User login
Apremilast for Behçet’s oral ulcers: Benefits maintained at 64 weeks
MADRID – of the long-term extension phase of the pivotal RELIEF trial, Alfred Mahr, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We now have strong evidence that apremilast is an effective and safe therapy to treat oral ulcers in patients with Behçet’s syndrome. I think this is a major advance in the field,” declared Dr. Mahr, a rheumatologist at St. Gallen (Switzerland) Cantonal Hospital.
Based largely upon the results of the 12-week, double-blind portion of the phase 3 RELIEF trial, the Food and Drug Administration approved apremilast (Otezla) for the treatment of oral ulcers in patients with Behçet’s disease in the summer of 2019.
The safety profile of the oral phosphodiesterase-4 inhibitor was as seen in other studies, including in patients with psoriatic arthritis, an FDA-approved indication for the drug since 2014. The main side effects in the long-term extension of RELIEF were diarrhea and nausea, typically mild or moderate in nature and roughly twice as frequent as in placebo-treated controls in the double-blind study phase.
“At the end of the day, at week 64, only 12% of patients treated with apremilast during the entire 64 weeks discontinued the drug due to a treatment-emergent adverse event, which I believe is a good indicator of the safety of this medication,” the rheumatologist said. “The overall feeling is that the benefit-to-risk ratio is very good and it’s a safe drug to prescribe.”
At the close of the initial 12-week, double-blind phase of RELIEF, 178 of the original 207 participants elected to enter the long-term extension, either staying on apremilast at 30 mg twice a day for an additional 52 weeks or switching to that regimen from placebo.
The focus of the long-term extension was on disease activity and quality of life outcomes. The results in patients who had switched from placebo to apremilast after 12 weeks proved to be reassuringly similar to outcomes in patients on the drug for the full duration. For example, the mean improvement on the patient-reported Behçet’s Syndrome Activity Scale was 18.6 points after 12 weeks of double-blind apremilast, 16.9 points after 64 weeks of continuous apremilast, and 16.8 points with 12 weeks of placebo followed by 52 weeks of active therapy.
After 12 weeks of double-blind apremilast, patients averaged a 3.4-point improvement on the Behçet’s Disease Quality of Life measure. After 64 weeks on the drug, the improvement over baseline was 3.6 points, while in the switch group it was 3.4 points. Similarly, on all three components of the SF-36 quality of life metric, the continuous apremilast group showed maintenance of effect from week 12 to week 64, while the placebo-to-apremilast group caught up. The same was true with regards to the Behçet’s Disease Current Activity Index, which encompasses measures of both the patient’s and clinician’s perception of disease activity.
At the outset of the RELIEF trial, participants averaged four oral ulcers. At week 64, the continuous apremilast group averaged 1.4 and the switch group 0.8, a nonsignificant difference.
Asked if apremilast had a favorable impact upon other manifestations of Behçet’s disease besides the oral ulcers, Dr. Mahr replied, “This is a very good question. People often wonder about it. We do, too. But this trial was not designed to capture less common manifestations of Behçet’s syndrome, such as genital ulcers. There have been some analyses done, but the number of patients who had genital ulcers at 12 weeks were very few. The same was true for eye manifestations. There was sort of a signal that it works, but we can’t prove it in a placebo-controlled trial.”
Dr. Mahr reported receiving research funding from and serving as a consultant to Celgene, the study sponsor.
MADRID – of the long-term extension phase of the pivotal RELIEF trial, Alfred Mahr, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We now have strong evidence that apremilast is an effective and safe therapy to treat oral ulcers in patients with Behçet’s syndrome. I think this is a major advance in the field,” declared Dr. Mahr, a rheumatologist at St. Gallen (Switzerland) Cantonal Hospital.
Based largely upon the results of the 12-week, double-blind portion of the phase 3 RELIEF trial, the Food and Drug Administration approved apremilast (Otezla) for the treatment of oral ulcers in patients with Behçet’s disease in the summer of 2019.
The safety profile of the oral phosphodiesterase-4 inhibitor was as seen in other studies, including in patients with psoriatic arthritis, an FDA-approved indication for the drug since 2014. The main side effects in the long-term extension of RELIEF were diarrhea and nausea, typically mild or moderate in nature and roughly twice as frequent as in placebo-treated controls in the double-blind study phase.
“At the end of the day, at week 64, only 12% of patients treated with apremilast during the entire 64 weeks discontinued the drug due to a treatment-emergent adverse event, which I believe is a good indicator of the safety of this medication,” the rheumatologist said. “The overall feeling is that the benefit-to-risk ratio is very good and it’s a safe drug to prescribe.”
At the close of the initial 12-week, double-blind phase of RELIEF, 178 of the original 207 participants elected to enter the long-term extension, either staying on apremilast at 30 mg twice a day for an additional 52 weeks or switching to that regimen from placebo.
The focus of the long-term extension was on disease activity and quality of life outcomes. The results in patients who had switched from placebo to apremilast after 12 weeks proved to be reassuringly similar to outcomes in patients on the drug for the full duration. For example, the mean improvement on the patient-reported Behçet’s Syndrome Activity Scale was 18.6 points after 12 weeks of double-blind apremilast, 16.9 points after 64 weeks of continuous apremilast, and 16.8 points with 12 weeks of placebo followed by 52 weeks of active therapy.
After 12 weeks of double-blind apremilast, patients averaged a 3.4-point improvement on the Behçet’s Disease Quality of Life measure. After 64 weeks on the drug, the improvement over baseline was 3.6 points, while in the switch group it was 3.4 points. Similarly, on all three components of the SF-36 quality of life metric, the continuous apremilast group showed maintenance of effect from week 12 to week 64, while the placebo-to-apremilast group caught up. The same was true with regards to the Behçet’s Disease Current Activity Index, which encompasses measures of both the patient’s and clinician’s perception of disease activity.
At the outset of the RELIEF trial, participants averaged four oral ulcers. At week 64, the continuous apremilast group averaged 1.4 and the switch group 0.8, a nonsignificant difference.
Asked if apremilast had a favorable impact upon other manifestations of Behçet’s disease besides the oral ulcers, Dr. Mahr replied, “This is a very good question. People often wonder about it. We do, too. But this trial was not designed to capture less common manifestations of Behçet’s syndrome, such as genital ulcers. There have been some analyses done, but the number of patients who had genital ulcers at 12 weeks were very few. The same was true for eye manifestations. There was sort of a signal that it works, but we can’t prove it in a placebo-controlled trial.”
Dr. Mahr reported receiving research funding from and serving as a consultant to Celgene, the study sponsor.
MADRID – of the long-term extension phase of the pivotal RELIEF trial, Alfred Mahr, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We now have strong evidence that apremilast is an effective and safe therapy to treat oral ulcers in patients with Behçet’s syndrome. I think this is a major advance in the field,” declared Dr. Mahr, a rheumatologist at St. Gallen (Switzerland) Cantonal Hospital.
Based largely upon the results of the 12-week, double-blind portion of the phase 3 RELIEF trial, the Food and Drug Administration approved apremilast (Otezla) for the treatment of oral ulcers in patients with Behçet’s disease in the summer of 2019.
The safety profile of the oral phosphodiesterase-4 inhibitor was as seen in other studies, including in patients with psoriatic arthritis, an FDA-approved indication for the drug since 2014. The main side effects in the long-term extension of RELIEF were diarrhea and nausea, typically mild or moderate in nature and roughly twice as frequent as in placebo-treated controls in the double-blind study phase.
“At the end of the day, at week 64, only 12% of patients treated with apremilast during the entire 64 weeks discontinued the drug due to a treatment-emergent adverse event, which I believe is a good indicator of the safety of this medication,” the rheumatologist said. “The overall feeling is that the benefit-to-risk ratio is very good and it’s a safe drug to prescribe.”
At the close of the initial 12-week, double-blind phase of RELIEF, 178 of the original 207 participants elected to enter the long-term extension, either staying on apremilast at 30 mg twice a day for an additional 52 weeks or switching to that regimen from placebo.
The focus of the long-term extension was on disease activity and quality of life outcomes. The results in patients who had switched from placebo to apremilast after 12 weeks proved to be reassuringly similar to outcomes in patients on the drug for the full duration. For example, the mean improvement on the patient-reported Behçet’s Syndrome Activity Scale was 18.6 points after 12 weeks of double-blind apremilast, 16.9 points after 64 weeks of continuous apremilast, and 16.8 points with 12 weeks of placebo followed by 52 weeks of active therapy.
After 12 weeks of double-blind apremilast, patients averaged a 3.4-point improvement on the Behçet’s Disease Quality of Life measure. After 64 weeks on the drug, the improvement over baseline was 3.6 points, while in the switch group it was 3.4 points. Similarly, on all three components of the SF-36 quality of life metric, the continuous apremilast group showed maintenance of effect from week 12 to week 64, while the placebo-to-apremilast group caught up. The same was true with regards to the Behçet’s Disease Current Activity Index, which encompasses measures of both the patient’s and clinician’s perception of disease activity.
At the outset of the RELIEF trial, participants averaged four oral ulcers. At week 64, the continuous apremilast group averaged 1.4 and the switch group 0.8, a nonsignificant difference.
Asked if apremilast had a favorable impact upon other manifestations of Behçet’s disease besides the oral ulcers, Dr. Mahr replied, “This is a very good question. People often wonder about it. We do, too. But this trial was not designed to capture less common manifestations of Behçet’s syndrome, such as genital ulcers. There have been some analyses done, but the number of patients who had genital ulcers at 12 weeks were very few. The same was true for eye manifestations. There was sort of a signal that it works, but we can’t prove it in a placebo-controlled trial.”
Dr. Mahr reported receiving research funding from and serving as a consultant to Celgene, the study sponsor.
REPORTING FROM EADV 2019
Dermatologists: Beware the ‘insulin ball’
LAS VEGAS – The patient, a 61-year-old man, came to see a dermatologist here about subcutaneous masses on his left arm, abdomen, and on both thighs.
It didn’t take long for Curt Samlaska, MD, of the University of Nevada, Reno, to link the masses to the patient’s daily regimen of seven insulin injections.
But diagnosing the condition required more than asking a few questions. At first, the man appeared to suffer from lipohypertrophy – a lump caused by an accumulation of fat at the site of insulin injections. But, Dr. Samlaska told colleagues, the patient had a different condition that’s barely been discussed in the dermatologic literature – insulin-derived amyloidosis, also known as “insulin ball.”
“It’s probably much more prevalent than we currently appreciate,” said Dr. Samlaska, who spoke in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Many cases are not [fully] evaluated and thought to be lipohypertrophy.”
Dr. Samlaska’s patient had suffered from diabetes since age 23 and tightly controls his blood sugar through seven daily injections. He injects short-acting insulin into his arms and abdomen, and long-acting insulin into his thighs.
