User login
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Measles
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
© 2020 Society of Hospital Medicine
A Plea to Reconsider the Diagnosis
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
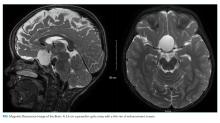
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
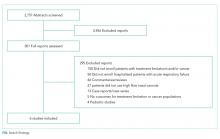
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
© 2019 Society of Hospital Medicine
Utility of ICD Codes for Stress Cardiomyopathy in Hospital Administrative Databases: What Do They Signify?
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
© 2020 Society of Hospital Medicine
Prediction of Disposition Within 48 Hours of Hospital Admission Using Patient Mobility Scores
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
© 2019 Society of Hospital Medicine
Recurrent Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema Refractory to Fresh Frozen Plasma
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
Millennials in Medicine: Cross-Trained Physicians Not Valued in Medical Marketplace
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
Millennials, defined as those born between 1981 and 1996, currently comprise 15% of all active physicians in the US.1,2 A recent survey found that nearly 4 of 5 US millennial physicians have a desire for cross-sectional work in areas beyond patient care, such as academic research, health care consulting, entrepreneurship, and health care administration.3
For employers and educators, a better understanding of these preferences, through consideration of the unique education and skill set of the millennial physician workforce, may lead to more effective recruitment of young physicians and improved health systems, avoiding a mismatch between health care provider skills and available jobs that can be costly for both employers and employees.4
This article describes how US millennial physicians are choosing to cross-train (obtaining multiple degrees and/or completing combined medical residency training) throughout undergraduate, medical, and graduate medical education. We also outline ways in which the current physician marketplace may not match the skills of this population and suggest some ways that health care organizations could capitalize on this trend toward more cross-trained personnel in order to effectively recruit and retain the next generation of physicians.
Millennial Education
Undergraduates
The number of interdisciplinary undergraduate majors increased by almost 250% from 1975 to 2000.5 In 2010, nearly 20% of US college students graduated with 2 majors, representing a 70% increase in double majors between 2001 and 2011.6,7 One emerging category of interdisciplinary majors in US colleges is health humanities programs, which have quadrupled since 2000.8
Medical school applicants and matriculants reflect this trend. Whereas in 1994, only 19% of applicants to medical school held nonscience degrees, about one-third of applicants now hold such degrees.9,10 We have found no aggregated data on double majors entering US medical schools, but public class profiles suggest that medical school matriculants mirror their undergraduate counterparts in their tendency to hold double majors. In 2016, for example, 15% of the incoming class at the University of Michigan Medical School was composed of double majors, increasing to over 25% in 2017.11
Medical Students
Early dual-degree programs in undergraduate medical training were reserved for MD/PhD programs.12 Most US MD/PhD programs (90 out of 151) now offer doctorates in social sciences, humanities, or other nontraditional fields of graduate medical study, reflecting a shift in interests of those seeking dual-degree training in undergraduate medical education.13 While only 3 MD/PhD programs in the 1970s included trainees in the social sciences, 17 such programs exist today.14
Interest in dual-degree programs offering master’s level study has also increased over the past decade. In 2017, 87 medical schools offered programs for students to pursue a master of public health (MPH) and 41 offered master of science degrees in various fields, up from 52 and 37 institutions, respectively in 2006.15 The number of schools offering combined training in nonscience fields has also grown, with 63 institutions now offering a master of business administration (MBA), nearly double the number offered in 2006.15 At some institutions more than 20% of students are earning a master’s degree or doctorate in addition to their MD degree.16
Residents
The authors found no documentation of US residency training programs, outside of those in the specialty of preventive medicine, providing trainees with formal opportunities to obtain an MBA or MPH prior to 2001.17 However, of the 510 internal medicine residency programs listed on the American Medical Association residency and fellowship database (freida.ama-assn.org), 45 identified as having established a pathway for residents to pursue an MBA, MPH, or PhD during residency.18
Over the past 20 years, combined residency programs have increased 49% (from 128 to 191), which is triple the 16% rate (1,350 to 1,562) of increase in programs in internal medicine, pediatrics, family medicine, psychiatry, and emergency medicine.19,20 A 2009 moratorium on the creation of new combined residency programs in psychiatry and neurology was lifted in 2016and is likely to increase the rate of total combined programs.21
The Table shows the number of categorical and combined residency programs available in 1996 and in 2016. Over 2 decades, 17 new specialty combinations became available for residency training. While there were no combined training programs within these 17 new combinations in 1996,there were 66 programs with these combinations in 2016.19,20
Although surgical specialties are notably absent from the list of combined residency options, likely due to the duration of surgical training, some surgical training programs do offer pathways that culminate in combined degrees,22 and a high number of surgery program directors agree that residents should receive formal training in business and practice management.23
The Medical Job Market
Although today’s young physicians are cross-trained in multiple disciplines, the current job market may not directly match these skill sets. Of the 7,235 jobs listed by the New England Journal of Medicine (NEJM) career center (www.nejmcareercenter.org/jobs), only 54 were targeted at those with combined training, the majority of which were aimed at those trained in internal medicine/pediatrics. Of the combined specialties in the Table, formal positions were listed for only 6.24 A search of nearly 1,500 federal medical positions on USAJOBS (www.usajobs.gov) found only 4 jobs that combined specialties, all restricted to internal medicine/pediatrics.25 When searching for jobs containing the terms MBA, MPH, and public health there were only 8 such positions on NEJM and 7 on USAJOBS.24,25 Although the totality of the medical marketplace may not be best encompassed by these sources, the authors believe NEJM and USAJOBS are somewhat representative of the opportunities for physicians in the US.
Medical jobs tailored to cross-trained physicians do not appear to have kept pace with the numbers of such specialists currently in medical school and residency training. Though millennials are cross-training in increasing numbers, we surmise that they are not doing so as a direct result of the job market.
Future Medicine
Regardless of the mismatch between cross-trained physicians and the current job market, millennials may be well suited for future health systems. In 2001, the National Academies of Sciences, Engineering and Medicine (NASEM) called for increasing interdisciplinary training and improving cross-functional team performance as a major goal for health care providers in twenty-first century health systems.26 NASEM also recommended that academic medical centers develop medical leaders who can manage systems changes required to enhance health, a proposal supported by the fact that hospitals with medically trained CEOs outperform others.27,28
Public Health 3.0, a federal initiative to improve and integrate public health efforts, also emphasizes cross-disciplinary teams and cross-sector partnerships,29 while the Centers for Medicare and Medicaid Services (CMS) has incentivized the development of interprofessional health care teams.30 While cross-training does not automatically connote interdisciplinary training, we believe that cross-training may reveal or develop an interdisciplinary mind-set that may support and embrace interdisciplinary performance. Finally, the US Department of Health and Human Services’ (HHS) Strategic Goals emphasize integrated care for vulnerable populations, something that cross-trained physicians may be especially poised to accomplish.31
A Path Forward
The education, training, and priorities of young physicians demonstrates career interests that diverge from mainstream, traditional options. Data provided herein describe the increasing rates at which millennial physicians are cross-training and have suggested that the current marketplace may not match the interests of this population. The ultimate question is where such cross-trained physicians fit into today’s (or tomorrow’s) health system?
It may be easiest to deploy cross-trained physicians in their respective clinical departments (eg, having a physician trained in internal medicine and pediatrics perform clinical duties in both a medicine department and a pediatrics department). But < 40% of dual-boarded physicians practice both specialties in which they’re trained, so other opportunities should be pursued.32,33 One strategy may be to embrace the promise of interdisciplinary care, as supported by Public Health 3.0 and NASEM.26,29 Our evidence may demonstrate that the interdisciplinary mind-set may be more readily evident in the millennial generation, and that this mind-set may improve interdisciplinary care.
As health is impacted both by direct clinical care as well as programs designed to address population health, cross-trained physicians may be better equipped to integrate aspects of clinical care spanning a variety of clinical fields as well as orchestrating programs designed to improve health at the population level. This mind-set may be best captured by organizations willing to adapt their medical positions to emphasize multidisciplinary training, skills, and capabilities. For example, a physician trained in internal medicine and psychiatry may have the unique training and skill-set to establish an integrated behavioral health clinic that crosses boundaries between traditional departments, emphasizing the whole health of the clinic’s population and not simply focusing on providing services of a particular specialty. Hiring cross-trained physicians throughout such a clinic may benefit the operations of the clinic and improve not only the services provided, but ultimately, the health of that clinic’s patients. By embracing cross-trained physicians, health care organizations and educators may better meet the needs of their employees, likely resulting in a more cost-effective investment for employers, employees, and the health system as a whole.4 Additionally, patient health may also improve.
There is evidence that cross-trained physicians are already likely to hold leadership positions compared with their categorically-trained counterparts, and this may reflect the benefits of an interdisciplinary mind-set.33 Perhaps a cross-trained physician is more likely to see beyond standard, specialty-based institutional barriers and develop processes and programs designed for overall patient benefit. Leadership is a skill that many millennials clearly wish to enhance throughout their career.34 Recruiting cross-trained physicians for leadership positions may reveal synergies between such training and an ability to lead health care organizations into the future.
Many millennial physicians are bringing a new set of skills into the medical marketplace. Health organizations should identify ways to recruit for these skills and deploy them within their systems in order to have more dedicated, engaged employees, more effective health systems, and ultimately, healthier patients.
Acknowledgments
Data from this analysis were presented at the 10th Consortium of Universities for Global Health conference in 2019.35
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
1. Dimock M. Defining generations: where millennials end and generation Z begins. http://www.pewresearch.org/fact-tank/2018/03/01/defining-generations-where-millennials-end-and-post-millennials-begin/. Published January 17, 2019. Accessed November 7, 2019.
2. IHS Inc. The complexities of physician supply and demand: projections from 2014 to 2025. Final report. https://www.modernhealthcare.com/assets/pdf/CH10888123.pdf. Published April 5, 2016. Accessed November 7, 2019.
