User login
Clinical Guideline Highlights for the Hospitalist: Therapeutic Monitoring of Vancomycin
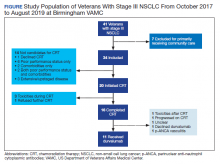
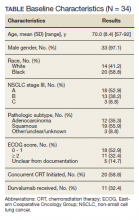
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
© 2020 Society of Hospital Medicine
Rethinking Hospital-Associated Disability for Patients With COVID-19
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
© 2020 Society of Hospital Medicine
Improving Patient Experience During the COVID-19 Pandemic: One Family’s Reflections
On March 11, 2020, the novel coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 On March 13, 2020, a national emergency was declared in the United States concerning the COVID-19 outbreak.2 Later that week, Mike Kueper, a 52-year-old previously healthy man and resident of the Indianapolis metropolitan area, became sick with what he would eventually learn was COVID-19. Prior to contracting the novel coronavirus, he had never had as much as an Emergency Department (ED) visit. He had never spent a night in a hospital. He and his sister, DeAnn Harvey, describe the events that followed.
DeAnn
As a 20-year veteran clinical child psychologist and mother of two teenagers, my first reaction to the governor’s call for state-wide lockdowns was that they sounded like an opportunity for time at home with my husband and children. I thought we would play games, watch movies, try new recipes, and get a much-needed reprieve from our hectic lives of sports schedules, homework, and social outings. Even a slowdown in my practice sounded good. Maybe I could finally finish those continuing education credits that were due for my upcoming license renewal. My greatest concerns about sheltering in place were about how I was going to structure my children’s online learning while at the same time getting into my office to manage my patients via telehealth. Unfortunately, this relaxed feeling was short-lived.
On March 20, 2020, a few days after the lockdown started, my brother Mike developed high fevers. During a virtual doctor visit, he was told that it could be COVID-19 and to self-quarantine. Our discussions turned to jokes about his lack of taste or smell. We had dropped off soup for him from a new recipe my daughter had tried. My son joked that Mike was lucky that he couldn’t taste it.
On the morning of March 28, my mother called to tell me that Mike needed to go to the ED. Because we needed to figure out which hospital would be the best for him and I didn’t want my children to worry too much, I jumped in my car and drove to our church parking lot. In between calls to area hospitals, I began praying for his health and for guidance and support from God. Mike, concerned about spreading the virus to the rest of the family, refused to let my parents or me drive him to the hospital.
Mike
I thought I had a regular cold, and then, once I had a temperature of 102 °F and night sweats, decided it was the flu. One night, I was so cold that I went to bed wearing winter gloves. After a virtual visit with a nurse, she said my symptoms did not sound like COVID, but recommended self-quarantine, just in case. On March 26, I noticed that my sense of taste and smell had disappeared completely, and it hurt to yawn or take deep breaths. By Saturday, March 28, I was getting sicker and was short of breath and very tired. My elderly parents wanted to drive me to the ED, but if it was COVID-19, I didn’t want them near me. After getting advice from my sister, I called a local hospital and asked if I could come into the ED. The person on the phone said if I got there within an hour, they would be able to take me. When I arrived, an aide came out to my car, put me in a protective gown and mask, and walked me in. Walking even this short distance was tiring, and from this moment, things get fuzzy. I only have glimpses of the next few days. At first, I was put into a negative pressure room. I spent the night in there. I remember talking to a doctor who asked if I had a living will. He recommended that I go on a ventilator. I asked him, “Do you expect me to die?”
I remember him saying, “That is always a possibility.”
DeAnn
Once Mike was admitted to the hospital, we didn’t hear from anyone for about 6 hours, and I started to panic. I called people I knew who worked in the hospital, and my friend who is an intensive care unit (ICU) nurse agreed to track him down. He was indeed admitted to the hospital and was receiving oxygen. When I finally got to talk to him later that night, Mike had difficulty completing sentences because he was so short of breath. I told him not to use his energy, and that if they would let me, I would be there by his side. I promised him that he was going to get through this. Around 1:30
Mike
I don’t remember much from the ICU, but I understand that it was touch and go at times. I knew I was on a ventilator, and I found out later that I was “proned’ for up to 16 hours. Being on the ventilator was horrible, but what was even worse was that, once I was off the vent and alone in my hospital room, I had no idea how I got there. I thought I had been in a plane crash. I wanted to check my phone to see where I was flying in from but couldn’t because I thought my phone had been hacked by terrorists. I had no idea what was real and what was not. It was extremely scary.
DeAnn
When I think about the doctor coming in to tell Mike they had to put him on the ventilator, my heart absolutely breaks. It hurts to think of him all alone, having to make this decision without any of his family there to support him. Neither he nor I wanted to think about it, but we knew there was the possibility that he would never come off the ventilator. We hadn’t had a chance to hug him or even see him for days before his admission. If he didn’t make it, we would never get one of his amazing “Uncle Mike” hugs again.
Our friend, the ICU nurse, made it a point to find out which nurse was assigned to Mike and made it a priority to gather information from that nurse daily, allowing our family to receive updates on Mike’s status 2-3 times a day. In addition, the ICU physician was in daily contact with my parents: however, it was still excruciating not being able to be there. I spent a lot of time pacing the house, not eating or sleeping, checking my phone for texts, fielding texts and calls from friends and family. I was unable to do even simple household tasks, and left laundry, cooking, and my kids’ online schooling to my husband.
Feeling so helpless, I turned to prayer. My close friends organized a daily prayer vigil at 7:30
Then, after 17 days, a miracle: he was taken off the ventilator and moved to the medical unit. Looking back, I think these are really the days that the presence of his family would have sped up his recovery. Mike was experiencing delirium and hallucinations as a result of illness, medications, and the time he spent in an induced coma. I wish I could have been there with him to be the one he asked if what he was experiencing was real or a hallucination. Then we could have laughed about it together; our family has always found that humor helps with healing.
Mike
I understand the purpose of the isolation, but it really did a number on my mind. I remember being in the ICU, having my catheter taken out, not knowing what was happening or how I ended up in the hospital. I was so confused and was seeing people who were not there. One morning, I woke up thinking I was in my house and I had stolen the hospital bed I was in. I was panicking and scheming about how I might get this hospital bed back to the hospital before anyone noticed. As I mentioned earlier, I thought I was on a plane that had been taken over by terrorists who were using us COVID patients as biological weapons. Then I thought agents of the Federal Bureau of Investigation were coming to interrogate me and that they were also looking for my sister. To protect DeAnn, the next time someone asked me the name of my sister, I told them, “Maria” (the name of my sister who passed away in 1991) rather than DeAnn, who is very much alive. Another time, I thought my grandmother and cousin had died in a plane crash. My cousin is a state representative in Illinois, so once I got my phone to work, I checked his Wikipedia page to see if there was a death date listed.
When I was less confused, I found it’s tough spending day after day lying in a hospital bed with no family member or friend to offer companionship, comfort, or clarity. Even though I was extremely weak and could barely walk, I was asking, daily, about when I was expected to be discharged. I had to get out.
One night, our friend the ICU nurse came into my room to sit with me and just talk. She spent about 30 minutes with me around 4:00 in the morning. It was wonderful. All I could think about was what a huge blessing it was. I don’t think she knows just how much that meant to me. More often, I would report some symptom of confusion or insomnia, and a nurse would offer me medications for sleep or pain. I did not want any more drugs in my system. Human contact would have been a far better treatment.
I was reluctant to ask for help when I needed something, like a trip to the bathroom or some ice water. When I did press the call button, I had to wait for the busy nurse or tech to put on all the protective gear, and then, when they left, watch them take all the steps to disinfect and rid themselves of the gear that they had just put on. Even so, I was excited when it came time to take my vital signs or administer medications because that meant human interaction, however brief (and even if it was 4:00 AM). I wanted to bathe or change gowns and/or socks, but I opted to wear the same gown and socks for over three-fourths of the time I was there because I did not want to burden the staff.
Video chat turned out to be one of the best tools for creating connection. I may have sobbed a few times when talking to my parents on FaceTime, but just seeing their faces made all the difference in the world.
Finally, on April 21, 25 days after I was led into the ED, I was discharged. As we reflect on this experience, my family and I have some recommendations for hospitals and health systems trying to make patient experience a priority during this pandemic:
Kueper Family Recommendations to Improve Patient Experience for Those With COVID-19
- Adopt a more systematic approach to communicating with patient families, which would greatly improve the connection between them and healthcare personnel. This is especially important for families when the patient is critically ill, and especially in times when the patient is in isolation. We were fortunate in that we received updates from nurses and physicians several times a day. This was partly due to the relationships or connections with staff members that existed previously or developed over the course of Mike’s stay. Staff members who became invested in Mike’s progress became part of his hospital “family.” Many people who have had a family member with COVID have not had this experience, nor did they have the opportunity to build relationships with the staff, which we felt were important to ensure good care and open and frequent communication with them and the patient. Therefore, we believe a more systematic approach toward communication (eg, “the team will call each day during multidisciplinary rounds at 11 AM,”) would greatly improve the connection between families and healthcare personnel.
- Allow visitation under certain conditions even while the patient is in isolation. Visitation would have been especially helpful once Mike was more awake but isolated and delirious. We know that these policies are difficult to create and navigate but believe that there should be allowance for some visitation when there is a clear clinical benefit (eg, delirium). Because Mike had little human contact the week after he was taken off the ventilator (eg, contact limited to nurses coming in to take vitals, once daily doctor visit), he had to navigate the hallucinations and delirium on his own. Even one family member by his side who could provide frequent feedback on reality would have helped to resolve the feelings of agitation and fear that can accompany delirium.
- Schedule more video chats. Even when Mike was on the ventilator, we found video chats to be an important way to understand his experience and connect with him. Although we know such chats are difficult for clinicians to schedule, it greatly improved the experience for us.
- Reassure patients that caring for them is not a burden and they should not hesitate to ask for help. Being contagious and believing you are a danger to others is a terrible feeling. No one on staff said or even implied that they were afraid to care for him, but Mike felt “dangerous” to the staff and as such hesitated to “burden” the clinicians with requests (eg, going to the bathroom, having a change of clothes). It is time-consuming and difficult to don PPE and the amount of effort it takes to enter the room is immediately obvious to the patient. Because of this, it is very important that the clinicians and staff reassure patients that it is part of the job and not a burden to come in and out of the room.
DeAnn
Having Mike alive and now home is an incredible gift. We are taking every chance we can to make up for the time that we could not see him and are so grateful for the hospital team that saved his life.
Mike
On April 21, I was discharged and sent home. Luckily, for about 2 weeks, I had a best friend, my brother, and DeAnn, separately, stay with me each night. This was a godsend as all made sure I was taking my medicine, eating, and doing my prescribed exercises. I am struggling with a long recovery. I used a walker for a while and had both a physical and occupational therapist visit me two to three times a week. I visit a neurologist for some of my symptoms that have not resolved, such as pain and atrophy in my right shoulder, hand tremors, and some numbness in my thighs. Thankfully, I was able to resume working from home, but even going up stairs causes me to become winded. I know that doctors don’t understand this disease very well, and neither do I. Sometimes I feel discouraged about how much it set me back physically. I wish things could have been different—that I could have avoided this disease altogether or had milder symptoms. But I am so grateful to be alive and so thankful for the doctors and nurses, as well as for my family, who could not be there physically during the hospitalization but did everything they could do to help me. Because of their love and support, I survived.
Disclosures
The authors have nothing to disclose.
1. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID–11 March 2020. Published March 11, 2020. Accessed August 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. Issued March 13, 2020. Accessed August 11, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
On March 11, 2020, the novel coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 On March 13, 2020, a national emergency was declared in the United States concerning the COVID-19 outbreak.2 Later that week, Mike Kueper, a 52-year-old previously healthy man and resident of the Indianapolis metropolitan area, became sick with what he would eventually learn was COVID-19. Prior to contracting the novel coronavirus, he had never had as much as an Emergency Department (ED) visit. He had never spent a night in a hospital. He and his sister, DeAnn Harvey, describe the events that followed.
DeAnn
As a 20-year veteran clinical child psychologist and mother of two teenagers, my first reaction to the governor’s call for state-wide lockdowns was that they sounded like an opportunity for time at home with my husband and children. I thought we would play games, watch movies, try new recipes, and get a much-needed reprieve from our hectic lives of sports schedules, homework, and social outings. Even a slowdown in my practice sounded good. Maybe I could finally finish those continuing education credits that were due for my upcoming license renewal. My greatest concerns about sheltering in place were about how I was going to structure my children’s online learning while at the same time getting into my office to manage my patients via telehealth. Unfortunately, this relaxed feeling was short-lived.
On March 20, 2020, a few days after the lockdown started, my brother Mike developed high fevers. During a virtual doctor visit, he was told that it could be COVID-19 and to self-quarantine. Our discussions turned to jokes about his lack of taste or smell. We had dropped off soup for him from a new recipe my daughter had tried. My son joked that Mike was lucky that he couldn’t taste it.
On the morning of March 28, my mother called to tell me that Mike needed to go to the ED. Because we needed to figure out which hospital would be the best for him and I didn’t want my children to worry too much, I jumped in my car and drove to our church parking lot. In between calls to area hospitals, I began praying for his health and for guidance and support from God. Mike, concerned about spreading the virus to the rest of the family, refused to let my parents or me drive him to the hospital.
Mike
I thought I had a regular cold, and then, once I had a temperature of 102 °F and night sweats, decided it was the flu. One night, I was so cold that I went to bed wearing winter gloves. After a virtual visit with a nurse, she said my symptoms did not sound like COVID, but recommended self-quarantine, just in case. On March 26, I noticed that my sense of taste and smell had disappeared completely, and it hurt to yawn or take deep breaths. By Saturday, March 28, I was getting sicker and was short of breath and very tired. My elderly parents wanted to drive me to the ED, but if it was COVID-19, I didn’t want them near me. After getting advice from my sister, I called a local hospital and asked if I could come into the ED. The person on the phone said if I got there within an hour, they would be able to take me. When I arrived, an aide came out to my car, put me in a protective gown and mask, and walked me in. Walking even this short distance was tiring, and from this moment, things get fuzzy. I only have glimpses of the next few days. At first, I was put into a negative pressure room. I spent the night in there. I remember talking to a doctor who asked if I had a living will. He recommended that I go on a ventilator. I asked him, “Do you expect me to die?”
I remember him saying, “That is always a possibility.”
DeAnn
Once Mike was admitted to the hospital, we didn’t hear from anyone for about 6 hours, and I started to panic. I called people I knew who worked in the hospital, and my friend who is an intensive care unit (ICU) nurse agreed to track him down. He was indeed admitted to the hospital and was receiving oxygen. When I finally got to talk to him later that night, Mike had difficulty completing sentences because he was so short of breath. I told him not to use his energy, and that if they would let me, I would be there by his side. I promised him that he was going to get through this. Around 1:30
Mike
I don’t remember much from the ICU, but I understand that it was touch and go at times. I knew I was on a ventilator, and I found out later that I was “proned’ for up to 16 hours. Being on the ventilator was horrible, but what was even worse was that, once I was off the vent and alone in my hospital room, I had no idea how I got there. I thought I had been in a plane crash. I wanted to check my phone to see where I was flying in from but couldn’t because I thought my phone had been hacked by terrorists. I had no idea what was real and what was not. It was extremely scary.
DeAnn
When I think about the doctor coming in to tell Mike they had to put him on the ventilator, my heart absolutely breaks. It hurts to think of him all alone, having to make this decision without any of his family there to support him. Neither he nor I wanted to think about it, but we knew there was the possibility that he would never come off the ventilator. We hadn’t had a chance to hug him or even see him for days before his admission. If he didn’t make it, we would never get one of his amazing “Uncle Mike” hugs again.
Our friend, the ICU nurse, made it a point to find out which nurse was assigned to Mike and made it a priority to gather information from that nurse daily, allowing our family to receive updates on Mike’s status 2-3 times a day. In addition, the ICU physician was in daily contact with my parents: however, it was still excruciating not being able to be there. I spent a lot of time pacing the house, not eating or sleeping, checking my phone for texts, fielding texts and calls from friends and family. I was unable to do even simple household tasks, and left laundry, cooking, and my kids’ online schooling to my husband.
Feeling so helpless, I turned to prayer. My close friends organized a daily prayer vigil at 7:30
Then, after 17 days, a miracle: he was taken off the ventilator and moved to the medical unit. Looking back, I think these are really the days that the presence of his family would have sped up his recovery. Mike was experiencing delirium and hallucinations as a result of illness, medications, and the time he spent in an induced coma. I wish I could have been there with him to be the one he asked if what he was experiencing was real or a hallucination. Then we could have laughed about it together; our family has always found that humor helps with healing.
Mike
I understand the purpose of the isolation, but it really did a number on my mind. I remember being in the ICU, having my catheter taken out, not knowing what was happening or how I ended up in the hospital. I was so confused and was seeing people who were not there. One morning, I woke up thinking I was in my house and I had stolen the hospital bed I was in. I was panicking and scheming about how I might get this hospital bed back to the hospital before anyone noticed. As I mentioned earlier, I thought I was on a plane that had been taken over by terrorists who were using us COVID patients as biological weapons. Then I thought agents of the Federal Bureau of Investigation were coming to interrogate me and that they were also looking for my sister. To protect DeAnn, the next time someone asked me the name of my sister, I told them, “Maria” (the name of my sister who passed away in 1991) rather than DeAnn, who is very much alive. Another time, I thought my grandmother and cousin had died in a plane crash. My cousin is a state representative in Illinois, so once I got my phone to work, I checked his Wikipedia page to see if there was a death date listed.
When I was less confused, I found it’s tough spending day after day lying in a hospital bed with no family member or friend to offer companionship, comfort, or clarity. Even though I was extremely weak and could barely walk, I was asking, daily, about when I was expected to be discharged. I had to get out.
One night, our friend the ICU nurse came into my room to sit with me and just talk. She spent about 30 minutes with me around 4:00 in the morning. It was wonderful. All I could think about was what a huge blessing it was. I don’t think she knows just how much that meant to me. More often, I would report some symptom of confusion or insomnia, and a nurse would offer me medications for sleep or pain. I did not want any more drugs in my system. Human contact would have been a far better treatment.
I was reluctant to ask for help when I needed something, like a trip to the bathroom or some ice water. When I did press the call button, I had to wait for the busy nurse or tech to put on all the protective gear, and then, when they left, watch them take all the steps to disinfect and rid themselves of the gear that they had just put on. Even so, I was excited when it came time to take my vital signs or administer medications because that meant human interaction, however brief (and even if it was 4:00 AM). I wanted to bathe or change gowns and/or socks, but I opted to wear the same gown and socks for over three-fourths of the time I was there because I did not want to burden the staff.
Video chat turned out to be one of the best tools for creating connection. I may have sobbed a few times when talking to my parents on FaceTime, but just seeing their faces made all the difference in the world.
Finally, on April 21, 25 days after I was led into the ED, I was discharged. As we reflect on this experience, my family and I have some recommendations for hospitals and health systems trying to make patient experience a priority during this pandemic:
Kueper Family Recommendations to Improve Patient Experience for Those With COVID-19
- Adopt a more systematic approach to communicating with patient families, which would greatly improve the connection between them and healthcare personnel. This is especially important for families when the patient is critically ill, and especially in times when the patient is in isolation. We were fortunate in that we received updates from nurses and physicians several times a day. This was partly due to the relationships or connections with staff members that existed previously or developed over the course of Mike’s stay. Staff members who became invested in Mike’s progress became part of his hospital “family.” Many people who have had a family member with COVID have not had this experience, nor did they have the opportunity to build relationships with the staff, which we felt were important to ensure good care and open and frequent communication with them and the patient. Therefore, we believe a more systematic approach toward communication (eg, “the team will call each day during multidisciplinary rounds at 11 AM,”) would greatly improve the connection between families and healthcare personnel.
- Allow visitation under certain conditions even while the patient is in isolation. Visitation would have been especially helpful once Mike was more awake but isolated and delirious. We know that these policies are difficult to create and navigate but believe that there should be allowance for some visitation when there is a clear clinical benefit (eg, delirium). Because Mike had little human contact the week after he was taken off the ventilator (eg, contact limited to nurses coming in to take vitals, once daily doctor visit), he had to navigate the hallucinations and delirium on his own. Even one family member by his side who could provide frequent feedback on reality would have helped to resolve the feelings of agitation and fear that can accompany delirium.
- Schedule more video chats. Even when Mike was on the ventilator, we found video chats to be an important way to understand his experience and connect with him. Although we know such chats are difficult for clinicians to schedule, it greatly improved the experience for us.
- Reassure patients that caring for them is not a burden and they should not hesitate to ask for help. Being contagious and believing you are a danger to others is a terrible feeling. No one on staff said or even implied that they were afraid to care for him, but Mike felt “dangerous” to the staff and as such hesitated to “burden” the clinicians with requests (eg, going to the bathroom, having a change of clothes). It is time-consuming and difficult to don PPE and the amount of effort it takes to enter the room is immediately obvious to the patient. Because of this, it is very important that the clinicians and staff reassure patients that it is part of the job and not a burden to come in and out of the room.
DeAnn
Having Mike alive and now home is an incredible gift. We are taking every chance we can to make up for the time that we could not see him and are so grateful for the hospital team that saved his life.
Mike
On April 21, I was discharged and sent home. Luckily, for about 2 weeks, I had a best friend, my brother, and DeAnn, separately, stay with me each night. This was a godsend as all made sure I was taking my medicine, eating, and doing my prescribed exercises. I am struggling with a long recovery. I used a walker for a while and had both a physical and occupational therapist visit me two to three times a week. I visit a neurologist for some of my symptoms that have not resolved, such as pain and atrophy in my right shoulder, hand tremors, and some numbness in my thighs. Thankfully, I was able to resume working from home, but even going up stairs causes me to become winded. I know that doctors don’t understand this disease very well, and neither do I. Sometimes I feel discouraged about how much it set me back physically. I wish things could have been different—that I could have avoided this disease altogether or had milder symptoms. But I am so grateful to be alive and so thankful for the doctors and nurses, as well as for my family, who could not be there physically during the hospitalization but did everything they could do to help me. Because of their love and support, I survived.
Disclosures
The authors have nothing to disclose.
On March 11, 2020, the novel coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 On March 13, 2020, a national emergency was declared in the United States concerning the COVID-19 outbreak.2 Later that week, Mike Kueper, a 52-year-old previously healthy man and resident of the Indianapolis metropolitan area, became sick with what he would eventually learn was COVID-19. Prior to contracting the novel coronavirus, he had never had as much as an Emergency Department (ED) visit. He had never spent a night in a hospital. He and his sister, DeAnn Harvey, describe the events that followed.
DeAnn
As a 20-year veteran clinical child psychologist and mother of two teenagers, my first reaction to the governor’s call for state-wide lockdowns was that they sounded like an opportunity for time at home with my husband and children. I thought we would play games, watch movies, try new recipes, and get a much-needed reprieve from our hectic lives of sports schedules, homework, and social outings. Even a slowdown in my practice sounded good. Maybe I could finally finish those continuing education credits that were due for my upcoming license renewal. My greatest concerns about sheltering in place were about how I was going to structure my children’s online learning while at the same time getting into my office to manage my patients via telehealth. Unfortunately, this relaxed feeling was short-lived.