The masses began appearing about 10 years ago, he told Dr. Samlaska, and he’s suffered more pain while injecting them over time. But the masses are easier to grasp during injections, and the patient’s body did not offer many other sites for injections.
, almost all in endocrinology journals. Ninety percent have a single lump, most commonly in the abdomen, and most have poor glycemic control, he said. (His patient is an outlier.)
Research suggests that insulin balls absorb about 34% of the insulin that’s injected, meaning that patients must inject more than usual to get the same effect. Be careful to advise patients about this, Dr. Samlaska said, because they might try alternative injection sites and get a sudden unexpected flood of insulin – potentially causing hypoglycemia.
He added that another drug – the HIV fusion inhibitor enfuvirtide – also has been linked to amyloidosis.
Pathology can offer insight into whether a mass is an insulin ball or a case of lipohypertrophy, he said. “They’re difficult to distinguish on clinical grounds,” he said, although lipohypertrophy masses are firmer, and they shrink when patients stop injecting insulin. Insulin balls do not.
The treatment for insulin balls is surgical excision, he said. “It’s very easy to do. With the extrusion technique, it comes out like a cheese, like a cyst.”
He said his patient was scheduled to soon undergo excision treatment.
Dr. Samlaska reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – The patient, a 61-year-old man, came to see a dermatologist here about subcutaneous masses on his left arm, abdomen, and on both thighs.
It didn’t take long for Curt Samlaska, MD, of the University of Nevada, Reno, to link the masses to the patient’s daily regimen of seven insulin injections.
But diagnosing the condition required more than asking a few questions. At first, the man appeared to suffer from lipohypertrophy – a lump caused by an accumulation of fat at the site of insulin injections. But, Dr. Samlaska told colleagues, the patient had a different condition that’s barely been discussed in the dermatologic literature – insulin-derived amyloidosis, also known as “insulin ball.”
“It’s probably much more prevalent than we currently appreciate,” said Dr. Samlaska, who spoke in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Many cases are not [fully] evaluated and thought to be lipohypertrophy.”
Dr. Samlaska’s patient had suffered from diabetes since age 23 and tightly controls his blood sugar through seven daily injections. He injects short-acting insulin into his arms and abdomen, and long-acting insulin into his thighs.
The masses began appearing about 10 years ago, he told Dr. Samlaska, and he’s suffered more pain while injecting them over time. But the masses are easier to grasp during injections, and the patient’s body did not offer many other sites for injections.
, almost all in endocrinology journals. Ninety percent have a single lump, most commonly in the abdomen, and most have poor glycemic control, he said. (His patient is an outlier.)
Research suggests that insulin balls absorb about 34% of the insulin that’s injected, meaning that patients must inject more than usual to get the same effect. Be careful to advise patients about this, Dr. Samlaska said, because they might try alternative injection sites and get a sudden unexpected flood of insulin – potentially causing hypoglycemia.
He added that another drug – the HIV fusion inhibitor enfuvirtide – also has been linked to amyloidosis.
Pathology can offer insight into whether a mass is an insulin ball or a case of lipohypertrophy, he said. “They’re difficult to distinguish on clinical grounds,” he said, although lipohypertrophy masses are firmer, and they shrink when patients stop injecting insulin. Insulin balls do not.
The treatment for insulin balls is surgical excision, he said. “It’s very easy to do. With the extrusion technique, it comes out like a cheese, like a cyst.”
He said his patient was scheduled to soon undergo excision treatment.
Dr. Samlaska reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – The patient, a 61-year-old man, came to see a dermatologist here about subcutaneous masses on his left arm, abdomen, and on both thighs.
It didn’t take long for Curt Samlaska, MD, of the University of Nevada, Reno, to link the masses to the patient’s daily regimen of seven insulin injections.
But diagnosing the condition required more than asking a few questions. At first, the man appeared to suffer from lipohypertrophy – a lump caused by an accumulation of fat at the site of insulin injections. But, Dr. Samlaska told colleagues, the patient had a different condition that’s barely been discussed in the dermatologic literature – insulin-derived amyloidosis, also known as “insulin ball.”
“It’s probably much more prevalent than we currently appreciate,” said Dr. Samlaska, who spoke in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Many cases are not [fully] evaluated and thought to be lipohypertrophy.”
Dr. Samlaska’s patient had suffered from diabetes since age 23 and tightly controls his blood sugar through seven daily injections. He injects short-acting insulin into his arms and abdomen, and long-acting insulin into his thighs.
The masses began appearing about 10 years ago, he told Dr. Samlaska, and he’s suffered more pain while injecting them over time. But the masses are easier to grasp during injections, and the patient’s body did not offer many other sites for injections.
, almost all in endocrinology journals. Ninety percent have a single lump, most commonly in the abdomen, and most have poor glycemic control, he said. (His patient is an outlier.)
Research suggests that insulin balls absorb about 34% of the insulin that’s injected, meaning that patients must inject more than usual to get the same effect. Be careful to advise patients about this, Dr. Samlaska said, because they might try alternative injection sites and get a sudden unexpected flood of insulin – potentially causing hypoglycemia.
He added that another drug – the HIV fusion inhibitor enfuvirtide – also has been linked to amyloidosis.
Pathology can offer insight into whether a mass is an insulin ball or a case of lipohypertrophy, he said. “They’re difficult to distinguish on clinical grounds,” he said, although lipohypertrophy masses are firmer, and they shrink when patients stop injecting insulin. Insulin balls do not.
The treatment for insulin balls is surgical excision, he said. “It’s very easy to do. With the extrusion technique, it comes out like a cheese, like a cyst.”
He said his patient was scheduled to soon undergo excision treatment.
Dr. Samlaska reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Bezafibrate beats placebo in pruritus of chronic cholestasis: The FITCH trial
BOSTON – Bezafibrate was superior to placebo for ameliorating pruritus in patients with chronic cholestatic liver diseases, investigators reported at the annual meeting of the American Association for the Study of Liver Diseases.
Improvements in itch were reported by four times as many patients treated with the peroxisome proliferator-activated receptor (PPAR) agonist, compared with those treated with placebo, according to results of the FITCH (Fibrates for cholestatic ITCH) trial.
That finding from FITCH is very encouraging for patients with this “vexing” clinical issue, which can be highly distressing and is a common feature of cholestatic liver diseases, said Michael R. Charlton, MBBS, FRCP, director of the Transplant Institute and hepatology chief at the University of Chicago.
“It’s generally a misery-making condition,” Dr. Charlton said in a podium discussion of the FITCH study results at the Liver Meeting 2019. “I had a patient tell me that they felt like the subject of Edvard Munch’s ‘Scream’ painting.”
As of this meeting, bezafibrate should be considered superior to placebo for treatment of pruritus in cholangiopathies and should be “considered as first-line treatment” for pruritus in primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), added Dr. Charlton, who was not involved in the study.
Investigators in FITCH recruited a total of 74 patients – all enrolled in the Netherlands or Spain – with cholestasis-induced pruritus who reported itch with an intensity of least 5 out of 10 on a visual analogue scale (VAS). Of the 70 patients who completed the trial, 44 had PSC, 24 had PBC, and 2 had secondary sclerosing cholangitis. Patients were randomly allocated to receive bezafibrate 400 mg once daily or placebo for 21 days.
The hypothesis was that PPAR agonist treatment would relieve itch by alleviating hepatobiliary inflammation and reducing formation of a biliary itch factor, according to the investigators, led by Elsemieke de Vries, MD, of the department of gastroenterology and hepatology, Amsterdam University Medical Centers.
“Guideline-approved pharmacological strategies show limited efficacy and can provoke serious side effects,” Dr. de Vries and coauthors said in the published abstract on the study.
The primary study endpoint, a 50% reduction in pruritus VAS score, was achieved in 45% of patients in the bezafibrate treatment arm (17 of 38 patients) versus just 11% in the placebo arm (4 of 36 patients; P = .003), according to updated results presented at the meeting.
The mean VAS score, comparable at baseline, was significantly lower in the bezafibrate group vs. the placebo group at day 21 (P < .001), the results showed.
Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
SOURCE: de Vries E et al. The Liver Meeting 2019. Abstract 13.
BOSTON – Bezafibrate was superior to placebo for ameliorating pruritus in patients with chronic cholestatic liver diseases, investigators reported at the annual meeting of the American Association for the Study of Liver Diseases.
Improvements in itch were reported by four times as many patients treated with the peroxisome proliferator-activated receptor (PPAR) agonist, compared with those treated with placebo, according to results of the FITCH (Fibrates for cholestatic ITCH) trial.
That finding from FITCH is very encouraging for patients with this “vexing” clinical issue, which can be highly distressing and is a common feature of cholestatic liver diseases, said Michael R. Charlton, MBBS, FRCP, director of the Transplant Institute and hepatology chief at the University of Chicago.
“It’s generally a misery-making condition,” Dr. Charlton said in a podium discussion of the FITCH study results at the Liver Meeting 2019. “I had a patient tell me that they felt like the subject of Edvard Munch’s ‘Scream’ painting.”
As of this meeting, bezafibrate should be considered superior to placebo for treatment of pruritus in cholangiopathies and should be “considered as first-line treatment” for pruritus in primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), added Dr. Charlton, who was not involved in the study.
Investigators in FITCH recruited a total of 74 patients – all enrolled in the Netherlands or Spain – with cholestasis-induced pruritus who reported itch with an intensity of least 5 out of 10 on a visual analogue scale (VAS). Of the 70 patients who completed the trial, 44 had PSC, 24 had PBC, and 2 had secondary sclerosing cholangitis. Patients were randomly allocated to receive bezafibrate 400 mg once daily or placebo for 21 days.
The hypothesis was that PPAR agonist treatment would relieve itch by alleviating hepatobiliary inflammation and reducing formation of a biliary itch factor, according to the investigators, led by Elsemieke de Vries, MD, of the department of gastroenterology and hepatology, Amsterdam University Medical Centers.
“Guideline-approved pharmacological strategies show limited efficacy and can provoke serious side effects,” Dr. de Vries and coauthors said in the published abstract on the study.
The primary study endpoint, a 50% reduction in pruritus VAS score, was achieved in 45% of patients in the bezafibrate treatment arm (17 of 38 patients) versus just 11% in the placebo arm (4 of 36 patients; P = .003), according to updated results presented at the meeting.
The mean VAS score, comparable at baseline, was significantly lower in the bezafibrate group vs. the placebo group at day 21 (P < .001), the results showed.
Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
SOURCE: de Vries E et al. The Liver Meeting 2019. Abstract 13.
BOSTON – Bezafibrate was superior to placebo for ameliorating pruritus in patients with chronic cholestatic liver diseases, investigators reported at the annual meeting of the American Association for the Study of Liver Diseases.
