide culturally responsive care to patients.
- A commitment to being culturally curious. We have to accept what the literature is sharing with us, that there is not a universal menopausal experience. We have for far too long applied this universal experience of menopause that has largely been based on White women to different racial and ethnic populations.
- Recognizing. I appreciate that my identity as a Black woman may be very different from other Black women in the room, or whatever their social identity. I am not expected to understand all of the others’ experiences, and I don’t expect that for you either.
- Acknowledge unconscious implicit biases. Acknowledge the groups to which you have a strong implicit bias, and allow it to drive you to reduce barriers to engaging with patients.
- Connecting with the individual patient. It is through a process of individuating that we learn from our patients’ unique characteristics, rather than relying on assumptions and stereotypes. We have a window of opportunity to see our patient and move beyond thinking of them in terms of racial and ethnic stereotypes or particular social groups. It is through this process of individualizing that we can seek answers to key questions.
The ultimate goal is to understand our individual patients’ perceptions, outlook on menopause, and contextual factors in their lives that influence the menopause journey.
CASE ENCOUNTER
I quickly look at the patient-filled form before I knock on the exam door, and I see that the patient has checked off that she has hot flashes, night sweats, and I make a mental note, she’s menopausal. I already have a preliminary plan to give this patient hormone therapy. I open the door, and I see that she’s Black. I know, based upon the data from SWAN and others, that her menopause means longer duration, more severe vasomotor symptoms. I have already teed up a prescription to go to the pharmacy.
The problem is, I have not even talked to her. She may actually nod her head, saying that she is going to go to the pharmacy, but she may never pick up that prescription. She likely leaves my office feeling unheard; her needs are unmet. I move onto the next patient. I feel good, but in actuality, I didn’t hear her. I have provided her bias and stereotyped care. I missed an opportunity to truly engage this patient and her care, and my good intentions of following the literature about her experience in menopause have contributed quite likely to her increased morbidity and mortality, her increased cardiovascular disease risk, all because I have not held space for her cultural identity.
References
- Harlow SD, Burnett-Bowie SM, Greendale GA, et al. Disparities in reproductive aging and midlife health between Black and White women: the Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health. 2022;8:3. doi: 10.1186/s40695-022-00073-y.
- Chlebowski RT, Aragaki AK, Anderson GL, et al. Forty-year trends in menopausal hormone therapy use and breast cancer incidence among postmenopausal black and white women. Cancer. 2020;126:2956-2964. doi: 10.1002/ cncr.32846.
- Weng HH, McBride CM, Bosworth HB, et al. Racial differences in physician recommendation of hormone replacement therapy. Prev Med. 2001;33:668673. doi: 10.1006/pmed.2001.0943.
- Williams M, Richard-Davis G, Williams PL, et al. A review of African American women’s experiences in menopause. Menopause. 2022;29:1331-1337. doi: 10.1097/GME.0000000000002060.
- Im EO. Ethnic differences in symptoms experienced during the menopausal transition. Health Care Women Int. 2009;30:339-355. doi: 10.1080/07399330802695002.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840-845. doi: 10.1161/CIRCULATIONAHA.106.642280.
- Liu B, Beral V, Balkwill A, et al; Million Women Study Collaborators. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ. 2008;337:a386. doi: 10.1136/bmj.a386.
- Renoux C, Dell’aniello S, Garbe E, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj. c2519.
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477. doi: 10.1001/jama.297.13.1465.
- Woods NF, Mitchell ES. Symptoms during the perimenopause: prevlance, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 suppl 12B:14-24. doi: 10.1016/j. amjmed.2005.09.031.
- Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159:1189-1199. doi: 10.1093/aje/kwh168.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531-539. doi: 10.1001/ jamainternmed.2014.8093.
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679-688. doi: 10.1002/1096-9861 (20000904)424:4<679::aid-cne9>3.0.co;2-l.
- Neal-Perry G. A phase 3, randomized, placebo-controlled, double-blind study to investigate the long-term safety and tolerability of fezolinetant in women seeking treatment for vasomotor symptoms associated with menopause (SKYLIGHT 4) – Abstract S-11. Paper presented at ENDO 2022. June 11, 2022.
- Crandall CJ, Diamant AL, Maglione M, et al. Genetic variation and hot flashes: a systematic review. J Clin Endocrinol Metab. 2020;105:e4907-e4957. doi: 10.1210/clinem/dgaa536.
Does subclinical hyperthyroidism raise fracture risk?
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
People with subclinical hyperthyroidism are at 34% greater risk of experiencing a fracture compared with those with normal thyroid function, new research shows.
The finding, from a study of nearly 11,000 middle-aged men and women followed for a median of 2 decades, “highlights a potential role for more aggressive screening and monitoring of patients with subclinical hyperthyroidism to prevent bone mineral disease,” the researchers wrote.
Primary care physicians “should be more aware of the risks for fracture among persons with subclinical hyperthyroidism in the ambulatory setting,” Natalie R. Daya, a PhD student in epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, and first author of the study, told this news organization.
Ms. Daya and her colleagues published their findings in JAMA Network Open.
Building on earlier findings
The results agree with previous work, including a meta-analysis of 13 prospective cohort studies of 70,289 primarily White individuals with an average age of 64 years, which found that subclinical hyperthyroidism was associated with a modestly increased risk for fractures, the researchers noted.
“Our study extends these findings to a younger, community-based cohort that included both Black and White participants, included extensive adjustment for potential confounders, and had a longer follow-up period (median follow-up of 21 years vs. 12 years),” they wrote.
The study included 10,946 participants in the Atherosclerosis Risk in Communities Study who were recruited in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis.
Baseline thyroid function was measured in blood samples collected during the second visit, which occurred between 1990 and 1992. No participants in the new analysis took thyroid medications or had a history of hospitalization for fractures at baseline, and all identified as Black or White. The mean age was 57 years, 24% were Black, and 54.3% were female.
Subclinical hyperthyroidism was defined as a thyrotropin level less than 0.56 mIU/L; subclinical hypothyroidism as a thyrotropin level greater than 5.1 mIU/L; and normal thyroid function as a thyrotropin level between 0.56 and 5.1 mIU/L, with normal free thyroxine levels of 0.85-1.4 ng/dL.
The vast majority (93%) of participants had normal thyroid function, 2.6% had subclinical hyperthyroidism, and 4.4% had subclinical hypothyroidism, according to the researchers.
Median follow-up was 21 years. The researchers identified 3,556 incident fractures, detected with hospitalization discharge codes through 2019 and inpatient and Medicare claims data through 2018, for a rate of 167.1 per 10,000 person-years.
Adjusted hazard ratios for fracture were 1.34 (95% confidence interval [CI], 1.09-1.65) for people with subclinical hyperthyroidism and 0.90 (95% CI, 0.77-1.05) for those with subclinical hypothyroidism, compared with those with normal thyroid function.
Most fractures occurred in either the hip (14.1%) or spine (13.8%), according to the researchers.
Limitations included a lack of thyroid function data during the follow-up period and lack of data on bone mineral density, the researchers wrote.
‘An important risk factor’
Endocrinologist Michael McClung, MD, founding and emeritus director of the Oregon Osteoporosis Center, Portland, who was not involved in the study, pointed out that both subclinical hypothyroidism and subclinical hyperthyroidism have been linked to greater risk for cardiovascular disease as well as fracture.
The new paper underscores that subclinical hyperthyroidism “should be included as an important risk factor” for fracture as well as cardiovascular risk, Dr. McClung said in an interview. In considering whether to treat osteoporosis, subclinical hyperthyroidism “may be enough to tip the balance in favor of pharmacological therapy,” he added.
Thyroid-stimulating hormone (TSH) tests to assess thyroid function are typically ordered only if a patient has symptoms of hyperthyroidism or hypothyroidism, Ms. Daya said. Depending on the cause and severity of a low TSH level, a physician may prescribe methimazole or radioactive iodine therapy to reduce the production of thyroxine, she said.
However, well-designed studies are needed to evaluate whether treatment of subclinical thyroid dysfunction reduces the risk for fracture or cardiovascular problems and assess downsides such as side effects, costs, and psychological harm, Dr. McClung noted.
The U.S. Preventive Services Task Force concluded in 2015 that the data were insufficient to recommend screening for thyroid dysfunction in adults without symptoms. As of a year ago, no new evidence has emerged to support an update, according to the task force’s website.
“Until those studies are available, selective screening of thyroid function should be considered in all patients undergoing risk assessment for cardiovascular disease or skeletal health,” Dr. McClung said.
The Atherosclerosis Risk in Communities Study has been funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and the U.S. Department of Health and Human Services. Ms. Daya and four study authors reported receiving NIH grants during the study period. Dr. McClung reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
USPSTF holds firm on postmenopausal hormone recommendations
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Hormone therapy–depression link may depend on mode of administration
An analysis of more than 800,000 women in Denmark offers more insight into the murky links between female hormones and midlife mental illness in women: It hints that hormone therapy (HT) may boost the risk of depression, have no effect, or lower it – all depending on how it’s administered and when.
Women who took systemic HT had a higher risk of depression from age 48 to 50 (adjusted hazard ratio, 1.50; 95% confidence interval, 1.24-1.81), researchers reported in JAMA Network Open. However, there was no overall link between depression and locally administered HT (aHR, 1.15; 95% CI, 0.70-1.87) – except when HT was begun between ages 54 and 60, when there were signs of a protective effect (aHR, 0.80; 95% CI, 0.70-0.91).
“Women in menopause who initiate systemically administered HT should be aware of depression as a potential adverse effect,” epidemiologist and study corresponding author Merete Osler, MD, PhD, DMSc, of Bispebjerg and Frederiksberg (Denmark) Hospitals and the University of Copenhagen, said in an interview. ”Further, women and clinicians alike should be aware of any misinterpretation of symptoms of depression as menopausal disturbances.”
Dr. Osler said the researchers launched the study to better understand potential hormone-depression links in light of suspicions that lower levels of estrogen in menopause may contribute to depression.
Several randomized clinical trials and cohort and cross-sectional studies have explored whether systemic HT affects depression during menopause, Dr. Osler said, “but the results from these studies have been inconsistent, and few have explored the role of the route of administration.”
For the new registry-based study, researchers retrospectively tracked all women in Denmark who were aged 45 between 1995 and 2017 without prior oophorectomy, certain kinds of cancer, prior use of HT, or ongoing depression.
During follow-up to a mean age of 56, 23% of the women began HT (at a median age of 55), and 1.6% were hospitalized for depression. Of those on HT, 65.8% received locally administered HT.
Researchers adjusted hazard ratios for a long list of factors such as educational level, marital status, number of still births or live births, prior use of hormonal contraceptives, several medical conditions, and prior depression.