On March 20, 2020, a few days after the lockdown started, my brother Mike developed high fevers. During a virtual doctor visit, he was told that it could be COVID-19 and to self-quarantine. Our discussions turned to jokes about his lack of taste or smell. We had dropped off soup for him from a new recipe my daughter had tried. My son joked that Mike was lucky that he couldn’t taste it.
On the morning of March 28, my mother called to tell me that Mike needed to go to the ED. Because we needed to figure out which hospital would be the best for him and I didn’t want my children to worry too much, I jumped in my car and drove to our church parking lot. In between calls to area hospitals, I began praying for his health and for guidance and support from God. Mike, concerned about spreading the virus to the rest of the family, refused to let my parents or me drive him to the hospital.
Mike
I thought I had a regular cold, and then, once I had a temperature of 102 °F and night sweats, decided it was the flu. One night, I was so cold that I went to bed wearing winter gloves. After a virtual visit with a nurse, she said my symptoms did not sound like COVID, but recommended self-quarantine, just in case. On March 26, I noticed that my sense of taste and smell had disappeared completely, and it hurt to yawn or take deep breaths. By Saturday, March 28, I was getting sicker and was short of breath and very tired. My elderly parents wanted to drive me to the ED, but if it was COVID-19, I didn’t want them near me. After getting advice from my sister, I called a local hospital and asked if I could come into the ED. The person on the phone said if I got there within an hour, they would be able to take me. When I arrived, an aide came out to my car, put me in a protective gown and mask, and walked me in. Walking even this short distance was tiring, and from this moment, things get fuzzy. I only have glimpses of the next few days. At first, I was put into a negative pressure room. I spent the night in there. I remember talking to a doctor who asked if I had a living will. He recommended that I go on a ventilator. I asked him, “Do you expect me to die?”
I remember him saying, “That is always a possibility.”
DeAnn
Once Mike was admitted to the hospital, we didn’t hear from anyone for about 6 hours, and I started to panic. I called people I knew who worked in the hospital, and my friend who is an intensive care unit (ICU) nurse agreed to track him down. He was indeed admitted to the hospital and was receiving oxygen. When I finally got to talk to him later that night, Mike had difficulty completing sentences because he was so short of breath. I told him not to use his energy, and that if they would let me, I would be there by his side. I promised him that he was going to get through this. Around 1:30
Mike
I don’t remember much from the ICU, but I understand that it was touch and go at times. I knew I was on a ventilator, and I found out later that I was “proned’ for up to 16 hours. Being on the ventilator was horrible, but what was even worse was that, once I was off the vent and alone in my hospital room, I had no idea how I got there. I thought I had been in a plane crash. I wanted to check my phone to see where I was flying in from but couldn’t because I thought my phone had been hacked by terrorists. I had no idea what was real and what was not. It was extremely scary.
DeAnn
When I think about the doctor coming in to tell Mike they had to put him on the ventilator, my heart absolutely breaks. It hurts to think of him all alone, having to make this decision without any of his family there to support him. Neither he nor I wanted to think about it, but we knew there was the possibility that he would never come off the ventilator. We hadn’t had a chance to hug him or even see him for days before his admission. If he didn’t make it, we would never get one of his amazing “Uncle Mike” hugs again.
Our friend, the ICU nurse, made it a point to find out which nurse was assigned to Mike and made it a priority to gather information from that nurse daily, allowing our family to receive updates on Mike’s status 2-3 times a day. In addition, the ICU physician was in daily contact with my parents: however, it was still excruciating not being able to be there. I spent a lot of time pacing the house, not eating or sleeping, checking my phone for texts, fielding texts and calls from friends and family. I was unable to do even simple household tasks, and left laundry, cooking, and my kids’ online schooling to my husband.
Feeling so helpless, I turned to prayer. My close friends organized a daily prayer vigil at 7:30
Then, after 17 days, a miracle: he was taken off the ventilator and moved to the medical unit. Looking back, I think these are really the days that the presence of his family would have sped up his recovery. Mike was experiencing delirium and hallucinations as a result of illness, medications, and the time he spent in an induced coma. I wish I could have been there with him to be the one he asked if what he was experiencing was real or a hallucination. Then we could have laughed about it together; our family has always found that humor helps with healing.
Mike
I understand the purpose of the isolation, but it really did a number on my mind. I remember being in the ICU, having my catheter taken out, not knowing what was happening or how I ended up in the hospital. I was so confused and was seeing people who were not there. One morning, I woke up thinking I was in my house and I had stolen the hospital bed I was in. I was panicking and scheming about how I might get this hospital bed back to the hospital before anyone noticed. As I mentioned earlier, I thought I was on a plane that had been taken over by terrorists who were using us COVID patients as biological weapons. Then I thought agents of the Federal Bureau of Investigation were coming to interrogate me and that they were also looking for my sister. To protect DeAnn, the next time someone asked me the name of my sister, I told them, “Maria” (the name of my sister who passed away in 1991) rather than DeAnn, who is very much alive. Another time, I thought my grandmother and cousin had died in a plane crash. My cousin is a state representative in Illinois, so once I got my phone to work, I checked his Wikipedia page to see if there was a death date listed.
When I was less confused, I found it’s tough spending day after day lying in a hospital bed with no family member or friend to offer companionship, comfort, or clarity. Even though I was extremely weak and could barely walk, I was asking, daily, about when I was expected to be discharged. I had to get out.
One night, our friend the ICU nurse came into my room to sit with me and just talk. She spent about 30 minutes with me around 4:00 in the morning. It was wonderful. All I could think about was what a huge blessing it was. I don’t think she knows just how much that meant to me. More often, I would report some symptom of confusion or insomnia, and a nurse would offer me medications for sleep or pain. I did not want any more drugs in my system. Human contact would have been a far better treatment.
I was reluctant to ask for help when I needed something, like a trip to the bathroom or some ice water. When I did press the call button, I had to wait for the busy nurse or tech to put on all the protective gear, and then, when they left, watch them take all the steps to disinfect and rid themselves of the gear that they had just put on. Even so, I was excited when it came time to take my vital signs or administer medications because that meant human interaction, however brief (and even if it was 4:00 AM). I wanted to bathe or change gowns and/or socks, but I opted to wear the same gown and socks for over three-fourths of the time I was there because I did not want to burden the staff.
Video chat turned out to be one of the best tools for creating connection. I may have sobbed a few times when talking to my parents on FaceTime, but just seeing their faces made all the difference in the world.
Finally, on April 21, 25 days after I was led into the ED, I was discharged. As we reflect on this experience, my family and I have some recommendations for hospitals and health systems trying to make patient experience a priority during this pandemic:
Kueper Family Recommendations to Improve Patient Experience for Those With COVID-19
- Adopt a more systematic approach to communicating with patient families, which would greatly improve the connection between them and healthcare personnel. This is especially important for families when the patient is critically ill, and especially in times when the patient is in isolation. We were fortunate in that we received updates from nurses and physicians several times a day. This was partly due to the relationships or connections with staff members that existed previously or developed over the course of Mike’s stay. Staff members who became invested in Mike’s progress became part of his hospital “family.” Many people who have had a family member with COVID have not had this experience, nor did they have the opportunity to build relationships with the staff, which we felt were important to ensure good care and open and frequent communication with them and the patient. Therefore, we believe a more systematic approach toward communication (eg, “the team will call each day during multidisciplinary rounds at 11 AM,”) would greatly improve the connection between families and healthcare personnel.
- Allow visitation under certain conditions even while the patient is in isolation. Visitation would have been especially helpful once Mike was more awake but isolated and delirious. We know that these policies are difficult to create and navigate but believe that there should be allowance for some visitation when there is a clear clinical benefit (eg, delirium). Because Mike had little human contact the week after he was taken off the ventilator (eg, contact limited to nurses coming in to take vitals, once daily doctor visit), he had to navigate the hallucinations and delirium on his own. Even one family member by his side who could provide frequent feedback on reality would have helped to resolve the feelings of agitation and fear that can accompany delirium.
- Schedule more video chats. Even when Mike was on the ventilator, we found video chats to be an important way to understand his experience and connect with him. Although we know such chats are difficult for clinicians to schedule, it greatly improved the experience for us.
- Reassure patients that caring for them is not a burden and they should not hesitate to ask for help. Being contagious and believing you are a danger to others is a terrible feeling. No one on staff said or even implied that they were afraid to care for him, but Mike felt “dangerous” to the staff and as such hesitated to “burden” the clinicians with requests (eg, going to the bathroom, having a change of clothes). It is time-consuming and difficult to don PPE and the amount of effort it takes to enter the room is immediately obvious to the patient. Because of this, it is very important that the clinicians and staff reassure patients that it is part of the job and not a burden to come in and out of the room.
DeAnn
Having Mike alive and now home is an incredible gift. We are taking every chance we can to make up for the time that we could not see him and are so grateful for the hospital team that saved his life.
Mike
On April 21, I was discharged and sent home. Luckily, for about 2 weeks, I had a best friend, my brother, and DeAnn, separately, stay with me each night. This was a godsend as all made sure I was taking my medicine, eating, and doing my prescribed exercises. I am struggling with a long recovery. I used a walker for a while and had both a physical and occupational therapist visit me two to three times a week. I visit a neurologist for some of my symptoms that have not resolved, such as pain and atrophy in my right shoulder, hand tremors, and some numbness in my thighs. Thankfully, I was able to resume working from home, but even going up stairs causes me to become winded. I know that doctors don’t understand this disease very well, and neither do I. Sometimes I feel discouraged about how much it set me back physically. I wish things could have been different—that I could have avoided this disease altogether or had milder symptoms. But I am so grateful to be alive and so thankful for the doctors and nurses, as well as for my family, who could not be there physically during the hospitalization but did everything they could do to help me. Because of their love and support, I survived.
Disclosures
The authors have nothing to disclose.
1. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID–11 March 2020. Published March 11, 2020. Accessed August 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. Issued March 13, 2020. Accessed August 11, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
1. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID–11 March 2020. Published March 11, 2020. Accessed August 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. Issued March 13, 2020. Accessed August 11, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
© 2020 Society of Hospital Medicine
Deployed: Pediatric Residents Caring for Adults During COVID-19’s First Wave in New York City
Stepping onto a busy coronavirus disease (COVID-19) unit for the first time can elicit trepidation for any medical provider. For a group of deployed pediatric residents at a New York City hospital in the spring of 2020, it was also the first time caring for adults since medical school. Imagine a pediatrician receiving this handoff: “77-year-old female with a history of diabetes, peripheral vascular disease, and COPD admitted with COVID-19 pneumonia, now intubated and proned with O2 saturations in the 80s. To do: DNR discussion.” General anxiety around COVID-19 was compounded by the discomfort of being thrust into adult medicine. But the doctoring instinct we have been honing throughout training kicked in, and we acted.
A NEW ORDER
As the COVID-19 crisis escalated in New York City, it became evident that staff from other specialties would be essential to manage the surge of patients. Hospital administrators selected a group of trainees for deployment based on their clinical experiences and willingness to volunteer. Almost overnight, a group of senior pediatric residents became adult providers, honoring the oath we each took to “remain a member of society with special obligations to all . . . fellow human beings.”¹
This health crisis brought different clinical disciplines together like never before. Entire wings of the hospital were converted into new COVID-19–dedicated wards and intensive care units (ICUs), and teams were built to optimize providers’ skills and capabilities. For example, one third-year pediatric resident was grouped with an outpatient endocrinologist—who had not practiced inpatient medicine in a decade—and a medicine intern. Hospitalists provided crucial support and guidance to these ward teams of deployed providers who were eager and willing to work but often not very knowledgeable about inpatient adult medicine.² In new ad hoc COVID-19 ICUs housed in other ICUs, where most pediatric residents were deployed, critical care attendings and neurointensivists led teams that also included anesthesiology, radiology, and neurosurgery residents, as well as nurses and advanced practice providers trained in various subspecialties of adult medicine.
PEDIATRICIANS IN AN ADULT WORLD
Although we wanted to help in any way we could, the prospect of entering this new world was incredibly daunting. We had not treated adults in several years, and during that time, our clinical experience with pediatric medicine greatly surpassed our adult training from medical school. We dug out materials on adult diseases, watched impromptu lectures on COVID-19 given by our critical care attendings, taught ourselves ventilator management in adults, and reviewed advanced cardiac life support (ACLS) protocols. But putting this all into practice was entirely different. Nothing can truly prepare you for arriving at the bedside of a hemodynamically unstable patient suffering from a virus that no one really understands.
When we arrived and introduced ourselves, we occasionally encountered surprise and curiosity from other providers. We felt that there was a perception that pediatricians do not often take care of critically ill or complex patients. Some of us were reluctant to disclose our specialty, lest it cloud perceptions of our capabilities. However, sick patients awaited us, so we got to work.
There was a steep learning curve over the first few days, from adjusting insulin for type 2 diabetes to troubleshooting renal replacement therapy issues. Accustomed to pediatric weight-based dosing, we were very anxious about ordering medications. The adult providers on our teams oriented us and helped us with many of these concerns. But the mystery of COVID-19 was a great equalizing force, leaving providers of every background with questions: Should we anticoagulate? How about steroids? Could this clinical change be another effect of the virus or a new infection?
We were pleasantly surprised that many aspects of our pediatric training proved beneficial in caring for adults. The focus on family-centered rounds and shared decision-making in pediatrics had imprinted on us the paramount importance of good communication. We were very cognizant of involving loved ones in discussions, now conducted by telephone or video call because infection-prevention guidelines precluded visitors. Family members were thankful for frequent updates, and as a result, largely embraced us as the doctors treating their loved ones. On one occasion, an internist, whose mother was a patient, was delighted to learn that the provider was a pediatric resident, saying, “I know you’ll take such good care of her.”
With the hospital inundated with sick adults, colleagues were grateful for our help. More so, they seemed appreciative of our compassion and ability to maintain a sense of humanity during the pandemonium of the pandemic despite feeling vulnerable, scared, and often powerless against COVID-19. In pediatrics, we do our best to truly engage with patients, from playing games with a 6-year-old with perforated appendicitis to holding and soothing a newborn in the neonatal ICU. We carried those skills over to the adult side. The team appreciated when a pediatric resident, with the help of an occupational therapist, used a letter board to communicate and receive assent for a tracheostomy from a nonsedated, intubated patient, directly answering the patient’s questions and addressing concerns rather than relying solely on a family member’s consent. And, though we had not previously led end-of-life discussions, we found that we were capable of doing so with the compassion instilled in us from our pediatric training. It had prepared us to face the universal challenge of communication in times of grief.
COVID-19 CHALLENGES
Besides grappling with our insecurity in treating adults, we, like all medical providers, had to balance our desire to provide care while keeping ourselves safe from COVID-19. To reduce our risk of exposure and preserve the dwindling supply of personal protective equipment (PPE), the flow of rounding, bedside care, and interventions was adapted to better cluster examinations, blood draws, and bedside tasks. Although efficient, this meant we did not enter rooms as frequently, creating an unfamiliar distance between provider and patient.³ We feared missing moments of clinical decompensation, and for pediatricians who value close patient contact, this made for a deeply uncomfortable reality.
We considered every plausible treatment for critically ill patients, sometimes unsure if they were beneficial or instead complicating the course further. Was lack of improvement a treatment failure or just the natural progression of this new illness? Unfortunately, most of the time, treatments were to no avail. Watching the respiratory, cardiovascular, renal, and neurologic devastation of COVID-19 on so many patients was horrifying. Seeing patients die without their loved ones beside them and at an alarmingly fast rate was simply crushing, as other trainees have similarly described.4 It was unlike anything we had ever experienced in pediatrics. Though we had begun to see a few pediatric COVID-19 patients in the hospital, their disease course was less severe. And, in the rare cases when pediatric patients die, they are almost invariably surrounded by family. One pediatric resident, who had never performed a single death examination before, did three in 1 week. It was emotionally trying, yet we had little time to mourn, as deathbeds were only briefly empty before the next gravely ill patients filled them.
Deployment took a toll on our bodies as well. We padded our faces to alleviate skin breakdown from 12-hour shifts spent entirely in N95 masks. We sanitized and washed our hands constantly, developing cracked skin and dermatitis, and showered meticulously after every shift. We isolated ourselves from our families and loved ones to protect them from the virus.
MOMENTS OF POSITIVITY
Despite these challenges, positive moments emerged. We worked with many wonderful colleagues from different disciplines we likely never would have met, let alone work alongside. We valued each other’s skills, talents, and knowledge. On an overnight shift in one of the ICUs, among the “ragtag team of deployees,” as one pediatric resident phrased it, each presented a topic from his or her respective specialty that might interest others. The pediatrician presented Kawasaki disease, as adult colleagues were beginning to ask questions about its cousin, the emerging multisystem inflammatory syndrome in children (MIS-C). This collegiality promoted a culture of collaboration and respect for other specialties that will hopefully continue.
A strong drive toward teamwork and shared responsibility flourished during deployment. No one was above any task. Residents and even fellows performed typical frontline tasks, such as ordering laboratory work and coordinating imaging. We all helped the proning team turn patients. Everyone shared insights, perspectives, and information gleaned from friends in different wards and hospitals and the ever-evolving literature. As we grappled with unpredictable disease courses, the traditional hierarchical roles of medicine—attending, fellow, resident—often blurred. We felt like we were all in this together.
Patient triumphs were celebrated. We danced with an 80-year-old patient admitted for almost 2 weeks when she was informed of her discharge and gave a standing ovation for a 91-year-old woman as she headed home. Music played over the hospital loudspeaker for every patient discharge. We also tried to create moments of lightheartedness. In the ICUs, we ate donated meals together and posed for pictures to express our gratitude to restaurants. Camaraderie blossomed during deployment.
ADVICE FOR THE FUTURE
Answering the call to help during the COVID-19 surge in New York City indelibly shaped our experiences as trainees and physicians. We will carry with us the lessons that we learned, both in the short term for the possible resurgence of cases and in the long term for ongoing patient care for the rest of our careers. For those residents who may be called upon next, the experience will be challenging, but rewarding. Each trainee has a foundation of knowledge, abilities, and instincts that will be useful, so trust in your training. Do not be afraid to ask questions or for help. You may be leaving your comfort zone, but you will not be alone, and families and other clinicians will be grateful to have you there. You are resilient, and you will make a difference.
Disclosures
The authors have nothing to disclose.
1. Lasagna L. Hippocratic oath—modern version. Published 1964. Accessed September 14, 2020. http://www.pbs.org/wgbh/nova/doctors/oath_modern.html
2. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314-315.https://doi.org/10.12788/jhm.3426
3. Cunningham CO, Diaz C, Slawek DE. COVID-19: the worst days of our careers. Ann Intern Med. 2020;172(11):764-765. https://doi.org/10.7326/M20-1715
4. Gallagher TH, Schleyer AM. “We signed up for this!”—student and trainee responses to the COVID-19 pandemic. N Engl J Med. 2020;382(25):e96. https://doi.org/10.1056/NEJMp2005234
Stepping onto a busy coronavirus disease (COVID-19) unit for the first time can elicit trepidation for any medical provider. For a group of deployed pediatric residents at a New York City hospital in the spring of 2020, it was also the first time caring for adults since medical school. Imagine a pediatrician receiving this handoff: “77-year-old female with a history of diabetes, peripheral vascular disease, and COPD admitted with COVID-19 pneumonia, now intubated and proned with O2 saturations in the 80s. To do: DNR discussion.” General anxiety around COVID-19 was compounded by the discomfort of being thrust into adult medicine. But the doctoring instinct we have been honing throughout training kicked in, and we acted.
A NEW ORDER
As the COVID-19 crisis escalated in New York City, it became evident that staff from other specialties would be essential to manage the surge of patients. Hospital administrators selected a group of trainees for deployment based on their clinical experiences and willingness to volunteer. Almost overnight, a group of senior pediatric residents became adult providers, honoring the oath we each took to “remain a member of society with special obligations to all . . . fellow human beings.”¹
This health crisis brought different clinical disciplines together like never before. Entire wings of the hospital were converted into new COVID-19–dedicated wards and intensive care units (ICUs), and teams were built to optimize providers’ skills and capabilities. For example, one third-year pediatric resident was grouped with an outpatient endocrinologist—who had not practiced inpatient medicine in a decade—and a medicine intern. Hospitalists provided crucial support and guidance to these ward teams of deployed providers who were eager and willing to work but often not very knowledgeable about inpatient adult medicine.² In new ad hoc COVID-19 ICUs housed in other ICUs, where most pediatric residents were deployed, critical care attendings and neurointensivists led teams that also included anesthesiology, radiology, and neurosurgery residents, as well as nurses and advanced practice providers trained in various subspecialties of adult medicine.
PEDIATRICIANS IN AN ADULT WORLD
Although we wanted to help in any way we could, the prospect of entering this new world was incredibly daunting. We had not treated adults in several years, and during that time, our clinical experience with pediatric medicine greatly surpassed our adult training from medical school. We dug out materials on adult diseases, watched impromptu lectures on COVID-19 given by our critical care attendings, taught ourselves ventilator management in adults, and reviewed advanced cardiac life support (ACLS) protocols. But putting this all into practice was entirely different. Nothing can truly prepare you for arriving at the bedside of a hemodynamically unstable patient suffering from a virus that no one really understands.
When we arrived and introduced ourselves, we occasionally encountered surprise and curiosity from other providers. We felt that there was a perception that pediatricians do not often take care of critically ill or complex patients. Some of us were reluctant to disclose our specialty, lest it cloud perceptions of our capabilities. However, sick patients awaited us, so we got to work.
There was a steep learning curve over the first few days, from adjusting insulin for type 2 diabetes to troubleshooting renal replacement therapy issues. Accustomed to pediatric weight-based dosing, we were very anxious about ordering medications. The adult providers on our teams oriented us and helped us with many of these concerns. But the mystery of COVID-19 was a great equalizing force, leaving providers of every background with questions: Should we anticoagulate? How about steroids? Could this clinical change be another effect of the virus or a new infection?
We were pleasantly surprised that many aspects of our pediatric training proved beneficial in caring for adults. The focus on family-centered rounds and shared decision-making in pediatrics had imprinted on us the paramount importance of good communication. We were very cognizant of involving loved ones in discussions, now conducted by telephone or video call because infection-prevention guidelines precluded visitors. Family members were thankful for frequent updates, and as a result, largely embraced us as the doctors treating their loved ones. On one occasion, an internist, whose mother was a patient, was delighted to learn that the provider was a pediatric resident, saying, “I know you’ll take such good care of her.”
With the hospital inundated with sick adults, colleagues were grateful for our help. More so, they seemed appreciative of our compassion and ability to maintain a sense of humanity during the pandemonium of the pandemic despite feeling vulnerable, scared, and often powerless against COVID-19. In pediatrics, we do our best to truly engage with patients, from playing games with a 6-year-old with perforated appendicitis to holding and soothing a newborn in the neonatal ICU. We carried those skills over to the adult side. The team appreciated when a pediatric resident, with the help of an occupational therapist, used a letter board to communicate and receive assent for a tracheostomy from a nonsedated, intubated patient, directly answering the patient’s questions and addressing concerns rather than relying solely on a family member’s consent. And, though we had not previously led end-of-life discussions, we found that we were capable of doing so with the compassion instilled in us from our pediatric training. It had prepared us to face the universal challenge of communication in times of grief.