3. Miller RN. Millennial physicians sound off on state of medicine today. https://wire.ama-assn.org/life-career/millennial-physicians-sound-state-medicine-today. Published March 27, 2017. Accessed November 7, 2019.
4. World Economic Forum. Matching skills and labour market needs: building social partnerships for better skills and better jobs. http://www3.weforum.org/docs/GAC/2014/WEF_GAC_Employment_MatchingSkillsLabourMarket_Report_2014.pdf. Published January 2014. Accessed November 7, 2019.
5. Brint SG, Turk-Bicakci L, Proctor K, Murphy SP. Expanding the social frame of knowledge: interdisciplinary, degree-granting fields in American Colleges and Universities, 1975–2000. Rev High Ed. 2009;32(2):155-183.
6. National Science Foundation. National survey of college graduates. https://www.nsf.gov/statistics/srvygrads. Updated February 2019. Accessed November 7, 2019.
7. Simon CC. Major decisions. New York Times. November 2, 2012. http://www.nytimes.com/2012/11/04/education/edlife/choosing-one-college-major-out-of-hundreds.html. Accessed November 7, 2019.
8. Berry SL, Erin GL, Therese J. Health humanities baccalaureate programs in the United States. http://www.hiram.edu/wp-content/uploads/2017/09/HHBP2017.pdf. Published September 2017. Accessed November 7, 2019.
9. Sorensen NE, Jackson JR. Science majors and nonscience majors entering medical school: acceptance rates and academic performance. NACADA J. 1997;17(1):32-41.
10. Association of American Medical Colleges. Table A-17: MCAT and GPAs for applicants and matriculants to U.S. medical schools by primary undergraduate major, 2019-2020. https://www.aamc.org/download/321496/data/factstablea17.pdf. Published October 16, 2019. Accessed November 7, 2019.
11. University of Michigan Medical School. Many paths, one destination: medical school welcomes its 170th class of medical students. https://medicine.umich.edu/medschool/news/many-paths-one-destination-medical-school-welcomes-its-170th-class-medical-students. Updated July 29, 2016. Accessed November 7, 2019.
12. Harding CV, Akabas MH, Andersen OS. History and outcomes of 50 years of physician-scientist training in medical scientist training programs. Acad Med. 2017; 92(10):1390-1398.
13. Association of American Medical Colleges. MD-PhD in “social sciences or humanities” and “other non-traditional fields of graduate study” - by school. https://students-residents.aamc.org/choosing-medical-career/careers-medical-research/md-phd-dual-degree-training/non-basic-science-phd-training-school/. Accessed November 8, 2019.
14. Holmes SM, Karlin J, Stonington SD, Gottheil DL. The first nationwide survey of MD-PhDs in the social sciences and humanities: training patterns and career choices. BMC Med Educ. 2017;17(1):60.
15. Association of American Medical Colleges Combined degrees and early acceptance programs. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/combined-degrees-and-early-acceptance-programs. Accessed November 8, 2019.
16. Tufts University School of Medicine. 2023 class profile. http://medicine.tufts.edu/Education/MD-Programs/Doctor-of-Medicine/Class-Profile. Published 2015. Accessed November 8, 2019.
17. Zweifler J, Evan R. Development of a residency/MPH program. Family Med. 2001;33(6):453-458.
18. American Medical Association. The AMA residency and fellowship database. http://freida.ama-assn.org/Freida. Accessed November 7, 2019.
19. National Resident Matching Program. NRMP data. http://www.nrmp.org/wp-content/uploads/2013/08/resultsanddata1996.pdf. Published March 1996. Accessed November 7, 2019.
20. Brotherton SE, Etzel SI. Graduate medical education, 2016-2017. JAMA. 2017;318(23):2368-2387.
21. American Board of Psychiatry and Neurology. Update for psychiatry GME programs on combined training program accreditation/approval February 2012. https://www.umassmed.edu/globalassets/neuropsychiatry/files/combined-program-letter.pdf. Accessed November 7, 2019.
22. Massachusetts General Hospital. Surgical residency program. https://www.massgeneral.org/surgery/education/residency.aspx?id=77. Accessed November 7, 2019.
23. Lusco VC, Martinez SA, Polk HC Jr. Program directors in surgery agree that residents should be formally trained in business and practice management. Am J Surg. 2005;189(1):11-13.
24. New England Journal of Medicine. NEJM CareerCenter. http://www.nejmcareercenter.org. Accessed November 7, 2019.
25. US Office of Personnel Management. USAJOBS. https://www.usajobs.gov. Accessed November 7, 2019.
26. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Published March 2001. Accessed November 7, 2019.
27. Kohn LT, ed; Committee on the Roles of Academic Health Centers in the 21st Century; Institute of Medicine of the National Academies. Academic Health Centers: Leading Change in the 21st Century. National Academy Press: Washington, DC; 2004.
28. Goodall AH. Physician-leaders and hospital performance: is there an association? http://ftp.iza.org/dp5830.pdf. Published July 2011. Accessed November 7, 2019.
29. US Department of Health and Human Services, Office of the Assistant Secretary for Health. Public health 3.0: a call to action to create a 21st century public health infrastructure. https://www.healthypeople.gov/sites/default/files/Public-Health-3.0-White-Paper.pdf. Accessed November 7, 2019.
30. Centers for Medicare and Medicaid Services. Health care innovation awards round one project profiles. http://innovation.cms.gov/files/x/hcia-project-profiles.pdf. Updated December 2013. Accessed November 7, 2019.
31. US Department of Health and Human Services. Strategic Objective 1.3: Improve Americans’ access to healthcare and expand choices of care and service options. https://www.hhs.gov/about/strategic-plan/strategic-goal-1/index.html#obj_1_3. Updated March 18, 2019. Accessed November 7, 2019.
32. Kessler CS, Stallings LA, Gonzalez AA, Templeman TA. Combined residency training in emergency medicine and internal medicine: an update on career outcomes and job satisfaction. Acad Emerg Med. 2009;16(9):894-899.
33. Summergrad P, Silberman E, Price LL. Practice and career outcomes of double-boarded psychiatrists. Psychosomatics. 2011;52(6):537-543.
34. Rigoni B, Adkins A. What millennials want from a new job. Harvard Business Rev. May 11, 2016. https://hbr.org/2016/05/what-millennials-want-from-a-new-job. Accessed November 7, 2019.
35. Jung P, Smith C. Medical millennials: a mismatch between training preferences and employment opportunities. Lancet Glob Health. 2019;7(suppl 1):S38.
The Worst and the Best of 2019
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
Readers may recall that at the end of each calendar as opposed to fiscal year—I know it is hard to believe time exists outside the Federal system—Federal Practitioner publishes my ethics-focused version of the familiar year-end roundup. This year I am reversing the typical order of most annual rankings by putting the worst first for 2 morally salient reasons.
The first is that, sadly, it is almost always easier to identify multiple incidents that compete ignominiously for the “worst” of federal health care. Even more disappointing, it is comparatively difficult to find stories for the “best” that are of the same scale and scope as the bad news. This is not to say that every day there are not individual narratives of courage and compassion reported in US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA), and hundreds more unsung heroes.
The second reason is that as human beings our psychology is such that we gravitate toward the worst things more powerfully and persistently than we do the best. This is in part why it is more difficult to find uplifting stories and why the demoralizing ones affect us so strongly. In an exhaustive review of the subject, psychologists Roy Baumeister and colleagues conclude that,
When equal measures of good and bad are present, however, the psychological effects of bad ones outweigh those of the good ones. This may in fact be a general principle or law of psychological phenomena, possibly reflecting the innate predispositions of the psyche or at least reflecting the almost inevitable adaptation of each individual to the exigencies of daily life.2
I am thus saving the best for last in the hope that it will be more memorable and impactful than the worst.
Unique to this year’s look-back, both the negative and the positive accounts come from the domain of end-of-life care. And unlike prior reviews where the lack of administrative vigilance and professional competence affected hundreds of patients, families, and staff, each of this year’s incidents involve a single patient.
An incident that occurred in September 2019 at a VA Community Living Center (CLC) in Georgia stood out in infamy apart from all others. It was the report of a veteran in a VA nursing home who had been bitten more than 100 times by ants crawling all over his room. He died shortly afterward. In a scene out of a horror movie tapping into the most primeval human fears, his daughter Laquana Ross described her father, a Vietnam Air Force veteran with cancer, to media and VA officials in graphic terms. “I understand mistakes happen,” she said. “I’ve had ants. But he was bit by ants two days in a row. They feasted on him.”3
In this new era of holding its senior executive service accountable, the outraged chair of the Senate Veterans Affairs Committee demanded that heads roll, and the VA acted rapidly to comply.4 The VA Central Office placed the network director on administrative leave, reassigned the chief medical officer, and initiated quality and safety reviews as well as an administrative investigative board to scrutinize how the parent Atlanta VA medical center managed the situation. In total, 9 officials connected to the incident were placed on leave. The VA apologized, with VA Secretary Robert Wilke zeroing in on the core values involved in the tragedy, “This is about basic humanity and dignity,” he said. “I don’t care what steps were taken to address the issues. We did not treat a vet with the dignity that he and his family deserved.”5 Yet it was the veteran’s daughter, with unbelievable charity, who asked the most crucial question that must be answered within the framework of a just culture if similar tragedies are not to occur in the future, “I know the staff, without a shadow of doubt, respected my dad and even loved him,” Ross said. “But what’s their ability to assess situations and fix things?”3
To begin to give Ms. Ross the answer she deserves, we must understand that the antithesis of love is not hate but indifference; of compassion, it is not cruelty but coldness. A true just culture reserves individual blame for those who have ill-will and adopts a systems perspective of organizational improvement toward most other types of errors.6 This means that the deplorable conditions in the CLC cannot be charged to the failure of a single staff member to fulfil their obligations but to collective collapse at many levels of the organization. Just culture is ethically laudable and far superior to the history in federal service of capricious punishment or institutional apathy that far too often were the default reactions to media exposures or congressional ire. Justice, though necessary, is not sufficient to achieve virtue. Those who work in health care also must be inspired to offer mercy, kindness, and compassion, especially in our most sacred privilege to provide care of the dying.