“We were surprised by our findings, which to some degree contradicted our prior hypothesis that systemic HT with estrogen would not be associated with first-time depression diagnosis in women aged 45 and above, while HT with progesterone would be associated with a slightly increased risk,” Dr. Osler said. “In our study, systemically administered HT was associated with an increased risk of depression with no difference between estrogen alone or in combination with progestin. As findings from previous studies have been inconsistent, our findings fit with some but not all previous studies.”
Why might the mode of administration make a difference? It’s possible that local administration may contribute less to the systemic circulation, Dr. Osler said, “or that menopausal symptoms including depression are more likely to be treated with systemic HT.”
As for age differences, Dr. Osler said “it is possible that women are more sensitive to the influence of HT on mood around menopause than at later ages. However, it should be noted that in the present study it was not possible to calculate precise risk estimates for use of systemic HT in menopausal women above age 54 because less than 1% initiated treatment with systemic HT after age 54 years.”
In an interview, psychiatrist Natalie Rasgon, MD, PhD, of Stanford (Calif.) University, who’s studied hormones and depression, said the study is “remarkably large and consistently executed.”
She cautioned, however, that the findings don’t prove any causality. “Saying that estrogen therapy or hormone therapy causes depression is patently incorrect.”
How can the findings be useful for medical professionals? “Women and physicians alike need to be very mindful of pre-existing mood disorders,” Dr. Rasgon said. “Women who in the past had anxiety disorders, mood swings, PTSD, or prior episodes of depression might have a differential response to hormone therapy in menopause.”
Also keep in mind, she said, that the transition from menopause to post menopause is “very volatile,” and depression may break through even in women undergoing treatment for the condition.
For her part, Dr. Osler said this study and others “emphasize the need for clinical guidelines to further consider the psychological side effects of systemic HT.”
Funding information was not provided. The study authors and Dr. Rasgon have no disclosures.
An analysis of more than 800,000 women in Denmark offers more insight into the murky links between female hormones and midlife mental illness in women: It hints that hormone therapy (HT) may boost the risk of depression, have no effect, or lower it – all depending on how it’s administered and when.
Women who took systemic HT had a higher risk of depression from age 48 to 50 (adjusted hazard ratio, 1.50; 95% confidence interval, 1.24-1.81), researchers reported in JAMA Network Open. However, there was no overall link between depression and locally administered HT (aHR, 1.15; 95% CI, 0.70-1.87) – except when HT was begun between ages 54 and 60, when there were signs of a protective effect (aHR, 0.80; 95% CI, 0.70-0.91).
“Women in menopause who initiate systemically administered HT should be aware of depression as a potential adverse effect,” epidemiologist and study corresponding author Merete Osler, MD, PhD, DMSc, of Bispebjerg and Frederiksberg (Denmark) Hospitals and the University of Copenhagen, said in an interview. ”Further, women and clinicians alike should be aware of any misinterpretation of symptoms of depression as menopausal disturbances.”
Dr. Osler said the researchers launched the study to better understand potential hormone-depression links in light of suspicions that lower levels of estrogen in menopause may contribute to depression.
Several randomized clinical trials and cohort and cross-sectional studies have explored whether systemic HT affects depression during menopause, Dr. Osler said, “but the results from these studies have been inconsistent, and few have explored the role of the route of administration.”
For the new registry-based study, researchers retrospectively tracked all women in Denmark who were aged 45 between 1995 and 2017 without prior oophorectomy, certain kinds of cancer, prior use of HT, or ongoing depression.
During follow-up to a mean age of 56, 23% of the women began HT (at a median age of 55), and 1.6% were hospitalized for depression. Of those on HT, 65.8% received locally administered HT.
Researchers adjusted hazard ratios for a long list of factors such as educational level, marital status, number of still births or live births, prior use of hormonal contraceptives, several medical conditions, and prior depression.
“We were surprised by our findings, which to some degree contradicted our prior hypothesis that systemic HT with estrogen would not be associated with first-time depression diagnosis in women aged 45 and above, while HT with progesterone would be associated with a slightly increased risk,” Dr. Osler said. “In our study, systemically administered HT was associated with an increased risk of depression with no difference between estrogen alone or in combination with progestin. As findings from previous studies have been inconsistent, our findings fit with some but not all previous studies.”
Why might the mode of administration make a difference? It’s possible that local administration may contribute less to the systemic circulation, Dr. Osler said, “or that menopausal symptoms including depression are more likely to be treated with systemic HT.”
As for age differences, Dr. Osler said “it is possible that women are more sensitive to the influence of HT on mood around menopause than at later ages. However, it should be noted that in the present study it was not possible to calculate precise risk estimates for use of systemic HT in menopausal women above age 54 because less than 1% initiated treatment with systemic HT after age 54 years.”
In an interview, psychiatrist Natalie Rasgon, MD, PhD, of Stanford (Calif.) University, who’s studied hormones and depression, said the study is “remarkably large and consistently executed.”
She cautioned, however, that the findings don’t prove any causality. “Saying that estrogen therapy or hormone therapy causes depression is patently incorrect.”
How can the findings be useful for medical professionals? “Women and physicians alike need to be very mindful of pre-existing mood disorders,” Dr. Rasgon said. “Women who in the past had anxiety disorders, mood swings, PTSD, or prior episodes of depression might have a differential response to hormone therapy in menopause.”
Also keep in mind, she said, that the transition from menopause to post menopause is “very volatile,” and depression may break through even in women undergoing treatment for the condition.
For her part, Dr. Osler said this study and others “emphasize the need for clinical guidelines to further consider the psychological side effects of systemic HT.”
Funding information was not provided. The study authors and Dr. Rasgon have no disclosures.
An analysis of more than 800,000 women in Denmark offers more insight into the murky links between female hormones and midlife mental illness in women: It hints that hormone therapy (HT) may boost the risk of depression, have no effect, or lower it – all depending on how it’s administered and when.
Women who took systemic HT had a higher risk of depression from age 48 to 50 (adjusted hazard ratio, 1.50; 95% confidence interval, 1.24-1.81), researchers reported in JAMA Network Open. However, there was no overall link between depression and locally administered HT (aHR, 1.15; 95% CI, 0.70-1.87) – except when HT was begun between ages 54 and 60, when there were signs of a protective effect (aHR, 0.80; 95% CI, 0.70-0.91).
“Women in menopause who initiate systemically administered HT should be aware of depression as a potential adverse effect,” epidemiologist and study corresponding author Merete Osler, MD, PhD, DMSc, of Bispebjerg and Frederiksberg (Denmark) Hospitals and the University of Copenhagen, said in an interview. ”Further, women and clinicians alike should be aware of any misinterpretation of symptoms of depression as menopausal disturbances.”
Dr. Osler said the researchers launched the study to better understand potential hormone-depression links in light of suspicions that lower levels of estrogen in menopause may contribute to depression.
Several randomized clinical trials and cohort and cross-sectional studies have explored whether systemic HT affects depression during menopause, Dr. Osler said, “but the results from these studies have been inconsistent, and few have explored the role of the route of administration.”
For the new registry-based study, researchers retrospectively tracked all women in Denmark who were aged 45 between 1995 and 2017 without prior oophorectomy, certain kinds of cancer, prior use of HT, or ongoing depression.
During follow-up to a mean age of 56, 23% of the women began HT (at a median age of 55), and 1.6% were hospitalized for depression. Of those on HT, 65.8% received locally administered HT.
Researchers adjusted hazard ratios for a long list of factors such as educational level, marital status, number of still births or live births, prior use of hormonal contraceptives, several medical conditions, and prior depression.
“We were surprised by our findings, which to some degree contradicted our prior hypothesis that systemic HT with estrogen would not be associated with first-time depression diagnosis in women aged 45 and above, while HT with progesterone would be associated with a slightly increased risk,” Dr. Osler said. “In our study, systemically administered HT was associated with an increased risk of depression with no difference between estrogen alone or in combination with progestin. As findings from previous studies have been inconsistent, our findings fit with some but not all previous studies.”
Why might the mode of administration make a difference? It’s possible that local administration may contribute less to the systemic circulation, Dr. Osler said, “or that menopausal symptoms including depression are more likely to be treated with systemic HT.”
As for age differences, Dr. Osler said “it is possible that women are more sensitive to the influence of HT on mood around menopause than at later ages. However, it should be noted that in the present study it was not possible to calculate precise risk estimates for use of systemic HT in menopausal women above age 54 because less than 1% initiated treatment with systemic HT after age 54 years.”
In an interview, psychiatrist Natalie Rasgon, MD, PhD, of Stanford (Calif.) University, who’s studied hormones and depression, said the study is “remarkably large and consistently executed.”
She cautioned, however, that the findings don’t prove any causality. “Saying that estrogen therapy or hormone therapy causes depression is patently incorrect.”
How can the findings be useful for medical professionals? “Women and physicians alike need to be very mindful of pre-existing mood disorders,” Dr. Rasgon said. “Women who in the past had anxiety disorders, mood swings, PTSD, or prior episodes of depression might have a differential response to hormone therapy in menopause.”
Also keep in mind, she said, that the transition from menopause to post menopause is “very volatile,” and depression may break through even in women undergoing treatment for the condition.
For her part, Dr. Osler said this study and others “emphasize the need for clinical guidelines to further consider the psychological side effects of systemic HT.”
Funding information was not provided. The study authors and Dr. Rasgon have no disclosures.
FROM JAMA NETWORK OPEN
Multiple menopause symptoms linked to increased cardiovascular risk
Up to 10 different menopausal symptoms were linked to an increased risk of cardiovascular disease when they were moderate to severe in women who initially had no evidence of cardiovascular disease, according to research presented at the North American Menopause Society annual meeting in Atlanta.
“The take-home message is that severe menopausal symptoms may increase the risk of cardiovascular disease,” Matthew Nudy, MD, an assistant professor of medicine at the Heart and Vascular Institute at Penn State University, Hershey, said in an interview about his findings. “Physicians and patients should be aware of this association. Women with severe symptoms may be more likely to see their physician, and this would be an ideal time to have their cardiovascular risk assessed.”
Margaret Nachtigall, MD, a clinical associate professor of obstetrics and gynecology at New York University and at NYU Langone Health, noted that these findings lined up with other studies showing an increased risk of cardiovascular disease in patients who have more symptoms, especially hot flashes.
“Other recent studies showed that an increase in severity of hot flush is associated with worse blood vessel function, leading to heart disease,” Dr. Nachtigall, who was not involved with the study, said in an interview. “The next step that makes sense is to try to eliminate these symptoms and hope that, in turn, would lower cardiovascular disease and improve survival.”
