User login
Your menopausal patient’s breast biopsy reveals atypical hyperplasia
A new series brought to you by the menopause experts

| Andrew M. Kaunitz, MD Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a NAMS Board member and certified menopause practitioner. He also serves on the OBG Management Board of Editors. |

| JoAnn V. Pinkerton, MD Professor, Department of Obstetrics and Gynecology, and Director, Division of Midlife Women’s Health, University of Virginia. Dr. Pinkerton is a North American Menopause Society (NAMS) past president and certified menopause practitioner. She also serves on the OBG Management Board of Editors. |

| James A. Simon, MD Clinical Professor, Department of Obstetrics and Gynecology, George Washington University, and Medical Director, Women’s Health & Research Consultants, Washington, DC. Dr. Simon is a NAMS past president, a certified menopause practitioner, and a certified clinical densitometrist. He also serves on the OBG Management Board of Editors. |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Agile, Bayer, Endoceutics, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Merck, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Atypical ductal hyperplasia
Your 56-year-old, married, white patient has been on hormone therapy (HT) since age 52 for the treatment of vasomotor symptoms. She is taking a low-dose oral estrogen and micronized progesterone combination as she has an intact uterus. Her family history is positive for breast cancer, as her mother was diagnosed at age 68.
Her most recent annual screening mammogram shows linear calcifications. Because fine, linear, branching or casting calcifications are worrisome for atypical ductal hyperplasia (ADH) or ductal carcinoma in situ, a biopsy is recommended.
She elects to wean off and discontinue HT during the evaluation of her abnormal mammogram. The mammographic-guided stereotactic biopsy reveals ADH. She undergoes an open excisional biopsy, the results of which reveal extensive ADH with negative margins.
Six weeks after a lumpectomy she returns to your office reporting moderate to severe hot flashes that occur seven to 10 times per day and impair her sleep, leading to fatigue and “brain fog.” In addition, she is noticing vaginal dryness and dyspareunia despite use of lubricants. She requests treatment for her symptoms and wonders if she can restart HT systemically or vaginally.
How do you manage her hot flashes?
What are the alternatives to HT for hot flashes?
Certain lifestyle changes have been reported to provide relief for hot flushes.1 These include:
- use of layered clothing
- maintenance of cool ambient temperature (particularly during sleep)
- consumption of cool foods or beverages
- relaxation techniques (such as deep breathing, or paced respirations, for 20 min three times per day).
Despite sparse data, avoiding triggers such as spicy or hot foods or alcohol may be helpful.
Therapies such as evening primrose oil, dong quai, ginseng, wild yam, magnet therapy, reflexology, and homeopathy have not been found more effective in treating hot flashes than placebo.2
Phytoestrogens (such as equol), acupuncture, yoga, and hypnosis continue to be tested in randomized trials with mixed results.
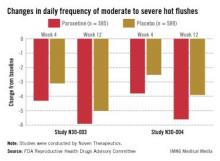
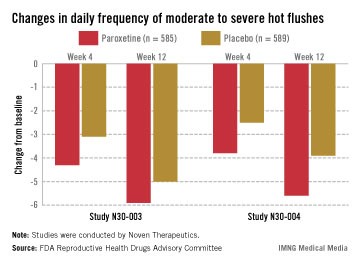
Off-label drug options offer modest help. There are currently no FDA-approved nonhormonal pharmaceutical options for relief of hot flashes; the gold standard for treatment remains estrogen therapy. For moderate to severe bothersome hot flashes, potentially effective drug therapies used off label include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and gabapentin (TABLE 1).2,4 In large, randomized, controlled trials, the following agents were modestly more effective than placebo: desvenlafaxine,5 low-dose paroxetine salt,6 escitalopram,7 and gastroretentive gabapentin.8 Participants in these trials included women with both spontaneous and surgically induced menopause.
Although sponsors have applied for approval for three of these agents, the FDA so far has declined to approve these agents for vasomotor treatment due to concerns about risks versus benefits. Benefits of these nonhormonal prescription therapies need to be weighed carefully against side effects, because the reduction in absolute hot flushes is modest.
Many small trials have assessed other medications and complementary and alternative therapies regarding management of menopausal symptoms. Most, however, are limited by small numbers of enrolled participants and shorter study duration (≤12 weeks). In addition, enrolled participants have variable numbers of hot flashes, often less than 14 per week.2,4
TABLE 1
Nonhormonal treatment of vasomotor symptoms
| Treatment | Study Design* | Findings |
|---|---|---|
| Complementary/alternative medicines (black cohosh, St. John’s Wort, red clover, acupuncture, exercise) | Duration: 4–52 wk; OL and RPL trials; entry criteria for most trials: >14 hot flashes/wk | Mixed results, mostly with no sustained improvement |
| SSRIs** (paroxetine, fluoxetine, sertraline, citalopram, escitalopram) | Duration: 4–36 wk; RPL trials with all agents; N = 20–90 in active arms; entry criteria for most trials: >14 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 28%–55% |
| SNRIs** (venlafaxine, desvenlafaxine) | Duration, 12–52 wk; RPL trials with all agents; N = 20–65 in VEN; N = 120–200 in DVS; Entry criteria >14 hot flashes/wk for VEN; >50/wk for DVS | Reduction in VMS (frequency, composite scores): 35%–58% for VEN, 55%–68% for DVS |
| Gabapentin** | Duration: 4–12 wk; RPL trials; N = 20–100; entry criteria for most trials: >14–50 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 50%–70% |
| *All studies of menopausal, nondepressed women. **Treatment is off label. Hall E, et al. Drugs. 2011;71:287-304. Reprinted with permission. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24, 2012. Accessed February 23, 2013.19 | ||
Can your patient restart HT? If so, should HT be offered vaginally or systemically?
Non-HT may be enough for vaginal dryness. Benefit has been shown with the use of vaginal moisturizers twice weekly and lubricants as needed for sexual activity.9 Therefore, the local application of daily lubricants, such as olive oil, along with the use of moisturizers with regular sexual intercourse may be enough to maintain vaginal health and function.
In randomized trials, phytoestrogens lack benefit data for vaginal atrophy. Small pilot studies of the effect of oral/ vaginal phytoestrogens on vaginal atrophy do not show any benefit on vaginal pH or vaginal maturation index and mixed improvement in vaginal dryness. In addition, no clear effect of these agents has been seen compared with placebo, except that there may be less progression of vaginal atrophy over time with phytoestrogens.10 It is possible that the benefits of phytoestrogens may take longer to take effect than the 12 weeks required to see an effect with HT.
Vaginal estrogen: limited safety data. No published clinical trials have assessed the impact of topical vaginal estrogen on risk of recurrence in breast cancer survivors, and concern exists because detectable estradiol levels have been reported in women who take aromatase inhibitors and have very atrophic vaginal mucosa.11 NAMS recommends that the discussion about vaginal estrogen be individualized between the patient, her provider, and her oncologist.12
Vaginal estrogen creams and tablets (Vagifem 10 μg per tablet) are often started daily for 2 weeks for a “priming dose” then dosed twice per week. To minimize systemic absorption, creams may be used externally or with smaller doses vaginally. The higher the dose or more frequent the use, the greater the risk of significant systemic absorption, particularly when the vagina is atrophic.13
Another option is the vaginal estradiol ring, which delivers a low dose (7.5 μg per day) for 90 days.14,15
Progestogen therapy is generally not needed when low-dose estrogen is administered locally to treat vaginal atrophy.12
A new oral option. In February 2013, ospemifene, a selective estrogen receptor modulator (SERM), was approved for pain with intercourse and vaginal dryness.
Related article: “New treatment option for vulvar and vaginal atrophy,” by Andrew M. Kaunitz, MD (May 2013)
There is concern with systemic estrogen use
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered. However, data from the Breast Cancer Surveillance Consortium9 showed that as postmenopausal HT use decreased (from 35% to 11% between 1999 and 2005), rates of ADH decreased from a peak of 5.5 per 10,000 mammograms in 1999 to 2.4 per 10,000 mammograms in 2005. Similarly, rates of invasive cancer with ADH decreased from a peak of 4.3 per 10,000 mammograms in 2003 to 3.3 per 10,000 mammograms in 2005. This finding—that rates of ADH and invasive breast cancer were significantly linked to postmenopausal use of HT—raises concern about using HT in women with prior ADH. Of note, the cancers linked with ADH were of lower grade and stage and more estrogen receptor–positive than cancers not linked with ADH.17
How do you manage your patient’s increased risk of breast cancer?
In examining the answer to this management point, we need to first ask and answer, “How does ADH develop?” and “What is her risk of developing breast cancer?”
The development of invasive breast cancer is believed to involve a complex, multistep process. Initially, there is disruption of normal cell development and growth, with overproduction of normal-looking cells (hyperplasia). These excess cells stack up and/or become abnormal. Then, there is continued change in appearance and multiplication, becoming ductal carcinoma in situ (noninvasive). If left untreated, the cells may develop into invasive cancer.18 See TABLE 2 for the relative risk of a patient with ADH developing breast cancer.10
Now, we can address, “How do you manage this patient’s increased risk of breast cancer?”
TABLE 2. Relative risk of developing breast cancer
| Risk | Relative Risk |
|---|---|
| Atypical ductal hyperplasia | 4-5 |
| Atypical ductal hyperplasia and positive family history | 6-8 |
More frequent breast screening!
- Clinical breast exams twice per year
- Screening mammograms annually
- Screening tomosynthesis (These are additional digital screening views which provide almost a 3D view.)
- Screening breast ultrasound
- Screening breast MRI — if she has a 20% lifetime risk of breast cancer (family history or genetic predisposition) (TABLE 3).
TABLE 3. ACS guidelines for screening breast MRI
| Risk | Recommendation |
|---|---|
| <15% lifetime risk | MRI not recommended |
| 15% to 20% lifetime risk | Talk about benefits and limitations of MRI screening |
| >20% lifetime risk | Annual mammogram and annual MRI alternating every 6 months |
Careful consideration of medications
Since it is possible that estrogen may fuel the growth of some breast cancers, avoiding systemic menopausal HT may be safest.
Counsel her about strategies to reduce breast cancer risk
These include:
- Lifestyle changes, including weight loss, exercise, and avoiding excess alcohol intake.
- Preventive medications. Tamoxifen or raloxifene (Evista) can be used for 5 years. These medications block estrogen from binding to the breast estrogen receptors. Another option is an aromatase inhibitor, which decreases estrogen production.
- Risk-reducing (prophylactic) mastectomy.
Management approach for this patient
This patient has had her ADH surgically excised. She will remain at higher risk for breast cancer and should consider strategies to decrease her risk, including lifestyle changes and the possible initiation of medications such as tamoxifen or raloxifene. New screening modalities, such as tomosynthesis or breast ultrasound, may be used to screen for breast cancer, and she may be a candidate for alternating mammograms and MRIs at 6-month intervals.
For her vaginal dryness, over-the-counter lubricants and moisturizers may be helpful. If not, topical or vaginal estrogen is available (as creams, tablets, or a ring) and provides primarily local benefit with limited systemic absorption.
For her bothersome hot flashes, if lifestyle changes don’t work, nonhormonal therapies can be offered off label, such as effexor, desvenlafaxine, gabapentin, or any of the SSRIs—including those tested in large, randomized, controlled trials, such as escitalopram and low-dose paroxetine salt, at low doses.
If she is taking tamoxifen, however, SSRIs such as paroxetine should be avoided due to P450 interaction.
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered, with education about the potential risks, as she is already at higher risk for breast cancer.
Acknowledgment
The author would like to thank Andrew M. Kaunitz, MD, for his forward thinking in helping to establish this new series on menopause.
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at [email protected] or send a Letter to the Editor to [email protected]
1. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11(1):11-33.
2. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health (Lond Engl). 2009;5(4):361-384.
3. Ghaleiha A, Jahangard L, Sherafat Z, et al. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blink, placebo-controlled, clinical study. Neuropsychiatr Dis Treat. 2012;8:407-412.
4. Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287-304.
5. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20(1):38-46.
6. Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: a 24-week randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371.-
7. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
8. Pinkerton JV, Kagan R, Portman D, Sathyanarayan RK, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the Breeze 3 study. Menopause. 2012;19(12):1377.-
9. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
10. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012]. J Steroid Biochem Mol Biol. 2012. doi: 10.1016/j.jsbmb.2012.12.004.
11. Moegele M, Buchholz S, Seitz S, Ortmann O. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397-402.
12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355-371.
13. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
14. Simon JA. Vulvovaginal atrophy: new and upcoming approaches. Menopause. 2009;16(1):5-7.
15. Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602-603.
16. Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17(4):403-408.
17. Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti D. Rates of atypical ductal hyperplasia declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822-2828.
18. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275-285.
19. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24 2012. Accessed February 23, 2013.
A new series brought to you by the menopause experts

| Andrew M. Kaunitz, MD Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a NAMS Board member and certified menopause practitioner. He also serves on the OBG Management Board of Editors. |

| JoAnn V. Pinkerton, MD Professor, Department of Obstetrics and Gynecology, and Director, Division of Midlife Women’s Health, University of Virginia. Dr. Pinkerton is a North American Menopause Society (NAMS) past president and certified menopause practitioner. She also serves on the OBG Management Board of Editors. |

| James A. Simon, MD Clinical Professor, Department of Obstetrics and Gynecology, George Washington University, and Medical Director, Women’s Health & Research Consultants, Washington, DC. Dr. Simon is a NAMS past president, a certified menopause practitioner, and a certified clinical densitometrist. He also serves on the OBG Management Board of Editors. |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Agile, Bayer, Endoceutics, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Merck, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Atypical ductal hyperplasia
Your 56-year-old, married, white patient has been on hormone therapy (HT) since age 52 for the treatment of vasomotor symptoms. She is taking a low-dose oral estrogen and micronized progesterone combination as she has an intact uterus. Her family history is positive for breast cancer, as her mother was diagnosed at age 68.
Her most recent annual screening mammogram shows linear calcifications. Because fine, linear, branching or casting calcifications are worrisome for atypical ductal hyperplasia (ADH) or ductal carcinoma in situ, a biopsy is recommended.
She elects to wean off and discontinue HT during the evaluation of her abnormal mammogram. The mammographic-guided stereotactic biopsy reveals ADH. She undergoes an open excisional biopsy, the results of which reveal extensive ADH with negative margins.
Six weeks after a lumpectomy she returns to your office reporting moderate to severe hot flashes that occur seven to 10 times per day and impair her sleep, leading to fatigue and “brain fog.” In addition, she is noticing vaginal dryness and dyspareunia despite use of lubricants. She requests treatment for her symptoms and wonders if she can restart HT systemically or vaginally.
How do you manage her hot flashes?
What are the alternatives to HT for hot flashes?
Certain lifestyle changes have been reported to provide relief for hot flushes.1 These include:
- use of layered clothing
- maintenance of cool ambient temperature (particularly during sleep)
- consumption of cool foods or beverages
- relaxation techniques (such as deep breathing, or paced respirations, for 20 min three times per day).
Despite sparse data, avoiding triggers such as spicy or hot foods or alcohol may be helpful.
Therapies such as evening primrose oil, dong quai, ginseng, wild yam, magnet therapy, reflexology, and homeopathy have not been found more effective in treating hot flashes than placebo.2
Phytoestrogens (such as equol), acupuncture, yoga, and hypnosis continue to be tested in randomized trials with mixed results.
Off-label drug options offer modest help. There are currently no FDA-approved nonhormonal pharmaceutical options for relief of hot flashes; the gold standard for treatment remains estrogen therapy. For moderate to severe bothersome hot flashes, potentially effective drug therapies used off label include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and gabapentin (TABLE 1).2,4 In large, randomized, controlled trials, the following agents were modestly more effective than placebo: desvenlafaxine,5 low-dose paroxetine salt,6 escitalopram,7 and gastroretentive gabapentin.8 Participants in these trials included women with both spontaneous and surgically induced menopause.
Although sponsors have applied for approval for three of these agents, the FDA so far has declined to approve these agents for vasomotor treatment due to concerns about risks versus benefits. Benefits of these nonhormonal prescription therapies need to be weighed carefully against side effects, because the reduction in absolute hot flushes is modest.
Many small trials have assessed other medications and complementary and alternative therapies regarding management of menopausal symptoms. Most, however, are limited by small numbers of enrolled participants and shorter study duration (≤12 weeks). In addition, enrolled participants have variable numbers of hot flashes, often less than 14 per week.2,4
TABLE 1
Nonhormonal treatment of vasomotor symptoms
| Treatment | Study Design* | Findings |
|---|---|---|
| Complementary/alternative medicines (black cohosh, St. John’s Wort, red clover, acupuncture, exercise) | Duration: 4–52 wk; OL and RPL trials; entry criteria for most trials: >14 hot flashes/wk | Mixed results, mostly with no sustained improvement |
| SSRIs** (paroxetine, fluoxetine, sertraline, citalopram, escitalopram) | Duration: 4–36 wk; RPL trials with all agents; N = 20–90 in active arms; entry criteria for most trials: >14 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 28%–55% |
| SNRIs** (venlafaxine, desvenlafaxine) | Duration, 12–52 wk; RPL trials with all agents; N = 20–65 in VEN; N = 120–200 in DVS; Entry criteria >14 hot flashes/wk for VEN; >50/wk for DVS | Reduction in VMS (frequency, composite scores): 35%–58% for VEN, 55%–68% for DVS |
| Gabapentin** | Duration: 4–12 wk; RPL trials; N = 20–100; entry criteria for most trials: >14–50 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 50%–70% |
| *All studies of menopausal, nondepressed women. **Treatment is off label. Hall E, et al. Drugs. 2011;71:287-304. Reprinted with permission. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24, 2012. Accessed February 23, 2013.19 | ||
Can your patient restart HT? If so, should HT be offered vaginally or systemically?
Non-HT may be enough for vaginal dryness. Benefit has been shown with the use of vaginal moisturizers twice weekly and lubricants as needed for sexual activity.9 Therefore, the local application of daily lubricants, such as olive oil, along with the use of moisturizers with regular sexual intercourse may be enough to maintain vaginal health and function.
In randomized trials, phytoestrogens lack benefit data for vaginal atrophy. Small pilot studies of the effect of oral/ vaginal phytoestrogens on vaginal atrophy do not show any benefit on vaginal pH or vaginal maturation index and mixed improvement in vaginal dryness. In addition, no clear effect of these agents has been seen compared with placebo, except that there may be less progression of vaginal atrophy over time with phytoestrogens.10 It is possible that the benefits of phytoestrogens may take longer to take effect than the 12 weeks required to see an effect with HT.
