User login
Addressing the Shortage of Physician Assistants in Medicine Clerkship Sites
The Federal Bureau of Labor Statistics projects 37% job growth for physician assistants (PAs) from 2016 to 2026, much greater than the average for all other occupations as well as for other medical professions.1 This growth has been accompanied by increased enrollment in medical (doctor of medicine [MD], doctor of osteopathic medicine) and nurse practitioner (NP) schools.2 Clinical teaching sites serve a crucial function in the training of all clinical disciplines. These sites provide hands-on and experiential learning in medical settings, necessary components for learners practicing to become clinicians. Significant PA program expansion has led to increased demand for clinical training, creating competition for sites and a shortage of willing and well-trained preceptors.3
This challenge has been recognized by PA program directors. In the Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey, PA program directors expressed concern about the adequacy of clinical opportunities for students, increased difficulty developing new core sites, and preserving existing core sites. In addition, they noted that a shortage of clinical sites was one of the greatest barriers to the PA programs’ sustained growth and success.4
Program directors also indicated difficulty securing clinical training sites in internal medicine (IM) and high rates of attrition of medicine clinical preceptors for their students.5 The reasons are multifold: increasing clinical demands, time, teaching competence, lack of experience, academic affiliation, lack of reimbursement, or compensation. Moreover, there is a declining number of PAs who work in primary care compared with specialty and subspecialty care, limiting the availability of clinical training preceptors in medicine and primary care.6-8 According to the American Academy of PAs (AAPA) census and salary survey data, the percentage of PAs working in the primary care specialties (ie, family medicine, IM, and general pediatrics) has decreased from > 47% in 1995 to 24% in 2017.9 As such, there is a need to broaden the educational landscape to provide more high-quality training sites in IM.
The postacute health care setting may address this training need. It offers a unique clinical opportunity to expose learners to a broad range of disease complexity and clinical acuity, as the percentage of patients discharged from hospitals to postacute care (PAC) has increased and care shifts from the hospital to the PAC setting.10,11 The longer PAC length of stay also enables learners to follow patients longitudinally over several weeks and experience interprofessional team-based care. In addition, the PAC setting offers learners the ability to acquire the necessary skills for smooth and effective transitions of care. This setting has been extensively used for trainees of nursing, pharmacy, physical therapy (PT) and occupational therapy (OT), speech-language pathology, psychology, and social work (SW), but few programs have used the PAC setting as clerkship sites for IM rotations for PA students. To address this need for IM sites, the VA Boston Healthcare System (VABHS), in conjunction with the Boston University School of Medicine Physician Assistant Program, developed a novel medicine clinical clerkship site for physician assistants in the PAC unit of the community living center (CLC) at VABHS. This report describes the program structure, curriculum, and participant evaluation results.
Clinical Clerkship Program
VABHS CLC is a 110-bed facility comprising 3 units: a 65-bed PAC unit, a 15-bed closed hospice/palliative care unit, and a 30-bed long-term care unit. The service is staffed continuously with physicians, PAs, and NPs. A majority of patients are admitted from the acute care hospital of VABHS (West Roxbury campus) and other regional VA facilities. The CLC offers dynamic services, including phlebotomy, general radiology, IV diuretics and antibiotics, wound care, and subacute PT, OT, and speech-language pathology rehabilitation. The CLC serves as a venue for transitioning patients from acute inpatient care to home. The patient population is often elderly, with multiple active comorbidities and variable medical literacy, adherence, and follow-up.
The CLC provides a diverse interprofessional learning environment, offering core IM rotations for first-year psychiatry residents, oral and maxillofacial surgery residents, and PA students. The CLC also has expanded as a clinical site both for transitions-in-care IM resident curricula and electives as well as a geriatrics fellowship. In addition, the site offers rotations for NPs, nursing, pharmacy, physical and occupational therapies, speech-language pathology, psychology, and SW.
The Boston University School of Medicine Physician Assistant Program was founded in 2015 as a master’s degree program completed over 28 months. The first 12 months are didactic, and the following 16 months are clinical training with 14 months of rotations (2 IM, family medicine, pediatrics, emergency medicine, general surgery, obstetrics and gynecology, psychiatry, neurology, and 5 elective rotations), and 2 months for a thesis. The program has about 30 students per year and 4 clerkship sites for IM.
Program Description
The VABHS medicine clerkship hosts 1 to 2 PA students for 4-week blocks in the PAC unit of the CLC. Each student rotates on both PA and MD teams. Students follow 3 to 4 patients and participate fully in their care from admission to discharge; they prepare daily presentations and participate in medical management, family meetings, chart documentation, and care coordination with the interprofessional team. Students are provided a physical examination checklist and feedback form, and they are expected to track findings and record feedback and goals with their supervising preceptor weekly. They also make formal case presentations and participate in monthly medicine didactic rounds available to all VABHS IM students and trainees via videoconference.
In addition, beginning in July 2017, all PA students in the CLC began to participate in a 4-week Interprofessional Curriculum in Transitional Care. The curriculum includes 14 didactic lectures taught by 16 interprofessional faculty, including medicine, geriatric, and palliative care physicians; PAs; social workers; physical and occupational therapists; pharmacists; and a geriatric psychologist. The didactics include topics on the interprofessional team, the care continuum, teams and teamwork, interdisciplinary coordination of care, components of effective transitions in care, medication reconciliation, approaching difficult conversations, advance care planning, and quality improvement. The goal of the curriculum is to provide learners the knowledge, skills, and dispositions necessary for high-quality transitional care and interprofessional practice as well as specific training for effective and safe transfers of care between clinical settings. Although PA students are the main participants in this curriculum, all other learners in the PAC unit are also invited to attend the lectures.
The unique attributes of this training site include direct interaction with supervising PAs and physicians, rather than experiencing the traditional teaching hierarchy (with interns, residents, fellows); observation of the natural progression of disease of both acute care and primary care issues due to the longer length of stay (2 to 6 weeks, where the typical student will see the same patient 7 to 10 times during their rotation); exposure to a host of medically complex patients offering a multitude of clinical scenarios and abnormal physical exam findings; exposure to a hospice/palliative care ward and end-of-life care; and interaction within an interprofessional training environment of nursing, pharmacy, PT, OT, speech-language pathology, psychology, and SW trainees.
Program Evaluation
At the end of rotations continuously through the year, PA students electronically complete a site evaluation from the Boston University School of Medicine Physician Assistant Program. The evaluation consists of 14 questions: 6 about site quality and 8 about instruction quality. The questions are answered on a 5-point Likert scale. Also included are 2 open-ended response questions that ask what they liked about the rotation and what they felt could be improved. Results are anonymous, de-identified and blinded both to the program as well as the clerkship site. Results are aggregated and provided to program sites annually. Responses are converted to a dichotomous variable, where any good or excellent response (4 or 5) is considered positive and any neutral or below (3, 2, 1) is considered a nonpositive response.
Results
The clerkship site has been operational since June 22, 2015. There have been 59 students who participated in the rotation. A different scale in these evaluations was used between June 22, 2015, and September 13, 2015. Therefore, 7 responses were excluded from the analysis, leaving 52 usable evaluations. The responses were analyzed both in total (for the CLC as well as other IM rotation sites) and by individual clerkship year to look for any trends over time: September 14, 2015, through April 24, 2016; April 25, 2016, through April 28, 2017; and May 1, 2017, through March 1, 2018 (Table).
Site evaluations showed high satisfaction regarding the quality of the physical environment as well as the learning environment. Students endorsed the PAC unit having resources and physical space for them, such as a desk and computer, opportunity for participation in patient care, and parking (100%; n = 52). Site evaluations revealed high satisfaction with the quality of teaching and faculty encouragement and support of their learning (100%; n = 52). The evaluations revealed that bedside teaching was strong (94%; n = 49). The students reported high satisfaction with the volume of patients provided (92%; n = 48) as well as the diversity of diagnoses (92%; n = 48).
There were fewer positive responses in the first 2 years of the rotation with regard to formal lectures (50% and 67%; 7/14 and 16/24, respectively). In the third year of the rotation, students had a much higher satisfaction rate (93%; 13/14). This increased satisfaction was associated with the development and incorporation of the Interprofessional Curriculum in Transitional Care in 2017.
Discussion
Access to high-quality PA student clerkship sites has become a pressing issue in recent years because of increased competition for sites and a shortage of willing and well-trained preceptors. There has been marked growth in schools and enrollment across all medical professions. The Accreditation Review Commission on Education for the PA (ARC-PA) reported that the total number of accredited entry-level PA programs in 2018 was 246, with 58 new accredited programs projected by 2022.12 The Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey reported a 66% increase in first-year enrollment in PA programs from 2002 to 2012.5 Programs must implement alternative strategies to attract clinical sites (eg, academic appointments, increased clinical resources to training sites) or face continued challenges with recruiting training sites for their students. Postacute care may be a natural extension to expand the footprint for clinical sites for these programs, augmenting acute inpatient and outpatient rotations. This implementation would increase the pool of clinical training sites and preceptors.
The experience with this novel training site, based on PA student feedback and evaluations, has been positive, and the postacute setting can provide students with high-quality IM clinical experiences. Students report adequate patient volume and diversity. In addition, evaluations are comparable with that of other IM site rotations the students experience. Qualitative feedback has emphasized the value of following patients over longer periods; eg, weeks vs days (as in acute care) enabling students to build relationships with patients as well as observe a richer clinical spectrum of disease over a less compressed period. “Patients have complex issues, so from a medical standpoint it challenges you to think of new ways to manage their care,” commented a representative student. “It is really beneficial that you can follow them over time.”
Furthermore, in response to student feedback on didactics, an interprofessional curriculum was developed to add formal structure as well as to create a curriculum in care transitions. This curriculum provided a unique opportunity for PA students to receive formal instruction on areas of particular relevance for transitional care (eg, care continuum, end of life issues, and care transitions). The curriculum also allows the interprofessional faculty a unique and enjoyable opportunity for interprofessional collaboration.
The 1 month PAC rotation is augmented with inpatient IM and outpatient family medicine rotations, consequently giving exposure to the full continuum of care. The PAC setting provides learners multifaceted benefits: the opportunity to strengthen and develop the knowledge, attitudes, and skills necessary for IM; increased understanding of other professions by observing and interacting as a team caring for a patient over a longer period as opposed to the acute care setting; the ability to perform effective, efficient, and safe transfer between clinical settings; and broad exposure to transitional care. As a result, the PAC rotation enhances but does not replace the necessary and essential rotations of inpatient and outpatient medicine.
Moreover, this rotation provides unique and core IM training for PA students. Our site focuses on interprofessional collaboration, emphasizing the importance of team-based care, an essential concept in modern day medicine. Formal exposure to other care specialties, such as PT and OT, SW, and mental health, is essential for students to appreciate clinical medicine and a patient’s physical and mental experience over the course of a disease and clinical state. In addition, the physical exam checklist ensures that students are exposed to the full spectrum of IM examination findings during their rotation. Finally, weekly feedback forms require students to ask and receive concrete feedback from their supervising providers.
Limitations
The generalizability of this model requires careful consideration. VABHS is a tertiary care integrated health care system, enabling students to learn from patients moving through multiple care transitions in a single health care system. In addition, other settings may not have the staffing or clinical volume to sustain such a model. All PAC clinical faculty teach voluntarily, and local leadership has set expectations for all clinicians to participate in teaching of trainees and PA students. Evaluations also note less diversity in the patient population, a challenge that some VA facilities face. This issue could be addressed by ensuring that students also have IM rotations at other inpatient medical facilities. A more balanced experience, where students reap the positive benefits of PAC but do not lose exposure to a diverse patient pool, could result. Furthermore, some of the perceived positive impacts also may be related to professional and personal attributes of the teaching clinicians rather than to the PAC setting.
Conclusion
PAC settings can be effective training sites for medicine clerkships for PA students and can provide high-quality training in IM as PA programs continue to expand. This setting offers students exposure to interprofessional, team-based care and the opportunity to care for patients with a broad range of disease complexity. Learning is further enhanced by the ability to follow patients longitudinally over their disease course as well as to work directly with teaching faculty and other interprofessional health care professionals. Evaluations of this novel clerkship experience have shown high levels of student satisfaction in knowledge growth, clinical skills, bedside teaching, and mentorship.
Acknowledgments
We thank Juman Hijab for her critical role in establishing and maintaining the clerkship. We thank Steven Simon, Matt Russell, and Thomas Parrino for their leadership and guidance in establishing and maintaining the clerkship. We thank the Boston University School of Medicine Physician Assistant Program Director Mary Warner for her support and guidance in creating and supporting the clerkship. In addition, we thank the interprofessional education faculty for their dedicated involvement in teaching, including Stephanie Saunders, Lindsay Lefers, Jessica Rawlins, Lindsay Brennan, Angela Viani, Eric Charette, Nicole O’Neil, Susan Nathan, Jordana Meyerson, Shivani Jindal, Wei Shen, Amy Hanson, Gilda Cain, and Kate Hinrichs.
1. US Department of Labor, Bureau of Labor Statistics. Occupational outlook handbook: physician assistants. https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Updated June 18, 2019. Accessed August 13, 2019.
2. Association of American Medical Colleges. 2019 update: the complexities of physician supply and demand: projections from 2017 to 2032. https://aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Published April 2019. Accessed August 15, 2019.
3. Glicken AD, Miller AA. Physician assistants: from pipeline to practice. Acad Med. 2013;88(12):1883-1889.
4. Erikson C, Hamann R, Levitan T, Pankow S, Stanley J, Whatley M. Recruiting and maintaining US clinical training sites: joint report of the 2013 multi-discipline clerkship/clinical training site survey. https://paeaonline.org/wp-content/uploads/2015/10/Recruiting-and-Maintaining-U.S.-Clinical-Training-Sites.pdf. Accessed August 13, 2019.
5. Physician Assistant Education Association. By the numbers: 30th annual report on physician assistant educational programs. 2015. http://paeaonline.org/wp-content/uploads/2016/12/2015-by-the-numbers-program-report-30.pdf. Published 2015. Accessed August 15, 2019.
6. Morgan P, Himmerick KA, Leach B, Dieter P, Everett C. Scarcity of primary care positions may divert physician assistants into specialty practice. Med Care Res Rev. 2017;74(1):109-122.
7. Coplan B, Cawley J, Stoehr J. Physician assistants in primary care: trends and characteristics. Ann Fam Med. 2013;11(1):75-79.
8. Morgan P, Leach B, Himmerick K, Everett C. Job openings for PAs by specialty. JAAPA. 2018;31(1):45-47.
9. American Academy of Physician Assistants. 2017 AAPA Salary Report. Alexandria, VA; 2017.
10. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
11. Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617.
12. Accreditation Review Commission on Education for the Physician Assistant. http://www.arc-pa.org/accreditation/accredited-programs. Accessed May 10, 2019.
The Federal Bureau of Labor Statistics projects 37% job growth for physician assistants (PAs) from 2016 to 2026, much greater than the average for all other occupations as well as for other medical professions.1 This growth has been accompanied by increased enrollment in medical (doctor of medicine [MD], doctor of osteopathic medicine) and nurse practitioner (NP) schools.2 Clinical teaching sites serve a crucial function in the training of all clinical disciplines. These sites provide hands-on and experiential learning in medical settings, necessary components for learners practicing to become clinicians. Significant PA program expansion has led to increased demand for clinical training, creating competition for sites and a shortage of willing and well-trained preceptors.3
This challenge has been recognized by PA program directors. In the Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey, PA program directors expressed concern about the adequacy of clinical opportunities for students, increased difficulty developing new core sites, and preserving existing core sites. In addition, they noted that a shortage of clinical sites was one of the greatest barriers to the PA programs’ sustained growth and success.4
Program directors also indicated difficulty securing clinical training sites in internal medicine (IM) and high rates of attrition of medicine clinical preceptors for their students.5 The reasons are multifold: increasing clinical demands, time, teaching competence, lack of experience, academic affiliation, lack of reimbursement, or compensation. Moreover, there is a declining number of PAs who work in primary care compared with specialty and subspecialty care, limiting the availability of clinical training preceptors in medicine and primary care.6-8 According to the American Academy of PAs (AAPA) census and salary survey data, the percentage of PAs working in the primary care specialties (ie, family medicine, IM, and general pediatrics) has decreased from > 47% in 1995 to 24% in 2017.9 As such, there is a need to broaden the educational landscape to provide more high-quality training sites in IM.
The postacute health care setting may address this training need. It offers a unique clinical opportunity to expose learners to a broad range of disease complexity and clinical acuity, as the percentage of patients discharged from hospitals to postacute care (PAC) has increased and care shifts from the hospital to the PAC setting.10,11 The longer PAC length of stay also enables learners to follow patients longitudinally over several weeks and experience interprofessional team-based care. In addition, the PAC setting offers learners the ability to acquire the necessary skills for smooth and effective transitions of care. This setting has been extensively used for trainees of nursing, pharmacy, physical therapy (PT) and occupational therapy (OT), speech-language pathology, psychology, and social work (SW), but few programs have used the PAC setting as clerkship sites for IM rotations for PA students. To address this need for IM sites, the VA Boston Healthcare System (VABHS), in conjunction with the Boston University School of Medicine Physician Assistant Program, developed a novel medicine clinical clerkship site for physician assistants in the PAC unit of the community living center (CLC) at VABHS. This report describes the program structure, curriculum, and participant evaluation results.
Clinical Clerkship Program
VABHS CLC is a 110-bed facility comprising 3 units: a 65-bed PAC unit, a 15-bed closed hospice/palliative care unit, and a 30-bed long-term care unit. The service is staffed continuously with physicians, PAs, and NPs. A majority of patients are admitted from the acute care hospital of VABHS (West Roxbury campus) and other regional VA facilities. The CLC offers dynamic services, including phlebotomy, general radiology, IV diuretics and antibiotics, wound care, and subacute PT, OT, and speech-language pathology rehabilitation. The CLC serves as a venue for transitioning patients from acute inpatient care to home. The patient population is often elderly, with multiple active comorbidities and variable medical literacy, adherence, and follow-up.
The CLC provides a diverse interprofessional learning environment, offering core IM rotations for first-year psychiatry residents, oral and maxillofacial surgery residents, and PA students. The CLC also has expanded as a clinical site both for transitions-in-care IM resident curricula and electives as well as a geriatrics fellowship. In addition, the site offers rotations for NPs, nursing, pharmacy, physical and occupational therapies, speech-language pathology, psychology, and SW.
The Boston University School of Medicine Physician Assistant Program was founded in 2015 as a master’s degree program completed over 28 months. The first 12 months are didactic, and the following 16 months are clinical training with 14 months of rotations (2 IM, family medicine, pediatrics, emergency medicine, general surgery, obstetrics and gynecology, psychiatry, neurology, and 5 elective rotations), and 2 months for a thesis. The program has about 30 students per year and 4 clerkship sites for IM.
Program Description
The VABHS medicine clerkship hosts 1 to 2 PA students for 4-week blocks in the PAC unit of the CLC. Each student rotates on both PA and MD teams. Students follow 3 to 4 patients and participate fully in their care from admission to discharge; they prepare daily presentations and participate in medical management, family meetings, chart documentation, and care coordination with the interprofessional team. Students are provided a physical examination checklist and feedback form, and they are expected to track findings and record feedback and goals with their supervising preceptor weekly. They also make formal case presentations and participate in monthly medicine didactic rounds available to all VABHS IM students and trainees via videoconference.
In addition, beginning in July 2017, all PA students in the CLC began to participate in a 4-week Interprofessional Curriculum in Transitional Care. The curriculum includes 14 didactic lectures taught by 16 interprofessional faculty, including medicine, geriatric, and palliative care physicians; PAs; social workers; physical and occupational therapists; pharmacists; and a geriatric psychologist. The didactics include topics on the interprofessional team, the care continuum, teams and teamwork, interdisciplinary coordination of care, components of effective transitions in care, medication reconciliation, approaching difficult conversations, advance care planning, and quality improvement. The goal of the curriculum is to provide learners the knowledge, skills, and dispositions necessary for high-quality transitional care and interprofessional practice as well as specific training for effective and safe transfers of care between clinical settings. Although PA students are the main participants in this curriculum, all other learners in the PAC unit are also invited to attend the lectures.
The unique attributes of this training site include direct interaction with supervising PAs and physicians, rather than experiencing the traditional teaching hierarchy (with interns, residents, fellows); observation of the natural progression of disease of both acute care and primary care issues due to the longer length of stay (2 to 6 weeks, where the typical student will see the same patient 7 to 10 times during their rotation); exposure to a host of medically complex patients offering a multitude of clinical scenarios and abnormal physical exam findings; exposure to a hospice/palliative care ward and end-of-life care; and interaction within an interprofessional training environment of nursing, pharmacy, PT, OT, speech-language pathology, psychology, and SW trainees.
Program Evaluation
At the end of rotations continuously through the year, PA students electronically complete a site evaluation from the Boston University School of Medicine Physician Assistant Program. The evaluation consists of 14 questions: 6 about site quality and 8 about instruction quality. The questions are answered on a 5-point Likert scale. Also included are 2 open-ended response questions that ask what they liked about the rotation and what they felt could be improved. Results are anonymous, de-identified and blinded both to the program as well as the clerkship site. Results are aggregated and provided to program sites annually. Responses are converted to a dichotomous variable, where any good or excellent response (4 or 5) is considered positive and any neutral or below (3, 2, 1) is considered a nonpositive response.
Results
The clerkship site has been operational since June 22, 2015. There have been 59 students who participated in the rotation. A different scale in these evaluations was used between June 22, 2015, and September 13, 2015. Therefore, 7 responses were excluded from the analysis, leaving 52 usable evaluations. The responses were analyzed both in total (for the CLC as well as other IM rotation sites) and by individual clerkship year to look for any trends over time: September 14, 2015, through April 24, 2016; April 25, 2016, through April 28, 2017; and May 1, 2017, through March 1, 2018 (Table).
Site evaluations showed high satisfaction regarding the quality of the physical environment as well as the learning environment. Students endorsed the PAC unit having resources and physical space for them, such as a desk and computer, opportunity for participation in patient care, and parking (100%; n = 52). Site evaluations revealed high satisfaction with the quality of teaching and faculty encouragement and support of their learning (100%; n = 52). The evaluations revealed that bedside teaching was strong (94%; n = 49). The students reported high satisfaction with the volume of patients provided (92%; n = 48) as well as the diversity of diagnoses (92%; n = 48).
There were fewer positive responses in the first 2 years of the rotation with regard to formal lectures (50% and 67%; 7/14 and 16/24, respectively). In the third year of the rotation, students had a much higher satisfaction rate (93%; 13/14). This increased satisfaction was associated with the development and incorporation of the Interprofessional Curriculum in Transitional Care in 2017.
Discussion
Access to high-quality PA student clerkship sites has become a pressing issue in recent years because of increased competition for sites and a shortage of willing and well-trained preceptors. There has been marked growth in schools and enrollment across all medical professions. The Accreditation Review Commission on Education for the PA (ARC-PA) reported that the total number of accredited entry-level PA programs in 2018 was 246, with 58 new accredited programs projected by 2022.12 The Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey reported a 66% increase in first-year enrollment in PA programs from 2002 to 2012.5 Programs must implement alternative strategies to attract clinical sites (eg, academic appointments, increased clinical resources to training sites) or face continued challenges with recruiting training sites for their students. Postacute care may be a natural extension to expand the footprint for clinical sites for these programs, augmenting acute inpatient and outpatient rotations. This implementation would increase the pool of clinical training sites and preceptors.
The experience with this novel training site, based on PA student feedback and evaluations, has been positive, and the postacute setting can provide students with high-quality IM clinical experiences. Students report adequate patient volume and diversity. In addition, evaluations are comparable with that of other IM site rotations the students experience. Qualitative feedback has emphasized the value of following patients over longer periods; eg, weeks vs days (as in acute care) enabling students to build relationships with patients as well as observe a richer clinical spectrum of disease over a less compressed period. “Patients have complex issues, so from a medical standpoint it challenges you to think of new ways to manage their care,” commented a representative student. “It is really beneficial that you can follow them over time.”
Furthermore, in response to student feedback on didactics, an interprofessional curriculum was developed to add formal structure as well as to create a curriculum in care transitions. This curriculum provided a unique opportunity for PA students to receive formal instruction on areas of particular relevance for transitional care (eg, care continuum, end of life issues, and care transitions). The curriculum also allows the interprofessional faculty a unique and enjoyable opportunity for interprofessional collaboration.
The 1 month PAC rotation is augmented with inpatient IM and outpatient family medicine rotations, consequently giving exposure to the full continuum of care. The PAC setting provides learners multifaceted benefits: the opportunity to strengthen and develop the knowledge, attitudes, and skills necessary for IM; increased understanding of other professions by observing and interacting as a team caring for a patient over a longer period as opposed to the acute care setting; the ability to perform effective, efficient, and safe transfer between clinical settings; and broad exposure to transitional care. As a result, the PAC rotation enhances but does not replace the necessary and essential rotations of inpatient and outpatient medicine.
Moreover, this rotation provides unique and core IM training for PA students. Our site focuses on interprofessional collaboration, emphasizing the importance of team-based care, an essential concept in modern day medicine. Formal exposure to other care specialties, such as PT and OT, SW, and mental health, is essential for students to appreciate clinical medicine and a patient’s physical and mental experience over the course of a disease and clinical state. In addition, the physical exam checklist ensures that students are exposed to the full spectrum of IM examination findings during their rotation. Finally, weekly feedback forms require students to ask and receive concrete feedback from their supervising providers.
Limitations
The generalizability of this model requires careful consideration. VABHS is a tertiary care integrated health care system, enabling students to learn from patients moving through multiple care transitions in a single health care system. In addition, other settings may not have the staffing or clinical volume to sustain such a model. All PAC clinical faculty teach voluntarily, and local leadership has set expectations for all clinicians to participate in teaching of trainees and PA students. Evaluations also note less diversity in the patient population, a challenge that some VA facilities face. This issue could be addressed by ensuring that students also have IM rotations at other inpatient medical facilities. A more balanced experience, where students reap the positive benefits of PAC but do not lose exposure to a diverse patient pool, could result. Furthermore, some of the perceived positive impacts also may be related to professional and personal attributes of the teaching clinicians rather than to the PAC setting.
Conclusion
PAC settings can be effective training sites for medicine clerkships for PA students and can provide high-quality training in IM as PA programs continue to expand. This setting offers students exposure to interprofessional, team-based care and the opportunity to care for patients with a broad range of disease complexity. Learning is further enhanced by the ability to follow patients longitudinally over their disease course as well as to work directly with teaching faculty and other interprofessional health care professionals. Evaluations of this novel clerkship experience have shown high levels of student satisfaction in knowledge growth, clinical skills, bedside teaching, and mentorship.
Acknowledgments
We thank Juman Hijab for her critical role in establishing and maintaining the clerkship. We thank Steven Simon, Matt Russell, and Thomas Parrino for their leadership and guidance in establishing and maintaining the clerkship. We thank the Boston University School of Medicine Physician Assistant Program Director Mary Warner for her support and guidance in creating and supporting the clerkship. In addition, we thank the interprofessional education faculty for their dedicated involvement in teaching, including Stephanie Saunders, Lindsay Lefers, Jessica Rawlins, Lindsay Brennan, Angela Viani, Eric Charette, Nicole O’Neil, Susan Nathan, Jordana Meyerson, Shivani Jindal, Wei Shen, Amy Hanson, Gilda Cain, and Kate Hinrichs.
The Federal Bureau of Labor Statistics projects 37% job growth for physician assistants (PAs) from 2016 to 2026, much greater than the average for all other occupations as well as for other medical professions.1 This growth has been accompanied by increased enrollment in medical (doctor of medicine [MD], doctor of osteopathic medicine) and nurse practitioner (NP) schools.2 Clinical teaching sites serve a crucial function in the training of all clinical disciplines. These sites provide hands-on and experiential learning in medical settings, necessary components for learners practicing to become clinicians. Significant PA program expansion has led to increased demand for clinical training, creating competition for sites and a shortage of willing and well-trained preceptors.3
This challenge has been recognized by PA program directors. In the Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey, PA program directors expressed concern about the adequacy of clinical opportunities for students, increased difficulty developing new core sites, and preserving existing core sites. In addition, they noted that a shortage of clinical sites was one of the greatest barriers to the PA programs’ sustained growth and success.4
Program directors also indicated difficulty securing clinical training sites in internal medicine (IM) and high rates of attrition of medicine clinical preceptors for their students.5 The reasons are multifold: increasing clinical demands, time, teaching competence, lack of experience, academic affiliation, lack of reimbursement, or compensation. Moreover, there is a declining number of PAs who work in primary care compared with specialty and subspecialty care, limiting the availability of clinical training preceptors in medicine and primary care.6-8 According to the American Academy of PAs (AAPA) census and salary survey data, the percentage of PAs working in the primary care specialties (ie, family medicine, IM, and general pediatrics) has decreased from > 47% in 1995 to 24% in 2017.9 As such, there is a need to broaden the educational landscape to provide more high-quality training sites in IM.
The postacute health care setting may address this training need. It offers a unique clinical opportunity to expose learners to a broad range of disease complexity and clinical acuity, as the percentage of patients discharged from hospitals to postacute care (PAC) has increased and care shifts from the hospital to the PAC setting.10,11 The longer PAC length of stay also enables learners to follow patients longitudinally over several weeks and experience interprofessional team-based care. In addition, the PAC setting offers learners the ability to acquire the necessary skills for smooth and effective transitions of care. This setting has been extensively used for trainees of nursing, pharmacy, physical therapy (PT) and occupational therapy (OT), speech-language pathology, psychology, and social work (SW), but few programs have used the PAC setting as clerkship sites for IM rotations for PA students. To address this need for IM sites, the VA Boston Healthcare System (VABHS), in conjunction with the Boston University School of Medicine Physician Assistant Program, developed a novel medicine clinical clerkship site for physician assistants in the PAC unit of the community living center (CLC) at VABHS. This report describes the program structure, curriculum, and participant evaluation results.
Clinical Clerkship Program
VABHS CLC is a 110-bed facility comprising 3 units: a 65-bed PAC unit, a 15-bed closed hospice/palliative care unit, and a 30-bed long-term care unit. The service is staffed continuously with physicians, PAs, and NPs. A majority of patients are admitted from the acute care hospital of VABHS (West Roxbury campus) and other regional VA facilities. The CLC offers dynamic services, including phlebotomy, general radiology, IV diuretics and antibiotics, wound care, and subacute PT, OT, and speech-language pathology rehabilitation. The CLC serves as a venue for transitioning patients from acute inpatient care to home. The patient population is often elderly, with multiple active comorbidities and variable medical literacy, adherence, and follow-up.
The CLC provides a diverse interprofessional learning environment, offering core IM rotations for first-year psychiatry residents, oral and maxillofacial surgery residents, and PA students. The CLC also has expanded as a clinical site both for transitions-in-care IM resident curricula and electives as well as a geriatrics fellowship. In addition, the site offers rotations for NPs, nursing, pharmacy, physical and occupational therapies, speech-language pathology, psychology, and SW.
The Boston University School of Medicine Physician Assistant Program was founded in 2015 as a master’s degree program completed over 28 months. The first 12 months are didactic, and the following 16 months are clinical training with 14 months of rotations (2 IM, family medicine, pediatrics, emergency medicine, general surgery, obstetrics and gynecology, psychiatry, neurology, and 5 elective rotations), and 2 months for a thesis. The program has about 30 students per year and 4 clerkship sites for IM.
Program Description
The VABHS medicine clerkship hosts 1 to 2 PA students for 4-week blocks in the PAC unit of the CLC. Each student rotates on both PA and MD teams. Students follow 3 to 4 patients and participate fully in their care from admission to discharge; they prepare daily presentations and participate in medical management, family meetings, chart documentation, and care coordination with the interprofessional team. Students are provided a physical examination checklist and feedback form, and they are expected to track findings and record feedback and goals with their supervising preceptor weekly. They also make formal case presentations and participate in monthly medicine didactic rounds available to all VABHS IM students and trainees via videoconference.
In addition, beginning in July 2017, all PA students in the CLC began to participate in a 4-week Interprofessional Curriculum in Transitional Care. The curriculum includes 14 didactic lectures taught by 16 interprofessional faculty, including medicine, geriatric, and palliative care physicians; PAs; social workers; physical and occupational therapists; pharmacists; and a geriatric psychologist. The didactics include topics on the interprofessional team, the care continuum, teams and teamwork, interdisciplinary coordination of care, components of effective transitions in care, medication reconciliation, approaching difficult conversations, advance care planning, and quality improvement. The goal of the curriculum is to provide learners the knowledge, skills, and dispositions necessary for high-quality transitional care and interprofessional practice as well as specific training for effective and safe transfers of care between clinical settings. Although PA students are the main participants in this curriculum, all other learners in the PAC unit are also invited to attend the lectures.
The unique attributes of this training site include direct interaction with supervising PAs and physicians, rather than experiencing the traditional teaching hierarchy (with interns, residents, fellows); observation of the natural progression of disease of both acute care and primary care issues due to the longer length of stay (2 to 6 weeks, where the typical student will see the same patient 7 to 10 times during their rotation); exposure to a host of medically complex patients offering a multitude of clinical scenarios and abnormal physical exam findings; exposure to a hospice/palliative care ward and end-of-life care; and interaction within an interprofessional training environment of nursing, pharmacy, PT, OT, speech-language pathology, psychology, and SW trainees.
Program Evaluation
At the end of rotations continuously through the year, PA students electronically complete a site evaluation from the Boston University School of Medicine Physician Assistant Program. The evaluation consists of 14 questions: 6 about site quality and 8 about instruction quality. The questions are answered on a 5-point Likert scale. Also included are 2 open-ended response questions that ask what they liked about the rotation and what they felt could be improved. Results are anonymous, de-identified and blinded both to the program as well as the clerkship site. Results are aggregated and provided to program sites annually. Responses are converted to a dichotomous variable, where any good or excellent response (4 or 5) is considered positive and any neutral or below (3, 2, 1) is considered a nonpositive response.