Improvements in itch were reported by four times as many patients treated with the peroxisome proliferator-activated receptor (PPAR) agonist, compared with those treated with placebo, according to results of the FITCH (Fibrates for cholestatic ITCH) trial.
That finding from FITCH is very encouraging for patients with this “vexing” clinical issue, which can be highly distressing and is a common feature of cholestatic liver diseases, said Michael R. Charlton, MBBS, FRCP, director of the Transplant Institute and hepatology chief at the University of Chicago.
“It’s generally a misery-making condition,” Dr. Charlton said in a podium discussion of the FITCH study results at the Liver Meeting 2019. “I had a patient tell me that they felt like the subject of Edvard Munch’s ‘Scream’ painting.”
As of this meeting, bezafibrate should be considered superior to placebo for treatment of pruritus in cholangiopathies and should be “considered as first-line treatment” for pruritus in primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), added Dr. Charlton, who was not involved in the study.
Investigators in FITCH recruited a total of 74 patients – all enrolled in the Netherlands or Spain – with cholestasis-induced pruritus who reported itch with an intensity of least 5 out of 10 on a visual analogue scale (VAS). Of the 70 patients who completed the trial, 44 had PSC, 24 had PBC, and 2 had secondary sclerosing cholangitis. Patients were randomly allocated to receive bezafibrate 400 mg once daily or placebo for 21 days.
The hypothesis was that PPAR agonist treatment would relieve itch by alleviating hepatobiliary inflammation and reducing formation of a biliary itch factor, according to the investigators, led by Elsemieke de Vries, MD, of the department of gastroenterology and hepatology, Amsterdam University Medical Centers.
“Guideline-approved pharmacological strategies show limited efficacy and can provoke serious side effects,” Dr. de Vries and coauthors said in the published abstract on the study.
The primary study endpoint, a 50% reduction in pruritus VAS score, was achieved in 45% of patients in the bezafibrate treatment arm (17 of 38 patients) versus just 11% in the placebo arm (4 of 36 patients; P = .003), according to updated results presented at the meeting.
The mean VAS score, comparable at baseline, was significantly lower in the bezafibrate group vs. the placebo group at day 21 (P < .001), the results showed.
Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
SOURCE: de Vries E et al. The Liver Meeting 2019. Abstract 13.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: Bezafibrate was superior to placebo for improving pruritus in chronic cholestatic liver diseases.
Major finding: A 50% reduction in pruritus visual analogue scale (VAS) score was achieved in 45% of patients in the bezafibrate treatment arm versus 11% in the placebo arm (P = .003).
Study details: Report on the randomized, placebo-controlled FITCH trial including 74 patients with cholestasis-induced pruritus.
Disclosures: Authors of the FITCH study reported disclosures related to Intercept Pharmaceuticals, Gilead, Takeda, Tillotts, Pliant, and Dr. Falk GmbH.
Source: de Vries E et al. The Liver Meeting 2019. Abstract 13.
Sweaty patient? Treatments require patient education
LAS VEGAS – at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
During an examination for another condition, he said, patients may be “sweating and dripping.” However, “you look over that diagnosis because that’s not what they’re there for,” said Dr. Desai, a dermatologist at the University of Texas Southwestern Medical Center in Dallas.
He described one of his patients, who only revealed that she suffered from “horrible, devastating” hyperhidrosis after he’d treated her for years for melasma. The sweating especially affected her because it prevented her from wearing the skin-exposing clothing of her Indian culture.
Delays in treatment are common in hyperhidrosis, which is believed to affect 5% of the world’s population. According to Dr. Desai, research suggests that 85% of patients with hyperhidrosis wait more than 3 years to bring it up with doctors, and half wait more than a decade.
There are many treatments for hyperhidrosis. Some are fairly simple: over-the-counter or prescription antiperspirants, said Dr. Desai, who likes the over-the-counter brand Certain Dri), iontophoresis (application of electric current), topical anticholinergics (including glycopyrronium tosylate cloth wipes, recently approved by the FDA for topical treatment of primary axillary hyperhidrosis for ages 9 years and older), and systemic management. Others are minimally invasive: Botox injections and the miraDry medical device (which relies on thermolysis). And surgical strategies may be an option for severe cases.
On its website, the International Hyperhidrosis Society provides a chart of options for hyperhidrosis in various parts of the body. Treatments tend to focus on the underarms, however, and “we’ve got huge unmet needs for patient options,” Dr. Desai said.
- During his presentation, he provided the following pearls regarding hyperhidrosis treatments:
- Distinguish between antiperspirants, which block sweating, and deodorants, which cover up body odor. “Sometimes I get caught up in the middle of a busy office visit and use these terms interchangeably. They’re really different, but patients and the public tend to equate those together,” he commented.
- Make sure patients understand how to properly use antiperspirants and explain that antiperspirants must be applied to dry skin. “Antiperspirant is forming a clog in the drain” to prevent the release of sweat, he said. “If you apply it to wet skin, you will block that chemical reaction in the duct.”
- Massage in the antiperspirant, he advises, and don’t occlude the skin. Apply twice daily, including before bedtime. “They can use antiperspirant on the hands and the bottom of the feet,”Dr. Desai said. “You want to ensure that they’re using the spray on the surface and in the web space. They can also use antiperspirants on the face, but avoid contact with the eyes.”
- Be careful if you prescribe glycopyrronium cloths off label. These wipes are helpful and they can be used outside the FDA-approved use in the underarms, said Dr. Desai, who said he has palmar hyperhidrosis and has successfully used them on his palms, but he hasn’t found them to be helpful on the soles of his feet.
Dr. Desai recommends 5-minute applications on the palms because the treatment can irritate the face and eyes.
Linda F. Stein Gold, MD, of Henry Ford Health System in Detroit, told the audience about the case of a teacher who touched his eyes after applying the treatment. He went to school, felt ill, and ended up in an emergency department because he had an enlarged pupil. “You just have to tell people this can happen,” she said.
Dr. Desai reported no relevant disclosures.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
During an examination for another condition, he said, patients may be “sweating and dripping.” However, “you look over that diagnosis because that’s not what they’re there for,” said Dr. Desai, a dermatologist at the University of Texas Southwestern Medical Center in Dallas.
He described one of his patients, who only revealed that she suffered from “horrible, devastating” hyperhidrosis after he’d treated her for years for melasma. The sweating especially affected her because it prevented her from wearing the skin-exposing clothing of her Indian culture.
Delays in treatment are common in hyperhidrosis, which is believed to affect 5% of the world’s population. According to Dr. Desai, research suggests that 85% of patients with hyperhidrosis wait more than 3 years to bring it up with doctors, and half wait more than a decade.
There are many treatments for hyperhidrosis. Some are fairly simple: over-the-counter or prescription antiperspirants, said Dr. Desai, who likes the over-the-counter brand Certain Dri), iontophoresis (application of electric current), topical anticholinergics (including glycopyrronium tosylate cloth wipes, recently approved by the FDA for topical treatment of primary axillary hyperhidrosis for ages 9 years and older), and systemic management. Others are minimally invasive: Botox injections and the miraDry medical device (which relies on thermolysis). And surgical strategies may be an option for severe cases.
On its website, the International Hyperhidrosis Society provides a chart of options for hyperhidrosis in various parts of the body. Treatments tend to focus on the underarms, however, and “we’ve got huge unmet needs for patient options,” Dr. Desai said.
- During his presentation, he provided the following pearls regarding hyperhidrosis treatments:
- Distinguish between antiperspirants, which block sweating, and deodorants, which cover up body odor. “Sometimes I get caught up in the middle of a busy office visit and use these terms interchangeably. They’re really different, but patients and the public tend to equate those together,” he commented.
- Make sure patients understand how to properly use antiperspirants and explain that antiperspirants must be applied to dry skin. “Antiperspirant is forming a clog in the drain” to prevent the release of sweat, he said. “If you apply it to wet skin, you will block that chemical reaction in the duct.”
- Massage in the antiperspirant, he advises, and don’t occlude the skin. Apply twice daily, including before bedtime. “They can use antiperspirant on the hands and the bottom of the feet,”Dr. Desai said. “You want to ensure that they’re using the spray on the surface and in the web space. They can also use antiperspirants on the face, but avoid contact with the eyes.”
- Be careful if you prescribe glycopyrronium cloths off label. These wipes are helpful and they can be used outside the FDA-approved use in the underarms, said Dr. Desai, who said he has palmar hyperhidrosis and has successfully used them on his palms, but he hasn’t found them to be helpful on the soles of his feet.
Dr. Desai recommends 5-minute applications on the palms because the treatment can irritate the face and eyes.
Linda F. Stein Gold, MD, of Henry Ford Health System in Detroit, told the audience about the case of a teacher who touched his eyes after applying the treatment. He went to school, felt ill, and ended up in an emergency department because he had an enlarged pupil. “You just have to tell people this can happen,” she said.
Dr. Desai reported no relevant disclosures.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
During an examination for another condition, he said, patients may be “sweating and dripping.” However, “you look over that diagnosis because that’s not what they’re there for,” said Dr. Desai, a dermatologist at the University of Texas Southwestern Medical Center in Dallas.
He described one of his patients, who only revealed that she suffered from “horrible, devastating” hyperhidrosis after he’d treated her for years for melasma. The sweating especially affected her because it prevented her from wearing the skin-exposing clothing of her Indian culture.
Delays in treatment are common in hyperhidrosis, which is believed to affect 5% of the world’s population. According to Dr. Desai, research suggests that 85% of patients with hyperhidrosis wait more than 3 years to bring it up with doctors, and half wait more than a decade.
There are many treatments for hyperhidrosis. Some are fairly simple: over-the-counter or prescription antiperspirants, said Dr. Desai, who likes the over-the-counter brand Certain Dri), iontophoresis (application of electric current), topical anticholinergics (including glycopyrronium tosylate cloth wipes, recently approved by the FDA for topical treatment of primary axillary hyperhidrosis for ages 9 years and older), and systemic management. Others are minimally invasive: Botox injections and the miraDry medical device (which relies on thermolysis). And surgical strategies may be an option for severe cases.
On its website, the International Hyperhidrosis Society provides a chart of options for hyperhidrosis in various parts of the body. Treatments tend to focus on the underarms, however, and “we’ve got huge unmet needs for patient options,” Dr. Desai said.
- During his presentation, he provided the following pearls regarding hyperhidrosis treatments:
- Distinguish between antiperspirants, which block sweating, and deodorants, which cover up body odor. “Sometimes I get caught up in the middle of a busy office visit and use these terms interchangeably. They’re really different, but patients and the public tend to equate those together,” he commented.
- Make sure patients understand how to properly use antiperspirants and explain that antiperspirants must be applied to dry skin. “Antiperspirant is forming a clog in the drain” to prevent the release of sweat, he said. “If you apply it to wet skin, you will block that chemical reaction in the duct.”
