User login
The frequency of influenza and bacterial coinfection
Treatment of patients admitted to the hospital with an upper respiratory infection is often complicated by the lack of diagnostics. Even in cases where a patient has a confirmed case of influenza, there is still the possibility that they may have, or become infected by, a secondary bacterial infection. This leads clinicians to treat patients empirically with antibiotics, which can result in unnecessary antibiotic use among patients without a bacterial infection.
Though antibiotics can be a lifesaving drug, their use is not without risks. Estimates suggest that 20% of patients taking common antibiotics experience some side effect. While most side effects are not life-threatening gastrointestinal effects, other nonnegligible side effects include anaphylactic shock, drug‐induced liver injury, increases in the risk of retinal detachment, serious arrhythmias, and superinfection with resistant bacteria. Antibiotics can also lead to secondary infections, such as Clostridium difficile.
Yet, despite these risks, there has been limited research on the percentage of patients with influenza who actually have a bacterial coinfection. A recent systematic review and meta-analysis conducted by my colleagues at Johns Hopkins University and the Center for Disease Dynamics, Economics & Policy examined the frequency of bacterial coinfection among hospitalized patients with influenza and identified the most common infecting bacterial species.
The findings, published in the journal Influenza and Other Respiratory Viruses, found that in the majority of studies, between 11% and 35% of patients with confirmed influenza had a bacterial coinfection. The most common coinfecting bacteria were found to be Streptococcus pneumoniae and Staphylococcus aureus. Combined, S. pneumoniae and S. aureus accounted for more than 60% of the identified coinfecting bacteria; however, many other bacterial species were found to cause infections as well.
The results suggest that while bacterial infection is common in influenza patients, only about a quarter of patients are likely to be infected. However, the studies were widely heterogeneous, both in patient makeup and results. Analyses of age, setting, enrollment year, study type, study size, and bacterial collection methods did not reveal a source for the heterogeneity in results. Thus, additional factors, such as patient comorbidities or prior antibiotic use, which could not be systematically assessed, may affect the likelihood of coinfection.
Given that the symptoms of influenza and bacterial infection often overlap, correctly diagnosing bacterial coinfection without a laboratory culture can present a challenge. This diagnostic uncertainty leads to significant overuse of antibiotics in patients with influenza alone. Most influenza cases will never result in serious bacterial infections (particularly in nonhospitalized patients), and thus a lot of antibiotic use is unnecessary. As mentioned earlier, unnecessary antibiotic use poses a nonnegligible risk to patients, but it also contributes significantly to rising rates of antibiotic resistance, a major public health issue.
The results from this study highlight that the majority of patients hospitalized with influenza are unlikely to be coinfected with a bacterial pathogen. Thus, it is important for clinicians to appropriately treat patients with antiviral drugs, and ensure that bacterial testing is done when presumptively starting patients on antibiotics. Based on the microbiology results, antibiotics can be stopped if no pathogen is identified or altered to be more appropriate depending on the pathogen found.
Although the findings of this study suggest we need a more thorough analysis of the issue, the results should still aid clinicians by improving their understanding of the likelihood of bacterial coinfection in hospitalized patients with influenza, and thus help them balance the need to minimize patient risks as well as the individual and societal risks of nonessential antibiotic use.
Eili Klein, PhD, is assistant professor in the department of emergency medicine at Johns Hopkins Medicine, Baltimore.
Treatment of patients admitted to the hospital with an upper respiratory infection is often complicated by the lack of diagnostics. Even in cases where a patient has a confirmed case of influenza, there is still the possibility that they may have, or become infected by, a secondary bacterial infection. This leads clinicians to treat patients empirically with antibiotics, which can result in unnecessary antibiotic use among patients without a bacterial infection.
Though antibiotics can be a lifesaving drug, their use is not without risks. Estimates suggest that 20% of patients taking common antibiotics experience some side effect. While most side effects are not life-threatening gastrointestinal effects, other nonnegligible side effects include anaphylactic shock, drug‐induced liver injury, increases in the risk of retinal detachment, serious arrhythmias, and superinfection with resistant bacteria. Antibiotics can also lead to secondary infections, such as Clostridium difficile.
Yet, despite these risks, there has been limited research on the percentage of patients with influenza who actually have a bacterial coinfection. A recent systematic review and meta-analysis conducted by my colleagues at Johns Hopkins University and the Center for Disease Dynamics, Economics & Policy examined the frequency of bacterial coinfection among hospitalized patients with influenza and identified the most common infecting bacterial species.
The findings, published in the journal Influenza and Other Respiratory Viruses, found that in the majority of studies, between 11% and 35% of patients with confirmed influenza had a bacterial coinfection. The most common coinfecting bacteria were found to be Streptococcus pneumoniae and Staphylococcus aureus. Combined, S. pneumoniae and S. aureus accounted for more than 60% of the identified coinfecting bacteria; however, many other bacterial species were found to cause infections as well.
The results suggest that while bacterial infection is common in influenza patients, only about a quarter of patients are likely to be infected. However, the studies were widely heterogeneous, both in patient makeup and results. Analyses of age, setting, enrollment year, study type, study size, and bacterial collection methods did not reveal a source for the heterogeneity in results. Thus, additional factors, such as patient comorbidities or prior antibiotic use, which could not be systematically assessed, may affect the likelihood of coinfection.
Given that the symptoms of influenza and bacterial infection often overlap, correctly diagnosing bacterial coinfection without a laboratory culture can present a challenge. This diagnostic uncertainty leads to significant overuse of antibiotics in patients with influenza alone. Most influenza cases will never result in serious bacterial infections (particularly in nonhospitalized patients), and thus a lot of antibiotic use is unnecessary. As mentioned earlier, unnecessary antibiotic use poses a nonnegligible risk to patients, but it also contributes significantly to rising rates of antibiotic resistance, a major public health issue.
The results from this study highlight that the majority of patients hospitalized with influenza are unlikely to be coinfected with a bacterial pathogen. Thus, it is important for clinicians to appropriately treat patients with antiviral drugs, and ensure that bacterial testing is done when presumptively starting patients on antibiotics. Based on the microbiology results, antibiotics can be stopped if no pathogen is identified or altered to be more appropriate depending on the pathogen found.
Although the findings of this study suggest we need a more thorough analysis of the issue, the results should still aid clinicians by improving their understanding of the likelihood of bacterial coinfection in hospitalized patients with influenza, and thus help them balance the need to minimize patient risks as well as the individual and societal risks of nonessential antibiotic use.
Eili Klein, PhD, is assistant professor in the department of emergency medicine at Johns Hopkins Medicine, Baltimore.
