User login
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
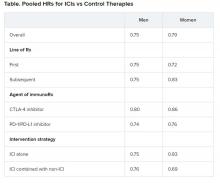
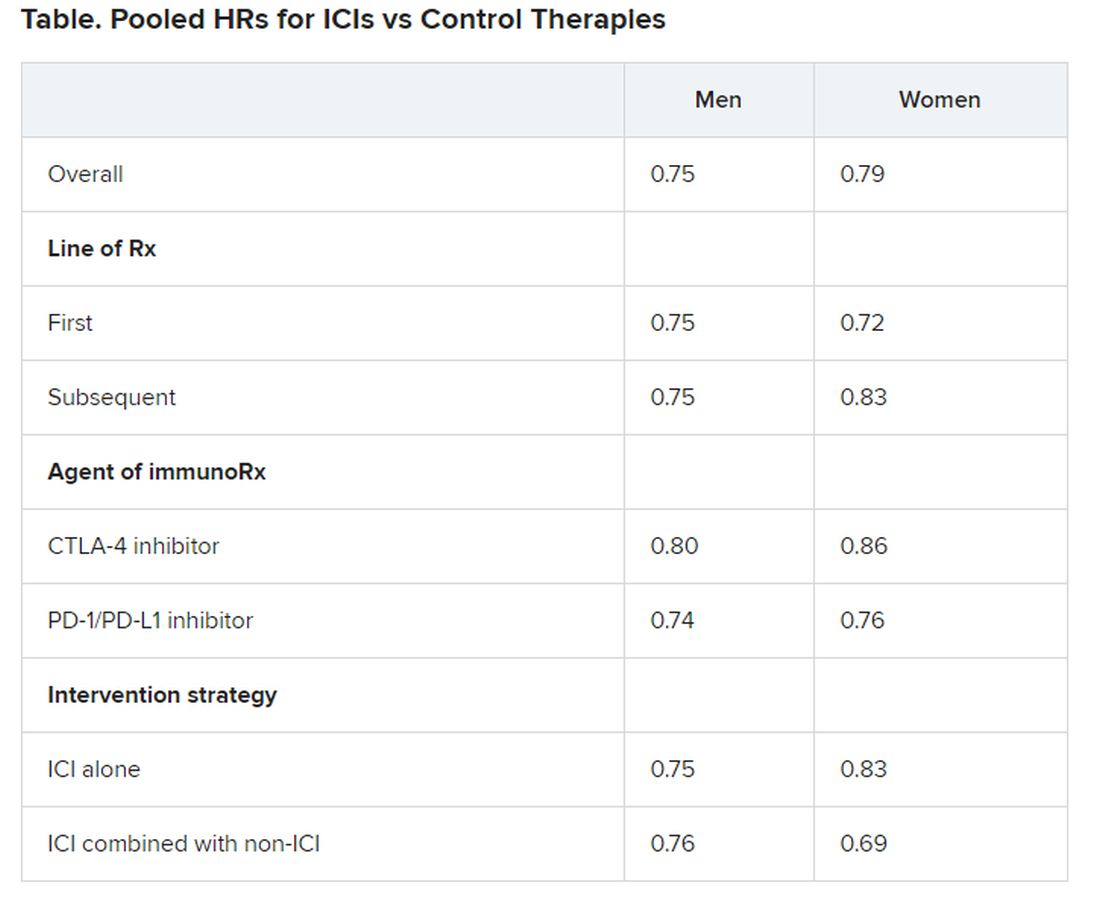
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Age, other risk factors predict length of MM survival
Younger age of onset and the use of autologous hematopoietic stem cell transplant (ASCT) treatment were key factors improving the length of survival of newly diagnosed, active multiple myeloma (MM) patients, according to the results of a retrospective analysis.
In addition, multivariable analysis showed that a higher level of blood creatinine, the presence of extramedullary disease, a lower level of partial remission, and the use of nonautologous hematopoietic stem cell transplantation were independent risk factors for shorter survival, according to Virginia Bove, MD, of the Asociación Espanola Primera en Socorros Mutuos, Montevideo, Uruguay and colleagues.
Dr. Bove and colleagues retrospectively analyzed clinical characteristics, response to treatment, and survival of 282 patients from multiple institutions who had active newly-diagnosed multiple myeloma. They compared the results between patients age 65 years or younger (53.2%) with those older than 65 years and assessed clinical risk factors, as reported online in Hematology, Transfusion, and Cell Therapy.
The main cause of death in all patients was MM progression and the early mortality rate was not different between the younger and older patients. The main cause of early death in older patients was infection, according to the researchers.
Multiple risk factors
“Although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior [overall survival] and [progression-free survival],” the researchers reported.
The overall response rate was similar between groups (80.6% vs. 81.4%; P = .866), and the overall survival was significantly longer in young patients (median, 65 months vs. 41 months; P = .001) and higher in those who received autologous hematopoietic stem cell transplantation.
Multivariate analysis was performed on data from the younger patients. The results showed that a creatinine level of less than or equal to 2 mg/dL (P = .048), extramedullary disease (P = .001), a lower VGPR (P = .003) and the use of nonautologous hematopoietic stem cell transplantation (P = .048) were all independent risk factors for shorter survival.
“Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Bove V et al. Hematol Transfus Cell Ther. 2020 Aug 20. doi: 10.1016/j.htct.2020.06.014.
Younger age of onset and the use of autologous hematopoietic stem cell transplant (ASCT) treatment were key factors improving the length of survival of newly diagnosed, active multiple myeloma (MM) patients, according to the results of a retrospective analysis.
In addition, multivariable analysis showed that a higher level of blood creatinine, the presence of extramedullary disease, a lower level of partial remission, and the use of nonautologous hematopoietic stem cell transplantation were independent risk factors for shorter survival, according to Virginia Bove, MD, of the Asociación Espanola Primera en Socorros Mutuos, Montevideo, Uruguay and colleagues.
Dr. Bove and colleagues retrospectively analyzed clinical characteristics, response to treatment, and survival of 282 patients from multiple institutions who had active newly-diagnosed multiple myeloma. They compared the results between patients age 65 years or younger (53.2%) with those older than 65 years and assessed clinical risk factors, as reported online in Hematology, Transfusion, and Cell Therapy.
The main cause of death in all patients was MM progression and the early mortality rate was not different between the younger and older patients. The main cause of early death in older patients was infection, according to the researchers.
Multiple risk factors
“Although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior [overall survival] and [progression-free survival],” the researchers reported.
The overall response rate was similar between groups (80.6% vs. 81.4%; P = .866), and the overall survival was significantly longer in young patients (median, 65 months vs. 41 months; P = .001) and higher in those who received autologous hematopoietic stem cell transplantation.
Multivariate analysis was performed on data from the younger patients. The results showed that a creatinine level of less than or equal to 2 mg/dL (P = .048), extramedullary disease (P = .001), a lower VGPR (P = .003) and the use of nonautologous hematopoietic stem cell transplantation (P = .048) were all independent risk factors for shorter survival.
“Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Bove V et al. Hematol Transfus Cell Ther. 2020 Aug 20. doi: 10.1016/j.htct.2020.06.014.