The researchers compared menopausal symptoms with cardiovascular outcomes and all-cause mortality in an observational cohort of 80,278 postmenopausal women for a median 8.2 years of follow-up. None of the women, all enrolled in the Women’s Health Initiative, had known cardiovascular disease at baseline. They had an average age of 63 years and average body mass index (BMI) of 25.9 at baseline. Most participants were White (86.7%), with 7% being Black and 4.1% Hispanic. Cardiovascular disease was a composite outcome that included hospitalized myocardial infarction, definite silent myocardial infarction, coronary death, stroke, congestive heart failure, angina, peripheral vascular disease, carotid artery disease, and coronary revascularization.
The researchers used a four-item Likert scale (0-3) to assess the severity of 15 symptoms experienced within the past 4 weeks at baseline: “night sweats, hot flashes, waking up several times at night, joint pain or stiffness, headaches or migraines, vaginal or genital dryness, heart racing or skipping beats, breast tenderness, dizziness, tremors (shakes), feeling tired, forgetfulness, mood swings, [feeling] restless or fidgety, and difficulty concentrating.”
The associations were adjusted for the following covariates: race/ethnicity, blood pressure, education, smoking status, bilateral oophorectomy, menopausal hormone therapy use (never/past/current), sleep duration, statin use, history of high cholesterol, aspirin use, use of antihypertensives, treated diabetes, and family history of heart attack. Continuous variables included age, age at menopause, BMI, blood pressure, and physical activity levels. Because of the high number of multiple comparisons, the researchers also used a Bonferroni correction to reduce the risk of spurious statistical significance.
The researchers found some clustering of symptoms. Among women who had at least two moderate or severe menopausal symptoms, more than half frequently woke up at night, had joint pain, or felt tired, the researchers reported. Those symptoms were also the most commonly reported ones overall. Younger women, between ages 50 and 59, were more likely than older women (60-79 years old) to experience vasomotor symptoms and all cognitive affective symptoms except forgetfulness.
The researchers identified 10 symptoms whose severity was significantly associated with cardiovascular disease. Compared to having no symptoms at all, the following moderate or severe symptoms were associated with an increased risk of a cardiovascular event after adjustment for covariates and corrected for multiple comparisons: night sweats – a 19% increased risk (P = .03), waking up several times at night – 11% increased risk (P = .05), joint pain or stiffness – 27% increased risk (P < .001), heart racing or skipping beats – 55% increased risk (P < .001), dizziness – 34% increased risk (P < .001), feeling tired – 35% increased risk (P < .001), forgetfulness – 25% increased risk (P < .001), mood swings – 21% increased risk (P = .02), feeling restless or fidgety – 29% increased risk (P < .001), and difficulty concentrating – 31% increased risk (P < .001)
In addition, all-cause mortality was associated with these symptoms when they were moderate or severe: heart racing or skipping beats (32% increased risk of all-cause mortality; hazard ratio, 1.32; P =.006), dizziness (HR, 1.58; P < .001), tremors (HR, 1.44; P < .001), feeling tired (HR, 1.26; P < .001), forgetfulness (HR, 1.29; P = .01), mood swings (HR, 1.35; P = .02), feeling restless or fidgety (HR, 1.35; P < .001), and difficulty concentrating (HR, 1.47; P < .001).
The symptom with the greatest association with all-cause mortality was dizziness, which was associated with an increased risk of 58% when rated moderate or severe. Any dizziness at all was linked to a 12% increased risk of cardiovascular disease, compared with no dizziness. Machine learning with the LASSO method determined that the symptoms most predictive of cardiovascular disease were dizziness, heart racing, feeling tired, and joint pain. The symptoms most associated with all-cause mortality, based on the machine learning algorithm, were dizziness, tremors, and feeling tired.
Dr. Nudy said that their study did not look at mitigation strategies. “Women should discuss with their physician the best methods for cardiovascular risk reduction,” he said. He also cautioned that severe menopausal symptoms can also indicate other health conditions that may require investigation.
“It is certainly possible some symptoms may represent other medical conditions we were unable to control for and may not be directly related to menopause,” such as autoimmune diseases, endocrine abnormalities, or subclinical cardiovascular disease, he said. Additional limitations of the study included an older cohort and retrospective assessment of menopausal symptoms only at baseline. In addition, ”we did not assess the cardiovascular risk among women whose symptoms persisted versus resolved during the study period,” Dr. Nudy said.
Dr. Nachtigall said a key message is that people who are experiencing these symptoms should try to get treatment for them and attempt to alleviate them, hopefully reducing the risk of heart disease and death.
”Estrogen treatment is one excellent option for some individuals and should be considered in the appropriate person,” Dr. Nachtigall said. “If estrogen treatment is to be considered, it should be given closer to menopause, within the first 10 years after menopause and in younger individuals (under 59) at start.”
Dr. Nachtigall referred to the NAMS 2022 position statement concluding that, for healthy women within 10 years of menopause who have bothersome menopause symptoms, “the benefits of hormone therapy outweigh its risks, with fewer cardiovascular events in younger versus older women.”
”Menopause and having menopausal symptoms is an opportunity for clinicians and patients to have a conversation about appropriate individualized management options,” Dr. Nachtigall said.
Women may also be able to mitigate their cardiovascular risk with regular exercise, eating a healthy diet, not smoking, and getting adequate sleep, Dr. Nachtigall said. But these healthy behaviors may not adequately treat moderate or severe menopausal symptoms.
“Some health care providers have said that because menopause happens naturally, individuals should just accept the symptoms and try to wait it out and not get treatment, but this study, as well as others, makes it clear that it actually may be beneficial to treat the symptoms,” Dr. Nachtigall said.
The research used no external funding. Dr. Nudy and Dr. Nachtigall had no disclosures.
Up to 10 different menopausal symptoms were linked to an increased risk of cardiovascular disease when they were moderate to severe in women who initially had no evidence of cardiovascular disease, according to research presented at the North American Menopause Society annual meeting in Atlanta.
“The take-home message is that severe menopausal symptoms may increase the risk of cardiovascular disease,” Matthew Nudy, MD, an assistant professor of medicine at the Heart and Vascular Institute at Penn State University, Hershey, said in an interview about his findings. “Physicians and patients should be aware of this association. Women with severe symptoms may be more likely to see their physician, and this would be an ideal time to have their cardiovascular risk assessed.”
Margaret Nachtigall, MD, a clinical associate professor of obstetrics and gynecology at New York University and at NYU Langone Health, noted that these findings lined up with other studies showing an increased risk of cardiovascular disease in patients who have more symptoms, especially hot flashes.
“Other recent studies showed that an increase in severity of hot flush is associated with worse blood vessel function, leading to heart disease,” Dr. Nachtigall, who was not involved with the study, said in an interview. “The next step that makes sense is to try to eliminate these symptoms and hope that, in turn, would lower cardiovascular disease and improve survival.”
The researchers compared menopausal symptoms with cardiovascular outcomes and all-cause mortality in an observational cohort of 80,278 postmenopausal women for a median 8.2 years of follow-up. None of the women, all enrolled in the Women’s Health Initiative, had known cardiovascular disease at baseline. They had an average age of 63 years and average body mass index (BMI) of 25.9 at baseline. Most participants were White (86.7%), with 7% being Black and 4.1% Hispanic. Cardiovascular disease was a composite outcome that included hospitalized myocardial infarction, definite silent myocardial infarction, coronary death, stroke, congestive heart failure, angina, peripheral vascular disease, carotid artery disease, and coronary revascularization.
The researchers used a four-item Likert scale (0-3) to assess the severity of 15 symptoms experienced within the past 4 weeks at baseline: “night sweats, hot flashes, waking up several times at night, joint pain or stiffness, headaches or migraines, vaginal or genital dryness, heart racing or skipping beats, breast tenderness, dizziness, tremors (shakes), feeling tired, forgetfulness, mood swings, [feeling] restless or fidgety, and difficulty concentrating.”
The associations were adjusted for the following covariates: race/ethnicity, blood pressure, education, smoking status, bilateral oophorectomy, menopausal hormone therapy use (never/past/current), sleep duration, statin use, history of high cholesterol, aspirin use, use of antihypertensives, treated diabetes, and family history of heart attack. Continuous variables included age, age at menopause, BMI, blood pressure, and physical activity levels. Because of the high number of multiple comparisons, the researchers also used a Bonferroni correction to reduce the risk of spurious statistical significance.
The researchers found some clustering of symptoms. Among women who had at least two moderate or severe menopausal symptoms, more than half frequently woke up at night, had joint pain, or felt tired, the researchers reported. Those symptoms were also the most commonly reported ones overall. Younger women, between ages 50 and 59, were more likely than older women (60-79 years old) to experience vasomotor symptoms and all cognitive affective symptoms except forgetfulness.
The researchers identified 10 symptoms whose severity was significantly associated with cardiovascular disease. Compared to having no symptoms at all, the following moderate or severe symptoms were associated with an increased risk of a cardiovascular event after adjustment for covariates and corrected for multiple comparisons: night sweats – a 19% increased risk (P = .03), waking up several times at night – 11% increased risk (P = .05), joint pain or stiffness – 27% increased risk (P < .001), heart racing or skipping beats – 55% increased risk (P < .001), dizziness – 34% increased risk (P < .001), feeling tired – 35% increased risk (P < .001), forgetfulness – 25% increased risk (P < .001), mood swings – 21% increased risk (P = .02), feeling restless or fidgety – 29% increased risk (P < .001), and difficulty concentrating – 31% increased risk (P < .001)
In addition, all-cause mortality was associated with these symptoms when they were moderate or severe: heart racing or skipping beats (32% increased risk of all-cause mortality; hazard ratio, 1.32; P =.006), dizziness (HR, 1.58; P < .001), tremors (HR, 1.44; P < .001), feeling tired (HR, 1.26; P < .001), forgetfulness (HR, 1.29; P = .01), mood swings (HR, 1.35; P = .02), feeling restless or fidgety (HR, 1.35; P < .001), and difficulty concentrating (HR, 1.47; P < .001).
The symptom with the greatest association with all-cause mortality was dizziness, which was associated with an increased risk of 58% when rated moderate or severe. Any dizziness at all was linked to a 12% increased risk of cardiovascular disease, compared with no dizziness. Machine learning with the LASSO method determined that the symptoms most predictive of cardiovascular disease were dizziness, heart racing, feeling tired, and joint pain. The symptoms most associated with all-cause mortality, based on the machine learning algorithm, were dizziness, tremors, and feeling tired.
