User login
Trends in COVID-19 Risk-Adjusted Mortality Rates
Early reports showed high mortality from coronavirus disease 2019 (COVID-19), while current United States data mortality rates are lower, raising hope that new treatments and management strategies have improved outcomes. For instance, Centers for Disease Control and Prevention data show that 6.7% of cases resulted in death in April, compared with 1.9% in September.1 However, the demographics of those infected have also changed, and more available testing may mean more comprehensive identification and earlier treatment. Nationally, for instance, the median age of confirmed cases was 38 years at the end of August, down from 46 years at the start of May.2 Therefore, whether decreasing COVID-19 mortality rates simply reflect changing demographics or represent actual improvements in clinical care is unknown. The objective of this analysis was to assess outcomes over time in a single health system, accounting for changes in demographics, clinical factors, and severity of disease at presentation.
METHODS
We analyzed monthly mortality rates for admissions between March 1 and August 31, 2020, in a single health system in New York City. Outcomes were obtained as of October 8, 2020. We included all hospitalizations of people 18 years and older with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified during the hospitalization or in the prior 2 weeks, excluding those admitted to hospice care. Patients with multiple hospitalizations (N=208 patients, 229 hospitalizations, 4.4%) were included repeatedly if they continued to have laboratory-confirmed disease. Patients without admission vital signs (N=28) were excluded. Mortality was defined as in-hospital death or discharge to hospice care. In-house laboratory testing began March 16 and all inpatients were tested for SARS-CoV-2 by April 1; elective surgeries resumed May 4-11 and were only conducted on confirmed SARS-CoV-2–negative patients.
All data were obtained from the electronic health record (Epic Systems, Verona, Wisconsin). Diagnosis codes were obtained from the problem list, past medical history, and billing codes. In addition, we used objective data such as hemoglobin A1c, ejection fraction, outpatient creatinine, and outpatient blood pressure to augment problem list diagnoses where relevant.
Based on prior literature, we constructed multivariable logistic regression models for mortality adjusting for age; sex; self-reported race and ethnicity; body mass index; smoking history; presence of hypertension, heart failure, hyperlipidemia, coronary artery disease, diabetes, cancer, chronic kidney disease, dementia, or pulmonary disease individually as dummy variables; and admission oxygen saturation, D-dimer, ferritin, and C-reactive protein.3-6 In the first model (C statistic 0.82), we did not include month of admission as a covariate and calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month to obtain the standardized mortality ratio (SMR) for each month. We then multiplied each period’s SMR by the overall average crude mortality to generate monthly adjusted mortality rates. We calculated Poisson control limits and indicated points outside the control limits as significantly different.
In a second model (C statistic 0.84), we included month as a covariate and calculated average marginal effects (AME) for each time period by using the margins library in R,7 which uses a discrete first-difference in predicted outcomes to obtain the AME. The average marginal effect represents the percentage point difference between the reference period (March) and a subsequent time period in probability of death or discharge to hospice, for equivalent patients. We obtained lower and upper confidence intervals for the AME using a bootstrapping approach described in Green.8 Finally, we conducted two sensitivity analyses: one, restricting the analysis to only those patients with principal diagnosis of COVID-19, sepsis, or respiratory disease (see Appendix A for complete list of codes) and one restricting the analysis to only those with length of stay of at least 3 days.
All statistical analyses were conducted with R, version 4.0.2. All analyses used 2-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The NYU institutional review board approved the study and granted a waiver of consent and a waiver of the Health Information Portability and Accountability Act.
RESULTS
We included 5,121 hospitalizations, of which 5,118 (99.94%) had known outcomes (death or hospital discharge). Peak hospitalizations occurred in late March to mid-April, which accounted for 53% of the hospitalizations. Median length of stay for patients who died or were discharged to hospice was 8 days (interquartile range, 4-15; max 140 days). The median age and the proportion male or with any comorbidity decreased over time (Table). For instance, the proportion with any chronic condition decreased from 81% in March to 72% in August.
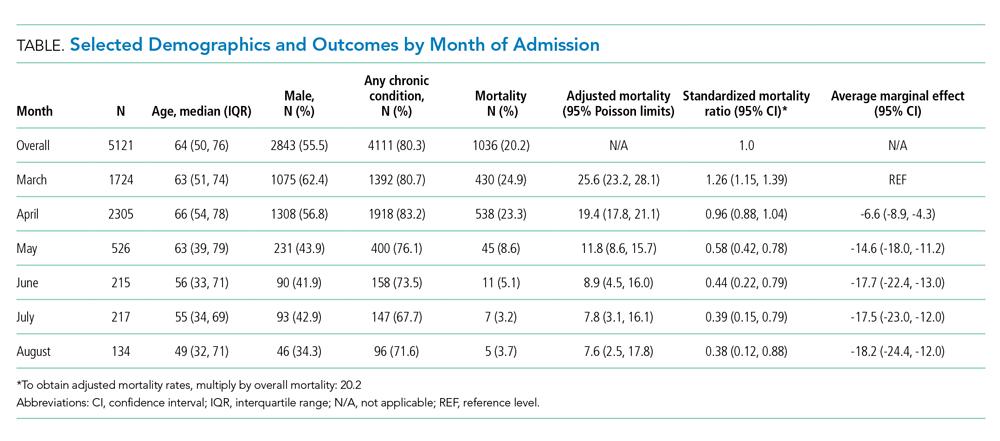
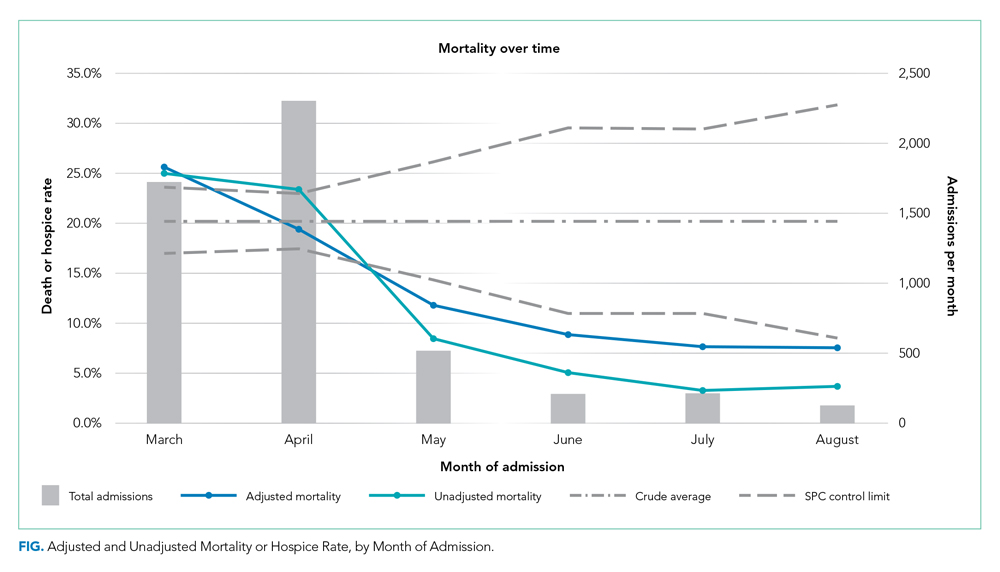
Adjusted mortality dropped each month, from 25.6% in March to 7.6% in August (Table and Figure). The SMR declined progressively over time, from 1.26 (95% CI, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August (Table). The adjusted average marginal effect was also significantly lower than in March in every subsequent month, reaching a maximum of an average 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August, accounting for changes in demographics and clinical severity (Table and Appendix B). The decrease in unadjusted mortality over time was observed across age groups (Appendix C).
Results of the two sensitivity analyses were similar (Appendices D and E), though attenuated in the case of the sepsis/respiratory cohort, with adjusted mortality falling from 31.4% to 14.4%, SMR decreasing from 1.28 (95% CI, 1.16-1.41) to 0.59 (95% CI, 0.16-1.50), and AME in August 17.0 percentage points (95% CI, 6.0-28.1).
DISCUSSION
In this study of COVID-19 mortality over 6 months at a single health system, we found that changes in demographics and severity of illness at presentation did not fully explain decreases in mortality seen over time. Even after risk adjustment for a variety of clinical and demographic factors, including severity of illness at presentation, mortality was significantly and progressively lower over the course of the study period.
Similar risk-adjusted results have been preliminarily reported among intensive care unit patients in a preprint from the United Kingdom.9 Incremental improvements in outcomes are likely a combination of increasing clinical experience, decreasing hospital volume, growing use of new pharmacologic treatments (such as systemic corticosteroids,10 remdesivir,11 and anticytokine treatments), nonpharmacologic treatments (such as placing the patient in the prone position, or proning, rather than on their back), earlier intervention, community awareness, and, potentially, lower viral load exposure from increased mask wearing and social distancing.12
Strengths of this study include highly detailed electronic health record data on hospitalizations at three different hospitals, a diverse patient population,6 near-complete study outcomes, and a lengthy period of investigation of 6 months. However, this study does have limitations. All patients were from a single geographic region and treated within a single health system, though restricting data to one system reduces institution-level variability and allows us to assess how care may have evolved with growing experience. Aggregating data from numerous health systems that might be at different stages of local outbreaks, provide different quality of care, and contribute different numbers of patients in each period introduces its own biases. We were also unable to disentangle different potential explanatory factors given the observational nature of the study. Residual confounding, such as a higher proportion of particularly frail patients admitted in earlier periods, is also a possibility, though the fact that we observed declines across all age groups mitigates this concern. Thresholds for hospital admission may also have changed over time with less severely ill patients being admitted in the later time periods. While changing admission thresholds could have contributed to higher survival rates in the latter portions of the study, our inclusion of several highly predictive clinical and laboratory results likely captured many aspects of disease severity.
CONCLUSION
In summary, data from one health system suggest that COVID-19 remains a serious disease for high-risk patients, but that mortality rates are improving over time.
1. CDC COVID Data Tracker. 2020. Centers for Disease Control and Prevention. Accessed October 14, 2020. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404-1409 http://dx.doi.org/0.15585/mmwr.mm6939e1
3. Lu L, Zhong W, Bian Z, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):318-e25. https://doi.org/10.1016/j.jinf.2020.07.002
4. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;Jun8:1-9. https://doi.org/10.1080/13685538.2020.1774748
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. https://doi.org/10.1016/j.jinf.2020.04.021
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966
7. margins: Marginal Effects for Model Objects [computer program]. Version R package version 0.3.232018. Accessed October 1, 2020. https://rdrr.io/cran/margins/
8. Greene WH. Econometric Analysis. 7th ed. Pearson; 2012.
9. Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints 2020. Preprint posted online August 11, 2020. https://doi.org/10.20944/preprints202008.0267.v1
10. Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Online first July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Enl J Med. 2020. Online first October 8, 2020. https://doi.org/10.1056/NEJMoa2007764
12. Gandhi M, Rutherford GW. Facial masking for Covid-19 - potential for “variolation” as we await a vaccine. N Engl J Med. 2020. Online first September 8, 2020. https://doi.org/10.1056/NEJMp2026913
Early reports showed high mortality from coronavirus disease 2019 (COVID-19), while current United States data mortality rates are lower, raising hope that new treatments and management strategies have improved outcomes. For instance, Centers for Disease Control and Prevention data show that 6.7% of cases resulted in death in April, compared with 1.9% in September.1 However, the demographics of those infected have also changed, and more available testing may mean more comprehensive identification and earlier treatment. Nationally, for instance, the median age of confirmed cases was 38 years at the end of August, down from 46 years at the start of May.2 Therefore, whether decreasing COVID-19 mortality rates simply reflect changing demographics or represent actual improvements in clinical care is unknown. The objective of this analysis was to assess outcomes over time in a single health system, accounting for changes in demographics, clinical factors, and severity of disease at presentation.
METHODS
We analyzed monthly mortality rates for admissions between March 1 and August 31, 2020, in a single health system in New York City. Outcomes were obtained as of October 8, 2020. We included all hospitalizations of people 18 years and older with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified during the hospitalization or in the prior 2 weeks, excluding those admitted to hospice care. Patients with multiple hospitalizations (N=208 patients, 229 hospitalizations, 4.4%) were included repeatedly if they continued to have laboratory-confirmed disease. Patients without admission vital signs (N=28) were excluded. Mortality was defined as in-hospital death or discharge to hospice care. In-house laboratory testing began March 16 and all inpatients were tested for SARS-CoV-2 by April 1; elective surgeries resumed May 4-11 and were only conducted on confirmed SARS-CoV-2–negative patients.
All data were obtained from the electronic health record (Epic Systems, Verona, Wisconsin). Diagnosis codes were obtained from the problem list, past medical history, and billing codes. In addition, we used objective data such as hemoglobin A1c, ejection fraction, outpatient creatinine, and outpatient blood pressure to augment problem list diagnoses where relevant.
Based on prior literature, we constructed multivariable logistic regression models for mortality adjusting for age; sex; self-reported race and ethnicity; body mass index; smoking history; presence of hypertension, heart failure, hyperlipidemia, coronary artery disease, diabetes, cancer, chronic kidney disease, dementia, or pulmonary disease individually as dummy variables; and admission oxygen saturation, D-dimer, ferritin, and C-reactive protein.3-6 In the first model (C statistic 0.82), we did not include month of admission as a covariate and calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month to obtain the standardized mortality ratio (SMR) for each month. We then multiplied each period’s SMR by the overall average crude mortality to generate monthly adjusted mortality rates. We calculated Poisson control limits and indicated points outside the control limits as significantly different.
In a second model (C statistic 0.84), we included month as a covariate and calculated average marginal effects (AME) for each time period by using the margins library in R,7 which uses a discrete first-difference in predicted outcomes to obtain the AME. The average marginal effect represents the percentage point difference between the reference period (March) and a subsequent time period in probability of death or discharge to hospice, for equivalent patients. We obtained lower and upper confidence intervals for the AME using a bootstrapping approach described in Green.8 Finally, we conducted two sensitivity analyses: one, restricting the analysis to only those patients with principal diagnosis of COVID-19, sepsis, or respiratory disease (see Appendix A for complete list of codes) and one restricting the analysis to only those with length of stay of at least 3 days.
All statistical analyses were conducted with R, version 4.0.2. All analyses used 2-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The NYU institutional review board approved the study and granted a waiver of consent and a waiver of the Health Information Portability and Accountability Act.
RESULTS
We included 5,121 hospitalizations, of which 5,118 (99.94%) had known outcomes (death or hospital discharge). Peak hospitalizations occurred in late March to mid-April, which accounted for 53% of the hospitalizations. Median length of stay for patients who died or were discharged to hospice was 8 days (interquartile range, 4-15; max 140 days). The median age and the proportion male or with any comorbidity decreased over time (Table). For instance, the proportion with any chronic condition decreased from 81% in March to 72% in August.

Adjusted mortality dropped each month, from 25.6% in March to 7.6% in August (Table and Figure). The SMR declined progressively over time, from 1.26 (95% CI, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August (Table). The adjusted average marginal effect was also significantly lower than in March in every subsequent month, reaching a maximum of an average 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August, accounting for changes in demographics and clinical severity (Table and Appendix B). The decrease in unadjusted mortality over time was observed across age groups (Appendix C).

Results of the two sensitivity analyses were similar (Appendices D and E), though attenuated in the case of the sepsis/respiratory cohort, with adjusted mortality falling from 31.4% to 14.4%, SMR decreasing from 1.28 (95% CI, 1.16-1.41) to 0.59 (95% CI, 0.16-1.50), and AME in August 17.0 percentage points (95% CI, 6.0-28.1).
DISCUSSION
In this study of COVID-19 mortality over 6 months at a single health system, we found that changes in demographics and severity of illness at presentation did not fully explain decreases in mortality seen over time. Even after risk adjustment for a variety of clinical and demographic factors, including severity of illness at presentation, mortality was significantly and progressively lower over the course of the study period.
Similar risk-adjusted results have been preliminarily reported among intensive care unit patients in a preprint from the United Kingdom.9 Incremental improvements in outcomes are likely a combination of increasing clinical experience, decreasing hospital volume, growing use of new pharmacologic treatments (such as systemic corticosteroids,10 remdesivir,11 and anticytokine treatments), nonpharmacologic treatments (such as placing the patient in the prone position, or proning, rather than on their back), earlier intervention, community awareness, and, potentially, lower viral load exposure from increased mask wearing and social distancing.12
Strengths of this study include highly detailed electronic health record data on hospitalizations at three different hospitals, a diverse patient population,6 near-complete study outcomes, and a lengthy period of investigation of 6 months. However, this study does have limitations. All patients were from a single geographic region and treated within a single health system, though restricting data to one system reduces institution-level variability and allows us to assess how care may have evolved with growing experience. Aggregating data from numerous health systems that might be at different stages of local outbreaks, provide different quality of care, and contribute different numbers of patients in each period introduces its own biases. We were also unable to disentangle different potential explanatory factors given the observational nature of the study. Residual confounding, such as a higher proportion of particularly frail patients admitted in earlier periods, is also a possibility, though the fact that we observed declines across all age groups mitigates this concern. Thresholds for hospital admission may also have changed over time with less severely ill patients being admitted in the later time periods. While changing admission thresholds could have contributed to higher survival rates in the latter portions of the study, our inclusion of several highly predictive clinical and laboratory results likely captured many aspects of disease severity.
CONCLUSION
In summary, data from one health system suggest that COVID-19 remains a serious disease for high-risk patients, but that mortality rates are improving over time.
Early reports showed high mortality from coronavirus disease 2019 (COVID-19), while current United States data mortality rates are lower, raising hope that new treatments and management strategies have improved outcomes. For instance, Centers for Disease Control and Prevention data show that 6.7% of cases resulted in death in April, compared with 1.9% in September.1 However, the demographics of those infected have also changed, and more available testing may mean more comprehensive identification and earlier treatment. Nationally, for instance, the median age of confirmed cases was 38 years at the end of August, down from 46 years at the start of May.2 Therefore, whether decreasing COVID-19 mortality rates simply reflect changing demographics or represent actual improvements in clinical care is unknown. The objective of this analysis was to assess outcomes over time in a single health system, accounting for changes in demographics, clinical factors, and severity of disease at presentation.
METHODS
We analyzed monthly mortality rates for admissions between March 1 and August 31, 2020, in a single health system in New York City. Outcomes were obtained as of October 8, 2020. We included all hospitalizations of people 18 years and older with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified during the hospitalization or in the prior 2 weeks, excluding those admitted to hospice care. Patients with multiple hospitalizations (N=208 patients, 229 hospitalizations, 4.4%) were included repeatedly if they continued to have laboratory-confirmed disease. Patients without admission vital signs (N=28) were excluded. Mortality was defined as in-hospital death or discharge to hospice care. In-house laboratory testing began March 16 and all inpatients were tested for SARS-CoV-2 by April 1; elective surgeries resumed May 4-11 and were only conducted on confirmed SARS-CoV-2–negative patients.
All data were obtained from the electronic health record (Epic Systems, Verona, Wisconsin). Diagnosis codes were obtained from the problem list, past medical history, and billing codes. In addition, we used objective data such as hemoglobin A1c, ejection fraction, outpatient creatinine, and outpatient blood pressure to augment problem list diagnoses where relevant.
Based on prior literature, we constructed multivariable logistic regression models for mortality adjusting for age; sex; self-reported race and ethnicity; body mass index; smoking history; presence of hypertension, heart failure, hyperlipidemia, coronary artery disease, diabetes, cancer, chronic kidney disease, dementia, or pulmonary disease individually as dummy variables; and admission oxygen saturation, D-dimer, ferritin, and C-reactive protein.3-6 In the first model (C statistic 0.82), we did not include month of admission as a covariate and calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month to obtain the standardized mortality ratio (SMR) for each month. We then multiplied each period’s SMR by the overall average crude mortality to generate monthly adjusted mortality rates. We calculated Poisson control limits and indicated points outside the control limits as significantly different.
In a second model (C statistic 0.84), we included month as a covariate and calculated average marginal effects (AME) for each time period by using the margins library in R,7 which uses a discrete first-difference in predicted outcomes to obtain the AME. The average marginal effect represents the percentage point difference between the reference period (March) and a subsequent time period in probability of death or discharge to hospice, for equivalent patients. We obtained lower and upper confidence intervals for the AME using a bootstrapping approach described in Green.8 Finally, we conducted two sensitivity analyses: one, restricting the analysis to only those patients with principal diagnosis of COVID-19, sepsis, or respiratory disease (see Appendix A for complete list of codes) and one restricting the analysis to only those with length of stay of at least 3 days.
All statistical analyses were conducted with R, version 4.0.2. All analyses used 2-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The NYU institutional review board approved the study and granted a waiver of consent and a waiver of the Health Information Portability and Accountability Act.
RESULTS
We included 5,121 hospitalizations, of which 5,118 (99.94%) had known outcomes (death or hospital discharge). Peak hospitalizations occurred in late March to mid-April, which accounted for 53% of the hospitalizations. Median length of stay for patients who died or were discharged to hospice was 8 days (interquartile range, 4-15; max 140 days). The median age and the proportion male or with any comorbidity decreased over time (Table). For instance, the proportion with any chronic condition decreased from 81% in March to 72% in August.

Adjusted mortality dropped each month, from 25.6% in March to 7.6% in August (Table and Figure). The SMR declined progressively over time, from 1.26 (95% CI, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August (Table). The adjusted average marginal effect was also significantly lower than in March in every subsequent month, reaching a maximum of an average 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August, accounting for changes in demographics and clinical severity (Table and Appendix B). The decrease in unadjusted mortality over time was observed across age groups (Appendix C).

Results of the two sensitivity analyses were similar (Appendices D and E), though attenuated in the case of the sepsis/respiratory cohort, with adjusted mortality falling from 31.4% to 14.4%, SMR decreasing from 1.28 (95% CI, 1.16-1.41) to 0.59 (95% CI, 0.16-1.50), and AME in August 17.0 percentage points (95% CI, 6.0-28.1).
DISCUSSION
In this study of COVID-19 mortality over 6 months at a single health system, we found that changes in demographics and severity of illness at presentation did not fully explain decreases in mortality seen over time. Even after risk adjustment for a variety of clinical and demographic factors, including severity of illness at presentation, mortality was significantly and progressively lower over the course of the study period.
Similar risk-adjusted results have been preliminarily reported among intensive care unit patients in a preprint from the United Kingdom.9 Incremental improvements in outcomes are likely a combination of increasing clinical experience, decreasing hospital volume, growing use of new pharmacologic treatments (such as systemic corticosteroids,10 remdesivir,11 and anticytokine treatments), nonpharmacologic treatments (such as placing the patient in the prone position, or proning, rather than on their back), earlier intervention, community awareness, and, potentially, lower viral load exposure from increased mask wearing and social distancing.12
Strengths of this study include highly detailed electronic health record data on hospitalizations at three different hospitals, a diverse patient population,6 near-complete study outcomes, and a lengthy period of investigation of 6 months. However, this study does have limitations. All patients were from a single geographic region and treated within a single health system, though restricting data to one system reduces institution-level variability and allows us to assess how care may have evolved with growing experience. Aggregating data from numerous health systems that might be at different stages of local outbreaks, provide different quality of care, and contribute different numbers of patients in each period introduces its own biases. We were also unable to disentangle different potential explanatory factors given the observational nature of the study. Residual confounding, such as a higher proportion of particularly frail patients admitted in earlier periods, is also a possibility, though the fact that we observed declines across all age groups mitigates this concern. Thresholds for hospital admission may also have changed over time with less severely ill patients being admitted in the later time periods. While changing admission thresholds could have contributed to higher survival rates in the latter portions of the study, our inclusion of several highly predictive clinical and laboratory results likely captured many aspects of disease severity.
CONCLUSION
In summary, data from one health system suggest that COVID-19 remains a serious disease for high-risk patients, but that mortality rates are improving over time.
1. CDC COVID Data Tracker. 2020. Centers for Disease Control and Prevention. Accessed October 14, 2020. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404-1409 http://dx.doi.org/0.15585/mmwr.mm6939e1
3. Lu L, Zhong W, Bian Z, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):318-e25. https://doi.org/10.1016/j.jinf.2020.07.002
4. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;Jun8:1-9. https://doi.org/10.1080/13685538.2020.1774748
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. https://doi.org/10.1016/j.jinf.2020.04.021
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966
7. margins: Marginal Effects for Model Objects [computer program]. Version R package version 0.3.232018. Accessed October 1, 2020. https://rdrr.io/cran/margins/
8. Greene WH. Econometric Analysis. 7th ed. Pearson; 2012.
9. Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints 2020. Preprint posted online August 11, 2020. https://doi.org/10.20944/preprints202008.0267.v1
10. Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Online first July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Enl J Med. 2020. Online first October 8, 2020. https://doi.org/10.1056/NEJMoa2007764
12. Gandhi M, Rutherford GW. Facial masking for Covid-19 - potential for “variolation” as we await a vaccine. N Engl J Med. 2020. Online first September 8, 2020. https://doi.org/10.1056/NEJMp2026913
1. CDC COVID Data Tracker. 2020. Centers for Disease Control and Prevention. Accessed October 14, 2020. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404-1409 http://dx.doi.org/0.15585/mmwr.mm6939e1
3. Lu L, Zhong W, Bian Z, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):318-e25. https://doi.org/10.1016/j.jinf.2020.07.002
4. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;Jun8:1-9. https://doi.org/10.1080/13685538.2020.1774748
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. https://doi.org/10.1016/j.jinf.2020.04.021
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966
7. margins: Marginal Effects for Model Objects [computer program]. Version R package version 0.3.232018. Accessed October 1, 2020. https://rdrr.io/cran/margins/
8. Greene WH. Econometric Analysis. 7th ed. Pearson; 2012.
9. Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints 2020. Preprint posted online August 11, 2020. https://doi.org/10.20944/preprints202008.0267.v1
10. Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Online first July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Enl J Med. 2020. Online first October 8, 2020. https://doi.org/10.1056/NEJMoa2007764
12. Gandhi M, Rutherford GW. Facial masking for Covid-19 - potential for “variolation” as we await a vaccine. N Engl J Med. 2020. Online first September 8, 2020. https://doi.org/10.1056/NEJMp2026913
© 2020 Society of Hospital Medicine
Clinical Progress Note: Decision-making for Tracheostomy Placement in Children With Neurological Impairment
Children with complex medical conditions are living longer, many with the help of interventions and technology, such as gastrostomy tubes, tracheostomies, ventilator support, and parenteral nutrition. Children with medical complexity and technology account for over 80% of hospital days in pediatric academic centers.1
Hospitalists need communication skills and clinical information to guide discussions with patients and families about whether to pursue these measures. Tracheostomy discussions can be particularly challenging. Over 4,000 infants and children undergo tracheostomy each year, with related hospital charges of more than $2 billion, a 30-day readmission rate of 24.9%, and a median length of stay for pneumonia or tracheitis of 4 days.2 There is limited research on prognosis, outcomes, decision-making, and effects on quality of life, especially in the population of children who have significant neurological impairment (NI) and/or progressive or deteriorating neurological conditions. Physician biases may also influence this discussion.
This article will examine the question: How can a hospitalist guide decision-making discussions with families about tracheostomy placement for children with NI? A literature search was performed on Medline and Web of Science using the key terms tracheostomy, prognosis, neurologically impaired children, and decision-making. Articles included were relevant to the clinical question and published in the last 5 years. One article was included outside this timeframe given the scarcity of data.
INDICATIONS FOR TRACHEOSTOMY
Indications for tracheostomy include airway obstruction and the need for prolonged ventilation support.3 The number of tracheostomies placed has been increasing over the last 30 years, especially at tertiary care centers.3 Primary indications for tracheostomy include prolonged ventilation particularly in the context of underlying conditions such as congenital or acquired respiratory disease, congenital or acquired neurologic disease, cardiopulmonary disease, and primary anatomic airway obstruction.3,4 Children who undergo tracheostomy often have multiple medical conditions that impact their overall health and prognosis, with 41% having three or more complex chronic health conditions.5 This article will focus on children who have a primary indication of NI and in whom tracheostomy is often used as a life-prolonging measure.
PROGNOSIS
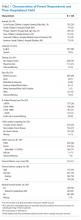
Discussions about tracheostomy should include information about risks, benefits, and prognosis. Prognosis discussions can be challenging given that many children for whom this intervention is being considered have multiple and complex medical conditions with uncertain or even known poor prognoses. Mortality rates ranging from 3% to 11% have been reported during the initial tracheostomy admission, with NI increasing the risk for mortality during the tracheostomy admission.5,6 Children with NI also have higher mortality beyond the initial hospital stay, lower decannulation rates, and more frequent admissions with longer lengths of stay than do children receiving a tracheostomy for upper airway obstruction (Table 1).6,7
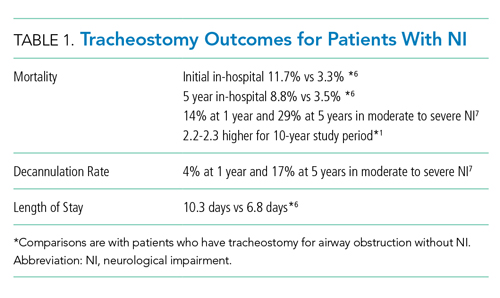
For most children in this population, prognosis is related more to the underlying disease process than to the risk of the surgery for tracheostomy placement itself. Discussions with families should include the anticipated prognosis of the underlying disease, as well as current available data on outcomes for children with neurological impairment who have undergone tracheostomy placement. Most patients who receive a tracheostomy are children with complex medical conditions who have an acute event that leads to airway compromise and respiratory failure underscoring the importance of advance care planning.5
GOALS OF CARE DISCUSSIONS
Clinicians face challenges when initiating advance care planning discussions, including prognostic uncertainty, the perception that families may not want to engage in these discussions, and the complexity and time these discussions can take. In one study of more than 300 chronically ill children, only 17% of parents had discussed advance directives, although 49% reported they would like to create one for their child.9 A small study found that, although parents find these discussions difficult, they also find them important. They value a step by step approach with consideration for hope and nonmedical concerns.10 Advance care planning discussions should be viewed as a time out to clarify what the family sees as the best path forward before initiation of a tracheostomy discussion and decision.
Determining goals of care is a cornerstone of any discussion about tracheostomy placement, especially when a child has a condition that is life limiting. The decision to pursue tracheostomy should involve shared decision-making. This decision-making process is the preferred communication model when multiple options could be pursued, each with its own risks and benefits.10
In this model of decision-making, the family’s goals and values should be determined in the context of the medical intervention that is being pursued. Medical information such as prognosis, risk, benefits, and impact of the intervention on quality of life should all be shared with the family. Ideally, shared decision-making allows the practitioner and family to make a decision together that matches the family’s goals and values with the best option available. If the family’s goal is to prolong life and they feel their child has good quality of life, tracheostomy placement may be the most appropriate option. However, it is also possible that the family’s goals may align more with less invasive treatment options or a transition to comfort care.
Discussions regarding goals of care can be challenging, and involving an interdisciplinary team and a Palliative Care consultant can be helpful.
WHAT PROVIDERS SAY, WHAT FAMILIES NEED TO HEAR
Research on what parents find helpful in discussions about tracheostomy is limited. One study of 56 caregivers found that parents did not feel they could make a “free choice” because the alternative to tracheostomy was death.11 In interviews with caregivers following tracheostomy, this same study found several themes in caregiver perspectives on their decision for tracheostomy (Table 2); caregivers saw a benefit to “health and well-being” from tracheostomy even though they reported feeling unprepared for the caregiving aspect at home or the potential negative side effects. Half the children in this study had a neurologic diagnosis, and only families who chose tracheostomy placement were included. To this author’s knowledge, there are currently no studies that look at decisional themes, satisfaction, or outcomes for families that chose to not pursue tracheostomy.
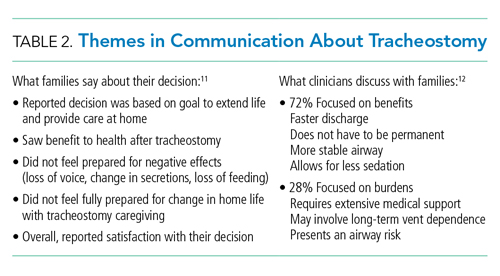
There is limited literature about how providers discuss tracheostomy. One single-center study of practitioners found that providers focused more often on the benefits of tracheostomy rather than burdens (72% vs 28%).12 A common benefit theme was the provider “suggesting life with a tracheostomy might not be as difficult as families fear in that the child may have the ability to regain speech, engage in normal activities, and have the tracheostomy reversed once the child’s health improved.” However, decannulation rates and recovery trajectories for children with NI do not support this general expectation (Table 1). These provider communication themes may help to explain the family’s perspective that they feel unprepared for the burdens of a tracheostomy or the intensity of home caregiving. Given the limited data, it is difficult to generalize. Comparing communication and decision-making themes side by side does draw attention to how providers might better communicate with families about this decision (Table 2).
The difficult aspects of caregiving deserve special attention. A study of 25 parents showed reduced parental quality of life after their child’s tracheostomy placement related to overwhelming medical care, fear of death of the child requiring constant vigilance, and financial and psychological stressors.13 Most (72%) families in this study reported decisional regret at 3 months.Resources and support for a child with this level of care varies based on the child’s community. Exploration and discussion of what is available for each family, including home nursing, respite, and/or a skilled nursing facility, should be completed prior to tracheostomy placement. Honest discussions about the potential effects of this intervention on the family’s life can help inform their decision.
Decision-making tools for tracheostomy could be valuable for both families and clinicians. These tools allow for a more systematic approach to the decision-making process that takes into account the multidimensional aspects of this decision. The “Child Tracheostomy Decision Guide,” published by the Winnipeg Regional Health Authority, is one available tool.14 This tool guides families through the factors that may affect their decision-making and includes thoughts about goals of care, quality of life, prognosis, care at home, and other options such as comfort care. The Courageous Parents Network has also developed parent videos giving the perspective of parents who have chosen or not chosen tracheostomy.15 Currently, there are no studies that examine the usefulness of decision-making tools.
GAPS IN LITERATURE
A common theme throughout the literature is the lack of a unifying classification system for reporting outcomes data. Each study utilizes different primary indications for tracheostomy and often different definitions for NI. There is very little literature that focuses specifically on outcomes for children with NI who receive tracheostomy as a life-prolonging measure. These gaps present challenges for obtaining meaningful prognosis data to share with families. Outcomes data for children who do not receive tracheostomy is also lacking. Additional studies on how families make this decision and their decisional satisfaction could help inform the decision-making process for both parents and clinicians. Research regarding the helpfulness and outcomes with decision-making tools would be useful.
CONCLUSIONS
Although there are limited data on outcomes specific to the children with NI and tracheostomy, existing literature shows a higher mortality, lower decannulation rate, higher hospitalization rate, and longer length of stay than that for children who receive tracheostomy for other indications. Tracheostomy is often a life-prolonging measure for children with NI. Shared decision-making should be the preferred communication process and include defining goals of care, as well as anticipated prognosis with balanced information about risks and benefits. Further research about the decision-making process and communication would be beneficial.
DISCLOSURE
Dr Shaw has nothing to disclose.
1. Children’s Hospital Association. Spend for children with dominant chronic diseases – The CARE award. Historical spending: 2012-2014. https://www.childrenshospitals.org/Care/Children-With-Medical-Complexity 2018
2. Russel CJ, Mack WJ, Schrager SM, Wu S. Care variations, length of stay and readmissions in children hospitalized for bacterial tracheostomy-associated respiratory infections. Hosp Pediatr. 2017;7(1):16-23. https://doi.org/10.1542/hpeds.2016-0104
3. McPherson ML, Shekerdemian L, Goldsworthy M, et al. A decade of pediatric tracheostomies: indications, outcomes, and long-term prognosis. Pediatr Pulmonol. 2017;52(7):946-953. https://doi.org/10.1002/ppul.23657
4. Gergin O, Adil EA, Kawai K, Watters K, Moritz E, Rahbar R. Indications of pediatric tracheostomy over the last 30 years: has anything changed? Int J Pediatr Otorhinolaryngol. 2016;87:144-147. https://doi.org/10.1016/j.ijporl.2016.06.018
5. Edwards J, Houtrow A, Lucas A, et al. Children and young adults who receive tracheostomies or were initiated on long-term ventilation in PICUs. Pediatr Crit Care Med. 2016;17(8):e324-334. https://doi.org/10.1097/pcc.0000000000000844
6. Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563-572. https://doi.org/10.1542/peds.2008-3491
7. Tsuboi N, Ide K, Nishimura N, Nakagawa S, Morimoto N. Pediatric tracheostomy: survival and long-term outcomes. Int J Pediatr Otorhinolaryngol. 2016;89:81-85. https://doi.org/10.1016/j.ijporl.2016.07.033
8. Liberman DB, Pham PK, Nager AL. Pediatric advance directives: parents’ knowledge, experience, and preferences. Pediatrics. 2014;134(2):e436-e443. https://doi.org/10.1542/peds.2013-3124
9. Lotz JD, Daxer M, Jox RJ, Borasio GD, Führer M. “Hope for the best, prepare for the worst”: a qualitative interview study on parents’ needs and fears in pediatric advance care planning. Palliat Med. 2017;31(8):764-771. https://doi.org/10.1177/0269216316679913
10. Nelson KE, Mahant S. Shared decision-making about assistive technology for the child with severe neurologic impairment. Pediatr Clin North Am. 2014;61(4):641-652. https://doi.org/10.1016/j.pcl.2014.04.001
11. Nageswaran S, Golden SL, Gower WA, King NMP. Caregiver perceptions about their decision to pursue tracheostomy for children with medical complexity. J Pediatr. 2018;203:354-360.e1. https://doi.org/10.1016/j.jpeds.2018.07.045
12. Hebert LM, Watson AC, Madrigal V, October TW. Discussing benefits and risks of tracheostomy: what physicians actually say. Pediatr Crit Care Med. 2017;18(12):e592-e597. https://doi.org/10.1097/PCC.0000000000001341
13. October T, Jones A, Michals H, Hebert L, Jiang J, Wang J. Parental conflict, regret, and short-term impact on quality of life in tracheostomy decision making. Pediatr Crit Care Med. 2020;21(2):136-142. https://doi.org/10.1097/PCC.0000000000002109
14. Winnipeg Regional Health Authority. Childhood Tracheostomy Decision Guide. Accessed August 15, 2019. https://www.wrha.mb.ca/extranet/eipt/files/EIPT-023-001.pdf
15. Courageous Parents Network. Tracheostomy Decision Making Videos. Accessed August 20, 2019. https://courageousparentsnetwork.org/video-library/decision-making/tracheostomy
Children with complex medical conditions are living longer, many with the help of interventions and technology, such as gastrostomy tubes, tracheostomies, ventilator support, and parenteral nutrition. Children with medical complexity and technology account for over 80% of hospital days in pediatric academic centers.1
Hospitalists need communication skills and clinical information to guide discussions with patients and families about whether to pursue these measures. Tracheostomy discussions can be particularly challenging. Over 4,000 infants and children undergo tracheostomy each year, with related hospital charges of more than $2 billion, a 30-day readmission rate of 24.9%, and a median length of stay for pneumonia or tracheitis of 4 days.2 There is limited research on prognosis, outcomes, decision-making, and effects on quality of life, especially in the population of children who have significant neurological impairment (NI) and/or progressive or deteriorating neurological conditions. Physician biases may also influence this discussion.
This article will examine the question: How can a hospitalist guide decision-making discussions with families about tracheostomy placement for children with NI? A literature search was performed on Medline and Web of Science using the key terms tracheostomy, prognosis, neurologically impaired children, and decision-making. Articles included were relevant to the clinical question and published in the last 5 years. One article was included outside this timeframe given the scarcity of data.
INDICATIONS FOR TRACHEOSTOMY
Indications for tracheostomy include airway obstruction and the need for prolonged ventilation support.3 The number of tracheostomies placed has been increasing over the last 30 years, especially at tertiary care centers.3 Primary indications for tracheostomy include prolonged ventilation particularly in the context of underlying conditions such as congenital or acquired respiratory disease, congenital or acquired neurologic disease, cardiopulmonary disease, and primary anatomic airway obstruction.3,4 Children who undergo tracheostomy often have multiple medical conditions that impact their overall health and prognosis, with 41% having three or more complex chronic health conditions.5 This article will focus on children who have a primary indication of NI and in whom tracheostomy is often used as a life-prolonging measure.
PROGNOSIS
Discussions about tracheostomy should include information about risks, benefits, and prognosis. Prognosis discussions can be challenging given that many children for whom this intervention is being considered have multiple and complex medical conditions with uncertain or even known poor prognoses. Mortality rates ranging from 3% to 11% have been reported during the initial tracheostomy admission, with NI increasing the risk for mortality during the tracheostomy admission.5,6 Children with NI also have higher mortality beyond the initial hospital stay, lower decannulation rates, and more frequent admissions with longer lengths of stay than do children receiving a tracheostomy for upper airway obstruction (Table 1).6,7

For most children in this population, prognosis is related more to the underlying disease process than to the risk of the surgery for tracheostomy placement itself. Discussions with families should include the anticipated prognosis of the underlying disease, as well as current available data on outcomes for children with neurological impairment who have undergone tracheostomy placement. Most patients who receive a tracheostomy are children with complex medical conditions who have an acute event that leads to airway compromise and respiratory failure underscoring the importance of advance care planning.5
GOALS OF CARE DISCUSSIONS
Clinicians face challenges when initiating advance care planning discussions, including prognostic uncertainty, the perception that families may not want to engage in these discussions, and the complexity and time these discussions can take. In one study of more than 300 chronically ill children, only 17% of parents had discussed advance directives, although 49% reported they would like to create one for their child.9 A small study found that, although parents find these discussions difficult, they also find them important. They value a step by step approach with consideration for hope and nonmedical concerns.10 Advance care planning discussions should be viewed as a time out to clarify what the family sees as the best path forward before initiation of a tracheostomy discussion and decision.
Determining goals of care is a cornerstone of any discussion about tracheostomy placement, especially when a child has a condition that is life limiting. The decision to pursue tracheostomy should involve shared decision-making. This decision-making process is the preferred communication model when multiple options could be pursued, each with its own risks and benefits.10
In this model of decision-making, the family’s goals and values should be determined in the context of the medical intervention that is being pursued. Medical information such as prognosis, risk, benefits, and impact of the intervention on quality of life should all be shared with the family. Ideally, shared decision-making allows the practitioner and family to make a decision together that matches the family’s goals and values with the best option available. If the family’s goal is to prolong life and they feel their child has good quality of life, tracheostomy placement may be the most appropriate option. However, it is also possible that the family’s goals may align more with less invasive treatment options or a transition to comfort care.
Discussions regarding goals of care can be challenging, and involving an interdisciplinary team and a Palliative Care consultant can be helpful.
WHAT PROVIDERS SAY, WHAT FAMILIES NEED TO HEAR
Research on what parents find helpful in discussions about tracheostomy is limited. One study of 56 caregivers found that parents did not feel they could make a “free choice” because the alternative to tracheostomy was death.11 In interviews with caregivers following tracheostomy, this same study found several themes in caregiver perspectives on their decision for tracheostomy (Table 2); caregivers saw a benefit to “health and well-being” from tracheostomy even though they reported feeling unprepared for the caregiving aspect at home or the potential negative side effects. Half the children in this study had a neurologic diagnosis, and only families who chose tracheostomy placement were included. To this author’s knowledge, there are currently no studies that look at decisional themes, satisfaction, or outcomes for families that chose to not pursue tracheostomy.