Treatment of patients admitted to the hospital with an upper respiratory infection is often complicated by the lack of diagnostics. Even in cases where a patient has a confirmed case of influenza, there is still the possibility that they may have, or become infected by, a secondary bacterial infection. This leads clinicians to treat patients empirically with antibiotics, which can result in unnecessary antibiotic use among patients without a bacterial infection.
Though antibiotics can be a lifesaving drug, their use is not without risks. Estimates suggest that 20% of patients taking common antibiotics experience some side effect. While most side effects are not life-threatening gastrointestinal effects, other nonnegligible side effects include anaphylactic shock, drug‐induced liver injury, increases in the risk of retinal detachment, serious arrhythmias, and superinfection with resistant bacteria. Antibiotics can also lead to secondary infections, such as Clostridium difficile.
Yet, despite these risks, there has been limited research on the percentage of patients with influenza who actually have a bacterial coinfection. A recent systematic review and meta-analysis conducted by my colleagues at Johns Hopkins University and the Center for Disease Dynamics, Economics & Policy examined the frequency of bacterial coinfection among hospitalized patients with influenza and identified the most common infecting bacterial species.
The findings, published in the journal Influenza and Other Respiratory Viruses, found that in the majority of studies, between 11% and 35% of patients with confirmed influenza had a bacterial coinfection. The most common coinfecting bacteria were found to be Streptococcus pneumoniae and Staphylococcus aureus. Combined, S. pneumoniae and S. aureus accounted for more than 60% of the identified coinfecting bacteria; however, many other bacterial species were found to cause infections as well.
The results suggest that while bacterial infection is common in influenza patients, only about a quarter of patients are likely to be infected. However, the studies were widely heterogeneous, both in patient makeup and results. Analyses of age, setting, enrollment year, study type, study size, and bacterial collection methods did not reveal a source for the heterogeneity in results. Thus, additional factors, such as patient comorbidities or prior antibiotic use, which could not be systematically assessed, may affect the likelihood of coinfection.
Given that the symptoms of influenza and bacterial infection often overlap, correctly diagnosing bacterial coinfection without a laboratory culture can present a challenge. This diagnostic uncertainty leads to significant overuse of antibiotics in patients with influenza alone. Most influenza cases will never result in serious bacterial infections (particularly in nonhospitalized patients), and thus a lot of antibiotic use is unnecessary. As mentioned earlier, unnecessary antibiotic use poses a nonnegligible risk to patients, but it also contributes significantly to rising rates of antibiotic resistance, a major public health issue.
The results from this study highlight that the majority of patients hospitalized with influenza are unlikely to be coinfected with a bacterial pathogen. Thus, it is important for clinicians to appropriately treat patients with antiviral drugs, and ensure that bacterial testing is done when presumptively starting patients on antibiotics. Based on the microbiology results, antibiotics can be stopped if no pathogen is identified or altered to be more appropriate depending on the pathogen found.
Although the findings of this study suggest we need a more thorough analysis of the issue, the results should still aid clinicians by improving their understanding of the likelihood of bacterial coinfection in hospitalized patients with influenza, and thus help them balance the need to minimize patient risks as well as the individual and societal risks of nonessential antibiotic use.
Eili Klein, PhD, is assistant professor in the department of emergency medicine at Johns Hopkins Medicine, Baltimore.
Flu vaccine prevented hospitalizations in patients 50 and older
The seasonal influenza vaccination reduced flu-related hospitalizations by 56.8% among people aged 50 and older during a recent flu season, according to a report published in Clinical Infectious Diseases.
Even in the oldest age group – the population with the highest risk of developing flu complications and perhaps the weakest immune response – influenza vaccination prevented serious complications, said Fiona P. Havers, MD, of the influenza division, Centers for Disease Control and Prevention, Atlanta, and her associates.
Data on vaccine efficacy in older adults are sparse, and randomized, placebo-controlled trials to gather evidence would be unethical. Dr. Havers and her colleagues studied the issue using a case-control design, focusing on community-dwelling adults aged 50 years and older during the 2010-2011 flu season. They identified 368 patients across 10 states who were hospitalized for polymerase chain reaction–confirmed influenza and matched them for age and county of residence with 773 control subjects.
Hospitalized case-patients were less likely to have been vaccinated (55%) than were control subjects (63%). Thus, the flu vaccine reduced the risk of hospitalization for influenza by 56.8% overall.
Vaccination reduced hospitalization for influenza by 63.9% in the youngest age group (50-64 years), by 61.0% in the intermediate age group (65-74 years), and by 57.3% in the oldest age group (75 years and older).
These results are similar to those reported in other studies assessing the same time period, including one that evaluated vaccine efficacy in ambulatory adults in the United States and Europe. They also are consistent with the results of observational studies performed during different flu seasons, the investigators said (Clin Infect Dis. 2016 Aug 2. doi: 10.1093/cid/ciw512).
Compared with control subjects, case-patients were more likely to be of nonwhite race, to be of Hispanic ethnicity, to have a lower income, to have had fewer years of education, to have two or more chronic health conditions, to have required recent hospitalization for respiratory problems, to have impaired mobility, and to have lower functional status.
“These findings support current U.S. recommendations for annual influenza vaccination in older adults, especially in adults aged 65 and older who are at higher risk of influenza-associated complications,” Dr. Havers and her associates said.
The Centers for Disease Control and Prevention supported the study. Dr. Havers reported having no relevant financial disclosures; one of her associates reported ties to Genentech, Merck, Novavax, and Pfizer.
The seasonal influenza vaccination reduced flu-related hospitalizations by 56.8% among people aged 50 and older during a recent flu season, according to a report published in Clinical Infectious Diseases.
Even in the oldest age group – the population with the highest risk of developing flu complications and perhaps the weakest immune response – influenza vaccination prevented serious complications, said Fiona P. Havers, MD, of the influenza division, Centers for Disease Control and Prevention, Atlanta, and her associates.
Data on vaccine efficacy in older adults are sparse, and randomized, placebo-controlled trials to gather evidence would be unethical. Dr. Havers and her colleagues studied the issue using a case-control design, focusing on community-dwelling adults aged 50 years and older during the 2010-2011 flu season. They identified 368 patients across 10 states who were hospitalized for polymerase chain reaction–confirmed influenza and matched them for age and county of residence with 773 control subjects.
Hospitalized case-patients were less likely to have been vaccinated (55%) than were control subjects (63%). Thus, the flu vaccine reduced the risk of hospitalization for influenza by 56.8% overall.
Vaccination reduced hospitalization for influenza by 63.9% in the youngest age group (50-64 years), by 61.0% in the intermediate age group (65-74 years), and by 57.3% in the oldest age group (75 years and older).