Vaginal estrogen: limited safety data. No published clinical trials have assessed the impact of topical vaginal estrogen on risk of recurrence in breast cancer survivors, and concern exists because detectable estradiol levels have been reported in women who take aromatase inhibitors and have very atrophic vaginal mucosa.11 NAMS recommends that the discussion about vaginal estrogen be individualized between the patient, her provider, and her oncologist.12
Vaginal estrogen creams and tablets (Vagifem 10 μg per tablet) are often started daily for 2 weeks for a “priming dose” then dosed twice per week. To minimize systemic absorption, creams may be used externally or with smaller doses vaginally. The higher the dose or more frequent the use, the greater the risk of significant systemic absorption, particularly when the vagina is atrophic.13
Another option is the vaginal estradiol ring, which delivers a low dose (7.5 μg per day) for 90 days.14,15
Progestogen therapy is generally not needed when low-dose estrogen is administered locally to treat vaginal atrophy.12
A new oral option. In February 2013, ospemifene, a selective estrogen receptor modulator (SERM), was approved for pain with intercourse and vaginal dryness.
Related article: “New treatment option for vulvar and vaginal atrophy,” by Andrew M. Kaunitz, MD (May 2013)
There is concern with systemic estrogen use
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered. However, data from the Breast Cancer Surveillance Consortium9 showed that as postmenopausal HT use decreased (from 35% to 11% between 1999 and 2005), rates of ADH decreased from a peak of 5.5 per 10,000 mammograms in 1999 to 2.4 per 10,000 mammograms in 2005. Similarly, rates of invasive cancer with ADH decreased from a peak of 4.3 per 10,000 mammograms in 2003 to 3.3 per 10,000 mammograms in 2005. This finding—that rates of ADH and invasive breast cancer were significantly linked to postmenopausal use of HT—raises concern about using HT in women with prior ADH. Of note, the cancers linked with ADH were of lower grade and stage and more estrogen receptor–positive than cancers not linked with ADH.17
How do you manage your patient’s increased risk of breast cancer?
In examining the answer to this management point, we need to first ask and answer, “How does ADH develop?” and “What is her risk of developing breast cancer?”
The development of invasive breast cancer is believed to involve a complex, multistep process. Initially, there is disruption of normal cell development and growth, with overproduction of normal-looking cells (hyperplasia). These excess cells stack up and/or become abnormal. Then, there is continued change in appearance and multiplication, becoming ductal carcinoma in situ (noninvasive). If left untreated, the cells may develop into invasive cancer.18 See TABLE 2 for the relative risk of a patient with ADH developing breast cancer.10
Now, we can address, “How do you manage this patient’s increased risk of breast cancer?”
TABLE 2. Relative risk of developing breast cancer
| Risk | Relative Risk |
|---|---|
| Atypical ductal hyperplasia | 4-5 |
| Atypical ductal hyperplasia and positive family history | 6-8 |
More frequent breast screening!
- Clinical breast exams twice per year
- Screening mammograms annually
- Screening tomosynthesis (These are additional digital screening views which provide almost a 3D view.)
- Screening breast ultrasound
- Screening breast MRI — if she has a 20% lifetime risk of breast cancer (family history or genetic predisposition) (TABLE 3).
TABLE 3. ACS guidelines for screening breast MRI
| Risk | Recommendation |
|---|---|
| <15% lifetime risk | MRI not recommended |
| 15% to 20% lifetime risk | Talk about benefits and limitations of MRI screening |
| >20% lifetime risk | Annual mammogram and annual MRI alternating every 6 months |
Careful consideration of medications
Since it is possible that estrogen may fuel the growth of some breast cancers, avoiding systemic menopausal HT may be safest.
Counsel her about strategies to reduce breast cancer risk
These include:
- Lifestyle changes, including weight loss, exercise, and avoiding excess alcohol intake.
- Preventive medications. Tamoxifen or raloxifene (Evista) can be used for 5 years. These medications block estrogen from binding to the breast estrogen receptors. Another option is an aromatase inhibitor, which decreases estrogen production.
- Risk-reducing (prophylactic) mastectomy.
Management approach for this patient
This patient has had her ADH surgically excised. She will remain at higher risk for breast cancer and should consider strategies to decrease her risk, including lifestyle changes and the possible initiation of medications such as tamoxifen or raloxifene. New screening modalities, such as tomosynthesis or breast ultrasound, may be used to screen for breast cancer, and she may be a candidate for alternating mammograms and MRIs at 6-month intervals.
For her vaginal dryness, over-the-counter lubricants and moisturizers may be helpful. If not, topical or vaginal estrogen is available (as creams, tablets, or a ring) and provides primarily local benefit with limited systemic absorption.
For her bothersome hot flashes, if lifestyle changes don’t work, nonhormonal therapies can be offered off label, such as effexor, desvenlafaxine, gabapentin, or any of the SSRIs—including those tested in large, randomized, controlled trials, such as escitalopram and low-dose paroxetine salt, at low doses.
If she is taking tamoxifen, however, SSRIs such as paroxetine should be avoided due to P450 interaction.
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered, with education about the potential risks, as she is already at higher risk for breast cancer.
Acknowledgment
The author would like to thank Andrew M. Kaunitz, MD, for his forward thinking in helping to establish this new series on menopause.
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at [email protected] or send a Letter to the Editor to [email protected]
A new series brought to you by the menopause experts

| Andrew M. Kaunitz, MD Professor and Associate Chairman, Department of Obstetrics and Gynecology, University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a NAMS Board member and certified menopause practitioner. He also serves on the OBG Management Board of Editors. |

| JoAnn V. Pinkerton, MD Professor, Department of Obstetrics and Gynecology, and Director, Division of Midlife Women’s Health, University of Virginia. Dr. Pinkerton is a North American Menopause Society (NAMS) past president and certified menopause practitioner. She also serves on the OBG Management Board of Editors. |

| James A. Simon, MD Clinical Professor, Department of Obstetrics and Gynecology, George Washington University, and Medical Director, Women’s Health & Research Consultants, Washington, DC. Dr. Simon is a NAMS past president, a certified menopause practitioner, and a certified clinical densitometrist. He also serves on the OBG Management Board of Editors. |
Disclosures
Dr. Kaunitz reports that his institution receives grant or research support from Agile, Bayer, Endoceutics, Teva, Medical Diagnostic Laboratories, and Noven, and that he is a consultant to Bayer, Merck, and Teva.
Dr. Pinkerton reports that her institution receives consulting fees from Pfizer, DepoMed, Shionogi, and Noven and multicenter research fees from DepoMed, Endoceutics, and Bionova.
Dr. Simon reports being a consultant to or on the advisory boards of Abbott Laboratories, Agile Therapeutics, Amgen, Ascend Therapeutics, BioSante, Depomed, Lelo, MD Therapeutics, Meda Pharmaceuticals, Merck, Noven, Novo Nordisk, Novogyne, Pfizer, Shionogi, Shippan Point Advisors LLC, Slate Pharmaceuticals, Sprout Pharmaceuticals, Teva, Warner Chilcott, and Watson. He also reports receiving (currently or in the past year) grant/research support from BioSante, EndoCeutics, Novo Nordisk, Novogyne, Palatin Technologies, Teva, and Warner Chilcott. He reports serving on the speakers bureaus of Amgen, Merck, Novartis, Noven, Novo Nordisk, Novogyne, Teva, and Warner Chilcott. Dr. Simon is currently the Chief Medical Officer for Sprout Pharmaceuticals.
CASE: Atypical ductal hyperplasia
Your 56-year-old, married, white patient has been on hormone therapy (HT) since age 52 for the treatment of vasomotor symptoms. She is taking a low-dose oral estrogen and micronized progesterone combination as she has an intact uterus. Her family history is positive for breast cancer, as her mother was diagnosed at age 68.
Her most recent annual screening mammogram shows linear calcifications. Because fine, linear, branching or casting calcifications are worrisome for atypical ductal hyperplasia (ADH) or ductal carcinoma in situ, a biopsy is recommended.
She elects to wean off and discontinue HT during the evaluation of her abnormal mammogram. The mammographic-guided stereotactic biopsy reveals ADH. She undergoes an open excisional biopsy, the results of which reveal extensive ADH with negative margins.
Six weeks after a lumpectomy she returns to your office reporting moderate to severe hot flashes that occur seven to 10 times per day and impair her sleep, leading to fatigue and “brain fog.” In addition, she is noticing vaginal dryness and dyspareunia despite use of lubricants. She requests treatment for her symptoms and wonders if she can restart HT systemically or vaginally.
How do you manage her hot flashes?
What are the alternatives to HT for hot flashes?
Certain lifestyle changes have been reported to provide relief for hot flushes.1 These include:
- use of layered clothing
- maintenance of cool ambient temperature (particularly during sleep)
- consumption of cool foods or beverages
- relaxation techniques (such as deep breathing, or paced respirations, for 20 min three times per day).
Despite sparse data, avoiding triggers such as spicy or hot foods or alcohol may be helpful.
Therapies such as evening primrose oil, dong quai, ginseng, wild yam, magnet therapy, reflexology, and homeopathy have not been found more effective in treating hot flashes than placebo.2
Phytoestrogens (such as equol), acupuncture, yoga, and hypnosis continue to be tested in randomized trials with mixed results.
Off-label drug options offer modest help. There are currently no FDA-approved nonhormonal pharmaceutical options for relief of hot flashes; the gold standard for treatment remains estrogen therapy. For moderate to severe bothersome hot flashes, potentially effective drug therapies used off label include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and gabapentin (TABLE 1).2,4 In large, randomized, controlled trials, the following agents were modestly more effective than placebo: desvenlafaxine,5 low-dose paroxetine salt,6 escitalopram,7 and gastroretentive gabapentin.8 Participants in these trials included women with both spontaneous and surgically induced menopause.
Although sponsors have applied for approval for three of these agents, the FDA so far has declined to approve these agents for vasomotor treatment due to concerns about risks versus benefits. Benefits of these nonhormonal prescription therapies need to be weighed carefully against side effects, because the reduction in absolute hot flushes is modest.
Many small trials have assessed other medications and complementary and alternative therapies regarding management of menopausal symptoms. Most, however, are limited by small numbers of enrolled participants and shorter study duration (≤12 weeks). In addition, enrolled participants have variable numbers of hot flashes, often less than 14 per week.2,4
TABLE 1
Nonhormonal treatment of vasomotor symptoms
| Treatment | Study Design* | Findings |
|---|---|---|
| Complementary/alternative medicines (black cohosh, St. John’s Wort, red clover, acupuncture, exercise) | Duration: 4–52 wk; OL and RPL trials; entry criteria for most trials: >14 hot flashes/wk | Mixed results, mostly with no sustained improvement |
| SSRIs** (paroxetine, fluoxetine, sertraline, citalopram, escitalopram) | Duration: 4–36 wk; RPL trials with all agents; N = 20–90 in active arms; entry criteria for most trials: >14 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 28%–55% |
| SNRIs** (venlafaxine, desvenlafaxine) | Duration, 12–52 wk; RPL trials with all agents; N = 20–65 in VEN; N = 120–200 in DVS; Entry criteria >14 hot flashes/wk for VEN; >50/wk for DVS | Reduction in VMS (frequency, composite scores): 35%–58% for VEN, 55%–68% for DVS |
| Gabapentin** | Duration: 4–12 wk; RPL trials; N = 20–100; entry criteria for most trials: >14–50 hot flashes/wk | Reduction in vasomotor symptoms (frequency, composite scores): 50%–70% |
| *All studies of menopausal, nondepressed women. **Treatment is off label. Hall E, et al. Drugs. 2011;71:287-304. Reprinted with permission. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24, 2012. Accessed February 23, 2013.19 | ||
Can your patient restart HT? If so, should HT be offered vaginally or systemically?
Non-HT may be enough for vaginal dryness. Benefit has been shown with the use of vaginal moisturizers twice weekly and lubricants as needed for sexual activity.9 Therefore, the local application of daily lubricants, such as olive oil, along with the use of moisturizers with regular sexual intercourse may be enough to maintain vaginal health and function.
In randomized trials, phytoestrogens lack benefit data for vaginal atrophy. Small pilot studies of the effect of oral/ vaginal phytoestrogens on vaginal atrophy do not show any benefit on vaginal pH or vaginal maturation index and mixed improvement in vaginal dryness. In addition, no clear effect of these agents has been seen compared with placebo, except that there may be less progression of vaginal atrophy over time with phytoestrogens.10 It is possible that the benefits of phytoestrogens may take longer to take effect than the 12 weeks required to see an effect with HT.
Vaginal estrogen: limited safety data. No published clinical trials have assessed the impact of topical vaginal estrogen on risk of recurrence in breast cancer survivors, and concern exists because detectable estradiol levels have been reported in women who take aromatase inhibitors and have very atrophic vaginal mucosa.11 NAMS recommends that the discussion about vaginal estrogen be individualized between the patient, her provider, and her oncologist.12
Vaginal estrogen creams and tablets (Vagifem 10 μg per tablet) are often started daily for 2 weeks for a “priming dose” then dosed twice per week. To minimize systemic absorption, creams may be used externally or with smaller doses vaginally. The higher the dose or more frequent the use, the greater the risk of significant systemic absorption, particularly when the vagina is atrophic.13
Another option is the vaginal estradiol ring, which delivers a low dose (7.5 μg per day) for 90 days.14,15
Progestogen therapy is generally not needed when low-dose estrogen is administered locally to treat vaginal atrophy.12
A new oral option. In February 2013, ospemifene, a selective estrogen receptor modulator (SERM), was approved for pain with intercourse and vaginal dryness.
Related article: “New treatment option for vulvar and vaginal atrophy,” by Andrew M. Kaunitz, MD (May 2013)
There is concern with systemic estrogen use
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered. However, data from the Breast Cancer Surveillance Consortium9 showed that as postmenopausal HT use decreased (from 35% to 11% between 1999 and 2005), rates of ADH decreased from a peak of 5.5 per 10,000 mammograms in 1999 to 2.4 per 10,000 mammograms in 2005. Similarly, rates of invasive cancer with ADH decreased from a peak of 4.3 per 10,000 mammograms in 2003 to 3.3 per 10,000 mammograms in 2005. This finding—that rates of ADH and invasive breast cancer were significantly linked to postmenopausal use of HT—raises concern about using HT in women with prior ADH. Of note, the cancers linked with ADH were of lower grade and stage and more estrogen receptor–positive than cancers not linked with ADH.17
How do you manage your patient’s increased risk of breast cancer?
In examining the answer to this management point, we need to first ask and answer, “How does ADH develop?” and “What is her risk of developing breast cancer?”
The development of invasive breast cancer is believed to involve a complex, multistep process. Initially, there is disruption of normal cell development and growth, with overproduction of normal-looking cells (hyperplasia). These excess cells stack up and/or become abnormal. Then, there is continued change in appearance and multiplication, becoming ductal carcinoma in situ (noninvasive). If left untreated, the cells may develop into invasive cancer.18 See TABLE 2 for the relative risk of a patient with ADH developing breast cancer.10
Now, we can address, “How do you manage this patient’s increased risk of breast cancer?”
TABLE 2. Relative risk of developing breast cancer
| Risk | Relative Risk |
|---|---|
| Atypical ductal hyperplasia | 4-5 |
| Atypical ductal hyperplasia and positive family history | 6-8 |
More frequent breast screening!
- Clinical breast exams twice per year
- Screening mammograms annually
- Screening tomosynthesis (These are additional digital screening views which provide almost a 3D view.)
- Screening breast ultrasound
- Screening breast MRI — if she has a 20% lifetime risk of breast cancer (family history or genetic predisposition) (TABLE 3).
TABLE 3. ACS guidelines for screening breast MRI
| Risk | Recommendation |
|---|---|
| <15% lifetime risk | MRI not recommended |
| 15% to 20% lifetime risk | Talk about benefits and limitations of MRI screening |
| >20% lifetime risk | Annual mammogram and annual MRI alternating every 6 months |
Careful consideration of medications
Since it is possible that estrogen may fuel the growth of some breast cancers, avoiding systemic menopausal HT may be safest.
Counsel her about strategies to reduce breast cancer risk
These include:
- Lifestyle changes, including weight loss, exercise, and avoiding excess alcohol intake.
- Preventive medications. Tamoxifen or raloxifene (Evista) can be used for 5 years. These medications block estrogen from binding to the breast estrogen receptors. Another option is an aromatase inhibitor, which decreases estrogen production.
- Risk-reducing (prophylactic) mastectomy.
Management approach for this patient
This patient has had her ADH surgically excised. She will remain at higher risk for breast cancer and should consider strategies to decrease her risk, including lifestyle changes and the possible initiation of medications such as tamoxifen or raloxifene. New screening modalities, such as tomosynthesis or breast ultrasound, may be used to screen for breast cancer, and she may be a candidate for alternating mammograms and MRIs at 6-month intervals.
For her vaginal dryness, over-the-counter lubricants and moisturizers may be helpful. If not, topical or vaginal estrogen is available (as creams, tablets, or a ring) and provides primarily local benefit with limited systemic absorption.
For her bothersome hot flashes, if lifestyle changes don’t work, nonhormonal therapies can be offered off label, such as effexor, desvenlafaxine, gabapentin, or any of the SSRIs—including those tested in large, randomized, controlled trials, such as escitalopram and low-dose paroxetine salt, at low doses.
If she is taking tamoxifen, however, SSRIs such as paroxetine should be avoided due to P450 interaction.
If her hot flashes remain persistent and bothersome, low-dose estrogen could be considered, with education about the potential risks, as she is already at higher risk for breast cancer.
Acknowledgment
The author would like to thank Andrew M. Kaunitz, MD, for his forward thinking in helping to establish this new series on menopause.
Suggest it to our expert panel. They may address your management dilemma in a future issue!
Email us at [email protected] or send a Letter to the Editor to [email protected]
1. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11(1):11-33.
2. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health (Lond Engl). 2009;5(4):361-384.
3. Ghaleiha A, Jahangard L, Sherafat Z, et al. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blink, placebo-controlled, clinical study. Neuropsychiatr Dis Treat. 2012;8:407-412.
4. Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287-304.
5. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20(1):38-46.
6. Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: a 24-week randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371.-
7. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
8. Pinkerton JV, Kagan R, Portman D, Sathyanarayan RK, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the Breeze 3 study. Menopause. 2012;19(12):1377.-
9. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
10. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012]. J Steroid Biochem Mol Biol. 2012. doi: 10.1016/j.jsbmb.2012.12.004.
11. Moegele M, Buchholz S, Seitz S, Ortmann O. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397-402.
12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355-371.
13. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
14. Simon JA. Vulvovaginal atrophy: new and upcoming approaches. Menopause. 2009;16(1):5-7.
15. Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602-603.
16. Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17(4):403-408.
17. Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti D. Rates of atypical ductal hyperplasia declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822-2828.
18. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275-285.
19. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24 2012. Accessed February 23, 2013.
1. North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11(1):11-33.
2. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health (Lond Engl). 2009;5(4):361-384.
3. Ghaleiha A, Jahangard L, Sherafat Z, et al. Oxybutynin reduces sweating in depressed patients treated with sertraline: a double-blink, placebo-controlled, clinical study. Neuropsychiatr Dis Treat. 2012;8:407-412.
4. Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287-304.
5. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20(1):38-46.
6. Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: a 24-week randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371.-
7. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267-274.
8. Pinkerton JV, Kagan R, Portman D, Sathyanarayan RK, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the Breeze 3 study. Menopause. 2012;19(12):1377.-
9. MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
10. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause [published online ahead of print December 25 2012]. J Steroid Biochem Mol Biol. 2012. doi: 10.1016/j.jsbmb.2012.12.004.