Dr. Nudy said that their study did not look at mitigation strategies. “Women should discuss with their physician the best methods for cardiovascular risk reduction,” he said. He also cautioned that severe menopausal symptoms can also indicate other health conditions that may require investigation.
“It is certainly possible some symptoms may represent other medical conditions we were unable to control for and may not be directly related to menopause,” such as autoimmune diseases, endocrine abnormalities, or subclinical cardiovascular disease, he said. Additional limitations of the study included an older cohort and retrospective assessment of menopausal symptoms only at baseline. In addition, ”we did not assess the cardiovascular risk among women whose symptoms persisted versus resolved during the study period,” Dr. Nudy said.
Dr. Nachtigall said a key message is that people who are experiencing these symptoms should try to get treatment for them and attempt to alleviate them, hopefully reducing the risk of heart disease and death.
”Estrogen treatment is one excellent option for some individuals and should be considered in the appropriate person,” Dr. Nachtigall said. “If estrogen treatment is to be considered, it should be given closer to menopause, within the first 10 years after menopause and in younger individuals (under 59) at start.”
Dr. Nachtigall referred to the NAMS 2022 position statement concluding that, for healthy women within 10 years of menopause who have bothersome menopause symptoms, “the benefits of hormone therapy outweigh its risks, with fewer cardiovascular events in younger versus older women.”
”Menopause and having menopausal symptoms is an opportunity for clinicians and patients to have a conversation about appropriate individualized management options,” Dr. Nachtigall said.
Women may also be able to mitigate their cardiovascular risk with regular exercise, eating a healthy diet, not smoking, and getting adequate sleep, Dr. Nachtigall said. But these healthy behaviors may not adequately treat moderate or severe menopausal symptoms.
“Some health care providers have said that because menopause happens naturally, individuals should just accept the symptoms and try to wait it out and not get treatment, but this study, as well as others, makes it clear that it actually may be beneficial to treat the symptoms,” Dr. Nachtigall said.
The research used no external funding. Dr. Nudy and Dr. Nachtigall had no disclosures.
Up to 10 different menopausal symptoms were linked to an increased risk of cardiovascular disease when they were moderate to severe in women who initially had no evidence of cardiovascular disease, according to research presented at the North American Menopause Society annual meeting in Atlanta.
“The take-home message is that severe menopausal symptoms may increase the risk of cardiovascular disease,” Matthew Nudy, MD, an assistant professor of medicine at the Heart and Vascular Institute at Penn State University, Hershey, said in an interview about his findings. “Physicians and patients should be aware of this association. Women with severe symptoms may be more likely to see their physician, and this would be an ideal time to have their cardiovascular risk assessed.”
Margaret Nachtigall, MD, a clinical associate professor of obstetrics and gynecology at New York University and at NYU Langone Health, noted that these findings lined up with other studies showing an increased risk of cardiovascular disease in patients who have more symptoms, especially hot flashes.
“Other recent studies showed that an increase in severity of hot flush is associated with worse blood vessel function, leading to heart disease,” Dr. Nachtigall, who was not involved with the study, said in an interview. “The next step that makes sense is to try to eliminate these symptoms and hope that, in turn, would lower cardiovascular disease and improve survival.”
The researchers compared menopausal symptoms with cardiovascular outcomes and all-cause mortality in an observational cohort of 80,278 postmenopausal women for a median 8.2 years of follow-up. None of the women, all enrolled in the Women’s Health Initiative, had known cardiovascular disease at baseline. They had an average age of 63 years and average body mass index (BMI) of 25.9 at baseline. Most participants were White (86.7%), with 7% being Black and 4.1% Hispanic. Cardiovascular disease was a composite outcome that included hospitalized myocardial infarction, definite silent myocardial infarction, coronary death, stroke, congestive heart failure, angina, peripheral vascular disease, carotid artery disease, and coronary revascularization.
The researchers used a four-item Likert scale (0-3) to assess the severity of 15 symptoms experienced within the past 4 weeks at baseline: “night sweats, hot flashes, waking up several times at night, joint pain or stiffness, headaches or migraines, vaginal or genital dryness, heart racing or skipping beats, breast tenderness, dizziness, tremors (shakes), feeling tired, forgetfulness, mood swings, [feeling] restless or fidgety, and difficulty concentrating.”
The associations were adjusted for the following covariates: race/ethnicity, blood pressure, education, smoking status, bilateral oophorectomy, menopausal hormone therapy use (never/past/current), sleep duration, statin use, history of high cholesterol, aspirin use, use of antihypertensives, treated diabetes, and family history of heart attack. Continuous variables included age, age at menopause, BMI, blood pressure, and physical activity levels. Because of the high number of multiple comparisons, the researchers also used a Bonferroni correction to reduce the risk of spurious statistical significance.
The researchers found some clustering of symptoms. Among women who had at least two moderate or severe menopausal symptoms, more than half frequently woke up at night, had joint pain, or felt tired, the researchers reported. Those symptoms were also the most commonly reported ones overall. Younger women, between ages 50 and 59, were more likely than older women (60-79 years old) to experience vasomotor symptoms and all cognitive affective symptoms except forgetfulness.
The researchers identified 10 symptoms whose severity was significantly associated with cardiovascular disease. Compared to having no symptoms at all, the following moderate or severe symptoms were associated with an increased risk of a cardiovascular event after adjustment for covariates and corrected for multiple comparisons: night sweats – a 19% increased risk (P = .03), waking up several times at night – 11% increased risk (P = .05), joint pain or stiffness – 27% increased risk (P < .001), heart racing or skipping beats – 55% increased risk (P < .001), dizziness – 34% increased risk (P < .001), feeling tired – 35% increased risk (P < .001), forgetfulness – 25% increased risk (P < .001), mood swings – 21% increased risk (P = .02), feeling restless or fidgety – 29% increased risk (P < .001), and difficulty concentrating – 31% increased risk (P < .001)
In addition, all-cause mortality was associated with these symptoms when they were moderate or severe: heart racing or skipping beats (32% increased risk of all-cause mortality; hazard ratio, 1.32; P =.006), dizziness (HR, 1.58; P < .001), tremors (HR, 1.44; P < .001), feeling tired (HR, 1.26; P < .001), forgetfulness (HR, 1.29; P = .01), mood swings (HR, 1.35; P = .02), feeling restless or fidgety (HR, 1.35; P < .001), and difficulty concentrating (HR, 1.47; P < .001).
The symptom with the greatest association with all-cause mortality was dizziness, which was associated with an increased risk of 58% when rated moderate or severe. Any dizziness at all was linked to a 12% increased risk of cardiovascular disease, compared with no dizziness. Machine learning with the LASSO method determined that the symptoms most predictive of cardiovascular disease were dizziness, heart racing, feeling tired, and joint pain. The symptoms most associated with all-cause mortality, based on the machine learning algorithm, were dizziness, tremors, and feeling tired.
Dr. Nudy said that their study did not look at mitigation strategies. “Women should discuss with their physician the best methods for cardiovascular risk reduction,” he said. He also cautioned that severe menopausal symptoms can also indicate other health conditions that may require investigation.
“It is certainly possible some symptoms may represent other medical conditions we were unable to control for and may not be directly related to menopause,” such as autoimmune diseases, endocrine abnormalities, or subclinical cardiovascular disease, he said. Additional limitations of the study included an older cohort and retrospective assessment of menopausal symptoms only at baseline. In addition, ”we did not assess the cardiovascular risk among women whose symptoms persisted versus resolved during the study period,” Dr. Nudy said.
Dr. Nachtigall said a key message is that people who are experiencing these symptoms should try to get treatment for them and attempt to alleviate them, hopefully reducing the risk of heart disease and death.
”Estrogen treatment is one excellent option for some individuals and should be considered in the appropriate person,” Dr. Nachtigall said. “If estrogen treatment is to be considered, it should be given closer to menopause, within the first 10 years after menopause and in younger individuals (under 59) at start.”
Dr. Nachtigall referred to the NAMS 2022 position statement concluding that, for healthy women within 10 years of menopause who have bothersome menopause symptoms, “the benefits of hormone therapy outweigh its risks, with fewer cardiovascular events in younger versus older women.”
”Menopause and having menopausal symptoms is an opportunity for clinicians and patients to have a conversation about appropriate individualized management options,” Dr. Nachtigall said.
Women may also be able to mitigate their cardiovascular risk with regular exercise, eating a healthy diet, not smoking, and getting adequate sleep, Dr. Nachtigall said. But these healthy behaviors may not adequately treat moderate or severe menopausal symptoms.
“Some health care providers have said that because menopause happens naturally, individuals should just accept the symptoms and try to wait it out and not get treatment, but this study, as well as others, makes it clear that it actually may be beneficial to treat the symptoms,” Dr. Nachtigall said.
The research used no external funding. Dr. Nudy and Dr. Nachtigall had no disclosures.
FROM NAMS 2022
Nicotine blocks estrogen production in women’s brains
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
Menopause an independent risk factor for schizophrenia relapse
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators studied a cohort of close to 62,000 people with SSDs, stratifying individuals by sex and age, and found that starting between the ages of 45 and 50 years – when the menopausal transition is underway – women were more frequently hospitalized for psychosis, compared with men and women younger than 45 years.
In addition, the protective effect of antipsychotic medication was highest in women younger than 45 years and lowest in women aged 45 years or older, even at higher doses.
“Women with schizophrenia who are older than 45 are a vulnerable group for relapse, and higher doses of antipsychotics are not the answer,” lead author Iris Sommer, MD, PhD, professor, department of neuroscience, University Medical Center of Groningen, the Netherlands, told this news organization.
The study was published online in Schizophrenia Bulletin.
Vulnerable period
There is an association between estrogen levels and disease severity throughout the life stages of women with SSDs, with lower estrogen levels associated with psychosis, for example, during low estrogenic phases of the menstrual cycle, the investigators note.
“After menopause, estrogen levels remain low, which is associated with a deterioration in the clinical course; therefore, women with SSD have sex-specific psychiatric needs that differ according to their life stage,” they add.
“Estrogens inhibit an important liver enzyme (cytochrome P-450 [CYP1A2]), which leads to higher blood levels of several antipsychotics like olanzapine and clozapine,” said Dr. Sommer. In addition, estrogens make the stomach less acidic, “leading to easier resorption of medication.”
As a clinician, Dr. Sommer said that she has “often witnessed a worsening of symptoms [of psychosis] after menopause.” As a researcher, she “knew that estrogens can have ameliorating effects on brain health, especially in schizophrenia.”
She and her colleagues were motivated to research the issue because there is a “remarkable paucity” of quantitative data on a “vulnerable period that all women with schizophrenia will experience.”
Detailed, quantitative data
The researchers sought to provide “detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding.”
