User login
Acute Abdominal Pain
Left Testicular Pain and Swelling
Rheumatoid Arthritis
In rheumatoid arthritis, imaging often helps contribute to early diagnosis, and can assess response to treatment. But all forms of imaging are not equal in RA.
While both ultrasound and MRI can visualize synovitis and erosions, only MRI can show osteitis.
Dr. Norman B. Gaylis, a practicing rheumatologist in Aventura, Fla., and the president of International Society of Extremity MRI in Rheumatology (ISEMIR), has been using imaging in his practice for many years.
He has a small-magnet (0.27 T) MRI extremity scanner, which he uses not only to diagnose RA but also to monitor patient response to treatment.
He also uses in-office ultrasound. “I think MRI and ultrasound are very different modalities. Everyone tries to compare them … but that's not how they're meant to be used. They should complement each other, not be alternatives.”
Here are Dr. Gaylis' thoughts on how different imaging modalities can help to better manage RA.
MRI
MRI is useful for much more than simply making an early diagnosis. MRI is also a powerful tool for helping to manage, maintain, and adjust a patient's treatment regimen.
We have 10 years' experience using MRI to evaluate and monitor rheumatoid arthritis changes. The detection of erosions that are not present on x-ray is not the exclusive reason for using MRI. Visualization of bone marrow edema is equally important, Dr. Gaylis noted.
In fact, osteitis and synovitis “are almost markers for disease activity in RA,” particularly in patients who appear to be free of inflammation by other indicators (sedimentation rates, CRP levels, and subjective perception of wellness). “On MRI, the presence of synovitis or osteitis may be a reason to continue treatment,” said Dr. Gaylis.
On the other hand, when no osteitis, synovitis, or active erosions are visible using MRI, it suggests the time has come to discontinue biologic treatment. If, on repeat MRI in 6-12 months, there are no signs of active disease, the patient is in remission. On the other hand, if there are signs of disease activity on repeat MRI, biologic treatment needs to be restarted.
It's generally adequate to repeat MRI scans on a yearly basis, regardless of the treatment. However, clinical or serologic findings may prompt rescanning sooner.
Some patients who are clinically and serologically in remission with no progression on MRI for several years of observation may not require continued yearly imaging, Dr. Gaylis said.
Getting paid for performing MRI scans for RA remains a challenge, largely because of the American College of Rheumatology white paper, “Evaluation of Low Field Extremity Magnetic Resonance Imaging (MRI),” which has been used as a basis to deny reimbursement.
In May 2008, however, the ACR issued a letter (see resources box), in which the organization stated that insurance companies should not be using the white paper to deny coverage or reimbursement for MRI scans to evaluate RA. “It's clear that MRI imaging, both high field and low field, is more sensitive than plain x-ray in detection of erosions and osteitis,” the letter stated.
“One still has to recognize that this is a problem with some insurance companies. There are other insurance companies that are very up to date with the literature,” he said. For the majority of insurance companies with which Dr. Gaylis works, “once I have documented the reason why, they are actually reimbursing.”
In-office MRI can be attractive to some rheumatologists. “The bulk of [RA] patients who need an MRI in a community environment would have to be referred to an MRI center,” said Dr. Gaylis. These facilities have a number of drawbacks. The first is scheduling, because RA patients must compete with patients from a number of other specialties.
In addition, these facilities typically use large, whole-body, high-field scanners. Positioning is very uncomfortable for RA patients in these large machines, which require hands above the head.
These scans are usually read by radiologists who do not have specific musculoskeletal training. These radiologists “are never going to have the ability to interpret and understand the nuances of RA findings to the same extent as someone who is a board-certified musculoskeletal radiologist, who is reading the scans of 100 RA patients a week,” he said.
Ultrasound
Ultrasound “definitely does help us see syno-vitis,” said Dr. Gaylis. “We know that clinically there may be no evidence of synovitis and yet in many cases the Doppler ultrasound imaging is going to be positive.” Ultrasound is very helpful in following disease activity by measuring synovial inflammation.
Ultrasound is a good option when you're not sure what is going on with your patient and you need to know right away if there is inflammation that needs to be addressed. “We use it in the in-between periods for that reason,” he said.
Ultrasound's convenience makes it an attractive imaging modality. “The beauty of ultrasound is that you can pull out the ultrasound machine while the patient is in the office. You can look at a few joints in the hand or the wrist or the elbow. You don't have to go through the process of MRI,” said Dr. Gaylis.
Ultrasound also can be used to visualize erosions, though this is very dependent on the experience of the technician and viewer. “Having experience in ultrasound is something that is critical for the management of these patients.”
Dr. Gaylis has an ultrasound technician in his office. He finds that it is easier to read the image if someone else is handling the technical component. He and the technologist will discuss the reading while it's being done. However, many rheumatologists perform their own ultrasound imaging.
It's almost mandatory to go to ultrasound courses to learn how to perform and read. He recommends 6 months of ultrasound practice without billing, just to become comfortable with the technique.
X-Ray
“X-rays don't help me much if at all in the diagnosis and management of rheumatoid arthritis, especially in the peripheral joints,” said Dr. Gaylis. X-ray doesn't help rheumatologists make biologic treatment choices for their patients with RA. “Changes occur so slowly on x-ray that it really just doesn't fit the rhythm of biologic therapy,” he said.
X-rays allow visualization of erosions and joint space narrowing. “I think if you were doing a SHARP score on every patient, then x-rays would have more validity because then you would be looking at joint-space narrowing and erosions in many joints. But that's a measurement used primarily in research alone and is totally impractical in clinical practice,” he said.
To view a video interview of Dr. Gaylis, go to
http://www.youtube.com/watch?v=8Q6iRPzJ3a8
Ultrasound is a good option when you're not sure what is going on with your patient and you need to know right away. DR. GAYLIS
T1 (left) and STIR imaging (right) reveal a profound, diffusely abnormal signal consistent with osteitis (arrows).
Near complete resolution of osteitis throughout the carpal bones and metacarpal bases (arrows) can be seen. Images courtesy Dr. Steven D. Needell
Resources
▸ ACR letter addressed to insurance companies clarifying the college's position on extremity MRI for rheumatologic conditions:
http://rheumatology.org/practice/advocacy/asc/letters/index.asp
▸ International Society of Extremity MRI in Rheumatology:
▸ American College of Radiology meetings:
www.acr.org/SecondaryMainMenuCategories/
Meetings andEvents.aspx
▸ American College of Radiology accreditation:
www.acr.org/accreditation/mri/mri_reqs.aspx
▸ Intersocietal Commission for the Accreditation of Magnetic Resonance Laboratories:
In rheumatoid arthritis, imaging often helps contribute to early diagnosis, and can assess response to treatment. But all forms of imaging are not equal in RA.
While both ultrasound and MRI can visualize synovitis and erosions, only MRI can show osteitis.
Dr. Norman B. Gaylis, a practicing rheumatologist in Aventura, Fla., and the president of International Society of Extremity MRI in Rheumatology (ISEMIR), has been using imaging in his practice for many years.
He has a small-magnet (0.27 T) MRI extremity scanner, which he uses not only to diagnose RA but also to monitor patient response to treatment.
He also uses in-office ultrasound. “I think MRI and ultrasound are very different modalities. Everyone tries to compare them … but that's not how they're meant to be used. They should complement each other, not be alternatives.”
Here are Dr. Gaylis' thoughts on how different imaging modalities can help to better manage RA.
MRI
MRI is useful for much more than simply making an early diagnosis. MRI is also a powerful tool for helping to manage, maintain, and adjust a patient's treatment regimen.
We have 10 years' experience using MRI to evaluate and monitor rheumatoid arthritis changes. The detection of erosions that are not present on x-ray is not the exclusive reason for using MRI. Visualization of bone marrow edema is equally important, Dr. Gaylis noted.
In fact, osteitis and synovitis “are almost markers for disease activity in RA,” particularly in patients who appear to be free of inflammation by other indicators (sedimentation rates, CRP levels, and subjective perception of wellness). “On MRI, the presence of synovitis or osteitis may be a reason to continue treatment,” said Dr. Gaylis.
On the other hand, when no osteitis, synovitis, or active erosions are visible using MRI, it suggests the time has come to discontinue biologic treatment. If, on repeat MRI in 6-12 months, there are no signs of active disease, the patient is in remission. On the other hand, if there are signs of disease activity on repeat MRI, biologic treatment needs to be restarted.
It's generally adequate to repeat MRI scans on a yearly basis, regardless of the treatment. However, clinical or serologic findings may prompt rescanning sooner.
Some patients who are clinically and serologically in remission with no progression on MRI for several years of observation may not require continued yearly imaging, Dr. Gaylis said.
Getting paid for performing MRI scans for RA remains a challenge, largely because of the American College of Rheumatology white paper, “Evaluation of Low Field Extremity Magnetic Resonance Imaging (MRI),” which has been used as a basis to deny reimbursement.
In May 2008, however, the ACR issued a letter (see resources box), in which the organization stated that insurance companies should not be using the white paper to deny coverage or reimbursement for MRI scans to evaluate RA. “It's clear that MRI imaging, both high field and low field, is more sensitive than plain x-ray in detection of erosions and osteitis,” the letter stated.
“One still has to recognize that this is a problem with some insurance companies. There are other insurance companies that are very up to date with the literature,” he said. For the majority of insurance companies with which Dr. Gaylis works, “once I have documented the reason why, they are actually reimbursing.”
In-office MRI can be attractive to some rheumatologists. “The bulk of [RA] patients who need an MRI in a community environment would have to be referred to an MRI center,” said Dr. Gaylis. These facilities have a number of drawbacks. The first is scheduling, because RA patients must compete with patients from a number of other specialties.
In addition, these facilities typically use large, whole-body, high-field scanners. Positioning is very uncomfortable for RA patients in these large machines, which require hands above the head.
These scans are usually read by radiologists who do not have specific musculoskeletal training. These radiologists “are never going to have the ability to interpret and understand the nuances of RA findings to the same extent as someone who is a board-certified musculoskeletal radiologist, who is reading the scans of 100 RA patients a week,” he said.
Ultrasound
Ultrasound “definitely does help us see syno-vitis,” said Dr. Gaylis. “We know that clinically there may be no evidence of synovitis and yet in many cases the Doppler ultrasound imaging is going to be positive.” Ultrasound is very helpful in following disease activity by measuring synovial inflammation.
Ultrasound is a good option when you're not sure what is going on with your patient and you need to know right away if there is inflammation that needs to be addressed. “We use it in the in-between periods for that reason,” he said.
Ultrasound's convenience makes it an attractive imaging modality. “The beauty of ultrasound is that you can pull out the ultrasound machine while the patient is in the office. You can look at a few joints in the hand or the wrist or the elbow. You don't have to go through the process of MRI,” said Dr. Gaylis.
Ultrasound also can be used to visualize erosions, though this is very dependent on the experience of the technician and viewer. “Having experience in ultrasound is something that is critical for the management of these patients.”
Dr. Gaylis has an ultrasound technician in his office. He finds that it is easier to read the image if someone else is handling the technical component. He and the technologist will discuss the reading while it's being done. However, many rheumatologists perform their own ultrasound imaging.
It's almost mandatory to go to ultrasound courses to learn how to perform and read. He recommends 6 months of ultrasound practice without billing, just to become comfortable with the technique.
X-Ray
“X-rays don't help me much if at all in the diagnosis and management of rheumatoid arthritis, especially in the peripheral joints,” said Dr. Gaylis. X-ray doesn't help rheumatologists make biologic treatment choices for their patients with RA. “Changes occur so slowly on x-ray that it really just doesn't fit the rhythm of biologic therapy,” he said.
X-rays allow visualization of erosions and joint space narrowing. “I think if you were doing a SHARP score on every patient, then x-rays would have more validity because then you would be looking at joint-space narrowing and erosions in many joints. But that's a measurement used primarily in research alone and is totally impractical in clinical practice,” he said.
To view a video interview of Dr. Gaylis, go to
http://www.youtube.com/watch?v=8Q6iRPzJ3a8
Ultrasound is a good option when you're not sure what is going on with your patient and you need to know right away. DR. GAYLIS
T1 (left) and STIR imaging (right) reveal a profound, diffusely abnormal signal consistent with osteitis (arrows).
Near complete resolution of osteitis throughout the carpal bones and metacarpal bases (arrows) can be seen. Images courtesy Dr. Steven D. Needell
Resources
▸ ACR letter addressed to insurance companies clarifying the college's position on extremity MRI for rheumatologic conditions:
http://rheumatology.org/practice/advocacy/asc/letters/index.asp
▸ International Society of Extremity MRI in Rheumatology:
▸ American College of Radiology meetings:
www.acr.org/SecondaryMainMenuCategories/
Meetings andEvents.aspx
▸ American College of Radiology accreditation:
www.acr.org/accreditation/mri/mri_reqs.aspx
▸ Intersocietal Commission for the Accreditation of Magnetic Resonance Laboratories:
In rheumatoid arthritis, imaging often helps contribute to early diagnosis, and can assess response to treatment. But all forms of imaging are not equal in RA.
While both ultrasound and MRI can visualize synovitis and erosions, only MRI can show osteitis.
Dr. Norman B. Gaylis, a practicing rheumatologist in Aventura, Fla., and the president of International Society of Extremity MRI in Rheumatology (ISEMIR), has been using imaging in his practice for many years.
He has a small-magnet (0.27 T) MRI extremity scanner, which he uses not only to diagnose RA but also to monitor patient response to treatment.
He also uses in-office ultrasound. “I think MRI and ultrasound are very different modalities. Everyone tries to compare them … but that's not how they're meant to be used. They should complement each other, not be alternatives.”
Here are Dr. Gaylis' thoughts on how different imaging modalities can help to better manage RA.
MRI
MRI is useful for much more than simply making an early diagnosis. MRI is also a powerful tool for helping to manage, maintain, and adjust a patient's treatment regimen.
We have 10 years' experience using MRI to evaluate and monitor rheumatoid arthritis changes. The detection of erosions that are not present on x-ray is not the exclusive reason for using MRI. Visualization of bone marrow edema is equally important, Dr. Gaylis noted.
In fact, osteitis and synovitis “are almost markers for disease activity in RA,” particularly in patients who appear to be free of inflammation by other indicators (sedimentation rates, CRP levels, and subjective perception of wellness). “On MRI, the presence of synovitis or osteitis may be a reason to continue treatment,” said Dr. Gaylis.
On the other hand, when no osteitis, synovitis, or active erosions are visible using MRI, it suggests the time has come to discontinue biologic treatment. If, on repeat MRI in 6-12 months, there are no signs of active disease, the patient is in remission. On the other hand, if there are signs of disease activity on repeat MRI, biologic treatment needs to be restarted.
It's generally adequate to repeat MRI scans on a yearly basis, regardless of the treatment. However, clinical or serologic findings may prompt rescanning sooner.
Some patients who are clinically and serologically in remission with no progression on MRI for several years of observation may not require continued yearly imaging, Dr. Gaylis said.
Getting paid for performing MRI scans for RA remains a challenge, largely because of the American College of Rheumatology white paper, “Evaluation of Low Field Extremity Magnetic Resonance Imaging (MRI),” which has been used as a basis to deny reimbursement.
In May 2008, however, the ACR issued a letter (see resources box), in which the organization stated that insurance companies should not be using the white paper to deny coverage or reimbursement for MRI scans to evaluate RA. “It's clear that MRI imaging, both high field and low field, is more sensitive than plain x-ray in detection of erosions and osteitis,” the letter stated.
“One still has to recognize that this is a problem with some insurance companies. There are other insurance companies that are very up to date with the literature,” he said. For the majority of insurance companies with which Dr. Gaylis works, “once I have documented the reason why, they are actually reimbursing.”
In-office MRI can be attractive to some rheumatologists. “The bulk of [RA] patients who need an MRI in a community environment would have to be referred to an MRI center,” said Dr. Gaylis. These facilities have a number of drawbacks. The first is scheduling, because RA patients must compete with patients from a number of other specialties.
In addition, these facilities typically use large, whole-body, high-field scanners. Positioning is very uncomfortable for RA patients in these large machines, which require hands above the head.
These scans are usually read by radiologists who do not have specific musculoskeletal training. These radiologists “are never going to have the ability to interpret and understand the nuances of RA findings to the same extent as someone who is a board-certified musculoskeletal radiologist, who is reading the scans of 100 RA patients a week,” he said.
Ultrasound
Ultrasound “definitely does help us see syno-vitis,” said Dr. Gaylis. “We know that clinically there may be no evidence of synovitis and yet in many cases the Doppler ultrasound imaging is going to be positive.” Ultrasound is very helpful in following disease activity by measuring synovial inflammation.
Ultrasound is a good option when you're not sure what is going on with your patient and you need to know right away if there is inflammation that needs to be addressed. “We use it in the in-between periods for that reason,” he said.
Ultrasound's convenience makes it an attractive imaging modality. “The beauty of ultrasound is that you can pull out the ultrasound machine while the patient is in the office. You can look at a few joints in the hand or the wrist or the elbow. You don't have to go through the process of MRI,” said Dr. Gaylis.
Ultrasound also can be used to visualize erosions, though this is very dependent on the experience of the technician and viewer. “Having experience in ultrasound is something that is critical for the management of these patients.”
Dr. Gaylis has an ultrasound technician in his office. He finds that it is easier to read the image if someone else is handling the technical component. He and the technologist will discuss the reading while it's being done. However, many rheumatologists perform their own ultrasound imaging.
It's almost mandatory to go to ultrasound courses to learn how to perform and read. He recommends 6 months of ultrasound practice without billing, just to become comfortable with the technique.
X-Ray
“X-rays don't help me much if at all in the diagnosis and management of rheumatoid arthritis, especially in the peripheral joints,” said Dr. Gaylis. X-ray doesn't help rheumatologists make biologic treatment choices for their patients with RA. “Changes occur so slowly on x-ray that it really just doesn't fit the rhythm of biologic therapy,” he said.
X-rays allow visualization of erosions and joint space narrowing. “I think if you were doing a SHARP score on every patient, then x-rays would have more validity because then you would be looking at joint-space narrowing and erosions in many joints. But that's a measurement used primarily in research alone and is totally impractical in clinical practice,” he said.
To view a video interview of Dr. Gaylis, go to
http://www.youtube.com/watch?v=8Q6iRPzJ3a8
Ultrasound is a good option when you're not sure what is going on with your patient and you need to know right away. DR. GAYLIS
T1 (left) and STIR imaging (right) reveal a profound, diffusely abnormal signal consistent with osteitis (arrows).
Near complete resolution of osteitis throughout the carpal bones and metacarpal bases (arrows) can be seen. Images courtesy Dr. Steven D. Needell
Resources
▸ ACR letter addressed to insurance companies clarifying the college's position on extremity MRI for rheumatologic conditions:
http://rheumatology.org/practice/advocacy/asc/letters/index.asp
▸ International Society of Extremity MRI in Rheumatology:
▸ American College of Radiology meetings:
www.acr.org/SecondaryMainMenuCategories/
Meetings andEvents.aspx
▸ American College of Radiology accreditation:
www.acr.org/accreditation/mri/mri_reqs.aspx
▸ Intersocietal Commission for the Accreditation of Magnetic Resonance Laboratories:
Acetabular Labral Tears
CTA Sees Plaque in Patients With Low Clinical Risk
BOSTON — Direct screening for atherosclerosis using CT coronary angiography may provide a more accurate cardiovascular risk picture than do routine clinical predictors, including the Framingham risk score.
However, the value of the imaging method in asymptomatic patients must be demonstrated in clinical trials before it can be used to modify therapy, said Dr. Benjamin Chow at the annual meeting of the American Society of Nuclear Cardiology.
Dr. Chow and associates conducted an imaging study that was designed to determine the prevalence of coronary atherosclerosis in patients with varying clinical predictors, as well as to identify the limitations of traditional cardiac risk factors for predicting individual atherosclerotic burden using computed tomographic angiography (CTA).
The study revealed evidence of calcific and noncalcific coronary atherosclerosis in a cohort of consecutive patients with low to intermediate Framingham risk scores (FRS).
This finding, together with the absence of atherosclerotic plaques in some patients with high FRS, suggests that the use of routine clinical predictors may be insufficient for identifying patients who might benefit from aggressive risk factor modification, Dr. Chow reported.
Of 1,247 consecutive patients who underwent CTA at the University of Ottawa (Ont.) Heart Institute between February 2006 and March 2008, Dr. Chow and his coinvestigators identified 554 patients (mean age, 55 years) who did not have a history of myocardial infarction, revascularization, or diabetes mellitus, and who were not on current statin therapy. Approximately half of the patients were men, and the mean body mass index was 28.5 kg/m
Using a 17-segment model of the coronary arteries to assess for the presence of calcific or noncalcific plaque, the investigators calculated a total plaque score by summing the number of coronary segments with visible atherosclerotic plaque. They calculated the FRS using age, sex, total cholesterol, high-density lipoprotein cholesterol, smoking history, and blood pressure.
Based on the FRS, 408 of the patients were considered to have a very low (5% or less) or low (10% or less) 10-year risk for cardiac events, whereas 93 patients had an intermediate risk (11%–19%) and 53 were considered high risk (20% or greater), said Dr. Chow. Of the patients in the very-low- and low-risk groups, more than half had visible evidence of atherosclerotic plaque on CTA, he said. Additionally, about 9% of patients in the high-risk category had no evidence of calcific or noncalcific plaques.
“Although the mean atherosclerotic plaque burden did increase with the 10-year Framingham risk, the correlation between [the FRS] and plaque was fair,” Dr. Chow reported. The findings suggest that, although the FRS is moderately predictive of plaque burden in this patient population, “it may underestimate total plaque burden,” he said.
The value of identifying subclinical coronary atherosclerosis through CTA has yet to be established in clinical trials, said Dr. Chow of the institute. “Although many would argue that more aggressive risk-factor modification is warranted for patients with evidence of coronary atherosclerosis, prospective studies are needed to determine whether modifying therapy [based on imaging evidence] is appropriate.”
Currently, the main suggested indication for CTA is for symptomatic patients or those with equivocal stress tests, Dr. Chow noted. CTA “is not currently indicated to screen for coronary atherosclerosis because the benefit of doing so has yet to be [proved].”
Dr. Chow reported no conflicts of interest with respect to his presentation.
BOSTON — Direct screening for atherosclerosis using CT coronary angiography may provide a more accurate cardiovascular risk picture than do routine clinical predictors, including the Framingham risk score.
However, the value of the imaging method in asymptomatic patients must be demonstrated in clinical trials before it can be used to modify therapy, said Dr. Benjamin Chow at the annual meeting of the American Society of Nuclear Cardiology.
Dr. Chow and associates conducted an imaging study that was designed to determine the prevalence of coronary atherosclerosis in patients with varying clinical predictors, as well as to identify the limitations of traditional cardiac risk factors for predicting individual atherosclerotic burden using computed tomographic angiography (CTA).
The study revealed evidence of calcific and noncalcific coronary atherosclerosis in a cohort of consecutive patients with low to intermediate Framingham risk scores (FRS).
This finding, together with the absence of atherosclerotic plaques in some patients with high FRS, suggests that the use of routine clinical predictors may be insufficient for identifying patients who might benefit from aggressive risk factor modification, Dr. Chow reported.
Of 1,247 consecutive patients who underwent CTA at the University of Ottawa (Ont.) Heart Institute between February 2006 and March 2008, Dr. Chow and his coinvestigators identified 554 patients (mean age, 55 years) who did not have a history of myocardial infarction, revascularization, or diabetes mellitus, and who were not on current statin therapy. Approximately half of the patients were men, and the mean body mass index was 28.5 kg/m
Using a 17-segment model of the coronary arteries to assess for the presence of calcific or noncalcific plaque, the investigators calculated a total plaque score by summing the number of coronary segments with visible atherosclerotic plaque. They calculated the FRS using age, sex, total cholesterol, high-density lipoprotein cholesterol, smoking history, and blood pressure.