11. Moegele M, Buchholz S, Seitz S, Ortmann O. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397-402.
12. North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355-371.
13. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. 2010;17(1):194-203.
14. Simon JA. Vulvovaginal atrophy: new and upcoming approaches. Menopause. 2009;16(1):5-7.
15. Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602-603.
16. Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2011;17(4):403-408.
17. Menes TS, Kerlikowske K, Jaffer S, Seger D, Miglioretti D. Rates of atypical ductal hyperplasia declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822-2828.
18. Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353(3):275-285.
19. Pinkerton JV, Shapiro M. The North American Menopause Society. Overview of available treatment options for VMS and VVA. Medscape Education Web site. http://www.medscape.org/viewarticle/763413. Published May 24 2012. Accessed February 23, 2013.
Prevention and treatment of osteoporosis
The National Osteoporosis Foundation released new 2013 guidelines for the treatment and management of osteoporosis for postmenopausal women and men over the age of 50 years.
Osteoporosis definition
Osteoporosis is defined by a bone mineral density (BMD) measurement (T score) less than or equal to 2.5 standard deviations (SD) below the mean for a young adult reference population, or the occurrence of a hip or vertebral fracture without preceding major trauma. Osteopenia is established by BMD testing showing a T score between 1.0-2.5 SD below a young adult reference population.
Assess patient’s risk for fracture
All postmenopausal women and men above age 50 years should be evaluated for risk of osteoporosis in order to determine the need for BMD testing and/or vertebral imaging. In addition, all patients should be evaluated for their risk of falling, since the majority of osteoporosis-related fractures occur because of a fall.
The WHO FRAX tool, may be used in order to calculate the 10-year probability of a hip fracture and the 10-year probability of a major osteoporotic fracture (clinical vertebral, hip, forearm or proximal humerus fracture). Risk of fracture can be calculated either with or without availability of BMD. The 10-year probability of fracture can be used to determine the need for pharmacologic treatment.
Diagnosis
Bone mineral density testing
Dual-energy x-ray absorptiometry (DXA) imaging of the hip and spine can diagnose or confirm osteoporosis. Testing should be considered in:
• Women aged 65 years and older and men 70 years of age and older, regardless of clinical risk factors.
• Patients of either sex who are aged between 50-69 years with clinical risk factors.
• Patients with a fracture after age 50 years.
• And patients with conditions (for example, rheumatoid arthritis) or on medications (for example, glucocorticoids) associated with low bone mass or bone loss.
Vertebral imaging
A single vertebral fracture increases the risk of subsequent vertebral and hip fractures, is consistent with the diagnosis of osteoporosis, and is an indication for pharmacologic treatment regardless of BMD. New to these guidelines is a recommendation for a proactive screening effort for vertebral fractures using lateral thoracic and lumbar spine x-ray or by lateral vertebral fracture assessment (VFA). Indications for vertebral imaging are:
• Women aged 65 years and older and men aged 70 years and older if T score is –1.5 or below.
• Women aged 70 years and men age 80 years and older.
• Postmenopausal women and men aged 50 years and older with a low trauma fracture.
• And/or postmenopausal women and men aged 50-69 years if there is height loss of 1.5 inches or more or ongoing long-term glucocorticoid treatment.
Markers of bone turnover
Biochemical markers of bone turnover are divided into two types:
• Markers of bone remodeling – serum C-telopeptide (CTx) and urinary N-telopeptide (NTx)
• Formation markers-serum bone-specific alkaline phosphatase (BSAP), osteocalcin (OC), and aminoterminal propeptide of type 1 procollagen (P1NP)]
Markers should be collected as fasting morning specimens and may be helpful in predicting risk of fracture and extend of fracture risk reduction when repeated after 3-6 months of pharmacologic therapy.
General recommendations
Vitamin D and calcium: A diet rich in vitamin D and calcium is an inexpensive way to prevent bone mineral density loss. Fruits, vegetables, low-fat dairy, and sunlight are great sources. If dietary supplementation is required, men aged 50-70 years should consume 1,000 mg of calcium/day and women over 51 years old should have 1,200 mg of calcium daily. Both men and women over 50 years should have 800-1,000 IU of vitamin D daily.
Treat vitamin D deficiencies: Supplementation should be adequate to achieve serum levels of 30ng/mL (75nmol/L).
Decreased alcohol use, smoking cessation, exercise, and fall prevention: Smoking cessation should be strongly advised. Moderate alcohol intake does not adversely affect bone and may be associated with lower fracture risk, though consuming more than three drinks daily may have an adverse effect on bone health and increases the risk of falling. Weight-bearing and muscle-strengthening exercise improves bone health and decreases the risk of falls. Home assessment for fall prevention for the elderly may decrease the risk of fracture.
Pharmacologic treatments
Treatment should be considered in postmenopausal women and men over 50 years with a hip or vertebral fracture; T score less than or equal to –2.5 at femoral neck, total hip or lumbar spine; low bone mass (T score between –1.0 and –2.5) and a 10-year probability of hip fracture greater than or equal to 3% or 10 year probability of major osteoporosis-related fracture greater than or equal to 20%. The antifracture benefits of medications have been studied primarily in postmenopausal women with osteoporosis. Pharmacologic therapy should not be considered life-long and that treatment decisions should be individualized. After 3-5 years of treatment a comprehensive risk assessment should be performed.
The bottom line
Identify risk factors for osteoporosis in postmenopausal women and men over the age of 50 years. Bone mineral density screening is an important part of fracture prevention, and vertebral imaging should now be considered as a part of osteoporosis screening. Pharmacologic treatment can be considered when a nontraumatic fracture is apparent; if the T score is less than or equal to –2.5; or for individuals with an elevated 10-year fracture risk based on WHO model.
• Source: Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Charles is a second year resident in the Family Medicine Residency Program at Abington Memorial Hospital.
The National Osteoporosis Foundation released new 2013 guidelines for the treatment and management of osteoporosis for postmenopausal women and men over the age of 50 years.
Osteoporosis definition
Osteoporosis is defined by a bone mineral density (BMD) measurement (T score) less than or equal to 2.5 standard deviations (SD) below the mean for a young adult reference population, or the occurrence of a hip or vertebral fracture without preceding major trauma. Osteopenia is established by BMD testing showing a T score between 1.0-2.5 SD below a young adult reference population.
Assess patient’s risk for fracture
All postmenopausal women and men above age 50 years should be evaluated for risk of osteoporosis in order to determine the need for BMD testing and/or vertebral imaging. In addition, all patients should be evaluated for their risk of falling, since the majority of osteoporosis-related fractures occur because of a fall.
The WHO FRAX tool, may be used in order to calculate the 10-year probability of a hip fracture and the 10-year probability of a major osteoporotic fracture (clinical vertebral, hip, forearm or proximal humerus fracture). Risk of fracture can be calculated either with or without availability of BMD. The 10-year probability of fracture can be used to determine the need for pharmacologic treatment.
Diagnosis
Bone mineral density testing
Dual-energy x-ray absorptiometry (DXA) imaging of the hip and spine can diagnose or confirm osteoporosis. Testing should be considered in:
• Women aged 65 years and older and men 70 years of age and older, regardless of clinical risk factors.
• Patients of either sex who are aged between 50-69 years with clinical risk factors.
• Patients with a fracture after age 50 years.
• And patients with conditions (for example, rheumatoid arthritis) or on medications (for example, glucocorticoids) associated with low bone mass or bone loss.
Vertebral imaging
A single vertebral fracture increases the risk of subsequent vertebral and hip fractures, is consistent with the diagnosis of osteoporosis, and is an indication for pharmacologic treatment regardless of BMD. New to these guidelines is a recommendation for a proactive screening effort for vertebral fractures using lateral thoracic and lumbar spine x-ray or by lateral vertebral fracture assessment (VFA). Indications for vertebral imaging are:
• Women aged 65 years and older and men aged 70 years and older if T score is –1.5 or below.
• Women aged 70 years and men age 80 years and older.
• Postmenopausal women and men aged 50 years and older with a low trauma fracture.
• And/or postmenopausal women and men aged 50-69 years if there is height loss of 1.5 inches or more or ongoing long-term glucocorticoid treatment.
Markers of bone turnover
Biochemical markers of bone turnover are divided into two types:
• Markers of bone remodeling – serum C-telopeptide (CTx) and urinary N-telopeptide (NTx)
• Formation markers-serum bone-specific alkaline phosphatase (BSAP), osteocalcin (OC), and aminoterminal propeptide of type 1 procollagen (P1NP)]
Markers should be collected as fasting morning specimens and may be helpful in predicting risk of fracture and extend of fracture risk reduction when repeated after 3-6 months of pharmacologic therapy.
General recommendations
Vitamin D and calcium: A diet rich in vitamin D and calcium is an inexpensive way to prevent bone mineral density loss. Fruits, vegetables, low-fat dairy, and sunlight are great sources. If dietary supplementation is required, men aged 50-70 years should consume 1,000 mg of calcium/day and women over 51 years old should have 1,200 mg of calcium daily. Both men and women over 50 years should have 800-1,000 IU of vitamin D daily.
Treat vitamin D deficiencies: Supplementation should be adequate to achieve serum levels of 30ng/mL (75nmol/L).
Decreased alcohol use, smoking cessation, exercise, and fall prevention: Smoking cessation should be strongly advised. Moderate alcohol intake does not adversely affect bone and may be associated with lower fracture risk, though consuming more than three drinks daily may have an adverse effect on bone health and increases the risk of falling. Weight-bearing and muscle-strengthening exercise improves bone health and decreases the risk of falls. Home assessment for fall prevention for the elderly may decrease the risk of fracture.
Pharmacologic treatments
Treatment should be considered in postmenopausal women and men over 50 years with a hip or vertebral fracture; T score less than or equal to –2.5 at femoral neck, total hip or lumbar spine; low bone mass (T score between –1.0 and –2.5) and a 10-year probability of hip fracture greater than or equal to 3% or 10 year probability of major osteoporosis-related fracture greater than or equal to 20%. The antifracture benefits of medications have been studied primarily in postmenopausal women with osteoporosis. Pharmacologic therapy should not be considered life-long and that treatment decisions should be individualized. After 3-5 years of treatment a comprehensive risk assessment should be performed.
The bottom line
Identify risk factors for osteoporosis in postmenopausal women and men over the age of 50 years. Bone mineral density screening is an important part of fracture prevention, and vertebral imaging should now be considered as a part of osteoporosis screening. Pharmacologic treatment can be considered when a nontraumatic fracture is apparent; if the T score is less than or equal to –2.5; or for individuals with an elevated 10-year fracture risk based on WHO model.
• Source: Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Charles is a second year resident in the Family Medicine Residency Program at Abington Memorial Hospital.
The National Osteoporosis Foundation released new 2013 guidelines for the treatment and management of osteoporosis for postmenopausal women and men over the age of 50 years.
Osteoporosis definition
Osteoporosis is defined by a bone mineral density (BMD) measurement (T score) less than or equal to 2.5 standard deviations (SD) below the mean for a young adult reference population, or the occurrence of a hip or vertebral fracture without preceding major trauma. Osteopenia is established by BMD testing showing a T score between 1.0-2.5 SD below a young adult reference population.
Assess patient’s risk for fracture
All postmenopausal women and men above age 50 years should be evaluated for risk of osteoporosis in order to determine the need for BMD testing and/or vertebral imaging. In addition, all patients should be evaluated for their risk of falling, since the majority of osteoporosis-related fractures occur because of a fall.
The WHO FRAX tool, may be used in order to calculate the 10-year probability of a hip fracture and the 10-year probability of a major osteoporotic fracture (clinical vertebral, hip, forearm or proximal humerus fracture). Risk of fracture can be calculated either with or without availability of BMD. The 10-year probability of fracture can be used to determine the need for pharmacologic treatment.
Diagnosis
Bone mineral density testing
Dual-energy x-ray absorptiometry (DXA) imaging of the hip and spine can diagnose or confirm osteoporosis. Testing should be considered in:
• Women aged 65 years and older and men 70 years of age and older, regardless of clinical risk factors.
• Patients of either sex who are aged between 50-69 years with clinical risk factors.
• Patients with a fracture after age 50 years.
• And patients with conditions (for example, rheumatoid arthritis) or on medications (for example, glucocorticoids) associated with low bone mass or bone loss.
Vertebral imaging
A single vertebral fracture increases the risk of subsequent vertebral and hip fractures, is consistent with the diagnosis of osteoporosis, and is an indication for pharmacologic treatment regardless of BMD. New to these guidelines is a recommendation for a proactive screening effort for vertebral fractures using lateral thoracic and lumbar spine x-ray or by lateral vertebral fracture assessment (VFA). Indications for vertebral imaging are:
• Women aged 65 years and older and men aged 70 years and older if T score is –1.5 or below.
• Women aged 70 years and men age 80 years and older.
• Postmenopausal women and men aged 50 years and older with a low trauma fracture.
• And/or postmenopausal women and men aged 50-69 years if there is height loss of 1.5 inches or more or ongoing long-term glucocorticoid treatment.
Markers of bone turnover
Biochemical markers of bone turnover are divided into two types:
• Markers of bone remodeling – serum C-telopeptide (CTx) and urinary N-telopeptide (NTx)
• Formation markers-serum bone-specific alkaline phosphatase (BSAP), osteocalcin (OC), and aminoterminal propeptide of type 1 procollagen (P1NP)]
Markers should be collected as fasting morning specimens and may be helpful in predicting risk of fracture and extend of fracture risk reduction when repeated after 3-6 months of pharmacologic therapy.
General recommendations
Vitamin D and calcium: A diet rich in vitamin D and calcium is an inexpensive way to prevent bone mineral density loss. Fruits, vegetables, low-fat dairy, and sunlight are great sources. If dietary supplementation is required, men aged 50-70 years should consume 1,000 mg of calcium/day and women over 51 years old should have 1,200 mg of calcium daily. Both men and women over 50 years should have 800-1,000 IU of vitamin D daily.
Treat vitamin D deficiencies: Supplementation should be adequate to achieve serum levels of 30ng/mL (75nmol/L).
Decreased alcohol use, smoking cessation, exercise, and fall prevention: Smoking cessation should be strongly advised. Moderate alcohol intake does not adversely affect bone and may be associated with lower fracture risk, though consuming more than three drinks daily may have an adverse effect on bone health and increases the risk of falling. Weight-bearing and muscle-strengthening exercise improves bone health and decreases the risk of falls. Home assessment for fall prevention for the elderly may decrease the risk of fracture.
Pharmacologic treatments
Treatment should be considered in postmenopausal women and men over 50 years with a hip or vertebral fracture; T score less than or equal to –2.5 at femoral neck, total hip or lumbar spine; low bone mass (T score between –1.0 and –2.5) and a 10-year probability of hip fracture greater than or equal to 3% or 10 year probability of major osteoporosis-related fracture greater than or equal to 20%. The antifracture benefits of medications have been studied primarily in postmenopausal women with osteoporosis. Pharmacologic therapy should not be considered life-long and that treatment decisions should be individualized. After 3-5 years of treatment a comprehensive risk assessment should be performed.
The bottom line
Identify risk factors for osteoporosis in postmenopausal women and men over the age of 50 years. Bone mineral density screening is an important part of fracture prevention, and vertebral imaging should now be considered as a part of osteoporosis screening. Pharmacologic treatment can be considered when a nontraumatic fracture is apparent; if the T score is less than or equal to –2.5; or for individuals with an elevated 10-year fracture risk based on WHO model.
• Source: Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Charles is a second year resident in the Family Medicine Residency Program at Abington Memorial Hospital.
Abdominal, thoracic CT scans reliably detect incidental low lumbar BMD
Abdominal and thoracic CT scans obtained for a variety of reasons, such as to assess pain, GI symptoms, or urinary tract complaints, also can be used "opportunistically" to examine lumbar bone mineral density and screen for occult osteoporosis, according to a report in the April 16 issue of the Annals of Internal Medicine.
Abdominal and thoracic CT scans done in routine practice happen to include imaging of the L1 level, which can easily be identified because it is the first non–rib-bearing vertebra. Such scans readily yield data on lumbar bone mineral density (BMD), which is a clinically useful way to diagnose or rule out osteoporosis, said Dr. Perry J. Pickhardt of the department of radiology and his associates at the University of Wisconsin, Madison.
It is important not to confuse this standard CT scanning with quantitative CT (QCT) scanning. QCT "is more labor-intensive; requires an imaging phantom or angle-corrected [region-of-interest] measurement of bone, muscle, and fat at multiple levels; and involves additional money, time, and radiation exposure," they explained.
Unlike dual-energy x-ray absorptiometry (DXA) screening or QCT assessment, "the method that we used requires a negligible amount of training and time; could be applied prospectively by the interpreting radiologist or retrospectively by a radiologist or even nonradiologist; adds no cost; and requires no additional patient time, equipment, software, or radiation exposure," the investigators wrote.
Such incidental CT scans can be assessed retrospectively because they are almost always stored indefinitely in electronic medical records, they noted.
Dr. Pickhardt and his colleagues evaluated CT-derived BMD assessment and compared it against DXA scanning of the hips and spine by identifying 1,867 adults who had undergone the two types of scanning within a 6-month period during the 10-year study interval. They retrieved and reviewed the images, paying particular attention to obvious moderate or severe compression deformities on the CT images, rather than to milder ones, "to avoid ambiguity related to more subjective borderline or mild compression deformities."
The study subjects had a total of 2,063 pairs of CT and DXA assessments that had been performed a median of 67 days apart. A total of 81% of these subjects were women, and the mean age was 59 years.
These patients had undergone abdominal or thoracic CT for a variety of clinical indications, most often for a suspected mass or an oncologic work-up (414 subjects), genitourinary problems (402 subjects), gastrointestinal symptoms (398 subjects), and/or unexplained abdominal pain or symptoms (374 subjects).
Approximately 55% of the CT scans involved intravenous contrast. The use of contrast had no effect on the interpretation of lumbar data on the scans.
The DXA screening identified 22.9% of the study subjects as osteoporotic, 44.8% as osteopenic, and 32.3% as having normal BMD. The CT scans were significantly more sensitive than DXA at distinguishing these three states.
In particular, CT scans identified 119 patients as having osteoporosis, with readily identifiable moderate or severe vertebral fractures, when DXA had classified 62 of these patients as having normal BMD (12 subjects) or only osteopenia (50 subjects).
"Our observations are consistent with prior studies documenting that many patients without osteoporosis diagnosed by DXA will sustain fragility fractures, and suggest that CT attenuation may be a more accurate risk predictor," Dr. Pickhardt and his associates wrote (Ann. Intern. Med. 2013:158:588-95).
If their findings are confirmed in other studies, it may become routine for all abdominal and thoracic CT scans performed for any reason to be used for lumbar BMD assessment as well. "In the future, it may even be possible to incorporate CT ... data into fracture risk assessment tools," they added.