Younger age of onset and the use of autologous hematopoietic stem cell transplant (ASCT) treatment were key factors improving the length of survival of newly diagnosed, active multiple myeloma (MM) patients, according to the results of a retrospective analysis.
In addition, multivariable analysis showed that a higher level of blood creatinine, the presence of extramedullary disease, a lower level of partial remission, and the use of nonautologous hematopoietic stem cell transplantation were independent risk factors for shorter survival, according to Virginia Bove, MD, of the Asociación Espanola Primera en Socorros Mutuos, Montevideo, Uruguay and colleagues.
Dr. Bove and colleagues retrospectively analyzed clinical characteristics, response to treatment, and survival of 282 patients from multiple institutions who had active newly-diagnosed multiple myeloma. They compared the results between patients age 65 years or younger (53.2%) with those older than 65 years and assessed clinical risk factors, as reported online in Hematology, Transfusion, and Cell Therapy.
The main cause of death in all patients was MM progression and the early mortality rate was not different between the younger and older patients. The main cause of early death in older patients was infection, according to the researchers.
Multiple risk factors
“Although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior [overall survival] and [progression-free survival],” the researchers reported.
The overall response rate was similar between groups (80.6% vs. 81.4%; P = .866), and the overall survival was significantly longer in young patients (median, 65 months vs. 41 months; P = .001) and higher in those who received autologous hematopoietic stem cell transplantation.
Multivariate analysis was performed on data from the younger patients. The results showed that a creatinine level of less than or equal to 2 mg/dL (P = .048), extramedullary disease (P = .001), a lower VGPR (P = .003) and the use of nonautologous hematopoietic stem cell transplantation (P = .048) were all independent risk factors for shorter survival.
“Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Bove V et al. Hematol Transfus Cell Ther. 2020 Aug 20. doi: 10.1016/j.htct.2020.06.014.
FROM HEMATOLOGY, TRANSFUSION, AND CELL THERAPY
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
COVID-19 impact: Less chemo, immune checkpoint inhibitors, and steroids
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
While neoadjuvant treatment recommendations were not strongly affected by the pandemic, about half of oncologists reported increased hesitancy over recommending frontline chemotherapy for metastatic disease, and a vast majority said they would recommend second- or third-line chemotherapy less often in the metastatic setting.
Most oncologists said they did not perform routine COVID-19 testing via reverse transcriptase–polymerase chain reaction (RT-PCR) before treating cancer patients. In fact, only 3% said they performed COVID-19 RT-PCR testing routinely.
Yüksel Ürün, MD, of Ankara (Turkey) University, and colleagues reported these findings in JCO Global Oncology.
The goal of the survey was to “understand readiness measures taken by oncologists to protect patients and health care workers from the novel coronavirus (COVID-19) and how their clinical decision-making was influenced by the pandemic,” the authors wrote.
The online survey was conducted among 343 oncologists from 28 countries. Responses were collected anonymously, a majority (71%) from university or academic centers, with 95% received between April 1 and April 29, 2020.
Use of telemedicine was common (80%) among respondents, as was use of surgical masks (90%) and personal protective equipment in general.
Only 33% of respondents described using N95 masks. However, the proportion of oncologists who had access to N95 masks while caring for patients known to have COVID-19, especially while doing invasive procedures such as intubation, bronchoscopy, and any airway-related manipulations, was not captured by the survey.
COVID testing and cancer treatment
Most respondents (58%) said they did not perform routine COVID-19 RT-PCR testing prior to administering systemic cancer treatment, with 39% stating they performed RT-PCR tests in selected patients, and 3% saying they performed such testing in all patients.
The survey indicated that hormonal treatments, tyrosine kinase inhibitors, and bone-modifying agents were considered relatively safe, but cytotoxic chemotherapy and immune therapies were not.
Nearly all oncologists said the pandemic would cause them to make no change to their recommendations regarding hormone therapy, and nearly 80% said they would make no changes regarding tyrosine kinase inhibitors or bone-modifying agents.
However, more than 90% of respondents said they would recommend cytotoxic chemotherapy less often, about 70% said they would recommend corticosteroids less often, and around 50% said they would recommend anti–programmed death-1/PD-ligand 1 or anti–cytotoxic T-lymphocyte–associated protein 4 antibodies less often.
The pandemic made most respondents more reluctant to recommend second- or third-line chemotherapy in the metastatic setting. About 80% and 70% of respondents, respectively, would recommend second- or third-line chemotherapy less often.
However, first-line chemotherapy for metastatic disease, as well as adjuvant and neoadjuvant therapy, were less affected. About 30% of respondents said they would recommend neoadjuvant therapy less often, and 50%-55% would recommend adjuvant therapy or frontline chemotherapy for metastatic disease less often.
Most respondents (78%) said they would use granulocyte colony–stimulating factor (G-CSF) more frequently during the pandemic.
The factors most likely to affect oncologists’ treatment decisions were patient age (81%) and concomitant disease (92%). Additionally, 80% of respondents’ treatment decisions were influenced by Eastern Cooperative Oncology Group performance status of 2 or higher, or the presence of chronic obstructive pulmonary disease.
Interpretation and implications
“These results highlight that, even in the early phases of COVID-19 – during which there was considerable uncertainty – basic core principles were guideposts for oncologists,” observed Aly-Khan Lalani, MD, of Juravinski Cancer Centre and McMaster University, Hamilton, Ont., who was not involved in this study.
“For example, [oncologists were] prioritizing strategies for treatments with the largest expected impact and carefully tailoring treatment according to patient comorbidities and performance status,” Dr. Lalani said.
Another oncologist who was not involved in the study expressed concern over reductions in adjuvant therapy supported by half of oncologists surveyed.
“Although benefits may be marginal in some cases, these are curative settings and especially warrant careful individual-level risk/benefit discussions,” said Kartik Sehgal, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston.
His concern extended as well to the small proportion (3%) of oncologists testing for COVID-19 in all patients. “Systematic testing is the need of the hour,” Dr. Sehgal said.
In their discussion of the findings, Dr. Ürün and colleagues noted a lack of consensus on monoclonal antibody and immunotherapy safety among surveyed oncologists. The steroids needed to manage severe immune-mediated toxicity with immune checkpoint inhibitors has led to some prescribing reluctance during the pandemic.
Immunosuppressive properties of immune checkpoint inhibitors also raise concern that they can increase COVID-19 severity. Studies are few, and findings to date are inconsistent with respect to the effect of immune checkpoint inhibitors on COVID-19 clinical course. However, a recently presented study suggested that immune checkpoint inhibitors do not increase the risk of death among cancer patients with COVID-19 (AACR: COVID-19 and Cancer, Abstract S02-01).
Dr. Ürün and colleagues noted that greater COVID-19 severity has been shown in patients with performance status greater than 1, hematologic malignancies, lung cancer, stage IV metastatic disease, chemotherapy within the prior 3 months, cancer treatment in the last 14 days, and the presence of chronic obstructive pulmonary disease. Nonmetastatic cancer has not been shown to affect COVID-19 severity, however.
Dr. Ürün and colleagues also underscored the need for research evidence to balance potential reductions in neutropenic complications with G-CSF (and therefore, reduced hospitalizations) with a theoretical risk of G-CSF–mediated pulmonary injury through its stimulation of an excessive immune response.
