User login
2018 Update on bone health
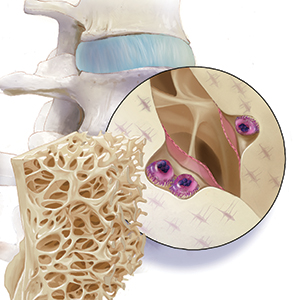
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As ObGyns, we are the first-line health care providers for our menopausal patients in terms of identifying, preventing, and initiating treatment for women at risk for fragility fractures. Osteoporosis is probably the most important risk factor for bone health, although sarcopenia, frailty, poor eyesight, and falls also play a significant role in bone health and fragility fracture.
In 2005, more than 2 million incident fractures were reported in the United States, with a total cost of $17 billion.1 By 2025, annual fractures and costs are expected to rise by almost 50%. People who are 65 to 74 years of age will likely experience the largest increase in fracture—greater than 87%.1
Findings from the Women’s Health Initiative study showed that the number of women who had a clinical fracture in 1 year exceeded all the cases of myocardial infarction, stroke, and breast cancer combined.2 Furthermore, the morbidity and mortality rates for fractures are staggering. Thirty percent of women with a hip fracture will be dead within 1 year.3 So, although many patients fear developing breast cancer, and cardiovascular disease remains the number 1 cause of death, the impact of maintaining and protecting bone health cannot be emphasized enough.

_
WHI incidental findings: Hormone-treated menopausal women had decreased hip fracture rate
Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938.
Manson and colleagues examined the total and cause-specific cumulative mortality of the 2 Women’s Health Initiative (WHI) hormone therapy trials. This was an observational follow-up of US multiethnic postmenopausal women aged 50 to 79 years (mean age at baseline, 63.4 years) enrolled in 2 randomized clinical trials between 1993 and 1998 and followed up through December 31, 2014. A total of 27,347 women were randomly assigned to treatment.
Treatment groups
Depending on the presence or absence of a uterus, women received conjugated equine estrogens (CEE, 0.625 mg/d) plus medroxyprogesterone acetate (MPA, 2.5 mg/d) (n = 8,506) or placebo (n = 8,102) for a median of 5.6 years or CEE alone (n = 5,310) versus placebo (n = 5,429) for a median of 7.2 years. All-cause mortality (the primary outcome) and cause-specific mortality (cardiovascular disease mortality, cancer mortality, and other major causes of mortality) were analyzed in the 2 trials pooled and in each trial individually.
All-cause and cause-specific mortality findings
Mortality follow-up was available for more than 98% of participants. During the cumulative 18-year follow-up, 7,489 deaths occurred. In the overall pooled cohort, all-cause mortality in the hormone therapy group was 27.1% compared with 27.6% in the placebo group (hazard ratio [HR], 0.99 [95% confidence interval (CI), 0.94–1.03]). In the CEE plus MPA group, the HR was 1.02 (95% CI, 0.96–1.08). For those in the CEE-alone group, the HR was 0.94 (95% CI, 0.88–1.01).
In the pooled cohort for cardiovascular mortality, the HR was 1.00 (95% CI, 0.92–1.08 [8.9% with hormone therapy vs 9.0% with placebo]). For total cancer mortality, the HR was 1.03 (95% CI, 0.95–1.12 [8.2% with hormone therapy vs 8.0% with placebo]). For other causes, the HR was 0.95 (95% CI, 0.88–1.02 [10.0% with hormone therapy vs 10.7% with placebo]). Results did not differ significantly between trials.
Key takeaway
The study authors concluded that among postmenopausal women, hormone therapy with CEE plus MPA for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years.
Postmenopausal hormone therapy is arguably the most effective “bone drug” available. While all other antiresorptive agents show hip fracture efficacy only in subgroup analyses of the highest-risk patients (women with established osteoporosis, who often already have pre-existing vertebral fractures), the hormone-treated women in the WHI—who were not chosen for having low bone mass (in fact, dual-energy x-ray absorptiometry [DXA] scores were not even recorded)—still had a statistically significant decrease in hip fracture as an adverse event when compared with placebo-treated women. Increasing data on the long-term safety of hormone therapy in menopausal patients will perhaps encourage its greater use from a bone health perspective.
Continue to: Appropriate to defer DXA testing to age 65...
Appropriate to defer DXA testing to age 65 when baseline FRAX score is below treatment level
Gourlay ML, Overman RA, Fine JP, et al; Women’s Health Initiative Investigators. Time to clinically relevant fracture risk scores in postmenopausal women. Am J Med. 2017;130:862.e15-862.e23.
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225-233.
Many clinicians used to (and still do) order bone mineral density (BMD) testing at 23-month intervals because that was what insurance would allow. Gourlay and colleagues previously published a study on BMD testing intervals and the time it takes to develop osteoporosis. I covered that information in previous Updates.4,5
To recap, Gourlay and colleagues studied 4,957 women, 67 years of age or older, with normal BMD or osteopenia and with no history of hip or clinical vertebral fracture or of treatment for osteoporosis; the women were followed prospectively for up to 15 years. The estimated time for 10% of women to make the transition to osteoporosis was 16.8 years for those with normal BMD, 4.7 years for those with moderate osteopenia, and 1.1 years for women with advanced osteopenia.
Today, FRAX is recommended to assess need for treatment
Older treatment recommendations involved determining various osteopenic BMD levels and the presence or absence of certain risk factors. More recently, the National Osteoporosis Foundation and many medical societies, including the American College of Obstetricians and Gynecologists, have recommended using the FRAX fracture prediction algorithm (available at https://www.sheffield.ac.uk/FRAX/) instead of T-scores to consider initiating pharmacotherapy.
The FRAX calculation tool uses information such as the country where the patient lives, age, sex, height, weight, history of previous fracture, parental fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, alcohol use of 3 or more units per day, and, if available, BMD determination at the femoral neck. It then yields the 10-year absolute risk of hip fracture and any major osteoporotic fracture for that individual or, more precisely, for an individual like that.
In the United States, accepted levels for cost-effective pharmacotherapy are a 10-year absolute risk of hip fracture of 3% or major osteoporotic fracture of 20%.
Continue to: Age also is a key factor in fracture risk assessment
Age also is a key factor in fracture risk assessment
Gourlay and colleagues more recently conducted a retrospective analysis of new occurrence of treatment-level fracture risk scores in postmenopausal women (50 years of age and older) before they received pharmacologic treatment and before they experienced a first hip or clinical vertebral fracture.
In 54,280 postmenopausal women aged 50 to 64 without a BMD test, the time for 10% to develop a treatment-level FRAX score could not be estimated accurately because of the rarity of treatment-level scores. In 6,096 women who had FRAX scores calculated with their BMD score, the estimated time to treatment-level FRAX was 7.6 years for those 65 to 69 and 5.1 years for 75 to 79 year olds. Furthermore, of 17,967 women aged 50 to 64 with a screening-level FRAX at baseline, only 100 (0.6%) experienced a hip or clinical vertebral fracture by age 65.
The investigators concluded that, “Postmenopausal women with sub-threshold fracture risk scores at baseline were unlikely to develop a treatment-level FRAX score between ages 50 and 64 years. After age 65, the increased incidence of treatment-level fracture risk scores, osteoporosis, and major osteoporotic fracture supports more frequent consideration of FRAX and bone mineral density testing.”
Many health care providers begin BMD testing early in menopause. Bone mass results may motivate patients to initiate healthy lifestyle choices, such as adequate dietary calcium, vitamin D supplementation, exercise, moderate alcohol use, smoking cessation, and fall prevention strategies. However, providers and their patients should be aware that if the fracture risk is beneath the threshold score at baseline, the risk of experiencing an osteoporotic fracture prior to age 65 is extremely low, and this should be taken into account before prescribing pharmacotherapy. Furthermore, as stated, FRAX can be performed without a DXA score. When the result is beneath a treatment level in a woman under 65, DXA testing may be deferred until age 65.