Results
The clerkship site has been operational since June 22, 2015. There have been 59 students who participated in the rotation. A different scale in these evaluations was used between June 22, 2015, and September 13, 2015. Therefore, 7 responses were excluded from the analysis, leaving 52 usable evaluations. The responses were analyzed both in total (for the CLC as well as other IM rotation sites) and by individual clerkship year to look for any trends over time: September 14, 2015, through April 24, 2016; April 25, 2016, through April 28, 2017; and May 1, 2017, through March 1, 2018 (Table).
Site evaluations showed high satisfaction regarding the quality of the physical environment as well as the learning environment. Students endorsed the PAC unit having resources and physical space for them, such as a desk and computer, opportunity for participation in patient care, and parking (100%; n = 52). Site evaluations revealed high satisfaction with the quality of teaching and faculty encouragement and support of their learning (100%; n = 52). The evaluations revealed that bedside teaching was strong (94%; n = 49). The students reported high satisfaction with the volume of patients provided (92%; n = 48) as well as the diversity of diagnoses (92%; n = 48).
There were fewer positive responses in the first 2 years of the rotation with regard to formal lectures (50% and 67%; 7/14 and 16/24, respectively). In the third year of the rotation, students had a much higher satisfaction rate (93%; 13/14). This increased satisfaction was associated with the development and incorporation of the Interprofessional Curriculum in Transitional Care in 2017.
Discussion
Access to high-quality PA student clerkship sites has become a pressing issue in recent years because of increased competition for sites and a shortage of willing and well-trained preceptors. There has been marked growth in schools and enrollment across all medical professions. The Accreditation Review Commission on Education for the PA (ARC-PA) reported that the total number of accredited entry-level PA programs in 2018 was 246, with 58 new accredited programs projected by 2022.12 The Joint Report of the 2013 Multi-Discipline Clerkship/Clinical Training Site Survey reported a 66% increase in first-year enrollment in PA programs from 2002 to 2012.5 Programs must implement alternative strategies to attract clinical sites (eg, academic appointments, increased clinical resources to training sites) or face continued challenges with recruiting training sites for their students. Postacute care may be a natural extension to expand the footprint for clinical sites for these programs, augmenting acute inpatient and outpatient rotations. This implementation would increase the pool of clinical training sites and preceptors.
The experience with this novel training site, based on PA student feedback and evaluations, has been positive, and the postacute setting can provide students with high-quality IM clinical experiences. Students report adequate patient volume and diversity. In addition, evaluations are comparable with that of other IM site rotations the students experience. Qualitative feedback has emphasized the value of following patients over longer periods; eg, weeks vs days (as in acute care) enabling students to build relationships with patients as well as observe a richer clinical spectrum of disease over a less compressed period. “Patients have complex issues, so from a medical standpoint it challenges you to think of new ways to manage their care,” commented a representative student. “It is really beneficial that you can follow them over time.”
Furthermore, in response to student feedback on didactics, an interprofessional curriculum was developed to add formal structure as well as to create a curriculum in care transitions. This curriculum provided a unique opportunity for PA students to receive formal instruction on areas of particular relevance for transitional care (eg, care continuum, end of life issues, and care transitions). The curriculum also allows the interprofessional faculty a unique and enjoyable opportunity for interprofessional collaboration.
The 1 month PAC rotation is augmented with inpatient IM and outpatient family medicine rotations, consequently giving exposure to the full continuum of care. The PAC setting provides learners multifaceted benefits: the opportunity to strengthen and develop the knowledge, attitudes, and skills necessary for IM; increased understanding of other professions by observing and interacting as a team caring for a patient over a longer period as opposed to the acute care setting; the ability to perform effective, efficient, and safe transfer between clinical settings; and broad exposure to transitional care. As a result, the PAC rotation enhances but does not replace the necessary and essential rotations of inpatient and outpatient medicine.
Moreover, this rotation provides unique and core IM training for PA students. Our site focuses on interprofessional collaboration, emphasizing the importance of team-based care, an essential concept in modern day medicine. Formal exposure to other care specialties, such as PT and OT, SW, and mental health, is essential for students to appreciate clinical medicine and a patient’s physical and mental experience over the course of a disease and clinical state. In addition, the physical exam checklist ensures that students are exposed to the full spectrum of IM examination findings during their rotation. Finally, weekly feedback forms require students to ask and receive concrete feedback from their supervising providers.
Limitations
The generalizability of this model requires careful consideration. VABHS is a tertiary care integrated health care system, enabling students to learn from patients moving through multiple care transitions in a single health care system. In addition, other settings may not have the staffing or clinical volume to sustain such a model. All PAC clinical faculty teach voluntarily, and local leadership has set expectations for all clinicians to participate in teaching of trainees and PA students. Evaluations also note less diversity in the patient population, a challenge that some VA facilities face. This issue could be addressed by ensuring that students also have IM rotations at other inpatient medical facilities. A more balanced experience, where students reap the positive benefits of PAC but do not lose exposure to a diverse patient pool, could result. Furthermore, some of the perceived positive impacts also may be related to professional and personal attributes of the teaching clinicians rather than to the PAC setting.
Conclusion
PAC settings can be effective training sites for medicine clerkships for PA students and can provide high-quality training in IM as PA programs continue to expand. This setting offers students exposure to interprofessional, team-based care and the opportunity to care for patients with a broad range of disease complexity. Learning is further enhanced by the ability to follow patients longitudinally over their disease course as well as to work directly with teaching faculty and other interprofessional health care professionals. Evaluations of this novel clerkship experience have shown high levels of student satisfaction in knowledge growth, clinical skills, bedside teaching, and mentorship.
Acknowledgments
We thank Juman Hijab for her critical role in establishing and maintaining the clerkship. We thank Steven Simon, Matt Russell, and Thomas Parrino for their leadership and guidance in establishing and maintaining the clerkship. We thank the Boston University School of Medicine Physician Assistant Program Director Mary Warner for her support and guidance in creating and supporting the clerkship. In addition, we thank the interprofessional education faculty for their dedicated involvement in teaching, including Stephanie Saunders, Lindsay Lefers, Jessica Rawlins, Lindsay Brennan, Angela Viani, Eric Charette, Nicole O’Neil, Susan Nathan, Jordana Meyerson, Shivani Jindal, Wei Shen, Amy Hanson, Gilda Cain, and Kate Hinrichs.
1. US Department of Labor, Bureau of Labor Statistics. Occupational outlook handbook: physician assistants. https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Updated June 18, 2019. Accessed August 13, 2019.
2. Association of American Medical Colleges. 2019 update: the complexities of physician supply and demand: projections from 2017 to 2032. https://aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Published April 2019. Accessed August 15, 2019.
3. Glicken AD, Miller AA. Physician assistants: from pipeline to practice. Acad Med. 2013;88(12):1883-1889.
4. Erikson C, Hamann R, Levitan T, Pankow S, Stanley J, Whatley M. Recruiting and maintaining US clinical training sites: joint report of the 2013 multi-discipline clerkship/clinical training site survey. https://paeaonline.org/wp-content/uploads/2015/10/Recruiting-and-Maintaining-U.S.-Clinical-Training-Sites.pdf. Accessed August 13, 2019.
5. Physician Assistant Education Association. By the numbers: 30th annual report on physician assistant educational programs. 2015. http://paeaonline.org/wp-content/uploads/2016/12/2015-by-the-numbers-program-report-30.pdf. Published 2015. Accessed August 15, 2019.
6. Morgan P, Himmerick KA, Leach B, Dieter P, Everett C. Scarcity of primary care positions may divert physician assistants into specialty practice. Med Care Res Rev. 2017;74(1):109-122.
7. Coplan B, Cawley J, Stoehr J. Physician assistants in primary care: trends and characteristics. Ann Fam Med. 2013;11(1):75-79.
8. Morgan P, Leach B, Himmerick K, Everett C. Job openings for PAs by specialty. JAAPA. 2018;31(1):45-47.
9. American Academy of Physician Assistants. 2017 AAPA Salary Report. Alexandria, VA; 2017.
10. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
11. Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617.
12. Accreditation Review Commission on Education for the Physician Assistant. http://www.arc-pa.org/accreditation/accredited-programs. Accessed May 10, 2019.
1. US Department of Labor, Bureau of Labor Statistics. Occupational outlook handbook: physician assistants. https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Updated June 18, 2019. Accessed August 13, 2019.
2. Association of American Medical Colleges. 2019 update: the complexities of physician supply and demand: projections from 2017 to 2032. https://aamc-black.global.ssl.fastly.net/production/media/filer_public/31/13/3113ee5c-a038-4c16-89af-294a69826650/2019_update_-_the_complexities_of_physician_supply_and_demand_-_projections_from_2017-2032.pdf. Published April 2019. Accessed August 15, 2019.
3. Glicken AD, Miller AA. Physician assistants: from pipeline to practice. Acad Med. 2013;88(12):1883-1889.
4. Erikson C, Hamann R, Levitan T, Pankow S, Stanley J, Whatley M. Recruiting and maintaining US clinical training sites: joint report of the 2013 multi-discipline clerkship/clinical training site survey. https://paeaonline.org/wp-content/uploads/2015/10/Recruiting-and-Maintaining-U.S.-Clinical-Training-Sites.pdf. Accessed August 13, 2019.
5. Physician Assistant Education Association. By the numbers: 30th annual report on physician assistant educational programs. 2015. http://paeaonline.org/wp-content/uploads/2016/12/2015-by-the-numbers-program-report-30.pdf. Published 2015. Accessed August 15, 2019.
6. Morgan P, Himmerick KA, Leach B, Dieter P, Everett C. Scarcity of primary care positions may divert physician assistants into specialty practice. Med Care Res Rev. 2017;74(1):109-122.
7. Coplan B, Cawley J, Stoehr J. Physician assistants in primary care: trends and characteristics. Ann Fam Med. 2013;11(1):75-79.
8. Morgan P, Leach B, Himmerick K, Everett C. Job openings for PAs by specialty. JAAPA. 2018;31(1):45-47.
9. American Academy of Physician Assistants. 2017 AAPA Salary Report. Alexandria, VA; 2017.
10. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6.
11. Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617.
12. Accreditation Review Commission on Education for the Physician Assistant. http://www.arc-pa.org/accreditation/accredited-programs. Accessed May 10, 2019.
Pseudo-Ludwig angina
An 83-year-old woman with hypertension, hypothyroidism, and a history of depression presented to the emergency department with acute shortness of breath and hypoxia. She was found to have submassive pulmonary embolism, and a heparin infusion was started immediately.
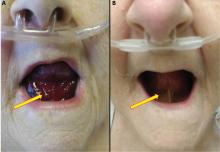
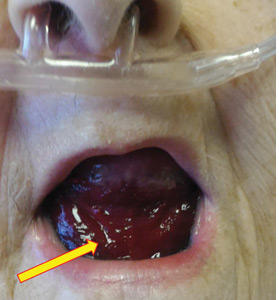
Urgent nasopharyngeal laryngoscopy revealed a hematoma at the base of her tongue that extended into the vallecula, piriform sinuses, and aryepiglottic fold, causing acute airway obstruction. These features combined with the supratherapeutic aPTT led to the diagnosis of pseudo-Ludwig angina.
DANGER OF RAPID AIRWAY COMPROMISE
Pseudo-Ludwig angina is a rare condition in which over-anticoagulation causes sublingual swelling leading to airway obstruction, whereas true Ludwig angina is an infectious regional suppuration of the neck.
Most reported cases of pseudo-Ludwig angina have resulted from overanticogulation with warfarin or warfarin-like substances (rodenticides), or from coagulopathy due to liver disease.1–3 Early recognition is essential to avoid airway compromise.
In our patient, all anticoagulation was discontinued, and she was intubated until the hematoma began to resolve, the aPTT returned to normal, and respiratory compromise improved. At follow-up 2 months later, the sublingual hematoma had completely resolved (Figure 1). And at a 6-month follow-up visit, the pulmonary embolism had resolved, and pulmonary pressures by 2-dimensional echocardiography were normal.
- Lovallo E, Patterson S, Erickson M, Chin C, Blanc P, Durrani TS. When is “pseudo-Ludwig’s angina” associated with coagulopathy also a “pseudo” hemorrhage? J Investig Med High Impact Case Rep 2013; 1(2):2324709613492503. doi:10.1177/2324709613492503
- Smith RG, Parker TJ, Anderson TA. Noninfectious acute upper airway obstruction (pseudo-Ludwig phenomenon): report of a case. J Oral Maxillofac Surg 1987; 45(8):701–704. pmid:3475442
- Zacharia GS, Kandiyil S, Thomas V. Pseudo-Ludwig's phenomenon: a rare clinical manifestation in liver cirrhosis. ACG Case Rep J 2014; 2(1):53–54. doi:10.14309/crj.2014.83
An 83-year-old woman with hypertension, hypothyroidism, and a history of depression presented to the emergency department with acute shortness of breath and hypoxia. She was found to have submassive pulmonary embolism, and a heparin infusion was started immediately.
Urgent nasopharyngeal laryngoscopy revealed a hematoma at the base of her tongue that extended into the vallecula, piriform sinuses, and aryepiglottic fold, causing acute airway obstruction. These features combined with the supratherapeutic aPTT led to the diagnosis of pseudo-Ludwig angina.
DANGER OF RAPID AIRWAY COMPROMISE
Pseudo-Ludwig angina is a rare condition in which over-anticoagulation causes sublingual swelling leading to airway obstruction, whereas true Ludwig angina is an infectious regional suppuration of the neck.
Most reported cases of pseudo-Ludwig angina have resulted from overanticogulation with warfarin or warfarin-like substances (rodenticides), or from coagulopathy due to liver disease.1–3 Early recognition is essential to avoid airway compromise.
In our patient, all anticoagulation was discontinued, and she was intubated until the hematoma began to resolve, the aPTT returned to normal, and respiratory compromise improved. At follow-up 2 months later, the sublingual hematoma had completely resolved (Figure 1). And at a 6-month follow-up visit, the pulmonary embolism had resolved, and pulmonary pressures by 2-dimensional echocardiography were normal.
An 83-year-old woman with hypertension, hypothyroidism, and a history of depression presented to the emergency department with acute shortness of breath and hypoxia. She was found to have submassive pulmonary embolism, and a heparin infusion was started immediately.
Urgent nasopharyngeal laryngoscopy revealed a hematoma at the base of her tongue that extended into the vallecula, piriform sinuses, and aryepiglottic fold, causing acute airway obstruction. These features combined with the supratherapeutic aPTT led to the diagnosis of pseudo-Ludwig angina.
DANGER OF RAPID AIRWAY COMPROMISE
Pseudo-Ludwig angina is a rare condition in which over-anticoagulation causes sublingual swelling leading to airway obstruction, whereas true Ludwig angina is an infectious regional suppuration of the neck.
Most reported cases of pseudo-Ludwig angina have resulted from overanticogulation with warfarin or warfarin-like substances (rodenticides), or from coagulopathy due to liver disease.1–3 Early recognition is essential to avoid airway compromise.
In our patient, all anticoagulation was discontinued, and she was intubated until the hematoma began to resolve, the aPTT returned to normal, and respiratory compromise improved. At follow-up 2 months later, the sublingual hematoma had completely resolved (Figure 1). And at a 6-month follow-up visit, the pulmonary embolism had resolved, and pulmonary pressures by 2-dimensional echocardiography were normal.
- Lovallo E, Patterson S, Erickson M, Chin C, Blanc P, Durrani TS. When is “pseudo-Ludwig’s angina” associated with coagulopathy also a “pseudo” hemorrhage? J Investig Med High Impact Case Rep 2013; 1(2):2324709613492503. doi:10.1177/2324709613492503
- Smith RG, Parker TJ, Anderson TA. Noninfectious acute upper airway obstruction (pseudo-Ludwig phenomenon): report of a case. J Oral Maxillofac Surg 1987; 45(8):701–704. pmid:3475442
- Zacharia GS, Kandiyil S, Thomas V. Pseudo-Ludwig's phenomenon: a rare clinical manifestation in liver cirrhosis. ACG Case Rep J 2014; 2(1):53–54. doi:10.14309/crj.2014.83
- Lovallo E, Patterson S, Erickson M, Chin C, Blanc P, Durrani TS. When is “pseudo-Ludwig’s angina” associated with coagulopathy also a “pseudo” hemorrhage? J Investig Med High Impact Case Rep 2013; 1(2):2324709613492503. doi:10.1177/2324709613492503
- Smith RG, Parker TJ, Anderson TA. Noninfectious acute upper airway obstruction (pseudo-Ludwig phenomenon): report of a case. J Oral Maxillofac Surg 1987; 45(8):701–704. pmid:3475442
- Zacharia GS, Kandiyil S, Thomas V. Pseudo-Ludwig's phenomenon: a rare clinical manifestation in liver cirrhosis. ACG Case Rep J 2014; 2(1):53–54. doi:10.14309/crj.2014.83
Are daily chest radiographs and arterial blood gas tests required in ICU patients on mechanical ventilation?
No, they are not required or needed, but daily radiography and arterial blood gas testing are common practice: eg, 60% of intensive care unit (ICU) patients get daily radiographs,1 even though results provide low diagnostic yield and are unlikely to alter patient management compared with testing only when indicated.
The Choosing Wisely campaign,2 a collaborative effort of a number of professional societies, advises against ordering these diagnostic tests daily because routine testing increases risks to patients and burdens the healthcare system. Instead, testing is recommended only in response to a specific clinical question, or when the test results will affect the patient’s treatment.
CHEST RADIOGRAPHS: DAILY VS CLINICALLY INDICATED
Chest radiographs enable practitioners to monitor the position of endotracheal tubes and central venous catheters, evaluate fluid status, follow up on abnormal findings, detect complications of procedures (such as a pneumothorax), and identify otherwise undetected conditions.
And daily chest radiographs often detect abnormalities. A 1991 study by Hall et al3 of 538 chest radiographs in 74 patients on mechanical ventilation reported that 30% of daily routine chest radiographs disclosed a new but minor finding (eg, a small change in endotracheal tube position or a small infiltrate). The new findings were major in 13 (17.6%) of the 74 patients (95% confidence interval [CI] 9%–26%). These included findings that required an immediate diagnostic or therapeutic intervention (eg, endotracheal tube below the tracheal carina, malposition of a catheter, pneumothorax, large pleural effusion).
But most studies say daily radiographs are not needed. In a large prospective study published in 2006, Graat et al4 evaluated the clinical value of 2,457 routine chest radiographs in 754 patients in a combined surgical and medical ICU. Daily chest radiographs revealed new or unexpected findings in 5.8% of cases, but only 2.2% warranted a change in therapy. No differences were found between the medical and surgical patients. The authors concluded that daily routine radiographs in ICU patients seldom reveal unexpected, clinically relevant abnormalities, and those findings rarely require urgent intervention.
A 2010 meta-analysis of 8 studies (7,078 patients) by Oba and Zaza5 compared on-demand and daily routine strategies of performing chest radiographs. They estimated that eliminating daily routine chest radiographs would not affect death rates in the hospital (odds ratio [OR] 1.02, 95% CI 0.89–1.17, P = .78) or the ICU (OR 0.92, 95% CI 0.76–1.11, P = .4). They also found no significant differences in length of stay or duration of mechanical ventilation. This meta-analysis suggests that routine radiographs can be eliminated without adversely affecting outcomes in ICU patients.
A larger meta-analysis (9 trials, 39,358 radiographs, 9,611 patients) published in 2012 by Ganapathy et al6 also found no harm associated with restrictive radiography protocols. These investigators compared a daily chest radiography protocol against a protocol based on clinical indications. The primary outcome was the mortality rate in the ICU; secondary outcomes were the mortality rate in the hospital, the length of stay in the ICU, and duration of mechanical ventilation. They found no differences between routine and restrictive strategies in terms of ICU mortality (risk ratio [RR] 1.04, 95% CI 0.84–1.28, P = .72), hospital mortality (RR 0.98, 95% CI 0.68–1.41, P = .91), or other secondary outcomes.
Clinically indicated testing is better
The conclusion from these studies is that routine chest radiographs in patients undergoing mechanical ventilation does not improve patient outcomes, and thus, a clinically indicated protocol is preferred.
Furthermore, routine daily radiographs have adverse effects such as more cumulative radiation exposure to the patient7 and greater risk of accidental removal of devices (eg, catheters, tubes).8 Another concern is a higher risk of hospital-associated infections from bacterial spread from caregivers’ hands.9
Finally, daily radiographs increase the use of healthcare resources and expenditures. In a 2011 study, Gershengorn et al1 estimated that adopting a clinically indicated radiography strategy could save more than $144 million annually in the United States.
The ACR agrees. Appropriateness criteria published by the American College of Radiology (ACR) in 201510 recommend against routine daily chest radiographs in the ICU, in keeping with the findings of the critical care community. The ACR recommends an initial radiograph at admission to the ICU. However, follow-up radiographs should be obtained only for specific clinical indications, including a change in the patient’s clinical condition or to check for proper placement of endotracheal or nasogastric or orogastric tubes, pulmonary arterial catheters, central venous catheters, chest tubes, and other life-support devices.
Ultrasonography as an alternative
Ultrasonography is widely available and provides an alternative to chest radiography for detecting significant abnormalities in patients on mechanical ventilation without exposing them to radiation and using relatively fewer resources.
A 2012 meta-analysis (8 studies, 1,048 patients) found that bedside ultrasonography reliably detects pneumothorax.11 It can also provide a rapid diagnosis of the cause of acute respiratory failure such as pneumonia or pulmonary edema.12 Ultrasonography, with the appropriate expertise, can also confirm the position of an endotracheal tube13 or central venous catheter.14
ARTERIAL BLOOD GAS TESTING: DAILY VS CLINICALLY INDICATED
Arterial blood gas testing has value for managing patients undergoing mechanical ventilation, and it is one of the most commonly performed diagnostic tests in the ICU. It provides reliable information about the patient’s oxygenation and acid-base status. It is commonly requested when changing ventilator settings.
Downsides. Arterial blood gas measurements account for 10% to 20% of the cost incurred during ICU stay.15 In addition, they require an arterial puncture—an invasive procedure associated with potentially serious complications such as occlusion of the artery, digital embolization leading to digital ischemia, local infection, pseudoaneurysm, hematoma, bleeding, and skin necrosis.
Is daily testing needed?
Guidelines say no. The 2013 American Association for Respiratory Care16 guidelines suggest that arterial blood gas testing should be based on the clinical assessment of the patient. They recommend blood gas analysis to evaluate the patient’s ventilatory status (reflected by the partial pressure of arterial carbon dioxide [PaCO2], acid-base status (reflected by pH), arterial oxygenation (partial pressure of arterial oxygen [PaO2] and oxyhemoglobin saturation), oxygen-carrying capacity, and whether the patient likely has an intrapulmonary shunt. They state that testing is useful to quantify the response to therapeutic or diagnostic interventions such as cardiopulmonary exercise testing, to monitor severity and progression of documented disease, and to assess the adequacy of circulatory response.
Studies agree
The ACR recommendation to test “as clinically indicated” is supported by studies showing that patient outcomes are not inferior for arterial blood gas testing when clinically indicated instead of daily, and that this practice is associated with fewer complications, less resource use, and reduced overall patient care costs.
A 2015 study compared the efficacy and safety of obtaining arterial blood gases based on clinical assessment vs daily in 300 critically ill patients.17 Overall, fewer samples were obtained per patient in the clinical assessment group than in the daily group (all patients 3.7 vs 5.5; ventilated patients 2.03 vs 6.12; P < .001 for both). In ventilated patients, there was a 60% decrease in arterial blood gas orders without affecting patient outcomes and safety, including a lower risk of complications and overall cost of care.
In another study, Martinez-Balzano et al18 evaluated the effect of guidelines they developed to optimize the use of arterial blood gas testing in their ICUs. These guidelines encouraged testing of arterial blood gases after an acute respiratory event or for a rational clinical concern, and discouraged testing for routine surveillance, after planned changes of positive end-expiratory pressure or inspired oxygen fraction on mechanical ventilation, for spontaneous breathing trials, or when a disorder was not suspected.
Compared with data collected before implementation, these guidelines reduced the number of arterial blood gas tests by 821.5 per month (41.5%), or approximately 1 test per patient per mechanical-ventilation day for each month (43.1%; P < .001). Appropriately indicated testing rose to 83.4% from a baseline of 67.5% (P = .002). Additionally, this approach was associated with saving 49 liters of blood, reducing ICU costs by $39,432, and freeing up 1,643 staff work hours for other tasks. There were no significant differences in days on mechanical ventilation, severity of illness, or mortality between the 2 periods.18
Extubation effects. Routine arterial blood gas testing has not been shown to affect extubation decisions in patients on mechanical ventilation. In a study of 83 patients who completed a spontaneous breathing trial (total of 100 trials), Salam et al19 found arterial blood gas values obtained during the trial did not change the extubation decision in 93% of the cases.
In a study of 54 extubations in 52 patients,20 65% of the extubations were performed without obtaining an arterial blood gas test after the patient completed a trial of spontaneous breathing. The extubation success rate was 94% for the entire group, and it was the same regardless of whether testing was done (94.7% vs 94.3%, respectively).
Alternatives to arterial blood gases
There are less-invasive means to obtain the information that comes from an arterial blood gas test.
Pulse oximetry is a rapid noninvasive tool that provides continuous assessment of peripheral arterial oxygen saturation as a surrogate marker for tissue arterial oxygenation. However, it cannot measure PaO2 or PaCO2.21
Transcutaneous carbon dioxide (PTCO2) monitoring is another continuous noninvasive alternative. The newer PTCO2 devices are useful in patients with acute respiratory failure and in critically ill patients on vasopressors or vasodilators. Studies have shown good correlation between PTCO2 and PaCO2.22,23
End-tidal carbon dioxide (PetCO2) is another alternative to estimate PaCO2. It can also be used to confirm endotracheal tube placement, during transportation, during procedures in which the patient is under conscious sedation, and to monitor the effectiveness of cardiopulmonary resuscitation and return of circulation after cardiac arrest. PetCO2 measurements are not as accurate as arterial blood gas testing owing to a difference of approximately 2 to 5 mm Hg between PaCO2 and PetCO2 in normal lungs due to alveolar dead space. This difference may be much higher depending on the clinical condition and the degree of alveolar dead space.21,24,25
Venous blood gases, which can be obtained from a peripheral or central venous catheter, are adequate to assess pH and partial pressure of carbon dioxide (PCO2) in hemodynamically stable patients. Walkey et al26 found that the accuracy of venous blood gas measurement to predict arterial blood gases was 90%. They recommended adjusting the venous pH up by 0.05 and the PCO2 down by 5 mm Hg to account for the positive bias of venous blood gases. A limitation of this method is that the values are not reliable in patients who are in shock.
These alternatives can be used as a substitute for daily arterial blood gases. However, in certain clinical scenarios, arterial blood gas measurement remains a necessary and useful clinical tool.
TAKE-HOME MESSAGE
Most scientific evidence suggests that chest radiographs and arterial blood gas measurement in patients undergoing mechanical ventilation—and critically ill, in general—are best done when clinically indicated rather than routinely on a daily basis. This will reduce cost and harm to patients that may result from these unnecessary tests and not adversely affect outcomes.
- Gershengorn HB, Wunsch H, Scales DC, Rubenfeld GD. Trends in use of daily chest radiographs among US adults receiving mechanical ventilation. JAMA Netw Open 2018; 1(4):e181119. doi:10.1001/jamanetworkopen.2018.1119
- American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/clinician-lists/critical-care-societies-collaborative-regular-diagnostic-tests. Accessed August 18, 2019.
- Hall JB, White SR, Karrison T. Efficacy of daily routine chest radiographs in intubated, mechanically ventilated patients. Crit Care Med 1991; 19(5):689–693. pmid:2026031
- Graat ME, Choi G, Wolthuis EK, et al. The clinical value of daily routine chest radiographs in a mixed medical-surgical intensive care unit is low. Crit Care 2006; 10(1):R11. doi:10.1186/cc3955
- Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology 2010; 255(2):386–395. doi:10.1148/radiol.10090946
- Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care 2012; 16(2):R68. doi:10.1186/cc11321
- Krishnan S, Moghekar A, Duggal A, et al. Radiation exposure in the medical ICU: predictors and characteristics. Chest 2018; 153(5):1160–1168. doi:10.1016/j.chest.2018.01.019
- Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet 2009; 374(9702):1687–1693. doi:10.1016/S0140-6736(09)61459-8
- Levin PD, Shatz O, Sviri S, et al. Contamination of portable radiograph equipment with resistant bacteria in the ICU. Chest 2009; 136(2):426–432. doi:10.1378/chest.09-0049
- Suh RD, Genshaft SJ, Kirsch J, et al. ACR Appropriateness Criteria® Intensive Care Unit Patients. J Thorac Imaging 2015; 30(6):W63–W65. doi:10.1097/RTI.0000000000000174
- Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012; 141(3):703–708. doi:10.1378/chest.11-0131
- Lichetenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134(1):117–125. doi:10.1378/chest.07-2800
- Das SK, Choupoo NS, Haldar R, Lahkar A. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta-analysis. Can J Anaesth 2015; 62(4):413–423. doi:10.1007/s12630-014-0301-z
- Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2017; 45(4):715–724. doi:10.1097/CCM.0000000000002188
- DellaVolpe JD, Chakraborti C, Cerreta K, et al. Effects of implementing a protocol for arterial blood gas use on ordering practices and diagnostic yield. Healthc (Amst) 2014; 2(2):130–135. doi:10.1016/j.hjdsi.2013.09.006
- Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry. Respir Care 2013; 58(10):1694–1703. doi:10.4187/respcare.02786
- Blum FE, Lund ET, Hall HA, Tachauer AD, Chedrawy EG, Zilberstein J. Reevaluation of the utilization of arterial blood gas analysis in the intensive care unit: effects on patient safety and patient outcome. J Crit Care 2015; 30(2):438.e1–e5. doi:10.1016/j.jcrc.2014.10.025
- Martínez-Balzano CD, Oliveira P, O’Rourke M, Hills L, Sosa AF; Critical Care Operations Committee of the UMass Memorial Healthcare Center. An educational intervention optimizes the use of arterial blood gas determinations across ICUs from different specialties: a quality-improvement study. Chest 2017; 151(3):579–585. doi:10.1016/j.chest.2016.10.035
- Salam A, Smina M, Gada P, et al. The effect of arterial blood gas values on extubation decisions. Respir Care 2003; 48(11):1033–1037. pmid:14585115
- Pawson SR, DePriest JL. Are blood gases necessary in mechanically ventilated patients who have successfully completed a spontaneous breathing trial? Respir Care 2004; 49(11):1316–1319. pmid:15507165
- Soubani AO. Noninvasive monitoring of oxygen and carbon dioxide. Am J Emerg Med 2001; 19(2):141–146. doi:10.1053/ajem.2001.21353
- Nicolini A, Ferrari MB. Evaluation of a transcutaneous carbon dioxide monitor in patients with acute respiratory failure. Ann Thorac Med 2011; 6(4):217–220. doi:10.4103/1817-1737.84776
- Bendjelid K, Schütz N, Stotz M, Gerard I, Suter PM, Romand JA. Transcutaneous PCO2 monitoring in critically ill adults: clinical evaluation of a new sensor. Crit Care Med 2005; 33(10):2203–2206. pmid:16215371
- Huttmann SE, Windisch W, Storre JH. Techniques for the measurement and monitoring of carbon dioxide in the blood. Ann Am Thorac Soc 2014; 11(4):645–652. doi:10.1513/AnnalsATS.201311-387FR
- McSwain SD, Hamel DS, Smith PB, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care 2010; 55(3):288–293. pmid:20196877
- Walkey AJ, Farber HW, O'Donnell C, Cabral H, Eagan JS, Philippides GJ. The accuracy of the central venous blood gas for acid-base monitoring. J Intensive Care Med 2010; 25(2):104–110. doi:10.1177/0885066609356164
No, they are not required or needed, but daily radiography and arterial blood gas testing are common practice: eg, 60% of intensive care unit (ICU) patients get daily radiographs,1 even though results provide low diagnostic yield and are unlikely to alter patient management compared with testing only when indicated.
The Choosing Wisely campaign,2 a collaborative effort of a number of professional societies, advises against ordering these diagnostic tests daily because routine testing increases risks to patients and burdens the healthcare system. Instead, testing is recommended only in response to a specific clinical question, or when the test results will affect the patient’s treatment.
CHEST RADIOGRAPHS: DAILY VS CLINICALLY INDICATED
Chest radiographs enable practitioners to monitor the position of endotracheal tubes and central venous catheters, evaluate fluid status, follow up on abnormal findings, detect complications of procedures (such as a pneumothorax), and identify otherwise undetected conditions.
And daily chest radiographs often detect abnormalities. A 1991 study by Hall et al3 of 538 chest radiographs in 74 patients on mechanical ventilation reported that 30% of daily routine chest radiographs disclosed a new but minor finding (eg, a small change in endotracheal tube position or a small infiltrate). The new findings were major in 13 (17.6%) of the 74 patients (95% confidence interval [CI] 9%–26%). These included findings that required an immediate diagnostic or therapeutic intervention (eg, endotracheal tube below the tracheal carina, malposition of a catheter, pneumothorax, large pleural effusion).
But most studies say daily radiographs are not needed. In a large prospective study published in 2006, Graat et al4 evaluated the clinical value of 2,457 routine chest radiographs in 754 patients in a combined surgical and medical ICU. Daily chest radiographs revealed new or unexpected findings in 5.8% of cases, but only 2.2% warranted a change in therapy. No differences were found between the medical and surgical patients. The authors concluded that daily routine radiographs in ICU patients seldom reveal unexpected, clinically relevant abnormalities, and those findings rarely require urgent intervention.