Finally, the authors urged oncologists to evaluate each proposed therapy’s risk/benefit ratio on an individual patient basis, and the team tasked the oncology community with gathering comprehensive, rigorous data.
There was no funding source declared for this study. Dr. Ürün and colleagues disclosed various relationships with many pharmaceutical companies, which included receiving research funding. Dr. Sehgal and Dr. Lalani reported no relevant conflicts.
SOURCE: Ürün Y et al. JCO Glob Oncol. 2020 Aug;6:1248-57.
FROM JCO GLOBAL ONCOLOGY
Elotuzumab-based therapy may benefit post-transplant response in multiple myeloma
Elotuzumab-based maintenance therapy may improve the posttransplant response for multiple myeloma (MM), according to the results of a small retrospective study at a single institution.
In addition, the therapies appear to be safely administered even to older patients because of the low rate of adverse effects, as indicated in a report published online in Blood Cells, Molecules and Diseases.
The researchers retrospectively evaluated the outcomes of seven MM patients who were started on elotuzumab-based maintenance (elotuzumab/lenalidomide/dexamethasone, elotuzumab/bortezomib/dexamethasone, or elotuzumab/bortezomib/methylprednisolone) following transplant, according to Xin Wang, MD, of the UMass Memorial Medical Center, Worcester, and colleagues.
The median age was 68 years (ranging from 56 years to 81 years) at the time of transplant, and median lines of induction therapy was 2; three patients (42.9%) had high-risk cytogenetics and five (71.4%) had stage II or greater disease at diagnosis.
Promising elotuzumab results
At a median follow-up of 24 months, five patients (71.4%) had improvement in their quality of response. Among all patients, there was a combined complete response (CR) or very good partial response (VGPR) rate increase from 57.1% to 100% (CR = 3, VGPR = 4). VGPR was defined by the researchers as an absence of abnormal immunofixation and soft tissue plasmacytoma without bone marrow biopsy.
All patients were alive without relapse or progression at the time of the final analysis. In terms of adverse effects, grade 3-4 events were observed in three (42.9%) of the patients. None of the patients discontinued the treatment because of intolerance, according to the researchers.
“Our study demonstrates that elotuzumab-based maintenance may deepen response post transplant in MM and can be safely administered even in older patients. Given its unique action and rare side effects, further studies of elotuzumab in the post-transplant setting are warranted,” the researchers concluded.
The study had no outside funding and the researchers reported that they had no disclosures.
SOURCE: Wang X et al. Blood Cells Mol Dis. 2020 Jul 28. doi: 10.1016/j.bcmd.2020.102482.
Elotuzumab-based maintenance therapy may improve the posttransplant response for multiple myeloma (MM), according to the results of a small retrospective study at a single institution.
In addition, the therapies appear to be safely administered even to older patients because of the low rate of adverse effects, as indicated in a report published online in Blood Cells, Molecules and Diseases.
The researchers retrospectively evaluated the outcomes of seven MM patients who were started on elotuzumab-based maintenance (elotuzumab/lenalidomide/dexamethasone, elotuzumab/bortezomib/dexamethasone, or elotuzumab/bortezomib/methylprednisolone) following transplant, according to Xin Wang, MD, of the UMass Memorial Medical Center, Worcester, and colleagues.
The median age was 68 years (ranging from 56 years to 81 years) at the time of transplant, and median lines of induction therapy was 2; three patients (42.9%) had high-risk cytogenetics and five (71.4%) had stage II or greater disease at diagnosis.
Promising elotuzumab results
At a median follow-up of 24 months, five patients (71.4%) had improvement in their quality of response. Among all patients, there was a combined complete response (CR) or very good partial response (VGPR) rate increase from 57.1% to 100% (CR = 3, VGPR = 4). VGPR was defined by the researchers as an absence of abnormal immunofixation and soft tissue plasmacytoma without bone marrow biopsy.
All patients were alive without relapse or progression at the time of the final analysis. In terms of adverse effects, grade 3-4 events were observed in three (42.9%) of the patients. None of the patients discontinued the treatment because of intolerance, according to the researchers.
“Our study demonstrates that elotuzumab-based maintenance may deepen response post transplant in MM and can be safely administered even in older patients. Given its unique action and rare side effects, further studies of elotuzumab in the post-transplant setting are warranted,” the researchers concluded.
The study had no outside funding and the researchers reported that they had no disclosures.
SOURCE: Wang X et al. Blood Cells Mol Dis. 2020 Jul 28. doi: 10.1016/j.bcmd.2020.102482.
Elotuzumab-based maintenance therapy may improve the posttransplant response for multiple myeloma (MM), according to the results of a small retrospective study at a single institution.
In addition, the therapies appear to be safely administered even to older patients because of the low rate of adverse effects, as indicated in a report published online in Blood Cells, Molecules and Diseases.
The researchers retrospectively evaluated the outcomes of seven MM patients who were started on elotuzumab-based maintenance (elotuzumab/lenalidomide/dexamethasone, elotuzumab/bortezomib/dexamethasone, or elotuzumab/bortezomib/methylprednisolone) following transplant, according to Xin Wang, MD, of the UMass Memorial Medical Center, Worcester, and colleagues.
The median age was 68 years (ranging from 56 years to 81 years) at the time of transplant, and median lines of induction therapy was 2; three patients (42.9%) had high-risk cytogenetics and five (71.4%) had stage II or greater disease at diagnosis.
Promising elotuzumab results
At a median follow-up of 24 months, five patients (71.4%) had improvement in their quality of response. Among all patients, there was a combined complete response (CR) or very good partial response (VGPR) rate increase from 57.1% to 100% (CR = 3, VGPR = 4). VGPR was defined by the researchers as an absence of abnormal immunofixation and soft tissue plasmacytoma without bone marrow biopsy.
All patients were alive without relapse or progression at the time of the final analysis. In terms of adverse effects, grade 3-4 events were observed in three (42.9%) of the patients. None of the patients discontinued the treatment because of intolerance, according to the researchers.
“Our study demonstrates that elotuzumab-based maintenance may deepen response post transplant in MM and can be safely administered even in older patients. Given its unique action and rare side effects, further studies of elotuzumab in the post-transplant setting are warranted,” the researchers concluded.
The study had no outside funding and the researchers reported that they had no disclosures.
SOURCE: Wang X et al. Blood Cells Mol Dis. 2020 Jul 28. doi: 10.1016/j.bcmd.2020.102482.
FROM BLOOD CELLS, MOLECULES AND DISEASES
FDA approves belantamab in relapsed/refractory multiple myeloma
The first-in-class drug belantamab mafodotin (Blenrep) has been granted an accelerated approval by the Food and Drug Administration for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
This follows a recommendation for approval on July 15 by an FDA advisory committee, which voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
The product has a novel mechanism of action: it targets B-cell maturation antigen (BCMA), a protein that is present on the surface of virtually all multiple myeloma cells but is absent from normal B cells.
The drug had already received an FDA breakthrough therapy designation, which facilitates the development of drugs that have shown clinical promise for conditions in which there is significant unmet need.
