User login
Same-day discharge for elective PCI shown safe in real-world analysis
Based on a large registry, there appears to be no adverse consequences for same-day discharge following an elective percutaneous cardiovascular intervention (PCI), according to an analysis of a nationwide registry.
“Our data suggest there has been no negative impact on patient outcomes as a result of increasing use of same-day discharge,” lead investigator Steven M. Bradley, MD, said in an interview.
The analysis was based on data on 819,091 patients who underwent an elective PCI procedure during July 2009–December 2017 in the National CathPCI Registry. During this period, the proportion of elective PCIs performed with same-day discharge rose from 4.5% to 28.6%, a fivefold gain, according to Dr. Bradley, an associate cardiologist at the Minneapolis Heart Institute, and colleagues.
Within this study, outcomes in 212,369 patients were analyzed through a link to Centers for Medicare & Medicaid Services data. Despite the growth in same-day discharge PCIs over the study period, there was no change in 30-day mortality rates while the rate of 30-day rehospitalization fell after risk adjustment.
These data are considered to have a message for routine practice, particularly for those hospitals that have been slow to move to same-day discharge for elective PCI when lack of complications makes this appropriate.
However, “this does not mean same-day discharge is safe for all patients,” Dr. Bradley cautioned, but these data suggest “there is a clear opportunity at sites with low rates” to look for strategies that allow patients to recover at home, which is preferred by many patients and lowers costs.
In 2009, the first year in which the data were analyzed, there was relatively little variation in the rate of same day discharge for elective PCI among the 1,716 hospitals that contributed patients to the registry. At that point, almost all hospitals had rates below 10%, according to the report published in JACC: Cardiovascular Interventions on Aug 2, 2021 .
From 2011 onward, there were progressive gains at most hospitals, with an even steeper rise beginning in 2014. By 2017, even though some hospitals were still performing almost no same-day discharge PCIs, many were discharging up to 40%, and the outliers were discharging nearly all.
Expressed in interquartiles at the hospital level, the range climbed from 0.0% to 4.7% in 2009 and reached 4.5% to 41.0% by 2017. For 2017, relative to 2009, this produced an odds ratio for same-day discharge that was more than fourfold greater, after adjustment for year and access site.
Access site was an important variable. For those undergoing PCI with radial access, the median same-day discharge rates climbed from 21.8% in 2009 to 58.3% in 2017. Same-day discharge rates for elective PCI performed by femoral access, already lower in 2009, have consistently lagged. By 2017, the median rate of same-day discharge for those undergoing PCI by the femoral route was less than half of that associated with radial access.
Despite the faster rise in same-day discharge and radial access over the course of the study, these were not directly correlated. In 2017, 25% of sites performing PCI by radial access were still discharging fewer than 10% of patients on the same day as their elective PCI.
Several previous studies have also found that same-day discharge can be offered selectively after elective PCI without adversely affecting outcomes, according to multiple citations provided by the authors. The advantage of early discharge includes both convenience for the patient and lower costs, with some of the studies attempting to quantify savings. In one, it was estimated that per-case savings from performing radial-access elective PCI with same-day discharge was nearly $3,700 when compared with transfemoral access and an overnight stay.
Radial access key to same-day success
An accompanying editorial by Deepak Bhatt, MD, and Jonathan G. Sung, MBChB, who are both interventional cardiologists at Brigham and Women’s Hospital, Boston, generally agreed with the premise that these data support judicious use of same-day discharge for elective PCI.
They pointed out limitations in the study, including its retrospective design and the inability to look at important outcomes other than mortality and 30-day rehospitalization, such as bleeding, that are relevant to the safety of early discharge, but concluded that same-day discharge, as well as radial access procedures, are underused.
“For uncomplicated elective PCI, we should aim for same-day discharge,” Dr. Bhatt said in an interview. He linked this to radial access.
“Radial access certainly facilitates same-day discharge, though even beyond that aspect, it should be the default route of vascular access whenever possible,” Dr. Bhatt said. Yet he was careful to say that neither same-day discharge nor radial access can be recommended in all patients. While the operator needs “to be comfortable” with a radial access approach, there are multiple factors that might preclude early discharge.
“Of course, if a long procedure, high contrast use, bleeding, a long travel distance to get home, etc. [are considered], then an overnight stay may be warranted,” he said.
Dr. Bradley advised centers planning to increase their same-day discharge rates for elective PCI to use a systematic approach.
“Sites should identify areas for opportunity in the use of same-day discharge and then track the implications on patient outcomes to ensure that the approach being used maintains high-quality care,” he said.
Dr. Bradley reported no potential conflicts of interest. Dr. Bhatt has received research funding from a large number of pharmaceutical and device manufacturers, including those that make products relevant to PCI.
Based on a large registry, there appears to be no adverse consequences for same-day discharge following an elective percutaneous cardiovascular intervention (PCI), according to an analysis of a nationwide registry.
“Our data suggest there has been no negative impact on patient outcomes as a result of increasing use of same-day discharge,” lead investigator Steven M. Bradley, MD, said in an interview.
The analysis was based on data on 819,091 patients who underwent an elective PCI procedure during July 2009–December 2017 in the National CathPCI Registry. During this period, the proportion of elective PCIs performed with same-day discharge rose from 4.5% to 28.6%, a fivefold gain, according to Dr. Bradley, an associate cardiologist at the Minneapolis Heart Institute, and colleagues.
Within this study, outcomes in 212,369 patients were analyzed through a link to Centers for Medicare & Medicaid Services data. Despite the growth in same-day discharge PCIs over the study period, there was no change in 30-day mortality rates while the rate of 30-day rehospitalization fell after risk adjustment.
These data are considered to have a message for routine practice, particularly for those hospitals that have been slow to move to same-day discharge for elective PCI when lack of complications makes this appropriate.
However, “this does not mean same-day discharge is safe for all patients,” Dr. Bradley cautioned, but these data suggest “there is a clear opportunity at sites with low rates” to look for strategies that allow patients to recover at home, which is preferred by many patients and lowers costs.
In 2009, the first year in which the data were analyzed, there was relatively little variation in the rate of same day discharge for elective PCI among the 1,716 hospitals that contributed patients to the registry. At that point, almost all hospitals had rates below 10%, according to the report published in JACC: Cardiovascular Interventions on Aug 2, 2021 .
From 2011 onward, there were progressive gains at most hospitals, with an even steeper rise beginning in 2014. By 2017, even though some hospitals were still performing almost no same-day discharge PCIs, many were discharging up to 40%, and the outliers were discharging nearly all.
Expressed in interquartiles at the hospital level, the range climbed from 0.0% to 4.7% in 2009 and reached 4.5% to 41.0% by 2017. For 2017, relative to 2009, this produced an odds ratio for same-day discharge that was more than fourfold greater, after adjustment for year and access site.
Access site was an important variable. For those undergoing PCI with radial access, the median same-day discharge rates climbed from 21.8% in 2009 to 58.3% in 2017. Same-day discharge rates for elective PCI performed by femoral access, already lower in 2009, have consistently lagged. By 2017, the median rate of same-day discharge for those undergoing PCI by the femoral route was less than half of that associated with radial access.
Despite the faster rise in same-day discharge and radial access over the course of the study, these were not directly correlated. In 2017, 25% of sites performing PCI by radial access were still discharging fewer than 10% of patients on the same day as their elective PCI.