- Massage in the antiperspirant, he advises, and don’t occlude the skin. Apply twice daily, including before bedtime. “They can use antiperspirant on the hands and the bottom of the feet,”Dr. Desai said. “You want to ensure that they’re using the spray on the surface and in the web space. They can also use antiperspirants on the face, but avoid contact with the eyes.”
- Be careful if you prescribe glycopyrronium cloths off label. These wipes are helpful and they can be used outside the FDA-approved use in the underarms, said Dr. Desai, who said he has palmar hyperhidrosis and has successfully used them on his palms, but he hasn’t found them to be helpful on the soles of his feet.
Dr. Desai recommends 5-minute applications on the palms because the treatment can irritate the face and eyes.
Linda F. Stein Gold, MD, of Henry Ford Health System in Detroit, told the audience about the case of a teacher who touched his eyes after applying the treatment. He went to school, felt ill, and ended up in an emergency department because he had an enlarged pupil. “You just have to tell people this can happen,” she said.
Dr. Desai reported no relevant disclosures.
SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Hot tips on uncovering the causes of sweating
LAS VEGAS – It is important to think outside the box and consider whether secondary causes of hyperhidrosis are at play when a patient complains of sweating too much, a dermatologist told his colleagues.
“Look at where the patient fits into the sweating paradigm,” advised Seemal R. Desai, MD, of University of Texas Southwestern Medical Center in Dallas, and consider factors such as where and how often patients are oversweating.
In cases of secondary hyperhidrosis – those that are caused by another condition – said Dr. Desai, who spoke in a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
According to Dr. Desai, the answers to several questions can help pinpoint a diagnosis of primary hyperhidrosis (also known as focal or primary focal hyperhidrosis) or secondary hyperhidrosis:
- Where does the sweating occur?
Sweating occurs over large parts of the body in patients with secondary hyperhidrosis, Dr. Desai said, although it is typically limited to certain areas, such as the armpits, palms, or soles in the primary form.
- When did the sweating begin?
When sweating begins in adulthood, he said, there’s a good chance that it has a secondary cause. Sweating that began in childhood is more likely to be the primary form.
- How does sweating occur at night?
Dr. Desai advised: “Ask about sleep patterns. Do you sweat during your sleep or wake up feeling like you’re sweating?” Sweating throughout a sleep cycle – not “night sweats” that are brief in nature – indicate a probable secondary cause, he said.
According to Dr. Desai, the causes of secondary hyperhidrosis are numerous, including hypoglycemia, neural tumors, and cardiovascular conditions. “Typically, if I’m trying to figure out why a patient is having generalized sweating, the No. 1 cause is medications.”
Dr. Desai reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It is important to think outside the box and consider whether secondary causes of hyperhidrosis are at play when a patient complains of sweating too much, a dermatologist told his colleagues.
“Look at where the patient fits into the sweating paradigm,” advised Seemal R. Desai, MD, of University of Texas Southwestern Medical Center in Dallas, and consider factors such as where and how often patients are oversweating.
In cases of secondary hyperhidrosis – those that are caused by another condition – said Dr. Desai, who spoke in a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
According to Dr. Desai, the answers to several questions can help pinpoint a diagnosis of primary hyperhidrosis (also known as focal or primary focal hyperhidrosis) or secondary hyperhidrosis:
- Where does the sweating occur?
Sweating occurs over large parts of the body in patients with secondary hyperhidrosis, Dr. Desai said, although it is typically limited to certain areas, such as the armpits, palms, or soles in the primary form.
- When did the sweating begin?
When sweating begins in adulthood, he said, there’s a good chance that it has a secondary cause. Sweating that began in childhood is more likely to be the primary form.
- How does sweating occur at night?
Dr. Desai advised: “Ask about sleep patterns. Do you sweat during your sleep or wake up feeling like you’re sweating?” Sweating throughout a sleep cycle – not “night sweats” that are brief in nature – indicate a probable secondary cause, he said.
According to Dr. Desai, the causes of secondary hyperhidrosis are numerous, including hypoglycemia, neural tumors, and cardiovascular conditions. “Typically, if I’m trying to figure out why a patient is having generalized sweating, the No. 1 cause is medications.”
Dr. Desai reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – It is important to think outside the box and consider whether secondary causes of hyperhidrosis are at play when a patient complains of sweating too much, a dermatologist told his colleagues.
“Look at where the patient fits into the sweating paradigm,” advised Seemal R. Desai, MD, of University of Texas Southwestern Medical Center in Dallas, and consider factors such as where and how often patients are oversweating.
In cases of secondary hyperhidrosis – those that are caused by another condition – said Dr. Desai, who spoke in a presentation at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
According to Dr. Desai, the answers to several questions can help pinpoint a diagnosis of primary hyperhidrosis (also known as focal or primary focal hyperhidrosis) or secondary hyperhidrosis:
- Where does the sweating occur?
Sweating occurs over large parts of the body in patients with secondary hyperhidrosis, Dr. Desai said, although it is typically limited to certain areas, such as the armpits, palms, or soles in the primary form.
- When did the sweating begin?
When sweating begins in adulthood, he said, there’s a good chance that it has a secondary cause. Sweating that began in childhood is more likely to be the primary form.
- How does sweating occur at night?
Dr. Desai advised: “Ask about sleep patterns. Do you sweat during your sleep or wake up feeling like you’re sweating?” Sweating throughout a sleep cycle – not “night sweats” that are brief in nature – indicate a probable secondary cause, he said.
According to Dr. Desai, the causes of secondary hyperhidrosis are numerous, including hypoglycemia, neural tumors, and cardiovascular conditions. “Typically, if I’m trying to figure out why a patient is having generalized sweating, the No. 1 cause is medications.”
Dr. Desai reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
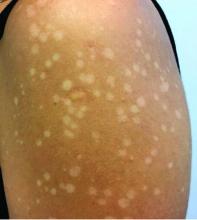
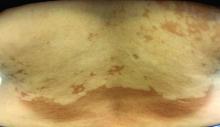
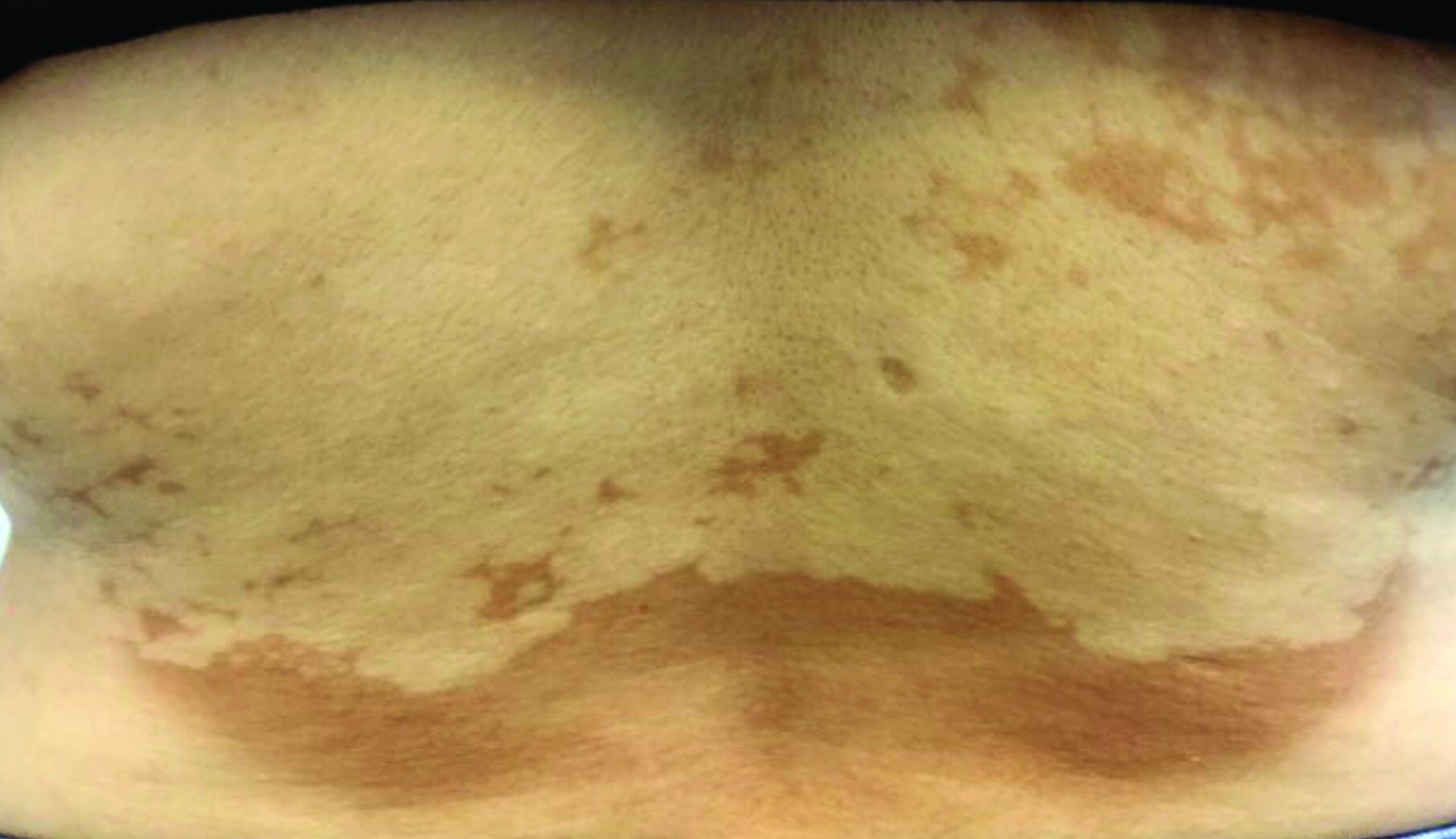
Asymptomatic hypopigmented macules and patches
, also known as Pityrosporum orbiculare or P. ovale. In its hyphal form, it produces skin lesions that appear as scaly, round or oval, hypopigmented, hyperpigmented, or pink macules or patches. Lesions are asymptomatic. The condition is more commonly seen in warm climates or during the summer months. Malassezia requires an oily environment for growth. Typically, TV appears in sebum-producing areas on the trunk. However, other sites may be affected such as the scalp, groin, and flexural areas. Infants may have facial lesions. Hypopigmentation may persist for months, even after lesions are treated, and takes time to resolve.