Belantamab mafodotin was recommended for conditional marketing approval in the European Union on July 24 and was accepted into the European Medicines Agency PRIME scheme for medicines that have potential to address unmet medical needs.
The new drug is indicated for patients with refractory or relapsed multiple myeloma who have already tried treatment with one of the three major classes of drugs, namely, an immunomolatory agent, a proteasome inhibitor, and a CD38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA comments. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
“While treatable, refractory multiple myeloma is a significant clinical challenge with poor outcomes for patients whose disease has become resistant to the current standard of care,” commented Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University, Atlanta, chair of the department of hematology and medical oncology at Emory, and a principal investigator for the clinical trial that led to the approval.
“Due to the limited options currently available, these patients are often retreated with drugs from the same classes after they relapse, which is why the approval of belantamab mafodotin, the first anti-BCMA therapy, is significant for both patients and physicians alike,” he said.
The product is an antibody-drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. It homes in on BCMA on myeloma cell surfaces. Once inside the myeloma cell, the cytotoxic agent is released, leading to apoptosis, the programmed death of the cancerous plasma cells.
Approval based on response rates
The accelerated approval from the FDA and the recommendation for conditional approval from the EMA are based on results for overall response rate and duration of response from a phase 2, open-label, randomized, two-arm study known as DREAMM-2. Both agencies said that they are waiting for further data on clinical benefit from ongoing trials.
The DREAMM-2 study investigated the efficacy and safety of two doses of belantamab mafodotin in multiple myeloma patients whose disease was still active after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results from this study were published in The Lancet Oncology in December. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The median duration of response had not been reached at the 6-month analysis, but for 73% of responders, DoR was ≥6 months.
The most commonly reported adverse events (≥20%) were keratopathy (changes in the cornea), decreased visual acuity, nausea, blurred vision, pyrexia, infusion-related reactions, and fatigue, the manufacturer notes.
Ocular toxicity
One of the most common adverse events with this product affects the eyes.
Ocular adverse reactions occurred in 77% of the 218 patients in the pooled safety population and included keratopathy (76%), changes in visual acuity (55%), blurred vision (27%), and dry eye (19%).
Corneal adverse events were monitored with eye exams prior to each dose, allowing dose reductions or interruptions as appropriate, the manufacturer noted. Patients also used preservative-free eyedrops. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the 2.5-mg/kg cohort.
Because of this ocular toxicity, the company has set up a risk evaluation and mitigation strategy (REMS) for the product. This requires education for all physicians who prescribe the product as well as their patients regarding the ocular risks associated with treatment. It also requires monitoring that includes regular ophthalmic examinations. Information about the scheme can be found at www.blenreprems.com.
At the FDA advisory committee meeting last month, one of the panelists, Gita Thanarajasingam, MD, an assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated but for ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some people than current treatments.
“It’s reasonable to leave open the option for decision making. Patients can express their values and preferences,” Dr. Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk-benefit discussion in a REMS program.”
Another panelist, Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it to them” with belantamab.
This article first appeared on Medscape.com.
The first-in-class drug belantamab mafodotin (Blenrep) has been granted an accelerated approval by the Food and Drug Administration for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
This follows a recommendation for approval on July 15 by an FDA advisory committee, which voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
The product has a novel mechanism of action: it targets B-cell maturation antigen (BCMA), a protein that is present on the surface of virtually all multiple myeloma cells but is absent from normal B cells.
The drug had already received an FDA breakthrough therapy designation, which facilitates the development of drugs that have shown clinical promise for conditions in which there is significant unmet need.
Belantamab mafodotin was recommended for conditional marketing approval in the European Union on July 24 and was accepted into the European Medicines Agency PRIME scheme for medicines that have potential to address unmet medical needs.
The new drug is indicated for patients with refractory or relapsed multiple myeloma who have already tried treatment with one of the three major classes of drugs, namely, an immunomolatory agent, a proteasome inhibitor, and a CD38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA comments. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
“While treatable, refractory multiple myeloma is a significant clinical challenge with poor outcomes for patients whose disease has become resistant to the current standard of care,” commented Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University, Atlanta, chair of the department of hematology and medical oncology at Emory, and a principal investigator for the clinical trial that led to the approval.
“Due to the limited options currently available, these patients are often retreated with drugs from the same classes after they relapse, which is why the approval of belantamab mafodotin, the first anti-BCMA therapy, is significant for both patients and physicians alike,” he said.
The product is an antibody-drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. It homes in on BCMA on myeloma cell surfaces. Once inside the myeloma cell, the cytotoxic agent is released, leading to apoptosis, the programmed death of the cancerous plasma cells.
Approval based on response rates
The accelerated approval from the FDA and the recommendation for conditional approval from the EMA are based on results for overall response rate and duration of response from a phase 2, open-label, randomized, two-arm study known as DREAMM-2. Both agencies said that they are waiting for further data on clinical benefit from ongoing trials.
The DREAMM-2 study investigated the efficacy and safety of two doses of belantamab mafodotin in multiple myeloma patients whose disease was still active after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results from this study were published in The Lancet Oncology in December. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The median duration of response had not been reached at the 6-month analysis, but for 73% of responders, DoR was ≥6 months.
The most commonly reported adverse events (≥20%) were keratopathy (changes in the cornea), decreased visual acuity, nausea, blurred vision, pyrexia, infusion-related reactions, and fatigue, the manufacturer notes.
Ocular toxicity
One of the most common adverse events with this product affects the eyes.
Ocular adverse reactions occurred in 77% of the 218 patients in the pooled safety population and included keratopathy (76%), changes in visual acuity (55%), blurred vision (27%), and dry eye (19%).
Corneal adverse events were monitored with eye exams prior to each dose, allowing dose reductions or interruptions as appropriate, the manufacturer noted. Patients also used preservative-free eyedrops. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the 2.5-mg/kg cohort.
Because of this ocular toxicity, the company has set up a risk evaluation and mitigation strategy (REMS) for the product. This requires education for all physicians who prescribe the product as well as their patients regarding the ocular risks associated with treatment. It also requires monitoring that includes regular ophthalmic examinations. Information about the scheme can be found at www.blenreprems.com.
At the FDA advisory committee meeting last month, one of the panelists, Gita Thanarajasingam, MD, an assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated but for ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some people than current treatments.
“It’s reasonable to leave open the option for decision making. Patients can express their values and preferences,” Dr. Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk-benefit discussion in a REMS program.”
Another panelist, Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it to them” with belantamab.
This article first appeared on Medscape.com.
The first-in-class drug belantamab mafodotin (Blenrep) has been granted an accelerated approval by the Food and Drug Administration for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
This follows a recommendation for approval on July 15 by an FDA advisory committee, which voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
The product has a novel mechanism of action: it targets B-cell maturation antigen (BCMA), a protein that is present on the surface of virtually all multiple myeloma cells but is absent from normal B cells.
The drug had already received an FDA breakthrough therapy designation, which facilitates the development of drugs that have shown clinical promise for conditions in which there is significant unmet need.
Belantamab mafodotin was recommended for conditional marketing approval in the European Union on July 24 and was accepted into the European Medicines Agency PRIME scheme for medicines that have potential to address unmet medical needs.
