User login
First North American clinical guidelines for hidradenitis suppurativa released
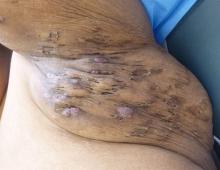
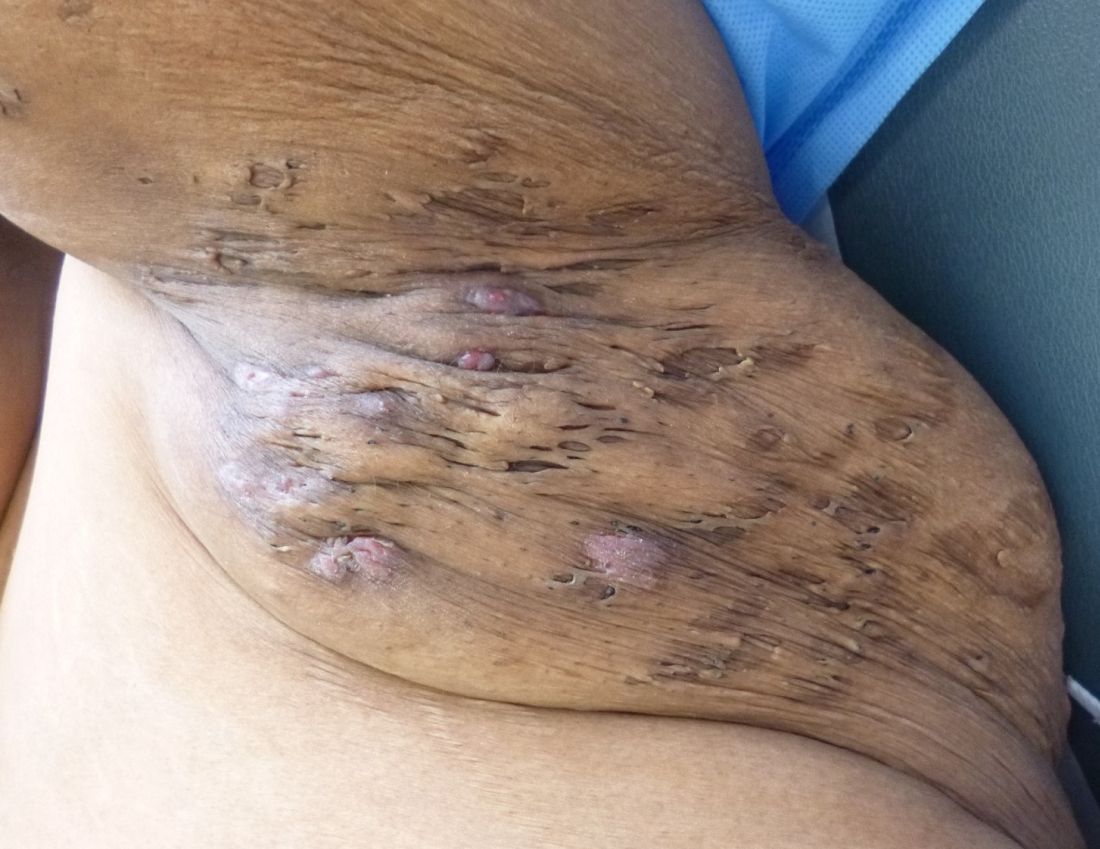
Rigorous evidence is unavailable for most interventions for treating hidradenitis suppurativa (HS), so management needs to be individualized, according to the
The guidelines were published in the Journal of the American Academy of Dermatology.
In an interview, Christopher Sayed, MD, cochair of the guidelines committee, of the department of dermatology at the University of North Carolina, Chapel Hill, said in an interview that the North American guidelines vary from the British Association of Dermatologists (Br J Dermatol. 2018 Dec 15. doi: 10.1111/bjd.17537) and European guidelines (J Eur Acad Dermatol Venereol. 2015 Apr;29[4]:619-44). For example, surgery is an active treatment option for various stages of the disorder in the North American guidelines, whereas surgery is considered a last-ditch effort in the British and European guidelines.
Surgical intervention is often needed “for patients to be the best they can be, and it can be difficult for medicine alone to fix [certain] patients,” Dr. Sayed said. This point that “using medical treatment alone or just surgical treatment alone often doesn’t lead to the best outcome” is stressed in the North American guidelines, he noted.
Limited evidence for high-level recommendations
Adalimumab (Humira), a tumor necrosis factor blocker, was the only therapy for which level 1A evidence is available, and this is because of its study in large-scale randomized controlled trials. (The Food and Drug Administration approved adalimumab in 2015 for treating moderate to severe HS.) Other biologic therapies such as infliximab, anakinra, and ustekinumab carry level 2B recommendations, which Dr. Sayed said will likely influence the availability of these therapies.
Similarly, Nd:YAG laser carries a level 2B recommendation, as does wide excision surgical intervention.
Many of the other treatment modalities explored in the guidelines are not well supported by the literature, according to Dr. Sayed . “The vast majority ... had category C recommendations,” he said. “Some things we tried to evaluate that, really, we can’t give any recommendation on at all because there was no evidence.” He noted that changes in lifestyle and dietary practices are issues patients with HS frequently bring up, but they carry very little evidence of benefit in the literature.
“Even things we use very commonly in HS are often supported by weak evidence because we have to rely on clinical experience. ... There’s just not funding for trials of older drugs or lifestyle interventions,” he said. Treatment mainstays such as tetracycline-class antibiotics, for example, similarly have no large-scale randomized controlled trials to support their use.
Consider comorbidity screening
Treatment is frequently complicated by patient comorbidities, including type 2 diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), and impaired sexual health, said Dr. Sayed.
“The disease leads to scarring and disfigurement, and can [have a] higher impact on quality of life than almost any other dermatologic disease if you compare them side by side using quality of life measures,” he noted. “We know these patients are more likely to have depression, there are high rates of suicide among these patients. A lot of that has to do with the fact that it’s a chronic disease where there is pain and disfigurement, and patients often grow very, very frustrated in part due to the disease.”
He advised consistent follow-up to ensure patients are not frustrated because of lack of perceived progress.
No one-size-fits-all approach
Dr. Sayed said individualized management is one of the most important parts of taking care of a patient with HS. For example, patients with Hurley stage 1 and Hurley stage 2 variations of the disease may present very differently, and that is the reason why the guidelines do not offer a stepwise treatment algorithm for the disease.
“[The guidelines] have many treatments that may overlap different stages of disease, where those things might need to be used together,” he said. “It takes a lot of discussion with patients about their treatment preferences and listening to whether their disease is progressing or not, whether or not it’s stable, and then trying to figure out whether things like surgery fit into the treatment strategy.”
Despite these recommendations, there may be situations where the answer is not listed in the guidelines. “The guidelines are based on evidence that’s available at the time we review the literature,” he said. “For many patients, they may fail every treatment that’s recommended within the guidelines. There will be times where you have to be creative for patients and go beyond what the guidelines can currently recommend and give patients the treatment that they need even when it seems like all options have been exhausted.”
