User login
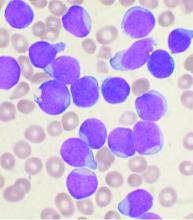
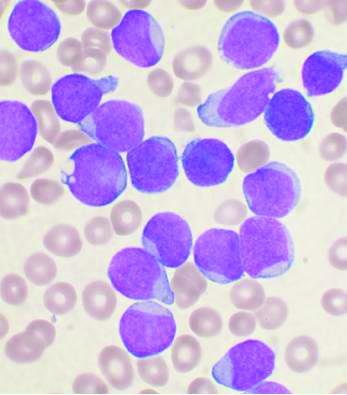
Compound CAR T – a double whammy with promise for AML
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
Six of eight relapsed/refractory acute myeloid leukemia patients, and one patient with accelerated phase chronic myelogenous leukemia, had no sign of residual disease 4 weeks after receiving compound CAR T therapy targeting both CD33 and CLL1.
Six patients moved on to subsequent hematopoietic stem cell transplantation (HSCT); the seventh responder withdrew from the study for personal reasons, according to a report at the virtual annual congress of the European Hematology Association.
Much work remains, but the initial results suggest that “CLL1-CD33 compound CAR T cell therapy could be developed as a bridge to transplant, a supplement to chemotherapy, or a standalone therapy for patients with acute myeloid leukemia” and other myeloid malignancies. The approach might also allow for reduced intensity conditioning or nonmyeloablative conditioning for HSCT, said lead investigator Fang Liu, MD, PhD, of the department of hematology at the Chengdu Military General Hospital, in Sichuan province, China.
It’s “a topic that will interest a lot of us.” For the first time, “a compound CAR with two independent CAR units induced remissions in AML,” said Pieter Sonneveld, MD, PhD, of the Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands, who introduced Dr. Liu’s presentation.
Chimeric antigen receptor (CAR) T cell therapy works well for B-cell malignancies, but translation to AML is “yet to be accomplished.” Meanwhile, despite progress against AML, about one-third of patients still relapse, “and prognosis for relapsed or refractory AML is dismal,” Dr. Liu and her team said.
CAR T is generally aimed against a single target, but AML bears heterogeneous cells that offset killing by single target therapies, resulting in disease relapse.
That problem suggested targeting multiple antigens simultaneously. CLL1 is an “ideal target,” Dr. Liu said, because the myeloid lineage antigen is highly expressed in AML, but absent in normal hematopoietic stem cells. CD33, meanwhile, is expressed on bulk AML cells in the majority of patients.
The CAR T cells were manufactured from autologous cells in eight of the subjects, and from a human leukocyte antigen-matched sibling donor cells for the ninth. The patients were lymphodepleted with fludarabine and cyclophosphamide, then infused with the therapeutic cells by a dose escalation at approximately 1-3 x 106/kg in a single or split dose.
On disease reevaluation within 4 weeks, seven of nine patients – all with relapsed or refractory disease after multiple conventional treatments – were minimal residual disease negative by flow cytometry. The other two had no response, one of whom was CD33 positive but CLL1 negative, “indicating the importance of [the] CLL1 target in CAR T treatment,” the investigators said.
All nine patients developed grade 4 pancytopenia; eight had cytokine release syndrome (CRS), which was grade 3 in two; and four subjects developed neurotoxicity, which was grade 3 in three.
Five subjects had mild liver enzyme elevations; four had a coagulation disorder; four developed diarrhea; three developed sepsis; two fungal infections; and three pneumonia. One subject had a skin rash and one developed renal insufficiency.
The adverse events resolved after treatment. “Early intervention with steroids had a positive effect on the reduction of CRS and neurotoxicity,” the team noted.
Of the six patients who went on to HCST, one had standard myeloablative conditioning, but the rest had reduced intensity conditioning. Five subjects successfully engrafted with persistent full chimerism, but one died of sepsis before engraftment.
The median age was 32 years. The median bone marrow blast count before treatment was 47%. Seven subjects had de novo AML; one – a 6-year-old girl – had juvenile myelomonocytic leukemia that transformed into AML; and one had accelerated phase chronic myelogenous leukemia.