These results are similar to those reported in other studies assessing the same time period, including one that evaluated vaccine efficacy in ambulatory adults in the United States and Europe. They also are consistent with the results of observational studies performed during different flu seasons, the investigators said (Clin Infect Dis. 2016 Aug 2. doi: 10.1093/cid/ciw512).
Compared with control subjects, case-patients were more likely to be of nonwhite race, to be of Hispanic ethnicity, to have a lower income, to have had fewer years of education, to have two or more chronic health conditions, to have required recent hospitalization for respiratory problems, to have impaired mobility, and to have lower functional status.
“These findings support current U.S. recommendations for annual influenza vaccination in older adults, especially in adults aged 65 and older who are at higher risk of influenza-associated complications,” Dr. Havers and her associates said.
The Centers for Disease Control and Prevention supported the study. Dr. Havers reported having no relevant financial disclosures; one of her associates reported ties to Genentech, Merck, Novavax, and Pfizer.
The seasonal influenza vaccination reduced flu-related hospitalizations by 56.8% among people aged 50 and older during a recent flu season, according to a report published in Clinical Infectious Diseases.
Even in the oldest age group – the population with the highest risk of developing flu complications and perhaps the weakest immune response – influenza vaccination prevented serious complications, said Fiona P. Havers, MD, of the influenza division, Centers for Disease Control and Prevention, Atlanta, and her associates.
Data on vaccine efficacy in older adults are sparse, and randomized, placebo-controlled trials to gather evidence would be unethical. Dr. Havers and her colleagues studied the issue using a case-control design, focusing on community-dwelling adults aged 50 years and older during the 2010-2011 flu season. They identified 368 patients across 10 states who were hospitalized for polymerase chain reaction–confirmed influenza and matched them for age and county of residence with 773 control subjects.
Hospitalized case-patients were less likely to have been vaccinated (55%) than were control subjects (63%). Thus, the flu vaccine reduced the risk of hospitalization for influenza by 56.8% overall.
Vaccination reduced hospitalization for influenza by 63.9% in the youngest age group (50-64 years), by 61.0% in the intermediate age group (65-74 years), and by 57.3% in the oldest age group (75 years and older).
These results are similar to those reported in other studies assessing the same time period, including one that evaluated vaccine efficacy in ambulatory adults in the United States and Europe. They also are consistent with the results of observational studies performed during different flu seasons, the investigators said (Clin Infect Dis. 2016 Aug 2. doi: 10.1093/cid/ciw512).
Compared with control subjects, case-patients were more likely to be of nonwhite race, to be of Hispanic ethnicity, to have a lower income, to have had fewer years of education, to have two or more chronic health conditions, to have required recent hospitalization for respiratory problems, to have impaired mobility, and to have lower functional status.
“These findings support current U.S. recommendations for annual influenza vaccination in older adults, especially in adults aged 65 and older who are at higher risk of influenza-associated complications,” Dr. Havers and her associates said.
The Centers for Disease Control and Prevention supported the study. Dr. Havers reported having no relevant financial disclosures; one of her associates reported ties to Genentech, Merck, Novavax, and Pfizer.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Seasonal influenza vaccination reduced flu-related hospitalizations by 56.8% in people aged 50 years and older.
Major finding: Vaccination reduced hospitalization for influenza by 63.9% in people aged 50-64 years, by 61.0% in those aged 65-74 years, and by 57.3% in those aged 75 years and older.
Data source: A retrospective case-control study involving 368 cases and 773 matched controls assessed during a single recent flu season.
Disclosures: The Centers for Disease Control and Prevention supported the study. Dr. Havers reported having no relevant financial disclosures; one of her associates reported ties to Genentech, Merck, Novavax, and Pfizer.
Summer flu? Think variant swine influenza virus infection
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Myth of the Month: Vaccinations in patients with Guillain-Barré syndrome
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Mortality rates higher among influenza B patients than influenza A patients
Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A, investigators found.
Among those with influenza B, patients aged 10-16 years were most likely to require ICU admission, suggesting this subpopulation may be a target for immunization programs.
The percentage of clinical cases attributed to influenza B range from less than 1% to 44%, according to data published by the Centers for Disease Control and Prevention. However, influenza B is considered less virulent and less capable of causing pandemics and has therefore been less studied and outcomes of its disease less characterized, Dat Tran, MD, MSc, of the Hospital for Sick Children in Canada and his associates reported (Pediatrics. 2016 August. doi: 10.1542/peds.2015-4643).
The purpose of this study was to further understand the prevalence and severity of influenza B cases in comparison with influenza A and to identify pediatric subpopulations most at risk for contracting influenza B.
Children aged 16 years or younger hospitalized from laboratory-confirmed influenza A or B from September 2004 to June 2013 (excluding the pandemic year 2009-2010) were identified through active surveillance of admissions at the 12 pediatric referral centers of the Canadian Immunization Monitoring Program Active (IMPACT), a national surveillance initiative. Information regarding demographics, health status, vaccination status, presenting signs and symptoms, illness severity and mortality, treatment regimens, and ICU admission were collected and analyzed.
Of 4,155 influenza-related admissions during this time period, influenza B accounted for 1,510 (36.3%) cases and influenza A accounted for 2,645 (63.7%) cases.
Children admitted with influenza B tended to be older with a median age 3.9 years (interquartile range, 1.4-7.2), compared with a median of 2 years (IQR, 0.6-4.8 years) for children admitted with influenza A.
Children admitted with influenza B, compared with influenza A, had higher odds of having a vaccine-indicated condition (odds ratio, 1.30; 95% confidence interval, 1.14-1.47) and lower odds of having no underlying medical condition (OR, 0.80; 95% CI, 0.71-0.91), Dr. Tran and his associates reported.
“Compared with influenza A cases, children admitted with influenza B had greater adjusted odds of presenting with headache, abdominal pain, and myalgia, ranging from 1.38 for abdominal pain to 3.19 for myalgia,” they added. “There were no significant differences in antiviral or antibiotic prescription or use between influenza A and B cases.”
There was no significant difference in the proportion of influenza A or B patients admitted to the ICU (12.7% vs. 12.6%). Rather, multivariate modeling identified age and presence of an underlying condition as independent predictors of ICU admission.
Finally, influenza-attributable mortality was significantly greater in children with influenza B (adjusted OR, 2.65; 95% CI, 1.18-5.94). Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. All-cause mortality followed a similar trend.
“Among hospitalized children, influenza A and B infections resulted in similar morbidity while mortality was greater for influenza B disease. Among healthy children hospitalized with influenza B, those aged 10-16 years were most likely to require ICU admission,” the investigators summarized.