Based on the FRS, 408 of the patients were considered to have a very low (5% or less) or low (10% or less) 10-year risk for cardiac events, whereas 93 patients had an intermediate risk (11%–19%) and 53 were considered high risk (20% or greater), said Dr. Chow. Of the patients in the very-low- and low-risk groups, more than half had visible evidence of atherosclerotic plaque on CTA, he said. Additionally, about 9% of patients in the high-risk category had no evidence of calcific or noncalcific plaques.
“Although the mean atherosclerotic plaque burden did increase with the 10-year Framingham risk, the correlation between [the FRS] and plaque was fair,” Dr. Chow reported. The findings suggest that, although the FRS is moderately predictive of plaque burden in this patient population, “it may underestimate total plaque burden,” he said.
The value of identifying subclinical coronary atherosclerosis through CTA has yet to be established in clinical trials, said Dr. Chow of the institute. “Although many would argue that more aggressive risk-factor modification is warranted for patients with evidence of coronary atherosclerosis, prospective studies are needed to determine whether modifying therapy [based on imaging evidence] is appropriate.”
Currently, the main suggested indication for CTA is for symptomatic patients or those with equivocal stress tests, Dr. Chow noted. CTA “is not currently indicated to screen for coronary atherosclerosis because the benefit of doing so has yet to be [proved].”
Dr. Chow reported no conflicts of interest with respect to his presentation.
BOSTON — Direct screening for atherosclerosis using CT coronary angiography may provide a more accurate cardiovascular risk picture than do routine clinical predictors, including the Framingham risk score.
However, the value of the imaging method in asymptomatic patients must be demonstrated in clinical trials before it can be used to modify therapy, said Dr. Benjamin Chow at the annual meeting of the American Society of Nuclear Cardiology.
Dr. Chow and associates conducted an imaging study that was designed to determine the prevalence of coronary atherosclerosis in patients with varying clinical predictors, as well as to identify the limitations of traditional cardiac risk factors for predicting individual atherosclerotic burden using computed tomographic angiography (CTA).
The study revealed evidence of calcific and noncalcific coronary atherosclerosis in a cohort of consecutive patients with low to intermediate Framingham risk scores (FRS).
This finding, together with the absence of atherosclerotic plaques in some patients with high FRS, suggests that the use of routine clinical predictors may be insufficient for identifying patients who might benefit from aggressive risk factor modification, Dr. Chow reported.
Of 1,247 consecutive patients who underwent CTA at the University of Ottawa (Ont.) Heart Institute between February 2006 and March 2008, Dr. Chow and his coinvestigators identified 554 patients (mean age, 55 years) who did not have a history of myocardial infarction, revascularization, or diabetes mellitus, and who were not on current statin therapy. Approximately half of the patients were men, and the mean body mass index was 28.5 kg/m
Using a 17-segment model of the coronary arteries to assess for the presence of calcific or noncalcific plaque, the investigators calculated a total plaque score by summing the number of coronary segments with visible atherosclerotic plaque. They calculated the FRS using age, sex, total cholesterol, high-density lipoprotein cholesterol, smoking history, and blood pressure.
Based on the FRS, 408 of the patients were considered to have a very low (5% or less) or low (10% or less) 10-year risk for cardiac events, whereas 93 patients had an intermediate risk (11%–19%) and 53 were considered high risk (20% or greater), said Dr. Chow. Of the patients in the very-low- and low-risk groups, more than half had visible evidence of atherosclerotic plaque on CTA, he said. Additionally, about 9% of patients in the high-risk category had no evidence of calcific or noncalcific plaques.
“Although the mean atherosclerotic plaque burden did increase with the 10-year Framingham risk, the correlation between [the FRS] and plaque was fair,” Dr. Chow reported. The findings suggest that, although the FRS is moderately predictive of plaque burden in this patient population, “it may underestimate total plaque burden,” he said.
The value of identifying subclinical coronary atherosclerosis through CTA has yet to be established in clinical trials, said Dr. Chow of the institute. “Although many would argue that more aggressive risk-factor modification is warranted for patients with evidence of coronary atherosclerosis, prospective studies are needed to determine whether modifying therapy [based on imaging evidence] is appropriate.”
Currently, the main suggested indication for CTA is for symptomatic patients or those with equivocal stress tests, Dr. Chow noted. CTA “is not currently indicated to screen for coronary atherosclerosis because the benefit of doing so has yet to be [proved].”
Dr. Chow reported no conflicts of interest with respect to his presentation.
Evaluation and management of pituitary incidentalomas
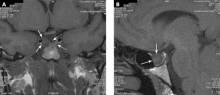
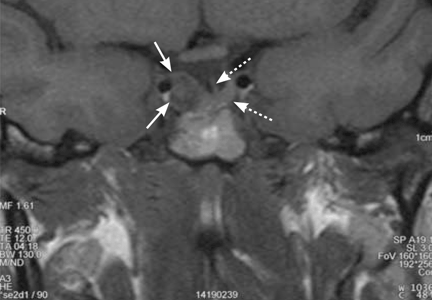
A 39-year-old woman is referred for evaluation of a pituitary mass, which was found on magnetic resonance imaging (MRI) performed because of persistent vertigo. The mass, measuring 1.1 by 1.0 cm, arises from the right portion of the sella turcica and does not reach the optic chiasm (Figure 1). It appears hypointense on MRI and enhances after contrast is given, suggesting it is a pituitary adenoma.
On physical examination she does not have any stigmata of Cushing syndrome or of acromegaly. Her blood pressure is 116/72 mm Hg and her heart rate is regular at 68 beats per minute. Her visual fields are normal as assessed by confrontation, and she has no galactorrhea.
How should this patient be evaluated?
BY DEFINITION, INCIDENTALOMAS ARE UNSUSPECTED
Pituitary “incidentalomas” are, by definition, masses that are discovered by computed tomography (CT) or MRI performed to evaluate unrelated disorders (such as head trauma), for cancer staging, or because of nonspecific symptoms such as dizziness and headache. In some series, headache was the most common reason for imaging studies that led to the discovery of pituitary incidentalomas.1
With more patients undergoing computed tomography (CT) and MRI, more incidentalomas are being discovered. Incidentally discovered pituitary adenomas accounted for 12% of the pituitary tumors in a series of 353 consecutive patients with a presumptive diagnosis of pituitary tumor at one institution over a 14-year period.2 Pituitary masses other than adenomas are discussed later in this paper.
Microadenomas are common, macroadenomas less so
Autopsy studies have revealed pituitary microadenomas (ie, < 10 mm in greatest dimension) in 3% to 27% of patients with no history of pituitary disorders. Macroadenomas (10 mm or larger), on the other hand, are found in fewer than 0.5% of people.3,4 Recently, a study of MRI in 2,000 healthy adult volunteers, age 45 to 97 years, found pituitary macroadenomas in 0.3%.5
Hall et al6 found that 10% of relatively young (< 60 years old) healthy volunteers harbored a pituitary microadenoma on pituitary MRI, but none had a macroadenoma. In a meta-analysis by Ezzat and colleagues,3 adenomas of all sizes were found in 1% to 40% of imaging or postmortem studies (for an average of 16.7%), but macroadenomas were found in only 0.16% to 0.2% of the population.
Although the natural history of pituitary incidentalomas is not well characterized, the numbers suggest that microadenomas rarely grow into macroadenomas.7 Another possibility is that most macroadenomas cause symptoms and therefore come to clinical attention, and thus are not incidentalomas per se.
THE INITIAL EVALUATION: TWO QUESTIONS
The initial approach to a patient with a pituitary incidentaloma should be guided by two questions:
- Is the tumor hormonally active?
- Is it causing a mass effect (ie, is it exerting pressure on adjacent structures)?
IS THE TUMOR HORMONALLY ACTIVE?
A careful history and physical examination may reveal overlooked symptoms or signs of hypersecretion of a specific hormone, which can be evaluated in detail to establish the diagnosis. However, most patients with pituitary incidentalomas have no symptoms, and for them there is no real consensus about the optimal workup strategy.
Prolactin excess
King et al8 calculated that the serum prolactin level is the single most cost-effective screening test for hormonal activity in patients with incidentally discovered pituitary microadenomas. They also suggested, however, that it may be cost-effective to measure multiple hormones in very anxious patients, since a negative test may provide reassurance and improve quality of life.
One should be careful in interpreting elevated prolactin levels in patients with pituitary incidentalomas, since a number of medications (eg, metoclopramide [Reglan], verapamil [Calan], phenothiazines) and disorders (eg, hypothyroidism, cirrhosis, renal failure) can cause mild to moderate elevations of prolactin. In general, a prolactin level of more than 200 ng/mL is almost always diagnostic of prolactinomas. In our experience, a prolactin level above 100 ng/mL is almost always due to a prolactin-secreting pituitary adenoma, except during pregnancy and in some patients who receive antipsychotics or metoclopramide. For these patients, if it is clinically safe to hold or switch medications, retesting after a drug holiday may prove useful and diagnostic.
Growth hormone excess
Growth hormone hypersecretion has been reported in patients with pituitary tumors who have no clinical stigmata of acromegaly.9,10 Moreover, acral changes may not correlate with the metabolic consequences of growth hormone excess.11 In a study by Reincke et al,12 one of 18 patients with pituitary incidentalomas and no apparent acromegalic features had a growth hormone-secreting pituitary adenoma. For this reason, looking for so-called silent growth hormone hypersecretion may be warranted in patients with pituitary tumors, especially in those with macroadenomas.9
The best initial test for growth hormone hypersecretion is the measurement of insulin-like growth factor-1 (IGF-1).13 A normal age- and sex-adjusted IGF-1 level almost always rules out acromegaly.
Further hormonal evaluation
Further hormonal evaluation should be guided by the clinical picture.
Cortisol. In a patient with excess weight gain, central obesity, proximal myopathy, and skin manifestations that suggest hypercortisolism, appropriate initial tests would be a midnight salivary cortisol level, an overnight 1-mg (low-dose) dexamethasone suppression test, or a 24-hour urinary free cortisol level.
Thyroid hormones. Patients with symptoms that suggest hyperthyroidism should have their thyroid-stimulating hormone (TSH; thyrotropin) and free thyroxine (T4) levels measured to rule out a TSH-secreting pituitary adenoma, a very rare tumor.
Gonadotropins. Screening for a gonadotropin-secreting pituitary adenoma by measuring follicle-stimulating hormone, luteinizing hormone, and gonadotropin alpha subunit is not routinely indicated, since almost all of such tumors are clinically silent and generally come to clinical attention only because of a mass effect (see below).
IS THERE A MASS EFFECT?
Pituitary macroadenomas can also cause problems via a mass effect. Examples: hypopituitarism, visual field defects (by compressing the optic chiasm), cranial neuropathy (eg, diplopia, eyelid ptosis secondary to lateral extension of the tumor into a cavernous sinus), and headache.
Hypopituitarism
Hypopituitarism can range from deficiency of one pituitary hormone to the loss of all anterior pituitary hormones (panhypopituitarism).
Hypopituitarism from a mass effect is rare in patients with microadenomas, but one or more anterior pituitary hormone deficiencies are found in more than 30% of patients with a pituitary macroadenoma.3,12,14 With some exceptions, including pituitary apoplexy, the loss of pituitary hormone secretion is slowly progressive; symptoms tend to be nonspecific and often are not noticed at first.
Increased intrasellar pressure may play a role in the pathogenesis of hypopituitarism in patients with pituitary masses.15 Blood flow through the portal vessels is decreased, possibly resulting in diminished delivery of hypo-thalamic hormones to pituitary cells or leading to variable ischemia or necrosis of the normal gland, or both.
All patients with a pituitary macroadenoma should undergo a hormonal evaluation to look for pituitary hormone deficiency.
Growth hormone, gonadotropin deficiencies. In general, pituitary hormone deficiencies from an expanding pituitary tumor tend to begin with growth hormone or the gonadotropins (luteinizing hormone and follicle-stimulating hormone), or both.
Low serum testosterone levels in men (estradiol in women) along with normal or low follicle-stimulating hormone and luteinizing hormone levels are consistent with gonadotropin deficiency in men and amenorrheic premenopausal women.
Failure of the follicle-stimulating hormone and luteinizing hormone levels to rise after menopause is also consistent with gonadotropin deficiency. The presence of regular menses almost always indicates a normal gonadotropin axis. In women with irregular menstruation, hormonal evaluation can be challenging for evaluation of the gonadotropin axis and usually is not indicated.
Patients with deficiencies of two or more pituitary axes and low IGF-1 levels can be presumed to have growth hormone deficiency and usually do not need dynamic testing. But when testing is indicated, the growth hormone axis is best evaluated by dynamic testing, using either a growth hormone-releasing hormone/ arginine stimulation test or the insulin tolerance test.
Thyroid deficiencies. As the tumor expands, deficiencies of thyrotropin and adrenocorticotropic hormone (ACTH) secretion may follow those of growth hormone and gonadotropins. In our experience, the thyrotropin axis is usually affected before the corticotropin axis.
To evaluate the thyrotropin axis, the serum thyrotropin level should be measured along with the free thyroxine level or the free thyroxine index. A low free thyroxine level with a low or normal thyrotropin level is consistent with secondary hypothyroidism. It is inappropriate to measure thyrotropin without also measuring thyroxine in a patient with pituitary disorder, since a normal thyrotropin level in a patient with hypopituitarism is not uncommon.
Adrenal insufficiency. The ACTH stimulation test or an early morning (8 am) plasma cortisol level are both reasonable initial tests to evaluate the hypothalamic-pituitary-adrenal axis. An early morning cortisol level lower than 3 μg/dL confirms adrenal insufficiency, while a value higher than 15 μg/dL makes the diagnosis highly unlikely. Cortisol levels in the range of 3 to 15 μg/dL are indeterminate and should be further evaluated by an ACTH stimulation test, which can be performed anytime during the day.
The standard-dose ACTH stimulation test uses an intravenous or intramuscular injection of 250 μg of cosyntropin (Cortrosyn; ACTH 1–24). A normal response is a plasma cortisol concentration higher than 18 μg/dL at 30 minutes.
The sensitivity of the ACTH stimulation test in detecting mild, partial adrenal insufficiency is higher if a lower dose of cosyntropin is used (1 μg intravenously). However, the low-dose test has a higher false-positive rate. In most clinical situations, the 30-minute cortisol value during a standard-dose ACTH stimulation test has a diagnostic accuracy close to that of the low-dose ACTH stimulation test.16 Patients with recent-onset ACTH deficiency (eg, in pituitary apoplexy or within 2 to 4 weeks following pituitary surgery) may have a normal response to the ACTH stimulation test, since their adrenal glands have not undergone sufficient atrophy and still respond to ACTH stimulation.
The insulin tolerance test is considered the gold standard for evaluating the hypothalamic-pituitary-adrenal axis, but it needs to be performed by an experienced clinician and is usually not needed for everyday clinical practice.
Visual field defects
Visual field loss generally begins in the superior temporal fields, which explains why the patient may not notice it at first. Then, with continued growth and compression, vision loss extends into the inferior temporal fields, then into the nasal fields as a late effect.
Because the patient may not notice the visual field defect, formal visual field testing is warranted if the tumor compresses or abuts the optic chiasm. While bitemporal hemianopia is the classic manifestation of chiasmal compression, variable visual field defects may occur depending on which portion of the optic apparatus is involved.
Cranial neuropathy
Abnormal eye movements, which may cause diplopia, result from extension of a pituitary tumor into one or both cavernous sinuses. Compression of the third (occulomotor, the cranial nerve most often affected), fourth (trochlear), and sixth (abducens) cranial nerves leads to eye movement deviations as well as eyelid ptosis due to third nerve dysfunction. Cranial neuropathy most commonly occurs in the setting of pituitary apoplexy (see below) but may occur without it.
Headache
Headache can be associated with pituitary tumors, but the underlying pathophysiology remains uncertain. Possible mechanisms include structural causes such as dural stretching or cavernous sinus invasion.17 Other possible mechanisms are an increase in the intrasellar pressure and tumor activity.15,18 The link between headache and tumor activity is supported by the observation that headaches resolve in some patients with acromegaly shortly after they start taking somatostatin analogues.19
Migraine may be the most common type of headache reported in patients with pituitary adenomas; however, short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) has also been reported.19
Of interest, there seems to be a strong association between pituitary-associated headache and a family history of headache.19 That said, headache is a common symptom in the general population, and establishing a cause-and-effect relationship prior to surgical removal of a pituitary tumor can be challenging. Approximately 50% of patients with headache who undergo an operation for a pituitary tumor have relief after surgery; however, 35% may not have relief, and up to 15% have a worsening of their headaches.19
OUR PATIENT’S HORMONAL EVALUATION
In the patient we described earlier, hormonal evaluation revealed the following:
- Prolactin 12.2 ng/mL (reference range 2–17.4)
- IGF-1 189 ng/mL (114–492)
- Thyrotropin 1.63 μU/mL (0.4–5.5)
- Free thyroxine index 9.5 μg/L (6–11)
- Maximum cortisol during a low-dose ACTH stimulation test 18.4 μg/dL. In short, all her test results were normal.
A formal visual field test was not performed, since the pituitary mass did not reach the optic chiasm (Figure 1).
ADENOMAS VS OTHER SELLAR MASSES
In some cases, it may be difficult to distinguish a nonadenomatous lesion from a nonfunctioning pituitary adenoma. However, several endocrine, radiographic, and neurologic features may help to differentiate pituitary tumors from other, less common sellar disorders.20
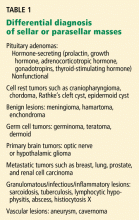
For instance, diabetes insipidus is extremely rare in patients with pituitary adenomas at presentation without significant suprasellar extension of the tumor. Therefore, its presence strongly suggests a nonpituitary cause such as hypophysitis, sarcoidosis, or a meta-static lesion.21
Some radiographic features that suggest sellar masses other than pituitary tumors include calcifications on CT in patients with craniopharyngiomas and meningiomas or a rapidly enlarging mass with lack of sellar enlargement (sellar remodeling), which suggests a metastatic lesion. While a dural tail sign (a linear enhanced structure or “tail” extending away from the tumor mass along the dural surface) may be seen with some meningiomas, peripheral enhancement of the dura is not specific for meningioma and may be seen with pituitary apoplexy as well.22,23
Cranial neuropathy is less common in patients with pituitary adenomas than in those with nonadenomatous masses (for example a metastasis or a meningioma), although the acute onset of cranial neuropathy often accompanies a hemorrhagic infarction of a preexisting pituitary adenoma (pituitary apoplexy).20
OUR RECOMMENDATIONS
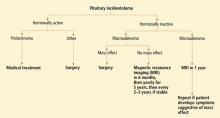
Our approach to a patient with a pituitary incidentaloma is summarized in Figure 4.
If the tumor is hormonally active
Prolactinoma is the exception. For this tumor, dopamine agonists can resolve symptoms and shrink the tumor in most cases. Even in patients with a visual field defect associated with a macroprolactinoma, vision usually improves within days after starting a dopamine agonist, before the tumor has observably shrunk. However, a follow-up visual field test is necessary 2 to 6 weeks after starting therapy to establish that the tumor is responding to therapy; if the tumor does not respond, surgery may be necessary.
If the tumor is hormonally inactive
If the tumor is hormonally inactive, its further evaluation depends on its size and whether there is a mass effect. In patients with a nonfunctioning pituitary macroadenoma, a comprehensive hormonal evaluation for hypopituitarism should be done. Patients with a visual field defect or cranial neuropathy should undergo surgical tumor resection. If there is no mass effect, observation may be an acceptable strategy. We, and others,1,25 recommend surgery for most patients with pituitary macroadenomas abutting the optic chiasm.
If the tumor is small
If the tumor is small (ie, a microadenoma), the risk of its growing is low. Three small studies followed such patients prospectively and found a 0 to 14% risk of tumor enlargement over a mean follow-up period of 1.8 to 6.7 years.12,25,26 While there is no consensus about how soon to follow up patients with nonfunctioning pituitary microadenomas, we obtain a follow-up MRI study in 1 year, with no further routine imaging if the tumor has remained stable, unless the patient develops symptoms or signs suggesting a mass effect.
If the tumor is large
If the tumor is large (ie, a macroadenoma), the risk of further growth is expected to be higher, since the tumor has already shown the propensity to grow. In the same three series discussed above, the risk of tumor growth for a pituitary macroadenomas was about 30% over the mean follow-up of 1.8 to 6.7 years.12,25,26
Furthermore, several recent studies have suggested a higher propensity to grow and to cause symptoms and signs than previously thought. For example, Karavitaki et al7 studied 24 patients who had nonfunctional macroadenomas and found that the 48-month probability of enlargement was 44%; of this group, 57% showed new or worsening visual field defects, and an additional 21% showed new chiasmatic compression without vision loss. Similarly, Arita and colleagues27 found that 21 (50%) of 42 nonfunctional adenomas (mean size 18.3 ± 7 mm) increased by at least 10% over an average of 32 months after the initial evaluation. Ten patients became symptomatic over a mean of about 5 years, with 4 of these 10 (9.5% of the entire cohort) suffering symptomatic pituitary apoplexy. Therefore, one may argue for surgery (especially in young patients) for pituitary macroadenomas even in the absence of mass effect.
We would obtain a follow-up MRI study at 6 months, then yearly for 5 years, and then every 2 to 3 years if the tumor is stable. Surgery would be indicated if there is evidence of tumor growth or a mass effect.
While tumor growth has been found to be independent of age in some studies,27 others have found longer tumor doubling time in patients older than 60 years.28
The risk of pituitary apoplexy
Pituitary apoplexy results from a hemorrhagic infarction of the tumor and manifests clinically as the sudden onset of severe headache, nausea, vomiting, vision loss, and cranial nerve palsies. While most cases of pituitary apoplexy are spontaneous, precipitating factors may include head injury, anticoagulant therapy, dopamine agonists, radiation therapy, or dynamic endocrine tests.29
It is important to educate patients and their families about the symptoms of pituitary apoplexy, especially patients with pituitary macroadenomas. If the condition is unrecognized and untreated, patients can develop hypotension and shock secondary to adrenal insufficiency, as well as irreversible vision loss or diplopia.
Surgery is generally recommended in cases of progressive vision loss or cranial neuropathy, preferably within 24 or 48 hours of onset if feasible, to minimize the risk of a permanent neurologic deficit.
Clinically significant pituitary apoplexy is rare in patients with pituitary microadenomas. In the study by Arita et al,27 the risk of pituitary apoplexy during 5 years of follow-up was 9.5%, and all of the tumors involved were macroadenomas. This rate is higher than in some other studies, in which the risk of apoplexy ranged from 0.4% to 7% during a mean follow-up of 2 to 6 years.1,25,30
CASE FOLLOW-UP
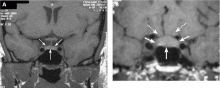
Since our patient had no evidence of hormonal hypersecretion or mass effect and no hypopituitarism, we asked her to return in 6 months. A repeat MRI study showed the tumor to be stable, with no evidence of growth. The patient was scheduled for a return visit in 1 year.
- Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol 2003; 149:123–127.
- Gsponer J, De Tribolet N, Déruaz JP, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (Baltimore) 1999; 78:236–269.
- Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer 2004; 101:613–619.
- Molitch ME, Russell EJ. The pituitary “incidentaloma.” Ann Intern Med 1990; 112:925–931.
- Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357:1821–1828.
- Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994; 120:817–820.
- Karavitaki N, Collison K, Halliday J, et al. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clin Endocrinol (Oxf) 2007; 67:938–943.
- King JT, Justice AC, Aron DC. Management of incidental pituitary microadenomas: a cost-effectiveness analysis. J Clin Endocrinol Metab 1997; 82:3625–3632.
- Klibanski A, Zervas NT, Kovacs K, Ridgway EC. Clinically silent hypersecretion of growth hormone in patients with pituitary tumors. J Neurosurg 1987; 66:806–811.
- Trouillas J, Sassolas G, Loras B, et al. Somatotropic adenomas without acromegaly. Pathol Res Pract 1991; 187:943–949.
- Cryer PE, Daughaday WH. Regulation of growth hormone secretion in acromegaly. J Clin Endocrinol Metab 1969; 29:386–393.