A 2010 meta-analysis of 8 studies (7,078 patients) by Oba and Zaza5 compared on-demand and daily routine strategies of performing chest radiographs. They estimated that eliminating daily routine chest radiographs would not affect death rates in the hospital (odds ratio [OR] 1.02, 95% CI 0.89–1.17, P = .78) or the ICU (OR 0.92, 95% CI 0.76–1.11, P = .4). They also found no significant differences in length of stay or duration of mechanical ventilation. This meta-analysis suggests that routine radiographs can be eliminated without adversely affecting outcomes in ICU patients.
A larger meta-analysis (9 trials, 39,358 radiographs, 9,611 patients) published in 2012 by Ganapathy et al6 also found no harm associated with restrictive radiography protocols. These investigators compared a daily chest radiography protocol against a protocol based on clinical indications. The primary outcome was the mortality rate in the ICU; secondary outcomes were the mortality rate in the hospital, the length of stay in the ICU, and duration of mechanical ventilation. They found no differences between routine and restrictive strategies in terms of ICU mortality (risk ratio [RR] 1.04, 95% CI 0.84–1.28, P = .72), hospital mortality (RR 0.98, 95% CI 0.68–1.41, P = .91), or other secondary outcomes.
Clinically indicated testing is better
The conclusion from these studies is that routine chest radiographs in patients undergoing mechanical ventilation does not improve patient outcomes, and thus, a clinically indicated protocol is preferred.
Furthermore, routine daily radiographs have adverse effects such as more cumulative radiation exposure to the patient7 and greater risk of accidental removal of devices (eg, catheters, tubes).8 Another concern is a higher risk of hospital-associated infections from bacterial spread from caregivers’ hands.9
Finally, daily radiographs increase the use of healthcare resources and expenditures. In a 2011 study, Gershengorn et al1 estimated that adopting a clinically indicated radiography strategy could save more than $144 million annually in the United States.
The ACR agrees. Appropriateness criteria published by the American College of Radiology (ACR) in 201510 recommend against routine daily chest radiographs in the ICU, in keeping with the findings of the critical care community. The ACR recommends an initial radiograph at admission to the ICU. However, follow-up radiographs should be obtained only for specific clinical indications, including a change in the patient’s clinical condition or to check for proper placement of endotracheal or nasogastric or orogastric tubes, pulmonary arterial catheters, central venous catheters, chest tubes, and other life-support devices.
Ultrasonography as an alternative
Ultrasonography is widely available and provides an alternative to chest radiography for detecting significant abnormalities in patients on mechanical ventilation without exposing them to radiation and using relatively fewer resources.
A 2012 meta-analysis (8 studies, 1,048 patients) found that bedside ultrasonography reliably detects pneumothorax.11 It can also provide a rapid diagnosis of the cause of acute respiratory failure such as pneumonia or pulmonary edema.12 Ultrasonography, with the appropriate expertise, can also confirm the position of an endotracheal tube13 or central venous catheter.14
ARTERIAL BLOOD GAS TESTING: DAILY VS CLINICALLY INDICATED
Arterial blood gas testing has value for managing patients undergoing mechanical ventilation, and it is one of the most commonly performed diagnostic tests in the ICU. It provides reliable information about the patient’s oxygenation and acid-base status. It is commonly requested when changing ventilator settings.
Downsides. Arterial blood gas measurements account for 10% to 20% of the cost incurred during ICU stay.15 In addition, they require an arterial puncture—an invasive procedure associated with potentially serious complications such as occlusion of the artery, digital embolization leading to digital ischemia, local infection, pseudoaneurysm, hematoma, bleeding, and skin necrosis.
Is daily testing needed?
Guidelines say no. The 2013 American Association for Respiratory Care16 guidelines suggest that arterial blood gas testing should be based on the clinical assessment of the patient. They recommend blood gas analysis to evaluate the patient’s ventilatory status (reflected by the partial pressure of arterial carbon dioxide [PaCO2], acid-base status (reflected by pH), arterial oxygenation (partial pressure of arterial oxygen [PaO2] and oxyhemoglobin saturation), oxygen-carrying capacity, and whether the patient likely has an intrapulmonary shunt. They state that testing is useful to quantify the response to therapeutic or diagnostic interventions such as cardiopulmonary exercise testing, to monitor severity and progression of documented disease, and to assess the adequacy of circulatory response.
Studies agree
The ACR recommendation to test “as clinically indicated” is supported by studies showing that patient outcomes are not inferior for arterial blood gas testing when clinically indicated instead of daily, and that this practice is associated with fewer complications, less resource use, and reduced overall patient care costs.
A 2015 study compared the efficacy and safety of obtaining arterial blood gases based on clinical assessment vs daily in 300 critically ill patients.17 Overall, fewer samples were obtained per patient in the clinical assessment group than in the daily group (all patients 3.7 vs 5.5; ventilated patients 2.03 vs 6.12; P < .001 for both). In ventilated patients, there was a 60% decrease in arterial blood gas orders without affecting patient outcomes and safety, including a lower risk of complications and overall cost of care.
In another study, Martinez-Balzano et al18 evaluated the effect of guidelines they developed to optimize the use of arterial blood gas testing in their ICUs. These guidelines encouraged testing of arterial blood gases after an acute respiratory event or for a rational clinical concern, and discouraged testing for routine surveillance, after planned changes of positive end-expiratory pressure or inspired oxygen fraction on mechanical ventilation, for spontaneous breathing trials, or when a disorder was not suspected.
Compared with data collected before implementation, these guidelines reduced the number of arterial blood gas tests by 821.5 per month (41.5%), or approximately 1 test per patient per mechanical-ventilation day for each month (43.1%; P < .001). Appropriately indicated testing rose to 83.4% from a baseline of 67.5% (P = .002). Additionally, this approach was associated with saving 49 liters of blood, reducing ICU costs by $39,432, and freeing up 1,643 staff work hours for other tasks. There were no significant differences in days on mechanical ventilation, severity of illness, or mortality between the 2 periods.18
Extubation effects. Routine arterial blood gas testing has not been shown to affect extubation decisions in patients on mechanical ventilation. In a study of 83 patients who completed a spontaneous breathing trial (total of 100 trials), Salam et al19 found arterial blood gas values obtained during the trial did not change the extubation decision in 93% of the cases.
In a study of 54 extubations in 52 patients,20 65% of the extubations were performed without obtaining an arterial blood gas test after the patient completed a trial of spontaneous breathing. The extubation success rate was 94% for the entire group, and it was the same regardless of whether testing was done (94.7% vs 94.3%, respectively).
Alternatives to arterial blood gases
There are less-invasive means to obtain the information that comes from an arterial blood gas test.
Pulse oximetry is a rapid noninvasive tool that provides continuous assessment of peripheral arterial oxygen saturation as a surrogate marker for tissue arterial oxygenation. However, it cannot measure PaO2 or PaCO2.21
Transcutaneous carbon dioxide (PTCO2) monitoring is another continuous noninvasive alternative. The newer PTCO2 devices are useful in patients with acute respiratory failure and in critically ill patients on vasopressors or vasodilators. Studies have shown good correlation between PTCO2 and PaCO2.22,23
End-tidal carbon dioxide (PetCO2) is another alternative to estimate PaCO2. It can also be used to confirm endotracheal tube placement, during transportation, during procedures in which the patient is under conscious sedation, and to monitor the effectiveness of cardiopulmonary resuscitation and return of circulation after cardiac arrest. PetCO2 measurements are not as accurate as arterial blood gas testing owing to a difference of approximately 2 to 5 mm Hg between PaCO2 and PetCO2 in normal lungs due to alveolar dead space. This difference may be much higher depending on the clinical condition and the degree of alveolar dead space.21,24,25
Venous blood gases, which can be obtained from a peripheral or central venous catheter, are adequate to assess pH and partial pressure of carbon dioxide (PCO2) in hemodynamically stable patients. Walkey et al26 found that the accuracy of venous blood gas measurement to predict arterial blood gases was 90%. They recommended adjusting the venous pH up by 0.05 and the PCO2 down by 5 mm Hg to account for the positive bias of venous blood gases. A limitation of this method is that the values are not reliable in patients who are in shock.
These alternatives can be used as a substitute for daily arterial blood gases. However, in certain clinical scenarios, arterial blood gas measurement remains a necessary and useful clinical tool.
TAKE-HOME MESSAGE
Most scientific evidence suggests that chest radiographs and arterial blood gas measurement in patients undergoing mechanical ventilation—and critically ill, in general—are best done when clinically indicated rather than routinely on a daily basis. This will reduce cost and harm to patients that may result from these unnecessary tests and not adversely affect outcomes.
No, they are not required or needed, but daily radiography and arterial blood gas testing are common practice: eg, 60% of intensive care unit (ICU) patients get daily radiographs,1 even though results provide low diagnostic yield and are unlikely to alter patient management compared with testing only when indicated.
The Choosing Wisely campaign,2 a collaborative effort of a number of professional societies, advises against ordering these diagnostic tests daily because routine testing increases risks to patients and burdens the healthcare system. Instead, testing is recommended only in response to a specific clinical question, or when the test results will affect the patient’s treatment.
CHEST RADIOGRAPHS: DAILY VS CLINICALLY INDICATED
Chest radiographs enable practitioners to monitor the position of endotracheal tubes and central venous catheters, evaluate fluid status, follow up on abnormal findings, detect complications of procedures (such as a pneumothorax), and identify otherwise undetected conditions.
And daily chest radiographs often detect abnormalities. A 1991 study by Hall et al3 of 538 chest radiographs in 74 patients on mechanical ventilation reported that 30% of daily routine chest radiographs disclosed a new but minor finding (eg, a small change in endotracheal tube position or a small infiltrate). The new findings were major in 13 (17.6%) of the 74 patients (95% confidence interval [CI] 9%–26%). These included findings that required an immediate diagnostic or therapeutic intervention (eg, endotracheal tube below the tracheal carina, malposition of a catheter, pneumothorax, large pleural effusion).
But most studies say daily radiographs are not needed. In a large prospective study published in 2006, Graat et al4 evaluated the clinical value of 2,457 routine chest radiographs in 754 patients in a combined surgical and medical ICU. Daily chest radiographs revealed new or unexpected findings in 5.8% of cases, but only 2.2% warranted a change in therapy. No differences were found between the medical and surgical patients. The authors concluded that daily routine radiographs in ICU patients seldom reveal unexpected, clinically relevant abnormalities, and those findings rarely require urgent intervention.
A 2010 meta-analysis of 8 studies (7,078 patients) by Oba and Zaza5 compared on-demand and daily routine strategies of performing chest radiographs. They estimated that eliminating daily routine chest radiographs would not affect death rates in the hospital (odds ratio [OR] 1.02, 95% CI 0.89–1.17, P = .78) or the ICU (OR 0.92, 95% CI 0.76–1.11, P = .4). They also found no significant differences in length of stay or duration of mechanical ventilation. This meta-analysis suggests that routine radiographs can be eliminated without adversely affecting outcomes in ICU patients.
A larger meta-analysis (9 trials, 39,358 radiographs, 9,611 patients) published in 2012 by Ganapathy et al6 also found no harm associated with restrictive radiography protocols. These investigators compared a daily chest radiography protocol against a protocol based on clinical indications. The primary outcome was the mortality rate in the ICU; secondary outcomes were the mortality rate in the hospital, the length of stay in the ICU, and duration of mechanical ventilation. They found no differences between routine and restrictive strategies in terms of ICU mortality (risk ratio [RR] 1.04, 95% CI 0.84–1.28, P = .72), hospital mortality (RR 0.98, 95% CI 0.68–1.41, P = .91), or other secondary outcomes.
Clinically indicated testing is better
The conclusion from these studies is that routine chest radiographs in patients undergoing mechanical ventilation does not improve patient outcomes, and thus, a clinically indicated protocol is preferred.
Furthermore, routine daily radiographs have adverse effects such as more cumulative radiation exposure to the patient7 and greater risk of accidental removal of devices (eg, catheters, tubes).8 Another concern is a higher risk of hospital-associated infections from bacterial spread from caregivers’ hands.9
Finally, daily radiographs increase the use of healthcare resources and expenditures. In a 2011 study, Gershengorn et al1 estimated that adopting a clinically indicated radiography strategy could save more than $144 million annually in the United States.
The ACR agrees. Appropriateness criteria published by the American College of Radiology (ACR) in 201510 recommend against routine daily chest radiographs in the ICU, in keeping with the findings of the critical care community. The ACR recommends an initial radiograph at admission to the ICU. However, follow-up radiographs should be obtained only for specific clinical indications, including a change in the patient’s clinical condition or to check for proper placement of endotracheal or nasogastric or orogastric tubes, pulmonary arterial catheters, central venous catheters, chest tubes, and other life-support devices.
Ultrasonography as an alternative
Ultrasonography is widely available and provides an alternative to chest radiography for detecting significant abnormalities in patients on mechanical ventilation without exposing them to radiation and using relatively fewer resources.
A 2012 meta-analysis (8 studies, 1,048 patients) found that bedside ultrasonography reliably detects pneumothorax.11 It can also provide a rapid diagnosis of the cause of acute respiratory failure such as pneumonia or pulmonary edema.12 Ultrasonography, with the appropriate expertise, can also confirm the position of an endotracheal tube13 or central venous catheter.14
ARTERIAL BLOOD GAS TESTING: DAILY VS CLINICALLY INDICATED
Arterial blood gas testing has value for managing patients undergoing mechanical ventilation, and it is one of the most commonly performed diagnostic tests in the ICU. It provides reliable information about the patient’s oxygenation and acid-base status. It is commonly requested when changing ventilator settings.
Downsides. Arterial blood gas measurements account for 10% to 20% of the cost incurred during ICU stay.15 In addition, they require an arterial puncture—an invasive procedure associated with potentially serious complications such as occlusion of the artery, digital embolization leading to digital ischemia, local infection, pseudoaneurysm, hematoma, bleeding, and skin necrosis.
Is daily testing needed?
Guidelines say no. The 2013 American Association for Respiratory Care16 guidelines suggest that arterial blood gas testing should be based on the clinical assessment of the patient. They recommend blood gas analysis to evaluate the patient’s ventilatory status (reflected by the partial pressure of arterial carbon dioxide [PaCO2], acid-base status (reflected by pH), arterial oxygenation (partial pressure of arterial oxygen [PaO2] and oxyhemoglobin saturation), oxygen-carrying capacity, and whether the patient likely has an intrapulmonary shunt. They state that testing is useful to quantify the response to therapeutic or diagnostic interventions such as cardiopulmonary exercise testing, to monitor severity and progression of documented disease, and to assess the adequacy of circulatory response.
Studies agree
The ACR recommendation to test “as clinically indicated” is supported by studies showing that patient outcomes are not inferior for arterial blood gas testing when clinically indicated instead of daily, and that this practice is associated with fewer complications, less resource use, and reduced overall patient care costs.
A 2015 study compared the efficacy and safety of obtaining arterial blood gases based on clinical assessment vs daily in 300 critically ill patients.17 Overall, fewer samples were obtained per patient in the clinical assessment group than in the daily group (all patients 3.7 vs 5.5; ventilated patients 2.03 vs 6.12; P < .001 for both). In ventilated patients, there was a 60% decrease in arterial blood gas orders without affecting patient outcomes and safety, including a lower risk of complications and overall cost of care.
In another study, Martinez-Balzano et al18 evaluated the effect of guidelines they developed to optimize the use of arterial blood gas testing in their ICUs. These guidelines encouraged testing of arterial blood gases after an acute respiratory event or for a rational clinical concern, and discouraged testing for routine surveillance, after planned changes of positive end-expiratory pressure or inspired oxygen fraction on mechanical ventilation, for spontaneous breathing trials, or when a disorder was not suspected.
Compared with data collected before implementation, these guidelines reduced the number of arterial blood gas tests by 821.5 per month (41.5%), or approximately 1 test per patient per mechanical-ventilation day for each month (43.1%; P < .001). Appropriately indicated testing rose to 83.4% from a baseline of 67.5% (P = .002). Additionally, this approach was associated with saving 49 liters of blood, reducing ICU costs by $39,432, and freeing up 1,643 staff work hours for other tasks. There were no significant differences in days on mechanical ventilation, severity of illness, or mortality between the 2 periods.18
Extubation effects. Routine arterial blood gas testing has not been shown to affect extubation decisions in patients on mechanical ventilation. In a study of 83 patients who completed a spontaneous breathing trial (total of 100 trials), Salam et al19 found arterial blood gas values obtained during the trial did not change the extubation decision in 93% of the cases.
In a study of 54 extubations in 52 patients,20 65% of the extubations were performed without obtaining an arterial blood gas test after the patient completed a trial of spontaneous breathing. The extubation success rate was 94% for the entire group, and it was the same regardless of whether testing was done (94.7% vs 94.3%, respectively).
Alternatives to arterial blood gases
There are less-invasive means to obtain the information that comes from an arterial blood gas test.
Pulse oximetry is a rapid noninvasive tool that provides continuous assessment of peripheral arterial oxygen saturation as a surrogate marker for tissue arterial oxygenation. However, it cannot measure PaO2 or PaCO2.21
Transcutaneous carbon dioxide (PTCO2) monitoring is another continuous noninvasive alternative. The newer PTCO2 devices are useful in patients with acute respiratory failure and in critically ill patients on vasopressors or vasodilators. Studies have shown good correlation between PTCO2 and PaCO2.22,23
End-tidal carbon dioxide (PetCO2) is another alternative to estimate PaCO2. It can also be used to confirm endotracheal tube placement, during transportation, during procedures in which the patient is under conscious sedation, and to monitor the effectiveness of cardiopulmonary resuscitation and return of circulation after cardiac arrest. PetCO2 measurements are not as accurate as arterial blood gas testing owing to a difference of approximately 2 to 5 mm Hg between PaCO2 and PetCO2 in normal lungs due to alveolar dead space. This difference may be much higher depending on the clinical condition and the degree of alveolar dead space.21,24,25
Venous blood gases, which can be obtained from a peripheral or central venous catheter, are adequate to assess pH and partial pressure of carbon dioxide (PCO2) in hemodynamically stable patients. Walkey et al26 found that the accuracy of venous blood gas measurement to predict arterial blood gases was 90%. They recommended adjusting the venous pH up by 0.05 and the PCO2 down by 5 mm Hg to account for the positive bias of venous blood gases. A limitation of this method is that the values are not reliable in patients who are in shock.
These alternatives can be used as a substitute for daily arterial blood gases. However, in certain clinical scenarios, arterial blood gas measurement remains a necessary and useful clinical tool.
TAKE-HOME MESSAGE
Most scientific evidence suggests that chest radiographs and arterial blood gas measurement in patients undergoing mechanical ventilation—and critically ill, in general—are best done when clinically indicated rather than routinely on a daily basis. This will reduce cost and harm to patients that may result from these unnecessary tests and not adversely affect outcomes.
- Gershengorn HB, Wunsch H, Scales DC, Rubenfeld GD. Trends in use of daily chest radiographs among US adults receiving mechanical ventilation. JAMA Netw Open 2018; 1(4):e181119. doi:10.1001/jamanetworkopen.2018.1119
- American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/clinician-lists/critical-care-societies-collaborative-regular-diagnostic-tests. Accessed August 18, 2019.
- Hall JB, White SR, Karrison T. Efficacy of daily routine chest radiographs in intubated, mechanically ventilated patients. Crit Care Med 1991; 19(5):689–693. pmid:2026031
- Graat ME, Choi G, Wolthuis EK, et al. The clinical value of daily routine chest radiographs in a mixed medical-surgical intensive care unit is low. Crit Care 2006; 10(1):R11. doi:10.1186/cc3955
- Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology 2010; 255(2):386–395. doi:10.1148/radiol.10090946
- Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care 2012; 16(2):R68. doi:10.1186/cc11321
- Krishnan S, Moghekar A, Duggal A, et al. Radiation exposure in the medical ICU: predictors and characteristics. Chest 2018; 153(5):1160–1168. doi:10.1016/j.chest.2018.01.019
- Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet 2009; 374(9702):1687–1693. doi:10.1016/S0140-6736(09)61459-8
- Levin PD, Shatz O, Sviri S, et al. Contamination of portable radiograph equipment with resistant bacteria in the ICU. Chest 2009; 136(2):426–432. doi:10.1378/chest.09-0049
- Suh RD, Genshaft SJ, Kirsch J, et al. ACR Appropriateness Criteria® Intensive Care Unit Patients. J Thorac Imaging 2015; 30(6):W63–W65. doi:10.1097/RTI.0000000000000174
- Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012; 141(3):703–708. doi:10.1378/chest.11-0131
- Lichetenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134(1):117–125. doi:10.1378/chest.07-2800
- Das SK, Choupoo NS, Haldar R, Lahkar A. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta-analysis. Can J Anaesth 2015; 62(4):413–423. doi:10.1007/s12630-014-0301-z
- Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2017; 45(4):715–724. doi:10.1097/CCM.0000000000002188
- DellaVolpe JD, Chakraborti C, Cerreta K, et al. Effects of implementing a protocol for arterial blood gas use on ordering practices and diagnostic yield. Healthc (Amst) 2014; 2(2):130–135. doi:10.1016/j.hjdsi.2013.09.006
- Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry. Respir Care 2013; 58(10):1694–1703. doi:10.4187/respcare.02786
- Blum FE, Lund ET, Hall HA, Tachauer AD, Chedrawy EG, Zilberstein J. Reevaluation of the utilization of arterial blood gas analysis in the intensive care unit: effects on patient safety and patient outcome. J Crit Care 2015; 30(2):438.e1–e5. doi:10.1016/j.jcrc.2014.10.025
- Martínez-Balzano CD, Oliveira P, O’Rourke M, Hills L, Sosa AF; Critical Care Operations Committee of the UMass Memorial Healthcare Center. An educational intervention optimizes the use of arterial blood gas determinations across ICUs from different specialties: a quality-improvement study. Chest 2017; 151(3):579–585. doi:10.1016/j.chest.2016.10.035
- Salam A, Smina M, Gada P, et al. The effect of arterial blood gas values on extubation decisions. Respir Care 2003; 48(11):1033–1037. pmid:14585115
- Pawson SR, DePriest JL. Are blood gases necessary in mechanically ventilated patients who have successfully completed a spontaneous breathing trial? Respir Care 2004; 49(11):1316–1319. pmid:15507165
- Soubani AO. Noninvasive monitoring of oxygen and carbon dioxide. Am J Emerg Med 2001; 19(2):141–146. doi:10.1053/ajem.2001.21353
- Nicolini A, Ferrari MB. Evaluation of a transcutaneous carbon dioxide monitor in patients with acute respiratory failure. Ann Thorac Med 2011; 6(4):217–220. doi:10.4103/1817-1737.84776
- Bendjelid K, Schütz N, Stotz M, Gerard I, Suter PM, Romand JA. Transcutaneous PCO2 monitoring in critically ill adults: clinical evaluation of a new sensor. Crit Care Med 2005; 33(10):2203–2206. pmid:16215371
- Huttmann SE, Windisch W, Storre JH. Techniques for the measurement and monitoring of carbon dioxide in the blood. Ann Am Thorac Soc 2014; 11(4):645–652. doi:10.1513/AnnalsATS.201311-387FR
- McSwain SD, Hamel DS, Smith PB, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care 2010; 55(3):288–293. pmid:20196877
- Walkey AJ, Farber HW, O'Donnell C, Cabral H, Eagan JS, Philippides GJ. The accuracy of the central venous blood gas for acid-base monitoring. J Intensive Care Med 2010; 25(2):104–110. doi:10.1177/0885066609356164
- Gershengorn HB, Wunsch H, Scales DC, Rubenfeld GD. Trends in use of daily chest radiographs among US adults receiving mechanical ventilation. JAMA Netw Open 2018; 1(4):e181119. doi:10.1001/jamanetworkopen.2018.1119
- American Board of Internal Medicine Foundation. Choosing Wisely. http://www.choosingwisely.org/clinician-lists/critical-care-societies-collaborative-regular-diagnostic-tests. Accessed August 18, 2019.
- Hall JB, White SR, Karrison T. Efficacy of daily routine chest radiographs in intubated, mechanically ventilated patients. Crit Care Med 1991; 19(5):689–693. pmid:2026031
- Graat ME, Choi G, Wolthuis EK, et al. The clinical value of daily routine chest radiographs in a mixed medical-surgical intensive care unit is low. Crit Care 2006; 10(1):R11. doi:10.1186/cc3955
- Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology 2010; 255(2):386–395. doi:10.1148/radiol.10090946
- Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care 2012; 16(2):R68. doi:10.1186/cc11321
- Krishnan S, Moghekar A, Duggal A, et al. Radiation exposure in the medical ICU: predictors and characteristics. Chest 2018; 153(5):1160–1168. doi:10.1016/j.chest.2018.01.019
- Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet 2009; 374(9702):1687–1693. doi:10.1016/S0140-6736(09)61459-8
- Levin PD, Shatz O, Sviri S, et al. Contamination of portable radiograph equipment with resistant bacteria in the ICU. Chest 2009; 136(2):426–432. doi:10.1378/chest.09-0049
- Suh RD, Genshaft SJ, Kirsch J, et al. ACR Appropriateness Criteria® Intensive Care Unit Patients. J Thorac Imaging 2015; 30(6):W63–W65. doi:10.1097/RTI.0000000000000174
- Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012; 141(3):703–708. doi:10.1378/chest.11-0131
- Lichetenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134(1):117–125. doi:10.1378/chest.07-2800
- Das SK, Choupoo NS, Haldar R, Lahkar A. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta-analysis. Can J Anaesth 2015; 62(4):413–423. doi:10.1007/s12630-014-0301-z
- Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2017; 45(4):715–724. doi:10.1097/CCM.0000000000002188
- DellaVolpe JD, Chakraborti C, Cerreta K, et al. Effects of implementing a protocol for arterial blood gas use on ordering practices and diagnostic yield. Healthc (Amst) 2014; 2(2):130–135. doi:10.1016/j.hjdsi.2013.09.006
- Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry. Respir Care 2013; 58(10):1694–1703. doi:10.4187/respcare.02786
- Blum FE, Lund ET, Hall HA, Tachauer AD, Chedrawy EG, Zilberstein J. Reevaluation of the utilization of arterial blood gas analysis in the intensive care unit: effects on patient safety and patient outcome. J Crit Care 2015; 30(2):438.e1–e5. doi:10.1016/j.jcrc.2014.10.025
- Martínez-Balzano CD, Oliveira P, O’Rourke M, Hills L, Sosa AF; Critical Care Operations Committee of the UMass Memorial Healthcare Center. An educational intervention optimizes the use of arterial blood gas determinations across ICUs from different specialties: a quality-improvement study. Chest 2017; 151(3):579–585. doi:10.1016/j.chest.2016.10.035
- Salam A, Smina M, Gada P, et al. The effect of arterial blood gas values on extubation decisions. Respir Care 2003; 48(11):1033–1037. pmid:14585115
- Pawson SR, DePriest JL. Are blood gases necessary in mechanically ventilated patients who have successfully completed a spontaneous breathing trial? Respir Care 2004; 49(11):1316–1319. pmid:15507165
- Soubani AO. Noninvasive monitoring of oxygen and carbon dioxide. Am J Emerg Med 2001; 19(2):141–146. doi:10.1053/ajem.2001.21353
- Nicolini A, Ferrari MB. Evaluation of a transcutaneous carbon dioxide monitor in patients with acute respiratory failure. Ann Thorac Med 2011; 6(4):217–220. doi:10.4103/1817-1737.84776
- Bendjelid K, Schütz N, Stotz M, Gerard I, Suter PM, Romand JA. Transcutaneous PCO2 monitoring in critically ill adults: clinical evaluation of a new sensor. Crit Care Med 2005; 33(10):2203–2206. pmid:16215371
- Huttmann SE, Windisch W, Storre JH. Techniques for the measurement and monitoring of carbon dioxide in the blood. Ann Am Thorac Soc 2014; 11(4):645–652. doi:10.1513/AnnalsATS.201311-387FR
- McSwain SD, Hamel DS, Smith PB, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care 2010; 55(3):288–293. pmid:20196877
- Walkey AJ, Farber HW, O'Donnell C, Cabral H, Eagan JS, Philippides GJ. The accuracy of the central venous blood gas for acid-base monitoring. J Intensive Care Med 2010; 25(2):104–110. doi:10.1177/0885066609356164
Often Off-label: Questionable Gabapentinoid Use Noted at Hospital Admission Warrants Deprescribing
Three years after gabapentin received US Food and Drug Administration (FDA) approval in 1990 for epilepsy, case reports and animal studies emerged announcing its potential in the treatment of pain syndromes through then-novel analgesic mechanisms.1 Fast forward 20 years to 2016: gabapentin and its close cousin, pregabalin, are internationally considered first-line agents for the treatment of neuropathic pain in guidelines from the Centers for Disease Control and Prevention, the Canadian Pain Society, and the National Institute for Health and Care Excellence. Gabapentin is the 10th most prescribed drug in the United States, and brand-name pregabalin sales were $4.4 billion USD, ranking 8th in invoice drug spending.2
The ascendancy of gabapentinoids as drugs of choice for pain, though, is fraught with controversy; yet, they were shepherded to commercial success. In 2004, the patent owner of gabapentin, Warner-Lambert (now owned by Pfizer), admitted guilt to charges that it violated federal regulations in its promotion: they encouraged off-label prescribing through paid physician-to-physician communications, publication of positive outcomes, and suppression of negative ones.3 Pfizer paid another settlement in 2009 for false claims about off-label indications for brand-name pregabalin.4
Mindful of historical biases, recent trials and meta-analyses have found less favorable outcomes for gabapentinoids in the treatment of off-label pain conditions and greater risks than previously reported. Cochrane reviews for gabapentin demonstrate efficacy only in postherpetic neuralgia (for which it has FDA approval) and diabetic peripheral neuropathy (for which it does not); pregabalin has efficacy in both these conditions as well as posttraumatic neuropathic pain and fibromyalgia (and FDA approval for all four). For other types of neuropathic pain, the evidence is of lower quality. Even for approved indications, the risk–benefit ratio is questionable, as the numbers needed to harm for dizziness and somnolence are similar to the numbers needed to treat for pain.5,6 Further, case–control studies have found increased odds of opioid-related death when gabapentinoids were coprescribed with opioids,7,8 prompting gabapentinoids to be reclassified as class C controlled substances in the UK as of April 2019.9
On this backdrop, Gingras and colleagues publish their retrospective cohort study on high-risk prescribing of these popular drugs in Montreal, Canada in this issue of Journal of Hospital Medicine.10 In their retrospective cohort study of 4,103 patients admitted to a clinical teaching unit, more than one in eight patients (13%) were being prescribed a gabapentinoid as an outpatient; chart review of the admission notes indicated that only 17% of them had an FDA-approved indication and 28% had no clear indication. Gabapentinoid users were more likely to be coprescribed an opioid than nonusers (28% vs 12%). There was no significant difference in length of stay or inpatient death between users and nonusers.
Gingras et al. thereby conclude that there is an opportunity to deprescribe on the basis of few gabapentinoid users having a documented indication and the recent research showing poten
First, the urgency of deprescribing in inpatient settings should be titrated to the degree of risk. When the reason for hospitalization is potentially an adverse drug effect, culprit medications posing a substantial and near-term risk of harm should be stopped, such as when patients on gabapentinoids present with major alteration of their mental status.
In less urgent circumstances, hospitalists should speak first with outpatient prescribers because they may have important contextual information (eg, indication, patient preference, failure of alternative therapies, etc.) about previous care that the inpatient clinician lacks. For gabapentinoids, it is easy to imagine how treated pain syndromes without objective markers of disease may escape notice by a hospitalist and remain undocumented, which may encourage erroneous deprescribing. If the shared decision between the patient and providers is to deprescribe, patients on high doses warrant a tapering schedule.11 Pharmacist consultation can help with this.
Second, before discharge, hospitalists should communicate their rationale for deprescribing medications to both patients and outpatient prescribers, especially if a prolonged tapering schedule is required. This type of communication occurred infrequently in this study: the reason for deprescribing a gabapentinoid was missing from the discharge summary 55% of the time. Without this, outpatient prescribers may simply reinitiate the medication after the patient is discharged.
To counter the overuse of gabapentinoids, hospitalists should look for opportunities to deprescribe them where there is concern about adverse events and when evidence-based indications do not exist. Successful deprescribing of these popular drugs will require deliberate collaboration and communication with the outpatient circle of care, as ongoing deprescribing ultimately depends on patients and outpatient prescribers agreeing to the change.
Disclosures
Dr. Steinman served as an unpaid expert witness in United States of America ex. Rel. David Franklin vs. Parke-Davis, Division of Warner-Lambert Company and Pfizer, Inc, litigation which alleged that the named pharmaceutical companies improperly marketed gabapentin for non-FDA-approved uses. Drs. Lam and Rochon have no conflicts of interest to declare.
Funding
Dr. Rochon is supported by the Retired Teachers of Ontario (RTO/ERO) Chair in Geriatric Medicine at the University of Toronto. Dr. Steinman is supported by the National Institute on Aging, US (K24AG049057 and P30AG044281).
1. Segal AZ, Rordorf G. Gabapentin as a novel treatment for postherpetic neuralgia. Neurology. 1996;46(4):1175-1176. https://doi.org/10.1212/WNL.46.4.1175.
2. Goodman CW, Brett AS. Gabapentin and pregabalin for pain — is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411-414. https://doi.org/10.1056/NEJMp1704633.
3. Steinman MA, Bero LA, Chren M-M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145(4):284. https://doi.org/10.7326/0003-4819-145-4-200608150-00008.
4. Department of Justice, Office of Public Affairs. Justice Department Announces Largest Health Care Fraud Settlement in Its History. https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history. Published September 2, 2009. Accessed April 12, 2019.
5. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938. https://doi.org/10.1002/14651858.CD007938.pub4.
6. Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1:CD007076. https://doi.org/10.1002/14651858.CD007076.pub3.
7. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case–control study. PLoS Med. 2017;14(10): e1002396. https://doi.org/10.1371/journal.pmed.1002396.
8. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732. https://doi.org/10.7326/M18-1136.
9. Mayor S. Pregabalin and gabapentin become controlled drugs to cut deaths from misuse. BMJ. 2018;363:k4364. https://doi.org/10.1136/bmj.k4364.
10. Gingras M-A, Lieu A, Papillon-Ferland L, Lee T, McDonald E. Retrospective cohort study of the prevalence of off-label gabapentinoid prescriptions in hospitalized medical patients. J Hosp Med. 2019;14(9):547-550. https://doi.org/10.12788/jhm.3203.
11. Parsons G. Guide to the management of gabapentinoid misuse. Prescriber. 2018;29(4):25-30. https://doi.org/10.1002/psb.1664.
Three years after gabapentin received US Food and Drug Administration (FDA) approval in 1990 for epilepsy, case reports and animal studies emerged announcing its potential in the treatment of pain syndromes through then-novel analgesic mechanisms.1 Fast forward 20 years to 2016: gabapentin and its close cousin, pregabalin, are internationally considered first-line agents for the treatment of neuropathic pain in guidelines from the Centers for Disease Control and Prevention, the Canadian Pain Society, and the National Institute for Health and Care Excellence. Gabapentin is the 10th most prescribed drug in the United States, and brand-name pregabalin sales were $4.4 billion USD, ranking 8th in invoice drug spending.2
The ascendancy of gabapentinoids as drugs of choice for pain, though, is fraught with controversy; yet, they were shepherded to commercial success. In 2004, the patent owner of gabapentin, Warner-Lambert (now owned by Pfizer), admitted guilt to charges that it violated federal regulations in its promotion: they encouraged off-label prescribing through paid physician-to-physician communications, publication of positive outcomes, and suppression of negative ones.3 Pfizer paid another settlement in 2009 for false claims about off-label indications for brand-name pregabalin.4
Mindful of historical biases, recent trials and meta-analyses have found less favorable outcomes for gabapentinoids in the treatment of off-label pain conditions and greater risks than previously reported. Cochrane reviews for gabapentin demonstrate efficacy only in postherpetic neuralgia (for which it has FDA approval) and diabetic peripheral neuropathy (for which it does not); pregabalin has efficacy in both these conditions as well as posttraumatic neuropathic pain and fibromyalgia (and FDA approval for all four). For other types of neuropathic pain, the evidence is of lower quality. Even for approved indications, the risk–benefit ratio is questionable, as the numbers needed to harm for dizziness and somnolence are similar to the numbers needed to treat for pain.5,6 Further, case–control studies have found increased odds of opioid-related death when gabapentinoids were coprescribed with opioids,7,8 prompting gabapentinoids to be reclassified as class C controlled substances in the UK as of April 2019.9
On this backdrop, Gingras and colleagues publish their retrospective cohort study on high-risk prescribing of these popular drugs in Montreal, Canada in this issue of Journal of Hospital Medicine.10 In their retrospective cohort study of 4,103 patients admitted to a clinical teaching unit, more than one in eight patients (13%) were being prescribed a gabapentinoid as an outpatient; chart review of the admission notes indicated that only 17% of them had an FDA-approved indication and 28% had no clear indication. Gabapentinoid users were more likely to be coprescribed an opioid than nonusers (28% vs 12%). There was no significant difference in length of stay or inpatient death between users and nonusers.
Gingras et al. thereby conclude that there is an opportunity to deprescribe on the basis of few gabapentinoid users having a documented indication and the recent research showing poten
First, the urgency of deprescribing in inpatient settings should be titrated to the degree of risk. When the reason for hospitalization is potentially an adverse drug effect, culprit medications posing a substantial and near-term risk of harm should be stopped, such as when patients on gabapentinoids present with major alteration of their mental status.
In less urgent circumstances, hospitalists should speak first with outpatient prescribers because they may have important contextual information (eg, indication, patient preference, failure of alternative therapies, etc.) about previous care that the inpatient clinician lacks. For gabapentinoids, it is easy to imagine how treated pain syndromes without objective markers of disease may escape notice by a hospitalist and remain undocumented, which may encourage erroneous deprescribing. If the shared decision between the patient and providers is to deprescribe, patients on high doses warrant a tapering schedule.11 Pharmacist consultation can help with this.
Second, before discharge, hospitalists should communicate their rationale for deprescribing medications to both patients and outpatient prescribers, especially if a prolonged tapering schedule is required. This type of communication occurred infrequently in this study: the reason for deprescribing a gabapentinoid was missing from the discharge summary 55% of the time. Without this, outpatient prescribers may simply reinitiate the medication after the patient is discharged.
To counter the overuse of gabapentinoids, hospitalists should look for opportunities to deprescribe them where there is concern about adverse events and when evidence-based indications do not exist. Successful deprescribing of these popular drugs will require deliberate collaboration and communication with the outpatient circle of care, as ongoing deprescribing ultimately depends on patients and outpatient prescribers agreeing to the change.
Disclosures
Dr. Steinman served as an unpaid expert witness in United States of America ex. Rel. David Franklin vs. Parke-Davis, Division of Warner-Lambert Company and Pfizer, Inc, litigation which alleged that the named pharmaceutical companies improperly marketed gabapentin for non-FDA-approved uses. Drs. Lam and Rochon have no conflicts of interest to declare.
Funding
Dr. Rochon is supported by the Retired Teachers of Ontario (RTO/ERO) Chair in Geriatric Medicine at the University of Toronto. Dr. Steinman is supported by the National Institute on Aging, US (K24AG049057 and P30AG044281).
Three years after gabapentin received US Food and Drug Administration (FDA) approval in 1990 for epilepsy, case reports and animal studies emerged announcing its potential in the treatment of pain syndromes through then-novel analgesic mechanisms.1 Fast forward 20 years to 2016: gabapentin and its close cousin, pregabalin, are internationally considered first-line agents for the treatment of neuropathic pain in guidelines from the Centers for Disease Control and Prevention, the Canadian Pain Society, and the National Institute for Health and Care Excellence. Gabapentin is the 10th most prescribed drug in the United States, and brand-name pregabalin sales were $4.4 billion USD, ranking 8th in invoice drug spending.2
The ascendancy of gabapentinoids as drugs of choice for pain, though, is fraught with controversy; yet, they were shepherded to commercial success. In 2004, the patent owner of gabapentin, Warner-Lambert (now owned by Pfizer), admitted guilt to charges that it violated federal regulations in its promotion: they encouraged off-label prescribing through paid physician-to-physician communications, publication of positive outcomes, and suppression of negative ones.3 Pfizer paid another settlement in 2009 for false claims about off-label indications for brand-name pregabalin.4
Mindful of historical biases, recent trials and meta-analyses have found less favorable outcomes for gabapentinoids in the treatment of off-label pain conditions and greater risks than previously reported. Cochrane reviews for gabapentin demonstrate efficacy only in postherpetic neuralgia (for which it has FDA approval) and diabetic peripheral neuropathy (for which it does not); pregabalin has efficacy in both these conditions as well as posttraumatic neuropathic pain and fibromyalgia (and FDA approval for all four). For other types of neuropathic pain, the evidence is of lower quality. Even for approved indications, the risk–benefit ratio is questionable, as the numbers needed to harm for dizziness and somnolence are similar to the numbers needed to treat for pain.5,6 Further, case–control studies have found increased odds of opioid-related death when gabapentinoids were coprescribed with opioids,7,8 prompting gabapentinoids to be reclassified as class C controlled substances in the UK as of April 2019.9
On this backdrop, Gingras and colleagues publish their retrospective cohort study on high-risk prescribing of these popular drugs in Montreal, Canada in this issue of Journal of Hospital Medicine.10 In their retrospective cohort study of 4,103 patients admitted to a clinical teaching unit, more than one in eight patients (13%) were being prescribed a gabapentinoid as an outpatient; chart review of the admission notes indicated that only 17% of them had an FDA-approved indication and 28% had no clear indication. Gabapentinoid users were more likely to be coprescribed an opioid than nonusers (28% vs 12%). There was no significant difference in length of stay or inpatient death between users and nonusers.
Gingras et al. thereby conclude that there is an opportunity to deprescribe on the basis of few gabapentinoid users having a documented indication and the recent research showing poten
First, the urgency of deprescribing in inpatient settings should be titrated to the degree of risk. When the reason for hospitalization is potentially an adverse drug effect, culprit medications posing a substantial and near-term risk of harm should be stopped, such as when patients on gabapentinoids present with major alteration of their mental status.
In less urgent circumstances, hospitalists should speak first with outpatient prescribers because they may have important contextual information (eg, indication, patient preference, failure of alternative therapies, etc.) about previous care that the inpatient clinician lacks. For gabapentinoids, it is easy to imagine how treated pain syndromes without objective markers of disease may escape notice by a hospitalist and remain undocumented, which may encourage erroneous deprescribing. If the shared decision between the patient and providers is to deprescribe, patients on high doses warrant a tapering schedule.11 Pharmacist consultation can help with this.
Second, before discharge, hospitalists should communicate their rationale for deprescribing medications to both patients and outpatient prescribers, especially if a prolonged tapering schedule is required. This type of communication occurred infrequently in this study: the reason for deprescribing a gabapentinoid was missing from the discharge summary 55% of the time. Without this, outpatient prescribers may simply reinitiate the medication after the patient is discharged.
To counter the overuse of gabapentinoids, hospitalists should look for opportunities to deprescribe them where there is concern about adverse events and when evidence-based indications do not exist. Successful deprescribing of these popular drugs will require deliberate collaboration and communication with the outpatient circle of care, as ongoing deprescribing ultimately depends on patients and outpatient prescribers agreeing to the change.
Disclosures
Dr. Steinman served as an unpaid expert witness in United States of America ex. Rel. David Franklin vs. Parke-Davis, Division of Warner-Lambert Company and Pfizer, Inc, litigation which alleged that the named pharmaceutical companies improperly marketed gabapentin for non-FDA-approved uses. Drs. Lam and Rochon have no conflicts of interest to declare.
Funding
Dr. Rochon is supported by the Retired Teachers of Ontario (RTO/ERO) Chair in Geriatric Medicine at the University of Toronto. Dr. Steinman is supported by the National Institute on Aging, US (K24AG049057 and P30AG044281).
1. Segal AZ, Rordorf G. Gabapentin as a novel treatment for postherpetic neuralgia. Neurology. 1996;46(4):1175-1176. https://doi.org/10.1212/WNL.46.4.1175.
2. Goodman CW, Brett AS. Gabapentin and pregabalin for pain — is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411-414. https://doi.org/10.1056/NEJMp1704633.
3. Steinman MA, Bero LA, Chren M-M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145(4):284. https://doi.org/10.7326/0003-4819-145-4-200608150-00008.
4. Department of Justice, Office of Public Affairs. Justice Department Announces Largest Health Care Fraud Settlement in Its History. https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history. Published September 2, 2009. Accessed April 12, 2019.
5. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938. https://doi.org/10.1002/14651858.CD007938.pub4.
6. Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1:CD007076. https://doi.org/10.1002/14651858.CD007076.pub3.
7. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case–control study. PLoS Med. 2017;14(10): e1002396. https://doi.org/10.1371/journal.pmed.1002396.
8. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732. https://doi.org/10.7326/M18-1136.
9. Mayor S. Pregabalin and gabapentin become controlled drugs to cut deaths from misuse. BMJ. 2018;363:k4364. https://doi.org/10.1136/bmj.k4364.
10. Gingras M-A, Lieu A, Papillon-Ferland L, Lee T, McDonald E. Retrospective cohort study of the prevalence of off-label gabapentinoid prescriptions in hospitalized medical patients. J Hosp Med. 2019;14(9):547-550. https://doi.org/10.12788/jhm.3203.
11. Parsons G. Guide to the management of gabapentinoid misuse. Prescriber. 2018;29(4):25-30. https://doi.org/10.1002/psb.1664.
1. Segal AZ, Rordorf G. Gabapentin as a novel treatment for postherpetic neuralgia. Neurology. 1996;46(4):1175-1176. https://doi.org/10.1212/WNL.46.4.1175.
2. Goodman CW, Brett AS. Gabapentin and pregabalin for pain — is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411-414. https://doi.org/10.1056/NEJMp1704633.
3. Steinman MA, Bero LA, Chren M-M, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med. 2006;145(4):284. https://doi.org/10.7326/0003-4819-145-4-200608150-00008.
4. Department of Justice, Office of Public Affairs. Justice Department Announces Largest Health Care Fraud Settlement in Its History. https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history. Published September 2, 2009. Accessed April 12, 2019.
5. Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938. https://doi.org/10.1002/14651858.CD007938.pub4.
6. Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1:CD007076. https://doi.org/10.1002/14651858.CD007076.pub3.
7. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case–control study. PLoS Med. 2017;14(10): e1002396. https://doi.org/10.1371/journal.pmed.1002396.
8. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732. https://doi.org/10.7326/M18-1136.
9. Mayor S. Pregabalin and gabapentin become controlled drugs to cut deaths from misuse. BMJ. 2018;363:k4364. https://doi.org/10.1136/bmj.k4364.
10. Gingras M-A, Lieu A, Papillon-Ferland L, Lee T, McDonald E. Retrospective cohort study of the prevalence of off-label gabapentinoid prescriptions in hospitalized medical patients. J Hosp Med. 2019;14(9):547-550. https://doi.org/10.12788/jhm.3203.
11. Parsons G. Guide to the management of gabapentinoid misuse. Prescriber. 2018;29(4):25-30. https://doi.org/10.1002/psb.1664.
© 2019 Society of Hospital Medicine
The Hospitalist Imperative: Standardizing Best Practice across Expanding Healthcare Networks
Rapid dissemination and adoption of evidence-based guidelines remains a challenge despite studies showing that key evidence-based care processes improve outcomes in sepsis and heart failure.1 Hospital medicine was virtually founded on the premise that hospitalists would be champions of delivering high-quality care. Hospitalists are now dealing with a new challenge—unprecedented growth of healthcare systems because of mergers and acquisitions. The year 2018 was a banner time for healthcare mergers and acquisitions, with a total of 1,182, up 14% from 2017.2 These are in response to the belief that healthcare systems may better navigate the mixed reimbursement models of fee-for-service and fee-for-value by achieving a larger patient base and economies of scale. Hospitalists must now achieve consistent, evidence-based standards of care across larger networks by educating their colleagues (often separated by large geographic areas) to manifest durable changes in their group practice with demonstrable improvement in patient outcomes and cost savings.
The study by Yurso et al. focused on implementing an education program, which included standardized learning through Clinical Performance and Value (CPV) vignettes with process measurement and feedback for sepsis and heart failure.3 Sepsis and heart failure have been a focus for treatment standardization because of the associated morbidity, mortality, and high cost of care. The study by Yurso et al. is a prospective quasi-controlled cohort of hospitalists in eight hospitals who were matched with comparator hospitalists in six nonparticipating hospitals across the AdventHealth system. Measurement and feedback were provided using CPV vignettes. Over two years, hospitalists who participated improved CPV scores by 8%, compliance with the utilization of the three-hour sepsis bundle from 46.0% to 57.5%, and orders of essential medical treatment elements for heart failure from 58.2% to 72.1%. In year one, the average length of stay (LOS) observed/expected (O/E) rates dropped by 8% for participating hospitalists compared with 2.5% in the comparator group. By year two, cost O/E rates improved slightly resulting in cost savings. The authors concluded that CPV case simulation-based measurement and feedback helped drive improvements in evidence-based care, which was associated with lower costs and shorter LOS.
While studies using traditional didactic CME struggle to demonstrate changes in practice leading to improved patient outcomes,4 the study by Yurso et al. gives a glimpse into how simulation can be used to help improve clinical performance and measure adherence to best practice. A remarkably similar study used CPV for simulated patients with serial performance measurement and feedback for heart failure and pneumonia. The study showed reduced practice variation between hospitalists at 11 hospitals across four states and decreased LOS and readmissions. However, the sole clinical outcome was no change in in-house mortality.5 Another study using CPV training in breast cancer treatment demonstrated increased adherence to evidence-based practice standards and decreased variation in care between providers across four states.6 Of note, this study did not include clinical outcomes. These studies collectively imply that simulation training with interactive learning, educational feedback, repetitive practice, and curriculum integration has shown modest success in creating practice change and improving adherence to best practice standards. However, they have minimal measures of patient outcomes and fairly simple analyses for cost savings. Because the education is computer-based and feedback can be performed remotely, it can be deployed across large and diverse growing healthcare systems. To really move the needle, future research in the field of simulation should identify optimal simulation methods and be designed with more rigor to include patient and cost outcomes.
At Intermountain Healthcare, hospitalist expansion occurred through a strategic realignment from the different geographic regions into the One Intermountain model. This model is built on the commitment that our patients will receive the same high-quality, high-value care wherever they walk through our doors. We have found four substantive changes have been particularly powerful in spurring a group practice mentality toward standardizing best practice. One, hospitalists are now aligned across the system under a single operational leadership structure that encourages combined efforts to share best practices and develop and deploy strategic initiatives around them. Two, hospitalists continue to build on a culture of quality and measure what matters to patients. While Intermountain Healthcare has a long history of using quality improvement to achieve better patient outcomes and lower costs,7 the new structure is allowing our group to test novel methods including redesigned education to see what actually improves adherence to best practice. Three, the group knows where the system’s reimbursement is coming from; Intermountain Healthcare has transitioned to a larger percentage of capitation,8 currently about 40%, with a strong commitment to partner with services geared to transition patients home quickly and keep them at home. Four, the organization has created a structure of accountability and reporting; an executive-sponsored systemwide operating model has been designed to cut through system barriers being identified by the frontline, allowing them to be rapidly surfaced and then solved at the executive level through daily huddles.9
Innovative educational programs such as the one described in the study by Yurso et al. that help the busy hospitalist achieve improved adherence to best practice are likely to be an important component leading to improved outcomes, but only after a group has been structured for success. As hospitalist groups continue to act as a single effector arm for high-value care, this will help meet the expectations of our patients and deliver on the promise of our field.
Disclosures: Dr. Srivastava is a physician founder of the I- PASS Patient Safety Institute. His employer, Intermountain Healthcare owns his equity in the I-PASS Patient Safety Institute. Dr. Srivastava is supported in part by the Children’s Hospital Association for his work as an executive council member of the Pediatric Research in Inpatient Settings (PRIS) network. Dr. Srivastava has received monetary awards, honorariums, and travel reimbursement from multiple academic and professional organizations for talks about pediatric hospitalist research networks and quality of care. All other authors have nothing to disclose. No funding was provided for this editorial.
Disclosures
The authors have no disclosures of financial conflicts of interest.
Funding
Dr. Walke was supported an award from the Health Resources and Services Administration Geriatric Workforce Enhancement Program to the University of Pennsylvania (U1QHP28720).
1. Seymour, CW, Geston F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. https://doi.org/10.1056/NEJMoa1703058.
2. Healthcare Finance. Lagasse J. Healthcare mergers and acquisitions had record year in 2018, up 14.4 percent.https://webcache.googleusercontent.com/search?q=cache:zoMrl9yoLokJ:https://www.healthcarefinancenews.com/news/healthcare-mergers-and-acquisitions-had-record-year-2018-144-percent+&cd=2&hl=en&ct=clnk&gl=us. Published January, 2019. Accessed April 26, 2019.
3. Yurso M, Box B, Burgon T, et al. Reducing unneeded clinical variation in sepsis and heart failure care to improve outcomes and reduce cost: a collaborative engagement with hospitalists in a multi-state system. J Hosp Med. 2019;14(9):542-546. https://doi.org/10.12788/jhm.3220.
4. Cervero RM, Gaines JK. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35(2):131-138. https://doi.org/10.1002/chp.21290.
5. Weems L, Strong J, Plummer D, et al. A quality collaboration in heart failure and pneumonia inpatient care at Novant Health: standardizing hospitalist practices to improve patient care and system performance. Jt Comm J Qual Patient Saf. 2019;45(3):199-206. https://doi.org/10.1016/j.jcjq.2018.09.005.
6. Peabody JW, Paculdo DR, Tamondong-Lachica D, et al. Improving clinical practice using a novel engagement approach; measurement, benchmarking and feedback; a longitudinal study. J Clin Med Res. 2016;8(9):633-640. https://doi.org/10.14740/jocmr2620w.
7. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358.
8. James BC, Poulsen GP. The case for capitation. Harv Bus Rev. 2016;94(7-8):102-111,134. PubMed
9. Harvard Business Review. Harrison M. How a U.S. Health Care System Uses 15-Minute Huddles to Keep 23 Hospitals Aligned. https://hbr.org/2018/11/how-a-u-s-health-care-system-uses-15-minute-huddles-to-keep-23-hospitals-aligned. Published November, 2019. Accessed May 16, 2019.
Rapid dissemination and adoption of evidence-based guidelines remains a challenge despite studies showing that key evidence-based care processes improve outcomes in sepsis and heart failure.1 Hospital medicine was virtually founded on the premise that hospitalists would be champions of delivering high-quality care. Hospitalists are now dealing with a new challenge—unprecedented growth of healthcare systems because of mergers and acquisitions. The year 2018 was a banner time for healthcare mergers and acquisitions, with a total of 1,182, up 14% from 2017.2 These are in response to the belief that healthcare systems may better navigate the mixed reimbursement models of fee-for-service and fee-for-value by achieving a larger patient base and economies of scale. Hospitalists must now achieve consistent, evidence-based standards of care across larger networks by educating their colleagues (often separated by large geographic areas) to manifest durable changes in their group practice with demonstrable improvement in patient outcomes and cost savings.
The study by Yurso et al. focused on implementing an education program, which included standardized learning through Clinical Performance and Value (CPV) vignettes with process measurement and feedback for sepsis and heart failure.3 Sepsis and heart failure have been a focus for treatment standardization because of the associated morbidity, mortality, and high cost of care. The study by Yurso et al. is a prospective quasi-controlled cohort of hospitalists in eight hospitals who were matched with comparator hospitalists in six nonparticipating hospitals across the AdventHealth system. Measurement and feedback were provided using CPV vignettes. Over two years, hospitalists who participated improved CPV scores by 8%, compliance with the utilization of the three-hour sepsis bundle from 46.0% to 57.5%, and orders of essential medical treatment elements for heart failure from 58.2% to 72.1%. In year one, the average length of stay (LOS) observed/expected (O/E) rates dropped by 8% for participating hospitalists compared with 2.5% in the comparator group. By year two, cost O/E rates improved slightly resulting in cost savings. The authors concluded that CPV case simulation-based measurement and feedback helped drive improvements in evidence-based care, which was associated with lower costs and shorter LOS.
While studies using traditional didactic CME struggle to demonstrate changes in practice leading to improved patient outcomes,4 the study by Yurso et al. gives a glimpse into how simulation can be used to help improve clinical performance and measure adherence to best practice. A remarkably similar study used CPV for simulated patients with serial performance measurement and feedback for heart failure and pneumonia. The study showed reduced practice variation between hospitalists at 11 hospitals across four states and decreased LOS and readmissions. However, the sole clinical outcome was no change in in-house mortality.5 Another study using CPV training in breast cancer treatment demonstrated increased adherence to evidence-based practice standards and decreased variation in care between providers across four states.6 Of note, this study did not include clinical outcomes. These studies collectively imply that simulation training with interactive learning, educational feedback, repetitive practice, and curriculum integration has shown modest success in creating practice change and improving adherence to best practice standards. However, they have minimal measures of patient outcomes and fairly simple analyses for cost savings. Because the education is computer-based and feedback can be performed remotely, it can be deployed across large and diverse growing healthcare systems. To really move the needle, future research in the field of simulation should identify optimal simulation methods and be designed with more rigor to include patient and cost outcomes.
At Intermountain Healthcare, hospitalist expansion occurred through a strategic realignment from the different geographic regions into the One Intermountain model. This model is built on the commitment that our patients will receive the same high-quality, high-value care wherever they walk through our doors. We have found four substantive changes have been particularly powerful in spurring a group practice mentality toward standardizing best practice. One, hospitalists are now aligned across the system under a single operational leadership structure that encourages combined efforts to share best practices and develop and deploy strategic initiatives around them. Two, hospitalists continue to build on a culture of quality and measure what matters to patients. While Intermountain Healthcare has a long history of using quality improvement to achieve better patient outcomes and lower costs,7 the new structure is allowing our group to test novel methods including redesigned education to see what actually improves adherence to best practice. Three, the group knows where the system’s reimbursement is coming from; Intermountain Healthcare has transitioned to a larger percentage of capitation,8 currently about 40%, with a strong commitment to partner with services geared to transition patients home quickly and keep them at home. Four, the organization has created a structure of accountability and reporting; an executive-sponsored systemwide operating model has been designed to cut through system barriers being identified by the frontline, allowing them to be rapidly surfaced and then solved at the executive level through daily huddles.9
Innovative educational programs such as the one described in the study by Yurso et al. that help the busy hospitalist achieve improved adherence to best practice are likely to be an important component leading to improved outcomes, but only after a group has been structured for success. As hospitalist groups continue to act as a single effector arm for high-value care, this will help meet the expectations of our patients and deliver on the promise of our field.
Disclosures: Dr. Srivastava is a physician founder of the I- PASS Patient Safety Institute. His employer, Intermountain Healthcare owns his equity in the I-PASS Patient Safety Institute. Dr. Srivastava is supported in part by the Children’s Hospital Association for his work as an executive council member of the Pediatric Research in Inpatient Settings (PRIS) network. Dr. Srivastava has received monetary awards, honorariums, and travel reimbursement from multiple academic and professional organizations for talks about pediatric hospitalist research networks and quality of care. All other authors have nothing to disclose. No funding was provided for this editorial.
Disclosures
The authors have no disclosures of financial conflicts of interest.
Funding
Dr. Walke was supported an award from the Health Resources and Services Administration Geriatric Workforce Enhancement Program to the University of Pennsylvania (U1QHP28720).
Rapid dissemination and adoption of evidence-based guidelines remains a challenge despite studies showing that key evidence-based care processes improve outcomes in sepsis and heart failure.1 Hospital medicine was virtually founded on the premise that hospitalists would be champions of delivering high-quality care. Hospitalists are now dealing with a new challenge—unprecedented growth of healthcare systems because of mergers and acquisitions. The year 2018 was a banner time for healthcare mergers and acquisitions, with a total of 1,182, up 14% from 2017.2 These are in response to the belief that healthcare systems may better navigate the mixed reimbursement models of fee-for-service and fee-for-value by achieving a larger patient base and economies of scale. Hospitalists must now achieve consistent, evidence-based standards of care across larger networks by educating their colleagues (often separated by large geographic areas) to manifest durable changes in their group practice with demonstrable improvement in patient outcomes and cost savings.
The study by Yurso et al. focused on implementing an education program, which included standardized learning through Clinical Performance and Value (CPV) vignettes with process measurement and feedback for sepsis and heart failure.3 Sepsis and heart failure have been a focus for treatment standardization because of the associated morbidity, mortality, and high cost of care. The study by Yurso et al. is a prospective quasi-controlled cohort of hospitalists in eight hospitals who were matched with comparator hospitalists in six nonparticipating hospitals across the AdventHealth system. Measurement and feedback were provided using CPV vignettes. Over two years, hospitalists who participated improved CPV scores by 8%, compliance with the utilization of the three-hour sepsis bundle from 46.0% to 57.5%, and orders of essential medical treatment elements for heart failure from 58.2% to 72.1%. In year one, the average length of stay (LOS) observed/expected (O/E) rates dropped by 8% for participating hospitalists compared with 2.5% in the comparator group. By year two, cost O/E rates improved slightly resulting in cost savings. The authors concluded that CPV case simulation-based measurement and feedback helped drive improvements in evidence-based care, which was associated with lower costs and shorter LOS.
While studies using traditional didactic CME struggle to demonstrate changes in practice leading to improved patient outcomes,4 the study by Yurso et al. gives a glimpse into how simulation can be used to help improve clinical performance and measure adherence to best practice. A remarkably similar study used CPV for simulated patients with serial performance measurement and feedback for heart failure and pneumonia. The study showed reduced practice variation between hospitalists at 11 hospitals across four states and decreased LOS and readmissions. However, the sole clinical outcome was no change in in-house mortality.5 Another study using CPV training in breast cancer treatment demonstrated increased adherence to evidence-based practice standards and decreased variation in care between providers across four states.6 Of note, this study did not include clinical outcomes. These studies collectively imply that simulation training with interactive learning, educational feedback, repetitive practice, and curriculum integration has shown modest success in creating practice change and improving adherence to best practice standards. However, they have minimal measures of patient outcomes and fairly simple analyses for cost savings. Because the education is computer-based and feedback can be performed remotely, it can be deployed across large and diverse growing healthcare systems. To really move the needle, future research in the field of simulation should identify optimal simulation methods and be designed with more rigor to include patient and cost outcomes.
At Intermountain Healthcare, hospitalist expansion occurred through a strategic realignment from the different geographic regions into the One Intermountain model. This model is built on the commitment that our patients will receive the same high-quality, high-value care wherever they walk through our doors. We have found four substantive changes have been particularly powerful in spurring a group practice mentality toward standardizing best practice. One, hospitalists are now aligned across the system under a single operational leadership structure that encourages combined efforts to share best practices and develop and deploy strategic initiatives around them. Two, hospitalists continue to build on a culture of quality and measure what matters to patients. While Intermountain Healthcare has a long history of using quality improvement to achieve better patient outcomes and lower costs,7 the new structure is allowing our group to test novel methods including redesigned education to see what actually improves adherence to best practice. Three, the group knows where the system’s reimbursement is coming from; Intermountain Healthcare has transitioned to a larger percentage of capitation,8 currently about 40%, with a strong commitment to partner with services geared to transition patients home quickly and keep them at home. Four, the organization has created a structure of accountability and reporting; an executive-sponsored systemwide operating model has been designed to cut through system barriers being identified by the frontline, allowing them to be rapidly surfaced and then solved at the executive level through daily huddles.9
Innovative educational programs such as the one described in the study by Yurso et al. that help the busy hospitalist achieve improved adherence to best practice are likely to be an important component leading to improved outcomes, but only after a group has been structured for success. As hospitalist groups continue to act as a single effector arm for high-value care, this will help meet the expectations of our patients and deliver on the promise of our field.
Disclosures: Dr. Srivastava is a physician founder of the I- PASS Patient Safety Institute. His employer, Intermountain Healthcare owns his equity in the I-PASS Patient Safety Institute. Dr. Srivastava is supported in part by the Children’s Hospital Association for his work as an executive council member of the Pediatric Research in Inpatient Settings (PRIS) network. Dr. Srivastava has received monetary awards, honorariums, and travel reimbursement from multiple academic and professional organizations for talks about pediatric hospitalist research networks and quality of care. All other authors have nothing to disclose. No funding was provided for this editorial.
Disclosures
The authors have no disclosures of financial conflicts of interest.
Funding
Dr. Walke was supported an award from the Health Resources and Services Administration Geriatric Workforce Enhancement Program to the University of Pennsylvania (U1QHP28720).
1. Seymour, CW, Geston F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. https://doi.org/10.1056/NEJMoa1703058.
2. Healthcare Finance. Lagasse J. Healthcare mergers and acquisitions had record year in 2018, up 14.4 percent.https://webcache.googleusercontent.com/search?q=cache:zoMrl9yoLokJ:https://www.healthcarefinancenews.com/news/healthcare-mergers-and-acquisitions-had-record-year-2018-144-percent+&cd=2&hl=en&ct=clnk&gl=us. Published January, 2019. Accessed April 26, 2019.
3. Yurso M, Box B, Burgon T, et al. Reducing unneeded clinical variation in sepsis and heart failure care to improve outcomes and reduce cost: a collaborative engagement with hospitalists in a multi-state system. J Hosp Med. 2019;14(9):542-546. https://doi.org/10.12788/jhm.3220.
4. Cervero RM, Gaines JK. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35(2):131-138. https://doi.org/10.1002/chp.21290.
5. Weems L, Strong J, Plummer D, et al. A quality collaboration in heart failure and pneumonia inpatient care at Novant Health: standardizing hospitalist practices to improve patient care and system performance. Jt Comm J Qual Patient Saf. 2019;45(3):199-206. https://doi.org/10.1016/j.jcjq.2018.09.005.
6. Peabody JW, Paculdo DR, Tamondong-Lachica D, et al. Improving clinical practice using a novel engagement approach; measurement, benchmarking and feedback; a longitudinal study. J Clin Med Res. 2016;8(9):633-640. https://doi.org/10.14740/jocmr2620w.
7. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358.
8. James BC, Poulsen GP. The case for capitation. Harv Bus Rev. 2016;94(7-8):102-111,134. PubMed
9. Harvard Business Review. Harrison M. How a U.S. Health Care System Uses 15-Minute Huddles to Keep 23 Hospitals Aligned. https://hbr.org/2018/11/how-a-u-s-health-care-system-uses-15-minute-huddles-to-keep-23-hospitals-aligned. Published November, 2019. Accessed May 16, 2019.
1. Seymour, CW, Geston F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. https://doi.org/10.1056/NEJMoa1703058.
2. Healthcare Finance. Lagasse J. Healthcare mergers and acquisitions had record year in 2018, up 14.4 percent.https://webcache.googleusercontent.com/search?q=cache:zoMrl9yoLokJ:https://www.healthcarefinancenews.com/news/healthcare-mergers-and-acquisitions-had-record-year-2018-144-percent+&cd=2&hl=en&ct=clnk&gl=us. Published January, 2019. Accessed April 26, 2019.
3. Yurso M, Box B, Burgon T, et al. Reducing unneeded clinical variation in sepsis and heart failure care to improve outcomes and reduce cost: a collaborative engagement with hospitalists in a multi-state system. J Hosp Med. 2019;14(9):542-546. https://doi.org/10.12788/jhm.3220.
4. Cervero RM, Gaines JK. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35(2):131-138. https://doi.org/10.1002/chp.21290.