“These children should be considered at high risk for complicated influenza B infection and be specifically targeted by immunization programs to receive influenza vaccination, and in particular, a [quadrivalent influenza vaccine],” they recommended.
This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A, investigators found.
Among those with influenza B, patients aged 10-16 years were most likely to require ICU admission, suggesting this subpopulation may be a target for immunization programs.
The percentage of clinical cases attributed to influenza B range from less than 1% to 44%, according to data published by the Centers for Disease Control and Prevention. However, influenza B is considered less virulent and less capable of causing pandemics and has therefore been less studied and outcomes of its disease less characterized, Dat Tran, MD, MSc, of the Hospital for Sick Children in Canada and his associates reported (Pediatrics. 2016 August. doi: 10.1542/peds.2015-4643).
The purpose of this study was to further understand the prevalence and severity of influenza B cases in comparison with influenza A and to identify pediatric subpopulations most at risk for contracting influenza B.
Children aged 16 years or younger hospitalized from laboratory-confirmed influenza A or B from September 2004 to June 2013 (excluding the pandemic year 2009-2010) were identified through active surveillance of admissions at the 12 pediatric referral centers of the Canadian Immunization Monitoring Program Active (IMPACT), a national surveillance initiative. Information regarding demographics, health status, vaccination status, presenting signs and symptoms, illness severity and mortality, treatment regimens, and ICU admission were collected and analyzed.
Of 4,155 influenza-related admissions during this time period, influenza B accounted for 1,510 (36.3%) cases and influenza A accounted for 2,645 (63.7%) cases.
Children admitted with influenza B tended to be older with a median age 3.9 years (interquartile range, 1.4-7.2), compared with a median of 2 years (IQR, 0.6-4.8 years) for children admitted with influenza A.
Children admitted with influenza B, compared with influenza A, had higher odds of having a vaccine-indicated condition (odds ratio, 1.30; 95% confidence interval, 1.14-1.47) and lower odds of having no underlying medical condition (OR, 0.80; 95% CI, 0.71-0.91), Dr. Tran and his associates reported.
“Compared with influenza A cases, children admitted with influenza B had greater adjusted odds of presenting with headache, abdominal pain, and myalgia, ranging from 1.38 for abdominal pain to 3.19 for myalgia,” they added. “There were no significant differences in antiviral or antibiotic prescription or use between influenza A and B cases.”
There was no significant difference in the proportion of influenza A or B patients admitted to the ICU (12.7% vs. 12.6%). Rather, multivariate modeling identified age and presence of an underlying condition as independent predictors of ICU admission.
Finally, influenza-attributable mortality was significantly greater in children with influenza B (adjusted OR, 2.65; 95% CI, 1.18-5.94). Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. All-cause mortality followed a similar trend.
“Among hospitalized children, influenza A and B infections resulted in similar morbidity while mortality was greater for influenza B disease. Among healthy children hospitalized with influenza B, those aged 10-16 years were most likely to require ICU admission,” the investigators summarized.
“These children should be considered at high risk for complicated influenza B infection and be specifically targeted by immunization programs to receive influenza vaccination, and in particular, a [quadrivalent influenza vaccine],” they recommended.
This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A, investigators found.
Among those with influenza B, patients aged 10-16 years were most likely to require ICU admission, suggesting this subpopulation may be a target for immunization programs.
The percentage of clinical cases attributed to influenza B range from less than 1% to 44%, according to data published by the Centers for Disease Control and Prevention. However, influenza B is considered less virulent and less capable of causing pandemics and has therefore been less studied and outcomes of its disease less characterized, Dat Tran, MD, MSc, of the Hospital for Sick Children in Canada and his associates reported (Pediatrics. 2016 August. doi: 10.1542/peds.2015-4643).
The purpose of this study was to further understand the prevalence and severity of influenza B cases in comparison with influenza A and to identify pediatric subpopulations most at risk for contracting influenza B.
Children aged 16 years or younger hospitalized from laboratory-confirmed influenza A or B from September 2004 to June 2013 (excluding the pandemic year 2009-2010) were identified through active surveillance of admissions at the 12 pediatric referral centers of the Canadian Immunization Monitoring Program Active (IMPACT), a national surveillance initiative. Information regarding demographics, health status, vaccination status, presenting signs and symptoms, illness severity and mortality, treatment regimens, and ICU admission were collected and analyzed.
Of 4,155 influenza-related admissions during this time period, influenza B accounted for 1,510 (36.3%) cases and influenza A accounted for 2,645 (63.7%) cases.
Children admitted with influenza B tended to be older with a median age 3.9 years (interquartile range, 1.4-7.2), compared with a median of 2 years (IQR, 0.6-4.8 years) for children admitted with influenza A.
Children admitted with influenza B, compared with influenza A, had higher odds of having a vaccine-indicated condition (odds ratio, 1.30; 95% confidence interval, 1.14-1.47) and lower odds of having no underlying medical condition (OR, 0.80; 95% CI, 0.71-0.91), Dr. Tran and his associates reported.
“Compared with influenza A cases, children admitted with influenza B had greater adjusted odds of presenting with headache, abdominal pain, and myalgia, ranging from 1.38 for abdominal pain to 3.19 for myalgia,” they added. “There were no significant differences in antiviral or antibiotic prescription or use between influenza A and B cases.”
There was no significant difference in the proportion of influenza A or B patients admitted to the ICU (12.7% vs. 12.6%). Rather, multivariate modeling identified age and presence of an underlying condition as independent predictors of ICU admission.
Finally, influenza-attributable mortality was significantly greater in children with influenza B (adjusted OR, 2.65; 95% CI, 1.18-5.94). Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. All-cause mortality followed a similar trend.
“Among hospitalized children, influenza A and B infections resulted in similar morbidity while mortality was greater for influenza B disease. Among healthy children hospitalized with influenza B, those aged 10-16 years were most likely to require ICU admission,” the investigators summarized.
“These children should be considered at high risk for complicated influenza B infection and be specifically targeted by immunization programs to receive influenza vaccination, and in particular, a [quadrivalent influenza vaccine],” they recommended.
This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
FROM PEDIATRICS
Key clinical point: Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A.
Major finding: Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. Influenza-attributable mortality was significantly greater in children with influenza B (adjusted odds ratio, 2.65, 95% confidence interval, 1.18-5.94).
Data source: An observational study of 4,155 children admitted to the hospital with influenza A or B during nonpandemic years between September 2004 and June 2013.
Disclosures: This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
LAIV no better than IIV for influenza protection in children
Live attenuated influenza vaccine (LAIV) was no more effective than inactivated influenza vaccine (IIV) in small, compact, rural communities, according to Mark Loeb, MD, and his associates.