COVID-19 CHALLENGES
Besides grappling with our insecurity in treating adults, we, like all medical providers, had to balance our desire to provide care while keeping ourselves safe from COVID-19. To reduce our risk of exposure and preserve the dwindling supply of personal protective equipment (PPE), the flow of rounding, bedside care, and interventions was adapted to better cluster examinations, blood draws, and bedside tasks. Although efficient, this meant we did not enter rooms as frequently, creating an unfamiliar distance between provider and patient.³ We feared missing moments of clinical decompensation, and for pediatricians who value close patient contact, this made for a deeply uncomfortable reality.
We considered every plausible treatment for critically ill patients, sometimes unsure if they were beneficial or instead complicating the course further. Was lack of improvement a treatment failure or just the natural progression of this new illness? Unfortunately, most of the time, treatments were to no avail. Watching the respiratory, cardiovascular, renal, and neurologic devastation of COVID-19 on so many patients was horrifying. Seeing patients die without their loved ones beside them and at an alarmingly fast rate was simply crushing, as other trainees have similarly described.4 It was unlike anything we had ever experienced in pediatrics. Though we had begun to see a few pediatric COVID-19 patients in the hospital, their disease course was less severe. And, in the rare cases when pediatric patients die, they are almost invariably surrounded by family. One pediatric resident, who had never performed a single death examination before, did three in 1 week. It was emotionally trying, yet we had little time to mourn, as deathbeds were only briefly empty before the next gravely ill patients filled them.
Deployment took a toll on our bodies as well. We padded our faces to alleviate skin breakdown from 12-hour shifts spent entirely in N95 masks. We sanitized and washed our hands constantly, developing cracked skin and dermatitis, and showered meticulously after every shift. We isolated ourselves from our families and loved ones to protect them from the virus.
MOMENTS OF POSITIVITY
Despite these challenges, positive moments emerged. We worked with many wonderful colleagues from different disciplines we likely never would have met, let alone work alongside. We valued each other’s skills, talents, and knowledge. On an overnight shift in one of the ICUs, among the “ragtag team of deployees,” as one pediatric resident phrased it, each presented a topic from his or her respective specialty that might interest others. The pediatrician presented Kawasaki disease, as adult colleagues were beginning to ask questions about its cousin, the emerging multisystem inflammatory syndrome in children (MIS-C). This collegiality promoted a culture of collaboration and respect for other specialties that will hopefully continue.
A strong drive toward teamwork and shared responsibility flourished during deployment. No one was above any task. Residents and even fellows performed typical frontline tasks, such as ordering laboratory work and coordinating imaging. We all helped the proning team turn patients. Everyone shared insights, perspectives, and information gleaned from friends in different wards and hospitals and the ever-evolving literature. As we grappled with unpredictable disease courses, the traditional hierarchical roles of medicine—attending, fellow, resident—often blurred. We felt like we were all in this together.
Patient triumphs were celebrated. We danced with an 80-year-old patient admitted for almost 2 weeks when she was informed of her discharge and gave a standing ovation for a 91-year-old woman as she headed home. Music played over the hospital loudspeaker for every patient discharge. We also tried to create moments of lightheartedness. In the ICUs, we ate donated meals together and posed for pictures to express our gratitude to restaurants. Camaraderie blossomed during deployment.
ADVICE FOR THE FUTURE
Answering the call to help during the COVID-19 surge in New York City indelibly shaped our experiences as trainees and physicians. We will carry with us the lessons that we learned, both in the short term for the possible resurgence of cases and in the long term for ongoing patient care for the rest of our careers. For those residents who may be called upon next, the experience will be challenging, but rewarding. Each trainee has a foundation of knowledge, abilities, and instincts that will be useful, so trust in your training. Do not be afraid to ask questions or for help. You may be leaving your comfort zone, but you will not be alone, and families and other clinicians will be grateful to have you there. You are resilient, and you will make a difference.
Disclosures
The authors have nothing to disclose.
Stepping onto a busy coronavirus disease (COVID-19) unit for the first time can elicit trepidation for any medical provider. For a group of deployed pediatric residents at a New York City hospital in the spring of 2020, it was also the first time caring for adults since medical school. Imagine a pediatrician receiving this handoff: “77-year-old female with a history of diabetes, peripheral vascular disease, and COPD admitted with COVID-19 pneumonia, now intubated and proned with O2 saturations in the 80s. To do: DNR discussion.” General anxiety around COVID-19 was compounded by the discomfort of being thrust into adult medicine. But the doctoring instinct we have been honing throughout training kicked in, and we acted.
A NEW ORDER
As the COVID-19 crisis escalated in New York City, it became evident that staff from other specialties would be essential to manage the surge of patients. Hospital administrators selected a group of trainees for deployment based on their clinical experiences and willingness to volunteer. Almost overnight, a group of senior pediatric residents became adult providers, honoring the oath we each took to “remain a member of society with special obligations to all . . . fellow human beings.”¹
This health crisis brought different clinical disciplines together like never before. Entire wings of the hospital were converted into new COVID-19–dedicated wards and intensive care units (ICUs), and teams were built to optimize providers’ skills and capabilities. For example, one third-year pediatric resident was grouped with an outpatient endocrinologist—who had not practiced inpatient medicine in a decade—and a medicine intern. Hospitalists provided crucial support and guidance to these ward teams of deployed providers who were eager and willing to work but often not very knowledgeable about inpatient adult medicine.² In new ad hoc COVID-19 ICUs housed in other ICUs, where most pediatric residents were deployed, critical care attendings and neurointensivists led teams that also included anesthesiology, radiology, and neurosurgery residents, as well as nurses and advanced practice providers trained in various subspecialties of adult medicine.
PEDIATRICIANS IN AN ADULT WORLD
Although we wanted to help in any way we could, the prospect of entering this new world was incredibly daunting. We had not treated adults in several years, and during that time, our clinical experience with pediatric medicine greatly surpassed our adult training from medical school. We dug out materials on adult diseases, watched impromptu lectures on COVID-19 given by our critical care attendings, taught ourselves ventilator management in adults, and reviewed advanced cardiac life support (ACLS) protocols. But putting this all into practice was entirely different. Nothing can truly prepare you for arriving at the bedside of a hemodynamically unstable patient suffering from a virus that no one really understands.
When we arrived and introduced ourselves, we occasionally encountered surprise and curiosity from other providers. We felt that there was a perception that pediatricians do not often take care of critically ill or complex patients. Some of us were reluctant to disclose our specialty, lest it cloud perceptions of our capabilities. However, sick patients awaited us, so we got to work.
There was a steep learning curve over the first few days, from adjusting insulin for type 2 diabetes to troubleshooting renal replacement therapy issues. Accustomed to pediatric weight-based dosing, we were very anxious about ordering medications. The adult providers on our teams oriented us and helped us with many of these concerns. But the mystery of COVID-19 was a great equalizing force, leaving providers of every background with questions: Should we anticoagulate? How about steroids? Could this clinical change be another effect of the virus or a new infection?
We were pleasantly surprised that many aspects of our pediatric training proved beneficial in caring for adults. The focus on family-centered rounds and shared decision-making in pediatrics had imprinted on us the paramount importance of good communication. We were very cognizant of involving loved ones in discussions, now conducted by telephone or video call because infection-prevention guidelines precluded visitors. Family members were thankful for frequent updates, and as a result, largely embraced us as the doctors treating their loved ones. On one occasion, an internist, whose mother was a patient, was delighted to learn that the provider was a pediatric resident, saying, “I know you’ll take such good care of her.”
With the hospital inundated with sick adults, colleagues were grateful for our help. More so, they seemed appreciative of our compassion and ability to maintain a sense of humanity during the pandemonium of the pandemic despite feeling vulnerable, scared, and often powerless against COVID-19. In pediatrics, we do our best to truly engage with patients, from playing games with a 6-year-old with perforated appendicitis to holding and soothing a newborn in the neonatal ICU. We carried those skills over to the adult side. The team appreciated when a pediatric resident, with the help of an occupational therapist, used a letter board to communicate and receive assent for a tracheostomy from a nonsedated, intubated patient, directly answering the patient’s questions and addressing concerns rather than relying solely on a family member’s consent. And, though we had not previously led end-of-life discussions, we found that we were capable of doing so with the compassion instilled in us from our pediatric training. It had prepared us to face the universal challenge of communication in times of grief.
COVID-19 CHALLENGES
Besides grappling with our insecurity in treating adults, we, like all medical providers, had to balance our desire to provide care while keeping ourselves safe from COVID-19. To reduce our risk of exposure and preserve the dwindling supply of personal protective equipment (PPE), the flow of rounding, bedside care, and interventions was adapted to better cluster examinations, blood draws, and bedside tasks. Although efficient, this meant we did not enter rooms as frequently, creating an unfamiliar distance between provider and patient.³ We feared missing moments of clinical decompensation, and for pediatricians who value close patient contact, this made for a deeply uncomfortable reality.
We considered every plausible treatment for critically ill patients, sometimes unsure if they were beneficial or instead complicating the course further. Was lack of improvement a treatment failure or just the natural progression of this new illness? Unfortunately, most of the time, treatments were to no avail. Watching the respiratory, cardiovascular, renal, and neurologic devastation of COVID-19 on so many patients was horrifying. Seeing patients die without their loved ones beside them and at an alarmingly fast rate was simply crushing, as other trainees have similarly described.4 It was unlike anything we had ever experienced in pediatrics. Though we had begun to see a few pediatric COVID-19 patients in the hospital, their disease course was less severe. And, in the rare cases when pediatric patients die, they are almost invariably surrounded by family. One pediatric resident, who had never performed a single death examination before, did three in 1 week. It was emotionally trying, yet we had little time to mourn, as deathbeds were only briefly empty before the next gravely ill patients filled them.
Deployment took a toll on our bodies as well. We padded our faces to alleviate skin breakdown from 12-hour shifts spent entirely in N95 masks. We sanitized and washed our hands constantly, developing cracked skin and dermatitis, and showered meticulously after every shift. We isolated ourselves from our families and loved ones to protect them from the virus.
MOMENTS OF POSITIVITY
Despite these challenges, positive moments emerged. We worked with many wonderful colleagues from different disciplines we likely never would have met, let alone work alongside. We valued each other’s skills, talents, and knowledge. On an overnight shift in one of the ICUs, among the “ragtag team of deployees,” as one pediatric resident phrased it, each presented a topic from his or her respective specialty that might interest others. The pediatrician presented Kawasaki disease, as adult colleagues were beginning to ask questions about its cousin, the emerging multisystem inflammatory syndrome in children (MIS-C). This collegiality promoted a culture of collaboration and respect for other specialties that will hopefully continue.
A strong drive toward teamwork and shared responsibility flourished during deployment. No one was above any task. Residents and even fellows performed typical frontline tasks, such as ordering laboratory work and coordinating imaging. We all helped the proning team turn patients. Everyone shared insights, perspectives, and information gleaned from friends in different wards and hospitals and the ever-evolving literature. As we grappled with unpredictable disease courses, the traditional hierarchical roles of medicine—attending, fellow, resident—often blurred. We felt like we were all in this together.
Patient triumphs were celebrated. We danced with an 80-year-old patient admitted for almost 2 weeks when she was informed of her discharge and gave a standing ovation for a 91-year-old woman as she headed home. Music played over the hospital loudspeaker for every patient discharge. We also tried to create moments of lightheartedness. In the ICUs, we ate donated meals together and posed for pictures to express our gratitude to restaurants. Camaraderie blossomed during deployment.
ADVICE FOR THE FUTURE
Answering the call to help during the COVID-19 surge in New York City indelibly shaped our experiences as trainees and physicians. We will carry with us the lessons that we learned, both in the short term for the possible resurgence of cases and in the long term for ongoing patient care for the rest of our careers. For those residents who may be called upon next, the experience will be challenging, but rewarding. Each trainee has a foundation of knowledge, abilities, and instincts that will be useful, so trust in your training. Do not be afraid to ask questions or for help. You may be leaving your comfort zone, but you will not be alone, and families and other clinicians will be grateful to have you there. You are resilient, and you will make a difference.
Disclosures
The authors have nothing to disclose.
1. Lasagna L. Hippocratic oath—modern version. Published 1964. Accessed September 14, 2020. http://www.pbs.org/wgbh/nova/doctors/oath_modern.html
2. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314-315.https://doi.org/10.12788/jhm.3426
3. Cunningham CO, Diaz C, Slawek DE. COVID-19: the worst days of our careers. Ann Intern Med. 2020;172(11):764-765. https://doi.org/10.7326/M20-1715
4. Gallagher TH, Schleyer AM. “We signed up for this!”—student and trainee responses to the COVID-19 pandemic. N Engl J Med. 2020;382(25):e96. https://doi.org/10.1056/NEJMp2005234
1. Lasagna L. Hippocratic oath—modern version. Published 1964. Accessed September 14, 2020. http://www.pbs.org/wgbh/nova/doctors/oath_modern.html
2. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314-315.https://doi.org/10.12788/jhm.3426
3. Cunningham CO, Diaz C, Slawek DE. COVID-19: the worst days of our careers. Ann Intern Med. 2020;172(11):764-765. https://doi.org/10.7326/M20-1715
4. Gallagher TH, Schleyer AM. “We signed up for this!”—student and trainee responses to the COVID-19 pandemic. N Engl J Med. 2020;382(25):e96. https://doi.org/10.1056/NEJMp2005234
© 2020 Society of Hospital Medicine
Professional Identity Formation During the COVID-19 Pandemic
In 1957, Merton wrote that the primary aim of medical education should be “to provide [learners] with a professional identity so that [they] come to think, act, and feel like a physician.”1 More than a half-century later, the Carnegie Foundation for the Advancement of Teaching echoed his sentiments in its landmark examination of the United States medical education system, which produced four key recommendations for curricular reform, including explicitly addressing professional identity formation (PIF).2 PIF is a process by which a learner transforms into a physician with the values, dispositions, and aspirations of the physician community.3 It is now recognized as crucial to developing physicians who can deliver high-quality care.2
Major changes to the learning environment can impact PIF. For example, when the Accreditation Committee for Graduate Medical Education duty-hour restrictions were implemented in 2003, several educators were concerned that the changes may negatively affect resident PIF,4 whereas others saw an opportunity to refocus curricular efforts on PIF.5 Medical education is now in the midst of another radical change with the novel coronavirus disease 2019 (COVID-19) pandemic. Over the past several months, we have begun to understand the pandemic’s effects on medical education in terms of learner welfare, educational experiences/value, innovation, and assessment.6-8 However, little has been published on the pandemic’s effect on PIF.9 We explore the impact of COVID-19 on physicians’ PIF and identify strategies to support PIF in physicians and other healthcare professionals during times of crisis.
SOCIALIZATION AND COMMUNITIES OF PRACTICE
PIF is dynamic and nonlinear, occurring at every level of the medical education hierarchy (medical student, resident, fellow, attending).10 Emphasis on PIF has grown in recent years as a response to the limitations of behavior-based educational frameworks such as competency-based medical education (CBME),3 which focuses on what the learner can “do.” PIF moves beyond “doing” to consider who the learner “is.”11 PIF occurs at the individual level as learners progress through multiple distinct identity stages during their longitudinal formation10,12-14 but also at the level of the collective. Socialization plays a crucial role; thus, PIF is heavily influenced by the environment, context, and other individuals.10
Medicine can be conceptualized as a community of practice, which is a sustaining network of individuals who share knowledge, beliefs, values, and experiences related to a common practice or purpose.15,16 In a community of practice, learning is social, includes knowledge that is tacit to the community, and is situated within the context in which it will be applied. PIF involves learners moving from “legitimate peripheral participation,” whereby they are accepted as novice community members, to “full participation,” which involves gaining competence in relevant tasks and internalizing community principles to become full partners in the community.13 Critical to this process is exposure to socializing agents (eg, attendings, nurses, peers), observation of community interactions, experiential learning in the clinical environment, and access to role models.10,16 Immersion in the clinical environment with other community members is thus crucial to PIF. This is especially important, as “medicine” is not truly a single community, but rather a “landscape of communities,” each with its own identity.17 Learners must therefore be immersed in many different clinical environments to experience the various communities within our field.
COVID-19 CHANGING THE LEARNING ENVIRONMENT
The pandemic is drastically altering the learning environment in medical education.8 Several institutions temporarily removed medical students from clinical rotations to reduce learner exposure and conserve personal protective equipment. Some residents were removed from nonessential clinical activities for similar reasons. Many attendings have been asked to work from home when not required to be present for clinical care duties. Common medical community activities, such as group meals and conferences, have been altered for physical distancing or simply canceled. Usual clinical care has rapidly evolved, with changes in rounding practices, a boon of telehealth, and cancellations of nonessential procedures. These necessary changes present constantly shifting grounds for anyone trying to integrate into a community and develop a professional identity.
Changes outside of the clinical learning environment are also affecting PIF. Social interactions, such as dinners and peer gatherings, occur via video conference or not at all. Most in-person contact happens with masks in place, physically distanced, and in smaller groups. Resident and student lounges are being modified to physically distance or reduce the number of occupants. There is often variable adherence, both intentional and unintentional, to physical distance and mask mandates, creating potential for confusion as learners try to internalize the values and norms of the medical community. Common professional rituals, such as white coat ceremonies, orientation events, and graduations, have been curtailed or canceled. Even experiences that are not commonly seen as social events but are important in the physician’s journey, such as the residency and fellowship application processes and standardized tests, are being transformed. These changes alter typical social patterns that are important in PIF and may adversely affect high-value social group interactions that serve as buffers against stressors during training.18
Finally, the pandemic has altered the timeline for many learners. Medical students at several institutions graduated early to join the workforce and help care for escalating numbers of patients during the pandemic.7 Some see the pandemic as a catalyst to move toward competency-based time-variable training, in which learners progress through training at variable rates depending on their individual performance and learning needs.19 These changes could shorten the amount of time some learners spend in a given role (eg, medical student, intern). In such situations, it is unclear whether a minimal maturational time is necessary for most learners to fully develop a professional identity.
SUPPORTING PIF DURING THE PANDEMIC
In 2019, Cruess et al published general principles for supporting PIF,17 which have been used to support PIF during the COVID-19 pandemic.20 In the Table, we describe these principles and provide examples of how to implement them in the context of the pandemic. We believe these principles are applicable for PIF in undergraduate medical education, graduate medical education, and faculty development programs. A common thread throughout the principles is that PIF is not a process that should be left to chance, but rather explicitly nurtured through systematic support and curricular initiatives.5 This may be challenging while the COVID-19 pandemic is sapping financial resources and requiring rapid changes to clinical systems, but given the central role PIF plays in physician development, it should be prioritized by educational leaders.
CREATING AND MAINTAINING A WELCOMING COMMUNITY: AN OPPORTUNITY
One of the final principles from Cruess et al is to create and maintain a welcoming community.17 This prompts questions such as: Is our community welcoming to everyone, where “everyone” really does mean everyone? Like other social structures, communities of practice tend to perpetuate existing power structures and inequities.17 It is no secret that medicine, like other professions, is riddled with inequities and bias based on factors such as race, gender, and socioeconomic status.21-23 The COVID-19 pandemic is likely exacerbating these inequities, such as the adverse impacts that are specifically affecting women physicians, who take on a disproportionate share of the child care at home.23 These biases impact not only the members of our professional community but also our patients, contributing to disparities in care and outcomes.
Physicians who have received inequitable treatment have laid bare the ways in which our communities of practice are failing them, and also outlined a better path on which to move forward.21,23 In addition to recruitment practices that promote diversity, meaningful programs should be developed to support inclusion, equity (in recognition, support, compensation), retention, and advancement. The disruption caused by COVID-19 can be a catalyst for this change. By taking this moment of crisis to examine the values and norms of medicine and how we systematically perpetuate harmful inequities and biases, we have an opportunity to deliberately rebuild our community of practice in a manner that helps shape the next generation’s professional identities to be better than we have been. This should always be the aim of education.
1. Merton RK. Some Preliminaries to a Sociology of Medical Education. Harvard University Press; 1957.
2. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. Jossey-Bass; 2010.
3. Irby DM, Hamstra SJ. Parting the clouds: three professionalism frameworks in medical education. Acad Med. 2016;91(12):1606-1611. https://doi.org/10.1097/ACM.0000000000001190
4. Reed DA, Levine RB, Miller RG, et al. Effect of residency duty-hour limits: views of key clinical faculty. Arch Intern Med. 2007;167(14):1487-1492. https://doi.org/10.1001/archinte.167.14.1487
5. Schumacher DJ, Slovin SR, Riebschleger MP, Englander R, Hicks PJ, Carraccio C. Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883-888. https://doi.org/10.1097/ACM.0b013e318257d57d
6. Anderson ML, Turbow S, Willgerodt MA, Ruhnke GW. Education in a crisis: the opportunity of our lives. J Hosp Med. 2020;15(5):287-291. https://doi.org/10.12788/jhm.3431
7. Kinnear B, Kelleher M, Olson AP, Sall D, Schumacher DJ. Developing trust with early medical school graduates during the COVID-19 pandemic. J Hosp Med. 2020;15(6):367-369. https://doi.org/10.12788/jhm.3463
8. Woolliscroft JO. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402
9. Cullum RJ, Shaughnessy A, Mayat NY, Brown ME. Identity in lockdown: supporting primary care professional identity development in the COVID-19 generation. Educ Prim Care. 2020;31(4):200-204. https://doi.org/10.1080/14739879.2020.1779616
10. Jarvis-Selinger S, Pratt DD, Regehr G. Competency is not enough: integrating identity formation into the medical education discourse. Acad Med. 2012;87(9):1185-1190. https://doi.org/10.1097/ACM.0b013e3182604968
11. Al‐Eraky M, Marei H. A fresh look at Miller’s pyramid: assessment at the ‘Is’ and ‘Do’ levels. Med Educ. 2016;50(12):1253-1257. https://doi.org/10.1111/medu.13101
12. Forsythe GB. Identity development in professional education. Acad Med. 2005;80(10 Suppl):S112-S117. https://doi.org/10.1097/00001888-200510001-0002913.