The authors report relationships with 3M, AbbVie, Adelphi Values, Amgen, BSN, Celgene, Chemocentryx, Coloplast, Galderma, Hidramed Solutions, Hollister, the HS Foundation, Incyte, InflaRx, Integra, Janssen, KCI Inc., Lenicura, Leo, Lilly, The Microdermis Corporation, Novartis, Pfizer, UCB, Valeant, and XBiotech both inside and outside the supported work.
SOURCES: Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.067; Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.068.
Rigorous evidence is unavailable for most interventions for treating hidradenitis suppurativa (HS), so management needs to be individualized, according to the
The guidelines were published in the Journal of the American Academy of Dermatology.
In an interview, Christopher Sayed, MD, cochair of the guidelines committee, of the department of dermatology at the University of North Carolina, Chapel Hill, said in an interview that the North American guidelines vary from the British Association of Dermatologists (Br J Dermatol. 2018 Dec 15. doi: 10.1111/bjd.17537) and European guidelines (J Eur Acad Dermatol Venereol. 2015 Apr;29[4]:619-44). For example, surgery is an active treatment option for various stages of the disorder in the North American guidelines, whereas surgery is considered a last-ditch effort in the British and European guidelines.
Surgical intervention is often needed “for patients to be the best they can be, and it can be difficult for medicine alone to fix [certain] patients,” Dr. Sayed said. This point that “using medical treatment alone or just surgical treatment alone often doesn’t lead to the best outcome” is stressed in the North American guidelines, he noted.
Limited evidence for high-level recommendations
Adalimumab (Humira), a tumor necrosis factor blocker, was the only therapy for which level 1A evidence is available, and this is because of its study in large-scale randomized controlled trials. (The Food and Drug Administration approved adalimumab in 2015 for treating moderate to severe HS.) Other biologic therapies such as infliximab, anakinra, and ustekinumab carry level 2B recommendations, which Dr. Sayed said will likely influence the availability of these therapies.
Similarly, Nd:YAG laser carries a level 2B recommendation, as does wide excision surgical intervention.
Many of the other treatment modalities explored in the guidelines are not well supported by the literature, according to Dr. Sayed . “The vast majority ... had category C recommendations,” he said. “Some things we tried to evaluate that, really, we can’t give any recommendation on at all because there was no evidence.” He noted that changes in lifestyle and dietary practices are issues patients with HS frequently bring up, but they carry very little evidence of benefit in the literature.
“Even things we use very commonly in HS are often supported by weak evidence because we have to rely on clinical experience. ... There’s just not funding for trials of older drugs or lifestyle interventions,” he said. Treatment mainstays such as tetracycline-class antibiotics, for example, similarly have no large-scale randomized controlled trials to support their use.
Consider comorbidity screening
Treatment is frequently complicated by patient comorbidities, including type 2 diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), and impaired sexual health, said Dr. Sayed.
“The disease leads to scarring and disfigurement, and can [have a] higher impact on quality of life than almost any other dermatologic disease if you compare them side by side using quality of life measures,” he noted. “We know these patients are more likely to have depression, there are high rates of suicide among these patients. A lot of that has to do with the fact that it’s a chronic disease where there is pain and disfigurement, and patients often grow very, very frustrated in part due to the disease.”
He advised consistent follow-up to ensure patients are not frustrated because of lack of perceived progress.
No one-size-fits-all approach
Dr. Sayed said individualized management is one of the most important parts of taking care of a patient with HS. For example, patients with Hurley stage 1 and Hurley stage 2 variations of the disease may present very differently, and that is the reason why the guidelines do not offer a stepwise treatment algorithm for the disease.
“[The guidelines] have many treatments that may overlap different stages of disease, where those things might need to be used together,” he said. “It takes a lot of discussion with patients about their treatment preferences and listening to whether their disease is progressing or not, whether or not it’s stable, and then trying to figure out whether things like surgery fit into the treatment strategy.”
Despite these recommendations, there may be situations where the answer is not listed in the guidelines. “The guidelines are based on evidence that’s available at the time we review the literature,” he said. “For many patients, they may fail every treatment that’s recommended within the guidelines. There will be times where you have to be creative for patients and go beyond what the guidelines can currently recommend and give patients the treatment that they need even when it seems like all options have been exhausted.”
The authors report relationships with 3M, AbbVie, Adelphi Values, Amgen, BSN, Celgene, Chemocentryx, Coloplast, Galderma, Hidramed Solutions, Hollister, the HS Foundation, Incyte, InflaRx, Integra, Janssen, KCI Inc., Lenicura, Leo, Lilly, The Microdermis Corporation, Novartis, Pfizer, UCB, Valeant, and XBiotech both inside and outside the supported work.
SOURCES: Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.067; Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.068.
Rigorous evidence is unavailable for most interventions for treating hidradenitis suppurativa (HS), so management needs to be individualized, according to the
The guidelines were published in the Journal of the American Academy of Dermatology.
In an interview, Christopher Sayed, MD, cochair of the guidelines committee, of the department of dermatology at the University of North Carolina, Chapel Hill, said in an interview that the North American guidelines vary from the British Association of Dermatologists (Br J Dermatol. 2018 Dec 15. doi: 10.1111/bjd.17537) and European guidelines (J Eur Acad Dermatol Venereol. 2015 Apr;29[4]:619-44). For example, surgery is an active treatment option for various stages of the disorder in the North American guidelines, whereas surgery is considered a last-ditch effort in the British and European guidelines.
Surgical intervention is often needed “for patients to be the best they can be, and it can be difficult for medicine alone to fix [certain] patients,” Dr. Sayed said. This point that “using medical treatment alone or just surgical treatment alone often doesn’t lead to the best outcome” is stressed in the North American guidelines, he noted.
Limited evidence for high-level recommendations
Adalimumab (Humira), a tumor necrosis factor blocker, was the only therapy for which level 1A evidence is available, and this is because of its study in large-scale randomized controlled trials. (The Food and Drug Administration approved adalimumab in 2015 for treating moderate to severe HS.) Other biologic therapies such as infliximab, anakinra, and ustekinumab carry level 2B recommendations, which Dr. Sayed said will likely influence the availability of these therapies.
Similarly, Nd:YAG laser carries a level 2B recommendation, as does wide excision surgical intervention.
Many of the other treatment modalities explored in the guidelines are not well supported by the literature, according to Dr. Sayed . “The vast majority ... had category C recommendations,” he said. “Some things we tried to evaluate that, really, we can’t give any recommendation on at all because there was no evidence.” He noted that changes in lifestyle and dietary practices are issues patients with HS frequently bring up, but they carry very little evidence of benefit in the literature.
“Even things we use very commonly in HS are often supported by weak evidence because we have to rely on clinical experience. ... There’s just not funding for trials of older drugs or lifestyle interventions,” he said. Treatment mainstays such as tetracycline-class antibiotics, for example, similarly have no large-scale randomized controlled trials to support their use.
Consider comorbidity screening
Treatment is frequently complicated by patient comorbidities, including type 2 diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), and impaired sexual health, said Dr. Sayed.
“The disease leads to scarring and disfigurement, and can [have a] higher impact on quality of life than almost any other dermatologic disease if you compare them side by side using quality of life measures,” he noted. “We know these patients are more likely to have depression, there are high rates of suicide among these patients. A lot of that has to do with the fact that it’s a chronic disease where there is pain and disfigurement, and patients often grow very, very frustrated in part due to the disease.”