For the study, vaccinations were given to children aged 36 months to 15 years living in Hutterite colonies in Canada. Hutterite colonies are isolated, their residents live communally, and influenza is prevalent, making them suited for this cluster randomized trial.
Of the 1,186 children included in the study, mean coverage was 76.7% in the LAIV group, compared with 72.4% in the IIV group. Incidence of influenza was 5.3% in the LAIV group and 5.2% in the IIV group. Compared to IIV, the hazard ratio for LAIV for influenza A or B was 1.03.
Children vaccinated with LAIV were at a higher risk for influenza A (hazard ratio, 1.62), but were at lower risk for influenza B (HR, 0.66). Influenza attack rates were similar in children younger and older than 6 years old. Adverse reactions were more likely in the IIV group than in the LAIV group, but no serious adverse events were reported.
“Although influenza transmission networks in Hutterite communities may differ from that in other communities, there are no data to confirm this. In fact, there may be more variability in social networks between urban and rural communities – or even among various urban communities – than between Hutterite and other (rural) communities. Even if variability exists, if a clear benefit of LAIV over IIV in reducing influenza-associated illness cannot be detected in this setting it is unlikely to be seen in other communities,” the investigators noted.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M16-0513).
Live attenuated influenza vaccine (LAIV) was no more effective than inactivated influenza vaccine (IIV) in small, compact, rural communities, according to Mark Loeb, MD, and his associates.
For the study, vaccinations were given to children aged 36 months to 15 years living in Hutterite colonies in Canada. Hutterite colonies are isolated, their residents live communally, and influenza is prevalent, making them suited for this cluster randomized trial.
Of the 1,186 children included in the study, mean coverage was 76.7% in the LAIV group, compared with 72.4% in the IIV group. Incidence of influenza was 5.3% in the LAIV group and 5.2% in the IIV group. Compared to IIV, the hazard ratio for LAIV for influenza A or B was 1.03.
Children vaccinated with LAIV were at a higher risk for influenza A (hazard ratio, 1.62), but were at lower risk for influenza B (HR, 0.66). Influenza attack rates were similar in children younger and older than 6 years old. Adverse reactions were more likely in the IIV group than in the LAIV group, but no serious adverse events were reported.
“Although influenza transmission networks in Hutterite communities may differ from that in other communities, there are no data to confirm this. In fact, there may be more variability in social networks between urban and rural communities – or even among various urban communities – than between Hutterite and other (rural) communities. Even if variability exists, if a clear benefit of LAIV over IIV in reducing influenza-associated illness cannot be detected in this setting it is unlikely to be seen in other communities,” the investigators noted.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M16-0513).
Live attenuated influenza vaccine (LAIV) was no more effective than inactivated influenza vaccine (IIV) in small, compact, rural communities, according to Mark Loeb, MD, and his associates.
For the study, vaccinations were given to children aged 36 months to 15 years living in Hutterite colonies in Canada. Hutterite colonies are isolated, their residents live communally, and influenza is prevalent, making them suited for this cluster randomized trial.
Of the 1,186 children included in the study, mean coverage was 76.7% in the LAIV group, compared with 72.4% in the IIV group. Incidence of influenza was 5.3% in the LAIV group and 5.2% in the IIV group. Compared to IIV, the hazard ratio for LAIV for influenza A or B was 1.03.
Children vaccinated with LAIV were at a higher risk for influenza A (hazard ratio, 1.62), but were at lower risk for influenza B (HR, 0.66). Influenza attack rates were similar in children younger and older than 6 years old. Adverse reactions were more likely in the IIV group than in the LAIV group, but no serious adverse events were reported.
“Although influenza transmission networks in Hutterite communities may differ from that in other communities, there are no data to confirm this. In fact, there may be more variability in social networks between urban and rural communities – or even among various urban communities – than between Hutterite and other (rural) communities. Even if variability exists, if a clear benefit of LAIV over IIV in reducing influenza-associated illness cannot be detected in this setting it is unlikely to be seen in other communities,” the investigators noted.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M16-0513).
FROM ANNALS OF INTERNAL MEDICINE
Model estimates risk of pneumonia after CABG
A model incorporating 17 easily obtainable preoperative variables may help clinicians estimate patients’ risk of developing pneumonia after undergoing coronary artery bypass graft surgery, according to a report published in Annals of Thoracic Surgery.
“This model may be used to inform clinician-patient decision making and to identify opportunities for mitigating a patient’s risk,” said Raymond J. Strobel, a medical student at the University of Michigan, Ann Arbor, and his associates.
Postoperative pneumonia is the most common hospital-acquired infection following CABG, and it raises mortality risk fourfold and increases length of stay threefold. But reliable estimation of patient risk of post-CABG pneumonia has been difficult because of its low relative incidence – roughly 3% – and because most studies of the disorder are nearly a decade out of date.
To devise a predictive model using current data, Mr. Strobel and his associates assessed numerous potential risk factors and outcomes for 16,084 consecutive patients undergoing CABG at all 33 cardiac centers across Michigan during a 3-year period. They identified 531 cases of post-CABG pneumonia (3.3%) in this cohort.
The investigators performed a univariate analysis to test the associations between pneumonia and numerous factors related to patient demographics, medical history, comorbid diseases, laboratory values, cardiac anatomy, cardiac function, pulmonary function, the CABG procedure, and the institution where the procedure was performed. Variables that were found to be significantly associated with pneumonia (though usually with small absolute magnitudes) were then assessed in a multivariate analysis, which was further refined to create the final model.
The final model includes 17 factors that clearly raise the risk of post-CABG pneumonia. These include an elevated leukocyte count; a decreased hematocrit; older patient age; comorbidities such as peripheral vascular disease, diabetes, and liver disease; markers of pulmonary impairment such as cigarette smoking, the need for home oxygen therapy, and chronic lung disease; markers of cardiac dysfunction such as a recent history of arrhythmia and decreased ejection fraction; and emergency or urgent rather than elective operative status.
“This model performs well and demonstrates robustness across important clinical subgroups and centers,” the investigators said (Ann Thorac Surg. 2016 Jun 1; doi: 10.1016/j.athoracsur.2016.03.074).
In particular, this study identified preoperative leukocytosis to be a significant predictor of post-CABG pneumonia across several subgroups of patients. “We speculate that patients presenting with an elevated white blood cell count before surgery may be mounting an immune response against a pathogen and that the insult of CABG significantly increases their odds of postoperative pneumonia. ... It may be prudent to delay surgery until the source of leukocytosis is satisfactorily investigated, if not identified and treated, or the leukocytosis has otherwise resolved,” Mr. Strobel and his associates noted.