The new drug is indicated for patients with refractory or relapsed multiple myeloma who have already tried treatment with one of the three major classes of drugs, namely, an immunomolatory agent, a proteasome inhibitor, and a CD38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA comments. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
“While treatable, refractory multiple myeloma is a significant clinical challenge with poor outcomes for patients whose disease has become resistant to the current standard of care,” commented Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University, Atlanta, chair of the department of hematology and medical oncology at Emory, and a principal investigator for the clinical trial that led to the approval.
“Due to the limited options currently available, these patients are often retreated with drugs from the same classes after they relapse, which is why the approval of belantamab mafodotin, the first anti-BCMA therapy, is significant for both patients and physicians alike,” he said.
The product is an antibody-drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. It homes in on BCMA on myeloma cell surfaces. Once inside the myeloma cell, the cytotoxic agent is released, leading to apoptosis, the programmed death of the cancerous plasma cells.
Approval based on response rates
The accelerated approval from the FDA and the recommendation for conditional approval from the EMA are based on results for overall response rate and duration of response from a phase 2, open-label, randomized, two-arm study known as DREAMM-2. Both agencies said that they are waiting for further data on clinical benefit from ongoing trials.
The DREAMM-2 study investigated the efficacy and safety of two doses of belantamab mafodotin in multiple myeloma patients whose disease was still active after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results from this study were published in The Lancet Oncology in December. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The median duration of response had not been reached at the 6-month analysis, but for 73% of responders, DoR was ≥6 months.
The most commonly reported adverse events (≥20%) were keratopathy (changes in the cornea), decreased visual acuity, nausea, blurred vision, pyrexia, infusion-related reactions, and fatigue, the manufacturer notes.
Ocular toxicity
One of the most common adverse events with this product affects the eyes.
Ocular adverse reactions occurred in 77% of the 218 patients in the pooled safety population and included keratopathy (76%), changes in visual acuity (55%), blurred vision (27%), and dry eye (19%).
Corneal adverse events were monitored with eye exams prior to each dose, allowing dose reductions or interruptions as appropriate, the manufacturer noted. Patients also used preservative-free eyedrops. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the 2.5-mg/kg cohort.
Because of this ocular toxicity, the company has set up a risk evaluation and mitigation strategy (REMS) for the product. This requires education for all physicians who prescribe the product as well as their patients regarding the ocular risks associated with treatment. It also requires monitoring that includes regular ophthalmic examinations. Information about the scheme can be found at www.blenreprems.com.
At the FDA advisory committee meeting last month, one of the panelists, Gita Thanarajasingam, MD, an assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated but for ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some people than current treatments.
“It’s reasonable to leave open the option for decision making. Patients can express their values and preferences,” Dr. Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk-benefit discussion in a REMS program.”
Another panelist, Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it to them” with belantamab.
This article first appeared on Medscape.com.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
ASCO says ‘no’ to home infusions of cancer treatment, with exceptions
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
OK to treat many cancer patients despite pandemic, says ESMO
Not all are highly vulnerable to COVID-19
Another important recommendation is to stop labeling all patients with cancer as being vulnerable to infection with the virus as it can lead to inappropriate care with potential negative outcomes.
“Although it was reasonable to adopt over-protective measures for our patients at the outbreak of a novel infective disease which was not previously observed in humans, we now need to step away from the assumption that all cancer patients are vulnerable to COVID-19,” said first author of the consensus article Giuseppe Curigliano, MD, PhD, of the European Institute of Oncology, Milan, Italy, in a statement. “The implications have been important because for some patients treatment was delayed or interrupted over the last few months, and I believe that we will see the impact of this over-precautionary approach in the...future.”
The recommendations were issued by the European Society of Medical Oncology (ESMO) to help guide physicians in “optimizing the pathway to cancer care” as well as to improve outcomes during the pandemic. The recommendations were published online July 31 in Annals of Oncology.
Studies have found that patients with cancer face a higher risk of serious complications and death if they develop COVID-19. Data from the COVID-19 and Cancer Consortium registry, for example, showed that patients with progressing cancer and COVID-19 infection had a fivefold increase in the risk of 30-day mortality compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
But while this may be true for some patients, Curigliano and colleagues emphasize that individuals with cancer are not a heterogeneous group and that the term “cancer” itself represents myriad different diseases. The European experts note that current evidence suggests many patients with solid tumors are not more vulnerable to serious complications than the general population.
Thus, cancer prognoses vary considerably, and addressing all patients with cancer as being “COVID-19-vulnerable is probably neither reasonable nor informative,” say the authors.
Dramatic changes were initiated in cancer management for all cancer types, nevertheless, and although these changes seemed reasonable in an acute pandemic situation, note the authors, they were made in the absence of strong supportive evidence. Attempts to define the individualized risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender, have been limited.
“Based on current evidence, only patients who are elderly, with multiple comorbidities, and receiving chemotherapy are vulnerable to the infection,” explained Curigliano.
However, on a positive note, a recently published prospective cohort study looked at approximately 800 patients with cancer – who had symptomatic COVID-19 – in the United Kingdom. The analysis showed no association at all between the risk for death and receiving chemotherapy or immunotherapy, points out Medscape commentator David Kerr, MD, of the University of Oxford, UK, in a recent commentary.
Key recommendations
An international consortium was established by ESMO, and the interdisciplinary expert panel consisted of 64 experts and one voting patient advocate. They agreed on 28 statements that can be used to help with many of the current clinical and technical areas of uncertainty that range from diagnosis to treatment decisions.
The following are several of the key recommendations:
- Patients with cancer who face the highest risk of severe COVID-19 are characterized by active and progressive cancer, advanced age, poor performance status, smoking status, comorbidities, and possibly type of cancer.
- Telehealth and digital health can be excellent tools for some types of care such as primary care triage and counseling, but meeting in person may be more effective for situations that include delivery of key cancer-related information and for patients with complex cancer needs.
- Prior to hospital admission, patients with cancer should be tested for COVID-19, if feasible, and if they are considered at high risk, regardless of symptoms or chest radiological findings.
- Patients with cancer and COVID-19 have a higher risk of thromboembolic events, and prophylaxis using low molecular weight or novel oral anticoagulants is recommended.
- Immune checkpoint inhibitors should not be withheld or delayed when there is a significant survival benefit, but use should be postponed in patients who test positive for COVID-19 until they recover.
- Use of high-dose steroids in patients with cancer infected with COVID-19 could potentially increase the risk of mortality, and a switch should be made to another immunosuppressant, if possible.
- The decision to use tyrosine kinase inhibitors (TKIs) of the PI3K/AKT/mTOR or RAS/RAF/MEK axis is complex, as they interfere with critical pathways involved in innate or adaptive immune responses. Stopping or withholding therapy depends on the risk-benefit balance, and the magnitude of benefit from the TKI needs to be considered.
The authors conclude that “ultimately, this set of statements will serve as a dynamic knowledge repository that will be better informed by accumulating data on SARS-CoV-2 biology, COVID-19 pandemic characteristics, on the risk of cancer patients for COVID-19 and its modulating factors, and finally, on optimal cancer care in the presence of the virus.”
No funding was reported for the current study. Several authors have disclosed relationships with industry, which are listed in the article.
This article first appeared on Medscape.com.