Several previous studies have also found that same-day discharge can be offered selectively after elective PCI without adversely affecting outcomes, according to multiple citations provided by the authors. The advantage of early discharge includes both convenience for the patient and lower costs, with some of the studies attempting to quantify savings. In one, it was estimated that per-case savings from performing radial-access elective PCI with same-day discharge was nearly $3,700 when compared with transfemoral access and an overnight stay.
Radial access key to same-day success
An accompanying editorial by Deepak Bhatt, MD, and Jonathan G. Sung, MBChB, who are both interventional cardiologists at Brigham and Women’s Hospital, Boston, generally agreed with the premise that these data support judicious use of same-day discharge for elective PCI.
They pointed out limitations in the study, including its retrospective design and the inability to look at important outcomes other than mortality and 30-day rehospitalization, such as bleeding, that are relevant to the safety of early discharge, but concluded that same-day discharge, as well as radial access procedures, are underused.
“For uncomplicated elective PCI, we should aim for same-day discharge,” Dr. Bhatt said in an interview. He linked this to radial access.
“Radial access certainly facilitates same-day discharge, though even beyond that aspect, it should be the default route of vascular access whenever possible,” Dr. Bhatt said. Yet he was careful to say that neither same-day discharge nor radial access can be recommended in all patients. While the operator needs “to be comfortable” with a radial access approach, there are multiple factors that might preclude early discharge.
“Of course, if a long procedure, high contrast use, bleeding, a long travel distance to get home, etc. [are considered], then an overnight stay may be warranted,” he said.
Dr. Bradley advised centers planning to increase their same-day discharge rates for elective PCI to use a systematic approach.
“Sites should identify areas for opportunity in the use of same-day discharge and then track the implications on patient outcomes to ensure that the approach being used maintains high-quality care,” he said.
Dr. Bradley reported no potential conflicts of interest. Dr. Bhatt has received research funding from a large number of pharmaceutical and device manufacturers, including those that make products relevant to PCI.
Based on a large registry, there appears to be no adverse consequences for same-day discharge following an elective percutaneous cardiovascular intervention (PCI), according to an analysis of a nationwide registry.
“Our data suggest there has been no negative impact on patient outcomes as a result of increasing use of same-day discharge,” lead investigator Steven M. Bradley, MD, said in an interview.
The analysis was based on data on 819,091 patients who underwent an elective PCI procedure during July 2009–December 2017 in the National CathPCI Registry. During this period, the proportion of elective PCIs performed with same-day discharge rose from 4.5% to 28.6%, a fivefold gain, according to Dr. Bradley, an associate cardiologist at the Minneapolis Heart Institute, and colleagues.
Within this study, outcomes in 212,369 patients were analyzed through a link to Centers for Medicare & Medicaid Services data. Despite the growth in same-day discharge PCIs over the study period, there was no change in 30-day mortality rates while the rate of 30-day rehospitalization fell after risk adjustment.
These data are considered to have a message for routine practice, particularly for those hospitals that have been slow to move to same-day discharge for elective PCI when lack of complications makes this appropriate.
However, “this does not mean same-day discharge is safe for all patients,” Dr. Bradley cautioned, but these data suggest “there is a clear opportunity at sites with low rates” to look for strategies that allow patients to recover at home, which is preferred by many patients and lowers costs.
In 2009, the first year in which the data were analyzed, there was relatively little variation in the rate of same day discharge for elective PCI among the 1,716 hospitals that contributed patients to the registry. At that point, almost all hospitals had rates below 10%, according to the report published in JACC: Cardiovascular Interventions on Aug 2, 2021 .
From 2011 onward, there were progressive gains at most hospitals, with an even steeper rise beginning in 2014. By 2017, even though some hospitals were still performing almost no same-day discharge PCIs, many were discharging up to 40%, and the outliers were discharging nearly all.
Expressed in interquartiles at the hospital level, the range climbed from 0.0% to 4.7% in 2009 and reached 4.5% to 41.0% by 2017. For 2017, relative to 2009, this produced an odds ratio for same-day discharge that was more than fourfold greater, after adjustment for year and access site.
Access site was an important variable. For those undergoing PCI with radial access, the median same-day discharge rates climbed from 21.8% in 2009 to 58.3% in 2017. Same-day discharge rates for elective PCI performed by femoral access, already lower in 2009, have consistently lagged. By 2017, the median rate of same-day discharge for those undergoing PCI by the femoral route was less than half of that associated with radial access.
Despite the faster rise in same-day discharge and radial access over the course of the study, these were not directly correlated. In 2017, 25% of sites performing PCI by radial access were still discharging fewer than 10% of patients on the same day as their elective PCI.
Several previous studies have also found that same-day discharge can be offered selectively after elective PCI without adversely affecting outcomes, according to multiple citations provided by the authors. The advantage of early discharge includes both convenience for the patient and lower costs, with some of the studies attempting to quantify savings. In one, it was estimated that per-case savings from performing radial-access elective PCI with same-day discharge was nearly $3,700 when compared with transfemoral access and an overnight stay.
Radial access key to same-day success
An accompanying editorial by Deepak Bhatt, MD, and Jonathan G. Sung, MBChB, who are both interventional cardiologists at Brigham and Women’s Hospital, Boston, generally agreed with the premise that these data support judicious use of same-day discharge for elective PCI.
They pointed out limitations in the study, including its retrospective design and the inability to look at important outcomes other than mortality and 30-day rehospitalization, such as bleeding, that are relevant to the safety of early discharge, but concluded that same-day discharge, as well as radial access procedures, are underused.
“For uncomplicated elective PCI, we should aim for same-day discharge,” Dr. Bhatt said in an interview. He linked this to radial access.
“Radial access certainly facilitates same-day discharge, though even beyond that aspect, it should be the default route of vascular access whenever possible,” Dr. Bhatt said. Yet he was careful to say that neither same-day discharge nor radial access can be recommended in all patients. While the operator needs “to be comfortable” with a radial access approach, there are multiple factors that might preclude early discharge.
“Of course, if a long procedure, high contrast use, bleeding, a long travel distance to get home, etc. [are considered], then an overnight stay may be warranted,” he said.
Dr. Bradley advised centers planning to increase their same-day discharge rates for elective PCI to use a systematic approach.
“Sites should identify areas for opportunity in the use of same-day discharge and then track the implications on patient outcomes to ensure that the approach being used maintains high-quality care,” he said.