- Reincke M, Allolio B, Saeger W, Menzel J, Winkelmann W. The ‘incidentaloma’ of the pituitary gland. Is neurosurgery required? JAMA 1990; 263:2772–2776.
- Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000; 85:526–529.
- Nammour GM, Ybarra J, Naheedy MH, Romeo JH, Aron DC. Incidental pituitary macroadenoma: a population-based study. Am J Med Sci 1997; 314:287–291.
- Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab 2000; 85:1789–1793.
- Mayenknecht J, Diederich S, Bahr V, Plockinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab 1998; 83:1558–1562.
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology 1993; 43:1678–1683.
- Abe T, Matsumoto K, Kuwazawa J, Toyoda I, Sasaki K. Headache associated with pituitary adenomas. Headache 1998; 38:782–786.
- Levy MJ, Matharu MS, Meeran K, Powell M, Goadsby PJ. The clinical characteristics of headache in patients with pituitary tumours. Brain 2005; 128:1921–1930.
- Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am 1999; 28:81–117.
- Gopan T, Toms SA, Prayson RA, Suh JH, Hamrahian AH, Weil RJ. Symptomatic pituitary metastases from renal cell carcinoma. Pituitary 2007; 10:251–259.
- Moore AF, Grinspoon SK. A dural tale. J Clin Endocrinol Metab 2007; 92:3367–3368.
- Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007; 27:525–551.
- Chanson P, Daujat F, Young J, et al. Normal pituitary hypertrophy as a frequent cause of pituitary incidentaloma: a follow-up study. J Clin Endocrinol Metab 2001; 86:3009–3015.
- Donovan LE, Corenblum B. The natural history of the pituitary incidentaloma. Arch Intern Med 1995; 155:181–183.
- Feldkamp J, Santen R, Harms E, Aulich A, Modder U, Scherbaum WA. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas—results of a prospective study. Clin Endocrinol (Oxf) 1999; 51:109–113.
- Arita K, Tominaga A, Sugiyama K, et al. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg 2006; 104:884–891.
- Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 2003; 98:359–365.
- Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry 2001; 71:542–545.
- Nishizawa S, Ohta S, Yokoyama T, Uemura K. Therapeutic strategy for incidentally found pituitary tumors (“pituitary incidentalomas”). Neurosurgery 1998; 43:1344–1348.
A 39-year-old woman is referred for evaluation of a pituitary mass, which was found on magnetic resonance imaging (MRI) performed because of persistent vertigo. The mass, measuring 1.1 by 1.0 cm, arises from the right portion of the sella turcica and does not reach the optic chiasm (Figure 1). It appears hypointense on MRI and enhances after contrast is given, suggesting it is a pituitary adenoma.
On physical examination she does not have any stigmata of Cushing syndrome or of acromegaly. Her blood pressure is 116/72 mm Hg and her heart rate is regular at 68 beats per minute. Her visual fields are normal as assessed by confrontation, and she has no galactorrhea.
How should this patient be evaluated?
BY DEFINITION, INCIDENTALOMAS ARE UNSUSPECTED
Pituitary “incidentalomas” are, by definition, masses that are discovered by computed tomography (CT) or MRI performed to evaluate unrelated disorders (such as head trauma), for cancer staging, or because of nonspecific symptoms such as dizziness and headache. In some series, headache was the most common reason for imaging studies that led to the discovery of pituitary incidentalomas.1
With more patients undergoing computed tomography (CT) and MRI, more incidentalomas are being discovered. Incidentally discovered pituitary adenomas accounted for 12% of the pituitary tumors in a series of 353 consecutive patients with a presumptive diagnosis of pituitary tumor at one institution over a 14-year period.2 Pituitary masses other than adenomas are discussed later in this paper.
Microadenomas are common, macroadenomas less so
Autopsy studies have revealed pituitary microadenomas (ie, < 10 mm in greatest dimension) in 3% to 27% of patients with no history of pituitary disorders. Macroadenomas (10 mm or larger), on the other hand, are found in fewer than 0.5% of people.3,4 Recently, a study of MRI in 2,000 healthy adult volunteers, age 45 to 97 years, found pituitary macroadenomas in 0.3%.5
Hall et al6 found that 10% of relatively young (< 60 years old) healthy volunteers harbored a pituitary microadenoma on pituitary MRI, but none had a macroadenoma. In a meta-analysis by Ezzat and colleagues,3 adenomas of all sizes were found in 1% to 40% of imaging or postmortem studies (for an average of 16.7%), but macroadenomas were found in only 0.16% to 0.2% of the population.
Although the natural history of pituitary incidentalomas is not well characterized, the numbers suggest that microadenomas rarely grow into macroadenomas.7 Another possibility is that most macroadenomas cause symptoms and therefore come to clinical attention, and thus are not incidentalomas per se.
THE INITIAL EVALUATION: TWO QUESTIONS
The initial approach to a patient with a pituitary incidentaloma should be guided by two questions:
- Is the tumor hormonally active?
- Is it causing a mass effect (ie, is it exerting pressure on adjacent structures)?
IS THE TUMOR HORMONALLY ACTIVE?
A careful history and physical examination may reveal overlooked symptoms or signs of hypersecretion of a specific hormone, which can be evaluated in detail to establish the diagnosis. However, most patients with pituitary incidentalomas have no symptoms, and for them there is no real consensus about the optimal workup strategy.
Prolactin excess
King et al8 calculated that the serum prolactin level is the single most cost-effective screening test for hormonal activity in patients with incidentally discovered pituitary microadenomas. They also suggested, however, that it may be cost-effective to measure multiple hormones in very anxious patients, since a negative test may provide reassurance and improve quality of life.
One should be careful in interpreting elevated prolactin levels in patients with pituitary incidentalomas, since a number of medications (eg, metoclopramide [Reglan], verapamil [Calan], phenothiazines) and disorders (eg, hypothyroidism, cirrhosis, renal failure) can cause mild to moderate elevations of prolactin. In general, a prolactin level of more than 200 ng/mL is almost always diagnostic of prolactinomas. In our experience, a prolactin level above 100 ng/mL is almost always due to a prolactin-secreting pituitary adenoma, except during pregnancy and in some patients who receive antipsychotics or metoclopramide. For these patients, if it is clinically safe to hold or switch medications, retesting after a drug holiday may prove useful and diagnostic.
Growth hormone excess
Growth hormone hypersecretion has been reported in patients with pituitary tumors who have no clinical stigmata of acromegaly.9,10 Moreover, acral changes may not correlate with the metabolic consequences of growth hormone excess.11 In a study by Reincke et al,12 one of 18 patients with pituitary incidentalomas and no apparent acromegalic features had a growth hormone-secreting pituitary adenoma. For this reason, looking for so-called silent growth hormone hypersecretion may be warranted in patients with pituitary tumors, especially in those with macroadenomas.9
The best initial test for growth hormone hypersecretion is the measurement of insulin-like growth factor-1 (IGF-1).13 A normal age- and sex-adjusted IGF-1 level almost always rules out acromegaly.
Further hormonal evaluation
Further hormonal evaluation should be guided by the clinical picture.
Cortisol. In a patient with excess weight gain, central obesity, proximal myopathy, and skin manifestations that suggest hypercortisolism, appropriate initial tests would be a midnight salivary cortisol level, an overnight 1-mg (low-dose) dexamethasone suppression test, or a 24-hour urinary free cortisol level.
Thyroid hormones. Patients with symptoms that suggest hyperthyroidism should have their thyroid-stimulating hormone (TSH; thyrotropin) and free thyroxine (T4) levels measured to rule out a TSH-secreting pituitary adenoma, a very rare tumor.
Gonadotropins. Screening for a gonadotropin-secreting pituitary adenoma by measuring follicle-stimulating hormone, luteinizing hormone, and gonadotropin alpha subunit is not routinely indicated, since almost all of such tumors are clinically silent and generally come to clinical attention only because of a mass effect (see below).
IS THERE A MASS EFFECT?
Pituitary macroadenomas can also cause problems via a mass effect. Examples: hypopituitarism, visual field defects (by compressing the optic chiasm), cranial neuropathy (eg, diplopia, eyelid ptosis secondary to lateral extension of the tumor into a cavernous sinus), and headache.
Hypopituitarism
Hypopituitarism can range from deficiency of one pituitary hormone to the loss of all anterior pituitary hormones (panhypopituitarism).
Hypopituitarism from a mass effect is rare in patients with microadenomas, but one or more anterior pituitary hormone deficiencies are found in more than 30% of patients with a pituitary macroadenoma.3,12,14 With some exceptions, including pituitary apoplexy, the loss of pituitary hormone secretion is slowly progressive; symptoms tend to be nonspecific and often are not noticed at first.
Increased intrasellar pressure may play a role in the pathogenesis of hypopituitarism in patients with pituitary masses.15 Blood flow through the portal vessels is decreased, possibly resulting in diminished delivery of hypo-thalamic hormones to pituitary cells or leading to variable ischemia or necrosis of the normal gland, or both.
All patients with a pituitary macroadenoma should undergo a hormonal evaluation to look for pituitary hormone deficiency.
Growth hormone, gonadotropin deficiencies. In general, pituitary hormone deficiencies from an expanding pituitary tumor tend to begin with growth hormone or the gonadotropins (luteinizing hormone and follicle-stimulating hormone), or both.
Low serum testosterone levels in men (estradiol in women) along with normal or low follicle-stimulating hormone and luteinizing hormone levels are consistent with gonadotropin deficiency in men and amenorrheic premenopausal women.
Failure of the follicle-stimulating hormone and luteinizing hormone levels to rise after menopause is also consistent with gonadotropin deficiency. The presence of regular menses almost always indicates a normal gonadotropin axis. In women with irregular menstruation, hormonal evaluation can be challenging for evaluation of the gonadotropin axis and usually is not indicated.
Patients with deficiencies of two or more pituitary axes and low IGF-1 levels can be presumed to have growth hormone deficiency and usually do not need dynamic testing. But when testing is indicated, the growth hormone axis is best evaluated by dynamic testing, using either a growth hormone-releasing hormone/ arginine stimulation test or the insulin tolerance test.
Thyroid deficiencies. As the tumor expands, deficiencies of thyrotropin and adrenocorticotropic hormone (ACTH) secretion may follow those of growth hormone and gonadotropins. In our experience, the thyrotropin axis is usually affected before the corticotropin axis.
To evaluate the thyrotropin axis, the serum thyrotropin level should be measured along with the free thyroxine level or the free thyroxine index. A low free thyroxine level with a low or normal thyrotropin level is consistent with secondary hypothyroidism. It is inappropriate to measure thyrotropin without also measuring thyroxine in a patient with pituitary disorder, since a normal thyrotropin level in a patient with hypopituitarism is not uncommon.
Adrenal insufficiency. The ACTH stimulation test or an early morning (8 am) plasma cortisol level are both reasonable initial tests to evaluate the hypothalamic-pituitary-adrenal axis. An early morning cortisol level lower than 3 μg/dL confirms adrenal insufficiency, while a value higher than 15 μg/dL makes the diagnosis highly unlikely. Cortisol levels in the range of 3 to 15 μg/dL are indeterminate and should be further evaluated by an ACTH stimulation test, which can be performed anytime during the day.
The standard-dose ACTH stimulation test uses an intravenous or intramuscular injection of 250 μg of cosyntropin (Cortrosyn; ACTH 1–24). A normal response is a plasma cortisol concentration higher than 18 μg/dL at 30 minutes.
The sensitivity of the ACTH stimulation test in detecting mild, partial adrenal insufficiency is higher if a lower dose of cosyntropin is used (1 μg intravenously). However, the low-dose test has a higher false-positive rate. In most clinical situations, the 30-minute cortisol value during a standard-dose ACTH stimulation test has a diagnostic accuracy close to that of the low-dose ACTH stimulation test.16 Patients with recent-onset ACTH deficiency (eg, in pituitary apoplexy or within 2 to 4 weeks following pituitary surgery) may have a normal response to the ACTH stimulation test, since their adrenal glands have not undergone sufficient atrophy and still respond to ACTH stimulation.
The insulin tolerance test is considered the gold standard for evaluating the hypothalamic-pituitary-adrenal axis, but it needs to be performed by an experienced clinician and is usually not needed for everyday clinical practice.
Visual field defects
Visual field loss generally begins in the superior temporal fields, which explains why the patient may not notice it at first. Then, with continued growth and compression, vision loss extends into the inferior temporal fields, then into the nasal fields as a late effect.
Because the patient may not notice the visual field defect, formal visual field testing is warranted if the tumor compresses or abuts the optic chiasm. While bitemporal hemianopia is the classic manifestation of chiasmal compression, variable visual field defects may occur depending on which portion of the optic apparatus is involved.
Cranial neuropathy
Abnormal eye movements, which may cause diplopia, result from extension of a pituitary tumor into one or both cavernous sinuses. Compression of the third (occulomotor, the cranial nerve most often affected), fourth (trochlear), and sixth (abducens) cranial nerves leads to eye movement deviations as well as eyelid ptosis due to third nerve dysfunction. Cranial neuropathy most commonly occurs in the setting of pituitary apoplexy (see below) but may occur without it.
Headache
Headache can be associated with pituitary tumors, but the underlying pathophysiology remains uncertain. Possible mechanisms include structural causes such as dural stretching or cavernous sinus invasion.17 Other possible mechanisms are an increase in the intrasellar pressure and tumor activity.15,18 The link between headache and tumor activity is supported by the observation that headaches resolve in some patients with acromegaly shortly after they start taking somatostatin analogues.19
Migraine may be the most common type of headache reported in patients with pituitary adenomas; however, short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) has also been reported.19
Of interest, there seems to be a strong association between pituitary-associated headache and a family history of headache.19 That said, headache is a common symptom in the general population, and establishing a cause-and-effect relationship prior to surgical removal of a pituitary tumor can be challenging. Approximately 50% of patients with headache who undergo an operation for a pituitary tumor have relief after surgery; however, 35% may not have relief, and up to 15% have a worsening of their headaches.19
OUR PATIENT’S HORMONAL EVALUATION
In the patient we described earlier, hormonal evaluation revealed the following:
- Prolactin 12.2 ng/mL (reference range 2–17.4)
- IGF-1 189 ng/mL (114–492)
- Thyrotropin 1.63 μU/mL (0.4–5.5)
- Free thyroxine index 9.5 μg/L (6–11)
- Maximum cortisol during a low-dose ACTH stimulation test 18.4 μg/dL. In short, all her test results were normal.
A formal visual field test was not performed, since the pituitary mass did not reach the optic chiasm (Figure 1).
ADENOMAS VS OTHER SELLAR MASSES
In some cases, it may be difficult to distinguish a nonadenomatous lesion from a nonfunctioning pituitary adenoma. However, several endocrine, radiographic, and neurologic features may help to differentiate pituitary tumors from other, less common sellar disorders.20
For instance, diabetes insipidus is extremely rare in patients with pituitary adenomas at presentation without significant suprasellar extension of the tumor. Therefore, its presence strongly suggests a nonpituitary cause such as hypophysitis, sarcoidosis, or a meta-static lesion.21
Some radiographic features that suggest sellar masses other than pituitary tumors include calcifications on CT in patients with craniopharyngiomas and meningiomas or a rapidly enlarging mass with lack of sellar enlargement (sellar remodeling), which suggests a metastatic lesion. While a dural tail sign (a linear enhanced structure or “tail” extending away from the tumor mass along the dural surface) may be seen with some meningiomas, peripheral enhancement of the dura is not specific for meningioma and may be seen with pituitary apoplexy as well.22,23
Cranial neuropathy is less common in patients with pituitary adenomas than in those with nonadenomatous masses (for example a metastasis or a meningioma), although the acute onset of cranial neuropathy often accompanies a hemorrhagic infarction of a preexisting pituitary adenoma (pituitary apoplexy).20
OUR RECOMMENDATIONS
Our approach to a patient with a pituitary incidentaloma is summarized in Figure 4.
If the tumor is hormonally active
Prolactinoma is the exception. For this tumor, dopamine agonists can resolve symptoms and shrink the tumor in most cases. Even in patients with a visual field defect associated with a macroprolactinoma, vision usually improves within days after starting a dopamine agonist, before the tumor has observably shrunk. However, a follow-up visual field test is necessary 2 to 6 weeks after starting therapy to establish that the tumor is responding to therapy; if the tumor does not respond, surgery may be necessary.
If the tumor is hormonally inactive
If the tumor is hormonally inactive, its further evaluation depends on its size and whether there is a mass effect. In patients with a nonfunctioning pituitary macroadenoma, a comprehensive hormonal evaluation for hypopituitarism should be done. Patients with a visual field defect or cranial neuropathy should undergo surgical tumor resection. If there is no mass effect, observation may be an acceptable strategy. We, and others,1,25 recommend surgery for most patients with pituitary macroadenomas abutting the optic chiasm.
If the tumor is small
If the tumor is small (ie, a microadenoma), the risk of its growing is low. Three small studies followed such patients prospectively and found a 0 to 14% risk of tumor enlargement over a mean follow-up period of 1.8 to 6.7 years.12,25,26 While there is no consensus about how soon to follow up patients with nonfunctioning pituitary microadenomas, we obtain a follow-up MRI study in 1 year, with no further routine imaging if the tumor has remained stable, unless the patient develops symptoms or signs suggesting a mass effect.
If the tumor is large
If the tumor is large (ie, a macroadenoma), the risk of further growth is expected to be higher, since the tumor has already shown the propensity to grow. In the same three series discussed above, the risk of tumor growth for a pituitary macroadenomas was about 30% over the mean follow-up of 1.8 to 6.7 years.12,25,26
Furthermore, several recent studies have suggested a higher propensity to grow and to cause symptoms and signs than previously thought. For example, Karavitaki et al7 studied 24 patients who had nonfunctional macroadenomas and found that the 48-month probability of enlargement was 44%; of this group, 57% showed new or worsening visual field defects, and an additional 21% showed new chiasmatic compression without vision loss. Similarly, Arita and colleagues27 found that 21 (50%) of 42 nonfunctional adenomas (mean size 18.3 ± 7 mm) increased by at least 10% over an average of 32 months after the initial evaluation. Ten patients became symptomatic over a mean of about 5 years, with 4 of these 10 (9.5% of the entire cohort) suffering symptomatic pituitary apoplexy. Therefore, one may argue for surgery (especially in young patients) for pituitary macroadenomas even in the absence of mass effect.
We would obtain a follow-up MRI study at 6 months, then yearly for 5 years, and then every 2 to 3 years if the tumor is stable. Surgery would be indicated if there is evidence of tumor growth or a mass effect.
While tumor growth has been found to be independent of age in some studies,27 others have found longer tumor doubling time in patients older than 60 years.28
The risk of pituitary apoplexy
Pituitary apoplexy results from a hemorrhagic infarction of the tumor and manifests clinically as the sudden onset of severe headache, nausea, vomiting, vision loss, and cranial nerve palsies. While most cases of pituitary apoplexy are spontaneous, precipitating factors may include head injury, anticoagulant therapy, dopamine agonists, radiation therapy, or dynamic endocrine tests.29
It is important to educate patients and their families about the symptoms of pituitary apoplexy, especially patients with pituitary macroadenomas. If the condition is unrecognized and untreated, patients can develop hypotension and shock secondary to adrenal insufficiency, as well as irreversible vision loss or diplopia.
Surgery is generally recommended in cases of progressive vision loss or cranial neuropathy, preferably within 24 or 48 hours of onset if feasible, to minimize the risk of a permanent neurologic deficit.
Clinically significant pituitary apoplexy is rare in patients with pituitary microadenomas. In the study by Arita et al,27 the risk of pituitary apoplexy during 5 years of follow-up was 9.5%, and all of the tumors involved were macroadenomas. This rate is higher than in some other studies, in which the risk of apoplexy ranged from 0.4% to 7% during a mean follow-up of 2 to 6 years.1,25,30
CASE FOLLOW-UP
Since our patient had no evidence of hormonal hypersecretion or mass effect and no hypopituitarism, we asked her to return in 6 months. A repeat MRI study showed the tumor to be stable, with no evidence of growth. The patient was scheduled for a return visit in 1 year.
A 39-year-old woman is referred for evaluation of a pituitary mass, which was found on magnetic resonance imaging (MRI) performed because of persistent vertigo. The mass, measuring 1.1 by 1.0 cm, arises from the right portion of the sella turcica and does not reach the optic chiasm (Figure 1). It appears hypointense on MRI and enhances after contrast is given, suggesting it is a pituitary adenoma.
On physical examination she does not have any stigmata of Cushing syndrome or of acromegaly. Her blood pressure is 116/72 mm Hg and her heart rate is regular at 68 beats per minute. Her visual fields are normal as assessed by confrontation, and she has no galactorrhea.
How should this patient be evaluated?
BY DEFINITION, INCIDENTALOMAS ARE UNSUSPECTED
Pituitary “incidentalomas” are, by definition, masses that are discovered by computed tomography (CT) or MRI performed to evaluate unrelated disorders (such as head trauma), for cancer staging, or because of nonspecific symptoms such as dizziness and headache. In some series, headache was the most common reason for imaging studies that led to the discovery of pituitary incidentalomas.1
With more patients undergoing computed tomography (CT) and MRI, more incidentalomas are being discovered. Incidentally discovered pituitary adenomas accounted for 12% of the pituitary tumors in a series of 353 consecutive patients with a presumptive diagnosis of pituitary tumor at one institution over a 14-year period.2 Pituitary masses other than adenomas are discussed later in this paper.
Microadenomas are common, macroadenomas less so
Autopsy studies have revealed pituitary microadenomas (ie, < 10 mm in greatest dimension) in 3% to 27% of patients with no history of pituitary disorders. Macroadenomas (10 mm or larger), on the other hand, are found in fewer than 0.5% of people.3,4 Recently, a study of MRI in 2,000 healthy adult volunteers, age 45 to 97 years, found pituitary macroadenomas in 0.3%.5
Hall et al6 found that 10% of relatively young (< 60 years old) healthy volunteers harbored a pituitary microadenoma on pituitary MRI, but none had a macroadenoma. In a meta-analysis by Ezzat and colleagues,3 adenomas of all sizes were found in 1% to 40% of imaging or postmortem studies (for an average of 16.7%), but macroadenomas were found in only 0.16% to 0.2% of the population.
Although the natural history of pituitary incidentalomas is not well characterized, the numbers suggest that microadenomas rarely grow into macroadenomas.7 Another possibility is that most macroadenomas cause symptoms and therefore come to clinical attention, and thus are not incidentalomas per se.
THE INITIAL EVALUATION: TWO QUESTIONS
The initial approach to a patient with a pituitary incidentaloma should be guided by two questions:
- Is the tumor hormonally active?
- Is it causing a mass effect (ie, is it exerting pressure on adjacent structures)?
IS THE TUMOR HORMONALLY ACTIVE?
A careful history and physical examination may reveal overlooked symptoms or signs of hypersecretion of a specific hormone, which can be evaluated in detail to establish the diagnosis. However, most patients with pituitary incidentalomas have no symptoms, and for them there is no real consensus about the optimal workup strategy.
Prolactin excess
King et al8 calculated that the serum prolactin level is the single most cost-effective screening test for hormonal activity in patients with incidentally discovered pituitary microadenomas. They also suggested, however, that it may be cost-effective to measure multiple hormones in very anxious patients, since a negative test may provide reassurance and improve quality of life.
One should be careful in interpreting elevated prolactin levels in patients with pituitary incidentalomas, since a number of medications (eg, metoclopramide [Reglan], verapamil [Calan], phenothiazines) and disorders (eg, hypothyroidism, cirrhosis, renal failure) can cause mild to moderate elevations of prolactin. In general, a prolactin level of more than 200 ng/mL is almost always diagnostic of prolactinomas. In our experience, a prolactin level above 100 ng/mL is almost always due to a prolactin-secreting pituitary adenoma, except during pregnancy and in some patients who receive antipsychotics or metoclopramide. For these patients, if it is clinically safe to hold or switch medications, retesting after a drug holiday may prove useful and diagnostic.