Not all are highly vulnerable to COVID-19
Not all are highly vulnerable to COVID-19
Another important recommendation is to stop labeling all patients with cancer as being vulnerable to infection with the virus as it can lead to inappropriate care with potential negative outcomes.
“Although it was reasonable to adopt over-protective measures for our patients at the outbreak of a novel infective disease which was not previously observed in humans, we now need to step away from the assumption that all cancer patients are vulnerable to COVID-19,” said first author of the consensus article Giuseppe Curigliano, MD, PhD, of the European Institute of Oncology, Milan, Italy, in a statement. “The implications have been important because for some patients treatment was delayed or interrupted over the last few months, and I believe that we will see the impact of this over-precautionary approach in the...future.”
The recommendations were issued by the European Society of Medical Oncology (ESMO) to help guide physicians in “optimizing the pathway to cancer care” as well as to improve outcomes during the pandemic. The recommendations were published online July 31 in Annals of Oncology.
Studies have found that patients with cancer face a higher risk of serious complications and death if they develop COVID-19. Data from the COVID-19 and Cancer Consortium registry, for example, showed that patients with progressing cancer and COVID-19 infection had a fivefold increase in the risk of 30-day mortality compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
But while this may be true for some patients, Curigliano and colleagues emphasize that individuals with cancer are not a heterogeneous group and that the term “cancer” itself represents myriad different diseases. The European experts note that current evidence suggests many patients with solid tumors are not more vulnerable to serious complications than the general population.
Thus, cancer prognoses vary considerably, and addressing all patients with cancer as being “COVID-19-vulnerable is probably neither reasonable nor informative,” say the authors.
Dramatic changes were initiated in cancer management for all cancer types, nevertheless, and although these changes seemed reasonable in an acute pandemic situation, note the authors, they were made in the absence of strong supportive evidence. Attempts to define the individualized risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender, have been limited.
“Based on current evidence, only patients who are elderly, with multiple comorbidities, and receiving chemotherapy are vulnerable to the infection,” explained Curigliano.
However, on a positive note, a recently published prospective cohort study looked at approximately 800 patients with cancer – who had symptomatic COVID-19 – in the United Kingdom. The analysis showed no association at all between the risk for death and receiving chemotherapy or immunotherapy, points out Medscape commentator David Kerr, MD, of the University of Oxford, UK, in a recent commentary.
Key recommendations
An international consortium was established by ESMO, and the interdisciplinary expert panel consisted of 64 experts and one voting patient advocate. They agreed on 28 statements that can be used to help with many of the current clinical and technical areas of uncertainty that range from diagnosis to treatment decisions.
The following are several of the key recommendations:
- Patients with cancer who face the highest risk of severe COVID-19 are characterized by active and progressive cancer, advanced age, poor performance status, smoking status, comorbidities, and possibly type of cancer.
- Telehealth and digital health can be excellent tools for some types of care such as primary care triage and counseling, but meeting in person may be more effective for situations that include delivery of key cancer-related information and for patients with complex cancer needs.
- Prior to hospital admission, patients with cancer should be tested for COVID-19, if feasible, and if they are considered at high risk, regardless of symptoms or chest radiological findings.
- Patients with cancer and COVID-19 have a higher risk of thromboembolic events, and prophylaxis using low molecular weight or novel oral anticoagulants is recommended.
- Immune checkpoint inhibitors should not be withheld or delayed when there is a significant survival benefit, but use should be postponed in patients who test positive for COVID-19 until they recover.
- Use of high-dose steroids in patients with cancer infected with COVID-19 could potentially increase the risk of mortality, and a switch should be made to another immunosuppressant, if possible.
- The decision to use tyrosine kinase inhibitors (TKIs) of the PI3K/AKT/mTOR or RAS/RAF/MEK axis is complex, as they interfere with critical pathways involved in innate or adaptive immune responses. Stopping or withholding therapy depends on the risk-benefit balance, and the magnitude of benefit from the TKI needs to be considered.
The authors conclude that “ultimately, this set of statements will serve as a dynamic knowledge repository that will be better informed by accumulating data on SARS-CoV-2 biology, COVID-19 pandemic characteristics, on the risk of cancer patients for COVID-19 and its modulating factors, and finally, on optimal cancer care in the presence of the virus.”
No funding was reported for the current study. Several authors have disclosed relationships with industry, which are listed in the article.
This article first appeared on Medscape.com.
Another important recommendation is to stop labeling all patients with cancer as being vulnerable to infection with the virus as it can lead to inappropriate care with potential negative outcomes.
“Although it was reasonable to adopt over-protective measures for our patients at the outbreak of a novel infective disease which was not previously observed in humans, we now need to step away from the assumption that all cancer patients are vulnerable to COVID-19,” said first author of the consensus article Giuseppe Curigliano, MD, PhD, of the European Institute of Oncology, Milan, Italy, in a statement. “The implications have been important because for some patients treatment was delayed or interrupted over the last few months, and I believe that we will see the impact of this over-precautionary approach in the...future.”
The recommendations were issued by the European Society of Medical Oncology (ESMO) to help guide physicians in “optimizing the pathway to cancer care” as well as to improve outcomes during the pandemic. The recommendations were published online July 31 in Annals of Oncology.
Studies have found that patients with cancer face a higher risk of serious complications and death if they develop COVID-19. Data from the COVID-19 and Cancer Consortium registry, for example, showed that patients with progressing cancer and COVID-19 infection had a fivefold increase in the risk of 30-day mortality compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
But while this may be true for some patients, Curigliano and colleagues emphasize that individuals with cancer are not a heterogeneous group and that the term “cancer” itself represents myriad different diseases. The European experts note that current evidence suggests many patients with solid tumors are not more vulnerable to serious complications than the general population.
Thus, cancer prognoses vary considerably, and addressing all patients with cancer as being “COVID-19-vulnerable is probably neither reasonable nor informative,” say the authors.
Dramatic changes were initiated in cancer management for all cancer types, nevertheless, and although these changes seemed reasonable in an acute pandemic situation, note the authors, they were made in the absence of strong supportive evidence. Attempts to define the individualized risk for a given patient, taking into account their primary tumor subtype, stage, age, and gender, have been limited.
“Based on current evidence, only patients who are elderly, with multiple comorbidities, and receiving chemotherapy are vulnerable to the infection,” explained Curigliano.
However, on a positive note, a recently published prospective cohort study looked at approximately 800 patients with cancer – who had symptomatic COVID-19 – in the United Kingdom. The analysis showed no association at all between the risk for death and receiving chemotherapy or immunotherapy, points out Medscape commentator David Kerr, MD, of the University of Oxford, UK, in a recent commentary.
Key recommendations
An international consortium was established by ESMO, and the interdisciplinary expert panel consisted of 64 experts and one voting patient advocate. They agreed on 28 statements that can be used to help with many of the current clinical and technical areas of uncertainty that range from diagnosis to treatment decisions.
The following are several of the key recommendations:
- Patients with cancer who face the highest risk of severe COVID-19 are characterized by active and progressive cancer, advanced age, poor performance status, smoking status, comorbidities, and possibly type of cancer.
- Telehealth and digital health can be excellent tools for some types of care such as primary care triage and counseling, but meeting in person may be more effective for situations that include delivery of key cancer-related information and for patients with complex cancer needs.