13. Cruess RL, Cruess SR, Boudreau JD, Snell L, Steinert Y. A schematic representation of the professional identity formation and socialization of medical students and residents: a guide for medical educators. Acad Med. 2015;90(6):718-725. https://doi.org/10.1097/ACM.0000000000000700
14. Kegan R. The Evolving Self: Problem and Process in Human Development. Harvard University Press; 1982.
15. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
16. Lave J, Wenger E. Situated Learning: Legitimate Peripheral Participation. Cambridge University Press; 1991.
17. Cruess SR, Cruess RL, Steinert Y. Supporting the development of a professional identity: general principles. Med Teach. 2019;41(6):641-649. https://doi.org/10.1080/0142159X.2018.1536260
18. Mavor KI, McNeill KG, Anderson K, Kerr A, O’Reilly E, Platow MJ. Beyond prevalence to process: the role of self and identity in medical student well‐being. Med Educ. 2014;48(4):351-360. https://doi.org/10.1111/medu.12375
19. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
20. Stetson GV, Kryzhanovskaya IV, Lomen‐Hoerth C, Hauer KE. Professional identity formation in disorienting times. Med Educ. 2020;54(8):765-766. https://doi.org/10.1111/medu.14202
21. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(9):572-573. https://doi.org/10.12788/jhm.3492
22. Beagan BL. Everyday classism in medical school: experiencing marginality and resistance. Med Educ. 2005;39(8):777-784. https://doi.org/10.1111/j.1365-2929.2005.02225.x
23. Jones Y, Durand V, Morton K, et al. Collateral damage: how COVID-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
In 1957, Merton wrote that the primary aim of medical education should be “to provide [learners] with a professional identity so that [they] come to think, act, and feel like a physician.”1 More than a half-century later, the Carnegie Foundation for the Advancement of Teaching echoed his sentiments in its landmark examination of the United States medical education system, which produced four key recommendations for curricular reform, including explicitly addressing professional identity formation (PIF).2 PIF is a process by which a learner transforms into a physician with the values, dispositions, and aspirations of the physician community.3 It is now recognized as crucial to developing physicians who can deliver high-quality care.2
Major changes to the learning environment can impact PIF. For example, when the Accreditation Committee for Graduate Medical Education duty-hour restrictions were implemented in 2003, several educators were concerned that the changes may negatively affect resident PIF,4 whereas others saw an opportunity to refocus curricular efforts on PIF.5 Medical education is now in the midst of another radical change with the novel coronavirus disease 2019 (COVID-19) pandemic. Over the past several months, we have begun to understand the pandemic’s effects on medical education in terms of learner welfare, educational experiences/value, innovation, and assessment.6-8 However, little has been published on the pandemic’s effect on PIF.9 We explore the impact of COVID-19 on physicians’ PIF and identify strategies to support PIF in physicians and other healthcare professionals during times of crisis.
SOCIALIZATION AND COMMUNITIES OF PRACTICE
PIF is dynamic and nonlinear, occurring at every level of the medical education hierarchy (medical student, resident, fellow, attending).10 Emphasis on PIF has grown in recent years as a response to the limitations of behavior-based educational frameworks such as competency-based medical education (CBME),3 which focuses on what the learner can “do.” PIF moves beyond “doing” to consider who the learner “is.”11 PIF occurs at the individual level as learners progress through multiple distinct identity stages during their longitudinal formation10,12-14 but also at the level of the collective. Socialization plays a crucial role; thus, PIF is heavily influenced by the environment, context, and other individuals.10
Medicine can be conceptualized as a community of practice, which is a sustaining network of individuals who share knowledge, beliefs, values, and experiences related to a common practice or purpose.15,16 In a community of practice, learning is social, includes knowledge that is tacit to the community, and is situated within the context in which it will be applied. PIF involves learners moving from “legitimate peripheral participation,” whereby they are accepted as novice community members, to “full participation,” which involves gaining competence in relevant tasks and internalizing community principles to become full partners in the community.13 Critical to this process is exposure to socializing agents (eg, attendings, nurses, peers), observation of community interactions, experiential learning in the clinical environment, and access to role models.10,16 Immersion in the clinical environment with other community members is thus crucial to PIF. This is especially important, as “medicine” is not truly a single community, but rather a “landscape of communities,” each with its own identity.17 Learners must therefore be immersed in many different clinical environments to experience the various communities within our field.
COVID-19 CHANGING THE LEARNING ENVIRONMENT
The pandemic is drastically altering the learning environment in medical education.8 Several institutions temporarily removed medical students from clinical rotations to reduce learner exposure and conserve personal protective equipment. Some residents were removed from nonessential clinical activities for similar reasons. Many attendings have been asked to work from home when not required to be present for clinical care duties. Common medical community activities, such as group meals and conferences, have been altered for physical distancing or simply canceled. Usual clinical care has rapidly evolved, with changes in rounding practices, a boon of telehealth, and cancellations of nonessential procedures. These necessary changes present constantly shifting grounds for anyone trying to integrate into a community and develop a professional identity.
Changes outside of the clinical learning environment are also affecting PIF. Social interactions, such as dinners and peer gatherings, occur via video conference or not at all. Most in-person contact happens with masks in place, physically distanced, and in smaller groups. Resident and student lounges are being modified to physically distance or reduce the number of occupants. There is often variable adherence, both intentional and unintentional, to physical distance and mask mandates, creating potential for confusion as learners try to internalize the values and norms of the medical community. Common professional rituals, such as white coat ceremonies, orientation events, and graduations, have been curtailed or canceled. Even experiences that are not commonly seen as social events but are important in the physician’s journey, such as the residency and fellowship application processes and standardized tests, are being transformed. These changes alter typical social patterns that are important in PIF and may adversely affect high-value social group interactions that serve as buffers against stressors during training.18
Finally, the pandemic has altered the timeline for many learners. Medical students at several institutions graduated early to join the workforce and help care for escalating numbers of patients during the pandemic.7 Some see the pandemic as a catalyst to move toward competency-based time-variable training, in which learners progress through training at variable rates depending on their individual performance and learning needs.19 These changes could shorten the amount of time some learners spend in a given role (eg, medical student, intern). In such situations, it is unclear whether a minimal maturational time is necessary for most learners to fully develop a professional identity.
SUPPORTING PIF DURING THE PANDEMIC
In 2019, Cruess et al published general principles for supporting PIF,17 which have been used to support PIF during the COVID-19 pandemic.20 In the Table, we describe these principles and provide examples of how to implement them in the context of the pandemic. We believe these principles are applicable for PIF in undergraduate medical education, graduate medical education, and faculty development programs. A common thread throughout the principles is that PIF is not a process that should be left to chance, but rather explicitly nurtured through systematic support and curricular initiatives.5 This may be challenging while the COVID-19 pandemic is sapping financial resources and requiring rapid changes to clinical systems, but given the central role PIF plays in physician development, it should be prioritized by educational leaders.

CREATING AND MAINTAINING A WELCOMING COMMUNITY: AN OPPORTUNITY
One of the final principles from Cruess et al is to create and maintain a welcoming community.17 This prompts questions such as: Is our community welcoming to everyone, where “everyone” really does mean everyone? Like other social structures, communities of practice tend to perpetuate existing power structures and inequities.17 It is no secret that medicine, like other professions, is riddled with inequities and bias based on factors such as race, gender, and socioeconomic status.21-23 The COVID-19 pandemic is likely exacerbating these inequities, such as the adverse impacts that are specifically affecting women physicians, who take on a disproportionate share of the child care at home.23 These biases impact not only the members of our professional community but also our patients, contributing to disparities in care and outcomes.
Physicians who have received inequitable treatment have laid bare the ways in which our communities of practice are failing them, and also outlined a better path on which to move forward.21,23 In addition to recruitment practices that promote diversity, meaningful programs should be developed to support inclusion, equity (in recognition, support, compensation), retention, and advancement. The disruption caused by COVID-19 can be a catalyst for this change. By taking this moment of crisis to examine the values and norms of medicine and how we systematically perpetuate harmful inequities and biases, we have an opportunity to deliberately rebuild our community of practice in a manner that helps shape the next generation’s professional identities to be better than we have been. This should always be the aim of education.
In 1957, Merton wrote that the primary aim of medical education should be “to provide [learners] with a professional identity so that [they] come to think, act, and feel like a physician.”1 More than a half-century later, the Carnegie Foundation for the Advancement of Teaching echoed his sentiments in its landmark examination of the United States medical education system, which produced four key recommendations for curricular reform, including explicitly addressing professional identity formation (PIF).2 PIF is a process by which a learner transforms into a physician with the values, dispositions, and aspirations of the physician community.3 It is now recognized as crucial to developing physicians who can deliver high-quality care.2
Major changes to the learning environment can impact PIF. For example, when the Accreditation Committee for Graduate Medical Education duty-hour restrictions were implemented in 2003, several educators were concerned that the changes may negatively affect resident PIF,4 whereas others saw an opportunity to refocus curricular efforts on PIF.5 Medical education is now in the midst of another radical change with the novel coronavirus disease 2019 (COVID-19) pandemic. Over the past several months, we have begun to understand the pandemic’s effects on medical education in terms of learner welfare, educational experiences/value, innovation, and assessment.6-8 However, little has been published on the pandemic’s effect on PIF.9 We explore the impact of COVID-19 on physicians’ PIF and identify strategies to support PIF in physicians and other healthcare professionals during times of crisis.
SOCIALIZATION AND COMMUNITIES OF PRACTICE
PIF is dynamic and nonlinear, occurring at every level of the medical education hierarchy (medical student, resident, fellow, attending).10 Emphasis on PIF has grown in recent years as a response to the limitations of behavior-based educational frameworks such as competency-based medical education (CBME),3 which focuses on what the learner can “do.” PIF moves beyond “doing” to consider who the learner “is.”11 PIF occurs at the individual level as learners progress through multiple distinct identity stages during their longitudinal formation10,12-14 but also at the level of the collective. Socialization plays a crucial role; thus, PIF is heavily influenced by the environment, context, and other individuals.10
Medicine can be conceptualized as a community of practice, which is a sustaining network of individuals who share knowledge, beliefs, values, and experiences related to a common practice or purpose.15,16 In a community of practice, learning is social, includes knowledge that is tacit to the community, and is situated within the context in which it will be applied. PIF involves learners moving from “legitimate peripheral participation,” whereby they are accepted as novice community members, to “full participation,” which involves gaining competence in relevant tasks and internalizing community principles to become full partners in the community.13 Critical to this process is exposure to socializing agents (eg, attendings, nurses, peers), observation of community interactions, experiential learning in the clinical environment, and access to role models.10,16 Immersion in the clinical environment with other community members is thus crucial to PIF. This is especially important, as “medicine” is not truly a single community, but rather a “landscape of communities,” each with its own identity.17 Learners must therefore be immersed in many different clinical environments to experience the various communities within our field.
COVID-19 CHANGING THE LEARNING ENVIRONMENT
The pandemic is drastically altering the learning environment in medical education.8 Several institutions temporarily removed medical students from clinical rotations to reduce learner exposure and conserve personal protective equipment. Some residents were removed from nonessential clinical activities for similar reasons. Many attendings have been asked to work from home when not required to be present for clinical care duties. Common medical community activities, such as group meals and conferences, have been altered for physical distancing or simply canceled. Usual clinical care has rapidly evolved, with changes in rounding practices, a boon of telehealth, and cancellations of nonessential procedures. These necessary changes present constantly shifting grounds for anyone trying to integrate into a community and develop a professional identity.
Changes outside of the clinical learning environment are also affecting PIF. Social interactions, such as dinners and peer gatherings, occur via video conference or not at all. Most in-person contact happens with masks in place, physically distanced, and in smaller groups. Resident and student lounges are being modified to physically distance or reduce the number of occupants. There is often variable adherence, both intentional and unintentional, to physical distance and mask mandates, creating potential for confusion as learners try to internalize the values and norms of the medical community. Common professional rituals, such as white coat ceremonies, orientation events, and graduations, have been curtailed or canceled. Even experiences that are not commonly seen as social events but are important in the physician’s journey, such as the residency and fellowship application processes and standardized tests, are being transformed. These changes alter typical social patterns that are important in PIF and may adversely affect high-value social group interactions that serve as buffers against stressors during training.18
Finally, the pandemic has altered the timeline for many learners. Medical students at several institutions graduated early to join the workforce and help care for escalating numbers of patients during the pandemic.7 Some see the pandemic as a catalyst to move toward competency-based time-variable training, in which learners progress through training at variable rates depending on their individual performance and learning needs.19 These changes could shorten the amount of time some learners spend in a given role (eg, medical student, intern). In such situations, it is unclear whether a minimal maturational time is necessary for most learners to fully develop a professional identity.
SUPPORTING PIF DURING THE PANDEMIC
In 2019, Cruess et al published general principles for supporting PIF,17 which have been used to support PIF during the COVID-19 pandemic.20 In the Table, we describe these principles and provide examples of how to implement them in the context of the pandemic. We believe these principles are applicable for PIF in undergraduate medical education, graduate medical education, and faculty development programs. A common thread throughout the principles is that PIF is not a process that should be left to chance, but rather explicitly nurtured through systematic support and curricular initiatives.5 This may be challenging while the COVID-19 pandemic is sapping financial resources and requiring rapid changes to clinical systems, but given the central role PIF plays in physician development, it should be prioritized by educational leaders.

CREATING AND MAINTAINING A WELCOMING COMMUNITY: AN OPPORTUNITY
One of the final principles from Cruess et al is to create and maintain a welcoming community.17 This prompts questions such as: Is our community welcoming to everyone, where “everyone” really does mean everyone? Like other social structures, communities of practice tend to perpetuate existing power structures and inequities.17 It is no secret that medicine, like other professions, is riddled with inequities and bias based on factors such as race, gender, and socioeconomic status.21-23 The COVID-19 pandemic is likely exacerbating these inequities, such as the adverse impacts that are specifically affecting women physicians, who take on a disproportionate share of the child care at home.23 These biases impact not only the members of our professional community but also our patients, contributing to disparities in care and outcomes.
Physicians who have received inequitable treatment have laid bare the ways in which our communities of practice are failing them, and also outlined a better path on which to move forward.21,23 In addition to recruitment practices that promote diversity, meaningful programs should be developed to support inclusion, equity (in recognition, support, compensation), retention, and advancement. The disruption caused by COVID-19 can be a catalyst for this change. By taking this moment of crisis to examine the values and norms of medicine and how we systematically perpetuate harmful inequities and biases, we have an opportunity to deliberately rebuild our community of practice in a manner that helps shape the next generation’s professional identities to be better than we have been. This should always be the aim of education.
1. Merton RK. Some Preliminaries to a Sociology of Medical Education. Harvard University Press; 1957.
2. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. Jossey-Bass; 2010.
3. Irby DM, Hamstra SJ. Parting the clouds: three professionalism frameworks in medical education. Acad Med. 2016;91(12):1606-1611. https://doi.org/10.1097/ACM.0000000000001190
4. Reed DA, Levine RB, Miller RG, et al. Effect of residency duty-hour limits: views of key clinical faculty. Arch Intern Med. 2007;167(14):1487-1492. https://doi.org/10.1001/archinte.167.14.1487
5. Schumacher DJ, Slovin SR, Riebschleger MP, Englander R, Hicks PJ, Carraccio C. Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883-888. https://doi.org/10.1097/ACM.0b013e318257d57d
6. Anderson ML, Turbow S, Willgerodt MA, Ruhnke GW. Education in a crisis: the opportunity of our lives. J Hosp Med. 2020;15(5):287-291. https://doi.org/10.12788/jhm.3431
7. Kinnear B, Kelleher M, Olson AP, Sall D, Schumacher DJ. Developing trust with early medical school graduates during the COVID-19 pandemic. J Hosp Med. 2020;15(6):367-369. https://doi.org/10.12788/jhm.3463
8. Woolliscroft JO. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402
9. Cullum RJ, Shaughnessy A, Mayat NY, Brown ME. Identity in lockdown: supporting primary care professional identity development in the COVID-19 generation. Educ Prim Care. 2020;31(4):200-204. https://doi.org/10.1080/14739879.2020.1779616
10. Jarvis-Selinger S, Pratt DD, Regehr G. Competency is not enough: integrating identity formation into the medical education discourse. Acad Med. 2012;87(9):1185-1190. https://doi.org/10.1097/ACM.0b013e3182604968
11. Al‐Eraky M, Marei H. A fresh look at Miller’s pyramid: assessment at the ‘Is’ and ‘Do’ levels. Med Educ. 2016;50(12):1253-1257. https://doi.org/10.1111/medu.13101
12. Forsythe GB. Identity development in professional education. Acad Med. 2005;80(10 Suppl):S112-S117. https://doi.org/10.1097/00001888-200510001-0002913.
13. Cruess RL, Cruess SR, Boudreau JD, Snell L, Steinert Y. A schematic representation of the professional identity formation and socialization of medical students and residents: a guide for medical educators. Acad Med. 2015;90(6):718-725. https://doi.org/10.1097/ACM.0000000000000700
14. Kegan R. The Evolving Self: Problem and Process in Human Development. Harvard University Press; 1982.
15. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
16. Lave J, Wenger E. Situated Learning: Legitimate Peripheral Participation. Cambridge University Press; 1991.
17. Cruess SR, Cruess RL, Steinert Y. Supporting the development of a professional identity: general principles. Med Teach. 2019;41(6):641-649. https://doi.org/10.1080/0142159X.2018.1536260
18. Mavor KI, McNeill KG, Anderson K, Kerr A, O’Reilly E, Platow MJ. Beyond prevalence to process: the role of self and identity in medical student well‐being. Med Educ. 2014;48(4):351-360. https://doi.org/10.1111/medu.12375
19. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
20. Stetson GV, Kryzhanovskaya IV, Lomen‐Hoerth C, Hauer KE. Professional identity formation in disorienting times. Med Educ. 2020;54(8):765-766. https://doi.org/10.1111/medu.14202
21. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(9):572-573. https://doi.org/10.12788/jhm.3492
22. Beagan BL. Everyday classism in medical school: experiencing marginality and resistance. Med Educ. 2005;39(8):777-784. https://doi.org/10.1111/j.1365-2929.2005.02225.x
23. Jones Y, Durand V, Morton K, et al. Collateral damage: how COVID-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
1. Merton RK. Some Preliminaries to a Sociology of Medical Education. Harvard University Press; 1957.
2. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. Jossey-Bass; 2010.
3. Irby DM, Hamstra SJ. Parting the clouds: three professionalism frameworks in medical education. Acad Med. 2016;91(12):1606-1611. https://doi.org/10.1097/ACM.0000000000001190
4. Reed DA, Levine RB, Miller RG, et al. Effect of residency duty-hour limits: views of key clinical faculty. Arch Intern Med. 2007;167(14):1487-1492. https://doi.org/10.1001/archinte.167.14.1487
5. Schumacher DJ, Slovin SR, Riebschleger MP, Englander R, Hicks PJ, Carraccio C. Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883-888. https://doi.org/10.1097/ACM.0b013e318257d57d
6. Anderson ML, Turbow S, Willgerodt MA, Ruhnke GW. Education in a crisis: the opportunity of our lives. J Hosp Med. 2020;15(5):287-291. https://doi.org/10.12788/jhm.3431
7. Kinnear B, Kelleher M, Olson AP, Sall D, Schumacher DJ. Developing trust with early medical school graduates during the COVID-19 pandemic. J Hosp Med. 2020;15(6):367-369. https://doi.org/10.12788/jhm.3463
8. Woolliscroft JO. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402
9. Cullum RJ, Shaughnessy A, Mayat NY, Brown ME. Identity in lockdown: supporting primary care professional identity development in the COVID-19 generation. Educ Prim Care. 2020;31(4):200-204. https://doi.org/10.1080/14739879.2020.1779616
10. Jarvis-Selinger S, Pratt DD, Regehr G. Competency is not enough: integrating identity formation into the medical education discourse. Acad Med. 2012;87(9):1185-1190. https://doi.org/10.1097/ACM.0b013e3182604968
11. Al‐Eraky M, Marei H. A fresh look at Miller’s pyramid: assessment at the ‘Is’ and ‘Do’ levels. Med Educ. 2016;50(12):1253-1257. https://doi.org/10.1111/medu.13101
12. Forsythe GB. Identity development in professional education. Acad Med. 2005;80(10 Suppl):S112-S117. https://doi.org/10.1097/00001888-200510001-0002913.
13. Cruess RL, Cruess SR, Boudreau JD, Snell L, Steinert Y. A schematic representation of the professional identity formation and socialization of medical students and residents: a guide for medical educators. Acad Med. 2015;90(6):718-725. https://doi.org/10.1097/ACM.0000000000000700
14. Kegan R. The Evolving Self: Problem and Process in Human Development. Harvard University Press; 1982.
15. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
16. Lave J, Wenger E. Situated Learning: Legitimate Peripheral Participation. Cambridge University Press; 1991.
17. Cruess SR, Cruess RL, Steinert Y. Supporting the development of a professional identity: general principles. Med Teach. 2019;41(6):641-649. https://doi.org/10.1080/0142159X.2018.1536260
18. Mavor KI, McNeill KG, Anderson K, Kerr A, O’Reilly E, Platow MJ. Beyond prevalence to process: the role of self and identity in medical student well‐being. Med Educ. 2014;48(4):351-360. https://doi.org/10.1111/medu.12375
19. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
20. Stetson GV, Kryzhanovskaya IV, Lomen‐Hoerth C, Hauer KE. Professional identity formation in disorienting times. Med Educ. 2020;54(8):765-766. https://doi.org/10.1111/medu.14202
21. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(9):572-573. https://doi.org/10.12788/jhm.3492
22. Beagan BL. Everyday classism in medical school: experiencing marginality and resistance. Med Educ. 2005;39(8):777-784. https://doi.org/10.1111/j.1365-2929.2005.02225.x
23. Jones Y, Durand V, Morton K, et al. Collateral damage: how COVID-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
© 2021 Society of Hospital Medicine
Creating Psychological Safety on Medical Teams in Times of Crisis
Hospitalized patients receive care via a team-based approach. Because of frequent turnover and constant changes in team members, medical teams require rapid establishment of psychological safety. Psychological safety, or “being able to show and employ one’s self without fear of negative consequences of self-image, status or career,”1 is at the core of successful team functioning. Google studied successful teams and found diverse personalities and skillsets work together most productively if they incorporate certain team dynamics; chief among these are focusing on shared values and psychological safety.2 Times of acute crisis, especially those in which clinicians are working in unfamiliar settings and with new teams, increase the need for psychological safety. During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, many hospitals responded by forming ad hoc teams of non-hospitalist clinicians, including redeployed outpatient physicians and subspecialists.3 Because this situation was an acute crisis in which strangers (some new to the field) were suddenly working side by side, it was an excellent example of a moment that required rapid establishment of psychological safety. As subsequent waves of COVID-19 arrive, this will likely occur again. In this perspective, we identify strategies that help to establish psychological safety on medical teams, aiming to increase the effectiveness of teams caring for hospitalized patients, enhance leaders’ abilities to improve team function, and allow for delivery of high-quality patient care.
WHY IS PSYCHOLOGICAL SAFETY IMPORTANT?
Psychological safety creates a nonthreatening team environment in which clinicians can ask questions and seek help with unfamiliar clinical scenarios. When psychological safety is present, the team dynamic encourages interpersonal risk-taking, improves learning, and increases the likelihood that team members will suggest new ideas.4 A culture of openness where people feel accepted and respected plays a vital role in helping people thrive in challenging and high-stakes work environments.5 In healthcare, team members who do not fear punishment for mistakes are more likely to disclose errors.6 Psychological safety has been associated with decreased anxiety in stressful situations, thereby freeing learners’ mental capacity to explore, innovate, and absorb new information.6
STRATEGIES TO IMPLEMENT PSYCHOLOGICAL SAFETY
Through a thorough literature review, we identified strategies that can increase psychological safety on clinical teams. We focused on strategies applicable to acute crises, like COVID-19, when dynamic teams and uncertainty are rife. These strategies primarily focus on “team leaders,” generally the attendings or senior residents, who influence the team’s culture.