There is limited literature about how providers discuss tracheostomy. One single-center study of practitioners found that providers focused more often on the benefits of tracheostomy rather than burdens (72% vs 28%).12 A common benefit theme was the provider “suggesting life with a tracheostomy might not be as difficult as families fear in that the child may have the ability to regain speech, engage in normal activities, and have the tracheostomy reversed once the child’s health improved.” However, decannulation rates and recovery trajectories for children with NI do not support this general expectation (Table 1). These provider communication themes may help to explain the family’s perspective that they feel unprepared for the burdens of a tracheostomy or the intensity of home caregiving. Given the limited data, it is difficult to generalize. Comparing communication and decision-making themes side by side does draw attention to how providers might better communicate with families about this decision (Table 2).
The difficult aspects of caregiving deserve special attention. A study of 25 parents showed reduced parental quality of life after their child’s tracheostomy placement related to overwhelming medical care, fear of death of the child requiring constant vigilance, and financial and psychological stressors.13 Most (72%) families in this study reported decisional regret at 3 months.Resources and support for a child with this level of care varies based on the child’s community. Exploration and discussion of what is available for each family, including home nursing, respite, and/or a skilled nursing facility, should be completed prior to tracheostomy placement. Honest discussions about the potential effects of this intervention on the family’s life can help inform their decision.
Decision-making tools for tracheostomy could be valuable for both families and clinicians. These tools allow for a more systematic approach to the decision-making process that takes into account the multidimensional aspects of this decision. The “Child Tracheostomy Decision Guide,” published by the Winnipeg Regional Health Authority, is one available tool.14 This tool guides families through the factors that may affect their decision-making and includes thoughts about goals of care, quality of life, prognosis, care at home, and other options such as comfort care. The Courageous Parents Network has also developed parent videos giving the perspective of parents who have chosen or not chosen tracheostomy.15 Currently, there are no studies that examine the usefulness of decision-making tools.
GAPS IN LITERATURE
A common theme throughout the literature is the lack of a unifying classification system for reporting outcomes data. Each study utilizes different primary indications for tracheostomy and often different definitions for NI. There is very little literature that focuses specifically on outcomes for children with NI who receive tracheostomy as a life-prolonging measure. These gaps present challenges for obtaining meaningful prognosis data to share with families. Outcomes data for children who do not receive tracheostomy is also lacking. Additional studies on how families make this decision and their decisional satisfaction could help inform the decision-making process for both parents and clinicians. Research regarding the helpfulness and outcomes with decision-making tools would be useful.
CONCLUSIONS
Although there are limited data on outcomes specific to the children with NI and tracheostomy, existing literature shows a higher mortality, lower decannulation rate, higher hospitalization rate, and longer length of stay than that for children who receive tracheostomy for other indications. Tracheostomy is often a life-prolonging measure for children with NI. Shared decision-making should be the preferred communication process and include defining goals of care, as well as anticipated prognosis with balanced information about risks and benefits. Further research about the decision-making process and communication would be beneficial.
DISCLOSURE
Dr Shaw has nothing to disclose.
Children with complex medical conditions are living longer, many with the help of interventions and technology, such as gastrostomy tubes, tracheostomies, ventilator support, and parenteral nutrition. Children with medical complexity and technology account for over 80% of hospital days in pediatric academic centers.1
Hospitalists need communication skills and clinical information to guide discussions with patients and families about whether to pursue these measures. Tracheostomy discussions can be particularly challenging. Over 4,000 infants and children undergo tracheostomy each year, with related hospital charges of more than $2 billion, a 30-day readmission rate of 24.9%, and a median length of stay for pneumonia or tracheitis of 4 days.2 There is limited research on prognosis, outcomes, decision-making, and effects on quality of life, especially in the population of children who have significant neurological impairment (NI) and/or progressive or deteriorating neurological conditions. Physician biases may also influence this discussion.
This article will examine the question: How can a hospitalist guide decision-making discussions with families about tracheostomy placement for children with NI? A literature search was performed on Medline and Web of Science using the key terms tracheostomy, prognosis, neurologically impaired children, and decision-making. Articles included were relevant to the clinical question and published in the last 5 years. One article was included outside this timeframe given the scarcity of data.
INDICATIONS FOR TRACHEOSTOMY
Indications for tracheostomy include airway obstruction and the need for prolonged ventilation support.3 The number of tracheostomies placed has been increasing over the last 30 years, especially at tertiary care centers.3 Primary indications for tracheostomy include prolonged ventilation particularly in the context of underlying conditions such as congenital or acquired respiratory disease, congenital or acquired neurologic disease, cardiopulmonary disease, and primary anatomic airway obstruction.3,4 Children who undergo tracheostomy often have multiple medical conditions that impact their overall health and prognosis, with 41% having three or more complex chronic health conditions.5 This article will focus on children who have a primary indication of NI and in whom tracheostomy is often used as a life-prolonging measure.
PROGNOSIS
Discussions about tracheostomy should include information about risks, benefits, and prognosis. Prognosis discussions can be challenging given that many children for whom this intervention is being considered have multiple and complex medical conditions with uncertain or even known poor prognoses. Mortality rates ranging from 3% to 11% have been reported during the initial tracheostomy admission, with NI increasing the risk for mortality during the tracheostomy admission.5,6 Children with NI also have higher mortality beyond the initial hospital stay, lower decannulation rates, and more frequent admissions with longer lengths of stay than do children receiving a tracheostomy for upper airway obstruction (Table 1).6,7

For most children in this population, prognosis is related more to the underlying disease process than to the risk of the surgery for tracheostomy placement itself. Discussions with families should include the anticipated prognosis of the underlying disease, as well as current available data on outcomes for children with neurological impairment who have undergone tracheostomy placement. Most patients who receive a tracheostomy are children with complex medical conditions who have an acute event that leads to airway compromise and respiratory failure underscoring the importance of advance care planning.5
GOALS OF CARE DISCUSSIONS
Clinicians face challenges when initiating advance care planning discussions, including prognostic uncertainty, the perception that families may not want to engage in these discussions, and the complexity and time these discussions can take. In one study of more than 300 chronically ill children, only 17% of parents had discussed advance directives, although 49% reported they would like to create one for their child.9 A small study found that, although parents find these discussions difficult, they also find them important. They value a step by step approach with consideration for hope and nonmedical concerns.10 Advance care planning discussions should be viewed as a time out to clarify what the family sees as the best path forward before initiation of a tracheostomy discussion and decision.
Determining goals of care is a cornerstone of any discussion about tracheostomy placement, especially when a child has a condition that is life limiting. The decision to pursue tracheostomy should involve shared decision-making. This decision-making process is the preferred communication model when multiple options could be pursued, each with its own risks and benefits.10
In this model of decision-making, the family’s goals and values should be determined in the context of the medical intervention that is being pursued. Medical information such as prognosis, risk, benefits, and impact of the intervention on quality of life should all be shared with the family. Ideally, shared decision-making allows the practitioner and family to make a decision together that matches the family’s goals and values with the best option available. If the family’s goal is to prolong life and they feel their child has good quality of life, tracheostomy placement may be the most appropriate option. However, it is also possible that the family’s goals may align more with less invasive treatment options or a transition to comfort care.
Discussions regarding goals of care can be challenging, and involving an interdisciplinary team and a Palliative Care consultant can be helpful.
WHAT PROVIDERS SAY, WHAT FAMILIES NEED TO HEAR
Research on what parents find helpful in discussions about tracheostomy is limited. One study of 56 caregivers found that parents did not feel they could make a “free choice” because the alternative to tracheostomy was death.11 In interviews with caregivers following tracheostomy, this same study found several themes in caregiver perspectives on their decision for tracheostomy (Table 2); caregivers saw a benefit to “health and well-being” from tracheostomy even though they reported feeling unprepared for the caregiving aspect at home or the potential negative side effects. Half the children in this study had a neurologic diagnosis, and only families who chose tracheostomy placement were included. To this author’s knowledge, there are currently no studies that look at decisional themes, satisfaction, or outcomes for families that chose to not pursue tracheostomy.