Continue to: USPSTF offers updated recommendations for osteoporosis screening
USPSTF offers updated recommendations for osteoporosis screening
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:2521-2531.
The 2018 updated osteoporosis screening recommendations from the United States Preventative Services Task Force (USPSTF) may seem contradictory to the conclusions of Gourlay and colleagues discussed above. They are not.
The USPSTF authors point out that by 2020, about 12.3 million US individuals older than 50 years are expected to have osteoporosis. Osteoporotic fractures (especially hip fractures) are associated with limitations in ambulation, chronic pain and disability, loss of independence, and decreased quality of life. In fact, 21% to 30% of people who sustain a hip fracture die within 1 year. As the US population continues to age, the potential preventable burden will likely increase.
_
Evidence on bone measurement tests, risk assessment tools, and drug therapy efficacy
The USPSTF conducted an evidence review on screening for and treatment of osteoporotic fractures in women as well as risk assessment tools. The task force found the evidence convincing that bone measurement tests are accurate for detecting osteoporosis and predicting osteoporotic fractures. In addition, there is adequate evidence that clinical risk assessment tools are moderately accurate in identifying risk of osteoporosis and osteoporotic fractures. Furthermore, there is convincing evidence that drug therapies reduce subsequent fracture rates in postmenopausal women.
The USPSTF recommends the following:
- For women aged 65 and older, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
- For women younger than 65 who are at increased risk for osteoporosis based on formal clinical risk assessment tools, screen for osteoporosis with bone measurement testing to prevent osteoporotic fractures.
We all agree that women older than 65 years of age should be screened with DXA measurements of bone mass. The USPSTF says that in women under 65, a fracture assessment tool like FRAX, which does not require bone density testing to yield an individual’s absolute 10-year fracture risk, should be used to determine if bone mass measurement by DXA is, in fact, warranted. This recommendation is further supported by the article by Gourlay and colleagues, in which women aged 50 to 64 with subthreshold FRAX scores had a very low risk of fracture prior to age 65.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
- Cauley JA, Wampler NS, Barnhart JM, et al; Women’s Health Initiative Observational Study. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women’s Health Initiative Observational Study. Osteoporos Int. 2008;19:1717-1723.
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579.
- Goldstein SR. Update on osteoporosis. OBG Manag. 2012;24:16-21.
- Goldstein SR. 2017 update on bone health. OBG Manag. 2017;29-32, 48.
Healthier lifestyle in midlife women reduces subclinical carotid atherosclerosis
Women who have a healthier lifestyle during the menopausal transition could significantly reduce their risk of cardiovascular disease, new research suggests.
Because women experience a steeper increase in CVD risk during and after the menopausal transition, researchers analyzed data from the Study of Women’s Health Across the Nation (SWAN), a prospective longitudinal cohort study of 1,143 women aged 42-52 years. The report is in JAHA: Journal of the American Heart Association.
The analysis revealed that women with the highest average Healthy Lifestyle Score – a composite score of dietary quality, levels of physical activity, and smoking – over 10 years of follow-up had a 0.024-mm smaller common carotid artery intima-media thickness and 0.16-mm smaller adventitial diameter, compared to those with the lowest average score. This was after adjustment for confounders and physiological risk factors such as ethnicity, age, menopausal status, body mass index, and cholesterol levels.
“Smoking, unhealthy diet, and lack of physical activity are three well-known modifiable behavioral risk factors for CVD,” wrote Dongqing Wang of the University of Michigan, Ann Arbor, and his coauthors. “Even after adjusting for the lifestyle-related physiological risk factors, the adherence to a healthy lifestyle composed of abstinence from smoking, healthy diet, and regular engagement in physical activity is inversely associated with atherosclerosis in midlife women.”
Women with higher average health lifestyle score also had lower levels of carotid plaque after adjustment for confounding factors, but this was no longer significant after adjustment for physiological risk factors.
The authors analyzed the three components of the healthy lifestyle score separately, and found that not smoking was strongly and significantly associated with lower scores for all three measures of subclinical atherosclerosis. Women who never smoked across the duration of the study had a 49% lower odds of having a high carotid plaque index compared with women who smoked at some point during the follow-up period.
The analysis showed an inverse association between average Alternate Healthy Eating Index score – a measure of diet quality – and smaller common carotid artery adventitial diameter, although after adjustment for BMI this association was no longer statistically significant. Likewise, the association between dietary quality and intima-media thickness was only marginally significant and lost that significance after adjustment for BMI.
Long-term physical activity was only marginally significantly associated with common carotid artery intima-media thickness, but this was not significant after adjustment for physiological risk factors. No association was found between physical activity and common carotid artery adventitial diameter or carotid plaque.
The authors said that 1.7% of the study population managed to stay in the top category for all three components of healthy lifestyle at all three follow-up time points in the study.
“The low prevalence of a healthy lifestyle in midlife women highlights the potential for lifestyle interventions aimed at this vulnerable population,” they wrote.
In particular, they highlighted abstinence from smoking as having the strongest impact on all three measures of subclinical atherosclerosis, which is known to affect women more than men. However, the outcomes from diet and physical activity weren’t so strong: The authors suggested that BMI could partly mediate the effects of healthier diet and greater levels of physical activity.
One strength of the study was its ethnically diverse population, which included African American, Chinese, and Hispanic women in addition to non-Hispanic white women. However, the study was not powered to examine the impacts ethnicity may have had on outcomes, the researchers wrote.
The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
SOURCE: Wang D et al. JAHA 2018 Nov. 28.
Women who have a healthier lifestyle during the menopausal transition could significantly reduce their risk of cardiovascular disease, new research suggests.
Because women experience a steeper increase in CVD risk during and after the menopausal transition, researchers analyzed data from the Study of Women’s Health Across the Nation (SWAN), a prospective longitudinal cohort study of 1,143 women aged 42-52 years. The report is in JAHA: Journal of the American Heart Association.
The analysis revealed that women with the highest average Healthy Lifestyle Score – a composite score of dietary quality, levels of physical activity, and smoking – over 10 years of follow-up had a 0.024-mm smaller common carotid artery intima-media thickness and 0.16-mm smaller adventitial diameter, compared to those with the lowest average score. This was after adjustment for confounders and physiological risk factors such as ethnicity, age, menopausal status, body mass index, and cholesterol levels.
“Smoking, unhealthy diet, and lack of physical activity are three well-known modifiable behavioral risk factors for CVD,” wrote Dongqing Wang of the University of Michigan, Ann Arbor, and his coauthors. “Even after adjusting for the lifestyle-related physiological risk factors, the adherence to a healthy lifestyle composed of abstinence from smoking, healthy diet, and regular engagement in physical activity is inversely associated with atherosclerosis in midlife women.”
Women with higher average health lifestyle score also had lower levels of carotid plaque after adjustment for confounding factors, but this was no longer significant after adjustment for physiological risk factors.
The authors analyzed the three components of the healthy lifestyle score separately, and found that not smoking was strongly and significantly associated with lower scores for all three measures of subclinical atherosclerosis. Women who never smoked across the duration of the study had a 49% lower odds of having a high carotid plaque index compared with women who smoked at some point during the follow-up period.
The analysis showed an inverse association between average Alternate Healthy Eating Index score – a measure of diet quality – and smaller common carotid artery adventitial diameter, although after adjustment for BMI this association was no longer statistically significant. Likewise, the association between dietary quality and intima-media thickness was only marginally significant and lost that significance after adjustment for BMI.
Long-term physical activity was only marginally significantly associated with common carotid artery intima-media thickness, but this was not significant after adjustment for physiological risk factors. No association was found between physical activity and common carotid artery adventitial diameter or carotid plaque.