He advised consistent follow-up to ensure patients are not frustrated because of lack of perceived progress.
No one-size-fits-all approach
Dr. Sayed said individualized management is one of the most important parts of taking care of a patient with HS. For example, patients with Hurley stage 1 and Hurley stage 2 variations of the disease may present very differently, and that is the reason why the guidelines do not offer a stepwise treatment algorithm for the disease.
“[The guidelines] have many treatments that may overlap different stages of disease, where those things might need to be used together,” he said. “It takes a lot of discussion with patients about their treatment preferences and listening to whether their disease is progressing or not, whether or not it’s stable, and then trying to figure out whether things like surgery fit into the treatment strategy.”
Despite these recommendations, there may be situations where the answer is not listed in the guidelines. “The guidelines are based on evidence that’s available at the time we review the literature,” he said. “For many patients, they may fail every treatment that’s recommended within the guidelines. There will be times where you have to be creative for patients and go beyond what the guidelines can currently recommend and give patients the treatment that they need even when it seems like all options have been exhausted.”
The authors report relationships with 3M, AbbVie, Adelphi Values, Amgen, BSN, Celgene, Chemocentryx, Coloplast, Galderma, Hidramed Solutions, Hollister, the HS Foundation, Incyte, InflaRx, Integra, Janssen, KCI Inc., Lenicura, Leo, Lilly, The Microdermis Corporation, Novartis, Pfizer, UCB, Valeant, and XBiotech both inside and outside the supported work.
SOURCES: Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.067; Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.068.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
A 60-year-old white male presented with a painful nodule on the right lateral thigh that had been present for years
Benign tumors consisting of glomus cells may be subdivided into two types: glomus tumors and glomuvenous malformations or glomangiomas. Glomus cells are modified smooth muscle cells that normally line the Sucquet-Hoyer canal, an arteriovenous fistula that is involved in temperature regulation in the digits.
. In women, lesions more frequently occur on the fingers (especially nail beds). Glomus tumors are firm subcutaneous nodules, often skin colored or bluish in color. Subungual tumors tend to appear bluish under the nail plate. Lesions are extremely tender or painful, with worse pain upon palpation. Occasionally, nontender lesions can be seen.
In children, multiple nontender lesions are called glomangiomas or glomuvenous malformations. They may be sporadic or can be inherited in an autosomal dominant fashion due to a mutation in glomulin on chromosome 1p21-p22. Multiple lesions may be scattered or grouped, often in a segmental distribution. Congenital lesions tend to be large, blue-purple in color with a cobblestone appearance. They are more superficial than venous malformations.
Histologically, a proliferation of blood vessels surrounded by glomus cells is seen. Glomus cells appear as monotonous cells with a dense, round nucleus and abundant pink cytoplasm. Glomus cells can also be appreciated single-filing through pale stroma, resembling strings of black pearls. Glomus cells stain positive for smooth muscle actin and vimentin.
The painful tumor differential diagnosis has been described in the literature by the mnemonic “LEND AN EGG:” leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumor, and granular cell tumor.
The malignant counterpart is glomangiosarcoma, which is a rare tumor. These lesions are often large and deeply located on the extremities. Histologically, sarcomatous areas are mixed with areas of benign glomus tumor.
Surgical excision is the treatment of choice for solitary glomus tumors to provide pain relief. Subungual tumors are more challenging due to their small size, but may be excised as well. Glomuvenous malformations may require different treatment modalities, such as surgery and laser, due to their larger size.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at www.mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Benign tumors consisting of glomus cells may be subdivided into two types: glomus tumors and glomuvenous malformations or glomangiomas. Glomus cells are modified smooth muscle cells that normally line the Sucquet-Hoyer canal, an arteriovenous fistula that is involved in temperature regulation in the digits.
. In women, lesions more frequently occur on the fingers (especially nail beds). Glomus tumors are firm subcutaneous nodules, often skin colored or bluish in color. Subungual tumors tend to appear bluish under the nail plate. Lesions are extremely tender or painful, with worse pain upon palpation. Occasionally, nontender lesions can be seen.
In children, multiple nontender lesions are called glomangiomas or glomuvenous malformations. They may be sporadic or can be inherited in an autosomal dominant fashion due to a mutation in glomulin on chromosome 1p21-p22. Multiple lesions may be scattered or grouped, often in a segmental distribution. Congenital lesions tend to be large, blue-purple in color with a cobblestone appearance. They are more superficial than venous malformations.
Histologically, a proliferation of blood vessels surrounded by glomus cells is seen. Glomus cells appear as monotonous cells with a dense, round nucleus and abundant pink cytoplasm. Glomus cells can also be appreciated single-filing through pale stroma, resembling strings of black pearls. Glomus cells stain positive for smooth muscle actin and vimentin.
The painful tumor differential diagnosis has been described in the literature by the mnemonic “LEND AN EGG:” leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumor, and granular cell tumor.
The malignant counterpart is glomangiosarcoma, which is a rare tumor. These lesions are often large and deeply located on the extremities. Histologically, sarcomatous areas are mixed with areas of benign glomus tumor.
Surgical excision is the treatment of choice for solitary glomus tumors to provide pain relief. Subungual tumors are more challenging due to their small size, but may be excised as well. Glomuvenous malformations may require different treatment modalities, such as surgery and laser, due to their larger size.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at www.mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Benign tumors consisting of glomus cells may be subdivided into two types: glomus tumors and glomuvenous malformations or glomangiomas. Glomus cells are modified smooth muscle cells that normally line the Sucquet-Hoyer canal, an arteriovenous fistula that is involved in temperature regulation in the digits.
. In women, lesions more frequently occur on the fingers (especially nail beds). Glomus tumors are firm subcutaneous nodules, often skin colored or bluish in color. Subungual tumors tend to appear bluish under the nail plate. Lesions are extremely tender or painful, with worse pain upon palpation. Occasionally, nontender lesions can be seen.
In children, multiple nontender lesions are called glomangiomas or glomuvenous malformations. They may be sporadic or can be inherited in an autosomal dominant fashion due to a mutation in glomulin on chromosome 1p21-p22. Multiple lesions may be scattered or grouped, often in a segmental distribution. Congenital lesions tend to be large, blue-purple in color with a cobblestone appearance. They are more superficial than venous malformations.
Histologically, a proliferation of blood vessels surrounded by glomus cells is seen. Glomus cells appear as monotonous cells with a dense, round nucleus and abundant pink cytoplasm. Glomus cells can also be appreciated single-filing through pale stroma, resembling strings of black pearls. Glomus cells stain positive for smooth muscle actin and vimentin.
The painful tumor differential diagnosis has been described in the literature by the mnemonic “LEND AN EGG:” leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumor, and granular cell tumor.
The malignant counterpart is glomangiosarcoma, which is a rare tumor. These lesions are often large and deeply located on the extremities. Histologically, sarcomatous areas are mixed with areas of benign glomus tumor.
Surgical excision is the treatment of choice for solitary glomus tumors to provide pain relief. Subungual tumors are more challenging due to their small size, but may be excised as well. Glomuvenous malformations may require different treatment modalities, such as surgery and laser, due to their larger size.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at www.mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
A 60-year-old white male presented with a painful nodule on the right lateral thigh that had been present for years. It has slowly been increasing in size over time.