5. Weems L, Strong J, Plummer D, et al. A quality collaboration in heart failure and pneumonia inpatient care at Novant Health: standardizing hospitalist practices to improve patient care and system performance. Jt Comm J Qual Patient Saf. 2019;45(3):199-206. https://doi.org/10.1016/j.jcjq.2018.09.005.
6. Peabody JW, Paculdo DR, Tamondong-Lachica D, et al. Improving clinical practice using a novel engagement approach; measurement, benchmarking and feedback; a longitudinal study. J Clin Med Res. 2016;8(9):633-640. https://doi.org/10.14740/jocmr2620w.
7. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358.
8. James BC, Poulsen GP. The case for capitation. Harv Bus Rev. 2016;94(7-8):102-111,134. PubMed
9. Harvard Business Review. Harrison M. How a U.S. Health Care System Uses 15-Minute Huddles to Keep 23 Hospitals Aligned. https://hbr.org/2018/11/how-a-u-s-health-care-system-uses-15-minute-huddles-to-keep-23-hospitals-aligned. Published November, 2019. Accessed May 16, 2019.
© 2019 Society of Hospital Medicine
Why Every Hospital Should (Must) Have an ACE Unit by 2040
Like the rest of the world, the United States is experiencing an aging boom. The number of adults aged 65 years or older is expected to grow from 49 million in 2016 to 82 million in 2040, indicating an increase of 67%. Even more impressively, the population of individuals aged 85 years or older is expected to increase by 129% to 14.6 million within this same time period.1 Considering that one in five Medicare Fee for Service beneficiaries are hospitalized at least once a year,2 hospitals can expect the number of adults over the age of 65 requiring acute care will substantially increase over the next 20 years. These demographic changes have important implications for the overall healthcare costs in the US. Of persons with the highest annual healthcare expenditures, 40% are 65 years of age or older. 3 Thus, optimizing the care of hospitalized older adults will remain a critical component in the management of healthcare costs in the next 20 years.
As such, the Acute Care for the Elderly (ACE) unit, an interprofessional model of care that has been shown to provide high-quality care to hospitalized older adults without increasing costs,4 will become an increasingly important component of acute care as the older adult population grows. In this edition of the Journal of Hospital Medicine, Brennan et al.5 describe a quality improvement initiative in which an interprofessional team that included a geriatric clinician, nurses, pharmacist, and chaplain developed a daily plan of care for ACE unit patients aged 70 years or older. The daily care plan, which focused on symptom management and advance care planning, was the nidus for collaboration between the hospital medicine attending and geriatrics team. Their results demonstrate that ACE unit patients had lower hospital costs and shorter lengths of stay (LOS) as compared with age-matched, usual-care patients despite having higher comorbidity scores. In addition, the greatest benefits were seen among persons in the highest quartile of the comorbidity score.
These results add to the small but consistent body of literature that demonstrates quality and cost benefits to the ACE unit care. Importantly, however, in contrast with the prior ACE unit studies in which persons with moderate risk were the ones to demonstrate the greatest benefits, Brennan et al.5 were able to demonstrate the greatest effect for the highest-need, highest-cost population. Reasons for this impressive effect may be attributed to this intervention’s specific emphasis on symptom management and estimated life expectancy. In an era when Medicare and other payers are looking to increase the value proposition in population health-based approaches by reducing high costs while preserving high quality, these findings represent an important example that merits a broader dissemination.
Of course, ACE units are not the only hospital-based programs that have shown to improve outcomes for older adults. The Hospital Elder Life Program (HELP) is an evidence-based delirium prevention intervention that has been shown to not only prevent delirium but also prevent cognitive and functional decline while decreasing hospital LOS, hospital falls, and sitter use.6 Moreover, similar to ACE units, HELP has been shown to reduce inhospital patient costs. Geriatrics surgery comanagement programs are another hospital-based intervention that has shown to improve outcomes for older surgical patients. Reductions in LOS, improved mobility, and higher discharge to home have been demonstrated in patients who have undergone spinal surgery.7 Decreased LOS and lower hospital costs have also been demonstrated among patients with hip fracture undergoing repair.8 Programs such as ACE units, HELP, and geriatric surgery comanagement are well aligned with the growing emphasis on value-based healthcare and will be especially needed by hospitals that strive to be high-reliability organizations as the number of adults aged 65 and older continues to grow. To date, few studies have explored the potential synergistic effects (or redundancies) of these programs and how to maximize the impact of these evidence-based interventions across healthcare systems with multiple hospitals that care for older adults from various socioeconomic and cultural backgrounds.
Looking toward the future, the implementation of ACE units and other innovative geriatric programs will equip hospitals to develop into Age-Friendly Health Systems (AFHS). AFHS is an initiative being led by the Institute for Healthcare Improvement, The John A. Hartford Foundation, the American Hospital Association, and the Catholic Health Association of the United States in partnership with several other leading healthcare organizations to provide high-value care to every older adult.9 AFHS provide care focused on the 4M framework—What Matters, Medications, Mobility, and Mentation. The goal is for 20% of hospitals and medical practices to join the AFHS initiative by 2020; to date, over 70 organizations nationwide have done so. Clearly, to reach this goal, and beyond, a greater collaboration between aging-focused interprofessional teams including geriatricians and hospitalists will be essential.
Given the aging demographic and rising healthcare costs, Brennan et al.’s work5 suggests that each hospital should have an ACE unit by 2040. Consistently, hospital care delivery has appropriately developed in response to the needs of the patient population served. Intensive care units (ICUs), dialysis units, and emergency rooms are just a few innovations that were adopted by hospitals to provide specialty care to individuals with complex acute illnesses. While technology within the ICU certainly plays a role in the care delivered in that setting, it could be argued that what makes the ICUs most effective is the cohorting of interprofessional expertise. Since the implementation of ICUs, the survival rate for critically ill patients has substantially improved and additional specialty units with an interprofessional team model, eg, cardiac care units, dialysis units, emergency rooms, etc., have followed suit. Specialty units have become a part of the fabric of acute care, so much so that it would be hard to imagine a modern hospital without an ICU, dialysis unit, or emergency room. The same should be true for ACE units. Even hospitals without geriatricians on site can use teleconferencing to successfully implement an ACE unit.10 We owe it to our older patients to transform our institutions into AFHS; implementing models of care proven to improve outcomes, such as the ACE unit, is one of the critical first steps.
Disclosures
The authors have no disclosures or financial conflicts of interest.
Funding
Dr. Walke was supported by an award from the Health Resources and Services Administration Geriatric Workforce Enhancement Program to the University of Pennsylvania (U1QHP28720
1. Administration for Community Living. Profile of older adults: 2017. https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OlderAmericansProfile.pdf Accessed April 22, 2019.
2. Gorina Y, Pratt LA, Kramarow EA, Elgaddal N. Hospitalization, readmission, and death experience of noninstitutionalized Medicare fee-for-service beneficiaries aged 65 and over. Hyattsville, MD: National Center for Health Statistics. 2015. PubMed
3. Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, Household Component 2015. https://meps.ahrq.gov/data_files/publications/st506/stat506.shtml Accessed April 1, 2019.
4. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older adults. N Engl J Med. 1995;332(20):1338-1344. https://doi.org/10.1056/NEJM199505183322006.
5. Brennan M, Knee A, Leahy E, et al. An acute care for elders QI program for complex, high cost patients yields savings for the system. J Hosp Med. 2019;14(9):527-533. https://doi.org/10.12788/jhm.3198.
6. Hospital Elder Life Program. https://www.hospitalelderlifeprogram.org/about/results/ Accessed May 6, 2019.
7. Adogwa O, Elsamadicy AA, Vuong VD, et al. Geriatric comanagement reduces perioperative complications and shortens duration of hospital stay after lumbar spine surgery: a prospective single-institution experience. J Neurosurg Spine. 2017;27(6):670-675. https://doi.org/10.3171/2017.5.SPINE17199.
8. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fracutues: a retrospective, controlled, cohort study. Geriatr Orthop Surg & Rehab.2013;4(1):10-15. https://doi.org/10.1177/2151458513495238.
9. Institute for Healthcare Improvement. http://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx. Accessed May 6, 2019.
10. Malone ML, Vollbrecht M, Stephenson J, Burke L, Pagel P, Goodwin JS. Acute Care for Elders (ACE) tracker and e-geriatrician: methods to disseminate ACE concepts to hospitals with no geriatricians on staff. J Am Geriatr Soc. 2010;58(1):161-167. https://doi.org/10.1111/j.1532-5415.2009.02624.x.
Like the rest of the world, the United States is experiencing an aging boom. The number of adults aged 65 years or older is expected to grow from 49 million in 2016 to 82 million in 2040, indicating an increase of 67%. Even more impressively, the population of individuals aged 85 years or older is expected to increase by 129% to 14.6 million within this same time period.1 Considering that one in five Medicare Fee for Service beneficiaries are hospitalized at least once a year,2 hospitals can expect the number of adults over the age of 65 requiring acute care will substantially increase over the next 20 years. These demographic changes have important implications for the overall healthcare costs in the US. Of persons with the highest annual healthcare expenditures, 40% are 65 years of age or older. 3 Thus, optimizing the care of hospitalized older adults will remain a critical component in the management of healthcare costs in the next 20 years.
As such, the Acute Care for the Elderly (ACE) unit, an interprofessional model of care that has been shown to provide high-quality care to hospitalized older adults without increasing costs,4 will become an increasingly important component of acute care as the older adult population grows. In this edition of the Journal of Hospital Medicine, Brennan et al.5 describe a quality improvement initiative in which an interprofessional team that included a geriatric clinician, nurses, pharmacist, and chaplain developed a daily plan of care for ACE unit patients aged 70 years or older. The daily care plan, which focused on symptom management and advance care planning, was the nidus for collaboration between the hospital medicine attending and geriatrics team. Their results demonstrate that ACE unit patients had lower hospital costs and shorter lengths of stay (LOS) as compared with age-matched, usual-care patients despite having higher comorbidity scores. In addition, the greatest benefits were seen among persons in the highest quartile of the comorbidity score.
These results add to the small but consistent body of literature that demonstrates quality and cost benefits to the ACE unit care. Importantly, however, in contrast with the prior ACE unit studies in which persons with moderate risk were the ones to demonstrate the greatest benefits, Brennan et al.5 were able to demonstrate the greatest effect for the highest-need, highest-cost population. Reasons for this impressive effect may be attributed to this intervention’s specific emphasis on symptom management and estimated life expectancy. In an era when Medicare and other payers are looking to increase the value proposition in population health-based approaches by reducing high costs while preserving high quality, these findings represent an important example that merits a broader dissemination.
Of course, ACE units are not the only hospital-based programs that have shown to improve outcomes for older adults. The Hospital Elder Life Program (HELP) is an evidence-based delirium prevention intervention that has been shown to not only prevent delirium but also prevent cognitive and functional decline while decreasing hospital LOS, hospital falls, and sitter use.6 Moreover, similar to ACE units, HELP has been shown to reduce inhospital patient costs. Geriatrics surgery comanagement programs are another hospital-based intervention that has shown to improve outcomes for older surgical patients. Reductions in LOS, improved mobility, and higher discharge to home have been demonstrated in patients who have undergone spinal surgery.7 Decreased LOS and lower hospital costs have also been demonstrated among patients with hip fracture undergoing repair.8 Programs such as ACE units, HELP, and geriatric surgery comanagement are well aligned with the growing emphasis on value-based healthcare and will be especially needed by hospitals that strive to be high-reliability organizations as the number of adults aged 65 and older continues to grow. To date, few studies have explored the potential synergistic effects (or redundancies) of these programs and how to maximize the impact of these evidence-based interventions across healthcare systems with multiple hospitals that care for older adults from various socioeconomic and cultural backgrounds.
Looking toward the future, the implementation of ACE units and other innovative geriatric programs will equip hospitals to develop into Age-Friendly Health Systems (AFHS). AFHS is an initiative being led by the Institute for Healthcare Improvement, The John A. Hartford Foundation, the American Hospital Association, and the Catholic Health Association of the United States in partnership with several other leading healthcare organizations to provide high-value care to every older adult.9 AFHS provide care focused on the 4M framework—What Matters, Medications, Mobility, and Mentation. The goal is for 20% of hospitals and medical practices to join the AFHS initiative by 2020; to date, over 70 organizations nationwide have done so. Clearly, to reach this goal, and beyond, a greater collaboration between aging-focused interprofessional teams including geriatricians and hospitalists will be essential.
Given the aging demographic and rising healthcare costs, Brennan et al.’s work5 suggests that each hospital should have an ACE unit by 2040. Consistently, hospital care delivery has appropriately developed in response to the needs of the patient population served. Intensive care units (ICUs), dialysis units, and emergency rooms are just a few innovations that were adopted by hospitals to provide specialty care to individuals with complex acute illnesses. While technology within the ICU certainly plays a role in the care delivered in that setting, it could be argued that what makes the ICUs most effective is the cohorting of interprofessional expertise. Since the implementation of ICUs, the survival rate for critically ill patients has substantially improved and additional specialty units with an interprofessional team model, eg, cardiac care units, dialysis units, emergency rooms, etc., have followed suit. Specialty units have become a part of the fabric of acute care, so much so that it would be hard to imagine a modern hospital without an ICU, dialysis unit, or emergency room. The same should be true for ACE units. Even hospitals without geriatricians on site can use teleconferencing to successfully implement an ACE unit.10 We owe it to our older patients to transform our institutions into AFHS; implementing models of care proven to improve outcomes, such as the ACE unit, is one of the critical first steps.
Disclosures
The authors have no disclosures or financial conflicts of interest.
Funding
Dr. Walke was supported by an award from the Health Resources and Services Administration Geriatric Workforce Enhancement Program to the University of Pennsylvania (U1QHP28720
Like the rest of the world, the United States is experiencing an aging boom. The number of adults aged 65 years or older is expected to grow from 49 million in 2016 to 82 million in 2040, indicating an increase of 67%. Even more impressively, the population of individuals aged 85 years or older is expected to increase by 129% to 14.6 million within this same time period.1 Considering that one in five Medicare Fee for Service beneficiaries are hospitalized at least once a year,2 hospitals can expect the number of adults over the age of 65 requiring acute care will substantially increase over the next 20 years. These demographic changes have important implications for the overall healthcare costs in the US. Of persons with the highest annual healthcare expenditures, 40% are 65 years of age or older. 3 Thus, optimizing the care of hospitalized older adults will remain a critical component in the management of healthcare costs in the next 20 years.
As such, the Acute Care for the Elderly (ACE) unit, an interprofessional model of care that has been shown to provide high-quality care to hospitalized older adults without increasing costs,4 will become an increasingly important component of acute care as the older adult population grows. In this edition of the Journal of Hospital Medicine, Brennan et al.5 describe a quality improvement initiative in which an interprofessional team that included a geriatric clinician, nurses, pharmacist, and chaplain developed a daily plan of care for ACE unit patients aged 70 years or older. The daily care plan, which focused on symptom management and advance care planning, was the nidus for collaboration between the hospital medicine attending and geriatrics team. Their results demonstrate that ACE unit patients had lower hospital costs and shorter lengths of stay (LOS) as compared with age-matched, usual-care patients despite having higher comorbidity scores. In addition, the greatest benefits were seen among persons in the highest quartile of the comorbidity score.
These results add to the small but consistent body of literature that demonstrates quality and cost benefits to the ACE unit care. Importantly, however, in contrast with the prior ACE unit studies in which persons with moderate risk were the ones to demonstrate the greatest benefits, Brennan et al.5 were able to demonstrate the greatest effect for the highest-need, highest-cost population. Reasons for this impressive effect may be attributed to this intervention’s specific emphasis on symptom management and estimated life expectancy. In an era when Medicare and other payers are looking to increase the value proposition in population health-based approaches by reducing high costs while preserving high quality, these findings represent an important example that merits a broader dissemination.
Of course, ACE units are not the only hospital-based programs that have shown to improve outcomes for older adults. The Hospital Elder Life Program (HELP) is an evidence-based delirium prevention intervention that has been shown to not only prevent delirium but also prevent cognitive and functional decline while decreasing hospital LOS, hospital falls, and sitter use.6 Moreover, similar to ACE units, HELP has been shown to reduce inhospital patient costs. Geriatrics surgery comanagement programs are another hospital-based intervention that has shown to improve outcomes for older surgical patients. Reductions in LOS, improved mobility, and higher discharge to home have been demonstrated in patients who have undergone spinal surgery.7 Decreased LOS and lower hospital costs have also been demonstrated among patients with hip fracture undergoing repair.8 Programs such as ACE units, HELP, and geriatric surgery comanagement are well aligned with the growing emphasis on value-based healthcare and will be especially needed by hospitals that strive to be high-reliability organizations as the number of adults aged 65 and older continues to grow. To date, few studies have explored the potential synergistic effects (or redundancies) of these programs and how to maximize the impact of these evidence-based interventions across healthcare systems with multiple hospitals that care for older adults from various socioeconomic and cultural backgrounds.
Looking toward the future, the implementation of ACE units and other innovative geriatric programs will equip hospitals to develop into Age-Friendly Health Systems (AFHS). AFHS is an initiative being led by the Institute for Healthcare Improvement, The John A. Hartford Foundation, the American Hospital Association, and the Catholic Health Association of the United States in partnership with several other leading healthcare organizations to provide high-value care to every older adult.9 AFHS provide care focused on the 4M framework—What Matters, Medications, Mobility, and Mentation. The goal is for 20% of hospitals and medical practices to join the AFHS initiative by 2020; to date, over 70 organizations nationwide have done so. Clearly, to reach this goal, and beyond, a greater collaboration between aging-focused interprofessional teams including geriatricians and hospitalists will be essential.
Given the aging demographic and rising healthcare costs, Brennan et al.’s work5 suggests that each hospital should have an ACE unit by 2040. Consistently, hospital care delivery has appropriately developed in response to the needs of the patient population served. Intensive care units (ICUs), dialysis units, and emergency rooms are just a few innovations that were adopted by hospitals to provide specialty care to individuals with complex acute illnesses. While technology within the ICU certainly plays a role in the care delivered in that setting, it could be argued that what makes the ICUs most effective is the cohorting of interprofessional expertise. Since the implementation of ICUs, the survival rate for critically ill patients has substantially improved and additional specialty units with an interprofessional team model, eg, cardiac care units, dialysis units, emergency rooms, etc., have followed suit. Specialty units have become a part of the fabric of acute care, so much so that it would be hard to imagine a modern hospital without an ICU, dialysis unit, or emergency room. The same should be true for ACE units. Even hospitals without geriatricians on site can use teleconferencing to successfully implement an ACE unit.10 We owe it to our older patients to transform our institutions into AFHS; implementing models of care proven to improve outcomes, such as the ACE unit, is one of the critical first steps.
Disclosures
The authors have no disclosures or financial conflicts of interest.
Funding
Dr. Walke was supported by an award from the Health Resources and Services Administration Geriatric Workforce Enhancement Program to the University of Pennsylvania (U1QHP28720
1. Administration for Community Living. Profile of older adults: 2017. https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OlderAmericansProfile.pdf Accessed April 22, 2019.
2. Gorina Y, Pratt LA, Kramarow EA, Elgaddal N. Hospitalization, readmission, and death experience of noninstitutionalized Medicare fee-for-service beneficiaries aged 65 and over. Hyattsville, MD: National Center for Health Statistics. 2015. PubMed
3. Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, Household Component 2015. https://meps.ahrq.gov/data_files/publications/st506/stat506.shtml Accessed April 1, 2019.
4. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older adults. N Engl J Med. 1995;332(20):1338-1344. https://doi.org/10.1056/NEJM199505183322006.
5. Brennan M, Knee A, Leahy E, et al. An acute care for elders QI program for complex, high cost patients yields savings for the system. J Hosp Med. 2019;14(9):527-533. https://doi.org/10.12788/jhm.3198.
6. Hospital Elder Life Program. https://www.hospitalelderlifeprogram.org/about/results/ Accessed May 6, 2019.
7. Adogwa O, Elsamadicy AA, Vuong VD, et al. Geriatric comanagement reduces perioperative complications and shortens duration of hospital stay after lumbar spine surgery: a prospective single-institution experience. J Neurosurg Spine. 2017;27(6):670-675. https://doi.org/10.3171/2017.5.SPINE17199.
8. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fracutues: a retrospective, controlled, cohort study. Geriatr Orthop Surg & Rehab.2013;4(1):10-15. https://doi.org/10.1177/2151458513495238.
9. Institute for Healthcare Improvement. http://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx. Accessed May 6, 2019.
10. Malone ML, Vollbrecht M, Stephenson J, Burke L, Pagel P, Goodwin JS. Acute Care for Elders (ACE) tracker and e-geriatrician: methods to disseminate ACE concepts to hospitals with no geriatricians on staff. J Am Geriatr Soc. 2010;58(1):161-167. https://doi.org/10.1111/j.1532-5415.2009.02624.x.
1. Administration for Community Living. Profile of older adults: 2017. https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OlderAmericansProfile.pdf Accessed April 22, 2019.
2. Gorina Y, Pratt LA, Kramarow EA, Elgaddal N. Hospitalization, readmission, and death experience of noninstitutionalized Medicare fee-for-service beneficiaries aged 65 and over. Hyattsville, MD: National Center for Health Statistics. 2015. PubMed
3. Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, Household Component 2015. https://meps.ahrq.gov/data_files/publications/st506/stat506.shtml Accessed April 1, 2019.
4. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older adults. N Engl J Med. 1995;332(20):1338-1344. https://doi.org/10.1056/NEJM199505183322006.
5. Brennan M, Knee A, Leahy E, et al. An acute care for elders QI program for complex, high cost patients yields savings for the system. J Hosp Med. 2019;14(9):527-533. https://doi.org/10.12788/jhm.3198.
6. Hospital Elder Life Program. https://www.hospitalelderlifeprogram.org/about/results/ Accessed May 6, 2019.
7. Adogwa O, Elsamadicy AA, Vuong VD, et al. Geriatric comanagement reduces perioperative complications and shortens duration of hospital stay after lumbar spine surgery: a prospective single-institution experience. J Neurosurg Spine. 2017;27(6):670-675. https://doi.org/10.3171/2017.5.SPINE17199.
8. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fracutues: a retrospective, controlled, cohort study. Geriatr Orthop Surg & Rehab.2013;4(1):10-15. https://doi.org/10.1177/2151458513495238.
9. Institute for Healthcare Improvement. http://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx. Accessed May 6, 2019.
10. Malone ML, Vollbrecht M, Stephenson J, Burke L, Pagel P, Goodwin JS. Acute Care for Elders (ACE) tracker and e-geriatrician: methods to disseminate ACE concepts to hospitals with no geriatricians on staff. J Am Geriatr Soc. 2010;58(1):161-167. https://doi.org/10.1111/j.1532-5415.2009.02624.x.
© 2019 Society of Hospital Medicine
Patient Perspective is Critical in Developing Interventions for Frequently Admitted Patients
In the context of rapidly rising healthcare costs and increasing disparities in health outcomes in the United States, there has been increasing interest in identifying and addressing the needs of our country’s most frequently admitted patients. These patients account for a disproportionate percentage of healthcare expenditures1-3; they also represent a vulnerable and high-risk population. Finding solutions to address the needs of these patients is important for the patients themselves and for the systems in which they receive care. The last 10-15 years have seen a proliferation of programs working to address the needs and contain the costs of frequently admitted patients,2,4-6 as well as increased interest in understanding the risk factors and drivers that lead to high utilization.
In this edition of the Journal of Hospital Medicine, O’Leary et al. report on their study of patients enrolled in the CHAMP program at Northwestern University, in which the authors elicit patients’ perceptions of factors contributing to the onset and continuation of high hospital use.7 The authors identify several themes, including the important role of psychological, social, and economic factors in course fluctuation, the perception of acute illness as uncontrollable and unpredictable, and a strong desire to avoid hospitalization. As a group, the themes suggest multiple strategies that may be of use in developing individualized plans for patients.
Several of the most commonly cited risk factors for high utilization—including mental health issues, housing insecurity or homelessness, and substance use2,3,8,9—did not emerge as themes identified by patients in this study as contributing to high hospital utilization. Although identified themes such as social support and psychological stress could certainly be related to these underlying risk factors, the risk factors themselves did not emerge. This is particularly notable in a population whose utilization is in line with other studies (participants had at least two unplanned 30-day inpatient readmissions within 12 months, and one readmission in the last six months, a referral, or at least three observation visits). In contrast to prior qualitative work with complex, high-needs patients,10 patients in this study did not identify difficult (or positive) relationships with care provider teams, or a history of early life trauma, as factors related to current utilization.
These findings raise several important questions. To what extent are frequently hospitalized patient populations comparable with each other? This is both a question about how populations are defined and a question about the inherent variability between populations (including geographic, social, socioeconomic, and other factors). It is not evident from the demographic information provided whether this population is fundamentally different from others that have been studied, or whether risk factors such as mental health issues, housing insecurity, substance abuse, and trauma history are present, but are just not identified by patients here as proximal contributors to their utilization. In either case, the findings raise important questions about the development of effective interventions for these patients. The discrepancies also highlight the utility of ascertaining and reporting the prevalence of these risk factors among study populations, ideally both among patients who opt in and those who opt out. Although obtaining this information adds an additional layer of complexity to data collection, this history, along with extended demographic data, would significantly improve our ability to assess the comparability of populations across studies. It would also help us understand whether perspectives of any specific groups of patients are not represented, due to frequent opting out of the study.
The fact that commonly identified risk factors for high utilization are not identified by patients in this study as contributing to their high hospital use highlights the importance of (1) including the patient perspective as an integral part of care plan and intervention development and (2) continuing local work aimed at understanding the risk factors and drivers of high utilization in specific populations. Many programs, including CHAMP at Northwestern and our own hospitalist-run program at Penn Medicine, work closely with patients to develop individualized care plans that aim to address the underlying drivers of high utilization. In our experience, a multidisciplinary committee reviewing patient cases has identified mental health conditions as likely drivers of frequent admissions in over 95% of program patients. In line with the findings here, however, patients themselves often do not see mental health as a significant contributor. If patients do not see factors such as mental health as important, this has significant implications for the development of interventions around these factors as part of a solution to high hospital use.
Patients are unlikely to respond to interventions targeting problems that they themselves do not identify as important. This is not to say that drivers such as mental health, housing instability, substance abuse, and behaviors rooted in childhood trauma cannot be addressed if they are not identified by a patient as problems. Rather, interventions must be sensitive to and developed within the context of the patient’s own perceptions and priorities. For any program aimed at addressing the underlying drivers of high utilization, therefore, it is critical to elicit individual patient perspectives and to incorporate them in the development of interventions tailored to a specific patient’s needs. This process not only informs the creation of an individualized care plan but also promotes engagement and builds trust.
In prior work,6 O’Leary et al. have joined others throughout the field in calling for standardized definitions of “high utilizers”; this is critical for our ability to compare study results across programs. However, standardizing definitions is just the first step. Individual site studies such as this are needed to help us understand which themes are universal, versus those that are population- and site-specific. They are also important for individual institutions in targeting, developing, and refining local interventions. As a whole, the results will help guide the development of best practices within the field and allow providers to better understand the needs of specific populations. This work is essential to our ability as providers, hospitals, and systems to develop effective interventions for individual patients in this heterogeneous, vulnerable, and high-risk population.
Disclosures
Dr. Knox and Dr. Greysen have nothing to disclose.
1. Stanton MW, Rutherford MK. The high concentration of U.S. health care expenditures. Research in Action Issue 19. 2005. Rockville, MD: Agency for Healthcare Research and Quality.
2. Center for Health Care Strategies (CHCS). “Super-utilizer summit: common themes from innovative complex care management programs.” CHCS. 2013.
3. Jiang H, Weiss A, Barrett M, Sheng M. Characteristics of hospital stays for super-utilizers by payer, 2012: Statistical Brief #190. PubMed
4. Bodenheimer T. Strategies to reduce costs and improve care for high-utilizing medicaid patients: reflections on pioneering programs. CHCS. 2013.
5. Hong C , Siegel A, Ferris T. Caring for high-need, high-cost patients: what makes for a successful care management program? New York (NY): Commonwealth Fund. 2014;19(1):1-19. PubMed
6. Goodwin A, Henschen BL, O’Dwyer LC, Nichols N, O’Leary KJ. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853-859. https://doi.org/10.12788/jhm.3090.
7. O’Leary K, Chapman M, Shandu F et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175.
8. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff. 2015;34(8):1312-1319. https://doi.org/10.1377/hlthaff.2014.1186.
9. Rinehart DJ, Oronce C, Durfee MJ, et al. Identifying subgroups of adult superutilizers in an urban safety-net system using latent class analysis: implications for clinical practice. Med Care. 2018;56(1):e1-e9. https://doi.org/10.1097/MLR.0000000000000628.
10. Mautner DB, Pang H, Brenner JC, et al. Generating hypotheses about care needs of high utilizers: lessons from patient interviews. Popul Health Manag. 2013;16(1):S26-S33. https://doi.org/10.1089/pop.2013.0033.
In the context of rapidly rising healthcare costs and increasing disparities in health outcomes in the United States, there has been increasing interest in identifying and addressing the needs of our country’s most frequently admitted patients. These patients account for a disproportionate percentage of healthcare expenditures1-3; they also represent a vulnerable and high-risk population. Finding solutions to address the needs of these patients is important for the patients themselves and for the systems in which they receive care. The last 10-15 years have seen a proliferation of programs working to address the needs and contain the costs of frequently admitted patients,2,4-6 as well as increased interest in understanding the risk factors and drivers that lead to high utilization.
In this edition of the Journal of Hospital Medicine, O’Leary et al. report on their study of patients enrolled in the CHAMP program at Northwestern University, in which the authors elicit patients’ perceptions of factors contributing to the onset and continuation of high hospital use.7 The authors identify several themes, including the important role of psychological, social, and economic factors in course fluctuation, the perception of acute illness as uncontrollable and unpredictable, and a strong desire to avoid hospitalization. As a group, the themes suggest multiple strategies that may be of use in developing individualized plans for patients.
Several of the most commonly cited risk factors for high utilization—including mental health issues, housing insecurity or homelessness, and substance use2,3,8,9—did not emerge as themes identified by patients in this study as contributing to high hospital utilization. Although identified themes such as social support and psychological stress could certainly be related to these underlying risk factors, the risk factors themselves did not emerge. This is particularly notable in a population whose utilization is in line with other studies (participants had at least two unplanned 30-day inpatient readmissions within 12 months, and one readmission in the last six months, a referral, or at least three observation visits). In contrast to prior qualitative work with complex, high-needs patients,10 patients in this study did not identify difficult (or positive) relationships with care provider teams, or a history of early life trauma, as factors related to current utilization.
These findings raise several important questions. To what extent are frequently hospitalized patient populations comparable with each other? This is both a question about how populations are defined and a question about the inherent variability between populations (including geographic, social, socioeconomic, and other factors). It is not evident from the demographic information provided whether this population is fundamentally different from others that have been studied, or whether risk factors such as mental health issues, housing insecurity, substance abuse, and trauma history are present, but are just not identified by patients here as proximal contributors to their utilization. In either case, the findings raise important questions about the development of effective interventions for these patients. The discrepancies also highlight the utility of ascertaining and reporting the prevalence of these risk factors among study populations, ideally both among patients who opt in and those who opt out. Although obtaining this information adds an additional layer of complexity to data collection, this history, along with extended demographic data, would significantly improve our ability to assess the comparability of populations across studies. It would also help us understand whether perspectives of any specific groups of patients are not represented, due to frequent opting out of the study.
The fact that commonly identified risk factors for high utilization are not identified by patients in this study as contributing to their high hospital use highlights the importance of (1) including the patient perspective as an integral part of care plan and intervention development and (2) continuing local work aimed at understanding the risk factors and drivers of high utilization in specific populations. Many programs, including CHAMP at Northwestern and our own hospitalist-run program at Penn Medicine, work closely with patients to develop individualized care plans that aim to address the underlying drivers of high utilization. In our experience, a multidisciplinary committee reviewing patient cases has identified mental health conditions as likely drivers of frequent admissions in over 95% of program patients. In line with the findings here, however, patients themselves often do not see mental health as a significant contributor. If patients do not see factors such as mental health as important, this has significant implications for the development of interventions around these factors as part of a solution to high hospital use.
Patients are unlikely to respond to interventions targeting problems that they themselves do not identify as important. This is not to say that drivers such as mental health, housing instability, substance abuse, and behaviors rooted in childhood trauma cannot be addressed if they are not identified by a patient as problems. Rather, interventions must be sensitive to and developed within the context of the patient’s own perceptions and priorities. For any program aimed at addressing the underlying drivers of high utilization, therefore, it is critical to elicit individual patient perspectives and to incorporate them in the development of interventions tailored to a specific patient’s needs. This process not only informs the creation of an individualized care plan but also promotes engagement and builds trust.
In prior work,6 O’Leary et al. have joined others throughout the field in calling for standardized definitions of “high utilizers”; this is critical for our ability to compare study results across programs. However, standardizing definitions is just the first step. Individual site studies such as this are needed to help us understand which themes are universal, versus those that are population- and site-specific. They are also important for individual institutions in targeting, developing, and refining local interventions. As a whole, the results will help guide the development of best practices within the field and allow providers to better understand the needs of specific populations. This work is essential to our ability as providers, hospitals, and systems to develop effective interventions for individual patients in this heterogeneous, vulnerable, and high-risk population.
Disclosures
Dr. Knox and Dr. Greysen have nothing to disclose.
In the context of rapidly rising healthcare costs and increasing disparities in health outcomes in the United States, there has been increasing interest in identifying and addressing the needs of our country’s most frequently admitted patients. These patients account for a disproportionate percentage of healthcare expenditures1-3; they also represent a vulnerable and high-risk population. Finding solutions to address the needs of these patients is important for the patients themselves and for the systems in which they receive care. The last 10-15 years have seen a proliferation of programs working to address the needs and contain the costs of frequently admitted patients,2,4-6 as well as increased interest in understanding the risk factors and drivers that lead to high utilization.