The authors said that 1.7% of the study population managed to stay in the top category for all three components of healthy lifestyle at all three follow-up time points in the study.
“The low prevalence of a healthy lifestyle in midlife women highlights the potential for lifestyle interventions aimed at this vulnerable population,” they wrote.
In particular, they highlighted abstinence from smoking as having the strongest impact on all three measures of subclinical atherosclerosis, which is known to affect women more than men. However, the outcomes from diet and physical activity weren’t so strong: The authors suggested that BMI could partly mediate the effects of healthier diet and greater levels of physical activity.
One strength of the study was its ethnically diverse population, which included African American, Chinese, and Hispanic women in addition to non-Hispanic white women. However, the study was not powered to examine the impacts ethnicity may have had on outcomes, the researchers wrote.
The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
SOURCE: Wang D et al. JAHA 2018 Nov. 28.
Women who have a healthier lifestyle during the menopausal transition could significantly reduce their risk of cardiovascular disease, new research suggests.
Because women experience a steeper increase in CVD risk during and after the menopausal transition, researchers analyzed data from the Study of Women’s Health Across the Nation (SWAN), a prospective longitudinal cohort study of 1,143 women aged 42-52 years. The report is in JAHA: Journal of the American Heart Association.
The analysis revealed that women with the highest average Healthy Lifestyle Score – a composite score of dietary quality, levels of physical activity, and smoking – over 10 years of follow-up had a 0.024-mm smaller common carotid artery intima-media thickness and 0.16-mm smaller adventitial diameter, compared to those with the lowest average score. This was after adjustment for confounders and physiological risk factors such as ethnicity, age, menopausal status, body mass index, and cholesterol levels.
“Smoking, unhealthy diet, and lack of physical activity are three well-known modifiable behavioral risk factors for CVD,” wrote Dongqing Wang of the University of Michigan, Ann Arbor, and his coauthors. “Even after adjusting for the lifestyle-related physiological risk factors, the adherence to a healthy lifestyle composed of abstinence from smoking, healthy diet, and regular engagement in physical activity is inversely associated with atherosclerosis in midlife women.”
Women with higher average health lifestyle score also had lower levels of carotid plaque after adjustment for confounding factors, but this was no longer significant after adjustment for physiological risk factors.
The authors analyzed the three components of the healthy lifestyle score separately, and found that not smoking was strongly and significantly associated with lower scores for all three measures of subclinical atherosclerosis. Women who never smoked across the duration of the study had a 49% lower odds of having a high carotid plaque index compared with women who smoked at some point during the follow-up period.
The analysis showed an inverse association between average Alternate Healthy Eating Index score – a measure of diet quality – and smaller common carotid artery adventitial diameter, although after adjustment for BMI this association was no longer statistically significant. Likewise, the association between dietary quality and intima-media thickness was only marginally significant and lost that significance after adjustment for BMI.
Long-term physical activity was only marginally significantly associated with common carotid artery intima-media thickness, but this was not significant after adjustment for physiological risk factors. No association was found between physical activity and common carotid artery adventitial diameter or carotid plaque.
The authors said that 1.7% of the study population managed to stay in the top category for all three components of healthy lifestyle at all three follow-up time points in the study.
“The low prevalence of a healthy lifestyle in midlife women highlights the potential for lifestyle interventions aimed at this vulnerable population,” they wrote.
In particular, they highlighted abstinence from smoking as having the strongest impact on all three measures of subclinical atherosclerosis, which is known to affect women more than men. However, the outcomes from diet and physical activity weren’t so strong: The authors suggested that BMI could partly mediate the effects of healthier diet and greater levels of physical activity.
One strength of the study was its ethnically diverse population, which included African American, Chinese, and Hispanic women in addition to non-Hispanic white women. However, the study was not powered to examine the impacts ethnicity may have had on outcomes, the researchers wrote.
The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
SOURCE: Wang D et al. JAHA 2018 Nov. 28.
FROM JAHA: JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: .
Major finding: Following a healthier diet and not smoking were significantly linked with lower subclinical carotid atherosclerosis in menopausal women.
Study details: A prospective, longitudinal cohort study of 1,143 women.
Disclosures: The Study of Women’s Health Across the Nation is supported by the National Institutes of Health. No conflicts of interest were declared.
Source: Wang D et al. JAHA 2018 Nov. 28.
Intimate partner violence and PTSD increase menopausal symptom risk
Intimate partner violence or sexual assault may have a significant effect on menopausal symptoms in women, according to a cohort study published in JAMA Internal Medicine.
Researchers analyzed data from 2,016 women aged 40 years or older who were enrolled in the observational Reproductive Risks of Incontinence Study; 40% were non-Latina white, 21% were black, 20% were Latina or Hispanic, and 19% were Asian. Of this cohort, 21% had experienced emotional intimate partner violence (IPV) – 64 (3.2%) in the past 12 months – 16% had experienced physical IPV, 14% had experienced both, and 19% reported sexual assault. More than one in five women (23%) met the criteria for clinically significant PTSD.
Women who had experienced emotional domestic abuse were 36% more likely to report difficulty sleeping, 50% more like to experience night sweats, and 60% more likely to experience pain with intercourse, compared with women who had not experienced any abuse.
Physical abuse was associated with 33% higher odds of night sweats, and sexual assault was associated with 41% higher odds of vaginal dryness, 42% higher odds of vaginal irritation, and 44% higher odds of pain with intercourse.
Women with clinically significant PTSD symptoms were significantly more likely to experience all the symptoms of menopause, including twofold higher odds of pain with intercourse and threefold higher odds of difficulty sleeping. When authors accounted for the effect of PTSD symptoms in the cohort, they found that only the association between emotional abuse and night sweats or pain with intercourse, and between sexual assault and vaginal dryness, remained independently significant.
Carolyn J. Gibson, PhD, MPH, of the San Francisco Veterans Affairs Health Care System, and coauthors said that the biological and hormonal changes that underpin menopausal symptoms, as well as health risk behaviors, cardiometabolic risk factors, and other chronic health conditions associated with menopause, all are impacted by trauma and its psychological effects.
“Chronic hyperarousal and hypervigilance, common in individuals who have experienced trauma and characteristic symptoms of PTSD, may affect sleep and symptom sensitivity,” they wrote.
The reverse is also true; that the symptoms of menopause can impact the symptoms of PTSD by affecting a woman’s sense of self-efficacy, interpersonal engagements, and heighten the stress associated with this period of transition.
“The clinical management of menopause symptoms may also be enhanced by trauma-informed care, including recognition of challenges that may impair efforts to address menopause-related concerns among women affected by trauma,” the authors wrote.
Clinicians also could help by providing education about the link between trauma and health, providing their patients with a safe and supportive treatment environment, and facilitating referrals for psychological or trauma-specific services when needed, they said.
The research was supported by the San Francisco Veterans Affairs Medical Center and Kaiser Permanente Northern California, and funded by the University of California San Francisco–Kaiser Permanente Grants Program for Delivery Science, the Office of Research on Women’s Health Specialized Center of Research, and grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
SOURCE: Gibson C et al. JAMA Intern Med. 2018 Nov 19. doi: 10.1001/jamainternmed.2018.5233.
An estimated 33% of women in the United States have been sexually assaulted, and an estimated 25% have experienced IPV, so be aware of how common this “wicked problem” is, the way it impacts health, and what role you can play in educating and helping patients by connecting them to available resources.
But that is not enough. Consider measures such as training yourself and staff in how to assess for IPV and sexual assault and use of EHR to integrate IPV assessment into routine clinical care, as well as developing protocols to be followed when a patient discloses IPV or sexual assault. A multidisciplinary approach also can help, including victim service advocates and behavioral health clinicians to provide care and support.