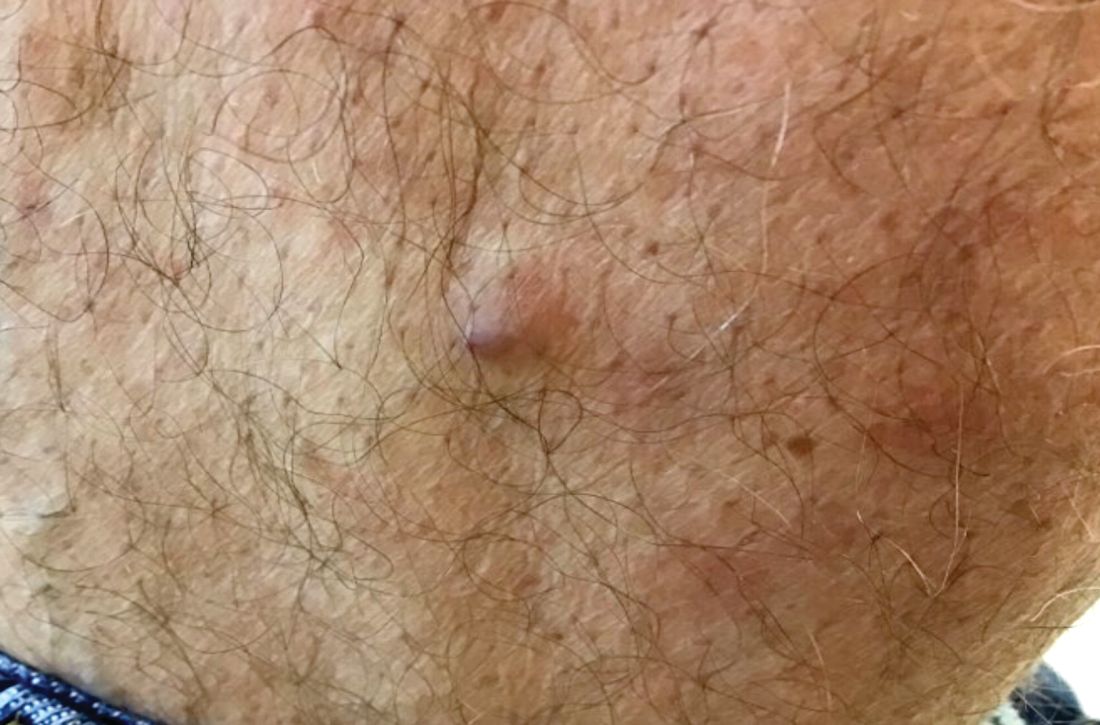
Hormonal management strategies for hidradenitis suppurativa target androgens
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
WASHINGTON – Hidradenitis suppurativa (HS) management should be individualized in patients, with consideration of their comorbidities, and therapies should be layered and rotated to improve efficacy, Ginette Okoye, MD, said at the annual meeting of the American Academy of Dermatology.
, spironolactone, and oral contraceptives, said Dr. Okoye, professor and chair of dermatology at Howard University, Washington. A patient’s comorbidities can help tailor which treatments to use, so if a patient with HS also has androgenetic alopecia, finasteride can be considered, while spironolactone, with or without an OC, can be considered for a patient with acne – and metformin can be considered for a patient with diabetes or prediabetes, or polycystic ovary syndrome (PCOS), she commented.
The main goal behind hormonal and metabolic therapies in patients with HS is to decrease androgens. Metformin, the oral hypoglycemic drug, reduces ovarian androgen production, and increases insulin-receptor sensitivity, and is an option for patients with HS, and can also treat comorbid conditions these patients tend to have, such as obesity, insulin resistance, and PCOS, she noted. Metformin dosing is 1,500 to 2,000 mg a day, starting at 500 mg per day with an evening meal, titrating up 500 mg every 2-4 weeks based on how patients tolerate side effects such as diarrhea, nausea, vomiting, and flatulence. Lactic acidosis is a less common side effect, but the risk increases for patients with renal and hepatic impairment or excessive alcohol intake, and for those who are undergoing a radiological procedure with contrast or who are over 65 years of age. While metformin alone, in her experience, does not make a big difference, it can be helpful when combined with other treatments such as antibiotics and biologics, and in patients with these comorbidities, she said.
Pregnant women with HS can benefit from treatment with metformin, but dermatologists should consult with the patient’s obstetrician-gynecologist as the medication is classified as pregnancy category B. In addition, metformin should not be given to patients with a glomerular filtration rate (GFR) less than 45 mL/min, and long-term use is associated with low vitamin B12 levels, she said.
“I often layer this with the antibiotic therapy, so my patient may be on clindamycin, rifampin, and metformin,” said Dr. Okoye. “If they are, you can give them a much lower dose of metformin since rifampin increases the plasma concentration of metformin.”
Patients with HS may also respond well to finasteride at doses between 1 mg and 5 mg once daily, an off-label use for this medication. Finasteride, which targets type 2 5-alpha-reductase, reduces the levels of dihydrotestosterone within hair follicles, which can improve HS symptoms, she said. However, she discusses potential side effects of finasteride use with patients, which include reduced libido, abnormal ejaculation, breast tenderness, prostate cancer, and depression. She also referred to postmarketing data suggesting that finasteride can lead to post-finasteride syndrome, characterized by symptoms that include depression and anhedonia, even long after stopping treatment, she said.
“I still think that it’s worth a try,” Dr. Okoye commented. “Many of our HS patients already are dealing with depression because of their disease. ... In 3 months, we talk about their symptoms, [and] make sure that they’re feeling okay before continuing.”
While finasteride is not appropriate for women of childbearing potential (pregnancy category X), it can be an option for women with HS who are of childbearing age but are not at risk for becoming pregnant, Dr. Okoye added, which can be determined by discussing a patient’s family planning goals. For example, she said, “if you have a woman of childbearing age but she’s in a same-sex relationship and has no intention of having children, then maybe finasteride is an option for her.”
The mineralocorticoid- and aldosterone-receptor antagonist spironolactone, used off label for acne treatment, also has antiandrogenic properties and is an option for patients with HS “at the higher end of the dosing spectrum” with 100-200 mg daily. However, Dr. Okoye referred to a recently published single-center retrospective study that showed a low daily dose of 75 mg was effective for HS (J Am Acad Dermatol. 2019 Jan;80[1]:114-9).
While spironolactone increases the risk of hyperkalemia, in patients with no preexisting renal disease under 50 years of age, monitoring is not necessary because there is little to no risk of clinical hyperkalemia in these patients, she said. Combining spironolactone or finasteride with OCs may increase antiandrogenic activity, she noted.
The data on effectiveness of hormonal contraceptives are mixed with regard to treatment of HS, with some studies showing benefit or worsening of the disease with OC use. “I think one of the reasons the data is so ‘dirty’ is because OCs range widely in terms of their ingredients and in terms of how androgenic their progesterones are,” Dr. Okoye commented.
OCs increase the risk of venous thromboembolism (VTE), but Dr. Okoye noted the risk is less than a patient would experience during pregnancy. “When you talk to dermatologists, there are two camps: some dermatologists who are very comfortable prescribing OCs, and dermatologists who prefer not to, given the risk of VTEs,” she said. However, risk should also be applied to patient population and location, she noted.