Dr. Bradley reported no potential conflicts of interest. Dr. Bhatt has received research funding from a large number of pharmaceutical and device manufacturers, including those that make products relevant to PCI.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Intracranial atherosclerosis finding on MRA linked to stroke
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
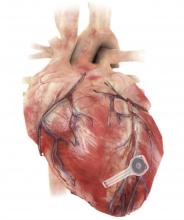
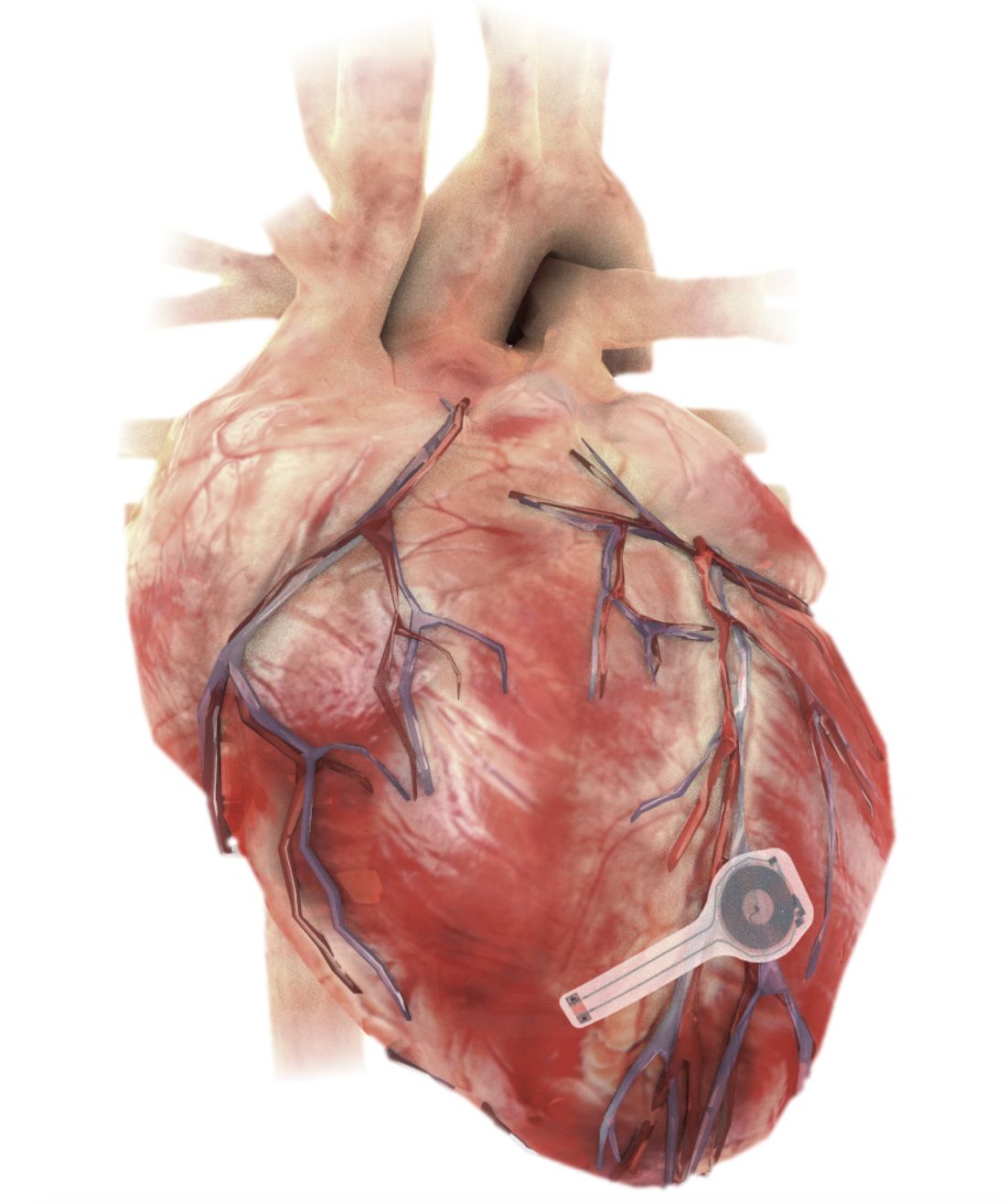
Dissolving pacemaker impressive in early research
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
A fully implantable, bioresorbable pacemaker has been developed that’s capable of sustaining heart rhythms in animal and human donor hearts before disappearing over 5-7 weeks.
Temporary pacing devices are frequently used after cardiac surgery but rely on bulky external generators and transcutaneous pacing leads that run the risk of becoming infected or dislodged and can damage the heart when removed if they’re enveloped in fibrotic tissue.
The experimental device is thin, powered without leads or batteries, and made of water-soluble, biocompatible materials, thereby bypassing many of the disadvantages of conventional temporary pacing devices, according to John A. Rogers, PhD, who led the device’s development and directs the Querrey Simpson Institute for Bioelectronics at Northwestern University in Chicago.
“The total material load on the body is very minimal,” he said in an interview. “The amount of silicon and magnesium in a multivitamin tablet is about 3,000 times more than the amount of those materials in our electronics. So you can think of them as a very tiny vitamin pill, in a sense, but configured with electronic functionality.”
Dr. Rogers and his team have a reputation for innovation in bioelectronic medicine, having recently constructed transient wireless devices to accelerate neuroregeneration associated with damaged peripheral nerves, to monitor critically ill neonates, and to detect early signs and symptoms associated with COVID-19.
Shortly after Dr. Rogers joined Northwestern, Rishi Arora, MD, a cardiac electrophysiologist and professor of medicine at Northwestern, reached out to discuss how they could leverage wireless electronics for patients needing temporary pacing.
“It was a natural marriage,” Dr. Arora said in an interview. “Part of the reason to go into the heart was because the cardiology group here at Northwestern, especially on the electrophysiology side, has been very involved in translational research, and John also had a very strong collaboration before he came here with Igor Efimov, [PhD, of George Washington University, Washington], a giant in the field in terms of heart rhythm research.”
Dr. Arora noted that the incidence of temporary pacing after cardiac surgery is at least 10% but can reach 20%. Current devices work well in most patients, but temporary pacing with epicardial wires can cause complications and, typically, work well only for a few days after cardiac surgery. Clinically, though, several patients need postoperative pacing support for 1-2 weeks.
“So if something like this were available where you could tack it onto the surface and forget it for a week or 10 days or 2 weeks, you’d be doing those 20% of patients a huge service,” he said.
Bioresorbable scaffold déjà vu?
The philosophy of “leave nothing behind” is nothing new in cardiology, with bioresorbable vascular scaffolds (BVS) gaining initial support as a potential solution to neoatherosclerosis and late-stent thrombosis in permanent metal stents. Failure to show advantages, and safety concerns such as in-scaffold thrombosis, however, led Abbott to stop global sales of the first approved BVS and Boston Scientific to halt its BVS program in 2017.
The wireless pacemaker, however, is an electrical device, not a mechanical one, observed Dr. Rogers. “The fact that it’s not in the bloodstream greatly lowers risks and, as I mentioned before, everything is super thin, low-mass quantities of materials. So, I guess there’s a relationship there, but it’s different in a couple of very important ways.”
As Dr. Rogers, Dr. Arora, Dr. Efimov, and colleagues recently reported in Nature Biotechnology, the electronic part of the pacemaker contains three layers: A loop antenna with a bilayer tungsten-coated magnesium inductive coil, a radiofrequency PIN diode based on a monocrystalline silicon nanomembrane, and a poly (lactide-co-glycolide) (PLGA) dielectric interlayer.
The electronic components rest between two encapsulation layers of PLGA to isolate the active materials from the surrounding biofluids during implantation, and connect to a pair of flexible extension electrodes that deliver the electrical stimuli to a contact pad sutured onto the heart. The entire system is about 16 mm in width and 15 mm in length, and weighs in at about 0.3 g.
The pacemaker receives power and control commands through a wireless inductive power transfer – the same technology used in implanted medical devices, smartphones, and radio-frequency identification tags – between the receiver coil in the device and a wand-shaped, external transmission coil placed on top of or within a few inches of the heart.