There is limited literature about how providers discuss tracheostomy. One single-center study of practitioners found that providers focused more often on the benefits of tracheostomy rather than burdens (72% vs 28%).12 A common benefit theme was the provider “suggesting life with a tracheostomy might not be as difficult as families fear in that the child may have the ability to regain speech, engage in normal activities, and have the tracheostomy reversed once the child’s health improved.” However, decannulation rates and recovery trajectories for children with NI do not support this general expectation (Table 1). These provider communication themes may help to explain the family’s perspective that they feel unprepared for the burdens of a tracheostomy or the intensity of home caregiving. Given the limited data, it is difficult to generalize. Comparing communication and decision-making themes side by side does draw attention to how providers might better communicate with families about this decision (Table 2).
The difficult aspects of caregiving deserve special attention. A study of 25 parents showed reduced parental quality of life after their child’s tracheostomy placement related to overwhelming medical care, fear of death of the child requiring constant vigilance, and financial and psychological stressors.13 Most (72%) families in this study reported decisional regret at 3 months.Resources and support for a child with this level of care varies based on the child’s community. Exploration and discussion of what is available for each family, including home nursing, respite, and/or a skilled nursing facility, should be completed prior to tracheostomy placement. Honest discussions about the potential effects of this intervention on the family’s life can help inform their decision.
Decision-making tools for tracheostomy could be valuable for both families and clinicians. These tools allow for a more systematic approach to the decision-making process that takes into account the multidimensional aspects of this decision. The “Child Tracheostomy Decision Guide,” published by the Winnipeg Regional Health Authority, is one available tool.14 This tool guides families through the factors that may affect their decision-making and includes thoughts about goals of care, quality of life, prognosis, care at home, and other options such as comfort care. The Courageous Parents Network has also developed parent videos giving the perspective of parents who have chosen or not chosen tracheostomy.15 Currently, there are no studies that examine the usefulness of decision-making tools.
GAPS IN LITERATURE
A common theme throughout the literature is the lack of a unifying classification system for reporting outcomes data. Each study utilizes different primary indications for tracheostomy and often different definitions for NI. There is very little literature that focuses specifically on outcomes for children with NI who receive tracheostomy as a life-prolonging measure. These gaps present challenges for obtaining meaningful prognosis data to share with families. Outcomes data for children who do not receive tracheostomy is also lacking. Additional studies on how families make this decision and their decisional satisfaction could help inform the decision-making process for both parents and clinicians. Research regarding the helpfulness and outcomes with decision-making tools would be useful.
CONCLUSIONS
Although there are limited data on outcomes specific to the children with NI and tracheostomy, existing literature shows a higher mortality, lower decannulation rate, higher hospitalization rate, and longer length of stay than that for children who receive tracheostomy for other indications. Tracheostomy is often a life-prolonging measure for children with NI. Shared decision-making should be the preferred communication process and include defining goals of care, as well as anticipated prognosis with balanced information about risks and benefits. Further research about the decision-making process and communication would be beneficial.
DISCLOSURE
Dr Shaw has nothing to disclose.
1. Children’s Hospital Association. Spend for children with dominant chronic diseases – The CARE award. Historical spending: 2012-2014. https://www.childrenshospitals.org/Care/Children-With-Medical-Complexity 2018
2. Russel CJ, Mack WJ, Schrager SM, Wu S. Care variations, length of stay and readmissions in children hospitalized for bacterial tracheostomy-associated respiratory infections. Hosp Pediatr. 2017;7(1):16-23. https://doi.org/10.1542/hpeds.2016-0104
3. McPherson ML, Shekerdemian L, Goldsworthy M, et al. A decade of pediatric tracheostomies: indications, outcomes, and long-term prognosis. Pediatr Pulmonol. 2017;52(7):946-953. https://doi.org/10.1002/ppul.23657
4. Gergin O, Adil EA, Kawai K, Watters K, Moritz E, Rahbar R. Indications of pediatric tracheostomy over the last 30 years: has anything changed? Int J Pediatr Otorhinolaryngol. 2016;87:144-147. https://doi.org/10.1016/j.ijporl.2016.06.018
5. Edwards J, Houtrow A, Lucas A, et al. Children and young adults who receive tracheostomies or were initiated on long-term ventilation in PICUs. Pediatr Crit Care Med. 2016;17(8):e324-334. https://doi.org/10.1097/pcc.0000000000000844
6. Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563-572. https://doi.org/10.1542/peds.2008-3491
7. Tsuboi N, Ide K, Nishimura N, Nakagawa S, Morimoto N. Pediatric tracheostomy: survival and long-term outcomes. Int J Pediatr Otorhinolaryngol. 2016;89:81-85. https://doi.org/10.1016/j.ijporl.2016.07.033
8. Liberman DB, Pham PK, Nager AL. Pediatric advance directives: parents’ knowledge, experience, and preferences. Pediatrics. 2014;134(2):e436-e443. https://doi.org/10.1542/peds.2013-3124
9. Lotz JD, Daxer M, Jox RJ, Borasio GD, Führer M. “Hope for the best, prepare for the worst”: a qualitative interview study on parents’ needs and fears in pediatric advance care planning. Palliat Med. 2017;31(8):764-771. https://doi.org/10.1177/0269216316679913
10. Nelson KE, Mahant S. Shared decision-making about assistive technology for the child with severe neurologic impairment. Pediatr Clin North Am. 2014;61(4):641-652. https://doi.org/10.1016/j.pcl.2014.04.001
11. Nageswaran S, Golden SL, Gower WA, King NMP. Caregiver perceptions about their decision to pursue tracheostomy for children with medical complexity. J Pediatr. 2018;203:354-360.e1. https://doi.org/10.1016/j.jpeds.2018.07.045
12. Hebert LM, Watson AC, Madrigal V, October TW. Discussing benefits and risks of tracheostomy: what physicians actually say. Pediatr Crit Care Med. 2017;18(12):e592-e597. https://doi.org/10.1097/PCC.0000000000001341
13. October T, Jones A, Michals H, Hebert L, Jiang J, Wang J. Parental conflict, regret, and short-term impact on quality of life in tracheostomy decision making. Pediatr Crit Care Med. 2020;21(2):136-142. https://doi.org/10.1097/PCC.0000000000002109
14. Winnipeg Regional Health Authority. Childhood Tracheostomy Decision Guide. Accessed August 15, 2019. https://www.wrha.mb.ca/extranet/eipt/files/EIPT-023-001.pdf
15. Courageous Parents Network. Tracheostomy Decision Making Videos. Accessed August 20, 2019. https://courageousparentsnetwork.org/video-library/decision-making/tracheostomy
1. Children’s Hospital Association. Spend for children with dominant chronic diseases – The CARE award. Historical spending: 2012-2014. https://www.childrenshospitals.org/Care/Children-With-Medical-Complexity 2018
2. Russel CJ, Mack WJ, Schrager SM, Wu S. Care variations, length of stay and readmissions in children hospitalized for bacterial tracheostomy-associated respiratory infections. Hosp Pediatr. 2017;7(1):16-23. https://doi.org/10.1542/hpeds.2016-0104
3. McPherson ML, Shekerdemian L, Goldsworthy M, et al. A decade of pediatric tracheostomies: indications, outcomes, and long-term prognosis. Pediatr Pulmonol. 2017;52(7):946-953. https://doi.org/10.1002/ppul.23657
4. Gergin O, Adil EA, Kawai K, Watters K, Moritz E, Rahbar R. Indications of pediatric tracheostomy over the last 30 years: has anything changed? Int J Pediatr Otorhinolaryngol. 2016;87:144-147. https://doi.org/10.1016/j.ijporl.2016.06.018
5. Edwards J, Houtrow A, Lucas A, et al. Children and young adults who receive tracheostomies or were initiated on long-term ventilation in PICUs. Pediatr Crit Care Med. 2016;17(8):e324-334. https://doi.org/10.1097/pcc.0000000000000844
6. Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563-572. https://doi.org/10.1542/peds.2008-3491
7. Tsuboi N, Ide K, Nishimura N, Nakagawa S, Morimoto N. Pediatric tracheostomy: survival and long-term outcomes. Int J Pediatr Otorhinolaryngol. 2016;89:81-85. https://doi.org/10.1016/j.ijporl.2016.07.033
8. Liberman DB, Pham PK, Nager AL. Pediatric advance directives: parents’ knowledge, experience, and preferences. Pediatrics. 2014;134(2):e436-e443. https://doi.org/10.1542/peds.2013-3124
9. Lotz JD, Daxer M, Jox RJ, Borasio GD, Führer M. “Hope for the best, prepare for the worst”: a qualitative interview study on parents’ needs and fears in pediatric advance care planning. Palliat Med. 2017;31(8):764-771. https://doi.org/10.1177/0269216316679913
10. Nelson KE, Mahant S. Shared decision-making about assistive technology for the child with severe neurologic impairment. Pediatr Clin North Am. 2014;61(4):641-652. https://doi.org/10.1016/j.pcl.2014.04.001
11. Nageswaran S, Golden SL, Gower WA, King NMP. Caregiver perceptions about their decision to pursue tracheostomy for children with medical complexity. J Pediatr. 2018;203:354-360.e1. https://doi.org/10.1016/j.jpeds.2018.07.045
12. Hebert LM, Watson AC, Madrigal V, October TW. Discussing benefits and risks of tracheostomy: what physicians actually say. Pediatr Crit Care Med. 2017;18(12):e592-e597. https://doi.org/10.1097/PCC.0000000000001341
13. October T, Jones A, Michals H, Hebert L, Jiang J, Wang J. Parental conflict, regret, and short-term impact on quality of life in tracheostomy decision making. Pediatr Crit Care Med. 2020;21(2):136-142. https://doi.org/10.1097/PCC.0000000000002109
14. Winnipeg Regional Health Authority. Childhood Tracheostomy Decision Guide. Accessed August 15, 2019. https://www.wrha.mb.ca/extranet/eipt/files/EIPT-023-001.pdf
15. Courageous Parents Network. Tracheostomy Decision Making Videos. Accessed August 20, 2019. https://courageousparentsnetwork.org/video-library/decision-making/tracheostomy
© 2020 Society of Hospital Medicine
Masks, Seat Belts, and the Politicization of Public Health
At the time this piece was written, 54 Florida hospitals reported no available intensive care unit (ICU) beds1; hospitals in Miami-Dade County even started sending patients to neighboring Broward County for care despite Broward County also reporting a hospital bed shortage. Patients might even have needed to be transferred further north to Palm Beach County.2 Miami-Dade County was diagnosing over 100 cases with SARS-CoV-2 per 100,000 residents per day at one point, with a test positivity rate of over 25% that suggests testing is inadequate and many more would-be positive tests are being missed.3 While certain parts of the United States seem to have gained some semblance of control over the novel coronavirus, Florida appears to be in a downward spiral of high infection rates and increasing hospitalizations.
It didn’t have to go this way.
According to Robert Redfield, MD, Director of the Centers for Disease Control and Prevention, wearing a mask significantly reduces SARS-CoV-2 transmission. If community masking were increased only modestly, disease transmission could be curtailed enough to prevent many stay-at-home orders and reduce losses of an estimated $1 trillion in gross domestic product4 while also providing incalculable improvements in morbidity and mortality. Some experts believe that, while wearing a mask can protect others, it can also protect the wearer.5
That masking should be universal has become the accepted public health sentiment during this pandemic. Yet at the time of writing this article, there was still no law mandating masks in Florida, perhaps due to a significant but vocal minority—those who have personal concerns about wearing a mask and little concern about transmitting the virus to other, more vulnerable populations. This was the reason that one of the authors (M.B.) campaigned tirelessly for mandatory masking at Palm Beach County Commission meetings, one of which made the international news because of the outrageous and seemingly heart-felt statements made by several antimask advocates.6
AN ORGANIZED AND OUTSPOKEN MINORITY
At the Palm Beach County Commission, organized antimask advocates arrived hours before the start of the meeting, coming in two buses. Because of social distancing guidelines and seating limitations, they were able to fill many of the open seats at the meeting, making it appear that the antimask advocates far outnumbered those in favor of mask laws. Despite their tactics of screaming and intimidation, a law mandating masks in the county passed unanimously, though medical exemptions for those with chronic obstructive pulmonary disease, asthma, or “other conditions that reduce breathing” and religious exemptions for “persons for whom wearing a facial covering conflicts with their religious beliefs or practices” were included.7 After the meeting, police escorts were required by those in favor of masks, while the county commissioners had to lock themselves behind chamber doors.
The antimask campaigners were already known to M.B., a teacher, from previous gatherings she had attended in support of firearm legislation aimed at reducing gun violence. The same antimask advocates at the County Commission meeting had previously gathered as counter-protesters at this prior event, heckling and threatening those advocating for improving gun safety through legislation such as background checks. While it should not be, mask wearing and the laws mandating it have become a question of politics rather than one based in scientific evidence.
POLITICIZATION OF PUBLIC HEALTH IN FLORIDA AND ITS CONSEQUENCES
The absence of mandatory masking laws in populations hesitant to wear them, combined with the rush to reopen businesses, resulted in increasing death rates in Florida, with 7-day averages continuing in an upward trajectory and over 7,000 deaths being reported as of August 3, 2020.8 A small glimmer of hope was raised on that day, when fewer than 100 deaths for the previous day were reported, although one wonders if the weekend’s Hurricane Isaias preparations may have delayed some reporting.
In the face of mounting death counts and increasingly stressed hospitals, Florida’s governor, Ron DeSantis, has not heeded calls to institute new regulations, instead deferring to localities. This is perhaps good news considering Georgia’s governor, Brian Kemp, has spoken out against local mask laws and has said that mandating wearing them, even at the local level, would be a “bridge too far.”9 Several Georgia municipalities defied the governor, passing mandatory masking for their populations anyway, prompting Governor Kemp to file a lawsuit against the city of Atlanta, which he subsequently dropped after a judge ordered the state and city into mediation.10
The idea that the state should create laws to regulate the health and safety of the population has been met with resistance in the past where there is a greater degree of libertarian and antipaternalistic thinking.11 Campaigning against public health laws is not a new phenomenon. In the 1970s and 1980s, mandatory seat belt laws were met with significant resistance by a vocal minority, with the most common predictors for opposing these laws noted as holding beliefs that seat belts were ineffective, inconvenient, or uncomfortable12—the same arguments that have been made against masks. Additionally, having lower educational attainment, less income, and younger age were predictors of being against mandatory seat belt laws.12
THE IMPORTANCE OF COMMUNITY ENGAGEMENT
In response to a vociferous and somewhat organized minority, which has, in many cases, intimidated state and local politicians into inaction, community organizers have put out the call for many more citizens to make their voices heard. This seemed to have had an impact on the Palm Beach County commissioners, one of whom tried to demonstrate that there was broad support for passing a mandatory masking law during the commission meeting by bringing a stack of printed-out communications he had received in favor of it. Community organizers and public health advocates generally have an easier time reaching local officials, whereas it can be more difficult to engage other government officials farther away in state capitals, especially in larger states such as Florida. The organizers can also appeal to the fact that the local officials must live in the communities they represent and do not want to suffer from the spread of SARS-CoV-2 and overflowing hospitals. While local officials may be ill equipped to handle a global pandemic, appealing to the community has been somewhat effective in putting pressure on these officials to get a patchwork of local laws, which hopefully will have an impact on Florida’s surge numbers.
In the absence of a statewide mandatory masking law in Florida, several municipalities have instituted their own restrictions. Counties with some of the largest cities, such as Miami, Fort Lauderdale, Tampa, and Orlando, have required that masks be worn in public since June or early July.13 These restrictions, however, were implemented later than states in the northeastern United States, which have required masks since April or May and before significant reopening of businesses took place, in contrast to the sequence observed in Florida.
In the absence of political leadership, Florida businesses are increasingly taking up the charge and mandating that employees work from home, while others are requiring that employees and customers wear masks. Following New York–based grocer Key Foods and national chains like Whole Foods, both of which have long required that Florida customers wear masks, Florida’s ubiquitous Publix Supermarkets mandated masks in over 800 of their stores beginning July 21.14
While individual businesses and localities should be commended for their efforts, unfortunately, this may not be enough to dampen the surge. A tool developed by Harvard-based researchers, has labeled Florida and several other neighboring states as having severe spread, necessitating the need for stay-at-home orders to be reinstated.15
CONCLUSION
Florida is currently a global epicenter for COVID-19 diagnoses, with the state reporting nearly 600,000 cases as of August 17,8 more than most countries with larger populations. Florida faces many barriers to gaining control over the virus, including a vocal and organized minority which has opposed public health measures, an unwilling state government and ill-equipped local officials, and an underfunded safety net if stay-at-home orders were to be issued. Appealing to the public and elected officials with science, sanity, and support for those who want to prevent the spread of COVID-19 may provide one solution for gaining some control over the pandemic.
Disclosures
The authors have nothing to disclose.
1. Hospital ICU Beds Census and Staffed Availability as Reported in ESS. My Florida. Accessed July 30, 2020. https://bi.ahca.myflorida.com/t/ABICC/views/Public/ICUBedsHospital
2. Goodman CK. Broward hospitals nearing capacity with overflow patients from Miami-Dade. South Florida Sun Sentinel. July 28, 2020. Accessed August 3, 2020. https://www.sun-sentinel.com/coronavirus/fl-ne-broward-hospitals-getting-overflow-20200728-akz7k5wmubb2billpnofsqtqdy-story.html
3. Miami-Dade County, FL. Covid Act Now. Accessed August 3, 2020. https://covidactnow.org/us/fl/county/miami_dade_county?s=790144
4. Brooks JT, Butler JC, Redfield RR. Universal masking to prevent SARS-CoV-2 transmission—the time is now. JAMA. Published online July 14, 2020. https://doi.org/10.1001/jama.2020.13107
5. Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;1-4. https://doi.org/10.1007/s11606-020-06067-8
6. ‘They want to throw God’s wonderful breathing system out.’ BBC News. June 25, 2020. Accessed August 3, 2020. https://www.bbc.com/news/av/world-us-canada-53174415/they-want-to-throw-god-s-wonderful-breathing-system-out
7. Palm Beach County Facial Coverings Frequently Asked Questions. Palm Beach County: Discover the Palm Beaches…the Best of Everything. Updated June 26, 2020. Accessed July 30, 2020. https://discover.pbcgov.org/PDF/COVID19/PBC-Facial-Coverings-FAQs.pdf
8. Florida COVID-19 Response. Accessed August 17, 2020. https://floridahealthcovid19.gov/
9. Flynn M, Iati M. Georgia Gov. Brian Kemp sues Atlanta over mask requirement as coronavirus surges in the state. Washington Post. July 16, 2020. Accessed August 3, 2020. https://www.washingtonpost.com/nation/2020/07/16/kemp-georgia-mask-mandates/
10. Jamerson J. Georgia Gov. Kemp drops lawsuit against Atlanta mayor over coronavirus restrictions. Wall Street Journal. August 13, 2020. Accessed August 17, 2020. https://www.wsj.com/articles/georgia-gov-kemp-drops-lawsuit-against-atlanta-mayor-over-coronavirus-restrictions-11597347685
11. Giubilini A, Savulescu J. Vaccination, risks, and freedom: the seat belt analogy. Public Health Ethics. 2019;12(3):237-249. https://doi.org/10.1093/phe/phz014
12. Morelock S, Hingson RW, Smith RA, Lederman RI. Mandatory seatbelt law support and opposition in New England—a survey. Public Health Rep. 1985;100(4):357-363.
13. Muller B. Most major Florida cities now require wearing face masks in public. News4Jax. June 19, 2020. Updated June 19, 2020. Accessed August 3, 2020. https://www.news4jax.com/news/local/2020/06/19/major-florida-cities-now-require-use-of-face-mask-in-public-places/
14. Ward B. Publix to mandate face masks for all stores starting next week. Tampa Bay Business Journal. July 16, 2020. Updated July 16, 2020. Accessed August 3, 2020. https://www.bizjournals.com/tampabay/news/2020/07/16/publix-to-mandate-face-masks-for-all-stores-starti.html
15. COVID Risk Levels Dashboard. Pandemics explained: unlocking evidence for better decision making. Accessed August 3, 2020. https://globalepidemics.org/key-metrics-for-covid-suppression/
At the time this piece was written, 54 Florida hospitals reported no available intensive care unit (ICU) beds1; hospitals in Miami-Dade County even started sending patients to neighboring Broward County for care despite Broward County also reporting a hospital bed shortage. Patients might even have needed to be transferred further north to Palm Beach County.2 Miami-Dade County was diagnosing over 100 cases with SARS-CoV-2 per 100,000 residents per day at one point, with a test positivity rate of over 25% that suggests testing is inadequate and many more would-be positive tests are being missed.3 While certain parts of the United States seem to have gained some semblance of control over the novel coronavirus, Florida appears to be in a downward spiral of high infection rates and increasing hospitalizations.
It didn’t have to go this way.
According to Robert Redfield, MD, Director of the Centers for Disease Control and Prevention, wearing a mask significantly reduces SARS-CoV-2 transmission. If community masking were increased only modestly, disease transmission could be curtailed enough to prevent many stay-at-home orders and reduce losses of an estimated $1 trillion in gross domestic product4 while also providing incalculable improvements in morbidity and mortality. Some experts believe that, while wearing a mask can protect others, it can also protect the wearer.5
That masking should be universal has become the accepted public health sentiment during this pandemic. Yet at the time of writing this article, there was still no law mandating masks in Florida, perhaps due to a significant but vocal minority—those who have personal concerns about wearing a mask and little concern about transmitting the virus to other, more vulnerable populations. This was the reason that one of the authors (M.B.) campaigned tirelessly for mandatory masking at Palm Beach County Commission meetings, one of which made the international news because of the outrageous and seemingly heart-felt statements made by several antimask advocates.6
AN ORGANIZED AND OUTSPOKEN MINORITY
At the Palm Beach County Commission, organized antimask advocates arrived hours before the start of the meeting, coming in two buses. Because of social distancing guidelines and seating limitations, they were able to fill many of the open seats at the meeting, making it appear that the antimask advocates far outnumbered those in favor of mask laws. Despite their tactics of screaming and intimidation, a law mandating masks in the county passed unanimously, though medical exemptions for those with chronic obstructive pulmonary disease, asthma, or “other conditions that reduce breathing” and religious exemptions for “persons for whom wearing a facial covering conflicts with their religious beliefs or practices” were included.7 After the meeting, police escorts were required by those in favor of masks, while the county commissioners had to lock themselves behind chamber doors.
The antimask campaigners were already known to M.B., a teacher, from previous gatherings she had attended in support of firearm legislation aimed at reducing gun violence. The same antimask advocates at the County Commission meeting had previously gathered as counter-protesters at this prior event, heckling and threatening those advocating for improving gun safety through legislation such as background checks. While it should not be, mask wearing and the laws mandating it have become a question of politics rather than one based in scientific evidence.
POLITICIZATION OF PUBLIC HEALTH IN FLORIDA AND ITS CONSEQUENCES
The absence of mandatory masking laws in populations hesitant to wear them, combined with the rush to reopen businesses, resulted in increasing death rates in Florida, with 7-day averages continuing in an upward trajectory and over 7,000 deaths being reported as of August 3, 2020.8 A small glimmer of hope was raised on that day, when fewer than 100 deaths for the previous day were reported, although one wonders if the weekend’s Hurricane Isaias preparations may have delayed some reporting.
In the face of mounting death counts and increasingly stressed hospitals, Florida’s governor, Ron DeSantis, has not heeded calls to institute new regulations, instead deferring to localities. This is perhaps good news considering Georgia’s governor, Brian Kemp, has spoken out against local mask laws and has said that mandating wearing them, even at the local level, would be a “bridge too far.”9 Several Georgia municipalities defied the governor, passing mandatory masking for their populations anyway, prompting Governor Kemp to file a lawsuit against the city of Atlanta, which he subsequently dropped after a judge ordered the state and city into mediation.10
The idea that the state should create laws to regulate the health and safety of the population has been met with resistance in the past where there is a greater degree of libertarian and antipaternalistic thinking.11 Campaigning against public health laws is not a new phenomenon. In the 1970s and 1980s, mandatory seat belt laws were met with significant resistance by a vocal minority, with the most common predictors for opposing these laws noted as holding beliefs that seat belts were ineffective, inconvenient, or uncomfortable12—the same arguments that have been made against masks. Additionally, having lower educational attainment, less income, and younger age were predictors of being against mandatory seat belt laws.12
THE IMPORTANCE OF COMMUNITY ENGAGEMENT
In response to a vociferous and somewhat organized minority, which has, in many cases, intimidated state and local politicians into inaction, community organizers have put out the call for many more citizens to make their voices heard. This seemed to have had an impact on the Palm Beach County commissioners, one of whom tried to demonstrate that there was broad support for passing a mandatory masking law during the commission meeting by bringing a stack of printed-out communications he had received in favor of it. Community organizers and public health advocates generally have an easier time reaching local officials, whereas it can be more difficult to engage other government officials farther away in state capitals, especially in larger states such as Florida. The organizers can also appeal to the fact that the local officials must live in the communities they represent and do not want to suffer from the spread of SARS-CoV-2 and overflowing hospitals. While local officials may be ill equipped to handle a global pandemic, appealing to the community has been somewhat effective in putting pressure on these officials to get a patchwork of local laws, which hopefully will have an impact on Florida’s surge numbers.
In the absence of a statewide mandatory masking law in Florida, several municipalities have instituted their own restrictions. Counties with some of the largest cities, such as Miami, Fort Lauderdale, Tampa, and Orlando, have required that masks be worn in public since June or early July.13 These restrictions, however, were implemented later than states in the northeastern United States, which have required masks since April or May and before significant reopening of businesses took place, in contrast to the sequence observed in Florida.
In the absence of political leadership, Florida businesses are increasingly taking up the charge and mandating that employees work from home, while others are requiring that employees and customers wear masks. Following New York–based grocer Key Foods and national chains like Whole Foods, both of which have long required that Florida customers wear masks, Florida’s ubiquitous Publix Supermarkets mandated masks in over 800 of their stores beginning July 21.14
While individual businesses and localities should be commended for their efforts, unfortunately, this may not be enough to dampen the surge. A tool developed by Harvard-based researchers, has labeled Florida and several other neighboring states as having severe spread, necessitating the need for stay-at-home orders to be reinstated.15
CONCLUSION
Florida is currently a global epicenter for COVID-19 diagnoses, with the state reporting nearly 600,000 cases as of August 17,8 more than most countries with larger populations. Florida faces many barriers to gaining control over the virus, including a vocal and organized minority which has opposed public health measures, an unwilling state government and ill-equipped local officials, and an underfunded safety net if stay-at-home orders were to be issued. Appealing to the public and elected officials with science, sanity, and support for those who want to prevent the spread of COVID-19 may provide one solution for gaining some control over the pandemic.
Disclosures
The authors have nothing to disclose.
At the time this piece was written, 54 Florida hospitals reported no available intensive care unit (ICU) beds1; hospitals in Miami-Dade County even started sending patients to neighboring Broward County for care despite Broward County also reporting a hospital bed shortage. Patients might even have needed to be transferred further north to Palm Beach County.2 Miami-Dade County was diagnosing over 100 cases with SARS-CoV-2 per 100,000 residents per day at one point, with a test positivity rate of over 25% that suggests testing is inadequate and many more would-be positive tests are being missed.3 While certain parts of the United States seem to have gained some semblance of control over the novel coronavirus, Florida appears to be in a downward spiral of high infection rates and increasing hospitalizations.
It didn’t have to go this way.
According to Robert Redfield, MD, Director of the Centers for Disease Control and Prevention, wearing a mask significantly reduces SARS-CoV-2 transmission. If community masking were increased only modestly, disease transmission could be curtailed enough to prevent many stay-at-home orders and reduce losses of an estimated $1 trillion in gross domestic product4 while also providing incalculable improvements in morbidity and mortality. Some experts believe that, while wearing a mask can protect others, it can also protect the wearer.5
That masking should be universal has become the accepted public health sentiment during this pandemic. Yet at the time of writing this article, there was still no law mandating masks in Florida, perhaps due to a significant but vocal minority—those who have personal concerns about wearing a mask and little concern about transmitting the virus to other, more vulnerable populations. This was the reason that one of the authors (M.B.) campaigned tirelessly for mandatory masking at Palm Beach County Commission meetings, one of which made the international news because of the outrageous and seemingly heart-felt statements made by several antimask advocates.6
AN ORGANIZED AND OUTSPOKEN MINORITY
At the Palm Beach County Commission, organized antimask advocates arrived hours before the start of the meeting, coming in two buses. Because of social distancing guidelines and seating limitations, they were able to fill many of the open seats at the meeting, making it appear that the antimask advocates far outnumbered those in favor of mask laws. Despite their tactics of screaming and intimidation, a law mandating masks in the county passed unanimously, though medical exemptions for those with chronic obstructive pulmonary disease, asthma, or “other conditions that reduce breathing” and religious exemptions for “persons for whom wearing a facial covering conflicts with their religious beliefs or practices” were included.7 After the meeting, police escorts were required by those in favor of masks, while the county commissioners had to lock themselves behind chamber doors.
The antimask campaigners were already known to M.B., a teacher, from previous gatherings she had attended in support of firearm legislation aimed at reducing gun violence. The same antimask advocates at the County Commission meeting had previously gathered as counter-protesters at this prior event, heckling and threatening those advocating for improving gun safety through legislation such as background checks. While it should not be, mask wearing and the laws mandating it have become a question of politics rather than one based in scientific evidence.
POLITICIZATION OF PUBLIC HEALTH IN FLORIDA AND ITS CONSEQUENCES
The absence of mandatory masking laws in populations hesitant to wear them, combined with the rush to reopen businesses, resulted in increasing death rates in Florida, with 7-day averages continuing in an upward trajectory and over 7,000 deaths being reported as of August 3, 2020.8 A small glimmer of hope was raised on that day, when fewer than 100 deaths for the previous day were reported, although one wonders if the weekend’s Hurricane Isaias preparations may have delayed some reporting.
In the face of mounting death counts and increasingly stressed hospitals, Florida’s governor, Ron DeSantis, has not heeded calls to institute new regulations, instead deferring to localities. This is perhaps good news considering Georgia’s governor, Brian Kemp, has spoken out against local mask laws and has said that mandating wearing them, even at the local level, would be a “bridge too far.”9 Several Georgia municipalities defied the governor, passing mandatory masking for their populations anyway, prompting Governor Kemp to file a lawsuit against the city of Atlanta, which he subsequently dropped after a judge ordered the state and city into mediation.10
The idea that the state should create laws to regulate the health and safety of the population has been met with resistance in the past where there is a greater degree of libertarian and antipaternalistic thinking.11 Campaigning against public health laws is not a new phenomenon. In the 1970s and 1980s, mandatory seat belt laws were met with significant resistance by a vocal minority, with the most common predictors for opposing these laws noted as holding beliefs that seat belts were ineffective, inconvenient, or uncomfortable12—the same arguments that have been made against masks. Additionally, having lower educational attainment, less income, and younger age were predictors of being against mandatory seat belt laws.12
THE IMPORTANCE OF COMMUNITY ENGAGEMENT
In response to a vociferous and somewhat organized minority, which has, in many cases, intimidated state and local politicians into inaction, community organizers have put out the call for many more citizens to make their voices heard. This seemed to have had an impact on the Palm Beach County commissioners, one of whom tried to demonstrate that there was broad support for passing a mandatory masking law during the commission meeting by bringing a stack of printed-out communications he had received in favor of it. Community organizers and public health advocates generally have an easier time reaching local officials, whereas it can be more difficult to engage other government officials farther away in state capitals, especially in larger states such as Florida. The organizers can also appeal to the fact that the local officials must live in the communities they represent and do not want to suffer from the spread of SARS-CoV-2 and overflowing hospitals. While local officials may be ill equipped to handle a global pandemic, appealing to the community has been somewhat effective in putting pressure on these officials to get a patchwork of local laws, which hopefully will have an impact on Florida’s surge numbers.
In the absence of a statewide mandatory masking law in Florida, several municipalities have instituted their own restrictions. Counties with some of the largest cities, such as Miami, Fort Lauderdale, Tampa, and Orlando, have required that masks be worn in public since June or early July.13 These restrictions, however, were implemented later than states in the northeastern United States, which have required masks since April or May and before significant reopening of businesses took place, in contrast to the sequence observed in Florida.
In the absence of political leadership, Florida businesses are increasingly taking up the charge and mandating that employees work from home, while others are requiring that employees and customers wear masks. Following New York–based grocer Key Foods and national chains like Whole Foods, both of which have long required that Florida customers wear masks, Florida’s ubiquitous Publix Supermarkets mandated masks in over 800 of their stores beginning July 21.14
While individual businesses and localities should be commended for their efforts, unfortunately, this may not be enough to dampen the surge. A tool developed by Harvard-based researchers, has labeled Florida and several other neighboring states as having severe spread, necessitating the need for stay-at-home orders to be reinstated.15
CONCLUSION
Florida is currently a global epicenter for COVID-19 diagnoses, with the state reporting nearly 600,000 cases as of August 17,8 more than most countries with larger populations. Florida faces many barriers to gaining control over the virus, including a vocal and organized minority which has opposed public health measures, an unwilling state government and ill-equipped local officials, and an underfunded safety net if stay-at-home orders were to be issued. Appealing to the public and elected officials with science, sanity, and support for those who want to prevent the spread of COVID-19 may provide one solution for gaining some control over the pandemic.
Disclosures
The authors have nothing to disclose.
1. Hospital ICU Beds Census and Staffed Availability as Reported in ESS. My Florida. Accessed July 30, 2020. https://bi.ahca.myflorida.com/t/ABICC/views/Public/ICUBedsHospital
2. Goodman CK. Broward hospitals nearing capacity with overflow patients from Miami-Dade. South Florida Sun Sentinel. July 28, 2020. Accessed August 3, 2020. https://www.sun-sentinel.com/coronavirus/fl-ne-broward-hospitals-getting-overflow-20200728-akz7k5wmubb2billpnofsqtqdy-story.html
3. Miami-Dade County, FL. Covid Act Now. Accessed August 3, 2020. https://covidactnow.org/us/fl/county/miami_dade_county?s=790144
4. Brooks JT, Butler JC, Redfield RR. Universal masking to prevent SARS-CoV-2 transmission—the time is now. JAMA. Published online July 14, 2020. https://doi.org/10.1001/jama.2020.13107
5. Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;1-4. https://doi.org/10.1007/s11606-020-06067-8
6. ‘They want to throw God’s wonderful breathing system out.’ BBC News. June 25, 2020. Accessed August 3, 2020. https://www.bbc.com/news/av/world-us-canada-53174415/they-want-to-throw-god-s-wonderful-breathing-system-out
7. Palm Beach County Facial Coverings Frequently Asked Questions. Palm Beach County: Discover the Palm Beaches…the Best of Everything. Updated June 26, 2020. Accessed July 30, 2020. https://discover.pbcgov.org/PDF/COVID19/PBC-Facial-Coverings-FAQs.pdf
8. Florida COVID-19 Response. Accessed August 17, 2020. https://floridahealthcovid19.gov/
9. Flynn M, Iati M. Georgia Gov. Brian Kemp sues Atlanta over mask requirement as coronavirus surges in the state. Washington Post. July 16, 2020. Accessed August 3, 2020. https://www.washingtonpost.com/nation/2020/07/16/kemp-georgia-mask-mandates/
10. Jamerson J. Georgia Gov. Kemp drops lawsuit against Atlanta mayor over coronavirus restrictions. Wall Street Journal. August 13, 2020. Accessed August 17, 2020. https://www.wsj.com/articles/georgia-gov-kemp-drops-lawsuit-against-atlanta-mayor-over-coronavirus-restrictions-11597347685
11. Giubilini A, Savulescu J. Vaccination, risks, and freedom: the seat belt analogy. Public Health Ethics. 2019;12(3):237-249. https://doi.org/10.1093/phe/phz014
12. Morelock S, Hingson RW, Smith RA, Lederman RI. Mandatory seatbelt law support and opposition in New England—a survey. Public Health Rep. 1985;100(4):357-363.
13. Muller B. Most major Florida cities now require wearing face masks in public. News4Jax. June 19, 2020. Updated June 19, 2020. Accessed August 3, 2020. https://www.news4jax.com/news/local/2020/06/19/major-florida-cities-now-require-use-of-face-mask-in-public-places/
14. Ward B. Publix to mandate face masks for all stores starting next week. Tampa Bay Business Journal. July 16, 2020. Updated July 16, 2020. Accessed August 3, 2020. https://www.bizjournals.com/tampabay/news/2020/07/16/publix-to-mandate-face-masks-for-all-stores-starti.html
15. COVID Risk Levels Dashboard. Pandemics explained: unlocking evidence for better decision making. Accessed August 3, 2020. https://globalepidemics.org/key-metrics-for-covid-suppression/
1. Hospital ICU Beds Census and Staffed Availability as Reported in ESS. My Florida. Accessed July 30, 2020. https://bi.ahca.myflorida.com/t/ABICC/views/Public/ICUBedsHospital
2. Goodman CK. Broward hospitals nearing capacity with overflow patients from Miami-Dade. South Florida Sun Sentinel. July 28, 2020. Accessed August 3, 2020. https://www.sun-sentinel.com/coronavirus/fl-ne-broward-hospitals-getting-overflow-20200728-akz7k5wmubb2billpnofsqtqdy-story.html
3. Miami-Dade County, FL. Covid Act Now. Accessed August 3, 2020. https://covidactnow.org/us/fl/county/miami_dade_county?s=790144
4. Brooks JT, Butler JC, Redfield RR. Universal masking to prevent SARS-CoV-2 transmission—the time is now. JAMA. Published online July 14, 2020. https://doi.org/10.1001/jama.2020.13107
5. Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;1-4. https://doi.org/10.1007/s11606-020-06067-8
6. ‘They want to throw God’s wonderful breathing system out.’ BBC News. June 25, 2020. Accessed August 3, 2020. https://www.bbc.com/news/av/world-us-canada-53174415/they-want-to-throw-god-s-wonderful-breathing-system-out
7. Palm Beach County Facial Coverings Frequently Asked Questions. Palm Beach County: Discover the Palm Beaches…the Best of Everything. Updated June 26, 2020. Accessed July 30, 2020. https://discover.pbcgov.org/PDF/COVID19/PBC-Facial-Coverings-FAQs.pdf
8. Florida COVID-19 Response. Accessed August 17, 2020. https://floridahealthcovid19.gov/
9. Flynn M, Iati M. Georgia Gov. Brian Kemp sues Atlanta over mask requirement as coronavirus surges in the state. Washington Post. July 16, 2020. Accessed August 3, 2020. https://www.washingtonpost.com/nation/2020/07/16/kemp-georgia-mask-mandates/
10. Jamerson J. Georgia Gov. Kemp drops lawsuit against Atlanta mayor over coronavirus restrictions. Wall Street Journal. August 13, 2020. Accessed August 17, 2020. https://www.wsj.com/articles/georgia-gov-kemp-drops-lawsuit-against-atlanta-mayor-over-coronavirus-restrictions-11597347685
11. Giubilini A, Savulescu J. Vaccination, risks, and freedom: the seat belt analogy. Public Health Ethics. 2019;12(3):237-249. https://doi.org/10.1093/phe/phz014
12. Morelock S, Hingson RW, Smith RA, Lederman RI. Mandatory seatbelt law support and opposition in New England—a survey. Public Health Rep. 1985;100(4):357-363.
13. Muller B. Most major Florida cities now require wearing face masks in public. News4Jax. June 19, 2020. Updated June 19, 2020. Accessed August 3, 2020. https://www.news4jax.com/news/local/2020/06/19/major-florida-cities-now-require-use-of-face-mask-in-public-places/
14. Ward B. Publix to mandate face masks for all stores starting next week. Tampa Bay Business Journal. July 16, 2020. Updated July 16, 2020. Accessed August 3, 2020. https://www.bizjournals.com/tampabay/news/2020/07/16/publix-to-mandate-face-masks-for-all-stores-starti.html
15. COVID Risk Levels Dashboard. Pandemics explained: unlocking evidence for better decision making. Accessed August 3, 2020. https://globalepidemics.org/key-metrics-for-covid-suppression/
© 2020 Society of Hospital Medicine
Grieving and Hospital-Based Bereavement Care During the COVID-19 Pandemic
As of July 25, 2020, there had been 146,073 deaths from COVID-19 in the United States and 641,273 worldwide, with a disproportionate number of deaths occurring in historically disadvantaged minority groups, specifically African Americans.1,2 The number of decedents will continue to increase over the coming months, even as the number of new COVID-19 cases decreases. Given that, for each death, five persons are believed to be significantly affected,3 the number of bereaved individuals whose loved ones died during the pandemic in the United States alone is likely to be in the millions.
COVID-19–related mortality has become a pressing public health issue, and as a result, support for bereaved family members, especially for minority populations, is also an important public health issue.4 It is likely that bereaved individuals are at greater risk of poor bereavement outcomes during the pandemic—irrespective of whether the death was a result of COVID-19—because of social isolation. This is particularly true if loved ones died in the hospital and, due to visitor restrictions, faced limited or no visitation. For many, bereavement will be affected by stay-at-home orders and social distancing restrictions that reduce access to emotional support and rituals, such as funerals, that usually provide comfort.5
Urgent attention is needed to support bereaved individuals, to flatten the curve of mental health disorders associated with the death of loved ones during the pandemic. Within a preventive model of care, we offer guidelines for how hospitals, longitudinal providers, and mental health clinicians can provide bereavement outreach to all individuals whose loved ones died during the COVID-19 pandemic.
PUBLIC HEALTH MODEL OF BEREAVEMENT SUPPORT
The provision of bereavement care, including the assessment of risk for poor bereavement outcomes, is an essential component of high-quality end-of-life care endorsed by the hospice and palliative care movement.6 However, the development of standardized bereavement services has lagged behind that of other components of palliative care, varying greatly by institution and provider.7 Approximately 10% to 20% of bereaved individuals experience psychiatric difficulties following the death of a loved one, including prolonged grief disorder, posttraumatic stress disorder, and major depressive disorder.8 Risk factors include a hospital-based death, death in an intensive care unit (ICU), sudden death, not being able to say goodbye, and a history of psychiatric disorders.8,9
One of the biggest barriers in providing standardized bereavement services is the lack of a systematic process to identify individuals at risk of poor bereavement outcomes.10 Aoun et al developed a public health model of bereavement support that comprises a three-tiered approach to risk and the corresponding need for support.11 They propose that the low-risk group, approximately 60% of bereaved individuals, would primarily need support from family and friends, the moderate-risk group (30%) would need support from the wider community, and the high-risk group (10%) would need support from mental health providers.
It is reasonable to assume that many individuals whose loved ones died during the pandemic will fall into a high-risk group for poor bereavement outcomes, as identified by Aoun et al.11 Given a higher than usual inpatient mortality due to COVID-19 for certain populations and that bereavement care is already underrecognized within healthcare systems, hospitals and other healthcare facilities and their providers need to fill this void.
EDUCATION, GUIDANCE, AND SUPPORT MODEL
We adopted an education, guidance, and support model of bereavement support in 2019.7 This model has been shown to positively affect the experience of bereaved individuals, especially because of condolences from providers and psycho-educational information about coping with grief.7 Each month, a list of deceased patients and family contacts is generated from a mortality review database,12 and bereavement packets are mailed to family members; the packet includes a condolence letter from senior management, a psycho-educational grief guide, and a list of community-based resources. A social worker is also available to provide telephone support and to assist with mental health referrals. For patients who died in the COVID-19–specific units, social work also provides support and outreach to families.
Psycho-Education
During the early weeks of the pandemic, a tip sheet—”Grieving during a pandemic”13— was created to include in the bereavement packet and for distribution to community organizations within the hospital’s geographical area. This tip sheet offers strategies to facilitate coping based on the psychological model of cognitive-behavioral therapy (CBT).14 Topics addressed include understanding the nature of grief, self-care, adapting bereavement rituals in light of social distancing, challenging unhelpful thinking patterns that might lead to feelings of guilt especially regarding the death of the patient, and ways to obtain support during the pandemic. The tip sheet was made available in Spanish, French, Chinese, Haitian Creole, Portuguese, Arabic, and Russian given that our mortality data, consistent with preliminary findings from New York State, suggested higher death rates among Black/African American and Hispanic/Latino groups, compared with historical mortality statistics.15
Virtual Support
As part of our bereavement response during the COVID-19 crisis, we have launched virtual bereavement support for families impacted by the pandemic. It is challenging to identify the optimal type of support and timing, given the reliance on virtual outreach without in-person screening. With the increased distress and trauma associated with deaths during the pandemic, one clinical challenge is managing emotions in a virtual group without access to the usual tools that clinicians rely on, such as reading body language. Following a graded exposure approach, a form of behavioral therapy,14 we recommend offering a psycho-educational seminar first in which facilitators can control the content and limit exposure of sharing stories from participants. For support groups (eg, 6 to 8 sessions), we recommend that participants be screened prior to assess their risk factors and readiness and provide individual therapist referrals as needed.10
Community Outreach
Many diverse communities have been affected significantly by COVID-19 and faced high mortality rates.16 We recognized that proactive bereavement outreach to these communities was essential. Grief guides and tip sheets in various languages were made available as part of our community outreach programs, which included vans traveling to severely affected communities and providing testing, masks, alcohol-based hand sanitizer, and written materials.
Education About Bereavement
Many clinicians and staff express feelings of inadequacy about providing bereavement outreach. Such feelings are not uncommon, especially because clinicians tend to receive little training in dealing with the emotional toll of patient deaths and bereavement care.17,18 These feelings are likely to be heightened during this pandemic given the increased exposure to patient deaths, concern for personal safety, and changed practices in providing care, including the need to socially distance. Providing support for clinicians to process their feelings about the death of patients is crucial.19 In addition to our Employee Assistance Program, psychosocial clinicians are facilitating weekly virtual support groups for providers to discuss the effects of the pandemic on their personal and professional lives.
Bereaved family members report they benefit from hearing from the clinical team and receiving condolences, which is seen as humanizing the physician-family relationship. This personal outreach is likely more important during this time because many providers will have interacted with family members virtually.7,20,21 To help facilitate offers of condolences, we developed the TEARS acronym to describe the components of a condolence call that can also be adapted for writing condolence cards (Table).
GUIDELINES
We recommend that hospitals and other healthcare facilities that might not have well-established bereavement programs consider adopting a building block approach to provide basic outreach to families of their deceased patients.7 Tapping into existing resources, the major components are as follows: (1) a letter of condolence from leadership, (2) psycho-educational information about grief, (3) a list of community/online resources, including information about local hospice bereavement programs and bereavement camps or programs for children, (4) offers of condolences from individual providers/teams, and (5) mental health outreach as indicated.
CONCLUSION
The COVID-19–related mortality, particularly among already vulnerable populations, coupled with the existing underrecognition of bereavement has created an urgent public health issue that needs to be addressed. Given that few institutions offer standardized bereavement follow-up, we believe that hospital providers and mental health clinicians need to take a proactive approach to providing bereavement outreach to families affected by death during the pandemic.
Acknowledgments
The authors would like to acknowledge the Brigham Health Bereavement Committee and the staff of Care Continuum Management and the Department of Community Outreach at Brigham and Women’s Hospital.
Disclosures
No competing financial interests relevant to this article exist for Dr Morris, Ms Paterson, and Dr Mendu. Dr Morris receives royalties for two self-help books about grief published by Robinson and Dr Mendu provides consulting services for Bayer AG unrelated to the content of this article.
1. Coronavirus Resource Center Covid-19: Case Tracker. Johns Hopkins University. Accessed July 25, 2020. https://coronavirus.jhu.edu/
2. Tappe A. America’s black and Hispanic communities are bearing the brunt of the coronavirus. CNN. April 21, 2020. Accessed June 7, 2020. https://www.cnn.com/2020/04/21/economy/coronavirus-burden-black-hispanic-workers/index.html
3. Shear K, Frank E, Houck PR, Reynolds CF 3rd. Treatment of complicated grief: a randomized controlled trial. JAMA. 2005;293(21):2601-2608. https://doi.org/10.1001/jama.293.21.2601
4. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/nejmsa2011686
5. Morris SE, Moment A, Thomas JD. Caring for bereaved family members during the COVID-19 pandemic: before and after the death of a patient. J Pain Symptom Manage. Published online May 7, 2020. https://doi.org/10.1016/j.jpainsymman.2020.05.002
6. National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 7, 2020. https://www.nationalcoalitionhpc.org/ncp
7. Morris SE, Block SD. Adding value to palliative care services: the development of an institutional bereavement program. J Palliat Med. 2015;18(11):915-922. https://doi.org/10.1089/jpm.2015.0080
8. Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370(9603):1960-1973. https://doi.org/10.1016/s0140-6736(07)61816-9
9. Kentish-Barnes N, Chaize M, Seegers V, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45(5):1341-1352. https://doi.org/10.1183/09031936.00160014
10. Morris SE, Anderson CM, Tarquini SJ, Block SD. A standardized approach to bereavement risk-screening: a quality improvement project. J Psychosoc Oncol. 2020;38(4):406-417. https://doi.org/10.1080/07347332.2019.1703065
11. Aoun SM, Breen LJ, Howting DA, Rumbold B, McNamara B, Hegney D. Who needs bereavement support? a population based survey of bereavement risk and support need. PLoS One. 2015;10(3):e0121101. https://doi.org/10.1371/journal.pone.0121101
12. Mendu ML, Lu Y, Petersen A, et al. Reflections on implementing a hospital-wide provider-based electronic inpatient mortality review system: lessons learnt. BMJ Qual Saf. 2020;29(4):304‐312. https://doi.org/10.1136/bmjqs-2019-009864
13. Morris SE. Grieving during a pandemic. Brigham and Women’s Hospital. Accessed July 25, 2020. https://www.brighamandwomens.org/covid-19/grieving-during-a-pandemic
14. Beck JS. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. Guilford Press; 2011.
15. Coronavirus Disease 2019 (COVID-19). Health Equity Considerations and Racial and Ethnic Minority Groups. Centers for Disease Control and Prevention. Updated July 24, 2020. Accessed July 25, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
16. Death rates in Mass. surged in areas already hard hit. Boston Globe. May 17, 2020. Accessed June 8, 2020. https://www.bostonglobe.com/2020/05/17/opinion/death-rate-mass-surged-areas-already-hard-hit/
17. Jackson VA, Sullivan AM, Gadmer NM, et al. “It was haunting…”: physicians’ descriptions of emotionally powerful patient deaths. Acad Med. 2005;80(7):648-656. https://doi.org/10.1097/00001888-200507000-00007
18. Morris S, Schaefer K, Rosowsky E. Primary care for the elderly bereaved: recommendations for medical education. J Clin Psychol Med Settings. 2018;25(4):463‐470. https://doi.org/10.1007/s10880-018-9556-9
19. Morris SE, Kearns JP, Moment A, Lee KA, deLima Thomas J. “Remembrance”: a self-care tool for clinicians. J Palliat Med. 2019;22(3):316-318. https://doi.org/10.1089/jpm.2018.0395
20. Morris SE, Nayak MM, Block SD. Insights from bereaved family members about end-of-life care and bereavement. J Palliat Med. Published online February 10, 2020. https://doi.org/10.1089/jpm.2019.0467
21. Kentish-Barnes N, Cohen-Solal Z, Souppart V, et al. “It was the only thing I could hold onto, but…”: receiving a letter of condolence after loss of a loved one in the ICU: a qualitative study of bereaved relatives’ experience. Crit Care Med. 2017;45(12):1965-1971. https://doi.org/10.1097/ccm.0000000000002687
As of July 25, 2020, there had been 146,073 deaths from COVID-19 in the United States and 641,273 worldwide, with a disproportionate number of deaths occurring in historically disadvantaged minority groups, specifically African Americans.1,2 The number of decedents will continue to increase over the coming months, even as the number of new COVID-19 cases decreases. Given that, for each death, five persons are believed to be significantly affected,3 the number of bereaved individuals whose loved ones died during the pandemic in the United States alone is likely to be in the millions.
COVID-19–related mortality has become a pressing public health issue, and as a result, support for bereaved family members, especially for minority populations, is also an important public health issue.4 It is likely that bereaved individuals are at greater risk of poor bereavement outcomes during the pandemic—irrespective of whether the death was a result of COVID-19—because of social isolation. This is particularly true if loved ones died in the hospital and, due to visitor restrictions, faced limited or no visitation. For many, bereavement will be affected by stay-at-home orders and social distancing restrictions that reduce access to emotional support and rituals, such as funerals, that usually provide comfort.5
Urgent attention is needed to support bereaved individuals, to flatten the curve of mental health disorders associated with the death of loved ones during the pandemic. Within a preventive model of care, we offer guidelines for how hospitals, longitudinal providers, and mental health clinicians can provide bereavement outreach to all individuals whose loved ones died during the COVID-19 pandemic.
PUBLIC HEALTH MODEL OF BEREAVEMENT SUPPORT
The provision of bereavement care, including the assessment of risk for poor bereavement outcomes, is an essential component of high-quality end-of-life care endorsed by the hospice and palliative care movement.6 However, the development of standardized bereavement services has lagged behind that of other components of palliative care, varying greatly by institution and provider.7 Approximately 10% to 20% of bereaved individuals experience psychiatric difficulties following the death of a loved one, including prolonged grief disorder, posttraumatic stress disorder, and major depressive disorder.8 Risk factors include a hospital-based death, death in an intensive care unit (ICU), sudden death, not being able to say goodbye, and a history of psychiatric disorders.8,9
One of the biggest barriers in providing standardized bereavement services is the lack of a systematic process to identify individuals at risk of poor bereavement outcomes.10 Aoun et al developed a public health model of bereavement support that comprises a three-tiered approach to risk and the corresponding need for support.11 They propose that the low-risk group, approximately 60% of bereaved individuals, would primarily need support from family and friends, the moderate-risk group (30%) would need support from the wider community, and the high-risk group (10%) would need support from mental health providers.
It is reasonable to assume that many individuals whose loved ones died during the pandemic will fall into a high-risk group for poor bereavement outcomes, as identified by Aoun et al.11 Given a higher than usual inpatient mortality due to COVID-19 for certain populations and that bereavement care is already underrecognized within healthcare systems, hospitals and other healthcare facilities and their providers need to fill this void.
EDUCATION, GUIDANCE, AND SUPPORT MODEL
We adopted an education, guidance, and support model of bereavement support in 2019.7 This model has been shown to positively affect the experience of bereaved individuals, especially because of condolences from providers and psycho-educational information about coping with grief.7 Each month, a list of deceased patients and family contacts is generated from a mortality review database,12 and bereavement packets are mailed to family members; the packet includes a condolence letter from senior management, a psycho-educational grief guide, and a list of community-based resources. A social worker is also available to provide telephone support and to assist with mental health referrals. For patients who died in the COVID-19–specific units, social work also provides support and outreach to families.
Psycho-Education
During the early weeks of the pandemic, a tip sheet—”Grieving during a pandemic”13— was created to include in the bereavement packet and for distribution to community organizations within the hospital’s geographical area. This tip sheet offers strategies to facilitate coping based on the psychological model of cognitive-behavioral therapy (CBT).14 Topics addressed include understanding the nature of grief, self-care, adapting bereavement rituals in light of social distancing, challenging unhelpful thinking patterns that might lead to feelings of guilt especially regarding the death of the patient, and ways to obtain support during the pandemic. The tip sheet was made available in Spanish, French, Chinese, Haitian Creole, Portuguese, Arabic, and Russian given that our mortality data, consistent with preliminary findings from New York State, suggested higher death rates among Black/African American and Hispanic/Latino groups, compared with historical mortality statistics.15
Virtual Support
As part of our bereavement response during the COVID-19 crisis, we have launched virtual bereavement support for families impacted by the pandemic. It is challenging to identify the optimal type of support and timing, given the reliance on virtual outreach without in-person screening. With the increased distress and trauma associated with deaths during the pandemic, one clinical challenge is managing emotions in a virtual group without access to the usual tools that clinicians rely on, such as reading body language. Following a graded exposure approach, a form of behavioral therapy,14 we recommend offering a psycho-educational seminar first in which facilitators can control the content and limit exposure of sharing stories from participants. For support groups (eg, 6 to 8 sessions), we recommend that participants be screened prior to assess their risk factors and readiness and provide individual therapist referrals as needed.10
Community Outreach
Many diverse communities have been affected significantly by COVID-19 and faced high mortality rates.16 We recognized that proactive bereavement outreach to these communities was essential. Grief guides and tip sheets in various languages were made available as part of our community outreach programs, which included vans traveling to severely affected communities and providing testing, masks, alcohol-based hand sanitizer, and written materials.
Education About Bereavement
Many clinicians and staff express feelings of inadequacy about providing bereavement outreach. Such feelings are not uncommon, especially because clinicians tend to receive little training in dealing with the emotional toll of patient deaths and bereavement care.17,18 These feelings are likely to be heightened during this pandemic given the increased exposure to patient deaths, concern for personal safety, and changed practices in providing care, including the need to socially distance. Providing support for clinicians to process their feelings about the death of patients is crucial.19 In addition to our Employee Assistance Program, psychosocial clinicians are facilitating weekly virtual support groups for providers to discuss the effects of the pandemic on their personal and professional lives.
Bereaved family members report they benefit from hearing from the clinical team and receiving condolences, which is seen as humanizing the physician-family relationship. This personal outreach is likely more important during this time because many providers will have interacted with family members virtually.7,20,21 To help facilitate offers of condolences, we developed the TEARS acronym to describe the components of a condolence call that can also be adapted for writing condolence cards (Table).
GUIDELINES
We recommend that hospitals and other healthcare facilities that might not have well-established bereavement programs consider adopting a building block approach to provide basic outreach to families of their deceased patients.7 Tapping into existing resources, the major components are as follows: (1) a letter of condolence from leadership, (2) psycho-educational information about grief, (3) a list of community/online resources, including information about local hospice bereavement programs and bereavement camps or programs for children, (4) offers of condolences from individual providers/teams, and (5) mental health outreach as indicated.
CONCLUSION
The COVID-19–related mortality, particularly among already vulnerable populations, coupled with the existing underrecognition of bereavement has created an urgent public health issue that needs to be addressed. Given that few institutions offer standardized bereavement follow-up, we believe that hospital providers and mental health clinicians need to take a proactive approach to providing bereavement outreach to families affected by death during the pandemic.
Acknowledgments
The authors would like to acknowledge the Brigham Health Bereavement Committee and the staff of Care Continuum Management and the Department of Community Outreach at Brigham and Women’s Hospital.
Disclosures
No competing financial interests relevant to this article exist for Dr Morris, Ms Paterson, and Dr Mendu. Dr Morris receives royalties for two self-help books about grief published by Robinson and Dr Mendu provides consulting services for Bayer AG unrelated to the content of this article.
As of July 25, 2020, there had been 146,073 deaths from COVID-19 in the United States and 641,273 worldwide, with a disproportionate number of deaths occurring in historically disadvantaged minority groups, specifically African Americans.1,2 The number of decedents will continue to increase over the coming months, even as the number of new COVID-19 cases decreases. Given that, for each death, five persons are believed to be significantly affected,3 the number of bereaved individuals whose loved ones died during the pandemic in the United States alone is likely to be in the millions.
COVID-19–related mortality has become a pressing public health issue, and as a result, support for bereaved family members, especially for minority populations, is also an important public health issue.4 It is likely that bereaved individuals are at greater risk of poor bereavement outcomes during the pandemic—irrespective of whether the death was a result of COVID-19—because of social isolation. This is particularly true if loved ones died in the hospital and, due to visitor restrictions, faced limited or no visitation. For many, bereavement will be affected by stay-at-home orders and social distancing restrictions that reduce access to emotional support and rituals, such as funerals, that usually provide comfort.5
Urgent attention is needed to support bereaved individuals, to flatten the curve of mental health disorders associated with the death of loved ones during the pandemic. Within a preventive model of care, we offer guidelines for how hospitals, longitudinal providers, and mental health clinicians can provide bereavement outreach to all individuals whose loved ones died during the COVID-19 pandemic.
PUBLIC HEALTH MODEL OF BEREAVEMENT SUPPORT
The provision of bereavement care, including the assessment of risk for poor bereavement outcomes, is an essential component of high-quality end-of-life care endorsed by the hospice and palliative care movement.6 However, the development of standardized bereavement services has lagged behind that of other components of palliative care, varying greatly by institution and provider.7 Approximately 10% to 20% of bereaved individuals experience psychiatric difficulties following the death of a loved one, including prolonged grief disorder, posttraumatic stress disorder, and major depressive disorder.8 Risk factors include a hospital-based death, death in an intensive care unit (ICU), sudden death, not being able to say goodbye, and a history of psychiatric disorders.8,9
One of the biggest barriers in providing standardized bereavement services is the lack of a systematic process to identify individuals at risk of poor bereavement outcomes.10 Aoun et al developed a public health model of bereavement support that comprises a three-tiered approach to risk and the corresponding need for support.11 They propose that the low-risk group, approximately 60% of bereaved individuals, would primarily need support from family and friends, the moderate-risk group (30%) would need support from the wider community, and the high-risk group (10%) would need support from mental health providers.
It is reasonable to assume that many individuals whose loved ones died during the pandemic will fall into a high-risk group for poor bereavement outcomes, as identified by Aoun et al.11 Given a higher than usual inpatient mortality due to COVID-19 for certain populations and that bereavement care is already underrecognized within healthcare systems, hospitals and other healthcare facilities and their providers need to fill this void.
EDUCATION, GUIDANCE, AND SUPPORT MODEL
We adopted an education, guidance, and support model of bereavement support in 2019.7 This model has been shown to positively affect the experience of bereaved individuals, especially because of condolences from providers and psycho-educational information about coping with grief.7 Each month, a list of deceased patients and family contacts is generated from a mortality review database,12 and bereavement packets are mailed to family members; the packet includes a condolence letter from senior management, a psycho-educational grief guide, and a list of community-based resources. A social worker is also available to provide telephone support and to assist with mental health referrals. For patients who died in the COVID-19–specific units, social work also provides support and outreach to families.
Psycho-Education
During the early weeks of the pandemic, a tip sheet—”Grieving during a pandemic”13— was created to include in the bereavement packet and for distribution to community organizations within the hospital’s geographical area. This tip sheet offers strategies to facilitate coping based on the psychological model of cognitive-behavioral therapy (CBT).14 Topics addressed include understanding the nature of grief, self-care, adapting bereavement rituals in light of social distancing, challenging unhelpful thinking patterns that might lead to feelings of guilt especially regarding the death of the patient, and ways to obtain support during the pandemic. The tip sheet was made available in Spanish, French, Chinese, Haitian Creole, Portuguese, Arabic, and Russian given that our mortality data, consistent with preliminary findings from New York State, suggested higher death rates among Black/African American and Hispanic/Latino groups, compared with historical mortality statistics.15
Virtual Support
As part of our bereavement response during the COVID-19 crisis, we have launched virtual bereavement support for families impacted by the pandemic. It is challenging to identify the optimal type of support and timing, given the reliance on virtual outreach without in-person screening. With the increased distress and trauma associated with deaths during the pandemic, one clinical challenge is managing emotions in a virtual group without access to the usual tools that clinicians rely on, such as reading body language. Following a graded exposure approach, a form of behavioral therapy,14 we recommend offering a psycho-educational seminar first in which facilitators can control the content and limit exposure of sharing stories from participants. For support groups (eg, 6 to 8 sessions), we recommend that participants be screened prior to assess their risk factors and readiness and provide individual therapist referrals as needed.10
Community Outreach
Many diverse communities have been affected significantly by COVID-19 and faced high mortality rates.16 We recognized that proactive bereavement outreach to these communities was essential. Grief guides and tip sheets in various languages were made available as part of our community outreach programs, which included vans traveling to severely affected communities and providing testing, masks, alcohol-based hand sanitizer, and written materials.
Education About Bereavement
Many clinicians and staff express feelings of inadequacy about providing bereavement outreach. Such feelings are not uncommon, especially because clinicians tend to receive little training in dealing with the emotional toll of patient deaths and bereavement care.17,18 These feelings are likely to be heightened during this pandemic given the increased exposure to patient deaths, concern for personal safety, and changed practices in providing care, including the need to socially distance. Providing support for clinicians to process their feelings about the death of patients is crucial.19 In addition to our Employee Assistance Program, psychosocial clinicians are facilitating weekly virtual support groups for providers to discuss the effects of the pandemic on their personal and professional lives.
Bereaved family members report they benefit from hearing from the clinical team and receiving condolences, which is seen as humanizing the physician-family relationship. This personal outreach is likely more important during this time because many providers will have interacted with family members virtually.7,20,21 To help facilitate offers of condolences, we developed the TEARS acronym to describe the components of a condolence call that can also be adapted for writing condolence cards (Table).
GUIDELINES
We recommend that hospitals and other healthcare facilities that might not have well-established bereavement programs consider adopting a building block approach to provide basic outreach to families of their deceased patients.7 Tapping into existing resources, the major components are as follows: (1) a letter of condolence from leadership, (2) psycho-educational information about grief, (3) a list of community/online resources, including information about local hospice bereavement programs and bereavement camps or programs for children, (4) offers of condolences from individual providers/teams, and (5) mental health outreach as indicated.
CONCLUSION
The COVID-19–related mortality, particularly among already vulnerable populations, coupled with the existing underrecognition of bereavement has created an urgent public health issue that needs to be addressed. Given that few institutions offer standardized bereavement follow-up, we believe that hospital providers and mental health clinicians need to take a proactive approach to providing bereavement outreach to families affected by death during the pandemic.
Acknowledgments
The authors would like to acknowledge the Brigham Health Bereavement Committee and the staff of Care Continuum Management and the Department of Community Outreach at Brigham and Women’s Hospital.
Disclosures
No competing financial interests relevant to this article exist for Dr Morris, Ms Paterson, and Dr Mendu. Dr Morris receives royalties for two self-help books about grief published by Robinson and Dr Mendu provides consulting services for Bayer AG unrelated to the content of this article.
1. Coronavirus Resource Center Covid-19: Case Tracker. Johns Hopkins University. Accessed July 25, 2020. https://coronavirus.jhu.edu/
2. Tappe A. America’s black and Hispanic communities are bearing the brunt of the coronavirus. CNN. April 21, 2020. Accessed June 7, 2020. https://www.cnn.com/2020/04/21/economy/coronavirus-burden-black-hispanic-workers/index.html
3. Shear K, Frank E, Houck PR, Reynolds CF 3rd. Treatment of complicated grief: a randomized controlled trial. JAMA. 2005;293(21):2601-2608. https://doi.org/10.1001/jama.293.21.2601
4. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/nejmsa2011686
5. Morris SE, Moment A, Thomas JD. Caring for bereaved family members during the COVID-19 pandemic: before and after the death of a patient. J Pain Symptom Manage. Published online May 7, 2020. https://doi.org/10.1016/j.jpainsymman.2020.05.002
6. National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 7, 2020. https://www.nationalcoalitionhpc.org/ncp
7. Morris SE, Block SD. Adding value to palliative care services: the development of an institutional bereavement program. J Palliat Med. 2015;18(11):915-922. https://doi.org/10.1089/jpm.2015.0080
8. Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370(9603):1960-1973. https://doi.org/10.1016/s0140-6736(07)61816-9
9. Kentish-Barnes N, Chaize M, Seegers V, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45(5):1341-1352. https://doi.org/10.1183/09031936.00160014
10. Morris SE, Anderson CM, Tarquini SJ, Block SD. A standardized approach to bereavement risk-screening: a quality improvement project. J Psychosoc Oncol. 2020;38(4):406-417. https://doi.org/10.1080/07347332.2019.1703065
11. Aoun SM, Breen LJ, Howting DA, Rumbold B, McNamara B, Hegney D. Who needs bereavement support? a population based survey of bereavement risk and support need. PLoS One. 2015;10(3):e0121101. https://doi.org/10.1371/journal.pone.0121101
12. Mendu ML, Lu Y, Petersen A, et al. Reflections on implementing a hospital-wide provider-based electronic inpatient mortality review system: lessons learnt. BMJ Qual Saf. 2020;29(4):304‐312. https://doi.org/10.1136/bmjqs-2019-009864
13. Morris SE. Grieving during a pandemic. Brigham and Women’s Hospital. Accessed July 25, 2020. https://www.brighamandwomens.org/covid-19/grieving-during-a-pandemic
14. Beck JS. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. Guilford Press; 2011.
15. Coronavirus Disease 2019 (COVID-19). Health Equity Considerations and Racial and Ethnic Minority Groups. Centers for Disease Control and Prevention. Updated July 24, 2020. Accessed July 25, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
16. Death rates in Mass. surged in areas already hard hit. Boston Globe. May 17, 2020. Accessed June 8, 2020. https://www.bostonglobe.com/2020/05/17/opinion/death-rate-mass-surged-areas-already-hard-hit/
17. Jackson VA, Sullivan AM, Gadmer NM, et al. “It was haunting…”: physicians’ descriptions of emotionally powerful patient deaths. Acad Med. 2005;80(7):648-656. https://doi.org/10.1097/00001888-200507000-00007
18. Morris S, Schaefer K, Rosowsky E. Primary care for the elderly bereaved: recommendations for medical education. J Clin Psychol Med Settings. 2018;25(4):463‐470. https://doi.org/10.1007/s10880-018-9556-9
19. Morris SE, Kearns JP, Moment A, Lee KA, deLima Thomas J. “Remembrance”: a self-care tool for clinicians. J Palliat Med. 2019;22(3):316-318. https://doi.org/10.1089/jpm.2018.0395
20. Morris SE, Nayak MM, Block SD. Insights from bereaved family members about end-of-life care and bereavement. J Palliat Med. Published online February 10, 2020. https://doi.org/10.1089/jpm.2019.0467
21. Kentish-Barnes N, Cohen-Solal Z, Souppart V, et al. “It was the only thing I could hold onto, but…”: receiving a letter of condolence after loss of a loved one in the ICU: a qualitative study of bereaved relatives’ experience. Crit Care Med. 2017;45(12):1965-1971. https://doi.org/10.1097/ccm.0000000000002687
1. Coronavirus Resource Center Covid-19: Case Tracker. Johns Hopkins University. Accessed July 25, 2020. https://coronavirus.jhu.edu/
2. Tappe A. America’s black and Hispanic communities are bearing the brunt of the coronavirus. CNN. April 21, 2020. Accessed June 7, 2020. https://www.cnn.com/2020/04/21/economy/coronavirus-burden-black-hispanic-workers/index.html
3. Shear K, Frank E, Houck PR, Reynolds CF 3rd. Treatment of complicated grief: a randomized controlled trial. JAMA. 2005;293(21):2601-2608. https://doi.org/10.1001/jama.293.21.2601
4. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/nejmsa2011686
5. Morris SE, Moment A, Thomas JD. Caring for bereaved family members during the COVID-19 pandemic: before and after the death of a patient. J Pain Symptom Manage. Published online May 7, 2020. https://doi.org/10.1016/j.jpainsymman.2020.05.002
6. National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 7, 2020. https://www.nationalcoalitionhpc.org/ncp
7. Morris SE, Block SD. Adding value to palliative care services: the development of an institutional bereavement program. J Palliat Med. 2015;18(11):915-922. https://doi.org/10.1089/jpm.2015.0080
8. Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370(9603):1960-1973. https://doi.org/10.1016/s0140-6736(07)61816-9
9. Kentish-Barnes N, Chaize M, Seegers V, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45(5):1341-1352. https://doi.org/10.1183/09031936.00160014
10. Morris SE, Anderson CM, Tarquini SJ, Block SD. A standardized approach to bereavement risk-screening: a quality improvement project. J Psychosoc Oncol. 2020;38(4):406-417. https://doi.org/10.1080/07347332.2019.1703065
11. Aoun SM, Breen LJ, Howting DA, Rumbold B, McNamara B, Hegney D. Who needs bereavement support? a population based survey of bereavement risk and support need. PLoS One. 2015;10(3):e0121101. https://doi.org/10.1371/journal.pone.0121101
12. Mendu ML, Lu Y, Petersen A, et al. Reflections on implementing a hospital-wide provider-based electronic inpatient mortality review system: lessons learnt. BMJ Qual Saf. 2020;29(4):304‐312. https://doi.org/10.1136/bmjqs-2019-009864
13. Morris SE. Grieving during a pandemic. Brigham and Women’s Hospital. Accessed July 25, 2020. https://www.brighamandwomens.org/covid-19/grieving-during-a-pandemic
14. Beck JS. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. Guilford Press; 2011.
15. Coronavirus Disease 2019 (COVID-19). Health Equity Considerations and Racial and Ethnic Minority Groups. Centers for Disease Control and Prevention. Updated July 24, 2020. Accessed July 25, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
16. Death rates in Mass. surged in areas already hard hit. Boston Globe. May 17, 2020. Accessed June 8, 2020. https://www.bostonglobe.com/2020/05/17/opinion/death-rate-mass-surged-areas-already-hard-hit/
17. Jackson VA, Sullivan AM, Gadmer NM, et al. “It was haunting…”: physicians’ descriptions of emotionally powerful patient deaths. Acad Med. 2005;80(7):648-656. https://doi.org/10.1097/00001888-200507000-00007
18. Morris S, Schaefer K, Rosowsky E. Primary care for the elderly bereaved: recommendations for medical education. J Clin Psychol Med Settings. 2018;25(4):463‐470. https://doi.org/10.1007/s10880-018-9556-9
19. Morris SE, Kearns JP, Moment A, Lee KA, deLima Thomas J. “Remembrance”: a self-care tool for clinicians. J Palliat Med. 2019;22(3):316-318. https://doi.org/10.1089/jpm.2018.0395
20. Morris SE, Nayak MM, Block SD. Insights from bereaved family members about end-of-life care and bereavement. J Palliat Med. Published online February 10, 2020. https://doi.org/10.1089/jpm.2019.0467
21. Kentish-Barnes N, Cohen-Solal Z, Souppart V, et al. “It was the only thing I could hold onto, but…”: receiving a letter of condolence after loss of a loved one in the ICU: a qualitative study of bereaved relatives’ experience. Crit Care Med. 2017;45(12):1965-1971. https://doi.org/10.1097/ccm.0000000000002687
© 2020 Society of Hospital Medicine
Clinical Progress Note: Procalcitonin in the Identification of Invasive Bacterial Infections in Febrile Young Infants
Febrile infants 60 days of age or younger pose a significant diagnostic challenge for clinicians. Most of these infants are well appearing and do not have localizing signs or symptoms of infection, yet they may have serious bacterial infections (SBI) such as urinary tract infection (UTI), bacteremia, and meningitis. While urinalysis is highly sensitive for predicting UTI,1 older clinical decision rules and biomarkers such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) lack both appropriate sensitivity and specificity for identifying bacteremia and meningitis (ie, invasive bacterial infection [IBI]),2,3 which affect approximately 2.4% and 0.9% of febrile infants during the first 2 months of life, respectively.4 The lack of accurate diagnostic markers can drive overuse of laboratory testing, antibiotics, and hospitalization despite the low rates of these infections. As a result, procalcitonin (PCT) has generated interest because of its potential to serve as a more accurate biomarker for bacterial infections. This review summarizes recent literature on the diagnostic utility of PCT in the identification of IBI in febrile young infants 60 days or younger.
MECHANISM OF PROCALCITONIN
Procalcitonin is undetectable in noninflammatory states but can be detected in the blood within 4 to 6 hours after initial bacterial infection.5 Its production is stimulated throughout various tissues of the body by cytokines such as interleukin-6 and tumor necrosis factor, which are produced in response to bacterial infections. Interferon-γ, which is produced in response to viral infections, attenuates PCT production. While these characteristics suggest promise for PCT as a more specific screening test for underlying bacterial infection, there are caveats. PCT levels are physiologically elevated in the first 48 hours of life and vary with gestational age, factors that should be considered when interpreting results.6 Additionally, PCT levels can rise in other inflammatory states such as autoimmune conditions and certain malignancies,5 though these states are unlikely to confound the evaluation of febrile young infants.
DIAGNOSTIC ACCURACY OF PROCALCITONIN
Because of PCT’s potential to be more specific than other commonly used biomarkers, multiple studies have evaluated its performance characteristics in febrile young infants. Gomez et al retrospectively evaluated 1,112 well-appearing infants younger than 3 months with fever without a source in seven European emergency departments (EDs).7 Overall, 23 infants (2.1%) had IBI (1 with meningitis). A PCT level of 0.5 ng/mL or greater was the only independent risk factor for IBI (adjusted odds ratio, 21.69; 95% CI, 7.93-59.28). Four infants with IBI had a PCT level less than 0.5 ng/mL, and none of these four had meningitis. PCT was superior to CRP, ANC, and WBC in detecting IBI (area under the curve [AUC], 0.825; 95% CI, 0.698-0.952). PCT was the also the best marker for identifying IBI among 451 infants with a normal urine dipstick and fever detected ≤6 hours before presentation (AUC, 0.819; 95% CI, 0.551-1.087).
In the largest prospective study to date evaluating the diagnostic accuracy of PCT in febrile young infants, Milcent et al studied 2,047 previously healthy infants aged 7-91 days admitted for fever from 15 French EDs.8 In total, 21 (1%) had an IBI (8 with meningitis). PCT performed better than CRP, ANC, and WBC for the detection of IBI with an AUC of 0.91 (95% CI, 0.83-0.99). In a multivariable model, a PCT level of 0.3 ng/mL or greater was the only independent risk factor for IBI with an adjusted odds ratio of 40.3 (95% CI, 5.0-332). Only one infant with IBI had a PCT level less than 0.3 ng/mL. This infant was 83 days old, had 4 hours of fever, and became afebrile spontaneously prior to the blood culture revealing Streptococcus pneumoniae. PCT also performed better than CRP in the detection of IBI in infants 7-30 days of age and those with fever for less than 6 hours, though both subgroups had small numbers of infants with IBI. The authors determined that a PCT level of 0.3 ng/mL was the optimal cutoff for ruling out IBI; this cutoff had a sensitivity of 90% and negative likelihood ratio (LR) of 0.1 (Table). In contrast, the more commonly studied PCT cutoff of 0.5 ng/mL increased the negative LR to 0.2. The authors suggested that PCT, when used in the context of history, exam, and tests such as urinalysis, could identify infants at low risk of IBI.
Gomez et al conducted a prospective, single-center study of well-appearing infants with fever without a source and negative urine dipsticks.9 They identified IBI in 9 of 196 infants (4.5%) 21 days or younger and 13 of 1,331 infants (1.0%) 22-90 days old. PCT was superior to CRP and ANC for IBI detection in both age groups. However, in infants 21 days or younger, both the positive and negative LRs for PCT levels of 0.5 ng/mL or greater were poor (Table). Differences in results from the prior two studies7,8 may be related to smaller sample size and differences in patient population because this study included infants younger than 7 days and a higher proportion of infants presenting within 6 hours of fever.
CLINICAL DECISION RULES
PCT has also been incorporated into clinical decision rules for febrile young infants, primarily to identify those at low risk of either IBI or SBI. The Step-by-Step approach10 classified well-appearing febrile infants 90 days or younger as having a high risk of IBI if they were ill appearing, younger than 21 days old, had a positive urine dipstick or a PCT level of 0.5 ng/mL or greater, and classified them as intermediate risk if they had a CRP level greater than 20 mg/L or ANC level greater than 10,000/µL. The remaining infants were classified as low risk and could be managed as outpatients without lumbar puncture or empiric antibiotics. Of note, derivation of this rule excluded patients with respiratory signs or symptoms. In a prospective validation study with 2,185 infants from 11 European EDs, 87 (4.0%) had an IBI (10 with bacterial meningitis). Sequentially identifying patients as high risk using general appearance, age, and urine dipstick alone identified 80% of infants with IBI and 90% of those with bacterial meningitis. The remaining case of meningitis would have been detected by an elevated PCT. A total of 7 of 991 infants (0.7%) classified as low risk had an IBI and none had meningitis. Six of these infants had a fever duration of less than 2 hours, which would not be enough time for PCT to rise. The Step-by-Step approach, with a sensitivity of 92% and negative LR of 0.17, performed well in the ability to rule out IBI.
A clinical prediction rule developed by the Pediatric Emergency Care Applied Research Network (PECARN) found that urinalysis, ANC, and PCT performed well in identifying infants 60 days or younger at low risk for SBI and IBI.11 This prospective observational study of 1,821 infants 60 days or younger in 26 US EDs found 170 (9.3%) with SBI and 30 (1.6%) with IBI; 10 had bacterial meningitis. Only one patient with IBI was classified as low risk, a 30-day-old whose blood culture grew Enterobacter cloacae and who had a negative repeat blood culture prior to antibiotic treatment. Together, a negative urinalysis, ANC of 4,090/µL or less, and PCT level of 1.71 ng/mL or less were excellent in predicting infants at low risk for both SBI and IBI, with a sensitivity of 97% and negative LR of 0.05 for the outcome of IBI. When applying these variables with “rounded cutoffs” of PCT levels less than 0.5 ng/mL (chosen by the authors because it is a more commonly used cutoff) and ANC of 4,000/µL or less to identify infants at low risk for SBI, their performance was similar to nonrounded cutoffs. Data for the rule with rounded cutoffs in identifying infants at low risk for IBI were not presented. The PECARN study was limited by the small numbers of infants with IBIs, and the authors recommended caution when applying the rule to infants 28 days or younger.
Older clinical decision rules without PCT, such as the Rochester and modified Philadelphia criteria, use clinical and laboratory features to assess risk of IBI.3 Recent studies have evaluated these criteria in cohorts with larger numbers of infants with IBI since the derivation studies included mostly infants with SBI and small numbers with IBI.3 Gomez et al demonstrated that the Rochester criteria had lower sensitivity and higher negative LR than the Step-by-Step approach in IBI detection.10 In a case-control study of 135 cases of IBI with 249 matched controls, Aronson et al reported that the modified Philadelphia criteria had higher sensitivity but lower specificity than the Rochester criteria for IBI detection.12 The ability of the Rochester and modified Philadelphia criteria to rule out IBI, as demonstrated by the negative LR (range 0.2-0.4), was inferior to the negative LRs documented by Milcent et al8 (PCT cutoff value of 0.3 ng/mL), the Step-by-Step approach,10 and the PECARN rule11 (range 0.05-0.17; Table). However, clinical decision rules with and without PCT suffer similar limitations in having poor specificity in identifying infants likely to have IBI.
GAPS IN THE LITERATURE
Several key knowledge gaps around PCT use for diagnosing neonatal infections exist. First, the optimal use of PCT in context with other biomarkers and clinical decision rules remains uncertain. A meta-analysis of 28 studies involving over 2,600 infants that compared PCT level (with and without CRP) with isolated CRP and presepsin levels found that PCT in combination with CRP had greater diagnostic accuracy than either PCT or CRP alone, which highlights a potential opportunity for prospective study.13 Second, more data are needed on the use of PCT in the ≤ 28-day age group given the increased risk of both IBI and neonatal herpes simplex virus infection (HSV), compared with that in the second month of life. Neonatal HSV poses diagnostic challenges because half of infants will initially present as afebrile,14 and delays in initiating antiviral treatment dramatically increase the risk of permanent disability or death.15 There have been no prospective studies evaluating PCT use as part of neonatal HSV evaluations.
CLINICAL APPLICATIONS AND CONCLUSIONS
In summary, PCT can play an important adjunctive diagnostic role in the evaluation of febrile young infants, especially during the second month of life when outpatient management is more likely to be considered. PCT is superior to other inflammatory markers in identifying IBI, though the optimal cutoffs to maximize sensitivity and specificity are uncertain. Its performance characteristics, both alone and within clinical decision rules, can help clinicians better identify children at low risk for IBI when compared with clinical decision rules without PCT. PCT measurement can help clinicians miss fewer infants with IBI and identify infants for whom safely doing less is an appropriate option, which can ultimately reduce costs and hospitalizations. PCT may be particularly helpful when the clinical history is difficult to assess or when other diagnostic test results are missing or give conflicting results. Centers that use PCT will need to ensure that results are available within a short turnaround time (a few hours) in order to meaningfully affect care. Future studies of PCT in febrile infant evaluations should focus on identifying optimal strategies for incorporating this biomarker into risk assessments that present information to parents in a way that enables them to understand their child’s risk of a serious infection.
1. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068
2. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
3. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1-297.
4. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190874. https://doi.org/10.1001/jamanetworkopen.2019.0874
5. Fontela PS, Lacroix J. Procalcitonin: is this the promised biomarker for critically ill patients? J Pediatr Intensive Care. 2016;5(4):162-171. https://doi.org/10.1055/s-0036-1583279
6. Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412(11-12):1053-1059. https://doi.org/10.1016/j.cca.2011.02.020
7. Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815-822. https://doi.org/10.1542/peds.2011-3575
8. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62-69. https://doi.org/10.1001/jamapediatrics.2015.3210
9. Gomez B, Diaz H, Carro A, Benito J, Mintegi S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch Dis Child. 2019;104(6):547-551. https://doi.org/10.1136/archdischild-2018-315397
10. Gomez B, Mintegi S, Bressan S, et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381
11. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
12. Aronson PL, Wang ME, Shapiro ED, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879
13. Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. https://doi.org/10.1186/s13054-018-2236-1
14. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166
15. Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med. 2013;18(2):e20. https://doi.org/10.1136/eb-2012-100674
Febrile infants 60 days of age or younger pose a significant diagnostic challenge for clinicians. Most of these infants are well appearing and do not have localizing signs or symptoms of infection, yet they may have serious bacterial infections (SBI) such as urinary tract infection (UTI), bacteremia, and meningitis. While urinalysis is highly sensitive for predicting UTI,1 older clinical decision rules and biomarkers such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) lack both appropriate sensitivity and specificity for identifying bacteremia and meningitis (ie, invasive bacterial infection [IBI]),2,3 which affect approximately 2.4% and 0.9% of febrile infants during the first 2 months of life, respectively.4 The lack of accurate diagnostic markers can drive overuse of laboratory testing, antibiotics, and hospitalization despite the low rates of these infections. As a result, procalcitonin (PCT) has generated interest because of its potential to serve as a more accurate biomarker for bacterial infections. This review summarizes recent literature on the diagnostic utility of PCT in the identification of IBI in febrile young infants 60 days or younger.
MECHANISM OF PROCALCITONIN
Procalcitonin is undetectable in noninflammatory states but can be detected in the blood within 4 to 6 hours after initial bacterial infection.5 Its production is stimulated throughout various tissues of the body by cytokines such as interleukin-6 and tumor necrosis factor, which are produced in response to bacterial infections. Interferon-γ, which is produced in response to viral infections, attenuates PCT production. While these characteristics suggest promise for PCT as a more specific screening test for underlying bacterial infection, there are caveats. PCT levels are physiologically elevated in the first 48 hours of life and vary with gestational age, factors that should be considered when interpreting results.6 Additionally, PCT levels can rise in other inflammatory states such as autoimmune conditions and certain malignancies,5 though these states are unlikely to confound the evaluation of febrile young infants.
DIAGNOSTIC ACCURACY OF PROCALCITONIN
Because of PCT’s potential to be more specific than other commonly used biomarkers, multiple studies have evaluated its performance characteristics in febrile young infants. Gomez et al retrospectively evaluated 1,112 well-appearing infants younger than 3 months with fever without a source in seven European emergency departments (EDs).7 Overall, 23 infants (2.1%) had IBI (1 with meningitis). A PCT level of 0.5 ng/mL or greater was the only independent risk factor for IBI (adjusted odds ratio, 21.69; 95% CI, 7.93-59.28). Four infants with IBI had a PCT level less than 0.5 ng/mL, and none of these four had meningitis. PCT was superior to CRP, ANC, and WBC in detecting IBI (area under the curve [AUC], 0.825; 95% CI, 0.698-0.952). PCT was the also the best marker for identifying IBI among 451 infants with a normal urine dipstick and fever detected ≤6 hours before presentation (AUC, 0.819; 95% CI, 0.551-1.087).
In the largest prospective study to date evaluating the diagnostic accuracy of PCT in febrile young infants, Milcent et al studied 2,047 previously healthy infants aged 7-91 days admitted for fever from 15 French EDs.8 In total, 21 (1%) had an IBI (8 with meningitis). PCT performed better than CRP, ANC, and WBC for the detection of IBI with an AUC of 0.91 (95% CI, 0.83-0.99). In a multivariable model, a PCT level of 0.3 ng/mL or greater was the only independent risk factor for IBI with an adjusted odds ratio of 40.3 (95% CI, 5.0-332). Only one infant with IBI had a PCT level less than 0.3 ng/mL. This infant was 83 days old, had 4 hours of fever, and became afebrile spontaneously prior to the blood culture revealing Streptococcus pneumoniae. PCT also performed better than CRP in the detection of IBI in infants 7-30 days of age and those with fever for less than 6 hours, though both subgroups had small numbers of infants with IBI. The authors determined that a PCT level of 0.3 ng/mL was the optimal cutoff for ruling out IBI; this cutoff had a sensitivity of 90% and negative likelihood ratio (LR) of 0.1 (Table). In contrast, the more commonly studied PCT cutoff of 0.5 ng/mL increased the negative LR to 0.2. The authors suggested that PCT, when used in the context of history, exam, and tests such as urinalysis, could identify infants at low risk of IBI.