“If you are in an area [where] you serve a patient population that has fewer options for access to care, and if you don’t prescribe the OCs, those patients have to wait several months before getting on therapy, said Dr. Okoye. “Maybe that’s a case where you might want to start the OC [with] one or two refills while they find an OB, but it’s really up to you and your risk aversion.”
Dietary factors may also contribute to HS, but more studies are needed to analyze how sugar and carbohydrates contribute to the condition. Instead of taking for granted that a patient will understand what reducing dietary carbohydrate and sugar intake means, Dr. Okoye said, “I like to get very specific; ask them what they’re drinking on a daily basis.”
With regard to weight loss, there is little to link significant weight loss and symptom improvement. However, weight loss could help with comorbid conditions in patients with HS, like metabolic syndrome, and subsequent skin reduction may reduce friction of intertriginous areas, she pointed out.
Dr. Okoye reports receiving grants and/or research funding from Eli Lilly.
EXPERT ANALYSIS FROM AAD 19
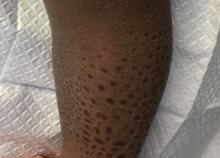
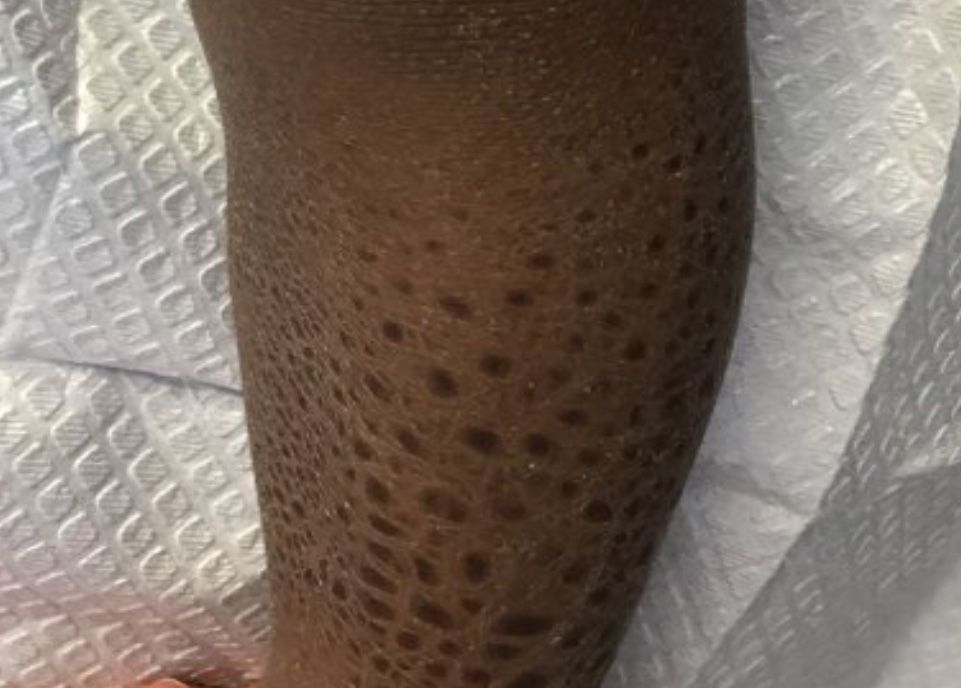
A 13-month-old, healthy black male presented with a 6-month history of dry, scaly skin on the body
Ichthyosis vulgaris
Ichthyoses describe a group of disorders of cornification in which the epidermis differentiates abnormally, leading to generalized scaling of the skin. Ichthyosis is derived from the Greek word for fish, “ichthys.” Ichthyosis vulgaris is the most common of these conditions and often presents in early childhood during the first year of life. It is inherited in an autosomal-dominant pattern. Skin is dry and scaly over the entire body, although the antecubital and popliteal fossa may be uninvolved. The scalp may be involved as well. Atopy and keratosis pilaris may be associated. By adulthood, symptoms tend to abate.
X-linked ichthyosis is an X-linked recessive trait, in which males are affected and mothers are carriers. The condition is caused by a deficiency of steroid sulfatase. This deficiency can result in low levels of estrogen during pregnancy in the mother of an affected fetus, hampering labor progression, and often requiring C-section. Children usually present before 3 months of age. Scales are large and dark. The antecubital and popliteal fossa are usually spared. The neck almost always is involved, coining the term “dirty neck disease.” Corneal opacities are present upon ophthalmologic examination. There is an increased risk of cryptorchidism and testicular cancer. Skin symptoms tend to worsen into adulthood.
Lamellar ichthyosis generally occurs at birth with a striking collodion-type membrane covering the body and underlying erythroderma, which then desquamates. Ectropion is usually present as well. Resulting scales are large and gray-brown. Lamellar ichthyosis is inherited in an autosomal recessive pattern. Mutations in transglutaminase 1 (TGM1), ALOXE3, ALOX12B, and ABCA12 genes have been implicated in this disorder.
Acquired ichthyosis can appear clinically similar to ichthyosis vulgaris. It occurs in patients with systemic diseases such as Hodgkin disease, non-Hodgkin lymphoma, mycosis fungoides, multiple myeloma, hypothyroidism, sarcoidosis, AIDS, and others.
improve hyperkeratosis. Urea-containing products can be helpful. Salicylic acid may be used but merit caution in children because of salicylate toxicity. Oral and topical retinoid can be helpful in lamellar ichthyosis.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Ichthyosis vulgaris
Ichthyoses describe a group of disorders of cornification in which the epidermis differentiates abnormally, leading to generalized scaling of the skin. Ichthyosis is derived from the Greek word for fish, “ichthys.” Ichthyosis vulgaris is the most common of these conditions and often presents in early childhood during the first year of life. It is inherited in an autosomal-dominant pattern. Skin is dry and scaly over the entire body, although the antecubital and popliteal fossa may be uninvolved. The scalp may be involved as well. Atopy and keratosis pilaris may be associated. By adulthood, symptoms tend to abate.
X-linked ichthyosis is an X-linked recessive trait, in which males are affected and mothers are carriers. The condition is caused by a deficiency of steroid sulfatase. This deficiency can result in low levels of estrogen during pregnancy in the mother of an affected fetus, hampering labor progression, and often requiring C-section. Children usually present before 3 months of age. Scales are large and dark. The antecubital and popliteal fossa are usually spared. The neck almost always is involved, coining the term “dirty neck disease.” Corneal opacities are present upon ophthalmologic examination. There is an increased risk of cryptorchidism and testicular cancer. Skin symptoms tend to worsen into adulthood.
Lamellar ichthyosis generally occurs at birth with a striking collodion-type membrane covering the body and underlying erythroderma, which then desquamates. Ectropion is usually present as well. Resulting scales are large and gray-brown. Lamellar ichthyosis is inherited in an autosomal recessive pattern. Mutations in transglutaminase 1 (TGM1), ALOXE3, ALOX12B, and ABCA12 genes have been implicated in this disorder.