State requirements for reporting partner and sexual violence differ, so be aware of your state laws.
A strength of this study is that it included emotional as well as physical IPV, which often is left out although it has serious impacts.
Rebecca C. Thurston, PhD, is from the department of psychiatry at the University of Pittsburgh, and Elizabeth Miller, MD, PhD, is from the division of adolescent and young adult medicine at the UPMC Children’s Hospital of Pittsburgh. These comments were taken from an accompanying editorial (JAMA Intern Med. 2018 Nov 19. doi: 10.1001/jamainternmed.2018.5242). Dr. Thurston declared research support from the National Institutes of Health and consultancies for Pfizer, Procter & Gamble, and MAS Innovations.
An estimated 33% of women in the United States have been sexually assaulted, and an estimated 25% have experienced IPV, so be aware of how common this “wicked problem” is, the way it impacts health, and what role you can play in educating and helping patients by connecting them to available resources.
But that is not enough. Consider measures such as training yourself and staff in how to assess for IPV and sexual assault and use of EHR to integrate IPV assessment into routine clinical care, as well as developing protocols to be followed when a patient discloses IPV or sexual assault. A multidisciplinary approach also can help, including victim service advocates and behavioral health clinicians to provide care and support.
State requirements for reporting partner and sexual violence differ, so be aware of your state laws.
A strength of this study is that it included emotional as well as physical IPV, which often is left out although it has serious impacts.
Rebecca C. Thurston, PhD, is from the department of psychiatry at the University of Pittsburgh, and Elizabeth Miller, MD, PhD, is from the division of adolescent and young adult medicine at the UPMC Children’s Hospital of Pittsburgh. These comments were taken from an accompanying editorial (JAMA Intern Med. 2018 Nov 19. doi: 10.1001/jamainternmed.2018.5242). Dr. Thurston declared research support from the National Institutes of Health and consultancies for Pfizer, Procter & Gamble, and MAS Innovations.
An estimated 33% of women in the United States have been sexually assaulted, and an estimated 25% have experienced IPV, so be aware of how common this “wicked problem” is, the way it impacts health, and what role you can play in educating and helping patients by connecting them to available resources.
But that is not enough. Consider measures such as training yourself and staff in how to assess for IPV and sexual assault and use of EHR to integrate IPV assessment into routine clinical care, as well as developing protocols to be followed when a patient discloses IPV or sexual assault. A multidisciplinary approach also can help, including victim service advocates and behavioral health clinicians to provide care and support.
State requirements for reporting partner and sexual violence differ, so be aware of your state laws.
A strength of this study is that it included emotional as well as physical IPV, which often is left out although it has serious impacts.
Rebecca C. Thurston, PhD, is from the department of psychiatry at the University of Pittsburgh, and Elizabeth Miller, MD, PhD, is from the division of adolescent and young adult medicine at the UPMC Children’s Hospital of Pittsburgh. These comments were taken from an accompanying editorial (JAMA Intern Med. 2018 Nov 19. doi: 10.1001/jamainternmed.2018.5242). Dr. Thurston declared research support from the National Institutes of Health and consultancies for Pfizer, Procter & Gamble, and MAS Innovations.
Intimate partner violence or sexual assault may have a significant effect on menopausal symptoms in women, according to a cohort study published in JAMA Internal Medicine.
Researchers analyzed data from 2,016 women aged 40 years or older who were enrolled in the observational Reproductive Risks of Incontinence Study; 40% were non-Latina white, 21% were black, 20% were Latina or Hispanic, and 19% were Asian. Of this cohort, 21% had experienced emotional intimate partner violence (IPV) – 64 (3.2%) in the past 12 months – 16% had experienced physical IPV, 14% had experienced both, and 19% reported sexual assault. More than one in five women (23%) met the criteria for clinically significant PTSD.
Women who had experienced emotional domestic abuse were 36% more likely to report difficulty sleeping, 50% more like to experience night sweats, and 60% more likely to experience pain with intercourse, compared with women who had not experienced any abuse.
Physical abuse was associated with 33% higher odds of night sweats, and sexual assault was associated with 41% higher odds of vaginal dryness, 42% higher odds of vaginal irritation, and 44% higher odds of pain with intercourse.
Women with clinically significant PTSD symptoms were significantly more likely to experience all the symptoms of menopause, including twofold higher odds of pain with intercourse and threefold higher odds of difficulty sleeping. When authors accounted for the effect of PTSD symptoms in the cohort, they found that only the association between emotional abuse and night sweats or pain with intercourse, and between sexual assault and vaginal dryness, remained independently significant.
Carolyn J. Gibson, PhD, MPH, of the San Francisco Veterans Affairs Health Care System, and coauthors said that the biological and hormonal changes that underpin menopausal symptoms, as well as health risk behaviors, cardiometabolic risk factors, and other chronic health conditions associated with menopause, all are impacted by trauma and its psychological effects.
“Chronic hyperarousal and hypervigilance, common in individuals who have experienced trauma and characteristic symptoms of PTSD, may affect sleep and symptom sensitivity,” they wrote.
The reverse is also true; that the symptoms of menopause can impact the symptoms of PTSD by affecting a woman’s sense of self-efficacy, interpersonal engagements, and heighten the stress associated with this period of transition.
“The clinical management of menopause symptoms may also be enhanced by trauma-informed care, including recognition of challenges that may impair efforts to address menopause-related concerns among women affected by trauma,” the authors wrote.
Clinicians also could help by providing education about the link between trauma and health, providing their patients with a safe and supportive treatment environment, and facilitating referrals for psychological or trauma-specific services when needed, they said.
The research was supported by the San Francisco Veterans Affairs Medical Center and Kaiser Permanente Northern California, and funded by the University of California San Francisco–Kaiser Permanente Grants Program for Delivery Science, the Office of Research on Women’s Health Specialized Center of Research, and grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
SOURCE: Gibson C et al. JAMA Intern Med. 2018 Nov 19. doi: 10.1001/jamainternmed.2018.5233.
Intimate partner violence or sexual assault may have a significant effect on menopausal symptoms in women, according to a cohort study published in JAMA Internal Medicine.
Researchers analyzed data from 2,016 women aged 40 years or older who were enrolled in the observational Reproductive Risks of Incontinence Study; 40% were non-Latina white, 21% were black, 20% were Latina or Hispanic, and 19% were Asian. Of this cohort, 21% had experienced emotional intimate partner violence (IPV) – 64 (3.2%) in the past 12 months – 16% had experienced physical IPV, 14% had experienced both, and 19% reported sexual assault. More than one in five women (23%) met the criteria for clinically significant PTSD.
Women who had experienced emotional domestic abuse were 36% more likely to report difficulty sleeping, 50% more like to experience night sweats, and 60% more likely to experience pain with intercourse, compared with women who had not experienced any abuse.
Physical abuse was associated with 33% higher odds of night sweats, and sexual assault was associated with 41% higher odds of vaginal dryness, 42% higher odds of vaginal irritation, and 44% higher odds of pain with intercourse.
Women with clinically significant PTSD symptoms were significantly more likely to experience all the symptoms of menopause, including twofold higher odds of pain with intercourse and threefold higher odds of difficulty sleeping. When authors accounted for the effect of PTSD symptoms in the cohort, they found that only the association between emotional abuse and night sweats or pain with intercourse, and between sexual assault and vaginal dryness, remained independently significant.
Carolyn J. Gibson, PhD, MPH, of the San Francisco Veterans Affairs Health Care System, and coauthors said that the biological and hormonal changes that underpin menopausal symptoms, as well as health risk behaviors, cardiometabolic risk factors, and other chronic health conditions associated with menopause, all are impacted by trauma and its psychological effects.