In this edition of the Journal of Hospital Medicine, O’Leary et al. report on their study of patients enrolled in the CHAMP program at Northwestern University, in which the authors elicit patients’ perceptions of factors contributing to the onset and continuation of high hospital use.7 The authors identify several themes, including the important role of psychological, social, and economic factors in course fluctuation, the perception of acute illness as uncontrollable and unpredictable, and a strong desire to avoid hospitalization. As a group, the themes suggest multiple strategies that may be of use in developing individualized plans for patients.
Several of the most commonly cited risk factors for high utilization—including mental health issues, housing insecurity or homelessness, and substance use2,3,8,9—did not emerge as themes identified by patients in this study as contributing to high hospital utilization. Although identified themes such as social support and psychological stress could certainly be related to these underlying risk factors, the risk factors themselves did not emerge. This is particularly notable in a population whose utilization is in line with other studies (participants had at least two unplanned 30-day inpatient readmissions within 12 months, and one readmission in the last six months, a referral, or at least three observation visits). In contrast to prior qualitative work with complex, high-needs patients,10 patients in this study did not identify difficult (or positive) relationships with care provider teams, or a history of early life trauma, as factors related to current utilization.
These findings raise several important questions. To what extent are frequently hospitalized patient populations comparable with each other? This is both a question about how populations are defined and a question about the inherent variability between populations (including geographic, social, socioeconomic, and other factors). It is not evident from the demographic information provided whether this population is fundamentally different from others that have been studied, or whether risk factors such as mental health issues, housing insecurity, substance abuse, and trauma history are present, but are just not identified by patients here as proximal contributors to their utilization. In either case, the findings raise important questions about the development of effective interventions for these patients. The discrepancies also highlight the utility of ascertaining and reporting the prevalence of these risk factors among study populations, ideally both among patients who opt in and those who opt out. Although obtaining this information adds an additional layer of complexity to data collection, this history, along with extended demographic data, would significantly improve our ability to assess the comparability of populations across studies. It would also help us understand whether perspectives of any specific groups of patients are not represented, due to frequent opting out of the study.
The fact that commonly identified risk factors for high utilization are not identified by patients in this study as contributing to their high hospital use highlights the importance of (1) including the patient perspective as an integral part of care plan and intervention development and (2) continuing local work aimed at understanding the risk factors and drivers of high utilization in specific populations. Many programs, including CHAMP at Northwestern and our own hospitalist-run program at Penn Medicine, work closely with patients to develop individualized care plans that aim to address the underlying drivers of high utilization. In our experience, a multidisciplinary committee reviewing patient cases has identified mental health conditions as likely drivers of frequent admissions in over 95% of program patients. In line with the findings here, however, patients themselves often do not see mental health as a significant contributor. If patients do not see factors such as mental health as important, this has significant implications for the development of interventions around these factors as part of a solution to high hospital use.
Patients are unlikely to respond to interventions targeting problems that they themselves do not identify as important. This is not to say that drivers such as mental health, housing instability, substance abuse, and behaviors rooted in childhood trauma cannot be addressed if they are not identified by a patient as problems. Rather, interventions must be sensitive to and developed within the context of the patient’s own perceptions and priorities. For any program aimed at addressing the underlying drivers of high utilization, therefore, it is critical to elicit individual patient perspectives and to incorporate them in the development of interventions tailored to a specific patient’s needs. This process not only informs the creation of an individualized care plan but also promotes engagement and builds trust.
In prior work,6 O’Leary et al. have joined others throughout the field in calling for standardized definitions of “high utilizers”; this is critical for our ability to compare study results across programs. However, standardizing definitions is just the first step. Individual site studies such as this are needed to help us understand which themes are universal, versus those that are population- and site-specific. They are also important for individual institutions in targeting, developing, and refining local interventions. As a whole, the results will help guide the development of best practices within the field and allow providers to better understand the needs of specific populations. This work is essential to our ability as providers, hospitals, and systems to develop effective interventions for individual patients in this heterogeneous, vulnerable, and high-risk population.
Disclosures
Dr. Knox and Dr. Greysen have nothing to disclose.
1. Stanton MW, Rutherford MK. The high concentration of U.S. health care expenditures. Research in Action Issue 19. 2005. Rockville, MD: Agency for Healthcare Research and Quality.
2. Center for Health Care Strategies (CHCS). “Super-utilizer summit: common themes from innovative complex care management programs.” CHCS. 2013.
3. Jiang H, Weiss A, Barrett M, Sheng M. Characteristics of hospital stays for super-utilizers by payer, 2012: Statistical Brief #190. PubMed
4. Bodenheimer T. Strategies to reduce costs and improve care for high-utilizing medicaid patients: reflections on pioneering programs. CHCS. 2013.
5. Hong C , Siegel A, Ferris T. Caring for high-need, high-cost patients: what makes for a successful care management program? New York (NY): Commonwealth Fund. 2014;19(1):1-19. PubMed
6. Goodwin A, Henschen BL, O’Dwyer LC, Nichols N, O’Leary KJ. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853-859. https://doi.org/10.12788/jhm.3090.
7. O’Leary K, Chapman M, Shandu F et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175.
8. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff. 2015;34(8):1312-1319. https://doi.org/10.1377/hlthaff.2014.1186.
9. Rinehart DJ, Oronce C, Durfee MJ, et al. Identifying subgroups of adult superutilizers in an urban safety-net system using latent class analysis: implications for clinical practice. Med Care. 2018;56(1):e1-e9. https://doi.org/10.1097/MLR.0000000000000628.
10. Mautner DB, Pang H, Brenner JC, et al. Generating hypotheses about care needs of high utilizers: lessons from patient interviews. Popul Health Manag. 2013;16(1):S26-S33. https://doi.org/10.1089/pop.2013.0033.
1. Stanton MW, Rutherford MK. The high concentration of U.S. health care expenditures. Research in Action Issue 19. 2005. Rockville, MD: Agency for Healthcare Research and Quality.
2. Center for Health Care Strategies (CHCS). “Super-utilizer summit: common themes from innovative complex care management programs.” CHCS. 2013.
3. Jiang H, Weiss A, Barrett M, Sheng M. Characteristics of hospital stays for super-utilizers by payer, 2012: Statistical Brief #190. PubMed
4. Bodenheimer T. Strategies to reduce costs and improve care for high-utilizing medicaid patients: reflections on pioneering programs. CHCS. 2013.
5. Hong C , Siegel A, Ferris T. Caring for high-need, high-cost patients: what makes for a successful care management program? New York (NY): Commonwealth Fund. 2014;19(1):1-19. PubMed
6. Goodwin A, Henschen BL, O’Dwyer LC, Nichols N, O’Leary KJ. Interventions for frequently hospitalized patients and their effect on outcomes: a systematic review. J Hosp Med. 2018;13(12):853-859. https://doi.org/10.12788/jhm.3090.
7. O’Leary K, Chapman M, Shandu F et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175.
8. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff. 2015;34(8):1312-1319. https://doi.org/10.1377/hlthaff.2014.1186.
9. Rinehart DJ, Oronce C, Durfee MJ, et al. Identifying subgroups of adult superutilizers in an urban safety-net system using latent class analysis: implications for clinical practice. Med Care. 2018;56(1):e1-e9. https://doi.org/10.1097/MLR.0000000000000628.
10. Mautner DB, Pang H, Brenner JC, et al. Generating hypotheses about care needs of high utilizers: lessons from patient interviews. Popul Health Manag. 2013;16(1):S26-S33. https://doi.org/10.1089/pop.2013.0033.
© 2019 Society of Hospital Medicine
Leadership & Professional Development: Searching for Ideas Close to Home
As hospitalists, many of us see things in our daily practice that help inform our efforts to improve quality of care, organizational efficiency, and medical education, and to reduce physician burnout. But many of those efforts, while well intended, lack rigorous empirical evaluation.
Indeed, it is the complexity of hospital care that leads scholars across many disciplines—including economics, epidemiology, and sociology—to look to hospital medicine as a place where “natural experimentation” can inform us about what works and doesn’t work in medical care. As a hospitalist and economist, I find that the very best of my ideas come from what I see in the hospital. And for many hospital-based clinicians and physician leaders, translating everyday insights into rigorous scientific explorations is not only feasible but is a natural extension of the curiosity that drives good clinical work. It is also a way to drive quality improvement.
Consider, for example, a question that hospitalists face every day: when to discharge a patient from the hospital. Hospital leaders and frontline clinicians are increasingly under pressure to discharge patients earlier and earlier, with some concerned that earlier discharge poses safety risks. Short of randomizing patients to earlier discharge and studying the effects on outcomes, how can a data-driven hospital leader identify which patients can be safely discharged earlier and how much earlier?
A simple observation of a practicing hospitalist could be a clue to elegantly and rigorously answering this question. It turns out that some patients happen to be hospitalized days before their birthday and it wouldn’t be absurd to think that a physician treating such a patient might be more likely to discharge that patient home on or before their birthday so they can celebrate it at home. The same might be true for patients who are in the hospital before an impending storm. Patient-level data could be used to assess whether length of stay is shorter for patients who are admitted to the hospital a few days before their birthday (or just before a storm), compared with otherwise similar patients admitted to the hospital several weeks earlier, and whether outcomes are any different, on average, or in specific subpopulations. For hospital leaders, this could not only be convincing “quasi-experimental” evidence that length of stay can be safely reduced, but it could also contribute to the scholarly literature.
How can hospitalists generate ideas like these, rigorously evaluate them, and translate them into practice? It turns out that examples such as these abound for the practicing hospitalist, yet few draw the link between these everyday phenomena and the larger question of how length of stay affects patient outcomes. To start, a systematic approach to generating ideas is important: “idea rounds”—a dedicated group discussion in which physicians and other providers brainstorm ideas for quality improvement—can leverage the wisdom of frontline clinicians. But, clever insights aren’t enough. Data and statistical expertise are needed, but with the growing use of electronic health record data and administrative data from large insurers, lack of data is less of a challenge. The larger challenge is data expertise. Data-driven hospital leaders should invest in personnel with statistical expertise to not only complement the scholarly endeavors of hospital medicine faculty, but also to conduct larger, more rigorous quality improvement studies. Particularly as hospitals are increasingly being measured and reimbursed on the basis of data-oriented quality-of-care metrics, it makes sense for hospital leaders to analogously invest in data infrastructure and the analytic capability to analyze that data. The innovation of this approach lies in the simple insight that the everyday activities of hospitalists can be used to answer interesting questions about what works, what doesn’t, and potentially why in healthcare.
Disclosures
Dr. Jena reports receiving consulting fees unrelated to this work from Pfizer, Hill Rom Services, Bristol Myers Squibb, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals, AstraZeneca, Celgene, Tesaro, Sanofi Aventis, Biogen, Precision Health Economics, and Analysis Group.
Funding
Support was provided by the Office of the Director, National Institutes of Health (1DP5OD017897, Dr. Jena).
As hospitalists, many of us see things in our daily practice that help inform our efforts to improve quality of care, organizational efficiency, and medical education, and to reduce physician burnout. But many of those efforts, while well intended, lack rigorous empirical evaluation.
Indeed, it is the complexity of hospital care that leads scholars across many disciplines—including economics, epidemiology, and sociology—to look to hospital medicine as a place where “natural experimentation” can inform us about what works and doesn’t work in medical care. As a hospitalist and economist, I find that the very best of my ideas come from what I see in the hospital. And for many hospital-based clinicians and physician leaders, translating everyday insights into rigorous scientific explorations is not only feasible but is a natural extension of the curiosity that drives good clinical work. It is also a way to drive quality improvement.
Consider, for example, a question that hospitalists face every day: when to discharge a patient from the hospital. Hospital leaders and frontline clinicians are increasingly under pressure to discharge patients earlier and earlier, with some concerned that earlier discharge poses safety risks. Short of randomizing patients to earlier discharge and studying the effects on outcomes, how can a data-driven hospital leader identify which patients can be safely discharged earlier and how much earlier?
A simple observation of a practicing hospitalist could be a clue to elegantly and rigorously answering this question. It turns out that some patients happen to be hospitalized days before their birthday and it wouldn’t be absurd to think that a physician treating such a patient might be more likely to discharge that patient home on or before their birthday so they can celebrate it at home. The same might be true for patients who are in the hospital before an impending storm. Patient-level data could be used to assess whether length of stay is shorter for patients who are admitted to the hospital a few days before their birthday (or just before a storm), compared with otherwise similar patients admitted to the hospital several weeks earlier, and whether outcomes are any different, on average, or in specific subpopulations. For hospital leaders, this could not only be convincing “quasi-experimental” evidence that length of stay can be safely reduced, but it could also contribute to the scholarly literature.
How can hospitalists generate ideas like these, rigorously evaluate them, and translate them into practice? It turns out that examples such as these abound for the practicing hospitalist, yet few draw the link between these everyday phenomena and the larger question of how length of stay affects patient outcomes. To start, a systematic approach to generating ideas is important: “idea rounds”—a dedicated group discussion in which physicians and other providers brainstorm ideas for quality improvement—can leverage the wisdom of frontline clinicians. But, clever insights aren’t enough. Data and statistical expertise are needed, but with the growing use of electronic health record data and administrative data from large insurers, lack of data is less of a challenge. The larger challenge is data expertise. Data-driven hospital leaders should invest in personnel with statistical expertise to not only complement the scholarly endeavors of hospital medicine faculty, but also to conduct larger, more rigorous quality improvement studies. Particularly as hospitals are increasingly being measured and reimbursed on the basis of data-oriented quality-of-care metrics, it makes sense for hospital leaders to analogously invest in data infrastructure and the analytic capability to analyze that data. The innovation of this approach lies in the simple insight that the everyday activities of hospitalists can be used to answer interesting questions about what works, what doesn’t, and potentially why in healthcare.
Disclosures
Dr. Jena reports receiving consulting fees unrelated to this work from Pfizer, Hill Rom Services, Bristol Myers Squibb, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals, AstraZeneca, Celgene, Tesaro, Sanofi Aventis, Biogen, Precision Health Economics, and Analysis Group.
Funding
Support was provided by the Office of the Director, National Institutes of Health (1DP5OD017897, Dr. Jena).
As hospitalists, many of us see things in our daily practice that help inform our efforts to improve quality of care, organizational efficiency, and medical education, and to reduce physician burnout. But many of those efforts, while well intended, lack rigorous empirical evaluation.
Indeed, it is the complexity of hospital care that leads scholars across many disciplines—including economics, epidemiology, and sociology—to look to hospital medicine as a place where “natural experimentation” can inform us about what works and doesn’t work in medical care. As a hospitalist and economist, I find that the very best of my ideas come from what I see in the hospital. And for many hospital-based clinicians and physician leaders, translating everyday insights into rigorous scientific explorations is not only feasible but is a natural extension of the curiosity that drives good clinical work. It is also a way to drive quality improvement.
Consider, for example, a question that hospitalists face every day: when to discharge a patient from the hospital. Hospital leaders and frontline clinicians are increasingly under pressure to discharge patients earlier and earlier, with some concerned that earlier discharge poses safety risks. Short of randomizing patients to earlier discharge and studying the effects on outcomes, how can a data-driven hospital leader identify which patients can be safely discharged earlier and how much earlier?
A simple observation of a practicing hospitalist could be a clue to elegantly and rigorously answering this question. It turns out that some patients happen to be hospitalized days before their birthday and it wouldn’t be absurd to think that a physician treating such a patient might be more likely to discharge that patient home on or before their birthday so they can celebrate it at home. The same might be true for patients who are in the hospital before an impending storm. Patient-level data could be used to assess whether length of stay is shorter for patients who are admitted to the hospital a few days before their birthday (or just before a storm), compared with otherwise similar patients admitted to the hospital several weeks earlier, and whether outcomes are any different, on average, or in specific subpopulations. For hospital leaders, this could not only be convincing “quasi-experimental” evidence that length of stay can be safely reduced, but it could also contribute to the scholarly literature.
How can hospitalists generate ideas like these, rigorously evaluate them, and translate them into practice? It turns out that examples such as these abound for the practicing hospitalist, yet few draw the link between these everyday phenomena and the larger question of how length of stay affects patient outcomes. To start, a systematic approach to generating ideas is important: “idea rounds”—a dedicated group discussion in which physicians and other providers brainstorm ideas for quality improvement—can leverage the wisdom of frontline clinicians. But, clever insights aren’t enough. Data and statistical expertise are needed, but with the growing use of electronic health record data and administrative data from large insurers, lack of data is less of a challenge. The larger challenge is data expertise. Data-driven hospital leaders should invest in personnel with statistical expertise to not only complement the scholarly endeavors of hospital medicine faculty, but also to conduct larger, more rigorous quality improvement studies. Particularly as hospitals are increasingly being measured and reimbursed on the basis of data-oriented quality-of-care metrics, it makes sense for hospital leaders to analogously invest in data infrastructure and the analytic capability to analyze that data. The innovation of this approach lies in the simple insight that the everyday activities of hospitalists can be used to answer interesting questions about what works, what doesn’t, and potentially why in healthcare.
Disclosures
Dr. Jena reports receiving consulting fees unrelated to this work from Pfizer, Hill Rom Services, Bristol Myers Squibb, Novartis, Amgen, Eli Lilly, Vertex Pharmaceuticals, AstraZeneca, Celgene, Tesaro, Sanofi Aventis, Biogen, Precision Health Economics, and Analysis Group.
Funding
Support was provided by the Office of the Director, National Institutes of Health (1DP5OD017897, Dr. Jena).
© 2019 Society of Hospital Medicine
Cognitive Biases Influence Decision-Making Regarding Postacute Care in a Skilled Nursing Facility
The combination of decreasing hospital lengths of stay and increasing age and comorbidity of the United States population is a principal driver of the increased use of postacute care in the US.1-3 Postacute care refers to care in long-term acute care hospitals, inpatient rehabilitation facilities, skilled nursing facilities (SNFs), and care provided by home health agencies after an acute hospitalization. In 2016, 43% of Medicare beneficiaries received postacute care after hospital discharge at the cost of $60 billion annually; nearly half of these received care in an SNF.4 Increasing recognition of the significant cost and poor outcomes of postacute care led to payment reforms, such as bundled payments, that incentivized less expensive forms of postacute care and improvements in outcomes.5-9 Early evaluations suggested that hospitals are sensitive to these reforms and responded by significantly decreasing SNF utilization.10,11 It remains unclear whether this was safe and effective.
In this context, increased attention to how hospital clinicians and hospitalized patients decide whether to use postacute care (and what form to use) is appropriate since the effect of payment reforms could negatively impact vulnerable populations of older adults without adequate protection.12 Suboptimal decision-making can drive both overuse and inappropriate underuse of this expensive medical resource. Initial evidence suggests that patients and clinicians are poorly equipped to make high-quality decisions about postacute care, with significant deficits in both the decision-making process and content.13-16 While these gaps are important to address, they may only be part of the problem. The fields of cognitive psychology and behavioral economics have revealed new insights into decision-making, demonstrating that people deviate from rational decision-making in predictable ways, termed decision heuristics, or cognitive biases.17 This growing field of research suggests heuristics or biases play important roles in decision-making and determining behavior, particularly in situations where there may be little information provided and the patient is stressed, tired, and ill—precisely like deciding on postacute care.18 However, it is currently unknown whether cognitive biases are at play when making hospital discharge decisions.
We sought to identify the most salient heuristics or cognitive biases patients may utilize when making decisions about postacute care at the end of their hospitalization and ways clinicians may contribute to these biases. The overall goal was to derive insights for improving postacute care decision-making.
METHODS
Study Design
We conducted a secondary analysis on interviews with hospital and SNF clinicians as well as patients and their caregivers who were either leaving the hospital for an SNF or newly arrived in an SNF from the hospital to understand if cognitive biases were present and how they manifested themselves in a real-world clinical context.19 These interviews were part of a larger qualitative study that sought to understand how clinicians, patients, and their caregivers made decisions about postacute care, particularly related to SNFs.13,14 This study represents the analysis of all our interviews, specifically examining decision-making bias. Participating sites, clinical roles, and both patient and caregiver characteristics (Table 1) in our cohort have been previously described.13,14
Analysis
We used a team-based approach to framework analysis, which has been used in other decision-making studies14, including those measuring cognitive bias.20 A limitation in cognitive bias research is the lack of a standardized list or categorization of cognitive biases. We reviewed prior systematic17,21 and narrative reviews18,22, as well as prior studies describing examples of cognitive biases playing a role in decision-making about therapy20 to construct a list of possible cognitive biases to evaluate and narrow these a priori to potential biases relevant to the decision about postacute care based on our prior work (Table 2).
We applied this framework to analyze transcripts through an iterative process of deductive coding and reviewing across four reviewers (ML, RA, AL, CL) and a hospitalist physician with expertise leading qualitative studies (REB).
Intercoder consensus was built through team discussion by resolving points of disagreement.23 Consistency of coding was regularly checked by having more than one investigator code individual manuscripts and comparing coding, and discrepancies were resolved through team discussion. We triangulated the data (shared our preliminary results) using a larger study team, including an expert in behavioral economics (SRG), physicians at study sites (EC, RA), and an anthropologist with expertise in qualitative methods (CL). We did this to ensure credibility (to what extent the findings are credible or believable) and confirmability of findings (ensuring the findings are based on participant narratives rather than researcher biases).
RESULTS
We reviewed a total of 105 interviews with 25 hospital clinicians, 20 SNF clinicians, 21 patients and 14 caregivers in the hospital, and 15 patients and 10 caregivers in the SNF setting (Table 1). We found authority bias/halo effect; default/status quo bias, anchoring bias, and framing was commonly present in decision-making about postacute care in a SNF, whereas there were few if any examples of ambiguity aversion, availability heuristic, confirmation bias, optimism bias, or false consensus effect (Table 2).
Authority Bias/Halo Effect
While most patients deferred to their inpatient teams when it came to decision-making, this effect seemed to differ across VA and non-VA settings. Veterans expressed a higher degree of potential authority bias regarding the VA as an institution, whereas older adults in non-VA settings saw physicians as the authority figure making decisions in their best interests.
Veterans expressed confidence in the VA regarding both whether to go to a SNF and where to go:
“The VA wouldn’t license [an SNF] if they didn’t have a good reputation for care, cleanliness, things of that nature” (Veteran, VA CLC)
“I just knew the VA would have my best interests at heart” (Veteran, VA CLC)
Their caregivers expressed similar confidence:
“I’m not gonna decide [on whether the patient they care for goes to postacute care], like I told you, that’s totally up to the VA. I have trust and faith in them…so wherever they send him, that’s where he’s going” (Caregiver, VA hospital)
In some cases, this perspective was closer to the halo effect: a positive experience with the care provider or the care team led the decision-makers to believe that their recommendations about postacute care would be similarly positive.
“I think we were very trusting in the sense that whatever happened the last time around, he survived it…they took care of him…he got back home, and he started his life again, you know, so why would we question what they’re telling us to do? (Caregiver, VA hospital)
In contrast to Veterans, non-Veteran patients seemed to experience authority bias when it came to the inpatient team.
“Well, I’d like to know more about the PTs [Physical Therapists] there, but I assume since they were recommended, they will be good.” (Patient, University hospital)
This perspective was especially apparent when it came to physicians:
“The level of trust that they [patients] put in their doctor is gonna outweigh what anyone else would say.” (Clinical liaison, SNF)
“[In response to a question about influences on the decision to go to rehab] I don’t…that’s not my decision to make, that’s the doctor’s decision.” (Patient, University hospital)
“They said so…[the doctor] said I needed to go to rehab, so I guess I do because it’s the doctor’s decision.” (Patient, University hospital)
Default/Status quo Bias
In a related way, patients and caregivers with exposure to a SNF seemed to default to the same SNF with which they had previous experience. This bias seems to be primarily related to knowing what to expect.
“He thinks it’s [a particular SNF] the right place for him now…he was there before and he knew, again, it was the right place for him to be” (Caregiver, VA hospital)
“It’s the only one I’ve ever been in…but they have a lot of activities; you have a lot of freedom, staff was good” (Patient, VA hospital)
“I’ve been [to this SNF] before and I kind of know what the program involves…so it was kind of like going home, not, going home is the wrong way to put it…I mean coming here is like something I know, you know, I didn’t need anybody to explain it to me.” (Patient, VA hospital)
“Anybody that’s been to [SNF], that would be their choice to go back to, and I guess I must’ve liked it that first time because I asked to go back again.” (Patient, University hospital)
Anchoring Bias
While anchoring bias was less frequent, it came up in two domains: first, related to costs of care, and second, related to facility characteristics. Costs came up most frequently for Veterans who preferred to move their care to the VA for cost reasons, which appeared in these cases to overshadow other considerations:
“I kept emphasizing that the VA could do all the same things at a lot more reasonable price. The whole purpose of having the VA is for the Veteran, so that…we can get the healthcare that we need at a more reasonable [sic] or a reasonable price.” (Veteran, CLC)
“I think the CLC [VA SNF] is going to take care of her probably the same way any other facility of its type would, unless she were in a private facility, but you know, that costs a lot more money.” (Caregiver, VA hospital)
Patients occasionally had striking responses to particular characteristics of SNFs, regardless of whether this was a central feature or related to their rehabilitation:
“The social worker comes and talks to me about the nursing home where cats are running around, you know, to infect my leg or spin their little cat hairs into my lungs and make my asthma worse…I’m going to have to beg the nurses or the aides or the family or somebody to clean the cat…” (Veteran, VA hospital)
Framing
Framing was the strongest theme among clinician interviews in our sample. Clinicians most frequently described the SNF as a place where patients could recover function (a positive frame), explaining risks (eg, rehospitalization) associated with alternative postacute care options besides the SNF in great detail.
“Aside from explaining the benefits of going and…having that 24-hour care, having the therapies provided to them [the patients], talking about them getting stronger, phrasing it in such a way that patients sometimes are more agreeable, like not calling it a skilled nursing facility, calling it a rehab you know, for them to get physically stronger so they can be the most independent that they can once they do go home, and also explaining … we think that this would be the best plan to prevent them from coming back to the hospital, so those are some of the things that we’ll mention to patients to try and educate them and get them to be agreeable for placement.” (Social worker, University hospital)
Clinicians avoided negative associations with “nursing home” (even though all SNFs are nursing homes) and tended to use more positive frames such as “rehabilitation facility.”
“Use the word rehab….we definitely use the word rehab, to get more therapy, to go home; it’s not a, we really emphasize it’s not a nursing home, it’s not to go to stay forever.” (Physical therapist, safety-net hospital)
Clinicians used a frame of “safety” when discussing the SNF and used a frame of “risk” when discussing alternative postacute care options such as returning home. We did not find examples of clinicians discussing similar risks in going to a SNF even for risks, such as falling, which exist in both settings.
“I’ve talked to them primarily on an avenue of safety because I think people want and they value independence, they value making sure they can get home, but you know, a lot of the times they understand safety is, it can be a concern and outlining that our goal is to make sure that they’re safe and they stay home, and I tend to broach the subject saying that our therapists believe that they might not be safe at home in the moment, but they have potential goals to be safe later on if we continue therapy. I really highlight safety being the major driver of our discussion.” (Physician, VA hospital)
In some cases, framing was so overt that other risk-mitigating options (eg, home healthcare) are not discussed.
“I definitely tend to explain the ideal first. I’m not going to bring up home care when we really think somebody should go to rehab, however, once people say I don’t want to do that, I’m not going, then that’s when I’m like OK, well, let’s talk to the doctors, but we can see about other supports in the home.” (Social worker, VA hospital)
DISCUSSION
In a large sample of patients and their caregivers, as well as multidisciplinary clinicians at three different hospitals and three SNFs, we found authority bias/halo effect and framing biases were most common and seemed most impactful. Default/status quo bias and anchoring bias were also present in decision-making about a SNF. The combination of authority bias/halo effect and framing biases could synergistically interact to augment the likelihood of patients accepting a SNF for postacute care. Patients who had been to a SNF before seemed more likely to choose the SNF they had experienced previously even if they had no other postacute care experiences, and could be highly influenced by isolated characteristics of that facility (such as the physical environment or cost of care).
It is important to mention that cognitive biases do not necessarily have a negative impact: indeed, as Kahneman and Tversky point out, these are useful heuristics from “fast” thinking that are often effective.24 For example, clinicians may be trying to act in the best interests of the patient when framing the decision in terms of regaining function and averting loss of safety and independence. However, the evidence base regarding the outcomes of an SNF versus other postacute options is not robust, and this decision-making is complex. While this decision was most commonly framed in terms of rehabilitation and returning home, the fact that only about half of patients have returned to the community by 100 days4 was not discussed in any interview. In fact, initial evidence suggests replacing the SNF with home healthcare in patients with hip and knee arthroplasty may reduce costs without worsening clinical outcomes.6 However, across a broader population, SNFs significantly reduce 30-day readmissions when directly compared with home healthcare, but other clinical outcomes are similar.25 This evidence suggests that the “right” postacute care option for an individual patient is not clear, highlighting a key role biases may play in decision-making. Further, the nebulous concept of “safety” could introduce potential disparities related to social determinants of health.12 The observed inclination to accept an SNF with which the individual had prior experience may be influenced by the acceptability of this choice because of personal factors or prior research, even if it also represents a bias by limiting the consideration of current alternatives.
Our findings complement those of others in the literature which have also identified profound gaps in discharge decision-making among patients and clinicians,13-16,26-31 though to our knowledge the role of cognitive biases in these decisions has not been explored. This study also addresses gaps in the cognitive bias literature, including the need for real-world data rather than hypothetical vignettes,17 and evaluation of treatment and management decisions rather than diagnoses, which have been more commonly studied.21
These findings have implications for both individual clinicians and healthcare institutions. In the immediate term, these findings may serve as a call to discharging clinicians to modulate language and “debias” their conversations with patients about care after discharge.18,22 Shared decision-making requires an informed choice by patients based on their goals and values; framing a decision in a way that puts the clinician’s goals or values (eg, safety) ahead of patient values (eg, independence and autonomy) or limits disclosure (eg, a “rehab” is a nursing home) in the hope of influencing choice may be more consistent with framing bias and less with shared decision-making.14 Although controversy exists about the best way to “debias” oneself,32 self-awareness of bias is increasingly recognized across healthcare venues as critical to improving care for vulnerable populations.33 The use of data rather than vignettes may be a useful debiasing strategy, although the limitations of currently available data (eg, capturing nursing home quality) are increasingly recognized.34 From a policy and health system perspective, cognitive biases should be integrated into the development of decision aids to facilitate informed, shared, and high-quality decision-making that incorporates patient values, and perhaps “nudges” from behavioral economics to assist patients in choosing the right postdischarge care for them. Such nudges use principles of framing to influence care without restricting choice.35 As the science informing best practice regarding postacute care improves, identifying the “right” postdischarge care may become easier and recommendations more evidence-based.36
Strengths of the study include a large, diverse sample of patients, caregivers, and clinicians in both the hospital and SNF setting. Also, we used a team-based analysis with an experienced team and a deep knowledge of the data, including triangulation with clinicians to verify results. However, all hospitals and SNFs were located in a single metropolitan area, and responses may vary by region or population density. All three hospitals have housestaff teaching programs, and at the time of the interviews all three community SNFs were “five-star” facilities on the Nursing Home Compare website; results may be different at community hospitals or other SNFs. Hospitalists were the only physician group sampled in the hospital as they provide the majority of inpatient care to older adults; geriatricians, in particular, may have had different perspectives. Since we intended to explore whether cognitive biases were present overall, we did not evaluate whether cognitive biases differed by role or subgroup (by clinician type, patient, or caregiver), but this may be a promising area to explore in future work. Many cognitive biases have been described, and there are likely additional biases we did not identify. To confirm the generalizability of these findings, they should be studied in a larger, more generalizable sample of respondents in future work.
Cognitive biases play an important role in patient decision-making about postacute care, particularly regarding SNF care. As postacute care undergoes a transformation spurred by payment reforms, it is more important than ever to ensure that patients understand their choices at hospital discharge and can make a high-quality decision consistent with their goals.
1. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. https://doi.org/10.1001/jamainternmed.2014.6383.
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. https://doi.org/10.1097/MLR.0000000000000359.
3. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617. https://doi.org/10.1001/jama.2018.2408.
4. Medicare Payment Advisory Commission June 2018 Report to Congress. http://www.medpac.gov/docs/default-source/reports/jun18_ch5_medpacreport_sec.pdf?sfvrsn=0. Accessed November 9, 2018.
5. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. https://doi.org/10.1002/jhm.2673.
6. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
7. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
8. Kennedy G, Lewis VA, Kundu S, Mousqués J, Colla CH. Accountable care organizations and post-acute care: a focus on preferred SNF networks. Med Care Res Rev MCRR. 2018;1077558718781117. https://doi.org/10.1177/1077558718781117.
9. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013;32(5):864-872. https://doi.org/10.1377/hlthaff.2012.1262.
10. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115.
11. Zhu JM, Patel V, Shea JA, Neuman MD, Werner RM. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff Proj Hope. 2018;37(8):1282-1289. https://doi.org/10.1377/hlthaff.2018.0257.
12. Burke RE, Ibrahim SA. Discharge destination and disparities in postoperative care. JAMA. 2018;319(16):1653-1654. https://doi.org/10.1001/jama.2017.21884.
13. Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017;65(11):2466-2472. https://doi.org/10.1111/jgs.14954.
14. Burke RE, Jones J, Lawrence E, et al. Evaluating the quality of patient decision-making regarding post-acute care. J Gen Intern Med. 2018;33(5):678-684. https://doi.org/10.1007/s11606-017-4298-1.
15. Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc. 2017;65(11):2459-2465. https://doi.org/10.1111/jgs.14988.
16. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff. 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155.
17. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Mak Int J Soc Med Decis Mak. 2015;35(4):539-557. https://doi.org/10.1177/0272989X14547740.
18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 Suppl 2:ii58-ii64. https://doi.org/10.1136/bmjqs-2012-001712.