The best of 2019 illustrates this distinction movingly. This account also involves a Vietnam veteran, this time a Marine also dying of cancer, which happened just about a month after the earlier report. To be transparent it occurred at my home VA medical center in New Mexico. I was peripherally involved in the case as a consultant but had no role in the wondrous things that transpired. The last wish of a patient dying in the hospice unit on campus was to see his beloved dog who had been taken to the local city animal shelter when he was hospitalized because he had no friends or family to look after the companion animal. A social worker on the palliative care team called the animal shelter and explained the patient did not have much time left but wanted to see his dog before he died. Working together with support from facility leadership, shelter workers brought the dog to visit with the patient for an entire day while hospice staff cried with joy and sadness.7
As the epigraph for this editorial from Dame Cicely Saunders, the founder of the modern hospice movement, says, the difference between unspeakable pain and meaningful suffering can be measured in the depth of compassion caregivers show to the dying. It is this quality of mercy that in one case condemns, and in the other praises, us all as health care and administrative professionals in the service of our country. Baumeister and colleagues suggest that the human tendency to magnify the bad and minimize the good in everyday myopia may in a wider vision actually be a reason for hope:
It may be that humans and animals show heightened awareness of and responded more quickly to negative information because it signals a need for change. Hence, the adaptiveness of self-regulation partly lies in the organism’s ability to detect when response modifications are necessary and when they are unnecessary. Moreover, the lessons learned from bad events should ideally be retained permanently so that the same dangers or costs are not encountered repeatedly. Meanwhile, good events (such as those that provide a feeling of satisfaction and contentment) should ideally wear off so that the organism is motivated to continue searching for more and better outcomes.2
Let us all take this lesson into our work in 2020 so that when it comes time to write this column next year in the chilling cold of late autumn there will be more stories of light than darkness from which to choose.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
1. Saunders C. The management of patients in the terminal stage. In: Raven R, ed. Cancer, Vol. 6. London: Butterworth and Company; 1960:403-417.
2. Baumeister RF, Bratslavasky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev General Psychol. 2001;5(4);323-370.
3. Knowles H. ‘They feasted on him’: Ants at VA nursing home bite a veteran 100 times before his death, daughter says. Washington Post. September 17, 2019. https://www.washingtonpost.com/health/2019/09/13/they-feasted-him-ants-va-nursing-home-bit-veteran-times-before-his-death-daughter-says. Accessed November 25, 2019.
4. Axelrod T. GOP senator presses VA after veteran reportedly bitten by ants in nursing home. https://thehill.com/homenews/senate/461196-gop-senator-presses-va-after-veteran-reportedly-bitten-by-ants-at-nursing. Published September 12, 2019. Accessed November 25, 2019.
5. Kime P. Nine VA leaders, staff placed on leave amid anti-bite scandal. https://www.military.com/daily-news/2019/09/17/nine-va-leaders-staff-placed-leave-amid-ant-bite-scandal.html. Published September 17, 2019. Accessed November 22, 2019.
6. Sculli GL, Hemphill R. Culture of safety and just culture. https://www.patientsafety.va.gov/docs/joe/just_culture_2013_tagged.pdf. Accessed November 22, 2019.
7. Hughes M. A Vietnam veteran in hospice care got to see his beloved dog one last time. https://www.cnn.com/2019/10/21/us/veteran-dying-wish-dog-trnd/index.html. Published October 21, 2019. Accessed November 22, 2019.
Leadership & Professional Development: Get to the “Both/And”
“For every complex problem there is a simple solution. And it’s wrong.”
—Anonymous as quoted in Barry Johnson’s Polarity Management1
Hospital medicine leaders often face what seem like unsolvable problems involving two opposing sides or viewpoints. Examples include individual versus team, margin versus mission, learner autonomy versus supervision, and customization versus standardization. Dr. Barry Johnson describes these dyads as polarities, which are two different values or points of view that are interdependent.1,2 Leaders who fail to realize this concept create a problem by artificially inserting the word ‘versus’ between the poles.
Polarities are not problems to be solved. How does one solve individual? Or team? How can a hospital have one without the other? When leaders treat polarities like problems to be solved, they typically crusade for one side over the other, until the losing side rises up for its own cause, causing a perpetual back and forth cycle described as an infinity loop where nobody is happy for long.1
How then can leaders avoid getting caught in this fruitless cycle?
Instead of trying to solve the unsolvable, learn to manage polarities. Polarity management seeks to maximize the best of both poles while minimizing the worst. Both sides of a polarity carry upsides and downsides. When leaders want change, or want to resist change, it is the fear of being caught in the downsides of the opposite pole that motivates behavior, and dominates conversation. The first step to changing this conversation is to introduce the concept to your team so they recognize polarities when they arise and model approaching issues in this manner.
Some issues truly are problems to be solved (for example, the ultrasound machine is broken and needs to be repaired), but many conflicts are polarities masquerading as problems. To identify polarities, ask two questions. (1) Is the situation ongoing? (2) Are there two interdependent poles? If yes, then the issue is a polarity. Ideal polarity management involves maximizing the upside values of both poles before potential conflict even begins. People often force themselves into unnecessary “either/or” mindsets rather than striving for “both/and”.
Here is a classic example in Hospital Medicine: Pole 1: customization Pole 2: standardization -- The Chief Medical Information Officer (CMIO) wants everyone to use the same electronic health record (EHR) template, while the hospitalist group wants to innovate templates using rapid cycles of change. Typical patterns of conflict: the CMIO releases a template and the hospitalists resent it, or the hospitalists each create their own notes but the CMIO bemoans the variability.
Once polarities are recognized, teams can draw a ‘polarity map’ to see the whole picture, identifying the upside values and downside fears of each pole.1,2 For example, standardization reduces unnecessary variation, but stifles innovation, while customization does the opposite. In fact, the upside values of one pole are usually the opposite of the downside fears of the other.
Leaders can actively engage people in both poles to make opposing views productive rather than destructive. The CMIO in our standardization/customization example could insist that everyone begins with the same template, but allow hospitalists to innovate to find a better way. Now the most resistant hospitalists become improvement agents. If a better way is found, then this becomes the new template that all hospitalists use, until the next better way is found. If an innovation is not an improvement, then hospitalists agree to return to the most recent successful template until a better way is found. This method of action and compromise produces both standardization and customization.
Using polarity management strategies does not guarantee success, but it can help engage all stakeholders, and break the frustrating cycle of repeatedly trying to solve the unsolvable.
Disclosures
The authors report no conflicts of interest or sources of funding.
1. Johnson B. Polarity management: Identifying and managing unsolvable problems. Human Resource Development; 1992.
2. Wesorick BL. Polarity thinking: An essential skill for those leading interprofessional integration. J Interprofessional Healthcare. 2014;1(1):12.
“For every complex problem there is a simple solution. And it’s wrong.”
—Anonymous as quoted in Barry Johnson’s Polarity Management1
Hospital medicine leaders often face what seem like unsolvable problems involving two opposing sides or viewpoints. Examples include individual versus team, margin versus mission, learner autonomy versus supervision, and customization versus standardization. Dr. Barry Johnson describes these dyads as polarities, which are two different values or points of view that are interdependent.1,2 Leaders who fail to realize this concept create a problem by artificially inserting the word ‘versus’ between the poles.
Polarities are not problems to be solved. How does one solve individual? Or team? How can a hospital have one without the other? When leaders treat polarities like problems to be solved, they typically crusade for one side over the other, until the losing side rises up for its own cause, causing a perpetual back and forth cycle described as an infinity loop where nobody is happy for long.1
How then can leaders avoid getting caught in this fruitless cycle?
Instead of trying to solve the unsolvable, learn to manage polarities. Polarity management seeks to maximize the best of both poles while minimizing the worst. Both sides of a polarity carry upsides and downsides. When leaders want change, or want to resist change, it is the fear of being caught in the downsides of the opposite pole that motivates behavior, and dominates conversation. The first step to changing this conversation is to introduce the concept to your team so they recognize polarities when they arise and model approaching issues in this manner.
Some issues truly are problems to be solved (for example, the ultrasound machine is broken and needs to be repaired), but many conflicts are polarities masquerading as problems. To identify polarities, ask two questions. (1) Is the situation ongoing? (2) Are there two interdependent poles? If yes, then the issue is a polarity. Ideal polarity management involves maximizing the upside values of both poles before potential conflict even begins. People often force themselves into unnecessary “either/or” mindsets rather than striving for “both/and”.
Here is a classic example in Hospital Medicine: Pole 1: customization Pole 2: standardization -- The Chief Medical Information Officer (CMIO) wants everyone to use the same electronic health record (EHR) template, while the hospitalist group wants to innovate templates using rapid cycles of change. Typical patterns of conflict: the CMIO releases a template and the hospitalists resent it, or the hospitalists each create their own notes but the CMIO bemoans the variability.
Once polarities are recognized, teams can draw a ‘polarity map’ to see the whole picture, identifying the upside values and downside fears of each pole.1,2 For example, standardization reduces unnecessary variation, but stifles innovation, while customization does the opposite. In fact, the upside values of one pole are usually the opposite of the downside fears of the other.
Leaders can actively engage people in both poles to make opposing views productive rather than destructive. The CMIO in our standardization/customization example could insist that everyone begins with the same template, but allow hospitalists to innovate to find a better way. Now the most resistant hospitalists become improvement agents. If a better way is found, then this becomes the new template that all hospitalists use, until the next better way is found. If an innovation is not an improvement, then hospitalists agree to return to the most recent successful template until a better way is found. This method of action and compromise produces both standardization and customization.
Using polarity management strategies does not guarantee success, but it can help engage all stakeholders, and break the frustrating cycle of repeatedly trying to solve the unsolvable.
Disclosures
The authors report no conflicts of interest or sources of funding.
“For every complex problem there is a simple solution. And it’s wrong.”