Growth hormone excess
Growth hormone hypersecretion has been reported in patients with pituitary tumors who have no clinical stigmata of acromegaly.9,10 Moreover, acral changes may not correlate with the metabolic consequences of growth hormone excess.11 In a study by Reincke et al,12 one of 18 patients with pituitary incidentalomas and no apparent acromegalic features had a growth hormone-secreting pituitary adenoma. For this reason, looking for so-called silent growth hormone hypersecretion may be warranted in patients with pituitary tumors, especially in those with macroadenomas.9
The best initial test for growth hormone hypersecretion is the measurement of insulin-like growth factor-1 (IGF-1).13 A normal age- and sex-adjusted IGF-1 level almost always rules out acromegaly.
Further hormonal evaluation
Further hormonal evaluation should be guided by the clinical picture.
Cortisol. In a patient with excess weight gain, central obesity, proximal myopathy, and skin manifestations that suggest hypercortisolism, appropriate initial tests would be a midnight salivary cortisol level, an overnight 1-mg (low-dose) dexamethasone suppression test, or a 24-hour urinary free cortisol level.
Thyroid hormones. Patients with symptoms that suggest hyperthyroidism should have their thyroid-stimulating hormone (TSH; thyrotropin) and free thyroxine (T4) levels measured to rule out a TSH-secreting pituitary adenoma, a very rare tumor.
Gonadotropins. Screening for a gonadotropin-secreting pituitary adenoma by measuring follicle-stimulating hormone, luteinizing hormone, and gonadotropin alpha subunit is not routinely indicated, since almost all of such tumors are clinically silent and generally come to clinical attention only because of a mass effect (see below).
IS THERE A MASS EFFECT?
Pituitary macroadenomas can also cause problems via a mass effect. Examples: hypopituitarism, visual field defects (by compressing the optic chiasm), cranial neuropathy (eg, diplopia, eyelid ptosis secondary to lateral extension of the tumor into a cavernous sinus), and headache.
Hypopituitarism
Hypopituitarism can range from deficiency of one pituitary hormone to the loss of all anterior pituitary hormones (panhypopituitarism).
Hypopituitarism from a mass effect is rare in patients with microadenomas, but one or more anterior pituitary hormone deficiencies are found in more than 30% of patients with a pituitary macroadenoma.3,12,14 With some exceptions, including pituitary apoplexy, the loss of pituitary hormone secretion is slowly progressive; symptoms tend to be nonspecific and often are not noticed at first.
Increased intrasellar pressure may play a role in the pathogenesis of hypopituitarism in patients with pituitary masses.15 Blood flow through the portal vessels is decreased, possibly resulting in diminished delivery of hypo-thalamic hormones to pituitary cells or leading to variable ischemia or necrosis of the normal gland, or both.
All patients with a pituitary macroadenoma should undergo a hormonal evaluation to look for pituitary hormone deficiency.
Growth hormone, gonadotropin deficiencies. In general, pituitary hormone deficiencies from an expanding pituitary tumor tend to begin with growth hormone or the gonadotropins (luteinizing hormone and follicle-stimulating hormone), or both.
Low serum testosterone levels in men (estradiol in women) along with normal or low follicle-stimulating hormone and luteinizing hormone levels are consistent with gonadotropin deficiency in men and amenorrheic premenopausal women.
Failure of the follicle-stimulating hormone and luteinizing hormone levels to rise after menopause is also consistent with gonadotropin deficiency. The presence of regular menses almost always indicates a normal gonadotropin axis. In women with irregular menstruation, hormonal evaluation can be challenging for evaluation of the gonadotropin axis and usually is not indicated.
Patients with deficiencies of two or more pituitary axes and low IGF-1 levels can be presumed to have growth hormone deficiency and usually do not need dynamic testing. But when testing is indicated, the growth hormone axis is best evaluated by dynamic testing, using either a growth hormone-releasing hormone/ arginine stimulation test or the insulin tolerance test.
Thyroid deficiencies. As the tumor expands, deficiencies of thyrotropin and adrenocorticotropic hormone (ACTH) secretion may follow those of growth hormone and gonadotropins. In our experience, the thyrotropin axis is usually affected before the corticotropin axis.
To evaluate the thyrotropin axis, the serum thyrotropin level should be measured along with the free thyroxine level or the free thyroxine index. A low free thyroxine level with a low or normal thyrotropin level is consistent with secondary hypothyroidism. It is inappropriate to measure thyrotropin without also measuring thyroxine in a patient with pituitary disorder, since a normal thyrotropin level in a patient with hypopituitarism is not uncommon.
Adrenal insufficiency. The ACTH stimulation test or an early morning (8 am) plasma cortisol level are both reasonable initial tests to evaluate the hypothalamic-pituitary-adrenal axis. An early morning cortisol level lower than 3 μg/dL confirms adrenal insufficiency, while a value higher than 15 μg/dL makes the diagnosis highly unlikely. Cortisol levels in the range of 3 to 15 μg/dL are indeterminate and should be further evaluated by an ACTH stimulation test, which can be performed anytime during the day.
The standard-dose ACTH stimulation test uses an intravenous or intramuscular injection of 250 μg of cosyntropin (Cortrosyn; ACTH 1–24). A normal response is a plasma cortisol concentration higher than 18 μg/dL at 30 minutes.
The sensitivity of the ACTH stimulation test in detecting mild, partial adrenal insufficiency is higher if a lower dose of cosyntropin is used (1 μg intravenously). However, the low-dose test has a higher false-positive rate. In most clinical situations, the 30-minute cortisol value during a standard-dose ACTH stimulation test has a diagnostic accuracy close to that of the low-dose ACTH stimulation test.16 Patients with recent-onset ACTH deficiency (eg, in pituitary apoplexy or within 2 to 4 weeks following pituitary surgery) may have a normal response to the ACTH stimulation test, since their adrenal glands have not undergone sufficient atrophy and still respond to ACTH stimulation.
The insulin tolerance test is considered the gold standard for evaluating the hypothalamic-pituitary-adrenal axis, but it needs to be performed by an experienced clinician and is usually not needed for everyday clinical practice.
Visual field defects
Visual field loss generally begins in the superior temporal fields, which explains why the patient may not notice it at first. Then, with continued growth and compression, vision loss extends into the inferior temporal fields, then into the nasal fields as a late effect.
Because the patient may not notice the visual field defect, formal visual field testing is warranted if the tumor compresses or abuts the optic chiasm. While bitemporal hemianopia is the classic manifestation of chiasmal compression, variable visual field defects may occur depending on which portion of the optic apparatus is involved.
Cranial neuropathy
Abnormal eye movements, which may cause diplopia, result from extension of a pituitary tumor into one or both cavernous sinuses. Compression of the third (occulomotor, the cranial nerve most often affected), fourth (trochlear), and sixth (abducens) cranial nerves leads to eye movement deviations as well as eyelid ptosis due to third nerve dysfunction. Cranial neuropathy most commonly occurs in the setting of pituitary apoplexy (see below) but may occur without it.
Headache
Headache can be associated with pituitary tumors, but the underlying pathophysiology remains uncertain. Possible mechanisms include structural causes such as dural stretching or cavernous sinus invasion.17 Other possible mechanisms are an increase in the intrasellar pressure and tumor activity.15,18 The link between headache and tumor activity is supported by the observation that headaches resolve in some patients with acromegaly shortly after they start taking somatostatin analogues.19
Migraine may be the most common type of headache reported in patients with pituitary adenomas; however, short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) has also been reported.19
Of interest, there seems to be a strong association between pituitary-associated headache and a family history of headache.19 That said, headache is a common symptom in the general population, and establishing a cause-and-effect relationship prior to surgical removal of a pituitary tumor can be challenging. Approximately 50% of patients with headache who undergo an operation for a pituitary tumor have relief after surgery; however, 35% may not have relief, and up to 15% have a worsening of their headaches.19
OUR PATIENT’S HORMONAL EVALUATION
In the patient we described earlier, hormonal evaluation revealed the following:
- Prolactin 12.2 ng/mL (reference range 2–17.4)
- IGF-1 189 ng/mL (114–492)
- Thyrotropin 1.63 μU/mL (0.4–5.5)
- Free thyroxine index 9.5 μg/L (6–11)
- Maximum cortisol during a low-dose ACTH stimulation test 18.4 μg/dL. In short, all her test results were normal.
A formal visual field test was not performed, since the pituitary mass did not reach the optic chiasm (Figure 1).
ADENOMAS VS OTHER SELLAR MASSES
In some cases, it may be difficult to distinguish a nonadenomatous lesion from a nonfunctioning pituitary adenoma. However, several endocrine, radiographic, and neurologic features may help to differentiate pituitary tumors from other, less common sellar disorders.20
For instance, diabetes insipidus is extremely rare in patients with pituitary adenomas at presentation without significant suprasellar extension of the tumor. Therefore, its presence strongly suggests a nonpituitary cause such as hypophysitis, sarcoidosis, or a meta-static lesion.21
Some radiographic features that suggest sellar masses other than pituitary tumors include calcifications on CT in patients with craniopharyngiomas and meningiomas or a rapidly enlarging mass with lack of sellar enlargement (sellar remodeling), which suggests a metastatic lesion. While a dural tail sign (a linear enhanced structure or “tail” extending away from the tumor mass along the dural surface) may be seen with some meningiomas, peripheral enhancement of the dura is not specific for meningioma and may be seen with pituitary apoplexy as well.22,23
Cranial neuropathy is less common in patients with pituitary adenomas than in those with nonadenomatous masses (for example a metastasis or a meningioma), although the acute onset of cranial neuropathy often accompanies a hemorrhagic infarction of a preexisting pituitary adenoma (pituitary apoplexy).20
OUR RECOMMENDATIONS
Our approach to a patient with a pituitary incidentaloma is summarized in Figure 4.
If the tumor is hormonally active
Prolactinoma is the exception. For this tumor, dopamine agonists can resolve symptoms and shrink the tumor in most cases. Even in patients with a visual field defect associated with a macroprolactinoma, vision usually improves within days after starting a dopamine agonist, before the tumor has observably shrunk. However, a follow-up visual field test is necessary 2 to 6 weeks after starting therapy to establish that the tumor is responding to therapy; if the tumor does not respond, surgery may be necessary.
If the tumor is hormonally inactive
If the tumor is hormonally inactive, its further evaluation depends on its size and whether there is a mass effect. In patients with a nonfunctioning pituitary macroadenoma, a comprehensive hormonal evaluation for hypopituitarism should be done. Patients with a visual field defect or cranial neuropathy should undergo surgical tumor resection. If there is no mass effect, observation may be an acceptable strategy. We, and others,1,25 recommend surgery for most patients with pituitary macroadenomas abutting the optic chiasm.
If the tumor is small
If the tumor is small (ie, a microadenoma), the risk of its growing is low. Three small studies followed such patients prospectively and found a 0 to 14% risk of tumor enlargement over a mean follow-up period of 1.8 to 6.7 years.12,25,26 While there is no consensus about how soon to follow up patients with nonfunctioning pituitary microadenomas, we obtain a follow-up MRI study in 1 year, with no further routine imaging if the tumor has remained stable, unless the patient develops symptoms or signs suggesting a mass effect.
If the tumor is large
If the tumor is large (ie, a macroadenoma), the risk of further growth is expected to be higher, since the tumor has already shown the propensity to grow. In the same three series discussed above, the risk of tumor growth for a pituitary macroadenomas was about 30% over the mean follow-up of 1.8 to 6.7 years.12,25,26
Furthermore, several recent studies have suggested a higher propensity to grow and to cause symptoms and signs than previously thought. For example, Karavitaki et al7 studied 24 patients who had nonfunctional macroadenomas and found that the 48-month probability of enlargement was 44%; of this group, 57% showed new or worsening visual field defects, and an additional 21% showed new chiasmatic compression without vision loss. Similarly, Arita and colleagues27 found that 21 (50%) of 42 nonfunctional adenomas (mean size 18.3 ± 7 mm) increased by at least 10% over an average of 32 months after the initial evaluation. Ten patients became symptomatic over a mean of about 5 years, with 4 of these 10 (9.5% of the entire cohort) suffering symptomatic pituitary apoplexy. Therefore, one may argue for surgery (especially in young patients) for pituitary macroadenomas even in the absence of mass effect.
We would obtain a follow-up MRI study at 6 months, then yearly for 5 years, and then every 2 to 3 years if the tumor is stable. Surgery would be indicated if there is evidence of tumor growth or a mass effect.
While tumor growth has been found to be independent of age in some studies,27 others have found longer tumor doubling time in patients older than 60 years.28
The risk of pituitary apoplexy
Pituitary apoplexy results from a hemorrhagic infarction of the tumor and manifests clinically as the sudden onset of severe headache, nausea, vomiting, vision loss, and cranial nerve palsies. While most cases of pituitary apoplexy are spontaneous, precipitating factors may include head injury, anticoagulant therapy, dopamine agonists, radiation therapy, or dynamic endocrine tests.29
It is important to educate patients and their families about the symptoms of pituitary apoplexy, especially patients with pituitary macroadenomas. If the condition is unrecognized and untreated, patients can develop hypotension and shock secondary to adrenal insufficiency, as well as irreversible vision loss or diplopia.
Surgery is generally recommended in cases of progressive vision loss or cranial neuropathy, preferably within 24 or 48 hours of onset if feasible, to minimize the risk of a permanent neurologic deficit.
Clinically significant pituitary apoplexy is rare in patients with pituitary microadenomas. In the study by Arita et al,27 the risk of pituitary apoplexy during 5 years of follow-up was 9.5%, and all of the tumors involved were macroadenomas. This rate is higher than in some other studies, in which the risk of apoplexy ranged from 0.4% to 7% during a mean follow-up of 2 to 6 years.1,25,30
CASE FOLLOW-UP
Since our patient had no evidence of hormonal hypersecretion or mass effect and no hypopituitarism, we asked her to return in 6 months. A repeat MRI study showed the tumor to be stable, with no evidence of growth. The patient was scheduled for a return visit in 1 year.
- Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol 2003; 149:123–127.
- Gsponer J, De Tribolet N, Déruaz JP, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (Baltimore) 1999; 78:236–269.
- Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer 2004; 101:613–619.
- Molitch ME, Russell EJ. The pituitary “incidentaloma.” Ann Intern Med 1990; 112:925–931.
- Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357:1821–1828.
- Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994; 120:817–820.
- Karavitaki N, Collison K, Halliday J, et al. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clin Endocrinol (Oxf) 2007; 67:938–943.
- King JT, Justice AC, Aron DC. Management of incidental pituitary microadenomas: a cost-effectiveness analysis. J Clin Endocrinol Metab 1997; 82:3625–3632.
- Klibanski A, Zervas NT, Kovacs K, Ridgway EC. Clinically silent hypersecretion of growth hormone in patients with pituitary tumors. J Neurosurg 1987; 66:806–811.
- Trouillas J, Sassolas G, Loras B, et al. Somatotropic adenomas without acromegaly. Pathol Res Pract 1991; 187:943–949.
- Cryer PE, Daughaday WH. Regulation of growth hormone secretion in acromegaly. J Clin Endocrinol Metab 1969; 29:386–393.
- Reincke M, Allolio B, Saeger W, Menzel J, Winkelmann W. The ‘incidentaloma’ of the pituitary gland. Is neurosurgery required? JAMA 1990; 263:2772–2776.
- Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000; 85:526–529.
- Nammour GM, Ybarra J, Naheedy MH, Romeo JH, Aron DC. Incidental pituitary macroadenoma: a population-based study. Am J Med Sci 1997; 314:287–291.
- Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab 2000; 85:1789–1793.
- Mayenknecht J, Diederich S, Bahr V, Plockinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab 1998; 83:1558–1562.
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology 1993; 43:1678–1683.
- Abe T, Matsumoto K, Kuwazawa J, Toyoda I, Sasaki K. Headache associated with pituitary adenomas. Headache 1998; 38:782–786.
- Levy MJ, Matharu MS, Meeran K, Powell M, Goadsby PJ. The clinical characteristics of headache in patients with pituitary tumours. Brain 2005; 128:1921–1930.
- Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am 1999; 28:81–117.
- Gopan T, Toms SA, Prayson RA, Suh JH, Hamrahian AH, Weil RJ. Symptomatic pituitary metastases from renal cell carcinoma. Pituitary 2007; 10:251–259.
- Moore AF, Grinspoon SK. A dural tale. J Clin Endocrinol Metab 2007; 92:3367–3368.
- Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007; 27:525–551.
- Chanson P, Daujat F, Young J, et al. Normal pituitary hypertrophy as a frequent cause of pituitary incidentaloma: a follow-up study. J Clin Endocrinol Metab 2001; 86:3009–3015.
- Donovan LE, Corenblum B. The natural history of the pituitary incidentaloma. Arch Intern Med 1995; 155:181–183.
- Feldkamp J, Santen R, Harms E, Aulich A, Modder U, Scherbaum WA. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas—results of a prospective study. Clin Endocrinol (Oxf) 1999; 51:109–113.
- Arita K, Tominaga A, Sugiyama K, et al. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg 2006; 104:884–891.
- Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 2003; 98:359–365.
- Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry 2001; 71:542–545.
- Nishizawa S, Ohta S, Yokoyama T, Uemura K. Therapeutic strategy for incidentally found pituitary tumors (“pituitary incidentalomas”). Neurosurgery 1998; 43:1344–1348.
- Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol 2003; 149:123–127.
- Gsponer J, De Tribolet N, Déruaz JP, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Medicine (Baltimore) 1999; 78:236–269.
- Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer 2004; 101:613–619.
- Molitch ME, Russell EJ. The pituitary “incidentaloma.” Ann Intern Med 1990; 112:925–931.
- Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357:1821–1828.
- Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994; 120:817–820.
- Karavitaki N, Collison K, Halliday J, et al. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clin Endocrinol (Oxf) 2007; 67:938–943.
- King JT, Justice AC, Aron DC. Management of incidental pituitary microadenomas: a cost-effectiveness analysis. J Clin Endocrinol Metab 1997; 82:3625–3632.
- Klibanski A, Zervas NT, Kovacs K, Ridgway EC. Clinically silent hypersecretion of growth hormone in patients with pituitary tumors. J Neurosurg 1987; 66:806–811.
- Trouillas J, Sassolas G, Loras B, et al. Somatotropic adenomas without acromegaly. Pathol Res Pract 1991; 187:943–949.
- Cryer PE, Daughaday WH. Regulation of growth hormone secretion in acromegaly. J Clin Endocrinol Metab 1969; 29:386–393.
- Reincke M, Allolio B, Saeger W, Menzel J, Winkelmann W. The ‘incidentaloma’ of the pituitary gland. Is neurosurgery required? JAMA 1990; 263:2772–2776.
- Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000; 85:526–529.
- Nammour GM, Ybarra J, Naheedy MH, Romeo JH, Aron DC. Incidental pituitary macroadenoma: a population-based study. Am J Med Sci 1997; 314:287–291.
- Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab 2000; 85:1789–1793.
- Mayenknecht J, Diederich S, Bahr V, Plockinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab 1998; 83:1558–1562.
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology 1993; 43:1678–1683.
- Abe T, Matsumoto K, Kuwazawa J, Toyoda I, Sasaki K. Headache associated with pituitary adenomas. Headache 1998; 38:782–786.
- Levy MJ, Matharu MS, Meeran K, Powell M, Goadsby PJ. The clinical characteristics of headache in patients with pituitary tumours. Brain 2005; 128:1921–1930.
- Freda PU, Post KD. Differential diagnosis of sellar masses. Endocrinol Metab Clin North Am 1999; 28:81–117.
- Gopan T, Toms SA, Prayson RA, Suh JH, Hamrahian AH, Weil RJ. Symptomatic pituitary metastases from renal cell carcinoma. Pituitary 2007; 10:251–259.
- Moore AF, Grinspoon SK. A dural tale. J Clin Endocrinol Metab 2007; 92:3367–3368.
- Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007; 27:525–551.
- Chanson P, Daujat F, Young J, et al. Normal pituitary hypertrophy as a frequent cause of pituitary incidentaloma: a follow-up study. J Clin Endocrinol Metab 2001; 86:3009–3015.
- Donovan LE, Corenblum B. The natural history of the pituitary incidentaloma. Arch Intern Med 1995; 155:181–183.
- Feldkamp J, Santen R, Harms E, Aulich A, Modder U, Scherbaum WA. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas—results of a prospective study. Clin Endocrinol (Oxf) 1999; 51:109–113.
- Arita K, Tominaga A, Sugiyama K, et al. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg 2006; 104:884–891.
- Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 2003; 98:359–365.
- Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry 2001; 71:542–545.
- Nishizawa S, Ohta S, Yokoyama T, Uemura K. Therapeutic strategy for incidentally found pituitary tumors (“pituitary incidentalomas”). Neurosurgery 1998; 43:1344–1348.
KEY POINTS
- Two key questions that must be answered when a pituitary incidentaloma is discovered are whether it is hormonally active and whether it is causing a mass effect (eg, a visual field defect due to pressure on the optic chiasm).
- Incidentalomas that are not hormonally active and that are not causing a mass effect can generally be managed by watchful waiting.
- Hormonally active prolactin-secreting tumors can be treated with dopamine agonists. Other hormonally active tumors and those that are causing a mass effect should be surgically removed.
- The risks of further tumor growth and of pituitary apoplexy are higher in tumors that are larger when discovered.
Hamstring Injuries
Stress SPECT MPI Has Prognostic Value in Practice
BOSTON — The prognostic performance of pharmacologic stress myocardial perfusion single-photon emission CT shown in academic studies generalizes to daily practice, a study has shown.
“The current prognostic literature indicates that [the imaging technology] is an effective tool for coronary artery disease risk stratification, but the majority of these studies come from single institutions using expert readers. We wanted to investigate the prognostic power of the perfusion imaging variables as read by local readers in many sites across the world,” Dr. James Udelson said at the annual meeting of the American Society of Nuclear Cardiology.
To do this, Dr. Udelson of Tufts University, Boston, along with lead investigator Dr. Rory Hachamovitch, who is in private practice in Los Angeles, and their colleagues, conducted a prospective study comprising 4,989 patients with known or suspected coronary artery disease recruited from 89 centers in eight countries during 20 consecutive work days. All of the patients had been referred for clinically indicated pharmacologic stress myocardial perfusion imaging by single-photon emission CT (PS SPECT MPI). Of the study population, 48% of the patients were tested in tertiary care centers, and 26% each underwent testing in private and community centers.
The investigators did not dictate the imaging protocol for the study; rather, “each center used its own standard protocol for stress isotope image acquisition,” said Dr. Udelson. “This was an effectiveness study of real life practices,” he added. Toward this end, approximately 150 local readers from the different sites interpreted the images and reported segmental scores using the 17-segment, 4-point scoring model from which nuclear variables were converted into percent myocardium ischemic and percent myocardium fixed, as has been done in the literature, he said.
The primary end point of the study was all-cause death, “with the simple goal of identifying the incremental value of perfusion imaging data over all other data,” said Dr. Udelson.
Using scripted phone calls to each site, the investigators followed the patients for revascularization and all-cause death for 1 year after the imaging study. Excluded from the study were 212 patients who had undergone early revascularization (within 90 days after testing) and thus were excluded from the final analysis. All-cause death was reported for 155 of the remaining patients, said Dr. Udelson. The all-cause death rate in patients with abnormal MPI was 3.7%, significantly higher than the 2.2% observed in patients with normal MPI, he said, noting that the risk of all-cause death was greatest in patients with both reversible and fixed perfusion defects (5.4%), compared with patients with fixed but no reversible defects (3.2%) and patients with reversible but no fixed defects (2.5%).
In addition to abnormal MPI, other predictors of all-cause death were history of heart failure, chronic obstructive pulmonary disease, and diabetes. Controlling for these clinical variables as well as for demographic and historical data, “the MPI results were highly predictive of 1-year all-cause death,” Dr. Udelson said.
In the final multivariate model, both the percent myocardium with reversible defects and percent with fixed defects had statistically significant increased hazard ratios, expressed per 1% change, Dr. Udelson explained. “For example, an increase in the percent myocardium reversible, or ischemic, of 3% would be associated with a 15% increased risk [of all-cause death].”