Acquired ichthyosis can appear clinically similar to ichthyosis vulgaris. It occurs in patients with systemic diseases such as Hodgkin disease, non-Hodgkin lymphoma, mycosis fungoides, multiple myeloma, hypothyroidism, sarcoidosis, AIDS, and others.
improve hyperkeratosis. Urea-containing products can be helpful. Salicylic acid may be used but merit caution in children because of salicylate toxicity. Oral and topical retinoid can be helpful in lamellar ichthyosis.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Ichthyosis vulgaris
Ichthyoses describe a group of disorders of cornification in which the epidermis differentiates abnormally, leading to generalized scaling of the skin. Ichthyosis is derived from the Greek word for fish, “ichthys.” Ichthyosis vulgaris is the most common of these conditions and often presents in early childhood during the first year of life. It is inherited in an autosomal-dominant pattern. Skin is dry and scaly over the entire body, although the antecubital and popliteal fossa may be uninvolved. The scalp may be involved as well. Atopy and keratosis pilaris may be associated. By adulthood, symptoms tend to abate.
X-linked ichthyosis is an X-linked recessive trait, in which males are affected and mothers are carriers. The condition is caused by a deficiency of steroid sulfatase. This deficiency can result in low levels of estrogen during pregnancy in the mother of an affected fetus, hampering labor progression, and often requiring C-section. Children usually present before 3 months of age. Scales are large and dark. The antecubital and popliteal fossa are usually spared. The neck almost always is involved, coining the term “dirty neck disease.” Corneal opacities are present upon ophthalmologic examination. There is an increased risk of cryptorchidism and testicular cancer. Skin symptoms tend to worsen into adulthood.
Lamellar ichthyosis generally occurs at birth with a striking collodion-type membrane covering the body and underlying erythroderma, which then desquamates. Ectropion is usually present as well. Resulting scales are large and gray-brown. Lamellar ichthyosis is inherited in an autosomal recessive pattern. Mutations in transglutaminase 1 (TGM1), ALOXE3, ALOX12B, and ABCA12 genes have been implicated in this disorder.
Acquired ichthyosis can appear clinically similar to ichthyosis vulgaris. It occurs in patients with systemic diseases such as Hodgkin disease, non-Hodgkin lymphoma, mycosis fungoides, multiple myeloma, hypothyroidism, sarcoidosis, AIDS, and others.
improve hyperkeratosis. Urea-containing products can be helpful. Salicylic acid may be used but merit caution in children because of salicylate toxicity. Oral and topical retinoid can be helpful in lamellar ichthyosis.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 13-month-old, healthy black male presented with a 6-month history of dry, scaly skin on the body, including scalp and extremities. His neck was unaffected. His mother reports an uneventful pregnancy and natural childbirth. He had been prescribed triamcinolone in the past for eczema.
Socioeconomic status affects scleroderma severity in African Americans
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
FROM ARTHRITIS CARE & RESEARCH
Resistant hypertension hits SLE patients hard
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
FROM ARTHRITIS CARE & RESEARCH
VIDEO: Immunomodulators for inflammatory skin diseases
WASHINGTON – During a session at the annual meeting of the American Academy of Dermatology, Adam Friedman, MD, presented on off-label use of immunomodulators for inflammatory skin diseases, the highlights of which he shared with fellow George Washington University dermatologist, A. Yasmine Kirkorian, MD, in an interview following the session.

Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington,
For example, as reflected in PubMed searches, low-dose naltrexone, which has to be compounded, is being used for such diseases as Hailey-Hailey and lichen planopilaris, said Dr. Friedman, who is using it for his mast cell activation syndrome patients. During the interview, he also describes his treatment approach for urticaria.
In his final remarks, Dr. Friedman encourages colleagues to “get creative,” publish, and talk about their experiences with off-label treatments in dermatology, citing the example of an article that mentioned using pioglitazone for lichen planopilaris. This article stimulated interest in using the type 2 diabetes agent pioglitazone to treat this skin disease, he notes.
Dr. Friedman and Dr. Kirkorian, a pediatric dermatologist at George Washington University and interim chief of pediatric dermatology at Children’s National in Washington had no relevant disclosures.
WASHINGTON – During a session at the annual meeting of the American Academy of Dermatology, Adam Friedman, MD, presented on off-label use of immunomodulators for inflammatory skin diseases, the highlights of which he shared with fellow George Washington University dermatologist, A. Yasmine Kirkorian, MD, in an interview following the session.

Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington,
For example, as reflected in PubMed searches, low-dose naltrexone, which has to be compounded, is being used for such diseases as Hailey-Hailey and lichen planopilaris, said Dr. Friedman, who is using it for his mast cell activation syndrome patients. During the interview, he also describes his treatment approach for urticaria.
In his final remarks, Dr. Friedman encourages colleagues to “get creative,” publish, and talk about their experiences with off-label treatments in dermatology, citing the example of an article that mentioned using pioglitazone for lichen planopilaris. This article stimulated interest in using the type 2 diabetes agent pioglitazone to treat this skin disease, he notes.
Dr. Friedman and Dr. Kirkorian, a pediatric dermatologist at George Washington University and interim chief of pediatric dermatology at Children’s National in Washington had no relevant disclosures.
WASHINGTON – During a session at the annual meeting of the American Academy of Dermatology, Adam Friedman, MD, presented on off-label use of immunomodulators for inflammatory skin diseases, the highlights of which he shared with fellow George Washington University dermatologist, A. Yasmine Kirkorian, MD, in an interview following the session.

Dr. Friedman, professor and interim chair of dermatology at George Washington University, Washington,
For example, as reflected in PubMed searches, low-dose naltrexone, which has to be compounded, is being used for such diseases as Hailey-Hailey and lichen planopilaris, said Dr. Friedman, who is using it for his mast cell activation syndrome patients. During the interview, he also describes his treatment approach for urticaria.
In his final remarks, Dr. Friedman encourages colleagues to “get creative,” publish, and talk about their experiences with off-label treatments in dermatology, citing the example of an article that mentioned using pioglitazone for lichen planopilaris. This article stimulated interest in using the type 2 diabetes agent pioglitazone to treat this skin disease, he notes.
Dr. Friedman and Dr. Kirkorian, a pediatric dermatologist at George Washington University and interim chief of pediatric dermatology at Children’s National in Washington had no relevant disclosures.
Immunomodulators for pediatric skin diseases
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
Consider individualized testosterone protocol for transgender acne
but patient compatibility will depend upon several factors, advised Jason A. Park and his associates in a research letter to the editor of the Journal of the American Academy of Dermatology.
In a multivariate logistic regression analysis, Mr. Park and his colleagues at Boston University sought to determine the timing of onset of acne in female-to-male transgender patients.
A total of 55 patients undergoing hormone therapy at the Center for Transgender Medicine and Surgery at Boston Medical Center between January 1, 2010, and December 31, 2017 were selected following a systematic chart review. Patients were excluded who were under the age of 18 years, who had been receiving testosterone therapy for less than 2 years, who presented with acne before start of treatment, or whose medical records were incomplete.
Given evidence in prior studies reporting on an association between elevated androgen levels and increased incidence of acne in this patient group, a median serum testosterone level of 630 ng/dL “was used to differentiate between higher and lower levels.”