A phase 1 trial is underway (NCT03795779).
The work was funded by iCell Gene Therapeutics. Several investigators were employees. Dr. Liu didn’t report any disclosures.
SOURCE: Liu F et al. EHA Congress. Abstract S149.
FROM EHA CONGRESS
Three-drug combo promising against high-risk CLL
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
FROM EHA CONGRESS
Can an app guide cancer treatment decisions during the pandemic?
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Risk index stratifies pediatric leukemia patients undergoing HSCT
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
FROM ASCO 2020
‘A good and peaceful death’: Cancer hospice during the pandemic
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Acute lymphoblastic leukemia can be successfully treated in the frail elderly
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Patient-focused precautions, testing help blunt pandemic effects on heme-onc unit
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Oncologists’ income and satisfaction are up
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Race and location appear to play a role in the incidence of CLL and DLBCL
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Universal CAR-T therapy produces CRs in relapsed/refractory T-ALL
according to initial findings from an ongoing study.
The first five patients enrolled in this first-in-human study received conditioning and an infusion of the premanufactured CD7-targeted CAR T-cell therapy, TruUCAR GC027.
All five patients achieved a complete remission (CR) or CR with incomplete count recovery (CRi), although one patient had a morphological relapse at 1 month.
Xinxin Wang, PhD, reported these results at the AACR Virtual Meeting I. Dr. Wang is employed by Gracell Biotechnologies in Shanghai, China, which is the company developing TruUCAR GC027.
The CAR T-cell therapy is manufactured using lentivirus and leukopaks from HLA-mismatched healthy donors, according to Dr. Wang. TruUCAR GC027 contains second-generation CAR T cells with genomic disruption of TCR-alpha and CD7 to help prevent graft-versus-host disease and fratricide.
TruUCAR GC027 was previously shown to expand and have antileukemic activity in a murine model, Dr. Wang noted.
Patients and treatment
The five patients in the phase 1 study had a median age of 24 years (range, 19 to 38 years). They had heavily pretreated T-ALL, with a median of 5 prior lines of therapy (range, 1-9). Baseline bone marrow tumor burden ranged from 4% to 80.2% (median, 38.2%).
None of the patients received prior allogeneic hematopoietic stem cell transplant.
All patients received a preconditioning chemotherapy regimen. One patient received TruUCAR GC027 at dose level 1 (6 x 106 cells/kg), three patients received dose level 2 (1 x 107 cells/kg), and one patient received dose level 3 (1.5 x 107 cells/kg) – each as a single infusion.
Expansion, response, and safety
“GC027 expansion, analyzed by flow [cytometry] was observed in most of the patients treated,” Dr. Wang said. “We started to see GC027 in the peripheral blood as early as day 5, with peaks around day 7-14.”
All five patients had a CR or CRi at the first postinfusion evaluation, which occurred at day 14 in four of the five patients. Four patients also achieved minimum residual disease (MRD) negativity by 1 month of follow-up and remained in MRD-negative CR at the February 6, 2020, data cutoff.
One patient achieved MRD-positive CR at day 14 but experienced morphological relapse at 1 month.
In the four patients with MRD-negative CR at 1 month, cellular expansion was observed as early as day 5 and continued for 2 weeks, but the patient who relapsed at day 29 showed no cellular expansion on flow cytometry, Dr. Wang said.
However, by a more sensitive quantitative polymerase chain reaction analysis, cellular expansion was observed in all five patients starting as early as day 1 after infusion, although the patient who relapsed had the shortest duration of expansion.
All patients developed cytokine release syndrome (CRS). Four patients experienced grade 3 CRS, and one experienced grade 4 CRS.
“The CRS was manageable and reversible,” Dr. Wang said, adding that none of the patients experienced neurotoxicity or graft-versus-host disease.
Prolonged cytopenia occurred in four patients, including one grade 1 case, two grade 3 cases, and one grade 4 case. Grade 3 pulmonary infections occurred in three patients, and grade 3 neutropenia occurred in all five patients.
‘Very impressive’ early results
Dr. Wang said the responses observed in this trial are notable because T-ALL constitutes 20%-25% of all adult ALL and 12%-15% of all pediatric ALL. T-ALL is highly aggressive, with event-free and overall survival of less than 25% in the relapsed setting. Dr. Wang noted that, despite the high unmet medical need and lack of treatment options for T-ALL, the development of novel immunotherapies has lagged.