—Anonymous as quoted in Barry Johnson’s Polarity Management1
Hospital medicine leaders often face what seem like unsolvable problems involving two opposing sides or viewpoints. Examples include individual versus team, margin versus mission, learner autonomy versus supervision, and customization versus standardization. Dr. Barry Johnson describes these dyads as polarities, which are two different values or points of view that are interdependent.1,2 Leaders who fail to realize this concept create a problem by artificially inserting the word ‘versus’ between the poles.
Polarities are not problems to be solved. How does one solve individual? Or team? How can a hospital have one without the other? When leaders treat polarities like problems to be solved, they typically crusade for one side over the other, until the losing side rises up for its own cause, causing a perpetual back and forth cycle described as an infinity loop where nobody is happy for long.1
How then can leaders avoid getting caught in this fruitless cycle?
Instead of trying to solve the unsolvable, learn to manage polarities. Polarity management seeks to maximize the best of both poles while minimizing the worst. Both sides of a polarity carry upsides and downsides. When leaders want change, or want to resist change, it is the fear of being caught in the downsides of the opposite pole that motivates behavior, and dominates conversation. The first step to changing this conversation is to introduce the concept to your team so they recognize polarities when they arise and model approaching issues in this manner.
Some issues truly are problems to be solved (for example, the ultrasound machine is broken and needs to be repaired), but many conflicts are polarities masquerading as problems. To identify polarities, ask two questions. (1) Is the situation ongoing? (2) Are there two interdependent poles? If yes, then the issue is a polarity. Ideal polarity management involves maximizing the upside values of both poles before potential conflict even begins. People often force themselves into unnecessary “either/or” mindsets rather than striving for “both/and”.
Here is a classic example in Hospital Medicine: Pole 1: customization Pole 2: standardization -- The Chief Medical Information Officer (CMIO) wants everyone to use the same electronic health record (EHR) template, while the hospitalist group wants to innovate templates using rapid cycles of change. Typical patterns of conflict: the CMIO releases a template and the hospitalists resent it, or the hospitalists each create their own notes but the CMIO bemoans the variability.
Once polarities are recognized, teams can draw a ‘polarity map’ to see the whole picture, identifying the upside values and downside fears of each pole.1,2 For example, standardization reduces unnecessary variation, but stifles innovation, while customization does the opposite. In fact, the upside values of one pole are usually the opposite of the downside fears of the other.
Leaders can actively engage people in both poles to make opposing views productive rather than destructive. The CMIO in our standardization/customization example could insist that everyone begins with the same template, but allow hospitalists to innovate to find a better way. Now the most resistant hospitalists become improvement agents. If a better way is found, then this becomes the new template that all hospitalists use, until the next better way is found. If an innovation is not an improvement, then hospitalists agree to return to the most recent successful template until a better way is found. This method of action and compromise produces both standardization and customization.
Using polarity management strategies does not guarantee success, but it can help engage all stakeholders, and break the frustrating cycle of repeatedly trying to solve the unsolvable.
Disclosures
The authors report no conflicts of interest or sources of funding.
1. Johnson B. Polarity management: Identifying and managing unsolvable problems. Human Resource Development; 1992.
2. Wesorick BL. Polarity thinking: An essential skill for those leading interprofessional integration. J Interprofessional Healthcare. 2014;1(1):12.
1. Johnson B. Polarity management: Identifying and managing unsolvable problems. Human Resource Development; 1992.
2. Wesorick BL. Polarity thinking: An essential skill for those leading interprofessional integration. J Interprofessional Healthcare. 2014;1(1):12.
© 2019 Society of Hospital Medicine
Clinical Progress Note: High Flow Nasal Cannula Therapy for Bronchiolitis Outside the ICU in Infants
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
© 2020 Society of Hospital Medicine
High-Flow Nasal Cannula Oxygen in Patients with Acute Respiratory Failure and Do-Not-Intubate or Do-Not-Resuscitate Orders: A Systematic Review
High-flow nasal cannula (HFNC) oxygen therapy is effective in treating adults with acute hypoxemic respiratory failure, and to a lesser extent acute hypercapnic respiratory failure.1-3 HFNC oxygen is capable of delivering oxygen with flows of 30-60 liters/minute, and can provide a high fraction of inspired oxygen, flush anatomic dead space, augment respiratory efforts, and provide mild continuous positive airway pressure effects. Several systematic reviews and meta-analyses have evaluated the effectiveness of HFNC oxygen and have shown modestly lower rates of intubation compared with conventional oxygen4,5 and similar intubation rates compared with noninvasive positive pressure ventilation.4-9 Although one randomized trial showed a lower risk of 90-day mortality for HFNC oxygen compared with either conventional oxygen or noninvasive positive pressure ventilation, several meta-analyses have shown no difference in intensive care unit (ICU) mortality.4,6,8,10 The majority of studies have shown improvements in oxygenation, comfort, dyspnea scores, and breathing pattern with the initiation of HFNC oxygen.6
While the evidence to support the use of HFNC oxygen in patients with nonhypercapnic acute hypoxemic respiratory failure is growing, this evidence is based on patients enrolled in clinical trials who have no treatment limitations and consent to intubation if necessary. Indeed, several, if not all, randomized trials evaluating HFNC oxygen excluded patients who had do-not-intubate (DNI) or do-not-resuscitate (DNR) orders.1,2,11 For patients with acute respiratory failure whose primary goal is not to extend life or utilize life support interventions such as invasive mechanical ventilation, HFNC oxygen may offer several benefits compared with other treatment options such as noninvasive positive pressure ventilation, conventional oxygen therapy, or palliative opioid therapy (Appendix Table 1). Determining which treatment options to use depends on the goals of care of the individual patient and the reasonable ability of a particular treatment to help the patient achieve those goals.
While a recent systematic review evaluated the existing evidence regarding the utility and outcomes of noninvasive positive pressure ventilation in adult patients with DNI orders,12 a systematic review evaluating the evidence and rationale for HFNC oxygen in patients with DNI and/or DNR orders is lacking. Assessing such evidence is necessary to help clinicians and patients determine appropriate treatment choices and establish research priorities. Therefore, our primary objective was to determine what were the following outcomes: mortality, dyspnea, work of breathing, opioid doses, and quality of life in patients who received HFNC oxygen for acute respiratory failure and had a DNI and/or DNR order.
METHODS
We conducted a systematic review of studies that evaluated patients who used HFNC oxygen for acute respiratory failure and had a DNI and/or DNR order. We reported the results using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements.13 This review was registered with the PROSPERO registry, CRD42017059914.
We included studies that enrolled patients who were (1) hospitalized, (2) >18 years old, (3) had an acute respiratory failure of any cause, (4) received HFNC oxygen, and (5) had a DNI or DNR or comfort measures only order. We included publications of all study designs (interventional, observational, and posthoc analyses) and all languages. We excluded studies that enrolled <5 patients. If necessary, we contacted the authors of the included studies for additional information.
Our search strategy included the following databases from inception to October 14, 2018: PubMed, MEDLINE, CINAHL, MICROMEDEX, EMBASE, Web of Science, and Scopus. The database-specific search strategy was developed using an experienced librarian (Appendix Table 2). In addition, we screened the reference lists of systematic reviews as well as the included studies to find additional relevant articles. Two authors (AM, MEW) independently assessed the inclusion criteria of the titles and abstracts that were identified in the search. In addition, these two authors abstracted relevant data of the included studies.
The primary outcomes were mortality, dyspnea and work of breathing, quality of life, and reduction of opioid doses. Secondary, posthoc, outcomes included the transition to noninvasive positive pressure ventilation (NPPV), tolerance of HFNC, adverse events, and quality of death in nonsurvivors. The risk of bias was evaluated using a modified Newcastle-Ottawa Quality Assessment Scale (Appendix Table 3).
RESULTS
Using the search strategy, we identified 2,757 citations and included 301 of these in the full-text review (Figure). We included six studies, which enrolled 293 patients in the final systematic review. Table 1 summarizes the characteristics of the included investigations, all of which were observational studies.15-20 The studies were conducted in the United States of America (n = 3), Europe (n = 2), and Asia (n = 1). Two studies were conducted in the general ICU populations and included patients with hypoxemic respiratory failure only. Four studies were conducted in cancer populations in the hospital wards or ICU and did not specify the type of respiratory failure (hypoxemic versus hypercapnic). Two studies included patients with DNI orders only.15,20 One study included patients with DNR orders only (DNI orders were excluded).17 Three studies included patients with both DNR and DNI orders.16,18,19 The numbers of enrolled patients with treatment limitations were generally low, with the two largest studies including 101 patients each on HFNC oxygen.18,19
Risk of Bias
All included studies had a high risk of bias (Table 2). A high risk of bias was suggested because the investigations were single-center studies with unclear patient selection methods, did not explicitly report how decisions to limit treatments were made, and did not explicitly differentiate and separately analyze patients with “comfort measures only” goals of care.
Mortality
The hospital mortality rates of patients with DNI and/or DNR orders receiving HFNC were variable and ranged from 40% to 87%. In the two studies enrolling general ICU patient populations, the hospital mortality rates ranged from 40% to 60%. In the four studies enrolling patients with active malignancy, the hospital mortality rates ranged from 75% to 87%. No studies compared mortality rates with and without DNI and/or DNR orders.
Dyspnea, Work of Breathing, and Reduction in Opioid Doses
The impact of HFNC oxygen on symptom relief was reported in one retrospective observational study (published as a conference abstract only to date), which compared the effect of HFNC oxygen (n = 101) with conventional oxygen (n = 110).18 At first evaluation after hospital admission to a palliative care unit (after the patients had previously been started on either conventional oxygen or high-flow oxygen), patients in the HFNC oxygen group had worse (higher) dyspnea scores compared with patients who used conventional oxygen (Edmonton Symptom Assessment Scale score of 7.5 versus 5, P < .001). At follow-up, approximately 24 hours after admission to the hospital palliative care unit, there was no difference in the change of dyspnea between the HFNC oxygen group (dyspnea score change of 0) and the conventional oxygen group (dyspnea score change of −1, P = .18. In the same study, there was also no significant difference in the morphine dose requirement in each group, and exact doses were not reported.