They drew on data from a nationwide, register-based cohort study of all hospitalized patients with SSD between 1972 and 2014 in Finland (n = 61,889), with follow-up from Jan. 1, 1996, to Dec. 31, 2017.
People were stratified according to age (younger than 45 years and 45 years or older), with the same person contributing person-time to both age groups. The cohort was also subdivided into 5-year age groups, starting at age 20 years and ending at age 69 years.
The primary outcome measure was relapse (that is, inpatient hospitalization because of psychosis).
The researchers focused specifically on monotherapies, excluding time periods when two or more antipsychotics were used concomitantly. They also looked at antipsychotic nonuse periods.
Antipsychotic monotherapies were categorized into defined daily doses per day (DDDs/d):
- less than 0.4
- 0.4 to 0.6
- 0.6 to 0.9
- 0.9 to less than 1.1
- 1.1 to less than 1.4
- 1.4 to less than 1.6
- 1.6 or more
The researchers restricted the main analyses to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone.
The turning tide
The cohort consisted of more men than women (31,104 vs. 30,785, respectively), with a mean (standard deviation) age of 49.8 (16.6) years in women vs. 43.6 (14.8) in men.
Among both sexes, olanzapine was the most prescribed antipsychotic (roughly one-quarter of patients). In women, the next most common antipsychotic was risperidone, followed by quetiapine and clozapine, whereas in men, the second most common antipsychotic was clozapine, followed by risperidone and quetiapine.
When the researchers compared men and women younger than 45 years, there were “few consistent differences” in proportions hospitalized for psychosis.
Starting at age 45 years and continuing through the oldest age group (65-69 years), higher proportions of women were hospitalized for psychosis, compared with their male peers (all Ps < .00001).
Women 45 or older had significantly higher risk for relapse associated with standard dose use, compared with the other groups.
When the researchers compared men and women older and younger than 45 years, women younger than 45 years showed lower adjusted hazard ratios (aHRs) at doses between of 0.6-0.9 DDDs/d, whereas for doses over 1.1 DDDs/d, women aged 45 years or older showed “remarkably higher” aHRs, compared with women younger than 45 years and men aged 45 years or older, with a difference that increased with increasing dose.
In women, the efficacy of the antipsychotics was decreased at these DDDs/d.
“We ... showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages,” the authors summarize. But after age 45 years, “the tide seems to turn for women,” compared with younger women and with men of the same age group.
One of several study limitations was the use of age as an estimation of menopausal status, they note.
Don’t just raise the dose
Commenting on the research, Mary Seeman, MD, professor emerita, department of psychiatry, University of Toronto, noted the study corroborates her group’s findings regarding the effect of menopause on antipsychotic response.
“When the efficacy of previously effective antipsychotic doses wanes at menopause, raising the dose is not the treatment of choice because it increases the risk of weight gain, cardiovascular, and cerebrovascular events,” said Dr. Seeman, who was not involved with the current research.
“Changing to an antipsychotic that is less affected by estrogen loss may work better,” she continued, noting that amisulpride and aripiprazole “work well post menopause.”
Additional interventions may include changing to a depot or skin-patch antipsychotic that “obviates first-pass metabolism,” adding hormone replacement or a selective estrogen receptor modulator or including phytoestrogens (bioidenticals) in the diet.
The study yields research recommendations, including comparing the effectiveness of different antipsychotics in postmenopausal women with SSDs, recruiting pre- and postmenopausal women in trials of antipsychotic drugs, and stratifying by hormonal status when analyzing results of antipsychotic trials, Dr. Seeman said.
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland. The Dutch Medical Research Association supported Dr. Sommer. Dr. Sommer declares no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Seeman declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCHIZOPHRENIA BULLETIN
Plant-based diet cut hot flashes 78%: WAVS study
Women eating a reduced-fat vegan diet combined with a daily serving of soybeans experienced a 78% reduction in frequency of menopausal hot flashes over 12 weeks, in a small, nonblinded, randomized-controlled trial.
“We do not fully understand yet why this combination works, but it seems that these three elements are key: avoiding animal products, reducing fat, and adding a serving of soybeans,” lead researcher Neal Barnard, MD, explained in a press release. “These new results suggest that a diet change should be considered as a first-line treatment for troublesome vasomotor symptoms, including night sweats and hot flashes,” added Dr. Barnard, who is president of the Physicians Committee for Responsible Medicine, and adjunct professor at George Washington University, Washington.
But, while “the findings from this very small study complement everything we know about the benefits of an excellent diet and the health benefits of soy,” they should be interpreted with some caution, commented Susan Reed, MD, president of the North American Menopause Society, and associate program director of the women’s reproductive research program at the University of Washington, Seattle.
For the trial, called WAVS (Women’s Study for the Alleviation of Vasomotor Symptoms), the researchers randomized 84 postmenopausal women with at least two moderate to severe hot flashes daily to either the intervention or usual diet, with a total of 71 subjects completing the 12-week study, published in Menopause. Criteria for exclusion included any cause of vasomotor symptoms other than natural menopause, current use of a low-fat, vegan diet that includes daily soy products, soy allergy, and body mass index < 18.5 kg/m2.
Participants in the intervention group were asked to avoid animal-derived foods, minimize their use of oils and fatty foods such as nuts and avocados, and include half a cup (86 g) of cooked soybeans daily in their diets. They were also offered 1-hour virtual group meetings each week, in which a registered dietitian or research staff provided information on food preparation and managing common dietary challenges.
Control group participants were asked to continue their usual diets and attend four 1-hour group sessions.
At baseline and then after the 12-week study period, dietary intake was self-recorded for 2 weekdays and 1 weekend day, hot flash frequency and severity was recorded for 1 week using a mobile app, and the effect of menopausal symptoms on quality of life was measured using the vasomotor, psychosocial, physical, and sexual domains of the Menopause-Specific Quality of Life (MENQOL) questionnaire.
Equol production was also assessed in a subset of 15 intervention and 12 control participants who had urinary isoflavone concentrations measured after eating half a cup (86 g) of soybeans twice daily for 3 days. This was based on the theory that diets such as the intervention in this study “seem to foster the growth of gut bacteria capable of converting daidzein to equol,” noted the authors. The ability to produce equol is detected more frequently in individuals following vegetarian diets than in omnivores and … has been proposed as a factor in soy’s apparent health benefits.”
The study found that total hot flash frequency decreased by 78% in the intervention group (P < .001) and 39% (P < .001) in the control group (between-group P = .003), and moderate to severe hot flashes decreased by 88% versus 34%, respectively (from 5.0 to 0.6 per day, P < .001 vs. from 4.4 to 2.9 per day, P < .001; between-group P < .001). Among participants with at least seven moderate to severe hot flashes per day at baseline, moderate to severe hot flashes decreased by 93% (from 10.6 to 0.7 per day) in the intervention group (P < .001) and 36% (from 9.0 to 5.8 per day) in the control group (P = .01, between-group P < .001). The changes in hot flashes were paralleled by changes in the MENQOL findings, with significant between-group differences in the vasomotor (P = 0.004), physical (P = 0.01), and sexual (P = 0.03) domains.
Changes in frequency of severe hot flashes correlated directly with changes in fat intake, and inversely with changes in carbohydrate and fiber intake, such that “the greater the reduction in fat intake and the greater the increases in carbohydrate and fiber consumption, the greater the reduction in severe hot flashes,” noted the researchers. Mean body weight also decreased by 3.6 kg in the intervention group and 0.2 kg in the control group (P < .001). “Equol-production status had no apparent effect on hot flashes,” they added.
The study is the second phase of WAVS, which comprises two parts, the first of which showed similar results, but was conducted in the fall, raising questions about whether cooler temperatures were partly responsible for the results. Phase 2 of WAVS enrolled participants in the spring “ruling out the effect of outside temperature,” noted the authors.
“Eating a healthy diet at midlife is so important for long-term health and a sense of well-being for peri- and postmenopausal women,” said Dr Reed, but she urged caution in interpreting the findings. “This was an unblinded study,” she told this news organization. “Women were recruited to this study anticipating that they would be in a study on a soy diet. Individuals who sign up for a study are hoping for benefit from the intervention. The controls who don’t get the soy diet are often disappointed, so there is no benefit from a nonblinded control arm for their hot flashes. And that is exactly what we saw here. Blinded studies can hide what you are getting, so everyone in the study (intervention and controls) has the same anticipated benefit. But you cannot blind a soy diet.”
Dr. Reed also noted that, while the biologic mechanism of benefit should be equol production, this was not shown – given that both equol producers and nonproducers in the soy intervention reported marked symptom reduction.
“Only prior studies with estrogen interventions have observed reductions of hot flashes of the amount reported here,” she concluded. “Hopefully future large studies will clarify the role of soy diet for decreasing hot flashes.”
Dr. Barnard writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources. Dr. Reed has no relevant disclosures.
Women eating a reduced-fat vegan diet combined with a daily serving of soybeans experienced a 78% reduction in frequency of menopausal hot flashes over 12 weeks, in a small, nonblinded, randomized-controlled trial.
“We do not fully understand yet why this combination works, but it seems that these three elements are key: avoiding animal products, reducing fat, and adding a serving of soybeans,” lead researcher Neal Barnard, MD, explained in a press release. “These new results suggest that a diet change should be considered as a first-line treatment for troublesome vasomotor symptoms, including night sweats and hot flashes,” added Dr. Barnard, who is president of the Physicians Committee for Responsible Medicine, and adjunct professor at George Washington University, Washington.
But, while “the findings from this very small study complement everything we know about the benefits of an excellent diet and the health benefits of soy,” they should be interpreted with some caution, commented Susan Reed, MD, president of the North American Menopause Society, and associate program director of the women’s reproductive research program at the University of Washington, Seattle.
For the trial, called WAVS (Women’s Study for the Alleviation of Vasomotor Symptoms), the researchers randomized 84 postmenopausal women with at least two moderate to severe hot flashes daily to either the intervention or usual diet, with a total of 71 subjects completing the 12-week study, published in Menopause. Criteria for exclusion included any cause of vasomotor symptoms other than natural menopause, current use of a low-fat, vegan diet that includes daily soy products, soy allergy, and body mass index < 18.5 kg/m2.
Participants in the intervention group were asked to avoid animal-derived foods, minimize their use of oils and fatty foods such as nuts and avocados, and include half a cup (86 g) of cooked soybeans daily in their diets. They were also offered 1-hour virtual group meetings each week, in which a registered dietitian or research staff provided information on food preparation and managing common dietary challenges.
Control group participants were asked to continue their usual diets and attend four 1-hour group sessions.