“Right now we’re almost at 15 inches, which I think is a very respectable distance for this particular piece of hardware, and clinically very doable,” observed Dr. Arora.
Competing considerations
Testing thus far shows effective ventricular capture across a range of frequencies in mouse and rabbit hearts and successful pacing and activation of human cardiac tissue.
In vivo tests in dogs also suggest that the system can “achieve the power necessary for operation of bioresorbable pacemakers in adult human patients,” the authors say.
Electrodes placed on the dogs’ legs showed a change in ECG signals from a narrow QRS complex (consistent with a normal rate sinus rhythm of 350-400 bpm) to a widened QRS complex with a shortened R-R interval (consistent with a paced rhythm of 400-450 bpm) – indicating successful ventricular capture.
The device successfully paced the dogs through postoperative day 4 but couldn’t provide enough energy to capture the ventricular myocardium on day 5 and failed to pace the heart on day 6, even when transmitting voltages were increased from 1 Vpp to more than 10 Vpp.
Dr. Rogers pointed out that a transient device of theirs that uses very thin films of silica provides stable intracranial pressure monitoring for traumatic brain injury recovery for 3 weeks before dissolving. The problem with the polymers used as encapsulating layers in the pacemaker is that even if they haven’t completely dissolved, there’s a finite rate of water permeation through the film.
“It turns out that’s what’s become the limiting factor, rather than the chemistry of bioresorption,” he said. “So, what we’re seeing with these devices beginning to degrade electrically in terms of performance around 5-6 days is due to that water permeation.”
Although it is not part of the current study, there’s no reason thin silica layers couldn’t be incorporated into the pacemaker to make it less water permeable, Dr. Rogers said. Still, this will have to be weighed against the competing consideration of stable operating life.
The researchers specifically chose materials that would naturally bioresorb via hydrolysis and metabolic action in the body. PLGA degrades into glycolic and lactic acid, the tungsten-coated magnesium inductive coil into Wox and Mg(OH)2, and the silicon nanomembrane radiofrequency PIN diode into Si(OH)4.
CT imaging in rat models shows the device is enveloped in fibrotic tissue and completely decouples from the heart at 4 weeks, while images of explanted devices suggest the pacemaker largely dissolves within 3 weeks and the remaining residues disappear after 12 weeks.
The researchers have started an investigational device exemption process to allow the device to be used in clinical trials, and they plan to dig deeper into the potential for fragments to form at various stages of resorption, which some imaging suggests may occur.
“Because these devices are made out of pure materials and they’re in a heterogeneous environment, both mechanically and biomechanically, the devices don’t resorb in a perfectly uniform way and, as a result, at the tail end of the process you can end up with small fragments that eventually bioresorb, but before they’re gone, they are potentially mobile within the body cavity,” Dr. Rogers said.
“We feel that because the devices aren’t in the bloodstream, the risk associated with those fragments is probably manageable but at the same time, these are the sorts of details that must be thoroughly addressed before trials in humans,” he said, adding that one solution, if needed, would be to encapsulate the entire device in a thin bioresorbable hydrogel as a containment vehicle.
Dr. Arora said they hope the pacemaker “will make patients’ lives a lot easier in the postoperative setting but, even there, I think one must remember current pacing technology in this setting is actually very good. So there’s a word of caution not to get ahead of ourselves.”
Looking forward, the excitement of this approach is not only in the immediate postop setting but in the transvenous setting, he said. “If we can get to the point where we can actually do this transvenously, that opens up a huge window of opportunity because there we’re talking about post-TAVR [transcatheter aortic valve replacement], post–myocardial infarction, etc.”
Currently, temporary transvenous pacing can be quite unreliable because of a high risk of dislodgement and infection – much higher than for surgical pacing wires, he noted.
“In terms of translatability to larger numbers of patients, the value would be huge. But again, a lot needs to be done before we can get there. But if it can get to that point, then I think you have a real therapy that could potentially be transformative,” Dr. Arora said.
Dr. Rogers reported support from the Leducq Foundation projects RHYTHM and ROI-HL121270. Dr. Arora has disclosed no relevant financial relationships. Coauthor disclosures are listed in the original article.
A version of this article first appeared on Medscape.com.
Five risk factors may predict thrombus on LAA occlusion implants
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
PCI after TAVR mostly succeeds, some risks identified
Coronary angiography and percutaneous coronary interventions (PCI) can be performed successfully after transcatheter aortic valve replacement in most cases, according to data drawn from an international registry that has collected more than 400 such cases.
Overall, reaccess coronary angiography was successful in about 99% of cases with type of prosthesis identified as the most important variable in predicting success, according to a multicenter investigating team led by Won-Keun Kim, MD, director of structural heart disease, Kerckhoff Heart Center, Bad Nauheim, Germany.
By type of prosthesis, Dr. Kim was referring to long versus short stent-frame prostheses (SFP). In the case of angiography of the right coronary artery, for example, success was achieved in 99.6% of those with a short SFP and 95.9% of those with a long SFP (P = .005).
The study was published online in JACC: Cardiovascular Interventions.
Based on these and previous data, “prosthetic choice will be the main decisive factor that affects coronary reaccess, and this decision is in the hands of the TAVR operator,” said Dr. Kim in an interview.
This does not preclude use of a long SFP in TAVR. For patients with increased likelihood of eventually requiring a coronary intervention after TAVR, such as those undergoing the procedure at a relatively young age, a short device appears to be preferable, but Dr. Kim emphasized that it is not the only consideration.
When performing TAVR, “the highest priority is to accomplish a safe procedure with a good immediate outcome,” he said, pointing out that angiographic reaccess and PCI are successfully achieved in most patients whether fitted with a short or long SFP.
“If for any reason I assume that the immediate outcome [after TAVR] might be better using a long SFP, I would not hesitate to use a long SFP,” said Dr. Kim, giving such examples as a need for resheathing or precise positioning.
Coronary reaccess has low relative priority
“Coronary reaccess is an important issue and there is an increasing awareness of this, but it has a lower priority” than optimizing TAVR success,” Dr. Kim explained.
The analysis of coronary angiographic reaccess was based on 449 TAVR patients from 25 sites who required reaccess angiography. The indication in most cases was an acute coronary syndrome, mostly non–ST-elevation myocardial infarction (STEMI, 79%). Of the remaining patients, about half had STEMIs and half had other acute cardiovascular situations. The median time interval from TAVR to need for coronary angiography was 311 days.
In all but 2.7%, diagnostic catheterization was performed initially. It was successful in 98.3% of the procedures in the right coronary artery, 99.3% of the left coronary artery, and 97.3% overall.
Of the 60% who underwent PCI, 9% were considered unsuccessful. The reasons included lack of reflow in eight cases and coronary access issues in six cases. A variety of other issues accounted for the remaining seven cases.
Technical success was achieved in 91.4% of native arteries. In the six cases in which engagement of the culprit vessel with a guiding catheter failed, three were converted to urgent coronary bypass grafting and three died in the hospital. Neither selective versus unselective guiding-catheter engagement nor long versus short SFP related to PCI success, but PCI was performed less commonly in the native coronary arteries of TAVR patients with a long rather than short SFP (49% vs. 57%).