Two studies reported improvement in oxygen saturation and respiratory rate after HFNC oxygen initiation (compared with before HFNC initiation).16,20 Oxygen saturation increased from 89% to 95%, P < .01, in one study and 92% to 97%, P < .01, in a second study. The respiratory rate decreased from 31 to 25 breaths/minute in one study, and from 28 to 25 breaths/minute in a second study (both P < .01).
Quality of Life
No studies evaluated the quality of life of survivors.
Secondary Outcomes
Transition to Noninvasive Positive Pressure Ventilation
The proportion of patients who transitioned from HFNC oxygen to NPPV was relatively low in the two studies that reported this outcome, ranging from 0%20 to 18%.16 In one observational study of a general ICU population, 9/50 (18%) of patients transitioned from HFNC oxygen to NPPV. There was no statistically significant difference in hospital mortality rates among those who progressed to NPPV (67%) versus those who did not progress to NPPV (58%), P = .72.
Tolerance of HFNC and Adverse Events
HFNC oxygen was generally well tolerated based on the assessment of three studies (Table 1). One study reported no adverse events,16 one study reported that HFNC oxygen had to be discontinued because of nasal discomfort in 1% of patients,19 and a second study reported that HFNC oxygen had to be discontinued because of agitation in 4% of patients.20
Quality of Death in Nonsurvivors
No studies evaluated the quality of death in those patients who died.
DISCUSSION
In this systematic review of six studies, all with a high risk of bias, a significant proportion of patients with a DNI and/or DNR order who used HFNC oxygen survived to hospital discharge. Oxygen saturation and respiratory rate consistently improved in the three studies that reported these outcomes. Only one study (published as a conference abstract only to date),18 however, measured patient-important outcomes related to symptom management and found no significant difference in dyspnea or morphine dose requirements in patients on HFNC oxygen compared with patients on conventional oxygen. HFNC oxygen was generally well tolerated and only had to be stopped in <5% of patients due to intolerance. We found no studies that assessed the quality of life in survivors or the quality of death in nonsurvivors.
Based on the limited evidence in the included studies, HFNC may be a viable treatment option for patients with preset treatment limitations who have acute respiratory failure—with potential benefits of improved oxygenation, decreased respiratory rates, and hospital survival in a proportion of patients. Nevertheless, this systematic review highlights the vast paucity of data available to guide the use of HFNC oxygen in patients with treatment limitations and acute respiratory failure. Only a few studies, which were at high risk of bias, have been conducted on this topic to date. There is an inadequate evidence base to evaluate the comparative effectiveness of HFNC oxygen (versus NPPV versus conventional oxygen versus palliative opioids) in patients with DNI orders or comfort measures only orders.
Our review included two studies that evaluated the comparative effectiveness of HFNC oxygen in patients with DNI and/or DNR orders. The first retrospective observational study compared HFNC oxygen with conventional oxygen in patients with DNR and DNI orders and malignancy—and found no change in dyspnea—but did note an increase in mortality with HFNC oxygen (76% versus 51%).18 The second observational study compared HFNC oxygen with NPPV in patients with DNR orders with malignancy noted no difference in mortality.17 In patients with full-code orders, systematic reviews have shown that HFNC oxygen (compared with conventional oxygen) was associated with possible reductions in intubation rates, respiratory rates, and improvements in oxygenation—with no difference in mortality, dyspnea, patient comfort, or ICU/hospital length of stay. Compared with NPPV, HFNC oxygen was associated with similar rates of intubation and mortality.4-6,21
Future studies in patients with acute respiratory failure and DNI and/or DNR orders should identify which treatment modality (HFNC oxygen compared with other modalities, such as NPPV, conventional oxygen, with or without palliative opioids) impacts outcomes, such as dyspnea reduction while maintaining an alert mental status, short- and long-term quality of life in survivors, and quality of death in nonsurvivors. Future studies should also identify the optimal treatment pathway to utilize when patients using HFNC oxygen fail this therapy (eg, transition to NPPV versus intensifying palliative opioids) as well as the optimal process to withdraw palliative HFNC oxygen.22 Identifying which patient populations may benefit from different treatment pathways should also be considered as different treatment strategies may be more beneficial in different patient populations (eg, based on cause and severity of acute respiratory failure). In addition, it should be noted that the primary goal of care might affect which outcomes are the most important to measure. While patients with comfort measures only, orders usually have a primary goal to prepare for a high-quality death, patients with DNI and/or DNR orders (but without comfort measures only orders) may have a primary goal to survive—but with the desire not to endure the high burden of intubation and mechanical ventilation if it became necessary. Finally, future studies should utilize high-quality study designs (eg, randomized controlled trials) that enable robust evaluation of comparative effectiveness of clinically relevant treatment strategies.
While several previous systematic reviews have evaluated the efficacy of HFNC in patients with acute respiratory failure without preset limitations on life support; to our knowledge, this is the first systematic review to assess outcomes in patients rigorously with preset treatment limitations. Our review is, however, limited by the high risk of bias of the studies that were included (single-center nature, retrospective observational study designs, small sample sizes, and lack of a description of how DNI and/or DNR statuses were determined) as well as the small number of studies available to be included.
CONCLUSIONS
This systematic review points to a significant evidence gap in our understanding of the role for HFNC oxygen (compared with other acceptable alternative treatment strategies) in adult patients with acute respiratory failure who have DNI and/or DNR orders. Further high-quality research is needed to explore these unanswered questions in an effort to best treat, guide, and engage in optimal end-of-life decision making among patients with acute respiratory failure.
1. Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Eng J Med. 2015;372(23):2185-2196. https://doi.org/ 10.1056/NEJMoa1503326.
2. Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331-2339. https://doi.org/ 10.1001/jama.2015.5213.
3. Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046-2056. https://doi.org/10.1111/crj.12772 28.
4. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest. 2017;151(4):764-775. https://doi.org/10.1016/j.chest.2017.01.004.
5. Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189(7):E260-E267. https://doi.org/10.1503/cmaj.160570.
6. Monro-Somerville T, Sim M, Ruddy J, Vilas M, Gillies MA. The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):e449-e456. https://doi.org/10.1097/CCM.0000000000002091.
7. Maitra S, Som A, Bhattacharjee S, Arora MK, Baidya DK. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care. 2016;35:138-144. https://doi.org/10.1016/j.jcrc.2016.05.013.
8. Nedel WL, Deutschendorf C, Moraes Rodrigues Filho E. High-flow nasal cannula in critically ill subjects with or at risk for respiratory failure: a systematic review and meta-analysis. Respir Care. 2017;62(1):123-132. https://doi.org/10.4187/respcare.04831.
9. Zhu Y, Yin H, Zhang R, Wei J. High-flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: a meta-analysis. J Crit Care. 2017;38:123-128. https://doi.org/10.1016/j.jcrc.2016.10.027.
10. Leeies M, Flynn E, Turgeon AF, et al. High-flow oxygen via nasal cannulae in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Syst Rev. 2017;6(1):202. https://doi.org/10.1186/s13643-017-0593-5.
11. Hernandez G, Vaquero C, Gonzalez P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-1361. https://doi.org/10.1001/jama.2016.2711.
12. Wilson ME, Majzoub AM, Dobler CC, et al. Noninvasive ventilation in patients with do-not-intubate and comfort-measures-only orders: a systematic review and meta-analysis. Crit Care Med. 2018. 46(8):1209-1216. https://doi.org/10.1097/CCM.0000000000003082.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
14. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. https://doi.org/10.1001/jama.283.15.2008.
15. Brugger SC, Rodriguez S, Domingo J, et al. High-flow nasal cannula therapy (HFNC) for patients with severe acute respiratory failure and do not intubate orders. Pilot study. Palliative Medicine. 2014;28(6):755.
16. Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care. 2013;58(4):597-600. https://doi.org/10.4187/respcare.01887.
17. Coudroy R, Jamet A, Petua P, Robert R, Frat JP, Thille AW. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care. 2016;6(1):45. https://doi.org/10.1186/s13613-016-0151-7.
18. Delgado-Guay MO, Rodriguez-Nunez A, Adegboyega OO, et al. Characteristics and outcomes of advanced cancer patients admitted to an acute palliative care unit (PCU) with severe dyspnea receiving high flow oxygen (HFO). Journal of Clinical Oncology Conference. 2015;33(29 SUPPL. 1):247.
19. Epstein AS, Hartridge-Lambert SK, Ramaker JS, Voigt LP, Portlock CS. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med. 2011;14(7):835-839. https://doi.org/10.1089/jpm.2011.0005.
20. Harada K, Kurosawa S, Hino Y, et al. Clinical utility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematological disease. Springerplus. 2016;5(1):512. https://doi.org/10.1186/s40064-016-2161-1.
21. Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563-572. https://doi.org/10.1007/s00134-019-05590-5.
22. Halpern SD, Hansen-Flaschen J. Terminal withdrawal of life-sustaining supplemental oxygen. JAMA. 2006;296(11):1397-1400. https://doi.org/10.1001/jama.296.11.1397.