Gomez et al conducted a prospective, single-center study of well-appearing infants with fever without a source and negative urine dipsticks.9 They identified IBI in 9 of 196 infants (4.5%) 21 days or younger and 13 of 1,331 infants (1.0%) 22-90 days old. PCT was superior to CRP and ANC for IBI detection in both age groups. However, in infants 21 days or younger, both the positive and negative LRs for PCT levels of 0.5 ng/mL or greater were poor (Table). Differences in results from the prior two studies7,8 may be related to smaller sample size and differences in patient population because this study included infants younger than 7 days and a higher proportion of infants presenting within 6 hours of fever.
CLINICAL DECISION RULES
PCT has also been incorporated into clinical decision rules for febrile young infants, primarily to identify those at low risk of either IBI or SBI. The Step-by-Step approach10 classified well-appearing febrile infants 90 days or younger as having a high risk of IBI if they were ill appearing, younger than 21 days old, had a positive urine dipstick or a PCT level of 0.5 ng/mL or greater, and classified them as intermediate risk if they had a CRP level greater than 20 mg/L or ANC level greater than 10,000/µL. The remaining infants were classified as low risk and could be managed as outpatients without lumbar puncture or empiric antibiotics. Of note, derivation of this rule excluded patients with respiratory signs or symptoms. In a prospective validation study with 2,185 infants from 11 European EDs, 87 (4.0%) had an IBI (10 with bacterial meningitis). Sequentially identifying patients as high risk using general appearance, age, and urine dipstick alone identified 80% of infants with IBI and 90% of those with bacterial meningitis. The remaining case of meningitis would have been detected by an elevated PCT. A total of 7 of 991 infants (0.7%) classified as low risk had an IBI and none had meningitis. Six of these infants had a fever duration of less than 2 hours, which would not be enough time for PCT to rise. The Step-by-Step approach, with a sensitivity of 92% and negative LR of 0.17, performed well in the ability to rule out IBI.
A clinical prediction rule developed by the Pediatric Emergency Care Applied Research Network (PECARN) found that urinalysis, ANC, and PCT performed well in identifying infants 60 days or younger at low risk for SBI and IBI.11 This prospective observational study of 1,821 infants 60 days or younger in 26 US EDs found 170 (9.3%) with SBI and 30 (1.6%) with IBI; 10 had bacterial meningitis. Only one patient with IBI was classified as low risk, a 30-day-old whose blood culture grew Enterobacter cloacae and who had a negative repeat blood culture prior to antibiotic treatment. Together, a negative urinalysis, ANC of 4,090/µL or less, and PCT level of 1.71 ng/mL or less were excellent in predicting infants at low risk for both SBI and IBI, with a sensitivity of 97% and negative LR of 0.05 for the outcome of IBI. When applying these variables with “rounded cutoffs” of PCT levels less than 0.5 ng/mL (chosen by the authors because it is a more commonly used cutoff) and ANC of 4,000/µL or less to identify infants at low risk for SBI, their performance was similar to nonrounded cutoffs. Data for the rule with rounded cutoffs in identifying infants at low risk for IBI were not presented. The PECARN study was limited by the small numbers of infants with IBIs, and the authors recommended caution when applying the rule to infants 28 days or younger.
Older clinical decision rules without PCT, such as the Rochester and modified Philadelphia criteria, use clinical and laboratory features to assess risk of IBI.3 Recent studies have evaluated these criteria in cohorts with larger numbers of infants with IBI since the derivation studies included mostly infants with SBI and small numbers with IBI.3 Gomez et al demonstrated that the Rochester criteria had lower sensitivity and higher negative LR than the Step-by-Step approach in IBI detection.10 In a case-control study of 135 cases of IBI with 249 matched controls, Aronson et al reported that the modified Philadelphia criteria had higher sensitivity but lower specificity than the Rochester criteria for IBI detection.12 The ability of the Rochester and modified Philadelphia criteria to rule out IBI, as demonstrated by the negative LR (range 0.2-0.4), was inferior to the negative LRs documented by Milcent et al8 (PCT cutoff value of 0.3 ng/mL), the Step-by-Step approach,10 and the PECARN rule11 (range 0.05-0.17; Table). However, clinical decision rules with and without PCT suffer similar limitations in having poor specificity in identifying infants likely to have IBI.
GAPS IN THE LITERATURE
Several key knowledge gaps around PCT use for diagnosing neonatal infections exist. First, the optimal use of PCT in context with other biomarkers and clinical decision rules remains uncertain. A meta-analysis of 28 studies involving over 2,600 infants that compared PCT level (with and without CRP) with isolated CRP and presepsin levels found that PCT in combination with CRP had greater diagnostic accuracy than either PCT or CRP alone, which highlights a potential opportunity for prospective study.13 Second, more data are needed on the use of PCT in the ≤ 28-day age group given the increased risk of both IBI and neonatal herpes simplex virus infection (HSV), compared with that in the second month of life. Neonatal HSV poses diagnostic challenges because half of infants will initially present as afebrile,14 and delays in initiating antiviral treatment dramatically increase the risk of permanent disability or death.15 There have been no prospective studies evaluating PCT use as part of neonatal HSV evaluations.
CLINICAL APPLICATIONS AND CONCLUSIONS
In summary, PCT can play an important adjunctive diagnostic role in the evaluation of febrile young infants, especially during the second month of life when outpatient management is more likely to be considered. PCT is superior to other inflammatory markers in identifying IBI, though the optimal cutoffs to maximize sensitivity and specificity are uncertain. Its performance characteristics, both alone and within clinical decision rules, can help clinicians better identify children at low risk for IBI when compared with clinical decision rules without PCT. PCT measurement can help clinicians miss fewer infants with IBI and identify infants for whom safely doing less is an appropriate option, which can ultimately reduce costs and hospitalizations. PCT may be particularly helpful when the clinical history is difficult to assess or when other diagnostic test results are missing or give conflicting results. Centers that use PCT will need to ensure that results are available within a short turnaround time (a few hours) in order to meaningfully affect care. Future studies of PCT in febrile infant evaluations should focus on identifying optimal strategies for incorporating this biomarker into risk assessments that present information to parents in a way that enables them to understand their child’s risk of a serious infection.
Febrile infants 60 days of age or younger pose a significant diagnostic challenge for clinicians. Most of these infants are well appearing and do not have localizing signs or symptoms of infection, yet they may have serious bacterial infections (SBI) such as urinary tract infection (UTI), bacteremia, and meningitis. While urinalysis is highly sensitive for predicting UTI,1 older clinical decision rules and biomarkers such as white blood cell (WBC) count, absolute neutrophil count (ANC), and C-reactive protein (CRP) lack both appropriate sensitivity and specificity for identifying bacteremia and meningitis (ie, invasive bacterial infection [IBI]),2,3 which affect approximately 2.4% and 0.9% of febrile infants during the first 2 months of life, respectively.4 The lack of accurate diagnostic markers can drive overuse of laboratory testing, antibiotics, and hospitalization despite the low rates of these infections. As a result, procalcitonin (PCT) has generated interest because of its potential to serve as a more accurate biomarker for bacterial infections. This review summarizes recent literature on the diagnostic utility of PCT in the identification of IBI in febrile young infants 60 days or younger.
MECHANISM OF PROCALCITONIN
Procalcitonin is undetectable in noninflammatory states but can be detected in the blood within 4 to 6 hours after initial bacterial infection.5 Its production is stimulated throughout various tissues of the body by cytokines such as interleukin-6 and tumor necrosis factor, which are produced in response to bacterial infections. Interferon-γ, which is produced in response to viral infections, attenuates PCT production. While these characteristics suggest promise for PCT as a more specific screening test for underlying bacterial infection, there are caveats. PCT levels are physiologically elevated in the first 48 hours of life and vary with gestational age, factors that should be considered when interpreting results.6 Additionally, PCT levels can rise in other inflammatory states such as autoimmune conditions and certain malignancies,5 though these states are unlikely to confound the evaluation of febrile young infants.
DIAGNOSTIC ACCURACY OF PROCALCITONIN
Because of PCT’s potential to be more specific than other commonly used biomarkers, multiple studies have evaluated its performance characteristics in febrile young infants. Gomez et al retrospectively evaluated 1,112 well-appearing infants younger than 3 months with fever without a source in seven European emergency departments (EDs).7 Overall, 23 infants (2.1%) had IBI (1 with meningitis). A PCT level of 0.5 ng/mL or greater was the only independent risk factor for IBI (adjusted odds ratio, 21.69; 95% CI, 7.93-59.28). Four infants with IBI had a PCT level less than 0.5 ng/mL, and none of these four had meningitis. PCT was superior to CRP, ANC, and WBC in detecting IBI (area under the curve [AUC], 0.825; 95% CI, 0.698-0.952). PCT was the also the best marker for identifying IBI among 451 infants with a normal urine dipstick and fever detected ≤6 hours before presentation (AUC, 0.819; 95% CI, 0.551-1.087).
In the largest prospective study to date evaluating the diagnostic accuracy of PCT in febrile young infants, Milcent et al studied 2,047 previously healthy infants aged 7-91 days admitted for fever from 15 French EDs.8 In total, 21 (1%) had an IBI (8 with meningitis). PCT performed better than CRP, ANC, and WBC for the detection of IBI with an AUC of 0.91 (95% CI, 0.83-0.99). In a multivariable model, a PCT level of 0.3 ng/mL or greater was the only independent risk factor for IBI with an adjusted odds ratio of 40.3 (95% CI, 5.0-332). Only one infant with IBI had a PCT level less than 0.3 ng/mL. This infant was 83 days old, had 4 hours of fever, and became afebrile spontaneously prior to the blood culture revealing Streptococcus pneumoniae. PCT also performed better than CRP in the detection of IBI in infants 7-30 days of age and those with fever for less than 6 hours, though both subgroups had small numbers of infants with IBI. The authors determined that a PCT level of 0.3 ng/mL was the optimal cutoff for ruling out IBI; this cutoff had a sensitivity of 90% and negative likelihood ratio (LR) of 0.1 (Table). In contrast, the more commonly studied PCT cutoff of 0.5 ng/mL increased the negative LR to 0.2. The authors suggested that PCT, when used in the context of history, exam, and tests such as urinalysis, could identify infants at low risk of IBI.