“The findings indicate that the perfusion imaging variables, as read by local readers from many sites across the world, have prognostic power incremental to all of the clinical data,” said Dr. Udelson. “This generalizability has not been shown for other imaging modalities.”
This study was funded by King Pharmaceuticals Research & Development Inc., for which Dr. Udelson and Dr. Hachamovitch are paid consultants.
The multicener, internationaltrial 'was an effectiveness study of real life practices.' DR. UDELSON
BOSTON — The prognostic performance of pharmacologic stress myocardial perfusion single-photon emission CT shown in academic studies generalizes to daily practice, a study has shown.
“The current prognostic literature indicates that [the imaging technology] is an effective tool for coronary artery disease risk stratification, but the majority of these studies come from single institutions using expert readers. We wanted to investigate the prognostic power of the perfusion imaging variables as read by local readers in many sites across the world,” Dr. James Udelson said at the annual meeting of the American Society of Nuclear Cardiology.
To do this, Dr. Udelson of Tufts University, Boston, along with lead investigator Dr. Rory Hachamovitch, who is in private practice in Los Angeles, and their colleagues, conducted a prospective study comprising 4,989 patients with known or suspected coronary artery disease recruited from 89 centers in eight countries during 20 consecutive work days. All of the patients had been referred for clinically indicated pharmacologic stress myocardial perfusion imaging by single-photon emission CT (PS SPECT MPI). Of the study population, 48% of the patients were tested in tertiary care centers, and 26% each underwent testing in private and community centers.
The investigators did not dictate the imaging protocol for the study; rather, “each center used its own standard protocol for stress isotope image acquisition,” said Dr. Udelson. “This was an effectiveness study of real life practices,” he added. Toward this end, approximately 150 local readers from the different sites interpreted the images and reported segmental scores using the 17-segment, 4-point scoring model from which nuclear variables were converted into percent myocardium ischemic and percent myocardium fixed, as has been done in the literature, he said.
The primary end point of the study was all-cause death, “with the simple goal of identifying the incremental value of perfusion imaging data over all other data,” said Dr. Udelson.
Using scripted phone calls to each site, the investigators followed the patients for revascularization and all-cause death for 1 year after the imaging study. Excluded from the study were 212 patients who had undergone early revascularization (within 90 days after testing) and thus were excluded from the final analysis. All-cause death was reported for 155 of the remaining patients, said Dr. Udelson. The all-cause death rate in patients with abnormal MPI was 3.7%, significantly higher than the 2.2% observed in patients with normal MPI, he said, noting that the risk of all-cause death was greatest in patients with both reversible and fixed perfusion defects (5.4%), compared with patients with fixed but no reversible defects (3.2%) and patients with reversible but no fixed defects (2.5%).
In addition to abnormal MPI, other predictors of all-cause death were history of heart failure, chronic obstructive pulmonary disease, and diabetes. Controlling for these clinical variables as well as for demographic and historical data, “the MPI results were highly predictive of 1-year all-cause death,” Dr. Udelson said.
In the final multivariate model, both the percent myocardium with reversible defects and percent with fixed defects had statistically significant increased hazard ratios, expressed per 1% change, Dr. Udelson explained. “For example, an increase in the percent myocardium reversible, or ischemic, of 3% would be associated with a 15% increased risk [of all-cause death].”
“The findings indicate that the perfusion imaging variables, as read by local readers from many sites across the world, have prognostic power incremental to all of the clinical data,” said Dr. Udelson. “This generalizability has not been shown for other imaging modalities.”
This study was funded by King Pharmaceuticals Research & Development Inc., for which Dr. Udelson and Dr. Hachamovitch are paid consultants.
The multicener, internationaltrial 'was an effectiveness study of real life practices.' DR. UDELSON
BOSTON — The prognostic performance of pharmacologic stress myocardial perfusion single-photon emission CT shown in academic studies generalizes to daily practice, a study has shown.
“The current prognostic literature indicates that [the imaging technology] is an effective tool for coronary artery disease risk stratification, but the majority of these studies come from single institutions using expert readers. We wanted to investigate the prognostic power of the perfusion imaging variables as read by local readers in many sites across the world,” Dr. James Udelson said at the annual meeting of the American Society of Nuclear Cardiology.
To do this, Dr. Udelson of Tufts University, Boston, along with lead investigator Dr. Rory Hachamovitch, who is in private practice in Los Angeles, and their colleagues, conducted a prospective study comprising 4,989 patients with known or suspected coronary artery disease recruited from 89 centers in eight countries during 20 consecutive work days. All of the patients had been referred for clinically indicated pharmacologic stress myocardial perfusion imaging by single-photon emission CT (PS SPECT MPI). Of the study population, 48% of the patients were tested in tertiary care centers, and 26% each underwent testing in private and community centers.
The investigators did not dictate the imaging protocol for the study; rather, “each center used its own standard protocol for stress isotope image acquisition,” said Dr. Udelson. “This was an effectiveness study of real life practices,” he added. Toward this end, approximately 150 local readers from the different sites interpreted the images and reported segmental scores using the 17-segment, 4-point scoring model from which nuclear variables were converted into percent myocardium ischemic and percent myocardium fixed, as has been done in the literature, he said.
The primary end point of the study was all-cause death, “with the simple goal of identifying the incremental value of perfusion imaging data over all other data,” said Dr. Udelson.
Using scripted phone calls to each site, the investigators followed the patients for revascularization and all-cause death for 1 year after the imaging study. Excluded from the study were 212 patients who had undergone early revascularization (within 90 days after testing) and thus were excluded from the final analysis. All-cause death was reported for 155 of the remaining patients, said Dr. Udelson. The all-cause death rate in patients with abnormal MPI was 3.7%, significantly higher than the 2.2% observed in patients with normal MPI, he said, noting that the risk of all-cause death was greatest in patients with both reversible and fixed perfusion defects (5.4%), compared with patients with fixed but no reversible defects (3.2%) and patients with reversible but no fixed defects (2.5%).
In addition to abnormal MPI, other predictors of all-cause death were history of heart failure, chronic obstructive pulmonary disease, and diabetes. Controlling for these clinical variables as well as for demographic and historical data, “the MPI results were highly predictive of 1-year all-cause death,” Dr. Udelson said.
In the final multivariate model, both the percent myocardium with reversible defects and percent with fixed defects had statistically significant increased hazard ratios, expressed per 1% change, Dr. Udelson explained. “For example, an increase in the percent myocardium reversible, or ischemic, of 3% would be associated with a 15% increased risk [of all-cause death].”
“The findings indicate that the perfusion imaging variables, as read by local readers from many sites across the world, have prognostic power incremental to all of the clinical data,” said Dr. Udelson. “This generalizability has not been shown for other imaging modalities.”
This study was funded by King Pharmaceuticals Research & Development Inc., for which Dr. Udelson and Dr. Hachamovitch are paid consultants.
The multicener, internationaltrial 'was an effectiveness study of real life practices.' DR. UDELSON
PCI or Drug Therapy: Consider Ischemic Burden
BOSTON — In the ongoing debate over whether patients with chronic, stable angina are better served by revascularization with percutaneous coronary intervention in addition to drug treatment or optimal medical therapy alone, the key variable appears to be ischemic burden, Dr. Daniel S. Berman reported at the annual meeting of the American Society of Nuclear Cardiology.
Last year, investigators in the Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation (COURAGE) trial reported that adding percutaneous coronary intervention (PCI) to optimal medical therapy in patients with stable coronary artery disease did not improve clinical end points, compared with optimal medical therapy alone (N. Engl. J. Med. 2007;356:1503–16). The results sparked a controversy that led some experts to conclude that PCI is overused and unnecessary in stable coronary disease.
More recently, however, a substudy of the COURAGE trial comprising 314 patients equally distributed between groups treated with PCI plus optical medical therapy and optimal medical therapy alone showed that the PCI strategy produced a greater ischemia reduction than the optimal medical therapy-only (OMT-only) intervention—particularly among patients with moderate to severe ischemia at baseline.
“Importantly, patients in both groups who experienced ischemia reduction had a significantly lower risk for death or myocardial infarction than patients without ischemia reduction, and the magnitude of residual ischemia was proportional to the overall risk of subsequent cardiac event,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Heart Center in Los Angeles.
The main COURAGE trial included 2,287 patients, with a history of angina or documented myocardial ischemia and at least one significant coronary lesion, who were stable on medical therapy. Participants were randomized to continue their medication alone or with PCI, and the study's combined end points were death or nonfatal myocardial infarction. The composite rates of death or nonfatal myocardial infarction over 4.6 years of follow-up were statistically similar in both groups, at 19.0% for the PCI group and at in18.5%, the patients who received only optimal medical therapy, showing no benefit of PCI over optical medical therapy in stable coronary artery disease.
In the nuclear imaging substudy, the 314 patients were equally distributed between the PCI and OMT groups and they were well matched with respect to demographics and risk factors, said Dr. Berman.
All of the patients were on medication for a mean 374 days from baseline and all underwent serial myocardial perfusion single-photon emission computed tomography (SPECT-MPI) studies 6–18 months following the baseline examination to assess the extent and severity of the perfusion defect in the global myocardium, he said.
With myocardial ischemia defined as the total perfusion deficit at stress minus the perfusion deficit at rest, 33% of patients in the PCI group and 20% in the OMT-only group showed a 5% or greater reduction in ischemia.
Among the patients in the imaging substudy with moderate to severe pretreatment ischemia, defined as a perfusion defect involving 10% or more of myocardium, “78% of the PCI patients demonstrated 5% improvement or greater, compared to 52% of the [OMT-only] patients,” Dr. Berman reported.
In considering these changes in terms of their relationship to subsequent outcomes, “we looked at the myocardial infarction rates in patients with and without ischemia reduction and determined that patients in both groups with 5% improvement in ischemia had approximately 50% lower cardiac event rate,” he said.
A similarly reduced cardiac event rate was observed in the 105 patients from both groups with moderate to severe ischemia and a greater than 5% reduction in ischemia observed post treatment, he said.
Although the substudy was not sufficiently powered to generalize that reducing ischemia will prevent later cardiac events, “we did see a striking relationship between amount of residual ischemia and the subsequent death or myocardial infarction rate,” Dr. Berman stated.
This observation is “definitely a hypothesis generator,” warranting a controlled trial comparing the PCI-based strategy with optimal medical therapy alone in patients with chronic stable angina who would be randomized based on the presence of moderate to severe ischemia, he said.
“We should be studying patients with 10% or more ischemia to determine if there is a subset of patients who would have improved angina and quality-of-life outcomes with revascularization.” The findings would be especially important to those patients with documented large amounts of jeopardized myocardia in whom medical therapy does not provide adequate relief, he concluded.
The COURAGE nuclear imaging substudy was supported by Bristol-Myers Squibb Medical Imaging and Astellas Healthcare.
COURAGE patients with moderate to severe ischemia showed greater improvement after PCIthan after OMT only. DR. BERMAN
The above SPECT-MPIimage shows the first and second stress myocardial perfusion from a patient in the nuclear substudy of the COURAGE trial who received optimal medical therapy only. Total perfusion deficit was reduced from 16% to 6%. Patients in both the PCI and OMT-only groups with 5% improvement in ischemia had 50% lower cardiac event rates. Images courtesy Dr. Daniel S. Berman
BOSTON — In the ongoing debate over whether patients with chronic, stable angina are better served by revascularization with percutaneous coronary intervention in addition to drug treatment or optimal medical therapy alone, the key variable appears to be ischemic burden, Dr. Daniel S. Berman reported at the annual meeting of the American Society of Nuclear Cardiology.
Last year, investigators in the Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation (COURAGE) trial reported that adding percutaneous coronary intervention (PCI) to optimal medical therapy in patients with stable coronary artery disease did not improve clinical end points, compared with optimal medical therapy alone (N. Engl. J. Med. 2007;356:1503–16). The results sparked a controversy that led some experts to conclude that PCI is overused and unnecessary in stable coronary disease.
More recently, however, a substudy of the COURAGE trial comprising 314 patients equally distributed between groups treated with PCI plus optical medical therapy and optimal medical therapy alone showed that the PCI strategy produced a greater ischemia reduction than the optimal medical therapy-only (OMT-only) intervention—particularly among patients with moderate to severe ischemia at baseline.
“Importantly, patients in both groups who experienced ischemia reduction had a significantly lower risk for death or myocardial infarction than patients without ischemia reduction, and the magnitude of residual ischemia was proportional to the overall risk of subsequent cardiac event,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Heart Center in Los Angeles.
The main COURAGE trial included 2,287 patients, with a history of angina or documented myocardial ischemia and at least one significant coronary lesion, who were stable on medical therapy. Participants were randomized to continue their medication alone or with PCI, and the study's combined end points were death or nonfatal myocardial infarction. The composite rates of death or nonfatal myocardial infarction over 4.6 years of follow-up were statistically similar in both groups, at 19.0% for the PCI group and at in18.5%, the patients who received only optimal medical therapy, showing no benefit of PCI over optical medical therapy in stable coronary artery disease.
In the nuclear imaging substudy, the 314 patients were equally distributed between the PCI and OMT groups and they were well matched with respect to demographics and risk factors, said Dr. Berman.
All of the patients were on medication for a mean 374 days from baseline and all underwent serial myocardial perfusion single-photon emission computed tomography (SPECT-MPI) studies 6–18 months following the baseline examination to assess the extent and severity of the perfusion defect in the global myocardium, he said.
With myocardial ischemia defined as the total perfusion deficit at stress minus the perfusion deficit at rest, 33% of patients in the PCI group and 20% in the OMT-only group showed a 5% or greater reduction in ischemia.
Among the patients in the imaging substudy with moderate to severe pretreatment ischemia, defined as a perfusion defect involving 10% or more of myocardium, “78% of the PCI patients demonstrated 5% improvement or greater, compared to 52% of the [OMT-only] patients,” Dr. Berman reported.
In considering these changes in terms of their relationship to subsequent outcomes, “we looked at the myocardial infarction rates in patients with and without ischemia reduction and determined that patients in both groups with 5% improvement in ischemia had approximately 50% lower cardiac event rate,” he said.
A similarly reduced cardiac event rate was observed in the 105 patients from both groups with moderate to severe ischemia and a greater than 5% reduction in ischemia observed post treatment, he said.
Although the substudy was not sufficiently powered to generalize that reducing ischemia will prevent later cardiac events, “we did see a striking relationship between amount of residual ischemia and the subsequent death or myocardial infarction rate,” Dr. Berman stated.
This observation is “definitely a hypothesis generator,” warranting a controlled trial comparing the PCI-based strategy with optimal medical therapy alone in patients with chronic stable angina who would be randomized based on the presence of moderate to severe ischemia, he said.
“We should be studying patients with 10% or more ischemia to determine if there is a subset of patients who would have improved angina and quality-of-life outcomes with revascularization.” The findings would be especially important to those patients with documented large amounts of jeopardized myocardia in whom medical therapy does not provide adequate relief, he concluded.
The COURAGE nuclear imaging substudy was supported by Bristol-Myers Squibb Medical Imaging and Astellas Healthcare.
COURAGE patients with moderate to severe ischemia showed greater improvement after PCIthan after OMT only. DR. BERMAN
The above SPECT-MPIimage shows the first and second stress myocardial perfusion from a patient in the nuclear substudy of the COURAGE trial who received optimal medical therapy only. Total perfusion deficit was reduced from 16% to 6%. Patients in both the PCI and OMT-only groups with 5% improvement in ischemia had 50% lower cardiac event rates. Images courtesy Dr. Daniel S. Berman
BOSTON — In the ongoing debate over whether patients with chronic, stable angina are better served by revascularization with percutaneous coronary intervention in addition to drug treatment or optimal medical therapy alone, the key variable appears to be ischemic burden, Dr. Daniel S. Berman reported at the annual meeting of the American Society of Nuclear Cardiology.
Last year, investigators in the Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation (COURAGE) trial reported that adding percutaneous coronary intervention (PCI) to optimal medical therapy in patients with stable coronary artery disease did not improve clinical end points, compared with optimal medical therapy alone (N. Engl. J. Med. 2007;356:1503–16). The results sparked a controversy that led some experts to conclude that PCI is overused and unnecessary in stable coronary disease.
More recently, however, a substudy of the COURAGE trial comprising 314 patients equally distributed between groups treated with PCI plus optical medical therapy and optimal medical therapy alone showed that the PCI strategy produced a greater ischemia reduction than the optimal medical therapy-only (OMT-only) intervention—particularly among patients with moderate to severe ischemia at baseline.
“Importantly, patients in both groups who experienced ischemia reduction had a significantly lower risk for death or myocardial infarction than patients without ischemia reduction, and the magnitude of residual ischemia was proportional to the overall risk of subsequent cardiac event,” said Dr. Berman, chief of cardiac imaging and nuclear cardiology at Cedars-Sinai Heart Center in Los Angeles.
The main COURAGE trial included 2,287 patients, with a history of angina or documented myocardial ischemia and at least one significant coronary lesion, who were stable on medical therapy. Participants were randomized to continue their medication alone or with PCI, and the study's combined end points were death or nonfatal myocardial infarction. The composite rates of death or nonfatal myocardial infarction over 4.6 years of follow-up were statistically similar in both groups, at 19.0% for the PCI group and at in18.5%, the patients who received only optimal medical therapy, showing no benefit of PCI over optical medical therapy in stable coronary artery disease.
In the nuclear imaging substudy, the 314 patients were equally distributed between the PCI and OMT groups and they were well matched with respect to demographics and risk factors, said Dr. Berman.
All of the patients were on medication for a mean 374 days from baseline and all underwent serial myocardial perfusion single-photon emission computed tomography (SPECT-MPI) studies 6–18 months following the baseline examination to assess the extent and severity of the perfusion defect in the global myocardium, he said.
With myocardial ischemia defined as the total perfusion deficit at stress minus the perfusion deficit at rest, 33% of patients in the PCI group and 20% in the OMT-only group showed a 5% or greater reduction in ischemia.
Among the patients in the imaging substudy with moderate to severe pretreatment ischemia, defined as a perfusion defect involving 10% or more of myocardium, “78% of the PCI patients demonstrated 5% improvement or greater, compared to 52% of the [OMT-only] patients,” Dr. Berman reported.
In considering these changes in terms of their relationship to subsequent outcomes, “we looked at the myocardial infarction rates in patients with and without ischemia reduction and determined that patients in both groups with 5% improvement in ischemia had approximately 50% lower cardiac event rate,” he said.
A similarly reduced cardiac event rate was observed in the 105 patients from both groups with moderate to severe ischemia and a greater than 5% reduction in ischemia observed post treatment, he said.
Although the substudy was not sufficiently powered to generalize that reducing ischemia will prevent later cardiac events, “we did see a striking relationship between amount of residual ischemia and the subsequent death or myocardial infarction rate,” Dr. Berman stated.
This observation is “definitely a hypothesis generator,” warranting a controlled trial comparing the PCI-based strategy with optimal medical therapy alone in patients with chronic stable angina who would be randomized based on the presence of moderate to severe ischemia, he said.
“We should be studying patients with 10% or more ischemia to determine if there is a subset of patients who would have improved angina and quality-of-life outcomes with revascularization.” The findings would be especially important to those patients with documented large amounts of jeopardized myocardia in whom medical therapy does not provide adequate relief, he concluded.
The COURAGE nuclear imaging substudy was supported by Bristol-Myers Squibb Medical Imaging and Astellas Healthcare.
COURAGE patients with moderate to severe ischemia showed greater improvement after PCIthan after OMT only. DR. BERMAN
The above SPECT-MPIimage shows the first and second stress myocardial perfusion from a patient in the nuclear substudy of the COURAGE trial who received optimal medical therapy only. Total perfusion deficit was reduced from 16% to 6%. Patients in both the PCI and OMT-only groups with 5% improvement in ischemia had 50% lower cardiac event rates. Images courtesy Dr. Daniel S. Berman
Using biochemical markers of bone turnover in clinical practice
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
MARKERS OF BONE FORMATION
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.
Collagen cross-links
Urinary pyridinoline and deoxypyridinoline are more specific markers of bone resorption.1
Pyridinolines are cross-linking amino acids that strengthen collagen fibrils in the extra-cellular matrix. They are found in the main fibril-forming collagens (types I, II, and III) of many tissues. Pyridinoline is the major chemical form, but deoxypyridinoline is also unusually abundant in bone collagen and hence is a relatively selective bone marker.
NTx. Since pyridinolines are not metabolized and are largely excreted as small peptides when produced by osteoclastic bone resorption, immunoassays have been developed that selectively measure cross-link-containing peptide fragments in urine and serum. The first was an assay that recognizes an N-telopeptide of collagen type I (NTx) in urine10 and serum.11 The recognized feature in this sequence is fully generated during the process of osteoclastic proteolysis and so requires no further metabolism by the liver or kidney for its production. Results from second-morning urine collections correlate well with those from 24-hour collections, which simplifies patient evaluation.
CTx. Several other assays target structural variants of a peptide sequence that originates from the carboxy-terminal cross-linking region of collagen type I (CTx).12,13
Other markers of bone resorption
Two enzymes found in osteoclasts have received attention as markers of osteoclast activity.
Serum tartrate-resistant acid phosphatase (TRAP) 5b has not been studied extensively in patients but appears to correlate with other markers of bone resorption.14
Serum cathepsin K is of interest because it is the primary proteolytic enzyme used by osteoclasts to degrade bone type I collagen during resorption. Several studies suggest it may be valuable as a marker of bone resorption,15 but more studies are required to evaluate its performance relative to established bone resorption markers.
Receptor activator of nuclear factor kappa (RANK), RANK ligand, and its decoy receptor osteoprotegerin are the pivotal regulators of osteoclast recruitment and activity.16 They may eventually be used as markers of bone metabolism, though the broad role of RANK ligand signaling in the immune system may limit its specificity.
FACTORS THAT INFLUENCE ASSAY RESULTS
To avoid being misled, clinicians who use biochemical markers of bone turnover should be familiar with factors that influence assay results.3
Diurnal and day-to-day variability
The most important biologic factors probably are diurnal and day-to-day variability in bone-forming and bone-resorbing activities. Levels of bone turnover markers are highest in the early morning and lowest in the afternoon and evening.
Levels of urinary markers can vary 20% to 30% from the highest to lowest value of the day. Serum markers change to a smaller degree except for serum CTx, which can vary by more than 60% during the day.17
In general, the day-to-day variability of urinary markers of bone resorption is similar in range to their diurnal variability. The serum markers of bone formation appear to vary less from day to day.
Eating, calcium intake
Blood for measurement of serum CTx should be taken in the morning after overnight fasting to avoid the large decrease that occurs after eating. An increase in calcium intake also can lower the levels of bone resorption markers, particularly in people whose calcium intake was previously low.18 Presumably, this effect is mediated by inhibition of parathyroid hormone secretion.
Sample handling
Improper collection and handling of specimens can seriously affect assay precision. The optimal time to collect samples is in the morning. Careful sample collection and storage are particularly important in measuring serum osteocalcin and TRAP. It is also important to use the same laboratory for serial measurements, since assay results can vary considerably among laboratories, even if they use identical methods.
BONE TURNOVER THROUGHOUT LIFE
In children, bone turnover can be more than 10 times greater than in adults because of three physiologic processes interacting in the skeleton: bone modeling, remodeling, and growth. Levels of bone formation and resorption markers therefore are much higher in children than in adults.19 Unfortunately, no studies have compared all the available markers in the same pediatric reference population.