“Chronic hyperarousal and hypervigilance, common in individuals who have experienced trauma and characteristic symptoms of PTSD, may affect sleep and symptom sensitivity,” they wrote.
The reverse is also true; that the symptoms of menopause can impact the symptoms of PTSD by affecting a woman’s sense of self-efficacy, interpersonal engagements, and heighten the stress associated with this period of transition.
“The clinical management of menopause symptoms may also be enhanced by trauma-informed care, including recognition of challenges that may impair efforts to address menopause-related concerns among women affected by trauma,” the authors wrote.
Clinicians also could help by providing education about the link between trauma and health, providing their patients with a safe and supportive treatment environment, and facilitating referrals for psychological or trauma-specific services when needed, they said.
The research was supported by the San Francisco Veterans Affairs Medical Center and Kaiser Permanente Northern California, and funded by the University of California San Francisco–Kaiser Permanente Grants Program for Delivery Science, the Office of Research on Women’s Health Specialized Center of Research, and grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
SOURCE: Gibson C et al. JAMA Intern Med. 2018 Nov 19. doi: 10.1001/jamainternmed.2018.5233.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Intimate partner violence increases the risk of menopausal symptoms.
Major finding:
Study details: A cohort study in 2,016 women aged 40 years and older.
Disclosures: The research was supported by the San Francisco Veterans Affairs Medical Center and Kaiser Permanente Northern California, and funded by the University of California San Francisco–Kaiser Permanente Grants Program for Delivery Science, the Office of Research on Women’s Health Specialized Center of Research, and grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
Source: Gibson C et al. JAMA Intern Med. 2018 Nov 19. doi:10.1001/jamainternmed.2018.5233.
Estetrol safely limited menopause symptoms in a phase 2b study
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
FROM NAMS 2018
Key clinical point: Estetrol relieved hot flashes without adversely affecting lipid markers.
Major finding: Estetrol 15 mg reduced vasomotor symptom severity by 28%.
Study details: Randomized, double-blind, placebo-controlled phase 2b dose-finding study of 200 women.
Disclosures: Dr. Utian reported financial relationships with several pharmaceutical companies, including Mithra, the sponsor of the study.
Source: Utian WH. NAMS 2018, Friday concurrent session 1.
Low sexual desire: Appropriate use of testosterone in menopausal women
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).
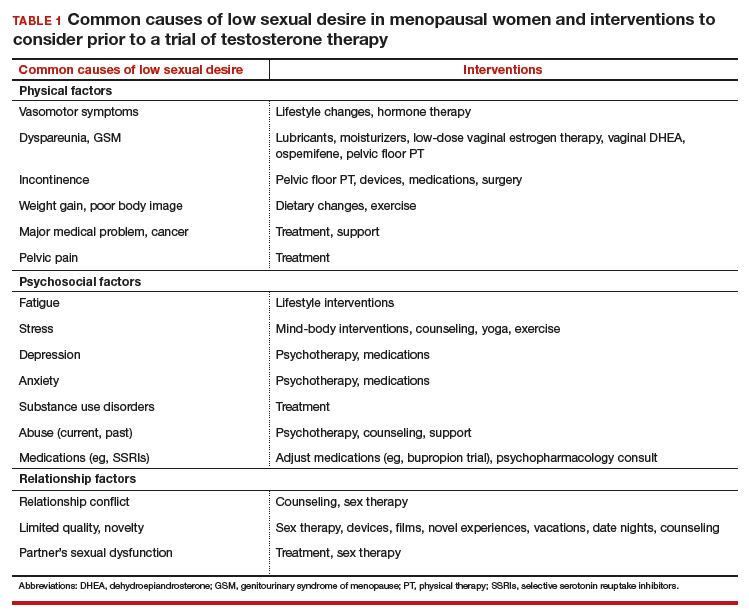
Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.
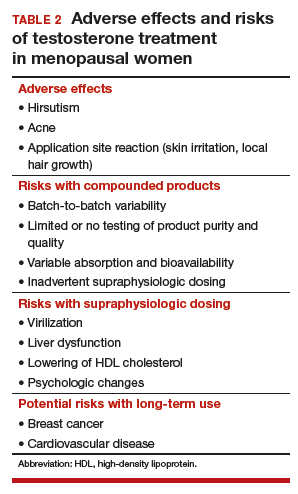
Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).

Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.

Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).

Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.

Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
FDA okays serum AMH assay to determine menopause status
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
New and promising GSM treatments, more clinical takeaways from NAMS 2018
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
No signal for CV, breast effects with bioidentical vaginal estrogen for dyspareunia
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
FROM NAMS 2018
Key clinical point: Safety data from clinical trials of a bioidentical vaginal estrogen for dyspareunia in menopausal women showed no signs of CV or breast risks.
Major finding: There were no cardiovascular events or thrombotic episodes among menopausal women with dyspareunia treated with TX-004HR.
Study details: Randomized, double-blind, placebo-controlled trial of 784 menopausal women with moderate to severe dyspareunia.
Disclosures: The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin reported financial relationships with several pharmaceutical companies, including TherapeuticsMD.
Source: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
With more mindfulness, menopausal symptoms wane
An observational Furthermore, mindfulness had the greatest positive effect on menopausal symptoms for those women with the highest self-reported stress levels.
“In this cross-sectional study, mindfulness was associated with lower menopausal symptom burden. In women with higher stress, the magnitude of association between mindfulness and menopausal symptoms appeared more robust,” said Richa Sood, MD, speaking at the annual meeting of the North American Menopause Society.
Menopausal symptoms can exist alongside many other midlife issues because women often are trying to keep many balls in the air: This age group may be facing aging parents, a household with teenagers, and work-related pressures, she noted.
Thus, menopausal symptoms can be amplified by stressors. New mood problems – or worsening of preexisting ones – can interfere with work productivity and negatively affect relationships. Life satisfaction can take a steep dive during midlife for some women, said Dr. Sood of the Mayo Clinic, Rochester, Minn.
Could mindfulness be effective for stress management in this complex landscape of physiological and lifespan changes? “Mindfulness is paying attention,” said Dr. Sood. Practitioners of mindfulness focus on purposeful attention, staying in the present moment, and avoiding judgment.
Mindfulness may work as a stress-management tool for a variety of reasons, said Dr. Sood. The technique can help retrain people with tendencies for emotional reactivity in stressful situations; additionally, the focus on the present and on observation, rather than judgment, may help avoid maladaptive rumination.
To see how mindfulness in everyday life could affect the burden of menopausal symptoms, Dr. Sood and her collaborators designed an observational, cross-sectional study of 1,744 women aged 40-64 years.
The investigators used three scales in their assessments. The first, the Menopause Rating Scale (MRS), is an 11-item scale ranging from 0 to 44 that assesses psychological, somatovegetative, and urogenital domains. The second, the Perceived Stress Scale 4 (PSS-4), is a four-item scale that is a global measure of stress over the last 4 weeks, with tallied scores in the 0-16 range. Finally, the Mindful Attention Awareness Scale (MAAS) is a 15-item scale that captures how frequently respondents are in a mindful state during their daily life, with higher scores meaning more mindfulness.
The 1,744 women were seen in a women’s health clinic over the period of one year. Participants were a mean 53.4 years old (standard deviation, 6.1 years). Almost all were white (93%), most were married (82.7%), and most also had at least a 4-year college degree (64.6%) and were employed (65.3%).
The investigators mapped each scale against each of the others, which yielded three plots. In the first, higher MAAS scores were correlated with lower MRS scores (correlation, –0.49; P less than .001), which means that more time in a mindful state was associated with less severe menopausal symptoms.