Gomez et al conducted a prospective, single-center study of well-appearing infants with fever without a source and negative urine dipsticks.9 They identified IBI in 9 of 196 infants (4.5%) 21 days or younger and 13 of 1,331 infants (1.0%) 22-90 days old. PCT was superior to CRP and ANC for IBI detection in both age groups. However, in infants 21 days or younger, both the positive and negative LRs for PCT levels of 0.5 ng/mL or greater were poor (Table). Differences in results from the prior two studies7,8 may be related to smaller sample size and differences in patient population because this study included infants younger than 7 days and a higher proportion of infants presenting within 6 hours of fever.
CLINICAL DECISION RULES
PCT has also been incorporated into clinical decision rules for febrile young infants, primarily to identify those at low risk of either IBI or SBI. The Step-by-Step approach10 classified well-appearing febrile infants 90 days or younger as having a high risk of IBI if they were ill appearing, younger than 21 days old, had a positive urine dipstick or a PCT level of 0.5 ng/mL or greater, and classified them as intermediate risk if they had a CRP level greater than 20 mg/L or ANC level greater than 10,000/µL. The remaining infants were classified as low risk and could be managed as outpatients without lumbar puncture or empiric antibiotics. Of note, derivation of this rule excluded patients with respiratory signs or symptoms. In a prospective validation study with 2,185 infants from 11 European EDs, 87 (4.0%) had an IBI (10 with bacterial meningitis). Sequentially identifying patients as high risk using general appearance, age, and urine dipstick alone identified 80% of infants with IBI and 90% of those with bacterial meningitis. The remaining case of meningitis would have been detected by an elevated PCT. A total of 7 of 991 infants (0.7%) classified as low risk had an IBI and none had meningitis. Six of these infants had a fever duration of less than 2 hours, which would not be enough time for PCT to rise. The Step-by-Step approach, with a sensitivity of 92% and negative LR of 0.17, performed well in the ability to rule out IBI.
A clinical prediction rule developed by the Pediatric Emergency Care Applied Research Network (PECARN) found that urinalysis, ANC, and PCT performed well in identifying infants 60 days or younger at low risk for SBI and IBI.11 This prospective observational study of 1,821 infants 60 days or younger in 26 US EDs found 170 (9.3%) with SBI and 30 (1.6%) with IBI; 10 had bacterial meningitis. Only one patient with IBI was classified as low risk, a 30-day-old whose blood culture grew Enterobacter cloacae and who had a negative repeat blood culture prior to antibiotic treatment. Together, a negative urinalysis, ANC of 4,090/µL or less, and PCT level of 1.71 ng/mL or less were excellent in predicting infants at low risk for both SBI and IBI, with a sensitivity of 97% and negative LR of 0.05 for the outcome of IBI. When applying these variables with “rounded cutoffs” of PCT levels less than 0.5 ng/mL (chosen by the authors because it is a more commonly used cutoff) and ANC of 4,000/µL or less to identify infants at low risk for SBI, their performance was similar to nonrounded cutoffs. Data for the rule with rounded cutoffs in identifying infants at low risk for IBI were not presented. The PECARN study was limited by the small numbers of infants with IBIs, and the authors recommended caution when applying the rule to infants 28 days or younger.
Older clinical decision rules without PCT, such as the Rochester and modified Philadelphia criteria, use clinical and laboratory features to assess risk of IBI.3 Recent studies have evaluated these criteria in cohorts with larger numbers of infants with IBI since the derivation studies included mostly infants with SBI and small numbers with IBI.3 Gomez et al demonstrated that the Rochester criteria had lower sensitivity and higher negative LR than the Step-by-Step approach in IBI detection.10 In a case-control study of 135 cases of IBI with 249 matched controls, Aronson et al reported that the modified Philadelphia criteria had higher sensitivity but lower specificity than the Rochester criteria for IBI detection.12 The ability of the Rochester and modified Philadelphia criteria to rule out IBI, as demonstrated by the negative LR (range 0.2-0.4), was inferior to the negative LRs documented by Milcent et al8 (PCT cutoff value of 0.3 ng/mL), the Step-by-Step approach,10 and the PECARN rule11 (range 0.05-0.17; Table). However, clinical decision rules with and without PCT suffer similar limitations in having poor specificity in identifying infants likely to have IBI.
GAPS IN THE LITERATURE
Several key knowledge gaps around PCT use for diagnosing neonatal infections exist. First, the optimal use of PCT in context with other biomarkers and clinical decision rules remains uncertain. A meta-analysis of 28 studies involving over 2,600 infants that compared PCT level (with and without CRP) with isolated CRP and presepsin levels found that PCT in combination with CRP had greater diagnostic accuracy than either PCT or CRP alone, which highlights a potential opportunity for prospective study.13 Second, more data are needed on the use of PCT in the ≤ 28-day age group given the increased risk of both IBI and neonatal herpes simplex virus infection (HSV), compared with that in the second month of life. Neonatal HSV poses diagnostic challenges because half of infants will initially present as afebrile,14 and delays in initiating antiviral treatment dramatically increase the risk of permanent disability or death.15 There have been no prospective studies evaluating PCT use as part of neonatal HSV evaluations.
CLINICAL APPLICATIONS AND CONCLUSIONS
In summary, PCT can play an important adjunctive diagnostic role in the evaluation of febrile young infants, especially during the second month of life when outpatient management is more likely to be considered. PCT is superior to other inflammatory markers in identifying IBI, though the optimal cutoffs to maximize sensitivity and specificity are uncertain. Its performance characteristics, both alone and within clinical decision rules, can help clinicians better identify children at low risk for IBI when compared with clinical decision rules without PCT. PCT measurement can help clinicians miss fewer infants with IBI and identify infants for whom safely doing less is an appropriate option, which can ultimately reduce costs and hospitalizations. PCT may be particularly helpful when the clinical history is difficult to assess or when other diagnostic test results are missing or give conflicting results. Centers that use PCT will need to ensure that results are available within a short turnaround time (a few hours) in order to meaningfully affect care. Future studies of PCT in febrile infant evaluations should focus on identifying optimal strategies for incorporating this biomarker into risk assessments that present information to parents in a way that enables them to understand their child’s risk of a serious infection.
1. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068
2. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
3. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1-297.
4. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190874. https://doi.org/10.1001/jamanetworkopen.2019.0874
5. Fontela PS, Lacroix J. Procalcitonin: is this the promised biomarker for critically ill patients? J Pediatr Intensive Care. 2016;5(4):162-171. https://doi.org/10.1055/s-0036-1583279
6. Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412(11-12):1053-1059. https://doi.org/10.1016/j.cca.2011.02.020
7. Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815-822. https://doi.org/10.1542/peds.2011-3575
8. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62-69. https://doi.org/10.1001/jamapediatrics.2015.3210
9. Gomez B, Diaz H, Carro A, Benito J, Mintegi S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch Dis Child. 2019;104(6):547-551. https://doi.org/10.1136/archdischild-2018-315397
10. Gomez B, Mintegi S, Bressan S, et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381
11. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
12. Aronson PL, Wang ME, Shapiro ED, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879
13. Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. https://doi.org/10.1186/s13054-018-2236-1
14. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166
15. Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med. 2013;18(2):e20. https://doi.org/10.1136/eb-2012-100674
1. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. https://doi.org/10.1542/peds.2017-3068
2. Cruz AT, Mahajan P, Bonsu BK, et al. Accuracy of complete blood cell counts to identify febrile infants 60 days or younger with invasive bacterial infections. JAMA Pediatr. 2017;171(11):e172927. https://doi.org/10.1001/jamapediatrics.2017.2927
3. Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1-297.
4. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190874. https://doi.org/10.1001/jamanetworkopen.2019.0874
5. Fontela PS, Lacroix J. Procalcitonin: is this the promised biomarker for critically ill patients? J Pediatr Intensive Care. 2016;5(4):162-171. https://doi.org/10.1055/s-0036-1583279
6. Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta. 2011;412(11-12):1053-1059. https://doi.org/10.1016/j.cca.2011.02.020
7. Gomez B, Bressan S, Mintegi S, et al. Diagnostic value of procalcitonin in well-appearing young febrile infants. Pediatrics. 2012;130(5):815-822. https://doi.org/10.1542/peds.2011-3575
8. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170(1):62-69. https://doi.org/10.1001/jamapediatrics.2015.3210
9. Gomez B, Diaz H, Carro A, Benito J, Mintegi S. Performance of blood biomarkers to rule out invasive bacterial infection in febrile infants under 21 days old. Arch Dis Child. 2019;104(6):547-551. https://doi.org/10.1136/archdischild-2018-315397
10. Gomez B, Mintegi S, Bressan S, et al. Validation of the “step-by-step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. https://doi.org/10.1542/peds.2015-4381
11. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342-351. https://doi.org/10.1001/jamapediatrics.2018.5501
12. Aronson PL, Wang ME, Shapiro ED, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879
13. Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316. https://doi.org/10.1186/s13054-018-2236-1
14. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166
15. Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med. 2013;18(2):e20. https://doi.org/10.1136/eb-2012-100674
© 2020 Society of Hospital Medicine
Association Between Bronchiolitis Patient Volume and Continuous Pulse Oximetry Monitoring in 25 Hospitals
Continuous pulse oximetry monitoring in children with bronchiolitis who don’t require supplemental oxygen is discouraged by practice guidelines and is recognized as a form of medical overuse.1-3 This practice can be associated with negative outcomes, including prolonged length of stay,4-6 increased cost of hospitalization,7 and alarm fatigue among nurses.8 Despite initiatives to reduce continuous pulse oximetry monitoring in stable patients with bronchiolitis,1,2 wide practice variation exists between hospitals.9,10 Previous studies have shown that higher prevalence of inpatient bronchiolitis admissions is associated with decreased utilization of unnecessary interventions.11 However, the relationship between pulse oximetry use and bronchiolitis prevalence has not been studied. The objective of this study is to test the hypothesis that hospital units with lower proportions of patients admitted for bronchiolitis and those with fewer general pediatrics patients relative to subspecialty patients would have higher rates of pulse oximetry overuse.
METHODS
Study Design
We conducted a substudy of the Pediatric Research in Inpatient Settings (PRIS) Network’s Eliminating Monitoring Overuse (EMO) pulse oximetry study,10,12 a 56-hospital cross-sectional study that used direct observation to measure the prevalence of continuous pulse oximetry monitoring in hospitalized infants with bronchiolitis who did not require supplemental oxygen between December 1, 2018, through March 31, 2019. This substudy was not included as part of the original aims of the project and was proposed as a separate analysis during data collection. For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study and served as the central IRB. The Research Ethics Board at University of Calgary also approved the study.
Site Selection
Hospitals with at least 60 observations were eligible for inclusion. Of the 32 hospitals that conducted the minimum observations, 25 agreed to participate (21 free-standing children’s hospitals, 3 children’s hospitals within general hospitals, and 1 community hospital).
Patient Population
The parent study included patients aged 8 weeks through 23 months with a primary diagnosis of bronchiolitis. Patients were included only if they were not receiving supplemental oxygen or nasal cannula flow at the time of data collection. The inclusion and exclusion criteria were for both the parent study and the substudy. Further inclusion and exclusion criteria have been described previously.10,12
Data Collection
In order to ascertain continuous pulse oximetry monitoring status, staff at each hospital performed observational rounds by walking to the bedside of each patient who met inclusion criteria. Additional methodology for the parent study has been published elsewhere.10,12
Bronchiolitis Admission Volume by Unit
Collaborators at each hospital gathered bronchiolitis census data from each unit that admitted patients with bronchiolitis. Units were identified prior to data collection and were characterized at the institution level based on previous local definitions. Each site was responsible for using institution-specific data collection methods for determining bronchiolitis and total admissions on each unit (eg, departmental reports or directly querying admissions data using International Classification of Diseases, Tenth Revision, diagnosis codes for bronchiolitis) over the same period as the parent study. Following data analysis, bronchiolitis admission burden was classified into five categories, based on less than 10%, 10% to less than 20%, 20% to less than 30%, 30% to less than 40%, or 40% or more of total admissions having a primary discharge diagnosis of bronchiolitis during the study period. This categorization allowed investigators to determine whether there was a dose-dependent response among categories.
Unit Composition
Site investigators also completed a survey identifying which patients were admitted to each unit (eg, general pediatrics only, medical subspecialty, surgical). Based on these results, units were further classified into seven types (Appendix Table). For the final analysis, units caring exclusively for general pediatrics patients were compared to all other unit types.
Analysis
Bronchiolitis admission burden and unit composition data were combined with observations of pulse oximetry monitoring use of patients not requiring supplemental oxygen from the parent study. We determined unadjusted observed monitoring proportions for each unit’s bronchiolitis admission burden category across all 25 hospitals. This was calculated as a simple proportion of the total number of observations during which patients were continuously monitored divided by the total number of observations performed within each unit’s admission category. We then calculated unadjusted odds ratios using the 40% and higher bronchiolitis admission burden category as a reference. We calculated similar proportions and odds ratios for the dichotomous unit composition variable. Next, we used mixed-effects logistic regression with a random intercept for each hospital to allow for differences in baseline monitoring rates, which varied widely between hospitals (2% to 92%),10 to calculate adjusted odds ratios for the unit’s admission category and unit’s composition. We also adjusted for the same covariates used in the primary study’s analysis (Table).10
RESULTS
We analyzed 2,366 observations of bronchiolitis patients from 25 hospitals. Most observations were concentrated in freestanding children’s hospitals (89%), and 50% were from hospitals with more than 250 pediatric beds. Observations were well distributed among the five categories of admit burden (Table).
In unadjusted regression, the relationship between admission burden and rate of pulse oximetry use did not appear to be dose-dependent, and 95% CIs were wide. We then analyzed the data accounting for baseline differences in hospital monitoring rates and adjusted for the covariates significantly associated with continuous pulse oximetry monitoring in the primary study’s analysis with use of a mixed-effects model. As shown in the Table, low-burden units in which bronchiolitis constituted less than 10% of total admissions had a 2.16-fold increased odds of unnecessary pulse oximetry monitoring compared to high-burden units in which bronchiolitis constituted 40% or more of total admissions (95% CI, 1.27-3.69; P = .01).
In examining the subspecialty unit composition, 596 observations (25.2%) were conducted on units exclusively caring for general pediatrics patients. In the mixed-effects model adjusted for bronchiolitis admission burden and the covariates used in the study’s primary analysis, units exclusively caring for general pediatrics patients did not have significantly different independent odds of pulse oximetry monitoring use compared to units with a mixed patient population (OR 1.01; 95% CI, 0.71-1.45; P = .95) (Appendix Table).
DISCUSSION
In this multicenter observational study of children hospitalized with bronchiolitis not concurrently receiving supplemental oxygen, units that only occasionally cared for bronchiolitis patients appeared to be more likely to overuse continuous pulse oximetry during bronchiolitis hospitalizations.
This finding was not immediately apparent when examining the raw data because of wide hospital-level variation in continuous pulse oximetry monitoring use. However, when the high degree of hospital-level variation in baseline overuse was accounted for with use of a random intercept for each hospital in the mixed-effects model, units that cared for higher proportions of bronchiolitis patients had significantly lower odds of continuous pulse oximetry monitoring use compared to units that cared for these infants infrequently.
As many institutions have subspecialized units to cultivate nursing expertise for care of certain diseases and patient populations, we hypothesized that units caring primarily for children on general pediatrics units would also have lower rates of monitoring overuse compared to mixed units. Interestingly, these units did not perform better, likely because potential cultural factors that might contribute to differences in monitoring are accounted for by bronchiolitis admission burden.
Our findings build on prior literature by demonstrating that unit-level, as well as hospital-level, factors appear to drive overuse in healthcare. A prior single-site retrospective cohort study demonstrated an association between higher prevalence of inpatient bronchiolitis and decreased use of unnecessary interventions such as laboratory and radiographic testing, as well as steroid and antibiotic administration.11 Although study of the relationship between volume and quality is not new to healthcare, to our knowledge, this study is the first to examine the relationship between pulse oximetry overuse in bronchiolitis and unit-level factors like admission burden and subspecialty composition.
There are several limitations. First, because the study population included only children not receiving supplemental oxygen, both the parent study and this substudy assumed that all observed use of pulse oximetry monitoring was overuse. In some cases, however, there may have been other compelling clinical reasons, institutional policies, or differences in pulse oximetry availability that were not captured during data collection or in our adjusted model. Second, hospitals used convenience sampling. It is possible this resulted in samples that were not representative of each unit’s underlying patient population or monitoring practice. In addition, not all of the 32 eligible sites were able to provide data related to hospital admissions at the unit level and thus are not included in our analysis. This remains a potential source of hospital-level selection bias.
CONCLUSION
These findings demonstrate that high bronchiolitis admission burden correlates with lower rates of unnecessary pulse oximetry monitoring in bronchiolitis. We speculate that these outcomes might reflect differing degrees of nursing comfort, expertise, and unit-level norms in caring for bronchiolitis patients, although our study was not designed to establish underlying causes. Identification of operating principles that underpin low pulse oximetry monitoring on high-burden units will provide guidance for decreasing unnecessary monitoring and will inform future studies seeking ways to discourage continuous pulse oximetry monitoring in low-risk infants. Given the institutional variation in monitoring rates, future studies examining both institution-wide and unit-level interventions will be necessary to decrease unnecessary pulse oximetry monitoring in bronchiolitis. Furthermore, these findings may be relevant to studying care quality in other disease processes, with bronchiolitis serving as a model illness for overuse.
Acknowledgments
The authors acknowledge the National Heart, Lung, and Blood Institute of the National Institutes of Health scientists who contributed their expertise to this project as part of the U01 Cooperative Agreement funding mechanism as federal employees conducting their official job duties: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD. The authors thank the executive council of the Pediatric Research in Inpatient Settings Network for their contributions to the early scientific development of this project. The network assessed a Collaborative Support Fee for access to the hospitals and support of this project.
The authors thank the PRIS Network collaborators for their major contributions to data collection (see Appendix).
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
3. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
4. Cunningham S, Rodriguez A, Adams T, et al; Bronchiolitis of Infancy Discharge Study (BIDS) group. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. https://doi.org/10.1016/s0140-6736(15)00163-4
5. Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. https://doi.org/10.1001/archpedi.158.6.527
6. Cunningham S, McMurray A. Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97(4):361-363. https://doi.org/10.1136/adc.2010.205211
7. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC; BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-172. https://doi.org/10.3310/hta19710
8. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851
10. Bonafide CP, Xiao R, Brady PW, et al; for the Pediatric Research in Inpatient Settings (PRIS) Network. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
11. Van Cleve WC, Christakis DA. Unnecessary care for bronchiolitis decreases with increasing inpatient prevalence of bronchiolitis. Pediatrics. 2011;128(5):e1106-e1112. https://doi.org/10.1542/peds.2011-0655
12. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. https://doi.org/10.1186/s40814-019-0453-2
Continuous pulse oximetry monitoring in children with bronchiolitis who don’t require supplemental oxygen is discouraged by practice guidelines and is recognized as a form of medical overuse.1-3 This practice can be associated with negative outcomes, including prolonged length of stay,4-6 increased cost of hospitalization,7 and alarm fatigue among nurses.8 Despite initiatives to reduce continuous pulse oximetry monitoring in stable patients with bronchiolitis,1,2 wide practice variation exists between hospitals.9,10 Previous studies have shown that higher prevalence of inpatient bronchiolitis admissions is associated with decreased utilization of unnecessary interventions.11 However, the relationship between pulse oximetry use and bronchiolitis prevalence has not been studied. The objective of this study is to test the hypothesis that hospital units with lower proportions of patients admitted for bronchiolitis and those with fewer general pediatrics patients relative to subspecialty patients would have higher rates of pulse oximetry overuse.
METHODS
Study Design
We conducted a substudy of the Pediatric Research in Inpatient Settings (PRIS) Network’s Eliminating Monitoring Overuse (EMO) pulse oximetry study,10,12 a 56-hospital cross-sectional study that used direct observation to measure the prevalence of continuous pulse oximetry monitoring in hospitalized infants with bronchiolitis who did not require supplemental oxygen between December 1, 2018, through March 31, 2019. This substudy was not included as part of the original aims of the project and was proposed as a separate analysis during data collection. For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study and served as the central IRB. The Research Ethics Board at University of Calgary also approved the study.
Site Selection
Hospitals with at least 60 observations were eligible for inclusion. Of the 32 hospitals that conducted the minimum observations, 25 agreed to participate (21 free-standing children’s hospitals, 3 children’s hospitals within general hospitals, and 1 community hospital).
Patient Population
The parent study included patients aged 8 weeks through 23 months with a primary diagnosis of bronchiolitis. Patients were included only if they were not receiving supplemental oxygen or nasal cannula flow at the time of data collection. The inclusion and exclusion criteria were for both the parent study and the substudy. Further inclusion and exclusion criteria have been described previously.10,12
Data Collection
In order to ascertain continuous pulse oximetry monitoring status, staff at each hospital performed observational rounds by walking to the bedside of each patient who met inclusion criteria. Additional methodology for the parent study has been published elsewhere.10,12
Bronchiolitis Admission Volume by Unit
Collaborators at each hospital gathered bronchiolitis census data from each unit that admitted patients with bronchiolitis. Units were identified prior to data collection and were characterized at the institution level based on previous local definitions. Each site was responsible for using institution-specific data collection methods for determining bronchiolitis and total admissions on each unit (eg, departmental reports or directly querying admissions data using International Classification of Diseases, Tenth Revision, diagnosis codes for bronchiolitis) over the same period as the parent study. Following data analysis, bronchiolitis admission burden was classified into five categories, based on less than 10%, 10% to less than 20%, 20% to less than 30%, 30% to less than 40%, or 40% or more of total admissions having a primary discharge diagnosis of bronchiolitis during the study period. This categorization allowed investigators to determine whether there was a dose-dependent response among categories.
Unit Composition
Site investigators also completed a survey identifying which patients were admitted to each unit (eg, general pediatrics only, medical subspecialty, surgical). Based on these results, units were further classified into seven types (Appendix Table). For the final analysis, units caring exclusively for general pediatrics patients were compared to all other unit types.
Analysis
Bronchiolitis admission burden and unit composition data were combined with observations of pulse oximetry monitoring use of patients not requiring supplemental oxygen from the parent study. We determined unadjusted observed monitoring proportions for each unit’s bronchiolitis admission burden category across all 25 hospitals. This was calculated as a simple proportion of the total number of observations during which patients were continuously monitored divided by the total number of observations performed within each unit’s admission category. We then calculated unadjusted odds ratios using the 40% and higher bronchiolitis admission burden category as a reference. We calculated similar proportions and odds ratios for the dichotomous unit composition variable. Next, we used mixed-effects logistic regression with a random intercept for each hospital to allow for differences in baseline monitoring rates, which varied widely between hospitals (2% to 92%),10 to calculate adjusted odds ratios for the unit’s admission category and unit’s composition. We also adjusted for the same covariates used in the primary study’s analysis (Table).10

RESULTS
We analyzed 2,366 observations of bronchiolitis patients from 25 hospitals. Most observations were concentrated in freestanding children’s hospitals (89%), and 50% were from hospitals with more than 250 pediatric beds. Observations were well distributed among the five categories of admit burden (Table).
In unadjusted regression, the relationship between admission burden and rate of pulse oximetry use did not appear to be dose-dependent, and 95% CIs were wide. We then analyzed the data accounting for baseline differences in hospital monitoring rates and adjusted for the covariates significantly associated with continuous pulse oximetry monitoring in the primary study’s analysis with use of a mixed-effects model. As shown in the Table, low-burden units in which bronchiolitis constituted less than 10% of total admissions had a 2.16-fold increased odds of unnecessary pulse oximetry monitoring compared to high-burden units in which bronchiolitis constituted 40% or more of total admissions (95% CI, 1.27-3.69; P = .01).
In examining the subspecialty unit composition, 596 observations (25.2%) were conducted on units exclusively caring for general pediatrics patients. In the mixed-effects model adjusted for bronchiolitis admission burden and the covariates used in the study’s primary analysis, units exclusively caring for general pediatrics patients did not have significantly different independent odds of pulse oximetry monitoring use compared to units with a mixed patient population (OR 1.01; 95% CI, 0.71-1.45; P = .95) (Appendix Table).
DISCUSSION
In this multicenter observational study of children hospitalized with bronchiolitis not concurrently receiving supplemental oxygen, units that only occasionally cared for bronchiolitis patients appeared to be more likely to overuse continuous pulse oximetry during bronchiolitis hospitalizations.
This finding was not immediately apparent when examining the raw data because of wide hospital-level variation in continuous pulse oximetry monitoring use. However, when the high degree of hospital-level variation in baseline overuse was accounted for with use of a random intercept for each hospital in the mixed-effects model, units that cared for higher proportions of bronchiolitis patients had significantly lower odds of continuous pulse oximetry monitoring use compared to units that cared for these infants infrequently.
As many institutions have subspecialized units to cultivate nursing expertise for care of certain diseases and patient populations, we hypothesized that units caring primarily for children on general pediatrics units would also have lower rates of monitoring overuse compared to mixed units. Interestingly, these units did not perform better, likely because potential cultural factors that might contribute to differences in monitoring are accounted for by bronchiolitis admission burden.
Our findings build on prior literature by demonstrating that unit-level, as well as hospital-level, factors appear to drive overuse in healthcare. A prior single-site retrospective cohort study demonstrated an association between higher prevalence of inpatient bronchiolitis and decreased use of unnecessary interventions such as laboratory and radiographic testing, as well as steroid and antibiotic administration.11 Although study of the relationship between volume and quality is not new to healthcare, to our knowledge, this study is the first to examine the relationship between pulse oximetry overuse in bronchiolitis and unit-level factors like admission burden and subspecialty composition.
There are several limitations. First, because the study population included only children not receiving supplemental oxygen, both the parent study and this substudy assumed that all observed use of pulse oximetry monitoring was overuse. In some cases, however, there may have been other compelling clinical reasons, institutional policies, or differences in pulse oximetry availability that were not captured during data collection or in our adjusted model. Second, hospitals used convenience sampling. It is possible this resulted in samples that were not representative of each unit’s underlying patient population or monitoring practice. In addition, not all of the 32 eligible sites were able to provide data related to hospital admissions at the unit level and thus are not included in our analysis. This remains a potential source of hospital-level selection bias.
CONCLUSION
These findings demonstrate that high bronchiolitis admission burden correlates with lower rates of unnecessary pulse oximetry monitoring in bronchiolitis. We speculate that these outcomes might reflect differing degrees of nursing comfort, expertise, and unit-level norms in caring for bronchiolitis patients, although our study was not designed to establish underlying causes. Identification of operating principles that underpin low pulse oximetry monitoring on high-burden units will provide guidance for decreasing unnecessary monitoring and will inform future studies seeking ways to discourage continuous pulse oximetry monitoring in low-risk infants. Given the institutional variation in monitoring rates, future studies examining both institution-wide and unit-level interventions will be necessary to decrease unnecessary pulse oximetry monitoring in bronchiolitis. Furthermore, these findings may be relevant to studying care quality in other disease processes, with bronchiolitis serving as a model illness for overuse.
Acknowledgments
The authors acknowledge the National Heart, Lung, and Blood Institute of the National Institutes of Health scientists who contributed their expertise to this project as part of the U01 Cooperative Agreement funding mechanism as federal employees conducting their official job duties: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD. The authors thank the executive council of the Pediatric Research in Inpatient Settings Network for their contributions to the early scientific development of this project. The network assessed a Collaborative Support Fee for access to the hospitals and support of this project.
The authors thank the PRIS Network collaborators for their major contributions to data collection (see Appendix).
Continuous pulse oximetry monitoring in children with bronchiolitis who don’t require supplemental oxygen is discouraged by practice guidelines and is recognized as a form of medical overuse.1-3 This practice can be associated with negative outcomes, including prolonged length of stay,4-6 increased cost of hospitalization,7 and alarm fatigue among nurses.8 Despite initiatives to reduce continuous pulse oximetry monitoring in stable patients with bronchiolitis,1,2 wide practice variation exists between hospitals.9,10 Previous studies have shown that higher prevalence of inpatient bronchiolitis admissions is associated with decreased utilization of unnecessary interventions.11 However, the relationship between pulse oximetry use and bronchiolitis prevalence has not been studied. The objective of this study is to test the hypothesis that hospital units with lower proportions of patients admitted for bronchiolitis and those with fewer general pediatrics patients relative to subspecialty patients would have higher rates of pulse oximetry overuse.
METHODS
Study Design
We conducted a substudy of the Pediatric Research in Inpatient Settings (PRIS) Network’s Eliminating Monitoring Overuse (EMO) pulse oximetry study,10,12 a 56-hospital cross-sectional study that used direct observation to measure the prevalence of continuous pulse oximetry monitoring in hospitalized infants with bronchiolitis who did not require supplemental oxygen between December 1, 2018, through March 31, 2019. This substudy was not included as part of the original aims of the project and was proposed as a separate analysis during data collection. For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study and served as the central IRB. The Research Ethics Board at University of Calgary also approved the study.
Site Selection
Hospitals with at least 60 observations were eligible for inclusion. Of the 32 hospitals that conducted the minimum observations, 25 agreed to participate (21 free-standing children’s hospitals, 3 children’s hospitals within general hospitals, and 1 community hospital).
Patient Population
The parent study included patients aged 8 weeks through 23 months with a primary diagnosis of bronchiolitis. Patients were included only if they were not receiving supplemental oxygen or nasal cannula flow at the time of data collection. The inclusion and exclusion criteria were for both the parent study and the substudy. Further inclusion and exclusion criteria have been described previously.10,12
Data Collection
In order to ascertain continuous pulse oximetry monitoring status, staff at each hospital performed observational rounds by walking to the bedside of each patient who met inclusion criteria. Additional methodology for the parent study has been published elsewhere.10,12
Bronchiolitis Admission Volume by Unit
Collaborators at each hospital gathered bronchiolitis census data from each unit that admitted patients with bronchiolitis. Units were identified prior to data collection and were characterized at the institution level based on previous local definitions. Each site was responsible for using institution-specific data collection methods for determining bronchiolitis and total admissions on each unit (eg, departmental reports or directly querying admissions data using International Classification of Diseases, Tenth Revision, diagnosis codes for bronchiolitis) over the same period as the parent study. Following data analysis, bronchiolitis admission burden was classified into five categories, based on less than 10%, 10% to less than 20%, 20% to less than 30%, 30% to less than 40%, or 40% or more of total admissions having a primary discharge diagnosis of bronchiolitis during the study period. This categorization allowed investigators to determine whether there was a dose-dependent response among categories.
Unit Composition
Site investigators also completed a survey identifying which patients were admitted to each unit (eg, general pediatrics only, medical subspecialty, surgical). Based on these results, units were further classified into seven types (Appendix Table). For the final analysis, units caring exclusively for general pediatrics patients were compared to all other unit types.
Analysis
Bronchiolitis admission burden and unit composition data were combined with observations of pulse oximetry monitoring use of patients not requiring supplemental oxygen from the parent study. We determined unadjusted observed monitoring proportions for each unit’s bronchiolitis admission burden category across all 25 hospitals. This was calculated as a simple proportion of the total number of observations during which patients were continuously monitored divided by the total number of observations performed within each unit’s admission category. We then calculated unadjusted odds ratios using the 40% and higher bronchiolitis admission burden category as a reference. We calculated similar proportions and odds ratios for the dichotomous unit composition variable. Next, we used mixed-effects logistic regression with a random intercept for each hospital to allow for differences in baseline monitoring rates, which varied widely between hospitals (2% to 92%),10 to calculate adjusted odds ratios for the unit’s admission category and unit’s composition. We also adjusted for the same covariates used in the primary study’s analysis (Table).10

RESULTS
We analyzed 2,366 observations of bronchiolitis patients from 25 hospitals. Most observations were concentrated in freestanding children’s hospitals (89%), and 50% were from hospitals with more than 250 pediatric beds. Observations were well distributed among the five categories of admit burden (Table).
In unadjusted regression, the relationship between admission burden and rate of pulse oximetry use did not appear to be dose-dependent, and 95% CIs were wide. We then analyzed the data accounting for baseline differences in hospital monitoring rates and adjusted for the covariates significantly associated with continuous pulse oximetry monitoring in the primary study’s analysis with use of a mixed-effects model. As shown in the Table, low-burden units in which bronchiolitis constituted less than 10% of total admissions had a 2.16-fold increased odds of unnecessary pulse oximetry monitoring compared to high-burden units in which bronchiolitis constituted 40% or more of total admissions (95% CI, 1.27-3.69; P = .01).
In examining the subspecialty unit composition, 596 observations (25.2%) were conducted on units exclusively caring for general pediatrics patients. In the mixed-effects model adjusted for bronchiolitis admission burden and the covariates used in the study’s primary analysis, units exclusively caring for general pediatrics patients did not have significantly different independent odds of pulse oximetry monitoring use compared to units with a mixed patient population (OR 1.01; 95% CI, 0.71-1.45; P = .95) (Appendix Table).
DISCUSSION
In this multicenter observational study of children hospitalized with bronchiolitis not concurrently receiving supplemental oxygen, units that only occasionally cared for bronchiolitis patients appeared to be more likely to overuse continuous pulse oximetry during bronchiolitis hospitalizations.
This finding was not immediately apparent when examining the raw data because of wide hospital-level variation in continuous pulse oximetry monitoring use. However, when the high degree of hospital-level variation in baseline overuse was accounted for with use of a random intercept for each hospital in the mixed-effects model, units that cared for higher proportions of bronchiolitis patients had significantly lower odds of continuous pulse oximetry monitoring use compared to units that cared for these infants infrequently.
As many institutions have subspecialized units to cultivate nursing expertise for care of certain diseases and patient populations, we hypothesized that units caring primarily for children on general pediatrics units would also have lower rates of monitoring overuse compared to mixed units. Interestingly, these units did not perform better, likely because potential cultural factors that might contribute to differences in monitoring are accounted for by bronchiolitis admission burden.
Our findings build on prior literature by demonstrating that unit-level, as well as hospital-level, factors appear to drive overuse in healthcare. A prior single-site retrospective cohort study demonstrated an association between higher prevalence of inpatient bronchiolitis and decreased use of unnecessary interventions such as laboratory and radiographic testing, as well as steroid and antibiotic administration.11 Although study of the relationship between volume and quality is not new to healthcare, to our knowledge, this study is the first to examine the relationship between pulse oximetry overuse in bronchiolitis and unit-level factors like admission burden and subspecialty composition.
There are several limitations. First, because the study population included only children not receiving supplemental oxygen, both the parent study and this substudy assumed that all observed use of pulse oximetry monitoring was overuse. In some cases, however, there may have been other compelling clinical reasons, institutional policies, or differences in pulse oximetry availability that were not captured during data collection or in our adjusted model. Second, hospitals used convenience sampling. It is possible this resulted in samples that were not representative of each unit’s underlying patient population or monitoring practice. In addition, not all of the 32 eligible sites were able to provide data related to hospital admissions at the unit level and thus are not included in our analysis. This remains a potential source of hospital-level selection bias.
CONCLUSION
These findings demonstrate that high bronchiolitis admission burden correlates with lower rates of unnecessary pulse oximetry monitoring in bronchiolitis. We speculate that these outcomes might reflect differing degrees of nursing comfort, expertise, and unit-level norms in caring for bronchiolitis patients, although our study was not designed to establish underlying causes. Identification of operating principles that underpin low pulse oximetry monitoring on high-burden units will provide guidance for decreasing unnecessary monitoring and will inform future studies seeking ways to discourage continuous pulse oximetry monitoring in low-risk infants. Given the institutional variation in monitoring rates, future studies examining both institution-wide and unit-level interventions will be necessary to decrease unnecessary pulse oximetry monitoring in bronchiolitis. Furthermore, these findings may be relevant to studying care quality in other disease processes, with bronchiolitis serving as a model illness for overuse.
Acknowledgments
The authors acknowledge the National Heart, Lung, and Blood Institute of the National Institutes of Health scientists who contributed their expertise to this project as part of the U01 Cooperative Agreement funding mechanism as federal employees conducting their official job duties: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD. The authors thank the executive council of the Pediatric Research in Inpatient Settings Network for their contributions to the early scientific development of this project. The network assessed a Collaborative Support Fee for access to the hospitals and support of this project.
The authors thank the PRIS Network collaborators for their major contributions to data collection (see Appendix).
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
3. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
4. Cunningham S, Rodriguez A, Adams T, et al; Bronchiolitis of Infancy Discharge Study (BIDS) group. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. https://doi.org/10.1016/s0140-6736(15)00163-4
5. Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. https://doi.org/10.1001/archpedi.158.6.527
6. Cunningham S, McMurray A. Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97(4):361-363. https://doi.org/10.1136/adc.2010.205211
7. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC; BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-172. https://doi.org/10.3310/hta19710
8. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851
10. Bonafide CP, Xiao R, Brady PW, et al; for the Pediatric Research in Inpatient Settings (PRIS) Network. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
11. Van Cleve WC, Christakis DA. Unnecessary care for bronchiolitis decreases with increasing inpatient prevalence of bronchiolitis. Pediatrics. 2011;128(5):e1106-e1112. https://doi.org/10.1542/peds.2011-0655
12. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. https://doi.org/10.1186/s40814-019-0453-2
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
3. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
4. Cunningham S, Rodriguez A, Adams T, et al; Bronchiolitis of Infancy Discharge Study (BIDS) group. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. https://doi.org/10.1016/s0140-6736(15)00163-4
5. Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. https://doi.org/10.1001/archpedi.158.6.527
6. Cunningham S, McMurray A. Observational study of two oxygen saturation targets for discharge in bronchiolitis. Arch Dis Child. 2012;97(4):361-363. https://doi.org/10.1136/adc.2010.205211
7. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC; BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-172. https://doi.org/10.3310/hta19710
8. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851
10. Bonafide CP, Xiao R, Brady PW, et al; for the Pediatric Research in Inpatient Settings (PRIS) Network. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
11. Van Cleve WC, Christakis DA. Unnecessary care for bronchiolitis decreases with increasing inpatient prevalence of bronchiolitis. Pediatrics. 2011;128(5):e1106-e1112. https://doi.org/10.1542/peds.2011-0655
12. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. https://doi.org/10.1186/s40814-019-0453-2
© 2020 Society of Hospital Medicine
Comparing Two Proximal Measures of Unrecognized Clinical Deterioration in Children
Unrecognized in-hospital clinical deterioration can lead to substantial morbidity and mortality.1 As a result, hospitals have implemented systems to identify and mitigate this form of potentially preventable harm.2-4 Cardiopulmonary arrest rates are useful metrics to evaluate the effectiveness of systems designed to identify and respond to deteriorating adult patients.5 Pediatric arrests outside of the intensive care unit (ICU) are rare; therefore, the identification of valid and more frequent proximal measures of deterioration is critical to the assessment of current systems and to guide future improvement efforts.6
Bonafide et al developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had an over 13-fold increased risk of in-hospital mortality.7 Implementation of a rapid response system was subsequently associated with a decrease in the trajectory of CDEs.2 At Cincinnati Children’s Hospital Medical Center (CCHMC), an additional proximal outcome measure was developed for unrecognized clinical deterioration: emergency transfers (ETs).8,9 An event meets criteria for an ET when the patient undergoes intubation, inotropic support, or three or more fluid boluses in the first hour after arrival or prior to ICU transfer.9 Recently, ETs were associated with an increased in-hospital mortality, ICU length of stay, and post-transfer hospital length of stay when compared with nonemergent transfers.10,11
While both CDEs and ETs were associated with adverse outcomes in children and may be modifiable through better rapid response systems, researchers have not previously compared the extent to which CDEs and ETs capture similar versus distinct events. Furthermore, the ability of focused situation awareness interventions to identify high-risk patients has not previously been assessed. Situation awareness is defined as the perception of elements in the environment, the comprehension of their meaning, and the projection of their status in the near future.12 Clinically, improved situation awareness can lead to earlier recognition of deterioration and a reduction in failure to rescue events.9 The objectives of this study were to (1) describe CDEs and ETs and assess for similarities, differences, and trends, and (2) evaluate the utility of situation awareness interventions to detect patients who experience these events.
METHODS
Setting and Inclusion Criteria
We conducted a retrospective cross-sectional study at CCHMC, a free-standing tertiary care children’s hospital. We included all patients cared for outside of the ICU during their hospitalization from January 2016 to July 2018. Transfer to the ICU included the pediatric and the cardiac ICUs.
Study Definitions
CDEs were events in which a patient received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of ICU transfer (Figure).7 ETs were events in which a patient underwent intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer (Figure).9 We examined two distinct situation awareness interventions: watcher identification and the pediatric early warning score (PEWS). A watcher is a situation awareness concern based on clinician perception, or “gut feeling,” that the patient is at high risk for deterioration.9,13 When clinicians designate a patient as a watcher in the electronic medical record, they establish an action plan, reassessment timeline, and objective criteria for activation of the rapid response team to assess the patient. Watcher patients are discussed at institution-wide safety huddles three times daily. The PEWS is a reproducible assessment of the patient’s status based on physiologic parameters, including behavior, cardiovascular, and respiratory assessments.3,4 At CCHMC, a Monaghan PEWS score is calculated with each assessment of vital signs.14 The bedside nurse calls the physician or advanced practice provider to assess the patient for a score of 4 or greater.
Event Identification and Classification
Two trained research nurses (C.F. and D.H.) manually reviewed all ICU transfers during the study period to determine if CDE criteria were met. Events meeting CDE criteria were classified as respiratory (requiring noninvasive or invasive ventilation), cardiac (requiring inotropes), or cardiopulmonary resuscitation (CPR) in which cardiac and respiratory interventions were initiated simultaneously. Additional information obtained included the time the patient met CDE criteria relative to the time of ICU transfer, watcher identification prior to the event, and the highest PEWS documented within 12 hours of the event. A physician (T.S.) performed manual chart review of each CDE as an additional validation step. ETs during the study period were obtained from an existing institutional database. ICU transfers meeting ET criteria are entered into this database in nearly real time by the inpatient nurse manager; this nurse attends all rapid response team calls and is aware of the disposition for each event. A physician (T.S.) performed manual chart review of each ET to determine event classification by intervention type, watcher identification, and the highest PEWS documented within 12 hours of the event. All CDEs and ETs were cross-referenced to determine overlap.
Outcome Measures and Statistical Analysis
The primary outcomes were CDEs and ETs, calculated as absolute counts and number of events per 10,000 non-ICU patient days. Events were classified by (1) category of intervention, (2) watcher identification prior to the event, and (3) PEWS of 4 or greater documented in the 12 hours prior to the event.
RESULTS
Incidence and Overlap of CDEs and ETs
There were 1,828 ICU transfers during the study period, of which 365 (20%) met criteria for a CDE, ET, or both. Among events captured, 359 (98.4%) met criteria for a CDE, occurring at a rate of 16.7 per 10,000 non-ICU patient days, and 88 (24.1%) met criteria for an ET, occurring at a rate of 4.1 per 10,000 non-ICU patient days (Table). Of the 88 ETs, 82 also met criteria for a CDE.
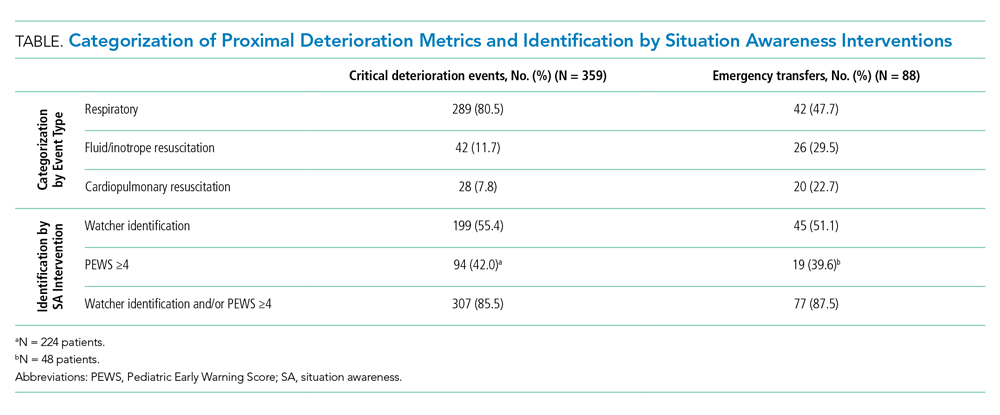
Timing and Categorization of CDEs and ETs
Despite the 12-hour time horizon, most CDEs (62.1%) met criteria within 1 hour of ICU transfer, and 79.9% met criteria within 3 hours (Figure). Respiratory events were most common for both CDEs (80.5%) and ETs (47.7%) (Table). Of respiratory CDEs, 67.4% required noninvasive ventilation, and 32.5% required invasive ventilation. Fluid or inotrope support were responsible for 11.7% of CDEs and nearly one-third of ETs; of note, the CDE definition does not include fluid boluses. Less than 10% of CDEs were characterized by CPR, whereas this accounted for 22.7% of ETs.