19. Hinds PS, Vogel RJ, Clarke-Steffen L. The possibilities and pitfalls of doing a secondary analysis of a qualitative data set. Qual Health Res. 1997;7(3):408-424. https://doi.org/10.1177/104973239700700306.
20. Magid M, Mcllvennan CK, Jones J, et al. Exploring cognitive bias in destination therapy left ventricular assist device decision making: a retrospective qualitative framework analysis. Am Heart J. 2016;180:64-73. https://doi.org/10.1016/j.ahj.2016.06.024.
21. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138. https://doi.org/10.1186/s12911-016-0377-1.
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22 Suppl 2:ii65-ii72. https://doi.org/10.1136/bmjqs-2012-001713.
23. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
24. Thinking, Fast and Slow. Daniel Kahneman. Macmillan. US Macmillan. https://us.macmillan.com/thinkingfastandslow/danielkahneman/9780374533557. Accessed February 5, 2019.
25. Werner RM, Konetzka RT, Coe NB. Does type of post-acute care matter? The effect of hospital discharge to home with home health care versus to skilled nursing facility. JAMA Intern Med. In press.
26. Jones J, Lawrence E, Ladebue A, Leonard C, Ayele R, Burke RE. Nurses’ role in managing “The Fit” of older adults in skilled nursing facilities. J Gerontol Nurs. 2017;43(12):11-20. https://doi.org/10.3928/00989134-20171110-06.
27. Lawrence E, Casler J-J, Jones J, et al. Variability in skilled nursing facility screening and admission processes: implications for value-based purchasing. Health Care Manage Rev. 2018. https://doi.org/10.1097/HMR.0000000000000225.
28. Ayele R, Jones J, Ladebue A, et al. Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc. 2019. https://doi.org/10.1111/jgs.15768.
29. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. https://doi.org/10.3928/19404921-20160120-01.
30. Konetzka RT, Perraillon MC. Use of nursing home compare website appears limited by lack of awareness and initial mistrust of the data. Health Aff Proj Hope. 2016;35(4):706-713. https://doi.org/10.1377/hlthaff.2015.1377.
31. Schapira MM, Shea JA, Duey KA, Kleiman C, Werner RM. The nursing home compare report card: perceptions of residents and caregivers regarding quality ratings and nursing home choice. Health Serv Res. 2016;51 Suppl 2:1212-1228. https://doi.org/10.1111/1475-6773.12458.
32. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. https://doi.org/10.1136/bmjqs-2016-005267.
33. Masters C, Robinson D, Faulkner S, Patterson E, McIlraith T, Ansari A. Addressing biases in patient care with the 5Rs of cultural humility, a clinician coaching tool. J Gen Intern Med. 2019;34(4):627-630. https://doi.org/10.1007/s11606-018-4814-y.
34. Burke RE, Werner RM. Quality measurement and nursing homes: measuring what matters. BMJ Qual Saf. 2019;28(7);520-523. https://doi.org/10.1136/bmjqs-2019-009447.
35. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378(3):214-216. https://doi.org/10.1056/NEJMp1712984.
36. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med. 2015;175(2):296-297. https://doi.org/10.1001/jamainternmed.2014.4298.
The combination of decreasing hospital lengths of stay and increasing age and comorbidity of the United States population is a principal driver of the increased use of postacute care in the US.1-3 Postacute care refers to care in long-term acute care hospitals, inpatient rehabilitation facilities, skilled nursing facilities (SNFs), and care provided by home health agencies after an acute hospitalization. In 2016, 43% of Medicare beneficiaries received postacute care after hospital discharge at the cost of $60 billion annually; nearly half of these received care in an SNF.4 Increasing recognition of the significant cost and poor outcomes of postacute care led to payment reforms, such as bundled payments, that incentivized less expensive forms of postacute care and improvements in outcomes.5-9 Early evaluations suggested that hospitals are sensitive to these reforms and responded by significantly decreasing SNF utilization.10,11 It remains unclear whether this was safe and effective.
In this context, increased attention to how hospital clinicians and hospitalized patients decide whether to use postacute care (and what form to use) is appropriate since the effect of payment reforms could negatively impact vulnerable populations of older adults without adequate protection.12 Suboptimal decision-making can drive both overuse and inappropriate underuse of this expensive medical resource. Initial evidence suggests that patients and clinicians are poorly equipped to make high-quality decisions about postacute care, with significant deficits in both the decision-making process and content.13-16 While these gaps are important to address, they may only be part of the problem. The fields of cognitive psychology and behavioral economics have revealed new insights into decision-making, demonstrating that people deviate from rational decision-making in predictable ways, termed decision heuristics, or cognitive biases.17 This growing field of research suggests heuristics or biases play important roles in decision-making and determining behavior, particularly in situations where there may be little information provided and the patient is stressed, tired, and ill—precisely like deciding on postacute care.18 However, it is currently unknown whether cognitive biases are at play when making hospital discharge decisions.
We sought to identify the most salient heuristics or cognitive biases patients may utilize when making decisions about postacute care at the end of their hospitalization and ways clinicians may contribute to these biases. The overall goal was to derive insights for improving postacute care decision-making.
METHODS
Study Design
We conducted a secondary analysis on interviews with hospital and SNF clinicians as well as patients and their caregivers who were either leaving the hospital for an SNF or newly arrived in an SNF from the hospital to understand if cognitive biases were present and how they manifested themselves in a real-world clinical context.19 These interviews were part of a larger qualitative study that sought to understand how clinicians, patients, and their caregivers made decisions about postacute care, particularly related to SNFs.13,14 This study represents the analysis of all our interviews, specifically examining decision-making bias. Participating sites, clinical roles, and both patient and caregiver characteristics (Table 1) in our cohort have been previously described.13,14
Analysis
We used a team-based approach to framework analysis, which has been used in other decision-making studies14, including those measuring cognitive bias.20 A limitation in cognitive bias research is the lack of a standardized list or categorization of cognitive biases. We reviewed prior systematic17,21 and narrative reviews18,22, as well as prior studies describing examples of cognitive biases playing a role in decision-making about therapy20 to construct a list of possible cognitive biases to evaluate and narrow these a priori to potential biases relevant to the decision about postacute care based on our prior work (Table 2).
We applied this framework to analyze transcripts through an iterative process of deductive coding and reviewing across four reviewers (ML, RA, AL, CL) and a hospitalist physician with expertise leading qualitative studies (REB).
Intercoder consensus was built through team discussion by resolving points of disagreement.23 Consistency of coding was regularly checked by having more than one investigator code individual manuscripts and comparing coding, and discrepancies were resolved through team discussion. We triangulated the data (shared our preliminary results) using a larger study team, including an expert in behavioral economics (SRG), physicians at study sites (EC, RA), and an anthropologist with expertise in qualitative methods (CL). We did this to ensure credibility (to what extent the findings are credible or believable) and confirmability of findings (ensuring the findings are based on participant narratives rather than researcher biases).
RESULTS
We reviewed a total of 105 interviews with 25 hospital clinicians, 20 SNF clinicians, 21 patients and 14 caregivers in the hospital, and 15 patients and 10 caregivers in the SNF setting (Table 1). We found authority bias/halo effect; default/status quo bias, anchoring bias, and framing was commonly present in decision-making about postacute care in a SNF, whereas there were few if any examples of ambiguity aversion, availability heuristic, confirmation bias, optimism bias, or false consensus effect (Table 2).
Authority Bias/Halo Effect
While most patients deferred to their inpatient teams when it came to decision-making, this effect seemed to differ across VA and non-VA settings. Veterans expressed a higher degree of potential authority bias regarding the VA as an institution, whereas older adults in non-VA settings saw physicians as the authority figure making decisions in their best interests.
Veterans expressed confidence in the VA regarding both whether to go to a SNF and where to go:
“The VA wouldn’t license [an SNF] if they didn’t have a good reputation for care, cleanliness, things of that nature” (Veteran, VA CLC)
“I just knew the VA would have my best interests at heart” (Veteran, VA CLC)
Their caregivers expressed similar confidence:
“I’m not gonna decide [on whether the patient they care for goes to postacute care], like I told you, that’s totally up to the VA. I have trust and faith in them…so wherever they send him, that’s where he’s going” (Caregiver, VA hospital)
In some cases, this perspective was closer to the halo effect: a positive experience with the care provider or the care team led the decision-makers to believe that their recommendations about postacute care would be similarly positive.
“I think we were very trusting in the sense that whatever happened the last time around, he survived it…they took care of him…he got back home, and he started his life again, you know, so why would we question what they’re telling us to do? (Caregiver, VA hospital)
In contrast to Veterans, non-Veteran patients seemed to experience authority bias when it came to the inpatient team.
“Well, I’d like to know more about the PTs [Physical Therapists] there, but I assume since they were recommended, they will be good.” (Patient, University hospital)
This perspective was especially apparent when it came to physicians:
“The level of trust that they [patients] put in their doctor is gonna outweigh what anyone else would say.” (Clinical liaison, SNF)
“[In response to a question about influences on the decision to go to rehab] I don’t…that’s not my decision to make, that’s the doctor’s decision.” (Patient, University hospital)
“They said so…[the doctor] said I needed to go to rehab, so I guess I do because it’s the doctor’s decision.” (Patient, University hospital)
Default/Status quo Bias
In a related way, patients and caregivers with exposure to a SNF seemed to default to the same SNF with which they had previous experience. This bias seems to be primarily related to knowing what to expect.
“He thinks it’s [a particular SNF] the right place for him now…he was there before and he knew, again, it was the right place for him to be” (Caregiver, VA hospital)
“It’s the only one I’ve ever been in…but they have a lot of activities; you have a lot of freedom, staff was good” (Patient, VA hospital)
“I’ve been [to this SNF] before and I kind of know what the program involves…so it was kind of like going home, not, going home is the wrong way to put it…I mean coming here is like something I know, you know, I didn’t need anybody to explain it to me.” (Patient, VA hospital)
“Anybody that’s been to [SNF], that would be their choice to go back to, and I guess I must’ve liked it that first time because I asked to go back again.” (Patient, University hospital)
Anchoring Bias
While anchoring bias was less frequent, it came up in two domains: first, related to costs of care, and second, related to facility characteristics. Costs came up most frequently for Veterans who preferred to move their care to the VA for cost reasons, which appeared in these cases to overshadow other considerations:
“I kept emphasizing that the VA could do all the same things at a lot more reasonable price. The whole purpose of having the VA is for the Veteran, so that…we can get the healthcare that we need at a more reasonable [sic] or a reasonable price.” (Veteran, CLC)
“I think the CLC [VA SNF] is going to take care of her probably the same way any other facility of its type would, unless she were in a private facility, but you know, that costs a lot more money.” (Caregiver, VA hospital)
Patients occasionally had striking responses to particular characteristics of SNFs, regardless of whether this was a central feature or related to their rehabilitation:
“The social worker comes and talks to me about the nursing home where cats are running around, you know, to infect my leg or spin their little cat hairs into my lungs and make my asthma worse…I’m going to have to beg the nurses or the aides or the family or somebody to clean the cat…” (Veteran, VA hospital)
Framing
Framing was the strongest theme among clinician interviews in our sample. Clinicians most frequently described the SNF as a place where patients could recover function (a positive frame), explaining risks (eg, rehospitalization) associated with alternative postacute care options besides the SNF in great detail.
“Aside from explaining the benefits of going and…having that 24-hour care, having the therapies provided to them [the patients], talking about them getting stronger, phrasing it in such a way that patients sometimes are more agreeable, like not calling it a skilled nursing facility, calling it a rehab you know, for them to get physically stronger so they can be the most independent that they can once they do go home, and also explaining … we think that this would be the best plan to prevent them from coming back to the hospital, so those are some of the things that we’ll mention to patients to try and educate them and get them to be agreeable for placement.” (Social worker, University hospital)
Clinicians avoided negative associations with “nursing home” (even though all SNFs are nursing homes) and tended to use more positive frames such as “rehabilitation facility.”
“Use the word rehab….we definitely use the word rehab, to get more therapy, to go home; it’s not a, we really emphasize it’s not a nursing home, it’s not to go to stay forever.” (Physical therapist, safety-net hospital)
Clinicians used a frame of “safety” when discussing the SNF and used a frame of “risk” when discussing alternative postacute care options such as returning home. We did not find examples of clinicians discussing similar risks in going to a SNF even for risks, such as falling, which exist in both settings.
“I’ve talked to them primarily on an avenue of safety because I think people want and they value independence, they value making sure they can get home, but you know, a lot of the times they understand safety is, it can be a concern and outlining that our goal is to make sure that they’re safe and they stay home, and I tend to broach the subject saying that our therapists believe that they might not be safe at home in the moment, but they have potential goals to be safe later on if we continue therapy. I really highlight safety being the major driver of our discussion.” (Physician, VA hospital)
In some cases, framing was so overt that other risk-mitigating options (eg, home healthcare) are not discussed.
“I definitely tend to explain the ideal first. I’m not going to bring up home care when we really think somebody should go to rehab, however, once people say I don’t want to do that, I’m not going, then that’s when I’m like OK, well, let’s talk to the doctors, but we can see about other supports in the home.” (Social worker, VA hospital)
DISCUSSION
In a large sample of patients and their caregivers, as well as multidisciplinary clinicians at three different hospitals and three SNFs, we found authority bias/halo effect and framing biases were most common and seemed most impactful. Default/status quo bias and anchoring bias were also present in decision-making about a SNF. The combination of authority bias/halo effect and framing biases could synergistically interact to augment the likelihood of patients accepting a SNF for postacute care. Patients who had been to a SNF before seemed more likely to choose the SNF they had experienced previously even if they had no other postacute care experiences, and could be highly influenced by isolated characteristics of that facility (such as the physical environment or cost of care).
It is important to mention that cognitive biases do not necessarily have a negative impact: indeed, as Kahneman and Tversky point out, these are useful heuristics from “fast” thinking that are often effective.24 For example, clinicians may be trying to act in the best interests of the patient when framing the decision in terms of regaining function and averting loss of safety and independence. However, the evidence base regarding the outcomes of an SNF versus other postacute options is not robust, and this decision-making is complex. While this decision was most commonly framed in terms of rehabilitation and returning home, the fact that only about half of patients have returned to the community by 100 days4 was not discussed in any interview. In fact, initial evidence suggests replacing the SNF with home healthcare in patients with hip and knee arthroplasty may reduce costs without worsening clinical outcomes.6 However, across a broader population, SNFs significantly reduce 30-day readmissions when directly compared with home healthcare, but other clinical outcomes are similar.25 This evidence suggests that the “right” postacute care option for an individual patient is not clear, highlighting a key role biases may play in decision-making. Further, the nebulous concept of “safety” could introduce potential disparities related to social determinants of health.12 The observed inclination to accept an SNF with which the individual had prior experience may be influenced by the acceptability of this choice because of personal factors or prior research, even if it also represents a bias by limiting the consideration of current alternatives.
Our findings complement those of others in the literature which have also identified profound gaps in discharge decision-making among patients and clinicians,13-16,26-31 though to our knowledge the role of cognitive biases in these decisions has not been explored. This study also addresses gaps in the cognitive bias literature, including the need for real-world data rather than hypothetical vignettes,17 and evaluation of treatment and management decisions rather than diagnoses, which have been more commonly studied.21
These findings have implications for both individual clinicians and healthcare institutions. In the immediate term, these findings may serve as a call to discharging clinicians to modulate language and “debias” their conversations with patients about care after discharge.18,22 Shared decision-making requires an informed choice by patients based on their goals and values; framing a decision in a way that puts the clinician’s goals or values (eg, safety) ahead of patient values (eg, independence and autonomy) or limits disclosure (eg, a “rehab” is a nursing home) in the hope of influencing choice may be more consistent with framing bias and less with shared decision-making.14 Although controversy exists about the best way to “debias” oneself,32 self-awareness of bias is increasingly recognized across healthcare venues as critical to improving care for vulnerable populations.33 The use of data rather than vignettes may be a useful debiasing strategy, although the limitations of currently available data (eg, capturing nursing home quality) are increasingly recognized.34 From a policy and health system perspective, cognitive biases should be integrated into the development of decision aids to facilitate informed, shared, and high-quality decision-making that incorporates patient values, and perhaps “nudges” from behavioral economics to assist patients in choosing the right postdischarge care for them. Such nudges use principles of framing to influence care without restricting choice.35 As the science informing best practice regarding postacute care improves, identifying the “right” postdischarge care may become easier and recommendations more evidence-based.36
Strengths of the study include a large, diverse sample of patients, caregivers, and clinicians in both the hospital and SNF setting. Also, we used a team-based analysis with an experienced team and a deep knowledge of the data, including triangulation with clinicians to verify results. However, all hospitals and SNFs were located in a single metropolitan area, and responses may vary by region or population density. All three hospitals have housestaff teaching programs, and at the time of the interviews all three community SNFs were “five-star” facilities on the Nursing Home Compare website; results may be different at community hospitals or other SNFs. Hospitalists were the only physician group sampled in the hospital as they provide the majority of inpatient care to older adults; geriatricians, in particular, may have had different perspectives. Since we intended to explore whether cognitive biases were present overall, we did not evaluate whether cognitive biases differed by role or subgroup (by clinician type, patient, or caregiver), but this may be a promising area to explore in future work. Many cognitive biases have been described, and there are likely additional biases we did not identify. To confirm the generalizability of these findings, they should be studied in a larger, more generalizable sample of respondents in future work.
Cognitive biases play an important role in patient decision-making about postacute care, particularly regarding SNF care. As postacute care undergoes a transformation spurred by payment reforms, it is more important than ever to ensure that patients understand their choices at hospital discharge and can make a high-quality decision consistent with their goals.
The combination of decreasing hospital lengths of stay and increasing age and comorbidity of the United States population is a principal driver of the increased use of postacute care in the US.1-3 Postacute care refers to care in long-term acute care hospitals, inpatient rehabilitation facilities, skilled nursing facilities (SNFs), and care provided by home health agencies after an acute hospitalization. In 2016, 43% of Medicare beneficiaries received postacute care after hospital discharge at the cost of $60 billion annually; nearly half of these received care in an SNF.4 Increasing recognition of the significant cost and poor outcomes of postacute care led to payment reforms, such as bundled payments, that incentivized less expensive forms of postacute care and improvements in outcomes.5-9 Early evaluations suggested that hospitals are sensitive to these reforms and responded by significantly decreasing SNF utilization.10,11 It remains unclear whether this was safe and effective.
In this context, increased attention to how hospital clinicians and hospitalized patients decide whether to use postacute care (and what form to use) is appropriate since the effect of payment reforms could negatively impact vulnerable populations of older adults without adequate protection.12 Suboptimal decision-making can drive both overuse and inappropriate underuse of this expensive medical resource. Initial evidence suggests that patients and clinicians are poorly equipped to make high-quality decisions about postacute care, with significant deficits in both the decision-making process and content.13-16 While these gaps are important to address, they may only be part of the problem. The fields of cognitive psychology and behavioral economics have revealed new insights into decision-making, demonstrating that people deviate from rational decision-making in predictable ways, termed decision heuristics, or cognitive biases.17 This growing field of research suggests heuristics or biases play important roles in decision-making and determining behavior, particularly in situations where there may be little information provided and the patient is stressed, tired, and ill—precisely like deciding on postacute care.18 However, it is currently unknown whether cognitive biases are at play when making hospital discharge decisions.
We sought to identify the most salient heuristics or cognitive biases patients may utilize when making decisions about postacute care at the end of their hospitalization and ways clinicians may contribute to these biases. The overall goal was to derive insights for improving postacute care decision-making.
METHODS
Study Design
We conducted a secondary analysis on interviews with hospital and SNF clinicians as well as patients and their caregivers who were either leaving the hospital for an SNF or newly arrived in an SNF from the hospital to understand if cognitive biases were present and how they manifested themselves in a real-world clinical context.19 These interviews were part of a larger qualitative study that sought to understand how clinicians, patients, and their caregivers made decisions about postacute care, particularly related to SNFs.13,14 This study represents the analysis of all our interviews, specifically examining decision-making bias. Participating sites, clinical roles, and both patient and caregiver characteristics (Table 1) in our cohort have been previously described.13,14
Analysis
We used a team-based approach to framework analysis, which has been used in other decision-making studies14, including those measuring cognitive bias.20 A limitation in cognitive bias research is the lack of a standardized list or categorization of cognitive biases. We reviewed prior systematic17,21 and narrative reviews18,22, as well as prior studies describing examples of cognitive biases playing a role in decision-making about therapy20 to construct a list of possible cognitive biases to evaluate and narrow these a priori to potential biases relevant to the decision about postacute care based on our prior work (Table 2).
We applied this framework to analyze transcripts through an iterative process of deductive coding and reviewing across four reviewers (ML, RA, AL, CL) and a hospitalist physician with expertise leading qualitative studies (REB).
Intercoder consensus was built through team discussion by resolving points of disagreement.23 Consistency of coding was regularly checked by having more than one investigator code individual manuscripts and comparing coding, and discrepancies were resolved through team discussion. We triangulated the data (shared our preliminary results) using a larger study team, including an expert in behavioral economics (SRG), physicians at study sites (EC, RA), and an anthropologist with expertise in qualitative methods (CL). We did this to ensure credibility (to what extent the findings are credible or believable) and confirmability of findings (ensuring the findings are based on participant narratives rather than researcher biases).
RESULTS
We reviewed a total of 105 interviews with 25 hospital clinicians, 20 SNF clinicians, 21 patients and 14 caregivers in the hospital, and 15 patients and 10 caregivers in the SNF setting (Table 1). We found authority bias/halo effect; default/status quo bias, anchoring bias, and framing was commonly present in decision-making about postacute care in a SNF, whereas there were few if any examples of ambiguity aversion, availability heuristic, confirmation bias, optimism bias, or false consensus effect (Table 2).
Authority Bias/Halo Effect
While most patients deferred to their inpatient teams when it came to decision-making, this effect seemed to differ across VA and non-VA settings. Veterans expressed a higher degree of potential authority bias regarding the VA as an institution, whereas older adults in non-VA settings saw physicians as the authority figure making decisions in their best interests.
Veterans expressed confidence in the VA regarding both whether to go to a SNF and where to go:
“The VA wouldn’t license [an SNF] if they didn’t have a good reputation for care, cleanliness, things of that nature” (Veteran, VA CLC)
“I just knew the VA would have my best interests at heart” (Veteran, VA CLC)
Their caregivers expressed similar confidence:
“I’m not gonna decide [on whether the patient they care for goes to postacute care], like I told you, that’s totally up to the VA. I have trust and faith in them…so wherever they send him, that’s where he’s going” (Caregiver, VA hospital)
In some cases, this perspective was closer to the halo effect: a positive experience with the care provider or the care team led the decision-makers to believe that their recommendations about postacute care would be similarly positive.
“I think we were very trusting in the sense that whatever happened the last time around, he survived it…they took care of him…he got back home, and he started his life again, you know, so why would we question what they’re telling us to do? (Caregiver, VA hospital)
In contrast to Veterans, non-Veteran patients seemed to experience authority bias when it came to the inpatient team.
“Well, I’d like to know more about the PTs [Physical Therapists] there, but I assume since they were recommended, they will be good.” (Patient, University hospital)
This perspective was especially apparent when it came to physicians:
“The level of trust that they [patients] put in their doctor is gonna outweigh what anyone else would say.” (Clinical liaison, SNF)
“[In response to a question about influences on the decision to go to rehab] I don’t…that’s not my decision to make, that’s the doctor’s decision.” (Patient, University hospital)
“They said so…[the doctor] said I needed to go to rehab, so I guess I do because it’s the doctor’s decision.” (Patient, University hospital)
Default/Status quo Bias
In a related way, patients and caregivers with exposure to a SNF seemed to default to the same SNF with which they had previous experience. This bias seems to be primarily related to knowing what to expect.
“He thinks it’s [a particular SNF] the right place for him now…he was there before and he knew, again, it was the right place for him to be” (Caregiver, VA hospital)
“It’s the only one I’ve ever been in…but they have a lot of activities; you have a lot of freedom, staff was good” (Patient, VA hospital)
“I’ve been [to this SNF] before and I kind of know what the program involves…so it was kind of like going home, not, going home is the wrong way to put it…I mean coming here is like something I know, you know, I didn’t need anybody to explain it to me.” (Patient, VA hospital)
“Anybody that’s been to [SNF], that would be their choice to go back to, and I guess I must’ve liked it that first time because I asked to go back again.” (Patient, University hospital)
Anchoring Bias
While anchoring bias was less frequent, it came up in two domains: first, related to costs of care, and second, related to facility characteristics. Costs came up most frequently for Veterans who preferred to move their care to the VA for cost reasons, which appeared in these cases to overshadow other considerations:
“I kept emphasizing that the VA could do all the same things at a lot more reasonable price. The whole purpose of having the VA is for the Veteran, so that…we can get the healthcare that we need at a more reasonable [sic] or a reasonable price.” (Veteran, CLC)
“I think the CLC [VA SNF] is going to take care of her probably the same way any other facility of its type would, unless she were in a private facility, but you know, that costs a lot more money.” (Caregiver, VA hospital)
Patients occasionally had striking responses to particular characteristics of SNFs, regardless of whether this was a central feature or related to their rehabilitation:
“The social worker comes and talks to me about the nursing home where cats are running around, you know, to infect my leg or spin their little cat hairs into my lungs and make my asthma worse…I’m going to have to beg the nurses or the aides or the family or somebody to clean the cat…” (Veteran, VA hospital)
Framing
Framing was the strongest theme among clinician interviews in our sample. Clinicians most frequently described the SNF as a place where patients could recover function (a positive frame), explaining risks (eg, rehospitalization) associated with alternative postacute care options besides the SNF in great detail.
“Aside from explaining the benefits of going and…having that 24-hour care, having the therapies provided to them [the patients], talking about them getting stronger, phrasing it in such a way that patients sometimes are more agreeable, like not calling it a skilled nursing facility, calling it a rehab you know, for them to get physically stronger so they can be the most independent that they can once they do go home, and also explaining … we think that this would be the best plan to prevent them from coming back to the hospital, so those are some of the things that we’ll mention to patients to try and educate them and get them to be agreeable for placement.” (Social worker, University hospital)
Clinicians avoided negative associations with “nursing home” (even though all SNFs are nursing homes) and tended to use more positive frames such as “rehabilitation facility.”
“Use the word rehab….we definitely use the word rehab, to get more therapy, to go home; it’s not a, we really emphasize it’s not a nursing home, it’s not to go to stay forever.” (Physical therapist, safety-net hospital)
Clinicians used a frame of “safety” when discussing the SNF and used a frame of “risk” when discussing alternative postacute care options such as returning home. We did not find examples of clinicians discussing similar risks in going to a SNF even for risks, such as falling, which exist in both settings.
“I’ve talked to them primarily on an avenue of safety because I think people want and they value independence, they value making sure they can get home, but you know, a lot of the times they understand safety is, it can be a concern and outlining that our goal is to make sure that they’re safe and they stay home, and I tend to broach the subject saying that our therapists believe that they might not be safe at home in the moment, but they have potential goals to be safe later on if we continue therapy. I really highlight safety being the major driver of our discussion.” (Physician, VA hospital)
In some cases, framing was so overt that other risk-mitigating options (eg, home healthcare) are not discussed.
“I definitely tend to explain the ideal first. I’m not going to bring up home care when we really think somebody should go to rehab, however, once people say I don’t want to do that, I’m not going, then that’s when I’m like OK, well, let’s talk to the doctors, but we can see about other supports in the home.” (Social worker, VA hospital)
DISCUSSION
In a large sample of patients and their caregivers, as well as multidisciplinary clinicians at three different hospitals and three SNFs, we found authority bias/halo effect and framing biases were most common and seemed most impactful. Default/status quo bias and anchoring bias were also present in decision-making about a SNF. The combination of authority bias/halo effect and framing biases could synergistically interact to augment the likelihood of patients accepting a SNF for postacute care. Patients who had been to a SNF before seemed more likely to choose the SNF they had experienced previously even if they had no other postacute care experiences, and could be highly influenced by isolated characteristics of that facility (such as the physical environment or cost of care).
It is important to mention that cognitive biases do not necessarily have a negative impact: indeed, as Kahneman and Tversky point out, these are useful heuristics from “fast” thinking that are often effective.24 For example, clinicians may be trying to act in the best interests of the patient when framing the decision in terms of regaining function and averting loss of safety and independence. However, the evidence base regarding the outcomes of an SNF versus other postacute options is not robust, and this decision-making is complex. While this decision was most commonly framed in terms of rehabilitation and returning home, the fact that only about half of patients have returned to the community by 100 days4 was not discussed in any interview. In fact, initial evidence suggests replacing the SNF with home healthcare in patients with hip and knee arthroplasty may reduce costs without worsening clinical outcomes.6 However, across a broader population, SNFs significantly reduce 30-day readmissions when directly compared with home healthcare, but other clinical outcomes are similar.25 This evidence suggests that the “right” postacute care option for an individual patient is not clear, highlighting a key role biases may play in decision-making. Further, the nebulous concept of “safety” could introduce potential disparities related to social determinants of health.12 The observed inclination to accept an SNF with which the individual had prior experience may be influenced by the acceptability of this choice because of personal factors or prior research, even if it also represents a bias by limiting the consideration of current alternatives.
Our findings complement those of others in the literature which have also identified profound gaps in discharge decision-making among patients and clinicians,13-16,26-31 though to our knowledge the role of cognitive biases in these decisions has not been explored. This study also addresses gaps in the cognitive bias literature, including the need for real-world data rather than hypothetical vignettes,17 and evaluation of treatment and management decisions rather than diagnoses, which have been more commonly studied.21
These findings have implications for both individual clinicians and healthcare institutions. In the immediate term, these findings may serve as a call to discharging clinicians to modulate language and “debias” their conversations with patients about care after discharge.18,22 Shared decision-making requires an informed choice by patients based on their goals and values; framing a decision in a way that puts the clinician’s goals or values (eg, safety) ahead of patient values (eg, independence and autonomy) or limits disclosure (eg, a “rehab” is a nursing home) in the hope of influencing choice may be more consistent with framing bias and less with shared decision-making.14 Although controversy exists about the best way to “debias” oneself,32 self-awareness of bias is increasingly recognized across healthcare venues as critical to improving care for vulnerable populations.33 The use of data rather than vignettes may be a useful debiasing strategy, although the limitations of currently available data (eg, capturing nursing home quality) are increasingly recognized.34 From a policy and health system perspective, cognitive biases should be integrated into the development of decision aids to facilitate informed, shared, and high-quality decision-making that incorporates patient values, and perhaps “nudges” from behavioral economics to assist patients in choosing the right postdischarge care for them. Such nudges use principles of framing to influence care without restricting choice.35 As the science informing best practice regarding postacute care improves, identifying the “right” postdischarge care may become easier and recommendations more evidence-based.36
Strengths of the study include a large, diverse sample of patients, caregivers, and clinicians in both the hospital and SNF setting. Also, we used a team-based analysis with an experienced team and a deep knowledge of the data, including triangulation with clinicians to verify results. However, all hospitals and SNFs were located in a single metropolitan area, and responses may vary by region or population density. All three hospitals have housestaff teaching programs, and at the time of the interviews all three community SNFs were “five-star” facilities on the Nursing Home Compare website; results may be different at community hospitals or other SNFs. Hospitalists were the only physician group sampled in the hospital as they provide the majority of inpatient care to older adults; geriatricians, in particular, may have had different perspectives. Since we intended to explore whether cognitive biases were present overall, we did not evaluate whether cognitive biases differed by role or subgroup (by clinician type, patient, or caregiver), but this may be a promising area to explore in future work. Many cognitive biases have been described, and there are likely additional biases we did not identify. To confirm the generalizability of these findings, they should be studied in a larger, more generalizable sample of respondents in future work.
Cognitive biases play an important role in patient decision-making about postacute care, particularly regarding SNF care. As postacute care undergoes a transformation spurred by payment reforms, it is more important than ever to ensure that patients understand their choices at hospital discharge and can make a high-quality decision consistent with their goals.
1. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. https://doi.org/10.1001/jamainternmed.2014.6383.
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. https://doi.org/10.1097/MLR.0000000000000359.
3. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617. https://doi.org/10.1001/jama.2018.2408.
4. Medicare Payment Advisory Commission June 2018 Report to Congress. http://www.medpac.gov/docs/default-source/reports/jun18_ch5_medpacreport_sec.pdf?sfvrsn=0. Accessed November 9, 2018.
5. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. https://doi.org/10.1002/jhm.2673.
6. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
7. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
8. Kennedy G, Lewis VA, Kundu S, Mousqués J, Colla CH. Accountable care organizations and post-acute care: a focus on preferred SNF networks. Med Care Res Rev MCRR. 2018;1077558718781117. https://doi.org/10.1177/1077558718781117.
9. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013;32(5):864-872. https://doi.org/10.1377/hlthaff.2012.1262.
10. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115.
11. Zhu JM, Patel V, Shea JA, Neuman MD, Werner RM. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff Proj Hope. 2018;37(8):1282-1289. https://doi.org/10.1377/hlthaff.2018.0257.
12. Burke RE, Ibrahim SA. Discharge destination and disparities in postoperative care. JAMA. 2018;319(16):1653-1654. https://doi.org/10.1001/jama.2017.21884.
13. Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017;65(11):2466-2472. https://doi.org/10.1111/jgs.14954.
14. Burke RE, Jones J, Lawrence E, et al. Evaluating the quality of patient decision-making regarding post-acute care. J Gen Intern Med. 2018;33(5):678-684. https://doi.org/10.1007/s11606-017-4298-1.
15. Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc. 2017;65(11):2459-2465. https://doi.org/10.1111/jgs.14988.
16. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff. 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155.
17. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Mak Int J Soc Med Decis Mak. 2015;35(4):539-557. https://doi.org/10.1177/0272989X14547740.
18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 Suppl 2:ii58-ii64. https://doi.org/10.1136/bmjqs-2012-001712.