A model incorporating 17 easily obtainable preoperative variables may help clinicians estimate patients’ risk of developing pneumonia after undergoing coronary artery bypass graft surgery, according to a report published in Annals of Thoracic Surgery.
“This model may be used to inform clinician-patient decision making and to identify opportunities for mitigating a patient’s risk,” said Raymond J. Strobel, a medical student at the University of Michigan, Ann Arbor, and his associates.
Postoperative pneumonia is the most common hospital-acquired infection following CABG, and it raises mortality risk fourfold and increases length of stay threefold. But reliable estimation of patient risk of post-CABG pneumonia has been difficult because of its low relative incidence – roughly 3% – and because most studies of the disorder are nearly a decade out of date.
To devise a predictive model using current data, Mr. Strobel and his associates assessed numerous potential risk factors and outcomes for 16,084 consecutive patients undergoing CABG at all 33 cardiac centers across Michigan during a 3-year period. They identified 531 cases of post-CABG pneumonia (3.3%) in this cohort.
The investigators performed a univariate analysis to test the associations between pneumonia and numerous factors related to patient demographics, medical history, comorbid diseases, laboratory values, cardiac anatomy, cardiac function, pulmonary function, the CABG procedure, and the institution where the procedure was performed. Variables that were found to be significantly associated with pneumonia (though usually with small absolute magnitudes) were then assessed in a multivariate analysis, which was further refined to create the final model.
The final model includes 17 factors that clearly raise the risk of post-CABG pneumonia. These include an elevated leukocyte count; a decreased hematocrit; older patient age; comorbidities such as peripheral vascular disease, diabetes, and liver disease; markers of pulmonary impairment such as cigarette smoking, the need for home oxygen therapy, and chronic lung disease; markers of cardiac dysfunction such as a recent history of arrhythmia and decreased ejection fraction; and emergency or urgent rather than elective operative status.
“This model performs well and demonstrates robustness across important clinical subgroups and centers,” the investigators said (Ann Thorac Surg. 2016 Jun 1; doi: 10.1016/j.athoracsur.2016.03.074).
In particular, this study identified preoperative leukocytosis to be a significant predictor of post-CABG pneumonia across several subgroups of patients. “We speculate that patients presenting with an elevated white blood cell count before surgery may be mounting an immune response against a pathogen and that the insult of CABG significantly increases their odds of postoperative pneumonia. ... It may be prudent to delay surgery until the source of leukocytosis is satisfactorily investigated, if not identified and treated, or the leukocytosis has otherwise resolved,” Mr. Strobel and his associates noted.
A model incorporating 17 easily obtainable preoperative variables may help clinicians estimate patients’ risk of developing pneumonia after undergoing coronary artery bypass graft surgery, according to a report published in Annals of Thoracic Surgery.
“This model may be used to inform clinician-patient decision making and to identify opportunities for mitigating a patient’s risk,” said Raymond J. Strobel, a medical student at the University of Michigan, Ann Arbor, and his associates.
Postoperative pneumonia is the most common hospital-acquired infection following CABG, and it raises mortality risk fourfold and increases length of stay threefold. But reliable estimation of patient risk of post-CABG pneumonia has been difficult because of its low relative incidence – roughly 3% – and because most studies of the disorder are nearly a decade out of date.
To devise a predictive model using current data, Mr. Strobel and his associates assessed numerous potential risk factors and outcomes for 16,084 consecutive patients undergoing CABG at all 33 cardiac centers across Michigan during a 3-year period. They identified 531 cases of post-CABG pneumonia (3.3%) in this cohort.
The investigators performed a univariate analysis to test the associations between pneumonia and numerous factors related to patient demographics, medical history, comorbid diseases, laboratory values, cardiac anatomy, cardiac function, pulmonary function, the CABG procedure, and the institution where the procedure was performed. Variables that were found to be significantly associated with pneumonia (though usually with small absolute magnitudes) were then assessed in a multivariate analysis, which was further refined to create the final model.
The final model includes 17 factors that clearly raise the risk of post-CABG pneumonia. These include an elevated leukocyte count; a decreased hematocrit; older patient age; comorbidities such as peripheral vascular disease, diabetes, and liver disease; markers of pulmonary impairment such as cigarette smoking, the need for home oxygen therapy, and chronic lung disease; markers of cardiac dysfunction such as a recent history of arrhythmia and decreased ejection fraction; and emergency or urgent rather than elective operative status.
“This model performs well and demonstrates robustness across important clinical subgroups and centers,” the investigators said (Ann Thorac Surg. 2016 Jun 1; doi: 10.1016/j.athoracsur.2016.03.074).
In particular, this study identified preoperative leukocytosis to be a significant predictor of post-CABG pneumonia across several subgroups of patients. “We speculate that patients presenting with an elevated white blood cell count before surgery may be mounting an immune response against a pathogen and that the insult of CABG significantly increases their odds of postoperative pneumonia. ... It may be prudent to delay surgery until the source of leukocytosis is satisfactorily investigated, if not identified and treated, or the leukocytosis has otherwise resolved,” Mr. Strobel and his associates noted.
FROM ANNALS OF THORACIC SURGERY
Key clinical point: A model incorporating 17 easily obtainable preoperative variables helps estimate patients’ risk of developing pneumonia after coronary artery bypass surgery.
Major finding: Seventeen factors clearly raise the risk of post-CABG pneumonia, including an elevated leukocyte count, a decreased hematocrit, cigarette smoking, and the need for home oxygen therapy.
Data source: A prospective observational cohort study assessing numerous risk factors in 16,084 CABG patients.
Disclosures: This study was funded in part by the U.S. Agency for Healthcare Research and Quality, Blue Cross and Blue Shield of Michigan, and Blue Care Network. The authors’ financial disclosures were not provided.
FDA approves generic version of Tamiflu
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
Influenza: A vaccine we love to hate
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.
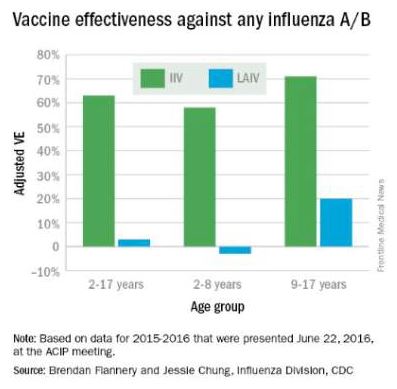
ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
ACIP votes to scrap LAIV vaccine for 2016-2017 influenza season
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices has voted to scrap the use of the live attenuated influenza vaccine for the 2016-2017 flu season.