The 30-day all-cause mortality in this series was 12.2%. The independent predictors were a history of diabetes and the occurrence of cardiogenic shock. In the PCI subgroup, these factors plus PCI success predicted 30-day mortality.
Strategies to improve reaccess not resolved
When performing TAVR, other factors that might influence subsequent PCI success includes commissural alignment and positioning, according to Dr. Kim. But he cautioned that there are a number of potential controversies when weighing how to improve chances of post-TAVR angiographic reaccess without compromising the success of valve replacement.
“Lower positioning facilitates coronary access, but unfortunately will increase rates of conduction disturbances,” he noted.
Overall, one of the main messages from this analysis is that “the fear of impaired coronary access [after TAVR] may well be disproportionate to the reality,” according to Neal S. Kleiman, MD, an interventional cardiologist at Houston Methodist DeBakey Heart and Vascular Center. Dr. Kleiman wrote an editorial on the registry findings in the same issue of JACC: Cardiovascular Interventions).
Yet, he agreed that the issue of angiographic reaccess after TAVR cannot be ignored. Although reaccess after TAVR has so far been “surprisingly rare,” Dr. Kleiman expects cases to increase as more younger patients undergo TAVR. He suggested that interventionalists will need consider this issue when performing TAVR, a point he reemphasized in an interview.
“It is still a concern when recommending TAVR to a patient and still poses challenges to device manufacturers,” said Dr. Kleiman, suggesting that “a new set of skills” will be required to perform TAVR that will optimize subsequent angiographic access and PCI.
Dr. Kim agreed. Ultimately, other challenges, such as PCI performed after TAVR-in-TAVR placement, are likely to further complicate this issue, but he, too, is looking to new devices to minimize the problems.
“It would be desirable to modify the design, especially of long SFPs, to improve access for PCI, and there are ongoing efforts of the manufacturers to achieve this,” Dr. Kim said.
Dr. Kim reported financial relationships with Abbot, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Lifesciences. Dr. Kleiman reported financial relationships with Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic.
Coronary angiography and percutaneous coronary interventions (PCI) can be performed successfully after transcatheter aortic valve replacement in most cases, according to data drawn from an international registry that has collected more than 400 such cases.
Overall, reaccess coronary angiography was successful in about 99% of cases with type of prosthesis identified as the most important variable in predicting success, according to a multicenter investigating team led by Won-Keun Kim, MD, director of structural heart disease, Kerckhoff Heart Center, Bad Nauheim, Germany.
By type of prosthesis, Dr. Kim was referring to long versus short stent-frame prostheses (SFP). In the case of angiography of the right coronary artery, for example, success was achieved in 99.6% of those with a short SFP and 95.9% of those with a long SFP (P = .005).
The study was published online in JACC: Cardiovascular Interventions.
Based on these and previous data, “prosthetic choice will be the main decisive factor that affects coronary reaccess, and this decision is in the hands of the TAVR operator,” said Dr. Kim in an interview.
This does not preclude use of a long SFP in TAVR. For patients with increased likelihood of eventually requiring a coronary intervention after TAVR, such as those undergoing the procedure at a relatively young age, a short device appears to be preferable, but Dr. Kim emphasized that it is not the only consideration.
When performing TAVR, “the highest priority is to accomplish a safe procedure with a good immediate outcome,” he said, pointing out that angiographic reaccess and PCI are successfully achieved in most patients whether fitted with a short or long SFP.
“If for any reason I assume that the immediate outcome [after TAVR] might be better using a long SFP, I would not hesitate to use a long SFP,” said Dr. Kim, giving such examples as a need for resheathing or precise positioning.
Coronary reaccess has low relative priority
“Coronary reaccess is an important issue and there is an increasing awareness of this, but it has a lower priority” than optimizing TAVR success,” Dr. Kim explained.
The analysis of coronary angiographic reaccess was based on 449 TAVR patients from 25 sites who required reaccess angiography. The indication in most cases was an acute coronary syndrome, mostly non–ST-elevation myocardial infarction (STEMI, 79%). Of the remaining patients, about half had STEMIs and half had other acute cardiovascular situations. The median time interval from TAVR to need for coronary angiography was 311 days.
In all but 2.7%, diagnostic catheterization was performed initially. It was successful in 98.3% of the procedures in the right coronary artery, 99.3% of the left coronary artery, and 97.3% overall.
Of the 60% who underwent PCI, 9% were considered unsuccessful. The reasons included lack of reflow in eight cases and coronary access issues in six cases. A variety of other issues accounted for the remaining seven cases.
Technical success was achieved in 91.4% of native arteries. In the six cases in which engagement of the culprit vessel with a guiding catheter failed, three were converted to urgent coronary bypass grafting and three died in the hospital. Neither selective versus unselective guiding-catheter engagement nor long versus short SFP related to PCI success, but PCI was performed less commonly in the native coronary arteries of TAVR patients with a long rather than short SFP (49% vs. 57%).
The 30-day all-cause mortality in this series was 12.2%. The independent predictors were a history of diabetes and the occurrence of cardiogenic shock. In the PCI subgroup, these factors plus PCI success predicted 30-day mortality.
Strategies to improve reaccess not resolved
When performing TAVR, other factors that might influence subsequent PCI success includes commissural alignment and positioning, according to Dr. Kim. But he cautioned that there are a number of potential controversies when weighing how to improve chances of post-TAVR angiographic reaccess without compromising the success of valve replacement.
“Lower positioning facilitates coronary access, but unfortunately will increase rates of conduction disturbances,” he noted.
Overall, one of the main messages from this analysis is that “the fear of impaired coronary access [after TAVR] may well be disproportionate to the reality,” according to Neal S. Kleiman, MD, an interventional cardiologist at Houston Methodist DeBakey Heart and Vascular Center. Dr. Kleiman wrote an editorial on the registry findings in the same issue of JACC: Cardiovascular Interventions).
Yet, he agreed that the issue of angiographic reaccess after TAVR cannot be ignored. Although reaccess after TAVR has so far been “surprisingly rare,” Dr. Kleiman expects cases to increase as more younger patients undergo TAVR. He suggested that interventionalists will need consider this issue when performing TAVR, a point he reemphasized in an interview.
“It is still a concern when recommending TAVR to a patient and still poses challenges to device manufacturers,” said Dr. Kleiman, suggesting that “a new set of skills” will be required to perform TAVR that will optimize subsequent angiographic access and PCI.
Dr. Kim agreed. Ultimately, other challenges, such as PCI performed after TAVR-in-TAVR placement, are likely to further complicate this issue, but he, too, is looking to new devices to minimize the problems.
“It would be desirable to modify the design, especially of long SFPs, to improve access for PCI, and there are ongoing efforts of the manufacturers to achieve this,” Dr. Kim said.
Dr. Kim reported financial relationships with Abbot, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Lifesciences. Dr. Kleiman reported financial relationships with Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic.
Coronary angiography and percutaneous coronary interventions (PCI) can be performed successfully after transcatheter aortic valve replacement in most cases, according to data drawn from an international registry that has collected more than 400 such cases.
Overall, reaccess coronary angiography was successful in about 99% of cases with type of prosthesis identified as the most important variable in predicting success, according to a multicenter investigating team led by Won-Keun Kim, MD, director of structural heart disease, Kerckhoff Heart Center, Bad Nauheim, Germany.