This should result in substantial savings in health care costs since osteoporosis will be diagnosed and treated earlier, before fractures occur, and since it also will reduce the number of costly DXA studies performed.
More than 80 million CT scans were performed in the United States in 2011, "most of which carry potentially useful information about BMD," the researchers noted.
The investigators are now turning their attention to using pelvic CT scans that were obtained for various clinical indications to assess hip BMD. "We are currently investigating the potential for deriving a DXA-equivalent T-score for the hips from standard pelvic CT scans by using a dedicated software tool," Dr. Pickhardt and his associates said.
This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
Dr. Pickhardt and his associates "have laid all the groundwork needed to justify using conventional CT imaging to detect incidental osteoporosis," said Dr. Sumit R. Majumdar and Dr. William D. Leslie.
Given the large number of such CT scans performed every year, "the idea of extracting more information from imaging data collected for other purposes holds merit," they said.
"It is now up to the rest of us to safely and cost-effectively translate this new knowledge into everyday clinical practice," Dr. Majumdar and Dr. Leslie said.
Dr. Majumdar is with the University of Alberta, Edmonton. Dr. Leslie is with the University of Manitoba, Winnipeg. Neither reported any financial conflicts of interest. These remarks were taken from their editorial, which accompanied Dr. Pickhardt’s report (Ann. Intern. Med. 2013;158:630-1).
Dr. Pickhardt and his associates "have laid all the groundwork needed to justify using conventional CT imaging to detect incidental osteoporosis," said Dr. Sumit R. Majumdar and Dr. William D. Leslie.
Given the large number of such CT scans performed every year, "the idea of extracting more information from imaging data collected for other purposes holds merit," they said.
"It is now up to the rest of us to safely and cost-effectively translate this new knowledge into everyday clinical practice," Dr. Majumdar and Dr. Leslie said.
Dr. Majumdar is with the University of Alberta, Edmonton. Dr. Leslie is with the University of Manitoba, Winnipeg. Neither reported any financial conflicts of interest. These remarks were taken from their editorial, which accompanied Dr. Pickhardt’s report (Ann. Intern. Med. 2013;158:630-1).
Dr. Pickhardt and his associates "have laid all the groundwork needed to justify using conventional CT imaging to detect incidental osteoporosis," said Dr. Sumit R. Majumdar and Dr. William D. Leslie.
Given the large number of such CT scans performed every year, "the idea of extracting more information from imaging data collected for other purposes holds merit," they said.
"It is now up to the rest of us to safely and cost-effectively translate this new knowledge into everyday clinical practice," Dr. Majumdar and Dr. Leslie said.
Dr. Majumdar is with the University of Alberta, Edmonton. Dr. Leslie is with the University of Manitoba, Winnipeg. Neither reported any financial conflicts of interest. These remarks were taken from their editorial, which accompanied Dr. Pickhardt’s report (Ann. Intern. Med. 2013;158:630-1).
Abdominal and thoracic CT scans obtained for a variety of reasons, such as to assess pain, GI symptoms, or urinary tract complaints, also can be used "opportunistically" to examine lumbar bone mineral density and screen for occult osteoporosis, according to a report in the April 16 issue of the Annals of Internal Medicine.
Abdominal and thoracic CT scans done in routine practice happen to include imaging of the L1 level, which can easily be identified because it is the first non–rib-bearing vertebra. Such scans readily yield data on lumbar bone mineral density (BMD), which is a clinically useful way to diagnose or rule out osteoporosis, said Dr. Perry J. Pickhardt of the department of radiology and his associates at the University of Wisconsin, Madison.
It is important not to confuse this standard CT scanning with quantitative CT (QCT) scanning. QCT "is more labor-intensive; requires an imaging phantom or angle-corrected [region-of-interest] measurement of bone, muscle, and fat at multiple levels; and involves additional money, time, and radiation exposure," they explained.
Unlike dual-energy x-ray absorptiometry (DXA) screening or QCT assessment, "the method that we used requires a negligible amount of training and time; could be applied prospectively by the interpreting radiologist or retrospectively by a radiologist or even nonradiologist; adds no cost; and requires no additional patient time, equipment, software, or radiation exposure," the investigators wrote.
Such incidental CT scans can be assessed retrospectively because they are almost always stored indefinitely in electronic medical records, they noted.
Dr. Pickhardt and his colleagues evaluated CT-derived BMD assessment and compared it against DXA scanning of the hips and spine by identifying 1,867 adults who had undergone the two types of scanning within a 6-month period during the 10-year study interval. They retrieved and reviewed the images, paying particular attention to obvious moderate or severe compression deformities on the CT images, rather than to milder ones, "to avoid ambiguity related to more subjective borderline or mild compression deformities."
The study subjects had a total of 2,063 pairs of CT and DXA assessments that had been performed a median of 67 days apart. A total of 81% of these subjects were women, and the mean age was 59 years.
These patients had undergone abdominal or thoracic CT for a variety of clinical indications, most often for a suspected mass or an oncologic work-up (414 subjects), genitourinary problems (402 subjects), gastrointestinal symptoms (398 subjects), and/or unexplained abdominal pain or symptoms (374 subjects).
Approximately 55% of the CT scans involved intravenous contrast. The use of contrast had no effect on the interpretation of lumbar data on the scans.
The DXA screening identified 22.9% of the study subjects as osteoporotic, 44.8% as osteopenic, and 32.3% as having normal BMD. The CT scans were significantly more sensitive than DXA at distinguishing these three states.
In particular, CT scans identified 119 patients as having osteoporosis, with readily identifiable moderate or severe vertebral fractures, when DXA had classified 62 of these patients as having normal BMD (12 subjects) or only osteopenia (50 subjects).
"Our observations are consistent with prior studies documenting that many patients without osteoporosis diagnosed by DXA will sustain fragility fractures, and suggest that CT attenuation may be a more accurate risk predictor," Dr. Pickhardt and his associates wrote (Ann. Intern. Med. 2013:158:588-95).
If their findings are confirmed in other studies, it may become routine for all abdominal and thoracic CT scans performed for any reason to be used for lumbar BMD assessment as well. "In the future, it may even be possible to incorporate CT ... data into fracture risk assessment tools," they added.
This should result in substantial savings in health care costs since osteoporosis will be diagnosed and treated earlier, before fractures occur, and since it also will reduce the number of costly DXA studies performed.
More than 80 million CT scans were performed in the United States in 2011, "most of which carry potentially useful information about BMD," the researchers noted.
The investigators are now turning their attention to using pelvic CT scans that were obtained for various clinical indications to assess hip BMD. "We are currently investigating the potential for deriving a DXA-equivalent T-score for the hips from standard pelvic CT scans by using a dedicated software tool," Dr. Pickhardt and his associates said.
This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
Abdominal and thoracic CT scans obtained for a variety of reasons, such as to assess pain, GI symptoms, or urinary tract complaints, also can be used "opportunistically" to examine lumbar bone mineral density and screen for occult osteoporosis, according to a report in the April 16 issue of the Annals of Internal Medicine.
Abdominal and thoracic CT scans done in routine practice happen to include imaging of the L1 level, which can easily be identified because it is the first non–rib-bearing vertebra. Such scans readily yield data on lumbar bone mineral density (BMD), which is a clinically useful way to diagnose or rule out osteoporosis, said Dr. Perry J. Pickhardt of the department of radiology and his associates at the University of Wisconsin, Madison.
It is important not to confuse this standard CT scanning with quantitative CT (QCT) scanning. QCT "is more labor-intensive; requires an imaging phantom or angle-corrected [region-of-interest] measurement of bone, muscle, and fat at multiple levels; and involves additional money, time, and radiation exposure," they explained.
Unlike dual-energy x-ray absorptiometry (DXA) screening or QCT assessment, "the method that we used requires a negligible amount of training and time; could be applied prospectively by the interpreting radiologist or retrospectively by a radiologist or even nonradiologist; adds no cost; and requires no additional patient time, equipment, software, or radiation exposure," the investigators wrote.
Such incidental CT scans can be assessed retrospectively because they are almost always stored indefinitely in electronic medical records, they noted.
Dr. Pickhardt and his colleagues evaluated CT-derived BMD assessment and compared it against DXA scanning of the hips and spine by identifying 1,867 adults who had undergone the two types of scanning within a 6-month period during the 10-year study interval. They retrieved and reviewed the images, paying particular attention to obvious moderate or severe compression deformities on the CT images, rather than to milder ones, "to avoid ambiguity related to more subjective borderline or mild compression deformities."
The study subjects had a total of 2,063 pairs of CT and DXA assessments that had been performed a median of 67 days apart. A total of 81% of these subjects were women, and the mean age was 59 years.
These patients had undergone abdominal or thoracic CT for a variety of clinical indications, most often for a suspected mass or an oncologic work-up (414 subjects), genitourinary problems (402 subjects), gastrointestinal symptoms (398 subjects), and/or unexplained abdominal pain or symptoms (374 subjects).
Approximately 55% of the CT scans involved intravenous contrast. The use of contrast had no effect on the interpretation of lumbar data on the scans.
The DXA screening identified 22.9% of the study subjects as osteoporotic, 44.8% as osteopenic, and 32.3% as having normal BMD. The CT scans were significantly more sensitive than DXA at distinguishing these three states.
In particular, CT scans identified 119 patients as having osteoporosis, with readily identifiable moderate or severe vertebral fractures, when DXA had classified 62 of these patients as having normal BMD (12 subjects) or only osteopenia (50 subjects).
"Our observations are consistent with prior studies documenting that many patients without osteoporosis diagnosed by DXA will sustain fragility fractures, and suggest that CT attenuation may be a more accurate risk predictor," Dr. Pickhardt and his associates wrote (Ann. Intern. Med. 2013:158:588-95).
If their findings are confirmed in other studies, it may become routine for all abdominal and thoracic CT scans performed for any reason to be used for lumbar BMD assessment as well. "In the future, it may even be possible to incorporate CT ... data into fracture risk assessment tools," they added.
This should result in substantial savings in health care costs since osteoporosis will be diagnosed and treated earlier, before fractures occur, and since it also will reduce the number of costly DXA studies performed.
More than 80 million CT scans were performed in the United States in 2011, "most of which carry potentially useful information about BMD," the researchers noted.
The investigators are now turning their attention to using pelvic CT scans that were obtained for various clinical indications to assess hip BMD. "We are currently investigating the potential for deriving a DXA-equivalent T-score for the hips from standard pelvic CT scans by using a dedicated software tool," Dr. Pickhardt and his associates said.
This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Major Finding: Conventional abdominal and CT scans performed for various indications were more accurate than DXA at assessing lumbar BMD and identifying occult osteoporosis.
Data Source: A 10-year cross-sectional study comparing incidental spine imaging on conventional CT scans against DXA screening in 1,867 adults from the general population.
Disclosures: This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
Early surgical menopause linked to cognitive decline
SAN DIEGO – Earlier age at surgical menopause may be associated with a steeper decline in cognitive function and increased Alzheimer’s disease–related neuropathologic scores, preliminary results from two longitudinal studies have shown.
"Our findings support a growing literature on the impact of surgical menopause on cognitive function, and add granularity to these outcome measures," Dr. Riley M. Bove said at the annual meeting of the American Academy of Neurology. "In light of the sometimes conflicting and sometimes controversial findings related to modifying factors such as hormone replacement therapy [HRT], we believe that ongoing investigations are warranted."
In an effort to determine the impact of reproductive decline on the spectrum of cognitive decline, Dr. Bove, a neurologist at Brigham and Women’s Hospital, Boston, and her associates studied 1,884 women enrolled in two ongoing longitudinal studies: the Memory and Aging Project (MAP), a study of older men and women in assisted living facilities that was launched in 1997, and the Religious Orders Study (ROS), a study of Catholic priests, nuns, and brothers that was launched in 1994.
"Observational studies have noted that the loss of estrogen associated with menopause, including surgical menopause, may be associated with cognitive decline," Dr. Bove said. "In animal models estrogen has been found to be neuroprotective. However, in humans the evidence is a lot more complex. The results of the Womens Health Initiative Memory Study a decade ago did find adverse effects of hormone replacement therapy initiated in women in their 60s. Since then a window of opportunity hypothesis has emerged, according to which HRT perimenopausally may be protective, but following this window, it may be neutral or even harmful. We aimed to look at longitudinal changes in cognition, risk of an Alzheimer’s diagnosis, and neuropathologic measures related to Alzheimer’s disease. Our hypothesis was that earlier surgical menopause is associated with earlier risk of cognitive decline."
Study participants, who have been followed for up to 19 years, underwent a baseline clinical and reproductive history, annual clinical and cognitive evaluations, and annual blood draws. The researchers examined the association between age at menarche and menopause, number of cycling years, and ever use and duration of HRT.
During annual cognitive tests, the researchers evaluated five composite domains that were weighted toward memory and categorized by factor analysis. The domains include episodic memory (seven tests), semantic memory (three tests), working memory (three tests), perceptual speed (two tests), and visuospatial ability (two tests). They also established a global cognition composite score, which was a sum of the 17 individual tests.
Dr. Bove and her associates considered a clinical diagnosis of Alzheimer’s based on clinical criteria and neuropathologic measures. The three Alzheimer’s disease–associated measures were neuritic plaques, neurofibrillary tangles, and diffuse plaques, as well as a global AD pathology score, which was an average of these three measures. Age at menopause was a continuous variable. All models controlled for age, smoking, and years of education.
Of the total women studied, all were free of dementia at enrollment, their mean age was 78 years, and 91% were non-Hispanic white. On average, compared with women in the ROS study, those in the MAP study were older (79.6 vs. 75.9 years, respectively); were less educated (14.1 vs. 17.9 years); were more likely to smoke (38% vs. 8%); had an earlier age at menarche (12.8 vs. 13.1); had a later age at menopause (47.3 vs. 46.6); and had a slightly higher number of cycling years (34.5 vs. 33.4).
Of the 1,884 women, 1,277 reported having natural menopause, and 607 reported having surgical menopause. More women in the surgical menopause group reported HRT use (53% vs. 27%, respectively). "Approximately 90% of HRT use was oral, so we collapsed all of the different forms of HRT use into one group," she explained.
Among women who had undergone surgical menopause, early age at menopause was associated with a faster decline in global cognition (P = .0007), as well as faster declines in episodic memory (P = .0003) and semantic memory (P = .0022). "We did not find the similar association in the natural menopause group," Dr. Bove said.
However, the researchers observed no significant association between age at surgical menopause and risk of clinical Alzheimer’s (P = .093) nor in the pathologic diagnosis of Alzheimer’s (P = .053). Early age at surgical menopause was associated with significantly lower scores in global AD pathology (P = .038) and neuritic plaques (P = .013) but not in neurofibrillary tangles (P = .138) or diffuse plaques (P = .490).
Dr. Bove also reported that there were no associations between HRT use ever vs. never in any of the outcomes examined, "even when HRT use was stratified according to its timing of initiation relative to menopause. Additionally, we did not find a significant association between duration of HRT use and any of the neurologic outcomes."
She acknowledged certain limitations of the study, including the fact that study participants were required to be nondemented at baseline. "This may have led to exclusion bias if there were early effects of menopause on cognitive function," Dr. Bove said. "The cognitive outcomes were weighted toward memory, and the reproductive histories were patient reported and retrospective, so there’s a limited definition of surgical menopause. We don’t have any information as to whether the oophorectomies were unilateral or bilateral, or the indication for the surgeries. There was also limited data about HRT use."
Dr. Bove said that she had no relevant financial disclosures.
SAN DIEGO – Earlier age at surgical menopause may be associated with a steeper decline in cognitive function and increased Alzheimer’s disease–related neuropathologic scores, preliminary results from two longitudinal studies have shown.
"Our findings support a growing literature on the impact of surgical menopause on cognitive function, and add granularity to these outcome measures," Dr. Riley M. Bove said at the annual meeting of the American Academy of Neurology. "In light of the sometimes conflicting and sometimes controversial findings related to modifying factors such as hormone replacement therapy [HRT], we believe that ongoing investigations are warranted."
In an effort to determine the impact of reproductive decline on the spectrum of cognitive decline, Dr. Bove, a neurologist at Brigham and Women’s Hospital, Boston, and her associates studied 1,884 women enrolled in two ongoing longitudinal studies: the Memory and Aging Project (MAP), a study of older men and women in assisted living facilities that was launched in 1997, and the Religious Orders Study (ROS), a study of Catholic priests, nuns, and brothers that was launched in 1994.
"Observational studies have noted that the loss of estrogen associated with menopause, including surgical menopause, may be associated with cognitive decline," Dr. Bove said. "In animal models estrogen has been found to be neuroprotective. However, in humans the evidence is a lot more complex. The results of the Womens Health Initiative Memory Study a decade ago did find adverse effects of hormone replacement therapy initiated in women in their 60s. Since then a window of opportunity hypothesis has emerged, according to which HRT perimenopausally may be protective, but following this window, it may be neutral or even harmful. We aimed to look at longitudinal changes in cognition, risk of an Alzheimer’s diagnosis, and neuropathologic measures related to Alzheimer’s disease. Our hypothesis was that earlier surgical menopause is associated with earlier risk of cognitive decline."
Study participants, who have been followed for up to 19 years, underwent a baseline clinical and reproductive history, annual clinical and cognitive evaluations, and annual blood draws. The researchers examined the association between age at menarche and menopause, number of cycling years, and ever use and duration of HRT.
During annual cognitive tests, the researchers evaluated five composite domains that were weighted toward memory and categorized by factor analysis. The domains include episodic memory (seven tests), semantic memory (three tests), working memory (three tests), perceptual speed (two tests), and visuospatial ability (two tests). They also established a global cognition composite score, which was a sum of the 17 individual tests.
Dr. Bove and her associates considered a clinical diagnosis of Alzheimer’s based on clinical criteria and neuropathologic measures. The three Alzheimer’s disease–associated measures were neuritic plaques, neurofibrillary tangles, and diffuse plaques, as well as a global AD pathology score, which was an average of these three measures. Age at menopause was a continuous variable. All models controlled for age, smoking, and years of education.
Of the total women studied, all were free of dementia at enrollment, their mean age was 78 years, and 91% were non-Hispanic white. On average, compared with women in the ROS study, those in the MAP study were older (79.6 vs. 75.9 years, respectively); were less educated (14.1 vs. 17.9 years); were more likely to smoke (38% vs. 8%); had an earlier age at menarche (12.8 vs. 13.1); had a later age at menopause (47.3 vs. 46.6); and had a slightly higher number of cycling years (34.5 vs. 33.4).
Of the 1,884 women, 1,277 reported having natural menopause, and 607 reported having surgical menopause. More women in the surgical menopause group reported HRT use (53% vs. 27%, respectively). "Approximately 90% of HRT use was oral, so we collapsed all of the different forms of HRT use into one group," she explained.
Among women who had undergone surgical menopause, early age at menopause was associated with a faster decline in global cognition (P = .0007), as well as faster declines in episodic memory (P = .0003) and semantic memory (P = .0022). "We did not find the similar association in the natural menopause group," Dr. Bove said.