ACIP’s interim recommendation guidance to the CDC is that “no live attenuated influenza vaccines (LAIV) should be used in any setting,” after reviewing data showing that for three consecutive influenza seasons, LAIV’s vaccine effectiveness (VE) against any flu virus was 3% (95% confidence interval, -49%-37%) in children aged 2-17 years. Meanwhile, injectable inactivated influenza vaccine (IIV) had a VE estimate of 63% (95% CI, 52%-72%) against any flu virus in that age group. Findings were similar across age groups.
CDC epidemiologist Brendan Flannery, Ph.D., painted a damning picture of the intranasal LAIV, presenting preliminary data from the U.S. Flu Vaccine Effectiveness Network for 2015-2016 that showed quadrivalent LAIV offered children “no significant protection against influenza A(H1N1)pdm09.” He also presented 2015-2016 national cohort data from Finland showing that unadjusted vaccine effectiveness (VE) against the H1N1 strain for LAIV in 2-year-olds was 47%, compared with 78% for IIV. Dr. Flannery also cited a series of other U.S. and international studies that did not support the use of LAIV, including unpublished Department of Defense 2015-2016 influenza season data for military families indicating that VE for LAIV against the strain last season was “insignificant” in children aged 2-17 years.
Data from an industry study of the quadrivalent LAIV FluMist (MedImmune) also indicated that in this cohort, for the 2015-2016 influenza season, LAIV underperformed compared with IIV, 46% vs. 65%.
Still more bad news for LAIV came from an English study published online just as the ACIP meeting was getting underway. Those data showed that from October 2015 through May 2016, LAIV in children aged 2-17 years had a VE of 57.6% against influenza A and B.
Debate over whether to pull support for LAIV entirely or to allow the use of it in certain circumstances – such as when a person declined a flu shot – hinged on the projected upheaval pulling LAIV is likely to cause.
Concerns were raised around what would happen in the case of LAIV vaccine orders already placed, over whether pulling LAIV would mean a shortage of other vaccine alternatives, and how communicating contingencies might only confuse the public, but several committee members spoke forcefully in favor of what they said the data compelled them to do.
“The science simply shows that LAIV has not worked for the past 3 years, whereas IIV has,” American Academy of Pediatrics Infectious Disease Committee’s Red Book editor, and AAP liaison to ACIP, Dr. David Kimberlin said before the vote.
Restricting but not prohibiting LAIV also would have allowed for an easier transition back to LAIV if the vaccine were successfully reformulated, ACIP Influenza Work Group Chair Dr. Ruth Karron said while presenting potential policy recommendations.
During the debate over whether to pull or limit support for the inhaled vaccine, Dr. Karron reminded the committee that at the time the Food and Drug Administration approved LAIV, serum antibody responses to both the tri- and quadrivalent vaccine were modest, prompting the FDA to call for effectiveness studies. “So, my question to the FDA now is, ‘What are their plans?’ ”
“We’ve seen the studies, but we’ve not really reviewed all the data,” Dr. Wellington Sun, the FDA’s ACIP liaison responded. “I think we have to acknowledge that LAIV has offered advantages over IIV in the past. At this point, we’re not ready to undertake a program for changing the prescriber’s information. We want to continue to work with MedImmune to find out the root cause of this phenomenon.”
“We’ve increased our research into understanding the biology of the H1N1pdm09 LAIV strains so we can improve their effectiveness in future seasons,” Dr. Chris Ambrose, vice president of U.S. medical affairs for infectious disease at MedImmune’s parent company AstraZeneca, told the committee.
“I think this is a very sad day for the influenza vaccination program,” ACIP chair Dr. Nancy Bennett said before adjourning the meeting. “We all had great hopes. It’s not over. If you’ve seen 1 influenza year, you’ve seen 1 influenza year. We may be back here next year having a very different discussion.”
The CDC is not obligated to adopt ACIP recommendations, but typically does. The committee also accepted a resolution to update the Vaccines for Children program in accordance with the recommendation to deny support for LAIV this flu season.
Thirteen members of the committee voted in favor of the recommendation. Dr. Edward Belongia abstained citing a conflict of interest, and Ms. Cynthia Pelligrini voted no, citing “insufficient time to consider this information.” The ICICLE trial NCT01997450, was sponsored by MedImmune, a subsidiary of AstraZeneca where Dr. Ambrose is vice president of U.S. medical affairs for infectious disease.
On Twitter @whitneymcknight
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices has voted to scrap the use of the live attenuated influenza vaccine for the 2016-2017 flu season.
ACIP’s interim recommendation guidance to the CDC is that “no live attenuated influenza vaccines (LAIV) should be used in any setting,” after reviewing data showing that for three consecutive influenza seasons, LAIV’s vaccine effectiveness (VE) against any flu virus was 3% (95% confidence interval, -49%-37%) in children aged 2-17 years. Meanwhile, injectable inactivated influenza vaccine (IIV) had a VE estimate of 63% (95% CI, 52%-72%) against any flu virus in that age group. Findings were similar across age groups.
CDC epidemiologist Brendan Flannery, Ph.D., painted a damning picture of the intranasal LAIV, presenting preliminary data from the U.S. Flu Vaccine Effectiveness Network for 2015-2016 that showed quadrivalent LAIV offered children “no significant protection against influenza A(H1N1)pdm09.” He also presented 2015-2016 national cohort data from Finland showing that unadjusted vaccine effectiveness (VE) against the H1N1 strain for LAIV in 2-year-olds was 47%, compared with 78% for IIV. Dr. Flannery also cited a series of other U.S. and international studies that did not support the use of LAIV, including unpublished Department of Defense 2015-2016 influenza season data for military families indicating that VE for LAIV against the strain last season was “insignificant” in children aged 2-17 years.
Data from an industry study of the quadrivalent LAIV FluMist (MedImmune) also indicated that in this cohort, for the 2015-2016 influenza season, LAIV underperformed compared with IIV, 46% vs. 65%.
Still more bad news for LAIV came from an English study published online just as the ACIP meeting was getting underway. Those data showed that from October 2015 through May 2016, LAIV in children aged 2-17 years had a VE of 57.6% against influenza A and B.
Debate over whether to pull support for LAIV entirely or to allow the use of it in certain circumstances – such as when a person declined a flu shot – hinged on the projected upheaval pulling LAIV is likely to cause.
Concerns were raised around what would happen in the case of LAIV vaccine orders already placed, over whether pulling LAIV would mean a shortage of other vaccine alternatives, and how communicating contingencies might only confuse the public, but several committee members spoke forcefully in favor of what they said the data compelled them to do.
“The science simply shows that LAIV has not worked for the past 3 years, whereas IIV has,” American Academy of Pediatrics Infectious Disease Committee’s Red Book editor, and AAP liaison to ACIP, Dr. David Kimberlin said before the vote.