One challenge is that T-ALL and normal T cells share common surface antigens, so targeted therapies for T-ALL will also target normal T cells. Another challenge is the potential contamination by malignant cells in autologous T-cell products, Dr. Wang said, noting that this can be avoided with universal CAR T cells.
Further, CD7 is a good target for T-ALL because it is expressed in more than 95% of T-ALL patients, she added.
“[TruUCAR GC027] demonstrated a very promising early response rate ... and showed a manageable toxicity profile at all three dose levels,” Dr. Wang said in closing, noting that further evaluation is warranted.
Indeed, the results of this next-generation CAR T-cell trial are “very impressive,” said invited discussant Yvonne Y. Chen, PhD, of the University of California, Los Angeles.
There have been concerns that “off-the-shelf” CAR T-cell products like TruUCAR GC027 might be limited by factors such as a reduced level of CAR T-cell persistence and therefore reduced efficacy leading to a need for repeat dosing, Dr. Chen noted. However, Dr. Wang and her colleagues showed a 100% CR/CRi rate with a single dose of CAR T cells and without graft-versus-host disease or neurotoxicity, Dr. Chen emphasized.
“I think it’s also important to note, however, that there’s quite a high incidence rate of grade 3 or higher toxicities, including CRS,” Dr. Chen said. “I suspect this may have something to do with the fairly high dosing levels used in this trial.”
The “big question,” however, is durability of the response, Dr. Chen said. “And this is something that the field will really watch as this trial progresses beyond the 7-month monitoring period ... reported today.”
Dr. Wang is an employee of Gracell Biotechnologies. Dr. Chen is cofounder of Kalthera Therapeutics and a scientific adviser for Gritstone Oncology and Notch Therapeutics.
SOURCE: Wang X et al. AACR 2020, Abstract CT052.
according to initial findings from an ongoing study.
The first five patients enrolled in this first-in-human study received conditioning and an infusion of the premanufactured CD7-targeted CAR T-cell therapy, TruUCAR GC027.
All five patients achieved a complete remission (CR) or CR with incomplete count recovery (CRi), although one patient had a morphological relapse at 1 month.
Xinxin Wang, PhD, reported these results at the AACR Virtual Meeting I. Dr. Wang is employed by Gracell Biotechnologies in Shanghai, China, which is the company developing TruUCAR GC027.
The CAR T-cell therapy is manufactured using lentivirus and leukopaks from HLA-mismatched healthy donors, according to Dr. Wang. TruUCAR GC027 contains second-generation CAR T cells with genomic disruption of TCR-alpha and CD7 to help prevent graft-versus-host disease and fratricide.
TruUCAR GC027 was previously shown to expand and have antileukemic activity in a murine model, Dr. Wang noted.
Patients and treatment
The five patients in the phase 1 study had a median age of 24 years (range, 19 to 38 years). They had heavily pretreated T-ALL, with a median of 5 prior lines of therapy (range, 1-9). Baseline bone marrow tumor burden ranged from 4% to 80.2% (median, 38.2%).
None of the patients received prior allogeneic hematopoietic stem cell transplant.
All patients received a preconditioning chemotherapy regimen. One patient received TruUCAR GC027 at dose level 1 (6 x 106 cells/kg), three patients received dose level 2 (1 x 107 cells/kg), and one patient received dose level 3 (1.5 x 107 cells/kg) – each as a single infusion.
Expansion, response, and safety
“GC027 expansion, analyzed by flow [cytometry] was observed in most of the patients treated,” Dr. Wang said. “We started to see GC027 in the peripheral blood as early as day 5, with peaks around day 7-14.”
All five patients had a CR or CRi at the first postinfusion evaluation, which occurred at day 14 in four of the five patients. Four patients also achieved minimum residual disease (MRD) negativity by 1 month of follow-up and remained in MRD-negative CR at the February 6, 2020, data cutoff.
One patient achieved MRD-positive CR at day 14 but experienced morphological relapse at 1 month.