Discuss Mistakes
Creating a culture in which openly discussing mistakes is normalized and learning is fostered is especially important for healthcare providers redeployed to COVID-19 wards. Acknowledging errors can be challenging, especially in medicine, because success is often celebrated.7 Creating an environment where discussing mistakes in a nonjudgmental manner is the norm helps people disclose and learn from errors. By modeling fallibility, team leaders can create an environment where learning from mistakes seems less threatening.8 Leaders can say, “I may miss something. I encourage all members of the team to share what they know.”9
Provide Frequent Updates and Seek Feedback
Information and guidelines are changing frequently as we learn more about the novel coronavirus. The barrage of new information and periodic policy changes can be disconcerting. Leaders can dispel some of the team’s anxiety by providing a unified message that distills new information into clear and essential updates.9 They can reassure the team that updates will be provided frequently, be honest about what is known, and offer some predictability by providing updates at set times via consistent forms of communication during times of crisis. They can show empathy by inquiring about individual worries and responding to concerns about changes that are being made in response to COVID-19.10 Leaders should routinely seek feedback. They can ask, “How are things going for you? What can we improve? What should we do differently? How can I make you feel more comfortable or help you learn more effectively?” Inviting input communicates that everyone’s opinion is respected and creates a climate where everyone feels comfortable asking questions or respectfully expressing diverging opinions.9
Foster Creativity and Seek New Ideas
Curiosity and creativity are associated with better group outcomes.11 Curiosity, or the motivation to learn and seek new ideas, improves individual and group dynamics by stimulating better job performance, inspiring leaders to discover more creative solutions, and encouraging employees to develop more trusting relationships, which makes them less likely to stereotype coworkers and patients as they ask questions and learn about others.12 People who approach situations with a more creative perspective are less likely to react defensively. Curious people tend to try to learn about and understand different points of view.13 For example, if an order is not placed for a patient, a curious team leader might think about why—was there disagreement on the order, confusion on how to place it, or was it inadvertently forgotten?—and be able help avoid similar scenarios in the future. This ability to see things from another person’s point of view helps individuals with diverse clinical, social, and ethnic backgrounds function as a harmonious team. An attitude of curiosity and interest in learning about what each person can contribute based on their unique training and personality will help well-functioning teams form in response to COVID-19. Openness to new ideas allows for more innovative solutions, which are important in times of crisis. To promote curiosity, team members should discuss differences and varied opinions openly. This open dialogue provides individuals opportunities to learn from each other.5
Build Connection and Trust
A culture of trust—the belief that others will act for the good of the team—helps create psychological safety.10 Leaders can build trust by making expectations clear, being consistent, being inclusive, and modeling behaviors they wish to encourage.6 Predictability reduces anxiety and promotes psychological safety.9 Defining goals and expectations helps people relax, ask questions, and focus on learning.14 In times of crisis, leaders can tell teams what changes to expect, spell out new priorities, and assign specific tasks to give people a way to contribute.15
Activities that create connection also build trust, enhancing the team’s sense of psychological safety. Shared experiences foster connections.2 Leaders can encourage team bonding by setting aside time to share stories and coping strategies.10 Chief residents in the early days of the pandemic found defining social distancing only as physical separation and focusing on emotional bonds helped maintain a sense of community. Debriefing about emotional patient encounters and discussing interesting clinical cases during video calls were ways to implement this strategy.10 Team members feel connected to each other and dedicated to their work if they focus on the meaning of the work to them, as well as its impact on society.2 This shared belief that what they are doing matters to their community helps bond them.2 Currently, the shared experience of treating a novel illness during the COVID-19 pandemic and the common goal of patient well-being unites healthcare providers across the globe. Leaders can create solidarity by emphasizing the shared identity of fighting COVID-19 and reminding teams of the impact of their work. Leaders can say, “Remember, we are here to improve patients’ health and form emotional bonds with people and their families.” These reminders have been shown to promote psychological safety and connection.16
Make Team Members Feel Valued
As many healthcare providers work harder or in unfamiliar environments during this pandemic, recognition of their efforts by leaders can be especially motivating and meaningful. When individuals on a team feel their work is valued, it helps create a sense of psychological safety.17 Employees feel valued when they believe their leaders care, they are in socially supportive environments, and are given resources for professional growth.17 Diversity and inclusion are also associated with feeling valued.17 During the height of the initial COVID-19 surge at our hospital, the chief of the Department of Medicine regularly sent messages and photographs of trainees and faculty, showing teams coming together during these unprecedented times. This boosted morale and created comradery and is an excellent example of a leader modeling inclusion.
Gratitude strengthens relationships and motivates people, especially when its expression is thoughtful and unique to individuals.18 Sincere compliments, acknowledgement of hard work, inclusiveness, and gratitude all contribute to team members feeling at ease and are key to leading, especially in times of crisis.17 Leaders can provide motivation by affirming the team’s ability to work together. Leaders can say, “I believe in each and every one of your capabilities—and I believe even more so in our joint capabilities. We can do this together.”19
CONCLUSION
Psychological safety is a powerful predictor of team performance, increased engagement, and satisfaction. It is critical for creating teams that can deal with uncertainty in high-risk situations, promoting a culture that is safe to acknowledge mistakes and take chances, which is important for optimal team functioning. Crises like the COVID-19 pandemic emphasize the need for psychologically safe team climates to promote learning, safe patient care, and team support. Hospitalists often care for patients when they are at their most vulnerable. Respecting and connecting with patients, through good and efficient teamwork, is important to providing effective care. The strategies suggested in this article strive to help hospitalists create a respectful culture to strengthen relationships with patients and colleagues in order to create an inclusive environment in times of crisis.
1. Kahn WA. Psychological conditions of personal engagement and disengagement at work. Acad Manage J. 1990;33(4):692-724. https://doi.org/10.5465/256287
2. Rozovsky J. The five keys to a successful Google team. re:Work. Posted November 17, 2015. Accessed October 11, 2020. https://rework.withgoogle.com/blog/five-keys-to-a-successful-google-team/
3. Hettle D, Sutherland K, Miles E, et al. Cross-skilling training to support medical redeployment in the COVID-19 pandemic. Future Healthc J. 2020:fhj.2020-0049. https://doi.org/10.7861/fhj.2020-0049
4. Edmondson AC, Lei Z. Psychological safety: the history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav. 2014;1(1):23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305
5. Edmondson A. Psychological safety and learning behavior in work teams. Admin Sci Quart.1999;44(2):350-383. https://doi.org/10.2307/2666999
6. Turner S, Harder N. Psychological safe environment: a concept analysis. Clin Simul Nurs. 2018;18:47-55. https://doi.org/10.1016/j.ecns.2018.02.004
7. Jug R, Jiang XS, Bean SM. Giving and receiving effective feedback: a review article and how-to guide. Arch Pathol Lab Med. 2019;143(2):244-250. https://doi.org/10.5858/arpa.2018-0058-RA
8. Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781. https://doi.org/10.1001/jama.1983.03340060055026
9. Edmondson AC, Woolley AW. Understanding outcomes of organizational learning interventions. In: Easterby-Smith M, Lyles M, eds. Blackwell Handbook of Organizational Learning and Knowledge Management. Blackwell Publishing; 2003.
10. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95(8):1152-1154. https://doi.org/10.1097/ACM.0000000000003419
11. Armstrong K. If you can’t beat it, join it: uncertainty and trust in medicine. Ann Intern Med. 2018;168(11):818-819. https://doi.org/10.7326/M18-0445
12. Gino F. The business case for curiosity. Harvard Bus Rev. 2018;96(5):48-57.
13. Kashdan TB, DeWall CN, Pond RS, et al. Curiosity protects against interpersonal aggression: cross-sectional, daily process, and behavioral evidence. J Pers. 2013;81(1):87-102. https://doi.org/10.1111/j.1467-6494.2012.00783.x
14. Epstein RM, Krasner MS. Physician resilience: what it means, why it matters, and how to promote it. Acad Med. 2013;88(3):301-303. https://doi.org/10.1097/ACM.0b013e318280cff0
15. Petriglieri G. The psychology behind effective crisis leadership. Harvard Bus Rev. Published April 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/the-psychology-behind-effective-crisis-leadership
16. Edmondson A. Building a psychologically safe workplace: TEDx Talk. May 4, 2014. Accessed October 11, 2020. https://youtube.com/watch?v=LhoLuui9gX8
17. Simpkin AL, Chang Y, Yu L, Campbell EG, Armstrong K, Walensky RP. Assessment of job satisfaction and feeling valued in academic medicine. JAMA Intern Med. 2019;179(7):992-994. https://doi.org/10.1001/jamainternmed.2019.0377
18. Nawaz S. In times of crisis, a little thanks goes a long way. Harvard Bus Rev. Published May 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/05/in-times-of-crisis-a-little-thanks-goes-a-long-way
19. Knight R. How to talk to your team when the future is uncertain. Harvard Bus Rev. Published April 20, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/how-to-talk-to-your-team-when-the-future-is-uncertain
Hospitalized patients receive care via a team-based approach. Because of frequent turnover and constant changes in team members, medical teams require rapid establishment of psychological safety. Psychological safety, or “being able to show and employ one’s self without fear of negative consequences of self-image, status or career,”1 is at the core of successful team functioning. Google studied successful teams and found diverse personalities and skillsets work together most productively if they incorporate certain team dynamics; chief among these are focusing on shared values and psychological safety.2 Times of acute crisis, especially those in which clinicians are working in unfamiliar settings and with new teams, increase the need for psychological safety. During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, many hospitals responded by forming ad hoc teams of non-hospitalist clinicians, including redeployed outpatient physicians and subspecialists.3 Because this situation was an acute crisis in which strangers (some new to the field) were suddenly working side by side, it was an excellent example of a moment that required rapid establishment of psychological safety. As subsequent waves of COVID-19 arrive, this will likely occur again. In this perspective, we identify strategies that help to establish psychological safety on medical teams, aiming to increase the effectiveness of teams caring for hospitalized patients, enhance leaders’ abilities to improve team function, and allow for delivery of high-quality patient care.
WHY IS PSYCHOLOGICAL SAFETY IMPORTANT?
Psychological safety creates a nonthreatening team environment in which clinicians can ask questions and seek help with unfamiliar clinical scenarios. When psychological safety is present, the team dynamic encourages interpersonal risk-taking, improves learning, and increases the likelihood that team members will suggest new ideas.4 A culture of openness where people feel accepted and respected plays a vital role in helping people thrive in challenging and high-stakes work environments.5 In healthcare, team members who do not fear punishment for mistakes are more likely to disclose errors.6 Psychological safety has been associated with decreased anxiety in stressful situations, thereby freeing learners’ mental capacity to explore, innovate, and absorb new information.6
STRATEGIES TO IMPLEMENT PSYCHOLOGICAL SAFETY
Through a thorough literature review, we identified strategies that can increase psychological safety on clinical teams. We focused on strategies applicable to acute crises, like COVID-19, when dynamic teams and uncertainty are rife. These strategies primarily focus on “team leaders,” generally the attendings or senior residents, who influence the team’s culture.
Discuss Mistakes
Creating a culture in which openly discussing mistakes is normalized and learning is fostered is especially important for healthcare providers redeployed to COVID-19 wards. Acknowledging errors can be challenging, especially in medicine, because success is often celebrated.7 Creating an environment where discussing mistakes in a nonjudgmental manner is the norm helps people disclose and learn from errors. By modeling fallibility, team leaders can create an environment where learning from mistakes seems less threatening.8 Leaders can say, “I may miss something. I encourage all members of the team to share what they know.”9
Provide Frequent Updates and Seek Feedback
Information and guidelines are changing frequently as we learn more about the novel coronavirus. The barrage of new information and periodic policy changes can be disconcerting. Leaders can dispel some of the team’s anxiety by providing a unified message that distills new information into clear and essential updates.9 They can reassure the team that updates will be provided frequently, be honest about what is known, and offer some predictability by providing updates at set times via consistent forms of communication during times of crisis. They can show empathy by inquiring about individual worries and responding to concerns about changes that are being made in response to COVID-19.10 Leaders should routinely seek feedback. They can ask, “How are things going for you? What can we improve? What should we do differently? How can I make you feel more comfortable or help you learn more effectively?” Inviting input communicates that everyone’s opinion is respected and creates a climate where everyone feels comfortable asking questions or respectfully expressing diverging opinions.9
Foster Creativity and Seek New Ideas
Curiosity and creativity are associated with better group outcomes.11 Curiosity, or the motivation to learn and seek new ideas, improves individual and group dynamics by stimulating better job performance, inspiring leaders to discover more creative solutions, and encouraging employees to develop more trusting relationships, which makes them less likely to stereotype coworkers and patients as they ask questions and learn about others.12 People who approach situations with a more creative perspective are less likely to react defensively. Curious people tend to try to learn about and understand different points of view.13 For example, if an order is not placed for a patient, a curious team leader might think about why—was there disagreement on the order, confusion on how to place it, or was it inadvertently forgotten?—and be able help avoid similar scenarios in the future. This ability to see things from another person’s point of view helps individuals with diverse clinical, social, and ethnic backgrounds function as a harmonious team. An attitude of curiosity and interest in learning about what each person can contribute based on their unique training and personality will help well-functioning teams form in response to COVID-19. Openness to new ideas allows for more innovative solutions, which are important in times of crisis. To promote curiosity, team members should discuss differences and varied opinions openly. This open dialogue provides individuals opportunities to learn from each other.5
Build Connection and Trust
A culture of trust—the belief that others will act for the good of the team—helps create psychological safety.10 Leaders can build trust by making expectations clear, being consistent, being inclusive, and modeling behaviors they wish to encourage.6 Predictability reduces anxiety and promotes psychological safety.9 Defining goals and expectations helps people relax, ask questions, and focus on learning.14 In times of crisis, leaders can tell teams what changes to expect, spell out new priorities, and assign specific tasks to give people a way to contribute.15
Activities that create connection also build trust, enhancing the team’s sense of psychological safety. Shared experiences foster connections.2 Leaders can encourage team bonding by setting aside time to share stories and coping strategies.10 Chief residents in the early days of the pandemic found defining social distancing only as physical separation and focusing on emotional bonds helped maintain a sense of community. Debriefing about emotional patient encounters and discussing interesting clinical cases during video calls were ways to implement this strategy.10 Team members feel connected to each other and dedicated to their work if they focus on the meaning of the work to them, as well as its impact on society.2 This shared belief that what they are doing matters to their community helps bond them.2 Currently, the shared experience of treating a novel illness during the COVID-19 pandemic and the common goal of patient well-being unites healthcare providers across the globe. Leaders can create solidarity by emphasizing the shared identity of fighting COVID-19 and reminding teams of the impact of their work. Leaders can say, “Remember, we are here to improve patients’ health and form emotional bonds with people and their families.” These reminders have been shown to promote psychological safety and connection.16
Make Team Members Feel Valued
As many healthcare providers work harder or in unfamiliar environments during this pandemic, recognition of their efforts by leaders can be especially motivating and meaningful. When individuals on a team feel their work is valued, it helps create a sense of psychological safety.17 Employees feel valued when they believe their leaders care, they are in socially supportive environments, and are given resources for professional growth.17 Diversity and inclusion are also associated with feeling valued.17 During the height of the initial COVID-19 surge at our hospital, the chief of the Department of Medicine regularly sent messages and photographs of trainees and faculty, showing teams coming together during these unprecedented times. This boosted morale and created comradery and is an excellent example of a leader modeling inclusion.
Gratitude strengthens relationships and motivates people, especially when its expression is thoughtful and unique to individuals.18 Sincere compliments, acknowledgement of hard work, inclusiveness, and gratitude all contribute to team members feeling at ease and are key to leading, especially in times of crisis.17 Leaders can provide motivation by affirming the team’s ability to work together. Leaders can say, “I believe in each and every one of your capabilities—and I believe even more so in our joint capabilities. We can do this together.”19
CONCLUSION
Psychological safety is a powerful predictor of team performance, increased engagement, and satisfaction. It is critical for creating teams that can deal with uncertainty in high-risk situations, promoting a culture that is safe to acknowledge mistakes and take chances, which is important for optimal team functioning. Crises like the COVID-19 pandemic emphasize the need for psychologically safe team climates to promote learning, safe patient care, and team support. Hospitalists often care for patients when they are at their most vulnerable. Respecting and connecting with patients, through good and efficient teamwork, is important to providing effective care. The strategies suggested in this article strive to help hospitalists create a respectful culture to strengthen relationships with patients and colleagues in order to create an inclusive environment in times of crisis.
Hospitalized patients receive care via a team-based approach. Because of frequent turnover and constant changes in team members, medical teams require rapid establishment of psychological safety. Psychological safety, or “being able to show and employ one’s self without fear of negative consequences of self-image, status or career,”1 is at the core of successful team functioning. Google studied successful teams and found diverse personalities and skillsets work together most productively if they incorporate certain team dynamics; chief among these are focusing on shared values and psychological safety.2 Times of acute crisis, especially those in which clinicians are working in unfamiliar settings and with new teams, increase the need for psychological safety. During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, many hospitals responded by forming ad hoc teams of non-hospitalist clinicians, including redeployed outpatient physicians and subspecialists.3 Because this situation was an acute crisis in which strangers (some new to the field) were suddenly working side by side, it was an excellent example of a moment that required rapid establishment of psychological safety. As subsequent waves of COVID-19 arrive, this will likely occur again. In this perspective, we identify strategies that help to establish psychological safety on medical teams, aiming to increase the effectiveness of teams caring for hospitalized patients, enhance leaders’ abilities to improve team function, and allow for delivery of high-quality patient care.
WHY IS PSYCHOLOGICAL SAFETY IMPORTANT?
Psychological safety creates a nonthreatening team environment in which clinicians can ask questions and seek help with unfamiliar clinical scenarios. When psychological safety is present, the team dynamic encourages interpersonal risk-taking, improves learning, and increases the likelihood that team members will suggest new ideas.4 A culture of openness where people feel accepted and respected plays a vital role in helping people thrive in challenging and high-stakes work environments.5 In healthcare, team members who do not fear punishment for mistakes are more likely to disclose errors.6 Psychological safety has been associated with decreased anxiety in stressful situations, thereby freeing learners’ mental capacity to explore, innovate, and absorb new information.6
STRATEGIES TO IMPLEMENT PSYCHOLOGICAL SAFETY
Through a thorough literature review, we identified strategies that can increase psychological safety on clinical teams. We focused on strategies applicable to acute crises, like COVID-19, when dynamic teams and uncertainty are rife. These strategies primarily focus on “team leaders,” generally the attendings or senior residents, who influence the team’s culture.
Discuss Mistakes
Creating a culture in which openly discussing mistakes is normalized and learning is fostered is especially important for healthcare providers redeployed to COVID-19 wards. Acknowledging errors can be challenging, especially in medicine, because success is often celebrated.7 Creating an environment where discussing mistakes in a nonjudgmental manner is the norm helps people disclose and learn from errors. By modeling fallibility, team leaders can create an environment where learning from mistakes seems less threatening.8 Leaders can say, “I may miss something. I encourage all members of the team to share what they know.”9
Provide Frequent Updates and Seek Feedback
Information and guidelines are changing frequently as we learn more about the novel coronavirus. The barrage of new information and periodic policy changes can be disconcerting. Leaders can dispel some of the team’s anxiety by providing a unified message that distills new information into clear and essential updates.9 They can reassure the team that updates will be provided frequently, be honest about what is known, and offer some predictability by providing updates at set times via consistent forms of communication during times of crisis. They can show empathy by inquiring about individual worries and responding to concerns about changes that are being made in response to COVID-19.10 Leaders should routinely seek feedback. They can ask, “How are things going for you? What can we improve? What should we do differently? How can I make you feel more comfortable or help you learn more effectively?” Inviting input communicates that everyone’s opinion is respected and creates a climate where everyone feels comfortable asking questions or respectfully expressing diverging opinions.9
Foster Creativity and Seek New Ideas
Curiosity and creativity are associated with better group outcomes.11 Curiosity, or the motivation to learn and seek new ideas, improves individual and group dynamics by stimulating better job performance, inspiring leaders to discover more creative solutions, and encouraging employees to develop more trusting relationships, which makes them less likely to stereotype coworkers and patients as they ask questions and learn about others.12 People who approach situations with a more creative perspective are less likely to react defensively. Curious people tend to try to learn about and understand different points of view.13 For example, if an order is not placed for a patient, a curious team leader might think about why—was there disagreement on the order, confusion on how to place it, or was it inadvertently forgotten?—and be able help avoid similar scenarios in the future. This ability to see things from another person’s point of view helps individuals with diverse clinical, social, and ethnic backgrounds function as a harmonious team. An attitude of curiosity and interest in learning about what each person can contribute based on their unique training and personality will help well-functioning teams form in response to COVID-19. Openness to new ideas allows for more innovative solutions, which are important in times of crisis. To promote curiosity, team members should discuss differences and varied opinions openly. This open dialogue provides individuals opportunities to learn from each other.5
Build Connection and Trust
A culture of trust—the belief that others will act for the good of the team—helps create psychological safety.10 Leaders can build trust by making expectations clear, being consistent, being inclusive, and modeling behaviors they wish to encourage.6 Predictability reduces anxiety and promotes psychological safety.9 Defining goals and expectations helps people relax, ask questions, and focus on learning.14 In times of crisis, leaders can tell teams what changes to expect, spell out new priorities, and assign specific tasks to give people a way to contribute.15
Activities that create connection also build trust, enhancing the team’s sense of psychological safety. Shared experiences foster connections.2 Leaders can encourage team bonding by setting aside time to share stories and coping strategies.10 Chief residents in the early days of the pandemic found defining social distancing only as physical separation and focusing on emotional bonds helped maintain a sense of community. Debriefing about emotional patient encounters and discussing interesting clinical cases during video calls were ways to implement this strategy.10 Team members feel connected to each other and dedicated to their work if they focus on the meaning of the work to them, as well as its impact on society.2 This shared belief that what they are doing matters to their community helps bond them.2 Currently, the shared experience of treating a novel illness during the COVID-19 pandemic and the common goal of patient well-being unites healthcare providers across the globe. Leaders can create solidarity by emphasizing the shared identity of fighting COVID-19 and reminding teams of the impact of their work. Leaders can say, “Remember, we are here to improve patients’ health and form emotional bonds with people and their families.” These reminders have been shown to promote psychological safety and connection.16
Make Team Members Feel Valued
As many healthcare providers work harder or in unfamiliar environments during this pandemic, recognition of their efforts by leaders can be especially motivating and meaningful. When individuals on a team feel their work is valued, it helps create a sense of psychological safety.17 Employees feel valued when they believe their leaders care, they are in socially supportive environments, and are given resources for professional growth.17 Diversity and inclusion are also associated with feeling valued.17 During the height of the initial COVID-19 surge at our hospital, the chief of the Department of Medicine regularly sent messages and photographs of trainees and faculty, showing teams coming together during these unprecedented times. This boosted morale and created comradery and is an excellent example of a leader modeling inclusion.