In puberty, bone growth accelerates, with an increase in bone turnover markers that reflects the effect of hormones that induce the growth spurt.1
Postmenopausal women who do not use hormone replacement therapy have higher levels of bone resorption and formation markers than premenopausal women.20 Levels in postmenopausal women on hormone replacement are no different than in premenopausal women.20,21 In postmenopausal women not on estrogen, urinary levels of NTx have been reported to discriminate between normal bone mineral density (lowest NTx levels), osteopenia, and osteoporosis (highest levels).22 Normal levels of NTx are found in a small percentage of women. This may be explained by the variable levels of serum estradiol in post-menopausal women.23
Elderly men, in contrast, have variable findings.24–28 However, accelerated bone turnover has been noted in men with full-blown hypogonadism caused by androgen suppression therapy.29
CLINICAL APPLICATIONS OF BONE TURNOVER MARKERS
In postmenopausal osteoporosis
Markers of bone formation are somewhat less likely to be elevated than markers of bone resorption, and if they are elevated, they decrease as expected in response to therapy that inhibits bone resorption, though more gradually and to a lesser extent than the resorption markers.31–35
To monitor bisphosphonate therapy. Antiresorptive drugs such as bisphosphonates reduce the risk of fracture, as they increase bone density and decrease the rate of bone resorption, as shown in many clinical trials.31–35 Because the rate of bone resorption reaches a nadir within 3 to 6 months of starting bisphosphonate therapy and because the increase in bone density after 1 year is quite modest (about 3%–4%), most of the decrease in vertebral fracture incidence, which becomes apparent during the first year of treatment, probably can be attributed more to normalization of bone resorption (and a less perforated structure) than to the increase in bone density.36 This would suggest that it is more appropriate to document that bone resorption has been inhibited than to measure bone density every year when following patients taking antiresorptive agents.
Furthermore, effective antiresorptive therapy reduces the levels of resorption markers by 50% to 70%,32–35 whereas after 1 year bone density has generally not increased more than the error of the bone density measurement. This observation has led to the suggestion that bone density measurements generally should not be done more often than every 2 years when following the effects of antiresorptive therapy. Even with a 20% to 30% day-to-day variation in levels of bone resorption markers, it is easier to document the efficacy of therapy with resorption biomarkers than with bone density.
To document compliance. Another reason to consider measuring a resorption marker (after 3 months of therapy) is to document compliance, a considerable problem in the treatment of an asymptomatic disorder.
To help decide when to restart bisphosphonate therapy. After long-term treatment with a bisphosphonate, the drug may be retained in the skeleton for years. This seems particularly true of alendronate (Fosamax).37 After 5 years of continuous alendronate treatment, bone resorption continues to be suppressed near the maximal level, in some patients for years after they stop taking the drug.38
Once the bone resorption marker begins to approach the pretreatment level, it would signal a possible need to restart the therapy. If a pretreatment level was not measured, an estimate of significant bone resorption would be signaled when the resorption marker is more than 20% above the mean premenopausal level. For urinary NTx this would be more than 42 nmol bone collagen equivalents/mmol creatinine.
In glucocorticoid-induced osteoporosis
Glucocorticoid therapy causes bone loss and an increased incidence of fractures when given in high doses or for prolonged periods by the oral, parenteral, or inhaled routes.41
The pathogenesis of the bone loss has been explored by measurements of bone turnover markers. During glucocorticoid therapy, levels of bone formation markers are generally low and those of bone resorption markers are either normal or low.42–44 Presumably, the reduction in bone resorption is not enough to overcome the reduction in bone formation, and bone loss ensues. In children, the effects on bone formation are particularly profound, as linear growth may be retarded.44
Giving a bisphosphonate during glucocorticoid therapy is quite effective in increasing bone density and preventing fractures.45–47 Patients who receive alendronate have lower levels of bone formation and resorption markers than do untreated subjects.45 Presumably, bone resorption is inhibited more than bone formation, accounting for the skeletal benefits.
In a recent study in patients with glucocorticoid-induced osteoporosis, bone mineral density of the lumbar spine increased more than twice as much with teriparatide than with alendronate over an 18-month period.48 As would be expected from the results of teriparatide therapy in postmenopausal osteoporosis, indices of both bone formation and resorption rose to a peak at 6 months, with formation greater than resorption.
In immobilization-induced osteoporosis
Studies of normal volunteers placed on bed rest indicate that urinary CTx and NTx excretion increase significantly after 24 hours, no doubt reflecting a rapid increase in osteoclast activity.49 In a 16-week study of bed rest in volunteers, markers of bone formation were reduced and markers of bone resorption increased, demonstrating the mechanisms for the profound and rapid loss of bone in immobilized patients.50
In a long-term cross-sectional study of paraplegic men with spinal cord injuries, bone turnover patterns changed over time.51 During the first year after injury, urinary deoxypyridinoline excretion was markedly elevated, whereas blood total alkaline phosphatase and osteocalcin levels were normal to slightly elevated. Over a 30-year period after injury, the bone resorption marker returned to normal levels in most patients and the bone formation markers were normal. Fracture incidence rose but leveled off after 20 years.
Bisphosphonate therapy in spinal cord injury patients reduces urinary NTx and prevents bone loss.52,53 These agents have also proven effective in reversing hypercalcemia in immobilized patients.54
In inflammatory bowel disease
Patients with inflammatory bowel disease, especially Crohn disease, have low bone mass and are at risk of fractures.55 These complications could be due to glucocorticoid therapy, hypogonadism, vitamin D defeciency, weight loss, and high circulating levels of bone-active cytokines released by inflammatory cells residing in the diseased intestine.
Bone formation markers have not been found to be outside the normal range, although both interleukin 1 and tumor necrosis factor alpha are known to inhibit bone formation.
Bisphosphonate treatment produces an increase in bone density concomitant with decreases in markers of bone resorption and formation.57,58 Of considerable interest is the observation that infliximab (anti-tumor necrosis factor alpha; Remicade) generally produces a rise in bone formation markers, with a smaller and inconsistent effect on bone resorption.59,60
In rheumatoid arthritis
The incidence of osteoporosis and fractures is also increased In patients with rheumatoid arthritis.61 As in patients with inflammatory bowel disease, a variety of factors can contribute to bone loss, including glucocorticoid therapy, hypogonadism, vitamin D deficiency, immobility, and elevated levels of bone-active cytokines.
Generally, studies have reported increased bone resorption based on type I collagen markers,62,63 whereas patients with osteoarthritis have levels of these bone resorption markers no different from those of control subjects.62 Although serum total TRAP protein is elevated in rheumatoid arthritis patients, this is probably due to the 5a isoform, the origin of which may be macrophages and dendritric cells.64
The influence of abnormalities in bone formation on bone loss is less clear. Levels of bone formation markers have been reported to be normal,65 elevated,66 or reduced.67
Treatment of rheumatoid arthritis with high-dose glucocorticoid pulse therapy is effective in controlling the symptoms and some manifestations of the immune system in patients with the disorder. The latter effect would be expected to have a beneficial effect on bone metabolism. This appears to be the case, as there are only transient decreases in bone formation markers and no significant reduction in bone density.68 Similarly, there is only a transient decrease in serum osteocalcin after an intra-articular injection of a glucocorticoid, and no effect on urinary pyridino-line.69
As would be expected, bisphosphonate therapy prevents bone loss in rheumatoid arthritis patients treated with glucocorticoids.70,71 Both oral and intravenous therapy decrease the levels of bone turnover markers.70–72 Infliximab therapy was shown to reduce the levels of bone resorption markers but not of PINP (a bone formation marker).73
In primary hyperparathyroidism
Hypersecretion of parathyroid hormone increases osteoclastic activity, with a secondary increase in osteoblastic activity. Bone loss may ensue and an increase in fracture incidence may be a consequence, particularly in post-menopausal women, who have the highest incidence of the disorder.74
Before screening chemistry panels became widely used during routine medical evaluations, it was not unusual to find elevated serum total alkaline phosphatase levels in patients discovered to have primary hyperparathyroidism. Today, this finding is not so common, as the disorder is diagnosed at a much earlier stage. Nevertheless, more specific and sensitive markers of bone turnover have made it possible to demonstrate the metabolic abnormalities that reflect the skeletal pathology in patients with primary hyperparathyroidism and its response to various therapies.75,76
On average, patients with untreated primary hyperparathyroidism have high levels of markers of bone resorption and formation, except in the mildest cases.73,74 Bone turnover returns to normal within 6 months to a year after successful parathyroidectomy.77,78 This response correlates with improvement in bone density, primarily in the lumbar spine.77,78
In patients who do not undergo surgery, alternative means of preventing bone loss include estrogen replacement in estrogendeficient postmenopausal women,76 bisphosphonates,79,80 and cinacalcet (Sensipar).81 Estrogen,76 raloxifene (Evista),82 and alendronate79,80 all reduce levels of bone resorption and formation markers, and estrogen76 and alendronate79,80 increase bone density. Although cinacalcet usually restores the serum calcium to the normal range and prevents bone loss, it only reduces serum parathyroid hormone levels by about 20%, and both bone resorption and formation markers increase above baseline.81 This could be related to fluctuations in serum parathyroid hormone that occur during each day of therapy.
In osteomalacia and rickets
Osteomalacia and rickets of any cause are characterized by increased osteoblastic activity. If the underlying cause is vitamin D deficiency, genetic or acquired defects in calcitriol synthesis, or vitamin D resistance, then hyper-parathyroidism with increased bone resorption is a secondary feature.
Serum total alkaline phosphatase activity has been a useful marker of disease activity for many years, although the newer markers, except for serum osteocalcin,83 are potentially more sensitive. The insensitivity of osteocalcin as an index of osteoblastic activity is unexplained but could be related to the state of differentiation of the osteoblasts. Bone resorption markers are elevated in vitamin D deficiency84 but are not widely used in clinical practice, as serum parathyroid hormone is an excellent indirect means of assessing the presence of increased bone resorption and the response to therapy.
In renal osteodystrophy
Bone disease associated with renal failure is termed renal osteodystrophy and is quite heterogeneous.85 Microscopic examination of a bone biopsy specimen is still considered the gold standard for diagnosis, and measurement in serum of intact parathyroid hormone is an important guide to diagnosis and response to therapy.
Nevertheless, recent studies suggest that serum markers of bone formation and resorption may be of additional help in assessing bone turnover.86 At present it is not certain whether any of the newer markers are superior to serum total alkaline phosphatase activity. Future studies that correlate bone histology with bone turnover markers should clarify the value of the various markers.
In cancer
Bone metastases are a common complication in cancer patients. They are classified as osteolytic, osteoblastic, or mixed on the basis of radiographic features. Biochemical markers of bone turnover have proven useful in assessing the magnitude of the metastases, the response to therapy, and even the prognosis for survival.87
Osteolytic metastases, which are common in breast cancer, are associated with increases in bone resorption markers, and after treatment with intravenous bisphosphonates the levels can decrease nearly 70%.88,89
Patients with higher levels of urinary NTx had a higher risk of skeletal complications and disease progression than patients with low levels across multiple tumor groups, including multiple myeloma.87
In osteoblastic metastases. Prostate cancer patients, who typically have predominantly osteoblastic lesions, have elevations of serum total alkaline phosphatase activity and other markers of bone formation.90 In addition, they have elevated bone resorption markers. Urinary NTx decreased markedly but serum bone-specific alkaline phosphatase decreased only slightly after treatment with intravenous zoledronic acid (Zometa),91 whereas androgen ablation therapy has inconsistent effects on bone turnover.92,93 High levels of these markers again predict poor prognosis.93,94
In hormone-suppression therapy. Two of the most successful cancer therapies, aromatase inhibitors for breast cancer95 and androgen ablation for prostate cancer,96 accelerate bone loss through marked suppression of gonadal steroids. Bone resorption and formation markers increase and bone loss ensues, with resorption exceeding formation. Estrogen suppression appears mainly responsible in both sexes, since raloxifene prevents bone loss in prostate cancer patients.97
Bisphosphonates are highly effective in preventing bone loss in either sex.98–100 A single infusion of zoledronic acid in androgen-ablated prostate cancer patients can prevent bone loss for at least 1 year.100
In Paget disease of bone
Paget disease of bone evolves over many years, from an early osteolytic phase to dominance of secondary osteoblastic activity. In patients with extensive polyostotic disease, bone resorption and formation marker levels may be higher than in almost any other skeletal disorder. An exception is serum osteocalcin,101 which once again usually does not accurately reflect the rate of bone formation.
Bisphosphonates, given orally or intravenously, produce an early decrease in bone resorption followed by a fall in bone formation.102 In clinical practice it appears adequate to use the least expensive test, serum total alkaline phosphatase activity, to assess disease activity and the response to therapy.103
Acknowledgments: Grant support to FRS from the Edythe and Eli Broad Foundation and Lois Rosen. Grant support to DRE from the National Institutes of Health (NIAMS: AR37318, AR36794).
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005; 2:504–517.
- Seibel MJ. Biochemical markers of bone turnover: part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006; 27:123–138.
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000; 26:103–109.
- Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996; 81:2264–2270.
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). 2004; 60:491–499.
- Cloos PA, Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004; 50:585–598.
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–469.
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992; 7:1251–1258.
- Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997; 43:2058–2063.
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–785.
- Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step, ELISA a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998; 44:2290–2300.
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004; 34:187–194.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget’s disease. Clin Lab. 2006; 52:1–10.
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(suppl 1):S1.
- Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999; 45:1858–1860.
- Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004; 15:290–294.
- Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007; 92:443–449.
- Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–3371.
- Prestwood KM, Pilbeam CC, Burleson JA, et al. The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab. 1994; 79:366–371.
- Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997; 157:1241–1245.
- Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra low-dose transdermal estradiol therapy on bone turnover and bone density in postmenopausal women. J Bone Miner Res. 2007; 22:1791–1797.
- Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274.
- Goemaere S, Van Pottelbergh I, Zmierczak H, et al. Inverse association between bone turnover rate and bone mineral density in community-dwelling men >70 years of age: no major role of sex steroid status. Bone. 2001; 29:286–291.
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001; 16:1642–1650.
- Scopacasa F, Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 2002; 57:M385–M391.
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 2007; 8:13.
- Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345:948–955.
- Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–1443.
- Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–1258.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004; 15:792–798.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
- Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000; 11:434–442.
- Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005; 20:962–970.
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005; 34:341–356.
- Ebeling PR, Erbas B, Hopper JL, Wark JD, Rubinfeld AR. Bone mineral density and bone turnover in asthmatics treated with long-term inhaled or oral glucocorticoids. J Bone Miner Res. 1998; 13:1283–1289.
- Siomou E, Challa A, Tzoufi M, Papadopoulou ZL, Lapatsanis PD, Siamopoulou A. Biochemical markers of bone metabolism in infants and children under intravenous corticosteroid therapy. Calcif Tissue Int. 2003; 73:319–325.
- Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002; 57:185–191.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001; 44:202–211.
- Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001; 69:242–247.
- Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003; 14:801–807.
- Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–2039.
- Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 2005; 57:31–36.
- Scheld K, Zittermann A, Heer M, et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001; 47:1688–1695.
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15:180–189.
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999; 80:243–251.
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007; 92:1385–1390.
- Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999; 80:998–1000.
- van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003; 125:1591–1597.
- Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000; 95:699–704.
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology. 2000; 119:639–646.
- Palomba S, Orio F, Manguso F, et al. Efficacy of risedronate administration in osteoporotic postmenopausal women affected by inflammatory bowel disease. Osteoporos Int. 2005; 16:1141–1149.
- Franchimont N, Putzeys V, Collette J, et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2004; 20:607–614.
- Ryan BM, Russel MG, Schurgers L, et al. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004; 20:851–857.
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006; 54:3104–3112.
- Wong PK, Young L, Vaile JH, et al. Telopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritis. Intern Med J. 2004; 34:539–544.
- Momohara S, Okamoto H, Yago T, et al. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005; 15:410–414.
- Janckila AJ, Neustadt DH, Nakasato YR, Halleen JM, Hentunen T, Yam LT. Serum tartrate-resistant acid phosphatase isoforms in rheumatoid arthritis. Clin Chim Acta. 2002; 320:49–58.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996; 55:288–293.
- Manrique F, Gamardo J, de Elguezabal K, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J Clin Rheumatol. 2003; 9:219–227.
- Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999; 24:381–385.
- Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methyl-prednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004; 31:1083–1087.
- Emkey RD, Lindsay R, Lyssy J, Weisberg JS, Dempster DW, Shen V. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996; 39:277–282.
- Lange U, Illgner U, Teichmann J, Schleenbecker H. Skeletal benefit after one year of risedronate therapy in patients with rheumatoid arthritis and glucocorticoid-induced osteoporosis: a prospective study. Int J Clin Pharmacol Res. 2004; 24:33–38.
- Tascioglu F, Colak O, Armagan O, Alatas O, Oner C. The treatment of osteoporosis in patients with rheumatoid arthritis receiving glucocorticoids: a comparison of alendronate and intranasal salmon calcitonin. Rheumatol Int. 2005; 26:21–29.
- Cremers SC, Lodder MC, Den Hartigh J, et al. Short term whole body retention in relation to rate of bone resorption and cartilage degradation after intravenous bisphosphonate (pamidronate) in rheumatoid arthritis. J Rheumatol. 2004; 31:1732–1737.
- Chopin F, Garnero P, Le Henanff A, et al. Long term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2007; 67:353–357.
- Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999; 14:1700–1707.
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:3487–3491.
- Orr-Walker BJ, Evans MC, Clearwater JM, Horne A, Grey AB, Reid IR. Effects of hormone replacement therapy on bone mineral density in postmenopausal women with primary hyperparathyroidism: four-year follow-up and comparison with healthy postmenopausal women. Arch Intern Med. 2000; 160:2161–2166.
- Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999; 25:237–244.
- Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5-year follow-up study. J Bone Miner Metab. 2007; 25:226–231.
- Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:581–587.
- Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3319–3325.
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005; 90:135–141.
- Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:1174–1178.
- Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000; 159:730–733.
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006; 368:48–52.
- Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004; 43:558–565.
- Malyszko J, Wolczynski S, Malyszko JS, Konstantynowicz J, Kaczmarski M, Mysliwiec M. Correlations of new markers of bone formation and resorption in kidney transplant recipients. Transplant Proc. 2003; 35:1351–1354.
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005; 23:4925–4935.
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997; 75:408–412.
- Coleman RE Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol 2002; 25(suppl 1):S25–S31.
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev 2006; 32(suppl 1):23–26.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468.
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998; 83:1561–1566.
- Johansen JS, Brasso K, Iversen P, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007; 13:3244–3249.
- Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006; 12:3361–3367.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006; 21:1215–1223.
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12:6315s–6319s.
- Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:3841–3846.
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007; 41:346–352.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007; 146:416–424.
- Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042.
- Kaddam IM, Iqbal SJ, Holland S, Wong M, Manning D. Comparison of serum osteocalcin with total and bone specific alkaline phosphatase and urinary hydroxyproline:creatinine ratio in patients with Paget’s disease of bone. Ann Clin Biochem. 1994; 31:327–330.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med. 2005; 353:898–908.
- Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004; 35:224–230.
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
MARKERS OF BONE FORMATION
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.
Collagen cross-links
Urinary pyridinoline and deoxypyridinoline are more specific markers of bone resorption.1
Pyridinolines are cross-linking amino acids that strengthen collagen fibrils in the extra-cellular matrix. They are found in the main fibril-forming collagens (types I, II, and III) of many tissues. Pyridinoline is the major chemical form, but deoxypyridinoline is also unusually abundant in bone collagen and hence is a relatively selective bone marker.
NTx. Since pyridinolines are not metabolized and are largely excreted as small peptides when produced by osteoclastic bone resorption, immunoassays have been developed that selectively measure cross-link-containing peptide fragments in urine and serum. The first was an assay that recognizes an N-telopeptide of collagen type I (NTx) in urine10 and serum.11 The recognized feature in this sequence is fully generated during the process of osteoclastic proteolysis and so requires no further metabolism by the liver or kidney for its production. Results from second-morning urine collections correlate well with those from 24-hour collections, which simplifies patient evaluation.
CTx. Several other assays target structural variants of a peptide sequence that originates from the carboxy-terminal cross-linking region of collagen type I (CTx).12,13
Other markers of bone resorption
Two enzymes found in osteoclasts have received attention as markers of osteoclast activity.
Serum tartrate-resistant acid phosphatase (TRAP) 5b has not been studied extensively in patients but appears to correlate with other markers of bone resorption.14
Serum cathepsin K is of interest because it is the primary proteolytic enzyme used by osteoclasts to degrade bone type I collagen during resorption. Several studies suggest it may be valuable as a marker of bone resorption,15 but more studies are required to evaluate its performance relative to established bone resorption markers.
Receptor activator of nuclear factor kappa (RANK), RANK ligand, and its decoy receptor osteoprotegerin are the pivotal regulators of osteoclast recruitment and activity.16 They may eventually be used as markers of bone metabolism, though the broad role of RANK ligand signaling in the immune system may limit its specificity.
FACTORS THAT INFLUENCE ASSAY RESULTS
To avoid being misled, clinicians who use biochemical markers of bone turnover should be familiar with factors that influence assay results.3
Diurnal and day-to-day variability
The most important biologic factors probably are diurnal and day-to-day variability in bone-forming and bone-resorbing activities. Levels of bone turnover markers are highest in the early morning and lowest in the afternoon and evening.
Levels of urinary markers can vary 20% to 30% from the highest to lowest value of the day. Serum markers change to a smaller degree except for serum CTx, which can vary by more than 60% during the day.17
In general, the day-to-day variability of urinary markers of bone resorption is similar in range to their diurnal variability. The serum markers of bone formation appear to vary less from day to day.
Eating, calcium intake
Blood for measurement of serum CTx should be taken in the morning after overnight fasting to avoid the large decrease that occurs after eating. An increase in calcium intake also can lower the levels of bone resorption markers, particularly in people whose calcium intake was previously low.18 Presumably, this effect is mediated by inhibition of parathyroid hormone secretion.
Sample handling
Improper collection and handling of specimens can seriously affect assay precision. The optimal time to collect samples is in the morning. Careful sample collection and storage are particularly important in measuring serum osteocalcin and TRAP. It is also important to use the same laboratory for serial measurements, since assay results can vary considerably among laboratories, even if they use identical methods.
BONE TURNOVER THROUGHOUT LIFE
In children, bone turnover can be more than 10 times greater than in adults because of three physiologic processes interacting in the skeleton: bone modeling, remodeling, and growth. Levels of bone formation and resorption markers therefore are much higher in children than in adults.19 Unfortunately, no studies have compared all the available markers in the same pediatric reference population.
In puberty, bone growth accelerates, with an increase in bone turnover markers that reflects the effect of hormones that induce the growth spurt.1
Postmenopausal women who do not use hormone replacement therapy have higher levels of bone resorption and formation markers than premenopausal women.20 Levels in postmenopausal women on hormone replacement are no different than in premenopausal women.20,21 In postmenopausal women not on estrogen, urinary levels of NTx have been reported to discriminate between normal bone mineral density (lowest NTx levels), osteopenia, and osteoporosis (highest levels).22 Normal levels of NTx are found in a small percentage of women. This may be explained by the variable levels of serum estradiol in post-menopausal women.23
Elderly men, in contrast, have variable findings.24–28 However, accelerated bone turnover has been noted in men with full-blown hypogonadism caused by androgen suppression therapy.29
CLINICAL APPLICATIONS OF BONE TURNOVER MARKERS
In postmenopausal osteoporosis
Markers of bone formation are somewhat less likely to be elevated than markers of bone resorption, and if they are elevated, they decrease as expected in response to therapy that inhibits bone resorption, though more gradually and to a lesser extent than the resorption markers.31–35
To monitor bisphosphonate therapy. Antiresorptive drugs such as bisphosphonates reduce the risk of fracture, as they increase bone density and decrease the rate of bone resorption, as shown in many clinical trials.31–35 Because the rate of bone resorption reaches a nadir within 3 to 6 months of starting bisphosphonate therapy and because the increase in bone density after 1 year is quite modest (about 3%–4%), most of the decrease in vertebral fracture incidence, which becomes apparent during the first year of treatment, probably can be attributed more to normalization of bone resorption (and a less perforated structure) than to the increase in bone density.36 This would suggest that it is more appropriate to document that bone resorption has been inhibited than to measure bone density every year when following patients taking antiresorptive agents.
Furthermore, effective antiresorptive therapy reduces the levels of resorption markers by 50% to 70%,32–35 whereas after 1 year bone density has generally not increased more than the error of the bone density measurement. This observation has led to the suggestion that bone density measurements generally should not be done more often than every 2 years when following the effects of antiresorptive therapy. Even with a 20% to 30% day-to-day variation in levels of bone resorption markers, it is easier to document the efficacy of therapy with resorption biomarkers than with bone density.