In the second plot, lower MRS scores were associated with lower PSS-4 scores (correlation, 0.55; P less than .001). The last plot mapped PSS-4 scores against MAAS scores, showing that higher MAAS scores were correlated with lower PSS-4 scores, which means that more time in a mindful state was also associated with less perceived stress (correlation, –0.53; P less than .001).
Most of these associations remained statistically significant after multivariable linear regression analysis and breaking out the subscales of the MRS. Only the association between higher MAAS scores and the somatovegetative domain of the PSS-4 lost significance (P = 0.44).
Next, Dr. Sood and her collaborators probed how higher perceived stress, as measured by higher PSS-4 scores, altered the effects that mindful activity had on menopausal symptoms (as measured by the MRS).
The effect of mindfulness became stronger in the milieu of higher perceived stress. At a relatively low PSS-4 value of 4, the MRS score dropped 1.53 points for each one-point increase in the MAAS total score. However, with a PSS-4 score of 12, the decrease in MRS was 2.27 points for each one-point increase in MAAS, and with the maximum perceived stress score of 16, the MRS fell 2.64 points for each one-point increase in the MAAS score.
These findings are set against the backdrop of previous literature linking mindfulness to positive health behaviors and outcomes, she said, noting that work looking specifically at mindfulness-based stress reduction in peri- and postmenopausal women showed a halving of symptoms and improved quality of life.
Dr. Sood said that the present study was observational only, noting that it looked only at time spent in a mindful state in an untrained cohort of women in midlife. “Trait, or dispositional, mindfulness appears to be protective against stress and symptoms in midlife women,” she commented. “More mindful women may be choosing to shift their attention to more pleasant aspects of life, rather than their symptoms.”
“If you allow me to speculate a bit,” Dr. Sood continued, “the underpinnings of psychological symptoms rest in threat-focused attention and emotional reactivity – so the mindfulness approach appears to fit very well to impact such a change.”
Mindfulness, she added, “might be a tool to impact the emotional component of the overall experience, thereby decreasing the total suffering.” However, she noted that what’s needed are studies with a more heterogeneous population, as well as ones designed to tease out causality. Still, “the current study adds to the wealth of data supporting the role of mindfulness in various settings for impacting positive change in health and behaviors.”
Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
SOURCE: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
An observational Furthermore, mindfulness had the greatest positive effect on menopausal symptoms for those women with the highest self-reported stress levels.
“In this cross-sectional study, mindfulness was associated with lower menopausal symptom burden. In women with higher stress, the magnitude of association between mindfulness and menopausal symptoms appeared more robust,” said Richa Sood, MD, speaking at the annual meeting of the North American Menopause Society.
Menopausal symptoms can exist alongside many other midlife issues because women often are trying to keep many balls in the air: This age group may be facing aging parents, a household with teenagers, and work-related pressures, she noted.
Thus, menopausal symptoms can be amplified by stressors. New mood problems – or worsening of preexisting ones – can interfere with work productivity and negatively affect relationships. Life satisfaction can take a steep dive during midlife for some women, said Dr. Sood of the Mayo Clinic, Rochester, Minn.
Could mindfulness be effective for stress management in this complex landscape of physiological and lifespan changes? “Mindfulness is paying attention,” said Dr. Sood. Practitioners of mindfulness focus on purposeful attention, staying in the present moment, and avoiding judgment.
Mindfulness may work as a stress-management tool for a variety of reasons, said Dr. Sood. The technique can help retrain people with tendencies for emotional reactivity in stressful situations; additionally, the focus on the present and on observation, rather than judgment, may help avoid maladaptive rumination.
To see how mindfulness in everyday life could affect the burden of menopausal symptoms, Dr. Sood and her collaborators designed an observational, cross-sectional study of 1,744 women aged 40-64 years.
The investigators used three scales in their assessments. The first, the Menopause Rating Scale (MRS), is an 11-item scale ranging from 0 to 44 that assesses psychological, somatovegetative, and urogenital domains. The second, the Perceived Stress Scale 4 (PSS-4), is a four-item scale that is a global measure of stress over the last 4 weeks, with tallied scores in the 0-16 range. Finally, the Mindful Attention Awareness Scale (MAAS) is a 15-item scale that captures how frequently respondents are in a mindful state during their daily life, with higher scores meaning more mindfulness.
The 1,744 women were seen in a women’s health clinic over the period of one year. Participants were a mean 53.4 years old (standard deviation, 6.1 years). Almost all were white (93%), most were married (82.7%), and most also had at least a 4-year college degree (64.6%) and were employed (65.3%).
The investigators mapped each scale against each of the others, which yielded three plots. In the first, higher MAAS scores were correlated with lower MRS scores (correlation, –0.49; P less than .001), which means that more time in a mindful state was associated with less severe menopausal symptoms.
In the second plot, lower MRS scores were associated with lower PSS-4 scores (correlation, 0.55; P less than .001). The last plot mapped PSS-4 scores against MAAS scores, showing that higher MAAS scores were correlated with lower PSS-4 scores, which means that more time in a mindful state was also associated with less perceived stress (correlation, –0.53; P less than .001).
Most of these associations remained statistically significant after multivariable linear regression analysis and breaking out the subscales of the MRS. Only the association between higher MAAS scores and the somatovegetative domain of the PSS-4 lost significance (P = 0.44).
Next, Dr. Sood and her collaborators probed how higher perceived stress, as measured by higher PSS-4 scores, altered the effects that mindful activity had on menopausal symptoms (as measured by the MRS).
The effect of mindfulness became stronger in the milieu of higher perceived stress. At a relatively low PSS-4 value of 4, the MRS score dropped 1.53 points for each one-point increase in the MAAS total score. However, with a PSS-4 score of 12, the decrease in MRS was 2.27 points for each one-point increase in MAAS, and with the maximum perceived stress score of 16, the MRS fell 2.64 points for each one-point increase in the MAAS score.
These findings are set against the backdrop of previous literature linking mindfulness to positive health behaviors and outcomes, she said, noting that work looking specifically at mindfulness-based stress reduction in peri- and postmenopausal women showed a halving of symptoms and improved quality of life.
Dr. Sood said that the present study was observational only, noting that it looked only at time spent in a mindful state in an untrained cohort of women in midlife. “Trait, or dispositional, mindfulness appears to be protective against stress and symptoms in midlife women,” she commented. “More mindful women may be choosing to shift their attention to more pleasant aspects of life, rather than their symptoms.”
“If you allow me to speculate a bit,” Dr. Sood continued, “the underpinnings of psychological symptoms rest in threat-focused attention and emotional reactivity – so the mindfulness approach appears to fit very well to impact such a change.”
Mindfulness, she added, “might be a tool to impact the emotional component of the overall experience, thereby decreasing the total suffering.” However, she noted that what’s needed are studies with a more heterogeneous population, as well as ones designed to tease out causality. Still, “the current study adds to the wealth of data supporting the role of mindfulness in various settings for impacting positive change in health and behaviors.”
Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
SOURCE: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
An observational Furthermore, mindfulness had the greatest positive effect on menopausal symptoms for those women with the highest self-reported stress levels.
“In this cross-sectional study, mindfulness was associated with lower menopausal symptom burden. In women with higher stress, the magnitude of association between mindfulness and menopausal symptoms appeared more robust,” said Richa Sood, MD, speaking at the annual meeting of the North American Menopause Society.
Menopausal symptoms can exist alongside many other midlife issues because women often are trying to keep many balls in the air: This age group may be facing aging parents, a household with teenagers, and work-related pressures, she noted.
Thus, menopausal symptoms can be amplified by stressors. New mood problems – or worsening of preexisting ones – can interfere with work productivity and negatively affect relationships. Life satisfaction can take a steep dive during midlife for some women, said Dr. Sood of the Mayo Clinic, Rochester, Minn.