Gratitude strengthens relationships and motivates people, especially when its expression is thoughtful and unique to individuals.18 Sincere compliments, acknowledgement of hard work, inclusiveness, and gratitude all contribute to team members feeling at ease and are key to leading, especially in times of crisis.17 Leaders can provide motivation by affirming the team’s ability to work together. Leaders can say, “I believe in each and every one of your capabilities—and I believe even more so in our joint capabilities. We can do this together.”19
CONCLUSION
Psychological safety is a powerful predictor of team performance, increased engagement, and satisfaction. It is critical for creating teams that can deal with uncertainty in high-risk situations, promoting a culture that is safe to acknowledge mistakes and take chances, which is important for optimal team functioning. Crises like the COVID-19 pandemic emphasize the need for psychologically safe team climates to promote learning, safe patient care, and team support. Hospitalists often care for patients when they are at their most vulnerable. Respecting and connecting with patients, through good and efficient teamwork, is important to providing effective care. The strategies suggested in this article strive to help hospitalists create a respectful culture to strengthen relationships with patients and colleagues in order to create an inclusive environment in times of crisis.
1. Kahn WA. Psychological conditions of personal engagement and disengagement at work. Acad Manage J. 1990;33(4):692-724. https://doi.org/10.5465/256287
2. Rozovsky J. The five keys to a successful Google team. re:Work. Posted November 17, 2015. Accessed October 11, 2020. https://rework.withgoogle.com/blog/five-keys-to-a-successful-google-team/
3. Hettle D, Sutherland K, Miles E, et al. Cross-skilling training to support medical redeployment in the COVID-19 pandemic. Future Healthc J. 2020:fhj.2020-0049. https://doi.org/10.7861/fhj.2020-0049
4. Edmondson AC, Lei Z. Psychological safety: the history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav. 2014;1(1):23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305
5. Edmondson A. Psychological safety and learning behavior in work teams. Admin Sci Quart.1999;44(2):350-383. https://doi.org/10.2307/2666999
6. Turner S, Harder N. Psychological safe environment: a concept analysis. Clin Simul Nurs. 2018;18:47-55. https://doi.org/10.1016/j.ecns.2018.02.004
7. Jug R, Jiang XS, Bean SM. Giving and receiving effective feedback: a review article and how-to guide. Arch Pathol Lab Med. 2019;143(2):244-250. https://doi.org/10.5858/arpa.2018-0058-RA
8. Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781. https://doi.org/10.1001/jama.1983.03340060055026
9. Edmondson AC, Woolley AW. Understanding outcomes of organizational learning interventions. In: Easterby-Smith M, Lyles M, eds. Blackwell Handbook of Organizational Learning and Knowledge Management. Blackwell Publishing; 2003.
10. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95(8):1152-1154. https://doi.org/10.1097/ACM.0000000000003419
11. Armstrong K. If you can’t beat it, join it: uncertainty and trust in medicine. Ann Intern Med. 2018;168(11):818-819. https://doi.org/10.7326/M18-0445
12. Gino F. The business case for curiosity. Harvard Bus Rev. 2018;96(5):48-57.
13. Kashdan TB, DeWall CN, Pond RS, et al. Curiosity protects against interpersonal aggression: cross-sectional, daily process, and behavioral evidence. J Pers. 2013;81(1):87-102. https://doi.org/10.1111/j.1467-6494.2012.00783.x
14. Epstein RM, Krasner MS. Physician resilience: what it means, why it matters, and how to promote it. Acad Med. 2013;88(3):301-303. https://doi.org/10.1097/ACM.0b013e318280cff0
15. Petriglieri G. The psychology behind effective crisis leadership. Harvard Bus Rev. Published April 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/the-psychology-behind-effective-crisis-leadership
16. Edmondson A. Building a psychologically safe workplace: TEDx Talk. May 4, 2014. Accessed October 11, 2020. https://youtube.com/watch?v=LhoLuui9gX8
17. Simpkin AL, Chang Y, Yu L, Campbell EG, Armstrong K, Walensky RP. Assessment of job satisfaction and feeling valued in academic medicine. JAMA Intern Med. 2019;179(7):992-994. https://doi.org/10.1001/jamainternmed.2019.0377
18. Nawaz S. In times of crisis, a little thanks goes a long way. Harvard Bus Rev. Published May 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/05/in-times-of-crisis-a-little-thanks-goes-a-long-way
19. Knight R. How to talk to your team when the future is uncertain. Harvard Bus Rev. Published April 20, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/how-to-talk-to-your-team-when-the-future-is-uncertain
1. Kahn WA. Psychological conditions of personal engagement and disengagement at work. Acad Manage J. 1990;33(4):692-724. https://doi.org/10.5465/256287
2. Rozovsky J. The five keys to a successful Google team. re:Work. Posted November 17, 2015. Accessed October 11, 2020. https://rework.withgoogle.com/blog/five-keys-to-a-successful-google-team/
3. Hettle D, Sutherland K, Miles E, et al. Cross-skilling training to support medical redeployment in the COVID-19 pandemic. Future Healthc J. 2020:fhj.2020-0049. https://doi.org/10.7861/fhj.2020-0049
4. Edmondson AC, Lei Z. Psychological safety: the history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav. 2014;1(1):23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305
5. Edmondson A. Psychological safety and learning behavior in work teams. Admin Sci Quart.1999;44(2):350-383. https://doi.org/10.2307/2666999
6. Turner S, Harder N. Psychological safe environment: a concept analysis. Clin Simul Nurs. 2018;18:47-55. https://doi.org/10.1016/j.ecns.2018.02.004
7. Jug R, Jiang XS, Bean SM. Giving and receiving effective feedback: a review article and how-to guide. Arch Pathol Lab Med. 2019;143(2):244-250. https://doi.org/10.5858/arpa.2018-0058-RA
8. Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781. https://doi.org/10.1001/jama.1983.03340060055026
9. Edmondson AC, Woolley AW. Understanding outcomes of organizational learning interventions. In: Easterby-Smith M, Lyles M, eds. Blackwell Handbook of Organizational Learning and Knowledge Management. Blackwell Publishing; 2003.
10. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95(8):1152-1154. https://doi.org/10.1097/ACM.0000000000003419
11. Armstrong K. If you can’t beat it, join it: uncertainty and trust in medicine. Ann Intern Med. 2018;168(11):818-819. https://doi.org/10.7326/M18-0445
12. Gino F. The business case for curiosity. Harvard Bus Rev. 2018;96(5):48-57.
13. Kashdan TB, DeWall CN, Pond RS, et al. Curiosity protects against interpersonal aggression: cross-sectional, daily process, and behavioral evidence. J Pers. 2013;81(1):87-102. https://doi.org/10.1111/j.1467-6494.2012.00783.x
14. Epstein RM, Krasner MS. Physician resilience: what it means, why it matters, and how to promote it. Acad Med. 2013;88(3):301-303. https://doi.org/10.1097/ACM.0b013e318280cff0
15. Petriglieri G. The psychology behind effective crisis leadership. Harvard Bus Rev. Published April 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/the-psychology-behind-effective-crisis-leadership
16. Edmondson A. Building a psychologically safe workplace: TEDx Talk. May 4, 2014. Accessed October 11, 2020. https://youtube.com/watch?v=LhoLuui9gX8
17. Simpkin AL, Chang Y, Yu L, Campbell EG, Armstrong K, Walensky RP. Assessment of job satisfaction and feeling valued in academic medicine. JAMA Intern Med. 2019;179(7):992-994. https://doi.org/10.1001/jamainternmed.2019.0377
18. Nawaz S. In times of crisis, a little thanks goes a long way. Harvard Bus Rev. Published May 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/05/in-times-of-crisis-a-little-thanks-goes-a-long-way
19. Knight R. How to talk to your team when the future is uncertain. Harvard Bus Rev. Published April 20, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/how-to-talk-to-your-team-when-the-future-is-uncertain
© 2021 Society of Hospital Medicine
Electronic Reminders Extend the Reach of Health Care
Many health care providers (HCPs) view the US Department of Veterans Affairs (VA) system of electronic reminders as a model. User experience and improvements that make clinical life easier (like automated text messaging, which requires no hands-on staff involvement) have brought more HCPs into the fold. And during a viral pandemic, preventive care is ever more important, as are the ways to provide it. But a recent Centers for Disease Control and Prevention (CDC) study shows some non-VA providers have some catching up to do.
Although the CDC researchers noted that electronic reminders can improve preventive and follow-up care, they also pointed out that HCPs must first have the computing capabilities to accomplish this. They analyzed 2017 data (the most recent available) from the National Electronic Health Records Survey of > 10,000 physicians and found only 65% of office-based physicians did.
Not surprisingly, practices that used electronic health record (EHR) systems were more than 3 times as likely to also have computerized capability to identify patients who needed preventive care or follow-up (71% vs 23% of practices without EHR). Primary care physicians were more likely than surgeons and other nonprimary care physicians to have the capability (73% vs 55% and 59%, respectively). Age also entered into it, with 70% of physicians aged between 45 and 54 years having the capability, compared with 57% of those aged 65 to 84 years. Offices with multiple physicians were more likely to have computerized capability.
The VA began using computerized clinical reminders 20 years ago to encourage patients to take better care of themselves to, for example, moderate alcohol use, manage cholesterol, or stop smoking. In 2006, the Veterans Health Information Systems and Technology Architecture (VistA) won an Innovations in American Government Award from Harvard University. The committee called VistA innovative because of its “unique linkage with standardized, consistent performance measurement.” VistA, the committee said, “substantially improves efficiency, reduces costs and demonstrably improves clinical decision-making.”
However, when the VA was getting its electronic reminder system up to speed, not all users were comfortable with it. Researchers who studied uptake of a system that sent reminders about lipid management to patients with ischemic heart disease found “substantial barriers” to implementation, including a possibly significant effect of “prior culture and attitudes” toward reminders.
Four years after the VA began using computerized reminders, attendees at “Camp CPRS,” a week-long meeting to train employees in the Computerized Patient Record System, were asked about facilitation and barriers. More than half of respondents could report at least 1 situation in which reminders helped them deliver care more effectively. But “[w]hile the potential benefits of such a system are significant,” the researchers said, “and in fact some VA hospitals are showing an increase in compliance with some best practices…it is generally understood that some providers within the VA do not use the clinical reminders.” Some HCPs said they were hard to use and cited insufficient training.
Experience and consistent use pay off, though. For instance, researchers from the VA Puget Sound Health Care System in Washington evaluated the effectiveness of an electronic clinical reminder for brief alcohol counseling at 8 VA sites. They wanted to determine how often the HCPs used the reminder, and whether it helped patients resolve unhealthy alcohol use. The study, involving 4,198 participants who screened positive for alcohol use, found 71% of the patients had the clinical reminder documented in the EHR—a high rate, the researchers noted, relative to other studies. The results were similar across the 2-year period, even in the first 8 months.
Sustainability also is a factor. At the time of their study, the researchers said, no health care system had achieved sustained implementation of brief alcohol counseling for patients who screened positive. Moreover, the patients who had reminders were significantly more likely to report having resolved unhealthy alcohol use at follow-up.
Do electronic daily reminders really improve adherence? Valentin Rivish, DNP, RN, NE-BC, telehealth specialist and facility e-consult coordinator with the Phoenix VA Health Care System in Arizona, wanted to see what evidence exists on telehealth adherence and utilization. He enlisted 40 veterans whose home-telehealth response rates were < 70%. Over 4 weeks, the veterans received an electronic daily reminder sent to their home-telehealth device, with the goal of having them respond daily.
As Rivish expected, daily reminders did improve adherence. After 4 weeks, 24 participants (60%) showed an increased response rate, and 14 (35%) achieved at least a 70% response rate pos-intervention. As a result, the Phoenix telehealth department has included the cost-effective intervention in its standard operating procedure.
The VA has continued to add to its repertoire of ways to stay in touch with patients. In 2018, for instance, it launched VEText, a text messaging appointment-reminder system. According to the Veterans Health Administration Office of Veterans Access to Care, in just the first few months more than 3.24 million patients had received VEText messages (and had canceled 319,504 appointments, freeing up time slots for other veterans).
This year, the VA, US Department of Defense, and US Coast Guard launched a joint health information exchange (HIE) that allows partners to quickly and securely share EHR data bidirectionally with participating community healthcare providers. To that end, the 46,000-member HIE is collaborating with the CommonWell Health Alliance, adding a nationwide network of more than 15,000 hospitals and clinics.
“As a clinician who is using the joint HIE, the more patient information I have access to, the more I can understand the full picture of my patients’ care and better meet their needs,” says Dr. Neil Evans, a VA primary care physician and clinical leader with the Federal Electronic Health Record Modernization office. “During the COVID-19 pandemic, efficient electronic health information is more important than ever.”
Many health care providers (HCPs) view the US Department of Veterans Affairs (VA) system of electronic reminders as a model. User experience and improvements that make clinical life easier (like automated text messaging, which requires no hands-on staff involvement) have brought more HCPs into the fold. And during a viral pandemic, preventive care is ever more important, as are the ways to provide it. But a recent Centers for Disease Control and Prevention (CDC) study shows some non-VA providers have some catching up to do.
Although the CDC researchers noted that electronic reminders can improve preventive and follow-up care, they also pointed out that HCPs must first have the computing capabilities to accomplish this. They analyzed 2017 data (the most recent available) from the National Electronic Health Records Survey of > 10,000 physicians and found only 65% of office-based physicians did.
Not surprisingly, practices that used electronic health record (EHR) systems were more than 3 times as likely to also have computerized capability to identify patients who needed preventive care or follow-up (71% vs 23% of practices without EHR). Primary care physicians were more likely than surgeons and other nonprimary care physicians to have the capability (73% vs 55% and 59%, respectively). Age also entered into it, with 70% of physicians aged between 45 and 54 years having the capability, compared with 57% of those aged 65 to 84 years. Offices with multiple physicians were more likely to have computerized capability.
The VA began using computerized clinical reminders 20 years ago to encourage patients to take better care of themselves to, for example, moderate alcohol use, manage cholesterol, or stop smoking. In 2006, the Veterans Health Information Systems and Technology Architecture (VistA) won an Innovations in American Government Award from Harvard University. The committee called VistA innovative because of its “unique linkage with standardized, consistent performance measurement.” VistA, the committee said, “substantially improves efficiency, reduces costs and demonstrably improves clinical decision-making.”
However, when the VA was getting its electronic reminder system up to speed, not all users were comfortable with it. Researchers who studied uptake of a system that sent reminders about lipid management to patients with ischemic heart disease found “substantial barriers” to implementation, including a possibly significant effect of “prior culture and attitudes” toward reminders.
Four years after the VA began using computerized reminders, attendees at “Camp CPRS,” a week-long meeting to train employees in the Computerized Patient Record System, were asked about facilitation and barriers. More than half of respondents could report at least 1 situation in which reminders helped them deliver care more effectively. But “[w]hile the potential benefits of such a system are significant,” the researchers said, “and in fact some VA hospitals are showing an increase in compliance with some best practices…it is generally understood that some providers within the VA do not use the clinical reminders.” Some HCPs said they were hard to use and cited insufficient training.
Experience and consistent use pay off, though. For instance, researchers from the VA Puget Sound Health Care System in Washington evaluated the effectiveness of an electronic clinical reminder for brief alcohol counseling at 8 VA sites. They wanted to determine how often the HCPs used the reminder, and whether it helped patients resolve unhealthy alcohol use. The study, involving 4,198 participants who screened positive for alcohol use, found 71% of the patients had the clinical reminder documented in the EHR—a high rate, the researchers noted, relative to other studies. The results were similar across the 2-year period, even in the first 8 months.
Sustainability also is a factor. At the time of their study, the researchers said, no health care system had achieved sustained implementation of brief alcohol counseling for patients who screened positive. Moreover, the patients who had reminders were significantly more likely to report having resolved unhealthy alcohol use at follow-up.
Do electronic daily reminders really improve adherence? Valentin Rivish, DNP, RN, NE-BC, telehealth specialist and facility e-consult coordinator with the Phoenix VA Health Care System in Arizona, wanted to see what evidence exists on telehealth adherence and utilization. He enlisted 40 veterans whose home-telehealth response rates were < 70%. Over 4 weeks, the veterans received an electronic daily reminder sent to their home-telehealth device, with the goal of having them respond daily.
As Rivish expected, daily reminders did improve adherence. After 4 weeks, 24 participants (60%) showed an increased response rate, and 14 (35%) achieved at least a 70% response rate pos-intervention. As a result, the Phoenix telehealth department has included the cost-effective intervention in its standard operating procedure.
The VA has continued to add to its repertoire of ways to stay in touch with patients. In 2018, for instance, it launched VEText, a text messaging appointment-reminder system. According to the Veterans Health Administration Office of Veterans Access to Care, in just the first few months more than 3.24 million patients had received VEText messages (and had canceled 319,504 appointments, freeing up time slots for other veterans).
This year, the VA, US Department of Defense, and US Coast Guard launched a joint health information exchange (HIE) that allows partners to quickly and securely share EHR data bidirectionally with participating community healthcare providers. To that end, the 46,000-member HIE is collaborating with the CommonWell Health Alliance, adding a nationwide network of more than 15,000 hospitals and clinics.
“As a clinician who is using the joint HIE, the more patient information I have access to, the more I can understand the full picture of my patients’ care and better meet their needs,” says Dr. Neil Evans, a VA primary care physician and clinical leader with the Federal Electronic Health Record Modernization office. “During the COVID-19 pandemic, efficient electronic health information is more important than ever.”
Many health care providers (HCPs) view the US Department of Veterans Affairs (VA) system of electronic reminders as a model. User experience and improvements that make clinical life easier (like automated text messaging, which requires no hands-on staff involvement) have brought more HCPs into the fold. And during a viral pandemic, preventive care is ever more important, as are the ways to provide it. But a recent Centers for Disease Control and Prevention (CDC) study shows some non-VA providers have some catching up to do.
Although the CDC researchers noted that electronic reminders can improve preventive and follow-up care, they also pointed out that HCPs must first have the computing capabilities to accomplish this. They analyzed 2017 data (the most recent available) from the National Electronic Health Records Survey of > 10,000 physicians and found only 65% of office-based physicians did.
Not surprisingly, practices that used electronic health record (EHR) systems were more than 3 times as likely to also have computerized capability to identify patients who needed preventive care or follow-up (71% vs 23% of practices without EHR). Primary care physicians were more likely than surgeons and other nonprimary care physicians to have the capability (73% vs 55% and 59%, respectively). Age also entered into it, with 70% of physicians aged between 45 and 54 years having the capability, compared with 57% of those aged 65 to 84 years. Offices with multiple physicians were more likely to have computerized capability.
The VA began using computerized clinical reminders 20 years ago to encourage patients to take better care of themselves to, for example, moderate alcohol use, manage cholesterol, or stop smoking. In 2006, the Veterans Health Information Systems and Technology Architecture (VistA) won an Innovations in American Government Award from Harvard University. The committee called VistA innovative because of its “unique linkage with standardized, consistent performance measurement.” VistA, the committee said, “substantially improves efficiency, reduces costs and demonstrably improves clinical decision-making.”
However, when the VA was getting its electronic reminder system up to speed, not all users were comfortable with it. Researchers who studied uptake of a system that sent reminders about lipid management to patients with ischemic heart disease found “substantial barriers” to implementation, including a possibly significant effect of “prior culture and attitudes” toward reminders.
Four years after the VA began using computerized reminders, attendees at “Camp CPRS,” a week-long meeting to train employees in the Computerized Patient Record System, were asked about facilitation and barriers. More than half of respondents could report at least 1 situation in which reminders helped them deliver care more effectively. But “[w]hile the potential benefits of such a system are significant,” the researchers said, “and in fact some VA hospitals are showing an increase in compliance with some best practices…it is generally understood that some providers within the VA do not use the clinical reminders.” Some HCPs said they were hard to use and cited insufficient training.
Experience and consistent use pay off, though. For instance, researchers from the VA Puget Sound Health Care System in Washington evaluated the effectiveness of an electronic clinical reminder for brief alcohol counseling at 8 VA sites. They wanted to determine how often the HCPs used the reminder, and whether it helped patients resolve unhealthy alcohol use. The study, involving 4,198 participants who screened positive for alcohol use, found 71% of the patients had the clinical reminder documented in the EHR—a high rate, the researchers noted, relative to other studies. The results were similar across the 2-year period, even in the first 8 months.
Sustainability also is a factor. At the time of their study, the researchers said, no health care system had achieved sustained implementation of brief alcohol counseling for patients who screened positive. Moreover, the patients who had reminders were significantly more likely to report having resolved unhealthy alcohol use at follow-up.
Do electronic daily reminders really improve adherence? Valentin Rivish, DNP, RN, NE-BC, telehealth specialist and facility e-consult coordinator with the Phoenix VA Health Care System in Arizona, wanted to see what evidence exists on telehealth adherence and utilization. He enlisted 40 veterans whose home-telehealth response rates were < 70%. Over 4 weeks, the veterans received an electronic daily reminder sent to their home-telehealth device, with the goal of having them respond daily.
As Rivish expected, daily reminders did improve adherence. After 4 weeks, 24 participants (60%) showed an increased response rate, and 14 (35%) achieved at least a 70% response rate pos-intervention. As a result, the Phoenix telehealth department has included the cost-effective intervention in its standard operating procedure.
The VA has continued to add to its repertoire of ways to stay in touch with patients. In 2018, for instance, it launched VEText, a text messaging appointment-reminder system. According to the Veterans Health Administration Office of Veterans Access to Care, in just the first few months more than 3.24 million patients had received VEText messages (and had canceled 319,504 appointments, freeing up time slots for other veterans).
This year, the VA, US Department of Defense, and US Coast Guard launched a joint health information exchange (HIE) that allows partners to quickly and securely share EHR data bidirectionally with participating community healthcare providers. To that end, the 46,000-member HIE is collaborating with the CommonWell Health Alliance, adding a nationwide network of more than 15,000 hospitals and clinics.
“As a clinician who is using the joint HIE, the more patient information I have access to, the more I can understand the full picture of my patients’ care and better meet their needs,” says Dr. Neil Evans, a VA primary care physician and clinical leader with the Federal Electronic Health Record Modernization office. “During the COVID-19 pandemic, efficient electronic health information is more important than ever.”