By type of prosthesis, Dr. Kim was referring to long versus short stent-frame prostheses (SFP). In the case of angiography of the right coronary artery, for example, success was achieved in 99.6% of those with a short SFP and 95.9% of those with a long SFP (P = .005).
The study was published online in JACC: Cardiovascular Interventions.
Based on these and previous data, “prosthetic choice will be the main decisive factor that affects coronary reaccess, and this decision is in the hands of the TAVR operator,” said Dr. Kim in an interview.
This does not preclude use of a long SFP in TAVR. For patients with increased likelihood of eventually requiring a coronary intervention after TAVR, such as those undergoing the procedure at a relatively young age, a short device appears to be preferable, but Dr. Kim emphasized that it is not the only consideration.
When performing TAVR, “the highest priority is to accomplish a safe procedure with a good immediate outcome,” he said, pointing out that angiographic reaccess and PCI are successfully achieved in most patients whether fitted with a short or long SFP.
“If for any reason I assume that the immediate outcome [after TAVR] might be better using a long SFP, I would not hesitate to use a long SFP,” said Dr. Kim, giving such examples as a need for resheathing or precise positioning.
Coronary reaccess has low relative priority
“Coronary reaccess is an important issue and there is an increasing awareness of this, but it has a lower priority” than optimizing TAVR success,” Dr. Kim explained.
The analysis of coronary angiographic reaccess was based on 449 TAVR patients from 25 sites who required reaccess angiography. The indication in most cases was an acute coronary syndrome, mostly non–ST-elevation myocardial infarction (STEMI, 79%). Of the remaining patients, about half had STEMIs and half had other acute cardiovascular situations. The median time interval from TAVR to need for coronary angiography was 311 days.
In all but 2.7%, diagnostic catheterization was performed initially. It was successful in 98.3% of the procedures in the right coronary artery, 99.3% of the left coronary artery, and 97.3% overall.
Of the 60% who underwent PCI, 9% were considered unsuccessful. The reasons included lack of reflow in eight cases and coronary access issues in six cases. A variety of other issues accounted for the remaining seven cases.
Technical success was achieved in 91.4% of native arteries. In the six cases in which engagement of the culprit vessel with a guiding catheter failed, three were converted to urgent coronary bypass grafting and three died in the hospital. Neither selective versus unselective guiding-catheter engagement nor long versus short SFP related to PCI success, but PCI was performed less commonly in the native coronary arteries of TAVR patients with a long rather than short SFP (49% vs. 57%).
The 30-day all-cause mortality in this series was 12.2%. The independent predictors were a history of diabetes and the occurrence of cardiogenic shock. In the PCI subgroup, these factors plus PCI success predicted 30-day mortality.
Strategies to improve reaccess not resolved
When performing TAVR, other factors that might influence subsequent PCI success includes commissural alignment and positioning, according to Dr. Kim. But he cautioned that there are a number of potential controversies when weighing how to improve chances of post-TAVR angiographic reaccess without compromising the success of valve replacement.
“Lower positioning facilitates coronary access, but unfortunately will increase rates of conduction disturbances,” he noted.
Overall, one of the main messages from this analysis is that “the fear of impaired coronary access [after TAVR] may well be disproportionate to the reality,” according to Neal S. Kleiman, MD, an interventional cardiologist at Houston Methodist DeBakey Heart and Vascular Center. Dr. Kleiman wrote an editorial on the registry findings in the same issue of JACC: Cardiovascular Interventions).
Yet, he agreed that the issue of angiographic reaccess after TAVR cannot be ignored. Although reaccess after TAVR has so far been “surprisingly rare,” Dr. Kleiman expects cases to increase as more younger patients undergo TAVR. He suggested that interventionalists will need consider this issue when performing TAVR, a point he reemphasized in an interview.
“It is still a concern when recommending TAVR to a patient and still poses challenges to device manufacturers,” said Dr. Kleiman, suggesting that “a new set of skills” will be required to perform TAVR that will optimize subsequent angiographic access and PCI.
Dr. Kim agreed. Ultimately, other challenges, such as PCI performed after TAVR-in-TAVR placement, are likely to further complicate this issue, but he, too, is looking to new devices to minimize the problems.
“It would be desirable to modify the design, especially of long SFPs, to improve access for PCI, and there are ongoing efforts of the manufacturers to achieve this,” Dr. Kim said.
Dr. Kim reported financial relationships with Abbot, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Lifesciences. Dr. Kleiman reported financial relationships with Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
DOACs linked to lower mortality than vitamin K antagonist: 3-year TAVR registry
Following a transcatheter aortic valve replacement (TAVR), direct oral anticoagulants (DOACs) are preferable to vitamin K antagonists (VKAs) in patients who are candidates for oral anticoagulants, according to data drawn from a large multicenter French TAVR registry.
When oral anticoagulation is appropriate following TAVR, such as in patients with atrial fibrillation, “DOACs are associated with improved survival and lower incidence of bleeding, compared to VKA,” reported a team of investigators led by Martine Gilard, MD, PhD, director of interventional cardiology, Brest (France) University Hospital Center.
The comparison, using propensity score matching, is not definitive, but it might be the best data currently available to support DOACs over VKA until a randomized trial is completed, according to Dr. Gilard, senior author of the newly published study.
Asked in an interview if DOACs should now be used preferentially after TAVR when patients are indicated for oral anticoagulation, Dr. Gilard replied, “My answer is yes.”
Of more than 24,000 TAVR patients in the French TAVI and FRANCE2 multicenter registries, which are linked to the French single-payer claims database (SNDS), 8,962 (36.4%) received an oral anticoagulant following their procedure. Of these, 2,180 (24.3%) received a DOAC and the remaining received VKA.
By linking data from the registries to the SNDS, outcomes were tracked. Propensity matching was employed to control for differences in baseline characteristics, including age, body mass index, functional class, diabetes, comorbidities, and past medical history.
On the primary endpoint of mortality at the end of 3 years, the rates were 35.6% and 31.2% for VKA and DOACs, respectively. This translated in a 37% greater hazard ratio for death among those treated with VKA (P < .005).
The rate of major bleeding, a secondary endpoint, was also higher (12.3% vs. 8.4%) and significantly different (HR, 1.65; P < .005) for VKA versus DOACs. The rates of ischemic stroke, acute coronary syndrome, and hemorrhagic stroke were all numerically higher in patients treated with VKA than DOACs, although none of these differences reached statistical significance.
Residual confounding cannot be discounted
“The large number of events allowed for taking into account a higher number of potential confounders with appropriate statistical power,” according to the authors. However, they acknowledged that residual confounding cannot be eliminated by propensity matching and conceded that prospective data are needed for a definitive comparison.
In an accompanying editorial, Daniele Giacoppo, MD, a cardiologist at Alto Vicentino Hospital, Santorso, Italy, enlarged on this point . In addition to the inherent limitations of retrospective data, he also noted that data from other studies addressing the same question have been inconsistent.
Of these studies, he pointed to the ATLANTIS trial, presented 2 months ago at the annual meeting of the American College of Cardiology. This study failed to show an advantage for the DOAC apixaban over VKA in TAVR patients for the primary composite outcome of time to death, myocardial infarction, systemic emboli, valve thrombosis, or major bleeding. Although this study was not limited to patients with an indication for oral anticoagulants, Dr. Giacoppo pointed out that there was no advantage, even among the subgroup of patients who did have an indication.