The differential diagnosis of hypopigmented lesions of tinea versicolor includes vitiligo, hypopigmented mycosis fungoides, progressive macular hypomelanosis (PMH), secondary syphilis, and pityriasis alba. Potassium hydroxide (KOH) preparations can be performed in the office for TV to reveal short, thick fungal hyphae with multiple spores, often referred to as “spaghetti and meatballs.” Use of a Wood’s light may aid in diagnosis. In TV, lesions may fluoresce yellow-green in adjacent follicles, unlike PMH, which characteristically show orange-red follicular fluorescence. A skin biopsy is necessary to rule out hypopgimented mycosis fungoides or syphilis. Histologically in TV, hyphae and spores will be present in the stratum corneum or in hair follicles. These are readily seen with PAS or GMS (Grocott methenamine silver) stains. There is usually no inflammation and skin appears “normal.” A biopsy was performed in this patient that revealed PAS positive hyphae.
Treatment for TV can be topical or systemic. Antifungal azole shampoo or creams, selenium sulfide shampoo, sulfur preparations, and allylamine creams have all been reported as useful treatments. Oral itraconazole or fluconazole are often given as systemic treatments. Monthly or weekly topical therapy may help prevent relapse.
This case and the photos were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
, also known as Pityrosporum orbiculare or P. ovale. In its hyphal form, it produces skin lesions that appear as scaly, round or oval, hypopigmented, hyperpigmented, or pink macules or patches. Lesions are asymptomatic. The condition is more commonly seen in warm climates or during the summer months. Malassezia requires an oily environment for growth. Typically, TV appears in sebum-producing areas on the trunk. However, other sites may be affected such as the scalp, groin, and flexural areas. Infants may have facial lesions. Hypopigmentation may persist for months, even after lesions are treated, and takes time to resolve.
The differential diagnosis of hypopigmented lesions of tinea versicolor includes vitiligo, hypopigmented mycosis fungoides, progressive macular hypomelanosis (PMH), secondary syphilis, and pityriasis alba. Potassium hydroxide (KOH) preparations can be performed in the office for TV to reveal short, thick fungal hyphae with multiple spores, often referred to as “spaghetti and meatballs.” Use of a Wood’s light may aid in diagnosis. In TV, lesions may fluoresce yellow-green in adjacent follicles, unlike PMH, which characteristically show orange-red follicular fluorescence. A skin biopsy is necessary to rule out hypopgimented mycosis fungoides or syphilis. Histologically in TV, hyphae and spores will be present in the stratum corneum or in hair follicles. These are readily seen with PAS or GMS (Grocott methenamine silver) stains. There is usually no inflammation and skin appears “normal.” A biopsy was performed in this patient that revealed PAS positive hyphae.
Treatment for TV can be topical or systemic. Antifungal azole shampoo or creams, selenium sulfide shampoo, sulfur preparations, and allylamine creams have all been reported as useful treatments. Oral itraconazole or fluconazole are often given as systemic treatments. Monthly or weekly topical therapy may help prevent relapse.
This case and the photos were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
, also known as Pityrosporum orbiculare or P. ovale. In its hyphal form, it produces skin lesions that appear as scaly, round or oval, hypopigmented, hyperpigmented, or pink macules or patches. Lesions are asymptomatic. The condition is more commonly seen in warm climates or during the summer months. Malassezia requires an oily environment for growth. Typically, TV appears in sebum-producing areas on the trunk. However, other sites may be affected such as the scalp, groin, and flexural areas. Infants may have facial lesions. Hypopigmentation may persist for months, even after lesions are treated, and takes time to resolve.
The differential diagnosis of hypopigmented lesions of tinea versicolor includes vitiligo, hypopigmented mycosis fungoides, progressive macular hypomelanosis (PMH), secondary syphilis, and pityriasis alba. Potassium hydroxide (KOH) preparations can be performed in the office for TV to reveal short, thick fungal hyphae with multiple spores, often referred to as “spaghetti and meatballs.” Use of a Wood’s light may aid in diagnosis. In TV, lesions may fluoresce yellow-green in adjacent follicles, unlike PMH, which characteristically show orange-red follicular fluorescence. A skin biopsy is necessary to rule out hypopgimented mycosis fungoides or syphilis. Histologically in TV, hyphae and spores will be present in the stratum corneum or in hair follicles. These are readily seen with PAS or GMS (Grocott methenamine silver) stains. There is usually no inflammation and skin appears “normal.” A biopsy was performed in this patient that revealed PAS positive hyphae.
Treatment for TV can be topical or systemic. Antifungal azole shampoo or creams, selenium sulfide shampoo, sulfur preparations, and allylamine creams have all been reported as useful treatments. Oral itraconazole or fluconazole are often given as systemic treatments. Monthly or weekly topical therapy may help prevent relapse.
This case and the photos were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Don’t leave dermatomyositis to the rheumatologists
LAS VEGAS – When she brings up dermatomyositis in the context of dermatology, Alisa Femia, MD, often hears from trainees and medical students who assume that this is a condition for rheumatologists to diagnose and treat. That’s not true: Dermatologists need to watch for this potentially fatal connective tissue disorder because they may see it first, she said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
“A fifth are clinically amyopathic,” and they may not initially see a rheumatologist, said Dr. Femia, director of inpatient dermatology at the department of dermatology, New York University. “They have normal muscle enzymes and no muscle weakness. It really puts them in our care as dermatologists.”
The challenge is that patient presentations can be subtle, but the stakes may be high, she added. “If we catch them early and treat some of these patients aggressively, we can save their lives.”
During the presentation, :
- Don’t rely on tests like biopsies to absolutely tell you what’s going on. “Clinical examination is the most important test to establish the diagnosis,” she said. “If you’re suspecting dermatomyositis ... get the patient in a gown [for a full-body exam] and look for all the signs that might not be as prominent as they are in our textbooks.”
- Look for the “butterfly” rash on the midface that is unique because it spares the nasolabial folds. “It looks like someone took an eraser and wiped out those areas,” Dr. Femia said. “This is important to help us nail down the diagnosis.”
- Other telltale signs, she said, include erythema on the extensor surfaces of digits, severe itching in the scalp, and a rash on the eyelids.
- Be aware of pulmonary involvement, including interstitial lung disease. In some cases, patients and their physicians wrongly believe that patients with dermatomyositis have asthma or pneumonia. “Pulmonologists are not necessarily aware of lung disease associated with dermatomyositis,” she said.
It’s wise to refer patients with dermatomyositis for malignancy and lung disease screening even if they don’t show signs of muscular involvement. “There’s no significant difference in rates of malignancy or lung disease in classic versus amyopathic dermatomyositis.”
Keep the MDA5 form of dermatomyositis in mind, Dr. Femia said. Anti–melanoma differentiation–associated gene 5 dermatomyositis is linked to rapidly progressive interstitial lung disease, alopecia, arthritis and oral lacerations. “Initially, it can have features of lupus, and the patient can be on immunosuppressive therapy, which mitigates diagnostic findings.”
Dr. Femia disclosed serving as principal investigator for a clinical trial in cutaneous dermatomyositis therapy.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – When she brings up dermatomyositis in the context of dermatology, Alisa Femia, MD, often hears from trainees and medical students who assume that this is a condition for rheumatologists to diagnose and treat. That’s not true: Dermatologists need to watch for this potentially fatal connective tissue disorder because they may see it first, she said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
“A fifth are clinically amyopathic,” and they may not initially see a rheumatologist, said Dr. Femia, director of inpatient dermatology at the department of dermatology, New York University. “They have normal muscle enzymes and no muscle weakness. It really puts them in our care as dermatologists.”
The challenge is that patient presentations can be subtle, but the stakes may be high, she added. “If we catch them early and treat some of these patients aggressively, we can save their lives.”
During the presentation, :
- Don’t rely on tests like biopsies to absolutely tell you what’s going on. “Clinical examination is the most important test to establish the diagnosis,” she said. “If you’re suspecting dermatomyositis ... get the patient in a gown [for a full-body exam] and look for all the signs that might not be as prominent as they are in our textbooks.”
- Look for the “butterfly” rash on the midface that is unique because it spares the nasolabial folds. “It looks like someone took an eraser and wiped out those areas,” Dr. Femia said. “This is important to help us nail down the diagnosis.”
- Other telltale signs, she said, include erythema on the extensor surfaces of digits, severe itching in the scalp, and a rash on the eyelids.
- Be aware of pulmonary involvement, including interstitial lung disease. In some cases, patients and their physicians wrongly believe that patients with dermatomyositis have asthma or pneumonia. “Pulmonologists are not necessarily aware of lung disease associated with dermatomyositis,” she said.
It’s wise to refer patients with dermatomyositis for malignancy and lung disease screening even if they don’t show signs of muscular involvement. “There’s no significant difference in rates of malignancy or lung disease in classic versus amyopathic dermatomyositis.”
Keep the MDA5 form of dermatomyositis in mind, Dr. Femia said. Anti–melanoma differentiation–associated gene 5 dermatomyositis is linked to rapidly progressive interstitial lung disease, alopecia, arthritis and oral lacerations. “Initially, it can have features of lupus, and the patient can be on immunosuppressive therapy, which mitigates diagnostic findings.”
Dr. Femia disclosed serving as principal investigator for a clinical trial in cutaneous dermatomyositis therapy.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – When she brings up dermatomyositis in the context of dermatology, Alisa Femia, MD, often hears from trainees and medical students who assume that this is a condition for rheumatologists to diagnose and treat. That’s not true: Dermatologists need to watch for this potentially fatal connective tissue disorder because they may see it first, she said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
“A fifth are clinically amyopathic,” and they may not initially see a rheumatologist, said Dr. Femia, director of inpatient dermatology at the department of dermatology, New York University. “They have normal muscle enzymes and no muscle weakness. It really puts them in our care as dermatologists.”
The challenge is that patient presentations can be subtle, but the stakes may be high, she added. “If we catch them early and treat some of these patients aggressively, we can save their lives.”
During the presentation, :
- Don’t rely on tests like biopsies to absolutely tell you what’s going on. “Clinical examination is the most important test to establish the diagnosis,” she said. “If you’re suspecting dermatomyositis ... get the patient in a gown [for a full-body exam] and look for all the signs that might not be as prominent as they are in our textbooks.”
- Look for the “butterfly” rash on the midface that is unique because it spares the nasolabial folds. “It looks like someone took an eraser and wiped out those areas,” Dr. Femia said. “This is important to help us nail down the diagnosis.”
- Other telltale signs, she said, include erythema on the extensor surfaces of digits, severe itching in the scalp, and a rash on the eyelids.
- Be aware of pulmonary involvement, including interstitial lung disease. In some cases, patients and their physicians wrongly believe that patients with dermatomyositis have asthma or pneumonia. “Pulmonologists are not necessarily aware of lung disease associated with dermatomyositis,” she said.
It’s wise to refer patients with dermatomyositis for malignancy and lung disease screening even if they don’t show signs of muscular involvement. “There’s no significant difference in rates of malignancy or lung disease in classic versus amyopathic dermatomyositis.”