19. Hinds PS, Vogel RJ, Clarke-Steffen L. The possibilities and pitfalls of doing a secondary analysis of a qualitative data set. Qual Health Res. 1997;7(3):408-424. https://doi.org/10.1177/104973239700700306.
20. Magid M, Mcllvennan CK, Jones J, et al. Exploring cognitive bias in destination therapy left ventricular assist device decision making: a retrospective qualitative framework analysis. Am Heart J. 2016;180:64-73. https://doi.org/10.1016/j.ahj.2016.06.024.
21. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138. https://doi.org/10.1186/s12911-016-0377-1.
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22 Suppl 2:ii65-ii72. https://doi.org/10.1136/bmjqs-2012-001713.
23. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
24. Thinking, Fast and Slow. Daniel Kahneman. Macmillan. US Macmillan. https://us.macmillan.com/thinkingfastandslow/danielkahneman/9780374533557. Accessed February 5, 2019.
25. Werner RM, Konetzka RT, Coe NB. Does type of post-acute care matter? The effect of hospital discharge to home with home health care versus to skilled nursing facility. JAMA Intern Med. In press.
26. Jones J, Lawrence E, Ladebue A, Leonard C, Ayele R, Burke RE. Nurses’ role in managing “The Fit” of older adults in skilled nursing facilities. J Gerontol Nurs. 2017;43(12):11-20. https://doi.org/10.3928/00989134-20171110-06.
27. Lawrence E, Casler J-J, Jones J, et al. Variability in skilled nursing facility screening and admission processes: implications for value-based purchasing. Health Care Manage Rev. 2018. https://doi.org/10.1097/HMR.0000000000000225.
28. Ayele R, Jones J, Ladebue A, et al. Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc. 2019. https://doi.org/10.1111/jgs.15768.
29. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. https://doi.org/10.3928/19404921-20160120-01.
30. Konetzka RT, Perraillon MC. Use of nursing home compare website appears limited by lack of awareness and initial mistrust of the data. Health Aff Proj Hope. 2016;35(4):706-713. https://doi.org/10.1377/hlthaff.2015.1377.
31. Schapira MM, Shea JA, Duey KA, Kleiman C, Werner RM. The nursing home compare report card: perceptions of residents and caregivers regarding quality ratings and nursing home choice. Health Serv Res. 2016;51 Suppl 2:1212-1228. https://doi.org/10.1111/1475-6773.12458.
32. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. https://doi.org/10.1136/bmjqs-2016-005267.
33. Masters C, Robinson D, Faulkner S, Patterson E, McIlraith T, Ansari A. Addressing biases in patient care with the 5Rs of cultural humility, a clinician coaching tool. J Gen Intern Med. 2019;34(4):627-630. https://doi.org/10.1007/s11606-018-4814-y.
34. Burke RE, Werner RM. Quality measurement and nursing homes: measuring what matters. BMJ Qual Saf. 2019;28(7);520-523. https://doi.org/10.1136/bmjqs-2019-009447.
35. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378(3):214-216. https://doi.org/10.1056/NEJMp1712984.
36. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med. 2015;175(2):296-297. https://doi.org/10.1001/jamainternmed.2014.4298.
1. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175(2):295-296. https://doi.org/10.1001/jamainternmed.2014.6383.
2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care. 2015;53(6):492-500. https://doi.org/10.1097/MLR.0000000000000359.
3. Werner RM, Konetzka RT. Trends in post-acute care use among medicare beneficiaries: 2000 to 2015. JAMA. 2018;319(15):1616-1617. https://doi.org/10.1001/jama.2018.2408.
4. Medicare Payment Advisory Commission June 2018 Report to Congress. http://www.medpac.gov/docs/default-source/reports/jun18_ch5_medpacreport_sec.pdf?sfvrsn=0. Accessed November 9, 2018.
5. Burke RE, Cumbler E, Coleman EA, Levy C. Post-acute care reform: implications and opportunities for hospitalists. J Hosp Med. 2017;12(1):46-51. https://doi.org/10.1002/jhm.2673.
6. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. https://doi.org/10.1001/jama.2016.12717.
7. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214-222. https://doi.org/10.1001/jamainternmed.2016.8263.
8. Kennedy G, Lewis VA, Kundu S, Mousqués J, Colla CH. Accountable care organizations and post-acute care: a focus on preferred SNF networks. Med Care Res Rev MCRR. 2018;1077558718781117. https://doi.org/10.1177/1077558718781117.
9. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013;32(5):864-872. https://doi.org/10.1377/hlthaff.2012.1262.
10. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115.
11. Zhu JM, Patel V, Shea JA, Neuman MD, Werner RM. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff Proj Hope. 2018;37(8):1282-1289. https://doi.org/10.1377/hlthaff.2018.0257.
12. Burke RE, Ibrahim SA. Discharge destination and disparities in postoperative care. JAMA. 2018;319(16):1653-1654. https://doi.org/10.1001/jama.2017.21884.
13. Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017;65(11):2466-2472. https://doi.org/10.1111/jgs.14954.
14. Burke RE, Jones J, Lawrence E, et al. Evaluating the quality of patient decision-making regarding post-acute care. J Gen Intern Med. 2018;33(5):678-684. https://doi.org/10.1007/s11606-017-4298-1.
15. Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc. 2017;65(11):2459-2465. https://doi.org/10.1111/jgs.14988.
16. Tyler DA, Gadbois EA, McHugh JP, Shield RR, Winblad U, Mor V. Patients are not given quality-of-care data about skilled nursing facilities when discharged from hospitals. Health Aff. 2017;36(8):1385-1391. https://doi.org/10.1377/hlthaff.2017.0155.
17. Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Mak Int J Soc Med Decis Mak. 2015;35(4):539-557. https://doi.org/10.1177/0272989X14547740.
18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 Suppl 2:ii58-ii64. https://doi.org/10.1136/bmjqs-2012-001712.
19. Hinds PS, Vogel RJ, Clarke-Steffen L. The possibilities and pitfalls of doing a secondary analysis of a qualitative data set. Qual Health Res. 1997;7(3):408-424. https://doi.org/10.1177/104973239700700306.
20. Magid M, Mcllvennan CK, Jones J, et al. Exploring cognitive bias in destination therapy left ventricular assist device decision making: a retrospective qualitative framework analysis. Am Heart J. 2016;180:64-73. https://doi.org/10.1016/j.ahj.2016.06.024.
21. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138. https://doi.org/10.1186/s12911-016-0377-1.
22. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22 Suppl 2:ii65-ii72. https://doi.org/10.1136/bmjqs-2012-001713.
23. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42(4):1758-1772. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
24. Thinking, Fast and Slow. Daniel Kahneman. Macmillan. US Macmillan. https://us.macmillan.com/thinkingfastandslow/danielkahneman/9780374533557. Accessed February 5, 2019.
25. Werner RM, Konetzka RT, Coe NB. Does type of post-acute care matter? The effect of hospital discharge to home with home health care versus to skilled nursing facility. JAMA Intern Med. In press.
26. Jones J, Lawrence E, Ladebue A, Leonard C, Ayele R, Burke RE. Nurses’ role in managing “The Fit” of older adults in skilled nursing facilities. J Gerontol Nurs. 2017;43(12):11-20. https://doi.org/10.3928/00989134-20171110-06.
27. Lawrence E, Casler J-J, Jones J, et al. Variability in skilled nursing facility screening and admission processes: implications for value-based purchasing. Health Care Manage Rev. 2018. https://doi.org/10.1097/HMR.0000000000000225.
28. Ayele R, Jones J, Ladebue A, et al. Perceived costs of care influence post-acute care choices by clinicians, patients, and caregivers. J Am Geriatr Soc. 2019. https://doi.org/10.1111/jgs.15768.
29. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. https://doi.org/10.3928/19404921-20160120-01.
30. Konetzka RT, Perraillon MC. Use of nursing home compare website appears limited by lack of awareness and initial mistrust of the data. Health Aff Proj Hope. 2016;35(4):706-713. https://doi.org/10.1377/hlthaff.2015.1377.
31. Schapira MM, Shea JA, Duey KA, Kleiman C, Werner RM. The nursing home compare report card: perceptions of residents and caregivers regarding quality ratings and nursing home choice. Health Serv Res. 2016;51 Suppl 2:1212-1228. https://doi.org/10.1111/1475-6773.12458.
32. Dhaliwal G. Premature closure? Not so fast. BMJ Qual Saf. 2017;26(2):87-89. https://doi.org/10.1136/bmjqs-2016-005267.
33. Masters C, Robinson D, Faulkner S, Patterson E, McIlraith T, Ansari A. Addressing biases in patient care with the 5Rs of cultural humility, a clinician coaching tool. J Gen Intern Med. 2019;34(4):627-630. https://doi.org/10.1007/s11606-018-4814-y.
34. Burke RE, Werner RM. Quality measurement and nursing homes: measuring what matters. BMJ Qual Saf. 2019;28(7);520-523. https://doi.org/10.1136/bmjqs-2019-009447.
35. Patel MS, Volpp KG, Asch DA. Nudge units to improve the delivery of health care. N Engl J Med. 2018;378(3):214-216. https://doi.org/10.1056/NEJMp1712984.
36. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med. 2015;175(2):296-297. https://doi.org/10.1001/jamainternmed.2014.4298.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Management of Acute Pancreatitis in the Pediatric Population
Pediatric acute pancreatitis is being diagnosed more commonly, affecting approximately one per 10,000 children annually with an estimated inpatient cost burden of $200 million per year.1,2 Common causes of pediatric acute pancreatitis include systemic illness, biliary disease, trauma, and medications; 13%-34% of cases are idiopathic.1 Currently, substantial variation exists in the clinical management of this condition.3,4 Hospitalists should familiarize themselves with the current literature, including the recent practice guideline by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN).2
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not graded, recommendation by expert consensus)
Recommendation 1. Diagnosis of acute pancreatitis in pediatric patients requires at least two of the following symptoms: abdominal pain compatible with acute pancreatitis, serum amylase and/or lipase values >3 times the upper limits of normal, and imaging findings consistent with acute pancreatitis.The most common symptoms of acute pancreatitis in children are epigastric or diffuse abdominal pain, vomiting, and irritability. Presentation varies by age, and diagnosis requires a high index of suspicion. Significant elevations in amylase and lipase levels are typically detected early in the disease course. The guideline does not specify a preferred serum biomarker in the diagnosis of pancreatitis but notes that lipase is more sensitive and specific than amylase, rises within 6 hours of symptoms, and stays elevated longer. Amylase levels rise faster but often normalize by 24 hours of symptom onset. Amylase and lipase can originate from extrapancreatic sources and may be elevated during acute illness in the absence of pancreatitis.
Laboratory testing to investigate the etiology of acute pancreatitis should include hepatic enzymes, bilirubin, triglyceride, and calcium levels. Although not typically necessary for diagnosis, imaging may demonstrate pancreatic edema or peripancreatic fluid, confirm disease complications, and identify obstructive causes. Transabdominal ultrasonography is indicated if biliary pancreatitis is suspected. Contrast-enhanced computed tomography should be considered for patients with severe presentation or deteriorating condition. Magnetic resonance cholangiopancreatography is useful in detecting pancreaticobiliary abnormalities.
Recommendation 2. Children with acute pancreatitis should be initially resuscitated with crystalloids, either with lactated Ringer’s or normal saline in the acute setting. These children should be provided 1.5-2 times maintenance intravenous fluids with monitoring of urine output over the next 24-48 hours.
Fluid resuscitation and maintenance are the current mainstays of therapy for pancreatitis. Prompt fluid administration corrects hypovolemia and may prevent potential complications. Early, aggressive fluid replacement in adults reduces the incidence of systemic inflammatory response syndrome and organ failure. Limited pediatric studies support correction of hypovolemia and/or circulatory compromise using 10-20 ml/kg boluses of isotonic crystalloid fluid. Although the literature is sparse regarding the rate of continued fluid replacement, the committee recommends patients receive 1.5-2 times maintenance intravenous fluid (IVF) with normal saline plus 5% dextrose for the first 24-48 hours. The rate of IVF administration should be adjusted based on volume status and urine output. IVF should be discontinued once the patient is able to maintain adequate hydration enterally. Cardiac, renal, and pulmonary complications of pancreatitis often present within the first 48 hours of illness and should prompt close monitoring with assessment of vital signs every four hours. The committee recommends monitoring serum electrolytes and renal function in the first 48 hours but does not offer guidance regarding the frequency of laboratory testing or the value of trending serum biomarkers.
Recommendation 3. Except in the presence of direct contraindications to use the gut, children with mild acute pancreatitis may benefit from early (within 48-72 hours of presentation) oral and enteral nutrition to decrease the length of stay (LOS) and the risk of organ dysfunction.
Adult studies suggest early enteral nutrition decreases complications and reduces LOS. Initiating enteral nutrition within 48 hours in children may have similar benefits. Several small pediatric studies have demonstrated a reduced LOS with early enteral feeds without an increase in complications. In a retrospective single-center study, children who were fed within the first 48 hours and received 1.5-2 times maintenance IVF had shorter LOS, less frequent intensive care admissions, and reduced severity of illness compared with those who were kept nil per os
Recommendation 4. Intravenous morphine or other opioids should be used for acute pancreatitis pain not responding to acetaminophen or nonsteroidal antiinflammatory drugs (NSAIDs).
Abdominal pain is the most common presenting symptom of pancreatitis, and pain control is an essential component of supportive care. There are no randomized trials identifying an optimal pain management regimen. The committee recommends the use of opioids for pain not controlled with acetaminophen and NSAIDs. Refractory pain may necessitate consultation with an acute pain specialist.
Recommendation 5. Routine use of prophylactic antibiotics, protease inhibitors, antioxidants, and probiotics is not recommended in acute pancreatitis.
Adult literature does not support routine use of antibiotics in acute pancreatitis, but their use may be beneficial in severe or recalcitrant cases. Pediatric literature neither confirms nor refutes this finding. The guideline does not recommend the use of antibiotics without signs of infection. Limited adult studies have shown protease inhibitors, antioxidants, and probiotics to be beneficial; however, no pediatric data support their use.
This guideline also discusses interventional and surgical procedures. Of note, biliary tract disease may necessitate endoscopic retrograde cholangiopancreatography or cholecystectomy. Such procedures should be considered in conjunction with subspecialty input.2
CRITIQUE
Methods in Preparing Guideline
The guideline development committee, funded by the NASPGHAN and the National Institutes of Diabetes and Digestive and Kidney Diseases, was composed of members of the NASPGHAN Pancreas Committee and included gastroenterologists from multiple sites.2 Topics were selected via group discussion, and Medline searches included both adult and pediatric literature. Preliminary recommendations were presented at the 2016 World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. Following revision, the 24 authors voted on each recommendation using a five-point Likert scale. A recommendation passed if 75% of the participants either agreed or strongly agreed with it. The authors reported no conflicts of interest.
Although the literature review was comprehensive, it lacked prospective pediatric studies and many of the recommendations were derived from adult research. The committee originally intended to grade the quality of evidence; however, the pediatric specific literature was underpowered and retrospective. Therefore, the committee opted to use consensus voting. The authors note that had the group used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, it would have returned grades of “low” or “very low” quality evidence.2 The Hungarian Pancreatic Study Group and the European Pancreatic Club published a consensus guideline on the management of pediatric acute pancreatitis shortly after the NASPGHAN guideline, which offers similar conclusions.2,6 The strength and generalizability of the NASPGHAN guideline are limited by its overreliance on adult literature, expert consensus, and small, retrospective pediatric studies to guide care.
AREAS OF FURTHER STUDY
This guideline highlights the need for pediatric research to guide the management of acute pancreatitis. The etiologies of pancreatitis in children are distinct from adults, where alcohol abuse and biliary disease are significant contributors.1 Furthermore, age and environmental factors influence the presentation and clinical course.1 Robust, prospective studies are needed to better understand the treatment outcomes of pediatric pancreatitis. Areas of further research include pediatric pancreatic severity scoring, ideal fluid composition and administration rate, enteral feed timing, optimal pain control, laboratory monitoring frequency, and adjuvant therapies.
Disclosures
Dr. Wall has nothing to disclose.
1. Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr. 2011;52(3):262–270. https://doi.org/10.1097/MPG.0b013e3182061d75.
2. Abu-El-Haija M, Kumar S, et al. The management of acute pancreatitis in the pediatric population: a clinical report from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition pancreas committee. J Pediatr Gastroenterol Nutr. 2018;66(1):159-176. https://doi.org/ 10.1097/MPG.0000000000001715.
3. Szabo, FK, Palermo, J, et al. Comparison of length of hospital stay of children admitted with acute pancreatitis among hospital services at a single pediatric tertiary care center [AGA Abstract Tu1144]. Gastroenterology. 2014;146(5):S–765. https://doi.org/10.1016/S0016-5085(14)62765-7.
4. Abu-El-Haija M, Lin TK, Palermo J. Update to the management of pediatric acute pancreatitis: highlighting areas in need of research. J Pediatr Gastroenterol Nutr. 2014;58:689–693. https://doi.org/ 10.1097/MPG.0000000000000360.
5. Szabo FK, Fei L, Cruz LA, et al. Early enteral nutrition and aggressive fluid resuscitation are associated with improved clinical outcomes in acute pancreatitis. J Pediatr. 2015;167(2):397–402e1. https://doi.org/10.1016/j.jpeds.2015.05.030.
6. Párniczky A, Abu-El-Haija M, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18(2):146-160. https://doi.org/10.1016/j.pan.2018.01.001.
Pediatric acute pancreatitis is being diagnosed more commonly, affecting approximately one per 10,000 children annually with an estimated inpatient cost burden of $200 million per year.1,2 Common causes of pediatric acute pancreatitis include systemic illness, biliary disease, trauma, and medications; 13%-34% of cases are idiopathic.1 Currently, substantial variation exists in the clinical management of this condition.3,4 Hospitalists should familiarize themselves with the current literature, including the recent practice guideline by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN).2
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not graded, recommendation by expert consensus)
Recommendation 1. Diagnosis of acute pancreatitis in pediatric patients requires at least two of the following symptoms: abdominal pain compatible with acute pancreatitis, serum amylase and/or lipase values >3 times the upper limits of normal, and imaging findings consistent with acute pancreatitis.The most common symptoms of acute pancreatitis in children are epigastric or diffuse abdominal pain, vomiting, and irritability. Presentation varies by age, and diagnosis requires a high index of suspicion. Significant elevations in amylase and lipase levels are typically detected early in the disease course. The guideline does not specify a preferred serum biomarker in the diagnosis of pancreatitis but notes that lipase is more sensitive and specific than amylase, rises within 6 hours of symptoms, and stays elevated longer. Amylase levels rise faster but often normalize by 24 hours of symptom onset. Amylase and lipase can originate from extrapancreatic sources and may be elevated during acute illness in the absence of pancreatitis.
Laboratory testing to investigate the etiology of acute pancreatitis should include hepatic enzymes, bilirubin, triglyceride, and calcium levels. Although not typically necessary for diagnosis, imaging may demonstrate pancreatic edema or peripancreatic fluid, confirm disease complications, and identify obstructive causes. Transabdominal ultrasonography is indicated if biliary pancreatitis is suspected. Contrast-enhanced computed tomography should be considered for patients with severe presentation or deteriorating condition. Magnetic resonance cholangiopancreatography is useful in detecting pancreaticobiliary abnormalities.
Recommendation 2. Children with acute pancreatitis should be initially resuscitated with crystalloids, either with lactated Ringer’s or normal saline in the acute setting. These children should be provided 1.5-2 times maintenance intravenous fluids with monitoring of urine output over the next 24-48 hours.
Fluid resuscitation and maintenance are the current mainstays of therapy for pancreatitis. Prompt fluid administration corrects hypovolemia and may prevent potential complications. Early, aggressive fluid replacement in adults reduces the incidence of systemic inflammatory response syndrome and organ failure. Limited pediatric studies support correction of hypovolemia and/or circulatory compromise using 10-20 ml/kg boluses of isotonic crystalloid fluid. Although the literature is sparse regarding the rate of continued fluid replacement, the committee recommends patients receive 1.5-2 times maintenance intravenous fluid (IVF) with normal saline plus 5% dextrose for the first 24-48 hours. The rate of IVF administration should be adjusted based on volume status and urine output. IVF should be discontinued once the patient is able to maintain adequate hydration enterally. Cardiac, renal, and pulmonary complications of pancreatitis often present within the first 48 hours of illness and should prompt close monitoring with assessment of vital signs every four hours. The committee recommends monitoring serum electrolytes and renal function in the first 48 hours but does not offer guidance regarding the frequency of laboratory testing or the value of trending serum biomarkers.
Recommendation 3. Except in the presence of direct contraindications to use the gut, children with mild acute pancreatitis may benefit from early (within 48-72 hours of presentation) oral and enteral nutrition to decrease the length of stay (LOS) and the risk of organ dysfunction.
Adult studies suggest early enteral nutrition decreases complications and reduces LOS. Initiating enteral nutrition within 48 hours in children may have similar benefits. Several small pediatric studies have demonstrated a reduced LOS with early enteral feeds without an increase in complications. In a retrospective single-center study, children who were fed within the first 48 hours and received 1.5-2 times maintenance IVF had shorter LOS, less frequent intensive care admissions, and reduced severity of illness compared with those who were kept nil per os
Recommendation 4. Intravenous morphine or other opioids should be used for acute pancreatitis pain not responding to acetaminophen or nonsteroidal antiinflammatory drugs (NSAIDs).
Abdominal pain is the most common presenting symptom of pancreatitis, and pain control is an essential component of supportive care. There are no randomized trials identifying an optimal pain management regimen. The committee recommends the use of opioids for pain not controlled with acetaminophen and NSAIDs. Refractory pain may necessitate consultation with an acute pain specialist.
Recommendation 5. Routine use of prophylactic antibiotics, protease inhibitors, antioxidants, and probiotics is not recommended in acute pancreatitis.
Adult literature does not support routine use of antibiotics in acute pancreatitis, but their use may be beneficial in severe or recalcitrant cases. Pediatric literature neither confirms nor refutes this finding. The guideline does not recommend the use of antibiotics without signs of infection. Limited adult studies have shown protease inhibitors, antioxidants, and probiotics to be beneficial; however, no pediatric data support their use.
This guideline also discusses interventional and surgical procedures. Of note, biliary tract disease may necessitate endoscopic retrograde cholangiopancreatography or cholecystectomy. Such procedures should be considered in conjunction with subspecialty input.2
CRITIQUE
Methods in Preparing Guideline
The guideline development committee, funded by the NASPGHAN and the National Institutes of Diabetes and Digestive and Kidney Diseases, was composed of members of the NASPGHAN Pancreas Committee and included gastroenterologists from multiple sites.2 Topics were selected via group discussion, and Medline searches included both adult and pediatric literature. Preliminary recommendations were presented at the 2016 World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. Following revision, the 24 authors voted on each recommendation using a five-point Likert scale. A recommendation passed if 75% of the participants either agreed or strongly agreed with it. The authors reported no conflicts of interest.
Although the literature review was comprehensive, it lacked prospective pediatric studies and many of the recommendations were derived from adult research. The committee originally intended to grade the quality of evidence; however, the pediatric specific literature was underpowered and retrospective. Therefore, the committee opted to use consensus voting. The authors note that had the group used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, it would have returned grades of “low” or “very low” quality evidence.2 The Hungarian Pancreatic Study Group and the European Pancreatic Club published a consensus guideline on the management of pediatric acute pancreatitis shortly after the NASPGHAN guideline, which offers similar conclusions.2,6 The strength and generalizability of the NASPGHAN guideline are limited by its overreliance on adult literature, expert consensus, and small, retrospective pediatric studies to guide care.
AREAS OF FURTHER STUDY
This guideline highlights the need for pediatric research to guide the management of acute pancreatitis. The etiologies of pancreatitis in children are distinct from adults, where alcohol abuse and biliary disease are significant contributors.1 Furthermore, age and environmental factors influence the presentation and clinical course.1 Robust, prospective studies are needed to better understand the treatment outcomes of pediatric pancreatitis. Areas of further research include pediatric pancreatic severity scoring, ideal fluid composition and administration rate, enteral feed timing, optimal pain control, laboratory monitoring frequency, and adjuvant therapies.
Disclosures
Dr. Wall has nothing to disclose.
Pediatric acute pancreatitis is being diagnosed more commonly, affecting approximately one per 10,000 children annually with an estimated inpatient cost burden of $200 million per year.1,2 Common causes of pediatric acute pancreatitis include systemic illness, biliary disease, trauma, and medications; 13%-34% of cases are idiopathic.1 Currently, substantial variation exists in the clinical management of this condition.3,4 Hospitalists should familiarize themselves with the current literature, including the recent practice guideline by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN).2
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not graded, recommendation by expert consensus)
Recommendation 1. Diagnosis of acute pancreatitis in pediatric patients requires at least two of the following symptoms: abdominal pain compatible with acute pancreatitis, serum amylase and/or lipase values >3 times the upper limits of normal, and imaging findings consistent with acute pancreatitis.The most common symptoms of acute pancreatitis in children are epigastric or diffuse abdominal pain, vomiting, and irritability. Presentation varies by age, and diagnosis requires a high index of suspicion. Significant elevations in amylase and lipase levels are typically detected early in the disease course. The guideline does not specify a preferred serum biomarker in the diagnosis of pancreatitis but notes that lipase is more sensitive and specific than amylase, rises within 6 hours of symptoms, and stays elevated longer. Amylase levels rise faster but often normalize by 24 hours of symptom onset. Amylase and lipase can originate from extrapancreatic sources and may be elevated during acute illness in the absence of pancreatitis.
Laboratory testing to investigate the etiology of acute pancreatitis should include hepatic enzymes, bilirubin, triglyceride, and calcium levels. Although not typically necessary for diagnosis, imaging may demonstrate pancreatic edema or peripancreatic fluid, confirm disease complications, and identify obstructive causes. Transabdominal ultrasonography is indicated if biliary pancreatitis is suspected. Contrast-enhanced computed tomography should be considered for patients with severe presentation or deteriorating condition. Magnetic resonance cholangiopancreatography is useful in detecting pancreaticobiliary abnormalities.
Recommendation 2. Children with acute pancreatitis should be initially resuscitated with crystalloids, either with lactated Ringer’s or normal saline in the acute setting. These children should be provided 1.5-2 times maintenance intravenous fluids with monitoring of urine output over the next 24-48 hours.
Fluid resuscitation and maintenance are the current mainstays of therapy for pancreatitis. Prompt fluid administration corrects hypovolemia and may prevent potential complications. Early, aggressive fluid replacement in adults reduces the incidence of systemic inflammatory response syndrome and organ failure. Limited pediatric studies support correction of hypovolemia and/or circulatory compromise using 10-20 ml/kg boluses of isotonic crystalloid fluid. Although the literature is sparse regarding the rate of continued fluid replacement, the committee recommends patients receive 1.5-2 times maintenance intravenous fluid (IVF) with normal saline plus 5% dextrose for the first 24-48 hours. The rate of IVF administration should be adjusted based on volume status and urine output. IVF should be discontinued once the patient is able to maintain adequate hydration enterally. Cardiac, renal, and pulmonary complications of pancreatitis often present within the first 48 hours of illness and should prompt close monitoring with assessment of vital signs every four hours. The committee recommends monitoring serum electrolytes and renal function in the first 48 hours but does not offer guidance regarding the frequency of laboratory testing or the value of trending serum biomarkers.
Recommendation 3. Except in the presence of direct contraindications to use the gut, children with mild acute pancreatitis may benefit from early (within 48-72 hours of presentation) oral and enteral nutrition to decrease the length of stay (LOS) and the risk of organ dysfunction.
Adult studies suggest early enteral nutrition decreases complications and reduces LOS. Initiating enteral nutrition within 48 hours in children may have similar benefits. Several small pediatric studies have demonstrated a reduced LOS with early enteral feeds without an increase in complications. In a retrospective single-center study, children who were fed within the first 48 hours and received 1.5-2 times maintenance IVF had shorter LOS, less frequent intensive care admissions, and reduced severity of illness compared with those who were kept nil per os
Recommendation 4. Intravenous morphine or other opioids should be used for acute pancreatitis pain not responding to acetaminophen or nonsteroidal antiinflammatory drugs (NSAIDs).
Abdominal pain is the most common presenting symptom of pancreatitis, and pain control is an essential component of supportive care. There are no randomized trials identifying an optimal pain management regimen. The committee recommends the use of opioids for pain not controlled with acetaminophen and NSAIDs. Refractory pain may necessitate consultation with an acute pain specialist.
Recommendation 5. Routine use of prophylactic antibiotics, protease inhibitors, antioxidants, and probiotics is not recommended in acute pancreatitis.
Adult literature does not support routine use of antibiotics in acute pancreatitis, but their use may be beneficial in severe or recalcitrant cases. Pediatric literature neither confirms nor refutes this finding. The guideline does not recommend the use of antibiotics without signs of infection. Limited adult studies have shown protease inhibitors, antioxidants, and probiotics to be beneficial; however, no pediatric data support their use.
This guideline also discusses interventional and surgical procedures. Of note, biliary tract disease may necessitate endoscopic retrograde cholangiopancreatography or cholecystectomy. Such procedures should be considered in conjunction with subspecialty input.2
CRITIQUE
Methods in Preparing Guideline
The guideline development committee, funded by the NASPGHAN and the National Institutes of Diabetes and Digestive and Kidney Diseases, was composed of members of the NASPGHAN Pancreas Committee and included gastroenterologists from multiple sites.2 Topics were selected via group discussion, and Medline searches included both adult and pediatric literature. Preliminary recommendations were presented at the 2016 World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. Following revision, the 24 authors voted on each recommendation using a five-point Likert scale. A recommendation passed if 75% of the participants either agreed or strongly agreed with it. The authors reported no conflicts of interest.
Although the literature review was comprehensive, it lacked prospective pediatric studies and many of the recommendations were derived from adult research. The committee originally intended to grade the quality of evidence; however, the pediatric specific literature was underpowered and retrospective. Therefore, the committee opted to use consensus voting. The authors note that had the group used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system, it would have returned grades of “low” or “very low” quality evidence.2 The Hungarian Pancreatic Study Group and the European Pancreatic Club published a consensus guideline on the management of pediatric acute pancreatitis shortly after the NASPGHAN guideline, which offers similar conclusions.2,6 The strength and generalizability of the NASPGHAN guideline are limited by its overreliance on adult literature, expert consensus, and small, retrospective pediatric studies to guide care.
AREAS OF FURTHER STUDY
This guideline highlights the need for pediatric research to guide the management of acute pancreatitis. The etiologies of pancreatitis in children are distinct from adults, where alcohol abuse and biliary disease are significant contributors.1 Furthermore, age and environmental factors influence the presentation and clinical course.1 Robust, prospective studies are needed to better understand the treatment outcomes of pediatric pancreatitis. Areas of further research include pediatric pancreatic severity scoring, ideal fluid composition and administration rate, enteral feed timing, optimal pain control, laboratory monitoring frequency, and adjuvant therapies.
Disclosures
Dr. Wall has nothing to disclose.
1. Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr. 2011;52(3):262–270. https://doi.org/10.1097/MPG.0b013e3182061d75.
2. Abu-El-Haija M, Kumar S, et al. The management of acute pancreatitis in the pediatric population: a clinical report from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition pancreas committee. J Pediatr Gastroenterol Nutr. 2018;66(1):159-176. https://doi.org/ 10.1097/MPG.0000000000001715.
3. Szabo, FK, Palermo, J, et al. Comparison of length of hospital stay of children admitted with acute pancreatitis among hospital services at a single pediatric tertiary care center [AGA Abstract Tu1144]. Gastroenterology. 2014;146(5):S–765. https://doi.org/10.1016/S0016-5085(14)62765-7.
4. Abu-El-Haija M, Lin TK, Palermo J. Update to the management of pediatric acute pancreatitis: highlighting areas in need of research. J Pediatr Gastroenterol Nutr. 2014;58:689–693. https://doi.org/ 10.1097/MPG.0000000000000360.
5. Szabo FK, Fei L, Cruz LA, et al. Early enteral nutrition and aggressive fluid resuscitation are associated with improved clinical outcomes in acute pancreatitis. J Pediatr. 2015;167(2):397–402e1. https://doi.org/10.1016/j.jpeds.2015.05.030.
6. Párniczky A, Abu-El-Haija M, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18(2):146-160. https://doi.org/10.1016/j.pan.2018.01.001.
1. Bai HX, Lowe ME, Husain SZ. What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr. 2011;52(3):262–270. https://doi.org/10.1097/MPG.0b013e3182061d75.
2. Abu-El-Haija M, Kumar S, et al. The management of acute pancreatitis in the pediatric population: a clinical report from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition pancreas committee. J Pediatr Gastroenterol Nutr. 2018;66(1):159-176. https://doi.org/ 10.1097/MPG.0000000000001715.
3. Szabo, FK, Palermo, J, et al. Comparison of length of hospital stay of children admitted with acute pancreatitis among hospital services at a single pediatric tertiary care center [AGA Abstract Tu1144]. Gastroenterology. 2014;146(5):S–765. https://doi.org/10.1016/S0016-5085(14)62765-7.
4. Abu-El-Haija M, Lin TK, Palermo J. Update to the management of pediatric acute pancreatitis: highlighting areas in need of research. J Pediatr Gastroenterol Nutr. 2014;58:689–693. https://doi.org/ 10.1097/MPG.0000000000000360.
5. Szabo FK, Fei L, Cruz LA, et al. Early enteral nutrition and aggressive fluid resuscitation are associated with improved clinical outcomes in acute pancreatitis. J Pediatr. 2015;167(2):397–402e1. https://doi.org/10.1016/j.jpeds.2015.05.030.
6. Párniczky A, Abu-El-Haija M, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18(2):146-160. https://doi.org/10.1016/j.pan.2018.01.001.
© 2019 Society of Hospital Medicine