However, the researchers observed no significant association between age at surgical menopause and risk of clinical Alzheimer’s (P = .093) nor in the pathologic diagnosis of Alzheimer’s (P = .053). Early age at surgical menopause was associated with significantly lower scores in global AD pathology (P = .038) and neuritic plaques (P = .013) but not in neurofibrillary tangles (P = .138) or diffuse plaques (P = .490).
Dr. Bove also reported that there were no associations between HRT use ever vs. never in any of the outcomes examined, "even when HRT use was stratified according to its timing of initiation relative to menopause. Additionally, we did not find a significant association between duration of HRT use and any of the neurologic outcomes."
She acknowledged certain limitations of the study, including the fact that study participants were required to be nondemented at baseline. "This may have led to exclusion bias if there were early effects of menopause on cognitive function," Dr. Bove said. "The cognitive outcomes were weighted toward memory, and the reproductive histories were patient reported and retrospective, so there’s a limited definition of surgical menopause. We don’t have any information as to whether the oophorectomies were unilateral or bilateral, or the indication for the surgeries. There was also limited data about HRT use."
Dr. Bove said that she had no relevant financial disclosures.
SAN DIEGO – Earlier age at surgical menopause may be associated with a steeper decline in cognitive function and increased Alzheimer’s disease–related neuropathologic scores, preliminary results from two longitudinal studies have shown.
"Our findings support a growing literature on the impact of surgical menopause on cognitive function, and add granularity to these outcome measures," Dr. Riley M. Bove said at the annual meeting of the American Academy of Neurology. "In light of the sometimes conflicting and sometimes controversial findings related to modifying factors such as hormone replacement therapy [HRT], we believe that ongoing investigations are warranted."
In an effort to determine the impact of reproductive decline on the spectrum of cognitive decline, Dr. Bove, a neurologist at Brigham and Women’s Hospital, Boston, and her associates studied 1,884 women enrolled in two ongoing longitudinal studies: the Memory and Aging Project (MAP), a study of older men and women in assisted living facilities that was launched in 1997, and the Religious Orders Study (ROS), a study of Catholic priests, nuns, and brothers that was launched in 1994.
"Observational studies have noted that the loss of estrogen associated with menopause, including surgical menopause, may be associated with cognitive decline," Dr. Bove said. "In animal models estrogen has been found to be neuroprotective. However, in humans the evidence is a lot more complex. The results of the Womens Health Initiative Memory Study a decade ago did find adverse effects of hormone replacement therapy initiated in women in their 60s. Since then a window of opportunity hypothesis has emerged, according to which HRT perimenopausally may be protective, but following this window, it may be neutral or even harmful. We aimed to look at longitudinal changes in cognition, risk of an Alzheimer’s diagnosis, and neuropathologic measures related to Alzheimer’s disease. Our hypothesis was that earlier surgical menopause is associated with earlier risk of cognitive decline."
Study participants, who have been followed for up to 19 years, underwent a baseline clinical and reproductive history, annual clinical and cognitive evaluations, and annual blood draws. The researchers examined the association between age at menarche and menopause, number of cycling years, and ever use and duration of HRT.
During annual cognitive tests, the researchers evaluated five composite domains that were weighted toward memory and categorized by factor analysis. The domains include episodic memory (seven tests), semantic memory (three tests), working memory (three tests), perceptual speed (two tests), and visuospatial ability (two tests). They also established a global cognition composite score, which was a sum of the 17 individual tests.
Dr. Bove and her associates considered a clinical diagnosis of Alzheimer’s based on clinical criteria and neuropathologic measures. The three Alzheimer’s disease–associated measures were neuritic plaques, neurofibrillary tangles, and diffuse plaques, as well as a global AD pathology score, which was an average of these three measures. Age at menopause was a continuous variable. All models controlled for age, smoking, and years of education.
Of the total women studied, all were free of dementia at enrollment, their mean age was 78 years, and 91% were non-Hispanic white. On average, compared with women in the ROS study, those in the MAP study were older (79.6 vs. 75.9 years, respectively); were less educated (14.1 vs. 17.9 years); were more likely to smoke (38% vs. 8%); had an earlier age at menarche (12.8 vs. 13.1); had a later age at menopause (47.3 vs. 46.6); and had a slightly higher number of cycling years (34.5 vs. 33.4).
Of the 1,884 women, 1,277 reported having natural menopause, and 607 reported having surgical menopause. More women in the surgical menopause group reported HRT use (53% vs. 27%, respectively). "Approximately 90% of HRT use was oral, so we collapsed all of the different forms of HRT use into one group," she explained.
Among women who had undergone surgical menopause, early age at menopause was associated with a faster decline in global cognition (P = .0007), as well as faster declines in episodic memory (P = .0003) and semantic memory (P = .0022). "We did not find the similar association in the natural menopause group," Dr. Bove said.
However, the researchers observed no significant association between age at surgical menopause and risk of clinical Alzheimer’s (P = .093) nor in the pathologic diagnosis of Alzheimer’s (P = .053). Early age at surgical menopause was associated with significantly lower scores in global AD pathology (P = .038) and neuritic plaques (P = .013) but not in neurofibrillary tangles (P = .138) or diffuse plaques (P = .490).
Dr. Bove also reported that there were no associations between HRT use ever vs. never in any of the outcomes examined, "even when HRT use was stratified according to its timing of initiation relative to menopause. Additionally, we did not find a significant association between duration of HRT use and any of the neurologic outcomes."
She acknowledged certain limitations of the study, including the fact that study participants were required to be nondemented at baseline. "This may have led to exclusion bias if there were early effects of menopause on cognitive function," Dr. Bove said. "The cognitive outcomes were weighted toward memory, and the reproductive histories were patient reported and retrospective, so there’s a limited definition of surgical menopause. We don’t have any information as to whether the oophorectomies were unilateral or bilateral, or the indication for the surgeries. There was also limited data about HRT use."
Dr. Bove said that she had no relevant financial disclosures.
AT THE 2013 AAN ANNUAL MEETING
Major finding: Among women who had undergone surgical menopause, early age at menopause was associated with a faster decline in global cognition (P = .0007) as well as faster declines in episodic memory (P = .0003) and semantic memory (P = .0022).
Data source: Findings from 1,884 women enrolled in two ongoing longitudinal studies: the Memory and Aging Project (MAP), a study of older men and women in assisted living facilities that was launched in 1997, and the Religious Orders Study (ROS), a study of Catholic priests, nuns, and brothers that was launched in 1994.
Disclosures: Dr. Bove said she had no relevant financial disclosures.
Being postmenopausal doubles hepatic steatosis risk
SAN FRANCISCO – Postmenopausal status is independently associated with a twofold increased risk of hepatic steatosis, the Dallas Heart Study has shown.
Among 1,018 women aged 30-65 years enrolled in the population-based study, 48% were postmenopausal. Their prevalence of hepatic steatosis as defined by greater than 5.5% hepatic fat content measured by magnetic resonance spectroscopy was 34%. In contrast, the prevalence was significantly less at 24% in the premenopausal women, Dr. Monika Sanghavi reported at the annual meeting of the American College of Cardiology.
The absolute hepatic triglyceride content in the postmenopausal cohort was 4.0%, significantly more than the 2.9% value in premenopausal women.
Of note, the prevalence of hepatic steatosis rose with greater time since the last menstrual period (LMP). The prevalence was 22% among women whose LMP was less than 2 months earlier, 31% in those whose LMP was 2-12 months earlier, and 35% in women whose LMP was more than 12 months prior, according to Dr. Sanghavi of the University of Texas Southwestern Medical Center, Dallas.
Women who were postmenopausal were, of course, substantially older on average than premenopausal women. However, they also had significantly higher average systolic blood pressure, 129 compared with 117 mm Hg; a greater prevalence of diabetes, 16% vs. 7%; a mean LDL cholesterol of 113 compared with 98 mg/dL, and an average serum triglyceride level of 107 mg/dL compared with 81 mg/dL in premenopausal women. In a multivariate analysis adjusted for these variables as well as smoking status, body mass index, and C-reactive protein level, being postmenopausal remained independently associated with a twofold increased likelihood of hepatic steatosis (odds ratio, 2.0).
Hepatic steatosis has come under increasing research scrutiny of late because it appears to be a marker of increased atherosclerotic risk. The liver abnormality is associated with the metabolic syndrome, but as the Dallas Heart Study data show, postmenopausal status confers an increased risk of hepatic steatosis through a mechanism independent of obesity, hyperlipidemia, and other conventional cardiovascular risk factors.
Dr. Sanghavi reported having no relevant financial conflicts.
SAN FRANCISCO – Postmenopausal status is independently associated with a twofold increased risk of hepatic steatosis, the Dallas Heart Study has shown.
Among 1,018 women aged 30-65 years enrolled in the population-based study, 48% were postmenopausal. Their prevalence of hepatic steatosis as defined by greater than 5.5% hepatic fat content measured by magnetic resonance spectroscopy was 34%. In contrast, the prevalence was significantly less at 24% in the premenopausal women, Dr. Monika Sanghavi reported at the annual meeting of the American College of Cardiology.
The absolute hepatic triglyceride content in the postmenopausal cohort was 4.0%, significantly more than the 2.9% value in premenopausal women.
Of note, the prevalence of hepatic steatosis rose with greater time since the last menstrual period (LMP). The prevalence was 22% among women whose LMP was less than 2 months earlier, 31% in those whose LMP was 2-12 months earlier, and 35% in women whose LMP was more than 12 months prior, according to Dr. Sanghavi of the University of Texas Southwestern Medical Center, Dallas.
Women who were postmenopausal were, of course, substantially older on average than premenopausal women. However, they also had significantly higher average systolic blood pressure, 129 compared with 117 mm Hg; a greater prevalence of diabetes, 16% vs. 7%; a mean LDL cholesterol of 113 compared with 98 mg/dL, and an average serum triglyceride level of 107 mg/dL compared with 81 mg/dL in premenopausal women. In a multivariate analysis adjusted for these variables as well as smoking status, body mass index, and C-reactive protein level, being postmenopausal remained independently associated with a twofold increased likelihood of hepatic steatosis (odds ratio, 2.0).
Hepatic steatosis has come under increasing research scrutiny of late because it appears to be a marker of increased atherosclerotic risk. The liver abnormality is associated with the metabolic syndrome, but as the Dallas Heart Study data show, postmenopausal status confers an increased risk of hepatic steatosis through a mechanism independent of obesity, hyperlipidemia, and other conventional cardiovascular risk factors.
Dr. Sanghavi reported having no relevant financial conflicts.
SAN FRANCISCO – Postmenopausal status is independently associated with a twofold increased risk of hepatic steatosis, the Dallas Heart Study has shown.
Among 1,018 women aged 30-65 years enrolled in the population-based study, 48% were postmenopausal. Their prevalence of hepatic steatosis as defined by greater than 5.5% hepatic fat content measured by magnetic resonance spectroscopy was 34%. In contrast, the prevalence was significantly less at 24% in the premenopausal women, Dr. Monika Sanghavi reported at the annual meeting of the American College of Cardiology.
The absolute hepatic triglyceride content in the postmenopausal cohort was 4.0%, significantly more than the 2.9% value in premenopausal women.
Of note, the prevalence of hepatic steatosis rose with greater time since the last menstrual period (LMP). The prevalence was 22% among women whose LMP was less than 2 months earlier, 31% in those whose LMP was 2-12 months earlier, and 35% in women whose LMP was more than 12 months prior, according to Dr. Sanghavi of the University of Texas Southwestern Medical Center, Dallas.
Women who were postmenopausal were, of course, substantially older on average than premenopausal women. However, they also had significantly higher average systolic blood pressure, 129 compared with 117 mm Hg; a greater prevalence of diabetes, 16% vs. 7%; a mean LDL cholesterol of 113 compared with 98 mg/dL, and an average serum triglyceride level of 107 mg/dL compared with 81 mg/dL in premenopausal women. In a multivariate analysis adjusted for these variables as well as smoking status, body mass index, and C-reactive protein level, being postmenopausal remained independently associated with a twofold increased likelihood of hepatic steatosis (odds ratio, 2.0).
Hepatic steatosis has come under increasing research scrutiny of late because it appears to be a marker of increased atherosclerotic risk. The liver abnormality is associated with the metabolic syndrome, but as the Dallas Heart Study data show, postmenopausal status confers an increased risk of hepatic steatosis through a mechanism independent of obesity, hyperlipidemia, and other conventional cardiovascular risk factors.
Dr. Sanghavi reported having no relevant financial conflicts.
AT ACC13
Major Finding: Postmenopausal women had a significantly greater prevalence of hepatic steatosis (34%) than did premenopausal women (24%). After multivariate adjustment, the risk of steatosis was doubled in postmenopausal women.
Data Source: The Dallas Heart Study, a multiethnic, population-based study, including 1,018 women aged 30-65.
Disclosures: The presenter reported having no relevant financial conflicts.
FDA panel rejects SSRI's approval as a hot flash treatment
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel recommended against approval of a low-dose formulation of paroxetine mesylate as a treatment for menopausal vasomotor symptoms, citing minimal benefits over placebo in clinical trials and the potential risks associated with treatment.
At the March 4 meeting, the FDA’s Reproductive Health Drugs Advisory Committee voted 10 to 4 that the overall risk-benefit profile of paroxetine did not support approval of the indication proposed by the manufacturer, Noven Therapeutics, as a treatment for moderate to severe vasomotor symptoms associated with menopause, at a dose of 7.5 mg once a day at bedtime.
The 7.5-mg dose is lower than the doses approved for psychiatric indications of paroxetine, which range from an initial dose of 10 mg to a maximum of 60 mg per day, depending on the indication.
Currently, paroxetine, a selective serotonin reuptake inhibitor (SSRI), is used off-label to treat vasomotor symptoms (VMS), and if approved, this could be the first nonhormonal treatment for VMS associated with menopause. Earlier in the day, the same panel voted against recommending approval for the antiepileptic drug gabapentin, another drug used off-label for VMS, for the same indication, citing the drug’s modest effects on VMS and its potential risks. In studies of both drugs, there was a marked placebo effect, which complicated the evaluations of the effectiveness.
In two pivotal studies conducted in the United States, 1,180 women (mean age, 54-55 years) with natural or surgical menopause who had at least 7 hot flashes per day or at least 50 per week were randomized to receive paroxetine or placebo, after a 12-week run-in period. Electronic diaries were used to document hot flashes. The primary endpoints were the mean change from baseline in hot flash frequency, and the mean change from baseline in hot flash severity, at weeks 4 and 12.
Those on paroxetine had statistically significant reductions in the daily number of hot flashes from baseline, compared with those on placebo at weeks 4 and 12 in both studies, and at week 24 in one of the two studies, which was a 24-week study. The reductions in the daily severity of hot flashes also were statistically significant at week 4 in both studies, and at week 12 in one study but not in the other study, according to the FDA.
However, the women on placebo had notable responses, not unexpected for a nonhormonal treatment, and the difference in the median number of hot flashes per day between the two groups was less than 2: In one study, the median number of hot flashes a day dropped by 4.29 by week 4 and 5.93 at week 12 among those on paroxetine, versus 3.13 and 5, respectively, among those on placebo. In the other study, the median number of hot flashes a day dropped by 3.79 at week 4 and 5.57 by week 12 among those on paroxetine, versus 2.5 and 3.86, respectively, among those on placebo.
The safety profile in the studies was consistent with the known safety profile of paroxetine, with no new or unexpected findings, according to Noven. Fatigue, nausea, and dizziness were the most common side effects in people treated with paroxetine, but they occurred mostly in the first 4 weeks of treatment and were mild to moderate Serious adverse events were higher among those on paroxetine (2.2% vs. 1.4%). In the 24-week study, there were three cases of suicidal ideation and one suicide attempt among those on paroxetine, all after 12 weeks of treatment. The one death in the studies was in a woman on paroxetine, but the death not considered related to the drug, according to the company.
Paroxetine was first approved in 1992 as paroxetine hydrochloride. Paroxetine mesylate was first approved in 2003 and is marketed as Pexeva; it is approved for major depressive disorder, obsessive compulsive disorder, panic disorder, and generalized anxiety disorder. As with all antidepressants, it has a boxed warning about the risk of suicidality and serotonin syndrome associated with treatment.
Noven refers to low-dose paroxetine as LDMP, for low-dose mesylate salt of paroxetine.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
psychiatric indications of paroxetine, selective serotonin reuptake inhibitor, SSRI,
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel recommended against approval of a low-dose formulation of paroxetine mesylate as a treatment for menopausal vasomotor symptoms, citing minimal benefits over placebo in clinical trials and the potential risks associated with treatment.
At the March 4 meeting, the FDA’s Reproductive Health Drugs Advisory Committee voted 10 to 4 that the overall risk-benefit profile of paroxetine did not support approval of the indication proposed by the manufacturer, Noven Therapeutics, as a treatment for moderate to severe vasomotor symptoms associated with menopause, at a dose of 7.5 mg once a day at bedtime.
The 7.5-mg dose is lower than the doses approved for psychiatric indications of paroxetine, which range from an initial dose of 10 mg to a maximum of 60 mg per day, depending on the indication.
Currently, paroxetine, a selective serotonin reuptake inhibitor (SSRI), is used off-label to treat vasomotor symptoms (VMS), and if approved, this could be the first nonhormonal treatment for VMS associated with menopause. Earlier in the day, the same panel voted against recommending approval for the antiepileptic drug gabapentin, another drug used off-label for VMS, for the same indication, citing the drug’s modest effects on VMS and its potential risks. In studies of both drugs, there was a marked placebo effect, which complicated the evaluations of the effectiveness.
In two pivotal studies conducted in the United States, 1,180 women (mean age, 54-55 years) with natural or surgical menopause who had at least 7 hot flashes per day or at least 50 per week were randomized to receive paroxetine or placebo, after a 12-week run-in period. Electronic diaries were used to document hot flashes. The primary endpoints were the mean change from baseline in hot flash frequency, and the mean change from baseline in hot flash severity, at weeks 4 and 12.
Those on paroxetine had statistically significant reductions in the daily number of hot flashes from baseline, compared with those on placebo at weeks 4 and 12 in both studies, and at week 24 in one of the two studies, which was a 24-week study. The reductions in the daily severity of hot flashes also were statistically significant at week 4 in both studies, and at week 12 in one study but not in the other study, according to the FDA.
However, the women on placebo had notable responses, not unexpected for a nonhormonal treatment, and the difference in the median number of hot flashes per day between the two groups was less than 2: In one study, the median number of hot flashes a day dropped by 4.29 by week 4 and 5.93 at week 12 among those on paroxetine, versus 3.13 and 5, respectively, among those on placebo. In the other study, the median number of hot flashes a day dropped by 3.79 at week 4 and 5.57 by week 12 among those on paroxetine, versus 2.5 and 3.86, respectively, among those on placebo.
The safety profile in the studies was consistent with the known safety profile of paroxetine, with no new or unexpected findings, according to Noven. Fatigue, nausea, and dizziness were the most common side effects in people treated with paroxetine, but they occurred mostly in the first 4 weeks of treatment and were mild to moderate Serious adverse events were higher among those on paroxetine (2.2% vs. 1.4%). In the 24-week study, there were three cases of suicidal ideation and one suicide attempt among those on paroxetine, all after 12 weeks of treatment. The one death in the studies was in a woman on paroxetine, but the death not considered related to the drug, according to the company.