Could mindfulness be effective for stress management in this complex landscape of physiological and lifespan changes? “Mindfulness is paying attention,” said Dr. Sood. Practitioners of mindfulness focus on purposeful attention, staying in the present moment, and avoiding judgment.
Mindfulness may work as a stress-management tool for a variety of reasons, said Dr. Sood. The technique can help retrain people with tendencies for emotional reactivity in stressful situations; additionally, the focus on the present and on observation, rather than judgment, may help avoid maladaptive rumination.
To see how mindfulness in everyday life could affect the burden of menopausal symptoms, Dr. Sood and her collaborators designed an observational, cross-sectional study of 1,744 women aged 40-64 years.
The investigators used three scales in their assessments. The first, the Menopause Rating Scale (MRS), is an 11-item scale ranging from 0 to 44 that assesses psychological, somatovegetative, and urogenital domains. The second, the Perceived Stress Scale 4 (PSS-4), is a four-item scale that is a global measure of stress over the last 4 weeks, with tallied scores in the 0-16 range. Finally, the Mindful Attention Awareness Scale (MAAS) is a 15-item scale that captures how frequently respondents are in a mindful state during their daily life, with higher scores meaning more mindfulness.
The 1,744 women were seen in a women’s health clinic over the period of one year. Participants were a mean 53.4 years old (standard deviation, 6.1 years). Almost all were white (93%), most were married (82.7%), and most also had at least a 4-year college degree (64.6%) and were employed (65.3%).
The investigators mapped each scale against each of the others, which yielded three plots. In the first, higher MAAS scores were correlated with lower MRS scores (correlation, –0.49; P less than .001), which means that more time in a mindful state was associated with less severe menopausal symptoms.
In the second plot, lower MRS scores were associated with lower PSS-4 scores (correlation, 0.55; P less than .001). The last plot mapped PSS-4 scores against MAAS scores, showing that higher MAAS scores were correlated with lower PSS-4 scores, which means that more time in a mindful state was also associated with less perceived stress (correlation, –0.53; P less than .001).
Most of these associations remained statistically significant after multivariable linear regression analysis and breaking out the subscales of the MRS. Only the association between higher MAAS scores and the somatovegetative domain of the PSS-4 lost significance (P = 0.44).
Next, Dr. Sood and her collaborators probed how higher perceived stress, as measured by higher PSS-4 scores, altered the effects that mindful activity had on menopausal symptoms (as measured by the MRS).
The effect of mindfulness became stronger in the milieu of higher perceived stress. At a relatively low PSS-4 value of 4, the MRS score dropped 1.53 points for each one-point increase in the MAAS total score. However, with a PSS-4 score of 12, the decrease in MRS was 2.27 points for each one-point increase in MAAS, and with the maximum perceived stress score of 16, the MRS fell 2.64 points for each one-point increase in the MAAS score.
These findings are set against the backdrop of previous literature linking mindfulness to positive health behaviors and outcomes, she said, noting that work looking specifically at mindfulness-based stress reduction in peri- and postmenopausal women showed a halving of symptoms and improved quality of life.
Dr. Sood said that the present study was observational only, noting that it looked only at time spent in a mindful state in an untrained cohort of women in midlife. “Trait, or dispositional, mindfulness appears to be protective against stress and symptoms in midlife women,” she commented. “More mindful women may be choosing to shift their attention to more pleasant aspects of life, rather than their symptoms.”
“If you allow me to speculate a bit,” Dr. Sood continued, “the underpinnings of psychological symptoms rest in threat-focused attention and emotional reactivity – so the mindfulness approach appears to fit very well to impact such a change.”
Mindfulness, she added, “might be a tool to impact the emotional component of the overall experience, thereby decreasing the total suffering.” However, she noted that what’s needed are studies with a more heterogeneous population, as well as ones designed to tease out causality. Still, “the current study adds to the wealth of data supporting the role of mindfulness in various settings for impacting positive change in health and behaviors.”
Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
SOURCE: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
FROM NAMS 2018
Key clinical point: Time spent in a mindful state was associated with fewer menopause symptoms.
Major finding: With maximum stress, each 1-point increase in mindfulness was associated with a 2.64-point drop in menopausal symptoms.
Study details: A cross-sectional, single-center study of 1,744 women aged 40-64 years.
Disclosures: Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
Source: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
For dyspareunia, intravaginal prasterone may work best soon after menopause
Neither age nor previous hormone therapy had statistically significant associations with the effect of intravaginal prasterone on dyspareunia severity, according to a new subgroup analysis of clinical trial data. In a trend that did not reach statistical significance, though, said David F. Archer, MD.
“This was an unexpected finding,” he said in an interview.
In a subgroup analysis of data from two clinical trials of intravaginal prasterone (Intrarosa), Dr. Archer and his colleagues sought to investigate whether age, time since menopause, or any previous use of hormone replacement therapy influenced prasterone’s efficacy in treating dyspareunia.
Dr. Archer and his collaborators pooled data from two prospective, randomized, double-blind, placebo-controlled trials (NCT02013544 and NCT01256684) of intravaginal prasterone dosed at 0.50%, 6.5 mg once daily for 12 weeks; he presented the subgroup analyses at the annual meeting of the North American Menopause Society in San Diego.
For each subgroup, Dr. Archer, a professor of obstetrics and gynecology at Eastern Virginia Medical School, Norfolk, and his coinvestigators compared the mean differences in dyspareunia severity score of women who received prasterone and those who received placebo.
All subgroup analyses used the endpoint of improvement in moderate to severe dyspareunia or whether dyspareunia was the most bothersome symptoms for the women participating in the study. The investigators began by looking at the subgroup of 460 women who were 56 years and older at baseline and compared them with the 180 younger participants.
The 283 older participants who received prasterone saw a decrease of 0.36 points in a dyspareunia severity score versus a 0.44 point decrease for the 123 women aged 55 and younger who received prasterone, a nonsignificant difference between subgroups. The decrease compared with placebo-takers was significant in both cases, however (P = .0003 and P =.0031, respectively).
Looking at time since menopause, Dr. Archer and his collaborators divided participants into 33 individuals who were 1 or 2 years post menopause, 86 women who were 3-5 years post menopause, and 521 women who had experienced menopause at least 6 years before study baseline.
In this analysis, 22 of the earliest postmenopause women received prasterone, seeing a 1.59 point drop in dyspareunia severity. For the 59 women in the prasterone study arms who were 3-5 year past menopause, the decrease from baseline was 0.59 points. Finally, among the 325 women who received prasterone and experienced menopause 6 or more years ago, the decrease was 0.27 points.
Although there was a numeric difference in the change in dyspareunia score severity among these groups, the differences were not statistically significant, said Dr. Archer. Again, though, those who took prasterone had a significant reduction in dyspareunia severity scores when compared with those taking placebo (P less than .0001, P = .0136, and P = .0024, respectively).
In the prasterone study arms, 184 had previously used hormone therapy, and 222 had not. After 12 weeks of intravaginal prasterone, there was no statistically significant difference between the two subgroups, with a decreases in dyspareunia severity scores of 0.45 and 0.32, respectively. The decreases in severity scores when compared with those among women who took placebo were again statistically significant for both subgroups, however (P = .0002 and P = .0057, respectively).
Prasterone is a steroid that is also known as dehydroepiandrosterone (DHEA) and is an endogenous hormone that is a precursor for estrogens and androgens. Prasterone’s mechanism of action to reduce vulvar and vaginal atrophy is not completely understood, according to the Food and Drug Administration.
“The nonstatistically significant smaller effect on dyspareunia observed when treatment is initiated after a longer period after menopause suggests that a longer treatment period could be needed to achieve optimal benefit and that treatment of dyspareunia should be initiated as early as possible after menopause,” said Dr. Archer.