At baseline and then after the 12-week study period, dietary intake was self-recorded for 2 weekdays and 1 weekend day, hot flash frequency and severity was recorded for 1 week using a mobile app, and the effect of menopausal symptoms on quality of life was measured using the vasomotor, psychosocial, physical, and sexual domains of the Menopause-Specific Quality of Life (MENQOL) questionnaire.
Equol production was also assessed in a subset of 15 intervention and 12 control participants who had urinary isoflavone concentrations measured after eating half a cup (86 g) of soybeans twice daily for 3 days. This was based on the theory that diets such as the intervention in this study “seem to foster the growth of gut bacteria capable of converting daidzein to equol,” noted the authors. The ability to produce equol is detected more frequently in individuals following vegetarian diets than in omnivores and … has been proposed as a factor in soy’s apparent health benefits.”
The study found that total hot flash frequency decreased by 78% in the intervention group (P < .001) and 39% (P < .001) in the control group (between-group P = .003), and moderate to severe hot flashes decreased by 88% versus 34%, respectively (from 5.0 to 0.6 per day, P < .001 vs. from 4.4 to 2.9 per day, P < .001; between-group P < .001). Among participants with at least seven moderate to severe hot flashes per day at baseline, moderate to severe hot flashes decreased by 93% (from 10.6 to 0.7 per day) in the intervention group (P < .001) and 36% (from 9.0 to 5.8 per day) in the control group (P = .01, between-group P < .001). The changes in hot flashes were paralleled by changes in the MENQOL findings, with significant between-group differences in the vasomotor (P = 0.004), physical (P = 0.01), and sexual (P = 0.03) domains.
Changes in frequency of severe hot flashes correlated directly with changes in fat intake, and inversely with changes in carbohydrate and fiber intake, such that “the greater the reduction in fat intake and the greater the increases in carbohydrate and fiber consumption, the greater the reduction in severe hot flashes,” noted the researchers. Mean body weight also decreased by 3.6 kg in the intervention group and 0.2 kg in the control group (P < .001). “Equol-production status had no apparent effect on hot flashes,” they added.
The study is the second phase of WAVS, which comprises two parts, the first of which showed similar results, but was conducted in the fall, raising questions about whether cooler temperatures were partly responsible for the results. Phase 2 of WAVS enrolled participants in the spring “ruling out the effect of outside temperature,” noted the authors.
“Eating a healthy diet at midlife is so important for long-term health and a sense of well-being for peri- and postmenopausal women,” said Dr Reed, but she urged caution in interpreting the findings. “This was an unblinded study,” she told this news organization. “Women were recruited to this study anticipating that they would be in a study on a soy diet. Individuals who sign up for a study are hoping for benefit from the intervention. The controls who don’t get the soy diet are often disappointed, so there is no benefit from a nonblinded control arm for their hot flashes. And that is exactly what we saw here. Blinded studies can hide what you are getting, so everyone in the study (intervention and controls) has the same anticipated benefit. But you cannot blind a soy diet.”
Dr. Reed also noted that, while the biologic mechanism of benefit should be equol production, this was not shown – given that both equol producers and nonproducers in the soy intervention reported marked symptom reduction.
“Only prior studies with estrogen interventions have observed reductions of hot flashes of the amount reported here,” she concluded. “Hopefully future large studies will clarify the role of soy diet for decreasing hot flashes.”
Dr. Barnard writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources. Dr. Reed has no relevant disclosures.
Women eating a reduced-fat vegan diet combined with a daily serving of soybeans experienced a 78% reduction in frequency of menopausal hot flashes over 12 weeks, in a small, nonblinded, randomized-controlled trial.
“We do not fully understand yet why this combination works, but it seems that these three elements are key: avoiding animal products, reducing fat, and adding a serving of soybeans,” lead researcher Neal Barnard, MD, explained in a press release. “These new results suggest that a diet change should be considered as a first-line treatment for troublesome vasomotor symptoms, including night sweats and hot flashes,” added Dr. Barnard, who is president of the Physicians Committee for Responsible Medicine, and adjunct professor at George Washington University, Washington.
But, while “the findings from this very small study complement everything we know about the benefits of an excellent diet and the health benefits of soy,” they should be interpreted with some caution, commented Susan Reed, MD, president of the North American Menopause Society, and associate program director of the women’s reproductive research program at the University of Washington, Seattle.
For the trial, called WAVS (Women’s Study for the Alleviation of Vasomotor Symptoms), the researchers randomized 84 postmenopausal women with at least two moderate to severe hot flashes daily to either the intervention or usual diet, with a total of 71 subjects completing the 12-week study, published in Menopause. Criteria for exclusion included any cause of vasomotor symptoms other than natural menopause, current use of a low-fat, vegan diet that includes daily soy products, soy allergy, and body mass index < 18.5 kg/m2.
Participants in the intervention group were asked to avoid animal-derived foods, minimize their use of oils and fatty foods such as nuts and avocados, and include half a cup (86 g) of cooked soybeans daily in their diets. They were also offered 1-hour virtual group meetings each week, in which a registered dietitian or research staff provided information on food preparation and managing common dietary challenges.
Control group participants were asked to continue their usual diets and attend four 1-hour group sessions.
At baseline and then after the 12-week study period, dietary intake was self-recorded for 2 weekdays and 1 weekend day, hot flash frequency and severity was recorded for 1 week using a mobile app, and the effect of menopausal symptoms on quality of life was measured using the vasomotor, psychosocial, physical, and sexual domains of the Menopause-Specific Quality of Life (MENQOL) questionnaire.
Equol production was also assessed in a subset of 15 intervention and 12 control participants who had urinary isoflavone concentrations measured after eating half a cup (86 g) of soybeans twice daily for 3 days. This was based on the theory that diets such as the intervention in this study “seem to foster the growth of gut bacteria capable of converting daidzein to equol,” noted the authors. The ability to produce equol is detected more frequently in individuals following vegetarian diets than in omnivores and … has been proposed as a factor in soy’s apparent health benefits.”
The study found that total hot flash frequency decreased by 78% in the intervention group (P < .001) and 39% (P < .001) in the control group (between-group P = .003), and moderate to severe hot flashes decreased by 88% versus 34%, respectively (from 5.0 to 0.6 per day, P < .001 vs. from 4.4 to 2.9 per day, P < .001; between-group P < .001). Among participants with at least seven moderate to severe hot flashes per day at baseline, moderate to severe hot flashes decreased by 93% (from 10.6 to 0.7 per day) in the intervention group (P < .001) and 36% (from 9.0 to 5.8 per day) in the control group (P = .01, between-group P < .001). The changes in hot flashes were paralleled by changes in the MENQOL findings, with significant between-group differences in the vasomotor (P = 0.004), physical (P = 0.01), and sexual (P = 0.03) domains.
Changes in frequency of severe hot flashes correlated directly with changes in fat intake, and inversely with changes in carbohydrate and fiber intake, such that “the greater the reduction in fat intake and the greater the increases in carbohydrate and fiber consumption, the greater the reduction in severe hot flashes,” noted the researchers. Mean body weight also decreased by 3.6 kg in the intervention group and 0.2 kg in the control group (P < .001). “Equol-production status had no apparent effect on hot flashes,” they added.
The study is the second phase of WAVS, which comprises two parts, the first of which showed similar results, but was conducted in the fall, raising questions about whether cooler temperatures were partly responsible for the results. Phase 2 of WAVS enrolled participants in the spring “ruling out the effect of outside temperature,” noted the authors.
“Eating a healthy diet at midlife is so important for long-term health and a sense of well-being for peri- and postmenopausal women,” said Dr Reed, but she urged caution in interpreting the findings. “This was an unblinded study,” she told this news organization. “Women were recruited to this study anticipating that they would be in a study on a soy diet. Individuals who sign up for a study are hoping for benefit from the intervention. The controls who don’t get the soy diet are often disappointed, so there is no benefit from a nonblinded control arm for their hot flashes. And that is exactly what we saw here. Blinded studies can hide what you are getting, so everyone in the study (intervention and controls) has the same anticipated benefit. But you cannot blind a soy diet.”
Dr. Reed also noted that, while the biologic mechanism of benefit should be equol production, this was not shown – given that both equol producers and nonproducers in the soy intervention reported marked symptom reduction.
“Only prior studies with estrogen interventions have observed reductions of hot flashes of the amount reported here,” she concluded. “Hopefully future large studies will clarify the role of soy diet for decreasing hot flashes.”
Dr. Barnard writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources. Dr. Reed has no relevant disclosures.
Early estrogen loss increases cardiovascular risk in women
The relationship between estrogen levels and heart health makes it particularly important for clinicians to be aware of those patients who might be at risk for cardiovascular disease despite not having other traditional risk factors, according to a presentation Oct. 12 at the North American Menopause Society annual meeting in Atlanta.
”Endogenous estrogens are protective for cardiovascular disease in premenopausal women,” Chrisandra L. Shufelt, MD, chair of the division of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Fla., told attendees. Yet, “a substantial population of young women are dying prematurely from cardiovascular disease,” with rates of cardiovascular death increasing in women aged 35-44 even as rates have decreased in postmenopausal women and in men. One potential reason may be premature estrogen loss.
Dr. Shufelt reminded attendees of four major causes of premature estrogen loss: Natural premature menopause, surgical menopause, chemotherapy-induced menopause, and premature ovarian insufficiency. But she would go on to discuss a less widely recognized condition, functional hypothalamic amenorrhea, that also may be contributing to increased cardiovascular risk.
First, Dr. Shufelt reviewed the evidence supporting the relationship between estrogen and cardiovascular health, starting with the Framingham study’s findings that cardiovascular disease is approximately two to four times more common in postmenopausal women than in premenopausal women, depending on the age range.
“Menopause at an early age, particularly under the age of 40, matters,” Dr. Shufelt said. “So we should be discussing this with our patients.”
Surgical menopause makes a difference to cardiovascular health as well, she said. In women under age 35, for example, the risk of a nonfatal heart attack in those with a bilateral oophorectomy was 7.7 times greater than in women who retained both ovaries and their uterus, and 1.5 times greater in women who had a hysterectomy without bilateral oophorectomy.
In a 2019 study, surgical premature menopause was associated with an 87% increased risk of heart disease even after researchers accounted for age, cardiovascular risk factors, and some forms of hormone therapy. The increased risk from natural premature menopause, on the other hand, was lower – a 36% increased risk of heart disease – compared with those producing endogenous hormones. Although randomized controlled trials are unavailable and unlikely to be done, the Nurses’ Health Study and the Danish Nurses Cohort Study, both observational studies, found that heart disease risk was diminished in those taking hormone therapy after surgical premature menopause.