Paroxetine was first approved in 1992 as paroxetine hydrochloride. Paroxetine mesylate was first approved in 2003 and is marketed as Pexeva; it is approved for major depressive disorder, obsessive compulsive disorder, panic disorder, and generalized anxiety disorder. As with all antidepressants, it has a boxed warning about the risk of suicidality and serotonin syndrome associated with treatment.
Noven refers to low-dose paroxetine as LDMP, for low-dose mesylate salt of paroxetine.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – The majority of a Food and Drug Administration advisory panel recommended against approval of a low-dose formulation of paroxetine mesylate as a treatment for menopausal vasomotor symptoms, citing minimal benefits over placebo in clinical trials and the potential risks associated with treatment.
At the March 4 meeting, the FDA’s Reproductive Health Drugs Advisory Committee voted 10 to 4 that the overall risk-benefit profile of paroxetine did not support approval of the indication proposed by the manufacturer, Noven Therapeutics, as a treatment for moderate to severe vasomotor symptoms associated with menopause, at a dose of 7.5 mg once a day at bedtime.
The 7.5-mg dose is lower than the doses approved for psychiatric indications of paroxetine, which range from an initial dose of 10 mg to a maximum of 60 mg per day, depending on the indication.
Currently, paroxetine, a selective serotonin reuptake inhibitor (SSRI), is used off-label to treat vasomotor symptoms (VMS), and if approved, this could be the first nonhormonal treatment for VMS associated with menopause. Earlier in the day, the same panel voted against recommending approval for the antiepileptic drug gabapentin, another drug used off-label for VMS, for the same indication, citing the drug’s modest effects on VMS and its potential risks. In studies of both drugs, there was a marked placebo effect, which complicated the evaluations of the effectiveness.
In two pivotal studies conducted in the United States, 1,180 women (mean age, 54-55 years) with natural or surgical menopause who had at least 7 hot flashes per day or at least 50 per week were randomized to receive paroxetine or placebo, after a 12-week run-in period. Electronic diaries were used to document hot flashes. The primary endpoints were the mean change from baseline in hot flash frequency, and the mean change from baseline in hot flash severity, at weeks 4 and 12.
Those on paroxetine had statistically significant reductions in the daily number of hot flashes from baseline, compared with those on placebo at weeks 4 and 12 in both studies, and at week 24 in one of the two studies, which was a 24-week study. The reductions in the daily severity of hot flashes also were statistically significant at week 4 in both studies, and at week 12 in one study but not in the other study, according to the FDA.
However, the women on placebo had notable responses, not unexpected for a nonhormonal treatment, and the difference in the median number of hot flashes per day between the two groups was less than 2: In one study, the median number of hot flashes a day dropped by 4.29 by week 4 and 5.93 at week 12 among those on paroxetine, versus 3.13 and 5, respectively, among those on placebo. In the other study, the median number of hot flashes a day dropped by 3.79 at week 4 and 5.57 by week 12 among those on paroxetine, versus 2.5 and 3.86, respectively, among those on placebo.
The safety profile in the studies was consistent with the known safety profile of paroxetine, with no new or unexpected findings, according to Noven. Fatigue, nausea, and dizziness were the most common side effects in people treated with paroxetine, but they occurred mostly in the first 4 weeks of treatment and were mild to moderate Serious adverse events were higher among those on paroxetine (2.2% vs. 1.4%). In the 24-week study, there were three cases of suicidal ideation and one suicide attempt among those on paroxetine, all after 12 weeks of treatment. The one death in the studies was in a woman on paroxetine, but the death not considered related to the drug, according to the company.
Paroxetine was first approved in 1992 as paroxetine hydrochloride. Paroxetine mesylate was first approved in 2003 and is marketed as Pexeva; it is approved for major depressive disorder, obsessive compulsive disorder, panic disorder, and generalized anxiety disorder. As with all antidepressants, it has a boxed warning about the risk of suicidality and serotonin syndrome associated with treatment.
Noven refers to low-dose paroxetine as LDMP, for low-dose mesylate salt of paroxetine.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
psychiatric indications of paroxetine, selective serotonin reuptake inhibitor, SSRI,
psychiatric indications of paroxetine, selective serotonin reuptake inhibitor, SSRI,
FROM AN FDA ADVISORY COMMITTEE MEETING
FDA panel recommends against gabapentin's approval for hot flashes
SILVER SPRING, MD. – Women need a nonhormonal treatment for moderate to severe vasomotor symptoms in menopause. But gabapentin is not that drug, according to the majority of a Food and Drug Administration advisory panel, who recommended against approval of a sustained release formulation of the antiepileptic drug on March 3.
At the meeting, the FDA’s Reproductive Health Drugs Advisory Committee voted 12-2 that the risk-benefit profile of gabapentin did not support approval of the proposed indication, citing the limited, modest effect on vasomotor symptoms in three clinical trials. Also working against recommendation of approval were the known risks of the medication, particularly effects on cognition and other central nervous system effects. The sustained release formulation, manufactured by Depomed Inc., stays in the stomach for 8-10 hours, prolonging release of the drug. Were it to be approved despite the panel’s recommendation, it would be the first nonhormonal drug approved for treating vasomotor symptoms. The manufacturer recommends that the drug be dosed by titration to a total daily dose of 1,800 mg, with a 600-mg dose taken with the morning meal and 1,200 mg taken with the evening meal.
"We all recognize the critical need for nonhormonal medications [for vasomotor symptoms], and our patients have clearly been asking for such a product," said the panel chair Julia Johnson, professor and chair of the department of obstetrics and gynecology, University of Massachusetts, Worcester. "However, the risk of the medication cannot be ignored for a treatment with marginal effectiveness on hot flashes and gabapentin’s adverse event profile, she noted.
Another panelist, Kathryn Curtis, Ph.D., in the Centers for Disease Control and Prevention’s division of reproductive health, said that despite the "huge" need for a nonhormonal treatment for vasomotor symptoms, the drug had a very limited, modest effect and its approval would be "misleading" to women who need the treatment.
Depomed conducted three phase III studies of postmenopausal women (mean ages 53-54 years), who had at least 7 moderate to severe hot flashes per day or at least 50 per week during the previous 30 days. The studies compared gabapentin with placebo. There was a statistically significant difference in the frequency of hot flashes among those treated with 1,800 mg/day, compared with those on placebo at week 4, but not at week 12, in all three studies. Changes from baseline in the severity of hot flashes was statistically significant at week 4 in all three studies and remained statistically significant in two of the three studies at week 12. But while statically significant, the benefits over placebo were modest.
The most common adverse events associated with treatment were dizziness and vertigo, and somnolence and sedation, as expected.
Gabapentin is widely prescribed off-label for vasomotor symptoms. The Depomed formulation was approved in 2011 for postherpetic neuralgia and is marketed as Gralise for this indication. The labeling includes a warning about suicidality risk, a class labeling for antiepileptic drugs.
The immediate-release formulation of gabapentin (Neurontin) was first approved in 1993 as an adjunctive treatment of partial seizures, and has been used off label for vasomotor symptoms. Gabapentin encarbil, a prodrug of gabapentin, marketed as Horizant, was approved in 2011 for restless legs syndrome and postherpetic neuralgia.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – Women need a nonhormonal treatment for moderate to severe vasomotor symptoms in menopause. But gabapentin is not that drug, according to the majority of a Food and Drug Administration advisory panel, who recommended against approval of a sustained release formulation of the antiepileptic drug on March 3.
At the meeting, the FDA’s Reproductive Health Drugs Advisory Committee voted 12-2 that the risk-benefit profile of gabapentin did not support approval of the proposed indication, citing the limited, modest effect on vasomotor symptoms in three clinical trials. Also working against recommendation of approval were the known risks of the medication, particularly effects on cognition and other central nervous system effects. The sustained release formulation, manufactured by Depomed Inc., stays in the stomach for 8-10 hours, prolonging release of the drug. Were it to be approved despite the panel’s recommendation, it would be the first nonhormonal drug approved for treating vasomotor symptoms. The manufacturer recommends that the drug be dosed by titration to a total daily dose of 1,800 mg, with a 600-mg dose taken with the morning meal and 1,200 mg taken with the evening meal.
"We all recognize the critical need for nonhormonal medications [for vasomotor symptoms], and our patients have clearly been asking for such a product," said the panel chair Julia Johnson, professor and chair of the department of obstetrics and gynecology, University of Massachusetts, Worcester. "However, the risk of the medication cannot be ignored for a treatment with marginal effectiveness on hot flashes and gabapentin’s adverse event profile, she noted.
Another panelist, Kathryn Curtis, Ph.D., in the Centers for Disease Control and Prevention’s division of reproductive health, said that despite the "huge" need for a nonhormonal treatment for vasomotor symptoms, the drug had a very limited, modest effect and its approval would be "misleading" to women who need the treatment.
Depomed conducted three phase III studies of postmenopausal women (mean ages 53-54 years), who had at least 7 moderate to severe hot flashes per day or at least 50 per week during the previous 30 days. The studies compared gabapentin with placebo. There was a statistically significant difference in the frequency of hot flashes among those treated with 1,800 mg/day, compared with those on placebo at week 4, but not at week 12, in all three studies. Changes from baseline in the severity of hot flashes was statistically significant at week 4 in all three studies and remained statistically significant in two of the three studies at week 12. But while statically significant, the benefits over placebo were modest.
The most common adverse events associated with treatment were dizziness and vertigo, and somnolence and sedation, as expected.
Gabapentin is widely prescribed off-label for vasomotor symptoms. The Depomed formulation was approved in 2011 for postherpetic neuralgia and is marketed as Gralise for this indication. The labeling includes a warning about suicidality risk, a class labeling for antiepileptic drugs.
The immediate-release formulation of gabapentin (Neurontin) was first approved in 1993 as an adjunctive treatment of partial seizures, and has been used off label for vasomotor symptoms. Gabapentin encarbil, a prodrug of gabapentin, marketed as Horizant, was approved in 2011 for restless legs syndrome and postherpetic neuralgia.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – Women need a nonhormonal treatment for moderate to severe vasomotor symptoms in menopause. But gabapentin is not that drug, according to the majority of a Food and Drug Administration advisory panel, who recommended against approval of a sustained release formulation of the antiepileptic drug on March 3.
At the meeting, the FDA’s Reproductive Health Drugs Advisory Committee voted 12-2 that the risk-benefit profile of gabapentin did not support approval of the proposed indication, citing the limited, modest effect on vasomotor symptoms in three clinical trials. Also working against recommendation of approval were the known risks of the medication, particularly effects on cognition and other central nervous system effects. The sustained release formulation, manufactured by Depomed Inc., stays in the stomach for 8-10 hours, prolonging release of the drug. Were it to be approved despite the panel’s recommendation, it would be the first nonhormonal drug approved for treating vasomotor symptoms. The manufacturer recommends that the drug be dosed by titration to a total daily dose of 1,800 mg, with a 600-mg dose taken with the morning meal and 1,200 mg taken with the evening meal.
"We all recognize the critical need for nonhormonal medications [for vasomotor symptoms], and our patients have clearly been asking for such a product," said the panel chair Julia Johnson, professor and chair of the department of obstetrics and gynecology, University of Massachusetts, Worcester. "However, the risk of the medication cannot be ignored for a treatment with marginal effectiveness on hot flashes and gabapentin’s adverse event profile, she noted.
Another panelist, Kathryn Curtis, Ph.D., in the Centers for Disease Control and Prevention’s division of reproductive health, said that despite the "huge" need for a nonhormonal treatment for vasomotor symptoms, the drug had a very limited, modest effect and its approval would be "misleading" to women who need the treatment.
Depomed conducted three phase III studies of postmenopausal women (mean ages 53-54 years), who had at least 7 moderate to severe hot flashes per day or at least 50 per week during the previous 30 days. The studies compared gabapentin with placebo. There was a statistically significant difference in the frequency of hot flashes among those treated with 1,800 mg/day, compared with those on placebo at week 4, but not at week 12, in all three studies. Changes from baseline in the severity of hot flashes was statistically significant at week 4 in all three studies and remained statistically significant in two of the three studies at week 12. But while statically significant, the benefits over placebo were modest.
The most common adverse events associated with treatment were dizziness and vertigo, and somnolence and sedation, as expected.
Gabapentin is widely prescribed off-label for vasomotor symptoms. The Depomed formulation was approved in 2011 for postherpetic neuralgia and is marketed as Gralise for this indication. The labeling includes a warning about suicidality risk, a class labeling for antiepileptic drugs.
The immediate-release formulation of gabapentin (Neurontin) was first approved in 1993 as an adjunctive treatment of partial seizures, and has been used off label for vasomotor symptoms. Gabapentin encarbil, a prodrug of gabapentin, marketed as Horizant, was approved in 2011 for restless legs syndrome and postherpetic neuralgia.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
FROM AN FDA ADVISORY COMMITTEE MEETING
UPDATE ON BREAST HEALTH
Women with ER-positive breast cancer may soon extend tamoxifen therapy to 10 years
Janelle Yates (February 2013)
Is overdiagnosis of breast cancer common among women screened
by mammography?
Andrew M. Kaunitz, MD (Examining the Evidence; January 2013)
Breast cancer genome analysis highlights 4 subtypes, link to
ovarian cancer
Janelle Yates (News for Your Practice; November 2012)
The effects of breast cancer on obstetric and gynecologic practices are pervasive. In this article, we touch on three aspects of breast cancer that are particularly relevant to the practicing ObGyn:
- the need to identify women at high risk for breast cancer and select those who would benefit from a discussion of the advantages and risks of chemoprophylaxis, which can reduce the likelihood of breast cancer by 50% or more
- the need for strategies to manage menopausal symptoms in the general population without increasing the risk of breast cancer. The traditional approach to this problem changed dramatically with the Women’s Health Initiative (WHI), which demonstrated an increased risk of breast cancer in women taking conjugated equine estrogen and progestin. The widely publicized initial findings of the estrogen-progestin arm of the WHI sharply contrast the equally relevant, somewhat unexpected, and less publicized results of the estrogen-alone arm, which demonstrated a substantial and statistically significant decrease in the incidence of breast cancer, even after estrogen was discontinued.
- the potential effects of breast cancer treatment on ovarian function in young women. This year, of the approximately 250,000 women who will be diagnosed with invasive breast cancer, more than 50,000 women will be of reproductive age. Most of these young women will require adjuvant chemotherapy; as a result, many will experience the premature onset of menopause. Along with the attendant loss of fertility these women will face, many will also develop distressing and life-altering menopausal symptoms. Management of these women before and after initiation of chemotherapy requires an understanding of both the expected effects of the chemotherapy and knowledge of how to actively manage these women with strategies to either prevent these events or to manage menopausal symptoms.
In women at normal risk for breast cancer, unopposed estrogen lowers the rate of the malignancy and the likelihood of mortality if the cancer occurs—but is not recommended as a prophylactic agent. Tamoxifen and other chemoprophylactic drugs can halve the rate of breast cancer in high-risk women but are not without drawbacks.
A look at the lower rate of breast cancer in the estrogen-alone arm of the WHI
Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
From 1993 through 1998, the WHI enrolled 10,739 postmenopausal women in the largest prospective trial evaluating the effect of hormone therapy (HT) on various clinical outcomes. The women were randomly allocated to three groups:
- conjugated estrogen with medroxyprogesterone acetate
- conjugated estrogen alone (in women with a prior hysterectomy)
- placebo.
The negative effects of estrogen plus progestin on the risk of breast cancer were the most widely discussed oucomes.1 Shortly after the findings from this arm of the study were published, the use of HT in the United States declined dramatically and unequivocally.2
In 2012, WHI published the results of the estrogen-alone arm in the British cancer specialty journal Lancet Oncology. As shown in the TABLE below, the incidence of breast cancer was statistically significantly lower (23%) in the estrogen group than in the placebo group. Women who were treated with estrogen alone were also 63% less likely to die of breast cancer, and all-cause mortality was 38% lower; both of these findings were statistically significant. Not only was there a significant reduction in the incidence of invasive breast cancer while the subjects were taking estrogen, but that reduction continued for a median of 4.7 years of follow-up after discontinuation of estrogen.
Breast cancer incidence and mortality in the estrogen-only arm of the WHI, compared with placebo*
| Event | Estrogen only (n = 5,310) | Placebo (n = 5,429) | Hazard ratio (95% confidence interval) |
|---|---|---|---|
| Invasive breast cancer | 151 (0.27%) | 199 (0.35%) | 0.77 (0.62–0.95) |
| Node-negative breast cancer | 88 (0.16%) | 134 (0.24%) | 0.67 (0.51–0.88) |
| Breast cancer mortality | 6 (0.009%) | 16 (0.024%) | 0.37 (0.13–0.91) |
| All-cause mortality | 30 (0.046%) | 50 (0.076%) | 0.62 (0.39–0.97) |
| * Median follow-up of 11.8 years | |||
The incidence figure is somewhat remarkable (199 in the placebo group versus 151 in the estrogen-alone group) in that it was nearly the exact reverse of the estrogen-progestin arm of the WHI trial (199 in the estrogen/progestin group vs 150 in the placebo group).3
Estrogen alone reduced both breast cancer incidence and breast cancer mortality while women were on therapy and for 5 years after discontinuing therapy. This finding should reassure women who have undergone hysterectomy, as well as their clinicians, that estrogen alone reduces the future likelihood of breast cancer. It should be noted that the effect of estrogen alone in women in higher-risk categories did not show a reduction in breast cancer, and for this reason, the authors cautioned against considering the use of estrogen alone in menopausal women as a breast cancer chemoprophylaxis agent.
All breast cancer chemoprophylactic agents carry risks as well as benefits
Goss P, Ingle J, Ales-Martinez J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391.
Cheung A, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomized controlled trial. Lancet Oncol. 2012;13(3):275–284.
Vogel V, Costantino J, Wickerham L, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res. 2010;3(6):696–706.
The number of new cases of breast cancer in the United States last year reached nearly a quarter-million. Clearly, reducing this number remains an important goal.4 Chemoprevention—the use of medication to reduce cancer risk—may be offered to women who are at high risk of developing breast cancer.
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial, tamoxifen (a selective estrogen-receptor modulator) was shown to reduce the risk of invasive breast cancer by 49% in a high-risk population, resulting in the FDA approving tamoxifen as the first drug for breast cancer prevention.5 The P-1 trial was followed by the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial, which demonstrated relative equivalence between the two medications as cancer prevention agents in menopausal women.6 Serious side effects of these medications limit their use among eligible women, although raloxifene seems to be associated with fewer adverse events. In the update of the STAR trial with an average of 81 months of follow-up, the risk ratio for adverse events (raloxifene:tamoxifen) was 0.75 for thromboembolic events, 0.55 for endometrial cancer, and 0.19 for uterine hyperplasia.