Keep the MDA5 form of dermatomyositis in mind, Dr. Femia said. Anti–melanoma differentiation–associated gene 5 dermatomyositis is linked to rapidly progressive interstitial lung disease, alopecia, arthritis and oral lacerations. “Initially, it can have features of lupus, and the patient can be on immunosuppressive therapy, which mitigates diagnostic findings.”
Dr. Femia disclosed serving as principal investigator for a clinical trial in cutaneous dermatomyositis therapy.
SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Rapid improvement seen with nemolizumab for prurigo nodularis in phase 2b study
MADRID – Nemolizumab, an investigational humanized monoclonal antibody targeting the interleukin-31 receptor alpha subunit, achieved rapid and clinically meaningful improvement in both itch and skin lesions of severe prurigo nodularis in a phase 2b, randomized trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We saw onset of pruritus improvement in week 1 and onset of lesion healing at week 4,” reported Dr. Stander, professor of dermatology and neurodermatology and head of the Center for Chronic Pruritus at the University of Münster (Germany).
The study results confirm IL-31 signaling as an important therapeutic target in prurigo nodularis and herald the arrival of nemolizumab as a promising potential therapy for severely affected patients, she added.
Prurigo nodularis is a chronic, highly pruritic disease that is difficult to treat and carries a high disease burden. While the disease’s pathogenesis is not completely understood, IL-31 is up-regulated in affected patients. And IL-31, a proinflammatory and immunomodulatory cytokine, is known to have a broad range of actions, including serving as a link between the immune and neural systems, as well as induction of itch and skin lesions.
Dr. Stander presented the results of a 20-center, double-blind, at weeks 0, 4, and 8, then followed off therapy out to week 18. These were severely affected patients: their mean weekly peak pruritus score on a 0-10 numeric rating scale was 8.4, with 7 being the accepted threshold for severe itch. The group had a mean Dermatologic Life Quality Index score of 16.4; 40% of patients had more than 100 nodules on their body, and the rest had 20-100.
The primary endpoint was the percentage decrease in the peak pruritus score from baseline to week 4, at which point they had only received one dose. The nemolizumab group averaged a 53.4% reduction, compared with 15.3% in placebo-treated controls. At week 12, a full month after the final injection, the split was 63.2% versus 20.2%. And at week 18, the nemolizumab group maintained a mean 58.2% reduction from baseline versus 20.9% in controls.
“The effect starts at week 1, with a 26% reduction in itch intensity in the nemolizumab group, compared to 6.7% with placebo,” the dermatologist observed.
The absolute decrease in weekly peak pruritus score at week 12 was 5.2 points with nemolizumab and 1.7 points with placebo.
Among the secondary endpoints was achievement of an Investigator Global Assessment score of 0-1, meaning clear or almost clear of skin lesions. The rate in the nemolizumab group climbed steadily from week 4 on, reaching 38.2% and still rising without a plateau at week 18, versus 5.6% in controls.
Another secondary endpoint was 75% or greater healing on the 7-item Prurigo Activity Scale. By week 4 there was already a statistically significant between-group difference: 23.5% versus 11.2%. Once again, in the nemolizumab group, this rate climbed without a plateau through the study’s end at week 18, by which point it was 44.1%, compared with 8.4% among those on placebo.
Scores on the Dermatologic Life Quality Index improved by an average of 10.2 points at week 4 in patients on nemolizumab, compared with 6 points among controls.
Self-reported sleep disturbance scores improved by 56% at week 4 in the nemolizumab group and 22.9% with placebo.
The safety profile of nemolizumab was similar to that of placebo, with roughly 5.7% of patients in each study arm withdrawing because of treatment-emergent adverse events. Unlike in the positive studies of the IL-31 inhibitor in patients with atopic dermatitis – another potential indication under active investigation – there was no signal of an increased risk of staphylococcal skin infections, conjunctivitis, or head and neck dermatitis in patients on nemolizumab for prurigo nodularis. Patients with comorbid atopic dermatitis were excluded from the prurigo nodularis trial in order to get a clearer picture of the biologic’s efficacy and safety specifically for that condition.
Dr. Stander reported serving as a consultant to Galderma, the study sponsor, as well as numerous other pharmaceutical companies.
MADRID – Nemolizumab, an investigational humanized monoclonal antibody targeting the interleukin-31 receptor alpha subunit, achieved rapid and clinically meaningful improvement in both itch and skin lesions of severe prurigo nodularis in a phase 2b, randomized trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We saw onset of pruritus improvement in week 1 and onset of lesion healing at week 4,” reported Dr. Stander, professor of dermatology and neurodermatology and head of the Center for Chronic Pruritus at the University of Münster (Germany).
The study results confirm IL-31 signaling as an important therapeutic target in prurigo nodularis and herald the arrival of nemolizumab as a promising potential therapy for severely affected patients, she added.
Prurigo nodularis is a chronic, highly pruritic disease that is difficult to treat and carries a high disease burden. While the disease’s pathogenesis is not completely understood, IL-31 is up-regulated in affected patients. And IL-31, a proinflammatory and immunomodulatory cytokine, is known to have a broad range of actions, including serving as a link between the immune and neural systems, as well as induction of itch and skin lesions.
Dr. Stander presented the results of a 20-center, double-blind, at weeks 0, 4, and 8, then followed off therapy out to week 18. These were severely affected patients: their mean weekly peak pruritus score on a 0-10 numeric rating scale was 8.4, with 7 being the accepted threshold for severe itch. The group had a mean Dermatologic Life Quality Index score of 16.4; 40% of patients had more than 100 nodules on their body, and the rest had 20-100.
The primary endpoint was the percentage decrease in the peak pruritus score from baseline to week 4, at which point they had only received one dose. The nemolizumab group averaged a 53.4% reduction, compared with 15.3% in placebo-treated controls. At week 12, a full month after the final injection, the split was 63.2% versus 20.2%. And at week 18, the nemolizumab group maintained a mean 58.2% reduction from baseline versus 20.9% in controls.
“The effect starts at week 1, with a 26% reduction in itch intensity in the nemolizumab group, compared to 6.7% with placebo,” the dermatologist observed.
The absolute decrease in weekly peak pruritus score at week 12 was 5.2 points with nemolizumab and 1.7 points with placebo.
Among the secondary endpoints was achievement of an Investigator Global Assessment score of 0-1, meaning clear or almost clear of skin lesions. The rate in the nemolizumab group climbed steadily from week 4 on, reaching 38.2% and still rising without a plateau at week 18, versus 5.6% in controls.
Another secondary endpoint was 75% or greater healing on the 7-item Prurigo Activity Scale. By week 4 there was already a statistically significant between-group difference: 23.5% versus 11.2%. Once again, in the nemolizumab group, this rate climbed without a plateau through the study’s end at week 18, by which point it was 44.1%, compared with 8.4% among those on placebo.
Scores on the Dermatologic Life Quality Index improved by an average of 10.2 points at week 4 in patients on nemolizumab, compared with 6 points among controls.
Self-reported sleep disturbance scores improved by 56% at week 4 in the nemolizumab group and 22.9% with placebo.
The safety profile of nemolizumab was similar to that of placebo, with roughly 5.7% of patients in each study arm withdrawing because of treatment-emergent adverse events. Unlike in the positive studies of the IL-31 inhibitor in patients with atopic dermatitis – another potential indication under active investigation – there was no signal of an increased risk of staphylococcal skin infections, conjunctivitis, or head and neck dermatitis in patients on nemolizumab for prurigo nodularis. Patients with comorbid atopic dermatitis were excluded from the prurigo nodularis trial in order to get a clearer picture of the biologic’s efficacy and safety specifically for that condition.
Dr. Stander reported serving as a consultant to Galderma, the study sponsor, as well as numerous other pharmaceutical companies.
MADRID – Nemolizumab, an investigational humanized monoclonal antibody targeting the interleukin-31 receptor alpha subunit, achieved rapid and clinically meaningful improvement in both itch and skin lesions of severe prurigo nodularis in a phase 2b, randomized trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
“We saw onset of pruritus improvement in week 1 and onset of lesion healing at week 4,” reported Dr. Stander, professor of dermatology and neurodermatology and head of the Center for Chronic Pruritus at the University of Münster (Germany).
The study results confirm IL-31 signaling as an important therapeutic target in prurigo nodularis and herald the arrival of nemolizumab as a promising potential therapy for severely affected patients, she added.
Prurigo nodularis is a chronic, highly pruritic disease that is difficult to treat and carries a high disease burden. While the disease’s pathogenesis is not completely understood, IL-31 is up-regulated in affected patients. And IL-31, a proinflammatory and immunomodulatory cytokine, is known to have a broad range of actions, including serving as a link between the immune and neural systems, as well as induction of itch and skin lesions.
Dr. Stander presented the results of a 20-center, double-blind, at weeks 0, 4, and 8, then followed off therapy out to week 18. These were severely affected patients: their mean weekly peak pruritus score on a 0-10 numeric rating scale was 8.4, with 7 being the accepted threshold for severe itch. The group had a mean Dermatologic Life Quality Index score of 16.4; 40% of patients had more than 100 nodules on their body, and the rest had 20-100.
The primary endpoint was the percentage decrease in the peak pruritus score from baseline to week 4, at which point they had only received one dose. The nemolizumab group averaged a 53.4% reduction, compared with 15.3% in placebo-treated controls. At week 12, a full month after the final injection, the split was 63.2% versus 20.2%. And at week 18, the nemolizumab group maintained a mean 58.2% reduction from baseline versus 20.9% in controls.
“The effect starts at week 1, with a 26% reduction in itch intensity in the nemolizumab group, compared to 6.7% with placebo,” the dermatologist observed.
The absolute decrease in weekly peak pruritus score at week 12 was 5.2 points with nemolizumab and 1.7 points with placebo.
Among the secondary endpoints was achievement of an Investigator Global Assessment score of 0-1, meaning clear or almost clear of skin lesions. The rate in the nemolizumab group climbed steadily from week 4 on, reaching 38.2% and still rising without a plateau at week 18, versus 5.6% in controls.
Another secondary endpoint was 75% or greater healing on the 7-item Prurigo Activity Scale. By week 4 there was already a statistically significant between-group difference: 23.5% versus 11.2%. Once again, in the nemolizumab group, this rate climbed without a plateau through the study’s end at week 18, by which point it was 44.1%, compared with 8.4% among those on placebo.
Scores on the Dermatologic Life Quality Index improved by an average of 10.2 points at week 4 in patients on nemolizumab, compared with 6 points among controls.
Self-reported sleep disturbance scores improved by 56% at week 4 in the nemolizumab group and 22.9% with placebo.