Identification of Events by Situation Awareness Interventions
The Table depicts the identification of events by watcher status and PEWS. All events were included for watcher identification, and events with a documented score in the 12 hours prior to transfer were included for PEWS. While half or less of the events were captured by watcher or PEWS separately, over 85% of events were captured by either one or both of the situation awareness interventions. The situation awareness interventions identified CDEs and ETs similarly.
DISCUSSION
This study is the first to classify and compare two proximal measures of clinical deterioration in children. Given that children with escalating respiratory symptoms are often treated successfully outside of the ICU, the findings that most events are respiratory in nature and occur within 1 hour of transfer are not unexpected. The analysis of situation awareness interventions suggests that neither watcher identification nor PEWS is independently sufficient to predict future deterioration. These findings support the necessity of both a clinician “gut feeling” and objective vital sign and physical exam findings to indicate a patient’s clinical status.9 Initiatives to improve the early recognition and mitigation of patient deterioration should focus on both tools to initiate an escalation of care, and work to understand gaps in these identification systems, which currently miss approximately 15% of acutely deteriorating patients. Although most patients had watcher identification or elevated PEWS prior to the event, they still required emergent life-sustaining care, which suggests that opportunities exist to improve mitigation and escalation pathways as a critical prevention effort.7,10
It is likely that CDEs and ETs are important outcome metrics in the evaluation of pediatric escalation systems, including rapid response systems.15 ETs are less common and more specific for unrecognized deterioration, which makes them a more feasible early metric for assessment. CDEs, which are likely more sensitive, may be useful in settings in which deterioration is rare or a more common outcome enhances power to detect the effect of interventions.10
This study has limitations and lends itself to future work. While CDEs and ETs are more common than cardiopulmonary arrest, they remain relatively uncommon. This was a single-site study at a large, tertiary care, free-standing children’s hospital, so generalizability to centers with different characteristics and patient populations may be limited. Future work should focus on comparing patient-level outcomes of CDEs and ETs, including length of stay and mortality. The determination of specific diagnoses and conditions associated with CDEs and ETs may inform targeted preventive improvement science interventions.
CONCLUSION
CDEs were roughly fourfold more common than ETs, with most CDEs occurring within 1 hour of ICU transfer. Most patients were identified by either watcher status or elevated PEWS, suggesting that these tools, when utilized as complementary situation awareness interventions, are important for identifying patients at risk for deterioration. Opportunities exist for improved escalation plans for patients identified as high-risk to prevent the need for emergent life-sustaining intervention.
1. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
2. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. https://doi.org/10.1001/jamapediatrics.2013.3266
3. Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. https://doi.org/10.1016/j.jcrc.2006.06.007
4. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? an observational cohort study. Intensive Crit Care Nurs. 2015;31(2):91-99. https://doi.org/10.1016/j.iccn.2014.01.001
5. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388
6. Feudtner C, Berry JG, Parry G, et al. Statistical uncertainty of mortality rates and rankings for children’s hospitals. Pediatrics. 2011;128(4):e966-e972. https://doi.org/10.1542/peds.2010-3074
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784
8. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747
9. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364
10. Hussain FS, Sosa T, Ambroggio L, Gallagher R, Brady PW. Emergency transfers: an important predictor of adverse outcomes in hospitalized children. J Hosp Med. 2019;14(8):482-485. https://doi.org/10.12788/jhm.3219
11. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2019;55(2):213-215. https://doi.org/10.1111/jpc.14185
12. Endsley MR. Toward a theory of situation awareness in dynamic systems. Human Factors. 1995;37(1):32-64. https://doi.org/10.1518/001872095779049543
13. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042
14. Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32-35. https://doi.org/10.7748/paed2005.02.17.1.32.c964
15. Subbe CP, Bannard-Smith J, Bunch J, et al. Quality metrics for the evaluation of Rapid Response Systems: proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1-12. https://doi.org/10.1016/j.resuscitation.2019.05.012
Unrecognized in-hospital clinical deterioration can lead to substantial morbidity and mortality.1 As a result, hospitals have implemented systems to identify and mitigate this form of potentially preventable harm.2-4 Cardiopulmonary arrest rates are useful metrics to evaluate the effectiveness of systems designed to identify and respond to deteriorating adult patients.5 Pediatric arrests outside of the intensive care unit (ICU) are rare; therefore, the identification of valid and more frequent proximal measures of deterioration is critical to the assessment of current systems and to guide future improvement efforts.6
Bonafide et al developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had an over 13-fold increased risk of in-hospital mortality.7 Implementation of a rapid response system was subsequently associated with a decrease in the trajectory of CDEs.2 At Cincinnati Children’s Hospital Medical Center (CCHMC), an additional proximal outcome measure was developed for unrecognized clinical deterioration: emergency transfers (ETs).8,9 An event meets criteria for an ET when the patient undergoes intubation, inotropic support, or three or more fluid boluses in the first hour after arrival or prior to ICU transfer.9 Recently, ETs were associated with an increased in-hospital mortality, ICU length of stay, and post-transfer hospital length of stay when compared with nonemergent transfers.10,11
While both CDEs and ETs were associated with adverse outcomes in children and may be modifiable through better rapid response systems, researchers have not previously compared the extent to which CDEs and ETs capture similar versus distinct events. Furthermore, the ability of focused situation awareness interventions to identify high-risk patients has not previously been assessed. Situation awareness is defined as the perception of elements in the environment, the comprehension of their meaning, and the projection of their status in the near future.12 Clinically, improved situation awareness can lead to earlier recognition of deterioration and a reduction in failure to rescue events.9 The objectives of this study were to (1) describe CDEs and ETs and assess for similarities, differences, and trends, and (2) evaluate the utility of situation awareness interventions to detect patients who experience these events.
METHODS
Setting and Inclusion Criteria
We conducted a retrospective cross-sectional study at CCHMC, a free-standing tertiary care children’s hospital. We included all patients cared for outside of the ICU during their hospitalization from January 2016 to July 2018. Transfer to the ICU included the pediatric and the cardiac ICUs.
Study Definitions
CDEs were events in which a patient received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of ICU transfer (Figure).7 ETs were events in which a patient underwent intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer (Figure).9 We examined two distinct situation awareness interventions: watcher identification and the pediatric early warning score (PEWS). A watcher is a situation awareness concern based on clinician perception, or “gut feeling,” that the patient is at high risk for deterioration.9,13 When clinicians designate a patient as a watcher in the electronic medical record, they establish an action plan, reassessment timeline, and objective criteria for activation of the rapid response team to assess the patient. Watcher patients are discussed at institution-wide safety huddles three times daily. The PEWS is a reproducible assessment of the patient’s status based on physiologic parameters, including behavior, cardiovascular, and respiratory assessments.3,4 At CCHMC, a Monaghan PEWS score is calculated with each assessment of vital signs.14 The bedside nurse calls the physician or advanced practice provider to assess the patient for a score of 4 or greater.
Event Identification and Classification
Two trained research nurses (C.F. and D.H.) manually reviewed all ICU transfers during the study period to determine if CDE criteria were met. Events meeting CDE criteria were classified as respiratory (requiring noninvasive or invasive ventilation), cardiac (requiring inotropes), or cardiopulmonary resuscitation (CPR) in which cardiac and respiratory interventions were initiated simultaneously. Additional information obtained included the time the patient met CDE criteria relative to the time of ICU transfer, watcher identification prior to the event, and the highest PEWS documented within 12 hours of the event. A physician (T.S.) performed manual chart review of each CDE as an additional validation step. ETs during the study period were obtained from an existing institutional database. ICU transfers meeting ET criteria are entered into this database in nearly real time by the inpatient nurse manager; this nurse attends all rapid response team calls and is aware of the disposition for each event. A physician (T.S.) performed manual chart review of each ET to determine event classification by intervention type, watcher identification, and the highest PEWS documented within 12 hours of the event. All CDEs and ETs were cross-referenced to determine overlap.
Outcome Measures and Statistical Analysis
The primary outcomes were CDEs and ETs, calculated as absolute counts and number of events per 10,000 non-ICU patient days. Events were classified by (1) category of intervention, (2) watcher identification prior to the event, and (3) PEWS of 4 or greater documented in the 12 hours prior to the event.
RESULTS
Incidence and Overlap of CDEs and ETs
There were 1,828 ICU transfers during the study period, of which 365 (20%) met criteria for a CDE, ET, or both. Among events captured, 359 (98.4%) met criteria for a CDE, occurring at a rate of 16.7 per 10,000 non-ICU patient days, and 88 (24.1%) met criteria for an ET, occurring at a rate of 4.1 per 10,000 non-ICU patient days (Table). Of the 88 ETs, 82 also met criteria for a CDE.

Timing and Categorization of CDEs and ETs
Despite the 12-hour time horizon, most CDEs (62.1%) met criteria within 1 hour of ICU transfer, and 79.9% met criteria within 3 hours (Figure). Respiratory events were most common for both CDEs (80.5%) and ETs (47.7%) (Table). Of respiratory CDEs, 67.4% required noninvasive ventilation, and 32.5% required invasive ventilation. Fluid or inotrope support were responsible for 11.7% of CDEs and nearly one-third of ETs; of note, the CDE definition does not include fluid boluses. Less than 10% of CDEs were characterized by CPR, whereas this accounted for 22.7% of ETs.

Identification of Events by Situation Awareness Interventions
The Table depicts the identification of events by watcher status and PEWS. All events were included for watcher identification, and events with a documented score in the 12 hours prior to transfer were included for PEWS. While half or less of the events were captured by watcher or PEWS separately, over 85% of events were captured by either one or both of the situation awareness interventions. The situation awareness interventions identified CDEs and ETs similarly.
DISCUSSION
This study is the first to classify and compare two proximal measures of clinical deterioration in children. Given that children with escalating respiratory symptoms are often treated successfully outside of the ICU, the findings that most events are respiratory in nature and occur within 1 hour of transfer are not unexpected. The analysis of situation awareness interventions suggests that neither watcher identification nor PEWS is independently sufficient to predict future deterioration. These findings support the necessity of both a clinician “gut feeling” and objective vital sign and physical exam findings to indicate a patient’s clinical status.9 Initiatives to improve the early recognition and mitigation of patient deterioration should focus on both tools to initiate an escalation of care, and work to understand gaps in these identification systems, which currently miss approximately 15% of acutely deteriorating patients. Although most patients had watcher identification or elevated PEWS prior to the event, they still required emergent life-sustaining care, which suggests that opportunities exist to improve mitigation and escalation pathways as a critical prevention effort.7,10
It is likely that CDEs and ETs are important outcome metrics in the evaluation of pediatric escalation systems, including rapid response systems.15 ETs are less common and more specific for unrecognized deterioration, which makes them a more feasible early metric for assessment. CDEs, which are likely more sensitive, may be useful in settings in which deterioration is rare or a more common outcome enhances power to detect the effect of interventions.10
This study has limitations and lends itself to future work. While CDEs and ETs are more common than cardiopulmonary arrest, they remain relatively uncommon. This was a single-site study at a large, tertiary care, free-standing children’s hospital, so generalizability to centers with different characteristics and patient populations may be limited. Future work should focus on comparing patient-level outcomes of CDEs and ETs, including length of stay and mortality. The determination of specific diagnoses and conditions associated with CDEs and ETs may inform targeted preventive improvement science interventions.
CONCLUSION
CDEs were roughly fourfold more common than ETs, with most CDEs occurring within 1 hour of ICU transfer. Most patients were identified by either watcher status or elevated PEWS, suggesting that these tools, when utilized as complementary situation awareness interventions, are important for identifying patients at risk for deterioration. Opportunities exist for improved escalation plans for patients identified as high-risk to prevent the need for emergent life-sustaining intervention.
Unrecognized in-hospital clinical deterioration can lead to substantial morbidity and mortality.1 As a result, hospitals have implemented systems to identify and mitigate this form of potentially preventable harm.2-4 Cardiopulmonary arrest rates are useful metrics to evaluate the effectiveness of systems designed to identify and respond to deteriorating adult patients.5 Pediatric arrests outside of the intensive care unit (ICU) are rare; therefore, the identification of valid and more frequent proximal measures of deterioration is critical to the assessment of current systems and to guide future improvement efforts.6
Bonafide et al developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had an over 13-fold increased risk of in-hospital mortality.7 Implementation of a rapid response system was subsequently associated with a decrease in the trajectory of CDEs.2 At Cincinnati Children’s Hospital Medical Center (CCHMC), an additional proximal outcome measure was developed for unrecognized clinical deterioration: emergency transfers (ETs).8,9 An event meets criteria for an ET when the patient undergoes intubation, inotropic support, or three or more fluid boluses in the first hour after arrival or prior to ICU transfer.9 Recently, ETs were associated with an increased in-hospital mortality, ICU length of stay, and post-transfer hospital length of stay when compared with nonemergent transfers.10,11
While both CDEs and ETs were associated with adverse outcomes in children and may be modifiable through better rapid response systems, researchers have not previously compared the extent to which CDEs and ETs capture similar versus distinct events. Furthermore, the ability of focused situation awareness interventions to identify high-risk patients has not previously been assessed. Situation awareness is defined as the perception of elements in the environment, the comprehension of their meaning, and the projection of their status in the near future.12 Clinically, improved situation awareness can lead to earlier recognition of deterioration and a reduction in failure to rescue events.9 The objectives of this study were to (1) describe CDEs and ETs and assess for similarities, differences, and trends, and (2) evaluate the utility of situation awareness interventions to detect patients who experience these events.
METHODS
Setting and Inclusion Criteria
We conducted a retrospective cross-sectional study at CCHMC, a free-standing tertiary care children’s hospital. We included all patients cared for outside of the ICU during their hospitalization from January 2016 to July 2018. Transfer to the ICU included the pediatric and the cardiac ICUs.
Study Definitions
CDEs were events in which a patient received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of ICU transfer (Figure).7 ETs were events in which a patient underwent intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer (Figure).9 We examined two distinct situation awareness interventions: watcher identification and the pediatric early warning score (PEWS). A watcher is a situation awareness concern based on clinician perception, or “gut feeling,” that the patient is at high risk for deterioration.9,13 When clinicians designate a patient as a watcher in the electronic medical record, they establish an action plan, reassessment timeline, and objective criteria for activation of the rapid response team to assess the patient. Watcher patients are discussed at institution-wide safety huddles three times daily. The PEWS is a reproducible assessment of the patient’s status based on physiologic parameters, including behavior, cardiovascular, and respiratory assessments.3,4 At CCHMC, a Monaghan PEWS score is calculated with each assessment of vital signs.14 The bedside nurse calls the physician or advanced practice provider to assess the patient for a score of 4 or greater.
Event Identification and Classification
Two trained research nurses (C.F. and D.H.) manually reviewed all ICU transfers during the study period to determine if CDE criteria were met. Events meeting CDE criteria were classified as respiratory (requiring noninvasive or invasive ventilation), cardiac (requiring inotropes), or cardiopulmonary resuscitation (CPR) in which cardiac and respiratory interventions were initiated simultaneously. Additional information obtained included the time the patient met CDE criteria relative to the time of ICU transfer, watcher identification prior to the event, and the highest PEWS documented within 12 hours of the event. A physician (T.S.) performed manual chart review of each CDE as an additional validation step. ETs during the study period were obtained from an existing institutional database. ICU transfers meeting ET criteria are entered into this database in nearly real time by the inpatient nurse manager; this nurse attends all rapid response team calls and is aware of the disposition for each event. A physician (T.S.) performed manual chart review of each ET to determine event classification by intervention type, watcher identification, and the highest PEWS documented within 12 hours of the event. All CDEs and ETs were cross-referenced to determine overlap.
Outcome Measures and Statistical Analysis
The primary outcomes were CDEs and ETs, calculated as absolute counts and number of events per 10,000 non-ICU patient days. Events were classified by (1) category of intervention, (2) watcher identification prior to the event, and (3) PEWS of 4 or greater documented in the 12 hours prior to the event.
RESULTS
Incidence and Overlap of CDEs and ETs
There were 1,828 ICU transfers during the study period, of which 365 (20%) met criteria for a CDE, ET, or both. Among events captured, 359 (98.4%) met criteria for a CDE, occurring at a rate of 16.7 per 10,000 non-ICU patient days, and 88 (24.1%) met criteria for an ET, occurring at a rate of 4.1 per 10,000 non-ICU patient days (Table). Of the 88 ETs, 82 also met criteria for a CDE.

Timing and Categorization of CDEs and ETs
Despite the 12-hour time horizon, most CDEs (62.1%) met criteria within 1 hour of ICU transfer, and 79.9% met criteria within 3 hours (Figure). Respiratory events were most common for both CDEs (80.5%) and ETs (47.7%) (Table). Of respiratory CDEs, 67.4% required noninvasive ventilation, and 32.5% required invasive ventilation. Fluid or inotrope support were responsible for 11.7% of CDEs and nearly one-third of ETs; of note, the CDE definition does not include fluid boluses. Less than 10% of CDEs were characterized by CPR, whereas this accounted for 22.7% of ETs.

Identification of Events by Situation Awareness Interventions
The Table depicts the identification of events by watcher status and PEWS. All events were included for watcher identification, and events with a documented score in the 12 hours prior to transfer were included for PEWS. While half or less of the events were captured by watcher or PEWS separately, over 85% of events were captured by either one or both of the situation awareness interventions. The situation awareness interventions identified CDEs and ETs similarly.
DISCUSSION
This study is the first to classify and compare two proximal measures of clinical deterioration in children. Given that children with escalating respiratory symptoms are often treated successfully outside of the ICU, the findings that most events are respiratory in nature and occur within 1 hour of transfer are not unexpected. The analysis of situation awareness interventions suggests that neither watcher identification nor PEWS is independently sufficient to predict future deterioration. These findings support the necessity of both a clinician “gut feeling” and objective vital sign and physical exam findings to indicate a patient’s clinical status.9 Initiatives to improve the early recognition and mitigation of patient deterioration should focus on both tools to initiate an escalation of care, and work to understand gaps in these identification systems, which currently miss approximately 15% of acutely deteriorating patients. Although most patients had watcher identification or elevated PEWS prior to the event, they still required emergent life-sustaining care, which suggests that opportunities exist to improve mitigation and escalation pathways as a critical prevention effort.7,10
It is likely that CDEs and ETs are important outcome metrics in the evaluation of pediatric escalation systems, including rapid response systems.15 ETs are less common and more specific for unrecognized deterioration, which makes them a more feasible early metric for assessment. CDEs, which are likely more sensitive, may be useful in settings in which deterioration is rare or a more common outcome enhances power to detect the effect of interventions.10
This study has limitations and lends itself to future work. While CDEs and ETs are more common than cardiopulmonary arrest, they remain relatively uncommon. This was a single-site study at a large, tertiary care, free-standing children’s hospital, so generalizability to centers with different characteristics and patient populations may be limited. Future work should focus on comparing patient-level outcomes of CDEs and ETs, including length of stay and mortality. The determination of specific diagnoses and conditions associated with CDEs and ETs may inform targeted preventive improvement science interventions.
CONCLUSION
CDEs were roughly fourfold more common than ETs, with most CDEs occurring within 1 hour of ICU transfer. Most patients were identified by either watcher status or elevated PEWS, suggesting that these tools, when utilized as complementary situation awareness interventions, are important for identifying patients at risk for deterioration. Opportunities exist for improved escalation plans for patients identified as high-risk to prevent the need for emergent life-sustaining intervention.
1. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
2. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. https://doi.org/10.1001/jamapediatrics.2013.3266
3. Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. https://doi.org/10.1016/j.jcrc.2006.06.007
4. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? an observational cohort study. Intensive Crit Care Nurs. 2015;31(2):91-99. https://doi.org/10.1016/j.iccn.2014.01.001
5. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388
6. Feudtner C, Berry JG, Parry G, et al. Statistical uncertainty of mortality rates and rankings for children’s hospitals. Pediatrics. 2011;128(4):e966-e972. https://doi.org/10.1542/peds.2010-3074
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784
8. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747
9. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364
10. Hussain FS, Sosa T, Ambroggio L, Gallagher R, Brady PW. Emergency transfers: an important predictor of adverse outcomes in hospitalized children. J Hosp Med. 2019;14(8):482-485. https://doi.org/10.12788/jhm.3219
11. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2019;55(2):213-215. https://doi.org/10.1111/jpc.14185
12. Endsley MR. Toward a theory of situation awareness in dynamic systems. Human Factors. 1995;37(1):32-64. https://doi.org/10.1518/001872095779049543
13. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042
14. Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32-35. https://doi.org/10.7748/paed2005.02.17.1.32.c964
15. Subbe CP, Bannard-Smith J, Bunch J, et al. Quality metrics for the evaluation of Rapid Response Systems: proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1-12. https://doi.org/10.1016/j.resuscitation.2019.05.012
1. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137-141. https://doi.org/10.1016/j.resuscitation.2004.03.005
2. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. https://doi.org/10.1001/jamapediatrics.2013.3266
3. Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271-278. https://doi.org/10.1016/j.jcrc.2006.06.007
4. Sefton G, McGrath C, Tume L, Lane S, Lisboa PJ, Carrol ED. What impact did a Paediatric Early Warning system have on emergency admissions to the paediatric intensive care unit? an observational cohort study. Intensive Crit Care Nurs. 2015;31(2):91-99. https://doi.org/10.1016/j.iccn.2014.01.001
5. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388
6. Feudtner C, Berry JG, Parry G, et al. Statistical uncertainty of mortality rates and rankings for children’s hospitals. Pediatrics. 2011;128(4):e966-e972. https://doi.org/10.1542/peds.2010-3074
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784
8. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747
9. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364
10. Hussain FS, Sosa T, Ambroggio L, Gallagher R, Brady PW. Emergency transfers: an important predictor of adverse outcomes in hospitalized children. J Hosp Med. 2019;14(8):482-485. https://doi.org/10.12788/jhm.3219
11. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2019;55(2):213-215. https://doi.org/10.1111/jpc.14185
12. Endsley MR. Toward a theory of situation awareness in dynamic systems. Human Factors. 1995;37(1):32-64. https://doi.org/10.1518/001872095779049543
13. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042
14. Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32-35. https://doi.org/10.7748/paed2005.02.17.1.32.c964
15. Subbe CP, Bannard-Smith J, Bunch J, et al. Quality metrics for the evaluation of Rapid Response Systems: proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1-12. https://doi.org/10.1016/j.resuscitation.2019.05.012
© 2020 Society of Hospital Medicine
Validity of Continuous Pulse Oximetry Orders for Identification of Actual Monitoring Status in Bronchiolitis
As part of improvement collaboratives that aimed to reduce overuse of continuous pulse oximetry in children hospitalized with bronchiolitis, researchers used the presence of an active order for it as a proxy for the actual use of such monitoring.1,2 With use of this proxy, investigators on a national study documented a high burden of continuous oximetry overuse (86.5% before quality improvement interventions and 45.5% after),1 but the validity of orders in representing actual monitoring practice is unknown. If the presence of an active pulse oximetry order accurately identifies infants on monitors, electronic health record data could inform epidemiologic estimates of monitoring overuse and measure the success of quality improvement and deimplementation interventions. Alternatively, if nurses commonly begin and/or discontinue pulse oximetry without updated orders, a pulse oximetry order would not be an accurate proxy, and additional data capture methods (eg, bedside observation or data capture from bedside monitors) would be needed.
Understanding the validity of orders for detection of actual use is critical because continuous pulse oximetry monitoring is considered an overused practice in pediatric acute viral bronchiolitis,3 and national guidelines recommend against its use in low-risk hospitalized children.4,5 Continuous monitoring may identify trivial, self-resolving oxygen desaturation and its use is not associated with improved outcomes.6-9 When self-resolving desaturations are treated with additional supplemental oxygen, hospital stays may be unnecessarily prolonged.10 In order to reduce unnecessary continuous pulse oximetry use, measurement of the extent of the overused practice is necessary. In this 56-hospital study,11 we aimed to determine the validity of using active continuous pulse oximetry orders instead of bedside observation of actual monitor use.
METHODS
Design
In this multicenter, repeated cross-sectional study, investigators used direct bedside observation to determine continuous pulse oximetry monitor use and then assessed whether an active continuous monitoring order was present in the electronic health record. The study took place during one bronchiolitis season, December 1, 2018, through March 31, 2019.
Setting and Patients
Investigators at 56 freestanding children’s hospitals, children’s hospitals within general hospitals, and community hospitals in the Pediatric Research in Inpatient Settings (PRIS) Network collected data on infants aged 8 weeks to 23 months who were hospitalized with bronchiolitis. As this work was a substudy of the larger Eliminating Monitor Overuse study, only infants not currently receiving supplemental oxygen were included.11 Investigators observed eligible infants outside of the intensive care unit on general hospital medicine units. We excluded infants born premature (documented prematurity of <28 weeks’ gestation or documented “premature” without a gestational age listed), as well as those with a home oxygen requirement, cyanotic congenital heart disease, pulmonary hypertension, tracheostomy, primary neuromuscular disease, immunodeficiency, or cancer.
Data Collection
Investigators used the electronic health record to identify eligible infants. Investigators entered patient rooms to confirm the infant was not on supplemental oxygen (hence confirming eligibility for the study) and determine if continuous pulse oximetry was actively in use by examining the monitor display for a pulse oximetry waveform. Investigators then confirmed if active orders for pulse oximetry were present in the patient’s chart. Per study design, site investigators aimed to observe approximately half of eligible infants during the day (10
Analysis
We excluded patients with conditional orders (eg, monitored only when certain conditions exist, such as when asleep) because of the time-varying and wide range of conditions that could be specified. Furthermore, conditional orders would not be useful as proxies to measure oximetry use because investigators would still need additional data (eg, bedside observation) to determine current monitoring status.
We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of active orders using the reference standard of direct bedside observation, as well as corresponding 95% CIs that accounted for within-hospital clustering. We calculated these test characteristics overall and as stratified across four age groups: 8 weeks to 5 months, 6 months to 11 months, 12 months to 17 months, and 18 months to 23 months. We also calculated the test characteristics for each hospital. We decided a priori that a PPV and NPV of 80% would represent a reasonable threshold to use active orders as a proxy in multicenter research. For hospital-level analyses we included only hospitals with 60 or more total observations and more than 15 observations with active orders for PPV and more than 15 observations without active orders for NPV. We used Stata (StataCorp LLC, College Station, Texas) version 15.1 for analysis.
For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study as the single reviewing IRB, and the remaining US sites established reliance agreements with the reviewing IRB. Research Ethics Boards at the Canadian sites (University of Calgary and The Hospital for Sick Children) also reviewed and approved the study. All sites granted waivers of consent, assent, parental permission, and HIPAA authorization.
RESULTS
Investigators completed 3,612 observations in 56 hospitals. This included 33 freestanding children’s hospitals, 14 hospitals within large general hospitals, and 9 community hospitals. Of 3,612 completed observations, on 631 occasions (17%) patients had conditional orders (eg, continuous monitoring only when sleeping) and were excluded from further analysis.
Most pulse oximetry–monitored infants did not have an active monitoring order (670 out of 1,309; sensitivity of 49%). Test characteristics, stratified by age group, are presented in the Table. Across all observations, the overall PPV was 77% (95% CI, 72-82), and the overall NPV was 69% (95% CI, 61-77). Variation of all test characteristics across age group was small (eg, the sensitivity ranged from 43% to 51%).
With inclusion of only those hospitals with sufficient observations, hospital-level variation in the PPV and NPV of using active orders was substantial (PPV range of 48% to 96% and NPV range of 30% to 98%). Only two hospitals had both a PPV and NPV for using monitor orders that exceeded the 80% threshold.
DISCUSSION
Active continuous pulse oximetry orders did not accurately represent actual monitoring status in this study. Monitoring orders alone frequently misrepresent true monitoring status and, as such, should be interpreted with caution in research or quality improvement activities. If more valid estimates of monitoring use and overuse are needed, potential measurement options include direct observation, as used in our study, as well as the use of more complex data streams such as the output of monitoring devices or pulse oximetry data in the electronic health record. In only two of the hospitals, using active continuous monitoring orders was a reasonable proxy for detecting actual monitor use. Monitoring orders could potentially be validly used for deimplementation efforts at those centers; other hospitals could consider targeted improvement efforts (eg, morning huddles examining the discordance between monitoring orders and monitoring status) to improve the accuracy of using continuous pulse oximetry orders.
We acknowledge several limitations of this study. Site investigators employed a convenience sampling approach, so it is possible that some investigators observed sicker or less sick infants. Although the PRIS network includes a geographically diverse group of North American hospitals, community hospitals were underrepresented in this study. Our results hence generalize more precisely to freestanding children’s hospitals than to community hospitals. We did not observe infants currently on supplemental oxygen, so we do not know to what degree using orders is valid in that context. We did not collect data on why actual monitoring status differed from monitoring orders and hence cannot quantify to what extent different factors (eg, nurse belief that monitors are a safety net or infants inadvertently left on monitors after a spot check pulse oximetry reading) contributed to this discordance. Finally, our study only examined one electronic health record variable—the presence of an active order. It may be that other variables in the health record (eg, minute-by-minute pulse oximetry values in a vital sign flowsheet) are much better proxies of actual continuous monitor use.
CONCLUSION
Using an active order for continuous pulse oximetry has poor sensitivity, PPV, and NPV for detecting true monitoring status at the bedside. Teams intending to measure the actual use of pulse oximetry should be aware of the limitations of using active orders alone as an accurate measure of pulse oximetry monitoring.
Acknowledgments
We thank the NHLBI scientists who contributed to this project as part of the U01 Cooperative Agreement funding mechanism: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD.
We thank the Executive Council of the PRIS Network for their contributions to the early scientific development of this project. We thank the PRIS site investigators for their major contributions to the Eliminating Monitor Overuse (EMO) Study data collection. Each listed collaborator is a group author for the PRIS Network in this manuscript. Their names can be found in the online supplemental information.
1. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1). https://doi.org/10.1542/peds.2015-0851
2. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
3. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
4. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
5. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
6. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
7. Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. https://doi.org/10.1016/s0140-6736(15)00163-4
8. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
9. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
10. Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. https://doi.org/10.1001/archpedi.158.6.527
11. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2
As part of improvement collaboratives that aimed to reduce overuse of continuous pulse oximetry in children hospitalized with bronchiolitis, researchers used the presence of an active order for it as a proxy for the actual use of such monitoring.1,2 With use of this proxy, investigators on a national study documented a high burden of continuous oximetry overuse (86.5% before quality improvement interventions and 45.5% after),1 but the validity of orders in representing actual monitoring practice is unknown. If the presence of an active pulse oximetry order accurately identifies infants on monitors, electronic health record data could inform epidemiologic estimates of monitoring overuse and measure the success of quality improvement and deimplementation interventions. Alternatively, if nurses commonly begin and/or discontinue pulse oximetry without updated orders, a pulse oximetry order would not be an accurate proxy, and additional data capture methods (eg, bedside observation or data capture from bedside monitors) would be needed.
Understanding the validity of orders for detection of actual use is critical because continuous pulse oximetry monitoring is considered an overused practice in pediatric acute viral bronchiolitis,3 and national guidelines recommend against its use in low-risk hospitalized children.4,5 Continuous monitoring may identify trivial, self-resolving oxygen desaturation and its use is not associated with improved outcomes.6-9 When self-resolving desaturations are treated with additional supplemental oxygen, hospital stays may be unnecessarily prolonged.10 In order to reduce unnecessary continuous pulse oximetry use, measurement of the extent of the overused practice is necessary. In this 56-hospital study,11 we aimed to determine the validity of using active continuous pulse oximetry orders instead of bedside observation of actual monitor use.
METHODS
Design
In this multicenter, repeated cross-sectional study, investigators used direct bedside observation to determine continuous pulse oximetry monitor use and then assessed whether an active continuous monitoring order was present in the electronic health record. The study took place during one bronchiolitis season, December 1, 2018, through March 31, 2019.
Setting and Patients
Investigators at 56 freestanding children’s hospitals, children’s hospitals within general hospitals, and community hospitals in the Pediatric Research in Inpatient Settings (PRIS) Network collected data on infants aged 8 weeks to 23 months who were hospitalized with bronchiolitis. As this work was a substudy of the larger Eliminating Monitor Overuse study, only infants not currently receiving supplemental oxygen were included.11 Investigators observed eligible infants outside of the intensive care unit on general hospital medicine units. We excluded infants born premature (documented prematurity of <28 weeks’ gestation or documented “premature” without a gestational age listed), as well as those with a home oxygen requirement, cyanotic congenital heart disease, pulmonary hypertension, tracheostomy, primary neuromuscular disease, immunodeficiency, or cancer.
Data Collection
Investigators used the electronic health record to identify eligible infants. Investigators entered patient rooms to confirm the infant was not on supplemental oxygen (hence confirming eligibility for the study) and determine if continuous pulse oximetry was actively in use by examining the monitor display for a pulse oximetry waveform. Investigators then confirmed if active orders for pulse oximetry were present in the patient’s chart. Per study design, site investigators aimed to observe approximately half of eligible infants during the day (10
Analysis
We excluded patients with conditional orders (eg, monitored only when certain conditions exist, such as when asleep) because of the time-varying and wide range of conditions that could be specified. Furthermore, conditional orders would not be useful as proxies to measure oximetry use because investigators would still need additional data (eg, bedside observation) to determine current monitoring status.
We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of active orders using the reference standard of direct bedside observation, as well as corresponding 95% CIs that accounted for within-hospital clustering. We calculated these test characteristics overall and as stratified across four age groups: 8 weeks to 5 months, 6 months to 11 months, 12 months to 17 months, and 18 months to 23 months. We also calculated the test characteristics for each hospital. We decided a priori that a PPV and NPV of 80% would represent a reasonable threshold to use active orders as a proxy in multicenter research. For hospital-level analyses we included only hospitals with 60 or more total observations and more than 15 observations with active orders for PPV and more than 15 observations without active orders for NPV. We used Stata (StataCorp LLC, College Station, Texas) version 15.1 for analysis.
For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study as the single reviewing IRB, and the remaining US sites established reliance agreements with the reviewing IRB. Research Ethics Boards at the Canadian sites (University of Calgary and The Hospital for Sick Children) also reviewed and approved the study. All sites granted waivers of consent, assent, parental permission, and HIPAA authorization.
RESULTS
Investigators completed 3,612 observations in 56 hospitals. This included 33 freestanding children’s hospitals, 14 hospitals within large general hospitals, and 9 community hospitals. Of 3,612 completed observations, on 631 occasions (17%) patients had conditional orders (eg, continuous monitoring only when sleeping) and were excluded from further analysis.
Most pulse oximetry–monitored infants did not have an active monitoring order (670 out of 1,309; sensitivity of 49%). Test characteristics, stratified by age group, are presented in the Table. Across all observations, the overall PPV was 77% (95% CI, 72-82), and the overall NPV was 69% (95% CI, 61-77). Variation of all test characteristics across age group was small (eg, the sensitivity ranged from 43% to 51%).