- Prior to hospital admission, patients with cancer should be tested for COVID-19, if feasible, and if they are considered at high risk, regardless of symptoms or chest radiological findings.
- Patients with cancer and COVID-19 have a higher risk of thromboembolic events, and prophylaxis using low molecular weight or novel oral anticoagulants is recommended.
- Immune checkpoint inhibitors should not be withheld or delayed when there is a significant survival benefit, but use should be postponed in patients who test positive for COVID-19 until they recover.
- Use of high-dose steroids in patients with cancer infected with COVID-19 could potentially increase the risk of mortality, and a switch should be made to another immunosuppressant, if possible.
- The decision to use tyrosine kinase inhibitors (TKIs) of the PI3K/AKT/mTOR or RAS/RAF/MEK axis is complex, as they interfere with critical pathways involved in innate or adaptive immune responses. Stopping or withholding therapy depends on the risk-benefit balance, and the magnitude of benefit from the TKI needs to be considered.
The authors conclude that “ultimately, this set of statements will serve as a dynamic knowledge repository that will be better informed by accumulating data on SARS-CoV-2 biology, COVID-19 pandemic characteristics, on the risk of cancer patients for COVID-19 and its modulating factors, and finally, on optimal cancer care in the presence of the virus.”
No funding was reported for the current study. Several authors have disclosed relationships with industry, which are listed in the article.
This article first appeared on Medscape.com.
Higher death rate seen in cancer patients with nosocomial COVID-19
, according to researchers.
In an observational study of patients with COVID-19 and cancer, 19% of patients had COVID-19 acquired during a non-COVID-related hospital stay, and 81% had community-acquired COVID-19.
At a median follow-up of 23 days, the overall mortality rate was 28%. However, the all-cause mortality rate in patients with nosocomial COVID-19 was more than double that of patients with community-acquired COVID-19, at 47% and 23%, respectively.
Arielle Elkrief, MD, of the University of Montreal, reported these results during the AACR virtual meeting: COVID-19 and Cancer.
“This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, at a rate of 19%,” Dr. Elkrief said. “This was associated with high mortality in both univariate and multivariate analyses.”
The study included 250 adults and 3 children with COVID-19 and cancer who were identified between March 3 and May 23, 2020. They ranged in age from 4 to 95 years, but the median age was 73 years.
All patients had either laboratory-confirmed (95%) or presumed COVID-19 (5%) and invasive cancer. The most common cancer types were similar to those seen in the general population. Lung and breast cancer were the most common, followed by lymphoma, prostate cancer, and colorectal cancer. Most patients were on active anticancer therapy, most often chemotherapy.
Most patients (n = 236) were residents of Quebec, but 17 patients were residents of British Columbia.
“It is important to note that Quebec was one of the most heavily affected areas in North America at the time of the study,” Dr. Elkrief said.
Outcomes by group
There were 206 patients (81%) who had community-acquired COVID-19 and 47 (19%) who had nosocomial COVID-19. The two groups were similar with respect to sex, performance status, and cancer stage. A small trend toward more patients on active therapy was seen in the nosocomial group, but the difference did not reach statistical significance.
The median overall survival was 27 days in the nosocomial group and 71 days in the community-acquired group (hazard ratio, 2.2; P = .002).
A multivariate analysis showed that nosocomial infection was “strongly and independently associated with death,” Dr. Elkrief said. “Other risk factors for poor prognosis included age, poor [performance] status, and advanced stage of cancer.”
There were no significant differences between the hospital-acquired and community-acquired groups for other outcomes, including oxygen requirements (43% and 47%, respectively), ICU admission (13% and 11%), need for mechanical ventilation (6% and 5%), or length of stay (median, 9.5 days and 8.5 days).
The low rate of ICU admission, considering the mortality rate of 28%, “could reflect that patients with cancer are less likely to be admitted to the ICU,” Dr. Elkrief noted.
Applying the findings to practice
The findings reinforce the importance of adherence to stringent infection control guidelines to protect vulnerable patients, such as those with cancer, Dr. Elkrief said.
In ambulatory settings, this means decreasing in-person visits through increased use of teleconsultations, and for those who need to be seen in person, screening for symptoms or use of polymerase chain reaction testing should be used when resources are available, she said.
“Similar principles apply to chemotherapy treatment units,” Dr. Elkrief said. She added that staff must avoid cross-contamination between COVID and COVID-free zones, and that “dedicated personnel and equipment should be maintained and separate between these two zones.
“Adequate protective personal equipment and strict hand hygiene protocols are also of utmost importance,” Dr. Elkrief said. “The threat of COVID-19 is not behind us, and so we continue to enforce these strategies to protect our patients.”
Session moderator Gypsyamber D’Souza, PhD, an infectious disease epidemiologist at Johns Hopkins University in Baltimore, raised the question of whether the high nosocomial infection and death rate in this study was related to patients having more severe disease because of underlying comorbidities.
Dr. Elkrief explained that the overall mortality rate was indeed higher than the 13% reported in other studies, and it may reflect an overrepresentation of hospitalized or more severely ill patients in the cohort.
However, the investigators made every effort to include all patients with both cancer and COVID-19 by using systematic screening of inpatient and outpatients lists and registries.
Further, the multivariate analysis included both inpatients and outpatients and adjusted for known negative prognostic factors for COVID-19 outcomes. These included increasing age, poor performance status, and different comorbidities.
The finding that nosocomial infection was an independent predictor of death “pushed us to look at nosocomial infection as a new independent risk factor,” Dr. Elkrief said.
Dr. Elkrief reported grant support from AstraZeneca. Dr. D’Souza did not report any disclosures.
SOURCE: Elkrief A et al. AACR: COVID and Cancer, Abstract S12-01.
, according to researchers.
In an observational study of patients with COVID-19 and cancer, 19% of patients had COVID-19 acquired during a non-COVID-related hospital stay, and 81% had community-acquired COVID-19.
At a median follow-up of 23 days, the overall mortality rate was 28%. However, the all-cause mortality rate in patients with nosocomial COVID-19 was more than double that of patients with community-acquired COVID-19, at 47% and 23%, respectively.
Arielle Elkrief, MD, of the University of Montreal, reported these results during the AACR virtual meeting: COVID-19 and Cancer.
“This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, at a rate of 19%,” Dr. Elkrief said. “This was associated with high mortality in both univariate and multivariate analyses.”
The study included 250 adults and 3 children with COVID-19 and cancer who were identified between March 3 and May 23, 2020. They ranged in age from 4 to 95 years, but the median age was 73 years.
All patients had either laboratory-confirmed (95%) or presumed COVID-19 (5%) and invasive cancer. The most common cancer types were similar to those seen in the general population. Lung and breast cancer were the most common, followed by lymphoma, prostate cancer, and colorectal cancer. Most patients were on active anticancer therapy, most often chemotherapy.
Most patients (n = 236) were residents of Quebec, but 17 patients were residents of British Columbia.
“It is important to note that Quebec was one of the most heavily affected areas in North America at the time of the study,” Dr. Elkrief said.