Guideline Concordance with Durvalumab in Unresectable Stage III Non-Small Cell Lung Cancer: A Single Center Veterans Hospital Experience
The US Food and Drug Administration (FDA) approved the use of durvalumab for patients with unresectable stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (CRT).1 After 2 randomized phase 3 studies in 2017 and 2018 showed significant progression-free and overall survival respectively,2,3 durvalumab became a category 1 recommendation for the above indication per National Comprehensive Cancer Network (NCCN) guidelines.4 Adherence to guidelines have been shown to improve patient survival across several cancer types.5-7 However, guideline adherence rates have been variable across health institutions. Therefore, further study is warranted to evaluate nonadherent practices with the goal of improving the quality of cancer care delivery.8,9
Stage III NSCLC is associated with poor survival rates.10 Concurrent CRT remains the standard of care in patients with good performance status based on clinical trial populations.4 Lung cancer remains a disease of the elderly, with a median age at diagnosis of 70 years.11 Discrepancies in the treatment of lung cancer in older adults can vary widely due to a lack of evidence surrounding the treatment in those who have comorbidities and poor performance status, widening the gap between clinical trial and real-world populations.11
A recent review by Passaro and colleagues revealed that at least 11 pivotal randomized controlled trials have shown the activity of immune checkpoint inhibitors (ICI) in locally advanced and metastatic lung cancer. However, these studies have mostly excluded patients with a performance status of the Eastern Cooperative Oncology Group (ECOG) level ≥ 2.11
Durvalumab is one of many new therapies to enter clinical practice to demonstrate survival benefit, but its use among veterans with stage III NSCLC in adherence with National Comprehensive Cancer Network (NCCN) guidelines was not robust at the Birmingham Veterans Affairs Medical Center (VAMC) in Alabama. Therefore, we decided to study the level of adherence and to identify barriers to conformity to the category 1 NCCN recommendations.
Methods
The Birmingham VAMC Outpatient Oncology Clinic billing data identified all individuals diagnosed with lung cancer treated between October 2017 and August 2019. Patients who did not have NSCLC that was stage III and unresectable were excluded from our study. Patients who did not receive a majority of their treatment at US Department of Veterans Affairs (VA) facilities were excluded as well. Each patient’s demographic, functional level, and tumor characteristics during the treatment planning phase and follow-up visits were obtained. Two investigators who evaluated health care provider documentation using the VA Computerized Patient Record System (CPRS) conducted chart reviews.
The primary outcomes were the proportion of patients who received concurrent CRT and the proportion who received durvalumab consolidation. Our chart review also categorized reasons for nonreceipt of concurrent CRT and subsequent durvalumab. Documented reasons for guideline discordancy were generated empirically and broadly. We noted if documentation was unclear and included reasons for why a veteran was not a candidate for CRT, the presence of toxicities associated with CRT, and a patient’s refusal for therapy despite medical advice. Descriptive data were analyzed for all clinical or demographic characteristics and outcomes.
This was considered an internal quality improvement initiative. As such, Birmingham VAMC did not require institutional review board approval for the study. The facility is accredited by the American College of Surgeons Commission on Cancer.
Results
A total of 41 veterans with stage III NSCLC were identified to have established care in the Birmingham VAMC Oncology Clinic between October 2017 and August 2019. Of these, 7 received the majority of their treatment from community-based non-VA facilities and 14 were not candidates for CRT and were excluded from this study.
The mean (SD) age of study participants was 70.0 (8.4) years (range, 57 to 92 years). Most of the study veterans (33; 97.1%) were male and 20 (58.8%) were African American (Table). Eighteen (53%) of study participants had clinical stage IIIa NSCLC; 19 (56%) showed a squamous subtype of NSCLC. A majority (53%) of the veterans studied were evaluated to be functionally fit with an ECOG status of 0 to 1, although documentation of ECOG status was lacking in 5 (14.7%) patients in the initial treatment planning visit records. It was unclear if performance status had been reevaluated and changes noted over the course of concurrent CRT.
CRT Patients
The relative distribution of veterans who underwent CRT for stage III NSCLC plus the reasons they did not receive guideline-based treatment with durvalumab is shown in the Figure. Fourteen patients (41%) were inappropriate candidates for CRT; the most common reason for this was their poor performance status upon initial evaluation and 3 patients (8.8%) in the study had extensive disease or were upstaged upon follow-up clinic visit.
Twenty (59%) veterans in the study initiated CRT. However, only 16 (47.1%) completed CRT. Those who dropped out of CRT did so because of toxicities that included various cytopenia, gastrointestinal toxicities due to radiation and/or chemotherapy, or failure to thrive.
Durvalumab Treatment
After initiation of CRT, 9 (26.5%) patients did not go on to receive durvalumab. Three patients (8.8%) suffered toxicities during CRT. One study patient was found to have a severe respiratory infection requiring intensive care unit admission. Another study patient was found to have a new sternal lesion on follow-up positron emission tomography. One declined because of a history of severe antineutrophil cytoplasmic antibodies vasculitis, which made durvalumab use unsafe. Three patients (8.8%) declined treatment with CRT or durvalumab because of personal preference. Documentation was unclear as to why durvalumab was prescribed to one patient who had completed CRT.
Discussion
NCCN guidelines on the use of durvalumab in NSCLC are based on the phase 3 PACIFIC placebo-controlled randomized clinical trial. This trial, which included only patients with documented performance status of ECOG 0 or 1, reported that grade 3 or 4 events occurred in 30.5% of patients randomized to consolidative durvalumab. Treatment was discontinued in 15.4% of patients due to adverse events.3
Our study examined consolidation therapy with durvalumab in patients with unresectable stage III NSCLC with an ECOG performance status of 0 to 1 who had not progressed after 2 or more cycles of definitive concurrent CRT.4 Patients with previous exposure to immunotherapy, a history of immunodeficiency, active infection, unresolved toxicity from CRT, autoimmune disease, and patients who received sequential CRT were excluded.2 Surprisingly, the adherence rate to guidelines was close to 100% with appropriate documentation and justification of CRT initiation and durvalumab use. Five (14.7%) of veterans with unresectable stage III NSCLC did not have clear documentation of ECOG status on initial visit and only 1 veteran who completed CRT did not have clear documentation as to why durvalumab was not provided. Unfortunately, 23 (68.6%) veterans in the study were unable to receive durvalumab, a potentially disease-modifying drug; nearly one-third (10) of veterans were deemed poor candidates for concurrent CRT despite the fact that 52.9% (18) of veterans in the study had a documented ECOG of 0 or 1 on initial evaluation.
Clinical Trials vs Real World
The heterogeneity between anticipated study populations, those who were able to receive durvalumab in the PACIFIC trial, compared with our observed real-world veteran population, likely stems from the lack of information about how comorbidity and fitness can affect the choice of therapeutic intervention in patients with lung cancer.12 In addition, older adults who participated in randomized controlled trials (RCTs) are not representative of the average older adult who presents to medical oncology clinics, making the application of guideline concordant care difficult.13
Similar real-world observations parallel to our analyses have confirmed, complemented and/or refuted findings of RCTs, and have helped impact the treatment of multiple acute and chronic conditions including influenza, cardiovascular disease, and diabetes.14
A component of socioeconomic barriers and access to supportive care played roles in the decisions of certain patients who chose not to undergo concurrent CRT despite medical advice. These 2 obstacles also affected the decision making for some in the study when considering the use of durvalumab (administered by a 60-minute IV infusion every 2 weeks for 1 year) per recommended guidelines.1 These hurdles need further study in the context of their effect on quality of life and the difficulties generated by various social determinants of health.
Limitations
Study limitations included the biased and confounding factors previously described about retrospective and nonrandomized observational studies that are controlled for during RCTs.15 Electronic health record data may have been incorrectly collected resulting in missing or wrong data points that affect the validity of our conclusion. Recall bias with regard to documentation by health care providers describing reasons why CRT or durvalumab were not initiated or the patient’s ability to recall previous treatments and report ECOG status or toxicities also may have impacted our findings. Comorbidities and poor performance status, frequently occurring among veterans, negatively impact cancer treatment decisions and may result in a detection bias. For example, tobacco use, cardiovascular disease, including heart failure, and chronic obstructive pulmonary disease, are notoriously higher in the US veteran population when compared with civilian cohorts.16-18 Also, veterans with poorly controlled depression and posttraumatic stress disorder resulting in functional impairment are a factor.19 Steps were taken to address some of these biases by performing repeat checks of tabulated data and employing 2 independent reviewers to evaluate all relevant clinical documentation, compare results, and reach a consensus.
Conlcusions
This retrospective analysis of adherence to category 1 NCCN guidelines for durvalumab use among patients at the Birmingham VAMC Oncology Clinic reinforced our practice and identified minor deficiencies in documentation that would impact future clinical visits. More importantly, it depicted the massive disparity in treatment candidacy among Birmingham veterans compared with clinical trial populations. Efforts will be made to address factors impacting a veteran’s candidacy for CRT and explore other variables such as socioeconomic barriers to treatment. Multiple complementary tools to assess patients’ frailty, such as the Charlson Comorbidity Index (CCI), are now being used for a variety of disorders including cancers. More robust data and standardization are needed to validate the use of these assessments in predicting response to immune checkpoint inhibitors.
Immune checkpoint inhibitors are currently being evaluated in stage III NSCLC studies and may be implemented as routine practice in the future.12 It is important to distinguish fit from frail veterans with lung cancer for treatment selection. We would like to see the expansion of the eligibility criteria for clinical trials to include patients with a performance status of ECOG 2 in order for results to be truly generalizable to the real-world population. Our hope is that such work will improve not only the quality of lung cancer care, but also the quality of care across multiple tumor types.
1. US Food and Drug Administration. FDA approves durvalumab after chemoradiation for unresectable stage II. Published February 20, 2018. Accessed October 9, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-after-chemoradiation-unresectable-stage-iii-nsclc
2. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi:10.1056/NEJMoa1709937
3. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi:10.1056/NEJMoa1809697
4. Ettinger DS, Wood DE, Aisner DL et al. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version8.2020. Updated September 15, 2020. Accessed October 9, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
5. Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220(5):940-950. doi:10.1016/j.jamcollsurg.2015.01.056
6. Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593-1601. doi:10.1002/cncr.27935
7. Schwentner L, Wöckel A, König J, et al. Adherence to treatment guidelines and survival in triple-negative breast cancer: a retrospective multi-center cohort study with 9,156 patients. BMC Cancer. 2013;13:487. Published 2013 Oct 21. doi:10.1186/1471-2407-13-487
8. Jazieh A, Alkaiyat MO, Ali Y, Hashim MA, Abdelhafiz N, Al Olayan A. Improving adherence to lung cancer guidelines: a quality improvement project that uses chart review, audit and feedback approach. BMJ Open Qual. 2019;8(3):e000436. Published 2019 Aug 26. doi:10.1136/bmjoq-2018-000436
9. Shaverdian N, Offin MD, Rimner A, et al. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiother Oncol. 2020;144:101-104. doi:10.1016/j.radonc.2019.11.015
10. National Cancer Institute. SEER cancer statistics review, 1975-2015, Table 15.1 cancer of the lung and bronchus. Accessed October 19, 2020 https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_15_lung_bronchus.pdf. Updated September 10, 2018
11. Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37(22):1863-1867. doi:10.1200/JCO.18.02118
12. Driessen EJM, Janssen-Heijnen MLG, Maas HA, Dingemans AC, van Loon JGM. Study protocol of the NVALT25-ELDAPT trial: selecting the optimal treatment for older patients with stage III non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e849-e852. doi:10.1016/j.cllc.2018.07.003
13. Schulkes KJ, Nguyen C, van den Bos F, van Elden LJ, Hamaker ME. Selection of Patients in Ongoing Clinical Trials on Lung Cancer. Lung. 2016;194(6):967-974. doi:10.1007/s00408-016-9943-7
14. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763-1774. doi:10.1007/s12325-018-0805-y
15. Garrison LP Jr, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report. Value Health. 2007;10(5):326-335. doi:10.1111/j.1524-4733.2007.00186.x
16. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
17. Shahoumian TA, Phillips BR, Backus LI. Cigarette smoking, reduction and quit attempts: prevalence among veterans with coronary heart disease. Prev Chronic Dis. 2016;13:E41. Published 2016 Mar 24. doi:10.5888/pcd13.150282
18. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
19. Kozel FA, Didehbani N, DeLaRosa B, et al. Factors impacting functional status in veterans of recent conflicts with PTSD. J Neuropsychiatry Clin Neurosci. 2016;28(2):112-117. doi:10.1176/appi.neuropsych.15070183
The US Food and Drug Administration (FDA) approved the use of durvalumab for patients with unresectable stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (CRT).1 After 2 randomized phase 3 studies in 2017 and 2018 showed significant progression-free and overall survival respectively,2,3 durvalumab became a category 1 recommendation for the above indication per National Comprehensive Cancer Network (NCCN) guidelines.4 Adherence to guidelines have been shown to improve patient survival across several cancer types.5-7 However, guideline adherence rates have been variable across health institutions. Therefore, further study is warranted to evaluate nonadherent practices with the goal of improving the quality of cancer care delivery.8,9
Stage III NSCLC is associated with poor survival rates.10 Concurrent CRT remains the standard of care in patients with good performance status based on clinical trial populations.4 Lung cancer remains a disease of the elderly, with a median age at diagnosis of 70 years.11 Discrepancies in the treatment of lung cancer in older adults can vary widely due to a lack of evidence surrounding the treatment in those who have comorbidities and poor performance status, widening the gap between clinical trial and real-world populations.11
A recent review by Passaro and colleagues revealed that at least 11 pivotal randomized controlled trials have shown the activity of immune checkpoint inhibitors (ICI) in locally advanced and metastatic lung cancer. However, these studies have mostly excluded patients with a performance status of the Eastern Cooperative Oncology Group (ECOG) level ≥ 2.11
Durvalumab is one of many new therapies to enter clinical practice to demonstrate survival benefit, but its use among veterans with stage III NSCLC in adherence with National Comprehensive Cancer Network (NCCN) guidelines was not robust at the Birmingham Veterans Affairs Medical Center (VAMC) in Alabama. Therefore, we decided to study the level of adherence and to identify barriers to conformity to the category 1 NCCN recommendations.
Methods
The Birmingham VAMC Outpatient Oncology Clinic billing data identified all individuals diagnosed with lung cancer treated between October 2017 and August 2019. Patients who did not have NSCLC that was stage III and unresectable were excluded from our study. Patients who did not receive a majority of their treatment at US Department of Veterans Affairs (VA) facilities were excluded as well. Each patient’s demographic, functional level, and tumor characteristics during the treatment planning phase and follow-up visits were obtained. Two investigators who evaluated health care provider documentation using the VA Computerized Patient Record System (CPRS) conducted chart reviews.
The primary outcomes were the proportion of patients who received concurrent CRT and the proportion who received durvalumab consolidation. Our chart review also categorized reasons for nonreceipt of concurrent CRT and subsequent durvalumab. Documented reasons for guideline discordancy were generated empirically and broadly. We noted if documentation was unclear and included reasons for why a veteran was not a candidate for CRT, the presence of toxicities associated with CRT, and a patient’s refusal for therapy despite medical advice. Descriptive data were analyzed for all clinical or demographic characteristics and outcomes.
This was considered an internal quality improvement initiative. As such, Birmingham VAMC did not require institutional review board approval for the study. The facility is accredited by the American College of Surgeons Commission on Cancer.
Results
A total of 41 veterans with stage III NSCLC were identified to have established care in the Birmingham VAMC Oncology Clinic between October 2017 and August 2019. Of these, 7 received the majority of their treatment from community-based non-VA facilities and 14 were not candidates for CRT and were excluded from this study.
The mean (SD) age of study participants was 70.0 (8.4) years (range, 57 to 92 years). Most of the study veterans (33; 97.1%) were male and 20 (58.8%) were African American (Table). Eighteen (53%) of study participants had clinical stage IIIa NSCLC; 19 (56%) showed a squamous subtype of NSCLC. A majority (53%) of the veterans studied were evaluated to be functionally fit with an ECOG status of 0 to 1, although documentation of ECOG status was lacking in 5 (14.7%) patients in the initial treatment planning visit records. It was unclear if performance status had been reevaluated and changes noted over the course of concurrent CRT.
CRT Patients
The relative distribution of veterans who underwent CRT for stage III NSCLC plus the reasons they did not receive guideline-based treatment with durvalumab is shown in the Figure. Fourteen patients (41%) were inappropriate candidates for CRT; the most common reason for this was their poor performance status upon initial evaluation and 3 patients (8.8%) in the study had extensive disease or were upstaged upon follow-up clinic visit.
Twenty (59%) veterans in the study initiated CRT. However, only 16 (47.1%) completed CRT. Those who dropped out of CRT did so because of toxicities that included various cytopenia, gastrointestinal toxicities due to radiation and/or chemotherapy, or failure to thrive.
Durvalumab Treatment
After initiation of CRT, 9 (26.5%) patients did not go on to receive durvalumab. Three patients (8.8%) suffered toxicities during CRT. One study patient was found to have a severe respiratory infection requiring intensive care unit admission. Another study patient was found to have a new sternal lesion on follow-up positron emission tomography. One declined because of a history of severe antineutrophil cytoplasmic antibodies vasculitis, which made durvalumab use unsafe. Three patients (8.8%) declined treatment with CRT or durvalumab because of personal preference. Documentation was unclear as to why durvalumab was prescribed to one patient who had completed CRT.
Discussion
NCCN guidelines on the use of durvalumab in NSCLC are based on the phase 3 PACIFIC placebo-controlled randomized clinical trial. This trial, which included only patients with documented performance status of ECOG 0 or 1, reported that grade 3 or 4 events occurred in 30.5% of patients randomized to consolidative durvalumab. Treatment was discontinued in 15.4% of patients due to adverse events.3
Our study examined consolidation therapy with durvalumab in patients with unresectable stage III NSCLC with an ECOG performance status of 0 to 1 who had not progressed after 2 or more cycles of definitive concurrent CRT.4 Patients with previous exposure to immunotherapy, a history of immunodeficiency, active infection, unresolved toxicity from CRT, autoimmune disease, and patients who received sequential CRT were excluded.2 Surprisingly, the adherence rate to guidelines was close to 100% with appropriate documentation and justification of CRT initiation and durvalumab use. Five (14.7%) of veterans with unresectable stage III NSCLC did not have clear documentation of ECOG status on initial visit and only 1 veteran who completed CRT did not have clear documentation as to why durvalumab was not provided. Unfortunately, 23 (68.6%) veterans in the study were unable to receive durvalumab, a potentially disease-modifying drug; nearly one-third (10) of veterans were deemed poor candidates for concurrent CRT despite the fact that 52.9% (18) of veterans in the study had a documented ECOG of 0 or 1 on initial evaluation.
Clinical Trials vs Real World
The heterogeneity between anticipated study populations, those who were able to receive durvalumab in the PACIFIC trial, compared with our observed real-world veteran population, likely stems from the lack of information about how comorbidity and fitness can affect the choice of therapeutic intervention in patients with lung cancer.12 In addition, older adults who participated in randomized controlled trials (RCTs) are not representative of the average older adult who presents to medical oncology clinics, making the application of guideline concordant care difficult.13
Similar real-world observations parallel to our analyses have confirmed, complemented and/or refuted findings of RCTs, and have helped impact the treatment of multiple acute and chronic conditions including influenza, cardiovascular disease, and diabetes.14
A component of socioeconomic barriers and access to supportive care played roles in the decisions of certain patients who chose not to undergo concurrent CRT despite medical advice. These 2 obstacles also affected the decision making for some in the study when considering the use of durvalumab (administered by a 60-minute IV infusion every 2 weeks for 1 year) per recommended guidelines.1 These hurdles need further study in the context of their effect on quality of life and the difficulties generated by various social determinants of health.
Limitations
Study limitations included the biased and confounding factors previously described about retrospective and nonrandomized observational studies that are controlled for during RCTs.15 Electronic health record data may have been incorrectly collected resulting in missing or wrong data points that affect the validity of our conclusion. Recall bias with regard to documentation by health care providers describing reasons why CRT or durvalumab were not initiated or the patient’s ability to recall previous treatments and report ECOG status or toxicities also may have impacted our findings. Comorbidities and poor performance status, frequently occurring among veterans, negatively impact cancer treatment decisions and may result in a detection bias. For example, tobacco use, cardiovascular disease, including heart failure, and chronic obstructive pulmonary disease, are notoriously higher in the US veteran population when compared with civilian cohorts.16-18 Also, veterans with poorly controlled depression and posttraumatic stress disorder resulting in functional impairment are a factor.19 Steps were taken to address some of these biases by performing repeat checks of tabulated data and employing 2 independent reviewers to evaluate all relevant clinical documentation, compare results, and reach a consensus.
Conlcusions
This retrospective analysis of adherence to category 1 NCCN guidelines for durvalumab use among patients at the Birmingham VAMC Oncology Clinic reinforced our practice and identified minor deficiencies in documentation that would impact future clinical visits. More importantly, it depicted the massive disparity in treatment candidacy among Birmingham veterans compared with clinical trial populations. Efforts will be made to address factors impacting a veteran’s candidacy for CRT and explore other variables such as socioeconomic barriers to treatment. Multiple complementary tools to assess patients’ frailty, such as the Charlson Comorbidity Index (CCI), are now being used for a variety of disorders including cancers. More robust data and standardization are needed to validate the use of these assessments in predicting response to immune checkpoint inhibitors.
Immune checkpoint inhibitors are currently being evaluated in stage III NSCLC studies and may be implemented as routine practice in the future.12 It is important to distinguish fit from frail veterans with lung cancer for treatment selection. We would like to see the expansion of the eligibility criteria for clinical trials to include patients with a performance status of ECOG 2 in order for results to be truly generalizable to the real-world population. Our hope is that such work will improve not only the quality of lung cancer care, but also the quality of care across multiple tumor types.
The US Food and Drug Administration (FDA) approved the use of durvalumab for patients with unresectable stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (CRT).1 After 2 randomized phase 3 studies in 2017 and 2018 showed significant progression-free and overall survival respectively,2,3 durvalumab became a category 1 recommendation for the above indication per National Comprehensive Cancer Network (NCCN) guidelines.4 Adherence to guidelines have been shown to improve patient survival across several cancer types.5-7 However, guideline adherence rates have been variable across health institutions. Therefore, further study is warranted to evaluate nonadherent practices with the goal of improving the quality of cancer care delivery.8,9
Stage III NSCLC is associated with poor survival rates.10 Concurrent CRT remains the standard of care in patients with good performance status based on clinical trial populations.4 Lung cancer remains a disease of the elderly, with a median age at diagnosis of 70 years.11 Discrepancies in the treatment of lung cancer in older adults can vary widely due to a lack of evidence surrounding the treatment in those who have comorbidities and poor performance status, widening the gap between clinical trial and real-world populations.11
A recent review by Passaro and colleagues revealed that at least 11 pivotal randomized controlled trials have shown the activity of immune checkpoint inhibitors (ICI) in locally advanced and metastatic lung cancer. However, these studies have mostly excluded patients with a performance status of the Eastern Cooperative Oncology Group (ECOG) level ≥ 2.11
Durvalumab is one of many new therapies to enter clinical practice to demonstrate survival benefit, but its use among veterans with stage III NSCLC in adherence with National Comprehensive Cancer Network (NCCN) guidelines was not robust at the Birmingham Veterans Affairs Medical Center (VAMC) in Alabama. Therefore, we decided to study the level of adherence and to identify barriers to conformity to the category 1 NCCN recommendations.
Methods
The Birmingham VAMC Outpatient Oncology Clinic billing data identified all individuals diagnosed with lung cancer treated between October 2017 and August 2019. Patients who did not have NSCLC that was stage III and unresectable were excluded from our study. Patients who did not receive a majority of their treatment at US Department of Veterans Affairs (VA) facilities were excluded as well. Each patient’s demographic, functional level, and tumor characteristics during the treatment planning phase and follow-up visits were obtained. Two investigators who evaluated health care provider documentation using the VA Computerized Patient Record System (CPRS) conducted chart reviews.