With inclusion of only those hospitals with sufficient observations, hospital-level variation in the PPV and NPV of using active orders was substantial (PPV range of 48% to 96% and NPV range of 30% to 98%). Only two hospitals had both a PPV and NPV for using monitor orders that exceeded the 80% threshold.
DISCUSSION
Active continuous pulse oximetry orders did not accurately represent actual monitoring status in this study. Monitoring orders alone frequently misrepresent true monitoring status and, as such, should be interpreted with caution in research or quality improvement activities. If more valid estimates of monitoring use and overuse are needed, potential measurement options include direct observation, as used in our study, as well as the use of more complex data streams such as the output of monitoring devices or pulse oximetry data in the electronic health record. In only two of the hospitals, using active continuous monitoring orders was a reasonable proxy for detecting actual monitor use. Monitoring orders could potentially be validly used for deimplementation efforts at those centers; other hospitals could consider targeted improvement efforts (eg, morning huddles examining the discordance between monitoring orders and monitoring status) to improve the accuracy of using continuous pulse oximetry orders.
We acknowledge several limitations of this study. Site investigators employed a convenience sampling approach, so it is possible that some investigators observed sicker or less sick infants. Although the PRIS network includes a geographically diverse group of North American hospitals, community hospitals were underrepresented in this study. Our results hence generalize more precisely to freestanding children’s hospitals than to community hospitals. We did not observe infants currently on supplemental oxygen, so we do not know to what degree using orders is valid in that context. We did not collect data on why actual monitoring status differed from monitoring orders and hence cannot quantify to what extent different factors (eg, nurse belief that monitors are a safety net or infants inadvertently left on monitors after a spot check pulse oximetry reading) contributed to this discordance. Finally, our study only examined one electronic health record variable—the presence of an active order. It may be that other variables in the health record (eg, minute-by-minute pulse oximetry values in a vital sign flowsheet) are much better proxies of actual continuous monitor use.
CONCLUSION
Using an active order for continuous pulse oximetry has poor sensitivity, PPV, and NPV for detecting true monitoring status at the bedside. Teams intending to measure the actual use of pulse oximetry should be aware of the limitations of using active orders alone as an accurate measure of pulse oximetry monitoring.
Acknowledgments
We thank the NHLBI scientists who contributed to this project as part of the U01 Cooperative Agreement funding mechanism: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD.
We thank the Executive Council of the PRIS Network for their contributions to the early scientific development of this project. We thank the PRIS site investigators for their major contributions to the Eliminating Monitor Overuse (EMO) Study data collection. Each listed collaborator is a group author for the PRIS Network in this manuscript. Their names can be found in the online supplemental information.
As part of improvement collaboratives that aimed to reduce overuse of continuous pulse oximetry in children hospitalized with bronchiolitis, researchers used the presence of an active order for it as a proxy for the actual use of such monitoring.1,2 With use of this proxy, investigators on a national study documented a high burden of continuous oximetry overuse (86.5% before quality improvement interventions and 45.5% after),1 but the validity of orders in representing actual monitoring practice is unknown. If the presence of an active pulse oximetry order accurately identifies infants on monitors, electronic health record data could inform epidemiologic estimates of monitoring overuse and measure the success of quality improvement and deimplementation interventions. Alternatively, if nurses commonly begin and/or discontinue pulse oximetry without updated orders, a pulse oximetry order would not be an accurate proxy, and additional data capture methods (eg, bedside observation or data capture from bedside monitors) would be needed.
Understanding the validity of orders for detection of actual use is critical because continuous pulse oximetry monitoring is considered an overused practice in pediatric acute viral bronchiolitis,3 and national guidelines recommend against its use in low-risk hospitalized children.4,5 Continuous monitoring may identify trivial, self-resolving oxygen desaturation and its use is not associated with improved outcomes.6-9 When self-resolving desaturations are treated with additional supplemental oxygen, hospital stays may be unnecessarily prolonged.10 In order to reduce unnecessary continuous pulse oximetry use, measurement of the extent of the overused practice is necessary. In this 56-hospital study,11 we aimed to determine the validity of using active continuous pulse oximetry orders instead of bedside observation of actual monitor use.
METHODS
Design
In this multicenter, repeated cross-sectional study, investigators used direct bedside observation to determine continuous pulse oximetry monitor use and then assessed whether an active continuous monitoring order was present in the electronic health record. The study took place during one bronchiolitis season, December 1, 2018, through March 31, 2019.
Setting and Patients
Investigators at 56 freestanding children’s hospitals, children’s hospitals within general hospitals, and community hospitals in the Pediatric Research in Inpatient Settings (PRIS) Network collected data on infants aged 8 weeks to 23 months who were hospitalized with bronchiolitis. As this work was a substudy of the larger Eliminating Monitor Overuse study, only infants not currently receiving supplemental oxygen were included.11 Investigators observed eligible infants outside of the intensive care unit on general hospital medicine units. We excluded infants born premature (documented prematurity of <28 weeks’ gestation or documented “premature” without a gestational age listed), as well as those with a home oxygen requirement, cyanotic congenital heart disease, pulmonary hypertension, tracheostomy, primary neuromuscular disease, immunodeficiency, or cancer.
Data Collection
Investigators used the electronic health record to identify eligible infants. Investigators entered patient rooms to confirm the infant was not on supplemental oxygen (hence confirming eligibility for the study) and determine if continuous pulse oximetry was actively in use by examining the monitor display for a pulse oximetry waveform. Investigators then confirmed if active orders for pulse oximetry were present in the patient’s chart. Per study design, site investigators aimed to observe approximately half of eligible infants during the day (10
Analysis
We excluded patients with conditional orders (eg, monitored only when certain conditions exist, such as when asleep) because of the time-varying and wide range of conditions that could be specified. Furthermore, conditional orders would not be useful as proxies to measure oximetry use because investigators would still need additional data (eg, bedside observation) to determine current monitoring status.
We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of active orders using the reference standard of direct bedside observation, as well as corresponding 95% CIs that accounted for within-hospital clustering. We calculated these test characteristics overall and as stratified across four age groups: 8 weeks to 5 months, 6 months to 11 months, 12 months to 17 months, and 18 months to 23 months. We also calculated the test characteristics for each hospital. We decided a priori that a PPV and NPV of 80% would represent a reasonable threshold to use active orders as a proxy in multicenter research. For hospital-level analyses we included only hospitals with 60 or more total observations and more than 15 observations with active orders for PPV and more than 15 observations without active orders for NPV. We used Stata (StataCorp LLC, College Station, Texas) version 15.1 for analysis.
For US sites, the Institutional Review Board (IRB) at Children’s Hospital of Philadelphia approved the study as the single reviewing IRB, and the remaining US sites established reliance agreements with the reviewing IRB. Research Ethics Boards at the Canadian sites (University of Calgary and The Hospital for Sick Children) also reviewed and approved the study. All sites granted waivers of consent, assent, parental permission, and HIPAA authorization.
RESULTS
Investigators completed 3,612 observations in 56 hospitals. This included 33 freestanding children’s hospitals, 14 hospitals within large general hospitals, and 9 community hospitals. Of 3,612 completed observations, on 631 occasions (17%) patients had conditional orders (eg, continuous monitoring only when sleeping) and were excluded from further analysis.
Most pulse oximetry–monitored infants did not have an active monitoring order (670 out of 1,309; sensitivity of 49%). Test characteristics, stratified by age group, are presented in the Table. Across all observations, the overall PPV was 77% (95% CI, 72-82), and the overall NPV was 69% (95% CI, 61-77). Variation of all test characteristics across age group was small (eg, the sensitivity ranged from 43% to 51%).

With inclusion of only those hospitals with sufficient observations, hospital-level variation in the PPV and NPV of using active orders was substantial (PPV range of 48% to 96% and NPV range of 30% to 98%). Only two hospitals had both a PPV and NPV for using monitor orders that exceeded the 80% threshold.
DISCUSSION
Active continuous pulse oximetry orders did not accurately represent actual monitoring status in this study. Monitoring orders alone frequently misrepresent true monitoring status and, as such, should be interpreted with caution in research or quality improvement activities. If more valid estimates of monitoring use and overuse are needed, potential measurement options include direct observation, as used in our study, as well as the use of more complex data streams such as the output of monitoring devices or pulse oximetry data in the electronic health record. In only two of the hospitals, using active continuous monitoring orders was a reasonable proxy for detecting actual monitor use. Monitoring orders could potentially be validly used for deimplementation efforts at those centers; other hospitals could consider targeted improvement efforts (eg, morning huddles examining the discordance between monitoring orders and monitoring status) to improve the accuracy of using continuous pulse oximetry orders.
We acknowledge several limitations of this study. Site investigators employed a convenience sampling approach, so it is possible that some investigators observed sicker or less sick infants. Although the PRIS network includes a geographically diverse group of North American hospitals, community hospitals were underrepresented in this study. Our results hence generalize more precisely to freestanding children’s hospitals than to community hospitals. We did not observe infants currently on supplemental oxygen, so we do not know to what degree using orders is valid in that context. We did not collect data on why actual monitoring status differed from monitoring orders and hence cannot quantify to what extent different factors (eg, nurse belief that monitors are a safety net or infants inadvertently left on monitors after a spot check pulse oximetry reading) contributed to this discordance. Finally, our study only examined one electronic health record variable—the presence of an active order. It may be that other variables in the health record (eg, minute-by-minute pulse oximetry values in a vital sign flowsheet) are much better proxies of actual continuous monitor use.
CONCLUSION
Using an active order for continuous pulse oximetry has poor sensitivity, PPV, and NPV for detecting true monitoring status at the bedside. Teams intending to measure the actual use of pulse oximetry should be aware of the limitations of using active orders alone as an accurate measure of pulse oximetry monitoring.
Acknowledgments
We thank the NHLBI scientists who contributed to this project as part of the U01 Cooperative Agreement funding mechanism: Lora Reineck, MD, MS, Karen Bienstock, MS, and Cheryl Boyce, PhD.
We thank the Executive Council of the PRIS Network for their contributions to the early scientific development of this project. We thank the PRIS site investigators for their major contributions to the Eliminating Monitor Overuse (EMO) Study data collection. Each listed collaborator is a group author for the PRIS Network in this manuscript. Their names can be found in the online supplemental information.
1. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1). https://doi.org/10.1542/peds.2015-0851
2. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
3. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
4. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
5. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
6. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
7. Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. https://doi.org/10.1016/s0140-6736(15)00163-4
8. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
9. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
10. Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. https://doi.org/10.1001/archpedi.158.6.527
11. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2
1. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1). https://doi.org/10.1542/peds.2015-0851
2. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
3. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
4. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064
5. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
6. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
7. Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386(9998):1041-1048. https://doi.org/10.1016/s0140-6736(15)00163-4
8. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
9. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
10. Schroeder AR, Marmor AK, Pantell RH, Newman TB. Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations. Arch Pediatr Adolesc Med. 2004;158(6):527-530. https://doi.org/10.1001/archpedi.158.6.527
11. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2
© 2020 Society of Hospital Medicine
Clinical Characteristics and Outcomes of Non-ICU Hospitalization for COVID-19 in a Nonepicenter, Centrally Monitored Healthcare System
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).
Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.
Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).

Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.

Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).

Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.

Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
© 2021 Society of Hospital Medicine
Financial Difficulties in Families of Hospitalized Children
Rising US healthcare costs coupled with high cost-sharing insurance plans have led to increased out-of-pocket healthcare expenditures, especially for those who are low income or in poorer health.1-7 Increased out-of-pocket expenditures can lead to “financial distress” (defined as the subjective level of stress felt toward one’s personal financial situation) and to “medical financial burden” (defined as the subjective assessment of financial problems relating specifically to medical costs). Financial distress and medical financial burden (defined together as “financial difficulty”) lead to impaired access and delayed presentation to care and treatment nonadherence in hopes of alleviating costs.8-12
Between 20% and 50% of families with children requiring frequent medical care report that their child’s healthcare has caused a financial difficulty.13,14 In addition to direct medical costs, these parents can also suffer from indirect costs of their child’s care, such as unemployment or missed work.15-17 Along with these families, families who are low income (generally defined as living below 200% of the Federal Poverty Level) also have higher absolute and relative out-of-pocket healthcare costs, and both groups are more likely to have unmet medical needs or to delay or forgo care.18-20 Medically complex children also represent an increasing percentage of patients admitted to children’s hospitals21,22 where their families may be more vulnerable to worsening financial difficulties caused by direct costs and income depletion—due to lost wages, transportation, and meals—associated with hospitalization.23
The hospitalized population can be readily screened and provided interventions. Although evidence on effective inpatient financial interventions is lacking, financial navigation programs piloted in the ambulatory setting that standardize financial screening and support trained financial navigators could prove a promising model for inpatient care.24-26 Therefore, understanding the prevalence of financial difficulties in this population and potential high-yield screening characteristics is critical in laying the groundwork for more robust in-hospital financial screening and support systems.
Our primary objective was to assess the prevalence of financial distress and medical financial burden in families of hospitalized children. Our secondary objective was to examine measurable factors during hospitalization that could identify families at risk for these financial difficulties to better understand how to target and implement hospital-based interventions.
METHODS
We conducted a cross-sectional survey at six university-affiliated children’s hospitals (Table 1). Each site’s institutional review board approved the study. All participants were verbally informed of the research goals of the study and provided with a research information document. Need for written informed consent was determined by each institutional review board.
Study enrollment occurred between October 2017 and November 2018, with individual sites having shorter active enrollment periods (ranging from 25 to 100 days) until sample size goals were met as explained below. Participants represented a convenience sample of parents or guardians (hereafter referred to only as “parents”), who were eligible for enrollment if their child was admitted to one of the six hospitals during the active enrollment period at that site. To avoid sampling bias, each site made an effort to enroll a consecutive sample of parents, but this was limited by resources and investigator availability. Parents were excluded if their child was admitted to a neonatal unit because of difficulty in complexity categorization and the confounding issue of mothers often being admitted simultaneously. There were no other unit-, diagnosis-, or service-based exclusions to participation. Parents were also excluded if their child was 18 years or older or if they themselves were younger than 18 years. Parents were approached once their child was identified for discharge from the hospital within 48 hours. Surveys were self-administered at the time of enrollment on provided electronic tablets. Participants at some sites were offered a $5 gift card as an incentive for survey completion.
The survey included a previously published financial distress scale (InCharge Financial Distress/Financial Wellbeing Scale [IFDFW])(Appendix).27 A question in addition to the IFDFW assessed whether families were currently experiencing financial burden from medical care28,29 and whether that burden was caused by their child (Appendix) because the IFDFW does not address the source of financial distress. The survey also included questions assessing perspectives on healthcare costs (data not presented here). The survey was refined through review by psychometric experts and members of the Family Advisory Council at the primary research site, which led to minor modifications. The final survey consisted of 40 items and was professionally translated into Spanish by a third-party company (Idem Translations). It was pilot tested by 10 parents of hospitalized children to assess for adequate comprehension and clarity; these parents were not included in the final data analysis.
Variables
The primary outcome variables were level of financial distress as defined by the IFDFW scale27 and the presence of medical financial burden. The IFDFW scale has eight questions answered on a scale of 1-10, and the final score is calculated by averaging these answers. The scale defines three categories of financial distress (high, 1-3.9; average, 4-6.9; low, 7-10); however, we dichotomized our outcome as high (<4) or not high (≥4). The outcome was analyzed as both continuous and dichotomous variables because small differences in continuous scores, if detected, may be less clinically relevant. Medical financial burden was categorized as child related, child unrelated, and none.
Our secondary aim was to identify predictors of financial distress and medical financial burden. The primary predictor variable of interest was the hospitalized child’s level of chronic disease (complex chronic disease, C-CD; noncomplex chronic disease, NC-CD; no chronic disease, no-CD) as categorized by the consensus definitions from the Center of Excellence on Quality of Care Measures for Children with Complex Needs (Appendix).30 We assigned level of chronic disease based on manual review of problem lists and diagnoses in the electronic health record (EHR) from up to 3 years prior. At sites with multiple researchers, the first five to ten charts were reviewed together to ensure consistency in categorization, but no formal assessment of interrater reliability was conducted. Other predictor variables are listed in Tables 2 and 3. Insurance payer was defined as “public” or “private” based on the documented insurance plan in the EHR. Patients with dual public and private insurance were categorized as public.
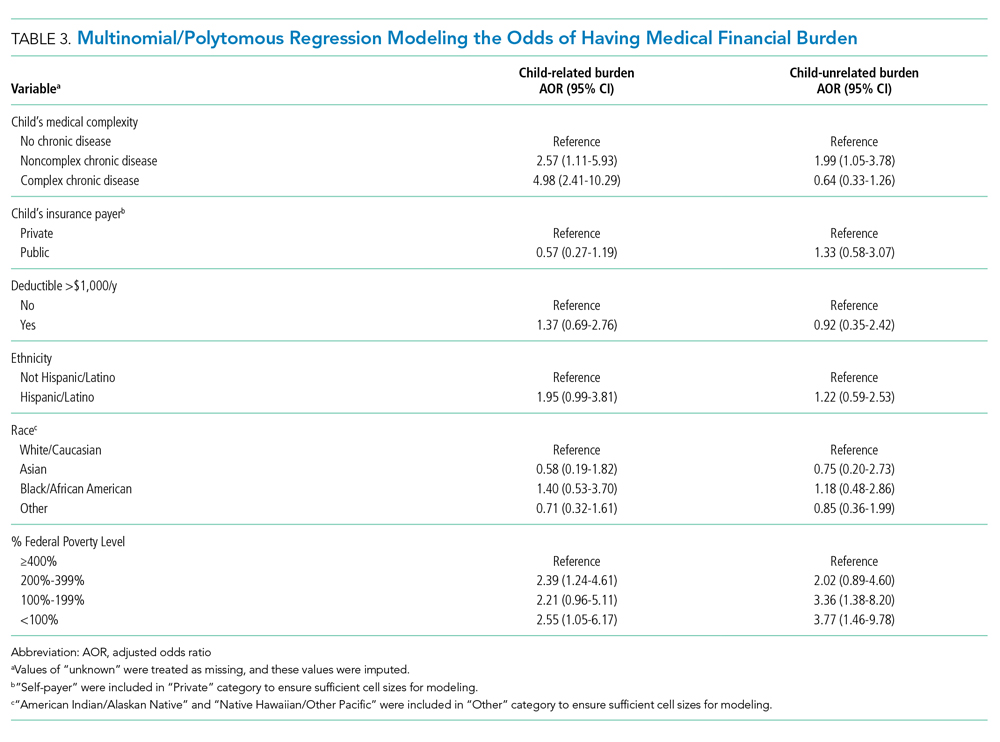
Statistical Analysis
We estimated sample size requirements using an expected mean IFDFW score with standard deviation of 5.7 ± 2 based on preliminary data from the primary study site and previously published data.27 We used a significance level of P = .05, power of 0.80, and an effect size of 0.5 points difference on the IFDFW scale between the families of children with C-CD and those with either NC-CD or no-CD. We assumed there would be unequal representation of chronic disease states, with an expectation that children with C-CD would make up approximately 40% of the total population.21,22,31 Under these assumptions, we calculated a desired total sample size of 519. This would also allow us to detect a 12% absolute difference in the rate of high financial distress between families with and without C-CD, assuming a baseline level of high financial distress of 30%.27 Our goal enrollment was 150 parents at the primary site and 75 parents at each of the other 5 sites.
We fit mixed effects logistic regression models to evaluate the odds of high financial distress and polytomous logistic regression models (for our three-level outcome) to evaluate the odds of having child-related medical financial burden vs having child-unrelated burden vs having no burden. We fit linear mixed effects models to evaluate the effect of chronic disease level and medical financial burden on mean IFDFW scores. Respondents who answered “I don’t know” to the medical financial burden question were aggregated with those who reported no medical financial burden. Models were fit as a function of chronic disease level, race, ethnicity, percentage of Federal Poverty Level (FPL), insurance payer, and having a deductible less than $1,000 per year. These models included a random intercept for facility. We also fit logistic regression models that used an interaction term between chronic disease level and percentage of FPL, as well as insurance payer and percentage of FPL, to explore potential effect modification between poverty and both chronic disease level and insurance payer on financial distress. For our models, we used the MICE package for multiple imputation to fill in missing data. We imputed 25 data sets with 25 iterations each and pooled model results using Rubin’s Rules.32 All analyses were performed in R 3.5.33
RESULTS
Of 644 parents who were invited to participate, 526 (82%) were enrolled. Participants and their hospitalized children were mostly White/Caucasian (69%) and not Hispanic/Latino (76%), with 34% of families living below 200% FPL and 274 (52%) having private insurance (Table 1). Of the hospitalized children, 225 (43%) were categorized as C-CD, 143 (27%) as NC-CD, and 157 (30%) as no-CD. All participants completed the IFDFW; however, there were five missing responses to the medical financial burden question. Table 1 lists missing demographic and financial difficulty data.
Financial Distress
The mean IFDFW score of all participants was 5.6 ± 2.1, with 125 having high financial distress (24%; 95% CI, 20-28) (Table 1). There was no difference in mean IFDFW scores among families of children with different chronic disease levels (Figure). On unadjusted and adjusted analyses, there was no association between level of chronic disease and high financial distress when C-CD and NC-CD groups were each compared with no-CD (Table 2). However, families living below 400% FPL (annual income of $100,400 for a family of four) were significantly more likely than families living at 400% FPL and above to have high financial distress. Families tended to have lower financial distress (as indicated by mean IFDFW scores) with increasing percentage of FPL; however, there were families in every FPL bracket who experienced high financial distress (Appendix Figure 1a). A secondary analysis of families below and those at or above 200% FPL did not find any significant interactions between percentage of FPL and either chronic disease level (P = .86) or insurance payer (P = .83) on financial distress.
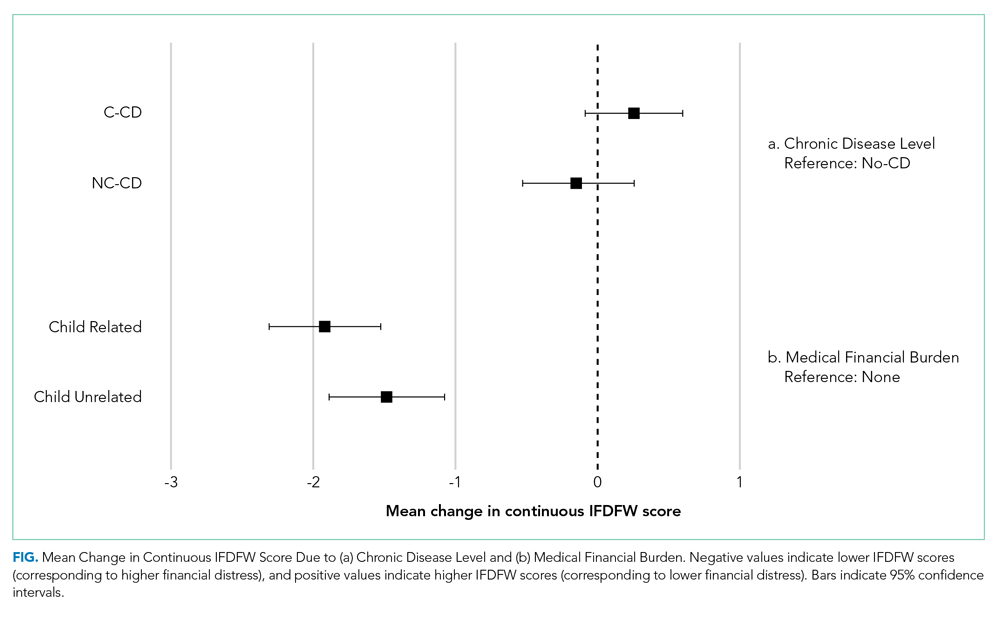
Medical Financial Burden
Overall, 160 parents (30%; 95% CI, 27-35) reported having medical financial burden, with 86 of those parents (54%) indicating their financial burden was related to their child’s medical care (Table 1). Compared with families with no such medical financial burden, respondents with medical financial burden, either child related or child unrelated, had significantly lower mean IFDFW scores (Figure), which indicates overall higher financial distress in these families. However, some families with low financial distress also reported medical financial burden.
Adjusted analyses demonstrated that, compared with families of children with no-CD, families of children with C-CD (adjusted odds ratio [AOR], 4.98; 95% CI, 2.41-10.29) or NC-CD (AOR, 2.57; 95% CI, 1.11-5.93) had significantly higher odds of having child-related medical financial burden (Table 3). Families of children with NC-CD were also more likely than families of children with no-CD to have child-unrelated medical burden (Table 3). Percentage of FPL was the only other significant predictor of child-related and child-unrelated medical financial burden (Table 3), but as with the distribution of financial distress, medical financial burden was seen across family income brackets (Appendix Figure 1b).
DISCUSSION
In this multicenter study of parents of hospitalized children, almost one in four families experienced high financial distress and almost one in three families reported having medical financial burden, with both measures of financial difficulty affecting families across all income levels. While these percentages are not substantially higher than those seen in the general population,27,34 70% of our population was composed of children with chronic disease who are more likely to have short-term and long-term healthcare needs, which places them at risk for significant ongoing medical costs.
We hypothesized that families of children with complex chronic disease would have higher levels of financial difficulties,13,35,36 but we found that level of chronic disease was associated only with medical financial burden and not with high financial distress. Financial distress is likely multifactorial and dynamic, with different drivers across various income levels. Therefore, while medical financial burden likely contributes to high financial distress, there may be other contributing factors not captured by the IFDFW. However, subjective medical financial burden has still been associated with impaired access to care.10,34 Therefore, our results suggest that families of children with chronic diseases might be at higher risk for barriers to consistent healthcare because of the financial burden their frequent healthcare utilization incurs.
Household poverty level was also associated with financial distress and medical financial burden, although surprisingly both measures of financial difficulty were present in all FPL brackets. This highlights an important reality that financial vulnerability extends beyond income and federally defined “poverty.” Non-income factors, such as high local costs of living and the growing problem of underinsurance, may significantly contribute to financial difficulty, which may render static financial metrics such as percentage of FPL insufficient screeners. Furthermore, as evidenced by the nearly 10% of our respondents who declined to provide their income information, this is a sensitive topic for some families, so gathering income data during admission could likely be a nonstarter.
In the absence of other consistent predictors of financial difficulty that could trigger interventions such as an automatic financial counselor consult, hospitals and healthcare providers could consider implementing routine non-income based financial screening questions on admission, such as one assessing medical financial burden, as a nondiscriminatory way of identifying at-risk families and provide further education and assistance regarding their financial needs. Systematically gathering this data may also further demonstrate the need for broad financial navigation programs as a mainstay in comprehensive inpatient care.
We acknowledge several limitations of this study. Primarily, we surveyed families prior to discharge and receipt of hospitalization-related bills, and these bills could contribute significantly to financial difficulties. While the families of children with chronic disease, who likely have recurrent medical bills, did not demonstrate higher financial distress, it is possible that the overall rate of financial difficulties would have been higher had we surveyed families several weeks after discharge. Our measures of financial difficulty were also subjective and, therefore, at risk for response biases (such as recall bias) that could have misestimated the prevalence of these problems in our population. However, published literature on the IFDFW scale demonstrates concordance between the subjective score and tangible outcomes of financial distress (eg, contacting a credit agency). The IFDFW scale was validated in the general population, and although it has been used in studies of medical populations,37-41 none have been in hospitalized populations, which may affect the scale’s applicability in our study. The study was also conducted only at university-affiliated children’s hospitals, and although these hospitals are geographically diverse, most children in the United States are admitted to general or community hospitals.31 Our population was also largely White, non-Hispanic/Latino, and English speaking. Therefore, our sample may not reflect the general population of hospitalized children and their families. We also assigned levels of chronic disease based on manual EHR review. While the EHR should capture each patient’s breadth of medical issues, inaccurate or missing documentation could have led to misclassification of complexity in some cases. Additionally, our sample size was calculated to detect fairly large differences in our primary outcome, and some of our unexpected results may have resulted from this study being underpowered for detection of smaller, but perhaps still clinically relevant, differences. Finally, we do not have data for several possible confounders in our study, such as employment status, health insurance concordance among family members, or sources of supplemental income, that may impact a family’s overall financial health, along with some potential important hospital-based screening characteristics, such as admitting service team or primary diagnosis.
CONCLUSION
Financial difficulties are common in families of hospitalized pediatric patients. Low-income families and those who have children with chronic conditions are at particular risk; however, all subsets of families can be affected. Given the potential negative health outcomes financial difficulties impose on families and children, the ability to identify and support vulnerable families is a crucial component of care. Hospitalization may be a prime opportunity to identify and support our at-risk families.
Acknowledgments
The authors would like to thank the parents at each of the study sites for their participation, as well as the multiple research coordinators across the study sites for assisting in recruitment of families, survey administration, and data collection. KT Park, MD, MS (Stanford University School of Medicine) served as an adviser for the study’s design.
Disclosures
All authors have no financial relationships or conflicts of interest relevant to this article to disclose.
1. Blumberg LJ, Waidmann TA, Blavin F, Roth J. Trends in health care financial burdens, 2001 to 2009. Milbank Q. 2014;92(1):88-113. https://doi.org/10.1111/1468-0009.12042
2. Claxton G, Rae M, Long M, et al. Employer Health Benefits, 2015 Annual Survey. Kaiser Family Foundation; 2015. http://files.kff.org/attachment/report-2015-employer-health-benefits-survey
3. Long M, Rae M, Claxton G, et al. Recent trends in employer-sponsored insurance premiums. JAMA. 2016;315(1):18. https://doi.org/10.1001/jama.2015.17349
4. Patients’ perspectives on health care in the United States: A look at seven states and the nation. Press release. NPR, Robert Wood Johnson Foundation, Harvard T.H. Chan School of Public Health; February 29, 2016. Accessed February 23, 2018. https://www.rwjf.org/en/library/research/2016/02/patients--perspectives-on-health-care-in-the-united-states.html
5. May JH, Cunningham PJ. Tough trade-offs: medical bills, family finances and access to care. Issue Brief Cent Stud Health Syst Change. 2004;(85):1-4.
6. Tu HT. Rising health costs, medical debt and chronic conditions. Issue Brief Cent Stud Health Syst Change. 2004;(88):1-5.
7. Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014;14:435. https://doi.org/10.1186/1472-6963-14-435
8. Choudhry NK, Saya UY, Shrank WH, et al. Cost-related medication underuse: prevalence among hospitalized managed care patients. J Hosp Med. 2012;7(2):104-109. https://doi.org/10.1002/jhm.948
9. QuickStats: percentage of persons of all ages who delayed or did not receive medical care during the preceding year because of cost, by U.S. Census region of residence—National Health Interview Survey, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(4):121. https://dx.doi.org/10.15585/mmwr.mm6604a9
10. Doty MM, Ho A, Davis K. How High Is Too High? Implications of High-Deductible Health Plans. The Commonwealth Fund; April 1, 2005. Accessed February 24, 2018. http://www.commonwealthfund.org/publications/fund-reports/2005/apr/how-high-is-too-high--implications-of-high-deductible-health-plans
11. Doty MM, Edwards JN, Holmgren AL. Seeing Red: American Driven into Debt by Medical Bills. The Commonwealth Fund; August 1, 2005. Accessed October 24, 2018. https://www.commonwealthfund.org/publications/issue-briefs/2005/aug/seeing-red-americans-driven-debt-medical-bills
12. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. https://doi.org/10.1093/jnci/djw205
13. Ghandour RM, Hirai AH, Blumberg SJ, Strickland BB, Kogan MD. Financial and nonfinancial burden among families of CSHCN: changes between 2001 and 2009-2010. Acad Pediatr. 2014;14(1):92-100. https://doi.org/10.1016/j.acap.2013.10.001
14. Thomson J, Shah SS, Simmons JM, et al. Financial and social hardships in families of children with medical complexity. J Pediatr. 2016;172:187-193.e1. https://doi.org/10.1016/j.jpeds.2016.01.049
15. Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2010;14(2):155-163. https://doi.org/10.1007/s10995-008-0434-1
16. Kuhlthau KA, Perrin JM. Child health status and parental employment. Arch Pediatr Adolesc Med. 2001;155(12):1346-1350. https://doi.org/10.1001/archpedi.155.12.1346
17. Witt WP, Gottlieb CA, Hampton J, Litzelman K. The impact of childhood activity limitations on parental health, mental health, and workdays lost in the United States. Acad Pediatr. 2009;9(4):263-269. https://doi.org/10.1016/j.acap.2009.02.008
18. Wisk LE, Witt WP. Predictors of delayed or forgone needed health care for families with children. Pediatrics. 2012;130(6):1027-1037. https://doi.org/10.1542/peds.2012-0668
19. Davidoff AJ. Insurance for children with special health care needs: patterns of coverage and burden on families to provide adequate insurance. Pediatrics. 2004;114(2):394-403. https://doi.org/10.1542/peds.114.2.394
20. Galbraith AA, Wong ST, Kim SE, Newacheck PW. Out-of-pocket financial burden for low-income families with children: socioeconomic disparities and effects of insurance. Health Serv Res. 2005;40(6 Pt 1):1722-1736. https://doi.org/10.1111/j.1475-6773.2005.00421.x
21. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
22. Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatrics. 2013;167(2):170-177. https://doi.org/10.1001/jamapediatrics.2013.432
23. Chang LV, Shah AN, Hoefgen ER, et al. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
24. Banegas MP, Dickerson JF, Friedman NL, et al. Evaluation of a novel financial navigator pilot to address patient concerns about medical care costs. Perm J. 2019;23:18-084. https://doi.org/10.7812/tpp/18-084
25. Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122-e129. https://doi.org/10.1200/jop.2017.024927
26. Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5 Suppl):S74-S79.
27. Prawitz AD, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. InCharge Financial Distress/Financial Well-Being Scale: Development, Administration, and Score Interpretation. J Financial Counseling Plann. 2006;17(1):34-50. https://doi.org/10.1037/t60365-000
28. Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief. 2014;(142):1-8.
29. Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood). 2011;30(2):322-331. https://doi.org/10.1377/hlthaff.2010.0584
30. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. https://doi.org/10.1542/peds.2013-3875
31. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987.
33. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/
34. Hamel L, Norton M, Pollitz K, Levitt L, Claxton G, Brodie M. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. Kaiser Family Foundation; January 5, 2016. Accessed February 26, 2019. https://www.kff.org/wp-content/uploads/2016/01/8806-the-burden-of-medical-debt-results-from-the-kaiser-family-foundation-new-york-times-medical-bills-survey.pdf
35. Witt WP, Litzelman K, Mandic CG, et al. Healthcare-related financial burden among families in the U.S.: the role of childhood activity limitations and income. J Fam Econ Issues. 2011;32(2):308-326. https://doi.org/10.1007/s10834-011-9253-4
36. Zan H, Scharff RL. The heterogeneity in financial and time burden of caregiving to children with chronic conditions. Matern Child Health J. 2015;19(3):615-625. https://doi.org/10.1007/s10995-014-1547-3
37. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135-1140. https://doi.org/10.1634/theoncologist.2014-0117
38. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199-1204. https://doi.org/10.1634/theoncologist.2015-0168
39. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281-288, 247-288. https://doi.org/10.1200/jop.2015.007401
40. Starkey AJ, Keane CR, Terry MA, Marx JH, Ricci EM. Financial distress and depressive symptoms among African American women: identifying financial priorities and needs and why it matters for mental health. J Urban Health. 2013;90(1):83-100. https://doi.org/10.1007/s11524-012-9755-x
41. Amanatullah DF, Murasko MJ, Chona DV, Crijns TJ, Ring D, Kamal RN. Financial distress and discussing the cost of total joint arthroplasty. J Arthroplasty. 2018;33(11):3394-3397. https://doi.org/10.1016/j.arth.2018.07.010
Rising US healthcare costs coupled with high cost-sharing insurance plans have led to increased out-of-pocket healthcare expenditures, especially for those who are low income or in poorer health.1-7 Increased out-of-pocket expenditures can lead to “financial distress” (defined as the subjective level of stress felt toward one’s personal financial situation) and to “medical financial burden” (defined as the subjective assessment of financial problems relating specifically to medical costs). Financial distress and medical financial burden (defined together as “financial difficulty”) lead to impaired access and delayed presentation to care and treatment nonadherence in hopes of alleviating costs.8-12
Between 20% and 50% of families with children requiring frequent medical care report that their child’s healthcare has caused a financial difficulty.13,14 In addition to direct medical costs, these parents can also suffer from indirect costs of their child’s care, such as unemployment or missed work.15-17 Along with these families, families who are low income (generally defined as living below 200% of the Federal Poverty Level) also have higher absolute and relative out-of-pocket healthcare costs, and both groups are more likely to have unmet medical needs or to delay or forgo care.18-20 Medically complex children also represent an increasing percentage of patients admitted to children’s hospitals21,22 where their families may be more vulnerable to worsening financial difficulties caused by direct costs and income depletion—due to lost wages, transportation, and meals—associated with hospitalization.23
The hospitalized population can be readily screened and provided interventions. Although evidence on effective inpatient financial interventions is lacking, financial navigation programs piloted in the ambulatory setting that standardize financial screening and support trained financial navigators could prove a promising model for inpatient care.24-26 Therefore, understanding the prevalence of financial difficulties in this population and potential high-yield screening characteristics is critical in laying the groundwork for more robust in-hospital financial screening and support systems.
Our primary objective was to assess the prevalence of financial distress and medical financial burden in families of hospitalized children. Our secondary objective was to examine measurable factors during hospitalization that could identify families at risk for these financial difficulties to better understand how to target and implement hospital-based interventions.
METHODS
We conducted a cross-sectional survey at six university-affiliated children’s hospitals (Table 1). Each site’s institutional review board approved the study. All participants were verbally informed of the research goals of the study and provided with a research information document. Need for written informed consent was determined by each institutional review board.
Study enrollment occurred between October 2017 and November 2018, with individual sites having shorter active enrollment periods (ranging from 25 to 100 days) until sample size goals were met as explained below. Participants represented a convenience sample of parents or guardians (hereafter referred to only as “parents”), who were eligible for enrollment if their child was admitted to one of the six hospitals during the active enrollment period at that site. To avoid sampling bias, each site made an effort to enroll a consecutive sample of parents, but this was limited by resources and investigator availability. Parents were excluded if their child was admitted to a neonatal unit because of difficulty in complexity categorization and the confounding issue of mothers often being admitted simultaneously. There were no other unit-, diagnosis-, or service-based exclusions to participation. Parents were also excluded if their child was 18 years or older or if they themselves were younger than 18 years. Parents were approached once their child was identified for discharge from the hospital within 48 hours. Surveys were self-administered at the time of enrollment on provided electronic tablets. Participants at some sites were offered a $5 gift card as an incentive for survey completion.
The survey included a previously published financial distress scale (InCharge Financial Distress/Financial Wellbeing Scale [IFDFW])(Appendix).27 A question in addition to the IFDFW assessed whether families were currently experiencing financial burden from medical care28,29 and whether that burden was caused by their child (Appendix) because the IFDFW does not address the source of financial distress. The survey also included questions assessing perspectives on healthcare costs (data not presented here). The survey was refined through review by psychometric experts and members of the Family Advisory Council at the primary research site, which led to minor modifications. The final survey consisted of 40 items and was professionally translated into Spanish by a third-party company (Idem Translations). It was pilot tested by 10 parents of hospitalized children to assess for adequate comprehension and clarity; these parents were not included in the final data analysis.
Variables
The primary outcome variables were level of financial distress as defined by the IFDFW scale27 and the presence of medical financial burden. The IFDFW scale has eight questions answered on a scale of 1-10, and the final score is calculated by averaging these answers. The scale defines three categories of financial distress (high, 1-3.9; average, 4-6.9; low, 7-10); however, we dichotomized our outcome as high (<4) or not high (≥4). The outcome was analyzed as both continuous and dichotomous variables because small differences in continuous scores, if detected, may be less clinically relevant. Medical financial burden was categorized as child related, child unrelated, and none.
Our secondary aim was to identify predictors of financial distress and medical financial burden. The primary predictor variable of interest was the hospitalized child’s level of chronic disease (complex chronic disease, C-CD; noncomplex chronic disease, NC-CD; no chronic disease, no-CD) as categorized by the consensus definitions from the Center of Excellence on Quality of Care Measures for Children with Complex Needs (Appendix).30 We assigned level of chronic disease based on manual review of problem lists and diagnoses in the electronic health record (EHR) from up to 3 years prior. At sites with multiple researchers, the first five to ten charts were reviewed together to ensure consistency in categorization, but no formal assessment of interrater reliability was conducted. Other predictor variables are listed in Tables 2 and 3. Insurance payer was defined as “public” or “private” based on the documented insurance plan in the EHR. Patients with dual public and private insurance were categorized as public.