Recommendations for premature or early menopause, from a wide range of different medical societies including NAMS, are that women without contraindications be given estrogen-based hormone therapy until the average age of natural menopause. Though not included in the same guidance, research has also shown that estrogen after oophorectomy does not increase the risk of breast cancer in women with a BRCA1 mutation, Dr. Shufelt said. Hormone therapy for premature or early menopause should adequately replace the levels women have lost and that means younger menopausal women often need higher doses than what older women receive, such as 2 mg/day of oral estradiol rather than the standard doses of 0.5 or 1 mg/day.
Functional hypothalamic amenorrhea and cardiovascular risk
Dr. Shufelt then discussed functional hypothalamic amenorrhea (hypogonadotropic hypogonadism), a common type of secondary amenorrhea that affects at least 1.4 million U.S. women. Diagnosis includes lack of a period for at least 3 months in someone who previously menstruated plus lab values below 50 pg/mL for estradiol, below 10 mIU/L for follicle stimulating hormone, and below 10 mIU/L for luteinizing hormone. Causes of this reversible form of infertility can include stress, overexercising, undereating, or some combination of these, plus an underlying genetic predisposition.
“After ruling out polycystic ovary syndrome, prolactinoma, and thyroid dysfunction, clinicians need to consider the diagnosis of hypothalamic amenorrhea,” Dr. Shufelt said. This condition goes beyond low estrogen levels: Women have elevated cortisol, low thyroid levels, low leptin levels, and increased ghrelin.
”This is not going away,” Dr. Shufelt said, sharing data on stress levels among U.S. adults, particularly Gen Z and millennial adults, noting that the ongoing “national mental health crisis” may be contributing to functional hypothalamic amenorrhea.
A 2020 substudy from the Nurses’ Health Study II found an increased risk of premature death in those who didn’t have a period or always had irregular periods starting as early as 14-17 years old. The increased risk of premature death rose with age in those with irregular or absent cycles – a 37% higher risk in 18- to 22-year-olds and a 39% increased risk in 29- to 46-year-olds.
But clinicians aren’t adequately identifying the “phenotype of the hypothalamic women,” Dr. Shufelt said, despite research showing overlap between hypothalamic amenorrhea and a higher risk of cardiovascular disease. Hypothalamic amenorrhea is so understudied that the last original research on the topic was in 2008, Dr. Shufelt said in an interview. ”No research except mine has been done to evaluate heart health in these young women,” she said.
Dr. Shufelt described a study she led involving 30 women with functional hypothalamic amenorrhea, 29 women with normal menstrual cycles, and 30 women who were recently menopausal and not on hormone therapy. The women with hypothalamic amenorrhea had average stress levels but their depression scores were higher than those of the other two groups.
The results showed that women with hypothalamic amenorrhea had lower estradiol and leptin levels and higher testosterone levels compared with the control group, and they had higher cortisol levels than those of both groups. Despite having similar body mass indexes as the control and menopausal groups, women with hypothalamic amenorrhea had lower blood pressure than that of the other two groups, yet they had higher cholesterol levels than those of the control group. EndoPAT© (Itamar Medical) testing showed that they had poor vascular function.
“In fact, one-third of the women [with hypothalamic amenorrhea] entered the trial with a diagnosis of what would be considered endothelial dysfunction,” Dr. Shufelt said. “Our results demonstrated significantly higher circulating levels of serum proinflammatory cytokines in the women with hypothalamic amenorrhea compared to eumenorrheic controls.”
Dr. Shufelt’s team then tested whether giving estradiol to the women with hypothalamic amenorrhea for 12 weeks would improve their vascular health, but they saw no significant differences between the women who received estrogen and those who received placebo.
“Endothelial function is partly mediated by estrogen, and it was expected that giving back estrogen would ‘fix’ the endothelium, but that is not what happened,” Nanette Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado at Denver, Aurora, said in interview. “The mechanisms that maintain vascular function in women are not limited to hormones,” said Dr. Santoro, who was not involved in Dr. Shufelt’s study but attended her lecture. “We need to think beyond the simple model of estrogen-good, no-estrogen-bad.”
Dr. Santoro noted how easy it is to overlook the women who may have cardiovascular risk because of hypothalamic amenorrhea.
“Because many women with functional hypothalamic amenorrhea are super athletic and do not have the typical features of people with cardiometabolic disease – such as glucose intolerance, obesity, abnormal cholesterol or triglycerides, or high blood pressure – clinicians tend to think of them as healthy and to think that simply giving back hormones will fix the problems with bone density and vascular function, but that is not enough,” Dr. Santoro said. “The cognitive-behavioral therapy model for treatment of women with functional hypothalamic amenorrhea addresses the stress-related factors that drive the disorder, and this needs to be considered the standard of care for treatment.”
Stephanie S. Faubion, MD, professor of medicine and director of Mayo Clinic’s Center for Women’s Health in Jacksonville, Fla., who was not involved in Dr. Shufelt’s presentation, also emphasized the importance of recognizing functional hypothalamic amenorrhea.
“This is an underrecognized entity to begin with, and the fact that these women appear to be at increased risk for vascular dysfunction and potentially increased risk for cardiovascular disease down the road makes it even more important for clinicians to identify them and provide interventions early on,” Dr. Faubion said in an interview. “These women need to be identified and the etiology of the amenorrhea addressed, whether it relates to overexercising, being underweight, or experiencing significant stressors that have led to the loss of menstrual cycles.”
Dr. Shufelt’s research was funded by the National Institutes of Health. She had no disclosures. Dr. Santoro is a member of the scientific advisory board for Astellas, Menogenix, Amazon Ember, and Que Oncology, and she consults for Ansh Labs. Dr. Faubion had no disclosures.
The relationship between estrogen levels and heart health makes it particularly important for clinicians to be aware of those patients who might be at risk for cardiovascular disease despite not having other traditional risk factors, according to a presentation Oct. 12 at the North American Menopause Society annual meeting in Atlanta.
”Endogenous estrogens are protective for cardiovascular disease in premenopausal women,” Chrisandra L. Shufelt, MD, chair of the division of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Fla., told attendees. Yet, “a substantial population of young women are dying prematurely from cardiovascular disease,” with rates of cardiovascular death increasing in women aged 35-44 even as rates have decreased in postmenopausal women and in men. One potential reason may be premature estrogen loss.
Dr. Shufelt reminded attendees of four major causes of premature estrogen loss: Natural premature menopause, surgical menopause, chemotherapy-induced menopause, and premature ovarian insufficiency. But she would go on to discuss a less widely recognized condition, functional hypothalamic amenorrhea, that also may be contributing to increased cardiovascular risk.
First, Dr. Shufelt reviewed the evidence supporting the relationship between estrogen and cardiovascular health, starting with the Framingham study’s findings that cardiovascular disease is approximately two to four times more common in postmenopausal women than in premenopausal women, depending on the age range.
“Menopause at an early age, particularly under the age of 40, matters,” Dr. Shufelt said. “So we should be discussing this with our patients.”
Surgical menopause makes a difference to cardiovascular health as well, she said. In women under age 35, for example, the risk of a nonfatal heart attack in those with a bilateral oophorectomy was 7.7 times greater than in women who retained both ovaries and their uterus, and 1.5 times greater in women who had a hysterectomy without bilateral oophorectomy.
In a 2019 study, surgical premature menopause was associated with an 87% increased risk of heart disease even after researchers accounted for age, cardiovascular risk factors, and some forms of hormone therapy. The increased risk from natural premature menopause, on the other hand, was lower – a 36% increased risk of heart disease – compared with those producing endogenous hormones. Although randomized controlled trials are unavailable and unlikely to be done, the Nurses’ Health Study and the Danish Nurses Cohort Study, both observational studies, found that heart disease risk was diminished in those taking hormone therapy after surgical premature menopause.
Recommendations for premature or early menopause, from a wide range of different medical societies including NAMS, are that women without contraindications be given estrogen-based hormone therapy until the average age of natural menopause. Though not included in the same guidance, research has also shown that estrogen after oophorectomy does not increase the risk of breast cancer in women with a BRCA1 mutation, Dr. Shufelt said. Hormone therapy for premature or early menopause should adequately replace the levels women have lost and that means younger menopausal women often need higher doses than what older women receive, such as 2 mg/day of oral estradiol rather than the standard doses of 0.5 or 1 mg/day.
Functional hypothalamic amenorrhea and cardiovascular risk
Dr. Shufelt then discussed functional hypothalamic amenorrhea (hypogonadotropic hypogonadism), a common type of secondary amenorrhea that affects at least 1.4 million U.S. women. Diagnosis includes lack of a period for at least 3 months in someone who previously menstruated plus lab values below 50 pg/mL for estradiol, below 10 mIU/L for follicle stimulating hormone, and below 10 mIU/L for luteinizing hormone. Causes of this reversible form of infertility can include stress, overexercising, undereating, or some combination of these, plus an underlying genetic predisposition.
“After ruling out polycystic ovary syndrome, prolactinoma, and thyroid dysfunction, clinicians need to consider the diagnosis of hypothalamic amenorrhea,” Dr. Shufelt said. This condition goes beyond low estrogen levels: Women have elevated cortisol, low thyroid levels, low leptin levels, and increased ghrelin.
”This is not going away,” Dr. Shufelt said, sharing data on stress levels among U.S. adults, particularly Gen Z and millennial adults, noting that the ongoing “national mental health crisis” may be contributing to functional hypothalamic amenorrhea.
A 2020 substudy from the Nurses’ Health Study II found an increased risk of premature death in those who didn’t have a period or always had irregular periods starting as early as 14-17 years old. The increased risk of premature death rose with age in those with irregular or absent cycles – a 37% higher risk in 18- to 22-year-olds and a 39% increased risk in 29- to 46-year-olds.
But clinicians aren’t adequately identifying the “phenotype of the hypothalamic women,” Dr. Shufelt said, despite research showing overlap between hypothalamic amenorrhea and a higher risk of cardiovascular disease. Hypothalamic amenorrhea is so understudied that the last original research on the topic was in 2008, Dr. Shufelt said in an interview. ”No research except mine has been done to evaluate heart health in these young women,” she said.
Dr. Shufelt described a study she led involving 30 women with functional hypothalamic amenorrhea, 29 women with normal menstrual cycles, and 30 women who were recently menopausal and not on hormone therapy. The women with hypothalamic amenorrhea had average stress levels but their depression scores were higher than those of the other two groups.