High-flow nasal cannula (HFNC) oxygen therapy is effective in treating adults with acute hypoxemic respiratory failure, and to a lesser extent acute hypercapnic respiratory failure.1-3 HFNC oxygen is capable of delivering oxygen with flows of 30-60 liters/minute, and can provide a high fraction of inspired oxygen, flush anatomic dead space, augment respiratory efforts, and provide mild continuous positive airway pressure effects. Several systematic reviews and meta-analyses have evaluated the effectiveness of HFNC oxygen and have shown modestly lower rates of intubation compared with conventional oxygen4,5 and similar intubation rates compared with noninvasive positive pressure ventilation.4-9 Although one randomized trial showed a lower risk of 90-day mortality for HFNC oxygen compared with either conventional oxygen or noninvasive positive pressure ventilation, several meta-analyses have shown no difference in intensive care unit (ICU) mortality.4,6,8,10 The majority of studies have shown improvements in oxygenation, comfort, dyspnea scores, and breathing pattern with the initiation of HFNC oxygen.6
While the evidence to support the use of HFNC oxygen in patients with nonhypercapnic acute hypoxemic respiratory failure is growing, this evidence is based on patients enrolled in clinical trials who have no treatment limitations and consent to intubation if necessary. Indeed, several, if not all, randomized trials evaluating HFNC oxygen excluded patients who had do-not-intubate (DNI) or do-not-resuscitate (DNR) orders.1,2,11 For patients with acute respiratory failure whose primary goal is not to extend life or utilize life support interventions such as invasive mechanical ventilation, HFNC oxygen may offer several benefits compared with other treatment options such as noninvasive positive pressure ventilation, conventional oxygen therapy, or palliative opioid therapy (Appendix Table 1). Determining which treatment options to use depends on the goals of care of the individual patient and the reasonable ability of a particular treatment to help the patient achieve those goals.
While a recent systematic review evaluated the existing evidence regarding the utility and outcomes of noninvasive positive pressure ventilation in adult patients with DNI orders,12 a systematic review evaluating the evidence and rationale for HFNC oxygen in patients with DNI and/or DNR orders is lacking. Assessing such evidence is necessary to help clinicians and patients determine appropriate treatment choices and establish research priorities. Therefore, our primary objective was to determine what were the following outcomes: mortality, dyspnea, work of breathing, opioid doses, and quality of life in patients who received HFNC oxygen for acute respiratory failure and had a DNI and/or DNR order.
METHODS
We conducted a systematic review of studies that evaluated patients who used HFNC oxygen for acute respiratory failure and had a DNI and/or DNR order. We reported the results using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements.13 This review was registered with the PROSPERO registry, CRD42017059914.
We included studies that enrolled patients who were (1) hospitalized, (2) >18 years old, (3) had an acute respiratory failure of any cause, (4) received HFNC oxygen, and (5) had a DNI or DNR or comfort measures only order. We included publications of all study designs (interventional, observational, and posthoc analyses) and all languages. We excluded studies that enrolled <5 patients. If necessary, we contacted the authors of the included studies for additional information.
Our search strategy included the following databases from inception to October 14, 2018: PubMed, MEDLINE, CINAHL, MICROMEDEX, EMBASE, Web of Science, and Scopus. The database-specific search strategy was developed using an experienced librarian (Appendix Table 2). In addition, we screened the reference lists of systematic reviews as well as the included studies to find additional relevant articles. Two authors (AM, MEW) independently assessed the inclusion criteria of the titles and abstracts that were identified in the search. In addition, these two authors abstracted relevant data of the included studies.
The primary outcomes were mortality, dyspnea and work of breathing, quality of life, and reduction of opioid doses. Secondary, posthoc, outcomes included the transition to noninvasive positive pressure ventilation (NPPV), tolerance of HFNC, adverse events, and quality of death in nonsurvivors. The risk of bias was evaluated using a modified Newcastle-Ottawa Quality Assessment Scale (Appendix Table 3).
RESULTS
Using the search strategy, we identified 2,757 citations and included 301 of these in the full-text review (Figure). We included six studies, which enrolled 293 patients in the final systematic review. Table 1 summarizes the characteristics of the included investigations, all of which were observational studies.15-20 The studies were conducted in the United States of America (n = 3), Europe (n = 2), and Asia (n = 1). Two studies were conducted in the general ICU populations and included patients with hypoxemic respiratory failure only. Four studies were conducted in cancer populations in the hospital wards or ICU and did not specify the type of respiratory failure (hypoxemic versus hypercapnic). Two studies included patients with DNI orders only.15,20 One study included patients with DNR orders only (DNI orders were excluded).17 Three studies included patients with both DNR and DNI orders.16,18,19 The numbers of enrolled patients with treatment limitations were generally low, with the two largest studies including 101 patients each on HFNC oxygen.18,19
Risk of Bias
All included studies had a high risk of bias (Table 2). A high risk of bias was suggested because the investigations were single-center studies with unclear patient selection methods, did not explicitly report how decisions to limit treatments were made, and did not explicitly differentiate and separately analyze patients with “comfort measures only” goals of care.
Mortality
The hospital mortality rates of patients with DNI and/or DNR orders receiving HFNC were variable and ranged from 40% to 87%. In the two studies enrolling general ICU patient populations, the hospital mortality rates ranged from 40% to 60%. In the four studies enrolling patients with active malignancy, the hospital mortality rates ranged from 75% to 87%. No studies compared mortality rates with and without DNI and/or DNR orders.
Dyspnea, Work of Breathing, and Reduction in Opioid Doses
The impact of HFNC oxygen on symptom relief was reported in one retrospective observational study (published as a conference abstract only to date), which compared the effect of HFNC oxygen (n = 101) with conventional oxygen (n = 110).18 At first evaluation after hospital admission to a palliative care unit (after the patients had previously been started on either conventional oxygen or high-flow oxygen), patients in the HFNC oxygen group had worse (higher) dyspnea scores compared with patients who used conventional oxygen (Edmonton Symptom Assessment Scale score of 7.5 versus 5, P < .001). At follow-up, approximately 24 hours after admission to the hospital palliative care unit, there was no difference in the change of dyspnea between the HFNC oxygen group (dyspnea score change of 0) and the conventional oxygen group (dyspnea score change of −1, P = .18. In the same study, there was also no significant difference in the morphine dose requirement in each group, and exact doses were not reported.
Two studies reported improvement in oxygen saturation and respiratory rate after HFNC oxygen initiation (compared with before HFNC initiation).16,20 Oxygen saturation increased from 89% to 95%, P < .01, in one study and 92% to 97%, P < .01, in a second study. The respiratory rate decreased from 31 to 25 breaths/minute in one study, and from 28 to 25 breaths/minute in a second study (both P < .01).
Quality of Life
No studies evaluated the quality of life of survivors.
Secondary Outcomes
Transition to Noninvasive Positive Pressure Ventilation
The proportion of patients who transitioned from HFNC oxygen to NPPV was relatively low in the two studies that reported this outcome, ranging from 0%20 to 18%.16 In one observational study of a general ICU population, 9/50 (18%) of patients transitioned from HFNC oxygen to NPPV. There was no statistically significant difference in hospital mortality rates among those who progressed to NPPV (67%) versus those who did not progress to NPPV (58%), P = .72.
Tolerance of HFNC and Adverse Events
HFNC oxygen was generally well tolerated based on the assessment of three studies (Table 1). One study reported no adverse events,16 one study reported that HFNC oxygen had to be discontinued because of nasal discomfort in 1% of patients,19 and a second study reported that HFNC oxygen had to be discontinued because of agitation in 4% of patients.20
Quality of Death in Nonsurvivors
No studies evaluated the quality of death in those patients who died.
DISCUSSION
In this systematic review of six studies, all with a high risk of bias, a significant proportion of patients with a DNI and/or DNR order who used HFNC oxygen survived to hospital discharge. Oxygen saturation and respiratory rate consistently improved in the three studies that reported these outcomes. Only one study (published as a conference abstract only to date),18 however, measured patient-important outcomes related to symptom management and found no significant difference in dyspnea or morphine dose requirements in patients on HFNC oxygen compared with patients on conventional oxygen. HFNC oxygen was generally well tolerated and only had to be stopped in <5% of patients due to intolerance. We found no studies that assessed the quality of life in survivors or the quality of death in nonsurvivors.
Based on the limited evidence in the included studies, HFNC may be a viable treatment option for patients with preset treatment limitations who have acute respiratory failure—with potential benefits of improved oxygenation, decreased respiratory rates, and hospital survival in a proportion of patients. Nevertheless, this systematic review highlights the vast paucity of data available to guide the use of HFNC oxygen in patients with treatment limitations and acute respiratory failure. Only a few studies, which were at high risk of bias, have been conducted on this topic to date. There is an inadequate evidence base to evaluate the comparative effectiveness of HFNC oxygen (versus NPPV versus conventional oxygen versus palliative opioids) in patients with DNI orders or comfort measures only orders.
Our review included two studies that evaluated the comparative effectiveness of HFNC oxygen in patients with DNI and/or DNR orders. The first retrospective observational study compared HFNC oxygen with conventional oxygen in patients with DNR and DNI orders and malignancy—and found no change in dyspnea—but did note an increase in mortality with HFNC oxygen (76% versus 51%).18 The second observational study compared HFNC oxygen with NPPV in patients with DNR orders with malignancy noted no difference in mortality.17 In patients with full-code orders, systematic reviews have shown that HFNC oxygen (compared with conventional oxygen) was associated with possible reductions in intubation rates, respiratory rates, and improvements in oxygenation—with no difference in mortality, dyspnea, patient comfort, or ICU/hospital length of stay. Compared with NPPV, HFNC oxygen was associated with similar rates of intubation and mortality.4-6,21
Future studies in patients with acute respiratory failure and DNI and/or DNR orders should identify which treatment modality (HFNC oxygen compared with other modalities, such as NPPV, conventional oxygen, with or without palliative opioids) impacts outcomes, such as dyspnea reduction while maintaining an alert mental status, short- and long-term quality of life in survivors, and quality of death in nonsurvivors. Future studies should also identify the optimal treatment pathway to utilize when patients using HFNC oxygen fail this therapy (eg, transition to NPPV versus intensifying palliative opioids) as well as the optimal process to withdraw palliative HFNC oxygen.22 Identifying which patient populations may benefit from different treatment pathways should also be considered as different treatment strategies may be more beneficial in different patient populations (eg, based on cause and severity of acute respiratory failure). In addition, it should be noted that the primary goal of care might affect which outcomes are the most important to measure. While patients with comfort measures only, orders usually have a primary goal to prepare for a high-quality death, patients with DNI and/or DNR orders (but without comfort measures only orders) may have a primary goal to survive—but with the desire not to endure the high burden of intubation and mechanical ventilation if it became necessary. Finally, future studies should utilize high-quality study designs (eg, randomized controlled trials) that enable robust evaluation of comparative effectiveness of clinically relevant treatment strategies.