Statistical Analysis
We estimated sample size requirements using an expected mean IFDFW score with standard deviation of 5.7 ± 2 based on preliminary data from the primary study site and previously published data.27 We used a significance level of P = .05, power of 0.80, and an effect size of 0.5 points difference on the IFDFW scale between the families of children with C-CD and those with either NC-CD or no-CD. We assumed there would be unequal representation of chronic disease states, with an expectation that children with C-CD would make up approximately 40% of the total population.21,22,31 Under these assumptions, we calculated a desired total sample size of 519. This would also allow us to detect a 12% absolute difference in the rate of high financial distress between families with and without C-CD, assuming a baseline level of high financial distress of 30%.27 Our goal enrollment was 150 parents at the primary site and 75 parents at each of the other 5 sites.
We fit mixed effects logistic regression models to evaluate the odds of high financial distress and polytomous logistic regression models (for our three-level outcome) to evaluate the odds of having child-related medical financial burden vs having child-unrelated burden vs having no burden. We fit linear mixed effects models to evaluate the effect of chronic disease level and medical financial burden on mean IFDFW scores. Respondents who answered “I don’t know” to the medical financial burden question were aggregated with those who reported no medical financial burden. Models were fit as a function of chronic disease level, race, ethnicity, percentage of Federal Poverty Level (FPL), insurance payer, and having a deductible less than $1,000 per year. These models included a random intercept for facility. We also fit logistic regression models that used an interaction term between chronic disease level and percentage of FPL, as well as insurance payer and percentage of FPL, to explore potential effect modification between poverty and both chronic disease level and insurance payer on financial distress. For our models, we used the MICE package for multiple imputation to fill in missing data. We imputed 25 data sets with 25 iterations each and pooled model results using Rubin’s Rules.32 All analyses were performed in R 3.5.33
RESULTS
Of 644 parents who were invited to participate, 526 (82%) were enrolled. Participants and their hospitalized children were mostly White/Caucasian (69%) and not Hispanic/Latino (76%), with 34% of families living below 200% FPL and 274 (52%) having private insurance (Table 1). Of the hospitalized children, 225 (43%) were categorized as C-CD, 143 (27%) as NC-CD, and 157 (30%) as no-CD. All participants completed the IFDFW; however, there were five missing responses to the medical financial burden question. Table 1 lists missing demographic and financial difficulty data.
Financial Distress
The mean IFDFW score of all participants was 5.6 ± 2.1, with 125 having high financial distress (24%; 95% CI, 20-28) (Table 1). There was no difference in mean IFDFW scores among families of children with different chronic disease levels (Figure). On unadjusted and adjusted analyses, there was no association between level of chronic disease and high financial distress when C-CD and NC-CD groups were each compared with no-CD (Table 2). However, families living below 400% FPL (annual income of $100,400 for a family of four) were significantly more likely than families living at 400% FPL and above to have high financial distress. Families tended to have lower financial distress (as indicated by mean IFDFW scores) with increasing percentage of FPL; however, there were families in every FPL bracket who experienced high financial distress (Appendix Figure 1a). A secondary analysis of families below and those at or above 200% FPL did not find any significant interactions between percentage of FPL and either chronic disease level (P = .86) or insurance payer (P = .83) on financial distress.

Medical Financial Burden
Overall, 160 parents (30%; 95% CI, 27-35) reported having medical financial burden, with 86 of those parents (54%) indicating their financial burden was related to their child’s medical care (Table 1). Compared with families with no such medical financial burden, respondents with medical financial burden, either child related or child unrelated, had significantly lower mean IFDFW scores (Figure), which indicates overall higher financial distress in these families. However, some families with low financial distress also reported medical financial burden.
Adjusted analyses demonstrated that, compared with families of children with no-CD, families of children with C-CD (adjusted odds ratio [AOR], 4.98; 95% CI, 2.41-10.29) or NC-CD (AOR, 2.57; 95% CI, 1.11-5.93) had significantly higher odds of having child-related medical financial burden (Table 3). Families of children with NC-CD were also more likely than families of children with no-CD to have child-unrelated medical burden (Table 3). Percentage of FPL was the only other significant predictor of child-related and child-unrelated medical financial burden (Table 3), but as with the distribution of financial distress, medical financial burden was seen across family income brackets (Appendix Figure 1b).
DISCUSSION
In this multicenter study of parents of hospitalized children, almost one in four families experienced high financial distress and almost one in three families reported having medical financial burden, with both measures of financial difficulty affecting families across all income levels. While these percentages are not substantially higher than those seen in the general population,27,34 70% of our population was composed of children with chronic disease who are more likely to have short-term and long-term healthcare needs, which places them at risk for significant ongoing medical costs.
We hypothesized that families of children with complex chronic disease would have higher levels of financial difficulties,13,35,36 but we found that level of chronic disease was associated only with medical financial burden and not with high financial distress. Financial distress is likely multifactorial and dynamic, with different drivers across various income levels. Therefore, while medical financial burden likely contributes to high financial distress, there may be other contributing factors not captured by the IFDFW. However, subjective medical financial burden has still been associated with impaired access to care.10,34 Therefore, our results suggest that families of children with chronic diseases might be at higher risk for barriers to consistent healthcare because of the financial burden their frequent healthcare utilization incurs.
Household poverty level was also associated with financial distress and medical financial burden, although surprisingly both measures of financial difficulty were present in all FPL brackets. This highlights an important reality that financial vulnerability extends beyond income and federally defined “poverty.” Non-income factors, such as high local costs of living and the growing problem of underinsurance, may significantly contribute to financial difficulty, which may render static financial metrics such as percentage of FPL insufficient screeners. Furthermore, as evidenced by the nearly 10% of our respondents who declined to provide their income information, this is a sensitive topic for some families, so gathering income data during admission could likely be a nonstarter.
In the absence of other consistent predictors of financial difficulty that could trigger interventions such as an automatic financial counselor consult, hospitals and healthcare providers could consider implementing routine non-income based financial screening questions on admission, such as one assessing medical financial burden, as a nondiscriminatory way of identifying at-risk families and provide further education and assistance regarding their financial needs. Systematically gathering this data may also further demonstrate the need for broad financial navigation programs as a mainstay in comprehensive inpatient care.
We acknowledge several limitations of this study. Primarily, we surveyed families prior to discharge and receipt of hospitalization-related bills, and these bills could contribute significantly to financial difficulties. While the families of children with chronic disease, who likely have recurrent medical bills, did not demonstrate higher financial distress, it is possible that the overall rate of financial difficulties would have been higher had we surveyed families several weeks after discharge. Our measures of financial difficulty were also subjective and, therefore, at risk for response biases (such as recall bias) that could have misestimated the prevalence of these problems in our population. However, published literature on the IFDFW scale demonstrates concordance between the subjective score and tangible outcomes of financial distress (eg, contacting a credit agency). The IFDFW scale was validated in the general population, and although it has been used in studies of medical populations,37-41 none have been in hospitalized populations, which may affect the scale’s applicability in our study. The study was also conducted only at university-affiliated children’s hospitals, and although these hospitals are geographically diverse, most children in the United States are admitted to general or community hospitals.31 Our population was also largely White, non-Hispanic/Latino, and English speaking. Therefore, our sample may not reflect the general population of hospitalized children and their families. We also assigned levels of chronic disease based on manual EHR review. While the EHR should capture each patient’s breadth of medical issues, inaccurate or missing documentation could have led to misclassification of complexity in some cases. Additionally, our sample size was calculated to detect fairly large differences in our primary outcome, and some of our unexpected results may have resulted from this study being underpowered for detection of smaller, but perhaps still clinically relevant, differences. Finally, we do not have data for several possible confounders in our study, such as employment status, health insurance concordance among family members, or sources of supplemental income, that may impact a family’s overall financial health, along with some potential important hospital-based screening characteristics, such as admitting service team or primary diagnosis.
CONCLUSION
Financial difficulties are common in families of hospitalized pediatric patients. Low-income families and those who have children with chronic conditions are at particular risk; however, all subsets of families can be affected. Given the potential negative health outcomes financial difficulties impose on families and children, the ability to identify and support vulnerable families is a crucial component of care. Hospitalization may be a prime opportunity to identify and support our at-risk families.
Acknowledgments
The authors would like to thank the parents at each of the study sites for their participation, as well as the multiple research coordinators across the study sites for assisting in recruitment of families, survey administration, and data collection. KT Park, MD, MS (Stanford University School of Medicine) served as an adviser for the study’s design.
Disclosures
All authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Rising US healthcare costs coupled with high cost-sharing insurance plans have led to increased out-of-pocket healthcare expenditures, especially for those who are low income or in poorer health.1-7 Increased out-of-pocket expenditures can lead to “financial distress” (defined as the subjective level of stress felt toward one’s personal financial situation) and to “medical financial burden” (defined as the subjective assessment of financial problems relating specifically to medical costs). Financial distress and medical financial burden (defined together as “financial difficulty”) lead to impaired access and delayed presentation to care and treatment nonadherence in hopes of alleviating costs.8-12
Between 20% and 50% of families with children requiring frequent medical care report that their child’s healthcare has caused a financial difficulty.13,14 In addition to direct medical costs, these parents can also suffer from indirect costs of their child’s care, such as unemployment or missed work.15-17 Along with these families, families who are low income (generally defined as living below 200% of the Federal Poverty Level) also have higher absolute and relative out-of-pocket healthcare costs, and both groups are more likely to have unmet medical needs or to delay or forgo care.18-20 Medically complex children also represent an increasing percentage of patients admitted to children’s hospitals21,22 where their families may be more vulnerable to worsening financial difficulties caused by direct costs and income depletion—due to lost wages, transportation, and meals—associated with hospitalization.23
The hospitalized population can be readily screened and provided interventions. Although evidence on effective inpatient financial interventions is lacking, financial navigation programs piloted in the ambulatory setting that standardize financial screening and support trained financial navigators could prove a promising model for inpatient care.24-26 Therefore, understanding the prevalence of financial difficulties in this population and potential high-yield screening characteristics is critical in laying the groundwork for more robust in-hospital financial screening and support systems.
Our primary objective was to assess the prevalence of financial distress and medical financial burden in families of hospitalized children. Our secondary objective was to examine measurable factors during hospitalization that could identify families at risk for these financial difficulties to better understand how to target and implement hospital-based interventions.
METHODS
We conducted a cross-sectional survey at six university-affiliated children’s hospitals (Table 1). Each site’s institutional review board approved the study. All participants were verbally informed of the research goals of the study and provided with a research information document. Need for written informed consent was determined by each institutional review board.
Study enrollment occurred between October 2017 and November 2018, with individual sites having shorter active enrollment periods (ranging from 25 to 100 days) until sample size goals were met as explained below. Participants represented a convenience sample of parents or guardians (hereafter referred to only as “parents”), who were eligible for enrollment if their child was admitted to one of the six hospitals during the active enrollment period at that site. To avoid sampling bias, each site made an effort to enroll a consecutive sample of parents, but this was limited by resources and investigator availability. Parents were excluded if their child was admitted to a neonatal unit because of difficulty in complexity categorization and the confounding issue of mothers often being admitted simultaneously. There were no other unit-, diagnosis-, or service-based exclusions to participation. Parents were also excluded if their child was 18 years or older or if they themselves were younger than 18 years. Parents were approached once their child was identified for discharge from the hospital within 48 hours. Surveys were self-administered at the time of enrollment on provided electronic tablets. Participants at some sites were offered a $5 gift card as an incentive for survey completion.
The survey included a previously published financial distress scale (InCharge Financial Distress/Financial Wellbeing Scale [IFDFW])(Appendix).27 A question in addition to the IFDFW assessed whether families were currently experiencing financial burden from medical care28,29 and whether that burden was caused by their child (Appendix) because the IFDFW does not address the source of financial distress. The survey also included questions assessing perspectives on healthcare costs (data not presented here). The survey was refined through review by psychometric experts and members of the Family Advisory Council at the primary research site, which led to minor modifications. The final survey consisted of 40 items and was professionally translated into Spanish by a third-party company (Idem Translations). It was pilot tested by 10 parents of hospitalized children to assess for adequate comprehension and clarity; these parents were not included in the final data analysis.
Variables
The primary outcome variables were level of financial distress as defined by the IFDFW scale27 and the presence of medical financial burden. The IFDFW scale has eight questions answered on a scale of 1-10, and the final score is calculated by averaging these answers. The scale defines three categories of financial distress (high, 1-3.9; average, 4-6.9; low, 7-10); however, we dichotomized our outcome as high (<4) or not high (≥4). The outcome was analyzed as both continuous and dichotomous variables because small differences in continuous scores, if detected, may be less clinically relevant. Medical financial burden was categorized as child related, child unrelated, and none.
Our secondary aim was to identify predictors of financial distress and medical financial burden. The primary predictor variable of interest was the hospitalized child’s level of chronic disease (complex chronic disease, C-CD; noncomplex chronic disease, NC-CD; no chronic disease, no-CD) as categorized by the consensus definitions from the Center of Excellence on Quality of Care Measures for Children with Complex Needs (Appendix).30 We assigned level of chronic disease based on manual review of problem lists and diagnoses in the electronic health record (EHR) from up to 3 years prior. At sites with multiple researchers, the first five to ten charts were reviewed together to ensure consistency in categorization, but no formal assessment of interrater reliability was conducted. Other predictor variables are listed in Tables 2 and 3. Insurance payer was defined as “public” or “private” based on the documented insurance plan in the EHR. Patients with dual public and private insurance were categorized as public.

Statistical Analysis
We estimated sample size requirements using an expected mean IFDFW score with standard deviation of 5.7 ± 2 based on preliminary data from the primary study site and previously published data.27 We used a significance level of P = .05, power of 0.80, and an effect size of 0.5 points difference on the IFDFW scale between the families of children with C-CD and those with either NC-CD or no-CD. We assumed there would be unequal representation of chronic disease states, with an expectation that children with C-CD would make up approximately 40% of the total population.21,22,31 Under these assumptions, we calculated a desired total sample size of 519. This would also allow us to detect a 12% absolute difference in the rate of high financial distress between families with and without C-CD, assuming a baseline level of high financial distress of 30%.27 Our goal enrollment was 150 parents at the primary site and 75 parents at each of the other 5 sites.
We fit mixed effects logistic regression models to evaluate the odds of high financial distress and polytomous logistic regression models (for our three-level outcome) to evaluate the odds of having child-related medical financial burden vs having child-unrelated burden vs having no burden. We fit linear mixed effects models to evaluate the effect of chronic disease level and medical financial burden on mean IFDFW scores. Respondents who answered “I don’t know” to the medical financial burden question were aggregated with those who reported no medical financial burden. Models were fit as a function of chronic disease level, race, ethnicity, percentage of Federal Poverty Level (FPL), insurance payer, and having a deductible less than $1,000 per year. These models included a random intercept for facility. We also fit logistic regression models that used an interaction term between chronic disease level and percentage of FPL, as well as insurance payer and percentage of FPL, to explore potential effect modification between poverty and both chronic disease level and insurance payer on financial distress. For our models, we used the MICE package for multiple imputation to fill in missing data. We imputed 25 data sets with 25 iterations each and pooled model results using Rubin’s Rules.32 All analyses were performed in R 3.5.33
RESULTS
Of 644 parents who were invited to participate, 526 (82%) were enrolled. Participants and their hospitalized children were mostly White/Caucasian (69%) and not Hispanic/Latino (76%), with 34% of families living below 200% FPL and 274 (52%) having private insurance (Table 1). Of the hospitalized children, 225 (43%) were categorized as C-CD, 143 (27%) as NC-CD, and 157 (30%) as no-CD. All participants completed the IFDFW; however, there were five missing responses to the medical financial burden question. Table 1 lists missing demographic and financial difficulty data.
Financial Distress
The mean IFDFW score of all participants was 5.6 ± 2.1, with 125 having high financial distress (24%; 95% CI, 20-28) (Table 1). There was no difference in mean IFDFW scores among families of children with different chronic disease levels (Figure). On unadjusted and adjusted analyses, there was no association between level of chronic disease and high financial distress when C-CD and NC-CD groups were each compared with no-CD (Table 2). However, families living below 400% FPL (annual income of $100,400 for a family of four) were significantly more likely than families living at 400% FPL and above to have high financial distress. Families tended to have lower financial distress (as indicated by mean IFDFW scores) with increasing percentage of FPL; however, there were families in every FPL bracket who experienced high financial distress (Appendix Figure 1a). A secondary analysis of families below and those at or above 200% FPL did not find any significant interactions between percentage of FPL and either chronic disease level (P = .86) or insurance payer (P = .83) on financial distress.

Medical Financial Burden
Overall, 160 parents (30%; 95% CI, 27-35) reported having medical financial burden, with 86 of those parents (54%) indicating their financial burden was related to their child’s medical care (Table 1). Compared with families with no such medical financial burden, respondents with medical financial burden, either child related or child unrelated, had significantly lower mean IFDFW scores (Figure), which indicates overall higher financial distress in these families. However, some families with low financial distress also reported medical financial burden.
Adjusted analyses demonstrated that, compared with families of children with no-CD, families of children with C-CD (adjusted odds ratio [AOR], 4.98; 95% CI, 2.41-10.29) or NC-CD (AOR, 2.57; 95% CI, 1.11-5.93) had significantly higher odds of having child-related medical financial burden (Table 3). Families of children with NC-CD were also more likely than families of children with no-CD to have child-unrelated medical burden (Table 3). Percentage of FPL was the only other significant predictor of child-related and child-unrelated medical financial burden (Table 3), but as with the distribution of financial distress, medical financial burden was seen across family income brackets (Appendix Figure 1b).
DISCUSSION
In this multicenter study of parents of hospitalized children, almost one in four families experienced high financial distress and almost one in three families reported having medical financial burden, with both measures of financial difficulty affecting families across all income levels. While these percentages are not substantially higher than those seen in the general population,27,34 70% of our population was composed of children with chronic disease who are more likely to have short-term and long-term healthcare needs, which places them at risk for significant ongoing medical costs.
We hypothesized that families of children with complex chronic disease would have higher levels of financial difficulties,13,35,36 but we found that level of chronic disease was associated only with medical financial burden and not with high financial distress. Financial distress is likely multifactorial and dynamic, with different drivers across various income levels. Therefore, while medical financial burden likely contributes to high financial distress, there may be other contributing factors not captured by the IFDFW. However, subjective medical financial burden has still been associated with impaired access to care.10,34 Therefore, our results suggest that families of children with chronic diseases might be at higher risk for barriers to consistent healthcare because of the financial burden their frequent healthcare utilization incurs.
Household poverty level was also associated with financial distress and medical financial burden, although surprisingly both measures of financial difficulty were present in all FPL brackets. This highlights an important reality that financial vulnerability extends beyond income and federally defined “poverty.” Non-income factors, such as high local costs of living and the growing problem of underinsurance, may significantly contribute to financial difficulty, which may render static financial metrics such as percentage of FPL insufficient screeners. Furthermore, as evidenced by the nearly 10% of our respondents who declined to provide their income information, this is a sensitive topic for some families, so gathering income data during admission could likely be a nonstarter.
In the absence of other consistent predictors of financial difficulty that could trigger interventions such as an automatic financial counselor consult, hospitals and healthcare providers could consider implementing routine non-income based financial screening questions on admission, such as one assessing medical financial burden, as a nondiscriminatory way of identifying at-risk families and provide further education and assistance regarding their financial needs. Systematically gathering this data may also further demonstrate the need for broad financial navigation programs as a mainstay in comprehensive inpatient care.
We acknowledge several limitations of this study. Primarily, we surveyed families prior to discharge and receipt of hospitalization-related bills, and these bills could contribute significantly to financial difficulties. While the families of children with chronic disease, who likely have recurrent medical bills, did not demonstrate higher financial distress, it is possible that the overall rate of financial difficulties would have been higher had we surveyed families several weeks after discharge. Our measures of financial difficulty were also subjective and, therefore, at risk for response biases (such as recall bias) that could have misestimated the prevalence of these problems in our population. However, published literature on the IFDFW scale demonstrates concordance between the subjective score and tangible outcomes of financial distress (eg, contacting a credit agency). The IFDFW scale was validated in the general population, and although it has been used in studies of medical populations,37-41 none have been in hospitalized populations, which may affect the scale’s applicability in our study. The study was also conducted only at university-affiliated children’s hospitals, and although these hospitals are geographically diverse, most children in the United States are admitted to general or community hospitals.31 Our population was also largely White, non-Hispanic/Latino, and English speaking. Therefore, our sample may not reflect the general population of hospitalized children and their families. We also assigned levels of chronic disease based on manual EHR review. While the EHR should capture each patient’s breadth of medical issues, inaccurate or missing documentation could have led to misclassification of complexity in some cases. Additionally, our sample size was calculated to detect fairly large differences in our primary outcome, and some of our unexpected results may have resulted from this study being underpowered for detection of smaller, but perhaps still clinically relevant, differences. Finally, we do not have data for several possible confounders in our study, such as employment status, health insurance concordance among family members, or sources of supplemental income, that may impact a family’s overall financial health, along with some potential important hospital-based screening characteristics, such as admitting service team or primary diagnosis.
CONCLUSION
Financial difficulties are common in families of hospitalized pediatric patients. Low-income families and those who have children with chronic conditions are at particular risk; however, all subsets of families can be affected. Given the potential negative health outcomes financial difficulties impose on families and children, the ability to identify and support vulnerable families is a crucial component of care. Hospitalization may be a prime opportunity to identify and support our at-risk families.
Acknowledgments
The authors would like to thank the parents at each of the study sites for their participation, as well as the multiple research coordinators across the study sites for assisting in recruitment of families, survey administration, and data collection. KT Park, MD, MS (Stanford University School of Medicine) served as an adviser for the study’s design.
Disclosures
All authors have no financial relationships or conflicts of interest relevant to this article to disclose.
1. Blumberg LJ, Waidmann TA, Blavin F, Roth J. Trends in health care financial burdens, 2001 to 2009. Milbank Q. 2014;92(1):88-113. https://doi.org/10.1111/1468-0009.12042
2. Claxton G, Rae M, Long M, et al. Employer Health Benefits, 2015 Annual Survey. Kaiser Family Foundation; 2015. http://files.kff.org/attachment/report-2015-employer-health-benefits-survey
3. Long M, Rae M, Claxton G, et al. Recent trends in employer-sponsored insurance premiums. JAMA. 2016;315(1):18. https://doi.org/10.1001/jama.2015.17349
4. Patients’ perspectives on health care in the United States: A look at seven states and the nation. Press release. NPR, Robert Wood Johnson Foundation, Harvard T.H. Chan School of Public Health; February 29, 2016. Accessed February 23, 2018. https://www.rwjf.org/en/library/research/2016/02/patients--perspectives-on-health-care-in-the-united-states.html
5. May JH, Cunningham PJ. Tough trade-offs: medical bills, family finances and access to care. Issue Brief Cent Stud Health Syst Change. 2004;(85):1-4.
6. Tu HT. Rising health costs, medical debt and chronic conditions. Issue Brief Cent Stud Health Syst Change. 2004;(88):1-5.
7. Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014;14:435. https://doi.org/10.1186/1472-6963-14-435
8. Choudhry NK, Saya UY, Shrank WH, et al. Cost-related medication underuse: prevalence among hospitalized managed care patients. J Hosp Med. 2012;7(2):104-109. https://doi.org/10.1002/jhm.948
9. QuickStats: percentage of persons of all ages who delayed or did not receive medical care during the preceding year because of cost, by U.S. Census region of residence—National Health Interview Survey, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(4):121. https://dx.doi.org/10.15585/mmwr.mm6604a9
10. Doty MM, Ho A, Davis K. How High Is Too High? Implications of High-Deductible Health Plans. The Commonwealth Fund; April 1, 2005. Accessed February 24, 2018. http://www.commonwealthfund.org/publications/fund-reports/2005/apr/how-high-is-too-high--implications-of-high-deductible-health-plans
11. Doty MM, Edwards JN, Holmgren AL. Seeing Red: American Driven into Debt by Medical Bills. The Commonwealth Fund; August 1, 2005. Accessed October 24, 2018. https://www.commonwealthfund.org/publications/issue-briefs/2005/aug/seeing-red-americans-driven-debt-medical-bills
12. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. https://doi.org/10.1093/jnci/djw205
13. Ghandour RM, Hirai AH, Blumberg SJ, Strickland BB, Kogan MD. Financial and nonfinancial burden among families of CSHCN: changes between 2001 and 2009-2010. Acad Pediatr. 2014;14(1):92-100. https://doi.org/10.1016/j.acap.2013.10.001
14. Thomson J, Shah SS, Simmons JM, et al. Financial and social hardships in families of children with medical complexity. J Pediatr. 2016;172:187-193.e1. https://doi.org/10.1016/j.jpeds.2016.01.049
15. Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2010;14(2):155-163. https://doi.org/10.1007/s10995-008-0434-1
16. Kuhlthau KA, Perrin JM. Child health status and parental employment. Arch Pediatr Adolesc Med. 2001;155(12):1346-1350. https://doi.org/10.1001/archpedi.155.12.1346
17. Witt WP, Gottlieb CA, Hampton J, Litzelman K. The impact of childhood activity limitations on parental health, mental health, and workdays lost in the United States. Acad Pediatr. 2009;9(4):263-269. https://doi.org/10.1016/j.acap.2009.02.008
18. Wisk LE, Witt WP. Predictors of delayed or forgone needed health care for families with children. Pediatrics. 2012;130(6):1027-1037. https://doi.org/10.1542/peds.2012-0668
19. Davidoff AJ. Insurance for children with special health care needs: patterns of coverage and burden on families to provide adequate insurance. Pediatrics. 2004;114(2):394-403. https://doi.org/10.1542/peds.114.2.394
20. Galbraith AA, Wong ST, Kim SE, Newacheck PW. Out-of-pocket financial burden for low-income families with children: socioeconomic disparities and effects of insurance. Health Serv Res. 2005;40(6 Pt 1):1722-1736. https://doi.org/10.1111/j.1475-6773.2005.00421.x
21. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
22. Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatrics. 2013;167(2):170-177. https://doi.org/10.1001/jamapediatrics.2013.432
23. Chang LV, Shah AN, Hoefgen ER, et al. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
24. Banegas MP, Dickerson JF, Friedman NL, et al. Evaluation of a novel financial navigator pilot to address patient concerns about medical care costs. Perm J. 2019;23:18-084. https://doi.org/10.7812/tpp/18-084
25. Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122-e129. https://doi.org/10.1200/jop.2017.024927
26. Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5 Suppl):S74-S79.
27. Prawitz AD, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. InCharge Financial Distress/Financial Well-Being Scale: Development, Administration, and Score Interpretation. J Financial Counseling Plann. 2006;17(1):34-50. https://doi.org/10.1037/t60365-000
28. Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief. 2014;(142):1-8.
29. Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood). 2011;30(2):322-331. https://doi.org/10.1377/hlthaff.2010.0584
30. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. https://doi.org/10.1542/peds.2013-3875
31. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987.
33. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/
34. Hamel L, Norton M, Pollitz K, Levitt L, Claxton G, Brodie M. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. Kaiser Family Foundation; January 5, 2016. Accessed February 26, 2019. https://www.kff.org/wp-content/uploads/2016/01/8806-the-burden-of-medical-debt-results-from-the-kaiser-family-foundation-new-york-times-medical-bills-survey.pdf
35. Witt WP, Litzelman K, Mandic CG, et al. Healthcare-related financial burden among families in the U.S.: the role of childhood activity limitations and income. J Fam Econ Issues. 2011;32(2):308-326. https://doi.org/10.1007/s10834-011-9253-4
36. Zan H, Scharff RL. The heterogeneity in financial and time burden of caregiving to children with chronic conditions. Matern Child Health J. 2015;19(3):615-625. https://doi.org/10.1007/s10995-014-1547-3
37. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135-1140. https://doi.org/10.1634/theoncologist.2014-0117
38. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199-1204. https://doi.org/10.1634/theoncologist.2015-0168
39. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281-288, 247-288. https://doi.org/10.1200/jop.2015.007401
40. Starkey AJ, Keane CR, Terry MA, Marx JH, Ricci EM. Financial distress and depressive symptoms among African American women: identifying financial priorities and needs and why it matters for mental health. J Urban Health. 2013;90(1):83-100. https://doi.org/10.1007/s11524-012-9755-x
41. Amanatullah DF, Murasko MJ, Chona DV, Crijns TJ, Ring D, Kamal RN. Financial distress and discussing the cost of total joint arthroplasty. J Arthroplasty. 2018;33(11):3394-3397. https://doi.org/10.1016/j.arth.2018.07.010
1. Blumberg LJ, Waidmann TA, Blavin F, Roth J. Trends in health care financial burdens, 2001 to 2009. Milbank Q. 2014;92(1):88-113. https://doi.org/10.1111/1468-0009.12042
2. Claxton G, Rae M, Long M, et al. Employer Health Benefits, 2015 Annual Survey. Kaiser Family Foundation; 2015. http://files.kff.org/attachment/report-2015-employer-health-benefits-survey
3. Long M, Rae M, Claxton G, et al. Recent trends in employer-sponsored insurance premiums. JAMA. 2016;315(1):18. https://doi.org/10.1001/jama.2015.17349
4. Patients’ perspectives on health care in the United States: A look at seven states and the nation. Press release. NPR, Robert Wood Johnson Foundation, Harvard T.H. Chan School of Public Health; February 29, 2016. Accessed February 23, 2018. https://www.rwjf.org/en/library/research/2016/02/patients--perspectives-on-health-care-in-the-united-states.html
5. May JH, Cunningham PJ. Tough trade-offs: medical bills, family finances and access to care. Issue Brief Cent Stud Health Syst Change. 2004;(85):1-4.
6. Tu HT. Rising health costs, medical debt and chronic conditions. Issue Brief Cent Stud Health Syst Change. 2004;(88):1-5.
7. Richman IB, Brodie M. A National study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res. 2014;14:435. https://doi.org/10.1186/1472-6963-14-435
8. Choudhry NK, Saya UY, Shrank WH, et al. Cost-related medication underuse: prevalence among hospitalized managed care patients. J Hosp Med. 2012;7(2):104-109. https://doi.org/10.1002/jhm.948
9. QuickStats: percentage of persons of all ages who delayed or did not receive medical care during the preceding year because of cost, by U.S. Census region of residence—National Health Interview Survey, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(4):121. https://dx.doi.org/10.15585/mmwr.mm6604a9
10. Doty MM, Ho A, Davis K. How High Is Too High? Implications of High-Deductible Health Plans. The Commonwealth Fund; April 1, 2005. Accessed February 24, 2018. http://www.commonwealthfund.org/publications/fund-reports/2005/apr/how-high-is-too-high--implications-of-high-deductible-health-plans
11. Doty MM, Edwards JN, Holmgren AL. Seeing Red: American Driven into Debt by Medical Bills. The Commonwealth Fund; August 1, 2005. Accessed October 24, 2018. https://www.commonwealthfund.org/publications/issue-briefs/2005/aug/seeing-red-americans-driven-debt-medical-bills
12. Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2016;109(2):djw205. https://doi.org/10.1093/jnci/djw205
13. Ghandour RM, Hirai AH, Blumberg SJ, Strickland BB, Kogan MD. Financial and nonfinancial burden among families of CSHCN: changes between 2001 and 2009-2010. Acad Pediatr. 2014;14(1):92-100. https://doi.org/10.1016/j.acap.2013.10.001
14. Thomson J, Shah SS, Simmons JM, et al. Financial and social hardships in families of children with medical complexity. J Pediatr. 2016;172:187-193.e1. https://doi.org/10.1016/j.jpeds.2016.01.049
15. Kuhlthau K, Kahn R, Hill KS, Gnanasekaran S, Ettner SL. The well-being of parental caregivers of children with activity limitations. Matern Child Health J. 2010;14(2):155-163. https://doi.org/10.1007/s10995-008-0434-1
16. Kuhlthau KA, Perrin JM. Child health status and parental employment. Arch Pediatr Adolesc Med. 2001;155(12):1346-1350. https://doi.org/10.1001/archpedi.155.12.1346
17. Witt WP, Gottlieb CA, Hampton J, Litzelman K. The impact of childhood activity limitations on parental health, mental health, and workdays lost in the United States. Acad Pediatr. 2009;9(4):263-269. https://doi.org/10.1016/j.acap.2009.02.008
18. Wisk LE, Witt WP. Predictors of delayed or forgone needed health care for families with children. Pediatrics. 2012;130(6):1027-1037. https://doi.org/10.1542/peds.2012-0668
19. Davidoff AJ. Insurance for children with special health care needs: patterns of coverage and burden on families to provide adequate insurance. Pediatrics. 2004;114(2):394-403. https://doi.org/10.1542/peds.114.2.394
20. Galbraith AA, Wong ST, Kim SE, Newacheck PW. Out-of-pocket financial burden for low-income families with children: socioeconomic disparities and effects of insurance. Health Serv Res. 2005;40(6 Pt 1):1722-1736. https://doi.org/10.1111/j.1475-6773.2005.00421.x
21. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122
22. Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatrics. 2013;167(2):170-177. https://doi.org/10.1001/jamapediatrics.2013.432
23. Chang LV, Shah AN, Hoefgen ER, et al. Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142(3):e20180195. https://doi.org/10.1542/peds.2018-0195
24. Banegas MP, Dickerson JF, Friedman NL, et al. Evaluation of a novel financial navigator pilot to address patient concerns about medical care costs. Perm J. 2019;23:18-084. https://doi.org/10.7812/tpp/18-084
25. Shankaran V, Leahy T, Steelquist J, et al. Pilot feasibility study of an oncology financial navigation program. J Oncol Pract. 2018;14(2):e122-e129. https://doi.org/10.1200/jop.2017.024927
26. Yezefski T, Steelquist J, Watabayashi K, Sherman D, Shankaran V. Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care. 2018;24(5 Suppl):S74-S79.
27. Prawitz AD, Garman ET, Sorhaindo B, O’Neill B, Kim J, Drentea P. InCharge Financial Distress/Financial Well-Being Scale: Development, Administration, and Score Interpretation. J Financial Counseling Plann. 2006;17(1):34-50. https://doi.org/10.1037/t60365-000
28. Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief. 2014;(142):1-8.
29. Galbraith AA, Ross-Degnan D, Soumerai SB, Rosenthal MB, Gay C, Lieu TA. Nearly half of families in high-deductible health plans whose members have chronic conditions face substantial financial burden. Health Aff (Millwood). 2011;30(2):322-331. https://doi.org/10.1377/hlthaff.2010.0584
30. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. https://doi.org/10.1542/peds.2013-3875
31. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; 1987.
33. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. https://www.R-project.org/
34. Hamel L, Norton M, Pollitz K, Levitt L, Claxton G, Brodie M. The Burden of Medical Debt: Results from the Kaiser Family Foundation/New York Times Medical Bills Survey. Kaiser Family Foundation; January 5, 2016. Accessed February 26, 2019. https://www.kff.org/wp-content/uploads/2016/01/8806-the-burden-of-medical-debt-results-from-the-kaiser-family-foundation-new-york-times-medical-bills-survey.pdf
35. Witt WP, Litzelman K, Mandic CG, et al. Healthcare-related financial burden among families in the U.S.: the role of childhood activity limitations and income. J Fam Econ Issues. 2011;32(2):308-326. https://doi.org/10.1007/s10834-011-9253-4
36. Zan H, Scharff RL. The heterogeneity in financial and time burden of caregiving to children with chronic conditions. Matern Child Health J. 2015;19(3):615-625. https://doi.org/10.1007/s10995-014-1547-3
37. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19(11):1135-1140. https://doi.org/10.1634/theoncologist.2014-0117
38. Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20(10):1199-1204. https://doi.org/10.1634/theoncologist.2015-0168
39. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract. 2016;12(3):e281-288, 247-288. https://doi.org/10.1200/jop.2015.007401
40. Starkey AJ, Keane CR, Terry MA, Marx JH, Ricci EM. Financial distress and depressive symptoms among African American women: identifying financial priorities and needs and why it matters for mental health. J Urban Health. 2013;90(1):83-100. https://doi.org/10.1007/s11524-012-9755-x
41. Amanatullah DF, Murasko MJ, Chona DV, Crijns TJ, Ring D, Kamal RN. Financial distress and discussing the cost of total joint arthroplasty. J Arthroplasty. 2018;33(11):3394-3397. https://doi.org/10.1016/j.arth.2018.07.010
© 2020 Society of Hospital Medicine