To document compliance. Another reason to consider measuring a resorption marker (after 3 months of therapy) is to document compliance, a considerable problem in the treatment of an asymptomatic disorder.
To help decide when to restart bisphosphonate therapy. After long-term treatment with a bisphosphonate, the drug may be retained in the skeleton for years. This seems particularly true of alendronate (Fosamax).37 After 5 years of continuous alendronate treatment, bone resorption continues to be suppressed near the maximal level, in some patients for years after they stop taking the drug.38
Once the bone resorption marker begins to approach the pretreatment level, it would signal a possible need to restart the therapy. If a pretreatment level was not measured, an estimate of significant bone resorption would be signaled when the resorption marker is more than 20% above the mean premenopausal level. For urinary NTx this would be more than 42 nmol bone collagen equivalents/mmol creatinine.
In glucocorticoid-induced osteoporosis
Glucocorticoid therapy causes bone loss and an increased incidence of fractures when given in high doses or for prolonged periods by the oral, parenteral, or inhaled routes.41
The pathogenesis of the bone loss has been explored by measurements of bone turnover markers. During glucocorticoid therapy, levels of bone formation markers are generally low and those of bone resorption markers are either normal or low.42–44 Presumably, the reduction in bone resorption is not enough to overcome the reduction in bone formation, and bone loss ensues. In children, the effects on bone formation are particularly profound, as linear growth may be retarded.44
Giving a bisphosphonate during glucocorticoid therapy is quite effective in increasing bone density and preventing fractures.45–47 Patients who receive alendronate have lower levels of bone formation and resorption markers than do untreated subjects.45 Presumably, bone resorption is inhibited more than bone formation, accounting for the skeletal benefits.
In a recent study in patients with glucocorticoid-induced osteoporosis, bone mineral density of the lumbar spine increased more than twice as much with teriparatide than with alendronate over an 18-month period.48 As would be expected from the results of teriparatide therapy in postmenopausal osteoporosis, indices of both bone formation and resorption rose to a peak at 6 months, with formation greater than resorption.
In immobilization-induced osteoporosis
Studies of normal volunteers placed on bed rest indicate that urinary CTx and NTx excretion increase significantly after 24 hours, no doubt reflecting a rapid increase in osteoclast activity.49 In a 16-week study of bed rest in volunteers, markers of bone formation were reduced and markers of bone resorption increased, demonstrating the mechanisms for the profound and rapid loss of bone in immobilized patients.50
In a long-term cross-sectional study of paraplegic men with spinal cord injuries, bone turnover patterns changed over time.51 During the first year after injury, urinary deoxypyridinoline excretion was markedly elevated, whereas blood total alkaline phosphatase and osteocalcin levels were normal to slightly elevated. Over a 30-year period after injury, the bone resorption marker returned to normal levels in most patients and the bone formation markers were normal. Fracture incidence rose but leveled off after 20 years.
Bisphosphonate therapy in spinal cord injury patients reduces urinary NTx and prevents bone loss.52,53 These agents have also proven effective in reversing hypercalcemia in immobilized patients.54
In inflammatory bowel disease
Patients with inflammatory bowel disease, especially Crohn disease, have low bone mass and are at risk of fractures.55 These complications could be due to glucocorticoid therapy, hypogonadism, vitamin D defeciency, weight loss, and high circulating levels of bone-active cytokines released by inflammatory cells residing in the diseased intestine.
Bone formation markers have not been found to be outside the normal range, although both interleukin 1 and tumor necrosis factor alpha are known to inhibit bone formation.
Bisphosphonate treatment produces an increase in bone density concomitant with decreases in markers of bone resorption and formation.57,58 Of considerable interest is the observation that infliximab (anti-tumor necrosis factor alpha; Remicade) generally produces a rise in bone formation markers, with a smaller and inconsistent effect on bone resorption.59,60
In rheumatoid arthritis
The incidence of osteoporosis and fractures is also increased In patients with rheumatoid arthritis.61 As in patients with inflammatory bowel disease, a variety of factors can contribute to bone loss, including glucocorticoid therapy, hypogonadism, vitamin D deficiency, immobility, and elevated levels of bone-active cytokines.
Generally, studies have reported increased bone resorption based on type I collagen markers,62,63 whereas patients with osteoarthritis have levels of these bone resorption markers no different from those of control subjects.62 Although serum total TRAP protein is elevated in rheumatoid arthritis patients, this is probably due to the 5a isoform, the origin of which may be macrophages and dendritric cells.64
The influence of abnormalities in bone formation on bone loss is less clear. Levels of bone formation markers have been reported to be normal,65 elevated,66 or reduced.67
Treatment of rheumatoid arthritis with high-dose glucocorticoid pulse therapy is effective in controlling the symptoms and some manifestations of the immune system in patients with the disorder. The latter effect would be expected to have a beneficial effect on bone metabolism. This appears to be the case, as there are only transient decreases in bone formation markers and no significant reduction in bone density.68 Similarly, there is only a transient decrease in serum osteocalcin after an intra-articular injection of a glucocorticoid, and no effect on urinary pyridino-line.69
As would be expected, bisphosphonate therapy prevents bone loss in rheumatoid arthritis patients treated with glucocorticoids.70,71 Both oral and intravenous therapy decrease the levels of bone turnover markers.70–72 Infliximab therapy was shown to reduce the levels of bone resorption markers but not of PINP (a bone formation marker).73
In primary hyperparathyroidism
Hypersecretion of parathyroid hormone increases osteoclastic activity, with a secondary increase in osteoblastic activity. Bone loss may ensue and an increase in fracture incidence may be a consequence, particularly in post-menopausal women, who have the highest incidence of the disorder.74
Before screening chemistry panels became widely used during routine medical evaluations, it was not unusual to find elevated serum total alkaline phosphatase levels in patients discovered to have primary hyperparathyroidism. Today, this finding is not so common, as the disorder is diagnosed at a much earlier stage. Nevertheless, more specific and sensitive markers of bone turnover have made it possible to demonstrate the metabolic abnormalities that reflect the skeletal pathology in patients with primary hyperparathyroidism and its response to various therapies.75,76
On average, patients with untreated primary hyperparathyroidism have high levels of markers of bone resorption and formation, except in the mildest cases.73,74 Bone turnover returns to normal within 6 months to a year after successful parathyroidectomy.77,78 This response correlates with improvement in bone density, primarily in the lumbar spine.77,78
In patients who do not undergo surgery, alternative means of preventing bone loss include estrogen replacement in estrogendeficient postmenopausal women,76 bisphosphonates,79,80 and cinacalcet (Sensipar).81 Estrogen,76 raloxifene (Evista),82 and alendronate79,80 all reduce levels of bone resorption and formation markers, and estrogen76 and alendronate79,80 increase bone density. Although cinacalcet usually restores the serum calcium to the normal range and prevents bone loss, it only reduces serum parathyroid hormone levels by about 20%, and both bone resorption and formation markers increase above baseline.81 This could be related to fluctuations in serum parathyroid hormone that occur during each day of therapy.
In osteomalacia and rickets
Osteomalacia and rickets of any cause are characterized by increased osteoblastic activity. If the underlying cause is vitamin D deficiency, genetic or acquired defects in calcitriol synthesis, or vitamin D resistance, then hyper-parathyroidism with increased bone resorption is a secondary feature.
Serum total alkaline phosphatase activity has been a useful marker of disease activity for many years, although the newer markers, except for serum osteocalcin,83 are potentially more sensitive. The insensitivity of osteocalcin as an index of osteoblastic activity is unexplained but could be related to the state of differentiation of the osteoblasts. Bone resorption markers are elevated in vitamin D deficiency84 but are not widely used in clinical practice, as serum parathyroid hormone is an excellent indirect means of assessing the presence of increased bone resorption and the response to therapy.
In renal osteodystrophy
Bone disease associated with renal failure is termed renal osteodystrophy and is quite heterogeneous.85 Microscopic examination of a bone biopsy specimen is still considered the gold standard for diagnosis, and measurement in serum of intact parathyroid hormone is an important guide to diagnosis and response to therapy.
Nevertheless, recent studies suggest that serum markers of bone formation and resorption may be of additional help in assessing bone turnover.86 At present it is not certain whether any of the newer markers are superior to serum total alkaline phosphatase activity. Future studies that correlate bone histology with bone turnover markers should clarify the value of the various markers.
In cancer
Bone metastases are a common complication in cancer patients. They are classified as osteolytic, osteoblastic, or mixed on the basis of radiographic features. Biochemical markers of bone turnover have proven useful in assessing the magnitude of the metastases, the response to therapy, and even the prognosis for survival.87
Osteolytic metastases, which are common in breast cancer, are associated with increases in bone resorption markers, and after treatment with intravenous bisphosphonates the levels can decrease nearly 70%.88,89
Patients with higher levels of urinary NTx had a higher risk of skeletal complications and disease progression than patients with low levels across multiple tumor groups, including multiple myeloma.87
In osteoblastic metastases. Prostate cancer patients, who typically have predominantly osteoblastic lesions, have elevations of serum total alkaline phosphatase activity and other markers of bone formation.90 In addition, they have elevated bone resorption markers. Urinary NTx decreased markedly but serum bone-specific alkaline phosphatase decreased only slightly after treatment with intravenous zoledronic acid (Zometa),91 whereas androgen ablation therapy has inconsistent effects on bone turnover.92,93 High levels of these markers again predict poor prognosis.93,94
In hormone-suppression therapy. Two of the most successful cancer therapies, aromatase inhibitors for breast cancer95 and androgen ablation for prostate cancer,96 accelerate bone loss through marked suppression of gonadal steroids. Bone resorption and formation markers increase and bone loss ensues, with resorption exceeding formation. Estrogen suppression appears mainly responsible in both sexes, since raloxifene prevents bone loss in prostate cancer patients.97
Bisphosphonates are highly effective in preventing bone loss in either sex.98–100 A single infusion of zoledronic acid in androgen-ablated prostate cancer patients can prevent bone loss for at least 1 year.100
In Paget disease of bone
Paget disease of bone evolves over many years, from an early osteolytic phase to dominance of secondary osteoblastic activity. In patients with extensive polyostotic disease, bone resorption and formation marker levels may be higher than in almost any other skeletal disorder. An exception is serum osteocalcin,101 which once again usually does not accurately reflect the rate of bone formation.
Bisphosphonates, given orally or intravenously, produce an early decrease in bone resorption followed by a fall in bone formation.102 In clinical practice it appears adequate to use the least expensive test, serum total alkaline phosphatase activity, to assess disease activity and the response to therapy.103
Acknowledgments: Grant support to FRS from the Edythe and Eli Broad Foundation and Lois Rosen. Grant support to DRE from the National Institutes of Health (NIAMS: AR37318, AR36794).
Although no guidelines to date recommend their widespread use in clinical practice, we believe they will eventually be accepted. For example, markers of bone resorption are excellent indices of disease activity in patients with osteoporosis due to menopause, immobilization, or autoimmune processes, as well as Paget disease of bone or bone metastases. Normalization of the test results can be used to help establish the efficacy of treatment.
Similarly, markers of bone formation are excellent indices of disease activity in Paget disease, osteomalacia and rickets, osteoblastic bone metastases, and to a lesser extent in renal osteodystrophy. Again, successful treatment is associated with normalization of the tests.
This review summarizes some aspects of bone physiology and the pathogenesis of various metabolic bone disorders as a guide for clinicians considering using biochemical markers of osteoblast and osteoclast activity in patient management.
MARKERS OF BONE FORMATION
Osteoblasts are mononuclear cells that attach to bone surfaces and form new bone, most commonly at sites that recently underwent resorption. They produce type I collagen and other matrix components of osteoid, and they also mineralize the osteoid with hydroxyapatite.
Growing children have many more osteoblasts than adults.5 In elderly women, osteoblasts may increase in number in response to the increase in bone resorption brought on by estrogen deficiency. In elderly men, osteoblast activity may decrease,6 possibly because of decreasing levels of serum insulin-like growth factor 1 and testosterone.7
Markers of bone formation are measured in serum. Some are enzymes or other proteins secreted by osteoblasts, others are byproducts of type I collagen deposition.
Total alkaline phosphatase
Alkaline phosphatase, introduced into clinical use in 1929, was the first biochemical marker of bone turnover and is still the one most widely used in clinical practice. This enzyme is found in the plasma membrane of osteoblasts and in cells of the liver, kidney, intestine, spleen, and placenta. Its function is still not precisely known, but it is thought to play a role in osteoid formation and mineralization.
Bone alkaline phosphatase
In normal adults, about half the alkaline phosphatase in the serum comes from bone.1 Because alkaline phosphatase from different types of cells differs in its carbohydrate content, workers have been able to develop relatively specific immunoassays for alkaline phosphatase from bone, although there still is cross-reactivity of up to 20% between the bone and liver enzymes.2
Osteocalcin
Osteocalcin is a large peptide that is synthesized by osteoblasts, odontoblasts, and some chondrocytes. It binds to hydroxyapatite, and much of it is deposited in the bone matrix. Because osteocalcin fragments are released from the bone matrix during resorption, assays for circulating osteocalcin and its fragments reflect both bone formation and resorption.8 The exact function of osteocalcin in bone is still unclear, but recent studies raise the surprising possibility that it is a hormone that influences energy metabolism by modulating the production and action of insulin.9
Procollagen type I propeptides
Procollagen type I propeptides are cleaved from the ends of the procollagen molecule and can be detected in the circulation.1 Those from the amino-terminal end are called PINPs; those from the carboxy-terminal end are called PICPs. Although these propeptides are also synthesized in the skin, tendons, ligaments, cornea, blood vessels, fibrocartilage, and many other tissues, their main source is bone. The level of each of the propeptides in blood is thought to reflect the amount of newly synthesized collagen.
MARKERS OF BONE RESORPTION
Osteoclasts are multinucleated cells that resorb bone. They initiate bone remodeling and help shape growing bone and so are more numerous in children. They also liberate skeletal calcium to maintain a normal serum calcium concentration.5 Postmenopausal women who are estrogen-deficient tend to produce more osteoclasts, which accounts for the bone loss that can occur after menopause.
Markers of bone resorption are measured in serum or urine. The most direct indicators are fragments of bone collagen produced by osteoclast activity.1
Hydroxyproline
Hydroxyproline is an amino acid common to and characteristic of all forms of collagen, and urinary hydroxyproline excretion is the oldest test of bone resorption. However, this test lacks specificity for bone resorption because excreted hydroxyproline also comes from other tissues, particularly from skin collagen (which can turn over rapidly in certain disorders), from newly synthesized collagen that is not incorporated into tissue, and from dietary collagen and gelatin. Because it is less specific than newer tests, it is no longer widely used.
Collagen cross-links
Urinary pyridinoline and deoxypyridinoline are more specific markers of bone resorption.1
Pyridinolines are cross-linking amino acids that strengthen collagen fibrils in the extra-cellular matrix. They are found in the main fibril-forming collagens (types I, II, and III) of many tissues. Pyridinoline is the major chemical form, but deoxypyridinoline is also unusually abundant in bone collagen and hence is a relatively selective bone marker.
NTx. Since pyridinolines are not metabolized and are largely excreted as small peptides when produced by osteoclastic bone resorption, immunoassays have been developed that selectively measure cross-link-containing peptide fragments in urine and serum. The first was an assay that recognizes an N-telopeptide of collagen type I (NTx) in urine10 and serum.11 The recognized feature in this sequence is fully generated during the process of osteoclastic proteolysis and so requires no further metabolism by the liver or kidney for its production. Results from second-morning urine collections correlate well with those from 24-hour collections, which simplifies patient evaluation.
CTx. Several other assays target structural variants of a peptide sequence that originates from the carboxy-terminal cross-linking region of collagen type I (CTx).12,13
Other markers of bone resorption
Two enzymes found in osteoclasts have received attention as markers of osteoclast activity.
Serum tartrate-resistant acid phosphatase (TRAP) 5b has not been studied extensively in patients but appears to correlate with other markers of bone resorption.14
Serum cathepsin K is of interest because it is the primary proteolytic enzyme used by osteoclasts to degrade bone type I collagen during resorption. Several studies suggest it may be valuable as a marker of bone resorption,15 but more studies are required to evaluate its performance relative to established bone resorption markers.
Receptor activator of nuclear factor kappa (RANK), RANK ligand, and its decoy receptor osteoprotegerin are the pivotal regulators of osteoclast recruitment and activity.16 They may eventually be used as markers of bone metabolism, though the broad role of RANK ligand signaling in the immune system may limit its specificity.
FACTORS THAT INFLUENCE ASSAY RESULTS
To avoid being misled, clinicians who use biochemical markers of bone turnover should be familiar with factors that influence assay results.3
Diurnal and day-to-day variability
The most important biologic factors probably are diurnal and day-to-day variability in bone-forming and bone-resorbing activities. Levels of bone turnover markers are highest in the early morning and lowest in the afternoon and evening.
Levels of urinary markers can vary 20% to 30% from the highest to lowest value of the day. Serum markers change to a smaller degree except for serum CTx, which can vary by more than 60% during the day.17
In general, the day-to-day variability of urinary markers of bone resorption is similar in range to their diurnal variability. The serum markers of bone formation appear to vary less from day to day.
Eating, calcium intake
Blood for measurement of serum CTx should be taken in the morning after overnight fasting to avoid the large decrease that occurs after eating. An increase in calcium intake also can lower the levels of bone resorption markers, particularly in people whose calcium intake was previously low.18 Presumably, this effect is mediated by inhibition of parathyroid hormone secretion.
Sample handling
Improper collection and handling of specimens can seriously affect assay precision. The optimal time to collect samples is in the morning. Careful sample collection and storage are particularly important in measuring serum osteocalcin and TRAP. It is also important to use the same laboratory for serial measurements, since assay results can vary considerably among laboratories, even if they use identical methods.
BONE TURNOVER THROUGHOUT LIFE
In children, bone turnover can be more than 10 times greater than in adults because of three physiologic processes interacting in the skeleton: bone modeling, remodeling, and growth. Levels of bone formation and resorption markers therefore are much higher in children than in adults.19 Unfortunately, no studies have compared all the available markers in the same pediatric reference population.
In puberty, bone growth accelerates, with an increase in bone turnover markers that reflects the effect of hormones that induce the growth spurt.1
Postmenopausal women who do not use hormone replacement therapy have higher levels of bone resorption and formation markers than premenopausal women.20 Levels in postmenopausal women on hormone replacement are no different than in premenopausal women.20,21 In postmenopausal women not on estrogen, urinary levels of NTx have been reported to discriminate between normal bone mineral density (lowest NTx levels), osteopenia, and osteoporosis (highest levels).22 Normal levels of NTx are found in a small percentage of women. This may be explained by the variable levels of serum estradiol in post-menopausal women.23
Elderly men, in contrast, have variable findings.24–28 However, accelerated bone turnover has been noted in men with full-blown hypogonadism caused by androgen suppression therapy.29
CLINICAL APPLICATIONS OF BONE TURNOVER MARKERS
In postmenopausal osteoporosis
Markers of bone formation are somewhat less likely to be elevated than markers of bone resorption, and if they are elevated, they decrease as expected in response to therapy that inhibits bone resorption, though more gradually and to a lesser extent than the resorption markers.31–35
To monitor bisphosphonate therapy. Antiresorptive drugs such as bisphosphonates reduce the risk of fracture, as they increase bone density and decrease the rate of bone resorption, as shown in many clinical trials.31–35 Because the rate of bone resorption reaches a nadir within 3 to 6 months of starting bisphosphonate therapy and because the increase in bone density after 1 year is quite modest (about 3%–4%), most of the decrease in vertebral fracture incidence, which becomes apparent during the first year of treatment, probably can be attributed more to normalization of bone resorption (and a less perforated structure) than to the increase in bone density.36 This would suggest that it is more appropriate to document that bone resorption has been inhibited than to measure bone density every year when following patients taking antiresorptive agents.
Furthermore, effective antiresorptive therapy reduces the levels of resorption markers by 50% to 70%,32–35 whereas after 1 year bone density has generally not increased more than the error of the bone density measurement. This observation has led to the suggestion that bone density measurements generally should not be done more often than every 2 years when following the effects of antiresorptive therapy. Even with a 20% to 30% day-to-day variation in levels of bone resorption markers, it is easier to document the efficacy of therapy with resorption biomarkers than with bone density.
To document compliance. Another reason to consider measuring a resorption marker (after 3 months of therapy) is to document compliance, a considerable problem in the treatment of an asymptomatic disorder.
To help decide when to restart bisphosphonate therapy. After long-term treatment with a bisphosphonate, the drug may be retained in the skeleton for years. This seems particularly true of alendronate (Fosamax).37 After 5 years of continuous alendronate treatment, bone resorption continues to be suppressed near the maximal level, in some patients for years after they stop taking the drug.38
Once the bone resorption marker begins to approach the pretreatment level, it would signal a possible need to restart the therapy. If a pretreatment level was not measured, an estimate of significant bone resorption would be signaled when the resorption marker is more than 20% above the mean premenopausal level. For urinary NTx this would be more than 42 nmol bone collagen equivalents/mmol creatinine.
In glucocorticoid-induced osteoporosis
Glucocorticoid therapy causes bone loss and an increased incidence of fractures when given in high doses or for prolonged periods by the oral, parenteral, or inhaled routes.41
The pathogenesis of the bone loss has been explored by measurements of bone turnover markers. During glucocorticoid therapy, levels of bone formation markers are generally low and those of bone resorption markers are either normal or low.42–44 Presumably, the reduction in bone resorption is not enough to overcome the reduction in bone formation, and bone loss ensues. In children, the effects on bone formation are particularly profound, as linear growth may be retarded.44
Giving a bisphosphonate during glucocorticoid therapy is quite effective in increasing bone density and preventing fractures.45–47 Patients who receive alendronate have lower levels of bone formation and resorption markers than do untreated subjects.45 Presumably, bone resorption is inhibited more than bone formation, accounting for the skeletal benefits.
In a recent study in patients with glucocorticoid-induced osteoporosis, bone mineral density of the lumbar spine increased more than twice as much with teriparatide than with alendronate over an 18-month period.48 As would be expected from the results of teriparatide therapy in postmenopausal osteoporosis, indices of both bone formation and resorption rose to a peak at 6 months, with formation greater than resorption.
In immobilization-induced osteoporosis
Studies of normal volunteers placed on bed rest indicate that urinary CTx and NTx excretion increase significantly after 24 hours, no doubt reflecting a rapid increase in osteoclast activity.49 In a 16-week study of bed rest in volunteers, markers of bone formation were reduced and markers of bone resorption increased, demonstrating the mechanisms for the profound and rapid loss of bone in immobilized patients.50
In a long-term cross-sectional study of paraplegic men with spinal cord injuries, bone turnover patterns changed over time.51 During the first year after injury, urinary deoxypyridinoline excretion was markedly elevated, whereas blood total alkaline phosphatase and osteocalcin levels were normal to slightly elevated. Over a 30-year period after injury, the bone resorption marker returned to normal levels in most patients and the bone formation markers were normal. Fracture incidence rose but leveled off after 20 years.
Bisphosphonate therapy in spinal cord injury patients reduces urinary NTx and prevents bone loss.52,53 These agents have also proven effective in reversing hypercalcemia in immobilized patients.54
In inflammatory bowel disease
Patients with inflammatory bowel disease, especially Crohn disease, have low bone mass and are at risk of fractures.55 These complications could be due to glucocorticoid therapy, hypogonadism, vitamin D defeciency, weight loss, and high circulating levels of bone-active cytokines released by inflammatory cells residing in the diseased intestine.
Bone formation markers have not been found to be outside the normal range, although both interleukin 1 and tumor necrosis factor alpha are known to inhibit bone formation.