While several previous systematic reviews have evaluated the efficacy of HFNC in patients with acute respiratory failure without preset limitations on life support; to our knowledge, this is the first systematic review to assess outcomes in patients rigorously with preset treatment limitations. Our review is, however, limited by the high risk of bias of the studies that were included (single-center nature, retrospective observational study designs, small sample sizes, and lack of a description of how DNI and/or DNR statuses were determined) as well as the small number of studies available to be included.
CONCLUSIONS
This systematic review points to a significant evidence gap in our understanding of the role for HFNC oxygen (compared with other acceptable alternative treatment strategies) in adult patients with acute respiratory failure who have DNI and/or DNR orders. Further high-quality research is needed to explore these unanswered questions in an effort to best treat, guide, and engage in optimal end-of-life decision making among patients with acute respiratory failure.
High-flow nasal cannula (HFNC) oxygen therapy is effective in treating adults with acute hypoxemic respiratory failure, and to a lesser extent acute hypercapnic respiratory failure.1-3 HFNC oxygen is capable of delivering oxygen with flows of 30-60 liters/minute, and can provide a high fraction of inspired oxygen, flush anatomic dead space, augment respiratory efforts, and provide mild continuous positive airway pressure effects. Several systematic reviews and meta-analyses have evaluated the effectiveness of HFNC oxygen and have shown modestly lower rates of intubation compared with conventional oxygen4,5 and similar intubation rates compared with noninvasive positive pressure ventilation.4-9 Although one randomized trial showed a lower risk of 90-day mortality for HFNC oxygen compared with either conventional oxygen or noninvasive positive pressure ventilation, several meta-analyses have shown no difference in intensive care unit (ICU) mortality.4,6,8,10 The majority of studies have shown improvements in oxygenation, comfort, dyspnea scores, and breathing pattern with the initiation of HFNC oxygen.6
While the evidence to support the use of HFNC oxygen in patients with nonhypercapnic acute hypoxemic respiratory failure is growing, this evidence is based on patients enrolled in clinical trials who have no treatment limitations and consent to intubation if necessary. Indeed, several, if not all, randomized trials evaluating HFNC oxygen excluded patients who had do-not-intubate (DNI) or do-not-resuscitate (DNR) orders.1,2,11 For patients with acute respiratory failure whose primary goal is not to extend life or utilize life support interventions such as invasive mechanical ventilation, HFNC oxygen may offer several benefits compared with other treatment options such as noninvasive positive pressure ventilation, conventional oxygen therapy, or palliative opioid therapy (Appendix Table 1). Determining which treatment options to use depends on the goals of care of the individual patient and the reasonable ability of a particular treatment to help the patient achieve those goals.
While a recent systematic review evaluated the existing evidence regarding the utility and outcomes of noninvasive positive pressure ventilation in adult patients with DNI orders,12 a systematic review evaluating the evidence and rationale for HFNC oxygen in patients with DNI and/or DNR orders is lacking. Assessing such evidence is necessary to help clinicians and patients determine appropriate treatment choices and establish research priorities. Therefore, our primary objective was to determine what were the following outcomes: mortality, dyspnea, work of breathing, opioid doses, and quality of life in patients who received HFNC oxygen for acute respiratory failure and had a DNI and/or DNR order.
METHODS
We conducted a systematic review of studies that evaluated patients who used HFNC oxygen for acute respiratory failure and had a DNI and/or DNR order. We reported the results using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements.13 This review was registered with the PROSPERO registry, CRD42017059914.
We included studies that enrolled patients who were (1) hospitalized, (2) >18 years old, (3) had an acute respiratory failure of any cause, (4) received HFNC oxygen, and (5) had a DNI or DNR or comfort measures only order. We included publications of all study designs (interventional, observational, and posthoc analyses) and all languages. We excluded studies that enrolled <5 patients. If necessary, we contacted the authors of the included studies for additional information.
Our search strategy included the following databases from inception to October 14, 2018: PubMed, MEDLINE, CINAHL, MICROMEDEX, EMBASE, Web of Science, and Scopus. The database-specific search strategy was developed using an experienced librarian (Appendix Table 2). In addition, we screened the reference lists of systematic reviews as well as the included studies to find additional relevant articles. Two authors (AM, MEW) independently assessed the inclusion criteria of the titles and abstracts that were identified in the search. In addition, these two authors abstracted relevant data of the included studies.
The primary outcomes were mortality, dyspnea and work of breathing, quality of life, and reduction of opioid doses. Secondary, posthoc, outcomes included the transition to noninvasive positive pressure ventilation (NPPV), tolerance of HFNC, adverse events, and quality of death in nonsurvivors. The risk of bias was evaluated using a modified Newcastle-Ottawa Quality Assessment Scale (Appendix Table 3).
RESULTS
Using the search strategy, we identified 2,757 citations and included 301 of these in the full-text review (Figure). We included six studies, which enrolled 293 patients in the final systematic review. Table 1 summarizes the characteristics of the included investigations, all of which were observational studies.15-20 The studies were conducted in the United States of America (n = 3), Europe (n = 2), and Asia (n = 1). Two studies were conducted in the general ICU populations and included patients with hypoxemic respiratory failure only. Four studies were conducted in cancer populations in the hospital wards or ICU and did not specify the type of respiratory failure (hypoxemic versus hypercapnic). Two studies included patients with DNI orders only.15,20 One study included patients with DNR orders only (DNI orders were excluded).17 Three studies included patients with both DNR and DNI orders.16,18,19 The numbers of enrolled patients with treatment limitations were generally low, with the two largest studies including 101 patients each on HFNC oxygen.18,19
Risk of Bias
All included studies had a high risk of bias (Table 2). A high risk of bias was suggested because the investigations were single-center studies with unclear patient selection methods, did not explicitly report how decisions to limit treatments were made, and did not explicitly differentiate and separately analyze patients with “comfort measures only” goals of care.
Mortality
The hospital mortality rates of patients with DNI and/or DNR orders receiving HFNC were variable and ranged from 40% to 87%. In the two studies enrolling general ICU patient populations, the hospital mortality rates ranged from 40% to 60%. In the four studies enrolling patients with active malignancy, the hospital mortality rates ranged from 75% to 87%. No studies compared mortality rates with and without DNI and/or DNR orders.
Dyspnea, Work of Breathing, and Reduction in Opioid Doses
The impact of HFNC oxygen on symptom relief was reported in one retrospective observational study (published as a conference abstract only to date), which compared the effect of HFNC oxygen (n = 101) with conventional oxygen (n = 110).18 At first evaluation after hospital admission to a palliative care unit (after the patients had previously been started on either conventional oxygen or high-flow oxygen), patients in the HFNC oxygen group had worse (higher) dyspnea scores compared with patients who used conventional oxygen (Edmonton Symptom Assessment Scale score of 7.5 versus 5, P < .001). At follow-up, approximately 24 hours after admission to the hospital palliative care unit, there was no difference in the change of dyspnea between the HFNC oxygen group (dyspnea score change of 0) and the conventional oxygen group (dyspnea score change of −1, P = .18. In the same study, there was also no significant difference in the morphine dose requirement in each group, and exact doses were not reported.
Two studies reported improvement in oxygen saturation and respiratory rate after HFNC oxygen initiation (compared with before HFNC initiation).16,20 Oxygen saturation increased from 89% to 95%, P < .01, in one study and 92% to 97%, P < .01, in a second study. The respiratory rate decreased from 31 to 25 breaths/minute in one study, and from 28 to 25 breaths/minute in a second study (both P < .01).
Quality of Life
No studies evaluated the quality of life of survivors.
Secondary Outcomes
Transition to Noninvasive Positive Pressure Ventilation
The proportion of patients who transitioned from HFNC oxygen to NPPV was relatively low in the two studies that reported this outcome, ranging from 0%20 to 18%.16 In one observational study of a general ICU population, 9/50 (18%) of patients transitioned from HFNC oxygen to NPPV. There was no statistically significant difference in hospital mortality rates among those who progressed to NPPV (67%) versus those who did not progress to NPPV (58%), P = .72.
Tolerance of HFNC and Adverse Events
HFNC oxygen was generally well tolerated based on the assessment of three studies (Table 1). One study reported no adverse events,16 one study reported that HFNC oxygen had to be discontinued because of nasal discomfort in 1% of patients,19 and a second study reported that HFNC oxygen had to be discontinued because of agitation in 4% of patients.20
Quality of Death in Nonsurvivors
No studies evaluated the quality of death in those patients who died.
DISCUSSION
In this systematic review of six studies, all with a high risk of bias, a significant proportion of patients with a DNI and/or DNR order who used HFNC oxygen survived to hospital discharge. Oxygen saturation and respiratory rate consistently improved in the three studies that reported these outcomes. Only one study (published as a conference abstract only to date),18 however, measured patient-important outcomes related to symptom management and found no significant difference in dyspnea or morphine dose requirements in patients on HFNC oxygen compared with patients on conventional oxygen. HFNC oxygen was generally well tolerated and only had to be stopped in <5% of patients due to intolerance. We found no studies that assessed the quality of life in survivors or the quality of death in nonsurvivors.
Based on the limited evidence in the included studies, HFNC may be a viable treatment option for patients with preset treatment limitations who have acute respiratory failure—with potential benefits of improved oxygenation, decreased respiratory rates, and hospital survival in a proportion of patients. Nevertheless, this systematic review highlights the vast paucity of data available to guide the use of HFNC oxygen in patients with treatment limitations and acute respiratory failure. Only a few studies, which were at high risk of bias, have been conducted on this topic to date. There is an inadequate evidence base to evaluate the comparative effectiveness of HFNC oxygen (versus NPPV versus conventional oxygen versus palliative opioids) in patients with DNI orders or comfort measures only orders.
Our review included two studies that evaluated the comparative effectiveness of HFNC oxygen in patients with DNI and/or DNR orders. The first retrospective observational study compared HFNC oxygen with conventional oxygen in patients with DNR and DNI orders and malignancy—and found no change in dyspnea—but did note an increase in mortality with HFNC oxygen (76% versus 51%).18 The second observational study compared HFNC oxygen with NPPV in patients with DNR orders with malignancy noted no difference in mortality.17 In patients with full-code orders, systematic reviews have shown that HFNC oxygen (compared with conventional oxygen) was associated with possible reductions in intubation rates, respiratory rates, and improvements in oxygenation—with no difference in mortality, dyspnea, patient comfort, or ICU/hospital length of stay. Compared with NPPV, HFNC oxygen was associated with similar rates of intubation and mortality.4-6,21
Future studies in patients with acute respiratory failure and DNI and/or DNR orders should identify which treatment modality (HFNC oxygen compared with other modalities, such as NPPV, conventional oxygen, with or without palliative opioids) impacts outcomes, such as dyspnea reduction while maintaining an alert mental status, short- and long-term quality of life in survivors, and quality of death in nonsurvivors. Future studies should also identify the optimal treatment pathway to utilize when patients using HFNC oxygen fail this therapy (eg, transition to NPPV versus intensifying palliative opioids) as well as the optimal process to withdraw palliative HFNC oxygen.22 Identifying which patient populations may benefit from different treatment pathways should also be considered as different treatment strategies may be more beneficial in different patient populations (eg, based on cause and severity of acute respiratory failure). In addition, it should be noted that the primary goal of care might affect which outcomes are the most important to measure. While patients with comfort measures only, orders usually have a primary goal to prepare for a high-quality death, patients with DNI and/or DNR orders (but without comfort measures only orders) may have a primary goal to survive—but with the desire not to endure the high burden of intubation and mechanical ventilation if it became necessary. Finally, future studies should utilize high-quality study designs (eg, randomized controlled trials) that enable robust evaluation of comparative effectiveness of clinically relevant treatment strategies.
While several previous systematic reviews have evaluated the efficacy of HFNC in patients with acute respiratory failure without preset limitations on life support; to our knowledge, this is the first systematic review to assess outcomes in patients rigorously with preset treatment limitations. Our review is, however, limited by the high risk of bias of the studies that were included (single-center nature, retrospective observational study designs, small sample sizes, and lack of a description of how DNI and/or DNR statuses were determined) as well as the small number of studies available to be included.
CONCLUSIONS
This systematic review points to a significant evidence gap in our understanding of the role for HFNC oxygen (compared with other acceptable alternative treatment strategies) in adult patients with acute respiratory failure who have DNI and/or DNR orders. Further high-quality research is needed to explore these unanswered questions in an effort to best treat, guide, and engage in optimal end-of-life decision making among patients with acute respiratory failure.
1. Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Eng J Med. 2015;372(23):2185-2196. https://doi.org/ 10.1056/NEJMoa1503326.
2. Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331-2339. https://doi.org/ 10.1001/jama.2015.5213.
3. Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046-2056. https://doi.org/10.1111/crj.12772 28.
4. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest. 2017;151(4):764-775. https://doi.org/10.1016/j.chest.2017.01.004.
5. Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189(7):E260-E267. https://doi.org/10.1503/cmaj.160570.
6. Monro-Somerville T, Sim M, Ruddy J, Vilas M, Gillies MA. The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):e449-e456. https://doi.org/10.1097/CCM.0000000000002091.
7. Maitra S, Som A, Bhattacharjee S, Arora MK, Baidya DK. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care. 2016;35:138-144. https://doi.org/10.1016/j.jcrc.2016.05.013.
8. Nedel WL, Deutschendorf C, Moraes Rodrigues Filho E. High-flow nasal cannula in critically ill subjects with or at risk for respiratory failure: a systematic review and meta-analysis. Respir Care. 2017;62(1):123-132. https://doi.org/10.4187/respcare.04831.
9. Zhu Y, Yin H, Zhang R, Wei J. High-flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: a meta-analysis. J Crit Care. 2017;38:123-128. https://doi.org/10.1016/j.jcrc.2016.10.027.
10. Leeies M, Flynn E, Turgeon AF, et al. High-flow oxygen via nasal cannulae in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Syst Rev. 2017;6(1):202. https://doi.org/10.1186/s13643-017-0593-5.
11. Hernandez G, Vaquero C, Gonzalez P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-1361. https://doi.org/10.1001/jama.2016.2711.
12. Wilson ME, Majzoub AM, Dobler CC, et al. Noninvasive ventilation in patients with do-not-intubate and comfort-measures-only orders: a systematic review and meta-analysis. Crit Care Med. 2018. 46(8):1209-1216. https://doi.org/10.1097/CCM.0000000000003082.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
14. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. https://doi.org/10.1001/jama.283.15.2008.
15. Brugger SC, Rodriguez S, Domingo J, et al. High-flow nasal cannula therapy (HFNC) for patients with severe acute respiratory failure and do not intubate orders. Pilot study. Palliative Medicine. 2014;28(6):755.
16. Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care. 2013;58(4):597-600. https://doi.org/10.4187/respcare.01887.
17. Coudroy R, Jamet A, Petua P, Robert R, Frat JP, Thille AW. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care. 2016;6(1):45. https://doi.org/10.1186/s13613-016-0151-7.
18. Delgado-Guay MO, Rodriguez-Nunez A, Adegboyega OO, et al. Characteristics and outcomes of advanced cancer patients admitted to an acute palliative care unit (PCU) with severe dyspnea receiving high flow oxygen (HFO). Journal of Clinical Oncology Conference. 2015;33(29 SUPPL. 1):247.
19. Epstein AS, Hartridge-Lambert SK, Ramaker JS, Voigt LP, Portlock CS. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med. 2011;14(7):835-839. https://doi.org/10.1089/jpm.2011.0005.
20. Harada K, Kurosawa S, Hino Y, et al. Clinical utility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematological disease. Springerplus. 2016;5(1):512. https://doi.org/10.1186/s40064-016-2161-1.
21. Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563-572. https://doi.org/10.1007/s00134-019-05590-5.
22. Halpern SD, Hansen-Flaschen J. Terminal withdrawal of life-sustaining supplemental oxygen. JAMA. 2006;296(11):1397-1400. https://doi.org/10.1001/jama.296.11.1397.
1. Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Eng J Med. 2015;372(23):2185-2196. https://doi.org/ 10.1056/NEJMoa1503326.
2. Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331-2339. https://doi.org/ 10.1001/jama.2015.5213.
3. Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046-2056. https://doi.org/10.1111/crj.12772 28.
4. Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest. 2017;151(4):764-775. https://doi.org/10.1016/j.chest.2017.01.004.
5. Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189(7):E260-E267. https://doi.org/10.1503/cmaj.160570.
6. Monro-Somerville T, Sim M, Ruddy J, Vilas M, Gillies MA. The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(4):e449-e456. https://doi.org/10.1097/CCM.0000000000002091.
7. Maitra S, Som A, Bhattacharjee S, Arora MK, Baidya DK. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care. 2016;35:138-144. https://doi.org/10.1016/j.jcrc.2016.05.013.
8. Nedel WL, Deutschendorf C, Moraes Rodrigues Filho E. High-flow nasal cannula in critically ill subjects with or at risk for respiratory failure: a systematic review and meta-analysis. Respir Care. 2017;62(1):123-132. https://doi.org/10.4187/respcare.04831.
9. Zhu Y, Yin H, Zhang R, Wei J. High-flow nasal cannula oxygen therapy vs conventional oxygen therapy in cardiac surgical patients: a meta-analysis. J Crit Care. 2017;38:123-128. https://doi.org/10.1016/j.jcrc.2016.10.027.
10. Leeies M, Flynn E, Turgeon AF, et al. High-flow oxygen via nasal cannulae in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Syst Rev. 2017;6(1):202. https://doi.org/10.1186/s13643-017-0593-5.
11. Hernandez G, Vaquero C, Gonzalez P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-1361. https://doi.org/10.1001/jama.2016.2711.
12. Wilson ME, Majzoub AM, Dobler CC, et al. Noninvasive ventilation in patients with do-not-intubate and comfort-measures-only orders: a systematic review and meta-analysis. Crit Care Med. 2018. 46(8):1209-1216. https://doi.org/10.1097/CCM.0000000000003082.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
14. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. https://doi.org/10.1001/jama.283.15.2008.
15. Brugger SC, Rodriguez S, Domingo J, et al. High-flow nasal cannula therapy (HFNC) for patients with severe acute respiratory failure and do not intubate orders. Pilot study. Palliative Medicine. 2014;28(6):755.
16. Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care. 2013;58(4):597-600. https://doi.org/10.4187/respcare.01887.
17. Coudroy R, Jamet A, Petua P, Robert R, Frat JP, Thille AW. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care. 2016;6(1):45. https://doi.org/10.1186/s13613-016-0151-7.
18. Delgado-Guay MO, Rodriguez-Nunez A, Adegboyega OO, et al. Characteristics and outcomes of advanced cancer patients admitted to an acute palliative care unit (PCU) with severe dyspnea receiving high flow oxygen (HFO). Journal of Clinical Oncology Conference. 2015;33(29 SUPPL. 1):247.
19. Epstein AS, Hartridge-Lambert SK, Ramaker JS, Voigt LP, Portlock CS. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med. 2011;14(7):835-839. https://doi.org/10.1089/jpm.2011.0005.
20. Harada K, Kurosawa S, Hino Y, et al. Clinical utility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematological disease. Springerplus. 2016;5(1):512. https://doi.org/10.1186/s40064-016-2161-1.
21. Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563-572. https://doi.org/10.1007/s00134-019-05590-5.
22. Halpern SD, Hansen-Flaschen J. Terminal withdrawal of life-sustaining supplemental oxygen. JAMA. 2006;296(11):1397-1400. https://doi.org/10.1001/jama.296.11.1397.
© 2019 Society of Hospital Medicine