Restricting but not prohibiting LAIV also would have allowed for an easier transition back to LAIV if the vaccine were successfully reformulated, ACIP Influenza Work Group Chair Dr. Ruth Karron said while presenting potential policy recommendations.
During the debate over whether to pull or limit support for the inhaled vaccine, Dr. Karron reminded the committee that at the time the Food and Drug Administration approved LAIV, serum antibody responses to both the tri- and quadrivalent vaccine were modest, prompting the FDA to call for effectiveness studies. “So, my question to the FDA now is, ‘What are their plans?’ ”
“We’ve seen the studies, but we’ve not really reviewed all the data,” Dr. Wellington Sun, the FDA’s ACIP liaison responded. “I think we have to acknowledge that LAIV has offered advantages over IIV in the past. At this point, we’re not ready to undertake a program for changing the prescriber’s information. We want to continue to work with MedImmune to find out the root cause of this phenomenon.”
“We’ve increased our research into understanding the biology of the H1N1pdm09 LAIV strains so we can improve their effectiveness in future seasons,” Dr. Chris Ambrose, vice president of U.S. medical affairs for infectious disease at MedImmune’s parent company AstraZeneca, told the committee.
“I think this is a very sad day for the influenza vaccination program,” ACIP chair Dr. Nancy Bennett said before adjourning the meeting. “We all had great hopes. It’s not over. If you’ve seen 1 influenza year, you’ve seen 1 influenza year. We may be back here next year having a very different discussion.”
The CDC is not obligated to adopt ACIP recommendations, but typically does. The committee also accepted a resolution to update the Vaccines for Children program in accordance with the recommendation to deny support for LAIV this flu season.
Thirteen members of the committee voted in favor of the recommendation. Dr. Edward Belongia abstained citing a conflict of interest, and Ms. Cynthia Pelligrini voted no, citing “insufficient time to consider this information.” The ICICLE trial NCT01997450, was sponsored by MedImmune, a subsidiary of AstraZeneca where Dr. Ambrose is vice president of U.S. medical affairs for infectious disease.
On Twitter @whitneymcknight
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices has voted to scrap the use of the live attenuated influenza vaccine for the 2016-2017 flu season.
ACIP’s interim recommendation guidance to the CDC is that “no live attenuated influenza vaccines (LAIV) should be used in any setting,” after reviewing data showing that for three consecutive influenza seasons, LAIV’s vaccine effectiveness (VE) against any flu virus was 3% (95% confidence interval, -49%-37%) in children aged 2-17 years. Meanwhile, injectable inactivated influenza vaccine (IIV) had a VE estimate of 63% (95% CI, 52%-72%) against any flu virus in that age group. Findings were similar across age groups.
CDC epidemiologist Brendan Flannery, Ph.D., painted a damning picture of the intranasal LAIV, presenting preliminary data from the U.S. Flu Vaccine Effectiveness Network for 2015-2016 that showed quadrivalent LAIV offered children “no significant protection against influenza A(H1N1)pdm09.” He also presented 2015-2016 national cohort data from Finland showing that unadjusted vaccine effectiveness (VE) against the H1N1 strain for LAIV in 2-year-olds was 47%, compared with 78% for IIV. Dr. Flannery also cited a series of other U.S. and international studies that did not support the use of LAIV, including unpublished Department of Defense 2015-2016 influenza season data for military families indicating that VE for LAIV against the strain last season was “insignificant” in children aged 2-17 years.
Data from an industry study of the quadrivalent LAIV FluMist (MedImmune) also indicated that in this cohort, for the 2015-2016 influenza season, LAIV underperformed compared with IIV, 46% vs. 65%.
Still more bad news for LAIV came from an English study published online just as the ACIP meeting was getting underway. Those data showed that from October 2015 through May 2016, LAIV in children aged 2-17 years had a VE of 57.6% against influenza A and B.
Debate over whether to pull support for LAIV entirely or to allow the use of it in certain circumstances – such as when a person declined a flu shot – hinged on the projected upheaval pulling LAIV is likely to cause.
Concerns were raised around what would happen in the case of LAIV vaccine orders already placed, over whether pulling LAIV would mean a shortage of other vaccine alternatives, and how communicating contingencies might only confuse the public, but several committee members spoke forcefully in favor of what they said the data compelled them to do.
“The science simply shows that LAIV has not worked for the past 3 years, whereas IIV has,” American Academy of Pediatrics Infectious Disease Committee’s Red Book editor, and AAP liaison to ACIP, Dr. David Kimberlin said before the vote.
Restricting but not prohibiting LAIV also would have allowed for an easier transition back to LAIV if the vaccine were successfully reformulated, ACIP Influenza Work Group Chair Dr. Ruth Karron said while presenting potential policy recommendations.
During the debate over whether to pull or limit support for the inhaled vaccine, Dr. Karron reminded the committee that at the time the Food and Drug Administration approved LAIV, serum antibody responses to both the tri- and quadrivalent vaccine were modest, prompting the FDA to call for effectiveness studies. “So, my question to the FDA now is, ‘What are their plans?’ ”
“We’ve seen the studies, but we’ve not really reviewed all the data,” Dr. Wellington Sun, the FDA’s ACIP liaison responded. “I think we have to acknowledge that LAIV has offered advantages over IIV in the past. At this point, we’re not ready to undertake a program for changing the prescriber’s information. We want to continue to work with MedImmune to find out the root cause of this phenomenon.”
“We’ve increased our research into understanding the biology of the H1N1pdm09 LAIV strains so we can improve their effectiveness in future seasons,” Dr. Chris Ambrose, vice president of U.S. medical affairs for infectious disease at MedImmune’s parent company AstraZeneca, told the committee.
“I think this is a very sad day for the influenza vaccination program,” ACIP chair Dr. Nancy Bennett said before adjourning the meeting. “We all had great hopes. It’s not over. If you’ve seen 1 influenza year, you’ve seen 1 influenza year. We may be back here next year having a very different discussion.”
The CDC is not obligated to adopt ACIP recommendations, but typically does. The committee also accepted a resolution to update the Vaccines for Children program in accordance with the recommendation to deny support for LAIV this flu season.
Thirteen members of the committee voted in favor of the recommendation. Dr. Edward Belongia abstained citing a conflict of interest, and Ms. Cynthia Pelligrini voted no, citing “insufficient time to consider this information.” The ICICLE trial NCT01997450, was sponsored by MedImmune, a subsidiary of AstraZeneca where Dr. Ambrose is vice president of U.S. medical affairs for infectious disease.
On Twitter @whitneymcknight
FROM AN ACIP MEETING