Data are supportive in absence of trial results
In general, Dr. Giacoppo agreed that the French registry are generally supportive of DOACs over VKA in TAVR patients with an indication for oral anticoagulation, but he cautioned that blanket statements are difficult. He anticipates better information from a randomized trial called ENVISAGE-TAVI AF, which is comparing edoxaban with VKA following TAVR in atrial fibrillation patients who have an indication for oral anticoagulation, but he indicated that some individualization of choice will be needed among those high or low relative risks of thrombotic events or bleeding.
“The concerns related to DOACs after TAVR are most confined to patients without an indication for oral anticoagulation,” Dr. Giacoppo said in an interview. In patients with an indication, “oral anticoagulation alone without antithrombotic therapy significantly reduced the risk of bleeding” in several studies, he added, citing in particular the POPular TAVI trial.
Issues about when to employ – or not employ – both oral anticoagulation and antithrombotic therapy based on such factors as bleeding risk remain unresolved, but “in aggregate, waiting for additional high-quality data, the use of a DOAC in patients with an indication for oral anticoagulation who underwent TAVR seems to be safe,” Dr. Giacoppo said. He thinks that the “higher predictability of DOACS compared to vitamin K antagonists might translate into lower bleeding rates over time in a real-world, unselected population.”
Benefit-to-risk ratio requires attention
A similar concern about balancing risks and benefits of oral anticoagulation in TAVR patients with an indication for oral anticoagulation was emphasized by Ron Waksman, MD, associate director, division of cardiology, MedStar Washington (D.C.) Hospital Center.
“The TAVR population is elderly in general and so are at high risk for bleeding with any additional anticoagulation,” Dr. Waksman said. He cited data that bring into question the utility of using a DOAC in TAVR patients without an additional indication for anticoagulation, but he believes DOACs do make sense in those who were on and had an indication for a DOAC even before TAVR.
Patients who had atrial fibrillation or another indication “should continue to take the DOAC after TAVR. This population can be assumed to have less bleeding risk as they are vetted as safe for DOACs before their TAVR procedure,” he said.
Although mortality was the primary endpoint of the French registry evaluation, it is the bleeding risk that is a dominant concern, according to Romain Didier, MD, PhD, the first author of this study who performed this work in collaboration with Dr. Gilard.
“We really believe that VKA use in real life after TAVR, even with INR monitoring, is associated with a higher risk of bleeding as compared to DOACs,” he said. It is for this reason that “we currently use DOACs as a first choice in patients who require anticoagulant after TAVR.”
Dr. Gilard, Dr. Didier, and Dr. Giacoppo reported no potential conflicts of interest. Dr. Waksman reported financial relationships with Amgen, AstraZeneca, Boston Scientific, Cardioset, Cardiovascular Systems, Chiesi, MedAlliance, Medtronic, and Pi-Cardia.
Following a transcatheter aortic valve replacement (TAVR), direct oral anticoagulants (DOACs) are preferable to vitamin K antagonists (VKAs) in patients who are candidates for oral anticoagulants, according to data drawn from a large multicenter French TAVR registry.
When oral anticoagulation is appropriate following TAVR, such as in patients with atrial fibrillation, “DOACs are associated with improved survival and lower incidence of bleeding, compared to VKA,” reported a team of investigators led by Martine Gilard, MD, PhD, director of interventional cardiology, Brest (France) University Hospital Center.
The comparison, using propensity score matching, is not definitive, but it might be the best data currently available to support DOACs over VKA until a randomized trial is completed, according to Dr. Gilard, senior author of the newly published study.
Asked in an interview if DOACs should now be used preferentially after TAVR when patients are indicated for oral anticoagulation, Dr. Gilard replied, “My answer is yes.”
Of more than 24,000 TAVR patients in the French TAVI and FRANCE2 multicenter registries, which are linked to the French single-payer claims database (SNDS), 8,962 (36.4%) received an oral anticoagulant following their procedure. Of these, 2,180 (24.3%) received a DOAC and the remaining received VKA.
By linking data from the registries to the SNDS, outcomes were tracked. Propensity matching was employed to control for differences in baseline characteristics, including age, body mass index, functional class, diabetes, comorbidities, and past medical history.
On the primary endpoint of mortality at the end of 3 years, the rates were 35.6% and 31.2% for VKA and DOACs, respectively. This translated in a 37% greater hazard ratio for death among those treated with VKA (P < .005).
The rate of major bleeding, a secondary endpoint, was also higher (12.3% vs. 8.4%) and significantly different (HR, 1.65; P < .005) for VKA versus DOACs. The rates of ischemic stroke, acute coronary syndrome, and hemorrhagic stroke were all numerically higher in patients treated with VKA than DOACs, although none of these differences reached statistical significance.
Residual confounding cannot be discounted
“The large number of events allowed for taking into account a higher number of potential confounders with appropriate statistical power,” according to the authors. However, they acknowledged that residual confounding cannot be eliminated by propensity matching and conceded that prospective data are needed for a definitive comparison.
In an accompanying editorial, Daniele Giacoppo, MD, a cardiologist at Alto Vicentino Hospital, Santorso, Italy, enlarged on this point . In addition to the inherent limitations of retrospective data, he also noted that data from other studies addressing the same question have been inconsistent.
Of these studies, he pointed to the ATLANTIS trial, presented 2 months ago at the annual meeting of the American College of Cardiology. This study failed to show an advantage for the DOAC apixaban over VKA in TAVR patients for the primary composite outcome of time to death, myocardial infarction, systemic emboli, valve thrombosis, or major bleeding. Although this study was not limited to patients with an indication for oral anticoagulants, Dr. Giacoppo pointed out that there was no advantage, even among the subgroup of patients who did have an indication.
Data are supportive in absence of trial results
In general, Dr. Giacoppo agreed that the French registry are generally supportive of DOACs over VKA in TAVR patients with an indication for oral anticoagulation, but he cautioned that blanket statements are difficult. He anticipates better information from a randomized trial called ENVISAGE-TAVI AF, which is comparing edoxaban with VKA following TAVR in atrial fibrillation patients who have an indication for oral anticoagulation, but he indicated that some individualization of choice will be needed among those high or low relative risks of thrombotic events or bleeding.
“The concerns related to DOACs after TAVR are most confined to patients without an indication for oral anticoagulation,” Dr. Giacoppo said in an interview. In patients with an indication, “oral anticoagulation alone without antithrombotic therapy significantly reduced the risk of bleeding” in several studies, he added, citing in particular the POPular TAVI trial.
Issues about when to employ – or not employ – both oral anticoagulation and antithrombotic therapy based on such factors as bleeding risk remain unresolved, but “in aggregate, waiting for additional high-quality data, the use of a DOAC in patients with an indication for oral anticoagulation who underwent TAVR seems to be safe,” Dr. Giacoppo said. He thinks that the “higher predictability of DOACS compared to vitamin K antagonists might translate into lower bleeding rates over time in a real-world, unselected population.”
Benefit-to-risk ratio requires attention
A similar concern about balancing risks and benefits of oral anticoagulation in TAVR patients with an indication for oral anticoagulation was emphasized by Ron Waksman, MD, associate director, division of cardiology, MedStar Washington (D.C.) Hospital Center.