Acne was found to develop in 9% of transgender men after 3 months and in 18% after 6 months; 38% of the subjects were found to have developed acne at some point during the study after 24 months of treatment. The authors found that acne was “significantly associated with serum testosterone levels higher than 630 ng/dL.” Increased body mass index (BMI), especially in those with positive smoking status, also was associated with an increased incidence of acne, the authors said.
According to several existing studies, transgender men undergoing testosterone therapy tend to develop increased sebum production and acne. Because the systemic and dermatologic virilization effects of testosterone are unpredictable once treatment has started, and because individual goals also are varied (from maximum virilization to only suppressing feminine secondary sex characteristics), Mr. Park and his colleagues suggested that customization may be ideal, provided they do not clash with individual patient transition goals, priorities, risk factors, and other comorbidities that may be present.
The study was funded by the Medical Student Summer Research Program at Boston University. The authors had no conflicts of interest to report.
SOURCE: Park JA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.12.040.
but patient compatibility will depend upon several factors, advised Jason A. Park and his associates in a research letter to the editor of the Journal of the American Academy of Dermatology.
In a multivariate logistic regression analysis, Mr. Park and his colleagues at Boston University sought to determine the timing of onset of acne in female-to-male transgender patients.
A total of 55 patients undergoing hormone therapy at the Center for Transgender Medicine and Surgery at Boston Medical Center between January 1, 2010, and December 31, 2017 were selected following a systematic chart review. Patients were excluded who were under the age of 18 years, who had been receiving testosterone therapy for less than 2 years, who presented with acne before start of treatment, or whose medical records were incomplete.
Given evidence in prior studies reporting on an association between elevated androgen levels and increased incidence of acne in this patient group, a median serum testosterone level of 630 ng/dL “was used to differentiate between higher and lower levels.”
Acne was found to develop in 9% of transgender men after 3 months and in 18% after 6 months; 38% of the subjects were found to have developed acne at some point during the study after 24 months of treatment. The authors found that acne was “significantly associated with serum testosterone levels higher than 630 ng/dL.” Increased body mass index (BMI), especially in those with positive smoking status, also was associated with an increased incidence of acne, the authors said.
According to several existing studies, transgender men undergoing testosterone therapy tend to develop increased sebum production and acne. Because the systemic and dermatologic virilization effects of testosterone are unpredictable once treatment has started, and because individual goals also are varied (from maximum virilization to only suppressing feminine secondary sex characteristics), Mr. Park and his colleagues suggested that customization may be ideal, provided they do not clash with individual patient transition goals, priorities, risk factors, and other comorbidities that may be present.
The study was funded by the Medical Student Summer Research Program at Boston University. The authors had no conflicts of interest to report.
SOURCE: Park JA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.12.040.
but patient compatibility will depend upon several factors, advised Jason A. Park and his associates in a research letter to the editor of the Journal of the American Academy of Dermatology.
In a multivariate logistic regression analysis, Mr. Park and his colleagues at Boston University sought to determine the timing of onset of acne in female-to-male transgender patients.
A total of 55 patients undergoing hormone therapy at the Center for Transgender Medicine and Surgery at Boston Medical Center between January 1, 2010, and December 31, 2017 were selected following a systematic chart review. Patients were excluded who were under the age of 18 years, who had been receiving testosterone therapy for less than 2 years, who presented with acne before start of treatment, or whose medical records were incomplete.
Given evidence in prior studies reporting on an association between elevated androgen levels and increased incidence of acne in this patient group, a median serum testosterone level of 630 ng/dL “was used to differentiate between higher and lower levels.”
Acne was found to develop in 9% of transgender men after 3 months and in 18% after 6 months; 38% of the subjects were found to have developed acne at some point during the study after 24 months of treatment. The authors found that acne was “significantly associated with serum testosterone levels higher than 630 ng/dL.” Increased body mass index (BMI), especially in those with positive smoking status, also was associated with an increased incidence of acne, the authors said.
According to several existing studies, transgender men undergoing testosterone therapy tend to develop increased sebum production and acne. Because the systemic and dermatologic virilization effects of testosterone are unpredictable once treatment has started, and because individual goals also are varied (from maximum virilization to only suppressing feminine secondary sex characteristics), Mr. Park and his colleagues suggested that customization may be ideal, provided they do not clash with individual patient transition goals, priorities, risk factors, and other comorbidities that may be present.
The study was funded by the Medical Student Summer Research Program at Boston University. The authors had no conflicts of interest to report.
SOURCE: Park JA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.12.040.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Patient, heal thyself!
Octavio has prostate cancer. His prostate growth is large but localized.
“What do your doctors suggest?” I asked him.
“They sent me to two specialists at the medical center,” he said. “One does robotic surgery, the other does radiation. Each one told me why they recommend their technique.”
“How will you decide?”
“I’ll do some reading,” he said.
“What about the doctor who sent you to them?”
“He hasn’t discussed the choice with me, just sent me to get opinions. I have to make up own mind.”
Out of training for some time, I gather from students and family medical interactions that patient autonomy is now a reigning principle. Here is one definition:
. Patient autonomy does allow for health care providers to educate the patient but does not allow the health care provider to make the decision for the patient.
This sounds sensible, even admirable: no more paternalistic physicians talking down to patients and ordering them around. Yet a closer look shows a contradiction:
1. The second sentence says that patient autonomy “does not allow the health care provider to make the decision for the patient.”
2. But the first one says that patients should decide, “without their health care provider trying to influence the decision.”
Is “trying to influence” the same as making the decision for the patient?
Some would argue that it is: The power discrepancy between the parties makes a doctor’s attempt to influence amount to coercion.
Do you agree, esteemed colleagues, those of you who, like me, treat patients all day? If the choice is between freezing an actinic keratosis, burning it, or using topical chemotherapy, do you just lay all three options out there and ask the patient to pick one? What if your patient works in public and doesn’t have 2 weeks to wait while the reaction to topical 5-fluorouracil that makes his skin look like raw lobster subsides? Can you point that out? Or would that be “trying to influence” and thus not allowed?
You and I can think of many other examples, about medical choices large and small, where we could pose similar questions. This is not abstract philosophy; it is what we do all day.
Look up robot-assisted surgery and radiation for prostate cancer. You will find proponents of both, each making claims concerning survival, recurrence, discomfort, complications. Which is more important – a 15% greater chance of living 2 years longer or a 22% lower risk of incontinence? Will reading such statistics make your choice easier? What if other studies show different numbers?
Octavio chose surgery. I asked him how he decided.
“I talked with an internist I know socially,” he said. “He shared his experience with patients he’s referred for my problem and advised surgery as the better choice. I also saw a story online about a lawyer who chose one method, then 5 years later had to do the other.”
Octavio is sophisticated and well read. He lives near Boston, the self-described hub of medical expertise and academic excellence. Yet he makes up his mind the way everybody does: by asking a trusted adviser, by hearing an arresting anecdote. It’s not science. It’s how people think.
You don’t have to be a behavioral psychologist to know how hard it is for patients, especially frightened ones, to interpret statistical variances or compare disparate categories. Which is better – shorter life with less pain or longer life with more? How much less? How much more? There are ways to address such questions, but having an expert, trusted, and sympathetic adviser is a pretty good way to start. Only an abstract ethicist with no practical exposure to (or sympathy with) actual existing patients and their actual existing providers could possibly think otherwise.