Outcomes by group
There were 206 patients (81%) who had community-acquired COVID-19 and 47 (19%) who had nosocomial COVID-19. The two groups were similar with respect to sex, performance status, and cancer stage. A small trend toward more patients on active therapy was seen in the nosocomial group, but the difference did not reach statistical significance.
The median overall survival was 27 days in the nosocomial group and 71 days in the community-acquired group (hazard ratio, 2.2; P = .002).
A multivariate analysis showed that nosocomial infection was “strongly and independently associated with death,” Dr. Elkrief said. “Other risk factors for poor prognosis included age, poor [performance] status, and advanced stage of cancer.”
There were no significant differences between the hospital-acquired and community-acquired groups for other outcomes, including oxygen requirements (43% and 47%, respectively), ICU admission (13% and 11%), need for mechanical ventilation (6% and 5%), or length of stay (median, 9.5 days and 8.5 days).
The low rate of ICU admission, considering the mortality rate of 28%, “could reflect that patients with cancer are less likely to be admitted to the ICU,” Dr. Elkrief noted.
Applying the findings to practice
The findings reinforce the importance of adherence to stringent infection control guidelines to protect vulnerable patients, such as those with cancer, Dr. Elkrief said.
In ambulatory settings, this means decreasing in-person visits through increased use of teleconsultations, and for those who need to be seen in person, screening for symptoms or use of polymerase chain reaction testing should be used when resources are available, she said.
“Similar principles apply to chemotherapy treatment units,” Dr. Elkrief said. She added that staff must avoid cross-contamination between COVID and COVID-free zones, and that “dedicated personnel and equipment should be maintained and separate between these two zones.
“Adequate protective personal equipment and strict hand hygiene protocols are also of utmost importance,” Dr. Elkrief said. “The threat of COVID-19 is not behind us, and so we continue to enforce these strategies to protect our patients.”
Session moderator Gypsyamber D’Souza, PhD, an infectious disease epidemiologist at Johns Hopkins University in Baltimore, raised the question of whether the high nosocomial infection and death rate in this study was related to patients having more severe disease because of underlying comorbidities.
Dr. Elkrief explained that the overall mortality rate was indeed higher than the 13% reported in other studies, and it may reflect an overrepresentation of hospitalized or more severely ill patients in the cohort.
However, the investigators made every effort to include all patients with both cancer and COVID-19 by using systematic screening of inpatient and outpatients lists and registries.
Further, the multivariate analysis included both inpatients and outpatients and adjusted for known negative prognostic factors for COVID-19 outcomes. These included increasing age, poor performance status, and different comorbidities.
The finding that nosocomial infection was an independent predictor of death “pushed us to look at nosocomial infection as a new independent risk factor,” Dr. Elkrief said.
Dr. Elkrief reported grant support from AstraZeneca. Dr. D’Souza did not report any disclosures.
SOURCE: Elkrief A et al. AACR: COVID and Cancer, Abstract S12-01.
, according to researchers.
In an observational study of patients with COVID-19 and cancer, 19% of patients had COVID-19 acquired during a non-COVID-related hospital stay, and 81% had community-acquired COVID-19.
At a median follow-up of 23 days, the overall mortality rate was 28%. However, the all-cause mortality rate in patients with nosocomial COVID-19 was more than double that of patients with community-acquired COVID-19, at 47% and 23%, respectively.
Arielle Elkrief, MD, of the University of Montreal, reported these results during the AACR virtual meeting: COVID-19 and Cancer.
“This is the first report that describes a high rate of hospital-acquired COVID-19 in patients with cancer, at a rate of 19%,” Dr. Elkrief said. “This was associated with high mortality in both univariate and multivariate analyses.”
The study included 250 adults and 3 children with COVID-19 and cancer who were identified between March 3 and May 23, 2020. They ranged in age from 4 to 95 years, but the median age was 73 years.
All patients had either laboratory-confirmed (95%) or presumed COVID-19 (5%) and invasive cancer. The most common cancer types were similar to those seen in the general population. Lung and breast cancer were the most common, followed by lymphoma, prostate cancer, and colorectal cancer. Most patients were on active anticancer therapy, most often chemotherapy.
Most patients (n = 236) were residents of Quebec, but 17 patients were residents of British Columbia.
“It is important to note that Quebec was one of the most heavily affected areas in North America at the time of the study,” Dr. Elkrief said.
Outcomes by group
There were 206 patients (81%) who had community-acquired COVID-19 and 47 (19%) who had nosocomial COVID-19. The two groups were similar with respect to sex, performance status, and cancer stage. A small trend toward more patients on active therapy was seen in the nosocomial group, but the difference did not reach statistical significance.
The median overall survival was 27 days in the nosocomial group and 71 days in the community-acquired group (hazard ratio, 2.2; P = .002).
A multivariate analysis showed that nosocomial infection was “strongly and independently associated with death,” Dr. Elkrief said. “Other risk factors for poor prognosis included age, poor [performance] status, and advanced stage of cancer.”
There were no significant differences between the hospital-acquired and community-acquired groups for other outcomes, including oxygen requirements (43% and 47%, respectively), ICU admission (13% and 11%), need for mechanical ventilation (6% and 5%), or length of stay (median, 9.5 days and 8.5 days).
The low rate of ICU admission, considering the mortality rate of 28%, “could reflect that patients with cancer are less likely to be admitted to the ICU,” Dr. Elkrief noted.
Applying the findings to practice
The findings reinforce the importance of adherence to stringent infection control guidelines to protect vulnerable patients, such as those with cancer, Dr. Elkrief said.
In ambulatory settings, this means decreasing in-person visits through increased use of teleconsultations, and for those who need to be seen in person, screening for symptoms or use of polymerase chain reaction testing should be used when resources are available, she said.
“Similar principles apply to chemotherapy treatment units,” Dr. Elkrief said. She added that staff must avoid cross-contamination between COVID and COVID-free zones, and that “dedicated personnel and equipment should be maintained and separate between these two zones.
“Adequate protective personal equipment and strict hand hygiene protocols are also of utmost importance,” Dr. Elkrief said. “The threat of COVID-19 is not behind us, and so we continue to enforce these strategies to protect our patients.”
Session moderator Gypsyamber D’Souza, PhD, an infectious disease epidemiologist at Johns Hopkins University in Baltimore, raised the question of whether the high nosocomial infection and death rate in this study was related to patients having more severe disease because of underlying comorbidities.
Dr. Elkrief explained that the overall mortality rate was indeed higher than the 13% reported in other studies, and it may reflect an overrepresentation of hospitalized or more severely ill patients in the cohort.
However, the investigators made every effort to include all patients with both cancer and COVID-19 by using systematic screening of inpatient and outpatients lists and registries.
Further, the multivariate analysis included both inpatients and outpatients and adjusted for known negative prognostic factors for COVID-19 outcomes. These included increasing age, poor performance status, and different comorbidities.
The finding that nosocomial infection was an independent predictor of death “pushed us to look at nosocomial infection as a new independent risk factor,” Dr. Elkrief said.
Dr. Elkrief reported grant support from AstraZeneca. Dr. D’Souza did not report any disclosures.
SOURCE: Elkrief A et al. AACR: COVID and Cancer, Abstract S12-01.
FROM AACR: COVID-19 AND CANCER