Dr. Archer reported grant support from and consulting relationships with several pharmaceutical companies, including Endoceutics, the producer of Intrarosa intravaginal prasterone.
Neither age nor previous hormone therapy had statistically significant associations with the effect of intravaginal prasterone on dyspareunia severity, according to a new subgroup analysis of clinical trial data. In a trend that did not reach statistical significance, though, said David F. Archer, MD.
“This was an unexpected finding,” he said in an interview.
In a subgroup analysis of data from two clinical trials of intravaginal prasterone (Intrarosa), Dr. Archer and his colleagues sought to investigate whether age, time since menopause, or any previous use of hormone replacement therapy influenced prasterone’s efficacy in treating dyspareunia.
Dr. Archer and his collaborators pooled data from two prospective, randomized, double-blind, placebo-controlled trials (NCT02013544 and NCT01256684) of intravaginal prasterone dosed at 0.50%, 6.5 mg once daily for 12 weeks; he presented the subgroup analyses at the annual meeting of the North American Menopause Society in San Diego.
For each subgroup, Dr. Archer, a professor of obstetrics and gynecology at Eastern Virginia Medical School, Norfolk, and his coinvestigators compared the mean differences in dyspareunia severity score of women who received prasterone and those who received placebo.
All subgroup analyses used the endpoint of improvement in moderate to severe dyspareunia or whether dyspareunia was the most bothersome symptoms for the women participating in the study. The investigators began by looking at the subgroup of 460 women who were 56 years and older at baseline and compared them with the 180 younger participants.
The 283 older participants who received prasterone saw a decrease of 0.36 points in a dyspareunia severity score versus a 0.44 point decrease for the 123 women aged 55 and younger who received prasterone, a nonsignificant difference between subgroups. The decrease compared with placebo-takers was significant in both cases, however (P = .0003 and P =.0031, respectively).
Looking at time since menopause, Dr. Archer and his collaborators divided participants into 33 individuals who were 1 or 2 years post menopause, 86 women who were 3-5 years post menopause, and 521 women who had experienced menopause at least 6 years before study baseline.
In this analysis, 22 of the earliest postmenopause women received prasterone, seeing a 1.59 point drop in dyspareunia severity. For the 59 women in the prasterone study arms who were 3-5 year past menopause, the decrease from baseline was 0.59 points. Finally, among the 325 women who received prasterone and experienced menopause 6 or more years ago, the decrease was 0.27 points.
Although there was a numeric difference in the change in dyspareunia score severity among these groups, the differences were not statistically significant, said Dr. Archer. Again, though, those who took prasterone had a significant reduction in dyspareunia severity scores when compared with those taking placebo (P less than .0001, P = .0136, and P = .0024, respectively).
In the prasterone study arms, 184 had previously used hormone therapy, and 222 had not. After 12 weeks of intravaginal prasterone, there was no statistically significant difference between the two subgroups, with a decreases in dyspareunia severity scores of 0.45 and 0.32, respectively. The decreases in severity scores when compared with those among women who took placebo were again statistically significant for both subgroups, however (P = .0002 and P = .0057, respectively).
Prasterone is a steroid that is also known as dehydroepiandrosterone (DHEA) and is an endogenous hormone that is a precursor for estrogens and androgens. Prasterone’s mechanism of action to reduce vulvar and vaginal atrophy is not completely understood, according to the Food and Drug Administration.
“The nonstatistically significant smaller effect on dyspareunia observed when treatment is initiated after a longer period after menopause suggests that a longer treatment period could be needed to achieve optimal benefit and that treatment of dyspareunia should be initiated as early as possible after menopause,” said Dr. Archer.
Dr. Archer reported grant support from and consulting relationships with several pharmaceutical companies, including Endoceutics, the producer of Intrarosa intravaginal prasterone.
Neither age nor previous hormone therapy had statistically significant associations with the effect of intravaginal prasterone on dyspareunia severity, according to a new subgroup analysis of clinical trial data. In a trend that did not reach statistical significance, though, said David F. Archer, MD.
“This was an unexpected finding,” he said in an interview.
In a subgroup analysis of data from two clinical trials of intravaginal prasterone (Intrarosa), Dr. Archer and his colleagues sought to investigate whether age, time since menopause, or any previous use of hormone replacement therapy influenced prasterone’s efficacy in treating dyspareunia.
Dr. Archer and his collaborators pooled data from two prospective, randomized, double-blind, placebo-controlled trials (NCT02013544 and NCT01256684) of intravaginal prasterone dosed at 0.50%, 6.5 mg once daily for 12 weeks; he presented the subgroup analyses at the annual meeting of the North American Menopause Society in San Diego.
For each subgroup, Dr. Archer, a professor of obstetrics and gynecology at Eastern Virginia Medical School, Norfolk, and his coinvestigators compared the mean differences in dyspareunia severity score of women who received prasterone and those who received placebo.
All subgroup analyses used the endpoint of improvement in moderate to severe dyspareunia or whether dyspareunia was the most bothersome symptoms for the women participating in the study. The investigators began by looking at the subgroup of 460 women who were 56 years and older at baseline and compared them with the 180 younger participants.
The 283 older participants who received prasterone saw a decrease of 0.36 points in a dyspareunia severity score versus a 0.44 point decrease for the 123 women aged 55 and younger who received prasterone, a nonsignificant difference between subgroups. The decrease compared with placebo-takers was significant in both cases, however (P = .0003 and P =.0031, respectively).
Looking at time since menopause, Dr. Archer and his collaborators divided participants into 33 individuals who were 1 or 2 years post menopause, 86 women who were 3-5 years post menopause, and 521 women who had experienced menopause at least 6 years before study baseline.
In this analysis, 22 of the earliest postmenopause women received prasterone, seeing a 1.59 point drop in dyspareunia severity. For the 59 women in the prasterone study arms who were 3-5 year past menopause, the decrease from baseline was 0.59 points. Finally, among the 325 women who received prasterone and experienced menopause 6 or more years ago, the decrease was 0.27 points.
Although there was a numeric difference in the change in dyspareunia score severity among these groups, the differences were not statistically significant, said Dr. Archer. Again, though, those who took prasterone had a significant reduction in dyspareunia severity scores when compared with those taking placebo (P less than .0001, P = .0136, and P = .0024, respectively).
In the prasterone study arms, 184 had previously used hormone therapy, and 222 had not. After 12 weeks of intravaginal prasterone, there was no statistically significant difference between the two subgroups, with a decreases in dyspareunia severity scores of 0.45 and 0.32, respectively. The decreases in severity scores when compared with those among women who took placebo were again statistically significant for both subgroups, however (P = .0002 and P = .0057, respectively).
Prasterone is a steroid that is also known as dehydroepiandrosterone (DHEA) and is an endogenous hormone that is a precursor for estrogens and androgens. Prasterone’s mechanism of action to reduce vulvar and vaginal atrophy is not completely understood, according to the Food and Drug Administration.
“The nonstatistically significant smaller effect on dyspareunia observed when treatment is initiated after a longer period after menopause suggests that a longer treatment period could be needed to achieve optimal benefit and that treatment of dyspareunia should be initiated as early as possible after menopause,” said Dr. Archer.
Dr. Archer reported grant support from and consulting relationships with several pharmaceutical companies, including Endoceutics, the producer of Intrarosa intravaginal prasterone.
FROM NAMS 2018
Key clinical point: Dyspareunia improvement was numerically, but not statistically, better soon after menopause.
Major finding: Dyspareunia scores dropped 1.59 points for those within 2 years of menopause, and 0.27 points for those 6 or more years post menopause.
Study details: Subgroup analysis of 640 postmenopausal women taking part in two clinical trials.
Disclosures: Dr. Archer reported receiving support from several pharmaceutical companies, including Endoceutics, the manufacturer of Intrarosa intravaginal prasterone.