Another drug used for cancer treatment has now entered the prevention scene. In 2011, the NCIC Clinical Trials Group Mammary Prevention.3 trial (NCIC CTG MAP.3) compared exemestane (an aromatase inhibitor) with placebo for menopausal women at high risk for breast cancer, demonstrating a 65% relative reduction in the incidence of invasive breast cancer. This study validated another option for cancer prevention in high-risk women, although its adoption is likely also to be limited by side effects, including vasomotor symptoms, a high rate of arthralgias, and vaginal dryness/dyspareunia. The greatest concern may be the potential effect on bone density. Though the rates of serious adverse events including fracture did not differ in the MAP.3 trial at 35 months of follow-up, women on exemestane had significantly larger losses of bone mineral density, compared with controls.
Chemoprophylaxis reduces the risk of breast cancer in high-risk women by about 50%. Who are good candidates for these medications? Based on these trials, menopausal women considered at high risk might include those with a Gail risk score of at least 1.66% (ie, risk of developing breast cancer in 5 years), age 60 years or older, and women with biopsy results demonstrating atypical hyperplasia or lobular carcinoma in situ (LCIS). (The Gail model is available at www.cancer.gov/bcrisktool.) Tamoxifen is the only option for premenopausal women age 35 and older. Those who have histologic markers of risk (atypical hyperplasia, LCIS) likely stand to derive the greatest benefit.4
Managing the reproductive health concerns of young women with breast cancer
Azim H, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31(1):73–79.
Howard-Anderson J, Ganz P, Bower J, Stanton A. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):1386–1405.
Of the approximately 230,000 new cases of invasive breast cancer identified in 2011, 50,430 cases involved women less than 50 years of age.4 For these women, the diagnosis of cancer raises multifaceted concerns, including the physical changes that accompany breast cancer treatment, concerns about recurrence and mortality, and significant sexual and reproductive consequences of treatment that alters ovarian function. Pregnancy-associated breast cancers (breast cancers diagnosed during pregnancy, lactation, and for 12 months postpartum) represent a small subset of these cancers and occur in about 1 in 3,000 pregnancies. One might anticipate that this rate will increase as women continue to delay childbearing, because pregnancy-associated breast cancers are more common in older women.
In the review article by Howard-Anderson and colleagues, the importance of these reproductive health consequences in young women diagnosed with breast cancer is highlighted. The women who transition to menopause as a result of chemotherapy (reported to range from 33%–73%) experience more symptoms, including hot flashes, night sweats, breast pain, vaginal dryness, and lack of sexual desire. Sixty-one percent of women younger than 40 years at diagnosis reported that they were concerned about menopause, and 30% reported that this concern influenced their treatment decisions. Thirty-nine percent of women in this group had major concerns about treatment-associated infertility, and only half of the women studied felt that their fertility concerns were adequately addressed.
On a positive note, for women who successfully achieve pregnancy after breast cancer, pregnancy outcomes appear to be similar to those of their nonpregnant peers. In the study by Azim and colleagues, women who became pregnant after a breast cancer diagnosis had disease-free survival that was statistically similar to that of matched women who did not have subsequent pregnancies. In addition, this outcome did not differ based on estrogen/progesterone receptor status (ER/PR positive or negative).
Both alkylating chemotherapeutic agents (eg, cyclophosphamide) and selective estrogen receptor modulating agents (for women with estrogen-receptor–positive tumors) are routine parts of adjuvant treatment for premenopausal women with invasive breast cancers.
These agents can have profound effects on both ovarian hormonal function and fertility. ObGyns and reproductive endocrinology/infertility specialists have a great opportunity to partner with our oncology colleagues to enhance the counseling that young women receive before, during, and after breast cancer treatment.
Women who are considering future childbearing should receive information about the impact of breast cancer treatment on fertility and options for fertility preservation prior to initiating treatment. For women who have completed childbearing, information on what to expect if menopause occurs and available options for symptom relief can be empowering as they make treatment decisions.
We want to hear from you! Tell us what you think.
1. Grady D. Study finds new risks in hormone therapy. New York Times. http://www.nytimes.com/2003/06/25/us/study-finds-new-risks-in-hormone-therapy.html?pagewanted=all&src=pm. Published June 25 2003. Accessed February 11, 2013.
2. Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47-53.
3. Chlebowski RT, Kuller LH, Prentice RL, et al. Women’s Health Initiative Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573-587.
4. American Cancer Society. Breast Cancer Facts and Figures 2011-2012. Atlanta, GA: American Cancer Society. http://www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-and-figures-2011-2012. Accessed February 11, 2013.
5. Fisher B, Constantino J, Wickerham L, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388.
6. Vogel V, Costantino J, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295(23):2727-2741.
Women with ER-positive breast cancer may soon extend tamoxifen therapy to 10 years
Janelle Yates (February 2013)
Is overdiagnosis of breast cancer common among women screened
by mammography?
Andrew M. Kaunitz, MD (Examining the Evidence; January 2013)
Breast cancer genome analysis highlights 4 subtypes, link to
ovarian cancer
Janelle Yates (News for Your Practice; November 2012)
The effects of breast cancer on obstetric and gynecologic practices are pervasive. In this article, we touch on three aspects of breast cancer that are particularly relevant to the practicing ObGyn:
- the need to identify women at high risk for breast cancer and select those who would benefit from a discussion of the advantages and risks of chemoprophylaxis, which can reduce the likelihood of breast cancer by 50% or more
- the need for strategies to manage menopausal symptoms in the general population without increasing the risk of breast cancer. The traditional approach to this problem changed dramatically with the Women’s Health Initiative (WHI), which demonstrated an increased risk of breast cancer in women taking conjugated equine estrogen and progestin. The widely publicized initial findings of the estrogen-progestin arm of the WHI sharply contrast the equally relevant, somewhat unexpected, and less publicized results of the estrogen-alone arm, which demonstrated a substantial and statistically significant decrease in the incidence of breast cancer, even after estrogen was discontinued.
- the potential effects of breast cancer treatment on ovarian function in young women. This year, of the approximately 250,000 women who will be diagnosed with invasive breast cancer, more than 50,000 women will be of reproductive age. Most of these young women will require adjuvant chemotherapy; as a result, many will experience the premature onset of menopause. Along with the attendant loss of fertility these women will face, many will also develop distressing and life-altering menopausal symptoms. Management of these women before and after initiation of chemotherapy requires an understanding of both the expected effects of the chemotherapy and knowledge of how to actively manage these women with strategies to either prevent these events or to manage menopausal symptoms.

In women at normal risk for breast cancer, unopposed estrogen lowers the rate of the malignancy and the likelihood of mortality if the cancer occurs—but is not recommended as a prophylactic agent. Tamoxifen and other chemoprophylactic drugs can halve the rate of breast cancer in high-risk women but are not without drawbacks.
A look at the lower rate of breast cancer in the estrogen-alone arm of the WHI
Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
From 1993 through 1998, the WHI enrolled 10,739 postmenopausal women in the largest prospective trial evaluating the effect of hormone therapy (HT) on various clinical outcomes. The women were randomly allocated to three groups:
- conjugated estrogen with medroxyprogesterone acetate
- conjugated estrogen alone (in women with a prior hysterectomy)
- placebo.
The negative effects of estrogen plus progestin on the risk of breast cancer were the most widely discussed oucomes.1 Shortly after the findings from this arm of the study were published, the use of HT in the United States declined dramatically and unequivocally.2
In 2012, WHI published the results of the estrogen-alone arm in the British cancer specialty journal Lancet Oncology. As shown in the TABLE below, the incidence of breast cancer was statistically significantly lower (23%) in the estrogen group than in the placebo group. Women who were treated with estrogen alone were also 63% less likely to die of breast cancer, and all-cause mortality was 38% lower; both of these findings were statistically significant. Not only was there a significant reduction in the incidence of invasive breast cancer while the subjects were taking estrogen, but that reduction continued for a median of 4.7 years of follow-up after discontinuation of estrogen.
Breast cancer incidence and mortality in the estrogen-only arm of the WHI, compared with placebo*
| Event | Estrogen only (n = 5,310) | Placebo (n = 5,429) | Hazard ratio (95% confidence interval) |
|---|---|---|---|
| Invasive breast cancer | 151 (0.27%) | 199 (0.35%) | 0.77 (0.62–0.95) |
| Node-negative breast cancer | 88 (0.16%) | 134 (0.24%) | 0.67 (0.51–0.88) |
| Breast cancer mortality | 6 (0.009%) | 16 (0.024%) | 0.37 (0.13–0.91) |
| All-cause mortality | 30 (0.046%) | 50 (0.076%) | 0.62 (0.39–0.97) |
| * Median follow-up of 11.8 years | |||
The incidence figure is somewhat remarkable (199 in the placebo group versus 151 in the estrogen-alone group) in that it was nearly the exact reverse of the estrogen-progestin arm of the WHI trial (199 in the estrogen/progestin group vs 150 in the placebo group).3
Estrogen alone reduced both breast cancer incidence and breast cancer mortality while women were on therapy and for 5 years after discontinuing therapy. This finding should reassure women who have undergone hysterectomy, as well as their clinicians, that estrogen alone reduces the future likelihood of breast cancer. It should be noted that the effect of estrogen alone in women in higher-risk categories did not show a reduction in breast cancer, and for this reason, the authors cautioned against considering the use of estrogen alone in menopausal women as a breast cancer chemoprophylaxis agent.
All breast cancer chemoprophylactic agents carry risks as well as benefits
Goss P, Ingle J, Ales-Martinez J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391.
Cheung A, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomized controlled trial. Lancet Oncol. 2012;13(3):275–284.
Vogel V, Costantino J, Wickerham L, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res. 2010;3(6):696–706.
The number of new cases of breast cancer in the United States last year reached nearly a quarter-million. Clearly, reducing this number remains an important goal.4 Chemoprevention—the use of medication to reduce cancer risk—may be offered to women who are at high risk of developing breast cancer.
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial, tamoxifen (a selective estrogen-receptor modulator) was shown to reduce the risk of invasive breast cancer by 49% in a high-risk population, resulting in the FDA approving tamoxifen as the first drug for breast cancer prevention.5 The P-1 trial was followed by the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial, which demonstrated relative equivalence between the two medications as cancer prevention agents in menopausal women.6 Serious side effects of these medications limit their use among eligible women, although raloxifene seems to be associated with fewer adverse events. In the update of the STAR trial with an average of 81 months of follow-up, the risk ratio for adverse events (raloxifene:tamoxifen) was 0.75 for thromboembolic events, 0.55 for endometrial cancer, and 0.19 for uterine hyperplasia.
Another drug used for cancer treatment has now entered the prevention scene. In 2011, the NCIC Clinical Trials Group Mammary Prevention.3 trial (NCIC CTG MAP.3) compared exemestane (an aromatase inhibitor) with placebo for menopausal women at high risk for breast cancer, demonstrating a 65% relative reduction in the incidence of invasive breast cancer. This study validated another option for cancer prevention in high-risk women, although its adoption is likely also to be limited by side effects, including vasomotor symptoms, a high rate of arthralgias, and vaginal dryness/dyspareunia. The greatest concern may be the potential effect on bone density. Though the rates of serious adverse events including fracture did not differ in the MAP.3 trial at 35 months of follow-up, women on exemestane had significantly larger losses of bone mineral density, compared with controls.
Chemoprophylaxis reduces the risk of breast cancer in high-risk women by about 50%. Who are good candidates for these medications? Based on these trials, menopausal women considered at high risk might include those with a Gail risk score of at least 1.66% (ie, risk of developing breast cancer in 5 years), age 60 years or older, and women with biopsy results demonstrating atypical hyperplasia or lobular carcinoma in situ (LCIS). (The Gail model is available at www.cancer.gov/bcrisktool.) Tamoxifen is the only option for premenopausal women age 35 and older. Those who have histologic markers of risk (atypical hyperplasia, LCIS) likely stand to derive the greatest benefit.4
Managing the reproductive health concerns of young women with breast cancer
Azim H, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31(1):73–79.
Howard-Anderson J, Ganz P, Bower J, Stanton A. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):1386–1405.
Of the approximately 230,000 new cases of invasive breast cancer identified in 2011, 50,430 cases involved women less than 50 years of age.4 For these women, the diagnosis of cancer raises multifaceted concerns, including the physical changes that accompany breast cancer treatment, concerns about recurrence and mortality, and significant sexual and reproductive consequences of treatment that alters ovarian function. Pregnancy-associated breast cancers (breast cancers diagnosed during pregnancy, lactation, and for 12 months postpartum) represent a small subset of these cancers and occur in about 1 in 3,000 pregnancies. One might anticipate that this rate will increase as women continue to delay childbearing, because pregnancy-associated breast cancers are more common in older women.
In the review article by Howard-Anderson and colleagues, the importance of these reproductive health consequences in young women diagnosed with breast cancer is highlighted. The women who transition to menopause as a result of chemotherapy (reported to range from 33%–73%) experience more symptoms, including hot flashes, night sweats, breast pain, vaginal dryness, and lack of sexual desire. Sixty-one percent of women younger than 40 years at diagnosis reported that they were concerned about menopause, and 30% reported that this concern influenced their treatment decisions. Thirty-nine percent of women in this group had major concerns about treatment-associated infertility, and only half of the women studied felt that their fertility concerns were adequately addressed.
On a positive note, for women who successfully achieve pregnancy after breast cancer, pregnancy outcomes appear to be similar to those of their nonpregnant peers. In the study by Azim and colleagues, women who became pregnant after a breast cancer diagnosis had disease-free survival that was statistically similar to that of matched women who did not have subsequent pregnancies. In addition, this outcome did not differ based on estrogen/progesterone receptor status (ER/PR positive or negative).
Both alkylating chemotherapeutic agents (eg, cyclophosphamide) and selective estrogen receptor modulating agents (for women with estrogen-receptor–positive tumors) are routine parts of adjuvant treatment for premenopausal women with invasive breast cancers.
These agents can have profound effects on both ovarian hormonal function and fertility. ObGyns and reproductive endocrinology/infertility specialists have a great opportunity to partner with our oncology colleagues to enhance the counseling that young women receive before, during, and after breast cancer treatment.
Women who are considering future childbearing should receive information about the impact of breast cancer treatment on fertility and options for fertility preservation prior to initiating treatment. For women who have completed childbearing, information on what to expect if menopause occurs and available options for symptom relief can be empowering as they make treatment decisions.
We want to hear from you! Tell us what you think.
Women with ER-positive breast cancer may soon extend tamoxifen therapy to 10 years
Janelle Yates (February 2013)
Is overdiagnosis of breast cancer common among women screened
by mammography?
Andrew M. Kaunitz, MD (Examining the Evidence; January 2013)
Breast cancer genome analysis highlights 4 subtypes, link to
ovarian cancer
Janelle Yates (News for Your Practice; November 2012)
The effects of breast cancer on obstetric and gynecologic practices are pervasive. In this article, we touch on three aspects of breast cancer that are particularly relevant to the practicing ObGyn:
- the need to identify women at high risk for breast cancer and select those who would benefit from a discussion of the advantages and risks of chemoprophylaxis, which can reduce the likelihood of breast cancer by 50% or more
- the need for strategies to manage menopausal symptoms in the general population without increasing the risk of breast cancer. The traditional approach to this problem changed dramatically with the Women’s Health Initiative (WHI), which demonstrated an increased risk of breast cancer in women taking conjugated equine estrogen and progestin. The widely publicized initial findings of the estrogen-progestin arm of the WHI sharply contrast the equally relevant, somewhat unexpected, and less publicized results of the estrogen-alone arm, which demonstrated a substantial and statistically significant decrease in the incidence of breast cancer, even after estrogen was discontinued.
- the potential effects of breast cancer treatment on ovarian function in young women. This year, of the approximately 250,000 women who will be diagnosed with invasive breast cancer, more than 50,000 women will be of reproductive age. Most of these young women will require adjuvant chemotherapy; as a result, many will experience the premature onset of menopause. Along with the attendant loss of fertility these women will face, many will also develop distressing and life-altering menopausal symptoms. Management of these women before and after initiation of chemotherapy requires an understanding of both the expected effects of the chemotherapy and knowledge of how to actively manage these women with strategies to either prevent these events or to manage menopausal symptoms.

In women at normal risk for breast cancer, unopposed estrogen lowers the rate of the malignancy and the likelihood of mortality if the cancer occurs—but is not recommended as a prophylactic agent. Tamoxifen and other chemoprophylactic drugs can halve the rate of breast cancer in high-risk women but are not without drawbacks.
A look at the lower rate of breast cancer in the estrogen-alone arm of the WHI
Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
From 1993 through 1998, the WHI enrolled 10,739 postmenopausal women in the largest prospective trial evaluating the effect of hormone therapy (HT) on various clinical outcomes. The women were randomly allocated to three groups:
- conjugated estrogen with medroxyprogesterone acetate
- conjugated estrogen alone (in women with a prior hysterectomy)
- placebo.
The negative effects of estrogen plus progestin on the risk of breast cancer were the most widely discussed oucomes.1 Shortly after the findings from this arm of the study were published, the use of HT in the United States declined dramatically and unequivocally.2
In 2012, WHI published the results of the estrogen-alone arm in the British cancer specialty journal Lancet Oncology. As shown in the TABLE below, the incidence of breast cancer was statistically significantly lower (23%) in the estrogen group than in the placebo group. Women who were treated with estrogen alone were also 63% less likely to die of breast cancer, and all-cause mortality was 38% lower; both of these findings were statistically significant. Not only was there a significant reduction in the incidence of invasive breast cancer while the subjects were taking estrogen, but that reduction continued for a median of 4.7 years of follow-up after discontinuation of estrogen.
Breast cancer incidence and mortality in the estrogen-only arm of the WHI, compared with placebo*
| Event | Estrogen only (n = 5,310) | Placebo (n = 5,429) | Hazard ratio (95% confidence interval) |
|---|---|---|---|
| Invasive breast cancer | 151 (0.27%) | 199 (0.35%) | 0.77 (0.62–0.95) |
| Node-negative breast cancer | 88 (0.16%) | 134 (0.24%) | 0.67 (0.51–0.88) |
| Breast cancer mortality | 6 (0.009%) | 16 (0.024%) | 0.37 (0.13–0.91) |
| All-cause mortality | 30 (0.046%) | 50 (0.076%) | 0.62 (0.39–0.97) |
| * Median follow-up of 11.8 years | |||
The incidence figure is somewhat remarkable (199 in the placebo group versus 151 in the estrogen-alone group) in that it was nearly the exact reverse of the estrogen-progestin arm of the WHI trial (199 in the estrogen/progestin group vs 150 in the placebo group).3
Estrogen alone reduced both breast cancer incidence and breast cancer mortality while women were on therapy and for 5 years after discontinuing therapy. This finding should reassure women who have undergone hysterectomy, as well as their clinicians, that estrogen alone reduces the future likelihood of breast cancer. It should be noted that the effect of estrogen alone in women in higher-risk categories did not show a reduction in breast cancer, and for this reason, the authors cautioned against considering the use of estrogen alone in menopausal women as a breast cancer chemoprophylaxis agent.