The results showed that women with hypothalamic amenorrhea had lower estradiol and leptin levels and higher testosterone levels compared with the control group, and they had higher cortisol levels than those of both groups. Despite having similar body mass indexes as the control and menopausal groups, women with hypothalamic amenorrhea had lower blood pressure than that of the other two groups, yet they had higher cholesterol levels than those of the control group. EndoPAT© (Itamar Medical) testing showed that they had poor vascular function.
“In fact, one-third of the women [with hypothalamic amenorrhea] entered the trial with a diagnosis of what would be considered endothelial dysfunction,” Dr. Shufelt said. “Our results demonstrated significantly higher circulating levels of serum proinflammatory cytokines in the women with hypothalamic amenorrhea compared to eumenorrheic controls.”
Dr. Shufelt’s team then tested whether giving estradiol to the women with hypothalamic amenorrhea for 12 weeks would improve their vascular health, but they saw no significant differences between the women who received estrogen and those who received placebo.
“Endothelial function is partly mediated by estrogen, and it was expected that giving back estrogen would ‘fix’ the endothelium, but that is not what happened,” Nanette Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado at Denver, Aurora, said in interview. “The mechanisms that maintain vascular function in women are not limited to hormones,” said Dr. Santoro, who was not involved in Dr. Shufelt’s study but attended her lecture. “We need to think beyond the simple model of estrogen-good, no-estrogen-bad.”
Dr. Santoro noted how easy it is to overlook the women who may have cardiovascular risk because of hypothalamic amenorrhea.
“Because many women with functional hypothalamic amenorrhea are super athletic and do not have the typical features of people with cardiometabolic disease – such as glucose intolerance, obesity, abnormal cholesterol or triglycerides, or high blood pressure – clinicians tend to think of them as healthy and to think that simply giving back hormones will fix the problems with bone density and vascular function, but that is not enough,” Dr. Santoro said. “The cognitive-behavioral therapy model for treatment of women with functional hypothalamic amenorrhea addresses the stress-related factors that drive the disorder, and this needs to be considered the standard of care for treatment.”
Stephanie S. Faubion, MD, professor of medicine and director of Mayo Clinic’s Center for Women’s Health in Jacksonville, Fla., who was not involved in Dr. Shufelt’s presentation, also emphasized the importance of recognizing functional hypothalamic amenorrhea.
“This is an underrecognized entity to begin with, and the fact that these women appear to be at increased risk for vascular dysfunction and potentially increased risk for cardiovascular disease down the road makes it even more important for clinicians to identify them and provide interventions early on,” Dr. Faubion said in an interview. “These women need to be identified and the etiology of the amenorrhea addressed, whether it relates to overexercising, being underweight, or experiencing significant stressors that have led to the loss of menstrual cycles.”
Dr. Shufelt’s research was funded by the National Institutes of Health. She had no disclosures. Dr. Santoro is a member of the scientific advisory board for Astellas, Menogenix, Amazon Ember, and Que Oncology, and she consults for Ansh Labs. Dr. Faubion had no disclosures.
The relationship between estrogen levels and heart health makes it particularly important for clinicians to be aware of those patients who might be at risk for cardiovascular disease despite not having other traditional risk factors, according to a presentation Oct. 12 at the North American Menopause Society annual meeting in Atlanta.
”Endogenous estrogens are protective for cardiovascular disease in premenopausal women,” Chrisandra L. Shufelt, MD, chair of the division of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Fla., told attendees. Yet, “a substantial population of young women are dying prematurely from cardiovascular disease,” with rates of cardiovascular death increasing in women aged 35-44 even as rates have decreased in postmenopausal women and in men. One potential reason may be premature estrogen loss.
Dr. Shufelt reminded attendees of four major causes of premature estrogen loss: Natural premature menopause, surgical menopause, chemotherapy-induced menopause, and premature ovarian insufficiency. But she would go on to discuss a less widely recognized condition, functional hypothalamic amenorrhea, that also may be contributing to increased cardiovascular risk.
First, Dr. Shufelt reviewed the evidence supporting the relationship between estrogen and cardiovascular health, starting with the Framingham study’s findings that cardiovascular disease is approximately two to four times more common in postmenopausal women than in premenopausal women, depending on the age range.
“Menopause at an early age, particularly under the age of 40, matters,” Dr. Shufelt said. “So we should be discussing this with our patients.”
Surgical menopause makes a difference to cardiovascular health as well, she said. In women under age 35, for example, the risk of a nonfatal heart attack in those with a bilateral oophorectomy was 7.7 times greater than in women who retained both ovaries and their uterus, and 1.5 times greater in women who had a hysterectomy without bilateral oophorectomy.
In a 2019 study, surgical premature menopause was associated with an 87% increased risk of heart disease even after researchers accounted for age, cardiovascular risk factors, and some forms of hormone therapy. The increased risk from natural premature menopause, on the other hand, was lower – a 36% increased risk of heart disease – compared with those producing endogenous hormones. Although randomized controlled trials are unavailable and unlikely to be done, the Nurses’ Health Study and the Danish Nurses Cohort Study, both observational studies, found that heart disease risk was diminished in those taking hormone therapy after surgical premature menopause.
Recommendations for premature or early menopause, from a wide range of different medical societies including NAMS, are that women without contraindications be given estrogen-based hormone therapy until the average age of natural menopause. Though not included in the same guidance, research has also shown that estrogen after oophorectomy does not increase the risk of breast cancer in women with a BRCA1 mutation, Dr. Shufelt said. Hormone therapy for premature or early menopause should adequately replace the levels women have lost and that means younger menopausal women often need higher doses than what older women receive, such as 2 mg/day of oral estradiol rather than the standard doses of 0.5 or 1 mg/day.
Functional hypothalamic amenorrhea and cardiovascular risk
Dr. Shufelt then discussed functional hypothalamic amenorrhea (hypogonadotropic hypogonadism), a common type of secondary amenorrhea that affects at least 1.4 million U.S. women. Diagnosis includes lack of a period for at least 3 months in someone who previously menstruated plus lab values below 50 pg/mL for estradiol, below 10 mIU/L for follicle stimulating hormone, and below 10 mIU/L for luteinizing hormone. Causes of this reversible form of infertility can include stress, overexercising, undereating, or some combination of these, plus an underlying genetic predisposition.
“After ruling out polycystic ovary syndrome, prolactinoma, and thyroid dysfunction, clinicians need to consider the diagnosis of hypothalamic amenorrhea,” Dr. Shufelt said. This condition goes beyond low estrogen levels: Women have elevated cortisol, low thyroid levels, low leptin levels, and increased ghrelin.
”This is not going away,” Dr. Shufelt said, sharing data on stress levels among U.S. adults, particularly Gen Z and millennial adults, noting that the ongoing “national mental health crisis” may be contributing to functional hypothalamic amenorrhea.
A 2020 substudy from the Nurses’ Health Study II found an increased risk of premature death in those who didn’t have a period or always had irregular periods starting as early as 14-17 years old. The increased risk of premature death rose with age in those with irregular or absent cycles – a 37% higher risk in 18- to 22-year-olds and a 39% increased risk in 29- to 46-year-olds.
But clinicians aren’t adequately identifying the “phenotype of the hypothalamic women,” Dr. Shufelt said, despite research showing overlap between hypothalamic amenorrhea and a higher risk of cardiovascular disease. Hypothalamic amenorrhea is so understudied that the last original research on the topic was in 2008, Dr. Shufelt said in an interview. ”No research except mine has been done to evaluate heart health in these young women,” she said.
Dr. Shufelt described a study she led involving 30 women with functional hypothalamic amenorrhea, 29 women with normal menstrual cycles, and 30 women who were recently menopausal and not on hormone therapy. The women with hypothalamic amenorrhea had average stress levels but their depression scores were higher than those of the other two groups.
The results showed that women with hypothalamic amenorrhea had lower estradiol and leptin levels and higher testosterone levels compared with the control group, and they had higher cortisol levels than those of both groups. Despite having similar body mass indexes as the control and menopausal groups, women with hypothalamic amenorrhea had lower blood pressure than that of the other two groups, yet they had higher cholesterol levels than those of the control group. EndoPAT© (Itamar Medical) testing showed that they had poor vascular function.
“In fact, one-third of the women [with hypothalamic amenorrhea] entered the trial with a diagnosis of what would be considered endothelial dysfunction,” Dr. Shufelt said. “Our results demonstrated significantly higher circulating levels of serum proinflammatory cytokines in the women with hypothalamic amenorrhea compared to eumenorrheic controls.”
Dr. Shufelt’s team then tested whether giving estradiol to the women with hypothalamic amenorrhea for 12 weeks would improve their vascular health, but they saw no significant differences between the women who received estrogen and those who received placebo.
“Endothelial function is partly mediated by estrogen, and it was expected that giving back estrogen would ‘fix’ the endothelium, but that is not what happened,” Nanette Santoro, MD, professor and chair of obstetrics and gynecology at the University of Colorado at Denver, Aurora, said in interview. “The mechanisms that maintain vascular function in women are not limited to hormones,” said Dr. Santoro, who was not involved in Dr. Shufelt’s study but attended her lecture. “We need to think beyond the simple model of estrogen-good, no-estrogen-bad.”
Dr. Santoro noted how easy it is to overlook the women who may have cardiovascular risk because of hypothalamic amenorrhea.
“Because many women with functional hypothalamic amenorrhea are super athletic and do not have the typical features of people with cardiometabolic disease – such as glucose intolerance, obesity, abnormal cholesterol or triglycerides, or high blood pressure – clinicians tend to think of them as healthy and to think that simply giving back hormones will fix the problems with bone density and vascular function, but that is not enough,” Dr. Santoro said. “The cognitive-behavioral therapy model for treatment of women with functional hypothalamic amenorrhea addresses the stress-related factors that drive the disorder, and this needs to be considered the standard of care for treatment.”
Stephanie S. Faubion, MD, professor of medicine and director of Mayo Clinic’s Center for Women’s Health in Jacksonville, Fla., who was not involved in Dr. Shufelt’s presentation, also emphasized the importance of recognizing functional hypothalamic amenorrhea.
“This is an underrecognized entity to begin with, and the fact that these women appear to be at increased risk for vascular dysfunction and potentially increased risk for cardiovascular disease down the road makes it even more important for clinicians to identify them and provide interventions early on,” Dr. Faubion said in an interview. “These women need to be identified and the etiology of the amenorrhea addressed, whether it relates to overexercising, being underweight, or experiencing significant stressors that have led to the loss of menstrual cycles.”
Dr. Shufelt’s research was funded by the National Institutes of Health. She had no disclosures. Dr. Santoro is a member of the scientific advisory board for Astellas, Menogenix, Amazon Ember, and Que Oncology, and she consults for Ansh Labs. Dr. Faubion had no disclosures.
FROM NAMS 2022