The safety profile of nemolizumab was similar to that of placebo, with roughly 5.7% of patients in each study arm withdrawing because of treatment-emergent adverse events. Unlike in the positive studies of the IL-31 inhibitor in patients with atopic dermatitis – another potential indication under active investigation – there was no signal of an increased risk of staphylococcal skin infections, conjunctivitis, or head and neck dermatitis in patients on nemolizumab for prurigo nodularis. Patients with comorbid atopic dermatitis were excluded from the prurigo nodularis trial in order to get a clearer picture of the biologic’s efficacy and safety specifically for that condition.
Dr. Stander reported serving as a consultant to Galderma, the study sponsor, as well as numerous other pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS
Violaceous papules on calf & foot
that usually presents on the distal lower extremities or feet as red to violaceous papules, patches, and plaques.
Clinically, lesions may look similar to Kaposi sarcoma (KS). It is considered to be a variant of stasis dermatitis or severe chronic venous stasis with a more exuberant vascular proliferation of preexisting vasculature. Although the exact etiology is unknown, it is thought that chronic edema, increased venous pressure, and tissue hypoxia may induce fibroblast and vascular proliferation.
It has also been described in association with vascular anomalies, such as Klippel-Trenaunay syndrome, Stewart-Bluefarb syndrome, and Prader-Labhart-Willi syndrome, and is caused by arteriovenous fistulae. Paralysis of lower extremities and amputation stumps are predisposing factors.
KS has four clinical variants: classic KS, African endemic KS, KS in immunocompromised patients, and AIDS-related epidemic KS. All types are caused by the human herpesvirus-8 (HHV-8). Violaceous lesions generally begin as macules and may progress to nodules or tumors.
Punch biopsies were performed in our patient. Histologically, thin-walled, dilated, capillary-like structures were present with a thin layer of surrounding pericytes with reactive fibrosis, hemorrhage, hemosiderin, and a scant chronic inflammatory cell infiltrate. The endothelial cells did not show atypia or mitotic activity. Endothelial cells were positive for CD31, CD34, and CD99. Pericytes and some of the endothelial cells were positive for actin and negative for D2-40, desmin, and HHV-8. In KS, vessels appear like slitlike or jagged spaces lined by spindled endothelial cells. Mild cytologic atypia is usually present. Endothelial cells are characteristically plump. A distinguishing feature in KS is the “promontory sign,” in which new vessels protrude into the vascular space. CD34 is usually negative and HHV-8 is positive.
Acroangiodermatitis of Mali may improve when the underlying venous insufficiency is addressed with compression stockings, pumps, or vascular intervention. Laser ablation of individual lesions has been described in the literature. Dapsone, oral erythromycin, and topical corticosteroids have been reported as helpful in some patients.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
that usually presents on the distal lower extremities or feet as red to violaceous papules, patches, and plaques.
Clinically, lesions may look similar to Kaposi sarcoma (KS). It is considered to be a variant of stasis dermatitis or severe chronic venous stasis with a more exuberant vascular proliferation of preexisting vasculature. Although the exact etiology is unknown, it is thought that chronic edema, increased venous pressure, and tissue hypoxia may induce fibroblast and vascular proliferation.
It has also been described in association with vascular anomalies, such as Klippel-Trenaunay syndrome, Stewart-Bluefarb syndrome, and Prader-Labhart-Willi syndrome, and is caused by arteriovenous fistulae. Paralysis of lower extremities and amputation stumps are predisposing factors.
KS has four clinical variants: classic KS, African endemic KS, KS in immunocompromised patients, and AIDS-related epidemic KS. All types are caused by the human herpesvirus-8 (HHV-8). Violaceous lesions generally begin as macules and may progress to nodules or tumors.
Punch biopsies were performed in our patient. Histologically, thin-walled, dilated, capillary-like structures were present with a thin layer of surrounding pericytes with reactive fibrosis, hemorrhage, hemosiderin, and a scant chronic inflammatory cell infiltrate. The endothelial cells did not show atypia or mitotic activity. Endothelial cells were positive for CD31, CD34, and CD99. Pericytes and some of the endothelial cells were positive for actin and negative for D2-40, desmin, and HHV-8. In KS, vessels appear like slitlike or jagged spaces lined by spindled endothelial cells. Mild cytologic atypia is usually present. Endothelial cells are characteristically plump. A distinguishing feature in KS is the “promontory sign,” in which new vessels protrude into the vascular space. CD34 is usually negative and HHV-8 is positive.
Acroangiodermatitis of Mali may improve when the underlying venous insufficiency is addressed with compression stockings, pumps, or vascular intervention. Laser ablation of individual lesions has been described in the literature. Dapsone, oral erythromycin, and topical corticosteroids have been reported as helpful in some patients.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
that usually presents on the distal lower extremities or feet as red to violaceous papules, patches, and plaques.
Clinically, lesions may look similar to Kaposi sarcoma (KS). It is considered to be a variant of stasis dermatitis or severe chronic venous stasis with a more exuberant vascular proliferation of preexisting vasculature. Although the exact etiology is unknown, it is thought that chronic edema, increased venous pressure, and tissue hypoxia may induce fibroblast and vascular proliferation.
It has also been described in association with vascular anomalies, such as Klippel-Trenaunay syndrome, Stewart-Bluefarb syndrome, and Prader-Labhart-Willi syndrome, and is caused by arteriovenous fistulae. Paralysis of lower extremities and amputation stumps are predisposing factors.
KS has four clinical variants: classic KS, African endemic KS, KS in immunocompromised patients, and AIDS-related epidemic KS. All types are caused by the human herpesvirus-8 (HHV-8). Violaceous lesions generally begin as macules and may progress to nodules or tumors.
Punch biopsies were performed in our patient. Histologically, thin-walled, dilated, capillary-like structures were present with a thin layer of surrounding pericytes with reactive fibrosis, hemorrhage, hemosiderin, and a scant chronic inflammatory cell infiltrate. The endothelial cells did not show atypia or mitotic activity. Endothelial cells were positive for CD31, CD34, and CD99. Pericytes and some of the endothelial cells were positive for actin and negative for D2-40, desmin, and HHV-8. In KS, vessels appear like slitlike or jagged spaces lined by spindled endothelial cells. Mild cytologic atypia is usually present. Endothelial cells are characteristically plump. A distinguishing feature in KS is the “promontory sign,” in which new vessels protrude into the vascular space. CD34 is usually negative and HHV-8 is positive.
Acroangiodermatitis of Mali may improve when the underlying venous insufficiency is addressed with compression stockings, pumps, or vascular intervention. Laser ablation of individual lesions has been described in the literature. Dapsone, oral erythromycin, and topical corticosteroids have been reported as helpful in some patients.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
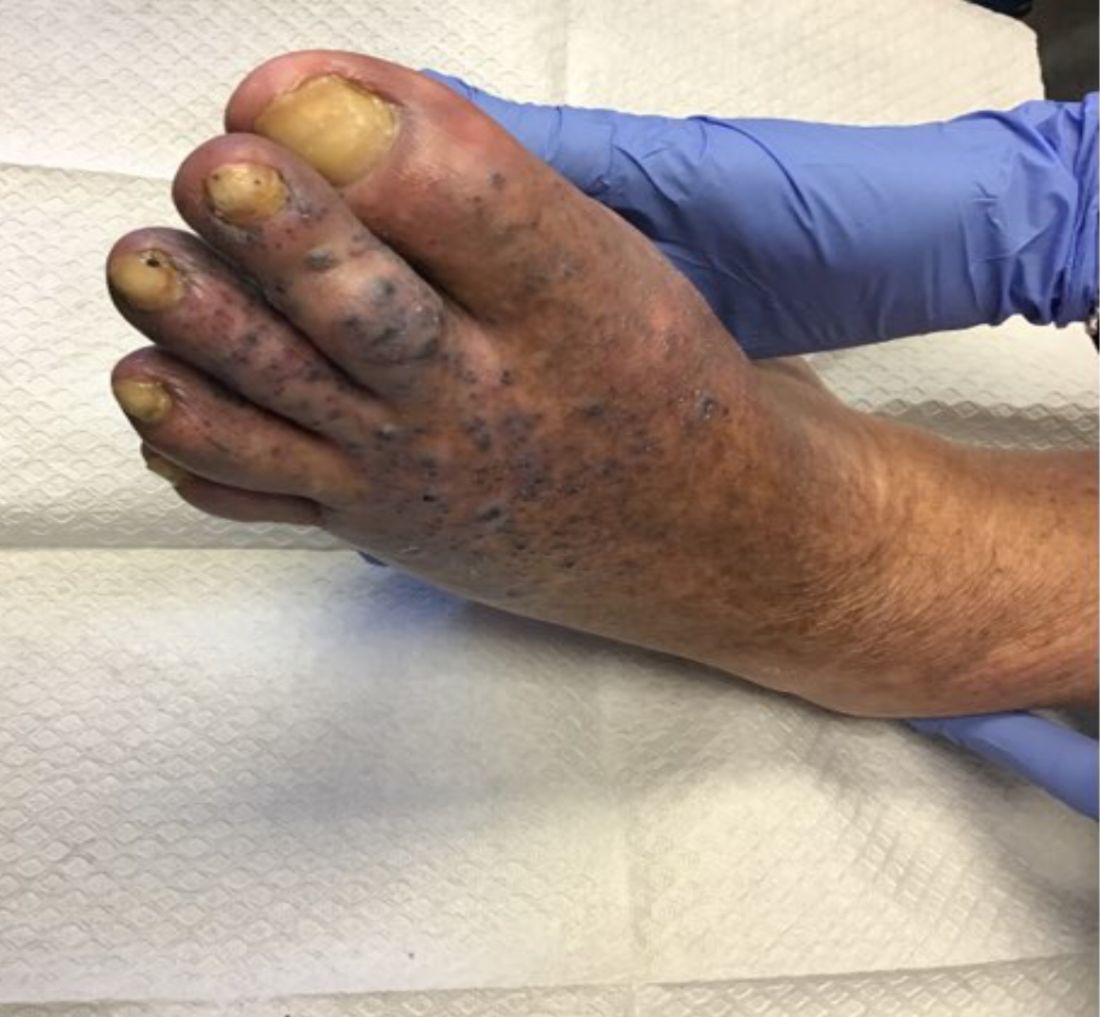
Spotting immunodeficiency in the pediatric dermatology clinic
SEATTLE – Immunodeficiency in children can look much like eczematous dermatitis. Be aware of this potential diagnosis.
“Although it is important to know these are extremely rare conditions, you don’t want to miss them because you can literally change that child’s life,” Markus Boos, MD, an assistant professor of pediatrics at the University of Washington, Seattle, said in an interview at the annual Coastal Dermatology Symposium.
He outlined some key clinical features and patient history that can raise a potential red flag.
“ and you really spend time looking at the morphology and distribution of the rash,” Dr. Boos said.