In the four patients with MRD-negative CR at 1 month, cellular expansion was observed as early as day 5 and continued for 2 weeks, but the patient who relapsed at day 29 showed no cellular expansion on flow cytometry, Dr. Wang said.
However, by a more sensitive quantitative polymerase chain reaction analysis, cellular expansion was observed in all five patients starting as early as day 1 after infusion, although the patient who relapsed had the shortest duration of expansion.
All patients developed cytokine release syndrome (CRS). Four patients experienced grade 3 CRS, and one experienced grade 4 CRS.
“The CRS was manageable and reversible,” Dr. Wang said, adding that none of the patients experienced neurotoxicity or graft-versus-host disease.
Prolonged cytopenia occurred in four patients, including one grade 1 case, two grade 3 cases, and one grade 4 case. Grade 3 pulmonary infections occurred in three patients, and grade 3 neutropenia occurred in all five patients.
‘Very impressive’ early results
Dr. Wang said the responses observed in this trial are notable because T-ALL constitutes 20%-25% of all adult ALL and 12%-15% of all pediatric ALL. T-ALL is highly aggressive, with event-free and overall survival of less than 25% in the relapsed setting. Dr. Wang noted that, despite the high unmet medical need and lack of treatment options for T-ALL, the development of novel immunotherapies has lagged.
One challenge is that T-ALL and normal T cells share common surface antigens, so targeted therapies for T-ALL will also target normal T cells. Another challenge is the potential contamination by malignant cells in autologous T-cell products, Dr. Wang said, noting that this can be avoided with universal CAR T cells.
Further, CD7 is a good target for T-ALL because it is expressed in more than 95% of T-ALL patients, she added.
“[TruUCAR GC027] demonstrated a very promising early response rate ... and showed a manageable toxicity profile at all three dose levels,” Dr. Wang said in closing, noting that further evaluation is warranted.
Indeed, the results of this next-generation CAR T-cell trial are “very impressive,” said invited discussant Yvonne Y. Chen, PhD, of the University of California, Los Angeles.
There have been concerns that “off-the-shelf” CAR T-cell products like TruUCAR GC027 might be limited by factors such as a reduced level of CAR T-cell persistence and therefore reduced efficacy leading to a need for repeat dosing, Dr. Chen noted. However, Dr. Wang and her colleagues showed a 100% CR/CRi rate with a single dose of CAR T cells and without graft-versus-host disease or neurotoxicity, Dr. Chen emphasized.
“I think it’s also important to note, however, that there’s quite a high incidence rate of grade 3 or higher toxicities, including CRS,” Dr. Chen said. “I suspect this may have something to do with the fairly high dosing levels used in this trial.”
The “big question,” however, is durability of the response, Dr. Chen said. “And this is something that the field will really watch as this trial progresses beyond the 7-month monitoring period ... reported today.”
Dr. Wang is an employee of Gracell Biotechnologies. Dr. Chen is cofounder of Kalthera Therapeutics and a scientific adviser for Gritstone Oncology and Notch Therapeutics.
SOURCE: Wang X et al. AACR 2020, Abstract CT052.
according to initial findings from an ongoing study.
The first five patients enrolled in this first-in-human study received conditioning and an infusion of the premanufactured CD7-targeted CAR T-cell therapy, TruUCAR GC027.
All five patients achieved a complete remission (CR) or CR with incomplete count recovery (CRi), although one patient had a morphological relapse at 1 month.
Xinxin Wang, PhD, reported these results at the AACR Virtual Meeting I. Dr. Wang is employed by Gracell Biotechnologies in Shanghai, China, which is the company developing TruUCAR GC027.
The CAR T-cell therapy is manufactured using lentivirus and leukopaks from HLA-mismatched healthy donors, according to Dr. Wang. TruUCAR GC027 contains second-generation CAR T cells with genomic disruption of TCR-alpha and CD7 to help prevent graft-versus-host disease and fratricide.
TruUCAR GC027 was previously shown to expand and have antileukemic activity in a murine model, Dr. Wang noted.
Patients and treatment
The five patients in the phase 1 study had a median age of 24 years (range, 19 to 38 years). They had heavily pretreated T-ALL, with a median of 5 prior lines of therapy (range, 1-9). Baseline bone marrow tumor burden ranged from 4% to 80.2% (median, 38.2%).