The primary outcomes were the proportion of patients who received concurrent CRT and the proportion who received durvalumab consolidation. Our chart review also categorized reasons for nonreceipt of concurrent CRT and subsequent durvalumab. Documented reasons for guideline discordancy were generated empirically and broadly. We noted if documentation was unclear and included reasons for why a veteran was not a candidate for CRT, the presence of toxicities associated with CRT, and a patient’s refusal for therapy despite medical advice. Descriptive data were analyzed for all clinical or demographic characteristics and outcomes.
This was considered an internal quality improvement initiative. As such, Birmingham VAMC did not require institutional review board approval for the study. The facility is accredited by the American College of Surgeons Commission on Cancer.
Results
A total of 41 veterans with stage III NSCLC were identified to have established care in the Birmingham VAMC Oncology Clinic between October 2017 and August 2019. Of these, 7 received the majority of their treatment from community-based non-VA facilities and 14 were not candidates for CRT and were excluded from this study.
The mean (SD) age of study participants was 70.0 (8.4) years (range, 57 to 92 years). Most of the study veterans (33; 97.1%) were male and 20 (58.8%) were African American (Table). Eighteen (53%) of study participants had clinical stage IIIa NSCLC; 19 (56%) showed a squamous subtype of NSCLC. A majority (53%) of the veterans studied were evaluated to be functionally fit with an ECOG status of 0 to 1, although documentation of ECOG status was lacking in 5 (14.7%) patients in the initial treatment planning visit records. It was unclear if performance status had been reevaluated and changes noted over the course of concurrent CRT.
CRT Patients
The relative distribution of veterans who underwent CRT for stage III NSCLC plus the reasons they did not receive guideline-based treatment with durvalumab is shown in the Figure. Fourteen patients (41%) were inappropriate candidates for CRT; the most common reason for this was their poor performance status upon initial evaluation and 3 patients (8.8%) in the study had extensive disease or were upstaged upon follow-up clinic visit.
Twenty (59%) veterans in the study initiated CRT. However, only 16 (47.1%) completed CRT. Those who dropped out of CRT did so because of toxicities that included various cytopenia, gastrointestinal toxicities due to radiation and/or chemotherapy, or failure to thrive.
Durvalumab Treatment
After initiation of CRT, 9 (26.5%) patients did not go on to receive durvalumab. Three patients (8.8%) suffered toxicities during CRT. One study patient was found to have a severe respiratory infection requiring intensive care unit admission. Another study patient was found to have a new sternal lesion on follow-up positron emission tomography. One declined because of a history of severe antineutrophil cytoplasmic antibodies vasculitis, which made durvalumab use unsafe. Three patients (8.8%) declined treatment with CRT or durvalumab because of personal preference. Documentation was unclear as to why durvalumab was prescribed to one patient who had completed CRT.
Discussion
NCCN guidelines on the use of durvalumab in NSCLC are based on the phase 3 PACIFIC placebo-controlled randomized clinical trial. This trial, which included only patients with documented performance status of ECOG 0 or 1, reported that grade 3 or 4 events occurred in 30.5% of patients randomized to consolidative durvalumab. Treatment was discontinued in 15.4% of patients due to adverse events.3
Our study examined consolidation therapy with durvalumab in patients with unresectable stage III NSCLC with an ECOG performance status of 0 to 1 who had not progressed after 2 or more cycles of definitive concurrent CRT.4 Patients with previous exposure to immunotherapy, a history of immunodeficiency, active infection, unresolved toxicity from CRT, autoimmune disease, and patients who received sequential CRT were excluded.2 Surprisingly, the adherence rate to guidelines was close to 100% with appropriate documentation and justification of CRT initiation and durvalumab use. Five (14.7%) of veterans with unresectable stage III NSCLC did not have clear documentation of ECOG status on initial visit and only 1 veteran who completed CRT did not have clear documentation as to why durvalumab was not provided. Unfortunately, 23 (68.6%) veterans in the study were unable to receive durvalumab, a potentially disease-modifying drug; nearly one-third (10) of veterans were deemed poor candidates for concurrent CRT despite the fact that 52.9% (18) of veterans in the study had a documented ECOG of 0 or 1 on initial evaluation.
Clinical Trials vs Real World
The heterogeneity between anticipated study populations, those who were able to receive durvalumab in the PACIFIC trial, compared with our observed real-world veteran population, likely stems from the lack of information about how comorbidity and fitness can affect the choice of therapeutic intervention in patients with lung cancer.12 In addition, older adults who participated in randomized controlled trials (RCTs) are not representative of the average older adult who presents to medical oncology clinics, making the application of guideline concordant care difficult.13
Similar real-world observations parallel to our analyses have confirmed, complemented and/or refuted findings of RCTs, and have helped impact the treatment of multiple acute and chronic conditions including influenza, cardiovascular disease, and diabetes.14
A component of socioeconomic barriers and access to supportive care played roles in the decisions of certain patients who chose not to undergo concurrent CRT despite medical advice. These 2 obstacles also affected the decision making for some in the study when considering the use of durvalumab (administered by a 60-minute IV infusion every 2 weeks for 1 year) per recommended guidelines.1 These hurdles need further study in the context of their effect on quality of life and the difficulties generated by various social determinants of health.
Limitations
Study limitations included the biased and confounding factors previously described about retrospective and nonrandomized observational studies that are controlled for during RCTs.15 Electronic health record data may have been incorrectly collected resulting in missing or wrong data points that affect the validity of our conclusion. Recall bias with regard to documentation by health care providers describing reasons why CRT or durvalumab were not initiated or the patient’s ability to recall previous treatments and report ECOG status or toxicities also may have impacted our findings. Comorbidities and poor performance status, frequently occurring among veterans, negatively impact cancer treatment decisions and may result in a detection bias. For example, tobacco use, cardiovascular disease, including heart failure, and chronic obstructive pulmonary disease, are notoriously higher in the US veteran population when compared with civilian cohorts.16-18 Also, veterans with poorly controlled depression and posttraumatic stress disorder resulting in functional impairment are a factor.19 Steps were taken to address some of these biases by performing repeat checks of tabulated data and employing 2 independent reviewers to evaluate all relevant clinical documentation, compare results, and reach a consensus.
Conlcusions
This retrospective analysis of adherence to category 1 NCCN guidelines for durvalumab use among patients at the Birmingham VAMC Oncology Clinic reinforced our practice and identified minor deficiencies in documentation that would impact future clinical visits. More importantly, it depicted the massive disparity in treatment candidacy among Birmingham veterans compared with clinical trial populations. Efforts will be made to address factors impacting a veteran’s candidacy for CRT and explore other variables such as socioeconomic barriers to treatment. Multiple complementary tools to assess patients’ frailty, such as the Charlson Comorbidity Index (CCI), are now being used for a variety of disorders including cancers. More robust data and standardization are needed to validate the use of these assessments in predicting response to immune checkpoint inhibitors.
Immune checkpoint inhibitors are currently being evaluated in stage III NSCLC studies and may be implemented as routine practice in the future.12 It is important to distinguish fit from frail veterans with lung cancer for treatment selection. We would like to see the expansion of the eligibility criteria for clinical trials to include patients with a performance status of ECOG 2 in order for results to be truly generalizable to the real-world population. Our hope is that such work will improve not only the quality of lung cancer care, but also the quality of care across multiple tumor types.
1. US Food and Drug Administration. FDA approves durvalumab after chemoradiation for unresectable stage II. Published February 20, 2018. Accessed October 9, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-after-chemoradiation-unresectable-stage-iii-nsclc
2. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi:10.1056/NEJMoa1709937
3. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi:10.1056/NEJMoa1809697
4. Ettinger DS, Wood DE, Aisner DL et al. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version8.2020. Updated September 15, 2020. Accessed October 9, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
5. Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220(5):940-950. doi:10.1016/j.jamcollsurg.2015.01.056
6. Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593-1601. doi:10.1002/cncr.27935
7. Schwentner L, Wöckel A, König J, et al. Adherence to treatment guidelines and survival in triple-negative breast cancer: a retrospective multi-center cohort study with 9,156 patients. BMC Cancer. 2013;13:487. Published 2013 Oct 21. doi:10.1186/1471-2407-13-487
8. Jazieh A, Alkaiyat MO, Ali Y, Hashim MA, Abdelhafiz N, Al Olayan A. Improving adherence to lung cancer guidelines: a quality improvement project that uses chart review, audit and feedback approach. BMJ Open Qual. 2019;8(3):e000436. Published 2019 Aug 26. doi:10.1136/bmjoq-2018-000436
9. Shaverdian N, Offin MD, Rimner A, et al. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiother Oncol. 2020;144:101-104. doi:10.1016/j.radonc.2019.11.015
10. National Cancer Institute. SEER cancer statistics review, 1975-2015, Table 15.1 cancer of the lung and bronchus. Accessed October 19, 2020 https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_15_lung_bronchus.pdf. Updated September 10, 2018
11. Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37(22):1863-1867. doi:10.1200/JCO.18.02118
12. Driessen EJM, Janssen-Heijnen MLG, Maas HA, Dingemans AC, van Loon JGM. Study protocol of the NVALT25-ELDAPT trial: selecting the optimal treatment for older patients with stage III non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e849-e852. doi:10.1016/j.cllc.2018.07.003
13. Schulkes KJ, Nguyen C, van den Bos F, van Elden LJ, Hamaker ME. Selection of Patients in Ongoing Clinical Trials on Lung Cancer. Lung. 2016;194(6):967-974. doi:10.1007/s00408-016-9943-7
14. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763-1774. doi:10.1007/s12325-018-0805-y
15. Garrison LP Jr, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report. Value Health. 2007;10(5):326-335. doi:10.1111/j.1524-4733.2007.00186.x
16. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
17. Shahoumian TA, Phillips BR, Backus LI. Cigarette smoking, reduction and quit attempts: prevalence among veterans with coronary heart disease. Prev Chronic Dis. 2016;13:E41. Published 2016 Mar 24. doi:10.5888/pcd13.150282
18. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
19. Kozel FA, Didehbani N, DeLaRosa B, et al. Factors impacting functional status in veterans of recent conflicts with PTSD. J Neuropsychiatry Clin Neurosci. 2016;28(2):112-117. doi:10.1176/appi.neuropsych.15070183
1. US Food and Drug Administration. FDA approves durvalumab after chemoradiation for unresectable stage II. Published February 20, 2018. Accessed October 9, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-after-chemoradiation-unresectable-stage-iii-nsclc
2. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi:10.1056/NEJMoa1709937
3. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. doi:10.1056/NEJMoa1809697
4. Ettinger DS, Wood DE, Aisner DL et al. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version8.2020. Updated September 15, 2020. Accessed October 9, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
5. Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220(5):940-950. doi:10.1016/j.jamcollsurg.2015.01.056
6. Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593-1601. doi:10.1002/cncr.27935
7. Schwentner L, Wöckel A, König J, et al. Adherence to treatment guidelines and survival in triple-negative breast cancer: a retrospective multi-center cohort study with 9,156 patients. BMC Cancer. 2013;13:487. Published 2013 Oct 21. doi:10.1186/1471-2407-13-487
8. Jazieh A, Alkaiyat MO, Ali Y, Hashim MA, Abdelhafiz N, Al Olayan A. Improving adherence to lung cancer guidelines: a quality improvement project that uses chart review, audit and feedback approach. BMJ Open Qual. 2019;8(3):e000436. Published 2019 Aug 26. doi:10.1136/bmjoq-2018-000436
9. Shaverdian N, Offin MD, Rimner A, et al. Utilization and factors precluding the initiation of consolidative durvalumab in unresectable stage III non-small cell lung cancer. Radiother Oncol. 2020;144:101-104. doi:10.1016/j.radonc.2019.11.015
10. National Cancer Institute. SEER cancer statistics review, 1975-2015, Table 15.1 cancer of the lung and bronchus. Accessed October 19, 2020 https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_15_lung_bronchus.pdf. Updated September 10, 2018
11. Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37(22):1863-1867. doi:10.1200/JCO.18.02118
12. Driessen EJM, Janssen-Heijnen MLG, Maas HA, Dingemans AC, van Loon JGM. Study protocol of the NVALT25-ELDAPT trial: selecting the optimal treatment for older patients with stage III non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e849-e852. doi:10.1016/j.cllc.2018.07.003
13. Schulkes KJ, Nguyen C, van den Bos F, van Elden LJ, Hamaker ME. Selection of Patients in Ongoing Clinical Trials on Lung Cancer. Lung. 2016;194(6):967-974. doi:10.1007/s00408-016-9943-7
14. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763-1774. doi:10.1007/s12325-018-0805-y
15. Garrison LP Jr, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force report. Value Health. 2007;10(5):326-335. doi:10.1111/j.1524-4733.2007.00186.x
16. Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709.
17. Shahoumian TA, Phillips BR, Backus LI. Cigarette smoking, reduction and quit attempts: prevalence among veterans with coronary heart disease. Prev Chronic Dis. 2016;13:E41. Published 2016 Mar 24. doi:10.5888/pcd13.150282
18. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
19. Kozel FA, Didehbani N, DeLaRosa B, et al. Factors impacting functional status in veterans of recent conflicts with PTSD. J Neuropsychiatry Clin Neurosci. 2016;28(2):112-117. doi:10.1176/appi.neuropsych.15070183
FIT unfit for inpatient, emergency settings
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Leadership & Professional Development: Fighting Reputational Inertia
“Becoming is better than being.”
—Carol Dweck
The words spoken about her in the staff meeting were flattering. She’d just been acknowledged with a departmental teaching award for the second year in a row. With only 3 years under her belt since completing training, the former chief resident was living up to all they’d anticipated.
Eager students requested to be on her team and colleagues delighted in sharing patients with her. “Great, as always,” her peers and learners said in hallways and evaluations. This would come to define her identity.
Things were going well. She was succeeding. But she began to wonder if this reciprocating engine of accolades represented who she truly was. Was she really that good? Was she an imposter? In her performance meetings, the feedback never wavered: “Great, as always.”
The following year she would leave for a different job.
THE THREAT OF REPUTATIONAL INERTIA
While specific plans for growth and improvement often get laid out for struggling colleagues and learners, far less effort is devoted to coaching high performers. Feedback that consists of nonspecific compliments may hinder potential, growth, and job satisfaction. We outline strategies for preventing this professional plateau in those you lead.
ENCOURAGE A GROWTH MINDSET
In Mindset: The New Psychology of Success, psychologist Carol Dweck describes how emphasis on qualities such as “being smart” or, in this example, “great,” underscores this “fixed mindset” that certain attributes are set in stone.1 Conversely, she defines the “growth mindset” as a belief that potential can be cultivated through efforts. Even when there aren’t obvious issues with performance, the failure, fine-tuning, and feedback necessary for resilience and, ultimately, sustained growth require intention.
Emphasize Effort
Instead of lauding an individual for being “great, as always,” consider focusing on the effort it required to get there. For example, regarding the aforementioned junior colleague who’d just won awards, a typical compliment might be: “Wow, you’re on fire!” An option, to promote a growth mindset, might be: “You work very hard at bedside teaching and innovative curriculum development. I’m happy to see that our learners and department have recognized your commitment and effort.” This language also affirms others and makes achievements seem attainable to all.
Provide Active Coaching
Identifying specific opportunities for development can challenge individuals to expand their skills. Even those who are doing well have room to become even better. Coproduction of new milestones that push beyond current comfort zones can acknowledge current achievements while encouraging continued growth—and make things personal. For example, encouraging an individual to apply to a national faculty development program, such as the Society of Hospital Medicine’s Academic Hospitalist Academy, could help them expand their skills and social network.
Offer Meaningful Feedback
Prioritizing feedback is essential for growth and peak performance. This can be particularly powerful when the observer moves beyond basic expectations to incorporate personal goals. Concrete feedback measured against individual potential then takes the place of nondescript compliments. For example, you could say: “Your teaching on systolic ejection murmurs was on target for the students. Next time I want to challenge you to broaden your teaching script to include points appropriate for more seasoned learners.” This feedback leaves them with a set of tailored “marching orders” to guide practice and improvement.
CONCLUSION
No matter where a person stands on the spectrum of performance, growth in medicine relies on deliberate practice, active coaching, meaningful feedback, and graduated opportunities. Even the most proficient among us can stagnate without these things. If we aren’t careful, this reputational inertia could amplify imposter syndrome, prevent individuals from achieving their full potential, and threaten faculty retention. Intentional work toward a growth mindset allows everyone to grow—and be seen.
Disclosures
The authors have nothing to disclose.
1. Dweck CS. Mindset: The New Psychology of Success. New York: Ballantine Books; 2008.
“Becoming is better than being.”
—Carol Dweck
The words spoken about her in the staff meeting were flattering. She’d just been acknowledged with a departmental teaching award for the second year in a row. With only 3 years under her belt since completing training, the former chief resident was living up to all they’d anticipated.
Eager students requested to be on her team and colleagues delighted in sharing patients with her. “Great, as always,” her peers and learners said in hallways and evaluations. This would come to define her identity.
Things were going well. She was succeeding. But she began to wonder if this reciprocating engine of accolades represented who she truly was. Was she really that good? Was she an imposter? In her performance meetings, the feedback never wavered: “Great, as always.”
The following year she would leave for a different job.
THE THREAT OF REPUTATIONAL INERTIA
While specific plans for growth and improvement often get laid out for struggling colleagues and learners, far less effort is devoted to coaching high performers. Feedback that consists of nonspecific compliments may hinder potential, growth, and job satisfaction. We outline strategies for preventing this professional plateau in those you lead.
ENCOURAGE A GROWTH MINDSET
In Mindset: The New Psychology of Success, psychologist Carol Dweck describes how emphasis on qualities such as “being smart” or, in this example, “great,” underscores this “fixed mindset” that certain attributes are set in stone.1 Conversely, she defines the “growth mindset” as a belief that potential can be cultivated through efforts. Even when there aren’t obvious issues with performance, the failure, fine-tuning, and feedback necessary for resilience and, ultimately, sustained growth require intention.
Emphasize Effort
Instead of lauding an individual for being “great, as always,” consider focusing on the effort it required to get there. For example, regarding the aforementioned junior colleague who’d just won awards, a typical compliment might be: “Wow, you’re on fire!” An option, to promote a growth mindset, might be: “You work very hard at bedside teaching and innovative curriculum development. I’m happy to see that our learners and department have recognized your commitment and effort.” This language also affirms others and makes achievements seem attainable to all.
Provide Active Coaching
Identifying specific opportunities for development can challenge individuals to expand their skills. Even those who are doing well have room to become even better. Coproduction of new milestones that push beyond current comfort zones can acknowledge current achievements while encouraging continued growth—and make things personal. For example, encouraging an individual to apply to a national faculty development program, such as the Society of Hospital Medicine’s Academic Hospitalist Academy, could help them expand their skills and social network.
Offer Meaningful Feedback
Prioritizing feedback is essential for growth and peak performance. This can be particularly powerful when the observer moves beyond basic expectations to incorporate personal goals. Concrete feedback measured against individual potential then takes the place of nondescript compliments. For example, you could say: “Your teaching on systolic ejection murmurs was on target for the students. Next time I want to challenge you to broaden your teaching script to include points appropriate for more seasoned learners.” This feedback leaves them with a set of tailored “marching orders” to guide practice and improvement.
CONCLUSION
No matter where a person stands on the spectrum of performance, growth in medicine relies on deliberate practice, active coaching, meaningful feedback, and graduated opportunities. Even the most proficient among us can stagnate without these things. If we aren’t careful, this reputational inertia could amplify imposter syndrome, prevent individuals from achieving their full potential, and threaten faculty retention. Intentional work toward a growth mindset allows everyone to grow—and be seen.
Disclosures
The authors have nothing to disclose.
“Becoming is better than being.”
—Carol Dweck
The words spoken about her in the staff meeting were flattering. She’d just been acknowledged with a departmental teaching award for the second year in a row. With only 3 years under her belt since completing training, the former chief resident was living up to all they’d anticipated.
Eager students requested to be on her team and colleagues delighted in sharing patients with her. “Great, as always,” her peers and learners said in hallways and evaluations. This would come to define her identity.
Things were going well. She was succeeding. But she began to wonder if this reciprocating engine of accolades represented who she truly was. Was she really that good? Was she an imposter? In her performance meetings, the feedback never wavered: “Great, as always.”
The following year she would leave for a different job.
THE THREAT OF REPUTATIONAL INERTIA
While specific plans for growth and improvement often get laid out for struggling colleagues and learners, far less effort is devoted to coaching high performers. Feedback that consists of nonspecific compliments may hinder potential, growth, and job satisfaction. We outline strategies for preventing this professional plateau in those you lead.
ENCOURAGE A GROWTH MINDSET
In Mindset: The New Psychology of Success, psychologist Carol Dweck describes how emphasis on qualities such as “being smart” or, in this example, “great,” underscores this “fixed mindset” that certain attributes are set in stone.1 Conversely, she defines the “growth mindset” as a belief that potential can be cultivated through efforts. Even when there aren’t obvious issues with performance, the failure, fine-tuning, and feedback necessary for resilience and, ultimately, sustained growth require intention.
Emphasize Effort
Instead of lauding an individual for being “great, as always,” consider focusing on the effort it required to get there. For example, regarding the aforementioned junior colleague who’d just won awards, a typical compliment might be: “Wow, you’re on fire!” An option, to promote a growth mindset, might be: “You work very hard at bedside teaching and innovative curriculum development. I’m happy to see that our learners and department have recognized your commitment and effort.” This language also affirms others and makes achievements seem attainable to all.
Provide Active Coaching
Identifying specific opportunities for development can challenge individuals to expand their skills. Even those who are doing well have room to become even better. Coproduction of new milestones that push beyond current comfort zones can acknowledge current achievements while encouraging continued growth—and make things personal. For example, encouraging an individual to apply to a national faculty development program, such as the Society of Hospital Medicine’s Academic Hospitalist Academy, could help them expand their skills and social network.
Offer Meaningful Feedback
Prioritizing feedback is essential for growth and peak performance. This can be particularly powerful when the observer moves beyond basic expectations to incorporate personal goals. Concrete feedback measured against individual potential then takes the place of nondescript compliments. For example, you could say: “Your teaching on systolic ejection murmurs was on target for the students. Next time I want to challenge you to broaden your teaching script to include points appropriate for more seasoned learners.” This feedback leaves them with a set of tailored “marching orders” to guide practice and improvement.
CONCLUSION
No matter where a person stands on the spectrum of performance, growth in medicine relies on deliberate practice, active coaching, meaningful feedback, and graduated opportunities. Even the most proficient among us can stagnate without these things. If we aren’t careful, this reputational inertia could amplify imposter syndrome, prevent individuals from achieving their full potential, and threaten faculty retention. Intentional work toward a growth mindset allows everyone to grow—and be seen.
Disclosures
The authors have nothing to disclose.
1. Dweck CS. Mindset: The New Psychology of Success. New York: Ballantine Books; 2008.
1. Dweck CS. Mindset: The New Psychology of Success. New York: Ballantine Books; 2008.
© 2020 Society of Hospital Medicine