“The TAVR population is elderly in general and so are at high risk for bleeding with any additional anticoagulation,” Dr. Waksman said. He cited data that bring into question the utility of using a DOAC in TAVR patients without an additional indication for anticoagulation, but he believes DOACs do make sense in those who were on and had an indication for a DOAC even before TAVR.
Patients who had atrial fibrillation or another indication “should continue to take the DOAC after TAVR. This population can be assumed to have less bleeding risk as they are vetted as safe for DOACs before their TAVR procedure,” he said.
Although mortality was the primary endpoint of the French registry evaluation, it is the bleeding risk that is a dominant concern, according to Romain Didier, MD, PhD, the first author of this study who performed this work in collaboration with Dr. Gilard.
“We really believe that VKA use in real life after TAVR, even with INR monitoring, is associated with a higher risk of bleeding as compared to DOACs,” he said. It is for this reason that “we currently use DOACs as a first choice in patients who require anticoagulant after TAVR.”
Dr. Gilard, Dr. Didier, and Dr. Giacoppo reported no potential conflicts of interest. Dr. Waksman reported financial relationships with Amgen, AstraZeneca, Boston Scientific, Cardioset, Cardiovascular Systems, Chiesi, MedAlliance, Medtronic, and Pi-Cardia.
Following a transcatheter aortic valve replacement (TAVR), direct oral anticoagulants (DOACs) are preferable to vitamin K antagonists (VKAs) in patients who are candidates for oral anticoagulants, according to data drawn from a large multicenter French TAVR registry.
When oral anticoagulation is appropriate following TAVR, such as in patients with atrial fibrillation, “DOACs are associated with improved survival and lower incidence of bleeding, compared to VKA,” reported a team of investigators led by Martine Gilard, MD, PhD, director of interventional cardiology, Brest (France) University Hospital Center.
The comparison, using propensity score matching, is not definitive, but it might be the best data currently available to support DOACs over VKA until a randomized trial is completed, according to Dr. Gilard, senior author of the newly published study.
Asked in an interview if DOACs should now be used preferentially after TAVR when patients are indicated for oral anticoagulation, Dr. Gilard replied, “My answer is yes.”
Of more than 24,000 TAVR patients in the French TAVI and FRANCE2 multicenter registries, which are linked to the French single-payer claims database (SNDS), 8,962 (36.4%) received an oral anticoagulant following their procedure. Of these, 2,180 (24.3%) received a DOAC and the remaining received VKA.
By linking data from the registries to the SNDS, outcomes were tracked. Propensity matching was employed to control for differences in baseline characteristics, including age, body mass index, functional class, diabetes, comorbidities, and past medical history.
On the primary endpoint of mortality at the end of 3 years, the rates were 35.6% and 31.2% for VKA and DOACs, respectively. This translated in a 37% greater hazard ratio for death among those treated with VKA (P < .005).
The rate of major bleeding, a secondary endpoint, was also higher (12.3% vs. 8.4%) and significantly different (HR, 1.65; P < .005) for VKA versus DOACs. The rates of ischemic stroke, acute coronary syndrome, and hemorrhagic stroke were all numerically higher in patients treated with VKA than DOACs, although none of these differences reached statistical significance.
Residual confounding cannot be discounted
“The large number of events allowed for taking into account a higher number of potential confounders with appropriate statistical power,” according to the authors. However, they acknowledged that residual confounding cannot be eliminated by propensity matching and conceded that prospective data are needed for a definitive comparison.
In an accompanying editorial, Daniele Giacoppo, MD, a cardiologist at Alto Vicentino Hospital, Santorso, Italy, enlarged on this point . In addition to the inherent limitations of retrospective data, he also noted that data from other studies addressing the same question have been inconsistent.
Of these studies, he pointed to the ATLANTIS trial, presented 2 months ago at the annual meeting of the American College of Cardiology. This study failed to show an advantage for the DOAC apixaban over VKA in TAVR patients for the primary composite outcome of time to death, myocardial infarction, systemic emboli, valve thrombosis, or major bleeding. Although this study was not limited to patients with an indication for oral anticoagulants, Dr. Giacoppo pointed out that there was no advantage, even among the subgroup of patients who did have an indication.
Data are supportive in absence of trial results
In general, Dr. Giacoppo agreed that the French registry are generally supportive of DOACs over VKA in TAVR patients with an indication for oral anticoagulation, but he cautioned that blanket statements are difficult. He anticipates better information from a randomized trial called ENVISAGE-TAVI AF, which is comparing edoxaban with VKA following TAVR in atrial fibrillation patients who have an indication for oral anticoagulation, but he indicated that some individualization of choice will be needed among those high or low relative risks of thrombotic events or bleeding.
“The concerns related to DOACs after TAVR are most confined to patients without an indication for oral anticoagulation,” Dr. Giacoppo said in an interview. In patients with an indication, “oral anticoagulation alone without antithrombotic therapy significantly reduced the risk of bleeding” in several studies, he added, citing in particular the POPular TAVI trial.
Issues about when to employ – or not employ – both oral anticoagulation and antithrombotic therapy based on such factors as bleeding risk remain unresolved, but “in aggregate, waiting for additional high-quality data, the use of a DOAC in patients with an indication for oral anticoagulation who underwent TAVR seems to be safe,” Dr. Giacoppo said. He thinks that the “higher predictability of DOACS compared to vitamin K antagonists might translate into lower bleeding rates over time in a real-world, unselected population.”
Benefit-to-risk ratio requires attention
A similar concern about balancing risks and benefits of oral anticoagulation in TAVR patients with an indication for oral anticoagulation was emphasized by Ron Waksman, MD, associate director, division of cardiology, MedStar Washington (D.C.) Hospital Center.
“The TAVR population is elderly in general and so are at high risk for bleeding with any additional anticoagulation,” Dr. Waksman said. He cited data that bring into question the utility of using a DOAC in TAVR patients without an additional indication for anticoagulation, but he believes DOACs do make sense in those who were on and had an indication for a DOAC even before TAVR.
Patients who had atrial fibrillation or another indication “should continue to take the DOAC after TAVR. This population can be assumed to have less bleeding risk as they are vetted as safe for DOACs before their TAVR procedure,” he said.
Although mortality was the primary endpoint of the French registry evaluation, it is the bleeding risk that is a dominant concern, according to Romain Didier, MD, PhD, the first author of this study who performed this work in collaboration with Dr. Gilard.
“We really believe that VKA use in real life after TAVR, even with INR monitoring, is associated with a higher risk of bleeding as compared to DOACs,” he said. It is for this reason that “we currently use DOACs as a first choice in patients who require anticoagulant after TAVR.”
Dr. Gilard, Dr. Didier, and Dr. Giacoppo reported no potential conflicts of interest. Dr. Waksman reported financial relationships with Amgen, AstraZeneca, Boston Scientific, Cardioset, Cardiovascular Systems, Chiesi, MedAlliance, Medtronic, and Pi-Cardia.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Gender pay gap most pronounced in procedural specialties
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
St. Jude to pay $27 million to end DOJ suit over faulty ICDs
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
CABANA: Ablation bests drugs for AFib in racial/ethnic minorities
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.