The distribution of the rash also can be distinctive. For example, hyper-IgE syndrome shows up as little red pus bumps that are widespread, but specifically occur on the face and other areas that usually aren’t affected eczematous dermatitis. “You should really focus on that, and not just assume that because something [like eczematous dermatitis] is common, everything has to be that,” Dr. Boos said at the meeting, which was jointly presented by the University of Louisville and Global Academy for Medical Education.
He also warned about a false positive. You may be alerted to high eosinophil and high IgE levels determined by a primary care physician’s tests, but these aren’t necessarily a strong indicator of hyper-IgE syndrome, he said. “Many inflammatory conditions in children have high levels of both those, so they aren’t a distinguishing feature of any one of them. You can reassure a family that the child doesn’t necessarily have hyper-IgE syndrome. There’s this leap [people take] because it sounds like the name, but it’s not a very specific marker of that particular condition.”
Patient history of an immunodeficiency patient in general obviously can include a history of infections, although a high rate of ear infections is pretty typical among children. The key is to ask yourself: “At what point does it seem like something that is beyond normal?” Dr. Boos said. Infections that required hospitalizations or were invasive or required antibiotics all are potential clues. Other factors to consider include growth and development issues such as frequent diarrhea or failure to thrive, or family members with frequent infections or who died prematurely.
Hyper-IgE patients also may have a prominent forehead and chin, deep-set eyes, broad nose, thickened facial skin, or a high arched palate. These physical features become more prominent by adolescence. For a reference for physical features go to https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/hyper-ige-syndrome.
Clinical features of various immunodeficiencies include the following:
- Papulopustular eruption with frequent infections and musculoskeletal changes. This presentation is suggestive of autosomal dominant hyper-IgE syndrome. These children have a “heterozygous mutation in the gene encoding the transcription factor STAT3,” according to the Immune Deficiency Foundation.
- Severe atopy with extensive warts/molluscum/herpes simplex virus. This presentation is suggestive of autosomal recessive hyper-IgE syndrome. These children have “mutations and deletions in the DOCK8 gene,” the Immune Deficiency Foundation asserts.
- Diffusely red baby. Consider immunodeficiency if the patient also has experienced failure to thrive and/or diarrhea, or has a history of infection. High IgE levels are not a strong signal of hyper-IgE syndrome.
- Severe eczematous (or psoriasiform) dermatitis with chronic diarrhea, failure to thrive, and diabetes or hypothyroidism. This presentation is suggestive of IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked).
- Atopic dermatitis with bloody diarrhea, thrombocytopenia, recurrent ear infections. This presentation is indicative of Wiskott-Aldrich syndrome.
Dr. Boos is personally familiar with primary immunodeficiencies because he works closely with an immunology clinic, which also means he has a lot of support. Most clinicians diagnosing these patients don’t. If you find yourself with a case, “call in the troops,” he advised. You should be connected to a rheumatologist when there’s evidence of autoimmune disease, and hematologists or oncologists for the treatment, which requires a bone marrow transplant in the case of autosomal recessive hyper-IgE syndrome. Otherwise treatment is largely supportive for this immunodeficiency.
Having that network can be invaluable in managing what can be a very complicated patient. “If you ever feel uncomfortable making a decision about their care, discussing it with those other providers can give you some peace of mind,” he said.
Dr. Boos disclosed that he is a clinical researcher for Regeneron. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – Immunodeficiency in children can look much like eczematous dermatitis. Be aware of this potential diagnosis.
“Although it is important to know these are extremely rare conditions, you don’t want to miss them because you can literally change that child’s life,” Markus Boos, MD, an assistant professor of pediatrics at the University of Washington, Seattle, said in an interview at the annual Coastal Dermatology Symposium.
He outlined some key clinical features and patient history that can raise a potential red flag.
“ and you really spend time looking at the morphology and distribution of the rash,” Dr. Boos said.
The distribution of the rash also can be distinctive. For example, hyper-IgE syndrome shows up as little red pus bumps that are widespread, but specifically occur on the face and other areas that usually aren’t affected eczematous dermatitis. “You should really focus on that, and not just assume that because something [like eczematous dermatitis] is common, everything has to be that,” Dr. Boos said at the meeting, which was jointly presented by the University of Louisville and Global Academy for Medical Education.
He also warned about a false positive. You may be alerted to high eosinophil and high IgE levels determined by a primary care physician’s tests, but these aren’t necessarily a strong indicator of hyper-IgE syndrome, he said. “Many inflammatory conditions in children have high levels of both those, so they aren’t a distinguishing feature of any one of them. You can reassure a family that the child doesn’t necessarily have hyper-IgE syndrome. There’s this leap [people take] because it sounds like the name, but it’s not a very specific marker of that particular condition.”
Patient history of an immunodeficiency patient in general obviously can include a history of infections, although a high rate of ear infections is pretty typical among children. The key is to ask yourself: “At what point does it seem like something that is beyond normal?” Dr. Boos said. Infections that required hospitalizations or were invasive or required antibiotics all are potential clues. Other factors to consider include growth and development issues such as frequent diarrhea or failure to thrive, or family members with frequent infections or who died prematurely.
Hyper-IgE patients also may have a prominent forehead and chin, deep-set eyes, broad nose, thickened facial skin, or a high arched palate. These physical features become more prominent by adolescence. For a reference for physical features go to https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/hyper-ige-syndrome.
Clinical features of various immunodeficiencies include the following:
- Papulopustular eruption with frequent infections and musculoskeletal changes. This presentation is suggestive of autosomal dominant hyper-IgE syndrome. These children have a “heterozygous mutation in the gene encoding the transcription factor STAT3,” according to the Immune Deficiency Foundation.
- Severe atopy with extensive warts/molluscum/herpes simplex virus. This presentation is suggestive of autosomal recessive hyper-IgE syndrome. These children have “mutations and deletions in the DOCK8 gene,” the Immune Deficiency Foundation asserts.
- Diffusely red baby. Consider immunodeficiency if the patient also has experienced failure to thrive and/or diarrhea, or has a history of infection. High IgE levels are not a strong signal of hyper-IgE syndrome.
- Severe eczematous (or psoriasiform) dermatitis with chronic diarrhea, failure to thrive, and diabetes or hypothyroidism. This presentation is suggestive of IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked).
- Atopic dermatitis with bloody diarrhea, thrombocytopenia, recurrent ear infections. This presentation is indicative of Wiskott-Aldrich syndrome.
Dr. Boos is personally familiar with primary immunodeficiencies because he works closely with an immunology clinic, which also means he has a lot of support. Most clinicians diagnosing these patients don’t. If you find yourself with a case, “call in the troops,” he advised. You should be connected to a rheumatologist when there’s evidence of autoimmune disease, and hematologists or oncologists for the treatment, which requires a bone marrow transplant in the case of autosomal recessive hyper-IgE syndrome. Otherwise treatment is largely supportive for this immunodeficiency.
Having that network can be invaluable in managing what can be a very complicated patient. “If you ever feel uncomfortable making a decision about their care, discussing it with those other providers can give you some peace of mind,” he said.
Dr. Boos disclosed that he is a clinical researcher for Regeneron. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – Immunodeficiency in children can look much like eczematous dermatitis. Be aware of this potential diagnosis.
“Although it is important to know these are extremely rare conditions, you don’t want to miss them because you can literally change that child’s life,” Markus Boos, MD, an assistant professor of pediatrics at the University of Washington, Seattle, said in an interview at the annual Coastal Dermatology Symposium.
He outlined some key clinical features and patient history that can raise a potential red flag.
“ and you really spend time looking at the morphology and distribution of the rash,” Dr. Boos said.
The distribution of the rash also can be distinctive. For example, hyper-IgE syndrome shows up as little red pus bumps that are widespread, but specifically occur on the face and other areas that usually aren’t affected eczematous dermatitis. “You should really focus on that, and not just assume that because something [like eczematous dermatitis] is common, everything has to be that,” Dr. Boos said at the meeting, which was jointly presented by the University of Louisville and Global Academy for Medical Education.
He also warned about a false positive. You may be alerted to high eosinophil and high IgE levels determined by a primary care physician’s tests, but these aren’t necessarily a strong indicator of hyper-IgE syndrome, he said. “Many inflammatory conditions in children have high levels of both those, so they aren’t a distinguishing feature of any one of them. You can reassure a family that the child doesn’t necessarily have hyper-IgE syndrome. There’s this leap [people take] because it sounds like the name, but it’s not a very specific marker of that particular condition.”
Patient history of an immunodeficiency patient in general obviously can include a history of infections, although a high rate of ear infections is pretty typical among children. The key is to ask yourself: “At what point does it seem like something that is beyond normal?” Dr. Boos said. Infections that required hospitalizations or were invasive or required antibiotics all are potential clues. Other factors to consider include growth and development issues such as frequent diarrhea or failure to thrive, or family members with frequent infections or who died prematurely.
Hyper-IgE patients also may have a prominent forehead and chin, deep-set eyes, broad nose, thickened facial skin, or a high arched palate. These physical features become more prominent by adolescence. For a reference for physical features go to https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/hyper-ige-syndrome.
Clinical features of various immunodeficiencies include the following:
- Papulopustular eruption with frequent infections and musculoskeletal changes. This presentation is suggestive of autosomal dominant hyper-IgE syndrome. These children have a “heterozygous mutation in the gene encoding the transcription factor STAT3,” according to the Immune Deficiency Foundation.
- Severe atopy with extensive warts/molluscum/herpes simplex virus. This presentation is suggestive of autosomal recessive hyper-IgE syndrome. These children have “mutations and deletions in the DOCK8 gene,” the Immune Deficiency Foundation asserts.
- Diffusely red baby. Consider immunodeficiency if the patient also has experienced failure to thrive and/or diarrhea, or has a history of infection. High IgE levels are not a strong signal of hyper-IgE syndrome.
- Severe eczematous (or psoriasiform) dermatitis with chronic diarrhea, failure to thrive, and diabetes or hypothyroidism. This presentation is suggestive of IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked).
- Atopic dermatitis with bloody diarrhea, thrombocytopenia, recurrent ear infections. This presentation is indicative of Wiskott-Aldrich syndrome.
Dr. Boos is personally familiar with primary immunodeficiencies because he works closely with an immunology clinic, which also means he has a lot of support. Most clinicians diagnosing these patients don’t. If you find yourself with a case, “call in the troops,” he advised. You should be connected to a rheumatologist when there’s evidence of autoimmune disease, and hematologists or oncologists for the treatment, which requires a bone marrow transplant in the case of autosomal recessive hyper-IgE syndrome. Otherwise treatment is largely supportive for this immunodeficiency.
Having that network can be invaluable in managing what can be a very complicated patient. “If you ever feel uncomfortable making a decision about their care, discussing it with those other providers can give you some peace of mind,” he said.
Dr. Boos disclosed that he is a clinical researcher for Regeneron. This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM