User login
Chasing Hope: When Are Requests for Hospital Transfer a Place for Palliative Care Integration?
“I don’t think she’ll ever make it home again.”
I stood at the nursing station with two staff nurses from our medical ward. The patient, a woman with metastatic cancer and acute respiratory failure, had just arrived by a medical helicopter from an out-of-state community hospital. At the previous hospital, days had stretched into weeks, and she was not getting better. Limited documentation told us that the patient’s family sought further options at our facility. Beyond that, we knew little about the conversations that had transpired. The transfer had been arranged and off she went, arriving on our helipad in worsening respiratory distress, now needing a higher level of supplemental oxygen and teetering on the edge of endotracheal intubation. Above all, she was extremely uncomfortable from shortness of breath, and an urgent palliative care consult was placed a few hours after she touched down.
I arrived at the bedside to meet the patient and family. In these circumstances, I often feel that a palliative care consult is not the miracle that patients seek, but the grim consolation prize behind door two of a tragic and exhausting game show of a hospital transfer. I read the family’s dismayed facial expressions as they gazed at my badge reading “Palliative Care” and imagined that they would have preferred a different answer to their question that led them here: “Is this all that can be done?”
The hope of patients and families is a precious thing. As a palliative care physician, I recognize that my role is never to take away hope but rather reframe the wish for recovery into the realm of the achievable. Some people can pivot when the hospital transfer happens. They feel that they have “done all they could” and searched the earth for the reversal of their medical circumstances. I empathize with the wish to try everything and respect the motivation that lies behind it. I also see the cost of chasing hope.
In 2017, 1.5 million patients were transferred, comprising 3.5% of all hospital admissions.1 Although the majority of hospital transfers are medically appropriate and hold the possibility of treatment options unavailable at smaller facilities, there are also significant drawbacks. Transfers have been identified as risky times for adverse events, are often expensive, and are associated with higher mortality and longer length of stay.2 There are often lapses in documentation and provider communication, and handoff practices vary widely between hospitals.3 Some transferred seriously ill patients become functionally trapped in the accepting hospital. Patients arrive too sick to undergo any meaningful disease-directed therapy and too medically tenuous to return to their home community.
When patients and families seem overly hopeful about what a transfer might provide, the request for transfer may indicate a deeper need for empathic understanding. Clinical conversations about “what can be done” typically focus on medical aspects and often miss a critical element—a complete exploration of a seriously ill patient’s prognostic awareness.
In palliative care, we use the term prognostic awareness to define patients’ dynamic understanding of their prognosis in terms of likely longevity and quality of life. Accurate prognostic awareness—where there is concordance between a patient’s worries and the medical facts as understood by their treatment team—has been associated with enhanced quality of life and mood when patients have enough emotional reserve to actively cope with the illness; for example, by reframing things in a positive light.4,5 However, when patients are struggling to cope, talking about the prognosis is hard for patients and for their clinicians, who often accurately perceive their patient’s struggle and delay conversations, hoping that more time will help their patient better adapt and prepare to talk about it.6,7 However, delaying conversations makes it even harder for patients and families to develop accurate prognostic awareness, leaving them unprepared when medical decisions arise.8 Such delays have a particularly strong impact in nononcology care, where a more unpredictable illness trajectory makes it even harder for patients to understand and prepare for what might happen.
When seriously ill patients and families consider transfer to a tertiary medical center in a situation of medical crisis, it can be a good time to pause. Palliative care specialists are trained to communicate around these difficult points of transition, but generalist clinicians already involved in the patient’s care can also sensitively explore patients’ prognostic awareness as it relates to the hospital transfer.9 In the Table, The phrases mentioned suggest language that is helpful in broaching such discussions, which assesses the patient’s illness understanding, hopes, and worries. Asking about patients’ hopes for their illness enables clinicians to quickly know some of their most important priorities. Giving patients the permission to be future-oriented and positive also supports them to cope in these challenging conversations. Asking patients to identify two or three hopes places their most optimistic hopes within a larger context and can lead to a discussion of the potential tradeoffs of the transfer.10 For example, the hope for a little more time from treatments available through transfer may be at odds with the hope to spend as much time as possible with family. Once the patient’s hopes are better understood, the clinician can then ask about worries. Most seriously ill patients are deeply (often silently) worried about the future, and when asked, can articulate worries about dying that can be the foundation for an honest conversation about the likely course of the hospital transfer.
With empathic assessment, several patients can speak honestly with their clinicians about their illness, including the pros and cons of hospital transfer. However, some continue to struggle, often asking clinicians to remain positive and not endorsing any worry. We describe these patients as having low prognostic awareness.11 With such patients, palliative care expertise may be needed to ensure that patients and their families have the information they need to engage in informed medical decision-making. There are emerging models for distance palliative care integration, which may be helpful in these situations.12 If these technologies became common in practice, frontline clinicians may increasingly find virtual consultation helpful in working with patients to develop more accurate prognostic awareness. There is also the possibility of clinician-to-clinician electronic consultation, or peer coaching, where the patient is not seen directly by the consultant, but expert advice is offered to the local provider.13 Although both these innovations offer service that currently may not be available in certain care settings, in-person consultation remains the gold standard. If a nuanced discussion cannot be had, palliative care expertise may be the reason for transfer.
Back at our hospital, I met with the patient and her partner. There were tradeoffs to be made and hard truths to be acknowledged. In this unfamiliar place, with caregivers she had met just hours before, the patient changed her resuscitation order to allow for a natural death. She passed away later that evening, surrounded by her immediate family but far away from the community that had held her throughout her illness. I reflect on the loss that can come with choosing “everything” when efforts are often better spent on ensuring comfort. Although hospital transfer is often the right answer for seriously ill patients seeking diagnostic and therapeutic options unavailable at their home medical centers, the question should also be an impetus for a nuanced assessment of patients’ prognostic awareness to prepare patients if things do not go as hoped or to enlist palliative care expertise for those struggling to cope. As there is a workforce shortage of palliative care providers, particularly in smaller and rural American hospitals, frontline hospitalist clinicians may find themselves increasingly playing a critical role in discussions where transfer is considered. By assessing patients’ prognostic awareness through thoughtful, compassionate inquiry in these moments of transition, we can support informed medical decision-making.
1. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. 2017;13(4):187-191. https://doi.org/10.1097/PTS.0000000000000148.
2. Ligtenberg JJ, Arnold LG, Stienstra Y, et al. Quality of inter‐hospital transport of critically ill patients: a prospective audit. Crit Care. 2005;9(4):R446-R451. https://doi.org/10.1097/PTS.0000000000000148.
3. Payne CE, Stein JM, Leong T, Dressler DD. Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925-932. https://doi.org/10.1136/bmjqs-2011-000308.
4. Nipp RD, Greer JA, El-Jawahri A, et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Onc. 2017;1(22):2551-2557. https://doi.org/10.1200/JCO.2016.71.3404.
5. El-Jawahri A, Traeger L, Park, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. https://doi.org/10.1002/cncr.28369.
6. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326. https://doi.org/10.1200/JCO.2010.32.4459.
7. Bernacki R, Paladino J, Neville BA, et al. Effect of the serious illness care program in outpatient oncology: a cluster randomized clinical trial. JAMA Intern Med. 2019;2019179(6):751-759. https://doi.org/10.1001/jamainternmed.2019.0077.
8. Jackson VA, Jacobsen J, Greer JA, et al. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900. https://doi.org/10.1089/jpm.2012.0547.
9. Lakin JR, Jacobsen J. Softening our approach to discussing prognosis. JAMA Intern Med. 2019;179(1):5-6. https://doi.org/10.1001/jamainternmed.2018.5786.
10. Nipp RD, El-Jawahri A, Fishbein JN, et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122(13):2110-2116. https://doi.org/10.1002/cncr.30025.
11. El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. https://doi.org/10.1002/cncr.28369.
12. Tasneem S, Kim A, Bagheri A, Lebret J. Telemedicine video visits for patients receiving palliative care: A qualitative study. Am J Hosp Palliat Care. 2019;36(9):789-794. https://doi.org/10.1177/1049909119846843.
13. Lustbader D, Mudra M, Romano C, et al. The impact of a home-based palliative care program in an accountable care organization. J Palliat Med. 2017;20:23-28. https://doi.org/10.1089/jpm.2016.0265.
“I don’t think she’ll ever make it home again.”
I stood at the nursing station with two staff nurses from our medical ward. The patient, a woman with metastatic cancer and acute respiratory failure, had just arrived by a medical helicopter from an out-of-state community hospital. At the previous hospital, days had stretched into weeks, and she was not getting better. Limited documentation told us that the patient’s family sought further options at our facility. Beyond that, we knew little about the conversations that had transpired. The transfer had been arranged and off she went, arriving on our helipad in worsening respiratory distress, now needing a higher level of supplemental oxygen and teetering on the edge of endotracheal intubation. Above all, she was extremely uncomfortable from shortness of breath, and an urgent palliative care consult was placed a few hours after she touched down.
I arrived at the bedside to meet the patient and family. In these circumstances, I often feel that a palliative care consult is not the miracle that patients seek, but the grim consolation prize behind door two of a tragic and exhausting game show of a hospital transfer. I read the family’s dismayed facial expressions as they gazed at my badge reading “Palliative Care” and imagined that they would have preferred a different answer to their question that led them here: “Is this all that can be done?”
The hope of patients and families is a precious thing. As a palliative care physician, I recognize that my role is never to take away hope but rather reframe the wish for recovery into the realm of the achievable. Some people can pivot when the hospital transfer happens. They feel that they have “done all they could” and searched the earth for the reversal of their medical circumstances. I empathize with the wish to try everything and respect the motivation that lies behind it. I also see the cost of chasing hope.
In 2017, 1.5 million patients were transferred, comprising 3.5% of all hospital admissions.1 Although the majority of hospital transfers are medically appropriate and hold the possibility of treatment options unavailable at smaller facilities, there are also significant drawbacks. Transfers have been identified as risky times for adverse events, are often expensive, and are associated with higher mortality and longer length of stay.2 There are often lapses in documentation and provider communication, and handoff practices vary widely between hospitals.3 Some transferred seriously ill patients become functionally trapped in the accepting hospital. Patients arrive too sick to undergo any meaningful disease-directed therapy and too medically tenuous to return to their home community.
When patients and families seem overly hopeful about what a transfer might provide, the request for transfer may indicate a deeper need for empathic understanding. Clinical conversations about “what can be done” typically focus on medical aspects and often miss a critical element—a complete exploration of a seriously ill patient’s prognostic awareness.
In palliative care, we use the term prognostic awareness to define patients’ dynamic understanding of their prognosis in terms of likely longevity and quality of life. Accurate prognostic awareness—where there is concordance between a patient’s worries and the medical facts as understood by their treatment team—has been associated with enhanced quality of life and mood when patients have enough emotional reserve to actively cope with the illness; for example, by reframing things in a positive light.4,5 However, when patients are struggling to cope, talking about the prognosis is hard for patients and for their clinicians, who often accurately perceive their patient’s struggle and delay conversations, hoping that more time will help their patient better adapt and prepare to talk about it.6,7 However, delaying conversations makes it even harder for patients and families to develop accurate prognostic awareness, leaving them unprepared when medical decisions arise.8 Such delays have a particularly strong impact in nononcology care, where a more unpredictable illness trajectory makes it even harder for patients to understand and prepare for what might happen.
When seriously ill patients and families consider transfer to a tertiary medical center in a situation of medical crisis, it can be a good time to pause. Palliative care specialists are trained to communicate around these difficult points of transition, but generalist clinicians already involved in the patient’s care can also sensitively explore patients’ prognostic awareness as it relates to the hospital transfer.9 In the Table, The phrases mentioned suggest language that is helpful in broaching such discussions, which assesses the patient’s illness understanding, hopes, and worries. Asking about patients’ hopes for their illness enables clinicians to quickly know some of their most important priorities. Giving patients the permission to be future-oriented and positive also supports them to cope in these challenging conversations. Asking patients to identify two or three hopes places their most optimistic hopes within a larger context and can lead to a discussion of the potential tradeoffs of the transfer.10 For example, the hope for a little more time from treatments available through transfer may be at odds with the hope to spend as much time as possible with family. Once the patient’s hopes are better understood, the clinician can then ask about worries. Most seriously ill patients are deeply (often silently) worried about the future, and when asked, can articulate worries about dying that can be the foundation for an honest conversation about the likely course of the hospital transfer.
With empathic assessment, several patients can speak honestly with their clinicians about their illness, including the pros and cons of hospital transfer. However, some continue to struggle, often asking clinicians to remain positive and not endorsing any worry. We describe these patients as having low prognostic awareness.11 With such patients, palliative care expertise may be needed to ensure that patients and their families have the information they need to engage in informed medical decision-making. There are emerging models for distance palliative care integration, which may be helpful in these situations.12 If these technologies became common in practice, frontline clinicians may increasingly find virtual consultation helpful in working with patients to develop more accurate prognostic awareness. There is also the possibility of clinician-to-clinician electronic consultation, or peer coaching, where the patient is not seen directly by the consultant, but expert advice is offered to the local provider.13 Although both these innovations offer service that currently may not be available in certain care settings, in-person consultation remains the gold standard. If a nuanced discussion cannot be had, palliative care expertise may be the reason for transfer.
Back at our hospital, I met with the patient and her partner. There were tradeoffs to be made and hard truths to be acknowledged. In this unfamiliar place, with caregivers she had met just hours before, the patient changed her resuscitation order to allow for a natural death. She passed away later that evening, surrounded by her immediate family but far away from the community that had held her throughout her illness. I reflect on the loss that can come with choosing “everything” when efforts are often better spent on ensuring comfort. Although hospital transfer is often the right answer for seriously ill patients seeking diagnostic and therapeutic options unavailable at their home medical centers, the question should also be an impetus for a nuanced assessment of patients’ prognostic awareness to prepare patients if things do not go as hoped or to enlist palliative care expertise for those struggling to cope. As there is a workforce shortage of palliative care providers, particularly in smaller and rural American hospitals, frontline hospitalist clinicians may find themselves increasingly playing a critical role in discussions where transfer is considered. By assessing patients’ prognostic awareness through thoughtful, compassionate inquiry in these moments of transition, we can support informed medical decision-making.
“I don’t think she’ll ever make it home again.”
I stood at the nursing station with two staff nurses from our medical ward. The patient, a woman with metastatic cancer and acute respiratory failure, had just arrived by a medical helicopter from an out-of-state community hospital. At the previous hospital, days had stretched into weeks, and she was not getting better. Limited documentation told us that the patient’s family sought further options at our facility. Beyond that, we knew little about the conversations that had transpired. The transfer had been arranged and off she went, arriving on our helipad in worsening respiratory distress, now needing a higher level of supplemental oxygen and teetering on the edge of endotracheal intubation. Above all, she was extremely uncomfortable from shortness of breath, and an urgent palliative care consult was placed a few hours after she touched down.
I arrived at the bedside to meet the patient and family. In these circumstances, I often feel that a palliative care consult is not the miracle that patients seek, but the grim consolation prize behind door two of a tragic and exhausting game show of a hospital transfer. I read the family’s dismayed facial expressions as they gazed at my badge reading “Palliative Care” and imagined that they would have preferred a different answer to their question that led them here: “Is this all that can be done?”
The hope of patients and families is a precious thing. As a palliative care physician, I recognize that my role is never to take away hope but rather reframe the wish for recovery into the realm of the achievable. Some people can pivot when the hospital transfer happens. They feel that they have “done all they could” and searched the earth for the reversal of their medical circumstances. I empathize with the wish to try everything and respect the motivation that lies behind it. I also see the cost of chasing hope.
In 2017, 1.5 million patients were transferred, comprising 3.5% of all hospital admissions.1 Although the majority of hospital transfers are medically appropriate and hold the possibility of treatment options unavailable at smaller facilities, there are also significant drawbacks. Transfers have been identified as risky times for adverse events, are often expensive, and are associated with higher mortality and longer length of stay.2 There are often lapses in documentation and provider communication, and handoff practices vary widely between hospitals.3 Some transferred seriously ill patients become functionally trapped in the accepting hospital. Patients arrive too sick to undergo any meaningful disease-directed therapy and too medically tenuous to return to their home community.
When patients and families seem overly hopeful about what a transfer might provide, the request for transfer may indicate a deeper need for empathic understanding. Clinical conversations about “what can be done” typically focus on medical aspects and often miss a critical element—a complete exploration of a seriously ill patient’s prognostic awareness.
In palliative care, we use the term prognostic awareness to define patients’ dynamic understanding of their prognosis in terms of likely longevity and quality of life. Accurate prognostic awareness—where there is concordance between a patient’s worries and the medical facts as understood by their treatment team—has been associated with enhanced quality of life and mood when patients have enough emotional reserve to actively cope with the illness; for example, by reframing things in a positive light.4,5 However, when patients are struggling to cope, talking about the prognosis is hard for patients and for their clinicians, who often accurately perceive their patient’s struggle and delay conversations, hoping that more time will help their patient better adapt and prepare to talk about it.6,7 However, delaying conversations makes it even harder for patients and families to develop accurate prognostic awareness, leaving them unprepared when medical decisions arise.8 Such delays have a particularly strong impact in nononcology care, where a more unpredictable illness trajectory makes it even harder for patients to understand and prepare for what might happen.
When seriously ill patients and families consider transfer to a tertiary medical center in a situation of medical crisis, it can be a good time to pause. Palliative care specialists are trained to communicate around these difficult points of transition, but generalist clinicians already involved in the patient’s care can also sensitively explore patients’ prognostic awareness as it relates to the hospital transfer.9 In the Table, The phrases mentioned suggest language that is helpful in broaching such discussions, which assesses the patient’s illness understanding, hopes, and worries. Asking about patients’ hopes for their illness enables clinicians to quickly know some of their most important priorities. Giving patients the permission to be future-oriented and positive also supports them to cope in these challenging conversations. Asking patients to identify two or three hopes places their most optimistic hopes within a larger context and can lead to a discussion of the potential tradeoffs of the transfer.10 For example, the hope for a little more time from treatments available through transfer may be at odds with the hope to spend as much time as possible with family. Once the patient’s hopes are better understood, the clinician can then ask about worries. Most seriously ill patients are deeply (often silently) worried about the future, and when asked, can articulate worries about dying that can be the foundation for an honest conversation about the likely course of the hospital transfer.
With empathic assessment, several patients can speak honestly with their clinicians about their illness, including the pros and cons of hospital transfer. However, some continue to struggle, often asking clinicians to remain positive and not endorsing any worry. We describe these patients as having low prognostic awareness.11 With such patients, palliative care expertise may be needed to ensure that patients and their families have the information they need to engage in informed medical decision-making. There are emerging models for distance palliative care integration, which may be helpful in these situations.12 If these technologies became common in practice, frontline clinicians may increasingly find virtual consultation helpful in working with patients to develop more accurate prognostic awareness. There is also the possibility of clinician-to-clinician electronic consultation, or peer coaching, where the patient is not seen directly by the consultant, but expert advice is offered to the local provider.13 Although both these innovations offer service that currently may not be available in certain care settings, in-person consultation remains the gold standard. If a nuanced discussion cannot be had, palliative care expertise may be the reason for transfer.
Back at our hospital, I met with the patient and her partner. There were tradeoffs to be made and hard truths to be acknowledged. In this unfamiliar place, with caregivers she had met just hours before, the patient changed her resuscitation order to allow for a natural death. She passed away later that evening, surrounded by her immediate family but far away from the community that had held her throughout her illness. I reflect on the loss that can come with choosing “everything” when efforts are often better spent on ensuring comfort. Although hospital transfer is often the right answer for seriously ill patients seeking diagnostic and therapeutic options unavailable at their home medical centers, the question should also be an impetus for a nuanced assessment of patients’ prognostic awareness to prepare patients if things do not go as hoped or to enlist palliative care expertise for those struggling to cope. As there is a workforce shortage of palliative care providers, particularly in smaller and rural American hospitals, frontline hospitalist clinicians may find themselves increasingly playing a critical role in discussions where transfer is considered. By assessing patients’ prognostic awareness through thoughtful, compassionate inquiry in these moments of transition, we can support informed medical decision-making.
1. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. 2017;13(4):187-191. https://doi.org/10.1097/PTS.0000000000000148.
2. Ligtenberg JJ, Arnold LG, Stienstra Y, et al. Quality of inter‐hospital transport of critically ill patients: a prospective audit. Crit Care. 2005;9(4):R446-R451. https://doi.org/10.1097/PTS.0000000000000148.
3. Payne CE, Stein JM, Leong T, Dressler DD. Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925-932. https://doi.org/10.1136/bmjqs-2011-000308.
4. Nipp RD, Greer JA, El-Jawahri A, et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Onc. 2017;1(22):2551-2557. https://doi.org/10.1200/JCO.2016.71.3404.
5. El-Jawahri A, Traeger L, Park, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. https://doi.org/10.1002/cncr.28369.
6. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326. https://doi.org/10.1200/JCO.2010.32.4459.
7. Bernacki R, Paladino J, Neville BA, et al. Effect of the serious illness care program in outpatient oncology: a cluster randomized clinical trial. JAMA Intern Med. 2019;2019179(6):751-759. https://doi.org/10.1001/jamainternmed.2019.0077.
8. Jackson VA, Jacobsen J, Greer JA, et al. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900. https://doi.org/10.1089/jpm.2012.0547.
9. Lakin JR, Jacobsen J. Softening our approach to discussing prognosis. JAMA Intern Med. 2019;179(1):5-6. https://doi.org/10.1001/jamainternmed.2018.5786.
10. Nipp RD, El-Jawahri A, Fishbein JN, et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122(13):2110-2116. https://doi.org/10.1002/cncr.30025.
11. El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. https://doi.org/10.1002/cncr.28369.
12. Tasneem S, Kim A, Bagheri A, Lebret J. Telemedicine video visits for patients receiving palliative care: A qualitative study. Am J Hosp Palliat Care. 2019;36(9):789-794. https://doi.org/10.1177/1049909119846843.
13. Lustbader D, Mudra M, Romano C, et al. The impact of a home-based palliative care program in an accountable care organization. J Palliat Med. 2017;20:23-28. https://doi.org/10.1089/jpm.2016.0265.
1. Hernandez-Boussard T, Davies S, McDonald K, Wang NE. Interhospital facility transfers in the United States: a nationwide outcomes study. J Patient Saf. 2017;13(4):187-191. https://doi.org/10.1097/PTS.0000000000000148.
2. Ligtenberg JJ, Arnold LG, Stienstra Y, et al. Quality of inter‐hospital transport of critically ill patients: a prospective audit. Crit Care. 2005;9(4):R446-R451. https://doi.org/10.1097/PTS.0000000000000148.
3. Payne CE, Stein JM, Leong T, Dressler DD. Avoiding handover fumbles: a controlled trial of a structured handover tool versus traditional handover methods. BMJ Qual Saf. 2012;21(11):925-932. https://doi.org/10.1136/bmjqs-2011-000308.
4. Nipp RD, Greer JA, El-Jawahri A, et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Onc. 2017;1(22):2551-2557. https://doi.org/10.1200/JCO.2016.71.3404.
5. El-Jawahri A, Traeger L, Park, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. https://doi.org/10.1002/cncr.28369.
6. Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319-2326. https://doi.org/10.1200/JCO.2010.32.4459.
7. Bernacki R, Paladino J, Neville BA, et al. Effect of the serious illness care program in outpatient oncology: a cluster randomized clinical trial. JAMA Intern Med. 2019;2019179(6):751-759. https://doi.org/10.1001/jamainternmed.2019.0077.
8. Jackson VA, Jacobsen J, Greer JA, et al. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900. https://doi.org/10.1089/jpm.2012.0547.
9. Lakin JR, Jacobsen J. Softening our approach to discussing prognosis. JAMA Intern Med. 2019;179(1):5-6. https://doi.org/10.1001/jamainternmed.2018.5786.
10. Nipp RD, El-Jawahri A, Fishbein JN, et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122(13):2110-2116. https://doi.org/10.1002/cncr.30025.
11. El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. https://doi.org/10.1002/cncr.28369.
12. Tasneem S, Kim A, Bagheri A, Lebret J. Telemedicine video visits for patients receiving palliative care: A qualitative study. Am J Hosp Palliat Care. 2019;36(9):789-794. https://doi.org/10.1177/1049909119846843.
13. Lustbader D, Mudra M, Romano C, et al. The impact of a home-based palliative care program in an accountable care organization. J Palliat Med. 2017;20:23-28. https://doi.org/10.1089/jpm.2016.0265.
© 2019 Society of Hospital Medicine
New Answers for Old Questions in the Treatment of Severe Infections from Injection Drug Use
As a result of the epidemic of opioid use disorder (OUD), there has been a secondary surge in hospitalizations for infectious complications of injection drug use (IDU).1,2 In the previous 10 years, there have been significant increases in IDU-associated human immunodeficiency virus (HIV)3 and hepatitis C virus (HCV)4 infection as well as increased hospitalizations from IDU-associated skin and soft tissue infections, osteomyelitis, septic arthritis, bacteremia, fungemia, and infective endocarditis in the United States.2,5-7 Patients admitted with IDU-associated infections have long lengths of stay, high rates of leaving against medical advice (AMA), readmission, and mortality.8-13 In a British cohort (median age 36 years), five-year mortality after an episode of IDU-associated endocarditis was 42%.14 Admissions for IDU-associated infections can be a troubling experience for both patients and providers alike.15 While management decisions of IDU-associated infectious syndromes have sometimes been based on emotion, dogma, and an often-stigmatizing approach toward people suffering from addiction,16 with a better understanding of addiction and effective treatments, as well as accumulating data in both addiction and infectious disease fields, it is an appropriate time to reevaluate the approach to treatment.
The goal of this review is to examine recent evidence and attempt to answer questions that frequently arise in the management of hospitalized patients with IDU-associated infections
KEY MANAGEMENT QUESTIONS IN THE INPATIENT MANAGEMENT OF INFECTIOUS COMPLICATIONS OF OUD
How Should OUD Be Managed in the Hospital?
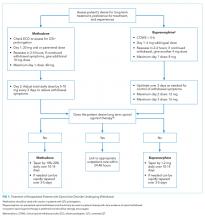
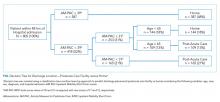
Management of an IDU-associated infection is incomplete without addressing the underlying addiction in some way. Addiction is highly undertreated among patients with IDU-associated infections, which may contribute to poor infection-related outcomes.8,13,17 Opioid agonist therapy (buprenorphine and methadone) to prevent withdrawal should be routinely offered to all patients with OUD including those with infectious complications of OUD to facilitate appropriate medical treatment and engage patients in long-term addiction treatment. Referral to addiction treatment has been associated with improved IDU-associated endocarditis mortality,18 and initiation of medications for OUD (MOUD) can be achieved successfully in the emergency department, inpatient wards, and specifically in patients admitted with IDU-associated endocarditis.19-21 Protocols and resources for inpatient management of withdrawal and initiation of MOUD are available along with telephone support services for providers seeking guidance on specific cases.21,22 Inpatient addiction consult services are an important resource for the management of hospitalized patients with addiction and are associated with increased completion of antibiotics, decreased AMA discharge, and increased rates of MOUD provision among patients with IDU-associated infections.12 However, when unavailable, initiation of opioid agonist therapy does not require an addiction specialist. Linkage to outpatient addiction care is ideal; however, opioid agonist therapy initiated in the hospital can be tapered prior to discharge if this is unavailable. Figure 1 outlines the initiation of methadone or buprenorphine for the treatment of both withdrawal and OUD in the inpatient setting.20,21
Who Can Prescribe Medications for Treatment of OUD in Hospitalized Patients?
Although buprenorphine prescribing in the outpatient setting requires certification, inpatient physicians are exempt from these requirements and can prescribe buprenorphine or methadone in the hospital setting.20 In the outpatient setting, buprenorphine prescription is restricted to providers with a Drug Addiction Treatment Act of 2000 (DATA 2000) waiver, also known as an “X-waiver”. X-waiver training is eight hours, and free web-based training is available.23 At the time of discharge, non-X-waivered physicians can prescribe up to 72 hours of buprenorphine as a bridge to follow-up with outpatient addiction services.24 In the outpatient setting, methadone can only be obtained through approved methadone maintenance programs (MMP); however, many such programs are often willing to do intakes on the same day or next day following hospital discharge.
Is It Safe to Place a Peripherally Inserted Central Catheter in a Patient Who Injects Drugs?
Many practitioners believe that IDU is an absolute contraindication to the use of peripherally inserted central catheters (PICC) for administration of antimicrobials; however, evidence of harm is lacking.25,26 In a review of outpatient parenteral antimicrobial therapy (OPAT) in patients with IDU, there were low overall rates of line-related adverse events and no significant difference in complications between IDU and non-IDU patients receiving OPAT.27 As with any medical intervention, risks and benefits must be balanced. Aside from patient comfort, a PICC allows patients to receive intravenous (IV) antimicrobials in a nonhospital setting, which may be more therapeutic for their addiction. Peripheral venous access can be difficult in patients with IDU who often have atrophic superficial veins. While often cited as a reason to avoid PICCs, there is no empirical evidence that PICC placement leads to increased drug use among people with OUD. Similarly, depriving a patient of a PICC does not prevent drug use, but it may prevent patients from completing infection treatment in a more acceptable setting. The most serious concern with a PICC is that if a patient injects drugs, transient bacteremia/fungemia could seed this prosthetic material and lead to worsening infection. Providers should employ a risk-based approach to the use of PICCs considering patient preferences, addiction disease activity, and stability of home environment weighed against the potential risks of prolonged hospitalization, clinic-based antibiotic infusions through a peripheral IV, or possibly suboptimal oral antimicrobial treatment.
What Is the Best Location for Patients to Receive Antibiotics for Their IDU-Associated Infection?
Antimicrobial treatment for severe IDU-associated infections such as endocarditis and osteomyelitis has traditionally included four- to six-week hospital admissions to complete the entirety of IV therapy. This practice has recently been called into question. Extended hospitalization for patients with IDU-associated infections—often not receiving evidence-based treatment for their addiction—can be a harrowing experience and may be antitherapeutic.15,28 Disposition decisions for patients with IDU-associated infections should involve risk stratification to assess addiction disease activity and take into account inpatient addiction treatment resources and patient preference, culture/availability of skilled nursing facilities (SNFs), and safety of the home environment.29 Some emerging models of care take advantage of long hospitalizations by engaging patients with comprehensive addiction services including substance use group meetings, counseling, and social resources. Another model using OPAT with intensive outpatient follow-up for both addiction and infection treatment showed similar infection outcomes, lower cost, and improved patient satisfaction compared with in-hospital treatment.30 When available, medical respite programs and OPAT-friendly residential addiction programs have shown success and financial savings as well.31,32 Still, many patients would prefer home OPAT, and there is evidence that home OPAT is no less successful than OPAT provided in an SNF.33 Despite this mounting evidence, there remains systemic stigmatization of people with OUD and inequity as many SNFs, and home infusion companies will not provide either MOUD or services to patients with OUD.34
Can Oral Antibiotics Be Used to Treat Severe Infections Due to IDU?
A general principle of infectious diseases is that oral antibiotics should be used whenever possible when presumed to be noninferior to IV alternatives. Accumulating evidence in the infectious disease literature suggests that there is a role for increasing the use of oral antibiotics for serious infections. Two recent pivotal randomized trials have questioned the dogma surrounding the use of IV antibiotics for the management of orthopedic infections and endocarditis. However, these studies included few patients with infections due to IDU.35,36 One study of oral antibiotics specifically in patients with IDU-associated infection showed that an all-oral regimen for the management of IDU-associated right-sided endocarditis was effective and well-tolerated.37 While oral antibiotics decrease the need for long-term hospitalization and OPAT, similar or even more intensive follow-up of these patients is required to ensure an appropriate response to treatment. Oral antibiotics should not be used to simply expedite discharge but instead should be done with careful planning and close follow-up.
When using oral antibiotics for severe infections, attempts should be made to use agents with the highest oral bioavailability, tolerability, and affordability. Antimicrobials with near-complete oral bioavailability include fluoroquinolones, triazoles, oxazolidinones (linezolid and tedizolid), clindamycin, trimethoprim-sulfamethoxazole, doxycycline, metronidazole, cefadroxil, and other select oral cephalosporins. One approach is to complete a short course of inpatient induction therapy with IV antimicrobials followed by consolidation therapy with oral antibiotics. In a study of uncomplicated Staphylococcus aureus bacteremia, a similar approach with initial IV therapy and oral linezolid follow-up treatment was noninferior to all-IV treatment.38 Decisions about the early transition to oral antimicrobials should be made in conjunction with infectious disease specialists where available.
What Is the Role of Long Half-Life IV Antibiotics for Treating IDU-Associated Infections?
Dalbavancin and oritavancin are extremely long half-life IV glycopeptide antibiotics for gram-positive bacterial infections that require, at most, weekly administration. These agents allow IV-equivalent antibiotics to be delivered without the need for daily infusions or PICCs. Currently, both are approved by the United States Food and Drug Administration only for skin and skin structure infections, but there are increasing reports of successful use in more severe infections including osteomyelitis, bacteremia, and endocarditis.39-42
Is Surgical Placement of Prosthetic Material Safe in Patients With IDU-Associated Infections?
When surgery for an IDU-associated infection has the potential to be acutely lifesaving, it should be offered. There is a concern that surgical interventions that require placement of prosthetic material might serve as a nidus of future infection in the setting of ongoing IDU. Although treatments for many substance use disorders are effective—particularly medications to treat OUD—addiction is a relapsing chronic condition, and at least, some future drug use is an expected part of the course. Research comparing outcomes after valve surgery between IDU and non-IDU-associated endocarditis patients shows no difference in short-term outcomes,44 but longer-term data show increased mortality between 60 and 180 days postoperatively, higher rates of valve-related complications, and up to 53% reinfection rates.10,45,46 These studies are limited by the lack of a nonsurgically treated control group and little information on the rate of addiction treatment, which may be protective against these negative outcomes. In contrast, another study found that surgery was the strongest predictor of survival among patients with IDU-associated endocarditis after a median of 3.6 years follow-up.18 Another consideration is that patients with IDU-associated infection tend to be younger, and despite advancements, many modern prostheses have a finite lifespan. When multiple surgical options exist, a procedure that avoids prosthetic material is preferred. For example, in a meta-analysis of studies of tricuspid valve endocarditis (41% IDU-associated), there was no mortality difference between valve repair compared with valve replacement, but there was a significantly lower rate of recurrent endocarditis among those with a repair only.47 Decisions about surgery must be individualized and consider a patient’s engagement in OUD treatment, social support, prior success with treatment, treatment and relapse prevention resources, and access to harm reduction interventions such as sterile syringes.
What Are Appropriate Harm Reduction Interventions for Patients Hospitalized With Infections Due to IDU?
A prolonged admission for IDU-associated infections is an opportunity to provide patients with education, health maintenance services, and secondary prevention interventions for both infection and overdose. Based on epidemiologic risk, patients should be screened for HIV, HCV, hepatitis B, syphilis, gonorrhea, and chlamydia. Patients should be vaccinated against hepatitis A, influenza, and tetanus (and pneumococcus if indicated), if unvaccinated or without vaccination records. Patients positive for HIV should be evaluated by an infectious disease specialist with consideration of the rapid initiation of antiretroviral therapy. Patients positive for HCV or hepatitis B should be referred for treatment in the outpatient setting. Patients without HIV should be educated about HIV preexposure prophylaxis and referred to outpatient services.
Harm reduction involves meeting patients where they are and providing services they are willing to accept to improve their health or prevent negative outcomes. One important strategy for reducing harm involves maintaining patients in care for their addiction and infection as much as possible, ideally avoiding AMA discharge. In one cohort of patients admitted with IDU-associated infections and OUD, 49% of those without an addiction medicine consult left AMA.12 If a patient plans to leave AMA, all efforts should be made to provide them with oral antibiotics that might be effective, even if suboptimal, for their infection. Hospitalists should consider documenting an oral “antibiotic contingency plan” that can be rapidly enacted if a patient is imminently leaving the hospital. They should be provided with outpatient follow-up appointments with infectious disease or primary care. All patients with IDU-associated infections should be discharged with naloxone, overdose prevention education, and community resources for addiction treatment and syringe exchange programs.
GENERAL APPROACH TO INPATIENT MANAGEMENT OF INFECTIOUS COMPLICATIONS OF OUD
Management of IDU-associated infection should be organized around a multidisciplinary framework with careful attention to infection treatment, OUD treatment, and harm reduction interventions (Figure 2). The first step in managing IDU-associated infections is recognizing addiction in the acute care setting. Substance use disorders, including OUD, are often unrecognized in patients presenting with IDU-associated infections.48 The Rapid Opioid Dependence Screen, a validated screening tool for OUD, can be quickly administered for all patients who present with endocarditis, bacteremia, skin and soft tissue infections, vertebral and epidural infections, and HIV and HCV infections.49 In addition to directly questioning patients about substance use, Figure 2 lists epidemiologic, physical exam, and laboratory findings that might suggest to the provider that OUD may be present.
The approach to infection management is similar to non-IDU-associated infections, including identifying a source, evaluating for complications and need for source control procedures, and administering antimicrobials. Management of the substance use disorder includes treatment of acute withdrawal, control of pain, initiation of MOUD when appropriate, and linkage to outpatient addiction treatment services in addition to harm reduction interventions.
CONCLUSION
Hospital admissions for infectious complications of IDU are increasingly common and are difficult experiences for both patients and providers. However, these hospitalizations serve as a “reachable moment” to engage patients with OUD into medical care and initiate holistic treatment of their infection and underlying substance use disorder.28,50
Disclosures
The authors both report no conflict of interest.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445-1452. https://doi.org/10.15585/mmwr.mm655051e1.
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
3. Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375(3):229-239. https://doi.org/10.1056/NEJMoa1515195.
4. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years — Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453-458.
5. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis. 2016;3(3):ofw157. https://doi.org/10.1093/ofid/ofw157.
6. Hartnett KP, Jackson KA, Felsen C, et al. Bacterial and fungal infections in persons who inject drugs - Western New York, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(26):583-586. https://doi.org/10.15585/mmwr.mm6826a2.
7. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2019.170(1):31-40. https://doi.org/10.7326/M18-2124,
8. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024.
9. Rabkin DG, Mokadam NA, Miller DW, Goetz RR, Verrier ED, Aldea GS. Long-term outcome for the surgical treatment of infective endocarditis with a focus on intravenous drug users. Ann Thorac Surg. 2012;93(1):51-57. https://doi.org/10.1016/j.athoracsur.2011.08.016.
10. Kim JB, Ejiofor JI, Yammine M, et al. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016;152(3):832-841.e1. https://doi.org/10.1016/j.jtcvs.2016.02.072
11. Leahey PA, LaSalvia MT, Rosenthal ES, Karchmer AW, Rowley CF. High morbidity and mortality among patients with sentinel admission for injection drug use-related infective endocarditis. Open Forum Infect Dis. 2019;6(4):ofz089. https://doi.org/10.1093/ofid/ofz089.
12. Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis. 2019;68(11):1935-1937. https://doi.org/10.1093/cid/ciy924.
13. Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated Staphylococcus aureus bacteremia. Open Forum Infect Dis. 2019;6(7):ofz289. https://doi.org/10.1093/ofid/ofz289.
14. Straw S, Baig MW, Gillott R, et al. Long-term outcomes are poor in intravenous drug users following infective endocarditis, even after surgery. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz869.
15. Bearnot BI, Mitton JA, Hayden M, Park ER. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: Results from a qualitative study. J Subst Abuse Treat. 2019;102:16-22. https://doi.org/10.1016/j.jsat.2019.04.008.
16. Hull SC, Jadbabaie F. When is enough enough? The dilemma of valve replacement in a recidivist intravenous drug user. Ann Thorac Surg. 2014;97(5):1486-1487. https://doi.org/10.1016/j.athoracsur.2014.02.010.
17. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/ADM.0000000000000454.
18. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open. 2018;1(7):e185220. https://doi.org/10.1001/jamanetworkopen.2018.5220.
19. Suzuki J. Medication-assisted treatment for hospitalized patients with intravenous-drug-use related infective endocarditis. Am J Addict. 2016;25(3):191-194. https://doi.org/10.1111/ajad.12349.
20. Theisen-Toupal J, Ronan MV, Moore A, Rosenthal ES. Inpatient management of opioid use disorder: a review for hospitalists. J Hosp Med. 2017;12(5):369-374. https://doi.org/10.12788/jhm.2731.
21. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. https://doi.org/10.1503/cmaj.160290.
22. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the improving addiction care team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
23. 8 Hour Online MAT Waiver Training. Providers Clinical Support System 2019; https://learning.pcssnow.org/p/onlinematwaiver. Accessed May 22, 2019.
24. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther. 2019;8(2):171-184. https://doi.org/10.1007/s40121-019-0247-0.
25. Rapoport AB, Fischer LS, Santibanez S, Beekmann SE, Polgreen PM, Rowley CF. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis. 2018;5(7):ofy132. https://doi.org/10.1093/ofid/ofy132.
26. Fanucchi L, Leedy N, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581-582. https://doi.org/10.1002/jhm.2582.
27. Suzuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. https://doi.org/10.1093/ofid/ofy194.
28. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an Experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4.
29. Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs requiring intravenous antibiotics may allow health systems to focus inpatient resources on those at greatest risk of ongoing drug use. Clin Infect Dis. 2019;68(6):1041-1043. https://doi.org/10.1093/cid/ciy722.
30. Fanucchi LC, Walsh SL, Thornton AC, Nuzzo PA, Lofwall MR. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis. 2019.pii:ciz654. https://doi.org/10.1093/cid/ciz654.
31. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. https://doi.org/10.1002/jhm.2597.
32. Jewell C, Weaver M, Sgroi C, Anderson K, Sayeed Z. Residential addiction treatment for injection drug users requiring intravenous antibiotics: a cost-reduction strategy. J Addict Med. 2013;7(4):271-276. https://doi.org/10.1097/ADM.0b013e318294b1eb.
33. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. https://doi.org/10.1093/ofid/ofy056.
34. Wakeman SE, Rich JD. Barriers to post-acute care for patients on opioid agonist therapy; an example of systematic stigmatization of addiction. J Gen Intern Med. 2017;32(1):17-19. https://doi.org/10.1007/s11606-016-3799-7.
35. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. Jan 31 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312.
36. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926.
37. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. Nov 4 1989;2(8671):1071-1073. https://doi.org/10.1016/s0140-6736(89)91083-0
38. Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy916.
39. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. https://doi.org/10.1093/ofid/ofy331.
40. Morata L, Cobo J, Fernandez-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother. 2019;63(5).pii. e02280-18. https://doi.org/10.1128/AAC.02280-18.
41. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis. 2018;67(5):795-798. https://doi.org/10.1093/cid/ciy279.
42. Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int J Infect Dis. 2019;81:210-214. https://doi.org/10.1016/j.ijid.2019.02.013.
43. Bryson-Cahn C, Beieler AM, Chan JD, Harrington RD, Dhanireddy S. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis. 2019;6(2):ofz028. https://doi.org/10.1093/ofid/ofz028.
44. Hall R, Shaughnessy M, Boll G, et al. Drug-use and post-operative mortality following valve surgery for infective endocarditis: a systematic review and meta-analysis. Clin Infect Dis. 2019;69(7):1120-1129. https://doi.org/10.1093/cid/ciy1064.
45. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100(3):875-882. https://doi.org/10.1016/j.athoracsur.2015.03.019.
46. Osterdal OB, Salminen PR, Jordal S, Sjursen H, Wendelbo O, Haaverstad R. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg. 2016;22(5):633-640. https://doi.org/10.1093/icvts/ivv397.
47. Yanagawa B, Elbatarny M, Verma S, et al. Surgical management of tricuspid valve infective endocarditis: a systematic review and meta-analysis. Ann Thorac Surg. 2018;106(3):708-714. https://doi.org/10.1016/j.athoracsur.2018.04.
48. Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis. 2019;68(7):116-1175. https://doi.org/10.1093/cid/ciy632.
49. Wickersham JA, Azar MM, Cannon CM, Altice FL, Springer SA. Validation of a brief measure of opioid dependence: the rapid opioid dependence screen (RODS). J Correct Health Care. 2015;21(1):12-26. https://doi.org/10.1177/1078345814557513.
50. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
As a result of the epidemic of opioid use disorder (OUD), there has been a secondary surge in hospitalizations for infectious complications of injection drug use (IDU).1,2 In the previous 10 years, there have been significant increases in IDU-associated human immunodeficiency virus (HIV)3 and hepatitis C virus (HCV)4 infection as well as increased hospitalizations from IDU-associated skin and soft tissue infections, osteomyelitis, septic arthritis, bacteremia, fungemia, and infective endocarditis in the United States.2,5-7 Patients admitted with IDU-associated infections have long lengths of stay, high rates of leaving against medical advice (AMA), readmission, and mortality.8-13 In a British cohort (median age 36 years), five-year mortality after an episode of IDU-associated endocarditis was 42%.14 Admissions for IDU-associated infections can be a troubling experience for both patients and providers alike.15 While management decisions of IDU-associated infectious syndromes have sometimes been based on emotion, dogma, and an often-stigmatizing approach toward people suffering from addiction,16 with a better understanding of addiction and effective treatments, as well as accumulating data in both addiction and infectious disease fields, it is an appropriate time to reevaluate the approach to treatment.
The goal of this review is to examine recent evidence and attempt to answer questions that frequently arise in the management of hospitalized patients with IDU-associated infections
KEY MANAGEMENT QUESTIONS IN THE INPATIENT MANAGEMENT OF INFECTIOUS COMPLICATIONS OF OUD
How Should OUD Be Managed in the Hospital?
Management of an IDU-associated infection is incomplete without addressing the underlying addiction in some way. Addiction is highly undertreated among patients with IDU-associated infections, which may contribute to poor infection-related outcomes.8,13,17 Opioid agonist therapy (buprenorphine and methadone) to prevent withdrawal should be routinely offered to all patients with OUD including those with infectious complications of OUD to facilitate appropriate medical treatment and engage patients in long-term addiction treatment. Referral to addiction treatment has been associated with improved IDU-associated endocarditis mortality,18 and initiation of medications for OUD (MOUD) can be achieved successfully in the emergency department, inpatient wards, and specifically in patients admitted with IDU-associated endocarditis.19-21 Protocols and resources for inpatient management of withdrawal and initiation of MOUD are available along with telephone support services for providers seeking guidance on specific cases.21,22 Inpatient addiction consult services are an important resource for the management of hospitalized patients with addiction and are associated with increased completion of antibiotics, decreased AMA discharge, and increased rates of MOUD provision among patients with IDU-associated infections.12 However, when unavailable, initiation of opioid agonist therapy does not require an addiction specialist. Linkage to outpatient addiction care is ideal; however, opioid agonist therapy initiated in the hospital can be tapered prior to discharge if this is unavailable. Figure 1 outlines the initiation of methadone or buprenorphine for the treatment of both withdrawal and OUD in the inpatient setting.20,21
Who Can Prescribe Medications for Treatment of OUD in Hospitalized Patients?
Although buprenorphine prescribing in the outpatient setting requires certification, inpatient physicians are exempt from these requirements and can prescribe buprenorphine or methadone in the hospital setting.20 In the outpatient setting, buprenorphine prescription is restricted to providers with a Drug Addiction Treatment Act of 2000 (DATA 2000) waiver, also known as an “X-waiver”. X-waiver training is eight hours, and free web-based training is available.23 At the time of discharge, non-X-waivered physicians can prescribe up to 72 hours of buprenorphine as a bridge to follow-up with outpatient addiction services.24 In the outpatient setting, methadone can only be obtained through approved methadone maintenance programs (MMP); however, many such programs are often willing to do intakes on the same day or next day following hospital discharge.
Is It Safe to Place a Peripherally Inserted Central Catheter in a Patient Who Injects Drugs?
Many practitioners believe that IDU is an absolute contraindication to the use of peripherally inserted central catheters (PICC) for administration of antimicrobials; however, evidence of harm is lacking.25,26 In a review of outpatient parenteral antimicrobial therapy (OPAT) in patients with IDU, there were low overall rates of line-related adverse events and no significant difference in complications between IDU and non-IDU patients receiving OPAT.27 As with any medical intervention, risks and benefits must be balanced. Aside from patient comfort, a PICC allows patients to receive intravenous (IV) antimicrobials in a nonhospital setting, which may be more therapeutic for their addiction. Peripheral venous access can be difficult in patients with IDU who often have atrophic superficial veins. While often cited as a reason to avoid PICCs, there is no empirical evidence that PICC placement leads to increased drug use among people with OUD. Similarly, depriving a patient of a PICC does not prevent drug use, but it may prevent patients from completing infection treatment in a more acceptable setting. The most serious concern with a PICC is that if a patient injects drugs, transient bacteremia/fungemia could seed this prosthetic material and lead to worsening infection. Providers should employ a risk-based approach to the use of PICCs considering patient preferences, addiction disease activity, and stability of home environment weighed against the potential risks of prolonged hospitalization, clinic-based antibiotic infusions through a peripheral IV, or possibly suboptimal oral antimicrobial treatment.
What Is the Best Location for Patients to Receive Antibiotics for Their IDU-Associated Infection?
Antimicrobial treatment for severe IDU-associated infections such as endocarditis and osteomyelitis has traditionally included four- to six-week hospital admissions to complete the entirety of IV therapy. This practice has recently been called into question. Extended hospitalization for patients with IDU-associated infections—often not receiving evidence-based treatment for their addiction—can be a harrowing experience and may be antitherapeutic.15,28 Disposition decisions for patients with IDU-associated infections should involve risk stratification to assess addiction disease activity and take into account inpatient addiction treatment resources and patient preference, culture/availability of skilled nursing facilities (SNFs), and safety of the home environment.29 Some emerging models of care take advantage of long hospitalizations by engaging patients with comprehensive addiction services including substance use group meetings, counseling, and social resources. Another model using OPAT with intensive outpatient follow-up for both addiction and infection treatment showed similar infection outcomes, lower cost, and improved patient satisfaction compared with in-hospital treatment.30 When available, medical respite programs and OPAT-friendly residential addiction programs have shown success and financial savings as well.31,32 Still, many patients would prefer home OPAT, and there is evidence that home OPAT is no less successful than OPAT provided in an SNF.33 Despite this mounting evidence, there remains systemic stigmatization of people with OUD and inequity as many SNFs, and home infusion companies will not provide either MOUD or services to patients with OUD.34
Can Oral Antibiotics Be Used to Treat Severe Infections Due to IDU?
A general principle of infectious diseases is that oral antibiotics should be used whenever possible when presumed to be noninferior to IV alternatives. Accumulating evidence in the infectious disease literature suggests that there is a role for increasing the use of oral antibiotics for serious infections. Two recent pivotal randomized trials have questioned the dogma surrounding the use of IV antibiotics for the management of orthopedic infections and endocarditis. However, these studies included few patients with infections due to IDU.35,36 One study of oral antibiotics specifically in patients with IDU-associated infection showed that an all-oral regimen for the management of IDU-associated right-sided endocarditis was effective and well-tolerated.37 While oral antibiotics decrease the need for long-term hospitalization and OPAT, similar or even more intensive follow-up of these patients is required to ensure an appropriate response to treatment. Oral antibiotics should not be used to simply expedite discharge but instead should be done with careful planning and close follow-up.
When using oral antibiotics for severe infections, attempts should be made to use agents with the highest oral bioavailability, tolerability, and affordability. Antimicrobials with near-complete oral bioavailability include fluoroquinolones, triazoles, oxazolidinones (linezolid and tedizolid), clindamycin, trimethoprim-sulfamethoxazole, doxycycline, metronidazole, cefadroxil, and other select oral cephalosporins. One approach is to complete a short course of inpatient induction therapy with IV antimicrobials followed by consolidation therapy with oral antibiotics. In a study of uncomplicated Staphylococcus aureus bacteremia, a similar approach with initial IV therapy and oral linezolid follow-up treatment was noninferior to all-IV treatment.38 Decisions about the early transition to oral antimicrobials should be made in conjunction with infectious disease specialists where available.
What Is the Role of Long Half-Life IV Antibiotics for Treating IDU-Associated Infections?
Dalbavancin and oritavancin are extremely long half-life IV glycopeptide antibiotics for gram-positive bacterial infections that require, at most, weekly administration. These agents allow IV-equivalent antibiotics to be delivered without the need for daily infusions or PICCs. Currently, both are approved by the United States Food and Drug Administration only for skin and skin structure infections, but there are increasing reports of successful use in more severe infections including osteomyelitis, bacteremia, and endocarditis.39-42
Is Surgical Placement of Prosthetic Material Safe in Patients With IDU-Associated Infections?
When surgery for an IDU-associated infection has the potential to be acutely lifesaving, it should be offered. There is a concern that surgical interventions that require placement of prosthetic material might serve as a nidus of future infection in the setting of ongoing IDU. Although treatments for many substance use disorders are effective—particularly medications to treat OUD—addiction is a relapsing chronic condition, and at least, some future drug use is an expected part of the course. Research comparing outcomes after valve surgery between IDU and non-IDU-associated endocarditis patients shows no difference in short-term outcomes,44 but longer-term data show increased mortality between 60 and 180 days postoperatively, higher rates of valve-related complications, and up to 53% reinfection rates.10,45,46 These studies are limited by the lack of a nonsurgically treated control group and little information on the rate of addiction treatment, which may be protective against these negative outcomes. In contrast, another study found that surgery was the strongest predictor of survival among patients with IDU-associated endocarditis after a median of 3.6 years follow-up.18 Another consideration is that patients with IDU-associated infection tend to be younger, and despite advancements, many modern prostheses have a finite lifespan. When multiple surgical options exist, a procedure that avoids prosthetic material is preferred. For example, in a meta-analysis of studies of tricuspid valve endocarditis (41% IDU-associated), there was no mortality difference between valve repair compared with valve replacement, but there was a significantly lower rate of recurrent endocarditis among those with a repair only.47 Decisions about surgery must be individualized and consider a patient’s engagement in OUD treatment, social support, prior success with treatment, treatment and relapse prevention resources, and access to harm reduction interventions such as sterile syringes.
What Are Appropriate Harm Reduction Interventions for Patients Hospitalized With Infections Due to IDU?
A prolonged admission for IDU-associated infections is an opportunity to provide patients with education, health maintenance services, and secondary prevention interventions for both infection and overdose. Based on epidemiologic risk, patients should be screened for HIV, HCV, hepatitis B, syphilis, gonorrhea, and chlamydia. Patients should be vaccinated against hepatitis A, influenza, and tetanus (and pneumococcus if indicated), if unvaccinated or without vaccination records. Patients positive for HIV should be evaluated by an infectious disease specialist with consideration of the rapid initiation of antiretroviral therapy. Patients positive for HCV or hepatitis B should be referred for treatment in the outpatient setting. Patients without HIV should be educated about HIV preexposure prophylaxis and referred to outpatient services.
Harm reduction involves meeting patients where they are and providing services they are willing to accept to improve their health or prevent negative outcomes. One important strategy for reducing harm involves maintaining patients in care for their addiction and infection as much as possible, ideally avoiding AMA discharge. In one cohort of patients admitted with IDU-associated infections and OUD, 49% of those without an addiction medicine consult left AMA.12 If a patient plans to leave AMA, all efforts should be made to provide them with oral antibiotics that might be effective, even if suboptimal, for their infection. Hospitalists should consider documenting an oral “antibiotic contingency plan” that can be rapidly enacted if a patient is imminently leaving the hospital. They should be provided with outpatient follow-up appointments with infectious disease or primary care. All patients with IDU-associated infections should be discharged with naloxone, overdose prevention education, and community resources for addiction treatment and syringe exchange programs.
GENERAL APPROACH TO INPATIENT MANAGEMENT OF INFECTIOUS COMPLICATIONS OF OUD
Management of IDU-associated infection should be organized around a multidisciplinary framework with careful attention to infection treatment, OUD treatment, and harm reduction interventions (Figure 2). The first step in managing IDU-associated infections is recognizing addiction in the acute care setting. Substance use disorders, including OUD, are often unrecognized in patients presenting with IDU-associated infections.48 The Rapid Opioid Dependence Screen, a validated screening tool for OUD, can be quickly administered for all patients who present with endocarditis, bacteremia, skin and soft tissue infections, vertebral and epidural infections, and HIV and HCV infections.49 In addition to directly questioning patients about substance use, Figure 2 lists epidemiologic, physical exam, and laboratory findings that might suggest to the provider that OUD may be present.
The approach to infection management is similar to non-IDU-associated infections, including identifying a source, evaluating for complications and need for source control procedures, and administering antimicrobials. Management of the substance use disorder includes treatment of acute withdrawal, control of pain, initiation of MOUD when appropriate, and linkage to outpatient addiction treatment services in addition to harm reduction interventions.
CONCLUSION
Hospital admissions for infectious complications of IDU are increasingly common and are difficult experiences for both patients and providers. However, these hospitalizations serve as a “reachable moment” to engage patients with OUD into medical care and initiate holistic treatment of their infection and underlying substance use disorder.28,50
Disclosures
The authors both report no conflict of interest.
As a result of the epidemic of opioid use disorder (OUD), there has been a secondary surge in hospitalizations for infectious complications of injection drug use (IDU).1,2 In the previous 10 years, there have been significant increases in IDU-associated human immunodeficiency virus (HIV)3 and hepatitis C virus (HCV)4 infection as well as increased hospitalizations from IDU-associated skin and soft tissue infections, osteomyelitis, septic arthritis, bacteremia, fungemia, and infective endocarditis in the United States.2,5-7 Patients admitted with IDU-associated infections have long lengths of stay, high rates of leaving against medical advice (AMA), readmission, and mortality.8-13 In a British cohort (median age 36 years), five-year mortality after an episode of IDU-associated endocarditis was 42%.14 Admissions for IDU-associated infections can be a troubling experience for both patients and providers alike.15 While management decisions of IDU-associated infectious syndromes have sometimes been based on emotion, dogma, and an often-stigmatizing approach toward people suffering from addiction,16 with a better understanding of addiction and effective treatments, as well as accumulating data in both addiction and infectious disease fields, it is an appropriate time to reevaluate the approach to treatment.
The goal of this review is to examine recent evidence and attempt to answer questions that frequently arise in the management of hospitalized patients with IDU-associated infections
KEY MANAGEMENT QUESTIONS IN THE INPATIENT MANAGEMENT OF INFECTIOUS COMPLICATIONS OF OUD
How Should OUD Be Managed in the Hospital?
Management of an IDU-associated infection is incomplete without addressing the underlying addiction in some way. Addiction is highly undertreated among patients with IDU-associated infections, which may contribute to poor infection-related outcomes.8,13,17 Opioid agonist therapy (buprenorphine and methadone) to prevent withdrawal should be routinely offered to all patients with OUD including those with infectious complications of OUD to facilitate appropriate medical treatment and engage patients in long-term addiction treatment. Referral to addiction treatment has been associated with improved IDU-associated endocarditis mortality,18 and initiation of medications for OUD (MOUD) can be achieved successfully in the emergency department, inpatient wards, and specifically in patients admitted with IDU-associated endocarditis.19-21 Protocols and resources for inpatient management of withdrawal and initiation of MOUD are available along with telephone support services for providers seeking guidance on specific cases.21,22 Inpatient addiction consult services are an important resource for the management of hospitalized patients with addiction and are associated with increased completion of antibiotics, decreased AMA discharge, and increased rates of MOUD provision among patients with IDU-associated infections.12 However, when unavailable, initiation of opioid agonist therapy does not require an addiction specialist. Linkage to outpatient addiction care is ideal; however, opioid agonist therapy initiated in the hospital can be tapered prior to discharge if this is unavailable. Figure 1 outlines the initiation of methadone or buprenorphine for the treatment of both withdrawal and OUD in the inpatient setting.20,21
Who Can Prescribe Medications for Treatment of OUD in Hospitalized Patients?
Although buprenorphine prescribing in the outpatient setting requires certification, inpatient physicians are exempt from these requirements and can prescribe buprenorphine or methadone in the hospital setting.20 In the outpatient setting, buprenorphine prescription is restricted to providers with a Drug Addiction Treatment Act of 2000 (DATA 2000) waiver, also known as an “X-waiver”. X-waiver training is eight hours, and free web-based training is available.23 At the time of discharge, non-X-waivered physicians can prescribe up to 72 hours of buprenorphine as a bridge to follow-up with outpatient addiction services.24 In the outpatient setting, methadone can only be obtained through approved methadone maintenance programs (MMP); however, many such programs are often willing to do intakes on the same day or next day following hospital discharge.
Is It Safe to Place a Peripherally Inserted Central Catheter in a Patient Who Injects Drugs?
Many practitioners believe that IDU is an absolute contraindication to the use of peripherally inserted central catheters (PICC) for administration of antimicrobials; however, evidence of harm is lacking.25,26 In a review of outpatient parenteral antimicrobial therapy (OPAT) in patients with IDU, there were low overall rates of line-related adverse events and no significant difference in complications between IDU and non-IDU patients receiving OPAT.27 As with any medical intervention, risks and benefits must be balanced. Aside from patient comfort, a PICC allows patients to receive intravenous (IV) antimicrobials in a nonhospital setting, which may be more therapeutic for their addiction. Peripheral venous access can be difficult in patients with IDU who often have atrophic superficial veins. While often cited as a reason to avoid PICCs, there is no empirical evidence that PICC placement leads to increased drug use among people with OUD. Similarly, depriving a patient of a PICC does not prevent drug use, but it may prevent patients from completing infection treatment in a more acceptable setting. The most serious concern with a PICC is that if a patient injects drugs, transient bacteremia/fungemia could seed this prosthetic material and lead to worsening infection. Providers should employ a risk-based approach to the use of PICCs considering patient preferences, addiction disease activity, and stability of home environment weighed against the potential risks of prolonged hospitalization, clinic-based antibiotic infusions through a peripheral IV, or possibly suboptimal oral antimicrobial treatment.
What Is the Best Location for Patients to Receive Antibiotics for Their IDU-Associated Infection?
Antimicrobial treatment for severe IDU-associated infections such as endocarditis and osteomyelitis has traditionally included four- to six-week hospital admissions to complete the entirety of IV therapy. This practice has recently been called into question. Extended hospitalization for patients with IDU-associated infections—often not receiving evidence-based treatment for their addiction—can be a harrowing experience and may be antitherapeutic.15,28 Disposition decisions for patients with IDU-associated infections should involve risk stratification to assess addiction disease activity and take into account inpatient addiction treatment resources and patient preference, culture/availability of skilled nursing facilities (SNFs), and safety of the home environment.29 Some emerging models of care take advantage of long hospitalizations by engaging patients with comprehensive addiction services including substance use group meetings, counseling, and social resources. Another model using OPAT with intensive outpatient follow-up for both addiction and infection treatment showed similar infection outcomes, lower cost, and improved patient satisfaction compared with in-hospital treatment.30 When available, medical respite programs and OPAT-friendly residential addiction programs have shown success and financial savings as well.31,32 Still, many patients would prefer home OPAT, and there is evidence that home OPAT is no less successful than OPAT provided in an SNF.33 Despite this mounting evidence, there remains systemic stigmatization of people with OUD and inequity as many SNFs, and home infusion companies will not provide either MOUD or services to patients with OUD.34
Can Oral Antibiotics Be Used to Treat Severe Infections Due to IDU?
A general principle of infectious diseases is that oral antibiotics should be used whenever possible when presumed to be noninferior to IV alternatives. Accumulating evidence in the infectious disease literature suggests that there is a role for increasing the use of oral antibiotics for serious infections. Two recent pivotal randomized trials have questioned the dogma surrounding the use of IV antibiotics for the management of orthopedic infections and endocarditis. However, these studies included few patients with infections due to IDU.35,36 One study of oral antibiotics specifically in patients with IDU-associated infection showed that an all-oral regimen for the management of IDU-associated right-sided endocarditis was effective and well-tolerated.37 While oral antibiotics decrease the need for long-term hospitalization and OPAT, similar or even more intensive follow-up of these patients is required to ensure an appropriate response to treatment. Oral antibiotics should not be used to simply expedite discharge but instead should be done with careful planning and close follow-up.
When using oral antibiotics for severe infections, attempts should be made to use agents with the highest oral bioavailability, tolerability, and affordability. Antimicrobials with near-complete oral bioavailability include fluoroquinolones, triazoles, oxazolidinones (linezolid and tedizolid), clindamycin, trimethoprim-sulfamethoxazole, doxycycline, metronidazole, cefadroxil, and other select oral cephalosporins. One approach is to complete a short course of inpatient induction therapy with IV antimicrobials followed by consolidation therapy with oral antibiotics. In a study of uncomplicated Staphylococcus aureus bacteremia, a similar approach with initial IV therapy and oral linezolid follow-up treatment was noninferior to all-IV treatment.38 Decisions about the early transition to oral antimicrobials should be made in conjunction with infectious disease specialists where available.
What Is the Role of Long Half-Life IV Antibiotics for Treating IDU-Associated Infections?
Dalbavancin and oritavancin are extremely long half-life IV glycopeptide antibiotics for gram-positive bacterial infections that require, at most, weekly administration. These agents allow IV-equivalent antibiotics to be delivered without the need for daily infusions or PICCs. Currently, both are approved by the United States Food and Drug Administration only for skin and skin structure infections, but there are increasing reports of successful use in more severe infections including osteomyelitis, bacteremia, and endocarditis.39-42
Is Surgical Placement of Prosthetic Material Safe in Patients With IDU-Associated Infections?
When surgery for an IDU-associated infection has the potential to be acutely lifesaving, it should be offered. There is a concern that surgical interventions that require placement of prosthetic material might serve as a nidus of future infection in the setting of ongoing IDU. Although treatments for many substance use disorders are effective—particularly medications to treat OUD—addiction is a relapsing chronic condition, and at least, some future drug use is an expected part of the course. Research comparing outcomes after valve surgery between IDU and non-IDU-associated endocarditis patients shows no difference in short-term outcomes,44 but longer-term data show increased mortality between 60 and 180 days postoperatively, higher rates of valve-related complications, and up to 53% reinfection rates.10,45,46 These studies are limited by the lack of a nonsurgically treated control group and little information on the rate of addiction treatment, which may be protective against these negative outcomes. In contrast, another study found that surgery was the strongest predictor of survival among patients with IDU-associated endocarditis after a median of 3.6 years follow-up.18 Another consideration is that patients with IDU-associated infection tend to be younger, and despite advancements, many modern prostheses have a finite lifespan. When multiple surgical options exist, a procedure that avoids prosthetic material is preferred. For example, in a meta-analysis of studies of tricuspid valve endocarditis (41% IDU-associated), there was no mortality difference between valve repair compared with valve replacement, but there was a significantly lower rate of recurrent endocarditis among those with a repair only.47 Decisions about surgery must be individualized and consider a patient’s engagement in OUD treatment, social support, prior success with treatment, treatment and relapse prevention resources, and access to harm reduction interventions such as sterile syringes.
What Are Appropriate Harm Reduction Interventions for Patients Hospitalized With Infections Due to IDU?
A prolonged admission for IDU-associated infections is an opportunity to provide patients with education, health maintenance services, and secondary prevention interventions for both infection and overdose. Based on epidemiologic risk, patients should be screened for HIV, HCV, hepatitis B, syphilis, gonorrhea, and chlamydia. Patients should be vaccinated against hepatitis A, influenza, and tetanus (and pneumococcus if indicated), if unvaccinated or without vaccination records. Patients positive for HIV should be evaluated by an infectious disease specialist with consideration of the rapid initiation of antiretroviral therapy. Patients positive for HCV or hepatitis B should be referred for treatment in the outpatient setting. Patients without HIV should be educated about HIV preexposure prophylaxis and referred to outpatient services.
Harm reduction involves meeting patients where they are and providing services they are willing to accept to improve their health or prevent negative outcomes. One important strategy for reducing harm involves maintaining patients in care for their addiction and infection as much as possible, ideally avoiding AMA discharge. In one cohort of patients admitted with IDU-associated infections and OUD, 49% of those without an addiction medicine consult left AMA.12 If a patient plans to leave AMA, all efforts should be made to provide them with oral antibiotics that might be effective, even if suboptimal, for their infection. Hospitalists should consider documenting an oral “antibiotic contingency plan” that can be rapidly enacted if a patient is imminently leaving the hospital. They should be provided with outpatient follow-up appointments with infectious disease or primary care. All patients with IDU-associated infections should be discharged with naloxone, overdose prevention education, and community resources for addiction treatment and syringe exchange programs.
GENERAL APPROACH TO INPATIENT MANAGEMENT OF INFECTIOUS COMPLICATIONS OF OUD
Management of IDU-associated infection should be organized around a multidisciplinary framework with careful attention to infection treatment, OUD treatment, and harm reduction interventions (Figure 2). The first step in managing IDU-associated infections is recognizing addiction in the acute care setting. Substance use disorders, including OUD, are often unrecognized in patients presenting with IDU-associated infections.48 The Rapid Opioid Dependence Screen, a validated screening tool for OUD, can be quickly administered for all patients who present with endocarditis, bacteremia, skin and soft tissue infections, vertebral and epidural infections, and HIV and HCV infections.49 In addition to directly questioning patients about substance use, Figure 2 lists epidemiologic, physical exam, and laboratory findings that might suggest to the provider that OUD may be present.
The approach to infection management is similar to non-IDU-associated infections, including identifying a source, evaluating for complications and need for source control procedures, and administering antimicrobials. Management of the substance use disorder includes treatment of acute withdrawal, control of pain, initiation of MOUD when appropriate, and linkage to outpatient addiction treatment services in addition to harm reduction interventions.
CONCLUSION
Hospital admissions for infectious complications of IDU are increasingly common and are difficult experiences for both patients and providers. However, these hospitalizations serve as a “reachable moment” to engage patients with OUD into medical care and initiate holistic treatment of their infection and underlying substance use disorder.28,50
Disclosures
The authors both report no conflict of interest.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445-1452. https://doi.org/10.15585/mmwr.mm655051e1.
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
3. Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375(3):229-239. https://doi.org/10.1056/NEJMoa1515195.
4. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years — Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453-458.
5. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis. 2016;3(3):ofw157. https://doi.org/10.1093/ofid/ofw157.
6. Hartnett KP, Jackson KA, Felsen C, et al. Bacterial and fungal infections in persons who inject drugs - Western New York, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(26):583-586. https://doi.org/10.15585/mmwr.mm6826a2.
7. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2019.170(1):31-40. https://doi.org/10.7326/M18-2124,
8. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024.
9. Rabkin DG, Mokadam NA, Miller DW, Goetz RR, Verrier ED, Aldea GS. Long-term outcome for the surgical treatment of infective endocarditis with a focus on intravenous drug users. Ann Thorac Surg. 2012;93(1):51-57. https://doi.org/10.1016/j.athoracsur.2011.08.016.
10. Kim JB, Ejiofor JI, Yammine M, et al. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016;152(3):832-841.e1. https://doi.org/10.1016/j.jtcvs.2016.02.072
11. Leahey PA, LaSalvia MT, Rosenthal ES, Karchmer AW, Rowley CF. High morbidity and mortality among patients with sentinel admission for injection drug use-related infective endocarditis. Open Forum Infect Dis. 2019;6(4):ofz089. https://doi.org/10.1093/ofid/ofz089.
12. Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis. 2019;68(11):1935-1937. https://doi.org/10.1093/cid/ciy924.
13. Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated Staphylococcus aureus bacteremia. Open Forum Infect Dis. 2019;6(7):ofz289. https://doi.org/10.1093/ofid/ofz289.
14. Straw S, Baig MW, Gillott R, et al. Long-term outcomes are poor in intravenous drug users following infective endocarditis, even after surgery. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz869.
15. Bearnot BI, Mitton JA, Hayden M, Park ER. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: Results from a qualitative study. J Subst Abuse Treat. 2019;102:16-22. https://doi.org/10.1016/j.jsat.2019.04.008.
16. Hull SC, Jadbabaie F. When is enough enough? The dilemma of valve replacement in a recidivist intravenous drug user. Ann Thorac Surg. 2014;97(5):1486-1487. https://doi.org/10.1016/j.athoracsur.2014.02.010.
17. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/ADM.0000000000000454.
18. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open. 2018;1(7):e185220. https://doi.org/10.1001/jamanetworkopen.2018.5220.
19. Suzuki J. Medication-assisted treatment for hospitalized patients with intravenous-drug-use related infective endocarditis. Am J Addict. 2016;25(3):191-194. https://doi.org/10.1111/ajad.12349.
20. Theisen-Toupal J, Ronan MV, Moore A, Rosenthal ES. Inpatient management of opioid use disorder: a review for hospitalists. J Hosp Med. 2017;12(5):369-374. https://doi.org/10.12788/jhm.2731.
21. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. https://doi.org/10.1503/cmaj.160290.
22. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the improving addiction care team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
23. 8 Hour Online MAT Waiver Training. Providers Clinical Support System 2019; https://learning.pcssnow.org/p/onlinematwaiver. Accessed May 22, 2019.
24. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther. 2019;8(2):171-184. https://doi.org/10.1007/s40121-019-0247-0.
25. Rapoport AB, Fischer LS, Santibanez S, Beekmann SE, Polgreen PM, Rowley CF. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis. 2018;5(7):ofy132. https://doi.org/10.1093/ofid/ofy132.
26. Fanucchi L, Leedy N, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581-582. https://doi.org/10.1002/jhm.2582.
27. Suzuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. https://doi.org/10.1093/ofid/ofy194.
28. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an Experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4.
29. Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs requiring intravenous antibiotics may allow health systems to focus inpatient resources on those at greatest risk of ongoing drug use. Clin Infect Dis. 2019;68(6):1041-1043. https://doi.org/10.1093/cid/ciy722.
30. Fanucchi LC, Walsh SL, Thornton AC, Nuzzo PA, Lofwall MR. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis. 2019.pii:ciz654. https://doi.org/10.1093/cid/ciz654.
31. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. https://doi.org/10.1002/jhm.2597.
32. Jewell C, Weaver M, Sgroi C, Anderson K, Sayeed Z. Residential addiction treatment for injection drug users requiring intravenous antibiotics: a cost-reduction strategy. J Addict Med. 2013;7(4):271-276. https://doi.org/10.1097/ADM.0b013e318294b1eb.
33. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. https://doi.org/10.1093/ofid/ofy056.
34. Wakeman SE, Rich JD. Barriers to post-acute care for patients on opioid agonist therapy; an example of systematic stigmatization of addiction. J Gen Intern Med. 2017;32(1):17-19. https://doi.org/10.1007/s11606-016-3799-7.
35. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. Jan 31 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312.
36. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926.
37. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. Nov 4 1989;2(8671):1071-1073. https://doi.org/10.1016/s0140-6736(89)91083-0
38. Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy916.
39. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. https://doi.org/10.1093/ofid/ofy331.
40. Morata L, Cobo J, Fernandez-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother. 2019;63(5).pii. e02280-18. https://doi.org/10.1128/AAC.02280-18.
41. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis. 2018;67(5):795-798. https://doi.org/10.1093/cid/ciy279.
42. Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int J Infect Dis. 2019;81:210-214. https://doi.org/10.1016/j.ijid.2019.02.013.
43. Bryson-Cahn C, Beieler AM, Chan JD, Harrington RD, Dhanireddy S. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis. 2019;6(2):ofz028. https://doi.org/10.1093/ofid/ofz028.
44. Hall R, Shaughnessy M, Boll G, et al. Drug-use and post-operative mortality following valve surgery for infective endocarditis: a systematic review and meta-analysis. Clin Infect Dis. 2019;69(7):1120-1129. https://doi.org/10.1093/cid/ciy1064.
45. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100(3):875-882. https://doi.org/10.1016/j.athoracsur.2015.03.019.
46. Osterdal OB, Salminen PR, Jordal S, Sjursen H, Wendelbo O, Haaverstad R. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg. 2016;22(5):633-640. https://doi.org/10.1093/icvts/ivv397.
47. Yanagawa B, Elbatarny M, Verma S, et al. Surgical management of tricuspid valve infective endocarditis: a systematic review and meta-analysis. Ann Thorac Surg. 2018;106(3):708-714. https://doi.org/10.1016/j.athoracsur.2018.04.
48. Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis. 2019;68(7):116-1175. https://doi.org/10.1093/cid/ciy632.
49. Wickersham JA, Azar MM, Cannon CM, Altice FL, Springer SA. Validation of a brief measure of opioid dependence: the rapid opioid dependence screen (RODS). J Correct Health Care. 2015;21(1):12-26. https://doi.org/10.1177/1078345814557513.
50. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445-1452. https://doi.org/10.15585/mmwr.mm655051e1.
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
3. Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375(3):229-239. https://doi.org/10.1056/NEJMoa1515195.
4. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years — Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453-458.
5. Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis. 2016;3(3):ofw157. https://doi.org/10.1093/ofid/ofw157.
6. Hartnett KP, Jackson KA, Felsen C, et al. Bacterial and fungal infections in persons who inject drugs - Western New York, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(26):583-586. https://doi.org/10.15585/mmwr.mm6826a2.
7. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2019.170(1):31-40. https://doi.org/10.7326/M18-2124,
8. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024.
9. Rabkin DG, Mokadam NA, Miller DW, Goetz RR, Verrier ED, Aldea GS. Long-term outcome for the surgical treatment of infective endocarditis with a focus on intravenous drug users. Ann Thorac Surg. 2012;93(1):51-57. https://doi.org/10.1016/j.athoracsur.2011.08.016.
10. Kim JB, Ejiofor JI, Yammine M, et al. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016;152(3):832-841.e1. https://doi.org/10.1016/j.jtcvs.2016.02.072
11. Leahey PA, LaSalvia MT, Rosenthal ES, Karchmer AW, Rowley CF. High morbidity and mortality among patients with sentinel admission for injection drug use-related infective endocarditis. Open Forum Infect Dis. 2019;6(4):ofz089. https://doi.org/10.1093/ofid/ofz089.
12. Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis. 2019;68(11):1935-1937. https://doi.org/10.1093/cid/ciy924.
13. Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated Staphylococcus aureus bacteremia. Open Forum Infect Dis. 2019;6(7):ofz289. https://doi.org/10.1093/ofid/ofz289.
14. Straw S, Baig MW, Gillott R, et al. Long-term outcomes are poor in intravenous drug users following infective endocarditis, even after surgery. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz869.
15. Bearnot BI, Mitton JA, Hayden M, Park ER. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: Results from a qualitative study. J Subst Abuse Treat. 2019;102:16-22. https://doi.org/10.1016/j.jsat.2019.04.008.
16. Hull SC, Jadbabaie F. When is enough enough? The dilemma of valve replacement in a recidivist intravenous drug user. Ann Thorac Surg. 2014;97(5):1486-1487. https://doi.org/10.1016/j.athoracsur.2014.02.010.
17. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13(1):69-74. https://doi.org/10.1097/ADM.0000000000000454.
18. Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open. 2018;1(7):e185220. https://doi.org/10.1001/jamanetworkopen.2018.5220.
19. Suzuki J. Medication-assisted treatment for hospitalized patients with intravenous-drug-use related infective endocarditis. Am J Addict. 2016;25(3):191-194. https://doi.org/10.1111/ajad.12349.
20. Theisen-Toupal J, Ronan MV, Moore A, Rosenthal ES. Inpatient management of opioid use disorder: a review for hospitalists. J Hosp Med. 2017;12(5):369-374. https://doi.org/10.12788/jhm.2731.
21. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. https://doi.org/10.1503/cmaj.160290.
22. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the improving addiction care team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
23. 8 Hour Online MAT Waiver Training. Providers Clinical Support System 2019; https://learning.pcssnow.org/p/onlinematwaiver. Accessed May 22, 2019.
24. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther. 2019;8(2):171-184. https://doi.org/10.1007/s40121-019-0247-0.
25. Rapoport AB, Fischer LS, Santibanez S, Beekmann SE, Polgreen PM, Rowley CF. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis. 2018;5(7):ofy132. https://doi.org/10.1093/ofid/ofy132.
26. Fanucchi L, Leedy N, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581-582. https://doi.org/10.1002/jhm.2582.
27. Suzuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. https://doi.org/10.1093/ofid/ofy194.
28. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an Experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4.
29. Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs requiring intravenous antibiotics may allow health systems to focus inpatient resources on those at greatest risk of ongoing drug use. Clin Infect Dis. 2019;68(6):1041-1043. https://doi.org/10.1093/cid/ciy722.
30. Fanucchi LC, Walsh SL, Thornton AC, Nuzzo PA, Lofwall MR. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis. 2019.pii:ciz654. https://doi.org/10.1093/cid/ciz654.
31. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. https://doi.org/10.1002/jhm.2597.
32. Jewell C, Weaver M, Sgroi C, Anderson K, Sayeed Z. Residential addiction treatment for injection drug users requiring intravenous antibiotics: a cost-reduction strategy. J Addict Med. 2013;7(4):271-276. https://doi.org/10.1097/ADM.0b013e318294b1eb.
33. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. https://doi.org/10.1093/ofid/ofy056.
34. Wakeman SE, Rich JD. Barriers to post-acute care for patients on opioid agonist therapy; an example of systematic stigmatization of addiction. J Gen Intern Med. 2017;32(1):17-19. https://doi.org/10.1007/s11606-016-3799-7.
35. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. Jan 31 2019;380(5):415-424. https://doi.org/10.1056/NEJMoa1808312.
36. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. https://doi.org/10.1056/NEJMoa1710926.
37. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. Nov 4 1989;2(8671):1071-1073. https://doi.org/10.1016/s0140-6736(89)91083-0
38. Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy916.
39. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. https://doi.org/10.1093/ofid/ofy331.
40. Morata L, Cobo J, Fernandez-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother. 2019;63(5).pii. e02280-18. https://doi.org/10.1128/AAC.02280-18.
41. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis. 2018;67(5):795-798. https://doi.org/10.1093/cid/ciy279.
42. Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int J Infect Dis. 2019;81:210-214. https://doi.org/10.1016/j.ijid.2019.02.013.
43. Bryson-Cahn C, Beieler AM, Chan JD, Harrington RD, Dhanireddy S. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis. 2019;6(2):ofz028. https://doi.org/10.1093/ofid/ofz028.
44. Hall R, Shaughnessy M, Boll G, et al. Drug-use and post-operative mortality following valve surgery for infective endocarditis: a systematic review and meta-analysis. Clin Infect Dis. 2019;69(7):1120-1129. https://doi.org/10.1093/cid/ciy1064.
45. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100(3):875-882. https://doi.org/10.1016/j.athoracsur.2015.03.019.
46. Osterdal OB, Salminen PR, Jordal S, Sjursen H, Wendelbo O, Haaverstad R. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg. 2016;22(5):633-640. https://doi.org/10.1093/icvts/ivv397.
47. Yanagawa B, Elbatarny M, Verma S, et al. Surgical management of tricuspid valve infective endocarditis: a systematic review and meta-analysis. Ann Thorac Surg. 2018;106(3):708-714. https://doi.org/10.1016/j.athoracsur.2018.04.
48. Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis. 2019;68(7):116-1175. https://doi.org/10.1093/cid/ciy632.
49. Wickersham JA, Azar MM, Cannon CM, Altice FL, Springer SA. Validation of a brief measure of opioid dependence: the rapid opioid dependence screen (RODS). J Correct Health Care. 2015;21(1):12-26. https://doi.org/10.1177/1078345814557513.
50. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
© 2019 Society of Hospital Medicine
Clinical Progress Note: Point-of-Care Ultrasound in the Evaluation of the Dyspneic Adult
Point-of-care ultrasound (POCUS) continues to gain traction in contemporary clinical practice both as a diagnostic tool and as an extension of the physical examination. Hospital Medicine (HM) lags behind Emergency Medicine (EM) and Critical Care (CC) in our uptake of such technology, although momentum is gaining. Leaders in HM have published frameworks for competency and credentialing, and the Society for Hospital Medicine has created a pathway for certification.1 POCUS use is the standard of care for several bedside procedures, but evidence for diagnostic applications is changing rapidly as the literature expands. However, the applicability of this evidence to HM patients can be challenging as most published studies are still from EM and CC settings. This Progress Note focuses on how a hospitalist might incorporate POCUS in the evaluation of adult patients with dyspnea. This topic was chosen after reviewing several relevant studies published in the past five years and recognizing the importance of dyspnea in HM. The Progress Note begins with a review of POCUS for undifferentiated dyspnea before exploring studies of common diagnoses that present with dyspnea, including pneumonia, pleural effusion, and acute decompensated heart failure (ADHF), aiming to update the knowledge of HM providers regarding this technology as well as to stimulate further study in this field.
SEARCH STRATEGY
In collaboration with an academic librarian in March 2019, PubMed was searched for studies published within the past five years using several MESH search terms for POCUS. The search was originally focused to the field of HM using specific search terms, but this yielded a very limited number of studies. Therefore, the search strategy was expanded to include EM and CC studies. This final search generated 346 papers that were supplemented with additional literature searches using references from studies found in the initial search.
UNDIFFERENTIATED DYSPNEA
Dyspnea is common in HM, both as the reason for a patient’s admission and as a symptom that develops during hospitalization such as after intravenous fluid resuscitation, a possible aspiration event, or central line placement. The differential diagnosis is broad, and multiple studies suggest that POCUS can aid in the evaluation of undifferentiated dyspnea while also being cost effective and avoiding the potential radiation of other testing modalities. The pulmonary POCUS evaluation incorporates a combination of several findings, including “A-lines” or horizontal artifacts from normal aerated lung; “B-lines”, vertical artifacts generated by extra-alveolar fluid, consolidation or “tissue-like pattern”; air bronchograms, consolidated lung surrounding airways; anechoic or hypoechoic areas in dependent zones of the lung; and the presence or absence of pleural sliding.2
In one prospective observational study of five internal medicine residents with no prior POCUS experience and three hours of training, the addition of handheld POCUS devices to usual clinical information improved the diagnostic accuracy for pneumonia, pulmonary edema, pleural effusion, and obstructive lung disease when evaluating patients with a primary complaint of dyspnea (area under the curve [AUC] 0.81 vs 0.87, P < .01).2 However, the largest improvements in the operating characteristics were observed with the two residents who received an extended two-week elective of training.
In another study of 383 consecutive patients presenting to the ED with dyspnea, physicians with basic and advanced POCUS training were blinded to all clinical information and recorded a diagnosis after performing a lung POCUS examination. The “ultrasound physician’s” diagnosis was then compared to the treating emergency department (ED) physician’s diagnosis using history, physical, and other diagnostic data. Lung POCUS had a sensitivity and a specificity of 87.6% and 96.2% for pulmonary edema, 85.7% and 99% for pneumonia, 98.2% and 67.3% for asthma/chronic obstructive pulmonary disease (COPD), 46.2% and 100% for pulmonary embolus (PE), and 71.4% and 100% for pneumothorax, respectively.3 The scanning protocol used, the BLUE (Bedside Lung Ultrasound Examination) protocol, was focused on ruling out significant pulmonary etiologies of dyspnea. The protocol classified the finding of normal lung ultrasound (A-line profile) as COPD or asthma since these conditions will have a normal sonographic appearance. This approach could lead to incorrect labeling of other extrapulmonary causes of dyspnea as COPD or asthma. The findings of this study suggest that POCUS is most effective at ruling in pulmonary edema and pneumonia while being most effective at ruling out asthma or COPD as causes of dyspnea. It is both sensitive and specific for pneumothorax. However, as other studies have found, the sensitivity of POCUS for COPD, asthma, and PE was inferior to traditional clinical evaluation.4 One of the few studies looking specifically at hospitalized ward patients compared a blinded lung POCUS diagnosis and a discharge clinical diagnosis classified as cardiac, pulmonary, or mixed dyspnea. The authors of that study found an “interstitial pattern” (two areas with more than two B-lines) in 94% of those classified as cardiac on discharge, but POCUS findings were less precise for those discharged with a pulmonary etiology of dyspnea.5 Identifying B-lines on lung POCUS appears to be helpful in rapidly differentiating cardiac from pulmonary etiologies of dyspnea.
An additional advantage of POCUS is that multiple organ systems can be evaluated in rapid succession when the etiology of dyspnea is unknown. In a smaller ED study of patients presenting with undifferentiated dyspnea, a diagnosis was recorded after history-taking and physical examination and then recorded again after lung, cardiac, and inferior vena cava POCUS. Clinician diagnostic accuracy improved from 53% to 77% with the use of POCUS (P = .003) compared with the final diagnosis.6 The treating physician’s primary impression changed in almost 50% of cases after using POCUS, most of which was driven by improved sensitivity and specificity of ADHF. In another study of 2,700 patients presenting to the ED with dyspnea, cardiopulmonary POCUS shortened the time to diagnosis (186 ± 72 minutes vs 24 ± 10 minutes, P = .025).4 These studies suggest that the use of POCUS in the initial evaluation of patients with undifferentiated dyspnea is a valuable tool with respect to diagnostic accuracy and timeliness.
PNEUMONIA
There are several different sonographic findings that can indicate pneumonia, such as consolidation or “hepatization”, the “shred” sign of an irregular border between consolidated lung and aerated lung, unilateral B-lines, and dynamic air bronchograms. Several recent systematic reviews and meta-analyses have investigated the operating characteristics of POCUS for the diagnosis of pneumonia. These reviews are limited by heterogeneity with respect to different patient populations, sonographers, and reference standards, but all three reviews found similar results, with the pooled AUC values ranging from 95% to 98%.7-9 This recent evidence along with other reviews suggests that lung ultrasound can serve as a primary diagnostic tool in pneumonia and is probably superior to chest radiography.
PLEURAL EFFUSION
Pleural effusions are observed with POCUS as anechoic or hypoechoic areas, generally in dependent lung zones. POCUS may provide additional benefit by better characterizing the effusion as having septations or floating fibrin strands. One recent systematic review and meta-analysis including 1,554 patients found that POCUS had excellent sensitivity and specificity (94% and 98%, respectively) in detecting pleural effusion versus chest radiography (51% and 91%, respectively), both compared with reference standard imaging such as computed tomography. The subgroup analysis found that sensitivity was higher for scanners who were intensivists or radiologists than for other physicians (97% vs 90%; P ≤ .001) and also found a nonstatistically significant trend toward reduced sensitivity when pocket-sized devices were used (90% vs 95%, P = .09).10
ACUTE DECOMPENSATED HEART FAILURE
It is extremely important to recognize that a POCUS finding of decreased left ventricular ejection fraction is not synonymous with a diagnosis of ADHF. Bedside providers can use POCUS to estimate cardiac function, but other clinical information is required to determine whether the syndrome of ADHF is present. In one study, examinations performed by 10 internists with approximately 18 hours of training in focused cardiac POCUS had a sensitivity and a specificity of 91% and 88%, respectively, for classifying left ventricular systolic function as normal or mildly, moderately, or severely depressed with “good/substantial” agreement (k = 0.77) compared with formal echocardiography.11 The presence of bilateral B-lines as a sign of pulmonary edema suggests accompanying functional decompensation. A meta-analysis of seven articles including 1075 patients in various clinical settings (ED, ICU, and inpatient wards) found a sensitivity of 94.1% and a specificity of 92.4% for using B-lines to diagnose acute cardiogenic pulmonary edema compared with the final clinical diagnosis.12 Al Deeb et al. examined 226 patients and found similar sensitivity (95.3%) and specificity (88.2%) for diagnosing acute cardiogenic pulmonary edema when nurses were trained to evaluate for bilateral B-lines in dyspneic patients admitted to the hospital, also compared with the adjudicated final diagnosis.13 Carlino et al. evaluated dyspneic patients using a three-minute pocket-sized device scan of the heart, lungs, and inferior vena cava and found that no single view offered a substantial improvement in diagnostic accuracy; however, the combination of bilateral B-lines and/or pleural effusion and either a dilated left atrium or left ventricular ejection fraction (LVEF) of <40% had a very high diagnostic accuracy (AUC 0.97).14 Russell et al. performed a secondary analysis of a prospective observational study of patients with dyspnea and found that a simple three-view scanning protocol looking for the presence of B-lines on the right and left anterior superior lung zones and an LVEF of <45% took an average of one minute and 32 seconds to perform and had 100% specificity for ADHF if all three were positive.15 Another recent systematic review and meta-analysis of six studies and 1,827 patients found a sensitivity of 88% (CI 75%-95%) for lung POCUS compared with a chest radiography at a sensitivity of 73% (70%-76%) for the diagnosis of ADHF.16 All these studies suggest that improving the diagnosis of ADHF does not require complex echocardiographic views and is probably more feasible and accessible than many expect.
SUMMARY
POCUS continues to show promise for evaluating patients with dyspnea. It is clear that adding a few POCUS examination maneuvers to a provider’s toolbox, such as looking for B-lines and overall cardiac function, can improve
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. Published online only January 2, 2019. https://doi.org/10.12788/jhm.3079.
2. Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9(9):594-597. https://doi.org/10.1002/jhm.2219.
3. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.02.028.
4. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295-1301. https://doi.org/10.1016/j.chest.2017.02.003.
5. Perrone T, Maggi A, Sgarlata C, et al. Lung ultrasound in internal medicine: a bedside help to increase accuracy in the diagnosis of dyspnea. Eur J Intern Med. 2017;46:61-65. https://doi.org/10.1016/j.ejim.2017.07.034.
6. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. https://doi.org/10.5811/westjem.2015.11.28525.
7. Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018;25(5):312-321. https://doi.org/10.1097/MEJ.0000000000000517.
8. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y
9. Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine . 2017;96(3):e5713. https://doi.org/10.1097/MD.0000000000005713.
10. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1-10.
11. Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92-99. https://doi.org/10.1002/jcu.22272.
12. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. https://doi.org/10.1111/acem.12435.
13. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea: a prospective observational study. Medicine (Baltimore). 2016;95(9):e2925. https://doi.org/10.1097/MD.0000000000002925.
14. Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the emergency department. Echocardiography (Mount Kisco, NY). 2018;35(6):785-791. https://doi.org/10.1111/echo.13851.
15. Russell FM, Ehrman RR. A modified lung and cardiac ultrasound protocol saves time and rules in the diagnosis of acute heart failure. J Emerg Med. 2017;52(6):839-845. https://doi.org/10.1016/j.jemermed.2017.02.003.
16. Maw AM, Hassanin A, Ho PM, et al. diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703. https://doi.org/10.1001/jamanetworkopen.2019.0703.
Point-of-care ultrasound (POCUS) continues to gain traction in contemporary clinical practice both as a diagnostic tool and as an extension of the physical examination. Hospital Medicine (HM) lags behind Emergency Medicine (EM) and Critical Care (CC) in our uptake of such technology, although momentum is gaining. Leaders in HM have published frameworks for competency and credentialing, and the Society for Hospital Medicine has created a pathway for certification.1 POCUS use is the standard of care for several bedside procedures, but evidence for diagnostic applications is changing rapidly as the literature expands. However, the applicability of this evidence to HM patients can be challenging as most published studies are still from EM and CC settings. This Progress Note focuses on how a hospitalist might incorporate POCUS in the evaluation of adult patients with dyspnea. This topic was chosen after reviewing several relevant studies published in the past five years and recognizing the importance of dyspnea in HM. The Progress Note begins with a review of POCUS for undifferentiated dyspnea before exploring studies of common diagnoses that present with dyspnea, including pneumonia, pleural effusion, and acute decompensated heart failure (ADHF), aiming to update the knowledge of HM providers regarding this technology as well as to stimulate further study in this field.
SEARCH STRATEGY
In collaboration with an academic librarian in March 2019, PubMed was searched for studies published within the past five years using several MESH search terms for POCUS. The search was originally focused to the field of HM using specific search terms, but this yielded a very limited number of studies. Therefore, the search strategy was expanded to include EM and CC studies. This final search generated 346 papers that were supplemented with additional literature searches using references from studies found in the initial search.
UNDIFFERENTIATED DYSPNEA
Dyspnea is common in HM, both as the reason for a patient’s admission and as a symptom that develops during hospitalization such as after intravenous fluid resuscitation, a possible aspiration event, or central line placement. The differential diagnosis is broad, and multiple studies suggest that POCUS can aid in the evaluation of undifferentiated dyspnea while also being cost effective and avoiding the potential radiation of other testing modalities. The pulmonary POCUS evaluation incorporates a combination of several findings, including “A-lines” or horizontal artifacts from normal aerated lung; “B-lines”, vertical artifacts generated by extra-alveolar fluid, consolidation or “tissue-like pattern”; air bronchograms, consolidated lung surrounding airways; anechoic or hypoechoic areas in dependent zones of the lung; and the presence or absence of pleural sliding.2
In one prospective observational study of five internal medicine residents with no prior POCUS experience and three hours of training, the addition of handheld POCUS devices to usual clinical information improved the diagnostic accuracy for pneumonia, pulmonary edema, pleural effusion, and obstructive lung disease when evaluating patients with a primary complaint of dyspnea (area under the curve [AUC] 0.81 vs 0.87, P < .01).2 However, the largest improvements in the operating characteristics were observed with the two residents who received an extended two-week elective of training.
In another study of 383 consecutive patients presenting to the ED with dyspnea, physicians with basic and advanced POCUS training were blinded to all clinical information and recorded a diagnosis after performing a lung POCUS examination. The “ultrasound physician’s” diagnosis was then compared to the treating emergency department (ED) physician’s diagnosis using history, physical, and other diagnostic data. Lung POCUS had a sensitivity and a specificity of 87.6% and 96.2% for pulmonary edema, 85.7% and 99% for pneumonia, 98.2% and 67.3% for asthma/chronic obstructive pulmonary disease (COPD), 46.2% and 100% for pulmonary embolus (PE), and 71.4% and 100% for pneumothorax, respectively.3 The scanning protocol used, the BLUE (Bedside Lung Ultrasound Examination) protocol, was focused on ruling out significant pulmonary etiologies of dyspnea. The protocol classified the finding of normal lung ultrasound (A-line profile) as COPD or asthma since these conditions will have a normal sonographic appearance. This approach could lead to incorrect labeling of other extrapulmonary causes of dyspnea as COPD or asthma. The findings of this study suggest that POCUS is most effective at ruling in pulmonary edema and pneumonia while being most effective at ruling out asthma or COPD as causes of dyspnea. It is both sensitive and specific for pneumothorax. However, as other studies have found, the sensitivity of POCUS for COPD, asthma, and PE was inferior to traditional clinical evaluation.4 One of the few studies looking specifically at hospitalized ward patients compared a blinded lung POCUS diagnosis and a discharge clinical diagnosis classified as cardiac, pulmonary, or mixed dyspnea. The authors of that study found an “interstitial pattern” (two areas with more than two B-lines) in 94% of those classified as cardiac on discharge, but POCUS findings were less precise for those discharged with a pulmonary etiology of dyspnea.5 Identifying B-lines on lung POCUS appears to be helpful in rapidly differentiating cardiac from pulmonary etiologies of dyspnea.
An additional advantage of POCUS is that multiple organ systems can be evaluated in rapid succession when the etiology of dyspnea is unknown. In a smaller ED study of patients presenting with undifferentiated dyspnea, a diagnosis was recorded after history-taking and physical examination and then recorded again after lung, cardiac, and inferior vena cava POCUS. Clinician diagnostic accuracy improved from 53% to 77% with the use of POCUS (P = .003) compared with the final diagnosis.6 The treating physician’s primary impression changed in almost 50% of cases after using POCUS, most of which was driven by improved sensitivity and specificity of ADHF. In another study of 2,700 patients presenting to the ED with dyspnea, cardiopulmonary POCUS shortened the time to diagnosis (186 ± 72 minutes vs 24 ± 10 minutes, P = .025).4 These studies suggest that the use of POCUS in the initial evaluation of patients with undifferentiated dyspnea is a valuable tool with respect to diagnostic accuracy and timeliness.
PNEUMONIA
There are several different sonographic findings that can indicate pneumonia, such as consolidation or “hepatization”, the “shred” sign of an irregular border between consolidated lung and aerated lung, unilateral B-lines, and dynamic air bronchograms. Several recent systematic reviews and meta-analyses have investigated the operating characteristics of POCUS for the diagnosis of pneumonia. These reviews are limited by heterogeneity with respect to different patient populations, sonographers, and reference standards, but all three reviews found similar results, with the pooled AUC values ranging from 95% to 98%.7-9 This recent evidence along with other reviews suggests that lung ultrasound can serve as a primary diagnostic tool in pneumonia and is probably superior to chest radiography.
PLEURAL EFFUSION
Pleural effusions are observed with POCUS as anechoic or hypoechoic areas, generally in dependent lung zones. POCUS may provide additional benefit by better characterizing the effusion as having septations or floating fibrin strands. One recent systematic review and meta-analysis including 1,554 patients found that POCUS had excellent sensitivity and specificity (94% and 98%, respectively) in detecting pleural effusion versus chest radiography (51% and 91%, respectively), both compared with reference standard imaging such as computed tomography. The subgroup analysis found that sensitivity was higher for scanners who were intensivists or radiologists than for other physicians (97% vs 90%; P ≤ .001) and also found a nonstatistically significant trend toward reduced sensitivity when pocket-sized devices were used (90% vs 95%, P = .09).10
ACUTE DECOMPENSATED HEART FAILURE
It is extremely important to recognize that a POCUS finding of decreased left ventricular ejection fraction is not synonymous with a diagnosis of ADHF. Bedside providers can use POCUS to estimate cardiac function, but other clinical information is required to determine whether the syndrome of ADHF is present. In one study, examinations performed by 10 internists with approximately 18 hours of training in focused cardiac POCUS had a sensitivity and a specificity of 91% and 88%, respectively, for classifying left ventricular systolic function as normal or mildly, moderately, or severely depressed with “good/substantial” agreement (k = 0.77) compared with formal echocardiography.11 The presence of bilateral B-lines as a sign of pulmonary edema suggests accompanying functional decompensation. A meta-analysis of seven articles including 1075 patients in various clinical settings (ED, ICU, and inpatient wards) found a sensitivity of 94.1% and a specificity of 92.4% for using B-lines to diagnose acute cardiogenic pulmonary edema compared with the final clinical diagnosis.12 Al Deeb et al. examined 226 patients and found similar sensitivity (95.3%) and specificity (88.2%) for diagnosing acute cardiogenic pulmonary edema when nurses were trained to evaluate for bilateral B-lines in dyspneic patients admitted to the hospital, also compared with the adjudicated final diagnosis.13 Carlino et al. evaluated dyspneic patients using a three-minute pocket-sized device scan of the heart, lungs, and inferior vena cava and found that no single view offered a substantial improvement in diagnostic accuracy; however, the combination of bilateral B-lines and/or pleural effusion and either a dilated left atrium or left ventricular ejection fraction (LVEF) of <40% had a very high diagnostic accuracy (AUC 0.97).14 Russell et al. performed a secondary analysis of a prospective observational study of patients with dyspnea and found that a simple three-view scanning protocol looking for the presence of B-lines on the right and left anterior superior lung zones and an LVEF of <45% took an average of one minute and 32 seconds to perform and had 100% specificity for ADHF if all three were positive.15 Another recent systematic review and meta-analysis of six studies and 1,827 patients found a sensitivity of 88% (CI 75%-95%) for lung POCUS compared with a chest radiography at a sensitivity of 73% (70%-76%) for the diagnosis of ADHF.16 All these studies suggest that improving the diagnosis of ADHF does not require complex echocardiographic views and is probably more feasible and accessible than many expect.
SUMMARY
POCUS continues to show promise for evaluating patients with dyspnea. It is clear that adding a few POCUS examination maneuvers to a provider’s toolbox, such as looking for B-lines and overall cardiac function, can improve
Point-of-care ultrasound (POCUS) continues to gain traction in contemporary clinical practice both as a diagnostic tool and as an extension of the physical examination. Hospital Medicine (HM) lags behind Emergency Medicine (EM) and Critical Care (CC) in our uptake of such technology, although momentum is gaining. Leaders in HM have published frameworks for competency and credentialing, and the Society for Hospital Medicine has created a pathway for certification.1 POCUS use is the standard of care for several bedside procedures, but evidence for diagnostic applications is changing rapidly as the literature expands. However, the applicability of this evidence to HM patients can be challenging as most published studies are still from EM and CC settings. This Progress Note focuses on how a hospitalist might incorporate POCUS in the evaluation of adult patients with dyspnea. This topic was chosen after reviewing several relevant studies published in the past five years and recognizing the importance of dyspnea in HM. The Progress Note begins with a review of POCUS for undifferentiated dyspnea before exploring studies of common diagnoses that present with dyspnea, including pneumonia, pleural effusion, and acute decompensated heart failure (ADHF), aiming to update the knowledge of HM providers regarding this technology as well as to stimulate further study in this field.
SEARCH STRATEGY
In collaboration with an academic librarian in March 2019, PubMed was searched for studies published within the past five years using several MESH search terms for POCUS. The search was originally focused to the field of HM using specific search terms, but this yielded a very limited number of studies. Therefore, the search strategy was expanded to include EM and CC studies. This final search generated 346 papers that were supplemented with additional literature searches using references from studies found in the initial search.
UNDIFFERENTIATED DYSPNEA
Dyspnea is common in HM, both as the reason for a patient’s admission and as a symptom that develops during hospitalization such as after intravenous fluid resuscitation, a possible aspiration event, or central line placement. The differential diagnosis is broad, and multiple studies suggest that POCUS can aid in the evaluation of undifferentiated dyspnea while also being cost effective and avoiding the potential radiation of other testing modalities. The pulmonary POCUS evaluation incorporates a combination of several findings, including “A-lines” or horizontal artifacts from normal aerated lung; “B-lines”, vertical artifacts generated by extra-alveolar fluid, consolidation or “tissue-like pattern”; air bronchograms, consolidated lung surrounding airways; anechoic or hypoechoic areas in dependent zones of the lung; and the presence or absence of pleural sliding.2
In one prospective observational study of five internal medicine residents with no prior POCUS experience and three hours of training, the addition of handheld POCUS devices to usual clinical information improved the diagnostic accuracy for pneumonia, pulmonary edema, pleural effusion, and obstructive lung disease when evaluating patients with a primary complaint of dyspnea (area under the curve [AUC] 0.81 vs 0.87, P < .01).2 However, the largest improvements in the operating characteristics were observed with the two residents who received an extended two-week elective of training.
In another study of 383 consecutive patients presenting to the ED with dyspnea, physicians with basic and advanced POCUS training were blinded to all clinical information and recorded a diagnosis after performing a lung POCUS examination. The “ultrasound physician’s” diagnosis was then compared to the treating emergency department (ED) physician’s diagnosis using history, physical, and other diagnostic data. Lung POCUS had a sensitivity and a specificity of 87.6% and 96.2% for pulmonary edema, 85.7% and 99% for pneumonia, 98.2% and 67.3% for asthma/chronic obstructive pulmonary disease (COPD), 46.2% and 100% for pulmonary embolus (PE), and 71.4% and 100% for pneumothorax, respectively.3 The scanning protocol used, the BLUE (Bedside Lung Ultrasound Examination) protocol, was focused on ruling out significant pulmonary etiologies of dyspnea. The protocol classified the finding of normal lung ultrasound (A-line profile) as COPD or asthma since these conditions will have a normal sonographic appearance. This approach could lead to incorrect labeling of other extrapulmonary causes of dyspnea as COPD or asthma. The findings of this study suggest that POCUS is most effective at ruling in pulmonary edema and pneumonia while being most effective at ruling out asthma or COPD as causes of dyspnea. It is both sensitive and specific for pneumothorax. However, as other studies have found, the sensitivity of POCUS for COPD, asthma, and PE was inferior to traditional clinical evaluation.4 One of the few studies looking specifically at hospitalized ward patients compared a blinded lung POCUS diagnosis and a discharge clinical diagnosis classified as cardiac, pulmonary, or mixed dyspnea. The authors of that study found an “interstitial pattern” (two areas with more than two B-lines) in 94% of those classified as cardiac on discharge, but POCUS findings were less precise for those discharged with a pulmonary etiology of dyspnea.5 Identifying B-lines on lung POCUS appears to be helpful in rapidly differentiating cardiac from pulmonary etiologies of dyspnea.
An additional advantage of POCUS is that multiple organ systems can be evaluated in rapid succession when the etiology of dyspnea is unknown. In a smaller ED study of patients presenting with undifferentiated dyspnea, a diagnosis was recorded after history-taking and physical examination and then recorded again after lung, cardiac, and inferior vena cava POCUS. Clinician diagnostic accuracy improved from 53% to 77% with the use of POCUS (P = .003) compared with the final diagnosis.6 The treating physician’s primary impression changed in almost 50% of cases after using POCUS, most of which was driven by improved sensitivity and specificity of ADHF. In another study of 2,700 patients presenting to the ED with dyspnea, cardiopulmonary POCUS shortened the time to diagnosis (186 ± 72 minutes vs 24 ± 10 minutes, P = .025).4 These studies suggest that the use of POCUS in the initial evaluation of patients with undifferentiated dyspnea is a valuable tool with respect to diagnostic accuracy and timeliness.
PNEUMONIA
There are several different sonographic findings that can indicate pneumonia, such as consolidation or “hepatization”, the “shred” sign of an irregular border between consolidated lung and aerated lung, unilateral B-lines, and dynamic air bronchograms. Several recent systematic reviews and meta-analyses have investigated the operating characteristics of POCUS for the diagnosis of pneumonia. These reviews are limited by heterogeneity with respect to different patient populations, sonographers, and reference standards, but all three reviews found similar results, with the pooled AUC values ranging from 95% to 98%.7-9 This recent evidence along with other reviews suggests that lung ultrasound can serve as a primary diagnostic tool in pneumonia and is probably superior to chest radiography.
PLEURAL EFFUSION
Pleural effusions are observed with POCUS as anechoic or hypoechoic areas, generally in dependent lung zones. POCUS may provide additional benefit by better characterizing the effusion as having septations or floating fibrin strands. One recent systematic review and meta-analysis including 1,554 patients found that POCUS had excellent sensitivity and specificity (94% and 98%, respectively) in detecting pleural effusion versus chest radiography (51% and 91%, respectively), both compared with reference standard imaging such as computed tomography. The subgroup analysis found that sensitivity was higher for scanners who were intensivists or radiologists than for other physicians (97% vs 90%; P ≤ .001) and also found a nonstatistically significant trend toward reduced sensitivity when pocket-sized devices were used (90% vs 95%, P = .09).10
ACUTE DECOMPENSATED HEART FAILURE
It is extremely important to recognize that a POCUS finding of decreased left ventricular ejection fraction is not synonymous with a diagnosis of ADHF. Bedside providers can use POCUS to estimate cardiac function, but other clinical information is required to determine whether the syndrome of ADHF is present. In one study, examinations performed by 10 internists with approximately 18 hours of training in focused cardiac POCUS had a sensitivity and a specificity of 91% and 88%, respectively, for classifying left ventricular systolic function as normal or mildly, moderately, or severely depressed with “good/substantial” agreement (k = 0.77) compared with formal echocardiography.11 The presence of bilateral B-lines as a sign of pulmonary edema suggests accompanying functional decompensation. A meta-analysis of seven articles including 1075 patients in various clinical settings (ED, ICU, and inpatient wards) found a sensitivity of 94.1% and a specificity of 92.4% for using B-lines to diagnose acute cardiogenic pulmonary edema compared with the final clinical diagnosis.12 Al Deeb et al. examined 226 patients and found similar sensitivity (95.3%) and specificity (88.2%) for diagnosing acute cardiogenic pulmonary edema when nurses were trained to evaluate for bilateral B-lines in dyspneic patients admitted to the hospital, also compared with the adjudicated final diagnosis.13 Carlino et al. evaluated dyspneic patients using a three-minute pocket-sized device scan of the heart, lungs, and inferior vena cava and found that no single view offered a substantial improvement in diagnostic accuracy; however, the combination of bilateral B-lines and/or pleural effusion and either a dilated left atrium or left ventricular ejection fraction (LVEF) of <40% had a very high diagnostic accuracy (AUC 0.97).14 Russell et al. performed a secondary analysis of a prospective observational study of patients with dyspnea and found that a simple three-view scanning protocol looking for the presence of B-lines on the right and left anterior superior lung zones and an LVEF of <45% took an average of one minute and 32 seconds to perform and had 100% specificity for ADHF if all three were positive.15 Another recent systematic review and meta-analysis of six studies and 1,827 patients found a sensitivity of 88% (CI 75%-95%) for lung POCUS compared with a chest radiography at a sensitivity of 73% (70%-76%) for the diagnosis of ADHF.16 All these studies suggest that improving the diagnosis of ADHF does not require complex echocardiographic views and is probably more feasible and accessible than many expect.
SUMMARY
POCUS continues to show promise for evaluating patients with dyspnea. It is clear that adding a few POCUS examination maneuvers to a provider’s toolbox, such as looking for B-lines and overall cardiac function, can improve
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. Published online only January 2, 2019. https://doi.org/10.12788/jhm.3079.
2. Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9(9):594-597. https://doi.org/10.1002/jhm.2219.
3. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.02.028.
4. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295-1301. https://doi.org/10.1016/j.chest.2017.02.003.
5. Perrone T, Maggi A, Sgarlata C, et al. Lung ultrasound in internal medicine: a bedside help to increase accuracy in the diagnosis of dyspnea. Eur J Intern Med. 2017;46:61-65. https://doi.org/10.1016/j.ejim.2017.07.034.
6. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. https://doi.org/10.5811/westjem.2015.11.28525.
7. Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018;25(5):312-321. https://doi.org/10.1097/MEJ.0000000000000517.
8. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y
9. Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine . 2017;96(3):e5713. https://doi.org/10.1097/MD.0000000000005713.
10. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1-10.
11. Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92-99. https://doi.org/10.1002/jcu.22272.
12. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. https://doi.org/10.1111/acem.12435.
13. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea: a prospective observational study. Medicine (Baltimore). 2016;95(9):e2925. https://doi.org/10.1097/MD.0000000000002925.
14. Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the emergency department. Echocardiography (Mount Kisco, NY). 2018;35(6):785-791. https://doi.org/10.1111/echo.13851.
15. Russell FM, Ehrman RR. A modified lung and cardiac ultrasound protocol saves time and rules in the diagnosis of acute heart failure. J Emerg Med. 2017;52(6):839-845. https://doi.org/10.1016/j.jemermed.2017.02.003.
16. Maw AM, Hassanin A, Ho PM, et al. diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703. https://doi.org/10.1001/jamanetworkopen.2019.0703.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. Published online only January 2, 2019. https://doi.org/10.12788/jhm.3079.
2. Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9(9):594-597. https://doi.org/10.1002/jhm.2219.
3. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.02.028.
4. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295-1301. https://doi.org/10.1016/j.chest.2017.02.003.
5. Perrone T, Maggi A, Sgarlata C, et al. Lung ultrasound in internal medicine: a bedside help to increase accuracy in the diagnosis of dyspnea. Eur J Intern Med. 2017;46:61-65. https://doi.org/10.1016/j.ejim.2017.07.034.
6. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. https://doi.org/10.5811/westjem.2015.11.28525.
7. Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018;25(5):312-321. https://doi.org/10.1097/MEJ.0000000000000517.
8. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y
9. Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine . 2017;96(3):e5713. https://doi.org/10.1097/MD.0000000000005713.
10. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1-10.
11. Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92-99. https://doi.org/10.1002/jcu.22272.
12. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. https://doi.org/10.1111/acem.12435.
13. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea: a prospective observational study. Medicine (Baltimore). 2016;95(9):e2925. https://doi.org/10.1097/MD.0000000000002925.
14. Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the emergency department. Echocardiography (Mount Kisco, NY). 2018;35(6):785-791. https://doi.org/10.1111/echo.13851.
15. Russell FM, Ehrman RR. A modified lung and cardiac ultrasound protocol saves time and rules in the diagnosis of acute heart failure. J Emerg Med. 2017;52(6):839-845. https://doi.org/10.1016/j.jemermed.2017.02.003.
16. Maw AM, Hassanin A, Ho PM, et al. diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703. https://doi.org/10.1001/jamanetworkopen.2019.0703.
© 2020 Society of Hospital Medicine
Missed Opportunities for Treatment of Opioid Use Disorder in the Hospital Setting: Updating an Outdated Policy
THE PROBLEM AND THE ROLE OF THE HOSPITALIST
Opioid use disorder (OUD) is a common, underrecognized, undertreated, and deadly medical condition. Although the focus of addressing the opioid epidemic has been centered in the outpatient setting, hospitalists play an important—and often underutilized—role in identifying OUD, initiating treatment, and assisting with linkage to longitudinal care after discharge.
Over the past 20 years, the annual rate of hospital discharges documenting OUD has quadrupled.1 During 2010-2016, the annual discharge rate for heroin overdoses increased by 23%.1 Although the total number of hospitalizations in the United States remained stable from 2002 to 2012, the number of admissions for opioid abuse or dependence increased from 301,707 to 520,275. More than 500,000 hospital admissions per year (1% of total nationwide hospitalizations) are now due primarily to OUD.2
Injection opioid use increases the risk of endocarditis, osteomyelitis, septic arthritis, and epidural abscesses, conditions that often prolong hospitalizations and frequently lead to readmissions. Admissions for OUD-related infections are rising at a startling rate. Between 2002 and 2012, the number of admissions for infections associated with OUDs had increased from 3,421 to 6,535.2 In addition to providing the opportunity to diagnose OUD, hospitalizations offer an ideal time to engage patients in OUD treatment and linkage to outpatient care.
Although we uniformly offer patients antibiotic treatment for acute infection, hospitalists should consistently incorporate treatment of OUD to address the root cause of these admissions. As infection is but one sequelae of the underlying disease of addiction, treating without medications for OUD (MOUD) would be akin to treating a diabetic foot ulcer with antibiotics and not providing medications to improve glycemic control. Omitting such addiction treatment can contribute to treatment failure and worse health outcomes. Among patients with endocarditis and an associated valve repair, those who continue injection drug use have a 10 times higher risk of death or reoperation between 90 and 180 days after repair than those not engaged in drug use.3
Despite data demonstrating the significant benefit and the minimal harm of MOUD, significant gaps remain in providing MOUD and linking patients from the hospital to community care.1,4 Hospital encounters are missed opportunities to provide life-saving MOUD treatment; the majority of patients with OUD do not receive evidence-based treatment while inpatient.5 Rosenthal et al. found that of 102 patients admitted with injection drug use-associated infective endocarditis from 2004 to 2014, only 8% received MOUD, and approximately half had a documentation of substance use treatment in their discharge worksheet.4 In Massachusetts, among individuals who experienced a nonfatal opioid overdose and had interaction with healthcare services, only 26% were on MOUD one year later.6 Based on our experience, a substantial proportion of patients with OUD do not seek or have access to medical care, acute care settings offer a critical opportunity to engage them in treatment for their addiction.
WHY SHOULD HOSPITALISTS INITIATE BUPRENORPHINE?
First, buprenorphine effectively treats withdrawal symptoms. Buprenorphine and methadone are superior to other medications in treating symptoms of withdrawal.7 If withdrawal symptoms are treated, patients are less likely to leave against medical advice8 and are more likely to complete treatment.
Second, MOUD is the standard of care for treating OUD.9 Medications include the full agonist methadone, the partial agonist buprenorphine, and the long-acting antagonist naltrexone. Although all these drugs are effective and legal to initiate for inpatients,6 this perspective focuses on buprenorphine in an effort to draw attention to associated policy barriers. Buprenorphine is the only MOUD that can be offered as office-based therapy by providers in the outpatient setting. Meta-analyses show that MOUD is associated with lower rates of mortality, illicit opioid use, HIV transmission, and violent crime and arrest.9
Third, MOUD treatment, rather than just referral, leads to higher long-term treatment success.10 When initiating buprenorphine in the hospital, treatment retention rates at one month were double that of referral alone. Six months after discharge, patients were five times more likely to remain engaged in treatment compared with those who received a detoxification protocol only.
Fourth, buprenorphine is not only effective, but it is also safe and has low risks of misuse. Because buprenorphine is a partial agonist, it has both a ceiling effect on respiratory depression (decreasing potential lethality) and on euphoria (decreasing the likelihood of misuse). Among individuals who took nonprescribed buprenorphine on the street, less than 7% reported taking it for any attempt at euphoria. Instead, people with OUD most often use nonprescribed or diverted buprenorphine to treat withdrawal symptoms.11
Fifth, buprenorphine treatment is associated with fewer hospital readmissions.12
Finally, initiating OUD treatment is feasible in the hospital setting. Any hospitalist can legally prescribe buprenorphine to treat opioid withdrawal for hospitalized patients admitted for medical or surgical reasons. A waiver is necessary only for prescribing at the time of hospital discharge for use in non-inpatient settings of care.
A POLICY BARRIER: THE X WAIVER
The United States Congress passed the Drug Addiction Treatment Act (DATA) of 2000, which codified the X waiver, in response to the growing opioid crisis. Only those providers with the DATA X waiver can write buprenorphine prescriptions to be filled in an outpatient pharmacy. To obtain an X waiver, physicians must complete an 8-hour course, whereas physician assistants and nurse practitioners must complete a 24-hour course. This training far exceeds any required training to prescribe opioids for pain.
Unfortunately, the X waiver requirement obstructs hospitalists from initiating buprenorphine in the inpatient setting in the following ways: (1) hospitalists often choose not to initiate chronic buprenorphine treatment if they lack the X waiver that would allow them to write the discharge prescription and/or (2) they are unable to identify a waivered provider in the community to continue the prescription. Unfortunately, only 6% of all medical practitioners are waivered to prescribe buprenorphine; greater than 40% of US counties are “buprenorphine deserts,” with no providers waivered to prescribe buprenorphine.13
To address the opioid crisis, we must rethink our current policies. The Department of Health and Human Services should eliminate the X waiver and allow any licensed physician, nurse practitioner, or physician assistant to prescribe buprenorphine.14 Recent American Medical Association Opioid Task Force recommendations have called to “remove… inappropriate administrative burdens or barriers that delay or deny care for FDA-approved medications used as part of medication-assisted treatment for OUD.”15 Legislation to remove the X wavier has been proposed in the United States.16
The removal of a buprenorphine waiver requirement has had success in other settings. The French deregulation of buprenorphine was associated with a reduction in opioid overdose deaths by 79%. Similar success in the United States would save an estimated 30,000 lives yearly.14 Removing the X waiver is an important step in empowering hospitalists to initiate MOUD for individuals in the hospital setting. Moreover, it opens the door to more outpatient primary care providers serving as community linkages for long-term addiction care.
NOT A PANACEA
Without the X waiver, the associated OUD training will no longer be required. This could have unintended consequences. For example, if hospitalists order buprenorphine while opioids remain active, precipitated withdrawal may ensue. Crucially, the current literature does not indicate that the required X waiver training improves knowledge, patient care, or outcomes.17 Nevertheless, MOUD and addiction training may help reduce knowledge gaps and empower providers to engage in productive conversations surrounding addiction. This highlights the crucial role of physician organizations, such as the Society of Hospital Medicine, in educating hospitalists about MOUD. (This organization, among others, has developed robust MOUD training.18)
It is also important to acknowledge that the waiver is only one obstacle. Other barriers have been identified in initiating buprenorphine, including access to treatment after discharge, access to social work support, and lack of EMR order sets, among others.19 Professional societies, hospitals, and hospitalists need to help address these barriers through ancillary support staff, quality improvement initiatives, and improved inpatient treatment of withdrawal with MOUD. This can be done successfully; one study found that 82% of hospitalized patients who engaged in a new transitional opioid program subsequently presented to outpatient opioid treatment.20 Novel interventions must be part of a hospital-wide approach to optimizing improved longitudinal treatment for patients suffering from addiction.
CONCLUSION
Hospitalization is an ideal opportunity for clinicians to diagnose and treat OUD in a population that often has not sought, or has fallen out of, addiction treatment. Hospitalists can and should initiate buprenorphine in appropriate inpatients and plan for their transition to chronic care. Eliminating the waiver in combination with designing innovative educational opportunities and systems approaches to provide better linkages to outpatient OUD treatment is needed to combat the opioid crisis. To enable more hospitalists to successfully initiate long-term buprenorphine therapy—and to enable more outpatient providers to continue prescriptions—we must eliminate the X waiver.
Disclosures
Dr. Wilson received honorarium from the American Society of Addiction Medicine for teaching and creating
1. Peterson C, Xu L, Florence C, Mack KA. Opioid-related US hospital discharges by type, 1993–2016. J Subst Abuse Treat. 2019;103:9-13. https://doi.org/10.1016/j.jsat.2019.05.003.
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff. 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
3. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100(3):875-882. https://doi.org/10.1016/j.athoracsur.2015.03.019.
4. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024.
5. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2017;13(1):62-64. https://doi.org/10.12788/jhm.2861.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Gowing L, Ali R, White JM, Mbewe D. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017;(2):CD002025. https://doi.org/10.1002/14651858.CD002025.pub5.
8. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59. https://doi.org/10.2105/AJPH.2015.302885.
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/NEJMra1604339.
10. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients. JAMA Intern Med. 2014;174(8):1369. https://doi.org/10.1001/jamainternmed.2014.2556.
11. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. Factors contributing to the rise of buprenorphine misuse: 2008-2013. Drug Alcohol Depend. 2014;142:98-104. https://doi.org/10.1016/j.drugalcdep.2014.06.005.
12. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/ADM.0000000000000499.
13. Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a dea waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Heal. 2019;35(1):108-112. https://doi.org/10.1111/jrh.12307.
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the x waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685.
15. American Medical Association Opioid Task Force. AMA Opioid Task Force recommendations offer roadmap to policymakers | American Medical Association. https://www.ama-assn.org/press-center/press-releases/ama-opioid-task-force-recommendations-offer-roadmap-policymakers. Accessed June 14, 2019.
16. Tonko P. H.R.2482: Mainstreaming Addiction Treatment Act of 2019. House Of Representatives (116th Congress); 2019. https://www.congress.gov/bill/116th-congress/house-bill/2482. Accessed July 10, 2019.
17. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382
18. Society of Hospital Medicine. Clinical Topics: Opioid Safety. https://www.hospitalmedicine.org/clinical-topics/opioid-safety/. Accessed October 24, 2019.
19. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. https://doi.org/10.1016/j.ajem.2019.02.025.
20. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. https://doi.org/10.1007/s11606-010-1311-3.
THE PROBLEM AND THE ROLE OF THE HOSPITALIST
Opioid use disorder (OUD) is a common, underrecognized, undertreated, and deadly medical condition. Although the focus of addressing the opioid epidemic has been centered in the outpatient setting, hospitalists play an important—and often underutilized—role in identifying OUD, initiating treatment, and assisting with linkage to longitudinal care after discharge.
Over the past 20 years, the annual rate of hospital discharges documenting OUD has quadrupled.1 During 2010-2016, the annual discharge rate for heroin overdoses increased by 23%.1 Although the total number of hospitalizations in the United States remained stable from 2002 to 2012, the number of admissions for opioid abuse or dependence increased from 301,707 to 520,275. More than 500,000 hospital admissions per year (1% of total nationwide hospitalizations) are now due primarily to OUD.2
Injection opioid use increases the risk of endocarditis, osteomyelitis, septic arthritis, and epidural abscesses, conditions that often prolong hospitalizations and frequently lead to readmissions. Admissions for OUD-related infections are rising at a startling rate. Between 2002 and 2012, the number of admissions for infections associated with OUDs had increased from 3,421 to 6,535.2 In addition to providing the opportunity to diagnose OUD, hospitalizations offer an ideal time to engage patients in OUD treatment and linkage to outpatient care.
Although we uniformly offer patients antibiotic treatment for acute infection, hospitalists should consistently incorporate treatment of OUD to address the root cause of these admissions. As infection is but one sequelae of the underlying disease of addiction, treating without medications for OUD (MOUD) would be akin to treating a diabetic foot ulcer with antibiotics and not providing medications to improve glycemic control. Omitting such addiction treatment can contribute to treatment failure and worse health outcomes. Among patients with endocarditis and an associated valve repair, those who continue injection drug use have a 10 times higher risk of death or reoperation between 90 and 180 days after repair than those not engaged in drug use.3
Despite data demonstrating the significant benefit and the minimal harm of MOUD, significant gaps remain in providing MOUD and linking patients from the hospital to community care.1,4 Hospital encounters are missed opportunities to provide life-saving MOUD treatment; the majority of patients with OUD do not receive evidence-based treatment while inpatient.5 Rosenthal et al. found that of 102 patients admitted with injection drug use-associated infective endocarditis from 2004 to 2014, only 8% received MOUD, and approximately half had a documentation of substance use treatment in their discharge worksheet.4 In Massachusetts, among individuals who experienced a nonfatal opioid overdose and had interaction with healthcare services, only 26% were on MOUD one year later.6 Based on our experience, a substantial proportion of patients with OUD do not seek or have access to medical care, acute care settings offer a critical opportunity to engage them in treatment for their addiction.
WHY SHOULD HOSPITALISTS INITIATE BUPRENORPHINE?
First, buprenorphine effectively treats withdrawal symptoms. Buprenorphine and methadone are superior to other medications in treating symptoms of withdrawal.7 If withdrawal symptoms are treated, patients are less likely to leave against medical advice8 and are more likely to complete treatment.
Second, MOUD is the standard of care for treating OUD.9 Medications include the full agonist methadone, the partial agonist buprenorphine, and the long-acting antagonist naltrexone. Although all these drugs are effective and legal to initiate for inpatients,6 this perspective focuses on buprenorphine in an effort to draw attention to associated policy barriers. Buprenorphine is the only MOUD that can be offered as office-based therapy by providers in the outpatient setting. Meta-analyses show that MOUD is associated with lower rates of mortality, illicit opioid use, HIV transmission, and violent crime and arrest.9
Third, MOUD treatment, rather than just referral, leads to higher long-term treatment success.10 When initiating buprenorphine in the hospital, treatment retention rates at one month were double that of referral alone. Six months after discharge, patients were five times more likely to remain engaged in treatment compared with those who received a detoxification protocol only.
Fourth, buprenorphine is not only effective, but it is also safe and has low risks of misuse. Because buprenorphine is a partial agonist, it has both a ceiling effect on respiratory depression (decreasing potential lethality) and on euphoria (decreasing the likelihood of misuse). Among individuals who took nonprescribed buprenorphine on the street, less than 7% reported taking it for any attempt at euphoria. Instead, people with OUD most often use nonprescribed or diverted buprenorphine to treat withdrawal symptoms.11
Fifth, buprenorphine treatment is associated with fewer hospital readmissions.12
Finally, initiating OUD treatment is feasible in the hospital setting. Any hospitalist can legally prescribe buprenorphine to treat opioid withdrawal for hospitalized patients admitted for medical or surgical reasons. A waiver is necessary only for prescribing at the time of hospital discharge for use in non-inpatient settings of care.
A POLICY BARRIER: THE X WAIVER
The United States Congress passed the Drug Addiction Treatment Act (DATA) of 2000, which codified the X waiver, in response to the growing opioid crisis. Only those providers with the DATA X waiver can write buprenorphine prescriptions to be filled in an outpatient pharmacy. To obtain an X waiver, physicians must complete an 8-hour course, whereas physician assistants and nurse practitioners must complete a 24-hour course. This training far exceeds any required training to prescribe opioids for pain.
Unfortunately, the X waiver requirement obstructs hospitalists from initiating buprenorphine in the inpatient setting in the following ways: (1) hospitalists often choose not to initiate chronic buprenorphine treatment if they lack the X waiver that would allow them to write the discharge prescription and/or (2) they are unable to identify a waivered provider in the community to continue the prescription. Unfortunately, only 6% of all medical practitioners are waivered to prescribe buprenorphine; greater than 40% of US counties are “buprenorphine deserts,” with no providers waivered to prescribe buprenorphine.13
To address the opioid crisis, we must rethink our current policies. The Department of Health and Human Services should eliminate the X waiver and allow any licensed physician, nurse practitioner, or physician assistant to prescribe buprenorphine.14 Recent American Medical Association Opioid Task Force recommendations have called to “remove… inappropriate administrative burdens or barriers that delay or deny care for FDA-approved medications used as part of medication-assisted treatment for OUD.”15 Legislation to remove the X wavier has been proposed in the United States.16
The removal of a buprenorphine waiver requirement has had success in other settings. The French deregulation of buprenorphine was associated with a reduction in opioid overdose deaths by 79%. Similar success in the United States would save an estimated 30,000 lives yearly.14 Removing the X waiver is an important step in empowering hospitalists to initiate MOUD for individuals in the hospital setting. Moreover, it opens the door to more outpatient primary care providers serving as community linkages for long-term addiction care.
NOT A PANACEA
Without the X waiver, the associated OUD training will no longer be required. This could have unintended consequences. For example, if hospitalists order buprenorphine while opioids remain active, precipitated withdrawal may ensue. Crucially, the current literature does not indicate that the required X waiver training improves knowledge, patient care, or outcomes.17 Nevertheless, MOUD and addiction training may help reduce knowledge gaps and empower providers to engage in productive conversations surrounding addiction. This highlights the crucial role of physician organizations, such as the Society of Hospital Medicine, in educating hospitalists about MOUD. (This organization, among others, has developed robust MOUD training.18)
It is also important to acknowledge that the waiver is only one obstacle. Other barriers have been identified in initiating buprenorphine, including access to treatment after discharge, access to social work support, and lack of EMR order sets, among others.19 Professional societies, hospitals, and hospitalists need to help address these barriers through ancillary support staff, quality improvement initiatives, and improved inpatient treatment of withdrawal with MOUD. This can be done successfully; one study found that 82% of hospitalized patients who engaged in a new transitional opioid program subsequently presented to outpatient opioid treatment.20 Novel interventions must be part of a hospital-wide approach to optimizing improved longitudinal treatment for patients suffering from addiction.
CONCLUSION
Hospitalization is an ideal opportunity for clinicians to diagnose and treat OUD in a population that often has not sought, or has fallen out of, addiction treatment. Hospitalists can and should initiate buprenorphine in appropriate inpatients and plan for their transition to chronic care. Eliminating the waiver in combination with designing innovative educational opportunities and systems approaches to provide better linkages to outpatient OUD treatment is needed to combat the opioid crisis. To enable more hospitalists to successfully initiate long-term buprenorphine therapy—and to enable more outpatient providers to continue prescriptions—we must eliminate the X waiver.
Disclosures
Dr. Wilson received honorarium from the American Society of Addiction Medicine for teaching and creating
THE PROBLEM AND THE ROLE OF THE HOSPITALIST
Opioid use disorder (OUD) is a common, underrecognized, undertreated, and deadly medical condition. Although the focus of addressing the opioid epidemic has been centered in the outpatient setting, hospitalists play an important—and often underutilized—role in identifying OUD, initiating treatment, and assisting with linkage to longitudinal care after discharge.
Over the past 20 years, the annual rate of hospital discharges documenting OUD has quadrupled.1 During 2010-2016, the annual discharge rate for heroin overdoses increased by 23%.1 Although the total number of hospitalizations in the United States remained stable from 2002 to 2012, the number of admissions for opioid abuse or dependence increased from 301,707 to 520,275. More than 500,000 hospital admissions per year (1% of total nationwide hospitalizations) are now due primarily to OUD.2
Injection opioid use increases the risk of endocarditis, osteomyelitis, septic arthritis, and epidural abscesses, conditions that often prolong hospitalizations and frequently lead to readmissions. Admissions for OUD-related infections are rising at a startling rate. Between 2002 and 2012, the number of admissions for infections associated with OUDs had increased from 3,421 to 6,535.2 In addition to providing the opportunity to diagnose OUD, hospitalizations offer an ideal time to engage patients in OUD treatment and linkage to outpatient care.
Although we uniformly offer patients antibiotic treatment for acute infection, hospitalists should consistently incorporate treatment of OUD to address the root cause of these admissions. As infection is but one sequelae of the underlying disease of addiction, treating without medications for OUD (MOUD) would be akin to treating a diabetic foot ulcer with antibiotics and not providing medications to improve glycemic control. Omitting such addiction treatment can contribute to treatment failure and worse health outcomes. Among patients with endocarditis and an associated valve repair, those who continue injection drug use have a 10 times higher risk of death or reoperation between 90 and 180 days after repair than those not engaged in drug use.3
Despite data demonstrating the significant benefit and the minimal harm of MOUD, significant gaps remain in providing MOUD and linking patients from the hospital to community care.1,4 Hospital encounters are missed opportunities to provide life-saving MOUD treatment; the majority of patients with OUD do not receive evidence-based treatment while inpatient.5 Rosenthal et al. found that of 102 patients admitted with injection drug use-associated infective endocarditis from 2004 to 2014, only 8% received MOUD, and approximately half had a documentation of substance use treatment in their discharge worksheet.4 In Massachusetts, among individuals who experienced a nonfatal opioid overdose and had interaction with healthcare services, only 26% were on MOUD one year later.6 Based on our experience, a substantial proportion of patients with OUD do not seek or have access to medical care, acute care settings offer a critical opportunity to engage them in treatment for their addiction.
WHY SHOULD HOSPITALISTS INITIATE BUPRENORPHINE?
First, buprenorphine effectively treats withdrawal symptoms. Buprenorphine and methadone are superior to other medications in treating symptoms of withdrawal.7 If withdrawal symptoms are treated, patients are less likely to leave against medical advice8 and are more likely to complete treatment.
Second, MOUD is the standard of care for treating OUD.9 Medications include the full agonist methadone, the partial agonist buprenorphine, and the long-acting antagonist naltrexone. Although all these drugs are effective and legal to initiate for inpatients,6 this perspective focuses on buprenorphine in an effort to draw attention to associated policy barriers. Buprenorphine is the only MOUD that can be offered as office-based therapy by providers in the outpatient setting. Meta-analyses show that MOUD is associated with lower rates of mortality, illicit opioid use, HIV transmission, and violent crime and arrest.9
Third, MOUD treatment, rather than just referral, leads to higher long-term treatment success.10 When initiating buprenorphine in the hospital, treatment retention rates at one month were double that of referral alone. Six months after discharge, patients were five times more likely to remain engaged in treatment compared with those who received a detoxification protocol only.
Fourth, buprenorphine is not only effective, but it is also safe and has low risks of misuse. Because buprenorphine is a partial agonist, it has both a ceiling effect on respiratory depression (decreasing potential lethality) and on euphoria (decreasing the likelihood of misuse). Among individuals who took nonprescribed buprenorphine on the street, less than 7% reported taking it for any attempt at euphoria. Instead, people with OUD most often use nonprescribed or diverted buprenorphine to treat withdrawal symptoms.11
Fifth, buprenorphine treatment is associated with fewer hospital readmissions.12
Finally, initiating OUD treatment is feasible in the hospital setting. Any hospitalist can legally prescribe buprenorphine to treat opioid withdrawal for hospitalized patients admitted for medical or surgical reasons. A waiver is necessary only for prescribing at the time of hospital discharge for use in non-inpatient settings of care.
A POLICY BARRIER: THE X WAIVER
The United States Congress passed the Drug Addiction Treatment Act (DATA) of 2000, which codified the X waiver, in response to the growing opioid crisis. Only those providers with the DATA X waiver can write buprenorphine prescriptions to be filled in an outpatient pharmacy. To obtain an X waiver, physicians must complete an 8-hour course, whereas physician assistants and nurse practitioners must complete a 24-hour course. This training far exceeds any required training to prescribe opioids for pain.
Unfortunately, the X waiver requirement obstructs hospitalists from initiating buprenorphine in the inpatient setting in the following ways: (1) hospitalists often choose not to initiate chronic buprenorphine treatment if they lack the X waiver that would allow them to write the discharge prescription and/or (2) they are unable to identify a waivered provider in the community to continue the prescription. Unfortunately, only 6% of all medical practitioners are waivered to prescribe buprenorphine; greater than 40% of US counties are “buprenorphine deserts,” with no providers waivered to prescribe buprenorphine.13
To address the opioid crisis, we must rethink our current policies. The Department of Health and Human Services should eliminate the X waiver and allow any licensed physician, nurse practitioner, or physician assistant to prescribe buprenorphine.14 Recent American Medical Association Opioid Task Force recommendations have called to “remove… inappropriate administrative burdens or barriers that delay or deny care for FDA-approved medications used as part of medication-assisted treatment for OUD.”15 Legislation to remove the X wavier has been proposed in the United States.16
The removal of a buprenorphine waiver requirement has had success in other settings. The French deregulation of buprenorphine was associated with a reduction in opioid overdose deaths by 79%. Similar success in the United States would save an estimated 30,000 lives yearly.14 Removing the X waiver is an important step in empowering hospitalists to initiate MOUD for individuals in the hospital setting. Moreover, it opens the door to more outpatient primary care providers serving as community linkages for long-term addiction care.
NOT A PANACEA
Without the X waiver, the associated OUD training will no longer be required. This could have unintended consequences. For example, if hospitalists order buprenorphine while opioids remain active, precipitated withdrawal may ensue. Crucially, the current literature does not indicate that the required X waiver training improves knowledge, patient care, or outcomes.17 Nevertheless, MOUD and addiction training may help reduce knowledge gaps and empower providers to engage in productive conversations surrounding addiction. This highlights the crucial role of physician organizations, such as the Society of Hospital Medicine, in educating hospitalists about MOUD. (This organization, among others, has developed robust MOUD training.18)
It is also important to acknowledge that the waiver is only one obstacle. Other barriers have been identified in initiating buprenorphine, including access to treatment after discharge, access to social work support, and lack of EMR order sets, among others.19 Professional societies, hospitals, and hospitalists need to help address these barriers through ancillary support staff, quality improvement initiatives, and improved inpatient treatment of withdrawal with MOUD. This can be done successfully; one study found that 82% of hospitalized patients who engaged in a new transitional opioid program subsequently presented to outpatient opioid treatment.20 Novel interventions must be part of a hospital-wide approach to optimizing improved longitudinal treatment for patients suffering from addiction.
CONCLUSION
Hospitalization is an ideal opportunity for clinicians to diagnose and treat OUD in a population that often has not sought, or has fallen out of, addiction treatment. Hospitalists can and should initiate buprenorphine in appropriate inpatients and plan for their transition to chronic care. Eliminating the waiver in combination with designing innovative educational opportunities and systems approaches to provide better linkages to outpatient OUD treatment is needed to combat the opioid crisis. To enable more hospitalists to successfully initiate long-term buprenorphine therapy—and to enable more outpatient providers to continue prescriptions—we must eliminate the X waiver.
Disclosures
Dr. Wilson received honorarium from the American Society of Addiction Medicine for teaching and creating
1. Peterson C, Xu L, Florence C, Mack KA. Opioid-related US hospital discharges by type, 1993–2016. J Subst Abuse Treat. 2019;103:9-13. https://doi.org/10.1016/j.jsat.2019.05.003.
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff. 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
3. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100(3):875-882. https://doi.org/10.1016/j.athoracsur.2015.03.019.
4. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024.
5. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2017;13(1):62-64. https://doi.org/10.12788/jhm.2861.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Gowing L, Ali R, White JM, Mbewe D. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017;(2):CD002025. https://doi.org/10.1002/14651858.CD002025.pub5.
8. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59. https://doi.org/10.2105/AJPH.2015.302885.
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/NEJMra1604339.
10. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients. JAMA Intern Med. 2014;174(8):1369. https://doi.org/10.1001/jamainternmed.2014.2556.
11. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. Factors contributing to the rise of buprenorphine misuse: 2008-2013. Drug Alcohol Depend. 2014;142:98-104. https://doi.org/10.1016/j.drugalcdep.2014.06.005.
12. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/ADM.0000000000000499.
13. Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a dea waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Heal. 2019;35(1):108-112. https://doi.org/10.1111/jrh.12307.
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the x waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685.
15. American Medical Association Opioid Task Force. AMA Opioid Task Force recommendations offer roadmap to policymakers | American Medical Association. https://www.ama-assn.org/press-center/press-releases/ama-opioid-task-force-recommendations-offer-roadmap-policymakers. Accessed June 14, 2019.
16. Tonko P. H.R.2482: Mainstreaming Addiction Treatment Act of 2019. House Of Representatives (116th Congress); 2019. https://www.congress.gov/bill/116th-congress/house-bill/2482. Accessed July 10, 2019.
17. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382
18. Society of Hospital Medicine. Clinical Topics: Opioid Safety. https://www.hospitalmedicine.org/clinical-topics/opioid-safety/. Accessed October 24, 2019.
19. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. https://doi.org/10.1016/j.ajem.2019.02.025.
20. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. https://doi.org/10.1007/s11606-010-1311-3.
1. Peterson C, Xu L, Florence C, Mack KA. Opioid-related US hospital discharges by type, 1993–2016. J Subst Abuse Treat. 2019;103:9-13. https://doi.org/10.1016/j.jsat.2019.05.003.
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff. 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
3. Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100(3):875-882. https://doi.org/10.1016/j.athoracsur.2015.03.019.
4. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. https://doi.org/10.1016/j.amjmed.2015.09.024.
5. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2017;13(1):62-64. https://doi.org/10.12788/jhm.2861.
6. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. https://doi.org/10.7326/M17-3107.
7. Gowing L, Ali R, White JM, Mbewe D. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017;(2):CD002025. https://doi.org/10.1002/14651858.CD002025.pub5.
8. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59. https://doi.org/10.2105/AJPH.2015.302885.
9. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368. https://doi.org/10.1056/NEJMra1604339.
10. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients. JAMA Intern Med. 2014;174(8):1369. https://doi.org/10.1001/jamainternmed.2014.2556.
11. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. Factors contributing to the rise of buprenorphine misuse: 2008-2013. Drug Alcohol Depend. 2014;142:98-104. https://doi.org/10.1016/j.drugalcdep.2014.06.005.
12. Moreno JL, Wakeman SE, Duprey MS, Roberts RJ, Jacobson JS, Devlin JW. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med. 2019;13(4):306-313. https://doi.org/10.1097/ADM.0000000000000499.
13. Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a dea waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Heal. 2019;35(1):108-112. https://doi.org/10.1111/jrh.12307.
14. Fiscella K, Wakeman SE, Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: x the x waiver. JAMA Psychiatry. 2019;76(3):229-230. https://doi.org/10.1001/jamapsychiatry.2018.3685.
15. American Medical Association Opioid Task Force. AMA Opioid Task Force recommendations offer roadmap to policymakers | American Medical Association. https://www.ama-assn.org/press-center/press-releases/ama-opioid-task-force-recommendations-offer-roadmap-policymakers. Accessed June 14, 2019.
16. Tonko P. H.R.2482: Mainstreaming Addiction Treatment Act of 2019. House Of Representatives (116th Congress); 2019. https://www.congress.gov/bill/116th-congress/house-bill/2482. Accessed July 10, 2019.
17. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382
18. Society of Hospital Medicine. Clinical Topics: Opioid Safety. https://www.hospitalmedicine.org/clinical-topics/opioid-safety/. Accessed October 24, 2019.
19. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. https://doi.org/10.1016/j.ajem.2019.02.025.
20. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. https://doi.org/10.1007/s11606-010-1311-3.
© 2019 Society of Hospital Medicine
Medical Comanagement of Hip Fracture Patients Is Not Associated with Superior Perioperative Outcomes: A Propensity Score-Matched Retrospective Cohort Analysis of the National Surgical Quality Improvement Project
Hip fractures are a large source of morbidity and mortality in the United States, with >1.5 million patients affected every year.1 These patients are primarily older adults with a significant burden of associated medical comorbidities.2 The outcomes of nonoperative management are poor with regard to mortality,3 although operative management of hip fractures remains associated with a high rate of morbidity and mortality compared with several other surgical procedures, substantial resources remain devoted to the operative repair of hip fractures and to process improvement strategies for perioperative care.
Medical comanagement involves having a second nonsurgical primary team—often an internist, a hospitalist, a geriatrician, or an anesthesiologist—who would follow the patient during the hip fracture admission, and provide daily care directed toward both the hip fracture and its associated management challenges and the patient’s underlying comorbidities. This includes taking a primary or shared role in daily rounding, writing progress notes, writing orders, managing medications and therapies, disposition planning, and discharge. One argument for this practice has centered around an efficiency proposition for surgeons to spend more of their time operating and less time in these tasks of acute care management. The primary argument, though, for medical comanagement has been an outcomes proposition that frail, elderly patients with significant medical comorbidities benefit from a nonsurgeon’s focused attention to their coexisting medical problems and the interaction with the surgical issues posed by operative intervention for hip fracture. A number of previous studies have demonstrated an association between comanagement and improved perioperative outcomes.4,5 However, the most convincing improvements in several studies have been process indicators (eg, time from admission to surgery, length of stay, nurse/surgeon satisfaction) without significant differences in mortality or major morbidity.6-8 Several studies were methodologically limited due to the use of historical controls,9,10 and several were conducted in focused clinical settings (eg, a single tertiary academic center), leaving uncertainty about external validity for other care environments.6,7 To our knowledge, comanagement has not been examined in the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) dataset of hip fracture patients.
The NSQIP database offers a unique tool for clinical outcomes research because its variables are prospectively collected by a trained clinical reviewer at each participating site. Data are deidentified and aggregated into a national database, which has grown from 121 participating sites in 2005 to 708 participating sites in 2017 and now contains data on more than 6.6 million patients. The targeted hip fracture participant use file (PUF) adds additional variables and is available beginning with 2016. Internal audits ensure a high level of data reliability.11 The NSQIP has compared favorably with single-institution morbidity and mortality conference systems,12 multi-institution clinical databases,13 and administrative databases14 in accurately capturing 30-day outcomes. Unlike other databases, outcomes are recorded within 30 days even if they occur after the initial postoperative discharge. Comanagement is a dedicated variable in the NSQIP hip fracture dataset.
This study sought to examine the effect of medical comanagement on perioperative outcomes in this contemporary NSQIP database.
METHODS
This study was exempt from the Institutional Review Board review because it uses deidentified data.
We used the targeted hip fracture NSQIP PUF for 2016-2017 to examine perioperative outcomes among patients undergoing hip fracture repair and assess the relationship with medical comanagement, which is a dedicated variable in the NSQIP hip fracture database. We included patients in the comanagement cohort if they received comanagement for part or all of their hip fracture hospitalization.
Demographic, comorbidity, and preoperative variables were examined between the two cohorts. Hypoalbuminemia, as a marker of malnutrition and frailty, was defined as a preoperative serum albumin level <3.5 g/dL, which has demonstrated independent predictive value for adverse outcomes in hip fracture patients in the NSQIP.15,16 Predicted morbidity and mortality rates are calculated as probabilities available for each patient in the PUF based on a NSQIP hierarchical regression analysis of patient-level factors to predict outcomes (eg, not including hospital or provider factors). We also examined the relationship in regard to participation in a standardized hip fracture program (SHFP), which is a multidisciplinary protocolized pathway for hip fracture patients that may include order sets, structured care coordination, involvement of multidisciplinary therapy personnel, and daily milestones and discharge criteria. Participation in an SHFP is recorded in the NSQIP and has demonstrated an association with significantly improved outcomes in this same dataset, the targeted hip fracture PUF.17
Logistic regression was performed using all baseline variables identified to be significantly different between the cohorts, as well as the following variables with a priori potential importance in predicting membership in the comanagement cohort: admission year, sex, American Society of Anesthesiologists (ASA) physical status ≥4, and participation in an SHFP. Propensity scores were calculated using the significant variables from this model (Table 1) and the abovementioned a priori potential confounders, and then propensity score matching was performed using a greedy matching algorithm (matching ratio 1:1, caliper width = 0.1 pooled standard deviations of the logit of the propensity score) to create comanagement and control cohorts for matched analysis.
The primary outcomes were 30-day mortality and a composite endpoint of major morbidity, including readmission, pulmonary complications (pneumonia, reintubation, prolonged mechanical ventilation, and pulmonary embolism [PE]), septic shock, stroke, myocardial infarction, cardiac arrest, or death. Secondary outcomes included postoperative length of stay, disposition at postoperative day 30, and process compliance measures (proportion of patients allowed to be weight-bearing as tolerated on postoperative day 1, and proportion of patients appropriately prescribed deep venous thrombosis [DVT] prophylaxis for 28 days, proportion of patients appropriately prescribed bone protective medication [eg, vitamin D, bisphosphonates, teriparatide, denosumab, and raloxifene] postoperatively).
Descriptive variables are reported as median (interquartile range) and number (percentage), unless otherwise noted. Continuous outcomes were compared using a Mann–Whitney–Wilcoxon test. Binary outcomes were compared using Fisher’s exact tests (or a Pearson’s Chi-square for more than two response levels) and odds ratios with 95% confidence intervals.
RESULTS
A total of 19,896 Hip fracture patients were categorized into a medical comanagement cohort of 17,600 (88.5%) patients and a cohort without comanagement of 2,296 patients (11.5%). Baseline characteristics of the two unadjusted cohorts before propensity score matching are presented in Table 2.
Patients in the comanagement cohort were older and sicker in terms of almost every comorbidity and condition evaluated (Table 2). These differences were also reflected in a higher predicted mortality by the NSQIP hierarchical regression-based equations for mortality (3.5% [1.7%-7.0%] vs 2.5% [0.9%-6.1%], P < .0001) and morbidity (9.1% [6.9%-12.5%] vs 8.5% [6.1%-12.1%], P ≤ .0001). As predicted, the observed, unadjusted rate of death in the comanagement cohort was higher than that in the cohort without comanagement: n = 1,210 (6.9%) vs n = 91 (4.0%), odds ratio (OR) 1.79: 1.44-2.22; P < .0001, as was the unadjusted rate of the composite endpoint of major morbidity: n = 3,425 (19.5%) vs n = 220 (9.6%), OR 2.28: 1.98-2.63, P < .0001. There was no difference in the prevalence of using an SHFP in the comanagement and noncomanagement cohorts (n = 9,441, 53.6% vs n = 1,232, 53.7%, P > .05).
Logistic regression modeling of the probability of membership in the comanagement cohort yielded satisfactory results (convergent model, null hypothesis rejected, area under the curve of the model receiver operating curve [AUROC] = 0.81). Propensity scores were calculated using the significant variables from this model, as detailed in Table 1. Propensity score matching was then performed with excellent results as follows: n = 2,278 of 2,296 (99.2%) potential pairs were successfully matched, residual absolute standardized difference = 0.0039 (99.7% reduction), variance ratio = 1.01. This satisfies the traditional criterion for a satisfactory variable balance in propensity score matching of a standardized difference ≤0.25 and a variance ratio between 0.5 and 2.0. It is also worthy of note that the propensity score matching process successfully eliminated the baseline difference in the NSQIP-predicted probability of mortality (2.7% [1.1%-5.8%] vs 2.5% [0.9%-6.2%], P = .15) and morbidity (8.6% [6.5%-11.7%] vs 8.6% [6.1%-12.2%], P = .80).
The characteristics of the propensity score-matched cohorts (n = 2,278 each) are shown in Table 3. Matching resulted in a satisfactory balance of measurable covariates between the two cohorts, with the exception of small (but statistically significant) differences in the prevalence of hypoalbuminemia and the distribution of fracture type.
The comanagement cohort did not experience superior results for either of the two primary outcomes mortality (OR 1.36: 1.02-1.81; P = .033) or in the composite endpoint of morbidity (OR 1.82: 1.52-2.20; P < .0001). The secondary outcomes of the two cohorts of patients are shown in Table 4. The comanagement cohort did not have superior outcomes in any variable examined, except for a slightly higher proportion of patients who were appropriately prescribed DVT prophylaxis. Despite the prophylaxis, the comanagement cohort did not have a smaller proportion of patients who experienced a DVT or PE.
Post hoc subgroup analysis was performed to assess whether comanagement demonstrated an association with improved outcomes depending on whether patients were or were not treated in an SHFP. This stratified analysis produced the same results as the primary analysis; ie, comanagement was not associated with improved outcomes in either subgroup.
DISCUSSION
The primary finding of this study is that even once propensity score matching eliminated nearly all discernible baseline differences between the cohorts of hip fracture patients with and without medical comanagement during their hospitalization, and comanagement was not associated with superior (and in fact was associated with still inferior) perioperative outcomes.
As is evident from the baseline differences shown in Table 2, medical comanagement is utilized in a patient population that has significant comorbidities and adverse patient factors. The NSQIP provides a robust opportunity to remove the effects of these confounding variables because of the richness of variables in the dataset. For instance, some studies used a summary score for patient frailty, which has been an apparent predictor of worse clinical outcomes in this population.18,19 The NSQIP analyzes each component of the frailty score (diabetic status, history of COPD or current pneumonia, congestive heart failure, hypertension requiring medication, and nonindependent functional status) as well as to add additional variables (eg, low serum albumin level) and propensity score matching on each of these variables individually.
It is also important to note that although prior analyses have demonstrated that SHFPs are associated with better outcomes in this database,17 comanagement did not correlate with the use of an SHFP, nor did comanagement demonstrate any association with better outcomes in the subgroup who participated in an SHFP or in the subgroup who did not.
This retrospective cohort analysis cannot, of course, demonstrate causation. Several limitations are worth noting. The ability to use any retrospective dataset depends on the quality of the variable definitions and the data quality contained in it. Although the NSQIP has demonstrated high validity and interobserver variability compared with other data sources, some imperfections and heterogeneity (for instance, in the way two different institutions may define comanagement) may be present.
It is important to note that any propensity score-matched analysis incurs the risk of residual/unmeasured confounding, since the power of this technique still depends on the presence of measured variables to match, and no match is ever perfect. For instance, some variables remain imperfectly balanced in the matched cohorts (eg, hypoalbuminemia and fracture type, Table 3). These differences may reach statistical significance because of large sample size without obvious clinical significance, but they illustrate the point that residual confounding may persist. It is also possible that some detection bias is present in the comanagement cohort, if dedicated comanagement personnel are more likely to diagnose complications (eg, pneumonia, PE) that require some clinical suspicion to be identified. We doubt that this plays a dominant role, for the NSQIP is relatively robust to this potential bias because of its rigorous process of relying on a trained clinical reviewer at each site (as opposed, for instance, to using billing codes), and several components of the composite morbidity endpoint (eg, reintubation, prolonged mechanical ventilation, stroke, cardiac arrest, or death) would be difficult to miss even if clinicians have low clinical suspicion or attentiveness. However, some potential remains.
It is also possible that comanagement is applied to sicker patients and functions more as a marker of that population than an intervention that improves results. To take a similar example, past literature has demonstrated a strong association between do-not-resuscitate (DNR) status and adverse outcomes.20-24 In all likelihood, the DNR status does not directly cause worse outcomes so much as it marks a sick and vulnerable population. Selection bias at the individual patient level may contribute to an association between comanagement and worse outcomes.
Similarly, institutions that routinely apply comanagement may care for a sicker patient population. To this end, institution-level variables may modulate the relationship between comanagement, SHFP participation, and outcomes. Comanagement and SHFP participation may cluster according to the surgeon, the institution, or the patient subtype (eg, ICU vs ward status). Unfortunately, individual hospital and surgeon identifiers are explicitly excluded from the publicly available NSQIP PUF to protect program and patient confidentiality, so that advanced hierarchical modeling techniques cannot explore these relationships with this dataset.
Beyond these limitations, one plausible explanation for the lack of an association between comanagement and improved outcomes is that standardization and other continuous quality improvement processes have already accomplished a great deal, and the addition of comanagement of individual patients is not having an appreciably positive additional impact. Although the acuity and prevalence of comorbidities in the hip fracture population are high, many of their issues may be stereotyped enough that thoughtful, well-designed algorithms and protocols may serve them nearly as well, if not better than individual comanagement.
This admittedly speculative explanation has significant implications for resource utilization and patient care. Medical comanagement involves a heavy investment of time, energy, and money on the part of a second medical team to deliberately duplicate some aspects of daily care with the intended goal of improving patient outcomes. The results of this study may provide motivation for efforts to hybridize or modify the involvement of comanaging physicians and teams—for instance, to guide and refine the creation and revision of SHFP protocols without providing daily comanagement to each individual patient and/or to implement more iterative, continuous process improvement initiatives.25 Our results may also help direct healthcare systems to focus elsewhere in the search for modifiable process and care delivery variables that can move the needle on the significant morbidity and mortality that still exist in this population.
1. Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St. Peter WL. Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis. 2013;62(4):747-754. https://doi.org/10.1053/j.ajkd.2013.02.368.
2. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. https://doi.org/10.1001/jama.2009.1462.
3. Chlebeck JD, Birch CE, Blankstein M, Kristiansen T, Bartlett CS, Schottel PC. Nonoperative geriatric hip fracture treatment is associated with increased mortality. J Orthop Trauma. 2019;33(7):346-350. https://doi.org/10.1097/BOT.0000000000001460.
4. Wu X, Tian M, Zhang J, et al. The effect of a multidisciplinary co-management program for the older hip fracture patients in Beijing: a “pre- and post-” retrospective study. Arch Osteoporos. 2019;14(1):43. https://doi.org/10.1007/s11657-019-0594-1.
5. Stephens JR, Chang JW, Liles EA, Adem M, Moore C. Impact of hospitalist vs. non-hospitalist services on length of stay and 30-day readmission rate in hip fracture patients. Hosp Pract. 2019;47(1):24-27. https://doi.org/10.1080/21548331.2019.1537850.
6. Phy MP, Vanness DJ, Melton LJ, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. https://doi.org/10.1001/archinte.165.7.796.
7. Batsis JA, Phy MP, Melton LJ, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. https://doi.org/10.1002/jhm.207
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016;136(10):1403-1409. https://doi.org/10.1007/s00402-016-2543-4.
10. Folbert EC, Hegeman JH, Vermeer M, et al. Improved 1-year mortality in elderly patients with a hip fracture following integrated orthogeriatric treatment. Osteoporos Int. 2017;28(1):269-277. https://doi.org/10.1007/s00198-016-3711-7.
11. Shiloach M, Frencher SK, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. https://doi.org/10.1016/j.jamcollsurg.2009.09.031.
12. Hutter MM, Rowell KS, Devaney LA, et al. Identification of surgical complications and deaths: an assessment of the traditional surgical morbidity and mortality conference compared with the American College of Surgeons-National Surgical Quality Improvement Program. J Am Coll Surg. 2006;203(5):618-624. https://doi.org/10.1016/j.jamcollsurg.2006.07.010.
13. Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University HealthSystem Consortium Clinical Database and the National Surgical Quality Improvement Program data set. Am J Med Qual. 2009;24(5):395-402. https://doi.org/10.1177/1062860609339936.
14. Yu P, Chang DC, Osen HB, Talamini MA. NSQIP reveals significant incidence of death following discharge. J Surg Res. 2011;170(2):e217-e224. https://doi.org/10.1016/j.jss.2011.05.040.
15. Wilson J, Lunati M, Grabel Z, Staley C, Schwartz A, Schenker M. Hypoalbuminemia is an independent risk factor for 30-day mortality, postoperative complications, readmission, and reoperation in the operative lower extremity orthopedic trauma patient. J Orthop Trauma. 2019;33(6):284-291. https://doi.org/10.1097/BOT.0000000000001448.
16. Bohl DD, Shen MR, Hannon CP, Fillingham YA, Darrith B, Della Valle CJ. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J Bone Jt Surg. 2017;99(24):2110-2118. https://doi.org/10.2106/JBJS.16.01620.
17. Arshi A, Rezzadeh K, Stavrakis AI, Bukata S V, Zeegen EN. Standardized hospital-based care programs improve geriatric hip fracture outcomes: an analysis of the ACS-NSQIP targeted hip fracture series. J Orthop Trauma. 2019;33(6): e223-e228. https://doi.org/10.1097/BOT.0000000000001443.
18. Traven SA, Reeves RA, Althoff AD, Slone HS, Walton ZJ. New 5-factor modified frailty index predicts morbidity and mortality in geriatric hip fractures. J Orthop Trauma. 2019;33(7):319-323. https://doi.org/10.1097/BOT.0000000000001455.
19. Wilson JM, Boissonneault AR, Schwartz AM, Staley CA, Schenker ML. Frailty and malnutrition are associated with inpatient post-operative complications and mortality in hip fracture patients. J Orthop Trauma. 2018;33(3):143-148. https://doi.org/10.1097/BOT.0000000000001386.
20. Brovman EY, Pisansky AJ, Beverly A, Bader AM, Urman RD. Do Not Resuscitate Status as an independent risk factor for patients undergoing surgery for hip fracture. World J Orthop. 2017;8(12):902-912. https://doi.org/10.5312/wjo.v8.i12.902.
21. Brovman EY, Walsh EC, Burton BN, et al. Postoperative outcomes in patients with a do-not-resuscitate (DNR) order undergoing elective procedures. J Clin Anesth. 2018;48:81-88. https://doi.org/10.1016/j.jclinane.2018.05.007.
22. Beverly A, Brovman EY, Urman RD. Comparison of postoperative outcomes in elderly patients with a do-not-resuscitate order undergoing elective and nonelective hip surgery. Geriatr Orthop Surg Rehabil. 2017;8(2):78-86. https://doi.org/10.1177/2151458516685826.
23. Maxwell BG, Lobato RL, Cason MB, Wong JK. Perioperative morbidity and mortality of cardiothoracic surgery in patients with a do-not-resuscitate order. PeerJ. 2014;2013(1):1-10. https://doi.org/10.7717/peerj.245.
24. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. https://doi.org/10.1001/archsurg.2011.69.
25. Brañas F, Ruiz-Pinto A, Fernández E, et al. Beyond orthogeriatric co-management model: benefits of implementing a process management system for hip fracture. Arch Osteoporos. 2018;13(1):81. https://doi.org/10.1007/s11657-018-0497-6.
Hip fractures are a large source of morbidity and mortality in the United States, with >1.5 million patients affected every year.1 These patients are primarily older adults with a significant burden of associated medical comorbidities.2 The outcomes of nonoperative management are poor with regard to mortality,3 although operative management of hip fractures remains associated with a high rate of morbidity and mortality compared with several other surgical procedures, substantial resources remain devoted to the operative repair of hip fractures and to process improvement strategies for perioperative care.
Medical comanagement involves having a second nonsurgical primary team—often an internist, a hospitalist, a geriatrician, or an anesthesiologist—who would follow the patient during the hip fracture admission, and provide daily care directed toward both the hip fracture and its associated management challenges and the patient’s underlying comorbidities. This includes taking a primary or shared role in daily rounding, writing progress notes, writing orders, managing medications and therapies, disposition planning, and discharge. One argument for this practice has centered around an efficiency proposition for surgeons to spend more of their time operating and less time in these tasks of acute care management. The primary argument, though, for medical comanagement has been an outcomes proposition that frail, elderly patients with significant medical comorbidities benefit from a nonsurgeon’s focused attention to their coexisting medical problems and the interaction with the surgical issues posed by operative intervention for hip fracture. A number of previous studies have demonstrated an association between comanagement and improved perioperative outcomes.4,5 However, the most convincing improvements in several studies have been process indicators (eg, time from admission to surgery, length of stay, nurse/surgeon satisfaction) without significant differences in mortality or major morbidity.6-8 Several studies were methodologically limited due to the use of historical controls,9,10 and several were conducted in focused clinical settings (eg, a single tertiary academic center), leaving uncertainty about external validity for other care environments.6,7 To our knowledge, comanagement has not been examined in the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) dataset of hip fracture patients.
The NSQIP database offers a unique tool for clinical outcomes research because its variables are prospectively collected by a trained clinical reviewer at each participating site. Data are deidentified and aggregated into a national database, which has grown from 121 participating sites in 2005 to 708 participating sites in 2017 and now contains data on more than 6.6 million patients. The targeted hip fracture participant use file (PUF) adds additional variables and is available beginning with 2016. Internal audits ensure a high level of data reliability.11 The NSQIP has compared favorably with single-institution morbidity and mortality conference systems,12 multi-institution clinical databases,13 and administrative databases14 in accurately capturing 30-day outcomes. Unlike other databases, outcomes are recorded within 30 days even if they occur after the initial postoperative discharge. Comanagement is a dedicated variable in the NSQIP hip fracture dataset.
This study sought to examine the effect of medical comanagement on perioperative outcomes in this contemporary NSQIP database.
METHODS
This study was exempt from the Institutional Review Board review because it uses deidentified data.
We used the targeted hip fracture NSQIP PUF for 2016-2017 to examine perioperative outcomes among patients undergoing hip fracture repair and assess the relationship with medical comanagement, which is a dedicated variable in the NSQIP hip fracture database. We included patients in the comanagement cohort if they received comanagement for part or all of their hip fracture hospitalization.
Demographic, comorbidity, and preoperative variables were examined between the two cohorts. Hypoalbuminemia, as a marker of malnutrition and frailty, was defined as a preoperative serum albumin level <3.5 g/dL, which has demonstrated independent predictive value for adverse outcomes in hip fracture patients in the NSQIP.15,16 Predicted morbidity and mortality rates are calculated as probabilities available for each patient in the PUF based on a NSQIP hierarchical regression analysis of patient-level factors to predict outcomes (eg, not including hospital or provider factors). We also examined the relationship in regard to participation in a standardized hip fracture program (SHFP), which is a multidisciplinary protocolized pathway for hip fracture patients that may include order sets, structured care coordination, involvement of multidisciplinary therapy personnel, and daily milestones and discharge criteria. Participation in an SHFP is recorded in the NSQIP and has demonstrated an association with significantly improved outcomes in this same dataset, the targeted hip fracture PUF.17
Logistic regression was performed using all baseline variables identified to be significantly different between the cohorts, as well as the following variables with a priori potential importance in predicting membership in the comanagement cohort: admission year, sex, American Society of Anesthesiologists (ASA) physical status ≥4, and participation in an SHFP. Propensity scores were calculated using the significant variables from this model (Table 1) and the abovementioned a priori potential confounders, and then propensity score matching was performed using a greedy matching algorithm (matching ratio 1:1, caliper width = 0.1 pooled standard deviations of the logit of the propensity score) to create comanagement and control cohorts for matched analysis.
The primary outcomes were 30-day mortality and a composite endpoint of major morbidity, including readmission, pulmonary complications (pneumonia, reintubation, prolonged mechanical ventilation, and pulmonary embolism [PE]), septic shock, stroke, myocardial infarction, cardiac arrest, or death. Secondary outcomes included postoperative length of stay, disposition at postoperative day 30, and process compliance measures (proportion of patients allowed to be weight-bearing as tolerated on postoperative day 1, and proportion of patients appropriately prescribed deep venous thrombosis [DVT] prophylaxis for 28 days, proportion of patients appropriately prescribed bone protective medication [eg, vitamin D, bisphosphonates, teriparatide, denosumab, and raloxifene] postoperatively).
Descriptive variables are reported as median (interquartile range) and number (percentage), unless otherwise noted. Continuous outcomes were compared using a Mann–Whitney–Wilcoxon test. Binary outcomes were compared using Fisher’s exact tests (or a Pearson’s Chi-square for more than two response levels) and odds ratios with 95% confidence intervals.
RESULTS
A total of 19,896 Hip fracture patients were categorized into a medical comanagement cohort of 17,600 (88.5%) patients and a cohort without comanagement of 2,296 patients (11.5%). Baseline characteristics of the two unadjusted cohorts before propensity score matching are presented in Table 2.
Patients in the comanagement cohort were older and sicker in terms of almost every comorbidity and condition evaluated (Table 2). These differences were also reflected in a higher predicted mortality by the NSQIP hierarchical regression-based equations for mortality (3.5% [1.7%-7.0%] vs 2.5% [0.9%-6.1%], P < .0001) and morbidity (9.1% [6.9%-12.5%] vs 8.5% [6.1%-12.1%], P ≤ .0001). As predicted, the observed, unadjusted rate of death in the comanagement cohort was higher than that in the cohort without comanagement: n = 1,210 (6.9%) vs n = 91 (4.0%), odds ratio (OR) 1.79: 1.44-2.22; P < .0001, as was the unadjusted rate of the composite endpoint of major morbidity: n = 3,425 (19.5%) vs n = 220 (9.6%), OR 2.28: 1.98-2.63, P < .0001. There was no difference in the prevalence of using an SHFP in the comanagement and noncomanagement cohorts (n = 9,441, 53.6% vs n = 1,232, 53.7%, P > .05).
Logistic regression modeling of the probability of membership in the comanagement cohort yielded satisfactory results (convergent model, null hypothesis rejected, area under the curve of the model receiver operating curve [AUROC] = 0.81). Propensity scores were calculated using the significant variables from this model, as detailed in Table 1. Propensity score matching was then performed with excellent results as follows: n = 2,278 of 2,296 (99.2%) potential pairs were successfully matched, residual absolute standardized difference = 0.0039 (99.7% reduction), variance ratio = 1.01. This satisfies the traditional criterion for a satisfactory variable balance in propensity score matching of a standardized difference ≤0.25 and a variance ratio between 0.5 and 2.0. It is also worthy of note that the propensity score matching process successfully eliminated the baseline difference in the NSQIP-predicted probability of mortality (2.7% [1.1%-5.8%] vs 2.5% [0.9%-6.2%], P = .15) and morbidity (8.6% [6.5%-11.7%] vs 8.6% [6.1%-12.2%], P = .80).
The characteristics of the propensity score-matched cohorts (n = 2,278 each) are shown in Table 3. Matching resulted in a satisfactory balance of measurable covariates between the two cohorts, with the exception of small (but statistically significant) differences in the prevalence of hypoalbuminemia and the distribution of fracture type.
The comanagement cohort did not experience superior results for either of the two primary outcomes mortality (OR 1.36: 1.02-1.81; P = .033) or in the composite endpoint of morbidity (OR 1.82: 1.52-2.20; P < .0001). The secondary outcomes of the two cohorts of patients are shown in Table 4. The comanagement cohort did not have superior outcomes in any variable examined, except for a slightly higher proportion of patients who were appropriately prescribed DVT prophylaxis. Despite the prophylaxis, the comanagement cohort did not have a smaller proportion of patients who experienced a DVT or PE.
Post hoc subgroup analysis was performed to assess whether comanagement demonstrated an association with improved outcomes depending on whether patients were or were not treated in an SHFP. This stratified analysis produced the same results as the primary analysis; ie, comanagement was not associated with improved outcomes in either subgroup.
DISCUSSION
The primary finding of this study is that even once propensity score matching eliminated nearly all discernible baseline differences between the cohorts of hip fracture patients with and without medical comanagement during their hospitalization, and comanagement was not associated with superior (and in fact was associated with still inferior) perioperative outcomes.
As is evident from the baseline differences shown in Table 2, medical comanagement is utilized in a patient population that has significant comorbidities and adverse patient factors. The NSQIP provides a robust opportunity to remove the effects of these confounding variables because of the richness of variables in the dataset. For instance, some studies used a summary score for patient frailty, which has been an apparent predictor of worse clinical outcomes in this population.18,19 The NSQIP analyzes each component of the frailty score (diabetic status, history of COPD or current pneumonia, congestive heart failure, hypertension requiring medication, and nonindependent functional status) as well as to add additional variables (eg, low serum albumin level) and propensity score matching on each of these variables individually.
It is also important to note that although prior analyses have demonstrated that SHFPs are associated with better outcomes in this database,17 comanagement did not correlate with the use of an SHFP, nor did comanagement demonstrate any association with better outcomes in the subgroup who participated in an SHFP or in the subgroup who did not.
This retrospective cohort analysis cannot, of course, demonstrate causation. Several limitations are worth noting. The ability to use any retrospective dataset depends on the quality of the variable definitions and the data quality contained in it. Although the NSQIP has demonstrated high validity and interobserver variability compared with other data sources, some imperfections and heterogeneity (for instance, in the way two different institutions may define comanagement) may be present.
It is important to note that any propensity score-matched analysis incurs the risk of residual/unmeasured confounding, since the power of this technique still depends on the presence of measured variables to match, and no match is ever perfect. For instance, some variables remain imperfectly balanced in the matched cohorts (eg, hypoalbuminemia and fracture type, Table 3). These differences may reach statistical significance because of large sample size without obvious clinical significance, but they illustrate the point that residual confounding may persist. It is also possible that some detection bias is present in the comanagement cohort, if dedicated comanagement personnel are more likely to diagnose complications (eg, pneumonia, PE) that require some clinical suspicion to be identified. We doubt that this plays a dominant role, for the NSQIP is relatively robust to this potential bias because of its rigorous process of relying on a trained clinical reviewer at each site (as opposed, for instance, to using billing codes), and several components of the composite morbidity endpoint (eg, reintubation, prolonged mechanical ventilation, stroke, cardiac arrest, or death) would be difficult to miss even if clinicians have low clinical suspicion or attentiveness. However, some potential remains.
It is also possible that comanagement is applied to sicker patients and functions more as a marker of that population than an intervention that improves results. To take a similar example, past literature has demonstrated a strong association between do-not-resuscitate (DNR) status and adverse outcomes.20-24 In all likelihood, the DNR status does not directly cause worse outcomes so much as it marks a sick and vulnerable population. Selection bias at the individual patient level may contribute to an association between comanagement and worse outcomes.
Similarly, institutions that routinely apply comanagement may care for a sicker patient population. To this end, institution-level variables may modulate the relationship between comanagement, SHFP participation, and outcomes. Comanagement and SHFP participation may cluster according to the surgeon, the institution, or the patient subtype (eg, ICU vs ward status). Unfortunately, individual hospital and surgeon identifiers are explicitly excluded from the publicly available NSQIP PUF to protect program and patient confidentiality, so that advanced hierarchical modeling techniques cannot explore these relationships with this dataset.
Beyond these limitations, one plausible explanation for the lack of an association between comanagement and improved outcomes is that standardization and other continuous quality improvement processes have already accomplished a great deal, and the addition of comanagement of individual patients is not having an appreciably positive additional impact. Although the acuity and prevalence of comorbidities in the hip fracture population are high, many of their issues may be stereotyped enough that thoughtful, well-designed algorithms and protocols may serve them nearly as well, if not better than individual comanagement.
This admittedly speculative explanation has significant implications for resource utilization and patient care. Medical comanagement involves a heavy investment of time, energy, and money on the part of a second medical team to deliberately duplicate some aspects of daily care with the intended goal of improving patient outcomes. The results of this study may provide motivation for efforts to hybridize or modify the involvement of comanaging physicians and teams—for instance, to guide and refine the creation and revision of SHFP protocols without providing daily comanagement to each individual patient and/or to implement more iterative, continuous process improvement initiatives.25 Our results may also help direct healthcare systems to focus elsewhere in the search for modifiable process and care delivery variables that can move the needle on the significant morbidity and mortality that still exist in this population.
Hip fractures are a large source of morbidity and mortality in the United States, with >1.5 million patients affected every year.1 These patients are primarily older adults with a significant burden of associated medical comorbidities.2 The outcomes of nonoperative management are poor with regard to mortality,3 although operative management of hip fractures remains associated with a high rate of morbidity and mortality compared with several other surgical procedures, substantial resources remain devoted to the operative repair of hip fractures and to process improvement strategies for perioperative care.
Medical comanagement involves having a second nonsurgical primary team—often an internist, a hospitalist, a geriatrician, or an anesthesiologist—who would follow the patient during the hip fracture admission, and provide daily care directed toward both the hip fracture and its associated management challenges and the patient’s underlying comorbidities. This includes taking a primary or shared role in daily rounding, writing progress notes, writing orders, managing medications and therapies, disposition planning, and discharge. One argument for this practice has centered around an efficiency proposition for surgeons to spend more of their time operating and less time in these tasks of acute care management. The primary argument, though, for medical comanagement has been an outcomes proposition that frail, elderly patients with significant medical comorbidities benefit from a nonsurgeon’s focused attention to their coexisting medical problems and the interaction with the surgical issues posed by operative intervention for hip fracture. A number of previous studies have demonstrated an association between comanagement and improved perioperative outcomes.4,5 However, the most convincing improvements in several studies have been process indicators (eg, time from admission to surgery, length of stay, nurse/surgeon satisfaction) without significant differences in mortality or major morbidity.6-8 Several studies were methodologically limited due to the use of historical controls,9,10 and several were conducted in focused clinical settings (eg, a single tertiary academic center), leaving uncertainty about external validity for other care environments.6,7 To our knowledge, comanagement has not been examined in the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) dataset of hip fracture patients.
The NSQIP database offers a unique tool for clinical outcomes research because its variables are prospectively collected by a trained clinical reviewer at each participating site. Data are deidentified and aggregated into a national database, which has grown from 121 participating sites in 2005 to 708 participating sites in 2017 and now contains data on more than 6.6 million patients. The targeted hip fracture participant use file (PUF) adds additional variables and is available beginning with 2016. Internal audits ensure a high level of data reliability.11 The NSQIP has compared favorably with single-institution morbidity and mortality conference systems,12 multi-institution clinical databases,13 and administrative databases14 in accurately capturing 30-day outcomes. Unlike other databases, outcomes are recorded within 30 days even if they occur after the initial postoperative discharge. Comanagement is a dedicated variable in the NSQIP hip fracture dataset.
This study sought to examine the effect of medical comanagement on perioperative outcomes in this contemporary NSQIP database.
METHODS
This study was exempt from the Institutional Review Board review because it uses deidentified data.
We used the targeted hip fracture NSQIP PUF for 2016-2017 to examine perioperative outcomes among patients undergoing hip fracture repair and assess the relationship with medical comanagement, which is a dedicated variable in the NSQIP hip fracture database. We included patients in the comanagement cohort if they received comanagement for part or all of their hip fracture hospitalization.
Demographic, comorbidity, and preoperative variables were examined between the two cohorts. Hypoalbuminemia, as a marker of malnutrition and frailty, was defined as a preoperative serum albumin level <3.5 g/dL, which has demonstrated independent predictive value for adverse outcomes in hip fracture patients in the NSQIP.15,16 Predicted morbidity and mortality rates are calculated as probabilities available for each patient in the PUF based on a NSQIP hierarchical regression analysis of patient-level factors to predict outcomes (eg, not including hospital or provider factors). We also examined the relationship in regard to participation in a standardized hip fracture program (SHFP), which is a multidisciplinary protocolized pathway for hip fracture patients that may include order sets, structured care coordination, involvement of multidisciplinary therapy personnel, and daily milestones and discharge criteria. Participation in an SHFP is recorded in the NSQIP and has demonstrated an association with significantly improved outcomes in this same dataset, the targeted hip fracture PUF.17
Logistic regression was performed using all baseline variables identified to be significantly different between the cohorts, as well as the following variables with a priori potential importance in predicting membership in the comanagement cohort: admission year, sex, American Society of Anesthesiologists (ASA) physical status ≥4, and participation in an SHFP. Propensity scores were calculated using the significant variables from this model (Table 1) and the abovementioned a priori potential confounders, and then propensity score matching was performed using a greedy matching algorithm (matching ratio 1:1, caliper width = 0.1 pooled standard deviations of the logit of the propensity score) to create comanagement and control cohorts for matched analysis.
The primary outcomes were 30-day mortality and a composite endpoint of major morbidity, including readmission, pulmonary complications (pneumonia, reintubation, prolonged mechanical ventilation, and pulmonary embolism [PE]), septic shock, stroke, myocardial infarction, cardiac arrest, or death. Secondary outcomes included postoperative length of stay, disposition at postoperative day 30, and process compliance measures (proportion of patients allowed to be weight-bearing as tolerated on postoperative day 1, and proportion of patients appropriately prescribed deep venous thrombosis [DVT] prophylaxis for 28 days, proportion of patients appropriately prescribed bone protective medication [eg, vitamin D, bisphosphonates, teriparatide, denosumab, and raloxifene] postoperatively).
Descriptive variables are reported as median (interquartile range) and number (percentage), unless otherwise noted. Continuous outcomes were compared using a Mann–Whitney–Wilcoxon test. Binary outcomes were compared using Fisher’s exact tests (or a Pearson’s Chi-square for more than two response levels) and odds ratios with 95% confidence intervals.
RESULTS
A total of 19,896 Hip fracture patients were categorized into a medical comanagement cohort of 17,600 (88.5%) patients and a cohort without comanagement of 2,296 patients (11.5%). Baseline characteristics of the two unadjusted cohorts before propensity score matching are presented in Table 2.
Patients in the comanagement cohort were older and sicker in terms of almost every comorbidity and condition evaluated (Table 2). These differences were also reflected in a higher predicted mortality by the NSQIP hierarchical regression-based equations for mortality (3.5% [1.7%-7.0%] vs 2.5% [0.9%-6.1%], P < .0001) and morbidity (9.1% [6.9%-12.5%] vs 8.5% [6.1%-12.1%], P ≤ .0001). As predicted, the observed, unadjusted rate of death in the comanagement cohort was higher than that in the cohort without comanagement: n = 1,210 (6.9%) vs n = 91 (4.0%), odds ratio (OR) 1.79: 1.44-2.22; P < .0001, as was the unadjusted rate of the composite endpoint of major morbidity: n = 3,425 (19.5%) vs n = 220 (9.6%), OR 2.28: 1.98-2.63, P < .0001. There was no difference in the prevalence of using an SHFP in the comanagement and noncomanagement cohorts (n = 9,441, 53.6% vs n = 1,232, 53.7%, P > .05).
Logistic regression modeling of the probability of membership in the comanagement cohort yielded satisfactory results (convergent model, null hypothesis rejected, area under the curve of the model receiver operating curve [AUROC] = 0.81). Propensity scores were calculated using the significant variables from this model, as detailed in Table 1. Propensity score matching was then performed with excellent results as follows: n = 2,278 of 2,296 (99.2%) potential pairs were successfully matched, residual absolute standardized difference = 0.0039 (99.7% reduction), variance ratio = 1.01. This satisfies the traditional criterion for a satisfactory variable balance in propensity score matching of a standardized difference ≤0.25 and a variance ratio between 0.5 and 2.0. It is also worthy of note that the propensity score matching process successfully eliminated the baseline difference in the NSQIP-predicted probability of mortality (2.7% [1.1%-5.8%] vs 2.5% [0.9%-6.2%], P = .15) and morbidity (8.6% [6.5%-11.7%] vs 8.6% [6.1%-12.2%], P = .80).
The characteristics of the propensity score-matched cohorts (n = 2,278 each) are shown in Table 3. Matching resulted in a satisfactory balance of measurable covariates between the two cohorts, with the exception of small (but statistically significant) differences in the prevalence of hypoalbuminemia and the distribution of fracture type.
The comanagement cohort did not experience superior results for either of the two primary outcomes mortality (OR 1.36: 1.02-1.81; P = .033) or in the composite endpoint of morbidity (OR 1.82: 1.52-2.20; P < .0001). The secondary outcomes of the two cohorts of patients are shown in Table 4. The comanagement cohort did not have superior outcomes in any variable examined, except for a slightly higher proportion of patients who were appropriately prescribed DVT prophylaxis. Despite the prophylaxis, the comanagement cohort did not have a smaller proportion of patients who experienced a DVT or PE.
Post hoc subgroup analysis was performed to assess whether comanagement demonstrated an association with improved outcomes depending on whether patients were or were not treated in an SHFP. This stratified analysis produced the same results as the primary analysis; ie, comanagement was not associated with improved outcomes in either subgroup.
DISCUSSION
The primary finding of this study is that even once propensity score matching eliminated nearly all discernible baseline differences between the cohorts of hip fracture patients with and without medical comanagement during their hospitalization, and comanagement was not associated with superior (and in fact was associated with still inferior) perioperative outcomes.
As is evident from the baseline differences shown in Table 2, medical comanagement is utilized in a patient population that has significant comorbidities and adverse patient factors. The NSQIP provides a robust opportunity to remove the effects of these confounding variables because of the richness of variables in the dataset. For instance, some studies used a summary score for patient frailty, which has been an apparent predictor of worse clinical outcomes in this population.18,19 The NSQIP analyzes each component of the frailty score (diabetic status, history of COPD or current pneumonia, congestive heart failure, hypertension requiring medication, and nonindependent functional status) as well as to add additional variables (eg, low serum albumin level) and propensity score matching on each of these variables individually.
It is also important to note that although prior analyses have demonstrated that SHFPs are associated with better outcomes in this database,17 comanagement did not correlate with the use of an SHFP, nor did comanagement demonstrate any association with better outcomes in the subgroup who participated in an SHFP or in the subgroup who did not.
This retrospective cohort analysis cannot, of course, demonstrate causation. Several limitations are worth noting. The ability to use any retrospective dataset depends on the quality of the variable definitions and the data quality contained in it. Although the NSQIP has demonstrated high validity and interobserver variability compared with other data sources, some imperfections and heterogeneity (for instance, in the way two different institutions may define comanagement) may be present.
It is important to note that any propensity score-matched analysis incurs the risk of residual/unmeasured confounding, since the power of this technique still depends on the presence of measured variables to match, and no match is ever perfect. For instance, some variables remain imperfectly balanced in the matched cohorts (eg, hypoalbuminemia and fracture type, Table 3). These differences may reach statistical significance because of large sample size without obvious clinical significance, but they illustrate the point that residual confounding may persist. It is also possible that some detection bias is present in the comanagement cohort, if dedicated comanagement personnel are more likely to diagnose complications (eg, pneumonia, PE) that require some clinical suspicion to be identified. We doubt that this plays a dominant role, for the NSQIP is relatively robust to this potential bias because of its rigorous process of relying on a trained clinical reviewer at each site (as opposed, for instance, to using billing codes), and several components of the composite morbidity endpoint (eg, reintubation, prolonged mechanical ventilation, stroke, cardiac arrest, or death) would be difficult to miss even if clinicians have low clinical suspicion or attentiveness. However, some potential remains.
It is also possible that comanagement is applied to sicker patients and functions more as a marker of that population than an intervention that improves results. To take a similar example, past literature has demonstrated a strong association between do-not-resuscitate (DNR) status and adverse outcomes.20-24 In all likelihood, the DNR status does not directly cause worse outcomes so much as it marks a sick and vulnerable population. Selection bias at the individual patient level may contribute to an association between comanagement and worse outcomes.
Similarly, institutions that routinely apply comanagement may care for a sicker patient population. To this end, institution-level variables may modulate the relationship between comanagement, SHFP participation, and outcomes. Comanagement and SHFP participation may cluster according to the surgeon, the institution, or the patient subtype (eg, ICU vs ward status). Unfortunately, individual hospital and surgeon identifiers are explicitly excluded from the publicly available NSQIP PUF to protect program and patient confidentiality, so that advanced hierarchical modeling techniques cannot explore these relationships with this dataset.
Beyond these limitations, one plausible explanation for the lack of an association between comanagement and improved outcomes is that standardization and other continuous quality improvement processes have already accomplished a great deal, and the addition of comanagement of individual patients is not having an appreciably positive additional impact. Although the acuity and prevalence of comorbidities in the hip fracture population are high, many of their issues may be stereotyped enough that thoughtful, well-designed algorithms and protocols may serve them nearly as well, if not better than individual comanagement.
This admittedly speculative explanation has significant implications for resource utilization and patient care. Medical comanagement involves a heavy investment of time, energy, and money on the part of a second medical team to deliberately duplicate some aspects of daily care with the intended goal of improving patient outcomes. The results of this study may provide motivation for efforts to hybridize or modify the involvement of comanaging physicians and teams—for instance, to guide and refine the creation and revision of SHFP protocols without providing daily comanagement to each individual patient and/or to implement more iterative, continuous process improvement initiatives.25 Our results may also help direct healthcare systems to focus elsewhere in the search for modifiable process and care delivery variables that can move the needle on the significant morbidity and mortality that still exist in this population.
1. Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St. Peter WL. Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis. 2013;62(4):747-754. https://doi.org/10.1053/j.ajkd.2013.02.368.
2. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. https://doi.org/10.1001/jama.2009.1462.
3. Chlebeck JD, Birch CE, Blankstein M, Kristiansen T, Bartlett CS, Schottel PC. Nonoperative geriatric hip fracture treatment is associated with increased mortality. J Orthop Trauma. 2019;33(7):346-350. https://doi.org/10.1097/BOT.0000000000001460.
4. Wu X, Tian M, Zhang J, et al. The effect of a multidisciplinary co-management program for the older hip fracture patients in Beijing: a “pre- and post-” retrospective study. Arch Osteoporos. 2019;14(1):43. https://doi.org/10.1007/s11657-019-0594-1.
5. Stephens JR, Chang JW, Liles EA, Adem M, Moore C. Impact of hospitalist vs. non-hospitalist services on length of stay and 30-day readmission rate in hip fracture patients. Hosp Pract. 2019;47(1):24-27. https://doi.org/10.1080/21548331.2019.1537850.
6. Phy MP, Vanness DJ, Melton LJ, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. https://doi.org/10.1001/archinte.165.7.796.
7. Batsis JA, Phy MP, Melton LJ, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. https://doi.org/10.1002/jhm.207
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016;136(10):1403-1409. https://doi.org/10.1007/s00402-016-2543-4.
10. Folbert EC, Hegeman JH, Vermeer M, et al. Improved 1-year mortality in elderly patients with a hip fracture following integrated orthogeriatric treatment. Osteoporos Int. 2017;28(1):269-277. https://doi.org/10.1007/s00198-016-3711-7.
11. Shiloach M, Frencher SK, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. https://doi.org/10.1016/j.jamcollsurg.2009.09.031.
12. Hutter MM, Rowell KS, Devaney LA, et al. Identification of surgical complications and deaths: an assessment of the traditional surgical morbidity and mortality conference compared with the American College of Surgeons-National Surgical Quality Improvement Program. J Am Coll Surg. 2006;203(5):618-624. https://doi.org/10.1016/j.jamcollsurg.2006.07.010.
13. Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University HealthSystem Consortium Clinical Database and the National Surgical Quality Improvement Program data set. Am J Med Qual. 2009;24(5):395-402. https://doi.org/10.1177/1062860609339936.
14. Yu P, Chang DC, Osen HB, Talamini MA. NSQIP reveals significant incidence of death following discharge. J Surg Res. 2011;170(2):e217-e224. https://doi.org/10.1016/j.jss.2011.05.040.
15. Wilson J, Lunati M, Grabel Z, Staley C, Schwartz A, Schenker M. Hypoalbuminemia is an independent risk factor for 30-day mortality, postoperative complications, readmission, and reoperation in the operative lower extremity orthopedic trauma patient. J Orthop Trauma. 2019;33(6):284-291. https://doi.org/10.1097/BOT.0000000000001448.
16. Bohl DD, Shen MR, Hannon CP, Fillingham YA, Darrith B, Della Valle CJ. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J Bone Jt Surg. 2017;99(24):2110-2118. https://doi.org/10.2106/JBJS.16.01620.
17. Arshi A, Rezzadeh K, Stavrakis AI, Bukata S V, Zeegen EN. Standardized hospital-based care programs improve geriatric hip fracture outcomes: an analysis of the ACS-NSQIP targeted hip fracture series. J Orthop Trauma. 2019;33(6): e223-e228. https://doi.org/10.1097/BOT.0000000000001443.
18. Traven SA, Reeves RA, Althoff AD, Slone HS, Walton ZJ. New 5-factor modified frailty index predicts morbidity and mortality in geriatric hip fractures. J Orthop Trauma. 2019;33(7):319-323. https://doi.org/10.1097/BOT.0000000000001455.
19. Wilson JM, Boissonneault AR, Schwartz AM, Staley CA, Schenker ML. Frailty and malnutrition are associated with inpatient post-operative complications and mortality in hip fracture patients. J Orthop Trauma. 2018;33(3):143-148. https://doi.org/10.1097/BOT.0000000000001386.
20. Brovman EY, Pisansky AJ, Beverly A, Bader AM, Urman RD. Do Not Resuscitate Status as an independent risk factor for patients undergoing surgery for hip fracture. World J Orthop. 2017;8(12):902-912. https://doi.org/10.5312/wjo.v8.i12.902.
21. Brovman EY, Walsh EC, Burton BN, et al. Postoperative outcomes in patients with a do-not-resuscitate (DNR) order undergoing elective procedures. J Clin Anesth. 2018;48:81-88. https://doi.org/10.1016/j.jclinane.2018.05.007.
22. Beverly A, Brovman EY, Urman RD. Comparison of postoperative outcomes in elderly patients with a do-not-resuscitate order undergoing elective and nonelective hip surgery. Geriatr Orthop Surg Rehabil. 2017;8(2):78-86. https://doi.org/10.1177/2151458516685826.
23. Maxwell BG, Lobato RL, Cason MB, Wong JK. Perioperative morbidity and mortality of cardiothoracic surgery in patients with a do-not-resuscitate order. PeerJ. 2014;2013(1):1-10. https://doi.org/10.7717/peerj.245.
24. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. https://doi.org/10.1001/archsurg.2011.69.
25. Brañas F, Ruiz-Pinto A, Fernández E, et al. Beyond orthogeriatric co-management model: benefits of implementing a process management system for hip fracture. Arch Osteoporos. 2018;13(1):81. https://doi.org/10.1007/s11657-018-0497-6.
1. Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St. Peter WL. Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis. 2013;62(4):747-754. https://doi.org/10.1053/j.ajkd.2013.02.368.
2. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. https://doi.org/10.1001/jama.2009.1462.
3. Chlebeck JD, Birch CE, Blankstein M, Kristiansen T, Bartlett CS, Schottel PC. Nonoperative geriatric hip fracture treatment is associated with increased mortality. J Orthop Trauma. 2019;33(7):346-350. https://doi.org/10.1097/BOT.0000000000001460.
4. Wu X, Tian M, Zhang J, et al. The effect of a multidisciplinary co-management program for the older hip fracture patients in Beijing: a “pre- and post-” retrospective study. Arch Osteoporos. 2019;14(1):43. https://doi.org/10.1007/s11657-019-0594-1.
5. Stephens JR, Chang JW, Liles EA, Adem M, Moore C. Impact of hospitalist vs. non-hospitalist services on length of stay and 30-day readmission rate in hip fracture patients. Hosp Pract. 2019;47(1):24-27. https://doi.org/10.1080/21548331.2019.1537850.
6. Phy MP, Vanness DJ, Melton LJ, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. https://doi.org/10.1001/archinte.165.7.796.
7. Batsis JA, Phy MP, Melton LJ, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. https://doi.org/10.1002/jhm.207
8. Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. https://doi.org/10.7326/0003-4819-141-1-200407060-00012.
9. Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016;136(10):1403-1409. https://doi.org/10.1007/s00402-016-2543-4.
10. Folbert EC, Hegeman JH, Vermeer M, et al. Improved 1-year mortality in elderly patients with a hip fracture following integrated orthogeriatric treatment. Osteoporos Int. 2017;28(1):269-277. https://doi.org/10.1007/s00198-016-3711-7.
11. Shiloach M, Frencher SK, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. https://doi.org/10.1016/j.jamcollsurg.2009.09.031.
12. Hutter MM, Rowell KS, Devaney LA, et al. Identification of surgical complications and deaths: an assessment of the traditional surgical morbidity and mortality conference compared with the American College of Surgeons-National Surgical Quality Improvement Program. J Am Coll Surg. 2006;203(5):618-624. https://doi.org/10.1016/j.jamcollsurg.2006.07.010.
13. Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University HealthSystem Consortium Clinical Database and the National Surgical Quality Improvement Program data set. Am J Med Qual. 2009;24(5):395-402. https://doi.org/10.1177/1062860609339936.
14. Yu P, Chang DC, Osen HB, Talamini MA. NSQIP reveals significant incidence of death following discharge. J Surg Res. 2011;170(2):e217-e224. https://doi.org/10.1016/j.jss.2011.05.040.
15. Wilson J, Lunati M, Grabel Z, Staley C, Schwartz A, Schenker M. Hypoalbuminemia is an independent risk factor for 30-day mortality, postoperative complications, readmission, and reoperation in the operative lower extremity orthopedic trauma patient. J Orthop Trauma. 2019;33(6):284-291. https://doi.org/10.1097/BOT.0000000000001448.
16. Bohl DD, Shen MR, Hannon CP, Fillingham YA, Darrith B, Della Valle CJ. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J Bone Jt Surg. 2017;99(24):2110-2118. https://doi.org/10.2106/JBJS.16.01620.
17. Arshi A, Rezzadeh K, Stavrakis AI, Bukata S V, Zeegen EN. Standardized hospital-based care programs improve geriatric hip fracture outcomes: an analysis of the ACS-NSQIP targeted hip fracture series. J Orthop Trauma. 2019;33(6): e223-e228. https://doi.org/10.1097/BOT.0000000000001443.
18. Traven SA, Reeves RA, Althoff AD, Slone HS, Walton ZJ. New 5-factor modified frailty index predicts morbidity and mortality in geriatric hip fractures. J Orthop Trauma. 2019;33(7):319-323. https://doi.org/10.1097/BOT.0000000000001455.
19. Wilson JM, Boissonneault AR, Schwartz AM, Staley CA, Schenker ML. Frailty and malnutrition are associated with inpatient post-operative complications and mortality in hip fracture patients. J Orthop Trauma. 2018;33(3):143-148. https://doi.org/10.1097/BOT.0000000000001386.
20. Brovman EY, Pisansky AJ, Beverly A, Bader AM, Urman RD. Do Not Resuscitate Status as an independent risk factor for patients undergoing surgery for hip fracture. World J Orthop. 2017;8(12):902-912. https://doi.org/10.5312/wjo.v8.i12.902.
21. Brovman EY, Walsh EC, Burton BN, et al. Postoperative outcomes in patients with a do-not-resuscitate (DNR) order undergoing elective procedures. J Clin Anesth. 2018;48:81-88. https://doi.org/10.1016/j.jclinane.2018.05.007.
22. Beverly A, Brovman EY, Urman RD. Comparison of postoperative outcomes in elderly patients with a do-not-resuscitate order undergoing elective and nonelective hip surgery. Geriatr Orthop Surg Rehabil. 2017;8(2):78-86. https://doi.org/10.1177/2151458516685826.
23. Maxwell BG, Lobato RL, Cason MB, Wong JK. Perioperative morbidity and mortality of cardiothoracic surgery in patients with a do-not-resuscitate order. PeerJ. 2014;2013(1):1-10. https://doi.org/10.7717/peerj.245.
24. Kazaure H, Roman S, Sosa JA. High mortality in surgical patients with do-not-resuscitate orders: analysis of 8256 patients. Arch Surg. 2011;146(8):922-928. https://doi.org/10.1001/archsurg.2011.69.
25. Brañas F, Ruiz-Pinto A, Fernández E, et al. Beyond orthogeriatric co-management model: benefits of implementing a process management system for hip fracture. Arch Osteoporos. 2018;13(1):81. https://doi.org/10.1007/s11657-018-0497-6.
© 2019 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Measles
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
© 2020 Society of Hospital Medicine
A Plea to Reconsider the Diagnosis
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
An eight-month-old unvaccinated boy presented to an emergency department (ED) with fever, neck pain, and lethargy. Examination of the cerebrospinal fluid (CSF) demonstrated hazy fluid with a white blood cell count of 3,906 cells/uL (90% polymorphonuclear cells, 6% lymphocytes, and 4% monocytes), 0 red blood cells/uL, protein of 40 mg/dL, and glucose of 56 mg/dL. No organisms were seen on Gram stain. Ceftriaxone and vancomycin were administered. CSF, blood, and urine cultures remained sterile; arbovirus serology was nonreactive, and polymerase chain reactions (PCRs) for enterovirus, Herpes simplex virus (HSV), Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenza were negative. His irritability improved, but his fevers continued. The antibiotics were stopped after 10 days of empiric treatment, and his fever resolved within 36 hours of cessation of antibiotics. He was diagnosed with aseptic meningitis and possible drug fever, attributed to either ceftriaxone or vancomycin.
There are many possibilities to consider in an unimmunized child with signs and symptoms of meningitis. The vaccine-preventable infections are ruled out in the setting of negative cultures and PCRs. While the most common etiology of aseptic meningitis is secondary to viral infections, the considerations of drug fever and aseptic meningitis deserve more attention. A thorough medication history should be taken as nonsteroidal anti-inflammatory drugs (NSAIDs) are relatively commonly linked to aseptic meningitis. Evaluation should focus on family history, medications, and exposures.
There was no family history of meningitis nor known exposures to mosquitos or ticks. The patient did not have a history of atypical or severe infections. He had one episode of acute otitis media that resolved without antibiotics. He had a history of delayed speech and was more irritable than his siblings.
Sixteen months later, at two years of age, he presented to his primary care physician in Wisconsin for evaluation of one day of fever and fussiness without rhinorrhea or cough. Examination showed enlarged tonsils without exudate or pharyngeal erythema. His tympanic membranes were normal, and the lung fields were clear. Two of his older siblings and his father had been diagnosed with streptococcal pharyngitis and were receiving antibiotic treatment. A rapid streptococcal antigen test was positive, and amoxicillin started.
Group A streptococcal (GAS) pharyngitis is an acute infection of the oropharynx or nasopharynx caused by Streptococcus pyogenes and is most common in school-aged children. GAS pharyngitis is less common at age two years unless there is definite exposure. The most frequent presentations in this age group (<3 years of age) include protracted nasal symptoms (congestion and rhinorrhea) and cough instead of a well-localized episode of pharyngitis.
The amoxicillin was continued for four days without improvement in fever or fussiness. His oral intake decreased, and he developed nonbilious, nonbloody emesis without diarrhea. He followed up with his pediatrician for the presumed streptococcal pharyngitis. Because of the previous concern for drug fever related to his ceftriaxone exposure, the amoxicillin was discontinued. Supportive care was recommended.
While viral infections remain the most likely etiology, noninfectious etiologies, such as vasculitis, should be considered. Kawasaki disease should be considered in any child with prolonged fever. Kawasaki disease can also cause aseptic meningitis that could provide an explanation for his original episode at eight months of age; nevertheless, it is rare for Kawasaki disease to recur.
Over the next three days, his temperature was as high as 38.8°C (101.8°F), he became more irritable, and his vomiting worsened; his family believed he had a headache. He was again seen by his pediatrician, now with eight days of fever. On examination, his oropharynx was mildly erythematous with palatal petechiae and 2+ tonsillar enlargement; shotty anterior cervical lymphadenopathy was present. Concern for incompletely treated streptococcal pharyngitis prompted prescription of azithromycin for five days.
This information does not change the differential diagnosis significantly. Azithromycin is as effective as beta lactams for the treatment of GAS pharyngitis if the GAS is susceptible to macrolides. Macrolide resistance rates vary between communities and have been as high as 15% in Wisconsin; knowledge of local resistance patterns is important.1
Despite the azithromycin, his symptoms worsened, and he became lethargic. The family believed the symptoms were similar to those during his previous episode of meningitis. They presented to an ED where he was febrile to 39.4°C (102.9°F) with a heart rate of 159 beats per minute and blood pressure of 113/84 mm Hg. His head circumference was 50.5 cm (97th percentile) compared with his weight of 10.8 kg (23.81 lbs; 22nd percentile). He was listless when undisturbed and irritable during the examination; his neck was supple and strong, and reflexes were normal. The remainder of his examination, including joints and skin, was normal. His white blood cell count was 18.6 K/uL, hemoglobin 11.8 g/dL, and platelets 401 K/uL. A chest radiograph was normal.
The patient is presenting on the 10th day of fever—a long time for any patient to remain febrile. Although most typically due to infectious etiologies, rheumatologic and oncologic diseases must be considered. It is important to characterize the pattern of fevers during the past 10 days and whether the patient has had similar febrile illnesses in the past. In this case, his past medical history substantially alters the differential diagnosis. The positive rapid strep test and history of recent strep pharyngitis are of uncertain importance, and the patient’s nonresponsiveness to antibiotics should raise concern for a second disease process (other than streptococcal infection) causing the fever. His unimmunized status changes the pretest probability of serious conditions such as bacterial meningitis caused by S. pneumoniae. A lumbar puncture should be performed, including an opening pressure; if the CSF again shows pleocytosis, but no infectious etiology is identified, then imaging of the brain (magnetic resonance imaging [MRI] or magnetic resonance angiogram) should be performed to evaluate for anatomic abnormalities.
CSF examination revealed 9,327 white blood cells/uL (82% polymorphonuclear cells, 1% lymphocytes, and 17% monocytes), 114 red blood cells/uL, protein of 87 mg/dL, and glucose of 63 mg/dL. Gram stain revealed no organisms. Ceftriaxone, vancomycin, and acyclovir were started, and he was transferred to a children’s hospital.
This history must be viewed through two alternate lenses: that the two episodes of meningitis are related or that they are unrelated. The finding of a neutrophil predominance in the CSF in the setting of aseptic (or nonbacterial) meningitis is less common than a lymphocytic predominance. Most commonly, aseptic meningitis is due to viral infection and is typically associated with a lymphocyte predominance, although a moderate neutrophil predominance can be seen in patients with enterovirus meningitis. Neutrophil-predominant aseptic meningitis can also accompany genetic auto-inflammatory syndromes (eg, familial Mediterranean fever and cryopyrin-associated periodic syndrome). This finding can also be seen in other noninfectious conditions such as neurosarcoidosis, Behçet’s disease, Cogan syndrome, and other vasculitides. Drug-induced aseptic meningitis can also cause neutrophil predominance. Additionally, the apparent neutrophil predominance could be explained if the patient had lymphopenia associated with primary or acquired immunodeficiency; therefore, the peripheral leukocyte differential obtained at the same time as the CSF should be evaluated. However, immunodeficiency is less likely given the patient’s lack of history of recurrent infections.
The main objective information added here is that the patient now has his second episode of likely aseptic meningitis with neutrophilic predominance, although it is possible that antibiotic therapy may have led to a false-negative CSF culture. However, this possible partial treatment was not a consideration in the first episode of meningitis. Having two similar episodes increases the likelihood that the patient has an underlying inflammatory/immune disorder, likely genetic (now termed “inborn errors of immunity”), or that there is a common exposure not yet revealed in the history (eg, drug-induced meningitis). Primary immunodeficiency is less likely than an autoinflammatory disease, considering the patient’s course of recovery with the first episode.
Further evaluation of the CSF did not reveal a pathogen. Bacterial CSF culture was sterile, and PCRs for HSV and enterovirus were negative.
The differential diagnosis is narrowing to include causes of recurrent, aseptic, neutrophilic meningitis. The incongruous head circumference and weight could be due to a relatively large head, a relatively low weight, or both. To interpret these data properly, one also needs to know the patient’s length, the trajectory of his growth parameters over time, and the parents’ heights and head circumferences. One possible scenario, considering the rest of the history, is that the patient has a chronic inflammatory condition of the central nervous system (CNS), leading to hydrocephalus and macrocephaly. It is possible that systemic inflammation could also lead to poor weight gain.
When considering chronic causes of aseptic meningitis associated with neutrophil predominance in the CSF, autoinflammatory disorders (cryopyrin-associated periodic syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease [NOMID], and chronic infantile neurological cutaneous articular syndrome [CINCA]) should be considered. The patient lacks the typical deforming arthropathy of the most severe NOMID/CINCA phenotype. If the brain imaging does not reveal another etiology, then genetic testing of the patient is indicated.
Because of the history of recurrent meningitis with marked neutrophilic pleocytosis, yet no evidence of infection given normal glucose, only mildly elevated protein, and no culture growth, an MRI of the brain was obtained. MRI revealed a sharply circumscribed, homogeneous, nonenhancing 2.6 cm diameter cystic suprasellar mass with a thin rim of capsular enhancement (Figure). The appearance was most consistent with an epidermoid cyst, a dermoid, Rathke’s cleft cyst (RCC), or, less likely, a craniopharyngioma. The recurrent aseptic meningitis was attributed to chemical meningitis secondary to episodic discharging of the tumor. There was no hydrocephalus on imaging, and the enlarged head circumference was attributed to large parental head circumference.
Antibiotics were discontinued and supportive care continued. A CSF cholesterol level of 4 mg/dL was found (normal range 0.6 ± 0.2 mg/dL) on the CSF from admission. Fevers and symptoms ultimately improved with 72 hours of admission. He was discharged with neurosurgical follow-up, and within a year, he developed a third episode of aseptic meningitis. He eventually underwent a craniotomy with near-total resection of the cyst. Histopathological analysis indicated the presence of an underlying RCC, despite initial clinical and radiographic suspicion of an epidermoid cyst. He recovered well with follow-up imaging demonstrating stable resolution of the RCC and no further incidents of aseptic meningitis in the 12 months since the surgery.
DISCUSSION
Aseptic meningitis is defined as meningitis with negative bacterial cultures from CSF and is habitually equated with viral meningitis.2 This erroneous equivalence may curb critical thinking about alternative diagnoses, as aseptic meningitis may also be associated with a wide range of both infectious and noninfectious etiologies (Table). A thorough history and physical examination are the essential first steps in determining the etiology of aseptic meningitis, as many of the listed etiologies can be effectively eliminated through the evaluation of risk factors and exposures. Laboratory evaluation of CSF including cell count with differential, glucose, and protein levels is required. Gram stain and culture should be obtained to evaluate for bacterial meningitis even if suspicion is low. Multiplex and dedicated PCRs to viral agents as well as a serologic test for arboviruses, are widely available. Multiple episodes of aseptic meningitis with HSV, known as Mollaret’s meningitis, or enterovirus, which is more common in males with X-linked agammaglobulinemia, should be considered in patients with recurrent disease. Imaging is not indicated for every patient with aseptic meningitis; however, if anatomic abnormalities or malignancy are suspected, or in the evaluation of recurrent disease, then an MRI of the brain should be considered.
This case highlights how the analysis of CSF pleocytosis is not always predictive of a specific underlying etiology. The classic teaching that viral meningitis is associated with lymphocytic pleocytosis is based on studies of mumps meningitis.3 It is important to recognize that a neutrophilic pleocytosis is also described in viral infections including those caused by an enterovirus, herpes simplex, and arboviruses.4,5 Moreover, while the magnitude of the neutrophilic pleocytosis should always raise suspicion of bacterial meningitis, it should also be associated with hypoglycorrhachia and elevated CSF protein levels. Antibiotic pretreatment of bacterial meningitis can alter CSF chemistries (raise CSF glucose levels and lower CSF protein levels), but chemistries are unlikely to return completely to normal.6 In this case, one clue that hinted toward a noninfectious etiology was the recurrence of relatively normal CSF glucose and protein levels in the setting of such a highly inflammatory pleocytosis on multiple occasions.
There is a wide spectrum of CNS mass lesions known for causing chemical meningitis including epidermoid, dermoid, craniopharyngiomas, and RCCs. While imaging can be suggestive, histological examination is often required to make a specific diagnosis. In this patient, the diagnosis of chemical meningitis secondary to a ruptured brain tumor was confirmed by MRI. CNS tumors that may cause aseptic meningitis are typically slow-growing lesions that cause symptoms due both to local growth and regional neurovascular compression. These masses can rupture and disseminate inflammatory contents into the subarachnoid space giving rise to chemical aseptic meningitis. Their contents may include materials rich in keratin, cholesterol, and lipids, which cause an intense sterile inflammatory reaction when discharged, possibly via cholesterol activation of the inflammasome.7,8 The subsequent inflammatory response produces a neutrophilic pleocytosis, often suggestive of bacterial meningitis, while simultaneously maintaining the near normalcy of the CSF glucose and protein levels. Elevated levels of CSF cholesterol may raise suspicion of the diagnosis. Not all discharging tumors result in purely chemical meningitis, as secondary bacterial meningitis with S. pneumoniae or other respiratory flora can coexist if cysts communicate with the respiratory tract.9
Rathke’s cleft is formed during the development of the pituitary gland by the evagination of oral ectoderm through the precursor of the oral cavity.10 The cleft gives rise to the endocrine cells of the anterior pituitary. It subsequently disconnects from the oral cavity and develops into the pars intermedia between the anterior and posterior pituitary. Cystic enlargement of Rathke’s cleft through epithelial proliferation and secondary secretions leads to the development of an RCC. RCCs are nonneoplastic lesions, and the majority are diagnosed incidentally. Asymptomatic RCCs are common and are detected in 13%-22% of routine autopsies.11 Symptomatic lesions may present with headaches due to mechanical effects on pain-sensitive dura or cranial nerves. Severe acute onset headaches may arise secondary to pituitary hemorrhage. RCCs can also cause ophthalmic or endocrinological impairment due to sellar compression. As in the present case, rarely cystic rupture and subarachnoid extravasation of epithelial-derived contents lead to a chemical aseptic meningitis.12
Surgical resection is indicated for symptomatic RCCs that exert neurologic, ophthalmic, or endocrinological symptoms.13 The surgical goal is the removal of the lesion and complete excision of the capsule unless it is extremely adherent to neurovascular structures. Surgical morbidity is related to the risk of hypopituitarism, visual decline, incomplete resection with lesion regrowth, and aseptic meningitis. Surgical approaches to this region are potentially complicated by proximity to optic nerves, pituitary glands, major arteries, and perforating vessels belonging to the circle of Willis. In addition, potential dehiscence of the skull base floor due to progressive cyst growth can give rise to a delayed risk of CSF leak and complicate surgical recovery. Surgery was indicated for this patient because of the parasellar location of his cyst placing him at risk for visual decline due to compression of the optic chiasm as well as pituitary dysfunction or obstructive hydrocephalus from ventricular compression.
This case is illustrative for learning because, at the outset, there were many possibilities to explore in an unimmunized child with meningitis. This patient’s neutrophilic cell count and partial antibiotic treatment only compounded the certainty of a bacterial etiology. However, further scrutiny of the history and laboratory parameters revealed the true underlying diagnosis of RCC. Ultimately, the plea to reconsider the pleocytosis was heard.
KEY LEARNING POINTS
- The CSF cell count and differential should be used in conjunction with CSF chemistries (glucose and protein) to raise or lower suspicion of bacterial meningitis.
- Aseptic meningitis is a syndrome and not a specific diagnosis. Clinicians should be alert to key aspects of the history and physical examination, which prompt consideration of noninfectious etiologies.
- Aseptic chemical meningitis secondary to discharging CNS tumors, including RCCs, should be considered in episodes of recurrent culture-negative meningitis.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
1. DeMuri GP, Sterkel AK, Kubica PA, Duster MN, Reed KD, Wald ER. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr Infect Dis J. 2017;36(3):342-344. https://doi.org/10.1097/INF.0000000000001442.
2. Lee BE, Davies HD. Aseptic meningitis. Curr Opin Infect Dis. 2007;20(3):272-277. https://doi.org/10.1097/QCO.0b013e3280ad4672.
3. Ritter BS. Mumps meningoencephalitis in children. J Pediatr. 1958;52(4):424-433. https://doi.org/10.1016/S0022-3476(58)80063-3.
4. Miller SA, Wald ER, Bergman I, DeBiasio R. Enteroviral meningitis in January with marked cerebrospinal fluid pleocytosis. Pediatr Infect Dis. 1986;5(6):706-707. https://doi.org/10.1097/00006454-198611000-00024.
5. Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77-81. https://doi.org/10.1016/j.ijid.2017.04.010.
6. Nigrovic LE, Malley R, Macias CG, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122(4):726-730. https://doi.org/10.1542/peds.2007-3275.
7. Cherian A, Baheti NN, Easwar HV, Nair DS, Iype T. Recurrent meningitis due to epidermoid. J Pediatr Neurosci. 2012;7(1):47-48. https://doi.org/10.4103/1817-1745.97624.
8. Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15(3):313. https://doi.org/10.1007/s11926-012-0313-z.
9. Kriss TC, Kriss VM, Warf BC. Recurrent meningitis: the search for the dermoid or epidermoid tumor. Pediatr Infect Dis J. 1995;14(8):697-700.
10. Bresson D, Herman P, Polivka M, Froelich S. Sellar lesions/pathology. Otolaryngol Clin North Am. 2016;49(1):63-93. https://doi.org/10.1016/j.otc.2015.09.004.
11. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary. 2004;7(3):131-137. https://doi.org/10.1007/s11102-005-1755-3.
12. Steinberg GK, Koenig GH, Golden JB. Symptomatic Rathke’s cleft cysts. Report of two cases. J Neurosurg. 1982;56(2):290-295. https://doi.org/10.3171/jns.1982.56.2.0290.
13. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. https://doi.org/10.3171/2011.5.FOCUS1183.
© 2019 Society of Hospital Medicine
Utility of ICD Codes for Stress Cardiomyopathy in Hospital Administrative Databases: What Do They Signify?
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
Stress cardiomyopathy (SCM), also known as takotsubo cardiomyopathy, is a nonischemic cardiomyopathy initially identified in Japan in 1990. In 2006, SCM gained an International Classification of Diseases code at the 9th Clinical Modification (ICD-9 CM). Subsequently, several epidemiological studies have used ICD codes to evaluate trends in the diagnosis of SCM;1-8 however, to our knowledge, no previous studies have validated ICD-9 or -10 codes using chart review. We aimed to determine the positive predictive value (PPV) and the limitations of these ICD codes among hospitalized patients.
METHODS
We performed a retrospective cohort study at a single tertiary care center, identifying all adults aged ≥18 years from 2010 to 2016 who were hospitalized with a first known diagnosis of SCM in our Electronic Health Records (EHR) system (Cerner, Stoltenberg Consulting, Inc., Bethel Park, Pennsylvania), which includes both inpatient and outpatient records. We included patients hospitalized with a principal or secondary ICD-9 discharge diagnosis code of 429.83 (for those hospitalized before October 2015) or an ICD-10 discharge diagnosis code of I51.81 (for those hospitalized from October 1, 2015 through December 2016). We excluded hospital readmissions and patients with recurrent SCM, but we could not administratively remove patients who carried a prior diagnosis of SCM made previously at other institutions. One investigator (KW) then reviewed our EHR for a documentation of SCM anywhere in the chart by performing a systematic review of discharge, admission, consultation, daily progress notes, as well as biomarkers, electrocardiograms, echocardiograms, and coronary angiograms. If the first reviewer did not find documentation of SCM anywhere in the EHR, this finding was confirmed by a second chart review by a cardiologist (QP).
Principal and secondary discharge diagnoses were entered into our administrative database by hospital coders using standard coding practices. Because ICD codes also record comorbidities that were present prior to admission, we determined whether each patient had a new diagnosis of SCM during the hospitalization. If not, we considered their ICD code as a preexisting comorbidity and labeled these as chronic cases.
We recorded age, sex, race, ethnicity, and frequency of echocardiogram and cardiac catheterization among all patients. To determine the burden of other comorbidities, we used the Charlson Comorbidity Index and the Elixhauser Comorbidity Index,9,10 but limited our reporting to comorbidities with >5% prevalence.
Our primary aim was to measure the PPV of these ICD codes to determine a diagnosis of SCM. This was done by dividing the total number of cases with a clinical documentation of SCM by the total number of patients with an ICD diagnosis of SCM. As secondary aims, we noted the percentage of new and chronic SCM, the proportion of patients who underwent echocardiography and/or cardiac catheterization and recorded the annual number of total cases of confirmed SCM from 2010 to 2016. Trends were evaluated using the Cochran-Armitage test. To better understand the difference between patients given a principal and secondary code for SCM, we compared these two groups using summary statistics using t tests and chi-squared tests as appropriate, noted the PPV, and determined the 95% confidence intervals of ICD codes in these subgroups. This study was approved by the institutional review board of Baystate Medical Center (#1109756-4). Statistical analysis was done using JMP version12.0.1 (SAS Institute, Cary, North Carolina, 2015).
RESULTS
During 2010-2016, a total of 592 patients with a first known ICD code in our EHR for SCM were hospitalized, comprising 242 (41.0%) with a principal diagnosis code. Upon chart review, we were unable to confirm a clinical diagnosis of SCM among 12 (2.0%) patients. In addition, 38 (6.4%) were chronic cases of SCM, without evidence of active disease at the time of hospitalization. In general, chronic cases typically carried an SCM diagnosis from a hospitalization at a non-Baystate hospital (outside our EHR), or from an outpatient setting. Occasionally, we also found cases where the diagnosis of SCM was mentioned but testing was not pursued, and the patient had no symptoms that were attributed to SCM. Overall use of echocardiogram and cardiac angiography was 91.5% and 66.8%, respectively, and was lower in chronic than in new cases of SCM.
Compared with patients with a secondary diagnosis code, patients with a principal diagnosis of SCM underwent more cardiac angiography and echocardiography (Table 1). When comparing the difference between those with principal and secondary ICD codes, we found that 237 (98%) vs 305 (87%) were new cases of SCM, respectively, and all 12 patients without any clinical diagnosis of SCM had been given a secondary ICD code. Between 2010 and 2016, we noted a significant increase in the number of cases of SCM (Cochrane–Armitage, P < .0001).
The overall PPV (95% CI) of either principal or secondary ICD codes for any form or presentation of SCM was 98.0% (96.4-98.8) with no difference in PPV between the coding systems (ICD-9, 66% of cases, PPV 98% [96.0-99.0] vs ICD-10, PPV 98% [94.9-99.2; P = .98]). Because all patients without a diagnosis of SCM were given secondary ICD codes, this changed the PPV (95% CI) for principal and secondary SCM to 100% (98.4-100.0) and 96.6% (94.1-98.0), respectively. When chronic cases were included as noncases, the PPV (95% CI) to detect a new case of SCM decreased to 97.9% (95.2-99.1) and 87.1% (83.0-90.2) for principal and secondary SCM, respectively (Table 1).
DISCUSSION
In this study, we found a strong relationship between the receipt of an ICD code for SCM and the clinical documentation of a diagnosis of SCM, with an overall PPV of 98%. The PPV was higher when the sample was limited to those assigned a principal ICD code for SCM, but it was lower when considering that some ICD codes represented chronic SCM from prior hospitalizations, despite our attempts to exclude these cases administratively prior to chart review. Furthermore, cardiac catheterization and echocardiography were used inconsistently and were less frequent among secondary compared with a principal diagnosis of SCM. Thus, although a principal ICD diagnosis code for SCM appears to accurately reflect a diagnosis of SCM, a secondary code for SCM appears less reliable. These findings suggest that future epidemiological studies can rely on principal diagnosis codes for use in research studies, but that they should use caution when including patients with secondary codes for SCM.
Our study makes an important contribution to the literature because it quantitates the reliability of ICD codes to identify patients with SCM. This finding is important because multiple studies have used this code to study trends in the incidence of this disease,1-8 and futures studies will almost certainly continue to do so. Our results also showed similar demographics and trends in the incidence of SCM compared with those of prior studies1-3,11 but additionally revealed that these codes also have some important limitations.
A key factor to remember is that neither a clinical diagnosis nor an ICD code at the time of hospital discharge is based upon formal diagnostic criteria for SCM. Importantly, all currently proposed diagnostic criteria require resolution of typical regional wall motion abnormalities before finalizing a research-grade diagnosis of SCM (Table 2).12,13 However, because the median time to recovery of ejection fraction in SCM is between three and four weeks after hospital discharge (with some recovery extending much longer),6 it is almost impossible to make a research-grade diagnosis of SCM after a three- to four-day hospitalization. Moreover, 33% of our patients did not undergo cardiac catheterization, 8.5% did not undergo echocardiography, and it is our experience that testing for pheochromocytoma and myocarditis is rarely done. Thus, we emphasize that ICD codes for SCM assigned at the time of hospital discharge represent a clinical diagnosis of SCM and not research-grade criteria for this disease. This is a significant limitation of prior epidemiologic studies that consider only the short time frame of hospitalization.
A limitation of our study is that we did not attempt to measure sensitivity, specificity, or the negative predictive value of these codes. This is because measurement of these diagnostic features would require sampling some of our hospital’s 53,000 annual hospital admissions to find cases where SCM was present but not recognized. This did not seem practical, particularly because it might also require directly overreading imaging studies. Moreover, we believe that for the purposes of future epidemiology research, the PPV is the most important feature of these codes because a high PPV indicates that when a principal ICD code is present, it almost always represents a new case of SCM. Other limitations include this being a single-center study; the rates of echocardiograms, cardiac angiography, clinical diagnosis, and coding may differ at other institutions.
In conclusion, we found a high PPV of ICD codes for SCM, particularly among patients with a principal discharge diagnosis of SCM. However, we also found that approximately 8% of cases were either wrongly coded or were chronic cases. Moreover, because of the need to document resolution of wall motion abnormalities, essentially no patients met the research-grade diagnostic criteria at the time of hospital discharge. Although this increases our confidence in the results of past studies, it also provides some caution to researchers who may use these codes in the future.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
1. Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53-63. https://doi.org/10.1016/j.ahj.2015.10.022.
2. Murugiah K, Wang Y, Desai NR, et al. Trends in short- and long-term outcomes for takotsubo cardiomyopathy among medicare fee-for-service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4(3):197-205. https://doi.org/10.1016/j.jchf.2015.09.013.
3. Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215-221. https://doi.org/10.1016/j.ahj.2012.04.010.
4. Smilowitz NR, Hausvater A, Reynolds HR. Hospital readmission following takotsubo syndrome. Eur Heart J Qual Care Clin Outcomes. 2018;5(2):114-120. https://doi.org/10.1093/ehjqcco/qcy045.
5. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako-Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. https://doi.org/10.1161/JAHA.118.009160.
6. Shaikh N, Sardar M, Jacob A, et al. Possible predictive factors for recovery of left ventricular systolic function in takotsubo cardiomyopathy. Intractable Rare Dis Res. 2018;7(2):100-105. https://doi.org/10.5582/irdr.2018.01042.
7. Shah M, Ram P, Lo KBU, et al. Etiologies, predictors, and economic impact of readmission within 1 month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;41(7):916-923. https://doi.org/10.1002/clc.22974.
8. Misumida N, Ogunbayo GO, Kim SM, Abdel-Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology. 2019;70(1):56-61. https://doi.org/10.1177/0003319718782049.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
10. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
11. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. Sep 3 2015;373(10):929-938. https://doi.org/10.1056/NEJMoa1406761.
12. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(16):1955-1971. https://doi.org/10.1016/j.jacc.2018.07.072.
13. Ghadri JR, Wittstein IS, Prasad A, et al. international expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39(22):2032-2046. https://doi.org/10.1093/eurheartj/ehy076.
© 2020 Society of Hospital Medicine
Prediction of Disposition Within 48 Hours of Hospital Admission Using Patient Mobility Scores
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
The loss of mobility during hospitalization is common and is an important reason why more than 40% of hospitalized Medicare patients require placement in a postacute facility.1,2 Discharge planning may be delayed when the medical team focuses on managing acute medical issues without recognizing a patient’s rehabilitation needs until near the time of discharge.3 For patients who require rehabilitation in a postacute facility, delays in discharge can exacerbate hospital-acquired mobility loss and prolong functional recovery.2,4 In addition, even small increases in length of stay have substantial financial impact.5 Increased efficiency in the discharge process has the potential to reduce healthcare costs, facilitate patient recovery, and reduce delays for new admissions awaiting beds.6 For effective discharge planning, a proactive, patient-centered, interdisciplinary approach that considers patient mobility status is needed.3
Systematic measurement of patient mobility that extends beyond evaluations by physical therapists is not common practice, but has the potential to facilitate early discharge planning.7,8 At our hospital, mobility assessment is performed routinely using a reliable and valid interdisciplinary assessment of mobility throughout the patient’s entire hospitalization.9 We recently showed that nurse-recorded mobility status within the first 24 hours of hospitalization was associated with discharge disposition,7 but a prediction tool to help aid clinicians in the discharge planning process would be more useful. In this study, we evaluated the predictive ability of a patient’s mobility score, obtained within 48 hours of hospital admission, to identify the need for postacute care in a diverse patient population.
METHODS
After receiving approval from the Johns Hopkins Institutional Review Board, we conducted analyses on a retrospective cohort of 821 admissions (777 unique patients admitted between January 1, 2017 and August 25, 2017) who were hospitalized for ≥72 hours on two inpatient units (medical and neurological/neurosurgical) at The Johns Hopkins Hospital (JHH). These units were chosen to reduce the potential for both selection and measurement bias. First, these units manage a diverse patient population that is likely to generalize to a general hospital population. Second, the nursing staff on these units has the most accurate and consistent documentation compliance for our predictor variable.
Mobility Measure
The Activity Measure for Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a measure of functional capacity. This short form is widely used and is nicknamed “6 clicks.” It has questions for six mobility tasks, and each question is scored on a four-point Likert scale.9 Patients do not have to attempt the tasks to be scored. Clinicians can score items using clinical judgement based on observation or discussion with the patient, family, or other clinicians. The interrater reliability is very good (Intraclass Correlation Coefficient = .85-.99)9 and construct validity has been demonstrated for the inpatient hospital population (AM-PAC IMSF correlations with: functional independence measure [FIM] = .65; Katz activities of daily living [ADL] = .80; 2-minute walk = .73; 5-times sit-to-stand = −.69).9 At JHH, the AM-PAC IMSF is scored at admission by nursing staff (>90% documentation compliance on the units in this study); these admission scores were used.
Outcome and Predictors
Discharge location (postacute care facility vs home) was the primary outcome in this study, as recorded in a discrete field in the electronic medical record (EMR). To ensure the validity of this measure, we performed manual chart audits on a sample of patients (n = 300). It was confirmed that the measure entered in the discrete field in the EMR correctly identified the disposition (home vs postacute care facility) in all cases. The primary predictor was the lowest AM-PAC IMSF score obtained within 48 hours after hospital admission, reflecting the patient’s capability to mobilize after hospital admission. Raw scores were converted to scale scores (0-100) for analysis.9 Additional predictors considered included: age, sex, race, and primary diagnosis, all of which were readily available from the EMR at the time of hospital admission. We then grouped the primary diagnosis into the following categories using ICD-10 codes upon admission: Oncologic, Progressive Neurological, Sudden Onset Neurological, and Medical/Other.
Statistical Analysis
We constructed a classification tree, a machine learning approach,10 to predict discharge placement (postacute facility vs home) based on the patients’ hospital admission characteristics and AM-PAC IMSF score. The prediction model was developed using the classification tree approach, as opposed to a logistic regression model. This approach allows for the inclusion of higher-order interactions (ie, interactions of more than two predictors) which would need to be explicitly specified otherwise and a priori we did not have strong evidence from prior studies to guide the model construction. The classification tree was constructed and evaluated by dividing our sample into a 70% training set and a 30% validation set using random sampling within key strata defined by age (<65 vs ≥65 years), gender, and quartile of the AM-PAC IMSF score. The classification tree was developed using the training set. Next, measures of predictive accuracy (ie, the proportion of correctly classified patients with placement in a postacute facility [sensitivity]) and the proportion of correctly classified patients not discharged to postacute care (ie, to home, specificity), were estimated by applying the validation set to the classification tree. The R statistical package rpart11 with procedure rpart was used to construct the classification tree using standard criteria for growing (Gini index10) and pruning (misclassification error estimated by leave-1-out cross-validation12) the tree.
RESULTS
DISCUSSION
Improving the efficiency of hospital discharge planning is of great interest to hospital-based clinicians and administrators. Identifying patients early who are likely to need placement in a postacute facility is an important first step. Using a reliable and valid nursing assessment tool of patient mobility to help with discharge planning is an attractive and feasible approach. The literature on predicting disposition is very limited and has focused primarily on patients with stroke or joint replacement.13,14 Previously, we used the same measure of mobility within 24 hours of admission to show an association with discharge disposition.7 Here, we expanded upon that prior research to include mobility assessment within a 48-hour window from admission in a diverse patient population. Using a machine learning approach, we were able to predict 73% of hospital discharges correctly using only the patient’s mobility score and age. Having tools such as this simple decision tree to identify discharge locations early in a patient’s hospitalization has the potential to increase efficiency in the discharge planning process.
Despite being able to classify the discharge disposition correctly for most patients, our sensitivity for predicting postacute care need was low. There are likely other patient and system factors that could be collected near the time of hospital admission, such as the patient’s prior level of function, the difference between function at baseline and admission, their prior living situation (eg, long term care, home environment), social support, and hospital relationships with postacute care facilities that may help to improve the prediction of postacute care placement.15 We recommend that future research consider these and other potentially important predictors. However, the specificity was high enough that all patients who score positive merit evaluation for possible postacute care. While our patient sample was diverse, it did not focus on some patients who may be more likely to be discharged to a postacute facility, such as the geriatric population. This may be a potential limitation to our study and will require this tool to be tested in more patient groups. A final limitation is the grouping of all potential types of postacute care into one category since important differences exist between the care provided at skilled nursing facilities with or without rehabilitation and inpatient acute rehabilitation. Despite these limitations, this study emphasizes the value of a systematic mobility assessment and provides a simple decision tree to help providers begin early discharge planning by anticipating patient rehabilitation needs.
Acknowledgments
The authors thank Christina Lin, MD and Sophia Andrews, PT, DPT for their assistance with data validation.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
1. Greysen SR, Patel MS. Annals for hospitalists inpatient notes-bedrest is toxic—why mobility matters in the hospital. Ann Intern Med. 2018;169(2):HO2-HO3. https://doi.org/10.7326/M18-1427.
2. Greysen SR, Stijacic Cenzer I, Boscardin WJ, Covinsky KE. Functional impairment: an unmeasured marker of Medicare costs for postacute care of older adults. J Am Geriatr Soc. 2017;65(9):1996-2002. https://doi.org/10.1111/jgs.14955.
3. Wong EL, Yam CH, Cheung AW, et al. Barriers to effective discharge planning: a qualitative study investigating the perspectives of frontline healthcare professionals. BMC Health Serv Res. 2011;11(1):242. https://doi.org/10.1186/1472-6963-11-242.
4. Greysen HM, Greysen SR. Mobility assessment in the hospital: what are the “next steps”? J Hosp Med. 2017;12(6):477-478. https://doi.org/10.12788/jhm.2759.
5. Lord RK, Mayhew CR, Korupolu R, et al. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717-724. https://doi.org/10.1097/CCM.0b013e3182711de2.
6. McDonagh MS, Smith DH, Goddard M. Measuring appropriate use of acute beds: a systematic review of methods and results. Health Policy. 2000;53(3):157-184. https://doi.org/10.1016/S0168-8510(00)00092-0.
7. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2019;179(1):118-120. https://doi.org/10.1001/jamainternmed.2018.5145.
8. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x.
9. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110.
10. Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
11. Therneau T, Atkinson B. rpart: recursive partitioning and regression trees. R package version. 2018;4:1-13. https://CRAN.R-project.org/package=rpart.
12. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer; 2001.
13. Stein J, Bettger JP, Sicklick A, Hedeman R, Magdon-Ismail Z, Schwamm LH. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil. 2015;96(2):210-217. https://doi.org/10.1016/j.apmr.2014.07.403.
14. Gholson JJ, Pugely AJ, Bedard NA, Duchman KR, Anthony CA, Callaghan JJ. Can we predict discharge status after total joint arthroplasty? A calculator to predict home discharge. J Arthroplasty. 2016;31(12):2705-2709. https://doi.org/10.1016/j.arth.2016.08.010.
15. Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289. https://doi.org/10.1186/s12913-019-4101-6.
© 2019 Society of Hospital Medicine
Recurrent Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema Refractory to Fresh Frozen Plasma
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
Angioedema induced by angiotensin-converting enzyme inhibitors (ACEIs) is present in from 0.1% to 0.7% of treated patients and more often involves the head, neck, face, lips, tongue, and larynx.1 ACEI-induced angioedema results from inhibition of angiotensin-converting enzyme (ACE), which results in reduced degradation and resultant accumulation of bradykinin, a potent inflammatory mediator.2
The treatment of choice is discontinuing all ACEIs; however, the patient may be at increased risk of a subsequent angioedema attack for many weeks.3 Antihistamines (H1 and H2 receptor blockade), epinephrine, and glucocorticoids are effective in allergic/histaminergic angioedema but are usually ineffective for hereditary angioedema or ACEI angioedema and are not recommended for acute therapy.4 Kallikrein-bradykinin pathway targeted therapies are now approved by the Food and Drug Administration (FDA) for hereditary angioedema attacks and have been studied for ACEI-induced angioedema. Ecallantide and icatibant inhibit conversion of precursors to bradykinin. Multiple randomized trials of ecallantide have not shown any advantage over traditional therapies.5 On the other hand, icatibant has shown resolution of angioedema in several case reports and in a randomized trial.6 Icatibant for ACEI-induced angioedema continues to be off-label because the data are conflicting.
Case Presentation
A 67-year-old man presented with a medical history of arterial hypertension (diagnosed 17 years previously), hypercholesterolemia, type 2 diabetes mellitus, alcohol dependence, and obesity. His outpatient medications included simvastatin, aripiprazole, losartan/hydrochlorothiazide, and amlodipine. He was voluntarily admitted for inpatient detoxification. After evaluation by the internist, medication reconciliation was done, and the therapy was adjusted according to medication availability. He reported having no drug allergies, and the losartan was changed for lisinopril. About 24 hours after the first dose of lisinopril, the patient developed swelling of the lips. Antihistamine and IV steroids were administered, and the ACEI was discontinued. His baseline vital signs were temperature 98° F, heart rate 83 beats per minute, respiratory rate 19 breaths per minute, blood pressure 150/94, and oxygen saturation 98% by pulse oximeter.
During the night shift the patient’s symptoms worsened, developing difficulty swallowing and shortness of breath. He was transferred to the medicine intensive care unit (MICU), intubated, and placed on mechanical ventilation to protect his airway. Laryngoscopic examination was notable for edematous tongue, uvula, and larynx. Also, the patient had mild stridor. His laboratory test results showed normal levels of complement, tryptase, and C1 esterase. On the fourth day after admission to MICU (Figure 1), the patient extubated himself. At that time, he did not present stridor or respiratory distress and remained at the MICU for 24 hours for close monitoring.
Thirty-six hours after self-extubation the patient developed stridor and shortness of breath at the general medicine ward. In view of his clinical presentation of recurrent ACEI-induced angioedema, the Anesthesiology Service was consulted. Direct visualization of the airways showed edema of the epiglottis and vocal cords, requiring nasotracheal intubation. Two units of fresh frozen plasma (FFP) were administered. Complete resolution of angioedema took at least 72 hours even after the administration of FFP. As part of the ventilator-associated pneumonia prevention bundle, the patient continued with daily spontaneous breathing trials. On the fourth day, he was he was extubated after a cuff-leak test was positive and his rapid shallow breathing index was adequate.
The cuff-leak test is usually done to predict postextubation stridor. It consists of deflating the endotracheal tube cuff to verify if gas can pass around the tube. Absence of cuff leak is suggestive of airway edema, a risk factor for postextubation stridor and failure of extubation. For example, if the patient has an endotracheal tube that is too large in relation to the patient’s airway, the leak test can result in a false negative. In this case, fiber optic visualization of the airway can confirm the endotracheal tube occluding all the airway even with the cuff deflated and without evidence of swelling of the vocal cords. The rapid shallow breathing index is a ratio of respiratory rate over tidal volume in liters and is used to predict successful extubation. Values < 105 have a high sensitivity for successful extubation.
The patient remained under observation for 24 hours in the MICU and then was transferred to the general medicine ward. Unfortunately, 36 hours after, the patient had a new episode of angioedema requiring endotracheal intubation and placement on mechanical ventilation. This was his third episode of angioedema; he had a difficult airway classified as a Cormack-Lehane grade 3, requiring intubation with fiber-optic laryngoscope. In view of the recurrent events, a tracheostomy was done several days later. Figure 2 shows posttracheostomy X-ray with adequate position of the tracheostomy tube.
The patient was transferred to the Respiratory Care Unit and weaned off mechanical ventilation. He completed an intensive physical rehabilitation program and was discharged home. On discharge, he was followed by the Otorhinolaryngology Service and was decannulated about 5 months after. After tracheostomy decannulation, he developed asymptomatic stridor. A neck computer tomography scan revealed soft tissue thickening at the anterior and lateral aspects of the proximal tracheal likely representing granulation tissue/scarring. The findings were consistent with proximal tracheal stenosis sequelae of tracheostomy and intubation. In Figure 3, the upper portion of the curve represents the expiratory limb of the forced vital capacity and the lower portion represents inspiration. The flow-volume loop graph showed flattening of the inspiratory limb. There was a plateau in the inspiratory limb, suggestive of limitation of inspiratory flow as seen in variable extrathoracic lesions, such as glotticstricture, tumors, and vocal cord paralysis.7 The findings on the flow-volume loop were consistent with the subglottic stenosis identified by laryngoscopic examination. The patient was reluctant to undergo further interventions.
Discussion
The standard therapy for ACEI-inducedangioedema continues to be airway management and discontinuation of medication. However, life-threatening progression of symptoms have led to the use of off-label therapies, including FFP and bradykinin receptor antagonists, such as icatibant, which has been approved by the FDA for the treatment of hereditary angioedema. Icatibant is expensive and most hospitals do not have access to it. When considering the bradykinin pathway for therapy, FFP is commonly used. The cases described in the literature that have reported success with the use of FFP have used up to 2 units. There is no reported benefit of its use beyond 2 units. The initial randomized trials of icatibant for ACEI angioedema showed decreased time of resolution of angioedema.6 However, repeated trials showed conflicting results. At Veterans Affairs Caribbean Healthcare System, this medication was not available, and we decided to use FFP to improve the patient’s symptoms.
The administration of 2 units of FFP has been documented on case reports as a method to decrease the time of resolution of angioedema and the risk of recurrence. The mechanism of action thought to be involved includes the degradation of bradykinin by the enzyme ACE into inactive peptides and by supplying C1 inhibitor.8 No randomized clinical trial has investigated the use of FFP for the treatment of ACEI-induced angioedema. However, a retrospective cohort study report compared patients who presented with acute (nonhereditary) angioedema and airway compromise and received FFP with patients who were not treated with FFP.9 The study suggested a shorter ICU stay in the group treated with FFP, but the findings did not present statistical outcomes.
Nevertheless, our patient had recurrent ACEI-induced angioedema refractory to FFP. In addition to ACE or kininase II, FFP contains high-molecular weight-kininogen and kallikrein, the substrates that form bradykinin, which explained the mechanism of worsening angioedema.10 No randomized trials have investigated the use of FFP for the treatment of bradykinin-induced angioedema nor the appropriate dose.
Conclusion
In view of the emerging case reports of the effectiveness of FFP, this case of refractory angioedema raises concern for its true effectiveness and other possible factors involved in the mechanism of recurrence. Probably it would be unwise to conduct randomized studies in clinical situations such as the ones outlined. A collection of case series where FFP administration was done may be a more reasonable source of conclusions to be analyzed by a panel of experts.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.
1. Sánchez-Borges M, González-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010;2(3):195-198.
2. Kaplan AP. Angioedema. World Allergy Organ J. 2008;1(6):103-113.
3. Moellman JJ, Bernstein JA, Lindsell C, et al; American College of Allergy, Asthma & Immunology (ACAAI); Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469-484.
4. LoVerde D, Files DC, Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725-735.
5. van den Elzen M, Go MFLC, Knulst AC, Blankestijn MA, van Os-Medendorp H, Otten HG. Efficacy of treatment of non-hereditary angioedema. Clinic Rev Allerg Immunol. 2018;54(3):412-431.
6. Bas M, Greve J, Stelter S, et al. A randomized trial of icatibant in ace-inhibitor–induced angioedema. N Engl J Med. 2015;372(5):418-425.
7. Diaz J, Casal J, Rodriguez W. Flow-volume loops: clinical correlation. PR Health Sci J. 2008;27(2):181-182.
8. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep. 2012;2012:pii:bcr2012006849.
9. Saeb A, Hagglund KH, Cigolle CT. Using fresh frozen plasma for acute airway angioedema to prevent intubation in the emergency department: a retrospective cohort study. Emerg Med Int. 2016;2016:6091510.
10. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). 2017;19(12):1377-1382.