Bisphosphonate treatment produces an increase in bone density concomitant with decreases in markers of bone resorption and formation.57,58 Of considerable interest is the observation that infliximab (anti-tumor necrosis factor alpha; Remicade) generally produces a rise in bone formation markers, with a smaller and inconsistent effect on bone resorption.59,60
In rheumatoid arthritis
The incidence of osteoporosis and fractures is also increased In patients with rheumatoid arthritis.61 As in patients with inflammatory bowel disease, a variety of factors can contribute to bone loss, including glucocorticoid therapy, hypogonadism, vitamin D deficiency, immobility, and elevated levels of bone-active cytokines.
Generally, studies have reported increased bone resorption based on type I collagen markers,62,63 whereas patients with osteoarthritis have levels of these bone resorption markers no different from those of control subjects.62 Although serum total TRAP protein is elevated in rheumatoid arthritis patients, this is probably due to the 5a isoform, the origin of which may be macrophages and dendritric cells.64
The influence of abnormalities in bone formation on bone loss is less clear. Levels of bone formation markers have been reported to be normal,65 elevated,66 or reduced.67
Treatment of rheumatoid arthritis with high-dose glucocorticoid pulse therapy is effective in controlling the symptoms and some manifestations of the immune system in patients with the disorder. The latter effect would be expected to have a beneficial effect on bone metabolism. This appears to be the case, as there are only transient decreases in bone formation markers and no significant reduction in bone density.68 Similarly, there is only a transient decrease in serum osteocalcin after an intra-articular injection of a glucocorticoid, and no effect on urinary pyridino-line.69
As would be expected, bisphosphonate therapy prevents bone loss in rheumatoid arthritis patients treated with glucocorticoids.70,71 Both oral and intravenous therapy decrease the levels of bone turnover markers.70–72 Infliximab therapy was shown to reduce the levels of bone resorption markers but not of PINP (a bone formation marker).73
In primary hyperparathyroidism
Hypersecretion of parathyroid hormone increases osteoclastic activity, with a secondary increase in osteoblastic activity. Bone loss may ensue and an increase in fracture incidence may be a consequence, particularly in post-menopausal women, who have the highest incidence of the disorder.74
Before screening chemistry panels became widely used during routine medical evaluations, it was not unusual to find elevated serum total alkaline phosphatase levels in patients discovered to have primary hyperparathyroidism. Today, this finding is not so common, as the disorder is diagnosed at a much earlier stage. Nevertheless, more specific and sensitive markers of bone turnover have made it possible to demonstrate the metabolic abnormalities that reflect the skeletal pathology in patients with primary hyperparathyroidism and its response to various therapies.75,76
On average, patients with untreated primary hyperparathyroidism have high levels of markers of bone resorption and formation, except in the mildest cases.73,74 Bone turnover returns to normal within 6 months to a year after successful parathyroidectomy.77,78 This response correlates with improvement in bone density, primarily in the lumbar spine.77,78
In patients who do not undergo surgery, alternative means of preventing bone loss include estrogen replacement in estrogendeficient postmenopausal women,76 bisphosphonates,79,80 and cinacalcet (Sensipar).81 Estrogen,76 raloxifene (Evista),82 and alendronate79,80 all reduce levels of bone resorption and formation markers, and estrogen76 and alendronate79,80 increase bone density. Although cinacalcet usually restores the serum calcium to the normal range and prevents bone loss, it only reduces serum parathyroid hormone levels by about 20%, and both bone resorption and formation markers increase above baseline.81 This could be related to fluctuations in serum parathyroid hormone that occur during each day of therapy.
In osteomalacia and rickets
Osteomalacia and rickets of any cause are characterized by increased osteoblastic activity. If the underlying cause is vitamin D deficiency, genetic or acquired defects in calcitriol synthesis, or vitamin D resistance, then hyper-parathyroidism with increased bone resorption is a secondary feature.
Serum total alkaline phosphatase activity has been a useful marker of disease activity for many years, although the newer markers, except for serum osteocalcin,83 are potentially more sensitive. The insensitivity of osteocalcin as an index of osteoblastic activity is unexplained but could be related to the state of differentiation of the osteoblasts. Bone resorption markers are elevated in vitamin D deficiency84 but are not widely used in clinical practice, as serum parathyroid hormone is an excellent indirect means of assessing the presence of increased bone resorption and the response to therapy.
In renal osteodystrophy
Bone disease associated with renal failure is termed renal osteodystrophy and is quite heterogeneous.85 Microscopic examination of a bone biopsy specimen is still considered the gold standard for diagnosis, and measurement in serum of intact parathyroid hormone is an important guide to diagnosis and response to therapy.
Nevertheless, recent studies suggest that serum markers of bone formation and resorption may be of additional help in assessing bone turnover.86 At present it is not certain whether any of the newer markers are superior to serum total alkaline phosphatase activity. Future studies that correlate bone histology with bone turnover markers should clarify the value of the various markers.
In cancer
Bone metastases are a common complication in cancer patients. They are classified as osteolytic, osteoblastic, or mixed on the basis of radiographic features. Biochemical markers of bone turnover have proven useful in assessing the magnitude of the metastases, the response to therapy, and even the prognosis for survival.87
Osteolytic metastases, which are common in breast cancer, are associated with increases in bone resorption markers, and after treatment with intravenous bisphosphonates the levels can decrease nearly 70%.88,89
Patients with higher levels of urinary NTx had a higher risk of skeletal complications and disease progression than patients with low levels across multiple tumor groups, including multiple myeloma.87
In osteoblastic metastases. Prostate cancer patients, who typically have predominantly osteoblastic lesions, have elevations of serum total alkaline phosphatase activity and other markers of bone formation.90 In addition, they have elevated bone resorption markers. Urinary NTx decreased markedly but serum bone-specific alkaline phosphatase decreased only slightly after treatment with intravenous zoledronic acid (Zometa),91 whereas androgen ablation therapy has inconsistent effects on bone turnover.92,93 High levels of these markers again predict poor prognosis.93,94
In hormone-suppression therapy. Two of the most successful cancer therapies, aromatase inhibitors for breast cancer95 and androgen ablation for prostate cancer,96 accelerate bone loss through marked suppression of gonadal steroids. Bone resorption and formation markers increase and bone loss ensues, with resorption exceeding formation. Estrogen suppression appears mainly responsible in both sexes, since raloxifene prevents bone loss in prostate cancer patients.97
Bisphosphonates are highly effective in preventing bone loss in either sex.98–100 A single infusion of zoledronic acid in androgen-ablated prostate cancer patients can prevent bone loss for at least 1 year.100
In Paget disease of bone
Paget disease of bone evolves over many years, from an early osteolytic phase to dominance of secondary osteoblastic activity. In patients with extensive polyostotic disease, bone resorption and formation marker levels may be higher than in almost any other skeletal disorder. An exception is serum osteocalcin,101 which once again usually does not accurately reflect the rate of bone formation.
Bisphosphonates, given orally or intravenously, produce an early decrease in bone resorption followed by a fall in bone formation.102 In clinical practice it appears adequate to use the least expensive test, serum total alkaline phosphatase activity, to assess disease activity and the response to therapy.103
Acknowledgments: Grant support to FRS from the Edythe and Eli Broad Foundation and Lois Rosen. Grant support to DRE from the National Institutes of Health (NIAMS: AR37318, AR36794).
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005; 2:504–517.
- Seibel MJ. Biochemical markers of bone turnover: part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006; 27:123–138.
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000; 26:103–109.
- Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996; 81:2264–2270.
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). 2004; 60:491–499.
- Cloos PA, Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004; 50:585–598.
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–469.
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992; 7:1251–1258.
- Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997; 43:2058–2063.
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–785.
- Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step, ELISA a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998; 44:2290–2300.
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004; 34:187–194.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget’s disease. Clin Lab. 2006; 52:1–10.
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(suppl 1):S1.
- Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999; 45:1858–1860.
- Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004; 15:290–294.
- Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007; 92:443–449.
- Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–3371.
- Prestwood KM, Pilbeam CC, Burleson JA, et al. The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab. 1994; 79:366–371.
- Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997; 157:1241–1245.
- Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra low-dose transdermal estradiol therapy on bone turnover and bone density in postmenopausal women. J Bone Miner Res. 2007; 22:1791–1797.
- Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274.
- Goemaere S, Van Pottelbergh I, Zmierczak H, et al. Inverse association between bone turnover rate and bone mineral density in community-dwelling men >70 years of age: no major role of sex steroid status. Bone. 2001; 29:286–291.
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001; 16:1642–1650.
- Scopacasa F, Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 2002; 57:M385–M391.
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 2007; 8:13.
- Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345:948–955.
- Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–1443.
- Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–1258.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004; 15:792–798.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
- Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000; 11:434–442.
- Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005; 20:962–970.
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005; 34:341–356.
- Ebeling PR, Erbas B, Hopper JL, Wark JD, Rubinfeld AR. Bone mineral density and bone turnover in asthmatics treated with long-term inhaled or oral glucocorticoids. J Bone Miner Res. 1998; 13:1283–1289.
- Siomou E, Challa A, Tzoufi M, Papadopoulou ZL, Lapatsanis PD, Siamopoulou A. Biochemical markers of bone metabolism in infants and children under intravenous corticosteroid therapy. Calcif Tissue Int. 2003; 73:319–325.
- Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002; 57:185–191.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001; 44:202–211.
- Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001; 69:242–247.
- Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003; 14:801–807.
- Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–2039.
- Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 2005; 57:31–36.
- Scheld K, Zittermann A, Heer M, et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001; 47:1688–1695.
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15:180–189.
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999; 80:243–251.
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007; 92:1385–1390.
- Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999; 80:998–1000.
- van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003; 125:1591–1597.
- Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000; 95:699–704.
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology. 2000; 119:639–646.
- Palomba S, Orio F, Manguso F, et al. Efficacy of risedronate administration in osteoporotic postmenopausal women affected by inflammatory bowel disease. Osteoporos Int. 2005; 16:1141–1149.
- Franchimont N, Putzeys V, Collette J, et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2004; 20:607–614.
- Ryan BM, Russel MG, Schurgers L, et al. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004; 20:851–857.
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006; 54:3104–3112.
- Wong PK, Young L, Vaile JH, et al. Telopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritis. Intern Med J. 2004; 34:539–544.
- Momohara S, Okamoto H, Yago T, et al. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005; 15:410–414.
- Janckila AJ, Neustadt DH, Nakasato YR, Halleen JM, Hentunen T, Yam LT. Serum tartrate-resistant acid phosphatase isoforms in rheumatoid arthritis. Clin Chim Acta. 2002; 320:49–58.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996; 55:288–293.
- Manrique F, Gamardo J, de Elguezabal K, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J Clin Rheumatol. 2003; 9:219–227.
- Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999; 24:381–385.
- Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methyl-prednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004; 31:1083–1087.
- Emkey RD, Lindsay R, Lyssy J, Weisberg JS, Dempster DW, Shen V. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996; 39:277–282.
- Lange U, Illgner U, Teichmann J, Schleenbecker H. Skeletal benefit after one year of risedronate therapy in patients with rheumatoid arthritis and glucocorticoid-induced osteoporosis: a prospective study. Int J Clin Pharmacol Res. 2004; 24:33–38.
- Tascioglu F, Colak O, Armagan O, Alatas O, Oner C. The treatment of osteoporosis in patients with rheumatoid arthritis receiving glucocorticoids: a comparison of alendronate and intranasal salmon calcitonin. Rheumatol Int. 2005; 26:21–29.
- Cremers SC, Lodder MC, Den Hartigh J, et al. Short term whole body retention in relation to rate of bone resorption and cartilage degradation after intravenous bisphosphonate (pamidronate) in rheumatoid arthritis. J Rheumatol. 2004; 31:1732–1737.
- Chopin F, Garnero P, Le Henanff A, et al. Long term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2007; 67:353–357.
- Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999; 14:1700–1707.
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:3487–3491.
- Orr-Walker BJ, Evans MC, Clearwater JM, Horne A, Grey AB, Reid IR. Effects of hormone replacement therapy on bone mineral density in postmenopausal women with primary hyperparathyroidism: four-year follow-up and comparison with healthy postmenopausal women. Arch Intern Med. 2000; 160:2161–2166.
- Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999; 25:237–244.
- Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5-year follow-up study. J Bone Miner Metab. 2007; 25:226–231.
- Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:581–587.
- Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3319–3325.
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005; 90:135–141.
- Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:1174–1178.
- Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000; 159:730–733.
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006; 368:48–52.
- Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004; 43:558–565.
- Malyszko J, Wolczynski S, Malyszko JS, Konstantynowicz J, Kaczmarski M, Mysliwiec M. Correlations of new markers of bone formation and resorption in kidney transplant recipients. Transplant Proc. 2003; 35:1351–1354.
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005; 23:4925–4935.
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997; 75:408–412.
- Coleman RE Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol 2002; 25(suppl 1):S25–S31.
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev 2006; 32(suppl 1):23–26.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468.
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998; 83:1561–1566.
- Johansen JS, Brasso K, Iversen P, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007; 13:3244–3249.
- Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006; 12:3361–3367.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006; 21:1215–1223.
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12:6315s–6319s.
- Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:3841–3846.
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007; 41:346–352.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007; 146:416–424.
- Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042.
- Kaddam IM, Iqbal SJ, Holland S, Wong M, Manning D. Comparison of serum osteocalcin with total and bone specific alkaline phosphatase and urinary hydroxyproline:creatinine ratio in patients with Paget’s disease of bone. Ann Clin Biochem. 1994; 31:327–330.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med. 2005; 353:898–908.
- Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004; 35:224–230.
- Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005; 2:504–517.
- Seibel MJ. Biochemical markers of bone turnover: part II: clinical applications in the management of osteoporosis. Clin Biochem Rev. 2006; 27:123–138.
- Glorieux FH, Travers R, Taylor A, et al. Normative data for iliac bone histomorphometry in growing children. Bone. 2000; 26:103–109.
- Clarke BL, Ebeling PR, Jones JD, et al. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996; 81:2264–2270.
- Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). 2004; 60:491–499.
- Cloos PA, Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004; 50:585–598.
- Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–469.
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992; 7:1251–1258.
- Clemens JD, Herrick MV, Singer FR, Eyre DR. Evidence that serum NTx (collagen-type I N-telopeptides) can act as an immunochemical marker of bone resorption. Clin Chem. 1997; 43:2058–2063.
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994; 79:780–785.
- Christgau S, Rosenquist C, Alexandersen P, et al. Clinical evaluation of the Serum CrossLaps One Step, ELISA a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem. 1998; 44:2290–2300.
- Hannon RA, Clowes JA, Eagleton AC, Al Hadari A, Eastell R, Blumsohn A. Clinical performance of immunoreactive tartrate-resistant acid phosphatase isoform 5b as a marker of bone resorption. Bone. 2004; 34:187–194.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget’s disease. Clin Lab. 2006; 52:1–10.
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 2007; 9(suppl 1):S1.
- Wichers M, Schmidt E, Bidlingmaier F, Klingmuller D. Diurnal rhythm of CrossLaps in human serum. Clin Chem. 1999; 45:1858–1860.
- Kenny AM, Prestwood KM, Biskup B, et al. Comparison of the effects of calcium loading with calcium citrate or calcium carbonate on bone turnover in postmenopausal women. Osteoporos Int. 2004; 15:290–294.
- Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007; 92:443–449.
- Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–3371.
- Prestwood KM, Pilbeam CC, Burleson JA, et al. The short-term effects of conjugated estrogen on bone turnover in older women. J Clin Endocrinol Metab. 1994; 79:366–371.
- Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997; 157:1241–1245.
- Huang AJ, Ettinger B, Vittinghoff E, Ensrud KE, Johnson KC, Cummings SR. Endogenous estrogen levels and the effects of ultra low-dose transdermal estradiol therapy on bone turnover and bone density in postmenopausal women. J Bone Miner Res. 2007; 22:1791–1797.
- Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998; 83:2266–2274.
- Goemaere S, Van Pottelbergh I, Zmierczak H, et al. Inverse association between bone turnover rate and bone mineral density in community-dwelling men >70 years of age: no major role of sex steroid status. Bone. 2001; 29:286–291.
- Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001; 16:1642–1650.
- Scopacasa F, Wishart JM, Need AG, Horowitz M, Morris HA, Nordin BE. Bone density and bone-related biochemical variables in normal men: a longitudinal study. J Gerontol A Biol Sci Med Sci 2002; 57:M385–M391.
- Nguyen TV, Meier C, Center JR, Eisman JA, Seibel MJ. Bone turnover in elderly men: relationships to change in bone mineral density. BMC Musculoskelet Disord 2007; 8:13.
- Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001; 345:948–955.
- Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–1443.
- Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004; 19:1250–1258.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004; 15:792–798.
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003; 18:1051–1056.
- Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004; 20:1291–1300.
- Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006; 296:2927–2938.
- Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000; 11:434–442.
- Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005; 20:962–970.
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am. 2005; 34:341–356.
- Ebeling PR, Erbas B, Hopper JL, Wark JD, Rubinfeld AR. Bone mineral density and bone turnover in asthmatics treated with long-term inhaled or oral glucocorticoids. J Bone Miner Res. 1998; 13:1283–1289.
- Siomou E, Challa A, Tzoufi M, Papadopoulou ZL, Lapatsanis PD, Siamopoulou A. Biochemical markers of bone metabolism in infants and children under intravenous corticosteroid therapy. Calcif Tissue Int. 2003; 73:319–325.
- Ahmed SF, Tucker P, Mushtaq T, Wallace AM, Williams DM, Hughes IA. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol (Oxf). 2002; 57:185–191.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001; 44:202–211.
- Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001; 69:242–247.
- Ringe JD, Dorst A, Faber H, Ibach K, Sorenson F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003; 14:801–807.
- Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007; 357:2028–2039.
- Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut. 2005; 57:31–36.
- Scheld K, Zittermann A, Heer M, et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin Chem. 2001; 47:1688–1695.
- Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15:180–189.
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999; 80:243–251.
- Gilchrist NL, Frampton CM, Acland RH, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007; 92:1385–1390.
- Massagli TL, Cardenas DD. Immobilization hypercalcemia treatment with pamidronate disodium after spinal cord injury. Arch Phys Med Rehabil. 1999; 80:998–1000.
- van Staa TP, Cooper C, Brusse LS, Leufkens H, Javaid MK, Arden NK. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003; 125:1591–1597.
- Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000; 95:699–704.
- Haderslev KV, Tjellesen L, Sorensen HA, Staun M. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology. 2000; 119:639–646.
- Palomba S, Orio F, Manguso F, et al. Efficacy of risedronate administration in osteoporotic postmenopausal women affected by inflammatory bowel disease. Osteoporos Int. 2005; 16:1141–1149.
- Franchimont N, Putzeys V, Collette J, et al. Rapid improvement of bone metabolism after infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2004; 20:607–614.
- Ryan BM, Russel MG, Schurgers L, et al. Effect of antitumour necrosis factor-alpha therapy on bone turnover in patients with active Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004; 20:851–857.
- van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006; 54:3104–3112.
- Wong PK, Young L, Vaile JH, et al. Telopeptides as markers of bone turnover in rheumatoid arthritis and osteoarthritis. Intern Med J. 2004; 34:539–544.
- Momohara S, Okamoto H, Yago T, et al. The study of bone mineral density and bone turnover markers in postmenopausal women with active rheumatoid arthritis. Mod Rheumatol. 2005; 15:410–414.
- Janckila AJ, Neustadt DH, Nakasato YR, Halleen JM, Hentunen T, Yam LT. Serum tartrate-resistant acid phosphatase isoforms in rheumatoid arthritis. Clin Chim Acta. 2002; 320:49–58.
- Lems WF, Gerrits MI, Jacobs JW, van Vugt RM, van Rijn HJ, Bijlsma JW. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996; 55:288–293.
- Manrique F, Gamardo J, de Elguezabal K, et al. Abnormalities of bone mineral density and bone metabolism in Venezuelan patients with rheumatoid arthritis. J Clin Rheumatol. 2003; 9:219–227.
- Garnero P, Jouvenne P, Buchs N, Delmas PD, Miossec P. Uncoupling of bone metabolism in rheumatoid arthritis patients with or without joint destruction: assessment with serum type I collagen breakdown products. Bone. 1999; 24:381–385.
- Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methyl-prednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol. 2004; 31:1083–1087.
- Emkey RD, Lindsay R, Lyssy J, Weisberg JS, Dempster DW, Shen V. The systemic effect of intraarticular administration of corticosteroid on markers of bone formation and bone resorption in patients with rheumatoid arthritis. Arthritis Rheum. 1996; 39:277–282.
- Lange U, Illgner U, Teichmann J, Schleenbecker H. Skeletal benefit after one year of risedronate therapy in patients with rheumatoid arthritis and glucocorticoid-induced osteoporosis: a prospective study. Int J Clin Pharmacol Res. 2004; 24:33–38.
- Tascioglu F, Colak O, Armagan O, Alatas O, Oner C. The treatment of osteoporosis in patients with rheumatoid arthritis receiving glucocorticoids: a comparison of alendronate and intranasal salmon calcitonin. Rheumatol Int. 2005; 26:21–29.
- Cremers SC, Lodder MC, Den Hartigh J, et al. Short term whole body retention in relation to rate of bone resorption and cartilage degradation after intravenous bisphosphonate (pamidronate) in rheumatoid arthritis. J Rheumatol. 2004; 31:1732–1737.
- Chopin F, Garnero P, Le Henanff A, et al. Long term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann Rheum Dis. 2007; 67:353–357.
- Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon W, Riggs B. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999; 14:1700–1707.
- Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996; 81:3487–3491.
- Orr-Walker BJ, Evans MC, Clearwater JM, Horne A, Grey AB, Reid IR. Effects of hormone replacement therapy on bone mineral density in postmenopausal women with primary hyperparathyroidism: four-year follow-up and comparison with healthy postmenopausal women. Arch Intern Med. 2000; 160:2161–2166.
- Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone. 1999; 25:237–244.
- Tamura Y, Araki A, Chiba Y, Mori S, Hosoi T, Horiuchi T. Remarkable increase in lumbar spine bone mineral density and amelioration in biochemical markers of bone turnover after parathyroidectomy in elderly patients with primary hyperparathyroidism: a 5-year follow-up study. J Bone Miner Metab. 2007; 25:226–231.
- Chow CC, Chan WB, Li JK, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:581–587.
- Khan AA, Bilezikian JP, Kung AW, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004; 89:3319–3325.
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005; 90:135–141.
- Rubin MR, Lee KH, McMahon DJ, Silverberg SJ. Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003; 88:1174–1178.
- Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000; 159:730–733.
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006; 368:48–52.
- Martin KJ, Olgaard K, Coburn JW, et al. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004; 43:558–565.
- Malyszko J, Wolczynski S, Malyszko JS, Konstantynowicz J, Kaczmarski M, Mysliwiec M. Correlations of new markers of bone formation and resorption in kidney transplant recipients. Transplant Proc. 2003; 35:1351–1354.
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005; 23:4925–4935.
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997; 75:408–412.
- Coleman RE Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol 2002; 25(suppl 1):S25–S31.
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev 2006; 32(suppl 1):23–26.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468.
- Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998; 83:1561–1566.
- Johansen JS, Brasso K, Iversen P, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res. 2007; 13:3244–3249.
- Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006; 12:3361–3367.
- Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res. 2006; 21:1215–1223.
- Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res 2006; 12:6315s–6319s.
- Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004; 89:3841–3846.
- Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007; 41:346–352.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007; 146:416–424.
- Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042.
- Kaddam IM, Iqbal SJ, Holland S, Wong M, Manning D. Comparison of serum osteocalcin with total and bone specific alkaline phosphatase and urinary hydroxyproline:creatinine ratio in patients with Paget’s disease of bone. Ann Clin Biochem. 1994; 31:327–330.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med. 2005; 353:898–908.
- Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004; 35:224–230.
KEY POINTS
- Biomarkers of bone formation and resorption reflect the overall osteoblastic and osteoclastic activity in the skeleton and in some situations may serve as surrogates for histologic examination of bone.
- Biomarkers of bone turnover can be used to document the effects of therapeutic agents in some patients with osteoporosis and possibly reduce the need for frequent bone density testing.
- In cancer patients with bone metastases, biomarkers of bone resorption provide evidence of the efficacy of antiresorptive therapy. The baseline levels also have prognostic value: patients with the highest levels have the worst prognosis.