“Let’s freeze those actinics off,” I suggest to a patient. “That won’t scar, you won’t need a dozen shots of lidocaine, and you won’t have to hide for 3 weeks.”
Did I influence her health care decision? Sure. Guilty as charged, with no apologies. When I am a patient, I want nothing less for myself: sympathetic, experienced guidance, shared by someone who knows me and appears to care one way or the other how I do.
Lord preserve us, doctors and patients both, from dogmatists who would demand otherwise.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Octavio has prostate cancer. His prostate growth is large but localized.
“What do your doctors suggest?” I asked him.
“They sent me to two specialists at the medical center,” he said. “One does robotic surgery, the other does radiation. Each one told me why they recommend their technique.”
“How will you decide?”
“I’ll do some reading,” he said.
“What about the doctor who sent you to them?”
“He hasn’t discussed the choice with me, just sent me to get opinions. I have to make up own mind.”
Out of training for some time, I gather from students and family medical interactions that patient autonomy is now a reigning principle. Here is one definition:
. Patient autonomy does allow for health care providers to educate the patient but does not allow the health care provider to make the decision for the patient.
This sounds sensible, even admirable: no more paternalistic physicians talking down to patients and ordering them around. Yet a closer look shows a contradiction:
1. The second sentence says that patient autonomy “does not allow the health care provider to make the decision for the patient.”
2. But the first one says that patients should decide, “without their health care provider trying to influence the decision.”
Is “trying to influence” the same as making the decision for the patient?
Some would argue that it is: The power discrepancy between the parties makes a doctor’s attempt to influence amount to coercion.
Do you agree, esteemed colleagues, those of you who, like me, treat patients all day? If the choice is between freezing an actinic keratosis, burning it, or using topical chemotherapy, do you just lay all three options out there and ask the patient to pick one? What if your patient works in public and doesn’t have 2 weeks to wait while the reaction to topical 5-fluorouracil that makes his skin look like raw lobster subsides? Can you point that out? Or would that be “trying to influence” and thus not allowed?
You and I can think of many other examples, about medical choices large and small, where we could pose similar questions. This is not abstract philosophy; it is what we do all day.
Look up robot-assisted surgery and radiation for prostate cancer. You will find proponents of both, each making claims concerning survival, recurrence, discomfort, complications. Which is more important – a 15% greater chance of living 2 years longer or a 22% lower risk of incontinence? Will reading such statistics make your choice easier? What if other studies show different numbers?
Octavio chose surgery. I asked him how he decided.
“I talked with an internist I know socially,” he said. “He shared his experience with patients he’s referred for my problem and advised surgery as the better choice. I also saw a story online about a lawyer who chose one method, then 5 years later had to do the other.”
Octavio is sophisticated and well read. He lives near Boston, the self-described hub of medical expertise and academic excellence. Yet he makes up his mind the way everybody does: by asking a trusted adviser, by hearing an arresting anecdote. It’s not science. It’s how people think.
You don’t have to be a behavioral psychologist to know how hard it is for patients, especially frightened ones, to interpret statistical variances or compare disparate categories. Which is better – shorter life with less pain or longer life with more? How much less? How much more? There are ways to address such questions, but having an expert, trusted, and sympathetic adviser is a pretty good way to start. Only an abstract ethicist with no practical exposure to (or sympathy with) actual existing patients and their actual existing providers could possibly think otherwise.
“Let’s freeze those actinics off,” I suggest to a patient. “That won’t scar, you won’t need a dozen shots of lidocaine, and you won’t have to hide for 3 weeks.”
Did I influence her health care decision? Sure. Guilty as charged, with no apologies. When I am a patient, I want nothing less for myself: sympathetic, experienced guidance, shared by someone who knows me and appears to care one way or the other how I do.
Lord preserve us, doctors and patients both, from dogmatists who would demand otherwise.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Octavio has prostate cancer. His prostate growth is large but localized.
“What do your doctors suggest?” I asked him.
“They sent me to two specialists at the medical center,” he said. “One does robotic surgery, the other does radiation. Each one told me why they recommend their technique.”
“How will you decide?”
“I’ll do some reading,” he said.
“What about the doctor who sent you to them?”
“He hasn’t discussed the choice with me, just sent me to get opinions. I have to make up own mind.”
Out of training for some time, I gather from students and family medical interactions that patient autonomy is now a reigning principle. Here is one definition:
. Patient autonomy does allow for health care providers to educate the patient but does not allow the health care provider to make the decision for the patient.
This sounds sensible, even admirable: no more paternalistic physicians talking down to patients and ordering them around. Yet a closer look shows a contradiction:
1. The second sentence says that patient autonomy “does not allow the health care provider to make the decision for the patient.”
2. But the first one says that patients should decide, “without their health care provider trying to influence the decision.”
Is “trying to influence” the same as making the decision for the patient?
Some would argue that it is: The power discrepancy between the parties makes a doctor’s attempt to influence amount to coercion.
Do you agree, esteemed colleagues, those of you who, like me, treat patients all day? If the choice is between freezing an actinic keratosis, burning it, or using topical chemotherapy, do you just lay all three options out there and ask the patient to pick one? What if your patient works in public and doesn’t have 2 weeks to wait while the reaction to topical 5-fluorouracil that makes his skin look like raw lobster subsides? Can you point that out? Or would that be “trying to influence” and thus not allowed?
You and I can think of many other examples, about medical choices large and small, where we could pose similar questions. This is not abstract philosophy; it is what we do all day.
Look up robot-assisted surgery and radiation for prostate cancer. You will find proponents of both, each making claims concerning survival, recurrence, discomfort, complications. Which is more important – a 15% greater chance of living 2 years longer or a 22% lower risk of incontinence? Will reading such statistics make your choice easier? What if other studies show different numbers?
Octavio chose surgery. I asked him how he decided.
“I talked with an internist I know socially,” he said. “He shared his experience with patients he’s referred for my problem and advised surgery as the better choice. I also saw a story online about a lawyer who chose one method, then 5 years later had to do the other.”
Octavio is sophisticated and well read. He lives near Boston, the self-described hub of medical expertise and academic excellence. Yet he makes up his mind the way everybody does: by asking a trusted adviser, by hearing an arresting anecdote. It’s not science. It’s how people think.
You don’t have to be a behavioral psychologist to know how hard it is for patients, especially frightened ones, to interpret statistical variances or compare disparate categories. Which is better – shorter life with less pain or longer life with more? How much less? How much more? There are ways to address such questions, but having an expert, trusted, and sympathetic adviser is a pretty good way to start. Only an abstract ethicist with no practical exposure to (or sympathy with) actual existing patients and their actual existing providers could possibly think otherwise.
“Let’s freeze those actinics off,” I suggest to a patient. “That won’t scar, you won’t need a dozen shots of lidocaine, and you won’t have to hide for 3 weeks.”
Did I influence her health care decision? Sure. Guilty as charged, with no apologies. When I am a patient, I want nothing less for myself: sympathetic, experienced guidance, shared by someone who knows me and appears to care one way or the other how I do.
Lord preserve us, doctors and patients both, from dogmatists who would demand otherwise.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].