All breast cancer chemoprophylactic agents carry risks as well as benefits
Goss P, Ingle J, Ales-Martinez J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391.
Cheung A, Tile L, Cardew S, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomized controlled trial. Lancet Oncol. 2012;13(3):275–284.
Vogel V, Costantino J, Wickerham L, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res. 2010;3(6):696–706.
The number of new cases of breast cancer in the United States last year reached nearly a quarter-million. Clearly, reducing this number remains an important goal.4 Chemoprevention—the use of medication to reduce cancer risk—may be offered to women who are at high risk of developing breast cancer.
In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial, tamoxifen (a selective estrogen-receptor modulator) was shown to reduce the risk of invasive breast cancer by 49% in a high-risk population, resulting in the FDA approving tamoxifen as the first drug for breast cancer prevention.5 The P-1 trial was followed by the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial, which demonstrated relative equivalence between the two medications as cancer prevention agents in menopausal women.6 Serious side effects of these medications limit their use among eligible women, although raloxifene seems to be associated with fewer adverse events. In the update of the STAR trial with an average of 81 months of follow-up, the risk ratio for adverse events (raloxifene:tamoxifen) was 0.75 for thromboembolic events, 0.55 for endometrial cancer, and 0.19 for uterine hyperplasia.
Another drug used for cancer treatment has now entered the prevention scene. In 2011, the NCIC Clinical Trials Group Mammary Prevention.3 trial (NCIC CTG MAP.3) compared exemestane (an aromatase inhibitor) with placebo for menopausal women at high risk for breast cancer, demonstrating a 65% relative reduction in the incidence of invasive breast cancer. This study validated another option for cancer prevention in high-risk women, although its adoption is likely also to be limited by side effects, including vasomotor symptoms, a high rate of arthralgias, and vaginal dryness/dyspareunia. The greatest concern may be the potential effect on bone density. Though the rates of serious adverse events including fracture did not differ in the MAP.3 trial at 35 months of follow-up, women on exemestane had significantly larger losses of bone mineral density, compared with controls.
Chemoprophylaxis reduces the risk of breast cancer in high-risk women by about 50%. Who are good candidates for these medications? Based on these trials, menopausal women considered at high risk might include those with a Gail risk score of at least 1.66% (ie, risk of developing breast cancer in 5 years), age 60 years or older, and women with biopsy results demonstrating atypical hyperplasia or lobular carcinoma in situ (LCIS). (The Gail model is available at www.cancer.gov/bcrisktool.) Tamoxifen is the only option for premenopausal women age 35 and older. Those who have histologic markers of risk (atypical hyperplasia, LCIS) likely stand to derive the greatest benefit.4
Managing the reproductive health concerns of young women with breast cancer
Azim H, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31(1):73–79.
Howard-Anderson J, Ganz P, Bower J, Stanton A. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):1386–1405.
Of the approximately 230,000 new cases of invasive breast cancer identified in 2011, 50,430 cases involved women less than 50 years of age.4 For these women, the diagnosis of cancer raises multifaceted concerns, including the physical changes that accompany breast cancer treatment, concerns about recurrence and mortality, and significant sexual and reproductive consequences of treatment that alters ovarian function. Pregnancy-associated breast cancers (breast cancers diagnosed during pregnancy, lactation, and for 12 months postpartum) represent a small subset of these cancers and occur in about 1 in 3,000 pregnancies. One might anticipate that this rate will increase as women continue to delay childbearing, because pregnancy-associated breast cancers are more common in older women.
In the review article by Howard-Anderson and colleagues, the importance of these reproductive health consequences in young women diagnosed with breast cancer is highlighted. The women who transition to menopause as a result of chemotherapy (reported to range from 33%–73%) experience more symptoms, including hot flashes, night sweats, breast pain, vaginal dryness, and lack of sexual desire. Sixty-one percent of women younger than 40 years at diagnosis reported that they were concerned about menopause, and 30% reported that this concern influenced their treatment decisions. Thirty-nine percent of women in this group had major concerns about treatment-associated infertility, and only half of the women studied felt that their fertility concerns were adequately addressed.
On a positive note, for women who successfully achieve pregnancy after breast cancer, pregnancy outcomes appear to be similar to those of their nonpregnant peers. In the study by Azim and colleagues, women who became pregnant after a breast cancer diagnosis had disease-free survival that was statistically similar to that of matched women who did not have subsequent pregnancies. In addition, this outcome did not differ based on estrogen/progesterone receptor status (ER/PR positive or negative).
Both alkylating chemotherapeutic agents (eg, cyclophosphamide) and selective estrogen receptor modulating agents (for women with estrogen-receptor–positive tumors) are routine parts of adjuvant treatment for premenopausal women with invasive breast cancers.
These agents can have profound effects on both ovarian hormonal function and fertility. ObGyns and reproductive endocrinology/infertility specialists have a great opportunity to partner with our oncology colleagues to enhance the counseling that young women receive before, during, and after breast cancer treatment.
Women who are considering future childbearing should receive information about the impact of breast cancer treatment on fertility and options for fertility preservation prior to initiating treatment. For women who have completed childbearing, information on what to expect if menopause occurs and available options for symptom relief can be empowering as they make treatment decisions.
We want to hear from you! Tell us what you think.
1. Grady D. Study finds new risks in hormone therapy. New York Times. http://www.nytimes.com/2003/06/25/us/study-finds-new-risks-in-hormone-therapy.html?pagewanted=all&src=pm. Published June 25 2003. Accessed February 11, 2013.
2. Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47-53.
3. Chlebowski RT, Kuller LH, Prentice RL, et al. Women’s Health Initiative Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573-587.
4. American Cancer Society. Breast Cancer Facts and Figures 2011-2012. Atlanta, GA: American Cancer Society. http://www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-and-figures-2011-2012. Accessed February 11, 2013.
5. Fisher B, Constantino J, Wickerham L, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388.
6. Vogel V, Costantino J, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295(23):2727-2741.
1. Grady D. Study finds new risks in hormone therapy. New York Times. http://www.nytimes.com/2003/06/25/us/study-finds-new-risks-in-hormone-therapy.html?pagewanted=all&src=pm. Published June 25 2003. Accessed February 11, 2013.
2. Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47-53.
3. Chlebowski RT, Kuller LH, Prentice RL, et al. Women’s Health Initiative Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573-587.
4. American Cancer Society. Breast Cancer Facts and Figures 2011-2012. Atlanta, GA: American Cancer Society. http://www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-and-figures-2011-2012. Accessed February 11, 2013.
5. Fisher B, Constantino J, Wickerham L, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388.
6. Vogel V, Costantino J, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295(23):2727-2741.
USPSTF recommends against postmenopausal vitamin D/calcium supplementation
The U.S. Preventive Services Task Force has recommended against vitamin D and calcium supplementation in healthy postmenopausal women, citing research showing that such supplementation increases the risk of kidney stones and does not protect against fractures in this population.
Specifically, the USPSTF recommended – with moderate certainty – against supplementation with doses of vitamin D at 400 IU or less, and calcium at 1,000 IU or less in noninstitutionalized postmenopausal women. The evidence with respect to higher doses is insufficient for making a recommendation, Dr. Virginia A. Moyer reported on behalf of the USPSTF.
The evidence is also insufficient to assess the balance of the benefits and harms of combined vitamin D and calcium supplementation for the primary prevention of fractures in men and in premenopausal women, according to the recommendation statement, which was published online in the Feb. 25 issue of Annals of Internal Medicine (doi:10.7326/
0003-4819-158-9-201305070-00605).
Based on previously released recommendation statements, however, the USPSTF does recommend vitamin D supplementation for the prevention of falls in community-dwelling adults aged 65 years or older who are at increased risk for falls, and recommends that women aged 65 years and older be screened for osteoporosis.
Younger women with a fracture risk that is equal to or greater than that of a 65-year-old white woman with no additional risk factors should also be screened.
The new recommendations apply to noninstitutionalized or community-dwelling asymptomatic adults without a history of fractures; they do not apply to persons with osteoporosis or vitamin D deficiency.
The USPSTF acknowledged that the health burden of fractures is substantial in the older population, with nearly half of all women over age 50 years experiencing an osteoporosis-related fracture during their lifetime, but the task force noted that the increased risk of renal stones demonstrated in participants in the Women’s Health Initiative study who were taking supplemental vitamin D and calcium was also substantial.
"One woman was diagnosed with a urinary tract stone for every 273 women who received supplementation over a 7-year follow-up period," according to the recommendation statement.
In developing the recommendations, the USPSTF commissioned two systematic reviews of the evidence from 16 available randomized controlled trials and an updated meta-analysis of vitamin D supplementation with or without calcium supplementation.
The members of the USPSTF said they had no relevant financial disclosures.
The USPSTF’s recommendation "must be interpreted in the light of ongoing disputes about the most effective method for assessing vitamin D deficiency, whether calcium and vitamin D supplements are needed by a large portion of the population, and what level of supplementation might best maximize benefits and minimize risks," according to Marion Nestle, Ph.D., and Malden C. Nesheim, Ph.D.
In particular, they cited the "conflicting perspectives" of the Institute of Medicine (IOM) and the Endocrine Society. Having determined that vitamin D and calcium deficiencies are generally not a serious problem in the United States, the IOM established average adult daily requirements for both, and has expressed concern about the possibility of adverse consequences from oversupplementation. Conversely, because vitamin D is a hormone, and supplementation must be considered a form of hormone replacement therapy, the Endocrine Society in 2011 made intake recommendations from a clinical endocrinology perspective based on the premise that vitamin D deficiencies are common among all age groups.
"The USPSTF’s recommendations can be understood as an attempt to clarify the present situation with respect to one specific outcome of supplementation. In doing so, its recommendations have a substantial advantage. They depend on hard endpoints – fractures – rather than on blood levels of 25-hydroxyvitamin D, at best an indirect measure of vitamin D adequacy. The USPSTF uses the same precautionary approach as did the IOM. In the absence of compelling evidence for benefit, taking supplements is not worth any risk, however small," they wrote.
Dr. Nestle and Dr. Nesheim noted that the USPSTF plans to publish further recommendations on the roles of vitamin D, and they urged the task force to "keep in mind the value of making a single recommendation ... that will encompass all potential benefits and risks" to avoid the confusion of multiple recommendations.
"While we wait for the results of further research, the USPSTF’s cautious, evidence-based advice should encourage clinicians to think carefully before advising calcium and vitamin D supplementation for healthy individuals," they concluded.
Dr. Nestle of New York University and Dr. Nesheim of Cornell University, Ithaca, N.Y., wrote their comments in an editorial responding to the USPSTF’s recommendations. Dr. Nestle disclosed some speakers fees and royalties from published books unrelated to this editorial and Dr. Nesheim disclosed royalties from a book unrelated to this editorial.
The USPSTF’s recommendation "must be interpreted in the light of ongoing disputes about the most effective method for assessing vitamin D deficiency, whether calcium and vitamin D supplements are needed by a large portion of the population, and what level of supplementation might best maximize benefits and minimize risks," according to Marion Nestle, Ph.D., and Malden C. Nesheim, Ph.D.
In particular, they cited the "conflicting perspectives" of the Institute of Medicine (IOM) and the Endocrine Society. Having determined that vitamin D and calcium deficiencies are generally not a serious problem in the United States, the IOM established average adult daily requirements for both, and has expressed concern about the possibility of adverse consequences from oversupplementation. Conversely, because vitamin D is a hormone, and supplementation must be considered a form of hormone replacement therapy, the Endocrine Society in 2011 made intake recommendations from a clinical endocrinology perspective based on the premise that vitamin D deficiencies are common among all age groups.
"The USPSTF’s recommendations can be understood as an attempt to clarify the present situation with respect to one specific outcome of supplementation. In doing so, its recommendations have a substantial advantage. They depend on hard endpoints – fractures – rather than on blood levels of 25-hydroxyvitamin D, at best an indirect measure of vitamin D adequacy. The USPSTF uses the same precautionary approach as did the IOM. In the absence of compelling evidence for benefit, taking supplements is not worth any risk, however small," they wrote.
Dr. Nestle and Dr. Nesheim noted that the USPSTF plans to publish further recommendations on the roles of vitamin D, and they urged the task force to "keep in mind the value of making a single recommendation ... that will encompass all potential benefits and risks" to avoid the confusion of multiple recommendations.
"While we wait for the results of further research, the USPSTF’s cautious, evidence-based advice should encourage clinicians to think carefully before advising calcium and vitamin D supplementation for healthy individuals," they concluded.
Dr. Nestle of New York University and Dr. Nesheim of Cornell University, Ithaca, N.Y., wrote their comments in an editorial responding to the USPSTF’s recommendations. Dr. Nestle disclosed some speakers fees and royalties from published books unrelated to this editorial and Dr. Nesheim disclosed royalties from a book unrelated to this editorial.
The USPSTF’s recommendation "must be interpreted in the light of ongoing disputes about the most effective method for assessing vitamin D deficiency, whether calcium and vitamin D supplements are needed by a large portion of the population, and what level of supplementation might best maximize benefits and minimize risks," according to Marion Nestle, Ph.D., and Malden C. Nesheim, Ph.D.
In particular, they cited the "conflicting perspectives" of the Institute of Medicine (IOM) and the Endocrine Society. Having determined that vitamin D and calcium deficiencies are generally not a serious problem in the United States, the IOM established average adult daily requirements for both, and has expressed concern about the possibility of adverse consequences from oversupplementation. Conversely, because vitamin D is a hormone, and supplementation must be considered a form of hormone replacement therapy, the Endocrine Society in 2011 made intake recommendations from a clinical endocrinology perspective based on the premise that vitamin D deficiencies are common among all age groups.
"The USPSTF’s recommendations can be understood as an attempt to clarify the present situation with respect to one specific outcome of supplementation. In doing so, its recommendations have a substantial advantage. They depend on hard endpoints – fractures – rather than on blood levels of 25-hydroxyvitamin D, at best an indirect measure of vitamin D adequacy. The USPSTF uses the same precautionary approach as did the IOM. In the absence of compelling evidence for benefit, taking supplements is not worth any risk, however small," they wrote.
Dr. Nestle and Dr. Nesheim noted that the USPSTF plans to publish further recommendations on the roles of vitamin D, and they urged the task force to "keep in mind the value of making a single recommendation ... that will encompass all potential benefits and risks" to avoid the confusion of multiple recommendations.
"While we wait for the results of further research, the USPSTF’s cautious, evidence-based advice should encourage clinicians to think carefully before advising calcium and vitamin D supplementation for healthy individuals," they concluded.
Dr. Nestle of New York University and Dr. Nesheim of Cornell University, Ithaca, N.Y., wrote their comments in an editorial responding to the USPSTF’s recommendations. Dr. Nestle disclosed some speakers fees and royalties from published books unrelated to this editorial and Dr. Nesheim disclosed royalties from a book unrelated to this editorial.
The U.S. Preventive Services Task Force has recommended against vitamin D and calcium supplementation in healthy postmenopausal women, citing research showing that such supplementation increases the risk of kidney stones and does not protect against fractures in this population.
Specifically, the USPSTF recommended – with moderate certainty – against supplementation with doses of vitamin D at 400 IU or less, and calcium at 1,000 IU or less in noninstitutionalized postmenopausal women. The evidence with respect to higher doses is insufficient for making a recommendation, Dr. Virginia A. Moyer reported on behalf of the USPSTF.
The evidence is also insufficient to assess the balance of the benefits and harms of combined vitamin D and calcium supplementation for the primary prevention of fractures in men and in premenopausal women, according to the recommendation statement, which was published online in the Feb. 25 issue of Annals of Internal Medicine (doi:10.7326/
0003-4819-158-9-201305070-00605).
Based on previously released recommendation statements, however, the USPSTF does recommend vitamin D supplementation for the prevention of falls in community-dwelling adults aged 65 years or older who are at increased risk for falls, and recommends that women aged 65 years and older be screened for osteoporosis.
Younger women with a fracture risk that is equal to or greater than that of a 65-year-old white woman with no additional risk factors should also be screened.
The new recommendations apply to noninstitutionalized or community-dwelling asymptomatic adults without a history of fractures; they do not apply to persons with osteoporosis or vitamin D deficiency.
The USPSTF acknowledged that the health burden of fractures is substantial in the older population, with nearly half of all women over age 50 years experiencing an osteoporosis-related fracture during their lifetime, but the task force noted that the increased risk of renal stones demonstrated in participants in the Women’s Health Initiative study who were taking supplemental vitamin D and calcium was also substantial.
"One woman was diagnosed with a urinary tract stone for every 273 women who received supplementation over a 7-year follow-up period," according to the recommendation statement.
In developing the recommendations, the USPSTF commissioned two systematic reviews of the evidence from 16 available randomized controlled trials and an updated meta-analysis of vitamin D supplementation with or without calcium supplementation.
The members of the USPSTF said they had no relevant financial disclosures.
The U.S. Preventive Services Task Force has recommended against vitamin D and calcium supplementation in healthy postmenopausal women, citing research showing that such supplementation increases the risk of kidney stones and does not protect against fractures in this population.
Specifically, the USPSTF recommended – with moderate certainty – against supplementation with doses of vitamin D at 400 IU or less, and calcium at 1,000 IU or less in noninstitutionalized postmenopausal women. The evidence with respect to higher doses is insufficient for making a recommendation, Dr. Virginia A. Moyer reported on behalf of the USPSTF.
The evidence is also insufficient to assess the balance of the benefits and harms of combined vitamin D and calcium supplementation for the primary prevention of fractures in men and in premenopausal women, according to the recommendation statement, which was published online in the Feb. 25 issue of Annals of Internal Medicine (doi:10.7326/
0003-4819-158-9-201305070-00605).
Based on previously released recommendation statements, however, the USPSTF does recommend vitamin D supplementation for the prevention of falls in community-dwelling adults aged 65 years or older who are at increased risk for falls, and recommends that women aged 65 years and older be screened for osteoporosis.
Younger women with a fracture risk that is equal to or greater than that of a 65-year-old white woman with no additional risk factors should also be screened.
The new recommendations apply to noninstitutionalized or community-dwelling asymptomatic adults without a history of fractures; they do not apply to persons with osteoporosis or vitamin D deficiency.
The USPSTF acknowledged that the health burden of fractures is substantial in the older population, with nearly half of all women over age 50 years experiencing an osteoporosis-related fracture during their lifetime, but the task force noted that the increased risk of renal stones demonstrated in participants in the Women’s Health Initiative study who were taking supplemental vitamin D and calcium was also substantial.
"One woman was diagnosed with a urinary tract stone for every 273 women who received supplementation over a 7-year follow-up period," according to the recommendation statement.
In developing the recommendations, the USPSTF commissioned two systematic reviews of the evidence from 16 available randomized controlled trials and an updated meta-analysis of vitamin D supplementation with or without calcium supplementation.
The members of the USPSTF said they had no relevant financial disclosures.
FROM ANNALS OF INTERNAL MEDICINE
STOP performing DXA scans in healthy, perimenopausal women
START counseling all women on lifestyle interventions to avoid fractures
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.
FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.

FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.

FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.