None of the patients received prior allogeneic hematopoietic stem cell transplant.
All patients received a preconditioning chemotherapy regimen. One patient received TruUCAR GC027 at dose level 1 (6 x 106 cells/kg), three patients received dose level 2 (1 x 107 cells/kg), and one patient received dose level 3 (1.5 x 107 cells/kg) – each as a single infusion.
Expansion, response, and safety
“GC027 expansion, analyzed by flow [cytometry] was observed in most of the patients treated,” Dr. Wang said. “We started to see GC027 in the peripheral blood as early as day 5, with peaks around day 7-14.”
All five patients had a CR or CRi at the first postinfusion evaluation, which occurred at day 14 in four of the five patients. Four patients also achieved minimum residual disease (MRD) negativity by 1 month of follow-up and remained in MRD-negative CR at the February 6, 2020, data cutoff.
One patient achieved MRD-positive CR at day 14 but experienced morphological relapse at 1 month.
In the four patients with MRD-negative CR at 1 month, cellular expansion was observed as early as day 5 and continued for 2 weeks, but the patient who relapsed at day 29 showed no cellular expansion on flow cytometry, Dr. Wang said.
However, by a more sensitive quantitative polymerase chain reaction analysis, cellular expansion was observed in all five patients starting as early as day 1 after infusion, although the patient who relapsed had the shortest duration of expansion.
All patients developed cytokine release syndrome (CRS). Four patients experienced grade 3 CRS, and one experienced grade 4 CRS.
“The CRS was manageable and reversible,” Dr. Wang said, adding that none of the patients experienced neurotoxicity or graft-versus-host disease.
Prolonged cytopenia occurred in four patients, including one grade 1 case, two grade 3 cases, and one grade 4 case. Grade 3 pulmonary infections occurred in three patients, and grade 3 neutropenia occurred in all five patients.
‘Very impressive’ early results
Dr. Wang said the responses observed in this trial are notable because T-ALL constitutes 20%-25% of all adult ALL and 12%-15% of all pediatric ALL. T-ALL is highly aggressive, with event-free and overall survival of less than 25% in the relapsed setting. Dr. Wang noted that, despite the high unmet medical need and lack of treatment options for T-ALL, the development of novel immunotherapies has lagged.
One challenge is that T-ALL and normal T cells share common surface antigens, so targeted therapies for T-ALL will also target normal T cells. Another challenge is the potential contamination by malignant cells in autologous T-cell products, Dr. Wang said, noting that this can be avoided with universal CAR T cells.
Further, CD7 is a good target for T-ALL because it is expressed in more than 95% of T-ALL patients, she added.
“[TruUCAR GC027] demonstrated a very promising early response rate ... and showed a manageable toxicity profile at all three dose levels,” Dr. Wang said in closing, noting that further evaluation is warranted.
Indeed, the results of this next-generation CAR T-cell trial are “very impressive,” said invited discussant Yvonne Y. Chen, PhD, of the University of California, Los Angeles.
There have been concerns that “off-the-shelf” CAR T-cell products like TruUCAR GC027 might be limited by factors such as a reduced level of CAR T-cell persistence and therefore reduced efficacy leading to a need for repeat dosing, Dr. Chen noted. However, Dr. Wang and her colleagues showed a 100% CR/CRi rate with a single dose of CAR T cells and without graft-versus-host disease or neurotoxicity, Dr. Chen emphasized.
“I think it’s also important to note, however, that there’s quite a high incidence rate of grade 3 or higher toxicities, including CRS,” Dr. Chen said. “I suspect this may have something to do with the fairly high dosing levels used in this trial.”
The “big question,” however, is durability of the response, Dr. Chen said. “And this is something that the field will really watch as this trial progresses beyond the 7-month monitoring period ... reported today.”
Dr. Wang is an employee of Gracell Biotechnologies. Dr. Chen is cofounder of Kalthera Therapeutics and a scientific adviser for Gritstone Oncology and Notch Therapeutics.
SOURCE: Wang X et al. AACR 2020, Abstract CT052.
FROM AACR 2020